Note: Descriptions are shown in the official language in which they were submitted.
CA 02725862 2010-12-17
PPC5337USNP1
PRODUCT DISPENSER AND ABSORBENT ARTICLE KIT
FIELD OF THE INVENTION
The present invention relates to a product dispenser for dispensing a personal
care
composition, and in particular for a product dispenser for dispensing and
applying such a
composition to a surface of the human body. The dispenser according to the
present invention is
particularly usefully for applying such a composition to the intimate area of
the body. The
present invention also relates to compositions for repelling fluid from a
surface, in particular a
surface of the human body. The compositions of the present invention comprise
a volatile
carrier, a powder-feel agent, and an ester, and are useful, for example, in
those areas of the
human body which are prone to wetness, such as the intimate area. The
compositions of the
present invention further tend to aid in preventing odor, skin irritation and
chafing associated
with exposure to moisture or fluid. The present invention further relates to a
kit including a
product dispenser of the type described above in combination with an absorbent
article.
BACKGROUND OF THE INVENTION
The intimate area and other surfaces of the body which come in regular contact
with fluid
can be a source of discomfort and embarrassment in both men and women. Such
discomfort and
embarrassment may be associated, for example, with adults that suffer from
incontinence, and the
regular monthly cycles of women during their reproductive years. In both these
cases, the
discomfort is generally related to irritation and the feeling of wetness and
the embarrassment is
usually due to the presence of odor. Also, in children, especially infants,
the intimate area can
become irritated due to the contact of urine and feces with the skin.
1
CA 02725862 2010-12-17
PPC5337USNP1
To overcome these issues, products have been developed which either absorb
fluids or
wick the fluid away from the body. Examples of such types of products include
sanitary
protection articles, diapers and incontinence products. Over the years,
several improvements have
been made to such products to aid in absorbing or wicking fluid, for example,
superabsorbent
material has been added to the constructions, new materials have been
developed for the cover
layer, and transfer layers added to help wick the fluid into the absorbent
layers. Additionally,
odor control agents have been incorporated to absorb or mask the odor.
Inclusion of fragrances
may also add additional odor control. All these improvements are based on an
external absorbent
product which wicks away the fluid or moisture.
Additionally, people for years have used products such as zinc oxide, oil or
petrolatum to
repel fluid from their skin. These products, while performing quite well at
providing a water-
proof barrier also left an undesirable sticky feel to the skin.
Examples of other compositions designed to overcome the sticky feel can be
found in US
6200964 and US 6384023 (both to Singleton et al.) and US 20060159645 (Miller
et al.). These
references all use a volatile liquid and a silicone polymer.
Nevertheless, applicants have recognized the need for compositions that are
more
effective at repelling fluid from a surface, such as the surface of a human
body than prior
compositions, and preferably overcome the sticky feel associated with prior
compositions as well.
In addition, applicants have recognized the need for a product dispenser that
can be used
effectively dispense and apply such compositions to the body in a discreet,
sanitary and simple
fashion.
2
CA 02725862 2010-12-17
PPC5337USNP1
SUMMARY OF THE INVENTION
In view of the foregoing the present invention provides, according to a first
aspect of the
invention, a kit including a product dispenser assembly and at least one
absorbent article, the
product dispenser assembly including a container housing having a head portion
and an internal
chamber for holding a composition, the head portion including a plurality of
apertures, a
moveable member arranged within the container housing that is selectively
moveable by a user
within the container to allow a user to selectively manipulate a volume of the
internal chamber,
the moveable member being adapted to enable a user to selectively deploy the
composition from
within the internal chamber, through the apertures, by reducing the volume
within the chamber, a
cap assembly structured and arranged to be placed on top of the head portion
of the container, the
cap assembly including an outer shell having a first open end and a second
closed end, a first
absorbent pad arranged on top of the head portion for receiving the
composition as it is deployed
from the container, the first removable pad being adapted to enable a user to
apply the
composition to the body and manually remove the pad from the head portion
after the
composition has been applied, a plurality of absorbent pads arranged in a
stacked configuration
within the outer shell, each of the plurality of pads being adapted to be
sequentially arranged on
top of the head portion after the first removable pad has been used to apply
the composition and
then removed from the head portion by the user.
The present invention provides, according to a second aspect of the invention,
a kit
including, a product dispenser assembly, and at least one absorbent article,
the product dispenser
assembly including a container housing having a head portion and an internal
chamber for
3
CA 02725862 2010-12-17
PPC5337USNP1
holding a composition, the head portion including at least one aperture, the
container housing
being structured and arranged to enable a user to selectively deploy the
composition from within
the internal chamber through the at least one aperture, a cap assembly
structured and arranged to
be placed on top of the head portion of the container, the cap assembly
including an outer shell
having a first open end and a second closed end, a first absorbent pad
arranged on top of the head
portion for receiving the composition as it is deployed from the container,
the first removable
pad being adapted to enable a user to apply the composition to the body and
manually remove
the pad from the head portion after the composition has been applied, a
plurality of absorbent
pads arranged in a stacked configuration within the outer shell, each of the
plurality of pads
being adapted to be sequentially arranged on top of the head portion after the
first removable pad
has been used to apply the composition and then removed from the head portion
by the user.
4
CA 02725862 2010-12-17
PPC5337USNP1
BRIEF DESCRIPTION OF THE DRAWINGS
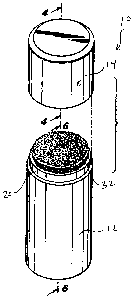
FIG. 1 is a perspective view of a product dispenser assembly according to a
first
embodiment the present invention;
FIG. 2 is a partially exploded view of the product dispenser assembly shown in
FIG. 1
showing the cap assembly removed from the container housing;
FIG. 3 is a detailed perspective view of the cap assembly showing the manner
in which a
plurality of absorbent pads are arranged within the outer shell of the cap
assembly;
FIG. 4 is a sectional view of the cap assembly taken along line 4-4 in FIG. 2;
FIG. 5 is a partially exploded perspective view showing the manner in which
one of the
absorbent pads is arranged on the head portion of the container housing;
FIG. 5a is a detailed perspective view of the head portion, according to one
embodiment
of the invention, showing the encircled region of head portion in FIG. 5;
FIG. 6 is a sectional view of the container housing and absorbent pad taken
along line 6-6
in FIG. 2;
FIG. 7 is a detailed sectional view of container housing and absorbent pad,
showing the
encircled portion in FIG. 6, and depicting the manner in which the composition
contained within
the container housing permeates through the absorbent pad during use of the
assembly;
FIG. 8 and 9 are partial sectional views of the cap assembly and container
housing
showing the manner in which a clean pad is retained on the head portion of
container housing
when the cap assembly is removed from the container housing;
5
CA 02725862 2010-12-17
PPC5337USNP1
FIG. 10 is a perspective view of an absorbent pad and container housing
according to an
alternate embodiment of the present invention;
FIG. 11 is a detailed sectional view taken along line 11-11 in FIG. 10 and
depicting the
manner in which the composition contained within the container housing passes
through the
apertures of the absorbent pad;
FIG. 12-14 are perspective views of a product dispenser assembly according to
a second
embodiment the present invention, depicting the manner in which an absorbent
pad can be
manually removed from the cap assembly and applied to the head por tion of the
container
housing.
15
6
CA 02725862 2010-12-17
PPC5337USNP 1
DETAILED DESCRIPTION OF THE INVENTION
It is believed that one skilled in the art can, based upon the description
herein, utilize the
present invention to its fullest extent. The following specific embodiments
are to be construed as
merely illustrative, and not limitative of the remainder of the disclosure in
any way whatsoever.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
the invention
belongs. Also, all publications, patent applications, patents, and other
references mentioned
herein are incorporated by reference.
All percentages listed in this specification are percentages by weight, unless
otherwise
specifically mentioned.
As used herein, the term "intimate area" shall mean the area near or between
the thighs,
including the crotch area of a human where body exudates, such as urine,
feces, vaginal
discharge, menstrual fluid, and the like, may be present. The intimate area
shall also include the
breasts. The intimate area is typically covered by undergarments or absorbent
articles.
As used herein the term "absorbent articles" includes articles such as diapers
(infant and
adult), sanitary napkins, shields, pantyliners, breast pads, and the like.
Referring now to the drawings, a product dispenser assembly 10 according to a
first
embodiment of the invention is shown in Figs. 1-11. As seen in Fig. 1, the
product dispenser
assembly 10, generally includes a container housing 12 and a removable cap
assembly 14 to
protect the composition contained within the housing 12.
As best seen in Fig. 6, the container housing 12 defines an internal chamber
16 adapted to
hold a composition 18. Preferably the composition 18 is in the form of a
liquid or gel. The
container housing 12 further includes a head portion 20 that includes a
plurality of apertures 22
7
CA 02725862 2010-12-17
PPC5337USNP1
as best seen in Figs. 6 and 7. As described in further detail below, the
plurality of apertures 22
permit a user to selectively deploy the composition from within the internal
chamber 16 through
the apertures 22.
As seen in Fig. 6, the product dispenser assembly 10 further includes a
moveable member
24 arranged within the container housing 12 and adapted to enable a user to
selectively
manipulate a volume of the internal chamber 16. By manipulating the volume of
the internal
chamber 16, and specifically by reducing the volume of the internal chamber, a
user can
selectively deploy the composition 18 through the apertures 22. A central
portion of the movable
member 24 is provided with a threaded coupling sleeve 26 for cooperation with
an elevator
screw 28. The lower end of the elevator screw 28 is axially fixed but
rotatable within an opening
29 of the container housing 12. The lower end of elevator screw 28 is
operatively coupled to a
knob 30. Rotation of the knob 30 permits the user to raise or lower the
moveable member 24
relative to the container housing 12. In this manner, the user can manually
control the volume
within the internal chamber 16 and thereby deploy the composition 18 from
within the chamber
16 through the apertures 22.
As best seen in Fig. 2 and Fig. 5, the product dispenser assembly 10 further
includes a
first absorbent pad 32 that is arranged on top of the head portion 20 of the
container housing 12.
As shown in Fig. 7, the first absorbent pad 32 is adapted to receive the
composition 18 as it is
deployed from the internal chamber 16 and through the apertures 22. As will be
described in
greater detail below, the absorbent pad 32 is adapted temporarily absorb and
retain the
composition to enable a user to apply the composition 18 to the body of the
user by placing the
pad 32 in contact with the body.
8
CA 02725862 2010-12-17
PPC5337USNP 1
As seen in Fig. 5a, a top surface 34 of the head portion 20 may be provided
with a
material including a plurality of hooks 36 adapted to retain the absorbent pad
32 in place while
the user applies the composition 18 to the body. The material including the
plurality of hooks 36
also permits a user to manually remove the absorbent pad 32 from the head
portion 20, and
dispose of the same, after the pad 32 has been used to apply the composition
18 to the body.
Particularly suitable hook materials of the type referred to above are
commercially available
from Velcro USA Inc., Manchester, New Hampshire. Other means may also be used
to retain
the pad absorbent pad 32 in place, such as certain adhesives, provided that
such means permit the
user to selectively remove the pad 32 after it has been used to apply the
composition 18 to the
body.
As shown in Fig. 1 the cap assembly 14 is structured and arranged to be placed
on top of
the head portion 20 of the container housing 12. As shown in Figs. 3 and 4,
the cap assembly 14
includes an outer shell 38 that has a first open end 40 and a second opposed
closed end 42. As
shown in Fig. 3, the outer shell 36 is structured and arranged to hold a
plurality of absorbent pads
44 that are arranged in a stacked configuration within the outer shell 36. As
shown in Fig. 4, the
plurality of pads 44 includes a lowermost pad 44a that is arranged closest to
the open end 40 of
the outer shell 38 and a plurality of pads 44b arranged on top of the
lowermost pad 44a. As
shown in Figs. 3 and 4, the outer shell 38 includes a rib structure 46 that
extends outwardly from
an internal surface 48 of the outer shell 38. As will be described in greater
detail below, the rib
structure.46 is adapted to retain the plurality of pads 44 within the cap
assembly 14 until such
time as each of the pads 44 is ready for use.
As shown in Fig. 4, the cap assembly 14 further includes a platform 48
arranged within
the outer shell 38. As will be described in greater detail below, the platform
48 is adapted to
9
CA 02725862 2010-12-17
PPC5337USNP 1
urge the plurality of absorbent pads 44 toward the open end 40 of the outer
shell 38. The cap
assembly 14 further includes a resilient member 50 that is arranged between
the outer shell 38
and the platform 48 and is adapted to urge the platform 48 towards the open
end 40 of the outer
shell 38. The resilient member 50 may comprise, for example, a spring having a
first end
coupled to the outer shell 38 and a second end coupled to the platform 48.
Other suitable
resilient member structures may alternatively be employed provided that they
effectively urge
the platform 46 towards the open end 40 of the outer shell 38.
The first absorbent pad 32 and each of the plurality of absorbent pads 44 are
preferably
formed from a fibrous material. Suitable fibrous materials include, without
limitation, woven,
nonwoven (oriented, e.g., via a carding process, or non-oriented), or knit
fabrics. The fibers may
be integrated into a nonwoven structure via, for example, needle punching,
through-air bonding,
hydro entangling, spun-bonding, chemical bonding (including adhesive bonding),
or mechanical
processing (such as embossing)..
The first absorbent pad 32 and each of the plurality of absorbent pads 44
preferably has a
thickness of about 0.5 mm to about 5 mm, more preferably from about 1 mm to
about 5 mm.
Preferably each absorbent pad has a basis between about 10 gsm (g/m2) and
about 450 gsm,
preferably between about 300 and about 400 gsm. The fibrous material forming
each absorbent
pad preferably includes rayon to provide softness and a strong, resilient
material such as an
olefin or polyester. One particularly suitable fibrous material is a needle-
punched blend of
staple-length 1.5 denier "TENCEL" rayon and staple-length 4-5 denier PET
available from
Precision Custom Coating, Totowa, NJ.
CA 02725862 2010-12-17
PPC5337USNP1
The container housing 12, removable cap assembly 14, moveable member 24,
elevator
screw 28, knob 30, as well as the constituent parts thereof, can be
constructed from any suitable
rigid material. Preferably the container housing 12, removable cap assembly
14, moveable
member 24, elevator screw 28, knob 30, as well as the constituent parts
thereof, are formed from
plastic by molding, although other suitable rigid materials could be used.
Referring to Figs. 10 and 11, the absorbent pad 32 may optionally be provided
with a
plurality of holes 33 intended to promote the flow of the composition through
and into the
absorbent pad 32 as shown in Fig. 11. In the specific embodiment of the
invention shown in
Figs. 10 and 11 the holes 33 are arranged such that they correspond in
location to the apertures
22 in the container housing 12. Of course, it is not required that the
plurality of holes 33 and
apertures 22 correspond exactly in number and/or location, provided that the
holes 33 and
apertures 22 promote the flow of the composition through and into the
absorbent pad 32.
The method of using the product dispenser assembly 10 according to the first
embodiment of the invention will now be described with reference to Figs. 1-9.
First the user
removes the cap assembly 14 from the container housing 12 as shown in Figs. 1
and 2. Once the
cap assembly 14 is removed from the container housing 12 the first absorbent
pad 32 is revealed
as shown in Fig. 2. Thereafter, the user can turn the knob 30 (Fig. 6) thereby
causing the
moveable member 24 to move in an upward direction thereby reducing the volume
of the
internal chamber 16. By reducing the volume of the internal chamber 16, the
composition 18 is
urged through the apertures 22 in the head portion 20 of the container housing
12, as shown in
Fig. 7. As the composition 18 is urged through the apertures 22 such
composition is then
absorbed by the first absorbent pad 32. Thereafter, the user may use the
product- dispenser
assembly 10 to apply the composition to the body by placing the first
absorbent pad 32 in surface
11
CA 02725862 2010-12-17
PPC5337USNP1
to surface contact with the body. The first absorbent pad 32 is retained in
place during
application of the composition 18 to the body by means of the material
including the plurality of
hooks 36 that is arranged on the top surface 34 of the head portion 20, as
seen in Fig. 5 and 5a.
After the first absorbent pad 32 is used to apply the composition 18 to the
body, the user may
manually remove the first absorbent pad 32 from the head portion 20 and
discard of the same.
Thereafter, the user places the cap assembly 14 back on the container housing
12 as shown in
Fig. 8. Once the cap assembly 14 is placed back on the container housing 12,
the lowermost pad
44a of the plurality of pads 44 is placed in abutment with the top surface 34
of the head portion,
and therefore in abutment with the material including the plurality of hooks
36, and the plurality
of hooks 36 engage lowermost pad 44a. Once the user is ready to use the
product dispenser
assembly 10 again, the user removes the cap assembly 14, as shown in Fig. 9.
Upon removal of
the cap assembly 14, the lowermost pad 44a is retained in place on the top
surface 34 of the head
portion 20 by the material including the plurality of hooks 36. In this manner
the lowermost pad
44a is automatically applied to, and retained on, the head portion 20 of the
container housing 12.
As shown in Fig. 9, as the cap assembly 14 is removed from the container
housing 12, the
platform 48 is urged toward the open end 40 of the outer shell 38 by the
resilient member 50. In
this way the remainder of the plurality of absorbent pads 44b are urged
towards the open end 40
of the outer shell 38. The absorbent pads 44b are retained within the cap
assembly 14 by the rib
structure 46. In this manner, a new clean absorbent pad 44 is always ready for
use when the user
removes the cap assembly 14.
A product dispenser assembly I Oa according to a second embodiment of the
invention is
shown in Figs. 12-14. The product dispenser assembly 1 Oa according to second
embodiment is
substantially identical to the product dispenser assembly l Ob described
above. However, the
12
CA 02725862 2010-12-17
PPC5337USNP1
product dispenser assembly 10a does not include the platform 48 or the
resilient member 50.
Rather, when the user desires to replace the used first absorbent pad 32, the
user simply manually
removes a new clean absorbent pad 44 from the cap assembly 14, as shown in
Fig. 13 and
manually applies the clean absorbent pad 44 to the head portion 20 of the
container housing 12
as shown in Fig. 14. To facilitate the manual removal of the clean absorbent
pad 44 from cap
assembly 14, the outer shell 38 is provided with an access port 52 as shown in
Figs. 12 and 13.
The product dispenser assembly 1 Oa is in all other respects identical to the
product dispenser 10
according to the first embodiment as described above.
The product dispenser assemblies described herein are preferred embodiments of
the
present invention, however other embodiments are possible within the scope and
spirit of the
claimed invention. For example, the container housing could be constructed as
a flexible tube
(e.g. like a toothpaste tube) to enable a user to manually deploy the
composition from within the
tube by squeezing the tube. Other embodiments of the invention, within the
spirit and scope of
the claimed invention, will be apparent to those of skill in the art based
upon the disclosure of the
invention made herein.
Personal Care Compositions
Personal care compositions particularly suitable for use with the dispenser
assembly
described above are described in detail below. Although preferred compositions
are described in
detail below, other liquid or gel compositions could be used with the
dispensing device described
above.
13
CA 02725862 2010-12-17
PPC5337USNP1
The composition of the present invention contains at least three components: a
volatile
cyclic silicone, a silicone-based powder-feel agent and an ester selected from
the group
consisting of compounds of Formula I, Formula II, Formula III, and
combinations of two or
more thereof-
0
F'
RI-C-O-R2 (I)
O O
II II
R3-C-O-R4-O-C-O-R5 (II)
O O
II II
R6- C- O - R7- O - C- O - R8 (III)
I
O
0=C
R9
wherein RI, R2, R3, R5, R6, R8 and R9 are independently linear or branched,
substituted or
unsubstituted, saturated or unsaturated, C3-C22 alkyl or alkenyl groups, R4 is
a linear or branched,
substituted or unsubstituted, saturated or unsaturated, C3-C22 alkylene or
alkenylene moiety, and
R7 is a linear or branched, substituted or unsubstituted, saturated or
unsaturated, C3-C22 moiety,
the composition being substantially anhydrous and the ester present in an
amount of about 5% or
less.
14
CA 02725862 2010-12-17
PPC5337USNP1
It has surprisingly been found that application of this composition results in
a greater
repulsion of fluid from the body than previously seen by other comparable
compositions. This
benefit is demonstrated by the measurement of the contact angle of water
placed on a surface that
has been treated with the composition in accord with the Contact Angle Test as
described herein
below. Applicants have discovered unexpectedly, that compositions of the
present invention
tend to exhibit a contact angle of 90 or greater. In certain preferred
embodiments, the
compositions exhibit a contact angle of 91 or greater and more preferably 92
or greater. In
certain particularly preferred embodiments, the compositions exhibit a contact
angle of 93 or
greater.
Applicants have also measured the Body Dryness Index associated with
compositions
and uses of the present invention. Applicants have discovered that the claimed
compositions
provide a significant body dryness as compared to other comparable
compositions.
Applicants have further recognized that in addition to unexpected fluid
repellency, the
compositions of the present invention may further be used on the body to
deliver an aesthetically
pleasing feel to the skin. Upon application to the skin, the composition
delivers a "powdery" feel
that is pleasing to the user and yet continues to deliver the benefit of
lubrication and slip between
the skin surface and other surfaces such as other skin surfaces or external
clothing.
Applicants have also measured the Body Dryness Index associated with
compositions
and uses of the present invention. Applicants have discovered that the claimed
compositions
provide a significant body dryness as compared to other comparable
compositions.
Any suitable volatile cyclic silicone carrier may be used in the present
invention. As
used herein the term "volatile" refers to those liquids that have a measurable
vapor pressure at
CA 02725862 2010-12-17
PPC5337USNP1
ambient temperature. Examples of suitable volatile cyclic silicone carriers
include
cyclomethicones of the formula:
CH3
Si-0
c
n
----------------------
wherein n=3 to 6. Examples of certain preferred volatile cyclic silicone
carriers include
decamethylcyclopentasiloxane, dodecamethylcyclohexasiloxane, and the like. A
particularly
preferred volatile cyclic silicone is decamethylcyclopentasiloxane. A variety
of commercially
available volatile, cyclic polydimethylsiloxanes include: Dow Corning DC 244
and DC 344
fluids (cyclotetrasiloxanes) and DC 245 and DC345 (cyclopentasiloxanes)
manufactured by Dow
Corning, Midland Mich.; Volatile Silicone 7158, 7207 and 7349 manufactured by
Momentive
Performance Materials, Tarrytown, NY and KF9937 and KF9945 manufactured by
Shin- Etsu
Silicones.
Any material that is capable of delivering a "powdery" feel when released onto
the skin
may be used in the present invention as a powder-feel agent. Suitable powder-
feel agents
include a variety of silicone polymers, gels, gums, particulate materials,
combinations of two or
more thereof, and the like.
Examples of silicone polymers useful as powder-feel agents in the present
invention
include crosslinked siloxane (e.g., dimethicone or dimethicone derivatives)
copolymers such as
stearyl methyl-dimethyl siloxane copolymer (Gransil SR-CYC, available from
Grant Industries,
Elmwood Park, N.J.); dimethicone/vinyldimethicone crosspolymers; Polysilicone-
11 (i.e., a
16
CA 02725862 2010-12-17
PPC5337USNP1
crosslinked silicone rubber formed by the reaction of vinyl terminated
silicone and
methylhydrodimethyl siloxane in the presence of cyclomethicone), cetearyl
dimethicone/vinyl
dimethicone crosspolymer (i.e., a copolymer of cetearyl dimethicone
crosslinked with vinyl
dimethyl polysiloxane), dimethicone/phenyl vinyl dimethicone crosspolymer
(i.e., copolymer of
dimethylpolysiloxane crosslinked with phenyl vinyl dimethylsiloxane), and
dimethicone/vinyl
dimethicone crosspolymer (i.e., copolymer of dimethylpolysiloxane crosslinked
with vinyl
dimethylsiloxane). More preferably, the compositions useful in the method of
this invention
include silicone elastomer blends. containing dimethicone/vinyldimethicone
crosspolymers (such
as those made by Dow Corning), dimethicone, cyclopentasiloxane, trisiloxane,
dimethicone and
hydrophobically-modified silica.
The silicone polymers may be of any suitable molecular weight. In certain
preferred
embodiments, the polymers have a weight average molecular weight in excess of
10,000 (e.g.,
between about 10,000 and 10,000,000).
Examples of suitable silicone gels include the following which are also
available
commercial from Grant Industries by the indicated tradename: cyclomethicone
(and)
polysilicone-11 (Gransil GCMS), cyclotetrasiloxane(D4) (and) petrolatum (and)
polysilicone-11
(Grangil PS-4), cyclopentasiloxane(D5) (and) petrolatum (and) polysilicone-11
(Gransil PS-5),
cyclopentasiloxane(D5) (and) dimethicone (and) polysilicone-11 (Gransil DMCM-
5),
cyclotetrasiloxane(D4) (and) dimethicone (and) polysilicone- 11 (Gransil DMCM-
4),
polysilicone-11 (and) isododecane (Gransil IDS), and cyclomethicone (and)
polysilicone- 11
(and) petrolatum (and) phytosphingosine (Gransil SPH). Other examples of such
gels, available
from General Electric, include cyclopentasiloxane (and) dimethicone/vinyl
dimethicone
crossploymer (SFE839). In general, the compositions set forth in U.S. Patent
No. 6,200,964 and
17
CA 02725862 2010-12-17
PPC5337USNP1
U.S. Patent No. 6,384,023, which are hereby incorporated herein by reference,
are suitable for
use in the methods of this invention.
Suitable silicone gels include silicone elastomer gels. The elastomer
chemically is a
crosslinked, 3-dimentional network of intertwined silicone polymers that swell
in the presence of
a carrier. Elastomers are not soluble in the carrier but swollen in the
carrier. Typically the
"effective" carrier solvent is a low molecular weight species that can migrate
into the
network. The crosslinking density of the elastomer can affect the "swelling"
efficiency;
generally, lower crosslinking density favors swelling (for a given carrier)
and gives a "wetter"
feel initially (sometimes could be sticky). Conversely a higher crosslinking
density elastomer
swells less and gives a "drier" skin feel initially. Most elastomer products
dry down to a
"powdery" after-feel particularly if the solvent is volatile. One non-limiting
example of a
suitable class of silicone elastomers is crosslinked organopolysiloxane (or
siloxane) elastomers,
which are generally described in U.S. patent application publication
US2003/0049212A1.
The crosslinked organopolysiloxane elastomers may be categorized as
emulsifying or
non-emulsifying. "Emulsifying," as used herein, means crosslinked
organopolysiloxane
elastomers having at least one polyoxyalkylene (e.g., polyoxyethylene or
polyoxypropylene) or
polyglycerin moiety. The polyoxyalkylene (e.g., polyoxyethylene or
polyoxypropylene) or
polyglycerin moiety may serve as the crosslinker within the elastomer.
Exemplary emulsifying
crosslinked organopolysiloxane elastomers are described in U.S. Pat. Nos.
5,412,004; 5,837,793,
and 5,811,487. Suitable emulsifying crosslinked organopolysiloxane elastomers
include
dimethicone/PEG-10 crosspolymers such as KSG 24; dimethicone/PEG-10
crosspolymers such
as KSG 21 and KSG 210; PEG-15/lauryl dimethicone crosspolymers such as KSG 31,
KG 32,
KSG 33, KSG 310, KG 320, KSG 330; PEG-15/lauryl dimethicone crosspolymers and
PEG-
18
CA 02725862 2010-12-17
PPC5337USNP1
10/lauryl dimethicone crosspolymers such as KSG 34 and KSG 340;
dimethicone/polyglycerine-
3 crosspolymers such as KSG-710; and lauryl dimethicone/polyglycerine-3
crosspolymers such
as KSG 810, KSG 820, KSG 830, and KSG 840. Also from Shin-Etsu are Silicone
Rubber
powder, KMP-400 type, Silicone resin powder, KMP-590, X-52-1631, Hybrid
silicone powders,
KSP-100, KSP-101 and KSP-300, etc.
"Non-emulsifying" means crosslinked organopolysiloxane elastomers are
essentially free
of polyoxyalkylene or polyglycerin moieties. Exemplary non-emulsifying
crosslinked siloxane
elastomers include the CTFA (Cosmetic, Toiletry, and Fragrance Association
International
Cosmetic Ingredient Dictionary and Handbook, 11<sup>th</sup> ed.) designated
dimethicone/vinyl
dimethicone crosspolymers supplied by a variety of suppliers including Dow
Coming (DC
9506), General Electric (SFE 839), Shin Etsu (KSG 15 and 16), and Grant
Industries (GRANSIL
RPS-NA) and dimethicone/phenyl vinyl dimethicone crosspolymer such as KSG 18
available
from Shin Etsu. Other exemplary non-emulsifying crosslinked siloxane elastomer
include the
CTFA designated dimethicone crosspolymers including Dow Corning. (DC 9040, DC
9041, DC
9045).
Also suitable are high molecular weight silicone gums with linear high
molecular
polymer "solids (gums)" which are soluble in a carrier and water-insoluble
silicones inclusive of
non-volatile polyalkyl and polyaryl siloxane gums and fluids, volatile cyclic
and linear
polyalkylsiloxanes, polyalkoxylated silicones, amino and quaternary ammonium
modified
silicones, rigid cross-linked and reinforced silicones and mixtures thereof.
E.g, Dimethiconol
(DC 2-9023 fluid), KF 8018 from shinetsu which is an amino gum in
cyclomethicone.
19
CA 02725862 2010-12-17
PPC5337USNP 1
The smooth soft, silky and powdery feel may also be achieved by using
particulate
materials. Typically, the particulate materials are free-flowing and solid
(i.e., the particles are not
hollow).
Suitable organic particulate materials include those available commercially
under the
tradenames as follows: those made of polymethylsilsesquioxane (e.g., Tospearl
145A available
from GE Toshiba Silicone Co., Ltd.), polyamide (e.g., nylon-12 and Orgasol
2002D Nat C05
available from Atofma), polyolefmes (e.g., Microthene FN5 10-00 available from
Equistar),
polyacrylates (e.g., ethylene acrylate copolymer, sold under the name FloBead
EA209 available
from Kobo), polymethacrylates (PMMA) (e.g., Micropearl M 100 available from
Seppic),
polystyrene (e.g., Dynospheres available from Dyno Particles),
polytetrafluoroethylene (PTFE),
polyurethanes, starch and starch derivatives, composite particles, and
mixtures thereof.
Copolymers derived from monomers of the aforementioned materials can also be
used. The
aforementioned polymers derived from carboxylic acid containing monomer
further include ester
and salts of the monomers.
Inorganic materials for improving skin feel include natural minerals such as
mica, talc,
and sericite, synthetic mica, synthetic sericite, plate-formed titanium oxide,
plate-formed silica,
plate-formed aluminum oxide, boron nitride, barium sulfate, plate-formed
titania-silica
composite oxide, and bismuth oxychloride. Further these inorganic particles
comprising those
described above as a base material and one or more inorganic oxides coating
the base material
such as titanium oxide, aluminum oxide, iron oxide, silicon dioxide, cerium
oxide, and
zirconium. The pure titanium or zinc oxides pigments may be coated with
compounds such as
amino acids such as lysine, silicones, lauroyl, collagen, polyethylene,
lecithin and ester oils. The
inorganic particles may be resin coated as cited in US patent application
2003/0171475. The
CA 02725862 2010-12-17
PPC5337USNP 1
resin is preferably one or more selected from the group consisting of
polyurethane, a styrene-but
adiene copolymer, an acrylonitrile-butadiene copolymer, a silicone-based
elastomer, and a
polyolefm-based elastomer.
According to certain preferred embodiments, the powder-feel agent is
preferably a
silicone-based powder feel agent such as a silicone polymer, silicone gels,
silicone gums,
hydrophobically-modified silica, combinations thereof, and the like. Preferred
silicone-based
powder feel agents include silicone elastomer blends and hydrophobic silica
blends,
combinations of two or more thereof, and the like.
Any suitable amounts of powder-feel agents may be used in the present
invention. In
certain embodiments, the powder feel agent is present in the composition in an
amount of about
65% or less by weight of the total composition. In certain. preferred
embodiments, the powder
feel agent is present in an amount of from about 5% to about 65%, more
preferably from about
8% to about 65%, more preferably from about 8% to about 60%, more preferably
from about 8%
to about 40%, and even more preferably from about 8% to about 30% by weight of
the total
composition.
Suitable esters for use in the present invention include those of Formula I,
Formula II,
Formula III:
0
11
RI-C-O-R2 (I)
0 0
11 11
R3-C-0-R4-O-C-O-R5 (II)
21
CA 02725862 2010-12-17
PPC5337USNP1
0 0
II II
R6-C-O-R7-O-C-O-R8 (III)
O
0=C
I
R9
wherein R1, R2, R3, R5, R6, R8 and R9 are independently linear or branched,
substituted or
unsubstituted, saturated or unsaturated, C3-C22 alkyl or alkenyl groups, R4 is
a linear or branched,
substituted or unsubstituted, saturated or unsaturated, C3-C22 alkylene or
alkenylene moiety, and
R7 is a linear or branched, substituted or unsubstituted, saturated or
unsaturated, C3-C22 moiety.
In certain preferred embodiments the ester of Formula I, Formula II, or
Formula III have a
viscosity ranging from about 10 to 1,000,000 centipoise at 25 C.
Examples of monoester oils of Formula I that may be used in the compositions
of the
invention include, but are not limited to, hexyldecyl benzoate, hexyl laurate,
hexadecyl
isostearate, hexydecyl laurate, hexyldecyl octanoate, hexyldecyl oleate,
hexyldecyl palmitate,
hexyldecyl stearate, hexyldodecyl salicylate, hexyl isostearate, butyl
acetate, butyl isostearate,
butyl oleate, butyl octyl oleate, cetyl palmitate, ceyl octanoate, cetyl
laurate, cetyl lactate, octyl
isonoonanoate, isostearyl isononanoate, isononyl isononanoate, cetyl
isononanoate, cetyl
stearate, stearyl lactate, stearyl octanoate, stearyl heptanoate, stearyl
stearate, and so on.
Suitable diesters of Formula II that may be used in the compositions of the
invention are
the reaction product of a dicarboxylic acid and an aliphatic or aromatic
alcohol, or a
22
CA 02725862 2010-12-17
PPC5337USNP1
monocarboxylic acid and an aliphatic or aromatic alcohol containing at least
two hydroxyl
groups. Preferably, one or more of the acid or alcohol is a fatty acid or
alcohol, i.e. contains 3-22
carbon atoms. The dicarboxylic acid may also be an alpha hydroxy acid.
Examples of diester oils
that may be used in the compositions of the invention include diisostearyl
malate, neopentyl
glycol dioctanoate, dibutyl sebacate, di-C12_13 alkyl malate, dicetearyl dimer
dilinoleate, dicetyl
adipate, diisocetyl adipate, diisononyl adipate, diisopropyl adipate,
diisostearyl dimer dilinoleate,
disostearyl fumarate, diisostearyl malate, isononyl isononanaote, isohexadecyl
stearate, and so
on.
Suitable triesters of Formula III comprise the reaction product of a
tricarboxylic acid and
an aliphatic or aromatic alcohol, or the reaction of an aliphatic or aromatic
alcohol having three
or more hydroxyl groups with mono-or dicarboxylic acids. Preferably, one or
more of the acid or
alcohol is a fatty acid or alcohol containing 3 to 22 carbon atoms. Examples
of triesters include
triarachidin, tributyl citrate, triisostearyl citrate, tri C12-13 alkyl
citrate, tricaprylin, tricaprylyl
citrate, tridecyl behenate, trioctyldodecyl citrate, tridecyl behenate,
tridecyl cocoate, tridecyl
isononanoate, and so on.
In certain preferred embodiments, the ester of the present invention is
selected from the
group consisting of octyl isononanoate, isopropyl palmitate, butyl stearate,
diisopropyl adipate,
triisostearyl citrate, and combinations of two or more thereof. In certain
more preferred
embodiments, the ester is selected from the group consisting of octyl
isononanoate, isopropyl
palmitate, butyl stearate, and combinations of two or more thereof. In certain
more preferred
embodiments, the ester comprises octyl isononanoate.
The ester selected from the group consisting of esters of Formula I, Formula
II, Formula
III, and combinations of two or more thereof may be present in the
compositions of the invention
23
CA 02725862 2010-12-17
PPC5337USNP1
in an amount of 5% or less by weight based on the total weight of composition.
In certain
preferred embodiments, the ester is present in an amount of from about 0.5 to
about 4.5%, more
preferably from about 1 to about 4%, more preferably from about 2-4%, and even
more
preferably from about 2-3%.
In certain preferred embodiments, the composition is substantially anhydrous.
As used
herein, the term "substantially anhydrous" means that the composition contains
less than 5% w/w
water. In more preferred embodiments, the composition contains less than 3%,
more preferably
less than 2%, more preferably less than 1 %, more preferably less than 0.5%
w/w water. In
certain preferred embodiments, the substantially anhydrous composition is an
anhydrous
composition (free of water).
The invention features a method of applying a cosmetic composition suitable
for
application to the skin, e.g., in the intimate area such as the perineum,
under the breasts or on the
thighs, of a subject in association with a cosmetically acceptable carrier.
The individual
components of the carrier are numerous and varied, but are also well known to
one skilled in the
art. In one aspect, the carrier comprises one or more of the members selected
from the group
consisting of acidifying agents, alkalizing agents, aerosol propellants,
antimicrobial agents,
antioxidants, buffering agents, chelating agents, coloring additives,
dermatologically active
agents, dispersing agents, emollients, emulsifying agents, humectants,
fragrances, masking
agents, preservatives, sugars, sunscreen agents, surfactants, suspending
agents, thickening
agents, an vehicles. These ingredients are discussed below. Examples of these
agents are listed
below as well as in the International Cosmetic Ingredient Dictionary and
Handbook, eds.
Wenninger and McEwen (The Cosmetic, Toiletry, and Fragrance Assoc.,
Washington, D.C.,
7<sup>th</sup> Edition, 1997) (hereinafter "ICT Handbook").
24
CA 02725862 2010-12-17
PPC5337USNP 1
Acidifying and alkalizing agents are preferably added to obtain the desired pH
of the
composition. Examples of acidifying agents included acetic acid, citric acid,
glacial acetic acid,
malic acid, and proprionic acid. Examples of alkalizing agent include edetol,
potassium
carbonate, potassium hydroxide, sodium borate, sodium carbonate, and sodium
hydroxide. Other
acidifying and alkalizing agents are listed on page 1653 of the ICT Handbook.
Aerosol propellants are used when the composition is to be administered as an
aerosol
under pressure. Examples of aerosol propellants include halogenated
hydrocarbons such as
dichlorodifluoromethane, dichlorotetrafluoroethane, and
trichloromonofluoromethane, nitrogen,
and volatile hydrocarbons such as butane, propane, isobutane, or mixtures
thereof. Other
propellants are listed on page 1655 of the ICT Handbook.
Anti-microbial agents are used when the area that the composition is to be
applied is
prone to microbial infection, e.g., by bacteria, fungal, or protozoa. Examples
of such agents
include benzyl alcohol, chlorobutanol, phenylethyl alcohol, phenylmercuric
acetate, potassium
sorbate, and sorbic acid, benzoic acid, butyl paraben, ethyl paraben, methyl
paraben, propyl
pareben, and sodium benzoate. Other anti-microbial agents are listed on page
1612 of the ICT
Handbook.
Antioxidants are used to protect ingredients of the composition from oxidizing
agents that
are included within or come in contact with the composition. Examples of
antioxidants include
water soluble antioxidants such as ascorbic acid, sodium sulfite,
metabisulfite, sodium bisulfite,
sodium formaldehyde, sulfoxylate, isoascorbic acid, isoascorbic acid, cysteine
hydrochloride,
1,4-diazobicyclo-(2,2,2)-octane, and mixtures thereof. Examples of oil-soluble
antioxidants
include ascorbyl palmitate, butytlated hydroxyanisole, butylated
hydroxytoluene, potassium
CA 02725862 2010-12-17
PPC5337USNP 1
propyl gallate, octyl gallate, dodecyl gallate, phenyl-alpha-napthyl-amine,
and tocopherols such
as alpha-tocopherol. Other antioxidants are listed on pages 1612-13 of the ICT
Handbook.
Coloring additives are used to add color to the composition. Examples of such
coloring
additives include titanium dioxide, yellow iron oxide, red iron oxide, black
iron oxide, caramel,
carmine, fluorescein derivatives, methoxsalen, trioxsalen, carbon black, azo
dyes, anthraquinone
dyes, blue azulenes, guajazulene, chamuzulene, erythrosin, bengal rose,
phloxin, cyanosin,
daphinin, eosin G, cosin l OB, and Acid Red 51. Other coloring agents are
listed on pages 1628-
30 of the ICT Handbook.
Dermatologically active agents include agents for treating wound healing,
inflammation,
acne, psoriasis, cutaneous aging, skin cancer, impetigo, herpes, chickenpox,
dermatitis, pain,
itching, and skin irritation. Examples of such dermatologically active agents
include
hydrocortisone, dexamethasone, panthenol, phenol, tetracycline hydrochloride,
yeast,
hexylresorcinol, lamin, kinetin, betamethagone, triamcinolone, fluocinolone,
methylprednisolone, retinoids such as retinol and retinoic acid, dapsone,
sulfasalazine,
resorcinol, salicylic acid, benzoyl peroxide, erythromycin-benzoyl peroxide,
erythromycin,
clindamycin, mupirocin, griseofulvin, azoles such as miconazole, econazole,
itraconazole,
fluconazole, and ketoconazole, ciclopirox, allylamines such as naftifine and
terfinafine,
acyclovir, famciclovir, valacyclovir, benzocaine, lidocaine, dibucaine,
pramoxine hydrochloride,
methyl salicylate, camphor, menthol, resocinol, and vitamins such as
tocopherol, tocopheryl
acetate, pentothenic acid, ascorbic acid, biotin, and retinoids such as
retinol, retinoic acid, retinal,
retinyl acetate, and retinyl palmitate, .alpha.-hydroxy acid, a.beta.-hydroxy
acid, or poly-
hydroxy acid such as glycolic acid, lactic acid, citric acid, malic acid, and
azaleic acid, and
26
CA 02725862 2010-12-17
PPC5337USNP1
sunless tanning agents such as 1,3-dihydroxyacetone and 1,3,4-trihydroxy-2-
butanone
(erythulose).
Examples of dispersing and suspending agents include quarternium- 18
hectorite,
polyhydroxy stearic acid, poligeenan and silicon dioxide. Other dispersing and
suspending
agents are listed on page 1690-91 of the ICT Handbook.
Emollients are agents that soften and smooth the skin. Examples of emollients
include
hydrocarbon oils and waxes (e.g., natural and synthetic waxes) such as mineral
oil, petrolatum,
microcrystaline wax, polyethylene, triglyceride esters such as those of castor
oil, cocoa butter,
safflower oil, cottonseed oil, corn oil, olive oil, cod liver oil, almond oil,
avocado oil, palm oil,
sesame oil, squalene, and soybean oil, acetylated monoglycerides, ethoxylated
glycerides, fatty
acids, alkyl esters of fatty acids, alkenyl esters of fatty acids, fatty
alcohols, fatty alcohol ethers,
ether-esters, lanolin and derivatives of lanolin, polyhydric alcohol esters,
wax esters such as
beeswax, vegetable waxes, phospholipids, and sterols. Other emollients are
listed on pages 1656-
61 of the ICT Handbook.
Emulsifying agents are used for preparing emulsions of the present invention.
Examples
of emulsifying agents used for preparing silicone-in-oil, or oil-in-silicone
emulsions include
cyclomethicone (and) dimethicone copolyol, dimethicone copolyol, cetyl
dimethicone copolyol,
Humectants are agents that promote the retention of moisture, e.g.,
moisturizers.
Examples of humectants include sorbitol, matricaria extract, aloe barbadensis
gel, glycerin,
glycereth 5 lactate, glycereth 7 triacetate, glycereth 7 diisononoate,
hexanetriol, hexylene glycol,
propylene glycol, dipropylene glycol, alkoxylated glucose, D-panthenol, 1-2-
pantandiol, 2-
methyl-1,3-propanediol, and derivatives thereof, and hyaluronic acid. Other
humectants are listed
on pages 1661-62 of the ICT Handbook.
27
CA 02725862 2010-12-17
PPC5337USNP1
Examples of fragrances include peppermint, rose oil, rose water, aloe vera,
clove oil,
menthol, camphor, eucalyptus oil, and other plant extracts. Certain fragrances
may require a
solubilizer, e.g., PPG-5-ceteareth-20. To eliminate certain odors from
compositions, masking
agents may be used. An example of a masking agent includes ethylene
brassylate. Other
fragrances and masking agents are listed on pages 1639-40 of the ICT Handbook.
Preservatives are used to protect the composition from degradation. Examples
of
preservatives include liquipar oil, phenoxyethanol, methyl paraben, propyl
paraben, butyl
paraben, isopropyl paraben, isobutyl paraben, dieizolidinyl urea,
imidazolidinyl urea, diazolindyl
urea, benzalkonium chloride, benzethonium chloride, phenol, and mixtures
thereof (e.g., liquipar
oil). Other preservatives are listed on pages 1654-55 of the ICT Handbook.
Surfactants are agents used to stabilize multi-component compositions, e.g.,
used as
wetting agents, antifoam agents, emulsifiers, dispersing agents, and
penetrants. Examples of
surfactants include methyl gluceth 20, decyl polyglucoside, lapyrium chloride,
laureth 4, laureth
9, monoethanolamine, nonoxynol 4, nonoxynol 9, nonoxynol 10, nonoxynol 15,
nonoxynol 30,
poloxalene, polyoxyl 8, 40, and 50 stearate, polysorbate 20, polysorbate 40,
polysorbate 60,
polysorbate 65, polysorbate 80, and polysorbate 85, sodium lauryl sulfate,
sorbitan and its
derivatives. Other surfactants are listed on page 1672-90 of the ICT Handbook.
The cosmetically acceptable carrier that may be in a number of different
delivery forms,
e.g., a spray, mist, aerosol, mousse, semi-solid cream, liquid such as a
solution, emulsion, or
suspension, lotion, gel, solid such as a powder, adherent stick, flexible
mask, self-hardening
liquid or gel, or other suitable forms intended to be applied to the skin of a
subject (e.g., a
human).
28
CA 02725862 2010-12-17
PPC5337USNP 1
The viscosity of the compositions of the present invention may be different
dependent
upon the type of formulation being prepared, e.g., a liquid formulation will
have a lower
viscosity than a gel or cream formulation. Typically, the viscosity of liquid
formulations of the
present invention will range from 5,000 to 25,000 cps. Bulking agents may be
used to increase
the viscosity of the composition.
The compositions of this invention may be prepared using methodology that is
well
known by an artisan of ordinary skill (e.g., by using well-known mixing and
blending
procedures).
29
CA 02725862 2010-12-17
PPC5337USNP1
EXAMPLES
The following is a description of the manufacture of certain compositions of
the present
invention. Other compositions of the invention can be prepared in an analogous
manner by a
person of ordinary skill in the art.
Test Procedure for Measuring Contact Angle (CAM)
Test fluid was made of the following mixture to simulate bodily fluids: 49.5%
of 0.9%
sodium chloride solution (V )VR catalog # VW 3257-7), 49.05% Glycerin (Emery
917), 1 %
Phenoxyethanol (Clariant Corporation Phenoxetol.TM.) and 0.45% Sodium Chloride
(Baker
sodium chloride crystal # 9624-05).
For each sample, a 2 A 2" x 2" sample film was created using Leneta 2A Opacity
charts,
BYK Gardner Opacity Drawdown Base, and a BYK Gardner wet film drawdown bar
with a
thickness of 1.5mil. The Opacity Drawdown Base was used to hold the opacity
charts at a
constant position during the drawdown application process. The drawdown
process involved
placing small amount of gels across the top of the opacity charts and using
the Drawdown bar to
vertically cover the chart with the gel. This vertically up-down motion was
carried out until the
gels formed a uniform film over the opacity charts. The charts were then cut
into2"x 2" film
strips, and weighed on a Mettler Toledo scale.
Advancing contact angles of each sample strip were measured using the Kruss DS
100
Drop Shape Analysis machine. Following the manual instructions of the machine,
the angle of
inclination on were tilted to 2 degrees; the third syringe was selected; the
drop type was changed
to sessile drop; and the drop subtype was changed to normal sessile drop. The
thickness of the
CA 02725862 2010-12-17
PPC5337USNP 1
needle was 0.509mm. Test fluid was placed in the machine and allowed to flush
through the
needle for a few minutes in order to remove flush the needle. The drop volume
was manually
controlled at a rate of 50p lminute. With the camera in focus, contact angles
of 4 sessile drops
were measured on each strip. The sample size of this experiment is 4 (n=4) and
Average Contact
Angle Measurement (CAM) was recorded.
Comparative Example 1
Contact Angle Testing was performed to determine the contact angle of a series
of esters
(Repellant Agents) labeled as comparative compositions C1-C5.
Table 1
Ester/ Contact
Exampl INCI Angle
e Repellant Name Supplier Location Measureme
Agent
nt
C1 HallStar Octyl Octyl HallStar Hackettstown,
Isononanoate isononanoa Co NJ 55.00
(Duck Oil) to
C2 Isopropyl HallStar Hackettstown
HallStar IPP palmitate Co , NJ 53.10
C3 HallStar BST Butyl HallStar Hackettstown 56 2
stearate Co , NJ
C4 Internatio
Ceraphyl Diisopropy nal Wayne, NJ 74.3
(DIPA) 1 adipate Specialty
Products
C5 Lubrizol
TISC Ester yl Triisostear stear Advanced Cleveland, OH 78.6
citrate
31
CA 02725862 2010-12-17
PPC5337USNP1
Duck oil is a long chain monoester with two branches. IPP is a long chain
monoester with a
single chain. BST is a long chain monoester, unbranched. DIPA is a branched
diester. TISC is
a large, branched triester. All of these esters alone have a contact angle of
less than 90 .
Example 1
A base formulation (B 1), several inventive examples (E 1-E5), and comparative
examples
(C6-C15) were prepared as described below. The CAM of each was measured and is
reported
below in Table 3.
A base formulation B 1 was prepared using the ingredients of Table 2.
Table 2 - Base Formulation (B1)
Function Trade INCI Name Supplier Address Conc.
Name w/w%
Volatilizing DC 245 Decamethylcyclopentasiloxane Dow Midland, 56.57
Agent Fluid Corning MI
Corp.
Powder USG-103 Dimethicone/vinyl Shin- Akron, 18.18
Feel dimethicone crosspolymer Etsu OH
Agents Silicone
of
America
Bulking DC 200 Dimethicone Dow Midland, 10.1
Agents Fluid, Corning MI
350 cSt Corp.
Bulking KF 8018 Aminopropyl dimethicone Shin- Akron, 7.07
Agents Etsu OH
Silicone
of
America
Powder KSP 100 Vinyl dimethicone/methicone Shin- Tokyo, 5.05
Feel , silsesquioxane crosspolymer Etsu Japan
Agents Chemical
Co.
Powder Cabosil Silica Cabot Somerset, 1.52
32
CA 02725862 2010-12-17
PPC5337USNP1
Feel M5 Corp. NJ
Agents
Powder Cabosil Dichlorodimethylsilane Cabot Somerset, 1.52
Feel TS 610 Corp. NJ
Agents
The ingredients were combined in the order they appear in the table into a
glass beaker, stirred
with a propeller mixer until the resultant composition was completely uniform.
The composition
was prepared at room temperature. The CAM of the composition B l was measured
to be 85.6 .
Compositions E1-E5 of the present invention and comparative compositions C6-
C16
were made by combining all of the ingredients from B 1 and an additional ester
(Repellant Agent)
as identified, and in the amount as indicated, in Table 4. The general
formulations of such
compounds were thus as indicated in Table 3.
Table 3
Function Trade INCI Name E1-E5 C6-C l 0 C l 1-C 15
Name
Volatilizi DC 245 Decamethyl- 56.0 53.737 50.909
ng Agent Fluid cyclopentasiloxane
Powder USG- Dimethicone/vinyl 18.0 17.273 16.364
Feel 103 dimethicone
Agents crosspolymer
Bulking DC 200 Dimethicone 5.0 4.798 4.545
Agents Fluid,
350 cSt
Bulking KF Aminopropyl 7.0 6.717 6.364
Agents 8018 dimethicone
Powder KSP Vinyl 10.0 9.596 9.091
Feel 100 dimethicone/methico
Agents ne silsesquioxane
crosspolymer
Powder Cabosil Silica 1.5 1.439 1.364
Feel M5
Agents
Powder Cabosil Dichlorodimethylsila 1.5 1.439 1.364
Feel TS 610 ne
Agents
33
CA 02725862 2010-12-17
PPC5337USNP 1
Repellant - - 1.0 5.0 10.0
Agent
from
Table 4
Compositions E1-E5 and C6-C15 were made as follows: the ratios of materials
specified
in Table 4 were measured out such that the total mixture weight was 100 grams.
The Repellant
Agents (esters) were added first to the Volatilizing Agents and mixed in a
beaker using a
propeller mixing blade at 100 RPM for 1-2 minutes. Powder Feel Agents were
then added and
mixed at 400-500 RPM until there were no visible clumps. CAM was measured for
each
resultant composition and reported in Table 4.
Table 4
Example Repellant Agent Concentration CAM
B1 Base Formulation - 85.60
El Octyl isononanoate 1 93.6
E2 HallStar IPP 1 93.6
E3 HallStar BST 1 93.8
E4 Ceraphyl (DIPA) 1 93.2
E5 TISC Ester 1 95.2
C6 Octyl isononanoate 5 82.0
C7 HallStar IPP 5 82.9
C8 HallStar BST 5 83.4
C9 Ceraphyl (DIPA) 5 84.9
CIO TISC Ester 5 86.7
C11 Octyl isononanoate 10 59.3
C12 HallStar IPP 10 73.5
C13 HallStar BST 10 79.6
C14 Ceraphyl (DIPA) 10 80.4
C15 TISC Ester 10 82.4
As illustrated in Table 4, adding less than 5% of the Repellant Agent to the
base
formulation resulted in a composition having a CAM over 90 . This is a
significant and
34
CA 02725862 2010-12-17
PPC5337USNP 1
surprising increase over the CAM of the base formulation (B1) or the Repellant
Agents alone
(see Table 1).
Example 2
Several compositions of the claimed invention (E6-E9) and comparative
compositions
(C 16-C27) were made and the CAM of each tested and reported in Table 5. Each
of
compositions E6-E9 and C16-C27 were made up of three components: the
Volatilizing Agent,
Powder Feel Agent, and Repellant Agent (Duck Oil (HallStar Octyl
Isononanoate)) as identified,
and in the amounts as listed, in Table 5. Such compositions were made as
follows: the Repellant
Agent was added first to the Volatilizing Agent and mixed in a beaker using a
propeller mixing
blade at 100 RPM for 1-2 minutes. The Powder Feel Agent was then added and
mixed at 400-
500 RPM until there were no visible clumps.
Table 5
Comp Volatilizing INCI VA Powder PFA RA CAM
Agent (VA) Conc Feel Con (Duck
Agent C. Oil -
w/w (PFA) w/w Hallstar
% % ) Conc.
w/w%
C16 IDD Isododecane 41.5 USG 57.5 1 78.3
(Isododecane) 103
C17 IDD Isododecane 75 KSP 24 1 48.1
100
C18 IDD Isododecane 91 TS 610 8 1 83.9
CA 02725862 2010-12-17
PPC5337USNP1
C19 IDD Isododecane 94 M5 5 1 12.6
C20 IHD Isohexadecane 41.5 USG 57.5 1 77.9
(Isohexadecane 103
C21 IHD Isohexadecane 75 KSP 24 1 51.2
100
C22 IHD Isohexadecane 91 TS 610 8 1 81.4
C23 IHD Isohexadecane 94 M5 5 1 14.2
C24 DC 200 (DC Dimethicone 41.5 USG 57.5 1 71.3
200 5 cST) 103
C25 DC 200 Dimethicone 75 KSP 24 1 54.5
100
C26 DC 200 Dimethicone 91 TS 610 8 1 85.1
C27 DC 200 Dimethicone 94 M5 5 1 21.3
E6 DC 245 Decamethyl- 41.5 USG 57.5 1 92.8
cyclopentasilox 103
ane
E7 DC 245 Decamethyl- 75 KSP 24 1 90.5
cyclopentasilox 100
ane
E8 DC 245 Decamethyl- 91 TS 610 8 1 96.4
cyclopentasilox
ane
E9 DC 245 Decamethyl- 94 M5 5 1 34.1
cyclopentasilox
ane
As illustrated in Table 5, the compositions comprising a volatile cyclic
silicone carrier,
ester, and certain powder feel agents tended to exhibit significantly higher
CAM than
36
CA 02725862 2010-12-17
PPC5337USNP1
comparative compositions. In particular, the compositions of the invention
exhibited CAM
values above 90 .
Example 3
Four compositions of the claimed invention (E10-E13) and a comparative
composition
(C28) were made and the CAM of each tested and reported in Table 6. Each of
the compositions
were made up of three components: the Volatilizing Agent, Powder Feel Agent,
and Repellant
Agent (Duck Oil) as identified, and in the amounts as listed, in Table 6. The
compositions were
made in the same manner as E6-E9.
15
37
CA 02725862 2010-12-17
PPC5337USNP1
Table 6
Comp. DC 245 Duck Powder Characteristics Conc. CAM
Conc. Oil - Feel (degrees)
w/w% Hallstar Agent
Conc.
w/w%
E10 41.5 1 USG 103 High MW silicone 57.5 92.8
elastomer/cross-linked
polymer gel in volatile
cyclic silicone solvent
Ell 34 1 USG 103 High MW silicone 65 93.8
elastomer/cross-linked
polymer gel in volatile
cyclic silicone solvent
E12 75 1 KSP 100 High MW silicone 24 90.5
cross-linked polymer
powder, thickener
E13 91 1 TS 620 Low MW hydrophobic 8 96.4
silica, thickener
C28 91-94 1 M5 Low MW untreated 5-8 Below
silica, thickener 90
As illustrated in Table 6, compositions comprising a volatile cyclic silicone
carrier, ester,
and certain powder feel agents tended to exhibit significantly higher CAM than
comparative
compositions. In particular, the compositions of the invention exhibited CAM
values above 90 .
Example 4
Six compositions of the claimed invention (E14-E19) and seven comparative
compositions (C29-C35) were made and the CAM of each tested and reported in
Table 7. Each
of the compositions were made up of three components: the Volatilizing Agent
(DC 245,
decamethylcyclopentasiloxane), Powder Feel Agent, and Repellant Agent (Duck
Oil - Hallstar)
as identified, and in the amounts as listed, in Table 7. The compositions were
made in the same
manner as E6-E9.
38
CA 02725862 2010-12-17
PPC5337USNP1
Table 7
Comp. DC 245 Duck Oil Powder Feel Powder Feel CAM
Conc. (Conc. Agent -Agent
w/w% w/w%) (Conc.
w/w%)
E14 91 1 TS 610 8 96.4
E15 41.5 1 USG 103 57.5 92.8
E16 75 1 KSP 100 24 90.5
C29 94 1 M5 5 34.1
E17 89 3 TS 610 8 93.2
E18 39.5 3 USG 103 57.5 90.1
C30 73 3 KSP 100 24 76.6
C31 92 3 M5 5 31.8
E19 88 4 TS 610 8 90.4
C32 38.5 4 USG 103 57.5 83.4
C33 72 4 KSP 100 24 53.4
C34 91 4 M5 5 32.1
C35 74 2 KSP 24 86.7
As illustrated in Table 7, compositions comprising a volatile cyclic silicone
carrier, ester,
and certain powder feel agents tended to exhibit significantly higher CAM than
comparative
compositions. In particular, the compositions of the invention exhibited CAM
values above 90 .
Example 5
The Body Dryness Index for the base formulation B1 and Examples El-E5 was
measured
in accord with the procedure below and is reported in Table 8.
Test Procedure for Body Dryness Index
Test fluid was made of the following mixture to simulate bodily fluids: 49.5%
of 0.9%
sodium chloride solution (V )VR catalog # VW 3257-7), 49.05% Glycerin (Emery
917), 1%
39
CA 02725862 2010-12-17
PPC5337USNP 1
Phenoxyethanol (Clariant Corporation Phenoxetol.TM.) and 0.45% Sodium Chloride
(Baker
sodium chloride crystal # 9624-05).
For each test composition (B1, E1-E5), a 2"x2" sample of hydrated V-skin
(Vitro-skin-
N-19 manufactured by IMS Inc, 70 Robinson Blvd, Orange, CT 06477; Telephone #
203-795-
9047) was cut and the weight recorded. This film sample provides a liquid
impervious surface
and represents the body in this test. The test composition, 0.5 grams thereof,
is spread uniformly
across the top surface of the film to form a test sample (Gardner Draw with
variable thickener
was used with 1.5mil set clearance is used to spread the test composition
evenly across the
hydrated V-skin to form a substantially uniform coated film on the V-skin). A
control sample
with no test composition applied thereto was prepared. All samples were
weighed and recorded
as initial weights. N=3
Using a Corning Syringe Pump, 10 mL of test fluid was dispensed onto the
surface of the
test samples and control sample. The test samples and control sample were then
allowed to rest
for 2 minutes. Each film was then tilted at a 45 incline for 1 minute to allow
excess fluid to drain
off. The weights were recorded as final weights N=3.
Calculating the Body Dryness Index
The Body Dryness Index (BDI) is based on the Body Wetness Index (BWI), which
is the
residual fluid on the skin (g) divided by the total weight of the fluid
dispensed.
Body Wetness Index (BWI) = residual fluid on skin (g)
Total weight of fluid dispensed (g)
Body Dryness Index (BDI) = 1
BWI
CA 02725862 2010-12-17
PPC5337USNP1
Table 8 represents the volume of test fluid absorbed by the V-skin uncoated or
coated
with the test composition for certain times. The values were generated by
subtracting the initial
weight(W;) from the final weight(Wf) for each time period.
Table 8
Sample W f-W; BDI
Control (uncoated 74
skin)
B1 1250
El 0.006 1667
E2 0.007 1429
E3 0.005 2000
E4 0.003 3333
E5 0.003 3333
As shown by the above data, surfaces coated with a layer of the test
composition containing a
repellent agent had a higher BDI that the surface coated with just the control
test formulation (no
repellent agent).
41
CA 02725862 2010-12-17
PPC5337USNP1
Personal Product Kit
According to another aspect of the invention, the present invention provides a
personal
product kit including in combination a product dispenser assembly as described
above, a
composition of the type described above provided within the product dispenser
assembly, and at
least one absorbent article. As used herein the term "absorbent articles"
includes articles such as
diapers (infant and adult), sanitary napkins, shields, pantyliners, breast
pads, and the like.
A specific example of a personal product kit according to the present
invention could
include, a product dispenser assembly as described above, a composition of the
type described
above adapted to be applied the vaginal area of a user's body, and at least
one sanitary napkin.
To use such a kit, a user would apply the composition to vaginal area using
the dispenser
assembly and then wear the sanitary napkin in a conventional fashion. The use
of the
composition in this manner would promote menstrual and/or urine flow into the
napkin and
thereby provide a clean dry feeling to the user. Kits according to the present
invention could of
course be provided with a plurality of absorbent articles, e.g. a plurality of
sanitary napkins, to
enable a user to replace the used absorbent after it has been soiled. Kits
according to the present
invention may alternative include other absorbent articles, such as
pantyliners or diapers, in
combination with a product dispenser assembly and a composition of the type
described above.
Another specific example of a personal product kit according to the present
invention
could include, a product dispenser assembly as described above, a composition
of the type
described above adapted to be applied the nipple area of a user's body, and at
least one breast
pad. To use such a kit, a user would apply the composition to nipple area
using the dispenser
assembly and then wear the breast pad. The use of the composition in this
manner would
promote the flow of fluid into the breast and thereby provide a clean dry
feeling to the user.
42