Note: Descriptions are shown in the official language in which they were submitted.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
PACKAGE WITH ENCLOSURE FOR DISPENSING
DOSES OF A MEDICAMENT
FIELD OF THE INVENTION
The present invention relates generally to packages for holding and dispensing
medicinal unit doses, and more particularly, is directed to a package for
dispensing a
single medicinal unit dose.
BACKGROUND OF THE INVENTION
Generally, medicinal units, such as tablets, capsules, caplets, films, gels or
the
like, are housed in a package, such as a plastic bottle that has an opening
which is
sufficiently large to accommodate and allow the flow of multiple medicinal
units
through the opening at one time in order to facilitate filling these
containers during
packaging line operations. Plastic bottles are inherently cheap and simple to
process on
packaging lines, making them a commonly used package for oral drug products.
Such packages, however, may render it difficult for consumers to limit
dispensing the desired number of medicinal units, particularly when the
consumer has
limited fine motor abilities. Consumers with limited fine motor abilities
include
patients, such as the elderly or those with neurological disorders (e.g.,
Parkinson's
disease). Consequently, such patients may undesirably dispense too many
medicinal
units from a package such as a bottle. Also of concern is spillage from the
undesirable
dispensing of too many medicinal units which may result whereby one or more
medicinal units drops to the floor and becomes lost, irretrievable, or
contaminated.
This may result in the loss of possibly very expensive medicinal units.
Although there are single unit dose dispensing packages available, for
example,
blister packs, these packs still present problems. Blister packs or cards
containing
pharmaceutical unit doses are sold with the blister pack as the primary
package which
holds the unit doses. During dispensing by patients with neurological
disorders such as
Parkinson's disease, there is still the problem that a unit dose will drop to
the floor or
otherwise become lost, irretrievable or contaminated.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
Thus, it would therefore be desirable to provide a package containing unit
doses,
which limits dispensing of medicinal unit doses one at a time while minimizing
the risk
of spillage.
SUMMARY OF THE INVENTION
Various embodiments of the present invention provide a package for dispensing
a single medicinal unit dose. The package may have a wall structure defining
an
enclosure; an access opening in the enclosure for retrieving a unit dose from
the
enclosure; and at least one medicament holder for holding the unit dose, the
at least one
medicament holder being mounted to the wall structure such that the unit dose
removed
from the medicament holder falls into and is captured by the enclosure, for
later
retrieval through the access opening.
Each medicament holder may include a blister for holding the unit dose. A
rupturable backing may be provided on an inner surface of the blister for
retaining the
unit dose in each the blister and there may be a plurality of blisters mounted
to the wall
structure. The medicament holder may be integrally formed with the wall
structure or
removable and replaced with a replacement medicament holder that has new set
up
doses of medicament. The medicament holder may include a blister for holding
the unit
dose, the blister being formed by a deformable portion of the wall structure,
and further
comprising a rupturable backing provided on an inner surface of the wall
structure in
association with each the blister for retaining the unit dose in the blister.
The wall
structure may include a removable closure cap or a cap that is attached to the
package.
The cap may be a senior friendly or child resistant cap. The wall structure
may include
at least one entry opening, and the at least one medicament holder is
permanently
secured to the wall structure in association with the at least one entry
opening. The at
least one medicament holder may include a blister for holding the unit dose
and a
rupturable backing provided on an inner surface of the blister for retaining
the unit dose
in the blister, the blister and rupturable backing being permanently secured
to the wall
structure for passing the unit dose from the blister through one the entry
opening into
the enclosure. An insert can have a plurality of the blisters therein, with
the rupturable
backing provided on an inner surface of the insert, the insert being
permanently secured
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
-3-
to the wall structure for passing the unit dose from the blister through one
the entry
opening into the enclosure, The blister may extend through one the entry
opening.
The wall structure may include at least one entry opening, and the at least
one
medicament holder is removably secured to the wall structure. The wall
structure may
further include at least one guide for guiding and mounting the at least one
medicament
holder such that each medicament holder is positioned in covering relation to
one the
entry opening. The medicament holder may include an insert having a plurality
of the
blisters therein, with the rupturable backing provided on an inner surface of
the insert,
the insert being removably mounted to the wall structure by the at least one
guide for
passing the unit dose from each the blister through one the entry opening into
the
enclosure. The insert may include a card insert, and the at least one guide
includes
parallel, spaced apart, L-shaped wails on an outer surface of the wall
structure for
slidably receiving the card insert to mount the card insert to the wall
enclosure. A label
and/or instructions on the use or dosing information may be included loosely
within the
package or contained on a wall structure.
Another embodiment provides for a package with a wall structure has a clam
shell package having a center section and first and second hinged side
sections hingedly
secured to opposite sides of the center section, the first side section
including at least
one medicament holder therein, and a container having entry openings and
mounted in
the second side section, such that when the clam shell package is closed, each
the
medicament holder is positioned in covering relation to one the entry opening,
the
container including the access opening. The wall structure can be formed by a
blank.
The medicament holder can have a deformable front wall which can be
depressed by a person's finger and a deformable rear wall for retaining the
unit dose in
the medicament holder, the deformable rear wall including an inwardly turned
section
which locks the unit dose in the medicament holder and which is turned
outwardly upon
deformation of the deforable front wall in order to release the unit dose
through the
deformable rear wall.
Additional embodiments of the present invention provide methods of reducing
the risk of a neurological patient dropping a single medicinal unit dose by
providing the.,
various packages of the present invention to a patient with a neurological
disease.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
.4..
Various embodiments provide a pharmaceutical product having at least one
medicament holder and a package for dispensing a single medicinal unit dose.
The
package may have a wall structure defining an enclosure; an access opening in
the
enclosure for retrieving a unit dose from the enclosure; and at least one
medicament
holder for holding the unit dose, the at least one medicament holder being
mounted to
the wall structure such that the unit dose removed from the medicament holder
falls
into and is captured by the enclosure, for later retrieval through the access
opening.
A single medicinal unit dose may be one medicament or multiple medicaments
and may be in any suitable pharmaceutical dosage form, such as but not limited
to, a
tablet, capsule, caplet and/or gelcaps. The single medicinal unit dose can
have one or
more medicaments such as one, two, three medicaments. For example, a single
medicinal unit dose may be I tablet or 2 tablets or 3 tablets, etc. The single
medicinal
unit dose may have at least one tablet, capsule, caplet or geicap or
combinations
thereof. The medicament can be the same or different dosage forms.
The medicament desirably contains an active pharmaceutical agent such as those
useful in treating people with diseases that may make the patient's hand shake
or
otherwise impeded the performance of one's hands, such as but not limited to a
those
actives useful in treating a neurological disease, such as Parkinson's
disease, epilepsy,
arthritis, turret's disease and the like.
Other embodiments provide for a pharmaceutical kit that includes a package for
dispensing a single medicinal unit dose and a plurality of medicament holders
which
house a plurality of pharmaceutical dosage forms. The kit may be sold together
so that
the medicament holders may be swapped in and out of the package as the dosage
forms
in the medicaments are finished.
Additional embodiments provide a blank for forming a package for dispensing a
single medicinal unit dose where the blank has a plurality of wall panels
connected
together for forming an enclosure with at least a closed bottom, and at least
one blister
for holding a unit dose incorporated into at least one of the wall panels of
the enclosure,
such that when the wall panels are connected together to form the enclosure, a
unit dose
removed f r o m one the. blister fil :trod is captured by the enclosure, for
later .
retrieval.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
-5-
The blank may further have a rupturabie backing provided on an inner surface
of the wall panel to each the blister for retaining a unit dose in each the
blister. There
may be a plurality of the blisters incorporated into at least one of the wall
panels. The
wall panels and the at least one blister may be made from the same material,
such as a
plastic material. The plurality of wall panels may include a front wall panel,
a rear wall
panel and two side wall panels connected together in series along score lines.
The
plurality of wall panels may include a bottom flap connected to a lower end of
one the
wall panel along a score line, and a top flap connected to an upper end of one
the wall
panel. The plurality of wall panels may include at least one bottom side flap
connected
to a lower end of at least one the wall panel and at least one top side flap
connected to
an upper end of at least one the wall panel.
Various embodiments of the present invention provide for a package for
dispensing a single medicinal unit dose. The package may include an enclosure,
an
access opening in the enclosure for retrieving a unit dose from the enclosure,
and at
least one medicament holder for holding the unit dose, such that the unit dose
removed
from the medicament holder falls into and is captured by the enclosure, for
later
retrieval through the access opening.
The medicament holder can be a blister that is integrally formed with the
package. Alternatively, the medicament holder can be a removable insert.
Multiple embodiments of the present invention provide a package in which an
insert such as a blister pack is integrated into the walls of the package.
Alternatively,
the insert may be removably insertable into the package. A unit dose of a
medicament
is capable of falling into the interior or enclosure of the package, thereby
preventing
loss thereof Various embodiments provide for safe, accurate, and/or low cost
delivery
of the single unit dose. Suitable delivery mechanisms include threaded
closures on a
neck finish, child resistant or senior friendly closures on a neck finish, a
dispensing
spout, a gable top, a sliding door, a tear strip, a locking flap, etc.
The package and the inserts or blisters may be made from the same material,
thereby assuring compliance with compatibility regulations. Desirably, a
package is
relatively incxpens ive.to manufacture.and easy to use.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
-6
In accordance with an aspect of the present invention, a package for
dispensing
a single medicinal unit dose; includes a plurality of wall panels forming an
enclosure,
an access opening in the enclosure for retrieving a unit dose from the
enclosure, and at
least one insert or blister for holding a unit dose incorporated into at least
one of the
wall panels of the package enclosure, such that a unit dose removed from one
blister
falls into and is captured by the enclosure thereby rendering the unit dose
ready for
retrieval through the access opening.
There may also be a rupturable backing provided on an inner surface of the
wall
panel to each blister for retaining a unit dose in each blister. There may be
a plurality of
the blisters incorporated into at least one of the wall panels of the
enclosure, and the
package may be a rectangular parallelepiped configuration. In addition, the
wall panels
and the at least one insert or blister may be made from the same material.
In various embodiments, the plurality of wall panels include a front wall
panel, a
rear wall panel and two side wall panels connected together in a rectangular
parallelepiped configuration which defines a lower opening thereof and an
upper end
thereof forming the access opening, a bottom flap for closing the lower
opening and a
top flap removably closing the access opening.
The above and other features of the invention will become readily apparent
from
the following detailed description thereof which is to be read in connection
with the
accompanying drawings.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
.7.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. I is a perspective view of a package for dispensing unit doses with
dosage
and spillage control according to the present invention;
Fig. 2 is a top plan view of a blank for forming the package of Fig. 1;
Fig. 3 is a cross-sectional view of the blank of Fig. 2, taken along line 3-3
thereof;
Fig. 4 is a side elevational view of the package of Fig. 2;
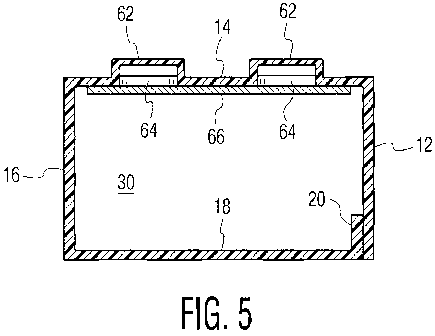
Fig. 5 is a cross-sectional view of the package of Fig. 1, taken along line 5-
5
thereof;
Fig. 6 is a perspective view of a card insert containing blisters;
Fig. 7 is a cross-sectional view similar to Fig. 5, showing a package
according to
another embodiment, for use with the card insert of Fig. 6;
Fig. 8 is perspective view of a package according to another embodiment of the
present invention;
Fig. 9 is perspective view of a package according to another embodiment of the
present invention;
Fig. 10 is perspective view of a bottle according to another embodiment of the
present invention;
Fig. I I is perspective view of a bottle according to another embodiment of
the
present invention;
Fig. 12 is front elevational view of a bottle according to another embodiment
of
the present invention;
Fig. 13 is top plan view of the bottle of Fig. 12;
Fig. 14 is a perspective view of the assembled clam shell package according to
another embodiment of the present invention;
Fig. 15 is a side elevational view of the clam shell package of Fig. 14 in an
opened configuration;
Fig. 16 is a side elevational view of the clam shell package of Fig. 14 in an
assembled condition;
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
Fig. 17 is a cross-sectional view of a modified blister arrangement in the non-
activated condition; and
Fig. 18 is a cross-sectional view of the modified blister arrangement in the
activated condition.
DETAILED DESCRIPTION
Referring to the drawings in detail, and initially to Figs. 1-3 thereof, a
receptacle
for dispensing unit doses with dosage and spillage control is formed from a
blank 11
which includes, in order, a first side wall panel 12, a front wall panel 14, a
second side
wall panel 16, a rear wall panel 18, and a securing tab panel 20, connected
together
respectively at longer sides thereof.
The receptacle can be any suitable shape such as a boxed shape, cylindrical
shape, triangular shape, rounded shape, etc. The outlet may be conical to
facilitate
removal of the medicament. The front wall panel can be any suitable shape,
including
rectangular, square, triangular, trapezoidal, etc. The wall panels can also
have rounded
edges.
Score lines 22, 24, 26 and 28 or the like are provided between adjacent
connected panels, namely, between first side wall panel 12 and front wall
panel 14,
between front wall panel 14 and second side wall panel 16, between second side
wall
panel 16 and rear wall panel 18 and between rear wall panel 18 and securing
tab panel
20, to enable blank 1 I to be folded along score lines 22, 24, 26 and 28 such
that each
panel is bent perpendicular to its adjacent panel.
The receptacle can made out of any suitable material, including paperboard,
cardboard, plastic, metal and the like. The receptacle may be reusable. In
such cases,
the medicament containing insert may be removed and replaced with another
insert.
One of the walls of the receptacle may be movable to allow for removal and
insertion of
the insert. The bottom wall, side wall or opening top may be removably
connected with
an appropriate latch or hinge mechanism.
Alternatively, rather than score lines, thinner diameter lines or V-shaped cut-
outs can provided in place thereof, and reference to score lines in the claims
is
intended to encompass all such variations. In this manner, securing tab panel
20 is
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
-9-
secured to first side wall panel 12 by an adhesive, as best shown in Fig. 5,
to form an
enclosure.
A bottom flap 30 is connected to one shorter side of rear wall panel 18 along
a
score line 32, and includes a tab 34 connected at the opposite end thereof
along a score
line 36. In addition, bottom side flaps 38 and 40 are connected to one shorter
side of
first and second side wall panels 12 and 16 along score lines 42 and 44,
respectively, on
the same side of blank I I as bottom flap 30.
When blank 11 has been folded along score tines 22, 24, 26 and 28 such that
each panel is bent perpendicular to its adjacent panel, and securing tab panel
20 is
secured to first side wall panel 12 by an adhesive to form an enclosure,
bottom side
flaps 38 and 40 are bent inwardly along score lines 42 and 44, and bottom flap
30 is
folded along score line 32 to a position overlapping bottom side flaps 38 and
40, and
which closes the lower opening of the enclosure. Tab 34 is folded along score
line 36
to be perpendicular to bottom flap 30 and thereby lie against the inner
surface of front
wall panel 14, thereby retaining bottom flap 30 in this position.
A top flap 46 is connected to one shorter side of front wall panel 14 along a
score line 48, on the opposite side of blank I I from bottom flap 30, and
includes a tab
50 connected at the opposite end thereof along a score line 52. In addition,
top side
flaps 54 and 56 are connected to the opposite shorter side of first and second
side wall
panels 12 and 16 along score lines 58 and 60, respectively, on the same side
of blank 1 I
as top flap 46.
When blank I 1 has been folded along score lines 22,24, 26 and 28 such that
each panel is bent perpendicular to its adjacent panel, and securing tab 20 is
secured to
first side wall panel 12 by an adhesive to form an enclosure, top side flaps
54 and 56 are
bent inwardly along score lines 58 and 60, and top flap 46 is folded along
score line 48
to a position overlapping top side flaps 54 and 56, and which closes the upper
opening
of the enclosure. Tab 50 is folded along score line 52 to be perpendicular to
top flap 46
and thereby lie against the inner surface of rear wall panel 18, thereby
retaining top flap
46 in this position. With this arrangement, package 10 forms a rectangular
parallelepiped configuration. Alternatively, the package may be any suitable
shape,
including triangular, circular, rectangular, conical and the like.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
DE06919
-to-
In accordance with other aspects of the present invention, blank 11 is formed
with a plurality of inserts, blisters or compartments 62 in front wall panel
14 and each
extending forwardly thereof, each for holding a single unit dose such as a
tablet,
capsule, caplet or the like, and each in alignment with an opening 64 (Figs. 3
and 5) in
front wall panel 14. Blisters 62 extend forwardly out of the plane of front
wall panel
14. A backing 66 can be made of foil, plastic, soft polymeric material or the
like, which
can be punctured, and is secured in covering relation to the rear or inner
surface of front
wall panel 14 to hold the unit doses in the individual blisters 62. It will be
appreciated
that the formation of blisters 62 is not limited to front wall panel 14, but
may be
provided in other wall panels, or in a plurality of the wall panels of package
10.
Blank 11, including blister inserts 62, may be made from one sheet of
material,
which may be a clear, fully colored or partially colored thermoformed plastic
material.
In use, a person merely presses on one of the blisters 62 to push the unit
dose
through backing 66, whereby the unit dose falls into the enclosure of package
10. The
unit dose can then be removed from package 10 by merely opening top flap 46
and top
side flaps 54 and 56. Alternatively, or in addition thereto, after a unit dose
has been
removed from its respective blister 62, dispensing of the unit dose from
package 10 can
be accomplished by means of a child-resistant closure, flap, funnel or spout
connected
with one or more wall panels or flaps, so that the patient receives the single
dose with
lowered chance of spillage.
It will be appreciated that a single unit dose can be dispensed into box
shaped
package 10. This means that the risk that the unit dose will drop, become lost
and
contaminated will be minimized. Advantageously, this bottle will help a person
with a
neurological disorder who attempts to remove a single unit dose from a bottle
or from a
conventional blister pack.
Additionally, an outer package or box for holding the blister pack may not be
needed, since blisters 62 are built directly into package 10, thereby reducing
the costs
and time of manufacture. This means of manufacture provides the advantages of
fewer
process steps, fewer errors and improved quality control. Because blisters 62
and
package 10 are made from the same material, manufacture thereof is less costly
and
easier, and a further benefit is derived because this assures compliance with
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
compatibility regulations. It will be appreciated that lower cost can be
achieved
because package 10 including blisters 62 can be manufactured from one
continuous roll
of plastic. In addition, the material can be made transparent so that the
patient is able to
observe the dose they are about to dispense.
It will also be appreciated that once the single dose is dispensed into the
chamber, access to or further dispensation of the dose into the patient's hand
or mouth
can be further controlled.
Thus, the present invention replaces the current blister pack to hand to mouth
dispensing method with a blister pack to receptacle to hand to mouth (or
directly from
receptacle to mouth), without adding any packaging compatibility issue.
It will be appreciated that reference to unit doses in the claims is intended
to
encompass all types of unit doses including but not limited to capsules,
tablets, colloids,
caplets, powders, liquids, etc.
Other embodiments of the present invention provide for a labeling either on
the
package or the blisters or the wails. Such labeling could identify the type of
drug
contained therein. Suitable labels include weeks, days of the week or
indications within
a day such as nighttime/PM or morning/AM that would be in accordance with the
desirable dosing regimens of the particular medicament(s) contained therein.
Other
embodiments include packages that include at least two active pharmaceutical
agents
either in separate dosage forms or in a combined dosage form. The package can
contain
labeling of the separate dosage forms with instructions of the appropriate use
and
dosing of such active pharmaceutical agents.
It will be appreciated that various modifications can be made to the present
invention.
For example, as shown in Fig. 6 and 7, rather than blisters 62 being formed as
part of the package, they can be formed as an insert that is assembled with
the package.
Specifically, referring first to Fig. 7, there is a shown a box shaped package
l0a in
which elements corresponding to box shaped package 10 are identified by the
same
numerals but with the letter 'a' appended thereto, and a detailed description
of the
common elements is not provided. Basically, box shaped package l0a is
identical to
box shaped package 10, except that blisters 62 are not formed thereon.
Instead,
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
12-
openings 68a are provided at positions of blisters 62 of box shaped package
10. An
insert card 70a is provided, having blisters or compartments 62a formed
thereon, each
extending forwardly thereof, each for holding a single unit dose such as a
tablet,
capsule, caplet or the like, and each in alignment with an opening 64a in
insert card 70a
for removal of the unit dose. Blisters 62a extend forwardly out of the plane
of insert
card 70a. A backing 66a can be made of foil, plastic, soft polymeric material
or the
like, which can be punctured, and is secured in covering relation to the rear
surface of
insert card 70a to hold the unit doses in the individual blisters 62a. Insert
card 70a,
including blisters 62a, may be made from one sheet of material, which may be a
clear,
fully colored or partially colored thermoformed plastic material.
With this arrangement, insert card 70a can be secured to the rear surface of
front
wall panel 14a such that blisters 62a extend out through openings 68a in front
wall
panel 14. Securement can be made by any suitable means, including adhesive,
ultrasonic welding, etc. Insert card 70a can be assembled with front wall
panel 14a of
the flattened blank 11, when the package 10 is fully assembled, or during any
step
therebetween. It will also be appreciated that insert card 70a is not limited
to assembly
with front wall panel 14, but may be provided in other wall panels, or in a
plurality of
the wall panels of package 1()a.
Although the present invention has been discussed with a package 10 of a
rectangular parallelepiped shape, the present invention is not limited
thereby. For
example, as shown in Fig. 8, a package IOb can have a cylindrical main body
section
72b on which blisters 62b are formed, and a conical spout section 74b secured
to the
upper end of main body section 72b. A lid flap 76b is secured at the upper
open end
78b of conical spout section 74b by any suitable means, such as a living hinge
80b. Lid
flap 76b can be secured to close open end 78b, for example, by a clasp (not
shown), a
friction fit, etc. To this end, the upper surface of lid flap 76b can have a
gripping
handle (not shown). Further, blisters 62b can be formed in the cylindrical
wall of main
body section 72b in a similar manner to blisters 62 of Figs. 1-5 or can be
formed on an
insert card in a similar manner to blisters 62a of Figs. 6 and 7.
Another example of a different shape is shown in Fig. 9 in which a package I0c
is formed in a conical shape by a conical wall 82c having an open lower end
which can
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
be closed by a bottom closure flap 84c and an open upper end 86c which can be
closed
by a lid flap 88e. Bottom closure flap 84c and lid flap 88c can be secured to
conical
wall 82c by any suitable means, such as a living hinge. Lid flap 88c and
bottom closure
flap 84c can be secured to close the upper and lower open ends, for example,
by a clasp
(not shown), a friction fit, etc. To this end, the upper surface of lid flap
88c and the
lower surface of bottom closure flap 84c can each have a gripping handle (not
shown).
Conical wall 82c has a plurality of openings 68c therein, similar to the
arrangement of Fig. 7, and a card insert 70c similar to card insert 70a of
Fig. 6 and
having a plurality of blisters 62c thereon with a backing sheet (not shown)
can be
assembled with conical wall 82c such that blisters 62c extend out through
openings
68c. Securement can be made by any suitable means, including adhesive,
ultrasonic
welding, etc. Insert card 70c can be assembled with conical wall 82c of a
flattened
blank thereof, when the package 10e is fully assembled, or during any step
therebetween.
It will be appreciated that the above embodiments have discussed the package
formed from a blank. However, package 10 can be a conventional plastic bottle
on
which the blisters are formed in the same manner as discussed in Figs. 1-5, or
as an
insert through openings in the bottle in the same manner as discussed in Figs.
6 and 7.
Referring now to Fig. 10, a bottle I Od as the package according to another
modification of the present invention includes a cylindrical main body 90d
having a
reduced diameter neck 92d at the upper end thereof, with a closure cap 94d
threadedly
received on neck 92d in a conventional manner. A plurality of openings 68d are
formed
in main body 90d. Individual blisters 62d, each having a unit dose thereon and
closed
by a backing (not shown), are permanently secured to the outer surface of main
body
90d in alignment with and in covering relation to openings 68d. When a person
pushes
on a blister 62d, the unit dose breaks through the backing and enters bottle I
Od through
the respective opening 68d.
Although the embodiment of Fig. 10 has been discussed with respect to unit
doses, such as capsules or tablets, this embodiment, as well as other
embodiments can
be used such that each blister 62d can hold a secondary ingredient, such as a
colorant,
sugar, etc., which when the blister 62d is burst, enters main body 90d which
is already
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
-14-
filled with a primary product or which is filled by the consumer just prior to
or after
bursting the blister 62d. Bottle I Od can then be shaken or stirred to mix the
components. In this manner, a consumer can change the contents of bottle I Od
to the
consumer's preference. For example, one blister can include a red colorant, a
second
blister a blue colorant, a third blister sugar, etc. In this manner, the
contents of the
primary product in bottle lOd can be adjusted in strength, color, sweetness,
etc.
It will be appreciated that the openings need not be formed in the main body
of
the bottle. For example, as shown in bottle l0e of Fig. 11, having a main body
90e, a
reduced diameter neck (not shown) and a closure cap 94e, blisters 62e can be
formed on
the upper surface of closure cap 94e in alignment with openings (not shown) in
closure
cap 94e.
Alternatively, as shown in Figs. 12 and 13, a bottle 1 Of has a main body 90f,
a
reduced diameter neck 92f and a closure cap 94f threaded on neck 921. A
plurality of
openings 68f are provided in main body 90f. In addition, L-shaped guides 96f
extend
down in parallel, spaced apart facing relation to each other on opposite sides
of
openings 68f. A bottom ledge 98f secured to the outer surface of main body 90f
connects the lower ends of L-shaped guides 96f and has two upstanding tabs
100f
thereon. A card insert 70f which is substantially identical to card insert 70a
has a
plurality of blisters 62f thereon, as well as two recesses I02f at the lower
edge thereof.
With this embodiment, it is only necessary to slide card insert 70f into L-
shaped guides
96f until recesses 102f receive tabs 100 The reason for tabs I00f and
recesses 102f is
to ensure accurate alignment of blisters 62f with openings 68f. However, it
will be
appreciated that tabs 100f and recesses 102f can be eliminated if the
tolerances of card
insert 70f in guides 96f are small. The advantage of this embodiment is that a
single
bottle can be used for a plurality of different medicines since it is only
necessary to slip
in a different card insert 70f containing a different medicine.
Referring now to Figs. 14-16, there is shown a package l Og according to
another embodiment of the present invention. Package IOg is formed in a molded
clam
shell type configuration with three sections, namely, a center triangular
section 104g
and two side sections 106g and l Ogg secured to opposite sides of triangular
section
I04g by living hinges 8Og or the like.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
One side section 106g is formed from two side wall panels I IOg and 112g
connected in a V-shaped formation, with side edges thereof closed by
triangular shaped
end panels 114g. Side wall panel I lOg includes a plurality of openings 68g
therein, and
a plurality of blisters 62g are formed on one side of side wall panel I I Og
in alignment
with openings 68g. Alternatively, as in the first embodiment of Figs. 1-5,
blisters 62g
can be integrally formed with side wall panel I I Og. A backing 66g of foil,
plastic, soft
polymeric material or the like, which can be punctured, is secured in covering
relation
to the opposite surface of side wall panel I I Og to hold the unit doses in
the individual
blisters 62. Backing 66g can be supplied from a roll 67g during manufacture
and then
be cut.
The other side section 108g is formed from two side wall panels 11 6g and 118g
connected in a V-shaped formation, with side edges thereof closed by
triangular shaped
end panels 120g. Side wall panel I 16g includes a curved wall section 122g for
receiving a bottle 124g having a main body 90g, a reduced diameter neck 92g
with
threads 93g thereon and a closure cap 94g threadedly received on neck 92g.
Curved
wall section 122g can have marketing information or different graphics
thereon.
Although not shown, the upper end of curved wall section 122g has an opening
(not
shown) for receiving neck 92g such that neck 92g and closure cap 94g extend
out of
curved wall section 122g. In this embodiment, it is preferred that one side
126g of
main body 90g of bottle 124g is flat so that openings 68g formed therein can
properly
align with blisters 62g in the assembled condition of package 10g. It will be
appreciated that bottle 124g will be rotated 90 degrees from the position
shown in Fig.
15 to be in facing relation to blisters 62g in the assembled condition, and
that bottle
124g is only shown in this rotated position to show openings 68g.
Thus, in this embodiment, after blisters 62g and backing 66g have been formed
on side wall panel 110g, bottle 124g is inserted into curved wall section
122g. Side
sections I06g and 108g are then pivoted about living hinges 80g until side
wall panels
I i Og and 116g are in abutting relation where they are secured together,
either
releasably, for example, in snap fitting manner, or permanently, for example,
by
adhesive, ultrasonic welding, etc.
CA 02726946 2010-12-03
WO 2009/149267 PCT/US2009/046274
-16-
In use, a person presses against a blister 62g, whereupon the unit dose
therein
breaks through backing 66g and falls through the respective opening 68g into
bottle
124g. Cap 94g is then removed and the unit dose can be easily retrieved.
It will further be appreciated that, while blisters 62-62g have been shown as
being closed by a backing 66-66g, each blister can be formed in any other
suitable
manner. For example, as shown in Figs. 17 and 18, a blister 62h is shown to be
backed
by a deformable non-foil, deformable backing 66h which extends away from
blister 62h
so as to increase the size of the cavity 63h between blister 62h and backing
66h.
However, backing 66h has an inwardly turned section 128h which extends into
the
cavity 63h and serves as a lock which holds the unit dose 130h in cavity 63h,
preferably
with pressure. The inwardmost wall 132h of inwardly turned section 128h has an
opening 134h therein.
Initially, inwardly turned section 128h holds unit dose 130h in cavity 63h
with
pressure. However, when a person's finger 136h presses against blister 62h,
unit dose
130h forces inwardly turned section 128 to turn outwardly, along with the
remainder of
backing 66h, and continued pressure forces unit dose 130h out through opening
134h.
Having described various embodiments of the invention with reference to the
accompanying drawings, it will be appreciated that the present invention is
not limited
to those precise embodiments and that various changes and modifications can be
effected therein by one of ordinary skill in the art without departing from
the scope or
spirit of the invention as defined by the appended claims.