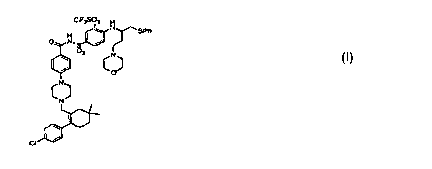Note: Descriptions are shown in the official language in which they were submitted.
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
A PROCESS FOR THE PREPARATION OF THE APOPTOSIS PROMOTER
ABT-263
FIELD OF THE INVENTION
The present invention relates to, among other things, novel compounds and
synthetic
processes, including those useful for making N-acylsulfonamide apoptosis
promoters.
BACKGROUND OF THE INVENTION
Novel N-acylsulfonamide apoptosis promoters are described in, for example, U.
S.
Patent Publication US2005/0159427A1, U. S. Patent 7,390,799 B2 (referred to
hereinafter as
the "'799 Patent") and elsewhere. Synthetic routes for the preparation of N-
acylsulfonamide
apoptosis promoters are described in the'799 Patent and K. Ding, et al.
(Synthesis, 2008, 15,
2398-2404).
SUMMARY OF THE INVENTION
The present invention provides, among other things, safe, efficient and cost-
effective
processes for making N-acylsulfonamide apoptosis promoters.
One aspect of this invention pertains to a process for making N-(4-(4-((2-(4-
chlorophenyl)-5, 5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin-1-
yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, an alkyl formate and a first base to
provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or
not isolating
the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, a second
base and a first silyl ether protecting group reagent to provide a first
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or not isolating
the first
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(c) reacting the first protected (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and 4-chlorophenyl magnesium bromide to provide the
first
protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the first protected (2E)-1-(4-chlorophenyl)-2-
(hydroxymethylene)-
4,4-dimethylcyclohexanol; and
1
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(d) reacting the first protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-
4,4-dimethylcyclohexanol and a first acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another aspect of this invention pertains to a process for making N-(4-(4-((2-
(4-
chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin- l -
yl)benzoyl)-4-(((l R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, an alkyl formate and a first base to
provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or
not isolating
the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, a second
base and a first silyl ether protecting group reagent to provide a first
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or not isolating
the first
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(c) reacting the first protected (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and 4-chlorophenyl magnesium bromide to provide the
first
protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanol; and
2
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
isolating or not isolating the first protected (2E)-1-(4-chlorophenyl)-2-
(hydroxymethylene)-
4,4-dimethylcyclohexanol; and
(d) reacting the first protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-
4,4-dimethylcyclohexanol and a first acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid;
(m) reacting 2-fluorobenzenesulfonyl chloride, and a first fluoride source to
provide 2-fluorobenzenesulfonyl fluoride and isolating or not isolating the 2-
fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and a second fluoride source to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene and
isolating or not isolating the 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and a
first NH3 source to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and
isolating or not isolating the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and a sixth base to provide 4-
(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
3
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another aspect of this invention pertains to a process for making N-(4-(4-((2-
(4-
chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin- l -
yl)benzoyl)-4-(((l R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, an alkyl formate and a first base to
provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or
not isolating
the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, a second
base and a first silyl ether protecting group reagent to provide a first
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or not isolating
the first
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(c) reacting the first protected (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and 4-chlorophenyl magnesium bromide to provide the
first
protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the first protected (2E)-1-(4-chlorophenyl)-2-
(hydroxymethylene)-
4,4-dimethylcyclohexanol; and
(d) reacting the first protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-
4,4-dimethylcyclohexanol and a first acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid;
4
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(r) reacting a first metal trifluoromethanesulfinate, a first aryl fluoride
source, and
a first catalyst to provide 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene and
isolating or not
isolating the 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and a
first NH3 source to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and
isolating or not isolating the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and a sixth base to provide 4-
(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another aspect of this invention pertains to a process for making N-(4-(4-((2-
(4-
chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoyl)-
4-(((lR)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and
isolating or
not isolating the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane to provide (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
5
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide to provide ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol;
(d) reacting (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
6
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another aspect of this invention pertains to a process for making N-(4-(4-((2-
(4-
chlorophenyl)-5, 5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin-1-
yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and
isolating or
not isolating the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane to provide (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide to provide ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol;
7
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(d) reacting (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid;
(m) reacting 2-fluorobenzenesulfonyl chloride, and tetra-n-butylammonium
fluoride to provide 2-fluorobenzenesulfonyl fluoride and isolating or not
isolating the 2-
fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and tris(dimethylamino)sulfonium difluorotrimethylsilicate to provide 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine to provide
4-(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
8
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another aspect of this invention pertains to a process for making N-(4-(4-((2-
(4-
chlorophenyl)-5, 5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin-1-
yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and
isolating or
not isolating the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane to provide (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide to provide ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
9
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
isolating or not isolating the (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol;
(d) reacting (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-I-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid;
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and copper(I) oxide to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine to provide
4-(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another aspect of this invention pertains to a process for making N-(4-(4-((2-
(4-
chlorophenyl)-5, 5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin-1-
yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a pharmaceutically
acceptable salt thereof,
comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and a
first coupling reagent with or without a fourth base and with or without an
auxiliary coupling
reagent, and isolating or not isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-
dimethyl-l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another aspect of this invention pertains to a process for making N-(4-(4-((2-
(4-
chlorophenyl)-5, 5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin-1-
yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
11
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a pharmaceutically
acceptable salt thereof,
comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another aspect of this invention pertains to a compound, or a pharmaceutically
acceptable salt thereof, chosen from (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, and (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone.
Another aspect of this invention pertains to 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-ene- l -carbaldehyde or a pharmaceutically acceptable salt thereof.
DETAILED DESCRIPTION OF THE INVENTION
Variable moieties are represented by identifiers (capital letters with
numerical and/or
alphabetical superscripts) and may be specifically embodied.
12
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
The term "alkyl," as used herein, means Ci-alkyl, C2-alkyl, C3-alkyl, C4-
alkyl,
C5-alkyl, and C6-alkyl.
The term "Ci-alkyl," as used herein, means methyl.
The term "C2-alkyl," as used herein, means ethyl.
The term "C3-alkyl," as used herein, means prop- l-yl and prop-2-yl
(isopropyl).
The term "C4-alkyl," as used herein, means but- l-yl, but-2-yl, 2-methylprop-l-
yl, and
2-methylprop-2-yl (tert-butyl).
The term "C5-alkyl," as used herein, means 2,2-dimethylprop-1-yl (neo-pentyl),
2-
methylbut-1-yl, 2-methylbut-2-yl, 3-methylbut-1-yl, 3-methylbut-2-yl, pent-l-
yl, pent-2-yl,
and pent-3-yl.
The term "C6-alkyl," as used herein, means 2,2-dimethylbut-1-yl, 2,3-
dimethylbut-l-
yl, 2,3-dimethylbut-2-yl, 3,3-dimethylbut-1-yl, 3,3-dimethylbut-2-yl, 2-
ethylbut-1-yl, hex-l-
yl, hex-2-yl, hex-3-yl, 2-methylpent-1-yl, 2-methylpent-2-yl, 2-methylpent-3-
yl, 3-
methylpent-l-yl, 3 -methylpent-2-yl, 3 -methylpent-3 -yl, 4-methylpent-l-yl,
and 4-
methylpent-2-yl.
The term "alcohol," as used herein, means methanol, ethanol, isopropanol,
tert-butanol, and the like or a mixture thereof.
The term, "bis-(2-fluorophenyl)iodonium alkyl-sulfonate," as used herein means
bis-
(2-fluorophenyl)iodonium methylsulfonate, bis-(2-fluorophenyl)iodonium
hexanesulfonate,bis-(2-fluorophenyl)iodonium dodecanesulfonate, bis-(2-
fluorophenyl)iodonium trifluromethylsulfonate, bis-(2-fluorophenyl)iodonium
allylsulfonate,
bis-(2-fluorophenyl)iodonium poly(vinyl)sulfonate and the like.
13
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
The term, "bis-(2-fluorophenyl)iodonium aryl-sulfonate," as used herein means
bis-
(2-fluorophenyl)iodonium benzenesulfonate, bis-(2-fluorophenyl)iodonium p-
toluenesulfonate, bis-(2-fluorophenyl)iodonium mesitylsulfonate, bis-(2-
fluorophenyl)iodonium naphthylsulfonate and the like.
The term, "bis-(2-fluorophenyl)iodonium cycloalkyl-sulfonate," as used herein
means
bis-(2-fluorophenyl)iodonium cyclopropylsulfonate, bis-(2-
fluorophenyl)iodonium
cyclohexylsulfonate and the like.
The term, "bis-(2-fluorophenyl)iodonium heterocycle-sulfonate," as used herein
means bis-(2-fluorophenyl)iodonium thiophenylsulfonate, bis-(2-
fluorophenyl)iodonium-2-
pyridylsulfonate, bis-(2-fluorophenyl)iodonium-3-pyridylsulfonate, bis-(2-
fluorophenyl)iodonium furfuryl-5-sulfonate, bis-(2-fluorophenyl)iodonium
indonylsulfonate,
and the like.
Compounds of this invention can have one or more than one asymmetrically
substituted carbon atoms in the R or S configuration. Compounds having
asymmetrically
substituted carbon atoms enriched with one configuration over the other are
assigned the
configuration which is present in the higher amount, preferably 85% to 95%
enrichment,
more preferably 95% to 99% enrichment, and still more preferably greater than
99%
enrichment. Accordingly, compounds of this invention can exist as enantiomers,
mixtures of
enantiomers, diastereomers having relative stereochemistry, diastereomers
having absolute
stereochemistry, diastereomers having at least one asymmetrically substituted
carbon atom
which is enriched in one configuration and at least one asymmetrically
substituted carbon
atom which is not enriched, and mixtures of the preceding.
Compounds of this invention can also have one or more than one carbon-carbon
double bond or carbon-nitrogen double bond. Accordingly, compounds of this
invention can
exist as geometric isomers of either Z or E configuration or as mixtures of
geometric isomers.
The terms "R", "S", "Z", and "E" are as defined by the IUPAC 1974
Recommendations for Section E, Fundamental Stereochemistry, Pure Appl. Chem.
(1976) 45,
13-10.
14
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Compounds of this invention may exist as acid addition salts or base addition
salts
and may be prepared during their isolation or following their purification.
Acid addition salts
of compounds are prepared by reaction with acid. For example, the acetate,
adipate, alginate,
bicarbonate, citrate, aspartate, benzoate, benzenesulfonate, bisulfate,
butyrate, camphorate,
camphorsufonate, citrate, digluconate, formate, fumarate, glycerophosphate,
glutamate,
hemisulfate, heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide,
lactobionate,
lactate, maleate, mesitylenesulfonate, methanesulfonate, naphthylenesulfonate,
nicotinate,
oxalate, pamoate, pectinate, persulfate, phosphate, picrate, propionate,
succinate, tartrate,
thiocyanate, trichloroacetate, trifluoroacetate, para-toluenesulfonate, and
undecanoate salts of
compounds of this invention are meant to be embraced thereby. Base addition
salts of
compounds of this invention may be prepared by reaction with a base such as
the hydroxide,
carbonate, bicarbonate, phosphate, hydrogen phosphate, or dihydrogen phosphate
of cations
such as calcium, iron, lithium, potassium, sodium or magnesium.
The term "isolating" as used herein, means separating a compound from a
solvent,
anti-solvent, or a mixture of solvent and anti-solvent to provide a solid,
semisolid or syrup.
This is typically accomplished by means such as centrifugation, filtration
with or without
vacuum, filtration under positive pressure, distillation, evaporation or a
combination thereof.
Isolating may or may not include purifying during which the chemical, chiral
or chemical and
chiral purity of the isolate is increased. Purifying is typically conducted by
means such as
crystallization, distillation, extraction, filtration through acidic, basic or
neutral alumina,
filtration through acidic, basic or neutral charcoal, column chromatography on
a column
packed with a chiral stationary phase, filtration through a porous paper,
plastic or glass
barrier, column chromatography on silica gel, ion exchange chromatography,
recrystallization, normal-phase high performance liquid chromatography,
reverse-phase high
performance liquid chromatography, trituration and the like.
The phrase "isolating or not isolating" as used herein, means that during the
practice
of this invention, it is optional to isolate a particular compound after each
step prior to the
next step. Such a decision can easily be made by one of ordinary skill in the
art, based on
stability, purity, solvent conditions of the next step, etc.
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
The exemplified compounds and intermediates were named using ACD/ChemSketch
Version 5.06 (05 June 2001, Advanced Chemistry Development Inc.,Toronto,
Ontario), or
ChemDraw Ver. 9Ø5 (CambridgeSoft, Cambridge, MA). N-(4-(4-((2-(4-
chlorophenyl)-
5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-l-yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-
yl)-l-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide was named as in the '799 Patent.
Synthetic routes to prepare 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, and 4-(4-((2-(4-chlorophenyl)-
5,5-
dimethylcyclohex- l -en- l -yl)methyl)piperazin- l -yl)benzoic acid,
intermediates in the
synthesis of apoptosis promoters are described in the '799 Patent, and K.
Ding, et al.
(Synthesis, 2008, 15, 2398-2404, referred to hereinafter as the Ding
reference). The `799
Patent describes a synthesis using trifluoromethyl iodide, a gas with toxicity
concerns. Also,
the subsequent oxidation step uses RuC13 and Na104, creating a highly
exothermic reaction.
The Ding reference describes numerous chemical steps to make 4-(4-((2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoic acid and lower
overall yield.
In addition, the very high reaction temperatures are not readily achievable or
ideal on a large
scale. Finally, the synthesis described in the Ding reference utilizes a
relatively expensive
starting material.
The present inventors previous explorations of the synthesis described in the
Ding, et
al. reference identified a number of problems. First of all, the chemistry
used is burdened
with a potential genotoxic liability due to brominated products created when
hydrobromic
and trifluoroacetic acids are used together. Also, wh I"' o acids s "61 as
~.._- a. 3 er user, hies ,%
... ,-,,.:\ \o,,~i' ov,;. .; ,_,:.
: :.......:.,..-.:.~ ...efhod\ \~, ,_.. ...._.,. .. i.., `:.. ; :, -
m,rit 3-11( process i time were o ise as Finally, poor physical properties of
isolated intermediates made filtrations slow and inefficient.
The present invention avoids these disadvantages.
EMBODIMENTS
One embodiment of this invention, therefore, pertains to a process for making
a
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, comprising:
16
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(a) reacting 4,4-dimethylcyclohexanone, an alkyl formate and a first base to
provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or
not isolating
the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone; and
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, a second
base and a first silyl ether protecting group reagent to provide a first
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or not isolating
the first
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone.
Another embodiment pertains to (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, and (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone, or a
pharmaceutically
acceptable salt thereof, prepared as described in the preceding embodiment.
Another embodiment pertains to the compound (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone or a pharmaceutically
acceptable salt
thereof.
Another embodiment pertains to (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, or a pharmaceutically
acceptable salt
thereof, for use in making N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-
cyclohex-l-en-1-
yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3 -(morpholin-4-yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide or
a pharmaceutically acceptable salt thereof.
Another embodiment pertains to (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, or a pharmaceutically
acceptable salt
thereof, for use in making compounds such as those described in, for example,
U. S. Patent
Publication US2005/0159427A1, the `799 Patent, and U.S. Provisional
Applications
61/145611, 61/120275, 61/181180, and 61/181203, which are hereby incorporated
by
reference.
17
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Another embodiment pertains to the compound (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or a pharmaceutically
acceptable salt
thereof.
Another embodiment pertains to (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or a pharmaceutically
acceptable salt
thereof, for use in making N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-
cyclohex-l-en-1-
yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3 -(morpholin-4-yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide or
a pharmaceutically acceptable salt thereof.
Another embodiment pertains to (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or a pharmaceutically
acceptable salt
thereof, for use in making compounds such as those described in, for example,
U. S. Patent
Publication US2005/0159427A1, the `799 Patent, and U.S. Provisional
Applications
61/145611, 61/120275, 61/181180, and 61/181203.
Another embodiment pertains to the compound (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone, or a
pharmaceutically
acceptable salt thereof.
Another embodiment pertains to (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-
4,4-dimethylcyclohexanone, or a pharmaceutically acceptable salt thereof, for
use in making
N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-
yl)methyl)piperazin- l -
yl)benzoyl)-4-(((1R)-3-(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide or a pharmaceutically acceptable
salt thereof.
Another embodiment pertains to (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-
4,4-dimethylcyclohexanone, or a pharmaceutically acceptable salt thereof, for
use in making
compounds such as those described in, for example, U. S. Patent Publication
US2005/0159427A1, the `799 Patent, and U.S. Provisional Applications
61/145611,
61/120275, 61/181180, and 61/181203.
18
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Compounds described in U. S. Patent Publication US2005/0159427A1, the `799
Patent, and U.S. Provisional Applications 61/145611, 61/120275, 61/181180, and
61/181203,
include 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)-N-
((4-(((1 R)-3-(dimethylamino)-1-((phenylthio)methyl)propyl)amino)-3-
nitrophenyl)sulfonyl)benzamide, 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-
l-en-l-
yl)methyl)piperazin-1-yl)-N-((4-(((1 R)-3 -(isopropyl(methyl)amino)- l -
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)phenyl)sulfonyl)benzamide ,
4-(4-((2-(4-chlorophenyl)-5,5 -dimethylcyclohex- l -en- l -yl)methyl)piperazin-
1-yl)-N-((5 -
(((1 R)-3-(isopropyl(methyl)amino)-1-((phenylthio)methyl)propyl)amino)-4-
nitrothien-2-
yl)sulfonyl)benzamide, 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)-N-((4-((3-morpholin-4-ylpropyl)amino)-3-
nitrophenyl)sulfonyl)-2-
phenoxybenzamide, 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)-N-((3-nitro-4-((tetrahydro-2H-pyran-4-
ylmethyl)amino)phenyl)sulfonyl)-2-(1H-pyrrolo(2,3-b)pyridin-5-yloxy)benzamide,
and the
like.
One embodiment of this invention, therefore, pertains to a process for making
2-(4-
chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(a) reacting 4,4-dimethylcyclohexanone, an alkyl formate and a first base to
provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or
not isolating
the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, a second
base and a first silyl ether protecting group reagent to provide a first
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or not isolating
the first
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(c) reacting the first protected (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and 4-chlorophenyl magnesium bromide to provide the
first
protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the first protected (2E)-1-(4-chlorophenyl)-2-
(hydroxymethylene)-
4,4-dimethylcyclohexanol; and
(d) reacting the first protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-
4,4-dimethylcyclohexanol and a first acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde.
19
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde or a pharmaceutically acceptable salt thereof.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, for use in making
N-(4-(4-((2-(4-
chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin- l -
yl)benzoyl)-4-(((l R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide or a pharmaceutically acceptable
salt thereof.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, for use in making
compounds
such as those described in, for example, U. S. Patent Publication
US2005/0159427A1, the
`799 Patent, and U.S. Provisional Applications 61/145611, 61/120275,
61/181180, and
61/181203.
Examples of alkyl formates useful for the practice of this invention are
methyl
formate, ethyl formate, n-propyl formate, tert-butyl formate and the like.
Examples of first bases useful for the practice of this invention are sodium
hydride,
sodium tert-butoxide, potassium tert-butoxide and the like.
Examples of second bases useful for the practice of this invention are
triethylamine,
2,6-lutidine, pyridine, imidazole, diisopropylethylamine, N-methylmorpholine,
dimethylaniline and the like.
Examples of first silyl ether protecting group reagents useful for the
practice of this
invention include trimethylchlorosilane, tert-butylchlorodimethylsilane,
triisopropylchlorosilane, tert-butylchlorodiphenylsilane, and the like.
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Examples of first protected (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanones
useful for the practice of this invention include (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone, and the like.
Examples of first protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanols useful for the practice of this invention include (2E)-
1 -(4-
chlorophenyl)-4,4-dimethyl-2-(((triisopropylsilyl)oxy)methylene)cyclohexanol,
(2E)-1-(4-
chlorophenyl)-4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanol,
(2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and the
like.
Examples of first acids useful for the practice of this invention are
tetra-n-butylammonium fluoride, trifluoroacetic acid, hydrochloric acid,
trifluoromethanesulfonic acid, sulfuric acid and the like.
Step (a) is typically conducted for about 6 to about 18 hours in a solvent
such as
tetrahydrofuran, N,N-dimethylformamide, mixtures thereof and the like.
Step (b) is typically conducted for about 4 to about 16 hours in solvents such
as
tetrahydrofuran, DMF, toluene, 2-methyltetrahydrofuran, ethyl acetate,
mixtures thereof and
the like.
Step (c) is typically conducted from about 2 to about 4 hours in a solvent
including
toluene, diethyl ether, tetrahydrofuran, N,N-dimethylformamide and the like or
mixtures
thereof.
Step (d) is typically conducted from about 1 to about 4 hours in solvents such
as
toluene, diethyl ether, tetrahydrofuran, N,N-dimethylformamide, water,
methanol, and the
like or mixtures thereof.
Still another embodiment pertains to a process for making ethyl 4-(4-((2-(4-
chlorophenyl)-5,5-/dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoate,
comprising:
21
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate.
Examples of first reducing agents useful for the practice of this invention
include
sodium triacetoxyborohydride and sodium cyanoborohydride.
Step (e) is typically conducted from about 10 to about 16 hours in solvents
such as
dichloromethane, acetonitrile, toluene, diethyl ether, tetrahydrofuran,
N,N-dimethylformamide, methyl tert-butyl ether, mixtures thereof and the like.
Still another embodiment pertains to ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoate prepared as
described in the
preceding embodiment.
Still another embodiment pertains to ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoate , or a
pharmaceutically
acceptable salt thereof, for use in making N-(4-(4-((2-(4-chlorophenyl)-5,5-
dimethyl-l-
cyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoyl)-4-(((lR)-3-(morpholin-4-yl)-
1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide , or
a pharmaceutically acceptable salt thereof.
Another embodiment pertains to ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoate, or a
pharmaceutically
acceptable salt thereof, for use in making compounds such as those described
in , for
example, U. S. Patent Publication US2005/0159427A1, the `799 Patent, and U.S.
Provisional
Applications 61/145611, 61/120275, 61/181180, and 61/181203.
Still another embodiment pertains to a process for making 4-(4-((2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoic acid, comprising:
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and a third base and isolating or not
isolating the 4-(4-((2-
(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoic
acid.
22
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Examples of third bases useful for the practice of this invention are sodium
hydroxide,
potassium hydroxide and the like.
Step (f) is typically conducted from about 10 hours to about 20 hours in
solvents such
as ethanol, tetrahydrofuran, heptanes, 2-methyltetrahydrofuran, water,
mixtures thereof and
the like.
Still another embodiment pertains to 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en-l-yl)methyl)piperazin-l-yl)benzoic acid, or a pharmaceutically acceptable
salt thereof,
prepared as described in the preceding embodiment.
Still another embodiment pertains to 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en-l-yl)methyl)piperazin-l-yl)benzoic acid, or a pharmaceutically acceptable
salt thereof,
for use in making N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide , or
a pharmaceutically acceptable salt thereof.
Another embodiment pertains to 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-
1-
en-l-yl)methyl)piperazin-l-yl)benzoic acid, or a pharmaceutically acceptable
salt thereof, for
use in making compounds such as those described in, for example, U. S. Patent
Publication
US2005/0159427A1, the `799 Patent, and U.S. Provisional Applications
61/145611,
61/120275, 61/181180, and 61/181203.
Still another embodiment of this invention, therefore, pertains to a process
for making
(2E)-4,4-dimethyl-2-(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium
tert-butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone
and
isolating or not isolating the (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone; and
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
23
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
triisopropylchlorosilane to provide (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone.
Another embodiment pertains to (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, and 2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone , or a
pharmaceutically
acceptable salt thereof, prepared as described in the preceding embodiment.
Still another embodiment of this invention pertains to a process for making 2-
(4-
chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and
isolating or
not isolating the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane to provide (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide to provide ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
24
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
(d) reacting (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde.
Still another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-
1-carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Still another embodiment pertains to a process for making ethyl 4-(4-((2-(4-
chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoate,
comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate.
Still another embodiment pertains to ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoate, or a
pharmaceutically
acceptable salt thereof, prepared as described in the preceding embodiment.
Still another embodiment pertains to a process for making 4-(4-((2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoic acid, comprising:
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and sodium hydroxide and isolating or not
isolating the 4-
(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-
yl)benzoic acid.
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Still another embodiment pertains to 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en-l-yl)methyl)piperazin-l-yl)benzoic acid, or a pharmaceutically acceptable
salt thereof,
prepared as described in the preceding embodiment.
Still another embodiment of this invention, therefore, pertains to a process
for making
(2E)-4,4-dimethyl-2-(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone at
about -10 C
to about 0 C and isolating or not isolating the (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone; and
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane at about -10 C to about 0 C to provide (2E)-4,4-
dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone.
Another embodiment pertains to (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, and 2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone, or a
pharmaceutically
acceptable salt thereof, prepared as described in the preceding embodiment.
Still another embodiment pertains to a process for making 2-(4-chlorophenyl)-
5,5-
dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium
tert-butoxide at about -10 C to about 0 C to provide (2E)-2-(hydroxymethylene)-
4,4-
26
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
dimethylcyclohexanone and isolating or not isolating the (2E)-2-
(hydroxymethylene)-4,4-
dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane at about -10 C to about 0 C to provide (2E)-4,4-
dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide at about -10 C to about 5 C to provide ((2E)-1-(4-
chlorophenyl)-4,4-
dimethyl-2-(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-
chlorophenyl)-4,4-
dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol, and
(d) reacting ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid at about 5 C to about 20 C to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-ene-l-carbaldehyde.
27
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Still another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-
1-carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Still another embodiment pertains to a process for making ethyl 4-(4-((2-(4-
chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoate,
comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride at about 15 C
to about
30 C and isolating or not isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en- l -yl)methyl)piperazin-l-yl)benzoate.
Still another embodiment pertains to ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoate, or a
pharmaceutically
acceptable salt thereof, prepared as described in the preceding embodiment.
Still another embodiment pertains to a process for making 4-(4-((2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoic acid, comprising:
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and sodium hydroxide at about 55 C to about
75 C, and
isolating or not isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-
l-en-1-
yl)methyl)piperazin-1-yl)benzoic acid.
Still another embodiment pertains to 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en-l-yl)methyl)piperazin-l-yl)benzoic acid, or a pharmaceutically acceptable
salt thereof,
prepared as described in the preceding embodiment.
Still another embodiment pertains to a process for making N-(4-(4-((2-(4-
chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin- l -
yl)benzoyl)-4-(((l R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide comprising:
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and a
28
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
first coupling reagent with or without a fourth base and with or without an
auxiliary coupling
reagent, and isolating or not isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-
dimethyl-l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment pertains to a process for making an apoptosis
promoter, or a
pharmaceutically acceptable salt thereof, such as those described in, for
example, U. S. Patent
Publication US2005/0159427A1, the `799 Patent, and U.S. Provisional
Applications
61/145611, 61/120275, 61/181180, and 61/181203, comprising:
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoic acid, a first appropriate benzenesulfonamide
and a first
coupling reagent with or without a fourth base and with or without an
auxiliary coupling
reagent, and isolating or not isolating the apoptosis promoter.
Examples of first coupling reagents are 1-ethyl-3-(3-(dimethylamino)propyl)-
carbodiimide hydrochloride, N,N'-dicyclohexylcarbodiimide, N,N'-
diisopropylcarbodiimide,
1,1'-carbonyldiimidazole and the like.
Examples of first auxiliary coupling reagents include 4-dimethylaminopyridine,
hydroxybenzotriazole, 1-hydroxy-7-aza-benzotriazole and the like.
Examples of fourth bases include 1,8-diazabicyclo(5.4.0)undec-7-ene, potassium
tert-
butoxide and the like.
Examples of apoptosis promoters include 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex- l -en-l-yl)methyl)piperazin-1-yl)-N-((4-(((1 R)-3-
(dimethylamino)-l -
((phenylthio)methyl)propyl)amino)-3-nitrophenyl)sulfonyl)benzamide, 4-(4-((2-
(4-
chlorophenyl)-5,5-dimethylcyclohex-l -en- l -yl)methyl)piperazin-1-yl)-N-((4-
(((1 R)-3-
29
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(isopropyl(methyl)amino)-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)phenyl)sulfonyl)benzamide , 4-(4-((2-(4-
chlorophenyl)-5,5-
dimethylcyclohex- l -en- l -yl)methyl)piperazin- l -yl)-N-((5-(((l R)-3-
(isopropyl(methyl)amino)-1-((phenylthio)methyl)propyl)amino)-4-nitrothien-2-
yl)sulfonyl)benzamide, 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)-N-((4-((3-morpholin-4-ylpropyl)amino)-3-
nitrophenyl)sulfonyl)-2-
phenoxybenzamide, 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)-N-((3-nitro-4-((tetrahydro-2H-pyran-4-
ylmethyl)amino)phenyl)sulfonyl)-2-(1H-pyrrolo(2,3-b)pyridin-5-yloxy)benzamide,
and the
like.
Examples of first appropriate benzenesulfonamides include 4-(((1R)-3-morpholin-
4-
yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
(R)-4-(4-(dimethylamino)-1-(phenylthio)butan-2-ylamino)-3-
nitrobenzenesulfonamide, and
the like.
Step (g) is typically conducted from about 36 to about 50 hours in solvents
such as
dichloromethane, acetonitrile, dioxane, tetrahydrofuran, mixtures thereof and
the like.
Still another embodiment pertains to a process for making an apoptosis
promoter, or a
pharmaceutically acceptable salt thereof, compounds such as those described
in, for
example, U. S. Patent Publication US2005/0159427A1, the `799 Patent, and U.S.
Provisional
Applications 61/145611, 61/120275, 61/181180, and 61/181203, comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoic acid, a first appropriate benzenesulfonamide
and a first
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
coupling reagent with or without a fourth base and with or without an
auxiliary coupling
reagent, and isolating or not isolating the apoptosis promoter.
Still another embodiment pertains to a process for making N-(4-(4-((2-(4-
chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoyl)-
4-(((1R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a pharmaceutically
acceptable salt thereof,
comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and a
first coupling reagent with or without a fourth base and with or without an
auxiliary coupling
reagent, and isolating or not isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-
dimethyl-l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment pertains to a process for making N-(4-(4-((2-(4-
chlorophenyl)-5, 5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin-1-
yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide comprising:
31
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoyl)-4-(((1R)-3-(morpholin-4-yl)-
l-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment pertains to a process for making an apoptosis
promoter, or a
pharmaceutically acceptable salt thereof, such as those described in, for
example, U. S. Patent
Publication US2005/0159427A1, the `799 Patent, and U.S. Provisional
Applications
61/145611, 61/120275, 61/181180, and 61/181203, comprising:
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, a first appropriate amine, 1-ethyl-3-(3-
(dimethylamino)propyl)carbodiimide hydrochloride and 4-dimethylaminopyridine,
and
isolating or not isolating the apoptosis promoter.
Still another embodiment pertains to a process for making an apoptosis
promoter, or a
pharmaceutically acceptable salt thereof, for use in making compounds, such as
those
described in, for example, U. S. Patent Publication US2005/0159427A1, the `799
Patent, and
U.S. Provisional Applications 61/145611, 61/120275, 61/181180, and 61/181203,
comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-I-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
32
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, a first appropriate amine, 1-ethyl-3-(3-
(dimethylamino)propyl)carbodiimide hydrochloride and 4-dimethylaminopyridine,
and
isolating or not isolating the apoptosis promoter.
Still another embodiment pertains to a process for making N-(4-(4-((2-(4-
chlorophenyl)-5, 5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin-1-
yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a pharmaceutically
acceptable salt thereof,
comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoyl)-4-(((1R)-3-(morpholin-4-yl)-
l-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
33
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Still another embodiment pertains to a process for making N-(4-(4-((2-(4-
chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin- l -
yl)benzoyl)-4-(((1 R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide comprising:
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-1-
yl)benzoyl)-
4-(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoyl)-4-(((1R)-3-(morpholin-4-yl)-
l-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment pertains to a process for making an apoptosis
promoter, or a
pharmaceutically acceptable salt thereof, such as those described in, for
example, U. S. Patent
Publication US2005/0159427A1, the `799 Patent, and U.S. Provisional
Applications
61/145611, 61/120275, 61/181180, and 61/181203,comprising:
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, a first appropriate amine, 1-ethyl-3-(3-
(dimethylamino)propyl)carbodiimide hydrochloride and 4-dimethylaminopyridine
at about
25 C to about 35 C, and isolating or not isolating the apoptosis promoter.
Still another embodiment pertains to a process for making an apoptosis
promoter, or a
pharmaceutically acceptable salt thereof, such as those described in, for
example, U. S. Patent
Publication US2005/0159427A1, the `799 Patent, and U.S. Provisional
Applications
61/145611, 61/120275, 61/181180, and 61/181203, comprising:
34
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride at about 15 C
to about
30 C and isolating or not isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en- l -yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-I-yl)benzoate and sodium hydroxide at about 55 C to about
75 C, and
isolating or not isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-
l-en-1-
yl)methyl)piperazin-1-yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, a first appropriate amine, 1-ethyl-3-(3-
(dimethylamino)propyl)carbodiimide hydrochloride and 4-dimethylaminopyridine
at about
25 C to about 35 C, and isolating or not isolating the apoptosis promoter.
Still another embodiment pertains to a process for making N-(4-(4-((2-(4-
chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoyl)-
4-(((1R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a pharmaceutically
acceptable salt thereof,
comprising:
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride at about 15 C
to about
C and isolating or not isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en- l -yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-I-yl)benzoate and sodium hydroxide at about 55 C to about
75 C, and
25 isolating or not isolating the 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-1-
yl)methyl)piperazin-1-yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
30 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -
yl)methyl)piperazin-1-yl)benzoyl)-
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
4-(((l R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoyl)-4-(((lR)-3-(morpholin-4-yl)-
1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, an alkyl formate and a first base to
provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or
not isolating
the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, a second
base and a first silyl ether protecting group reagent to provide a first
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or not isolating
the first
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(c) reacting the first protected (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and 4-chlorophenyl magnesium bromide to provide the
first
protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the first protected (2E)-1-(4-chlorophenyl)-2-
(hydroxymethylene)-
4,4-dimethylcyclohexanol; and
(d) reacting the first protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-
4,4-dimethylcyclohexanol and a first acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
36
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin-l-yl)benzoyl)-4-(((lR)-3-(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and
isolating or
not isolating the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane to provide (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
37
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide to provide ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol;
(d) reacting (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
38
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide at about -10 C to about 0 C to provide (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and isolating or not isolating the (2E)-2-
(hydroxymethylene)-4,4-
dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane at about -10 C to about 0 C to provide (2E)-4,4-
dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide at about -10 C to about 5 C to provide ((2E)-1-(4-
chlorophenyl)-4,4-
dimethyl-2-(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-
chlorophenyl)-4,4-
dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
39
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol, and
(d) reacting (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid at about 5 C to about 20 C to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-ene-l-carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride at about 15 C
to about
30 C and isolating or not isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en- l -yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-I-yl)benzoate and aqueous sodium hydroxide at about 55 C
to about
75 C, and isolating or not isolating the 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-
1-yl)methyl)piperazin-1-yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -
yl)methyl)piperazin-1-yl)benzoyl)-
4-(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
One embodiment of this invention, therefore, pertains to a process for making
2-(4-
chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(h) reacting 4,4-dimethylcyclohexanone, diethoxycarbenium fluoroborate and a
fifth base to provide (2-(diethoxymethyl)-4,4-dimethylcyclohexanone and
isolating or not
isolating the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone;
(i) reacting the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone and 4-
chlorophenyl magnesium bromide to provide 1-(4-chlorophenyl)-2-
(diethoxymethyl)-4,4-
dimethylcyclohexanol and isolating or not isolating the 1-(4-chlorophenyl)-2-
(diethoxymethyl)-4,4-dimethylcyclohexanol; and
(j) reacting the 1-(4-chlorophenyl)-2-(diethoxymethyl)-4,4-
dimethylcyclohexanol
and a second acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, for use in making
N-(4-(4-((2-(4-
chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin- l -
yl)benzoyl)-4-(((l R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a pharmaceutically
acceptable salt thereof.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, for use in making
compounds
such as those described in, for example, U. S. Patent Publication
US2005/0159427A1, the
`799 Patent, and U.S. Provisional Applications 61/145611, 61/120275,
61/181180, and
61/181203.
Examples of fifth bases useful for the practice of this invention include N,N-
diisopropylethylamine, 1,8-diazabicyclo(5.4.0)undec-7-ene and the like.
41
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Examples of second acids useful for the practice of this invention include
aqueous
hydrochloric acid, sulfuric acid and the like.
Step (h) is typically conducted for about 1 to about 3 hours in a solvent such
as
dichloromethane and the like.
Step (i) is typically conducted for about 1 to about 3 hours in a solvent such
as
tetrahydrofuran, diethyl ether, mixtures thereof and the like.
Step (j) is typically conducted for about 15 to about 20 hours in a solvent
such as
tetrahydrofuran, diethyl ether, mixtures thereof and the like.
Still another embodiment of this invention, therefore, pertains to a process
for making
2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(h) reacting 4,4-dimethylcyclohexanone, diethoxycarbenium fluoroborate and
N,N-diisopropylethylamine to provide (2-(diethoxymethyl)-4,4-
dimethylcyclohexanone and
isolating or not isolating the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone;
(i) reacting the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone and 4-
chlorophenyl magnesium bromide to provide 1-(4-chlorophenyl)-2-
(diethoxymethyl)-4,4-
dimethylcyclohexanol and isolating or not isolating the 1-(4-chlorophenyl)-2-
(diethoxymethyl)-4,4-dimethylcyclohexanol; and
(j) reacting the 1-(4-chlorophenyl)-2-(diethoxymethyl)-4,4-
dimethylcyclohexanol
and aqueous hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-1-
carbaldehyde and isolating or not isolating the 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-1-
ene-l-carbaldehyde.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Still another embodiment of this invention, therefore, pertains to a process
for making
2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(h) reacting 4,4-dimethylcyclohexanone, diethoxycarbenium fluoroborate and
N,N-diisopropylethylamine at about -60 C to -90 C to provide (2-
(diethoxymethyl)-4,4-
42
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
dimethylcyclohexanone and isolating or not isolating the 2-(diethoxymethyl)-
4,4-
dimethylcyclohexanone;
(i) reacting the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone and 4-
chlorophenyl magnesium bromide at about -60 C to -10 C to provide 1-(4-
chlorophenyl)-2-
(diethoxymethyl)-4,4-dimethylcyclohexanol and isolating or not isolating the 1-
(4-
chlorophenyl)-2-(diethoxymethyl)-4,4-dimethylcyclohexanol; and
(j) reacting the 1-(4-chlorophenyl)-2-(diethoxymethyl)-4,4-
dimethylcyclohexanol
and aqueous hydrochloric acid at about 50 C to 80 C to provide 2-(4-
chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-ene-l-carbaldehyde.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(h) reacting 4,4-dimethylcyclohexanone, diethoxycarbenium fluoroborate and a
fifth base to provide (2-(diethoxymethyl)-4,4-dimethylcyclohexanone and
isolating or not
isolating the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone;
(i) reacting the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone and 4-
chlorophenyl magnesium bromide to provide 1-(4-chlorophenyl)-2-
(diethoxymethyl)-4,4-
dimethylcyclohexanol and isolating or not isolating the 1-(4-chlorophenyl)-2-
(diethoxymethyl)-4,4-dimethylcyclohexanol;
(j) reacting the 1-(4-chlorophenyl)-2-(diethoxymethyl)-4,4-
dimethylcyclohexanol
and a second acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
43
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin-l-yl)benzoyl)-4-(((lR)-3-(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(h) reacting 4,4-dimethylcyclohexanone, diethoxycarbenium fluoroborate and
N,N-diisopropylethylamine to provide (2-(diethoxymethyl)-4,4-
dimethylcyclohexanone and
isolating or not isolating the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone;
(i) reacting the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone and 4-
chlorophenyl magnesium bromide to provide 1-(4-chlorophenyl)-2-
(diethoxymethyl)-4,4-
dimethylcyclohexanol and isolating or not isolating the 1-(4-chlorophenyl)-2-
(diethoxymethyl)-4,4-dimethylcyclohexanol;
(j) reacting the 1-(4-chlorophenyl)-2-(diethoxymethyl)-4,4-
dimethylcyclohexanol
and aqueous hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-1-
carbaldehyde and isolating or not isolating the 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-1-
ene- l -carbaldehyde;
44
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-I-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(h) reacting 4,4-dimethylcyclohexanone, diethoxycarbenium fluoroborate and
N,N-diisopropylethylamine at about -60 C to -90 C to provide (2-
(diethoxymethyl)-4,4-
dimethylcyclohexanone and isolating or not isolating the 2-(diethoxymethyl)-
4,4-
dimethylcyclohexanone;
(i) reacting the 2-(diethoxymethyl)-4,4-dimethylcyclohexanone and 4-
chlorophenyl magnesium bromide at about -60 C to -10 C to provide 1-(4-
chlorophenyl)-2-
(diethoxymethyl)-4,4-dimethylcyclohexanol and isolating or not isolating the 1-
(4-
chlorophenyl)-2-(diethoxymethyl)-4,4-dimethylcyclohexanol;
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(j) reacting the 1-(4-chlorophenyl)-2-(diethoxymethyl)-4,4-
dimethylcyclohexanol
and aqueous hydrochloric acid at about 50 C to 80 C to provide 2-(4-
chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride at about 15 C
to about
30 C and isolating or not isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en- l -yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide at about 55 C
to about
75 C, and isolating or not isolating the 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-
1-yl)methyl)piperazin-1-yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -
yl)methyl)piperazin-1-yl)benzoyl)-
4-(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
One embodiment of this invention, therefore, pertains to a process for making
2-(4-
chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(k) reacting 4,4-dimethylcyclohexanone, and phosphorus oxychloride to provide
2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde and isolating or not isolating
the 2-chloro-
5,5-dimethylcyclohex-l-enecarbaldehyde; and
46
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(1) reacting the 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde, 4-
chlorophenylboronic acid, a first phase transfer catalyst, a fifth base, and a
first palladium
catalyst to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde and
isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, for use in making
N-(4-(4-((2-(4-
chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -yl)methyl)piperazin- l -
yl)benzoyl)-4-(((l R)-
3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide , or a pharmaceutically
acceptable salt
thereof.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde , or a pharmaceutically acceptable salt thereof, for use in
making compounds
such as those described in, for example, U. S. Patent Publication
US2005/0159427A1, the
`799 Patent, and U.S. Provisional Applications 61/145611, 61/120275,
61/181180, and
61/181203.
Examples of first phase transfer catalysts useful for the practice of this
invention
include tetrabutyl ammonium bromide, tetrapropyl ammonium bromide, tributyl
benzyl
ammonium chloride, tetraethyl ammonium bromide, tetraoctyl ammonium bromide,
tetra
butyl ammonium hydrogen sulphate, benzyl trimethyl ammonium chloride, benzyl
triethyl
ammonium chloride, tetrabutyl ammonium acetate, tetrabutyl ammonium iodide,
and the like.
Examples of fifth bases useful for the practice of this invention include
potassium
carbonate, potassium phosphate, potassium fluoride, potassium t-butoxide and
the like.
Examples of first palladium catalysts useful for the practice of this
invention include
palladium (II) acetate, tris(dibenzylideneacetone)dipalladium(0),
47
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
tetrakis(triphenylphosphine)palladium(O), (1,1'-
bis(diphenylphosphino)ferrocene)dichloropalladium(II), and the like.
Step (k) is typically conducted for about 15 to about 20 hours in a solvent
such as
dichloromethane, N,N-dimethylformamide, mixtures thereof and the like.
Step (1) is typically conducted for about 5 to about 10 hours in a solvent
such as water,
or mixtures of water and one or more organic solvents such as toluene,
methylene chloride,
DMF, and the like.
Another embodiment of this invention, therefore, pertains to a process for
making
2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(k) reacting 4,4-dimethylcyclohexanone, and phosphorus oxychloride to provide
2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde and isolating or not isolating
the 2-chloro-
5,5-dimethylcyclohex-l-enecarbaldehyde; and
(1) reacting the 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde, 4-
chlorophenylboronic acid, tetrabutyl ammonium bromide, potassium carbonate,
and
palladium (II) acetate to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-l-
carbaldehyde and isolating or not isolating the 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-1-
ene-l-carbaldehyde.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Another embodiment of this invention, therefore, pertains to a process for
making 2-
(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde, comprising:
(k) reacting 4,4-dimethylcyclohexanone and phosphorus oxychloride at about
20 C to 45 C to provide 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde and
isolating or
not isolating the 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde; and
(1) reacting the 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde, 4-
chlorophenylboronic acid, tetrabutyl ammonium bromide, potassium carbonate,
and
palladium (II) acetate at about 20 C to 45 C to provide 2-(4-chlorophenyl)-5,5-
48
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde.
Another embodiment pertains to 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-1-
carbaldehyde, or a pharmaceutically acceptable salt thereof, prepared as
described in the
preceding embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(k) reacting 4,4-dimethylcyclohexanone, and phosphorus oxychloride to provide
2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde and isolating or not isolating
the 2-chloro-
5,5-dimethylcyclohex- l -enecarbaldehyde;
(1) reacting the 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde, 4-
chlorophenylboronic acid, a first phase transfer catalyst, a fifth base, and a
first palladium
catalyst to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde and
isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
49
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(k) reacting 4,4-dimethylcyclohexanone, and phosphorus oxychloride to provide
2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde and isolating or not isolating
the 2-chloro-
5,5-dimethylcyclohex- l -enecarbaldehyde;
(1) reacting the 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde, 4-
chlorophenylboronic acid, tetrabutyl ammonium bromide, potassium carbonate,
and
palladium (II) acetate to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-l-
carbaldehyde and isolating or not isolating the 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-1-
ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(k) reacting 4,4-dimethylcyclohexanone and phosphorus oxychloride at about
C to 45 C to provide 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde and
isolating or
not isolating the 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde;
(1) reacting the 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde, 4-
15 chlorophenylboronic acid, tetrabutyl ammonium bromide, potassium carbonate,
and
palladium (II) acetate at about 20 C to 45 C to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
20 ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride at about 15
C to about
C and isolating or not isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en- l -yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide at about 55 C
to about
25 75 C, and isolating or not isolating the 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-
1-yl)methyl)piperazin-1-yl)benzoic acid; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
30 1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -
yl)methyl)piperazin-1-yl)benzoyl)-
51
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
4-(((l R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoyl)-4-(((lR)-3-(morpholin-4-yl)-
1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
One embodiment of this invention, therefore, pertains to a process for making
4-
(((1 R)-3-morpholin-4-yl-l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(m) reacting 2-fluorobenzenesulfonyl chloride, and a first fluoride source to
provide 2-fluorobenzenesulfonyl fluoride and isolating or not isolating the 2-
fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and a second fluoride source to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene and
isolating or not isolating the 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and a
first NH3 source to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and
isolating or not isolating the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and a sixth base to provide 4-
(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, prepared as described in the
preceding embodiment.
52
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, for use in making N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoyl)-4-(((1R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide
or a salt thereof.
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, for use in making compounds such as
those
described in , for example, U. S. Patent Publication US2005/0159427A1, the
`799 Patent, and
U.S. Provisional Applications 61/145611, 61/120275, 61/181180, and 61/181203.
Examples of first fluoride sources useful for the practice of this invention
include
tetra-n-butylammonium fluoride and potassium fluoride, and mixtures thereof.
Examples of second fluoride sources useful for the practice of this invention
include
tris(dimethylamino)sulfonium difluorotrimethylsilicate, tetra-n-butylammonium
fluoride,
cesium fluoride, tetrabutylammonium triphenyldifluorosilicate, and
tetrabutylammonium
triphenyldifluorsilicate.
Examples of first NH3 sources useful for the practice of this invention
include
aqueous ammonium hydroxide, ammonia in methanol, ammonium carbamate, ammonia
in
isopropyl alcohol, and hexamethyldisilazane.
Examples of sixth bases useful for the practice of this invention include
triethylamine
and the like.
Step (m) is typically conducted for about 30 minutes to about 2 hours in a
solvent
such as tetrahydrofuran, acetonitrile, water, mixtures thereof and the like.
53
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Step (n) is typically conducted for about 30 minutes to about 2 hours in a
solvent such
as dichloromethane, tetrahydrofuran, toluene, dimethoxyethane, N-methyl-2-
pyrrolidone
mixtures thereof and the like.
Step (o) is typically conducted for about 15 to about 25 hours.
Step (p) is typically conducted for about 30 minutes to about 24 hours in a
solvent
such acetonitrile, tetrahydrofuran, ethyl acetate, isopropyl alcohol,
isopropyl acetate,
dimethoxyethane, dichloromethane, toluene, mixtures thereof and the like.
Step (q) is typically conducted for about 12 to about 24 hours in a solvent
such as
ethyl acetate, tetrahydrofuran, 2-methyl tetrahydrofuran, mixtures thereof and
the like.
Another embodiment of this invention pertains to a process for making 4-(((1R)-
3-
morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(m) reacting 2-fluorobenzenesulfonyl chloride, and tetra-n-butylammonium
fluoride to provide 2-fluorobenzenesulfonyl fluoride and isolating or not
isolating the 2-
fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and tris(dimethylamino)sulfonium difluorotrimethylsilicate to provide 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine to provide
4-(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
54
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, prepared as described in the
preceding embodiment.
Another embodiment of this invention pertains to a process for making 4-(((1R)-
3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(m) reacting 2-fluorobenzenesulfonyl chloride, and tetra-n-butylammonium
fluoride at about -10 C to about 10 C to provide 2-fluorobenzenesulfonyl
fluoride and
isolating or not isolating the 2-fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and tris(dimethylamino)sulfonium difluorotrimethylsilicate at about room
temperature to
provide 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene and isolating or not
isolating the 1-
fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid at about 110 C to about 130 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonyl chloride and isolating or not
isolating the 4-
fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide at about -5 C to about -60 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl- 1-((phenylthio)methyl)propylamine and triethylamine at about
40 C to about
50 C to provide 4-(((1R)-3-morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-
3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, prepared as described in the
preceding embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(m) reacting 2-fluorobenzenesulfonyl chloride, and a first fluoride source to
provide 2-fluorobenzenesulfonyl fluoride and isolating or not isolating the 2-
fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and a second fluoride source to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene and
isolating or not isolating the 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and a
first NH3 source to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and
isolating or not isolating the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and a sixth base to provide 4-
(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
56
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(m) reacting 2-fluorobenzenesulfonyl chloride, and tetra-n-butylammonium
fluoride to provide 2-fluorobenzenesulfonyl fluoride and isolating or not
isolating the 2-
fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and tris(dimethylamino)sulfonium difluorotrimethylsilicate to provide 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine to provide
4-(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
57
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(m) reacting 2-fluorobenzenesulfonyl chloride, and tetra-n-butylammonium
fluoride at about 0 C to about 10 C to provide 2-fluorobenzenesulfonyl
fluoride and isolating
or not isolating the 2-fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and tris(dimethylamino)sulfonium difluorotrimethylsilicate at about room
temperature to
provide 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene and isolating or not
isolating the 1-
fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid at about 110 C to about 130 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonyl chloride and isolating or not
isolating the 4-
fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide at about -5 C to about -60 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine at about 40
C to about
50 C to provide 4-(((1R)-3-morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-
3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
58
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -
yl)methyl)piperazin-1-yl)benzoyl)-
4-(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
One embodiment of this invention, therefore, pertains to a process for making
4-
(((1 R)-3-morpholin-4-yl-l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(r) reacting a first metal trifluoromethanesulfinate, a first aryl fluoride,
and a first
catalyst to provide 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene and
isolating or not
isolating the 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and a
first NH3 source to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and
isolating or not isolating the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and a sixth base to provide 4-
(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
59
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, prepared as described in the
preceding embodiment.
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide for use
in making N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3 -(morpholin-4-yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof.
Another embodiment pertains to 4-(((1R)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, for use in making compounds such as
those
described in , for example, U. S. Patent Publication US2005/0159427A1, the
`799 Patent, and
U.S. Provisional Applications 61/145611, 61/120275, 61/181180, and 61/181203.
Examples of first catalysts useful for the practice of this invention include
Cul, Cu20,
CuC1, copper (I) trifluoromethanesulfonate benzene complex, copper triflate,
CuSCN, copper
acetate, and palladium(II) acetate alone or in combination with ligands such
as
tetramethylethylenediamine, 4,4'-ditert-butyl-2,2'-dipyridyl, 1,10-
phenanthroline, 2,9-
dimethyl-1,10-phenanthroline, 3,4,7,8-tetramethyl-1,10-phenanthroline, 4,7-
diphenyl-1,10-
phenanthrolines, 2-acetylcyclohexanone, N,N-diethylsalicylamide, triphenyl
phosphine, tri-
tert-butylphosphine, 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl, 1,1'-
bis(diphenylphosphino)ferrocene, 2-(di-t-butylphosphino)biphenyl, triphenyl
phosphite,
triphenylphosphine oxide, dimethylmethylphosphonate, 1,3-bis(2,4,6-
trimethylphenyl)-1,3-
dihydro-2H-imidazol-2-ylidene, 1,3-bis(2,6-diisopropylphenyl)-imidazol-2-
ylidene, 1,3-
bis(l -adamantyl)imidazol-2-ylidene, 1,3-di-tert-butylimidazol-2-ylidene and
the like.
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Examples of first metal trifluoromethanesulfinates useful for the practice of
this
invention include potassium trifluoromethanesulfinate, lithium
trifluoromethanesulfinate,
rubidium trifluoromethanesulfinate, cesium trifluoromethanesulfinate, and the
like.
Examples of first aryl fluoride sources useful for the practice of this
invention include
mesityl-2-fluorophenyl iodonium triflate, bis-(2-fluorophenyl)iodonium alkyl-
sulfonates, bis-
(2-fluorophenyl)iodonium aryl-sulfonates, bis-(2-fluorophenyl)iodonium
cycloalkyl-
sulfonates, bis-(2-fluorophenyl)iodonium heterocycle-sulfonates, bis-(2-
fluorophenyl)iodonium tetrafluoroborates, bis-(2-fluorophenyl)iodonium
hexafluorophosphates, and the like.
Step (r) is typically conducted for about 10 hours to about 20 hours in a
solvent such
as N,N-dimethylformamide, dimethylsulfoxide, dimethylacetamide, 1,4-dioxane,
1,2-
dimethoxyethane, tetrahydrofuran, 1-methyl-2-pyrrolidinone, diglyme, methyl
tert-butyl
ether, methylene dichloride, chloroform, carbon tetrachloride, acetone, ethyl
acetate,
isopropyl acetate, acetonitrile, benzonitrile, toluene, benzene, mesitylene,
xylenes, anisole,
chlorobenzenes, fluorobenzenes, hexanes, heptanes, pentanes, methanol,
ethanol, propanol,
butanol, tert-butanol, hexanol, octanol, pyridines, tri-butylamines, mixtures
thereof and the
like.
Another embodiment of this invention pertains to a process for making 4-(((1R)-
3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and copper(I) oxide to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
61
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine to provide
4-(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, prepared as described in the
preceding embodiment.
Another embodiment of this invention pertains to a process for making 4-(((1R)-
3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and copper(I) oxide at about 0 C to about 110 C to provide 1-
fluoro-2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid at about 110 C to about 130 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonyl chloride and isolating or not
isolating the 4-
fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide at about -5 C to about -60 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl- 1-((phenylthio)methyl)propylamine and triethylamine at about
40 C to about
50 C to provide 4-(((1R)-3-morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-
3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
62
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Another embodiment pertains to 4-(((lR)-3-morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or a
pharmaceutically acceptable salt thereof, prepared as described in the
preceding embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and a first catalyst to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene
and isolating or not isolating the 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and a
first NH3 source to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and
isolating or not isolating the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and a sixth base to provide 4-
(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
63
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-l-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and copper(I) oxide to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine to provide
4-(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoyl)-4-(((1R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
64
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and copper(I) oxide at about 0 C to about 110 C to provide 1-
fluoro-2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid at about 110 C to about 130 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonyl chloride and isolating or not
isolating the 4-
fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide at about -5 C to about -60 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine at about 40
C to about
50 C to provide 4-(((1R)-3-morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-
3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -
yl)methyl)piperazin-1-yl)benzoyl)-
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
4-(((l R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex-l-en-l-yl)methyl)piperazin-l-yl)benzoyl)-4-(((lR)-3-(morpholin-4-yl)-
1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, an alkyl formate and a first base to
provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or
not isolating
the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, a second
base and a first silyl ether protecting group reagent to provide a first
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or not isolating
the first
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(c) reacting the first protected (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and 4-chlorophenyl magnesium bromide to provide the
first
protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the first protected (2E)-1-(4-chlorophenyl)-2-
(hydroxymethylene)-
4,4-dimethylcyclohexanol; and
(d) reacting the first protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-
4,4-dimethylcyclohexanol and a first acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
66
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid;
(m) reacting 2-fluorobenzenesulfonyl chloride, and a first fluoride source to
provide 2-fluorobenzenesulfonyl fluoride and isolating or not isolating the 2-
fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and a second fluoride source to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene and
isolating or not isolating the 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and a
first NH3 source to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and
isolating or not isolating the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and a sixth base to provide 4-
(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
67
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-l-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and
isolating or
not isolating the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane to provide (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide to provide ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
(d) reacting (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
68
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin- l -yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid;
(m) reacting 2-fluorobenzenesulfonyl chloride, and tetra-n-butylammonium
fluoride to provide 2-fluorobenzenesulfonyl fluoride and isolating or not
isolating the 2-
fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Ruppert's reagent
(CH3SiCF3),
and tris(dimethylamino)sulfonium difluorotrimethylsilicate to provide 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine to provide
4-(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
69
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide at about -10 C to about 0 C to provide (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and isolating or not isolating the (2E)-2-
(hydroxymethylene)-4,4-
dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane at about -10 C to about 0 C to provide (2E)-4,4-
dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
magnesium bromide at about -10 C to about 5 C to provide ((2E)-1-(4-
chlorophenyl)-4,4-
dimethyl-2-(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-
chlorophenyl)-4,4-
dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol, and
(d) reacting ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid at about 5 C to about 20 C to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-ene-l-carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride at about 15 C
to about
30 C and isolating or not isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en- l -yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide at about 55 C
to about
75 C, and isolating or not isolating the 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-
1-yl)methyl)piperazin-1-yl)benzoic acid;
(m) reacting 2-fluorobenzenesulfonyl chloride, and tetra-n-butylammonium
fluoride at about 0 C to about 10 C to provide 2-fluorobenzenesulfonyl
fluoride and isolating
or not isolating the 2-fluorobenzenesulfonyl fluoride;
(n) reacting the 2-fluorobenzenesulfonyl fluoride, Rupert's reagent
(CH3SiCF3),
and tris(dimethylamino)sulfonium difluorotrimethylsilicate at about room
temperature to
provide 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene and isolating or not
isolating the 1-
fluoro-2-((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid at about 110 C to about 130 C to provide 4-fluoro-3-
71
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
((trifluoromethyl)sulfonyl)benzenesulfonyl chloride and isolating or not
isolating the 4-
fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide at about -5 C to about -60 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl- 1-((phenylthio)methyl)propylamine and triethylamine at about
40 C to about
50 C to provide 4-(((1R)-3-morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-
3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5 -dimethyl- l -cyclohex- l -en- l -
yl)methyl)piperazin-1-yl)benzoyl)-
4-(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
72
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(a) reacting 4,4-dimethylcyclohexanone, an alkyl formate and a first base to
provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or
not isolating
the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone, a second
base and a first silyl ether protecting group reagent to provide a first
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone and isolating or not isolating
the first
protected (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(c) reacting the first protected (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone and 4-chlorophenyl magnesium bromide to provide the
first
protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the first protected (2E)-1-(4-chlorophenyl)-2-
(hydroxymethylene)-
4,4-dimethylcyclohexanol; and
(d) reacting the first protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-
4,4-dimethylcyclohexanol and a first acid to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex- l -ene- l -carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and a first reducing agent and isolating or not
isolating the
ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -en-l-
yl)methyl)piperazin- l -
yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and an aqueous third base, and isolating or
not isolating
the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-
1-yl)benzoic
acid;
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and a first catalyst to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene
and isolating or not isolating the 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and a
first NH3 source to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and
isolating or not isolating the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
73
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and a sixth base to provide 4-
(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, a first
coupling reagent, and, optionally; a first auxiliary coupling reagent and
isolating or not
isolating the N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3 -(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide to provide (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone and
isolating or
not isolating the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane to provide (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
74
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide to provide ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
(d) reacting (2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid to provide 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-
carbaldehyde
and isolating or not isolating the 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-
ene-1-
carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride and isolating
or not
isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-I-yl)benzoate and aqueous sodium hydroxide, and isolating
or not
isolating the 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-
yl)benzoic acid;
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and copper(I) oxide to provide 1-fluoro-2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid to provide 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
and isolating
or not isolating the 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl
chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine to provide
4-(((lR)-3-
morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, 1-
ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine and isolating or not isolating the N-(4-(4-((2-(4-
chlorophenyl)-5,5-
dimethyl-l -cyclohex-l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-
(morpholin-4-yl)-
1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-1-yl)benzoyl)-4-(((1 R)-3-(morpholin-4-
yl)- l -
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
Still another embodiment of this invention pertains to a process for making N-
(4-(4-
((2-(4-chlorophenyl)-5,5-dimethyl- l -cyclohex- l -en-l-yl)methyl)piperazin-1-
yl)benzoyl)-4-
(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, comprising:
(a) reacting 4,4-dimethylcyclohexanone, ethyl formate and potassium tert-
butoxide at about -10 C to about 0 C to provide (2E)-2-(hydroxymethylene)-4,4-
76
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
dimethylcyclohexanone and isolating or not isolating the (2E)-2-
(hydroxymethylene)-4,4-
dimethylcyclohexanone;
(b) reacting the (2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone,
triethylamine and trimethylchlorosilane, tert-butylchlorodimethylsilane, or
triisopropylchlorosilane at about -10 C to about 0 C to provide (2E)-4,4-
dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and isolating or
not
isolating the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-
4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-
(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone;
(c) reacting the (2E)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanone, (2E)-4,4-dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanone, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-4,4-dimethylcyclohexanone and 4-
chlorophenyl
magnesium bromide at about -10 C to about 5 C to provide ((2E)-1-(4-
chlorophenyl)-4,4-
dimethyl-2-(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-
chlorophenyl)-4,4-
dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol; and
isolating or not isolating the ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol, and
(d) reacting ((2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol, (2E)-1-(4-chlorophenyl)-4,4-
dimethyl-2-
(((trimethylsilyl)oxy)methylene)cyclohexanol, or (2E)-2-(((tert-
butyl(dimethyl)silyl)oxy)methylene)-1-(4-chlorophenyl)-4,4-
dimethylcyclohexanol and
hydrochloric acid at about 5 C to about 20 C to provide 2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-ene-l-carbaldehyde and isolating or not isolating the 2-(4-
chlorophenyl)-
5,5-dimethylcyclohex-l-ene-l-carbaldehyde;
(e) reacting 2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde,
ethyl 4-piperazin-l-ylbenzoate and sodium triacetoxyborohydride at about 15 C
to about
77
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
30 C and isolating or not isolating the ethyl 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-
1-en- l -yl)methyl)piperazin-l-yl)benzoate;
(f) reacting ethyl 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-l-yl)benzoate and aqueous sodium hydroxide at about 55 C
to about
75 C, and isolating or not isolating the 4-(4-((2-(4-chlorophenyl)-5,5-
dimethylcyclohex-l-en-
1-yl)methyl)piperazin-1-yl)benzoic acid;
(r) reacting sodium trifluoromethanesulfinate, bis-(2-fluorophenyl)iodonium
tetrafluoroborate and copper(I) oxide at about 0 C to about 110 C to provide 1-
fluoro-2-
((trifluoromethyl)sulfonyl)benzene and isolating or not isolating the 1-fluoro-
2-
((trifluoromethyl)sulfonyl)benzene;
(o) reacting 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene, and chlorosulfonic
acid at about 110 C to about 130 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonyl chloride and isolating or not
isolating the 4-
fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride;
(p) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride,
and
aqueous ammonium hydroxide at about -5 C to about -60 C to provide 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide;
(q) reacting 4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, (1R)-3-
morpholin-4-yl-1-((phenylthio)methyl)propylamine and triethylamine at about 40
C to about
50 C to provide 4-(((1R)-3-morpholin-4-yl-l-((phenylthio)methyl)propyl)amino)-
3-
((trifluoromethyl)sulfonyl)benzenesulfonamide and isolating or not isolating
the 4-(((1R)-3-
morpholin-4-yl- l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide; and
(g) reacting 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-l-
yl)methyl)piperazin-1-yl)benzoic acid, 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide,
1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride, and
4-dimethylaminopyridine at about 25 C to about 35 C and isolating or not
isolating the N-(4-
(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-cyclohex-l-en-l-yl)methyl)piperazin-1-
yl)benzoyl)-
4-(((1 R)-3-(morpholin-4-yl)-1-((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide.
78
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Still another embodiment pertains to N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-
l-
cyclohex- l -en- l -yl)methyl)piperazin-l-yl)benzoyl)-4-(((l R)-3-(morpholin-4-
yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide, or
a pharmaceutically acceptable salt thereof, prepared as described in the
preceding
embodiment.
The following schemes and examples are presented to provide what is believed
to be
the most useful and readily understood description of procedures and
conceptual aspects of
this invention.
SCHEMES
Scheme 1
CI
O ~O `o
(8)o'er (9) i O O
CI
CO2H
O CI H H NN" (4A)
O O H N
(10) (4) N
(1A)
CI
CI (6)
O
OH OPG OH
OPG
(1) (2) (3)
Scheme 1 shows three synthetic routes used to prepare the aldehyde
intermediate (2-
(4-chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde ) (4) from (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone (IA). The aldehyde intermediate
(4) can
then be reacted with ethyl 4-(piperazin-l-yl)benzoate (4A) to provide ethyl 4-
(4-((2-(4-
chlorophenyl)-5,5-dimethylcyclohex-l-en-l-yl)methyl)piperazin-1-yl)benzoate,
which is
79
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
hydrolyzed to provide 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-1-
yl)methyl)piperazin-1-yl)benzoic acid (6).
One such route involves reacting 4,4-dimethylcyclohexanone (IA) and an alkyl
formate such as but not limited to ethyl formate, in the presence of a base
such as but not
limited to potassium t-butoxide to provide (2E)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanone (1). The reaction is typically performed below room
temperature in
a solvent such as but not limited to tetrahydrofuran, before warming to room
temperature and
stirring overnight. (2E)-2-(Hydroxymethylene)-4,4-dimethylcyclohexanone (1) is
then
reacted with a base such as but not limited to triethylamine and a silyl ether
protecting group
reagent such as but not limited to triisopropylsilylchloride to provide the
protected (2E)-2-
(hydroxymethylene)-4,4-dimethylcyclohexanone (2). The reaction is typically
performed
below room temperature in a solvent such as but not limited to 2-
methyltetrahydrofuran. (4-
Chlorophenyl)magnesium bromide is then added to a solution of the protected
alcohol (2) to
provide the protected (2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-
dimethylcyclohexanol (3). The reaction is typically performed below room
temperature in a
solvent such as but not limited to tetrahydrofuran, toluene, or mixtures
thereof. The protected
(2E)-1-(4-chlorophenyl)-2-(hydroxymethylene)-4,4-dimethylcyclohexanol (3) can
be treated
with an acid such as but not limited to HC1, to provide the aldehyde
intermediate (2-(4-
chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde) (4).
Another route involves reacting dimethylcyclohexanone (IA) with a prepared
solution
of diethoxycarbenium fluoroborate to provide 2-(diethoxymethyl)-4,4-
dimethylcyclohexanone (8). The reaction is typically performed below room
temperature in
a solvent such as but not limited to methylene chloride. Chlorophenyl
magnesium bromide is
then added to a solution of 2-(diethoxymethyl)-4,4-dimethylcyclohexanone (8)
to provide 1-
(4-chlorophenyl)-2-(diethoxymethyl)-4,4-dimethylcyclohexanol (9). The reaction
is typically
performed below room temperature in a solvent such as but not limited to
tetrahydrofuran. 1-
(4-Chlorophenyl)-2-(diethoxymethyl)-4,4-dimethylcyclohexanol (9) can be
treated with an
acid such as but not limited to HC1, to provide the aldehyde intermediate (2-
(4-chlorophenyl)-
5,5-dimethylcyclohex-l-ene-l-carbaldehyde) (4).
Still another route involves reacting dimethylcyclohexanone (IA) with POC13 to
provide 2-chloro-5,5-dimethylcyclohex-l-enecarbaldehyde (10). The reaction is
typically
performed below room temperature in a solvent such as but not limited to
methylene
chloride, N,N-dimethylformamide, or mixtures thereof. The aldehyde
intermediate (2-(4-
chlorophenyl)-5,5-dimethylcyclohex-l-ene-l-carbaldehyde) (4) can be prepared
from 2-
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
chloro-5,5-dimethylcyclohex-l-enecarbaldehyde (10) by reacting the latter with
4-
chlorophenylboronic acid, a phase transfer catalyst such as but not limited to
tetrabutyl
ammonium bromide, a palladium catalyst such as but not limited to palladium
(II) acetate in
the presence of a base such as but not limited to K2C03. The reaction is
typically performed
above room temperature in a solvent such as but not limited to water.
Scheme 2
/
CF3SO2 H
N S
SOZCI SOZF SOZCF3 SOZCF3 SOZCF3 ~ /
H2NO2 S
F F F
F F CN
I / CI02S I / HzNOzS (15) T 01 (17)
(12) (13)
(11)
FizN S,0
F F 1 BF4-
6-1-6 Cod
(18) (16)
Scheme 2 shows two routes used to make 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide (17).
One route involves reacting commercially available 2-fluorobenzenesulfonyl
chloride (11)
with TBAF (tetra-n-butylammonium fluoride) to provide 2-fluorobenzenesulfonyl
fluoride
(12). The reaction is typically performed below room temperature in a solvent
such as but
not limited to tetrahydrofuran. 2-Fluorobenzenesulfonyl fluoride (12) can be
converted to 1-
fluoro-2-((trifluoromethyl)sulfonyl)benzene (13) by reaction with Ruppert's
reagent
(CH3SiCF3) catalyzed by (((CH3)2N)3S)+(F2Si(CH3)3)-, also known as TASF. The
reaction is
typically performed at about room temperature in a solvent such as but not
limited to
tetrahydrofuran, dichloromethane, toluene, dimethoxyethane, or mixtures
thereof. 1-Fluoro-
2-((trifluoromethyl)sulfonyl)benzene (13) can be reacted with chlorosulfonic
acid to provide
4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride (14). The
reaction is typically
performed at an elevated temperature before cooling for the addition of
S02C12, used to
quench the chlorosulfonic acid. 4-Fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide
(15) can be prepared from 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
(14) by reacting the latter with aqueous ammonium hydroxide. The reaction is
typically
performed in a solvent such as acetonitrile, tetrahydrofuran, ethyl acetate,
isopropyl alcohol,
isopropyl acetate, dimethoxyethane, dichloromethane, toluene, or mixtures
thereof. (1R)-3-
81
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
morpholin-4-yl-1-((phenylthio)methyl)propylamine (16), prepared as described
in United
States Patent 7,390,799 B2, can be reacted with 4-fluoro-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide (15) in the presence of a base
such as but not
limited to triethylamine, to provide 4-(((1R)-3-morpholin-4-yl-l-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide (17).
The reaction is typically performed at elevated temperatures in a solvent such
as but not
limited to ethyl acetate.
Another route involves first reacting a reagent such as bis-(2-
fluorophenyl)iodonium
tetrafluoroborate (J. Org. Chem. 2008, 73, 4602) and a reagent such as sodium
trifluoromethanesulfinate in the presence of a Cu(I)/Cu(III) or Pd(II)/Pd(IV)
catalyst to
provide 1-fluoro-2-((trifluoromethyl)sulfonyl)benzene (13). The reaction is
typically
performed at elevated temperatures in a solvent such as but not limited to N,N-
dimethylformamide, dimethylsulfoxide, 1,4-dioxane, 1,2-dimethoxyethane,
tetrahydrofuran,
1-methyl-2-pyrrolidinone, diglyme and CH2C12. Catalysts include but are not
limited to Cul,
Cu20, CuC1, copper (I) trifluoromethanesulfonate benzene complex, CuSCN
Cu(OAc),
Pd(OAc)2 alone or in combination with ligands such as
tetramethylethylenediamine, 4,4'-
ditert-butyl-2,2'-dipyridyl, phenanthrolines, 2-acetylcyclohexanone, N,N-
diethylsalicylamide, phosphines and the like. Other metals, such as nickel,
cadmium, cobalt,
tin, iron, rhodium, iridium, and platinum, or combinations thereof either in
the metallic form,
as salts, or complexes may also prove effective in this chemistry.
Scheme 3
CO H CF3SO2 H
z ~ N SPh
H / O N S I /
CF3SO2 H I O2
N I \ N S I \ N
NJ + H2NO2S" v ~r 3. Cod
N
J (17) (N)
J
CI ~ ~ ~ C
O N
(6)
I~
CI /
(7)
As shown in Scheme 3, 4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-l-en-1-
yl)methyl)piperazin-l-yl)benzoic acid (6) can be reacted with 4-(((1R)-3-
morpholin-4-yl-1-
((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide (17) to
82
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
provide a compound such as N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l-
cyclohex-l-en-1-
yl)methyl)piperazin- l -yl)benzoyl)-4-(((l R)-3-(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide (7)
using methods known in the art, widely available in the literature, and as
described in the
example section herein.
EXPERIMENTALS
Compounds of this invention may be made by synthetic chemical processes,
examples
of which are shown herein. It is meant to be understood that the order of the
steps in the
processes may be varied, reagents, solvents, and reaction conditions may be
substituted for
those specifically mentioned, and vulnerable moieties may be protected and
deprotected, as
necessary, by NH, C(O)OH, OH, SH protecting groups.
The following abbreviations have the meanings indicated. ADDP means
1,l'-(azodicarbonyl)dipiperidine; AD-mix-(3 means a mixture of (DHQD)2PHAL,
K3Fe(CN)6,
K2C03, and K2SO4; 9-BBN means 9-borabicyclo(3.3.1)nonane; Boc means
tert-butoxycarbonyl; (DHQD)2PHAL means hydroquinidine 1,4-phthalazinediyl
diethyl
ether; DBU means 1,8-diazabicyclo(5.4.0)undec-7-ene; DIBAL means
diisobutylaluminum
hydride; DIEA means diisopropylethylamine; DMAP means N,N-
dimethylaminopyridine;
DMF means N,N-dimethylformamide; dmpe means 1,2-bis(dimethylphosphino)ethane;
DMSO means dimethylsulfoxide; dppb means 1,4-bis(diphenylphosphino)-butane;
dppe
means 1,2-bis(diphenylphosphino)ethane; dppf means 1,1'-
bis(diphenylphosphino)ferrocene;
dppm means 1,1 -bis(diphenylphosphino)methane; EDAC=HClmeans 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; Fmoc means
fluorenylmethoxycarbonyl; HATU means O-(7-azabenzotriazol-l-yl)-N,N'N'N'-
tetramethyluronium hexafluorophosphate; HMPA means hexamethylphosphoramide;
IPA
means isopropyl alcohol; MP-BH3 means macroporous triethylammonium
methylpolystyrene
cyanoborohydride; TEA means triethylamine; TFA means trifluoroacetic acid; THE
means
tetrahydrofuran; NCS means N-chlorosuccinimide; NMM means N-methylmorpholine;
NMP
means N-methylpyrrolidine; PPh3 means triphenylphosphine.
EXAMPLE 1
83
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(2E)-2-(hydroxymethylene)-4,4-dimethylcyclohexanone
4, 4-Dimethylcyclohexanone (20 g), ethyl formate ( 15.8 g, 1.35 eq) and
tetrahydrofuran (100ml) were charged in a 250 ml flask and the mixture was
cooled to 0-5
C. Potassium t-butoxide (1M in tetrahydrofuran 191 mL, 1.2 equiv) was slowly
added while
maintaining the temperature below 0 C. The reaction mixture was mixed at -5 to
+5 C and
monitored for completion by GC analysis. After mixing overnight, no starting
material was
observed. Water (140 mL) was slowly added, and the resulting mixture was
extracted with
heptane (150 mL). The pH of the aqueous layer was adjusted with 6 M HC1 to pH
= 1-2 (-40
g HC1) and the extracted with toluene (80 mL). The organic layer was washed
with 20%
aqueous NaC1(2 x 140 mL). The product solution in toluene was used directly in
the next
step within 12 hours (storage at 0 C).
EXAMPLE 2
(2E)-4,4-dimethyl-2-(((triisopropylsilyl)oxy)methylene)cyclohexanone
EXAMPLE 1 (21 g as an 18% solution in toluene) was charged to a 1 L flask
followed by 2-methyltetrahydrofuran (300 mL). The reaction mixture was cooled
to between
0-5 C, and triethylamine (20.67 g, 1.5 equiv) was charged maintaining a
reaction
temperature between 0-5 C. Triisopropylsilylchloride (TIPSCI) (28.9 g, 1.1
equiv) was
slowly charged, maintaining a reaction temperature between 0-5 C. The
reaction mixture
was mixed at a temperature range between 0-5 C and monitored by GC for
completion.
Heptane (430 mL) was added, and temperature was adjusted to 10 C. Water (300
mL) was
added, maintaining an internal temperature below 15 C. The reaction mixture
was mixed
thoroughly, and the layers were separated. The organic layer was washed with
water (2 x 200
mL), 20% aqueous NaC1(200 mL) and 20% aqueous NaC1(360 mL). The organic layer
was
distilled to approximately 100 mL and chased with toluene (280 mL). The title
compound
was stored as a toluene solution in the refrigerator. 1H NMR (400 MHz, CDC13)
6 1.03 (s, 6
H) 1.07 (d, J=5.76 Hz, 14 H) 1.10 (s, 10 H) (with overlapping residual solvent
resonances)
1.23 (dd, J=14.55, 6.59 Hz, 3 H) 1.26 - 1.29 (m, 2 H) (residual solvent) 1.64
(t, J=7.00 Hz, 2
H) 2.06 (s, 1 H), 2.26 - 2.28 (m, 2 H) 2.38 (t, J=7.00 Hz, 2 H) 7.55 (t,
J=1.92 Hz, 1 H) ppm.
Mass spec: DCI/ NH3 M+1 = 311 amu; M + NH3 = 328 amu.
EXAMPLE 2A
(2E)-4,4-dimethyl-2-(((trimethylsilyl)oxy)methylene)cyclohexanone
84
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
Example 1 (0.5 g, 1 eq), 0.47m1 of triethylamine (1.05 eq) and 5 ml of
anhydrous
tetrahydrofuran were mixed together in a round bottom flask under N2.
Trimethylsilyl
chloride (0.43 ml, 1.05 eq) was added drop wise at ambient temperature, and
the mixture
stirred at ambient temperature for 18 hours. The reaction was quenched with 10
ml of 1 N
HC1 and 10 ml of ethyl acetate. The organic layer was isolated and
concentrated down and
the residue was purified by column chromatography using 5% heptane in ethyl
acetate.
EXAMPLE 3
(2E)-1-(4-chlorophenyl)-4,4-dimethyl-2-
(((triisopropylsilyl)oxy)methylene)cyclohexanol
To EXAMPLE 2 (26 g, as a solution in toluene) was added to anhydrous
tetrahydrofuran (65 mL). The resulting mixture was cooled to -5 to 0 C. A
separate flask
was charged with anhydrous tetrahydrofuran (65 mL) and a 1 M solution of (4-
chlorophenyl)magnesium bromide (172 mL, 2.05 equiv). The reagent mixture was
cooled to
-5 to 0 C. The mixture of EXAMPLE 2 in toluene was slowly added to the
Grignard
reagent, maintaining a reaction temperature below 5 C. The reaction was
monitored by
HPLC and was determined to be complete in about one hour. The reaction mixture
was
quenched slowly with methanol (91 mL), maintaining an internal temperature
below 5 C.
EXAMPLE 4
2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -ene- l -carbaldehyde
The solution from EXAMPLE 3 was slowly treated with 2N HC1(240 mL),
maintaining an internal temperature below 20 C. The contents were mixed at 20
C for 10-12
hours, and the mixture was monitored by GC analysis. Isopropyl acetate (130
mL) was
charged to the reaction mixture, and the layers were mixed and separated. The
organic layer
was washed with water (45 mL), 1% aqueous NaHCO3 (45 mL), and 20% aqueous
NaC1(2 x
45 mL). The product mixture was azeotropically dried through a solvent
exchange with
toluene (125 g) to obtain a 32% solution of the title compound. 1H NMR (400
MHz, DMSO-
d6) 6 0.95 (s, 6 H) 1.48 (t, J=6.45 Hz, 2 H) 2.03 (t, J=1.92 Hz, 2 H) 2.53 -
2.57 (m, J=6.38,
6.38, 2.20, 2.06 Hz, 2 H) 7.36 (ddd, J=8.75, 2.54, 2.30 Hz, 2 H) 7.47 (ddd,
J=8.78, 2.54, 2.26
Hz, 2 H) 9.37 (s, 1 H) ppm. Mass Spec: DCI/ NH3 M+l = 248 amu; M+NH3 = 266
amu.
EXAMPLE 4
2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -ene- l -carbaldehyde
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
EXAMPLE 9 was dissolved in 50m1 tetrahydrofuran, and 40 ml 6N aqueous HC1 was
added. The mixture was heated to 65 C for 17 hours. The reaction mixture was
cooled,
neutralized to pH-7 with 18% Na2CO3, extracted with ether, and washed with
aqueous NaCl.
The organics were concentrated to give the title compound. 1H NMR (400 MHz,
DMSO-d6)
6 0.95 (s, 6 H) 1.48 (t, J=6.45 Hz, 2 H) 2.03 (t, J=1.92 Hz, 2 H) 2.53 - 2.57
(m, J=6.38, 6.38,
2.20, 2.06 Hz, 2 H) 7.36 (ddd, J=8.75, 2.54, 2.30 Hz, 2 H) 7.47 (ddd, J=8.78,
2.54, 2.26 Hz, 2
H) 9.37 (s, 1 H) ppm. Mass Spec: DCI/ NH3 M+l = 248 amu; M+NH3 = 266 amu.
EXAMPLE 4
2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -ene- l -carbaldehyde
EXAMPLE 10 (1.00 eq.), 4-chlorophenylboronic acid (1.11 eq.), tetrabutyl
ammonium bromide (0.99 eq.), palladium (II) acetate (0.021 eq.), K2C03 (2.45
eq.) and H2O
(10.0 vol.) were added together and mixed. The mixture was degassed under a
cycle of high
vacuum and nitrogen purge for at least three times and then warmed up to 45
C. The
reaction mixture was stirred at 45 C under nitrogen purge about 6 hours. The
reaction
mixture was cooled to room temperature and diluted with H2O (15.0 vol.). The
diluted
mixture was extracted with CH2C12 (9.0 vol. x 3). The CH2C12 extracts are
combined and
washed with H2O (9.0 vol. x 3) and then dried over Na2SO4. The solid Na2SO4
was filtered
off and rinsed with CH2C12 (5.0 vol.). The filtrate and rinse were combined
and
concentrated. 1H NMR (400 MHz, DMSO-d6) 6 0.95 (s, 6 H) 1.48 (t, J=6.45 Hz, 2
H) 2.03
(t, J=1.92 Hz, 2 H) 2.53 - 2.57 (m, J=6.38, 6.38, 2.20, 2.06 Hz, 2 H) 7.36
(ddd, J=8.75, 2.54,
2.30 Hz, 2 H) 7.47 (ddd, J=8.78, 2.54, 2.26 Hz, 2 H) 9.37 (s, 1 H) ppm. Mass
Spec: DCI/
NH3 M+1 = 248 amu; M+NH3 = 266 amu.
EXAMPLE 4
2-(4-chlorophenyl)-5,5-dimethylcyclohex-l -ene- l -carbaldehyde
Example 1 (2.0 g, 1 eq), 2.2 ml of triethylamine (1.2 eq) and 30 ml of
anhydrous
tetrahydrofuran were mixed together in a round bottom flask under N2. tert-
Butylchlorodimethylsilane (2.35 g, 1.2 eq) was added drop wise at ambient
temperature, and
the mixture was stirred at ambient temperature for 1 hour. The mixture was
cooled to 0 C and
28.6 ml of 1 M 4-chlorophenyl magnesium bromide in tetrahydrofuran was added
slowly.
After the addition was complete, the reaction mixture was stirred at 0 C for
30 minutes. The
reaction was quenched with 90 ml of 1 N aqueous HC1 and 90 ml of ethyl
acetate. The
86
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
organic layer was isolated and concentrated, and the residue was purified by
column
chromatography using 5% heptane in ethyl acetate.
EXAMPLE 5
ethyl 4-(4-((2-(4-chlorophenyl)-5,5 -dimethylcyclohex- l -en-l-
yl)methyl)piperazin- l -
yl)benzoate
Ethyl 4-piperazin-1-ylbenzoate (14 g) was treated with sodium
triacetoxyborohydride
(23 g) and then tetrahydrofuran (187 g), stirred for 15 minutes, treated with
EXAMPLE 4
toluene solution (42.1g, 32.5 % aldehyde) over 20-30 minutes and stirred for 6
hours with
evolution of hydrogen gas. After 12 hours, no starting material was detected
by HPLC.
Methyl tent-butyl ether (70 g) was added, and the reaction was quenched with
20% aqueous
ammonium chloride (170 g). Again, hydrogen gas evolved. Both layers were mixed
for 30
minutes and separated. The extract was washed with water (135 g), concentrated
to about
115 ml, diluted with of tetrahydrofuran (125 g), concentrated to 115 ml,
charged with
tetrahydrofuran (125 g), concentrated to 115 ml, and charged with of ethanol
(220 g), causing
a solid to form. The slurry was concentrated to approximately 70 ml, charged
with ethanol
(165 g), and mixed for 2-3 hours (liquor concentration was 3.01 mg/mL) and
filtered. The
solid was washed with ethanol (2 x 77 g) and dried in a vacuum oven at 40 C
for 10 hours. A
mixture of the solid and tetrahydrofuran (41 g) was heated at 50 C for 30
minutes, treated
with ethanol (146 g), stirred for 1 hour, cooled to 20 C (liquor concentration
was
5.12 mg/mL), and filtered. The solid was washed with ethanol (73 g) and dried
in a vacuum
oven at 40 C for 10 hours. A mixture of the solid and tetrahydrofuran (37 g)
was heated to
50 C for 30 minutes, treated with of ethanol (133 g), stirred for 1 hour,
cooled to 20 C (liquor
concentration was 5.1 mg/mL) and filtered. The solid was washed once with
ethanol (67 g)
and dried in the vacuum oven at 40 C for 10 hours.
EXAMPLE 6
4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex- l -en-l -yl)methyl)piperazin-1-
yl)benzoic
acid
2-Methyltetrahydrofuran (20 g) and heptanes (63 g) were stirred to homogeneity
for
use as a product rinse. NaC1(48 g) was dissolved into water (146 g) for use in
a work-up.
KH2PO4 (40 g) and NaC1(51 g) was dissolved into water (291 g) for use in the
work-up. A
87
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
solution of NaOH (13 g) was dissolved in water (113 g), treated with EXAMPLE 5
and 2-
methyltetrahydrofuran (125 g), stirred for 30 minutes, adjusted to 25-30 C,
treated with
ethanol (81 g), transferred to a flask while maintaining the internal
temperature less than
35 C, adjusted to 65 5 C, stirred for at least 16 hours, until the reaction
was complete by
HPLC analysis (<1% EXAMPLE 5), cooled to 20 C, and settled for about 30
minutes. The
lower layer was removed after sampling to assay for product loss. Part of the
monobasic
phosphate solution (219 g) was transferred to the reactor, and the contents
were mixed for 30
minutes and settled for 30 minutes. The lower layer was removed after sampling
to assay for
product loss after pH determination. About half of the 25 wt % aqueous NaCl
solution (91 g)
was charged to the reactor, and the contents were stirred for at least 15
minutes and settled for
minutes. The lower layer was transferred to drums after sampling for product
losses, and
the remaining 25 wt % NaCl solution (about 97 g) was charged to the reactor.
The contents
were mixed for at least 15 minutes and allowed to settle for at least 1 hour.
The lower layer
was transferred to drums after sampling for product losses. 2-
Methyltetrahydrofuran (125 g)
15 was charged to the reactor, and the contents were stirred for at least 10
minutes. The product
solution was transferred through a nylon filter into the rinsed reactor using
2-
methyltetrahydrofuran to rinse the canisters (4 g 2-methyltetrahydrofuran per
canister) and
distilled under vacuum at a jacket temperature of about 40 C to about 214 ml.
While
maintaining vacuum and a volume of about 214 ml, 2-methyltetrahydrofuran (375
g) was
added in portions to azeotropically dry the product solution. This solution
was sampled
during the distillation to determine moisture content (target <1 % water). The
solution was
concentrated under vacuum to approximately 73 ml, and the internal temperature
was
adjusted to about 45 C (target: 40 C). The contents were stirred for 1 hour,
treated with
heptanes (172 g) over 30 minutes, and stirred at about 45 C (target:40 C) for
2 hours and
about 25 C (target: 20 C) for 2 hours. The product suspension was distilled
under vacuum to
140 ml, and the internal temperature was adjusted to about 25 C (target: 20
C). The mixture
was vacuum filtered, and the liquors were recycled one time to rinse the
flask. The filtrate
was rinsed with a portion of the heptane/ 2-methyltetrahydrofuran solution (41
g), allowing
the rinse to sit on the cake for at least 15 minutes before applying vacuum.
The rinse was
repeated with the remaining heptane/2-methyltetrahydrofuran solution (about 22
g), again
allowing the rinse to sit on the cake for at least 15 minutes before applying
vacuum. The
liquors and rinses were sampled separately for product loss assays. The filter
pot was purged
for at least 2 hours with nitrogen, and the product was transferred to a dryer
and dried under
vacuum with a nitrogen bleed at less than or equal to 40 C (target: 35 C), for
at least 12
88
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
hours.
EXAMPLE 7
N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-l -cyclohex-l -en- l -
yl)methyl)piperazin-l -
yl)benzoyl)-4-(((1R)-3-(morpholin-4-yl)-1-
((phenylsulfanyl)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide
A mixture of 4-(((lR)-3-morpholin-4-yl-1-((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide (8.575 g) (EXAMPLE 17), EXAMPLE
6 (6.4
g), 4-dimethylaminopyridine (4.4 g), 1-ethyl-3-(3-
(dimethylamino)propyl)carbodiimide
hydrochloride (4.1 g) and dichloromethane (80 g) was heated at 30 C, cooled to
room
temperature, quenched with N,N-dimethylethylenediamine (1.1 g), distilled to
30 ml, treated
with water (5.4 g) and ethyl acetate (97 g), distilled to 65 ml, treated with
water (5.4 g) and
ethyl acetate (97 g), distilled to 80 ml and treated with 10% acetic
acid/0.75% brine (182 g)
and ethyl acetate (65 g). The layers were separated, and the extract was
washed with 10%
aqueous acetic acid/0.75% brine (182 g), 25% aqueous K2HPO4 (180 g) and pH 7
buffer
solution (163 g), concentrated to 35 ml and chase distilled with ethyl acetate
(120 g, 120 g
and 60 g) with concentration to 35 ml after each addition. The extract was
then treated with
ethyl acetate (60 g), and the solution was diluted with ethanol (71 g) and
polish-filtered
through a polypropylene 0.5 m filter into a reactor with an ethyl acetate (20
g) rinse. In a
separate reactor, HC1(2.8 g) in ethanol (80 g) was prepared and polish-
filtered through a
separate filter and housing into the reactor. The polish filtration of the
solution removed
residual phosphate salts from the final extraction. The solution was
concentrated to about
150 ml and maintained at that level while an additional chase of ethanol (160
g) was
conducted, heated at 45 C, treated with seeds (90 mg) in ethanol (1 g),
stirred for 12 hours,
cooled to 20 C and stirred for another 4 hours. Analysis of the filtrates
indicated the
crystallization was complete. The slurry was filtered, and the solids were
rinsed with ethanol
(2 x 57 g). The rinses were applied in a slurry fashion with no vacuum,
(contact time 15-25
minutes for each) then removed by vacuum filtration. The wet cake was sampled
for
impurities to determine if a recrystallization would be necessary. The solids
were dried under
vacuum and nitrogen at 50 C for 3 days. Analysis of the dryer sample (GPAS
residual
solvents method) showed that drying was complete. 1H NMR (400 MHz, methanol-
d4) 6
ppm1.08(s,5H)1.57(t,J=6.38Hz,2H)2.11(s,2H)2.26-2.34(m,1H)2.40(t,J=5.69
Hz, 2 H) 3.18 - 3.27 (m, 4 H) 3.42 (dd, J=14.48, 4.87 Hz, 2 H) 3.69 (s, 2 H)
3.87 (s, 2 H) 4.14
89
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
(s,1H)6.99(td,J=6.07,2.54Hz,3H)7.11-7.19(m,5H)7.29-7.32(m,2H)7.37-7.41
(m, 2 H) 7.76 (d, J=9.06 Hz, 2 H) 8.07 (dd, J=9.19, 2.33 Hz, 1 H) 8.29 (d,
J=2.20 Hz, 1 H).
EXAMPLE 8
2-(diethoxymethyl)-4,4-dimethylcyclohexanone
To 34 ml of HC(OCH2CH3)3 (0.2mol) at -38 C was added drop wise a solution of
31
ml BF3(OCH2CH3)2 in 90 ml CH2C12 (0.24 mol) over 15 minutes at about -30 C.
The
mixture was warmed to 0 C and mixed for 10 minutes to form diethoxycarbenium
fluoroborate. The mixture was cooled to -78 C, and 12.6 g
dimethylcyclohexanone in 40m1
CH2C12 (0.1 mol) was added, followed by drop wise addition of 52 ml N,N-
diisopropylethylamine(0.3 mol) over 30 minutes. The mixture stirred at -78 C
for 1.5 hours.
The cold reaction was quenched into 1000 g aqueous 6.5% NaHCO3.
Dichloromethane
(250m1) was added and the mixture was stirred vigorously for 10 minutes. The
organics were
washed with 400 g cold aqueous I% H2SO4 and then with 400 g cold water. The
organics
were dried over MgSO4, filtered, and the filtrate was concentrated to give the
title compound.
GCMS: 183(ms/z).
EXAMPLE 9
1-(4-chlorophenyl)-2-(diethoxymethyl)-4,4-dimethylcyclohexanol
EXAMPLE 8 was dissolved in 200 ml tetrahydrofuran, cooled to -78 C, and 195 ml
1 M chlorophenyl magnesium bromide (0.2 mol) was added over 25 minutes. The
mixture
was mixed at about -20 C for 2 hours. The reaction mixture was quenched with
200g 20%
aqueous NH4C1 at about -7 C, extracted with 400m1 ethyl acetate, then washed
with 20%
aqueous NH4C1, 5% aqueous NaHCO3, and 25% aqueous NaCl. The organics were
concentrated to provide the title compound.
EXAMPLE 10
2-chloro-5,5-dimethylcyclohex- l -enecarbaldehyde
A solution of N,N-dimethylformamide (1.37 eq.) in CH2C12 (1.5 vol.) was cooled
to
0 C and then POC13 (1.25 eq.) was added slowly to the N,N-dimethylformamide
solution at
below 25 C. The resulting solution was allowed to warm up to room
temperature. A
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
solution of 4, 4 -dimethylcyclohexanone (1.00 eq.) in CH2Cl2 (1.4 vol.) was
added slowly to
the above solution at below 30 C, and then the reaction mixture was stirred
at 45 C
overnight. The reaction mixture was quenched into a cooled 10% sodium acetate
solution
(30.0 vol.) at below 15 C, followed by a CH2Cl2 rinse (4.6 vol.). The
quenched mixture was
stirred while allowing it to warm up to room temperature. The organic layer
was separated
and then aqueous layer was extracted with CH2Cl2 (7.6 vol. x 3). The organic
layer and
CH2Cl2 extracts were combined and concentrated to give a solution and the
solution was used
in the next step without purification. 1H NMR (400 MHz, CDC13) 6 10.1 (1H, s,
CHO)
iH NMR (400 MHz, CDC13) 6 10.1 (1H, s, CHO), 2.61-2.57 (2H, m), 2.09 (2H, s),
1.54-1.51
(2H, m), 0.95 (6H, s).
EXAMPLE 11
2-fluorobenzenesulfonyl chloride
The title compound was purchased commercially from Sigma-Aldrich.
EXAMPLE 12
2-fluorobenzenesulfonyl fluoride
A 100-L round bottomed flask fitted with overhead stirrer, nitrogen inlet,
temperature
probe and ice bath was charged with 2-fluorobenzenesulfonyl chloride (50 g)
and
tetrahydrofuran (220 g). The solution was cooled down to -5 C with an ice
bath.
Tetrabutylammonium fluoride (270 mL of 1M in tetrahydrofuran solution) was
added slowly
to the reactor. The internal temperature was kept less than 8 C. After the
addition was
completed, HPLC showed the reaction was completed. The reaction was quenched
by slowly
adding 250 g of water. Then 430 g of toluene was added to the reaction
mixture. The layers
were separated. The organic layer was washed with 250 g of water twice. The
organic layer
was distilled down under vacuum with the bath temperature at 48 C. 1H NMR (400
MHz,
CDC13) 6 8.01-7.96 (m, 1H), 7.76-7.83 (m, 1H), 7.32-7.44 (m, 2H).
EXAMPLE 13
1 -fluoro-2-((trifluoromethyl)sulfonyl)benzene
The solution of EXAMPLE 12 (44.3g) in a solution of 450 ml was added to a
round
bottomed flask fitted with overhead stirrer, nitrogen inlet, cold-water bath
and temperature
probe. It was cooled down to 12 C. (((CH3)2N)3S)+(F2Si(CH3)3)-, also known as
TASF (6.1
91
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
g), was added in one portion. Ruppert's reagent (370 g, CH3SiCF3) was charged
slowly to
the reaction mixture while maintaining the internal temperature less than 23
C. The reaction
was completed after addition. Water (220 g) was added to quench the reaction,
the layers
were separated, and the organic layer was washed twice with 220 g of water.
The organic
solution was distilled under vacuum with the bath temperature at 50 C.
Heptanes (500 g)
were added to the residue, and the mixture was distilled down until the
solvent was removed.
1H NMR (400 MHz, CDC13) 6 8.01-8.05 (m, I H), 7.84-7.87 (m, I H), 7.44-7.48
(m, I H),
7.36-7.39 (m 1H).
EXAMPLE 13
1 -fluoro-2-((trifluoromethyl)sulfonyl)benzene
A 1 L three-neck round bottom flask equipped with a magnetic stir bar and a J-
Chemthermocouple was charged with sodium trifluoromethanesulfinate (9.29 g,
59.5 mmol),
bis-(2-fluorophenyl)iodonium tetrafluoroborate (18.5 g, 45.8 mmol) (J. Org.
Chem. 2008, 73,
4602) and copper(I) oxide (0.131 g, 0.92 mmol). The flask was purged with
nitrogen for 30
minutes. Degassed N,N-dimethylformamide (225 mL) was added to the flask and
the
reaction mixture was stirred at 50 C for 16 hours under a positive atmosphere
of nitrogen.
The reaction mixture was cooled to the room temperature, diluted with 500 mL
isopropyl
acetate and transferred to a 2 L reparatory funnel. The organic layer was
washed with 500
mL of 5% aqueous K2C03 solution. The organic layer was washed with water (500
mL x 2)
followed by a wash with 250 mL brine. The organic layer was dried over
magnesium sulfate,
filtered through diatomaceous earth and concentrated in vacuo to obtain the
title compound.
The product was further purified by distillation. 1H NMR (500 MHz, CDC13) 6
8.03 (t, J=
7.3, 1H), 7.85 (dd, J= 7.5, 13.2, 1H), 7.45 (t, J= 7.7, 1H), 7.36 (t, J= 9.1,
1H). 19F NMR
(470 MHz, CDC13) 6 77.4 (d, J= 9.4, 3F), d 102.7 (m, 1F). HPLC: Zorbax SB-C18
4.6 x 150
mm, 3.5 gm, method: 90% of 0.1% H3PO4 and 10% CH3CN, ramped to 90% CH3CN over
7
minutes, held for 3 minutes, ramped to 10% CH3CN over 1 minutes; peak at 6.58
minutes.
EXAMPLE 13
1 -fluoro-2-((trifluoromethyl)sulfonyl)benzene
Mesityl-2-fluorophenyl iodonium triflate (Sanford M et al. JACS, 2005, 127,
7330.and Widdowson, D. et al. Tetrahedron Letters 2000, 41, 5393.) (0.1 g,
0.204 mmol),
sodium trifluoromethanesulfinate (0.035 g, 0.224 mmol) and copper(I) oxide
(2.92 mg, 0.020
92
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
mmol) were weighed into a reactor equipped with a magnetic stir bar. N,N-
dimethylformamide (1 mL) was added, the reactor was capped and heated at 25 C
for 24
hours. HPLC analysis of the crude reaction mixture indicated the formation of
the title
compound.
EXAMPLE 14
4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonyl chloride
EXAMPLE 13 (80 g) was charged to a reactor fitted with overhead stirrer,
nitrogen
inlet, condenser, scrubber, temperature probe and heating bath. Chlorosulfonic
acid (307 g)
was added slowly through an additional funnel. The mixture was then heated up
to 120 C
and held for 22 hours at 120 C. The mixture was cooled down to room
temperature. Thionyl
chloride (118 g) was added to the reaction mixture in one portion at ambient
temperature.
The mixture was stirred at 25 C for 24 hours. A sample was pulled and HPLC
showed the
reaction was completed with 1.1 % of the sulfonic acid left. Isopropyl acetate
(775 g) was
cooled down to -50 C. Water (600 g) was charged to isopropyl acetate
solution.. The
reaction mixture was transferred slowly to the isopropyl acetate /ice mixture
through an
additional funnel. The internal temperature increased to 0.8 C during the
addition.
The cold reaction mixture was warmed up 15 C and layers were separated. The
organic layer
was used in the next step (EXAMPLE 15) without isolation.
EXAMPLE 15
4-fluoro-3-((trifluoromethyl)sulfonyl)benzenesulfonamide
The solution from EXAMPLE 14 was charged into a flask fitted with overhead
stirrer,
nitrogen inlet, temperature probe and cooling bath. The solution was cooled
down to -50 C
using an acetone/dry ice bath. Ammonium hydroxide (238 g) solution was slowly
added to
the reaction mixture through an additional funnel. The internal temperature
was kept at about
-40 C. HPLC showed the reaction was completed. The solution was cooled down to
-60 C.
6 N HC1(600 g) was added to the reaction mixture slowly to keep the
temperature under
-35 C. The mixture was warmed up to room temperature. The layers were
separated, and the
organic layer was washed twice with 375 g of 4 N HC1. The organic layer was
distilled down
under vacuum with bath temperature from 40 to 50 C. Toluene (700 g) was added
to the
residue, and the mixture was stirred at room temperature for an hour. The
mixture was
filtered and washed with toluene to obtain the title compound.
93
CA 02727932 2010-12-10
WO 2009/155386 PCT/US2009/047723
EXAMPLE 16
(1 R)-3-morpholin-4-yl- l -((phenylthio)methyl)propylamine
The title compound can be prepared as described in United States Patent
7,390,799 B2.
EXAMPLE 17
4-(((1 R)-3-morpholin-4-yl-l -((phenylthio)methyl)propyl)amino)-3-
((trifluoromethyl)sulfonyl)benzenesulfonamide
The title compound can be prepared as described in United States Patent
7,390,799 B2.
The preceding is meant to be illustrative of this invention and not limiting.
Obvious
variations and changes are meant to be within the scope of this invention, as
defined in the
claims.
94