Note: Descriptions are shown in the official language in which they were submitted.
CA 02728167 2010-12-15
Description
Ni-BASED SINGLE CRYSTAL SUPERALLOY AND COMPONENT USING THE SAME
AS SUBSTRATE
Technical Field
The present invention relates to an Ni-based single crystal superalloy
comprising
Al, Ta, W, Re, Cr, Ru and Nb as the main alloying elements, and to a component
comprising it as the substrate, and in particular, the invention relates to a
technology for
improving the high-temperature creep property of the superalloy and improving
the
environmental resistance such as the high-temperature corrosion resistance
thereof.
Background Art
Typical compositions of an Ni-based single crystal superalloy developed as a
material for high-temperature turbine blades/nozzle guide vanes such as
aircraft, gas
turbines and others are, for example, those shown in Table 1.
Table 1
Alloy Element (wt.%)
Generation
Name Co Cr Mo W Al 11 Nb Ta Hf Re Ru Ni
PWA1480 5.0 10.0 - 4.0 5.0 1.5 - 12.0 - -
- balance
PWA1484 10.0 5.0 2.0 6.0 5.6 - - 9.0 0.1 3.0 balance
MX-4
Fourth 16.5 2.0 2.0 6.0 5.6 - - 8.3 0.2 6.0 3.0 balance
PWA1497
Second CMSX-4 9.6 6.4 0.6 6.4 5.6 1.0 - 6.5 0.1 3.0 - balance
Third CMSX-10X 3.3 2.3 0.4 5.5 5.7 0.3 0.1 8.4 0.03 6.3 - balance
Rene'N4 8.0 9.0 2.0 6.0 3.7 4.2 0.5 4.0 - - - balance
Rene'N5 8.0 7.0 2.0 5.0 6.2 - - 7.0 0.2 3.0 - balance
Third Rene'N6 13.0 4.0 1.0 6.0 6.0 - 0.3 7.0 0.2 5.0 - balance
RR3010 3.1 1.7 0.5 5.5 5.9 0.1 0.1 8.5 - 6.8 - balance
Fourth 3B 12.5 5.0
5.5 5.7 0.5 - 8.0 0.15 6.0 3.0 balance
The above-mentioned Ni-based single crystal superalloy is obtained through
solution treatment at a predetermined temperature followed by aging treatment.
The alloy
is a so-called precipitation-hardened alloy, and has a morphology where a
precipitation
1
CA 02728167 2010-12-15
phase, y'-phase is precipitated in the matrix phase, y-phase.
Of the alloys shown in Table 1, CMSX-2 (by Canon Muskegon, see Patent
Reference 1) is a first-generation alloy; CMSX-4 (by Canon Muskegon, see
Patent
Reference 2) is a second-generation alloy; Rene'N6 (by General Electric, see
Patent
Reference 3) and CMSX-10K (by Canon Muskegon, see Patent Reference 4) are
third-generation alloys; 3B and MX-4 (by General Electric, see Patent
Reference 5) are
fourth-generation alloys.
The above-mentioned first-generation alloy CMSX-2 and second-generation alloy
CMSX-4 are, though comparable thereto in point of creep strength at low
temperatures,
inferior to the third-generation alloys in point of creep strength at high
temperatures, since a
large quantity of eutectic y'-phase remains therein even after high-
temperature solution
treatment.
The above-mentioned third-generation Rene'N6 and CMSX-10K are alloys that
are intended to have more increased creep strength at high temperatures than
the
second-generation alloys. However, since the compositional ratio of Re (5% by
mass or
more) is over the Re solid solution limit in the matrix phase (y-phase), the
excessive Re
may compound with the other elements to form a so-called TCP phase
(topologically close
packed phase) through precipitation at high temperatures, therefore bringing
about a
problem in that the amount of the TCP phase increases in long-term use at high
temperatures and the creep strength of the alloy is thereby lowered.
For improving the creep strength of the Ni-based single crystal superalloy, it
will be
effective to make the lattice constant of the precipitation phase (y'-phase)
slightly lower
than the lattice constant of the matrix phase (y-phase); however, since the
lattice constant
of each phase greatly changes depending on the compositional ratio of the
alloying
elements, it used to be difficult to precisely control the lattice constant
and to improve the
creep strength. In consideration of the above-mentioned situation, the present
inventors
have already proposed an Ni-based single crystal superalloy of which the
strength is
improved by significantly preventing the precipitation of the TCP phase
therein at high
temperatures (Patent References 6, 7).
In general, in case where the above-mentioned Ni-based single crystal
superalloy
having a high strength at high temperatures is used as a material for high-
temperature
turbine blades/nozzle guide vanes such as aircraft, gas turbines or the like,
the alloy is
exposed to oxygen-containing, high-temperature combustion gas for a long
period of time,
and therefore, along with the above-mentioned strength improvement at high
temperatures,
the oxidation resistance and the corrosion resistance at high temperatures are
also
important performance factors of the Ni-based single crystal superalloy that
should not be
2
CA 02728167 2010-12-15
,
overlooked. None of the above-mentioned patent references have Examples
relating to
concrete oxidation resistance; but some of them have a qualitative description
indicating
the effectiveness of Cr, Hf, Ta and the like for oxidation resistance.
However, Ru that
shows a remarkable effect for strength improvement at high temperatures is, on
the other
hand, said to lower the oxidation resistance and the corrosion resistance at
high
temperature (Patent Reference 8). In Fig. 1, the data of the creep rupture
lifetime at
1100 C and 137 MP and the oxidation resistance at 1100 C of typical various
existing
alloys are plotted. Rene'N5 and CMSX-4 shows considerably excellent oxidation
resistance; however, these existing alloys could have improved oxidation
resistance owing
to the high Cr content therein, but their creep life at high temperatures is
insufficient. On
the other hand, the MX-4 alloy is known as a fourth-generation alloy having
considerably
excellent high-temperature heat resistance, but its oxidation resistance at
high
temperatures is poor. General Electric has proposed a coating system including
diffusion
barrier coating for improving the oxidation resistance of MX-4 (Patent
Reference 6). As
seen in these examples, it is difficult to develop an Ni-based single crystal
superalloy
satisfying both life/strength and oxidation resistance at high temperatures,
and this will be
an important technical theme toward industrialization of heat-resistant alloys
in future.
Patent Reference 1: USP 4,582,548
Patent Reference 2: USP 4,643,782
Patent Reference 3: USP 5,455,120
Patent Reference 4: USP 5,366,695
Patent Reference 5: USP 5,151,249
Patent Reference 6: USP 6,966,956
Patent Reference 7: EP 1,262,569
Patent Reference 8: USP 6,921,586
Disclosure of Invention
Problems to be solved by the Invention
Specifically, an object of the present invention is to provide a high-
performance
Ni-based single crystal superalloy well balanced in two features of high-
temperature
strength and oxidation resistance at high temperatures in practical use.
Another object of
the invention is to provide an Ni-based single crystal superalloy still having
sufficient
characteristics even in "heat treatment window" that should not be overlooked
in practical
use.
Means for Solving the Problems
3
CA 02728167 2010-12-15
To attain the above-mentioned objects, the invention employs the following
constitution.
The first aspect of invention is characterized in that the ingredients have a
composition containing, as ratio by mass, from 5.0% by mass to 7.0% by mass of
Al, from
4.0% by mass to 8.0% by mass of Ta, from 0% by mass to 2.0% by mass of Mo,
from
3.0% by mass to 8.0% by mass of W, from 3.0% by mass to 8.0% by mass of Re,
from 0%
by mass to 0.50% by mass of Hf, from 3.0% by mass to 7.0% by mass of Cr, from
0% by
mass to 9.9% by mass of Co, from 1.0% by mass to 14.0% by mass of Ru, and from
0.1%
by mass to 4.0% by mass of Nb, with the balance of Ni and inevitable
impurities.
The second aspect of the invention is characterized in that the ingredients
have a
composition containing, as ratio by mass, from 5.0% by mass to 7.0% by mass of
Al, from
4.0% by mass to 10.0% by mass of Ta, from 0% by mass to less than 1.1% by mass
of Mo,
from 3.0% by mass to 6.0% by mass of W, from 3.0% by mass to 8.0% by mass of
Re,
from 0% by mass to 0.50% by mass of Hf, from 3.0% by mass to 7.0% by mass of
Cr, from
0% by mass to 9.9% by mass of Co, from 1.0% by mass to 8.0% by mass of Ru, and
from
0.1% by mass to 4.0% by mass of Nb, with the balance of Ni and inevitable
impurities.
The third aspect of the invention is characterized in that the ingredients
have a
composition containing, as ratio by mass, from 5.0% by mass to 7.0% by mass of
Al, from
4.0% by mass to 8.0% by mass of Ta, from 0% by mass to less than 1.1% by mass
of Mo,
from 3.0% by mass to less than 6.0% by mass of W, from 3.0% by mass to 8.0% by
mass
of Re, from 0% by mass to less than 0.12% by mass of Hf, from 3.0% by mass to
7.0% by
mass of Cr, from 0% by mass to 9.9% by mass of Co, from 1.0% by mass to 8.0%
by
mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb, with the balance of
Ni and
inevitable impurities.
The for the aspect of the invention is characterized in that the ingredients
have a
composition containing, as ratio by mass, from 5.0% by mass to 7.0% by mass of
Al, from
4.0% by mass to 8.0% by mass of Ta, from 0% by mass to less than 1.1% by mass
of Mo,
from 3.0% by mass to less than 6.0% by mass of W, from 5.8% by mass to 8.0% by
mass
of Re, from 0% by mass to less than 0.12% by mass of Hf, from 3.0% by mass to
7.0% by
mass of Cr, from 0% by mass to 9.9% by mass of Co, from 1.0% by mass to 8.0%
by
mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb, with the balance of
Ni and
inevitable impurities.
The fifth aspect of the invention is characterized in that the ingredients
have a
composition containing, as ratio by mass, from 5.0% by mass to 7.0% by mass of
Al, from
4.0% by mass to 8.0% by mass of Ta, from 0% by mass to less than 1.1% by mass
of Mo,
from 3.0% by mass to less than 6.0% by mass of W, from 5.8% by mass to 8.0% by
mass
4
CA 02728167 2010-12-15
of Re, from 0% by mass to less than 0.12% by mass of Hf, from 3.0% by mass to
7.0% by
mass of Cr, from 0% by mass to 9.9% by mass of Co, from 4.1% by mass to 8.0%
by
mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb, with the balance of
Ni and
inevitable impurities.
The sixth aspect of the invention is characterized in that the ingredients
have a
composition containing, as ratio by mass, from 5.0% by mass to 7.0% by mass of
Al, from
4.0% by mass to less than 6.0% by mass of Ta, from 0% by mass to less than
1.1% by
mass of Mo, from 3.0% by mass to less than 6.0% by mass of W, from 5.8% by
mass to
8.0% by mass of Re, from 0% by mass to less than 0.12% by mass of Hf, from
3.0% by
mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of Co, from 4.1%
by
mass to 8.0% by mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb, with
the
balance of Ni and inevitable impurities.
The seventh aspect of the invention is characterized in that the ingredients
have a
composition containing, as ratio by mass, from 5.0% by mass to 7.0% by mass of
Al, from
4.0% by mass to less than 6.0% by mass of Ta, from 0% by mass to less than
1.1% by
mass of Mo, from 3.0% by mass to less than 6.0% by mass of W, from 5.8% by
mass to
8.0% by mass of Re, from 0.0% by mass to less than 0.12% by mass of Hf, from
3.0% by
mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of Co, from 4.1%
by
mass to 8.0% by mass of Ru, and from more than 1.0% by mass to 3.0% by mass of
Nb,
with the balance of Ni and inevitable impurities.
The eighth aspect of the invention is characterized in that the ingredients
have a
composition containing, as ratio by mass, from 5.0% by mass to 7.0% by mass of
Al, from
4.0% by mass to less than 6.0% by mass of Ta, from 0% by mass to less than
1.1% by
mass of Mo, from 4.0% by mass to less than 5.0% by mass of W, from 5.8% by
mass to
8.0% by mass of Re, from 0.0% by mass to less than 0.12% by mass of Hf, from
3.0% by
mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of Co, from 4.1%
by
mass to 8.0% by mass of Ru, and from more than 1.0% by mass to 3.0% by mass of
Nb,
with the balance of Ni and inevitable impurities.
The ninth aspect of the invention is the Ni-based single crystal superalloy of
any
one of the invention afore-mentioned, which further contains Ti in an amount,
as ratio by
mass, of at most 2.0% by mass.
The tenth aspect of the invention is the Ni-based single crystal superalloy of
any
one of the invention afore-mentioned, which further contains at least any one
of B, C, Si, Y,
La, Ce, V and Zr.
The eleventh aspect of the invention is the Ni-based single crystal superalloy
of
any one of the invention afore-mentioned, wherein, when the lattice constant
of the matrix
CA 02728167 2014-03-19
phase is represented by al and the lattice constant of the precipitation phase
is
represented by a2, then the relation of al and a2 satisfies 0.992a1 a2 < al .
The twelfth aspect of the invention is an alloy component comprising an Ni-
based
single crystal superalloy as the substrate, wherein the Ni-based single
crystal superalloy is
the Ni-based single crystal superalloy of any of the invention afore-
mentioned.
In an embodiment, the Ni-based single crystal super alloy of the invention
comprises Al, Ta a Ni-based single crystal superalloy comprising Al, Ta, W,
Re, Cr, Ru and
Nb as the main additive elements, wherein the superalloy contains, as ratio by
mass:
from 5.0% by mass to 7.0% by mass of Al,
from 4.0% by mass to 8.0% by mass of Ta,
from 0% by mass to less than 1.1% by mass of Mo,
from 3.0% by mass to less than 6.0% by mass of W,
from 5.8% by mass to 8.0% by mass of Re,
from 0% by mass to less than 0.12% by mass of Hf,
from 3.0% by mass to 7.0% by mass of Cr,
from 0% by mass to 9.9% by mass of Co,
from 5.0% by mass to 8.0% by mass of Ru, and
from 0.1% by mass to 4.0% by mass of Nb,
with the balance of Ni and inevitable impurities.
Advantage of the Invention
Use of the above-mentioned Ni-based single crystal superalloy system enables,
in
principle, suppression of TCP phase precipitation in use at high temperatures
that causes
strength reduction, by addition of Ru thereto; and by defining the
compositional ratio of the
other alloying elements to fall within the optimum range as in the above to
thereby control
the lattice constant of the mother phase (7-phase) and the lattice constant of
the
precipitation phase (y'-phase) to the optimum value, an alloy excellent in
high-temperature
strength can be provided.
On the other hand, however, it is known that Ru lowers oxidation resistance
and
corrosion resistance at high temperatures. The present invention is directed
to
optimization of the composition for improving the above-mentioned high-
temperature
strength and to improvement of the oxidation resistance of the substrate
itself of the
Ni-based single crystal superalloy, and the inventors have found the
practicable Ni-based
single crystal superalloy well balanced in both the strength and the oxidation
resistance at
high temperatures by further optimizing the compositional ratio of Ru and
other alloying
elements.
6
CA 02728167 2014-03-19
Specifically, in the above-mentioned Ni-based single crystal superalloy
system, in
case where the ingredients have a composition containing, as ratio by mass,
5.6% by
mass of Al, 5.6% by mass of Ta, 1.0% by mass of Mo, 4.8% by mass of W, 6.4% by
mass
of Re, 0.10% by mass of Hf, 4.6% by mass of Cr, 5.6% by mass of Co, 5.0% by
mass of
Ru and 1.1% by mass of Nb with the balance of Ni and inevitable impurities,
the creep
rupture lifetime of the alloy at 1,100 C and 137 MPa is about 1,400 hours; and
in a
high-temperature oxidation acceleration test by a cycle at 1,100 C for 1.0
hour, the alloy
undergoes little mass change up to 50 cycles.
The above-mentioned Ni-based single crystal superalloy system may further
contain, as ratio by mass, from 0% by mass to 2.0% by mass of Ti.
The above-mentioned Ni-based single crystal superalloy system may contain at
least one of B, C, Si, Y, La, Ce, V and Zr.
In this case, the individual ingredients are preferably, as ratio by mass, at
most
0.05% by mass of B, at most 0.15% by mass of C, at most 0.1% by mass of Si, at
most
6a
CA 02728167 2010-12-15
0.1% by mass of Y, at most 0.1% by mass of La, at most 0.1% by mass of Ce, at
most 1%
by mass of V and at most 0.1% by mass of Zr.
Further, the Ni-based single crystal superalloy of the invention is the
above-mentioned Ni-based single crystal superalloy wherein al representing the
lattice
constant of the matrix phase and a2 representing the lattice constant of the
precipitation
phase satisfy 0.992a1 a2 < al .
Best Mode for Carrying out the Invention
Embodiments of the invention are described in detail hereinunder.
The Ni-based single crystal superalloy of the invention is an alloy containing
Al, Ta,
W, Re, Cr, Ru and Nb as the main additives and containing Mo, Hf and Co as
regulative
additive elements.
The Ni-based single crystal superalloy of the invention is characterized in
that the
ingredients have a composition containing, as ratio by mass, from 5.0% by mass
to 7.0%
by mass of Al, from 4.0% by mass to 8.0% by mass of Ta, from 0% by mass to
2.0% by
mass of Mo, from 3.0% by mass to 8.0% by mass of W, from 3.0% by mass to 8.0%
by
mass of Re, from 0% by mass to 0.50% by mass of Hf, from 3.0% by mass to 7.0%
by
mass of Cr, from 0% by mass to 9.9% by mass of Co, from 1.0% by mass to 14.0%
by
mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb, with the balance of
Ni and
inevitable impurities.
The Ni-based single crystal superalloy of the invention is characterized in
that the
ingredients have a composition containing, as ratio by mass, from 5.0% by mass
to 7.0%
by mass of Al, from 4.0% by mass to 10.0% by mass of Ta, from 0% by mass to
less than
1.1% by mass of Mo, from 3.0% by mass to 6.0% by mass of W, from 3.0% by mass
to
8.0% by mass of Re, from 0% by mass to 0.50% by mass of Hf, from 3.0% by mass
to
7.0% by mass of Cr, from 0% by mass to 9.9% by mass of Co, from 1.0% by mass
to 8.0%
by mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb, with the balance
of Ni and
inevitable impurities.
The Ni-based single crystal superalloy of the invention is characterized in
that the
ingredients have a composition containing, as ratio by mass, from 5.0% by mass
to 7.0%
by mass of Al, from 4.0% by mass to 8.0% by mass of Ta, from 0% by mass to
less than
1.1% by mass of Mo, from 3.0% by mass to less than 6.0% by mass of W, from
3.0% by
mass to 8.0% by mass of Re, from 0% by mass to less than 0.12% by mass of Hf,
from
3.0% by mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of Co,
from 1.0%
by mass to 8.0% by mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb,
with the
balance of Ni and inevitable impurities.
7
CA 02728167 2010-12-15
The Ni-based single crystal superalloy of the invention is characterized in
that the
ingredients have a composition containing, as ratio by mass, from 5.0% by mass
to 7.0%
by mass of Al, from 4.0% by mass to 8.0% by mass of Ta, from 0% by mass to
less than
1.1% by mass of Mo, from 3.0% by mass to less than 6.0% by mass of W, from
5.8% by
mass to 8.0% by mass of Re, from 0% by mass to less than 0.12% by mass of Hf,
from
3.0% by mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of Co,
from 1.0%
by mass to 8.0% by mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb,
with the
balance of Ni and inevitable impurities.
The Ni-based single crystal superalloy of the invention is characterized in
that the
ingredients have a composition containing, as ratio by mass, from 5.0% by mass
to 7.0%
by mass of Al, from 4.0% by mass to 8.0% by mass of Ta, from 0% by mass to
less than
1.1% by mass of Mo, from 3.0% by mass to less than 6.0% by mass of W, from
5.8% by
mass to 8.0% by mass of Re, from 0% by mass to less than 0.12% by mass of Hf,
from
3.0% by mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of Co,
from 4.1%
by mass to 8.0% by mass of Ru, and from 0.1% by mass to 4.0% by mass of Nb,
with the
balance of Ni and inevitable impurities.
The Ni-based single crystal superalloy of the invention is characterized in
that the
ingredients have a composition containing, as ratio by mass, from 5.0% by mass
to 7.0%
by mass of Al, from 4.0% by mass to less than 6.0% by mass of Ta, from 0% by
mass to
less than 1.1% by mass of Mo, from 3.0% by mass to less than 6.0% by mass of
W, from
5.8% by mass to 8.0% by mass of Re, from 0% by mass to less than 0.12% by mass
of Hf,
from 3.0% by mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of
Co, from
4.1% by mass to 8.0% by mass of Ru, and from 0.1% by mass to 4.0% by mass of
Nb,
with the balance of Ni and inevitable impurities.
The Ni-based single crystal superalloy of the invention is characterized in
that the
ingredients have a composition containing, as ratio by mass, from 5.0% by mass
to 7.0%
by mass of Al, from 4.0% by mass to less than 6.0% by mass of Ta, from 0% by
mass to
less than 1.1% by mass of Mo, from 3.0% by mass to less than 6.0% by mass of
W, from
5.8% by mass to 8.0% by mass of Re, from 0.0% by mass to less than 0.1% by
mass of Hf,
from 3.0% by mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of
Co, from
4.1% by mass to 8.0% by mass of Ru, and from more than 1.0% by mass to 3.0% by
mass of Nb, with the balance of Ni and inevitable impurities.
The Ni-based single crystal superalloy of the invention is characterized in
that the
ingredients have a composition containing, as ratio by mass, from 5.0% by mass
to 7.0%
by mass of Al, from 4.0% by mass to less than 6.0% by mass of Ta, from 0% by
mass to
less than 1.1% by mass of Mo, from 4.0% by mass to less than 5.0% by mass of
W, from
8
CA 02728167 2010-12-15
5.8% by mass to 8.0% by mass of Re, from 0.0% by mass to less than 0.1% by
mass of Hf,
from 3.0% by mass to 7.0% by mass of Cr, from 0% by mass to 9.9% by mass of
Co, from
4.1% by mass to 8.0% by mass of Ru, and from more than 1.0% by mass to 3.0% by
mass of Nb, with the balance of Ni and inevitable impurities.
The above-mentioned alloys all have an austenite phase, 'y-phase (matrix
phase),
and an intermediate order phase, y'-phase (precipitation phase) dispersed and
precipitated
in the matrix phase. The y'-phase mainly comprises an intermetallic compound
represented by Ni3A1, and the y'-phase improves the high-temperature strength
of the
Ni-based single crystal superalloy.
Cr is an element excellent in oxidation resistance, and improves the
high-temperature corrosion resistance of the Ni-based single crystal
superalloy.
The compositional ratio of Cr is preferably within a range of from 3.0% by
mass to
7.0% by mass, more preferably within a range of from 3.5% by mass to 6.5% by
mass,
most preferably within a range of from 4.0% by mass to 6.0% by mass.
When the compositional ratio of Cr is less than 3.0% by mass, then it is
unfavorable since the desired high-temperature corrosion resistance could not
be secured;
but when the compositional ratio of Cr is more than 7.0% by mass, it is
unfavorable since
the y'-phase precipitation is suppressed and harmful phases such as es-phase,
j_i-phase
and the like would be formed to lower the high-temperature strength.
Mo dissolves in the matrix phase, y-phase in the co-presence of W and Ta,
thereby
increasing the high-temperature strength of the alloy, and contributes toward
the
high-temperature strength through precipitation hardening. In addition, Mo
greatly
contributes toward the lattice misfit and the dislocation network distance (to
be mentioned
below) that are the characteristics of the present alloy.
The compositional ratio of Mo is preferably within a range of from 0.0% by
mass to
2.0% by mass, more preferably within a range of from 0.0% by mass to less than
1.1% by
mass.
When the compositional ratio of Mo is more than 2.0% by mass, then it is
unfavorable since the desired oxidation resistance characteristic at high
temperatures
could not be secured in the composition range of the Ni-based single crystal
superalloy
exemplified in the above.
W increases the high-temperature strength of the alloy owing to the effect of
solid
solution strengthening and precipitation hardening in the co-presence of Ta
and Mo as so
mentioned in the above. When the compositional ratio of W is less than 3.0% by
mass,
then it is unfavorable since the desired high-temperature strength could not
be secured;
but when the compositional ratio of W is too large, it is also unfavorable
since the
9
CA 02728167 2010-12-15
high-temperature corrosion resistance would lower. The compositional ratio of
W is
preferably within a range of from 3.0% by mass to 8.0% by mass, more
preferably within a
range of from 3.0% by mass to 6.0% by mass, most preferably within a range of
from 4.0%
by mass to 5.0% by mass.
Ta increases the high-temperature strength of the alloy owing to the effect of
solid
solution strengthening and precipitation hardening in the co-presence of W and
Mo as so
mentioned in the above, and it partly acts on the y'-phase for precipitation
hardening to
thereby increase the high-temperature strength.
The compositional ratio of Ta is preferably within a range of from 4.0% by
mass to
8.0% by mass.
When the compositional ratio of Ta is less than 4.0% by mass, then it is
unfavorable since the desired high-temperature strength could not be secured;
but when
the compositional ratio of Ta is more than 10.0% by mass, it is also
unfavorable since
o--phase and pt-phase would be formed to lower the high-temperature strength.
In
addition, in practical use, when the compositional ratio of Ta is 8.0% by mass
or more, then
it is unfavorable since the density of the Ni-based single crystal superalloy
would increase.
The most preferred compositional ratio of Ta is within a range of from 4.0% by
mass to less
than 6.0% by mass.
Al compounds with Ni and forms an intermetallic compound represented by Ni3A1
to form the y'-phase that is finely and uniformly dispersed and precipitated
in the matrix
phase in a fraction ratio by volume of from 60 to 70%, thereby increasing the
high-temperature strength of the alloy.
The compositional ratio of Al is preferably within a range of from 5.0% by
mass to
7.0% by mass.
When the compositional ratio of Al is less than 5.0% by mass, then it is
unfavorable since the precipitation amount of the y'-phase would be low and
the desired
high-temperature strength of the alloy could not be secured; but when the
compositional
ratio of Al is more than 7.0% by mass, then it is also unfavorable since a
large quantity of
coarse y'-phase called eutectic y'-phase would be formed to disable the
solution treatment
and the alloy could not secure sufficient high-temperature strength.
Hf is an antioxidation-enhancing element. The compositional ratio of Hf is
preferably within a range of from 0.00% by mass to 0.50% by mass, most
preferably within
a range of from 0.01% by mass to less than 0.12% by mass. When the
compositional
ratio of Hf is less than 0.01% by mass, then it is unfavorable since the
antioxidation-enhancing effect could not be secured. However, depending on the
content
of Al and/or Cr, the compositional ratio of Hf may be from 0% by mass to less
than 0.01%
CA 02728167 2010-12-15
by mass, as the case may be. When the compositional ratio of Hf is too large,
it is
unfavorable as often causing local melting to lower the high-temperature
strength of the
alloy.
Co expands the solid solution limit of Al, Ta and others in the matrix phase
at high
temperatures thereby to disperse and precipitate fine y'-phase through heat
treatment to
increase the high-temperature strength of the alloy.
The compositional ratio of Co is preferably within a range of from 0.0% by
mass to
9.9% by mass, more preferably within a range of from 0.1% by mass to 9.9% by
mass.
When the compositional ratio of Co is less than 0.1% by mass, then it is
unfavorable since
the y'-phase precipitation would be insufficient and the desired high-
temperature strength
could not be secured. However, depending on the content of Al and/or Ta, the
compositional ratio of Co may be 0% by mass or less than 0.1% by mass, as the
case may
be.
When the compositional ratio of Co is more than 9.9% by mass, then it is
unfavorable
since the balance with the other elements such as Al, Ta, Mo, W, Hf, Cr and
others may be
lost and some harmful phases may precipitate to lower the high-temperature
strength of
the alloy.
Re dissolves in the matrix phase, y-phase to improve the high-temperature
strength of the alloy through solid solution strengthening. In addition, it
has another effect
of enhancing the corrosion resistance. On the other hand, addition of too much
Re would
lower the high-temperature strength as causing precipitation of the harmful
phase, TCP
phase at high temperatures.
The compositional ratio of Re is preferably within a range of from 3.0% by
mass to
8.0% by mass, more preferably from 5.8% by mass to 8.0% by mass.
When the compositional ratio of Re is less than 3.0% by mass, then it is
unfavorable since the solid solution strengthening for the y-phase would be
insufficient and
the desired high-temperature strength could not be secured. When the
compositional
ratio of Re is more than 8.0% by mass, then it is also unfavorable since the
TCP phase
would precipitate at high temperatures to lower the high-temperature strength
and since
the increase in the amount of expensive Re would cause the increase in the
alloy material
cost.
Ru prevents the precipitation of the TCP phase, thereby improving the
high-temperature strength of the alloy.
The compositional ratio of Ru is preferably within a range of from 1.0% by
mass to
14.0% by mass, more preferably within a range of from 1.0% by mass to 8.0% by
mass.
Even more preferably, the compositional ratio of Ru is within a range of from
4.1% by mass
to 8.0% by mass.
11
CA 02728167 2010-12-15
When the compositional ratio of Ru is less than 1.0% by mass, then the TCP
phase would precipitate at high temperatures and sufficient high-temperature
strength
could not be secured. Further, when the compositional ratio of Ru is less than
4.1% by
mass, then the high-temperature strength of the alloy would be lower than that
of the case
where the compositional ratio of Ru is not lower than 4.1% by mass. When the
compositional ratio of Ru is more than 8.0% by mass, then it is unfavorable
since 6-phase
would precipitate and the high-temperature strength of the alloy would be
thereby lowered.
In addition, the increase in the amount of expensive Ru would cause the
increase in the
alloy material cost, which is unfavorable from the viewpoint of the practical
use of the alloy.
The compositional ratio of Nb is preferably within a range of from 0.1% by
mass to
4.0% by mass, more preferably within a range of from more than 1.0% by mass to
3.0% by
mass. Nb is an element favorable in the point of lowering the density of the
Ni-based
single crystal superalloy; however, when the compositional ratio of Nb is 3.0%
by mass or
more, then it is unfavorable since the harmful phase would be readily formed
at high
temperatures.
In the invention, the compositional ratio of Al, Ta, Mo, W, Hf, Cr. Co, Re, Nb
and Ni
is controlled to be an optimum one to thereby make the lattice misfit and the
dislocation
network distance (to be mentioned below) that are computed from the lattice
constant of
the y-phase and the lattice constant of the y'-phase, fall within an optimum
range to
increase the high-temperature strength of the alloy, and TCP phase
precipitation may be
prevented by addition of Ru. In particular, defining the compositional ratio
of Al, Cr, Ta and
Mo to fall within the above-mentioned compositional range makes it possible to
lower the
alloy production cost. Further, the invention facilitates increase in the
specific strength
and definition of the lattice misfit and the dislocation network distance to
be the optimum
value.
In addition, in a service environment at high temperatures of from 1273K (1000
C)
to 1373K (1100 C), when the lattice constant of the crystal that constitutes
the matrix
phase, y-phase is represented by al and the lattice constant of the crystal
constituting the
precipitation phase, y'-phase is by a2, the relation between al and a2
preferably satisfies
a2 < a 1 .
In the following description, the percentage of al to the difference between
the
lattice constant al of the crystal of the mother phase and the lattice
constant a2 of the
crystal of the precipitation phase {(a2 - al )/al x 100 (%)} is referred to as
"lattice misfit".
When the lattice misfit range is more negative so far as the coherency of the
matrix phase, y-phase and the precipitation phase, y'-phase is kept well, then
the
dislocation network distance could be smaller therefore bringing about the
effect of
12
CA 02728167 2010-12-15
improving the creep strength of the alloy.
The lattice misfit is less than 0%, preferably at most -0.1%, more preferably
at
most -0.15%.
However, when the numerical value of the lattice misfit is too much shifted to
negativity, the coherency could not be maintained and the performance of the
alloy would
worsen; and therefore, preferably, the value is at least -1%, more preferably -
0.8%, even
more preferably -0.7%.
In other words, the relation between the lattice constant a2 of the crystal of
the
precipitation phase and the lattice constant al of the crystal of the matrix
phase is 0.990a1
a2 < al , preferably 0.992a1 a2 0.999a1, more preferably 0.993a1 a2 0.9985a1.
In case where the lattice constants of the two are in the relation as above,
the
precipitation phase could form and grow in the matrix phase by heat treatment
to
continuously extend in the perpendicular direction relative to the loading
direction thereto,
and therefore, the dislocation defects migration in the alloy microstructure
is suppressed
under stress, and the creep strength of the alloy is thereby increased. For
controlling the
lattice constant al and the lattice constant a2 to be in the above-mentioned
relation, the
composition of the alloying elements of the Ni-based single crystal superalloy
must be
suitably controlled.
The Ni-based single crystal superalloy may further contain Ti. In this case,
the
compositional ratio of Ti is preferably within a range of from 0% by mass to
2.0% by mass.
When the compositional ratio of Ti is more than 2.0% by mass, then it is
unfavorable since
harmful phases would precipitate to lower the high-temperature strength of the
alloy.
Regarding the compositional ratio of Ta, Nb and Ti, when the total of these
(Ta +
Nb + Ti) is from 4.0% by mass to 10.0% by mass, then the high-temperature
strength of
the alloy could be increased.
The Ni-based single crystal superalloy may contain, for example, B, C, Si, Y,
La,
Ce, V, Zr and the like, in addition to inevitable impurities. In case where
the alloy contains
at least one of B, C, Si, Y, La, Ce, V and Zr, the compositional ratio of the
individual
ingredients is preferably such that B is at most 0.05% by mass, C is at most
0.15% by
mass, Si is at most 0.1% by mass, Y is at most 0.1% by mass, La is at most
0.1% by mass,
Ce is at most 0.1% by mass, V is at most 1% by mass, Zr is at most 0.1% by
mass.
When the compositional ratio of the individual ingredients is more than the
above-mentioned range, then it is unfavorable since harmful phases would
precipitate to
lower the high-temperature strength of the alloy.
Some existing Ni-based single crystal superalloys undergo reverse partition,
but
the Ni-based single crystal alloy of the invention does not undergo reverse
partition.
13
CA 02728167 2010-12-15
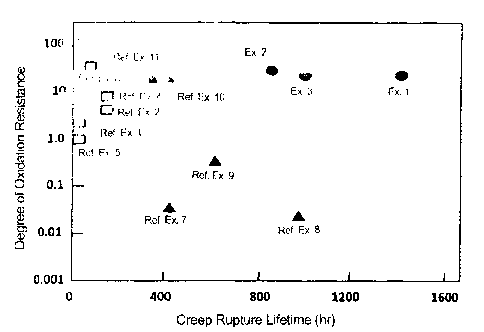
The creep rupture lifetime and the oxidation resistance of the Ni-based
single-crystal superalloy of the invention described hereinabove are shown in
Fig. 1 along
with the characteristics of various typical existing alloys therein. It is
obvious that, as
compared with Rene'N5, CMSX-4 and MX-4 alloys, the Ni-based single crystal
superalloy
of the invention has extremely excellent characteristics of creep life and
oxidation
resistance at high temperatures.
The degree of oxidation resistance on the vertical axis in Fig. 1 is defined
by the
following formula. In general, in case where samples of Ni-based single
crystal
superalloys are oxidized and their oxidation is promoted at high temperatures,
the mass of
some alloys temporarily increases by oxidation but then decreases, and that of
other alloys
gradually decreases after the start of oxidation. The formula applies to all
cases and
indicates the oxidation resistance of the alloys.
Degree of Oxidation Resistance = log[1/w1 x 11(1w50 -
wherein w1 means the mass increase in one cycle (mg/cm2),
and w50 - w1 means the mass change from 1 cycle up to 50 cycles (mg/cm2).
Examples
The effect of the invention is described below with reference to the following
Examples.
Using a vacuum melting furnace, various types of Ni-based single crystal
superalloy melts were prepared, and the alloy melts were cast into plural
alloy ingots each
having a different composition. The compositional ratios of the alloys of the
invention
(Examples 1 to 3), as well as those of six typical existing heat-resistant
alloys (Reference
Examples 1 to 6) and four types of fourth-generation and fifth-generation heat-
resistant
alloys for which the present applicant already filed patent applications
(Reference
Examples 7 to 11) (Patent References 6 and 7) are shown in Table 2.
14
CA 02728167 2010-12-15
Table 2
Constitutive Element (wt.%)
Sample
Co Cr Mo W Al Ti Nb Ta Hf Re Ru Ni
Ex. 1 5.6 4.6 1.0 5.1 5.5 - 1.2 5.6 0.1
6.5 5.0 balance
Ex. 2 8.0 4.6 1.0 4.8 5.6 - 1.2 5.6 0.1
6.4 5.0 balance
Ex. 3 5.6 4.8 0.8 5.2 5.6 - 1.2 5.6 0.1
6.4 5.0 balance
Ref. Ex. 1 5.0 10.0 - 4.0 5.0 1.5 - 12.0 - - -
balance
Ref. Ex. 2 10.0 5.0 2.0 6.0 5.6 - - 9.0 0.1 3.0 -
balance
Ref. Ex. 3 16.5 2.0 2.0 6.0 5.6 - -
8.3 0.2 6.0 3.0 balance
Ref. Ex. 4 9.6 6.4 0.6 6.4 5.6 1.0 - 6.5 0.1 3.0 -
balance
Ref. Ex. 5 8.0 9.0 2.0 6.0 3.7 4.2 0.5 4.0 - - -
balance
Ref. Ex. 6 8.0 7.0 2.0 5.0 6.2 - - 7.0 0.2 3.0 -
balance
Ref. Ex. 7 5.8 2.9 2.9 5.9 5.9 - -
5.9 0.1 4.9 2.0 balance
Ref. Ex. 8 5.6 2.8 2.8 5.6 5.6 - - 5.6 0.1
6.9 5.0 balance
Ref. Ex. 9 5.6 4.6 2.4 5.0 5.6 - -
5.6 0.1 6.4 5.0 balance
Ref. Ex. 10 12.0 4.6 1.0 4.8 5.6 - 1.2 5.6 0.1
6.4 5.0 balance
Ref. Ex. 11 8.0 7.0 0.0 4.8 5.6 - 1.2 5.6 0.1
6.4 5.0 balance
Ex. Example Ref. Ex. Reference Example
Next, the alloy ingot was processed for solution treatment and for aging
treatment,
and the alloy microstructure was observed with a scanning electronic
microscope (SEM).
For the solution treatment of the alloys of Examples 1 to 3 and Reference
Examples 7 to
11, they were kept at 1573K (1300 C) for 1 hour, then heated up to 1603K (1330
C) and
kept as such for 5 hours. The aging treatment was continuous treatment of
primary aging
treatment at 1273K to 1423K (1000 C to 1150 C) for 4 hours followed by
secondary aging
treatment at 1143K (870 C) for 20 hours. The existing alloys of Reference
Examples 1 to
6 were processed for solution treatment and aging treatment under known
conditions for
each alloy. As a result, no TCP phase was confirmed in the microstructure of
every
sample.
Fig. 2 is the transmission electromicroscopic picture of the Ni-based single
crystal
alloy of Example 1 that was processed for solution treatment at 1335 C for 18
hours
followed by aging treatment at 1150 C. Network dislocations are observed and
the
network distance is about 0.32 m, which indicates favorable Ni-based single
crystal
alloys.
Next, the solution-treated and aging-treated samples were tested in a creep
test.
CA 02728167 2010-12-15
In the creep test, each sample (Examples 1 to 3 and Reference Examples 1 to
11) was
tested at the temperature and under the stress shown in Table 3, and the creep
rupture
lifetime thereof was recorded. The results are shown in Table 3.
Further, the solution-treated and aging-treated samples were tested in an
oxidation resistance test. Regarding the oxidation resistance test condition,
each sample
was exposed to air at a high temperature of 1150 C for 1 hour as one cycle and
the mass
change thereof was measured. The degree of oxidation resistance after 50
cycles is
shown in Table 3.
Table 3
Creep Rupture Degree of Oxidation
Sample Type of Alloy
Lifetime Resistance
Ex. 1 1400(h) 19.553
Ex. 2 857(h) 24.105
Ex. 3 986(h) 20.364
Ref. Ex. 1 17.8(h) 2.099 (PWA1480)
Ref. Ex. 2 141(h) 3.998 (PWA1484)
Ref. Ex. 3 142(h) 0.008 (MX-4)
Ref. Ex. 4 139(h) 4.276 (CMSX-4)
Ref. Ex. 5 31(h) 0.892 (Rene' N4)
Ref. Ex. 6 89(h) 33.449 (Rene'N5)
Ref. Ex. 7 412(h) 0.032
previously filed for patent application
Ref. Ex. 8 967(h) 0.021
previously filed for patent application
Ref. Ex. 9 608(h) 0.361
previously filed for patent application
Ref. Ex. 10 443(h) 21.843
previously filed for patent application
Ref. Ex. 11 382(h) 20.364
previously filed for patent application
Creep Rupture Lifetime: Test result at 1373K (1100 C) under 137 MPa.
Ex. Example Ref. Ex. Reference Example
In Fig. 1, the heat-resistant alloys of the invention (Examples 1 to 3),
various
typical existing practical alloys (Reference Examples 1 to 6) and heat-
resistant alloys
already proposed by the present inventors (Reference Examples 7 to 11) (Patent
References 6 and 7) were compared with each other in point of their
properties, creep
rupture lifetime at 1100 C and under 137 MP and oxidation resistance at 1150
C. Typical
existing practical alloys are poor in the high temperature mechanical
strength; and the
16
CA 02728167 2010-12-15
alloys already proposed by the inventors are obviously more excellent than the
practical
alloys in point of the high temperature mechanical strength, but some of them
are not
always sufficient in point of the oxidation resistance. Though the data
thereof are not
plotted, the degree of oxidation resistance of the existing alloy MX-4 of
Reference 3 was at
most 0.01, and was extremely lower than that of the other alloy systems. The
results
shown in Fig. 1 suggest that the alloy system of the invention is extremely
excellent both in
the mechanical strength and the oxidation resistance at the high temperature
as compared
with the above-mentioned existing alloys.
Fig. 3 comparatively shows the mass change of the alloy of Example 1 and the
alloy of Reference Example 4 in a cyclic oxidation test where the alloys were
exposed to
air at a high temperature of 1100 C for 1 hour as one cycle, and repeatedly
for a total of
about 600 cycles. The results indicate that the alloy of the invention has
much more
excellent oxidation resistance than the existing alloy CMSX-4 that is
generally known to
have excellent oxidation resistance.
Fig. 4 shows the observation of the surface of the alloy of Example 1 exposed
to
air at 1100 C for 1 hour. The alloy surface has a multilayer structure of
plural dense and
thin layers including a protective alumina layer, which indicates excellent
oxidation
resistance of the alloy.
The lattice misfit value CYO of the alloy of Example 1 and that of the typical
existing
alloy CMSX-4 (Reference Example 4) were determined through computation, and
were
-0.28 and -0.14, respectively. The alloy of Example 1 was better for the
smaller
dislocation network distance and the consequent improvement of the creep
strength of the
alloy with maintaining the coherency between the matrix phase, 7-phase and the
precipitation phase, y'-phase.
Fig. 5 shows the data of heat-treatment window of the alloy of Example 1 and
the
practical alloy of Reference Example 4. The heat-treatment window of the alloy
of
Example 1 and that of the practical alloy of Reference Example 4 were 47 C and
28 C,
respectively. The alloy of the invention has a broader heat-treatment window
than the
practical alloy of Reference Example 4 with no problem in the industrial blade
casting
process of producing it, and is expected to have an extremely high blade yield
in the
casting process.
Brief Description of Drawings
Fig. 1 is a view of comparing the heat-resistant alloys of the invention
(Examples
1 to 3), typical existing practical alloys (Reference Examples 1 to 6) and
alloys for which
the present inventors already filed patent application (Reference Examples 7
to 11) with
17
CA 02728167 2010-12-15
each other in point of the creep rupture lifetime at 1100 C and under 137 MP
and the
oxidation resistance at 1150 C.
Fig. 2 is a transmission electromicroscopic picture of the solution-treated
and
aging-treated, Ni-based single crystal alloy of Example 1.
Fig. 3 is a view showing the mass change of the alloy of Example 1 and the
practical alloy of Reference Example 4 exposed to air at a high temperature of
1100 C for
1 hour as one cycle and repeatedly for a total of about 600 cycles.
Fig. 4 is a photographic picture to observe the surface of the alloy of
Example 1
exposed to air at 1100 C for 1 hour.
Fig. 5 shows thermal analysis results for heat-treatment window of the alloy
of
Example 1 and the practical alloy of Reference Example 4.
18