Note: Descriptions are shown in the official language in which they were submitted.
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
ZERO PLATINUM GROUP METAL CATALYSTS
TECHNICAL FIELD OF THE INVENTION
[01] The present invention relates to catalysts which are free of any platinum
group
metals for reducing emissions of nitrous oxide, carbon monoxide, hydrocarbons,
and
sulfur in exhaust streams.
BACKGROUND OF THE INVENTION
[02] Catalysts in catalytic converters have been used to decrease the
pollution caused
by exhaust from various sources, such as automobiles, utility plants,
processing and
manufacturing plants, airplanes, trains, all terrain vehicles, boats, mining
equipment, and
other engine-equipped machines. A common catalyst used in this way is the
three-way
catalyst ("TWC"). The TWC works by converting carbon monoxide, hydrocarbons,
and
nitrogen oxides into less harmful compounds or pollutants. Specifically, a TWC
works
by simultaneously reducing the nitrogen oxides to nitrogen and oxygen,
oxidizing carbon
monoxide to less harmful carbon dioxide, and oxidizing unburnt hydrocarbons to
carbon
dioxide and water. The prior art TWC is made using at least some platinum
group
metals. Platinum group metals are defined in this specification to mean
platinum,
palladium, ruthenium, iridium, osmium, and rhodium in this application unless
otherwise
stated.
[03] With the ever stricter standards for acceptable emissions, the demand on
platinum
group metals continues to increase due to their efficiency in removing
pollutants from
exhaust. However, this demand along with other demands for platinum group
metals
places a strain on the supply of platinum group metals, which in turn drives
up the cost of
platinum group metals and therefore catalysts and catalytic converters.
Therefore, there
is a need for a catalyst that does not require platinum group metals, and has
a similar or
better efficiency as the prior art catalysts.
-1-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
SUMMARY OF THE INVENTION
[04] The present invention pertains to a catalyst system comprising a
substrate and a
washcoat, wherein the catalyst system is substantially free of platinum group
metals. The
washcoat comprises at least one oxide solid, wherein the oxide solid is
selected from the
group consisting of a carrier material oxide, a catalyst, and a mixture
thereof. The carrier
material oxide comprises one or more selected from the group consisting of an
oxygen
storage material, aluminum oxide, doped aluminum oxide, spinel, delafossite,
lyonsite,
garnet, perovskite, pyrochlore, doped ceria, fluorite, zirconium oxide, doped
zirconia,
titanium, tin oxide, silicon dioxide, and mixtures thereof. The catalyst
comprises one or
more selected from the group consisting of a ZPGM transition metal catalyst, a
mixed
metal oxide catalyst, a zeolite catalyst, and mixtures thereof. The oxygen
storage
material comprises one or more selected from the group consisting of cerium,
zirconium,
lanthanum, yttirum, lanthanides, actinides, and mixtures thereof. The catalyst
system
may optionally comprise an overcoat comprising at least one oxide solid,
wherein the
overcoat oxide solid comprises one or more selected from the group consisting
of a
carrier material oxide, a catalyst, and mixtures thereof.
[05] The present invention also pertains to a catalyst system comprising a
substrate, a
washcoat, and an overcoat, wherein the catalyst system is substantially free
of platinum
group metals. The washcoat comprises one or more selected from the group
consisting of
a carrier material oxide, ceramic, and mixtures thereof. The overcoat
comprises a
catalyst. The catalyst of the overcoat comprises one or more selected from the
group
consisting of a ZPGM transition metal catalyst, a mixed metal oxide catalyst,
a zeolite
catalyst, and mixtures thereof. The catalyst system may further comprise one
or more
selected from the group consisting of a perovskite, a spinel, a lyonsite, an
oxygen storage
material, alumina, and mixtures thereof.
[06] A ZPGM transition metal catalyst comprises one or more transition metals.
A
mixed metal oxide catalyst comprises a mixed metal oxide and at least one
transition
metal, wherein the mixed metal oxide comprises one or more selected from the
group
consisting of alkali metals, alkaline earth metals, lanthanides, actinides,
and mixtures
-2-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
thereof. A zeolite catalyst comprises at least one zeolite and at least one
transition metal.
The zeolite comprises one or more selected from the group consisting of ZSM5,
heulandite, chabazite, and mixtures thereof. The transition metal comprises
one or more
selected from the group consisting of chromium, gallium, manganese, iron,
cobalt, nickel,
copper, niobium, molybdenum, tungsten, silver, and mixtures thereof
[07] The present invention also pertains to a method of making a catalyst
system by
impregnation, comprising depositing a washcoat on a substrate and treating the
washcoat
and the substrate to convert metal salts into metal oxides, wherein the
catalyst system is
substantially free of platinum group metals. The washcoat comprises at least
one oxide
solid, wherein the oxide solid comprises one or more selected from the group
consisting
of a carrier material oxide, a catalyst, and mixtures thereof. The method may
further
comprise after treating, depositing an overcoat on the washcoat and treating
the overcoat
and washcoat. The overcoat comprises at least one oxide solid, wherein the
oxide solid
comprises one or more selected from the group consisting of a carrier material
oxide, a
catalyst, and mixtures thereof.
[08] The present invention also pertains to a method of making a catalyst
system by
precipitation, comprising precipitating a transition metal salt on a washcoat,
treating the
precipitated transition metal salt and the washcoat, depositing the
precipitated transition
metal salt and the washcoat on a substrate, and treating the precipitated
transition metal
salt and the washcoat on the substrate, wherein the catalyst system is
substantially free of
platinum group metals. The transition metal salt comprises at least one
transition metal
and at least one carrier material oxide. The method may further comprise after
treating
the precipitated transition metal salt and the washcoat on the substrate,
depositing an
overcoat on the treated precipitated transition metal salt and the washcoat,
and treating
the overcoat, the treated precipitated transition metal salt and the washcoat.
[09] The present invention also pertains to a method of making a catalyst
system by
co-milling, comprising milling together a catalyst and at least one carrier
material oxide,
depositing the milled catalyst in the form of a washcoat on to a substrate;
and treating the
substrate and the washcoat, wherein the catalyst system is substantially free
of platinum
-3-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
group metals. The method may further comprise depositing an overcoat on the
washcoat
and treating the overcoat and the washcoat. The overcoat comprises at least
one oxide
solid, wherein the oxide solid comprises one or more selected from the group
consisting
of a carrier material oxide, a catalyst, and mixtures thereof.
[10] The present invention also pertains to a method of reducing pollutants
including,
but not limited to nitrogen oxide, carbon monoxide, hydrocarbons, and sulfur
emitted in
exhaust comprising flowing exhaust substantially through a catalyst system as
described
herein and reducing the pollutants in the exhaust.
[11] The present invention also pertains to a catalyst system comprising a
first catalyst
system and a second catalyst system. The first catalyst system comprises a
substrate and
a washcoat, wherein the washcoat comprises at least one oxide solid and
wherein the first
catalyst system is substantially free of platinum group metals. The second
catalyst
system comprises at least one platinum group metal. The first and second
catalyst
systems are in series in any order, wherein at least a substantial portion of
a gas stream
passes through the first catalyst and the second catalyst sequentially. More
than a first
and second catalyst system may be used in a catalyst system, e.g. a third
catalyst system
or more.
BRIEF DESCRIPTION OF THE FIGURES
[12] Fig. 1 shows a schematic of Architecture 1 for the catalyst systems of
the present
invention;
[13] Fig. 2 shows a schematic of Architecture 2 for the catalyst systems of
the present
invention;
[14] Fig. 3 shows a schematic of Architecture 3 for the catalyst systems of
the present
invention;
[15] Fig. 4 shows the pore volume results for fresh catalyst systems ZPGM-1
through
ZPGM-5;
-4-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[16] Fig. 5 shows the pore volume results for aged catalyst systems ZPGM-1
through
ZPGM-5;
[17] Fig. 6 shows the surface area summary for fresh and aged catalyst systems
ZPGM-1 through ZPGM-5;
[18] Fig. 7 shows the x-ray diffraction analysis of a ZPGM-1 catalyst system
(fresh
and aged Ce0.6La0.4Mn0.6Cu0.4O, powders);
[19] Fig. 8 shows the x-ray diffraction analysis of a ZPGM-2 catalyst system
(fresh
and aged);
[20] Fig. 9 shows the x-ray diffraction analysis of a ZPGM-3 catalyst system
(fresh
and aged);
[211 Fig. 10 shows the x-ray diffraction analysis of a ZPGM-4 catalyst system
(fresh
and aged);
[22] Fig. 11 shows the x-ray diffraction analysis of a ZPGM-5 catalyst system
(fresh
and aged);
[23] Fig. 12 shows the x-ray diffraction analysis of a ZPGM-6 catalyst system
(fresh
and aged);
[24] Fig. 13 shows the sweep test results for a ZPGM-1 catalyst system (fresh
and
aged);
[25] Fig. 14 shows the sweep test results for a ZPGM-2 catalyst system (fresh
and
aged);
[26] Fig. 15 shows the sweep test results for a ZPGM-3 catalyst system (fresh
and
aged);
-5-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[27] Fig. 16 shows the sweep test results for a ZPGM-4 catalyst system (fresh
and
aged);
[28] Fig. 17 shows the sweep test results for a ZPGM-5 catalyst system (fresh
and
aged);
[29] Fig. 18 shows the sweep test results for a ZPGM-6 catalyst system (fresh
and
aged);
[30] Fig. 19 shows the results of light off tests for an example of a Type D
ZPGM
transition metal catalyst;
[31 ] Fig. 20 shows the results of light off tests for an example of a Type D/
Type H
ZPGM transition metal catalyst;
[32] Fig. 21 shows the results of light off tests for an example of a Type D/
Type H
ZPGM transition metal catalyst;
[33] Fig. 22 shows the results of light off tests for an example of a Type F
mixed metal
oxide catalyst;
[34] Fig. 23 shows the results of light off tests for an example of a Type F
mixed metal
oxide catalyst;
[35] Fig. 24 shows the results of light off tests for an example of a Type F
mixed metal
oxide catalyst;
[36] Fig. 25 shows the results of light off tests for an example of a Type G
ZPGM
transition metal catalyst;
[37] Fig. 26 shows the results of light off tests for an example of a Type G
ZPGM
transition metal catalyst;
-6-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[38] Fig. 27 shows the results of light off tests for an example of a Type G/
Type D
ZPGM transition metal catalyst;
[39] Fig. 28 shows the results of light off tests for an example of a Type G/
Type D
ZPGM transition metal catalyst;
[40] Fig. 29 shows the results of ramp light off tests for an example of a
Type D
ZPGM transition metal catalyst;
[41] Fig. 30 shows the results of ramp light off tests for an example of a
Type I;
[42] Fig. 31 shows light off test results for architecture 3;
[43] Fig. 32 shows the results of a light-off test for a ZPGM-1 catalyst
system (fresh
and aged);
[44] Fig. 33 shows the results of a light-off test for a ZPGM-2 catalyst
system (fresh
and aged);
[45] Fig. 34 shows the results of a light-off test for a ZPGM-3 catalyst
system (fresh
and aged);
[46] Fig. 35 shows the results of a light-off test for a ZPGM-4 catalyst
system (fresh
and aged);
[47] Fig. 36 shows the results of a light-off test for a ZPGM-5 catalyst
system (fresh
and aged); and
[48] Fig. 37 shows the results of a light-off test for a ZPGM-6 catalyst
system (fresh
and aged).
DEFINITIONS
[49] The following definitions are provided to clarify the invention.
-7-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[50] The term "catalyst system" is defined in this specification to mean a
substrate, a
washcoat, and optionally an overcoat as illustrated by Architecture 1,
Architecture 2, or
Architecture 3 as set forth in Fig. 1, 2, and 3, respectively.
[51] The term "substrate" is defined in this specification to mean any
material known
in the art for supporting a catalyst and can be of any shape or configuration
that yields a
sufficient surface area for the deposit of the washcoat and/or overcoat,
including, but not
limited to a honeycomb, pellets, or beads.
[52] The term "washcoat" is defined in this specification to mean a coating
comprising
one or more oxide solids that is coupled with a substrate.
[53] The term "overcoat" is defined in this specification to mean a coating
comprising
one or more oxide solids that is coupled with a substrate and a washcoat.
[54] The term "oxide solid" is defined in this specification to mean one or
more
selected from the group consisting of a carrier material oxide, a catalyst,
and mixtures
thereof.
[55] The term "carrier material oxide" is defined in this specification to
mean materials
used for providing a surface for at least one catalyst and comprises one or
more selected
from the group consisting of oxygen storage material, aluminum oxide, doped
aluminum
oxide, spinel, delafossite, lyonsite, garnet, perovksite, pyrochlore, doped
ceria, fluorite,
zirconium oxide, doped zirconia, titanium oxide, tin oxide, silicon dioxide,
zeolite, and
mixtures thereof.
[56] The term "oxygen storage material" is defined in this specification to
mean
materials that can take up oxygen from oxygen-rich feed streams and release
oxygen to
oxygen-deficient feed streams. The oxygen storage material comprises one or
more
oxides selected from the group consisting of cerium, zirconium, lanthanum,
yttrium,
lanthanides, actinides, and mixtures thereof.
-8-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[57] The term "catalyst" is defined in this specification to mean a catalyst
for
decreasing the amount of nitrogen oxide, hydrocarbon, carbon monoxide, and/or
sulfur
that is free of platinum group metals, preferably completely free of platinum
group
metals.
[58] The term "ZPGM Transition Metal Catalyst" is defined in this
specification to
mean a catalyst comprising one or more transition metals.
[59] The term "Mixed Metal Oxide Catalyst" is defined in this specification to
mean a
catalyst comprising at least one transition metal and at least one other
metal.
[60] The term "Zeolite Catalyst" is defined in this specification to mean a
catalyst
comprising at least one zeolite and at least one transition metal.
[61 ] The term "transition metal" is defined in this specification to mean the
transition
metals of the periodic table excluding the platinum group metals, which are
scandium,
titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc,
yttrium,
zirconium, niobium, molybdenum, technetium, ruthenium, silver, cadmium,
hafnium,
tantalum, tungsten, rhenium, gold, mercury, rutherfordium, dubnium,
seaborgium,
bohrium, hassium, meitnerium, ununnilium, unununium, ununbium, and gallium.
[62] The term "copper" is defined in this specification to mean copper, copper
complexes, copper atoms, or any other copper compounds known in the art.
[63] The term "free" is defined in this specification to mean substantially
free or
completely free.
[64] The term "impregnation component" is defined in this specification to
mean one
or more components added to a washcoat and/or overcoat to yield a washcoat
and/or
overcoat comprising a catalyst. The impregnation component comprises one or
more
selected from the group consisting of a transition metal, alkali and alkaline
earth metal,
cerium, lanthanum, yttrium, lanthanides, actinides, and mixtures thereof.
-9-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[65] The term "depositing," "deposited," or "deposit(s)" is defined in this
specification
to include, without limitation, placing, adhering, curing, coating (such as
vacuum
coating), spraying, dipping, painting and any known process for coating a film
on a
substrate.
[66] The term "treating," "treated," or "treatment" is defined in this
specification to
include, without limitation, precipitation, drying, firing, heating,
evaporating, calcining,
or mixtures thereof.
[67] The term "platinum group metals" is defined in this specification to mean
platinum, palladium, ruthenium, iridium, osmium, and rhodium.
[68] The term "coupled with" is defined in this specification to mean the
washcoat
and/or overcoat is in a relationship with the substrate or each other, such
that they may be
directly in contact with each other; or they may be associated with each
other, but there
may be something in between each of them, e.g. the overcoat may be coupled
with a
substrate, but a washcoat may be in between the substrate and the overcoat.
[69] Examples of catalyst systems are denoted by "ZPGM" and a number, e.g.
"ZPGM-1". Examples of catalysts are denoted by "Type" and a letter, e.g. "Type
A".
[70] All percentages discussed herein are weight percent unless otherwise
indicated.
All ratios discussed herein are weight ratios unless otherwise indicated.
DETAILED DESCRIPTION
[71 ] The catalyst system of the present invention is free of platinum group
metals;
decreases the amount of at least one of carbon monoxide, nitrogen oxides,
hydrocarbon,
and sulfur emissions; and comprises one or more catalysts.
-10-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
Substrates
[72] The substrate of the present invention may be, without limitation, a
refractive
material, a ceramic substrate, a honeycomb structure, a metallic substrate, a
ceramic
foam, a metallic foam, a reticulated foam, or suitable combinations, where the
substrate
has a plurality of channels and at least the required porosity. Porosity is
substrate
dependent as is known in the art. Additionally, the number of channels may
vary
depending upon the substrate used as is known in the art. The channels found
in a
monolith substrate are described in more detail below. The type and shape of a
suitable
substrate would be apparent to one of ordinary skill in the art. Preferably,
all of the
substrates, either metallic or ceramic, offer a three-dimensional support
structure.
[73] In one embodiment, the substrate may be in the form of beads or pellets.
The
beads or pellets may be formed from, without limitation, alumina, silica
alumina, silica,
titania, mixtures thereof, or any suitable material. In another embodiment,
the substrate
may be, without limitation, a honeycomb substrate. The honeycomb substrate may
be a
ceramic honeycomb substrate or a metal honeycomb substrate. The ceramic
honeycomb
substrate may be formed from, for example without limitation, sillimanite,
zirconia,
petalite, spodumene (lithium aluminum silicate), magnesium silicates, mullite,
alumina,
cordierite (e.g. Mg2A14Si5O18), other alumino-silicate materials, silicon
carbide,
aluminum nitride, or combinations thereof. Other ceramic substrates would be
apparent
to one of ordinary skill in the art.
[74] If the substrate is a metal honeycomb substrate, the metal may be,
without
limitation, a heat-resistant base metal alloy, particularly an alloy in which
iron is a
substantial or major component. The surface of the metal substrate may be
oxidized at
elevated temperatures above about 10000 C to improve the corrosion resistance
of the
alloy by forming an oxide layer on the surface of the alloy. This oxide layer
on the
surface of the alloy may also enhance the adherence of a washcoat to the
surface of the
monolith substrate.
-11-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[75] In one embodiment, the substrate may be a monolithic carrier having a
plurality of
fine, parallel flow passages extending through the monolith. The passages can
be of any
suitable cross-sectional shape and/or size. The passages may be, for example
without
limitation, trapezoidal, rectangular, square, sinusoidal, hexagonal, oval, or
circular,
although other shapes are also suitable. The monolith may contain from about 9
to about
1200 or more gas inlet openings or passages per square inch of cross section,
although
fewer passages may be used.
[76] The substrate can also be any suitable filter for particulates. Some
suitable forms
of substrates may include, without limitation, woven filters, particularly
woven ceramic
fiber filters, wire meshes, disk filters, ceramic honeycomb monoliths, ceramic
or metallic
foams, wall flow filters, and other suitable filters. Wall flow filters are
similar to
honeycomb substrates for automobile exhaust gas catalysts. They may differ
from the
honeycomb substrate that may be used to form normal automobile exhaust gas
catalysts
in that the channels of the wall flow filter may be alternately plugged at an
inlet and an
outlet so that the exhaust gas is forced to flow through the porous walls of
the wall flow
filter while traveling from the inlet to the outlet of the wall flow filter.
Washcoats
[77] According to an embodiment, at least a portion of the catalyst of the
present
invention may be placed on the substrate in the form of a washcoat. The oxide
solids in
the washcoat may be one or more carrier material oxide, one or more catalyst,
or a
mixture of carrier material oxide(s) and catalyst(s). Carrier material oxides
are normally
stable at high temperatures (>I 000' C) and under a range of reducing and
oxidizing
conditions. A preferable oxygen storage material is a mixture of ceria and
zirconia; more
preferably a mixture of (1) ceria, zirconia, and lanthanum or (2) ceria,
zirconia,
neodymium, and praseodymium.
[78] According to an embodiment, if a catalyst of the present invention
comprises at
least one oxygen storage material, the catalyst may comprise about 10 to about
90 weight
percent oxygen storage material, preferably about 20 to about 80 weight
percent, more
-12-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
preferably about 40 to about 75 weight percent. The weight percent of the
oxygen
storage material is on the basis of the oxides.
[79] Various amounts of any of the washcoats of the present invention may be
coupled
with a substrate, preferably an amount that covers most of, or all of, the
surface area of a
substrate. In an embodiment, about 80 g/L to about 250 g/L of a washcoat may
be
coupled with a substrate.
[80] In an embodiment, a washcoat may be formed on the substrate by suspending
the
oxide solids in water to form an aqueous slurry and depositing the aqueous
slurry on the
substrate as a washcoat.
[81 ] Other components may optionally be added to the aqueous slurry. Other
components such as acid or base solutions or various salts or organic
compounds may be
added to the aqueous slurry to adjust the rheology of the slurry and/or
enhance binding of
the washcoat to the substrate. Some examples of compounds that can be used to
adjust
the rheology include, but are not limited to, ammonium hydroxide, aluminum
hydroxide,
acetic acid, citric acid, tetraethylammonium hydroxide, other
tetralkylammonium salts,
ammonium acetate, ammonium citrate, glycerol, commercial polymers such as
polyethylene glycol, polyvinyl alcohol and other suitable polymers.
[82] The slurry may be placed on the substrate in any suitable manner. For
example,
without limitation, the substrate may be dipped into the slurry, or the slurry
may be
sprayed on the substrate. Other methods of depositing the slurry onto the
substrate
known to those skilled in the art may be used in alternative embodiments. If
the substrate
is a monolithic carrier with parallel flow passages, the washcoat may be
formed on the
walls of the passages. Gas flowing through the flow passages can contact the
washcoat
on the walls of the passages as well as materials that are supported on the
washcoat.
[83] It is believed that the oxygen storage material may improve the rheology
of the
washcoat slurry. Such an improvement may be seen in process control and/or
manufacture of the catalyst system. The enhanced rheology of the washcoat
slurry that
- 13 -
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
may be due to the presence of the oxygen storage material may enhance the
adhesion of
the washcoat slurry to the substrate.
Catalyst System Architecture
[84] The catalyst system of the present invention may have one of the
following three
architectures. In one embodiment, a catalyst system may comprise a substrate
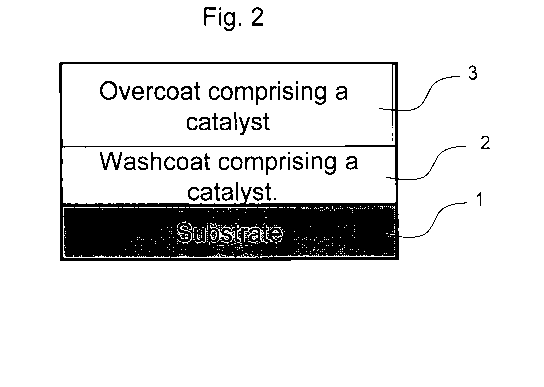
(1) and a
washcoat (2), wherein the washcoat comprises at least one catalyst. See Fig. 1
(Architecture 1). In another embodiment, a catalyst system may comprise a
substrate (1),
a washcoat (2), and an overcoat (3), wherein the washcoat (2) and overcoat (3)
each
comprise at least one catalyst. See Fig. 2 (Architecture 2). In another
embodiment, a
catalyst system may comprise a substrate (1), a washcoat (2), and an overcoat
(3),
wherein the overcoat (3) comprises at least one catalyst, but the washcoat (2)
is free of
catalyst, preferably completely free. See Fig. 3 (Architecture 3). The
washcoat (2) of the
third catalyst system architecture comprises a carrier material oxide or
mixtures thereof.
Other components known to one of ordinary skill in the art may be included.
[85] The Architectures depicted in Figs. 1-3 show how the layers are applied
in order,
but the end product may not have the layers as depicted due to, without
limitation, the
reactions that may occur between the layers.
[86] In the event that a washcoat (2) or an overcoat (3) with a catalyst is
required, the
washcoat (2) may be deposited in three different ways. First, depositing all
desired
components in one step. Or second, depositing components without a catalyst,
then
separately depositing at least one impregnation component and heating (this
separate
deposit is also referred to as an impregnation step). The impregnation
component
comprises, without limitation, transition metals, alkali and alkaline earth
metals, cerium,
lanthanum, yttrium, lanthanides, actinides, or mixtures thereof. The
impregnation step
converts metal salts into metal oxides creating a washcoat (2) comprising a
catalyst.
Third, depositing all desired components at once, including metal salts and
then heating
to convert the metals salts to metal oxides.
-14-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[87] The overcoat (3) is typically applied after treating the washcoat (2),
but treating is
not required prior to application of the overcoat (3) in every embodiment.
Preferably, the
overcoat (3) is applied after the washcoat (2).
[88] According to an embodiment, a catalyst system comprises a substrate (1)
and one
or more catalyst selected from the group consisting of a ZPGM transition metal
catalyst,
a mixed metal oxide catalyst, and a zeolite catalyst.
ZPGM Transition Metal Catalyst
[89] According to an embodiment, a catalyst system of the present invention
comprises
a ZPGM transition metal catalyst. A ZPGM transition metal catalyst comprises
one or
more transition metals. Preferably the transition metal is copper, nickel,
iron, manganese,
silver, cobalt, tungsten, niobium, molybdenum, or chromium; more preferably
copper,
nickel, iron, or manganese; most preferably copper, nickel, or cobalt.
[90] According to an embodiment, the ZPGM transition metal catalyst optionally
comprises one or more of a carrier material oxide. Preferably the catalyst
comprises a
perovskite, a spinel, a lyonsite, an oxygen storage material, alumina, or
mixtures thereof;
more preferably a spinel, an oxygen storage material, alumina, or mixtures
thereof, most
preferably at least one spinel and at least one oxygen storage material, or
alumina and at
least one oxygen storage material.
[91] If a catalyst of the present invention comprises at least one oxygen
storage
material, the catalyst may comprise about 10 to about 90 weight percent oxygen
storage
material, preferably about 20 to about 80 weight percent, more preferably
about 40 to
about 75 weight percent. The weight percent of the oxygen storage material is
on the
basis of the oxides.
[92] With any of the catalyst systems described herein, the catalysts may
optionally
further comprise one or more of a transition metal, alkaline earth metal,
ceria, and
-15-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
mixtures thereof. Preferably, the transition metal is iron, manganese, or
mixtures thereof
Preferably, the alkaline earth metal is magnesium, barium, or mixtures
thereof.
[93] According to an embodiment, the catalyst, referred to as "Type H",
comprises at
least one transition metal and at least one carrier material oxide. The
transition metals
may be a single transition metal, or a mixture of transition metals which
includes, but is
not limited to, chromium, manganese, iron, cobalt, nickel, copper, silver,
niobium,
molybdenum, and tungsten. The preferred transition metals are copper, nickel
and cobalt.
The total amount of the transition metal(s) are present in about 5% to about
50% by
weight of the total catalyst weight and may be present in any ratio of
transitional metals.
[94] According to an embodiment, the catalyst, referred to as "Type D",
comprises
copper and one or more carrier material oxides. Optionally, additional
transition metals
may be included. The copper may be applied through impregnation as discussed
herein.
The copper in the catalyst may be present in about 5% to about 50% by weight,
preferably about 5% to about 30%, more preferably about 15% by weight.
[95] According to an embodiment, a catalyst system, referred to as "ZPGM-6",
comprises a substrate, a washcoat, and an overcoat. The substrate comprises
cordierite.
The washcoat comprises a spinel and at least one oxygen storage material,
preferably the
oxygen storage material is a mixture of cerium, zirconium, and lanthanum. The
spinel in
this embodiment comprises magnesium aluminum oxides. Additionally, the oxygen
storage material and the spinet may be present in the washcoat in a ratio of
40 to about 60
by weight. If an impregnation step is required, copper, cerium, zirconium, and
lanthanum
may be added and heated to convert metal salts into metal oxides that create a
washcoat
comprising the catalyst. The overcoat comprises copper oxide, a spinel, and at
least one
oxygen storage material, preferably the oxygen storage material comprises a
mixture of
cerium, zirconium, neodymium, and praseodymium. The spinet in this embodiment
comprises magnesium aluminum oxides. The spinel and oxygen storage material of
the
overcoat may be present in the overcoat in a ratio of about 60 to about 40.
The copper in
the overcoat is present in about 5% to about 50%, preferably about 10% to
about 16% by
weight.
-16-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[96] According to an embodiment, a catalyst system, referred to as "ZPGM-5",
comprises a substrate, a washcoat, and an overcoat. The substrate comprises
cordierite.
The washcoat comprises lanthanum-doped aluminum oxide and at least one oxygen
storage material, preferably the oxygen storage material comprises a mixture
of cerium,
zirconium, neodymium, and praseodymium. Additionally, the oxygen storage
material
and the lanthanum-doped aluminum oxide may be present in the washcoat in a
ratio of
about 40 to about 60. The optional impregnation components comprise copper,
cerium,
zirconium, and lanthanum. The overcoat comprises copper oxide, lanthanum-
stabilized
aluminum oxide, and at least one oxygen storage material, preferably the
oxygen storage
material comprises a mixture of cerium, zirconium, neodymium, and
praseodymium.
The aluminum oxide and oxygen storage material of the overcoat may be present
in the
overcoat in a ratio of about 75 to about 25. The copper in the overcoat is
present in about
5% to about 50%, preferably about 15% by weight.
[97] According to an embodiment, a catalyst system, referred to as "ZPGM-4",
comprises a substrate, a washcoat, and an overcoat. The washcoat comprises tin
aluminum oxide and at least one oxygen storage material, preferably the oxygen
storage
material comprises a mixture of cerium, zirconium, neodymium, and
praseodymium.
The tin aluminum oxide and the oxygen storage material may be present in the
washcoat
in a ratio of from about 25:75 to about 75:25, preferably in a ratio of about
60 to about
40. The optional impregnation components comprise copper, cerium, zirconium,
and
lanthanum. The overcoat comprises aluminum, copper, and at least one oxygen
storage
material, preferably the oxygen storage material comprises a mixture of
cerium,
zirconium, and lanthanum. The aluminum oxide and oxygen storage material may
be
present in the overcoat in a ratio of about 60 to about 40. According to an
embodiment,
there is about 5% to about 30% copper by weight in the overcoat, preferably
about 10%
to about 20%, more preferably about 12%.
[98] According to an embodiment, a catalyst system, referred to as "ZPGM-3",
comprises a substrate and a washcoat. The washcoat comprises copper, tin
aluminum
oxide, and at least one oxygen storage material, preferably the oxygen storage
material
comprises a mixture of cerium, zirconium, neodymium, and praseodymium. The tin
-17-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
aluminum oxide and the oxygen storage material may be present in the washcoat
in a
ratio of about 60 to about 40. If an impregnation step is used, the
impregnation
components comprise copper, cerium, zirconium, and lanthanum. The cerium,
zirconium, and lanthanum may be present in the washcoat in a ratio of about 60
to about
30 to about 10. The washcoat may comprise additional transition metals.
According to
an embodiment, there is about 5% to about 30% copper by weight in the
washcoat,
preferably about 10% to about 20%, more preferably about 12%.
[99] According to an embodiment, a catalyst system, referred to as "ZPGM-2",
comprises a substrate and a washcoat. The washcoat may comprise, without
limitation,
copper, aluminum oxide, and at least one oxygen storage material, preferably
the oxygen
storage material is a mixture of cerium, zirconium, and lanthanum. The
aluminum oxide
and the oxygen storage material may be present in the washcoat in a ratio of
about 60 to
about 40. The copper in the washcoat may be about 5% to about 20% copper by
weight,
preferably about 8%. The washcoat coat may optionally comprise additional
transitional
metals and/or ceria.
[100] According to an embodiment, a catalyst system, referred to as "ZPGM-1 ",
comprises a substrate and a washcoat. The washcoat comprises at least one
carrier
material oxide and a perovskite; preferably the carrier material oxide
comprises an
oxygen storage material, more preferably comprises one or more selected from
the group
consisting of cerium, zirconium, lanthanum, neodymium, praseodymium, and
mixtures
thereof; and the perovskite preferably is a mixture of cerium, lanthanum,
manganese and
copper, having the specific formula Ce0.6La0.4Mn0.6Cuo.403.
[101 ]According to an embodiment, the catalyst, referred to as "Type A",
comprises at
least one transition metal, at least one alkaline earth metal, cerium, and at
least one carrier
material oxide. The transition metal, alkaline earth metal and cerium are
present in about
5% to about 50% by weight in any ratio of the three components. Preferably,
the alkaline
earth metals comprise one or more selected from the group consisting of
magnesium,
calcium, barium, and strontium. The transition metals may be a single
transition metal,
-18-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
or a mixture of transition metals which include, but is not limited to,
chromium,
manganese, iron, cobalt, nickel, copper, niobium, molybdenum, and tungsten.
[ 102] According to an embodiment, the catalyst, referred to as "Type C",
comprises at
least one transition metal, at least one alkaline earth metal, and at least
one carrier
material oxide. The transition metal may be a single transition metal, or a
mixture of
transition metals which include, but is not limited to, chromium, manganese,
iron, cobalt,
nickel, copper, niobium, molybdenum, tungsten, and silver. The alkaline earth
metal may
be, but is not limited to, magnesium, calcium, barium or strontium. The
preferred
transition metals are copper, nickel, and cobalt, while the preferred alkaline
earth metals
are barium and strontium. The alkaline earth metal and the transition metal
may be
present in a molar ratio of about 1:10 to 1:1 and at about 2% to about 50%
weight of the
catalyst.
[103] According to an embodiment, the catalyst, referred to as "Type E",
comprises at
least one transition metal and a perovskite having the formula ABO3. The
transition
metal may be, but is not limited to, copper, nickel, cobalt, manganese, iron,
chromium,
niobium, molybdenum, tungsten, and silver. Preferably, the transition metals
are copper,
nickel, and/or cobalt. "A" comprises lanthanum, cerium, magnesium, calcium,
barium,
strontium, lanthanides, actinides, or a mixture thereof. "B" comprises iron,
manganese,
copper, nickel, cobalt, cerium, or mixtures thereof. The transition metal(s)
is present in
about 2% to about 30% by weight.
[ 104] According to one embodiment, the Type E catalyst comprises a perovskite
(ABO3), at least one transition metal, and at least one a carrier material
oxide. The
transition metal may be a single transition metal, or a mixture of transition
metals which
includes, but is not limited to, chromium, manganese, iron, cobalt, nickel,
copper,
niobium, molybdenum, tungsten, silver, or mixtures thereof. The perovskite and
transition metal are present in about 5% to about 50% by weight.
[105] According to an embodiment, the catalyst, referred to as "Type G",
comprises at
least one transition metal and a spinet having the formula AB204. The
transition metal
-19-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
may be, but is not limited to, copper, nickel, cobalt, manganese, iron,
chromium,
niobium, molybdenum, tungsten, and silver. The preferred transition metals
include,
copper, nickel, and cobalt; more preferably copper. "A" and "B" each comprise
aluminum, magnesium, manganese, gallium, nickel, copper, cobalt, iron,
chromium,
niobium, titanium, tin, or mixtures thereof. A preferred spinel is MgA12O4.
The
transition metal(s) are present in about 2% to about 30% by weight.
[ 106] According to one embodiment, the Type G catalyst comprises a spinel
(AB204), a
transition metal, and a carrier material oxide. The transition metal may be a
single
transition metal, or a mixture of transition metals which includes, but is not
limited to,
chromium, manganese, iron, cobalt, nickel, copper, niobium, molybdenum,
tungsten,
and/or silver. A preferred spinel is MgA12O4. The spinel and transition
metal(s) are
present in about 5% to about 50% by weight.
Mixed Metal Oxide Catalyst
[107] According to an embodiment, a catalyst may be a mixed metal oxide
catalyst,
which comprises at least one transition metal and at least one other metal.
The other
metals of the mixed metal oxide may include, but are not limited to alkali and
alkaline
earth metal, lanthanides, or actinides. For example, the mixed metal oxide may
be a
spinel, a perovskite, a delafossite, a lyonsite, a garnet, or a pyrochlore.
[108] According to an embodiment, the catalyst, referred to as "Type B",
comprises a
perovskite having the formula ABO3 or a related structure with the general
formula Aa_
xBxMOb, wherein "a" is 1 or 2, "b" is 3 when "a" is 1 or "b" is 4 when "a" is
2, and "z" is
a number defined by 0.1 <x<0.7. "A" comprises lanthanum, lanthanides,
actinides,
cerium, magnesium, calcium, barium, strontium, or mixtures thereof. "B"
comprises a
single transition metal, or a mixture of transition metals including but not
limited to iron,
manganese, copper, nickel, cobalt, and cerium, or mixture thereof. According
to an
embodiment, the catalyst may have the formula AMni_xCuxO3, wherein "A" is
lanthanum, cerium, barium, strontium, a lanthanide, or an actinide and "x" is
0 to 1.
-20-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[ 109] According to another embodiment, the Type B catalyst may have the
formula
ACeI_XCuxO3, wherein "A" is barium, strontium, or calcium, and "x" is 0 to 1.
According
to an embodiment, about l Og/L to about 180g/L of the formula ABO3 may be
coupled
with the substrate.
[110] According to one embodiment, the Type B catalyst comprises a perovskite
(ABO3) or related structure (with general formula Aa_xBxMOb) and one or more
of a
carrier material oxide. The perovskite or related structure is present in
about 5% to about
50% by weight.
[I I I] According to an embodiment, the catalyst, referred to as "Type F",
comprises a
spinel having the formula AB204. "A" and "B" of the formula is aluminum,
magnesium,
manganese, gallium, nickel, copper, cobalt, iron, chromium, titanium, tin, or
mixtures
thereof.
[112] According to an embodiment, the Type F catalyst comprises a spinel and a
carrier
material oxide. The spinel is present in about 5% to about 50% by weight.
Zeolite Catalyst
[113]According to an embodiment, a catalyst may be a zeolite catalyst
comprising a
zeolite or mixture of zeolites and at least one transition metal. A zeolite is
mixed
aluminosillicates with regular interconnected pores. The zeolite includes, but
is not
limited to ZSM5, heulandite, chabazite, or mixtures thereof, preferably ZSM5.
According to an embodiment, the catalyst, referred to as "Type I" comprises at
least one
transition metal impregnated into a zeolite or mixtures of zeolite. The
transition metal(s)
may be a single transition metal or a mixture of transition metal which
includes, but is not
limited to, chromium, gallium, manganese, iron, cobalt, nickel, copper,
niobium,
molybdenum, tungsten, and silver. Preferably, the transition metals are
selected from the
group consisting of copper, nickel, gallium, cobalt, and mixtures thereof. The
transition
metals may be present in about 3% to about 25% by weight in any ratio of
transition
metals.
-21-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[11_'4]According to an embodiment, the catalysts of the present invention may
reduce
pollutants emitted from exhaust. This is done by passing exhaust substantially
through a
catalyst system, such that the flowing exhaust reduces the pollutants. The
exhaust
includes, but is not limited to exhaust from an automobile, vehicle, factory,
train,
airplane, building, and laboratory. Pollutants are any compounds, substances,
gases, or
waste that causes damage to water, air, land, and any other part of the
environment,
including carbon monoxide, hydrocarbons, nitrogen oxides, and sulfur.
[115]The catalysts of the present invention to decrease the amount of nitrogen
oxide
emissions. For example: NO +V2024 NO2 and 6NO2 + 8NH3 4 7N2 + 12H2O. The
catalyst also decreases the amount of the unburned hydrocarbons and carbon
monoxide
by oxidizing them. For example: 2CXHy + (2x+y/2)02 --> 2xCO2 + yH2O or 2CO +
02
- 2CO2. The catalysts may also decrease the amount of sulfur emissions.
[116] According to an embodiment, a catalyst system comprises a first catalyst
system
and a second catalyst system. The first catalyst system may be any catalyst
described
herein. The second catalyst system comprises a catalyst comprising at least
one platinum
group metal, wherein the catalyst may comprise any platinum group metal known
in the
art, including, but not limited to mixtures of platinum group metals and
carrier material
oxides. The first catalyst system and the second catalyst system may be in an
orientation
such that a gas stream is capable of passing through the first catalyst system
followed by
the second catalyst system in series or vice versa. Further, a catalyst system
may
comprise more than a first and a second catalyst system, e.g. a third catalyst
system.
Preparation of a Zero Platinum Group Metal Catalyst by Impregnation
[117]A washcoat having the properties discussed herein may be prepared by
methods
well known in the art. The washcoat may comprise any of the catalysts and/or
additional
components described herein. The washcoat is deposited on a substrate and is
treated.
The treating is done at a temperature between 300 C and 700 C, preferably
about 550
C. The treating may last from about 2 to about 6 hours, preferably about 4
hours. After
the washcoat and the substrate are treated, they are cooled to about room
temperature.
-22-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
After the washcoat and the substrate are cooled, the washcoat is impregnated
with at least
one impregnation component. The impregnation component includes, without
limitation,
a transition-metal salt or salts being dissolved in water and impregnated on
the washcoat.
Following the impregnation step, the washcoat with the impregnation components
are
treated. The treating may be performed at about 300 C to about 700 C,
preferably about
550 C. The treating may last from about 2 to about 6 hours, preferably about
4 hours.
[ 118] According to an embodiment, the substrate, the washcoat, and the
impregnation
components may be treated to form the catalyst composition before or after the
washcoat
and/or the impregnation components are added to the substrate. In an
embodiment, the
washcoat and the impregnation component may be treated before coating.
[119] The impregnation method may be performed on an overcoat. After
depositing the
overcoat, the overcoat is impregnated with at least one impregnation
component. The
impregnation component includes, without limitation, a transition-metal salt
or salts
being dissolved in water and impregnated on the overcoat. Following the
impregnation
step, the overcoat with the impregnation components are treated. The treating
may be
performed at about 300 C to about 700 C, preferably about 550 C. The
treating may
last from about 2 hours to about 6 hours, preferably about 4 hours.
Preparation of a Zero Platinum Group Metal Catalyst by Precipitation
[120] The method of precipitation includes precipitating a transition metal
salt or salts
on a washcoat. The transition metal salt or salts may be precipitated with,
but is not
limited to NH4OH, (NH4)2CO3, tetraethylammonium hydroxide, other
tetralkylammonium salts, ammonium acetate, or ammonium citrate. The washcoat
may
be any washcoat described herein. Next, the precipitated transition metal salt
or salts and
washcoat are treated. The treating may be from about 2 hours to about 24
hours. Next,
the precipitated transition metal salt or salts and the washcoat are deposited
on a substrate
followed by treating for about 2 hours to about 6 hours, preferably about 4
hours at a
temperature of about 300 C to about 700 C, preferably about 550 C.
Optionally, after
treating, an overcoat may be deposited on the treated precipitated transition
metal salt or
-23-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
salts and washcoat and treated again. The overcoat may be treated for about 2
hours to
about 6 hours, preferably about 4 hours and at a temperature of about 300 C
to about
700 C, preferably about 550 C.
Preparation of a Zero Platinum Group Metal Catalyst by Co-Milling
[121]A catalyst and a carrier material oxide are milled together. The catalyst
can be
synthesized by any chemical technique such as, but not limited to solid-state
synthesis,
precipitation, or any other technique known in the art. The milled catalyst
and carrier
material oxide are deposited on a substrate in the form of a washcoat and then
treated.
The treatment may be from about 2 hours to about 6 hours, preferably about 4
hours and
at a temperature of about 300 C to about 700 C, preferably about 550 C.
Optionally,
an overcoat may be deposited on the treated catalyst after cooling to about
room
temperature. The overcoat, washcoat and substrate are treated for about 2
hours to about
6 hours, preferably about 4 hours and at a temperature of 300 C to about 700
C,
preferably about 550 C.
[122] The following examples are intended to illustrate, but not to limit, the
scope of the
invention. It is to be understood that other procedures known to those skilled
in the art
may alternatively be used.
[123] Example 1: Pore Volume and Surface Area Measurements for Zero Platinum
Group Metal Catalysts
[124] Fig. 4 shows the measured pore volume for the fresh catalyst systems
ZPGM-1
through ZPGM-5 and Fig. 5 shows the measured pore volume for the aged catalyst
systems ZPGM-I through ZPGM-5. The aged catalyst systems were aged at 950 C
for
16 hours with 10% H2O and air. The y-axis on the right side of Fig. 4 is for
the pore
volume (cm3/g) of ZPGM-1 only.
[125] The pore volumes were measured using a Micromeritics (Norcross, GA)
TriStar
3000 gas adsorption analyzer at 77K. The pore volumes were obtained from the
nitrogen
adsorption isotherms using the Barrett-Joiner-Halenda (BJH) method (E. P.
Barrett, L. G.
-24-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
Joyner, P. P. Halenda, "The determination of pore volume and area
distributions in
porous substances. I. Computations from nitrogen isotherms," J. Am. Chem. Soc.
(1951),
73, 373-380).
[126] The results in Figs. 4 and 5 show that the pore volume decreases for all
the
catalyst systems (ZPGM-1 through ZPGM-5) upon aging. The average pore volume
for
the fresh ZPGM-1 decreases from 0.106 cm3/g to 0.017 cm3/g for the.aged
catalyst.
Similarly, the average pore volume for the fresh ZPGM-2 decreases from 0.173
cm3/g to
0.116 cm3/g for the aged catalyst. Again, the average pore volume for the
fresh ZPGM-3
decreases from 0.107 cm3/g to 0.010 cm3/g for the aged catalyst. The average
pore
volume for the fresh ZPGM-4 decreases from 0.190 cm3/g to 0.142 cm3/g for the
aged
catalyst. The average pore volume for the fresh ZPGM-5 decreases from 0.213
cm3/g to
0.122 cm3/g for the aged catalyst.
[127]Examplle 2: Surface Area Analysis for Fresh and Aged Catalyst Systems
ZPGM-
1 through ZPGM-5
[128] The surface areas for the fresh and aged ZPGM catalyst systems are
presented in
Fig. 6. The aged catalyst systems were aged at 950 C for 16 hours with 10%
H2O and
air.
[129] The surface areas were measured using a Micromeritics (Norcross, GA)
TriStar
3000 gas adsorption analyzer at 77K. The surface areas were calculated using
the BET
(Brunauer, Emmitt and Teller) method (S. Brunauer, P. H. Emmett and E. Teller,
J. Am.
Chem. Soc., 1938, 60, 309).
[130] The results in Figure 6 show that the surface area decreases for all
catalyst
systems (ZPGM-1 through ZPGM-5) upon aging. The surface area decreases from
18.72
m2/g for the fresh ZPGM-1 to 2.76 m2/g for the aged catalyst. Similarly, the
surface area
decreases from 38.60 m2/g for the fresh ZPGM-2 to 15.48 m2/g for the aged
catalyst. The
surface area decreases from 30.78 m2/g for the fresh ZPGM-3 to 16.71 m2/g for
the aged
catalyst. The surface area decreases from 46.95 m2/g for the fresh ZPGM-4 to
22.06 m2/g
-25-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
for the aged catalyst. The surface area decreases from 53.45 m2/g for the
fresh ZPGM-5
to 24.02 m2/g for the aged catalyst.
[1311 Example 3: X-ray diffraction analysis for ZPGM Transition Metal
Catalysts
[132] Fig. 7-12 show the X-ray diffraction (XRD) patterns of fresh and aged
catalyst
systems ZPGM-1 through ZPGM-6; the aged catalyst systems were aged at 950 C
for 16
hrs with 10% H2O and air.
[133]The XRD analysis was conducted to determine the crystalline phases
present for
each catalyst system. The XRD patterns were measured on a Rigaku powder
diffractometer (MiniFlexTM) using Cu Ka radiation in the 2-theta range of 20-
70 with a
step size of 0.05 and a dwell time of 2s. The tube voltage and current were
set at 40 kV
and 30 mA, respectively. The resulting diffraction patterns were analyzed
using the
International Centre for Diffraction Data (ICDD) database.
[134] Fig. 7 shows the XRD spectra of the fresh and aged ZPGM-1 catalyst
system,
Ce0.6La0.4Mn0.6Cu0.403, shows the presence of the perovskite (open circles)
and fluorite
(filled squares) structures. The fluorite and the perovskite structures are
larger in the
aged sample as evidenced by the sharper peaks.
[135] Fig. 8 shows the XRD patterns of fresh and aged ZPGM-2 catalyst system,
8% Cu
impregnated on A1203 + Ceo.64Zr0.21 Lao. 1502 (60:40 weight ratio of A1203 to
Ceo.64Zro.21Lao. 1502) (160g/ml). The XRD spectrum of the fresh ZPGM-2
catalyst system
shows the presence of the fluorite structure (open squares), alumina (A) and
CuO (filled
circles). The aged ZPGM-2 catalyst system shows fluorite (open squares),
CuAl2O4
(filled diamonds) and alumina (A). The fluorite structure is larger in the
aged sample as
evidenced by the sharper peaks.
[136] Fig. 9 shows the XRD patterns of fresh and aged ZPGM-3 catalyst system,
8% Cu
+ 6.1% Ce + 2.4% Zr + 1.5% La impregnated on 15% Sn-A1203 +
Ceo.6Zro.3Ndo.o5Pro.0502
(60:40 weight ratio of Sn-Al203 to Ceo.6Zro.3Ndo.o5Pro.0502) (200g/L). The XRD
spectrum
of the fresh ZPGM-3 catalyst system shows the presence of the fluorite
structure (open
-26-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
circles), Zr02 (open squares), alumina (A) and CuO (filled circles). The aged
ZPGM-3
catalyst system shows fluorite (open circles), Zr02 (open squares), Sn02
(filled circles),
CuA12O4 (filled diamonds) and alumina (A). The cordierite peak in the aged
sample is
from the substrate. During the aging the tin oxide dissociates from the
alumina, the Cu
reacts with the A1203 to form CuA12O4.
Fig. 10 shows the XRD patterns of fresh and aged ZPGM-4 catalyst system, which
is
composed of an overcoat containing 12% Cu impregnated on Ceo.6Zro.21 Lao.1502
+ A1203
(60:40 weight ratio of Ce0.6Zro.21Lao. 1502 to A1203) and a washcoat
containing 8% Cu +
6.1% Ce + 2.4 % Zr + 1.5% La impregnated impregnated on 15% Sn-A1203 +
Ceo.6Zro.3Ndo.o5Pro.0502 (60:40 weight ratio of Sn-A1203 to
Ce0.6Zro.3Ndo.o5Pro.0502)= The
XRD spectrum of the fresh ZPGM-4 catalyst system shows the presence of the
fluorite
structure (filled circles), Ce02 (open squares), alumina (A) and CuO (filled
squares). The
aged ZPGM-4 catalyst system shows fluorite (filled circles), CeO2 (open
squares), Sn02
(open circles), CuAl2O4 (filled diamonds) and alumina (A). During the aging
the tin
oxide dissociates from the alumina, the Cu reacts with the A1203 to form
CuAl2O4.
[137]Fig. 11 shows the XRD patterns of fresh and aged ZPGM-5 catalyst system,
which is composed of an overcoat containing 12.4% CuO impregnated on La-A1203
+
Ce0.6Zro.3Ndo.osPro.0502 (25:75 weight ratio of La-A1203 to
Ce0.6Zro.3Ndo.o5Pro.o502) (65
g/L) and a washcoat containing 8% Cu + 6.1% Ce + 2.4% Zr + 1.5% La impregnated
on
La-A1203 + Ceo.6Zro.3Ndo.o5Pro.0502 (60:40 weight ratio of La-A1203 to
Ce0.6Zr0.3Ndo.o5Pro.0502) (180 g/L). The XRD spectrum of the fresh ZPGM-5
catalyst
system shows the presence of the fluorite structure (filled circles) and
alumina (A). The
aged ZPGM-5 catalyst system shows fluorite (filled circles), CuA12O4 (filled
diamonds)
and alumina (A). During the aging the Cu reacts with the A1203 to form
CuA1204.
[138] Fig. 12 shows the XRD patterns of fresh and aged ZPGM-6 catalyst system,
which is composed of an overcoat containing 10% Cu +12 % Ce impregnated on
MgA12O4 + 16% Cu impregnated on Ce0.6Zro.3Ndo.o5Pro.o502 (60:40 weight ratio
of Ce
impregnated on MgAl2O4 to 16% Cu impregnated on Ce0.6Zro.3Ndo.o5Pro.0502) (65
g/L)
and a washcoat containing 4% Cu + 6.1 % Ce + 2.4 % Zr + 1.5% La impregnated on
-27-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
MgAl2O4 + Ce0.64Zro,2I Lao.1502 (60:40 weight ratio of MgA12O4 to Ce0.64Zro.2I
Lao. 1502)
(180 g/L). The XRD spectrum of the fresh ZPGM-6 catalyst system shows the
presence
of two fluorite structures (filled and open circles), and MgA12O4 (open
diamonds). The
aged ZPGM-6 catalyst system shows two fluorite structures (filled and open
circles),
MgA12O4 (open diamonds), CuA12O4 (filled diamonds), and CuO (filled squares).
During
the aging the CZL and CuO became more crystalline, and some CuA12O4 formed.
[139]Example 4: Sweep test for Catalyst Systems ZPGM-1 through ZPGM-6
[ 140] Figs. 13 - 18 show the sweep test results for catalyst systems ZPGM-1
through
ZPGM-6 (as described above in Examples 3-8), respectively. The sweep test was
performed with an inlet temperature of 600 C, an air/fuel span of 0.2 and a
cycle
frequency of 1 Hz. A sweep test indicates the catalyst performance at various
R-values
(moles of reductant divided by moles of oxidant). High conversions over a
large range of
R-values indicate a promising catalyst because it can perform well under rich
(R-values >
1) and lean (R-values < 1) engine conditions. The aged catalyst systems were
aged at
1050 C for 10 hrs cycling between a 56 second rich segment and a 4 second lean
segment.
[141]Fig. 13 shows the sweep test results for the fresh and aged ZPGM-1
catalyst
system. The sweep results for the fresh catalyst show that the CO conversion
decreases
with R-values >1.05, while the hydrocarbon (HC) conversion decreases with
increasing
R-values. The NO conversion increases with R-value > 0.85. The catalytic
properties for
CO, hydrocarbons and NO decrease after aging; the NO conversion is < 5% over
the
entire R-value range tested. The CO conversion of the aged ZPGM-1 decreases
with
increasing R-value. The HC conversion for the aged ZPGM-1 is best for R-values
between 0.95 and 1.05.
[142] Fig. 14 shows the sweep test results for the fresh and aged ZPGM-2
catalyst
system. The sweep results for the fresh catalyst show that the CO conversion
decreases
with R-values >1.05, while the hydrocarbon (HC) conversion decreases with
increasing
R-values. The NO conversion increases with R-value > 0.85. The catalytic
properties for
-28-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
CO, hydrocarbons and NO decrease after aging. The CO and HC conversions of the
aged
ZPGM-2 decrease with increasing R-value. The NO conversion is the highest at
R=0.85,
for the aged ZPGM-2 catalyst system.
[143] Fig. 15 shows the sweep test results for the fresh and aged ZPGM-3
catalyst
system. The sweep results for the fresh catalyst show that the CO conversion
decreases
with R-values >1.05, while the hydrocarbon (HC) conversion decreases with
increasing
R-values. The NO conversion increases with increasing R-values. The catalytic
properties for CO, hydrocarbons and NO decrease after aging. The CO and HC
conversions of the aged ZPGM-3 decrease with increasing R-value. The NO
conversion
for the aged ZPGM-3 increases with R-values > 0.95.
[144] Fig. 16 shows the sweep test results for the fresh and aged ZPGM-4
catalyst
system. The sweep results for the fresh catalyst show that the CO conversion
decreases
with R-values >0.975, while the hydrocarbon (HC) conversion decreases with
increasing
R-values. The NO conversion increases with increasing R-values. The catalytic
properties for CO, hydrocarbons and NO decrease after aging. The CO and HC
conversions of the aged ZPGM-4 decrease with increasing R-value. The NO
conversion
for the aged ZPGM-4 increases with R-values > 0.95.
[145]Fig. 17 shows the sweep test results for the fresh and aged ZPGM-5
catalyst
system. The sweep results for the fresh catalyst show that the CO conversion
decreases
with R-values >0.975, while the hydrocarbon (HC) conversion decreases with
increasing
R-values. The NO conversion increases with increasing R-values. The catalytic
properties for CO, hydrocarbons and NO decrease after aging. The CO and HC
conversions of the aged ZPGM-5 decrease with increasing R-value. The NO
conversion
for the aged ZPGM-5 increases with R-values > 1.05.
[146]Fig. 18 shows the sweep test results for the fresh and aged ZPGM-6
catalyst
system. The sweep results for the fresh catalyst show that the CO conversion
decreases
with R-values >0.975, while the hydrocarbon (HC) conversion decreases with
increasing
R-values. The NO conversion increases with increasing R-values. The catalytic
-29-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
properties for CO, hydrocarbons and NO decrease after aging. The CO and HC
conversions of the aged ZPGM-6 decrease with increasing R-value. The NO
conversion
for the aged ZPGM-6 increases with R-values > 0.975.
[147] Example 5 = Light-off test for Type D or Type H ZPGM Transition Metal
Catalysts
[ 148] Figs. 19 - 21 show the light-off test results for examples of Type D or
Type H
ZPGM Transition Metal Catalysts. It should be noted that a catalyst may fall
into one or
more types, such as here, where the catalyst is both Type D and Type H. A
light-off test
was performed on aged (800 C for 16 hours, composed of a 56 second rich
segment and a
4 second lean segment) catalysts of the present invention. The test was
performed by
increasing the temperature from about 100 C to 640 C at R-value = 1.05 and R-
value =
1.5. The light-off test measures the conversions of nitrogen oxide, carbon
monoxide, and
hydrocarbons as a function of the catalyst system temperature. For a specific
temperature, a higher conversion signifies a more efficient catalyst.
Conversely, for a
specific conversion, a lower temperature signifies a more efficient catalyst.
[149] Fig. 19 shows the results for Type D/H catalyst with a composition of
16% Cu /
Ceo.3Zro.6Ndo.05Pro.05O2. It should be noted that a catalyst may fall into one
or more types,
such as here, where the catalyst is both Type D and Type H. The light-off test
at R =
1.05 shows that the catalyst has T50 for CO at 267 C and a T50 for HC at 525
C. The
maximum conversion for NO is about 2% at 640 C. Increasing the R-value to 1.5
improves the NO conversion, but the CO and HC performance deteriorates. The
light-off
test at R = 1.5 shows that the catalyst has T50s for CO and HC decrease to 323
C and
595 C, respectively. The NO light-off at R = 1.5 shows a T50 of 494 C.
[150] Fig. 20 shows the results for Type D/H catalyst with a composition of
12% Cu /
Ce0.6Zr0.3La0,1 O2. It should be noted that a catalyst may fall into one or
more types, such
as here, where the catalyst is both Type D and Type H. The light-off test at R
= 1.05
shows that the catalyst has T50 for CO at 237 C and a T50 for HC at 543 C. The
maximum
conversion for NO is about 4% at 640 C. Increasing the R-value to 1.5 improves
the NO
-30-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
conversion, but the CO and HC performance deteriorates. The light-off test at
R = 1.5
shows that the catalyst has T50s for CO and HC decrease to 329 C and 611 C,
respectively. The NO light-off at R = 1.5 shows a T50 of 515 C.
[151 ] Fig. 21 shows the results for Type D/H catalyst with a composition of
10% Cu +
12% Ce / La-A12O3. It should be noted that a catalyst may fall into one or
more types,
such as here, where the catalyst is both Type D and Type H. The light-off test
at R =
1.05 shows that the catalyst has T50 for CO at 298 C and a T50 for HC at 546
C. The
maximum conversion for NO is about 3% at 640 C. Increasing the R-value to 1.5
improves the NO conversion, but the CO and HC performance deteriorates. The
light-off
test at R = 1.5 shows that the catalyst has T50s for CO and HC decrease to 325
C and
598 C, respectively. The NO light-off at R = 1.5 shows a T50 of 461 C.
[152] Example 6: Light-off test for Type F ZPGM Transition Metal Catalysts
[153] Figs. 22 -24 show the light-off test results for examples of Type F
catalyst. A
light-off test was performed on aged (800 C for 16 hours, composed of a 56
second rich
segment and a 4 second lean segment) catalysts of the present invention. The
test was
performed by increasing the temperature from about 100 C to 640 C at R-value =
1.05
and R-value = 1.5. The light-off test measures the conversions of nitrogen
oxide, carbon
monoxide, and hydrocarbons as a function of the catalyst system temperature.
For a
specific temperature, a higher conversion signifies a more efficient catalyst.
Conversely,
for a specific conversion, a lower temperature signifies a more efficient
catalyst.
[154] Fig. 22 shows the results for Type F catalyst with a composition of
CuLa0004Al1.9604. The light-off test at R = 1.05 shows that the catalyst has
T50 for CO at
334 C. The maximum conversions for NO and HC at 640 C are about 6% and 38%,
respectively. Increasing the R-value to 1.5 improves the NO conversion, but
the CO and
HC performance deteriorates. The light-off test at R = 1.5 shows that the
catalyst has T5o
for CO decreases to about 453 C. The NO light-off at R = 1.5 shows a T50 of
521 C.
While, the maximum conversion for HC is about 16% at 640 C.
-31-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[155] Fig. 23 shows the results for Type F catalyst with a composition of
Cu0.5Fe0.5La0.04A11.9604. The light-off test at R = 1.05 shows that the
catalyst has T50 for
CO at 346 C and a T50 for HC at 535 C. The maximum NO conversion is about 1%
at
640 C. Increasing the R-value to 1.5 improves the NO conversion, but the CO
and HC
performance deteriorates. The light-off test at R = 1.5 shows that the
catalyst has T50s for
CO and HC decrease to 368 C and 588 C, respectively. The NO light-off at R =
1.5
shows a T50 of 491 C.
[ 156] Fig. 24 shows the results for Type F catalyst with a composition of
CuLa0.04A11.47Mn0.49O4. The light-off test at R = 1.05 shows that the catalyst
has T50 for
CO at 371 C. The maximum conversions for NO and HC at 640 C are about 2% and
27%, respectively. Increasing the R-value to 1.5 improves the NO conversion,
but the
CO and HC performance deteriorates. The light-off test at R = 1.5 shows that
the catalyst
has T50 for CO decreases to about 479 C. While, the maximum conversions for NO
and
HC are each about 16% at 640 C.
[157]Example 7: Light-off test for Type G ZPGM Transition Metal Catalysts
[158] Figs. 25 -28 show the light-off test results for examples of Type G/Type
D
catalyst. It should be noted that a catalyst may fall into one or more types,
such as here,
where the catalyst is both Type G and Type D. A light-off test was performed
on aged
(800 C for 16 hours, composed of a 56 second rich segment and a 4 second lean
segment)
catalysts of the present invention. The test was performed by increasing the
temperature
from about 100 C to 640 C at R-value = 1.05 and R-value = 1.5. The light-off
test
measures the conversions of nitrogen oxide, carbon monoxide, and hydrocarbons
as a
function of the catalyst system temperature. For a specific temperature, a
higher
conversion signifies a more efficient catalyst. Conversely, for a specific
conversion, a
lower temperature signifies a more efficient catalyst.
[159] Fig. 25 shows the results for Type G/Type D catalyst with a composition
of 10%
Ag / Cu0.5Feo.5L404A11.9604. It should be noted that a catalyst may fall into
one or more
types, such as here, where the catalyst is both Type G and Type D. The light-
off test at R
-32-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
= 1.05 shows that the catalyst has T50 for CO at 383 C. The maximum
conversions for
NO and HC at 640 C are about 1% and 33%, respectively. Increasing the R-value
to 1.5
improves the NO conversion, but the CO and HC performance deteriorates. The
light-off
test at R = 1.5 shows that the catalyst has T50 for CO decreases to about 394
C. The NO
light-off at R = 1.5 shows a T50 of 485 C. While, the maximum conversion for
HC is
about 16% at 640 C.
[160] Fig. 26 shows the results for Type G/Type D catalyst with a composition
of 10%
Cu / CuLa0.04Al1.96O4. It should be noted that a catalyst may fall into one or
more types,
such as here, where the catalyst is both Type G and Type D. The light-off test
at R =
1.05 shows that the catalyst has T50 for CO at 272 C and a T50 for HC at 464
C. There is
no measured NO conversion up to 640 C. Increasing the R-value to 1.5 improves
the NO
conversion, but the CO and HC performance deteriorates. The light-off test at
R = 1.5
shows that the catalyst has T50s for CO and HC decrease to 375 C and 565 C,
respectively. The NO light-off at R = 1.5 shows a T50 of 500 C.
[ 161 ] Fig. 27 shows the results for Type G/Type D catalyst with a
composition of 20%
CuO / MgLa0.04Al1.96O4. It should be noted that a catalyst may fall into one
or more
types, such as here, where the catalyst is both Type G and Type D. The light-
off test at R
= 1.05 shows that the catalyst has T50 for CO at 305 C and a T50 for HC at 513
C. The
maximum NO conversion is about I% at 640 C. Increasing the R-value to 1.5
improves
the NO conversion, but the CO and HC performance deteriorates. The light-off
test at R
= 1.5 shows that the catalyst has T50s for CO and HC decrease to 412 C and 587
C,
respectively. The NO light-off at R = 1.5 shows a T50 of 478 C.
[162] Fig. 28 shows the results for Type G/Type D catalyst with a composition
of 10%
Cu + 12% Ce / MgLa0.04A11.96O4. It should be noted that a catalyst may fall
into one or
more types, such as here, where the catalyst is both Type G and Type D. The
light-off
test at R = 1.05 shows that the catalyst has T50 for CO at 302 C and a T50 for
HC at
506 C. The maximum NO conversion is about 2% at 640 C. Increasing the R-value
to
1.5 improves the NO conversion, but the CO and HC performance deteriorates.
The
-33-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
light-off test at R = 1.5 shows that the catalyst has T50s for CO and HC
decrease to 338 C
and 585 C, respectively. The NO light-off at R = 1.5 shows a T50 of 461 C.
[163] Example 8: Light-off test for Type D ZPGM Transition Metal Catalysts
[164] Fig. 29 shows the light-off test results for an example of Type D
catalyst. A light-
off test was performed on aged (800 C for 16 hours, composed of a 56 second
rich
segment and a 4 second lean segment) catalysts of the present invention. The
test was
performed by increasing the temperature from about 100 C to 640 C at R-value =
1.05
and R-value = 1.5. The light-off test measures the conversions of nitrogen
oxide, carbon
monoxide, and hydrocarbons as a function of the catalyst system temperature.
For a
specific temperature, a higher conversion signifies a more efficient catalyst.
Conversely,
for a specific conversion, a lower temperature signifies a more efficient
catalyst.
[165] Fig. 29 shows the results for Type D catalyst with a composition of 12%
CuO /
(Ce0.6Zr0.3La0.102 + MgLa0.04A11.9604 (40:60)). The light-off test at R = 1.05
shows that
the catalyst has T50s for CO at 258 C, for HC at 381 C, and for NO at 519 C.
Increasing
the R-value to 1.5 improves the NO conversion, but the CO and HC performance
deteriorates. The light-off test at R = 1.5 shows that the catalyst has T50s
for CO and HC
decrease to 316 C and 464 C, respectively. The NO light-off at R = 1.5 shows a
T50 of
375 C.
[166]Example 9: Light-off test for Type I Zeolite Catalyst
[167] Fig. 30 shows the light-off test results for an example of Type I
Zeolite catalyst.
A light-off test was performed on a fresh catalyst of the present invention.
The test was
performed by increasing the temperature from about 100 C to 640 C at R-value =
1.05.
The light-off test measures the conversions of nitrogen oxide, carbon
monoxide, and
hydrocarbons as a function of the catalyst system temperature. For a specific
temperature, a higher conversion signifies a more efficient catalyst.
Conversely, for a
specific conversion, a lower temperature signifies a more efficient catalyst.
-34-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[168] Fig. 30 shows the results for Type I catalyst with a composition of 5%
Ga + 8%
Cu / (ZSM-5). The light-off test at R = 1.05 shows that the catalyst has T50s
for CO at
376 C, for HC at 319 C, and for NO at 343 C.
[169]Example 10: Light-off test for Architecture Type 3. which comprises a
substrate,
a washcoat, and an overcoat, wherein the overcoat comprises at least one
catalyst, but the
washcoat does not.
[ 170] Fig. 31 shows the light-off test results for an example of Architecture
Type 3
Catalyst, which comprises a substrate, a washcoat, and an overcoat, wherein
the overcoat
comprises at least one catalyst, but the washcoat does not (washcoat comprises
La-A1203
+ Ceo.6Zro.3Ndo.o5Pro.o5O2; 60:40; 100 g/L and overcoat comprises 12% Cu on
Ceo.6Zro.3Ndo.o5Pro.05O2; 150g/L). A light-off test was performed on aged (800
C for 16
hours, composed of a 56 second rich segment and a 4 second lean segment)
catalysts of
the present invention. The test was performed by increasing the temperature
from about
100 C to 640 C at R-value = 1.05 and R-value = 1.5. The light-off test
measures the
conversions of nitrogen oxide, carbon monoxide, and hydrocarbons as a function
of the
catalyst system temperature. For a specific temperature, a higher conversion
signifies a
more efficient catalyst. Conversely, for a specific conversion, a lower
temperature
signifies a more efficient catalyst.
[171]The light-off test at R = 1.05 shows that the catalyst has T50 for CO at
314 C and
a T50 for HC at 464 C. The maximum NO conversion is about 6% at 640 C.
Increasing
the R-value to 1.5 improves the NO conversion, but the HC performance
deteriorates.
The light-off test at R = 1.5 shows that the catalyst has T50s for CO and HC
decrease to
316 C and 566 C, respectively. The NO light-off at R = 1.5 shows a T50 of 453
C.
[172] Example 11: Light-off test for Catalyst Systems ZPGM-1 through ZPGM-6
(fresh and aged)
[173] Figs. 32 - 37 show the light-off test results for ZPGM-1 through ZPGM-6.
A
light-off test was performed on fresh and aged (1050 C for 10 hrs cycling
between a 56
second rich segment and a 4 second lean segment) catalysts of the present
invention. The
-35-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
test was performed by increasing the temperature from about 100 C to 640 C at
R-value
= 1.05. The plotted temperatures in the figures were measured at the middle of
the
catalyst. The light-off test measures the conversions of nitrogen oxide,
carbon monoxide,
and hydrocarbons as a function of the catalyst system temperature. For a
specific
temperature, a higher conversion signifies a more efficient catalyst.
Conversely, for a
specific conversion, a lower temperature signifies a more efficient catalyst.
[ 174] Fig. 32 shows the light-off results at R = 1.05 for fresh and aged ZPGM-
1 catalyst
system (Ce0.6La0.4Mn0.6Cu0.4O3). The light-off test for the fresh catalyst
system shows
that the CO and HC exhibit Tsos at 288 C and at 503 C, respectively. The
maximum NO
conversion is about 19% at 600 C. After aging, the catalyst performance
decreases for
CO, HC and NO. The aged catalyst shows a T50 for CO at about 600 C. The
maximum
conversions for HC and NO are 19% and 2%, respectively, at 600 C.
[175] Fig. 33 shows the light-off results at R = 1.05 for fresh and aged ZPGM-
2 catalyst
system (8% Cu impregnated on A1203 + Ceo_64Zro.2ILao. 1502 (60:40 weight ratio
of A1203
to Ceo.64Zro.2ILao.15O2)). The light-off test for the fresh catalyst system
shows that the CO
and HC exhibit T50s at 205 C and at 389 C, respectively. The maximum NO
conversion
is about 22% at 600 C. After aging, the catalyst performance decreases for CO,
HC and
NO. The maximum conversions for CO, HC and NO are 27%, 24% and 3%,
respectively,
at 600 C.
[176] Fig. 34 shows the light-off results at R = 1.05 for fresh and aged ZPGM-
3 catalyst
system (8% Cu + 6.1% Ce + 2.4 % Zr + 1.5% La impregnated on 15% Sn-Al203 +
Ceo.6Zr0..3Ndo.o5Pro.o5O2 (60:40 weight ratio of Sn-A12O3 to
Ceo.6Zro..3Ndo.o5Pro.o5O2))=
The light-off test for the fresh catalyst system shows that the CO, HC and NO
exhibit
T50s at 205 C, at 389 C, and 651 C, respectively. After aging, the catalyst
performance
decreases for CO, HC and NO. The aged catalyst shows a T50 for CO and HC at
about
599 C and 651 C, respectively. The maximum conversion for NO is 5% at 700 C.
[177] Fig. 35 shows the light-off results at R = 1.05 for fresh and aged ZPGM-
4 catalyst
system (overcoat containing 12% Cu impregnated on Ceo.64Zr0.21Lao. 1502 +
A1203 (60:40
-36-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
weight ratio of Ceo.64Zro.21Lao. 1502 to A1203) and a washcoat containing 8%
Cu + 6.1%
Ce + 2.4 % Zr + 1.5% La impregnated impregnated on 15% Sn-A1203 +
Ceo.6Zr0.3Ndo.o5Pro.05O2 (60:40 weight ratio of Sn-A1203 to
Ce0.6Zro.3Ndo.o5Pro.0502)). The
light-off test for the fresh catalyst system shows that the CO, HC and NO
exhibit T50s at
254 C, at 442 C, and 636 C, respectively. After aging, the catalyst
performance
decreases for CO, HC and NO. The aged catalyst shows a T50 for CO and HC at
about
462 C and 604 C, respectively. The maximum conversion for NO is about 30% at
770 C.
[178] Fig. 36 shows the light-off results at R = 1.05 for fresh and aged ZPGM-
5 catalyst
system (overcoat containing 12.4% CuO impregnated on La-A12O3 +
Ceo.6Zro.3Ndo.05Pro.0502 (25:75 weight ratio of La-A12O3 to
Ceo.6Zro.3Ndo.o5Pro.o5O2) and a
washcoat containing 8% Cu + 6.1% Ce + 2.4 % Zr + 1.5% La impregnated on La-
A1203
+ Ce0.6Zr0.3Ndo.o5Pro.05O2 (60:40 weight ratio of La-A12O3 to
Ce0.6Zr0.3Ndo.o5Pro.o5O2))=
The light-off test for the fresh catalyst system shows that the CO, HC and NO
exhibit
T50s at 262 C, at 449 C, and 608 C, respectively. After aging, the catalyst
performance
decreases for CO, HC and NO. The aged catalyst shows a T50 for CO and HC at
about
571 C and 654 C, respectively. The maximum conversion for NO is about I% at
700 C.
[179]Fig. 37 shows the light-off results at R = 1.05 for fresh and aged ZPGM-6
catalyst
system (overcoat containing 10% Cu +12 % Ce impregnated on MgA12O4 + 16% Cu
impregnated on Ce0.6Zr0.3Ndo.05Pr0.05O2 (60:40 weight ratio of Ce impregnated
on
MgA12O4 to 16% Cu impregnated on Ce0.6Zro.3Ndo.o5Pro.05O2) (65 g/L) and a
washcoat
containing 4% Cu + 6.1 % Ce + 2.4 % Zr + 1.5% La impregnated on MgA12O4 +
Ceo.64Zr0.21Lao. 1502 (60:40 weight ratio of MgA12O4 to Ceo.64Zro.21 Lao. 150
2)). The light-
off test for the fresh catalyst system shows that the CO, HC and NO exhibit
T50s at
262 C, at 463 C, and 622 C, respectively. After aging, the catalyst
performance
decreases for CO, HC and NO. The aged catalyst shows a T50 for CO and HC at
about
425 C and 613 C, respectively. The maximum conversion for NO is about 23% at
730 C.
-37-
CA 02729235 2010-12-23
WO 2009/158009 PCT/US2009/003800
[ 180] Although the present invention has been described in terms of specific
embodiments, changes and modifications can be made without departing from the
scope
of the invention which is intended to be defined only by the scope of the
claims. All
references cited herein are hereby incorporated by reference in their
entirety, including
any references cited therein.
-38-