Note: Descriptions are shown in the official language in which they were submitted.
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 1 --
GASOLINE COMPOSITIONS
Field of the Invention
The present invention relates to an oxygenate
composition suitable for use in gasoline.
Background of the Invention
Esters are known components for use in fragrance and
flavouring applications. In particular, ethyl valerate
(also called ethyl pentanoate) is an ester commonly used
in fragrance and flavouring applications.
JP57-115490-Al (K.K. My-Skincare-Laboratories &
Daikyu K.K.) discloses a kerosene deodoriser containing 1
kind or 2 or more kinds of lower fatty acid esters.
JP07-018269-Al (Riken Koryo Kogyo K.K.) discloses
fuel additives for suppressing the unpleasant odour
characteristic of the fuel produced during incomplete
combustion of said fuel. Gasoline is disclosed as one
possible fuel for the fuel additives disclosed therein,
and included in the list of compounds disclosed as being
suitable for use as the fuel additive are a range of
esters. JP07-018269-Al mentions in passing that the fuel
additives may be added to the fuel as they are or they
can be added with other solvents, however, no details
concerning which combinations of fuels, fuel additives
and solvents would be applicable is disclosed therein.
WO 01/36354 Al (Ronyak) discloses compositions
containing an odour-emitting hydrocarbonaceous material
and an odour-suppressing amount of an aldehyde or a
ketone, and a carboxylic acid ester. Gasoline is listed
as one of many possible hydrocarbonaceous materials.
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
2 -
US 2,228,662 and US 2,334,006 (Standard Oil Company)
discloses the addition of esters to motor fuels
consisting essentially of branched chain paraffin
hydrocarbons and having a relatively high anti-knock
value to increase the anti-knock quality thereof.
US 2001/0034966 Al discloses a method of reducing
the vapour pressure of a C3 to C12 hydrocarbon-based
motor fuel mixture containing 0.1 to 20 % by volume of
ethanol for conventional spark ignition internal
combustion engines, wherein, in addition to an ethanol
component (b) and a C3 to C12 hydrocarbon component (a),
an oxygen-containing additive (c) selected from at least
one of the following types of compounds: alcohol other
than ethanol, ketone, ether, ester, hydroxy ketone,
ketone ester, and a heterocyclic containing oxygen, is
used in the fuel mixture in an amount of at least 0.05 by
volume of the total fuel.
Due to environmental concerns, there is a growing
demand for the use of bio-components, i.e. components
derived from a biological source, in gasoline.
Ethanol is a well-known bin-component currently used
in gasoline, however, it has been observed that the
addition of ethanol to base gasoline has the effect of
increasing the E70 and E100 of the formulated gasoline
relative to the base gasoline. Therefore, in order to
include significant quantities of ethanol in gasoline,
the base gasoline to which it is added has to be
specially formulated in order for the formulated gasoline
to meet gasoline specifications around the world.
It has now been found that blends of certain
oxygenates can be prepared that can be blended with base
gasoline to provide a gasoline composition without
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 3 -
significantly altering the E70 and E100 value of the base
gasoline.
Summary of the Invention
The present invention provides a gasoline
composition comprising:
(i) base gasoline; and
(ii) a composition comprising component A and at least
one component selected from categories (a) and (b) below:
(a) component B, and
(b) one component selected from components C, D and E,
wherein:
component A is an ester or mixture of esters having
formula I:
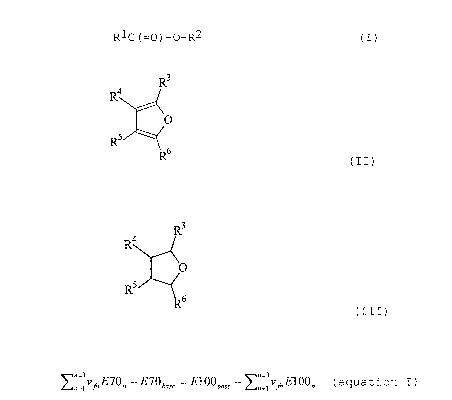
R1C (=O) -O-R2 ( I )
wherein R1 is selected from a C1-6 alkyl group and R2 is
selected from a C1-4 alkyl group and wherein the total
number of carbon atoms in R1 and R2 is in the range of
from 5-9, with the proviso that component A has a boiling
point or boiling point range within the temperature range
of from 90 to 200 C;
component B is ethanol;
component C is a compound of formula II or formula III:
R3
4
O
R5
R6
(II)
R3
R4
410
RS
R6
(III)
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 4 -
wherein the R3, R4, R5 and R6 groups are independently
selected from hydrogen and C1_6 hydrocarbyl groups, with
the proviso that component C has a boiling point or
boiling point range of at most 110 C;
component D is butanol; and
component E is an ether of the general formula IV:
R7-0-C(Me)3 (IV)
wherein R7 is selected from methyl, ethyl or mixtures
thereof,
wherein the concentration of the components in the
composition is calculated using the following equation
(equation I):
n=1v~,E70n -E7Ob,,e =E10Obas, -~n_1v El00, (equation I)
wherein:
n = 1 is component B,
n = 2 is component A,
n = 3 is any one of components C, D or E,
vtn is the volume fraction of the component n = 1, 2 or 3
in the composition comprising component A and at least
one component selected from components B, C, D and E,
E70n is the blending E70 value of the component
represented by n,
E100, is the blending E100 value of the component
represented by n,
E70base is in the range of from 10 to 55 %vol., and
E100base is in the range of from 35 to 75 ovol..
The present invention further provides a process for
the preparation of a gasoline composition according to
the present invention, said process comprising bringing
into admixture with the base gasoline, a composition
comprising component A and at least one component
selected from categories (a) and (b) below:
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
-
(a) component B, and
(b) one component selected from components C, D and E.
The present invention yet further provides a process
for the preparation of a gasoline composition according
5 to the present invention, said process comprising
bringing into admixture with the base gasoline, component
A and at least one component selected from categories (a)
and (b) below:
(a) component B, and
(b) one component selected from components C, D and E.
The present invention yet further provides a method
of operating a spark-ignition internal combustion engine,
which comprises bringing into the combustion chambers of
said engine a gasoline composition according to the
present invention.
Detailed Description of the Invention
The composition of the present invention comprises
component A and at least one component selected from
components B, C, D and E.
The composition of the present invention preferably
comprises component A and at least one component selected
from categories (a) and (b) below:
(a) component B, and
(b) one component selected from components C, D and E.
i.e. the composition of the present invention
preferably comprises any of the following mixtures of
components A, B, C, D and E:
= Component A and component B;
= Component A and component C;
= Component A and component D;
Component A and component E;
= Component A, component B and component C;
= Component A, component B and component D; and
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
6 -
Component A, component B and component E.
In one specific embodiment of the present invention,
the composition of the present invention comprises
component A and one component selected from components C,
D and E, and optionally comprises component B.
Component A is an ester or mixture of esters having
formula I:
R1C (=O) -O-R2 ( I )
wherein R1 is selected from a Cl_6 alkyl group and
R2 is selected from a C1-4 alkyl group and wherein the
total number of carbon atoms in R1 and R2 is in the range
of from 5-9, with the proviso that component A has a
boiling point or boiling point range within the
temperature range of from 90 to 200 C;
Preferably, R1 is selected from a C2_5 alkyl group,
more preferably a C3-5 alkyl group, and especially a C4
alkyl group.
Preferably R2 is selected from a C1-4 alkyl group,
more preferably a C1_3 alkyl group, and especially a C2
alkyl group.
Preferably the total number of carbon atoms in R1
and R2 is in the range of from 5-8, more preferably in
the range of from 6-7.
The boiling point or boiling point range of
component A is preferably within the temperature range of
from 95 to 180 C, more preferably within the temperature
range of from 100 to 170 C.
Examples of particularly suitable esters include
methyl valerate (methyl pentanoate), ethyl valerate
(ethyl pentanoate), propyl valerate (propyl pentanoate),
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 7 -
methyl hexanoate, ethyl hexanoate and propyl hexanoate
and mixtures thereof.
Most preferably component A is ethyl valerate.
Component B is ethanol.
Component C is a compound or mixture of compounds
having formula II or formula III:
R3
R
O
RS
R6
(I1)
R4
O
R6
R6
(1zx)
wherein the R3, R4, R5 and R6 groups are
independently selected from hydrogen and C1..6 hydrocarbyl
groups, with the proviso that component C has a boiling
point or boiling point range of at most 110 C.
Preferably, one or two of the R3, R4, R5 and R6
groups are independently selected from C1-6 hydrocarbyl
groups, with the remaining R3, R4, R5 and R6 groups being
hydrogen. More preferably, the R4 and R5 groups are
hydrogen and the R3 and R6 groups are independently
selected from hydrogen and C1_6 hydrocarbyl groups, with
at least one of the R3 and R6 groups being a 1-6
hydrocarbyl group.
Preferably, the C1_6 hydrocarbyl groups are C1-6
alkyl groups, more preferably methyl, ethyl and propyl
groups.
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
8 -
The boiling point or boiling point range of
component C is preferably within the temperature range at
most 105 C, more preferably at most 100 C. Typically,
the boiling point or boiling point range of component C
is within the range of from 40 to 110 C, more typically
within the temperature range of from 50 to 105 C, most
typically within the temperature range of from 60 to
100 C.
Examples of suitable compounds according to formula
11 include 2-methyl furan, 3-methyl furan, 2-ethyl furan,
3-ethyl furan, 2,5-dimethyl furan, 2,5-diethyl furan and
2-methyl-S-ethyl furan, and mixtures thereof. Examples
of suitable compounds according to formula III include 2-
methyl tetrahydrofuran, 3-methyl tetrahydrofuran, 2-ethyl
tetrahydrofuran, 3-ethyl tetrahydrofuran, 2,5-dimethyl
tetrahydrofuran, 2,5-diethyl tetrahydrofuran and 2-
methyl-S-ethyl tetrahydrofuran, and mixtures thereof.
Most preferably component C is selected from 2-
methyl furan, 2,5-dimethyl furan and mixtures thereof.
Component D is butanol.
Component E is an ether of the general formula IV.
R7-O-C(Me)3 (IV)
Wherein R7 is selected from methyl, ethyl or
mixtures thereof.
The composition of the present invention is suitable
for blending with a base gasoline to form a gasoline
composition.
Components A, B, C and D can be derived from a
biological source using methods known in the art,
therefore compositions according to the present invention
may be partially or entirely derived from a biological
source material and therefore be included in a gasoline
composition as a biofuel component. Preferably, at least
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
9 -
one of components A to D is derived from a biological
source material.
Advantageously, by varying the relative
concentrations of the at least two different components
in the composition of the present invention, it allows
the formation of a gasoline component that has a reduced
impact on the Dry Vapour Pressure Equivalent (DVPE) (EN
130161), E70 (%vol. evaporated at 70 C, as determined
by EN ISO 3405) and E100 (%vol. evaporated at 100 C, as
determined by EN ISO 3405) of the base gasoline to which
it is to be blended, compared to the blending of a
concentration equal to the concentration of the
composition of the present invention of any of the
individual components.
It has been found that for a given E70 and E100 of
the base gasoline, a composition according to the present
invention may be blended that will not significantly
alter the E70 and E100 values in the formed gasoline
composition. By the term "not significantly alter the
E70 and E100 values" it is meant that both the E70 value
and the E100 value of the formulated gasoline composition
is maintained within 25 %, preferably within 20 %, more
preferably within 15 of both the E70 value and the
E100 value of the base gasoline, and the value of E70 +
E100 will be maintained within 15 preferably within
10 %, more preferably within 5 % of the value of E70 +
E100 of the base gasoline.
The concentrations of the two or three components of
the composition of the present invention can be
calculated using the following equation (equation I):
,n aVf,E70n -E7Qbase =E100base -YnwiV Eloo, (equation I)
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
wherein:
n = 1 is component B,
n = 2 is component A,
n = 3 is any one of components C, D or E,
5 vfr, is the volume fraction of the component n = 1, 2 or 3
in a the composition comprising component A and at least
one component selected from components B, C, D and E,
E70õ is the blending E70 value of the component
represented by n,
10 E100, is the blending E100 value of the component
represented by n,
E70base is in the range of from 10 to 55 %vol., and
E100bdSe is in the range of from 35 to 75 %vol..
The E70base value is preferably in the range of from
14 to 51 %vol., more preferably in the range of from 14
to 50 %vol., and most preferably in the range of from 20
to 50 %vol.. In one embodiment of the present invention,
the E70base value is in the range of from 20 to 48 %vol..
In an alternative embodiment of the present invention,
the E70ba.se value is in the range of from 22 to 50 %vol..
It is especially preferred that the E70baSe value is the
E70 value of the base gasoline.
The E100base value is preferably in the range of from
40 to 72 %vol., more preferably in the range of from 40
to 71 %vol., and most preferably in the range of from 46
to 71 %vol.. It is especially preferred that the E100base
value is the E100 value of the base gasoline.
The blending E70õ and E100õ values for components A,
B, C, D and E are average values determined from data
collected on base fuels containing the single oxygenate
component (n = A, B, C, D or E) added across a range of
blend ratios. The E70õ and E100õ values are determined
according to equations II and III below:
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 11 -
E70n _ E70blend -E70ba,Se(1-vf) (equation II)
vfn r
E100blend - '100base`1-Vfn)
E100n= (equation III)
Vf,
wherein:
n is component A, B, C, D or E
vfõ is the volume fraction of the component A, B, C, D or
E when combined with a base gasoline
E70base is the E70 value of the base gasoline used
El00base is the E100 value of the base gasoline used
E70b1end is the E70 value of the base gasoline combined
with component A, B, C, D or E, and
E.OOb1e,d is the E100 value of the base gasoline combined
with component A, B, C, D or E.
Currently, the EN228 gasoline specification
specifies that the E70 value is in the range of from 20
to 50 %vol., specifically for summer gasoline the E70
value is in the range of from 20 to 48 %vol. and for
winter gasoline E70 value is in the range of from 22 to
50 %vol., and the E100 value is in the range of from 46
to 71 %vol.. Therefore, the E70ba$e value and the El00base
value are conveniently in the ranges specified in the
EN228 gasoline specification.
Thus, the composition of the present invention may
be blended with a base gasoline that complies with
current gasoline specifications (e.g. EN228) in relation
to DVPE, E70 and E100, to form a gasoline composition
which still complies with same gasoline specification
relating to DVPE, E70 and E100.
Usefully, because at least one of components A to E
can be derived from a biological source material and the
fact that compositions according to the present invention
may be blended with a base gasoline without significantly
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 12 -
altering the E70 and E100 values, the composition of the
present invention can be used in order to maximize the
bio-energy content of a gasoline composition.
The compositions of the present invention typically
have high RON (Research Octane Number) and MON (Motor
Octane Number) values, and therefore may be also be used
to increase the RON and/or MON of a base gasoline.
The present invention also provides a gasoline
composition comprising:
(i) base gasoline; and
(ii) a composition comprising component A and at least
one component selected from components B, C, D and E, as
described above.
The gasoline composition according to the present
invention may be prepared by blending the base gasoline
with component A and at least one component selected from
components B, C, D and E. The order in which the base
gasoline and components A to E are combined is not
critical. Therefore, the gasoline composition of the
present invention can also be described as comprising:
(i) base gasoline; and
(ii) component A and at least one component selected from
components B, C, D and E, as described above.
The preferred relative concentrations of components
A to E in the gasoline composition are as described above
and are calculated on the basis of a composition
comprising component A and at least one component
selected from components B, C, D and E, in the absence of
the base gasoline, whether or not such a composition is
prepared prior to combining components A to E with the
base gasoline.
The concentration, based on the overall gasoline
composition, of the composition comprising component A
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 13 --
and at least one component selected from components B, C,
D and E, as described above, which can be blended with
the base gasoline to form a gasoline composition
according to the present invention preferably accords
with one of parameters (i) to (v) below, or a combination
of one of parameters (i) to (v) and one of parameters
(vi) to (x) :-
(i) at most 40 %vol.;
(ii) at most 35 %vol.;
(iii) at most 30 nvol.;
(iv) at most 25 %vol.;
(v) at most 20 %vol.;
with features (i), (ii), (iii), (iv) and (v) being
progressively more preferred; and
(vi) at least 0.5 %vol.;
(vii) at least 1.0 %vol.;
(viii) at least 2.0 %vol.;
(ixi) at least 3.0 %vol.;
(x) at least 5.0 %vol.;
with features (vi), (vii), (viii), (ix) and (x) being
progressively more preferred.
The concentration of the composition comprising
component A and at least one component selected from
components B, C, D and B, is calculated on the basis of a
composition comprising component A and at least one
component selected from components B, C, D and E, in the
absence of the base gasoline, whether or not such a
composition is prepared prior to combining components A
to B with the base gasoline.
Ranges having a combination of any feature selected
from (i) through (v) above and any feature selected from
(vi) through (x) above are particularly applicable in the
gasoline compositions provided by present invention.
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 14 -
Examples of specific combinations of the above features
include (i) and (vi), (ii) and (vii), (iii) and (viii),
(iv) and (ix), and (v) and (x), respectively being
progressively more preferred.
The base gasoline to which the composition of the
present invention can be blended with may be any gasoline
suitable for use in an internal combustion engine of the
spark-ignition (petrol) type known in the art.
The base gasoline typically comprises mixtures of
hydrocarbons boiling in the range from 25 to 230 C (EN-
ISO 3405), the optimal ranges and distillation curves
typically varying according to climate and season of the
year. The hydrocarbons in a gasoline base fuel may be
derived by any means known in the art, conveniently the
hydrocarbons may be derived in any known manner from
straight-run gasoline, synthetically-produced aromatic
hydrocarbon mixtures, thermally or catalytically cracked
hydrocarbons, hydro-cracked petroleum fractions,
catalytically reformed hydrocarbons-or mixtures of these.
The specific distillation curve, hydrocarbon
composition, research octane number (RON) and motor
octane number (MON) of the gasoline base fuel are not
critical.
Conveniently, the research octane number (RON) of
the gasoline base fuel may be in the range of from 80 to
110, preferably from 90 to 105, more preferably from 93
to 102, most preferably from 94 to 100 (EN 25164); the
motor octane number (MON) of the gasoline base fuel may
suitably be in the range of from 70 to 110, preferably
from 75 to 105, more preferably from 80 to 100, most
preferably from 84 to 95 (EN 25163).
Typically, gasoline base fuels comprise components
selected from one or more of the following groups;
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
saturated hydrocarbons, olefinic hydrocarbons, aromatic
hydrocarbons, and oxygenated hydrocarbons. Conveniently,
the gasoline base fuel may comprise a mixture of
saturated hydrocarbons, olefinic hydrocarbons, aromatic
5 hydrocarbons, and, optionally, oxygenated hydrocarbons.
Typically, the olefinic hydrocarbon content of the
gasoline base fuel is in the range of from 0 to 40
percent by volume based on the gasoline base fuel;
preferably, the olefinic hydrocarbon content of the
10 gasoline base fuel is in the range of from 0 to 30
percent by volume based on the gasoline base fuel...
Typically, the aromatic hydrocarbon content of the
gasoline base fuel is in the range of from 0 to 70
percent by volume based on the gasoline base fuel;
15 preferably, the aromatic hydrocarbon content of the
gasoline base fuel is in the range of from 10 to 60
percent by volume based on the gasoline base fuel.
The benzene content of the gasoline base fuel is at
most 10 percent by volume, more preferably at most 5
percent by volume, especially at most 1 percent by volume
based on the gasoline base fuel.
Typically, the saturated hydrocarbon content of the
gasoline base fuel is at least 40 percent by volume based
on the gasoline base fuel; preferably, the saturated
hydrocarbon content of the gasoline base fuel is in the
range of from 40 to 80 percent by volume based on the
gasoline base fuel.
The gasoline base fuel preferably has a low or ultra
low sulphur content, for instance at most 1000 ppmw
(parts per million by weight), preferably no more than
500 ppmw, more preferably no more than 100, even more
preferably no more than 50 and most preferably no more
than even 10 ppmw.
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 16 -
The gasoline base fuel also preferably has a low
total lead content, such as at most 0.005 g/l, most
preferably being lead free - having no lead compounds
added thereto (i.e. unleaded).
When the gasoline comprises oxygenated hydrocarbons,
at least a portion of non-oxygenated hydrocarbons will be
substituted for oxygenated hydrocarbons.
The oxygenated hydrocarbons that may be included in
the gasoline base fuel are oxygenated components other
than components A to E described herein. If the base
gasoline contains an oxygenated component of the type
described by components A to E, then this component is to
be considered as a component of the composition according
to the present invention and the relative quantities of
the other components A to E will be adjusted accordingly.
Examples of suitable gasoline base fuels include
gasoline base fuels which have an olefinic hydrocarbon
content of from 0 to 20 percent by volume (ASTM D1319),
an oxygen content of from 0 to 5 percent by weight
(EN 1601), an aromatic hydrocarbon content of from 0 to
50 percent by volume (ASTM D1319) and a benzene content
of at most 1 percent by volume.
Whilst not critical to the present invention, the
gasoline base fuel or the gasoline composition of the
present invention may conveniently additionally include
one or more fuel additive. The concentration and nature
of the fuel additive(s) that may be included in the
gasoline base fuel or the gasoline composition of the
present invention is not critical. Non-limiting examples
of suitable types of fuel additives that can be included
in the gasoline base fuel or the gasoline composition of
the present invention include anti-oxidants, corrosion
inhibitors, detergents, dehazers, antiknock additives,
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 17 -
metal deactivators, valve-seat recession protectant
compounds, dyes, friction modifiers, carrier fluids,
diluents and markers. Examples of suitable such
additives are described generally in
US Patent No. 5,855,629.
Conveniently, the fuel additives can be blended with
one or more diluents or carrier fluids, to form an
additive concentrate, the additive concentrate can then
be admixed with the gasoline composition or gasoline base
fuel.
The (active matter) concentration of any additives
present in the gasoline base fuel or the gasoline
composition is preferably up to 1 percent by weight, more
preferably in the range from 5 to 1000 ppmw,
advantageously in the range of from 75 to 300 ppmw, such
as from 95 to 150 ppmw.
A gasoline composition according to the present
invention may be prepared by a process which comprises
bringing into admixture with the base gasoline, a
composition comprising component A and at least one of
components B, C, D and E, and optionally other
conventional gasoline components, such as one or more
fuel additives. As explained above, it is not critical
that the composition comprising component A and at least
one of components B, C, D and E is formed prior to
blending with the base gasoline, provided that component
A and at least one of components B to E are brought into
admixture with the base gasoline (i.e. the composition
may formed in-situ).
Therefore, the present invention provides a process
for the preparation of a gasoline composition as
described above, said process comprising bringing into
admixture with the base gasoline, a composition
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 18 -
comprising component A and at least one component
selected from categories (a) and (b) below:
(a) component B, and
(b) one component selected from components C, D and E.
Alternatively, the present invention provides a
process for the preparation of a gasoline composition as
described above, said process comprising bringing into
admixture with the base gasoline, component A and at
least one component selected from categories (a) and (b)
below:
(a) component B, and
(b) one component selected from components C, D and E.
If the gasoline composition additionally comprises
one or more fuel additives, then the one or more fuel
additive, or the additive concentrate, may be admixed
with one or more of the constituents of the gasoline
composition (e.g. component A, component B, component C,
component D, component E, or the composition comprising
component A and at least one component selected from
categories (a) and (b) as described above, and the base
gasoline) or with the gasoline composition itself. If
the one or more fuel additive is added to more than one
of the constituents of the gasoline composition, then the
fuel additive added to each of the constituents of the
gasoline composition may be the same or different.
The present invention also provides a method of
operating a spark-ignition internal combustion engine,
which comprises bringing into the combustion chambers of
said engine a gasoline composition as defined above.
The present invention will be further understood
from the following examples. Unless otherwise indicated,
parts and percentages (concentration) are by volume
(%v/v) and temperatures are in degrees Celsius ( C).
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
19
Examples
Comparative Examples A to C
The base gasoline used in comparative examples A to
C was an EN 228 unleaded gasoline having the specific
properties detailed in Table 1 below:
Table 1
Property
RON 96.1
MON 85.1
RVP (kPa) 81.7
Density (kg/m3) 742.6
FBP ( C) 202.0
Residue (%v) 1.0
E70 (%v) 29.6
E100 (%v) 48.7
E150 (%v) 85.6
The base gasoline described in Table 1 above and
blends of the base gasoline with 10 %vol. and 20 %vol.
ethyl valerate (EV), based on the volume of the
formulated gasoline composition, were prepared.
The properties of each of the gasoline compositions
are provided in Table 2 below.
Table 2
Base
Gasoline EV RVP E70 E100 E70 +
Example (%v/v) (%v/v) (kPa) (%v) (%v) E100
A 100 0 81.7 29.6 48.7 78.3
B 90 10 76.2 25.4 42.6 68
C 80 20 70.1 21.8 37.2 59
Comparative Examples D to G
The base gasoline used in comparative examples D to G was
an EN 228 unleaded gasoline having the specific
properties detailed in Table 3 below:
CA 02729353 2010-12-23
PCT/EP2009/058227
WO 2010/000760 2 0 -
Table 3
Property
RON 92.2
MON 83.0
Density (kg/m) 740.9
FBP ( C) 193.4
E70 (%v) 36.2
E100 (%v) 61.8
E150 (%v) 89.4
The base gasoline described in Table 3 above and
blends of the base gasoline with 5 %vol., 10 %vol. and
20 %vol. ethanol (EtOH), based on the volume of the
formulated gasoline composition, were prepared.
The properties of each of the gasoline compositions
are provided in Table 4 below.
Table 4
Base
Gasoline EtOH E70 E100 E70 +
Example (%v/v) (%v/v) (%v) (ov) E100
D 100 0 36.2 61.8 89.4
E 95 5 44.7 65.2 91.4
F 90 10 55.2 66.0 90.7
G 80 20 74.8 74.8 91.6
Comparative Example H and Examples 1 to 16
The properties several gasoline compositions
containing compositions according to the present
invention are given below.
The base gasoline used in the following examples was
an EN 228 unleaded gasoline having the specific
properties detailed in Table 5 below.
CA 02729353 2010-12-23
WO 2010/000760 - 2 - PCT/EP2009/058227
1
Table 5
Property
RON 95.7
MON 85.2
RVP (kPa) 88.9
Density (kg/m3) 731.5
IBP ( C) 27.2
FBP ( C) 199.5
Residue (%v) 1.0
Recovery (%v) 94
Loss (%v) 5.0
10% evap ( C) 40.6
20% evap ( C) 51.7
30% evap ( C) 64.2
40'W evap ( C) 78.2
50% evap ( C) 92. 4
60% evap ( C) 105.6
70% evap ( C) 117.8
80% evap ( C) 133.4
90% evap ( C) 156.2
95% evap ( C) 173.1
E70 (%v) 34.3
E100 (%v) 55.5
E120 (%v) 71.6
E150 (%v) 87.5
E180 (%v) 96.4
The ethyl valerate used was supplied by Sigma-
Aldrich and had a purity of 99%.
The ethanol (anhydrous) used was supplied by Sigma-
Aldrich and had a purity of >99%.
The 2-methyl furan used was supplied by Sigma-
Aldrich and had a purity of 99%.
To prepare the gasoline compositions, four separate
compositions according to the present invention were
prepared and are detailed in Table 6 below.
CA 02729353 2010-12-23
PCT/EP2009/058227
WO 2010/000760 - 2 2 -
Table 6
Example Composition Ethyl Valerate Ethanol 2-Methyl
(%v/v) (%v/v) Furan
(%v/v)
1 Oxl 42 0 58
2 0x2 51 10 39
3 0x3 60 20 20
4 0x4 70 30 0
Using the above four oxygenate compositions (Ox1 to
Ox4), twelve different gasoline compositions were
prepared by admixing each of the above oxygenate
compositions (Oxl, 0x2, 0x3 and Ox4) individually with
the base gasoline detailed in Table 5, at 5 %vol.,
%vol. and 20 %vol. concentrations based on the volume
of the formulated gasoline composition.
The properties of each of the gasoline compositions
are provided in Table 7 below.
Table 7
Base Oxygenate (%v/v) E70
Gasoline RVP E70 E100 +
Example (%v/v) Oxl Ox2 0x3 Ox4 (kPa) (%v) (%v) E100
H 100 88.9 34.3 55.5 89.8
5 95 5 87.4 35 56.6 91.6
6 95 5 90.2 34 55.4 89.4
7 95 5 90.3 33.7 54.3 89.0
8 95 5 92.2 34.3 53.9 88.2
9 90 10 84.2 33.5 56.3 89.8
10 90 10 87.5 34.1 55.2 89.3
11 90 10 88.8 34.5 53.9 88.4
12 90 10 87.9 34.9 52.2 87.1
13 80 20 76.6 32.3 56.9 89.2
14 80 20 82.2 33.9 54.8 88.7
80 20 82.9 34.7 51.5 86.2
16 80 20 83.5 37.5 51.3 88.8
CA 02729353 2010-12-23
WO 2010/000760 PCT/EP2009/058227
- 23 -
It can clearly be seen that the E70, E100 and the
E70 + E100 values of the gasoline compositions according
to the present invention are not significantly altered
from the E70 and E100 values of the base gasoline
(comparative Example H), and the E70 and E100 values of
the gasoline compositions according to the present
invention are well within the current EN 228 gasoline
specifications.