Note: Descriptions are shown in the official language in which they were submitted.
CA 02729718 2011-01-27
TITLE
ANTIFUNGAL COMPOSITIONS
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of United States Provisional
Application
No_ 61/298,672, filed January 27, 2010, the disclosure of which is
incorporated herein
by reference.
BACKGROUND OF THE INVENTION
[0002] This invention relates in general to antifungal compositions, and in
particular to antifungal compositions that can be added to provide mold
resistance to
building materials and other materials.
[00031 Mold problems can arise in interior living spaces- In the past 30 years
as
buildings have become better insulated and more energy efficient there has
been an
increase in mold problems due to trapped moisture. Building materials such as
wood,
insulation materials and wallboard used in building construction are
susceptible to
mold and fungal growth in moist environments or when exposed to wetting.
Fluffed
cellulose fiber, shredded paper, low density fiberboard used as insulating
materials
and. nonwoven webs, such as paper, used to cover gypsum wallboard, are all
prime
growth media for fungi. Several species of mold and fungus that grow in these
environments are toxic to humans. A number of health risks such as
occupational
asthma, rhinoconjunctivitis, hypersensitivity pneumonitis and organic dust
toxic
syndrome (ODTS), aspergillosis and histoplasma have been linked to antigens
generated by fungi. Diseases associated with inhalation of fungal spores can
include
toxic pneumonitis, hypersensitivity pneumonitis, tremors, chronic fatigue
syndrome,
kidney failure, and cancer.
10004] The current mold resistant wallboard technology (DensArmor Georgia
Pacific) consists of a fiberglass based product that has limitations in
implementation
due to its cost and its density. The shell of DensArmor is composed of
fiberglass.
1
CA 02729718 2011-01-27
This makes it weigh more than regular wallboard which uses paper as the shell.
An
ideal mold resistant drywall would be of similar construction as standard
drywall but
be able to resist mold growth. Ecology Coatings, Inc. has developed a coating
for
drywall that needs to be cured by exposure to UV light.
[0005] It would be desirable to provide an antifungal composition that confers
mold resistance to building materials such as loose fill and dense packed
cellulose
insulation, wallboards and to other materials.
SUMMARY OF THE INVENTION
[0006] In one embodiment, an antifungal composition comprises a borate
fungicide
and an ion-exchange type antimicrobial agent with two or more different metal
ions.
The combination of the metal ions and the borate provide a synergistic effect
in fungal
resistance.
[00071 In another embodiment, an antifungal composition comprises an ion-
exchange type antimicrobial agent with borate ions and two or more different
metal
ions. The combination of the borate ions and the metal ions provide a
synergistic
effect in fungal resistance.
[0008] A fungus resistant building material comprises a building. material
susceptible to fungus growth and an antifungal composition comprising a borate
fungicide and an ion-exchange type antimicrobial agent with two or more
different
metal ions. The combination of the metal ions and the borate provide a
synergistic
effect in fungal resistance.
[0009] Various aspects of this invention will become apparent to those skilled
in
the art from the following detailed description of the preferred embodiments.
BRIEF DESCRIPTION OF THE DRAWING
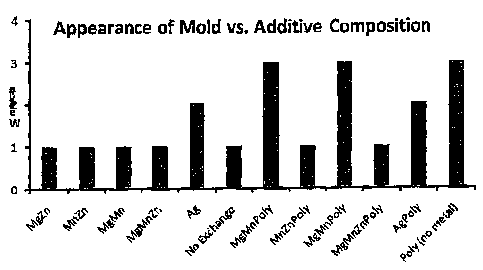
[00101 Figure I is a bar graph showing the week at which mold growth was first
observed based on paper handsheet compositions in testing done with different
additives-
2
CA 02729718 2011-01-27
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[00111 The present development relates to an antifungal composition that
comprises a borate fungicide and an ion-exchange type antimicrobial agent with
two
or more different metal ions. The combination of the metal ions and the borate
provide a synergistic effect in fungal resistance. The antifungal composition
can be
effective in providing resistance to one or more types of fungus, such as
molds, yeasts,
mushrooms and other eukaryotic organisms.
[0012] Any suitable borate fungicide can. be used in the composition. Borate
fungicides are well known in the industry and include, for example, boric
acid, sodium
borate, borax, sodium tetraborate decahydrate, sodium tetraborate
pentahydrate,
disodium octaborate tetrahydrate, disodium octoborate, sodium metaborate,
sodium
perborate, sodium perborate tetrabydrate, sodium pentaborate decahydrate, and
the
like. In apart icular embodiment, the borate fungicide is sodium perborate
tetrahydratc, which is available commercially as Polybor from U.S. Borax
Inc.,
Valencia, California-
[00131 The ion-exchange type antimicrobial agent can be any suitable type.
These
agents typically comprise an inorganic ion-exchange carrier and one or more
antimicrobial metals and/or metal ions, most preferably one or more
antimicrobial
metal ions. The inorganic ion-exchange carrier is preferably an ion-exchange
type
ceramic particle wherein antimicrobial metal ions have been exchanged
(replaced) for
other non-antimicrobially effective ions in the ceramic particles or a
combination of
the foregoing with an antimicrobial metal salt. Suitable ceramic particles
include, but
are not limited to zeolites, hydroxyapatite, zirconium phosphates and other
ion-
exchange ceramics, and come in many forms and types, including natural and
synthetic forms.
[00141 The term "zeolite" is used here in its generic sense and refers to
crystalline
inorganic molecular sieves such as aluminophophates, silicon aluminum
phosphates,
microporous borosilicates, titanosilicates and tatanioaluminosilicates, as
well as
rnieroporous aluminosilicates and their silica analogs, having a framework
structure
consisting of nanopores and interconnected cavities which can be occupied by
ions
3
CA 02729718 2011-01-27
and/or water molecules, both of which have considerable freedom of movement
permitting ion exchange and reversible dehydration. In contrast to amorphous
materials these crystalline structures contain regular arrays of
intracrystalline pores
(zianopores) and voids of uniform dimensions. A typical naturally occurring
zeolite is
the mineral clinoptilolite with formula (Ca, Na, l( 2.3AI3(AI,Si)2Si13O3fi
12(H2O). As
used herein, "ion exchanged zeolite" refers-to a natural or synthetic zeolite
that has'
been modified by an ion exchange process to increase the content of one or
more
metal ions in the zeolite.
[0015] The ion-exchange type antimicrobial agent is modified by exchange with
two or more different metal ions. The combination of the metal ions and the
borate
provide a synergistic effect in fungal resistance. For example, as described
in the
experiments below, it was discovered that the combination of magnesium ions
and
manganese ions and the borate provide a synergistic effect in fungal
resistance. Using
the ASTM D6523 Standard Test Method for Resistance to Growth of Mold it has
been
demonstrated that this combination is an unexpectedly effective mold
inhibitor.
However, other combinations of ions may also provide such a synergistic
effect.
[00161 The experiments below describe the use of silver and zinc ions as well
as
magnesium and manganese. Some other metal ions known for use in ion exchange
materials include, but are not limited to, copper, gold, mercury, tin, lead,
iron, cobalt,
nickel, arsenic, antimony, bismuth, barium, cadmium, chromium and thallium.
[0017] In an alternative embodiment, instead of using a borate fungicide along
with
the ion-exchange type antimicrobial agent, the antifungal composition can
comprise
an ion-exchange type antimicrobial agent having borate ions in addition to the
metal
ions. Any suitable borate ions can be used.
[0018] Other embodiments of this technology could include a binder, such as
nanocellulose or other natural or synthetic binder, to aid in incorporating
and retaining
the antifungal composition into or onto the material to which it is applied.
(00191 The antifungal composition can have any suitable effectiveness against
fungi. In certain embodiments, the antifungal composition scores a 10 in the
ASTM
D3273 test for mold resistance. Also, in certain embodiments, the antifungal
4
CA 02729718 2011-01-27
composition is effective to prevent mold growth onset at least about 3 weeks
according to mold resistance test ASTM D6523. Such testing is described in the
experiments below.
[0020] The antifungal composition can be used in many different applications
where it is desirable to prevent fungal growth. The antifungal composition may
be
applied as a coating or may be incorporated into a material. Such applications
can
involve applying the antifungal composition in or on many different materials-
Such
materials may include, for example, wood, paper, metal, plastic, glass, or
fabric/textile. In certain embodiments, the antifungal composition is
incorporated into
or coated onto any number of inorganic materials, especially those employed in
building construction, like wood, paper, fluffed cellulose fiber, fiberboard,
cements,
mortar, grout, plaster, and the like, or in the manufacture of building
materials, such as
ceramics and cements for tiles, In certain embodiments, the antifungal
composition is
used as a building material additive, for example, in wallboard of any type,
more
particularly in the paper cover of drywall or in cellulose insulation
formulations - both
loose fill and dense packed products.
[0021] The antifungal composition can be used in any amount suitable for
providing fungal resistance. For example, the composition may be used in an
amount
of from about 1% to about 30% by weight of the material when used as an
additive, or
by weight of a coating when applied as a coating-
[0022] Development. and Test of the Innovation
[0023] The main goal of this innovation was to identify a zeolite formulation
which
would inhibit mold growth when incorporated into paper. The scope of work
comprised two parts. Part I tested pre-screening of ion exchanged zeolite
candidates
for mold inhibition on paper handsheets. The best mold inhibitor candidates
were
chosen for a second round of tests, Part II. Combinations of two metals and
metals
with Polybor were utilized in making handsheets which were then tested for
mold.
inhibition. Finally, a sample of "drywall paper" with the best performing
zeolite was
CA 02729718 2011-01-27
submitted to an ASTM lab in order to test its effectiveness under the ASTM
mold
resistance test (ASTM D6523).
[0024] Part I.
[0025] The testing was performed in two stages. The first stage involved
preparing
a series of handsheets which incorporated a range of ceolite/metal
compositions.
[0026], Handsheet Compositions
Hand Sheet Mold Test Key:
Sheet Composition
A Ag/OLIN
B Fe/CLIN
C Mg/CLIN
D Mn/CLIN
E Zn/CLIN
F Ti/CLIN (#3- normal pH, exchanged in ethanol, CLIN normal)
G Ti/CLIN (#2- low pH, exchanged in water, CLIN damaged?)
II Zn/50% exchange
I Ag/FAU
J CLIN (unexchanged)
K BLANK
L Polybor
CLIN: clinoptilolite
FAU: faujasite
Polybor: spray-on boric acid compound
[0027] Mold Test Results
[0028] Inhibition of mold growth was conducted in Dr. Jody Jellison's lab at
the
University of Maine. Dr_ Jellison is an expert in mold microbiology and
performs
consulting work for private industry. The choice of mold species was
recommended
by her and Zeomatrix' experimental design was modified by her to best suit
the'
facilities available and her knowledge of best known practices. The test
utilized four
mold spores common to domestic environments.
6
CA 02729718 2011-01-27
[0029] The mold species used in this study are as follows:
Aspergillus niger ATCC 6275
- isolated from leather
Aspergillus niger ATCC 9642
- isolated from wireless radio equipment, New South Wales, Australia
Cladosporium minourae ATCC 52853
-isolated from rotting wood, Japan
Penicillium funiculosum ATCC 11797
- isolated from mercury-treated fabric,.Maryland
[0030] A cocktail of these organisms were prepared from individual cultures
obtained from ATCC (American Type Culture Collection). Careful preparation of
the
cocktail required that each mold species be present at the same concentration
(steps 1
through 4). This process required four weeks. Paper samples (5 each) were 4.0
cm
diameter circles cut from handsheets and placed in a randomized fashion 4 at a
time
onto agar plates (step 5). Each plate was inoculated with mold spores by
spraying
with 10 mL of the spore cocktail (step 6). The plates were incubated at 25 C
in humid
conditions for 4 weeks. The plates were photographed each week (step 8). The
ability of the paper samples to inhibit mold growth nearby was determined by
measuring zones of partial and total inhibition (step 9)"
1. Molds Grown on Potato Dextrose Agar (PDA)
2. Spores Collected Using Minimal Salts Solution
3. Spores Counted Using l-Iemocytometer
4. 105 Spores from Each Mold Added to Spore.Cocktail
5. Plates Assembled Using Minimal Salts Solution And Samples
6. 10 mL Spore Cocktail Sprayed onto Each Plate Using Minimal Salts Agar
7. Plates Incubated At 25 C In Humid Conditions for Four-Weeks
8. Plates Were Photographed Regularly
9. Zones of Total and Partial Inhibition Were Measured
[0031] Results:
[0032] Zones of inhibition showed that handsbeets containing zeolites
exchanged
with silver, and magnesium in combination with boric acid were effective in
7
CA 02729718 2011-01-27
preventing mold from growing near the paper samples. Samples containing
silver,
natural zeolite, zinc, and boric acid demonstrated some effectiveness at
inhibiting
mold growth on the paper samples.
[0033] Part II.
[0034] Part II of this research effort involved three different exchanged
zeolites
which exhibited mold inhibition in Part I - Ag, Mg and Zn- exchanged. The Ag-
exchanged sample was used as a control and is not a good candidate for a
commercial
product. However, both the Mg and Zn samples were tested in the follow-on
study.
[00351 Mold Study
[0036] As before, zeolite samples were exchanged by Zeomatrix and handsheets
incorporating these zeolites were prepared by the Process Development Center
(PDC)
at UM. The handsheets were divided up into samples as shown in Table 1 and
submitted to Jody Jellison for incubation with the test mold spores. The mold
testing
was conducted for 8 weeks and the results are in Figure 1.
Table I. Handsheet Sample Compositions
Hands-beet Sam 1e Number Exchanged Ions Composition
A M Zn 1:1
B MnZn 1:1
C Mn 1:1
D MgMnZn 1:1:1
E Ag Control
P No exchange Zeolite Control
G MMn 1:1, 3.5% Polybor
H MnZn. 1:1, 4.7% Pol bor
I MgMn 1:1, 3.0% Pol bor
J MgMnZn I- L 1, 4.1% Polybor
K Ag 3.6% Polybor
L No exchange 5.0% Polybor
[0037] The exchanged zeolites in the preceding table were incorporated into
handsheets
in the amounts indicated (1:1, 300rng_300mg). The Polybor was applied as a
spray-on
solution at the various levels indicated. The Polybor is simply a boric acid
solution comprised
of boron, oxygen, and sodium.
8
CA 02729718 2011-01-27
[0038] Mold Test
[0039] The mold test was conducted as previously described (Part I). The
plates
were prepared in a double blind study where the biology researchers were
unaware of
the different compositions of samples A-L. Different samples were randomly
grouped.
on the 5 plates in sets of three in order to test 5 replicates of each
composition. The
results of the replicates are summarized in the table in Table 11-
Table II. Summary of Initial Mold Growth for Replicate Samples.
Groai F tst
;" :weexr"I :~C X X X I X X X
--- ' x I_ x
NOM AtwccX4ev-ovearmc ir typolu airu,eormo- rve kaes-Yimmoldgrna
0 f 0 0 O 3 I 0 3 1 2 3 4 3
HMIMw;l6ne, 0 0 0 0 3 -I 0 -3--~ 2 4-.-_.
gowdh21Wccr61
- -- - -= --I - -- -=-- =--- - ---- -- '
s- Wei, 0- I. 0 0 0 4 1 0 3 3 4 3 4 3
Acplir~tcsrilh=m ---~ -~ --0 __j---_p - - =-0-- q- 0 4--~ 3 - ~- - 4---,-` _ -
4 - --- 3' ... ...: : ... .. . .
. I I
I , I
~aapt6ca~ac wfth,iol .0 .. ~.~_ 0- i 0 4 i 0 s 13 _4
19v'pyp~ at WeeK z ~.-
-[0040] Summary of Innovation Test
[00411 The Polybor definitely had a positive effect on the mold resistance
properties. The best overall performers (all replicates over eight weeks) were
the ones
containing either magnesium plus manganese, or silver, plus Polybor- However,
the
mg:mn plus polybor samples (I and G) were more resistant to mold growth onset
than
the Ag -- Polybor as shown in Figure 1. This composition (Mg:Mn + polybor) was
selected for the drywall test panel. There was a definite synergistic effect
of the two
metals (Mg and Mn) in combination with Polybor. The result is non-obvious
9
CA 02729718 2011-01-27
considering that the addition of zinc (MgMnZnP) did not inhibit mold growth
more
than no exchange at all.
[00421 The drywall panel was submitted to Accugen Laboratory in Illinois for
the ASTM
D3273 test. This test showed that the MgMn Polybor treated drywall sample
scored a "10"
out of 10, or the highest score for mold resistance,
[0043] The principle and mode of operation of this invention have been
explained
and illustrated in its preferred embodiment. However, it must be understood
that this
invention may be practiced otherwise than as specifically explained and
illustrated
without departing from its spirit or scope.