Note: Descriptions are shown in the official language in which they were submitted.
CA 02729959 2012-12-12
50795-49
1
. TARGETED REAGENT INJECTION FOR SLAG CONTROL FROM
COMBUSTION OF COALS HIGH IN IRON AND/OR CALCIUM
DESCRIPTION
Background of the Invention
[00011 The invention relates to a process that increases the output of a
combustor fired with
coal having high iron and/or calcium content, by reducing the tendency of slag
to form on heat
exchange surfaces, changing the nature of the slag to make it easier to remove
and actually
removing slag.
[0002] Combustion of coal, like other fossil fuels, is invariably less
efficient than desired and
can be a source of pollution. Maintaining . combustor operation at high
efficiency and
controlling the quality of the emissions is essential for maintaining the
energy needed to
power .our economy while preserving the quality of the air we require for
survival. Because
efficiency and emissions are interrelated and some technological solutions
have been shown to
be competitive with each other, it has been difficult to achieve both.
Economic operation of
power plants and incinerators is in the public interest, and new technologies
are essential to
this effort.
- [0003] Fuel selection plays an important role in mitigating some
pollution problems, but it
cannot eliminate them. Some coals, such as certain Appalachian and Illinois
Basin bituminous
coals, are important in many plants designed for coal where economics limits
other options.
The tendency to form slag and the properties of the slag for such high iron
content. coals have
been a major concern of combustion engineers and plant operators for decades.
There are a
number of factors that impact the physical and chemical properties of slag.
See, for example,
Combustion Fossil Power, 1991, Joseph G. Singer, P.E., editor, Chapter 3,
Combustion
Engineering. However, as the industry stands today, there is a compromise
between selection
=
CA 02729959 2013-10-30
= 50795-49
2
of low-cost coal and the actual economics of energy production where slagging
becomes a
problem. Slag accumulation is a problem that causes decreased heat transfer
and often leads to
long periods of downtime for cleaning.
[0004] An interrelated problem with coal is that large amounts of ash and fine
particulates are
formed that must be captured and disposed of. The art has used additives to
control slag
formation and properties, but the additives can stress the solids recovery
systems employed in
terms of sheer volume. Accordingly, optimum slag control has often been
compromised
because the solids recovery system could not effectively remove all of the
solids necessary.
This is especially a problem with older plants where increasing the solids
collection capacity
is not an option.
[0005] Making the problem more complex is the fact that coals react
differently to additives
as a function of their composition. As a general rule, there are no known
formulae that make it
possible to address all different coal compositions with suitable additives at
effective levels
that can be adequately handled by solids recovery equipment. The discovery of
individual
coal composition and additive regimens are highly sought after to assure that
economical
power can be supplied while generating sufficient revenues for effective
pollution control.
[0006] There is a need for an improved process that more effectively controls
slagging,
especially with problem fuels, such as coals with sulfur contents that cause
them to play an
increased role in slagging and also those having high iron and/or calcium
contents, to improve
boiler efficiency and economics.
Disclosure of Invention
[0007] It is an object of some embodiments of the invention to provide an
improved
technology for slag control in combustors utilizing fuels tending toward the
production of
slag.
[0008] It is another object of some embodiments to provide a process to
control slag from the
combustion of coal with high iron and/or calcium contents while reducing
chemical
utilization.
CA 02729959 2013-10-30
50795-49
3
[0009] It is another object of some embodiments to provide a process to remove
slag from
boiler heat exchange surfaces due to the combustion of coal with high iron
and/or calcium
contents while reducing chemical utilization.
[0010] A yet further but more specific object of some embodiments is to
provide a process to
more effectively control slag by decreasing the amount of downtime associated
with slag
removal.
[0011] It is a more specific object of some embodiments of some aspects of the
invention to
achieve the above objects while at the same time improving combustor
efficiency.
[0012] These and other objects may be achieved by the present invention in at
least its
preferred aspects which provides an improved process for slag control in
combustors burning
slag-forming coal with high iron and/or calcium content.
[0013] In one aspect, the invention provides a process for reducing slag
cohesiveness and/or
adhesiveness in a combustor, thereby decreasing the rate of fouling,
comprising: combusting a
slag-forming coal, having high iron and/or calcium content, with an overall
excess of oxygen;
moving the resulting combustion gases though heat exchange equipment under
conditions
which cause cooling of slag formed by burning the coal; and prior to contact
with said heat
exchange equipment, introducing aqueous aluminum trihydroxide in amounts and
with droplet
sizes and concentrations effective to decrease the rate of fouling, and
preferably, increase the
friability of the resulting slag.
[0014] In one preferred aspect, the aluminum trihydroxide reagent is
introduced in the form of
an aqueous liquid and computational fluid dynamics is employed to determine
flow rates and
select reagent introduction rates, reagent introduction location(s), reagent
concentration,
reagent droplet size and/or reagent momentum.
[0015] In another preferred aspect, magnesium hydroxide is introduced as an
aqueous slurry
along with the slurry of aluminum trihydroxide.
[0016] In another aspect the invention provides a process for cleaning furnace
surfaces having
a slag buildup, by introducing aqueous aluminum trihydroxide in amounts and
with droplet
CA 02729959 2014-06-19
50795-49
4
sizes and concentrations effective to contact for fine particulates resulting
from drying the slurry
to contact existing slag deposits.
[0017] In another aspect, the invention provides a process a cleaning and
maintenance of a
combustor comprising a regimen of initial dosing of from about 3 to 6 pounds
of ATH per ton of
coal and about 1 to 2 pounds of Mg(OH)2 per ton of coal for a time sufficient
to reduce slag,
followed by a reduced reducing the dosing of from about 10 to about 50% of the
initial values for
maintaining the combustor clean and operating efficiently.
[0017A] According to one aspect of the present invention, there is provided a
process for reducing
slag cohesiveness and/or adhesiveness in a combustor, thereby decreasing the
rate of fouling,
comprising: combusting a slag-forming coal, having an iron content of greater
than about 15%
based on the weight of the ash and expressed as Fe203 and/or a calcium content
of greater than 5%
based on the weight of the ash and expressed as CaO, with an overall excess of
oxygen; moving
the resulting combustion gases though heat exchange equipment under conditions
which cause
cooling of slag formed by burning the fuel; and prior to contact with said
heat exchange
equipment in the combustor, introducing into hot combustion gases in the
combustor an aqueous
liquid aluminum trihydroxide reagent in amounts and with droplet sizes and
concentrations
effective to form nano-sized particles of under 200 nanometers in the hot
combustion gases and to
decrease the rate of fouling by slag and to increase the friability of
resulting slag.
[001713] According to another aspect of the present invention, there is
provided a process for
removing slag deposits in a combustor burning coal, comprising: introducing
into hot combustion
gases in the combustor, aqueous aluminum trihydroxide in amounts and with
droplet sizes and
concentrations effective to remove slag deposits.
[0017C] According to still another aspect of the present invention, a process
for cleaning and
maintenance of a boiler burning coal having an iron content of greater than
about 15% based on
the weight of the ash and expressed as Fe203 and/or a calcium content of
greater than 5% based on
the weight of the ash and expressed as CaO, comprising a regimen of initial
dosing of from
about 3 to 6 pounds of an aqueous slurry of aluminum trihydroxide per ton of
coal and about 1
to 2 pounds of an aqueous slurry of magnesium hydroxide per ton of coal for a
time sufficient to
reduce slag,
CA 02729959 2013-10-30
50795-49
4a
followed by reducing the dosing of from about 10 to about 50% of the initial
values by weight
for maintaining the combustor clean and operating efficiently, wherein the
aqueous aluminum
trihydroxide and magnesium hydroxide are introduced with droplet sizes and
concentrations
effective to form nano-sized particles of under 200 nanometers and to decrease
the rate of
fouling by slag.
[0018] Other preferred aspects and their advantages are set out in the
description which follows.
Brief Description of the Drawings
[0019] The invention will be better understood and its advantages will become
more apparent
when the following detailed description is read in conjunction with the
accompanying
drawings, in which:
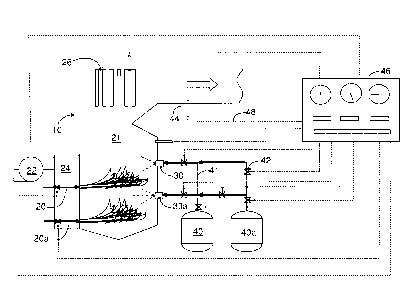
[0020] Fig. 1 is a schematic view of one embodiment of the invention.
[0021] Fig. 2 is a photograph of a slag sample obtained after operation for 24
hours of
aluminum trihydroxide into a combustor operated on a high iron content coal as
set out in
Example 2 below.
Detailed Description of the Invention
[0022] Reference will first be made to Fig. 1, which is a schematic view of
one embodiment of
the invention. Fig. 1 shows a large combustor 10 of the type used for
producing steam for
electrical power generation, process steam, heating or incineration. Coal is
fed by burners 20 and
20a and burned with air in a combustion zone 21. It is an advantage of the
invention that coal
that is high in iron (e.g., iron contents of greater than about 15%, e.g.,
from about 20 to 35%,
based on the weight of the ash and expressed as Fe2O3) and/or calcium content
(e.g., calcium
contents of greater than 5%, e.g., from about 10 to 25%, based on the weight
of the ash and
expressed as CaO). It is also an advantage of the invention that slag can be
effectively
CA 02729959 2011-01-05
WO 2010/006325
PCT/US2009/050354
controlled even for coals having significant sulfur contents, e.g., above
about 1% and in the
range of from about 3 to about 5%. Here, and throughout this description, all
parts and
percentages are by weight.
[0023] Air for combustion, supplied by fan 22 and ductwork 24, is preferably
preheated by a
gas-to-gas heat exchangers (not shown) which transfer heat from ductwork (not
shown) at the
exit end of the combustor. Hot combustion gases rise and flow past heat
exchangers 26, which
transfer heat from the combustion gases to water for the generation of steam.
Other heat
exchangers, including an economizer (downstream and not shown) may also be
provided
according to the design of the particular boiler. Slag left untreated would
tend to form on these
heat exchanger surfaces, which are positioned within specific combustors based
on design
considerations important to individual locations. It is an advantage of the
present invention
that modeling techniques, such as computational fluid dynamics, are employed
to initially
direct treatment chemicals (especially, those identified as effective for
particular types of coal
according to the invention) to the optimum locations for reducing and/or
controlling slag
buildup and maintaining efficient operation of the boiler.
[0024] A series of suitable, preferably air assisted atomizing, nozzles in
each of nozzle banks
30 and 30a are provided for introducing aluminum trihydroxide alone or with
magnesium
hydroxide slurry from vessels 40 and 40a respectively. Both the ATH and the
magnesium
hydroxide are preferably aqueous, as slurries and/or solutions as appropriate.
Supply lines
(e.g., 41) are shown as double lines in the drawing. Valves (e.g., 42) are
represented by the
common symbol ( ), and temperature sensors (e.g., 44) are represented by the
common
symbol ( ). Both valves 42 and temperature sensors 44 are connected to
controller 46 via
electrical leads (e.g., 48) shown in dotted lines. These valves, temperature
sensors and leads
are illustrative only, and the skilled worker using the principles outlined
herein will place
them strategically to provide appropriate control signals and responses. The
controller 46 can
be a general purpose digital computer programmed in accord with a
predetermined control
regimen with both feed forward and feedback features.
[0025] Aluminum trihydroxide (Al(OH)3), which has been found effective
according to the
invention for greatly lessening the deposition of slag or cleaning deposited
slag from
troublesome coal types, is also known under other names such as ATH, aluminum
hydroxide
CA 02729959 2011-01-05
WO 2010/006325
PCT/US2009/050354
6
and hydrated alumina. Regardless of the form of aluminum trihydroxide raw
material, it is
preferred that it is mixed with water for introduction from tank 40 through
associated lines
41, with or without chemical stabilizers, to concentrations suitable for
storage and handling,
e.g., at least about 25%, and preferably at least about 65%, solids by weight.
[0026] As will be described, the concentration and flow rates will be
initially determined by
modeling to assure that the proper amount of chemical is supplied to the
correct location in the
combustor in the correct physical form to achieve the desired results of
reduced slagging and
ease of clean up. For use in the process, it is diluted as determined, e.g.,
by computational
fluid dynamics (CFD) to within the range of from about 0.1 to about 10 %, more
narrowly
from about 1 to about 5 %. When the aqueous aluminum trihydroxide contacts the
hot gases in
the combustor, it is believed to be reduced to very small particles, e.g.,
nano-sized particles,
e.g., under 200 nanometers and preferably below about 100 nanometers. Median
particle sizes
of from 50 to about 150 nanometers are useful ranges for the process of the
invention. To
approach this size, it is important that the ATH be introduced with water. The
small particles
are believed to disrupt the normal crystalline or glass that forms the slag.
Regardless of the
mechanism involved it is a distinct advantage of the invention that the slag
that does form is
highly friable and breaks easily with brushing and can be crushed by hand.
[0027] It is a significant advantage of the invention that the friability of
slag that is formed is
increased, making it easier to remove. The invention also slows or eliminates
the buildup of
slag. Advantageously, at high doses, the invention can actually remove slag
that has already
formed. By the term "increase the friability of the slag" it is meant that the
slag after treatment
requires less force per unit area to crush than slag formed under the same
conditions without
the treatment. By the term "remove slag" it is meant that the weight of the
slag adhering to
boiler, particularly heat exchange, surfaces is reduced from initial values by
the treatment of
the invention. There are several additional and attendant advantages of the
invention,
including the reduction of SO 3 for high sulfur coals, the reduction of the
pressure drop across
heat exchange apparatus, the ability to use lower cost coal, lower CO
generation, lower CO2
generation due to increased fuel consumption, better heat transfer, less down
time, higher
throughput, cleaning on line, cleaner heat exchange surfaces, ability to clean
the whole
combustor, and the ability to run at all loads with greater efficiency.
CA 02729959 2011-01-05
WO 2010/006325
PCT/US2009/050354
7
[0028] The process for most coals works best with a combination of ATH and
magnesium
hydroxide. While some coals, e.g., with low silicate compositions can be
burned with reduced
problems attributed to slag, the use of magnesium hydroxide, at least
initially, is preferred.
The magnesium hydroxide reagent can preferably be prepared from brines
containing calcium
and other salts, usually from underground brine pools or seawater. Dolomitic
lime is mixed
with these brines to form calcium chloride solution and magnesium hydroxide
which is
precipitated and filtered out of the solution. This fortn of magnesium
hydroxide can be mixed
with water, with or without stabilizers, to concentrations suitable for
storage and handling,
e.g., from 25 to 65% solids by weight. For use in the process, it is diluted
as determined by
computational fluid dynamics (CFD) to within the range of from 0.1 to 10%,
more narrowly
from Ito 5%. When it contacts the effluent in combustor, it is believed
reduced to nano-sized
particles, e.g., under 200 nanometers and preferably below about 100
nanometers. Median
particle sizes of from SO to about 150 nanometers are useful ranges for the
process of the
invention. Other forms of MgO can also be employed where necessary or desired,
e.g., "light
burn" or "caustic" can be employed where it is available in the desired
particle size range.
[0029] To best achieve these effects, the invention will preferably take
advantage of CFD to
project initial flow rates and select initial reagent introduction rates,
reagent introduction
location(s), reagent concentration, reagent droplet size and reagent momentum.
CFD is a well
understood science, and it is utilized with full benefit in this case, where
it is desired to supply
a minimum amount of chemical for maximum effect.
[0030] It is noted as highly significant that the amount of chemical will be
substoichiometric
in terms of affecting the fusion point of the slag ¨ often considered to be
the controlling factor
in slag control. According to the present invention, there is good evidence
besides the
relatively small amount of reagent employed that the results of the invention
are due to a
physical disruption of slag formation with possible boundary chemical and
kinetic effects not
explained by the literature.
[0031] Testing has shown that initial feed rates determined by CFD can be
utilized with good
effect and then adjusted based on observed results. As a guide to feed rates,
the initial feed
rate for the best economics for combustors operating similar to the one
exemplified below can
be up to about 6 pounds of ATH(as dry active ATH) or 8 pounds (as a 65-70%
slurry) per ton
CA 02729959 2011-01-05
WO 2010/006325
PCT/US2009/050354
8
of coal. For example, when added as a preferred 70% slurry, amounts of from
about Ito about
6 pounds of slurry will be effective (more narrowly, e.g., about 2 to about 3
pounds of slurry).
It is preferred to also use up to about 2 pounds of Mg(OH)2 slurry (at about
50 - 60% solids)
per ton of coal. For example, when added as a preferred 60% slurry, amounts of
from about
0.5 to about 2 pounds of Mg(OH)2 slurry per ton of coal, e.g., from about 0.7
to about 1
pounds of Mg(OH)2 slurry per ton of coal can be utilized. The slurries are
diluted as
necessary, typically to a solids concentration of from about 5% for smaller
applications to
about 35% or more.
[0032] The weight of the slag adhering to a combustor, particularly heat
exchange, surfaces is
effectively reduced from initial values by the treatment of the invention,
especially when the
ATH and Mg(OH)., are used at high concentrations within the above ranges,
e.g., from about 3
to 6 pounds of ATH per ton of coal and about 1 to 2 pounds of Mg(OH)7 per ton
of coal. This
ability to remove slag provides the ability to provide a cleaning and
maintenance regimen
wherein the initial dosing is as just mentioned for removing slag, with the
dosing then reduced
to from about 10 to about 50% of the initial values for maintaining the
combustor clean and
operating efficiently.
[0033] It is essential for optimum slag remediation according to the
invention, that the correct
initial concentrations, rates and introduction rates be calculated and
employed for the effective
physical form of aluminum trihydroxide, and preferably, optionally magnesium
hydroxide, to
be introduced into the hot combustion gases in chamber 20 to enable the
chemical to be added
with the desired effect. The implementation of CFD to the invention can be
accomplished as
set out in U. S. Patent No. 7,162,960 to Smyrniotis, et al. Particulate
removal equipment (not
shown) can be employed to remove particulates prior to passing the effluent up
the stack.
[0034] In another alternate form of the invention, combustion catalysts and or
effluent
treatment chemicals can be added to the fuel, combustion zone or otherwise as
described, for
example in U. S. Patent No. 7,162,960 to Smyrniotis, et al.
[0035] The following examples are presented to further explain and illustrate
the invention
and are not to be taken as limiting in any regard. Unless otherwise indicated,
all parts and
percentages are by weight.
CA 02729959 2011-01-05
WO 2010/006325
PCT/US2009/050354
9
Example 1
[0036] This example illustrates introduction of aluminum trihydroxide into a
furnace burning
540 tons of coal per day. The coal is a blend of Illinois basin and
Appalachian bituminous
coals, giving the following analysis as combined:
Sample
2 3
Moisture, % 11.28 10.85 10.19
Ash, % 14.91 13.63 13.91
Volatile Matter, % 36.03 35.04
Fixed Carbon, % 39.49 40.86
Total, % 100 100
Sulfur, % 3.95 4.44
HHV, BTU/lb 10,742 10,730
[0037] For the test Al(OH)3 (aluminum trihydroxide slurry or ATH for short) is
fed as a
70% by weight aqueous slurry at a rate of 5 pounds slurry per ton of coal
consumed from
two banks of three air-cooled nozzles positioned on the wall opposite of two
banks of
pulverized coal burners ¨ one bank at an elevation between the two burners and
one bank
at an elevation above the uppermost coal burners. The slurry is diluted to a
concentration
of 35 weight % ATH. The density of the ATH slurry before dilution is about 14
pounds/gallon, meaning that the feed rate is about 193 gallons per day (about
5 pounds per
ton of coal) for ATH slurry.
[0038] Based on this test, it is estimated that an effective feed rate for
this particular
combustor will be from about 1 to about 6 pounds of ATH slurry per ton of
coal, e.g.,
about 2 to about 3 pounds per ton.
CA 02729959 2012-12-12
50795-49
Example 2
[0039] This example illustrates the effect. of introducing Mg(OH)2 (magnesium
hydroxide)
into a furnace burning 540 tons of coal per day in addition to the aluminum
trihydroxide
fed in Example 1. The coal was a blend of Illinois basin and Appalachian
bituminous
coals, as illustrated in Example 1.
[0040] The magnesium hydroxide was fed as a slurry at 2 lbs of 50 to 60 weight
% slurry
per ton of coal consumed. Density of the magnesium hydroxide slurry was
approximately
12 lbs/gallon. Therefore, the feed rate was about 90 gallons per day for the
Mg(OH)2
slurry. As before, we fed the aluminum trihydroxide slurry at about 5 pounds
of slurry per
ton of coal consumed. The density of the ATH was about 14 pounds/gallon,
making the
feed rate about 193 gallons per day for ATH.
[0041] Based on this test, we estimate optimal feed rate for the best
economics for the this
particular combustor to be about 0.5 to about 2 pounds Mg(OH)2 slurry per ton
of coal
(e.g., about 1 pound per ton) plus from about 1 to about 6 pounds ATH slurry
per ton (e.g.,
about 2 to about 3 pounds per ton). Fig. 2 is a photograph of a slag sample
obtained after
operation for 24 hours of ATH feed only. The slag was unexpectedly friable.
[0042] The above description is for the purpose of teaching the person of
ordinary skill in the
art how to practice the invention. It is not intended to detail all of those
obvious modifications
and variations, which will become apparent to the skilled worker upon reading
the description.