Note: Descriptions are shown in the official language in which they were submitted.
CA 02732486 2011-02-23
RECOVERY METHOD FOR COBALT
TECHNICAL FIELD OF THE INVENTION
(0001) The present invention relates to a recovery method for cobalt. The
present invention relates to a recovery method for electrolytic cobalt from
cobalt solution produced as a solution after removing copper from acid
solution mainly containing copper by the combined method of solution
extraction and adsorption.
BACKGROUND OF THE INVENTION
(0002) Cobalt is used in alloy material and lithium-ion battery. Though it is
industrially important, the fact is that cobalt consumed in Japan is mostly
imported. Accordingly, even if it is low-concentrated, a recovering metal
cobalt from a cobalt-containing solution is significant.
(0003) Generally, cobalt is produced as a by-product of copper, nickel ore and
the like, and it is in the oxide form or sulfide form and it contains impurity
elements other than target metal. In the case of obtaining electrolytic cobalt
by electrowinning, it is known that quality of the electrolytic cobalt
deteriorates because of electrocrystallization of a metal, such as copper,
being
more base than cobalt if electrolysis solution contains the metal being more
base than cobalt. Accordingly, in order to obtain high-quality electrolytic
cobalt, it is necessary to remove impurity elements from electrolysis
solution.
(0004) As a general method for removing copper from a cobalt-containing acid
solution containing highly-concentrated copper, a method of sulfidizing
treatment and then sulfidizing precipitation is used. Copper can be
selectively removed by the method. However, in the method, toxic gas is
i
CA 02732486 2011-02-23
generated, medical agent is very expensive, recovering step for precipitated
copper is necessary and then the method has disadvantages on the cost front.
As the other methods, there is a method in which metal iron and metal
aluminum are added and copper in the solution is separated and removed as
metal by cementation. In the method, copper can be removed because it
reduces to metal easily. However, the solution is contaminated by added
metals and then it is necessary to remove the added metals.
(0005) Recently, copper can be-removed by solvent extraction as disclosed in
Japanese Patent Application Laid-open Publication No.11-50167 (patent
document 1).
(0006) However, the solvent extraction is used for a solution containing
relatively low-concentrated copper as impurities and the document does not
disclose a separation of copper from acid aqueous solution in which
concentration rate of Cu/Co is more or equal to 5 and copper is contained in
high concentration.
(Patent documents 1) Japanese Patent Application Laid-open Publication
No.11-50167
SUMMARY OF THE INVENTION
(0007) The present invention aims to recover highly-pure cobalt efficiently
from acid aqueous solution in which at least copper and cobalt are contained.
(0008) The inventors have studied to cope with the requirements, and have
found out the following inventions.
(1) A recovery method for cobalt, wherein copper is removed from acid
2
CA 02732486 2012-09-27
aqueous solution in which copper and cobalt are contained and concentration
ratio of Cu/Co is more or equal to 5, by a solvent extraction using cationic
exchange extracting agent and a subsequent adsorption using cationic
exchange resin, and then cobalt is recovered as electrolytic cobalt by a
solvent
extraction and a subsequent electrowinning, and
(1) the acid aqueous solution contains more or equal to 10g/ L of
copper and less or equal to 5g/L of cobalt,
(2) the cationic exchange extracting agent is an oxime series extract
agent, and
(3) the cationic exchange resin is an acid chelate resin.
(0009) (2) The recovery method for cobalt of (1), wherein the acid aqueous
solution derives from wet processing of copper ore and the solution contains
more or equal to 15g/ L of chlorine.
(0010) (3) The recovery method for cobalt of (1) or (2), wherein the
concentration of copper is reduced to a level in which the concentration ratio
of Cu/Co is less than 1 / 10000 in the copper-removed solution by the
combination treatment of the solvent extraction and the resin adsorption.
(0011) (4) The recovery method for cobalt of any one of (1) to (3), wherein
the
solvent extraction of copper is conducted at pH 1 to 3.
(0012) (5) The recovery method for cobalt of any one of (1) to (4), wherein
the
electrolytic copper is produced by an electrowinning of copper removed by the
solvent extraction.
(0013) (6) The recovery method for cobalt of any one of (1) to (5), wherein
the
3
CA 02732486 2011-02-23
acid aqueous solution contains calcium and/or zinc and the calcium and/or
zinc are removed by the solvent extraction at pH 1 to 3.
(0014) (7) The recovery method for cobalt of (6), wherein the solution is
passed through the resin without extra pretreatments after the solvent
extraction of copper or calcium and/or zinc.
ADVANTAGEOUS EFFECT OF THE INVENTION
(0015) The present invention can produce highly-pure cobalt at lower cost
than ever before from previously little-used acid aqueous solution in which a
concentration of copper is more or equal to 10g/L, a concentration of cobalt
is less or equal to 5g/L and concentration rate of Cu/Co is more or equal to
S.
Therefore, an industrial value of the present invention is very high.
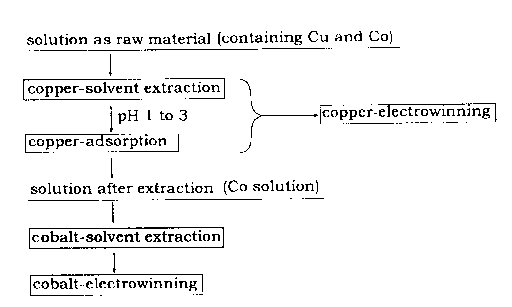
BRIEF DESCRIPTION OF DRAWINGS
Fig. 1 indicates a process flow chart of one embodiment of the present
invention.
Fig.2 indicates a process flow chart of another embodiment of the present
invention.
Fig.3 indicates effects of pH on extraction performance of copper on the basis
of results in example 1.
Fig.4 indicates effects of pH on extraction performance of calcium on the
basis of results in example 3.
Fig.5 indicates effects of pH on extraction performance of zinc on the basis
of
results in example 4.
4
CA 02732486 2011-02-23
DESCRIPTION OF THE PREFERRED EMBODIMENTS
(0016) An intended solution of the present invention is an acid aqueous
solution containing copper and containing cobalt in low concentration. More
specifically, it is an acid aqueous solution in which a concentration of
copper
is more or equal to lOg/L, a concentration of cobalt is less or equal to 5g/L
and concentration rate of Cu/Co is more or equal to 5.
Removing copper by solvent extraction
Procedures of the solvent extraction may comply with an ordinary method.
For example, the acid aqueous solution (aqueous phase) is contacted with an
oxime series extract agent (organic phase), they are agitated and mixed with a
mixer typically and then copper is reacted with the extract agent. In the
case of using LIX984N as the extract agent, an extract pH is 1.0 to 3.0,
preferably 2.0 to 3Ø The solvent extraction is preferably conducted in room
temperature (15 to 25 C) to 60 C and in atmosphere pressure in terms of
keeping qualities of the extract agent.
As the other extract agent, LIX84, LIX860, LIX984N and the like (trade
names by Henkel corporation), Acorga OPT5510 (trade name) which is
prepared by using 5-nonylsalicylaldoxime and the like can be used
particularly.
(0017) Removing copper by adsorption
In the solvent extraction, copper cannot be sufficiently removed and more
or equal to a few mg/L of copper remains in the solution after extraction.
Copper is metal that is more base than cobalt. If a cobalt electrolysis is
conducted to the solution after extraction, a low-grade electrolytic cobalt
containing copper is produced. Accordingly, it is necessary to conduct
adsorption after the solvent extraction and remove copper by the adsorption
CA 02732486 2011-02-23
to a level in which the concentration rate of Cu/Co is less or equal to
1 / 10000 in order to improve quality of electrolytic cobalt.
Procedures of the adsorption may comply with an ordinary method. For
example, column method may be used. An acidic chelate resin is filled in
column, the acid aqueous solution containing metal ions is passed through
the column and then copper is reacted with the resin. Contact temperature
with the resin is room temperature (for example, 15 to 25 C) to 100 C.
The chelate resin may be, for example, UR-10S or UR-40H (trade names by
Unitika corporation) that has iminodi acetic acid as functional group.
By the above method, copper can be recovered from the solution
sufficiently.
(0018) Recover of cobalt
Cobalt solution can be produced as the solution after extraction of copper
and it can be recovered by a combination of a solvent extraction and an
electrowinning.
(0019) Recover of electrolytic copper
Copper extracted into the organic phase by the solvent extraction is
back-extracted with sulfuric acid via simple washing and then copper sulfate
solution can be produced. Electrolytic copper can be produced by
electrolysis of the copper sulfate solution. The organic phase in which
copper is removed can be used in the solvent extraction repeatedly.
(0020) Removal of calcium and zinc
If the solution of the present invention contains calcium, it can be removed
by the solvent extraction. For example, the acid aqueous solution (aqueous
phase) containing calcium is contacted with non-chelated extract agent
6
CA 02732486 2011-02-23
(organic phase), they are agitated and mixed with a mixer typically and then
calcium is reacted with the extract agent. In the case of using DP-8R
(Daihachi Chemicals) as the non-chelated extract agent, an extract pH of
calcium is 1.0 to 3.0, preferably 1.5 to 3.0, and an extract pH of zinc is 1.0
to
3.0, preferably 2.0 to 3Ø In the light of degradation control of the extract
agent, the solvent extraction is preferably conducted at room temperature (15
to 25 C) to 60 C in atmosphere pressure.
The non-chelated extract agent other than those above may be, for example,
PC-88A (Daihachi Chemicals) that is an acidic phosphoric acid series extract
agent.
Examples
(0021) Examples of the present invention are described below. However, the
present invention is not limited to these examples.
(0022) Example 1: Removal method of copper by oxime series extract agent
The solvent extraction of copper, wherein LIX984N is used as the oxime
series extract agent, is described below as an example.
A solution containing 20g/L of copper and 1.5g/L of cobalt was prepared
and it was used as the solution before extraction.
LIX984N was diluted to 20 vol.% by adding Isoper M.
The solution before extraction and the extract agent were agitated together
in volume rate 1:2 at room temperature in atmosphere pressure for 15
minutes with change in pH 0.5 to 3, and then they were left at rest for 15
minutes for solid-liquid separation.
After the solid-liquid separation, concentrations of copper and cobalt in the
aqueous phase (the solution after extraction) were estimated. A result of this
example is shown in Figure 3.
7
CA 02732486 2011-02-23
(0023) As shown in Figure 3, cobalt was barely extracted and copper was
selectively extracted and separated in each pH, and specifically in more or
equal to pH 1.5, concentration of copper contained in the solution after
extraction was less or equal to 100mg/L. Accordingly, it is understood that
copper can be extracted and separated by controlling pH of the solution.
However, precipitates are produced in pH 4 or more and then copper can be
removed more effectively, preferably in pH 2.0 to 3Ø
(0024) Example 2: Adsorption of copper by acidic chelate resin
Two types of simulated solution, wherein one was conducted extraction of
Ca and Zn and the other was not conducted the extraction, were prepared as
the solution before adsorption. UR-10S (Unitika corporation) was used as
an acidic chelate resin. The resin was deaerated and 20mL of the deaerated
resin was filled in column, and then the solution was passed through the
column in the condition of LV 1. Concentrations of the solution before and
after adsorption are shown in Table 1.
8
CA 02732486 2011-02-23
(0025)
Table 1
Cu Co Zn Ca Cu/Co
m L m L m L m L
Solution before
adsorption
(without 800 1590 30.5 53.4 0.5
extraction of Ca
and Zn)
Solution after < 0.1 1310 28.6 53.2 < 0.0001
adsorption
Solution before
adsorption 60 1390 1.3 < 0.1 0.04
(with extraction
of Ca and Zn)
Solution after < 0.1 1110 0.8 < 0.1 < 0.0001
adsorption
(0026) As shown in this example, copper was adsorbed to the resin and
concentration ratio of Cu/Co was less than 1/10000. Accordingly, it is
understood that cobalt solution can be produced by removing copper with
resin adsorption even if the extraction of Ca and Zn is conducted.
(0027) Example 3: Removal method of calcium by solvent extraction
The solvent extraction of calcium, wherein DP-8R (Daihachi Chemicals) is
used as the non-chelated extract agent, is described below as an example.
A solution containing lg/L of calcium, 100mg/L of copper and 1.5g/L of
cobalt was prepared and it was used as the solution before extraction.
DP-8R was diluted to 20 vol.% by adding Isoper M. The solution before
extraction and the extract agent were agitated together in volume rate 1:1 at
room temperature in atmosphere pressure for 15 minutes with change in pH
0.5 to 3, and then they were left at rest for 15 minutes for solid-liquid
separation.
After the solid-liquid separation, concentrations of calcium, copper and
9
CA 02732486 2011-02-23
cobalt in the aqueous phase (the solution after extraction) were estimated. A
result of this example is shown in Figure 4.
(0028) As shown in Figure 4, cobalt was barely extracted and calcium was
selectively extracted and separated in each pH, and specifically in more or
equal to pH 1.0, concentration of calcium contained in the solution after
extraction was less or equal to 150mg/ L. Further, copper is also extracted
with calcium. Accordingly, purer cobalt solution can be produced. Calcium
can be removed more effectively, preferably in pH 1.5 to 3Ø
(0029) Example 4: Removal method of zinc by solvent extraction
The solvent extraction of zinc, wherein DP-8R (Daihachi Chemicals) is used
as the non-chelated extract agent, is described below as an example. A
solution containing 40mg/L of zinc, 400mg/L of calcium and 1OOmg/L of
copper was prepared and it was used as the solution before extraction.
DP-8R was diluted to 10 vol.% by adding Isoper M. The solution before
extraction and the extract agent were agitated together in volume rate 1:1 at
room temperature in atmosphere pressure for 15 minutes with change in pH
0.5 to 3, and then they were left at rest for 15 minutes for solid-liquid
separation. After the solid-liquid separation, concentrations of zinc, calcium
and copper in the aqueous phase (the solution after extraction) were
estimated. A result of this example is shown in Figure S.
(0030) As shown in Figure 5, zinc and calcium were extracted according to a
rise of pH, and specifically in more or equal to pH 2.0, concentration of zinc
contained in the solution after extraction was less or equal to 5mg/L, and
concentration of calcium contained in the solution after extraction was less
or equal to 1Omg/L. Accordingly, it is understood that zinc and calcium can
CA 02732486 2011-02-23
be extracted and separated by controlling pH of the solution. Further,
concentration of copper was less than 20mg/ L. In the case of recovering
cobalt by electrolysis, an existence of copper is undesirable and then it is
significant to remove copper in this step.
(0031) Example 5: Electrolysis method for cobalt
The electrolysis method for cobalt, wherein energization for 40 hours in the
condition of current density 200A/m2 to the solution produced by the method
conducted according to the inventions of claims 1 to 7, is described below as
an example. An assay result of electrolytic cobalt produced by the referential
or experimental values of quality is shown in Table 2.
(0032)
Table 2
Co Fe Cu Ni Zn
Liquid
composition 49.4 < 0.01 < 0.001 0.02 < 0.001
before electrolysis
(g/L)
Quality of
electrolytic cobalt 99.8 < 0.01 < 0.01 < 0.15 < 0.005
referential
Quality of
electrolytic cobalt 99.9 < 0.01 < 0.01 < 0.01 < 0.001
(experimental)
(The experimental value of cobalt was calculated by subtracting percentage of
impurities from 100%.)
(0033)
As shown in this example 5, highly-pure electrolytic cobalt, wherein
impurities are very few, was produced by removing impurities by conducting
the method of the inventions of claims 1 to 7.
11