Note: Descriptions are shown in the official language in which they were submitted.
CA 02734549 2011-02-16
- 1 -
Anti-idiotypic antibodies which neutralize the inhibitory
activity of an inhibitory antibody directed against the
Cl domain of Factor VIII
Prior art and introduction
The present invention is related to a monoclonal
anti-idiotypic antibody directed against a Factor VIII
inhibitory antibody which binds to the C1 domain of
Factor VIII, as well as to a cell line producing this
monoclonal anti-idiotypic antibody , to the use of this
monoclonal anti-idiotypic antibody as medicament, and
more particularly, to the use thereof for manufacturing a
medicament for the treatment of haemophilia A.
Haemophilia A is a hereditary disease linked to an
anomaly of chromosome X, which displays itself in the
affected person by an inability to coagulate. This
disease is the result of mutations on the gene of a
protein involved in coagulation, the Factor VIII (FVIII)
protein, which determine either a total absence of Factor
VIII in the blood, or a partial deficit thereof.
Haemophilia A is the most common of insufficiencies
affecting blood coagulation : in France 1 male in 5000 is
affected, which represents 80 % of patients suffering
from haemophilia. The other type of haemophilia,
haemophilia B, affects 20 % of patients suffering from
haemophilia ; it is caused by a deficiency in an other
clotting factor, known as Factor IX.
Present treatment of heamophilia (type A or B)
consists of intravenous administration of the deficient
or absent clotting factor. In France, Factor VIII for the
treatment of haemophiliacs is available in form of blood
derived medicaments provided by the Laboratoire Frangais
du Fractionnement et des Biotechnologies (LFB) or by
international pharmaceutical laboratories, as well as in
CA 02734549 2011-02-16
- 2 -
form of recombinant medicaments prepared by genetic
engineering methods. Effectively, the DNA coding Factor
VIII has been isolated and expressed in mammalian cells
(Wood et al., Nature (1984) 312 : 330-337), and its amino
acid sequence was deduced from cDNA.
Secreted Factor VIII (FVIII) is a glycoprotein with
a molecular weight of 300 Kda (2332 amino acids), and
plays a key role in the activation of intrinsic
coagulation pathway. Inactive FVIII consists of six
regions : Al (residues 1-372), A2 (residues 373-740), B
(residues 741-1648), A3 (residues 1690-2019), Cl
(residues 2020-2172) and C2 (residues 2173-2332), from
the N-terminal extremity to the C-terminal extremity.
After being secreted, FVIII interacts with the von
Willebrand Factor (vWF), which protects the FVIII against
plasma proteases. FVIII dissociates from vWF upon
cleavage by thrombin. This cleavage results in the
elimination of the B domain and the formation of a
heterodimer. FVIII circulates in plasma in this form.
This heterodimer consists of a heavy chain (Al, A2) and
of a light chain (A3, Cl, C2).
When FVIII is infused to a haemophiliac patient, it
binds to the von Willebrand Factor in the blood
circulation of the patient. Activated Factor VIII acts as
a co-factor of activated Factor IX, accelerating the
conversion of Factor X into activated Factor X. Activated
Factor X converts prothrombin into thrombin. Then the
thrombin converts fibrinogen into fibrin, and clotting
occurs.
The major problem encountered with Factor VIII
administration is the appearance of antibodies directed
against Factor VIII in the patient, referred to as
inhibiting antibodies >>. These antibodies neutralize
the procoagulant activity of Factor VIII, which is
inactivated as soon as infused. Thus, the administered
CA 02734549 2011-02-16
- 3 -
clotting factor is destroyed before bleeding can be
stopped, which leads to a serious complication thus
causing the treatment to be ineffective. Further, some
genetically non-haemophiliac patients may develop
inhibitors against endogenous Factor VIII : this is
called acquired haemophilia.
Studies have shown that the anti-Factor VIII immune
response is of the polyclonal IgG type belonging mostly
to the IgG4 and IgGl sub-class, and more rarely to IgG2.
The IgG3 subclass is never represented. The light chain
is often of Kappa type. The overrepresentation of IgG4 is
more pronounced with heamophiliacs having a long-term
established inhibitor. The C2 and A2 domains of the FVIII
molecule are the favoured targets of the immune response
although, in some cases, antibodies directed against the
A3 domain are detected. When plasma of haemophiliac
patients is passed through an immunoadsorption column
with immobilized FVIII, it is possible to purify total
anti-FVIII antibodies. The recovered amounts are often
higher than 100 pg per 10 mg of total IgGs (Gilles JG et
al. (1993) Blood ; 82 : 2452-2461) . An animal model has
been developed to study the formation of inhibitors of
Factor VIII ; rats immunized with human recombinant
Factor VIII show a rapid immune response of the
polyclonal type (Jarvis et al., Thromb Haemost. 1996 Feb
; 75(2):318-25). The mechanisms by which anti-Factor VIII
antibodies interfere with function of Factor VIII are
numerous, and include interference with the proteolytic
cleavage of Factor VIII and with the interaction of
Factor VIII with different partners, such as von
Willebrand Factor (vWF), phospholipids (PL), Factor IX,
activated Factor X (FXa) or APC (Activated Protein C).
Several treatments allowing attenuation of the
consequences of this immune response are available, such
as for example treatments involving desmopressin, which
CA 02734549 2011-02-16
- 4 -
is a synthetic hormone stimulating the production of
Factor VIII, coagulation promoting agents, such as
concentrates of prothrombin complexes or concentrates of
activated prothrombin complexes, recombinant Factor VIIa,
plasmapheresis and infusions of large or intermediary
amounts of Factor VIII. Nevertheless, these methods are
very expensive and of low efficacy.
Because of the complexity of the in vivo analysis
of this immune polyclonal response, monoclonal antibodies
directed against certain domains of Factor VIII have been
isolated by some research teams. Thus, a human monoclonal
antibody of the IgG4kappa type, LE2E9, has been isolated.
This antibody is directed against the Cl domain of Factor
VIII and inhibits the cofactor activity of Factor VIII
and its binding to the von Willebrand Factor (Jacquemin
et al., (2000) Blood 95:156-163). In the same way, a
human monoclonal antibody directed against the C2 domain
of Factor VIII, referred to as BO2C11 (IgG4kappa),
produced from a library of memory B cells of a patient
suffering from haemophilia A with inhibitors, has been
isolated (Jacquemin et al., Blood 1998 Jul 15;92 (2):496-
506). BO2C11 recognizes the C2 domain of Factor VIII, and
inhibits its binding to von Willebrand Factor and to
phospholipids. It completely inhibits the procoagulation
activity of native and activated Factor VIII. A further
example of monoclonal antibody is the BOIIB2 antibody
directed against the A2 domain of Factor VIII. The BOIIB2
antibody inhibits 99 % of Factor VIII activity. By
binding to the A2 domain, it can interfere with and
inhibit the binding of FIXa, which contains a low
affinity binding site within this region of FVIII, and
thus inhibits the enzyme activity of FIXa. The second
conceivable way of action is its interference with the
equilibrium between the heterodimeric form (A2:A1 and
A3:C1:C2) of FVIII and the heterotrimeric form (A2 and Al
CA 02734549 2011-02-16
- 5 -
and A3:C1:C2) of FVIII by accelerating the dissociation
of the A2 domain of these complexes, rendering them non-
functional. (Ananyeva NM et al., (2004) Blood Coagul
Fibrinolysis. Mar. 15(2):109-24. Review).
With the aid of these new tools, a further, more
recent strategic struggle against the Factor VIII
inhibitor antibodies has considered administering anti-
idiotypic antibodies (antibodies having the ability to
interact with the variable region of other antibodies)
neutralizing the inhibitor antibodies (Saint-Remy JM at
al., (1999) Vox Sang ; 77 (suppl 1) : 21-24). A mouse
anti-idiotypic antibody, known as 14C12, disclosed in the
document WO 2004/014955, neutralizes in vivo, in a dose-
dependent manner, the inhibitory properties of the anti-
Factor VIII target antibody (monoclonal antibody BO2C11),
which is directed against the C2 domain of Factor VIII.
The anti-Factor VIII immune response being polyclonal,
mouse anti-idiotypic antibodies directed against the A2
domain of Factor VIII have also been developed (and
described in the patent application FR 05 08320). The A2
domain is a domain of 43 kD, the function of which is not
well known but it has been demonstrated that inhibitory
antibodies directed against the A2 domain of Factor VIII
inhibit the function of Factor VIIIa by inhibiting the
conversion of the complex FXase/FX in the transition
state (Lollar et al., J Clin Invest. 1994 Jun;
93(6):2497-504, Fay et al., J Biol Chem. 1996 ; 271(11)
6027-6032).
However, the immune response directed against
Factor VIII is polyclonal, and, therefore, implies that
inhibitory antibodies are directed against domains
different from the A2 and C2 domains. Indeed, even if the
study of epitopic specificities of anti-Factor VIII
antibodies has shown that the majority of the inhibitors
recognize limited zones of the Factor VIII molecule,
CA 02734549 2011-02-16
- 6 -
located on the A2 domain of the heavy chain and/or on the
C2 domain of the light chain, other epitopes are
sometimes recognized. Indeed, some plasmas from patients
contain antibodies capable to bind to the C1 domain of
the light chain of Factor VIII (Moreau et al., 2000 ;
95(11) :3435-441 ; Jacquemin et al,. 2000 ; 95(1) :156-
162).
Thus, there is always a need for further tools
enabling neutralization of other Factor VIII inhibitory
antibodies directed against other domains of Factor VIII,
in order to more completely neutralize the anti-Factor
VIII polyclonal responses of haemophiliac patients.
Thus, the Applicant has attempted to develop a
novel tool for treating haemophilia A enabling
neutralization of inhibitory antibodies directed against
the Cl domain of Factor VIII.
DETAILED DESCRIPTION OF THE INVENTION
Thus, a first object of the invention relates to a
monoclonal anti-idiotypic antibody directed against a
Factor VIII human inhibitory antibody, the inhibitory
antibody being directed against the C1 domain of Factor
VIII, this anti-idiotypic antibody having at least one
CDR region (Complementarity Determining Region) of each
of the light chains of said antibody, in which the
peptide sequence has at least 70% identity to a sequence
selected from the sequences SEQ ID NO : 12, SEQ ID NO :
13, SEQ ID NO : 14 and at least one CDR region of each of
the heavy chains of said antibody, in which the peptide
sequence has at least 70% identity to a sequence selected
from the sequences SEQ ID NO : 9, SEQ ID NO : 10, SEQ ID
NO : 11.
The concerned CDR regions are the CDR1 and/or CDR2
and/or CDR3 regions.
The sequences SEQ ID NO : 9, SEQ ID NO : 10, SEQ ID
CA 02734549 2011-02-16
- 7 -
NO : 11, SEQ ID NO : 12, SEQ ID NO : 13 and SEQ ID NO
14, are defined according to Kabat [Kabat et al.,
"Sequences of Proteins of Immunological Interest", NIH
Publication, 91-3242 (1991)].
In a particularly advantageous embodiment, the
identity with each of the above-mentioned sequences is at
least 800, preferably at least 90%, 95%, 99%, and more
preferably 100%. The percentage of identity is calculated
by aligning the two sequences to be compared and by
counting the number of positions having an identical
amino acid, this number being divided by the total number
of amino acids of the sequence. In any case, these
sequence differences do not affect at all either the
affinity of the monoclonal antibody for its target, or
its functionality.
Inhibitory antibodies >> or o inhibitors >> of
Factor VIII refers to antibodies which inhibit all or a
part of the procoagulant activity of Factor VIII, namely
by binding thereto, and particularly an anti-Factor VIII
antibody the epitope of which is located on Factor VIII.
Advantageously, the antibody of the invention has the
ability to neutralize at least 20%, advantageously at
least 30%, advantageously at least 40%, advantageously at
least 50%, advantageously at least 60%, and in an even
more advantageous way, at least 70%, 80%, 90%, 99% or
100% of the coagulation inhibitory activity of inhibitory
antibodies directed against the Cl domain of Factor VIII,
which are the targets of the anti-idiotypic monoclonal
antibodies of the invention. This ability to neutralize
the coagulation inhibitory activity of inhibitory
antibodies can be determined by measuring the activity of
Factor VIII in the presence of an inhibitory antibody and
of an anti-idiotypic antibody in an assay such as the
Factor VIII chromogen test >> (Jacquemin et al. (1998)
Blood 92. 494-506).
CA 02734549 2011-02-16
- 8 -
The expression o anti-idiotypic antibody >> refers
to an antibody directed against the variable region of
the target inhibitory antibodies. In a particular aspect
of the invention, the anti-idiotypic antibody of the
invention is directed against inhibitory antibodies, of
which the variable domain of the heavy chain of said
antibody is related to the germ line DP-10. Such
inhibitory antibodies can be obtained from humans (for
example from serum of patients containing inhibitory
antibodies) or other animal species such as mouse, horse,
goat, non-human primates, taken from a non-limiting list,
by immunization with Factor VIII or fragments derived
from Factor VIII, and more particularly with a fragment
comprising all or a part of the Cl domain.
Advantageously, the target inhibitory antibody of
the anti-idiotypic antibody of the invention recognizes
the Cl domain in its native configuration.
Advantageously, the target inhibitory antibody of the
anti-idiotypic antibody of the invention does not
recognize the same domain being a R2150H mutation.
The monoclonal anti-idiotypic antibody of the
invention can be of human or animal origin. In addition,
it can be obtained using a variety of different methods.
For example, cells producing anti-idiotypic antibodies
can be obtained from peripheral blood lymphocytes of
patients having anti-Factor VIII inhibitory antibodies or
from healthy persons. These cells can be immortalized by
use of techniques well known to those skilled in the art
and selected with regard to the ability of the produced
anti-idiotypic antibodies to neutralize inhibitory
antibodies directed against Factor VIII. A further method
for producing the monoclonal anti-idiotypic antibody of
the invention is through the immunization of animals,
advantageously mice, by injection of Factor VIII
inhibitory antibodies directed against the Cl domain of
CA 02734549 2011-02-16
- 9 -
Factor VIII, then by fusion of spleen lymphocytes with a
myeloma cell line, advantageously mouse myeloma, followed
by the identification and the cloning of cell cultures
producing the anti-idiotypic antibodies directed against
the Factor VIII inhibitory antibodies.
In a preferred embodiment of the invention, each
CDR region of the light chains of the anti-idiotypic
antibody of the invention contains a peptide sequence
having at least 70% identity with the sequences
respectively identified as SEQ ID NO : 12, SEQ ID NO : 13
and SEQ ID NO : 14, and each CDR region of each of the
heavy chains of said antibody contains a peptide sequence
having at least 70% identity with the sequences
respectively identified as SEQ ID NO : 9, SEQ ID NO : 10
and SEQ ID NO : 11.
Thus, the CDRl region of each of the light chains
of the antibody of the invention contains a peptide
sequence having at least 70% identity to the sequence SEQ
ID NO : 12, the latter having the amino acid sequence Arg
Ala Ser Ser Ser Val Ser Tyr Met Asn, the CDR2 region of
each of the light chains of the antibody of the invention
contains a peptide sequence having at least 70% identity
to the sequence SEQ ID NO : 13, the latter having the
amino acid sequence Ala Thr Ser Asn Leu Ala Ser, the CDR3
region of each of the light chains of the antibody of the
invention contains a peptide sequence having at least 70%
identity to the sequence SEQ ID NO : 14, the latter
having the amino acid sequence Gln Gln Trp Ser Ser Asn
Pro Pro Met Leu Thr, and the CDR1 region of each of the
heavy chains of the antibody of the invention contains a
peptide sequence having at least 70% identity to the
sequence SEQ ID NO : 9, the latter having the amino acid
sequence Gly Tyr Thr Phe Thr Thr Tyr Trp Met His, the
CDR2 region of each of the heavy chains of the antibody
of the invention contains a peptide sequence having at
CA 02734549 2011-02-16
- 10 -
least 70% identity to the sequence SEQ ID NO : 10, the
latter having the amino acid sequence Tyr Ile Asn Pro Thr
Ser Gly Tyr Thr Glu Tyr Asn Gln Asn Phe Lys Asp, and the
CDR3 region of each of the heavy chains of the antibody
of the invention contains a peptide sequence having at
least 70% identity to the sequence SEQ ID NO : 11, the
latter having the amino acid sequence Ser Gly Ala Tyr Tyr
Arg Tyr Asp Asp Ala Met Asp Ser. In a particularly
advantageous way, the identity to each of the above-
mentioned sequences is at least 80%, preferably at least
90%, 95%, 99% and more preferably 100%.
Advantageously, the variable region of each of the light
chains of the monoclonal anti-idiotypic antibody of the
invention is coded by a nucleic acid sequence having at
least 70% identity to the nucleic acid sequence SEQ ID
NO : 16, the latter having the following nucleic acid
sequence :
caaattgttc tctcccagtc tccagcaatc ctgtctgcat ctccagggga
gaaggtcaca atgacttgca gggccagctc aagtgtaagt tacatgaact
ggtatcagca gaagccagga tcctccccca aaccctggat ttatgccaca
tccaacctgg cttctggagt ccctgctcgc ttcagtggca gtgggtctgg
gacctcttat tctctcacaa tcagcagagt ggaggctgaa gatgctgcca
cttattactg ccagcagtgg agtagtaacc cacccatgct cacgttcggt
gctgggacca agctggagct gaaac, and the variable region of
each of the heavy chains of the monoclonal anti-idiotypic
antibody is coded by a nucleic acid sequence having at
least 70% identity to the nucleic acid sequence SEQ ID
NO : 15, the latter having the following nucleic acid
sequence :
caggtccagc ttcagcagtc tggggctgaa ctggcaaaac ctggggcctc
agtgaagatg tcctgcaagg cttctggcta cacctttact acctactgga
tgcactggat aaaacagagg cctggacagg atctggaatg gattggatac
attaatccta cctctggtta tactgagtac aatcagaact tcaaggacaa
ggccacattg actgcagaca aatcctccag cacagcctac atgcaactga
acagcctgac atctgaggac tctgcagtct atttctgtgc aagatcgggg
CA 02734549 2011-02-16
- 11 -
gcctactata ggtacgacga tgctatggac tcctggggtc aaggaacctc
agtcaccgtc tcctcag.
For the purposes of the invention, a signal peptide
can be added to sequences SEQ ID NO : 15 and SEQ ID NO :
16 to yield respectively, for example, the sequences SEQ
ID NO : 1 and SEQ ID NO : 2, wherein neither the activity
nor the specificity of the antibody of the invention are
affected by such a signal peptide.
The sequence of SEQ ID NO : 1 correspond to the following
nucleic acid sequence :
atgggatgga gctggatctt tctcttcctg ttttcagtaa ctgcaggtgt
ccactcccag gtccagcttc agcagtctgg ggctgaactg gcaaaacctg
gggcctcagt gaagatgtcc tgcaaggctt ctggctacac ctttactacc
tactggatgc actggataaa acagaggcct ggacaggatc tggaatggat
tggatacatt aatcctacct ctggttatac tgagtacaat cagaacttca
aggacaaggc cacattgact gcagacaaat cctccagcac agcctacatg
caactgaaca gcctgacatc tgaggactct gcagtctatt tctgtgcaag
atcgggggcc tactataggt acgacgatgc tatggactcc tggggtcaag
gaacctcagt caccgtctcc tcag.
The sequence of SEQ ID NO : 2 correspond to the following
nucleic acid sequence :
atggattttc aggtgcagat tttcagcttc ctgctattca gtgcctcagt
cataatgtcc agaggacaaa ttgttctctc ccagtctcca gcaatcctgt
ctgcatctcc aggggagaag gtcacaatga cttgcagggc cagctcaagt
gtaagttaca tgaactggta tcagcagaag ccaggatcct cccccaaacc
ctggatttat gccacatcca acctggcttc tggagtccct gctcgcttca
gtggcagtgg gtctgggacc tcttattctc tcacaatcag cagagtggag
gctgaagatg ctgccactta ttactgccag cagtggagta gtaacccacc
catgctcacg ttcggtgctg ggaccaagct ggagctgaaa c.
In a particularly advantageous way, the sequence
identity is at least 80%, and preferably from at least 95
to 99%. The percentage of identity is calculated by
alignment of 2 sequences to be compared and by counting
the number of positions containing an identical
CA 02734549 2011-02-16
- 12 -
nucleotide, this number is divided by the total number of
nucleotides of the sequence. Genetic code degeneration
leads to the fact that the same amino acid can be coded
by several triplets of different nucleotides. In any
case, neither the affinity of the monoclonal antibody for
its target nor its ability to neutralize the inhibitory
activity of the target inhibitory antibodies are at all
affected by these sequence differences.
In a preferred aspect of the invention, the
variable region of each of the light chains of the
monoclonal anti-idiotypic antibody is coded by the
nucleic acid sequence SEQ ID NO : 16, and the variable
region of each of the heavy chains of the monoclonal
anti-idiotypic antibody is coded by the nucleic acid
sequence SEQ ID NO : 15.
In an advantageous manner, the peptide sequence of each
of the variable regions of the light chains of the
antibody of the invention is a sequence having at least
70% identity, and advantageously at least 80% or 90%, and
yet more advantageously at least 99% identity to the
sequence SEQ ID NO : 18, the latter having the following
amino acid sequence
Gln Ile Val Leu Ser Gln Ser Pro Ala Ile Leu Ser Ala Ser
Pro Gly Glu Lys Val Thr Met Thr Cys Arg Ala Ser Ser Ser
Val Ser Tyr Met Asn Trp Tyr Gln Gln Lys Pro Gly Ser Ser
Pro Lys Pro Trp Ile Tyr Ala Thr Ser Asn Leu Ala Ser Gly
Val Pro Ala Arg Phe Ser Gly Ser Gly Ser Gly Thr Ser Tyr
Ser Leu Thr Ile Ser Arg Val Glu Ala Glu Asp Ala Ala Thr
Tyr Tyr Cys Gln Gln Trp Ser Ser Asn Pro Pro Met Leu Thr
Phe Gly Ala Gly Thr Lys Leu Glu Leu Lys.
For the purposes of the invention, a signal peptide
can be added to the sequences SEQ ID NO : 17 and SEQ ID
NO : 18 in order to yield, for example, respectively, the
sequences SEQ ID NO : 3 and SEQ ID NO : 4, neither the
activity nor the specificity of the antibody of the
CA 02734549 2011-02-16
- 13 -
invention are affected at by such a signal peptide.
Advantageously, the peptide sequence of each of the
variable regions of the heavy chains of the antibody of
the invention is a sequence having at least 70% identity,
and advantageously at least 80% or 90%, and yet more
advantageously at least 99% identity to the sequence SEQ
ID NO : 3, the latter having the following amino acid
sequence :
Met Gly Trp Ser Trp Ile Phe Leu Phe Leu Phe Ser Val Thr
Ala Gly Val His Ser Gln Val Gln Leu Gln Gln Ser Gly Ala
Glu Leu Ala Lys Pro Gly Ala Ser Val Lys Met Ser Cys Lys
Ala Ser Gly Tyr Thr Phe Thr Thr Tyr Trp Met His Trp Ile
Lys Gln Arg Pro Gly Gln Asp Leu Glu Trp Ile Gly Tyr Ile
Asn Pro Thr Ser Gly Tyr Thr Glu Tyr Asn Gln Asn Phe Lys
Asp Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser Thr Ala
Tyr Met Gln Leu Asn Ser Leu Thr Ser Glu Asp Ser Ala Val
Tyr Phe Cys Ala Arg Ser Gly Ala Tyr Tyr Arg Tyr Asp Asp
Ala Met Asp Ser Trp Gly Gln Gly Thr Ser Val Thr Val Ser
Ser.
In a particularly advantageous manner, the peptide
sequence of each of the light chains of the antibody of
the invention is a sequence having at least 70% identity,
and advantageously at least 80% or 90%, and yet more
advantageously at least 99% identity to the sequence SEQ
ID NO : 4, and the peptide sequence of each of the heavy
chains of the antibody of the invention is a sequence
having at least 70% identity, and advantageously at least
80% or 90%, and yet more advantageously, at least 99%
identity to the sequence SEQ ID NO : 3.
Preferably, the peptide sequence of each of the light
chains of the antibody of the invention is the sequence
SEQ ID NO : 4, the latter having the following amino acid
sequence
Met Asp Phe Gln Val Gln Ile Phe Ser Phe Leu Leu Phe Ser
Ala Ser Val Ile Met Ser Arg Gly Gln Ile Val Leu Ser Gln
CA 02734549 2011-02-16
- 14 -
Ser Pro Ala Ile Leu Ser Ala Ser Pro Gly Glu Lys Val Thr
Met Thr Cys Arg Ala Ser Ser Ser Val Ser Tyr Met Asn Trp
Tyr Gln Gln Lys Pro Gly Ser Ser Pro Lys Pro Trp Ile Tyr
Ala Thr Ser Asn Leu Ala Ser Gly Val Pro Ala Arg Phe Ser
Gly Ser Gly Ser Gly Thr Ser Tyr Ser Leu Thr Ile Ser Arg
Val Glu Ala Glu Asp Ala Ala Thr Tyr Tyr Cys Gln Gln Trp
Ser Ser Asn Pro Pro Met Leu Thr Phe Gly Ala Gly Thr Lys
Leu Glu Leu Lys.
Preferably, the peptide sequence of each of the
light chains of the antibody of the invention is the
sequence SEQ ID NO : 3.
The peptide sequence deduced from the sequence SEQ ID
NO : 16 is the sequence SEQ ID NO : 18, and the peptide
sequence deduced from the sequence SEQ ID NO : 15 is the
sequence SEQ ID NO : 17, the latter having the following
amino acid sequence
Gln Val Gln Leu Gln Gln Ser Gly Ala Glu Leu Ala Lys Pro
Gly Ala Ser Val Lys Met Ser Cys Lys Ala Ser Gly Tyr Thr
Phe Thr Thr Tyr Trp Met His Trp Ile Lys Gln Arg Pro Gly
Gln Asp Leu Glu Trp Ile Gly Tyr Ile Asn Pro Thr Ser Gly
Tyr Thr Glu Tyr Asn Gln Asn Phe Lys Asp Lys Ala Thr Leu
Thr Ala Asp Lys Ser Ser Ser Thr Ala Tyr Met Gln Leu Asn
Ser Leu Thr Ser Glu Asp Ser Ala Val Tyr Phe Cys Ala Arg
Ser Gly Ala Tyr Tyr Arg Tyr Asp Asp Ala Met Asp Ser Trp
Gly Gln Gly Thr Ser Val Thr Val Ser Ser. Preferably, the
variable region of each of the light chains of the
monoclonal anti-idiotypic antibody of the invention has
the peptide sequence SEQ ID NO : 18, and the variable
region of each of the heavy chains of the monoclonal
anti-idiotypic antibody of the invention has the peptide
sequence SEQ ID NO : 17.
In a preferred manner, the target inhibitory
antibody of the anti-idiotypic antibody of the invention
is the antibody RHD5 deposited at the Belgian Co-
ordinated Collections of Microorganisms/Plasmid
CA 02734549 2011-02-16
- 15 -
Collection (BCCM/LMBP), Laboratorium voor Moleculaire
Biologie, University of Ghent, Technologiepark 297, B-
9052 Zwijnaarede, Belgium, in August 2004, by the Collen
Research Foundation, under the accession number LMBP
6165CB. This antibody, as well as its nucleotide and
peptide sequences are described in the patent application
WO 2005/016455. The antibody RHD5 is a human monoclonal
IgGl antibody directed against the Cl domain of Factor
VIII produced initially from lymphocytes of a patient
suffering from haemophilia A, namely an acquired severe
haemophilia A with a high level of inhibitors. This
antibody belongs to the sub-class IgGl, and originates
from the germ line DP-10. The epitope recognized by said
antibody on Factor VIII is the Cl domain in its native
configuration, but not the same domain with a R2150H
mutation. The antibody RHD5 can inhibit up to 98% of
Factor VIII activity.
The antibody of the invention refers also to any
modified antibody having the features of the invention,
in which one or more amino acid(s) have been substituted
or deleted. Such a substitution or deletion can be
located on any position in the molecule. In the case
where several amino acids have been substituted or
deleted, any combination of substitution or deletion can
be considered. Such sequence modifications of the
variable regions of the antibody of the invention can be
carried out in order to increase the number of residues
likely to come into contact with the anti-idiotypic
antibody of the invention and with the target inhibitor
antibody.
In one embodiment of the invention, the anti-
idiotypic antibody is a mouse antibody.
Advantageously, this mouse monoclonal anti-
idiotypic antibody is a IgGlkappa.
Preferably, the monoclonal antibody of the
CA 02734549 2011-02-16
- 16 -
invention is a chimeric antibody. By the expression
Chimeric antibody >> it is to be understood that it
refers to an antibody in which the variable regions of
the light chains and of the heavy chains belong to a
different species than the constant regions of the light
chains and of the heavy chains. Thus, the antibody of the
invention, also contains the constant regions of light
and heavy chains belonging to a non-murine species. In
this regard, all non-murine mammalian families and
species are capable of being used, and in particular, for
example, man, monkey, muridae (except the mouse), suidae,
bovidae, equidae, felidae, canidae, as well as birds.
The chimeric antibodies of the invention can be
constructed using standard techniques for recombinant
DNA, well known by those skilled in the art, and more
particularly through the use of the o chimeric >> antibody
construction techniques described, for example by
Morrison et al., Proc. Natl. Acad. Sci. U.S.A., 81. pp.
6851-55 (1984), where use is made of recombinant DNA
technology to replace the constant region of a heavy
chain and/or the constant region of a light chain of an
antibody originating from a non-human mammal with the
corresponding regions of a human immunoglobulin.
In a particular aspect of the invention, the
antibody of the invention is a human hybrid antibody,
that is to say a chimeric antibody, the constant part of
which is human. This embodiment of the invention enables
a reduction in the immunogenicity of the antibody in
humans, and thereby improves its efficacy upon
therapeutic administration to man.
Advantageously, the antibody of the invention is a
humanized antibody. Such an antibody can be obtained by
association of one or more CDR region(s) (Complementarity
Determining Region) of a monoclonal antibody of a non-
human species with human framework regions (highly
CA 02734549 2011-02-16
- 17 -
conserved regions of variable regions, known as
frameworks), such a manufacturing process being taught in
the state of the art (Jones et al., Nature (1986)
321:522 ; Riechmann et al., Nature (1988) 332:323). Such
a humanized antibody directed against the variable domain
of inhibitory antibodies recognizing the Cl domain of
FVIII can contain human framework regions and one or more
CDR regions of the sequences SEQ ID NO : 9, SEQ ID NO :
10, SEQ ID NO : 11, SEQ ID NO : 12, SEQ ID NO : 13, SEQ
ID NO : 14. A particular humanized antibody of the
invention is a humanized antibody directed against the
variable domain of inhibitory antibodies recognizing the
Cl domain of FVIII, the CDR regions of which are regions
of sequence SEQ ID NO : 9, SEQ ID NO : 10, SEQ ID NO
11, SEQ ID NO : 12, SEQ ID NO : 13, SEQ ID NO : 14.
In an advantageous way, the monoclonal anti-
idiotypic antibody of the invention is the antibody 18B6
produced by the hybridoma 18B6 deposited under the
registration number CNCM 1-3559, on January 24th, 2006,
at the Collection Nationale de Cultures de
Microorganismes (CNCM, 25 rue du Docteur Roux, 75724
Paris Cedex 15). The variable region of each of the light
chains of the monoclonal anti-idiotypic antibody 18B6 is
coded by the nucleic acid sequence SEQ ID NO : 16, and
the variable region of each of the heavy chains of the
monoclonal anti-idiotypic antibody 18B6 is coded by the
nucleic acid sequence SEQ ID NO : 15. The method for
obtaining the hybridoma 18B6 is described in the
Examples >> section of the present document.
The monoclonal anti-idiotypic antibody of the
invention refers also to any antibody comprising
fragments of the antibody 18B6, and more particularly any
antibody comprising the variable region of the light
chain and/or the variable region of the heavy chain of
the antibody 18B6, or any fragment of the variable region
CA 02734549 2011-02-16
- 18 -
of the light chain and/or the variable region of the
heavy chain of the antibody 18B6. By the expression
Fragments >>, it is meant a F(ab')2 fragment or a Fab'
fragment or a Fab fragment or a CDR region or any
modified version of any of these fragments or region.
In a particular embodiment of the invention, the
monoclonal anti-idiotypic antibody of the invention is a
F(ab')2 fragment or a Fab' fragment or a Fab fragment or
a CDR region or any modified version of any of these
fragments or region. The enzymatic digestion of
immunoglobulins with papain generates 2 identical
fragments called o Fab fragment >> (Fragment Antigen
Binding), and a Fc fragment (crystallizable fraction).
The Fc fragment is the support for the effector functions
of immunoglobulins.
Using pepsin digestion, a F(ab')2 fragment is
generated where both Fab fragments remain linked by two
disulfide bonds, and the Fc fragment is split into
several peptides. The F(ab')2 fragment is formed by two
Fab' fragments (one Fab' fragment consisting of a Fab and
a hinge region), linked by intercatenary disulfide bonds
in order to form a F(ab')2.
Such fragments, which contain the binding site of
the antibody, may have lost some of the properties of a
whole antibody from which they are derived, such as the
ability to activate the complement or to bind the Fcgamma
receptors. However, these fragments have not lost the
ability of the whole antibody to neutralize the inhibitor
antibody. Thus, the invention refers also to the F(ab')2,
Fab', Fab fragments, or to the CDR region or any modified
version of any of these fragments or region of the
antibody 18B6. Particularly, these fragments have
preserved the ability of the whole antibody to neutralize
RHD5 antibodies.
A further object of the invention is a stable cell
CA 02734549 2011-02-16
- 19 -
line producing an antibody such as described above. The
stable cell line of the invention can be of human or
animal origin. The stable cell line of the invention can
originate from human immortalized cells. In a further
embodiment of the invention, this cell line can originate
from immortalized cells of animal origin, for example
mice. A preferred example of a cell line obtained in this
embodiment of the invention is the line 18B6, deposited
at CNCM under the number 1-3559. In a further embodiment,
the stable cell line of the invention is a line which has
integrated a genetic construction allowing the expression
of the antibody of the invention at the desired point of
the genome. The step consisting of obtaining such a cell
is a stable transfection. This step can be applied to any
type of cells as long as they can be maintained in in
vitro culture. Stable transfection requires integration
of the genetic construction, which can be carried out by
homologous recombination or randomly. The presence of a
positive selection cassette in the genetic construction
comprising the gene of interest which confers antibiotic
resistance to the cell, for example, attests to the
insertion of the transgene into the cell genome. As
result of a sub-cloning step, a long term producer cell
line is obtained from the antibody of the invention, for
example 18B6, which can be maintained in in vitro
culture.
The stable cell line expressing an antibody of the
invention can be selected from the group consisting of a
human cell line, a rodent cell line, for example a mouse
cell line, SP2/0, YB2/0, IR983F, a human myeloma such as
Namalwa, or any other cell of human origin such as PERC6,
CHO cell lines, namely CHO-K-l, CHO-LeclO, CHO-Lecl, CHO-
Lec13, CHO Pro-5, CHO dhfr- (CHO DX Bll, CHO DG44), or
further cell lines selected from Wil-2, Jurkat, Vero,
Molt-4, COS-7, 293-HEK, BHK, K6H6, NSO, SP2/0-Ag 14 and
CA 02734549 2011-02-16
- 20 -
P3X63Ag8.653.
A further particular subject matter of the
invention is the hybridoma 18B6 deposited under the
registration number CNCM 1-3559 at the Collection
Nationale de Cultures de Microorganismes (CNCM). The
variable region of each of the light chains of the
monoclonal anti-idiotypic antibody produced by the
hybridoma 18B6 is coded by the nucleic acid sequence SEQ
ID NO : 16, and the variable region of each of the heavy
chains of the monoclonal anti-idiotypic antibody
produced by the hybridoma 18B6 is coded by the nucleic
acid sequence SEQ ID NO : 15. The antibody produced by
the hybridoma 18B6 is the antibody 18B6, and a method for
obtaining the hybridoma 18B6 is described in the
"Examples" Section of the present document.
A further subject matter of the invention is a DNA
fragment of the sequence SEQ ID NO : 15 encoding the
variable region of the heavy chain of the antibody of the
invention such as previously described. This DNA fragment
can be inserted into a vector enabling the expression of
a polypeptide, preferably of an antibody, the variable
region of the heavy chain of said antibody is coded by
the nucleic acid sequence SED ID NO : 15, the derived
peptide sequence of which is the sequence SEQ ID NO : 17,
in order to be introduced and maintained in a host cell.
This vector enables the expression of this foreign
nucleic acid fragment in the host cell because it
contains the sequences (promoter, polyadenylation
sequence, selection gene) essential for this expression.
Such vectors are well known to those skilled in the art,
and can be an adenovirus, a retrovirus, a plasmid or a
bacteriophage, this list is not being limitative. In
addition, any mammalian cell can be used as the host
cell, that is as the cell expressing the polypeptide or
the antibody of the invention, for example SP2/0, YB2/0,
CA 02734549 2011-02-16
- 21 -
IR983F, a human myeloma such as Namalwa, or any other
cell of human origin such as PERC6,CHO cell lines, namely
CHO-K-l, CHO-LeclO, CHO-Lecl, CHO-Lecl3, CHO Pro-5, CHO
dhfr-(CHO DX Bll, CHO DG44), or other lines selected from
Wil-2, Jurkat, Vero, Molt-4, COS-7, 293-HEK, BHK, K6H6,
NSO, SP2/0-Ag 14 and P3X63Ag8.653.
A further object of the invention is a DNA fragment
of the sequence SEQ ID NO : 16 coding the variable region
of the light chain of an antibody of the invention such
as previously described. This DNA fragment can be
inserted into a vector enabling the expression of a
polypeptide, preferably of an antibody, the variable
region of the light chain of said antibody is coded by
the nucleic acid sequence SED ID NO : 16, the deduced
peptide sequence thereof is the sequence SEQ ID NO : 18,
in order to be introduced into and maintained in a host
cell. This vector enables the expression of this foreign
nucleic acid fragment in the host cell because it
contains the sequences (promoter, polyadenylation
sequence, selection gene) essential for this expression.
Such vectors are well known to those skilled in the art,
and can be an adenovirus, a retrovirus, a plasmide or a
bacteriophage, this list not being limitative. In
addition, any mammalian cell can be used as host cell,
that is as the cell expressing the polypeptide or the
antibody of the invention, for example SP2/0, YB2/0,
IR983F, a human myeloma as Namalwa, or any other cell of
human origin as PERC6, CHO cell lines, especially CHO-K-
1, CHO-LeclO, CHO-Lecl, CHO-Lecl3, CHO Pro-5, CHO dhfr-
(CHO DX B11, CHO DG44), or other lines selected from Wil-
2, Jurkat, Vero, Molt-4, COS-7, 293-HEK, BHK, K6H6, NSO,
SP2/0-Ag 14 and P3X63Ag8.653.
A further object of the invention is a
pharmaceutical composition comprising an antibody of the
invention and at least an excipient and/or at least one
CA 02734549 2011-02-16
- 22 -
pharmaceutically acceptable carrier. Preferably, the
monoclonal anti-idiotypic antibody contained in the
pharmaceutical composition of the invention is the
antibody 18B6, a fragment or a region derived from 18B6,
or even a chimeric or humanized antibody comprising the
variable regions or the CDRs of 18B6, and such as
previously described in the present document. The
pharmaceutical composition of the invention can be
formulated into any excipient which can be tolerated by a
patient to be treated. Examples of such excipients
include water, saline solutions, Ringer's solution,
dextrose solutions, and any other suitable aqueous
physiological solution. The excipient can also contain
low amounts of additives, such as substances increasing
the isotonicity and the stability of the composition.
Such excipients include phosphate buffer, bicarbonate
buffer, and Tris buffer. Such excipients are well known
to those skilled in the art. Standard formulations can be
in the form of liquids for injection or solid
formulations which can be resuspended in a suitable
liquid prior to administration. The useful carriers for
preparing the pharmaceutical composition of the invention
advantageously have the function of increasing the half-
life of the therapeutic composition in the animal or
patient, or enabling the controlled release of the active
ingredient. Such carriers can be organic and synthetic
polymers and further chemical compounds capable of
disseminating the medicaments at a normal rate or
disseminating them only in certain environments, and can
also be liposomes, this list being not limitative.
Advantageously, the pharmaceutical composition of
the invention, moreover, comprises at least an anti-
idiotypic antibody directed against the inhibitory
antibody binding to a domain different from the Cl domain
of Factor VIII. This other antibody can be an anti-
CA 02734549 2011-02-16
- 23 -
idiotypic antibody directed against an inhibitor antibody
binding to the Al, or A3, or B, A2 or C2 domains of
Factor VIII. Indeed, a patient suffering from haemophilia
A, having developed inhibitory antibodies, exhibits most
frequently several types of inhibitory antibodies. In
addition, the amounts and the nature of the different
types of inhibitory antibodies are not fixed but may
change during the patient's life. The different
inhibitory antibodies of a same patient are thus directed
against the different domains of Factor VIII, and it is
particularly advantageous to treat the patient not with
one but with several types of anti-idiotypic antibody,
directed against the different inhibitory antibodies.
Preferably, the pharmaceutical composition
comprises a monoclonal anti-idiotypic antibody directed
against an inhibitory antibody binding to the C2 domain
of Factor VIII and/or an inhibitory antibody binding to
the A2 domain of Factor VIII, and the monoclonal antibody
of the invention. Indeed, the A2 and C2 domains are the
main targets of the anti-Factor VIII immune reaction.
Thus, a pharmaceutical composition comprising a mixture
of anti-idiotypic antibodies directed against inhibitory
.antibodies binding to the Cl domain of Factor VIII and of
anti-idiotypic antibodies directed against inhibitory
antibodies binding to the C2 domain, enables
neutralization of at least 70%, and advantageously at
least 80% or 90% of all inhibitory antibodies present in
a patient. In a preferred embodiment of the invention,
the pharmaceutical composition of the invention comprises
the antibody 14C12 (deposited under the number LMBP
5878CB at the Belgian Coordinated Collections of
Microorganisms) and/or the antibody 30D1 (deposited at
CNCM under the number I-3450). In a further preferred
embodiment of the invention, the pharmaceutical
composition comprises the chimeric antibody 14C12
CA 02734549 2011-02-16
- 24 -
deposited at the CNCM under the number 1-3510 and/or a
chimeric or humanized antibody derived from the antibody
30D1, that is an antibody comprising the variable regions
of the antibody 30D1.
A further object of the invention is the use of the
antibody of the invention as a medicament.
A further object of the invention is the use of the
antibody of the invention for manufacturing a medicament.
Advantageously, such a medicament is used for reducing
and/or preventing and/or treating bleeding in a patient
suffering from haemophilia comprising inhibitory
antibodies directed against the Cl domain of Factor VIII.
A further object of the invention is the use of the
antibody of the invention for manufacturing a medicament
intended for the treatment of type A haemophilia.
Advantageously, the thus treated type A haemophilia
is a haemophilia with inhibitors. This type of
haemophilia treated with the antibody of the invention
can be inborn or acquired. By neutralizing the inhibitory
antibodies, the antibody of the invention makes treatment
by injection of Factor VIII to a patient effective, since
the activity of Factor VIII is no longer inhibited by
inhibitory antibodies.
A further object of the invention is the use of the
antibody of the invention for neutralisation of the in
vitro or in vivo inhibitory activity of an inhibitory
antibody directed against the Cl domain of Factor VIII.
This process can be carried out in order to deplete the
inhibitory antibodies directed against the C1 domain of
Factor VIII from the blood of a patient, and afterwards
to re-inject the treated blood to said patient.
A further object of the invention is related to a
medicament comprising an antibody of the invention,
preferentially the antibody 18B6.
A further object of the invention is the use of the
CA 02734549 2011-02-16
- 25 -
antibody for adsorption of the inhibitory antibodies, by
way of example in order to purify Factor VIII inhibitory
antibodies.
Finally, a further object of the invention is the
use of the antibody of the invention for detection and/or
purification of Factor VIII inhibitory antibodies. The
general processes carrying out such methods of detection
and purification are well known to those skilled in the
art. By way of example, the use of an immuno-purification
column containing beads with the antibody of the
invention grafted on their surface, can be mentioned.
Only the molecules recognized by the antibody will affix
themselves to the beads. The others will pass through the
column. In order to recover the molecule, an increase of
the ionic strength of the solvent is sufficient.
Further aspects and advantages of the invention
will be described in the following examples, which are to
be considered by way of illustration and not of
limitation to the scope of the invention.
Description of Figures
Figure 1 : increase (mean value) for the 4 mice in the
binding of anti-idiotypic antibodies to RHD5.
Figure 2 : direct binding of the anti-idiotypic antibody
18B6 to the insolubilized antibody RHD5.
Figure 3 : inhibition of the binding of the antibody RHD5
to insolubilized recombinant FVIII (recFVIII).
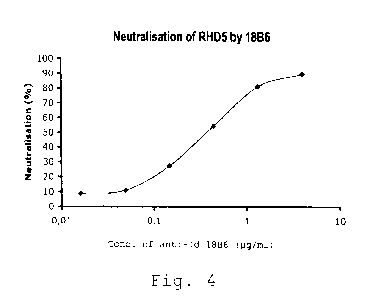
Figure 4 : neutralisation of RHD5 by 18B6.
ExAMPLEs
Example 1 : Production of a human monoclonal antibody
directed against the Cl domain of Factor VIII (<< anti-C1
antibody >>)
The human lymphoblastoid cell line RHD5 described
here below was obtained by immortalization of B
lymphocytes of a patient suffering from acquired
CA 02734549 2011-02-16
- 26 -
haemophilia A having developed an immune response to
Factor VIII, according to the procedure described in the
document Jacquemin et al. (1998), Blood 92, 496-506 and
in the patent application WO 2005/016455.
The cell line producing the monoclonal anti-C1 RHD5
antibody was deposited at the Belgian Co-ordinated
Collections of Microorganisms/Plasmid Collection
(BCCM/LMBP), Laboratorium voor Moleculaire Biologie,
University of Ghent, Technologiepark 297, B-9052
Zwijnaarede, Belgium, in August 2004, by the Collen
Research Foundation, under the accession number LMBP
6165CB.
The nucleotide sequence of the variable region of the
heavy chain of the RHD5 antibody is sequence SEQ ID NO :
5, the latter having the following nucleic acid
sequence :
atggactgga cctggaggtt cctctttgtg gtggcagcag ctgcaggtgt
ccagtcccag gtgcagctgg tgcagtctgg ggctgaggtg aagaagcccg
ggtcgtcggt gatggtctcc tgcaaggctt ctggaggcac cttcagcagc
tttggtatca gctgggtgcg acaggcccct ggacaagggc ttgagtgggt
gggagggatc atccctatct ttggtacagc aaacaccgca cggaacttcc
agaatagagt caccattacc gcggacgaat tcacgagcac agcctacata
cgactgagga gcctgagatc tgaagatacg gccgtgtatt actgtgtcgg
cggtcgagat gcctacagct ttgatggttt tgatgtctgg ggccaaggga
caatggtcac cgtctcttca g,
and the nucleotide sequence of the variable region of the
light chain of the RHD5 antibody is sequence SEQ ID NO :
6, the latter having the following nucleic acid
sequence :
atggcatgga tccctctctt cctcggcgtc cttgtttact gcacaggatc
cgtggcctcc tctgggctga ctcagccaca ctcagtgtcc gtgtccccag
gacagacagc caacatcacc tgctctagag ataagttggg tcataaattt
gcttcctggt atcaacagaa gccaggccag tcccctgctc ttctcatcta
tcaagacagc aagcggccct cagggatccc tgagcgattc tctggctcca
actctgggaa cacagccact ctgaccatca gcgggaccca ggctatggat
CA 02734549 2011-02-16
- 27 -
gaggctgact attactgtca ggcgtgggac aacaccactg ccgtattcgg
cggagggacc aagttgacag tcctaagtca gccca.
The peptide sequence corresponding to the sequence SEQ ID
NO : 5 is sequence SEQ ID NO : 7, the latter having the
following amino acid sequence :
Met Asp Trp Thr Trp Arg Phe Leu Phe Val Val Ala Ala Ala
Ala Gly Val Gin Ser Gin Val Gin Leu Val Gin Ser Gly Ala
Glu Val Lys Lys Pro Gly Ser Ser Val Met Val Ser Cys Lys
Ala Ser Gly Gly Thr Phe Ser Ser Phe Gly Ile Ser Trp Val
Arg Gin Ala Pro Gly Gin Gly Leu Glu Trp Val Gly Gly Ile
Ile Pro Ile Phe Gly Thr Ala Asn Thr Ala Arg Asn Phe Gin
Asn Arg Val Thr Ile Thr Ala Asp Glu Phe Thr Ser Thr Ala
Tyr Ile Arg Leu Arg Ser Leu Arg Ser Glu Asp Thr Ala Val
Tyr Tyr Cys Val Gly Gly Arg Asp Ala Tyr Ser Phe Asp Gly
Phe Asp Val Trp Gly Gin Gly Thr Met Val Thr Val Ser Ser,
and the peptide sequence corresponding to the sequence
SEQ ID NO : 6 is sequence SEQ ID NO 8, the latter
having the following amino acid sequence
Met Ala Trp Ile Pro Leu Phe Leu Gly Val Leu Val Tyr Cys
Thr Gly Ser Val Ala Ser Ser Gly Leu Thr Gin Pro His Ser
Val Ser Val Ser Pro Gly Gin Thr Ala Asn Ile Thr Cys Ser
Arg Asp Lys Leu Gly His Lys Phe Ala Ser Trp Tyr Gin Gin
Lys Pro Gly Gin Ser Pro Ala Leu Leu Ile Tyr Gin Asp Ser
Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Gly Ser Asn
Ser Gly Asn Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala
Met Asp Glu Ala Asp Tyr Tyr Cys Gin Ala Trp Asp Asn Thr
Thr Ala Val Phe Gly Gly Gly Thr Lys Leu Thr Val Leu Ser
Gin Pro.
Optionally, antibodies exhibiting the required
properties can be produced by immunization of animals. In
this case, human Factor VIII is injected into mice with
an adjuvant. Monoclonal anti-human antibodies are
obtained by fusion of spleen lymphocytes with a mouse
myeloma cell line. Cell supernatants producing the anti-
Factor VIII antibodies are identified and cloned by
CA 02734549 2011-02-16
- 28 -
limiting dilution. A general description of such methods
can be found in Current Protocols in Immunology,
Chapter 2, John Wiley & Sons, Inc, 1994 >>. Further
selections of inhibitors exhibiting the desired
properties are described hereafter.
Example 2 : Production of anti-idiotypic antibody 18B6
I. Mice immunization
Four 6 week old Balb/c female mice were sub-
cutaneously injected (SC) thrice in the footpad, with 10
pg of the human anti-Cl domain of FVIII RHD5 antibody
suspended in a complete Freund's adjuvant (ACF) (1st
immunization) then in an incomplete Freund's adjuvant
(AIF).
The first bloodletting (bloodletting 0) was
performed prior to immunization (bleeding Day 0 (DO)),
then the injections and bloodletting proceeded as follows
Dl: Injection N 1 (10 pg of RHD5 antibody in the
presence of complete Freund's adjuvant)
D15: Bloodletting N l
D16: Injection N 2 (10 pg of RHD5 antibody in the
presence of incomplete Freund's adjuvant)
D28: Bloodletting N 2
D29: Injection N 3 (10 pg of RHD5 antibody in the
presence of incomplete Freund's adjuvant)
D44: Bloodletting N 3
II. Evaluation of the immune response of mice
In order to evaluate the presence of the anti-RHD5
antibodies in the different bloodlettings, an ELISA assay
with direct binding is performed. To this end, either the
RHD5 antibody, or a control IgGl at 3 pg/ml were
insolubilized, 50 p1/well, un Glycine buffer, over night
at 4 C (Glycine buffer = O.1M Glycine, 0.17M NaCl, pH
9.2). Three washings are performed with PBS/Tween (PBS =
140.0 mM NaCl, 2.6 mM KC1, 1.4 mM KH2PO4, 8.1 nM
CA 02734549 2011-02-16
- 29 -
Na2HPO4. 2H2O, pH 7 . 4) . The system is left at saturation
for 30 minutes at room temperature (RT) with 100 pl/well
of Magic Buffer (Magic Buffer = 50mM Tris, 0.17M NaCl, 1%
BSA, pH 7.2). Afterwards, the bloodlettings were diluted
to 1/10, 1/100, 1/1000 and 1/10000 in Magic Buffer and
incubated for 2 hours at room temperature (50pl/well).
Then, 3 washings are carried out in PBS/Tween.
Subsequently, the system is incubated with a 1 leg/ml
solution of goat polyclonal mouse anti-IgG antibodies
labelled with HRP (horseradish peroxidase) (Bio-Rad) for
2 hours at room temperature (50p1/well) (dilution in
Magic Buffer) . Then, the system is washed 3 times with
PBS/Tween, and revelation is carried out with a chromogen
(Ortho-phenyl diamine) and the intensity of the obtained
coloration is read using a reader with filters
corresponding to wavelengths 490/650 nm (reader Emax
Molecular Devithese, Sunnyvale, CA).
CA 02734549 2011-02-16
- 30 -
Result of optical densities obtained with the control
IgGl:
Dilution 1000X Mouse 1 Mouse 2 Mouse 3 Mouse 4
Bloodletting 0 0.031 0.019 0.018 0.018
Bloodletting 1 0.019 0.020 0.025 0.028
Bloodletting 2 0.026 0.023 0.169 0.045
Bloodletting 3 0.027 0.063 0.150 0.024
Table 1
Result of optical densities obtained with RHD5:
Dilution 1000X Mouse 1 Mouse 2 Mouse 3 Mouse 4
Bloodletting 0 0.047 0.020 0.012 0.018
Bloodletting 1 0.446 0.188 0.142 0.157
Bloodletting 2 0.632 0.685 0.648 0.911
Bloodletting 3 0.570 0.708 0.778 0.852
Table 2
The results obtained are depicted in Figure 1.
Conclusion : Each mouse responded correctly and reacted
similarly to the injection of the RHD5 Fab fragment. In
an arbitrary manner, mouse n 4 was selected to carry out
the fusion.
III. Fusion and screening
The fusion of spleen lymphocytes of mouse n 4 with
cells of a myeloma SP2/0 was carried out. The fusion was
carried out in a conventional way for those skilled in
the art (J.G. Gilles et al., Blood (2004) 103 : 2617-23 ;
P. Cornelis, << Les anticorps monoclonaux >>, Revue IRE,
vol. 7, N 4, 1983).
The cells were successively expanded in a DMEM
medium (Dulbecco's Modified Eagle Medium) containing
hypoxanthine and thymidine according to the principle of
limit dilutions and the clones tested positive detected
in direct binding ELISA assay, such as previously
described in point II.
CA 02734549 2011-02-16
- 31 -
The specificity of the binding was confirmed by
insolubilizing a human IgGl antibody having an irrelevant
specificity, and produced in the Laboratory.
In order to determine hybridoma stability, the
epitope screening tests(tests 1 to 3) were repeated
during clones expansion, in different volumes of medium
from 200 pl to 5 ml.
Test 1 = measurement in well of 200 pi
Test 2 = measurement in well of 1 ml
Test 3 = measurement in bottle of 5 ml
Results obtained during different epitope screenings are
resumed in the following Table :
Screening Screening Screening Screening Inhibition: Neutralization:
1 2 3 3 /IgGl Elisa functional test
lAl + - - -
1F3 + + + - + (92.5%) + (100%)
2A1 + - - -
2C9 + + + +
3G9 + + + +
4B7 + + - -
4B10 + + + +
4D5 + + + +
5B11 + + + - + (93.6%) + (100%)
5E1 + - +/- +
5G3 + + + +
5H7 + - - +
5H8 + + +/- +
6A9 + + + +
6E1 + + +/-- +
6H7 + + + +
6H8 + + + +
9D2 + - - +
10D2 + + + - + (93.6%) + (100%)
10G8 + + + - -
11C5 + + + - -
11D7 + - - -
CA 02734549 2011-02-16
- 32 -
11G3 + + + +
12D7 + + + - -
12G3 + - - -
12H12 + + + - -
13A1 + + + - + (90.9%) + (95.6%)
13C3 + + + - + (93.6%) + (100%)
13D7 + + + -
13H5 + + +/- +
14H1 + + - -
14D11 + + +/-- +
14F11 + + + - + (83.6%) + (100%)
14H2 + + + +
14H5 + + + +
15B4 + + - -
15F6 + - - +
16B4 + + + - + (94%) + (90.9%)
16F6 + + + - -
17A5 + + + +
17C4 + + + - + (92.4%) + (100%)
18A5 + + + - -
18A9 + + + - -
18B6 + + + - + (93.1%) + (100%)
18C4 + + + +
19C4 + + + +
19G3 + + - +/-
20A7 + + + +
20C4 + - - -
20G3 + - - -
21D8 + + + - + (93.8%) -
22H7 + - + +
23A7 + + + - -
23E3 + + - -
23G2 + - +/- - -
24D5 + + + - +/- (56.3%) +/- (76.9%)
24D12 + + + +
24E3 + - - -
CA 02734549 2011-02-16
- 33 -
128C10 + - - -
Table 3
IV. Inhibition assay with culture supernatants
As shown in Table 3, a test of inhibition was
carried out with the culture supernatants. This assay was
carried out in order to select, from the clones, the
anti-idiotypic antibodies which exactly recognize an
epitope determinant located at the paratope level of the
RHD5 Ab. The anti-idiotypic antibodies were tested in an
ELISA assay for inhibition of binding of the RHD5 to the
insolubilized FVIII.
Recombinant Factor VIII (recFVIII) (Baxter) at 2
pg/ml in a glycine buffer, 50 pl/well, was insolubilized,
then left for 2 hours at room temperature. The RHD5
antibody (or an irrelevant IgGl) at 0.6 pg/ml final
concentration was pre-incubated over 2 hours with the
culture supernatants in a dilution 1/1, 1/2 and 1/4 in
Magic Buffer. The wells were washed 3 times with a
PBS/Tween buffer, then saturated with 100 pl/well of
Magic Buffer (30 min at room temperature) . Afterwards,
the culture supernatant was incubated with 50 pl of RHD5
(or irrelevant IgGl) (2 hours at room temperature, Magic
Buffer), then, 3 washings were performed. The RHD5
antibodies bound to insolubilized recFVIII were detected
by addition of 50 p1/well of a mouse polyclonal human
anti-IgG HRP-labelled (Southern Biotechnology) antibodies
solution of 1 pg/ml in Magic Buffer. Three successive
washings were carried out with PBS/Tween, then revelation
carried out with a chromogen (OPD ortho-phenyl diamine)
and a reading of the obtained coloration intensity with a
reader having filters corresponding to wavelengths
490/650 nm (reader Emax Molecular Devithese, Sunnyvale,
CA).
Conclusions
CA 02734549 2011-02-16
- 34 -
As shown in Table 3, 11 clones are able to
specifically inhibit the RHD5 antibody binding to
insolubilized recFVIII. (N.B. : A negative value either
expresses the possibility of binding to an external
region of the paratope, or reflects an insufficient
concentration of the Ab in the culture supernatant.
However, with respect to the number of positives, the
negative wells were eliminated from the following tests).
V. Functional test with culture suprenatants
measurement of neutralisation of RHD5 antibody
inhibitory activity (anti-Factor VIII)
The RHD5 antibody is incubated at a concentration
of 1 pg/ml with supernatants of different clones selected
during the test of inhibition (diluted 3 times, 6 times,
12 times and 24 times) in Magic Buffer at 37 C. After 30
min, the FVIII Kogenate (Bayer) at 0.5 U/ml final was
added, then a complementary incubation of 30 min at 37 C
was carried out. The samples were diluted 30X in Magic
Buffer, then the reagents of the chromogenic DADE test
(Factor VIII chromogenic, Dade Behring Gmbh, Marburg,
Germany) were added following the manufacturer's
instructions.
As shown in the Table 3, 10 clones are able to
neutralize the inhibitory activity of the RHD5 Ab.
Antibody 18B6 was selected to be used in the following
experiences, as a function of the results and
neutralisation curves.
VI. Extensive production of the selected 18B6 anti-RHD5
clone
The anti-idiotypic antibody 18B6 was produced in a
DMEM culture medium. This production was followed by
purification on a Protein G affinity column (which
enables purification, then concentration of the
antibodies, and thus to ascertain further the obtained
CA 02734549 2011-02-16
- 35 -
anti-idiotypic antibody specificity).
Purification : 18B6 : production of 8 ml at 8.48 mg/ml
VII. Specificity evaluation
The various preparations were evaluated with an
ELISA following the same protocol as described in points
II. and IV.
1. ELISA assay : direct binding of the anti-idiotypic
antibody 18B6 to insolubilized antibody RHD5
The direct binding of the anti-idiotypic antibody
18B6 to the insolubilized antibody RHD5 is illustrated in
Figure 2. The curve shows that the binding of antibody
18B6 to RHD5 is dose-dependent.
2. ELISA assay : inhibition of antibody RHD5 binding to
insolubilized recombinant FVIII.
The inhibition of RHD5 antibody binding to
insolubilized recombinant FVIII was measured according to
the protocol described in point IV. The concentration of
used RHD5 is equal to 2 pg/ml.
Conc. 18B6 Inhibition of
ug/ml binding (%)
50 96.1
97.2
12.5 96.9
6.25 96.7
3.12 94.1
1.56 90.3
0.78 50.3
0.39 9.2
0.195 0
0.098 0
20 Table 4
The results are shown in Figure 3. A 50% inhibition
of RHD5 binding to FVIII is obtained at a molar ratio
CA 02734549 2011-02-16
- 36 -
RHD5/18B6 of 2.5, while an equimolar ratio inhibits 92%
of this binding.
3. Functional test : measurement of the neutralisation of
RHD5 antibody inhibitory activity (anti-FVIII)
The protocol is the same as described in point V
with a final RHD5 concentration of 0.4 pg/ml and a curve
of purified anti-idiotypic antibody from 4 to 0.002 pg/ml
final concentration.
The results are given in the following Table 5
Conc. anti- Neutralisation
Id (pg/ml) (%)
4 89.5
1.33 81.2
0.44 54.3
0.148 27.4
0.049 11
0.0165 8.8
0.0055 6.7
0.00183 8.9
0.0006 6.7
0.0002 0
Table 5
The results are illustrated in Figure 4. A 50 %
neutralisation of RHD5 inhibitory activity is obtained at
an equimolar RHD5/18B6 ratio.
4. Measurement of the binding kinetics of anti-idiotypic
antibody 18B6 with the Surface plasmon resonance
Biacor >> method
The binding kinetics of the anti-idiotypic antibody
18B6 to inhibitor RHD5 antibody was evaluated by use of
the << Surface plasmon resonance Biacore >> method using
the Pharmacia Biosensor BlAcore (Pharmacia Biosensor AB,
Uppsala, Sweden). The RHD5 antibodies were immobilized on
CA 02734549 2011-02-16
- 37 -
the activated surface of a CM5 probe. The anti-idiotypic
antibodies 18B6 were infused in different RHD5
concentrations immobilized on the surface of the probe.
The association and dissociation constants were
determined :
Ka (M-1S-1) = 4,26 x 103
Kd (S-1) = 1.45 x 10-5
KD :M. 3, 4 x 10-9
5. Characterization of the anti-idiotypic antibody 18B6
sub-class
In order to determine the sub-class of the antibody
18B6, the IsoStrip system by Roche was used (colorimetric
strip). The antibody 18B6 was identified as a IgGl Kappa.
VIII. Sequence of the antibody 18B6
In order to carry out sequencing, mRNA of hybridoma
producing the anti-idiotypic antibody 18B6 was isolated,
using a Quick Prep Micro mRNA Purification Kit (Amersham
Pharmacia Biotech, Uppsala, Sweden). The cDNA was
synthesized by use of First-strand cDNA Synthesis Kit
(Amersham Pharmacia Biotech). The cDNA encoding the heavy
chain (VH) and the light chain (VL) was amplified by PCR
(Polymerase Chain Reaction) using specific primers
corresponding to different families of genes potentially
found in the mouse. The PCR products were isolated from
an agarose gel 1.5% by means of QIA quick Gel Extraction
Kit (Qiagen, Hilden, Germany) and cloned with pGEM-T Easy
Vector system (Promega, Madison, WI). Plasmidic DNA of
positive colonies was isolated by means of High Pure
Plasmid Isolation Kit (Roche Diagnostics, Mannheim,
Germany) and sequenced in both directions with Seqenase
(US Biochemical, Cleveland, OH).
IX. Particular properties of the 18B6 antibody
CA 02734549 2011-02-16
- 38 -
Antibody 18B6 completely inhibits RHD5 antibody
binding to its antigen, Factor VIII. The RHD5 antibody
carries an idiotype complementary to that of 18B6.
The binding of an antibody to the antigen involves
an interface of mutual recognition of 6 to 12 angstroms2'
corresponding to a great number of amino acids which
associate one with another by hydrogen bonds, hydrophobic
or polar attraction and VanderWals bridges.
At a functional level, when an antibody completely
inhibits the binding of an antibody to the antigen, this
implies that the inhibiting antibody carries an "internal
image" of the antigen, that is, a three-dimensional
structure mimicking the 3-D structure of the antigen.
Although the primary structure (amino acids
sequence) of the 18B6 antibody shows low identity to the
Cl domain of Factor VIII, the alignment of secondary
structures of the 18B6 antibody with that of the Cl
domain of FVIII, the antigenic target of the RHD5
antibody, and three-dimensional modelling of the 18B6
indicate, by superposition with the 3-D structure of the
Cl domain, that the variable part of the light chain (VL)
of 18B6 represents an internal image of the Cl domain.
This observation confers on the 18B6 antibody a
particular, novel and not foreseeable property. In other
words, any attempt to generate antibodies similar to 18B6
by immunization with an antibody such as RHD5 does not
yield de facto antibodies identical to 18B6.