Note: Descriptions are shown in the official language in which they were submitted.
CA 02734980 2013-03-05
DESCRIPTION
PEARLITE RAIL HAVING SUPERIOR ABRASION RESISTANCE AND
EXCELLENT TOUGHNESS
TECHNICAL FIELD
[0001]
The present invention relates to a pearlite rail used for freight railways in
overseas in which both the abrasion resistance (wear resistance) and toughness
are
improved at the head portion.
BACKGROUND ART
[0002]
In conjunction with economic development, new development of natural
resources, such as coal or the like, is progressing. Specifically, mining is
underway at
regions with a severe natural environment which have not so far been
developed.
Accordingly, the track environment is becoming remarkably severe in overseas
freight
railways used to transport natural resources. There is a demand for rails to
have
toughness or the like in regions with cold weather in addition to higher wear
resistance
than ever. In such circumstances, there is a demand to develop rails having
higher wear
resistance and higher toughness than those of presently-used high-strength
rails.
[0003]
CA 02734980 2011-02-22
2
In general, it is known that the refinement of a pearlite structure,
specifically,
grain refining in an austenite structure which is yet to be transformed into
pearlite or the
refinement of pearlite blocks is effective to improve the toughness of a
pearlite steel. In
order to achieve grain refining in an austenite structure, during a hot
rolling, the rolling
temperature is decreased and the rolling reduction rate is increased and,
furthermore, a
heat treatment by low-temperature reheating after hot rolling of rails is
implemented. In
addition, in order to achieve the refinement of a pearlite structure, pearlite
transformation
starting from the inside of austenite grains is accelerated by utilizing
transformation nuclei
or the like.
However, in the manufacturing of rails, from the viewpoint of ensuring
formability during the hot rolling, there are limitations on a decrease in the
rolling
temperature and an increase in the rolling reduction rate; and thereby,
sufficient refinement
of austenite grains could not be achieved. In addition, with regard to the
pearlite
transformation from the inside of austenite grains by utilizing transformation
nuclei, there
are problems in that the amount of transformation nuclei is difficult to
control, and the
pearlite transformation from the inside of grains is not stable; and thereby,
sufficient
refinement of a pearlite structure could not be achieved.
[0004]
Due to these problems, a method has been applied to fundamentally improve the
toughness of rails having a pearlite structure in which low-temperature
reheating is
conducted after hot rolling a rail, and then pearlite transformation is
performed by
accelerated cooling so as to refine a pearlite structure. However, recently,
rails have been
made to include a high content of carbon for improving the wear resistance;
and therefore,
there is a problem in that coarse carbides remain inside austenite grains
during the
above-described low-temperature reheating treatment, which lowers the
ductility and
CA 02734980 2011-02-22
3
toughness of a pearlite structure after the accelerated cooling. In addition,
since this
method includes reheating, there is another problem in regard to economic
efficiency, such
as a high manufacturing cost, a low productivity or the like.
[0005]
Consequently, there is a demand to develop a method for manufacturing a
high-carbon steel rail that ensures the formability during rolling and refines
the pearlite
structure after hot rolling. In order to solve this problem, methods for
manufacturing a
high-carbon steel rail shown below have been developed. The major
characteristics of
those methods for manufacturing a rail are that the fact that austenite grains
in a
high-carbon steel are easily recrystallized at a relatively low temperature
and even with a
small rolling reduction rate is utilized so as to refine the pearlite
structure. As a result,
fine grains with similar grain diameters are obtained by continuous rolling
under a small
rolling reduction rate; and thereby, the ductility and toughness of a pearlite
steel is
improved (for example, Patent Documents 1, 2 and 3).
[0006]
Patent Document 1 discloses that a rail having high ductile can be provided by
conducting 3 or more continual passes of rolling with a predetermined interval
of time in
the finish rolling of a high carbon steel rail.
Patent Document 2 discloses that a rail having superior wear resistance and
high
toughness can be provided by conducting two or more continual passes of
rolling with a
predetermined interval of time in the finish rolling of a high carbon steel
rail, and
furthermore, conducting accelerated cooling after the continuous rolling.
Patent Document 3 discloses that a rail having superior wear resistance and
high
toughness can be provided by conducting cooling between passes of rolling in
the finish
rolling of a high-carbon steel rail, and conducting accelerated cooling after
the continuous
CA 02734980 2011-02-22
4
rolling.
[0007]
The technologies disclosed by Patent Documents 1 to 3 can achieve the
refinement of an austenite structure at a certain level and exhibit a slight
improvement in
toughness by the combination of the temperature, the number of rolling passes,
and the
interval of time between passes during the continuous hot rolling. However,
there is a
problem in that these technologies do not exhibit any effects in regard to
fracture starting
from inclusions present inside the steel; and thereby, the toughness is not
fundamentally
improved.
[0008]
Furthermore, grain growth rate of an austenite structure is fast in a high-
carbon
steel. As a result, grains of an austenite structure which are refined by
rolling grow after
the rolling; and therefore, there is a problem in that the toughness of a heat-
treated rail is
not improved even in the case where accelerated cooling is conducted.
[0009]
Considering these circumstances, the addition of Ca, the reduction of the
oxygen
content, and the reduction of the Al content have been studied in order to
suppress the
generation of typical inclusions in rails, that is, MnS or A1203. The
characteristics of
these manufacturing methods are that MnS is changed to CaS by adding Ca in the
preliminary treatment of hot metal so as to become harmless, and furthermore,
the oxygen
content is reduced as much as possible by adding deoxidizing elements or
applying a
vacuum treatment so as to reduce the amount of inclusions in molten steel, and
technologies of which have been studied (for example, Patent Documents 4, 5
and 6).
[0010]
The technology in Patent Document 4 discloses a method for manufacturing a
CA 02734980 2011-02-22
.
high-carbon silicon-killed high-cleanliness molten steel in which the added
amount of Ca
is optimized to fix S as CaS; and thereby, the amount of elongated MnS-based
inclusions
is reduced. In this technology, S which segregates and concentrates in a
solidification
process reacts with Ca which similarly segregates and concentrates or calcium
silicate
5 generated in the molten steel; and thereby, S is sequentially fixed as
CaS. As a result, the
generation of elongated MnS inclusions is suppressed.
[0011]
The technology in Patent Document 5 discloses a method for manufacturing a
high-carbon high-cleanliness molten steel in which the amount of MnO
inclusions is
reduced; and thereby, the amount of elongated MnS inclusions precipitated from
MnO is
reduced. In this technology, a steel is tapped in a non-deoxidized or weakly
deoxidized
state after being melted in an atmosphere refining furnace, and then a vacuum
treatment is
conducted at a degree of vacuum of 1 Ton or less so as to make the dissolved
oxygen
content be in a range of 30 ppm or less. Next, Al and Si are added, and then
Mn is added.
Thereby, the number of secondary deoxidization products is reduced which will
become
crystallization nuclei of MnS that crystalizes out in finally solidified
portions, and the
concentration of MnO in oxides is lowered. Thereby, the crystallization of MnS
is
suppressed.
[0012]
The technology in Patent Document 6 discloses a method for manufacturing a
high-carbon high-cleanliness molten steel with reduced amounts of oxygen and
Al in the
molten steel. In this technology, a rail having superior damage resistance can
be
manufactured by limiting the total amount of oxygen based on the relationship
between
the total oxygen value in oxide-based inclusions and the damage property.
Furthermore,
the damage resistance of rails can be further improved by limiting the amount
of
CA 02734980 2011-02-22
6
. solid-soluted Al or the composition of inclusions in a preferable range.
[0013]
The above-described technologies disclosed in Patent Documents 4 to 6 control
the configurations and amounts of MnS and Al-based inclusions generated in a
bloom
stage. However, the configuration of inclusions is altered during hot rolling
in the rolling
of rails. In particular, Mn sulfide-based inclusions elongated in the
lengthwise direction
by rolling act as the starting points of fracture in rails; and therefore,
there is a problem in
that the damage resistance or toughness of rails cannot be stably improved in
the case
where only the inclusions in the bloom stage is controlled.
[0014]
In addition, the application of precipitates has been studied in order to
suppress
the grain growth of an austenite structure after hot rolling. The
characteristics of this
manufacturing method are that alloy elements are added and carbonitrides are
precipitated
so as to pin an austenite structure; and thereby, grain growth is suppressed.
Consequently,
a heat-treated structure is refined, and toughness is improved (for example,
Patent
Document 7).
In the technology of Patent Document 7, V and Nb are added, and carbonitrides
of V and Nb are precipitated. Furthermore, accelerated cooling is conducted
depending
on the added amounts of V and Nb, and the grain growth of an austenite
structure after hot
rolling is controlled; and thereby, a pearlite structure is refined and the
toughness of a rail
is improved.
[0015]
In the technology disclosed in Patent Document 7, alloy elements are added and
carbonitrides are precipitated so as to pin an austenite structure; and
thereby, grain growth
is suppressed. However, the amount of the generated carbonitrides of the alloy
elements
CA 02734980 2011-02-22
7
gretly varies depending on the rolling temperature and the rolling reduction
rate. As a
result, a huge variation occurs in the effects of suppressing the grain
growth, and
coarsening of crystal grains occurs partially. Therefore, there is a problem
in that the
damage resistance and the toughness of rails cannot be stably improved by the
carbonitrides of alloy elements alone.
In addition, the technology disclosed in Patent Document 7 just achieves the
refinement of an austenite structure. This technology has no effect on damages
due to
Mn sulfide-based inclusions elongated in the lengthwise direction by rolling;
and therefore,
there is a problem in that the damage resistance and the toughness of rails
cannot be stably
improved.
[0016]
Furthermore, in the technologies disclosed in Patent Documents 4 to 7,
embrittlement occurs in a structure due to the alteration in the components of
a steel,
particularly, the alteration of components mixed therein as impurities.
Therefore, there is
a problem in that the damage resistance and the toughness of rails cannot be
stably
improved by controlling inclusions due to the addition of alloy elements and
the reduction
of the oxygen content, and by refining an austenite structure due to the
application of
precipitates.
[0017]
From such circumstances, it has become desirable to provide a pearlite rail
having
superior wear resistance and toughness in which both the wear resistance and
damage
resistance of a pearlite structure are improved.
PRIOR ART DOCUMENTS
Patent Documents
CA 02734980 2011-02-22
8
[0018]
Patent Document 1: Japanese Unexamined Patent Application Publication No.
H07-173530
Patent Document 2: Japanese Unexamined Patent Application Publication No.
2001-234238
Patent Document 3: Japanese Unexamined Patent Application Publication No.
2002-226915
Patent Document 4: Japanese Unexamined Patent Application Publication No.
H05-171247
Patent Document 5: Japanese Unexamined Patent Application Publication No.
H05-263121
Patent Document 6: Japanese Unexamined Patent Application Publication No.
2001-220651
Patent Document 7: Japanese Unexamined Patent Application Publication No.
2007-291413
DISCLOSURE OF THE INVENTION
Problems to be Solved by the Invention
[0019]
The present invention has been made in consideration of the above problems,
and
the object of the present invention is to provide a pearlite rail in which
both wear
resistance and toughness are improved at the head portion that are
particularly in demand
as a rail for freight railways in overseas.
Means for Solving the Problems
CA 02734980 2014-01-29
9
[0020]
The present invention has the following features.
A pearlite rail according to the present invention consists of a steel
including, in
terms of percent by mass, C: 0.65% to 1.20%, Si: 0.05% to 2.00%, Mn: 0.05% to
2.00%, P
0.0150%, S 0.0100%, Ca: 0.0005% to 0.0200%, and Fe and inevitable impurities
as
the balance. In a head portion of the rail, a head surface portion which
ranges from
surfaces of head corner portions and a head top portion to a depth of 10 mm
has a pearlite
structure, and a hardness Hv of the pearlite structure is in a range of 320 to
500. Mn
sulfide-based inclusions having major lengths in a range of 10 to 100 !Am are
present at an
amount per unit area in a range of 10 to 200/mm2 in a cross-section (a cross-
section
parallel to the longitudinal direction of the rail) taken along a lengthwise
direction in the
pearlite structure.
The present invention further provides a pearlite rail having a steel
composition
comprising: in terms of percent by mass,
C: 0.65 to 1.20%;
Si: 0.05 to 2.00%;
Mn: 0.05 to 2.00%;
P 0.0150%;
S 0.0100%;
Ca: 0.0005 to 0.0200%; and
Fe and inevitable impurities as the balance,
wherein, a ratio of S/Ca is in a range of 0.45 to 3.00, among a head portion
of the rail, in a head
surface portion which ranges from surfaces of head corner portions and a head
top portion to a depth
of 10 mm or in a portion which ranges from the surfaces of the head corner
portions and the head top
CA 02734980 2013-03-05
9a
portion to a depth of 20 mm, 95% or more of a metallographic structure is a
pearlite
structure,
a hardness Hv of the pearlite structure is in a range of 320 to 500, and
Mn sulfide-based inclusions having major lengths in a range of 10 to 100 pm
are
present at an amount per unit area in a range of 10 to 200/mm2 in a cross-
section taken
from a portion ranging from the surface of the rail head portion to a depth of
3 mm to 10
mm along a lengthwise direction in the pearlite structure.
Here, Hv refers to the Vickers hardness defined by JIS B7774.
In the pearlite rail according to the present invention, the steel may further
include, in terms of percent by mass, either one or both of Mg: 0.0005 to
0.0200% and Zr:
0.0005 to 0.0100%, and Mg-based oxides, Zr oxides, and Mn sulfide-based
inclusions
having grain diameters in a range of 5 nm to 100 nm may be present at an
amount per unit
area in a range of 500 to 50,000/mm2 in a transverse cross-section (a cross-
section parallel
to the width direction of the rail) in the pearlite structure.
The steel may further include, in terms of percent by mass, one or more of
steel
components described in the following (1) to (9).
(1) Co: 0.01% to 1.00%
(2) either one or both of Cr: 0.01% to 2.00% and Mo: 0.01% to 0.50%
(3) either one or both of V: 0.005% to 0.50% and Nb: 0.002% to 0.050%
(4) B: 0.0001% to 0.0050%
CA 02734980 2011-02-22
(5) Cu: 0.01% to 1.00%
(6)Ni: 0.01% to 1.00%
(7) Ti: 0.0050% to 0.0500%
(8) Al: more than 0.0100% to 1.00%
5 (9)N: 0.0060 to 0.0200%
Effects of the Invention
[0021]
In accordance with the present invention, the components, structure and
hardness
10 of a rail steel are controlled, and, in addition, the contents of P and
S are reduced, Ca is
added, and the number of Mn sulfide-based inclusions is controlled. Thereby,
the wear
resistance and toughness of a pearlite structure are improved; and as a
result, it is possible
to improve the usable period of a rail, particularly, for freight railways in
overseas
(overseas freight railways). Furthermore, it is possible to further improve
the toughness
of the pearlite structure by adding Mg and Zr and controlling the number of
fine Mn
sulfide-based inclusions and Mg and Zr-based oxides; and as a result, it is
possible to
further improve the usable period.
BRIEF DESCRIPTION OF THE DRAWINGS
[0022]
FIG 1 is a view showing nominal designations of portions in a transverse
cross-section (a cross-section perpendicular to the lengthwise direction) of
the rail steel
according to the present invention.
FIG 2 is a view showing the effects of the addition of Ca and the addition of
Mg
and Zr on the relationship between the amount of S and the impact value which
are results
CA 02734980 2013-03-05
11
obtained by melting steels in which the amount of S is altered, the amount of
P is in a range
of 0.0150% or less, the amount of carbon is 1.00%, and Ca, Mg and Zr are
added, conducting
a laboratory melting and rolling test that simulates equivalent rolling
conditions for rails, and
conducting an impact test.
FIG. 3 is a view showing the observation location of Mn sulfide-based
inclusions in
the rail steel according to an embodiment of the present invention.
FIG. 4 is a view showing the observation location of Mn sulfide-based
inclusions,
Mg-based oxides and Zr oxides in the rail steel according to an embodiment of
the present
invention.
FIG. 5 is a view showing the location where the specimens are taken for the
wear
test.
FIG. 6 is a view showing the outline of the wear test.
FIG. 7 is a view showing the location where the specimens are taken for the
impact
test.
FIG. 8 is a view showing the relationship between the amount of carbon and the
amount of wear in the results of the wear test of the rail steels according to
the present
invention and the comparative rail steels (Steel Nos. 48, 50, 51, 52, 53, 64,
66 and 67).
FIG 9 is a view showing the relationship between the amount of carbon and the
impact value in the results of the impact test of the rail steels according to
the present
invention and the comparative rail steels (Steel Nos. 49, 51, 53, 65, 66 and
68).
FIG. 10 is a view showing the relationship between the amount of carbon and
the
impact value in the results of the impact test of the rail steels according to
the present
invention and the comparative rail steels (Steel Nos. 54 to 63 and rails with
the added
amounts of P, S and Ca outside the ranges of the present invention).
FIG. 11 is a view showing the relationship between the amount of carbon and
the
impact value in the results of the impact test of the rail steels according to
the present
CA 02734980 2011-02-22
12
. invention (Steel Nos. 11 to 13, 18 to 20,24 to 26,29 to 31,33 to 35,36 to
38 and 45 to
47).
BEST MODE FOR CARRYING OUT THE INVENTION
[0023]
Hereinafter, as embodiments to carry out the present invention, pearlite rails
with
superior wear resistance and toughness will be described in detail. Here, the
units of the
contents of alloy elements are % by mass, and, hereinafter, expressed simply
as %.
FIG. 1 shows a cross-section perpendicular to the lengthwise direction of the
pearlite rail having superior wear resistance and toughness according to the
present
invention. A rail head portion 3 includes a head top portion 1 and head corner
portions 2
situated at both ends of the head top portion 1. One of the head corner
portions 2 is a
gauge corner (G C.) portion that mainly comes into contact with wheels.
A portion ranging from surfaces of the head corner portions 2 and the head top
portion 1 to a depth of 10 mm is called a head surface portion 3a (diagonal
solid line area).
In addition, a portion ranging from the surfaces of the head corner portions 2
and the head
top portion 1 to a depth of 20 mm is given a reference number 3b (diagonal
dotted line
area).
[0024]
At first, the inventors of the present invention studied a steel component
system
having a bad effect on the toughness of rails. A test melting and a hot
rolling test which
simulated the equivalent hot rolling conditions for rails were conducted using
steels of
which the contents of P and S were varied while utilizing steels having a
varied amount of
carbon as a base; and thereby, prototypes of rails were manufactured. Then,
the impact
values of the prototypes were measured by an impact test, and the effects of
the contents
CA 02734980 2011-02-22
13
= of P and S on the impact values were studied.
As a result, with regard to pearlite steels having Hv levels of 320 to 500, it
was
observed that the impact values were improved in the case where both the
contents of P
and S were reduced to a certain level or less.
Furthermore, as a result of studying the optimal contents of P and S, it was
observed that the impact values were greatly improved in the case where both
the contents
of P and S were reduced to a certain level or less.
[0025]
Next, the inventors of the present invention attempted to clarify the factors
dominating the impact values in order to further improve the impact values of
rails. As a
result, it was observed that rails having low impact values included a lot of
Mn
sulfide-based inclusions elongated in the lengthwise direction by hot rolling,
and these
inclusions acted as starting points of fracture.
Then, the inventors of the present invention clarified the generation
mechanism
of Mn sulfide-based inclusions elongated in the lengthwise direction. When
manufacturing rails, a bloom is reheated to a temperature in a range of 1200 C
to 1300 C,
and then the bloom is subjected to hot rolling. The inventors have
investigated the
relationship between the hot rolling conditions and the configuration of MnS.
As a result,
it was observed that, in the case where the rolling temperature was high or in
the case
where the rolling reduction rate was high during rolling, plastic deformation
of soft Mn
sulfide-based inclusions easily occurred; and thereby, the Mn sulfide-based
inclusions
were easily elongated in the lengthwise direction of rails.
[0026]
In view of these circumstances, the inventors of the present inventions
studied
methods to suppress the elongation of Mn sulfide-based inclusions. As a result
of
CA 02734980 2011-02-22
14
= conducting test melting and a hot rolling test, it was observed that Mn
sulfide-base
inclusions were generated from various kinds of oxides as nuclei. Furthermore,
as a
result of investigating the hardness of oxides and the configurations of Mn
sulfide-based
inclusions, it was observed that the elongation could be suppressed by
hardening
inclusions which acted as the nuclei of the Mn sulfide-based inclusions.
Furthermore, the inventors of the present invention studied hard inclusions
which
acted as the nuclei of Mn sulfide-based inclusions. As a result of conducting
a laboratory
test using oxides with a high melting point, it was found that Ca with a
relative high
melting point formed sulfides and oxides, and formed CaO-CaS aggregates. In
addition,
the inventors have found that, since CaS has a high consistency with Mn
sulfide-based
inclusions, Mn sulfide-based inclusions were efficiently generated in the
aggregates of the
oxides and sulfides of Ca (CaO-CaS).
Here, the consistency refers to a difference of lattice constants (interatomic
distance) on crystal planes in the crystal structures of two metals. The
smaller the
difference is, the higher the consistency is. That is, it is considered that
two metals are
easily bonded.
[0027]
Next, the inventors of the present invention conducted test melting and a hot
rolling test using steels including Ca in order to verify the above
observation. As a result,
it was observed that Mn sulfide-based inclusions generated from the aggregates
of the
oxides and sulfides of Ca (CaO-CaS) acting as the nuclei were rarely elongated
after hot
rolling; and consequently, the number of Mn sulfide-based inclusions elongated
in the
lengthwise direction was decreased.
Furthermore, as a result of conducting an impact test using the steels, it was
observed that, with regard to steels in which Ca was added and the number of
elongated
CA 02734980 2011-02-22
- Mn 'sulfide-based inclusions was small, the occurrence of fracture
starting from the
elongated Mn sulfide-based inclusions was decreased; and as a result, the
impact values
were improved.
[0028]
5 In addition, in order to further suppress the elongation of Mn sulfide-
based
inclusions, the inventors of the present invention studied the relationship
between the
added amount of Ca and the added amount of S which enable oxides and sulfides
to form
aggregates by conducting test melting and a hot rolling test. As a result, it
was observed
that an appropriate number of Ca sulfides were generated and finely dispersed
by
10 controlling the ratio of the added amount of S and the added amount of
Ca; and
consequently, it was possible to further suppress the elongation of Mn sulfide-
based
inclusions after hot rolling.
[0029]
Furthermore, in addition to the suppressing of generation of elongated Mn
15 sulfide-based inclusions having a bad effect on the toughness, the
inventors of the present
invention studied methods that suppress the grain growth of an austenite
structure after hot
rolling by using Mn sulfide-based inclusions and oxides. As a result of test
melting and a
hot rolling test, it was found that it is necessary to finely disperse nano-
sized oxides and
Mn sulfide-based inclusions, instead of alloy elements formerly used, in an
austenite
structure as pinning elements in order to stably suppress the grain growth of
the austenite
structure.
In view of these circumstances, the inventors of the present invention studied
methods that finely disperse oxides and Mn sulfide-based inclusions. As a
result, it was
observed that the oxides of Mg and Zr did not aggregate, but were finely and
uniformly
dispersed. Furthermore, it was observed that, since both Mg-based oxides and
Zr oxides
CA 02734980 2011-02-22
16
= have a good consistency with Mn sulfide-based inclusions, Mn sulfide-
based inclusions
were also finely dispersed with the fine oxides as the nuclei.
[0030]
Next, the inventors of the present invention conducted a hot rolling test
using
steels including Mg and Zr. As a result, it was observed that nano-sized
oxides and Mn
sulfide-based inclusions were finely dispersed, and the grain growth of an
austenite
structure after hot rolling could be suppressed. Furthermore, as a result of
conducting an
impact test using these steels, it was observed that impact values were
improved by the
refinement of a pearlite structure in the steels including Mg and Zr.
[0031]
The inventors of the present invention conducted a test melting of
experimental
steels by preparing steels including carbon at a content of 1.00% and P at a
content in a
range of 0.0150% or less, adding various contents of S, and further adding Ca,
Mg and Zr.
Next, the inventors conducted a laboratory rolling test which simulated the
equivalent
rolling conditions for rails so as to manufacture prototypes of rails. Then,
the impact
values of the prototypes were measured by an impact test, and the effects of
the amount of
S and the effects of the addition of Ca, Mg and Zr on the impact values were
studied.
Here, the hardness of the materials was set to an Hv level of 400 by
controlling heat
treatment conditions.
[0032]
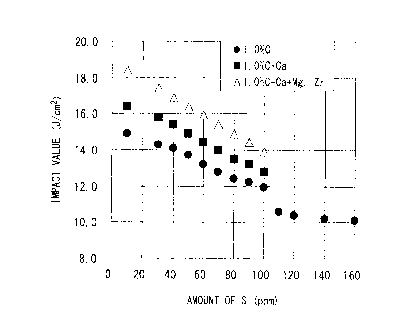
FIG 2 shows the relationship between the amount of S (ppm) and the impact
value. With regard to the steels including C at a content of 1.00% (.marks),
it was
observed that, in the case where the content of P was in a range of 0.0150% or
less, the
impact values were improved if the content of S was reduced to 0.0100% or
less. In
addition, from the results of the steels including Ca (a marks), it was
observed that the
CA 02734980 2011-02-22
17
- generation of the elongated Mn sulfide-based inclusions were suppressed
by the addition
of Ca; and thereby, the impact values were improved. Furthermore, from the
results of
the steels including Ca, Mg and Zr (A marks), it was observed that nano-sized
oxides and
Mn sulfide-based inclusions were finely dispersed by adding Mg and Zr together
with Ca;
and thereby, the impact values were remarkably improved.
[0033]
Based on the above-described study results, the present invention with the
above-described features has been completed. The features of the present
invention will
be described hereinafter.
(1) The reason why the chemical components of the steels are limited:
The reason why the chemical components of the steels are limited within the
above-described numeric ranges in the pearlite rail according to the present
invention will
be described in detail.
C is an effective element that accelerates pearlite transformation and ensures
wear
resistance. In the case where the amount of C is less than 0.65%, in the
present
component system, it is not possible to maintain a minimum level of strength
or wear
resistance required for rails. In addition, in the case where the amount of C
exceeds
1.20%, a large amount of coarse proeutectoid cementite structure is generated;
and thereby,
wear resistance or toughness is degraded. Therefore, the amount of C is
limited to be in
a range of 0.65% to 1.20%. Here, it is preferable that the amount of C is in a
range of
0.90% or more in order to sufficiently ensure wear resistance.
[0034]
Si is an essential element as a deoxidizing material. In addition, Si is an
element
that increases the hardness (strength) of a rail head portion by solid
solution strengthening
in the ferrite phase in a pearlite structure. Furthermore, Si is an element
that suppresses
CA 02734980 2011-02-22
18
- the generation of proeutectoid cementite structures in hypereutectoid
steels; and thereby, a
decrease in toughness is suppressed. However, in the case where the amount of
Si is less
than 0.05%, it is not possible to sufficiently expect such effects. In
addition, in the case
where the amount of Si exceeds 2.00%, a number of surface defects are
generated during
[0035]
Mn is an element that increases hardenability and refines pearlite lamellar
spacing; and thereby, the hardness of the pearlite structure is ensured and
wear resistance
is improved. However, in the case where the amount of Mn is less than 0.05%,
such
[0036]
P is an element inevitably included in steels. The amount of P has a
relationship
with toughness, and, if the amount of P increases, the pearlite structure is
embrittled due to
CA 02734980 2011-02-22
19
= Therefore, the amount of P is desirably small in order to improve
toughness. As a result
of experimentally observing the relationship between the impact value and the
amount of P,
it was observed that, in the case where the amount of P was reduced to 0.0150%
or less,
the segregation of P was remarkably reduced, the embrittlement of the pearlite
structure
which was the starting point of fracture was suppressed; and thereby, impact
values were
greatly improved. From these results, the amount of P is limited to be in a
range of
0.0150% or less. The lower limit of the amount of P is not specified; however,
about
0.0020% is considered to be the lower limit of the amount of P when actually
manufacturing rails in view of dephosphorization capability in a refining
process.
Meanwhile, a treatment for lowering the P amount (reduction of the amount of
P)
is not only accompanied by an increase in refining costs but also by
degradation of
productivity. As a result, in consideration of economic efficiency, it is
preferable that the
amount of P is in a range of 0.0030% to 0.0100% in order to stably improve
impact
values.
[0037]
S is an element inevitably included in steels. The amount of S has a
relationship
with toughness, and if the amount of S increases, stress concentration occurs
due to the
coarsening of MnS or the increase of density of MnS; and thereby, brittle
fracture, that is,
rail damage is easy to occur. Therefore, the amount of S is desirably small in
order to
improve toughness. As a result of experimentally observing the relationship
between the
impact value and the amount of S, it was observed that, if the amount of S was
reduced to
0.0100% or less, the amount of Mn sulfide-based inclusions generated which was
the
starting point of fracture was reduced, and furthermore, the embrittlement of
the pearlite
structure was suppressed by the suppression of the elongation of Mn sulfide-
based
inclusions or the refinement of Mn sulfide-based inclusions due to the
addition of Ca, Zr,
CA 02734980 2011-02-22
or Mg. As a result, the impact value was greatly improved. From these results,
the
amount of S is limited to be in a range of 0.0100% or less. The lower limit of
the amount
of S is not specified; however, about 0.0010% is considered to be the lower
limit of the
amount of S when actually manufacturing rails in view of desulfurization
capability in a
5 refining process.
Meanwhile, a treatment for lowering the S amount (reduction of the amount of
S)
is not only accompanied by an increase in refining costs but also by
degradation of
productivity. As a result, in consideration of economic efficiency, it is
preferable that the
amount of S is in a range of 0.0060% or less in order to suppress generation
of elongated
10 Mn sulfide-based inclusions and stably improve impact values.
In addition, in order to further improve impact values, it is preferable that
the
amount of S is in a range of 0.0020% to 0.0035% in order to stably generate
fine Mn
sulfide-based inclusions which pin the austenite structure and to suppress the
generation of
elongated Mn sulfide-based inclusions.
15 [0038]
Ca is a deoxidizing and desulfurizing element, and aggregates of the oxides
and
sulfides of calcium (CaO-CaS) are generated by the addition of Ca. These
aggregates act
as nuclei for the generation of Mn sulfide-based inclusions; and thereby, the
elongation of
Mn sulfide-based inclusions is suppressed after hot rolling. Furthermore, nano-
sized Mn
20 sulfide-based inclusions are formed from these aggregates as nuclei
(formed by utilizing
the aggregates as nuclei). Ca is an element having such functional effects. In
the case
where the amount of Ca is less than 0.0005%, such effects become small, and
the
aggregates cannot sufficiently act as nuclei for the generation of Mn sulfide-
based
inclusions. In the case where the amount of Ca exceeds 0.0200%, the amount of
independent hard CaO which does not act as the nuclei for Mn sulfide-based
inclusions is
CA 02734980 2011-02-22
21
= increased depending on the amount of oxygen in a steel. As a result, the
toughness of a
rail steel is greatly degraded. Therefore, the amount of Ca is limited to be
in a range of
0.0005% to 0.0200%.
Meanwhile, it is preferable that the amount of Ca is in a range of 0.0015% to
0.0150% in order to improve impact values by stably suppressing the generation
of
elongated Mn sulfide-based inclusions and by suppressing in advance the
generation of
hard CaO which does not act as the nuclei for Mn sulfide-based inclusions and
is harmful
to toughness. In addition, in order to further improve impact values, it is
necessary to
stably generate fine Mn sulfide-based inclusions which pin the austenite
structure so as to
suppress the coarsening of Mn sulfide-based inclusions. Therefore, it is more
preferable
that the amount of Ca is in a range of 0.0020% to 0.0080%.
[0039]
As described above, S and Ca generate the aggregates of the oxides and
sulfides
(CaO-CaS). These aggregates act as nuclei for Mn sulfide-based inclusions; and
therefore, the aggregates greatly affect the elongation of Mn sulfide-based
inclusions.
Therefore, it is important to control the amount of S and the amount of Ca. In
view of
these circumstances, steels with varied amounts of S and Ca were test-melted,
and a hot
rolling test was conducted. As a result, it was found that, in the case where
the ratios of
the amount of Ca to the amount of S (S/Ca) were within a specific range, an
appropriate
number of the oxides and sulfides of Ca were generated and finely dispersed;
and thereby,
it was possible to further suppress the elongation of Mn sulfide-based
inclusions after hot
rolling.
Specifically, in the case where the value of S/Ca is less than 0.45, the
amount of
independent hard CaO which does not act as nuclei for Mn sulfide-based
inclusions is
slightly increased. As a result, there are cases in which the toughness of
rail steels is
CA 02734980 2011-02-22
22
degraded. In the case where the value of S/Ca exceeds 3.00, the number of the
aggregates of sulfides (CaO-CaS) which act as nuclei for Mn sulfide-based
inclusions is
reduced; and thereby, the elongation of Mn sulfide-based inclusions is
promoted. As a
result, there are cases in which the toughness of rail steels is degraded.
Therefore, it is
preferable that the ratio of S/Ca is in a range of 0.45 to 3.00.
[0040]
The present invention preferably includes either one or both of Mg and Zr.
Mg is a deoxidizing element that is mainly bonded with 0 to form a complex of
fine nano-sized oxides (MgO) and sulfides (MgS). Nano-sized Mn sulfide-based
inclusions are formed from the complexes as nuclei (formed by utilizing the
complexes as
nuclei). As a result, the grain growth of an austenite structure after hot
rolling is
suppressed; and thereby, the structure of rail steel is refined. As a result,
it is possible to
improve the toughness of a pearlite structure. However, in the case where the
amount of
Mg is less than 0.0005%, the generated amount of the complexes of fine oxides
(MgO)
and sulfides (MgS) is small; and thereby, the effect of suppressing the grain
growth of an
austenite structure after hot rolling cannot be sufficiently obtained. In the
case where the
amount of Mg exceeds 0.0200%, the coarse oxides of Mg are generated; and
thereby, the
toughness of rails is degraded, and simultaneously, fatigue damage occurs from
the coarse
oxides. Therefore, the amount of Mg is limited to be in a range of 0.0005% to
0.0200%.
Here, it is preferable that the amount of Mg is in a range of 0.0010% to
0.0050%
in order to improve impact values by sufficiently ensuring the generated
amount of fine
oxides (MgO) which pin an austenite structure and the generated amount of the
complexes
of the oxides (MgO) and sulfides (MgS) which form nano-sized Mn sulfide-based
inclusions, and by sufficiently suppressing the generation of coarse oxides
which are
harmful to fatigue damage.
CA 02734980 2011-02-22
23
[0041]
Zr is a deoxidizing element that is mainly bonded with 0 so as to form fine
nano-sized oxides (Zr02). These oxides are dispersed finely and uniformly, and
furthermore, nano-sized Mn sulfide-based inclusions are formed from the oxides
as nuclei
(formed by utilizing the oxides as nuclei). As a result, the grain growth of
an austenite
structure after hot rolling is suppressed; and thereby, the structure of a
rail steel is refined.
As a result, it is possible to improve the toughness of a pearlite structure.
However, in
the case where the amount of Zr is less than 0.0005%, the generated amount of
fine oxides
(Zr02) is small; and thereby, the effect of suppressing the grain growth of an
austenite
structure after hot rolling cannot be sufficiently obtained. In the case where
the amount
of Zr exceeds 0.0100%, the coarse oxides of Zr are generated; and thereby, the
toughness
of rails is degraded, and simultaneously, fatigue damage occurs from the
coarse
precipitates. Therefore, the amount of Zr added is limited to be in a range of
0.0005% to
0.0100%.
Meanwhile, it is preferable that the amount of Mg is in a range of 0.0010% to
0.0050% in order to improve impact values by sufficiently ensuring the
generated amount
fine oxides (Zr02) which pin an austenite structure and the generated amount
of oxides
(Zr02) which form nano-sized Mn sulfide-based inclusions, and by sufficiently
suppressing the generation of coarse oxides which are harmful to fatigue
damage.
[0042]
If necessary, rails manufactured in the above-described component composition
preferably include one or more elements selected from the group consisting of
Co, Cr, Mo,
V, Nb, B, Cu, Ni, Ti, Al and N for the purpose of the improvement in the
hardness
(strength) of a pearlite structure or a proeutectoid ferrite structure, the
improvement in
toughness, the prevention of softening in weld heat-affected zones, and the
control of the
CA 02734980 2011-02-22
24
= cross-sectional hardness distribution inside the rail head portion.
[0043]
Hereinafter, the main purposes and functional effects of the addition of the
above-described elements will be shown.
Co refines a lamellar structure in a rolling contact surface and decreases
ferrite
grain diameter; and thereby, the wear resistance of a pearlite structure is
increased. Cr
and Mo increase the equilibrium transformation point, and mainly refine
pearlite lamellar
spacing; and thereby, the hardness of a pearlite structure is ensured. V and
Nb generate
carbides and nitrides in a hot rolling process and a subsequent cooling
process; and
thereby, the growth of austenite grains= is suppressed. Furthermore, V and Nb
precipitate
and harden in a ferrite structure and a pearlite structure; and thereby, the
toughness and
hardness of a pearlite structure are improved. In addition, V and Nb stably
generate
carbides and nitrides; and thereby, the softening of welded joint heat-
affected zones is
prevented.
B reduces the dependency of the pearlite transformation temperature on a
cooling
rate; and thereby, the hardness distribution in the rail head portion is made
uniform. Cu
is solid-solubilized in a ferrite structure and in a ferrite phase in a
pearlite structure; and
thereby, the hardness of the pearlite structure is increased. Ni improves the
toughness
and hardness of a ferrite structure and a pearlite structure, and
simultaneously, Ni prevents
the softening of welded joint heat-affected zones. Ti refines the structure in
weld
heat-affected zones and prevents the embrittlement of welded joint heat-
affected zones.
Al raises the eutectoid transformation temperature to a higher temperature,
and increases
the hardness of a pearlite structure. N segregates in austenite grain
boundaries; and
thereby, pearlite transformation is accelerated. In addition, N refines the
size of pearlite
blocks; and thereby, toughness is improved.
CA 02734980 2011-02-22
[0044]
Hereinafter, the reason why the amounts of these components are limited will
be
described in detail.
Co is solid-solubilized in a ferrite phase in a pearlite structure. Thereby,
fine
5 ferrite structure formed by the contact with wheels at the rolling
contact surface of the rail
head portion is further refined; and as a result, wear resistance is improved.
In the case
where the amount of Co is less than 0.01%, the refinement of ferrite structure
is not
achieved; and therefore, it is not possible to expect the effect of improving
the wear
resistance. In addition, even in the case where the amount of Co exceeds
1.00%, the
10 above-described effect is saturated; and therefore, the refinement of
ferrite structure
corresponding to the added amount of Co is not achieved. In addition, an
increase in the
cost for adding alloy elements degrades economic efficiency. Therefore, the
amount of
Co is limited to be in a range of 0.01% to 1.00%.
[0045]
15 Cr increases the equilibrium transformation temperature, and
consequently Cr
refines ferrite structure and pearlite structure; and thereby, Cr contributes
to an increase of
hardness (strength). At the same time, Cr strengthens cementite phase; and
thereby, the
hardness (strength) of pearlite structure is improved. However, in the case
where the
amount of Cr is less than 0.01%, such an effect becomes small, and the effect
of
20 improving the hardness of a rail steel is not observed at all. In the
case where Cr is
excessively added at an amount of more than 2.00%, hardenability is increased,
and
martensite structure is generated. Thereby, spalling damage starting from the
martensite
structure occurs in the head corner portions and the head top portion; and as
a result,
resistance to surface damages is degraded. Therefore, the amount of Cr is
limited to be
25 in a range of 0.01% to 2.00%.
CA 02734980 2011-02-22
26
[0046]
Mo, similarly to Cr, increases the equilibrium transformation temperature, and
consequently Mo refines ferrite structure and pearlite structure; and thereby,
Mo
contributes to an increase of hardness (strength). Therefore, Mo is an element
that
improves hardness (strength). However, in the case where the amount of Mo is
less than
0.01%, such an effect becomes small, and the effect of improving the hardness
of rail
steels is not observed at all. In the case where Mo is excessively added at an
amount of
more than 0.50%, transformation rate is remarkably degraded. Thereby, spalling
damage
starting from the martensite structure occurs in the head corner portions and
the head top
portion; and as a result, resistance to surface damages is degraded.
Therefore, the
amount of Mo is limited to be in a range of 0.01% to 0.50%.
[0047]
V refines austenite grains due to the pinning effect of V carbides and V
nitrides in
the case where a heat treatment is conducted at high temperatures.
Furthermore, V
increases the hardness (strength) of ferrite structure and pearlite structure
due to the
precipitation hardening of V carbides and V nitrides generated in the cooling
process after
hot rolling, and simultaneously, V improves toughness. V is an effective
element to
obtain those effects. In addition, in heat-affected portions that are reheated
to a
temperature in a range of Acl or less, V is an effective element to prevent
the softening of
welded joint heat-affected zones by generating V carbides and V nitrides in a
relatively
high temperature range. However, in the case where the amount of V is less
than 0.005%,
such an effect cannot be sufficiently expected, and the improvement in the
hardness and
the toughness of the ferrite structure and the pearlite structure is not
observed. In the
case where the amount of V exceeds 0.50%, the precipitation hardening of the
carbides
and nitrides of V becomes excessive, and the toughness of the ferrite
structure and the
CA 02734980 2011-02-22
27
pearlite structure is degraded. Thereby, spalling damage occurs in the head
corner
portions and the head top portion; and as a result, resistance to surface
damages is
degraded. Therefore, the amount of V is limited to be in a range of 0.005% to
0.50%.
[0048]
Nb, similarly to V, refines austenite grains due to the pinning effect of Nb
carbides and Nb nitrides in the case where a heat treatment is conducted at
high
temperatures. Furthermore, Nb increases the hardness (strength) of ferrite
structure and
pearlite structure due to the precipitation hardening of Nb carbides and Nb
nitrides
generated in the cooling process after hot rolling, and simultaneously, Nb
improves
toughness. Nb is an effective element to obtain those effect. In addition, in
heat-affected portions that are reheated to a temperature in a range of Acl or
less, Nb is an
effective element to prevent the softening of welded joint heat-affected zones
by stably
generating the carbides of Nb and the nitrides of Nb from a low temperature
range to a
high temperature range. However, in the case where the amount of Nb is less
than
0.002%, such an effect cannot be expected, and the improvement in the hardness
and the
the toughness of the ferrite structure and the pearlite structure is not
observed. In the
case where the amount of Nb exceeds 0.050%, the precipitation hardening of the
carbides
and nitrides of Nb becomes excessive, and the toughness of ferrite structure
and the
pearlite structure is degraded. Thereby, spalling damage occurs in the head
corner
portions and the head top portion; and as a result, resistance to surface
damages is
degraded. Therefore, the amount of Nb is limited to be in a range of 0.002% to
0.050%.
[0049]
B forms iron borocarbides (Fe23(CB)6) in austenite grain boundaries, and B
accelerates pearlite transformation. This effect of accelerating pearlite
transformation
reduces the dependency of the pearlite transformation temperature on a cooling
rate; and
CA 02734980 2011-02-22
28
. thereby, more uniform hardness distribution is achieved from the head
surface portion to
the inside portion of a rail. Therefore, it is possible to extend the usable
period of the rail.
In the case where the amount of B is less than 0.0001%, those effects are not
sufficient,
and improvement of the hardness distribution in the rail head portion is not
observed. In
the case where the amount of B exceeds 0.0050%, coarse iron borocarbides are
generated;
and thereby, toughness is degraded. Therefore, the amount of B is limited to
be in a
range of 0.0001% to 0.0050%.
[0050]
Cu is an element that is solid-solubilized in a ferrite structure and in a
ferrite
phase in a pearlite structure, and Cu improves the hardness (strength) of the
pearlite
structure due to solid solution strengthening. In the case where the amount of
Cu is less
than 0.01%, those effects cannot be expected. In the case where the amount of
Cu
exceeds 1.00%, martensite structure, which is harmful to toughness, is
generated by the
remarkable improvement of hardenability. Thereby, spalling damage occurs in
the head
corner portions and the head top portion; and as a result, resistance to
surface damages is
degraded. Therefore, the amount of Cu is limited to be in a range of 0.01% to
1.00%.
[0051]
Ni is an element that improves toughness of a ferrite structure and a pearlite
structure, and simultaneously, Ni increases hardness (strength) by solid
solution
strengthening. Furthermore, Ni finely precipitates intermetallic compound of
Ni3Ti,
which is a complex compound with Ti, in weld heat-affected zones; and thereby,
softening
is suppressed by precipitation strengthening. In the case where the amount of
Ni is less
than 0.01%, those effects are extremely small. In the case where the amount of
Ni
exceeds 1.00%, toughness of a ferrite structure and a pearlite structure is
remarkably
degraded. Thereby, spalling damage occurs in the head corner portions and the
head top
CA 02734980 2011-02-22
29
= portion; and as a result, resistance to surface damages is degraded.
Therefore, the
amount of Ni is limited to be in a range of 0.01% to 1.00%.
[0052]
Ti is an effective element that refines the structure of heat-affected zones
which
are heated to an austenite range by utilizing the fact that carbides of Ti and
nitrides of Ti,
which are precipitated during the reheating in welding, are not melted; and
thereby, Ti
prevents the embrittlement of welded joint portions. However, in the case
where the
amount of Ti is less than 0.0050%, those effects are small, and in the case
where the
amount of Ti exceeds 0.0500%, coarse carbides of Ti and nitrides of Ti are
generated; and
thereby, toughness of a rail is degraded. At the same time, fatigue damage
occurs due to
coarse precipitates. Therefore, the amount of Ti is limited to be in a range
of 0.0050% to
0.050%.
[0053]
Al is an essential element as a deoxidizing material. In addition, Al is an
element that raises the eutectoid transformation temperature to a higher
temperature, and
Al contributes to an increase in the hardness (strength) of a pearlite
structure. In the case
where the amount of Al is 0.0100% or less, those effects are small. In the
case where the
amount of Al exceeds 1.00%, it becomes difficult to solid-solubilize Al in a
steel; and
thereby, coarse alumina-based inclusions are generated. Thereby, toughness of
a rail is
degraded, and simultaneously, fatigue damage occurs due to coarse
precipitates.
Furthermore, oxides are generated during welding; and thereby, weldability is
degraded
remarkably. Accordingly, the amount of Al is limited to be in a range of more
than
0.0100% to 1.00%.
[0054]
N segregates in austenite grain boundaries; and thereby, N accelerates ferrite
CA 02734980 2011-02-22
traniformation and pearlite transformation from the austenite grain
boundaries. As a
result, the size of pearlite blocks is mainly refined; and thereby, it is
possible to improve
toughness. However, in the case where the amount of N is less than 0.0060%,
those
effects are small. In the case where the amount of N exceeds 0.0200%, it
becomes
5 difficult to solid-solubilize N in a steel. As a result, air bubbles
which act as the starting
points of fatigue damage are generated; and thereby, fatigue damage occurs
inside the rail
head portion. Therefore, the amount of N is limited to be in a range of
0.0060% to
0.0200%.
[0055]
10 (2) The reasons why the regions and hardness of pearlite structure in
the rail head
surface portion 3a are limited:
Next, the reasons why the head surface portion 3a of a rail includes a
pearlite
structure and the hardness Hv thereof is limited to be in a range of 320 to
500 will be
described.
15 At first, the reason why the hardness Hv of a pearlite structure is
limited to be in a
range of 320 to 500 will be described.
In the present component system, in the case where the hardness Hv of the
pearlite structure is less than 320, it becomes difficult to ensure the wear
resistance of the
head surface portion 3a of the rail; and thereby, the usable period of the
rail is reduced.
20 In addition, flaking damage occurs in the rolling contact surface due to
plastic
deformation; and thereby, the resistance to surface damages in the rail head
surface portion
3a is greatly degraded. In addition, in the case where the hardness Hv of a
pearlite
structure exceeds 500, the toughness of the pearlite structure is greatly
degraded; and
thereby, the damage resistance in the rail head surface portion 3a is
degraded. Therefore,
25 the hardness Hv of the pearlite structure is limited to be in a range of
320 to 500.
CA 02734980 2011-02-22
31
,
= [0056]
Next, the reason why a range necessary to include a pearlite structure having
a
hardness Hy in a range of 320 to 500 is limited to the head surface portion 3a
of a rail steel
will be described.
Here, the head surface portion 3a of a rail refers to, as shown in FIG 1, a
portion
ranging from surfaces of the head corner portions 2 and the head top portion 1
to a depth
of 10 mm (diagonal solid line area). If a pearlite structure having the above-
described
components is disposed in the head surface portion 3a, abrasion due to the
contact with
wheels is suppressed; and thereby, the wear resistance of the rail is
improved.
[0057]
In addition, it is preferable to dispose a pearlite structure having a
hardness fly in
a range of 320 to 500 in a portion 3b ranging from the surfaces of the head
corner portions
2 and the head top portion 1 to a depth of 20 mm, that is, at least in the
diagonal dotted
line area in FIG 1. Thereby, wear resistance is further ensured even in the
case where
abrasion occurs in the deeper inside of the rail head portion due to the
contact with
wheels; and thereby, the usable period of rails is improved. Therefore, it is
preferable to
dispose a pearlite structure having a hardness Hy in a range of 320 to 500 at
or in the
vicinity of the surface of the rail head portion 3, with which the wheels
mainly contact,
and other portions may be a metallographic structure other than the pearlite
structure.
Meanwhile, with regard to a method to obtain a pearlite structure having a
hardness Hy in a range of 320 to 500 at or in the vicinity of the surface of
the rail head
portion 3, as described below, it is preferable to conduct an accelerated
cooling on a rail
head portion 3 including an austenite region in a high-temperature state after
hot rolling or
reheating.
[0058]
CA 02734980 2013-03-05
32
Among the rail head portion 3 in the present invention, it is preferable that
the
metallographic structure in the head surface portion 3a or in the portion 3b
which ranging
to a depth of 20 mm and including the head surface portion 3a consists of the
above-described pearlite structure. However, depending on the component
compositions
of a rail and the conditions of heat treatments and manufacturing methods,
there are cases
in which the pearlite structure is mixed with proeutectoid ferrite structure,
proeutectoid
cementite structure, bainite structure and martensite structure at a small
amount, for
example, an area ratio of 5% or less. Even in the case where the above-
described
structures are contained at a content of 5% or less, these structures do not
have a major
adverse affect on the wear resistance and the toughness of the rail head
portion 3.
Therefore, the above-described pearlite structure may include structures mixed
with
proeutectoid ferrite structure, proeutectoid cementite structure, bainite
structure,
martensite structure or the like at an area ratio of 5% or less.
In other words, among the rail head portion 3 in the present invention, 95% or
more of the metallographic structure in the head surface portion 3a or the
portion 3b
ranging to a depth of 20 mm and including the head surface portion 3a needs to
be a
pearlite structure, and it is preferable that 98% or more of the
metallographic structure in
the head portion be a pearlite structure in order to sufficiently ensure wear
resistance and
toughness.
Meanwhile, in the columns of 'Microstructure' in Tables 1 and 2 below, the
description 'small amount' refers to a content of 5% or less, and structures
other than a
pearlite structure without the description 'small amount' mean that the
structures are
included at an amount of more than 5% (out of the range of the present
invention).
[00591
(3) The reason why the number (per unit area) of Mn sulfide-based inclusions
CA 02734980 2011-02-22
33
' having major axes (major lengths) in a range of 10 pm to 100 in is limited:
The reason why, in the present invention, the length of the major axis (major
length) of Mn sulfide-based inclusions in an arbitrary cross-section taken
along the
lengthwise direction, which are evaluation subjects, is limited to be in a
range of 10 p.m to
100 pm will be described in detail.
As a result of investigating the length of the major axis of Mn sulfide-based
inclusions and the actual damage performance of actual rails (damage status
when actually
using rails), in the present component system, it was observed that the
fracture of rails
occurred from the end portions of Mn sulfide-based inclusions, at which stress
concentration occurred. In view of these circumstances, steels were test-
melted to
include Mn sulfide-based inclusions having various lengths of the major axis,
and a hot
rolling test was conducted. As a result, it was observed that there was a good
relationship between the number of Mn sulfide-based inclusions having lengths
of the
major axis in a range of 10 p.m to 100 pm and the damage resistance of the
rail.
Consequently, the length of the major axis of Mn sulfide-based inclusions
eligible for the
evaluation subjects to count the numbers is limited to be in a range of 10 pm
to 100 p.m.
[0060]
Meanwhile, Mn sulfide-based inclusions having a long length of the major axis,
in which stress concentration occurs remarkably, have a large effect on damage
resistance,
and Mn sulfide-based inclusions having a short length of the major axis have a
small
effect on the damage resistance. However, in the steel according to the
present invention,
there are a small number of Mn sulfide-based inclusions having a length
exceeding 100
pm, which are not suitable to identify the characteristics of the steels. And
Mn
sulfide-based inclusions having a length of less than 10 p.m have a small
effect on the
CA 02734980 2011-02-22
34 =
' damage resistance. Therefore, Mn sulfide-based inclusions having the above-
described
lengths of the major axis (major lengths) are used as evaluation subjects.
[0061]
Next, the reason why the number (per unit area) of Mn sulfide-based inclusions
having major lengths in a range of 10 p.m to 100 p.m in an arbitrary cross-
section taken
along the lengthwise direction (a cross-section parallel to the longitudinal
direction of a
rail) is limited to be in a range of 10 /mm2 to 200 /mm2 will be described in
detail.
In the case where the total number (per unit area) of Mn sulfide-based
inclusions
having major lengths in a range of 10 pm to 100 pm exceeds 200 /min2, in the
present
component system, the number of Mn sulfide-based inclusions becomes excessive;
and
thereby, the possibility of rail damage increases due to the generation of
stress
concentration at or in the vicinity of the inclusions. Even in terms of the
mechanical
characteristics of the steel, it is not possible to improve the impact value.
In the case
where the total number (per unit area) of Mn sulfide-based inclusions having
major
lengths in a range of 10 pm to 100 p.m is less than 10 /mm2, in the present
component
system, trap sites which absorb inevitable hydrogen remaining in the steel are
remarkably
reduced. Thereby, the possibility of inducing hydrogenous defects (hydrogen
embrittlment) increases; and thereby, the damage resistance of the rail is
impaired. As a
result, the total number (per unit area) of Mn sulfide-based inclusions having
major
lengths in a range of 10 pm to 100 j.tm is limited to be in a range of 10 /mm2
to 200 /mm2.
Meanwhile, in the present limitation, the Mn sulfide-based inclusions refer to
both of Mn sulfide-based inclusions generated from aggregates of oxides and
sulfides of
calcium (CaO-CaS) as nuclei and other Mn sulfide-based inclusions as
evaluation
subjects.
CA 02734980 2013-03-05
[0062]
In addition, with regard to the number of Mn sulfide-based inclusions, a
sample is
taken from a cross-section taken along the lengthwise direction of the rail
head portion 3,
in which the rail damage becomes obvious as shown in FIG. 3, and the
measurement of
5 sulfide-based inclusions is conducted. The cross-section in the
lengthwise direction of
the rail of each of the taken samples is mirror-polished, and Mn sulfide-based
inclusions
are investigated on an arbitrary cross-section with an optical microscope.
Then, the
number of inclusions having the above-limited sizes is counted and calculated
as the
number per unit cross-section area. The typical value of each rail steel is
obtained from
10 the average value of the numbers per unit cross-section area of these 20
viewing fields.
The location (portion) to be used to investigate Mn sulfide-based inclusions
is not
particularly limited; however, it is preferable to observe a portion ranging
from the surface
of the rail head portion 3, which acts as the starting point of damage, to a
depth of 3 to 10
mm.
15 In addition, in order to stably improve fracture resistance of a rail
by
further decreasing the effect of Mn sulfide-based inclusions which act as the
starting
points of fracture and by suppressing hydrogenous defects in advance, it is
preferable to
control the total number (per unit area) of Mn sulfide-based inclusions having
major
lengths in a range of 10 wn to 100 !dm to be in a range of 20/mm2 to 180/mm2.
20 [0063]
(4) The reason why the number (per unit area) of Mg-based oxides, Zr oxides
and
Mn sulfide-based inclusions having grain diameters in a range of 5 nm to 100
nm is
limited:
In the present invention, it is preferable that Mg-based oxides, Zr oxides,
and Mn
25 sulfide-based inclusions having grain diameters in a range of 5 nm to
100 nm are present
CA 02734980 2011-02-22
36
. at an amount per unit area in a range of 500/mm2 to 50,000/mm2 in an
arbitrary transverse
cross-section.
The reason why the grain diameters of Mg-based oxides, Zr oxides and Mn
sulfide-based inclusions, which are evaluation subjects, is limited to be in a
range of 5 nm
to 100 nm will be described in detail.
In the case where the grain diameters of Mg-based oxides, Zr oxides and Mn
sulfide-based inclusions is in a range of from 5 nm to 100 nm, a sufficient
pinning effect is
obtained in grain boundaries when they are generated in an austenite
structure. Thereby,
it was observed that, without adversely affecting the damage resistance of a
rail,
consequently, a pearlite structure was refined; and thereby, toughness was
reliably
improved. Therefore, the grain diameters of Mg-based oxides, Zr oxides and Mn
sulfide-based inclusions eligible for the evaluation subjects is limited to be
in a range of 5
nm to 100 nm.
Meanwhile, with regard to the pinning effect, the more inclusions having fine
grain diameters are present, the larger the effect becomes. However, with
regard to
Mg-based oxides, Zr oxides and Mn sulfide-based inclusions having grain
diameters in a
range of less than 5 nm, it is extremely difficult to measure the number
thereof. In
addition, with regard to Mg-based oxides, Zr oxides and Mn sulfide-based
inclusions
having grain diameters in a range of more than 100 nm, the above-described
pinning effect
cannot be obtained. Therefore, Mg-based oxides, Zr oxides and Mn sulfide-based
inclusions having the above-described grain diameters are used as evaluation
subjects.
[0064]
Next, regarding the preferable configurations, the reason why the amount
(number) (per nu-n2) of Mg-based oxides, Zr oxides and Mn sulfide-based
inclusions
having grain diameters in a range of 5 nm to 100 nm in an arbitrary cross-
section in the
CA 02734980 2011-02-22
37
lengthwise direction is limited to be in a range of 500 to 50,000 will be
described in detail.
In the case where the total number (per unit area) of Mg-based oxides, Zr
oxides
and Mn sulfide-based inclusions having grain diameters in a range of 5 nm to
100 nm is
less than 500/mm2, the pinning effect is not sufficiently obtained in an
austenite structure
after hot rolling. As a result, a pearlite structure becomes coarsened, and
toughness of
the rail is not improved. In the case where the total number (per unit area)
of Mg-based
oxides, Zr oxides and Mn sulfide-based inclusions having grain diameters in a
range of 5
nm to 100 nm exceeds 50,000/mm2, precipitation occurs excessively, and a
pearlite
structure becomes embrittled; and thereby, the toughness of the rail is
degraded.
Therefore, the total number (per unit area) of Mg-based oxides, Zr oxides and
Mn
sulfide-based inclusions having grain diameters in a range of 5 nm to 100 nm
is limited to
be in a range of 500/mm2 to 50,000/mm2.
[0065]
Meanwhile, in the present limitation, the Mg-based oxides and the Zr oxides
refer
to oxides partially including complex oxides such as Mn sulfide or the like.
In addition,
the Mn sulfide-based inclusions refer to inclusions generated from fine oxides
such as Mg
oxides, Zr oxides, Ca oxides or the like, as nuclei.
The grain diameter and the number of the Mg-based oxides, the Zr oxides and
the
Mn sulfide-based inclusions are observed and measured in the following manner.
At first,
a thin film is taken from an arbitrary transverse cross-section shown in FIG.
4, and the thin
film is observed at a magnification of 50,000 to 500,000 using a transmission
electron
microscope. The grain diameter of precipitates is obtained by measuring the
area of each
precipitate through observation and calculating the diameter of a circle
having the same
area as that of the precipitate.
The precipitates are observed at 20 viewing fields, and the number of
precipitates
CA 02734980 2011-02-22
38
, having diameters in a predetermined range of 5 nm to 100 nrn is counted,
and the number
per unit area is calculated from the counted number. The typical value of a
rail steel is
obtained from the average value of these 20 viewing fields. Meanwhile, the
location
(portion) to be used to investigate the Mg-based oxides, the Zr oxides, and
the Mn
sulfide-based inclusions is not particularly limited; however, it is
preferable to observe a
portion ranging from the surface of the rail head surface portion 3a to a
depth of 3 mm to
mm, which requires toughness.
[00661
(5) Method for manufacturing the rail steel (rail) according to the present
10 invention:
The method for manufacturing the rail steel according to the present invention
including the above-described component composition and microstructure is not
particularly limited; however, in general, the rail steel is manufactured by
the following
method. At first, melting is conducted so as to obtain molten steel with a
commonly
used melting furnace such as a converter furnace, an electric furnace or the
like. Then,
the molten steel is subjected to an ingot-making and blooming method or a
continuous
casting method so as to manufacture a bloom (a steel ingot) for rolling.
Furthermore, the
bloom is reheated to 1200 C or more, and then, the bloom is subjected to
several passes of
hot rolling, and molded into rails. Thereafter, heat treatments (reheating and
cooling) are
conducted so as to manufacture a rail.
In particular, in the hot metal step, general desulfurization and
dephosphorization
are conducted (dephosphorization and desulfurization treatment), and
furthermore,
sufficient desulfurization and dephosphorization are conducted in a commonly
used
melting furnace such as a converter furnace, an electric furnace or the like
(dephosphorization and desulfurization treatment). Next, Ca is added to
control Mn
CA 02734980 2011-02-22
39
= sulfide-based inclusions. Furthermore, according to necessity, Mg and Zr
are added to
finely disperse nano-sized oxides and Mn sulfide-based inclusions.
The details of the manufacturing conditions will be shown below.
[0067]
In the hot metal step, it is preferable to conduct general dephosphorization
treatment and desulfurization treatment in a careful manner to achieve the
reduction of the
amounts of P and S.
Regarding desulfurization, it is preferable to add CaO slowly and sufficiently
in a
hot-metal ladle (a preceding step of refining in a converter furnace), and to
eject CaS as
slag.
Meanwhile, the addition of CaO is a method conducted in the case where S is
reduced from a hot metal having an extremely large amount of S. Unlike the
addition of
CaO-Si alloy, which is added to generate aggregates of oxides and sulfides of
calcium
(CaO-CaS), as described below, this method has no influence.
Regarding dephosphorization, it is preferable, in refining in a converter
furnace,
to eject slag in the middle of refining in order to prevent P from being
melted again from
the slag including P (P205 or the like) separated by dephosphorization.
[0068]
Next, Ca is added so as to control Mn sulfide-based inclusions.
It is preferable to add Ca in a refining process prior to casting. A
preferable
adding method of Ca is either adding Ca alloy (Ca-Si alloy or the like) wires
or Ca alloy
ingots in a ladle or injecting a Ca alloy powder.
As the Ca alloy, a Ca-Si alloy (50Ca-50Si or the like), a Fe-Si-Ca alloy
(Fe-30Si-30Ca or the like) and a Ni-Ca alloy (90Ni-10Ca or the like) are used.
Since the
CA 02734980 2011-02-22
- slag on the surface of the molten steel is involved into the molten
steel; and thereby, the
purity of the molten steel is degraded. In addition, the yield rate becomes
low.
Consequently, the addition of a Ca alloy, for example, a Ca-Si alloy is widely
conducted.
Compared with pure Ca, the activity of Ca is lowered in the Ca alloy.
Therefore, in the
5 case of adding the Ca alloy, vaporization during the addition becomes
relatively gentle,
and the yield rate is also improved.
The lower the concentration of Ca in the alloy is, the more the yield rate is
improved, and the generation of splashing during the addition is also
suppressed.
Therefore, the low concentration of Ca in the alloy is preferable. However,
since
10 elements other than Ca (Si or the like) are included in the case where
the concentration of
Ca is low, it is necessary to carefully select the composition of the Ca
alloy.
[0069]
In order to prevent the aggregation or segregation of the aggregates of the
oxides
and sulfides of calcium (CaO-CaS), it is preferable to stir the molten steel
via Ar bubbling
15 or the like in the ladle after the addition of the Ca alloy so as to
make the concentration of
Ca uniform and to float large-sized inclusions. In the case where an amount of
the
molten steel is 200 t or more, it is preferable to conduct the stirring for
about 5 minutes to
10 minutes. Excessive stirring causes the aggregation of inclusions; and
thereby, the
inclusions coarsen. Therefore, excessive stirring is not preferable.
20 From the viewpoint of ensuring the yield rate of Ca, it is
advantageous to perform
the addition of a Ca alloy at the final stage of a refining process. Ca may be
added to a
tundish in a casting process, instead of the refining process. It is necessary
to adjust the
addition rate of a Ca alloy depending on the throughput during casting (the
casting amount
per hour). In this case, since the stirring of the molten steel after the
addition of Ca is
25 conducted inside the tundish or a casting mold, the uniformity of the
concentration of Ca
CA 02734980 2011-02-22
41
is slightly worse than that in the case of adding Ca in the ladle. Therefore,
it is preferable
to stir the molten steel during solidification via an electromagnetic force or
the like in
order to prevent the aggregation or segregation of the aggregates of the
oxides and sulfides
of calcium (CaO-CaS) in the casting step. In addition, it is preferable to
optimize the
shape of a casting nozzle in order to control the flow of the molten steel
during the
casting.
Furthermore, in order to efficiently generate CaS having a high consistency
with
Mn sulfide-based inclusions, it is preferable to adjust the amount of oxygen
in the molten
steel so as to suppress the generation of an excessive amount of CaO. In order
to adjust
the amount of oxygen in advance, it is preferable to deoxidize the molten
steel in advance
via Al, Si or the like.
[0070]
In addition, in order to finely disperse fine nano-sized oxides and Mn
sulfide-based inclusions, it is preferable to add pure metallic Mg, an Mg
alloy (Fe-Si-Mg,
Fe-Mn-Mg, Fe-Si-Mn-Mg and Si-Mg) or a Zr alloy (Fe-Si-Zr, Fe-Mn-Mg-Zr and
Fe-Si-Mn-Mg-Zr) in a molten-steel ladle at high temperatures after general
refining or in a
tundish during casting. Furthermore, it is preferable to stir the molten steel
during
solidification via an electromagnetic force or the like in order to prevent
the aggregation or
segregation in the casting step. In addition, it is preferable to optimize the
shape of a
casting nozzle in order to control the flow of the molten steel during the
casting.
Here, although the order of adding Ca, Mg and Zr is not clearly described, in
a
high-carbon steel including a small amount of oxygen, it is preferable to add
Ca having a
relatively weak oxidizing power at first, and then to add Mg and Zr having
strong
oxidizing powers in order to generate oxides of Ca, Mg and Zr with a good
efficiency.
[0071]
CA 02734980 2013-03-05
42
In hot rolling, the temperature at which the final molding is conducted is
preferably in a range of 900 C to 1000 C from the viewpoint of ensuring the
shape and
material.
In addition, regarding the heat treatment after the hot rolling, it is
preferable to
conduct accelerated cooling on a rail head portion 3 at high temperatures
including
austenite regions after hot rolling or reheating in order to obtain a pearlite
structure with a
hardness Hv of 320 to 500 in the rail head portion 3. As the accelerated
cooling method,
by conducting the heat treatment (and cooling) with a method described in
Patent
Document 8 (Japanese Unexamined Patent Application, Publication No. 1-108-
246100),
Patent Document 9 (Japanese Unexamined Patent Application, Publication No.
H09-111352), it is possible to obtain a structure and hardness in
predetermined ranges.
Here, in order to conduct the heat treatment with reheating after the rolling
of the
rail, it is preferable to heat the rail head portion or the entire rail with a
flame or induction
heating.
EXAMPLES
[0072]
Next, examples of the present invention will be described.
Tables 1 to 6 show the chemical components of tested rail steels. Here, the
balance consists of Fe and inevitable impurities. Rail steels having the
component
compositions shown in Tables 1 to 6 were manufactured in the following manner.
Dephosphorization and desulfurization were conducted in a hot metal step, and,
furthermore, sufficient dephosphorization and desulfurization were conducted
in a
commonly used melting furnace such as a converter furnace, an electric furnace
or the like
CA 02734980 2011-02-22
43
= so as to obtain molten steel. Ca was added to the molten steel so as to
control Mn
sulfide-based inclusions, or Mg and Zr were further added so as to finely
disperse
nano-sized oxides and Mn sulfide-based inclusions. Then, a steel ingot was
manufactured by a continuous casting method, and hot rolling was conducted on
the steel
ingot. Thereafter, a heat treatment was conducted so as to manufacture a rail.
[0073]
0
0 Table 1
I.)
-1
L..,
.
.1,.
l0 Chemical components
(mass %)
0
0 Rail Steel
Co, Cr, Mo, V, Nb, S/Ca
I.) C Si Mn P S
Ca Mg, Zr
0
B, Cu, Ni, Ti, Al, N
H
H
I
0 1 0.65 0.25 0.80 0.0100 0.0050
0.0020 Mg: 0.0020 Cu: 0.15 2.50
I.)
1
I.)
I.)
2 1.20 0.25 0.80 0.0100 0.0050 0.0020 Mg: 0.0020
Cu: 0.15 2.50
3 0.85 0.05 0.60 0.0120 0.0070 0.0080 -
0.88
4 0.85 2.00 0.60 0.0120 0.0070 0.0080
0.88
0020
.41.
4=.
5 0.90 0.30 0.05 0.0060 0.0040 0.0060 Mg: 0.
Cr: 0.25 0.67
Zr: 0.0012
Rail steels
Mg: 0.0020
6 0.90 0.30 2.00 0.0060 0.0040 0.0060
Cr: 0.25 0.67
of the
Zr: 0.0012
present
7 1.00 0.50 1.00 0.0150 0.0030 0.0100 -
0.30
invention
8 1.00 0.50 1.00 0.0020 0.0030 0.0100 -
0.30
9 1.10 0.50 0.70 0.0150 0.0100 0.0120 Zr: 0.0015
0.83
10 1.10 0.50 0.70 0.0020 0.0010 0.0120 Zr: 0.0015
0.08
11 0.95 0.95 0.80 0.0070 0.0030 0.0005 -
Ti: 0.01 6.00
12 0.95 0.95 0.80 0.0070 0.0030 0.0200 -
Ti: 0.01 0.15
,
[0074]
Table 2
Chemical components (mass %)
Rail Steel
Co, Cr, Mo, V, Nb, S/Ca
C Si Mn P S Ca
Mg, Zr
B, Cu, Ni, Ti, Al, N
13 0.65 0.30 0.75 0.0080 0.0050 0.0190 -
0.26
14 0.65 0.30 0.75 0.0080 0.0050 0.0035
1.43
0012
15 0.65 0.30 0.75 0.0080 0.0050 0.0035 Mg: 0.
1.43 0
Zr: 0.0015
4=. 2
16 0.70 0.30 0.75 0.0040 0.0060 0.0020 Zr: 0.0020
3.00 (.,-. ....,
a,
l0
CO
17 0.70 1.25 0.20 0.0140 0.0020 0.0040 -
Ni: 0.25 0.50 0
I.)
0
Rail steel
H
s
H
I
18 0.75 0.50 1.00 0.0130 0.0060 0.0008 -
Nb: 0.01 7.50
of the
0
I.)
I
present
I.)
19 0.75 0.50 1.00 0.0130 0.0060 0.0080 -
Nb: 0.01 0.75 "
invention
20 0.75 0.50 1.00 0.0130 0.0060 0.0080 Mg: 0.0050
Nb: 0.01 0.75
21 0.80 0.40 1.10 0.0100 0.0100 0.0020 -
5.00
22 0.80 0.40 1.10 0.0100 0.0060 0.0020 -
3.00
23 0.80 0.40 1.10 0.0100 0.0020 0.0020 -
1.00
24 0.85 0.55 0.85 0.0060 0.0080 0.0009 -
8.89
[0075]
Table 3
Chemical components (mass %)
Rail Steel C Si Mn P S Ca
Mg, Zr Co, Cr, Mo, V, Nb, S/Ca
B, Cu, Ni, Ti, Al, N
25 0.85 0.55 0.85 0.0060 0.0080 0.0050
1.60
0040
26 0.85 0.55 0.85 0.0060 0.0080 0.0050
Mg: 0. 1.60
Zr: 0.0025
27 0.90 0.30 1.25 0.0050 0.0095 0.0140
Zr: 0.0050 0.68 n
0
28 0.90 0.30 1.25 0.0050 0.0095 0.0140
Zr: 0.0050 Co: 0.30 0.68
UJ
FP
l0
CO
29 0.95 0.95 0.80 0.0070 0.0030 0.0005
- Ti: 0.01 6.00 0
I.)
0
Rail steels
H
H
30 0.95 0.95 0.80 0.0070 0.0030 0.0030
Ti: 0.01 1.00 1
of the
0
I.)
I
present
Mg: 0.0020 K)
31 0.95 0.95 0.80 0.0070 0.0030 0.0030
Ti: 0.01 1.00 "
invention
Zr: 0.0030
32 0.95 0.25 1.20 0.0095 0.0095 0.0150
- Mo: 0.02 0.63
33 1.00 0.50 0.70 0.0040 0.0080 0.0009
- Cr: 0.20 8.89
34 1.00 0.50 0.70 0.0040 0.0080 0.0045
- Cr: 0.20 1.78
35 1.00 0.50 0.70 0.0040 0.0080 0.0045
Mg: 0.0050 Cr: 0.20 1.78
36 1.05 0.10 0.90 0.0050 0.0025 0.0160
- Al: 0.0080 0.16
[0076]
Table 4
Chemical components (mass %)
Rail Steel C Si Mn P S Ca
Mg, Zr Co, Cr, Mo, V, Nb, S/Ca
B, Cu, Ni, Ti, Al, N
37 1.05 0.10 0.90 0.0050 0.0025 0.0030
Al: 0.0080 0.83
0050
38 1.05 0.10 0.90 0.0050 0.0025 0.0030
Mg: 0. Al: 0.0080 0.83
Zr: 0.0010
39 1.05 0.85 0.80 0.0030 0.0040 0.0050
Mg: 0.0007 B: 0.0020, Ti: 0.01 0.80 n
0
0005
.,.1
40 1.10 0.50 0.70 0.0040 0.0050 0.0040
Mg: 0.0005
125 us,
Zr: 0.0005
a,
l0
CO
0020
0
41 1.10 0.50 0.70 0.0040 0.0050 0.0040
Mg: 0.0020
1.25 I.)
Rail steels
Zr: 0.0020 0
H
of the Mg:
0.0080 H
i
42 1.10 0.50 0.70 0.0040 0.0050 0.0040
present
Zr: 0.0080.
I\) 0
N)
1
I.)
invention
I.)
43 1.15 0.35 1.35 0.0040 0.0070 0.0040
1.75
44 1.15 0.95 0.90 0.0050 0.0090 0.0020
Mg: 0.0020 V: 0.02 4.50
45 1.20 1.25 0.45 0.0020 0.0060 0.0010-
N: 0.0080 6.00
46 1.20 1.25 0.45 0.0020 0.0060 0.0035-
N: 0.0080 1.71
_
0010
47 1.20 1.25 0.45 0.0020 0.0060 0.0035
Mg: 0. N: 0.0080 1.71
Zr: 0.0030
[0077]
Table 5
Chemical components (mass %)
Rail Steel
Co, Cr, Mo, V, Nb, S/Ca
C Si Mn P S Ca
Mg, Zr
B, Cu, Ni, Ti, Al, N
48 0.60 0.25 0.80 0.0100 0.0050 0.0020
Mg: 0.0020 Cu: 0.15 2.50
49 1.30 0.25 0.80 0.0100 0.0050 0.0020
Mg: 0.0020 Cu: 0.15 2.50
0
50 0.85 0.01 0.60 0.0120 0.0070 0.0080
- 0.88
41, 2
00 ....,
us,
51 0.85 2.50 0.60 0.0120 0.0070 0.0080
0.88
,0
0
0
0020 I.)
52 0.90 0.30 0.01 0.0060 0.0040 0.0060
Mg: 0.0020
Cr: 0.25
0.67 0
Zr: 0.0012 H
,
Comparative Mg: 0.0020 0
_
53 0.90 0.30 2.30 0.0060 0.0040 0.0060
Cr: 0.25 0.67 I\),
rail steels
Zr: 0.0012 I.)
I.)
54 1.00 0.50 1.00 0.0250 0.0030 0.0100
- 0.30
55 1.10 0.50 0.70 0.0150 0.0240 0.0120
Zr: 0.0015 2.00
56 0.95 0.95 0.80 0.0070 0.0030 0.0001
- Ti: 0.01 30.00
57 0.95 0.95 0.80 0.0070 0.0030 0.0300
- Ti: 0.01 0.10
58 0.65 0.30 0.75 0.0160 0.0050 0.0035
- 1.43
0
0[0078]
I.)
.
-I
us,
a,
Table 6
0
0
I.) Chemical components
(mass %)
0
H
H Rail Steel
Co, Cr, Mo, V, Nb, SiCa
C Si Mn P S
Ca Mg, Zr
1
0
B, Cu, Ni, Ti, Al, N
I.)
1
I.)
" 59 0.75 0.50 1.00 0.0180 0.0150
0.0004 - Nb: 0.01 37.50
60 0.85 0.55 0.85 0.0060 0.0120
0.0050 - 2.40
61 0.95 0.95 0.80 0.0170 0.0030
0.0002 - Ti: 0.01 15.0
4,
62 1.05 0.10 0.90 0.0050 0.0025
0.0210 - Al: 0.0080 0.12
63 1.20 1.25 0.45 0.0190 0.0130
0.0035 - N: 0.0090 3.71
Comparative
rail steels
64 0.65 0.30 0.45 0.0080 0.0050
0.0010 - 5.00
65 1.20 0.50 0.45 0.0020 0.0060
0.0050 - N: 0.0080 1.20
66 0.95 1.20 1.20 0.0070 0.0030
0.0080 - Ti: 0.01 0.38
-
67 0.85 0.30 0.30 0.0060 0.0080
0.0025 - 3.20
68 1.05 1.00 1.35 0.0050 0.0025
0.0030- Al: 0.0080 0.83
CA 02734980 2013-03-05
[0079]
(a) The measurement of the number of Mn sulfide-based inclusions
FIG. 3 shows a location at which Mn sulfide-based inclusions were observed in
the rail steel.
5 As shown in FIG 3, among cross-sections taken along the lengthwise
direction of
the obtained rail steel, a sample was cut off from a portion ranging from the
surface of the
rail head portion to a depth of 3 to 10 mm including the head surface portion
3a. Then,
the number (per unit area) (inclusions/mm2) of Mn sulfide-based inclusions
having major
lengths (lengths of major axes) in a range of 10 p.m to 100 lam was obtained
by the
10 above-described method.
[0080]
(b) The measurement of the number of Mn sulfide-based inclusions, Mg-based
oxides and Zr oxides
FIG. 4 shows a location at which Mn sulfide-based inclusions, Mg-based oxides
15 and Zr oxides were observed in the rail steel.
As shown in FIG 4, among transverse cross-sections of the obtained rail steel,
a
sample was cut off from a portion ranging from the surface of the rail head
portion to a
depth of 3 to 10 mm including the head surface portion 3a. Then, the number
(per unit
area) (inclusions/mm2) of Mg-based oxides, Zr oxides and Mn sulfide-based
inclusions
20 having grain diameters in a range of 5 nm to 100 nm was obtained by the
above-described
method.
[0081]
(c) The observation of the microstructure and the measurement of the hardness
of
the head surface portion 3a
25 A sample was cut off from a portion situated at a depth of 4 mm from
the surface
CA 02734980 2013-03-05
51
of the rail head portion 3. Thereafter, a surface to be observed was polished,
and then the
surface was etched with nital etching fluid. The microstructure in the surface
to be
observed was observed using an optical microscope in accordance with JIS G
0551.
In addition, in accordance with JIS B7774, the Vickers hardness Hv of the cut-
off
sample was measured. Here, the Vickers hardness was measured while a diamond
indenter was loaded on the sample at a load of 98 N (10 kgf). The Vickers
hardness is
expressed as (Hv, 98N) in Tables.
The obtained results are shown in Tables 7 to 12. Here, in Tables, the 'Head
portion material *1' refers to a material in a portion situated at a depth of
4 mm from the
surface of the rail head portion 3.
[0082]
Table 7
Number of Mg-based oxides, Zr
Head portion material *1
Number of Mn sulfide-based
inclusions having major oxides and Mn sulfide-based
Rail Steel inclusions having grain
Hardness
lengths in a range of 10 gm to
Microstructure
100 gm (/mm2) diameters in a range of 5 nm to
(Hv, 98N)
100 rim (imm2)
Pearlite + small amount of
1 50 3800
320
proeutectoid ferrite
Pearlite + small amount of
2 50 3800
400
proeutectoid cementite
0
I.)
3 100 - Pearlite
330 -I
us,
a,
Pearlite + small amount of
0
4 100 -
460 0
martensite
I.)
0
5 70 5600 Pearlite
320 LA H
t,)
Rail steels of
Pearlite + small amount of 0
"
the present 6 70 5600
460 '
I.)
martensite
I.)
invention
7 30 - Pearlite
440
8 30 - Pearlite
440
9 150 3500 Pearlite
420
10 30 3500 Pearlite
420
11 200 - Pearlite
430
12 10 - Pearlite
430
[0083]
Table 8
Number of Mg-based oxides, Zr
Head portion material *1
Number of Mn sulfide-based
inclusions having major oxides and Mn sulfide-based
Rail Steel inclusions having grain
Hardness
Microstructure
lengths in a range of 10 ilm to
100 pm (imm2) diameters in a range of 5 urn to
(Hv, 98N)
100 nm (/mm2)
13 15 -
Pearlite 350
14 70 -
Pearlite 350
0
15 60 6800
Pearlite 350 0
I.)
-1
L.,
16 100 4000
Pearlite 350 l0
CO
0
17 12 -
Pearlite 370 "
0
cp. H
tk.) HI
Rail steels of 18 190 -
Pearlite + small amount of bainite 390 0
"
the present
I
I.)
K)
invention 19 90 -
Pearlite + small amount of bainite 390
20 80 17000
Pearlite + small amount of bainite 390
21 180 -
Pearlite 400
22 100 -
Pearlite 400
23 20 -
Pearlite 400
24 180-
Pearlite
400
[0084]
Table 9
Number of Mg-based oxides, Zr
Head portion material *1
Number of Mn sulfide-based
oxides and Mn sulfide-based
inclusions having major
Rail Steel inclusions having grain
Hardness
lengths in a range of 10 pm to
Microstructure
100 2M 0131112)
diameters in a range of 5 rim to
(Hv, 98N)
1
100 nm (imm2)
25 140 -
Pearlite 400
26 130 30000
Pearlite 400
0
27 170 18000
Pearlite 420 0
I.)
-1
28 170 19000
Pearlite 420 us,
a,
l0
CO
29 190 -
Pearlite 430 - 0
I.)
0
Rail steels of 30 140
- Pearlite 430 t.., H
4=,
0
the present 31 130
19000 Pearlite 430 I.)
1
I.)
invention
I.)
Pearlite + small amount of
32 170 -
450
martensite
33 195 -
Pearlite 425
34 150 -
Pearlite 425
35 130 15000
Pearlite 425
36 18 -
Pearlite 375
[0085]
Table 10
Number of Mg-based oxides, Zr
Head portion material *1
Number of Mn sulfide-based
inclusions having major oxides and Mn sulfide-based
Rail Steel inclusions having grain
Hardness
Microstructure
lengths in a range of 10 gm to
diameters in a range of 5 nm to
(Hv, 98N)
100 gm (1mm2)
100 nm (/m2)
37 100 - Pearlite
375
38 80 26000 Pearlite
375 0
39 60 635 Pearlite
460 0
I.)
-1
L.,
40 90 1200 Pearlite
445
co
0
41 80 13000 Pearlite
445 N)
0
(.1) H
LA H
Rail steels of 42 50 45000
Pearlite 445 I0
I.)
the present
I
I.)
Pearlite + small amount of
I.)
invention 43 120 -
500
proeutectoid cementite
44 150 4500 Pearlite
450
Pearlite + small amount of
45 190 -
445
proeutectoid cementite
Pearlite + small amount of
46 90 -
445
proeutectoid cementite
Pearlite + small amount of
47 70 12000
445
proeutectoid cementite
[0086]
Table 11
Number of Mn sulfide-based Number of Mg-based oxides, Zr
Head portion material *1
inclusions having major lengths oxides and Mn sulfide-based
Rail Steel
Hardness
in a range of 10 pm to 100 pm inclusions having grain diameters in
Microstructure
(/mm2) a range of 5 nrn to 100 nm
(/mm2) (Hv, 98N)
48 50 3800 Pearlite +
proeutectoid ferrite 300
Pearlite + proeutectoid
49 50 3800
420
cementite
0
50 100 -
Pearlite 310
0
I.)
51 100 - Pearlite +
martensite 550 -1
L.,
.1,.
,0
52 70 5600
Pearlite 280 0
0
I.)
53 70 5600 Pearlite +
martensite 580 0
CA H
01 H
.
I
Comparative 54 30 -
Pearlite 440 0
I.)
1
rail steels
"
300
"
55 (number of inclusions increase -
Pearlite 420
--> toughness decreases)
230
56 (number of inclusions increase -
Pearlite 430
¨> toughness decreases)
57 (CaO generates ¨> toughness -
Pearlite 430
decreases)
58 70 -
Pearlite 350
'
[0087]
=
Table 12
Number of Mn sulfide-based Number of Mg-based oxides, Zr
Head portion material *1
inclusions having major oxides and Mn sulfide-based
Rail Steel
Hardness
lengths in a range of 10 gm to inclusions having grain diameters in
Microstructure
100 gm (/mm2) a range of 5 nm to 100
urn(hrim2) (Hv, 98N)
220
Pearlite + small amount of
59 (number of inclusions increase -
390
bainite
¨> toughness decreases)
60 140 Pearlite
400 n
0
210
"
-1
L.,
61 (number of inclusions increase Pearlite
430 a,
,0
0
¨> toughness decreases)
0
8
"
0
H
62 (CaO generates ¨> toughness - Pearlite
375 (A H
===4 I
0
decreases)
I.)
1
Comparative
I.)
rail steels 63 90 -
Pearlite + small amount of
445
I.)
proeutectoid cementite
64 70 - Pearlite +
proeutectoid ferrite 320
65 90 - Pearlite +
proeutectoid
370
cementite
66 140 - Pearlite +
martensite 490
67 140 - Pearlite
300
68 100 - Pearlite
520
CA 02734980 2011-02-22
58
= [0088]
(d) Wear test
FIG 5 shows a location from which a test specimen for the wear test was taken,
and the numeric values in the drawing show dimensions (mm).
As shown in FIG 5, a disk-like test specimen was cut off from a portion
including
the head surface portion 3a in the rail steel. Then, as shown in FIG 6, two
opposing
rotation axes were prepared, the disk-like test specimen (rail test specimen
4) was
disposed at one of the rotation axis, and an opponent material 5 was disposed
at the other
rotation axis. The rail test specimen 4 and the opponent material 5 were
brought into
contact in a state where a predetermined load was applied to the rail test
specimen 4. In
such a state, the two rotation axes were rotated at a predetermined speed
while cooling the
test specimen by supplying a compressed air from a cooling nozzle 6. Then,
after
rotating the axes 700,000 times, the reduced amount (abraded amount) of the
weight of the
rail test specimen 4 was measured.
The conditions for the wear test are shown below.
Testing machine: Nishihara-type wear testing machine (refer to FIG 6)
Shape of test specimen: Disk-like test specimen (outer diameter: 30 mm,
thickness: 8 mm)
Location from which the test specimen is taken: 2 mm below the surface of the
rail head portion (refer to FIG 5)
Test load: 686 N (contact surface pressure 640 MPa)
Sliding ratio: 20 %
Opponent material: pearlite steel (Hv 380)
Atmosphere: in the atmosphere (air)
Cooling: Forcible cooling by a compressed air (flow rate: 100 N1/min)
CA 02734980 2011-02-22
59
Number of repetitions: 700,000
[0089]
(e) Impact test of the head portion
FIG 7 shows a location from which a test specimen for the impact test was
taken.
As shown in FIG 7, a test specimen was cut off along the rail width direction
(transverse cross-section) in the transverse cross-section of the rail steel
so that a portion
including the head surface portion 3a forms the bottom of a notch. Then, the
obtained
test specimen was subjected to an impact test under the following conditions;
and thereby,
impact values (J/cm2) were measured.
Testing machine: Impact testing machine
Shape of test specimen: 2 mm U notch in JIS No. 3
Location from which the test specimen is taken: 2 mm below the surface of the
rail head portion (refer to FIG 7)
Testing temperature: normal temperature (20 C)
[0090]
The obtained results are shown in Tables 13 to 15. Here, in Tables, the 'Wear
test results *2' refer to the results of the above-described wear test, and
the reduced
amount (g) of the weight of the rail test specimen 13 is expressed as the
abraded amount.
The 'Impact test results *3' refer to the results of the above-described
impact test of the
In the present evaluation, a case where an abraded amount was in a range of
1.5 g
or less after the 700,000 times rotation was evaluated to have an excellent
wear resistance.
Since the impact values measured at 20 C are greatly varied with the amount of
carbon in
CA 02734980 2011-02-22
. and the relative merits of the impact values were evaluated among the
rail steels having
the same amount of carbon.
[0091]
Table 13
Impact test results *3
Wear test results *2
Rail Steel
(g, 700,000 times) Impact value (J/cm2)
1 1.45 37.0
2 0.35 10.0
3 1.25 19.0
4 1.10 17.0
5 1.00 16.0
6 0.91 14.5
7 0.62 12.5
8 0.63 16.0
9 0.46 11.3
10 0.45 13.0
11 0.80 13.0
Rail steels of the 12 0.81 12.0
present
invention 13 1.35 33.0
14 1.33 34.5
15 1.37 38.5
16 1.25 29.0
17 1.22 26.0
18 1.18 25.0
19 1.19 27.0
20 1.18 31.0
21 1.05 18.5
22 1.04 19.5
23 1.06 22.5
24 0.95 19.5
5
CA 02734980 2011-02-22
61
. [0092]
Table 14
Impact test results *3
Wear test results *2
Rail Steel
(g, 700,000 times) Impact value
(J/cm2)
25 0.94 20.5
26 0.94 25.0
27 0.86 18.0
28 0.70 18.5
29 0.75 14.0
30 0.74 15.5
31 0.75 18.5
32 0.72 14.2
33 0.60 12.5
34 0.62 14.0
Rail steels of the 35 0.60 16.0
present 36 0.64 12.0
invention
37 0.63 13.5
38 0.63 16.0
39 0.45 13.5
40 0.44 12.5
41 0.43 14.0
42 0.44 16.0
43 0.30 11.0
44 0.32 12.0
45 0.25 10.0
46 0.26 11.5
47 0.27 14.0
[0093]
CA 02734980 2011-02-22
62
Table 15
Impact test results *3
Wear test results *2
Rail Steel
(g, 700,000 times) Impact value (J/cm2)
48 2.30 (greatly abraded) 37.0
49 0.30 5.0
(impact value is lowered)
50 1.65 (greatly abraded) 18.0
51 1.80 (greatly abraded) 4.5
(impact value is lowered)
52 1.62 (greatly abraded) 16.0
53 1.90 (greatly abraded) 4.0
(impact value is lowered)
54 0.62 9.0
55 0.46 7.5
56 0.75 9.5
57 0.75 8.0
Comparative rail
58 1.35 29.0
steels
59 1.18 20.0
60 0.95 14.0
61 0.75 9.8
62 0.64 9.0
63 0.25 7.0
64 2.00 (greatly abraded) 35.0
65 0.40 6.0
(impact value is lowered)
66 1.90 (greatly abraded) 4.0
(impact value is lowered)
67 1.75 (greatly abraded) 18.0
68 0.40 7.0
(impact value is lowered)
[0094]
(1) Rails according to the present invention (47 rails), Steel Nos. 1 to 47
CA 02734980 2011-02-22
63
= Steel Nos. 3, 4, 7, 8, 11 to 14, 17 to 19,21 to 25, 29, 30, 32 to 34, 36,
37, 43, 45
and 46: pearlite rails having superior wear resistance and toughness which
have the
chemical compositions within the above-described limited range of the present
invention
and of which the number of Mn sulfide-based inclusions having major lengths
(lengths of
major axes) in a range of 10 i_tm to 100 m, the microstructure of the rail
head portion and
the hardness are within the limited ranges of the present invention.
Steel Nos. 1, 2, 5, 6, 9, 10, 15, 16, 20, 26 to 28, 31, 35, 38 to 42, 44 and
47:
pearlite rails having superior wear resistance and toughness which have the
chemical
compositions within the above-described limited range of the present invention
and of
which the number of Mn sulfide-based inclusions having major lengths (lengths
of major
axes) in a range of 10 mm to 100 Jim, the number of Mg-based oxides, Zr oxides
and Mn
sulfide-based inclusions having grain diameters in a range of 5 nm to 100 nm,
the
microstructure of the rail head portion and the hardness are within the
limited ranges of
the present invention.
[0095]
(2) Comparative rails (21 rails), Steel Nos. 48 to 68
Steel Nos. 48 to 53: rails of which the amounts of C, Si and Mn are outside
the
ranges of the present invention.
Steel Nos. 54 to 55: rails of which the amounts of P and S are outside the
ranges
of the present invention.
Steel Nos. 56 to 57: rails of which the amount of Ca is outside the range of
the
present invention.
Steel Nos. 58 to 63: rails of which the amounts of P, S and Ca are outside the
range of the present invention.
Steel Nos. 64 to 66: rails of which the chemical compositions are within the
range
CA 02734980 2011-02-22
64
= of the present invention; however, the microstructure of the head portion
does not fulfill
the above-described features of the present invention.
Steel Nos. 67 to 68: rails of which the chemical compositions are within the
range
of the present invention; however, the hardness of the head portion is outside
the
above-described range of the present invention.
[0096]
As shown in Tables 1 to 15, compared with the comparative rail steels (Steel
Nos.
48 to 53), the rail steels according to the present invention (Steel Nos. 1 to
47) include C,
Si and Mn at contents within the limited ranges of the present invention.
Therefore, it is
possible to stably obtain a pearlite structure having a hardness within the
limited range of
the present invention without generating eutectoid ferrite structure,
eutectoid cementite
structure and martensite structure, which adversely affect the wear resistance
and the
toughness.
Compared with the comparative rail steels (Steel Nos. 64 to 68), the rail
steels
according to the present invention (Steel Nos. 1 to 47) include a pearlite
structure in the
microstructure of the head portion, and the hardness of the pearlite structure
is within the
limited range of the present invention. As a result, it is possible to improve
the wear
resistance and the toughness of the rail.
[0097]
FIG 8 shows the results of the wear test of the rail steels according to the
present
invention (Steel Nos. 1 to 47) and Comparative rail steels (Steel Nos. 48, 50,
51, 52, 53,
64, 66 and 67).
In the case where C, Si and Mn are included at amounts within the limited
range
of the present invention, the generation of eutectoid ferrite structure and
martensite
structure, which adversely affect the wear resistance, is prevented, and in
addition, the
CA 02734980 2011-02-22
hardness is within the limited range of the present invention. Thereby, it is
possible to
greatly improve the wear resistance with any amount of carbon.
[0098]
FIG 9 shows the results of the impact test of the rail steels according to the
5 present invention (Steel Nos. 1 to 47) and Comparative rail steels (Steel
Nos. 49, 51, 53,
65, 66 and 68).
In the case where C, Si and Mn are included at amounts within the limited
range
of the present invention, the generation of eutectoid cementite structure and
martensite
structure, which adversely affect the toughness, is prevented, and in
addition, the hardness
10 is within the limited range of the present invention. Thereby, it is
possible to greatly
improve the toughness with any amount of carbon.
[0099]
As shown in FIG 10, compared with the comparative rail steels (Steel Nos. 54
to
63), the rail steels according to the present invention (Steel Nos. 1 to 47)
include P, S and
15 Ca at amounts within the limited ranges of the present invention.
Thereby, it is possible
to greatly improve the toughness of the pearlite rails with any amount of
carbon.
Furthermore, as shown in FIG 11, the rail steels according to the present
invention (Steel Nos. 11 to 13, 18 to 20,24 to 26,29 to 31,33 to 35,36 to 38
and 45 to
47) include Ca, and furthermore, the added amount of Ca is optimized. Thereby,
Mn
20 sulfide-based inclusions are controlled so that the number thereof is
within the limited
range of the present invention. As a result, it is possible to improve the
toughness of the
pearlite rail. In addition, in the case where Mg and Zr are added, oxides and
Mn
sulfide-based inclusions are finely dispersed so that the number of Mg-based
oxides, Zr
oxides and Mn sulfide-based inclusions is made to be in a range of 500/mm2 to
25 50,000/nun2. Thereby, it is possible to further improve the toughness of
the pearlite rail.
CA 02734980 2011-02-22
66
INDUSTRIAL APPLICABILITY
[0100]
The pearlite rail according to the present invention has wear resistance and
toughness superior to those of a high-strength rail in current use. Therefore,
the present
invention can be preferably applied to rails used in an extremely severe track
environment,
such as rails for freight railways that transport natural resources mined from
regions with
severe natural environments.
Brief Description of Symbols
=
[0101]
1: head top portion
2: head corner portion
3: rail head portion
3a: head surface portion
3b: a portion ranging from surfaces of head corner portions and a head top
portion to a depth of 20 mm
4: rail test specimen
5: opposing material
6: nozzle for cooling