Note: Descriptions are shown in the official language in which they were submitted.
CA 02735235 2013-07-08
1
DRUG DELIVERY IMPLANTS
[0001]
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0002] The present invention relates to implants, and, more particularly, to
orthopaedic
implants.
2. Description of the Related Art
[0003] Orthopaedic implants include short-term implants, long-term implants,
and non-
permanent implants. Short-term implants include implants for the treatment of
infection.
Long-term implants include total implants for total hip, knee, shoulder, and
elbow joints.
Non-permanent implants include trauma products such as nails, plates, and
external fixation
devices.
[0004] Regarding short-term implants, when tissue, especially bone,
surrounding an
orthopaedic implant becomes infected, that implant must typically be removed,
the infection
must be eliminated, and a new implant (revision implant) is then implanted.
The span of time
between implant removal and revision implantation can be from several weeks
(about 4
weeks) to a few months (approximately 3 months). During this time surgeons
currently have
two basic options: create temporary implants during surgery with antibiotic
bone cement
(created with or without the aid of a mold) or use a preformed antibiotic bone
cement
temporary implant (e.g. Exactech's InterSpace TM Hip and Knee). In either
case, antibiotic
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
2
bone cement is used to deliver antibiotics directly to the site of the
infection in the bone. The
patient also typically receives IV antibiotics. The shortcomings of such
implants are the
limited duration in which they deliver a clinically relevant dose of
antibiotics, the lack of
ability to change antibiotic type or dose during the 4-12 week treatment time,
and the limited
patient mobility, range of motion, and weight bearing that they allow.
[0005] Further, antibiotic cements typically provide useful local antibiotic
levels for a
duration of less than one week. The treatment time is frequently 6 to 8 weeks.
However,
beyond one week, the antibiotic cement implants provide no useful amount of
antibiotics.
[0006] Further, infections can be caused by a great number of bacteria,
viruses, yeast, etc.
The effectiveness of various antibiotics depends greatly upon what in
particular has caused
the infection. Thus, in order to treat an infection most effectively, the
cause of that infection
must be known. The results of cell cultures give this information and indicate
which
antibiotic and dose will most effectively treat the infection. The samples for
culturing are
usually collected during surgery. The results of the culture are not known
until several days
after the surgery. Since the type of antibiotic cement used in current
temporary implants
must be chosen at or before the time of surgery, the information gained from
the cultures
cannot be applied to the antibiotics used at the infection site.
[0007] Further, one key to a patient recovering from joint surgery with full
range of motion
in that joint is to encourage movement of that joint. This helps to prevent
the formation of
scar tissue and stiffening of tissue around the joint. The current options for
temporary
implants allow limited range of motion and weight bearing at best.
[0008] Regarding long-term implants, with regard to bone ingrowth, bone
ingrowth into a
porous material is sometimes required to provide stability or fixation of an
implant to the
bone. Examples of this include porous coatings on total joint components,
fusion devices
(i.e., spinal fusion devices), and bone augmentation components (i.e., tibial
wedges).
[0009] With regard to resorbtion, resorbtion can occur in the region
surrounding a total
joint implant for a number of reasons and can lead to implant loosening and
subsequent
revision surgery. Some causes of resorbtion include: (1) Stress shielding ¨
Bone tissue
requires loading to remain strong and healthy. If an implant does not properly
transfer loads
to the surrounding bone, regions of bone can resorb; (2) Lysis due to wear
particles ¨
Osteolysis and resorbtion are frequently caused by the body's reaction to wear
particles
created by the bearing of one total joint component on another; (3)
Osteoporosis or other
bone disorders ¨ bone metabolic disorders can also cause the resorbtion of
bone.
[0010] With regard to oncology, localized delivery of oncological drugs in the
region of
tumors may improve results in slowing/halting tumor growth. The ability for
localized
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
3
delivery may also lessen the need/dose of systemic drugs, resulting in fewer
side effects.
[0011] Regarding non-permanent implants (i.e., trauma implants), such non-
permanent
implants include nails, plates, and external fixation devices. Nails are
temporary,
intramedullary devices. They are typically used to treat traumatic fracture.
The risk of
infection can be high especially in the case of open fractures. With regard to
oncology, nails
can be used to treat fractures associated with bone tumors. They can also be
used to help
prevent a fracture where cancer has weakened bone. Plates treat many of the
same
indications as nails; however plates are applied to the outside of the bone.
External fixation
devices are a temporary implant that is used to stabilize a fracture. These
can be used for
days to months. External fixation devices typically include several pins fixed
in the bone and
extending through the skin to a rigid plate, ring, rod, or similar stabilizing
device. These
devices carry the added risk of infection due to their extending through the
skin. Bacteria can
travel along the pins directly to the soft tissue and bone.
[0012] Further, orthopaedic implants include internal fixation devices and
porous devices.
Internal fixation devices include, but are not limited to, screws and anchors.
[0013] What is needed in the art is an orthopaedic implant which includes a
reservoir and a
plurality of channels leading from the reservoir to deliver at least one
therapeutic agent
locally to bone or surrounding soft tissue, the orthopaedic implant being an
internal fixation
device and/or a porous device.
SUMMARY OF THE INVENTION
[0014] The present invention provides an orthopaedic implant which includes a
reservoir
and a plurality of channels leading from the reservoir to deliver at least one
therapeutic agent
locally to bone or surrounding soft tissue, the orthopaedic implant being an
internal fixation
device and/or a porous device.
[0015] The invention in one form is directed to an orthopaedic implant system,
including
an orthopaedic implant implantable at a selected location within a corporeal
body and
configured for delivering at least one therapeutic agent to the corporeal
body, the implant
defining a reservoir and a plurality of channels, the reservoir configured for
receiving the at
least one therapeutic agent, the plurality of channels configured for
conveying the at least one
therapeutic agent from the reservoir to a treatment site relative to the
corporeal body, the
implant being at least one of an internal fixation device and a porous device.
[0016] The invention in another form is directed to a method of using an
orthopaedic
implant system, the method including the steps of: providing an orthopaedic
implant defining
a reservoir and a plurality of channels, the implant being at least one of an
internal fixation
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
4
device and a porous device; implanting the implant at a selected location
within the corporeal
body; receiving at least one therapeutic agent in the reservoir; conveying the
at least one
therapeutic agent from the reservoir to a treatment site relative to the
corporeal body via the
plurality of channels; and delivering the at least one therapeutic agent to
the corporeal body.
[0017] The invention in another form is directed to a method of using an
orthopaedic
implant, the method including the steps of: providing an orthopaedic implant
body defining
at least one pathway; receiving at least one therapeutic agent by the implant
body; implanting
the orthopaedic implant at a selected location within a corporeal body;
conveying the at least
one therapeutic agent from the implant body to a treatment site relative to
the corporeal body
via the at least one pathway using pressure generated by the corporeal body to
mechanically
force the at least one therapeutic agent from the implant body to the
treatment site.
[0018] The invention in another form is directed to a method of using an
orthopaedic
implant, the method including the steps of: providing an orthopaedic implant
defining a
reservoir and a plurality of channels; implanting the implant at a selected
location within a
corporeal body, the implant being implanted into soft tissue of the corporeal
body; receiving
at least one therapeutic agent in the reservoir; conveying the at least one
therapeutic agent
from the reservoir to a treatment site relative to the corporeal body via said
plurality of
channels; and delivering the at least one therapeutic agent to the corporeal
body.
[0019] An advantage of the present invention is that it provides an
orthopaedic implant that
allows for the delivery of drugs directly to the bone and/or surrounding soft
tissue.
[0020] Another advantage of the present invention is that it provides a
temporary or short-
term implant that would allow for the delivery of antibiotics directly to the
bone and
surrounding tissue.
[0021] Yet another advantage of the present invention is that it would allow
for post-
operative injections of antibiotics into the implant, thereby allowing for the
delivery of
multiple antibiotics throughout treatment.
[0022] Yet another advantage of the present invention is that the implant
according to the
present invention allows for the delivery of the correct dose of antibiotics,
continuously for
any length of time required.
[0023] Yet another advantage of the present invention is that is provides an
orthopaedic
implant which can deliver a therapeutic agent locally to bone or surrounding
soft tissue as
long as the implant remains implanted in a corporeal body.
[0024] Yet another advantage of the present invention is that it provides a
long-term
implant which would allow drugs to be delivered directly to the bone and
surrounding tissue
(or to any specific location).
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
[0025] Yet another advantage of the present invention is that, with regard to
enhancing
bone ingrowth and combating resorbtion, it provides that bone growth
stimulators can be
injected intraoperatively or postoperatively to enhance or speed bone ingrowth
into porous
material (i.e., porous coatings on total joint components; fusion devices,
i.e., spinal fusion
devices; bone augmentation components, i.e., tibial wedges); these drugs could
also be
injected months to years post-operatively, using an implant according to the
present
invention, to combat bone resorbtion due to such causes as stress-shielding,
osteolysis, or
bone metabolic disorders.
[0026] Yet another advantage of the present invention is that, with regard to
oncology, the
present invention provides an implant that would similarly allow for delivery
of drugs to
some or all tissue surrounding the implant.
[0027] Yet another advantage of the present invention is that it would allow
antibiotics to
be delivered to the bone surrounding the nail of the present invention as a
preventative or to
treat an infection if one develops.
[0028] Yet another advantage of the present invention is that it provides a
non-permanent
implant, such as a nail according to the present invention, which can provide
the delivery of
bone growth stimulators directly to the region of bone fracture(s); such
delivery of bone
growth stimulators can be advantageous in difficult cases such as non-unions,
bony defects,
and osteotomies.
[0029] Yet another advantage of the present invention is that it provides a
non-permanent
implant, such as a nail according to the present invention, which can provide
localized
delivery of oncological drugs in the region of tumors which may improve
results in
slowing/halting tumor growth; this ability for localized delivery provided by
the present
invention may also lessen the need/dose of systemic drugs, resulting in fewer
side effects.
[0030] Yet another advantage of the present invention is that it provides an
external
fixation device that would allow antibiotics or other anti-infective agents to
be provided to
the bone and soft tissue surrounding the pins.
BRIEF DESCRIPTION OF THE DRAWINGS
[0031] The above-mentioned and other features and advantages of this
invention, and the
manner of attaining them, will become more apparent and the invention will be
better
understood by reference to the following description of embodiments of the
invention taken
in conjunction with the accompanying drawings, wherein:
[0032] Fig. 1 is a schematic representation of a sectional view of a short-
term femoral hip
implant according to the present invention;
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
6
[0033] Fig. 2 is a schematic representation of a sectional view of a short-
term femoral hip
implant system according to the present invention;
[0034] Fig. 3 is a schematic representation of a sectional view of a short-
term acetabular
cup implant according to the present invention;
[0035] Fig. 4 is a schematic representation of a top view of a short-term
femoral knee
implant according to the present invention;
[0036] Fig. 5 is a schematic representation of a sectional view of the short-
term femoral
knee implant taken along line 5-5 in Fig. 4;
[0037] Fig. 6 is a schematic representation of a top view of a short-term
femoral knee
implant according to the present invention;
[0038] Fig. 7 is a schematic representation of a front view of short-term
femoral knee
implant;
[0039] Fig. 8 is a schematic representation of a sectional view of a short-
term tibial knee
implant;
[0040] Fig. 9 is a schematic representation of a side view of a long-term
femoral hip
implant system according to the present invention;
[0041] Fig. 10 is a schematic representation of a sectional view of the long-
term femoral
hip implant of Fig. 9;
[0042] Fig. 11 is a schematic representation of a top view of a long-term
femoral knee
implant according to the present invention;
[0043] Fig. 12 is a schematic representation of a sectional view of the long-
term femoral
knee implant taken along line 12-12 in Fig. 11;
[0044] Fig. 13 is a schematic representation of a top view of a long-term
femoral knee
implant system according to the present invention;
[0045] Fig. 14 is a schematic representation of a side view of a long-term
femoral knee
implant system according to the present invention, the long-term femoral
implant being
attached to a femur;
[0046] Fig. 15 is a schematic representation of a side view of a long-term
femoral hip
implant system according to the present invention;
[0047] Fig. 16 is a schematic representation of a sectional view of the long-
term femoral
hip implant system of Fig. 15 taken along line 16-16;
[0048] Fig. 17 is a schematic representation of a sectional view of an
orthopaedic nail
according to the present invention;
[0049] Fig. 18 is a schematic representation of a sectional view of an
orthopaedic plate
according to the present invention;
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
7
[0050] Fig. 19 is a schematic representation of a sectional view of an
external fixation
device according to the present invention;
[0051] Fig. 20 is a schematic representation of a sectional view of an
orthopaedic implant
system including a therapeutic agent cartridge;
[0052] Fig. 21 is a schematic representation of a sectional view of an
orthopaedic implant
of Fig. 20 without the therapeutic agent cartridge inserted therein;
[0053] Fig. 22 is a schematic representation of a side view of an orthopaedic
implant that is
entirely porous;
[0054] Fig. 23 is a schematic representation of a side view of an orthopaedic
implant that is
entirely porous and includes a reservoir and drug delivery channels according
to the present
invention;
[0055] Fig. 24 is a schematic representation of a sectional view of an
orthopaedic implant
that is partially porous;
[0056] Fig. 25 is a schematic representation of a sectional view of an
orthopaedic implant
that is partially porous and includes a reservoir and drug delivery channels
according to the
present invention;
[0057] Fig. 26 is a schematic representation of a sectional view of an
orthopaedic implant
that is partially porous and includes a reservoir and drug delivery channels
according to the
present invention;
[0058] Fig. 27 is a schematic representation of a sectional view of an
orthopaedic implant
system according to the present invention including a sponge-like material;
[0059] Fig. 28 is a schematic representation of an orthopaedic implant system
according to
the present invention;
[0060] Fig. 29 is a schematic representation of an orthopaedic implant system
according to
the present invention.
[0061] Fig. 30 is a schematic representation of a sectional view of an
internal fixation
device, in the form of a bone screw, according to the present invention;
[0062] Fig. 31 is a schematic representation of a side view of the bone screw
of Fig. 30
inserted into a femur;
[0063] Fig. 32 is a schematic representation of a sectional view of an
orthopaedic implant
system according to the present invention including a bone screw and a
reservoir attached
thereto;
[0064] Fig. 33 is a schematic representation of a sectional view of an
orthopaedic implant
system according to the present invention including a bone screw and a
reservoir attached
thereto;
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
8
[0065] Fig. 34 is a schematic representation of a sectional view of an
orthopaedic implant
system according to the present invention including a bone screw, a catheter,
and a remote
reservoir;
[0066] Fig. 35 is a schematic representation of a sectional view of a bone
screw according
to the present invention;
[0067] Fig. 36 is a schematic representation of a sectional view of a bone
screw according
to the present invention;
[0068] Fig. 37 is a schematic representation of a side view of a bone screw
according to the
present invention;
[0069] Fig. 38 is a schematic representation of a side view of a bone screw
according to the
present invention;
[0070] Fig. 39 is a schematic representation of a sectional view of a bone
screw according
to the present invention;
[0071] Fig. 40 is a schematic representation of a sectional view of a bone
screw according
to the present invention;
[0072] Fig. 41 is a schematic representation of a sectional view of a bone
screw according
to the present invention;
[0073] Fig. 42 is a schematic representation of a partially sectional view of
an orthopaedic
implant system according to the present invention including a bone screw, an
attachment
device, a catheter, and a port;
[0074] Fig. 43 is a schematic representation of a partially sectional view of
an orthopaedic
implant system according to the present invention including a bone screw, an
attachment
device, a catheter, and a port;
[0075] Fig. 44 is a schematic representation of a perspective view of an
orthopaedic
implant according to the present invention implanted in a femur;
[0076] Fig. 45 is a schematic representation of a sectional view of the
orthopaedic implant
of Fig. 44 implanted in a femur;
[0077] Fig. 46 is a schematic representation of a sectional view of two
orthopaedic
implants of Fig. 44 implanted respectively in a femur and a pelvis;
[0078] Fig. 47 is a schematic representation of a sectional view of an
orthopaedic implant
according to the present invention;
[0079] Fig. 48 is a perspective view of an orthopaedic implant according to
the present
invention;
[0080] Fig. 49 is a bottom view of the orthopaedic implant of Fig. 48;
[0081] Fig. 50 is a schematic representation of a sectional view an
orthopaedic implant
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
9
according to the present invention implanted in a corporeal body;
[0082] Fig. 51 is a schematic representation of a sectional view of an
orthopaedic implant
according to the present invention;
[0083] Fig. 52 is a schematic representation of a sectional view an
orthopaedic implant
according to the present invention implanted in a corporeal body; and
[0084] Fig. 53 is a schematic representation of a perspective view of a porous
sheet to be
rolled into a screw according to the present invention;
[0085] Fig. 54 is a schematic representation of an end view of the sheet of
Fig. 53 during
the rolling process;
[0086] Fig. 55 is a schematic representation of a sectioned end view of the
sheet of Fig. 53
after the rolling process;
[0087] Fig. 56 is a schematic representation of the sheet of Fig. 53 after the
rolling process;
[0088] Fig. 57 is a schematic representation of a perspective view of a
spiraled band of
material;
[0089] Fig. 58 is a schematic representation of a perspective view of screw
layers exploded
from one another according to the present invention;
[0090] Fig. 59 is a schematic representation of a side view of a screw
according to the
present invention;
[0091] Fig. 60 is a schematic representation of a side view of a screw
according to the
present invention;
[0092] Fig. 61 is a schematic representation of a screw blank according to the
present
invention;
[0093] Fig. 62 is a schematic representation of a sheet showing raised threads
formed prior
to rolling;
[0094] Fig. 63 is a schematic representation of a sheet showing threads formed
by material
removal prior to rolling;
[0095] Fig. 64 is a schematic representation of a plan view of a sheet showing
threads
formed prior to stacking;
[0096] Fig. 65 is a schematic representation of a perspective view of a thread
prior to
assembly to a screw blank; and
[0097] Fig. 66 is a schematic representation of an end view of a screw
according to the
present invention.
[0098] Corresponding reference characters indicate corresponding parts
throughout the
several views. The exemplifications set out herein illustrate embodiments of
the invention,
and such exemplifications are not to be construed as limiting the scope of the
invention in any
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
manner.
DETAILED DESCRIPTION OF THE INVENTION
[0099] Referring now to the drawings, and more particularly to Fig. 1, there
is shown an
orthopaedic implant system 30 according to the present invention which
generally includes an
orthopaedic implant 32 implantable at a selected location within a corporeal
body 34 and
configured for delivering at least one therapeutic agent 36 to the corporeal
body 34. The
implant 32 includes at least one reservoir 38 and a plurality of channels 40.
The reservoir 38
is configured for receiving at least one therapeutic agent 36 and can be
configured for being
refilled with the therapeutic 36 agent after the implant 32 has been implanted
in the corporeal
body 34. Channels 40 form pathways for the therapeutic agent 36 to move from
the reservoir
38 to a treatment site 42 relative to the corporeal body 34. Each pathway
formed by a
channel 40 is an interior space formed by the walls of channel 40. Channel 40
can, for
example, have a circular, square, or some other cross-sectional shape. Thus,
channels 40 are
configured for conveying at least one therapeutic agent 36 from reservoir 38
to treatment site
42 relative to corporeal body 34.
[00100] Fig. 1 shows two reservoirs 38 and a plurality of channels 40 running
from each
reservoir 38. The implant according to the present invention (i.e., implant
232) may include
only one reservoir (i.e., reservoir 238). The reservoirs 38 of Fig. 1 can
optionally hold
different therapeutic agents 36 at the same time; stated another way, each
reservoir 38 can
hold a different therapeutic agent 36, or each reservoir 38 can hold at least
two therapeutic
agents 36. Thus, the implant according to the present invention is configured
for delivering a
plurality of therapeutic agents to the corporeal body via the reservoir and
the plurality of
channels; examples of such implants include implant 32 (Fig. 1) and implant
232 (Fig. 3).
Further, implant 32 may be formed such that no seal or seal cap is formed over
any of
channels 40 prior to release of any therapeutic agent 36.
[00101] A corporeal body herein means the physical body of a human being or of
an
animal (i.e., a veterinary patient). Thus, a corporeal body is one of flesh
and bones. The
corporeal body can be alive or dead. The corporeal body can also be referred
to as a patient
body herein, which includes both human and veterinary "patients", alive or
dead.
"Therapeutic agent" is a general term and includes, but is not limited to,
pharmaceuticals and
biologics (i.e., biological matter). Therapeutic agents can be variously
referred to herein,
without limitation, as drugs, pharmaceuticals, medicinal agents, or biologics.
Therapeutic
agents can be formed, for example, as a liquid, a solid, a capsule, or a bead.
[00102] Further, Fig. 1 shows that implant 32 includes a body 44 implantable
at the
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
11
selected location. Body 44 defines reservoir 38 and channels 40 and includes
an exterior
surface 46. The reservoir of the present invention can be a cavity or an
enclosed pocket
(closed but for channels extending to the surface of the body of the implant)
formed by the
body of the implant. The reservoir can be formed by the core (i.e., the
central interior
portion) of the body, rather than in the exterior surface of the body. The
reservoir can occupy
a substantial portion of the core but yet still have elongate channels running
from the
reservoir to the exterior surface. Reservoir 38 can be a cavity in body 44.
Reservoir 38 is not
necessarily a through-hole through body 44. Channels 40 fluidly communicate
reservoir 38
with exterior surface 46 and thereby forms the pathways for the at least one
therapeutic agent
36 to move from reservoir 38 to exterior surface 46. That is, channels 40
fluidly
communicate reservoir 38 with exterior surface 46 and thereby convey at least
one
therapeutic agent 36 from reservoir 38 to exterior surface 46. Fig. 1 shows
the body 44 of the
implant 32 being the implant 32 itself.
[00103] Further, Fig. 1 shows that implant 32 is formed as a hip prosthesis
and that
corporeal body 34 is formed as a hip. More specifically, Fig. 1 shows a
sectional view of a
short-term femoral hip implant 32 (which is one type of orthopaedic implant)
which forms
part of the upper femur (or, thighbone) and is thus load-bearing. The body 44
of the femoral
hip prosthesis 32 of Fig. 1 (the body 44 and the femoral hip prosthesis 32
being coextensive
relative to one another and thus being the same structural member in Fig. 1)
includes a stem
(the downward extending portion of implant 32 in Fig. 1) which can be inserted
into the
upper femur of a body 34 and a femoral head (the ball portion of implant 32 in
Fig. 1) which
is received by and mates with an acetabulum (i.e., the patient's natural
acetabulum, or a
prosthetic acetabular cup). Fig. 1 shows that both the stem and the femoral
head include
reservoirs 38 and a plurality of channels 40 running from the respective
reservoirs 38 to the
exterior surface 46 of the implant 32. Depending upon the size of reservoir 38
relative to
exterior surface 46 and/or the nearness of reservoir 38 to exterior surface
46, channels 40 can
be formed as holes or apertures in body 44. In use, therapeutic agent 36 is
inserted in
reservoirs 38 prior to and/or after implantation of implant in body 34.
Therapeutic agent 36
can then migrate into channels 40 and travel via channels 40 to exterior
surface 46 (channels
40 forming holes in exterior surface 46). Therapeutic agent 36 exits channels
40 and contacts
treatment site 42, which can be for example bone or soft tissue (it is
understood that "bone"
includes bone tissue). Optionally, reservoir 38 can be refilled with
therapeutic agent 36 (the
same or a different therapeutic agent 36) as implant 32 remains implanted in
corporeal body
34.
[00104] The orthopaedic implant of the present invention can be, for example,
a
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
12
prosthesis, a nail, a plate, or an external fixation device formed as an
implantable pin. Figs.
1-16 and 20-27 shows orthopaedic implants which are prostheses. A prosthesis
is an implant
that substitutes for or supplements a missing or defective part of the
corporeal body. Fig. 17
shows an orthopaedic implant which is a nail. Fig. 18 shows an orthopaedic
implant which is
a plate. Fig. 19 shows an orthopaedic implant which is an external fixation
device with an
implantable pin.
[00105] Fig. 2 shows another embodiment of the orthopaedic implant according
to the
present invention. Structural features in Fig. 2 corresponding to similar
features in Fig. 1
have reference characters raised by a multiple of 100. Short-term orthopaedic
implant system
130 includes a short-term prosthetic implant 132 and an attachment feature
150. Body 144
defines reservoir 138 and channels 140 running from reservoir 138 to exterior
surface 146.
Attachment feature 150 is for attaching a port (not shown in Fig. 2) thereto.
The attachment
feature 150 can be a tubular element. The attachment feature 150 and the port
can be used to
refill the reservoir 138 with a therapeutic agent. Upon filling reservoir 138
with the
therapeutic agent (either initially and/or as a refill) via attachment feature
150, the therapeutic
agent can move from the reservoir 138 to the treatment site via channels 140.
[00106] Fig. 3 shows another embodiment of the orthopaedic implant according
to the
present invention. Structural features in Fig. 3 corresponding to similar
features in prior
figures have reference characters raised by a multiple of 100. Fig. 3 shows a
sectional view
of another short-term hip implant 232. Prosthetic implant 232 is formed as an
acetabular cup,
which receives a femoral head. The body 244 of the acetabular cup 232 is the
acetabular cup
232 in Fig. 3. Body 244 defines reservoir 238 and a plurality of channels 240
running from
reservoir 238 to exterior surface 246. Upon filling reservoir 238 with the
therapeutic agent
(either initially and/or as a refill), the therapeutic agent moves from the
reservoir 238 to the
treatment site via channels 240.
[00107] Figs. 4-8 show additional embodiments of orthopaedic implants
according to the
present invention. More specifically, Figs. 4-8 show short-term orthopaedic
implants formed
as prosthetic knee implants, both femoral and tibial prosthetic knee implants.
Structural
features in Figs. 4-7 corresponding to similar features in prior figures have
reference
characters raised by a multiple of 100. Figs 4 and 5 show that the body 344 of
implant 332 is
the femoral knee implant 332. Body 344 includes a lower portion (the generally
U-shaped
piece in Fig. 5) and an optional stem (the vertical, upstanding piece atop the
lower portion in
Fig. 5). Both the lower portion and the stem include drug reservoirs 338 and
drug delivery
channels/holes 340 communicating the respective reservoir 338 with exterior
surface 346 to
deliver the therapeutic agent(s) in the reservoirs 338 to the treatment
site(s) 342. Fig. 6
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
13
shows a top view of femoral knee implant 432 similar to the implant 332 shown
in Fig. 5.
Channels 440 are shown as exit holes in exterior surface 446 of the lower
portion. The circle
in Fig. 6 represents an optional, upstanding stem 452. Fig. 7 shows a front
view of short-term
femoral knee implant 532 marked with lettering which is more radiopaque than
the implant
body 544 so that the letters are visible on an X-ray or fluoroscope, as shown
in Fig. 7. Upon
filling the reservoirs for Figs. 5 and 6 with the therapeutic agent (either
initially and/or as a
refill), the therapeutic agent can move from these reservoirs to the treatment
site via channels
340, 440.
[00108] Fig. 8 shows a sectional view of a short-term tibial knee implant 632
according to
the present invention. Structural features in Fig. 8 corresponding to similar
features in prior
figures have reference characters raised by a multiple of 100. The body 644 of
implant 632 is
the tibial knee implant 632. Body 644 includes a tibial tray (the generally
horizontal piece in
Fig. 8) and an optional stem (the generally vertical piece below the
horizontal piece in Fig.
8). Both the lower portion and the stem define drug reservoirs 638 and drug
delivery
channels/holes 640 communicating the respective reservoir 638 with exterior
surface 646 to
deliver the therapeutic agent(s) to the treatment site(s) 642. Upon filling
reservoir 638 with
the therapeutic agent (either initially and/or as a refill), the therapeutic
agent can move from
the reservoir 638 to the treatment site 642 via channels 640.
[00109] The implants according to the present invention shown in Figs. 1-8 are
thus short-
term implants that can be used, for example, to treat infections within a
corporeal body. Such
short-term or temporary implants allow for the delivery of therapeutic agents,
such as
antibiotics, directly to the bone of a corporeal body and to surrounding
tissue.
[00110] A device such as a port could be used to allow for post-operative
injections of
antibiotics into the implant. (See Fig. 2). This would allow for the delivery
of multiple
antibiotics throughout treatment. Reservoirs and/or channels in the implant
would allow the
antibiotics from these injections to be delivered over a time-period from
hours to weeks.
(Figs. 1-8). Injection intervals of approximately a week would likely be well-
accepted
clinically. The drugs could be delivered to all bone and soft tissue
surrounding the implant or
only to specific locations. Variations of this concept would allow for a range
of joint
mobility from no motion at the joint to the mobility typical of a permanent
total joint. These
short-term implants can be held in the bone with a loose press-fit or with
antibiotic or
standard bone cement. In the case of bone cement, cement restrictors would
also be included
in the technology to prevent cement from sealing over the drug delivery holes.
[00111] Antibiotic cements typically provide useful local antibiotic levels
for a duration of
less than one week. The treatment time is frequently six to eight weeks.
However, beyond
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
14
one week, the antibiotic cement implants provide no useful amount of
antibiotics. The
implant according to the present invention, by contrast, allows the delivery
of the correct dose
of antibiotics continuously for any length of time required. Through a feature
such as a port
attached to the implant of the present invention, the implant reservoir can be
refilled as often
as necessary to provide the proper drug dosing.
[00112] The implant of the present invention allows for any number of
antibiotics to be
used at any time during treatment. An initial antibiotic can be used at the
time of surgery. If
the cell cultures indicate that a different antibiotic or dose would be more
effective, that
change in treatment regimen can be made in accordance with the present
invention.
[00113] A short-term femoral hip implant, as discussed above, can include a
stem and a
separate head or could be a one-piece construction. Multiple sizes of stem and
head size
could be accommodated. A separate acetabular component could be provided, as
discussed
above. The femoral head could mate with a short-term acetabular component or
with the
patient's acetabulum. (See Figs. 1-3). According to the present invention,
drugs can be
delivered to the acetabulum through the head of the femoral component if an
acetabular
component is not used (See Fig. 1) or through the acetabular component if one
is used (See
Fig. 3).
[00114] A short term knee implant can include a one-piece tibial component
(combining
the two pieces of a standard total knee replacement) and a one- or two-piece
femoral
component (the two-piece design would combine the condyles and stem). The
present
invention provides multiple sizes of tibia components and of stem and condyles
(either
combined as one piece or separate). (See Figs. 4-8). Similar components are
provided for
shoulder, elbow, and other joints, according to the present invention.
[00115] Since the implants of Figs. 1-8 are designed for short-term use, the
short-term
implants of the present invention can include markings which are both visible
on the implant
surface by the naked eye and visible by X-ray, as indicated above. These
markings would
clearly indicate that the implants are intended for short-term use only. (See
Fig. 7).
[00116] The present invention provides an orthopaedic implant system (whether
short-
term, long-term, or non-permanent implants) which provide for continuously
delivering drugs
to a point near the implant or to the entire region surrounding the implant
for extended
periods of time. The implants according to the present invention shown in
Figs. 9-16 are
long-term implants. Such implants can be used, for example, as total hip,
knee, shoulder, and
elbow joints within a patient body. The long-term implants of the present
invention have a
basic similarity with the short-term implants described above. Thus,
structural features in
Figs. 9-16 corresponding to similar features in prior figures have reference
characters raised
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
by multiples of 100. Thus, similar to the short-term implants described above,
the present
invention further provides a long-term implant which would allow drugs to be
delivered
directly to the bone and surrounding tissue (or to any specific location). A
device such as a
port could be used to allow for post-operative injections of drugs into the
long-term implant.
(See Fig. 14). This would allow for the delivery of any number of drugs
throughout treatment
and allow for the refilling of drugs to provide proper drug dosing throughout
treatment.
Reservoirs and/or channels in the long-term implant according to the present
invention would
allow the drugs from these injections to be delivered over a time period from
hours to weeks.
(See Figs. 9-16). The drugs could be delivered to all bone and soft tissue
surrounding the
implant or only to specific locations.
[00117] Figs. 9 and 10 show a long-term femoral hip prosthetic implant system
730
according to the present invention. Structural features in Figs. 9 and 10
corresponding to
similar features in prior figures have reference characters raised by
multiples of 100. System
730 includes a long-term femoral hip prosthetic implant 732 and a porous
surface 754
attached to the exterior surface 746. Similar to the short-term implants
discussed above,
implant has a body 744 defining a drug reservoir 738 and a plurality of drug
delivery
channels 740 running from the reservoir 738 to the exterior surface 746 so as
to deliver a
therapeutic agent(s) to a treatment site in the corporeal body. Porous surface
754 is
configured for receiving bone and/or tissue ingrowth therein. Such ingrowth is
shown by
arrow 756 in Fig. 9. The porous surface 754 can be variously referred to as a
porous
member, a porous pad, or a scaffold. Drug delivery channels 740 can be routed
by or through
body 744 so as to avoid the ingrowth region. Stated another way, channels 740
can be routed
by or through body 744 so as to avoid releasing therapeutic agents into porous
surface 754.
By contrast, channels 740 can be routed by or through body 744 so as to
release drugs
through the ingrowth porous surface 754. Fig. 9 shows channels 740 which avoid
releasing
drugs into porous surface 754. Upon filling reservoir 738 with the therapeutic
agent (either
initially and/or as a refill), the therapeutic agent can move from the
reservoir 738 to the
treatment site via channels 740.
[00118] Figs. 11 and 12 show a long-term femoral knee implant according to the
present
invention. Structural features in Figs. 11 and 12 corresponding to similar
features in prior
figures have reference characters raised by multiples of 100. The body 844 of
implant 832 is
the femoral knee implant 832. Body 844 includes a lower portion (the generally
U-shaped
piece in Fig. 12) and an optional stem (the vertical, upstanding piece atop
the lower portion in
Fig. 12). Both the lower portion and the stem include drug reservoirs 838. The
stem further
includes drug delivery channels/holes 840 communicating the respective
reservoir 838 with
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
16
exterior surface 846 to deliver the therapeutic agent(s) to the treatment
site(s) 846. The lower
portion also includes at least one drug delivery channel 840 leading from
reservoir to a
treatment site. Upon filling reservoir 838 with the therapeutic agent (either
initially and/or as
a refill), the therapeutic agent can move from the reservoir 838 to the
treatment site via
channels 840.
[00119] Fig. 13 shows a long-term femoral knee implant system 930 according to
the
present invention. Structural features in Fig. 13 corresponding to similar
features in prior
figures have reference characters raised by multiples of 100. System 930
includes a
prosthetic implant 932 similar to the implant 832 of Fig. 12 but with a
plurality of ingrowth
porous surfaces 954 attached to the body 944 of implant 932. Each porous
surface 954 is
configured for receiving bone and/or tissue ingrowth therein. Further, while
the reservoir
cannot be seen in Fig. 13, a drug delivery channel 940 leading from the drug
reservoir is
shown in Fig. 13. The reservoir of Fig. 13 can be situated just under exterior
surface 946 as
reservoir 838 is shown in Fig. 12. Channel 940 routes around (and thereby
avoids) ingrowth
pads 954. Upon filling the reservoir of implant 932 with the therapeutic agent
(either initially
and/or as a refill), the therapeutic agent can move from the reservoir of
implant 932 to the
treatment site via channels 940.
[00120] Fig. 14 shows a long-term femoral knee implant system 1030 according
to the
present invention. Structural features in Fig. 14 corresponding to similar
features in prior
figures have reference characters raised by multiples of 100. System 1030
includes a
prosthetic implant 1032 similar to the implant 832 of Fig. 12. Implant 1032 is
attached to a
femur 1035. The system 1030 further includes an attachment feature or element
1050 (such
as a tubular element) for an injection port 1058, an injection port 1058, a
catheter 1060, and a
reservoir 1062 remote to the implant 1032. The injection port is provided for
additional
refilling of drugs into the implant 1032, which includes at least one channel
for routing the
therapeutic agent to the treatment site. Since an external reservoir 1062 is
attached to implant
1032, implant body 1044 may or may not define an additional internal
reservoir. Upon filling
the internal reservoir of implant 1032 with the therapeutic agent (either
initially and/or as a
refill) via attachment element 1050, injection port 1058, catheter 1060, and
external reservoir
1062, the therapeutic agent can move from the reservoir of implant 1032 to the
treatment site
via the drug delivery channels. If implant 1032 does not have an internal
reservoir, then the
therapeutic agent moves to the treatment site via the drug delivery channels
from external
reservoir 1062 via catheter 1060, injection port 1058, and attachment element
1050.
[00121] Figs. 15 and 16 show a long-term femoral hip implant system 1130
according to
the present invention. Structural features in Figs. 15 and 16 corresponding to
similar features
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
17
in prior figures have reference characters raised by multiples of 100. Fig. 15
shows long-
term femoral hip implant system 1130 including a long-term femoral hip
prosthetic implant
1132 and an ingrowth porous surface 1154. Fig. 16 shows a first porous surface
1154 on the
top (as oriented in Fig. 16) of the implant body 1144 or substrate 1144 (in
each of the figures,
the body 1144 can also be referred to as a substrate) and a second porous
surface 1154 on the
bottom (as oriented in Fig. 16) of the body 1144. Porous surfaces 1154 are
configured for
receiving bone and/or tissue ingrowth therein, as shown by arrow 1156. While
Fig. 16 shows
some space between porous surfaces 1154 and body 1144, it is understood that
this space is
for illustrative purposes and that porous surfaces 1154 can be flush with body
1144 but for
any adhesive that may be used to attach surfaces 1154 with exterior surface
1146 of body
1144. Each porous surface 1154 includes a first side 1164 attached to exterior
surface 1146
of body 1144 and a second side 1166 opposing said first side 1164. Each porous
surface
1154 includes a through-hole 1168 running from first side 1164 to second side
1166.
Through-hole 1168 is configured for communicating the therapeutic agent 1136
from first
side 1164 to second side 1166 and thereby for communicating the therapeutic
agent 1136 to
the treatment site 1142. The through-holes 1168 in porous surfaces 1154 lead
to surface
channels 1170 and sub-surface channels 1172, respectively. Channels 1170 and
1172 can
function essentially the same as channels 40 in that they are drug delivery
channels. Fig. 16
shows a reservoir 1138 and connecting channels 1140 in broken lines; for, it
is understood
that such a reservoir 1138 and connecting channels 1140 (connecting reservoir
1138 with
channels 1170 and/or 1172) may not be visible in this section, or,
alternatively, that such a
reservoir 1138 and connecting channels 1140 can be optional (stated another
way, the implant
1132 would not contain such an interior reservoir 1138 and connecting channels
1140 leading
from the reservoir 1138 to the surface channels 1170 or the sub-surface
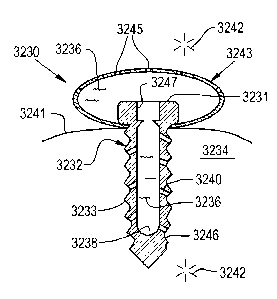
channels 1172).
[00122] Further, Fig. 16 shows that exterior surface 1146 of body 1144 can
define a
surface channel 1170 which is in communication with and cooperates with
channel 1140 and
through-hole 1168 of porous surface 1154 to provide the therapeutic agent 1136
from the
reservoir 1138 to the treatment site 1142. Fig. 16 shows a plurality of such
surface channels
1170, each of which can optionally be connected to reservoir 1138 via a
respective
connecting channel 1140, as discussed above. If implant 1132 has reservoir
1138 and
connecting channels 1140, then upon filling reservoir 1138 with the
therapeutic agent (either
initially or as a refill), the therapeutic agent can move from reservoir 1138
to the treatment
site via the channels 1140 and 1170. If implant 1132 does not have reservoir
1138 and
connecting channels 1140, then surface channels 1170 can be filled with the
therapeutic agent
(either initially and/or as a refill) and the therapeutic agent moves via
surface channels 1170,
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
18
through through-holes 1168, to the treatment site 1142. The therapeutic agent
can also be
provided to the bone and/or tissue growing into porous surface 1154.
[00123] Further, Fig. 16 shows that channels 1140 running from reservoir 1138
can
connect to the sub-surface channels 1172. Sub-surface channels 1172 and
through-holes
1168 in porous surface 1154 are aligned with and cooperate with one another to
provide the
therapeutic agent 1136 from the reservoir 1138 to the treatment site 1142.
Holes 1174 (which
can also be considered as channels of the present invention, like channels 40)
are also
provided in body 1144 leading from subsurface channels 1172 to exterior
surface 1146.
These holes 1174 can be considered to be part of the respective channels 1140
and 1172.
[00124] Figs. 15 and 16 thus also show an orthopaedic implant system 1130
including an
orthopaedic implant 1132 and a porous surface 1154. The orthopaedic implant
1132 includes
a body 1144 implantable at a selected location within a corporeal body 1134
and configured
for delivering a therapeutic agent 1136 to corporeal body 1134. Body 1144 of
implant 1132
includes an exterior surface 1146 defining a plurality of surface channels
1170 and, as
discussed above, can have an absence of a therapeutic agent reservoir 1138.
The broken lines
of the reservoir 1138 in Fig. 16, as stated above, indicates that the
reservoir 1138 is optional.
The plurality of surface channels 1170 are configured for receiving, holding,
delivering, and
being refilled with the therapeutic agent 1136 after implant 1132 has been
implanted in
corporeal body 1134. Orthopaedic implant 1132 is a prosthesis. Alternatively,
implant 1132
can be formed as a nail (Fig. 17), a plate (Fig. 18), or an external fixation
device with an
implantable pin (Fig. 19). Porous surface 1154 is attached to exterior surface
1146. Porous
surface 1154 is configured for receiving at least one of bone and tissue
ingrowth therein, as
shown by arrow 1156. As discussed above, porous surface 1154 includes a first
side 1164
attached to exterior surface 1146 and a second side 1166 opposing first side
1164. Porous
surface 1154 includes a plurality of through-holes 1168 running from first
side 1164 to
second side 1166. The plurality of surface channels 1170 communicate and
cooperate with
the plurality of through-holes 1168 to provide the therapeutic agent 1136 from
the plurality of
surface channels 1170, then to first side 1164 of porous surface 1154, and
then to second
side 1166 of porous surface 1154. Surface channels 1170 can be filled with the
therapeutic
agent (either initially and/or as a refill) and the therapeutic agent 1136
moves via surface
channels 1170, through through-holes 1168, to the treatment site 1142.
[00125] Thus, the present invention could be applied to long-term implants
with any type
of porous coating or surface or to cemented implants. Drugs could be delivered
through the
porous coatings or be routed to regions without porous coatings (as disclosed
above),
depending on the requirements. (See Figs. 9, 10, 13, 15, and 16). For delivery
through the
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
19
porous coatings, channels can be created on the surface of the implant
substrate (the solid
material of the implant to which the porous surface is attached ¨ see Fig. 14)
or below the
surface, as disclosed above relative to Figs. 15 and 16. For surface channels,
holes can be
drilled through the porous surface to the surface channels to create a path
through which the
drugs can be delivered. For sub-surface channels, holes must be drilled from
the surface of
the substrate (the body of the implant) to the sub-surface channels to create
paths for drugs to
be delivered. (See Fig. 16). This drilling can occur prior to attaching the
porous
coating/surface or after the porous coating/surface is attached. If this
drilling occurs after the
porous coating/surface is attached, the holes will be created through the
porous
coating/surface and the substrate/body surface. (See Fig. 16).
[00126] Cement restrictors can also be used according to the present invention
to prevent
cement from sealing over the drug delivery holes. The present invention can be
applied to all
types of total joint implants, such as total hip components, total knee
components, total
shoulder components, and total elbow components.
[00127] With regard to enhancing bone ingrowth and combating resorbtion, bone
growth
stimulators can be injected intraoperatively or postoperatively to enhance or
speed bone
ingrowth into porous material (i.e., porous coatings or pads or surfaces on
total joint
components, on fusion devices (i.e., spinal fusion devices), or on bone
augmentation
components (i.e., tibial wedges)). These drugs could also be injected months
to years post-
operatively, using a long-term implant according to the present invention, to
combat bone
resorbtion due to such causes as stress-shielding, osteolysis, or bone
metabolic disorders.
[00128] With regard to oncology, the implant of the present invention would
similarly
allow for delivery of drugs to some or all tissue surrounding the implant. The
implants of the
present invention may be cemented. The present invention provides a way to
route the drugs
around the regions of cement and provides a way for preventing the cement from
sealing over
the drug delivery holes.
[00129] The implants according to the present invention shown in Figs. 17-19
are non-
permanent implants. Such implants can be trauma products, such as nails,
plates, and
external fixation devices. The non-permanent implants of the present invention
are not
necessarily limited to these devices. The non-permanent implants of the
present invention
have a basic similarity with the short-term and long-term implants described
above. Thus,
structural features in Figs. 17-19 corresponding to similar features in Fig. 1
have reference
characters raised by multiples of 100. Thus, similar to the short-term and
long-term implants
described above, the present invention further provides a non-permanent
implant which
would allow drugs to be delivered directly to the bone and surrounding tissue
(or to any
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
specific location). Reservoirs and/or channels in the non-permanent implant
according to the
present invention would allow the drugs to be delivered to the treatment site
and could be
refilled. A nail according to the present invention is shown in Fig. 17. A
plate according to
the present invention is shown in Fig. 18. An external fixation device
according to the
present invention is shown in 19.
[00130] Nails are temporary, intramedullary devices. They are typically used
to treat
traumatic fracture. The risk of infection can be high especially in the case
of open fractures.
The present invention would allow antibiotics to be delivered to the bone
surrounding the nail
as a preventative or to treat an infection if one develops.
[00131] With regard to bone growth, in the case of fractures, there are
instances in which
the delivery of bone growth stimulators directly to the region of the
fracture(s) would be
beneficial. This is especially true in difficult cases such as non-unions,
bony defects, and
osteotomies. The nail according to the present invention would allow for such
delivery bone
growth stimulators directly to the region of the fracture(s).
[00132] With regard to oncology, nails can be used to treat fractures
associated with bone
tumors. They can also be used to help prevent a fracture where cancer has
weakened bone.
The nail according to the present invention provides for localized delivery of
oncological
drugs in the region of tumors which may improve results in slowing/halting
tumor growth.
This ability for localized delivery provided by the nail according to the
present invention may
also lessen the need/dose of systemic drugs, resulting in fewer side effects.
[00133] Fig. 17 shows an orthopaedic nail 1232 implantable in the corporeal
body.
Structural features in Fig. 17 corresponding to similar features in prior
figures have reference
characters raised by multiples of 100. Nail 1232 includes a body 1244 defining
a reservoir
1238 and a drug delivery channel 1240 leading from drug reservoir 1238 to
exterior surface
1246 of nail 1232. The present invention thus provides an orthopaedic nail
1232 with a drug
delivery portion, which is similar to that, for instance, for long-term
implants, such as a
femoral hip implant (such as a hip stem). This design allows drugs to be
delivered directly to
all areas of the bone or to any specific location. (Fig. 17). A device such as
a port could be
used to allow for post-operative injections of drugs into the nail 1232. This
would allow for
the delivery of any number of drugs throughout treatment. Reservoirs 1238
and/or channels
1240 in the nail 1232 would allow the drugs from these injections to be
delivered over a time
period from hours to weeks. Thus, upon filling reservoir 1238 with the
therapeutic agent
(either initially and/or as a refill), the therapeutic agent can move from the
reservoir 1238 to
the treatment site via channels 1240. The drugs could be delivered to all bone
tissue
surrounding the implant or only to specific locations. All types of nails
could utilize this
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
21
technology, including antegrade and retrograde versions of femoral, tibial,
and humeral nails.
[00134] Orthopaedic plates treat many of the same indications as nails;
however, plates are
applied to the outside of the bone. Plates offer the same opportunities for
delivering drugs
locally. Since nails are intramedullary, they can be used to deliver drugs,
according to the
present invention, primarily to the bone tissue. Since plates are applied to
the outside of the
bone, they can be used to deliver drugs, according to the present invention,
to both bone and
soft tissues. Examples of potential soft tissue treatments benefited by
localized drug delivery
include the enhancement of soft tissue ingrowth or healing, the prevention of
infection by the
delivery of antibiotics, and the treatment of nearby soft tissue tumors with
localized delivery
of oncological drugs.
[00135] Fig. 18 shows an orthopaedic plate 1332 that is implantable in a
corporeal body.
Structural features in Fig. 18 corresponding to similar features in prior
figures have reference
characters raised by multiples of 100. Plate 1332 includes a body 1344
defining a reservoir
1338 and a drug delivery channel 1340 leading from drug reservoir 1338 to
exterior surface
1346 of plate 1332. Upon filling reservoir 1338 with the therapeutic agent
(either initially
and/or as a refill), the therapeutic agent can move from the reservoir 1338 to
the treatment
site via channels 1340.
[00136] Thus, the drug delivery portion of plate is similar to that for
orthopaedic nails
according to the present invention. Plate allows drugs to be delivered
directly to the bone and
surrounding tissue (or to any specific location). A device such as a port
could be used to
allow for post-operative injections of drugs into plate. This would allow for
the delivery any
number of drugs throughout treatment. Reservoirs 1338 and/or channels 1340 in
the plate
implant 1332 allow the drugs from these injections to be delivered over a time-
period from
hours to weeks. The drugs could be delivered to all bone and soft tissue
surrounding the plate
implant 1332 or only to specific locations.
[00137] External fixation devices are temporary implants that are used to
stabilize a
fracture. These external fixation devices can be used for days to months.
External fixation
devices typically include several pins fixed in the bone and extending through
the skin to a
rigid plate, ring, rod, or similar stabilizing device. These devices carry the
added risk of
infection considering that the pins extend through the skin. Bacteria can
travel along the pins
directly to the soft tissue and bone. The present invention can be applied to
external fixation
devices. Thus, antibiotics or other anti-infective agents can be provided to
the bone and soft
tissue surrounding the pins. (Fig. 19). An external reservoir could be used to
supply/pump
antibiotics to the bone and soft tissue.
[00138] Fig. 19, for instance, shows an external fixation device 1432
according to the
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
22
present invention which is a trauma device. Structural features in Fig. 19
corresponding to
similar features in prior figures have reference characters raised by
multiples of 100.
External fixation device 1432 includes an implantable pin 1476, a sheath 1478
coupled with
pin 1476, and a reservoir 1480 coupled with sheath 1478, pin 1476 defining a
plurality of
channels 1440. More specifically, pin 1476 includes a wall 1482 defining an
inner spatial
area 1484 and a plurality of drug delivery channels 1440 or holes 1440.
Connected to the
outer circumference of the pin 1476 is sheath 1478, which can be coaxial with
pin 1476.
Sheath 1478 serves to prevent drugs from exiting that portion of the external
fixation device
1432 which is outside of the skin 1434. To the right (as oriented on the page
of Fig. 19) of
the wall of skin 1434 is space that is external to the corporeal body.
Further, drug reservoir
1480 is attached to sheath 1478. Drug reservoir 1480 is shaped to allow
attachment of the
external fixation device 1432 to external fixation rods and/or plates (not
shown). The
therapeutic agent moves from drug reservoir 1480 to the inner spatial area
1484 of pin 1476,
through channels/holes 1440 in pin wall 1482, and to the treatment site. Thus,
upon filling
reservoir 1480 with the therapeutic agent (either initially and/or as a
refill), the therapeutic
agent can move from the reservoir 1480 to the treatment site 1442 via inner
spatial area 1484
and channel(s) 1440.
[00139] Shortcomings of temporary bone cement implants used to treat
infections are
discussed above. An additional shortcoming includes the difficulty of
delivering adequate
quantities of therapeutic agents through such implants to bone due to lack of
blood flow.
Figs. 20-27 provide orthopaedic drug delivery implants which address this
shortcoming.
More specifically, Figs. 20-21 provide therapeutic agent delivery via a
removable and
replaceable cartridge. Further, Figs. 22-26 provide therapeutic agent delivery
via leaching
through an implant that is partially or totally porous. Further, Fig. 27
provides a modified
reservoir design. The designs shown in Figs. 20-27 can be used in short-term,
long-term, or
non-permanent orthopaedic implants. Structural features in Figs. 20-27
corresponding to
similar features in prior figures have reference characters raised by
multiples of 100.
[00140] Figs. 20 and 21 show an orthopaedic implant system 1530 including an
orthopaedic implant 1532 and a cartridge 1586. More specifically, Fig. 20
shows cartridge
1586 inserted in implant 1532. Fig. 21, however, shows implant 1532 with
cartridge 1586
removed. Implant 1532 is formed as, for example, a short-term femoral hip
prosthetic
implant 1532. Implant 1532 is implanted in corporeal body 1534. Implant 1532
is defined
by its body 1544. Body 1544 defines a reservoir 1538 and a plurality of
channels 1540
running from the reservoir 1538 to the exterior surface 1546 of body 1544.
Cartridge 1586 is
inserted into and thus received by reservoir 1538, which serves as a housing
for cartridge
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
23
1586. Thus, reservoir 1538, as a housing for cartridge 1586, may be shaped to
matingly
accommodate and connect to cartridge 1586. Reservoir 1538 can be generally cup-
shaped
and thus be open to exterior surface 1546 (and thus reservoir 1538 can
essentially be a blind
hole in exterior surface 1546) so as to receive cartridge 1586. Cartridge 1586
contains at
least one therapeutic agent 1536, which is shown in broken lines in Fig. 20.
Cartridge 1586 is
configured for releasing the therapeutic agent 1536 (shown as a circle in
cartridge 1586) into
reservoir 1538 and/or at least one channel 1540 such that the therapeutic
agent 1536 moves
away from reservoir 1538 in at least one channel 1540 and thus to exterior
surface 1546 of
body 1544. Cartridge 1586 is removable from reservoir 1538 and is replaceable
with another
cartridge 1586 after implant 1532 has been implanted in the corporeal body.
The first
cartridge 1586 is replaced when it is empty of the therapeutic agent (or when
it has otherwise
released the desired amount of therapeutic agent from the first cartridge
1586). The second
cartridge 1586, which replaces the empty first cartridge 1586, is full (or has
the desired
amount of therapeutic agent therein) of therapeutic agent when it is inserted
into reservoir
1538 and thereby replaces first cartridge 1586. Thus, the refilling of
reservoir 1538 in system
1530 occurs by replacing first cartridge 1586 with a second cartridge 1586.
[00141] Thus, system 1530 can have implant body 1544 and a replaceable portion
or
cartridge 1586. (Figs. 20-21). Replaceable cartridge 1586, as stated, contains
therapeutics.
Upon implantation, the surgeon can decide with what therapeutics to fill
cartridge 1586.
Over time, cartridge 1586 can be replaced with a new cartridge 1586 filled
with the same
therapeutic as before or a different therapeutic. Ideally, cartridge
replacement would occur as
a minor outpatient procedure.
[00142] The replaceable cartridge may be optionally formed relative to the
implant. As a
first option, the cartridge may be considered a distinct device relative to
the implant but
which can be directly attached to the implant, as shown in Fig. 20. As a
second option, the
cartridge may be considered a portion of the implant which can be detached
from the implant
body. As a third option, the cartridge may be a second replaceable implant
located within the
patient body away from the first implant (i.e., the femoral hip implant) but
connected to the
first implant, such as via a catheter. As a fourth option, the cartridge may
be a device that is
situated external to the patient body, while the implant (i.e., the femoral
hip implant) is
implanted in the patient body.
[00143] Figs. 22-26 show implants that are partially or totally porous to
facilitate
therapeutic agent delivery via leaching through the respective implant. In
much the same
manner of powder metallurgy bearings that are self-lubricating, therapeutic
agents may be
delivered to the patient body from an implant that is partially or totally
porous. (Figs. 22-26).
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
24
Therapeutics will leach from the porous portions of the implant to the body.
Such implants
may also contain drug delivery channels, reservoirs, and the various ways of
recharging
therapeutics as previously discussed herein. Figs. 22 and 23 each shows a
femoral hip
prosthetic implant 1632 in which the entire body 1644 of the implant 1632 is
porous to
facilitate leaching of therapeutic agents therefrom. Pores are labeled as
1690. The implant
1632 of Fig. 22, however, does not necessarily include a drug reservoir or
drug delivery
channels in addition to the pores. Rather, it is understood that the pores and
the connections
between the pores form the reservoir and the channels according to the present
invention.
The "connections" between the pores (1) can be formed by elongate channels
extending
between the pores, or (2) can be formed simply by the interconnection of
adjacent pores
which are adjoined and open to one another (no additional elongate channel
would extend
between the pores in the second example). In use, the therapeutic agent, such
as a liquid
therapeutic agent, can be pumped into one or more pores of implant 1632; then,
the
therapeutic agent leaches out through the pores (and any additional connecting
elongate
channels) to the exterior surface or otherwise to the treatment site. Thus,
the therapeutic
agent is delivered via the pores 1690 of implant 1632 to the treatment site,
which can be
within or outside of the pores 1690. By contrast, Fig. 23 shows a drug
reservoir 1638 and
drug delivery channels 1640 embedded in or defined by the body 1644 of the
implant 1632.
Thus, upon filling reservoir 1638 with the therapeutic agent (either initially
and/or as a refill),
the therapeutic agent can move from the reservoir 1638 to the treatment site
via channels
1640. Figs. 24-26 each shows a femoral hip prosthetic implant 1732 in which a
portion of the
body 1744 of the implant 1732 is porous to facilitate leaching of therapeutic
agents
therefrom. The porous portion of body 1744 is labeled as 1790. The implant
1732 of Fig. 24,
however, does not include in addition thereto a drug reservoir or drug
delivery channels; but,
as stated above relative to implant 1632 and Fig. 24, one or more pores, as
well as the
connections between the pores ("connections", as explained above) can form the
reservoir of
the channels according to the present invention. Thus, the therapeutic agent
can be delivered
via the porous portion 1790 to the treatment site, which can be within or
outside of the porous
portion 1790. By contrast, the implants 1732 of Figs. 25 and 26 do include in
addition
thereto a drug reservoir 1738 and drug delivery channels 1740. Fig. 25 shows
the reservoir
1738 embedded in or defined by the porous portion 1790 of the body 1744 of the
implant
1732 and drug delivery channels 1740 at least partially embedded in or defined
by the porous
portion 1790 of the body 1744 of the implant 1732. Thus, upon filling
reservoir 1738 with
the therapeutic agent (either initially and/or as a refill), the therapeutic
agent can move from
the reservoir 1738 to the treatment site (which can be either within or
outside of the porous
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
portion 1790) via channels 1740. Fig. 26 shows that the reservoir 1738 is not
located in the
porous portion 1790 and shows the drug delivery channels 1740 at least in part
leading to the
porous portion 1790. Thus, upon filling reservoir 1738 with the therapeutic
agent (either
initially and/or as a refill), the therapeutic agent can move from the
reservoir 1738 to the
treatment site (which can be either within or outside of the porous portion
1790) via channels
1740.
[00144] Fig. 27 shows an orthopaedic implant system 1830 with a femoral hip
prosthetic
implant 1832 and a sponge-like or spongy material or element 1892. Similar to
the implants
discussed above, the body 1844 of the implant 1832 defines a drug reservoir
1838 and drug
delivery channels 1840 leading from the reservoir 1838 to the exterior surface
1846 of the
body 1844. The reservoir 1838 contains or houses the spongy element 1892. The
purpose of
this material is to control dispersion of the therapeutic agents from the
reservoir 1838 into the
drug delivery channels 1840, to keep bone and tissue from growing into and
filling the
reservoir 1838, and/or to stiffen the implant 1832. Upon filling reservoir
1838 with the
therapeutic agent (either initially and/or as a refill) and having positioned
sponge-like
material 1892 in reservoir 1838, the therapeutic agent can move from the
reservoir 1838 (and
thus also from spongy element 1892) to the treatment site via channels 1840.
Depending
upon the outcome desired, the material of the sponge-like element 1892 can be
a number of
possibilities. For example, if the sponge 1892 is to remain in reservoir 1838
for a long time,
then a Polyvinyl Alcohol (PVA) or Ivalon sponge, for example, can be used. On
the other
hand, if the sponge 1892 is to last a shorter amount of time, then a collagen
based material
(i.e., Instat, by Johnson and Johnson, for example) or a gelatin sponge (i.e.,
Gelfoam, by
Pfizer, for example), for example, can be used. These examples of the sponge
1892 are
provided by way of example, and not by way of limitation.
[00145] Any of the devices according to the present invention described above
can include
a single or multiple attachment features (such as connections for catheters or
ports) and a
single or multiple sets of reservoirs and/or channels. The same therapeutic
agent can be used
in all reservoirs/channels, or several therapeutic agents can be used at one
time. Separate
reservoirs/channels allow each of the therapeutic agents to be delivered to a
specific location
on the implant, if desired.
[00146] Any of the internal (implanted) devices according to the present
invention
described above can include an internal reservoir (contained within the
implant) in
conjunction with delivery channels/paths to allow for short- and/or long-term
delivery of the
therapeutic agents. If an internal reservoir does not exist, the implant can
contain delivery
channels/paths to allow for the dispersion of the therapeutic agent.
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
26
[00147] According to the present invention, therapeutic agents can be
introduced into the
delivery channels/paths and/or implant reservoir of the implant of the present
invention by
one or more of the following ways:
a. Direct interface between a delivery vessel (such as a hypodermic syringe).
b. Direct attachment of a drug pump, external reservoir (external to the
implant, but
can be located internally or externally to the patient), and/or port to the
implant; that is, a
drug pump, external reservoir, and/or port can be attached directly to the
implant. A catheter
can be, but is not necessarily, located between the drug pump, external
reservoir, and/or port
and the implant. The therapeutic agent is then introduced into one of these
intermediary
devices by, for example, a hypodermic syringe. The therapeutic agent is then
transferred to
the implant delivery channels/paths and/or implant reservoir.
c. A drug pump, reservoir, and/or port can be implanted in the body in another
location remote to the implant and/or can be connected to the implant by, for
example, a
delivery tube or catheter. Fig. 28 shows schematically this option for an
orthopaedic implant
system. According to system 1930, a reservoir 1994, a pump 1995, and a port
1996 are
implanted under the skin of a patient body 1934 remote from implant 1932 and
are shown
connected via an implanted catheter 1998 to the reservoir 1938 of the implant
1932. The
reservoir 1994, pump 1995, and port 1996 are thereby configured for delivering
the
therapeutic agent (shown by arrow 1936, which also shows the direction of
travel of the
therapeutic agent) from the reservoir 1994 to the treatment site 1942 via the
implant 1932.
Stated another way, the pump 1995 and port 1996 can cooperate with the
reservoir 1938 to
deliver the therapeutic agent 1936 via the catheter 1998 to the reservoir 1938
defined by the
body of the implant 1932. The body of implant 1932 can define channels, either
sub-surface
or surface channels, running from reservoir 1938 to the exterior surface of
implant 1932. The
implant 1932 is an orthopaedic implant, such as a prosthesis, a nail, a plate,
an implanted pin
of an external fixation device, an internal fixation device, a porous device,
a bladder, a
spongy element, an implant implantable in soft tissue, or any other
orthopaedic implant.
d. A drug pump, reservoir, and/or port can be located external to the body and
connected to the implant by, for example, a delivery tube or catheter. The
main difference
between the example of this subparagraph and the example of subparagraph c of
this
paragraph is that the catheter runs from one location inside the body to
another location
inside the body in the example of subparagraph c of this paragraph, while the
catheter runs
from outside of the body to the implant inside the body in the example of this
subparagraph.
Fig. 29 shows schematically this option for an orthopaedic implant system.
According to
system 2030, a reservoir 2094, a pump 2095, and a port 2096 are not implanted
under the
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
27
skin of a patient body 2034 but are shown connected to the reservoir 2038 of
the implant
2032 by a transcutaneous (passing, entering, or made by penetration through
the skin)
catheter 2098. The reservoir 2094, pump 2095, and port 2096 are thereby
configured for
delivering the therapeutic agent (shown by arrow 2036, which also shows the
direction of
travel of the therapeutic agent) from the reservoir 2094 to the treatment site
2042 via the
implant 2032. Stated another way, the pump 2095 and port 2096 can cooperate
with the
reservoir 2094 to deliver the therapeutic agent 2036 via the catheter 2098 to
the reservoir
2038 defined by the body of the implant 2032. The body of implant 2032 can
define
channels, either sub-surface or surface channels, running from reservoir 2038
to the exterior
surface of implant 2032. The implant 2032 is an orthopaedic implant, such as a
prosthesis, a
nail, a plate, an implanted pin of an external fixation device, an internal
fixation device, a
porous device, a bladder, a spongy element, an implant implantable in soft
tissue, or any
other orthopaedic implant.
e. A catheter runs from outside the body to the implant inside the body but
would not
include a pump, a reservoir, or a port being attached to the outside end of
the catheter (the
outside end being the end opposite the end which is attached to the implant).
[00148] The orthopaedic implants of the present invention can be applied in
conjunction
with any currently available designs, including porous coatings, and can also
be used in
conjunction with cemented implants.
[00149] The present invention further provides a method of using an
orthopaedic implant
system, such as system 30. The method includes the steps of: implanting an
orthopaedic
implant 32 at a selected location within a corporeal body 34, implant 32
including a reservoir
38 and a plurality of channels 40; receiving at least one therapeutic agent 36
in reservoir 38;
conveying at least one therapeutic agent 36 from reservoir 38 to a treatment
site 42 relative to
corporeal body 34 via channels 40; and delivering at least one therapeutic
agent 42 to
corporeal body 34. As discussed above, the implant according to the present
invention is a
prosthesis, a nail, a plate, or an external fixation device with an implanted
pin. Implant 32
includes a body 44 which is implanted at the selected location, body 44
defining reservoir 38
and channels 40 and including an exterior surface 46, channels 40 fluidly
communicating
reservoir 38 with exterior surface 46 and thereby conveying therapeutic agent
36 from
reservoir 38 to exterior surface 46. The method can include attaching a porous
surface 1154
to exterior surface 1146, porous surface 1154 receiving bone and/or tissue
ingrowth 1156
therein, porous surface 1154 including a first side 1164 attached to exterior
surface 1146 and
a second side 1166 opposing first side 1164, porous surface 1154 including a
through-hole
1168 running from first side 1164 to second side 1166, through-hole 1168
communicating at
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
28
least one therapeutic agent 1136 from first side 1164 to second side 1166 and
thereby
communicating at least one therapeutic agent 1136 to treatment site 1142.
Exterior surface
1146 can define a surface channel 1170, surface channel 1170 being in
communication with
and cooperating with at least one channel 1140 and at least one through-hole
1168 and
thereby providing at least one therapeutic agent 1136 from reservoir 1138 to
treatment site
1142. At least one channel 40 can be a sub-surface channel 1172, sub-surface
channel 1172
and through-hole 1168 being aligned with and cooperating with one another and
thereby
providing at least one therapeutic agent 1136 from reservoir 1138 to treatment
site 1142. The
method can include implanting a second reservoir 1994, a pump 1995, and/or a
port 1996 in
corporeal body 1934 remote from implant 1932, connecting second reservoir
1994, pump
1995, and/or port 1996 to reservoir 1938 of implant 1932 by at least one
catheter 1998
implanted in corporeal body 1934, and delivering at least one therapeutic
agent 1936 to
treatment site 1942 via implant 1932, catheter 1998, and second reservoir
1994, pump 1995,
and/or port 1996. The method can include providing a second reservoir 2094, a
pump 2095,
and/or a port 2096 which is not implanted in corporeal body 2034, connecting
second
reservoir 2094, pump 2095, and/or port 2096 to reservoir 2038 of implant 2032
by at least
one transcutaneous catheter 2098, and delivering at least one therapeutic
agent 2036 to
treatment site 2042 via implant 2032, catheter 2098, and second reservoir
2094, pump 2095,
and/or port 2096. The method can include inserting a cartridge 1586 into
reservoir 1538,
cartridge 1586 containing at least one therapeutic agent 1536 and releasing at
least one
therapeutic agent 1536 into reservoir 1538 and/or at least one channel 1540
such that at least
one therapeutic agent 1536 moves away from reservoir 1538 in at least one
channel 1540,
removing cartridge 1586 from reservoir 1538 after implant 1532 has been
implanted in
corporeal body 1534, and replacing cartridge 1586 with another cartridge 1586
after implant
1532 has been implanted in corporeal body 1534. The method can include
providing a
spongy element 1892, reservoir 1838 containing spongy element 1892. Body 1644,
1744 of
implant 1632, 1732 can be partially or completely porous. External fixation
device 1432 can
include implantable pin 1476, a sheath 1478 coupled with pin 1476, and
reservoir 1480
coupled with sheath 1478, pin 1476 defining a plurality of channels 1440.
Implant may
include only one reservoir. The method can include refilling reservoir 38 with
at least one
therapeutic agent 36 after implant 32 has been implanted in corporeal body 34.
The method
can include delivering a plurality of therapeutic agents 36 to corporeal body
34 via reservoir
38 and channels 40 of implant 32.
[00150] Fig. 30 shows an orthopaedic implant system 3030 according to the
present
invention. Structural features in Figs. 30-31 corresponding to similar
features in prior figures
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
29
have reference characters raised by multiples of 100. System 3030 includes an
orthopaedic
implant 3032 which is implantable at a selected location within corporeal body
3034 and is
configured for delivering at least one therapeutic agent 3036 to corporeal
body 3034. Implant
3032 defines reservoir 3038 and channels 3040. Reservoir 3038 is configured
for receiving
at least one therapeutic agent 3036. Channels 3040 are configured for
conveying at least one
therapeutic agent 3036 from reservoir 3038 to treatment site 3042 relative to
corporeal body
3034. Reservoir 3038 can receive therapeutic agent 3036 before or after
implant 3032 is
implanted in corporeal body 3034. Advantageously, as with the implants
described above,
implant 3032 can be advantageously simply pumped with a therapeutic agent
3036, which
can be a liquid, into the reservoir 3038, the therapeutic agent 3036 then
migrating through the
channels 3040 to the exterior surface 3046 or otherwise to the treatment site
3042. The
orthopaedic implant of the present invention is an internal fixation device
3032 and/or a
porous device 3532. Upon filling reservoir 3038 with the therapeutic agent
3036 (either
initially and/or as a refill, before or after implantation), the therapeutic
agent 3036 can move
from the reservoir 3038 to the treatment site via channels 3040.
[00151] An internal fixation device is a device which attaches something to
the skeleton, a
bone, of the corporeal body. An internal fixation device includes, but is not
limited to, a bone
screw, a bone anchor, a bone tack, a bone graft, or a bone plug. A bone screw,
for example,
can be used to fix soft tissue (i.e., muscles, ligaments) to bone, or to fix
bone to bone. An
internal fixation device can be implanted within the corporeal body. Such
internal fixation
devices may include threads for affixation; alternatively, such internal
fixation devices may
include barbs (rather than threads) to provide the affixation, may have a
smooth shaft with
blades at the end of the shaft (the barbs providing the affixation), or may
form a press fit
with, for example, bone. These examples of the device and the usages of the
device are
provided by way of example and not by way of limitation.
[00152] Fig. 30 shows internal fixation device 3032 as a bone screw 3032 which
includes
an exterior surface 3046, a head 3031, and a threaded section 3033. Head 3031
includes at
least one channel 3040. Threaded section 3033 includes at least one channel
3040. Channels
3040 fluidly communicate reservoir 3038 with exterior surface 3046 and thereby
are
configured for conveying at least one therapeutic agent 3036 from reservoir
3038 to exterior
surface 3046. Fig. 30 thus shows that at least one therapeutic agent 3036 can
be delivered
through channels 3040 in threads 3033 and/or through the delivery channel 3040
in head
3031. Implant 3032 can include only one reservoir 3038. A similar design can
be applied to,
for example, a bone anchor.
[00153] Fig. 31 shows bone screw 3032 in use in a knee. Fig. 31 shows
schematically a
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
knee joint including a femur 3035, a tibia 3037, and a joint capsule 3039.
Bone screw 3032
is placed in the femur 3035 within the joint capsule 3039. As such, the
reservoir 3038 of
bone screw 3032 can be filled with at least one therapeutic agent 3036, which
can then flow
through the channels 3040 and thereby be delivered directly into the joint
capsule 3039
and/or to the femur 3035.
[00154] Fig. 32 shows an orthopaedic implant system 3230 according to the
present
invention. Structural features in Figs. 32-33 corresponding to similar
features in prior figures
have reference characters raised by multiples of 100. System 3230 includes an
internal
fixation device (i.e., bone screw 3232) implanted in a bone 3241 and a second
reservoir 3243
implantable at the selected location within corporeal body 3234. Second
reservoir 3243
includes a plurality of delivery holes 3245 configured for delivering at least
one therapeutic
agent 3236 to corporeal body 3234. Fig. 32 shows internal fixation device as a
bone screw
3232 including a head 3231, a threaded section 3233, and an exterior surface
3246. Second
reservoir 3243 surrounds and is attached to head 3231. Head 3231 includes an
ingress
channel 3247 configured for conveying at least one therapeutic agent 3236 from
second
reservoir 3243 to reservoir 3238 of bone screw 3232. Threaded section 3233
includes
channels 3240. Channels 3240 fluidly communicate reservoir 3238 of bone screw
3232 with
exterior surface 3246 and thereby are configured for conveying at least one
therapeutic agent
3236 from reservoir 3238 of bone screw 3232 to exterior surface 3246. Second
reservoir
3243 can be filled with therapeutic agent 3236 and then communicate
therapeutic agent 3236
to reservoir 3238. Alternatively, reservoir 3238 can be filled with
therapeutic agent 3236 and
then communicate therapeutic agent 3236 to second reservoir 3243.
Alternatively,
therapeutic agent 3236 in reservoir 3238 and second reservoir 3243 can flow
from reservoir
3238 to second reservoir 3243, or vice versa, depending upon the balance of
pressures in the
reservoir 3238 and the second reservoir 3243. Thus, therapeutic agents 3236
can be delivered
through threads 3233 of screw 3232 and/or through reservoir 3238. An anchor or
a bone plug
can be used in place of the bone screw 3232. Second reservoir 3243 can also be
referred to as
a bladder or balloon. A syringe can be used to fill or refill reservoir 3238
and/or second
reservoir 3243. Upon filling reservoir 3238 and/or second reservoir 3243 with
the therapeutic
agent 3236 (either initially and/or as a refill, before or after
implantation), the therapeutic
agent 3236 can move from the second reservoir 3243 and/or reservoir 3238 to
the treatment
site 3242 via channels 3240 and/or holes 3245.
[00155] Fig. 32 shows second reservoir 3243 can be elastic and thereby be
configured for
expelling at least one therapeutic agent 3236 through holes 3245 and/or into
ingress channel
3247. That is, as second reservoir 3243 is filled beyond its elastic yield
point, the pressure
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
31
created by second reservoir 3243 can force therapeutic agent 3236 out through
holes 3245
and into ingress channel 3247. Therapeutic agent 3236 can flow from ingress
channel to
reservoir 3238 and then through channels 3240 to exterior surface 3246 and to
a treatment
site 3242. Alternatively, Fig. 33 shows second reservoir 3243 can be rigid
(not elastic) and
can form a permeable membrane configured for controllably releasing at least
one therapeutic
agent 3236 therefrom. System 3230 in Fig. 33 is substantially identical to
system 3230 in
Fig. 32 but for second reservoir 3243, as explained herein. Arrows in Fig. 33
show
therapeutic agent 3236 flowing through the permeable membrane of second
reservoir 3243.
As a permeable membrane, second reservoir 3243 can be made of a material that
inherently
has permeability without having to form holes therein in a separate
manufacturing step.
Upon filling reservoir 3238 and/or second reservoir 3243 with the therapeutic
agent 3236
(either initially and/or as a refill, before or after implantation), the
therapeutic agent 3236 can
move from the second reservoir 3243 and/or reservoir 3238 to the treatment
site 3242 via
channels 3240 and/or holes 3245.
[00156] Fig. 34 shows an orthopaedic implant system 3430 according to the
present
invention. Structural features in Fig. 34 corresponding to similar features in
prior figures
have reference characters raised by multiples of 100. System 3430 includes an
internal
fixation device (i.e., bone screw 3432) implanted in a femur 3435 of a knee
joint including
the femur 3435 and the tibia 3437. Skin of corporeal body 3434 is also shown
in Fig. 34.
System 3430 further includes a second reservoir 3462 and a tubular element
3498 (i.e.,
catheter). Second reservoir 3462 is implanted within corporeal body 3434
remote from bone
screw 3432. Tubular element 3498 is implanted within corporeal body 3434. Bone
screw
3432 includes an exterior surface 3446. Second reservoir 3462 is coupled with
bone screw
3432 via tubular element 3498 and is thereby configured for delivering at
least one
therapeutic agent to exterior surface 3446 via tubular element 3498, the
reservoir, and the
channels. The reservoir and channels of bone screw 3432 are not shown in Fig.
34, but it is
understood that bone screw 3432 includes a reservoir and channels like those
of bone screw
3032. Tubular element 3498 can be coupled with the head of bone screw using,
for example,
an interference fit; an alternative way of attachment is shown below. Fig. 34
shows bone
screw 3432 placed inside the joint capsule 3439. Therapeutic agents are
contained in second
reservoir 3462, are delivered to screw 3432, and are eluted or otherwise
passed from screw
3432 into the femur 3435 and/or the joint capsule 3439. Second reservoir 3462
can be placed
within soft tissue, rather than at the knee where the bone is close to the
skin. Upon filling
reservoir 3462 with the therapeutic agent (either initially and/or as a
refill, before or after
implantation), the therapeutic agent can move from the reservoir 3462 to the
treatment site
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
32
via bone screw 3432. Second reservoir 3462 can be filled prior to or after
implantation of
reservoir 3462 and/or bone 3432, initially and/or as a refill.
[00157] Figs. 9, 13, 15-16, 22-26, 35-36, 40, 44-49, and 51 show porous
devices according
to the present invention. The porous device according to the present invention
can be a
screw, as discussed below, but can be other orthopaedic implants as well. For
instance, the
porous device can be other internal fixation devices. Further, the porous
device according to
the present invention can be natural or artificial bone grafts.
[00158] Thus, the present invention further provides porous screws and screws
that can
deliver therapeutic agents. Further, the present invention provides a porous
screw for
attaching various soft tissues to bone, and/or for attaching bone to bone,
and/or for delivering
therapeutic agents (for example biologics or drugs) to soft tissue and/or
bone. Potential uses
include, but are not limited to, ACL and PCL reconstruction, medial collateral
ligament
repair, lateral collateral ligament repair, posterior oblique ligament repair,
iliotibial band
tenodesis reconstruction, patellar ligament and tendon repair, pedicle screws
for spine repair,
bone fracture fixation screw, and drug eluting implant (non-load bearing) for
delivery of
therapeutics.
[00159] An embodiment of the present invention provides an orthopaedic screw
having a
plurality of regions, at least one of which may be porous. The orthopaedic
screw includes a
head, a tip and at least one thread. The porosity of the screw of the present
invention can
vary within the part or region, including changes in pore shape, size and
density. These
characteristics can vary along the length of the screw axis and/or radially
(from the outer
diameter to the axis).
[00160] The orthopaedic screw of the present invention may further include at
least one
solid region formed of any implantable polymer, reinforced polymer or metal.
The solid
region of material may be, for example, at the outer portion of the threads
and the leading tip
of the screw due to the high stresses present during insertion. The solid
region may further
include the head of the orthopaedic screw of the present invention.
[00161] The materials to create the orthopaedic screw of the present invention
can be any
implantable polymer, metal or ceramic, or any combination thereof. Possible
polymers
include polyetheretherketone (PEEK), polyetherketone (PEK),
polyaryletherketone (PAEK),
polyethylene, and resorbable polymers such as polylactic acid (PLA) and
polyglycolic acid
(PGA).
[00162] The thread of the orthopaedic screw of the present invention may be
continuous or
discontinuous and be a single or multiple lead thread. The inventive screw may
further be
cannulated or non-cannulated.
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
33
[00163] The orthopaedic screw of the present invention may further be used to
locally
deliver therapeutic agents that promote positive tissue response (e.g.
increased growth rate,
decreased inflammatory response). Such therapeutic agents include, but are not
limited to,
hydroxyapatite, drugs and biologics.
[00164] Another embodiment of the orthopaedic screw of the present invention
provides
for immediate delivery of a therapeutic agent through channels and/or holes
and reservoirs
for long-term delivery of a therapeutic agent. Access to the delivery
channels, holes and/or
reservoirs may be gained by provision of a self-sealing polymer diaphragm
which can allow
for direct interface with a needle at the time of surgery of post-surgery.
Alternatively, a
removable cap made of PEEK or other implantable material may provide access to
and seal
the medicine delivery features of the inventive screw.
[00165] An advantage of the present invention is that the porous nature of the
inventive
orthopaedic screw and the ability to deliver therapeutic agents to the
surrounding tissue
promotes successful tissue integration. Such local delivery of therapeutic
agents can aid in
such issues as improving the attachment strength of soft tissue to bone in
reconstructive
surgeries, improving the attachment strength of bone to screw, and strengthen
bone in
osteoarthritic or osteoporotic patients. Another advantage is that the
orthopaedic screw of the
present invention can effectively be utilized for long term or short term
delivery of
therapeutic agents. Another advantage is that the therapeutic agent can be pre-
loaded into
the device at the factory or loaded by the surgeon before, during, or after
surgery.
[00166] The present invention provides a device which can have a porous nature
and
which has the ability to deliver therapeutic agents. The porous nature of the
device of the
present invention and the ability of the device of the present invention to
deliver therapeutic
agents therethrough promotes successful bone and/or soft tissue integration.
[00167] The present invention provides a screw that is porous and/or can
deliver
therapeutic agents to the surrounding tissue. The materials to create this
screw can be any
implantable polymer, metal or ceramic or combinations of these. Possible
polymers include
PEEK (Poly(etheretherketone)), PEK (Poly(etherketone)), PAEK
(poly(aryletherketone)),
polyethylene, and resorbable polymers such as PLA (Poly(lactic acid)) and PGA
(poly(glycolic acid)). Likely first candidates are PEEK, reinforced PEEK
(reinforcing
materials include but are not limited to carbon fiber/particles/nanotubes,
barium sulfate,
zirconia) and titanium/titanium alloys. The screw of the present invention can
include the
ability to deliver therapeutic agents (such as drugs or biologics) to the
surrounding tissue.
The therapeutic agent can be selected by the surgeon before the surgery, at
the time of
surgery, or at any point in time thereafter. In addition, the therapeutic
agent can be pre-
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
34
loaded into the device at the factory through currently acceptable practices
or loaded by the
surgeon before, during, or after surgery (as a follow-up procedure).
[00168] The screw of the present invention can be porous but does not need to
be porous.
[00169] Structural features in Figs. 35-43 and 53-66 corresponding to similar
features in
prior figures have reference characters raised by multiples of 100. Screw 3532
of the present
invention can be fully porous or have select regions of solid material. Fig.
35 shows a
completely porous screw. The reservoir of screw 3532 of the present invention
is formed by
one or more pores 3590. The channels 3540 of screw 3532 of the present
invention are
formed by the pores 3590 themselves; more specifically, the interconnected
pores 3590 form
the channels 3540 to the exterior surface 3546 of screw 3532. Such a porous
screw can be
pumped, for instance, with a therapeutic agent, such as a liquid therapeutic
agent; the
therapeutic agent can then leach out through the interconnected pores 3590 to
the exterior
surface 3546 of implant to a treatment site 3542.
[00170] Further, Fig. 36 shows that screw 3532 may include porous region 3590
and a
solid region 3593 of material at the outer portion of threads 3533 and leading
tip 3549 of
screw 3532. The solid region 3593 of material at the outer portion of threads
3533 and
leading tip 3549 of screw 3532 may be desired due to the high stresses these
regions can see
during screw insertion (see Fig. 36). In addition, a very rough porous
structure on the outer
portion of the threads can cause insertion of the screw to be difficult due to
its potential to
grab versus slide past or cut through bone/soft tissue. Head 3531 of screw
3532 may be
solid. This solid material can be formed of any implantable polymer,
reinforced polymer, or
metal. To fill or partially fill implant 3532, an attachment device, a
catheter, and a port can
be used, for example, as shown in Figs. 42 and 43.
[00171] Thread 3533 can be continuous (see Fig. 37) or discontinuous (see Fig.
38) and be
a single or multiple lead thread. The porosity of the screw 3532 can vary
within the
region(s), including changes in pore shape, size, and density. These
characteristics can vary
along the length of the screw axis and/or radially (from the outer diameter to
the axis).
Another way of improving integration of the surrounding tissue is to deliver
therapeutic
agents that promote positive tissue response (e.g. increased growth rate,
decreased
inflammatory response). The orthopaedic screw of the present invention can be
used to
locally deliver such therapeutic agents to the tissue surrounding the device.
Such local
delivery of therapeutic agents can aid in such issues as improving the
attachment strength of
soft tissue to bone in reconstructive surgeries, improving the attachment
strength of bone to
the screw, and strengthen bone in osteoarthritic or osteoporotic patients.
Therapeutic agents
include, but are not limited to, hydroxyapatite, drugs, and biologics.
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
[00172] Screws allowing for localized delivery of therapeutic agents,
according to the
present invention, can be, but need not be, porous. Porous screws according to
the present
invention can, but need not, allow for localized delivery of therapeutic
agents.
[00173] Screw 3532 can contain reservoirs 3538 for the long-term delivery of
the
therapeutic agents, as illustrated in Fig. 40 and/or channels/holes 3540, as
illustrated in Fig.
39, for immediate, local delivery of therapeutic agents. Screw 3532 can
further include a
plurality of interconnected pores 3590 allowing for local delivery of a
therapeutic agent to the
surrounding tissue, as shown in Fig. 40. These options are described as
follows:
1. Long term delivery.
a. Reservoirs. One or more reservoirs 3538 can allow for the long-term (hours
to
weeks) delivery of the therapeutic agents. Access to delivery channels 3540,
reservoir 3538, etc. of screw 3532 is gained by several ways including:
i. Self-sealing polymer diaphragm 3551 can allow for direct interface
with a needle at the time of surgery or post-surgery (see Fig. 40).
ii. A removable cap 3553 made of PEEK or another implantable material
can also provide access to the therapeutic agent delivery features and
seal these features after delivery of the therapeutic agent (Fig. 41). A
tool that facilitates insertion of the screw could also aide in assembling
cap 3553 to the screw.
b. Connect to another device. Access to the therapeutic agent delivery
features
of the screw can be provided by interfacing screw 3532 with a device designed
to deliver therapeutic agents from subcutaneous to elsewhere in the body (e.g.
a port that is frequently used to deliver therapeutic agents from sub-skin to
a
vein deeper in the chest cavity). The last option can include attachment
feature 3555 on screw 3532 that directly interfaces with port 3596, interfaces
with catheter 3598 (which interfaces with the port 3596), or interfaces with
an
additional component, which can be attached to screw 3532 to interface with
port 3596 or catheter 3598. (See Figs. 42 and 43). Fig. 43 shows an
alternative
attachment feature 3555. Port 3596 can have a septum (the center circle of
port 3596) for receiving an injection of a therapeutic agent.
2. Immediate delivery. No reservoir is required for this approach, although a
reservoir
can be provided. The access means of the reservoir design above (self-healing
or self-
sealing polymer diaphragm 3551 and removable cap 3553) can also be used to
access
delivery channels 3540 in this design. This design can also include a simple
interface
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
36
with a delivery tool. An example of this is a simple slip fit between a
delivery needle
and the screw's cannula.
A given screw can contain any or all of these options. Upon filling reservoir
3538 of
screw 3532 (whether screw 3532 is porous or not) with the therapeutic agent
(either initially
and/or as a refill, before or after implantation), the therapeutic agent can
move from the
reservoir 3538 (or from the pores where the filling with the therapeutic agent
occurred) to the
treatment site via bone screw 3532.
[00174] Cannulation. The screws can be cannulated or non-cannulated.
[00175] Sections (A) through (E) are discussed immediately below. These
sections are as
follows: (A) manufacturing options for making the porous screw according to
the present
invention; (B) how to bond parts containing polymer(s); (C) how to bond
metal/metal alloy
parts; (D) manufacturing options for making screw threads of a screw according
to the
present invention; and (E) and manufacturing options for cannulation according
to the present
invention. Sections (A) through (E) are discussed in reference to forming a
screw according
to the present invention. It is understood, however, that the discussion can
be applied or
adapted as necessary to other internal fixation devices and/or porous devices.
[00176] A. Porous Structure ¨ Manufacturing Options According to the Present
Invention
The porous structure of the present invention can be manufactured using a
variety of
methods. These manufacturing options according to the present invention
include seven
options as follows:
1. Rolled. A porous sheet can be, for example, rolled into a screw. This is
essentially
the reverse of making a radial, spiral cut that is parallel to the axis of the
screw.
Layers of different materials can be combined in this process. This process
involves
the following:
a. Make a porous sheet with holes in a pattern so that they line up when
rolled.
b. Roll sheet (see Figs. 53-56. Fig. 53 shows a porous sheet 5332 according to
the present invention to be rolled into a screw. Fig. 54 shows an end view of
sheet 5332 during the rolling process. Fig. 55 shows a sectioned end view of
the final product, formed as a screw 5332. Fig. 56 shows the sheet 5332 with
a center 5365 formed as a cannula (an open hole through the screw axis), or a
porous rod, or a solid rod.). This step can be performed with or without the
aid of a center mandrel or rod.
1. The sheet can be rolled without the aid of any center mandrels. This
can create a cannulated screw. A biocompatible pin/rod can be
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
37
inserted in any center hole and bonded to the screw to create a non-
cannulated screw.
2. The sheet can be rolled around a removable mandrel. This can create
a cannulated screw. A biocompatible pin/rod can be inserted in any
center hole and bonded to the screw to create a non-cannulated
screw.
3. Alternately the sheet can be rolled around and bonded to a
biocompatible rod, creating a non-cannulated screw.
c. Bond the rolled material.
2. Spiraled layers. This method is similar to the rolled approach, but this
method
involves bands of material that are wrapped around one another. The main
difference
between this method and that of rolling is that in this method, the bands of
material
5732 translate along the axis while they are wrapped (see Fig. 57. Fig. 57
shows an
example of a spiraled band of material, the material not having pores). Bands
of
several materials can be combined and intertwined. All bands can have the same
direction and pitch of winding or different directions and pitches. These
bands can be
wrapped around a mandrel 5765 that is later removed to aid in bonding and to
create a
cannula. They can also be wrapped around a pin 5765 which they are then bonded
to,
creating a non-cannulated screw. An alternate option for creating a non-
cannulated
screw is to create the screw with or without the aid of a mandrel, then insert
and bond
a pin within the center hole of the screw.
3. Layered/stacked. Make a number of layers that are stacked and bonded to
create the
screw. These layers can be parallel to one another. The faces of the layers
are
perpendicular to the axis of the screw, parallel to it, or any other angle of
orientation.
To reduce secondary operations, alignment of one layer to another may be
desirable.
Alignment of layer to layer can be achieved by such ways as alignment fixtures
that
line up the center cannula (if the screw is cannulated) of each layer to one
another (by
way of a pin for example), fixtures or implant components/features that align
pore or
thread features to one another, or fixtures or implant components/features
that align
features on the outer diameter of each layer to one another. Features can also
be
created within a given layer to aid in alignment and/or assembly (such as
grooves and
mating protrusions). Figs. 58-60 show the stacked manufacturing method. Fig.
58
shows layers 5867 of the screw 5832 exploded from one another and stacking in
the
direction of the arrows. Fig. 59 shows a side view of screw 5932 with stacked
layers
5967 perpendicular to the longitudinal axis of screw 5832. Fig. 60 shows a
side view
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
38
of screw 6032 with stacked layers 6067 parallel to the longitudinal axis of
screw
6032.
Note: The holes shown in Figs. 58-60 can be created by, for example, laser
cutting,
punching, etching, electrical discharge machining, plasma etching,
electroforming, electron
beam machining, water jet cutting, stamping, or machining. For polymer based
materials,
they can be created as the sheets are created by, for example, extruding,
injection molding, or
hot stamping.
4. Dissolvable material.
a. One method involves creating a mixture of powdered implantable material
(e.g. PEEK) and a powder (e.g. salt) that is soluble in something in which the
implantable material is not soluble (such as water, isopropyl alcohol for the
PEEK example). The mixture is then heated to bond the implantable particles
together. Pressure can also be applied to aid in the bonding of particle to
particle. Heat can be created by convection or other ways (such as coating the
powder with a material that absorbs a given range of energy waves ¨ such as
laser waves ¨ and causes heating. (e.g. Clearweld coating by Gentex
Corporation)). Finally, dissolve away the filler to create the porous
implantable material. This method can create net shape parts or raw material
shapes from which individual parts can be created.
b. Another method involves mixing an implantable polymer with a dissolvable
material such as described above. The mixture is then pelletized and then
injection molded to an intermediary or the final part shape. The filler is
dissolved away to create the porous implantable polymer.
5. Stereolithography.
6. Laser or electron beam sintering of powdered material.
7. A combination of the above methods: for example, using the dissolvable
method to
create microporous sheets of PEEK, then stamping larger pores and stacking to
create
a screw.
[00177] B. How to bond parts containing polymer(s)
Options for bonding processes
1. Heat. Heat can be generated in several ways:
a. Ultrasonic welding ¨ use ultrasonic waves to create heat at the interface
of
layers.
b. Heat staking ¨ use a heated tool to cause melting between the layers.
c. Vibratory welding.
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
39
d. Laser welding.
e. Convection ¨ use an oven to create heat to cause bonding.
f. Intermediary layer ¨ for example, use a material that can absorb energy
waves
that pass through the polymer (for example PEEK) without causing damage.
The absorbed energy will cause localized heating. An example of such a
coating is Clearweld by Gentex Corporation. The laser waves that
Clearweld absorbs pass through the PEEK without causing damage, allowing
the layers to be melted together without large scale damage to the PEEK.
2. Chemical.
a. Adhesives ¨ a secondary material (such as adhesive) can be used to bond the
material.
b. Solvent bonding ¨ a material in which the polymer or reinforced polymer is
soluble can be applied to the sheet surfaces allowing multiple surfaces to be
bonded to one another.
c. Overmolding ¨ overmolding of the polymer or reinforced polymer can provide
a chemical bonding
3. Mechanical.
a. Overmolding ¨ overmolding of a polymer or reinforced polymer can create a
mechanical lock between components on a micro or macro scale (microscale ¨
the molded material locks with surface asperities of the existing material.
Macroscale ¨ features such as tongue-groove connections or undercuts). The
overmolded material can be a separate component from the layers or one layer
can be overmolded onto another layer.
b. Features are provided within the layers or by a separate component which
provides a mechanical lock ¨ e.g. a pin, snap lock connection, dove-tail,
tongue-groove, rivet, melting tabs to create a mechanical lock, etc.
c. Some adhesives provide a mechanical bond in addition to or instead of a
chemical bond.
4. Combinations of any/all of the above methods.
Order of processes
1. Bond all layers together at once ¨ especially attractive for methods
utilizing energy
waves to trigger bonding (e.g. Clearweld coating by Gentex Corporation or
ultraviolet light curable adhesives).
2. Simultaneously bond and roll/stack layers at once ¨ again, may be
especially
attractive for methods utilizing energy waves to trigger bonding (e.g. if
light cannot
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
penetrate all layers of a rolled design in order to activate an adhesive, the
rolling
operation could take place in a light box allowing for a continuous rolling
and
adhesive curing operation.
3. Roll/stack layers and bond in increments. This could add a single
layer at a time
or multiple layers.
[00178] C. How to bond metal/metal alloy parts
Options for bonding processes
1. Heat.
a. Laser welding ¨ layers can be laser welded in a number of locations. Two or
more layers or wraps of material can be welded together at once depending on
the size of the part and alignment of the pores (the laser can access several
layers to be bonded through the porosity).
b. Spot welding ¨ traditional spot welding can be used to bond two or more
layers/wraps of material.
c. Diffusion bonding/sintering.
d. Vibratory welding.
e. Ultrasonic welding.
2. Adhesives.
3. Mechanical ways. Features are provided within the layers or by a separate
component
which provides a mechanical lock ¨ e.g. a pin, snap lock connection, dove-
tail,
tongue-groove, rivet, melting tabs to create a mechanical lock etc.
4. Overmolding with an implantable polymer. Overmolding of PEEK or another
implantable polymer can create a mechanical lock between components on a micro
or
macro scale (microscale ¨ the molded material locks with surface asperities of
the
existing material. Macroscale ¨ features such as tongue-groove connections or
undercuts). The overmolded material can be a separate component from the
layers or
one layer can be overmolded onto another layer.
Order of processes
As with the polymer materials discussed above, two or more layers of metal can
be
bonded during increments or as a continuous stacking/bonding process.
[00179] D. Making threads ¨ Manufacturing Options According to the Present
Invention
1. Form the threads after the layers have been bonded to create a screw blank
(see Fig.
61. Fig. 61 shows the screw blank 6132 of the stacked type.).
a. Machine the threads
b. Hot form the threads with a mold
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
41
2. Form threads in the sheets prior to bonding.
a. Rolling method: The material will not actually create the complete thread
shape until the sheets are formed into the final shape. Continuous or
discontinuous threads can be created. Design options for this method include
creating raised material that forms the threads (see Fig. 62) or removing
material to leave the thread material (see Fig. 63). The raised material in
the
first method can be created by way of machining, laser ablation, hot stamping,
hot or cold forming, chemical etching, electro-discharge machining and
similar methods. The material of the second method can be removed by way
of machining, laser cutting, stamping, etching, punching, electro-discharge
machining, water jet cutting, electron beam machining or other means. Fig. 62
shows a sheet 6232 according to the present invention having raised threads
6233 formed prior to rolling. Fig. 62 shows raised material to form threads
6233. The bottom portion of Fig. 62 (below the broken lines) shows a top
view of the sheet 6232 prior to rolling. The top portion of Fig. 62 (above the
broken lines) shows a side view (more precisely, an edge view) of the sheet
6232 prior to rolling. The threads of the bottom portion and top portion of
Fig. 62 align with one another per the broken lines, which show the
correspondence between the bottom and top portions of Fig. 62. Fig. 63
shows a sheet 6332 showing threads 6333 formed by material removal prior to
rolling. In Fig. 63, D is screw major diameter, t is sheet thickness, and p is
screw pitch. Fig. 63 shows a vertical tab T and a horizontal tab T (as
oriented
on the drawing page), one or both of which may be removable. Porous region
is labeled as 6390, the circles showing pores. An open area (no material) is
labeled as A. The area labeled as B shows a thread region which may be solid
or porous or may gradually change from solid to porous starting at the tab and
moving inward to the porous region 6390. The sheet 6332 may be rolled and
bonded to make screw 6332.
b. Stacking method: Continuous or discontinuous threads can also be created by
this method. The 'ears' of material in each layer 6467 form the threads 6433
when the layers are stacked (see Fig. 64). These can be created by way of
machining, hot stamping, hot or cold forming, dies/punches, chemical etching,
electro-discharge machining and similar methods. Fig. 64 shows preformed
threads 6433 in one layer 6467 of a stacked part. Stated another way, Fig. 64
shows a sheet showing threads 6433 formed prior to stacking.
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
42
3. Add separate threads ¨ Threads can be formed separately and attached to the
screw
blank. Separate threads can look like 6533 in Fig. 65. The material for these
threads
can include: biocompatible polymers, reinforced biocompatible polymers and/or
biocompatible metals. The attachment ways for these threads include:
a. Mechanical attachment ¨ press/interference fit, tabs.
b. Overmolding ¨ mold the solid, porous, or reinforced polymer screw inside of
the solid threads or mold the porous, solid or reinforced polymer threads onto
the already formed screw.
c. Adhesive or solvent bonding.
[00180] E. Cannulation ¨ Manufacturing Options According to the Present
Invention
With any of the manufacturing methods, screws can be created with or without a
cannula.
1. Cannulated.
a. Rolling method. In this method, it can be desirable to wind the material
around a mandrel that is at the center of the screw, running along its axis.
This
mandrel can be removed to leave an open cannula (see Fig. 66). Fig. 66 shows
a screw 6632 with an open cannula after the mandrel is removed during the
rolling method.
b. Layered method. A center hole at the axis of each layer is created to form
the
cannula when they are stacked together.
2. Non-cannulated.
a. Rolled method.
i. The sheet can also be bonded to the mandrel, with the mandrel forming
a portion of the implant. This mandrel can be solid or porous and of
any implantable material such as PEEK or titanium.
ii. In addition, the material can be formed around a removable mandrel,
creating a cannula. This cannula can be then be filled with a
biocompatible material that is attached/bonded to the screw.
b. Layered method. The layers that are stacked to create the screw can have
solid material in place of the holes that would create the cannula.
Alternately,
they can have cut-outs creating the cannula and this cannula can be filled
with
a biocompatible material that is attached/bonded to the screw.
[00181] Structural features in Figs. 44-49 corresponding to similar features
in prior figures
have reference characters raised by multiples of 100. The porous device can
also be formed
as a bone graft. It is understood that "bone graft" refers to either a natural
bone graft or an
artificial bone graft. A natural bone graft is taken from an alive donor or a
dead donor. A
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
43
natural bone graft can be taken from the corporeal body which is to receive
the implanted
bone graft, taken from another human being, or taken from an animal (such as
autografts,
allografts, and xenografts). Figs. 44-45 show a natural bone graft, formed as
a bone plug
4432, which is implanted in the distal end of the femur 4435 for delivering at
least one
therapeutic agent 4436. The bone plug 4432 is placed in the distal
intramedulllary canal of
the femur 4435. The bone plug 4432 includes an artificially formed reservoir
4438,
artificially formed channels 4440, and an exterior surface 4446. It is
understood that such a
natural bone plug 4432 likely has naturally formed pores, which possibly can
receive bone
and/or soft tissue ingrowth therein (as shown by arrow 4456). Fig. 46 shows
two such bone
plugs 4432 for delivering therapeutic agent(s) 4436. The bone plugs 4432 are
shown placed
in bone near the joint space. Fig. 46 shows the hip joint capsule 4439. One
bone plug 4432
is implanted in the pelvic bone 4457. The other bone plug 4432 is implanted in
the femoral
head of the femur 4435. Bone plug 4432 can deliver therapeutic agents(s)
directly within the
joint capsule 4439 and/or to the bone 4435, 4457. Fig. 47 shows a bone graft
4732 without
artificially formed reservoir and channels; rather, bone graft 4732 in Fig. 47
includes a
plurality of naturally formed pores 4790 which form the reservoir and the
plurality of
channels. The reservoir 4738 is formed by one or more such pores 4790. The
channels 4740
are formed by the interconnection between the pores 4790 to the exterior
surface 4746. Fig.
47 shows bone and/or soft tissue ingrowth by arrow 4756. Figs. 48-49 show an
artificially
formed bone graft 4832, which can also be referred to as a porous surface or
scaffold. The
scaffold 4832 is shown in a simplified form, for illustrative purposes. The
scaffold 4832 has
two layers, each layer having a plurality of pores 4890. The pores 4890 of
each layer are
offset with each other in a way that still forms pathways or channels 4840
extending between
the top and bottom sides of the scaffold. One or more pores 4890 of the
scaffold 4832 can
form the reservoir 4838, the interconnected pores 4890 also forming the
channels 4840.
Bone and/or soft tissue ingrowth is shown by arrow 4856. Fig. 49 shows the
bottom side of
the scaffold 4832 shown in Fig. 48. Thus, the dashes in Fig. 49 show the pores
4890 from the
top layer of the scaffold 4832 in Fig. 48. The circles which have a solid line
defining its
entire perimeter are pores 4890 formed in the bottom layer of the scaffold
4832 shown in Fig.
48. The scaffold 4832 can be made of metal, polymer, or ceramic. Further,
Figs. 48-49 show
an inflow of a liquid or fluid therapeutic agent (using arrow 4836) which is
provided (i.e., by
a pumping action) to the implant 4832, the therapeutic agent 4836 then
leaching out through
the pores 4890 and/or channels 4840 to the exterior surface 4846 of the
implant 4832 to the
treatment site. Upon filling reservoirs of implants 4432, 4732, 4832 with the
therapeutic
agent (either initially and/or as a refill, before or after implantation), the
therapeutic agent can
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
44
move from the reservoir to the treatment site via the implant 4432, 4732,
4832.
[00182] The present invention further provides a method of using an
orthopaedic implant
system 3030, 3530. The method includes the steps of: providing an orthopaedic
implant
3032, 3532 defining a reservoir 3038, 3538 and a plurality of channels 3040,
3540, implant
3032, 3532 being at least one of an internal fixation device 3032 and a porous
device 3532;
implanting implant 3032, 3532 at a selected location within corporeal body
3034, 3534;
receiving at least one therapeutic agent 3036, 3536 in reservoir 3038, 3538;
conveying at
least one therapeutic agent 3036, 3536 from reservoir 3038, 3538 to a
treatment site 3042,
3542 relative to corporeal body 3034, 3534 via channels 3040, 3540; and
delivering at least
one therapeutic agent 3036, 3536 to corporeal body 3034, 3534.
[00183] Internal fixation device 3032 includes an exterior surface 3046, the
plurality of
channels 3040 fluidly communicating reservoir 3038 with exterior surface 3046
and thereby
conveying at least one therapeutic agent 3036 from reservoir 3038 to exterior
surface 3046.
Internal fixation device 3032 is a bone screw 3032 including an exterior
surface 3046, a head
3031, and a threaded section 3033, head 3031 including at least one channel
3040, threaded
section 3033 including at least one channel 3040, channels 3040 fluidly
communicating
reservoir 3038 with exterior surface 3046 and thereby conveying at least one
therapeutic
agent 3036 from reservoir 3038 to exterior surface 3046.
[00184] The method can further include implanting a second reservoir 3243 at
the selected
location within corporeal body 3234, delivering at least one therapeutic agent
3236 to
corporeal body 3234 via a plurality of holes 3245 in second reservoir 3243,
internal fixation
device 3232 including an exterior surface 3246, second reservoir 3243 at least
partially
surrounding and being attached to internal fixation device 3232, internal
fixation device 3232
including an ingress channel 3247 conveying at least one therapeutic agent
3236 from second
reservoir 3243 to reservoir 3238 of internal fixation device 3232, plurality
of channels 3240
fluidly communicating reservoir 3238 of internal fixation device 3232 with
exterior surface
3246 and thereby conveying at least one therapeutic agent 3236 from reservoir
3238 of
internal fixation device 3232 to exterior surface 3246. Internal fixation
device 3232 can be a
bone screw 3232 including a head 3231, a threaded section 3233, and an
exterior surface
3246, second reservoir 3243 surrounding and being attached to head 3231, head
3231
including ingress channel 3247 conveying at least one therapeutic agent 3236
from second
reservoir 3243 to reservoir 3238 of bone screw 3232, threaded section 3233
including
channels 3240, channels 3240 fluidly communicating reservoir 3238 of bone
screw 3232 with
exterior surface 3246 and thereby conveying at least one therapeutic agent
3236 from
reservoir 3238 of bone screw 3232 to exterior surface 3246. Second reservoir
3243 can be
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
elastic and thereby expel at least one therapeutic agent 3236 through holes
3245 and/or into
ingress channel 3247. Second reservoir 3243 can be rigid and form a permeable
membrane
which controllably releases at least one therapeutic agent 3236 therefrom.
[00185] The method can further include implanting a second reservoir 3462
within
corporeal body 3434 remote from internal fixation device 3432, implanting a
tubular element
3498 within corporeal body 3434, second reservoir 3462 coupled with internal
fixation
device 3232 via tubular element 3498 and thereby delivering at least one
therapeutic agent
3436 to an exterior surface 3446 of internal fixation device 3434 via tubular
element 3498,
reservoir of device 3432, and channels of device 3432. Internal fixation
device 3432 is a
bone screw 3432.
[00186] Porous device 3532 is partially porous (Fig. 36) or completely porous
(Fig. 35).
Porous device can be a natural or an artificial bone graft 4732, 4832
including a plurality of
pores 4790, 4890 forming reservoir 4738, 4838 and channels 4740, 4840, porous
device
4732, 4832 receiving bone and/or soft tissue ingrowth 4756, 4856 therein.
Porous device can
be a natural bone graft 4432 which is configured for being implanted in a bone
4435 and for
delivering at least one therapeutic agent 4436 directly within a joint capsule
4439 and/or to
bone 4435.
[00187] The implant according to the present invention may include only one
internal
reservoir. The method can further include refilling reservoir 3038 with at
least one
therapeutic agent 3036 after implant 3032 has been implanted in corporeal body
3034. The
method can further include delivering a plurality of therapeutic agents 3036
to corporeal body
3034 via reservoir 3038 and channels 3040. Reservoir 3038 receives at least
one therapeutic
agent 3036 after implant 3032 has been implanted in corporeal body 3034 and
then
communicates at least one therapeutic agent 3036 via channels 3040 to
treatment site 3042.
[00188] The present invention further provides a method of using an
orthopaedic implant,
the method including the steps of: providing an orthopaedic implant body 5044
defining at
least one pathway 5040 (shown by arrow 5040); receiving at least one
therapeutic agent 5036
by implant body 5044; implanting the orthopaedic implant 5032 at a selected
location within
a corporeal body 5034; conveying at least one therapeutic agent 5036 from
implant body
5044 to a treatment site 5042 relative to corporeal body 5034 via at least one
pathway 5044
using pressure generated by corporeal body 5034 to mechanically force at least
one
therapeutic agent 5036 from implant body 5044 to treatment site 5042.
Structural features in
Figs. 50-51 corresponding to similar features in prior figures have reference
characters raised
by multiples of 100. Implant body 5044 can be an elastic bladder.
Alternatively, the implant
implanted as shown in Fig. 50 can be a spongy element 5132 including a
plurality of pores
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
46
5190, the at least one pathway 5140 formed by at least one pore 5190. Fig. 50
shows an
orthopaedic implant 5032 according to the present invention formed as a
bladder or reservoir.
Implant 5032 thus forms an internal reservoir 5038. Reservoir 5032 has holes
or channels
5040 for delivering the therapeutic agent 5036 to the treatement site 5042.
Fig. 51 shows a
spongy element 5132 includes an implant body 5144 with interconnected pores
5190 forming
at least one channel 5140. Depending upon the outcome desired, the material of
the spongy
or sponge-like element 5132 can be a number of possibilities. For example, if
the sponge
5132 is to remain implanted for a long time, then a Polyvinyl Alcohol (PVA) or
Ivalon
sponge, for example, can be used. On the other hand, if the sponge 5132 is to
last a shorter
amount of time, then a collagen based material (i.e., Instat, by Johnson and
Johnson, for
example) or a gelatin sponge (i.e., Gelfoam, by Pfizer, for example), for
example, can be
used. These examples of the sponge 5132 are provided by way of example, and
not by way
of limitation. Fig. 50 shows implant 5032 implanted under the quadriceps
tendon 5059, as
well as femur 5035, tibia 5037, and patella 5061. The forces experienced
during walking and
other motion can be used to force the therapeutic agent(s) 5036 from the
implant 5032. Upon
filling reservoir 5038, 5138 with the therapeutic agent (either initially
and/or as a refill,
before or after implantation), the therapeutic agent can move from the
reservoir 5038, 5138 to
the treatment site via channels 5040, 5140.
[00189] The present invention further provides a method of using an
orthopaedic implant
5232, the method including the steps of: providing an orthopaedic implant 5232
defining a
reservoir 5238 and a plurality of channels 5240; implanting implant 5232 at a
selected
location within a corporeal body 5234, the implant 5232 being implanted into
soft tissue 5263
of corporeal body 5234; receiving at least one therapeutic agent 5236 in
reservoir 5238;
conveying at least one therapeutic agent 5236 from reservoir 5238 to a
treatment site 5242
relative to corporeal body 5234 via plurality of channels 5240; and delivering
at least one
therapeutic agent 5236 to corporeal body 5234. Such an implant 5232 can be a
reservoir, a
bladder, a balloon, a sponge, a cloth, or any other orthopaedic implant
configured for being
implanted into soft tissue 5263. Soft tissue 5263 refers to bodily tissue
other than bone.
Implant 5232 is implanted into soft tissue 5263 such as a muscle, a ligament,
a tendon, a joint
capsule, a fibrous tissue, fat, a membrane, and/or cartilage of said corporeal
body.
Orthpaedic implant 5232 according to the present invention and soft tissue
5263 are shown
schematically in Fig. 52. Structural features in Fig. 52 corresponding to
similar features in
prior figures have reference characters raised by multiples of 100. Upon
filling reservoir
5238 with the therapeutic agent (either initially and/or as a refill, before
or after
implantation), the therapeutic agent can move from the reservoir 5238 to the
treatment site
CA 02735235 2011-02-24
WO 2010/025378 PCT/US2009/055380
47
via channels 5240.
[00190] The present invention thus provides a drug delivery implant configured
for
delivering therapeutic agents for the treatment of osteoarthritis or other
diseases. The implant
can deliver one or more therapeutic agents to the targeted joint capsule. Such
an implant can
be applied to any joint. Such joints include, but are not necessarily limited
to, hip, knee,
ankle, wrist, facets, and joints within the hand and foot.
[00191] The implants according to the present invention are placed in or near
the targeted
joint space to allow for the delivery of therapeutic agents to the joint
space. Generally, the
implant is designed to hold fast to the bone and/or soft tissue near the
target joint, provide a
reservoir to hold therapeutic agents that will be delivered to the region over
a period of days,
weeks, or months, and deliver those agents at a desired rate. In addition,
these devices can
allow the implant to be filled at the time of surgery and/or at any time after
surgery and allow
the surgeon to select one or more therapeutic agents to be delivered at any of
these times.
[00192] The general shape of the implant according to the present invention
can be that of
a screw (see Fig. 30), reservoir, bladder, balloon, plug, anchor, sponge
and/or cloth. Implants
can be combinations of these possibilities, such as a screw with a balloon
attached to the
screw head (see Figs. 32 and 33) or a screw or anchor attached to a porous
balloon via a
catheter (see Fig. 34).
[00193] The implants according to the present invention can be placed directly
in the joint
space, in the bone surrounding the joint (see Figs. 31, 32, 33, 34, 44, 45,
46), in the soft tissue
surrounding the joint (see Figs. 51-52), or in combinations of these (see Fig.
34).
[00194] The goal is to deliver therapeutic agents to the joint space. This can
be
accomplished by delivering the agents directly to the joint space, to the bone
and/or soft
tissue surrounding the joint, or to a combination of these. (See Figs. 31-34,
44-46, 50, 52).
[00195] Transport of the therapeutic agents to the tissue can be achieved by,
for example,
osmosis, diffusion, or pressure. An example of a device which uses osmosis is
a rigid
reservoir with a permeable membrane to allow for the controlled release of the
therapeutic
agent. An example of a device which uses diffusion is a rigid reservoir with
delivery
channels (see Fig. 30). An example of a device which uses pressure is an
elastic bladder
which is filled with therapeutic agents beyond its elastic yield point and
which has holes in
the bladder. The force of the bladder contracting will force the therapeutic
agent through the
holes into the tissue (see Fig. 32). Another example of a device which uses
pressure to
deliver therapeutic agents is one that utilizes the forces generated within a
joint and the
surrounding tissue to force the therapeutic agent from the bladder into the
surrounding tissue.
For example, a bladder with delivery pores can be implanted between the
quadriceps tendon
CA 02735235 2013-07-08
48
and the femur (see Fig. 50, and optionally Fig. 34).
[00196] Each of the implants according to the present invention (including
those
described above) is configured for delivering a plurality of therapeutic
agents to the
corporeal body via the respective reservoir and channels. Further, with
respect to each of
the implants (including those described above) according to the present
invention, the
respective reservoir(s) is configured for receiving at least one therapeutic
agent after the
implant has been implanted in the corporeal body and then communicating at
least one
therapeutic agent via the respective plurality of channels to the treatment
site. Further,
with respect to each of the implants according to the present invention
(including those
described above), the respective reservoir(s) is configured for being refilled
with at least
one therapeutic agent after the implant has been implanted in the corporeal
body (see, for
example, Figs. 40-43). Thus, each of the implants according to the present
invention
(including those described above) can be filled with the therapeutic agent,
such as a
liquid, before or after implantation and can be refilled after implantation in
a simple
manner using for instance a pump and a catheter, as described above.
[00197] The scope of the claims should not be limited by the preferred
embodiments
set forth in the description, but should be given the broadest interpretation
consistent with
the description as a whole.