Note: Descriptions are shown in the official language in which they were submitted.
CA 02736441 2011-04-06
PYRAZOLYLTHIAZOLE COMPOUNDS AS AF508-CYSTIC FIBROSIS TRANSMEMBRANE
CONDUCTANCE REGULATOR CORRECTORS
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH
This invention was made with government support under grant nos. DK072517 and
GM076151 awarded by the National Institutes of Health and grant nos. CHE-
0614756, CHE-
0449845, and CHE-9808183 awarded by the National Science Foundation. The
government has
certain rights in the invention.
FIELD
The present disclosure relates to corrector compounds and methods for
correcting
cellular processing of mutant cystic fibrosis transmembrane conductance
regulator protein.
BACKGROUND
The cystic fibrosis transmembrane conductance regulator protein (CFTR) is a
cAMP
activated chloride ion (CI-) channel responsible for Cl- transport. CFTR is
expressed in epithelial
cells in mammalian airways, intestine, pancreas and testis. It is there where
CFTR provides a
pathway for the movement of Cl- ions across the apical membrane and a key
point at which to
regulate the rate of transepithelial salt and water transport. Hormones, such
as a (3-adrenergic
agonist, or toxins, such as cholera toxin, lead to an increase in cAMP,
activation of cAMP-
dependent protein kinase, and phosphorylation of the CFTR Cl- channel, which
causes the
channel to open. An increase in the concentration of Ca2+ in a cell can also
activate different
apical membrane channels. Phosphorylation by protein kinase C can either open
or shut Cf
channels in the apical membrane.
Dysfunction of CFTR is associated with a wide spectrum of disease, including
cystic
fibrosis (CF) and with some forms of male infertility, polycystic kidney
disease and secretory
diarrhea. CF is a hereditary disease that mainly affects the lungs and
digestive system, causing
progressive disability and early death. With an average life expectancy of
around 31 years, CF is
one of the most common life-shortening, childhood-onset inherited diseases.
This disease is
caused by mutation of the gene encoding CFTR, and is autosomal recessive. The
most common
CFTR mutation, deletion of phenylalanine-508 (AF508-CFTR), is present in at
least one allele in
about 90 % of CF patients (Egan et al., (2004) Science 304:600-602). AF508-
CFTR causes Cf
impermeability because it is not processed correctly, causing it to be
retained at the endoplasmic
reticulum (rather than the plasma membrane). AF508-CFTR also has reduced
intrinsic Cf
conductance relative to wild type CFTR.
Strategies have been investigated to correct the defects in AF508-CFTR
cellular
processing and intrinsic function in cells. Cell growth at low temperature (<
30 C) (Denning et al.,
(1992) Nature 358,761-764) or with high concentrations of chemical chaperones
such as glycerol
(Sato et al., (1996) J. Biol. Chem. 271,635-638; Brown, et al., (1996) Cell
Stress & Chaperones 1,
1 17-125) corrects partially defective AF508-CFTR cellular processing by a
mechanism that may
1
CA 02736441 2011-04-06
involve improved protein folding and stability (Sharma et al., (2001) J. Biol.
Chem. 276, 8942-
8950). A sustained increase in intracellular calcium concentration by
thapsigargin also corrects
defective AF508-CFTR processing (Egan et al., (2002) Nature Med. 8,485-492),
possibly by
interfering with Interactions with molecular chaperones. Compounds like
phenylbutryate facilitate
AF508-CFTR cellular processing by altering chaperone function andlor
transcriptional
enhancement (Rubenstein et al., (2000) Am. J. Physiol. 278, C259-C267; Kang et
al., (2002)
Proc. Natl. Acad. Sci. U.S.A. 99, 838-843). Although these approaches provide
insight into
mechanisms of AF508-CFTR retention at the endoplasmic reticulum, they probably
do not offer
clinically-useful therapies.
AF508-CFTR has significantly impaired channel activity even when present at
the cell
plasma membrane (Dalemans et al., (1991) Nature 354, 526-528). Cell-attached
patchclamp
measurements showed reduced AF508-CFTR open channel probability and prolonged
closed
times even with maximal cAMP stimulation (Haws et al., (1996) Am. J. Physiol.
270, C1544-
C1555; Hwang et al., (1997) Am. J. Physiol. 273, C988-C998). Patch-clamp
measurements in
excised membranes indicated 7-fold reduced AF508-CFTR activation after
phosphorylation
compared to wildtype CFTR. Relatively high concentrations of the flavone
genistein (>50 pM,
Hwang, et al., (1997) Am. J. PhysioL 273, C988-C998; Wang et al., (2000) J.
Physiol. 524,637-
638) or the xanthine isobutylmethylxanthine (>1 mM, Drurnrn et al., (1991)
Science 254, 1797-
1799) in combination with cAMP agonists increase AF508-CFTR channel activity.
Again, these
studies have not offered any clinically useful therapies.
There is accordingly still a need for compounds that can correct cellular
processing or
folding of mutant CFTR, e.g., AF508-CTFR, and methods of using such compounds
for the study
and treatment of CF and the treatment and control of other secretory
disorders. The present
disclosure addresses these needs, as well as others.
SUMMARY
The present disclosure provides compositions, pharmaceutical preparations and
methods
for increasing activity (e.g., ion transport) of a mutant-cystic fibrosis
transmembrane conductance
regulator protein ("mutant-CFTR") that are useful for the study and treatment
of cystic fibrosis
("CF"). The compositions and pharmaceutical preparations may comprise one or
more
compounds of the present disclosure, or an analog or derivative thereof.
The present disclosure provides a compound of formula (I):
R2
O N-N O
Rt 4 s X
FIN
N (I)
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
2
CA 02736441 2011-04-06
R' is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, cycloalkyl, substituted
cycloalkyl, heteroaryl, substituted
heteroaryl, heterocyclyl, and substituted heterocyclyl;
R2 is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, cycloalkyl, substituted
cycloalkyl, heteroaryl, substituted
heteroaryl, heterocyclyl, and substituted heterocyclyl; and
X is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl, substituted
alkynyl, aryl, substituted aryl, cycloalkyl, substituted cycloalkyl,
heteroaryl, substituted heteroaryl,
heterocyclyl, substituted heterocyclyl, alkoxy, substituted alkoxy, amino,
substituted amino, and
hydroxyl.
The present disclosure provides a compound of formula (II):
R2
p HN-N O
R1
HN X
N (II)
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
R1 is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, cycloalkyl, substituted
cycloalkyl, heteroaryl, substituted
heteroaryl, heterocyclyl, and substituted heterocyclyl;
R2 is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, cycloalkyl, substituted
cycloalkyl, heteroaryl, substituted
heteroaryl, heterocyclyl, and substituted heterocyclyl; and
X is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl, substituted
alkynyl, aryl, substituted aryl, cycloalkyl, substituted cycloalkyl,
heteroaryl, substituted heteroaryl,
heterocyclyl, substituted heterocyclyl, alkoxy, substituted alkoxy, amino,
substituted amino, and
hydroxyl.
The present disclosure provides pharmaceutical compositions comprising an
effective
amount of a disclosed compound. The pharmaceutical compositions can include at
least one of a
pharmaceutically acceptable carrier, a pharmaceutically acceptable diluent, a
pharmaceutically
acceptable excipient, and a pharmaceutically acceptable adjuvant.
The present disclosure provides methods of treating a subject having a
condition
associated with mutant-CFTR, which involves administering to the subject a
therapeutically
effective amount of a pharmaceutical composition comprising a disclosed
compound. The
present disclosure provides a method of increasing ion permeability of a cell
producing a mutant-
CFTR protein, which involves contacting the cell with an effective amount of a
composition
comprising a disclosed compound so as to increase CFTR-mediated ion
permeability of the cell.
The present disclosure provides kits containing one or more compositions
comprising a
disclosed compound, as well as methods of preparing the compositions.
3
CA 02736441 2011-04-06
Advantages of the compounds and compositions include improved drug like
properties
such as increased potency and solubility, as well as expanded diversity for
generating additional
corrector compounds. The compounds also are useful in the study of mutant-CFTR
related
disorders. Thus the present disclosure addresses many unmet needs in the
development and
use of mutant-CFTR corrector compounds. These and other objects and advantages
of the
present disclosure will be apparent from the detailed description below.
BRIEF DESCRIPTION OF FIGURES
Figure 1. Dose-response relation for increased I- influx in AF508-CFTR cells
treated with pyrazolylthiazoles: 14g (in solid) and 14h (in dashed).
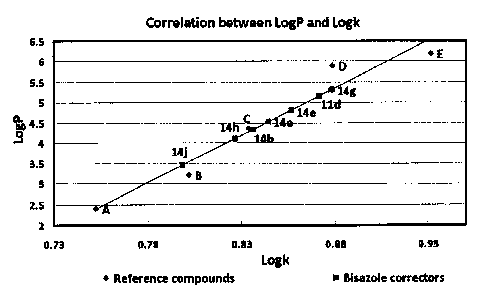
Figure 2. Standard calibration curve correlating experimentally determined
capacity factor k with IogP. Data are shown for reference compounds (+: A = 4-
chlorophenol, B =
2,4-dichlorophenol, C = 3,4,5-trichlorophenol, D = pentachlorophenol, E = p,p'-
DDT), Compounds
11 d/14a/14b/14e/14g114h114j.
DETAILED DESCRIPTION
The present disclosure is based on the discovery of pyrazolylthiazole and
related
compounds that correct cellular processing or folding of mutant cystic
fibrosis transmembrane
conductance regulator protein ("mutant-CFTR") with high nanomolar potency, and
that exhibit a
broad range of one or more other properties that find use in the study and
treatment of disorders
related to mutant-CFTR, such as cystic fibrosis ("CF").
The compounds share a pyrazolylthiazolyl core which includes a pyrazole ring
that can
accommodate attachment of functionalities on the core ring.
By exploiting the chemical and structural aspects in the design, and synthesis
and
screening of compound libraries of the present disclosure, features for
optimization of compounds
containing a pyrazolylthiazolyl core structural motif have been identified.
The compounds of the
present disclosure include one or more of such features so as to impart a
pharmacological or
biological property that benefits the compound's manufacture, handling,
potency, selectivity,
and/or pharmacokinetic parameters. The present disclosure also includes
compounds with
features useful in the study of mutant-CFTR.
As such, the present disclosure provides novel compounds, compositions and
pharmaceutical preparations that correct cellular processing or folding of
mutant-CFTR (e.g.,
AF508-CFTR). The present disclosure also features methods of use of such
compositions in the
treatment of a subject for CF, as well as increasing activity of mutant CFTR
in a cell, e.g., by
correcting cellular processing or folding of mutant CFTR, as well as kits and
compound libraries
useful for the study and treatment of CF.
Before the present invention and specific exemplary embodiments of the
invention are
described, it is to be understood that this invention is not limited to
particular embodiments
described, as such may, of course, vary. It is also to be understood that the
terminology used
4
CA 02736441 2011-04-06
herein is for the purpose of describing particular embodiments only, and is
not intended to be
limiting, since the scope of the present invention will be limited only by the
appended claims.
Where a range of values is provided, it is understood that each intervening
value, to the
tenth of the unit of the lower limit unless the context clearly dictates
otherwise, between the upper
and lower limit of that range and any other stated or intervening value in
that stated range is
encompassed within the embodiments. The upper and lower limits of these
smaller ranges may
independently be included in the smaller ranges is also encompassed within the
embodiments,
subject to any specifically excluded limit in the stated range. Where the
stated range includes one
or both of the limits, ranges excluding either both of those included limits
are also included in the
embodiments.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although any methods and materials similar or equivalent to those
described herein can
also be used in the practice or testing of the present invention, exemplary
methods and materials
are now described.
All publications and patents cited in this specification are herein
incorporated by
reference as if each individual publication or patent were specifically and
individually indicated to
be incorporated by reference and are incorporated herein by reference to
disclose and describe
the methods and/or materials in connection with which the publications are
cited. The citation of
any publication is for its disclosure prior to the filing date and should not
be construed as an
admission that the present invention is not entitled to antedate such
publication by virtue of prior
invention. Further, the dates of publication provided may be different from
the actual publication
dates which may need to be independently confirmed.
It must be noted that as used herein and in the appended claims, the singular
forms "a",
"and", and "the" include plural referents unless the context clearly dictates
otherwise. Thus, for
example, reference to a "compound" includes a plurality of such compounds and
equivalents
thereof known to those skilled in the art, and so forth.
Terms
When describing the compounds, pharmaceutical compositions containing such
compounds and methods of using such compounds and compositions, the following
terms have
the following meanings unless otherwise indicated. It should also be
understood that any of the
moieties defined forth below may be substituted with a variety of
substituents, and that the
respective definitions are intended to include such substituted moieties
within their scope. By way
of non-limiting example, such substituents may include e.g. halo (such as
fluoro, chloro, bromo), -
ON, -CF3i -OH, -OCF3, C2.6 alkenyl, C3_6 alkynyl, C1_6 alkoxy, aryl and di-
C1.6 alkylamino.
"Acyl" refers to a radical -C(O)R, where R is hydrogen, alkyl, cycloalkyl,
cycloheteroalkyl,
aryl, arylalkyl, heteroalkyl, heteroaryl or heteroarylalkyl as defined herein.
Representative
examples include, but are not limited to, formyl, acetyl, cylcohexylcarbonyl,
cyclohexylmethylcarbonyl, benzoyl, benzylcarbonyl and the like.
5
CA 02736441 2011-04-06
"Acylamino" refers to a radical -NR'C(O)R, where R' is hydrogen, alkyl,
cycloalkyl,
cycloheteroalkyl, aryl, arylalkyl, heteroalkyl, heteroaryl, heteroarylalkyl
and R is hydrogen, alkyl,
alkoxy, cycloalkyl, cycloheteroalkyl, aryl, arylalkyl, heteroalkyl, heteroaryl
or heteroarylalkyl, as
defined herein. Representative examples include, but are not limited to,
formylamino,
acetylamino, cyclohexylcarbonylamino, cyclohexylmethyl-carbonylamino,
benzoylamino,
benzylcarbonylamino and the like.
"Acyloxy" refers to the group -OC(O)H, -OC(O)-alkyl, -OC(O)-aryl or -OC(O)-
cycloalkyl.
"Aliphatic" refers to hydrocarbyl organic compounds or groups characterized by
a
straight, branched or cyclic arrangement of the constituent carbon atoms and
an absence of
aromatic unsaturation. Aliphatics include, without limitation, alkyl,
alkylene, alkenyl, alkenylene,
alkynyl and alkynylene. Aliphatic groups typically have from 1 or 2 to 6 or 12
carbon atoms. The
simplest aliphatic compound is methane and its chemically bonded form methyl
(e.g., CH4, CH3-,
-CH2-, -CH(R)-, -C(R;)(R;;)-). Aliphatics include saturated and unsaturated
compounds. Lower
aliphatics typically refer to shorter aliphatic compounds having from 1 to 6
carbon atoms.
"Alkanoyl" or "acyl" as used herein refers to the group -C(O)H or -C(O)-alkyl.
"Alkenyl" refers to monovalent olefinically unsaturated hydrocarbyl groups
having up to
about 11 carbon atoms, particularly, from 2 to 8 carbon atoms, and more
particularly, from 2 to 6
carbon atoms, which can be straight-chained or branched and having at least 1
and particularly
from 1 to 2 sites of olefinic unsaturation. Particular alkenyl groups include
ethenyl (-CH=CH2), n-
propenyl (-CH2CH=CH2), isopropenyl (-C(CH3)=CH2), vinyl and substituted vinyl,
and the like.
"Alkenylene" refers to divalent olefinically unsaturated hydrocarbyl groups
particularly
having up to about 11 carbon atoms and more particularly 2 to 6 carbon atoms
which can be
straight-chained or branched and having at least 1 and particularly from 1 to
2 sites of olefinic
unsaturation. This term is exemplified by groups such as ethenylene (-CH=CH-),
the propenylene
isomers (e.g., -CH=CHCH2- and -C(CH3)=CH- and -CH=C(CH3)-) and the like.
"Alkoxy" refers to the group -0-alkyl. Particular alkoxy groups include, by
way of
example, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, tert-butoxy, sec-
butoxy, n-pentoxy,
n-hexoxy, 1,2-dimethyl butoxy, and the like.
"Alkoxyamino" refers to a radical -N(H)O-alkyl or -N(H)O-cycloalkyl as defined
herein.
"Alkoxycarbonyl" refers to a radical -C(O)-alkoxy where alkoxy is as defined
herein.
"Alkoxycarbonylamino" refers to the group -NRC(O)OR' where R is hydrogen,
alkyl, aryl
or cycloalkyl, and R' is alkyl or cycloalkyl.
"Alkyl" refers to monovalent saturated aliphatic hydrocarbyl groups
particularly having up
to about 11 carbon atoms, more particularly as a lower alkyl, from 1 to 8
carbon atoms and still
more particularly, from 1 to 6 carbon atoms. The hydrocarbon chain may be
either straight-
chained or branched. This term is exemplified by groups such as methyl, ethyl,
n-propyl,
isopropyl, n-butyl, iso-butyl, tert-butyl, n-hexyl, n-octyl, tert-octyl and
the like. The term "lower
alkyl" refers to alkyl groups having 1 to 6 carbon atoms. The term "alkyl"
also includes
"cycloalkyls" as defined below.
6
CA 02736441 2011-04-06
"Alkylamino" refers to a radical alkyl-NRR', wherein each of R and R' are
independently
selected from hydrogen and alkyl.
"Alkylarylamino" refers to a radical -NRR' where R represents an alkyl or
cycloalkyl group
and R' is an aryl as defined herein.
"Alkylene" refers to divalent saturated aliphatic hydrocarbyl groups
particularly having up
to about 11 carbon atoms and more particularly 1 to 6 carbon atoms which can
be straight-
chained or branched. This term is exemplified by groups such as methylene (-
CH2-), ethylene (-
CH2CH2-), the propylene isomers (e.g., -CH2CH2CH2- and -CH(CH3)CH2-) and the
like.
"Alkylthio" refers to a radical -S-alkyl or -S-cycloalkyl group as defined
herein that may be
optionally substituted as defined herein. Representative examples include, but
are not limited to,
methylthio, ethylthio, propylthio, butylthio, and the like.
"Alkynyl" refers to acetylenically unsaturated hydrocarbyl groups particularly
having up to
about 11 carbon atoms and more particularly 2 to 6 carbon atoms which can be
straight-chained
or branched and having at least 1 and particularly from 1 to 2 sites of
alkynyl unsaturation.
Particular non-limiting examples of alkynyl groups include acetylenic, ethynyl
(-C=CH), propargyl
(-CH2C=CH), and the like.
"Amide" refers to the radical -NHC(O)- or -C(O)NH2.
"Amino" refers to the radical -NH2.
"Aminocarbonyl" refers to the group -C(O)NRR where each R is independently
hydrogen,
alkyl, aryl or cycloalkyl, or where the R groups are joined to form an
alkylene group.
"Aminocarbonylamino" refers to the group -NRC(O)NRR where each R is
independently
hydrogen, alkyl, aryl or cycloalkyl, or where two R groups are joined to form
an alkylene group.
"Aminocarbonyloxy" refers to the group -OC(O)NRR where each R is independently
hydrogen, alkyl, aryl or cycloalky, or where the R groups are joined to form
an alkylene group.
"Aralkyl" or "arylalkyl" refers to an alkyl group, as defined above,
substituted with one or
more aryl groups, as defined above.
"Aromatic" refers to a mono- or polycyclic aromatic hydrocarbon group, and may
include
one or more heteroatoms in the aromatic ring or ring system termed a
heteroaromatic. Also
referred to as "aromatic ring" or "aromatic ring system." Simple aromatics
comprise from 3-14
carbons, examples of which include arsindole, benzene, benzothiophene,
benzo[c]thiophene,
benzimidazole, benzoxazole, benzisoxazole, benzothiazole, carbazole, (3-
carboline, chromane,
chromene, cinnoline, furan, imidazole, indazole, indole, indoline, indolizine,
isobenzofuran,
isochromene, isoindole, isoindoline, isoquinoline, isothiazole, isoxazole,
oxadiazole, oxazole,
perimidine, phenanthridine, phenanthroline, phenazine, phthalazine, pteridine,
purine, pyran,
pyrazine, pyrazole, pyridazine, pyridine, pyrimidine, pyrrole, pyrrolizine,
purine quinazoline,
quinoline, quinolizine, quinoxaline, tetrazole, thiadiazole, thiazole,
thiophene, triazole,
[2,4,6]triazine and xanthene, as well as fused ring systems such as acridine,
anthracene,
cinnoline, naphthalene, naphthyridine, quinoline, isoquinoline, quinoxaline
and quinazoline.
"Aryl" refers to any functional group or substituent derived from a simple
aromatic ring by
removal of a hydrogen atom from a carbon atom of a parent aromatic ring
system. Typical aryl
7
CA 02736441 2011-04-06
groups comprises from 6 to 14 carbon atoms. Examples include the radicals of
arsindole,
benzene, benzothiophene, benzo[c]thiophene, benzimidazole, benzoxazole,
benzisoxazole,
benzothiazole, carbazole, R-carboline, chromane, chromene, cinnoline, furan,
imidazole,
indazole, indole, indoline, indolizine, isobenzofuran, isochromene, isoindole,
isoindoline,
isoquinoline, isothiazole, isoxazole, oxadiazole, oxazole, perimidine,
phenanthridine,
phenanthroline, phenazine, phthalazine, pteridine, purine, pyran, pyrazine,
pyrazole, pyridazine,
pyridine, pyrimidine, pyrrole, pyrrolizine, purine quinazoline, quinoline,
quinolizine, quinoxaline,
tetrazole, thiadiazole, thiazole, thiophene, triazole, [2,4,6]triazine and
xanthene, as well as fused
ring systems such as acridine, anthracene, cinnoline, naphthalene,
naphthyridine, quinoline,
isoquinoline, quinoxaline and quinazoline. Examples of radicals denoted by the
term "aryl" that
are of particular interest include: phenyl, furyl, pyrrolyl, pyrrolidinyl,
imidazolyl, isoxazolyl, triazolyl,
thiadiazolyl, oxadiazolyl, tetrazolyl, thiatriazolyl, oxatriazolyl, pyridyl,
pyrimidyl, pyrazinyl,
pyridazinyl, oxazinyl, triazinyl, thiadiazinyl tetrazolo, 1,5-[b]pyridazinyl
and purinyl, as well as
benzo-fused derivatives, for example, benzoxazolyl, benzthiazolyl,
benzimidazolyl and indolyl.
"Arylalkyloxy" refers to an -0-arylalkyl radical where arylalkyl is as defined
herein.
"Arylamino" refers to the group aryl-NRR', wherein each of R and Rare
independently
selected from hydrogen, aryl and heteroaryl.
"Aryloxy" refers to -O-aryl groups wherein "aryl" is as defined herein.
"Arylsulfonyl" refers to a radical -S(O)2R where R is an aryl or heteroaryl
group as defined
herein.
"Azide" refers to N3 or its radical -N3 (also referred to as "azido").
"Carbamoyl" refers to the radical -C(O)N(R)2 where each R group is
independently
hydrogen, alkyl, cycloalkyl or aryl, as defined herein, which may be
optionally substituted as
defined herein.
"Carbonyl" refers to the radical -C(O)-.
"Carboxy" refers to the radical -C(O)OH (also referred to as "carboxyl").
"Cyano" refers to the radical -CN.
"Cycloalkenyl" refers to cyclic hydrocarbyl groups having from 3 to 10 carbon
atoms and
having a single cyclic ring or multiple condensed rings, including fused and
bridged ring systems
and having at least one and particularly from 1 to 2 sites of olefinic
unsaturation. Such
cycloalkenyl groups include, by way of example, single ring structures such as
cyclohexenyl,
cyclopentenyl, cyclopropenyl, and the like.
"Cycloalkyl" refers to cyclic hydrocarbyl groups having from 3 to about 10
carbon atoms
and having a single cyclic ring or multiple condensed rings, including fused
and bridged ring
systems, which optionally can be substituted with from 1 to 3 alkyl groups.
Such cycloalkyl groups
include, by way of example, single ring structures such as cyclopropyl,
cyclobutyl, cyclopentyl,
cyclooctyl, 1 -methylcyclopropyl, 2-methylcyclopentyl, 2-methylcyclooctyl, and
the like, and
multiple ring structures such as adamantanyl, and the like.
"Cycloheteroalkyl" refers to a stable heterocyclic non-aromatic ring and fused
rings
containing one or more heteroatoms independently selected from N, 0 and S. A
fused
8
CA 02736441 2011-04-06
heterocyclic ring system may include carbocyclic rings and need only include
one heterocyclic
ring. Examples of heterocyclic rings include, but are not limited to,
piperazinyl, homopiperazinyl,
piperidinyl and morpholinyl, which can be optionally substituted with one or
more groups selected
from the group consisting of acyl, acylamino, acyloxy, alkoxy, substituted
alkoxy, alkoxycarbonyl,
alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino,
am inocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano, cycloalkyl,
substituted cycloalkyl,
halogen, hydroxyl, keto, nitro, thioalkoxy, substituted thioalkoxy,
thioaryloxy, thioketo, thiol, alkyl-
S(O)-, aryl-S(O)-, alkyl-S(O)2- and aryl-S(O)2-. Substituting groups include
carbonyl or
thiocarbonyl which provide, for example, lactam and urea derivatives. In the
examples, M is CR7,
NR3,0, or S; Q is O, NR3 or S.
"Dialkylamino" means a radical -NRR' where R and R' independently represent an
alkyl,
substituted alkyl, aryl, substituted aryl, cycloalkyl, substituted cycloalkyl,
cycloheteroalkyl,
substituted cycloheteroalkyl, heteroaryl, or substituted heteroaryl group as
defined herein.
"Halo" or "halogen" refers to fluoro, chloro, bromo and iodo. Halo groups can
be either
fluoro or chloro.
"Hetero" when used to describe a compound or a group present on a compound
means
that one or more carbon atoms in the compound or group have been replaced by a
nitrogen,
oxygen, or sulfur heteroatom. Hetero may be applied to any of the hydrocarbyl
groups described
above such as alkyl, e.g. heteroalkyl, cycloalkyl, e.g. cycloheteroalkyl,
aryl, e.g. heteroaryl,
cycloalkenyl, cycloheteroalkenyl, and the like having from 1 to 5, and
especially from 1 to 3
heteroatoms.
"Heterocycle," "heterocyclic," "heterocycloalkyl," and "heterocyclyl" refer to
a saturated or
unsaturated group having a single ring or multiple condensed rings, including
fused bridged and
Spiro ring systems, and having from 3 to 15 ring atoms, including 1 to 4
hetero atoms. These
hetero atoms are selected from the group consisting of nitrogen, sulfur, or
oxygen, wherein, in
fused ring systems, one or more of the rings can be cycloalkyl, aryl, or
heteroaryl, provided that
the point of attachment is through the non-aromatic ring. In certain
embodiments, the nitrogen
and/or sulfur atom(s) of the heterocyclic group are optionally oxidized to
provide for the N-oxide, -
S(O)-, or -SO2- moieties.
"Heteroaryl" by itself or as part of another substituent, refers to a
monovalent
heteroaromatic radical derived by the removal of one hydrogen atom from a
single atom of a
heteroaromatic ring system. Typical heteroaryl groups include, but are not
limited to, groups
derived from acridine, arsindole, carbazole, (3-carboline, chromane, chromene,
cinnoline, furan,
imidazole, indazole, indole, indoline, indolizine, isobenzofuran, isochromene,
isoindole,
isoindoline, isoquinoline, isothiazole, isoxazole, naphthyridine, oxadiazole,
oxazole, perimidine,
phenanthridine, phenanthroline, phenazine, phthalazine, pteridine, purine,
pyran, pyrazine,
pyrazole, pyridazine, pyridine, pyrimidine, pyrrole, pyrrolizine, quinazoline,
quinoline, quinolizine,
quinoxaline, tetrazole, thiadiazole, thiazole, thiophene, triazole, xanthene,
benzodioxole and the
like. In certain embodiments, the heteroaryl group is from 5-20 membered
heteroaryl. In certain
embodiments, the heteroaryl group is from 5-10 membered heteroaryl. In certain
embodiments,
9
CA 02736441 2011-04-06
heteroaryl groups are those derived from thiophene, pyrrole, benzothiophene,
benzofuran, indole,
pyridine, quinoline, imidazole, oxazole and pyrazine.
"Hydroxyl" refers to the radical -OH.
"Nitro" refers to the radical -NO2.
"Phenyl" (often abbreviated as -Ph) is the aryl form of benzene with the
functional group,
and has the formula -C6H5, where the six carbon atoms are arranged in an
aromatic ring
structure.
"Substituted" refers to a group in which one or more hydrogen atoms are each
independently replaced with the same or different substituent(s). Typical
substituents include, but
are not limited to, -X, -R 14, -0-, =0, -OR14, -SR14, -S-, =S, -NR 74R15,
=NR14, -CX3, -CF3, -CN,
-OCN, -SCN, -NO, -NO2, =N2, -N3, -S(0)20, -S(O)2OH, -S(O)2R14, -OS(02)O
OS(O)2R14,
-P(O)(O-)2, -P(O)(OR14)(O-), -OP(O)(OR14)(OR15), -C(O)R14, -C(S)R14, -
C(O)OR14, -C(O)NR14R1s
-C(O)O C(S)OR14, -NR16C(O)NR14R15, -NR 16C(S)NR14R15, -NR 17C(NR'6)NR14R15 and
-C(NR16)NR14R15, where each X is independently a halogen, and where" R74",
"Rt5", "R16", and
"R17" are independently hydrogen, alkyl, substituted alkyl, aryl, arylalkyl,
cycloalkyl,
cycloheteroalkyl, substituted cycloheteroalkyl, heteroalkyl, substituted
heteroalkyl, heteroaryl,
substituted heteroaryl, heteroarylalkyl, substituted heteroarylalkyl, -
NR1'R19, -C(O)R18 or
-S(O)2R18 or optionally R18 and R19 together with the atom to which they are
both attached form a
cycloheteroalkyl or substituted cycloheteroalkyl ring, and where "R'8", "R19",
and "R 22" are each
independently selected from the group consisting of hydrogen, substituted or
unsubstituted alkyl,
substituted or unsubstituted aryl, substituted or unsubstituted arylalkyl,
substituted or
unsubstituted cycloalkyl, substituted or unsubstituted cycloheteroalkyl,
substituted or
unsubstituted heteroalkyl, substituted or unsubstituted heteroaryl, and
substituted or
unsubstituted heteroarylalkyl.
"Substituted aliphatic" includes those groups recited in the definition of
"substituted"
herein, and particularly refers to aliphatic group having 1 or more
substituents, for instance from 1
to 5 substituents, and particularly from 1 to 3 substituents, selected from
the group consisting of
selected from the group consisting of acyl, acylamino, acyloxy, alkoxy,
substituted alkoxy,
alkoxycarbonyl, alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino, aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano,
cycloalkyl,
substituted cycloalkyl, halogen, hydroxyl, keto, nitro, thioalkoxy,
substituted thioalkoxy,
thioaryloxy, thioketo, thiol, alkyl-S(O)-, aryl-S(O)-, alkyl-S(O)2- and aryl-
S(O)2-.
"Substituted alkenyl" includes those groups recited in the definition of
"substituted"
herein, and particularly refers to an alkenyl group having 1 or more
substituents, for instance from
1 to 5 substituents, and particularly from 1 to 3 substituents, selected from
the group consisting of
acyl, acylamino, acyloxy, aliphatic, substituted aliphatic, alkoxy,
substituted alkoxy,
alkoxycarbonyl, alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino, aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano,
cycloalkyl,
substituted cycloalkyl, halogen, hydroxyl, keto, nitro, thioalkoxy,
substituted thioalkoxy,
thioaryloxy, thioketo, thiol, alkyl-S(O)-, aryl-S(O)-, alkyl-S(O)2- and aryl-
S(O)2--
CA 02736441 2011-04-06
"Substituted alkoxy" includes those groups recited in the definition of
"substituted" herein,
and particularly refers to an alkoxy group having 1 or more substituents, for
instance from 1 to 5
substituents, and particularly from 1 to 3 substituents, selected from the
group consisting of acyl,
acylamino, acyloxy, aliphatic, substituted aliphatic, alkoxy, substituted
alkoxy, alkoxycarbonyl,
alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino,
aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano, cycloalkyl,
substituted cycloalkyl,
halogen, heteroaryl, hydroxyl, keto, nitro, thioalkoxy, substituted
thioalkoxy, thioaryloxy, thioketo,
thiol, alkyl-S(O)-, aryl-S(O)-, alkyl-S(O)2- and aryl-S(O)2-.
"Substituted alkyl" includes those groups recited in the definition of
"substituted" herein,
and particularly refers to an alkyl group having 1 or more substituents, for
instance from 1 to 5
substituents, and particularly from 1 to 3 substituents, selected from the
group consisting of acyl,
acylamino, acyloxy, aliphatic, substituted aliphatic, alkenyl, substituted
alkenyl, alkynyl,
substituted alkynyl, alkoxy, substituted alkoxy, alkoxycarbonyl,
alkoxycarbonylamino, amino,
substituted amino, aminocarbonyl, aminocarbonylamino, aminocarbonyloxy, aryl,
aryloxy, azido,
carboxyl, cyano, cycloalkyl, substituted cycloalkyl, halogen, hydroxyl,
heteroaryl, keto, nitro,
thioalkoxy, substituted thioalkoxy, thioaryloxy, thioketo, thiol, alkyl-S(O)-,
aryl-S(O)-, alkyl-S(O)2-,
and aryl-S(O)2-.
"Substituted alkylene" includes those groups recited in the definition of
"substituted"
herein, and particularly refers to an alkylene group having 1 or more
substituents, for instance
from 1 to 5 substituents, and particularly from 1 to 3 substituents, selected
from the group
consisting of acyl, acylamino, acyloxy, aliphatic, substituted aliphatic,
alkoxy, substituted alkoxy,
alkoxycarbonyl, alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino, aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano,
halogen, hydroxyl,
keto, nitro, thioalkoxy, substituted thioalkoxy, thioaryloxy, thioketo, thiol,
alkyl-S(O)-, aryl-S(O)-,
alkyl-S(O)2- and aryl-S(O) 2--
"Substituted alkynyl" includes those groups recited in the definition of
"substituted"
herein, and particularly refers to an alkynyl group having 1 or more
substituents, for instance from
1 to 5 substituents, and particularly from 1 to 3 substituents, selected from
the group consisting of
acyl, acylamino, acyloxy, aliphatic, substituted aliphatic, alkoxy,
substituted alkoxy,
alkoxycarbonyl, alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino, aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano,
cycloalkyl,
substituted cycloalkyl, halogen, hydroxyl, keto, nitro, thioalkoxy,
substituted thioalkoxy,
thioaryloxy, thioketo, thiol, alkyl-S(O)-, aryl-S(O)-, alkyl-S(O)2- and aryl-
S(0)2--"Substituted amino" includes those groups recited in the definition of
"substituted" herein,
and particularly refers to the group -N(R)2 where each R is independently
selected from the group
consisting of hydrogen, aliphatic, substituted aliphatic, alkyl, substituted
alkyl, alkenyl, substituted
alkenyl, alkynyl, substituted alkynyl, aryl, substituted aryl, cycloalkyl,
substituted cycloalkyl, and
where both R groups are joined to form an alkylene group.
"Substituted aryl" includes those groups recited in the definition of
"substituted" herein,
and particularly refers to an aryl group that may optionally be substituted
with 1 or more
11
CA 02736441 2011-04-06
substituents, for instance from 1 to 5 substituents, particularly 1 to 3
substituents, selected from
the group consisting of acyl, acylamino, acyloxy, aliphatic, substituted
aliphatic, alkenyl,
substituted alkenyl, alkoxy, substituted alkoxy, alkoxycarbonyl, alkyl,
substituted alkyl, alkynyl,
substituted alkynyl, amino, substituted amino, aminocarbonyl,
aminocarbonylamino,
aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano, cycloalkyl,
substituted cycloalkyl,
halogen, hydroxyl, nitro, thioalkoxy, substituted thioalkoxy, thioaryloxy,
thiol, alkyl-S(O)-, aryl-
S(O)-, alkyl-S(O)2- and aryl-S(O)2-. May include heteroaryls and substituted
heteroaryls in which
one or more carbon atoms of the aromatic ring system is replaced by a group
selected from N, 0
and S. Examples of substituents of particular interest are from one to three
halo, trihalomethyl,
amino, protected amino, amino salts, mono-substituted amino, disubstituted
amino, carboxy,
protected carboxy, carboxylate salts, hydroxy, protected hydroxy, salts of a
hydroxy group, lower
alkoxy, lower allcylthio, alkyl, substituted alkyl, cycloallyl, substituted
cycloalkyl, (cycloallcyl)alkyl,
substituted (cycloalkyl)allyl, phenyl, substituted phenyl, phenylalkyl, and
(substituted phenyl)allyl.
Substituents for the heteroaryl group are as heretofore defined, or in the
case of trihalomethyl,
can be trifluoromethyl, trichloromethyl, tribromomethyl, or triiodomethyl. As
used in conjunction
with the above substituents for heteroaryl.
"Substituted cycloalkenyl" includes those groups recited in the definition of
"substituted"
herein, and particularly refers to a cycloalkenyl group having 1 or more
substituents, for instance
from 1 to 5 substituents, and particularly from 1 to 3 substituents, selected
from the group
consisting of acyl, acylamino, acyloxy, aliphatic, substituted aliphatic,
alkoxy, substituted alkoxy,
alkoxycarbonyl, alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino, aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano,
cycloalkyl,
substituted cycloalkyl, halogen, hydroxyl, keto, nitro, thioalkoxy,
substituted thioalkoxy,
thioaryloxy, thioketo, thiol, alkyl-S(O)-, aryl-S(O)-, alkyl-S(O)2- and aryl-
S(O)2-.25 "Substituted cycloalkyl" includes those groups recited in the
definition of "substituted"
herein, and particularly refers to a cycloalkyl group having 1 or more
substituents, for instance
from 1 to 5 substituents, and particularly from 1 to 3 substituents, selected
from the group
consisting of acyl, acylamino, acyloxy, aliphatic, substituted aliphatic,
alkoxy, substituted alkoxy,
alkoxycarbonyl, alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino, aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano,
cycloalkyl,
substituted cycloalkyl, halogen, hydroxyl, keto, nitro, thioalkoxy,
substituted thioalkoxy,
thioaryloxy, thioketo, thiol, alkyl-S(O)-, aryl-S(O)-, alkyl-S(O)2- and aryl-
S(O)2-.
"Substituted phenyl" includes those groups recited in the definition of
"substituted" herein,
and particularly refers to a phenyl group that may optionally be substituted
with 1 or more
substituents, for instance from 1 to 5 substituents, particularly 1 to 3
substituents. Substituents of
the phenyl group include those selected from the group consisting of acyl,
acylamino, acyloxy,
alkenyl, substituted alkenyl, aliphatic, substituted aliphatic, alkoxy,
substituted alkoxy,
alkoxycarbonyl, alkyl, substituted alkyl, alkynyl, substituted alkynyl, amino,
substituted amino,
aminocarbonyl, aminocarbonylamino, aminocarbonyloxy, aryl, aryloxy, azido,
carboxyl, cyano,
cycloalkyl, substituted cycloalkyl, halogen, hydroxyl, nitro, thioalkoxy,
substituted thioalkoxy,
12
CA 02736441 2011-04-06
thioaryloxy, thiol, alkyl-S(O)-, aryl-S(O)-, alkyl-S(O)2- and aryl-S(O)2-.
Substituents of the phenyl
group include those that form a fused phenyl ring system in which a
heterocycle ring is fused to
the phenyl ring, and the heterocycle contains one or more heteroatoms
independently selected
from N, 0 and S. Substituents of the phenyl group of particular interest are
selected from the
group consisting of halogen, hydroxy, protected hydroxy, amino, protected
amino, amide,
protected amide, thiol, protected thiol, cyano, nitro, azido, trifluoromethyl,
C, to C7 alkyl, C, to C7
alkoxy, C, to C7 acyl, C1 to C7 acyloxy, carboxy, oxycarboxy, protected
carboxy, carboxymethyl,
protected carboxymethyl, hydroxymethyl, protected hydroxymethyl, amino,
protected amino,
(monosubstituted)amino, protected (monosubstituted)amino,
(disubstituted)amino, carboxamide,
protected carboxarnide, N-(C1 to C6 alkyl)carboxamide, protected N-( C, to C6
allyl)carboxamide,
N,N-di(C1 to C6 allyl)carboxamide, trifluoromethyl, N-(( C, to C6
alkyl)sulfonyl)amino, N-
(pl'-enylsulfonyl)amino or phenyl, substituted or unsubstituted, such that,
for example, a biphenyl
or naphthyl group results. Examples of substituted phenyls include a mono- or
di(halo)phenyl
group such as 2,3 or 4-chlorophenyl, 2,6-dichlorophenyl, 2,5-dichlorophenyl,
3,4-dichlorophenyl,
2,3 or 4-bromophenyl, 3,4-dibromophenyl, 3-chloro-4-fluorophenyl, 2,3 or 4-
fluorophenyl and the
like; a mono or di(hydroxy)phenyl group such as 2,3, or 4-hydroxyphenyl, 2,4-
dihydroxyphenyl,
the protected-hydroxy derivatives thereof and the like; a nitrophenyl group
such as 2,3, or 4-
nitrophenyl; a cyanophenyl group, for example, 2,3 or 4-cyanophenyl; a mono-
or di(alkyl)phenyl
group such as 2, 3, or 4-methylphenyl, 2,4-dimethylphenyl, 2, 3 or 4-(iso-
propyl)phenyl, 2,3, or 4-
ethylphenyl, 2,3 or 4-(n-propyl)phenyl and the like; a mono or
di(alkoxy)phenyl group, for
example, 2,6-dimethoxyphenyl, 2,3 or 4-(isopropoxy)phenyl, 2,3 or 4-(t-
butoxy)phenyl, 3-ethoxy-
4-methoxyphenyl and the like; 2,3 or 4-trifluoromethylphenyl; a mono- or
dicarboxyphenyl or
(protected carboxy)phenyl group such as 2,3 or 4-carboxyphenyl or 2,4-
dibrotected
carboxy)phenyl; a mono- or di(hydroxymethyl)phenyl or (protected hydroxymethyl
)phenyl such
as 2,3 or 4-(protected hydroxymethyl )phenyl or 3,4-di(hydroxymethyl )phenyl;
a mono- or
di(aminomethyl)phenyl or (protected aminomethyl)phenyl such as 2,3 or 4-
(aminomethyl)phenyl
or 2,4-(protected aminomethyl)phenyl; or a mono- or dim-
(methylsulfonylamino))phenyl such as
2,3 or 4-(N-(methylsulfonylamino))phenyl. Also, the term "substituted phenyl"
represents
disubstituted phenyl groups wherein the substituents are different, for
example, 3-methyl-4-
hydroxyphenyl, 3-chloro-4-hydroxyphenyl, 2-methoxy-4-bromophenyl, 4-ethyl-2-
hydroxyphenyl, 3-
hydroxy-4-nitrophenyl, 2-hydroxy-4-chlorophenyl and the like
"Substituted thioalkoxy" includes those groups recited in the definition of
"substituted"
herein, and particularly refers to a thioalkoxy group having 1 or more
substituents, for instance
from 1 to 5 substituents, and particularly from 1 to 3 substituents, selected
from the group
consisting of acyl, acylamino, acyloxy, aliphatic, substituted aliphatic,
alkoxy, substituted alkoxy,
alkoxycarbonyl, alkoxycarbonylamino, amino, substituted amino, aminocarbonyl,
aminocarbonylamino, aminocarbonyloxy, aryl, aryloxy, azido, carboxyl, cyano,
cycloalkyl,
substituted cycloalkyl, halogen, hydroxyl, keto, nitro, thioalkoxy,
substituted thioalkoxy,
thioaryloxy, thioketo, thiol, alkyl-S(O)-, aryl-S(O)-, alkyl-S(0)2- and aryl-
S(0)2-
13
CA 02736441 2011-04-06
"Sulfanyl" refers to the radical -SH. "Substituted sulfanyl" refers to a
radical such as -SR
wherein R is any substituent described herein.
"Sulfone" refers to the group -SO2R. In particular embodiments, R is selected
from H,
lower alkyl, alkyl, aryl and heteroaryl.
"Sulfonyl" refers to the divalent radical -S(02)-. "Substituted sulfonyl"
refers to a radical
such as R-(02)S- wherein R is any substituent described herein.
"Aminosulfonyl" refers to the
radical H2N(02)S-, and "substituted aminosulfonyl" refers to a radical such as
R2N(02)S- wherein
each R is independently any substituent described herein.
"Thioalkoxy" refers to the group -S-alkyl.
"Thioaryloxy" refers to the group -S-aryl.
"Thioketo" refers to the group =S.
"Thiol" refers to the group -SH.
One having ordinary skill in the art will recognize that the maximum number of
heteroatoms in a stable, chemically feasible heterocyclic ring, whether it is
aromatic or non
aromatic, is determined by the size of the ring, the degree of unsaturation
and the valence of the
heteroatoms. In general, a heterocyclic ring may have one to four heteroatoms
so long as the
heteroaromatic ring is chemically feasible and stable.
A "mutant cystic fibrosis transmembrane conductance regulator protein" or
"mutant-
CFTR" is the protein that results from a mutation, e.g., deletion mutation,
insertion mutation, or
point (substitution) mutation of the CFTR gene product relative to wildtype. A
"mutant cystic
fibrosis transmembrane conductance regulator protein", or "mutant-CFTR" refers
to a
dysfunctional CFTR as compared to a functional (e.g., wildtype) CFTR, where
the dysfunction
can encompass one or more of the following: (i) aberrant CFTR production
(e.g., at the level of
transcription or translation); (ii) aberrant folding and/or trafficking; (iii)
abnormal regulation of
conductance; (iv) decreases in chloride conductance; (v) reduction in
synthesis; and the like. A
"mutant-CFTR gene" is a gene, or coding sequence, which encodes a mutant-CFTR.
For the
purposes of this application, the terms "genome" and "gene" are used
interchangeably, e.g.
"genome that encodes mutant-CFTR" and "gene that encodes mutant-CFTR".
A "gating defective mutant cystic fibrosis transmembrane conductance regulator
protein"
or "gating defective mutant-CFTR" is a mutant-CFTR that is present on the cell
surface and is
defective in gating of ions through the channel (e.g., regulation of ion
transport). Thus, as used
herein a "gating defective mutant-CFTR" encompasses dysfunctions associated
with (i) abnormal
regulation of conductance; and or (ii) decreases in chloride conductance.
A "mutant-CFTR protein-mediated condition" means any condition, disorder or
disease,
or symptom of such condition, disorder, or disease that results from or is
correlated to the
presence of a mutant-CFTR, e.g., AF508-CFTR, e.g., chloride ion impermeability
caused by
reduced activity of AF508-CFTR in ion transport relative to a wild-type CFTR.
A "mutant-CFTR
protein-mediated condition" encompasses conditions in an affected subject
which are associated
with the presence of a AF508-CFTR mutation on at least one allele, thus
including subjects that
carry a AF508-CFTR mutation on both alleles as well as heterozygous subjects
having two
14
CA 02736441 2011-04-06
different mutant forms of CFTR, e.g., a subject with one copy of AF508-CFTR
and a copy of
different form of CFTR, e.g., a non-mutant CFTR or a different mutant CFTR.
Such conditions,
disorders, diseases, or symptoms thereof are treatable by specific activation
of mutant-CFTR
activity, e.g., activation of mutant-CFTR ion transport. AF508-CFTR is
correlated to the presence
of cystic fibrosis (CF), and a description of this disease, including its
symptoms, is found in
Accession No. 602421 (entitled cystic fibrosis transmembrane conductance
regulator; CFTR),
and Accession No. 2 19700 (entitled Cystic fibrosis; CF) of the Online
Mendelian Inheritance of
Man database, as found at the world wide website of the National Institute of
Health at
ncbi.nlm.nih.gov. Symptoms of mutant-CFTR protein-mediated conditions include
meconium
ileus, liver disease including biliary tract obstruction and stenosis,
pancreatic insufficiency,
pulmonary disease including chronic Pseudomonas aeruginosa infections and
other infections of
the lung, infertility associated with abnormal vas deferens development or
abnormal cervical
mucus, and carcinoma including adenocarcinoma. In certain embodiments,
subjects that have a
mutant-CFTR protein-mediated condition are homozygous for a gene encoding a
AF508-CFTR
protein. In certain embodiments, subjects that have a mutant-CFTR protein-
mediated condition
are heterozygous for a gene encoding a AF508-CFTR protein.
A "AF508-cystic fibrosis transmembrane conductance regulator protein" or
"AF508-
CFTR" is the protein that results from the deletion of a phenylalanine residue
at amino acid
position 508 of the CFTR gene product. A "AF508-CFTR gene" is a gene, or
coding sequence,
which encodes AF508-CFTR. A AF508-CFTR gene usually results from deletion of
three
nucleotides corresponding to the phenylalanine residue at amino acid position
508 of the
encoded CFTR gene product. For the purposes of this application, the terms
"genome" and
"gene" are used interchangeably, e.g. "genome that encodes AF508-CFTR and
"gene that
encodes AF508-CFTR". For an example of a gene that encodes AF508-CFTR, see,
e.g. WO
91102796.
A "mutant-CFTR activator" as used herein is a compound that increases the
level of ion
transport by a mutant-CFTR relative to ion transport in the absence of the
compound, and
particularly with respect to transport of chloride ions. CFTR activators of
the embodiments are
those that are specific mutant-CFTR activators, e.g., compounds that activate
mutant-CFTR
activity rather than affecting CFTR cellular misprocessing. Mutant-CFTR
activators are usually
high-affinity mutant-CFTR activators, e.g., have an affinity for mutant-CFTR
of at least about one
micromolar, about one to five micromolar, about 200 nanomolar to one
micromolar, about 50
nanomolar to 200 nanomolar, or below 50 nanomolar.
A "gating defective mutant-CFTR activator" as used herein is a compound that
increases
the level of ion transport by a gating defective mutant-CFTR relative to ion
transport in the
absence of the compound, and particularly with respect to transport of
chloride ions. CFTR
activators of the embodiments are those that are specific gating defective
mutant-CFTR
activators, e.g., compounds that activate gating defective mutant-CFTR
activity rather than
affecting, for example, CFTR cellular misprocessing. Gating defective mutant-
CFTR activators
are usually high-affinity activators of gating defective mutant-CFTRs, e.g.,
have an affinity for a
CA 02736441 2011-04-06
gating defective mutant-CFTR (e.g., AF508-CFTR, G551D-CFTR, G1349D-CFTR, or
D1152H-
CFTR) of at least about one micromolar, about one to five micromolar, about
200 nanomolar to
one micromolar, about 50 nanomolar to 200 nanomolar, or below 50 nanomolar.
A "AF508-CFTR activator" as used herein is a compound that increases the level
of ion
transport by AF508-CFTR relative to ion transport in the absence of the
compound, and
particularly with respect to transport of chloride ions. CFTR activators of
the embodiments are
those that are specific AF508-CFTR activators, e.g., compounds that activate
AF508-CFTR
activity rather than affecting CFTR cellular misprocessing. AF508-CFTR
activators are usually
high-affinity AF508-CFTR activators, e.g., have an affinity for AF508-CFTR of
at least about one
micromolar, about one to five micromolar, about 200 nanomolar to one
micromolar, about 50
nanomolar to 200 nanomolar, or below 50 nanomolar.
As used herein and in the cystic fibrosis field a "potentiator" refers to a
compound that
increases a basal level of ion transport by a mutant-CFTR (e.g,. AF508-CFTR,
G551 D-CFTR,
G1349D-CFTR, or D1152H-CFTR), where the mutant CFTR (in the absence of the
compound)
exhibits aberrantly low levels of ion transport relative to wildtype CFTR. As
such, a "mutant-CFTR
potentiator" refers to a potentiator compound that provides for increased
level of ion transport by
a mutant-CFTR relative to ion transport capability of the mutant-CFTR in the
absence of the
compounds.
As used herein and in the cystic fibrosis field a "mutant-CFTR corrector" is a
compound
that increases the level of ion transport by a mutant-CFTR relative to ion
transport in the absence
of the compound by correcting the underlying defect of the CFTR polypeptide,
e.g., a defect that
results from post-translational mis-processing (e.g., misfolding). CFTR
correctors of the
embodiments are those that facilitate correction of specific mutant-CFTRs.
Mutant-CFTR
correctors are usually exhibit high affinity for one or more mutant-CFTRs,,
e.g., have an affinity
for mutant-CFTR of at least about one micromolar, about one to five
micromolar, about 200
nanomolar to one micromolar, about 50 nanomolar to 200 nanomolar, or below 50
nanomolar.
As used herein, a "mutant-CFTR corrector-potentiator" is a compound that
exhibits both
mutant-CFTR corrector and potentiator activity, and usually exhibit high
affinity for one or more
mutant-CFTRs, e.g., have an affinity for mutant-CFTR of at least about one
micromolar, about
one to five micromolar, about 200 nanomolar to one micromolar, about 50
nanomolar to 200
nanomolar, or below 50 nanomolar.
The term "analog" or "analogue" refers to without limitation any compound
which has
structural similarity to the parent compound and would be expected, by one
skilled in the art, to
exhibit the same or similar utility as the parent compound.
The term "derivative" refers to without limitation any compound which has a
structure
derived from the structure of the parent compound and whose structure is
sufficiently similar to
those disclosed herein and based upon that similarity, would be expected, by
one skilled in the
art, to exhibit the same or similar activities and utilities as the parent
compound.
The term "effective amount" of a compound as provided herein is intended to
mean a
sufficient amount of the compound to provide the desired utility. The term
"therapeutically
16
CA 02736441 2011-04-06
effective amount" or "efficacious amount" means the amount of a compound that,
when
administered to a mammal or other subject for treating a disease, is
sufficient to effect such
treatment for the disease. The "therapeutically effective amount" will vary
depending on the
compound, the disease and its severity and the age, weight, etc., of the
subject to be treated.
Thus, as will be pointed out below, the exact amount required will vary from
subject to subject,
depending on the species, age, and general condition of the subject, the
severity of the condition
or disease that is being treated, the particular compound used, its mode of
administration, and
the like. Thus, it is not possible to specify an exact "effective amount."
However, an appropriate
effective amount may be determined by one of ordinary skill in the art using
only routine
experimentation.
"Functional group" refers to atoms or small groups of atoms (two to four) that
exhibit a
characteristic reactivity when treated with certain reagents, and are attached
to the carbon
backbone of organic molecules. The same functional group will undergo the same
or similar
chemical reaction(s) regardless of the size of the molecule it is a part of.
Examples of functional
groups include halogen, hydroxy, carboxy, ester, thioester, amino, oxime,
hydrazone, thiol, azide,
nitro, nitroso, aldehyde and ketone. The functional groups can be protected or
unprotected,
activated or unactivated.
The term "in combination with" as used herein refers to uses where, for
example, the first
compound is administered during the entire course of administration of the
second compound;
where the first compound is administered for a period of time that is
overlapping with the
administration of the second compound, e.g. where administration of the first
compound begins
before the administration of the second compound and the administration of the
first compound
ends before the administration of the second compound ends; where the
administration of the
second compound begins before the administration of the first compound and the
administration
of the second compound ends before the administration of the first compound
ends; where the
administration of the first compound begins before administration of the
second compound begins
and the administration of the second compound ends before the administration
of the first
compound ends; where the administration of the second compound begins before
administration
of the first compound begins and the administration of the first compound ends
before the
administration of the second compound ends. As such, "in combination" can also
refer to regimen
involving administration of two or more compounds. "In combination with" as
used herein also
refers to administration of two or more compounds which may be administered in
the same or
different formulations, by the same of different routes, and in the same or
different dosage form
type.
The term "isolated" means that a compound which has been substantially
separated
from, or enriched relative to, other compounds with which it occurs in nature.
"Isolated" also
refers to the state of a compound separated from all or some of the components
that accompany
it during manufacture (e.g., chemical synthesis, recombinant expression,
culture medium, and the
like). Isolated compounds may be present as stereoisomers, and in particular,
diastereomers as
well as their racemic and resolved, enantiomerically pure forms and salts
thereof. Typically, an
17
CA 02736441 2011-04-06
isolated compound is substantially pure when it is at least 50% to 60%, by
weight, free from
organic molecules with which it is naturally associated or with which it is
associated during
manufacture. Generally, the preparation is at least 75%, more usually at least
90%, and generally
at least 99%, by weight, of the compound of interest. A substantially pure
compound can be
obtained, for example, by extraction from a natural source (e.g., bacteria),
by chemically
synthesizing a compound, or by a combination of purification and chemical
modification. A
substantially pure compound can also be obtained by, for example, enriching a
sample having a
particular isomer of a compound of interest. Purity can be measured by any
appropriate method,
e.g., chromatography, mass spectroscopy, HPLC analysis, etc.
The term "optional" or "optionally" means that the subsequently described
event,
circumstance, feature or element may, but need not, occur, and that the
description includes
instances where the event or circumstance occurs and instances in which it
does not. For
example, "heterocyclo group optionally mono- or di- substituted with an alkyl
group" means that
the alkyl may, but need not, be present, and the description includes
situations where the
heterocyclo group is mono- or disubstituted with an allyl group and situations
where the
heterocyclo group is not substituted with the alkyl group.
The term "organic group" and "organic radical" means any carbon containing
group,
including hydrocarbon groups that are classified as an aliphatic group, cyclic
group, aromatic
group, functionalized derivatives thereof and/or various combination thereof.
The terms "monosubstituted" refers to group with one substituent,
"disubstituted" refers to
group with two substituents, "trisubstituted" refers a group with three
substituents, and so forth.
For example, a (monosubstituted)amino refers to an amino group with one
substituent, whereas a
(disubstituted)amino refers to an amino group with two substituents, and
whereas a
(trisubstituted)amino refers to an amino group with three substitutents. When
two or more
substituents are present, they can be the same or different.
The term "pharmaceutically acceptable" refers to a material that is not
biologically or
otherwise undesirable, i.e., the material is of a medically acceptable quality
and composition that
may be administered to an individual along with the selected active
pharmaceutical ingredient
without causing any undesirable biological effects or interacting in a
deleterious manner with any
of the other components of the pharmaceutical composition in which it is
contained.
The term "pharmaceutically acceptable excipient" as used herein refers to any
suitable
substance which provides a pharmaceutically acceptable vehicle for
administration of a
compound(s) of interest to a subject. "Pharmaceutically acceptable excipient"
can encompass
substances referred to as pharmaceutically acceptable diluents,
pharmaceutically acceptable
additives and pharmaceutically acceptable carriers. For example, a
"pharmaceutically acceptable
excipient," "pharmaceutically acceptable diluent," "pharmaceutically
acceptable carrier," and
"pharmaceutically acceptable adjuvant" includes excipient, diluent, carrier,
and adjuvant that are
useful in preparing a pharmaceutical composition that are generally safe, non-
toxic and neither
biologically nor otherwise undesirable, and include an excipient, diluent,
carrier, and adjuvant that
18
CA 02736441 2011-04-06
are acceptable for veterinary use as well as human pharmaceutical use, and may
include both
one and more than one such excipient, diluent, carrier, and adjuvant.
The term "physiological conditions" is meant to encompass those conditions
compatible
with living cells, e.g., predominantly aqueous conditions of a temperature,
pH, salinity, etc. that
are compatible with living cells.
The term "pharmaceutical composition" is meant to encompass a composition
suitable for
administration to a subject, such as a mammal, especially a human. In general
a "pharmaceutical
composition" is sterile, and preferably free of contaminants that are capable
of eliciting an
undesirable response within the subject (e.g., the compound(s) in the
pharmaceutical
composition is pharmaceutical grade). Pharmaceutical compositions can be
designed for
administration to subjects or patients in need thereof via a number of
different routes of
administration including oral, buccal, rectal, parenteral, intraperitoneal,
intradermal, intracheal and
the like.
The term "pharmaceutically acceptable derivatives" of a compound include
salts, esters,
enol ethers, enol esters, acetals, ketals, orthoesters, hemiacetals,
hemiketals, acids, bases,
solvates, hydrates or prodrugs thereof. Such derivatives may be readily
prepared by those of skill
in this art using known methods for such derivatization. The compounds
produced may be
administered to animals or humans without substantial toxic effects and either
are
pharmaceutically active or are prodrugs.
The term "pharmaceutically acceptable salt" of a compound means a salt that is
pharmaceutically acceptable and that possesses the desired pharmacological
activity of the
parent compound. Such salts include: (1) acid addition salts, formed with
inorganic acids such as
hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric
acid, and the like; or
formed with organic acids such as acetic acid, propionic acid, hexanoic acid,
cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic
acid, succinic acid,
malic acid, maleic acid, fumaric acid, tartaric acid, citric acid, benzoic
acid, 3-(4-
hydroxybenzoyl )benzoic acid, cinnamic acid, mandelic acid, methanesulfonic
acid,
ethanesulfonic acid, 1,2-ethanedisulfonic acid, 2-hydroxyethanesulfonic acid,
benzenesulfonic
acid, 4-chlorobenzenesulfonic acid, 2-naphthalenesulfonic acid, 4-
toluenesulfonic acid,
camphorsulfonic acid, glucoheptonic acid, 4,4'-methylenebis-(3-hydroxy-2-ene-1-
carboxylic acid),
3-phenylpropionic acid, trimethylacetic acid, tertiary butylacetic acid,
lauryl sulfuric acid, gluconic
acid, glutamic acid, hydroxynaphthoic acid, salicylic acid, stearic acid,
muconic acid, and the like;
or (2) salts formed when an acidic proton present in the parent compound
either is replaced by a
metal ion, e.g., an alkali metal ion, an alkaline earth ion, or an aluminum
ion; or coordinates with
an organic base such as ethanolamine, diethanolamine, triethanolarnine,
tromethamine, N-
methyiglucamine, and the like.
The terms "polypeptide" and "protein", used interchangeably herein, refer to a
polymeric
form of amino acids of any length, which can include coded and non-coded amino
acids,
chemically or biochemically modified or derivatized amino acids, and
polypeptides having
modified peptide backbones. The term includes fusion proteins, including, but
not limited to,
19
CA 02736441 2011-04-06
fusion proteins with a heterologous amino acid sequence, fusions with
heterologous and
homologous leader sequences, with or without N-terminal methionine residues;
immunologically
tagged proteins; fusion proteins with detectable fusion partners, e.g., fusion
proteins including as
a fusion partner a fluorescent protein, (3-galactosidase, luciferase, etc.;
and the like. Polypeptides
may be of any size, and the term "peptide" refers to polypeptides that are 8-
50 residues (e.g., 8-
20 residues) in length.
The term "protecting group" means a chemical group introduced into a molecule
by
chemical modification of a functional group in order to protect or shield the
functional group from
its normal chemical reactivity. Protecting groups, their addition and removal
are well known (W.
Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-
Interscience, New York,
2005). Removal of the protecting group generates the original functional
group, which may be
referred to as an "unprotected group".
The term "prodrugs" means any compound that releases an active parent drug in
vivo
when such prodrug is administered to a mammalian subject. Prodrugs of a
compound are
prepared by modifying functional groups present in the compound in such a way
that the
modifications may be cleaved in vivo to release the parent compound. Prodrugs
include
compounds wherein a hydroxy, amino, or sulfhydryl group in the compound is
bonded to any
group that may be cleaved in vivo to regenerate the free hydroxyl, amino, or
sulfhydryl group,
respectively. Examples of prodrugs include, but are not limited to esters
(e.g., acetate, formate,
and benzoate derivatives), carbamates (e.g., N,N-dimethylaminocarbonyl) of
hydroxy functional
groups in the compounds, and the like.
The term "racemic" means a mixture containing approximately equal proportions
of
enantiomers.
The terms "subject," "host," "patient," and "individual" are used
interchangeably herein to
refer to any mammalian subject for whom diagnosis or therapy is desired,
particularly humans.
Other subjects may include cattle, dogs, cats, guinea pigs, rabbits, rats,
mice, horses, and so on.
Non-human animal models, particularly mammals, e.g. primate, murine,
lagomorpha, etc. may be
used for experimental investigations.
As used herein, the terms "determining," "measuring," and "assessing," and
"assaying"
are used interchangeably and include both quantitative and qualitative
determinations.
The term "stereo isomer" means a compound with the same chemical formula and
bond
structure as a reference compound, but the geometrical positioning of atoms
and functional
groups in space differs. This class of isomers includes "enantiomers" in which
different isomers
are non-superimposable mirror-images of each other, and diastereomers when
they are not.
Enantiomers can be designated by "(+)-" versus "(-)-" when based on optical
properties, or "(R)-"
versus "(S)-" and or "D-" versus "L-" when based on geometric properties. For
example, "D-
enantiomer" and "L-enantiomer" refer to the enantiomers of a chiral system,
based on the actual
geometry of each enantiomer. In the context of amino acids, the enantiomer
with geometry
based on a naturally occurring amino acid is the L-enantiomer, whereas and the
enantiomer
based on a non-naturally occurring amino acid is the D-enantiomer. The term
"diastereomer"
CA 02736441 2011-04-06
refers to rotational or conformational steroisomers (`rotational isomers" or
"rotomers"; and
"conformational isomers" or "conformers") when the isomers can interconvert by
chemical bond
rotations, or cis-trans isomerism ("cis-trans isomers") when this is not
possible. Stereoisomers
also include "tautomers" which are structural isomers of the same chemical
substance that
spontaneously interconvert with each other, even when pure. Thus unless
indicated otherwise,
the description or naming of a particular compound in the specification and
claims is intended to
include both individual enantiomers and mixtures, racemic or otherwise,
thereof. The methods for
the determination of stereochemistry and the separation of stereoisomers are
well-known in the
art (see, e.g., the discussion in Chapter 4 of "Advanced Organic Chemistry",
4th edition J. March,
John Wiley and Sons, New York, 1992).
The term "treating" or "treatment" of a condition or disease includes: (1)
preventing at
least one symptom of the conditions, i.e., causing a clinical symptom to not
significantly develop
in a mammal that may be exposed to or predisposed to the disease but does not
yet experience
or display symptoms of the disease, (2) inhibiting the disease, i.e.,
arresting or reducing the
development of the disease or its symptoms, or (3) relieving the disease,
i.e., causing regression
of the disease or its clinical symptoms.
The term "unit dosage form" refers to physically discrete units suitable as
unitary dosages
for human and animal subjects, each unit containing a predetermined quantity
of compounds of
the embodiments calculated in an amount sufficient to produce the desired
effect in association
with a pharmaceutically acceptable diluent, carrier or vehicle. The
specifications for the novel unit
dosage forms of the embodiments depend on the particular compound employed and
the effect to
be achieved, and the pharmacodynamics associated with each compound in the
host.
It is understood that in all substituted groups defined above, polymers
arrived at by
defining substituents with further substituents to themselves (e.g.,
substituted aryl having a
substituted aryl group as a substituent which is itself substituted with a
substituted aryl group,
which is further substituted by a substituted aryl group, etc.) are not
intended for inclusion herein.
In such cases, the maximum number of such substitutions is three. For example,
serial
substitutions of substituted aryl groups are limited to substituted aryl-
(substituted aryl)-substituted
aryl.
It is further noted that the claims may be drafted to exclude any optional or
alternative
element. As such, this statement is intended to serve as antecedent basis for
use of such
exclusive terminology as "solely", "only" and the like in connection with the
recitation of claim
elements, or the use of a "negative" limitation.
In describing the embodiments, the structure of the compounds will be
described first.
Then, pharmaceutical formulations containing the compounds will be discussed,
followed by a
description of their methods of use, and kits.
Compounds
The present disclosure provides compounds and compositions containing them
that
correct cellular processing or folding of mutant-CFTR, such as AF508-CFTR, and
methods of
their use in treatment of mutant-CFTR-mediated diseases and conditions, e.g.,
cystic fibrosis.
21
CA 02736441 2011-04-06
Such compounds also find use in the study of CFTR ion transport, particularly
that of AF508-
CFTR.
In one embodiment, the present disclosure provides high-affinity small-
molecule
compounds that increase chloride ion (CI-) conductance in cellular processing
and folding
defective mutant-CFTRs, such as OF508-CFTR. The compounds comprise a
pyrazolylthiazolyl
core and multiple diversity points of substituents.
The compositions of the present disclosure include compounds of Formulae I-
VII, shown
below. Pharmaceutical compositions and methods of the present disclosure also
contemplate
compounds of Formulae I-VII.
Formula I
The present disclosure provides a compound of formula (I):
R2
O N-N O
Ri S X
HN-<
N (I)
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
R' is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, cycloalkyl, substituted
cycloalkyl, heteroaryl, substituted
heteroaryl, heterocyclyl, and substituted heterocyclyl;
R2 is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, cycloalkyl, substituted
cycloalkyl, heteroaryl, substituted
heteroaryl, heterocyclyl, and substituted heterocyclyl; and
X is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl, substituted
alkynyl, aryl, substituted aryl, cycloalkyl, substituted cycloalkyl,
heteroaryl, substituted heteroaryl,
heterocyclyl, substituted heterocyclyl, alkoxy, substituted alkoxy, amino,
substituted amino, and
hydroxyl.
Formula 11
The present disclosure provides a compound of formula (II):
R2
~O HN-N O
R1 S X
HN - \
N (II)
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
R' is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, cycloalkyl, substituted
cycloalkyl, heteroaryl, substituted
heteroaryl, heterocyclyl, and substituted heterocyclyl;
22
CA 02736441 2011-04-06
R2 is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl,
substituted alkynyl, aryl, substituted aryl, cycloalkyl, substituted
cycloalkyl, heteroaryl, substituted
heteroaryl, heterocyclyl, and substituted heterocyclyl; and
X is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
alkynyl, substituted
alkynyl, aryl, substituted aryl, cycloalkyl, substituted cycloalkyl,
heteroaryl, substituted heteroaryl,
heterocyclyl, substituted heterocyclyl, alkoxy, substituted alkoxy, amino,
substituted amino, and
hydroxyl.
In Formulae (I) and (II), R' is selected from alkyl, substituted alkyl,
alkenyl, substituted
alkenyl, alkynyl, substituted alkynyl, aryl, substituted aryl, cycloalkyl,
substituted cycloalkyl,
heteroaryl, substituted heteroaryl, heterocyclyl, and substituted
heterocyclyl.
In certain embodiments, R1 is selected from alkyl, substituted alkyl, alkenyl,
substituted
alkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclyl, and substituted
heterocyclyl. In certain embodiments, R' is selected from alkyl, substituted
alkyl, alkenyl,
substituted alkenyl, aryl, substituted aryl, heteroaryl, and substituted
heteroaryl. In certain
embodiments, R' is selected from alkyl, substituted alkyl, aryl, and
substituted aryl.
In certain embodiments, R1 is alkyl. In certain embodiments, R' is substituted
alkyl. In
certain embodiments, R' is alkenyl. In certain embodiments, R1 is substituted
alkenyl. In certain
embodiments, R' is alkynyl. In certain embodiments, R1 is substituted alkynyl.
In certain
embodiments, R1 is aryl. In certain embodiments, R' is substituted aryl. In
certain embodiments,
R1 is cycloalkyl. In certain embodiments, R1 is substituted cycloalkyl. In
certain embodiments, R1
is heteroaryl. In certain embodiments, R' is substituted heteroaryl. In
certain embodiments, R' is
heterocyclyl. In certain embodiments, R1 is substituted heterocyclyl.
In Formulae (I) and (II), R2 is selected from alkyl, substituted alkyl,
alkenyl, substituted
alkenyl, alkynyl, substituted alkynyl, aryl, substituted aryl, cycloalkyl,
substituted cycloalkyl,
heteroaryl, substituted heteroaryl, heterocyclyl, and substituted
heterocyclyl.
In certain embodiments, R2 is selected from alkyl, substituted alkyl, alkenyl,
substituted
alkenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl,
heterocyclyl, and substituted
heterocyclyl. In certain embodiments, R2 is selected from alkyl, substituted
alkyl, alkenyl,
substituted alkenyl, aryl, and substituted aryl.
In certain embodiments, R2 is alkyl. In certain embodiments, R2 is substituted
alkyl. In
certain embodiments, R2 is alkenyl. In certain embodiments, R2 is substituted
alkenyl. In certain
embodiments, R2 is alkynyl. In certain embodiments, R2 is substituted alkynyl.
In certain
embodiments, R2 is aryl. In certain embodiments, R2 is substituted aryl. In
certain embodiments,
R2 is cycloalkyl. In certain embodiments, R2 is substituted cycloalkyl. In
certain embodiments, R2
is heteroaryl. In certain embodiments, R2 is substituted heteroaryl. In
certain embodiments, R2 is
heterocyclyl. In certain embodiments, R2 is substituted heterocyclyl.
In Formulae (I) and (II), X is selected from alkyl, substituted alkyl,
alkenyl, substituted
alkenyl, alkynyl, substituted alkynyl, aryl, substituted aryl, cycloalkyl,
substituted cycloalkyl,
23
CA 02736441 2011-04-06
heteroaryl, substituted heteroaryl, heterocyclyl, substituted heterocyclyl,
alkoxy, substituted
alkoxy, amino, substituted amino, and hydroxyl.
In certain embodiments, X is selected from alkyl, substituted alkyl, aryl,
substituted aryl,
cycloalkyl, substituted cycloalkyl, heteroaryl, substituted heteroaryl,
heterocyclyl, substituted
heterocyclyl, alkoxy, substituted alkoxy, amino, substituted amino, and
hydroxyl. In certain
embodiments, X is selected from aryl, substituted aryl, heteroaryl,
substituted heteroaryl,
heterocyclyl, substituted heterocyclyl, alkoxy, substituted alkoxy, amino,
substituted amino, and
hydroxyl. In certain embodiments, X is selected from heterocyclyl, substituted
heterocyclyl,
alkoxy, substituted alkoxy, amino, substituted amino, and hydroxyl.
In certain embodiments, X is alkyl. In certain embodiments, X is substituted
alkyl. In
certain embodiments, X is alkenyl. In certain embodiments, X is substituted
alkenyl. In certain
embodiments, X is alkynyl. In certain embodiments, X is substituted alkynyl.
In certain
embodiments, X is aryl. In certain embodiments, X is substituted aryl. In
certain embodiments, X
is cycloalkyl. In certain embodiments, X is substituted cycloalkyl. In certain
embodiments, X is
heteroaryl. In certain embodiments, X is substituted heteroaryl. In certain
embodiments, X is
heterocyclyl. In certain embodiments, X is substituted heterocyclyl. In
certain embodiments, X is
alkoxy. In certain embodiments, X is substituted alkoxy. In certain
embodiments, X is amino. In
certain embodiments, X is substituted amino. In certain embodiments, X is
hydroxyl.
Formula III
The present disclosure provides a compound of formula (III):
R2
O N-N O
R14 S X
HN
N (III)
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
R1 is selected from alkyl, substituted alkyl, aryl, and substituted aryl;
R2 is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
aryl, and
substituted aryl; and
X is selected from heterocyclyl, substituted heterocyclyl, amino, substituted
amino, and
hydroxyl.
Formula IV
The present disclosure provides a compound of formula (IV):
R2
O HN-N O
R14 S X
HN <,
N (IV)
24
CA 02736441 2011-04-06
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
R' is selected from alkyl, substituted alkyl, aryl, and substituted aryl;
R2 is selected from alkyl, substituted alkyl, alkenyl, substituted alkenyl,
aryl, and
substituted aryl; and
X is selected from heterocyclyl, substituted heterocyclyl, alkoxy, substituted
alkoxy,
amino, substituted amino, and hydroxyl.
In formulae (III) and (IV), R' is selected from alkyl, substituted alkyl,
aryl, and substituted
aryl. In certain embodiments, R1 is alkyl. In certain embodiments, R1 is
substituted alkyl. In
certain embodiments, R1 is aryl. In certain embodiments, R1 is substituted
aryl.
In formulae (III) and (IV), R2 is selected from alkyl, substituted alkyl,
alkenyl, substituted
alkenyl, aryl, and substituted aryl. In certain embodiments, R2 is alkyl. In
certain embodiments,
R2 is substituted alkyl. In certain embodiments, R2 is alkenyl. In certain
embodiments, R2 is
substituted alkenyl. In certain embodiments, R2 is aryl. In certain
embodiments, R2 is substituted
aryl.
In formulae (III) and (IV), X is selected from heterocyclyl, and substituted
heterocyclyl,
alkoxy, substituted alkoxy, amino, substituted amino, and hydroxyl. In certain
embodiments, X is
heterocyclyl. In certain embodiments, X is substituted heterocyclyl. In
certain embodiments, X is
alkoxy. In certain embodiments, X is substituted alkoxy. In certain
embodiments, X is amino. In
certain embodiments, X is substituted amino. In certain embodiments, X is
hydroxyl.
Formula V
The present disclosure provides a compound of formula (V):
R2
O HN-N O
R14 S X
HN \
N (V)
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
R1 is selected from alkyl and substituted alkyl;
R2 is selected from alkyl, substituted alkyl, alkenyl, and substituted
alkenyl; and
X is selected from amino and substituted amino.
In formula (V), R1 is selected from alkyl and substituted alkyl. In certain
embodiments, R1
is alkyl. In certain embodiments, R1 is substituted alkyl. In certain
embodiments, R1 is a tert-butyl
group.
In formula (V), R2 is selected from alkyl, substituted alkyl, alkenyl, and
substituted
alkenyl. In certain embodiments, R2 is alkyl. In certain embodiments, R2 is
substituted alkyl. In
CA 02736441 2011-04-06
certain embodiments, R2 is alkenyl. In certain embodiments, R2 is substituted
alkenyl. In certain
embodiments, R2 is allyl. In certain embodiments, R2 is benzyl. In certain
embodiments, R2 is
-CH2C6H3(2-OMe/4-CI).
In formula (V), X is selected from amino and substituted amino. In certain
embodiments,
X is amino. In certain embodiments, X is substituted amino. In certain
embodiments, X is
NHC6H4(4-OMe). In certain embodiments, X is NHCH2C6H5. In certain embodiments,
X is
N(CH2CH2)20.
Formula Vl
The present disclosure provides a compound of formula (VI):
R2
~p HN-N O
R1 g X
HN--< ~
N (VI)
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
R1 is selected from alkyl and substituted alkyl;
R2 is selected from alkyl, substituted alkyl, alkenyl, and substituted
alkenyl; and
X is selected from alkoxy, substituted alkoxy, amino, and substituted amino.
In formula (VI), R' is selected from alkyl and substituted alkyl. In certain
embodiments,
R' is alkyl. In certain embodiments, R1 is substituted alkyl. In certain
embodiments, R' is a tert-
butyl group.
In formula (VI), R2 is selected from alkyl, substituted alkyl, alkenyl, and
substituted
alkenyl. In certain embodiments, R2 is alkyl. In certain embodiments, R2 is
substituted alkyl. In
certain embodiments, R2 is alkenyl. In certain embodiments, R2 is substituted
alkenyl. In certain
embodiments, R2 is benzyl.
In formula (VI), X is selected from alkoxy, amino and substituted amino. In
certain
embodiments, X is alkoxy. In certain embodiments, X is amino. In certain
embodiments, X is
substituted amino. In certain embodiments, X is ethoxy.
Formula Vll
The present disclosure provides a compound of formula (VII):
R2
O N-N 0
R14 S X
HN~
N (VII)
or salts, solvates, hydrates, and prodrug forms thereof, and stereoisomers
thereof,
wherein:
26
CA 02736441 2011-04-06
R' is selected from alkyl and substituted alkyl;
R2 is selected from aryl and substituted aryl; and
X is selected from amino and substituted amino.
In formula (VII), R' is selected from alkyl and substituted alkyl. In certain
embodiments,
R' is alkyl. In certain embodiments, R' is substituted alkyl. In certain
embodiments, R' is a tert-
butyl group.
In formula (VII), R2 is selected from aryl and substituted aryl. In certain
embodiments, R2
is aryl. In certain embodiments, R2 is substituted aryl. In certain
embodiments, R2 is C6H4(4-Br).
In formula (VII), X is selected from amino and substituted amino. In certain
embodiments, X is amino. In certain embodiments, X is substituted amino. In
certain
embodiments, X is NHC6H4(4-OMe).
Particular compounds of interest are illustrated in the following tables.
27
CA 02736441 2011-04-06
R,2
O N-N O
R1 S
HN X
N (I)
Compound R R X
lla C(CH3)3 CH2CH=CH2 NHC6H4(4-OMe)
lib C(CH3)3 CH2CH=CH2 NHCH2C6H5
lic C(CH3)3 CH2CH=CH2 N(CH2CH2)20
lid C(CH3)3 C6H4(4-Br) NHC6H4(4-OMe)
lie C(CH3)3 C6H4(4-Br) NHCH2C6H5
lit C(CH3)3 C6H4(4-Br) N(CH2CH2)20
llg C(CH3)3 CH2C6H5 NHC6H4(4-OMe)
11 h C(CH3)3 CH2C6H5 NHCH2C6H5
i i i C(CH3)3 CH2C6H5 N(CH2CH2)20
l ij C(CH3)3 CH2CH2OH NHC6H4(4-OMe)
1l k C(CH3)3 CH2CH2OH NHCH2C6H5
ill C(CH3)3 CH2CH2OH N(CH2CH2)20
u rn C6H5 CH2CH=CH2 NH(CH2)OH
l l n C6H5 C6H4(4-Br) NH(CH2)OH
llo C6H5 CH2C6H5 NH(CH2)OH
11P C6H5 CH2CH2OH NH(CH2)OH
llq C6H5 CH2CH=CH2 NHC6H4(4-OMe)
11 r C6H5 C6H4(4-Br) NHC6H4(4-OMe)
his C6H5 CH2C6H5 NHC6H4(4-OMe)
lit C6H5 CH2CH2OH NHC6H4(4-OMe)
llu C6H5 CH2CH=CH2 N(CH2CH2)20
liv C6H5 C6H4(4-Br) N(CH2CH2)20
11w C6H5 CH2C6H5 N(CH2CH2)20
l i x C6H5 CH2CH2OH N(CH2CH2)20
12a C(CH3)3 CH2CH=CH2 OH
12b C(CH3)3 C6H4(4-Br) OH
12c C(CH3)3 CH2C6H5 OH
12d C6H5 CH2CH=CH2 OH
12e C6H5 C6H4(4-Br) OH
12f C6H5 CH2C6H5 OH
12g C6H5 CH2CH2OH OH
28
CA 02736441 2011-04-06
R2
O HN-N 0
R14 S X
HN-<, ~
N (II)
Compound R1 R 2 X
10b C(CH3)3 CH2C6H5 OEt
14a C(CH3)3 CH2CH=CH2 NHC6H4(4-OMe)
14b C(CH3)3 CH2CH=CH2 NHCH2C6H5
14c C(CH3)3 CH2CH=CH2 N(CH2CH2)20
14d C(CH3)3 CH2C6H5 NHC6H4(4-OMe)
14e C(CH3)3 CH2C6H5 NHCH2C6H5
14f C(CH3)3 CH2C6H5 N(CH2CH2)20
14g C(CH3)3 CH2C6H3(2-OMe/4-Cl) NHC6H4(4-OMe)
14h C(CH3)3 CH2C6H3(2-OMe/4-Cl) N(CH2CH2)20
14i C(CH3)3 CH2C6H3(2-OMe/4-Cl) NHCH2C6H5
14j C(CH3)3 CH2C6H3(2-OMe/4-CI) NH(CH2)OH
15a C(CH3)3 CH2CH=CH2 OH
15b C(CH3)3 CH2C6H5 OH
Particular compound of interest, and salts or solvates or stereoisomers
thereof, include:
Compounds 1Ob, 11d, 14a, 14b, 14e, 14g, 14h, and 14j.
Particular compound of interest, and salts or solvates or stereoisomers
thereof, include:
Compounds 14a, 14e, 14g, and 14h.
Analog and Derivative Compounds
Also provided by the present disclosure are analogs and derivatives of the
subject
compounds described above. The terms "analog" and "derivative" refers to a
molecule which is
structurally similar or has the same function or activity as the subject
pyrazolylthiazolyl-containing
compounds. Such analogs and derivatives of the subject compounds can be
screened for
efficiency in binding to and modulating the activity of a mutant CFTR, such as
AF508-CFTR.
In some embodiments, in silico modeling can be used to screen libraries of
analog or
derivative compounds. For example, protein-ligand docking can be used to
predict the position
and orientation of a ligand (a small molecule) when it is bound to a protein
such as a mutant-
CFTR. Docking techniques can be for a variety of purposes, most notably in the
virtual screening
of large databases of available chemicals in order to select likely drug
candidates. An exemplary
in silico modeling program suitable for use with the subject method is the
PREDICTTM 3D
Modeling Technology (Predix Pharmaceuticals, Woburn MA), described in greater
detail in
Becker et al., PNAS 101(3 1): 11304-1 1309 (2004).
Pharmaceutical Preparations
Also provided by the present disclosure are pharmaceutical preparations of the
subject
compounds described above. Pharmaceutically acceptable derivatives include
those that retain
29
CA 02736441 2011-04-06
the essential characteristic of the parent compound, namely, the ability to
activate a mutant-
CFTR, such as AF508-CFTR. The pharmaceutically acceptable derivatives may
further include
one or more of additional features that impart a pharmacological or biological
property that
benefits the compound's manufacture, handling, potency, selectivity, and / or
pharmacokinetic
parameters.
The subject compounds can be incorporated into a variety of formulations for
therapeutic
administration by a variety of routes. More particularly, the compounds of the
present
embodiments can be formulated into pharmaceutical compositions by combination
with
appropriate, pharmaceutically acceptable carriers, diluents, excipients andlor
adjuvants, and may
be formulated into preparations in solid, semi-solid, liquid or gaseous forms,
such as tablets,
capsules, powders, granules, ointments, solutions, suppositories, injections,
inhalants and
aerosols. The formulations may be designed for administration to subjects or
patients in need
thereof via a number of different routes, including oral, buccal, rectal,
parenteral, intraperitoneal,
intradermal, intratracheal, etc., administration.
Pharmaceutically acceptable excipients usable with the embodiments, such as
vehicles,
adjuvants, carriers or diluents, are readily available to the public.
Moreover, pharmaceutically
acceptable auxiliary substances, such as pH adjusting and buffering agents,
tonicity adjusting
agents, stabilizers, wetting agents and the like, are readily available to the
public.
Suitable excipient vehicles are, for example, water, saline, dextrose,
glycerol, ethanol, or
the like, and combinations thereof. In addition, if desired, the vehicle may
contain minor amounts
of auxiliary substances such as wetting or emulsifying agents or pH buffering
agents. Actual
methods of preparing such dosage forms are known, or will be apparent, to
those skilled in the
art. See, e.g., Remington's Pharmaceutical Sciences, Mack Publishing Company,
Easton,
Pennsylvania, 17th edition, 1985; Remington: The Science and Practice of
Pharmacy, A.R.
Gennaro, (2000) Lippincott, Williams & Wilkins. The composition or formulation
to be
administered will, in any event, contain a quantity of the agent adequate to
achieve the desired
state in the subject being treated.
Dosage forms
In pharmaceutical dosage forms, the subject compounds of the embodiments may
be
administered in the form of their pharmaceutically acceptable salts, or they
may also be used
alone or in appropriate association, as well as in combination, with other
pharmaceutically active
compounds. The following methods and excipients are merely exemplary and are
in no way
limiting.
The agent can be administered to a host using any available conventional
methods and
routes suitable for delivery of conventional drugs, including systemic or
localized routes. In
general, routes of administration contemplated by the embodiments include, but
are not
necessarily limited to, enteral, parenteral, or inhalational routes, such as
intrapulmonary or
intranasal delivery.
Conventional and pharmaceutically acceptable routes of administration include
intranasal, intrapulmonary intramuscular, intratracheal, intratumoral,
subcutaneous, intradermal,
CA 02736441 2011-04-06
topical application, intravenous, rectal, nasal, oral and other parenteral
routes of administration.
Routes of administration may be combined, if desired, or adjusted depending
upon the agent
andlor the desired effect. The composition can be administered in a single
dose or in multiple
doses.
In one embodiment of particular interest, the compounds of the embodiments are
administered in aerosol formulation via intrapulmonary inhalation. The
compounds of the
embodiments can be formulated into pressurized acceptable propellants such as
dichlorodifluoromethane, propane, nitrogen and the like.
Mechanical devices designed for intrapulmonary delivery of therapeutic
products, include
but are not limited to nebulizers, metered dose inhalers, and powder inhalers,
all of which are
familiar to those of skill in the art. Specific examples of commercially
available devices suitable for
the practice of the embodiments are the Ultravent nebulizer, manufactured by
Mallinckrodt, Inc.,
St. Louis, Mo.; the Acorn 11 nebulizer, manufactured by Marquest Medical
Products, Englewood,
Colo.; the Ventolin metered dose inhaler, manufactured by Glaxo Inc., Research
Triangle Park,
North Carolina; the Spinhaler powder inhaler, manufactured by Fisons Corp.,
Bedford, Mass.; the
"standing cloud" device of Inhale Therapeutic Systems, Inc., San Carlos,
Calif.; the AIR inhaler
manufactured by Alkennes, Cambridge, Mass.; and the AERx pulmonary drug
delivery system
manufactured by Aradigm Corporation, Hayward, Calif. Of particular interest
are the PARI LC
PLUS , the PARI LC STAR , and the PARI BABYTM nebulizers by PARI Respiratory
Equipment,
Inc., Monterey, Calif.
Formulations for use with a metered dose inhaler device may generally comprise
a finely
divided powder. This powder may be produced by lyophilizing and then milling a
liquid conjugate
formulation and may also contain a stabilizer such as human serum albumin
(HSA). Typically,
more than 0.5% (wlw) HSA is added. Additionally, one or more sugars or sugar
alcohols may be
added to the preparation if necessary. Examples include lactose maltose,
mannitol, sorbitol,
sorbitose, trehalose, xylitol, and xylose. The amount added to the formulation
can range from
about 0.01 to 200% (wlw), preferably from approximately 1 to 50%, of the
conjugate present.
Such formulations may then lyophilized and milled to the desired particle
size.
The properly sized particles may then suspended in a propellant with the aid
of a
surfactant. The propellant may be any conventional material employed for this
purpose, such as a
chlorofluorocarbon, a hydrochlorofluorocarbon, a hydrofluorocarbon, or a
hydrocarbon, including
trichlorofluoromethane, dichlorodifluoromethane, dichlorotetrafluoroethanol,
and 1,1,1,2-
tetrafluoroethane, or combinations thereof. Suitable surfactants may include
sorbitan trioleate and
soya lecithin. Oleic acid may also be useful as a surfactant. This mixture may
then loaded into the
delivery device. An example of a commercially available metered dose inhaler
suitable.for use in
the embodiments is the Ventolin metered dose inhaler, manufactured by Glaxo
Inc., Research
Triangle Park, N.C.
Formulations for powder inhalers may comprise a finely divided dry powder
containing
conjugate and may also include a bulking agent, such as lactose, sorbitol,
sucrose, or mannitol in
amounts which facilitate dispersal of the powder from the device, e.g., 50% to
90% by weight of
31
CA 02736441 2011-04-06
the formulation. The particles of the powder may have aerodynamic properties
in the lung
corresponding to particles with a density of about 1 g/cm2 having a median
diameter less than 10
micrometers, preferably between 0.5 and 5 micrometers, most preferably of
between 1.5 and 3.5
micrometers. An example of a powder inhaler suitable for use in accordance
with the teachings
herein is the Spinhaler powder inhaler, manufactured by Fisons Corp., Bedford,
Mass. The
powders for these devices may be generated andlor delivered by methods
disclosed in U.S. Pat.
No. 5,997,848, U.S. 5,993,783, U.S. 5,985,248, U.S. 5,976574, U.S. 5,922,354,
U.S. 5,785,049
and U.S. 5,654,007.
For oral preparations, the subject compounds can be used alone or in
combination with
appropriate additives to make tablets, powders, granules or capsules, for
example, with
conventional additives, such as lactose, mannitol, corn starch or potato
starch; with binders, such
as crystalline cellulose, cellulose derivatives, acacia, corn starch or
gelatins; with disintegrators,
such as corn starch, potato starch or sodium carboxymethylcellulose; with
lubricants, such as talc
or magnesium stearate; and if desired, with diluents, buffering agents,
moistening agents,
preservatives and flavoring agents.
Parenteral routes of administration other than inhalation administration
include, but are
not necessarily limited to, topical, transdermal, subcutaneous, intramuscular,
intraorbital,
intracapsular, intraspinal, intrasternal, and intravenous routes, i.e., any
route of administration
other than through the alimentary canal. Parenteral administration can be
carried to effect
systemic or local delivery of the agent. Where systemic delivery is desired,
administration
typically involves invasive or systemically absorbed topical or mucosal
administration of
pharmaceutical preparations.
Methods of administration of the agent through the skin or mucosa include, but
are not
necessarily limited to, topical application of a suitable pharmaceutical
preparation, transdermal
transmission, injection and epidermal administration. For transdermal
transmission, absorption
promoters or iontophoresis are suitable methods. lontophoretic transmission
may be
accomplished using commercially available "patches" which deliver their
product continuously via
electric pulses through unbroken skin for periods of several days or more.
The subject compounds of the embodiments can be formulated into preparations
for
injection by dissolving, suspending or emulsifying them in an aqueous or
nonaqueous solvent,
such as vegetable or other similar oils, synthetic aliphatic acid glycerides,
esters of higher
aliphatic acids or propylene glycol; and if desired, with conventional
additives such as solubilizers,
isotonic agents, suspending agents, emulsifying agents, stabilizers and
preservatives.
The agent can also be delivered to the subject by enteral administration.
Enteral routes of
administration include, but are not necessarily limited to, oral and rectal
(e.g., using a
suppository) delivery.
Furthermore, the subject compounds can be made into suppositories by mixing
with a
variety of bases such as emulsifying bases or water-soluble bases. The
compounds of the
embodiments can be administered rectally via a suppository. The suppository
can include
32
CA 02736441 2011-04-06
vehicles such as cocoa butter, carbowaxes and polyethylene glycols, which melt
at body
temperature, yet are solidified at room temperature.
Dosages
Depending on the subject and condition being treated and on the administration
route,
the subject compounds may be administered in dosages of, for example, 0.1 pg
to 10 mg/kg body
weight per day. The range is broad, since in general the efficacy of a
therapeutic effect for
different mammals varies widely with doses typically being 20, 30 or even 40
times smaller (per
unit body weight) in man than in the rat. Similarly the mode of administration
can have a large
effect on dosage. Thus, for example, oral dosages may be about ten times the
injection dose.
Higher doses may be used for localized routes of delivery.
A typical dosage may be a solution suitable for intravenous administration; a
tablet taken
from two to six times daily, or one time-release capsule or tablet taken once
a day and containing
a proportionally higher content of active ingredient, etc. The time-release
effect may be obtained
by capsule materials that dissolve at different pH values, by capsules that
release slowly by
osmotic pressure, or by any other known means of controlled release.
Those of skill in the art will readily appreciate that dose levels can vary as
a function of
the specific compound, the severity of the symptoms and the susceptibility of
the subject to side
effects. Preferred dosages for a given compound are readily determinable by
those of skill in the
art by a variety of means.
Although the dosage used will vary depending on the clinical goals to be
achieved, a
suitable dosage range is one which provides up to about 1 pg to about 1,000 pg
or about 10,000
pg of subject composition of the to reduce a symptom in a subject animal.
Unit dosage forms for oral or rectal administration such as syrups, elixirs,
and
suspensions may be provided wherein each dosage unit, for example,
teaspoonful, tablespoonful,
tablet or suppository, contains a predetermined amount of the composition
containing one or
more compounds of the embodiments. Similarly, unit dosage forms for injection
or intravenous
administration may comprise the compound (s) in a composition as a solution in
sterile water,
normal saline or another pharmaceutically acceptable carrier.
Combination therapy
For use in the subject methods, the subject compounds may be formulated with
or
otherwise administered in combination with other pharmaceutically active
agents, including other
CFTR-activating agents. The subject compounds may be used to provide an
increase in the
effectiveness of another chemical, such as a pharmaceutical (e.g., other CFTR-
activating agents,
or agents potentiate gating defective mutant-CFTR), or a decrease in the
amount of another
chemical, such as a pharmaceutical (e.g., other CFTR-activating agents), that
is necessary to
produce the desired biological effect.
Examples of other CFTR activating agents include, but are not limited to,
enhancers of
intracellular cAMP levels, such as for example, but not limited to, forskolin,
rolipram, 8-bromo-
cAMP, theophylline, papaverine, cAMP and salts, analogs, or derivatives
thereof. Other examples
include beta agonists, tobramycin (TOBI , Chiron Inc., Emeryville, Calif.) and
curcumin (Egan et
33
CA 02736441 2011-04-06
al., (2004) Science 304:600-603). The compounds of the embodiments may also be
used in
combination with specific mutant CFTR activators, such as correctors and/or
potentiators.
Examples of mutant-CFTR potentiating agents include, but are not limited to,
phenylglycine
containing compounds and sulfonamide containing compounds described in WO
2005/120497
and co-pending US provisional patent application serial no. 60/980,387, filed
October 16, 2007,
entitled "Compounds Having Activity In Increasing Ion Transport By Mutant-CFTR
And Uses
Thereof", each of which are incorporated herein by reference in its entirety.
The compounds described above may also be combined with other therapies for
CF,
including oral corticosteroids, ibuprofen, ribovarin or antibiotics such as
dicloxacillin,
cephalosporin, cephalexin,erythromycin, amoxicillin-clavulanate, ampicillin,
tetracycline,
trim ethoprim-suIfamethoxazole, chloramphenicol ciprofloxacin, tobramycin,
gentamicin,
cephalosporins, monobactams and the like.
The compounds described herein for use in combination therapy with the
compounds of
the embodiments may be administered by the same route of administration (e.g.
intrapulmonary,
oral, enteral, etc.) that the 'compounds are administered. In the alternative,
the compounds for
use in combination therapy with the compounds of the embodiments may be
administered by a
different route of administration that the compounds are administered.
Methods
Methods for increasing chloride ion permeability of a mutant-CFTR cell
The present disclosure provides methods for increasing ion permeability of a
cell that
produces mutant-CFTR protein, with cells having a folding or processing
defective mutant-CFTR
being of interest, with cells having a AF508-CFTR being of particular
interest. In general, the
method involves contacting the cell with a compound in an amount effective to
correct the folding
or processing defect of a mutant-CFTR protein and increase ion permeability of
the cell. In some
embodiments, the cell contains a recombinant expression cassette that encodes
said mutant-
CFTR protein. In other embodiments, the cell contains a genome that encodes
said mutant-CFTR
protein. In yet other embodiments, the mutant-CFTR is a OF508-CFTR. In another
embodiment of
interest, a compound of the embodiments is used in the method in combination
with a second
mutant-CFTR activator or potentiator.
The present disclosure provides methods for treating a subject having a
condition
associated with mutant-CFTR, which involves administering to the subject a
therapeutically
effective amount of a pharmaceutical composition of the embodiments. The
present disclosure
also provides a method of increasing ion permeability of a cell producing a
mutant-CFTR protein,
which involves contacting the cell with an effective amount of the
pharmaceutical composition of
the embodiments so as to increase CFTR-mediated ion permeability of the cell.
In some
embodiments, the condition is cystic fibrosis. In some embodiments, the
subject, after treatment,
has a decrease in mucous or bacterial titer in their lungs, a decrease in
coughing or wheezing, a
decrease in pancreatic insufficiency, or a decrease in electrolyte levels in
their sweat. In some
embodiments, the subject is a non-human animal. In some embodiments, the
subject is human.
34
CA 02736441 2011-04-06
In embodiments of particular interest, the animal is a mammal. In some
embodiments, the
mutant-CFTR is a AF508-CFTR.
In many embodiments, the mutant-CFTR protein is present on the plasma membrane
of
the cell. Methods of detecting mutant-CFTR protein presence on the plasma
membrane are well
known in the art and can include but are not limited to, for example, labeling
a molecule that binds
to CFTR protein with a fluorescent, chemical or biological tag. Examples of
molecules that bind to
CFTR protein include, without limitation, antibodies (monoclonal and
polyclonal), FAB fragments,
humanized antibodies and chimeric antibodies. For an example of an antibody
that binds to
CFTR protein, see, e.g. U.S. Patent No. 6,201,107.
In many embodiments, the cell has increased permeability to chloride ions, and
the
contacting of the cell with a compound of the embodiments, particularly when
provided in
combination with another mutant-CFTR activator or potentiator, increases the
rate of chloride ion
transport across the plasma membrane of the cell. Contacting the cell with a
compound of the
embodiments usually increases the activity of mutant-CFTR protein to increase
ion transport.
In most embodiments, the ion transport activity of mutant-CFTR, or the
permeability of a
cell to ions, is increased by up to about 10%, by up to about 20%, by up to
about 50%, by up to
about 100%, by up to about 150%, by up to about 200%, by up to about 300%, by
up to about
400%, by up to about 500%, by up to about 800%, or up to about 1000% or more.
In certain
embodiments, where there is no detectable ion transport activity of mutant-
CFTR or permeability
of a cell to ions, contacting of the cell with a compound of the embodiments
causes detectable
activity of mutant-CFTR or permeability of a cell to ions.
Activation of mutant-CFTR and/or ion permeability may be measured using any
convenient methods that may use molecular markers, e.g., a halide sensitive
GFP or another
molecular marker (e.g., Galietta et al., (2001) FEBS Lett. 499,220-224), patch
clamp assays, and
short circuit assays.
Suitable cells include those cells that have an endogenous or introduced
mutant-CFTR
gene. Suitable cells include mammalian cell systems (e.g., COS, CHO, BHK, 293,
3T3 cells etc.)
harboring constructs that have an expression cassette for expression of mutant-
CFTR. The cell
used in the subject methods may be a cell present in vivo, ex vivo, or in
vitro. As used herein, the
term "expression cassette" is meant to denote a genetic sequence, e.g. DNA or
RNA, that codes
for mutant-CFTR protein, e.g., AF508-CFTR. Methods of introducing an
expression cassette into
a cell are well known in the art. See for example, Sambrook et al., Molecular
Cloning: A
Laboratory Manual. Cold Spring Harbor Laboratory Press, NY, Vols. 1-3 (1989).
Methods of treating cystic fibrosis
The present disclosure also provides methods of treating a subject having a
condition
associated with mutant-CFTR, e.g., cystic fibrosis. In general, the method
involves administering
to the subject a compound of the embodiments in an amount effective to
activate a mutant-CFTR
protein to increase ion transport and thereby treat the condition. In an
embodiment of particular
interest, a compound of the embodiments is administered in combination with a
second mutant-
CFTR activator or potentiator, e.g., a compound that enhances intracellular
cAMP, e.g., forskolin
CA 02736441 2011-04-06
or a potentiator compound, such as the phenyiglycine and sulfonamide
containing potentiator
compound described in WO 2005/120497 and co-pending US provisional patent
application serial
no. 60/980,387, filed October 16, 2007, entitled "Compounds Having Activity In
Increasing Ion
Transport By Mutant-CFTR And Uses Thereof", each of which are incorporated
herein by
reference in its entirety.
The compounds disclosed herein are useful in the treatment of a mutant-CFTR
mediated
condition, e.g., any condition, disorder or disease, or symptom of such
condition, disorder, or
disease, that results from the presence and/or activity of mutant-CFTR as
compared to wild-type
CFTR, e.g., activity of mutant-CFTR in ion transport. Such conditions,
disorders, diseases, or
symptoms thereof are amenable to treatment by correction of cellular
processing or folding of a
mutant-CFTR, e.g., activation of mutant-CFTR chloride transport. Cystic
fibrosis, a hereditary
condition associated with a mutant-CFTR, e.g., AF508-CFTR, is an example of a
condition that is
treatable using the compounds of the embodiments. Use of the compounds of the
embodiments
in combination with a second mutant CFTR activator or potentiator is of
particular interest,
including a corrector compound of the embodiments.
The above methods may be used to treat CF and its symptoms in humans or in
animals.
Several animal models for CF are known in the art. For example, Engelhardt et
at. (J. Clin. Invest.
90: 2598-2607, 1992) developed an animal model of the human airway, using
bronchial
xenografts engrafted on rat tracheas and implanted into nude mice. More
recently transgenic
models of cystic fibrosis have been produced (e.g., Clarke et al., Science
257: 1125-1 128, 1992;
Dorin et al., Nature 359: 21 1-215, 1992). With the recent advances of nuclear
transfer and stem
cell transformation technologies, the alteration of a wild type CFTR gene in
an animal to make it
into a mutant-CFTR gene is possible for a wide variety of animals.
Many of these animals show human CF symptoms. In particular, many of these
animals
showed measurable defects in ion permeability of airway and intestinal
epithelia, similar to those
demonstrable in human CF tissues, and a susceptibility to bacterial infection.
Furthermore, most
of the deficient mice had intestinal pathology similar to that of meconium
ileus. Also, there
appeared to be no prenatal loss from litters produced from crosses between
heterozygotes.
Animals suitable for treatment using the subject methods include any animal
with a
mutant-CFTR related condition, particularly a mammal, e.g., non-human primates
(e.g., monkey,
chimpanzee, gorilla, and the like), rodents (e.g., rats, mice, gerbils,
hamsters, ferrets, and the
like), lagomorphs, swine (e.g., pig, miniature pig), equine, canine, feline,
and the like. Large
animals are of particular interest. Transgenic mammals may also be used, e.g.
mammals that
have a chimeric gene sequence. Methods of making transgenic animals are well
known in the art,
see, for example, U.S. Patent No. 5,614,396. For an example of a transgenic
mouse with a CFTR
defect, see e.g. WO 94104669.
Such animals may be tested in order to assay the activity and efficacy of the
subject
compounds. Improvement in lung function can be assessed by, for example,
monitoring prior to
and during therapy the subject's forced vital capacity (FVC), carbon monoxide
diffusing capacity
36
CA 02736441 2011-04-06
(DLco), andlor room air PO2 >55 mmHg at rest. Significant improvements in one
or more of these
parameters are indicative of efficacy. It is well within the skill of the
ordinary healthcare worker
(e.g., clinician) provide adjust dosage regimen and dose amounts to provide
for optimal benefit to
the patient according to a variety of factors (e.g., patient dependent factors
such as the severity of
the disease and the like), the compound administered, and the like).
Subjects suitable for treatment
Subjects suitable for treatment with a method of the embodiments include
individuals
having mutant-CFTR protein-mediated condition disorder or disease, or symptom
of such
condition, disorder, or disease that results from or is correlated to the
presence of a mutant-
CFTR, usually two alleles of the mutant CFTR. Moreover, subjects suitable for
treatment with a
method of the embodiments include individuals with CF. Of particular interest
in many
embodiments is the treatment of humans with CF.
Symptoms of mutant-CFTR protein-mediated conditions include meconium ileus,
liver
disease including biliary tract obstruction and stenosis, pancreatic
insufficiency, pulmonary
disease including chronic Pseudomonas aeruginosa infections and other
infections of the lung,
infertility associated with abnormal vas deferens development or abnormal
cervical mucus, and
carcinoma including adenocarcinoma.
The compounds of the embodiments affect the ion transport capability of the
mutant-
CFTR by increasing the reduced level of ion transport mediated by a mutant-
CFTR, such as the
AF508-CFTR. As such, the corrector compounds of the embodiments have
particular clinical
utility in treating a subset of CF patients that have mutations in the CFTR
gene that results a
mutant-CFTR that is expressed in the plasma membrane and has reduced chloride
conductance
capability due to folding or cellular processing (i.e., the mutant-CFTR is
folding or cellular
processing defective). As such, the corrector compounds of the embodiments
have clinical utility
in treating CF patients having a folding or cellular processing mutant-CFTR,
such as SF508-
CFTR. In addition, the corrector compounds of the embodiments also have
clinical utility in
treating CF patients when used in conjunction with compounds that activate or
potentiate a gating
mutant-CFTR, such as AF508-CFTR, G551 D-CFTR, G1349D-CFTR, or D1152H-CFTR.
CFTR mutations associated with CF are well known in the art. These mutations
can be
classified in five general categories with respect to the CFTR protein. These
classes of CFTR
dysfunction include limitations in CFTR production (e.g., transcription and/or
translation) (Class I),
aberrant folding and/or trafficking (Class II), abnormal regulation of
conduction (Class III),
decreases in chloride conduction (Class IV), and reductions in synthesis
(Class V). Due to the
lack of functional CFTR, Class I, II, and III mutations are typically
associated with a more severe
phenotype in CF (i.e. pancreatic insufficiency) than the Class IV or V
mutations, which may have
very low levels of functional CFTR expression. A listing of the different
mutations that have been
identified in the CFTR gene is as found at the world-wide website of the
Cystic Fibrosis Mutation
Database at genet.sickkids.on.ca/cgi-bin/WebObjects/MUTATION, specifically
incorporated by
reference herein in its entirety.
37
CA 02736441 2011-04-06
A subject suitable for treatment with a method of the embodiments may be
homozygous
for a specific mutant-CFTR, i.e. homozygous subjects with two copies of a
specific mutant-CFTR,
e.g., AF508-CFTR. In addition, subjects suitable for treatment with a method
of the embodiments
may also be compound heterozygous for two different CFTR mutants, i.e.,
wherein the genome of
the subjects includes two different mutant forms of CFTR, e.g., a subject with
one copy of AF508-
CFTR and a copy of different mutant form of CFTR.
In certain embodiments, the mutant-CFTR polypeptide is AF508-CFTR. In certain
embodiments, the mutant-CFTR polypeptide is G551 D-CFTR. In certain
embodiments, the
mutant-CFTR polypeptide is G1349D-CFTR. In certain embodiments, the mutant-
CFTR
polypeptide is D152H-CFTR. The present disclosure, however, should not be
construed to be
limited solely to the treatment of CF patients having this mutant form of
CFTR. Rather, the
present disclosure should be construed to include the treatment of CF patients
having other
mutant forms of CFTR with similar characteristics, that result in expression
of the mutant-CFTR in
the plasma membrane and has reduced chloride conductance capability or has
abnormal
regulation of conductance.
Kits & Systems
Also provided are kits and systems that find use in practicing the subject
methods, as
described above. The kits typically contain unit doses of the subject
compounds, usually in oral or
injectable doses. For example, kits and systems for practicing the subject
methods may include
one or more pharmaceutical formulations that include corrector compound of the
embodiments,
and optionally one or more additional components. As such, in certain
embodiments the kits may
include a single pharmaceutical composition present as one or more unit
dosages. In yet other
embodiments, the kits may include two or more separate pharmaceutical
compositions, as well as
be part of a system. The term "system" as employed herein refers to a
collection of components
or agents present in single or disparate compositions that are brought
together for the purpose of
practicing the subject methods. This includes systems libraries of the
compounds of the
embodiments as well as individual compounds of the embodiments.
Thus the kits can include one or more of, depending upon the intended use of
the kit, the
compositions described herein, such as: a corrector compound of the
embodiments. Other
optional components of the kit include: buffers, delivery vehicles, delivery
systems etc., for
administering a corrector compound, and/or for performing a diagnostic assay.
The various
components of the kit may be present in separate containers or certain
compatible components
may be pre-combined into a single container, as desired. The kits also may
include one or more
additional pharmaceuticals or agents for treating a mutant-CFTR.
In addition to the above components, the subject kits may further include
instructions for
practicing the subject methods, such as an informational package insert
describing the use and
attendant benefits of the drugs in treating pathological condition of
interest. These instructions
may be present in the kits in a variety of forms, one or more of which may be
present in or on the
kit. One form in which these instructions may be present is as printed
information on a suitable
medium or substrate, e.g., a piece or pieces of paper on which the information
is printed, in or on
38
CA 02736441 2011-04-06
the packaging of the kit, in a package insert, etc. Yet another means would be
a computer
readable medium, e.g., diskette, CD, etc., on which the information has been
recorded. Yet
another means that may be present is a website address which may be used via
the internet to
access the information at a removed site. Any convenient means may be present
in the kits.
In a specific embodiment, a kit is provided for use in treating a subject
suffering from
cystic fibrosis. This kit includes a pharmaceutical composition comprising
corrector compound of
the embodiments and instructions for the effective use of the pharmaceutical
composition in a
method of treating a subject suffering from cystic fibrosis. Such instructions
may include not only
the appropriate handling properties, dosing regiment and method of
administration, and the like,
but further include instructions to optionally screen and type the subject for
mutant-CFTR (e.g.,
AF508-CFTR, G551 D-CFTR, G1349D-CFTR, or D1152H-CFTR). This aspect assists the
practitioner of the kit in tracking or gauging the potential responsiveness of
the subject to
treatment with a composition of the embodiments. In another embodiment, the
kit includes one or
more corrector compositions that are detectably labeled.
EXAMPLES
The following examples are put forth so as to provide those of ordinary skill
in the art with
a complete disclosure and description of how to make and use the present
embodiments, and are
not intended to limit the scope of what the inventors regard as their
invention nor are they
intended to represent that the experiments below are all or the only
experiments performed.
Efforts have been made to ensure accuracy with respect to numbers used (e.g.
amounts,
temperature, etc.) but some experimental errors and deviations should be
accounted for. Unless
indicated otherwise, parts are parts by weight, molecular weight is weight
average molecular
weight, temperature is in degrees Centigrade, and pressure is at or near
atmospheric. Thus it is
understood that the examples and embodiments described herein are for
illustrative purposes
only and that various modifications or changes in light thereof will be
suggested to persons skilled
in the art and are to be included within the spirit and purview of this
application and scope of the
appended claims.
All publications, patents, and patent applications cited herein are hereby
incorporated by
reference in their entirety for all purposes.
Chemistry
General and integrated synthetic methods to access a series of functionally
and
regioisomerically diversified pyrazolylthiazoles is disclosed herein.
39
CA 02736441 2011-04-06
Scheme 1. Synthesis of Ny-substituted pyrazolylthiazoles.
O
a: R1 = C(CH3)3
HCID H2N S ~ b: R1 = C6H5
N
6
1(a)
O O O
1 1
OEt
R HN~S (-~ R HN-<S
N N
7a,b 8a,b
R2N,N 0 (c)
R1--~ /S NR3R3' R2 N
HNN ~(c{) R1 S N~ C02Et
11 or (f) HN-<\
N 2
(e) 9 R
R2N-N 0 + nI' C02Et
-~ S 2H R1 S
R1 CO
HN-<\
HN--<\ N
N
12
Reagents and conditions: (a) R'CO2H, CDI, DMF, 85 C (b) Diethyl oxalate,
LHMDS, THF, -78 C (c)
R2NHNH2, EtOH (d) R3R"NH, AIMe3, DCM, 0 C (e) NaOH, THF/H20 (f) Ethanol
amine, MW, 160 C, 30
5 Min.
As shown in Scheme 1, synthetic effort began with conversion of the amino
group of 6
into amide 7 through a CDI-mediated coupling reaction, which occurred in
greater than 79% yield.
Amide 7 was then subjected to Claisen reaction with diethyl oxalate in the
presence of 2.1
10 equivalents of LHMDS, giving 1,3-diketone 8 in excellent yield (>90%).
Next, the condensation
reaction between 8 and substituted hydrazines proceeded with excellent
selectivity to deliver the
Ny regioisomers 9 in good yield (>87%; 9:10>10:1). A minor by- product was
found to be 7,
indicating that the 2,4-dioxobutanoate moiety is susceptible to a reverse
Claisen reaction in the
presence of hydrazine nucleophiles. The excellent regioselectivity of this
condensation reaction
reflects the fact that the two carbonyl groups in 8 have very different
electrophilicity. From
pyrazolylthiazole 9, the final diversification in this series was achieved by
converting the ester
moiety into the corresponding amide (-+11) via aminolysis in good yield (>73%)
or acid (-+ 12) by
saponification. Thirty-nine Ny-substituted pyrazolylthiazoles were prepared
(see Table 1) by
varying three diversity points (two R1 inputs, four R2 inputs, and four NR3R3'
inputs).
40
CA 02736441 2011-04-06
Table 1. Ny-Substituted pyrazolylthiazole analogs.
R2
N-N 3 O
RI-~ S X
NH-<,
N
R1 = C(CH3)3, C6H5
R2 = CH2CH=CH2, C6H4(4-Br), CH2C6H5,
(CH2)20H
X = OEt, NHC6H4(4-OMe), NHCH2C6H5,
NH(CH2)20H, N(CH2CH2)20, OH
Compd R1 2 X Compd R2 X
9a C(CH3)3 CH2CH=CH2 OEt lim C6H5 CH2CH=CH2 NH(CH2)OH
9b C(CH3)3 C6H4(4-Br) OEt lin C6H5 C6H4(4-Br) NH(CH2)OH
9c C(CH3)3 CH2C6H5 OEt 110 C6H5 CH2C6H5 NH(CH2)OH
9d C(CH3)3 CH2CH2OH OEt lip C6H5 CH2CH2OH NH(CH2)OH
9e C6H5 CH2CH=CH2 OEt iiq C6H5 CH2CH=CH2 NHC6H4(4-OMe)
9f C6H5 C6H4(4-Br) OEt lir C6H5 C6H4(4-Br) NHC6H4(4-OMe)
9g C6H5 CH2C6H5 OEt ils C6H5 CH2C6H5 NHC6H4(4-OMe)
9h C6H5 CH2CH2OH OEt lit C6H5 CH2CH2OH NHC6H4(4-OMe)
iia C(CH3)3 CH2CH=CH2 NHC6H4(4-OMe) iiu C6H5 CH2CH=CH2 N(CH2CH2)20
lib C(CH3)3 CH2CH=CH2 NHCH2C6H5 liv C6H5 C6H4(4-Br) N(CH2CH2)20
llc C(CH3)3 CH2CH=CH2 N(CH2CH2)20 llw C6H5 CH2C6H5 N(CH2CH2)20
lid C(CH3)3 C6H4(4-Br) NHC6H4(4-OMe) llx C6H5 CH2CH2OH N(CH2CH2)20
lie C(CH3)3 C6H4(4-Br) NHCH2C6H5 12a C(CH3)3 CH2CH=CH2 OH
11f C(CH3)3 C6H4(4-Br) N(CH2CH2)20 12b C(CH3)3 C6H4(4-Br) OH
lug C(CH3)3 CH2C6H5 NHC6H4(4-OMe) 12c C(CH3)3 CH2C6H5 OH
llh C(CH3)3 CH2C6H5 NHCH2C6H5 12d C6H5 CH2CH=CH2 OH
iii C(CH3)3 CH2C6H5 N(CH2CH2)20 12e C6H5 C6H4(4-Br) OH
1i j C(CH3)3 CH2CH2OH NHC6H4(4-OMe) 12f C6H5 CH2C6H5 OH
ilk C(CH3)3 CH2CH2OH NHCH2C6H5 12g C6H5 CH2CH2OH OH
iii C CH3 3 CH2CH2OH N CH2CH2 20
41
CA 02736441 2011-04-06
Scheme 2. Synthesis of N(3-substituted pyrazolylthiazole.
8a,b a: R1 = C(CH3)3
(a) R2 b: R1 = C61-15
H
R1S N ~ C02Et R1 /O S N ~ C02Et
N e N
13a,b 10
(c) or (d/ \e)
R2 R2
e N O N-N O
O S NR3R3' I/ OH
x 4HNC-<,
,
I
N N
14 15
Reagents: (a) NH2NH2=H2O, EtOH (b) R2Br, K2CO3, Acetone, 60 C (c) R3R3NH,
AIMe3, DCM, MW, 100 C
(d) NH2(CH2)20H, EtOH, MW 180 C (e) NaOH, THF/H20.
Scheme 2 shows a synthetic route to access the N(3-substituted pyrazole
regioisomer.
First, 8 was reacted with hydrazine monohydrate to deliver pyrazole 13 in 63%
yield. The N(3
position of 13 was then selectively alkylated, giving mainly 10 (R = H) in 59-
66% yield when R1 _
pivaloyl (10:9 = 8:1). The major by-product of this reaction is N-alkylation
of the amide moiety
leading to formation of bis-alkylated product (10: R = R2). This complication
was serious with the
benzamide analog of 13 (R1 = C6H5) that the major product was, in fact, the
bisalkylation product
(10: R = R2); the desired mono-alkylated product (10: R = H) was obtained in
20% yield.
Therefore, final diversification on the N(3-substituted isomers was mainly
focused on pivaloyl
amide analogs of 10 and included hydrolysis or aminolysis, such as microwave
irradiation in the
AIMe3-mediated aminolysis, to deliver 14 and 15, respectively. In total,
fifteen N(3-substituted
pyrazolylthiazoles were prepared for this focused library (see Table 2).
42
CA 02736441 2011-04-06
Table 2. N(3-Substituted pyrazolylthiazole analogs.
R2
N,N 5 0
O ~
R1 S X
HN-<,
N
Rt = C(CH3)3
R2 = CH2CH=CH2, C6H4(4-Br), CH2C6H3(2-OMe/4-Cl)
X = OEt, NHC6H4(4-OMe), NHCH2C6H5,
N(CH2CH2)20, NH(CH2)20H, OH
Compd R' R2 X Compd R1 R2 X
10a C(CH3)3 CH2CH=CH2 OEt 14f C(CH3)3 CH2C6H5 N(CH2CH2)20
10b C(CH3)3 CH2C6H5 OEt 14g C(CH3)3 CH2C6H3(2-OMe/4-CI) NHC6H4(4-OMe)
10c C(CH3)3 CH2C6H3(2-OMe/4-CI) OEt 14h C(CH3)3 CH2C6H3(2-OMe/4-CI)
N(CH2CH2)20
14a C(CH3)3 CH2CH=CH2 NHC6H4(4-OMe) 141 C(CH3)3 CH2C6H3(2-OMe/4-CI) NHCH2C6H5
14b C(CH3)3 CH2CH=CH2 NHCH2C6H5 14j C(CH3)3 CH2C6H3(2-OMe/4-CI) NH(CH2)20H
14c C(CH3)3 CH2CH=CH2 N(CH2CH2)20 15a C(CH3)3 CH2CH=CH2 OH
14d C(CH3)3 CH2C6H5 NHC6H4(4-OMe) 15b C(CH3)3 CH2C6H5 OH
14e C CH 3 CH2CH NHCH2C H
Structure-Activity Relationships
The Ny- and N(3-regioisomeric pyrazolylthiazoles were assayed for AF508-CFTR
corrector activity. An established cell-based corrector assay was used in
which I- influx was
measured in FRT cells coexpressing human AF508-CFTR and the I--sensitive
fluorescent sensor
YFP-H1 48Q/11 52L. Following 24 h incubation with test compounds, I- influx
was determined from
the kinetics of YFP-H1 48Q/11 52L quenching in response to I- addition in
cells treated with a cAMP
agonist and the potentiator genistein. Out of the fifty-four compounds tested,
eight had significant
corrector activity as judged by concentration-dependent increases in I- influx
as exemplified in
Figure 1 for 14g and 14h.
Structures for these compounds as well as their EC50 and Vmax values from ion
influx data
are summarized in Table 3. Their EC50 values range from 0.93 to 8.5 M while
increasing F influx
up to 7.3 pM/s (note: increased F influx is a quantitative measure of
effective AF508-CFTR
corrector activity). As illustrated in Table 3, pyrazolylthiazole 14h is a
corrector with good activity
among the eight hits. Of the eight active compounds, 10b, an ester, and 11d,
Ny-substituted
pyrazole, are less effective in the assay used as pyrazolylthiazole
correctors. Considering that
ester-containing pyrazolylthiazoles 9 and 10 are inactive in the assay used
except for 10b, the
carboxamide group at C5 of the pyrazole ring seems to be a determinant of
activity. Another
observation from Table 3, that shows the eight hits, is that the majority of
active pyrazolylthiazoles
are N(3-substituted pyrazole isomers; there is an active Ny-substituted
corrector (11d); and there
is an active ester corrector (10b).
43
CA 02736441 2011-04-06
Table 3. Pyrazolylthiazole AF508-CFTR corrector activity.
corrector corrector
structure number ECFn (uM)a Vmax1L~
N-N OJ
O
S 1/
HN~N I 10b 8.5 2.6
Br
N-N HN a OMe
/ O
OHN \NI 11d 3.4 2.5
-
N-N HN a OMe
HN--1,N I / O
14a 0.93 0.5
c / \
N-N HN
O
HN~N 14b 3.0 1.4
N,N HN
/
O S O
HN~N 14e 0.75 1.0
OMe
cI \ J
N-N HN OMe
O S I /
O
HN-<\
14g 1.0 6.1
OMe
CI \ (0
N_N N
/
O
O HN~N I 14h 1.0 7.26
/ OMe
CI \
~OH
N_N HN
O /S O
HN~\N 14j 3.0 2.4
a Concentration where the increased I- influx is 50% of Vmax=
b Vmax is maximum increase in f influx due to compound effect.
44
CA 02736441 2011-04-06
LogP measurements
LogP represents a compound's partition coefficient log value determined from
octanol
versus water, where a smaller logP correlates with better water solubility.
LogP is a well-
established parameter for the ADME profiling as it has implications in
solubility, absorption,
distribution, metabolism, and excretion - which are important for orally
administered drugs - and,
according Lipinski's rule of 5, should generally be <5 for good
bioavailability.
LogP can be related to the experimentally determined capacity factor k by
measuring the
retention time of the compound using reverse-phase HPLC. A standard
calibration curve is
constructed using compounds with known logP values and experimentally
determined logk values
(see diamond data points in Figure 2); this standard calibration curve is then
used to correlate
logk with logP for each pyrazolylthiazole corrector. These data for 11 b and
14a/b/e/g/h/j are
depicted in Figure 2. Most of the active pyrazolylthiazole correctors (squares
in Figure 2) have
logP values of less than 5. The most active pyrazolylthiazole, 14h, has a logP
value of 4.1 and
14j has a logP of 3.5.
Experimental Section
Ye et al., J. Med. Chem. 2010, 53, 3773-3781 is hereby incorporated by
reference in its
entirety.
All purchased starting materials and regents were used without further
purification.
Product purification was performed either on an automated flash chromatography
system
(Combiflash by Teledyne: 35 min of elution with linear gradient from 100%
hexane to 100%
EtOAc solvent) with silica gel columns or on an HPLC system [Waters:1 5 mUmin
flow rate, linear
gradient elution with 0.1% TFA-containing H20/MeCN from 5-95% MeCN in 20 min.
Xterra Prep
MS C18 OBD column (19 mm x 100 mm) and dual wavelength absorbance detector].
NMR
spectra ('H at 600 MHz; 13C at 151 MHz) were recorded in CDCI3 solvent on a
Varian 600.
Chemical shifts are expressed in parts per million relative to internal TMS or
solvent. Coupling
constants are expressed in units of hertz (Hz). Splitting patterns are
designated as s (singlet), d
(doublet), t (triplet), q (quartet), m (multiplet), and bs (broad singlet).
LC/MS (Waters Micromass
ZQ) specifications are as follows: electrospray (+) ionization, mass ranging
from 100 to 900 Da,
20-V cone voltage. LC: Xterra MS C18 column (2.1 mm x 50mm x 3.5 pm), 0.2
mL/min
water/acetonitrile (containing 0.1% TFA), 30 min linear gradient 0-100%
acetonitrile. The LC/MS
UV detector is a diode array with 200-400nm wave length range. Purity is based
on the peak area
percentage of the UV diode array signals. Compound purities by RP-HPLC were
2:95%.
A508-CFTR corrector activity assay.
FRT epithelial cells stably coexpressing human AF508-CFTR and the high-
sensitivity
halide-sensing fluorescent protein YFP-H148Q/1152L were used as described
previously. Cells
were grown at 370 C (95% air / 5% CO2) for 24 h and then incubated for 16 - 20
h with 50 pL of
medium containing the test compound. At the time of the assay, cells were
washed with PBS and
then incubated with PBS containing forskolin (20 M) and genistein (50 M) for
20 min.
Measurements were carried out using aFLUOstar fluorescence plate reader
(Optima; BMG
CA 02736441 2011-04-06
LABTECH Gmbh) equipped with 500 10 nm excitation and 535 15 nm emission
filters
(Chroma Technology Corp.). Each well was assayed individually for F influx by
recording
fluorescence continuously (200 ms per point) for 2 s (baseline) and then for
12 s after rapid (<1
second) addition of 165 pL PBS in which 137 mM Cl- was replaced by F. Initial
F influx rate was
computed exponential regression. All experiments contained negative control
(DMSO vehicle)
and positive control [N-(2-(5-chloro-2-methoxyphenylamino)-4'-methyl-4,5'-
bithiazol-2'-
yl)benzamide].
1-(2-Amino-4-methylthiazol-5-yl)ethanone HCI (6).
Thiazole 6 was prepared as described in Hantzsch A., Thiazoles from thiamides.
Justus
Liebigs Ann. Chem. 1889, 250, 257-73, which is hereby incorporated by
reference in its entirety.
General procedure A: Preparation of 8 via CDI-mediated Amide Formation.
Carboxylic acid (1.75 equiv) was dissolved in DMF (3.3 mUmmol of carboxylic
acid) and
carbonyldiimidazole (CDI; 1.75 equiv) was added slowly to manage CO2
evolution. After all the
CDI had been added, the solution was stirred for an additional 15 min at which
point thiazole HCI
salt 6 (1 equiv). The reaction mixture was warmed to 85 C and stirred for 20
h. After the reaction
was complete, the reaction mixture was cooled to room temperature and poured
into water (17
mL/mmol of carboxylic acid) to precipitate the product, which was then
collected by filtration,
washed with water (3x200m1), and dried under vacuum at 100 C for 18 h to
deliver 7.
N-(5-acetyl-4-methylthiazol-2-yi)pivalamide (7a).
Pivalic acid (5.0 g, 49 mmol) was reacted with the thiazole=HCI 6 (5.4 g, 28
mmol) by
general procedure A and 7a was obtained as an off-white solid (5.3 g, 79%). 1H
NMR (600 MHz,
CDCI3) b 9.06 (s, 1 H), 2.64 (s, 3H), 2.51 (s, 3H), 1.34 (s, 9H); 13C NMR (151
MHz, CDC13) 6
190.66, 176.46, 158.72, 155.21, 125.30, 39.29, 30.44, 27.16 18.08; LC/MS: cal.
[M+H+]= 241.10,
found 241.12.
(Z)-Ethyl 2-hydroxy-4-(4-methyl-2-pivalamidothiazol-5-yl)-4-oxobut-2-enoate
(8a).
To a solution of LHMDS (1 M; 50.3 mL, 50.3 mmol) in THE (50 mL) cooled to -78
C was
slowly added 7a (5.5 g, 22.9 mmol) in dry THE (100 mL) via a syringe and the
mixture was stirred
for 30 min. Diethyl oxalate (3.7 mL, 27.4 mmol) was added quickly in one
portion and the
resulting mixture was stirred at -78 C for 2 h before being being allowed to
warm to room
temperature for another 2 h. When the reaction was completed, water (50 mL)
and 1 N aq. HCI
(50 mL) were sequentially added to the reaction mixture and the product was
extracted with
EtOAc (3 x 100 mL). The combined organic extract was washed with brine twice
and dried over
MgSO4. Filtration and solvent removal under vacuum delivered the crude product
which was
purified on a silica gel column with automated flash chromatography (solvent
system: gradient
hexane/ethyl acetate) to give 8 as a yellow solid (7.22 g, 92%). 1H NMR (600
MHz, CDCI3) 6 9.08
(s, 1 H), 6.70 (s, 1 H), 4.39 (q, J = 6, 2H), 2.71 (s, 3H), 1.40 (t, J = 6,
3H), 1.35 (s, 9H); 13C NMR
(151 MHz, CDCI33) 6 186.24, 176.56, 165.29, 162.01, 160.00, 157.46, 123.06,
101.63, 62.57,
39.33, 27.08, 18.60, 14.12; LC/MS: ESI-MS, cal. [M+H']= 341.12, found 341.04.
46
CA 02736441 2011-04-06
General Procedure B: Preparation of Ny-Substituted Pryazoles, 9 via
Cyclocondensation
with 8.
A mixture of substituted hydrazine (or hydrazine hydrochloride; 1.05
equivalent) and 8 in
absolute ethanol (3.3 mL/mmol of 8) was stirred at room temperature for 18 h.
After the starting
material was consumed as indicated by TLC, the solvent was removed by
rotoevaporation and
the concentrate was extracted with ethyl acetate (3.3 mL/mmol of 8) and washed
with water. The
ethyl acetate layer was dried over Na2SO4, filtered, and concentrated under
reduced pressure to
deliver the crude Ny-substituted pyrazole product, which was then purified by
silica gel column
chromatography (Combiflash).
Ethyl 1-(4-bromophenyl)-5-(4-methyl-2-pivalamidothiazol-5-yl)-1 H-pyrazole-3-
carboxylate
(9b).
Para-bromophenylhydrazine (0.14 g, 0.64 mmol) was reacted with 8a (0.217 g,
0.63
mmol) by general procedure B. After purification, an off-white product was
obtained (0.27 g,
87%). 1H NMR (600 MHz, CDCI3) 6 8.80 (s, 1H), 7.50 (d, J=6, 2H), 7.27 (d, J=
6, 2H), 7.02 (s,
1 H), 4.46 (q, J = 6, 2H), 2.01 (s, 3H), 1.42 (t, J = 6, 3H), 1.33 (s, 9H);
13C NMR (151 MHz, CDCI3)
6 176.30, 162.19, 158.25, 147.48, 144.95, 138.29, 135.34, 132.52, 126.59,
122.64, 113.14,
112.69, 61.58, 39.34, 27-37,15.90, 14.61; LC/MS: cal. [M+H+] and [M+2+H]
=491.08 and
493.08, found 490.97 and 492.86.
General Procedure C: Preparation of 11a-I via Aminolysis of Ny-substituted
Pyrazole Ester
9.
Pyrazolylthiazole 9 (1 equiv) was dissolved in dry DCM (15 mUmmol of 9) and
cooled to
0 C for 10 min. AIMe3 (2.0 equiv) in hexane (1.0 M) was added and the
resulting mixture was
stirred at room temperature for 12 h. When the reaction was complete, water
was added followed
by 0.1 N aq. HCI to neutralized the mixture which was then extracted with
EtOAc. The EtOAc layer
was dried over Na2SO4, filtered, and concentrated under reduced pressure to
give the crude
amide 10, which was then purified by silica gel column chromatography with
Combiflash.
1-(4-Bromophenyl)-N-(4-methoxyphenyl)-5-(4-methyl-2-pivalamidothiazol-5-yl)-1
H-pyrazole-
3-carboxamide (11d).
Pyrazolylthiazole 9b (100 mg, 0.20 mmol) was reacted with anisidine (28 mg,
0.22 mmol)
by general procedure C and an off-white solid product was obtained (85 mg, 73%
yield). 1H NMR
(600 MHz, CDCI3) 6 8.84 (s, 1 H), 8.64 (s, 1 H), 7.61 (d, J = 9.0, 2H), 7.54
(d, J = 8.8, 2H), 7.28 (d,
J = 8.8, 2H), 7.11 (s, 1 H), 6.91 (d, J = 9.0, 2H), 3.82 (s, 3H), 2.05 (d, J =
5.6, 3H), 1.32 (s, 9H);
13C NMR (151 MHz, CDCI3) 6 176.29, 159.15, 158.35, 156.63, 147.90, 147.53,
138.28, 136.09,
132.67, 131.03, 126.38, 122.64, 121.74, 114.46, 112.81, 111.50, 77.43, 77.22,
77.01, 55.71,
39.34, 27.36, 15.93; LC/MS: cal. [M+H+] = 568.10, found 568.10.
Synthesis of ethyl 3-(4-methyl-2-pivalamidothiazol-5-yl)-1 H-pyrazole-5-
carboxylate (13).
2,4-Dioxo-4-(thiazol-5-yl)butanoate 8 (2.0 g, 5.9 mmol) and hydrazine hydrate
(0.40 mL,
6.4 mmol) were dissolved in absolute ethanol (20 mL) and the mixture was
stirred at room
temperature for 20 h. When the reaction was over, the reaction mixture was
concentrated to half
volume by rotoevaporation and the resulting solid was collected by filtration.
This solid residue
47
CA 02736441 2011-04-06
was washed with cold ethanol (x3), dried under vacuum, and used in the next
step without further
purification. A small portion of product remained in the ethanol filtrate,
which was concentrated
under reduced pressure and the resulting solid collected. The two portions of
product were
combined and weighed (1.66 g, 83%).'H NMR (600 MHz, DMSO+CDCI3) 6 13.79 and
11.99 (s
and s, 1 H), 11.77 (s, 1 H), 6.97 and 6.85 (s and s, 1 H), 4.34 and 4.29 (q
and q, J = 7.1, 2H), 2.46
and 2.37 (s and s, 3H), 1.31 (triplet overlapping, J= 7.1, 3H), 1.23 (s, 9H);
13 C NMR (151 MHz,
DMSO+CDCI3) 6 176.83(minor) and 176.53, 161.81 (minor) and 158.79,
156.90(minor) and
156.00, 144.96 (minor) and 144.87, 143.11(minor) and 143.07, 135.30(minor) and
134.57,
116.84, 106.91 and 106.22(minor), 60.93, and 60.15(minor), 40.05(minor) and
38.74, 26.59, 16.38
and 15.92(minor), 14.24 (minor) and 14.15; LC/MS: cal. [M+H+] = 337.13, found
337.05.
General procedure D: Preparation of 10 via Alkylation of the Pyrazolylthiazole
13.
Pyrazolylthiazole 13 (1 equiv), K2CO3 (0.750.85 equiv) and an alkylating agent
were
dissolved in acetone (3.3 mUmmol of 13). The mixture was then refluxed (60 C)
for 24 h. When
the reaction was complete, the reaction mixture was cooled to room temperature
and
concentrated under reduced pressure. Water was added to the resulting mixture,
which was then
extracted with EtOAc (3x). The organic extracts were combined, dried over
MgSO4, filtered, and
concentrated by rotoevaporation. The resulting crude product was then purified
by silica gel
chromatography (Combiflash; Hexane/EtOAc gradient elution).
Ethyl 1-ally)-3-(4-methyl-2-pivalamidothiazol-5-yl)-1 H-pyrazole-5-carboxylate
(10a, R=H,
R2=Allyl).
Allyl bromide (0.172 mL, 1.98 mmol) was reacted with pyrazolylthiazole 13 (0.5
g, 1.49
mmol) by general procedure D. Product 10a was obtained after purification
(0.33 g, 59% yield).
'H NMR (600 MHz, CDCI3) 6 8.71 (s, 1 H), 6.95 (s, 1 H), 6.04 (m, 1 H), 5.19
(m, 3H), 5.14 (dd, J =
1.1, 17.0, 1 H), 4.37 (q, J = 7.1 2H), 2.52 (s, 3H), 1.39 (t, J = 7.1, 3H),
1.33 (s, 9H);13C NMR (151
MHz, CDCI3) 6 175.75, 159.36, 155.65, 143.29, 143.19,133.10, 133.09, 118.20,
117.74, 109.61,
61.21, 54.15, 39.11, 27.21, 16.35, 14.23; LC/MS: cal. [M+H+]=377.17, found
377.13.
Ethyl 1-benzyl-3-(4-methyl-2-pivalamidothiazol-5-yl)-1 H-pyrazole-5-
carboxylate (10a, R=H,
R2=Bn).
Benzyl chloride (0.12 mL, 0.96 mmol) was reacted with pyrazolylthiazole 13
(0.27 g, 0.80
mmol) by general procedure D. Product 10b was obtained after purification
(0.23 g, 66%). 1H
NMR (600 MHz, CDCI3) 6 8.74 (s, 1 H), 7.31 (m, 5H), 6.96 (s, 1 H), 5.76 (s,
2H), 4.33 (q, J = 7.1,
2H), 2.51 (t, J = 7.1 3H), 1.33 (s, 9H); 13C NMR (151 MHz, CDCI3) 6 175.98,
159.62, 155.77,
143.82, 143.61, 137.08, 133.27, 128.89, 128.70, 128.46, 128.06, 128.05,
127.96, 118.51, 109.99,
61.40, 60.59, 55.22, 39.31, 27.43, 21.26, 16.73, 14.41, 0.21; LC/MS: cal.
[M+H+] = 427.18, found
427.08.
Ethyl 1-(5-chloro-2-methoxybenzyl)-3-(4-methyl-2-pivalamidothiazol-5-yl)-1 H-
pyrazole-5-
carboxylate (10a, R=H, R2=CH2(2-MeO-5-Clphenyl)).
2-(Bromomethyl)-4-chloro-1-methoxybenzene (0.37 g, 1.57 mmol) was reacted with
13
(0.5 g, 1.49 mmol) by general procedure D. Product 10c was obtained after
purification (0.37 g,
51 %).1 H NMR (600 MHz, CDCI3) 6 8.70 (s, 1 H), 7.17 (dd, J = 2.6, 8.7, 1 H),
7.02 (s, 1 H), 6.79 (d,
48
CA 02736441 2011-04-06
J = 8.7, 1 H), 6.59 (d, J = 2.6, 1 H), 5.76 (s, 2H), 4.32 (q, J = 7.1, 2H),
3.86 (s, 3H), 2.53 (s, 3H),
1.34 (t, J= 7.1, 3H), 1.32 (s, 9H); 13C NMR (151 MHz, CDC13)
6175.90,159.47,155.74,155.25,
144.02, 143.96, 134.06, 128.38, 128.00, 127.27, 125.79, 118.39, 111.59,
110.01, 61.48, 56.01,
50.28, 39.31, 27.45, 16.75, 14.36; LC/MS: purity and Calculated [M+H`] and
[M+2+H+] =491.15
and 493.14, found 491.10 and 493.08.
General Procedure E: Preparation of 14a-i via Aminolysis of 10 with non-
alcoholic amines.
To a solution of ethyl ester 10 (1 equiv) in dry DCM (10 mUmmol of 10), which
was
chilled at 0 C in a sealed microwave reaction vessel, was added 2.0 M
trimethyl aluminum (1.2
equiv) in hexanes. The resulting mixture was stirred at 0 C for 10 min and
then the amine
reactant (1.2 equiv) in dry DCM (1 mUmmol of 10) was injected into the
mixture. The reaction
tube was then mounted to the microwave reactor and irradiated with microwave
at 100 C for 40
min. After cooling, water and 0.1 N aq. HCI were added to the reaction mixture
sequentially to
neutralize the solution. DCM extraction (3x), drying over MgSO4, filtration,
and rotoevaporation
gave a residue which purified by silica gel chromatography (CombiFlash).
1-Allyl-N-(4-methoxyphenyl)-3-(4-methyl-2-pivalamidothiazol-5-yl)-1 H-pyrazole-
5-
carboxamide (14a).
Ethyl ester 10a (100 mg, 0.27 mmol) was reacted with anisidine (40 mg, 33
mmol) by
general procedure E and gave 14a (45 mg, 67%). 1H NMR (600 MHz, CDC13) b 8.80
(s, 1H), 7.83
(s, 1 H), 7.50 (d, J = 8.7, 2H), 6.91 (d, J = 9.0, 2H), 6.73 (s, 1 H), 6.08
(ddd, J = 5.8, 10.9, 16.1,
1H), 5.23-5.10 (m, 4H), 3.81 (s, 3H), 2.51 (s, 3H), 1.32 (s, 9H); 13C NMR (151
MHz, CDC13) b
176.02, 157.72, 157.19, 155.66, 143.92, 143.35, 136.48, 133.56, 130.21,
124.50, 122.59, 118.31,
118.09, 114.53, 105.41, 77.46, 77.25, 77.04, 55.74, 54.19, 39.33, 27.44,
16.71; LC/MS: cal.
[M+H'] = 454.19, found 454.16.
1-allyl-N-benzyl-3-(4-methyl-2-pivalamidothiazol-5-yl)-1 H-pyrazole-5-
carboxamide (14b).
Ethyl ester 10a (100 mg, 0.27 mmol) was reacted with benzylamine (36 uL, 33
mmol) by
general procedure E and gave 14b (85 mg, 91% yield). 1H NMR (600 MHz, CDCI3) 6
8.93 (s, 1H),
7.30 (m, 4H), 6.87 (t, J = 5.6, 1 H), 6.64 (s, 1 H), 6.02 (ddt, J = 5.7, 11.3,
17.0, 1 H), 5.12 (m, 4H),
4.59 (d, J= 5.8, 2H), 2.46 (s, 3H), 1.29 (t, J= 2.9, 9H); 13C NMR (151 MHz,
CDCI3) 6 176.09,
159.69, 155.66, 143.74, 143.20, 137.89, 136.22, 133.66, 128.98, 127.98,
127.89, 118.34, 117.82,
105.52, 54.04, 43.75, 39.28, 27.38, 16.63; LC/MS: cal. [M+H']=438.20, found
438.13.
Synthesis of N,1-dibenzyl-3-(4-methyl-2-pivalamidothiazol-5-yl)-1 H-pyrazole-5-
carboxamide
(14e).
Ethyl ester 1Ob (100 mg, 0.23 mmol), benzylamine (0.51 mL, 4.7 mmol), and
sodium
cyanide (5.3 mg, 0.1 mmol) were mixed in MeOH (8 mL) and refluxed for 18 h.
Upon cooling, the
methanol was removed by rotoevaporation and the residue was taken up in EtOAc
(50 mL),
wahed with water (50 mL; 3x) and 0.1N aq. HCl (50 mL), dried over Na2SO4,
filtered, and
concentrated under reduced pressure. The concentrate was purified by silica
gel chromatography
(Combiflash), giving pure product of 14e (31.9 mg, 34.41%). 'H NMR (600 MHz,
CDCI3) 6 8.71 (s,
1 H), 7.37-7.23 (m, 1 OH), 6.58 (s, 1 H), 6.24 (t, J = 5.6, 1 H), 5.79 (s,
2H), 4.58 (d, J = 5.8, 2H), 2.50
(s, 3H), 1.32 (s, 9H); 13C NMR (151 MHz, CDCI3) 6 175.94, 159.67, 155.63,
143.79, 143.36,
49
CA 02736441 2011-04-06
137.70, 137.31, 136.12, 129.07, 128.71, 128.33, 128.00, 127.99, 127.94,
118.46, 105.32, 54.98,
43.80, 39.31, 27.44, 16.74; LC/MS: cal. [M+H']= 488.21, found 488.20.
1-(5-chloro-2-methoxybenzyl)-N-(4-methoxyphenyl)-3-(4-methyl-2-
pivalamidothiazol-5-yl)-
1H-pyrazole-5-carboxamide (14g).
Ethyl ester 10c(30 mg, 0.061 mmol) was reacted with anisidine (12 mg, 0.098
mmol) by
gerenal procedure E to give 14g (26 mg, 75%). 'H NMR (600 MHz, CDCI3) 5 8.83
(s, 1 H), 7.76
(s, 1 H), 7.48 (d, J = 8.5, 2H), 7.16 (dd, J = 2.6, 8.7, 1 H), 6.89 (d, J =
9.0, 2H), 6.79 (d, J = 2.5,
1 H), 6.75 (d, J = 8.7, 2H), 5.78 (s, 2H), 3.80 (s, 3H), 3.78 (s, 3H), 2.51
(s, 3H), 1.32 (s, 9H). 13C
NMR (151 MHz, CDCI3) 6 176.00, 157.68, 157.18, 155.73, 155.47, 144.06, 143.84,
130.26,
128.52, 128.15, 127.91, 125.76, 122.42, 118.27, 114.53, 111.67, 105.38, 55.97,
55.71, 49.90,
39.32, 27.43, 16.71; LC/MS: cal. [M+H+]=568.18, found 568.17.
N-(5-(1-(5-chloro-2-methoxybenzyl)-5-(morpholine-4-carbonyl)-1 H-pyrazol-3-yl)-
4-
methylthiazol-2-yl)pivalamide (14h).
Ethyl ester 10c (30 mg, 0.061 mmol) was reacted with morpholine (7 uL, 0.091
mmol) by
gerenal procedure E to give 14h (28 mg, 87%). 1H NMR (600 MHz, CDCI3) 6 8.74
(s, 1H), 7.19
(dd, J = 2.6, 8.7, 1 H), 6.86 (d, J = 2.6, 1 H), 6.77 (d, J = 8.7, 1 H), 6.38
(d, J = 9.6, 1 H), 5.50 (s,
2H), 3.79 (d, J = 12.0, 3H), 3.66 (d, J = 24.8, 4H), 3.45 (s, 4H), 2.50 (s,
3H), 1.31 (s, 9H); 13C
NMR (151 MHz, CDCI3) 6 175.95, 160.92, 155.76, 155.55, 143.95, 143.31, 136.09,
128.96,
128.83, 127.45, 125.67, 118.40, 111.95, 105.67, 66.82, 56.16, 49.29, 39.31,
27.43, 16.72;
LC/MS: cal. [M+H'] = 532.18, found 532.14.
General procedure F for Preparation of amide 11rn-p and 14j: Aminolysis of 9
or 10 with
alcoholic amine.
Pyrazolylthiazole 9 or 13 was dissolved in dry ethanol (4 mL) in a 10 mL
microwave
reaction tube. Ethanolamine (20 equivalent) was added to the solution, the
tube was sealed,
placed in a microwave reactor, and the reaction mixture was heated at 180 C
for 30 minutes.
After the tube had cooled, ethanol was removed under reduced pressure, aq.
NH4CI was added,
and the mixture was extracted with chloroform (x3). The chloroform extracts
were combined and
the solvent removed under reduced pressure to give a crude product, which was
purified with
HPLC.
1-(5-ch to ro-2-methoxybenzyl)-N-(2-hyd roxyethyl)-3-(4-methyl-2-
pivalamidothiazol-5-yl)-1 H-
pyrazole-5-carboxamide (14j).
Following general procedure F, 14j was obtained in 33% yield. 1H NMR (600 MHz,
CDCI3) 6 7.19 (dd, J = 2.6, 8.7, 1 H), 6.80 (d, J = 8.8, 1 H), 6.73 (d, J =
2.6, 1 H), 6.72 (s, 1 H), 6.52
(s, 1 H), 5.76 (s, 2H), 3.85 (s, 3H), 3.82 (t, J = 5.4, 2H), 3.59 (dd, J =
5.5, 10.3, 2H), 2.60 (s, 3H),
1.37 (s, 9H); 13C NMR (151 MHz, CDCI3) 6 178.26, 160.36, 159.63, 155.31,
140.97, 137.49,
134.35, 128.57, 127.81, 127.17, 125.52, 117.96, 111.65, 104.79, 77.35, 77.22,
77.01, 76.80,
61.75, 55.85, 49.93, 42.12, 39.94, 26.56, 13.43; LC/MS: cal. [M+H`] = 506.16,
found 506.15.