Note: Descriptions are shown in the official language in which they were submitted.
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
PYRIMIDINONE DERIVATIVES FOR USE AS MEDICAMENTS
Field of the Invention
The present invention is directed to substituted pyrimidinone and/or
tetrahydropyrimidinone compounds and their uses as therapeutic agents,
especially
PDE4 inhibitors.
Background of the Invention
The Inflammatory Response (Inflammation)
Inflammation is an essential localized host response to invading
microorganisms or
tissue injury which involves cells of the immune system. The classic signs of
inflammation include redness (erythema), swelling (edema), pain and increased
heat
production (pyrema) at the site of injury. The inflammatory response allows
the body to
specifically recognize and eliminate an invading organism and/or repair tissue
injury.
Many of the acute changes at the site of inflammation are either directly or
indirectly
attributable to the massive influx of leukocytes (e.g., neutrophils,
eosinophils,
lymphocytes, monocytes) which is intrinsic to this response. Leukocytic
infiltration and
accumulation in tissue results in their activation and subsequent release of
inflammatory
mediators such as LTB4, prostaglandins, TNF-a, IL-1(3, IL-8, IL-5, IL-6,
histamine,
proteases and reactive oxygen species for example.
Normal inflammation is a highly regulated process that is tightly controlled
at several
levels for each of the cell types involved in the response. For example,
expression of the
pro-inflammatory cytokine TNF-a is controlled at the level of gene expression,
translation, post-translational modification and release of the mature form
from the cell
membrane. Many of the proteins up-regulated during inflammation are controlled
by the
transcription factor, NF-KB. Pro-inflammatory responses are normally countered
by
endogenous anti-inflammatory mechanisms such as generation of IL-10 or IL-4.,A
characteristic of a normal inflammatory response is that it is temporary in
nature and is
followed by a resolution phase which brings the state of the tissue back to
its prior
condition. The resolution phase is thought to involve up-regulation of anti-
inflammatory
mechanisms, such as IL-10, as well as down-regulation of the proinflammatory
processes.
1
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Inflammatory Disease
Inflammatory disease occurs when an inflammatory response is initiated that is
inappropriate and/or does not resolve in the normal manner but rather persists
and
results in a chronic inflammatory state. Inflammatory disease may be systemic
(e.g.
lupus) or localized to particular tissues or organs and exerts an enormous
personal and
economic burden on society. Examples of some of the most common and
problematic
inflammatory diseases are rheumatoid arthritis, inflammatory bowel disease,
psoriasis,
asthma, chronic obstructive pulmonary disease, emphysema, colitis and ischemia-
reperfusion injury.
A common underlying theme in inflammatory disease is a perturbation of the
cellular
immune response that results in recognition of host proteins (antigens) as
foreign. Thus
the inflammatory response becomes misdirected at host tissues with effector
cells
targeting specific organs or tissues often resulting in irreversible damage.
The self-
recognition aspect of auto-immune disease is often reflected by the clonal
expansion of
T-cell subsets characterized by a particular T-cell receptor (TCR) subtype in
the disease
state. Often inflammatory disease is also characterized by an imbalance in the
levels of
T-helper (Th) subsets (i.e., Thl cells vs. Th2 cells).
Therapeutic strategies aimed at curing inflammatory diseases usually fall into
one of two
categories: (a) down-modulation of processes that are up-regulated in the
disease state
or (b) up-regulation of anti-inflammatory pathways in the affected cells or
tissues. Most
regimes currently employed in the clinic fall into the first category. Some
examples of
which are corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs).
Many of the tissue, cellular and biochemical processes which are perturbed in
inflammatory disease have been elucidated and this has allowed the development
of
experimental models or assays to mimic the disease state. These in-vitro
assays
enable selection and screening of compounds with a high probability of
therapeutic
efficacy in the relevant inflammatory disease. Thus, currently employed assays
used to
model the importance of the activated leukocytes in the development of acute
inflammation and maintenance of the chronic inflammatory state are assays
monitoring
leukocyte chemotaxis and cellular degranulation and cytokine synthesis and
reactive
oxygen species (ROS) production assays in vitro. Since a result of acute or
chronic
neutrophil activation is release of ROS with resultant tissue damage, an assay
for
scavengers of ROS allows detection of compounds with potential therapeutic
efficacy.
2
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Cellular assays to detect inhibitors of TNF-a release from stimulated
macrophage or
monocytic cells are an important component of an in vitro model for
inflammation as this
cytokine is upregulated and has been shown to contribute to the pathology in
many
inflammatory diseases. Since elevated cAMP in affected cells has been shown to
modulate or dampen the inflammatory response, monitoring cellular cyclic AMP
(cAMP)
levels, and the activity of pathways controlling cAMP levels allows for the
detection of
potential anti-inflammatory compounds. Assays may include monitoring the level
of
cAMP itself, phosphodiesterase activity, or changes in cAMP response element
(CRE)-
luciferase activity.
Cyclic Nucleotide Messengers and Phosphodiesterases
The cyclic nucleotides, cyclic adenosine monophosphate (CAMP) and cyclic
guanosine
monophosphate (cGMP), play a key role in regulating cell function and
phosphodiesterases (PDEs) provide the main route for the degradation of cyclic
nucleotides. cAMP is now known to control the functional and genomic responses
for a
variety of cellular functions triggered by a wide array of receptors (Beavo,
J.A. and
Brunton, L.L., Nat. Rev. Mol. Cell Biol., 3, 710-718 (2002)). Local control of
cAMP
signalling is affected by a complex pattern of localized synthesis, by
adenylate cyclise
(AC), and by phosphodiesterase (PDE)-mediated enzymatic degradation.
The PDEs are a family of enzymes that catalyze the hydrolysis of 3',5'-cyclic
nucleotides
to 5' nucleoside monophosphates, including the conversion of cAMP to AMP and
cGMP
to GMP. PDE enzymes are collectively grouped as a superfamily of eleven
different, but
homologous, gene-families with a highly conserved catalytic domain (Soderling,
S.H. and
Beavo, J.A., Curr. Opin. Cell Biol., 12, 174-179 (2000)). At present twenty-
one different
mammalian PDE genes have been identified. Many of these genes are expressed in
multiple isoforms either by differing initiation sequences or splicing
patterns.
Differentiation of the enzymes can be achieved on the basis of substrate
specificity,
kinetic properties and sensitivity to regulatory molecules. PDEs in families
5, 6 and 9
specifically catalyze the hydrolysis of cGMP while PDEs 4, 7 and 8 are
specific for
cAMP. Enzymes belonging to the other PDE families (1, 2, 3, 10 and 11)
catalyze the
hydrolysis of both cAMP and cGMP with differing kinetics. Different PDE
isozymes can
have specific tissue, cellular and subcellular distributions and more than one
type of PDE
is usually present in any given cell. The types of PDEs= expressed in a cell,
together with
3
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
their relative proportions and subcellular localization, control the cyclic
nucleotide
phenotype of that cell.
The PDE4 enzyme is responsible for selective, high affinity hydrolytic
degradation of the
second messenger cAMP, has a low Michaelis constant and is sensitive to
inhibition by
rolipram. The PDE4 enzyme family consists of four genes, which produce 4
isoforms of
the PDE4 enzyme (PDE4A, PDE4B, PDE4C, and PDE4D) (Wang et al., "Expression,
Purification, and Characterization of human cAMP Specific Phosphodiesterase
(PDE4)
Subtypes A, B, C, and D, Biochem", Biophys. Res. Comm., 234, 320-324 (1997)).
Moreover, various splice variants of each PDE4 isoform have been identified
and play a
role in the compartmentalized cAMP signalling in cells (Houslay, M.D.,
Schafer, P., and
Zhang, K.Y., Drug Discov. Today, 15;10(22):1503-19 (2005)). Recently, a number
of
selective PDE4 inhibitors have been discovered to have beneficial
pharmacological
effects resulting from PDE4 inhibition as shown in a variety of disease models
(Torphy et
al., Environ. Health Perspect., 102 Suppl. 10, 79-84, 1994; Duplantier et al.,
J. Med.
Chem., 39 120-125 (1996); Schneider et al., Pharmacol. Biochem. Behav., 50,
211-217
(1995); Banner and Page, Br. J. Pharmacol., 114, 93-98 (1995); Barnette et
al., J.
Pharmacol. Exp. Ther., 273, 674-679 (1995); Wright et al., "Differential in
vivo and in vitro
bronchorelaxant activities of CP-80633, a selective phosphodiesterase 4
inhibitor," Can.
J. Physiol. Pharmacol., 75, 1001-1008 (1997); Manabe et al., "Anti-
inflammatory and
bronchodilator properties of KF19514, a phosphodiesterase 4 and 1 inhibitor,"
Eur. J.
Pharmacol., 332, 97-107 (1997); and Ukita et al., "Novel, potent, and
selective
phosphodiesterase-4 inhibitors as antiasthmatic agents: synthesis and
biological
activities of a series of 1-py_ridylnaphthalene derivatives," J. Med. Chem.,
42, 1088-1099
(1999)). Therefore, considerable interest exists in the discovery of
additional selective
inhibitors of PDE4.
Regulation of cAMP activity is important in many biological processes,
including
inflammation, depression and cognitive function. Chronic inflammation is a
multitude of
heterogeneous diseases characterized in part by activation of multiple
inflammatory
cells, particularly cells of lymphoid lineage (including T lymphocytes) and
myeloid lineage
(including granulocytes, macrophages, and monocytes). Activation of these
inflammatory
cells results in production and release of proinflammatory mediators,
including cytokines
and chemokines, such as tumor necrosis factor (TNF) and interleukin-1 (IL-1).
Discovery
of a molecule that suppresses or inhibits such cellular activation and
proinflammatory
mediator release would be useful in the therapeutic treatment of inflammatory
diseases.
Elevated cAMP levels suppress inflammatory cell activation. Increased cAMP
levels
4
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
associated with PDE4 inhibition has therefore become a valid potential
therapeutic
approach to control inflammatory responses and disorders (Beavo et al.,
"Cyclic
Nucleotide Phosphodiesterases: Structure, Regulation and Drug Action," Wiley
and
Sons, Chichester, pp. 3-14 (1990); Torphy et al., Drug News and Perspectives,
6, pp.
203-214 (1993); Giembycz et al., Clin. Exp. Allergy, 22, pp. 337-344 (1992);
and Sanz,
M.J., Cortijo, J., Morcillo, E.J., Pharmacol Ther. 106(3):269-97 (2005)).
PDE4 inhibitors have recently shown clinical utility in mitigating the effects
of the chronic
pulmonary inflammatory diseases of asthma and chronic obstructive pulmonary
disease
(COPD). Roflumilast, a selective PDE4 inhibitor, demonstrated improvements in
measures of airway function (forced expiratory volume in 1 second; FEV1, and
peak
expiratory flow; PEF) in mild asthmatics in a recently published clinical
trial of 12 weeks
duration (Bateman et al., Ann. Allergy Asthma Immunol., 96(5): 679-86 (2006)).
A
separate study with roflumilast also demonstrated improvements in airway hyper-
responsiveness (AHR) to direct histamine provocation in a similar group of
mild
asthmatics in response to allergen challenge (Louw et al., Respiration, Sept.
5 2006).
Recently published results of a long term (6 month) study of cilomilast
treatment in
patients with COPD indicated that treatment with a selective PDE4 inhibitor
arrested
airway function (FEV1) decline in these patients and positively affected their
quality of life
as measured by the St. Georges Respiratory Questionnaire (Rennard et al.,
Chest,
129(1) 65-66 (2006)).
The clinical usefulness of PDE4 inhibition has also been demonstrated in
disorders of the
central nervous system. PDE4 inhibition by rolipram improves cognitive
function in
rodents and was developed as an antidepressant in humans. cAMP acts as a
second
messenger for neurotransmitters, and thus mediates their cellular responses.
The
therapeutic effects of PDE4 inhibitors in cognition and depression likely
originate from
enhancement of the cAMP-dependent cellular responses.
The listing or discussion of an apparently prior-published document in this
specification
should not necessarily be taken as an acknowledgement that the document is
part of the
state of the art or is common general knowledge.
International patent application WO 2007/081570 discloses various compounds
that may
be useful in the treatment of cholesterol-related diseases. However, there is
no
disclosure that such compounds may be useful as phosphodiesterase 4
inhibitors, and
5
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
therefore in the treatment of inflammation. Further, there is no specific
disclosure of
substituted pyrimidinones and tetrahydropyrimidinones.
International patent application WO 2006/124874 discloses a broad range of
inter alia
heterocyclic compounds that may be of use as inhibitors of B-Raf, and
therefore of use in
the treatment of cancer. There is no specific disclosure in that document of
substituted
pyrimidinones and tetrahydropyrimidinones.
US patents/applications US 6,162,927, US 2002/0055457, US 7,208,517 and US
2007/0203124, international patent applications WO 2002/11713, WO 2002/011713,
WO
99/006397, WO 96/006095, WO 97/030045, WO 02/017912, WO 2005/115389 and WO
95/028926 and European patent EP 299 549 all disclose various compounds,
including
heterocycles, which may be useful as medicaments. However, there is no
disclosure in
any of these documents of substituted pyrimidinones and/or
tetrahydropyrimidinones.
Further, US patent applications US 2003/0186943 and US 2004/0224316 and
international patent applications WO 00/14083, WO 2004/031149, WO 2007/137181,
WO 2004/091609 and WO 2004/016227 disclose inter alia piperidinones that may
be
useful in the treatment of inflammation-based diseases. However, these
documents do
not disclose substituted pyrimidinones and/or tetrahydropyrimidinones.
International patent application WO 01/68600 discloses various compounds,
including
pyrrolidinones and tetrahydropyrimidinoes, which may be useful in the
treatment of
inflammation-based diseases. However, there is no disclosure of pyrimidinones
and
tetrahydropyrimidinones substituted (via a linker) with certain heteroaryl or
heterocycloalkyl groups.
International patent application WO 2007/110793 discloses various compounds,
including piperidinones that may be useful as PDE inhibitors. However, there
is no
3o disclosure in that document of pyrimidinones.
6
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Disclosure of the Invention
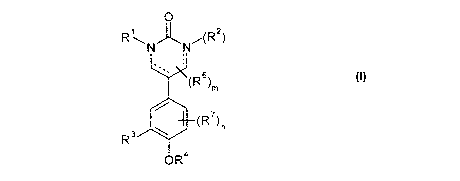
According to the invention, there is now provided a compound of formula (I),
0
RNN -(R2
(R6)
M
(R) n
R3
OR4
wherein:
the dotted lines each independently represent an optional bond (and when the
dotted line
between the carbon and nitrogen is present, then R2 is absent, and when the
dotted line
between the carbon and nitrogen is absent, then R2 is present);
m represents 0, 1, 2, 3, 4 or 5;
n represents 0, 1, 2 or 3;
at least one of R1 and, if present, R2 represents -A'-Tz-B' and the other (if
present)
represents R5;
R3 represents hydrogen, -OR4a, C,_12 alkyl (optionally substituted by one or
more
substituents selected from =0 and X) or -B2;
R4 and R41 independently represent hydrogen, C1.12 alkyl (optionally
substituted by one or
more substituents selected from =0 and X2) or -B3;
R5 represents hydrogen, C1_12 alkyl (optionally substituted by one or more
substituents
selected from =0 and X3) or -B 3a;
each R6 and each R7 independently represent X4, C1.12 alkyl (optionally
substituted by
one or more substituents selected from =0 and X5) or -B4; or
7
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
any two R6 groups may be linked together to form a further ring, which is
formed either
by the two relevant groups being linked together by a direct bond or C1.5
alkylene;
A' represents C1_12 alkylene (optionally substituted by one or more
substituents selected
from =0 and X6);
TZ represents a direct bond, -N(R")- or -C(O)N(R12)_;
R"`' and R"'2 independently represent hydrogen, C1_12 alkyl (optionally
substituted by one
or more substituents selected from X) or -B5;
B1 represents:
1) a monocyclic 5-membered.heteroaryl group;
2) a polycyclic heteroaryl group;
3) a polycyclic aryl group; or
4) a heterocycloalkyl group,
all four of which are optionally and independently substituted with one or
more
substituents selected from X6 and, in the case of heterocycloalkyl or any non-
aromatic
rings of a polycyclic aryl or heteroaryl group, =O;
B2, B3 and B3a independently represent aryl (optionally substituted by one or
more
substituents selected from X9), heterocycloalkyl (optionally substituted by
one or more
substituents selected from =0 and X10) or heteroaryl (optionally substituted
by one or
more substituents selected from X");
B4 and B5 independently represent heterocycloalkyl (optionally substituted by
one or
more substituents selected from =0 and X12);
X1, X2, X3, X4, X5, X6, X7, X8, X9, X10 X" and X12 independently represent B6,
halo, -CN,
-NO2, -Si(R8a)3, -OR 9a, -OC(O)-R 9b, -N(R9c)R9d, -C(O)R9e, -C(O)OR9f, -
C(O)N(R99)R9h,
-N(R9i)C(O)OR11b, -N(R9')C(O)R8c, -N(R9k)S(O)tR8d -S(O)tORBe, -S(O)pR8f,
-S(O)tN(R9m)R9n, -N(R9p)C(O)N(R9')R9', -N(R9S)S(O)tOR89, -OC(O)N(R9t)R9u
and/or
-OS(O)tR8h;
R8a, R8b, R8d, R8f, R89 and R8h independently represent C1_12 alkyl optionally
substituted by
one or more substituents selected from =0 and E';
8
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Be 9b 9d 9e 9n 9k 9n 9G 9r Rsc RR9a RR9c RRR9f R9g RR9f R9j RR9m RR9p RRR
R9t
and R9u independently represent hydrogen or C1.12 alkyl optionally substituted
by one or
more substituents selected from =0 and E2; or
any pair of R9C and R9d, R99 and R9h, R9m and R9n, R9q and R9r, and R9t and
R9u may be
linked together with the nitrogen atom to which they are attached to form a 3-
to 8-
membered ring, optionally containing one or more (e.g. one or two)
unsaturations (e.g.
double bonds), optionally containing one or two (e.g. one) further heteroatoms
(preferably selected from nitrogen and oxygen), and which ring is optionally
substituted
by one or more substituents selected from =0, halo and C1_6 alkyl optionally
substituted
by one or more halo atoms;
B6 represents C1_12 alkyl, heterocycloalkyl (which latter two groups are
optionally
substituted by one or substituents selected from =0 and E3), aryl or
heteroaryl (which
latter two groups are optionally substituted by one or substituents selected
from E4);
t represents, at each occurrence when used herein, 1 or 2;
p represents 0, 1 or 2;
E', E2, E3 and E4 independently represent halo, -CN, -NO2, -OR10a, -OC(O)-R'0b
-N(R1oC)Riod -C(O)R1oe, -C(O)OR10f, -C(O)N(R1o9)R1Oh _N(R10)C(O)OR'1a,
-N(R'oj)C(0)R11b _N(Rbok)S(O)t1R11o -S(O)t,OR11d -S(O)p1R>>e
_S(O)t1N(Riom)RiOn
-N(R10p)C(O)N(R10q)R10r, -N(R10S)S(O)t1OR11f, -OC(O)N(R10)R10u, -OS(O)t1R1
and/or
-Si(R11h)3;
Rtoa Riob Rtoc Rlod Rioe R1of R109 Rton R10 R10j R1ok Worn Rton Rio Rioq Rior
R10s
Riot R10u R1 lb and R"d independently represent hydrogen or C1.3 alkyl
optionally
substituted by one or more halo atoms;
3o R"a, R11C, R7e, R"f R119 and R1 1h independently represent C1.3 alkyl
optionally
substituted by one or more halo atoms;
t1 represents, at each occurrence when used herein, 1 or 2;
p1 represents 0, 1 or 2,
or a pharmaceutically acceptable salt thereof,
9
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
which compounds are hereinafter referred to as the "compounds of the
invention".
Pharmaceutically-acceptable salts include acid addition salts and base
addition salts.
Such salts may be formed by conventional means, for example by reaction of a
free acid
or a free base form of a compound of formula I with one or more equivalents of
an
appropriate acid or base, optionally in a solvent, or in a medium in which the
salt is
insoluble, followed by removal of said solvent, or said medium, using standard
techniques (e.g. in vacuo, by freeze-drying or by filtration). Salts may also
be prepared
by exchanging a counter-ion of a compound of the invention in the form of a
salt with
another counter-ion, for example using a suitable ion exchange resin.
Compounds of the invention may contain double bonds and may thus exist as E
(entgegen) and Z (zusammen) geometric isomers about each individual double
bond. All
such isomers and mixtures thereof are included within the scope of the
invention.
Compounds of the invention may also exhibit tautomerism. All tautomeric forms
and
mixtures thereof are included within the scope of the invention.
Compounds of the invention may also contain one or more asymmetric carbon
atoms
and may therefore exhibit optical and/or diastereoisomerism. Diastereoisomers
may be
separated using conventional techniques, e.g. chromatography or fractional
crystallisation. The various stereoisomers may be isolated by separation of a
racemic or
other mixture of the compounds using conventional, e.g. fractional
crystallisation or
HPLC, techniques. Alternatively the desired optical isomers may be made by
reaction of
the appropriate optically active starting materials under conditions which
will not cause
racemisation or epimerisation (i.e. a 'chiral pool' method), by reaction of
the appropriate
starting material with a 'chiral auxiliary' which can subsequently be removed
at a suitable
stage, by derivatisation (i.e. a resolution, including a dynamic resolution),
for example
with a homochiral acid followed by separation of the diastereomeric
derivatives by
conventional means such as chromatography, or by reaction with an appropriate
chiral
reagent or chiral catalyst all under conditions known to the skilled person.
All
stereoisomers and mixtures thereof are included within the scope of the
invention.
Unless otherwise specified, Cl., alkyl, and C1_q alkylene, groups (where q is
the upper
limit of the range), defined herein may be straight-chain or, when there is a
sufficient
number (i.e. a minimum of two or three, as appropriate) of carbon atoms, be
branched-
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
chain, and/or cyclic (so forming, in the case of alkyl, a C3_q cycloalkyl
group or, in the
case of alkylene, a C3_q cycloalkylene group). Further, when there is a
sufficient number
(i.e. a minimum of four) of carbon atoms, such groups may also be part cyclic.
Further,
unless otherwise specified, such alkyl groups may also be saturated or, when
there is a
sufficient number (i.e. a minimum of two) of carbon atoms and unless otherwise
specified, be unsaturated (forming, for example, in the case of alkyl, a C2.q
alkenyl or a
C2-q alkynyl group or, in the case of alkylene, a C2_q alkenylene or a C2_q
alkynylene
group). In the case of alkylene groups, it is preferred that they are acyclic
and/or
straight-chain, but may be saturated or unsaturated.
The term "halo", when used herein, includes fluoro, chloro, bromo and iodo.
Heterocycloalkyl groups that may be mentioned include non-aromatic monocyclic,
bicyclic and tricyclic (e.g. monocyclic or bicyclic) heterocycloalkyl groups
(which groups
may further be bridged) in which at least one (e.g. one to four) of the atoms
in the ring
system is other than carbon (i.e. a heteroatom), and in which the total number
of atoms
in the ring system is between three and twelve (e.g. between five and ten).
Further, such
heterocycloalkyl groups may be saturated or unsaturated containing one or more
double
and/or triple bonds, forming for example a C2_q heterocycloalkenyl (where q is
the upper
limit of the range) or a C7_q heterocycloalkynyl group. C2_q heterocycloalkyl
groups that
may be mentioned include 7-azabicyclo-[2.2.1]heptanyl, 6-
azabicyclo[3.1.1]heptanyl, 6-
azabicyclo[3.2. 1 ]-octanyl, 8-azabicyclo[3.2.1]octanyl, aziridinyl,
azetidinyl,
dihydropyranyl, dihydropyridyl, dihydropyrrolyl (including 2,5-
dihydropyrrolyl), dioxolanyl
(including 1,3-dioxolanyl), dioxanyl (including 1,3-dioxanyl and 1,4-
dioxanyl), dithianyl
(including 1,4-dithianyl), dithiolanyl (including 1,3-dithiolanyl),
imidazolidinyl, imidazolinyl,
morpholinyl, 7-oxabicyclo[2.2.1 ]heptanyl, 6-oxabicyclo[3.2.1 ]-octanyl,
oxetanyl, oxiranyl,
piperazinyl, piperidinyl, pyranyl, pyrazolidinyl, pyrrolidinonyl,
pyrrolidinyl, pyrrolinyl,
quinuclidinyl, sulfolanyl, 3-sulfolenyl, tetrahydropyranyl, tetrahydrofuranyl,
tetrahydropyridyl (such as 1,2,3,4-tetrahydropyridyl and 1,2,3,6-
tetrahydropyridyl),
thietanyl, thiiranyl, thiolanyl, thiomorpholinyl, trithianyl (including 1,3,5-
trithianyl), tropanyl
and the like. Substituents on heterocycloalkyl groups may, where appropriate,
be
located on any atom in the ring system including a heteroatom. Further, in the
case
where the substituent is another cyclic compound, then the cyclic compound may
be
attached through a single atom on the heterocycloalkyl group, forming a so-
called
"spiro"-compound. The point of attachment of heterocycloalkyl groups may be
via any
atom in the ring system including (where appropriate) a heteroatom (such as a
nitrogen
atom), or an atom on any fused carbocyclic ring that may be present as part of
the ring
11
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
system. Heterocycloalkyl groups may also be in the N- or S- oxidised form.
Most
preferably, heterocycloalkyl groups that may be mentioned include 5- or 6-
membered
monocyclic heterocycloalkyl groups.
For the avoidance of doubt, the term "bicyclic" (e.g. when employed in the
context of
heterocycloalkyl groups) refers to groups in which the second ring of a two-
ring system is
formed between two adjacent atoms of the first ring. Bicyclic also includes
bridged
bicyclic groups. The term "bridged" (e.g. when employed in the context of
heterocycloalkyl groups) refers to monocyclic or bicyclic groups in which two
non-
adjacent atoms are linked by either an aikylene or heteroalkylene chain (as
appropriate).
Aryl groups that may be mentioned include C6_14 (such as C6.13 (e.g. C6-1o))
aryl groups.
Such groups may be polycyclic (e.g. monocyclic or bicyclic) and have between 6
and 14
ring carbon atoms, in which at least one ring is aromatic. C6.14 aryl groups
include
phenyl, naphthyl and the like, such as 1,2,3,4-tetrahydronaphthyl, indanyl,
indenyl and
fluorenyl. The point of attachment of aryl groups may be via any atom of the
ring system.
However, when aryl groups are bicyclic or tricyclic, they are linked to the
rest of the
molecule via an aromatic ring.
Heteroaryl groups that may be mentioned include those which have between 5 and
14
(e.g. 10) members. Such groups may be monocyclic, bicyclic or tricyclic,
provided that at
least one of the rings is aromatic and wherein at least one (e.g. one to four)
of the atoms
in the ring system is other than carbon (i.e. a heteroatom). Heteroaryl groups
that may
be mentioned include acridinyl, benzimidazolyl, benzodioxanyl,
benzodioxepinyl,
benzodioxolyl (including 1,3-benzodioxolyl), benzofuranyl, benzofurazanyl,
benzothiazolyl, benzoxadiazolyl (including 2,1,3-benzoxadiazolyl),
benzoxazinyl
(including 3,4-dihydro-2H-1,4-benzoxazinyl), benzoxazolyl, benzomorpholinyl,
benzoselenadiazolyl (including 2,1,3-benzoselenadiazotyl), benzothiadiazolyl
(including
2,1,3-benzothiadiazolyl), benzothienyl, carbazolyl, chromanyl, cinnolinyl,
furanyl,
imidazolyl, imidazopyridyl (including imidazo[4,5-b]pyridyl, imidazo[5,4-
b]pyridyl and
imidazo[1,2-a]pyridyl), indazolyl, indolinyl, indolyl, isobenzofuranyl,
isochromanyl,
isoindolinyl, isoindolyl, isoquinolinyl, isothiaziolyl, isothiochromanyl,
isoxazolyl,
naphthyridinyl (including 1,6-naphthyridinyl or, preferably, 1,5-
naphthyridinyl and 1,8-
naphthyridinyl), oxadiazolyl (including 1,3,4-oxadiazolyl), oxazolyl,
phenazinyl,
phenothiazinyl, phthalazinyl, pteridinyl, purinyl, pyrazinyl, pyrazolyl,
pyridazinyl, pyridyl,
pyrimidinyl, pyrrolyl, quinazolinyl, quinolinyl, quinolizinyl, quinoxalinyl,
tetrahydroiso-
quinolinyl (including 1,2,3,4-tetrahydroisoquinolinyl and 5,6,7,8-
tetrahydroisoquinolinyl),
12
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
tetrahydroquinolinyl (including 1,2,3,4-tetrahydroquinolinyl and 5,6,7,8-
tetrahydroquinolinyl), tetrazolyl, thiadiazolyl (including 1,3,4-
thiadiazolyl), thiazolyl,
oxazolopyridyl (including oxazolo[4,5-b]pyridyl, oxazolo[5,4-b]pyridyl and, in
particular,
oxazolo[4,5-c]pyridyl and oxazolo[5,4-c]pyridyl), thiazolopyridyl (including
thiazolo[4,5-
b]pyridyl, thiazolo[5,4-b]pyridyl and, in particular, thiazolo[4,5-c]pyridyl
and thiazolo[5,4-
c]pyridyl), thiochromanyl, thienyl, triazolyl (including 1,2,3-triazolyl and
1,2,4-triazolyl)
and the like. Substituents on heteroaryl groups may, where appropriate, be
located on
any atom in the ring system including a heteroatom. The point of attachment of
heteroaryl groups may be via any atom in the ring system including (where
appropriate)
a heteroatom (such as a nitrogen atom), or an atom on any fused carbocyclic
ring that
may be present as part of the ring system. However, when heteroaryl groups are
polycyclic, they are preferably linked to the rest of the molecule via an
aromatic ring.
Heteroaryl groups may also be in the N- or S- oxidised form.
Heteroatoms that may be mentioned include phosphorus, silicon, boron,
tellurium,
selenium and, preferably, oxygen, nitrogen and sulphur.
For the avoidance of doubt, in cases in which the identity of two or more
substituents in a
compound of the invention may be the same, the actual identities of the
respective
substituents are not in any way interdependent. For example, in the situation
in which B2
and B3 both represent an aryl group optionally substituted as hereinbefore
defined, the
aryl groups in question may be the same or different. Similarly, when groups
are
substituted by more than one substituent as defined herein, the identities of
those
individual substituents are not to be regarded as being interdependent. For
example,
when X' represents two optional substituents, the identities of the two X'
groups are not
to be regarded as being interdependent. Likewise, when B2 represents e.g. an
aryl
group substituted by one or more (e.g. two) X9 groups, the identities of the
two X9 groups
are not to be regarded as being interdependent.
For the avoidance of doubt, where it is stated that the dotted lines may each
independently represent an optional bond, we mean that when the optional bond
is
present (together with the existing bond represented by an unbroken line) so
forming a
carbon-nitrogen double bond or carbon-carbon double bond (as appropriate).
Similarly,
where the optional bond represented by the dotted line is absent, only a
single bond
between the relevant carbon-nitrogen and carbon-carbon bond (represented by
the
unbroken line) is present. Hence, the following compounds of formula I are
included:
13
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
O 0
RNNR
2 2
RN~N R R RI N N N IN
(Rsm \
(R6)m \ (R~m (R%
(R7), (R)n
R3 R3 R 3 R3
OR4 OR4 OR4 OR4
la Ib Ic Id
For the avoidance of doubt, where the dotted line between carbon and the
nitrogen to
which R2 is bound represents a bond (so forming a double bond between carbon
and the
nitrogen to which R2 is bound), then R2 is absent. Similarly, where the dotted
line
between carbon and the nitrogen to which R2 is bound does not represent a bond
(thus
resulting in a single bond between carbon and the nitrogen to which R2 is
bound), then
R2 is present.
Particularly preferred compounds of formula I, include those of formula Ic
depicted
above, i.e. the following compounds in which the dotted line that is not
attached to a
nitrogen atom represents a double bond:
0 p
RN NCR 2 RN,J'N, R2
(R)m (R)m
(R)n (R)n
R3 R3
OR4 OR4
which the skilled person will appreciate may be depicted as either one of the
above two
compounds (in view of the fact that it is specified herein that either one of
R1 and, when
present, R2 may represent certain/the same integers).
For the avoidance of doubt, -(R7), represents between one and three optional
(i.e. R7
may not be present) substituents (as n may be 0, 1, 2 or 3), which may be
attached to
any one of the three free positions of the requisite benzene ring of the
compound of
formula I (to which -(R 7)n is bound). Similarly, -(R)m represents between one
and five
optional (i.e. R6 may not be present) substituents, which may be attached to
any one of
the free positions of the ring to which -(R6)m ring is attached, as permitted
by the
standard valencies of the relevant atoms in the rings.
14
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
For the avoidance of doubt, when a term such as "X' to X12i is employed
herein, this will
be understood by the skilled person to mean X', X2, X3, X4, X5, X6, X7, X8,
X9, X10, X" and
X12 inclusively.
Compounds of the invention that may be mentioned include those in which:
R80 represents C1_12 alkyl optionally substituted by one or more substituents
selected
from =0 and E';
R"d represents C1.3 alkyl optionally substituted by one or more halo atoms;
when an alkyl group mentioned herein is substituted with halo, then that halo
group is
preferably fluoro;
C1.12 alkyl groups mentioned herein are more preferably C1_6 alkyl groups.
Other compounds of the invention that may be mentioned include those in which:
there is a maximum of one R6 group (i.e. m represents 0 or 1) present;
there are not two R6 groups attached to the same carbon atom (of the requisite
pyrimidinone ring of formula I);
any two R6 groups present on the same carbon atom are not linked together;
any two R6 groups are not linked together; and/or
m represents 0 (i.e. there are no R6 substituents present).
It is stated above that compounds of the invention that may be mentioned
include those
in which there are not two R6 groups attached to the same carbon atom and any
two R6
groups present on the same carbon atom are not linked together; hence, in this
instance
there is no tetra-substituted carbon atom in the main pyrimidinone ring of the
compound
of formula I.
Preferred compounds of the invention that may be mentioned include those in
which:
the dotted lines either both represent bonds or, more preferably, are both not
present (in
another preferred embodiment, one double bond is present between two carbon
atoms
of the requisite pyrimidinone ring of the compound of formula I);
when B' represents a polycyclic aryl group, then it is preferably bicyclic
(e.g. a naphthyl
or tetrahydronaphthyl group), in which the point of attachment (to the Tz
moiety) may be
via an aromatic or non-aromatic ring;
when B1 represents a polycyclic (e.g. bicyclic) heteroaryl group, the ring
attached to the
Tz group is a six-membered or, more preferably, five-membered heterocycloalkyl
or
heteroaryl ring of the polycycle;
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
B1 preferably represents a monocyclic 5-membered heteroaryl group; a
polycyclic (e.g.
bicyclic) heteroaryl group; or a heterocycloalkyl group, all of which are
optionally
substituted as defined herein;
when R3 is -OR 4a, at least one of -R4 and -R 4a is other than acyclic C1_12
alkyl (e.g.
methyl);
when R3 is -OR 4a, at least one of -R4 and -R 41 is cycloalkyl or
heterocycloalkyl as defined
herein;
when R3 is -OR 4a, at least one of -R4 and -R 4a (e.g. R4a) is other than
acyclic C1_12 alkyl
(for example, R4a is cycloalkyl or heterocycloalkyl as defined herein) and the
other (e.g.
R4) represents acyclic C1.12 (e.g. C1.6) alkyl.
Preferred compounds of the invention include those in which:
m represents 3, preferably, 2, or, more preferably, 0 or 1;
n represents 2 or, preferably, 0 or 1;
R' represents -A'-Tz-B';
R3 represents hydrogen, C1.12 alkyl (optionally substituted by one or more
substituents
selected from =0 and X') or, preferably, -OR4a;
R4 and R41 independently represent C1.12 alkyl (optionally substituted by one
or more
substituents selected from =0 and X2) or -B3;
when R4 and R4a independently represent C1.12 alkyl, then they may represent
acyclic
C1.6 alkyl or, preferably, a C3.8 (e.g. C5_6) cycloalkyl group (both of which
may be
optionally substituted as defined herein);
for instance, certain compounds of formula I in which one of R4 and R4a is
acyclic (e.g.
acyclic C1_6 alkyl as defined herein) and the other is acyclic (e.g. acyclic
C1.6 alkyl as
defined herein) or, preferably, cyclic (e.g. a C3.8 (e.g. C5_6) cycloalkyl
group), i.e. most
preferably, one of R4 and R4a is acyclic and the other is preferably cyclic;
R41 more preferably represents a 5- or 6-membered (e.g. 5-membered)
heterocycloalkyl
group (e.g. in which the heterocycloalkyl group contains two or preferably one
heteroatom, preferably selected from nitrogen or, especially oxygen) or, R4a
more
preferably represents C3_8 cycloalkyl (e.g. C5_6 cycloalkyl), which
heterocycloalkyl and
cycloalkyl groups are optionally substituted as hereinbefore defined, but
which are
preferably unsubstituted;
R4 more preferably represents C1_12 alkyl, such as acyclic C1_6 alkyl (e.g.
C1.3 alkyl, scuh
as methyl), which group may be substituted as defined herein, but is
preferably
unsubstituted;
R5 represents C1.6 (e.g. C1.3) alkyl (optionally substituted by one or more
substituents
selected from =0 and X) or, preferably, hydrogen;
16
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
each R6 and each R7 independently represent X4 or C1_6 (e.g. C1_3) alkyl
(optionally
substituted by one or more substituents selected from =0 and X);
any two R6 groups are not linked together;
A' represents C1.6 (e.g. C1.3) alkylene (e.g. methylene) (optionally
substituted by one or
more substituents selected from =0 and X6; but preferably unsubstituted);
R"'' and RW2 independently represent C1.3 alkyl (e.g. methyl) or, preferably,
hydrogen;
B1 represents a 5-membered heteroaryl group or, preferably, a polycyclic (e.g.
bicyclic)
heteroaryl group (both of which are) optionally substituted with one or more
substituents
selected from X8;
1o when B1 represents a 5-membered heteroaryl group, then it preferably
contains one or
two heteroatoms, preferably selected from nitrogen, oxygen and sulfur (more
preferably,
it contains at least one nitrogen heteroatom and preferably another one
heteroatom, i.e.
two heteroatoms in total);
when B1 represents a 5-membered heteroaryl group, then it may be
unsubstituted, but is
preferably substituted by one or more (e.g. one or two) X8 substituents (e.g.
e.g. halo
(e.g. F or Cl), C1.3 alkyl (e.g. CH3 or CF3) or -OR9e (e.g. OCH3 or OCF3), or,
more
preferably, the X8 substituents are e.g. halo or C1.3 alkyl, e.g. chloro or
methyl);
when B' represents a polycyclic (e.g. bicyclic) heteroaryl group, then the
point of
attachment of that polycyclic heteroaryl group to the Tz group is via a
heterocyclic (e.g.
heteroaromatic) ring of that polycyclic group (most preferably the point of
attachment is
via a 5-membered heteroaromatic ring);
B2 B3 B3a independently represent phenyl (optionally substituted by one or
more
substituents selected from X), a 5- or 6-membered heterocycloalkyl group
(optionally
substituted by one or more substituents selected from =0 and X10) or a 5- or 6-
membered heteroaryl group (optionally substituted by one or more substituents
selected
from X");
when B2, B3 and B3a represent a 5- or 6-membered heterocycloalkyl or
heteroaryl group,
then, in each case, the heteroatom(s) is/are preferably selected from oxygen
and
nitrogen (further, in the case where B2, B3 and B3a represent heteroaryl, then
the
3o heteroatom(s) may also be selected from sulfur);
when B2, B3 and B3a represent a 5- or 6-membered heterocycloalkyl or
heteroaryl group,
then those groups contain two or, preferably, one heteroatom(s);
B4 and B5 independently represent a 5- or 6-membered heterocycloalkyl group
(optionally substituted by one or more substituents selected from =0 and X12);
when B4 and B5 represent a 5- or 6-membered heterocycloalkyl group, then the
heteroatom(s) is/are preferably selected from oxygen and nitrogen;
17
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
when B4 and B5 represent a 5- or 6-membered heterocycloalkyl group, then those
groups
contain two or, preferably, one heteroatom(s);
B3 represents a five-membered heteroaryl or heterocycloalkyl group, in which
the
heteroatom is preferably oxygen (so forming, e.g. a furanyl or
tetrahydrofuranyl group);
X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X" and X12 independently represent
B6, preferably,
-C(O)OR9f, -S(O)tN(R9m)R9n, -N(R9k)S(O)tR8d and/or, more preferably, -CN, -
NO2, halo
(e.g. fluoro), -OR9a, -N(R9c)R9d, -C(O)N(R99)R9h and/or -N(R9j)C(O)Rsc;
X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X" and X12 (especially X8)
independently
represents, at each occurrence when used herein, B6, -CN, -NO2, halo (e.g.
fluoro),
-OR 9a, -N(R9c)R9d, -C(O)N(R99)R9' and/or -N(R9j)C(O)R8c;
RBa, R8b, Red, R8e, R8f, R89 and R8h independently represent C1_6 (e.g. C1.3)
alkyl optionally
substituted by one or more substituents selected from E';
RBc, R9a, R9b, R9c, R9d, R9e, R9f, R99, R9h, R9', R9j, R9k, R9m, R9n, R9P,
R9G, R91, R9s, R9t and
R9u independently represent hydrogen or C1.6 (e.g. C1.4 or, preferably, C1.3)
alkyl (e.g. t-
butyl or, preferably, methyl) optionally substituted by one or more
substituents selected
from E2; or
any pair of R9c and R9d, R99 and R9h, R9rti and R9n, R9q and R9r, and R9t and
R9u may be
linked together with the nitrogen atom to which they are attached to form a 5-
or 6-
membered ring, optionally containing one or two double bonds, optionally
containing one
further nitrogen or oxygen heteroatom, and which ring is optionally
substituted by one or
more substituents selected from fluoro, =0 and C1_3 alkyl optionally
substituted by one or
more fluoro atoms (more preferably, any R9 pair are not linked together);
B6 represents (acyclic or, e.g. preferably, cyclic) C3.8 alkyl, 5- or 6-
membered
heterocycloalkyl (both of which are optionally substituted by one or more E3
substituents), preferably, heteroaryl or, more preferably, aryl (e.g. phenyl),
which latter
two groups are optionally substituted by one or more E4 substituents;
E', E2, E3 and E4 independently represent -N(R10k)S(O)t1R1I , -
S(O)t,N(R10m)R1On
preferably, -NO2, -C(O)OR10f, or, more preferably, halo, -CN, -OR1oa -
N(R1oc)R1od
-C(O)N(R109)R1 Oh and/or -N(R10j)C(O)R1'b (particularly preferred groups, e.g.
E2 groups,
include -C(O)N(R109)R1 oh);
R1oa R1ob, R1oc, R1od R10e R10f R109 R1oh R10' , R10j R10k, R10m R1on R10 ,
R1oq R10r R105
R1of R1ou and Rub independently represent hydrogen, -CH3 or -CF3 (e.g. R10 and
R1oh
independently represent hydrogen);
R1 la R11c R1 1d R1 le R11', R119 and R1 1h independently represent -CH3 or -
CF3.
18
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
When B' represents a monocyclic 5-membered heteroaryl group, preferred groups
include represents pyrazolyl, thiazolyl, furanyl, imidazolyl and oxazolyl.
Particularly
preferred groups are 1,3-dimethyl-pyrazol-5-yl and 2-chloro-thiazol-5-yl.
When B' represents a polycyclic (e.g. bicyclic) heteroaryl group, preferred
groups include
optionally substituted (e.g. by X8) benzoxazolyl (e.g. 2-benzoxazolyl),
benzimidazolyl
(e.g. 2-benzimidazolyl), benzofuranyl (e.g. 2-benzofuranyl), indolyl (e.g. 3-
indolyl),
benzothienyl (e.g. 3-benzothienyl), benzothiazolyl (e.g. 2-benzothiazolyl),
benzotriazolyl
(e.g. benzo-1,2-3-triazol-1-yl) and oxazolopyridinyl (e.g. oxazolo[5,4-
b]pyridinyl,
oxazolo[5,4-c]pyridinyl, oxazolo[4,5-b]pyridinyl or oxazolo[4,5-c]pyridinyl).
Particularly
preferred bicyclic heteroaryl groups include benzimidazolyl (e.g. 2-
benzimidazolyl) or,
preferably, benzoxazolyl (e.g. 2-benzoxazolyl) groups. Particularly preferred
groups are
5-fluoro-benzoxazol-2-yl, 1-[-C(O)Ot-butyl]-benzimidazol-2-yl, 5-methoxy-
benzofuran-2-
yl, unsubstituted benzimidazol-2-yl, 4-fluoro-benzoxazol-2-yl, 7-fluoro-
benzoxazol-2-yl, 6-
fluoro-benzoxazol-2-yl, 5-fluoro-benzoxazol-2-yl, 4-cyano-benzoxazol-2-yl and
7-cyano-
benzoxazol-2-yl.
Preferred optional substituents on B1 groups include -C(O)O-C,_4 alkyl (e.g.
-C(O)O-t-butyl); or, preferably, C1.4 alkyl (e.g. methyl) optionally
substituted by one or
more halo atoms (so forming, for example, a difluoromethyl or trifluoromethyl
group);
halo (e.g. chloro or fluoro); -CN; and -O-C1 ~ alkyl (e.g. methoxy) optionally
substituted by
one or more substituents selected from -C(O)N(R18)2 (in which R18 is
preferably
hydrogen; so forming, for example an acetamidoxy substituent) or, more
preferably, halo
(so forming, for example, a difluoromethoxy or trifluoromethoxy group).
Particularly
preferred such substituents include fluoro atoms. Further, such substituents
may, for
example, when substituted on a benzimidazolyl (e.g. 2-benzimidazolyl) or
benzoxazolyl
(e.g. 2-benzoxazolyl) group, be in the 4- to 7- (e.g. 4-, 7- or, preferably, 5-
) position.
More preferred compounds of the invention include those in which:
m represents 0;
n represents 0;
the dotted lines both represent bonds (i.e. there are two double bonds in the
requisite 6-
membered ring of formula I, thereby forming a pyrimidinone), are both not
present (i.e.
there are only single bonds in the requisite 6-membered ring of formula I,
thereby
forming a tetrahydropyrimidinone), or, preferably, one of the dotted lines
(e.g. the dotted
line between two carbon atoms) represents a bond (i.e. such that there is one
double
19
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
bond present between two carbon atoms in the requisite 6-membered ring of
formula I,
thereby forming a 2-oxo-2,3-dihydro-pyrimidin-1 -yl group);
R3 represents -OR 41;
R4a preferably represents furanyl (e.g. 3-furanyl), tetrahydrofuranyl (e.g. 3-
tetrahydrofuranyl) or, more preferably, cyclopentyl;
R4 represents trifluoromethyl, difluoromethyl or, preferably, methyl;
R5 represents H;
A' represents -CH2-;
Tz represents a direct bond;
1o B' (preferably unsubstituted or, more preferably, substituted with at least
one group
selected from X8) represents pyrazolyl (e.g. 5-pyrazolyl) or, preferably,
benzofuranyl (e.g.
3-benzofuranyl or 2-benzofuranyl), benzoxazolyl (e.g. 2-benzoxazolyl),
benzimidazolyl
(e.g. 2-benzimidazolyl), thienyl (e.g. 2-thienyl), furanyl (e.g. 2-furanyl),
imidazolyl (e.g. 2-
imidazolyl), oxazolyl (e.g. 2-oxazolyl), thiazolyl (e.g. e.g. 5-thiazolyl or,
preferably, 2-
thiazolyl), indofyl (e.g. 3-indolyl or 2-indolyl),, benzothienyl (e.g. 3-
benzothienyl or 2-
benzothienyl) or benzotriazolyl (e.g. 1 -benzotriazolyl or 2-benzotriazolyl);
X$ represents C,_2 alkyl (e.g. methyl), preferably, -C(O)O-C1_4 alkyl (e.g. -
C(O)O-t-butyl);
or, more preferably, -OCH3; halo (e.g. -F and/or -Cl); -CH3; -NO2; -CN; -CF3
and
-O-CH2-C(=O)-NH2.
Particularly preferred compounds of the invention include those in the
following list:
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(7-methoxy-2-
benzofurylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(7-fluoro-2-
benzofurylmethyl)tetrahydropyri-
midin-2-one;
1-(2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5,6-dimethyl-2-
benzimidazolylmethyl)tetra-
hydropyrimidin-2-one;
1-(2-benzofurylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)tetrahydropyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(2-th ienylmethyf )tetrahydropyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(2-furylmethyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-imidazolyimethyl)tetrahydropyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(1-methyl-2-
imidazolylmethyl)tetrahydropyrimi-
din-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-oxazolylmethyl)tetrahydropyrimidin-2-
one;
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-thiazolylmethyl)tetrahydropyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(3-indolylmethyl)tetrahydropyrimidin-2-
one;
1-(3-benzothienylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
1-(2-benzothiazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methyl-2-
benzoxazolylmethyl)tetrahydro-
pyrimidin-2-one;
1-(6-chloro-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-methyl-2-
benzoxazolylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(6-methyl-2-
benzoxazolylmethyl)tetrahydro-
pyrimidin-2-one;
1-(1-benzotriazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-nitro-2-benzoxazolylmethyl)tetra hyd
ropy rim i-
din-2-one;
1-(5-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-fluoro-2-
benzoxazolylmethyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-trifluoromethyl-2-
benzoxazolylmethyI)tetra-
hydropyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-methyl-2-
benzoxazolylmethyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methoxy-2-
benzoxazolylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-methoxy-2-
benzoxazolylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-fluoro-2-
benzoxazolylmethyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-fluoro-2-
benzoxazolylmethyl)tetrahydropyri-
midin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyri-
midin-2-one;
21
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
1-(7-chloro-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-trifluoromethyl-2-
benzoxazolylmethyl)tetra-
hydropyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(5-methoxy-2-
benzofurylmethyl)tetrahydropyri-
midin-2-one;
1-(2-benzimidazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(1-methyl-2-
benzimidazolylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(5,6-dichloro-2-
benzimidazolylmethyl)tetra-
hydropyrimidin-2-one;
1-(4-acetamidoxy-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetra-
hydropyrimidin-2-one;
31(7-acetamidoxy-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetra-
hydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
difluoromethoxyphenyl)tetra-
hydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmeth yl)-5-(3-cyclopentyloxy-4-trifluorometh
oxyphenyl)tetra-
hydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-d ifIuoromethoxy-3-(3-
tetrahydrofuranyloxy)-
phenyl]tetrahydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-methoxy-3-(3-
tetrahydrofuranyloxy)phenyl]-
tetrahydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-trifluoromethoxy-3-(3-
tetrahydrofuranyloxy)-
phenyl]tetrahydropyrimidin-2-one.
Further particularly preferred compounds of the invention include those in the
following
list:
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-methoxy-2-benzofurylmethyl)pyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-fluoro-2-benzofurylmeth yl)pyrimidin-
2-one;
1-(2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5,6-dimethyl-2-
benzimidazolylmethyl)pyrimidin-
2-one;
1-(2-benzofurylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-thienylmethyl)pyrimidin-2-one;
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-furylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-imid azolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(1-methyl-2-imidazolylmethyl)pyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-oxazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-thiazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(3-indolylmethyl)pyrimidin-2-one;
1-(3-benzothienylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one;
1-(2-benzothiazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methyl-2-
benzoxazolylmethyl)pyrimidin-2-
one;
1-(6-chloro-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-methyl-2-
benzoxazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(6-methyl-2-
benzoxazolylmethyl)pyrimidin-2-
one;
1-(1-benzotriazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-nitro-2-benzoxazolylmethyl)pyrimidin-
2-one;
1-(5-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-
2-one
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-fluoro-2-
benzoxazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-trifluoromethyl-2-
benzoxazolylmethyl)pyrimi-
din-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-methyl-2-
benzoxazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methoxy-2-
benzoxazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-methoxy-2-
benzoxazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-fluoro-2-
benzoxazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-fluoro-2-
benzoxazolylmethyl)pyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-
2-one
1-(7-chloro-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-trifluoromethyl-2-
benzoxazolylmethyl)pyrimi-
din-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methoxy-2-benzofurylmethyl)pyrimidin-
2-one;
1-(2-benzimidazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-
one;
2 3
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(1 -methyl-2-
benzimidazolylmethyl)pyrimid in-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5,6-dichloro-2-benzimidazolylmeth
yl)pyrimidin-
2-one;
1-(4-acetamidoxy-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)pyrimi-
din-2-one;
1-(7-acetamidoxy-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)pyrimi-
din-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
difluoromethoxyphenyl)pyrimi-
din-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
trifluoromethoxyphenyl)pyrimi-
din-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-difluoromethoxy-3-(3-
furanyloxy)phenyl]pyrimidin-
2-one;
1 -(4-cya no-2-benzoxazo lylmethyl)-5-[4-methoxy-3-(3-fu
ranyloxy)phenyl]pyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-trifluoromethoxy-3-(3-
furanyioxy)phenyl]pyrimi-
din-2-one.
Particularly preferred compounds of the invention include those of the
examples
described hereinafter.
Compounds of the invention may be made in accordance with techniques that are
well
known to those skilled in the art, for example as described hereinafter.
According to a further aspect of the invention there is provided a process for
the
preparation of a compound of formula I which process comprises:
(i) reaction of a compound of formula II,
24
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
O
HNAN ,(R
(R6)m I I
R3
O R4
or a protected derivative thereof, wherein R2, R3, R4, R6, R7 and the dotted
lines are as
hereinbefore defined, with a compound of formula IV,
B'-TZ-A'-L' I I I
wherein B', TZ and A' are as hereinbefore defined, and L' represents a
suitable leaving
group, such as a sulfonate group or, more preferably an iodo, bromo or chloro
group, in
the presence of a base, such as a strong base, for instance an alkali metal-
based base
such as NaH and/or KO-tent-butyl, optionally in the presence of an additive
(for example,
a sodium or potassium co-ordinating agent, such as a crown ether (e.g. 15-
crown-5)), for
example in the presence of a suitable solvent, such as a polar aprotic solvent
(e.g.
tetrahydrofuran or diethyl ether), for example at sub-ambient temperatures
(e.g. 0 C to
-78 C) under an inert atmosphere. The skilled person will appreciate that the
base may
need to be added to the compound of formula II before the addition of the
compound of
formula III. Further, one leaving group may be converted into another leaving
group (e.g.
into a stronger/better leaving group in the compound of formula III, for
instance by iodide
exchange, e.g. by adding an iodide source (e.g. KI) to a compound of formula
Ill in which
L' is chloro, thereby exchanging the chloride with iodide);
(ii) for compounds of formula I in which the dotted lines are not present and
R2 is present
and is not H, reaction of a compound of formula I in which the dotted lines
are not
present and R2 is H with a compound of formula IV,
Rea-L2 IV
wherein Rea represents R2 as hereinbefore defined provided that it does not
represent H,
and L2 represents a suitable leaving group, such as one hereinbefore defined
in respect
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
of L', under suitable conditions, such as those hereinbefore described in
respect of
process step (i);
(iii) for compounds of formula I in which the dotted lines are not present and
R2 is H,
reduction of a compound of formula I in which the dotted lines represent bonds
(so
forming double bonds in the compound of formula I) or protected derivatives
thereof, for
example under standard conditions, such as under hydrogenation reaction
conditions
(e.g. catalytic hydrogenation conditions in the presence of a precious metal
catalyst, e.g.
Pd/C);
(iv) for compounds of formula I in which one or both of the dotted lines
represent bonds
(so forming one or two double bonds in the compound of formula I),
dehydrogenation or
oxidation of a compound of formula I in which one or both of the dotted lines
are not
present and R2 is H, or protected derivatives thereof, for example under
standard
conditions, such as in the presence of a suitable reagent (e.g. DDQ (2,3-
dichloro-5,6-
dicyano-l,4-benzoquinone)) and/or by heating in the presence of Pd/C;
(v) for compounds of formula I wherein the dotted lines represent bonds,
reaction of a
compound of formula V,
Yla 0
(R6)M1
V
(R)n
R3 \
OR 4
or a protected derivative thereof, wherein R3, R4 R6 R7 and n are as
hereinbefore
defined, and ml is 0, 1 or 2 (the skilled person will appreciate that -(R6)m
represents two
optional R6 substituents, and that the structure of the compound of formula V
dictates
that these substituents may only be positioned at the carbonyl carbon and or
in the (3
position relative to the carbonyl carbon) and y1a is -OH or -NYaYb, where ya
and Yb are
independently alkyl (e.g. C,_12 alkyl, including cycloalkyl),
heterocycloalkyl, aryl and/or
heteroaryl, or Ya and yb may be joined to form a ring optionally containing
one or more
3o additional heteroatom, with a compound of formula VI,
26
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
0
B TZIANNH VI
H 2
or a protected derivative thereof, wherein B1, Tz and A' are as hereinbefore
defined,
under suitable conditions, for example under acid reaction conditions (e.g. in
the
presence of a hydrogen halide (e.g. HCl) optionally in a suitable solvent,
such as an
alcoholic solvent, e.g. ethanol);
(vi) for compounds of formula I where the dotted lines represent bonds,
reaction of a
compound of formula VII,
O
Vi l
(R7)n
R3
OR 4
or a protected derivative thereof, wherein R3, R4, R7 and m are as
hereinbefore defined,
with a compound of formula VI as hereinbefore defined and in the presence of a
suitable
reagent such as an ester (e.g. C1.6 ester) of formic acid (e.g. methyl or
ethyl formate) or a
suitable equivalent thereof (e.g. triethyl orthoformate) under conditions
known to one
skilled in the art, such as standard Aldol-type reaction conditions,
conditions such as
those hereinbefore defined in respect of process step (v) or, when e.g.
triethyl formate is
employed under acidic reaction conditions;
(vii) for compounds of formula I in which the dotted lines do not represent
bonds and R2
is H, intramolecular reaction of a compound of formula VIII,
O
L3 N'J~ NH Vlll
'
1 1
ATZ.B
(R7)n
R3
OR4
27
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
or a protected derivative thereof, wherein L3 represents a suitable leaving
group as
hereinbefore defined in respect of L', and R3, R4, R7 B', Tz, A' and n are as
hereinbefore
defined, under suitable conditions, such as those hereinbefore described in
respect of
process step (i);
(viii) for compounds of formula I in which the dotted lines do not represent
bonds,
reaction of a compound of formula IX,
'
R R2
NH HNC
(R6)n, IX
(R7)n
R3
OR 4
or a protected derivative thereof, where R1, R3, R4, R6, R7, m and n are as
hereinbefore
defined, and R2 is present and is as hereinbefore defined, with a compound of
formula X,
O
L4 L5 X
where L4 and L5 independently represent a suitable leaving group, such as -O-
C,_6 alkyl
(e.g. -OEt), a heterocycle (e.g. imidazole) wherein the heterocycle is bound
to the
carbonyl group at the heteroatom (e.g. 1,1'-carbonyldiimidazole) or a chloro
group (e.g.
phosgene, or a suitable phosgene derivative such as triphosgene), optionally
in the
presence of a suitable base, such as an amine base (e.g. pyridine), for
example
optionally in the presence of a suitable solvent, such as a polar aprotic
solvent (e.g.
toluene, preferably, tetrahydrofuran or diethyl ether);
(ix) for compounds of formula I, reaction of a compound of formula XI,
28
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
0
RNAN(R2)
Y (R6)m XI
L 5a
or a protected derivative thereof, where R', R2 R6, m and the dotted lines are
as
hereinbefore defined and L5a represents a suitable leaving group, such as one
hereinbefore defined in respect of L5, e.g. chloro, bromo, ibdo, a sulfonate
group (e.g.
-OS(O)2CF3, -OS(O)2CH3, -OS(O)2PhMe or a nonaflate), -B(OH)2, -B(ORwX)2, -
Sn(R")3,
diazonium salts, or a similar group known to the skilled person (in which each
RWX
independently represents a C1-6 alkyl group, or, in the case of -B(OR"'X)2,
the respective
R'"X groups may be linked together to form a 4- to 6-membered cyclic group
(such as a
4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl group)), and L5a preferably
represents
-B(OH)2, with a compound of formula XII,
L6
(R7)n XII
R3
OR4
or a protected derivative thereof, where R3, R4, R7 and n are as hereinbefore
defined,
and L6 represents a suitable leaving group such as chloro, bromo, iodo, a
sulfonate
group (e.g. -OS(O)2CF3, -OS(O)2CH3, -OS(O)2PhMe or a nonaflate), -B(OH)2, -
B(OR"'X)2,
-Sn(R"'X)3 or diazonium salts, in which each RWX independently represents a
C1_6 alkyl
group, or, in the case of -B(OR')2, the respective Rv" groups may be linked
together to
form a 4- to 6-membered cyclic group (such as a 4,4,5,5-tetramethyl-1,3,2-
dioxaboroian-
2-yl group), and L6 preferably represents bromo (the skilled person will also
appreciate
that L5 and L6 should be mutually compatible, and may also be interchanged),
for
example, in the presence of a suitable catalyst system, e.g. a metal (or a
salt or complex
thereof) such as Cul, Pd/C, PdCI2, Pd(OAc)2, Pd(Ph3P)2CI2, Pd(Ph3P)4,
Pd2(dba)3 or NiCl2
and a ligand such as t-Bu3P, (C6Hõ)3P, Ph3P, AsPh3, P(o-Tol)3, 1,2-
bis(diphenylphosphino)ethane, 2,2'-bis(di-tert-butylphosphino)-1,1'-biphenyl,
2,2'-
bis(diphenylphosphino)-1, 1'-bi-naphthyl, 1,1'-
bis(diphenylphosphinoferrocene), 1,3-
bis(di phenyl-phosphino) propane, xantphos, or a mixture thereof, together
with a suitable
base such as, Na2CO3, K3P04, Cs2CO3, NaOH, KOH, K2CO3, CsF, Et3N, (i-Pr)2NEt,
t-
BuONa or t-BuOK (or mixtures thereof) in a suitable solvent such as dioxane,
toluene,
29
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
ethanol, dimethylformamide, ethylene glycol dimethyl ether, water,
dimethylsulfoxide,
acetonitrile, dimethylacetamide, N-methylpyrrolidinone, tetrahydrofuran or
mixtures
thereof. The reaction may also be carried out for example at room temperature
or above
(e.g. at a high temperature such as the reflux temperature of the solvent
system) or using
microwave irradiation;
(x) compounds of formula I, particularly those in which R3 represents -OR4a in
which R4a
is other than hydrogen, reaction of a compound of formula XIII,
O
RNAN(R2)
(R6)m XIII
(R)n
L7
L
or a protected derivative thereof, wherein R', R2, R6, R7, m, n and the dotted
lines are as
hereinbefore defined and L7 represents Lx or R3 as hereinbefore defined, and
L8
represents Lx or -OR4 as hereinbefore defined, and Lx represents a suitable
leaving
group such as chloro, bromo, iodo, a sulfonate group (e.g. -OS(O)2CF3, -
OS(O)2CH3, -
OS(O)2PhMe or a nonaflate), -B(OH)2, -B(OR")2, -Sn(R")3 or diazonium salts, in
which
each R" independently represents a C1.6 alkyl group with a compound of formula
XIV,
R4x-OH XIV
wherein R4x represents R4 or R4a as required/appropriate, under suitable
conditions, for
example optionally in the presence of an appropriate metal catalyst (or a salt
or complex
thereof) such as Cu, Cu(OAc)2, Cul (or Cul/diamine complex), copper
tris(triphenyl-
phosphine)bromide, Pd(OAc)2, Pd2(dba)3 or NiCl2 and an optional additive such
as Ph3P,
2,2'-bis(diphenylphosphino)-1,1'-binaphthyl, xantphos, Nal or an appropriate
crown ether
such as 18-crown-6-benzene, in the presence of an appropriate base such as
NaH, Et3N,
pyridine, N,N'-dimethylethylenediamine, Na2CO3, K2CO3, K3PO4, Cs2CO3, t-BuONa
or t-
BuOK (or a mixture thereof, optionally in the presence of 4A molecular
sieves), in a
suitable solvent (e.g. dichloromethane, dioxane, toluene, ethanol,
isopropanol,
dimethylformamide, ethylene glycol, ethylene glycol dimethyl ether, water,
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
dimethylsulfoxide, acetonitrile, dimethylacetamide, N-methylpyrrolidinone,
tetrahydrofuran or a mixture thereof) or in the absence of an additional
solvent when the
reagent may itself act as a solvent (e.g. when -R 4x represents R4 and R4
represents
methyl). This reaction may be carried out at room temperature or above (e.g.
at a high
temperature, such as the reflux temperature of the solvent system that is
employed) or
using microwave irradiation;
(xi) for compounds of formula I in which R3 represents -OR 4a in which-R41 is
other than
hydrogen and/or where R4 is other than hydrogen, reaction of a corresponding
compound of formula I in which R3 represents -OH and/or R4 represents
hydrogen, with a
compound of formula XV,
R4Y-L9 XV
wherein R4Y represents R4 or R4a as required/appropriate, and L9 represents a
suitable
leaving group such as one defined hereinbefore in respect of L1, under
suitable reaction
conditions, for example such as those hereinbefore described in respect of
process step
(i);
(xii) for compounds of formula I in which TZ represents -N(R"r1)-, reaction of
a compound
of formula XVI,
L10
O
IN N_(R
(R6)m XVI
(R7n
R3
O R4
or a protected derivative thereof, wherein L10 represents a suitable leaving
group, such
as one hereinbefore defined in respect of L1 and R2, R3, R4, R6, R7, m, n and
the dotted
lines are as hereinbefore defined, with a compound of formula XVII,
H-Za XVI l
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
wherein Za represents -N(R")-B', and R"'' and B' are as hereinbefore defined,
under
suitable conditions, for example those hereinbefore described in respect of
process step
(i);
(xiii) for compounds of formula I in which Tz represents -C(O)-N(Rw2)-,
reaction of a
compound of formula XVIII,
HO O
T O
A1NN,(R2)
(R6)m XVII I
(R) n
R 3
OR4
or a protected derivative thereof (e.g. an ester derivative), wherein the
dotted lines, R2,
R3, R4, R5, R6, R7, A', m, n and the dotted lines are as hereinbefore defined,
with a
compound of formula XIX,
H-Zb XIX
wherein Zb represents -N(Rw2)-B', and R"'2 and B1 are as hereinbefore defined,
under
standard amide coupling reaction conditions, for example in the presence of a
suitable
coupling reagent (e.g. 1,1'-carbonyldiimidazole, N,N'-
dicyclohexylcarbodiimide, 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide (or hydrochloride thereof), N,N'-
disuccinimidyl
carbonate, benzotriazol-1-yloxytris(dimethylamino)phosphonium
hexafluorophosphate, 2-
(1 H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate,
benzotriazol-1-
yloxytris-pyrrolidinophosphonium hexafluorophosphate, bromo-tris-
pyrrolidinophosponium hexafluorophosphate, 2-(1 H-benzotriazol-1 -yl)-1, 1,3,3-
tetramethyluronium tetra-fluorocarbonate, 1-cyclohexyl-carbodiimide-3-
propyloxymethyl
polystyrene, O-(7-azabenzotriazol-1-yl)-N,N,N,N tetramethyluronium
hexafluorophosphate and/or O-benzotriazol-1-yl-N, N, N',N'-tetramethyluronium
tetrafluoroborate), optionally in the presence of a suitable base (e.g. sodium
hydride,
32
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
sodium bicarbonate, potassium carbonate, pyridine, triethylamine,
dimethylaminopyridine, diisopropylamine, sodium hydroxide, potassium tert-
butoxide
and/or lithium diisopropylamide (or variants thereof), an appropriate solvent
(e.g.
tetrahydrofuran, pyridine, toluene, dichloromethane, chloroform, acetonitrile,
dimethylformamide, trifIuoromethylbenzene, dioxane or triethylamine) and a
further
additive (e.g. 1-hydroxybenzotriazole hydrate). Alternatively, the carboxylic
acid group of
the compound of formula XVIII may be converted under standard conditions to
the
corresponding acyl chloride (e.g. in the presence of SOCI2 or oxalyl
chloride), which acyl
chloride is then reacted with a compound of formula XIX, for example under
similar
conditions to those mentioned above.
Compounds of formula If wherein the dotted lines represent bonds may be
prepared by
reaction of a compound of formula V, preferably wherein Y' represents -OH,
with urea
under conditions hereinbefore described in respect of process step (v).
Compounds of formula V in which Y' represents -OH may be prepared by
hydrolysis of a
compound of formula V wherein Y' represents -NYaYb, under conditions known to
those
skilled in the art, for example in the presence of an aqueous base (e.g.
aqueous NaOH)
and optionally in the presence of a suitable solvent or solvent mixture (e.g.
ethanol and
water).
Compounds of formula V in which Y' represents -OH may also be prepared by
reaction
of a compound of formula VII with a suitable ester of formic acid (e.g.methyl
or ethyl
formate) or the like, for example under reaction conditions known to those
skilled in the
art, such as those hereinbefore described in respect of preparation of
compounds of
formula I (process step (v)).
Compounds of formula V, in which Y' represents -NYaYb, wherein Ya and yb are
both
methyl, may be prepared by reaction of a compound of formula VII with DMF
(which, the
skilled person will understand, may also be used as a solvent or co-solvent),
under
conditions known to those skilled in the art, for example in the presence of
POC13 and
optionally in the presence of a suitable solvent or solvent mixture.
Compounds of formula VI may be prepared by reaction of urea with a compound of
formula III under conditions known to those skilled in the art, for example
those described
hereinbefore in respect of process step (i).
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
I Compounds of formula Vlll wherein L3 represents an iodo, chloro or,
preferably, a bromo
group, may be prepared by reaction of a compound of formula XX,
O
HO N)~ NH XX
H
A TZ.B
R7)n
R3 \
OR4
wherein R3, R4, R5, R7, A', Tz, B1 and n are as hereinbefore defined, with a
suitable
halogenating agent, for example, where L3 represents a bromo group, reaction
with CBr4
and PPh3 in the presence of a suitable solvent (e.g. dichioromethane).
Compounds of formula Vill wherein L3 represents a sulfonate group may be
prepared by
reaction of a compound of formula XX as hereinbefore defined, or a suitable
protected
derivative thereof, with a suitable sulfonyl chloride, for example
trifluoromethane
sulfonylchloride or p-toluene sulfonylchloride, optionally in the presence of
a suitable
amine base (e.g. pyridine or triethyl amine) and in the presence of a suitable
solvent (e.g.
dichloromethane).
Compounds of formula XVIII may be prepared by reaction of a compound of
formula II as
hereinbefore defined, with a compound of formula XXI,
L"-A'-C(O)OH XXI
or a protected derivative (e.g. ester) thereof, wherein L" represents a
suitable leaving
group, for example one hereinbefore defined in respect of L' (e.g. bromo) and
A' is as
hereinbefore defined, under standard reaction conditions known to those
skilled in the
art, for example such as those hereinbefore defined in respect of preparation
of
compounds of formula I (process step (i) above).
Compounds of formula XX may be prepared by reaction of a compound of formula
XXII,
34
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
HO NH2 XXII
(R),
R 3
OR 4
or a protected derivative thereof, with a compound of formula XXIII,
B'-TZ
A'-N=C=O XXIII
wherein R3, R4, R7, A', Tz, B' and n are as hereinbefore defined, optionally
in the
presence of a suitable solvent (e.g. tetrahydrofuran) and under conditions
known to one
skilled in the art.
Compounds of formula XXII may be prepared by reduction of a compound of
formula
XXIV,
HO N3 XXIV
R)n
R3 \
OR4
wherein R3, R4, R7 and n are as hereinbefore described, under conditions known
to one
skilled in the art, for example by reaction with a suitable reagent, for
example a suitable
reagent (e.g. triphenylphosphine), optionally in presence of a suitable
solvent (e.g. THF).
Compounds of formula XXIV may be prepared by reaction of a compound of formula
XXV,
;5
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
H O O H XXV
R7)
R3
OR4
or a suitably protected derivative thereof (e.g. a mono 0-protected
derivative), wherein
R3, R4, R' and n are as hereinbefore defined, with
A a suitable sulfonating agent (e.g. p-toluenesulfonyl chloride), under
conditions
known to one skilled in the art, for example in the presence of a suitable
base
(e.g. pyridine), a suitable catalyst (e.g. DMAP) and a suitable solvent (e.g.
THE or
DCM); followed by
B a suitable source of an azide nucleophile, for example an azide salt (e.g.
sodium
azide), under conditions known to one skilled in the art, for example in the
presence of a suitable solvent (e.g. DMF) and optionally in the presence of a
suitable metal ion complexing agent, for example a crown ether (e.g. 15-crown-
5).
Compounds of formula XXV may be prepared by reduction of:
(a) a compound of formula XXVI,
0
HO OH XXVI
(R),
R3 \
OR4 ; or
(b) a compound of formula XXVIA,
36
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
0 0
HO OH XXVIA
(R),
R3
OR4
or a suitably protected derivative thereof (e.g. ester), wherein, in both
cases, R3, R4, R7
and n are as hereinbefore defined, under conditions known to one skilled in
the art, for
example in the presence of a suitable reducing agent, such as a suitable
borane or
complex thereof (e.g. BH3=THF) and in the presence of a suitable solvent (e.g.
THF).
Compounds of formula XXII may also be obtained by reaction of a compound of
formula
XXVI as hereinbefore defined, or preferably a suitably protected derivative
thereof, for
example, an ester derivative (e.g. a methyl ester), with:
A a suitable sulfonating agent (e.g. p-toluenesulfonyl chloride, so forming a
tosylate
group; alternatively, the skilled person will appreciate that corresponding
compounds in which the tosylate group is replaced with a different leaving
group,
such as chloro, bromo or iodo, may also be employed), under conditions known
to one skilled in the art, for example in the presence of a suitable base
(e.g.
pyridine), a suitable catalyst (e.g. DMAP) and a suitable solvent (e.g. THE or
DCM); followed by
B a suitable source of an azide nucleophile, for example an azide salt (e.g.
sodium
azide), under conditions known to one skilled in the art, for example in the
presence of a suitable solvent (e.g. DMF) and optionally in the presence of a
suitable metal ion complexing agent, for example a crown ether (e.g. 15-crown-
5); followed by
C a suitable reducing agent (e.g. lithium aluminum hydride (LiAIH4)), under
conditions known to one skilled in the art, for example in the presence of a
suitable solvent (e.g. tetrahydrofuran).
Suitably protected derivatives (e.g. O-benzylated derivatives) of compounds of
formula
XXVI may be prepared by reaction of a compound of formula XXVII,
37
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
0
U XXVII
3 (R7),
R
OR 4
wherein U represents -ORu or -N(Ru')Ru2, in which Ru, Ru' and RU2
independently
represent hydrogen, C1.12 alkyl or aryl (which latter two groups may be
optionally
substituted by one or more substituents selected from a substituent such as
one
hereinbefore defined by X' and, in the case of C1_12 alkyl, =0), or, Ru' and R
U2 may be
linked together to form an optionally substituted (e.g. by one or more
substituents
selected from a substituent such as one hereinbefore defined by X1, aryl and
heteroaryl),
and R3, R4, R7 and n are as hereinbefore defined. Preferably Ru' and R U2 do
not
represent hydrogen. When Ru' and R U2 are linked together, they may together
form the
following group (i.e. U may represent the following group):
0
N
or an enantiomer thereof (or another suitable chiral derivative thereof, such
as one based
on Evans' chiral auxiliary), with formaldehyde, paraformaldehyde or a suitably
protected
derivative (e.g. an O-benzylated derivative) of a compound of formula XXVIII,
HO-CH2-CI XXVII I
or a derivative thereof, under suitable conditions known to one skilled in the
art, for
example those hereinbefore defined in respect of process step (i), followed by
hydrolysis
under suitable conditions as known to one skilled in the art, for example in
the presence
of an aqueous base such as sodium hydroxide.
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Compounds of formula XXVIA may be prepared by reaction of a compound of
formula
XXVII (as hereinbefore defined) or a compound of formula XXIX (as defined
hereinafter)
with a compound of formula XXVIIIA,
HO-C(O)-Cl XXVIIIA
for example under reaction conditions known to those skilled in the art, such
as those
hereinbefore described in respect of preparation of compounds of formula XXVI.
Alternatively, compounds of formula XXVIA may be prepared by reaction of a
compound
corresponding to a compound of formula Xll but in which L6 represents a metal-
containing group, such as Li, MgBr, ZnCI or the like (which may be prepared by
reaction
of a corresponding compound of formula XII in which L6 represents halo, by
e.g.
Iithiation, a Grignard-forming reaction, followed by, if necessary, metal-
exchange
reactions and the like) with a compound of formula XXVIIIB,
HO-C(O)-C(H)(W")-C(O)OH XXVIIIB
wherein W" represents a suitable leaving group, such as chioro (or the like),
for example
under reaction conditions known to those skilled in the art, e.g. such as
those catalytic
reaction conditions described in respect of preparation of compounds of
formula I
(process step (ix)).
Compounds of formula XXVII may be prepared by reaction of a compound of
formula
XXIX,
O
HOH XXIX
r
(R7~n
R3 ~
O R4
wherein R3, R4, R7 and n are as hereinbefore defined, with a compound of
formula XXX,
39
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
H
N O
XXX
under suitable conditions known to one skilled in the art, such as those
hereinbefore
described in respect of process step (xiii).
Compounds of formulae IV, VII, X, XI, XII, XIII, XV, XVI, XXVIIIA, XXVIIIB,
XXIX and
XXX (and also others, e.g. certain compounds of formulae VI and IX) may be
commercially available, are known in the literature, or may be obtained either
by analogy
with the processes described herein, or by conventional synthetic procedures,
in
accordance with standard techniques, from.available starting materials using
appropriate
reagents and reaction conditions. In this respect, the skilled person may
refer to inter
alia "Comprehensive Organic Synthesis" by B. M. Trost and I. Fleming, Pergamon
Press,
1991.
The substituents either in final compounds of the invention or in relevant
intermediates
(as appropriate) may be modified one or more times, after or during the
processes
described above by way of methods that are well known to those skilled in the
art.
Examples of such methods include substitutions, reductions, oxidations,
alkylations,
acylations, hydrolyses, esterifications, etherifications, halogenations or
nitrations. Such
reactions may result in the formation of a symmetric or asymmetric final
compound of the
invention or intermediate. In this respect, the skilled person may also refer
to
"Comprehensive Organic Functional Group Transformations" by A. R. Katritzky,
O. Meth-
Cohn and C. W. Rees, Pergamon Press, 1995. Specific transformation steps that
may
be mentioned include the conversion of one L6 group (in the compound of
formula XII)
into another L6 group (e.g. the conversion of one halo group, such as chloro,
into another
halo group, such as iodo, for example by reaction in the presence of potassium
iodide),
or even the conversion of a hydroxy group to a boronic acid group. Other
transformation
steps include the reduction of a nitro group to an amino group, the hydrolysis
of a nitrile
group to a carboxylic acid group, and standard nucleophilic aromatic
substitution
reactions.
As stated herein, the skilled person will also appreciate that chiral groups
may be
employed in order to obtain optically active compounds of the invention or
intermediates
thereof. For example, optically active compounds of formula XXVII may be
employed
(e.g. a variant based on Evan's chiral auxiliary).
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
It will also be appreciated by those skilled in the art that in the process
described below
the functional groups of intermediate compounds may need to be protected by
suitable
protecting groups. Such functional groups include hydroxy, amino, mercapto and
carboxylic acid. Suitable protecting groups for hydroxy include trialkylsilyl
or
diarylalkylsilyl (e.g., t-butyldimethylsilyl, t-butyldiphenylsilyl or
trimethylsilyl),
tetrahydropyranyl, benzyl, methyl and the like. Suitable protecting groups for
amino,
amidino and guanidino include t-butoxycarbonyl, benzyloxycarbonyl, and the
like.
Suitable protecting groups for mercapto include -C(O)-R" (where R" is alkyl,
aryl or
aralkyl), p-methoxybenzyl, trityl and the like. Suitable protecting groups for
carboxylic
acid include alkyl, aryl or aralkyl esters. Further, a carbonyl group may be
protected as
the silyl enol ether, which may be introduced under standard conditions, and
converted
back to the enolate (or carbonyl compound) by reaction in the presence of
fluoride ions
(or a suitable source thereof).
Protecting groups may be added or removed in accordance with standard
techniques (for
example a methyl protecting group on a hydroxy group may be removed by
reaction in
the presence of a suitable 'cleaving reagent' such as BBr3), which, are known
to one
skilled in the art and as described herein. The use of protecting groups is
described in
detail in Green, T.W. and P.G.M. Wuts, Protective Groups in Organic Synthesis
(1999),
3rd Ed., Wiley.
The protecting group may also be a polymer resin such as a Wang resin or a 2-
chlorotrityl-chloride resin.
Medical and Pharmaceutical Uses
Compounds of the invention are indicated as pharmaceuticals. According to a
further
aspect of the invention there is provided a compound of the invention, as
hereinbefore
3o defined, for use as a pharmaceutical.
Although compounds of the invention may possess pharmacological activity as
such,
certain pharmaceutically-acceptable (e.g. "protected") derivatives of
compounds of the
invention may exist or be prepared which may not possess such activity, but
may be
administered parenterally or orally and thereafter be metabolised in the body
to form
compounds of the invention. Such compounds (which may possess some
pharmacological activity, provided that such activity is appreciably lower
than that of the
41
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
"active" compounds to which they are metabolised) may therefore be described
as
"prodrugs" of compounds of the invention.
By "prodrug of a compound of the invention", we include compounds that form a
compound of the invention, in an experimentally-detectable amount, within a
predetermined time (e.g. about 1 hour), following oral or parenteral
administration. All
prodrugs of the compounds of the invention are included within the scope of
the
invention.
Furthermore, certain compounds of the invention may possess no or minimal
pharmacological activity as such, but may be administered parenterally or
orally, and
thereafter be metabolised in the body to form compounds (e.g. compounds of the
invention) that possess pharmacological activity as such. Such compounds
(which also
includes compounds that may possess some pharmacological activity, but that
activity is
appreciably lower than that of the "active" compounds of the invention to
which they are
metabolised), may also be described as "prodrugs".
Thus, the compounds of the invention are useful because they possess
pharmacological
activity, and/or are metabolised in the body following oral or parenteral
administration to
form compounds which possess pharmacological activity.
According to a further aspect of the invention there is provided a
pharmaceutical
composition/formulation including a compound of the invention as hereinbefore
defined
in admixture with a pharmaceutically acceptable adjuvant, carrier, diluent or
excipient.
Depending on e.g. potency and physical characteristics of the compound of the
invention
(i.e. active ingredient), pharmaceutical formulations that may be mentioned
include those
in which the active ingredient is present in at least 1 % (or at least 10%, at
least 30% or at
least 50%) by weight. That is, the ratio of active ingredient to the other
components (i.e.
the addition of adjuvant, diluent and carrier) of the pharmaceutical
composition is at least
1:99 (or at least 10:90, at least 30:70 or at least 50:50) by weight.
Such compositions/formulations may be prepared in accordance with standard
and/or
accepted pharmaceutical practice.
42
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Compounds of the invention will normally be administered orally,
intravenously,
subcutaneously, buccally, rectally, dermally, nasally, tracheally,
bronchially, sublingually,
by any other parenteral route or via inhalation, in a pharmaceutically
acceptable dosage
form.
Compounds of the invention may be administered alone, but are preferably
administered
by way of known pharmaceutical formulations, including tablets, capsules or
elixirs for
oral administration, suppositories for rectal administration, sterile
solutions or
suspensions for parenteral or intramuscular administration, and the like.
The invention further provides a process for the preparation of a
pharmaceutical
composition/formulation, as hereinbefore defined, which process comprises
bringing into
association a compound of the invention, as hereinbefore defined, or a
pharmaceutically
acceptable derivative (e.g. salt) thereof, with a pharmaceutically-acceptable
adjuvant,
carrier, diluent or excipient.
Compounds of the invention may be useful as inhibitors of certain enzymes such
as a
cyclic AMP phosphodiesterase, a phosphodiesterase 7; a phosphodiesterase 4; a
phosphodiesterase 3; or a cyclic GMP phosphodiesterase. In particular,
compounds of
the invention may be useful as inhibitors of a phosphodiesterase 7 and,
particularly, a
phosphodiesterase 4.
Accordingly, compounds of the invention may therefore be useful in treating or
preventing inflammatory diseases or conditions in a patient. Hence, in another
aspect,
this invention is directed to methods for treating or preventing an
inflammatory disease or
condition in a mammal, preferably a human, wherein the method comprises
administering to the mammal in need thereof a therapeutically effective amount
of a
compound of the invention as hereinbefore described.
3o The term "inflammation" will be understood by those skilled in the art to
include any
condition characterised by a localised or a systemic protective response,
which may be
elicited by physical trauma, infection, chronic diseases, such as those
mentioned
hereinbefore, and/or chemical and/or physiological reactions to external
stimuli (e.g. as
part of an allergic response). Any such response, which may serve to destroy,
dilute or
sequester both the injurious agent and the injured tissue, may be manifest by,
for
example, heat, swelling, pain, redness, dilation of blood vessels and/or
increased blood
43
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
flow, invasion of the affected area by white blood cells, loss of function
and/or any other
symptoms known to be associated with inflammatory conditions.
The term "inflammation" will thus also be understood to include any
inflammatory
disease, disorder or condition per se, any condition that has an inflammatory
component
associated with it, and/or any condition characterised by inflammation as a
symptom,
including inter alia acute, chronic, ulcerative, specific, allergic and
necrotic inflammation,
and other forms of inflammation known to those skilled in the art. The term
thus also
includes, for the purposes of this invention, inflammatory pain and pain
generally.
Where a condition has an inflammatory component associated with it, or a
condition
characterised by inflammation as a symptom, the skilled person will appreciate
that
compounds of the invention may be useful in the treatment of the inflammatory
symptoms and/or the inflammation associated with the condition.
The inflammatory condition or disease may be an autoimmune condition or
disease; the
inflammatory condition or disease may involve acute or chronic inflammation of
bone
and/or cartilage compartments of joints; the inflammatory condition or disease
may be an
arthritis selected from rheumatoid arthritis, gouty arthritis or juvenile
rheumatoid arthritis;
the inflammatory condition or disease may be a respiratory disorder selected
from
asthma or a chronic obstructive pulmonary disease (COPD, e.g., emphysema or
chronic
bronchitis); the condition or disease may be associated with the disregulation
of T-cells;
the condition or disease may be associated with elevated levels of
inflammatory
cytokines (e.g., wherein the inflammatory cytokine is IL-2, or wherein the
inflammatory
cytokine is IFN-y, or wherein the inflammatory cytokine is TNF-a); the
inflammatory
condition or disease may be multiple sclerosis; the inflammatory condition or
disease
may be pulmonary sarcadosis.; the inflammatory condition or disease may be
ocular
inflammation or allergy; the inflammatory condition or disease may be an
inflammatory
bowel disease (e.g., Crohn's disease or ulcerative colitis); and the
inflammatory condition
or disease may be an inflammatory cutaneous disease (e.g., psoriasis or
dermatitis).
Compounds of the invention may be useful in modulating intracellular cyclic
adenosine
5'-monophosphate levels within a mammal, preferably a human. Hence, in another
aspect, this invention is directed to methods for modulating intracellular
cyclic adenosine
5'-monophosphate levels within a mammal, preferably a human, wherein the
method
comprises administering to the mammal in need thereof an amount of a compound
of the
invention (e.g. those hereinbefore defined) or a pharmaceutical
formulation/composition
44
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
of the invention as hereinbefore described effective to modulate the
intracellular cyclic
adenosine 5'-monophosphate levels of the mammal. The mammal, preferably a
human,
may have an inflammatory condition or disease (for example one defined
herein).
Compounds of the invention may be useful in treating or preventing a disease
or
condition in a mammal, preferably a human, where the disease or condition is
associated
with pathological conditions that are modulated by inhibiting enzymes
associated with
secondary cellular messengers. Hence, in another aspect, this invention is
directed to
methods for treating or preventing a disease or condition in a mammal,
preferably a
human, wherein the method comprises administering to the mammal in need
thereof a
therapeutically effective amount of a compound of the invention or a
pharmaceutical
formulation/composition of the invention as hereinbefore described, and the
disease or
condition is associated with pathological conditions that are modulated by
inhibiting
enzymes associated with secondary cellular messengers. Such enzymes (that may
be
inhibited) may be a cyclic AMP phosphodiesterase; a phosphodiesterase 7; a
phosphodiesterase 4; a phosphodiesterase 3; or a cyclic GMP phosphodiesterase.
Further, more than one type of enzyme may be inhibited, for instance, the
enzymes may
be both phosphodiesterase 4 and phosphodiesterase 3. In particular, the enzyme
that
may be inhibited is a phosphodiesterase 7 or, preferably, a phosphodiesterase
4.
Compounds of the invention may be useful in treating or preventing
uncontrolled cellular
proliferation in a mammal, preferably a human. Hence, in another aspect, this
invention
is directed to methods for treating or preventing uncontrolled cellular
proliferation in a
mammal, preferably a human, wherein the method comprises administering to the
mammal in need thereof a therapeutically effective amount (e.g. an amount
effective to
treat or prevent uncontrolled cellular) of a compound of the invention or a
pharmaceutical
formulation/composition of the invention as hereinbefore described. The
uncontrolled
cellular proliferation may be caused by a cancer selected from leukaemia and
solid
tumors.
Compounds of the invention may be useful in treating or preventing transplant
rejection
in a mammal, preferably a human. Hence, in another aspect, this invention is
directed to
methods for treating or preventing transplant rejection in a mammal,
preferably a human,
wherein the method comprises administering to the mammal in need thereof a
therapeutically effective amount (e.g. an amount effective to treat or prevent
transplant
rejection in the mammal) of a compound of the invention. The rejection may be
due to
graft versus host disease.
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Compounds of the invention may be useful in treating or preventing conditions
associated with the central nervous system (CNS) in a mammal, preferably a
human.
Hence, in another aspect, this invention is directed to methods for treating
or preventing
conditions associated with the central nervous system in a mammal, preferably
a human,
wherein the method comprises administering to the mammal in need thereof a
therapeutically effective amount (e.g. an amount effective to treat or prevent
conditions
associated with the central nervous system (CNS) in the mammal) of a compound
of the
invention as described above (e.g. those hereinbefore defined) or a
pharmaceutical
formulation/composition of the invention as hereinbefore described. The
condition
associated with the central nervous system (CNS) may be depression.
These and other aspects and embodiments of the present invention will be
apparent
upon reference to the following detailed description. To this end, various
references are
set forth herein which describe in more detail certain procedures, compounds
and/or
formulations/compositions, and are hereby incorporated by reference in their
entirety.
In particular, compounds of the invention are inhibitors of PDE7 and,
preferably, PDE4.
As used herein, the terms "disease" and "condition" may be used
interchangeably or
may be different in that the particular malady or condition may not have a
known
causative agent (so that etiology has not yet been worked out) and it is
therefore not yet
recognised as a disease but only as an undesirable condition or syndrome,
wherein a
more-or-less specific set of symptoms have been identified by clinicians.
Thus, the compounds and compositions of the invention may be used to treat
inflammation, including both acute and chronic inflammation as well as certain
proliferative disorders (cancers). As used herein, inflammation includes,
without
limitation, ankylosing spondylitis, arthritis (e.g. juvenile arthritis and
rheumatoid arthritis),
asthma, COPD, chronic bronchitis, respiratory distress syndrome, rhinitis,
allergic rhinitis,
Crohn's disease, nephritis, eczema, dermatitis (e.g. atopic dermatitis),
urticaria,
conjunctivitis, ulcerative colitis, rheumatoid arthritis, osteoarthritis,
eosinophilic
gastrointestinal disorders, vascular disease, diabetes mellitus, fibromyalgia
syndrome,
gout, inflammations of the brain (including multiple sclerosis, AIDS dementia,
Lyme
encephalopathy, herpes encephalitis, Creutzfeld-Jakob disease, and cerebral
toxoplasmosis), emphysema, inflammatory bowel disease, irritable bowel
syndrome,
ischemia-reperfusion injury juvenile erythematosus pulmonary sarcoidosis,
Kawasaki
46
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
disease, osteoarthritis, pelvic inflammatory disease, psoriatic arthritis,
psoriasis,
tissue/organ transplant, scleroderma, spondyloarthropathies, systemic lupus
erythematosus, pulmonary sarcoidosis, ulcerative colitis, viral infections
(i.e.
inflammation associated with a viral infection) (e.g. influenza, common cold,
herpes
zoster, hepatitis C and AIDS), bacterial infections (i.e. inflammation
associated with a
bacterial infection), and any other disease with an inflammatory component. As
used
herein, proliferative disorders includes, without limitation, all cancers,
leukemias and
solid tumors that are susceptible to undergoing differentiation or apoptosis
upon
interruption of their cell cycle. As stated herein, the compounds and
compositions of the
invention may also be useful for treating diseases associated with the central
nervous
system. Such diseases include cognitive function, Alzheimer's disease and
other
neurodegenerative disorders, learning and memory disorders.
Compounds of the invention may inhibit disease induction, for example in the
models in
the biological examples, at doses of less than 500 mg/kg. The Biological
Examples
below outline some, but not all, of the preclinical models that may be used to
support the
claims of this patent. For instance, compounds of the examples are tested in
the
Biological Example 1, and are found to exhibit at least 50% inhibition of PDE4
at a
concentration of 10 pM or below (and more preferably at a concentration of 0.3
M or
below).
Compounds of the invention may also be combined with other therapeutic agents
that
are useful in the treatment of the conditions described herein. For instance,
the
compounds of the invention may be combined with other compounds that may be
useful
in the treatment of:
i) an inflammatory disorder;
ii) a disorder in which the modulation of intracellular cyclic adenosine 5'-
monophosphate
levels within a mammal is desired and/or required, which disorder may be an
inflammatory disorder;
iii) a disorder associated with pathological conditions that are modulated by
inhibiting
enzymes associated with secondary cellular messengers (e.g. a cyclic AMP
phosphodiesterase; a phosphodiesterase 4; a phosphodiesterase 3; a cyclic GMP
phosphodiesterase; or both phosphodiesterase 4 and phosphodiesterase 3), which
disorder may be an inflammatory disorder (it is most preferred that compounds
of the
invention are combined (an) inhibitor(s) of PDE7 or, in particular, (an)
inhibitor(s) of
PDE4);
iv) transplant rejection in a mammal;
47
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
v) uncontrolled cellular proliferation; and/or
vi) a disorder associated with the central nervous system.
According to a further aspect of the invention, there is provided a
combination product
comprising:
(A) a compound of the invention as hereinbefore defined; and
(B) another therapeutic agent that is useful in the treatment of i), ii),
iii), iv), v) or vi)
above (e.g. a therapeutic agent that is useful in the treatment of an
inflammatory
disorder),
wherein each of components (A) and (B) is formulated in admixture with a
pharmaceutically-acceptable adjuvant, diluent, carrier or excipient.
Such combination products provide for the administration of a compound of the
invention
in conjunction with the other therapeutic agent, and may thus be presented
either as
separate formulations, wherein at least one of those formulations comprises a
compound
of the invention, and at least one comprises the other therapeutic agent, or
may be
presented (i.e. formulated) as a combined preparation (i.e. presented as a
single
formulation including a compound of the invention and the other therapeutic
agent).
Thus, there is further provided:
(1) a pharmaceutical formulation/composition including a compound of the
invention, as
hereinbefore defined, another therapeutic agent that is useful in the
treatment of i), ii), iii),
iv), v) or vi) above (e.g. a therapeutic agent that is useful in the treatment
of an
inflammatory disorder), and a pharmaceutically-acceptable adjuvant, diluent,
carrier or
excipient; and
(2) a kit of parts comprising components:
(a) a pharmaceutical formulation/composition including a. compound of the
invention, as hereinbefore defined, in admixture with a pharmaceutically-
acceptable adjuvant, diluent, carrier or excipient; and
(b) a pharmaceutical formulation/composition including another therapeutic
agent
that is useful in the treatment of i), ii), iii), iv), v) or vi) above (e.g. a
therapeutic
agent that is useful in the treatment of an inflammatory disorder) in
admixture with
a pharmaceutically-acceptable adjuvant, diluent, carrier or excipient,
48
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
which components (a) and (b) are each provided in a form that is suitable for
administration in conjunction with the other.
The invention further provides a process for the preparation of a combination
product as
hereinbefore defined, which process comprises bringing into association a
compound of
the invention, as hereinbefore defined, or a pharmaceutically acceptable
derivative (e.g.
salt) thereof with another therapeutic agent that is useful in the treatment
of i), ii), iii), iv),
v) or vi) above (e.g. a therapeutic agent that is useful in the treatment of
an inflammatory
disorder), and at least one pharmaceutically-acceptable adjuvant, diluent,
carrier or
excipient.
By "bringing into association", we mean that the two components are rendered
suitable
for administration in conjunction with each other.
Thus, in relation to the process for the preparation of a kit of parts as
hereinbefore
defined, by bringing the two components "into association with" each other, we
include
that the two components of the kit of parts may be:
(i) provided as separate formulations (i.e. independently of one another),
which are
subsequently brought together for use in conjunction with each other in
combination
therapy; or
(ii) packaged and presented together as separate components of a "combination
pack"
for use in conjunction with each other in combination therapy.
The compounds of the invention, or their pharmaceutically acceptable salts,
are
administered in a therapeutically effective amount, which will vary depending
upon a
variety of factors including the activity of the specific compound employed;
the metabolic
stability and length of action of the compound; the age, body weight, general
health, sex,
and diet of the patient; the mode and time of administration; the rate of
excretion; the
drug combination; the severity of the particular disease or condition; and the
subject
undergoing therapy.
The term "effective amount" refers to an amount of a compound, which confers a
therapeutic effect on the treated. patient. The effect may be objective (i.e.
measurable by
some test or marker) or subjective (i.e. the subject gives an indication of or
feels an
effect).
Compounds of the invention may be administered at varying doses. Oral,
pulmonary
49
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
and topical dosages may range from between about 0.01 mg/kg of body weight per
day
(mg/kg/day) to about 100 mg/kg/day, preferably about 0.01 to about 10
mg/kg/day, and
more preferably about 0.1 to about 5.0 mg/kg/day. For e.g. oral
administration, the
compositions typically contain between about 0.01 mg to about 500 mg, and
preferably
between about 1 mg to about 100 mg, of the active ingredient. Intravenously,
the most
preferred doses will range from about 0.001 to about 10 mg/kg/hour during
constant rate
infusion. Advantageously, compounds may be administered in a single daily
dose, or the
total daily dosage may be administered in divided doses of two, three or four
times daily.
When a pharmaceutical composition containing a compound of the invention is
employed, it shall contain an appropriate amount/concentration/ratio of the
active
ingredient.
The ranges of effective doses provided herein are not intended to be limiting
and
represent preferred dose ranges. However, the most preferred dosage will be
tailored to
the individual subject, as is understood and determinable by one skilled in
the relevant
arts. (see, e.g., Berkow et al., eds., The Merck Manual, 16th edition, Merck
and Co.,
Rahway, N.J., 1992; Goodmanetna., eds.,Goodman and Cilman's The
Pharmacological
Basis of Therapeutics, 10th edition, Pergamon Press, Inc., Elmsford, N.Y.,
(2001);
Avery's Drug Treatment: Principles and Practice of Clinical Pharmacology and
Therapeutics, 3rd edition, ADIS Press, LTD., Williams and Wilkins, Baltimore,
MD.
(1987), Ebadi, Pharmacology, Little, Brown and Co., Boston, (1985); Osolci
al., eds.,
Remington's Pharmaceutical Sciences, 18th edition, Mack Publishing Co.,
Easton, PA
(1990); Katzung, Basic and Clinical Pharmacology, Appleton and Lange, Norwalk,
CT
(1992)).
The physician, or the skilled person, will be able to determine the actual
dosage and/or
route of administration which will be most suitable for an individual patient,
which is likely
to vary with the route of administration, the type and severity of the
condition that is to be
treated, as well as the species, age, weight, sex, renal function, hepatic
function and
3o response of the particular patient to be treated. The above-mentioned
dosages are
exemplary of the average case; there can, of course, be individual instances
where
higher or lower dosage ranges are merited, and such are within the scope of
this
invention.
Compounds of the invention may have the advantage that they are effective
inhibitors
(and hence particularly effective in the treatment of the conditions described
herein), and
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
in particular effective PDE inhibitors (such as PDE7 inhibitors and especially
effective
PDE4 inhibitors).
Compounds of the invention may also have the advantage that they may be more
efficacious than, be less toxic than, be longer acting than, be more potent
than, produce
fewer side effects than, be more easily absorbed than, and/or have a better
pharmacokinetic profile (e.g. higher oral bioavailability and/or lower
clearance) than,
and/or have other useful pharmacological, physical, or chemical properties
over,
compounds known in the prior art, whether for use in the above-stated
indications or
otherwise.
Biological Examples
In vitro Inhibition of PDE4 phosphodiesterases
PDE4 U937 cytoplasmic extracts are prepared by a modified procedure of the
assay
described in MacKenzie, S.J. and Houslay, M.D., "Action of rolipram on
specific PDE4
cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-
element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase
in
U937 monocytic cells", Biochem J. (2000), 347(Pt 2):571-8, by lysis of U937
cells
(ATCC: Catalogue No. CRL-159) in M-PER Lysis buffer (Pierce) containing 10%
protease inhibitor cocktail (Sigma). The cell lysates are then centrifuged at
30,000 rpm
for 15 minutes at 4 C. The supernatants are aliquoted and stored at -80 C.
PDE4 has
been shown to be the predominant cyclic nucleotide phosphodieterase activity
in U937
cells.
An alternative source of PDE4 enzymes is from recombinant human PDE4 obtained
from
baculovirus-SF9 cells expression system. cDNA containing PDE4D1 is cloned into
a
baculovirus vector, insect cells (SF9) are then infected and cells cultured to
express the
PDE4 protein. The cells are lysed and used directly in assay or partially
purified using
standard procedures. The process can be used for other PDE4 and PDE enzymes.
Compounds of the invention are evaluated for inhibitory activity against PDE4
enzymes
by the following assay Method A or B.
Method A:
PDE4 assay based on modified procedure of Phosphodiesterase [3H]cAMP SPA
Enzyme
;i
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Assay (Amersham Biosciences, code TRKQ 7090). In this assay, PDE4 enzymes
converts [3H]cAMP to [3H]5'-AMP. The assay is quenched by the addition of SPA
yttrium
silicate beads which preferentially bind linear nucleotides over cyclic
nucleotides in the
presence of zinc sulphate. The amount of [3H]5'-AMP formed is proportional to
the PDE4
activity, hence PDE4 inhibitors would decrease the amount of [3H]5'-AMP
formed.
Reactions are performed in duplicate by the addition of 10 pl PDE4 enzyme
(U937 lysate
or recombinant hPDE4) to 20 pL of assay mix and 20 pL of test compounds in
Isoplates
(Wallac) for 30 minutes, at 37 C. The final assay mixture contained: 50 mM
Tris (pH
7.5), 8.3 mM MgCl2, 1.7 mM EGTA and [3H]cAMP (0.025 pCi) (Amersham). Assay is
terminated by addition 25 pL SPA beads. The plate is sealed, shaken for 1
minute and
then allowed to settle 30 minutes and the cpm is determined using a Wallac
Micobeta.
Method B:
PDE4 assay based on modified procedure of Thompson and Appleman (Biochemistry
(1971); 10; 311-316). In this assay, PDE4 enzymes converts [3H]cAMP to [3H]5'-
AMP.
The [3H]5'-AMP is then converted to [3H]adenosine and phosphate by
nucleotidase. The
amount of [3H]adenosine formed is proportional to the PDE4 activity, hence
PDE4
inhibitors would decrease the amount off H]adenosine formed.
PDE reactions are performed for 30 minutes at 37 C in 100 pL volumes in 1 pM
cAMP,
0.05 pCi [3H]cAMP (Amersham), 0.5 U/mL 5'-nucleotidase (Sigma), 50 mM Tris, 10
mM
MgCl2 pH 7.5. Reactions are performed in duplicate. Reactions are terminated
by
boiling for 2 minutes at 100 C followed by the addition of 200 pL Dowex 1-8
400 Cl-
anion exchange resin in a ratio of 1 resin:2 methanol:1 H2O. Samples are mixed
by
inversion and then allowed to settle for 2-3 hours. An aliquot of 75 pL is
transferred to
Isoplates (Wallac), 150 pL of scintillation fluid added and the plate sealed
and shaken for
minutes. The cpm is determined using a Wallac Micobeta.
Compounds of invention are dissolved in 100% DMSO and diluted such that the
final
3o DMSO concentration in the assay does not exceed 1% to avoid affecting the
PDE4
activity. PDE4 enzyme is added in quantities such that less than 15% of
substrate is
consumed (linear assay conditions). Test compounds are assayed at 6-8
concentrations
ranging from 0.1 nM to 30 pM and IC50 values are determined from the
concentration
curves by nonlinear regression analysis (GraphPad Prism 4).
Compounds of the invention, when tested in these assays, demonstrate the
ability to
inhibit PDE4 phosphodiesterase activity.
52
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Measurement of Cyclic AMP PDE7 activity
The PDE7 assay is based on a modified procedure if the phosphodiesterase
[3H]cAMP
SPA Enzyme Assay (Amersham Biosciences code TRKQ 7090). In this assay, PDE7
enzyme(s) convert [3H]cAMP to [3H]5'-AMP. The assay is quenched by the
addition of
ice-cold SPA yttrium silicate beads which preferentially binds linear
nucleides, eg 5'-
AMPover cycling nucleotides in the presence of zinc sulphate. The amount of
[3H]5'-
AMP formed is proportional to the activity of the PDE7, and hence inhibitors
of the
enzyme would decrease the amount of [3H]5'-AMP formed.
Reactions are performed in duplicate by the addition of 15 pL of PDE7
(Baculovirus
lysate) to 10 pL of assay mix and 25 pL test compounds in 96-well flat-bottom
plate for
60 min at ambient temperature. The Assay mixture contains 50 mM Tris (pH 7.5),
8.3
mM MgCl2, 1.7 mM EGTA and [3H]cAMP (0.025 pCi) (Amersham). The assay is
terminated by addition 25 pL SPA beads. The plate is sealed, shaken for 1
minute and
then allowed to settle for 20 to 45 minutes and the cpm is determined using a
Packard
Topcount Scintillation counter.
Compounds of the invention may inhibit PDE7, as demonstrated by the above
assay.
PBMC cell assay
Peripheral blood mononuclear cells (PBMC) were isolated from healthy
volunteers and
dissolved in RPMI 1640 to a final cell concentration of 1,33x106 cells/mL.
0,2% Fetal
bovine serum (FBS) was added to the cell suspension.
1. The PBMC cells in 384 well microtiter plate (100 000 cells/ well) were
induced
with 2 mg/mL lipopolysaccharide (LPS) giving a final concentration of 10
pg/mL.
2. IC5o curves were run in duplicate with 10 different concentrations of
compound.
1,5 pL of compound in DMSO were added to each well.
3. The cells were incubated with substance for 18 h at 37 C and 5% CO2 in a
humidified chamber.
4. Incubation was terminated at -80 C for at least one hour.
5. 10 pL of assay solution is transferred into a low volume 384-well plate.
TNF-a
was detected according to Cisbio's TNF-a HTRF assay (Cisbio, ref no
62TNFPEB). The cell assay was incubated with 5 pL of each HTRF reagent
during 3 h. The amount of TNF-a was detected on a Tecan Saphire 2.
53
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
6. IC50 curves were fitted with GraphPad Prism software.
Examples
Chemicals employed in the synthesis of the compounds in the examples may be
commercially available from, e.g. Sigma-Aldrich Fine Chemicals or Acros or
Int. Alfa
Aesar, Menai Organics, Chembrige and Matrix Scientific.
The invention is illustrated by way of the following examples, in which the
following
abbreviations may be employed:
aq aqueous
Boc tert-butyloxycarbonyl
conc concentrated
DCM dichloromethane
DMSO dimethylsulphoxide
DMF N,N-dimethylformamide
EtOAc ethyl acetate
EtOH ethanol
MeCN acetonitrile
MeOH methanol
MS mass spectrometry
NMR nuclear magnetic resonance
rt room temperature
Pd/C Palladium on activated carbon
Intermediate A
5-(3-Cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one
(a) 2-(3-Cyclopentyloxy-4-methoxyphenyl)-3-(dimethylamino)acrylaldehyde
Me2N
H
A -r-
\
0
MeO
POC13 (15 g, 9.3 mL, 100 mmol) was added to a solution of 2-(3-cyclopentyloxy-
4-
methoxyphenyi)acetic acid (10 g, 40 mmol) in DMF (50 mL). The mixture was
stirred at
70 C for 18 h. After cooling to rt the mixture was poured into a stirred aq
solution of
54
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
K2CO3 (2 M, 200 mL, 400 mmol). NaOH (10 g, 250 mmol) was added in portions to
the
stirred mixture. After the NaOH had dissolved, the mixture was extracted with
toluene.
The combined extracts were washed with brine, dried over Na2SO4 and
concentrated to
give the sub-title compound which was used in the next step without further
purification.
MS (m/z): 290 (M+H+).
1 H NMR (DMSO-d6, 400 MHz): 5 8.93 (s, 1 H), 7.08 (br. s, 1 H), 6.87 (d, 1 H),
6.60 (d, 1 H),
6.57 (dd, 1H), 4.75-4.79 (m, 1H), 3.73 (s, 3H), 2.80 (br. s, 6H), 1.87-1.77
(m, 2H), 1.75-
1.65 (m, 4H), 1.60-1.50 (m, 2H).
(b) 5-(3-Cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one
H
MeO
A solution of HCI in EtOH (1.25 M, 48 mL, 60 mmol) was added to a mixture of
the 2-(3-
cyclopentyloxy-4-methoxyphenyl)-3-(dimethylamino)acrylaldehyde from the
previous
step, urea (4.8 g, 80 mmol) and EtOH (200 mL). The mixture was stirred at 70
C for 3 h.
After cooling to rt the mixture was concentrated and the product was
precipitated by
addition of water (-20 mL). Recrystallization from EtOH gave the sub-title
compound.
Yield 4.3 g (38 % from 2-(3-cyclopentyloxy-4-methoxyphenyl)acetic acid).
MS (m/z): 287 (M+H+).
1H NMR (DMSO-d6, 400 MHz): 6 12.14 (s, 1H), 8.56 (br. s, 2H), 7.15 (d, 1H),
7.10 (dd,
1 H), 6.98 (d, 1 H), 4.95-4.90 (m, 1 H), 3.75 (s, 3H), 1.95-1.84 (m, 2H), 1.77-
1.67 (m, 4H),
1.62-1.52 (m, 2H).
Intermediate B
5-(3-Cyclopentyloxy-4-methoxyphenyl)tetrahydropyrimidin-2-one
HNINH
I\
O
MeO
A mixture of 5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one (1.97 g, 6.87
mmol),
Pd/C (10 %, 500 mg) and EtOH (200 mL) was hydrogenated at ambient temperature
and
pressure for 2 d. The mixture was filtered through Celite and concentrated to
give the
sub-title compound. Yield 1.91 g (96 %).
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
MS (m/z): 291 (M+H+),
1H NMR (CDC13, 400 MHz): 6 6.82 (d, 1 H), 6.75-6.71 (m, 2H), 5.48 (d, 2H),
4.77-4.72 (m,
1H), 3.82 (s, 3H), 3.47-3.37 (m, 4H), 3.16-3.08 (m, 1H), 1.96-1.78 (m, 6H),
1.66-1.56 (m,
2H).
Intermediate C
5-Fluoro-2-iodomethylbenzoxazole
The title compound was prepared as described in international patent
application
W02008/110793, section "Synthetic Preparation of Intermediates of Formula
III",
Preparation Route 1.
Intermediate D
tert-Butyl 2-iodomethylbenzimidazole-1-carbox Irate
The title compound was prepared as described in international patent
application
W02008/110793, section "Synthetic Preparation of Intermediates of Formula
III",
Preparation Route 8.
Intermediate E
2-Bromometh yl-5-methoxybenzofuran
The title compound was prepared as described in international patent
application
W02008/110793, section "Synthetic Preparation of Intermediates of Formula
III",
Preparation Route 11.
Intermediate F
4-Fluoro-2-iodomethylbenzoxazole
The title compound was prepared in accordance with international patent
application
W02008/110793, section "Synthetic Preparation of Intermediates of Formula
III",
Preparation Route 1, from 2-amino-3-fluorophenol.
Intermediate G
7-Fluoro-2-iodomethylbenzoxazole
The title compound was prepared as described in international patent
application
W02008/110793, section "Synthetic Preparation of Intermediates of Formula
III",
Preparation Route 1.
56
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Intermediate H
6-Fluoro-2-iodomethylbenzoxazole
The title compound was prepared in accordance with international patent
application
W020081110793, section "Synthetic Preparation of Intermediates of Formula
III",
Preparation Route 1, from 2-amino-5-fluorophenol.
Intermediate I
2-(Iodomethyl)benzoxazole-4-carbonitrile
The title compound was prepared as described in international patent
application
W02008/110793, section "Synthetic Preparation of Intermediates of Formula
III",
Preparation Route 5.
Intermediate J
2-(Iodomethyl)benzoxazole-7-carbonitrile
The title compound was prepared in accordance with international patent
application
W02008/110793, section "Synthetic Preparation of Intermediates of Formula
III",
Preparation Route 5, from 2-hydroxybenzonitrile.
Example 1
5-(3-Cyclopentyloxy-4-methoxyphenyl)-1-(5-fluorobenzoxazol-
2_ylmethyl)pyrimidin-2-one
NaH (60 % suspention in oil, 20 mg, 0.5 mmol) was added to a solution of
Intermediate A
(115 mg, 0.4 mmol) in DMF (10 mL) at rt. After stirring for 1 h, Intermediate
C (139 mg,
0.5 mmol) was added and the mixture was stirred at rt for 1 h. Ice-cold water
was added
and the mixture was extracted with DCM. The combined extracts were washed with
brine, dried over Na2SO4 and concentrated. Crystallization of the residue from
MeCN
gave the sub-title compound. Yield and spectroscopic data are listed in Table
1.
Example 2
5-(3-Cyclopentyloxy-4-methoxyphenyl)-1-(5-fluorobenzoxazol-2-ylmethyl)tetra-
hydropyrimidin-2-one
A mixture of the 5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(5-fluorobenzoxazol-2-
ylmethyl)pyrimidin-2-one (87 mg, 0.2 mmol, see Example 1), Pd/C (10 %, 50 mg)
and
EtOH (50 mL) was hydrogenated at ambient temperature and pressure for 2 d. The
mixture was filtered through Celite and concentrated. Crystallization of the
residue gave
the title compound. Yield and spectroscopic data are listed in Table 1.
57
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Example 3
tert-Butyl 2-(5-(3-cyclopentyloxy-4-methoxyphenyl)-2-oxopyrimidin-1-
ylmethyl)benzimidazole-1-carboxylate
The title compound was prepared in accordance with Example 1 from
Intermediates A
and D. Yield and spectroscopic data are listed in Table 1.
Example 4
tert-Butyl 2-(5-(3-cyclopentyloxy-4-methoxvphenyl)-2-oxotetrahydropyrimidin-l -
yl-
methyl)benzimidazole-1-carboxylate
The title compound was prepared in accordance with Example 2 from tert-butyl 2-
(5-(3-
cyclopentyloxy-4-methoxyphenyl)-2-oxopyrimidin-1-ylmethyl)benzimidazole-1-
carboxylate (see Example 3). Yield and spectroscopic data are listed in Table
1.
Example 5
5-(3-Cyclopentyloxy-4-methoxvphenyl)-1-(5-methoxybenzofuran-2-ylmethyl)-
tetrahydropyrimidin-2-one
The title compound was prepared in accordance with Example 1 from
Intermediates B
and E. Yield and spectroscopic data are listed in Table 1.
Example 6
1-(Benzimidazol-2-yimethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one
Trifluoroacetic acid (1.5 mL) was added to a mixture of tert-butyl 2-((5-(3-
cyclopentyloxy-
4-methoxyphenyl)-2-oxotetrahydropyrimidin-1-yl)methyl)benzimidazole-1-
carboxylate
(190 mg, 0.37 mmol, see Example 4) and DCM (15 ml-) and the mixture was
stirred at rt
for 4 h. Concentration gave the title compound. Yield and spectroscopic data
are listed in
Table 1.
58
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Example 7
5-(3-(Cyclopentyloxy)-4-methoxvphenvl)-1-((4-fluorobenzoxazol-2-yl)meth
ll)pyrimidin-2-
one
The title compound was prepared in accordance with Example 1 from
Intermediates A
and F. Yield and spectroscopic data are listed in Table 1.
Example 8
5-(3-(Cyclopentyloxy)-4-methoxvphenvl)-1-((7-fluorobenzoxazol-2- l)y
methyl)pyrimidin-2-
one
The title compound was prepared in accordance with Example 1 from
Intermediates A
and G. Yield and spectroscopic data are listed in Table 1.
Example 9
5-(3-(Cyclopentyloxy)-4-methoxvphenvl)-1-((4-fluorobenzoxazol-2-yl)methyl)-
tetrahydropyrimidin-2-one
The title compound was prepared in accordance with Example 2 from 5-(3-
(cyclopentyloxy)-4-methoxyphenyl)-1-((4-fluorobenzoxazol-2-yl)methyl)pyrimidin-
2-one
(see Example 7). Yield and spectroscopic data are listed in Table 1.
Example 10
5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((6-fluorobenzoxazol-2-
yI)methyl)pyrimidin-2-
one
The title compound was prepared in accordance with Example 1 from
Intermediates A
and H. Yield and spectroscopic data are listed in Table 1.
3o Example 11
5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((7-fluorobenzoxazol-2-yl)methyl)-
tetrahydropyrimidin-2-one
The title compound was prepared in accordance with Example 2 from 5-(3-
(cyclopentyloxy)-4-methoxyphenyl)-1 -((7-fluorobenzoxazol-2-
yl)methyl)pyrimidin-2-one
(see Example 8). Yield and spectroscopic data are listed in Table 1.
59
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Example 12
5-(3-(Cvclopentvloxv)-4-methoxyphenvl)-1-((6-fluorobenzoxazol-2-yl)methyl)-
tetrahydropyrimidin-2-one
The title compound was prepared in accordance with Example 2 from 5-(3-
(cyciopentyloxy)-4-methoxyphenyl)-1-((6-fluorobenzoxazol-2-yl)methyl)pyrimidin-
2-one
(see Example 10). Yield and spectroscopic data are listed in Table 1.
Examples 13 and 14
(R)- and (S)-5-(3-Cvclopentvloxv-4-methoxyphenvl)-1-(5-fluorobenzoxazol-2yl-
methyl)pyrimidin-2-ones
The title compounds where prepared by separation of racemic 5-(3-
cyclopentyloxy-4-
methoxyphenyl)-1-(5-fluorobenzoxazol-2-ylmeth ylpyrimidin-2-one (see Example
1) using
preparative HPLC (column: Phenomenex Lux 5p Cellulose-2; eluent:
isopropanol:isohexane, 10:90). The compound of Example 13 had lower retention
time.
The spectroscopic data of the enantiomers are identical to those of the
racemate (see
Example 1). The absolute configurations were not determined.
Example 15
5-(3-(Cvclopentvloxv)-4-methoxyphenvl)-1-((1 3-dimethylpyrazol-5-
yl)methyl)pyrimidin-2-
one
KI (1.2 g, 7.2 mmol) was added to a solution of 5-chloromethyl-1,3-
dimethylpyrazole (415
mg, 2.9 mmol) in DMF (10 mL) and the mixture was stirred at rt for 30 min.
Intermediate
A (822 mg, 2.9 mmol) followed by NaH (60 % suspention in oil, 287 mg, 7.2
mmol) were
added and the mixture was stirred at rt for 1 h. The mixture was quenched with
icecold
water (20 mL) and extracted with DCM (3x20 mL). The combined extracts were
washed
with brine (20 mL), dried over Na2SO4 and concentrated. Crystallization from
MeCN gave
the title compound. Yield and spectroscopic data are listed in Table 1.
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Example 16
5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((1 3-dimethylpyrazol-5- Iy )methyl)-
tetrahydropyrimidin-2-one
The title compound was prepared in accordance with Example 2 from 5-(3-
(cyclopentyloxy)-4-methoxyphenyl)-1-((1,3-dimethylpyrazol-5-
yl)methyl)pyrimidin-2-one
(see Example 15). Yield and spectroscopic data are listed in Table 1.
Example 17
1-((2-Chlorothiazol-5-yl)methyl)-5-(3-(cyclopentyloxy)-4-
methoxyphenyl)pyrimidin-2-one
The title compound was prepared in accordance with Example 15 from
Intermediate A
and 2-chloro-5-(chloromethyl)thiazole. Yield and spectroscopic data are listed
in Table 1.
Example 18
2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxopyrimidin-1 -
yl)methyl)benzoxazole-4-
carbonitrile
The title compound was prepared in accordance with Example 1 from
Intermediates A
and I. Yield and spectroscopic data are listed in Table 1.
Examples 19 and 20
2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxo-2,3-dihydropyrimidin-l-yl)-
methyl)benzoxazole-4-carbonitrile and 2-((5-(3-(cyclopentyloxy)-4-
methoxyphenyl)-2-
oxotetrahydropyrimidin-i-yl)methyl)benzoxazole-4-carbonitrile
A mixture of the title compounds was prepared in accordance with Example 2
from 2-((5-
(3-(cyclopentyloxy)-4-methoxyphenyl)-2-oxopyrimidin-1-yl)methyl)benzoxazole-4-
carbonitrile (see Example 18). The title compounds were obtained by separation
using
preparative HPLC (column: Agilent XDB-C18; eluent: MeCN:water gradient, 5:95 -
100:0). Yields and spectroscopic data are listed in Table 1.
Example 21
2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxopyrimidin-1-
yl)methyl)benzoxazole-7-
carbonitrile
The title compound was prepared in accordance with Example 1 from
Intermediates A
and J. Yield and spectroscopic data are listed in Table 1.
61
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Examples 22 and 23
(5-(3-(Cyclopentyloxy)-4-methox yphenyl)-2-oxo-2 3-dihydropyrimidin-1-yl)-
methyl)benzoxazole-7-carbonitrile and 2-((5-(3-(cyclopentyloxy)-4-
methoxyphenyl)-2-
oxotetrahydropyrimidin-1-yl)methyl)benzoxazole-7-carbonitrile
A mixture of the title compounds was prepared in accordance with Example 2
from 2-((5-
(3-(cyclopentyloxy)-4-methoxyphenyl)-2-oxopyrimidin-1-yl)methyl)benzoxazole-7-
carbonitrile (see Example 21). The title compounds were obtained by separation
using
preparative HPLC (column: Agilent XDB-C18; eluent: MeCN:water, gradient 5:95 -
100:0). Yields and spectroscopic data are listed in Table 1.
Table 1.
Chemical structure MS (m/z) Yield (%)
Ex. Name
NMR
0
Nk N
F
~
436 (M+H+) 40
1 o10
MeO
1 5-(3-Cyclopentyloxy-4-methoxyphenyl)-1-(5-fluorobenzoxazol-2-yl-
methyl)pyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) 6 9.06 (d, 1 H), 8.72 (d, 1 H), 7.80 (dd,
1 H), 7.62 (dd, 1 H), 7.27 (ddd, 1 H), 7.19 (d, 1 H), 7.16 (dd, 1 H), 7.04 (d,
1H), 5.48 (s, 2H), 4.94-4.89 (m, 1H), 3.77 (s, 3H), 1.96-1.85 (m, 2H),
1.77-1.67 (m, 4H), 1.62-1.52 (m, 2H)
NINH
F / o
2 440 (M+H+) 41
o10
MeO
5-(3-Cyclopentyloxy-4-methoxyphenyl)-1-(5-fluorobenzoxazol-2-yl-
methyl)tetrahydropyrimidin-2-one
62
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Chemical structure JMS (m/z) Yield (%)
Ex. Name
NMR
'H NMR (DMSO-d6, 400 MHz) 8 7.73 (dd, 1 H), 7.60 (dd, 1 H), 7.24
(ddd, 1 H), 6.89 (d, 1 H), 6.87 (d, 1 H), 6.81 (dd, 1 H), 6.70 (d, 1 H), 4.82
(d, 1 H), 4.79-4.74 (m, 1 H), 4.68 (d, 1 H), 3.70 (s, 3H), 3.59 (dd, 1 H),
3.51 (ddd, I H), 3.38-3.26 (m, 2H), 3.24-3.16 (m, I H), 1.90-1.80 (m,
2H), 1.74-1.63 (m, 4H), 1.59-1.50 (m, 2H)
~Of N j
~~j 417 (M-Boc+H) 74
,0
0
MeO
3 tert-Butyl 2-(5-(3-cyclopentyloxy-4-methoxyphenyl)-2-oxopyrimidin-1-
ylmethyl)benzimidazole-1-carboxylate
' H NMR (DMSO-d6, 400 MHz) 6 9.07 (d, 1H), 8.62 (d, I H), 7.94 (d,
1H), 7.63 (d, 1H), 7.40 (ddd, 1H), 7.32 (ddd, 1H), 7.19-7.15 (m, 2H),
7.03 (d, 1H), 5.63 (s, 2H), 4.93-4.88 (m, 1H), 3.77 (s, 3H), 1.95-1.85
(m, 2H), 1.77-1.67 (m, 4H), 1.73 (s, 9H), 1.60-1.52 (m, 2H)
o~o
o
N-111-,~ N~NH
6-N
421 (M-Boc+H+) 99
o
We
tert-Butyl 2-(5-(3-cyclopentyloxy-4-methoxyphenyl)-2-oxotetrahydro-
4 pyrimidin-1-y1methyl)benzimidazole-1-carboxylate
'H NMR (DMSO-d6, 400 MHz) 8 7.93-7.89 (m, 1 H), 7.71-7.67 (m, 1 H),
7.38-7.30 (m, 2H), 6.91 (d, 1H), 6.88 (d, 1H), 6.82 (dd, 1H), 6.57 (d,
1 H), 4.93 (d, 1 H), 4.88 (d, 1 H), 4.81-4.76 (m, 1 H), 3.70. (s, 3H), 3.71-
3.65 (m, 1H), 3.55-3.49 (m, 1H), 3.41-3.26 (m, 3H), 1.90-1.81 (m, 2H),
1.75-1.64 (m, 4H), 1.67 (s, 9H), 1.59-1.50 (m, 2H)
63
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Chemical structure MS (m/z) Yield (%)
Ex. Name
NMR
NNH
Me0 / , O
451 (M+H+) 14
I~ 10
0
MeO
5-(3-Cyclopentyloxy-4-methoxyphenyl)-1-(5-methoxybenzofuran-2-yl-
methyl)tetrahydropyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) S 7.41 (d, 1H), 7.06 (d, 1H), 6.87-6.82
(m, 3H), 6.77 (dd, 1 H), 6.63 (s, 1 H), 6.58 (d, 1 H), 4.75-4.70 (m, 1 H),
4.66 (d, 1 H), 4.51 (d, 1 H), 3.75 (s, 3H), 3.69 (s, 3H), 3.42-3.26 (m,
2H), 3.34-3.28 (m, 1 H), 3.27-3.21 (m, 1 H), 3.15-3.07 (m, 1 H), 1.86-
1.77 (m, 2H), 1.73-1.60 (m, 4H), 1.57-1.48 (m, 2H)
N NNH
NH
(~ 421 (M+H+) 99
1 01/
MeO
6 1 -(Benzimidazol-2-ylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)-
tetrahydropyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) 5 7.82-7.77 (m, 2H), 7.55-7.50 (m, 2H),
6.91 (d, 1 H), 6.89 (d, 1 H), 8.87 (d, 1 H), 6.80 (dd, 1 H), 4.90 (d, 1 H),
4.86 (d, 1 H), 4.79-4.74 (m, 1 H), 3.71 (s, 3H), 3.68 (dd, 1 H), 3.57 (ddd,
1 H), 3.40-3.25 (m, 3H), 1.90-1.80 (m, 2H), 1.75-1.64 (m, 4H), 1.60-
1.50 (m, 2H)
F NI N
/ \ o 1
7 I ,0 436 (M+H+) 50
o
MeO
5-(3-(Cyclopentyloxy)-4-methoxyphenyi)-1-((4-fiuorobenzoxazol-2-yl)-
methyl)pyrimidin-2-one
64
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Chemical structure MS (m/z) yield (%)
Ex. Name
NMR
'H NMR (DMSO-d6, 400 MHz) 6 9.08 (d, 1 H), 8.73 (d, 1 H), 7.64 (dd,
1 H), 7.45 (td, 1 H), 7.27 (ddd, 1 H), 7.20 (d, 1 H), 7.18 (dd, 1 H), 7.05 (d,
1H), 5.51 (s, 2H), 4.95-4.90 (m, 1H), 3.78 (s, 3H), 1.97-1.85 (m, 2H),
1.78-1.67 (m, 4H), 1.62-1.52 (m, 2H)
F 436 (M+H+) 20
o
MeO
8 5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((7-fluorobenzoxazol-2-yl)-
methyl)pyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) 6 9.08 (d, 1 H), 8.73 (d, 1 H), 7.58 (dd,
1 H), 7.43-7.34 (m, 2H), 7.20 (d, 1 H), 7.18 (dd, 1 H), 7.05 (d, 1 H), 5.52
(s, 2H), 4.94-4.89 (m, 1 H), 3.78 (s, 3H), 1.97-1.85 (m, 2H), 1.78-1.67
(m, 4H), 1.62-1.52 (m, 2H)
" N NH
440 (M+H+) 98
Meo
9 5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((4-fluorobenzoxazol-2-yI)-
methyl)tetrahydropyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) 6 7.57 (dd, 1 H), 7.40 (td, 1 H), 7.24
(ddd, 1 H), 6.90 (d, 1 H), 6.86 (d, 1 H), 6.81 (dd, 1 H) 6.72 (d, 1 H), 4.85
(d, 1 H), 4.79-4.74 (m, 1 H), 4.71 (d, 1 H), 3.70 (s, 3H), 3.60 (dd, 1 H),
3.53 (ddd, 1 H), 3.33 (dd, 1 H), 3.31-3.27 (m, 1 H), 3.24-3.17 (m, 1 H),
1.89-1.80 (m, 2H), 1.74-1.63 (m, 4H), 1.58-1.50 (m, 2H)
N
1
436 (M+H+) 32
F I \ r-\
MeO
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Chemical structure MS (m/z) Yield (%)
Ex. Name
NMR
5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((6-fluorobenzoxazol-2-yI)-
methyl)pyrimidin-2-one
' H NMR (DMSO-d6, 400 MHz) S 9.07 (d, 1 H), 8.72 (d, 1 H), 7.78 (dd,
1 H), 7.75 (dd. 1 H), 7.27 (dd, 1 H), 7.25 (dd, 1 H), 7.19 (d, 1 H), 7.16 (d,
I H), 7.05 (d, 1 H), 5.47 (s, 1 H), 4.94-4.90 (m, 1 H), 3.78 (s, 1 H), 1.96-
1.87 (m, 2H), 1.78-1.68 (m, 4H), 1.62-1.53 (m, 2H)
NNH
440 (M+H+) 99
F
MeO
11 5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((7-fluorobenzoxazol-2-yl)-
methyl)tetrahydropyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) 5 7.57 (dd, 1 H), 7.37 (td, 1 H), 7.32
(ddd, 1 H), 6.90 (d, 1 H), 6.86 (d, 1 H), 6.81 (dd, 1 H), 6.72 (d, 1 H); 4.87
(d, 1 H), 4.79-4.74 (m, 1 H), 4.72 (d, 1 H), 3.70 (s, 3H), 3.60 (dd, 1 H),
3.53 (ddd, 1 H), 3.38-3.32 (m, 1 H), 3.32-3.26 (m, 1 H), 3.25-3.17 (m,
1 H), 1.90-1.79 (m, 2H), 1.74-1.62 (m, 4H), 1.60-1.50 (m, 2H)
NNH
440 (M+H+) 99
F r\
Meo
12 5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((6-fluorobenzoxazol-2-yI)-
methyl)tetrahydropyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) S 7.73 (dd, 1 H), 7.69 (dd, 1 H), 7.23
(ddd, 1 H), 6.89 (d, 1 H), 6.86 (d, 1 H), 6.80 (dd, 1 H), 6.70 (d, 1 H), 4.83
(d, 1 H), 4.79-4.74 (m, 1 H), 4.68 (d, 1 H), 3.70 (s, 3H), 3.57 (dd, 1 H),
3.51 (ddd, 1 H), 3.37-3.32 (m, 1 H), 3.32-3.26 (m, 1 H), 3.23-3.16 (m,
1H), 1.90-1.79 (m, 2H), 1.74-1.63 (m, 4H), 1.59-1.50 (m, 2H)
66
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Chemical structure MS (m/z) JYield (%)
Ex. Name
NMR
N
N-N
395 (M+H+) 50
I~ ,0
o
MeO
15 5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((1,3-dimethylpyrazol-5-yf)-
methyl)pyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) 6 8.95 (d, 1 H), 8.56 (d, 1 H), 7.14 (d,
1 H), 7.12 (dd, 1 H), 7.02 (d, 1 H), 5.99 (s, 1 H), 5.13 (s, 2H), 4.92-4.90
(m, 1 H), 3.82 (s, 3H), 3.76 (s, 3H), 2.06 (s, 3H), 1.94-1.86 (m, 2H),
1.76-1.68 (m, 4H), 1.63-1.54 (m, 2H)
NNH
N-
399 (M+H+) 99
1\ O
~ o
MeO
16 5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-1-((1,3-dimethyIpyrazol-5-yl)-
methyl)tetrahydropyrimidin-2-one
'H NMR (DMSO-d6, 400 MHz) 6 6.85 (d, 1 H), 6.84 (d, 1 H), 6.76 (dd,
1 H), 6.55 (d, 1 H), 5.90 (s, 1 H), 4.75-4.73 (m, 1 H), 4.45 (s, 2H), 3.69
(s, 3H), 3.66 (s, 3H), 3.31-3.19 (m, 4H), 3.09-3.02 (m, 1H), 2.06 (s,
3H), 1.88-1.80 (m, 2H), 1.74-1.62 (m, 4H), 1.59-1.51 (m, 2H)
N'Y'NIP
H
ci 418 (M+H+) 13
I~
MeO
17 1-((2-Chlorothiazof-5-yf)methyl)-5-(3-(cyclopentyloxy)-4-methoxy-
phenyl)pyrimidin-2-one
' H NMR (DMSO-d6, 400 MHz) 6 8.97 (d, 1 H), 8.68 (d, 1 H), 7.82 (s,
1 H), 7.14 (d, 1 H), 7.11 (dd, 1 H), 7.02 (d, 1 H), 5.26 (s, 2H), 4.93-4.88
(m, 1 H), 3.77 (s, 3H), 1.95-1.85 (m, 2H), 1.78-1.68 (m, 4H), 1.63-1.53
(m, 2H)
67
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Chemical structure MS (m/z) Yield (%)
Ex. Name
NMR
NN
- 443 (M+H+) 36
I\ O
~ o
MeO
18 2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxopyrimidin-l-yl)-
methyl)benzoxazole-4-carbonitrile
'H NMR (DMSO-d6, 400 MHz) 6 9.09 (d, 1 H), 8.74 (d, 1 H), 8.17 (dd,
1 H), 7.92 (dd, 1 H), 7.60 (dd, 1 H), 7.21 (d, 1 H), 7.19 (dd, 1 H), 7.05 (d,
1 H), 5.57 (s, 2H), 4.95-4.90 (m, 1 H), 3.78 (s, 3H), 1.97-1.86 (m, 2H),
1.78-1.67 (m, 4H), 1.63-1.52 (m, 2H)
N NH
- 445 (M+H+) 16 JD o
MeO
19 2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxo-2,3-dihydro-
pyrimidin-1-yl)methyl)benzoxazole-4-carbonitrile
'H NMR (DMSO-d6, 400 MHz) 6 8.76 (d, 1 H), 8.15 (dd, 1 H), 7.90 (dd,
1 H), 7.57 (dd, 1 H), 6.89 (d, 1 H), 6.87 (d, 1 H), 6.76 (dd, 1 H), 6.63 (d,
1 H), 4.93 (s, 2H), 4.87-4.82 (m, 1 H), 4.32 (s, 2H), 3.71 (s, 3H), 1.90-
1.80 (m, 2H), 1.75-1.65 (m, 4H), 1.59-1.50 (m, 2H)
N NH
20 - 447 (M+H+) 21
p~o
MeO
2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxotetrahydropyrimidin-
1-yi)methyl)benzoxazole-4-carbonitrile
68
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Chemical structure MS (m/z) Yield (%)
Ex. Name
NMR
'H NMR (DMSO-d6, 400 MHz) 8 8.11 (dd, 1 H), 7.89 (dd, 1 H), 7.56 (dd,
1 H), 6.89 (d, 1 H), 6.87 (d, 1 H), 6.83 (dd, 1 H), 6.74 (d, 1 H), 4.89 (d,
1 H), 4.79-4.74 (m, 1 H), 4.77 (d, 1 H), 3.70 (s, 3H), 3.61 (dd, 1 H), 3.55
(ddd, 1 H), 3.39-3.31 (m, 1 H), 3.31-3.27 (m, 1 H), 3.26-3.18 (m, 1 H),
1.90-1.80 (m, 2H), 1.74-1.62 (m, 4H), 1.59-1.50 (m, 2H)
N
443 (M+H+) 99
N
O
Meo
21 2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxopyrimidin-1-yl)-
methyl)benzoxazole-7-carbonitrile
'H NMR (DMSO-d6, 400 MHz) S 9.08 (d, 1 H), 8.73 (d, 1 H), 8.12 (dd,
1 H), 7.95 (dd, 1 H), 7.57 (t, 1 H), 7.19 (d, 1 H), 7.17 (dd, 1 H), 7,05 (d,
1 H), 5.57 (s, 2H), 4.94-4.89 (m, 1 H), 3.78 (s, 3H), 1.95-1.86 (m, 2H),
1.78-1.67 (m, 4H), 1.62-1.53 (m, 2H)
N~NI NH
445 (M+H+) 37
N I
\
\ o~
Meo
22 2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxo-2,3-dihydro-
pyrimidin-l-yl)methyl)benzoxazole-7-carbonitrile
'H NMR (DMSO-d6, 400 MHz) 8 8.75 (d, 1 H), 8.12 (dd, 1 H), 7.91 (dd,
1 H), 7.56 (t, 1 H), 6.89 (d, 1 H), 6.86 (d, 1 H), 6.74 (dd, 1 H), 6.63 (d,
1 H), 4.93 (s, 2H), 4.87-4.82 (m, 1 H), 4.43 (s, 2H), 3.72 (s, 3H), 1.90-
1.80 (m, 2H), 1.75-1.65 (m, 4H), 1.58-1.50 (m, 2H)
N~~NNH
o
23 447 (M+H+) 50
N
\\ \ Jam/
Meo
69
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
Chemical structure MS (m/z) Yield (%)
Ex. Name
NMR
2-((5-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-oxotetrahydropyrimidin-
1-yl)methyl)benzoxazole-7-carbonitrile
'H NMR (DMSO-d6, 400 MHz) 8 8.11 (dd, 1 H), 7.90 (dd, 1 H), 7.55 (t,
1 H), 6.89 (d, 1 H), 6.87 (d, 1 H), 6.82 (dd, 1 H), 6.74 (d, 1 H), 4.90 (d,
1 H), 4.79-4.75 (m, 1 H), 4.77 (d, 1 H), 3.70 (s, 3H), 3.61 (dd, 1 H), 3.55
(ddd, 1 H), 3.38-3.31 (m, 1 H), 3.31-3.27 (m. 1 H), 3.25-3.18 (m, 1 H),
1.90-1.80 (m, 2H), 1.74-1.63 (m, 4H), 1.59-1.49 (m, 2H)
Example 24
The following examples/compounds of the invention are prepared in accordance
with the
techniques described herein:
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(7-methoxy-2-
benzofurylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-fluoro-2-
benzofurylmethyl)tetrahydropyri-
midin-2-one;
1-(2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5,6-dimethyl-2-
benzimidazolylmethyl)tetra-
hydropyrimidin-2-one;
1-(2-benzofurylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)tetrahydropyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-thienylmethyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(2-furylmethyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-imidazolylmethyl)tetrahydropyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(1 -methyl-2-
imidazolylmethyl)tetrahydropyrimi-
din-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-oxazolylmethyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-thiazolylmethyl)tetrahydropyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(3-indolylmethyl)tetrahydropyrimidin-2-
one;
1-(3-benzothienylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
1-(2-benzothiazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
5-(3-cyciopentyioxy-4-methoxyphenyl)-1-(5-methyl-2-
benzoxazolylmethyl)tetrahydro-
pyrimidin-2-one;
1-(6-chloro-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-methyl-2-
benzoxazolylmethyi)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(6-methyl-2-
benzoxazolylmethyl)tetrahydro-
pyrimidin-2-one;
1-(1-benzotriazoiylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-nitro-2-
benzoxazolylmethyl)tetrahydropyrimi-
din-2-one;
1 -(5-cya no-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahyd ropy ri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-fiuoro-2-
benzoxazolylmethyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-trifluoromethyl-2-
benzoxazolylmethyl)tetra-
hydropyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-methyl-2-
benzoxazolylmethyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methoxy-2-
benzoxazolylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-methoxy-2-
benzoxazoiylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyioxy-4-methoxyphenyl)-1-(4-fiuoro-2-
benzoxazolylmethyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-fiuoro-2-
benzoxazolyimethyl)tetrahydropyri-
midin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyri-
midin-2-one;
1-(7-chloro-2-benzoxazolylmethyl)-5-(3-cyciopentyioxy-4-
methoxyphenyl)tetrahydropyri-
midin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-trifluoromethyl-2-
benzoxazolylmethyl)tetra-
hydropyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methoxy-2-
benzofurylmethyl)tetrahydropyri-
midin-2-one;
71
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
1-(2-benzimidazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetrahydropyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(1 -methyl-2-
benzimidazolylmethyl)tetrahydro-
pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(5,6-dichloro-2-
benzimidazolylmethyl)tetra-
hydropyrimidin-2-one;
1-(4-acetamidoxy-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetra-
hydropyrimidin-2-one;
31(7-acetamidoxy-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)tetra-
hydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
difluoromethoxyphenyl)tetra-
hydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
trifluoromethoxyphenyl)tetra-
hydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-difluoromethoxy-3-(3-
tetrahydrofuranyloxy)-
phenyl]tetrahydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-methoxy-3-(3-
tetrahydrofuranyloxy)phenyl]-
tetrahydropyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-trifluoromethoxy-3-(3-
tetrahydrofuranyloxy)-
phenyl]tetrahydropyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-methoxy-2-benzofuryimethyl)pyrimid
in-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(7-fluoro-2-benzofurylmethyl)pyrimidin-
2-one;
1-(2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5,6-dimethyl-2-
benzimidazolylmethyl)pyrimidin-
2-one;
1-(2-benzofurylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-thienylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-furylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-imidazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(1-methyl-2-imidazolylmethyl)pyrimidin-
2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-oxazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(2-thiazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(3-indolylmethyl)pyrimidin-2-one;
1-(3-benzothienylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-one;
1-(2-benzothiazoiylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methyl-2-benzoxazolylmethyl)
pyrimidin-2-
one;
72
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
1-(6-chloro-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-methyl-2-
benzoxazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(6-methyl-2-
benzoxazolylmethyl)pyrimidin-2-
one;
1-(1-benzotriazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-nitro-2-benzoxazolylmethyl)pyrimidin-
2-one;
1-(5-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-
2-one
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-fluoro-2-
benzoxazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-trifluoromethyl-2-
benzoxazolylmethyl)pyrimi-
din-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-methyl-2-
benzoxazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methoxy-2-
benzoxazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-methoxy-2-
benzoxazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-fluoro-2-
benzoxazolylmethyl)pyrimidin-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(7-fluoro-2-
benzoxazolylmethyl)pyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-
2-one
1-(7-chloro-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyI)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(4-trifluoromethyl-2-
benzoxazolylmethyl)pyrimi-
din-2-one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5-methoxy-2-benzofurylmethyl)pyrimidin-
2-one;
1-(2-benzimidazolylmethyl)-5-(3-cyclopentyloxy-4-methoxyphenyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1 -(1-methyl-2-
benzimidazolylmethyl)pyrimidin-2-
one;
5-(3-cyclopentyloxy-4-methoxyphenyl)-1-(5,6-dichloro-2-
benzimidazolylmethyl)pyrimidin-
2-one;
1-(4-acetamidoxy-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)pyrimi-
din-2-one;
1-(7-acetamidoxy-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
methoxyphenyl)pyrimi-
din-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
difluoromethoxyphenyl)pyrimi-
din-2-one;
73
CA 02736970 2011-03-11
WO 2010/029299 PCT/GB2009/002169
1-(4-cyano-2-benzoxazolylmethyl)-5-(3-cyclopentyloxy-4-
trifluoromethoxyphenyl)pyrimi-
din-2-one;
1-(4-cyano-2-benzoxazolylmethyl)-5-[4-difluoro methoxy-3-(3-
furanyloxy)phenyl]pyrimidin-
2-one;
1 -(4-cyano-2-benzoxazo lylmethyl)-5-[4-methoxy-3-(3-fu
ranyloxy)phenyl]pyrimidin-2-one;
1-(4-cyano-2-benzoxazolylmeth yl)-5-[4-trifluorometh oxy-3-(3-
furanyloxy)phenyl]pyrimi-
din-2-one.
Example 25
1o Title compounds of the Examples were tested in a biological test described
above
(PBMC cell assay) and were found to inhibit PDE-4. Thus, when the total
concentration
of title compounds in the assay was 10 NM, the following %-inhibition values
where
obtained:
Example % inhibition
1 74
2 77
3 82
4 88
5 81
Example 26
Title compounds of the Examples were tested in the biological test described
above and
were found to inhibit PDE-4. Thus, the following IC50 values where obtained:
Example IC50 Example IC50
1 3700 13 870
2 540 14 670
3 1600 15 12000
4 530 16 9400
5 160 17 3300
6 520 18 250
7 1300 19 14
8 3200 20 87
9 350 21 160
10 2500 22 12
11 290 23 130
12 1000
74