Note: Descriptions are shown in the official language in which they were submitted.
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
METHODS AND KITS FOR TREATING CLUSTER HEADACHE
DISORDERS
Background of the Invention
In general, the invention relates to therapies for cluster headache
disorders.
Also known as "suicide headaches," cluster headaches are characterized
by an extreme level of pain. Cluster headache disorders often manifest as
recurring bouts of frequent attacks, called cluster periods, which may last
from
weeks to months. In episodic cluster headache disorder, cluster periods are
interrupted by periods of remission when the attacks stop completely. In
chronic cluster headache disorder, attacks continue unremittingly or with
infrequent or brief remission periods.
Cluster headache disorders are not per se life-threatening; however, they
are traumatic and debilitating. Although treatments exist, including abortive
treatments (e.g., oxygen and sumatriptan) for stopping an initiated cluster
headache attack and suppressive treatments (e.g., corticosteroids and lithium)
for decreasing the frequency or severity of attacks, in some subjects, usually
those with the chronic form, the disorder is medically intractable, with
patients
responding poorly or not at all to treatment. Thus, there remains a need for
effective therapies for cluster headache disorders, particularly for chronic
cluster headache disorder.
Summary of the Invention
The invention features methods and kits for treating a recurrent cluster
headache disorder in a subject and for extending the period of remission in a
subject with a cluster headache disorder by administering bromolysergide.
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
In a first aspect, the invention features a method of treating a recurrent
cluster headache disorder in a subject in need thereof by administering 2-
bromolysergic acid diethylamide, or a pharmaceutically acceptable salt
thereof,
to the subject in an amount sufficient to treat the recurrent cluster headache
disorder.
The invention also features a method of treating a chronic cluster
headache disorder in a subject in need thereof by administering 2-
bromolysergic acid diethylamide, or a pharmaceutically acceptable salt
thereof,
to the subject in an amount sufficient to treat the chronic cluster headache
disorder.
The invention further features a method of treating an episodic cluster
headache disorder in a subject in need thereof by administering 2-
bromolysergic acid diethylamide, or a pharmaceutically acceptable salt
thereof,
to the subject in an amount sufficient to treat the episodic cluster headache
disorder.
In a related aspect the invention features a method of extending the
remission period of a subject with a cluster headache disorder, the method
including administering 2-bromolysergic acid diethylamide, or a
pharmaceutically acceptable salt thereof, to the subject while the subject is
in
remission in an amount sufficient to extend the remission period. In certain
embodiments, the cluster headache disorder is episodic cluster headache
disorder. In other embodiments, the cluster headache disorder is chronic
cluster
headache disorder. In particular embodiments, the subject is in remission, but
is experiencing autonomic symptoms characteristic of an impending cluster
headache attack.
In any of the above methods, the 2-bromolysergic acid diethylamide can
be administered orally, subcutaneously, intravenously, or intramuscularly.
2
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
In any of the above methods, the method can include administering to
the subject a dose of from 20 to 50 jig/kg of 2-bromolysergic acid
diethylamide,
two doses of from 20 to 50 tig/kg of 2-bromolyscrgic acid diethylamide
administered over a period of 2 to 7 days, or three doses of from 20 to 50
g/kg
of 2-bromolysergic acid diethylamide administered over a period of 4 to 20
days.
In certain embodiments of the above methods, the method can include
administering to the subject a unit dosage form including from 1.5 to 5 mg of
2-
bromolysergic acid diethylamide. For example, the unit dosage form can
include from 1.5 to 3.5 mg, 1.5 to 2.5 mg, 2.0 to 4.5 mg, 2.5 to 4.5 mg, 2.0
to
3.5 mg, 2.5 to 3.5 mg, 3.0 to 5.0 mg, 3.0 to 4.5 mg, 2.0 to 5.0 mg, or 2.0 to
4.0
mg of 2-bromolysergic acid diethylamide. In particular embodiments, two
doses of the unit dosage form are administered to the subject over a period of
2
to 7 days. In still other embodiments, three doses of the unit dosage form are
administered to the subject over a period of 4 to 20 days. For example, three
doses of a unit dosage form including from 1.5 to 3 mg of 2-bromolyscrgic acid
diethylamide can be administered to the subject over a period of 7 to 12 days.
In one particular embodiment, three doses of a unit dosage form including from
1.5 to 3 mg of 2-bromolysergic acid diethylamide is administered to the
subject
once every five days over a period of 11 days.
In a particular embodiment of any of the above methods, the subject has
a cluster headache disorder that is refractory to one or more prophylactic
therapies. For example, the cluster headache disorder can be refractory to
treatment using corticosteroids (e.g., hydrocortisone, prednisone,
methylprednisolone, triamcinolone, bethamethasone, and dexamethasone,
among other corticosteroids); tricyclic antidepressants (e.g., amitriptyline,
amoxapine, clomipramine, desipramine, dothiepin, doxepin, imipramine,
lofepramine, maprotiline, mianserin, mirtazapine, nortriptyline, octriptyline,
oxaprotiline, protriptyline, and trimipramine, among other tricyclic
3
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
antidepressants); calcium channel blockers (nifedipine, amlodipine,
felodipine,
flunarizine, isradipine, nicardipine, diltiazem, verapamil, and bepridil,
among
other calcium channel blockers); beta blockers (e.g., propanolol, nadolol,
timolol, pindolol, labetolol, metoprolol, atenolol, esmolol, acebutolol,
carvedilol, bopindolol, carteolol, oxprenolol, penbutolol, medroxalol,
bucindolol, levobutolol, metipranolol, bisoprolol, nebivolol, betaxolol,
celiprolol, solralol, and propafenone, among other beta blockers);
anticonvulsants (e.g., valproate and topiramate, among other anticonvulsants);
methysergide; and/or lithium.
In a related aspect, the invention features a kit including: (i) a
pharmaceutical composition including 2-bromolysergic acid diethylamide, or a
pharmaceutically acceptable salt thereof; and (ii) instructions for
administering
the composition to a subject for the treatment of a recurrent cluster headache
disorder.
The invention further features a kit including: (i) a pharmaceutical
composition including 2-bromolysergic acid diethylamide, or a
pharmaceutically acceptable salt thereof; and (ii) instructions for
administering
the composition to a subject for the treatment of chronic cluster headache
disorder.
The invention also features a kit including: (i) a pharmaceutical
composition including 2-bromolysergic acid diethylamide, or a
pharmaceutically acceptable salt thereof; and (ii) instructions for
administering
the composition to a subject for the treatment of episodic cluster headache
disorder.
The invention further features a kit including: (i) a pharmaceutical
composition including 2-bromolysergic acid diethylamide, or a
pharmaceutically acceptable salt thereof; and (ii) instructions for
administering
the composition to a subject for extending the remission period of a subject
4
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
with a cluster headache disorder. In certain embodiments, the cluster headache
disorder is episodic cluster headache disorder or chronic cluster headache
disorder.
In certain embodiments of the above kits, the pharmaceutical
composition is a unit dosage form (e.g., a tablet, capsule, or caplet)
including
1.5 to 5 mg of 2-bromolysergic acid diethylamide, or a pharmaceutically
acceptable salt thereof.
In a particular embodiment of the above kits, the kit of the invention
includes from two to four doses (e.g., two, three, or four doses) of the
pharmaceutical composition and instructions for administering the doses to the
subject over a period of 4 to 20 days, or any other dosing regimen described
herein.
As used herein, "bromolysergide" refers to the compound 2-
bromolysergic acid diethylamide or a pharmaceutically acceptable salt of 2-
bromolysergic acid diethylamide.
By a "cluster headache disorder" is meant a disorder characterized by
frequent attacks of short lasting, severe, uniform, unilateral head pain
associated with autonomic symptoms occurring ipsilateral to the pain (e.g.
lacrimation and/or nasal congestion) (see, for example, Headache
Classification
Committee of the International Headache Society (2004) The International
Classification of Headache Disorders, 2nd edn. Cephalalgia 24 Suppl 1: 1-160).
The pain is generally intense and is often described as a burning, boring,
stabbing or piercing sensation. For example, a subject with a cluster headache
disorder can have at least 2, 3, 4, or 5 attacks of severe unilateral orbital,
supraorbital, and/or temporal pain lasting 15-180 minutes each and having an
average frequency of from one every other day to 8 per day if left untreated.
The attacks, referred to herein as "cluster headache attacks" or simply
"attacks," are associated with one or more of the following autonomic
symptoms occurring ipsilateral to the pain: conjunctival injection and/or
5
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
lacrimation; nasal congestion and/or rhinorrhoea; eyelid edema; forehead and
facial sweating; and miosis and/or ptosis. The attacks may also be associated
with a sense of restlessness or agitation. During part (but less than half) of
the
time-course of a cluster period, attacks may be less severe and/or of shorter
or
longer duration. During part (but less than half) of the time-course of a
cluster
period, attacks may be less frequent. Attacks usually occur in series (cluster
periods) lasting for weeks or months separated by remission periods usually
lasting weeks, months, or years. During a cluster period, attacks occur
regularly and may be provoked by alcohol, histamine, or nitroglycerin. Pain is
maximal orbitally, supraorbitally, temporally, or in any combination of these
sites, but may spread to other regions of the head. Pain almost invariably
recurs
on the same side during an individual cluster period. During the worst
attacks,
the intensity of pain is excruciating. Patients are usually unable to lie down
and
characteristically pace the floor. Cluster headache disorders include, but are
not limited to, cluster headache with coexistent trigeminal neuralgia (cluster-
tic
syndrome), episodic cluster headache disorder, and chronic cluster headache
disorder. Cluster headache is typically diagnosed by a well characterized
clinical presentation.
By "episodic cluster headache disorder" is meant a disorder in which the
subject meets the criteria for cluster headache disorder and experiences at
least
two cluster periods lasting 7 to 365 days cumulative separated by pain-free
remission periods of about one month or longer. For example, a subject with
episodic cluster headache disorder may experience an attack once every other
day for a period of three weeks, followed by a remission period lasting two
months before the occurrence of another cluster period. Subjects with episodic
cluster headache disorder typically have cluster periods lasting between 2
weeks and 3 months.
6
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
By "chronic cluster headache disorder" is meant a disorder in which the
subject meets the criteria for cluster headache disorder and experiences
attacks
unremittingly or with infrequent or short remission periods of less than about
one month cumulative over a period of a year. For example, a subject with
chronic cluster headache disorder may experience an attack once every other
day for a period of 200 days, followed by a pain free remission period of 15
days, followed by one or more attacks daily for 150 days. Chronic cluster
headache disorder may arise de novo or evolve from the episodic cluster
headache disorder.
By a "recurrent cluster headache disorder" is meant chronic cluster
headache disorder or episodic cluster headache disorder.
By "remission period" is meant a period of at least two days during
which a subject with a cluster headache disorder does not experience any
cluster headache attacks. The remission period may be, e.g., 2, 10, 30, or 90
days, or 1, 2, or 10 years.
By "extending the remission period" is meant delaying the occurrence of
a cluster period in a subject with a cluster headache disorder who is in
remission and undergoing a therapy of the invention relative to the length of
remission period that would have been experienced by the subject in the
absence of treatment, or relative to the average length of remission
experienced
by a matched set of subjects with a cluster headache disorder of similar type
and severity who are not undergoing a therapy of the invention. The delay
extending the remission period may be, e.g., at least 2, 7, 30, 90, or 180
days.
Thus, in the claims and embodiments, "extending the remission period"
involves the administration to a subject who is already in remission for
prophylactic purposes.
As used herein, the teflil "autonomic symptoms" refers to a change in an
autonomic function in a subject. Examples of autonomic symptoms include,
but are not limited to, postural hypotension, increased forehead and facial
7
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
sweating, dry eyes, dry mouth, and failure of accommodation (e.g., dilated
pupils or miosis), ptosis, lacrimation, eyelid edema, and/or rhinorrhea.
As used herein, "treating" refers to administering a pharmaceutical
composition for therapeutic purposes. "Therapeutic treatment" refers to
providing treatment to a subject suffering from a disorder to ameliorate the
disorder and improve the subject's condition. Thus, in the claims and
embodiments, treating a subject with a cluster headache disorder involves the
administration of a composition to the subject for therapeutic purposes.
The term "administration" or "administering" refers to a method of
giving a dose of a pharmaceutical composition to a patient, where the method
is, e.g., oral, topical, subcutaneous, by inhalation, intravenous,
intraperitoneal,
intracerebroventricular, intrathecal, or intramuscular. The preferred method
of
administration can vary depending on, e.g., the components of the
pharmaceutical composition, the general health of the patient, and the
severity
of the disorder.
As used herein, the terms "an amount sufficient" and "sufficient
amount" refer to the amount of bromolysergide required to achieve therapeutic
effects in a subject diagnosed with a cluster headache disorder. Therapeutic
effects can include, but are not limited to, (i) diminution of pain during a
cluster
period, (ii) inducing early remission from cluster period, (iii) decreasing
the
frequency of acute attacks during a cluster period, (iv) converting a chronic
cluster headache disorder to an episodic cluster headache disorder, (v)
extending the remission period of a subject between cluster periods, (vi)
reducing the severity of one or more non-pain symptoms associated with an
attack (i.e., ipsilateral conjunctival injection and/or lacrimation;
ipsilateral nasal
congestion and/or rhinorrhoea; ipsilateral eyelid edema; ipsilateral forehead
and
facial sweating; ipsilateral miosis and/or ptosis; and a sense of restlessness
or
agitation), or (vii) reducing sensitivity to agents, such as alcohol,
histamine, and
nitroglycerin, which can provoke a cluster headache attack. The amount
8
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
sufficient used to practice the invention for therapeutic treatment of
conditions
caused by, or contributed to by, a cluster headache disorder can vary
depending
upon the manner of administration, the age, body weight, and general health of
the subject. Ultimately, the attending physician will decide the appropriate
amount and dosage regimen. Such amount is referred to as an "amount
sufficient."
Other features and advantages of the invention will be apparent from the
Drawings, Detailed Description, and the claims.
Brief Description of the Drawings
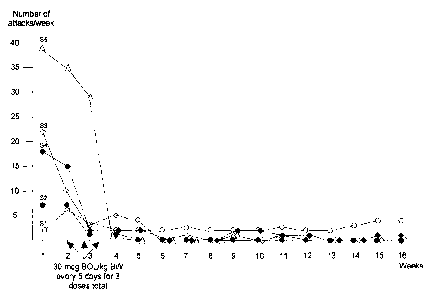
Figure 1 is a graph depicting the change in the number of cluster attacks
experienced by five subjects following, and just prior to, bromolysergide
administration (3 x 30 tg/kg; see Example 3). For these five treatment-
refractory cluster headache patients, the results show that three single doses
of
bromolysergide over a 10 day period can either break a cluster headache cycle
or considerably improve the frequency and intensity of attacks, even resulting
in changing from a chronic state to an episodic form with remission extending
for months.
Detailed Description
The invention features methods and kits for treating a recurrent cluster
headache disorder in a subject and for extending the period of remission in a
subject with a cluster headache disorder by administering bromolysergide.
9
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
Bromolysergide
Also known as bromo-LSD, BOL, BOL 148, (8(3)-2-bromo-9,10-
didehydro-N,N-diethy1-6-methylergoline-8-carboxamide, 2-bromo-N,N-diethyl
D-lysergamide, and bromolysergide, the compound 2-bromolysergic acid
diethylamide has the following structure:
131
____________________________________________ CH,
H C-N
CH3
100 'CH3
HN
Br
The synthesis of 2-bromolysergic acid diethylamide has been described by
Troxler and Hofmann (Hely. Chim. Acta 40:2160 (1957)).
Pharmaceutically acceptable salts of 2-bromolysergic acid diethylamide
can also be useful in the methods and kits of the invention. Salts that can be
used in the preparation of bromolysergide include non-toxic acid addition
salts
or metal complexes that are commonly used in the pharmaceutical industry.
Examples of acid addition salts include organic acids, e.g., acetic, lactic,
pamoic, maleic, citric, malic, ascorbic, succinic, benzoic, palmitic, suberic,
salicylic, tartaric, methanesulfonic, toluenesulfonic, or trifluoroacetic
acids;
polymeric acids, e.g., tannic acid or carboxymethyl cellulose; and inorganic
acids, e.g., hydrochloric acid, hydrobromic acid, sulfuric acid, or phosphoric
acid. Metal complexes include, e.g., calcium, zinc, and iron.
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
Formulations and Administration
Formulations of bromolysergide can include, but are not limited to,
solutions, suspensions, tablets, or capsules. Methods well known in the art
for
making formulations are found, for example, in "Remington: The Science and
Practice of Pharmacy" (21st ed.) ed. A.R. Gennaro, 2005, Lippincott,
Philadelphia, PA. The concentration of the compound in the formulation will
vary depending upon a number of factors, including the volume to be
administered and the route of administration. The dosage of drug to be
administered is likely to depend on such variables as the severity of the
disorder, the overall health status of the particular subject, the formulation
of
the compound, and its route of administration.
Effective dosage ranges for bromolysergide are generally between about
10 vig/kg and about 100 vtg/kg body weight. Desirably, a dose of between
about 20 ig/kg to about 50 pg/kg body weight is administered. For example, a
dose of 2.4 mg may be administered to a 80 kg adult to deliver about 30 fig/kg
bromolysergide. Bromolysergide can be administered to a subject by a route
that is suitable for the formulation, e.g., by oral, subcutaneous,
intravenous, or
intramuscular administration. Several doses may be administered on different
days (e.g., as described in the examples).
11
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
Therapy
Bromolysergide can be administered to treat a subject with a recurrent
cluster headache disorder. The recurrent cluster headache disorder may be
episodic or chronic. In this embodiment of the invention, the therapeutic
effects of administration of bromolysergide can include diminution or
elimination of the pain experienced by the subject during an attack, a
decrease
in frequency of attacks during the cluster period, or amelioration of other
symptoms associated with cluster headache disorders. Applicants have
discovered that bromolysergide need not be administered continually as the
therapeutic effects of administration can persist for several weeks or several
months following administration (see, for instance, Examples 1 and 3).
Bromolysergide can also be administered to a subject with a cluster
headache disorder for the purpose of extending the period of remission. For
example, the bromolysergide can be administered to extend the period of
remission indefinitely (e.g., extend the period of remission more than 2
months,
4 months, or 1 year). In some instances, the bromolysergide is administered
while the subject is experiencing autonomic symptoms characteristic of an
impending cluster headache attack.
Kits
Bromolysergide can be packaged in a kit for carrying out the methods of
the invention. The kit may be manufactured as a single use unit dose for one
patient, multiple uses for a particular patient; or the kit may contain
multiple
doses suitable for administration to multiple patients ("bulk packaging"). The
kit components may be assembled in cartons, blister packs, bottles, tubes, and
the like. Kits may also include instructions for administering the
pharmaceutical compositions using any indication and/or dosing regimen
described herein.
12
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
The following examples are put forth so as to provide those of ordinary
skill in the art with a complete disclosure and description of how the methods
and kits claimed herein are used, and are intended to be purely exemplary of
the
invention and are not intended to limit the scope of what the inventors regard
as
their invention.
Example 1. Treatment of chronic cluster headache disorder with
bromolysergide.
The desired amount of bromolysergide (hydrochloride salt) was mixed
with dextrose and placed into gelatin capsules for administration to subjects
suffering from chronic cluster headache disorder.
Bromolysergide was administered to two adult subjects diagnosed with
chronic cluster headache disorder during a cluster period. A dose of 2 mg of 2-
bromolysergic acid dethylamide (30 lig/kg body weight) was administered
orally to subject 1 three times, once every five days. A dose of 2.5 mg of 2-
bromolysergic acid dethylamide (approximately 30 pg/kg body weight) was
administered orally to subject 2 three times, once every five days.
Subjects were asked to rate their symptoms following administration of
bromolysergide.
Subject 1 rated the results as "minimally improved" (Clinical Global
Impressions Scale), reporting a 30% reduction in pain and 30% reduction of
attack frequency for ten weeks. After ten weeks, the cluster headache disorder
of subject 1 reverted to its pre-treatment form.
Subject 2 rated the results as "marked/very much improved" (Clinical
Global Impressions Scale), and reported complete or nearly complete remission
of symptoms. The improvement in subject 2 was so substantial that the subject
no longer met criteria set forth by the International Headache Society for
initial
13
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
diagnosis with chronic cluster headache disorder. Subject 2 remained free of
attacks for more than 3 months (at last follow-up), despite having suffered
from
unremitting chronic cluster headache disorder for more than 6 years.
Example 2. Extending the remission period of a cluster headache disorder.
Bromolysergide can also be administered to subjects diagnosed with a
cluster headache disorder to extend the remission period.
A subject who is diagnosed with episodic cluster headache disorder and
is in remission is selected for treatment with bromolysergide. After 6 months
of remission, the subject experiences an autonomic symptom characteristic of
an impending cluster headache attack, e.g., miosis and/or rhinorrhea, and
receives three doses of bromolysergide (3 x 1.5 mg administered orally), the
first dose on the day the subject first experiences the autonomic symptoms
(day
0), the second dose five days later (on day 5), and the third dose ten days
following the appearance of the autonomic symptom. With therapeutic
intervention, the impending cluster headache attack may be aborted and the
remission period may last an additional 1, 2, 3, 4, 5, or 6 months.
A subject who is diagnosed with chronic cluster headache disorder and
is in remission is selected for treatment with bromolysergide. After a cluster
period lasting 20 months, the subject goes into remission. On day 3, day 8,
and
day 13 of the remission period, the subject receives 3 mg bromolysergide
administered orally. With therapeutic intervention, the remission period may
be
extended for several weeks or several months.
As a result of the therapeutic intervention, both subjects can benefit from
an extended remission period, i.e., an absence of cluster headache attacks for
a
period longer than the period would have been in the absence of therapeutic
intervention.
14
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
Example 3. Treatment of chronic cluster headache disorder with
bromolysergide in treatment-refractory cluster headache patients.
Patients referred to the Pain Clinic at the Hannover Medical School were
identified with cluster headache if they met the respective diagnostic
criteria of
the international classification of headache disorders (Headache
Classification
Committee of the International Headache Society (2004) The International
Classification of Headache Disorders, 2nd edn. Cephalalgia 24 Suppl 1:1-160).
In addition, all patients were non-responders to verapamil and other
prophylactic medications (i.e., refractory to the prophylactic medications
listed
in Table 1 for each patient). In accordance with German national and local
ethics committee rules, all patients signed an informed consent, in which the
patients declared their agreement to participate in this project on the
compassionate use of bromolysergide for cluster headache. Patients kept a
standardized daily diary of cluster headache symptoms starting at least two
weeks prior to bromolysergide administration. Bromolysergide (hydrochloride
salt) was manufactured by THCphann GmbH (Frankfurt am Main, Germany).
A purity of >99.2% was identified by HPLC and other analytical tests.
Bromolysergide hydrochloride 30 jig per Kg body weight was dissolved into
distilled water and then given once every 5 days for a total of 3 doses per
os.
Alterations in consciousness, thought disturbances, and vital signs (blood
pressure, heart rate) were measured during a three to four hour observational
period following administration. Patients were asked to continue completing
daily headache diaries for the next months or until they experience 3 days of
attacks of a starting new cluster series.
The results are summarized below in Table 1 and Figure 1. One patient
(S2) with episodic cluster headache and four patients with the chronic form
participated. All but one patient (S1) had symptoms for more than 10 years.
Patient S2 noted the termination of his cluster period and a long-lasting
remission period of six months (at last follow-up) and continuing. Patients S3
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
and S5 reported a pronounced reduction of attack frequency, which included a
continuous greater than 4-week period of attack quiescence indicating the
transition from a chronic to an episodic form. Patient S4 did not show a
continuous 4-week break of attacks but nevertheless did show a profound
reduction of attack frequency. In addition, patients S3 and S4 showed such
reductions of pain intensity during their remaining occasional attacks that
they
no longer administered an acute intervention as they had prior to receiving
the
bromolysergide treatment. Although patient S1 did not show this pronounced
attack reduction similar to the other 4 patients, he indicated a decrease of
attack
intensity of about 30% within the first 4 months. It is likely relevant that
patient S1 continued to drink alcohol (contrary to advice), a known and
common trigger for attacks.
No changes to heart rate and blood pressure were observed during
bromolysergide treatment. Most of the patients recorded some kind of "flabby"
or "light drunk" feelings. Patient S2 noted a "funny" feeling, tense muscles,
and sweating of his hands. These mild subjective effects lasted from one to
two
hours. No visual hallucinations or distortions occurred nor was there any
evidence of delusional thinking or overt psychosis.
For the first five treatment-refractory cluster headache patients enrolled,
the non-hallucinogen bromolysergide was quite effective at breaking cluster
headache cycle and not just a single headache attack. The results show that
three single doses of bromolysergide within 10 days can either break a cluster
headache cycle or considerably improve the frequency and intensity of attacks,
even resulting in changing from a chronic state to an episodic form with
remission extending for many months or longer. Except for very mild
alterations of subjective state and mild to no sympathetic reactions for about
2
hours, no other side effects were observed.
16
CA 02737135 2011-03-14
WO 2010/033392
PCT/US2009/055971
Interestingly, patient S4's cluster headache was refractory to
methysergide, which the patient stopped taking in 1978. While bromolysergide
is non-hallucinogenic, methysergide in supra-therapeutic doses is
hallucinogenic (i.e., ca. 4 mg methysergide is estimated to be equivalent to
25
micgrograms LSD; see Abrahamson et al., J. Asthma Res. 3:81 (1965)).
Methysergide is used for the prophylactic treatment of cluster headache
disorders. It is typically administered daily and, over time, the longstanding
user is at risk to develop the very serious medical complication of fibrotic
reactions (retroperitoneal, pulmonary, pleural, and cardiac) although these
are
rare (see Kudrow L: Comparative results of prednisone, methysergide, and
lithium therapy in cluster headache, in Greene R (ed): Current Concepts in
Migraine Research. New York: Raven Press, pp. 159-163 (1978); Kudrow L.
Cluster headache: mechanisms and management. New York: Oxford University
Press, (1980); Zakrzewska J.M., Br J. Oral Maxillofac. Surg. 39:103 (2001);
Curran et al., Res. Clin. Stud. Headache 1:74 (1967); Krabbe A., Cephalalgia
9:404 (1989); and Graham et al., N. Eng. J. Med. 274:359 (1966)).
The current standard of care involves interventions that break single
headache attacks and reduce pain duration, frequency, and intensity of attack
cycles, but without approved treatments that extend remission. In contrast, we
have demonstrated that treatment with bromolysergide can break a cluster
headache cycle, convert a chronic cluster headache disorder into an episodic
cluster headache disorder, and extend the remission period of a subject
suffering from a cluster headache disorder.
17
CA 02737135 2016-02-04
'
'
Table I. Patient information and clinical aspects.
Subject S1 S2 S3 S4 . S5
..
Sex (in/t) m m m m m
Age (years) 46 28 47 . 41 41
Body weight (kg) 83 68 106 105 ' 74
Body height (crn) 180 168 188 195 174
_
Years of illness 3 10 10 33 32
, ___
Cranial side of left right right right right
,
attacks ,
Cluster headache chronic episodic chronic chronic chronic
form since 2005 since 2001 since 2007
Attacks per weekb 6 1 7 10 . 15 19 _
Mean intensity of 8.4 83 5.5 6.4 7.0
attacks (VAS)b
Treatments (acute) sumatriptan oxy. gcn Frovatriptane oxygen
oxygen
i,n. oxygen sumatriptan ,
, ______________________________________________________________ --.--i
Treatments verapatnil verapamil verapamil s.c. verapamil verapanul
(prophylactic) 240 mg/d 240 med 240 mg/d 320 mg/dd 960 rngtde_
BOL (30 ug/kg)I '2.5 mg , 2.0mg 3.1 mg 3.1 mg _ 2.2 mg
Side effectsg "flabby "funny "slightly "slightly "slightly
feeling" feeling" tipsy" 11.P.sY" tipsy"
Vital sins unchan _ ed unchan:ed unchanged unchanged unchanged
_
a. Left (from I 999-2005). right (since 2005).
b. In the preassessment week.
c. Up to T1D 2.5 mg.
d. Taken for 3 months. Also refractory to methysergide (taken for 1 year in
1978);
prednisonc (only for 5 days); and lithium for 3 months.
e. Also prednisone (only 14 4 days); lithium for 3 days; and doxepine (10 mg).
f. Bromolyscrgide (BOL) administered three times within 10 days (e.g., on
days 1, 5,
and 10).
g. Each of the subjects reported experiencing a side effect for about 2
hours.
, .
Other Embodiments
18
CA 02737135 2016-02-04
While the invention has been described in connection with specific
embodiments thereof, it will be understood that it is capable of further
modifications and this application is intended to cover any variations, uses,
or
adaptations of the invention following, in general, the principles of the
invention and including such departures from the present disclosure that come
within known or customary practice within the art to which the invention
pertains and may be applied to the essential features hereinbeforc set forth,
and
follows in the scope of the claims.
19