Note: Descriptions are shown in the official language in which they were submitted.
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
ANTIBODY FORMULATION
I. INTRODUCTION
100011 The present disclosure relates to liquid formulations of antibodies
or fragments
thereof that specifically bind to a human !COS polypeptide, exhibit increased
in vivo ADCC
activity and undergo reversible self-association in solution, which
formulations exhibit stability,
low to undetectable levels of antibody fragmentation, low to undetectable
levels of aggregation,
and very little to no loss of the biological activities of the antibodies,,
even during long periods of
storage. The present disclosure also relates to methods of preventing,
treating, managing or
ameliorating symptoms associated with an ICOS mediated disease or disorder
(for example, but
not limited to, systemic lupus erythematosus, myosins, multiple sclerosis,
scleroderrna,
inflammatory bowel disease, insulin dependent diabetes mellitus, psoriasis,
autoimmune
thyroiditis, rheumatoid arthritis and glomerulonephritis, transplant
rejection, graft versus host
disease) utilizing high concentration liquid formulations of antibodies or
fragments thereof that
specifically bind to a human ICOS polypeptide and exhibit increased in vivo
ADCC activity.
2. BACKGROUND
100021 ICOS is a type I transmembrane protein comprising an extracellular
(1g)V4ike
domain. 1COS serves as the receptor for the 117h co-stimulatory molecule. ICOS
expression is
low on naive human T cells but becomes upregulated within hours after TCR
engagement.
ICOS expression persists on activated T cells subpopulations such as Thi, Th2,
and Th17 CD4'
cells.
[00031 Given that 1COS expression is concentrated on activated I helper
cell
populations, the therapeutic use of an anti-ICOS antibody with enhanced
effector function holds
the promise of improving the efficacy of treatment and prevention of T cell-
mediated diseases
and disorders, such as, but not limited to, chronic infection, autoimmuite
disease or disorder,
inflammatory disease or disorder, graft-versus-host disease (GVHD), transplant
rejection, and
T cell proliferative disorder using therapeutic anti-ICOS antibodies NO th
enhanced effector
function.
[00041 Currently, many antibodies are provided as lyophilized formulations.
Lyophilized
formulations of antibodies have a number of limitations, including a prolonged
process for
lyophilization and resulting high cost for manufacturing. In addition, a
lyophilized formulation
has to be reconstituted aseptically and accurately by healthcare practitioners
prior to
administering to patients. 'thus, a need exists for liquid formulations of
antibodies, in particular,
1
CA 02743469 2016-06-13
' 51332-100
anti-human ICOS antibodies, at a concentration comparable to or higher than
the reconstituted
lyophilized formulations so that there is no need to reconstitute the
formulation prior to
administration. This allows healthcare practitioners much quicker and easier
administration of
antibodies to a patient
10095] Prior liquid antibody preparations have short shelf lives and
may lose biological
activity of the antibodies resulting from chemical and physical instabilities
during the storage.
Chemical instability may be caused by deamidation, racernization, hydrolysis,
midation, beta
elimination or disulfide exchange, and physical instability may be caused by
antibody
denaturation, aggregation, precipitation or adsorption. Among those,
anregation, deamidation
and oxidation are known to be the most common causes of the antibody
degradation (Wang et
al., 1988, J qfParenteral Science & Technology 42(Suppl):S4-S26; Cleland et
al., 1993, Critical
Reviews in :Therapeutic Drug Carrier .5tweins 10(0:307-377).. Thus, there is a
need for. a stable
liquid formulation of antibodies, in particular, stable liquid anti-human ICOS
antibodies,
3. SUMMARY
10006] The present disclosure relates to sterile, stable aqueous
formulations comprising
an antibody or fragment thereof that specifically binds human 'COS, has.
enhanced effector
functions and undergoes reversible self-association in solution. In one
embodiment, the present
disclosure provides a formulation of an anti-ICOS antibody described in US
Patent Application
Publication US 2008/0279851A1. hi a specific embodiment, a formulation of the
disclosure comprises an
anti-human ICOS antibody comprising an Fe region having complex N-glycoside-
linked sugar chains in
which fucose is not bound to N-aeetylglucosarnine in the reducing end in the
sugar chain. In
another embodiment a formulation of the disclosure comprises an anti-human
ICOS antibody'
comprising a heavy chain sequence of SEQ NO:6 and a light chain sequence o SEQ
ID
NO;]. In a further embodiment, a formulation described herein comprises an
anti-human ICOS
antibody that undergoes reversible self-association in solution, wherein at
least 10 mole percent
of the antibody exists as a trimer in PBS at 10 mg/m1 antibody concentration
at 37T, and
wherein the reversible self-association does not induce aggregate formation.
In one embodiment,
a formulation of the disclosure is provided in a pre-filled syringe,
[00011 The present disclosure provides methods of stabilizing an anti-
human ICOS
antibody or fragment thereof.
1000S] The present disclosure further relates to processes of making
a sterile, stable
aqueous formulation comprising an antibody or fragment thereof that
specifically binds human
'COS.
81619315
[0009] The present disclosure also encompasses methods of preventing,
managing,
treating or ameliorating an inflammatory disease or disorder, an autoimmune
disease or disorder, a
proliferative disease, a T cell proliferative disease, an infection, a disease
or disorder associated
with or characterized by aberrant expression and/or activity of ICOS, a
disease or disorder
associated with or characterized by aberrant expression and/or activity of the
ICOS receptor,
or one or more symptoms thereof, said methods comprising administering to a
subject in need
thereof a prophylactically or therapeutically effective amount of an anti-
human ICOS antibody
formulation. The present disclosure also relates to methods of treating or
preventing
T cell-mediated diseases and disorders, such as, but not limited to, chronic
infection,
autoimmune disease or disorder, inflammatory disease or disorder, graft-versus-
host disease
(GVHD), transplant rejection, and T cell proliferative disorder using
formulations comprising
anti-ICOS antibodies with enhanced effector function.
[0009a] In an embodiment, the invention provides a sterile, stable aqueous
formulation
comprising an antibody that specifically binds human ICOS, wherein a) the
antibody comprises an
Fc region having complex N-glycoside-linked sugar chains in which fucose is
not bound to N-
acetylglucosamine in the reducing end in the sugar chain; b) the antibody
undergoes reversible self-
association in solution, wherein the reversible self-association does not
induce aggregate
formation; c) at least 10 mole percent of the antibody exists as a trimer in
PBS at 10 mg/ml
antibody concentration at 37 C; d) the formulation comprises between 5 mg/ml
and 10 mg/ml of
the antibody; e) the aqueous formulation comprises 80 mM NaCI; 10 mM
histidine; 4 % trehalose;
and 0.02 ')/0 polysorbate 80, when wherein the pH of the formulation is 6.0;
and f) the antibody
comprises a heavy chain sequence of SEQ ID NO: 6 and a light chain sequence of
SEQ ID NO: 1.
[0009b] In another embodiment, the invention provides a pharmaceutical unit
dosage form
for parenteral administration to a human which comprises an antibody
formulation as described
herein in a suitable container.
[0009c] In another embodiment, the invention provides use of the
formulation as described
herein for the treatment of an autoimmune disease or disorder in a human.
[0009d] In another embodiment, the invention provides use of the
formulation as described
herein for the treatment or prevention of rejection in a human transplant
patient.
3
CA 2743469 2018-04-26
81619315
[0009e] In another embodiment, the invention provides use of the
formulation as described
herein for the treatment of an inflammatory disease or disorder in a human.
1000911 In another embodiment, the invention provides use of the
formulation as described
herein for the depletion of ICOS expressing T cells in a human patient.
[0009g] In another embodiment, the invention provides use of the
formulation as described
herein for the disruption of germinal center architecture in a secondary
lymphoid organ of a primate.
3.1. DEFINITIONS
[0010] All formulations of antibodies and/or antibody fragments that
specifically bind to
an antigen of interest (e.g., ICOS) are herein collectively referred to as
"formulations of the
disclosure", "liquid formulations of the disclosure", "high concentration
stable liquid formulations
of the disclosure", "antibody liquid formulations of the disclosure", or
"antibody formulations of
the disclosure".
[0011] As used herein, the terms "antibody" and "antibodies"
(immunoglobulins)
encompass monoclonal antibodies (including full-length monoclonal antibodies),
polyclonal
antibodies, multispecific antibodies (e.g., bispecific antibodies) formed from
at least two intact
antibodies, human antibodies, humanized antibodies, camelised antibodies,
chimeric antibodies,
single-chain Fvs (scFv), single-chain antibodies, single domain antibodies,
domain antibodies,
Fab fragments, F(ab')2 fragments, antibody fragments that exhibit the desired
biological activity,
disulfide-linked Fvs (sdFv), and anti-idiotypic (anti-Id) antibodies
(including, e.g., anti-Id
antibodies to antibodies of the disclosure), intrabodies, and epitope-binding
fragments of any of
the above. In particular, antibodies include immunoglobulin molecules,
biologically active
fragments of the disclosed molecules and immunologically active fragments of
immunoglobulin
molecules, i.e., molecules that contain an antigen-binding site.
Immunoglobulin molecules can be
of any type (e.g., IgG, IgE, IgM, IgD, IgA and IgY), class (e.g., IgGl, IgG2,
IgG3, IgG4, IgAl
and IgA2) or subclass.
[0012] Native antibodies are usually heterotetrameric glycoproteins of
about
150,000 daltons, composed of two identical light (L) chains and two identical
heavy (H) chains.
Each light chain is linked to a heavy chain by one covalent disulfide bond,
while the number of
3a
CA 2743469 2018-04-26
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
disulfide linkages varies between the heavy chains of different immunoglobuhn
isotypes. Each
heavy and light chain also has regularly spaced intrachain disulfide bridges.
Each heavy chain
has at one end a variable domain (VII) followed by a number of constant
domains. Each light
chain has a variable domain at one end (VI) and a constant domain at its other
end; the constant
domain of the light chain is aligned with the first constant domain of th.e
heavy chain, and the
light chain variable domain is aligned with the variable domain of the heavy
chain. Light chains
are classified as either lambda chains or kappa chains based on the amino acid
sequence of the
light chain constant region. The variable domain of a kappa light chain may
also be denoted
herein as VIC The term ''variable region" may also be used to describe the
variable domain of a
heavy chain or light chain. Particular amino acid residues are believed to
form an interface
between the light and heavy chain variable domains. Such antibodies may be
derived from any
mammal, including, but not limited to, humans, monkeys, pigs, horses, rabbits,
dogs, cats, mice,
etc.
[00131 The term "variable" refers to the fact that certain portions of the
variable domains
differ extensively in sequence among antibodies and are responsible for the
binding specificity of
each particular antibody for its particular antigen. However, the variability
is not evenly
distributed through the variable domains of antibodies. It is concentrated in
segments called
Complementarily Determining Regions (CDRs) both in the light chain and the
heavy chain
variable domains. The more highly conserved portions of the variable domains
are called the
framework regions (FW). The variable domains of native heavy and light chains
each comprise
four FW regions, largely adopting a 0-sheet configuration, connected by three
CDRs, which
form loops connecting, and in some cases forming part of, the 0-sheet
structure. The CDRs in
each chain are held together in close proximity by the FW regions and, with
the CDRs from the
other chain, contribute to the formation of the antigen-binding site of
antibodies (see, Kabat et
al., Sequences of Proteins of Immunological Interest, 5th Ed. Public Health
Service, National
Institutes of Health, Bethesda, MD (1991)). The constant domains are generally
not involved
directly in antigen binding, but may influence antigen binding affinity and
may exhibit various
effector functions, such as participation of the antibody in ADCC, CDC,
antibody-dependent
phagocytosis andior apoptosis.
100141 The term "hypervariable region" when used herein refers to the amino
acid
residues of an antibody which are associated with its binding to antigen. The
hypervariable
regions encompass the amino acid residues of the "complementarily determining
regions.' or
"CDRs" (e.g., residues 24-34 (Li), 50-56 (L2) and 89-97 (L3) of the light
chain variable domain
and residues 31-35 (HI), 50-65 (112) and 95-102 (H3) of the heavy chain
variable domain;
Kabat et at. Sequences of Proteins of Immunological Interest, 5th Ed. Public
Health Service,
4
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
National hist Lutes of Health, Bethesda, MD (1991)) and/or those residues from
a "hypervariable
loop" (e.g., residues 26-32 (U ), 50-52 (L2) and 91-96 (L3) in the light chain
variable domain
and 26-32 (HI), 53-55 (112) and 96-101 (H3) in the heavy chain variable
domain; Chothia and
Lesk, J. Mol. Biol., 196:901-917(1987)1. "Framework" or "FW" residues are
those variable
domain residues flanking the CDRs. FW residues are present in chimeric,
humanized, human,
domain antibodies, diabodies, vaccibodies, linear antibodies, and bispecific
antibodies.
100151 As used herein "Fc region" includes the polypeptides comprising the
constant
region of an antibody excluding the first constant region immunoglobulin
domain. Thus Fe
refers to the last two constant region immunoglobulin domains of Ig.A.,1gD,
and IgG, and the last
three constant region immunoglobulin domains of lgE and 1gM, and the flexible
hinge N-
terminal to these domains. For IRA and 143M Fe may include the1 chain. For
IRO, Fe comprises
immunoglobulin domains Cgamma2 and Cgarnma3 (C72 and (43) and the hinge
between
Cgammal (C71) and Cgamma2 (C72). Although the boundaries of the Fe region may
vary, the
human IgG heavy chain Fe region is usually defined to comprise residues C226
or P230 to its
carboxyl-terminus, wherein the numbering is according to the EU index as in
Kabat et al. (1991,
NIH Publication 91-3242, National Technical Information Service, Springfield,
VA). The "EU
index as set forth in Kabat" refers to the residue numbering of the human !gal
EU antibody as
described in Kabat et al. supra. Fe may refer to this region in isolation, or
this region in the
context of an antibody, antibody fragment, or Fe fusion protein. An Fe variant
protein may be an
antibody, Fe fusion, or any protein or protein domain that comprises an Fe
region. Particularly
preferred are proteins comprising variant Fe regions, Which are non-naturally
occurring variants
of an Fe region. The amino acid sequence of a non-naturally occurring Fe
region (also referred
to herein as a "variant Fc region") comprises a substitution, insertion and/or
deletion of at least
one amino acid residue compared to the wild type amino acid sequence. Any new
amino acid
residue appearing in the sequence of a variant Fe region as a result of an
insertion or substitution
may be referred to as a non-naturally occurring amino acid residue. Note:
Polymorphisms have
been observed at a number of Fe positions, including but not limited to Kabat
270, 272, 312,
315, 356, and 358, and thus slight differences between the presented sequence
and sequences in
the prior art may exist.
[00161 The term -monoclonal antibody" as used herein refers to an antibody
obtained
from a population of substantially homogeneous antibodies, i.e., the
individual antibodies
comprising the population are identical except for possible naturally
occurring mutations that
may be present in minor amounts. Monoclonal antibodies are highly specific,
being directed
against a single antigenic site. Furthermore, in contrast to conventional
(polyelonal) antibody
preparations which typically include different antibodies directed against
different determinants
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
(epitopes). each monoclonal antibody is directed against a single determinant
on the antigen. In
addition to their specificity, monoclonal antibodies are advantageous in that
they can be
synthesized by hybridoma cells that are uncontaminated by other
inununoglobulin producing
cells. Alternative production methods are known to those trained in the art,
for example, a
.monoclonal antibody may be produced by cells stably or transiently
transfected with the heavy
and light chain genes encoding the monoclonal antibody.
(00171 The modifier "monoclonal" indicates the character of the antibody as
being
obtained from a substantially homogeneous population of antibodies, and is not
to be construed
as requiring engineering of the antibody by any particular method. The term
"monoclonal" is
used herein to refer to an antibody that is derived from a clonal population
of cells, including any
eukaryotic, prokaryotic, or phage clone, and not the method by which the
antibody was
engineered. For example, the monoclonal antibodies to be used in accordance
with the present
disclosure may be made by the hybridoma method first described by Kohler et
al., Nature,
256:495 (1975), or may be made by any recombinant DNA method (see, e.g., U.S.
Patent No.
4,816,567), including isolation from phage antibody libraries using the
techniques described in
Clackson et al., Nature, 352:624-628 (1991) and Marks et al., J. Mol. Biol.,
222:581-597 (1991),
for example. These methods can be used to produce monoclonal mammalian,
chimeric,
humanized, human, domain antibodies, diabodies, vaecibodies, linear
antibodies, and bispecific
antibodies.
100181 A "human antibody" can be an antibody derived from a human or an
antibody
obtained from a transgenic organism that has been "engineered" to produce
specific human
antibodies in response to antigenic challenge and can be produced by any
method known in the
art In certain techniques, elements of the human heavy and light chain loci
are introduced into
strains of the organism derived from emblyonie stem cell lines that contain
targeted disruptions
of the endogenous heavy chain and light chain loci. The transgenic organism
can synthesize
human antibodies specific for human antigens, and the organism can be used to
produce human
antibody-secreting hy,'bridomas. A human antibody can also be an antibody
wherein the heavy
and light chains are encoded by a nucleotide sequence derived from one or more
sources of
human DNA. A fully human antibody also can be constructed by genetic or
chromosomal
transfection methods, as well as phage display technology, or in vitro
activated [COS expressing
T cells, all of which are known in the art
100191 "Antibody-dependent cell-mediated eytotoxicity" and "ADCC" refer to
a cell-
mediated reaction in which non-specific cytotoxic cells (e.g., Natural Killer
(NK) cells,
neutrophils, and macrophages) recognize bound antibody on a target cell and
subsequently cause
Iysis of the target cell. In one embodiment, such cells are human cells. While
not wishing to be
6
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
limited to any particular mechanism of action, these cytotoxic cells that
mediate .ADCC
generally express Fe receptors (FtRs). The primary cells for mediating ADCC,
NK cells,
express FeyRIII, whereas rnonocytes express FcyRI, FcyRII, FcyR111 and/or
FcyRIV. FeR
expression on hematopoietic cells is summarized in Ravetch and Kinet, Annu.
Rev. linmunol.,
9:457-92 (1991). To assess ADCC activity of a molecule, an in vitro ADCC
assay, such as that
described in U.S. Patent No. 5,500,362 or 5,82.1337 may be performed. Useful
effector cells for
such assays include peripheral blood mononuclear cells (PBMC) and Natural
Killer (NK) cells.
Alternatively, or additionally, ADCC activity of the molecules of interest may
be assessed in
vivo, e.g., in an animal model such as that disclosed in Clynes et al., Proc.
Natl. Acad. Sci.
(USA), 95:652-656(1998).
100201 "Complement dependent cytotoxicity" or "CDC" refers to the ability
of a
molecule to initiate complement activation and lyse a target in the presence
of complement. The
complement activation pathway is initiated by the binding of the first
component of the
complement system (Clq) to a molecule (e.g., an antibody) complexed with a
cognate antigen.
To assess complement activation, a CDC assay, e.g., as described in Gazzano-
Santaro at al., J.
Immtmol. Methods, 202:163 (1990, may be performed.
100211 "Antibody-dependent phagocytosis" or "opsonization" as used herein
refers to the
cell-mediated reaction wherein nonspecific cytotoxic cells that express FcyRs
recognize bound
antibody on a target cell and subsequently cause phagocytosis of the target
cell.
"Effector cells" are leukocytes which express one or more FeRs and perform
effector functions.
The cells express at least FcyRI, FCyRII, FcyR111 and/or FcyRIV and carry out
ADCC effector
function. Examples of human leukocytes which mediate ADCC include peripheral
blood
mononuclear cells (PBMC), natural killer (NK) cells, monocytes, cytotoxic T
cells and
neutrophils.
100221 The terms "Fe receptor" or "FcR" are used to describe a receptor
that binds to the
Fe region elan antibody. In one embodiment, the .FcR is a native sequence
human FcR.
Moreover, in certain embodiments, the FcR. is one which binds an IgG antibody
(a gamma
receptor) and includes receptors of the FcyRI, FcyRlL FcyRIII, and FcyRIV
subclasses, including
allelic variants and alternatively spliced forms of these receptors. FeyR11
receptors include
FcyRIIA (an "activating receptor") and FcyRIIB (an "inhibiting receptor"),
which have similar
amino acid sequences that differ primarily in the cytoplasmic domains thereof
Activating
receptor FcyRIIA contains an imimmoreceptor tyrosine-based activation motif
(ITAM) in its
cytoplasmic domain. Inhibiting receptor FcyRIIR contains an immunoreceptor
tyrosine-based
inhibition motif (ITIM) in its cytoplasmic domain. (See, Daeron, Annu. Rev.
Immunol., 15:203-
234(1997)). FcRs are reviewed in Ravetch and Kinet, Annu. Rev. Immunol..,
9:457-92 (1991);
7
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Capel et. at, Immunomethods. 4:25-34 (1994); and de Haas et al., J. Lab. Clin.
Med., 126:330-41
(1995). Other Fats, including those to be identified in the future, are
encompassed by the term
"FcR" herein. The term also includes the neonatal receptor. FcRn, which is
responsible for the
transfer of maternal IgGs to the fetus (Guyer et at, Immunol., 117:587 (1976)
and Kim et at, J.
Immunot, 24:249(1994)).
(00231 "Affinity" of an antibody for an epitope to be used in the
treatment(s) described
herein is a term well understood in the art and means the extent, or strength,
of binding of
antibody to epitope. Affinity may be measured and/or expressed in a number of
ways known in
the art, including, but not limited to, equilibrium dissociation constant (KD
or Kd), apparent
equilibrium dissociation constant (KU or Kd'), and 1050 (amount needed to
effect 50%
inhibition in a competition assay). It is understood that, for purposes of
this disclosure, an
affinity is an average affinity for a given population of antibodies which
bind to an epitope.
Values of KO reported herein in terms of mg 1gG per ml, or mgiml, indicate mg
It4 per mi. of
serum, although plasma can be used. When antibody affinity is used as a basis
for
administration of the treatment methods described herein, or selection for the
treatment methods
described herein, antibody affinity can be measured before and/or dining
treatment, and the
values obtained can be used by a clinician in assessing whether a human
patient is an appropriate
candidate for treatment
100241 As used herein, the term "avidity" is a measure of the overall
binding strength
(i.e., both antibody arms) with which an antibody binds an antigen. Antibody
avidity can be
determined by measuring the dissociation of the antigen-antibody bond in
antigen excess using
any means known in the art, such as, but not limited to, by the modification
of indirect
fluorescent antibody as described by Gray et al., J. Virol. Meth., 44:11-24.
(1993).
100251 An "epitope" is a term well understood in the an and means any
chemical moiety
that exhibits specific binding to an antibody. An "antigen" is a. moiety or
molecule that contains
an epitope, and, as such, also specifically binds to antibody.
100261 The term "antibody half-life" as used herein means a pharmacokinetic
property of
an antibody that is a measure of the mean survival time of antibody molecules
following their
administration. Antibody half-life can be expressed as the time required to
eliminate 50 percent
of a known quantity ofimmunoglobulin from the patient's body or a specific
compartment
thereof, for example, as measured in serum or plasma, i.e., circulating half-
life, or in other
tissues. Half-life may vary from one immunoglobulin or class of immunoglobulin
to another. In
general, an increase in antibody half-life results in an increase in mean
residence time (MRT) in
circulation for the antibody administered.
8
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
100271 The term "isotype" refers to the classification of an antibody's
heavy or light
chain constant region. The constant domains of antibodies are not involved in
binding to
antigen, but exhibit various effector functions. Depending on the amino acid
sequence of the
heavy chain constant region, a given human antibody or immunoglobtdin can be
assigned to one
of five major classes or irnmunoglobulins: IgA, 1gD, IgE, IgG, and Ig.M.
Several of these classes
may be further divided into subclasses (isotypes), e.g., IgG1 (gamma 1),1gG2
(gamma 2), laG3
(gamma 3), and IgG4 (gamma 4), and IgAl and lgA2. The heavy chain constant
regions that
correspond to the different classes of immunoglobulins are called a, 8, c, 7,
and p,, respectively.
The structures and three-dimensional configurations of different classes of
immunoglobutins are
well-known. Of the various human immunoglobul in classes, only human !gal,
102. IgG3,
IgG4, and IgM are known to activate complement. Human IRG1 and Ig03 are known
to mediate
AMC in humans. Human light chain constant regions may be classified into two
major classes,
kappa and lambda
[00281 As used herein, the term "immunogenicity" means that a compound is
capable of
provoking an immune response (stimulating production of specific antibodies
and/or
proliferation of specific T cells).
100291 As used herein, the term "antigenicity" means that a compound is
recognized by
an antibody or may bind to an antibody and induce an immune response.
The term "excipient" as used herein refers to an inert substance which is
commonly used as a diluent, vehicle, preservative, binder or stabilizing agent
for drugs which
imparts a. beneficial physical property to a formulation, such as increased
protein stability,
increased protein solubility, and decreased viscosity. Examples of excipiems
include, but are not
limited to, proteins (for example, but not limited to, serum albumin), amino
acids (for example,
but not limited to, aspartic acid, glutamic acid, lysine, arginine, glycine),
surfactants (for
example, but not limited to, SDS. Tween 20, Tween 80, polysorbate and nonionic
surfactants),
saccharides (for example, but not limited to, glucose, sucrose, maltose and
trehalose), polyols
(for example, but not limited to, mannitol and sorbitol), fatty acids and
phospholipids (for
example, but not limited to, alkyl sulfonates and caprylate). For additional
information
regarding excipients, see Remington's Pharmaceutical Sciences (by Joseph P.
Remington, 18th
ed., Mack Publishing Co., Easton, PA), which is incorporated herein in its
entirety.
100311 The phrase "pharmaceutically acceptable" as used herein means
approved by a
regulatory agency of the Federal or a state government, or listed in the U.S.
Pharmacopeia,
European Pharmacopia or other generally recognized pharmacopeia for use in
animals, and more
particularly in humans.
9
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
100321 The terms "stability" and "stable" as used herein in the context of
a liquid
formulation comprising an antibody (including antibody fragment thereof) that
specifically binds
to an antigen of interest (eg., ICOS) refer to the resistance of the antibody
(including antibody
fragment thereof) in the formulation to aggregation, degradation or
fragmentation under given
manufacture, preparation, transportation and storage conditions. The "stable"
formulations of
the disclosure retain biological activity under given manufacture,
preparation, transportation and
storage conditions. The stability of said antibody (including antibody
fragment thereof) can be
assessed by degrees of aggregation, degradation or fragmentation, as measured
by HPSEC,
reverse phase chromatography, static light scattering (SLS), Dynamic Light
Scattering (DIS),
Fourier Transform infrared Spectroscopy (FTIR), circular dichroism (CD), urea
unfolding
techniques, intrinsic tryptophan fluorescence, differential scanning
calorimetry, and/or ANS
binding techniques, compared to a reference formulation. For example, a
reference formulation
may be a reference standard frozen at -10 C consisting of 10 inglml of an
antibody (inducting
antibody fragment thereof) (for example, but not limited to, an antibody
comprising a heavy
chain sequence of SEQ ID NO:6, a light chain sequence of SEQ ID NO:1 and an Fc
region
having complex N-glycoside-linked sugar chains in which fucose is not bound to
N-
acetylglucosamine in the reducing end in the sugar chain) in 10 mM histidine,
pH 6.0-6.5 that
contains 80 mM NaCl, 4% trehalose and 0.02% polysorbate 80, which reference
formulation
regularly gives a single monomer peak (e.g., 97% area) by HPSEC. The overall
stability of a
formulation comprising an antibody (including antibody fragment thereof) can
be assessed by
various immunological assays including, for example, ELISA and
radioimmunoassay using
isolated antigen molecules.
100331 The phrase "low to undetectable levels of aggregation" as used
herein refers to
samples containing no more than about 5%, no more than about 4%, no more than
about 3%, no
more than about 2%, no more than about .1% and no more than about 0,5%
aggregation by
weight of protein as measured by high performance size exclusion
chromatography (tIPSEC) or
static light scattering (SLS) techniques.
100341 The term -low to undetectable levels of fragmentation" as used
herein refers to
samples containing equal to or more than about 80%, about 85%, about 90%,
about 95%, about
98% or about 99% of the total protein, for example, in a single peak as
determined by HPSEC or
reverse phase chromatography, or in two peaks (e.g, heavy- and belt-chains)
(or as many peaks
as there are subunits) by reduced Capillary Gel Electrophoresis (rCGE),
representing the non-
degraded antibody or a non-degraded fragment thereof, and containing no other
single peaks
having more than about 5%, more than about 4%, more than about 3%, more than
about 2%,
more than about 1%, or more than about 0.5% of the total protein in each. The
term "reduced
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Capillary Gel Electrophoresis" as used herein refers to capillary gel
electrophoresis under
reducing conditions sufficient to reduce disulfide bonds in an antibody.
100351 As used herein, the terms "disorder" and "disease" are used
interchangeably to
refer to a condition in a subject in which the subject differs from a healthy,
unaffected subject.
In particular_ the term "autoimmune disease" is used interchangeably with the
term "autoimmune
disorder" to refer to a condition in a subject characterized by cellular,
tissue and/or organ injury
caused by an immunologic reaction of the subject to its own cells, tissues
and/or organs. The
term "inflammatory disease" is used interchangeably with the term
"inflammatory disorder" to
refer to a condition in a subject characterized by inflammation, for example,
but not limited to,
chronic inflammation. Autoimmtme disorders may or may not be associated with
inflammation.
Moreover, inflammation may or may not be caused by an autoimmune disorder.
Certain
conditions may be characterized as more than one disorder. For example,
certain conditions may
be characterized as both autoimmune and inflammatory disorders.
100361 The terms "therapies" and "therapy" can refer to any protocol(s),
method(s),
andlor agent(s) that can be used in the prevention, treatment and/or
management of a disease or
disorder.
100371 Ily the terms "treat," "treating" or "treatment or (or grammatically
equivalent
terms) it is meant that the severity of the subject's condition is reduced or
at least partially
improved or ameliorated and/or that some alleviation, mitigation or decrease
in at least one
clinical symptom is achieved and/or there is an inhibition or delay in the
progression of the
condition and/or prevention or delay of the onset of a disease or illness.
Thus, the terms
"treating" or "treatment of' (or grammatically equivalent terms) refer to both
prophylactic and
therapeutic treatment regimes.
100381 As used herein, the terms "manage," "managing," and "management"
refer to the
beneficial effects that a subject derives from a therapy (e.g., a prophylactic
or therapeutic agent),
which does not result in a cure of the disease. In certain embodiments, a
subject is administered
one or more therapies (e.g., one or more prophylactic or therapeutic agents)
to "manage" a
disease so as to prevent the progression or worsening of the disease.
100391 As used herein, the terms "prevent," "preventing," and '`prevention"
refer to the
inhibition of the development or onset of disease or disorder, or the
prevention of the recurrence,
onset, or development of one or more symptoms of a disease or disorder in a
subject resulting
from the administration of a therapy (e.g., a prophylactic or therapeutic
agent), or the
administration of a combination of therapies (e.g., a combination of
prophylactic or therapeutic
agents).
11
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
100401 As used herein, the terms "prophylactic agent" and "prophylactic
agents" refer to
any agent(s) which can be used in the prevention of the onset, recurrence or
development of a
disease or disorder. In certain embodiments, the term "prophylactic agent"
refers to an antibody
that specifically binds to human ICOS. [n certain other embodiments, the term
"prophylactic
agent" refers to an agent other than an antibody that specifically binds to
human ICOS. in
certain embodiments, a prophylactic agent is an agent which is known to be
useful to or has been
or is currently being used to prevent or impede the onset, development,
progression and/or
severity of a disease or disorder.
10041.1 As used herein, the term "immunomodulatoty wit" and variations
thereof
including, but not limited to, immunomodulatory agents, immunomodulants or
immunomodulatory drugs, refer to an went that modulates a host's immune
system. In a
specific embodiment, an immunomodulatory agent is an agent that shifts one
aspect of a
subject's immune response. In certain embodiments, an i minunotnodulatory
agent is an agent
that. inhibits or reduces a subject's immune system (i.e., an
immunosuppressant agent). In
certain other embodiments, an immunomodulatoty agent is an agent that
activates or increases a
subject's immune system (i.e., an immun.ostimulatory agent). In accordance
with the disclosure,
an i inmunomodulatory agent used in the combination therapies of the
disclosure does not include
an antibody of the disclosure. Immunorriodulatory agents include, but are not
limited to, small
molecules, peptides, polypeptides, proteins, nucleic acids (for example, but
not limited to. DNA
and RNA nucleotides including, but not limited to, antisense nucleotide
sequences, triple helices,
RN Ai, and nucleotide sequences encoding biologically active proteins,
polypeptides or peptides),
antibodies, synthetic or natural inorganic molecules, mimetic agents, and
synthetic or natural
organic molecules.
100411 As used herein, a "sufficient amount' or "an amount sufficient. to"
achieve a
particular result refers to an amount of an antibody or composition of the
disclosure that is
effective to produce a desired effect, which is optionally a therapeutic
effect (i.e.. by
administration of a therapeutically effective amount). For example, a
"sufficient amount" or "an
amount sufficient to" can be an amount that is effective to deplete ICOS
expressing T cells.
100431 A "therapeutically effective" amount as used herein is an amount
that provides
some improvement or benefit to the subject. Stated in another way, a
"therapeutically effective"
amount is an amount that provides some alleviation, mitigation, and/or
decrease in at least one
clinical symptom. Clinical symptoms associated with the disorders that can be
treated by the
methods of the disclosure are well-known to those skilled in the art. Further,
those skilled in the
art will appreciate that the therapeutic effects need not be complete or
curative, as long as some
benefit is provided to the subject.
12
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
[00441 A "therapeutically effective dosage" of an anti-ICOS antibody of the
disclosure
results in a decrease in severity of at least one disease symptom, an increase
in frequency and
duration at' disease symptom-free periods, or a prevention of impairment or
disability due to the
disease affliction. For example, in the case of systemic lupus erythematosus
(SLE), a
therapeutically effective dose prevents further deterioration of at least one
physical symptom
associated with SLE, such as, for example, pain or fatigue. A therapeutically
effective dose also
prevents or delays onset of SLE, such as may be desired when early or
preliminaty signs of the
disease are present. Likewise it includes delaying chronic progression
associated with SLE.
Laboratory tests utilized in the diagnosis of SLE include chemistries,
hematology, serology and
radiology. Accordingly. any clinical or biochemical assay that monitors any of
the foregoing
may be used to determine whether a particular treatment is a therapeutically
effective dose for
treating SLE One of ordinary skill in the art would be able to determine such
amounts based on
such factors as the subject's size, the severity of the subject's symptoms,
and the particular
composition or route of administration selected.
100451 As used herein, the term *subject" includes any human or nonhuman
animal. The
term "nonhuman animal" includes all vertebrates, for example, but not limited
to, mammals and
non-mammals, such as nonhuman primates, sheep, dogs, cats, horses, cows,
chickens,
amphibians, reptiles, etc.
100461 As used herein, the terms "non-responsive" and refractory" describe
patients
treated with a currently available therapy (e.g., prophylactic or therapeutic
agent) for a disease or
disorder. Such patients likely suffer from severe, persistently active disease
and require
additional therapy to ameliorate the symptoms associated with the disorder.
100471 Concentrations, amounts, cell counts, percentages and other
numerical values
may be presented herein in a range format. It is also to be understood that
such range format is
used merely for convenience and brevity and should be interpreted flexibly to
include not only
the numerical values explicitly recited as the limits of the range but also to
include all the
individual numerical values or sub-ranges encompassed within that range as if
each numerical
value and sub-range is explicitly recited.
4. BRIEF DESCRIPTION OF THE DRAWINGS
100481 For the purpose orillustrating representative embodiments of the
disclosure,
drawings are provided herein.
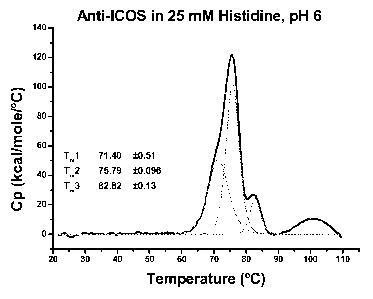
100491 Figure I.. DSC profile of the 136 anti-ICOS antibody in 25 rnIVI
histidine (pH
(.0).
13
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
100501 Figure 2. Effect of pH on thermal stability of the 136 anti-ICOS
antibody.
Tiyptophan -fluorescence intensity profiles (measured at 330 nm) as a function
of temperature are
shown. Try ptophan fluorescence intensity profile measurements were performed
at various pHs.
100511 Figure 3. pH dependence of the colloidal stability of anti -ICOS
formulations.
The 350nrn absorption of formulations with various pHs as a function of
temperature is shown.
[00521 Figure 4. Schematics of the use of colloidal stability measurement
.for excipient
screening.
100531 Figure 5. Single excipient screening: Effect of polysorbate,
trehalose, sucrose
and lysine on colloidal stability of 136 formulations.
100541 Figure 6. Single excipient screening: Effect of increasing NaC1
concentration on
colloidal stability of 136 formulations.
100551 Figure 7. Single excipient screening: Effect of increasing NaCI or
arginine
concentration on colloidal stability of 136 formulations.
100561 Figure 8. Excipient screening: Effect of the combination of
trehalose and
arginine on colloidal stability of .136 formulations.
100571 Figure 9. Stability of 136 anti-ICOS antibody formulations. The
stability of the
antibody formulations was ascertained by SEC. Chart displays the percent (,/o)
monomer content
of the formulation, as determined by SEC, after storage at 40 C.
100581 Figure .10. Stability of .136 anti-ICOS antibody formulations. The
stability of the
antibody formulations comprising 90 rrigtml 136, 10 mtvl histidine (pH 6.0),
4% trehalose and
either 80mM Nan (A) or 100 mkt arginine HC1 (B) was ascertained by SEC. The
formulations
were stored at 40 C for 21 days prior to performing SEC analysis. SEC protein
elution profiles
are shown.
100591 Figure 11. Effect of polysorbate 80 on the stability of 136 anti4COS
antibody
formulations. The stability of 136 formulations (105 mg/nil 136, 10 mM
histidine (pH 6.0), 80
mM NaC1) comprising 0 0.02 % or 0.05 µ?4, polysorbate 80 was ascertained
following storage
at 40 C. Chart displays the percent (%) monomer content of the formulation, as
determined by
SEC, at various time points.
100601 Figure 12. Effect of polysorbate 80 on the stability of 136 anti-
ICOS antibody
formulations. The stability of .136 formulations (105 nighnl 136, 10 iriM
histidine (pH 6.0), 80
mM NaCI) comprising 0 %, 0.02 or 0.05 % polysorbate 80 was ascertained
following storage
at 40 C. Chart displays the percent (%) fragment content of the formulation,
as determined by
SEC, at various time points.
100611 Figure 13. Effect of polysorbate 80 on the stability of 136 anti-
ICOS antibody
formulations. The stability of 136 formulations (105 mg/ml 136, 10 mM
histidine (pH 6.0), 80
14
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
mM NaCI) comprising 0 %, 0.02 % or 0.05 % polysorbate 80 was ascertained
following storage
at 40 C. Chart displays the percent (i%) dimer content of the formulation, as
determined by SEC,
at various time points.
100621 Figure 14. Stability of a 136 anti-ICOS antibody formulation stored
at 2-8,25 or
40 C. The stability of the 136 formulation comprising 105 inging 136õ 10 rnM
histidine (pH
6.0), 80 rnM Naa and 0.02 % polysorbate 80 was ascertained following storage
at 2-8, 25 or
40 C. Chart displays the percent (%) monomer content of the formulation, as
determined by
SEC, at various time points.
100631 Figure 15. A) BlAcore binding affinity of the fucosylated and
afucosylated anti-
ICOS MAb to mouse FcgRIV. B) Immuno-phenotype characterization in the steady
state of
ICOS expression on splenic naive and T helper memory cells (central and
effector). C) Fucose
free anti4COS MAb (IgG2a-aFtic) mediates more effective depletion of ICOS
bearing T
Pharmacodynamic analysis of splenic helper central and effector memory ICOS
bearing T cells
upon one single intraperitoneal injection of the indicated anti-ICOS MAbs into
naive Balt&
mice (250 tg/animal).
100641 Figure 16. Anti-1COS MAb (IgG2a-aFuc) reduces graft versus host
scleroderma
clinical score. Mean clinical disease score following biweekly treatment
(starting time: day 8)
with anti-mouse ICOS 102a-aFtic or isotype control MAb (n=10) is shown.
Baseline skin scores
measurements were obtained on study day 6. (*p<0.05, "p<0.005)
100651 Figure 17. Anti-ICOS MAb mediates effective elimination of ICOS
bearing TFH
and inhibits the expansion of germinal center B cells. Immunophenotype
analysis of spleen,
lymph node and peripheral blood Th memo*, (A) and Th memory ICOS+ cells (B, C)
(gated as
indicated in Fig. IC) isolated from Bathic control mice and from rag2
deficient mice treated with
either anti-ICOS or isotype control MAb. D) Anti-ICOS therapy prevents the
expansion of TFH
cells. While anti-ICOS MAb does not alter the overall number of total splenic
B cells (CD] 9+)
(E), it significantly inhibits the TFH-mediated expansion of germinal center B
cells (F).
Depletion of !COS bearing T cells does not perturb the overall CD4+ ((3) and
CD8+ (H) T cell
compartments.
100661 Figure 18. Histology of R.A.G2-/- spleen and kidney from an isotype
control MAb
treated animal (A, E,) and anti-ICOS MAb treated animal (C). Higher
magnification (x200) of
the spleen demonstrates lack of germinal center formation in anti-ICOS-treated
animals (D)
compared to the isotype (B). Original magnification, x100; inset x1000.
100671 Figure .19. Treatment with anti-ICOS MAb significantly inhibits the
GvHD-SSc
skin pathology. Histology of back skin from either Balbic (A, B), or RAG2-/-
mice grafted with
sphmocytes at 4 weeks from isotype control MAb group (C, D) and anti-ICOS MAb
treated
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
group (E, F) is shoun. Tissue sections were stained with either hematoxylin
and eosin stain (top
row) or Masson's Trichrome stain (bottom row). Original magnifications, x200,
100681 Figure 20. ICOS MAb treatment impacts I helper- and TFH-associated
genes
and the autoimmune-gene fingerprint in the skin.
100691 Figure 21. Effect of concentration on Hydrodynamic Diameter of the
136 anti-
'COS antibody. in the figure closed triangle represents data obtained with the
136 anti-ICOS
antibody and closed circle represents data obtained with a non interacting
monoclonal antibody
(mAbB).
100701 Figure 22. Effect of sodium chloride concentration on the 136 anti-
ICOS
antibody RSA at 23'12. (closed circle) and 37 C (closed triangle),
100711 Figure 23. Effect of pH on the 136 anti -ICOS antibody RSA. Data
obtained with
a control non-interacting antibody )mAbB) is also shown.
100721 Figure 24. Effect of temperature on the 136 anti-ICOS antibody RSA.
mAbB is
a non-interacting control antibody.
100731 Figure 25. Effect of Temperature on the .136 anti-ICOS antibody
Dissociation
Kinetics.
5. DETAILED DESCRIPTION
100741 Characterization of the physico-chemical properties of the 136 anti-
ICOS
antibody led to the surprising discovery that the antibody undergoes
reversible self-association in
solution. The observed reversible self-association of the 136 antibody is
unique in that it does
not lead to aggregate formation. Because of the self-association, a
significant fraction of the 136
antibody exists as a Winer in solution. Additional experimental work
demonstrated that the
equilibrium between the monomer and winter form of 136 in solution is
influenced by antibody
concentration, temperature, ionic strength and pH. For example, at least 10
mole percent of the
136 antibody exists as a hinter in PBS at 10 mu/m1 antibody concentration at
37 C. Described
herein are stable liquid formulations comprising an antibody that specifically
binds human ICOS
and undergoes reversible self-association in solution.
100751 The present disclosure relates to stable liquid formulations of
antibodies or
fragments thereof that specifically bind to ICOS, undergo reversible self-
association in solution
and have an enhanced effector function (e.g., antibody-dependent cellular
cytotoxicity (ADCC)õ
complement-dependent cell-mediated cytotoxidty (CDC), and/or antibody-
dependent
phagocytosis). In certain embodiments, a stable liquid formulation of an anti-
human ICOS
antibody or a fragment thereof is suitable for parenteral administration to a
human subject. In a
16
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
specific embodiment, a stable liquid formulation of the disclosure is suitable
for subcutaneous
administration to a human subject.
5.1. Antibody Formulations
100761 In specific embodiments, the present disclosure encompasses stable
liquid
formulations of antibodies that. specifically bind to human ICOS, undergo
reversible self-
association in solution and have an enhanced effector function (e.g., antibody-
dependent cellular
cytotoxicity (ADCC), complement-dependent cell-mediated cytotoxicity (CDC),
and/or
antibody-dependent phwacytosis), wherein the formulations exhibit low to
undetectable levels
of antibody aggregation and/or fragmentation with very little to no loss of
the biological
activities during manufacture, preparation, transportation, and long periods
of storage. The
present disclosure also encompasses stable liquid formulations of antibodies
that specifically
bind to human ICOS, undergo reversible self-association in solution ,have an
enhanced effector
function and have increased in vivo half-lives, said formulations exhibiting
low to undetectable
levels of antibody aggregation and/or fragmentation, and very little to no
loss of the biological
activities of the antibodies during manufacture, preparation, transportation,
and long periods of
storage, in specific embodiments, a formulation of the disclosure comprises an
anti-human
ICOS antibody having increased in vivo ADCC activity, said formulation
exhibiting low to
undetectable levels of antibody aggregation and/or fragmentation, and very
little to no loss of the
biological activities of the antibodies during manufacture, preparation,
transportation, and long
periods of storage.
100771 In one embodiment, a liquid formulation of the disclosure is an
aqueous
formulation. In a specific embodiment, a liquid formulation of the disclosure
is an aqueous
formulation wherein the aqueous carrier is distilled water.
100781 In one embodiment, a formulation of the disclosure is sterile.
[00791 In one embodiment, a formulation of the disclosure is homogeneous.
100801 In one embodiment, a formulation of the disclosure is isotonic.
100811 The present disclosure provides stable high concentration liquid
formulations
comprising an anti-ICOS antibody having an enhanced effector function. In one
embodiment, a
formulation of the disclosure comprises an anti-ICOS antibody described in US
Patent
Application 12/116,512.
100821 In one embodiment, a formulation of the disclosure comprises an anti-
ICOS
antibody or a fragment thereof, wherein said antibody or a fragment thereof
comprises a VII
domain having the amino acid sequence of SEQ NO:7 and a VI, domain having the
amino
acid sequence of sEQ ID NO:2. In a specific embodiment, a formulation of the
disclosure
17
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
comprises an anti-ICOS antibody comprising a heavy chain having the amino acid
sequence of
SEQ ID NO:6 and a light chain having the amino acid sequence of SEC! ID NO: 1.
In a specific
embodiment, a formulation of the disclosure comprises an anti-human ICOS
antibody
comprising an Fc region having complex N-glycoside-linked sugar chains in
which fucose is not
bound to N-acetylglucosamine in the reducing end in the sugar chain.
[00831 The disclosure encompasses stable liquid formulations comprising a
single
antibody of interest (including antibody fragment thereof), for example, an
antibody that
specifically binds to an ICOS polypeptide. The disclosure also encompasses
stable liquid
formulations comprising two or more antibodies of interest (including antibody
fragments
thereof), for example, antibodies that specifically bind to an ICOS
polypeptide(s).
100841 In one embodiment, a formulation of' the disclosure comprises at
least about
1 mg/rril, at least about 5 ing/ml, at least about 10 mg/ml, at least about 20
mg/ml, at least about
30 mg/m], at least about 40 mg/nil, at least about 50 mg/nil, at least about
60 mg/mi. at least
about 70 mg/ml, at least about 80 mg/ml, at least about 90 inging, at least
about 100 ing/ml, at
least about 110 mg/ml, at least about 120 mg/ml, at least about .130 mg/ml, at
least about
140 ingtml, at least about 150 nighnl, at least about 160 mg/nil, at least
about 170 mg/ml, at least
about 180 mg/ml, at least about 190 mg/ml, at least about 200 mg/ml, at least
about 250 mg/ml,
or at least about 3(X) mg/nil of an anti-ICOS antibody or a fragment thereof.
In a specific
embodiment, a formulation of the disclosure comprises at least about 5 me/m1
of an anti-ICOS
antibody of a fragment thereof In a specific embodiment, a formulation of the
disclosure
comprises at least about 10 mg/m1 of an anti-ICOS antibody of a fragment
thereof In a specific
embodiment, a formulation of the disclosure comprises at least about 15 ing/m1
or an anti-ICOS
antibody of a fragment thereof. In a specific embodiment, a formulation of the
disclosure
comprises at least about 100 mg/m1 of an anti-ICOS antibody of a fragment
thereof. In a specific
embodiment, a formulation of the disclosure comprises at least about 125 mg/ml
of an anti4COS
antibody of a fragment thereof In a specific embodiment, a formulation of the
disclosure
comprises at least about 130 mg/m1 of an anti-ICOS antibody of a fragment
thereof. In a specific
embodiment, a formulation of the disclosure comprises at least about 150
rnWirl of an anti-ICOS
antibody of a fragment thereof In a specific embodiment, a formulation of the
disclosure
comprises at least about 90 mg/m1 of an anti-ICOS antibody of a fragment
thereof. In another
embodiment, a formulation of the disclosure comprises between about 1 rngiml
and about
20 mg/nal, between about 5 mg/m1 and about 20 ingirril, between about] inglml
and about
25 mgind, between about 1 mg/m1 and about 200 mg/ml, between about 25 mg/m1
and about
200 nig/nil, between about 50 mg/m1 and about 200 mg/ml, between about 75
mg/m1 and about
200 mg/ml, between about: 100 .mg/nil and about 200 mg/ml, between about 125
mg/m1 and
18
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
about 200 Ing/ml, between about 150 mg/m' and about 200 mg/ml, between about
25 mg/rill and
about 150 mg/ml, between about 50 ing/m1 and about 150 inglird, between about
75 nig/m1 and
about 150 mg/nil, between about 100 mg/m.1 and about 150 inWml, between about
125 mg/nil
and about 150 ma/nil, between about 25 mg/m1 and about .125 mg/ml, between
about 50
and about 125 mg/nil, between about 75 mg/mi. and about 125 ingimi, between
about 100 mg/m1
and about 125 mg/nil, between about 25 mg/m1 and about 100 mg/ml, between
about 50 mertil
and about 100 riving, between about 75 inalml and about 100 mg/m1, between
about 25 mg/nil
and about 75 menal, between about 50 and about 75
mg/ml, or between about 25 me/ml
and about 50 memi of an anti-ICOS antibody or a fragment thereof. In a
specific embodiment,. a
fomiulation of the disclosure comprises between about 5 mg/m1 and about 20
mWm1 of an anti-
ICOS antibody or a fragment thereof In a specific embodiment, a formulation of
the disclosure
comprises between about 90 mg/m1 and about 110 mg/m1 of an anti-IC OS antibody
or a
fragment thereof. In a specific embodiment, a formulation of the disclosure
comprises between
about 100 memi and about 210 nigiml of an anti-1COS antibody or a fragment
thereof. In a
further embodiment, a formulation described herein comprises about 1 mg/nil,
about 2 mg/ml,
about 3 .rngitril, about 4 ingiml, about 5 .mg/ml, about 10 mg/ml, about 15
mg/ml, about
20 mWtril, about 30 inviml, about 40 mg/ml, about 50 mg/ml, about 60 mg/nil,
about 70 mg/ml.
about 80 about 90
mg/nil, about 100 mg/ml, about 110 !mini, about 120 mg/ml, about
130 menil, about 140 mg/ml, about 150 mg/ml, about 160 mg/ml, about .170
mg/ml, about
180 mg/ml, about 190 mg/ml, about 200 mg/ml, about 250 mg/ml, or about 300
mg/m1 of an
anti-ICOS antibody or a fragment thereof. In a specific embodiment, a
formulation of the
disclosure comprises about 5 ing/m1 of an anti-1COS antibody or a fragment
thereof. In a
specific embodiment, a formulation of the disclosure comprises about 10 mg/m1
of an anti-ICOS
antibody or a fragment thereof. In a specific embodiment, a formulation of the
disclosure
comprises about 15 maiml of an anti-ICOS antibody or a fragment thereof. In a
specific
embodiment, a formulation of the disclosure comprises about 100 mg/m1 of an
anti-ICOS
antibody or a fragment thereof. In a specific embodiment, a formulation of the
disclosure
comprises about 125 mg/m1 of an anti-ICOS antibody or a fragment thereof In a
specific
embodiment, a formulation of the disclosure comprises about 130 mg/m1 of an
anti-ICOS
antibody or a fragment thereof. In a specific embodiment, a formulation of the
disclosure
comprises about .150 mg/ml of an anti-ICOS antibody or a fragment thereof. In
a specific
embodiment, a formulation of the disclosure comprises about 200 mg/m1 of an
anti-1COS
antibody or a fragment thereof. In a specific embodiment, a formulation of the
disclosure
comprises the anti-ICOS antibody comprising a heavy chain sequence of SEQ ID
NO:6, a light
chain sequence of SEQ ID NO:1 and an Fe region having complex N-
glycoside4inked sugar
19
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
chains in which fucose is not bound to N-actitylghicosamine in the reducing
end in the sugar
chain.
100851 in one embodiment, a formulation of the disclosure comprises at
least 1 mg/ml, at
least 5 mg/rril, at least 10 mg/ml, at least 20 mg/nil, at least 30 nigimi, at
least 40 mg/ml, at least
50 mg/ml, at least 60 mg/nil, at least 70 ingiml, at least 80 mg/ml, at least
90 ing/ml, at least
100 merril, at least 110 inglnil, at least .120 mg/ml, at least 130 rnelini,
at least 140 nig/ml, at
least 150 nig/ml, at least 160 Mg/MI, at least 170 mg/ml, at least 180 at
least 190 ing/ml,
at least 200 mg/ml, at least 250 mg/ml, or at least 300 mg/m.1 of an anti-ICOS
antibody or a
fragment thereof In a specific embodiment, a formulation of the disclosure
comprises at least
mg/m1 of an anti-ICOS antibody of a fragment thereof. In a specific
embodiment, a
thrmulation of the disclosure comprises at least 10 mg/nil of an anti-ICOS
antibody of a
fragment thereof. In a specific embodiment, a formulation of the disclosure
comprises at least
mg/nil of an anti-ICOS antibody of a fragment thereof In a specific embodiment
a
formulation of the disclosure comprises at least 100 trig/ml of an anti-ICOS
antibody of a
fragment thereof in a specific embodiment, a formulation of the disclosure
comprises at least
125 mg/m1 of an anti-ICOS antibody of a fragment thereof. In a specific
embodiment, a
formulation of the disclosure comprises at least 150 mg/m1 of an anti-KOS
antibody of a
fragment thereof. In a specific embodiment, a formulation of the disclosure
comprises at least
175 mg/nil of an anti-ICOS antibody of a fragment thereof In a specific
embodiment, a
formulation of the disclosure comprises at least 200 mg/m1 of an ariti4COS
antibody of a
fragment thereof. in another embodiment, a formulation of the disclosure
comprises between
1 mg/m1 and 20 mg/nil, between 5 mg/m! and 20 mg/nil, between 1 mg/m1 and 25
mg/m!,
between 1 mg/nil and 200 rng/rnl, between 25 rrig/m1 and 200 mg/nil, between
50 mg/m1 and
200 mg/mi. between 75 mg/m1 and 200 mg/ml, between 100 mg/nil and 200 mg/ml,
between
125 mg/m! and 200 mg/ml, between 150 mg/ini and 200 mg/ml, between 25 mg/mi
and
150 mg/nil, between 50 inglml and 150 mg/ml, between 75 mg/m1 and 150 mg/ml.
between
100 mg/m1 and 150 mg/nil, between 125 mg/m1 and 1.50 mg/nil, between 25 mg/rni
and
125 mentl, between 50 mg/nil and 125 mg/MI, between 75 mg/nil and 125 mg, miõ
between
100 mg/m1 and 125 mg/ml, between 25 mg/m1 and 100 mg/ml, between 50 nigiml and
100 mg/nil, between 75 -mglin.1 and 100 rug/ml, between 25 mg/nil and 75
mg/nil, between
50 mg/ml and 75 mg/ml, or between 25 mg/m1 and 50 mg/nil of an anti-1COS
antibody or a
fragment thereof In a specific embodiment, a formulation of the disclosure
comprises between
5 and 20 mg/ml of an anti-ICOS antibody or a fragment thereof. In a
specific
embodiment, a formulation of the disclosure comprises between 90 ing/m1 and
110 inglinl of an
anti-IC OS antibody or a fragment thereof. In a specific embodiment, a
formulation of the
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
disclosure comprises between 100 inglinl and 210 Ing/mi of an anti-ICOS
antibody or a fragment
thereof In a further embodiment, a formulation described herein comprises 1
mgirni, 2 nigirnl,
3 mg/ml, 4 mg/ml, 5 mg/nil, 10 rnsiml, 15 mg/ml, 20 30 nwiml, 40 mg/ml, 50
mg/ml,
60 mg/int 70 mg/ml, 80 mg/ml, 90 mg/ml, 100 rriglml, 110 mg/ml, 120 mg/nil,
130 mg/int
140 mernl, 150 mg/nil, 160 mg/ml, 170 mg/ml, 180 trigiml, 190 mg/ml, 200
mg/mi. 250 mg/To!,
or 300 mg/m1 of an anti-1COS antibody or a fragment thereof. In a specific
embodiment, a.
formulation of the disclosure comprises 10 ingtml of an anti-ICOS antibody or
a fragment
thereof In a specific embodiment, a formulation of the disclosure comprises
100 inghnl of an
rmti-ICOS antibody or a fragment thereof. In a specific embodiment, a
formulation of the
disclosure comprises 125 ming of an anti-ICOS antibody or a fragment thereof
In a specific
embodiment, a formulation of the disclosure comprises 150 ingiml of an anti-
ICOS antibody or a
fragment thereof. In a specific embodiment, a formulation of the disclosure
comprises
175 mg/ml of an anti-ICOS antibody or a fragment thereof In a specific
embodiment, a
formulation of the disclosure comprises 200 inglinl of an anti-ICOS antibody
or a fragment
thereof. In a specific embodiment, a formulation of the disclosure comprises
the anti-ICOS
antibody comprising a heavy chain sequence of S.EQ ID NO:6, alight chain
sequence of SEQ ID
NO:1 and an Fe region having complex N-glycoside-linked sugar chains in which
fucose is not
bound to N-acetylglucosamine in the reducing end in the sugar chain.
100861 Optionally, the formulations of the disclosure may further comprise
common
excipients and/or additives such as buffering agents, saccharides, salts and
surfactants.
Additionally or alternatively, the formulations of the disclosure may further
comprise common
excipients and/or additives, such as, but not limited to, solubilizers,
diluents, binders, stabilizers,
salts, lipophilic solvents, amino acids, chelators, preservatives, or the
like.
100871 In certain embodiments, the buffering agent is selected from the
group consisting
of histidine, citrate, phosphate, glycine, and acetate. In other embodiments
the saccharide
excipient is selected from the group consisting of trehalose, sucrose,
mannitol, maltose and
raffinose. in still other embodiments the surfactant is selected from the
group consisting of
polysorbate 20, polysorbate 40, polysorbate 80, and Pluronic F68. In yet other
embodiments the
salt is selected from the group consisting of NaCI, KC1, MgCl2, and CaCl2
100881 Optionally, the formulations of the disclosure may further comprise
other
common auxiliary components, such as, but not limited to, suitable excipients,
polyols,
soluhilizers. diluents, binders, stabilizers, lipophilic solvents, chelators,
preservatives, or the like.
100891 The formulations of the disclosure include a buffering or pH
adjusting agent to
provide improved pH control. In one embodiment, a formulation of the
disclosure has a pli of
between about 3.0 and about 9.0, between about 4.0 and about 8.0, between
about 5.0 and about
21
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
8.0, between about 5.0 and about 7Ø between about 5.0 and about 6.5, between
about 5.5 and
about 8.0, between about 5.5 and about 7.0, or between about 5.5 and about
6.5. In a further
embodiment, a formulation of the disclosure has a pH of about 3.0, about 3.5,
about 4.0, about
4.5, about 5.0, about 5.1, about 5.2, about 5.3, about 5.4, about 5.5, about
5.6, about 5.7, about
5.8, about 5.9, about 6.0, about 6.1, about 6.2, about 6.3, about 6.4, about
6.5, about 6.6, about
6.7, about 6.8, about 6.9, about 7.0, about 7.5, about $.0, about 8.5, or
about 9Ø In a specific
embodiment, a formulation of the disclosure has a pH of about 6Ø
100901 The formulations of the disclosure include a buffering or pH
adjusting agent to
provide improved pH control. In one embodiment, a formulation of the
disclosure has a pH of
between 3.0 and 9.0, between 4.0 and 8.0, between 5.0 and 8.0, between 5.0 and
7Ø between 5.0
and 6.5, between 5.5 and 8.0, between 5.5 and 7.0, or between 5.5 and 6.5. In
a further
embodiment, a formulation of the disclosure has a pH of 3.0, 3.5, 4.0,4.5,
5.0, 5.1, 5.2, 5.3, 5.4,
5.5, 5.6, 5.7. 5.8, 5.9, 6.0,6.1, 6.2, 6.3, 6.4, 6.5, 6.6, 6.7, 6.8, 6.9, 7.0,
7.5,, 8.0, 8.5, or 9Ø In a
specific embodiment, a formulation of the disclosure has a pH of 6Ø
100911 The pti of the formulation generally should not be equal to the
isoelectric point of
the particular antibody (including antibody fragment thereof) to be used in
the formulation (for
example, but not limited to, the isoelectric point of the anti-1COS antibody
comprising a heavy
chain sequence of SEQ ID NO:6, alight chain sequence of SEQ ED NO:1 and an Fe
region
having complex N-glycoside-linked sugar chains in which fucose is not bound to
N-
acetylglucosamine in the reducing end in the sugar chain) and may range from
about. 4.0 to about
8.0, or may range from about 5.5 to about 6.5.
100921 Typically, the buffering agent is a salt prepared from an organic or
inorganic acid
or base. Representative buffering agents include, but are not limited to,
organic acid salts such
as salts of citric acid, ascorbic acid, gluconic acid, carbonic acid, tartaric
acid, succinic acid,
acetic acid, or phthalic acid; Tris, tromethamine hydrochloride, or phosphate
buffers. In
addition, amino acid components can also function in a buffering capacity.
Representative
amino acid components which may be utilized in the formulations of the
disclosure as buffering
agents include, but are not limited to, glycine and histidine. In certain
embodiments, the
buffering agent is selected from the group consisting of histidine citrate,
phosphate, glycine, and
acetate. In a specific embodiment, the buffering agent is histidine. In
another specific
embodiment, the buffering agent is citrate. The purity of the buffering agent
should be at least
98%, or at least 99%, or at least 99.5%. As used herein, the term purity" in
the context of
histidine refers to chemical purity of histidine as understood in the art,
e.g, as described in The
Merck Index, 13th ed., O'Neil et al. ed. (Merck & Co., 2001).
22
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00931 Buffering agents are typically used at concentrations between about
1 mM and
about 200 mM or any ranee or value therein, depending on the desired ionic
strength and the
buffering capacity required. The usual concentrations of conventional
buffering agents
employed in parenteral formulations can be found in: Pharmaceutical Dosage
Form: Parenteral
Medications, Volume I , 2nd Edition, Chapter 3, p. 194, De Luca and Boylan,
"Formulation of
Small Volume Parenteralsff, Table 5: Commonly used additives in Parenteral
Products. In one
embodiment, the buffering agent is at a concentration of about 1 mM, or of
about 5 niM, or of
about 10 triM, or of about 15 mM, or of about 20 mM, or of about 25 mM, or of
about 30 mM, or
of about 35 rnM, or of about 40 mM, or of about 45 mM, or of about 50 mM, or
of about 60 mM,
or of about 70 inM, or of about 80 rnM, or of about 90 mM, or of about 100 mM.
In one
embodiment, the buffering agent is at a concentration of 1 mM, or of 5 mM, or
of 10 mM, or of
15 mM, or of 20 m.M, or of 25 mM, or of 30 mM. or o35 mM, or of 40 mM, or of
45 mM, or of
50 mM, or of 60 mM, or of 70 mIVI, or of 80 mM, or of 90 mM, or of 100 mM. in
a specific
embodiment, the buffering agent is at a concentration of between about 5 mM
and about 50 mM.
In another specific embodiment, the buffering agent is at a concentration of
between 5 mM and
20 MM.
100941 Buffering agents are typically used at concentrations between 1 mM
and 200 mM
or any range or value therein, depending on the desired ionic strength and the
buffering capacity
required. The usual concentrations of conventional buffering agents employed
in parenteral
formulations can be found in: Pharmaceutical Dosage Form: Parenteral
Medications, Volume 1,
ri Edition. Chapter 5, p. 194, De Luca and Boylan, ?!Formulation of Small
Volume Paremeralsff,
Table 5: Commonly used additives in Parenteral Products. In one embodiment,
the buffering
agent is at a concentration of 1 mM, or of 5 mkt, or of 10 mM, or of 15 mM, or
of 20 mM., or of
25 mM, or of 30 mM, or of 35 raM, or of 40 m.M., or of 45 inM, or of 50 .m/q,
or of 60 mM, or of
70 rrtM, or of 80 mM, or of 90 mM, or of IOU mM. in one embodiment, the
buffering agent is at
a concentration of! mM, or of 5 mM, or of 10 mM, or of 15 mM, or of 20 mM, or
of 25 niM, or
of 30 mM, or of 35 mM, or of 40 mM, or of 45 mM, or of 50 mM, or of 60 mM, or
of 70 mM, or
of 80 mM, or of 90 mM, or of 100 mM. in a specific embodiment, the buffering
agent is at a
concentration of between 5 m114 and 50 mM. In another specific embodiment, the
buffering
agent is at a concentration of between 5 mM and 20 mM.
[0095[ In certain embodiments, a formulation of the disclosure comprises a
buffering
agent In one embodiment, said buffering agent is selected from the group
consisting of
histidine, citrate, phosphate, glycine, and acetate. In a specific embodiment,
a formulation of the
disclosure comprises histidine as a buffering agent.
23
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
100961 In one embodiment, a formulation of the disclosure comprises at
least about
1 .mM, at least about 5 mM, at least about 10 mM. at least about 20 mM, at
least about 30 mM., at
least about 40 mM, at least about 50 mM, at least about 75 mM, at least about
100 mM, at least
about 150 mM, or al least about 200 mM histidine. In another embodiment, a
formulation of the
disclosure comprises between about 1 mM and about 200 mM, between about 1 mM
and about
150 triM, between about 1 mM and about 100 mM. between about] mM and about 75
inM,
between about 10 mkt and about 200 mM, between about 10 mM and about 150 mM,
between
about 10 iniM and about 100 mM, between about 10 riiM and about 75 mM, between
about 10
mM and about 50 mM, between about 10 mM and about 40 mM, between about 10 mM
and
about 30 mM, between about 20 mM and about 75 mM, between about 20 mM and
about
50 rnM, between about 20 mkt and about 40 mM, or between about 20 riiM and
about 30 mM
histidine. In a further embodiment of the disclosure comprises about 1 mM,
about 5 mM, about
mM, about 20 mM. about 25 mM, about 30 mlvl, about 35 mM, about 40 mMõ about
45 niM,
about 50 mM, about 60 mM, about 70 mM, about 80 TIM, about 90 mM, about 100
mM, about
150 mM, or about 200 mM histidine. In a specific embodiment, a formulation of
the disclosure
comprises about .10 mM histidine.
100971 in one embodiment, a formulation of the disclosure comprises at
least 1 mM, at
least 5 mM, at least 10 mM, at least 20 mM, at least 30 mM, at least 40 mM, at
least 50 mM, at
least 75 mM, at least 100 mM, at least 150 mM, or at least 200 mM histidine.
In another
embodiment, a formulation of the disclosure comprises between 1 mM and 200 mM,
between 1
mM and 150 mM, between I mM and .100 mkt, between 1 mM and 75 mM. between 10
mM and
200 mM, between 10 mls4 and 150 VIM, between 10 mM and 100 mM, between 10 mM
and
75 mM, between 10 mivi and 50 mM, between 10 mM and 40 mM. between 10 mM and
30 mM,
between 20 mM and 75 mM, between 20 mM and 50 mM, between 20 mM and 40 mM, or
between 20 mM and 30 mkt histidine. In a further embodiment of the disclosure
comprises
1 inM, 5 mM, 10 mM, 20 mM, 25 mM, 30 mM, 35 mM, 40 mM, 45 mM, 50 mM, 60 mM,
70 mM, 80 mM, 90 mM, 100 raki, 150 mM, or 200 mM histidine. In a specific
embodiment, a
formulation of the disclosure comprises 10 mM histidine.
100981 hi certain embodiments, the formulations of the disclosure comprise
a
carbohydrate excipient. Carbohydrate excipients can act, e.g., as viscosity
enhancing agents,
stabilizers, bulking agents, solubihring agents, and/or the like. Carbohydrate
excipiarts are
generally present at between about 1% and about 99% by weight or volume. In
one
embodiment, the carbohydrate excipient is present at between about 0.1% and
about 20%. In
another embodiment, the carbohydrate excipient is present at between about
0.1% and about
15%. In a specific embodiment, the carbohydrate excipient is present at
between about 0.1% and
24
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
about 5%, or between about] A, and about 20%, or between about 5% and about
15%, or
between about 8% and about 10%, or between about 10% and about 15%, or between
about 15%
and about 20%. In another specific embodiment, the carbohydrate excipient is
present at
between 0.1% and 20%, or between 5% and 15%, or between 8% and 10%, or between
10% and
15%, or between 15% and 20%. In still another specific embodiment, the
carbohydrate excipient
is present at between about 0.1% and about 5%. In still another specific
embodiment, the
carbohydrate excipient is present at between about 5% and about 10%. In yet
another specific
embodiment, the carbohydrate excipient is present at between about 15% and
about 20%. In still
other specific embodiments, the carbohydrate excipient is present at 1%, or at
1.5%, or at 2%, or
at 2.5%, or at 3%, oral 4%, or at 5%. or at 10%, or at 15%, or at 20%.
100991 In certain embodiments, the formulations of the disclosure comprise
a
carbohydrate excipient. Carbohydrate excipients can act, e.g., as viscosity
enhancing agents,
stabilizers, bulking agents, solubilizing agents, and/or the like.
Carbohydrate excipients are
generally present at between 1% and 990,'o by weight or volume. In one
embodiment, the
carbohydrate excipient is present at between 0.1% and 20%. In another
embodiment, the
carbohydrate excipient is present at between 0.1% and 15%. In a specific
embodiment; the
carbohydrate excipient is present at between 0.1% and 5%, or between 1% and
20%, or between
5% and 15%, or between 8% and 10%, or between 10% and 15%, or between 15% and
20%. In
another specific embodiment, the carbohydrate excipient is present at between
0.1% and 20%, or
between 5% and .15%, or between 8% and 1.0%, or between 10% and 15%, or
between 15% and
20% In still another specific embodiment: the carbohydrate excipient is
present at between
0.1% and 5%. In still another specific embodiment, the carbohydrate excipient
is present at
between 5% and 10%. In yet another specific embodiment, the carbohydrate
excipient is present
at between .15% and 20%. In still other specific embodiments, the carbohydrate
excipient is
present at 1%, or at 1.5%, or at 2%, or at 2.5%, or at 3%, or at 4%, or at 5%,
or at 10%, or at
15%, or at 20%.
1001001 Carbohydrate excipients suitable for use in the formulations of the
disclosure
include, for example, monosaccharides such as fructose, maltose, galactose,
glucose, 1) -
man n os e, sorbose, and the like; disaccharides, such as lactose, sucrose,
trehalose, cellobiose, and
the like: polysaccharides, such as raffinose, melezitose, rnaltodextrins,
dextrans, starches, and the
like; and alditols, such as mannitol, sorbitol (glucitol) and the
like. In one embodiment, the carbohydrate excipients -for use in the present
disclosure are
selected from the group consisting of, sucrose, trehalose, lactose, mannitol,
and raffinose. In a
specific embodiment, the carbohydrate excipient is trehalose. In another
specific embodiment,
the carbohydrate excipient is mannitol. In yet another specific embodiment,
the carbohydrate
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
excipient is sucrose. In still another specific embodiment, the carbohydrate
excipient is
raffinose. The purity of the carbohydrate excipient should be at least 98%, or
at least 99%, or at
least 99.5%.
100101] in one embodiment, a formulation of the disclosure comprises at
least about .1%,
at least about 2%, at least about 4%, at least about 8%, at least about 20%,
at least about 30%, or
at least about 40% trehalose. In another embodiment, a formulation of the
disclosure comprises
between about 1% and about 40%, between about 1% and about 30%, between about
1% and
about 20%, between about 2% and about 40%, between about 2% and about 30%,
between about
2% and about 20%, between about 4% and about 40%, between about 4% and about
30%, or
between about 4% and about 20% trehalose. In a further embodiment, a
formulation of the
disclosure comprises about 1%, about 2%, about 4%, about 8%, about 20%, about
30%, or about
40% trehalose. In a specific embodiment, a formulation of the disclosure
comprises about 4%
trehalose.
PO1021 In one embodiment, a formulation of the disclosure comprises at
least 1%, at least
2%, at least 4%, at least 8%, at least 20%, at least 30%, or at least 40%
trehalose. In another
embodiment, a formulation of the disclosure comprises between I% and 40%,
between 1% and
30%, between 1% and 20%, between 2% and 40%, between 2% and 30%, between 2%
and 20%,
between 4% and 40%, between 4% and 30%, or between 4% and 20% trehalose. In a
further
embodiment, a formulation of the disclosure comprises 1%, 2%, 4%, 8%, 20%,
30%, or 40%
trehalose. In a specific embodiment, a formulation of the disclosure comprises
4 % trehalose.
[001031 In one embodiment, a formulation of the disclosure comprises an
excipient. In a
specific embodiment, a formulation of the disclosure comprises at least one
excipient selected
from the group consisting of: sugar, salt, surfactant amino acid, poly ol,
chelating agent,
emulsifier and preservative. In one embodiment, a formulation of the
disclosure comprises a
salt. In one embodiment, a formulation of the disclosure comprises a salt
selected from the
group consisting of NaCl, Ka, CaCl2, and MgCl2. In a specific embodiment, a
formulation of
the disclosure comprises Naa.
[00104) hi one embodiment, a formulation of the disclosure comprises at
least about
mM, at least about -25 mM, at least about 50 mM, at least about 75 mM, at
least about 80 mM,
at least about 100 mM, at least about 125 mM, at least about 150 mM, at least
about 175 mM. at
least about 200 mM, or at least about 300 rn1V1 sodium chloride. In a further
embodiment, a
-fomulation described herein comprises between about 10 mfvf and about 300 mM,
between
about 10 triM and about 200 mM, between about 10 mlvi and about 175 inM,
between about
10 mM and about 150 niM, between about 25 mM and about 300 niM, between about
25 mM
and about 200 mM, between about 25 mM and about 175 mM, between about 25 mM
and about
26
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
150 niM, between about 50 triM and about 300 mkt, between about 50 mM and
about 200 mM,
between about 50 mM and about 175 triM, between about 50 mM and about 150 mM,
between
about 75 mM and about 300 mM, between about 75 mM and about 200 mM, between
about
75 mM and about 175 mM, between about 75 mM and about 150 mM, between about
100 mM
and about 300 mM, between about 100 mM and about 200 mkt, between about 1.00
mM and
about 175 triM, or between about 100 mM and about 150 Mkt sodium chloride. in
a further
embodiment, a formulation of the disclosure comprises about 10 mM. about 25
mM, about 50
mkt, about 75 mM, about 80 mkt, about 100 mM, about 125 mM, about 150 mM,
about
175 mM, about 200 mM., or about 300 mM sodium chloride. In a specific
embodiment, a
formulation of the disclosure comprises 80 mM sodium chloride.
1001051 in one embodiment, a .formulation of' the disclosure comprises at
least 10 mM, at
least 25 mM, at least 50 mM, at least 75 mM, at least 80 mM, at least .100 mM,
at least 125 ;TIM,
at least 150 mM, at least 175 ink4. at least 200 mM. or at least 300 mM sodium
chloride. In a
further embodiment, a formulation described herein comprises between 10 mM and
300 mM,
between 10 mM and 200 mtki, between 10 mM and 175 mM, between 10 mM and 150
mM,
between 25 mM and 300 mkt, between 25 mM and 200 mM, between 25 mM and 175 mM,
between 25 mkt and 150 mM, between 50 mM and 300 mM, between 50 mM and 200 mM,
between 50 mM and 175 mM, between 50 mM and 150 mM, between 75 mkt and 300 mM,
between 75 triM and 200 .mM, between 75 mkt and 175 naM, between 75 mM and 150
mM,
between 100 mM and 300 mM, between 100 .mM and 200 mM, between 100 rriM and
1.73 mM,
or between 100 mM and 150 mM sodium chloride. in a further embodiment, a
formulation of
the disclosure comprises 10 mM. 25 mM, 50 mM, 75 mM, 80 mM, 100 mM, 125 mkt,
150 rriM,
175 mM, 200 mM, or 300 trikl sodium chloride. In a specific embodiment, a
formulation of the
disclosure comprises 80 mM sodium chloride.
100106] The formulations of the disclosure may further comprise a
surfactant. The term
"surfactant" as used herein refers to organic substances having amphipathic
structures; namely,
they are composed of groups of opposing solubility tendencies, typically an
oil-soluble
hydrocarbon chain and a water-soluble ionic group. Surfactants can be
classified, depending on
the charge of the surface-active moiety, into anionic, cationic, and nonionic
surfactants.
Surfactants are often used as wetting, emulsifying, solubilizing, and
dispersing agents for various
pharmaceutical compositions and preparations of biological materials.
Pharmaceutically
acceptable surfactants like polysorbates (e.g polysorbates 20 or 80);
polyoxamers (e.g.
poloxamer 188); Triton; sodium octyl glycoside; lautyl-, myristyl-, linoleyl-,
or stearyl-
sulfobetaine; myristyl-, linoleyl- or steatyl-sarcosine; linoleyl-,
myristyl-, or cetyl-
betaine; lauroamidopropyl-, cocarnidopropyl-, linoleamidopropyl-,
myristamidopropyl-,
27
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
palmidopropyl-, or isosteammidopropyl-betaine (e.g. latiroamidoproff1);
myristamidopropyl-,
palmidopropyl-, or isostearamidoprowl-dimethylamine; sodium methyl cocoyl-, or
disodium
methyl oleyl-tawate; and the MONAQUA.TK series (Mona Industries, Inc.,
Paterson, NJ.),
polyethyl glycol, polypropyl glycol, and copolymers of ethylene and propylene
glycol (e.g
Pluronics, PF68 etc), can optionally be added to the formulations of the
disclosure to reduce
aggregation. Surfactants are particularly useful if a pump or plastic
container is used to
administer the formulation. The presence of a pharmaceutically acceptable
surfactant mitigates
the propensity for the protein to aggregate. In a specific embodiment, the
formulations of the
disclosure comprise a polysorbate which is at a concentration ranging from
between about
0.001% to about 1%, or about 0.001% to about 0.1%, or about 0.01% to about
0.1%. In other
specific embodiments, the formulations of the disclosure comprise a
polysorbate which is at a
concentration of 0.001%, or 0.002%, or 0.003%, or 0.004%, or 0.005%, or
0.006%, or 0.007%,
or 0.008%, or 0.009%, or 0.01%, or 0.015%, or 0.02%. In another specific
embodiment, the
polysorbate is polysorbate-80. In a specific embodiment, the formulations of
the disclosure
comprise a polysorbate which is at a concentration ranging from between 0.001%
and 1%, or
0.001% and 0.1%, or 0.01% and 0.1%. In other specific embodiments, the
formulations of the
disclosure comprise a polysorbate which is at a concentration of 0.001%, or
0.002%, or 0.003%,
or 0.004%, or 0.005%, or 0.006%, or 0.007%, Of 0.008%, or 0.009%, or 0.01%, or
0.015%, or
0.02%. In another specific embodiment, the polysorbate is polysorbate-80.
1001071 In one embodiment, a formulation of the disclosure comprises a
surfactant. In
one embodiment, a formulation of the disclosure comprises Polysorbine 20,
Polysorbate 40,
Polysorbate 60, or Polysorbate 80. In a specific embodiment, a formulation of
the disclosure
comprises Polysorbate 80.
1001041 In one embodiment, a formulation of the disclosure comprises at
least about
0.001%, at least about 0.002%, at least about 0.005%, at least about 0.01%, at
least about 0.02%,
at least about 0.05%, at least about 0. 1%, at least about 0.2%, or at least
about 0.5% Polysorbate
80. In another embodiment, a formulation of the disclosure comprises between
about 0.001%
and about 0.5%, between about 0.001% and about 0.2%, between about 0.001% and
about 0.1%,
between about 0.001% and about 0.05%, between about 0.002% and about 0.5%,
between about
0.002% and about 0.2%, between about 0.002% and about 0.1%, between about
0.002% and
about 0.05%, between about 0.005% and about 0.5%, between about 0.005% and
about 0.2%,
between about 0.005% and about 0.1%, between about 0.005% and about 0.05%,
between about
0.01% and about 0.5%, between about 0.01% and about 0.2%, between about 0.01%
and about
0.1%, or between about 0.01% and about 0.05% Polysorbate 80. In a further
embodiment, a
formulation of the disclosure comprises about 0.001%, about 0.002%, about
0.005%, about
28
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
0.01%, about 0.02%, about 0.05%. about 0.1%, about 0.2%, and about 0.5%
Polysorbate 80. In
a specific embodiment, a formulation of the disclosure comprises about 0.02%
Polysorbate 80.
In a specific embodiment, a formulation of the disclosure comprises about 004%
Polysorbate
80. In a specific embodiment, a formulation of the disclosure comprises about
0.05%
Polysorbate 80.
(001091 In one embodiment, a formulation of the disclosure comprises at
least 0.001%, at
least 0.002%, at least 0.005%, at least 0.01%, at least 0.02%, at least 0.05%,
at least 0. 1%, at
least 0.2%, or at least 0.5% Polysorbate 80. In another embodiment, a
formulation of the
disclosure comprises between 0.001% and 0.5%. between 0.001% and 0.2%, between
0.001%
and 0.1%, between 0.001% and 0.05%, between 0.002% and 0.5%, between 0,002%
and 0.2%,
between 0.002% and 0.1%, between 0.002% and 0.05%, between 0.005% and 0.5%,
between
0.005% and 0.2%, between 0.005% and 0.1%, between 0.005% and 0.05%, between
0.01% and
0.5%, between 0.01% and 0.2%, between 0.01% and 0.1%, or between 0.01% and
0.05%
Polysorbate 80. In a further embodiment, a formulation of the disclosure
comprises 0.001%,
0.002%, 0.005%, 0.01%, 0.02%, 0.05%, 0.1%, 0.2%, and 0.5% Polysorbate 80. In a
specific
embodiment, a formulation of the disclosure comprises 0.02% Polysorbate 80. In
a specific
embodiment, a formulation of the disclosure comprises 0.04% Polysorbate 80. In
a specific
embodiment, a formulation of the disclosure comprises 0.05% Polysorbate 80.
1001101 Optionally, the formulations of the disclosure may further comprise
other
common excipients and/or additives including, but not limited to, diluents,
binders, stabilizers,
lipophilic solvents, preservatives, adjuvants, or the like. Pharmaceutically
acceptable excipients
and/or additives may be used in the formulations of the disclosure. Commonly
used
excipientsladditives, such as pharmaceutically acceptable chelators (for
example, but not limited
to, EDTA. DTPA or EGTA) can optionally be added to the formulations of the
disclosure to
reduce aggregation. These additives are particularly useful if a pump or
plastic container is used
to administer the formulation.
1001111 Preservatives, such as phenol, m-cresol, p-cresol, o-cresol,
cblorocresol, benzyl
alcohol, phanylmercuric nitrite, pherioxyethanol, formaldehyde, chlorobutanol,
magnesium
chloride (for example, but not limited to, hexahydrate), rdItylparaben
(methyl, ethyl, propyl,
butyl and the like), benzalkonium chloride, benzethonium chloride, sodium
dehydroacetate and
thimerosal, or mixtures thereof can optionally be added to the formulations of
the disclosure at
any suitable concentration such as between about 0.001% to about 5%, or any
range or value
therein. The concentration of preservative used in the formulations of the
disclosure is a
concentration sufficient to yield an anti-microbial effect. Such
concentrations are dependent on
the preservative selected and are readily determined by the skilled artisan.
29
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[001121 Other contemplated excipiaitsladditives, which may be utilized in
the
formulations of the disclosure include, for example, flavoring agents,
antimicrobial agents,
sweeteners, antioxidants, antistatic agents, lipids such as phospholipids or
fatty acids, steroids
such as cholesterol, protein excipients such as serum albumin (human serum
albumin (1-1SA),
recombinant human albumin (rI-1A)), gelatin, casein, salt-forming counterions
such as sodium
and the like. These and additional known pharmaceutical euipients and/or
additives suitable for
use in the formulations of the disclosure are known in the art, e.g, as listed
in "Remington: The
Science & Practice of Pharmacy", 21 ed., Lippincott Williams & Wilkins,
(2005), and in the
"Physician's Desk Reference", 60th ed., Medical Economics, Montvale, NI
(2005).
Pharmaceutically acceptable carriers can be routinely selected that are
suitable for the mode of
administration, solubility and/or stability of Fe variant protein as well
known in the art or as
described herein.
[001131 It will be understood by one skilled in the art that the
formulations of the
disclosure may be isotonic with human blood, that is the formulations of the
disclosure have
essentially the same osmotic pressure as human blood. Such isotonic
formulations will generally
have an osmotic pressure from about 250 mOSm to about 350 mOSm. Isotonicity
can be
measured by, for example, using a vapor pressure or ice-freezing type
osmometer. Tonicity of a
formulation is adjusted by the use of tonicity modifiers. "Tonicity modifiers"
are those
pharmaceutically acceptable inert substances that can be added to the
formulation to provide an
isotonicity of the formulation. Tonicity modifiers suitable for this
disclosure include, but are not
limited to, saccharides, salts and amino acids.
[001141 In certain embodiments, the formulations of the present disclosure
have an
osmotic pressure from about 100 mOSm to about 1200 mOSm, or from about 200
xn0Sm to
about 1000 mOSm, or from about 200 .mOSm to about 800 mOSm, or from about 200
mOSm to
about 600 mOSm, or from about 250 mOSm to about 500 mOSm, or from about 250
mOSm to
about 400 mOSm, or from about 250 mOSm to about 350 mOSm.
1001151 In certain embodiments, the formulations of the present disclosure
have an
osmotic pressure from 100 mOSm to 12(X) mOSm, or from 200 mOSm to 1000 mOSin,
or from
200 mOSm to Stk mOSm, or from 200 mOSm to 600 mOSm, or from 250 mOSm to 500
mOSm, or from 250 mOSm to 400 mOSm, or from 250 mOSm to 350 mOSm.
[001161 Concentration of any one or any combination of various components
of the
formulations of the disclosure is adjusted to achieve the desired tonicity of
the final formulation.
For example, the ratio of the carbohydrate excipient to antibody may be
adjusted according to
methods known in the art (e.g., U.S. Patent No. 4685,940). In certain
embodiments, the molar
ratio of the carbohydrate excipient to antibody may be from about 100 moles to
about 1000
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
moles of carbohydrate excipient to about 1 mole of antibody, or from about
2(K) moles to about
6000 moles of carbohydrate excipient to about 1 mole of antibody, or from
about 100 moles to
about 510 moles of carbohydrate excipient to about 1 mole of antibody, or from
about 100 moles
to about 600 moles of carbohydrate excipient to about 1 mole of antibody.
1001171 Concentration of ally one or any combination of various components
of the
formulations of the disclosure isadjusted to achieve the desired tonicity of
the final formulation.
For example, the ratio of the carbohydrate excipient to antibody may be
adjusted according to
methods known in the art (e.g., U.S. Patent No. 6,685,940). In certain
embodiments, the molar
ratio of the carbohydrate excipient to antibody may be from 100 moles to .1000
moles of
carbohydrate excipient to 1 mole of antibody, or from 200 moles to 6000 moles
of carbohydrate
excipient to 1 mole of antibody, or ftom 100 moles to 510 moles of
carbohydrate excipient to 1
mole of antibody, or from 100 moles to 600 moles of carbohydrate excipient to
I mole of
antibody.
[001 IS) The desired isotonicity of the final formulation may also be
achieved by adjusting
the salt concentration of the formulations. Salts that are pharmaceutically
acceptable and
suitable for this disclosure as tonicity modifiers include, but are not
limited to, sodium chloride,
sodium succinate, sodium sulfate, potassium chloride. magnesium chloride,
magnesium sulfate,
and calcium chloride. In specific embodiments, formulations of the disclosures
comprise NaCl,
MgCl. and/or CaCl2. In one entbodiment, concentration of NaCl is between about
75 mM and
about 150 triM. In another embodiment, concentration of MgCl2 is between about
1 mM and
about 100 mlvt. Amino acids that are pharmaceutically acceptable and suitable
for this
disclosure as tonicity modifiers include, but are not limited to, proline,
alanine. L-aminine,
asparagine, L-aspartic acid, glycine, serine, lysine, and histidine.
1001.191 In one embodiment, a -forrntdation of the disclosure comprises
histidine, sodium
chloride, trehalose, and Polysorbate 80. In one embodiment., a formulation of
the disclosure
comprises sodium chloride, trehalose, and Polysorbate 80. In one embodiment, a
formulation of
the disclosure comprises histidine, trehalose, and Polysorbate 80. In one
embodiment, a
formulation of the disclosure comprises histidine, sodium chloride, and
Polysorbate 80. In one
embodiment, a formulation of the disclosure comprises histidine, sodium
chloride, and trehalose.
In one embodiment a formulation of the disclosure comprises histidine and
sodium chloride. In
one embodiment_ a formulation of the disclosure comprises histidine arid
trehalose. In one
embodiment, a formulation of the disclosure comprises histidine and
Polysorbate 80. In one
embodiment, a formulation of the disclosure comprises sodium chloride and
trehalose. In one
embodiment, a formulation of the disclosure comprises sodium chloride and
Polysorbate 80. In
one embodiment, a formulation of the disclosure comprises trehalose, and
Polysorbate 80.
11
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
1001201 in one embodiment, a formulation of the disclosure comprises
histidine, sodium
chloride, trehalose and Polysorbate 80. In one embodiment, a fomulation of the
disclosure
comprises between about 5 mM and about 100 mM histidine, between about 10 mM
and about
300 niM sodium chloride, between about 0.3% and about 10% trehalose, and
between about
0.005% and about 0.1% Polysorbate 80, wherein said formulation has a pH of
between about 5.0
and about 7Ø In another embodimcnt, a formulation of the disclosure
comprises between about
mM and about 50 mM histidine, between about 50 mM and about 200 MI11 sodium
chloride,
between about 1% and about 8% trehalose, and between about 0.01% and about
0.05%
Polysorbate 80, wherein said formulation has a pH of between about 53 and
about 6.5. In a
further embodiment, a formulation of the disclosure comprises about 10 mM
histidine, about
80 mM sodium chloride, about 4% trehalose and about 0.02% Polysorbate 80,
wherein said
formulation has a pH of about 6Ø
1001211 In one embodiment, a formulation of the disclosure comprises
histidine, sodium
chloride, trehalose and Polysorbate 80. In one embodiment, a formulation of
the disclosure
comprises between 5 m114 and 100 mM histidine, between 10 inkl and 300 rtiM
sodium chloride,
between 1% and 10% trehalose, and between 0.005% and 0.1% Polysorbate 80,
wherein said
formulation has a pH of between 5.0 and 7Ø In another embodiment, a
formulation of the
disclosure comprises between 5 mM and 50 mM histidine, between 50 m114 and 200
mM sodium
chloride, between 1% and 6% trehalose, and between 0.01% and 0.05% Polysorbate
80, wherein
said formulation has a pH of between 5.5 and 6.5. In a further embodiment, a
formulation of the
disclosure comprises 10 mM histidineõ 80 m114 sodium chloride, 4% trehalose
and 0.02%
Polysorbate 80, wherein said formulation has a pH of 6Ø
1001221 In one embodiment; a formulation of the disclosure consists of
between about
20 mg/ml and about 150 mg/m1 anti-ICOS antibody, about 10 mM histidine, about
80 mM
sodium chloride, about 4% trehalose and about 0.02% Polysorbate 80, wherein
said formulation
has a pH of about 6Ø In another embodiment; a formulation of the disclosure
consists of about
50 mg/m1 an ti-ICOS antibody, about 10 rtiM histidine, about 80 mM sodium
chloride, about 4%
trehalose and about 0,02% Polysorbate 80, wherein said formulation has a pH of
about 6Ø In a
further embodiment, a formulation of the disclosure consists of about 100
mg/m1 anti-ICUS
antibody, about 10 mM histidine, about 80 rriM sodium chloride, about 4%
trehalose and about
0.02% Polysorhate 80, wherein said formulation has a pH of about 6Ø In a
further embodiment,
a formulation of the disclosure consists of about 110 mg/m1 anti-ICOS
antibody, about 10 mt14
histidine, about 80 mM sodium chloride, about 4% trehalose and about 0.02%
Polysorbate 80,
wherein said formulation has a pH of about 6Ø In a farther embodiment, a
formulation of the
disclosure consists of about 120 mg/ml anti-ICOS antibody, about 10 mkt
histidine, about
32
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
80 mM sodium chloride, about 4% trehalose and about 0.02% Polysorbate 80,
wherein said
formulation has a pH of about 6Ø In a further embodiment, a formulation of
the disclosure
consists of about 130 nigirril anti-ICOS antibody, about 10 mM histidine,
about 80 mM sodium
chloride, about 4% trehalose and about 0.02% Polysorbate 80, wherein said
formulation has a pH
of about 6Ø In a specific embodiment, a formulation of the disclosure
comprises the anti-ICOS
antibody comprising a heavy chain sequence of SEQ ID NO:6, alight chain
sequence of SEQ ID
NO: .1 and an Fc region having complex N-glycoside-linked sugar chains in
which Incase is not
bound to N-acetylgtucosamine in the reducing end in the sugar chain.
1001.231 In one embodiment, a formulation of the disclosure consists of
between 20 ingiml
and 150 mg/m1 anti-ICOS antibody, 10 mM histidine, 80 mM sodium chloride, 4%
trehalose and
0.02% Polysorbate 80, wherein said formulation has a pH of 6Ø In another
embodiment, a
formulation of the disclosure consists of 50 mg/nil an ti-ICOS antibody, 10 mM
hisfidine, 80 mIVI
sodium chloride, 4% trehalose and 0.02% Polysorbate 80, wherein said
formulation has a pH of
6Ø In a further embodiment, a formulation of the disclosure consists of 100
mg/m1 anti-ICOS
antibody, 10 m114 histidine, 80 mM sodium chloride, 4% trehalose and 0.02%
Polysorbate 80,
wherein said formulation has a pH of 6Ø In a further embodiment, a
formulation of the
disclosure consists of 110 mg/m1 anti-ICOS antibody, 10 mM histidine, 80 mM
sodium chloride,
4% trehalose and 0.02% Polysorbate 80, wherein said formulation has a pH of
6Ø In a further
embodiment, a formulation of the disclosure consists of .120 Ingtml anti-ICOS
antibody, 10 mM
histidine, 80 mM sodium chloride, 4% trehalose and 0.02% Polysorbate 80,
wherein said
formulation has a pH of 6Ø In a further embodiment, a formulation of the
disclosure consists of
130 mg/ ml anu-ICOS antibody, 10 mM histidine, 80 mM sodium chloride, 4%
trehalose and
0.02% Polysorbate 80, wherein said formulation has a pH of 6Ø In a specific
embodiment, a
formulation of the disclosure comprises the anti-ICOS antibody comprising a
heavy chain
sequence of SEQ .11) NO:6, a light chain sequence of SEQ NO:1 and an Fc region
having
complex N-glycoside-linked sugar chains in which fucose is not bound to N-
acetylglucosamine
in the reducing end in the sugar chain.
1001241 In one embodiment, a formulation of the disclosure consists of
between about
mg/nil and about 20 mg/a anti-ICOS antibody, about 10 mM histidine, about 80
m114 sodium
chloride, about 4% trehalose and about 0.02% Polysorbate 80, wherein said
formulation has a pH
of about 6Ø In another embodiment, a formulation of the disclosure consists
of about 5 .mg/ml
anti-ICOS antibody, about 10 mM histidine, about 80 mM sodium chloride, about
4% trehalose
and about 0.02% Polysorbate 80, wherein said formulation has a pH of about
6Ø In a further
embodiment, a formulation of the disclosure consists of about 10 mg/ml anti -
ICOS antibody,
about 10 inlvl histidine, about 80 mM sodium chloride, about 4% trehalose and
about 0.02%
33
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
Polysorbate 80, wherein said formulation has a pH of about 6Ø In a fiather
embodiment a
-formulation of the disclosure consists of about 15 ing/m1 anti4COS antibody,
about 10 m1s4
histidine, about 80 mM sodium chloride, about 4% trehalose and about 0.02%
Polysorbate 80,
wherein said formulation has a pH of about 6Ø In a specific embodiment, a
formulation of the
disclosure comprises the anti-ICOS antibody comprising a heavy chain sequence
of SEQ ID
NO:6, a. light chain sequence of SEQ ID NO:1 and an Fc region having. complex
N-glycoside-
linked sugar chains in which fucose is not bound to N-acetylglucosamine in the
reducing end in
the sugar chain.
1001251 In one embodiment, a formulation of the disclosure consists of
between 5 mg/m1
and 20 engtml anti-ICOS antibody. 10 mM histidine, 80 mM sodium chloride, 4%
trehalose and
0.02% Polysorbate 80, wherein said tbrinulation has a pH of 6Ø In another
embodiment, a
formulation of the disclosure consists of 5 mg/m.1 anti-ICOS antibody, .10 mM
histidine, 80 mM
sodium chloride, 4% trehalose and 0.02% Polysorbate 80, wherein said
formulation has a pH of
6Ø In a further embodiment, a formulation of the disclosure consists of 10
mg/ml anti-ICOS
antibody, 10 m114 histidine, 80 mM sodium chloride, 4% trehalose and 0.02%
Polysorbate 80,
wherein said formulation has a pH of 6Ø In a further embodiment, a
formulation of the
disclosure consists of 20 mg/nil anti-ICOS antibody, 10 mM histidine, 80 mM
sodium chloride,
4% trehalose and 0.02% Polysorbate 80, wherein said formulation has a pH of
6Ø In a specific
embodiment, a formulation of the disclosure comprises the anti-ICOS antibody
comprising a
heavy chain sequence of SEQ ID NO:6, a light chain sequence of SEQ. ID NO:1
and an Fe
region having complex N-glycoside-linked sugar chains in which fucose is not
bound to N-
acetylglucosamine in the reducing end in the sugar chain.
1001.261 In one embodiment the formulations of the disclosure are pyrogen-
free
formulations which are substantially free of endotoxi re; and/or related
pyrogenic substances.
Endotoxins include toxins that are confined inside a microorganism and are
released only when
the microorganisms are broken down or die. Pyrogenic substances also include
fever-inducing,
thermostable substances (glycoproteins) from the outer membrane of bacteria
and other
microorganisms. Both of these substances can cause fever, hypotension and
shock if
administered to humans. Due to the potential harmful effects, even low amounts
of endotoxins
must be removed from intravenously administered pharmaceutical drug solutions.
The Food &
Drug Administration ("FDA") has set an upper limit of 5 endotoxin units (EU)
per dose per
kilogram body weight in a single one hour period for intravenous drug
applications (The United
States Pharmacopeia" Convention, Phamiacopeial Forum 26 (1):223 (2000)). When
therapeutic
proteins are administered in amounts of several hundred or thousand milligrams
per kilogram
body weight, as can be the case with antibodies, even trace amounts of harmful
and dangerous
14
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
endotoxin must be removed. In certain specific embodiments, the endotoxin and
pyrogen levels
in the composition are less then .10 EU/mg, or less then 5 EUInigõ or less
then I EU/mg, or less
then 0.1 ELT/mg, or less then 0.01 EU/mg, or less then 0.001 EU/mg.
1001271 When used for in viva administration, the formulations of the
disclosure should be
sterile. The formulations of the disclosure may be sterilized by various
sterilization methods,
including sterile filtration, radiation, etc. In one embodiment. the antibody
formulation is filter-
sterilized with a westernized 0.22-micron filter. Sterile compositions for
injection can be
formulated according to conventional pharmaceutical practice as described in
"Remington: The
Science & Practice of Pharmacy", 214 ed., Lippincott Williams & Wilkins,
(2005). Formulations
comprising antibodies, such as those disclosed herein, ordinarily will be
stored in lyophilized
.form or in solution. It is contemplated that sterile compositions comprising
antibodies are placed
into a container having a sterile access port, for example, an intravenous
solution bag or vial
having an adapter that allows retrieval of the formulation, such as a stopper
pierceable by a
hypodermic injection needle. In one embodiment, a composition of the
disclosure is provided as
a pre-filled syringe.
5.2.Stability of Formulations
1001281 In one embodiment-, a formulation of the disclosure comprises an
antibody or
fragment thereof that is susceptible to aggregation, fragmentation and/or
dearnidation.
1001291 In one embodiment, a formulation of the disclosure stabilizes an
anti-ICOS
antibody. In one embodiment, a formulation of the disclosure prevents
aggregation of an anti-
ICOS antibody or fragment thereof. In another embodiment, a formulation of the
disclosure
prevents fragmentation of an anti-ICOS antibody or fragment thereof. In a
specific embodiment,
a formulation of the disclosure comprises the anti-ICOS antibody comprising a
heavy chain
sequence of SEQ ID NO:6, a light chain sequence of SEQ ID NO: I and an Fc
region having
complex N-glycoside-linked sugar chains in which fucose is not bound to N-
acetylglucosmnine
in the reducing end in the sugar chain.
1001301 The present disclosures provide stable liquid formulations
comprising anti-1COS
antibodies of the disclosure. The stability of said antibody can be assessed
by degrees of
aggregation, degradation or fragmentation, as measured by FIPSEC, reverse
phase
chromatography, static light scattering (SLS), Dynamic Light Scattering (DLS),
Fourier
Transform Infrared Spectroscopy (FTIR), circular dichroism (CD), urea.
unfolding techniques,
intrinsic untophan fluorescence, differential scanning calorimetry, and/or ANS
binding
techniques, compared to a reference formulation comprising a reference
antibody. For example,
a reference formulation may be a reference standard frozen at -70T consisting
of It) mg/nd of a
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
reference antibody (including antibody fragment thereof) (for example, but not
limited to. the
136 anti-1COS antibody comprising an Fc region having complex N-glycoside-
linked Mar
chains in which fucose is not bound to N-acetylglucosamine in the reducing end
in the sugar
chain) in 10 mM histidine (pH 6.0) that contains 80 mM NaCl, 4% trehalose and
0.02%
polysorbate 80, which reference formulation regularly gives a single monomer
peak (e.g., 95%
area) by .HPSEC. In certain embodiments, a reference formulation is identical
to the formulation
whose stability is tested; the reference formulation may be stored frozen at -
70 C during the
stability testing to preserve the reference formulation in its original
condition. For example, the
reference standard for assessing any loss of ICOS antigen binding activity in
a formulation
stored at 40 C may be the identical formulation stored at -70 C for 30 days.
The overall stability
of a formulation comprising an antibody (including antibody fragment thereof)
may also be
assessed by various immunological assays including, for example, .FELISA. and
radioimmunoassay using isolated antigen molecules. Furthermore, the stability
of a formulation
comprising an antibody may also be assessed using various assays designed to
measure a
functional characteristic of the antibody, for example, assays designed to
measure antigen
binding affinity, in vitro ADCC activity, in vivo depletion activity, in vitro
CDC activity.
1001311 In one embodiment, a formulation of the disclosure is stable upon
storage at about
40 C for at least about I week, at least about 2 weeks, at least about 3
weeks, or at least about 4
weeks. In one embodiment, a formulation of the disclosure is stable upon
storage at about 40"C
for at least about 1 month, at least about 2 months, at least about 3 months,
at least about 4
months, at least about 5 months, or at least about 6 months. In a specific
embodiment. a
formulation of the disclosure is stable upon storage in a pre-filled syringe.
1001321 In one embodiment, a formulation of the disclosure is stable upon
storage at about
.5 C. for at least about 1 month, at least about 2 months, at least about 3
months, at least about 4
months, at least about 5 months, at least about 6 months, at least about 7
months, at least about 8
months, at least about 9 months, at least about 10 months, at least About 11
months, or at least
about 12 months. In one embodiment, a formulation of the disclosure is stable
upon storage at
about 5 C for at least about 1 year, at least about 2 years, at least about 3
years, at least about 4
years, at least about 5 years, at least about 6 years, at least about 7 years,
at least about 8 yews, at
least about 9 years, at least about 10 years, at least about 11 years, or at
least about 12 years. In a
specific embodiment, a formulation of the disclosure is stable upon storage in
a pre-filled
syringe.
1001331 In one embodiment, a formulation of the disclosure is stable upon
storage at about
40 C for about 1 week, about 2 weeks, about 3 weeks, or about 4 weeks. In one
embodiment, a
formulation of the disclosure is stable upon storage at about 40 C for about 1
month, about 2
36
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
months, about 3 months, about 4 months, about 3 months, or about 6 months. In
a specific
embodiment, a formulation of the disclosure is stable upon storage in a pre-
filled syringe.
1001341 In one embodiment, a formulation of the disclosure is stable upon
storage at about
C for about I month, about 2 months, about 3 months, about 4 months, about 5
months, about
6 months, about 7 months, about 8 months, about 9 months, about 10 months,
about 11 months,
or about 12 months. In one embodiment, a formulation of the disclosure is
stable upon storage at
about 5 C for about I year, about 2 years, about 3 years, about 4 years, about
5 years, about 6
years, about 7 years, about 8 years, about 9 years, about 10 years, about 11
years, or about 12
years. In a specific embodiment, a formulation of the disclosure is stable
upon storage in a pm-
filled syringe.
1001351 In one embodiment, a formulation ofthe disclosure comprises an anti-
ICOS
antibody that has a ICOS binding activity that is at least 50%, at least 60%,
at least 70%õ at least
80%, at least 90%, at least 95%, or at least 99% of the ICOS binding activity
of a reference
antibody, wherein said formulation was stored at about 40 C for about I week,
about 2 weeks,
about 3 weeks, or about 4 weeks. In one embodiment, a formulation of the
disclosure comprises
an anti-ICOS antibody that has a ICOS binding activity that is at least 50%,
at least 60%, at least
70%, at least 80%, at least 90%, at least 95%, or at least 99% of the ICOS
binding activity of a
reference antibody, wherein said formulation was stored at about 40 C for
about 1 month, about
2 months, about 3 months, about 4 months, about 5 months, or about 6 months.
In a specific
embodiment, a formulation of the disclosure comprises an anti4COS antibody
comprising a
heavy chain sequence of SEQ ID NO:6, a light chain sequence of SEQ ID NO: I
and an Fc
region having complex N-glycoside-linIsed sugar chains in which fucose is not
bound to N-
acetylglucosamine in the reducing end in the sugar chain.
1001361 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody that has a ICOS binding activity that is at least 50%, at least 60%,
at least 70%, at least
80%, at least 90%, at least 95%, or at least 99% of the ICOS binding activity
of a reference
antibody, wherein said formulation was stored at about 25 C for about 1 week,
about 2 weeks,
about 3 weeks, or about 4 weeks. In one embodiment, a formulation of the
disclosure comprises
an anti-ICOS antibody that has a ICOS binding activity that is at least 50%,
at least 60%, at least
70%, at least 80%, at least 90%, at least 95%, or at least 99% of the ICOS
binding activity of a
reference antibody, wherein said formulation was stored at about 25 C for
about I month, about
2 months, about 3 months, about 4 months, about 5 months, or about 6 months.
In a specific
embodiment, a formulation of the disclosure comprises an anti-ICOS antibody
comprising a
heavy chain sequence of SEQ ID NO:6, a light chain sequence of SEQ ID NO:1 and
an Fc
37
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
region having complex N-glycoside-linked sugar chains in which fucose is not
bound to N-
aeetylglucosamine in the reducing end in the sugar chain.
1001371 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody that has a ICOS binding activity that is at least 50%, at least 60%,
at least 70%, at least
80%, at least 90%, at least 95%, or at least 99% of the ICOS binding activity
of a reference
antibody, wherein said formulation was stored at about 5 C for about 1 month,
about 2 months,
about 3 months, about 4 months, about 5 months, about 6 months, about 7
months, about 8
months, about 9 months, about 10 months, about 11 months, or about 12 months.
In one
embodiment, a formulation of the disclosure comprises an anti-ICOS antibody
that has a ICOS
binding activity that is at least 50%, at least 60%, at least 70%. at least 80
A, at least 90%. at least
95%, or at least 99% of the 'COS binding activity of a reference antibody,
wherein said
formulation was stored at about 5 C for about I year, about 2 years, about 3
years, about 4 years,
about 5 years, about 6 years, about 7 years, about 8 years, about 9 years,
about 10 years, about 11
years, or about 12 years. In a specific embodiment, a formulation of the
disclosure is stored in a
pre-filled syringe. In a specific embodiment, a formulation of the disclosure
comprises an anti -
'COS antibody comprising a heavy chain sequence of SEQ ID NO:6, a light chain
sequence of
SEQ ID NO:1 and an Fc region having complex N-glycoside-linked sugar chains in
which
fucose is not bound to N-acetylglucosamine in the reducing end in the sugar
chain.
loom] In one embodiment, a ft-mutation of the disclosure comprises an anti-
ICOS
antibody, wherein the antibody loses no more than about 50%, no more than
about 40%, no more
than about 30%, no more than about 20%, no more than about 10%, no more than
about 5%, or
no more than about 1% of its ICOS binding activity during storage of the
formulation at about
40 C. for about 1 week, about 2 weeks, about 3 weeks. or about 4 weeks. In one
embodiment. a
formulation of the disclosure comprises an anti-ICOS antibody, wherein the
antibody loses no
more than about 50%, no more than about 40%, no more than about 30%, no more
than about
20%, no more than about 10%, no more than about 5%, or no more than about 1%
of its ICOS
binding activity during storage of the formulation at about 40 C for about 1
month, about 2
months, about 3 months, about 4 months, about 5 months, or about 6 months. In
a specific
embodiment, a formulation of the disclosure comprises an anti-ICOS antibody
comprising a
heavy chain sequence of SEQ ID NO:6, alight chain sequence of SEQ ID NO:1 and
an Fe
region having complex N-glycoside-linked sugar chains in which fucose is not
bound to N-
acetylglucosamine in the reducing end in the sugar chain.
1001391 in one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein the antibody loses no more than about 50%, no more than
about 40%, no more
than about 30%, no more than about 20%, no more than about 10%, no more than
about 5%, or
38
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
no more than about % of its ICOS binding activity during storage of the
formulation at about
2.5 C. for about I week, about 2 weeks, about 3 weeks, or about 4 weeks. In
one embodiment. a
formulation of the disclosure comprises an anti-ICOS antibody, wherein the
antibody loses no
more than about 50%, no more than about 40%, no more than about 30%, no more
than about
20%, no more than about 10%, no more than about 5%, or no more than about 1%
of its ICOS
binding activity during storage of the formulation at about 25 C for about 1
month, about 2
months, about 3 months, about 4 months, about 5 months, or about 6 months. In
a specific
embodiment, a formulation of the disclosure comprises an anti-ICOS antibody
comprising a
heavy chain sequence of SEQ ID NO:6, alight chain sequence of SEQ ID NO:1 and
an Fc
region having complex N-glycoside-linked sugar chains in which fucose is not
bound to N-
acetylglucosamine in the reducing end in the sugar chain.
1001401 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein the antibody loses no more than about 50%, no more than
about 40%, no more
than about 30%. no more than about 20%, no more than about 10%, no more than
about 5%, or
no more than about 1% of its ICOS binding activity during storage of the
formulation at about
C for about I month, about 2 months, about 3 months, about 4 months, about 5
months, about
6 months, about 7 months, about 8 months, about 9 months, about 10 months,
about Ii months.
or about 12 months. In one embodiment, a formulation of the disclosure
comprises an anti-ICOS
antibody, wherein the antibody loses no more than about 50%, no more than
about 40%, no more
than about 30%, no more than about 20%, no more than about 10%, no more than
about 5%, or
no more than about I% of its ICOS binding activity during storage of the
formulation at about
.5 C' for about I year, about 2 years, about 3 years, about 4 years, about 5
years, about 6 years,
about 7 years, about 8 years, about 9 years, about 10 years, about 11 years,
or about 12 years. In
a specific ernbodi meat, a formulation of the disclosure is stored in a pre-
filled syringe. In a
specific embodiment, a formulation of the disclosure comprises an anti-ICOS
antibody
comprising a heavy chain sequence of SEQ ID NO:6, alight chain sequence of SEQ
ID NO:1
and an Pc region having complex N-glycoside-linked sugar chains in which
fucose is not bound
to N-atetylglucosamine in the reducing end in the sugar chain.
1001411 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein said antibody -retains at least 50%, at least 60%, at least
70%, at least 80%, at
least 90%, at least 95%, or at least 99% of binding ability to a human ICOS
compared to a
reference antibody representing the antibody prior to the storage at about 40
C for at least about
1 week, at least about 2 weeks. at least about 3 weeks, or at least about 4
weeks. In one
embodiment, a formulation of the disclosure comprises an anti-ICOS antibody,
wherein said
antibody retains at least 50%, at least 60%, at least 70%, at least 80%, at
least 90%, at least 95%,
39
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
or at least 99% of binding ability to a human ICOS compared to a reference
antibody
representing the antibody prior to the storage at about 40 C for at least
about 1 month, at least
about 2 months, at least about 3 months, at least about 4 months, at least
about 5 months, or at
least about 6 months. In a specific embodiment, a formulation of the
disclosure is stored in a
pre-filled syringe. In a specific embodiment, a formulation of the disclosure
comprises the anti-
ICOS antibody comprising a heavy chain sequence of SEQ ID NO:6, a. light chain
sequence of
SEQ ID NO:1 and an Fc region having complex N-glycoside-linked sugar chains in
which
fucose is not bound to N-acetylglucosamine in the reducing end in the sugar
chain.
1001421 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein said antibody retains at least 50%, at least 60%, at least
70%, at least 80%, at
least 90%, at least 95%, or at least 99% of binding ability to a human ICOS
compared to a
reference antibody representing the antibody prior to the storage at about 5 C
for at least about 1
month at least about 2 months, at least about 3 months, at least about 4
months, at least about 5
months, at least about 6 months, at least about 7 months, at least about 8
months, at least about 9
months, at least about 10 months, at least about 1.1 months, or at least about
12 months. In one
embodiment, a formulation of the disclosure comprises an anti-ICOS antibody,
wherein said
antibody retains at least 50%, at least 60%, at least 70%, at least 80%, at
least 9094, at least 93%,
or at least 99% of binding ability to a human ICOS compared to a reference
antibody
representing the antibody prior to the storage at about 5 C for at least about
1 year, at least about
2 years, at least about 3 years, at least about 4 years, at least about 5
years, at least about 6 years,
at least about 7 years, at least about 8 years, at least about 9 years, at
least about 10 years, at least
about 11 years, or at least about 12 years. In a specific embodiment, a
formulation of the
disclosure is stored in a pre-filled syringe. In a specific embodiment, a
formulation of the
disclosure comprises the anti-ICOS antibody comprising a heavy chain sequence
of SEQ
Na6, a light chain sequence of SEQ ID NO:1 and an Fe region having complex N-
glycoside-
linked sugar chains in which fucose is not bound to N-acetylglucosamine in the
reducing end in
the sugar chain.
1001431 In one embodiment, a formulation of the disclosure comprises anti-
ICOS
antibody, wherein said antibody retains at least 50%, at least 60%, at least
70%, at least 80%, at
least 90%, at least 95%, or at least 99% of binding ability to a human ICOS
compared to a
reference antibody representing the antibody prior to the storage at about 40
C for about] week,
about 2 weeks, about 3 weeks, or about 4 weeks. In one embodiment, a
formulation of the
disclosure comprises anti-ICOS antibody, wherein said antibody retains at
least 50%, at least
60%, at least 70%, at least 80%, at least 90%, at least 95%, or at least 99%
of binding ability to a
human ICOS compared to a reference antibody representing the antibody prior to
the storage at
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
about 40 C for about 1 month, about 2 months, about 3 months, about 4 months,
about 5 months,
or about 6 months. In a specific embodiment, a formulation of the disclosure
is stored in a pre-
filled syringe. In a specific embodiment, a formulation of the disclosure
comprises the anti-
ICOS antibody comprising a heavy chain sequence of SEQ ID NO:6, a light chain
sequence of
SEQ ID NO:1 and an Fc region having complex N-glycoside-linked sugar chains in
which
fume is not bound to N-acetylglucosamine in the reducing end in the sugar
chain.
1001441 In one embodiment, a formulation of the disclosure comprises anti-
ICOS
antibody, wherein said antibody retains at least 50%, at least 60%, at least
70%, at least 80 4, at
least 90%, at least 95%, or at least 99% of binding ability to a human ICOS
compared a
reference antibody representing the antibody prior to the storage at about 5 C
for about 1 month,
about 2 months, about 3 months, about 4 months, about 5 months, about 6
months, about 7
months, about 8 months, about 9 months, about 10 months, about 11 months, or
about 12
months. In one embodiment, a formulation of the disclosure comprises anti -
ICOS antibody,
wherein said antibody retains at least 50%, at least 60%, at least 70%, at
least 80%, at least 90%,
at least 95%, or at least 99% of binding ability to a human ICOS compared to a
reference
antibody representing the antibody prior to the storage at about 5 C for about
1 year, about 2
years. about 3 years, about 4 years, about 5 years, about 6 years, about 7
years, about 8 years,
about 9 years, about 10 years, about 11 years, or about 12 years. In a
specific embodiment, a
tbrmulation of the disclosure is stored in a pre-filled syringe. in a specific
embodiment-, a
tbrmulation of the disclosure comprises the anti-ICOS antibody comprising a
heavy chain
sequence of SEQ NO:6, alight chain sequence of SEQ ID 10:1 and an Fc region
having
complex N-glycoside-linked sugar chains in which fucose is not bound to N-
acetylglucosamine
in the reducing end in the sugar chain.
1001451 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody forms an aggregate as determined by HPSEC
upon storage
at about 40 C for at least about 1 week, at least about 2 wwks, at least about
3 weeks, or at least
about 4 weeks. In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody forms an aggregate as determined by
liFSEC upon storage
at about 40 C for at least about 1 month, at least about 2 months, at least
about 3 months, at least
about 4 months, at least about 5 months, or at least about 6 months. In a
specific embodiment, a.
formulation of the disclosure is stored in a pre-filled syringe. In a specific
embodiment, a
formulation of the disclosure comprises the anti-ICOS antibody comprising a
heavy chain
sequence of SEQ ID NO:6, alight chain sequence of SEQ ID NO:1 and an Fc region
having
41
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
complex N-glycoside-linked sugar chains in which fucose is not bound to N-
acerOglucosamine
in the reducing end in the sugar chain.
1001461 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody forms an aggregate as determined by HPSEC
upon storage
at about 5 C for at least about 1 month, at least about 2 months, at least
about 3 months, at least
about 4 months, at least about 5 months, at least about 6 months, at least
about 7 months, at least
about 8 months, at least about 9 months, at least about 10 months, at least
about 1.1 months, or at
least about 12 months. In one embodiment, a formulation of the disclosure
comprises an anti-
1COS antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%,
less than 5%,
less than 7% or less than 10% of said antibody forms an aggregate as
determined by HPSEC
upon storage at about 5 C for at least about year, at least about 2 y ears, at
least about 3 years,
at least about 4 years, at least about 5 years, at least about 6 years, at
least about 7 years, at least
about 8 years, at least about 9 years, at least about 10 years, at least about
11 years, or at least
about 12 years. In a specific embodiment, a formulation of the disclosure is
stored in a pre-filled
syringe. In a specific embodiment, a formulation of the disclosure comprises
the anh-ICOS
antibody comprising a heavy chain sequence of SEQ ID NO:6, a light chain
sequence of SEQ ID
NO:1 and an Fe region having complex N-glycoside-linked sugar chains in which
fueose is not
bound to N-acetylglucosamine in the reducing end in the sugar chain.
1001471 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody forms an aggregate as determined by
HPSEC. upon storage
at about 40 C for about 1 week, about 2 weeks, about 3 weeks, or about 4
weeks. In one
embodiment, a formulation of the disclosure comprises an anti-ICOS antibody,
wherein less than
I c.Yki, less than 2%, less than 3%, less than 4%, less than 5%, less than 7%
or less than 10% of said
antibody forms an aggregate as determined by HPSEC upon storage at about 40 C
for about 1
month. about 2 months, about 3 months, about 4 months, about 5 months, or
about 6 months. In
a specific embodiment, a formulation of the disclosure is stored in a pre-
filled syringe. In a
specific embodiment, a formulation of the disclosure comprises the anti-ICOS
antibody
comprising a heavy chain sequence of SEQ H3 NO:6, alight chain sequence of SEQ
ID NO:1
and an Fe region having complex N-glycoside-linked sugar chains in which
fueose is not bound
to N-acetylglucosamine in the reducing end in the sugar chain.
1001481 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody forms an aggregate as determined by HPSEC
upon storage
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
at about 5 C for about 1 month, about 2 months, about 3 months, about. 4
months, about 5
months, about 6 months, about 7 months, about months, about 9 months, about 10
months,
about 11 months, or about 12 months. in one embodiment, a formulation of the
disclosure
comprises an anti-ICOS antibody, wherein less than 1%, less than 2%, less than
3%, less than
4%,, less than 5%, less than 7% or less than 10% of said antibody forms an
aggregate as
determined by HPSK upon storage at about 5 C for about I year, about 2 years,
about 3 years,
about 4 years, about 5 years, about 6 years, about 7 years, about 8 years,
about 9 years, about 10
years, about 11 years, or about 12 years. in a specific embodiment, a
formulation of the
disclosure is stored in a pre-filled syringe. in a specific embodiment, a
formulation of the
disclosure comprises the anti-KOS antibody comprising a heavy chain sequence
of SEQ ID
NO:6, alight chain sequence of SEQ NO:I and an Fc region having complex N-
glycoside-
linked sugar chains in which fucose is not bound to N-acetylglucosamine in the
reducing end in
the sugar chain.
W1491 In one embodiment. a formulation of the disclosure comprises an anti-
1COS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody is fragmented as determined by RP-HPLC
upon storage at
about 40 C for at least about I week, at least about 2 weeks, at least about 3
weeks, or at least
about 4 weeks. In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody is fragmented as determined by RP.;11PLC
upon storage at
about 40 C for at least about 1 month, at least about 2 months, at least about
3 months, at least
about 4 months, at least about 5 months, or at least about 6 months. In a
specific embodiment. a
formulation of the disclosure is stored in a pre-filled syringe. In a specific
embodiment, a
formulation of the disclosure comprises the anti-ICOS antibody comprising a
heavy chain
sequence of SEQ 'ID NO:6, a light chain sequence of SEQ ID NO: I and an Fc
region having
complex N-glycoside-linked sugar chains in which fucose is not bound to N-
acetylglucosamine
in the reducing end in the sugar chain.
[00150) In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody is fragmented as determined by RP-11NX
upon storage at
about 5 C for at least about 1 month, at least about 2 months. at least about
3 months, at least
about 4 months, at least about 5 months, at least about 6 months, at least
about 7 months, at least
about 8 months, at least about 9 months, at least about 10 months, at least
about 11 months, or at
least about 12 months. In one embodiment, a formulation of the disclosure
comprises an anti-
ICOS antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%,
lass than 5%,
43
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
less than 7% or less than 10% of said antibody is fragmented as determined by
RP-HPLC upon
storage at about 5 C for at least about 1 year, at least about 2 years, at
least about 3 years, at least
about 4 years, at least about 5 years, at least about 6 years, at least about
7 years, at least about 8
years, at least about 9 years, at least about 10 years, at least about 11
years, or at least about 12
years. In a specific embodiment, a formulation of the disclosure is stored in
a pre-filled syringe.
In a specific embodiment, a formulation of the disclosure comprises the anti-
1COS antibody
comprising a heavy chain sequence of SEQ ID NO:6, alight chain sequence of SEQ
NO:1
and an Fc region having complex N-glycoside-linked sugar chains in which
lucose is not bound
to N-acetylglucosamine in the reducing end in the sugar chain.
1001511 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 5%, less than
7% or less than 10% of said antibody is fragmentt...d as determined by RP4-
IPLC upon storage at
about 40 C for about 1 week, about 2 weeks, about 3 weeks, or about 4 weeks.
In one
embodiment, a formulation of the disclosure comprises an anti -ICOS antibody,
wherein less than
1%, less than 2%, less than 3%, less than 4%, less than 5%, less than 7% or
less than 10% of said
antibody is fragmented as determined by RP-HPLC upon storage at about 40 C for
about 1
month, about 2 months, about 3 months, about 4 months, about 3 months, or
about 6 months.
In a specific embodiment, a formulation of the disclosure is stored in a pre-
filled syringe In a
specific embodiment, a formulation of the disclosure comprises the anti-ICOS
antibody
comprising a heavy chain sequence of SEQ. ID NO:6, a light chain sequence of
SEQ ID NO:1
and an .Fc region having complex N-glycoside-linked sugar chains in which
fucose is not bound
to N-acetylglucosamine in the reducing end in the sugar chain.
1001521 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody, wherein less than 1%, less than 2%, less than 3%, less than 4%, less
than 3%, less than
7% or less than 10% of said antibody is fragmented as determined by RP-Fine
upon storage at
about 5 C for about 1 month, about 2 months, about 3 months, about 4 months,
about 5 months,
about 6 months, about 7 months, about 8 months, about 9 months, about 10
months, about 11
months, or about 12 months. In one embodiment, a formulation of the disclosure
comprises an
anti-ECOS antibody, wherein less than 1%, less than 2%, less than 3%, less
than 4%, less than
5%, less than 7% or less than 10% of said antibody is fragmented as determined
by RP-11PLC
upon storage at about 5 C for about 1 year, about 2 years, about 3 years,
about 4 years, about 3
years, about 6 years, about 7 years, about 8 years, about 9 years, about 10
years, about 11 years,
or about 12 years. In a specific embodiment, a formulation of the disclosure
is stored in a pre-
filled syringe. In a specific embodiment, a formulation of the disclosure
comprises the anti-
ICOS antibody comprising a heavy chain sequence of SEQ ID NO:6, a light chain
sequence of
44
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
SEQ ID NO:! and an Fc region having complex N-glycoside-linked sugar chains in
which
-fticose is not bound to N-acetylglucosamine in the reducing end in the sugar
chain.
1001531 In one embodiment, a formulation of the disclosure is clear and
colorless as
determined by visual inspection upon storage at about 40 C for at least about
l week, at least
about 2 weeks, at least about 3 weeks, or at least about 4 weeks. in one
embodiment, a
formulation of the disclosure is Clear and colorless as determined by visual
inspection upon
storage at about 40 C for at least about I month, at least about 2 months, at
least about 3 months,
at least about 4 months, at least about 5 months, or at least about 6 months.
In a specific
embodiment, a formulation of the disclosure is stored in a pre-filled syringe.
In a specific
embodiment, a formulation of the disclosure comprises the anti-ICOS antibody
comprising a
heavy chain sequence of SEQ ID NO:6, alight chain sequence of SEQ. ID NO:1 and
an Fc
region having complex. N-glycoside-linked sugar chains in which fucose is not
bound to N-
acetylglucosamine in the reducing end in the sugar chain.
100154) In one embodiment, a formulation of the disclosure is clear and
colorless as
determined by visual inspection upon storage at about 5 C for at least about 1
month, at least
about 2 months, at least about 3 months, at least about 4 months, at least
about 5 months, at least
about 6 months, at least about 7 months, at least about 8 months, at least
about 9 months, at least
about 10 months, at least about 11 months, or at least about 12 months. In one
embodiment, a
formulation of' the disclosure is clear and colorless as determined by visual
inspection upon
storage at about 5 C for at least about 1 year, at least about 2 years, at
least about 3 years, at least
about 4 years, at least about 5 years, at least about 6 years, at least about
7 years, at least about 8
years, at least about 9 years, at least about 10 years, at least about II
years, or at least about 12
years. In a specific embodiment, a formulation of the disclosure comprises the
anti-ICOS
antibody comprising a heavy chain sequence of SEQ ID NO:6, a light chain
sequence of SEQ ID
NO:] and an Fe region having complex N-glycoside-linked sugar chains in which
.fticose is not
bound to N-acetylglucosamine in the reducing end in the sugar chain.
1001551 In one embodiment, a formulation of the disclosure is clear and
colorless as
determined by visual inspection upon storage at About 40 C for about I week,
about 2 weeks,
about 3 weeks, or about 4 weeks. In one embodiment, a formulation of the
disclosure is clear
and colorless as determined by visual inspection upon storage at about 40 C
for about: l month,
about 2 months, about 3 months, about 4 months, about 5 months, or about 6
months. In a
specific embodiment, a formulation of the disclosure is stored in a pre-filled
syringe. In a
specific embodiment, a formulation of the disclosure comprises the anti-ICOS
antibody
comprising a heavy chain sequence of SEQ ID NO:6, a light chain sequence of
SEQ ID NO: I
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
and an Fc region having complex N-glycoside-linked sugar chains in which
fucose is not bound
to N-acet);Iglucosarnine in the reducing end in the sugar chain.
1001561 In one embodiment, a formulation of the disclosure is clear and
colorless as
determined by visual inspection upon storage at about 5 C for about 1 month,
about 2 months,
about 3 months, about 4 months, about 5 months, about: 6 months, about 7
months, about 8
months, about 9 months, about 10 months, about 11 months, or about 12 months.
In one
embodiment, a formulation of the disclosure is clear and colorless as
determined by visual
inspection upon storage at about 5 C for about 1 year, about 2 years, about 3
years, about 4
years, about 5 years, about 6 years, about 7 years, about 8 years, about 9
years, about 10 years,
about 11 years, or about 12 years. In a specific embodiment, a formulation of
the disclosure is
stored in a pre-filled syringe. In a specific embodiment., a formulation of
the disclosure
comprises the anti-ICOS antibody comprising a heavy chain sequence of SEQ ID
NO:6, a light
chain sequence of SEQ ID NO:1 and an Pc region having complex N-glycoside-
linked sugar
chains in which fucose is not bound to N-acetylglucosamine in the reducing end
in the sugar
chain.
1001511 In certain embodiments, the formulations of the disclosure maintain
improved
aggregation profiles upon storage, for example, for extended periods (for
example, but not
limited to I week, 1 month, 6 months, 1 year, 2 years, 3 years or 5 years) at
room temperature or
4 C or for periods (such as, but not limited to 1 week, 2 weeks, 3 weeks, 1
month, 2 months, 3
months, or 6 months) at elevated temperatures such as 38 C-42 C. In certain
embodiments, the
formulations maintain improved aggregation profiles upon storage while exposed
to light or
stored in the dark in a variety of humidity conditions including but not
limited to a relative
humidity of up to 10%, or up to 20%, or up to 30%, or up to 40%, or up to 50%,
or up to 60%, or
up to 70%, or up to 80%, or up to 90%, or up to 100%. it will be understood in
the art that the
term "ambient" conditions generally refers to temperatures of about 20 C at a
relative humidity
of between 10% and 60% with exposure to light. Similarly, temperatures between
about 2 C.
and about 8 'V at a relative humidity of less then about 10% are collectively
referred to as "4 C"
or "5 "C", temperatures between about 23 C and about 27 C. at a relative
humidity of about
60% are collectively referred to as "25 'V" and temperatures between about 38
C. and about 42
"C at a relative humidity of about 75% are collectively referred to as "40 C."
In a specific
embodiment, a formulation of the disclosure is stored in a pre-filled syringe.
1001581 in certain embodiments, after storage at 4 C for at least one
month, the
formulations of the disclosure comprise (or consists of as the aggregate
fraction) a particle
profile of less than about 3.4 E +5 particles/ml of diameter 2-4 pm, less than
about 4.0 E
particles/ml of diameter 4-10 pm, less than about 4.2 E +3 particles/till of
diameter 10-20 pm,
46
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
less than about 5.0 E +2 particles/m1 of diameter 20-30 gm, less than about
7.5 E +1 particles/nil
of diameter 30-40 rim, and less than about 9.4 particles/nil of diameter 40-60
im as determined
by a particle inultisizer. in certain embodiments, the formulations of the
disclosure contain no
detectable particles greater than 40 tun, or greater than 30 pm. In a specific
embodiment, a
formulation of the disclosure is stored in a pre-filled syringe.
(001591 Numerous methods useful for determining the degree of aggregation,
and/or types
and/or sizes of aggregates present in a protein formulation (e.g., antibody
formulation of the
disclosure) are known in the art, including but not limited to, size exclusion
chromatography
(SEC), high performance size exclusion chromatography (HPSEC), static light
scattering (SIS),
Fourier Transform infrared Spectroscopy (FTIR), circular dichroism (CD), urea-
induced protein
unfolding techniques, intrinsic tryptophan fluorescence, differential scanning
calorimeny, and I-
anilino-8-naphthalenesullonic acid (ANS) protein binding techniques. For
example, size
exclusion chromatography (SEC) may be performed to separate molecules on the
basis of their
size, by passing the molecules over a column packed with the appropriate
resin, the larger
molecules (e.g aggregates) will elute before smaller molecules (e.g.
monomers). The molecules
are generally detected by UV absorbance at 280 nm and may be collected for
further
characterization. High pressure liquid chromatographic columns are often
utilized for SEC
analysis (HP-SEC). Specific SEC methods are detailed in the section entitled
"Examples" infra.
Alternatively, analytical ultracentrifugation. (AUC) may be utilized. AUC is
an orthogonal
technique which determines the sedimentation coefficients (reported in
Svedberg, S) of
macromolecules in a liquid sample. Like SEC, AUC is capable of separating and
detecting
antibody fragments/aggregates from monomers and is further able to provide
information on
molecular mass. Protein aggregation in the formulations may also be
characterized by particle
counter analysis using a coulter counter or by turbidity measurements using a
turbidimeter.
Turbidity is a measure of the amount by which the particles in a solution
scatter light and, thus,
may be used as a general indicator of protein aggregation. In addition, non-
reducing
polyacrylainide gel electrophoresis (PAGE) or capillary gel electrophoresis
(CGE) may be used
to characterize the aggregation and/or fragmentation state of antibodies or a.
fragment thereof in a
formulation of the disclosure.
1001.601 In one embodiment; a formulation of the disclosure is for
parenteral
administration. In one embodiment, a formulation of the disclosure is an
injectable formulation.
in one embodiment, a. formulation of the disclosure is for intravenous,
subcutaneous, or
intramuscular administration. In a specific embodiment, a formulation of the
disclosure
comprises an anti-ICOS antibody wherein said formulation is for subcutaneous
injection. In a
specific embodiment, a formulation of the disclosure is provided in a pre-
filled syringe. In a
47
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
specific embodiment, a formulation of the disclosure comprises the anti-ICOS
antibody
comprising a heavy chain sequence of SEQ IDNO:6, a light chain sequence of SEQ
ID NO:1
and an Fc region having complex N-glycoside-linked wear chains in which fucose
is not bound
to N-acetylglucosamine in the reducing end in the sugar chain.
1001611 In one embodiment, a formulation of the disclosure is for
intravenous
administration 'wherein said foffnulation comprises between about 20 mg/m1 and
about
40 ming of an anti-ICOS antibody or a fragment thereof In a specific
embodiment, a
formulation of the disclosure comprises the anti-ICOS antibody comprising a
heavy chain
sequence of SEQ NO:6, alight chain sequence of SEQ ID NO:.1 and an Fc region
having
complex N-glycoside-linked sugar chains in which fucose is not bound to N-
acetylglucosamine
in the reducing end in the sugar chain.
1001621 In one embodiman, a formulation of the disclosure is for
subcutaneous
administration wherein said formulation comprises between about 70 mg/ml and
about
250 mg/m1 of an anti-ICOS antibody or a fragment. thereof In a specific
embodiment, a
formulation of the disclosure is provided in a pre-filled syringe. In a
specific embodiment, a
formulation of the disclosure comprises the anti-ICOS antibody comprising a
heavy chain
sequence of SEQ II.) NO:6, a light chain sequence of SEQ ID NO:1 and an Fc
region having
complex N-glycoside-linked sugar chains in which fucose is not bound to N-
acetylglucosamine
in the reducing end in the sugar chain.
1001631 In one embodiment, a formulation of the disclosure is for aerosol
administration.
[001641 The present disclosure also provides a pharmaceutical unit dosage
form suitable
for parenteral administration to a human which comprises an anti-ICOS antibody
formulation in
a suitable container. In one embodiment, a pharmaceutical unit dosage of the
disclosure
comprises an intravenously, subcutaneously, or intramuscularly delivered anti-
ICOS antibody
formulation. In another embodiment, a pharmaceutical unit dosage of the
disclosure comprises
aerosol delivered anti-ICOS antibody formulation. In a specific embodiment, a
pharmaceutical
unit dosage of the disclosure comprises a subcutaneously delivered anti-ICOS
antibody
formulation. In another embodimea a pharmaceutical unit dosage of the
disclosure comprises
an aerosol delivered anti-ICOS antibody formulation. In a further embodiment,
a pharmaceutical
unit dosage of the disclosure comprises an intranasally administered anti-ICOS
antibody
formulation. In one embodiment, a suitable container is a pre-filled syringe.
In a specific
embodiment, a formulation of the disclosure comprises the anti-ICOS antibody
comprising a
heavy chain sequence of SEQ NO:6, a light chain sequence of SEQ ID NO:1 and an
Fc
region having complex N-glycoside-linked sugar chains in which fucose is not
bound to N-
acetylglucosamine in the reducing end in the sugar chain.
48
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
1001651 in one embodiment, a formulation of the disclosure is provided in a
sealed
container. In a specific embodiment, a formulation of the disclosure is
provided in a pre-filled
syringe. In a specific embodiment, a formulation of the disclosure comprises
the anti-ICOS
antibody comprising a heavy chain sequence of SEQ. ID NO:6, a light chain
sequence of SEQ. ID
NO:1 and an Fe region having complex N-glycoside-linked sugar chains in which
fucose is not
bound to N-acetylelucosamine in the reducing end in the sugar chain.
[001661 The present disclosure further provided a kit comprising an anti-
ICUS antibody
formulation of the disclosure.
1.061.671 The present disclosure also relates to methods of treating and
preventing T cell-
mediated diseases and disorders, such as, but not limited to, chronic
infection, autoimmune
disease or disorder, inflammatory disease or disorder, graft-versus-host
disease (GVEID),
transplant rejection, and I cell proliferative disorder in a human, comprising
administering to a
human in need thereof a formulation comprising an anti-ICOS antibody with
enhanced effector
function (e.g., antibody-dependent cellular cytotoxicity (ADCC), complement-
dependent cell-
mediated cytotoxicity (CDC), and/or antibody-dependent phagocytosis) in an
amount sufficient
to deplete circulating ICOS expressing cells. In a particular aspect, the
present disclosure also
concerns methods of treating and preventing 1' cell-mediated diseases and
disorders, such as, but
not limited to, chronic infection, autoimmune disease or disorder,
inflammatory disease or
disorder, grallsversus-host disease (GVilD), transplant rejection, and T cell
proliferative disorder
in a human comprising administration of a therapeutically effective regimen of
an anti-ICOS
antibody with enhanced effector function, which is of the IgGI or 1gG3 human
isotype.
[00168j The present disclosure also provides methods of preventing,
managing, treating or
ameliorating an inflammatory disease or disorder, an autoimmune disease Or
disorder, a
proliferative disease, an infection, a disease or disorder associated with or
characterized by
aberrant expression and/or activity of !COS, a disease or disorder associated
with or
characterized by aberrant expression and/or activity of the ICOS receptor, or
one or more
symptoms thereof.
[001691 In one embodiment, a method of the disclosure comprises
administering to a
subject in need thereof a prophylactically or therapeutically effective amount
of an anti-ICOS
antibody formulation. In one embodiment, a method of the disclosure is for the
prevention,
treatment, management or amelioration of a disease or disorder selected from
the group
consisting of multiple sclerosis, inflammatory bowel disease, insulin
dependent diabetes
mellitus, psoriasis, autoimmune thyroiditis, rheumatoid arthritis,
glomerulonephritis, systemic
lupus erythematosus, idiopathic inflammatory inyopathies (IIM),
derinatomyositis (DM),
polyirssositis (PM), and inclusion body myositis (II3M). In a specific
embodiment, a method of
49
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
the disclosure is for the prevention, treatment, management or amelioration of
systemic lupus
eiythematosus. In a specific embodiment, a method of the disclosure is for the
prevention,
treatment, management or amelioration of psoriasis. In a specific embodiment,
a method of the
disclosure is for the prevention, treatment, management or amelioration of
autoimmune diabetes.
In another embodiment, a method of the disclosure is for the prevention,
treatment, management
or amelioration of transplant rejection or graft versus host disease. In a
further embodiment. a
method of the disclosure is for the prevention, treatment, management or
amelioration of
idiopathic inflammatory myopathies (JIM), dermatomyositis (DM), polytnyositis
(PM), and
inclusion body myositis (IBM).
1001701 In one embodiment, a method of the disclosure for the prevention.
treatment,
management or amelioration of a disease or disorder further comprises
administering to said
subject a prophylactically or therapeutically effective amount of a
prophylactic or therapeutic
agent other than an antibody or antibody fragment that specifically binds to
ICOS.
(00171) In one embodiment, a method of the disclosure for the prevention,
treatment,
management or amelioration of a disease or disorder further comprises
administering to said
subject a prophylactically or therapeutically effective amount of a
prophylactic or therapeutic
agent other than an antibody or antibody fragment that specifically binds
ICOS, Nvherein said
prophylactic or therapeutic agent is an anti-inflammatory agent,
inunimomodulatory agent, anti-
angiogenic agent, or anti-cancer agent.
5.3.Antibodies Useful in the Formulations of the Disclosure
(001721 The present disclosure provides formulations of antibodies that
specifically bind
to human ICOS and have an enhanced effector function. In one embodiment, a
formulation of
the disclosure comprises an anti-ICOS antibody with enhanced effector
function, such as, but not
limited to, enhanced ADCC, enhanced CDC, and enhanced antibody-dependent
phagocytosis. In
a specific embodiment, a formulation of the disclosure comprises an anti-human
ICOS antibody
with enhanced ADCC activity. These antibodies can be used .for therapeutic,
including
prophylactic, purposes, for example in situations where the production or
expression of ICOS is
associated with pathological symptoms. Such antibodies can also be used for
the diagnosis of
various diseases or for the study of the evolution of such diseases.
[001731 The antibodies useful in the present disclosure include, but are
not limited to,
monoclonal antibodies, synthetic antibodies, multispecific antibodies
(including hi-specific
antibodies), human antibodies, humanized antibodies, chimeric antibodies,
single-chain Fvs
(scFv) (including bi-specific scFvs), single chain antibodies, Fab fragments,
F(ab') fragments,
disulfide-linked Fvs (sdFv), and epitope-binding fragments of any of the
above. In particular,
CA 02743469 2016-06-13
51332-100
antibodies of the present disclosure include immunoglobulin molecules and
immunologically
active portions of immunoelobulin molecules, i.e., molecules that contain an
antigen binding site
that specifically binds to an antigen. The immunoglobulin molecules of the
disclosure can be of
any type (e.g., IgG, IsEõ IgM, IgD, IeA and IgY), class (es., IgG. leG2, eG3,
feat, leAi and
IgA.2) or subclass of inimunoglobulin molecule.
[001741 The antibodies useful in the present disclosure may be from any
animal origin
including birds and mammals (for example, but not limited to, human, murine,
donkey, sheep,
rabbit, goat, guinea pia, camel, horse, or chicken). In specific embodiments,
the antibodies are
human or humanized monoclonal antibodies.
1001751 The antibodies useltil in the present disclosure may be
monospecific, bispecific,
trispecific or of greater multispecificity. M.ultispecific antibodies may
specifically bind to
different epitopes of a polypeptide or may specifically hind to both a
polypeplide as well a
heterologous epitope, such as a heterologous polypeptide or solid support
material. See, e.g.,
International Publication Nos. WO 93/17715, WO 92108802, WO 91/00360, and WO
92105793:
Tuft, et al., 1991, I. Immunol. 147:60-69; U.S. Patent Nos. 4,474,893,
4,7.1081, 4,925,648,
5,573,920, and 5,601,81.9; and Kostelny et al., 1992, J. limminol. 148:1547-
1553,
[001761 The antibodies useful in the present disclosure can be single-
chain antibodies.
The design and construction of a single-chain antibody is described in Marasco
et al, 1993, Proc
Nail Aced Sci 90:7889-7893.
[001771 The present disclosure provides formulations of an hbodies that
specifically bind
to human1COS and have an enhanced effector function. In one embodiment a
formulation of
the disclosure comprises an anti-ICOS antibody with enhanced effector
function, such as, but not
limited to, enhanced ADCC, enhanced CDC, and enhanced antibody-dependent
phanocytosis.
1001.781 The present disclosure further provides formulations of anti-
ICOS antibodies that
efficiently depletel.COS expressing cells in a mouse xenograft model system.
In one
embodiment, administration of one or more therapeutic doses of an anti-ICOS
antibody
'formulation of the disclosure may achieve at least about 20%, at least about
30%, at least about
40%, at least about 50%, at least about 60%. at least about 70%, at least
about 80%, at least
about 90%, at least about 95%, at least about 97%, at least about 99%, or at
least about 100%
depletion of 1COS expressing cells in a mouse xenograft model system,
[001791 The present disclosure further provides formulations of anti-
ICOS antibodies that
efficiently deplete 1C.05 expressing cells in a transgertic mouse model
system. In one
embodiment, administration of one or more therapeutic doses of an anti-ICOS
antibody
formulation of the disclosure may achieve at least about 20%, at least about
30%, at least about
40%, at least about 50%, at least about 60%, at least about 70%, at least
about 80%, at least
51
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
about 90%, at least about 95%, at least about 97%, at least about 99%, or at
least about 100%
depletion of ICOS expressing cells in a transgenic mouse model system.
1001801 The present disclosure also provides formulations of anti-ICOS
antibodies that
efficiently deplete ICOS expressing cells in a primate (non-human primate or
human). In one
embodiment, administration of one or more therapeutic doses of an anti-ICOS
antibody
formulation of the disclosure may achieve at least about 20%, at least about
30%, at least about
40%, at least about 50%, at least about WA, at least about 70%, at least about
80%, at least
about 90%, at least about 95%, at least about 97%, at least about 99%, or at
least about 100%
depletion of ICOS expressing cells in a primate (non-human primate or human).
1001811 The present disclosure also provides formulations of anti-ICOS
antibodies that
efficiently deplete ICOS expressing T cells in a primate (non-human primate or
human). In one
embodiment, administration of one or more therapeutic doses of an anti-ICOS
antibody
formulation of the disclosure may achieve at least about 20%, at least about
30%, at least about
40%, at least about 50%, at least about 60%, at least about 70%, at least
about 80%, at least
about 90%, at least about 95%, at least about 97%, at least about 99%, or at
least about 100%
depletion of ICOS expressing T cells in a primate (non-human primate or
human).
1001821 The present disclosure also provides formulations of anti-1COS
antibodies that
efficiently deplete ICOS expressing T helper cells in a primate (non-human
primate or human).
In one embodiment, administration of one or more therapeutic doses of an anti-
ICOS antibody
formulation of the disclosure may achieve at least about 20%, at least about
30%, at least about
40%, at least about 50%, at least about 60%, at least about 70%, at least
about 80%, at least
about 90%, at least about 95%, at least about 97%, at least about 99%, or at
least about 100%
depletion of ICOS expressing T helper cells in a primate (non-human primate or
human).
10018:31 The present disclosure also provides formulations of anti-ICOS
antibodies that
efficiently deplete 'ICOS expressing Thl cells in a primate (non-human primate
or human). In
one embodiment, administration of one or more therapeutic doses of an anti-
ICOS antibody
formulation of the disclosure may achieve at least about 20%, at least about
30%, at least about
40%, at least about 50%, at least about 60%, at least about 70%, at least
about 80%, at least
about 90%, at least about 95%, at least about 97%, at least about 99%, or at
least about 100%
depletion of ;COS expressing Th.1 cells in a primate (non-human primate or
human).
1001841 The present disclosure also provides fomadations of anti-ICOS
antibodies that
efficiently deplete !COS expressing Th2 cells in a primate (non-human primate
or human). In
one embodiment, administration of one or more therapeutic doses of an anti-
ICOS antibody
.formulation of the disclosure may achieve at least about 20%, at least about
30%, at least about
40%, at least about 50%, at least about 60%, at least about 70%, at least
about 80%, at least
CA 02743469 2011-05-11
WO 2010/056804
PCTIUS2009/064127
about 90%, at least about 95%, at least about 97%, at least about 99%, or at
least about 100%
depletion of ICOS expressing Th2 cells in a primate (non-human primate or
human).
1001851 The present disclosure also provides formulations of anti-ICOS
antibodies that
efficiently deplete ICOS expressing Th17 cells in a primate (non-human primate
or human). In
one embodiment, administration of one or more therapeutic doses of an anti-
ICOS antibody
formulation of the disclosure may achieve at least about 20%, at least about
30%, at least about
40%, at least about 50%, at least about 60%, at least about 70%, at least
about 80%, at least
about 90%, at least about 95%, at least about 97%, at least about 99%, or at
least about 100%
depletion of ICOS expressing Th17 cells in a primate (non-human primate or
human).
1001861 The present disclosure also provides formulations of anti-ICOS
antibodies that
efficiently deplete ICOS ekpressing memory helper T cells in a primate (non-
human primate or
human). In one embodiment, administration of one or more therapeutic doses of
an anti-.ICOS
antibody formulation of the disclosure may achieve at least about 20%, at
least about 30%., at
least about 40%, at least about 50%, at least about 60%, at least about 70%,
at least about 80%,
at least about 90%, at least about 95%, at least about 97%, at least about
99%, or at least about
100% depletion of ICOS expressing .memory helper T cells in a primate (non-
human primate or
human).
1001871 Depletion of a particular cell type may lead to the depletion of a
secreted product
of said cell type. For example, depletion of Th17 cells using an effector
function enhanced anti-
ICOS antibody of the disclosure may lead to depletion of 1L-.17. The present
disclosure also
provides formulations of anti-1COS antibodies that efficiently deplete IL-17
in a. primate (non-
human primate or human). In one embodiment, administration of one or more
therapeutic doses
of an anti-ICOS antibody formulation of the disclosure may achieve at least
about 20%, at least
about 30%, at least about 40%, at least about 50%, at least about 60%, at
least about 70%, at
least about 80%, at least about 90%, at least about 95%, at least about 97%,
at least about 99%,
or at least about 100% depletion of 1.1.-17 in a primate (non-human primate or
human).
1001881 The present disclosure also provides formulations of anti-ICOS
antibodies that
efficiently deplete 1L-2 in a primate (non-human primate or human). In one
embodiment,
administration of one or more therapeutic doses of an anti-ICOS antibody
formulation of the
disclosure may achieve at least about 20%, at least about 30%, at least about
40%, at least about
50%, at least about 60%, at least about 70%, at least. about 80510, at. least
about 90%, at least
about 95%, at least about 97%, at least about 99%, or at least about 100%
depletion of 1L-2 in a
primate (non-human primate or human).
1001891 The present disclosure provides formulations of anti-ICOS
antibodies that upon
administration efficiently prevent germinal center formation in a secondaiy
lymphoid organ of a
53
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
primate (non-human primate or human). In one embodiment, the secondary
lymphoid organ is a
lymph node. In another embodiment, the secondary lymphoid organ is the spleen.
In a further
embodiment, the secondary lymphoid organ is the tonsil In one embodiment, the
secondary
lymphoid organ is a mesenteric lymph node.
1001901 The present disclosure also provides formulations of anti-ICOS
antibodies that
upon administration efficiently disrupt germinal center architecture in a
secondary lymphoid
organ of a primate (non-human primate or human). In one embodiment, the
secondary lymphoid
organ is a lymph node. In another embodiment, the secondary lymphoid organ is
the spleen. In
a further embodiment, the secondary lymphoid organ is the tonsil. In one
embodiment, the
secondary lymphoid organ is a mesenteric lymph node.
1001911 The present disclosure also provides formulations of anti -ICOS
antibodies that
upon administration efficiently deplete germinal center B cells from a
secondary lymphoid organ
in a primate (non-human primate or human). In one embodiment, the secondary
lymphoid organ
is a lymph node In another embodiment, the secondary lymphoid organ is the
spleen. In a
further embodiment, the seconders, lymphoid organ is the tonsil. In one
embodiment, the
secondary lymphoid organ is a mesenteric lymph node.
1001921 The present disclosure also provides formulations of anti-ICOS
antibodies that
upon administration efficiently deplete circulating class switched B cells in
a primate (non-
human primate or human). In one embodiment, the administration of one or more
therapeutic
doses of an anti-ICOS antibody formulation of the disclosure depletes
circulating class switched
B cells in a primate (non-human primate or human) for at least I day, at
least. 2 days at least 5
days, at least I week, at least 2 weeks, at least 3 weeks, at least I month,
at least 2 months, at
least 3 months, at least 4 months, at least 5 months, at least 6 months. at
least 9 months.
Depletion of circulating class switched B cells is considered to
"substantially persist" during the
time period following the administration done or more doses of anti-ICOS
antibody when the
number of circulating class switched B cells is at least 10% lower in the
antibody treated sample
than the number of circulating class switched B cells in the untreated control
sample.
1001931 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody that mediates antibody-dependent cellular cytotoxicity (ADCC),
complement-
dependent cell-mediated cytotoxicity (CDC), and/or antibody-dependent
phagocytosis. In one
embodiment, an anti-ICOS antibody of the disclosure mediates antibody-
dependent cellular
cytotoxicity (ADCC) and/or antibody-dependent phagocytosis.. In one
embodiment, an anti-
ICOS antibody of the disclosure has enhanced antibody-dependent cellular
cytotoxicity (ADCC).
1001941 In one embodiment, a formulation of the disclosure comprises an
anti-ICOS
antibody comprising a variant Fc region that mediates enhanced antibody-
dependent cellular
54
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
cytotoxicity (ADCC). In a further embodiment, an anti-ICOS antibody of the
disclosure
comprises a variant Fc region comprising at least one substitution of an amino
acid residue
selected from the group consisting of: residue 239, 330, and 332, wherein the
amino acid residue
positions are determined according to the EU convention. In a specific
embodiment, an anti-
ICOS antibody of the disclosure comprises a variant Fe region comprising at
least: on amino acid
substitution selected from the group consisting of: S239D, A330L, and 1332E:
wherein the
amino acid residue positions are determined according to the 'EU convention.
In a further
embodiment, an anti-ICOS antibody of the disclosure comprises at least one
amino acid residue
selected from the group consisting of: D at position 239, L at position 330,
and E at position 332;
wherein the amino acid residue positions are determined according to the ELT
convention.
1001951 hi one embodiment, a formulation ofthe disclosure comprises an anti-
ICOS
antibody having an engineered Fe region comprising at least one engineered
glycofortn, wherein
said engineered Fe region mediates enhanced antibody-dependent cellular
cytotoxicity (ADCC).
In one embodiment, an anti-ICOS antibody of the disclosures comprises an
engineered Fe region
Jacking glycosylation. In one embodiment, an anti-ICOS antibody of the
disclosure comprises
an engineered Fe region having complex N-glycoside-linked sugar chains linked
to Asa297 in
which fucose is not bound to N-acetylglueosamine in the reducing end.
1001961 In certain embodiments, a formulation of the disclosure comprises
an anti-ICOS
antibody having a variant Fe region that has a higher affinity for an Fe
binding protein such as,
but not limited to, Fe receptor, Clq than a wild type Fe region. In one
embodiment, an anti-
ICOS antibody of the disclosure comprises a variant Fe region that has higher
affinity for the
FeyRIIIA receptor protein than a wild type Fe region.
1001971 In certain embodiments, a formulation of the disclosure comprises
an anti4COS
antibody having an engineered Fe region comprising at least one engineered
glycol-0m wherein
said engineered Fe region has a higher affinity for an Fe binding protein such
as, but not limited
to. Fe receptor, CI q than a wild type Pc region. In one embodiment, an anti-
ICOS antibody of
the disclosure comprises an engineered Fe region comprising at least one
engineered glyco.form,
wherein said engineered Fc region has higher affinity for the FcyltiTIA
receptor protein than a
wild type Fe region.
1001981 in one embodiment, an anti-ICOS antibody of the disclosure
comprises a variant
Pc region. In another embodiment, an anti-ICOS antibody of the disclosure
comprises a variant
Pc region that has an altered affinity for an Fc ligand protein. In a further
embodiment, an anti-
ICOS antibody of the disclosure comprises a variant Fe region that has an
altered affinity for an
Fe ligand selected from the group consisting of: FcyRIA, FeYRIIA, Fcy1111B,
.FeyRIIIA,
FcyRIIIB, FeyRIV, and Clq. In a specific embodiment-, an anti4COS antibody of
the disclosure
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
comprises a variant Fc region that has an altered affinity for the FcyRIIIA
protein. In a further
embodiment, an anti-ICOS antibody of the disclosure comprises a variant Fc
region that has an
altered affinity for the Cl q protein. In a specific embodiment, an Fe ligand
protein may be a
mouse, human or primate (e.g., cynomolgu.$) Fe ligand protein.
1001991 in one embodiment, an anti-ICOS antibody of the disclosure
comprises a variant
Fc region that has an increased affinity for an Fe ligand protein. In a
further embodiment, an
anti -ICOS antibody of the disclosure comprises a variant Fe region that has
an increased affinity
for an Fc ligand selected from the group consisting of: Fe7RIA, FeyRilA.
FcyRIIB,
FcyRIV, and C lg. In a specific embodiment, an anti-ICOS antibody of the
disclosure
comprises a variant Fc region that has an increased affinity for the FcyRIIIA
protein. In a further
embodiment, an anti-1COS antibody of the disclosure comprises a variant Fe
region that has an
increased affinity for the Clq protein. In a specific embodiment, an Fe ligand
protein may be a
mouse, human or primate (e.g., cynornolgus) Fe ligand protein.
1002001 In one embodiment, an anti-ICOS antibody of the disclosure
comprises a variant
Pc region wherein said variant Fc region comprises at least one amino acid
substitution, insertion
or deletion. In another embodiment, an anti-ICOS antibody of the disclosure
comprises a
variant Fc region comprising at least one amino acid substitution, insertion
or deletion wherein
said at least one amino acid residue substitution, insertion or deletion
results in an increased
affinity for an Fc ligand selected from the group consisting of: FcyRIA,
FeyRTIA, FcyRIIB,
'Fel/R.111A, FcyRIIIB, FcyRIV, and Clq. In a specific embodiment, an anti-ICOS
antibody of the
disclosure comprises a variant Fc region comprising at least one amino acid
substitution,
insertion or deletion wherein said at least one amino acid residue
substitution, insertion or
deletion results in an increased affinity for the FcyR111A protein. In a
further embodiment, an
anti-ICOS antibody of the disclosure comprises a valiant Fc region comprising
at least one
amino acid substitution, insertion or deletion wherein said at least one amino
acid residue
substitution, insertion or deletion results in an increased affinity for the
Clq protein. In a
specific embodiment, an Fc ligand protein may be a mouse, human or primate
(e.g.. cynomolgus)
Fc ligand protein.
1002011 in one embodiment, an anti-ICOS antibody of the disclosure
comprises a variant
Fe region comprising at least one amino acid substitution, insertion or
deletion wherein said at
least one amino acid residue is selected from the group consisting of: residue
239, 330, and 332,
wherein amino acid residues are nwnbered following the Ell index. In another
embodiment, an
anti-ICOS antibody of the disclosure comprises a variant Fe region comprising
at least one
amino acid substitution, insertion or deletion wherein said at least one
substituted, inserted or
deleted amino acid residue is selected from the group consisting of: residue
239, 330, and 332,
56
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
wherein amino acid residues are numbered following the EU index. In a further
embodiment, an
anti-ICOS antibody described herein comprises a variant Fe region comprising
at least one
amino acid substitution wherein said at least one substituted amino acid
residue is selected from
the group consisting of residue 239, 330, and 332, wherein amino acid residues
are numbered
following the EU index. In another embodiment, an anti-ICOS antibody described
herein
comprises a variant Fc region comprising at least one amino acid substitution
wherein said at
least one amino acid substitution is selected from the group consisting of:
S239D, A330L,
A330Y, and 1332E, wherein amino acid residues are numbered following the EU
index. In a
specific embodiment; an anti-ICOS an of the disclosure comprises a variant
Fe region
comprising the S239D, A330L, and 1332E amino acid substitutions, wherein amino
acid residues
are numbered following the EU index.
1002021 In one embodimaiL an anti-ICOS antibody of the disclosure comprises
a variant
Fc region comprising at least one of the amino acid residues selected from the
group consisting
of: D at residue 239. E at residue 239. L at residue 330, Y at residue 330, E
at residue 332, and D
at residue 332, wherein amino acid residues are numbered following the EU
index. In a specific
embodiment, an anti-ICOS antibody of the disclosure comprises a variant Fe
region comprising
D at residue 239. L at residue 330, and E at residue 332, wherein amino acid
residues are
numbered following the EU index.
1002031 in one embodiment, an anti-ICOS antibody of the disclosure
comprises an
engineered Fe region wherein the engineered Fe region comprises a
posttrarislational
modification that is different from that of the parental anti-ICOS antibody.
In a specific
embodiment, an anti-ICOS antibody of the disclosure comprises an engineered Fc
region
wherein said engineered Fe region comprises complex N-glycoside-linked sugar
chains in which
fueose is not bound to N-acewlglucosamine in the reducing end in the sugar
chain.
1002041 in one embodiment, an anti-ICOS antibody of the disclosure
comprises an
engineered Fe region that has an altered affinity for an Fe ligand protein. In
a further
embodiment, an anti-ICOS antibody of the disclosure comprises an engineered Fc
region that has
an altered affinity for an Fe ligand selected from the group consisting of
FeyRI A, Fr..7RIIA,
Fcylt1113, FcyRIIIA, FeyRiliB. FeyltIV, and Clq. In a specific embodiment, an
anti-ICOS
antibody of the disclosure comprises an engineered Fe region that has an
altered affinity for the
FcyRIIIA protein. In a further embodiment, an anti-1COS antibody of the
disclosure comprises
an engineered Fe region that has an altered affinity for the CI q protein.
1002051 in one embodiment, an anti-ICOS antibody of the disclosure
comprises an
engineered Fe region that has an increased affinity for an Fc ligand protein.
In a further
embodiment, an anti-ICOS antibody of the disclosure comprises an engineered Fe
region that has
57
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
an increased affinity for an Fe ligand selected from the group consisting of:
Fes/REA, FcyRIIA,
FcyR11B, Fey12111A, FeyRIIEB, FeyRIV, and Clq. In a specific embodiment, an
anti-ICOS
antibody of the disclosure comprises an engineered Fe region that has an
increased affinity for
the FeYRIIIA protein. In a further embodiment, an anti-ICOS antibody of the
disclosure
comprises an engineered Fe region that has an increased affinity for the CI q
protein.
(00206) In one embodiment. an anti-ICOS antibody of the disclosure
comprises an
engineered Fe region wherein said engineered Fc region comprises a reduced
level of fucose
compared to a native antibody. In another embodiment, an anti-1COS antibody of
the disclosure
comprises an engineered Fe region comprising a reduced level of fucose,
wherein said reduction
in fucose level results in an increased affinity for an Fe ligand selected
from the group consisting
of: FeyRIA, FeyRI1A, FeyRIEB, FeyRIIIA, FeyRIIEB, FeyRIV, and Cl q. In a
specific
embodiment, an anti-ICOS antibody of the disclosure comprises an engineered Fc
region
comprising a reduced level of fucose, wherein said reduction in fucose level
results in an
increased affinity for the FeyRill A protein. In a further embodiment, an anti-
ICOS antibody of
the disclosure comprises an engineered Fe region comprising a reduced level of
fucose, wherein
said reduction in fucose level results in an increased affinity for the CI q
protein.
1002071 Anti-ICOS antibodies described herein comprise Fe regions having a
high binding
affinity for the human FcyRIIIA protein. In one embodiment, an anti-ICOS
antibody of the
disclosure comprises an Fe region that has an affinity constant or Ka (Like)
of at least 103 M'1,
at least 5 X l& Nfl, at least 104M-1, at least 5 X 104 NO, at least 1051144,
at least 5 X105 M"1, at
least 106 NT% at least 5 X 106 M"1, at least 107.1W1, at least 5 X 107M, at
least 108 .Nfi, at least 5
X 108 Ncl, at least 109 M-1, at least 5 X10,9 MS', at least 1019 M-1, at least
5 X 1010 M4, at least
ion M-1, at least 5 X lOn M. at least 1012 Nfl, or at least 5 X 1012 M'1. In
another embodiment,
an anti-ICOS antibody of the disclosure comprises an Fe region that has a
dissociation constant
or Li (koffik.õ) of less than 5x10-3 M, less than 10'3 M, less than 5x I 0"4
M, less than 10'4M, less
than 5x104 M. less than 10 M, less than 5x10"6 M, less than l0 M, less than
5x10.7 M, less
than 104 M, less than 5x10"8 NI, less than 104 M, less than 5x10-9 M. less
than 10'9 M, less than
.5x1049 M. less than 1018M, less than 5.x.10-11 M, less than 1(111 M, less
than 5x10'12 M, or less
than 10-12 M.
(00208) An antibody used in accordance with a method described herein may
comprise an
Fe region that binds to human FeyR11.1.A with a dissociation constant (I(d) or
less than 3000 nM,
less than 25(X) nM, less than 2000 nM, less than 1500 nM, less than 1000 nM,
less than 750 nM,
less than 500 nM, less than 250 nM, less than 200 nM, less than 150 nM, less
than 100 nM, less
than 75 nM, less than 50 tiM, less than 25 nM, less than 10 nM, less than 5
nM, less than 1 nM
58
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
as assessed using a method described herein or known to one of skill in the
art (e.g., a BlAcore
assay, ELISA) (Biacore International AB, Uppsala, Sweden). In a specific
embodiment, an
antibody used in accordance with a method described herein may comprise an Fc
region that
binds to human FcyRIIIA with a dissociation constant (Kd) of between 1 to 3000
nM, I to 30(X)
nM, Ito 2000 riM, Ito .1500 riM, 1 to I0(X) nM, .110 750 nM, 1 to 500 riM, Ito
250 nM, I to
100 nM, I to 50 nM, I to 25 nM, 1 to 10 nM as assessed using a method
described herein or
known to one of skill in the art (e.g., a BIAcore assay. ELISA). In another
embodiment, an anti-
'COS antibody used in accordance with a method described herein may comprise
an Fc region
that binds to human FcyRIIIA with a dissociation constant (K49 of 500 nM, 250
nM, 100 nM, 75
nM, 50 nM, 25 nM, 10 nM or I 1M as assessed using a method described herein or
known to one
of skill in the art (e.g., a BIAcore assay, ELISA).
[00209i Anti-ICOS
antibodies described herein comprise Fe regions having a high binding
affinity for the non-human primate (e.g., cynomolgus) feyRIIIA protein. In one
embodiment, an
anti-ICOS antibody of the disclosure comprises an Fc region that has an
affinity constant or Ki
(k.,/k) of at least 103 M4, at least 5 X 103 M4, at least 104 M4, at least 5 X
104 M4, at least 105
WI, at least 5 X 1()5 WI, at least 106M4, at least 5 X 106 M4, at least 107 M.
at least 5 X 107
WI, at least 108 M4, at least 5 X 108.M4, at least 109 NC'. at least 5 .X1(9
at least 10" M4, at
least 5 X 10" M4, at least 10" 1v14, at least 5 X 10" hf 1, at least 1012
Tv14, oral least 5 X 1012
IVF1. In another embodiment, an anti-ICOS antibody of the disclosure comprises
an Fe region
that has a dissociation constant or Kd (k)nikõõ) of less than 5x10 M. lass
than 10 M, less than
5x1(14 M, less than 1 M, less than 5x10-5 M, less than. 10 M, less than 5x.10-
6 M, less than IV
6 M. less than 5x10 M. less than 10M, less than 5x10 M. less than le Mõ less
than 5x10"9
M, less than 10'9 M. less than 5x1049 M. less than 104 Mõ less than 5x104 M,
less than 1041M,
less than 5x104 2M, or less than 1042M.
1002i0j An
antibody used in accordance with a method described herein may comprise an
Fe region that binds to non-human primate (e.g., cynomolgus) FeyRillA with a
dissociation
constant (Kd) of less than 3000 nM, less than 2500 nM, less than 2000 nM, less
than 15(X) nM,
less than I0(X) rtM, less than 750 nM, less than 500 nM, less than 250 nM,
less than 2(X) nM, less
than 150 nM, less than 100 nM, less than 75 nM, less than 50 nM, less than 25
nM, less than 10
nM, less than 5 nM, less than 1 nM as assessed using a method described herein
or known to one
of skill in the art (e.g a BlAcore assay, ELBA) (Biacore international AB,
Uppsala, Sweden).
In a specific embodiment, an antibody used in accordance with a method
described herein may
comprise an Fe region that binds to non-human primate (e.g., cynomolgus)
FeyRIIIA with a
dissociation constant: (Kd) of between 1 to 3000 nM. I. to 30(X) rtM, 1 to
2000 nM, .1 to 1500 nM,
39
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
1:01000 tiM, I to 750 nM, 1 to 500 nM. 1 to 250 nM, 1. to 10t) nM, 1 to 50 nM,
1 to 25 nM, Ito
nM as assessed using a method described herein or known to one of skill in the
art (e.g., a
BlAcore assay, ELISA). In another embodiment, an anti4COS antibody used in
accordance
with a method described herein may comprise an Fc region that hinds to non-
human primate
cynomolgus) FcyRIIIA with a dissociation constant (Kd) of 500 nM, 250 riM, 100
nM, 75
nM, 50111K 25 nM, 10 nM or 1 nM as assessed using a method described herein or
known to one
of skill in the art (e.g., a BlAcore assay, ELISA).
10021.11 Anti-ICOS antibodies described herein comprise Fc regions having a
high binding
affinity for the mouse FcyRillA protein. In one embodiment, an anti-ICOS
antibody of the
disclosure comprises an Fc region that has an affinity constant or K. (4.1.0
of at least 1()3
at least 5 X 103 M4, at least 104M-1, at least 5 X 104 M-1, at least 105 MT%
at least 5 X 105 IS11, at
least 106 M-1, at least 5 X 106 M'1, at least 107M', at least 5 X 107M4, at
least le M"1, at least 5
X 108M-1, at least 109 M. at least 5 X 109 M4, at least 101 M. at least 5 X
1019 M4, at least
1011M-1, at least 5 X 10" M-1, at least 1012 M-1, or at least 5 X 1012M-1. In
another embodiment,
an anti-ICOS antibody of the disclosure comprises an Fc region that has a
dissociation constant
or K4 (k.sik..) of less than 5x1041M, less than 103 M, less than 5x104 M, less
than 10-4 M, less
than 5x10 "5M, less than 10-5 M, less than 5x1e M, less than 104 M, less than
5x10-7 M, less
than 1(17M, less than 5x104 M, less than le M. less than 5x109 M, less thanI0-
9M, less than
5x10-1(' M, less than 10-19 M. less than 5x10"11 M, less than 1041 M. less
than 5x I (112 M, or less
than 1042 M.
1002121 An antibody used in accordance with a method described herein may
comprise an
Fe region that binds to mouse FeyRIIIA with a dissociation constant (K,d) of
less than 3000 nM,
less than 2500 nM, less than 20(X) nIVI, less than 1500 nM, less than 1000 nM,
less than 750 nM,
less than 500 nM, less than 250 riM, less than 2()0 riM, less than 150 nM,
less than 100 nM, less
than 75 nM, less than 50 aM, less than 25 rtM, less than 10 nM, less than 5
n114, less than 1 nM
as assessed using a method described herein or known to one of skill in the
art (e.g., a BIAcore
assay-, ELISA) (Biaeore International AB, Uppsala, Sweden). In a specific
embodiment, an
antibody used in accordance with a method described herein may comprise an Pc
region that
binds to mouse FeyRIIIA with a dissociation constant (.1(d) of between Ito
3000 nM, I. to 3000
nM, Ito 2000 nM, Ito 1500 nM, Ito 1000 nM, Ito 750 nM, Ito 500 nM, 1 to 250
riM, Ito
100 nM, 1 to 50 nM, 1 to 25 -nM, I to 10 nM as assessed using a method
described herein or
known to one of skill in the art (e.g , a B1Acore assay, ELISA). In another
embodiment, an anti-
IC:OS antibody used in accordance with a method described herein may comprise
an Fc region
that binds to mouse FcyRIIIA with a dissociation constant (Kd) of 500 nM, 250
nM, 100 nMõ 75
CA 02743469 2016-06-13
= 5 1 3 3 2-1 0 0
riM, 50 nM, 25 TIM, 10 nM or 1 tiM as assessed using a method described herein
or known to one
of skill in the art (e.g , a BlAcore assay, ELISA).
1002131 The present disclosure provides formulations of antibodies
that specifically bind
to human ICOS and have an enhanced effector function. In one embodiment, a
formulation of
the disclosure comprises an anti-ICOS antibody with enhanced effector
function, such as, but not
limited to, enhanced ADCC, enhanced CDC, and enhanced antibody-dependent
phagoqtosis. In
one embodiment, a formulation of the disclosure comprises an anti-ICOS
antibody disclosed in US
Patent Application Publication US 2008/0279851A1, filed on May 7, 2008. In one
embodiment,
anti-ICOS antibodies of the disclosure comprise one, two, three, four, five or
all six of the CDRs of
JMAb-136 (see, US Patent 6,803,039).
1002141 The amino acid sequences for CDRI, CD.R2, and CDR3 of the
heavy chain
variable region of IMAb-136 defined according to Kabat are identified as SEQ
ID .N0:8, SEQ
ID .N0:9, and SEQ ID NO:10, respectively. The amino acid sequences for CDR1.
CDR2 and
CD1i3 of the light chain variable region of JMAb-136 defined according to
Kabat are identified
as SEQ ID NO:3õ SEQ ID NO:4, and SEQ ID NO:5, respectively.
1002151 Kabat numbering is based on the seminal work of Kabat et al,
(1991) Sequences
qfProtents of Inuntatological Interest, Publication No. 91-3242, published as
a three volume set
by the National Institutes of Health, National Technical Information Service
(hereinafter
"Kale), Kabat provides multiple sequence alignments of immunoglobulin chains
from
numerous species antibody isotypes. The aligned sequences are numbered
according to a single
numbering system, the Kabat numbering system. The Kabat sequences have been
updated since
the 1991 publication and are available as an electronic sequence. database
(latest downioadable
version 1997). Any immunoglobulin sequence can be numbered according to Kabat
by
performing an alignment with the Kabat reference sequence. Accordingly, the
Kabat numbering
system provides a uniform system for numbering immunoglobulin chains. Unless
indicated
otherwise, all immunoglobulin amino acid sequences described herein are
numbered according
to the Kabat numbering system. Similarly, all single amino acid positions
referred to herein are
numbered according to the Kabat numbering system,
[00216j In certain embodiments, an anti-ICOS antibody of the
disclosure may comprise a
heavy chain variable region, VI-I, comprising at least one CDR having the
amino acid sequence
selected from the group consisting of SEQ ID NO:8, SEQ ID NO:9, and SEQ ID
NO:10. In
certain embodiments, an anti4COS antibody of the disclosure may comprise a VI-
I domain
having the amino acid sequence of SEQ ID NO:7.
100217] In certain embodiments, an anti-ICOS antibody described herein
may comprise a
light chain variable region. VK, comprising at least one CDR having an amino
acid sequence
61
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
selected from the group consisting a SEQ ID NO:3, SEQ NO:4, and SEQ ID NO:5.
in
certain embodiments, an anti-ICOS antibody of the disclosure may comprise a.
Vic domain
having the amino acid sequence of SEQ II) NO:2.
1002181 in one embodiment, an anti-ICOS antibody of the disclosure
comprises a VK
domain having the amino acid sequence of SEQ 11) NO:2 and further comprises a
VII domain
having the amino acid sequence of SEQ ID NO:7.
1002191 The present disclosure encompasses antibodies that bind to human
ICOS,
comprising derivatives of the VII domain, VU CDRI, VU CDR2, VU CDR3, VK
domain, VK
CDR1, VK. CDR2, or VK CDR3 described herein that may bind to human ICOS.
Standard
techniques known to those of skill in the art can be used to introduce
mutations (e.g, additions,
deletions, and/or substitutions) in the nucleotide sequence encoding an
antibody, including, for
example, site-directed mutagenesis and PCR-mediated mutagenesis that are
routinely used to
generate amino acid substitutions. In one embodiment, the VII and/or VK CDR
derivatives may
include less than 25 amino acid substitutions, less than 20 amino acid
substitutions, less than 15
amino acid substitutions, less than 10 amino acid substitutions, less than 5
amino acid
substitutions, less than 4 amino acid substitutions, less than 3 amino acid
substitutions, less than
2 amino acid substitutions, or I amino acid substitution relative to the
original VII and/or VK
CDRs of the Mb-136 anti-ICOS antibody. In another embodiment, the VU and/or VK
CDR
derivatives may have conservative amino acid substitutions (e.g. supra) made
at one or more
predicted non-essential amino acid residues (i.e., amino acid residues which
are not critical for
the antibody to specifically bind to human ICOS). Mutations can also be
introduced randomly
along all or part of the VH and/or VK CDR coding sequences, such as by
saturation
mutagenesis, and the resultant mutants can be screened for biological activity
to identify mutants
that retain activity. Following mutagenesis, the encoded antibody can be
expressed and the
activity of the antibody can be determined.
1002201 The present disclosure further encompasses antibodies that bind to
human ICOS,
said antibodies or antibody fragments comprising one or more CDRs wherein said
CDRs
comprise an amino acid sequence that is at least 45%, at least 50%, at least
55%, at least 603, at
least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%, or at
least 99% identical to the amino acid sequence of one or more CDRs of the
ilvlab-136 anti-ICOS
antibody. The percent identity of two amino acid sequences can be determined
by any method
known to one skilled in the an. including, but not limited to, BLAST protein
searches.
1002211 The present disclosure further encompasses antibodies that bind to
human ICOS,
said antibodies or antibody fragments comprising a VII and/or a VK domain
wherein said VII
and/or VK domains comprise an amino acid sequence that is at least 45%, at
least 50%, at least
62
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
55%, at least 60%. at least 65%, at least 70%, at least 75%, at least 80%, at
least 85%, at least
90%, at least 95%, or at least 99% identical to the amino acid sequence of the
VFI and VI(
domain of the Atlab-136 anti-ICOS antibody. The percent identity of two amino
acid sequences
can be determined by any method known to one skilled in the art, including,
but not limited to.
BLAST protein searches.
1002221 In one embodiment, an anti-ICOS antibody of the disclosure may bind
to human
ICOS with an affinity comparable to that of the A/lab-136 anti-ICOS antibody,
1002231 In one embodiment, an anti-ICOS antibody ofthe disclosure
specifically binds the
same epitope of ICOS as the A/lab-136 anti-ICOS antibody.
1002241 In one embodiment, an anti-ICOS antibody specifically competes the
iMab-136
anti -ICOS antibody for ICOS binding. The competition assay may be performed
using any
binding assay known in the art, for example, but not: limited to ELBA. assay,
radioimmunoassay,
and flow cytometry.
1002251 The disclosure further provides polynucleotides comprising a
nucleotide sequence
encoding an anti-ICOS antibody with enhanced effector function. The disclosure
also
encompasses polynucleotides that hybridize under stringent or lower stringency
hybridization
conditions, as defined herein, to polynucleotides that encode an anti-ICOS
antibody with
enhanced effector function.
1002261 Another embodiment of the disclosure is a vector comprising one or
more
nucleotide sequences encoding an anti-ICOS antibody with enhanced effector
function.
1002271 The present disclosure further relates to an isolated cell
comprising a vector
wherein said vector comprises one or more nucleotide sequences encoding an
anti-ICOS
antibody with enhanced effector function.
1002281 Anti-ICOS antibodies of the disclosure include those of the IgGI,
IgG2, IgG3, or
1804 human isotype.
1002291 The present disclosure relates to anti-ICOS antibodies with
enhanced effector
function, as well as to compositions comprising those antibodies. In certain
embodiments, an
anti-ICOS antibody of the disclosure may mediate antigen-dependent-cell-
mediated- cytotoxicity
(ADCC). In other embodiments, the present disclosure is directed toward
compositions
comprising an anti-ICOS antibody of the Ig0.1 and/or 1g03 human isotype, as
well as to an anti-
ICOS antibody of the IgG2 and/or IgG4 human isotype, that may mediate human
ADCC. CDC,
and/or antibody-dependent phagocytosis.
1002301 Anti-ICOS antibodies described herein may have a high binding
affinity for the
human [COS antigen. For example, an antibody described herein may have an
association rate
constant: or 4. rate (antibody (Ab) + antigen (Ad --)Ab-A,,,g) of at least 2 X
1053M-Is4, at least
63
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
X 1.05NT1s4, at least 104 M4s4, at least 5 X 106Nlls"1, at least107M4s4, at
least 5 .X 107N/Ils"
1, or at least 104s.
1002311 in another embodiment, an anti-ICOS antibody may have a kõfr rate
((Ab-
Aok-orLanti' 'way (Ab) + antigen (Ag)) of less than 5x104 s4, less than WI
s"1, less than RI 02
s4, less than 10'284, less than 5x1.(I3s-1, less than 10-384, less than
.5x104s.1, or less than I 04 s'1*
In a another embodiment an antibody of the disclosure has a koff of less than
5x104 s4, less than
104 s'1, less than 5x10'6s4. less than 10'6s4, less than 5x107s1, less than
DIY's.", less than 5x10
8.4 I, less than 104 s-1, less than 5x10-9s4, less than le s4, or less than
1048s4.
1002321 In another embodiment; an anti4COS antibody may have an affinity
constant or
(kon/Kar) of at least 102M", at least 5X 102 N11, at least 103 M4, s least 5 X
103M', at least
104 M4, at least 5 X 104M4, at least 105 M4, at least 5 X 105 M4, at least 106
Nfl, at least 5 X
106 M4, at least 1.07 M4, at least 5 X. 107M4, at least 108 NI1, at least 5 X.
108 M. at least 109
1, at least 5.X109 M.4, at least 101 M4, at least 5X 10 Nil, at least 1011
N44, at least 5 X 1011
M4, at least 1012 N11, at least 5 X 1012 M4, at least 10 NC, at least S X1013
M4, at least. 1014 NI
',at least 5 X 1014 M4, at least le M4, or at least 5 X1015 M4. In yet another
embodiment, an
anti4COS antibody may have a dissociation constant or Kd 0.-00(00 of less than
5x102 M, lass
than 10*2 M, less than 5x10"3 M. less than u.r3 M, less than 5x10"4 M. less
than 104 M, less than
5x1115 M, less than 104 M. less than 5x10'8 M, less than le M. less than 5x107
M, less than 10"
7 M, less than 5x104 Ni, less than 10.8 Ni. less than $x10-9 Ni, less than 10-
9 M, less than 5x1 04
M. less than 10'10 M, less than 5x104' M, less than 10'11M, less than 5x102 M,
less than 1042
Ni, less than 5x1043M, less than 1043 M. less than 5x1044 M. less than 1044M,
less than 5x1015
M, or less than 1045 Ni.
1002331 An antibody used in accordance with a method described herein may
immtmospecifically bind to ICOS and may have a dissociation constant (Kd) of
less than 3000
pM, less than 2500 OW, less than 2000 pM, less than .1500 pM, less than 1000
pM, less than 750
pM, less than 500 pM, less than 250 pM, less than 200 pM, less than 150 pM,
less than 100 pM,
less than 75 pM as assessed using a method described herein or known to one of
skill in the art
(e.g., a BIAcore assay, HASA) (Biacore International AB, Uppsala, Sweden). In
a specific
embodiment, an antibody used in accordance with a method described herein may
immtmospecifically bind to a human ICOS antigen and may have a dissociation
constant (1(4) of
between 25 to 3400 pM, 25 to 3000 pM, 25 to 2500 pM, 25 to 2000 pM, 25 to
1500p, 25 to
1000 pM, 25 to 750 OM, 25 to 500 pM, 25 to 250 pM, 25 to 100 04, 25 to 75 pM,
25 to 50 pM
as assessed using a method described herein or known to one of skill in the
art (e.g., a BlAcore
assay, ELISA). In another embodiment; an anti-ICOS antibody used in accordance
with a
method described herein may immunospecifically bind to ICOS and may have a
dissociation
64
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
constant (Kd) of 500 pM, 100 pM, 75 pM or 50 pM as assessed using a method
described herein
or known to one of skill in the art (e.g., a BlAcore assay, ELISA).
1002341 The antibodies that specifically bind to ICOS include derivatives
that are
modified, i.e., by the covalent attachment of any type of molecule to the
antibody such that
covalent attachment does not eliminate binding to human ICOS. For example, but
not by way of
limitation, the antibody derivatives include antibodies that have been
modified, for example, but
not limited to, by glycosylation, acetylation, pevlation, phosphotylation,
amidation,
derivalization by known protecting/blocking groups, proteolytic cleavage,
linkage to a cellular
ligand or other protein, etc. Any of numerous chemical modifications may be
carried out by
known techniques, including, but not limited to, specific chemical cleavage,
acetylation,
fonnylation, metabolic synthesis of tunicamycin, etc. Additionally, the
derivative may contain
one or more non-classical amino acids.
1002351 The formulations of antibodies of the present disclosure that
specifically bind to
human ICOS may be monospecific, bispecific, trispecific or of greater
multispecificity.
Multispecific antibodies may be specific for different epitopes of human ICOS
or may be
specific for both human ICOS as well as for a heterologous epitope, such as a
heterologous
polypeptide or solid support material.
5.4. Monoclonal Anti-ICOS Antibodies
1002361 A monoclonal anti-ICOS antibody exhibits binding specificity to
human ICOS
antigen and may mediate human ADCC, CDC and/or antibody-dependent
phagocytosis. Such
an antibody can be generated using a wide variety of techniques known in the
art including the
use of hybridom.a, recombinant, and phage display technologies, Or a
combination thereof.
Antibodies are highly specific, being directed against a single antigenic
site. An engineered anti-
ICOS antibody can be produced by any means known in the art, including, but
not limited to,
those techniques described below and improvements to those techniques. Large-
scale high-yield
production typically involves culturing a host cell that produces the
engineered anfi-ICOS
antibody and recovering the anti-ICOS antibody .from the host cell culture.
Hybridoma Technique
1002371 Monoclonal antibodies can be produced using hybridoma techniques
including
those known in the art and taught, for example, in Harlow etal.. Antibodies: A
Laboratory
Manual, (Cold Spring Harbor Laboratory Press, 2nd ed. 1988); Harnmerling et
at, in
Monoclonal Antibodies and T cell Hybridomas, 563-68.1 (Elsevier, N.Y., 1981)
(said references
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
incorporated herein hy reference in their entireties). For example, in the
hybridoma method, a
mouse or other appropriate host animal, such as a hamster or macaque monkey,
is immunized to
elicit lymphocytes that produce or are capable of producing antibodies that
will specifically bind
to the protein used for immunization. Lymphocytes may also be immunized in
vitro.
Lymphocytes then are fused with myeloma cells using a suitable fusing agent,
such as
polyethylene glycol, to -form a hybridoma cell (Goding, Monoclonal Antibodies:
Principles and
Practice, pp. 59-103 (Academic Press, 1986)).
1002381 The hybridoma cells thus prepared are seeded and grown in a
suitable culture
medium that contains one or more substances that inhibit the growth or
survival of the tmfused,
parental myelorna cells. For example, if the parental myeloma cells lack the
enzyme
hypoxanthine guanine phosphoribosyl transferase (FIGPRT or HPRT), the culture
medium for
the hybridomas typically will include hypoxanthine, arninopterin, and
thymidine (HAT medium),
which substances prevent the growth of HGPRT-deficient cells.
100239) Specific embodiments employ inyeloma cells that fuse efficiently,
support stable
high-level production of antibody by the selected antibody-producing cells,
and are sensitive to a
medium such as HAT medium. Among these myeloma cell lines are murine rweloma
such as those derived from MOPC-21 and MPC-11 mouse tumors available from the
Salk
Institute Cell Distribution Center, San Diego, CA, USA, and SP-2 or X63-
Ag8.653 cells
available from the American Type Culture Collection, Rockville, MD, USA. Human
myelorna
and mouse-human heteromyeloma cell lines also have been described for the
production of
human monoclonal antibodies (KozborõI. Inununot, 133:3001 (1984); Brodeur et
al.,
Monoclonal Antibody Production Techniques and Applications, pp. 51-63 (Marcel
Dekker, Inc.,
New York, 1987)).
1002401 Culture medium in which hybridoma cells are growing is assayed for
production
of monoclonal antibodies directed against the human 1COS antigen. The binding
specificity of
monoclonal antibodies produced by hybridoma cells can be determined by
immunoprecipitation
or by an in vitro binding assay, such as radioimmunoassay (RIA) or enzyme-
linked
immtmoabsorbent assay (ELISA).
[002411 After hybridoma cells are identified that produce antibodies of the
desired
specificity, affinity, and/or activity, the clones may be subcloned by
limiting dilution procedures
and grown by standard methods (Goding,Monoclonal Antibodies: Principles and
Practice, pp.
59-.103 (Academic Press, 1986)). Suitable culture media for this purpose
include, for example,
D-MEM or RPM' 1640 medium. In addition, the hybridoma cells may be grown in
vivo as
ascites tumors in an animal.
66
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
1002421 The monoclonal antibodies secreted by the subclones are suitably
separated from
the culture medium, ascites .fluid, or serum by conventional inununoglobulin
purification
procedures such as, for example, protein A-Sepharose, hydroxylapatite
chromatography, gel
electrophoresis, dialysis, or affinity chromatography.
5.4.2. Recombinant DNA Techniques
100243.1 DNA encoding an anti-ICOS antibody described herein is readily
isolated and
sequenced using conventional procedures (e.g., by using oligonucleotide probes
that are capable
of binding specifically to genes encoding the heavy and light chains of anti-
ICOS antibodies).
The hybridoma cells serve as a source of such DNA. Once isolated, the DNA may
be placed
into expression vectors, which are then transfected into host cells such as E.
coil cells, simian
COS cells, Chinese hamster ovary (CHO) cells, or inyeloma cells that do not
otherwise produce
immunoalobulin protein, to obtain the synthesis of anti-ICOS antibodies in the
recombinant host
cells.
1002441 In phage display methods, functional antibody domains are displayed
on the
surface of phage particles which carry the polynucleotide sequences encoding
them. In
particular, DNA sequences encoding lerfl and VI, domains are amplified from
animal cDNA
libraries (e.g., human or xnurine cDN A libraries of affected tissues). The
DNA encoding the Vu
and VI, domains are recombined together with a say linker by PCR and cloned
into a phagemid
vector. The vector is electroporated into E. colt and the E coif is infected
with helper phage.
PliaL.,0e used in these methods is typically filamentous phage including fd
and MI3 and the Vì
and Viõ. domains are usually recombinantly fused to either the phage gene III
or gene VIII. Phage
expressing an antigen-binding domain that binds to a particular antigen can be
selected or
identified with antigen, e.g., using labeled antigen or antigen bound or
captured to a solid surface
or bead. Examples of .phage display methods that can be used to make the
antibodies of the
present disclosure include those disclosed in Brinkman et at, .1995,./.
Immunal. Methtxls,
182:41-50; Ames etal., 1995, J. Ninunot Methods, 184:177-186; =Kettleborough
et al., 1994,
Eur. J. Nominal., 24:952-958; Persic et al., 1997, Gene, 187:9-18; Burton
etal., 1994, Advances
in Immunology, 57:19.1-280; International Application No. PCT/GB91/01 134;
International
Publication Nos. WO 90/02809, WO 911.10737, WO 92/01.047, WO 92118619, WO
93/11236,
WO 95/15982, WO 95/2040.1, and W097/13844; and U.S. Patent Nos. 5,698,426,
5,223,409,
5,403,484, 5,580,717, 5,427,908, 5,750,753, 5,821,047, 5,571,698, 5,427,908,
5,516,637,
5,780,225, 5,658,727, 5,733,743, and 5,969,108; each of which is incorporated
herein by
reference in its entirety.
67
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
[002451 As described in the above references, after phage selection, the
antibody coding
regions from the phage can be isolated and used to generate whole antibodies,
including human
antibodies, or any other desired antigen-binding fragment, and expressed in
any desired host,
including mammalian cells, insect cells, plant cells, yeast, and bacteria,
e.g., as described below.
Techniques to recombinantly produce Fab, Fab' and F(abt)2 fragments can also
be employed
using methods known in the art such as those disclosed in PCT Publication No.
WO 92/22324;
Mu11ina' ci al., 1992, Bionehniques, 12(6):864-869; Sawai etal.. 1995, AIN,
34:26-34; and
Better et al., 1988, Science, 240;104.1-1043 (said references incorporated by
reference in their
entireties).
1002461 Antibodies may be isolated from antibody phage libraries generated
using the
techniques described in McCafferty et al., Nature. 348552-554(1990). Clackson
et al, Nature,
352:624-628 (1991). Marks et al., Mol. Biol., 222:581-597 (1991) describe the
isolation of
murine and human antibodies, respectively, using phage libraries. Chain
shuffling can be used in
the production of high affinity (nM range) human antibodies (Marks etal..
Bial'eehnologv,
10:779-783 (1992)), as well as combinatorial infection and in vivo
recombination as a strategy
for constructing very large phage libraries (Waterhouse etal., Nucl. Acids.
Res., 21:2265-2266
(1993)). Thus, these techniques are viable alternatives to traditional
monoclonal antibody
hybridorna techniques for isolation of anti-ICOS antibodies.
1002471 To generate whole antibodies, PCR primers including VII or VI,
nucleotide
sequences, a restriction site, and a flanking sequence to protect the
restriction site can be used to
amplify the VII or VL sequences in scFv clones. Utilizing cloning techniques
known to those of
skill in the art, the PCR amplified VH domains can be cloned into vectors
expressing a heavy
chain constant region, e.g, the human gamma 4 constant region, and the 'PCR
amplified VI,
domains can be cloned into vectors expressing a light chain constant regionõ
e.g., human kappa
or lambda constant regions. The vectors for expressing the VII or VL domains
may comprise an
EF-la promoter, a secretion signal, a cloning site for the variable domain,
constant domains, and
a selection marker such as neomycin. The VH and VI. domains may also be cloned
into one
vector expressing the necessaty constant regions. The heavy chain conversion
vectors and light
chain conversion vectors are -then co-transfected into cell lines to generate
stable or transient cell
lines that express full-length antibodies, e.g., IgG, using techniques known
to those of skill in the
art.
[00248J The DNA also may be modified, for example, by substituting the
coding sequence
for human heavy and light chain constant domains in place of the homologous
marine sequences
(U.S. Patent No. 4,816,567; Morrison eral., Proc. Nall. .Acad. Set. USA,
81:6851 (1984)), or by
68
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
covalently joining to the immunoglobulin coding sequence all or part of the
coding sequence for
a non-immunoglobulin polypeptide.
5.5. Chimeric Antibodies
1002491 The anti-ICOS antibodies herein specifically include chimeric
antibodies
(inununoglobulins) in which a portion of the heavy and/or light chain is
identical with or
homologous to corresponding sequences in antibodies derived from a particular
species or
belonging to a particular antibody class or subclass, while another portion of
the chain(s) is
identical with or homologous to corresponding sequences in antibodies derived
from another
species or belonging to another antibody class or subclass, as well as
fragments of such
antibodies, so long as they exhibit the desired biological activity (U.S.
Patent No. 4,816,567;
Morrison et al., Proc.... Natl. Acad. Sci. (ISA, 81:6851-6855 (1984)).
Chimeric antibodies of
interest herein include "primatized" antibodies comprising variable domain
antigen-binding
sequences derived from a nonhuman primate (e.g., Old World Monkey, such as
baboon, rhesus
or cynomolgus Illoakey) and human constant region sequences (U.S. Patent No.
5,693,780).
5.6. Altered/Mutant Antibodies
1002501 Anti-ICOS antibodies of compositions and methods described herein
can be
mutant antibodies. As used herein, "antibody mutant" or "altered antibody"
refers to an amino
acid sequence variant of an anti-ICOS antibody wherein one or more of the
amino acid residues
of an anti-ICOS antibody have been modified. The modifications to the amino
acid sequence of
an anti4COS antibody include modifications to the sequence that may improve
affinity or
avidity of the antibody for its antigen, and/or modifications to the Fc
portion of the antibody that
may improve effector function.
1002511 The present disclosure therefore relates to anti -ICOS antibodies
with enhanced
effector function disclosed herein as well as altered/mutant derivatives
thereof including, but not
limited to ones exhibiting altered human !COS binding characteristics; e.g.
altered association
constants koN, dissociation constants Lyn:, and/or equilibrium constant or
binding affinity, i(f).
In certain embodiments the Ko of an anti-ICOS antibody described herein, or an
altered/mutant
derivative thereof, for human ICOS may be no more than about .104M, 104M,
104M, or lem,
Methods and reagents suitable for determination of such binding
characteristics of an antibody of
the present disclosure, or an altered/mutant derivative thereof, are known in
the art and/or are
commercially available (se above and, e.g., U.S. Patent No. 6,849,425, U.S.
Patent No.
6,632,926, U.S. Patent No. 6,294,391, and U.S. Patent No. 6,143,574, each of
which is hereby
69
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
incorporated by reference in its entirety). Moreover, equipment and software
designed for such
kinetic analyses are commercially available (e.g. Biacore A100, and Biacore
2000
instruments; Biacore International AB, Uppsala, Sweden).
1002521 The modifications may be made to any known anti-ICOS antibodies or
anti4COS
antibodies identified as described herein. Such altered antibodies necessarily
have less than
100% sequence identity or similarity with a known anti-1COS antibody. By way
of example, an
altered antibody may have an amino acid sequence that. is within the range of
from about 25% to
about 95% identical or similar to the amino acid sequence of either the heavy
or light chain
variable domain of an anti-ICOS antibody as described herein. An altered
antibody may have an
amino acid sequence having at least 25%, 35%, 45%, 55%, 65%. 75%, 80%, 85%.
90%, or 95%
amino acid sequence identity or similarity with the amino acid sequence of
either the heavy or
light chain variable domain of an anti-ICOS antibody as described herein. In
another
embodiment, an altered antibody may have an amino acid sequence having at
least 25%, 35%,
45%, 55%, 65%, 75%, 80%, 85%, 90%, or 95% amino acid sequence identity or
similarity with
the amino acid sequence of the lieaNy chain CDR1. CDR2, or CDR3 of an anti-
ICOS antibody as
described herein. In one embodiment, an altered antibody may maintain human
ICOS binding
capability. In certain embodiments, an anti-ICOS antibody as described herein
may comprise a
VH that is at least or about 10%, 15%, 20%, 25%õ 30%, 35%, 40%, 45%, 50%, 55%,
60%, 65%,
70%, 75%, WM 85%, 90%, 95"4 or more identical to the amino acid sequence of
SEQ NO:7.
1002531 In another embodiment. an altered antibody may have an amino acid
sequence
having at least 25%, 35%. 45%, 55%, 65%, 75%, 80%, 85%, 90%, or 95% amino acid
sequence
identity or similarity with the amino acid sequence of the light chain CDR1,
CDR2, or CDR3 of
an anti-ICOS antibody as described herein. In certain embodiments, an anti-
ICOS antibody of
the disclosure may comprise a VI. that is at least or about 10%, 1.5%, 20%,
25%, 30%, 35%,
40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95% or more identical
to an
amino acid sequence of SEQ Hji NO:2.
1002541 Identity or similarity with respect to a sequence is defined herein
as the
percentage of amino acid residues in the candidate sequence that are identical
(i.e., same residue)
or similar (i.e., amino acid residue from the same group based on common side-
chain properties,
see below) with anti-ICOS antibody residues, after aligning the sequences and
introducing gaps,
if necessary, to achieve the maximum percent sequence identity. None of N-
terminal, C-
terminal, or internal extensions, deletions, or insertions into the antibody
sequence outside of the
variable domain shall be construed as affecting sequence identity or
similarity.
1002551 '14 identity," as known in the art, is a measure of the
relationship between two
polynucleotides or two polypeptides, as determined by comparing their
sequences. In general,
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
the two sequences to be compared are aligned to give a maximum correlation
between the
sequences. The alignment of the two sequences is examined and the number of
positions giving
an exact amino acid or nucleotide correspondence between the two sequences
determined,
divided by the total length of the alignment and multiplied by 100 to give a %
identity figure.
This % identity figure may be determined over the whole length of the
sequences to be
compared, which is particularly suitable for sequences of the same or very
similar length and
which are highly homologous, or over shorter defined lengths, which is more
suitable for
sequences of unequal length or which have a lower level of homology.
1002561 For example, sequences can be aligned with the software clustalw
under Unix
which generates a. file with an "aln" extension, this file can then be
imported into the Bioedit
program (Hall, T.A. 1999, RtoE'dit: a user:friendly biological sequence
all,gninem editor and
analysis program for Windows 95/98/N7INucl. Acids. Symp. Ser. 41:95-98) which
opens the .aln
file. In the Bioedit window, one can choose individual sequences (two at a
time) and alignment
them. ibis method allows for comparison of the entire sequence.
1002571 Methods for comparing the identity of two or more sequences are
well known in
the art. Thus for instance, programs are available in the Wisconsin Sequence
Analysis Package,
version 9.1 (Devereux J. et al, Nucleic Acids Res., 12:387-395, 1984,
available from Genetics
Computer Group, Madison, WI, USA). The determination of percent identity
between two
sequences can be accomplished using a mathematical algorithm. For example, the
programs
BESTF1T and GAP, may be used to determine the % identity between two
polynucleotides and
the % identity between two polypeptide sequences. BEsmn uses the "local
homology"
algorithm of Smith and Waterman (Advances in Applied Mathematics, 2:482-489,
1981) and
finds the best single region of similarity between two sequences. BESTF1T is
more suited to
comparing two polynucleotide or two polypeptide sequences which are dissimilar
in length, the
program assuming that the shorter sequence represents a portion of the longer.
In comparison,
GAP aligns two sequences finding a "maximum similarity" according to the
algorithm of
Neddleman and Wunsch (J. Ma B/W., 48:443-354, 197(i). GAP is more suited to
comparing
sequences which are approximately the same length and an alignment is expected
over the entire
length. Preferably the parameters "Gap Weight" and "Length Weight" used in
each program are
50 and 3 for polynucleotides and 12 and 4 for polypeptides, respectively.
Preferably (3.10 identities
and similarities are determined when the two sequences being compared are
optimally aligned.
1002581 Other programs for determining identity and/or similarity between
sequences are
also known in the art, for instance the BLAST family of programs (Karlin &
Altschul, 1990,
Proc. Nod. Acad. Sbc. USA, 87:2264-2268, modified as in Karlin & Altschul,
1993, Proc. Nail
Acad. Sci. USA, 90:5873-5877, available from the National Center for
Biotechnology
71
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Information (NCB), Bethesda, MD, USA and accessible through the home page of
the NCB! at
www.ncbi.nlm.nih.gov). These programs are non-limiting examples of a
mathematical
algorithm utilized for the comparison of two sequences. Such an algorithm is
incorporated into
the NBLAST and XBLAST programs of Altschul et al., .1990, j .Mol. Biol.,
215:403-410.
BLAST nucleotide searches can be performed with the NBLAST program, score
.100,
wordlength 12 to obtain nucleotide sequences homologous to a nucleic acid
molecule encoding
all or a portion if an anti-ICOS antibody of the disclosure. BLAST protein
searches can be
performed with the XBLAST program, score 50, wordlength 3 to obtain amino acid
sequences homologous to a protein molecule of the disclosure. To obtain gapped
alignments for
comparison purposes, Gapped BLAST can be utilized as described in Altschul
etal., 1997,
Nucleic Acids Res., 25:3389-3402. PSI-Blast can also be used to perform an
iterated search
which detects distant relationships between molecules (id.). When utilizing
BLAST, Gapped
BLAST, and PSI-Blast programs, the default parameters of the respective
programs (e.g.,
XBLAST and NBLAST) can be used. See, http://www.ncbi.nlm.nih.gov.
[002591 Another non-limiting example of a program for determining identity
and/or
similarity between sequences known in the art is FASTA (Pearson W. R. and
Lipman D.J., Proc.
Natl. .Acad. Sc!. USA, 85:2444-2448, 1988, available as part of the Wisconsin
Sequence Analysis
Package). Preferably the BLOSUM62 amino acid substitution matrix (Henikoff S.
and Henikoff
.1,G., Proc., Nail, Acad. Sc!. USA, 89:10915-10919, 1992) is used in
polypeptide sequence
comparisons including where nucleotide sequences are first translated into
amino acid sequences
before comparison
[002601 Yet another non-limiting example of a program known in the art for
determining
identity andlor similatity between amino acid sequences is Seq Web Software (a
web-based
interface to the GCG Wisconsin Package: Gap program) which is utilized with
the default
algorithm and parameter settings of the program b1osum62, gap weight 8, length
weight 2.
1002611 The percent identity between two sequences can be determined using
techniques
similar to those described above, with or without allowing gaps. In
calculating percent identity,
typically exact matches are counted.
[002621 Preferably the program BESTF1T is used to determine the % identity
of a query
polynucleotide or a polypeptide sequence with respect to a poly,mucleotide or
a polypeptide
sequence of the present disclosure, the query and the reference sequence being
optimally aliened
and the parameters of the program set at the default value.
1002631 To generate an altered antibody, one or more amino acid alterations
(e.g,
substitutions) are introduced in one Or more of the hypervariable regions of
the species-
dependent antibody. One or more alterations (e.g., substitutions) of framework
region residues
72
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
may also be introduced in an anti-ICOS antibody where these result in an
improvement in the
binding affinity of the antibody mutant for the antigen from the second
mammalian species.
Examples of framework region residues to modify include those which non-
covalently bind
antigen directly (Amit et al., Science, 233:747-753 (1986)); interact
withletTect the conformation
of a CDR (Chothia et at Mot Biol., 196:901-917 (1987)); and/or participate in
the VL-VH
interface (EP 239 400131). In certain embodiments, modification done or more
of such
framework region residues results in an enhancement of the binding affinity of
the antibody for
the antigen from the second mammalian species. For example, from about one to
about five
framework residues may be altered in this embodiment of the disclosure.
Sometimes, this may
be sufficient to yield an antibody mutant suitable for use in preclinical
trials, even where none of
the hypervariable region residues have been altered. Normally, however, an
altered antibody
will comprise additional hypervariable region alteration(s).
1002641 The hypervariable region residues which are altered may be changed
randomly.
especially where the starting binding affinity den anti-1COS antibody for the
enliven from the
second mammalian species is such that such randomly produced altered antibody
can be readily
screened.
1002651 One useful procedure for generating such an altered antibody is
called "alanine
scanning mutagenesis" (Cunningham and Wells, .Science, 244:1081-1085 (1989)).
Here, one or
more of the hypervariable region residue(s) are replaced by alanine or
polyalanine residue(s) to
affect the interaction of the amino acids with the antigen from the second
mammalian species.
Those hypervariable region residue(s) demonstrating functional sensitivity to
the substitutions
then are refined by introducing additional or other mutations at or for the
sites of substitution.
Thus, while the site for introducing an amino acid sequence variation is
predetermined, the
nature of the mutation per se need not be predetermined. The Ala-mutants
produced this way are
screened for their biological activity as described herein.
1002661 Another procedure for generating such an altered antibody involves
affinity
maturation using phan display (Hawkins et al., .11/fal. Biol., 254:889-8%
(1992) and Lowman
et al., Biochemistry, 30(45):10832-10837 (1991)). Briefly, several
hypervariable region sites
(e.g, 6-7 sites) are mutated to generate all possible amino acid substitutions
at each site. The
antibody mutants thus generated are displayed in a monovalent fashion from
filamentous phage
particles as fusions to the gene 111 product of M.13 packaged within each
particle. The phage-
displayed mutants are then screened for their biological activity (e.g.,
binding affinity) as herein
disclosed.
1002671 Mutations in antibody sequences may include substitutions,
deletions, including
internal deletions, additions, including additions yielding fusion proteins,
or conservative
73
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
substitutions of amino acid residues within and/or adjacent to the amino acid
sequence, but that
result in a "silent" change, in that the change produces a functionally
equivalent anti-ICOS
antibody. Conservative amino acid substitutions may be made on the basis of
similarity in
polarity, charge, solubility, hydrophobicity, hydrophilicity, and/or the
amphipathic nature of the
residues involved. For example, non-polar (hydrophobic) amino acids include
alanine leucine,
isoleucine. valine, prohne, phenylalanin.e, nyptophan, and methionine; polar
neutral amino acids
include glycine, serine, threonine, cysteine, tyrosine, asparagine, and
glutamine; positively
charged (basic) amino acids include arginine, lysine, and histidine; and
negatively charged
(acidic) amino acids include aspartic acid and glutamic acid. In addition,
glycine and proline are
residues that can influence chain orientation. Non-conservative substitutions
will entail
exchanging a member of one of these classes for a member of another class.
Furthermore, if
desired., non-classical amino acids or chemical amino acid analogs can be
introduced as a
substitution or addition into the antibody sequence. Non-classical amino acids
include. but are
not limited to, the 1)-isomers of the common amino acids, CA -amino isobutyric
acid, 4-
aminobutyric acid, Abu, 2-amino butyric acid, 7-Abu, a-.Ahx, 6-amino hexanoic
acid, Aib, 2-
amino isobutyric acid, 3-amino propionic acid, o.mithine, norleticine,
norvaline, hydroxyproline,
sarcosine, citruiline, cysteic akid, t-btitylglycine, t-butylalanine,
phenylglycine,
cyclohexylalanine, 0-alanine, fluoro-amino acids, designer amino acids such as
0-methyl amino
acids, Ca-methyl amino acids, Nix-methyl amino acids. and amino acid analogs
in general.
1002681 In another embodiment. the sites selected for modification are
affinity matured
using phase display (see above).
1002691 Any technique for mutagenesis known in the art can be used to
modify individual
nucleotides in a DNA sequence, for purposes of making amino acid
substitution(s) in the
antibody sequence, or for creating/deleting restriction sites to facilitate
further manipulations.
Such techniques include, but are not limited to, chemical mutagenesis, in
vitro site-directed
mutagenesis (Kunkel, Proc. Natl. Acad. Sci. /.N4, 82:488 (1985); Hutchinson,
C. eral., J. Biol.
Chem., 253:6551 (1978)), oligonucleotide-direcied mutagenesis (Smith, Ann.
Rev. Genet,
19:423-463 (1985); Hill et al.,.Methods Enzymol., 155:558-568 (1987)), PCR-
based overlap
extension (Ho et al., Gene, 77:51-59(1989)). PCR-based inegaprimer mutagenesis
(Sarkar et al.,
Motechniques, 8:404-407 (.1990)), etc. Modifications can be confirmed by
double-stranded
dideoxy DNA sequencing.
1002701 In certain embodiments of the disclosure, an anti-ICOS antibody can
be modified
to produce fusion proteins; i.e., the antibody, or a fragment thereof, fused
to a heterologous
protein, polypeptide or peptide. In certain embodiments, the protein fused to
the portion of an
anti-IC OS antibody is an enzyme component of Antibody-Directed Enzyme Prodrug
Therapy
74
CA 02743469 2016-06-13
' 51332-100
(ADEPT). Examples of other proteins or poly peptides that can be engineered as
a fusion protein
with art anti-ICOS antibody include, but are not limited to toxins such as
ricin, abrin,
ribonuclease, DNase I, Staphylococcal enterotoxin-A, pokeweed anti-viral
protein, aelonin,
diphtheria toxin, Pseudomonas exotoxin, and Pseudomonas endotoxin. See, for
example, Pastan
ei al., Cell, 47:641 (1986), and Goldenberg et al, Cancer Journal for
Clinicians, 44:43 (1994).
.Enzymatically active toxins and fragments thereof which can be used include
diphtheria A chain,
non-binding active fragments of diphtheria toxin, exotoxin A chain (from
Pseudonyms
aertiginosa), ricin A chain, ab.rin A chain, modeccin A chain, alpha-
sarcitnAleurues.lbrdli
proteins, dianthin proteins. Phytolaca americana proteins (PAP1, PAM, and PAP-
S), momordica
charantia inhibitor, cumin, crotin, sapaonaria officinalis inhibitor, gelonin,
mitogellin,
restrictocin, phenomycin, enortryein and the tricothecenes. See, for example,
WO 93/21232
published October 28, 1993.
[002711 Additional fusion proteins may be generated through the
techniques of gene-
shuffling, motif-shuffling, exon-shufflina, andlor codon-shuftIinu.
(collectively referred to as
"DNA shuffling"). DNA shuffling may be employed to alter the activities of the
anti -ICOS
antibody or fragments thereof (e.g., an antibody or a fragment thereof with
higher affinities and
lower dissociation rates). See, generally, U.S. Patent Nos, 5,605,793;
5,811,238; 5,830,721;
5,834,252; and 5,837,458, and Patten et al., 1997, -C"..l'irrr. ()pinion
Biatecimol., 8724-33;
Harayama, 1998, Trends Biotechnol. 16(2):76-82, Hansson et at., 1999,J. /viol.
BioL,
287:265-76; and Lorenzo and Blasco, 1998, Biotechniques 24(2):308-313). The
antibody can
further be a binding-domain immunoglobulin fusion protein as described in U.S.
Publication
20030118592, U.S. Publication 200330133939, and PCT Publication WO 02/056910,
all
to Ledbelter et al..
5.7. Domain Antibodies
[002721 Anti-1COS antibodies of compositions and methods of the
disclosure can be
domain antibodies, e.g., antibodies containing the small functional binding
units of antibodies,
corresponding to the variable regions of the heavy (VH) or light (VI) chains
of human antibodies.
Examples of domain antibodies include, but are not limited to, those available
from Domantis
Limited (Cambridge, UK) and Domantis Inc. (Cambridge, MA, USA) that are
specific to
therapeutic targets (see, for example, W0041058821, W004/0030.19; U.S. Patent
Nos.
6,291,158; 6,582,915; 6,696,2.45; and 6,593,081). Commercially available
libraries of domain
antibodies can be used to identify anti-1COS domain antibodies. In certain
embodiments, anti-
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
ICOS antibodies comprise an ICOS functional binding unit and an Fe gamma
receptor functional
binding unit.
1002731 in one embodiment, an anti-ICOS domain antibody may comprise any
one of, or
any combination of the CDRs of the heavy or light chains of the iMab-136
monoclonal antibody.
1002741 in another embodiment, an anti-ICOS domain antibody may comprise
Vil CDR3
of Jklab-I 36 -together with any combination of the CDRs comprised by the
heavy or light chains
variable regions of the iMab-136 monoclonal antibody. An anti-ICOS domain
antibody may
also comprise VK. CDR3 of iMab-136 together with any combination of the CDRs
comprised by
the heavy or light chains variable regions of the .11V16-136 monoclonal
antibody.
1002751 in yet another embodiment, an anti-ICOS domain antibody may
comprise "VH
CDR3 of iMah-136. An anti-ICOS domain antibody may also comprise VK CDR3 of
ilviab-
136.
5.8. Diabodies
1002761 in certain embodiments of the disclosure, anti-ICOS antibodies are
"diabodies".
The term "diabodies" refers to small antibody fragments with two antigen-
binding sites, which
fragments comprise a heavy chain variable domain NO connected to a light chain
variable
domain (VE) in the same polypeptide chain (VII-V1.). By using a linker that is
too short to allow
pairing between the two domains on the same chain, the domains are forced to
pair with the
complementary domains of another chain and create two antigen-binding sites.
Diaboclies are
described more fully in, for example, EP 404,097; WO 93/11161; and Hollinger
el al., Proc.
Natl. Acad. Sc!. au, 90:6444-6448 (1993).
5.9. Vaccibodies
1002771 in certain embodiments of the disclosure, anti-ICOS antibodies are
Vaccibodies.
Vaccibodies are dimeric polypeptides. Each monomer of a vaccibody consists of
a scFv with
specificity for a surface molecule on APC connected through a hinge region and
a Cy3 domain to
a second scFv. In other embodiments of the disclosure, vaccibodies containing
as one of the
scFv's an anti-ICOS antibody fragment may be used to juxtapose those ICOS
expressing cells to
be destroyed and an effector cell that mediates ADCC. For example, see. Bogen
et al.,U.S.
Patent Application Publication No. 20040253238.
76
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
5.10. Linear Antibodies
1002781 In certain embodiments of the disclosure, anti-1COS antibodies are
linear
antibodies. Linear antibodies comprise a pair of tandem Fd segments (Vir-Cm-Vu-
Cm) which
form a pair of antigen-binding regions. Linear antibodies can be bispecific or
monospecific.
See. Zapata et al., Protein Eng., 8(10); 1057-1062 (1995).
5.11. Parent Antibody
1002791 In certain embodiments of the disclosure, an anti-ICOS antibody is
a parent
antibody. .A "parent antibody" is an antibody comprising an amino acid
sequence which may
lack, or may be deficient in, one or more amino acid residues in or adjacent
to one or more
hypervariable regions thereof compared to an altered/mutant antibody as herein
disclosed. Thus,
the parent antibody may have a shorter hypervariable region Than the
corresponding
hypervariable region of an antibody mutant as herein disclosed. The parent
polypeptide may
comprise a native antibody sequence (i.e., a naturally occurring, including
anaturally occurring
allelic variant) or an antibody sequence with pre-existing amino acid sequence
modifications
(such as other insertions, deletions and/or substitutions) of a naturally
occurring sequence. The
parent antibody may be a humanized antibody or a human antibody.
5.12. Antibody Fragments
1002801 "Antibody fragments' comprise a portion of a full-length antibody.
generally the
antigen binding or variable region thereof. Examples of antibody fragments
include Fab, Fab ,
Nab )2, and Fv fragments; diabodies; linear antibodies; single-chain antibody
molecules; and
muhispecific antibodies formed from antibody fragments.
1002811 Traditionally, these fragments were derived via proteolytic
digestion of intact
antibodies (see, e.g.. Morimoto et al., journal of Biochemical and Biophysical
itleihodv, 24:107-
117 (1992) and Brennan et al, Science, 229:81 (1985)). However, these
fragments can now be
produced directly by recombinant host cells. For example, the antibody
fragments can be
isolated from the antibody phage libraries discussed above. Fab' -SH fragments
can also be
directly recovered from E coil and chemically coupled to form F(ab )2
fragments (Carter etal.,
Bioffechnology, 10:163-167 (1992)). According to another approach, F(ab )2
fragments can be
isolated directly from recombinant host cell culture. Other techniques for the
production of
antibody fragments will be apparent to the skilled practitioner. In other
embodiments, the
77
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
antibody of choice is a single-chain Fv fragment (scFv). See, for example, WO
93/16185. In
certain embodiments, the antibody is not a Fab fragment.
5.13. Bispecifie Antibodies
1002821 Bispecific antibodies are antibodies that have binding
specificities for at least two
different epitopes. Exemplary bispecific antibodies may bind to two different
epitopes of the
ICOS expressing T cell surface marker. Other such antibodies may bind a first
ICOS expressing
T cell marker and further bind a second ICOS expressing T cell surface marker.
An anti-ICOS
expressing T cell marker binding arm may also be combined with an arm Which
binds to a
triggering molecule on a leukocyte such as a T cell receptor molecule (e.g.,
CD2 or CD3 or Fe
receptors for IgG (FcyR), so as to focus cellular defense mechanisms to the
ICOS expressing
Bispecific antibodies may also be used to localize cytotoxic agents to the
ICOS expressing
cell. These antibodies possess an ICOS expressing T cell marker-binding arm
and an arm
which binds the cytotoxic agent (e.g., saporin, anti-interferon-a, vinca
alkaloid, ricin A chain,
methola-exate or radioactive isotope haptea Bispecific antibodies can be
prepared as Nil-
length antibodies or antibody fragments (e.g.,.E(ab ): bispecific antibodies).
1002831 Methods for making bispecifie antibodies are known in the art.
(See, for example.
Millstein el aL, .Nature, 305:517-539 (1983); Traunecker et al., F.,1080 J.,
10:3655-3659 (1991);
Suresh etal.. Alethadv in linzpnolagv, 121:210 (1986); Kostelny et at., J.
inununol.,
148(5)1547-1553 (1992): Hollinger ei at.. Proc. Nail Acad. Sci. USA, 90:6444-
6448(1993);
Gruber ei Minima, 152:5368(1994); U.S. Patent Nos. 4..474.893;4,714.681:
4,925,648;
3,573,920; 5,601,81; 95,731,168; 4,676,980: and 4,676,980, WO 94/04690; WO
91/00360; WO
92/200373; WO 93/17715; WO 92/08802; and .EP 03089.)
1002841 in one embodiment, where an anti-ICOS antibody of compositions and
methods
of the disclosure is bispecitic, the anti-KOS antibody may be human or
humanized and may
have specificity for human ICOS and an epitope on a I cell or may be capable
abinding to a
human effector cell such as, for example, a monocyte/macrophage and/or a
natural killer cell to
effect cell death.
544. Variant Fc Regions
100285) The present disclosure provides formulation of proteins comprising
a variant Fe
region. That is, a non-naturally occurring Fe region, for example an Fe region
comprising one or
more non-naturally occurring amino acid residues. Also encompassed by the
variant Fe regions
78
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
of present disclosure are Fe regions which comprise amino acid deletions,
additions andlor
modifications.
1002861 it will be understood that Fe region as used herein includes the
polypeptides
comprising the constant region of an antibody excluding the first constant
region
immunoglobulin domain. Thus Fe refers to the last two constant region
immunoglobulin
domains of IgA.,1gD, and JgG. and the last three constant region
immunoglobulin domains of
IgE and IgM, and the flexible hinge N-terminal to these domains. For IgeN and
1gM Fc may
include the .1 chain. For IgG, Fe comprises immunoglobulin domains Comma2 and
Cgamma3
(C72 and Cy3) and the hinge between Cgarrunal (Cy I) and Cgamma2 (Cy2).
Although the
boundaries of the Fc region may vary, the human 1gG heavy chain Fe region is
usually defined to
comprise residues C226 or P230 to its carboxyl-terminus, wherein the numbering
is according to
the Eli index as in Kabat et al. (.1991, NFU Publication 91-3242, National
Technical Information
Service, Springfield, VA). The "EU index as set forth in )(that" refers to the
residue numbering
of the humanigG1 EU antibody as described in Kabat et at. supra. Fe may refer
to this region in
isolation, or this region in the context of an antibody, antibody fragment, or
Fc fusion protein.
An Fe variant protein may be an antibody, Fe fusion, or any protein or protein
domain that
comprises an Fc region including, but not limited to, proteins comprising
variant Fe regions,
which are non naturally occurring variants of an Fe. Note: Polymorphisms have
been observed
at a number of Fe positions, including but not limited to Kabat 270, 272, 312,
315, 356, and 358,
and thus slight differences between the presented sequence and sequences in
the prior art may
exist.
1002871 The present disclosure encompasses Fc variant proteins which have
altered
binding properties for an Fc I igand (e.g., an Fe receptor, Clq) relative to a
comparable molecule
(e.g., a protein having the same amino acid sequence except having a wild type
Fc region).
Examples of binding properties include but are not limited to, binding
specificity, equilibrium
dissociation constant (K0), dissociation and association rates (kat and ki,õ
respectively), binding
affinity and/or avidity. It is generally understood that a binding molecule
(e.g., an Fe variant
protein such as an antibody) with a low .K0 may be preferable to a binding
molecule with a high
KD. However, in some instances the value of the k. or koff may be more
relevant than the value
of the KD. One skilled in the art can determine which kinetic parameter is
most important for a
given antibody application.
1002881 The affinities and binding properties of an Fe domain for its
ligand may be
determined by a variety of in vitro assay methods (biochemical or
immunological based assays)
known in the art for determining Fc-Feylk interactions, i.e., specific binding
of an Fc region to an
FeyR including but not limited to, equilibrium methods (e.g., enzyme-linked
immunoabsorbent
79
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
assay (ELISA), or radioimmunoassay (RIA)), or kinetics (e.g.. BIACORDe
analysis), and other
methods such as indirect binding assays, competitive inhibition assays,
fluorescence resonance
energy transfer (FRET), gel electrophoresis and chromatography (e.g., gel
filtration). These and
other methods may utilize a label on one or more of the components being
examined and/or
employ a. variety of detection methods including but not limited to
chromogenic, fluorescmt,
luminescent, or isotopic labels. A detailmd description of binding affinities
and kinetics can be
found in Paul. WE., ed., Fundamental immunology, 4th Ed., Lippincott-Raven,
Philadelphia
(1999), which .focuses on anti body-immunogen interactions.
1002891 In one embodiment, the Fc variant protein has enhanced binding to
one or more
Fc ligand relative to a comparable molecule. In another embodiment, the Fc
variant protein has
an affinity for an Fc ligand that is at least 2 fold, or at least 3 fold, or
at least 5 fold, or at least 7
fold, or a least 10 fold, or at least 20 fold, or at least 30 fold, or at
least 40 fold, or at least 50
fold, or at least 60 fold, or at least 70 fold, or at least 80 fold, or at
least 90 fold, or at least .100
fold, or at least 200 fold greater than that of a comparable molecule. In a
specific embodiment,
the Fc variant protein has enhanced binding to an Fc receptor. In another
specific embodiment,
the Fc variant protein has enhanced binding to the Fe receptor Fel/Rink In
still another specific
embodiment, the Fe variant protein has enhanced binding to the Fc receptor
FcRn. In yet
another specific embodiment, the Fc variant protein has enhanced binding to
Ciq relative to a
comparable molecule.
1002901 The serum half-life of proteins comprising Fe regions may be
increased by
increasing the binding affinity of the Fe region for Fan. In one embodiment,
the Fc variant
protein has enhanced serum half life relative to comparable molecule.
1002911 "Antibody-dependent cell-mediated cytotoxicity" or "ADCC" refers to
a .form of
cytotoxicity in which secreted 1g bound onto Fc receptors (FcRs) present on
certain cytotoxic
cells (e.g., Natural Killer (NK) cófls, nentrophils, and macrophages) enables
these cytotoxic
effector cells to bind specifically to an antigen-bearing target cell and
subsequently kill the target
cell with cytotoxins. Specific high-affinity igG antibodies directed to the
surface of target cells
"arm" the cytotoxic cells and are absolutely required for such killing. Lysis
of the target cell is
extracellidar, requires direct cell-to-cell contact, and does not involve
complement. It is
contemplated that, in addition to antibodies, other proteins comprising Fc
regions, specifically Fe
fusion proteins, having the capacity to bind specifically to an antigen-
bearing target cell will be
able to effect cell-mediated cytotoxicity. For simplicity, the cell-mediated
cytotoxicity resulting
from the activity of an Fc fusion protein is also referred to herein as ADCC
activity.
1002921 The ability of any particular Fe variant protein to mediate lysis
of the target cell
by ADCC can be assayed. To assess ADCC activity an Fc variant protein of
interest is added to
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
target cells in combination with immune effector cells, which may be activated
by the antigen
antibody complexes resulting in cytolysis of the target cell. Cytolysis is
generally detected by the
release of label (e.g. radioactive substrates, fluorescent dyes or natural
intracellular proteins)
from the lysed cells. Useful effector cells for such assays include peripheral
blood mononuclear
cells (PBMC) and Natural Killer (NK) cells. Specific examples of in vitro
ADCC: assays are
described in Vvrisecarver et al., 1985 79:277-282; Bruggemann et al., 1987,3
Exp Med .166:1351-
1361; Wilkinson etal., 2001, 3 Immunol Methods 258:183-191; Patel et al., 1995
.1 Immunol
Methods 184:29-38. ADCC activity of the Fc variant protein of interest may
also be assessed in
vivo, e.g., in an animal model such as that disclosed in Clynes et al., 1998,
Proc. Mu!. Acad. Sci.
USA 95:652-656.
1002931 In one embodiment, an Fe variant protein has enhanced ADCC activity
relative to
a comparable molecule. In a specific embodiment, an Fe variant protein has
ADCC activity that
is at least 2 fold, or at least 3 fold, or at least 5 fold or at least 10 fold
or at least 50 fold or at least
100 fold greater than that of a comparable molecule. In another specific
embodiment, an Fe
variant protein has enhanced binding to the Fe receptor FcyRIIIA and has
enhanced ADCC
activity relative to a comparable molecule. In other embodiments, the Fc
variant protein has
both enhanced ADCC activity and enhanced serum half life relative to a
comparable molecule.
I002941 "Complement dependent cy totaxicity" and "CDC* refer to the lysing
of a target
cell in the presence of complement The complement activation pathway is
initiated by the
binding of the first component of the complement system (C10 to a molecule, an
antibody for
example, complexed with a cognate antigen. To assess complement activation, a
CDC assay,
e.g. as described in Gazzano-Santoro et al., 1996,3. Immunol. Methods,
202:163, may be
-performed. In one embodiment. an Fe variant protein has enhanced CDC activity
relative to a
comparable molecule. In a specific embodiment, an Fe variant protein has CDC
activity that is
at least 2 fold, or at least 3 fold, or at least 5 fold or at least 10 fold or
at least 50 fold or at least
100 fold greater than that of a comparable molecule. In other embodiments, the
Fe variant
protein has both enhanced CDC activity and enhanced serum half life relative
to a comparable
molecule.
1002951 In one embodiment, the present disclosure provides compositions,
wherein the Fc
region comprises a non naturally occurring amino acid residue at one or more
positions selected
from the group consisting of 234, 235, 236, 237, 238, 239, 240, 241, 243, 244,
245, 247, 251,
252, 254, 255, 256, 262, 263, 264, 265, 266, 267, 268, 269, 279, 280, 284,
292, 296, 297, 298,
299, 305, 313, 316, 325, 326, 327, 328, 329, 330, 332, 333, 334, 339, 341,
343, 370, 373, 378,
392, 416, 419, 421,440 and 443 as numbered by the Eli index as set forth in
Kabat. Optionally,
the Fe region may comprise a non naturally occurring amino acid residue at
additional and/or
81
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
alternative positions known to one skilled in the art (see, e.g., U.S. Patents
5,624,821; 6,277,375;
6,737,056; PCT Patent Publications WO 01/58957; WO 02/06919; WO 041016750; WO
04/029207; WO 04/035152; WO 04/074455; WO 04/099249; WO 04/063351; WO
05/070963;
WO 05/040217, WO 05/092925 and WO 06/020114).
1002%1 In a specific embodiment, the present disclosure provides an Fe
variant protein
composition, wherein the Fe region comprises at least one non naturally
occurring amino acid
residue selected from the group consisting of 2341), 234E, 234N, 234Q, 234T,
2341-1, 234Y,
2341, 234V, 234F, 235A, 2350, 235k, 235W, 235P, 235S, 235N, 235Q, 2351, 23511,
235Y,
2351, 235V, 235F, 236E, 2391), 239E, 239N, 239Q, 23917, 2'39T, 239H, 239Y,
2401, 240A,
240T, 240M, 241W, 2411,, 241Y, 241E, 241 R. 243W, 2431 243Y, 243R, 243Q, 244H,
245A,
2411, 247V, 2470, 251F, 252Y, 254T, 2551, 256E, 256M, 2621, 262A, 2621, 262E,
2631,
263A, 263T, 263M, 2641, 2641,264W, 2641', 264R, 264F, 264M, 264Y, 264E, 2650,
265N,
265Q, 265Y, 265F, 265V, 2651 2651, 265H, 265T, 2661, 266A 2661, 266M, 267Q,
2671,,
268E, 26911, 269Y, 269F, 269R, 270E, 280A, 284M, 292P, 2921, 296E, 296Q, 2960,
296N,
296S, 2961, 2961, 2961, 29611,269G. 291S, 2970, 297E, 29811, 2981, 298T, 298F,
2991, 2991,
299A, 299S, 299V, 29911, 299F, 299E, 3051, 313F, 3161), 325Q, 3251, 3251,
3251), 325E, 325A,
325T, 325V, 32511, 3270., 327W, 327N, 3271, 328S, 328M, 3281), 328E, 328N,
328Q, 328F,
3281, 328V, 3281', 328H, 328A, 329F, 329H, 329Q, 330K, 330G, 330T, 330C, 3301,
330Y,
330V, 3301, 330F, 330R, 33011, 3321), 332S, 332W, 332F, 332E, 332N, 332Q,
332T, 33211,
332Y. 332A, 3391', 370E, 370N, 3781), 3921', 3961, 416G, 41911,421K, 440Yand
434W as
numbered by the EU index as set forth in Kabat. Optionally, the Fe region may
comprise
additional and/or alternative non naturally occurring amino acid residues
known to one skilled in
the art (see, e.g., U.S. Patents 5,624,821; 6,277,375; 6,737,056; PCT Patent
Publications WO
01/58957; WO 02/06919; WO 04/016750; WO 041029207; WO 04/035152 and WO
05/040217).
1002971 in another embodiment, the present disclosure provides an Fe
variant protein
composition, wherein the Pc region comprises at least a non naturally
occurring amino acid at
one or more positions selected from the group consisting of 239, 330 and 332.
as numbered by
the EU index as set forth in Kabat. In a specific embodiment, the present
disclosure provides an
Fe variant protein formulation, wherein the Fe region comprises at least one
non naturally
occurring amino acid selected from the group consisting of 2391), 3301 and
332E, as numbered
by the EU index as set forth in Kabat. Optionally, the Fe region may further
comprise additional
non naturally occurring amino acid at one or more positions selected from the
group consisting
of 252, 254, and 256, as numbered by the EU index as set forth in Kabat. In a
specific
embodiment, the present disclosure provides an Fe variant protein formulation,
wherein the Fe
region comprises at least one non naturally occurring amino acid selected from
the group
82
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
consisting of 2391), 330L and 332E, as numbered by the EU index as set forth
in Kabat and at
least one non naturally occurring amino acid at one or more positions are
selected from the group
consisting of 252Y, 254T and 256E, as numbered by the EU index as set forth in
Kabat.
1002981 in one
embodiment, the Fe variants of the present disclosure may be combined
with other known Pc variants such as those disclosed in Ghetie et al., 1997,
Nat Biotech. 15:637-
40; Duncan et al, 1988, Nature 332:563-564; Lund et al., 1991, J. Immunol
147:2657-2662;
Lund et at. 1992. Mol Immunol 29:53-59; Alegre et al, 1994, Transplantation
57:1537-1543;
Hutchins et al., 1995, Proc.! Natl. Acad Sc! USA 92:11980-11984; Jefferis et
al, 1995, Immunol
Lett. 44:111-117; Lund et al., 1995, Faseb J 9:115-119; Jefferis et al, 1996,
Immunol Lett
54:101-104; Lund et al, 1996. J Immunol 157:4963-4969; Armour et al., 1999,
Eur J Immunol
29:2613-2624; Idusonie et al, 2000, J Immunol 164:4178-4184; Reddy et al,
2000, J Immunol
164:1925-1933; Xu et al., 2000, Cell Immunol 200:16-26; idusogie et al, 2001,3
Immunol
166:2571-2575; Shields etal., 2001, 3 Biol Chem 276:6591-6604; Jefferis et al,
2002, Immunol
Lett 82:57-65; Presta et al., 2002, Biochem Sege Trans 30:487-490); U.S.
Patent Nos. 5,624,821;
5,885,573; 5,677,425; 6,165,745; 6,277,375; 5,869,046; 6,121,022; 5,624,821;
5,648,260;
6,528,624; 6,194,551; 6,737,056; 6,821,505; 6,277,375; U.S. Patent Publication
Nos.
2004/0002587 and PCT Publications WO 94/29351; WO 99/58572; WO 00/42072; WO
021060919; WO 041029207; WO 04/099249; WO 041063351. Also encompassed by the
present
disclosure are Pc regions which comprise deletions, additions and/or
modifications. Still other
.modifications/substitutions/additions/deletions of the Pc domain will be
readily apparent to one
skilled in the art.
[00299j Methods for
generating non naturally occurring Pc regions are known in the art.
For example, amino acid substitutions and/or deletions can be generated by
mulagenesis
methods, including, but not limited to, site- directed mutagenesis (Kunkel,
Proc. Natl. Acad. Sri.
USA 82:488-492 (1985)). PCR mutagenesis (Higuchi, in "PCR Protocols: A Guide
to Methods
and Applications", Academic Press, San Diego, pp. 177-183(1990)), and cassette
mutagenesis
(Wells et al., Gene 34:315-323 (1985)). Preferably, site-directed mutagenesis
is performed by the
overlap-extension PCR method (Higuchi, in "PCR Technology: Principles and
Applications for
DNA Amplification", Stockton Press, New York, pp. 61-70(1989)). The technique
of overlap-
extension PCR (Higuchi, ibid.) can also be used to introduce any desired
mutation(s) into a target
sequence (the starting DNA). For example. the first round of PCR in the
overlap- extension
method involves amplifying the target sequence with an outside primer (printer
.1) and an
internal mutagenesis primer (primer 3), and separately with a second outside
printer (primer 4)
and an internal primer (printer 2), yielding two PCR segments (segments A and
B). The internal
mutagenesis primer (primer 3) is designed to contain mismatches to the target
sequence
83
CA 02743469 2016-06-13
= 51332-100
specifying the desired mutation(s). In the second round of PCR, the products
of the first round of
PCR (segments A and .13) are amplified by VCR. using the two outside primers
(primers 1 and 4).
The resulting full-length PCR segment (segment C) is digested with restriction
enzymes and the
resulting restriction fragment is cloned into an appropriate vector. As the
first step of
motagenesis, the starting DNA (e.g., encoding an Fe fusion protein, an
antibody or simply an Fe
region), is operably cloned into a minagenesis vector. The primers are
designed to reflect the
desired amino acid substitution. Other methods useful for the generation of
variant Fc regions
are known in the art (see, e.g., U.S. Patent Nos. 5,624,821; 5,885,573;
5,677,425: 6,165,745;
6,277,375; 5,869,046; 6,121,022; 5,624,821; 5,648,260; 6,528,624; 6,194,551;
6,737,056;
6,821,505; 6,277,375; U.S. Patent Publication Nos, 2004/0002587 and PCT
Publications WO
94/29351; WO 99/58572; WO 0(1/42072; WO 02/060919; WO 04/029207; WO 04/099249;
WO
04/063351).
[003001 In some embodiments, an Fc variant protein comprises one or
more engineered
glycoforms, i.e., a carbohydrate composition that is COS' Went:- attached to
the molecule
comprising an Fe region. Engineered glycoforms may be useful for a variety of
purposes,
including but not limited to enhancing or reducing effector function.
Engineered glycofortns may
be generated by any method known to one skilled in the art, for example by
using engineered or
variant expression strains, by co-expression with one or more enzymes, for
example DI N-
acetylglucosaminyltransferase HI (cinTI1 1), by expressing a molecule
comprising an Fc region
in various organisms or cell lines from various organisms, or by modifying
carbohydrate(s) after
the molecule comprising Fc region has been expressed. Methods for generating
engineered
glycoforms are known in the art, and include but are not limited to those
described in Umana et
al, 1.999, Nat. Biotechnol 17:176-180; Davies et al., 20017 Biotechnol Bioeng
74:288-294;
Shields et al, 2002, J Biol Chem 277:26733-26740; Shinkawa et al.,, 2003, J
Biol Chem
278:3466-3473) U.S. Pat No 6,602,684; U.S. Patent Application Publication US
2003/0157108 Al;
U.S. Patent Application Publication US 2003/0003097A1; PCT WO 00/61739A1;
PCT WO 01/292246A1; PCT WO 02/311140A1; PCT WO 02/30954A1; Potelligentrm
technology
(Biowa, Inc. Princeton, N.J.); GlycoMAbrm gIycosylation engineering technology
(GLYCART
.biotechnology AG, Zurich, Switzerland). See. e.g., WO 00061739; EA01229125;
US 20030115614;
Okazaki et al., 2004, JMB, 336: 1239-49.
5.1.5. Glycosylation Of Antibodies
[003011 In still another embodiment, the ,glycosylation of antibodies
utilized in accordance
with the disclosure is modified_ For e.xample, an aglycosylated antibody can
be made (i.e., the
antibody lacks alycosylation). Glycosylation can be altered to, for example,
increase the affinity
of the antibody for a target antigen. Such carbohydrate modifications can be
accomplished by.
84
CA 02743469 2016-06-13
51332-100
for example, altering one or more sites of glycosylation within the antibody
sequence. For
example, one or more amino acid substitutions can be made that result in
elimination of one or
more variable region framework glycosylation sites to thereby eliminate
glycosylation at that
site. Such aglycosylation may increase the affinity of the antibody for
antigen. Such an
approach is described in further detail in U.S. Patent Nos. 5,714,350 and
6,350,861. One or
more amino acid substitutions can also be made. that result in elimination of
a glycosylation site
present in the Fc retort. (e.g.. Aspararj..ne 297 of IgG). Fu.rthennore,
aglycosylated antibodies
may be produced in bacterial cells which lack the necessary glycosylation
machinery.
1003021 An. antibody can also be made that has an altered type of
glycosylation, such as a
hypofucosylated antibody having reduced amounts of fucosyl residues or an
antibody having
increased bisecting GicNAc structures. Such altered elycosylatio.n patterns
have been
demonstrated to increase the ADCC ability of antibodies. Such carbohydrate
modifications can
be accomplished by, for example, expressing the antibody in a host cell with
altered
glycosylation machinery. Celts with altered glycosylation machinery have been
described in the
art and can be used as host cells in which to express recombinant antibodies
of the disclosure to
thereby produce an antibody with altered glycosylation. See, for example,
Shields, P.L. et al.
(2002)1. Biol. Chem. 277:26733-26740; Umana et al. (1999) Nat. Biotech. 17:176-
1, as well as,
'U.S. Patent No: US 6,946,292; European Patent No: EP 1,176,195; PCT
Publications WO
03/035835: WO 99/54342.
1003031 Antibodies with altered glycosylation pattern may also be
generated using lower
eukaryotic host cells comprising modified glycosylation machinery as described
in U.S. Patent
No. US7029872, US Patent Publication US20060148035AI.
5.16. Engineering Effector Function
1003041 It may be desirable to modify an anti-ICOS antibody of the
disclosure with
respect to effector function, so as to enhance the effectiveness of the
antibody in treating T cell-
mediated diseases, for example. For example, cysteine residue(s) may be
introduced in the Fc
region, thereby allowing interchain disulfide bond formation in this region.
The homodimeric
antibody thus generated may have improved internalization capability and/or
increased
complement-mediated cell killing and/or antibody-dependent cellular
cytoinxicity (ADCC)
and/or antibody dependent phagocytosis. See, Caron et Exp
176:1191-1.195 (1.992)
and Shopes, B., 1.48:2918-2922(1992). Homodimeric antibodies with
enhanced
anti-tumor activity may also be prepared using heterobifunctional cross-
linkers as described in
Wolff et al., Cancer Research, 53:2560-2565 (1993). An antibody can also be
engineered Which
CA 02743469 2016-06-13
- 51332-100
has dual Fc regions and may thereby have enhanced. complement lysis, antibody-
dependent
pliagocytosis and/or ADCC capabilities. See, Stevenson et al.,..4nti-Cancer
Drug Design, 1219-
230 (1989).
1003051 Other methods of engineering Fe regions of antibodies so as to
alter effector
functions are known in the art (e.g., U.S. Patent Publication No. 20040/85045
and PCT
Publication No. WO 2004/016750, both to Koenig et al., which describe altering
the Fc region to
enhance the binding affinity for Fcy1/1113 as compared with the binding
affinity for F0yRII.A;
see, also, PCT Publication Nos, WO 99/58572 to Armour et al., WO 99/51642 to
Edusogie curL.
and U S 6,395,272 to Deo et al.). Methods of modifying the Fc region to
decrease binding affinity
to FcyRIIB are also known in the art (e.g., U S. Patent Publication No
20010036459 and
PCT Publication No. WO 01/79299, both to Ravetch et al.). Modified antibodies
having variant
Fc regions with enhanced binding affinity for FcyRIIIA and/or FcyRIIA as
compared with a
= wildtype Fc region have also been described (e.g., PCT Publication Nos.
WO 2004/063351, to
Stavenhagen et al.).
t003061 In vitro assays known in the art can be used to determine
whether anti-ICOS
antibodies used in compositions and methods of the disclosure are capable of
mediating ADCC,
CDC, and/or antibody-depenedent phagocytosis, such as those described herein.
5.17. Manufacture/Production of Anti-ECOS Antibodies
[003071 Once a desired anti-1COS antibody is engineered, the anti-ICOS
antibody can be
produced on a commercial scale using methods that are well-known in the art
for large scale
manufacturing of antibodies. For example, this can be accomplished using
recombinant
expressing systems such as, but not limited to, those described below. The
antibodies (including
antibody fragments thereof) that specifically bind to an. antigen can be
produced by any method
known in the art for the synthesis of antibodies, in particular, by chemical
synthesis or by
recombinant expression techniques (see, US Patent Application 12/116,512).
5.18. Recombinant Expression Systems
1003081 Recombinant expression of an antibody or variant thereof,
generally requires
construction of an apression vector containing a polynucleotide that encodes
the antibody.
Once a polynucleotide encoding an antibody molecule or a heavy or light chain
of an antibody,
or portion thereof, has been obtained, the vector for the production of the
antibody molecule may
86
CA 02743469 2011-05-11
WO 2010/056804
PCTIUS2009/064127
be produced by recombinant DNA technology using techniques well-known in the
art. See, e.g..
U.S. Patent No. 6,331,415, which is incorporated herein by reference in its
entirety. Thus,
methods for preparing a protein by expressing a polynucleotide containing an
antibody encoding
nucleotide sequence are described herein. Methods which are well-known to
those skilled in the
art can be used to construct expression vectors containing antibody coding
sequences and
appropriate transcriptional and translational control signals. These methods
include, for
example, in vitro recombinant. DNA techniques, synthetic techniques, and in
viw) genetic
recombination. The disclosure, thus, provides replicable vectors comprising a
nucleotide
sequence encoding an antibody molecule, a heavy or light chain of an antibody,
a heavy or light
chain variable domain of an antibody or a portion thereof, or a heavy or light
chain CDR,
operably linked to a promoter. Such vectors may include the nucleotide
sequence encoding the
constant; region of the antibody molecule (see, e.g., International
Publication Nos. WO 86/05807
and WO 89/01036; and U.S. Patent No. 5,122.464) and the variable domain of the
antibody may
be cloned into such a vector for expression of the entire heavy, the entire
light chain, or both the
entire heavy and light chains.
1003091 In another embodiment; ariti-ICOS antibodies can be made using
targeted
homologous recombination to produce all or portions of the anti-ICOS
antibodies (see, U.S.
Patent Nos. 6,063,630, 6,187,305, and 6,692,737). In certain embodiments, anti-
ICOS
antibodies can be made using random recombination techniques to produce all or
portions of the
anti-ICOS antibodies (see, U.S. Patent Nos. 6,361,972, 6,524,818, 6,541,221,
and 6,623,958).
Anti-ICOS antibodies can also be produced in cells expressing an antibody from
a genomic
sequence of the cell comprising a modified immunoglobulin locus using Cre-
mediated site-
specific homologous recombination (see, U.S. Patent No. 6,091,001). The host
cell line may be
derived from human or nonhuman species including but not limited to mouse, and
Chinese
hamster. Where human or humanized antibody production is desired, the host
cell line should be
a human cell line. These methods may advantageously be used to engineer stable
cell lines
which permanently express the antibody molecule.
[00310j Once the expression vector is transferred to a host cell by
conventional
techniques, the transfected cells are then cultured by conventional techniques
to produce an
antibody. Thus, the disclosure includes host cells containing a polynucleotide
encoding an
antibody of the disclosure or fragments thereof, or a heavy or light chain
thereof, or portion
thereof, or a single-chain antibody of the disclosure, operably 'linked to a
heterologous promoter.
In certain embodiments for the expression of double-chained antibodies,
vectors encoding both
the heavy and light chains may be co-expressed in the host cell for expression
of the entire
immunoglobulin molecule, as detailed below.
87
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[003111 A -variety of host-expression vector systems may be utilized to
express an anti-
1COS antibody or portions thereof that can be used in the engineering and
generation of anti-
1COS antibodies (see, e.g., U.S. Patent No. 5,807,715). For example, mammalian
cells such as
Chinese hamster ovary cells (CHO), in conjunction with a vector such as the
major intermediate
early gene promoter element from human cytomegalovirus is an effective
expression system for
antibodies (Foeckina c/ al.. Gene, 45:101 (1986); and Cockett etal.,
BiotTechnology, 8;2
(1990)). In addition, a host cell strain may be chosen which modulates the
expression of inserted
antibody sequences, or modifies and processes the antibody tame product in the
specific fashion
desired. Such modifications (e.g., glyaxsylation) and processing (e.g,
cleavage) of protein
products may be important for the function of the protein. Different host
cells have characteristic
and specific mechanisms for the post-translational processing and modification
of proteins and
gene products. Appropriate cell lines or host systems can be chosen to ensure
the correct
modification and processing of the antibody or portion thereof expressed. To
this end,
eukaryotic host cells which possess the cellular machinery for proper
processing of the primary
transcript, alycosylation, and phosphorylation of the gene product may be
used. Such
mammalian host cells include but are not limited to CHO, VERY, BHK, Hela, COS,
MDCK,
293, 3T3, WI 38. BT483, Hs578T, HTB2, BT20 and T47D, NSO (a murine myeloma
cell line
that does not endogenously produce any functional immunoglobulin chains),
CRL7030 and
lisS78Bst cells.
1003121 In one embodiment, human cell lines developed by immortalizing
human
lymphocytes can be used to recombinantly produce monoclonal human anti-1COS
antibodies. In
one embodiment, the human cell line PER.C6. (Cnicell, Netherlands) can be used
to
recombinantly produce monoclonal human anti-ICOS antibodies.
1003131 In bacterial systems, a number of expression vectors may be
advantageously
selected depending upon the use intended for the antibody molecule being
expressed. For
example, when a large quantity of such an antibody is to be produced, for the
generation of
pharmaceutical compositions comprising an ami-ICOS antibody, vectors which
direct the
expression of high levels of fusion protein products that are readily purified
may be desirable.
Such vectors include, but are not limited to, the E. coif expression vector
pUR278 (Ruther ei at,
EMBO, 12:1791 (1983)), in which the antibody coding sequence may be ligated
individually into
the vector in frame with the lac Z coding region so that a fusion protein is
produced.; p.1N vectors
(Inouye & Inouye, 198.5,Nueleic Acids Res. 13:3101-3109 (1985); Van .Heeke &
Schuster, 1989,
Bid (hem., 24:5503-5509(1989)); and the like. pC1EX vectors may also be used
to express
.foreign -polypeptides as fusion proteins with glutathione-S-transferase
((1ST). In general, such
fusion proteins are soluble and can easily be purified from lysed cells by
adsorption and binding
88
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
to glutathione-agarose affinity matrix followed by elution in the presence of
free glutathione.
The pGEX vectors are designed to introduce a thrombin and/or factor Xa
protease cleavage sites
into the expressed polypeptide so that the cloned target gene product can be
released from the
GST moiety.
[003141 In an insect system, Atatwapha ctilijitrnico nuclear polyhedrosis
virus (AcNPV)
is used as a vector to express foreign genes. The virus grows in
...Vockipterafrugiperdo cells.
The antibody coding sequence may be cloned individually into non-essential
regions (for
example, the polyhedrin gene) of the virus and placed under control of an
AcNPV promoter (for
example, the polyhedrin promoter).
1003151 In mammalian host cells, a number of virus based expression systems
may be
utilized In cases where an adenovirus is used as an expression vector, the
antibody coding
sequence of interest may be ligated to an adenovirus transcription/translation
control complex,
e.g, the late promoter and tripartite leader sequence. This chimeric gene may
then be inserted in
the adenovirus genome by in vitro or in vivo recombination. Insertion into a
non-essential region
of the viral genome (e.g., region El or E3) will result in a recombinant virus
that is viable and
capable of expressing the antibody molecule in infected hosts (e.g., see,
Logan 8µ. Shenk, Proc.!.
Nat!. Acad. Sri USA, 81:355-359 (1984)). Specific initiation signals may also
be required for
efficient translation of inserted antibody coding sequences. These signals
include the ATG
initiation codon and adjacent sequences. Furthermore, the initiation codon
should generally be
in frame with the reading frame of the desired coding sequence to ensure
translation of the entire
insert. These exogenous translational control signals and initiation codons
can be of a variety of
origins, both natural arid synthetic. The efficiency of expression may be
enhanced by the
inclusion of appropriate transcription enhancer elements, transcription
terminators, etc. (see, e.g,
Bitiner et al.. Methods in Enzymol., .153:51-544(1987)).
1003161 Stable expression can be used for long-term, high-yield production
of
recombinant proteins. For example, cell lines which stably express the
antibody molecule may
be generated. Host cells can be transformed with an appropriately engineered
vector comprising
expression control elements (e.g. promoter, enhancer, transcription
terminators,
polyadenylation sites, etc.), and a selectable marker gene. Following the
introduction of the
foreign DNA, cells may be allowed to grow for 1-2 days in an enriched media,
and then are
switched to a selective media. The selectable marker in the recombinant
plasmid confers
resistance to the selection and allows cells that stably integrated the
plasmid into their
chromosomes to grow and form foci which in turn can be cloned and expanded
into cell lines.
Plasmids that encode an anti-ICOS antibody can be used to introduce the
geriecDNA into any
cell line suitable for production in culture.
89
CA 02743469 2016-06-13
s 5 1 3 32-1 00
1003171 A number of selection systems may be used, including, but. not
limited to, the
herpes simplex virus thymidine kit:lase (Wittier et al., Cell, 11:223 (1
977)), hypoxanthineguanine
phosphoribosyhransferase (Szybalska & Szybalski, Proc. Not/, Acad. Sc!. UM,
48:202 (1992)),
and adenine phosphoribosyltransferase (Lowy et (11,, Cell, 22:8-17 (1980))
genes can be
employed in tIC. hgprf or apercells, respectively. Also, -tmtimetabolite
resistance can. be used as
the basis of selection for the following genes: &tit', which confers
resistance to methotrexate
(Wigler e.t Natl. Acad. Sci. USA, 77:357 (1980); O'Hare et al, Proc. Nail.
Axil SW, USA,
78:1527 (1981)); gpt, which confers resistance to mycophenolic acid (Mulligan
& Berg, Proc.
Nag Acid Sc!. USA, 78:2072 (1981)); neo, which confers resistance to the
aminoglycoside G-
418 (Wu and Wu, Biorheropy 3;87-95 (1991); Tolstoshev. Ann. Rev. Pharmacol.
32:573-596(1993); Mulligan, Science 260:926-932 (1993); and Morgan and
Anderson, Ann.
Rev. Biochem. 62:191-217 (1993); May, T113 TECH 11(5):155-2 15 (1993)); and
hygro, which
confers resistance to hyeromycin (Santerre et al.. Gene, 30:147 (1984)),
Methods commonly
known in the art of recombinant DNA technology may be routinely applied to
select the desired
recombinant clone, and such methods are described, for example, in Ausubel el
al. (eds.),
Current Protocols in Molecular Biology, John Wiley & Sons, NY (1993);
Kriegler, Gene
Transfer and Expression, A Laboratory Manual, Stockton Press, NY (1990); and
in Chapters 12
and 13. Draccpcill ei at (eds.), Current Protocols in Human Genetics, John
Wiley & Sons, NY
(1994); Colberre-Garapin Cl al., 1981, /Yr& Biol., 150:1.
[003181 The expression levels of an antibody molecule can be increased
by vector
amplification (for a review, see, Bebbington and Hentschel, The use of vectors
based on gene
ampiffication for the expression of cloned genes in mammalian cells in DNA
cloning, Vol. 3.
Academic Press, New York (1987)). When a ,marker in the vector system
expressing antibody is
amplifiable, increase in the level of inhibitor present in culture of host
cell will increase the
number of copies of the marker gene. Since the amplified region is associated
with the antibody
gene, production of the antibody will also increase (Crouse et al, Mai. Cell.
Biol., 3;257 (1983)).
Antibody expression levels may be amplified through the use recombinant
methods and tools
known to those skilled in the art of recombinant protein production, including
technologies that
remodel surrounding chromatin and enhance transeene expression in the form of
an active
artificial transcriptional domain_
1003191 The host cell may be co-transfected with two expression
vectors, the first vector
encoding a heavy chain derived po/ypeptide and the second vector encoding a
light chain derived
polypeptide. The two vectors may contain identical, or different selectable
markers. A single
vector which encodes, and is capable of expressing, both heavy and light chain
polypeptid.es may
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
also be used. In such situations, the light chain should be placed 5' to the
heavy chain to avoid
an excess of toxic free heavy chain (Proudfoot. Nature 322:562-65 (1986); and
Kohler, 1980,
Pro'. . Natl. Acad. Set. USA, 77:2197 (1980)). The coding sequences for the
heavy and light
chains may comprise cDNA or genomie DNA.
1003201 Once an antibody molecule has been produced by recombinant
expression, it may
be purified by any method known in the art for purification of an
immunoglobulin molecule. for
example, by chromatography (e.g., ion exchange, affinity, particularly by
affinity for the specific
antigens Protein A or Protein CI, and sizing column chromatography),
centrifugation, differential
solubility, or by any other standard technique for the purification of
proteins. Further, the
antibodies of the present disclosure or fragments thereof may be fused to
heterologous
poly peptide sequences described herein or otherwise known in the art to
facilitate purification.
5.19. Antibody Purification And Isolation
1003211 When using recombinant techniques, the antibody can be produced
intrac,ellularly,
in the periplastnic space, or directly secreted into the medium. lithe
antibody is pmduced
iniracelltil arty, as a first step, the particulate debris, either host cells
or lysed fragments, is
removed, for example, by centrifugation or ultrafiltration. Carter et at,
BialTechnology, 10:163-
167 (1992) describe a procedure for isolating antibodies which are secreted
into the peiiplasmie
space of E coil. Briefly, cell paste is thawed in the presence of sodium
acetate (pH 3.5), EDTA,
and phenylmethylsulfonylfluoride (PMSF) over about 30 min. Cell debris can be
removed by
centrifugation. Where the antibody mutant is secreted into the medium,
supernatants from such
expression systems are generally first concentrated using a commercially
available protein
concentration filter, for example, an .Amicon or Millipore Pellicon
ultmfiltration unit. A protease
inhibitor such as PMSF may be included in any of the foregoing steps to
inhibit proteolysis and
antibiotics may be included to prevent the growth of adventitious
contaminants.
[003221 The antibody composition prepared from the cells can be purified
using, for
example, hydroxylapafite chromatography, hydrophobic interaction
chromatography, ion
exchange chromatography, gel electrophoresis, dialysis, and/or affinity
chromatography either
alone or in combination with other purification steps. The suitability of
protein A as an affinity
ligand depends on the species and isotype of any immunoglobulin Fe domain that
is present in
the antibody mutant. Protein A can be used to purify antibodies that are based
on human yl, 72,
or y 4 heavy chains (Lindrnark etal.,J. Immunal. Methods, 62:1-13 (.1983)).
Protein 0 is
recommended for all mouse isotypes and for human y3 (Cuss eral.. 1914130 .1.,
5:15671575
(1986)). The matrix to which the affinity ligand is attached is most often
agarose, but other
matrices are available. Mechanically stable matrices such as controlled pore
glass or
91
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
poly(styrenedivinyl)benzene allow for faster flow rates and shorter processing
times than can be
achieved with agarose. Where the antibody comprises a CH 3 domain, the
Bakerbond ABX resin
(J.T. Baker, Phillipsburg, NJ) is useful for purification. Other techniques
for protein purification
such as fractionation on an ion-exchange column, ethanol precipitation,
Reverse Phase IIPLC,
chromatography on silica, chromatography on heparin, SEPHAROSE chromatography
on an
anion or cation exchange resin (such as a polyaspartic acid column),
chromatofocusing, SOS-
PAGE, and ammonium sulfate precipitation are also available depending on the
antibody to be
recovered.
1003231 Following any preliminary purification step(s), the mixture
comprising the
antibody of interest and contaminants may be subjected to low pH hydrophobic
interaction
chromatography using an elution butler at a pH between about 2.5-4.5, and
performed at low salt
concentrations (e.g., from about 0-0.25 M salt).
5.20. Therapeutic Anti-ICOS Antibodies
1003241 An anti-ICOS antibody used in compositions and methods of the
disclosure may
be a human antibody or ahurnanized antibody that may mediate T cell lineage
ADCC, antibody-
dependent phagocytosis and/or CDC, or can be selected from known anti-ICOS
antibodies that
may mediate T lineage cell ADCC, antibody-dependent phagocytosis and/or CDC.
In certain
embodiments, anti-ICOS antibodies can be chimeric antibodies. In certain
embodiments, an
ariti4COS antibody can be a monoclonal human humanized, or chimeric an ti-ICOS
antibody.
An anti-ICOS antibody used in compositions and methods of the disclosure may
be a human
antibody or a humanized antibody of the Igal or 1143 human isotype or any
IgC11 or IgC13 allele
found in the human population. In other embodiments, an anti-ICOS antibody
used in
compositions and methods of the disclosure can be a. human antibody or a
humanized antibody
of the IgG2 or 104 human isotype or any 402 or IgG4 allele found in the human
population.
[00325i While such antibodies can he generated using the techniques
described above, in
other embodiments of the disclosure, the human IMab-136 anti-ICOS antibody
(see, US Patent
6,803,039) can be modified to generate an anti-ICOS antibody with enhanced
effector function
such as. but not limited to, ADCC, antibody-dependent phagocytosis and/or CDC.
For example,
known anti-ICOS antibodies that can be used include, but are not limited to,
anti-human ICOS
monoclonal antibodies disclosed in US Patent 6,803,039, and clone ISA-3
(eBioscience, US).
1003261 In certain embodiments, the antibody is an isotype switched variant
of a known
antibodv (e.g., to an IgGI or IgG3 human isotype) such as those described
above.
[003271 An anti-ICOS antibodies used in compositions and methods of the
disclosure can
be naked antibodies, immunoconjugates or fusion proteins. Anti-ICOS antibodies
described
92
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
above for use in compositions and methods of the disclosure may be able to
reduce or deplete
ICOS expressing I cells and circulating immunoglobulirt in a human treated
therewith.
Depletion of T cells can be in circulating T cells, or in particular tissues
such as, but not limited
to, bone marrow, spleen, gut-associated lymphoid tissues, andior lymph nodes.
Such depletion
may be achieved via various mechanisms such as antibody-dependent cell-
mediated cytotoxicity
(ADCC), and/or antibody dependent phagocytosis, and/or by blocking of !COS
interaction with
its intended ligand, and/or complement dependent cytotoxicity (CDC). By
"depletion" of T cells
it is meant a reduction in circulating ICOS expressing I cells and/or 1COS
expressing T cells in
particular tissue(s) by at least about 25%, 40%, 50%, 65%, 75%. 80%, 85%, 90%,
95% or more.
in particular embodiments, virtually all detectable "COS expressing T cells
are depleted from the
circulation and/or particular tissue(s). By "depletion" of circulating
iirmiunoglobulin (Ig) it is
meant a reduction by at least about 25%, 40%, 50%, 65%, 75%, 80%, 85%, 90%,
95% or more.
In particular embodiments, virtually all detectable Ig is depleted from the
circulation.
5.21. Screening Of Antibodies For Human !COS Binding
1003281 Binding assays can be used to identify antibodies that bind the
human ICOS
antigen. Binding assays may be performed either as direct binding assays or as
competition-
binding assays. Binding can be detected using standard ELISA or standard Flow
(..ytometty
assays. In a direct binding assay, a candidate antibody is tested for binding
to human !COS
antigen. In certain embodiments, the screening assays comprise, in a second
step, determining
the ability to of an antibody to induce downstream signaling events in T cells
expressing human
ICOS. Competition-binding assays, on the other hand, assess the ability of a
candidate antibody
to compete with a known anti-ICOS antibody or other compound that binds human
ICOS.
1003291 In a direct binding assay, the human ICOS antigen is contacted with
a candidate
antibody under conditions that allow binding of the candidate antibody to the
human ICOS
antigen. The binding may take place in solution or on a solid surface. The
candidate antibody
may have been previously labeled for detection. Any detectable compound can be
used for
labeling, such as ,but not limited to, a luminescent, -fluorescent, or
radioactive isotope or group
containing same, or a nonisotopic label, such as an enzyme or dye. After a
period of incubation
sufficient for binding to take place, the reaction is exposed to conditions
and manipulations that
remove excess or non-specifically bound antibody. Typically, it involves
washing with an
appropriate buffer. Finally, the presence of an ICOS-antibody complex is
detected.
1003301 In a competition-binding assay, a candidate antibody is evaluated
tbr its ability to
inhibit or displace the binding of a known anti-ICOS antibody (or other
compound) to the human
ICOS antigen. A labeled known binder ofiCOS may be mixed with the candidate
antibody, and
9:1
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
placed under conditions in which the interaction between them would normally
occur, with and
without the addition of the candidate antibody. The amount of labeled known
binder of !COS
that binds the human ICOS may be compared to the amount bound in the presence
or absence of
the candidate antibody.
1003311 In one embodiment. the binding assay is carried out with one or
more components
immobilized on a solid surface to facilitate antibody antigen complex
formation and detection.
In VariOUS embodiments, the solid support could be, but is not restricted to,
poly vinylidene
fluoride ,polycarbonate, polystyrene, polypropylene, polyethylene, glass,
nitrocellulose, dextran,
nylon, polyacrylamide and agarose. The support configuration can include
beads, membranm,
microparticles, the interior surface of a reaction vessel such as a microliter
plate, test tube or
other reaction vessel. The immobilization of human ICOS, or other component,
can be achieved
through covalent or non-covalent attachments. In one embodiment, the
attachment may be
indirect, i.e., through an attached antibody. In another embodiment, the human
ICOS antigen
and negative controls are tagged with an epitope, such as glutathione S-
transferase ((IST) so that
the attachment to the solid surface can be mediated by a commercially
available antibody such as
anti-OST (Santa Cruz Biotechnology).
1003321 For example, such an affinity binding assay may be performed using
the human
ICOS antigen which is immobilized to a solid support. Typically, the non-
mobilized component
of the binding reaction, in this case the candidate anti-ICOS antibody, is
labeled to enable
detection. .A variety of labeling methods are available and may be used, such
as luminescent,
chromophore, fluorescent, or radioactive isotope or group containing same, and
nonisotopic
labels, such as enzymes or dyes. In one embodiment, the candidate anti-ICOS
antibody is
labeled with a .fluorophore such as fluorescein isothiocyanate (FITC,
available from Sigma
Chemicals, St. Louis). Such an affinity binding assay may be performed using
the human ICOS
antigen immobilized on a solid surface. Anti-ICOS antibodies are then
incubated with the
antigen and the specific binding of antibodies is detected by methods known in
the art including,
but not limited to, BiaCore Analyses, FLISA, FMET and RIA methods.
1003331 Finally, the label remaining on the solid surface may be detected
by any detection
method known in the art. For example, if the candidate anti-ICOS antibody is
labeled with a
fluorophore, a fluotimeter may be used to detect complexes.
1003341 The human ICOS antigen can be added to binding assays in the form
of intact
cells that express human ICOS antigen, or isolated membranes containing human
ICOS antigen.
Thus, direct binding to human ICOS antigen may be assayed in intact cells in
culture or in
animal models in the presence and absence of the candidate anti-ICOS antibody.
A labeled
candidate anti-ICOS antibody may be mixed with cells that express human ICOS
antigen, or
94
CA 02743469 2011-05-11
WO 2010/056804 PCTIUS2009/064127
with crude extracts obtained from such cells, and the candidate anti-ICOS
antibody may be
added. Isolated membranes may be used to identify candidate anti-ICOS
antibodies that interact
with human !COS. For example, in a typical experiment using isolated
membranes, cells may be
genetically engineered to express human ICOS antigen. Membranes can be
harvested by
standard techniques and used in an in Wm binding assay. Labeled candidate anti-
ICOS antibody
(e.g, fluorescent labeled antibody) is bound to the membranes and assayed for
specific activity;
specific binding is determined by comparison with binding assays performed in
the presence of
excess unlabeled (cold) candidate anti-ICOS antibody. Soluble human ICOS
antigen may also
be recombinantly expressed and utilized in non-cell based assays to identify
antibodies that bind
to human ICOS antigen. The recombinantly expressed human ICOS polypeptides can
be used in
the non-cell based screening assays. Peptides corresponding to one or more of
the binding
portions of human ICOS antigen, or fusion proteins containing one or more of
the binding
portions of human ICOS antigen can also be used in non-cell based assay
systems to identify
antibodies that bind to portions of human ICOS antigen. In non-cell based
assays the
recombinantly expressed human ICOS is attached to a solid substrate such as a
test tube,
microtiter well or a column, by means well-known to those in the art (see.
Ausubel et 01., supra).
The test antibodies are then assayed for their ability to bind to human ICOS
antigen.
1003351 The binding reaction may also be carried out in solution. In this
assay, the labeled
component is allowed to interact with its binding partner(s) in solution. If
the size differences
between the labeled component and its binding partner(s) permit such a
separation, the
separation can be achieved by passing the products of the binding reaction
through an uhrafilter
whose pores allow passage of unbound labeled component but not of its binding
partner(s) or of
labeled component bound to its partner(s). Separation can also be achieved
using any reagent
capable of capturing a binding partner of the labeled component from solution,
such as an
antibody against the binding partner and so on.
1003361 In another specific embodiment, the solid support is membrane
containing human
ICOS antigen attached to a microti ter dish. Candidate antibodies, for
example, can bind cells
that express library antibodies cultivated under conditions that allow
expression of the library
members in the microliter dish. Library members that bind to the human ICOS
are harvested.
Such methods, are generally described by way of example in Parmley and Smith,
1988, Gene,
73:305-31.8; Fowlke,s etal.. 1992, BioTeehniques, .13:422-427; PCT Publication
No.
W094/1831 8; and in references cited hereinabove. Antibodies identified as
binding to human
1COS antigen can be of any of the types or modifications of antibodies
described above.
91
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
5.21.1. Screening Of Antibodies For Human ADCC Effector Function
M03371 Antibodies of the human IgG class, which have functional
characteristics such a
long half-life in serum and the ability to mediate various effector functions
are used in certain
embodiments of the disclosure (Monoclonal Antibodies; Principles and
.Applications, Wiley-
Liss, Inc., Chapter 1 (1)95)). The human IgG class antibody is farther
classified into the
following 4 subclasses: 1gal 1g02,1g03 and IgG4. A large number of studies
have so far been
conducted for ADCC and CDC as effector functions of the lgG class antibody,
and it has been
reported that among antibodies of the human IgG class, the IgG1 subclass has
the highest ADCC
activity and CDC activity in humans (Chemical Immunology, 65, 88 (1997)).
(00338) Expression of ADCC activity and CDC activity of the human 1g01
subclass
antibodies generally involves binding of the Fc region of the antibody to a
receptor for an
antibody (hereinafter referred to as "Fe)'R") existing on the surface of
effector cells such as killer
cells, natural killer cells or activated macrophages. Various complement
components can be
bound. Regarding the binding, it has been suggested that several amino acid
residues in the
hinge region and the second domain of C region (hereinafter referred to as
"Cy2 domain") of the
antibody are important (Eur. J. Immunot , 23,1098 (1993), Immunology, 86, 319
(1995),
Chemical Immunology, 65, 88 (1997)) and that a sugar chain in the Cy2 domain
(Chemical
Immunology, 65, 88 (1997)) is also important.
1003391 Anti-K:OS antibodies can be modified with respect to effector
function, e.g., so as
to enhance ADCC and/or complement dependent cytotoxicity (CDC) of the
antibody. This may
be achieved by introducing one or more amino acid substitutions in the Fe
region of an antibody.
Cysteine residue(s) may also be introduced in the Fe region, allowing for
interchain disulfide
bond formation in this region. In this way a homodime,ric antibody can be
generated that may
have improved internalization capability and or increased complement-mediated
cell killing and
ADCC (Caron el al, Exp Med., 176:1191-1195 (1992) and Shapes,
Immunol.,148:2918-
2922 (1992)). Heterobifunctional cross-linkers can also be used to generate
homodimeric
antibodies with enhanced anti-tumor activity (Wolff et al, Cancer Research,
53:2560-2565
(1993)). Antibodies can also be engineered to have two or more Fc regions
resulting in
enhanced complement lysis and ADCC capabilities (Stevenson et al, Ann-Cancer
Drug Design,
(3)219-230 (1989)).
(00340J Other methods of engineering Fe regions of antibodies so as to
alter effector
functions are known in the art (e.g., U.S. Patent Publication No. 20040185045
and PCT
Publication No. WO 2004/016750, both to Koenig et al., which describe altering
the Fe region to
enhance the binding affinity for FeyRIIB as compared with the binding affinity
for FCIRIIA; see
also PCT Publication Nos. WO 99/58572 to Armour et at WO 99/51642 to ldusogie
ei al., and
96
CA 02743469 2016-06-13
s 51332-100
U.S. 6,395,272 to Deo et aL the disclosures of which are incorporated herein
in their entireties).
Methods of modifying the fc region to decrease binding affinity to FeyRIIB are
also known in
the art (e.g., U.S. Patent Publication No, 20010036459 and PCT Publication No.
WO 01179299,
both to .Ravetch
Modified antibodies having variant Fc regions with enhanced binding affinity
for
and/or Fey.RIIA as compared with a. wildtype Fe region have also been
described (e.g., PCT
Publication No. WO 20041063351, to Stavenhagen et' al).
1003411 At least four different types of Feyll. have been found, which
are respectively
called Fey RI (CD64), FeyRII ((I)32), FcyR11.1 (CD16), and Fc7R1.V. In human,
FcyRIE and
FcyRlIl are 'further classified into Fe-,eRlia and FcylITIli, and FcyR11Ia.
and FcTR11.11). respectively.
FcliR is a membrane protein belonging to the immunoglobulin superfamily,
Fc1RiI, FcTRIII, and
FeyRIV have an a chain having an extracellular region containing two
imminoglobillin-like
domains, FcyR1 has an a. chain having an extracellulat region containing three
immunottlobulin-
like domains, as a constituting component, and the a chain is involved in the
IgG binding
activity. In addition, Fc'yRi and FcyRIII have ay chain or C. chain as a
constituting component
which has a signal transduction function in association with the a chain
(Anna. Rev. linfnunotõ
18, 709 (2000), Annti. Rev. Immunot, 19, 275 (2001)). FcyRIV has been
described by Brehm et
al., Clin. invest Med., (Canada) 27:31) (2004).
1003421 To assess ADCC activity of an anti-ICOS antibody of interest,
an in vitro ADCC
assay can be used, such as that described_ in U.S. Patent No. 5,500,362 or
5,821,337. The assay
may also be .perforrned using a commercially available kit, e.g. CytoTox 96 ft
(Prornega).
Useful effector cells for such assays include, but. are not limited to
peripheral blood mononuclear
cells (PBMC), Natural Killer (NK) cells, and NK cell lines. NK. cell lines
expressing a
transeenic Fe receptor (e.g. CD16) and associated signaling polypeptide (e.g.
KeR1-1) may also
serve as effector cells (see, e.g. WO 2006/023148 A2 to Campbell). For
example, the ability of
any particular antibody to mediate iysis of the target cell by complement
activation and/or
ADCC can be assayed. The cells of interest are grown and labeled in vitra, the
antibody is added
to the cell culture in combination with immune cells which may be activated by
the antigen
antibody complexes; i.e., effector cells involved in the ADCC response. The
antibody can also
be tested for complement: activation. In either case, qtolysis of the target:
cells is detected by the
release of label from the lysed cells. The extent of target cell lysis may
also be determined by
detecting the release of cytoplasmic proteins (e.g. LDH) into the supernatant.
In fact, antibodies
can be screened using the patient's own serum as a source of complement and/or
immune cells.
97
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
The antibodies that are capable of mediating human ADCC in the in vitro test
can then be used
therapeutically in that particular patient ADCC activity of the molecule of
interest may also be
assessed in vivo, e.g., in an animal model such as that disclosed in Clynes et
al., Proc.. Nail.
Acad. Sci. (USA) 95:652-656 (1998). Moreover, techniques for modulating (i.e.,
increasing or
decreasing) the level of ADCC, and optionally CDC activity, of an antibody are
well-known in
the art. See, e.g., U.S. Patent No. 6,194,551. Antibodies of the present
disclosure may be
capable or may have been modified to have the ability of inducing ADCC and/or
CDC. Assays
to determine ADCC function can be practiced using human effector cells to
assess human ADCC
function. Such assays may also include those intended to screen for antibodies
that induce,
mediate, enhance, block cell death by necrotic and/or apoptotic mechanisms.
Such methods
including assays utilizing viable dyes, methods of detecting and analyzing
easpases, and assays
measuring DNA breaks can be used to assess the apoptotic activity of cells
cultured in vitro with
an anti-1COS antibody of interest.
(00343) For example, Annexin V or Ta-mediated dUTP nick-end labeling
(TUNEL)
assws can be carried out as described in Decker etal.. Blood (USA) 103:2718-
2725 (2004) to
detect apoptotic activity. The TUNE!, assay involves culturing the cell of
interest with
fluorescein-labeled dUTP for incorporation into DNA strand breaks. The cells
are then
processed for analysis by flow cytometry. The Annexin V assay detects the
appearance of
phosp.hatidylserine (PS) on the outside of the plasma membrane of apoptotic
cells using a
fluorescein-conjugated Annexin V that specifically recognizes the exposed PS
molecules.
Concurrently, a viable dye such as propidium iodide can be used to exclude
late apoptotic cells.
The cells are stained with the labeled Annexin V and are analyzed by flow cy
tometry.
5.22. I mmunoconjugates And Fusion Proteins
[00344j According to certain aspects of the disclosure, therapeutic agents
or toxins can be
conjugated to anti-1COS antibodies for use in compositions and methods of the
disclosure. In
certain embodiments, these conjugates can be generated as fusion proteins.
Examples of
therapeutic agents and toxins include, but are not limited to. members of the
enediyne -family of
molecules, such as calicheamicin and esperamicia Chemical toxins can also be
taken from the
group consisting of duocarmycin (see, e.g., U.S. Patent No. 5,703,080 and U.S.
Patent No.
4,923,990), methotrexate, doxorubicin, melp.halan, chlorambucil, A.RA-C,
vindesine, mitomycin
C, cis-platinum, etoposi de, bleomycin and 5-fluorouracil. Examples of
chemotherapeutic agents
also include Adriamycin, Doxonibiein, 5-Fluorouracil, Cytosine arabinoside
(Ara-C),
Cyclophosphamide, Thiotepa, Taxotere (docetaxel), Busulfan, Cyto.xin, Taxol,
Methotrexate,
Cisplatin, Melphalan, Vinblastine, Bleotnycin, Etoposide, Ifosfamide,
Mitomycin C,
98
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Mitoxantrone, Vincreistirie, Vinorelbine, Carboplatin, Teniposide,
Dauriotnycin, Cartninotnycin,
Aminopterin, Dactinomycin, Mitomycinsõ Esperamicins (See, U.S. Patent No.
4,675,187),
Melphalan, and other related nitrogen mustards.
1003451 In certain embodiments, anti-ICOS antibodies are conjugated to a
cytostatic,
cytotoxic or immunosuppressive agent wherein the cytotoxic agent is selected
from the group
consisting of an enediyne. a lexitropsin. a duocarrnycin, a taxane. a
pttromycin, a dolastatin, a
maytansinoid, and a vinca Alkaloid. In certain, more specific embodiments, the
qtotaxic agent is
paclitaxel, docetaxel, CC-1065, SN-38, topotecan, morpholino-doxorubicin,
rhizoxin,
cyanomorpholino-doxorubicin, dolastatin-10, echinomycin, combretastatin,
calicheamicin,
maytansine, DM-I, auristatin E, AEB, AEVB, AEFP, MMAE (see. US Patent
Application No.
10/983340. or netropsin.
1003461 In certain embodiments, the cytotoxic agent dap anti-ICOS antibody-
cytotoxic
agent conjugate of the disclosure is an anti-nibulin agent. In specific
embodiments, the cytotoxic
agent is selected from the group consisting of a Ainca alkaloid, a
podaphyllotoxin, a taxane, a
baccatin derivative, a ciyptophysin, a maytstsinoid, a combretastatin, and a
dolastatin. In other
embodiments, the cytotoxic agent is vincristine, vinblastine, vindesine,
vinorelbine, VP-16,
camptodiecin, paclitaxel, clocetaxel, epithilone A, epithilone B, nocodazole,
coichicine, colcimid,
estramustine, cemadotin, discodennolide, maytansine, DM-1. AEFP, auristatin E.
AEB, AEVB,
.AEFP, MMAE or eleutherobin.
1003471 In specific embodiments, an anti-ICOS antibody is conjugated to the
cytotoxic
agent via a linker, wherein the linker is peptide linker. In other
embodiments, an anti-ICOS
antibody is conjugated to the cytotoxic agent via a linker, wherein the linker
is a sal-cit linker, a
phe-.lys linker, a hydra2one linker, or a disulfide linker.
1003481 In certain embodiments, the anti-ICOS antibody of an anti-ICOS
antibody-
cytotoxic agent conjugate is conjugated to the cytotoxic agent via a linker,
wherein the linker is
hydrolysabie at a pH of less than 5.5. in a specific embodiment the linker is
hydrolyzable at a pH
of less than 5Ø
1003491 In certain embodiments, the anti -ICOS antibody of an anti-ICOS
antibody-
cytotoxic agent conjugate is conjugated to the cytotoxic agent via a linker,
wherein the linker is
cleavable by a protease. In a specific embodiment, the protease is a
lysosornal protease. In other
embodiments, the protease is, inter al ia, a membrane-associated protease, an
intracellular
protease, or an endosomal protease.
1003501 Other toxins that can be used in immunoconjugates of the disclosure
include
poisonous lectins, plant toxins such as dein, abrin, modeccin, batulina, and
diphtheria toxins. Of
course, combinations of the various toxins could also be coupled to one
antibody molecule
99
CA 02743469 2011-05-11
WO 2010/056804
PCTIUS2009/064127
thereby accommodating variable cytotoxicity. Illustrative of toxins which are
suitably employed
in combination therapies of the disclosure are ricin, abrin, ribonuclease,
DNase I, Staphylococcal
enterotoxin-A, pokeweed anti-viral protein, gelonin, diphtheria toxin,
Pseudomonas exotoxin,
and Pseudomonas endotoxin. See, for example, Pastan et aL,Cell, 47:641 (1986),
and
Goldenberg et al., Cancer Join-nal fir Clinicians, 44:43 (1994). Enzymatically
active toxins and
fragments thereof winch can be used include diphtheria A chain, non-binding
active fragments of
diphtheria toxin, exotoxin A chain (from l'setitionionas aerttginosa), richt A
chain, abrin A
chain, modeccin A chain, alpha-sarcin, AFeuritesf.srdii proteins, dian thin
proteins, Phytolaca
americana proteins (PAP1, PAP!!, and PAP-S). Mornordico charantia inhibitor,
cumin, crotin.
Sapaonaria teicinalis inhibitor, gelonin, mitogellin, restrictocin,
phenomycin, enomycin and the
tricothecenes. See, for example, WO 93/21232 published October 28, 1993.
1003511 Suitable toxins and chemotherapeutic agents are described in
Remington's
Pharmaceutical Sciences, 19th Ed. (Mack Publishing Co. 1995), and in Goodman
And Gilman's
The Pharmacological Basis of Therapeutics, 7th Ed. (MacMillan Publishing Co.
1985). Other
suitable toxins and/or chemotherapeutic agents are known to those of skill in
the art.
1003521 The present disclosure further encompasses antibodies (including
antibody
fragments or variants thereol) comprising or conjugated to a radioactive agent
suitable for
diagnostic purposes. Examples of suitable radioactive materials include, but
are not limited to,
iodine ("31, 1.125I, 1311), carbon (t), sulfur (35S), tritiurn (313), indium
(I Ilnõ "21n, 113'In,
technetium (95tc, Tc), thallium (201Ti), gallium (68011,67Ga), palladium (I
3Pd),
molybdenum (9N4o), xenon (1"Xe), fluorine (18F), ""Stn, 1771,th "90d, 149Pm,
14 La, 175Yb,
166Ho,9`)Y, "Se, I'Re. '"Re, l'"Pr,l("Rh, and 97Ru.
1003531 Further, an arni-ICOS antibody of the disclosure (including an scEv
or other
molecule comprising, or alternatively consisting of, antibody fragments or
variants thereof), may
be coupled or conjugated to a radioactive metal ion utilized for therapeutic
purposes. Examples
of suitable radioactive ions include, but are not limited to, alpha-emitters
such as 21.ti, or other
radioisotopes such as 1 3Pd, I"Xe, 6aGe, "Co,
657,n, 85Sr. 32P, 35S, 90Y, 153SM, 1$3Gd, 169Yb,
"Cr. Mn. "Se, 113 Sn, 9 Y, "Tin, '.Re, "Re and 166Ho. In specific embodiments,
an antibody
or fragment thereof is attached to macrocyclic chelators that chelate
radiometal ions, including
but not limited to, '771,u, 90Y, 76611o, and '"Stn., to polypeptides. In
specific embodiments, the
macrocyclic chelator is 1,4õ7,10-tetraazacyclod- odecane-N,N,N",Nm-tetraacetic
acid (DOTA).
In other specific embodiments, the DOTA is attached to an antibody of the
disclosure or
fragment thereof via a linker molecule. Examples of linker molecules useful
for conjugating
DOTA to a poly-peptide are commonly known in the art--see, for example,
DeNardo et al., Clin
Cancer Res 4(10):2483-90, .1998; Peterson et al., Bioconjug Chem 1.0(4):553-7,
1999; and
lot)
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Zimmerman et al., Nucl Med Biol 26(8):943-50, 1999 which are hereby
incorporated by
reference in their entirety.
[00354] An anti-ICOS antibody of the present disclosure may also be used in
ADEPT by
conjugating the antibody to a prodrug-activating enzyme which converts a
prodrug (e.g., a
peptidyl chemotherapeutic agent, see, W081/01145) to an active anti-cancer
drug. See, for
example, WO 88/07378 and U.S. Patent No. 4,975,278. The enzyme component of
the
immunoconjugate useful for ADEPT includes any enzyme capable of acting on a
prodrug in
such a way so as to covert it into its more active, cytotoxic form.
[00355] Enzymes that are useful in the method of this disclosure include,
but are not
limited to, alkaline phosphatase useful for converting phosphate-containing
prodrugs into free
drugs; arylsulfatase useful for converting sulfate-containing prodrugs into
free drugs; cytosine
deaminase useful for converting non-toxic 5-fluorocytosine into the anti-
cancer drug, 5-
fluorouracil; proteases, such as serratia protease, thermolysin, subtilisin,
carboxypeptidases and
cathepsins (such as cathepsins B and L), that are useful for converting
peptide-containing
prodrugs into free drugs; D-alanylcarboxypeptidases, useful for converting
prodrugs that contain
D-amino acid substituents; carbohydrate-cleaving enzymes such as P-
galactosidase and
neuraminidase useful for converting glycosylated prodrugs into free drugs; P-
lactamase useful
for converting drugs derivatized with a-lactams into free drugs; and
penicillin amidases, such as
penicillin V amidase or penicillin G amidase, useful for converting drugs
derivatized at their
amine nitrogens with phenoxyacetyl or phenylacetyl groups, respectively, into
free drugs.
Antibodies with enzymatic activity, also known in the art as "abzymes," can be
used as well to
convert the prodrugs into free active drugs (see, e.g., Massey, Nature 328:457-
458 (1987)).
Antibody-abzyme conjugates can be prepared as described herein for delivery of
the abzyme as
desired to portions of a human affected by an ICOS expressing T cell
malignancy.
[00356] Antibodies of this disclosure may be covalently bound to the
enzymes by
techniques well-known in the art such as the use of the heterobifunctional
cross linking reagents
discussed above. Fusion proteins comprising at least the antigen-binding
region of an anti-ICOS
antibody linked to at least a functionally active portion of an enzyme may
also be constructed
using recombinant DNA techniques well-known in the art (see, e.g., Neuberger
et al., Nature,
312:604-608 (1984)).
[00357] Covalent modifications of an anti-1COS antibody are included within
the scope of
this disclosure. They may be made by chemical synthesis or by enzymatic or
chemical cleavage
of the antibody, if applicable. Other types of covalent modifications of an
anti-ICOS antibody
are introduced into the molecule by reacting targeted amino acid residues of
the antibody with an
101
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
organic derivatizing agent that is capable of reacting with selected side
chains or the N- or C-
terminal residues.
[00358] Cysteinyl residues most commonly are reacted with a-haloacetates
(and
corresponding amines), such as chloroacetic acid or chloroacetamide, to give
carboxymethyl or
carboxyamidomethyl derivatives. Similarly, iodo-reagents may also be used.
Cysteinyl residues
also are derivatized by reaction with bromotrifluoroacetone, a-bromo-13-(5-
imidozoyl)propionic
acid, chloroacetyl phosphate, N-alkylmaleimides, 3-nitro-2-pyridyl disulfide,
methyl 2-pyridyl
disulfide, p-chloromercuribenzoate, 2-chloromercuri-4-nitrophenol, or chloro-7-
nitrobenzo-2-
oxa-1,3-diazole.
[00359] Histidyl residues are derivatized by reaction with
diethylpyrocarbonate at pH
5.5-7.0 because this agent is relatively specific for the histidyl side chain.
Para-bromophenacyl
bromide also is useful; the reaction can be performed in 0.1 M sodium
cacodylate at pH 6Ø
[00360] Lysyl and amino-terminal residues are reacted with succinic or
other carboxylic
acid anhydrides. Derivatization with these agents has the effect of reversing
the charge of the
lysinyl residues. Other suitable reagents for derivatizing a-amino-containing
residues and/or
C-amino-containing residues include imidoesters such as methyl picolinimidate,
pyridoxal
phosphate, pyridoxal, chloroborohydride, trinitrobenzenesulfonic acid, 0-
methylisourea,
2,4-pentancdione, and transaminase-catalyzed reaction with glyoxylate.
[00361] Arginyl residues are modified by reaction with one or several
conventional
reagents, among them phenylglyoxal, 2,3-butanedione, 1,2-cyclohexanedione, and
ninhydrin.
Derivatization of arginyl residues generally requires that the reaction be
performed in alkaline
conditions because of the high pKa of the guanidine functional group.
Furthermore, these
reagents may react with the C-amino groups of lysine as well as the arginine
epsilon-amino
group.
[00362] The specific modification of tyrosyl residues may be made, with
particular
interest in introducing spectral labels into tyrosyl residues by reaction with
aromatic diazonium
compounds or tetranitromethane. Most commonly, N-acetylimidizole and
tetranitromethane are
used to form 0-acetyl tyrosyl species and 3-nitro derivatives, respectively.
Tyrosyl residues are
iodinated using 1251 or 1311 to prepare labeled proteins for use in
radioimmunoassay.
[00363] Carboxyl side groups (aspartyl or glutamyl) are selectively
modified by reaction
with carbodiimides (R--N=C=N--R.), where R and R' arc different alkyl groups,
such as 1-
cyclohexy1-3-(2-morpholinyl-- 4-ethyl) carbodiimide or 1-ethyl-3-(4-azonia-4,4-
dimethylpentyl)
carbodiimide. Furthermore, aspartyl and glutamyl residues are converted to
asparaginyl and
glutaminyl residues by reaction with ammonium ions.
102
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00364] Glutaminyl and asparaginyl residues are frequently deamidated to
the
corresponding glutamyl and aspartyl residues, respectively. These residues are
deamidated
under neutral or basic conditions. The deamidated form of these residues falls
within the scope
of this disclosure.
[00365] Other modifications include hydroxylation of proline and lysinc,
phosphorylation
of hydroxyl groups of seryl or threonyl residues, methylation of the a-amino
groups of lysine,
arginine, and histidine side chains (T.E. Creighton, Proteins: Structure and
_Molecular
Properties, W.H. Freeman & Co., San Francisco, pp. 79-86 (1983)), acetylation
of the N-
terminal amine, and amidation of any C-terminal carboxyl group.
[00366] Another type of covalent modification involves chemically or
enzymatically
coupling glycosides to the antibody. These procedures are advantageous in that
they do not
require production of the antibody in a host cell that has glycosylation
capabilities for N- or 0-
linked glycosylation. Depending on the coupling mode used, the sugar(s) may be
attached to (a)
arginine and histidine, (b) free carboxyl groups, (c) free sulfhydryl groups
such as those of
cysteine, (d) free hydroxyl groups such as those of serine, threonine, or
hydroxyproline, (e)
aromatic residues such as those of phenylalanine, tyrosine, or tryptophan, or
(f) the amide group
of glutamine. These methods are described in WO 87/05330 published 11 Sep.
1987, and in
Aplin and Wriston, CRC Crit. Rev. Biochem., pp. 259-306 (1981).
5.23. Chemotherapeutic Combinations
[00367] According to the disclosure, cancer or one or more symptoms thereof
may be
prevented, treated, managed or ameliorated by the administration of an anti-
ICOS antibody
formulation in combination with the administration of one or more therapies
such as, but not
limited to, chemotherapies, radiation therapies, hormonal therapies, and/or
biological
therapies/immunotherapies.
[00368] In a specific embodiment, methods of the disclosure encompass the
administration of one or more angiogenesis antagonists such as but not limited
to: Angiostatin
(plasminogen fragment); antiangiogenic antithrombin III; Angiozyme; ABT-627;
Bay 12-9566;
Benefin; Bevacizumab; BMS-275291; cartilage-derived inhibitor (CDI); CAI; CD59
complement fragment; CEP-7055; Col 3; Combretastatin A-4; Endostatin (collagen
XVIII
fragment); Fibronectin fragment; Gro-beta; Halofuginone; Heparinases; Heparin
hexasaccharide
fragment; HMV833; Human chorionic gonadotropin (hCG); IM-862; Interferon
alpha/beta/gamma; Interferon inducible protein (1P-10); Interleukin-12;
Kringle 5 (plasminogen
fragment); Marimastat; Metalloproteinase inhibitors (TIMPs); 2-
Methoxyestradiol; MMI 270
(CGS 27023A); MoAb IMC-1C11; Neovastat; NM-3; Panzem; PI-88; Placental
ribonuclease
103
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
inhibitor; Plasminogen activator inhibitor; Platelet factor-4 (PF4);
Prinomastat; Prolactin 16kD
fragment; Proliferin-related protein (PRP); PTK 787/ZK 222594; Retinoids;
Solimastat;
Squalamine; SS 3304; SU 5416; SU6668; SU11248; Tetrahydrocortisol-S;
tetrathiomolybdate;
thalidomide; Thrombospondin-1 (TSP-1); TNP-470; Transforming growth factor-
beta (TGF-b);
Vasculostatin; Vasostatin (calreticulin fragment); ZD6126; ZD 6474; farncsyl
transferase
inhibitors (FTI); and bisphosphonates (such as but are not limited to,
alendronate, clodronate,
etidronate, ibandronate, pamidronate, risedronate, tiludronate, and
zoledronate).
[00369] In a specific embodiment, methods of the disclosure encompass the
administration of one or more immunomodulatory agents, such as but not limited
to,
chemotherapeutic agents and non-chemotherapeutic immunomodulatory agents. Non-
limiting
examples of chemotherapeutic agents include methotrexate, cyclosporin A,
leflunomide,
cisplatin, ifosfamide, taxanes such as taxol and paclitaxol, topoisomerase I
inhibitors (e.g.,
CPT-11, topotecan, 9-AC, and GG-211), gemcitabine, vinorelbine, oxaliplatin, 5-
fluorouracil (5-
FU), leucovorin, vinorelbine, temodal, cytochalasin B, gramicidin D, emetine,
mitomycin,
etoposide, tenoposide, vincristine, vinblastine, colchicin, doxorubicin,
daunorubicin, dihydroxy
anthracin dione, mitoxantrone, mithramycin, actinomycin D, 1-
dehydrotestosterone,
glucocorticoids, procaine, tetracaine, lidocaine, propranolol, and puromycin
homologues, and
cytoxan. Examples of non-chemotherapcutic immunomodulatory agents include, but
are not
limited to, anti-T cell receptor antibodies (e.g., anti-CD4 antibodies (e.g.,
cM-T412 (Boeringer),
IDEC-CE9.1t (IDEC and SKB), mAB 4162W94, Orthoclone and OKTcdr4a (Janssen-
Cilag)),
anti-CD3 antibodies (e.g., Nuvion (Product Design Labs), OKT3 (Johnson &
Johnson), or
Rituxan (IDEC)), anti-CD5 antibodies (e.g., an anti-CD5 ricin-linked
immunoconjugatc), anti-
CD7 antibodies (e.g., CHH-380 (Novartis)), anti-CD8 antibodies, anti-CD40
ligand monoclonal
antibodies (e.g., IDEC-131 (IDEC)), anti-CD52 antibodies (e.g., CAMPATH 1H
(Ilex)), anti-
CD2 antibodies (e.g., MEDI-507 (MedImmune, Inc., International Publication
Nos. WO
02/098370 and WO 02/069904), anti-CD11a antibodies (e.g., Xanelim
(Genentech)), and anti-B7
antibodies (e.g., IDEC-114) (IDEC)); anti-cytokine receptor antibodies (e.g.,
anti-IFN receptor
antibodies, anti-IL-2 receptor antibodies (e.g., Zenapax (Protein Design
Labs)), anti-IL-4
receptor antibodies, anti-IL-6 receptor antibodies, anti-IL-10 receptor
antibodies, and anti-IL-12
receptor antibodies), anti-cytokine antibodies (e.g., anti-IFN antibodies,
anti-'TNE-ci antibodies,
anti-IL-113 antibodies, anti-1L-6 antibodies, anti-IL-8 antibodies (e.g., ABX-
IL-8 (Abgenix)),
anti-IL-12 antibodies and anti-IL-23 antibodies)); CTLA4-immunoglobulin; LFA-
3TIP (Biogen,
International Publication No. WO 93/08656 and U.S. Patent No. 6,162,432);
soluble cytokine
receptors (e.g., the extracellular domain of a TNF-o, receptor or a fragment
thereof, the
extracellular domain of an 1L-113 receptor or a fragment thereof, and the
extracellular domain of
104
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
an IL-6 receptor or a fragment thereof); cytokines or fragments thereof (e.g.,
interleukin (IL)-2,
IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-15, IL-23,
TNF-a, TNE-13,
interferon (IFN)-a, IFN-I3, IFN-7, and GM-CSF); and anti-cytokine antibodies
(e.g., anti-IL-2
antibodies, anti-IL-4 antibodies, anti-IL-6 antibodies, anti-IL-10 antibodies,
anti-IL-12
antibodies, anti-IL-15 antibodies, anti-TNF-a antibodies, and anti-IFN-y
antibodies), and
antibodies that immunospecifically bind to tumor-associated antigens (e.g.,
Herceptink). In
certain embodiments, an immunomodulatory agent is an immunomodulatory agent
other than a
chemotherapeutic agent. In other embodiments an immunomodulatory agent is an
immunomodulatory agent other than a cytokine or hcmapoictic such as IL-1, 1L-
2, IL-4, 1L-12,
IL-15, TNF, IFN-a, IFN-I3, IFN-y, M-CSF, G-CSF, IL-3 or erythropoietin. In yet
other
embodiments, an immunomodulatory agent is an agent other than a
chemotherapeutic agent and
a cytokine or hemapoietic factor.
[00370] In a specific embodiment, methods of the disclosure encompass the
administration of one or more anti-inflammatory agents, such as but not
limited to, non-steroidal
anti-inflammatory drugs (NSAIDs), steroidal anti-inflammatory drugs, beta-
agonists,
anticholingeric agents, and methyl xanthines. Examples of NSAIDs include, but
are not limited
to, aspirin, ibuprofen, celecoxib (CELEBREXTm), diclofenac (VOLTARENTm),
etodolac
(LODINE'm), fenoprofen (NALFON 'm), indomethacin (INDOCIN'm), ketoralac
(TORADOLTm), oxaprozin (DAYPROTm), nabumentone (RELAFENTm), sulindac
(CLINORILTm), tolmentin (TOLECTINTm), rofecoxib (VIOXXTm), naproxen (ALEVETM,
NAPROSYNTm), ketoprofen (ACTRONTm) and nabumetone (RELAFENTm). Such NSAIDs
function by inhibiting a cyclooxygenase enzyme (e.g., COX-1 and/or COX-2).
Examples of
steroidal anti-inflammatory drugs include, but are not limited to,
glucocorticoids, dexamethasone
(DECADRONTm), cortisone, hydrocortisone, prednisone (DELTASONETm),
prednisolone,
triamcinolone, azulfidine, and eicosanoids such as prostaglandins,
thromboxanes, and
leukotrienes.
[00371] In another specific embodiment, methods of the disclosure encompass
the
administration of one or more antiviral agents (e.g., amantadine, ribavirin,
rimantadine,
acyclovir, famciclovir, foscarnet, ganciclovir, trifluridine, vidarabine,
didanosine, stavudine,
zalcitabine, zidovudine, interferon), antibiotics (e.g., dactinomycin
(formerly actinomycin),
bleomycin, mithramycin, and anthramycin (AMC)), anti-emetics (e.g.,
alprazolam,
dexamethoasone, domperidone, dronabinol, droperidol, granisetron, haloperidol,
haloperidol,
iorazepam, methylprednisolone, metoclopramide, nabilone, ondansetron,
prochlorperazine), anti-
fungal agents (e.g., amphotericin, clotrimazole, econazole, fluconazole,
flucytosine, griseofulvin,
itraconazole, ketoconazole, miconazole and nystatin), anti-parasite agents
(e.g., dehydroemetinc,
105
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
diloxanide furoate, emetine, mefloquine, melarsoprol, metronidazole,
nifurtimox, paromomycin,
pentabidine, pentamidine isethionate, primaquine, quinacrine, quinidine) or a
combination
thereof.
[00372] Specific examples of anti-cancer agents that can be used in various
embodiments
of the disclosure, including pharmaceutical compositions and dosage forms and
kits, include, but
are not limited to: acivicin; aclarubicin; acodazole hydrochloride; acronine;
adozelesin;
aldesleukin; altretamine; ambomycin; ametantrone acetate; aminoglutethimide;
amsacrine;
anastrozole; anthramycin; asparaginase; asperlin; azacitidine; azetepa;
azotomycin; batimastat;
benzodepa; bicalutamide; bisantrene hydrochloride; bisnafide dimesylate;
bizelesin; bleomycin
sulfate; brequinar sodium; bropirimine; busulfan; cactinomycin; calusterone;
caracemide;
carbetimer; carboplatin; carmustine; carubicin hydrochloride; carzelesin;
cedefingol;
chlorambucil; cirolemycin; cisplatin; cladribine; crisnatol mesylate;
cyclophosphamide;
cytarabine; dacarbazine; dactinomycin; daunorubicin hydrochloride; decitabine;
dexormaplatin;
dezaguanine; dezaguanine mesylate; diaziquone; docetaxel; doxorubicin;
doxorubicin
hydrochloride; droloxifene; droloxifene citrate; dromostanolone propionate;
duazomycin;
edatrexate; eflornithine hydrochloride; elsamitrucin; enloplatin; enpromate;
epipropidine;
epirubicin hydrochloride; erbulozole; esorubicin hydrochloride; estramustine;
estramustine
phosphate sodium; ctanidazole; etoposide; etoposide phosphate; etoprine;
fadrozole
hydrochloride; fazarabine; fenretinide; floxuridine; fludarabine phosphate;
fluorouracil;
flurocitabine; fosquidone; fostriecin sodium; gemcitabine; gemcitabine
hydrochloride;
hydroxyurea; idarubicin hydrochloride; ifosfamide; ilmofosine; interleukin II
(including
recombinant interleukin II, or rIL2), interferon alpha-2a; interferon alpha-
2b; interferon alpha-nl
; interferon alpha-n3; interferon beta-I a; interferon gamma-I b; iproplatin;
irinotecan
hydrochloride; lanreotide acetate; letrozole; leuprolide acetate; liarozole
hydrochloride;
lometrexol sodium; lomustine; losoxantrone hydrochloride; masoprocol;
maytansine;
mechlorethamine hydrochloride; megestrol acetate; melengestrol acetate;
melphalan; menogaril;
mercaptopurine; methotrexate; methotrexate sodium; metoprine; meturedepa;
mitindomide;
mitocarcin; mitocromin; mitogillin; mitomalcin; mitomycin; mitosper; mitotane;
mitoxantrone
hydrochloride; mycophenolic acid; nocodazole; nogalamycin; ormaplatin;
oxisuran; paclitaxel;
pegaspargase; peliomycin; pentamustine; peplomycin sulfate; perfosfamide;
pipobroman;
piposulfan; piroxantrone hydrochloride; plicamycin; plomestane; porfimer
sodium;
porfiromycin; prednimustine; procarbazine hydrochloride; puromycin; puromycin
hydrochloride;
pyrazofurin; riboprine; rogletimide; safingol; safingol hydrochloride;
semustine; simtrazene;
sparfosate sodium; sparsomycin; spirogermanium hydrochloride; spiromustine;
spiroplatin;
streptonigrin; streptozocin; sulofenur; talisomycin; tecogalan sodium;
tegafur; teloxantrone
106
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
hydrochloride; temoporfin; teniposide; teroxirone; testolactone; thiamiprine;
thioguanine;
thiotepa; tiazofurin; tirapazamine; toremifene citrate; trestolone acetate;
triciribine phosphate;
trimetrexate; trimetrexate glucuronate; triptorelin; tubulozole hydrochloride;
uracil mustard;
uredepa; vapreotide; verteporfin; vinblastine sulfate; vincristine sulfate;
vindesine; vindesine
sulfate; vinepidine sulfate; vinglycinate sulfate; vinicurosine sulfate;
vinorelbine tartrate;
vinrosidine sulfate; vinzolidine sulfate; vorozole; zeniplatin; zinostatin;
zorubicin hydrochloride.
Other anti-cancer drugs include, but are not limited to: 20-epi-1,25
dihydroxyvitamin D3;
5-ethynyluracil; abiraterone; aclarubicin; acylfulvene; adecypenol;
adozelesin; aldesleukin;
ALL-TK antagonists; altretaminc; ambamustine; amidox; amifostinc;
aminolevulinic acid;
amrubicin; amsacrine; anagrelide; anastrozole; andrographolide; angiogenesis
inhibitors;
antagonist D; antagonist G; antarelix; anti-dorsalizing morphogenetic protein-
1; antiandrogen,
prostatic carcinoma; antiestrogen; antineoplaston; antisense oligonucleotides;
aphidicolin
glycinate; apoptosis gene modulators; apoptosis regulators; apurinic acid; ara-
CDP-DL-PTBA;
arginine deaminase; asulacrine; atamestane; atrimustine; axinastatin 1;
axinastatin 2; axinastatin
3; azasetron; azatoxin; azatyrosine; baccatin III derivatives; balanol;
batimastat; BCR/ABL
antagonists; benzochlorins; benzoylstaurosporine; beta lactam derivatives;
beta-alethine;
betaclamycin B; betulinic acid; bFGF inhibitor; bicalutamide; bisantrene;
bisaziridinylspermine;
bisnafide; bistratene A; bizelesin; breflate; bropiriminc; budotitane;
buthioninc sulfoximinc;
calcipotriol; calphostin C; camptothecin derivatives; canarypox IL-2;
capecitabine;
carboxamide-amino-triazole; carboxyamidotriazole; CaRest M3; CARN 700;
cartilage derived
inhibitor; carzelesin; casein kinase inhibitors (ICOS); castanospermine;
cecropin B; cetrorelix;
chlorins; chloroquinoxaline sulfonamide; cicaprost; cis-porphyrin; cladribinc;
clomifene
analogues; clotrimazole; collismycin A; collismycin B; combretastatin A4;
combretastatin
analogue; conagenin; crambescidin 816; crisnatol; cryptophycin 8; cryptophycin
A derivatives;
curacin A; cyclopentanthraquinones; cycloplatam; cypemycin; cytarabine
ocfosfate; cytolytic
factor; cytostatin; dacliximab; decitabine; dehydrodidemnin B; deslorelin;
dexamethasone;
dexifosfamide; dexrazoxane; dexverapamil; diaziquone; didemnin B; didox;
diethylnorspermine;
dihydro-5-azacytidine; dihydrotaxol, 9-; dioxamycin; diphenyl spiromustine;
docetaxel;
docosanol; dolasetron; doxifluridine; droloxifene; dronabinol; duocarmycin SA;
ebselen;
ecomustine; edelfosine; edrecolomab; eflornithine; elemene; emitefur;
epirubicin; epristeride;
estramustine analogue; estrogen agonists; estrogen antagonists; etanidazole;
etoposidc
phosphate; exemestane; fadrozole; fazarabine; fenretinide; filgrastim;
finasteride; flavopiridol;
flezelastine; fluasterone; fludarabine; fluorodaunorunicin hydrochloride;
forfenimex; formestane;
fostriecin; fotemustine; gadolinium texaphyrin; gallium nitrate; galocitabine;
ganirelix;
gclatinase inhibitors; gemcitabine; glutathione inhibitors; hepsulfam;
heregulin; hexamethylene
107
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
bisacetamide; hypericin; ibandronic acid; idarubicin; idoxifene; idramantone;
ilmofosine;
ilomastat; imidazoacridones; imiquimod; immunostimulant peptides; insulin-like
growth factor-1
receptor inhibitor; interferon agonists; interferons; interleukins;
iobenguane; iododoxorubicin;
ipomeanol, 4-; iroplact; irsogladine; isobengazole; isohomobalicondrin B;
itasetron;
jasplakinolide; kahalalide F; lamellarin-N triacetate; lanrcotide; leinamycin;
lenograstim;
lentinan sulfate; leptolstatin; letrozole; leukemia inhibiting factor;
leukocyte alpha interferon;
leuprolide+estrogen+progesterone; leuprorelin; levamisole; liarozole; linear
polyamine analogue;
lipophilic disaccharide peptide; lipophilic platinum compounds; lissoclinamide
7; lobaplatin;
lombricine; lometrexol; lonidamine; losoxantronc; HMG-CoA reductasc inhibitor
(such as but
not limited to, Lovastatin, Pravastatin, Fluvastatin, Statin, Simvastatin, and
Atorvastatin);
loxoribine; lurtotecan; lutetium texaphyrin; lysofylline; lytic peptides;
maitansine; mannostatin
A; marimastat; masoprocol; maspin; matrilysin inhibitors; matrix
metalloproteinase inhibitors;
menogaril; merbarone; meterelin; methioninase; metoclopramide; MIF inhibitor;
mifepristone;
miltefosine; mirimostim; mismatched double stranded RNA; mitoguazone;
mitolactol;
mitomycin analogues; mitonafide; mitotoxin fibroblast growth factor-saporin;
mitoxantrone;
mofarotene; molgramostim; monoclonal antibody, human chorionic gonadotrophin;
monophosphoryl lipid A+myobacterium cell wall sk; mopidamol; multiple drug
resistance gene
inhibitor; multiple tumor suppressor 1-based therapy; mustard anticancer
agent; mycaperoxide
B; mycobacterial cell wall extract; myriaporone; N-acetyldinaline; N-
substituted benzamides;
nafarelin; nagrestip; naloxone+pentazocine; napavin; naphterpin; nartograstim;
nedaplatin;
nemorubicin; neridronic acid; neutral endopeptidase; nilutamide; nisamycin;
nitric oxide
modulators; nitroxidc antioxidant; nitrullyn; 06-benzylguanine; octreotide;
okicenonc;
oligonucleotides; onapristone; ondansetron; ondansetron; oracin; oral cytokine
inducer;
ormaplatin; osaterone; oxaliplatin; oxaunomycin; paclitaxel; paclitaxel
analogues; paclitaxel
derivatives; palauamine; palmitoylrhizoxin; pamidronic acid; panaxytriol;
panomifene;
parabactin; pazelliptine; pegaspargase; peldesine; pentosan polysulfate
sodium; pentostatin;
pentrozole; perflubron; perfosfamide; perillyl alcohol; phenazinomycin;
phenylacetate;
phosphatase inhibitors; picibanil; pilocaipine hydrochloride; pirarubicin;
piritrexim; placetin A;
placetin B; plasminogen activator inhibitor; platinum complex; platinum
compounds;
platinum-triamine complex; porfimer sodium; porfiromycin; prednisone; propyl
bis-acridone;
prostaglandin J2; proteasome inhibitors; protein A-based immune modulator;
protein kinase C
inhibitor; protein kinase C inhibitors, microalgal; protein tyrosine
phosphatase inhibitors; purine
nucleoside phosphorylase inhibitors; putpurins; pyrazoloacridine;
pyridoxylated hemoglobin
polyoxyethylene conjugate; raf antagonists; raltitrexed; ramosetron; ras
farnesyl protein
transferase inhibitors; ras inhibitors; ras-GAP inhibitor; retelliptine
demethylatcd; rhenium Re
108
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
186 etidronate; rhizoxin; ribozymes; RII retinamide; rogletimide; rohitukine;
romurtide;
roquinimex; rubiginone Bl; ruboxyl; safingol; saintopin; SarCNU; sarcophytol
A; sargramostim;
Sdi 1 mimetics; semustine; senescence derived inhibitor 1; sense
oligonucleotides; signal
transduction inhibitors; signal transduction modulators; single chain antigen
binding protein;
sizofiran; sobuzoxane; sodium borocaptate; sodium phenylacetate; solvcrol;
somatomedin
binding protein; sonermin; sparfosic acid; spicamycin D; spiromustine;
splenopentin;
spongistatin 1; squalamine; stem cell inhibitor; stem-cell division
inhibitors; stipiamide;
stromelysin inhibitors; sulfinosine; superactive vasoactive intestinal peptide
antagonist;
suradista; suramin; swainsonine; synthetic glycosaminoglycans; tallimustine;
tamoxifen
methiodide; tauromustine; tazarotene; tecogalan sodium; tegafur;
tellurapyrylium; telomerase
inhibitors; temoporfin; temozolomide; teniposide; tetrachlorodecaoxide;
tetrazomine;
thaliblastine; thiocoraline; thrombopoietin; thrombopoietin mimetic;
thymalfasin; thymopoietin
receptor agonist; thymotrinan; thyroid stimulating hormone; tin ethyl
etiopurpurin; tirapazamine;
titanocene bichloride; topsentin; toremifene; totipotent stem cell factor;
translation inhibitors;
tretinoin; triacetyluridine; triciribine; trimetrexate; triptorelin;
tropisetron; turosteride; tyrosine
kinase inhibitors; tyrphostins; UBC inhibitors; ubenimex; urogenital sinus-
derived growth
inhibitory factor; urokinase receptor antagonists; vapreotide; variolin B;
vector system,
erythrocyte gene therapy; velaresol; veramine; verdins; verteporfin;
vinorelbine; vinxaltine;
Vitaxink; vorozole; zanoterone; zeniplatin; zilascorb; and zinostatin
stimalamer. Additional
anti-cancer drugs are 5-fluorouracil and leucovorin. These two agents may be
useful when used
in methods employing thalidomide and a topoisomerase inhibitor. In specific
embodiments, an
anti-cancer agent is not a chemotherapeutic agent.
[00373] In more particular embodiments, the present disclosure also
comprises the
administration of an anti-ICOS antibody formulation in combination with the
administration of
one or more therapies such as, but not limited to, anti-cancer agents such as
those disclosed in
Table 1, for the treatment of breast, ovary, melanoma, prostate, colon and
lung cancers as
described above. When used in a combination therapy, the dosages and/or the
frequency of
administration listed in Table 1 may be decreased.
[00374] Table 1. Anti-cancer agents
Therapeutic Dose/Administration/Formulation
Agent
doxorubicin ! Intravenous 60-75 mg/m2 on Day 1 ! 21
day intervals
hydrochloride
(Adriamycin
RDF and
109
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Therapeutic Dose/Administration/Formulation
Agent
Adriamycin
PFSg
epirubicin Intravenous 100-120 mg/m2 on Day 1 of 3-4 week cycles
hydrochloride each cycle or
(EllenceTm) divided equally and given on
Days 1-8 of the cycle
I fluorousacil Intravenous How supplied:
mL and 10 mL vials
(containing 250 and 500 mg
.=
flourouracil respectively)
docetaxel Intravenous 60- 100 mg/m2 over 1 hour Once every 3 weeks
(Taxoteret)
paclitaxel __ rIntravenous 175 mg/m2 over 3 hours
Every 3 weeks for
(Taxo1R) 4 courses (administered
sequentially to
doxorubicin-containing
combination
chemotherapy)
tamoxifen citrate Oral 20-40 mg Daily
(Nolvadexg) (tablet) Dosages greater than 20 mg
should be given in divided
doses (morning and evening)
leucovorin intravenous How supplied: Dosage is unclear from
calcium for or 350 mg vial text. PDR 3610
injection intramuscula
r injection
luprolide acetate single 1 mg (0.2 mL or 20 unit Once a day
Lupron0) subcutaneou mark)
s injection
flutamide Oral 50 mg 3 times a day at 8 hour
(Eulexing) (capsule) (capsules contain 125 mg intervals (total daily
flutamide each) dosage 750 mg)
nilutamide Oral 300 mg or 150 mg 300 mg once a day for 30
(Nilandront) (tablet) (tablets contain 50 or 150 mg days followed by 150
mg
nilutamidc each) once a day
bicalutamidc Oral 50 mg Once a day
(Casodexg) (tablet) (tablets contain 50 mg
bicalutamide each)
progesterone Injection USP in sesame oil 50 mg/mL
ketoconazole Cream 2% cream applied once or
(Nizoralk) twice daily depending on
Isymptoms
prednisonc Oral Initial dosage may vary from
110
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Therapeutic Dose/Administration/Formulation
Agent
(tablet) 5 mg to 60 mg per day
depending on the specific
disease entity being treated.
estramustine Oral 14 mg/ kg of body weight Daily given in 3 or 4
phosphate (capsule) (i.e. one 140 mg capsule for divided doses
sodium each 10 kg or 22 lb of body
(Emcyt ) weight)
etoposide or Intravenous 5 mL of 20 mg/ mL solution
VP-16
(100 mg)
dacarbazine Intravenous 2-4.5 mg/kg Once a day for 10 days.
(DTIC-Dome ) May be repeated at 4 week
intervals
polifeprosan 20 wafer placed 8 wafers, each containing 7.7
with carmustine in resection mg of carmustine, for a total
implant (BCNU) cavity of 61.6 mg, if size and shape
(nitrosourea) of resection cavity allows
(Gliadelt)
cisplatin Injection [nia in PDR 861]
How supplied:
solution of 1 mg/mL in
multi-dose vials of 50mL
and 100mL
mitomycin Injection supplied in 5 mg and 20 mg
vials (containing 5 mg and
20 mg mitomycin)
gemcitabine HC1 Intravenous For NSCLC- 2 schedules 4 week schedule-
(Gemzar ) have been investigated and Days 1,8 and 15 of each
the optimum schedule has 28-day cycle. Cisplatin
not been determined intravenously at 100
4 week schedule- mg/m2 on day 1 after the
administration intravenously infusion of Gemzar.
at 1000 mg/m2 over 30 3 week schedule-
minutes on 3 week schedule- Days 1 and 8 of each 21
Gemzar administered day cycle. Cisplatin at
intravenously at 1250 mg/m2 dosage of 100 mg/m2
over 30 minutes administered
intravenously after
administration of Gemzar
on day 1.
carboplatin Intravenous Single agent therapy: Every
4 weeks
(Paraplatin0) 360 mg/m7 I.V. on day 1
(infusion lasting 15 minutes
or longer)
Other dosage calculations:
= Combination therapy with
111
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Therapeutic Dose/Administration/Formulation
Agent
! cyclophosphamide, Dose
adjustment
recommendations, Formula
dosing, etc.
ifosamide Intravenous 1.2 g/m2 daily 5 consecutive days
(IfexCz,) Repeat every 3 weeks or
after recovery from
hematologic toxicity
topotecan Intravenous 1.5 mg/m2 by intravenous 5 consecutive days,
hydrochloride infusion over 30 minutes starting on day 1 of 21
day
(Hycamting) daily course
Bisphosphonates Intravenous 60 mg or 90 mg single
Pamidronate or Oral infusion over 4 - 24 hours to
correct hypercalcemia in
Alendronate take with
cancer patients
Risedronate 6-8 oz
water. 5 mg/d daily for 2 years and
then 10 mg/d for 9 month to
prevent or control bone
resorption.
5.0 mg to prevent or control
bone resorption.
Lovastatin Oral 10 - 80 mg/day in single or
(MevacorTm) two divided dose.
[00375] The disclosure also encompasses administration of an anti-
ICOSantibody
formulation of the disclosure in combination with radiation therapy comprising
the use of x-rays,
gamma rays and other sources of radiation to destroy the cancer cells. In
particular
embodiments, the radiation treatment is administered as external beam
radiation or teletherapy
wherein the radiation is directed from a remote source. In other embodiments,
the radiation
treatment is administered as internal therapy or brachytherapy wherein a
radiaoactive source is
placed inside the body close to cancer cells or a tumor mass.
[00376] Cancer therapies and their dosages, routes of administration and
recommended
usage are known in the art and have been described in such literature as the
Physician's Desk
Reference (56th ed., 2002).
5.24. Antibodies Having Increased Half-Lives
[00377] The present disclosure provides for formulations of antibodies and
antibody
fragments that specifically bind to an antigen of interest (e.g., ICOS) which
have an extended
half-life in vivo. In particular, the present disclosure provides formulations
of antibodies and
112
CA 02743469 2016-06-13
51332-100
antibody fragments that specifically bind to an antigen of interest (e.g.,
TOGS) which have a half-
life in a mammal (for example, but not limited to, a human), of greater than 3
days, greater than
7 days, greater than 10 days, greater than 15 days, greater than 25 days,
greater than 30 days,
greater than 35 days, greater than 40 days, greater than 45 days, greater than
2 months, greater
than 3 months, greater than 4 months, or greater than 5 months.
[00378] To prolong the serum circulation of antibodies (for example,
but not limited to,
monoclonal antibodies and single chain antibodies) or antibody fragments (for
example, but not
limited to, Fab fragments) in vivo, for example, inert polymer molecules such
as high molecular
weight polyethyleneglycol (PEG) can be attached to the antibodies (including
antibody
fragments thereof) with or without a multifunctional linker either through
site-specific
conjugation of the PEG to the N¨ or C-terminus of the antibodies or via
epsilon-amino groups
present on lysine residues. Linear or branched polymer derivatization that
results in minimal
loss of biological activity will be used. The degree of conjugation can be
closely monitored by
SDS-PAGE and mass spectrometry to ensure proper conjugation of PEG molecules
to the
antibodies. Unreacted PEG can be separated from antibody-PEG conjugates by
size-exclusion or
by ion-exchange chromatography. PEG-derivatized antibodies (including antibody
fragments
thereof) can be tested for binding activity as well as for in vivo efficacy
using methods known to
those of skill in the art, for example, by immunoassays described herein.
[00379] Antibodies having an increased half-life in vivo can also be
generated introducing
one or more amino acid modifications (i.e., substitutions, insertions or
deletions) into an IgG
constant domain, or Fcrin binding fragment thereof (e.g., Fe or hinge-Fe
domain fragment). See,
e.g., International Publication No. WO 98/23289; International Publication No.
WO 97/34631;
and U.S. Patent No. 6,277,375.
[00380] Further, antibodies (including antibody fragments thereof) can
be conjugated to
albumin in order to make the antibody (including antibody fragment thereof)
more stable in vivo
or have a longer half life in vivo. The techniques are well known in the art,
see e.g., International
Publication Nos. WO 93/15199, WO 93/15200, and WO 01/77137; and European
Patent No. EP
413, 622.
5.25. Methods Of Preparing the Antibody Formulations
[00381] The present disclosure provides methods for preparing liquid
formulations of
antibodies or derivatives, analogues, or fragments thereof that specifically
bind to an antigen of
interest (e.g., human ICOS polypeptide). The methods for preparing liquid
formulations of the
present disclosure may comprise: purifying the antibody (including antibody
fragment thereof)
from conditioned medium (either single lots or pooled lots of medium) and
concentrating a
113
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
fraction of the purified antibody (including antibody fragment thereof) to a
final concentration of
about 15 mg/ml, about 20 mg/ml, about 30 mg/ml, about 40 mg/ml, about 50
mg/ml, about 60
mg/ml, about 70 mg/ml, about 80 mg/ml, about 90 mginal, about 100 mg/ml, about
150 mg/ml,
about 175 mg/ml, about 200 mg/ml, about 250 mg/ml, or about 300 mg/ml.
Conditioned
medium containing the antibody (including antibody fragment thereof), for
example, an antibody
that specifically binds to ICOS may be subjected to CUNO filtration and the
filtered antibody is
subjected to HS50 cation exchange chromatography. The fraction from the HS50
cation
exchange chromatography is then subjected to low pH treatment followed by MEP
Hypercel
chromatography. The fraction from the MEP Hypercel chromatography is subject
to
nanofiltration. The purified antibody or a fragment thereof obtained after
nanofiltration is then
subjected to diafiltration and ultrafiltration to buffer exchange and
concentrate into the
formulation buffer using the same membrane.
[00382] The liquid
formulations of the present disclosure can be prepared as unit dosage
forms by preparing a vial containing an aliquot of the liquid formulation for
a one-time use. For
example, a unit dosage per vial may contain 1 ml, 2 ml, 3 ml, 4 ml, 5 ml, 6
ml, 7 ml, 8 ml, 9 ml,
ml, 15 ml, or 20 ml of different concentrations of an antibody (including
antibody fragment
thereof) that specifically binds to ICOS ranging from about 10 mg/ml to about
300 mg/ml. If
necessary, these preparations can be adjusted to a desired concentration by
adding a sterile
diluent to each vial. In a specific embodiment, the liquid formulations of the
present disclosure
are formulated into single dose vials as a sterile liquid that contains 10 mM
histidine buffer at pH
6.0, 80 mM NaC1, 4% trehalose and 0.02% polysorbate 80. Each 1.0 mL of
solution contains
100 mg of the antibody (including antibody fragment thereof). In one
embodiment, the antibody
(including antibody fragment thereof) of the disclosure is supplied at 100
mg/ml in 3 cc USP
Type I borosilicate amber vials (West Pharmaceutical Services - Part No. 6800-
0675). The
target fill volume is 1.2 mL.
[00383] The liquid
formulations of the present disclosure can be prepared as unit dosage
forms by preparing a pre-filled syringe containing an aliquot of the liquid
formulation for a one-
time use. For example, a unit dosage per pre-filled syringe may contain 0.1m1,
0.2 ml, 0.3 ml,
0.4 ml, 0.5 ml, 0.6 ml, 0.7 ml, 0.8 ml, 0.9 ml, 1 ml, 2 ml, 3 ml, 4 ml, 5 ml,
6 ml, 7 ml, 8 ml, 9 ml,
10 ml, 15 ml, or 20 ml of different concentrations of an antibody (including
antibody fragment
thereof) that specifically binds to 'COS ranging from about 10 mg/ml to about
300 mg/ml. In a
specific embodiment, the liquid formulations of the present disclosure are
formulated into single
dose pre-filled syringes as a sterile liquid that contains 10 mM histidine
buffer at pH 6.0, 80 mM
NaC1, 4% trehalose and 0.02% polysorbate 80. Each 1.0 mL of solution contains
100 mg of the
antibody (including antibody fragment thereof).
114
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00384] The liquid formulations of the present disclosure may be sterilized
by various
sterilization methods, including sterile filtration, radiation, etc. In a
specific embodiment, the
diafiltrated antibody formulation is filter-sterilized with a presterilized
0.2 micron filter.
Sterilized liquid formulations of the present disclosure may be administered
to a subject to
prevent, treat and/or manage a disease or disorder associated with or
characterized by aberrant
expression and/or activity of ICOS, a disease or disorder associated with or
characterized by
aberrant expression and/or activity of ICOS receptor, an autoimmune disease or
disorder, an
inflammatory disease or disorder, a T cell proliferative disease or disorder,
a malignancy, a T cell
malignancy, transplant rejection, graft versus host disease, or one or more
symptoms thereof.
[00385] Although the disclosure is directed to liquid non-lyophilized
formulations, it
should be noted for the purpose of equivalents that the formulations of the
disclosure may be
lyophilized if desired. Thus, the disclosure encompasses lyophilized forms of
the formulations
of the disclosure.
5.26. Methods Of Monitoring The Stability And Aggregation Of Antibody
Formulations
[00386] There are various methods available for assessing the stability of
protein
formulations, including antibody formulations, based on the physical and
chemical structures of
the proteins as well as on their biological activities. For example, to study
denaturation of
proteins, methods such as charge-transfer absorption, thermal analysis,
fluorescence
spectroscopy, circular dichroism (CD), NMR, reducing capillary gel
electrophoresis (rCGE) and
high performance size exclusion chromatography (HPSEC), tangential flow
filtration (TFF),
static light scattering (SLS), Fourier Transform Infrared Spectroscopy (FTIR),
urea-induced
protein unfolding techniques, intrinsic tryptophan fluorescence, differential
scanning
calorimetry, and 1-anilino-8-naphthalenesulfonic acid (ANS) protein binding
techniques are
available. See, for example, Wang et al., 1988, J. of Parenteral Science &
Technology
42(Suppl):54-526.
[00387] rCGE and HPSEC are the most common and simplest methods to assess
the
formation of protein aggregates, protein degradation, and protein
fragmentation. Accordingly,
the stability of the liquid formulations of the present disclosure may be
assessed by these
methods.
[00388] For example, the stability of the liquid formulations of the
present disclosure may
be evaluated by HPSEC, wherein the percent area of the peaks represents the
non-degraded
antibody or non-degraded antibody fragments. In particular, approximately 250
jig of the
antibody (including antibody fragment thereof) (approximately 25 !al of a
liquid formulation
comprising 10 mg/ml said antibody or antibody fragment) is injected onto a
TosoH Biosep TSK
115
CA 02743469 2016-06-13
51332-100
G3000SW)c column (7.8 min x 30 cm) fitted with a TSK SW xl guard column (6.0
mm CX 4.0
cm). The antibody (including antibody fragment thereof) is eluted
isocratically with 0.1 M
disodium phosphate containing 0.1 M sodium sulfate and 0.05% sodium azide, at
a flow rate of
0.8 to 1.0 ml/min. Eluted protein is detected using UV absorbance at 280 nm.
Reference
standards are run in the assay as controls, and the results are reported as
the area percent of the
product monomer peak compared to all other peaks excluding the included volume
peak
observed at approximately 12 to 14 minutes. Peaks eluting earlier than the
monomer peak are
recorded as percent aggregate.
[00389] The liquid formulations of the present disclosure exhibit low
to undetectable
levels of aggregation as measured by any of the methods described above, that
is, no more than
5%, no more than 4%, no more than 3%, no more than 2%, no more than 1%, and no
more than
0.5% aggregate by weight protein, and low to undetectable levels of
fragmentation, that is, 80%
or higher, 85% or higher, 90% or higher, 95% or higher, 98% or higher, or 99%
or: higher, or
99.5% or higher of the total peak area in the peak(s) representing intact
antibodies (including
antibody fragments thereof). When SDS-PAGE is used to measure antibody
fragmentation, the
density or the radioactivity of each band stained or labeled with radioisotope
can be measured
and the % density or % radioactivity of the band representing non-degraded
antibodies
(including antibody fragments thereof) can be obtained.
[003901 The stability of the liquid formulations of the present
disclosure can be also
assessed by any assays which measure the biological activity of the antibody
in the formulation.
The biological activities of antibodies include, but are not limited to,
antigen-binding activity,
blocking of ligand-receptor interaction, and so forth (see infra). Antigen-
binding activity of the
antibodies (including antibody fragments thereof) can be measured by any
method known to
those skilled in the art, including but not limited to ELISA,
radioinamunoassay, Western blot,
and the like. Also see Harlow et al., Antibodies: A Laboratory Manual,
(Cold Spring Harbor Laboratory Press, 2nd ed. 1988). An ELISA
based assay, e.g., may be used to compare the ability of an antibody
(including antibody
fragments thereof) to specifically bind to an ICOS polypeptide to that of a
reference standards
antibody.
[00391] The purity of the liquid antibody formulations of the
disclosure may be measured
by any method well-known to one of skill in the art such as, for example, but
not limited to,
HPSEC. The sterility of the liquid antibody formulations may be assessed by
any method well-
known to one of skill in the art such as, e.g.: sterile soybean-casein digest
medium and fluid
thioglycollate medium are inoculated with a test liquid antibody formulation
by filtering the
liquid antibody formulation through a sterile filter having a nominal porosity
of 0.45 p.m. When
116
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
using the SterisureTM or SteritestTM method, each filter device is aseptically
filled with
approximately 100 ml of sterile soybean-casein digest medium or fluid
thioglycollate medium.
When using the conventional method, the challenged filter is aseptically
transferred to 100 ml of
sterile soybean-casein digest medium or fluid thioglycollate medium. The media
are incubated
at appropriate temperatures and observed three times over a 14 day period for
evidence of
bacterial or fungal growth.
5.27.Methods Of Administering The Antibody Formulations
[00392] The disclosure provides methods of prevention, treatment and/or
management of
a disorder, for example, a disease or disorder associated with or
characterized by aberrant
expression and/or activity of ICOS, a disease or disorder associated with or
characterized by
aberrant expression and/or activity of ICOS receptor, an autoimmune disease or
disorder, an
inflammatory disease or disorder, a T cell proliferative disease or disorder,
a malignancy, a T cell
malignancy, transplant rejection, graft versus host disease, or one or more
symptoms thereof by
administrating to a subject of an effective amount of liquid formulations of
the disclosure.
Various delivery systems are known and can be used to administer a liquid
formulation of the
present disclosure or a prophylactic or therapeutic agent. Methods of
administering antibody
liquid formulations of the present disclosure or a therapy (e.g., a
prophylactic or therapeutic
agent) include, but are not limited to, parenteral administration (e.g.,
intradermal, intramuscular,
intraperitoneal, intravenous and, and subcutaneous), epidural administration,
topical
administration, and mucosa' administration (for example, but not limited to,
intranasal and oral
routes). In a specific embodiment, liquid formulations of the present
disclosure are administered
intramuscularly, intravenously, or subcutaneously. In one embodiment, the
liquid formulations
of the disclosure are administered subcutaneously. The formulations may be
administered by
any convenient route, for example by infusion or bolus injection, by
absorption through
epithelial or mucocutaneous linings (e.g., oral mucosa, rectal and intestinal
mucosa, etc.) and
may be administered together with other biologically active agents.
Administration can be
systemic or local.
[00393] The disclosure also provides that a liquid formulation of the
present disclosure is
packaged in a hermetically sealed container such as an ampoule or sachette
indicating the
quantity of antibody (including antibody fragment thereof). In one embodiment,
a liquid
formulation of the present disclosure is in a hermetically sealed container
indicating the quantity
and concentration of the antibody (including antibody fragment thereof). In
one embodiment, a
liquid formulation of the present disclosure is supplied in a hermetically
sealed container and
comprises about 10 mg/ml, about 15 mg/ml, about 20 mg/ml, about 30 mg/ml,
about 40 mg/ml,
117
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
about 50 mg/ml, about 60 mg/ml, about 70 mg/ml, about 80 mg/ml, about 90
mg/ml, about 100
mg/ml, about 110 mg/ml, about 120 mg/ml, about 130 mg/ml, about 150 mg/ml,
about 175
mg/ml, about 200 mg/ml, about 250 mg/ml, or about 300 mg/ml of an antibody
(including
antibody fragment thereof) that specifically binds to human ICOS, in a
quantity of about 1 ml,
about 2 ml, about 3 ml, about 4 ml, about 5 ml, 6 about ml, about 7 ml, about
8 ml, about 9 ml,
about 10 ml, about 15 ml, or about 20 ml. In a specific embodiment of the
disclosure, a liquid
formulation of the disclosure is supplied in a hermetically sealed container
and comprises at least
about 15 mg/ml, at least about 20 mg/ml, at least about 25 mg/ml, at least
about 50 mg/ml, at
least about 100 mg/ml, at least about 110 mg/ml, at least about 120 mg/ml, at
least about 130
mg/ml, at least about 150 mg/ml, at least about 175 mg/ml, at least about 200
mg/ml, at least
about 250 mg/m1 or at least about 300 mg/ml of an antibody (including antibody
fragment
thereof) that specifically binds to human ICOS (for example, but not limited
to, or an antigen-
binding fragment thereof) for intravenous injections, and at least about 15
mg/ml, at least about
20 mg/ml, at least about 50 mg/ml, at least about 80 mg/ml, at least about 100
mg/ml, at least
about 110 mg/ml, at least about 120 mg/ml, at least about 130 mg/ml, at least
about 150 mg/ml,
at least about 175 mg/ml, at least about 200 mg/ml, at least about 250 mg/ml
or at least about
300 mg/ml of an antibody (including antibody fragment thereof) that
specifically binds to human
ICOS (for example, but not limited to, or a fragment thereof) for repeated
subcutaneous
administration.
[00394] The amount of a liquid formulation of the present disclosure which
will be
effective in the prevention, treatment and/or management of a disease or
disorder associated with
or characterized by aberrant expression and/or activity of ICOS, a disease or
disorder associated
with or characterized by aberrant expression and/or activity of ICOS receptor,
an autoimmune
disease or disorder, an inflammatory disease or disorder, a T cell
proliferative disease or
disorder, a malignancy, a T cell malignancy, transplant rejection, graft
versus host disease, or
one or more symptoms thereof can be determined by standard clinical techniques
well-known in
the art or described herein. The precise dose to be employed in the
formulation will also depend
on the route of administration, and the seriousness of the inflammatory
disorder, or autoimmune
disorder, and should be decided according to the judgment of the practitioner
and each patient's
circumstances. Effective doses may be extrapolated from dose-response curves
derived from in
vitro or animal model test systems.
5.28. PHARMACEUTICAL FORMULATIONS
[00395] The disclosure also relates to immunotherapeutic formulations and
methods for
the treatment of T cell-mediated diseases and disorders in human subjects,
such as, but not
118
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
limited to, chronic infection, autoimmune disease or disorder, inflammatory
disease or disorder,
graft-versus-host disease (GVHD), transplant rejection, and T cell
proliferative disorder in
human subjects, using therapeutic antibodies that bind to the ICOS antigen and
mediate human
ADCC.
[00396] The present disclosure relates to pharmaceutical formulations
comprising effector
function enhanced anti-ICOS antibodies of the IgG1 or IgG3 human isotype. The
present
disclosure also relates to pharmaceutical formulations comprising human or
humanized
anti-ICOS antibodies of the IgG2 or IgG4 human isotype that mediate human
ADCC. In certain
embodiments, the present disclosure also relates to pharmaceutical
formulations comprising
monoclonal anti-ICOS antibodies with enhanced effector.
[00397] Therapeutic formulations and regimens are described for treating
human subjects
diagnosed with autoimmune diseases, such as, but not limited to, systemic
lupus erythematosus,
rheumatoid arthritis, immune thrombocytopenic purpura (ITP), diabetes,
psoriasis, and
hypersensitivity reactions (e.g., allergies, hay fever, asthma, and acute
edema cause type I
hypersensitivity reactions). The present disclosure also relates to
formulations and regimens for
the treatment of human subjects diagnosed with chronic inflammatory diseases,
such as, but not
limited to, inflammatory bowel disease (Crohn's disease and ulcerative
colitis), Grave's disease,
Hashimoto's thyroiditis, and diabetes mellitus.
[00398] Therapeutic formulations and regimens are described for treating
human subjects
diagnosed with T cell malignancies that derive from ICOS expressing T cells
and their
precursors.
[00399] In particular embodiments, a formulation of the disclosure
comprises an anti-
ICOS antibody that may mediate ADCC, complement-dependent cellular
cytotoxicity, or
antibody-dependent phagocytosis. formulations and methods of the present
disclosure also have
the advantage of targeting a narrower population of T cells than other T cell
directed
immunotherapies. For example, formulations of the present disclosure may be
effective to
specifically target activated T cells, for example, but not limited to,
activated T cells.
Accordingly, methods and formulations of the disclosure may be effective to
reduce or deplete
circulating activated CD4+ T cells as well as activated CD8+ T cells.
[00400] Accordingly, in one aspect, the disclosure provides anti-ICOS
antibody
formulations for the treatment and prevention of GVHD and graft rejection,
which are associated
with fewer and/or less severe complications than less-targeted therapeutic
agents and regimens.
In one embodiment, formulations and methods of the disclosure are used with
lower doses of
traditional therapeutic agents than would be possible in the absence of the
methods and
formulations of the disclosure. In another embodiment, formulations and
methods of the
119
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
disclosure obviate the need for a more severe form of therapy, such as
radiation therapy,
high-dose chemotherapy, or splenectomy.
[00401] In certain embodiments, anti-ICOS antibody formulations may be
administered to
a transplant recipient patient prior to or following transplantation, alone or
in combination with
other therapeutic agents or regimens for the treatment or prevention of GVHD
and graft
rejection. For example, anti-ICOS antibody formulations may be used to deplete
activated T
cells from a transplant recipient prior to or following transplantation of an
allogeneic graft.
Anti-ICOS antibody formulations may also be used to deplete activated T cells
from the graft ex
vivo, prior to transplantation, or in the donor, or as prophylaxis against
GVHD and graft
rejection.
5.29. PHARMACEUTICAL FORMULATIONS, ADMINISTRATION AND DOSING
[00402] Pharmaceutical formulations of the disclosure contain as the active
ingredient
anti-ICOS antibodies with enhanced effector function. The formulations contain
naked antibody,
immunoconjugate, or fusion protein in an amount effective for producing the
desired response in
a unit of weight or volume suitable for administration to a human patient, and
are preferably
sterile. The response can, for example, be measured by determining the
physiological effects of
the anti-ICOS antibody formulation, such as, but not limited to, T cell
depletion, IL-17 depletion,
regression of a T cell malignancy, or decrease of disease symptoms. Other
assays will be known
to one of ordinary skill in the art and can be employed for measuring the
level of the response
(for example, but not limited to SLEDAL BILAG, PRO). Additional assays that
may be used to
monitor response include, but are not limited to, immunohistochemistry of
tissue biopsy (e.g.,
skin biopsy), 1COS mRNA expression in tissue sample (e.g., skin biopsy, tonsil
biopsy, blood),
flow cytometry of blood cells, microarray analysis of tissue sample (e.g.,
skin biopsy, blood),
proteomics analysis of tissue sample (e.g., skin biopsy, blood), antibody
array analysis, SNP
analysis.
5.29.1. ADMINISTRATION AND DOSING
[00403] Administration of formulations of the disclosure to a human patient
can be by any
route, including but not limited to intravenous, intradermal, transdermal,
subcutaneous,
intramuscular, inhalation (e.g., via an aerosol), buccal (e.g., sub-lingual),
topical (i.e., both skin
and mucosal surfaces, including airway surfaces), intrathecal, intraarticular,
intraplural,
intracerebral, intra-arterial, intraperitoneal, oral, intralymphatic,
intranasal, rectal or vaginal
administration, by perfusion through a regional catheter, or by direct
intralesional injection. In
120
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
one embodiment, formulations of the disclosure are administered by intravenous
push or
intravenous infusion given over defined period (e.g., 0.5 to 2 hours).
Formulations of the
disclosure can be delivered by peristaltic means or in the form of a depot,
although the most
suitable route in any given case will depend, as is well known in the art, on
such factors as the
species, age, gender and overall condition of the subject, the nature and
severity of the condition
being treated and/or on the nature of the particular formulation (i.e.,
dosage, formulation) that is
being administered. In particular embodiments, the route of administration is
via bolus or
continuous infusion over a period of time, once or twice a week. In other
particular
embodiments, the route of administration is by subcutaneous injection,
optionally once, twice,
three times or four times monthly. In one embodiment, formulations, and/or
methods of the
disclosure are administered on an outpatient basis.
[00404] In certain embodiments, the dose of a formulation comprising anti-
ICOS antibody
is measured in units of mg/kg of patient body weight. In other embodiments,
the dose of a
formulation comprising anti-ICOS antibody is measured in units of mg/kg of
patient lean body
weight (i.e., body weight minus body fat content). In yet other embodiments,
the dose of a
formulation comprising anti-ICOS antibody is measured in units of mg/m2 of
patient body
surface area. In yet other embodiments, the dose of a formulation comprising
anti-ICOS
antibody is measured in units of mg per dose administered to a patient. Any
measurement of
dose can be used in conjunction with formulations and methods of the
disclosure and dosage
units can be converted by means standard in the art.
[00405] Those skilled in the art will appreciate that dosages can be
selected based on a
number of factors including the age, sex, species and condition of the subject
(e.g., stage of
disease), the desired degree of cellular depletion, the disease to be treated
and/or the particular
antibody or antigen-binding fragment being used and can be determined by one
of skill in the art.
For example, effective amounts of formulations of the disclosure may be
extrapolated from
dose-response curves derived in vitro test systems or from animal model (e.g.,
the cotton rat or
monkey) test systems. Models and methods for evaluation of the effects of
antibodies are known
in the art (Wooldridge et al., Blood, 89(8): 2994-2998 (1997)), incorporated
by reference herein
in its entirety). In certain embodiments, for particular ICOS expressing T
cell malignancies,
therapeutic regimens standard in the art for antibody therapy can be used with
formulations and
methods of the disclosure.
[00406] Examples of dosing regimens that can be used in methods of the
disclosure
include, but are not limited to, daily, three times weekly (intermittent),
weekly, bi-weekly,
monthly, bi-monthly, or quarterly (once every three month). In certain
embodiments, dosing
regimens include, but arc not limited to, monthly dosing or dosing every 6-8
weeks.
121
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00407] Those skilled in the art will appreciate that dosages are generally
higher and/or
frequency of administration greater for initial treatment as compared with
maintenance regimens.
[00408] In some embodiments of the disclosure, anti-ICOS antibodies bind to
ICOS
expressing T cells and may result in efficient (i.e., at low dosage) depletion
of ICOS expressing
T cells (as described herein). In certain embodiments, dosages of the antibody
(optionally in a
pharmaceutically acceptable carrier as part of a pharmaceutical formulation)
are at least about
0.0005, 0.001, 0.05, 0.075, 0.1, 0.25, 0.375, 0.5, 1, 2.5, 5, 10, 20, 37.5, or
50 mg/m2 and/or less
than about 500, 475, 450, 425, 400, 375, 350, 325, 300, 275, 250, 225, 200,
175, 150, 125, 100,
75, 60, 50, 37.5, 20, 15, 10, 5, 2.5, 1, 0.5, 0.375, 0.1, 0.075 or 0.01 mg/m2.
In certain
embodiments, the dosage is between about 0.0005 to about 200 mg/m2, between
about 0.001 and
150 mg/m2. between about 0.075 and 125 mg/m2, between about 0.375 and 100
mg/m2, between
about 2.5 and 75 mg/m2, between about 10 and 75 mg/m2, and between about 20
and 50 mg/m2.
In related embodiments, the dosage of anti-ICOS antibody used is at least
about 0.1, 0.2, 0.3, 0.4,
0.5, 0.6, 0.7, 0.8, 0.9, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7,
7.5, 8, 8.5, 9, 9.5, 10, 10.5, 11,
11.5, 12, 12.5, 13, 13.5, 14, 14.5, 15, 15.5, 16, 16.5, 17, 17.5, 18, 18.5,
19, 19.5, 20, 20.5 mg/kg
of body weight of a patient. In certain embodiments, the dose of naked anti-
ICOS antibody used
is at least about Ito 10,5 to 15, 10 to 20, or 15 to 25 mg/kg of body weight
of a patient. In
certain embodiments, the dose of anti-ICOS antibody used is at least about 1
to 20, 3 to 15, or 5
to 10 mg/kg of body weight of a patient. In other embodiments, the dose of
anti-ICOS antibody
used is at least about 5, 6, 7, 8,9, or 10 mg/kg of body weight of a patient.
In certain
embodiments, a single dosage unit of the antibody (optionally in a
pharmaceutically acceptable
carrier as part of a pharmaceutical formulation) can be at least about 0.5, 1,
2, 4, 6, 8, 10, 12, 14,
16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46, 48, 50, 52,
54, 56, 58, 60, 62, 64, 66,
68, 70, 72, 74, 76, 78, 80, 82, 84, 86, 88, 90, 92, 94, 96, 98, 100, 102, 104,
106, 108, 110, 112,
114, 116, 118, 120, 122, 124, 126, 128, 130, 132, 134, 136, 138, 140, 142,
144, 146, 148, 150,
152, 154, 156, 158, 160, 162, 164, 166, 168, 170, 172, 174, 176, 178, 180,
182, 184, 186, 188,
190, 192, 194, 196, 198, 200, 204, 206, 208, 210, 212, 214, 216, 218, 220,
222, 224, 226, 228,
230, 232, 234, 236, 238, 240, 242, 244, 246, 248, or 250 micrograms/m2. In
other embodiments,
dose is up to 100 mg per single dosage unit.
[00409] In some embodiments of methods of this disclosure, antibodies
and/or
formulations of this disclosure can be administered at a dose lower than about
375 mg/m2; at a
dose lower than about 37.5 mg/m2; at a dose lower than about 0.375 mg/m2;
and/or at a dose
between about 0.075 mg/m2 and about 125 mg/m2. In certain embodiments of
methods of the
disclosure, dosage regimens comprise low doses, administered at repeated
intervals. For
example, in one embodiment, formulations of the disclosure can be administered
at a dose lower
122
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
than about 375 mg/m2 at intervals of approximately every 1, 2, 3, 4, 5, 6, 7,
8, 9, 10, 14, 15, 20,
21, 25, 30, 35, 40, 45, 50, 60, 70, 80, 90, 100, 120, 125, 150, 175, or 200
days.
[00410] The specified dosage can result in ICOS expressing T cell depletion
in the human
treated using formulations and methods of the disclosure for a period of at
least about 1, 2, 3, 5,
7, 10, 14, 20, 30, 45, 60, 75, 90, 120, 150 or 180 days or longer. In certain
embodiments of
methods of the disclosure, ICOS expressing T cells are depleted by at least
30%, 40%, 50%,
60%, 70%, 80%, 90%, or 100% in comparison to ICOS expressing T cell levels in
the patient
being treated before use of formulations and methods of the disclosure. In
other embodiments of
methods of the disclosure, ICOS expressing T cells are depleted by at least
30%, 40%, 50%,
60%, 70%, 80%, 90%, or 100% in comparison to typical standard ICOS expressing
T cell levels
for humans. In related embodiments, the typical standard ICOS expressing T
cell levels for
humans are determined using patients comparable to the patient being treated
with respect to age,
sex, weight, and other factors.
[00411] In certain embodiments of the disclosure, a dosage of about 125
mg/m2 or less of
an antibody or antigen-binding fragment results in ICOS expressing T cell
depletion for a period
of at least about 7, 14, 21, 30, 45, 60, 90, 120, 150, or 200 days. In another
representative
embodiment, a dosage of about 37.5 mg/m2 or less depletes ICOS expressing T
cells for a period
of at least about 7, 14, 21, 30, 45, 60, 90, 120, 150, or 200 days. In still
other embodiments, a
dosage of about 0.375 mg/m2 or less results in depletion of ICOS expressing T
cells for at least
about 7, 14, 21, 30, 45 or 60 days. In another embodiment, a dosage of about
0.075 mg/m7 or
less results in depletion of ICOS expressing T cells for a period of at least
about 7, 14, 21, 30, 45,
60, 90, 120, 150, or 200 days. In yet other embodiments, a dosage of about
0.01 mg/m2, 0.005
mg/m2 or even 0.001 mg/m2 or less results in depletion of ICOS expressing T
cells for at least
about 3, 5, 7, 10, 14, 21, 30, 45, 60, 90, 120, 150, or 200 days. According to
these embodiments,
the dosage can be administered by any suitable route, but is optionally
administered by a
subcutaneous route.
[00412] As another aspect, the disclosure provides the discovery that ICOS
expressing T
cell depletion and/or treatment of T cell-mediated disorders can be achieved
at lower dosages of
antibody or antibody fragments than employed in currently available methods.
Thus, in another
embodiment, the disclosure provides a method of depleting ICOS expressing T
cells and/or
treating a T cell-mediated disorder, comprising administering to a human, an
effective amount of
an antibody that specifically binds to ICOS, wherein a dosage of about 500,
475, 450, 425, 400,
375, 350, 325, 300, 275, 250, 225, 200, 175, 150, 125, 100, 75, 60, 50, 37.5,
20, 10, 5, 2.5, 1,
0.5, 0.375, 0.25, 0.1, 0.075, 0.05, 0.001,0.0005 mg/m2 or less results in a
depletion of ICOS
expressing T cells (circulating and/or tissue ICOS expressing T cells) of 25%,
35%, 50%, 60%,
123
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
75%, 80%, 85%, 90%, 95%, 98% or more for a period at least about 3, 5, 7, 10,
14, 21, 30, 45,
60, 75, 90, 120, 150, 180, or 200 days or longer. In representative
embodiments, a dosage of
about 125 mg/m2 or 75 mg/m2 or less results in at least about 50%, 75%, 85% or
90% depletion
of ICOS expressing T cells for at least about?, 14, 21, 30, 60, 75, 90, 120,
150 or 180 days. In
other embodiments, a dosage of about 50, 37.5 or 10 mg/m2 results in at least
about a 50%, 75%,
85% or 90% depletion of ICOS expressing T cells for at least about?, 14, 21,
30, 60, 75, 90, 120
or 180 days. In still other embodiments, a dosage of about 0.375 or 0.1 mg/m2
results in at least
about a 50%, 75%, 85% or 90% depletion of ICOS expressing T cells for at least
about 7, 14, 21,
30, 60, 75 or 90 days. In further embodiments, a dosage of about 0.075, 0.01,
0.001, or 0.0005
mg/m2 results in at least about a 50%, 75%, 85% or 90% depletion of ICOS
expressing T cells
for at least about 7, 14, 21, 30 or 60 days.
[00413] In certain embodiments of the disclosure, the dose can be escalated
or reduced to
maintain a constant dose in the blood or in a tissue, such as, but not limited
to, bone marrow. In
related embodiments, the dose is escalated or reduced by about 2%, 5%, 8%,
10%, 15%, 20%,
30%, 40%, 50%, 60%, 70%, 80%, 90%, and 95% in order to maintain a desired
level of an
antibody of formulations and methods of the disclosure.
[00414] In certain embodiments, the dosage can be adjusted and/or the
infusion rate can
be reduced based on patient's immunogenic response to formulations and methods
of the
disclosure.
[00415] For formulations of the antibodies, proteins, polypeptides,
peptides and fitsion
proteins encompassed by the disclosure, the dosage administered to a patient
may be calculated
using the patient's weight in kilograms (kg) multiplied by the dose to be
administered in mg/kg.
The required volume (in mL) to be given is then determined by taking the mg
dose required
divided by the concentration of the antibody formulation. The final calculated
required volume
will be obtained by pooling the contents of as many vials as are necessary
into syringe(s) to
administer the antibody formulation of the disclosure. The final calculated
required volume will
be obtained by pooling the contents of as many vials as are necessary into
syringe(s) to
administer the drug. A maximum volume of 2.0 mL of the antibody formulation
can be injected
per site. The dose (in mL) can be calculated using the following formula: Dose
(mL) =
[volunteer weight] (kg) x [dose] mg/kg 100 mg/mL of the antibody
formulation. Generally,
human antibodies have a longer half-life within the human body than antibodies
from other
species due to the immune response to the foreign polypeptidcs. Thus, lower
dosages of human
antibodies and less frequent administration is often possible. Further, the
dosage, volume and
frequency of administration of liquid formulations of the present disclosure
may be reduced by
increasing the concentration of an antibody (including antibody fragment
thereof) in the
124
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
formulations, increasing affinity and/or avidity of the antibody (including
antibody fragment
thereof), and/or increasing the half-life of the antibody (including antibody
fragment thereof).
[00416] In a specific embodiment, the dosage administered to a patient will
be calculated
using the patient's weight in kilograms (kg) multiplied by the dose to be
administered in mg/kg.
The required volume (in mL) to be given is then determined by taking the mg
dose required
divided by the concentration of the antibody (including antibody fragment
thereof) in the
formulations (100 mg/mL). The final calculated required volume may be obtained
by pooling
the contents of as many vials as are necessary into syringe(s) to administer
the drug. A
maximum volume of 2.0 mL of antibody (including antibody fragment thereof) in
the
formulations can be injected per site.
[00417] In one embodiment, 0.01 to 20 mg/kg/week, 0.01 to 10 mg/kg/week,
0.01 to 5
mg/week, 0.01 to 2 mg/week, 0.01 to 1 mg/week, 0.01 to 0.5 mg/week, 0.01 to
0.2 mg/week,
0.01 to 0.1 mg/week of an antibody (including antibody fragment thereof) that
specifically binds
to human ICOS (for example, but not limited to, or a fragment thereof) in a
liquid formulation of
the disclosure is administered to a subject with an inflammatory disorder, an
autoimmune
disorder or a malignancy. In another embodiment, 0.01 to 20 mg/kg/month, 0.01
to 10
mg/kg/month, 0.01 to 5 mg/month, 0.01 to 2 mg/month, 0.01 to 1 mg/month, 0.01
to 0.5
mg/month, 0.01 to 0.2 mg/month, 0.01 to 0.1 mg/month of an antibody (including
antibody
fragment thereof) that specifically binds to human ICOS (for example, but not
limited to, or a
fragment thereof) in a liquid formulation of the disclosure is administered to
a subject with an
inflammatory disorder, an autoimmune disorder or a malignancy. In a further
embodiment, 0.01
to 20 mg/kg/2 month, 0.01 to 10 mg/kg/2 month, 0.01 to 5 mg/2 month, 0.01 to 2
mg/2 month,
0.01 to 1 mg/2 month, 0.01 to 0.5 mg/2 month, 0.01 to 0.2 mg/2 month, 0.01 to
0.1 mg/2 month
of an antibody (including antibody fragment thereof) that specifically binds
to human ICOS (for
example, but not limited to, or a fragment thereof) in a liquid formulation of
the disclosure is
administered to a subject with an inflammatory disorder, an autoimmune
disorder or a
malignancy. In another embodiment, a subject is administered one or more doses
of a
prophylactically or therapeutically effective amount of a liquid formulation
of the disclosure,
wherein the prophylactically or therapeutically effective amount is not the
same for each dose.
[00418] In one embodiment, a liquid formulation of the disclosure is
administered in a
dosing regimen that maintains the plasma concentration of the antibody
specific for human 1COS
at a desirable level (e.g., from about 0.001 to about 100 ug/m1), which
continuously depletes
ICOS expressing cells. In a specific embodiment, the plasma concentration of
the antibody is
maintained at about 0.001 jig/ml, about 0.01 Wm], about 0.1 jig/ml, about 0.2
jig/nil, about 0.5
ug/ml, about 1 jig/ml, about 2 ug/ml, about 3 g/ml, about 4 jig/ml, about 5
jig/ml, about 6
125
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
jig/ml, about 7 jig/ml, about 8 jig/ml, about 9 jig/ml, about 10 g/ml, about
15 g/ml, about 20
jig/ml, about 25 jig/ml, about 30 jig/ml, about 35 g/ml, about 40 g/ml,
about 45 jig/m1 or about
50 jig/mi. The plasma concentration that is desirable in a subject will vary
depending on several
factors, including but not limited to, the nature of the disease or disorder,
the severity of the
disease or disorder and the condition of the subject. Such dosing regimens are
especially
beneficial in prevention, treatment and/or management of a chronic disease or
disorder.
[00419] In another embodiment, a human subject is administered one or more
doses of a
prophylactically or therapeutically effective amount of an antibody that
specifically binds to
human ICOS in a liquid formulation of the disclosure, wherein the dose of a
prophylactically or
therapeutically effective amount of the antibody in the liquid formulation of
the disclosure
administered to said subject is increased by, e.g., about 0.01 jig/kg, about
0.02 jig/kg, about 0.04
pg/kg, about 0.05 jig/kg, about 0.06 jig/kg, about 0.08 og/kg, about 0.1
jig/kg, about 0.2 lug/kg,
about 0.25 kg/kg, about 0.5 jig/kg, about 0.75 jig/kg, about 1 jig/kg, about
1.5 jig/kg, about 2
jig/kg, about 4 jig/kg, about 5 jig/kg, about 10 jig/kg, about 15 jig/kg,
about 20 jig/kg, about 25
jig/kg, about 30 jig/kg, about 35 jig/kg, about 40 jig/kg, about 45 jig/kg,
about 50 jig/kg, about 55
jig/kg, about 60 jig/kg, about 65 jig/kg, about 70 jig/kg, about 75 jig/kg,
about 80 jig/kg, about 85
jig/kg, about 90 jig/kg, about 95 jig/kg, about 100 jig/kg, or about 125
jig/kg, as treatment
progresses.
[00420] In another embodiment, a subject (e.g., a human) is administered
one or more
doses of a prophylactically or therapeutically effective amount of an antibody
that specifically
binds to human ICOS in a liquid formulation of the disclosure, wherein the
dose of a
prophylactically or therapeutically effective amount of the antibody in the
liquid formulation of
the disclosure administered to said subject is decreased by, e.g., about 0.01
jig/kg, about 0.02
jig/kg, about 0.04 jig/kg, about 0.05 jig/kg, about 0.06 vg/kg, about 0.08
jig/kg, about 0.1 jig/kg,
about 0.2 jig/kg, about 0.25 jig/kg, about 0.5 jig/kg, about 0.75 jig/kg,
about 1 jig/kg, about 1.5
jig/kg, about 2 jig/kg, about 4 jig/kg, about 5 lag/kg, about 10 lag/kg, about
15 jig/kg, about 20
jig/kg, about 25 jig/kg, about 30 jig/kg, about 35 jig/kg, about 40 jig/kg,
about 45 jig/kg, about 50
jig/kg, about 55 jig/kg, about 60 jig/kg, about 65 jig/kg, about 70 jig/kg,
about 75 jig/kg, about 80
jig/kg, about 85 jig/kg, about 90 jig/kg, about 95 jig/kg, about 100 jig/kg,
or about 125 jig/kg, as
treatment progresses.
[00421] The dosages of prophylactic or therapeutic agents are described in
the Physicians'
Desk Reference (60th ed., 2006).
126
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
5.29.2. TOXICITY TESTING
[00422] The tolerance, toxicity and/or efficacy of the formulations and/or
treatment
regimens of the present disclosure can be determined by standard
pharmaceutical procedures in
cell cultures or experimental animals, e.g., for determining the LD50 (the
dose lethal to 50% of
the population), the ED50 (the dose therapeutically effective in 50% of the
population), and ICSO
(the dose effective to achieve a 50% inhibition). In one embodiment, the dose
is a dose effective
to achieve at least a 60%, 70%, 80%, 900,/0,
95%, or 99% depletion of circulating ICOS
expressing T cells. The dose ratio between toxic and therapeutic effects is
the therapeutic index
and it can be expressed as the ratio LD50/ED50. Therapies that exhibit large
therapeutic indices
may be preferred. While therapies that exhibit toxic side effects may be used,
care should be
taken to design a delivery system that targets such agents to ICOS-expressing
cells in order to
minimize potential damage to ICOS negative cells and, thereby, reduce side
effects.
[00423] Data obtained from the cell culture assays and animal studies can
be used in
formulating a range of dosages of the formulations and/or treatment regimens
for use in humans.
The dosage of such agents may lie within a range of circulating concentrations
that include the
ED50 with little or no toxicity. The dosage may vary within this range
depending upon the
dosage form employed and the route of administration utilized. For any therapy
used in methods
of the disclosure, a therapeutically effective dose can be estimated by
appropriate animal models.
Depending on the species of the animal model, the dose can be scaled for human
use according
to art-accepted formulas, for example, as provided by Freireich et al.,
Quantitative comparison of
toxicity of anticancer agents in mouse, rat, monkey, dog, and human, Cancer
Chemotherapy
Reports, NCI 1966 40:219-244. Data obtained from cell culture assays can be
useful for
predicting potential toxicity. Animal studies can be used to formulate a
specific dose to achieve
a circulating plasma concentration range that includes the IC50 (i.e., the
concentration of the test
compound that achieves a half-maximal inhibition of symptoms) as determined in
cell culture.
Such information can be used to more accurately determine useful doses in
humans. Plasma
drug levels may be measured, for example, by high performance liquid
chromatography, EL1SA,
or by cell based assays.
5.30. THERAPEUTIC USES
[00424] Formulations comprising an anti-ICOS antibody with enhanced
effector function
may be used for the treatment of autoimmune diseases, such as systemic lupus
erythematosus,
rheumatoid arthritis, multiple sclerosis, diabetes, immune thrombocytopenic
purpura (ITP), and
psoriasis; chronic inflammatory diseases, such as inflammatory bowel disease
(Crohn's disease
127
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
and ulcerative colitis), Grave's disease, Hashimoto's thyroiditis, and
diabetes mellitus. Anti-
ICOS formulations described herein may also be used to alleviate toxic shock
syndrome,
inflammatory bowel disease, allosensitization due to blood transfusions, T-
cell dependent B-cell-
mediated diseases, and the treatment of graft vs. host disease. In addition,
formulations and
methods of the disclosure may be useful in therapeutic indications that call
for the inhibition or
enhancement of antibody production.
[00425] Formulations comprising an anti-ICOS antibody with enhanced
effector function
may also be used as immunosuppressive agents for bone marrow and organ
transplantation and
may be used to prolong graft survival. Such formulations may provide
significant advantages
over existing treatment. Bone marrow and organ transplantation therapy must
contend with T-
cell-mediated rejection of the foreign cells or tissue by the host. Present
therapeutic regimens for
inhibiting T-cell-mediated rejection involve treatment with the drugs
cyclosporine or FK506.
While drugs are effective, patients suffer from serious side effects,
including hepatotoxicity,
nephrotoxicity, and neurotoxicity. The target for the cyclosporin/FK506 class
of therapeutics is
calcineurin, a phosphatase with ubiquitous expression. Since ICOS expression
is restricted to T-
cells, depletion of ICOS expressing T cells may lack the severe side effects
observed with the
use of the present immunotherapeutic agents.
[00426] Hypersensitivity is a normally beneficial immune response that is
exaggerated or
inappropriate, and leads to inflammatory reactions and tissue damage.
Hypersensitivity reactions
which are antibody-mediated may be particularly susceptible to antagonism by
depletion of
ICOS expressing cells. Allergies, hay fever, asthma, and acute edema cause
type I
hypersensitivity reactions, and these reactions may be suppressed by depletion
of ICOS
expressing cells.
[00427] Diseases that cause antibody-mediated hypersensitivity reactions,
including
systemic lupus erythematosus, arthritis (rheumatoid arthritis, reactive
arthritis, psoriatic arthritis),
nephropathies (glomerulonephritis, membranous, mesangiocapillary, focal
segmental, focal
necrotizing, crescentic, proliferative--tubulopathies), skin disorders
(pemphigus and pemphigoid,
erythema nodosum), endocrinopathies (thyroiditis--Grave's, Hashimoto's--
insulin dependent
diabetes mellitus), various pneumopathies (especially extrinsic alveolitis),
various
vasculopathies, coeliac disease, with aberrant production of IgA, many anemias
and
thrombocytopenias, Guillain-Barre Syndrome, and myasthenia gravis, may be
treated using
formulations comprising an anti-ICOS antibody with enhanced effector function.
[00428] In addition, lymphoproliferative disorders, such as multiple
myeloma,
Waldenstrom's macroglobulinemia, and crioglobulinemias may be inhibited by
administering a
formulation comprising an anti-ICOS antibody with enhanced effector function.
Additionally,
128
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
graft versus host disease, an "artificial" immune disorder, may benefit from
the depletion of
ICOS expressing cells.
[00429] The ICOS dependent co-stimulatory pathway is involved in regulating
IgE
production. IgE is an immunoglobulin isotype specifically involved in
mediating allergic
responses such as asthma, food allergies, hay fever, type 1 hypersensitivity
and sinus
inflammation. Upon exposure to an allergen, a process involving T-cell and B
cell collaboration
results in B cell production of IgE specific for the allergen. Allergen-
specific IgE released into
the circulation by B cells bind to mast cells and basophils through the high
affinity IgE receptor
(FceRI). Mast cells and basophils to which IgE is bound become sensitized and
subsequent
exposure to the allergen results in cross-linking of the surface receptors and
release of
histamines.
[00430] The disclosure provides for the use of an anti-ICOS antibody to
regulate IgE
production and to prevent or treat IgE-mediated disorders. By way of example,
such disorders
include allergic responses such as asthma, food allergies, hay fever,
hypersensitivity, and sinus
inflammation. In one embodiment, an anti-ICOS antibody of the disclosure is
used to partially
or completely inhibit IgE production. An anti-ICOS antibody of the disclosure
may be used
separately, or in combination, in a treatment regimen for decreasing IgE
levels.
[00431] The disclosure also provides for the use of an anti-ICOS antibody
in combination
with an IgE antagonist to partially or completely inhibit IgE production and
to prevent and/or
treat disorders characterized by excessive or inappropriate IgE production. As
used herein the
term "IgE antagonist" refers to a compound capable of disrupting or blocking
the interaction of
IgE with its high affinity receptor FceRI on cells such that the response to
allergen stimulus is
attenuated or eliminated. Antagonists include an anti-IgE antibody and
fragments thereof,
soluble FceRI receptor and fragments thereof, anti- FceRI antibody and
fragments thereof, IgE
variants and fragments thereof, IgE binding peptides, FceRI receptor binding
peptides, and small
molecules capable of binding to IgE or competing with IgE for binding to FceRI
receptor. An
anti-ICOS antibody of the disclosure may also be used with in combination with
antihistamines,
allergen desensitization, reduction in exposure to allergen and the like for
treatment of allergic
disorders.
[00432] The disclosure also provides for the prevention and/or treatment of
asthma
comprising administering an anti-ICOS antibody of the disclosure alone or in
conjunction with
one or more agents for treating asthma. Examples of such agents include
bronchodilators (anti-
cholinergic agents, .beta-2 adrenergic receptor agonists, lenkotriene D-4
antagonists, neurokinin
antagonists, potassium channel openers, substance P antagonists, thromboxane A-
2 antagonists,
and xanthines), anti-inflammatories (5-lipoxygcnasc inhibitors, 5-lipoxygenase
activating protein
129
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
inhibitors, phosphodiesterase IV inhibitors, platelet activating factor
antagonists, respiratory
NSAIDS, steroids, and tyrosine kinase inhibitors), cytokine inhibitors (CD4,
IL-4 and IL-5
inhibitors) and IgE antagonists as set forth above.
[00433] Formulations and methods according to this disclosure are able to
control
(suppress or stimulate) proliferation of ICOS expressing cells or production
of cytokine (for
example, IL-17) by ICOS expressing cells, thereby enabling suppression of
various pathological
conditions and treatment or prevention of various disorders caused by diverse
physiological
phenomena related to signal transduction mediated by ICOS.
[00434] Formulations comprising an anti-1COS antibody of this disclosure
enables
suppression, prevention and/or treatment of, for example, but not limited to,
rheumatoid arthritis,
multiple sclerosis, autoimmune thyroiditis, allergic contact-type dermatitis,
chronic
inflammatory dermatosis (e.g., lichen planus), systemic lupus erythematosus,
insulin-dependent
diabetes mellitus, psoriasis, autoimmune or allergic disorders, autoimmune
disease and delayed
allergy caused by cellular immunity; arthropathia (for example, but not
limited to, rheumatoid
arthritis (RA) and osteoarthritis (OA)), inflammation (e.g., hepatitis), graft
versus host reaction
(GVH reaction), graft versus host disease (GVHD), immune rejection
accompanying
transplantation of a tissue (e.g., skin, cornea, bone) or organ (e.g., liver,
heart, lung, kidney,
pancreas), immune response triggered by a foreign antigen or autoantigen (for
example,
production of antibodies against said antigen, cell proliferation, production
of cytokines), and
disorders caused by the abnormal intestinal immunity (e.g., inflammatory
intestinal disorders,
Crohn's disease, ulcerative colitis, alimentary allergy).
[00435] Furthermore, formulations and methods described herein may be
utilized for the
suppression/treatment of transplant rejection or GVHD in combination with
known
immunosuppressive agents such as inhibitors of cytokine transcription (e.g.,
cyclosporin A,
tacrolimus), nucleotide synthesis (e.g., azathiopurine, mycophenolate
mofetil), growth factor
signal transduction (e.g., sirolimus, rapamycin), and the T cell interleukin 2
receptor (e.g.,
daclizumab, basiliximab). In a particular embodiment, an immunosuppressant
agent used in
combination with formulations and methods of the disclosure includes one or
more of the
following: adriamycin, azathiopurine, busulfan, cyclophosphamide, cyclosporin
A ("CyA"),
cytoxin, fludarabine, 5-fluorouracil, methotrexate, mycophenolate mofetil
(MOFETIL),
nonsteroidal anti-inflammatories (NSAIDs), rapamycin, and tacrolimus (FK506).
[00436] The formulations and methods of the present disclosure can be
applied to
inflammatory disease for example, inflammation accompanying various arthritis
(for example,
rheumatoid arthritis, osteoarthritis), pneumonia, hepatitis (including viral
hepatitis),
inflammation accompanying infectious diseases, inflammatory bowel diseases,
intestinal
130
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
enteritis, nephritis (e.g., glomerular nephritis, nephrofibrosis), gastritis,
angiitis, pancreatitis,
peritonitis, bronchitis, myocarditis, cerebritis, inflammation in postischemic
reperfusion injury
(myocardial ischemic reperfusion injury), inflammation attributed to immune
rejection after
transplantation of tissue and organ, burn, various skin inflammation
(psoriasis, allergic contact-
type dermatitis, lichen planus), inflammation in multiple organ failure,
inflammation after
operation of PTCA or PTCR, and inflammation accompanying arteriosclerosis, and
autoimmune
thyroiditis.
[00437] Formulations of the disclosure comprising an anti-ICOS antibody
with enhanced
effector function as an active ingredient may be used to inhibit, treat and/or
prevent a variety of
diseases, for example, but not limited to rheumatoid arthritis, multiple
sclerosis, autoimmune
thyroiditis, allergic contact dermatitis, lichen planus, systemic lupus
erythematosus, insulin
dependent diabetes mellitus, psoriasis, autoimmune diseases or allergic
diseases, delayed
allergies mediated by cellular immunity; arthropathies (e.g., rheumatoid
arthritis (RA),
osteoarthritis (OA)), inflammation (e.g., hepatitis), graft versus host
reaction (GVH reaction),
graft versus host disease (GVHD), immunorejection associated with
transplantation of tissues
(e.g., skin, cornea and bone) or organs (e.g., liver, heart, lung, kidney,
pancreas), inflammatory
bowel disease, Crohn's disease, ulcerative colitis, and alimentary allergy.
[00438] The formulations in accordance with the present disclosure make it
possible to
treat or prevent some inflammations for which various steroidal drugs are used
as anti-
inflammatory drugs, for example, inflammation associated with various
arthritides (e.g.,
rheumatoid arthritis, osteoarthritis), pneumonia, hepatitis (including viral
hepatitis),
inflammation associated with infectious diseases, inflammatory bowel disease,
enteritis,
nephritis, glomerular nephritis, inflammation associated with kidney fibrosis,
gastritis, vasculitis,
pancreatitis, peritonitis, bronchitis, myocarditis, encephalitis, inflammation
associated with
ischemia-reperfusion injury, myocardial ischemia-reperfusion injury,
inflammation associated
with immunorejection after transplantation of tissues or organs, psoriasis,
allergic contact
dermatitis, lichen planus, inflammation associated with multiple organ
failure, inflammation
after operation of PTCA or PTCR, inflammation associated with atherosclerosis,
and
autoimmune thyroiditis.
5.31. TRANSPLANTATION
[00439] According to certain aspects of the disclosure, the treatment
regimen and dose
used with formulations and methods of the disclosure is chosen based on a
number of factors
including, for example, clinical manifestation that place a patient at risk
for developing
transplant rejection, or clinical evidence that such a rejection is
developing.
131
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00440] The present disclosure provides formulations, methods and regimens
effective to
reduce the incidence, severity, or duration of GVHD, a rejection episode, or
post-transplant
lymphoproliferative disorder. In certain embodiments, formulations and methods
of the
disclosure are effective to attenuate the host response to ischemic
reperfusion injury of a solid
tissue or organ graft. In one embodiment, formulations and methods of the
disclosure are
effective to prolong survival of a graft in a transplant recipient.
[00441] The present disclosure encompasses grafts that are autologous,
allogeneic, or
xenogeneic to the recipient. The types of grafts encompassed by the disclosure
include tissue
and organ grafts, including but not limited to, bone marrow grafts, peripheral
stem cell grafts,
skin grafts, arterial and venous grafts, pancreatic islet cell grafts, and
transplants of the kidney,
liver, pancreas, thyroid, and heart. The terms "graft" and "transplant" are
used interchangeably
herein. In one embodiment, the autologous graft is a bone marrow graft, an
arterial graft, a
venous graft or a skin graft. In one embodiment, the allograft is a bone
marrow graft, a corneal
graft, a kidney transplant, a pancreatic islet cell transplant, or a combined
transplant of a kidney
and pancreas. In one embodiment, the graft is a xenograft, wherein the
possible animal donors
include, but are not limited to pigs. The formulations and methods of the
present disclosure may
also be used to suppress a deleterious immune response to a non-biological
graft or implant,
including but not limited to an artificial joint, a stent, or a pacemaker
device.
[00442] Anti-ICOS antibodies, formulations, and methods of the disclosure
may be used
to treat or prevent GVHD, rejection, or post-transplant lymphoproliferative
disorder without
regard to the particular indications initially giving rise to the need for the
transplant or the
particular type of tissue transplanted.
[00443] Therapeutic formulations and regimens of the present disclosure are
described for
treating human subjects diagnosed with autoimmune diseases or disorders,
including but not
limited to, rheumatoid arthritis, SLE, ITP, pemphigus-related disorders,
diabetes, and
scleroderma.
[00444] Appropriate treatment regimens can be determined by one of skill in
the art for
the particular patient or patient population. In particular embodiments, the
treatment regimen is
a pre-transplant conditioning regimen, a post-transplant maintenance regimen,
or post-transplant
treatment regimen for an acute or a chronic rejection. In certain embodiments,
the particular
regimen is varied for a patient who is assessed as being at a high or
intermediate risk of
developing a rejection response, compared with the regimen for a patient who
is assessed as
being at a low risk of rejection.
[00445] In certain embodiments, the particular regimen is varied according
to the stage of
rejection, with more aggressive therapy being indicated for patients at later
stages of rejection.
132
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
The stages of humoral rejection may be classified according to the knowledge
and skill in the art.
For example, the stages of humoral rejection may be classified as one of
stages Ito IV according
to the following criteria: Stage I Latent Response, characterized by
circulating anti-donor
alloantibodies, especially anti-HLA antibodies; Stage TI Silent Reaction,
characterized by
circulating anti-donor alloantibodies, especially anti-HLA antibodies, and C4d
deposition, but
without histologic changes or graft dysfunction; Stage III Subclinical
Rejection: characterized by
circulating anti-donor alloantibodies, especially anti-HLA antibodies, C4d
deposition, and tissue
pathology, but without graft dysfunction; Stage IV Humoral Rejection:
characterized by
circulating anti-donor alloantibodies, especially anti-HLA antibodies, C4d
deposition, tissue
pathology, and graft dysfunction.
[00446] Anti-ICOS antibodies, formulations and methods of the disclosure
may be
practiced to treat or prevent GVHD, rejection, or post-transplantation
lymphoproliferative
disorders, either alone or in combination with other therapeutic agents or
treatment regimens.
Other therapeutic regimens for the treatment or prevention of GVHD, rejection,
or
post-transplantation lymphoproliferative disorders may comprise, for example,
one or more of
anti-lymphocyte therapy, steroid therapy, antibody depletion therapy,
immunosuppression
therapy, and plasmapheresis.
[00447] Anti-lymphocyte therapy may comprise the administration to the
transplant
recipient of anti-thymocyte globulins, also referred to as thymoglobulin. Anti-
lymphocyte
therapy may also comprise the administration of one or more monoclonal
antibodies directed
against T cell surface antigens. Examples of such antibodies include, without
limitation,
OKT3 ' (muromonab-CD3), CAMPATH'm-1H (alemtuzumab), CAMPATH'm -1G,
CAMPATHTm -1M, SIMULECTTm (basiliximab), and ZENAPAXTM (daclizumab). In a
specific
embodiment, the anti-lymphocyte therapy comprises one or more antibodies
directed against B
cells, including, without limitation, RITUXANTm (rituximab).
[00448] Steroid therapy may comprise administration to the transplant
recipient of one or
more steroids selected from the group consisting of cortisol, prednisone,
methyl prednisolone,
dexamethazone, and indomethacin. One or more of the steroids may be
corticosteroids,
including without limitation, cortisol, prednisone, and methylpreclnisolone.
[00449] Antibody depletion therapy may include, for example, administration
to the
transplant recipient of intravenous immunoglobulin. Antibody depletion therapy
may also
comprise immunoadsorption therapy applied to the graft ex vivo, prior to
transplantation.
Immunoadsorption may be accomplished using any suitable technique, for
example, protein A
affinity, or antibody based affinity techniques using antibodies directed
against T cell or B cell
133
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
surface markers such as anti-CD3 antibodies, anti-CD19 antibodies, anti-CD20
antibodies, and
anti-CD22 antibodies.
[00450] Immunosuppression therapy may comprise the administration of one or
more
immunosuppressive agents such as inhibitors of cytokine transcription (e.g.,
cyclosporin A,
tacrolimus), nucleotide synthesis (e.g., azathiopurine, mycophenolatc
mofetil), growth factor
signal transduction (e.g., sirolimus, rapamycin), and the T cell interleukin 2
receptor (e.g.,
daclizumab, basiliximab). In a particular embodiment, an immunosuppressant
agent used in
combination with formulations and methods of the disclosure includes one or
more of the
following: adriamycin, azathiopurine, busulfan, cyclophosphamidc, cyclosporin
A ("CyA"),
cytoxin, fludarabine, 5-fluorouracil, methotrexate, mycophenolate mofetil
(MOFETIL),
nonsteroidal anti-inflammatories (NSAIDs), rapamycin, and tacrolimus (FK506).
Immunosuppressive agents may also comprise inhibitors of complement, for
example, soluble
complement receptor-1, anti-CS antibody, or a small molecule inhibitor of Cis,
for example as
described in Buerke et al. (J. Immunol., 167:5375-80 (2001).
[00451] In one embodiment, formulations and methods of the disclosure are
used in
combination with one or more therapeutic regimens for suppressing rejection,
including, without
limitation, tacrolimus and mycophenolate mofetil therapy, immunoadsorpti on,
intravenous
immunoglobulin therapy, and plasmapheresis.
5.32. INFLAMMATORY DISORDER
[00452] Anti-ICOS antibodies of the disclosure may be administered to a
subject in need
thereof to prevent, manage, treat or ameliorate an inflammatory disorder
(e.g., asthma) or one or
more symptoms thereof. Formulations of the disclosure may also be administered
in
combination with one or more other therapies, preferably therapies useful for
the prevention,
management, treatment or amelioration of an inflammatory disorder (including,
but not limited
to the prophylactic or therapeutic agents listed herein) to a subject in need
thereof to prevent,
manage, treat or ameliorate an inflammatory disorder or one or more symptoms
thereof In a
specific embodiment, the disclosure provides a method of preventing, managing,
treating or
ameliorating an inflammatory disorder or one or more symptoms thereof, said
method
comprising administering to a subject in need thereof a dose of a
prophylactically or
therapeutically effective amount of an anti-ICOS antibody of the disclosure.
In another
embodiment, the disclosure provides a method of preventing, managing, treating
or ameliorating
an inflammatory disorder or one or more symptoms thereof, said method
comprising
administering to a subject in need thereof a dose of a prophylactically or
therapeutically effective
amount of an effector function enhanced anti-ICOS antibody of the disclosure
and a dose of a
134
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
prophylactically or therapeutically effective amount of one or more therapies
(e.g., prophylactic
or therapeutic agents) other than antibodies (including antibody fragments
thereof) that
immunospecifically bind to an ICOS polypeptide.
[00453] The
disclosure provides methods for managing, treating or ameliorating one or
more symptoms of an inflammatory disorder in a subject refractory to
conventional therapies
(e.g., methotrexate and a TNF-alpha antagonist (e.g., REMICADETivi or
ENBRELTm)) for such
an inflammatory disorder, said methods comprising administering to said
subject a dose of a
prophylactically or therapeutically effective amount of an effector function
enhanced anti-ICOS
antibody of the disclosure. The disclosure also provides methods for managing,
treating or
ameliorating one or more symptoms of an inflammatory disorder in a subject
refractory to
existing single agent therapies for such an inflammatory disorder, said
methods comprising
administering to said subject a dose of a prophylactically or therapeutically
effective amount of
an effector function enhanced anti-ICOS antibody of the disclosure and a dose
of a
prophylactically or therapeutically effective amount of one or more therapies
(e.g., prophylactic
or therapeutic agents) other than antibodies (including antibody fragments
thereof) that
immunospecifically bind to an ICOS polypeptide. The disclosure also provides
methods for
managing or treating an inflammatory disorder by administering an effector
function enhanced
anti-ICOS antibody of the disclosure in combination with any other treatment
to patients who
have proven refractory to other treatments but are no longer on these
treatments. The disclosure
also provides alternative methods for the treatment of an inflammatory
disorder where another
therapy has proven or may prove too toxic, i.e., results in unacceptable or
unbearable side
effects, for the subject being treated. For example, a formulation of the
disclosure may be
administered to a subject, wherein the subject is refractory to a TNF
antagonist or methotrexate.
Further, the disclosure provides methods for preventing the recurrence of an
inflammatory
disorder in patients that have been treated and have no disease activity by
administering an
effector function enhanced anti-ICOS antibody of the disclosure.
[00454] Inflammatory
disorders that can be treated by the methods encompassed by the
disclosure include, but are not limited to, asthma, encephalitis, inflammatory
bowel disease,
chronic obstructive pulmonary disease (COPD), allergic disorders, septic
shock, pulmonary
fibrosis, undifferentiated spondyloarthropathy, undifferentiated arthropathy,
arthritis,
osteoarthritis, spondyloarthropathies (e.g., psoriatic arthritis, ankylosing
spondylitis, Reiter's
Syndrome (reactive arthritis), inflammatory osteolysis, Wilson's disease and
chronic
inflammation resulting from chronic viral or bacteria infections. As described
herein, some
autoimmune disorders are associated with an inflammatory condition.
135
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00455] Anti-inflammatory therapies and their dosages, routes of
administration and
recommended usage are known in the art and have been described in such
literature as the
Physician's Desk Reference (61th ed., 2007).
5.32.1.Anti-Inflammatory Therapies
[00456] The present disclosure provides methods of preventing, managing,
treating or
ameliorating an inflammatory disorder or one or more symptoms thereof, said
methods
comprising administering to a subject in need thereof an effector function
enhanced anti-ICOS
antibody of the disclosure and one or more therapies (e.g., prophylactic or
therapeutic agents
other than antibodies (including antibody fragments thereof) that
immunospecifically bind to an
ICOS polypeptide. Any agent or therapy which is known to be useful, or which
has been used or
is currently being used for the prevention, management, treatment or
amelioration of an
inflammatory disorder or one or more symptoms thereof can be used in
combination with an
effector function enhanced anti-ICOS antibody of the disclosure in accordance
with the
disclosure described herein.
[00457] Any anti-inflammatory agent, including agents useful in therapies
for
inflammatory disorders, well-known to one of skill in the art can be used in
the formulations and
methods of the disclosure. Non-limiting examples of anti-inflammatory agents
include non-
steroidal anti-inflammatory drugs (NSAIDs), steroidal anti-inflammatory drugs,
anticholinergics
(e.g., atropine sulfate, atropine methylnitrate, and ipratropium bromide
(ATROVENTTm)), beta2-
agonists (e.g., abuterol (VENTOLINTm and PROVENTILTm), bitolterol
(TORNALATETm),
levalbuterol (XOPONEXTm), metaproterenol (ALUPENTTm), pirbuterol (mAxAm_fm),
terbutlaine (BRETHAIRETm and BRETHINETm), albuterol (PROVENTILTm, REPETABSTm,
and VOLMAXTm), formoterol (FORADIL AEROLIZERTm), and salmeterol (SEREVENTM and
SEREVENT DISKUSTm)), and methylxanthines (e.g., theophylline (UINIPHYLTM, THEO-
DURim, SLO-BID im, AND TEHO-421m)). Examples of NSAIDs include, but are not
limited to,
aspirin, ibuprofen, celecoxib (CELEBREXTm), diclofenac (VOLTARENTm), etodolac
(LODINETm), fenoprofen (NALFONTm), indomethacin (INDOCINTm), ketoralac
(TORADOLTm), oxaprozin (DAYPROTm), nabumcntone (RELAFEN Tim), sulindac
(CLINORILTm), tolmentin (TOLECTINTm), rofecoxib (VIOXXTm), naproxen (ALEVETM,
NAPROSYNTm), ketoprofen (ACTRONTm) and nabumetone (RELAFENTm). Such NSAIDs
function by inhibiting a cyclooxgenase enzyme (e.g., COX- l and/or COX-2).
Examples of
steroidal anti-inflammatory drugs include, but arc not limited to,
glucocorticoids, dexamethasonc
(DECADRONTm), corticosteroids (e.g., methylprednisolone (MEDROLTm)),
cortisone,
hydrocortisone, prednisone (PREDNISONETM and DELTASONETm), prednisolone
136
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
(PRELONETM and PEDIAPREDTm), triamcinolone, azulfidine, and inhibitors of
eicosanoids
(e.g., prostaglandins, thromboxanes, and leukotrienes).
[00458] In one embodiment, an effective amount of one or more formulations
of the
disclosure is administered in combination with a mast cell protease inhibitor
to a subject at risk
of or with an inflammatory disorder. In another embodiment, the mast cell
protease inhibitor is a
tryptase kinase inhibitor, such as, but not limited to GW-45, GW-58, and
genisteine. In a
specific embodiment, the mast cell protease inhibitor is phosphatidylinositide-
3' (P13)-kinase
inhibitors, such as, but not limited to calphostin C. In another embodiment,
the mast cell
protease inhibitor is a protein kinase inhibitor such as, but not limited to
staurosporine. In one
embodiment, the mast cell protease inhibitor is administered locally to the
affected area.
[00459] Specific examples of immunomodulatory agents which can be
administered in
combination with an effector function enhanced anti-ICOS antibody of the
disclosure to a subject
with an inflammatory disorder include, but are not limited to, methothrexate,
leflunomide,
cyclophosphamide, cytoxan, Immuran, cyclosporine A, minocycline, azathioprine,
antibiotics
(e.g., FK506 (tacrolimus)), methylprednisolone (MP), corticosteroids,
steroids, myeophenolate
mofetil, rapamycin (sirolimus), mizoribine, deoxyspergualin, brequinar,
malononitriloamindes
(e.g., leflunamide), anti-T cell receptor antibodies (e.g., anti-CD4
antibodies (e.g., cM-T412
(Boeringer), IDEC-CE9.1® (IDEC and SKB), mAB 4162W94, Orthoclone and
OKTcdr4a
(Janssen-Cilag)), anti-CD3 antibodies (e.g., Nuvion (Product Design Labs),
OKT3 (Johnson &
Johnson), or Rituxan (IDEC)), anti-CD5 antibodies (e.g., an anti-CD5 ricin-
linked
immunoconjugate), anti-CD7 antibodies (e.g., CHH-380 (Novartis)), anti-CD8
antibodies, anti-
CD40 ligand monoclonal antibodies (e.g., IDEC-131 (IDEC)), anti-CD52
antibodies (e.g.,
CAMPATH 1H (Ilex)), anti-CD2 antibodies (e.g., MEDI-507 (Medimmune, Inc.,
International
Publication Nos. WO 02/098370 and WO 02/069904), anti-CD1la antibodies (e.g.,
Xanelim
(Genentech)), and anti-B7 antibodies (e.g., IDEC-114) (IDEC)); anti-cytokine
receptor
antibodies (e.g., anti-IFN receptor antibodies, anti-IL-2 receptor antibodies
(e.g., Zenapax
(Protein Design Labs)), anti-IL-4 receptor antibodies, anti-IL-6 receptor
antibodies, anti-IL-10
receptor antibodies, and anti-IL-12 receptor antibodies), anti-cytokine
antibodies (e.g., anti-IFN
antibodies, anti-TNF-alpha antibodies, anti-IL-lbeta antibodies, anti-IL-6
antibodies, anti-IL-8
antibodies (e.g., ABX-IL-8 (Abgenix)), and anti-IL-12 antibodies)); CTLA4-
immunoglobulin;
LFA-3TIP (Biogen, International Publication No. WO 93/08656 and U.S. Pat. No.
6,162,432);
soluble cytokine receptors (e.g., the extracellular domain of a TNF-alpha
receptor or a fragment
thereof, the extracellular domain of an IL-lbeta receptor or a fragment
thereof, and the
extracellular domain of an IL-6 receptor or a fragment thereof); cytokines or
fragments thereof
(e.g., interleukin (IL)-2, 1L-3, IL-4, 1L-5, IL-6, IL-7, 1L-8, 1L-9, IL-10, IL-
11, IL-112, IL-15,
137
CA 02743469 2016-06-13
" 51332-100
TNF-alpha, TNF'-beta, interferon (aN)-alpha, IFN-beta, IFN-gamma, and GM-CSF);
and anti-
cytolcine antibodies (e.g., anti-IL-2 antibodies, anti-IL-4 antibodies, anti-
1L-6 antibodies, anti-IL-
9 antibodies, anti-IL-10 antibodies, anti-IL-12 antibodies, anti-IL-15
antibodies, anti-1L17
antibodies, anti-TNF-alpha antibodies, and anti-IFN-gamma antibodies).
[004601 Any TNF-alpha antagonist well-known to one of skill in the
art can be used in the
formulations and methods of the disclosure. Non-limiting examples of TNF-alpha
antagonists
which can be administered in combination with an effector function enhanced
anti-ICOS
antibody of the disclosure to a subject with an inflammatory disorder include
proteins,
polypeptides, peptides, fusion proteins, antibodies (e.g., human, humanized,
chimeric,
monoclonal, polyclonal, Fvs, ScFvs, Fab fragments, F(ab)2 fragments, and
antigen-binding
fragments thereof) such as antibodies that immunospecifically bind to 1NF-
alpha, nucleic acid
molecules (e.g., antisense molecules or triple helices), organic molecules,
inorganic molecules,
and small molecules that blocks, reduces, inhibits or neutralizes the
function, activity and/or
expression of TNF-alpha. In various embodiments, a TNF-alpha antagonist
reduces the function,
activity and/or expression of TNF-alpha by at least 10%, at least 15%, at
least 20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%, at
least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95% or at
least 99% relative to a control such as phosphate buffered saline (PBS).
Examples of antibodies
that immunospecifically bind to TNF-alpha include, but are not limited to,
infliximab
(REMICADETm; Centacor), D2E7 (Abbott Laboratories/Knoll Pharmaceuticals Co.,
Mt Olive,
NJ.), CDP571 which is also known as HUMICADETm and CDP-870 (both of
Celltech/Pharmacia, Slough, U.K.), and TN3-19.12 (Williams et al., 1994, Proc.
Natl. Acad. Sci.
USA 91: 2762-2766; Thorbecke et al., 1992, Proc. Natl. Acad. Sci. USA 89:7375-
7379). The
present disclosure also encompasses the use of antibodies that
immunospecifically bind to TNF-
alpha disclosed in the following U.S. Patents in the formulations and methods
of the disclosure:
5,136,021; 5,147,638; 5,223,395; 5,231,024; 5,334,380; 5,360,716; 5,426,181;
5,436,154;
5,610,279; 5,644,034; 5,656,272; 5,658,746; 5,698,195; 5,736,138; 5,741,488;
5,808,029;
5,919,452; 5,958,412; 5,959,087; 5,968,741; 5,994,510;
6,036,978; 6,114,517; and 6,171,787. Examples of soluble TNF-
alpha receptors include, but are not limited to, sTNF-R1 (Amgen), etanercept
(ENl3RELTm;
Immunex) and its rat homolog RENBRELThl, soluble inhibitors of TNF-alpha
derived from
TNFrI, TNFrIE (Kohno et al., 1990, Proc. Natl. Acad. Sci. USA 87:8331-8335),
and TNF-alpha
Inh (Seckinger et al, 1990, Proc. Natl. Acad. Sci. USA 87:5188-5192).
[00461] Other INF-alpha antagonists encompassed by the disclosure
include, but are not
limited to, IL-10, which is known to block TNF-alpha production via interferon
gamma-
138
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
activated macrophages (Oswald et al. 1992, Proc. Natl. Acad. Sci. USA 89:8676-
8680), INFR-
IgG (Ashkenazi et al., 1991, Proc. Natl. Acad. Sci. USA 88:10535-10539), the
murine product
TBP-1 (Serono/Yeda), the vaccine CytoTAb (Protherics), antisense molecule
104838 (ISIS), the
peptide RDP-58 (SangStat), thalidomide (Celgene), CDC-801 (Celgene), DPC-333
(Dupont),
VX-745 (Vertex), AG1X-4207 (AtheroGenics), 1TF-2357 (Italfarmaco), NP1-13021-
31 (Nereus),
SC10-469 (Scios), TACE targeter (Immunix/AHP), CLX-120500 (Calyx),
Thiazolopyrim
(Dynavax), auranofin (Ridaura) (SmithKline Beecham Pharmaceuticals),
quinacrine (mepacrine
dichlorohydrate), tenidap (Enablex), Melanin (Large Scale Biological), and
anti-p38 MAPK
agents by Uriach.
[00462] Non-limiting examples of anti-inflammatory agents which can be
administered in
combination with an effector function enhanced anti-ICOS antibody of the
disclosure to a subject
with an inflammatory disorder include non-steroidal anti-inflammatory drugs
(NSAIDs),
steroidal anti-inflammatory drugs, beta-agonists, anticholingeric agents, and
methyl xanthines.
Examples of NSAIDs include, but are not limited to, aspirin, ibuprofen,
celecoxib
(CELEBREXim), diclofenac (VOLTAREN1m), etodolac (LODINE1m), fenoprofen
(NALFONTm), indomethacin (INDOCINTm), ketoralac (TORADOLTm), oxaprozin
(DAYPROTm), nabumentone (RELAFENTm), sulindac (CLINORILTm), tolmentin
(TOLECT1NTm), rofecoxib (V1OXXTm), naproxen (ALEVETm, NAPROSYNTm), ketoprofen
(ACTRONTm) and nabumetone (RELAFENTm). Such NSAIDs function by inhibiting a
cyclooxgenase enzyme (e.g., COX-1 and/or COX-2). Examples of steroidal anti-
inflammatory
drugs include, but are not limited to, glucocorticoids, dexamethasone
(DECADRONTm),
cortisone, hydrocortisone, prednisone (DELTASONETm), prednisolone,
triamcinolone,
azulfidine, and eicosanoids such as prostaglandins, thromboxanes, and
leukotrienes.
[00463] In specific embodiments, patients with osteoarthritis are
administered a
prophylactically or therapeutically effective amount of an effector function
enhanced anti-ICOS
antibody of the disclosure in combination with other agents or therapies
useful for osteoarthritis
prevention, treatment, management or amelioration including but not limited
to: analgesics (non-
limiting examples are acetaminophen, in a dose up to 4000 mg/d; phenacetin;
and tramadol, in a
daily dose in the range of 200 to 300 mg); NSAIDs (non-limiting examples
include but not
limited to, aspirin, diflunisal, diclofenac, etodolac, fenamates, fenoprofen,
flurbiprofen,
ibuprofen, indomethacin, ketoprofen, methylsalicylatc, ncbumetone, naproxin,
oxaprazin,
phenylbutazone, piroxicam, sulindac, and tolmetin. Low dose NSAIDs are
preferred, e.g.,
ibuprofen at 1200 mg/d, naproxen at 500 mg/d. A gastroprotective agent, e.g.,
misoprostol,
famotidine or omeprazole, is preferred to use concurrently with a NSAID);
nonacetylated
salicylates including but not limited to salsalate; cyclooxygenase (Cox)-2-
specific inhibitors
139
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
(CSIs), including but not limited to, celecoxib and rofecoxib; intra- or
periarticular injection of a
depot glucocorticoid preparation; intra-articular injection of hyaluronic
acid; capsaicin cream;
copious irrigation of the osteroarthritis knee to flush out fibrin, cartilage
shards and other debris;
and joint replacement surgery. Formulations and methods of the disclosure can
also be used in
combination with other nonpharmacologic measures in prevention, treatment,
management and
amelioration of osteoarthritis including but not limited to: reduction of
joint loading (non-
limiting examples are correction of poor posture, support for excessive lumbar
lordosis, avoid
excessive loading of the involved joint, avoid prolonged standing, kneeling
and squatting);
application of heat to the affected joint; aerobic exercise and other physical
therapies.
[00464] In specific embodiments, patients with rheumatoid arthritis are
administered a
prophylactically or therapeutically effective amount of an effector function
enhanced anti-ICOS
antibody of the disclosure in combination with other agents or therapies
useful in prevention,
treatment, management and amelioration of rheumatoid arthritis including but
not limited to:
NSAIDs (non-limiting examples include but not limited to, aspirin, diflunisal,
diclofenac,
etodolac, fenamates, fenoprofen, flurbiprofen, ibuprofen, indomethacin,
ketoprofen,
methylsalicylate, nebumetone, naproxin, oxaprazin, phenylbutazone, piroxicam,
sulindac, and
tolmetin.); analgesics (non-limiting examples are acetaminophen, phenacetin
and tramadol);
CSIs including but not limited to, celecoxib and rofccoxib; glucocorticoids
(preferably low-dose
oral glucocorticoids, e.g., <7.5 mg/d prednisone, or monthly pulses with high-
dose
glucocorticoids, or intraarticular glucocorticoids); disease-modifying
antirheumatic drugs
(DMARDs) including but not limited to, methotrexate (preferably given
intermittent low dose,
e.g., 7.5-30 mg once weekly), gold compounds (e.g., gold salts), D-
penicillamine, the
antimalarials (e.g., chloroquine), and sulfasalazine; TNF-alpha neutralizing
agents including but
not limited to, etanercept and infliximab; immunosuppressive and cytotoxic
agents (examples
include but not limited to, azathioprine, leflunomide, cyclosporine, and
cyclophosphamide), and
surgery (examples include but not limited to, arthroplasties, total joint
replacement,
reconstructive hand surgery, open or arthroscopic synovectomy, and early
tenosynovectomy of
the wrist). The formulations and methods of the disclosure may also be used in
combination
with other measures in prevention, treatment, management and amelioration of
the rheumatoid
arthritis including but not limited to: rest, splinting to reduce unwanted
motion of inflamed joint,
exercise, used of a variety of orthotic and assistive devices, and other
physical therapies. The
formulations and methods of the disclosure may also be used in combination
with some
nontraditional approaches in prevention, treatment, management and
amelioration of rheumatoid
arthritis including but not limited to, diets (e.g., substituting omega-3
fatty acids such as
140
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
eicosapentaenoic acid found in certain fish oils for dietary omega-6 essential
fatty acids found in
meat), vaccines, hormones and topical preparations.
[00465] In specific embodiments, patients with chronic obstructive
pulmonary disease
(COPD) are administered a prophylactically or therapeutically effective amount
of an effector
function enhanced anti-ICOS antibody of the disclosure in combination with
other agents or
therapies useful in prevention, treatment, management and amelioration of COPD
including but
not limited to: bronchodilators including but not limited to, short- and long-
acting beta2 -
adrenergic agonists (examples of short-acting beta2 agonist include but not
limited to, albuterol,
pirbutcrol, terbutaline, and metaproterenol; examples of long-acting beta2
agonist include but not
limited to, oral sustained-release albuterol and inhaled salmeterol),
anticholinergics (examples
include but not limited to ipratropium bromide), and theophylline and its
derivatives (therapeutic
range for theophylline is preferably 10-20 µg/mL); glucocorticoids;
exogenous alphalAT
(e.g., alphalAT derived from pooled human plasma administered intravenously in
a weekly dose
of 60 mg/kg); oxygen; lung transplantation; lung volume reduction surgery;
endotracheal
intubation, ventilation support; yearly influenza vaccine and pneumococcal
vaccination with 23-
valent polysaccharide; exercise; and smoking cessation.
[00466] In specific embodiments, patients with asthma are administered a
prophylactically
or therapeutically effective amount of an effector function enhanced anti-1COS
antibody of the
disclosure in combination with an effective amount of one or more other agents
useful for
asthma therapy. Non-limiting examples of such agents include adrenergic
stimulants (e.g.,
catecholamines (e.g., epinephrine, isoproterenol, and isoetharine),
resorcinols (e.g.,
metaproterenol, terbutalinc, and fenoterol), and saligenins (e.g.,
salbutamol)), adrenocorticoids,
blucocorticoids, corticosteroids (e.g., beclomethadonse, budesonide,
flunisolide, fluticasone,
triamcinolone, methylprednisolone, prednisolone, and prednisone), other
steroids, beta2-agonists
(e.g., albtuerol, bitolterol, fenoterol, isoetharine, metaproterenol,
pirbuterol, salbutamol,
terbutaline, formoterol, salmeterol, and albutamol terbutaline), anti-
cholinergics (e.g.,
ipratropium bromide and oxitropium bromide), IL-4 antagonists (including
antibodies), IL-5
antagonists (including antibodies), IL-9 antagonists (including antibodies),
IL-13 antagonists
(including antibodies), IL_17 antagonists (including antibodies), PDE4-
inhibitor, NF-Kappa-beta
inhibitor, VLA-4 inhibitor, CpG, anti-CD23, selectin antagonists (TBC 1269),
mast cell protease
inhibitors (e.g., tryptase kinase inhibitors (e.g., GW-45, GW-58, and
gcnisteine),
phosphatidylinositide-3' (P13)-kinase inhibitors (e.g., calphostin C), and
other kinase inhibitors
(e.g., staurosporine) (see Temkin et al., 2002 J Immunol 169(5):2662-2669;
Vosseller et al.,
1997 Mol. Biol. Cell 8(5):909-922; and Nagai et al., 1995 Biochem Biophys Res
Commun
208(2):576-581)), a C3 receptor antagonists (including antibodies),
immunosuppressant agents
141
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
(e.g., methotrexate and gold salts), mast cell modulators (e.g., cromolyn
sodium (INTALTm) and
nedocromil sodium (TILADElm)), and mucolytic agents (e.g., acetylcysteine)).
In a specific
embodiment, the anti-inflammatory agent is a leukotriene inhibitor (e.g.,
montelukast
(SINGULAIRTm), zafirlukast (ACCOLATETm), pranlukast (ONONTm), or zileuton
(ZYFLOTm)).
[00467] In specific embodiments, patients with allergy are administered a
prophylactically
or therapeutically effective amount of an effector function enhanced anti-ICOS
antibody of the
disclosure in combination with an effective amount of one or more other agents
useful for allergy
therapy. Non-limiting examples of such agents include antimediator drugs
(e.g., antihistamine),
corticosteroids, decongestants, sympathomimetic drugs (e.g., alpha-adrenergic
and .beta-
adrenergic drugs), TNX901 (Leung et al., N Engl J 348(11):986-993 (2003)),
IgE
antagonists (e.g., antibodies rhuMAb-E25 omalizumab (see Finn et al., 2003 J
Allergy Clin
Immuno 111(2):278-284; Con-en et al., 2003 J Allergy Clin Immuno 111(1):87-90;
Busse and
Neaville, 2001 Curr Opin Allergy Clin Immuno 1(1):105-108; and Tang and
Powell, 2001, Eur J
Pediatr 160(12): 696-704), HMK-12 and 6HD5 (see Miyajima et al., 2202 Int Arch
Allergy
Immuno 128(1):24-32), and mAB Hu-901 (see van Neerven et al., 2001 Int Arch
Allergy Immuno
124(1-3):400), theophylline and its derivatives, glucocorticoids, and
immunotherapies (e.g.,
repeated long-term injection of allergen, short course desensitization, and
venom
immunotherapy).
5.33. AUTOIMMUNE DISEASE
[00468] According to certain aspects of the disclosure, the treatment
regimen and dose
used with formulations and methods of the disclosure is chosen based on a
number of factors
including, but not limited to, the stage of the autoimmune disease or disorder
being treated.
Appropriate treatment regimens can be determined by one of skill in the art
for particular stages
of an autoimmune disease or disorder in a patient or patient population. Dose
response curves
can be generated using standard protocols in the art in order to determine the
effective amount of
formulations of the disclosure for treating patients having different stages
of an autoimmune
disease or disorder. In general, patients having more activity of a autoimmune
disease or
disorder will require higher doses and/or more frequent doses which may be
administered over
longer periods of time in comparison to patients having less activity of an
autoimmune disease or
disorder.
[00469] Anti-ICOS antibodies, formulations and methods may be practiced to
treat an
autoimmune disease or disorder. The term "autoimmune disease or disorder"
refers to a
condition in a subject characterized by cellular, tissue and/or organ injury
caused by an
142
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
immunologic reaction of the subject to its own cells, tissues and/or organs.
The term
"inflammatory disease" is used interchangeably with the term "inflammatory
disorder" to refer to
a condition in a subject characterized by inflammation, including, but not
limited to chronic
inflammation. Autoimmune disorders may or may not be associated with
inflammation.
Moreover, inflammation may or may not be caused by an autoimmune disorder.
Thus, certain
disorders may be characterized as both autoimmune and inflammatory disorders.
Exemplary
autoimmune diseases or disorders include, but are not limited to: alopecia
areata, ankylosing
spondylitis, antiphospholipid syndrome, autoimmune Addison's disease,
autoimmune diseases of
the adrenal gland, autoimmune hemolytic anemia, autoimmune hepatitis,
autoimmune oophoritis
and orchitis, autoimmune thrombocytopenia, Behcet's disease, bullous
pemphigoid,
cardiomyopathy, celiac sprue-dermatitis, chronic fatigue immune dysfunction
syndrome
(CFIDS), chronic inflammatory demyelinating polyneuropathy, Churg-Strauss
syndrome,
cicatrical pemphigoid, CREST syndrome, cold agglutinin disease, Crohn's
disease, discoid
lupus, essential mixed cryoglobulinemia, diabetes, eosinophilic fascites,
fibromyalgia-fibromyositis, glomerulonephritis, Graves' disease, Guillain-
Barre, Hashimoto's
thyroiditis, Henoch-Schonlein purpura, idiopathic pulmonary fibrosis,
idiopathic/autoimmune
thrombocytopenia purpura (ITP), IgA neuropathy, juvenile arthritis, lichen
planus, lupus
erthematosus, Moniere's disease, mixed connective tissue disease, multiple
sclerosis, type 1 or
immune-mediated diabetes mellitus, myasthenia gravis, pemphigus -related
disorders (e.g.,
pemphigus vulgaris), pernicious anemia, polyarteritis nodosa, polychrondritis,
polyglandular
syndromes, polymyalgia rheumatica, polymyositis and dermatomyositis, primary
agammaglobulincmia, primary biliary cirrhosis, psoriasis, psoriatic arthritis,
Raynauld's
phenomenon, Reiter's syndrome, Rheumatoid arthritis, sarcoidosis, scleroderma,
Sjogren's
syndrome, stiff-man syndrome, systemic lupus erythematosus (SLE), Sweet's
syndrome, Still's
disease, lupus erythematosus, takayasu arteritis, temporal arteristis/ giant
cell arteritis, ulcerative
colitis, uveitis, vasculitides such as dermatitis herpetiformis vasculitis,
vitiligo, and Wegener's
granulomatosis. Examples of inflammatory disorders include, but are not
limited to, asthma,
encephalitis, inflammatory bowel disease, chronic obstructive pulmonary
disease (COPD),
allergic disorders, septic shock, pulmonary fibrosis, undifferentiated
spondyloarthropathy,
undifferentiated arthropathy, arthritis, inflammatory osteolysis, graft versus
host disease,
urticaria, Vogt-Koyanagi-Hareda syndrome and chronic inflammation resulting
from chronic
viral or bacteria infections.
143
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
5.33.1.Autoimmune Disorder Treatment
[00470] An effector function enhanced anti-ICOS antibody of the disclosure
may be
administered to a subject in need thereof to prevent, manage, treat or
ameliorate an autoimmune
disorder or one or more symptoms thereof. Formulations of the disclosure may
also be
administered in combination with one or more other therapies, preferably
therapies useful for the
prevention, management or treatment of an autoimmune disorder (including, but
not limited to
the prophylactic or therapeutic agents) to a subject in need thereof to
prevent, manage, treat or
ameliorate an autoimmune disorder or one or more symptoms thereof. In a
specific embodiment,
the disclosure provides a method of preventing, managing, treating or
ameliorating an
autoimmune disorder or one or more symptoms thereof, said method comprising
administering
to a subject in need thereof a dose of a prophylactically or therapeutically
effective amount of an
effector function enhanced anti-ICOS antibody of the disclosure. In another
embodiment, the
disclosure provides a method of preventing, managing, treating or ameliorating
an autoimmune
disorder or one or more symptoms thereof, said method comprising administering
to a subject in
need thereof a dose of a prophylactically or therapeutically effective amount
of an effector
function enhanced anti-ICOS antibody of the disclosure and a dose of a
prophylactically or
therapeutically effective amount of one or more therapies (e.g., prophylactic
or therapeutic
agents) other than antibodies (including antibody fragments thereof) that
immunospecifically
bind to an ICOS polypeptide.
[00471] The disclosure provides methods for managing, treating or
ameliorating an
autoimmune disorder or one or more symptoms thereof in a subject refractory to
conventional
therapies for such an autoimmune disorder, said methods comprising
administering to said
subject a dose of a prophylactically or therapeutically effective amount of an
effector function
enhanced anti-ICOS antibody of the disclosure. The disclosure also provides
methods for
managing, treating or ameliorating an autoimmune disorder or one or more
symptoms thereof in
a subject refractory to existing single agent therapies for such an autoimmune
disorder, said
methods comprising administering to said subject a dose of a prophylactically
or therapeutically
effective amount of an effector function enhanced anti-ICOS antibody of the
disclosure and a
dose of a prophylactically or therapeutically effective amount of one or more
therapies (e.g.,
prophylactic or therapeutic agents) other than antibodies (including antibody
fragments thereof)
that immunospecifically bind to an ICOS polypeptide. The disclosure also
provides methods for
managing, treating or ameliorating an autoimmune disorder or one or more
symptoms thereof by
administering an effector function enhanced anti-ICOS antibody of the
disclosure in combination
with any other treatment to patients who have proven refractory to other
treatments but are no
longer on these treatments. The disclosure also provides alternative methods
for the
144
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
management or treatment of an autoimmune disorder where another therapy has
proven or may
prove too toxic, i.e., results in unacceptable or unbearable side effects, for
the subject being
treated. Particularly, the disclosure provides alternative methods for the
management or
treatment of an autoimmune disorder where the patient is refractory to other
therapies. Further,
the disclosure provides methods for preventing the recurrence of an autoimmunc
disorder in
patients that have been treated and have no disease activity by administering
an effector function
enhanced anti-ICOS antibody of the disclosure.
[00472] Examples of autoimmune disorders that can be treated by the methods
of the
disclosure include, but are not limited to, alopecia greata, ankylosing
spondylitis,
antiphospholipid syndrome, autoimmune Addison's disease, autoimmune diseases
of the adrenal
gland, autoimmune hemolytic anemia, autoimmune hepatitis, autoimmune
oophoritis and
orchitis, autoimmune thrombocytopenia, Behcet's disease, bullous pemphigoid,
cardiomyopathy,
celiac sprue-dermatitis, chronic fatigue immune dysfunction syndrome (CFIDS),
chronic
inflammatory demyelinating polyneuropathy, Churg-Strauss syndrome, cicatrical
pemphigoid,
CREST syndrome, cold agglutinin disease, Crohn's disease, discoid lupus,
essential mixed
cryoglobulinemia, fibromyalgia-fibromyositis, glomerulonephritis, Graves'
disease, Guillain-
Barre, Hashimoto's thyroiditis, idiopathic pulmonary fibrosis, idiopathic
thrombocytopeni a
putpura IgA neuropathy, juvenile arthritis, lichen planus, lupus
erythematosus, Mnire's
disease, mixed connective tissue disease, multiple sclerosis, type 1 or immune-
mediated diabetes
mellitus, myasthenia gravis, pemphigus vulgaris, pernicious anemia,
polyarteritis nodosa,
polychrondritis, polyglandular syndromes, polymyalgia rheumatica, polymyositis
and
dermatomyositis, primary agammaglobulincmia, primary biliary cirrhosis,
psoriasis, psoriatic
arthritis, Raynauld's phenomenon, Reiter's syndrome, Rheumatoid arthritis,
sarcoidosis,
scleroderma, Sjogren's syndrome, stiff-man syndrome, systemic lupus
erythematosus, lupus
erythematosus, takayasu arteritis, temporal arteristis/giant cell arteritis,
ulcerative colitis, uveitis,
vasculitides such as dermatitis herpetiformis vasculitis, vitiligo, and
Wegener's granulomatosis.
[00473] Autoimmune therapies and their dosages, routes of administration
and
recommended usage are known in the art and have been described in such
literature as the
Physician's Desk Reference (61th ed., 2007).
5.33.2.Autoimmune Disorder Therapies
[00474] The present disclosure provides methods of preventing, managing,
treating or
ameliorating an autoimmune disorder or one or more symptoms thereof, said
methods
comprising administering to a subject in need thereof an effector function
enhanced anti-ICOS
antibody of the disclosure and one or more therapies (e.g., prophylactic or
therapeutic agents)
145
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
other than antibodies (including antibody fragments thereof) that
immunospecifically bind to an
ICOS polypeptide. Any agent or therapy which is known to be useful, or which
has been used or
is currently being used for the prevention, management, treatment or
amelioration of an
autoimmune disorder or one or more symptoms thereof can be used in combination
with an
effector function enhanced anti-1COS antibody of the disclosure in accordance
with the
disclosure described herein. Examples of such agents include, but are not
limited to,
immunomodulatory agents, anti-inflammatory agents and TNF-alpha antagonists.
Specific
examples of immunomodulatory agents, anti-inflammatory agents and TNF-alpha
antagonists
which can be used in combination with an effector function enhanced anti-ICOS
antibody of the
disclosure for the prevention, management, treatment or amelioration of an
autoimmune disorder
are disclosed herein.
[00475] In specific embodiments, patients with multiple sclerosis (MS) are
administered a
prophylactically or therapeutically effective amount of an effector function
enhanced anti-ICOS
antibody of the disclosure in combination with other agents or therapies
useful in prevention,
treatment, management and amelioration of MS including but not limited to: IFN-
betalb
(Betaseron) (e.g., 8.0 million international unites (MIU) is administered by
subcutaneous
injection every other day); IFN-betal a (Avonex) (e.g., 6.0 MU is administered
by intramuscular
injection once every week); glatiramcr acetate (Copaxonc) (e.g., 20 mg is
administered by
subcutaneous injection every day); mitoxantrone (e.g., 12 mg/m2 is
administered by intravenous
infusion every third month); azathioprine (e.g., 2-3 mg/kg body weight is
administered orally
each day); methotrexate (e.g., 7.5 mg is administered orally once each week);
cyclophosphamide; intravenous immunoglobulin (e.g., 0.15-0.2 g/kg body weight
administered
monthly for up to 2 years); glucocorticoids; methylprednisolone (e.g.,
administered in bimonthly
cycles at high doses); 2-chlorodeoxyadenosine (cladribine); baclofen (e.g., 15
to 80 mg/d in
divided doses, or orally in higher doses up to 240 mg/d, or intrathecally via
an indwelling
catheter); cycloenzaprine hydrochloride (e.g., 5-10 mg bid or tid); clonazepam
(e.g., 0.5 to 1.0
mg tid, including bedtime dose); clonidine hydrochloride (e.g., 0.1 to 0.2 mg
tid, including a
bedtime dose); carbamazepine (e.g., 100-1200 mg/d in divided, escalating
doses); gabapentin
(e.g., 300-3600 mg/d); dilantin (e.g., 300-400 mg/d); amitriptyline (e.g., 25-
150 mg/d); baclofen
(e.g., 10-80 mg/d); primidone (e.g., 125-250 mg bid or tid); ondansetron
(e.g., 4 to 8 mg bid or
tid); isoniazid (e.g., up to 1200 mg in divided doses); oxybutynin (e.g., 5 mg
bid or tid);
tolterodine (e.g., 1-2 mg bid); propantheline (e.g., 7.5 to 15 mg qid);
bethanecol (e.g., 10-50 mg
tid or qid); terazosin hydrochloride (e.g., 1-5 mg at bedtime); sildenafil
citrate (e.g., 50-100 mg
po pm); amantading (e.g., 100 mg bid); pemoline (e.g., 37.5 mg bid); high dose
vitamins;
calcium orotate; gancyclovir; antibiotic; and plasma exchange.
146
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00476] In specific embodiments, patients with psoriasis are administered a
prophylactically or therapeutically effective amount of an effector function
enhanced anti-ICOS
antibody of the disclosure in combination with other agents or therapies
useful in prevention,
treatment, management and amelioration of psoriasis including but not limited
to: topical steroid
cream or ointment; tar (examples including but not limited to, Estar,
Psorigel, Fototar cream, and
LCD 10% in Nutraderm lotion or mixed directly with triamcinolone 0.1% cream);
occlusion;
topical vitamin D analogue (a non-limiting example is calcipotriene ointment);
ultraviolet light;
PUVA (psoralen plus ultraviolet A); methotrexate (e.g., up to 25 mg once
weekly or in divided
doses every 12 hours for three doses once a week); synthetic retinoid (a non-
limiting examples is
etretinate, e.g., in dosage of 0.5-1 mg/kg/d); immunomodulatory therapy (a non-
limiting example
is cyclosporine); sulfasalazine (e.g., in dosages of 1 g three times daily).
[00477] In specific embodiments, patients with Crohn's disease are
administered a
prophylactically or therapeutically effective amount of an effector function
enhanced anti-ICOS
antibody of the disclosure in combination with other agents or therapies
useful in prevention,
treatment, management and amelioration of Crohn's disease including but not
limited to:
antidiarrheals (e.g., loperamide 2-4 mg up to 4 times a day, diphenoxylate
with atropine 1 tablet
up to 4 times a day, tincture of opium 8-15 drops up to 4 times a day,
cholestyramine 2-4 g or
colestipol 5 g once or twice daily), antispasmodics (e.g., propantheline 15
mg, dicyclomine 10-
20 mg, or hyoscyamine 0.125 mg given before meals), 5-aminosalicylic acid
agents (e.g.,
sulfasalazine 1.5-2 g twice daily, mesalamine (ASACOLTM) and its slow release
form
(PENTASATm), especially at high dosages, e.g., PENTASATm 1 g four times daily
and
ASACOLTM 0.8-1.2 g four times daily), corticosteroids, immunomodulatory drugs
(e.g.,
azathioprine (1-2 mg/kg), mercaptopurine (50-100 mg), cyclosporine, and
methotrexate),
antibiotics, TNF inhibitors (e.g., inflixmab (REMICADETm)), immunosuppressive
agents (e.g.,
tacrolimus, mycophenolate mofetil, and thalidomide), anti-inflammatory
cytokines (e.g., IL-10
and IL-11), nutritional therapies, enteral therapy with elemental diets (e.g.,
Vivonex for 4
weeks), and total parenteral nutrition.
[00478] In specific embodiments, patients with lupus erythematosus are
administered a
prophylactically or therapeutically effective amount of an effector function
enhanced anti-ICOS
antibody of the disclosure in combination with other agents or therapies
useful in prevention,
treatment, management and amelioration of lupus crythematosus including but
not limited to:
antimalarials (including but not limited to, hydroxychloroquine);
glucocorticoids (e.g., low dose,
high dose, or high-dose intravenous pulse therapy can be used);
immunosuppressive agents
(including but not limited to, cyclophosphamide, chlorambucil, and
azanthioprine); cytotoxic
agents (including but not limited to methotrexate and mycophenolate mofetil);
androgenic
147
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
steroids (including but not limited to danazol); anticoagulants (including but
not limited to
warfarin); and B-lymphocyte stimulator inhibitor (e.g. belimumab). In specific
embodiments,
patients with lupus erythematosus are administered a prophylactically or
therapeutically effective
amount of a formulation described herein in combination with belimumab.
[00479] The antibody formulations of the disclosure or combination
therapies of the
disclosure may be used as the first, second, third, fourth, or fifth therapy
to prevent, manage,
treat, and/or ameliorate an autoimmune disorder or one or more symptom thereof
The
disclosure also includes methods of preventing, treating, managing, and/or
ameliorating an
autoimmunc disorder or one or more symptoms thereof in a patient undergoing
therapy for other
disease or disorder. The disclosure encompasses methods of preventing,
managing, treating,
and/or ameliorating an autoimmune disorder or one or more symptoms thereof in
a patient before
any adverse effects or intolerance to therapies other than antibodies of the
disclosure develops.
The disclosure also encompasses methods of preventing, treating, managing,
and/or ameliorating
an autoimmune disorder or a symptom thereof in refractory patients. The
disclosure
encompasses methods for preventing, treating, managing, and/or ameliorating a
proliferative
disorder or a symptom thereof in a patient who has proven refractory to
therapies other than
antibodies, formulations, or combination therapies of the disclosure. The
determination of
whether a patient is refractory can be made either in vivo or in vitro by any
method known in the
art for assaying the effectiveness of a treatment of autoimmune disorders,
using art-accepted
meanings of "refractory" such a context. In certain embodiments, a patent with
an autoimmune
disorder is refractory to a therapy when one or more symptoms of an autoimmune
disorder is not
prevented, managed, and/or alleviated. The disclosure also encompasses methods
of preventing,
managing, treating, and/or ameliorating an autoimmune disorder or a symptom
thereof in
patients who are susceptible to adverse reactions to conventional therapies.
[00480] The present disclosure encompasses methods for preventing,
treating, managing,
and/or ameliorating an autoimmune disorder or one or more symptoms thereof as
an alternative
to other conventional therapies. In specific embodiments, the patient being
managed or treated
in accordance with the methods of the disclosure is refractory to other
therapies or is susceptible
to adverse reactions from such therapies. The patient may be a person with a
suppressed
immune system (e.g., post-operative patients, chemotherapy patients, and
patients with
immunodeficiency disease, patients with broncho-pulmonary dysplasia, patients
with congenital
heart disease, patients with cystic fibrosis, patients with acquired or
congenital heart disease, and
patients suffering from an infection), a person with impaired renal or liver
function, the elderly,
children, infants, infants born prematurely, persons with neuropsychiatric
disorders or those who
take psychotropic drugs, persons with histories of seizures, or persons on
medication that would
148
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
negatively interact with conventional agents used to prevent, manage, treat,
or ameliorate an
autoimmune disease or disorder.
[00481] Autoimmune therapies and their dosages, routes of administration
and
recommended usage are known in the art and have been described in such
literature as the
Physician's Desk Reference (61th ed., 2007).
5.33.3. Diagnosis of Autoimmune Diseases or Disorders
[00482] The diagnosis of an autoimmune disease or disorder is complicated
in that each
type of autoimmune disease or disorder manifests differently among patients.
This heterogeneity
of symptoms means that multiple factors are typically used to arrive at a
clinical diagnosis.
Generally, clinicians use factors, such as, but not limited to, the presence
of autoantibodies,
elevated cytokine levels, specific organ dysfunction, skin rashes, joint
swelling, pain, bone
remodeling, and/or loss of movement as primarily indicators of an autoimmune
disease or
disorder. For certain autoimmune diseases or disorders, such as RA and SLE,
standards for
diagnosis are known in the art. For certain autoimmune diseases or disorders,
stages of disease
have been characterized and are well known in the art. These art recognized
methods for
diagnosing autoimmune diseases and disorders as well as stages of disease and
scales of activity
and/or severity of disease that are well known in the art can be used to
identify patients and
patient populations in need of treatment for an autoimmune disease or disorder
using
formulations and methods of the disclosure.
5.33.4. Clinical criteria for diagnosing autoimmune diseases or disorders
[00483] Diagnostic criteria for different autoimmune diseases or disorders
are known in
the art. Historically, diagnosis is typically based on a combination of
physical symptoms. More
recently, molecular techniques such as gene-expression profiling have been
applied to develop
molecular definitions of autoimmune diseases or disorders. Exemplary methods
for clinical
diagnosis of particular autoimmune diseases or disorders are provided below.
Other suitable
methods will be apparent to those skilled in the art.
[00484] In certain embodiments , patients with low levels of autoimmune
disease activity
or patients with an early stage of an autoimmune disease (for diseases where
stages are
recognized) can be identified for treatment using anti-ICOS antibody
formulations and methods .
The early diagnosis of autoimmune disease is difficult due to the general
symptoms and overlap
of symptoms among diseases. In such embodiments, a patient treated at an early
stage or with
low levels of an autoimmune disease activity has symptoms comprising at least
one symptom of
an autoimmune disease or disorder. In related embodiments, a patient treated
at an early stage or
149
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
with low levels of an autoimmune disease has symptoms comprising at least 1,
2, 3, 4, 5, 6, 7, 8,
9, 10, 11, 12, 13, 14, or 15 symptoms of an autoimmune disease or disorder.
The symptoms may
be of any autoimmune diseases and disorders or a combination thereof. Examples
of
autoimmune disease and disorder symptoms are described below.
5.34. IMMUNOTHERAPEUTIC PROTOCOLS
[00485] Anti-ICOS antibody formulations used in the therapeutic
regimen/protocols,
referred to herein as "anti-ICOS immunotherapy" can be naked antibodies,
immunoconjugates
and/or fusion proteins. Formulations of the disclosure can be used as a single
agent therapy or in
combination with other therapeutic agents or regimens. Anti-ICOS antibodies or
immunoconjugates can be administered prior to, concurrently with, or following
the
administration of one or more therapeutic agents. Therapeutic agents that can
be used in
combination therapeutic regimens with formulations of the disclosure include
any substance that
inhibits or prevents the function of cells and/or causes destruction of cells.
Examples include,
but are not limited to, radioactive isotopes, chemotherapeutic agents, and
toxins such as
enzymatically active toxins of bacterial, fungal, plant or animal origin, or
fragments thereof.
[00486] The therapeutic regimens described herein, or any desired treatment
regimen can
be tested for efficacy using a transgenic animal model which expresses human
ICOS antigen in
place of native ICOS antigen. Thus, an anti-ICOS antibody treatment regimen
can be tested in
an animal model to determine efficacy before administration to a human.
5.35. ANTI-ICOS IMMUNOTHERAPY
[00487] In accordance with the present disclosure "anti-ICOS immunotherapy"
encompasses the administration of any of the anti-ICOS antibodies of the
disclosure in
accordance with any therapeutic regimen described herein. Anti-ICOS antibodies
can be
administered as naked antibodies, or immunoconjugates or fusion proteins. In
one embodiment,
a human subject having a T cell-mediated disease or disorder can be treated by
administering an
anti-ICOS antibody capable to mediate human ADCC.
[00488] Antibodies of IgG1 or IgG3 human isotypes are in some cases
preferred for
therapy. However, the IgG2 or IgG4 human isotypes can be used as well,
provided they have the
relevant effector function, for example human ADCC. Such effector function can
be assessed by
measuring the ability of the antibody in question to mediate target cell lysis
by effector cells in
vitro or in vivo.
150
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00489] In one embodiment, the dose of antibody used should be sufficient
to deplete
circulating ICOS expressing T cells. Progress of the therapy can be monitored
in the patient by
analyzing blood samples. Other signs of clinical improvement can be used to
monitor therapy.
[00490] Methods for measuring depletion of ICOS expressing T cells that can
be used in
connection with formulations and methods of the disclosure are well known in
the art and
include, but are not limited to the following embodiments. In one embodiment,
circulating ICOS
expressing T cells depletion can be measured with flow cytometry using a
reagent other than an
anti-ICOS antibody that binds to ICOS expressing T cells to define the amount
of ICOS
expressing T cells. In another embodiment, 1COS expressing T cell depletion
can be measured
by immunochemical staining to identify ICOS expressing T cells. In such
embodiments, ICOS
expressing T cells or tissues or serum comprising ICOS expressing T cells
extracted from a
patient can be placed on microscope slides, labeled and examined for presence
or absence. In
related embodiments, a comparison is made between ICOS expressing T cells
extracted prior to
therapy and after therapy to determine differences in the presence of ICOS
expressing T cells.
[00491] In embodiments of the disclosure where an anti-ICOS antibody is
administered as
a single agent therapy, the disclosure contemplates use of different treatment
regimens.
[00492] According to certain aspects of the disclosure, an anti-ICOS
antibody used in
formulations and methods of the disclosure, is a naked antibody. In related
embodiments, the
dose of naked anti-ICOS antibody used is at least about 0.1, 0.2, 0.3, 0.4,
0.5, 0.6, 0.7, 0.8, 0.9, 1,
1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5, 10, 10.5,
11, 11.5, 12, 12.5, 13, 13.5,
14, 14.5, 15, 15.5, 16, 16.5, 17, 17.5, 18, 18.5, 19, 19.5, 20, 20.5 mg/kg of
body weight of a
patient. In certain embodiments, the dose of naked anti-ICOS antibody used is
at least about 1 to
10,5 to 15, 10 to 20, or 15 to 25 mg/kg of body weight of a patient. In
certain embodiments, the
dose of naked anti-ICOS antibody used is at least about 1 to 20, 3 to 15, or 5
to 10 mg/kg of
body weight of a patient. In other embodiments, the dose of naked anti-ICOS
antibody used is at
least about 5, 6, 7, 8, 9, or 10 mg/kg of body weight of a patient.
[00493] In certain embodiments, the dose comprises about 375 mg/m2 of anti-
ICOS
antibody administered weekly for about 1, 2, 3, 4, 5, 6, 7 or 8 consecutive
weeks. In certain
embodiments, the dose is at least about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12,
13, 14, or 15 mg/kg of
body weight of the patient administered weekly for about 1, 2, 3, 4, 5, 6, 7
or 8 consecutive
weeks.
[00494] The exemplary doses of anti-ICOS antibody described above can be
administered
as described herein. In one embodiment, the above doses are single dose
injections. In other
embodiments, the doses are administered over a period of time. In other
embodiments, the doses
are administered multiple times over a period of time. The period of time may
be measured in
151
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
days, weeks, or months. Multiple doses of an anti-ICOS antibody can be
administered at
intervals suitable to achieve a therapeutic benefit while balancing toxic side
effects. For
example, where multiple doses are used, it may be preferred to time the
intervals to allow for
recovery of the patient's monocyte count prior to the repeat treatment with
antibody. This
dosing regimen will optimize the efficiency of treatment, since the monocyte
population reflects
ADCC function in the patient.
[00495] In certain embodiments, formulations of the disclosure are
administered to a
human patient as long as the patient is responsive to therapy. In other
embodiments,
formulations of the disclosure are administered to a human patient as long as
the patient's
disease does not progress. In related embodiments, formulations of the
disclosure are
administered to a human patient until a patient's disease does not progress or
has not progressed
for a period of time, then the patient is not administered formulations of the
disclosure unless the
disease reoccurs or begins to progress again. If disease progression stops or
reverses, then he
patient will not be administered formulations of the disclosure until that
patient relapses, i.e., the
disease being treated reoccurs or progresses. Upon this reoccurrence or
progression, the patient
can be treated again with the same dosing regimen initially used or using
other doses described
above.
[00496] In certain embodiments, formulations of the disclosure can be
administered as a
loading dose followed by multiple lower doses (maintenance doses) over a
period of time. In
such embodiments, the doses may be timed and the amount adjusted to maintain
effective ICOS
expressing T cell depletion. In certain embodiments, the loading dose is about
10, 11, 12, 13, 14,
15, 16, 17, or 18 mg/kg of patient body weight and the maintenance dose is at
least about 5 to 10
mg/kg of patient body weight. In other embodiments, the maintenance dose is
administered at
intervals of every 7, 10, 14 or 21 days.
[00497] The antibody compositions of the disclosure can be used in the
treatment of
autoimmune diseases, such as systemic lupus erythematosus (SLE), multiple
sclerosis (MS),
inflammatory bowel disease (IBD; including Crohn's Disease, Ulcerative Colitis
and Celiac's
Disease), insulin dependent diabetes mellitus (IDDM), psoriasis, autoimmune
thyroiditis,
rheumatoid arthritis (RA) and glomerulonephritis. Furthermore, the antibody
compositions of
the disclosure can be used for inhibiting or preventing transplant rejection
or in the treatment of
graft versus host disease (GVHD).
[00498] The liquid formulations of the present disclosure may be used
locally or
systemically in the body as a therapeutic. The formulations of the present
disclosure may also be
utilized in combination with one or more other therapies (e.g., one or more
other prophylactic or
therapeutic agents). When one or more other therapies (e.g., prophylactic or
therapeutic agents)
152
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
are used, they can be administered separately, in any appropriate form and by
any suitable route.
Therapeutic or prophylactic agents include, but are not limited to, small
molecules, synthetic
drugs, peptides, polypeptides, proteins, nucleic acids (for example, but not
limited to, DNA and
RNA nucleotides including, but not limited to, antisense nucleotide sequences,
triple helices,
RNAi, and nucleotide sequences encoding biologically active proteins,
polypeptides or peptides)
antibodies, synthetic or natural inorganic molecules, mimetic agents, and
synthetic or natural
organic molecules.
[00499] Any therapy (e.g., prophylactic or therapeutic agents) which is
known to be
useful, or which has been used or is currently being used for the prevention,
treatment and/or
management of one or more symptoms associated with a disease or disorder
associated with or
characterized by aberrant expression and/or activity of ICOS, a disease or
disorder associated
with or characterized by aberrant expression and/or activity of the ICOS
receptor or one or more
subunits thereof, an autoimmune disease, transplant rejection, graft versus
host disease can be
used in combination with the liquid antibody formulations of the present
disclosure in
accordance with the disclosure described herein. See, e.g., Gilman et al.,
Goodman and
Gilman's: The Pharmacological Basis of Therapeutics, Tenth Ed., McGraw-Hill,
New York,
2001; The Merck Manual of Diagnosis and Therapy, Berkow, M.D. et al. (eds.),
17th Ed., Merck
Sharp & Dohme Research Laboratories, Rahway, NJ, 1999; and Cecil Textbook of
Medicine,
20th Ed., Bennett and Plum (eds.), W.B. Saunders, Philadelphia, 1996 for
information regarding
therapies, in particular prophylactic or therapeutic agents, which have been
or are currently being
used for preventing, treating and/or managing diseases or disorders associated
with or
characterized by aberrant expression and/or activity of ICOS, diseases or
disorders associated
with or characterized by aberrant expression and/or activity of the ICOS
receptor or one or more
subunits thereof, autoimmune diseases, inflammatory diseases, or one or more
symptoms
thereof. Examples of prophylactic and therapeutic agents include, but are not
limited to,
immunomodulatory agents, anti-inflammatory agents (for example, but not
limited to,
adrenocorticoids, corticosteroids (for example, but not limited to,
beclomethasone, budesonide,
flunisolide, fluticasone, triamcinolone, methlyprednisolone, prednisolone,
prednisone,
hydrocortisone), glucocorticoids, steroids, non-steriodal anti-inflammatory
drugs (for example,
but not limited to, aspirin, ibuprofen, diclofenac, and COX-2 inhibitors), and
leukotreine
antagonists (for example, but not limited to, montelukast, methyl xanthines,
zafirlukast, and
zileuton), beta2-agonists (for example, but not limited to, albuterol,
biterol, fenoterol, isoetharie,
metaproterenol, pirbuterol, salbutamol, terbutalin formoterol, salmeterol, and
salbutamol
terbutaline), anticholinergic agents (for example, but not limited to,
ipratropium bromide and
oxitropium bromide), sulphasalazine, penicillamine, dapsone, antihistamines,
anti-malarial
153
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
agents (for example, but not limited to, hydroxychloroquine), anti-viral
agents, and antibiotics
(for example, but not limited to, dactinomycin (formerly actinomycin),
bleomycin, erythomycin,
penicillin, mithramycin, and anthramycin (AMC)).
[00500] A liquid formulation of the disclosure may be administered to a
human
concurrently with one or more other therapies (e.g., one or more other
prophylactic or
therapeutic agents). The term "concurrently" is not limited to the
administration of prophylactic
or therapeutic agents/therapies at exactly the same time, but rather it is
meant that a liquid
formulation of the disclosure and the other agent/therapy are administered to
a mammal in a
sequence and within a time interval such that the antibody (including antibody
fragment thereof)
that specifically binds to ICOS contained in the liquid formulation can act
together with the other
agent/therapy to provide an increased benefit than if they were administered
otherwise.
[00501] In various embodiments, a liquid formulation of the disclosure and
one or more
other therapies (e.g., one or more other prophylactic or therapeutic agents),
are administered less
than 1 hour apart, at about 1 hour apart, at about 1 hour to about 2 hours
apart, at about 2 hours
to about 3 hours apart, at about 3 hours to about 4 hours apart, at about 4
hours to about 5 hours
apart, at about 5 hours to about 6 hours apart, at about 6 hours to about 7
hours apart, at about 7
hours to about 8 hours apart, at about 8 hours to about 9 hours apart, at
about 9 hours to about 10
hours apart, at about 10 hours to about 11 hours apart, at about 11 hours to
about 12 hours apart,
no more than 24 hours apart or no more than 48 hours apart. In specific
embodiments, a liquid
formulation of the disclosure and one or more other therapies are administered
within the same
patient visit. In other embodiments, a liquid formulation of the disclosure
and one or more other
therapies are administered at about 2 to 4 days apart, at about 4 to 6 days
apart, at about 1 week
part, at about 1 to 2 weeks apart, or more than 2 weeks apart. In specific
embodiments, a liquid
formulation of the disclosure and one or more other therapies are administered
in a time frame
where both agents are still active. One skilled in the art would be able to
determine such a time
frame by determining the half-life of the administered agents.
[00502] In certain embodiments, a liquid formulation of the disclosure and
one or more
other therapies (e.g., one or more other prophylactic or therapeutic agents),
are cyclically
administered to a subject. Cycling therapy involves the administration of a
first agent for a
period of time, followed by the administration of a second agent and/or third
agent for a period
of time and repeating this sequential administration. Cycling therapy can
reduce the
development of resistance to one or more of the therapies, avoid or reduce the
side effects of one
of the therapies, and/or improves the efficacy of the treatment.
[00503] In other embodiments, liquid formulation of the disclosure and one
or more other
therapies (e.g., prophylactic or therapeutic agents) are administered in
metronomic dosing
154
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
regimens, either by continuous infusion or frequent administration without
extended rest periods.
Such metronomic administration can involve dosing at constant intervals
without rest periods.
Typically the prophylactic or therapeutic agents, in particular cytotoxic
agents, are used at lower
doses. Such dosing regimens encompass the chronic daily administration of
relatively low doses
for extended periods of time. In specific embodiments, the use of lower doses
can minimize
toxic side effects and eliminate rest periods. In certain embodiments, the
prophylactic and
therapeutic agents are delivered by chronic low-dose or continuous infusion
ranging from about
24 hours to about 2 days, to about 1 week, to about 2 weeks, to about 3 weeks
to about 1 month
to about 2 months, to about 3 months, to about 4 months, to about 5 months, to
about 6 months.
[00504] In one embodiment, a liquid formulation of the disclosure is
administered in a
dosing regimen that maintains the plasma concentration of the antibody
(including antibody
fragment thereof) specific for ICOS at a desirable level (e.g., about 0.1 to
about 100 g/m1),
which maintains depletion of ICOS expressing cells. In a specific embodiment,
the plasma
concentration of the antibody (including antibody fragment thereof) is
maintained at 0.001
tigiml, 0.005 g/ml, 0.01 ug/ml, 0.05 g/ml, 0.1 g/ml, 0.2 g/ml, 0.5 g/ml,
1 g/ml, 2 g/ml, 3
lug/ml, 4 lug/ml, 5 g/ml, 6 tg/ml, 7 tg/ml, 8 tg/ml, 9 g/ml, 10 pg/ml, 15
g/ml, 20 lag/ml, 25
jig/ml, 30 pg/ml, 35 g/ml, 40 g/ml, 45 g/m1 or 50 g/ml. The plasma
concentration that is
desirable in a subject will vary depending on several factors, including but
not limited to, the
nature of the disease or disorder, the severity of the disease or disorder and
the condition of the
subject. Such dosing regimens are especially beneficial in prevention,
treatment and/or
management of a chronic disease or disorder.
[005051 In one embodiment, a liquid formulation of the disclosure is
administered to a
subject with a disease or disorder associated with or characterized by
aberrant expression and/or
activity of ICOS, a disease or disorder associated with or characterized by
aberrant expression
and/or activity of the ICOS receptor or one or more subunits thereof, an
autoimmune disease, a
malignant disease, transplant rejection, graft versus host disease, or one or
more symptoms
thereof using a dosing regimen that maintains the plasma concentration of the
an antibody
(including antibody fragment thereof) that specifically binds to ICOS at a
level that maintains at
least 40%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90% or at least 95% depletion of ICOS
expressing cells. In a
specific embodiment, the plasma concentration of the an antibody (including
antibody fragment
thereof) that specifically binds to ICOS is maintained at about 0.001 g/m1 to
about 100 g/m1 in
a subject with a disease or disorder associated with or characterized by
aberrant expression
and/or activity of ICOS, a disease or disorder associated with or
characterized by aberrant
expression and/or activity of the ICOS receptor or one or more subunits
thereof, an autoimmunc
155
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
disease, a malignancy, transplant rejection, graft versus host disease, or one
or more symptoms
thereof.
[00506] In some embodiments, a liquid formulation of the disclosure is
administered
intermittently to a subject, wherein the liquid formulation comprises an
antibody (including
antibody fragment thereof) conjugated to a moiety.
[00507] When used in combination with other therapies (e.g., prophylactic
and/or
therapeutic agents) the liquid formulations of the disclosure and the other
therapy can act
additively or synergistically. The disclosure contemplates administration of a
liquid formulation
of the disclosure in combination with other therapies (e.g., prophylactic or
therapeutic agents) by
the same or different routes of administration, for example, but not limited
to, oral and
parenteral. In certain embodiments, when a liquid formulation of the
disclosure is administered
concurrently with one or more therapies (e.g., prophylactic or therapeutic
agents) that potentially
produce adverse side effects (including, but not limited to, toxicity), the
therapies (e.g.,
prophylactic or therapeutic agents) can advantageously be administered at a
dose that falls below
the threshold that the adverse side effect is elicited.
5.36. COMBINATION WITH CHEMOTHERAPEUTIC AGENTS
[00508] Anti-ICOS immunotherapy (using naked antibody, immunoconjugates, or
fusion
proteins) can be used in conjunction with other therapies including but not
limited to,
chemotherapy, radioimmunotherapy (RIT), chemotherapy and external beam
radiation
(combined modality therapy, CMT), or combined modality radioimmunotherapy
(CMRIT) alone
or in combination, etc. In certain embodiments, an anti-ICOS antibody therapy
of the present
disclosure can be administered in conjunction with CHOP
(Cyclophosphamide-Hydroxydoxorubicin-Oncovin (vincristine)-Prednisolone) As
used herein,
the term "administered in conjunction with" means that an anti-ICOS
immunotherapy can be
administered before, during, or subsequent to the other therapy employed.
[00509] In certain embodiments, an anti-ICOS immunotherapy is in
conjunction with a
cytotoxic radionuclide or radiotherapeutic isotope. For example, an alpha-
emitting isotope such
as 225Ac, 224Ac, 21 lAt, 212Bi, 213Bi, 212pb,
224Ra, or 223Ra. The cytotoxic radionuclide may also be
a beta-emitting isotope such as 186Re, 188Re, 90y, 1311, 67c,u, 171m, 153sm,
166= .t-t0,
or 64Cu. Further,
the cytotoxic radionuclide may emit Auger and low energy electrons and include
the isotopes
1251, 123=I or 77
Br. In other embodiments the isotope may be I98Au, 32P, and the like. In
certain
embodiments, the amount of the radionuclide administered to the subject is
between about 0.001
mCi/kg and about 10 mCi/kg.
156
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00510] In some embodiments, the amount of the radionuclide administered to
the subject
is between about 0.1 mCiikg and about 1.0 mCi/kg. In other embodiments, the
amount of the
radionuclide administered to the subject is between about 0.005 mCi/kg and 0.1
mCi/kg.
[00511] In certain embodiments, an anti-ICOS immunotherapy is in
conjunction with a
chemical toxin or chemotherapeutic agent. The chemical toxin or
chemotherapeutic agent may
be selected from the group consisting of an enediyne such as calicheamicin and
esperamicin;
duocarmycin, methotrexate, doxorubicin, melphalan, chlorambucil, ARA-C,
vindesine,
mitomycin C, cis-platinum, etoposide, bleomycin and 5-fluorouracil.
[00512] Suitable chemical toxins or chemotherapeutic agents that can be
used in
combination therapies with an anti-ICOS immunotherapy include members of the
enediyne
family of molecules, such as calicheamicin and esperamicin. Chemical toxins
can also be taken
from the group consisting of duocarmycin (see, e.g., U.S. Pat. No. 5,703,080
and U.S. Pat. No.
4,923,990), methotrexate, doxorubicin, melphalan, chlorambucil, ARA-C,
vindesine, mitomycin
C, cis-platinum, etoposide, bleomycin and 5-fluorouracil. Examples of
chemotherapeutic agents
also include Adriamycin, Doxorubicin, 5-Fluorouracil, Cytosine arabinoside
("Ara-C"),
Cyclophosphamide, Thiotepa, Taxotere (docetaxel), Bus ulfan, Cytoxin, Taxol,
Methotrexate,
Cisplatin, Melphalan, Vinblastine, Bleomycin, Etoposide, Ifosfamide, Mitomycin
C,
Mitoxantrone, Vincreistine, Vinorelbine, Carboplatin, Teniposide, Daunomycin,
Carminomycin,
Aminopterin, Dactinomycin, Mitomycins, Esperamicins (see, U.S. Pat. No.
4,675,187),
Melphalan and other related nitrogen mustards.
[00513] In other embodiments, for example, "CVB" (1.5 g/m2
cyclophosphamide,
200-400 mg/m2 etoposide, and 150-200 mg/m2 carmustine) can be used in
combination therapies
of the disclosure. CVB is a regimen used to treat non-Hodgkin's lymphoma.
Patti et al., Eur. J.
Haematol. 51:18 (1993). Other suitable combination chemotherapeutic regimens
are
well-known to those of skill in the art. See, for example, Freedman et al.,
"Non-Hodgkin's
Lymphomas," in CANCER MEDICINE, VOLUME 2, 3rd Edition, Holland et al. (eds.),
pp.
2028-2068 (Lea & Febiger 1993). As an illustration, first generation
chemotherapeutic regimens
for treatment of intermediate-grade non-Hodgkin's lymphoma include C-MOPP
(cyclophosphamide, vincristine, procarbazine and prednisone) and CHOP
(cyclophosphamide,
doxorubicin, vincristine, and prednisone). A useful second generation
chemotherapeutic
regimen is m-BACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide,
vincristine,
dexamethasone and leucovorin), while a suitable third generation regimen is
MACOP-B
(methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone,
bleomycin and
leucovorin). Additional useful drugs include phenyl butyrate and brostatin-1.
In a multimodal
therapy, both chemotherapeutic drugs and cytokines are co-administered with an
antibody,
157
CA 02743469 2011-05-11
WO 2010/056804 PCT/1JS2009/064127
immunoconjugate or fusion protein according to the present disclosure. The
cytokines,
chemotherapeutic drugs and antibody, immunoconjugate or fusion protein can be
administered in
any order, or together.
[00514] Other toxins that may be used in formulations and methods of the
disclosure
include poisonous lectins, plant toxins such as ricin, abrin, modeccin,
botulina and diphtheria
toxins. Of course, combinations of the various toxins could also be coupled to
one antibody
molecule thereby accommodating variable cytotoxicity. Illustrative of toxins
which are suitably
employed in combination therapies of the disclosure are ricin, abrin,
ribonuclease, DNase I,
Staphylococcal cnterotoxin-A, pokeweed antiviral protein, gclonin, diphtherin
toxin,
Pseudomonas exotoxin, and Pseudomonas endotoxin. See, for example, Pastan et
al., Cell
47:641 (1986), and Goldenberg et al., Cancer Journal for Clinicians 44:43
(1994).
Enzymatically active toxins and fragments thereof which can be used include
diphtheria A chain,
nonbinding active fragments of diphtheria toxin, exotoxin A chain (from
Pseudomonas
aeruginosa), ricin A chain, abrin A chain, modeccin A chain, alpha-sarcin,
Aleuritesfordii
proteins, dianthin proteins, Phytolaca americana proteins (PAPI, PAPII, and
PAP-S),
momordica charantia inhibitor, curcin, crotin, sapaonaria officinalis
inhibitor, gelonin,
mitogellin, restrictocin, phenomycin, enomycin and the tricothecenes. See, for
example, WO
93/21232 published October 28, 1993.
[00515] Suitable toxins and chemotherapeutic agents are described in
REMINGTON'S
PHARMACEUTICAL SCIENCES, 19th Ed. (Mack Publishing Co. 1995), and in GOODMAN
AND GILMAN'S THE PHARMACOLOGICAL BASIS OF THERAPEUTICS, 7th Ed.
(MacMillan Publishing Co. 1985). Other suitable toxins and/or chemotherapeutic
agents are
known to those of skill in the art.
[00516] An anti-ICOS immunotherapy of the present disclosure may also be in
conjunction with a prodrug-activating enzyme which converts a prodrug (e.g., a
peptidyl
chemotherapeutic agent, see, W081/01145) to an active anti-cancer drug. See,
for example, WO
88/07378 and U.S. Patent No. 4,975,278. The enzyme component of such
combinations includes
any enzyme capable of acting on a prodrug in such a way so as to covert it
into its more active,
cytotoxic form. The term "prodrug" as used in this application refers to a
precursor or derivative
form of a pharmaceutically active substance that is less cytotoxic to tumor
cells compared to the
parent drug and is capable of being enzymatically activated or converted into
the more active
parent form. See, e.g., Wilman, "Prodrugs in Cancer Chemotherapy" Biochemical
Society
Transactions, 14, pp. 375-382, 615th Meeting Belfast (1986) and Stella et al.,
"Prodrugs: A
Chemical Approach to Targeted Drug Delivety," Directed Drug Delivery,
Borchardt et al. (ed.),
pp. 247-267, Humana Press (1985). Prodrugs that can be used in combination
with anti-1COS
158
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
antibodies include, but are not limited to, phosphate-containing prodrugs,
thiophosphate-containing prodrugs, sulfate-containing prodrugs, peptide-
containing prodrugs,
D-amino acid-modified prodrugs, glycosylated prodrugs, a-lactam-containing
prodrugs,
optionally substituted phenoxyacetamide-containing prodrugs or optionally
substituted
phenylacetamidc-containing prodrugs, 5-fluorocytosinc and other 5-
fluorouridine prodrugs
which can be converted into the more active cytotoxic free drug. Examples of
cytotoxic drugs
that can be derivatized into a prodrug form for use in this disclosure
include, but are not limited
to, those chemotherapeutic agents described above.
[00517] In certain embodiments, administration of formulations and methods
of the
disclosure may enable the postponement of toxic therapy and may help avoid
unnecessary side
effects and the risks of complications associated with chemotherapy and delay
development of
resistance to chemotherapy. In certain embodiments, toxic therapies and/or
resistance to toxic
therapies is delayed in patients administered formulations and methods of the
disclosure delay
for up to about 6 months, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 years.
5.37. COMBINATION WITH THERAPEUTIC ANTIBODIES
[00518] An anti-ICOS immunotherapy described herein may be administered in
combination with other antibodies, including, but not limited to, anti-CD19
mAb, anti-CD52
mAb, anti-CD22 antibody, and anti-CD20 antibodies, such as RITUXANTm (C2B8;
RITUXIMABTm; IDEC Pharmaceuticals). Other examples of therapeutic antibodies
that can be
used in combination with antibodies of the disclosure or used in formulations
of the disclosure
include, but are not limited to, HERCEPTINTm (Trastuzumab; Genentech),
MYLOTARGTm
(Gemtuzumab ozogamicin; Wyeth Pharmaceuticals), CAMPATH'm (Alemtuzumab;
Berlex),
ZEVALINTM (Ipritumomab tiuxetan; Biogen Idec), BEXXARTm (Tositumomab;
GlaxoSmithKline Corixa), ERBITUXTm (Cetuximab; Imclone), and AVASTINTm
(Bevacizumab; Genentech).
5.38. COMBINATION COMPOUNDS THAT ENHANCE MONOCYTE
OR MACROPHAGE FUNCTION
[00519] In certain embodiments of methods of the disclosure, a compound
that enhances
monocyte or macrophage function (e.g., at least about 25%, 50%, 75%, 85%, 90%,
95% or
more) can be used in conjunction with an anti-1COS immunothcrapy. Such
compounds are
known in the art and include, without limitation, cytokines such as
interleukins (e.g., IL-12), and
interferons (e.g., alpha or gamma interferon).
159
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00520] The compound that enhances monocyte or macrophage function or
enhancement
can be formulated in the same pharmaceutical formulation as the antibody,
immunoconjugate or
antigen-binding fragment. When administered separately, the antibody/fragment
and the
compound can be administered concurrently (within a period of hours of each
other), can be
administered during the same course of therapy, or can be administered
sequentially (i.e., the
patient first receives a course of the antibody/fragment treatment and then a
course of the
compound that enhances macrophage/monocyte function or vice versa). In such
embodiments,
the compound that enhances monocyte or macrophage function is administered to
the human
subject prior to, concurrently with, or following treatment with other
therapeutic regimens and/or
formulations of the disclosure. In one embodiment, the human subject has a
blood leukocyte,
monocyte, neutrophil, lymphocyte, and/or basophil count that is within the
normal range for
humans. Normal ranges for human blood leukocytes (total) is about 3.5- about
10.5 (109/L).
Normal ranges for human blood neutrophils is about 1.7- about 7.0 (109/L),
monocytes is about
0.3- about 0.9 (109/L), lymphocytes is about 0.9- about 2.9 (109/L), basophils
is about 0- about
0.3 (109/L), and eosinophils is about 0.05- about 0.5 (109/L). In other
embodiments, the human
subject has a blood leukocyte count that is less than the normal range for
humans, for example at
least about 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, or 0.8 (109/L)
leukocytes.
5.39. COMBINATION WITH IMMUNOREGULATORY AGENTS
[00521] The anti-ICOS immunotherapy of the present disclosure may also be
in
conjunction with an immunoregulatory agent. The term "immunoregulatory agent"
as used
herein for combination therapy refers to substances that act to suppress,
mask, or enhance the
immune system of the host.
[00522] Examples of immunomodulatory agents include, but are not limited
to,
proteinaceous agents such as cytokines, peptide mimetics, and antibodies
(e.g., human,
humanized, chimeric, monoclonal, polyclonal, Fvs, ScFvs, Fab or F(ab)2
fragments or epitope
binding fragments), nucleic acid molecules (e.g., antisense nucleic acid
molecules, RNAi and
triple helices), small molecules, organic compounds, and inorganic compounds.
In particular,
immunomodulatory agents include, but are not limited to, methothrexate,
leflunomide,
cyclophosphamide, cytoxan, Immuran, cyclosporine A, minocycline, azathioprine,
antibiotics
(e.g., FK506 (tacrolimus)), methylprednisolone (MP), corticosteroids,
steriods, mycophenolate
mofetil, rapamycin (sirolimus), mizoribine, deoxyspergualin, brequinar,
malononitriloamindes
(e.g., leflunamide), T cell receptor modulators, and cytokine receptor
modulators. Examples of
immunosupressant, include, but are not limited to, mycophenolate mofetil
(CELLCEPTTm), D-
160
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
penicillamine (CUPRIMINETm, DEPENTm), methotrexate (RHEUMATREXTm, TREXALLTm),
and hydroxychloroquine sulfate (PLAQUENILim).
[00523] Immunomodulatory agents would also include substances that suppress
cytokine
production, downregulate or suppress self-antigen expression, or mask the MHC
antigens.
Examples of such agents include 2-amino-6-aryl-5-substituted pyrimidines (see,
U.S. Pat. No.
4,665,077), azathioprine (or cyclophosphamide, if there is an adverse reaction
to azathioprine);
bromocryptine; glutaraldehyde (which masks the MHC antigens, as described in
U.S. Pat. No.
4,120,649); anti-idiotypic antibodies for MHC antigens and MHC fragments;
cyclosporin A;
steroids such as glucocorticosteroids, e.g., prednisone, methylprednisolone,
and dexamethasone;
cytokine or cytokine receptor antagonists including anti-interferon-gamma, -
beta, or -alpha
antibodies; anti-tumor necrosis factor-alpha antibodies; anti-tumor necrosis
factor-beta
antibodies; anti-interleukin-2 antibodies and anti-IL-2 receptor antibodies;
anti-L3T4 antibodies;
heterologous anti-lymphocyte globulin; pan-T antibodies, preferably anti-CD3
or anti-
CD4/CD4a antibodies; soluble peptide containing a LFA-3 binding domain (WO
90/08187
published Jul. 26, 1990); streptokinase; TGF-.beta.; streptodomase; RNA or DNA
from the host;
FK506; RS-61443; deoxyspergualin; rapamycin; T-cell receptor (U.S. Pat. No.
5,114,721); 1-
cell receptor fragments (Offner et al., Science 251:430-432 (1991); WO
90/11294; and WO
91/01133); and T-Cell receptor antibodies (EP 340,109) such as T10B9.
[00524] Examples of cytokines include, but are not limited to lymphokines,
monokines,
and traditional polypeptide hormones. Included among the cytokines are growth
hormone such
as human growth hormone, N-methionyl human growth hormone, and bovine growth
hormone;
parathyroid hormone; thyroxine; insulin; proinsulin; relaxin; prorelaxin;
glycoprotein hormones
such as follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH),
and luteinizing
hormone (LH); hepatic growth factor; fibroblast growth factor; prolactin;
placental lactogen;
tumor necrosis factor-alpha; mullerian-inhibiting substance; mouse
gonadotropin-associated
peptide; inhibin; activin; vascular endothelial growth factor; integrin;
thrombopoiotin (TP0);
nerve growth factors such as NGF-alpha; platelet-growth factor; transforming
growth factors
(TGFs) such as TGF-alpha and TGF-alpha; insulin-like growth factor-I and -II;
erythropoietin
(EPO); osteoinductive factors; interferons; colony stimulating factors (CSFs)
such as
macrophage-CSF (M-CSF); granulocyte-macrophage-CgP (GM-CSP); and granulocyte-
CSF (G-
CSF); interleukins (ILs) such as IL-1, IL-la, 1L-2, 1L-3, IL-4, 1L-5, IL-6, IL-
7, 1L-8, IL-9, IL-11,
IL-12, IL-15; a tumor necrosis factor such as TNF-alpha or TNF-beta; and other
polypeptide
factors including LIF and kit ligand (KL). As used herein, the term cytokine
includes proteins
from natural sources or from recombinant cell culture and biologically active
equivalents of the
native sequence cytokines. In certain embodiments, the methods further include
administering to
161
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
the subject one or more immunomodulatory agents, preferably a cytokine.
Preferred cytokines
are selected from the group consisting of interleukin-1 (IL-1), IL-2, IL-3, IL-
12, IL-15, IL-18, G-
CSF, GM-CSF, thrombopoietin, and gamma interferon.
[00525] In certain embodiments, the immunomodulatory agent is a cytokine
receptor
modulator. Examples of cytokine receptor modulators include, but are not
limited to, soluble
cytokine receptors (e.g., the extracellular domain of a TNF-alpha receptor or
a fragment thereof,
the extracellular domain of an IL- lbeta receptor or a fragment thereof, and
the extracellular
domain of an IL-6 receptor or a fragment thereof), cytokines or fragments
thereof (e.g.,
interleukin (1L)-2, 1L-3, 1L-4, IL-5, 1L-6, IL-7, IL-8, 1L-9, 1L-10, 1L-11, IL-
12, 1L-15, TNF-
alpha, TNF-beta, interferon (IFN)-alpha, IFN-beta, IFN-gamma, and GM-CSF),
anti-cytokine
receptor antibodies (e.g., anti-IL-2 receptor antibodies, anti-IL-4 receptor
antibodies, anti-IL-6
receptor antibodies, anti-IL-10 receptor antibodies, and anti-IL-12 receptor
antibodies), anti-
cytokine antibodies (e.g., anti-IFN receptor antibodies, anti-TNF-alpha
antibodies, anti-IL- lbeta
antibodies, anti-IL-6 antibodies, anti-IL-9, anti-IL-17 antibodies,
antibodies, and anti-IL-12
antibodies). In a specific embodiment, a cytokine receptor modulator is IL-4,
IL-10, or a
fragment thereof. In another embodiment, a cytokine receptor modulator is an
anti-IL-lbeta
antibody, anti-IL-6 antibody, anti-IL-12 receptor antibody, anti-TNF-alpha
antibody. In another
embodiment, a cytokine receptor modulator is the extracellular domain of a TNF-
alpha receptor
or a fragment thereof. In certain embodiments, a cytokine receptor modulator
is not a TNF-alpha
antagonist.
[00526] In certain embodiments, the immunomodulatory agent is a T cell
receptor
modulator. Examples of T cell receptor modulators include, but are not limited
to, anti-T cell
receptor antibodies (e.g., anti-CD4 antibodies (e.g., cM-T412 (Boeringer),
IDEC-CE9.1 (IDEC
and SKB), mAB 4162W94, Orthoclone and OKTcdr4a (Janssen-Cilag)), anti-CD3
antibodies,
anti-CD5 antibodies (e.g., an anti-CD5 ricin-linked immunoconjugate), anti-CD7
antibodies
(e.g., CHH-380 (Novartis)), anti-CD8 antibodies, anti-CD40 ligand monoclonal
antibodies, anti-
CD52 antibodies (e.g., CAMPATH 1H (Ilex)), anti-CD2 monoclonal antibodies) and
CTLA4-
immunoglobulin.
[00527] In certain embodiments, the immunomodulatory agent is a TNF-alpha
antagonist.
Examples of TNF-alpha antagonists include, but are not limited to, antibodies
(e.g., infliximab
(REMICADETm; Centocor), D2E7 (Abbott Laboratories/Knoll Pharmaceuticals Co.,
Mt. Olive,
N.J.), CDP571 which is also known as HUMIRATm and CDP-870 (both of
Cellteclv'Pharmacia,
Slough, U.K.), and TN3-19.12 (Williams et al., 1994, Proc. Natl. Acad. Sci.
USA 91: 2762-
2766; Thorbecke et al., 1992, Proc. Natl. Acad. Sci. USA 89:7375-7379))
soluble TNF-alpha
receptors (e.g., sTNF-R1 (Amgen), etanercept (ENBRELTM; Immunex) and its rat
homolog
162
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
RENBRELTM, soluble inhibitors of INF-alpha derived from INFrI, TNFrII (Kohno
et al., 1990,
Proc. Natl. Acad. Sci. USA, 87:8331-8335), and TNF-alpha Inh (Seckinger et al,
1990, Proc.
Natl. Acad. Sci. USA, 87:5188-5192)), IL-10, TNFR-IgG (Ashkenazi et al., 1991,
Proc. Natl.
Acad. Sci. USA, 88:10535-10539), the murine product TBP-1 (Serono/Yeda), the
vaccine
CytoTAb (Protherics), antisense molecule 104838 (ISIS), the peptide RDP-58
(SangStat),
thalidomide (Celgene), CDC-801 (Celgene), DPC-333 (Dupont), VX-745 (Vertex),
AGIX-4207
(AtheroGenics), ITF-2357 (Italfarmaco), NPI-13021-31 (Nereus), SCIO-469
(Scios), TACE
targeter (Immunix/AHP), CLX-120500 (Calyx), Thiazolopyrim (Dynavax), auranofin
(Ridaura)
(SmithKline Beecham Pharmaceuticals), quinacrine (mepacrine dichlorohydrate),
tenidap
(Enablex), Melanin (Large Scale Biological), and anti-p38 MAPK agents by
Uriach.
[00528] An anti-ICOS immunotherapy may also be in conjunction with an
immunoregulatory agent. In this approach, a chimeric, human or humanized anti-
ICOS antibody
can be used. The term "immunoregulatory agent" as used herein for combination
therapy refers
to substances that act to suppress, mask, or enhance the immune system of the
host. This would
include substances that suppress cytokine production, downregulate or suppress
self-antigen
expression, or mask the MHC antigens. Examples of such agents include
2-amino-6-aryl-5-substituted pyrimidines (see, U.S. Pat. No. 4,665,077),
azathioprine (or
cyclophosphamidc, if there is an adverse reaction to azathioprine);
bromocryptine;
glutaraldehyde (which masks the MHC antigens, as described in U.S. Pat. No.
4,120,649);
anti-idiotypic antibodies for MHC antigens and MHC fragments; cyclosporin A;
steroids such as
glucocorticosteroids, e.g., prednisone, methylprednisolone, and dexamethasone;
cytokine or
cytokine receptor antagonists including anti-interferon-y, -13, or -a
antibodies; anti-tumor necrosis
factor-a antibodies; anti-tumor necrosis factor-13 antibodies; anti-
interleukin-2 antibodies and
anti-IL-2 receptor antibodies; anti-L3T4 antibodies; heterologous anti-
lymphocyte globulin;
pan-T antibodies, for example anti-CD3 or anti-CD4/CD4a antibodies; soluble
peptide
containing a LFA-3 binding domain (WO 90/08187 published Jul. 26, 1990);
streptokinase;
TGF-13; streptodornase; RNA or DNA from the host; FK506; RS-61443;
deoxyspergualin;
rapamycin; T-cell receptor (U.S. Pat. No. 5,114,721); T-cell receptor
fragments (Offner et al.,
Science 251:430-432 (1991); WO 90/11294; and WO 91/01133); and T-cell receptor
antibodies
(EP 340,109) such as T10B9. Examples of cytokines include, but are not limited
to
lymphokines, monokincs, and traditional polypeptidc hormones. Included among
the cytokines
are growth hormone such as human growth hormone, N-methionyl human growth
hormone, and
bovine growth hormone; parathyroid hormone; thyroxine; insulin; proinsulin;
relaxin; prorelaxin;
glycoprotein hormones such as follicle stimulating hormone (FSH), thyroid
stimulating hormone
(TSH), and luteinizing hormone (LH); hepatic growth factor; fibroblast growth
factor; prolactin;
163
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
placental lactogen; tumor necrosis factor -a; mullerian-inhibiting substance;
mouse
gonadotropin-associated peptide; inhibin; activin; vascular endothelial growth
factor; integrin;
thrombopoiotin (TP0); nerve growth factors such as NGF-a; platelet-growth
factor;
transforming growth factors (TGFs) such as TGF-a and TGF- a; insulin-like
growth factor-I and
-11; erythropoietin (EPO); osteoinductive factors; interferons; colony
stimulating factors (CSFs)
such as macrophage-CSF (M-CSF); granulocyte-macrophage-CgP (GM-CSP); and
granulocyte-CSF (G-CSF); interleukins (ILs) such as IL-1, IL-la, IL-2, 1L-3,
IL-4, IL-5, IL-6,
IL-7, IL-8, IL-9, IL-1 I, IL-12, IL-15; a tumor necrosis factor such as TNF-a
or TNF-I3; and other
polypeptide factors including LIF and kit ligand (KL). As used herein, the
term cytokine
includes proteins from natural sources or from recombinant cell culture and
biologically active
equivalents of the native sequence cytokines. In certain embodiments, the
methods further
include administering to the subject one or more immunomodulatory agents, for
example a
cytokine. Suitable cytokines may be selected from the group consisting of
interleukin-1 (IL-1),
IL-2, IL-3, IL-12, IL-15, IL-18, G-CSF, GM-CSF, thrombopoietin, and y
interferon.
[00529] These immunoregulatory agents are administered at the same time or
at separate
times from anti-ICOS antibodies. The preferred immunoregulatory agent will
depend on many
factors, including the type of disorder being treated, as well as the
patient's history, but the agent
frequently may be selected from cyclosporin A, a glucocorticosteroid (for
example prednisone or
methylprednisolone), azathioprine, bromocryptine, heterologous anti-lymphocyte
globulin, or a
mixture thereof.
5.40. COMBINATION WITH OTHER THERAPEUTIC AGENTS
[00530] Agents that act on the tumor neovasculature can also be used in
conjunction with
anti-ICOS immunotherapy and include tubulin-binding agents such as
combrestatin A4 (Griggs
etal., Lancet Oncol. 2:82. (2001)) and angiostatin and endostatin (reviewed in
Rosen,
Oncologist 5.20 (2000), incorporated by reference herein). Immunomodulators
suitable for use
in combination with anti-ICOS antibodies include, but are not limited to, of a-
interferon,
7-interferon, and tumor necrosis factor alpha (TNFa). In certain embodiments,
the therapeutic
agents used in combination therapies using formulations and methods of the
disclosure are
peptides.
[00531] In certain embodiments, an anti-ICOS immunotherapy is in
conjunction with one
or more calicheamicin molecules. The calicheamicin family of antibiotics are
capable of
producing double-stranded DNA breaks at sub-picomolar concentrations.
Structural analogues
of calicheamicin which may be used include, but are not limited to, 711, 7 21
, 731, N-acetyl- 71I,
164
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
PSAG and 011 Hinman et al., Cancer Research 53:3336-3342 (1993) and Lode et
al., Cancer
Research 58: 2925-2928 (1998)).
[00532] In certain embodiments, a treatment regimen includes compounds that
mitigate
the cytotoxic effects of an anti-ICOS antibody formulation. Such compounds
include analgesics
(e.g., acetaminophen), bisphosphonates, antihistamines (e.g., chlorphcniramine
malcate), and
steroids (e.g., dexamethasone, retinoids, deltoids, betamethasone, cortisol,
cortisone, prednisone,
dehydrotestosterone, glucocorticoids, mineralocorticoids, estrogen,
testosterone, progestins).
[00533] In certain embodiments, the therapeutic agent used in combination
with an
anti-ICOS immunotherapy is a small molecule (i.e., inorganic or organic
compounds having a
molecular weight of less than about 2500 daltons). For example, libraries of
small molecules
may be commercially obtained from Specs and BioSpecs B.V. (Rijswijk, The
Netherlands),
Chembridge Corporation (San Diego, CA), Comgenex USA Inc. (Princeton, NJ), and
Maybridge
Chemicals Ltd. (Cornwall PL34 OHW, United Kingdom).
[00534] In certain embodiments an anti-ICOS immunotherapy can be
administered in
combination with an anti-bacterial agent. Non-limiting examples of anti-
bacterial agents include
proteins, polypeptides, peptides, fusion proteins, antibodies, nucleic acid
molecules, organic
molecules, inorganic molecules, and small molecules that inhibit and/or reduce
a bacterial
infection, inhibit and/or reduce the replication of bacteria, or inhibit
and/or reduce the spread of
bacteria to other cells or subjects. Specific examples of anti-bacterial
agents include, but are not
limited to, antibiotics such as penicillin, cephalosporin, imipenem,
axtreonam, vancomycin,
cycloserine, bacitracin, cblorampbenicol, erythromycin, clindamycin,
tetracycline, streptomycin,
tobramycin, gentamicin, amikacin, kanamycin, neomycin, spectinomycin,
trimethoprim,
norfloxacin, rifampin, polymyxin, amphotericin B, nystatin, ketocanazole,
isoniazid,
metronidazole, and pentamidine.
[00535] In certain embodiments an anti-ICOS immunotherapy can be
administered in
combination with an anti-fungal agent. Specific examples of anti-fungal agents
include, but are
not limited to, azole drugs (e.g., miconazole, ketoconazole (NIZORAC),
caspofungin acetate
(CANCIDAS ), imidazole, triazoles (e.g., fluconazole (DIFLUCAN )), and
itraconazole
(SPORANOX )), polyene (e.g., nystatin, amphotericin B (FUNGIZONE ),
amphotericin B lipid
complex ("ABLC") (ABELCET ), amphotericin B colloidal dispersion ("ABCD")
(AMPHOTEC ), liposomal amphotcricin B (AMBISONER)), potassium iodide (K1),
pyrimidine
(e.g., flucytosine (ANCOBON ), and voriconazole (VFEND )). Administration of
anti bacterial
and anti-fungal agents can mitigate the effects or escalation of infectious
disease that may occur
in methods of the disclosure where a patient's ICOS expressing T cells are
significantly depleted.
165
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00536] In certain embodiments of the disclosure, an anti-ICOS
immunotherapy can be
administered in combination with one or more of the agents described above to
mitigate the toxic
side effects that may accompany administration of formulations of the
disclosure. In other
embodiments, an anti-ICOS immunotherapy can be administered in combination
with one or
more agents that are well known in the art for use in mitigating the side
effects of antibody
administration, chemotherapy, toxins, or drugs.
[00537] In embodiments of the disclosure where an anti-ICOS immunotherapy
is
administered in combination with another antibody or antibodies and/or agent,
the additional
antibody or antibodies and/or agents can be administered in any sequence
relative to the
administration of the antibody of this disclosure. For example, the additional
antibody or
antibodies can be administered before, concurrently with, and/or subsequent to
administration of
an anti-ICOS antibody or immunoconjugate to the human subject. The additional
antibody or
antibodies can be present in the same pharmaceutical formulation as an
antibody of the
disclosure, and/or present in a different pharmaceutical formulation. The dose
and mode of
administration of an antibody of this disclosure and the dose of the
additional antibody or
antibodies can be the same or different, in accordance with any of the
teachings of dosage
amounts and modes of administration as provided in this application and as are
well known in
the art.
5.41. USE OF ANTI-ICOS ANTIBODIES IN DIAGNOSING T CELL MALIGNANCIES
[00538] The present disclosure also encompasses anti-ICOS antibodies, and
formulations
thereof, that immunospecifically bind to the human ICOS antigen, which anti-
ICOS antibodies
are conjugated to a diagnostic or detectable agent. In certain embodiments,
the antibodies are
anti-ICOS antibodies with enhanced effector function. Such anti-ICOS
antibodies can be useful
for monitoring or prognosing the development or progression of a T cell
malignancy as part of a
clinical testing procedure, such as determining the efficacy of a particular
therapy. Such
diagnosis and detection can be accomplished by coupling an anti-ICOS antibody
that
immunospecifically binds to the human ICOS antigen to a detectable substance
including, but not
limited to, various enzymes, such as but not limited to, horseradish
peroxidase, alkaline
phosphatase, beta-galactosidase, or acetylcholinesterase; prosthetic groups,
such as but not
limited to, streptavidinlbiotin and avidin/biotin; fluorescent materials, such
as but not limited to,
umbelliferone, fluorescein, fluorescein isothiocynate, rhodamine,
dichlorotriazinylamine
fluorescein, dansyl chloride or phycoerythrin; luminescent materials, such as
but not limited to,
luminol; bioluminescent materials, such as but not limited to, luciferase,
luciferin, and aequorin;
radioactive materials, such as but not limited to iodine (1311, 1251, 1231,
121
carbon (14C), sulfur
166
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
(35S), tritium (3H), indium (115In, 1131n,
) and technetium (99Tc), thallium (201To,
gallium (68Ga, 67Ga), palladium (1 3Pd), molybdenum (99Mo), xenon (133Xe),
fluorine ("F),
153Sm, 177Lu, 159Gd, 149pm, 14.0La, myb, 16611o, 90y, 4-7sc, 186Re, 188Re, 142-
r,
P 105Rh,97Ru, 686e,
57 co, 65zn, 83sr, 32p, 153Gd,
Y D 51Cr, 541'v1n, 75Se, 113Sn, and 117Tin; positron emitting metals
using various positron emission tomographies, noradioactive paramagnetic metal
ions, and
molecules that are radiolabelled or conjugated to specific radioisotopes. Any
detectable label
that can be readily measured can be conjugated to an anti-ICOS antibody and
used in diagnosing
T cell malignancies. The detectable substance may be coupled or conjugated
either directly to an
antibody or indirectly, through an intermediate (such as, for example, a
linker known in the art)
using techniques known in the art. See, e.g., U.S. Patent No. 4,741,900 for
metal ions which can
be conjugated to antibodies for use as a diagnostics according to the present
disclosure. In
certain embodiments, the disclosure provides for diagnostic kits comprising an
anti-ICOS
antibody conjugated to a diagnostic or detectable agent.
5.42. USE OF ANTI-ICOS ANTIBODIES IN MONITORINIG IMMUNE RECONSTITUION
[00539] The present disclosure also encompasses anti-ICOS antibodies, and
formulations
thereof, that immunospecifically bind to the human ICOS antigen, which anti-
ICOS antibodies
are conjugated to a diagnostic or detectable agent. Such anti-ICOS antibodies
can be useful for
monitoring immune system reconstitution following immunosuppressive therapy or
bone
marrow transplantation. Such monitoring can be accomplished by coupling an
anti-ICOS
antibody that immunospecifically binds to the human ICOS antigen to a
detectable substance
including, but not limited to, various enzymes, such as, but not limited to,
horseradish
peroxidase, alkaline phosphatase, beta-galactosidase, or acetylcholinesterase;
prosthetic groups,
such as, but not limited to, streptavidinlbiotin and avidin/biotin;
fluorescent materials, such as,
but not limited to, umbelliferone, fluorescein, fluorescein isothiocynate,
rhodamine,
dichlorotriazinylamine fluorescein, dansyl chloride or phycoerythrin;
luminescent materials,
such as, but not limited to, luminol; bioluminescent materials, such as, but
not limited to,
luciferase, luciferin, and aequorin; radioactive materials, such as, but not
limited to, iodine (1311,
1251, 123=,
1211,), carbon (14C), sulfur (35S), tritium (3H), indium ('''In, tzIn, 11
m and
technetium (99Tc), thallium (201Ti), gallium (68Ga, 67Ga), palladium (1 3Pd),
molybdenum (99Mo),
xenon (133Xe), fluorine (8F), 153Sm, 177Lu, 159Gd, 149pm, 140La, 175yb, 166H0,
90y, 47sc, 186Re,
188,, 142,, 105Rh 97Ru 68Ge 57Co 65Zn 85Sr, 32P 153Gd 169Yb 51Cr, 54Mr1 "Se,
113Sn and
rr, , , , , ,
"Tin; positron-emitting metals using various positron-emission tomographies,
noradioactive
paramagnetic metal ions, and molecules that are radiolabelled or conjugated to
specific
radioisotopes. Any detectable label that can be readily measured can be
conjugated to an
167
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
anti-ICOS antibody and used in diagnosing an autoimmune disease or disorder.
The detectable
substance may be coupled or conjugated either directly to an antibody or
indirectly, through an
intermediate (such as, for example, a linker known in the art) using
techniques known in the art.
See, e.g., U.S. Patent No. 4,741,900 for metal ions which can be conjugated to
antibodies for use
as a diagnostics according to the present disclosure. In certain embodiments,
the disclosure
provides for diagnostic kits comprising an anti-ICOS antibody conjugated to a
diagnostic or
detectable agent.
5.43. USE OF ANTI-ICOS ANTIBODIES E\T DIAGNOSING AUTOIMMUNE DISEASES
OR DISORDERS
[00540] The present disclosure also encompasses anti-ICOS antibodies, and
formulations
thereof, that immunospecifically bind to the human ICOS antigen, which anti-
ICOS antibodies
are conjugated to a diagnostic or detectable agent. In certain embodiments,
the antibodies are
anti-ICOS antibodies with enhanced effector function. Such anti-ICOS
antibodies can be useful
for monitoring or prognosing the development or progression of an autoimmune
disease or
disorder as part of a clinical testing procedure, such as determining the
efficacy of a particular
therapy. Such diagnosis and detection can be accomplished by coupling an anti-
ICOS antibody
that immunospecifically binds to the human ICOS antigen to a detectable
substance including,
but not limited to, various enzymes, such as but not limited to, horseradish
peroxidase, alkaline
phosphatase, beta-galactosidase, or acetylcholinesterase; prosthetic groups,
such as but not
limited to, streptavidinlbiotin and avidin/biotin; fluorescent materials, such
as but not limited to,
umbelliferone, fluorescein, fluorescein isothiocynate, rhodamine,
dichlorotriazinylamine
fluorescein, dansyl chloride or phycoerythrin; luminescent materials, such as
but not limited to,
luminol; bioluminescent materials, such as but not limited to, luciferase,
luciferin, and aequorin;
radioactive materials, such as but not limited to iodine (1311, 1251, 1231,
1214 carbon (14C), sulfur
(35S), tritium (3H), indium (115In, ) and technetium (99Tc), thallium
(2otTi),
gallium (68Ga, 67Ga), palladium (1 3Pd), molybdenum (99Mo), xenon (133Xe),
fluorine ("F),
153sm, 177Lm 159Gd, 149pm, 140La, 175-Th, 166H0, 90y, 47se, 186Re, 188Re,
142pr, 105- ,
Rh 97Rtl, 686e,
57CO, 65Zn, 85Sr, 32P, 1513Gd, I69Yb, 5ICr, 54Mn, 755e, "35n, and II7Tin;
positron emitting metals
using various positron emission tomographies, noradioactive paramagnetic metal
ions, and
molecules that are radiolabelled or conjugated to specific radioisotopes. Any
detectable label
that can be readily measured can be conjugated to an anti-ICOS antibody and
used in diagnosing
an autoimmune disease or disorder. The detectable substance may be coupled or
conjugated
either directly to an antibody or indirectly, through an intermediate (such
as, for example, a
linker known in the art) using techniques known in the art. See, e.g.,U U.S.
Patent No. 4,741,900
168
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
for metal ions which can be conjugated to antibodies for use as a diagnostics
according to the
present disclosure. In certain embodiments, the disclosure provides for
diagnostic kits
comprising an anti-ICOS antibody conjugated to a diagnostic or detectable
agent.
5.44. Kits
[00541] The disclosure provides a pharmaceutical pack or kit comprising one
or more
containers filled with a liquid formulation of the disclosure. In one
embodiment, a container
filled with a liquid formulation of the disclosure is a pre-filled syringe. In
a specific
embodiment, the liquid formulations of the disclosure comprise antibodies
(including antibody
fragments thereof) recombinantly fused or chemically conjugated to another
moiety, including
but not limited to, a heterologous protein, a heterologous polypeptide, a
heterologous peptide, a
large molecule, a small molecule, a marker sequence, a diagnostic or
detectable agent, a
therapeutic moiety, a drug moiety, a radioactive metal ion, a second antibody,
and a solid
support. The disclosure also provides a pharmaceutical pack or kit comprising
in one or more
first containers a liquid formulation of the disclosure and in one or more
second containers one
or more other prophylactic or therapeutic agents useful for the prevention,
management or
treatment of a disease or disorder, for example, a disease or disorder
associated with or
characterized by aberrant expression and/or activity of ICOS, a disease or
disorder associated
with or characterized by aberrant expression and/or activity of ICOS receptor,
an autoimmune
disease or disorder, an inflammatory disease or disorder, a T cell
proliferative disease or
disorder, a T cell malignancy, transplant rejection, graft versus host
disease, or one or more
symptoms thereof. In a specific embodiment, the liquid formulations of the
disclosure are
formulated in single dose vials as a sterile liquid containing 10 mM histidine
buffer at p1-I 6.0, 80
mM NaCl, 4% trehalose and 0.02% Polysorbate 80. The formulations of the
disclosure may be
supplied in 3 cc USP Type I borosilicate amber vials (West Pharmaceutical
Serices - Part No.
6800-0675) with a target volume of 1.2 mL. Optionally associated with such
container(s) can be
a notice in the form prescribed by a governmental agency regulating the
manufacture, use or sale
of pharmaceuticals or biological products, which notice reflects approval by
the agency of
manufacture, use or sale for human administration. In another embodiment, a
formulation of the
disclosure may be supplied in a pre-filled syringe.
[00542] In one embodiment, a container filled with a liquid formulation of
the disclosure
is a pre-filled syringe. Any pre-filled syringe known to one of skill in the
art may be used in
combination with a liquid formulation of the disclosure. Pre-filled syringes
that may be used are
described in, for example, but not limited to, PCT Publications W005032627,
W008094984,
W09945985, W003077976, US Patents US6792743, US5607400, US5893842, US7081107,
169
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
US7041087, US5989227, US6807797, US6142976, US5899889, US Patent Publications
US20070161961A1, US20050075611A1, US20070092487A1, US20040267194A1,
11S20060129108A1. Pre-filled syringes may be made of various materials. In one
embodiment
a pre-filled syringe is a glass syringe. In another embodiment a pre-filled
syringe is a plastic
syringe. One of skill in the art understands that the nature and/or quality of
the materials used
for manufacturing the syringe may influence the stability of a protein
formulation stored in the
syringe. For example, it is understood that silicon based lubricants deposited
on the inside
surface of the syringe chamber may affect particle formation in the protein
formulation. In one
embodiment, a pre-filled syringe comprises a silicone based lubricant. In one
embodiment, a
pre-filled syringe comprises baked on silicone. In another embodiment, a pre-
filled syringe is
free from silicone based lubricants. One of skill in the art also understands
that small amounts of
contaminating elements leaching into the formulation from the syringe barrel,
syringe tip cap,
plunger or stopper may also influence stability of the formulation. For
example, it is understood
that tungsten introduced during the manufacturing process may adversely affect
formulation
stability. In one embodiment, a pre-filled syringe may comprise tungsten at a
level above 500
ppb. In another embodiment, a pre-filled syringe is a low tungsten syringe. In
another
embodiment, a pre-filled syringe may comprise tungsten at a level between
about 500 ppb and
about 10 ppb, between about 400 ppb and about 10 ppb, between about 300 ppb
and about 10
ppb, between about 200 ppb and about 10 ppb, between about 100 ppb and about
10 ppb,
between about 50 ppb and about 10 ppb, between about 25 ppb and about 10 ppb.
[00543] The present disclosure provides kits that can be used in the above
methods. In
one embodiment, a kit comprises a liquid formulation of the disclosure, in one
or more
containers. In another embodiment, a kit comprises a liquid formulation of the
disclosure, in one
or more containers, and one or more other prophylactic or therapeutic agents
useful for the
prevention, management or treatment of a disease or disorder associated with
or characterized by
aberrant expression and/or activity of ICOS, a disease or disorder associated
with or
characterized by aberrant expression and/or activity of ICOS receptor, an
autoimmune disease or
disorder, an inflammatory disease or disorder, a T cell proliferative disease
or disorder, a T cell
malignancy, transplant rejection, graft versus host disease, or one or more
symptoms thereof. In
a specific embodiment, the antibody (including antibody fragments thereof)
included in said
liquid formulations is an antigen-binding fragment. The kit may further
comprise instructions
for preventing, treating and/or managing a disorder (e.g., using the liquid
formulations of the
disclosure alone or in combination with another prophylactic or therapeutic
agent), as well as
side effects and dosage information for method of administration.
170
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
5.45.Articles of Manufacture
[00544] The present disclosure also encompasses a finished packaged and
labeled
pharmaceutical product. This article of manufacture includes the appropriate
unit dosage form in
an appropriate vessel or container such as a glass vial, pre-filled syringe or
other container that is
hermetically sealed. The unit dosage form is provided as a sterile particulate
free solution
comprising an anti-ICOS antibody that is suitable for parenteral
administration.
[00545] In one embodiment, the unit dosage form is suitable for
intravenous,
intramuscular, intranasal, oral, topical or subcutaneous delivery. Thus, the
disclosure
encompasses sterile solutions suitable for each delivery route.
[00546] As with any pharmaceutical product, the packaging material and
container are
designed to protect the stability of the product during storage and shipment.
Further, the
products of the disclosure include instructions for use or other informational
material that advise
the physician, technician or patient on how to appropriately prevent or treat
the disease or
disorder in question. In other words, the article of manufacture includes
instruction means
indicating or suggesting a dosing regimen including, but not limited to,
actual doses, monitoring
procedures, and other monitoring information.
[00547] Specifically, the disclosure provides an article of manufacture
comprising
packaging material, such as a box, bottle, tube, vial, container, pre-filled
syringe, sprayer,
insufflator, intravenous (i.v.) bag, envelope and the like; and at least one
unit dosage form of a
pharmaceutical agent contained within said packaging material, wherein said
pharmaceutical
agent comprises a liquid formulation containing an antibody. The packaging
material includes
instruction means which indicate that said antibody can be used to prevent,
treat and/or manage
one or more symptoms associated with a disease or disorder associated with or
characterized by
aberrant expression and/or activity of ICOS, a disease or disorder associated
with or
characterized by aberrant expression and/or activity of ICOS receptor, an
autoimmune disease or
disorder, an inflammatory disease or disorder, a T cell proliferative disease
or disorder, a T cell
malignancy, transplant rejection, graft versus host disease, or one or more
symptoms thereof by
administering specific doses and using specific dosing regimens as described
herein.
[00548] The disclosure also provides an article of manufacture comprising
packaging
material, such as a box, bottle, tube, vial, container, pre-filled syringe,
sprayer, insufflator,
intravenous (i.v.) bag, envelope and the like; and at least one unit dosage
form of each
pharmaceutical agent contained within said packaging material, wherein one
pharmaceutical
agent comprises a liquid formulation containing an antibody that specifically
binds to ICOS and
the other pharmaceutical agent comprises a prophylactic or therapeutic agent
other than an
171
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
antibody that specifically binds to ICOS, and wherein said packaging material
includes
instruction means which indicate that said agents can be used to prevent,
treat and/or manage one
or more symptoms associated with a disease or disorder associated with or
characterized by
aberrant expression and/or activity of ICOS, a disease or disorder associated
with or
characterized by aberrant expression and/or activity of ICOS receptor, an
autoimmune disease or
disorder, an inflammatory disease or disorder, a T cell proliferative disease
or disorder, a T cell
malignancy, transplant rejection, graft versus host disease, or one or more
symptoms thereof by
administering specific doses and using specific dosing regimens as described
herein.
[00549] The present disclosure provides that the adverse effects that may
be reduced or
avoided by the methods of the disclosure are indicated in informational
material enclosed in an
article of manufacture for use in preventing, treating and/or managing one or
more symptoms
associated with an autoimmune disorder, an inflammatory disorder, a malignancy
or an infection.
Adverse effects that may be reduced or avoided by the methods of the
disclosure include, but are
not limited to, vital sign abnormalities (fever, tachycardia, bardycardia,
hypertension,
hypotension), hematological events (anemia, lymphopenia, leukopenia,
thrombocytopenia),
headache, chills, dizziness, nausea, asthenia, back pain, chest pain (chest
pressure), diarrhea,
myalgia, pain, pruritus, psoriasis, rhinitis, sweating, injection site
reaction, and vasodilatation.
[00550] Further, the information material enclosed in an article of
manufacture described
herein can indicate that foreign proteins may also result in allergic
reactions, including
anaphylaxis, or cytosine release syndrome. The information material should
indicate that
allergic reactions may exhibit only as mild pruritic rashes or they may be
severe such as
erythroderma, Stevens-Johnson syndrome, vasculitis, or anaphylaxis. The
information material
should also indicate that anaphylactic reactions (anaphylaxis) are serious and
occasionally fatal
hypersensitivity reactions. Allergic reactions including anaphylaxis may occur
when any foreign
protein is injected into the body. They may range from mild manifestations
such as urticaria or
rash to lethal systemic reactions. Anaphylactic reactions occur soon after
exposure, usually
within 10 minutes. Patients may experience paresthesia, hypotension, laryngeal
edema, mental
status changes, facial or pharyngeal angioedema, airway obstruction,
bronchospasm, urticaria
and pruritus, serum sickness, arthritis, allergic nephritis,
glomerulonephritis, temporal arthritis,
or eosinophilia.
5.46. Specific embodiments
[00551] 1. A sterile, stable aqueous formulation comprising an antibody
that specifically
binds human ICOS, wherein the antibody comprises an Fc region having complex N-
glycoside-
172
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
linked sugar chains in which fucose is not bound to N-acetylglucosamine in the
reducing end in
the sugar chain.
[00552] 2. The formulation of embodiment 1, wherein said antibody was not
subjected to
lyophilization.
[00553] 3. The formulation of embodiment 1, wherein said antibody is from
an
immunoglobulin type selected from the group consisting of IgA, IgE, IgM, IgD,
IgY and IgG.
[00554] 4. The formulation of embodiment 1, wherein said antibody is of the
IgGl, IgG2,
IgG3, or IgG4 human isotype.
[00555] 5. The formulation of embodiment 1, wherein said antibody is a
murine antibody,
a chimeric antibody, a humanized antibody or a human antibody.
[00556] 6. The formulation of any one of embodiments 1 to 5, wherein said
antibody
comprises a heavy chain variable sequence of SEQ ID NO:7.
[00557] 7. The formulation of any one of embodiments 1 to 5, wherein said
antibody
comprises a light chain variable sequence of SEQ ID NO:2.
[00558] 8. The formulation of any one of embodiments 1 to 5, wherein said
antibody
comprises a heavy chain variable sequence of SEQ ID NO:7 and a light chain
variable sequence
of SEQ ID NO:2.
[00559] 9. The formulation of any one of embodiments 1 to 5, wherein said
antibody
comprises a heavy chain sequence of SEQ ID NO:6 and a light chain sequence of
SEQ ID NO: 1.
[00560] 10. The formulation of any one of embodiments 1 to 9, wherein the
concentration
of said antibody is at least about 1 mg/ml, at least about 2 mg/ml, at least
about 3 mg/ml, at least
about 4 mg/ml, at least about 5 mg/ml, at least about 10 mg/ml, at least about
15 mg/ml, at least
about 20 mg/ml, at least about 25 mg/ml, at least about 30 mg/ml, at least
about 40 mg/ml, at
least about 50 mg/ml, at least about 60 mg/ml, at least about 70 mg/ml, at
least about 80 mg/ml,
at least about 90 mg/ml, at least about 100 mg/ml, at least about 120 mg/ml,
at least about
150 mg/ml, at least about 160 mg/ml, at least about 180 mg/ml, at least about
200 mg/ml, at least
about 250 mg/ml, or at least about 300 mg/ml.
[00561] 11A. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is at least about 1 mg/ml.
[00562] 11B. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is at least about 2 mg/ml.
[00563] 11C. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is at least about 3 mg/ml.
[00564] 11D. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is at least about 4 mg/ml.
173
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00565] 11E. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is at least about 5 mg/ml.
[00566] 11F. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is at least about 10 mg/ml.
[00567] 11G. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is at least about 20 mg/ml.
[00568] 11H. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is at least about 50 mg/ml.
[00569] 111. The formulation of any one of embodiments 1 to 9, wherein the
concentration
of said antibody is at least about 100 mg/ml.
[00570] 12. The formulation of any one of embodiments 1 to 9, wherein the
concentration
of said antibody is at least about 125 mg/ml.
[00571] 13. The formulation of any one of embodiments 1 to 9, wherein the
concentration
of said antibody is least about 150 mg/ml.
[00572] 14. The formulation of any one of embodiments 1 to 9, wherein the
concentration
of said antibody is at least about 175 mg/ml.
[00573] 15. The formulation of any one of embodiments 1 to 9, wherein the
concentration
of said antibody is at least about 200 mg/ml
[00574] 16A. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is between about 1 mg/ml and about 50 mg/ml.
[00575] 16B. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is between about 1 mg/ml and about 20 mg/ml.
[00576] 16C. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is between about 5 mg/ml and about 15 mg/ml.
[00577] 16D. The formulation of any one of embodiments 1 to 9, wherein the
concentration of said antibody is between about 90 mg/ml and about 250 mg/ml.
[00578] 17. The formulation of any one of embodiments 1 to 9, wherein the
concentration
of said antibody is of between about 110 mg/ml and about 250 mg/ml.
[00579] 18. The formulation of any one of embodiments 1 to 17, wherein said
formulation
further comprises at least about one buffering component.
[00580] 19. The formulation of any one of embodiments 1 to 18, wherein said
formulation
further comprises at least about one excipient.
[00581] 20. The formulation of embodiments 18 or 19, wherein said buffering
component
is selected from the group consisting of histidine, citrate, phosphate,
glycine, and acetate.
174
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00582] 21. The formulation of embodiments 18 or 19, wherein said buffering
component
is histidine.
[00583] 22. The formulation of embodiment 21, wherein said histidine is at
a
concentration from about 1 nM to about 200 nM.
[00584] 23. The formulation of embodiment 21, wherein said histidine is at
a
concentration from about 1 nM to about 50 nM.
[00585] 24. The formulation of embodiment 21, wherein said histidine is at
a
concentration from about 5 nM to about 20 nM.
[00586] 25. The formulation of embodiment 21, wherein said histidine is at
a
concentration of about 10 nM, about 15 nM or about 20 nM.
[00587] 26. The formulation of embodiment 19, wherein said excipient is a
saccharide.
[00588] 27. The formulation of embodiment 26, wherein said saccharide is a
disaccharide.
[00589] 28. The formulation of embodiment 27, wherein said disaccharide is
trehalose or
sucrose.
[00590] 29. The formulation of embodiment 27, wherein said disaccharide is
trehalose.
[00591] 30. The formulation of embodiment 29, wherein said trehalose is at
a
concentration from about 1% to about 40%.
[00592] 31. The formulation of embodiment 29, wherein said trehalose is at
a
concentration from about 2% to about 20%.
[00593] 32. The formulation of embodiment 29, wherein said trehalose is at
a
concentration from about 2% to about 10%.
[00594] 33. The formulation of embodiment 29, wherein said trehalose is at
a
concentration of about 2%, about 4% or about 8%.
[00595] 34. The formulation of embodiment 19, wherein said excipient is a
salt.
[00596] 35. The formulation of embodiment 34, wherein said salt is sodium
chloride.
[00597] 36. The formulation of embodiment 35, wherein said sodium chloride
is at a
concentration from about 50 mM to about 200 mM.
[00598] 37. The formulation of embodiment 35, wherein said sodium chloride
is at a
concentration of about 70 mM, about 80 mM, about 90 mM, about 100 mM, about
120 mM, or
about 150 mM.
[00599] 38. The formulation of embodiment 19, wherein said excipient is a
surfactant.
[00600] 39. The formulation of embodiment 38, wherein said surfactant is a
polysorbate.
[00601] 40. The formulation of embodiment 39, wherein said polysorbate is
polysorbate 20 or polysorbate 80.
175
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00602] 41. The formulation of embodiment 39, wherein said polysorbate is
polysorbate 80.
[00603] 42. The formulation of embodiment 41, wherein said polysorbate 80
is at a
concentration from about 0.001% to about 2%.
[00604] 43. The formulation of embodiment 41, wherein said polysorbate 80
is at a
concentration of about 0.01%, about 0.02%, about 0.04% or about 0.08%.
[00605] 44. The formulation of any one of embodiments 1 to 43, wherein said
formulation
has a pH of between about 5.5 and about 6.5.
[00606] 45. The formulation of any one of embodiments 1 to 43, wherein said
formulation
has a pH of about 6Ø
[00607] 46. The formulation of any one of embodiments 1 to 45, wherein said
formulation
is isotonic.
[00608] 47. The formulation of any one of embodiments 1 to 46, wherein said
formulation
is stable upon storage at 40 C for at least about 4 weeks.
[00609] 48. The formulation of any one of embodiments 1 to 46, wherein said
formulation
is stable upon storage at 5 C for at least about 3 months.
[00610] 49. The formulation of any one of embodiments Ito 46, wherein said
formulation
is stable upon storage at 5 C for at least about 12 months.
[00611] 50. The formulation of any one of embodiments 1 to 46, wherein said
antibody
retains at least about 80% of binding ability to a human ICOS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 40 C for
at least about 4
weeks.
[00612] 51. The formulation of any one of embodiments 1 to 46, wherein said
antibody
retains at least about 80% of binding ability to a human ICOS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
[00613] 52. The formulation of any one of embodiments 1 to 46, wherein said
antibody
retains at least about 80% of binding ability to a human ICOS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00614] 53. The formulation of any one of embodiments 1 to 46, wherein said
antibody
retains at least about 90% of binding ability to a human ICOS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 40 C for
at least about 4
weeks.
176
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00615] 54. The formulation of any one of embodiments 1 to 46, wherein said
antibody
retains at least about 90% of binding ability to a human ICOS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
[00616] 55. The formulation of any one of embodiments 1 to 46, wherein said
antibody
retains at least about 90% of binding ability to a human ICOS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00617] 56. The formulation of any one of embodiments 1 to 46, wherein said
antibody
retains at least about 95% of binding ability to a human ICOS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 40 C for
at least about 4
weeks.
[00618] 57. The formulation of any one of embodiments 1 to 46, wherein said
antibody
retains at least about 95% of binding ability to a human ICOS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
[00619] 58. The formulation of any one of embodiments Ito 46, wherein said
antibody
retains at least about 95% of binding ability to a human 1COS polypeptide
compared to a
reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00620] 59. The formulation of any one of embodiments 1 to 46, wherein said
antibody is
susceptible to aggregation, or fragmentation.
[00621] 60. The formulation of any one of embodiments 1 to 46, wherein less
than about
2% of said antibody forms an aggregate upon storage at 40 C for at least about
4 weeks as
determined by as determined by HPSEC.
[00622] 61. The formulation of any one of embodiments 1 to 46, wherein less
than about
2% of said antibody forms an aggregate upon storage at 5 C for at least about
3 months as
determined by HPSEC.
[00623] 62. The formulation of any one of embodiments 1 to 46, wherein less
than about
2% of said antibody forms an aggregate upon storage at 5 C for at least about
12 months as
determined by HPSEC.
[00624] 63. The formulation of any one of embodiments 1 to 46, wherein less
than about
5% of said antibody is fragmented upon storage at 40 C for at least about 4
weeks as determined
by RP-HPLC.
177
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00625] 64. The formulation of any one of embodiments 1 to 46, wherein less
than about
5% of said antibody is fragmented upon storage at 5 C for at least about 3
months as determined
by RP-HPLC.
[00626] 65. The formulation of any one of embodiments Ito 46, wherein less
than about
5% of said antibody is fragmented upon storage at 5 C for at least about 12
months as
determined by RP-HPLC.
[00627] 66. The formulation of any one of embodiments 1 to 65, wherein said
formulation
is an injectable formulation.
[00628] 67. The formulation of embodiment 66, wherein said formulation is
suitable for
intravenous, subcutaneous, or intramuscular administration.
[00629] 68. The formulation of embodiment 67, wherein said formulation is
suitable for
intravenous administration and the antibody or antibody fragment concentration
is from about
20 mg/m1 to about 40 mg/ml.
[00630] 69. The formulation of embodiment 67, wherein said formulation is
suitable for
subcutaneous administration and the antibody or antibody fragment
concentration is from about
70 mg/ml to about 250 mg/ml.
[00631] 70. The formulation of any one of embodiments I to 65, wherein said
formulation
is suitable for aerosol administration.
[00632] 71. A pharmaceutical unit dosage form suitable for parenteral
administration to a
human which comprises an antibody formulation of any one of embodiments 1 to
65 in a suitable
container.
[00633] 72. The pharmaceutical unit dosage form of embodiment 71, wherein
the antibody
formulation is administered intravenously, subcutaneously, or intramuscularly.
[00634] 73. A pharmaceutical unit dosage form suitable for aerosol
administration to a
human which comprises an antibody formulation of any one of embodiments 1 to
65 in a suitable
container.
[00635] 74. The pharmaceutical unit dosage of embodiment 73, wherein the
antibody
formulation is administered intranasally.
[00636] 75. A sealed container containing the formulation of any one of
embodiments 1 to
74.
[00637] 76. A kit comprising the formulation of any one of embodiments 1 to
74.
[00638] 77. A method of treating an autoimmune disease or disorder in a
human,
comprising administering to a human in need thereof a therapeutically-
effective amount of the
formulation of any one of embodiments 1 to 74.
178
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00639] 78. The method of embodiment 77, wherein the autoimmune disease or
disorder
is SLE or scleroderma.
[00640] 79. A method of treating or preventing rejection in a human
transplant patient,
comprising administering to a human in need thereof a therapeutically-
effective amount of the
formulation of any one of embodiments 1 to 74.
[00641] 80. A method of treating a T cell malignancy in a human comprising
administering to a human in need thereof a therapeutically-effective amount of
the formulation
of any one of embodiments 1 to 74.
[00642] 81. A method of treating an inflammatory disease or disorder in a
human,
comprising administering to a human in need thereof a therapeutically-
effective amount of the
formulation of any one of embodiments 1 to 74.
[00643] 82. The method of embodiment 81, wherein the inflammatory disease
or disorder
is myositis.
[00644] 83. The method of embodiment 82, wherein the myositis is inclusion-
body
myositis (IBM), polymyositis (PM) or dermatomyositis (DM).
[00645] 84. A method of depleting ICOS expressing T cells in a human
patient comprising
administering to a human in need thereof a therapeutically-effective amount of
the formulation
of any one of embodiments 1 to 74.
[00646] 85. The method of embodiment 84, wherein the depletion
substantially persists
for at least about 1, at least about 2, at least about 3 or at least about 4
weeks following the
administration of the antibody.
[00647] 86. The method of embodiment 84, wherein at least about 95% of the
T cells are
depleted.
[00648] 87. The method of embodiment 84, wherein the ICOS expressing T cell
is a
memory T cell.
[00649] 88. The method of embodiment 84, wherein the ICOS expressing T cell
is a
circulating T cell.
[00650] 89. A method of disrupting germinal center architecture in a
secondary lymphoid
organ of a primate, comprising administering an effective amount of the
formulation of any one
of embodiments 1 to 74.
[00651] 90. The method of embodiment 89, wherein the primate is a non-human
primate.
[00652] 91. A method of depleting germinal center B cells from a secondary
lymphoid
organ of a primate comprising administering an effective amount of the
formulation of any one
of embodiments I to 74.
[00653] 92. The method of embodiment 91, wherein the primate is a non-human
primate.
179
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00654] 93. The method of embodiment 91, wherein the primate is a human.
[00655] 94. The method of embodiment 91, wherein the depletion
substantially persists
for at least about 1, at least about 2, at least about 3 or at least about 4
weeks following the
administration of the antibody.
[00656] 95. A method of depleting circulating class switched B cells in a
primate
comprising administering an effective amount of the formulation of any one of
embodiments 1 to
74.
[00657] 96. The method of embodiment 95, wherein the primate is a non-human
primate.
[00658] 97. The method of embodiment 95, wherein the primate is a human.
[00659] 98. The method of embodiment 95, wherein the depletion
substantially persists
for at least about 1, at least about 2, at least about 3 or at least about 4
weeks following the
administration of the antibody.
[00660] 99. The method of embodiment 95, wherein at least about 95% of the
circulating
class switched B cells are depleted.
[00661] 100. A sterile, stable aqueous formulation comprising an anti-human
ICOS
antibody, and further comprising histidine, sodium chloride, trehalose or
polysorbate 80, wherein
the antibody comprises a heavy chain sequence of SEQ ID NO:6, a light chain
sequence of SEQ
ID NO:1 and an Fe region having complex N-glycosidc-linked sugar chains in
which fucosc is
not bound to N-acetylglucosamine in the reducing end in the sugar chain.
[00662] 101. A sterile, stable aqueous formulation comprising an anti-human
ICOS
antibody, and further comprising histidine, sodium chloride, trehalose and
polysorbate 80,
wherein the antibody comprises a heavy chain sequence of SEQ ID NO:6, a light
chain sequence
of SEQ ID NO:1 and an Fe region having complex N-glycoside-linked sugar chains
in which
fucose is not bound to N-acetylglucosamine in the reducing end in the sugar
chain.
[00663] 102A. The formulation of embodiment 101, wherein said formulation
comprises
between about 1 mg/m1 and about 20 mg/ml of the anti-human ICOS antibody,
between about
1 mM and about 100 mM histidine, between about 1% and about 40% trehalose and
about
between about 0.001% and about 5% polysorbate 80 and wherein the pH of said
formulation is
between about 5 and about 7.
[00664] 102B. The formulation of embodiment 101, wherein said formulation
comprises
between about 50 mg/ml and about 150 mg/ml of the anti-human ICOS antibody,
between about
1 mM and about 100 mM histidine, between about 1% and about 40% trehalose and
about
between about 0.001% and about 5% polysorbate 80 and wherein the pH of said
formulation is
between about 5 and about 7.
180
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00665] 103A. The formulation of embodiment 101, wherein said formulation
comprises
between about 5 mg/ml and about 15 mg/ml of the anti-human ICOS antibody,
between about
mM and about 25 mM histidine, between about 2% and about 15% trehalose and
between
about 0.005% and about 1% polysorbate 80 and wherein the pH of said
formulation is between
about 5.5 and about 6.5.
[00666] 103B. The formulation of embodiment 101, wherein said formulation
comprises
between about 80 mg/m1 and about 120 mg/ml of the anti-human ICOS antibody,
between about
5 mM and about 25 mM histidine, between about 2% and about 15% trehalose and
between
about 0.005% and about 1% polysorbatc 80 and wherein the pH of said
formulation is between
about 5.5 and about 6.5.
[00667] 104A. The formulation of embodiment 101, wherein said formulation
comprises
about 10 mg/ml of the anti-human ICOS antibody, about 10 mM histidine, about
4% trehalose
and about 0.02% polysorbate 80 and wherein the pH of said formulation is about
6.
[00668] 104B. The formulation of embodiment 101, wherein said formulation
comprises
about 100 mg/m1 of the anti-human ICOS antibody, about 10 mM histidine, about
4% trehalose
and about 0.02% polysorbate 80 and wherein the pH of said formulation is about
6.
[00669] 105. The formulation of any one of embodiments 101 to 104, wherein
said
formulation is isotonic.
[00670] 106. The formulation of any one of embodiments 101 to 104, wherein
said
formulation is stable upon storage at 40 C for at least about 4 weeks.
[00671] 107. The formulation of any one of embodiments 101 to 104, wherein
said
formulation is stable upon storage at 5 C for at least about 3 months.
[00672] 108. The formulation of any one of embodiments 101 to 104, wherein
said
formulation is stable upon storage at 5 C for at least about 12 months.
[00673] 109. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 80% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 40 C
for at least about 4
weeks.
[00674] 110. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 80% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
[00675] 111. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 80% of binding ability to a human ICOS
polypeptide compared to
181
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00676] 112. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 90% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 40 C
for at least about 4
weeks.
[00677] 113. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 90% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
[00678] 114. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 90% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00679] 115. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 95% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 40 C
for at least about 4
weeks.
[00680] 116. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 95% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
[00681] 117. The formulation of any one of embodiments 101 to 104, wherein
said
antibody retains at least about 95% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00682] 118. The formulation of any one of embodiments 101 to 104, wherein
said
antibody is susceptible to aggregation or fragmentation.
[00683] 119. The formulation of any one of embodiments 101 to 104, wherein
less than
about 2% of said antibody forms an aggregate upon storage at 40 C for at least
about 4 weeks as
determined by as determined by HPSEC.
[00684] 120. The formulation of any one of embodiments 101 to 104, wherein
less than
about 2% of said antibody forms an aggregate upon storage at 5 C for at least
about 3 months as
determined by HP SEC.
182
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00685] 121. The formulation of any one of embodiments 101 to 104, wherein
less than
about 2% of said antibody forms an aggregate upon storage at 5 C for at least
about 12 months
as determined by HPSEC.
[00686] 122. The formulation of any one of embodiments 101 to 104, wherein
less than
about 5% of said antibody is fragmented upon storage at 40 C for at least
about 4 weeks as
determined by RP-HPLC.
[00687] 123. The formulation of any one of embodiments 101 to 104, wherein
less than
about 5% of said antibody is fragmented upon storage at 5 C for at least about
3 months as
determined by RP-HPLC.
[00688] 124. The formulation of any one of embodiments 101 to 104, wherein
less than
about 5% of said antibody is fragmented upon storage at 5 C for at least about
12 months as
determined by RP-HPLC.
[00689] 125. The formulation of any one of embodiments 101 to 104, wherein
said
formulation is clear and colorless upon storage at 5 C for at least about 3
months as determined
by visual inspection.
[00690] 126. The formulation of any one of embodiments 101 to 104, wherein
said
formulation is clear and colorless upon storage at 5 C for at least about 12
months as determined
by visual inspection.
[00691] 127. The formulation of any one of embodiments 101 to 126, wherein
said
formulation is an injectable formulation.
[00692] 128. The formulation of embodiment 127, wherein said formulation is
suitable for
intravenous, subcutaneous, or intramuscular administration.
[00693] 129. The formulation of embodiment 128, wherein said formulation is
suitable for
intravenous administration.
[00694] 130. The formulation of embodiment 128, wherein said formulation is
suitable for
subcutaneous administration.
[00695] 131. The formulation of any one of embodiments 101 to 126, wherein
said
formulation is suitable for aerosol administration.
[00696] 132. A process for the preparation of a formulation according to
any one of
embodiments 101 to 126, comprising:
[00697] a) concentrating the anti-human 1COS antibody solution to between
about
mg/m1 and about 50 mg/ml;
[00698] b) diafiltering said concentrated antibody with a solution
comprising histidine.
[00699] 133. The process of embodiment 132 further comprising:
183
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00700] (c) concentrating the antibody diafiltered with a solution
comprising histidine to
between about 50 mg/ml and about 250 mg/m1;
[00701] (d) admixing the concentrated antibody solution with at least about
one solution
comprising at least about one excipient.
[00702] 134. A method for stabilizing an anti-human 1COS antibody
comprising
combining said antibody with histidine-HC1, sodium chloride, trehalose and
polysorbate 80 at a
pH of 6, wherein the antibody comprises a heavy chain sequence of SEQ ID NO:6,
a light chain
sequence of SEQ ID NO:1 and an Fc region having complex N-glycoside-linked
sugar chains in
which fucose is not bound to N-acetylglucosamine in the reducing end in the
sugar chain.
[00703] 135. The method of embodiment 134, wherein the antibody
concentration is
between about 80 mg/ml and about 120 mg/ml.
[00704] 136. A pharmaceutical unit dosage form suitable for parenteral
administration to a
human which comprises an antibody formulation of any one of embodiments 101 to
131 in a
suitable container.
[00705] 137. The pharmaceutical unit dosage form of embodiment 136, wherein
the
antibody formulation is administered intravenously, subcutaneously, or
intramuscularly.
[00706] 138. A pharmaceutical unit dosage form suitable for aerosol
administration to a
human which comprises an antibody formulation of any one of embodiments 101 to
131 in a
suitable container.
[00707] 139. The pharmaceutical unit dosage of embodiment 138, wherein the
antibody
formulation is administered intranasally.
[00708] 140. A sealed container containing the formulation of any one of
embodiments
101 to 131.
[00709] 141. A kit comprising the formulation of any one of embodiments 101
to 131.
[00710] 142. A method of treating an autoimmune disease or disorder in a
human,
comprising administering to a human in need thereof a therapeutically-
effective amount of the
formulation of any one of embodiments 101 to 131.
[00711] 143. The method of embodiment 142, wherein the autoimmune disease
or
disorder is SLE or scleroderma.
[00712] 144. A method of treating or preventing rejection in a human
transplant patient,
comprising administering to a human in need thereof a therapeutically-
effective amount of the
formulation of any one of embodiments 101 to 131.
[00713] 145. A method of treating a T cell malignancy in a human comprising
administering to a human in need thereof a therapeutically-effective amount of
the formulation
of any one of embodiments 101 to 131.
184
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00714] 146. A method of treating an inflammatory disease or disorder in a
human,
comprising administering to a human in need thereof a therapeutically-
effective amount of the
formulation of any one of embodiments 101 to 131.
[00715] 147. The method of embodiment 180, wherein the inflammatory disease
or
disorder is myositis.
[00716] 148. The method of embodiment 147, wherein the myositis is
inclusion-body
myositis (IBM), polymyositis (PM) or dermatomyositis (DM).
[00717] 149. A method of depleting ICOS expressing T cells in a human
patient
comprising administering to a human in need thereof a therapeutically-
effective amount of the
formulation of any one of embodiments 101 to 131.
[00718] 150. The method of embodiment 149, wherein the depletion
substantially persists
for at least about 1, at least about 2, at least about 3 or at least about 4
weeks following the
administration of the antibody.
[00719] 151. The method of embodiment 149, wherein at least about 95% of
the T cells
are depleted.
[00720] 152. The method of embodiment 149, wherein the ICOS expressing T
cell is a
memory T cell.
[00721] 153. The method of embodiment 149, wherein the 1COS expressing T
cell is a
circulating T cell.
[00722] 154. A method of disrupting germinal center architecture in a
secondary lymphoid
organ of a primate, comprising administering an effective amount of the
formulation of any one
of embodiments 101 to 131.
[00723] 155. The method of embodiment 149, wherein the primate is a non-
human
primate.
[00724] 156. A method of depleting germinal center B cells from a secondary
lymphoid
organ of a primate comprising administering an effective amount of the
formulation of any one
of embodiments 101 to 131.
[00725] 157. The method of embodiment 156, wherein the primate is a non-
human
primate.
[00726] 158. The method of embodiment 156, wherein the primate is a human.
[00727] 159. The method of embodiment 156, wherein the depletion
substantially persists
for at least about 1, at least about 2, at least about 3 or at least about 4
weeks following the
administration of the antibody.
185
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00728] 160. A method of depleting circulating class switched B cells in a
primate
comprising administering an effective amount of the formulation of any one of
embodiments 101
to 131.
[00729] 161. The method of embodiment 194, wherein the primate is a non-
human
primate.
[00730] 162. The method of embodiment 194, wherein the primate is a human.
[00731] 163. The method of embodiment 194, wherein the depletion
substantially persists
for at least about 1, at least about 2, at least about 3 or at least about 4
weeks following the
administration of the antibody.
[00732] 164. The method of embodiment 194, wherein at least about 95% of
the
circulating class switched B cells are depleted.
[00733] 165. The formulation of any one of embodiments 1 to 70 or 101 to
131, wherein
said formulation is a pharmaceutically acceptable formulation.
[00734] 166. The formulation of any one of embodiments 66, 67, 69, 127, 128
or 130
wherein the formulation is in a pre-filled syringe.
[00735] 167. The formulation of embodiment 166, wherein the pre-filled
syringe
comprises a needle.
[00736] 168. The formulation of embodiment 166, wherein the pre-filled
syringe is a
plastic syringe or a glass syringe.
[00737] 169. The formulation of embodiment 166, wherein the pre-filled
syringe is a
plastic syringe.
[00738] 170. The formulation of embodiment 168, wherein the pre-filled
syringe is a glass
syringe.
[00739] 171. The formulation of the embodiment 166, wherein the pre-filled
syringe is
made of materials that are substantially free from tungsten.
[00740] 172. The formulation of embodiment 166, wherein the pre-filled
syringe is
substantially free from silicone.
[00741] 173. The formulation of embodiment 166, wherein the pre-filled
syringe does not
comprise a silicone based lubricant.
[00742] 174. The formulation of embodiment 166, wherein the pre-filled
syringe
comprises a plunger having a fluoropolymer resin disk.
[00743] 175. The pharmaceutical unit dosage of any one of embodiments 71,
72, 136 or
137, wherein the suitable container is a pre-filled syringe.
[00744] 176. The pharmaceutical unit dosage of embodiment 172, wherein the
pre-filled
syringe comprises a needle.
186
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00745] 177. The pharmaceutical unit dosage of embodiment 172, wherein the
pre-filled
syringe is a plastic syringe or a glass syringe.
[00746] 178. The pharmaceutical unit dosage of embodiment 178, wherein the
pre-filled
syringe is a plastic syringe.
[00747] 179. The pharmaceutical unit dosage of embodiment 178, wherein the
pre-filled
syringe is a glass syringe.
[00748] 180. The pharmaceutical unit dosage of the embodiment 172, wherein
the pre-
filled syringe is made of materials that are substantially free from tungsten.
[00749] 181. The pharmaceutical unit dosage of embodiment 172, wherein the
pre-filled
syringe is substantially free from silicone.
[00750] 182. The pharmaceutical unit dosage of embodiment 172, wherein the
pre-filled
syringe does not comprise a silicone based lubricant.
[00751] 183. The pharmaceutical unit dosage of embodiment 172, wherein the
pre-filled
syringe comprises a plunger having a fluoropolymer resin disk.
[00752] 184. The sealed container of any one of embodiments 75 or 140,
wherein the
sealed container is in a pre-filled syringe.
[00753] 185. The sealed container of embodiment 184, wherein the pre-filled
syringe
comprises a needle.
[00754] 186. The sealed container of embodiment 184, wherein the pre-filled
syringe is a
plastic syringe or a glass syringe.
[00755] 187. The sealed container of embodiment 186, wherein the pre-filled
syringe is a
plastic syringe.
[00756] 188. The sealed container of embodiment 186, wherein the pre-filled
syringe is a
glass syringe.
[00757] 189. The sealed container of the embodiment 184, wherein the pre-
filled syringe
is made of materials that are substantially free from tungsten.
[00758] 190. The sealed container of embodiment 184, wherein the pre-filled
syringe is
substantially free from silicone.
[00759] 191. The sealed container of embodiment 184, wherein the pre-filled
syringe does
not comprise a silicone based lubricant.
[00760] 192. The sealed container of embodiment 184, wherein the pre-filled
syringe
comprises a plunger, wherein the plunger comprises a fluoropolymer resin disk.
[00761] 193. The kit of any one of embodiments 76 or 141, wherein the kit
comprises a
pre-filled syringe.
187
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00762] 194. The kit of embodiment 193, wherein the pre-filled syringe
comprises a
needle.
[00763] 195. The kit of embodiment 193, wherein the pre-filled syringe is a
plastic syringe
or a glass syringe.
[00764] 196. The kit of embodiment 195, wherein the pre-filled syringe is a
plastic
syringe.
[00765] 197. The kit of embodiment 195, wherein the pre-filled syringe is a
glass syringe.
[00766] 198. The kit of the embodiment 193, wherein the pre-filled syringe
is made of
materials that are substantially free from tungsten.
[00767] 199. The kit of embodiment 193, wherein the pre-filled syringe is
substantially
free from silicone.
[00768] 200. The kit of embodiment 193, wherein the pre-filled syringe does
not comprise
a silicone based lubricant.
[00769] 201. The kit of embodiment 193, wherein the pre-filled syringe
comprises a
plunger, wherein the plunger comprises a fluoropolymer resin disk.
[00770] 202. A pre-filled syringe containing a sterile, stable aqueous
formulation
comprising an anti-human ICOS antibody, and further comprising histidine,
sodium chloride,
trehalose or polysorbate 80, wherein the antibody comprises a heavy chain
sequence of SEQ ID
NO:6, a light chain sequence of SEQ ID NO:1 and an Fc region having complex N-
glycoside-
linked sugar chains in which fucose is not bound to N-acetylglucosamine in the
reducing end in
the sugar chain.
[00771] 203. The pre-filled syringe of embodiment 202, wherein the pre-
filled syringe
comprises a needle.
[00772] 204. The pre-filled syringe of embodiment 202, wherein the pre-
filled is sealed.
[00773] 205. The pre-filled syringe of embodiment 202, wherein the pre-
filled syringe is a
plastic syringe or a glass syringe.
[00774] 206. The pre-filled syringe of embodiment 211, wherein the pre-
filled syringe is a
plastic syringe.
[00775] 207. The pre-filled syringe of embodiment 211, wherein the pre-
filled syringe is a
glass syringe.
[00776] 208. The pre-filled syringe of embodiment 202, wherein the pre-
filled syringe is
made of materials that are substantially free from tungsten.
[00777] 209. The pre-filled syringe of embodiment 202, wherein the pre-
filled syringe is
substantially free from silicone.
188
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
[00778] 210. The pre-filled syringe of embodiment 202, wherein the pre-
filled syringe
does not comprise a silicone based lubricant.
[00779] 211. The pre-filled syringe of embodiment 202, wherein the pre-
filled syringe
comprises a plunger having a fluoropolymer resin disk.
[00780] 212. The pre-filled syringe of embodiment 202, wherein the syringe
is a plastic
syringe substantially free from silicone and tungsten comprising a plunger
having a
fluoropolymer resin disk.
[00781] 213. The pre-filled syringe of any one of embodiments 202-212,
wherein said
formulation comprises the anti-human ICOS antibody, histidine, sodium
chloride, trehalose and
polysorbate 80.
[00782] 214. The pre-filled syringe of embodiment 202, wherein said
formulation
comprises between about 50 mg/ml and about 150 mg/m1 of the anti-human ICOS
antibody,
between about 1 mM and about 100 mM histidine, between about 10 mM and about
200 mM
NaCl, between about 1% and about 40% trehalose and between about 0.001% and
about 5%
polysorbate 80 and wherein the pH of said formulation is between about 5 and
about 7.
[00783] 215. The pre-filled syringe of embodiment 202, wherein said
formulation
comprises between about 80 mg/m1 and about 120 mg/ml of the anti-human ICOS
antibody,
between about 1 mM and about 50 mM histidine, between about 50 mM and about
150 mM
NaCl, between about 1% and about 20% trehalose and between about 0.005% and
about 1%
polysorbate 80 and wherein the pH of said formulation is between about 5.5 and
about 6.5.
[00784] 216. The pre-filled syringe of embodiment 202, wherein said
formulation
comprises about 100 mg/m1 of the anti-human ICOS antibody, about 10 mM
histidine, about 80
mM NaC1, about 4% trehalose and between about 0.01% and about 0.05%
polysorbate 80 and
wherein the pH of said formulation is about 6.
[00785] 217. The pre-filled syringe of embodiment 202, wherein said
formulation
comprises about 100 mg/m1 of the anti-human ICOS antibody, about 10 mM
histidine, about 80
mM NaC1, about 4% trehalose and about 0.02% polysorbate 80 and wherein the pH
of said
formulation is about 6.
[00786] 218. The pre-filled syringe of any one of embodiments 202 to 217,
wherein said
formulation is isotonic.
[00787] 219. The pre-filled syringe of any one of embodiments 202 to 217,
wherein said
formulation is stable upon storage at 40 C for at least about 4 weeks.
[00788] 220. The pre-filled syringe of any one of embodiments 202 to 217,
wherein said
formulation is stable upon storage at 5 C for at least about 3 months.
189
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
[00789] 221. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
formulation is stable upon storage at 5 C for at least about 12 months.
[00790] 222. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 80% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 40 C
for at least about 4
weeks.
[00791] 223. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 80% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
[00792] 224. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 80% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00793] 225. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 90% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 40 C
for at least about 4
weeks.
[00794] 226. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 90% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
[00795] 227. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 90% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00796] 228. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 95% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 40 C
for at least about 4
weeks.
[00797] 229. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 95% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 3
months.
190
CA 02743469 2011-05-11
WO 2010/056804
PCT/US2009/064127
[00798] 230. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody retains at least about 95% of binding ability to a human ICOS
polypeptide compared to
a reference antibody representing the antibody prior to the storage at 5 C for
at least about 12
months.
[00799] 231. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
antibody is susceptible to aggregation or fragmentation.
[00800] 232. The pre-
filled syringe of any one of embodiments 202 to 217, wherein less
than about 2% of said antibody forms an aggregate upon storage at 40 C for at
least about 4
weeks as determined by as determined by HPSEC.
[00801] 233. The pre-
filled syringe of any one of embodiments 202 to 217, wherein less
than about 2% of said antibody forms an aggregate upon storage at 5 C for at
least about 3
months as determined by HPSEC.
[00802] 234. The pre-
filled syringe of any one of embodiments 202 to 217, wherein less
than about 2% of said antibody forms an aggregate upon storage at 5 C for at
least about 12
months as determined by HPSEC.
[00803] 235. The pre-
filled syringe of any one of embodiments 202 to 217, wherein less
than about 5% of said antibody is fragmented upon storage at 40 C for at least
about 4 weeks as
determined by RP-HPLC.
[00804] 236. The pre-
filled syringe of any one of embodiments 202 to 217, wherein less
than about 5% of said antibody is fragmented upon storage at 5 C for at least
about 3 months as
determined by RP-HPLC.
[00805] 237. The pre-
filled syringe of any one of embodiments 202 to 217, wherein less
than about 5% of said antibody is fragmented upon storage at 5 C for at least
about 12 months as
determined by RP-HPLC.
[00806] 238. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
formulation is clear and colorless upon storage at 5 C for at least about 3
months as determined
by visual inspection.
[00807] 239. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
formulation is clear and colorless upon storage at 5 C for at least about 12
months as determined
by visual inspection.
[00808] 240. The pre-
filled syringe of any one of embodiments 202 to 217, wherein said
formulation is substantially free from particulates upon storage at 5 C for at
least about 3 months
as determined by visual inspection.
191
CA 02743469 2016-06-13
' 51332-100
[00809] 241. The pre-filled syringe of any one of embodiments 202 to
217, wherein said
formulation is substantially free from particulates upon storage at 5 C for at
least about 12
months as determined by visual inspection.
[00810] 242. The pre-filled syringe of any one of embodiments 202 to
217, wherein said
formulation is an injectable formulation.
[00811] 243. The pre-filled syringe of embodiment 242, wherein said
formulation is
suitable for subcutaneous or intramuscular.
[00812] 244. The pre-filled syringe of embodiment 243, wherein said
formulation is
suitable for subcutaneous administration.
[00813] 245. The pre-filled syringe of any one of embodiments 202 to
244, wherein said
formulation is a pharmaceutically acceptable formulation.
[00814] 246. A kit comprising the pre-filled syringe of any one of
embodiments 202 to
245.
[00815] Those skilled in the art will recognize, or be able to
ascertain using no more than
routine experimentation, many equivalents to the specific embodiments of the
disclosure
described herein. Such equivalents are intended to be encompassed by the
following claims.
[00816]
[00817] Citation or discussion of a reference herein shall not be
construed as an admission
that such is prior art to the present disclosure.
6. EXAMPLES
[00818] These examples are provided for the purpose of illustration
only and the
disclosure should in no way be construed as being limited to these examples
but rather should be
construed to encompass any and all variations which become evident as a result
of the teachings
provided herein.
[00819] The following section describes the development of formulations
comprising an
anti-human ICOS antibody. Unless stated otherwise, experimental results
presented here were
generated using the 136 anti-human ICOS antibody comprising a heavy chain
sequence of SEQ
ID NO:6, a light chain sequence of SEQ ID NO:1 and an Fc region having complex
N-glycoside-
linked sugar chains in which fucose is not bound to N-acetylglucosamine in the
reducing end in
the sugar chain (see, US Patent Application Publication US 2008/0279851A1,
filed on May 7, 2008).
192
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
6.1. Experimental methods
[00820] Purified anti-human ICOS antibody may be generated following
standard
industrial scale protocols described herein. Protein concentration may be
estimated from optical
density measurement at 280 nm.
[00821] Purified anti-human ICOS antibody is nanofiltered using a Planova
20N filter to
remove particulate matter. Anti-human ICOS antibody formulations is prepared
using
Tangential Flow Filtration (TFF). The nanofiltered anti-human ICOS antibody is
concentrated to
approximately 25 mg/ml on a Millipore Labscale TFF device. The anti-human ICOS
antibody is
then 5x diafiltered into the appropriate buffer (e.g., 10 mM histidine-HCL (pH
6.0), 80 mM
NaCl). Once the buffer exchange is complete, the antibody is concentrated to
approximately
150 mg/ml. Excipients are introduced by spiking the concentrated antibody
preparation with the
appropriate concentrated stock solutions. For example, a final concentration
of 4% Trehalose is
achieved by adding 11 ml of 10 mM histidine-HCl, 80 mM NaCl, 40% Trehalose (pH
6.0) to
every 100 ml of concentrated antibody preparation. Multiple excipients may be
introduced in
consecutive steps. For example, a final concentration of 0.02% Polysorbate 80
is introduced
after the addition of Trehalose by diluting 100 fold a 10 mM histidine-HC1 (pH
6.0), 80 mM
NaC1, 4% Trehalose, 2% Polysorbate 80 stock solution with the Trehalose
containing antibody
preparation. The final antibody concentration of is adjusted to 100+5 mg/ml
with the final
formulation buffer (e.g., 10 mM histidine-HCl (pH 6.0), 80 mM NaCl. 4%
Trehalose, 0.02%
Polysorbate 80).
[00822] The following section describes methods that may be used to
characterize the
formulation comprising 100 mg/m1 anti-ICOS antibody in 10 mM Histidine (pH
6.0), 80 mM
NaCl, 4% Trehalose, 0.02% Polysorbate 80 in a sterile aqueous solution.
[00823] Formulation stability is tested by analyzing the physical
properties of single dose
aliquots stored for extended periods of time. Some aliquots are stored under
temperatures
recommended for clinical storage (5 C). Other aliquots are stored under
elevated temperature
(25 C or 40 C) to simulate the effects of very long term storage.
[00824] Additional storage conditions that may affect stability of a
formulation include,
but are not limited to, light intensity, light wavelength, humidity, vial
composition, and stopper
composition. The effect of these parameters on formulation stability may also
be determined
using the methods described herein.
[00825] Size exclusion chromatography may be utilized to measure the amount
of
antibody aggregates (e.g., dimmers) and the extent of fragmentation in the
formulation. SEC
may be performed using the Agilcnt 1100 Series High Performance Liquid
Chromatography
193
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
(HPLC) system as follows. Samples are diluted to 10 mg/ml. 25 .1 diluted
sample containing
250 ug protein is injected onto a TSK-Gel 3000 column (size 7.8 mm x 30.0 cm;
Tosoh
Biosciences Corporation). Protein elution profile is determined by following
the eluate's optical
density at 280 nm. Data analysis may be performed using ChemStation (Agilent)
auto
integration parameters.
[00826] Reversed Phase High Performance Liquid Chromatography (RP-HPLC) may
also
be used to determine the amount of antibody fragments in the formulation. RP-
HPLC is
performed using the Agilent 1100 Series High Performance Liquid Chromatography
(HPLC)
system. Samples are analysed on a PLRP-S (8 um, 4000 A, 2.0 x 150 mm) column
from
Michrom Bioresources. Protein elution profile is determined by following the
eluate's optical
density at 280 nm. Data analysis may be performed using ChemStation (Agilent)
auto
integration parameters.
[00827] Ion exchange chromatography (IEC) may be employed to measure charge
isoform
heterogeneity of in various formulations. Agilent 1100 Series High Performance
Liquid
Chromatography (HPLC) systems may be used for this analysis. Samples are
analysed on a
Propac WCX-10G (4 x 250mm) Analytical Column (Dionex). Data analysis is
performed using
the ChemStation (Agilent) auto integration parameters.
[00828] Visual inspection: color, clarity, and amount of particulates in a
given formulation
are determined by inspecting the sample with a naked eye.
6.1.1. Size exclusion chromatography (SEC)
[00829] Size exclusion chromatography may be performed to analyze the
antibody
formulation for the presence of antibody aggregates and fragments. The test
samples are injected
onto a high resolution size exclusion column (e.g., G3000 SWxL 5 lam, 300 A,
7.8 x 300 mm,
Toso Haas). The mobile phase is 0.1 M di-sodium phosphate, 0.1 M sodium
sulphate and 0.05
')/0 sodium azide (pH 6.7), running isocratically at a flow rate of 0.25 - 1.0
mL/min. Eluted
protein may be detected by UV absorbance at 280 nm and collected for further
characterization.
The relative amount of any protein species detected is reported as the area
percent of the product
peak as compared to the total area of all other detected peaks excluding the
initial excluded
volume peak. Peaks eluting earlier than the antibody monomer peak are recorded
in the
aggregate percentile, while peaks eluting later than the antibody monomer
peak, but earlier than
the buffer peak, are recorded in the fragment percentile. The hydrodynamic
radius and
molecular weight of the individual peaks may be obtained with a coupled
multiangle light
scattering detector.
[00830] SEC may be used to monitor antibody aggregate formation and
antibody
fragmentation in a formulations stored for extended time periods (e.g.,
multiple measurements
194
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
performed over 9 months). The formulation may be stored at different
temperature ranges (e.g.,
2-8 C, 20-24 C and 38-42 C). Temperature ranges above the proposed clinical
storage
temperature (2-8 C) are used to stress the formulation with the goal of
simulating the effects of
storage beyond 9 months. The ratio of fragments and aggregates is expected to
increase over
time; this increase is likely to be accelerated at elevated temperatures. A
finding that
fragmentation and aggregation rates are constant within each temperature range
would show that
higher storage temperatures accurately simulate an accelerated time scale.
[00831] The logarithm of the estimated rates of fragmentation/aggregation
(log(rate)) may
also be determined. A finding that the log(rate) shows a linear dependence to
the reciprocal of
the storage temperature (1/T (1(4) would allow the investigator to predict the
rate of
aggregation/fragmentation of the formulation at any temperature or, more
importantly, the
formulation characteristics at any time at a given temperature.
[00832] In situations where the chromatography peaks corresponding to
aggregates and
fragments are not be sufficiently distinct from each other, or from the
monomer peak (e.g., at low
relative levels of aggregates/fragments), SEC may not serve as an accurate
measure of
fragmentation/ aggregation.
6.1.2. Analytical ultracentrifugation
[00833] Analytical ultracentrifugation (AUC) may also be used to
characterize the
antibody formulation for the presence of antibody aggregates and fragments.
AUC is an
orthogonal technique which determines the sedimentation coefficients (reported
in Svedberg, S)
of macromolecules in a liquid sample. Like SEC, AUC is capable of separating
and detecting
antibody fragments/aggregates from monomers and is further able to provide
information on
molecular mass. Compared to SEC, AUC eliminates the possibility of aggregate
loss due to
solid-phase interaction and is better able to resolve differing species of a
given macromolecule.
[00834] Sedimentation velocity experiments may be performed using an
analytical
ultracentrifuge, for example, Beckman Optima XL-A. Test samples are diluted to
an antibody
concentration of 0.5 mg/ml with reference buffer (e.g., 20 mM citric acid, 100
mM NaCl, 1.5%
mannitol, 50 [EM diethylenetriamine-pentaacetic acid, 0.02% Polysorbate 80, pH
6.0). 415 ill of
the diluted antibody sample and 412 jil or the reference buffer is loaded into
a 12 mm centrifuge
cell in the sample and reference channels, respectively. Loaded cells are
placed into an AN-50Ti
analytical rotor and equilibrated to 25 C. Samples are scanned at 280 nm with
a rotor speed of
42000 rpm at full vacuum. A total of 80 scans for each cell are collected for
analysis. The first
scan for each sample is excluded from downstream data processing to avoid
artifacts caused by
meniscus.
195
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
[00835] The data is analyzed using the c(s) method developed by Peter Shuck
at N.I.H.
and the SEDFIT (version 8.8) program with implemented c(s). Using the c(s)
method, raw data
scans are directly fit to a Lamm function of S in order to derive a
distribution of sedimentation
coefficients. The parameters used for the fitting procedure are resolution,
400; confidence
interval, 0.75; grid size, 1000; partial specific volume, 0.7245; buffer
density, 1.000; and buffer
viscosity, 0.1002. Frictional ratio, meniscus and bottom positions are set as
fitted parameters.
Time independent noise is also fitted. The detected peaks are integrated and
classified as
follows: from 0 to 6 S, fragments; from 6 to 9 S, monomer; and from 9 to 20 S,
aggregates.
[00836] AUC may be used to characterize antibody formulations with low
relative levels
of aggregation and fragmentation. AUC may be able to better resolve antibody
fragments and
aggregates from the monomer species in situations that are beyond the
resolution capabilities of
SEC. peaks. AUC estimates of the molecular mass of an aggregate peak may also
be used as an
indicator of its composition (e.g., dimers vs. higher multimers).
[00837] Compared to SEC. AUC may also able to better resolve differing
species of a
given macromolecule. It is, however, necessary to establish first the proper
sample dilution rate,
as the noise/signal ratio of AUC is dependent on the antibody concentration in
the sample.
6.1.3. Turbidity measurement:
[00838] Protein aggregation in the antibody formulation may also be
characterized by
turbidity measurement. Turbidity is a measure of the amount by which the
particles in a solution
scatter light and, thus, may be used as a general indicator of protein
aggregation or denaturation.
Elevated turbidity may indicate a higher level of aggregation or an increased
number/ increased
size of particles.
[00839] Turbidity measurement may be performed with a turbidimeter (e.g.,
2100AN or
2100N, Hatch) following the manufacturer's instructions. Approximately 3 to 4
ml of
formulation sample is transferred into a glass test tube and degassed for 2
minutes using an in-
line vacuum system. The degassed sample is then placed into a turbidimeter
(e.g., 2100AN or
2100N, Hatch) sample compartment at room temperature for analysis. The
turbidimeter is
calibrated with STABLCALk Stabilized Formazin Turbidity standard (Hatch) at
40, 200, 1000
and 4000 NTU (nephelometric turbidity unit) and verified by analyzing control
suspensions of
formazin at 3, 6, 18, 30 and 60 NTU.
6.1.4. Particle Count
[00840] The number and size of particles in a particular formulation may be
determined
using a particle counter (e.g., Beckman Coulter Multisizer 3) according to the
manufacturer's
instruction.
196
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
6.1.5. Viscosity profile
[00841] Viscosities of antibody formulations may be measured using a
viscometer (e.g.,
ViscoLab 4000 Viscometer System from Cambridge Applied Systems equipped with a
ViscoLab
Piston (0.3055", 1-20 cP)). The viscometer is calibrated before use with the
appropriate
standards (e.g., S6S Reference Standard from Koehler Instrument Company,
Inc.). The
viscometer is connected to a water bath to equilibrate the system to 20 C.
Piston is checked
using S6S viscosity reference standard (8.530 cP @ 20.00 C). Piston is also
checked using
RODI H20 (1.00 cP @ 20.0 C). The piston is cleaned and rinsed thoroughly with
soap and
water between measurements of each different solution type. Subsequently the
system is cooled
to < 2 C. Once the system temperature is at or below 2 C, sample is loaded
into the chamber
and the piston is lowered into the sample. After sample is equilibrated to the
temperature of the
chamber, measurement is initiated. The temperature is increased at 1 C
increments every 7-10
minutes to a final temperature of? 25 C. The viscosity result is recorded
immediately prior to
increasing the temperature. The piston remains in motion during measurements
to minimize the
need for re-equilibration.
6.1.6. Differential Scanning Ca lorimetry
[00842] Differential Scanning Calorimetry (DSC) may be used to ascertain
changes over
time in the thermal stability of an antibody in a particular formulation.
Thermal melting
temperatures (T,õ) are determined with a differential scanning calorimeter
(e.g., VP-DSC from
MicroCal, LLC) following the manufacturer's instruction. In one example, VP-
DSC is used at a
scan rate of 1.0 C/min and with a temperature range of 25 ¨120 C. A filter
period of 8 seconds
is used along with a 5 minute pre-scan thermostating. Samples are prepared by
dialysis into 25
mM Histidine-HC1, pH 6 using Pierce dialysis cups (3.5 kD). Average Mab
concentrations are
500 g/mL as determined by A280. Melting temperatures are determined following
the
manufacturer's instructions using software supplied with the system.
6.1.7. Liquid Chromatography Mass Spectrometry (LC-MS)
[00843] Liquid Chromatography Mass Spectrometry (LC-MS) may be used to
characterize a degradation fragment detected by SEC or AUC in the antibody
formulation.
[00844] Peak SEC column fractions containing the degradation fragment are
collected and
digested with N-Glycosidase F, also known as PNGase F, at 37 C overnight.
PNGase F is an
amidase used to deglycosylate protein samples. The enzyme cleaves between the
innermost
GlcNAc and asparagine residues of high mannose, hybrid and complex
oligosaccharides on N-
linked glycoproteins. The deglycosylatcd samples mixed with a reducing buffer
(e.g., 2.5
mg/mL DTT, 6.0 M guanindine HC1, pH 8.2) and kept at 56 C in a water bath for
60 minutes.
197
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Neat 4-vinylpyridine (e.g., Aldrich Chem. Co., WI) is then added to the
sample, and the reaction
mixture is held at ambient temperature for 30 minutes. The deglycosylated,
reduced and
alkylated sample is immediately loaded onto a reversed phase column in order
to separate the
modified samples from the reactants.
[00845] Dcglycosylated, reduced, and alkylated samples are fractionated
using a reversed
phase column (e.g., Jupiter 5um C4, 300 A, 250 x 2.00 mm, Phenomenex) with a
binary gradient
HPLC system (Agilent 1100). Mobile phase A consists of 30% acetonitrile in
water with 0.1%
trifluoroacetic acid and mobile phase B consists of 50% acetonitrile in water
with 0.1%
trifluoroacctic acid. The samples are separated using a linear gradient of 30-
50% acetonitrile in
water, over 16 min. with a flow rate of approximately 200 i.tL/min. The column
effluent is
directed to a UV detector and then split 1:1, one half going through a
switching valve on an Ion
Trap mass spectrometer (e.g., LTQ, ThermoElectro, San Jose, CA), and the
remaining half to
waste.
[00846] The ion-trap mass spectrometer is calibrated before the
experimental run using a
mixture of caffeine, L-methionyl-arginyl-phenylalanyl-alanine acetateT120, and
Ultramark 162.
The Electrospray Ionisation Mass Spectrometry (ESI-MS) data is acquired in
positive ESI full
scan mode. The BioWork deconvolution program (ThermoFinnigan) may be used to
reconstruct
the mass spectra and obtain the molecular masses of the peptides/proteins from
their original
mass spectra. The mass data subsequently is used to determine the identity of
the degradation
fragment.
6.1.8. Isoelectric Focusing Gel Electrophoresis
[00847] Isoelectric point measurements of may be used to ascertain the
antibody's
chemical stability in a given formulation. Isoelectric points are determined
using a Pharmacia
Biotech Multiphor 2 electrophoresis system with a multi temp 3 refrigerated
bath recirculation
unit and an EPS 3501 XL power supply. Pre-cast ampholine gels (Amersham
Biosciences, pI
range 2.5-10) are loaded with 5 pg of protein. Broad range pI marker standards
(Amersham, pI
range 3-10, 8 4) are used to determine relative pI for the Mabs.
Electrophoresis is performed at
1500 V, 50 mA for 105 minutes. The gel is fixed using a Sigma fixing solution
(5x) diluted with
purified water to lx. Staining is performed overnight at room temperature
using Simply Blue
stain (Invitrogen). Destaining is carried out with a solution of 25% ethanol,
8% acetic acid and
67% purified water. Isoelectric points are determined using a Bio-Rad
Densitometer relative to
calibration curves of the standards.
6.1.9. Disulfide bond determination
[00848] Disulfide bond determination protocols may be used to monitor the
stability of
disulfide bridge crosslinks in a particular antibody formulation. Antibody
samples are
198
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
denatured, for example, in 10 mM phosphate buffer, 250 mM NaCl, 5 mM NEM, 6 M
Guanidine, pH 7.0 at 37 C for 1 to 3 hr. The denatured samples are diluted 6
fold with 100 mM
phosphate buffer, 0.1 mM EDTA, pH 7.0, to which Endoproteinase Lys-C (e.g.,
Roche) is added
at a 1:10 enzyme to protein ratio. The reaction mixtures are incubated at 37
C for 16 to 24
hours. In half of the reaction mixture disulfide bridges are reduced by adding
5-104 of 100
mM DTT followed by incubation at 37 'V for 1 hr. Lys-C digested samples are
fractionated by
reverse-phase HPLC (e.g., Phenomenex Jupiter 5m C18 column; 250 x 2.1 mm).
Eluant is
analyzed by an UV-detector and an in-line LCQ or LTQ Ion Trap mass
spectrometer (e.g.,
ThermoElectron). The RP-HPLC mobile phase A is 0.1% TFA in H20 and mobile
phase B is
0.1% TFA in acetonitrile. The peptides are eluted at a flow rate of 0.2 mL/min
with the
following step gradient: 1) 0-2 min, 5% Mobile Phase B; 2) 2-32 min, 5-20%
Mobile Phase B; 3)
32-132 min, 20-40% Mobile Phase B; 4) 132-152 min, 40-60% Mobile Phase B;
5)152-155 min,
60-95% Mobile Phase B.
[00849] The ion-trap mass spectrometer is calibrated before the
experimental run using a
mixture of caffeine, L-methionyl-arginyl-phenylalanyl-alanine acetate1120, and
Ultramark 162.
The Electrospray Ionisation Mass Spectrometry (ESI-MS) data is acquired in
positive ESI full
scan mode. The BioWork deconvolution program (ThermoFinnigan) may be used to
reconstruct
the mass spectra and obtain the molecular masses of the peptides from their
original mass
spectra. Comparison of the mass data acquired using the DTT reduced and non-
reduced samples
allows the identification of the disulfide crosslinked peptides.
6.1.10. Binding affinity characterization
[00850] Binding affinity of monoclonal antibody recovered form the
formulation
following long term storage (e.g., 1 month at 40 C or 6 months at 5 C) may be
determined by
surface plasmon resonance (see, e.g., Jonsson et al., Biotechniques 11(5):620-
627 (1991); Johne,
B., Molecular Biotechnology 9(1):65-71(1989)) using a BlAcore 3000 instrument
(BlAcore,
Inc., Piscataway, NJ). antibody is captured on a Prot-G coated CMS chip. A
Prot-G coated
CMS chip with captured isotype control human-IgG (Sigma) antibody is used for
reference
purposes. ICOS-Fc fusion protein dissolved in HBS-EP running buffer is passed
over the chip at
a rate of 25 ul/min. 5 minutes of association time is followed by a 10 minute
dissociation period.
Independent measurements are performed by exposing the chips to different
concentrations of
ICOS-Fc (e.g. concentrations between 10 nM and 80 nM). Chips are regenerated
by a 0.4
minute wash with 20 mM NaOH 400mM NaCl at a flow rate of 100u1/min. Once the
entire
data set is collected, the resulting binding curves are globally fitted to a
1:1 Langmuir binding
model using BIAevaluation software (BlAcore, Inc., Piscataway, NJ). This
algorithm calculates
both the association rate (kW and the dissociation rate (koff), from which the
apparent
199
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
equilibrium binding constant, KD, is deduced as the ratio of the two rate
constants, korf Icon. A
more detailed explanation of how the individual rate constants are derived can
be found in the
BIAevaluation Software Handbook (BIAcore, Inc., Piscataway, NJ).
6.2. Formulation development
[00851] The physicochemical properties of the afucosylated 136 antibody
were
characterized as follows. The DSC profile of the afucosylated 136 was
determined as described
above. DSC measurements were performed in 25 mM histidine (pH 6.0) buffer. The
DSC
profile of the afucosylated 136 antibody (Figure 1) is essentially identical
to that of the
fucosylated parent antibody.
[00852] The effect of pH on the thermal stability of the 136 antibody was
ascertained by
measuring tryptophan fluorescence as a function of temperature at various pHs.
A representative
sample of the experimental results is shown in Figure 2. The 136 antibody
appeared stable in the
pH 5-7 range.
[00853] The effect of formulation pH on colloidal stability was assessed by
measuring
turbidity (A350 nm) of various 136 formulations as a function of temperature.
A representative
sample of the experimental results is shown in Figure 3. The 136 antibody
appeared stable in the
pH range of 4-7.
[00854] A high throughput screen was designed to identify excipients that
can stabilize the
136 antibody. A metastable control buffer (20 mM phosphate at pH 7.2) was
selected for the
screen based on the pH sensitivity of the 136 antibody. Excipients were
screened by comparing
the colloidal stability of 136 in the control buffer to the colloidal
stability of 136 in a buffer
comprising a single additional excipient. Changes in colloidal stability were
followed by
measuring the turbidity (A 350 nm) of the formulation over time. Stabilizing
excipients
decelerate or eliminate the increase in turbidity over time while
destabilizing excipients
accelerate the increase in turbidity. A representative result is shown in
Figure 4. Turbidity
changes in a buffer comprising a stabilizing excipients were slowed down or
eliminated
compared to the rate of turbidity change observed in the control buffer. The
high throughput
screen identified histidine, citrate, arginine, lysine ,sodium chloride and
trehalose as stabilizing
excipients for the 136 antibody (Figures 5-7). Additional experiments were
performed to
ascertain the effect of combinations of these excipients on 136 stability
(Figure 8). The most
pronounced stabilizing effect was observed with 100 mM arginine or lysine when
used in
combination with 4% trehalose.
[00855] Based on the results of the high throughput screen three candidate
formulations
were selected for long term stability experiments. Formulation 1 comprised 10
mM histidine
200
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
(pH 6.0); Formulation 2 comprised 10 mM histidine (pH 6.0) and 150 mM NaCl;
Formulation 3
comprised 10 mM histidine (pH 6.0), 100 mM arginine-HC1 and 4% trehalose. The
stability of
the 136 antibody (60 mg/m1) in these formulations was assessed by measuring
the monomer
concentration following storage at 40 C. Monomer concentration was determined
by SEC as
described herein. An example of the stability results obtained are shown in
Figure 9. The results
confirmed that the addition of arginine/trehalose or NaCl increased the
stability of the 136
antibody. Arginine/trehalose had a stronger stabilizing effect than that of
NaCl alone.
[00856] The stability of 136 antibody was also ascertained in lysine
containing
formulations. 136 antibody recovered form Formulation 2 (10 mM histidine (pH
6.0) and 150
mM NaCl) and Formulation 4 (10 mM histidine (pH 6.0), 100 mM lysine-HC1 and 4%
trehalose)
following 1 month incubation at 40 C was analyzed on HP-SEC. Representative
elution profiles
are shown in Figure10. The main monomer peak in the elution profile of 136
recovered form
Formulation 4 displays a pronounced shoulder. The main monomer peak of the 136
antibody
recovered form arginine containing formulations also displayed the same
shoulder. The presence
of the shoulder indicates monomer/dimer mixture formation in lysine and
arginine containing
formulations. Such a shoulder is not detected in the elution profile of 136
antibody recovered
from Formulation 2.
[00857] A formulation comprising 10 rrtM histidine (pH 6.0), 80 mM NaCl, 4
% trehalose
and 0%, 0.02% or 0.05% polysorbate was selected for further stability studies.
The aggregation
and fragmentation profile of 136 in these formulation following storage at 5
C, 2 C or 40 C was
determined using methods described herein. Representative results from these
experiments are
presented in Figures 11-15. Data shown demonstrates that the formulation
comprising 100
mg/ml, 10 mM histidine (pH 6.0), 80 mM NaCl, 4% trehalose and 0.02%
polysorbate 80 is
highly stable liquid formulation.
6.3. Formulation stability in pre-filled syringes.
[00858] The stability of a formulation comprising 100 mg/m1 antibody, 10 mM
histidine,
80 mM NaCl, 4% trehalose and 0.02% PS-80 at pH 6.0 may be tested in pre-filled
syringes.
Stability testing is performed by loading 1 ml of the formulation into a
syringe and storing the
formulation-filled syringe at 5 C, 25 C or 40 C for extended periods of time.
Formulation
stability is analysed using the analytical methods described herein. Particle
formation, a key
determinant of formulation stability in pre-filled syringes, is assessed by
visual inspection.
Protein aggregation and/or fragmentation is assessed by subjecting the
formulation recovered
from the syringe to an analytical method known to one of skill (e.g., SEC).
201
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
6.4. Depletion of ICOS bearing T cells prevents disease in a GvH model of
scleroderma.
[00859] In this study, the function of ICOS in the pathogenesis of a graft-
versus-host
disease (GvHD) mouse model of scleroderma (SSc) was investigated using a
glycoengineered
anti-mouse ICOS MAb with enhanced antibody-dependent cellular cytotoxicity
(ADCC). An
afucosylated rat anti-murine ICOS monoclonal antibody (IgG2a) directed against
the ligand
binding domain of murine ICOS was produced in a fucosyltransferase 8-deficient
Chinese
Hamster Ovary (CHO) producer cell line (BioWa Potelligent Technology). The
activity of the
afucosylated anti-mouse ICOS MAb, was evaluated in a murine GvHD model, which
recapitulates key aspects of human SSc, including inflammation, fibrosis, and
vasculopathy.
[00860] Dosing of the anti-ICOS-aFuc MAb reduced severity and incidence of
dermal
lesions when compared to isotype control MAb and control syngeneic graft. Mean
clinical scores
were significantly reduced in anti-ICOS-treated groups compared to isotype
control MAb-treated
mice, as early as 11 days post-graft (3.4-fold, p<0.05), and thereafter up to
4 weeks post graft
(8.1-fold, p<0.005). The anti-ICOS-aFuc MAb also prevented the disease-
associated
accumulation of TFH cells and the associated expansion of germinal center B
cells and
immunoglobulin secreting B cells. There were no ICOS MAb-related clinical
signs or changes in
body weight in animals during the study.
[00861] The results indicate that ICOS plays an important role in dermal
pathology of
murine GvHD-SSc, as depletion of ICOS+ T cells reduced the overall clinical
disease score. The
identification of dysregulated ICOS+ TFH cells in GvHD-SSc underscores their
critical function
in driving the generation of pathogenic B cells into germinal center B cells
in secondary
lymphoid tissues and in the differentiation of immunoglobulin secreting B
cells in the skin.
Importantly, treatment with the anti-mouse ICOS-aFuc MAb resulted in a
significant reduction
of the clinical signs of disease.
[00862] BIAcore binding affinity of the fucosylated and afucosylated anti-
ICOS MAb to
mouse FcgRIV is shown in Fig. 15A. Enhanced binding of ICOS MAb Fe to the
FcgRIV
expressed on effector cells was expected to increase antibody-dependent
cellular cytotoxicity
(ADCC). Figure 15B shows the immuno-phenotype characterization in the steady
state of ICOS
expression on naïve splenic naïve and T helper memory cells (central and
effector). Splenocyte
isolated from naive Balb/c mice were processed and stained with the indicated
markers to
identify the expression profile of ICOS on T helper cell subpopulations.
Fucose free anti-ICOS
MAb (IgG2a-aFuc) mediated more effective depletion of ICOS bearing T cells
(Figure 15C).
Pharmacodynamic analysis of splenic helper central and effector memory ICOS
bearing T cells
202
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
was determined upon one single intraperitoneal injection of the indicated anti-
ICOS MAbs into
naïve Balb/c mice (250 ug/animal).
[00863] The GvH model used in this study was described in Zhou L., J Invest
Dermatol,
2006, 126(2): 305-14. Balb/c hosts were grafted with T cells from a BlOD2
donor. Control
animals were grafted with syngcnic Balb/c T cells. Antibody was administered
at days 7, 14 and
21 post-graft. Administration of anti-ICOS MAb (IgG2a-aFuc) reduced graft
versus host
scleroderma clinical score (Figure 16). Mean clinical disease score was
evaluated following
biweekly treatment (starting time: day 8) with anti-mouse ICOS IG2a-aFuc or
isotype control
MAb (n=10). Baseline skin scores measurements were obtained on study day 6,
and biweekly
until study day 26, when animals were euthanized for tissue harvest. Skin was
scored as follows:
0= normal; 1 = lesion <1 cm2; 2= lesion 1-2 cm2; 3 = lesion > 2 cm2.
Extremities (ear, tail,
paws) appearing scaly were given a score of 0.3, for a maximum total score of
3.9 per animal
(*p<0.05, "p<0.005).
[00864] Anti-ICOS MAb mediated effective elimination of ICOS bearing TFH
and
inhibited the expansion of germinal center B cells. Figure 17 shows the
immunophenotype
analysis of spleen, lymph node and peripheral blood Th memory and Th memory
ICOS+ cells
(gated as indicated in Fig. IC) isolated from Balb/c control mice and from
rag2 deficient mice
treated with either anti-ICOS or isotype control MAb. Anti-ICOS therapy
prevents the expansion
of TFH cells (Figure 17D). While anti-ICOS MAb does not alter the overall
number of total
splenic B cells (CD19+) (Figure 17E), it significantly inhibited the TFH-
mediated expansion of
germinal center B cells (Figure 17F). Depletion of ICOS bearing T cells did
not perturb the
overall CD4+ (Figure 17G) and CD8+ (Figure 17H) T cell compartments.
[00865] Histology of RAG2-/- spleen and kidney from an isotype control MAb
treated
animal and anti-ICOS MAb treated animal is shown in Figure 18. Higher
magnification (x200)
of the spleen demonstrates lack of germinal center formation in anti-ICOS-
treated animals
compared to the isotype. In the kidney, there was moderate perivascular
cuffing (E) with
lymphocytes admixed with fewer plasma cells (F, inset). Original
magnification, x100; inset
x1000.
[00866] Treatment with anti-ICOS MAb significantly inhibited the
development of
GvHD-SSc skin pathology. Histology of back skin from either Balb/c, or RAG2-/-
mice grafted
with splenocytes at 4 weeks from isotype control MAb group and anti-ICOS MAb
treated group
is shown in Figure 19. Hematoxylin and eosin stain (Figure 19 A, C and E) of
skin representing
2/10 animals in the isotype group MAb demonstrates marked deep dermal
inflammation with
infiltration of lymphocytes, scattered neutrophils and macrophages within
increased collagenous
matrix. Diffusely, the epidermis is thickened with apoptosis and necrosis of
individual basal cells
203
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
and within the inner root sheath of hair follicles. Masson's Trichrome stain
(Figure 19 B, D and
F) demonstrates increased immature collagen within the dermis of the isotype
group. There was
minimal to no skin inflammation in the anti-ICOS MAb treated animals. Original
magnifications, x200.
[00867] ICOS MAb treatment impacted expression of T helper- and TFH-
associatcd genes
and the autoimmune-gene fingerprint in the skin. Total RNA was extracted from
skin biopsies
and preamplification of cDNA and real-time PCR were prepared using the TaqMan
PreAmp
Master Mix Kit (Applied Biosystems). Skin RNA samples, collected from Balb/c
control mice
and from Rag2 knockout mice treated with anti-ICOS or isotypc control MAb,
were run in
triplicate using TaqMan Gene Expression Assays in the BioMark 48.48 Dynamic
Array chips
(Fluidigm Corp). Delta-delta Cts (AACt) were calculated using the mean of the
3 reference genes
(GAPDH, 18S, ACTB) and a calibrator sample, and were converted to fold
expression change by
the 2¨AACt formula. Results are presented in Figure 20.
6.5. Reversible self-association of the 136 anti-ICOS antibody
[00868] Analytical ultracentrifugation analysis performed on 136 anti-ICOS
antibody
showed that the major peak of was broader than observed for a typical IgG. At
protein
concentrations of 0.1 mg/mL, 0.5 mg/mL, and 2.0 mg/mL, at 25 C in PBS, size
distribution
analysis showed a broadening of the peak and shift to higher S (sedimentation
coefficient;
Svedberg) values with increasing protein concentration, which is indicative of
self-association.
Sedimentation equilibrium experiments showed that the stoichiometry of the
self-association
best fit a monomer-trimer equilibrium model, with an association constant of
2.5 x 108 M-2.
[00869] Dynamic Light Scattering (DLS) was used to study 136 anti-ICOS
antibody self-
association and to screen for conditions/excipients to prevent or minimize
self association. The
DLS system determines the size of a particle by first measuring the Brownian
motion, which is
the random movement of particles suspended in a fluid. When a coherent and
monochromatic
light beam passes through a colloidal dispersion, the particles scatter the
light and because of
Brownian motion results in time-dependent fluctuation in the scattered
intensity. Analysis of the
time dependence of the intensity fluctuation can yield the diffusion
coefficient of the particles
which is dependent on the size of the particle. Hydrodynamic radius of the
particles is then
calculated from the diffusion coefficient via Stokes Einstein equation.
204
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
6.5.1. Methods
[00870] Dynamic Light Scattering: The protein size distribution and hence
molecular size
were monitored by dynamic light scattering (DLS) using a Zetasizer Nano ZS
(Malvern
Instruments, Malvern, PA). This instrument incorporated noninvasive
backscattering optics that
could measure protein sizes in the range of 0.6 nm to 6 jim. DLS measures the
time-dependent
fluctuations in the intensity of scattered light due to Brownian motion of the
protein molecules.
The analysis of these intensity fluctuations enabled the determination of the
diffusion
coefficients of particles, which were mathematically converted to an average
apparent
hydrodynamic diameter of an equivalent sphere using the Strokes Einstein
relationship. The
diffusion coefficient was calculated from the time correlation function. To
understand the
reversible self-association of proteins, the time-dependent autocorrelation
function of the
photocurrent was acquired every 10 seconds, with 15-18 acquisitions for each
run. The sample
solution was illuminated using a 633 nm laser, and the intensity of scattered
light was measured
at an angle of 173 degrees. After correcting for viscosity, refractive index
and absorbance, the
DLS measurements provided accurate estimates of both hydrodynamic diameter and
its Gaussian
distribution, which was used to monitor potential self-association behavior.
[00871] Data Analysis to Calculate Percent Monomer and Trimer Fraction:
Sedimentation equilibrium experiments showed that the stoichiometry of the
self-association
best fit a monomer-trimer equilibrium model. Hydrodynamic size obtained by DLS
under
various conditions was fitted to a monomer-trimer equilibrium to derive an
association constant
from which the mole percent of self-associated species was calculated under
various conditions.
Apparent size in DLS measurements was taken to be a weighted average of that
of monomer (9.0
nm) and trimer (35.6 nm), multiplied by their respective fractional presence
(from 0 to 1,
depending on the solution conditions). As the total concentration is known,
the concentration of
monomer equals:
[RH Obs RH Monomer]
"'Monomer PTotal D
1-111/¨Trimer RH¨Monomer]
The concentration of trimer is then:
P = [PMtal] [PMonomer]}
Trfmer
3
And the association constant (in M-2) is:
[PIHmer]
([PMonomerpi
205
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
Where, PTotal is 136 anti-ICOS antibody concentration in M, R H-Obs is
observed hydrodynamic
diameter in nm, R H-Monomer is hydrodynamic diameter of Monomer and R H-Trimer
is
hydrodynamic diameter of Trimer.
6.5.2. Data and Discussion
[00872] Reversible Self-Association (RSA) of proteins may result from
relatively weak
non-covalent protein interactions which is usually charge and hydrophobic
interactions. Since
the system is reversible, there will be equilibrium between the monomer and
higher order forms
and this equilibrium can be shifted depending on solution conditions. The
following section
describes the effect of changing protein concentration, pH, ionic strength and
temperature on self
association of 136 anti-ICOS antibody will be discussed.
6.5.2.1. Development of DLS as an effective tool to study RSA in 136 anti-ICOS
antibody
Correlation between DLS and AUC
[00873] Monoclonal antibodies (MW¨ 150 kDa) typically have a hydrodynamic
diameter
of 9-12 nm and a Gaussian distribution of ¨ 3 nm. A larger hydrodynamic
diameter and a wider
Gaussian distribution can each be indicative of self-association. Hence
hydrodynamic diameter
was monitored under various solution conditions to determine self association
behavior of 136
anti-ICOS antibody. Before studying the effect of various solution conditions
on RSA of 136
anti-ICOS antibody using DLS, we established the correlation between DLS and
well established
orthogonal technique (to study RSA) like AUC.
6.5.2.2.Hydrodynamic size of the 136 anti-ICOS antibody under various
conditions.
Effect of concentration
[00874] AUC analysis of the 136 anti-ICOS antibody had showed a broadening
of the
peak and shift to higher S (sedimentation coefficient; Svedberg) values with
increasing protein
concentration. In order to confirm these observations and also check if
similar self-association
can be picked up by DLS, the hydrodynamic diameter of the 136 anti-ICOS
antibody was
determined at various protein concentrations. Similar to AUC (an increase in
weight average
sedimentation coefficient) an increase in hydrodynamic diameter at higher
protein concentration
would be expected if significant self-association is present. Figure 21 shows
the hydrodynamic
diameter of the 136 anti-ICOS antibody over a wide range of concentrations. A
concentration
dependent increase in hydrodynamic diameter was observed in the wide range
studied. This
strongly indicates that the 136 anti-ICOS antibody is undergoing RSA at high
protein
206
CA 02743469 2011-05-11
WO 2010/056804 PCT/US2009/064127
concentration. Where as, a control non interacting monoclonal antibody of
similar size showed
no change in hydrodynamic diameter at the same concentration.
Effect of pH and Ionic strength
[00875] The 136 anti-ICOS antibody RSA was sensitive to increasing ionic
strength
(increasing NaCl concentration) and pH, suggesting the importance of charge
interactions.
Figure 22 shows the hydrodynamic size of the 136 anti-ICOS antibody for a
fixed concentration
(10 mg/ml) in presence of increasing NaC1 concentrations.
[00876] DLS measurements of the 136 anti-ICOS antibody at various pH
revealed that the
extent of self association also increased with pH (Figure 23). Significant
impact of both pH and
ionic strength suggested the importance of charge interactions in the 136 anti-
ICOS antibody
RSA.
Effect of Temperature
[00877] The 136 anti-ICOS antibody hydrodynamic size were determined under
various
temperatures to understand the effect of temperature on the 136 anti-ICOS
antibody RSA. Fig 24
shows the hydrodynamic size of the 136 anti-ICOS antibody at various
temperatures at pH 6 and
pH 7.2 along with a control antibody (mAbB).
Kinetics of Temperature induced Dissociation
[00878] The kinetics of dilution- or temperature-induced dissociation of
the 136 anti-
ICOS antibody was measured using two methods: rapid dilution by static light
scattering, and
temperature shift by DLS. Rapid dilution experiments with single angle light
scattering
detection demonstrated that the rate of dissociation is rapid with a half-time
lower than the
detection limit of 0.1 second. The kinetics of temperature-induced
dissociation of the 136 anti-
ICOS antibody self-association (10 mg/mL, in PBS) as measured by DLS is
presented in. With
a temperature increase from 25 C to 37 C, a substantial decrease in the
hydrodynamic diameter
was observed within the first measurable time point of 1 minute. A similar
decrease in the
hydrodynamic diameter was observed upon a temperature increase from 4 C to 37
C within the
first measurable time point of 3 minutes. These results indicated that
temperature-induced
dissociation was rapid.
6.5.2.3. Level of the 136 anti-ICOS antibody Self-association under various
solution condition
[00879] Dynamic light scattering studies showed an increase in the 136 anti-
ICOS
antibody hydrodynamic size (indicative of self-association) at higher
concentration, lower
temperature, higher pH, and increased salt concentration. Also, analytical
ultracentrifugation data
suggested a monomer-trimer equilibrium. Therefore, the 136 anti-ICOS antibody
hydrodynamic
207
CA 02743469 2016-06-13
^ 51332-100
size obtained under various condition was fitted to a monomer-trimer
equilibrium to derive an
association constant from which the mole percent of self-associated species
was calculated under
various conditions. Modeling was used to determine the mole percent of self-
associated 136 anti-
ICOS antibody from the DLS derived association binding affinities, as a
function of protein
concentration.
Table 2. Mole percent of self-associated 136 anti-ICOS antibody from
the DLS.
Fornmiation buffer comprises 10 mM histidine (pH 6.0), 80 mM NaC1, 4%
trehalose and 0.02%
polysorbate 80.
Protein Conc. Buffer Temperature Percent Monomer
Formulation Buffer 23 C 80
1 Formulation Buffer 23 C 99
0.01 Formulation Buffer 23 C 100
10 PBS 37 C 85
1 PBS 37 C 99
0.01 PBS 37 C 100
[00880] In conclusion, the reversible self-association of the 136 anti-
ICOS antibody
observed by analytical ultracentrifugation analysis was dependent on protein
concentration and
temperature. The kinetic studies indicated that rapid dissociation occurred
upon dilution and at
increased temperature. The reversible self-association did not induce
formation of aggregates.
On the basis of the rapid dilution experiments and modeling calculations, upon
subcutaneous
injection of the highest clinical dose (3 mg), the rapid dilution and
temperature equilibration to
37 C (body temperature) will result in a primarily monomeric form of the 136
anti-ICOS
antibody
[00881] Whereas, particular embodiments of the disclosure have been
described above for
purposes of description, it will be appreciated by those skilled in the art
that numerous variations
of the details may be made without departing from the disclosure as described
in the appended
claims.
[00882]
208
CA 02743469 2011-05-11
SEQUENCE LISTING IN ELECTRONIC FORM
In accordance with Section 111(1) of the Patent Rules, this
description contains a sequence listing in electronic form in ASCII
text format (file: 51332-100 Seq 02-MAY-11 vl.txt).
A copy of the sequence listing in electronic form is available from
the Canadian Intellectual Property Office.
The sequences in the sequence listing in electronic form are
reproduced in the following table.
SEQUENCE TABLE
<110> MedImmune LLC
Sathish, Hasige
Shah, Ambarish
Carlesso, Gianluca
Delaney, Tracy
<120> Antibody Formulation
<130> IC320PCT
<140> PCT/U52009/064127
<141> 2009-11-12
<150> 61/113,796
<151> 2008-11-12
<150> 61/249,365
<151> 2009-10-07
<160> 10
<170> PatentIn version 3.5
<210> 1
<211> 214
<212> PRT
<213> Homo sapiens
<400> 1
Asp Ile Gin Met Thr Gin Ser Pro Ser Ser Val Ser Ala Ser Val Gly
1 5 10 15
Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gin Gly Ile Ser Arg Leu
20 25 30
Leu Ala Trp Tyr Gin Gin Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile
35 40 45
Tyr Val Ala Ser Ser Leu Gin Ser Gly Val Pro Ser Arg Phe Ser Gly
50 55 60
Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gin Pro
65 70 75 80
208a
CA 02743469 2011-05-11
Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ala Asn Ser Phe Pro Trp
85 90 95
Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys Arg Thr Val Ala Ala
100 105 110
Pro Ser Val Phe Ile Phe Pro Pro Ser Asp Glu Gln Leu Lys Ser Gly
115 120 125
Thr Ala Ser Val Val Cys Leu Leu Asn Asn Phe Tyr Pro Arg Glu Ala
130 135 140
Lys Val Gln Trp Lys Val Asp Asn Ala Leu Gln Ser Gly Asn Ser Gln
145 150 155 160
Glu Ser Val Thr Glu Gln Asp Ser Lys Asp Ser Thr Tyr Ser Leu Ser
165 170 175
Ser Thr Leu Thr Leu Ser Lys Ala Asp Tyr Glu Lys His Lys Val Tyr
180 185 190
Ala Cys Glu Val Thr His Gln Gly Leu Ser Ser Pro Val Thr Lys Ser
195 200 205
Phe Asn Arg Gly Glu Cys
210
<210> 2
<211> 107
<212> PRT
<213> Homo sapiens
<400> 2
Asp Ile Gln Met Thr Gln Ser Pro Her Her Val Ser Ala Ser Val Gly
1 5 10 15
Asp Arg Val Thr Ile Thr Cys Arg Ala Ser Gln Gly Ile Ser Arg Leu
20 25 30
Leu Ala Trp Tyr Gln Gln Lys Pro Gly Lys Ala Pro Lys Leu Leu Ile
35 40 45
Tyr Val Ala Ser Ser Leu Gln Ser Gly Val Pro Ser Arg Phe Ser Gly
50 55 60
Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Ser Leu Gln Pro
65 70 75 80
Glu Asp Phe Ala Thr Tyr Tyr Cys Gln Gln Ala Asn Ser Phe Pro Trp
85 90 95
Thr Phe Gly Gln Gly Thr Lys Val Glu Ile Lys
100 105
<210> 3
<211> 11
<212> PRT
<213> Homo sapiens
<400> 3
Arg Ala Ser Gln Gly Ile Ser Arg Leu Leu Ala
1 5 10
<210> 4
<211> 7
<212> PRT
<213> Homo sapiens
20 8b
CA 02743469 2011-05-11
<400> 4
Val Ala Ser Ser Leu Gin Ser
1 5
<210> 5
<211> 9
<212> PRT
<213> Homo sapiens
<400> 5
Gin Gin Ala Asn Ser Phe Pro Trp Thr
1 5
<210> 6
<211> 455
<212> PRT
<213> Homo sapiens
<400> 6
Gin Val Gin Leu Val Gin Ser Gly Ala Glu Val Lys Lys Pro Gly Ala
1 5 10 15
Ser Val Lys Val Ser Cys Lys Ala Ser Gly Tyr Thr Phe Thr Gly Tyr
20 25 30
Tyr Met His Trp Val Arg Gin Ala Pro Gly Gin Gly Leu Glu Trp Met
35 40 45
Gly Trp Ile Asn Pro His Ser Gly Gly Thr Asn Tyr Ala Gin Lys Phe
50 55 60
Gin Gly Arg Val Thr Met Thr Arg Asp Thr Ser Ile Ser Thr Ala Tyr
65 70 75 80
Met Glu Leu Ser Arg Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Thr Tyr Tyr Tyr Asp Ser Ser Gly Tyr Tyr His Asp Ala Phe
100 105 110
Asp Ile Trp Gly Gln Gly Thr Met Val Thr Val Ser Ser Ala Ser Thr
115 120 125
Lys Gly Pro Ser Val Phe Pro Leu Ala Pro Ser Ser Lys Ser Thr Ser
130 135 140
Gly Gly Thr Ala Ala Leu Gly Cys Leu Val Lys Asp Tyr Phe Pro Glu
145 150 155 160
Pro Val Thr Val Ser Trp Asn Ser Gly Ala Leu Thr Ser Gly Val His
165 170 175
Thr Phe Pro Ala Val Leu Gin Ser Ser Gly Leu Tyr Ser Leu Ser Ser
180 185 190
Val Val Thr Val Pro Ser Ser Ser Leu Gly Thr Gin Thr Tyr Ile Cys
195 200 205
Asn Val Asn His Lys Pro Ser Asn Thr Lys Val Asp Lys Arg Val Glu
210 215 220
Pro Lys Ser Cys Asp Lys Thr His Thr Cys Pro Pro Cys Pro Ala Pro
225 230 235 240
Glu Leu Leu Gly Gly Pro Ser Val Phe Leu Phe Pro Pro Lys Pro Lys
245 250 255
Asp Thr Leu Met Ile Ser Arg Thr Pro Glu Val Thr Cys Val Val Val
260 265 270
Asp Val Ser His Glu Asp Pro Glu Val Lys Phe Asn Trp Tyr Val Asp
275 280 285
208c
CA 02743469 2011-05-11
Gly Val Glu Val His Asn Ala Lys Thr Lys Pro Arg Glu Glu Gin Tyr
290 295 300
Asn Ser Thr Tyr Arg Val Val Ser Val Leu Thr Val Leu His Gin Asp
305 310 315 320
Trp Leu Asn Gly Lys Glu Tyr Lys Cys Lys Val Ser Asn Lys Ala Leu
325 330 335
Pro Ala Pro Ile Glu Lys Thr Ile Ser Lys Ala Lys Gly Gin Pro Arg
340 345 350
Glu Pro Gin Val Tyr Thr Leu Pro Pro Ser Arg Glu Glu Met Thr Lys
355 360 365
Asn Gin Val Ser Leu Thr Cys Leu Val Lys Gly Phe Tyr Pro Ser Asp
370 375 380
Ile Ala Val Glu Trp Glu Ser Asn Gly Gin Pro Glu Asn Asn Tyr Lys
385 390 395 400
Thr Thr Pro Pro Val Leu Asp Ser Asp Gly Ser Phe Phe Leu Tyr Ser
405 410 415
Lys Leu Thr Val Asp Lys Ser Arg Trp Gin Gin Gly Asn Val Phe Ser
420 425 430
Cys Ser Val Met His Glu Ala Leu His Asn His Tyr Thr Gin Lys Ser
435 440 445
Leu Ser Leu Ser Pro Gly Lys
450 455
<210> 7
<211> 125
<212> PRT
<213> Homo sapiens
<400> 7
Gin Val Gin Leu Val Gin Ser Gly Ala Glu Val Lys Lys Pro Gly Ala
1 5 10 15
Ser Val Lys Val Ser Cys Lys Aid Ser Gly Tyr Thr Phe Thr Gly Tyr
20 25 30
Tyr Met His Trp Val Arg Gin Ala Pro Gly Gin Gly Leu Glu Trp Met
35 40 45
Gly Trp Ile Asn Pro His Ser Gly Gly Thr Asn Tyr Ala Gin Lys Phe
50 55 60
Gin Gly Arg Val Thr Met Thr Arg Asp Thr Ser Ile Ser Thr Ala Tyr
65 70 75 80
Met Glu Leu Ser Arg Leu Arg Ser Asp Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Thr Tyr Tyr Tyr Asp Ser Ser Gly Tyr Tyr His Asp Ala Phe
100 105 110
Asp Ile Trp Gly Gin Gly Thr Met Val Thr Val Ser Ser
115 120 125
<210> E3
<211> 5
<212> PRT
<213> Homo sapiens
<400> 8
Gly Tyr Tyr Met His
1 5
208d
CA 02743469 2011-05-11
<210> 9
<211> 17
<212> PRT
<213> Homo sapiens
<400> 9
Trp Ile Asn Pro His Ser Gly Gly Thr Asn Tyr Ala Gin Lys Phe Gin
1 5 10 15
Gly
<210> 10
<211> 16
<212> PRT
<213> Homo sapiens
<400> 10
Thr Tyr Tyr Tyr Asp Ser Ser Gly Tyr Tyr His Asp Ala Phe Asp Ile
1 5 10 15
208e