Note: Descriptions are shown in the official language in which they were submitted.
CA 02743826 2011-05-16
WO 2010/065130 PCT/US2009/006387
INDUSTRIAL AND AUTOMOTIVE GREASE AND PROCESS FOR ITS
MANUFACTURE
FIELD OF THE INVENTION
10011 This invention relates to a lubricating grease composition
suitable for
industrial and automotive uses, and a process for its manufacture. In
particular,
the invention relates to a premium multipurpose grease composition exhibiting
favorable water resistant properties, high and low temperature performance,
and
which is suitable for use in both industrial and automotive applications.
BACKGROUND OF THE INVENTION
[002] In North America and other northern climates, it is desirable for a
lubricating grease to exhibit good performance over a wide range of
temperatures. In addition, industrial greases often require good performance
in
wet environments. Testing methods and performance criteria established by the
National Lubricating Grease Institute (NLGI) have become industry-wide
accepted standards. These standards include greases for use in automotive
applications. It is therefore desirable for lubricating grease to meet NLGI
grade
classification. Preferably, such greases should be multipurpose, being
suitable
_ _
for industrial applications and meet NLGI grade classification for automotive
_
_ _ _
application.
[003] There is a need to improve the water resistance and oxidation life of
commercial premium greases that exhibit good high and low temperature
performance. There is a further need to provide enhanced life expectancy and
better overall performance in wet applications over current lubricants and
CA 02743826 2011-05-16
WO 2010/065130
PCT/US2009/006387
improved performance in relation to low temperature torque and fretting wear.
Accordingly, embodiments of this invention satisfy these needs.
SUMMARY OF THE INVENTION
10041 In one embodiment, this invention relates to a premium multipurpose
lubricating grease suitable for industrial and automotive uses, and a process
for
making the same. In this embodiment, a lubricating grease is disclosed
comprising:
(a) at least one Group I oil;
(b) at least one Group II oil;
(c) a hydrophilic copolymer; and
(d) a soap thickener,
wherein the thickener is dispersed into the at least one Group I oil during a
cooking phase and the at least one Group II oil is introduced during a
finishing
phase.
[005] In a second embodiment, a method for making a lubricating grease is
disclosed. The method comprises: obtaining at least one Group I oil, obtaining
at least one Group II oil, obtaining i hydfophili& c-opolym&r, obtaining a
soap
thickener, and mixing the at least one Group I oil, the least one Group II
oil, the
polymer and the soap thickener to form a grease wherein the thickener is
dispersed into the at least one Group I oil during a cooking phase and at
least one
Group II oil is introduced during a finishing phase.
2
CA 02743826 2016-03-07
BRIEF DESCRIIMON OF "1-1 1E 1)RA WINGS
[006] Fig. 1 is a graph illustrating the favorable low temperature torque
properties of the inventive example over the comparative examples, as measured
by ASTM D4693;
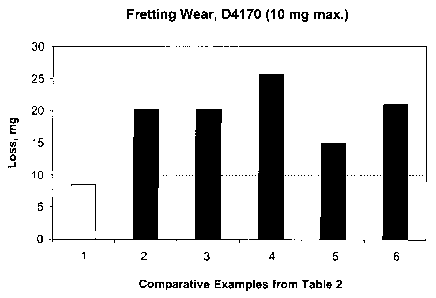
[007] Fig. 2 is a graph illustrating the favorable fretting wear properties
of the
inventive example over the comparative examples, as measured by ASTM
D4170;
[008] Fig. 3 is a graph illustrating the favorable wheel bearing life
properties
of the inventive example over the comparative examples, as measured by ASTM
D3527;
[009] Fig. 4 is a graph illustrating the favorable water spray off
properties of
the inventive example over the comparative examples, as measured by ASTM
D4049.
DETAILED DESCRIPTION OF THE PREFERRED FM,1301)1mtNrs
[010] The present invention will be described in connection with its
preferred
embodiments. However, to the extent that the following description is specific
to a particular embodiment or a particular use of the invention, this is
intended to
be illustrative only. On the contrary, it is intended to cover all
alternatives,
modifications, and equivalents. The scope of the claims should not be limited
by
particular embodiments set forth herein, but should be construed in a manner
consistent with the specification as a whole.
3
CA 02743826 2011-05-16
WO 2010/065130 PCT/US2009/006387
[011] In one embodiment, we have invented novel greases that are suitable
for use in industrial applications, and at the same time meets NLGI grade
classification for use in automotive applications. The compositions of the
greases and methods of manufacturing the greases are disclosed herein.
10121 Various lubricating oils can be employed in preparing the grease
compositions of the present invention. Applicants have found that using oils
of a
certain type during the cooking phase of the grease preparation, and oils of a
different type during the finishing phase achieved a grease with favorable
properties. Another embodiment of the present invention is the inclusion of a
polymer that imparts excellent water resistance properties without
compromising
the low temperature performance of the grease. Applicants have found that
using a hydrophilic polymer provided favorable properties.
[013] Groups I, II, III, IV and V are broad categories of base oil stocks
developed and defined by the American Petroleum Institute (API Publication
1509; www.API.org) to create guidelines for lubricant base oils. Group I base
stocks generally have a viscosity index of between about 80 to 120 and contain
greater than about 0.03% sulfur and/or less than about 90% saturates. Group II
base stocks generally have a viscosity index of between about 80 to 120, and
contain less than or equal to about 0.03% sulfur and greater than or equal to
about 90% saturates. Group III stock generally has a viscosity index greater
than -
about 120 and contains less than or equal to about 0.03 % sulfur and greater
than
about 90% saturates. Group IV includes polyalphaolefins (PAO). Group V base
stocks include base stocks not included in Groups I-IV. Table 1 summarizes
properties of each of these five groups.
4
CA 02743826 2011-05-16
WO 2010/065130
PCT/US2009/006387
Table 1: Base Stock Properties
Saturates Sulfur Viscosity Index
Group I <90% and/or > 0.03% and 80 and < 120
Group II 90% and 0.03% and 80 and < 120
Group III 90% and 0.03% and 120
Group IV Polyalphaolefins (PAO)
Group V All other base oil stocks not included in Groups I, II, III, or
IV
[014] In a preferred embodiment, the base stocks include at least one base
stock of synthetic oils. Synthetic oil for purposes of this application shall
include all oils that are not naturally occurring mineral oils.
[015] In general, lubricating oils will typically comprise between 50 - 90
wt%
of the overall grease composition. These oils will typically combine to
provide
an overall viscosity of the grease in the range of ISO 100 to ISO 320. The
preferred viscosity for the present invention is between ISO 150 to ISO 275,
with ISO 220 being the most preferred.
[016] Lubricating oils used during the cooking and finishing phases can be
either mineral or synthetic. Mineral oils can be any conventionally refined
base
stocks derived from paraffinic, naphthenic and mixed based crudes. Synthetic
lubricating oils that can be used include esters of glycols such as a Co oxo
acid
diester of tetraethylene glycol, or complex esters such as one formed from 1
mole of sebacic acid and 2 moles of tetraethylene glycol and 2 moles of 2-
ethylhexanoic acid. Other synthetic oils that can be used include synthetic
hydrocarbons such as polyalphaolefins; alkyl benzenes, e.g. alkylate bottoms
from the alkylation of benzene with tetrapropylene, or the copolymers of
ethylene and propylene; silicon oil, e.g. ethyl phenyl polysiloxanes, methyl
CA 02743826 2011-05-16
WO 2010/065130
PCT/US2009/006387
polysiloxanes, etc.; polyglycol oils, e.g. those obtained by condensing butyl
alcohol with propylene oxide; carbonate esters, e.g. the product of reacting
C8
oxo alcohol with ethyl carbonate to form a half ester followed by reaction of
the
latter with tetraethylene glycol, etc. Other suitable synthetic oils include
the
polyphenyl esters, e.g. those having from about 3 to 7 ether linages and about
4
to 8 phenyl groups.
10171 The lubricating oils used as the base stock in the cooking phase of
the
manufacturing process is preferably selected from Group I and Group V oils.
These oils will have a preferred viscosity in the range of 200 to 1400 cSt at
40 C, with a range of 200 to 500 cSt at 40 C being most preferred. A
combination of heavy naphthenic oil and a bright stock was preferred as the
base
stock during the cooking phase, with 5 - 15 wt% heavy naphthenic oil and 30 -
40 wt% bright stock being the preferred amounts. The use of Group II oils
during the cooking phase should be limited or avoided altogether. Group II
oils
may be combined with other lubricating oils during the finishing phase. A
combination of 5 - 10 wt% of an ISO 68 Group I and 20 - 30 wt% of an ISO 100
Group II was preferred during the finishing phase. Various other oils in
smaller
amounts may also be incorporated during the finishing phase.
10181 The grease composition will also contain a thickener dispersed in
the
¨ lubricating oil during the cooking phase to-form a base grease. The
thickener - -
will typically comprise between 5% and 15% of the overall grease composition
weight. The particular thickener employed is not critical and can vary broadly
provided that it is effectively water insoluble. For example, the thickener
may
be based on aluminum, barium, calcium or lithium soaps, or their complexes.
Soap thickeners may be derived from a wide range of animal oils, vegetable
oils
and greases, as well as the fatty acids derived therefrom. Carbon black,
silica,
and clays may be used as well as dyes, polyureas and other organic thickeners.
6
CA 02743826 2016-03-07
Pyrrolidone-based thickeners can also be used. Preferred thickeners are based
on lithium soap, calcium soap, their complexes, or mixtures thereof
Particularly
preferred is a lithium or lithium complex thickener derived from reacting a C-
18
fatty acid (12-hydroxy stearic acid) and a C-9 dicarboxylic acid (azelaic
acid)
with lithium hydroxide monohydrate. Canadian Patent 996537 provides a
process for making this preferred thickener.
[019] In one inventive embodiment, during the cooking phase of a preferred
embodiment of the grease preparation, a mixture of Group I and Group V oils
and a lithium soap of a C12 to C24 hydroxy fatty acid is first prepared. Then
a C2
to C12 aliphatic carboxylic acid is added to that mixture and converted to its
dilithium soap under conditions that are suitable for the formation of a
complex
between the lithium soap of the dicarboxylic acid and the lithium soap of the
hydroxy fatty acid. While the lithium soap of the hydroxy fatty acid could be
preformed and then dispersed in the lubricating oil medium, it is generally
more
expedient to prepare that soap in situ in the lubricating oil by neutralizing
the
hydroxy fatty acid with lithium base. The usual procedure during the cooking
phase is to charge into the grease kettle the Group I and Group V oils and to
then
add the hydroxy fatty acid. The mixture of fatty acid and oil is heated
sufficiently to bring about the dissolving action, e.g. at about 180 to 200 F.
Then a concentrated aqueous solution of the lithium base is added, usually in
an
amount slightly in excess of that required to neutralize the acid. The
temperature
at this stage of the cooking phase is usually between 200 and 210 F. The rate
of
addition of the lithium base may be varied. It is possible at this stage to
proceed
with the addition of the dicarboxylic acid and its subsequent neutralization
to its
dilithium soap, but this will require the neutraliztion to be conducted slowly
or
stepwise so as to ensure complexing of the two soaps with each other before
the
complete neutralization of the dicarboxylic acid has been brought about.
7
CA 02743826 2011-05-16
WO 2010/065130
PCT/US2009/006387
Accordingly, before proceeding with the addition of dicarboxylic acid and
conversion to its dilithium soap, it is preferred that the temperature of the
mixture of the Group I and Group V oils and lithium soap of the hydroxy fatty
acid be raised to between 250 and 300 F. This is done in order to bring about
a
substantial dehydration of the mixture, such as, the removal of 70 to 100% of
the
water. As noted in Canadian Patent 996537, substantial dehydration at this
stage
also promotes the subsequent complexing reaction during the neutralization of
the dicarboxylic acid. After substantial dehydration has been brought about,
the
mixture is cooled to between 230 and 240 F and the dicarboxylic acid is added
to the mixture. The mixture is stirred in order to bring about proper
dispersion
of the acid throughout the mixture and the concentrated aqueous solution of
lithium base is then added to convert the dicarboxylic acid to its dilithium
soap.
Similarly with the neutralization of the fatty acid, the amount of lithium
base
added at this stage is slightly in excess of the amount required to neutralize
both
acid groups of the dicarboxylic acid. The temperature during this stage should
preferably be maintained between 210 and 230 F, and more preferably between
220 to 230 F.
10201 After all of the lithium base has been added to complete the
neutralization of the dicarboxylic acid, the temperature of the grease mixture
is
once again raised in order to bring about dehydration. Preferably this will
take
place atabout 280 to 300 F. Following dehydration of the- mixture, in order to
-
ensure optimal thickener dispersion, the temperature of the mixture should
further be raised to preferably between 380 and 400 F. The soap stock is then
cooled during the finishing phase of the grease preparation. Finishing oils,
including Group II oils and various other lubricating oils, may be added into
the
mixture at this point. Mixing may continue until the grease has reached
ambient
temperatures. When the temperature has been lowered to about 150 F, other
8
CA 02743826 2016-03-07
grease additives can be introduced as would be understood by persons skilled
in
the art.
[021] As mentioned previously, one embodiment contemplates the inclusion
of a polymer. Various polymers may be used in greases, although the precise
impact of any given polymer on a given grease cannot be predicted. Applicants
have found that the use of a hydrophilic copolymer was important in achieving
excellent water resistance properties. In a preferred embodiment of the
present
invention, maleic anhydride styrene esterified copolymer is used, with the
preferred amount being between 2 and 6 wt% of the overall grease. The
polymer may be incorporated during either the cooking phase or finishing phase
of the grease preparation.
[022] The preferred styrene malcic anhydride ester (SMAE) copolymer is
unique from other polymer examples because it contains oxygen groups. The
structure of the SMAE copolymer (shown below) has exposed hydroxyl and
carbonyl groups that can act as hydrogen bond donors (former) and acceptors
(latter). As a result, the SMAE copolymer is more hydrophilic than strictly
hydrocarbon-based copolymers such as styrene isoprene and styrene isobutylene.
Eq. 1
OH OCnH
0
CH, CH¨CH, -CH¨ CnH2rv-i OH
¨ I
'H
tr.
CH¨CH 1
Or' MEK, ET 3N. Ar 2 reflux 48 hr
SMA SMA Ester
[023] Equation 1 shows the chemical structure of the SMA ester and the
esterification of styrene maleic anhydride copolymer to form a SMA ester and
9
CA 02743826 2011-05-16
WO 2010/065130 PCT/US2009/006387
the resulting chemical structure of the SMA ester. The grease structure is a
type
of soap. The ability of the soap to dissolve in waters varies. Preferably, the
grease soap should not readily dissociate in contact with water.
[024] The grease soap structure is held together with a variety of bonds,
including ionic bonds with the metal, hydrogen bonds within the oxygen-rich
triglyceride and the ester function of 12-hydroxy stearic acid (once
incorporated
into the structure) and van der Waals interactions between the C-C side
chains.
When a grease is exposed to water, bond networks may be disrupted and the
grease's structural stability may be compromised. This can result in poor
performance in water resistance tests.
[025] A polymer that incorporates or binds water molecules into its
structure
may enhance the water resistance performance of a grease. Hydrogen bonding
capability present in certain copolymers. For example, SMAE can improve their
ability to incorporate or bind water molecules into their respective
structures.
The water resistance performance of a grease may be improved where the
copolymer provides preferential binding of the water, such as, the attraction
of
water to the copolymer, through hydrogen bonding, is stronger than the
attraction of water to the grease structure.
[026]
The grease may also contain-sthall amounts of supplemental - additives, -
which include antioxidants, anti-wear agents and other additives. Specific
antioxidants employed are not critical and can vary broadly to achieve
favorable
properties. A combination of a Group II oil and diphenylamine antioxidant was
found to enhance the oxidation life of the grease, while achieving good high
temperature performance. Antioxidants will typically comprise less than 5 wt%
of the overall grease composition. The total amount of all additives,
including
the antioxidant, will typically be between 2 - lOwt% of the overall grease. A
CA 02743826 2011-05-16
WO 2010/065130
PCT/US2009/006387
person skilled in the art will recognize the benefits of adding specific
additives
to the grease disclosed herein to achieve favorable properties.
[027] This invention will be further understood by reference to the
following
tables and examples, which describes the preferred embodiment of the
"Invention". Figures 1 through 4, collectively, illustrate the better overall
performance of the inventive example 1 over the comparative examples with
respect to low temperature torque, fretting water, wheel bearing life, and
water
spray off. The data, illustrated in bar chart form in the figures, is shown in
chart
form in Table 2 below.
Examples
[028] The examples in table 2 below disclose various screening tests for
the
influence of the base oils and thickener on overall grease performance. The
grease performance tests include water spray-off, low temperature torque,
fretting wear and wheel bearing life.
[029] The results in Table 2 demonstrate that changes to the oils in either
the
cooking phase or the finishing phase will not yield a grease meeting all of
the
necessary NLGI performance criteria for automotive use.
11
CA 027 43826 2011-05-16
WO 2010/065130
PCT/US2009/006387
Table 2
EXAMPLE # 1 2 3 4 5 6
9
Invention 8 IP2007279/
12-52-07 1P2007281/ B GR1004- 13-3 11-
01-07
BATCH # 1P2007455 GR1004-152 1P2007193 158
1P2007443 1P2007411
TOTAL BASE OILS
-
ISO 100 Group ll 24 0 43.5 36.4 33.3 27.8
Heavy Naphthenic 10.2 11.7 0 7.8 10.4 32.9
ISO 68 Group I 8.7 16.6 0 0 0 0
Bright Stock 35.6 47.5 30.3 32.3 33.5 17.1
ISO 22 Naphthenic 0 4.1 0 0 0 0
Kinematic Viscosity@40 C, cst 206 238 185 211 223
201
VI 93 91 98 94 93 79
'Aniline Pt, C 119 115 125 122 122 112
COOK OILS
. ISO 100 Group II 0 0 43.5 35.9 0 0
.
Heavy Naphthenic 10.2 11.7 0 7.8 10.4 32.9
ISO 68 Group I 0 11.8 0 0 0 0
Bright Stock 31.7 13.2 0 0 33.5 11.9
ISO 22 Naphthenic 0 4.1 0 0 0 0
Kinematic Viscosity@40 C, cst 405 163 104 123 440
306
VI 89 83 99 92 89 60
Aniline Pt, C 119 107 124 119 120 102
FINISHING OILS
ISO 100 Group II 24 0 0 0.9 33.3 27.8
Heavy Naphthenic 0 0 0 0 0 0
ISO 68 Group I 8.7 4.8 0 0 0 0
Bright Stock 3.9 34.3 30.3 32.3 0 5.2
ISO 22 Naphthenic 0 0 0 0 0 0
Kinematic Viscosity@40 C, cst,
calc 105 350 183 448 104 128
Aniline Pt, C, calc 120 124 125 127 124 125
- _ADDITIVES _
Antioxidant (diphenylamine) 1.5 1.5 1.5 1.5 1.5 1.5
Polymer A 3 . 3 3 3 3 3
Polymer B 0 0 . 0 0 0 0.3
PERFORMANCE
Water Spray-off, D4049, wt%
(35 max.) 26 31 37 67 35 21
Low Temp Torque, D4693, n-m
(15.5 max.) 11.4 11.9 8.2 9.8 9.6 17.2
Fretting Wear, D4170, mg
(10 max.) 8.5 20.3 20.3 25.7 15 21
Wheel Bearing Life, 03527, hr
(125 mm.) 153 47 130 150
12
CA 02743826 2011-05-16
WO 2010/065130 PCT/US2009/006387
10011 Table 3 below discloses screening test for the influence of the
polymer
selection and concentration on overall grease performance. These tests
includes water
spray-off, wet roll, water washout, low temperature torque, fretting wear,
wheel bearing
life and apparent viscosity.
10021 The
results in Table 3 demonstrate that using 3 wt% Polymer A or maleic
anhydride styrene ester copolymer exhibits excellent water resistance
performance and .
also meets the other key performance parameters.
Table 3
EXAMPLE # 1 7 8 9 10 11 12
BATCH # IOL Invention GR1004 GR1004
11- GR1004 GR1003 GR1003
target 12-57-07 -151 -150 01-07 -22 -743
-741
BASE OILS
ISO 100 Group II 24 24 94 27.8 32.9
Heavy Naphthenic 10.2 10.2 10.2 32.9
ISO 68 Group I 8.7 8.7 8.7
Bright Stock 35.6 35.6 35.6 17.1 39.8 36.7 36.7
IOS 100 Group I 30.0
29.7
ADDITIVES
Polymer A 3 0 6 3 2.8
Polymer B 0 0 0 0.3 0.5
Polymer C -
9.2
Polymer D
Polymer E 4.5
4.5 .
PERFORMANCE
Water Spray-off, D4049 wt% 35 max 26 40 21 21 23
96 24
Wet roll, PQP3.05-1977, 24h, 0 to 35 35 31 28 9 61 '
-38 50
60C. Delta pen
Water Washout, Dl 264, wt% 3 max 2.1 2.4
Low Temp Torque, D4693, 15.5 11.4 13.0 9.23 17.2 12.4
n-m max
Fretting Wear, D4170, mg 10 max 8.5 9.0 10.7 21 9.3
Wheel Bearing Life D3527, hr 125 min 153 150
Apparent Viscosity, D1092 @ - 3500 2720 3600
IOC, cP @ 20s-I max
13
SUBSTITUTE SHEET (RULE 26)
CA 02743826 2011-05-16
WO 2010/065130
PCT/US2009/006387
Table 3¨ Continued
EXAMPLE # 13 14 15 16 17 18
BATCH # GR1003- GR1003 GR1003 GR1003 GR1003 GR1003
742 -702 -701 -861 -862 -863
BASE OILS
ISO 100 Group II
Heavy Naphthenic
ISO 68 Group I
Bright Stock 37.0 39.9 39.9 26 25.9 26.3
IOS 100 Group I 32.0 32.7 32.7 31.0 30.9 31.4
ADDITIVES
Polymer A
Polymer B
Polymer C 0.58 0.29 0
Polymer D 6.4 0.9 0.43
Polymer E 4.5 4.0 4.0 4.0
PERFORMANCE
Water Spray-off, D4049 24 37 35 25 17 29
wt%
Wet roll, PQP3.05-1977, 40 30 -20 -5 -47 4
24h, 60C. Delta pen
Water Washout, D1264, 1.5 2.2 1.7
wt%
Low Temp Torque, 21.1 26.8 20.5
D4693, n-m
Fretting Wear, D4170, mg 10.1 14.5 15.6
Wheel Bearing Life
D3527, hr
Apparent Viscosity, 4832 4375 4150
D1092 @ -10C, cP @
20s-1
14
SUBSTITUTE SHEET (RULE 26)
CA 02743826 2011-05-16
WO 2010/065130 PCT/US2009/006387
Legend 3a (Base Oil) Legend 3b (Polymer)
Polymer Polymer type
Base Oil Typical viscosity @ 40 C, cSt
ISO 100 Group 100
Polymer A maleic anhydride styrene ester
copolymer
II
Heavy Naphthenic 290 - 390 Polymer B styrene isobutylene
copolymer
ISO 68 Group I 68 Polymer C hydrogenated styrene-
isoprene copolymer
Bright Stock 480 Polymer D hydrogenated styrene-
isoprene star copolymer
ISO 100 Group I 100 Polymer E functionalized olefin
copolymer
=
SUBSTITUTE SHEET (RULE 26)
CA 027 4382 6 2011-05-16
WO 2010/065130 PCT/US2009/006387
10321 Table 4 discloses screening tests for the influence of various
antioxidants on grease performance for wheel bearing life. The results of
Table
4 demonstrate that a combination of an ISO 100 Group II base oil and a
diphenylamine antioxidant achieved good high temperature performance and
oxidation life.
Table 4
EXAMPLE # 1 19 20 21 22
Invention GR1004- GR1004- GR1004- Batch 8
BATCH # 12-52-07 156-2 156-3 156-1
GR1004-152
BASE OILS
ISO 100 Group II 24 24 24 24 0
Heavy Naphthenic 10.2 10.2 10.2 10.2 11.7
ISO 68 Group I 8.7 8.7 8.7 8.7 16.6
Bright Stock 35.6 35.6 35.6 35.6 47.5
ISO 22 Naphthenic 0 0 0 0 4.1
ADDITIVES
Polymer A 3 3 3 3 3
Antioxidant (diphenylamine) 1.5 0.76 0.30 0 1.5
PERFORMANCE
Wheel Bearing Life, D3527,
hr (125 min.) 153 134 130 80 47
LEGEND
, Base Oil Typical viscosity @40 C, cSt
ISO 100 Group II 100
Heavy Naphthenic 290 -390
ISO 68 Group I 60
Bright Stock 480
ISO 22 Naphthenic 20
16