Note: Descriptions are shown in the official language in which they were submitted.
CA 02745090 2016-04-07
WO 2010/077328
PCT/US2009/006677
SYSTEM AND/OR METHOD FOR GLUCOSE SENSOR CALIBRATION
BACKGROUND
Field:
The subject matter disclosed herein relates to calibration of glucose sensors
for use in
glucose monitoring systems, for example.
Information:
Over the years, body characteristics have been determined by obtaining a
sample of
bodily fluid. For example, diabetics often test for blood glucose levels.
Traditional blood
glucose determinations have utilized a painful finger prick using a lancet to
withdraw a small
blood sample. This results in discomfort from the lancet as it contacts nerves
in the
subcutaneous tissue. The pain of lancing and the cumulative discomfort from
multiple needle
pricks is a strong reason why patients fail to comply with a medical testing
regimen used to
determine a change in a body characteristic over a period of time. Although
non-invasive
systems have been proposed, or are in development, none to date have been
commercialized that
are effective and provide accurate results. In addition, all of these systems
are designed to
provide data at discrete points and do not provide continuous data to show the
variations in the
characteristic between testing times.
A variety of implantable electrochemical sensors have been developed for
detecting
and/or quantifying specific agents or compositions in a patient's blood. For
instance, glucose
sensors are being developed for use in obtaining an indication of blood
glucose levels in a
diabetic patient. Such readings are useful in monitoring and/or adjusting a
treatment regimen
which typically includes the regular administration of insulin to the patient.
Thus, blood glucose
readings improve medical therapies with semi-automated medication infusion
pumps of the
external type, as generally described in U.S. Pat. Nos. 4,562,751; 4,678,408;
and 4,685,903; or
automated implantable medication infusion pumps, as generally described in
U.S. Pat. No.
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
4,573,994. Typical thin film sensors are described in commonly assigned U.S.
Pat. Nos.
5,390,671; 5,391,250; 5,482,473; and 5,586,553. See also U.S. Pat. No.
5,299,571.
SUMMARY
Briefly, one embodiment relates to a method, system and/or apparatus for
obtaining samples of an electrical signal generated by a sensor, said samples
having sample
values associated with measurements of a blood-glucose concentration;
individually weighting at least some of said sample values according to a
function of blood
glucose reference samples associated with said sample values; and
estimating a relationship of sample values with said blood-glucose
concentration based, at least
in part, on said individually weighted samples.
In another implementation estimating said relationship comprises estimating a
linear
relationship between said sample values and said blood-glucose concentration
based, at least in
part, on a linear regression of said weighted samples and associated blood-
glucose reference
values. Here, for example, such estimating said linear relationship may
further comprise
calculating a linear regression sensitivity ratio based, at least in part, on
said weighted samples
and associated blood-glucose reference values; selecting an offset based, at
least in part, on said
calculated linear regression sensitivity ratio; and calculating a modified
linear regression
sensitivity ratio based, at least in part, on said selected offset, said
weighted samples and said
associated blood-glucose reference values.
In another particular implementation the function of blood glucose reference
samples is
based, at least in part, on a measure of statistical dispersion of said sample
values as function of
associated blood glucose reference samples. Here, for example,
said measure of statistical dispersion may comprise a variance and/or
approximation of a
variance of said sample values as a function of said associated blood glucose
reference samples.
Alternatively, the function may comprise an inverse of said measure of
statistical dispersion of
said sample values. In yet another alternative, the method includes estimating
a linear
relationship of said measure of statistical dispersion of said sample values
versus blood glucose
concentration; and deriving the function based, at least in part, on said
linear relationship.
In another particular implementation, individually weighting said at least
some of said
sample values further comprises further weighting said samples based on how
recently said
samples are obtained.
2
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
In another particular implementation, the method includes detecting a failure
of said
sensor based, at least in part, on a change in said estimated relationship.
In another particular implementation, the method includes calibrating
measurements from
said sensor for measuring a blood-glucose concentration based, at least in
part, on said estimated
relationship.
In another particular implementation, individually weighting said at least
some of said
sample values comprises weighting said at least some of said sample values
according to a
decreasing function of blood glucose reference values associated with said
weighted samples.
Particular embodiments may be directed to an article comprising a storage
medium
including machine-readable instructions stored thereon which, if executed by a
computing
platform, are directed to enable the computing platform to execute at least a
portion of the
aforementioned method according to one or more of the particular
aforementioned
implementations. In other particular embodiments, a sensor adapted generate
one or more
signals responsive to a blood glucose concentration in a body while a
computing platform is
adapted to perform the aforementioned method according to one or more of the
particular
aforementioned implementations based upon the one or more signals generated by
the sensor.
BRIEF DESCRIPTION OF THE FIGURES
Non-limiting and non-exhaustive features will be described with reference to
the
following figures, wherein like reference numerals refer to like parts
throughout the various
figures.
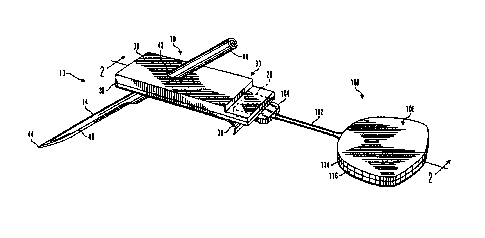
FIG. 1 is a is a perspective view illustrating a subcutaneous glucose sensor
insertion set
and glucose monitor device in accordance with an embodiment;
FIG. 2 is a cross-sectional view of the sensor set and glucose monitor device
as shown
along the line 2--2 of FIG. 1;
FIG. 3 is a cross-sectional view of a slotted insertion needle used in the
insertion set of
FIGS. land 2;
FIG. 4 is a cross-sectional view as shown along line 4--4 of FIG. 3;
FIG. 5 is a cross-sectional view as shown along line 5--5 of FIG. 3;
FIG. 6 is a partial cross-sectional view corresponding generally with the
encircled region
6 of FIG. 2;
FIG. 7 is a cross-sectional view as shown along line 7--7 of FIG. 2;
3
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
FIGs. 8a through 8c are diagrams showing a relationship between sampled
values,
interval values and memory storage values according to an embodiment;
FIG. 9 is a chart showing clipping limits according to an embodiment;
FIG. 10 is a sample computer screen shot image of a post processor analysis of
glucose
monitor data according to an embodiment;
FIG. 11 is a chart illustrating the pairing of a blood glucose reference
reading with
glucose monitor data according to an embodiment;
FIG. 12 is a chart illustrating an example of a single-point calibration
according to an
embodiment;
FIG. 13 is a block diagram illustrating a single-point calibration technique
according to
an embodiment;
FIG. 14 is a chart illustrating an example of a linear regression calibration
according to an
embodiment;
FIG. 15a is a flow diagram illustrating a calibration process according to an
embodiment;
FIG. 15b is a plot of sensor measurements versus reference blood samples
according to
an embodiment;
FIG. 15c is a plot of an inverse variance of sensor measurements versus blood
glucose
concentration according to an embodiment;
FIG. 15d is a plot illustrating a linear best fit of a standard deviation of
sensor
measurements versus blood glucose concentration according to an embodiment;
FIG. 15e is a plot of a function for obtaining weights to be applied to sensor
sample
values according to an embodiment;
FIG. 16 is a flowchart of a self-adjusting calibration technique in accordance
with an
embodiment;
FIGS. 17a and 17b are charts illustrating an example of the self-adjusting
calibration
technique according to an embodiment; and
FIGS. 18a and 18b are further charts illustrating an example of the self-
adjusting
calibration technique according to an embodiment.
DETAILED DESCRIPTION
Reference throughout this specification to "one embodiment" or "an embodiment"
means
that a particular feature, structure, or characteristic described in
connection with the embodiment
is included in at least one embodiment of claimed subject matter. Thus, the
appearances of the
4
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
phrase "in one embodiment" or "an embodiment" in various places throughout
this specification
are not necessarily all referring to the same embodiment. Furthermore, the
particular features,
structures, or characteristics may be combined in one or more embodiments.
Systems for monitoring glucose in the body, for the treatment of diabetes for
example,
typically employ one or more glucose sensors to measure a blood-glucose
concentration. For
example, such sensors may be adapted to generate one or more electrical
signals having a value
(e.g., voltage and/or current level) that is related to such a blood-glucose
concentration. Such a
measurement of a blood-glucose concentration may then be used for any one of
several
applications such as, for example, monitoring a blood-glucose concentration
for a diabetes
patient.
Over time and/or with normal wear and usage of a glucose sensor, such a
relationship
between a value of a signal generated by the glucose monitoring blood sensor
and actual
measured blood glucose concentration may change. Accordingly, calibration of
the signal
generated by such a glucose monitoring with reference samples of blood-glucose
concentration
may enable an accurate estimate of a relationship between signal values
generated by a glucose
sensor and blood-glucose concentration, leading to more effective applications
of glucose
sensors and better treatment of diabetes patients.
As shown in the drawings for purposes of illustration, embodiments are
directed to
calibration methods for a glucose monitor that is coupled to a sensor set to
provide continuous
data recording of readings of glucose levels from a sensor for a period of
time. In one particular
implementation, a sensor and monitor provide a glucose sensor and a glucose
monitor for
determining glucose levels in the blood and/or bodily fluids of a user.
However, it will be
recognized that further embodiments may be used to determine the levels of
other body
characteristics including, for example, analytes or agents, compounds or
compositions, such as
hormones, cholesterol, medications concentrations, viral loads (e.g., HIV),
bacterial levels, or the
like without deviating from claimed subject matter. In particular
implementations, a glucose
sensor is primarily adapted for use in subcutaneous human tissue. However, in
still further
embodiments, one or more sensors may be placed in other tissue types, such as
muscle, lymph,
organ tissue, veins, arteries or the like, and used in animal tissue to
measure body characteristics.
Embodiments may record readings from the sensor on an intermittent, periodic,
on-demand,
continuous, or analog basis.
According to an embodiment, a blood glucose concentration in fluid may be
measured
based upon values of a sampled sensor signal. Also, as discussed below, it can
be observed in
5
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
particular embodiments that the accuracy of such measurements used to measure
blood glucose
concentration may decrease with increases in blood glucose concentration.
Accordingly, as
illustrated below, in estimating a blood glucose response of a particular
sensor, measurements
taken at lower blood glucose concentrations may be more heavily weighted than
samples take a
higher blood glucose concentrations.
Briefly, in one particular embodiment, an electrical signal generated by a
sensor may be
sampled to provide sample values associated with a blood-glucose
concentration. Uncertainty
values may be associated with individual ones of the measurements based, at
least in part, on
blood-glucose reference values associated with the measurements. At least some
of the sample
values are weighted according to a decreasing function of uncertainty values
associated with the
sample values. A relationship of sample values with blood-glucose
concentration may then be
determined based, at least in part, on the individually weighted sample
values. It should be
understood, however, this is merely an example embodiment and claimed subject
matter is not
limited in this respect.
FIGS. 1-7 illustrate a glucose monitor system 1 for use with calibration
methods
described herein. Glucose monitor system 1, in accordance with one particular
implementation,
includes a subcutaneous glucose sensor set 10 and a glucose monitor 100. Here,
glucose
monitor 100 may be of the type described in U.S. Patent Application Serial No.
60/121,664, filed
on Feb. 25, 1999, entitled "Glucose Monitor System." In alternative
embodiments, the glucose
monitor is of the type described in U.S. Patent No. 7,324,012.
In one particular application, glucose monitor 100 may be worn by a user while
connected to a surface mounted glucose sensor set 10 attached to the user's
body by an
electrically conductive cable 102, of the type described in U.S. Patent
Application Serial No.
60/121,656, filed on Feb. 25, 1999, entitled "Test Plug and Cable for a
Glucose Monitor." In one
embodiment, a sensor interface may be configured in the form of a jack to
accept different types
of cables that provide adaptability of the glucose monitor 100 to work with
different types of
subcutaneous glucose sensors and/or glucose sensors placed in different
locations of a user's
body. However, in alternative embodiments, such a sensor interface may be
permanently
connected to the cable 102. In additional alternative embodiments, a
characteristic monitor may
be connected to one or more sensor sets to record data of one or more body
characteristics from
one or more locations on or in a user's body.
According to an embodiment, glucose sensor set 10 may be of a type described
in U.S.
Patent Application Serial No. 60/121,655, filed on Feb. 25, 1999, entitled
"Glucose Sensor Set",
6
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
or U.S. patent application Ser. No. 08/871,831, filed on Jun. 9, 1997,
entitled "Insertion Set For
A Transcutaneous Sensor." Glucose sensor 12 may be of a type described in U.S.
patent
application Ser. No. 09/101,218, filed on Feb. 25, 1999, entitled "Glucose
Sensor", or described
in commonly assigned U.S. Pat. Nos. 5,390,671; 5,391,250; 5,482,473; and
5,586,553; extends
from the glucose sensor set 10 into a user's body with electrodes 20 of the
glucose sensor 12
terminating in the user's subcutaneous tissue. See also U.S. Pat. No.
5,299,571. However, in
alternative embodiments, glucose sensor 12 may use other types of sensors,
such as chemical
based, optical based, or the like. In further alternative embodiments, sensors
may be of a type
that is used on the external surface of the skin or placed below the skin
layer of the user for
detecting body characteristics.
According to an embodiment, glucose monitor 100 may be capable of recording
and
storing data as it is received from glucose sensor 12, and may include either
a data port (not
shown) or wireless transmitter and/or receiver (also not shown) for
transferring data to and/or
from a data processor 200 such as a computer, communication station, a
dedicated processor
designed specifically to work with the glucose monitor, or the like. In a
particular
implementation, glucose monitor 100 may comprise a glucose monitor as
described in U.S.
Patent No. 7,324,012.
In particular applications, glucose monitor system 1 may reduce inconvenience
by
separating complicated monitoring process electronics into two separate
devices; the glucose
monitor 100, which attaches to the glucose sensor set 10; and the data
processor 200, which
contains the software and programming instructions to download and evaluate
data recorded by
the glucose monitor 100. In addition, the use of multiple components (e.g.,
glucose monitor 100
and data processor 200) may facilitate upgrades or replacements, since one
module, or the other,
can be modified, re-programmed, or replaced without requiring complete
replacement of the
monitor system 1. Further, the use of multiple components can improve the
economics of
manufacturing, since some components may require replacement on a more
frequent basis, sizing
requirements may be different for each module, different assembly environment
requirements,
and modifications can be made without affecting the other components.
Glucose monitor 100 may take raw glucose sensor data from glucose sensor 12
and
assess such sensor data in real-time and/or stores it for later processing or
downloading to data
processor 200, which in turn may analyze, display, and log the received data.
Data processor
200 may utilize the recorded data from the glucose monitor 100 to analyze and
review a blood
glucose history. In particular embodiments, glucose monitor 100 is placed into
a corn-station
7
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
which facilitates downloading data to a personal computer for presentation to
a physician.
Software may be used to download such data, create a data file, calibrate the
data, and display
such data in various formats including charts, forms, reports, graphs, tables,
lists and/or the like.
In further embodiments, glucose monitor system 1 may be used in a hospital
environment and/or
the like.
In alternative embodiments, glucose monitor 100 may include at least portions
of the
software described as contained within the data processor 200 above. Glucose
monitor 100 may
further contain software enabling calibration of glucose sensor signals,
display of a real-time
blood glucose value, a showing of blood glucose trends, activate alarms and
the like. A glucose
monitor with these added capabilities is useful for patients that might
benefit from real-time
observations of their blood glucose characteristics even while they're not in
close proximity to a
computer, communication device and/or dedicated independent data processor.
As shown in FIG. 2, data processor 200 may include a display 214 adapted to
display
calculated results of raw glucose sensor data received via a download from
glucose monitor 100.
Results and information displayed may include, but is not limited to, trending
information of a
characteristic (e.g., rate of change of glucose), graphs of historical data,
average characteristic
levels (e.g., glucose), stabilization and calibration information, raw data,
tables (showing raw
data correlated with the date, time, sample number, corresponding blood
glucose level, alarm
messages, and more) and/or the like. Alternative embodiments may include an
ability to scroll
through raw data. Display 214 may also be used in conjunction with buttons
(not shown) on the
data processor 200, computer, communication station, characteristic monitor
and/or or the like,
to program or update data.
Glucose monitor 100 may be combined with other medical devices to accept other
patient
data through a common data network and/or telemetry system. Glucose monitor
100 may be
combined with a blood glucose meter to directly import or correlate glucose
calibration reference
values such as described in U.S. patent application Ser. No. 09/334,996, filed
Jun. 17, 1999,
entitled "Characteristic Monitor With A Characteristic Meter and Method Of
Using The Same."
Glucose monitor 100 may also be combined with semi-automated medication
infusion pumps of
the external type, as described according to particular embodiments in U.S.
Pat. Nos. 4,562,751;
4,678,408; and 4,685,903; or automated implantable medication infusion pumps,
as described
according to particular embodiments in U.S. Pat. No. 4,573,994. Glucose
monitor 100 may
record data from the infusion pumps and/or may process data from both the
glucose sensor 12
and an infusion pump to establish a closed loop system to control the infusion
pump based, at
8
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
least in part, on glucose sensor measurements. In other embodiments, other
body characteristics
are monitored, and the monitor may be used to provide feedback in a closed
loop system to
control a drug delivery rate. In further alternative embodiments, glucose
monitor 100 can be
combined with a glucose sensor set 10 as a single unit.
Glucose sensors may be replaced periodically to avoid infection, decaying
enzyme
coating and therefore sensor sensitivity, deoxidization of the electrodes,
and/or the like. Here, a
user may disconnect glucose sensor set 10 from cable 102 and glucose monitor
100. A needle 14
may be used to install another glucose sensor set 10, and then the needle 14
may be removed.
Further description of the needle 14 and sensor set 10 according to particular
embodiments may
be found in U.S. Pat. Nos. 5,586,553; 6,368,141 and 5,951,521.
A user may connect connection portion 24 of glucose sensor set 10 through
cable 102 to
glucose monitor 100, so that glucose sensor 12 can then be used over a
prolonged period of time.
An initial reading may be downloaded from the glucose sensor set 10 and
glucose monitor 100 to
data processor 200, to verify proper operation of glucose sensor 10 and
glucose monitor 100. In
particular embodiments, glucose sensor set 10 may provide data to glucose
monitor 100 for one
to seven days before replacement. Glucose sensors 12 may last in the user's
body for longer or
shorter periods of time depending on the quality of the installation,
cleanliness, the durability of
the enzyme coating, deoxidization of the sensor, user's comfort, and the like.
After installation into the body, glucose sensor 12 may be initialized to
achieve a steady
state of operation before starting a calibration process. In a particular
implementation, power
supplied by three series silver oxide 357 battery cells 110 in glucose monitor
100 may be used to
speed the initialization of glucose sensor 12. Alternatively, other power
supplies may be used
such as, different battery chemistries including lithium, alkaline, or the
like, and different
numbers of batteries, solar cells, a DC converter plugged into an AC socket
(provided with
proper electrical isolation), and/or the like.
The use of an initialization process can reduce the time for glucose sensor 12
stabilization
from several hours to an hour or less, for example. One particular
initialization procedure uses a
two step process. First, a high voltage (e.g., between 1.0-1.1 volts--although
other voltages may
be used) may be applied between electrodes 20 of the sensor 12 for one to two
minutes (although
different time periods may be used) to allow sensor 12 to stabilize. Then, a
lower voltage (e.g.,
between 0.5-0.6 volts--although other voltages may be used) may be applied for
the remainder of
the initialization process (e.g., 58 minutes or less). Other
stabilization/initialization procedures
using differing currents, currents and voltages, different numbers of steps,
or the like, may be
9
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
used. Other embodiments may omit such an initialization/stabilization process,
if not required
by a particular body characteristic sensor or if timing is not a factor.
Alternatively, a
characteristic monitor or data processor 200 may apply an algorithm to the
sensor data to
determine whether initial transients have sufficiently diminished and the
sensor is at a
significantly stable state to begin calibration.
In particular embodiments, data may not be considered valid until a sensor
initialization
event flag (ESI) is set in data indicating that stabilization is complete. In
one particular
implementation, stabilization may be complete after 60 minutes or when a user
enters a sensor
initialization flag using one or more buttons on the glucose monitor 100.
Following completion
of stabilization/initialization, glucose monitor 100 may be calibrated to
accurately interpret
readings from the newly installed glucose sensor 12.
Beginning with the stabilization process, glucose monitor 100 may measure a
continuous
electrical current signal (ISIG) generated by glucose sensor 12 in connection
with a
concentration of glucose present in the subcutaneous tissue of the user's
body. In particular
embodiments, glucose monitor 100 may sample the ISIG from glucose sensor 12 at
a sampling
rate of once every 10 seconds, for example, as shown in FIGS. 8a-c. Examples
of sampled
values are labeled A-AD in FIG. 8a. At an interval rate of once per minute,
the highest and
lowest of the sampled values (shown in FIG. 8a as circled sampled values A, E,
G, I, M, R, V,
W, Y, and AB) are ignored, and the remaining four sampled values from an
interval are averaged
to create interval values (shown in FIG. 8b as values F', L', R', X', and
AD'). At a glucose
monitor memory storage rate of once every five minutes, the highest and lowest
of the interval
values (shown in FIG. 8b as values L' and X') are ignored and the remaining
three interval values
are averaged and stored in a glucose monitor memory as memory values (shown in
FIG. 8c as
point AD"). The memory values are retained in memory and may be downloaded to
data
processor 200. Such memory values may be used to calibrate glucose monitor 100
and/or post
processor 200 and to analyze blood glucose levels. The sampling rate, interval
rate and the
memory storage rate may be varied as necessary to capture data with sufficient
resolution to
observe transients or other changes in the data depending on the rate at which
sensor values can
change, which is affected by the sensor sensitivity, the body characteristic
being measured, the
.. physical status of the user, and the like. In other embodiments, all of the
sampled values are
included in the average calculations of memory storage values. In alternative
embodiments,
more or less sampled values or interval values are ignored depending on the
signal noise, sensor
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
stability, or other causes of undesired transient readings. Finally, in still
other embodiments, all
sampled values and/or interval values are stored in memory.
Clipping limits may be used to limit a signal magnitude variation from one
value to the
next thereby reducing the effects of extraneous data, outlying data points, or
transients. In
.. particular embodiments, clipping limits may be applied to interval values.
For instance, interval
values that are above a maximum clipping limit or below a minimum clipping
limit may be
replaced with the nearest clipping limit value.
In alternative embodiments, interval values that are outside of clipping
limits may be
ignored and not used to calculate a subsequent memory storage value. In
particular
implementations, detection of interval values outside of clipping limits may
be considered a
calibration cancellation event. In further particular embodiments, a
calibration cancellation
event may be recognized if more than one value is deemed outside of clipping
limits.
(Calibration cancellation events are discussed below).
In particular embodiments, clipping limits may be shifted after each data
point. Here,
clipping limits may be set to a level based, at least in part, on an
acceptable amount of change
from a previous interval value to a present interval value, which is affected
by sensor sensitivity,
signal noise, signal drift, and/or the like. In particular implementations,
clipping limits may be
calculated for a current interval based on the magnitude of the previous
interval value. For
example, for a previous interval value from zero up to but not including 15
Nano-Amps, clipping
limits may be set at plus and minus 0.5 Nano-Amps about the previous interval
value. For a
previous interval value from 15 Nano-Amps up to but not including 25 Nano-
Amps, clipping
limits may be set at plus and minus 3% of the previous interval value, about
the previous interval
value. For a previous interval value from 25 Nano-Amps up to but not including
50 Nano-Amps,
clipping limits may be set at plus and minus 2% of the previous interval
value, about the
previous interval value. For a previous interval value of 50 Nano-Amps and
greater, clipping
limits may be set at plus and minus 1% about the previous interval value. In
alternative
embodiments, different clipping limits may be used and claimed subject matter
is not limited in
this respect.
FIG. 9 shows a clipping limit example according to a particular embodiment in
which a
previous interval value 500, associated with interval N-1, has a magnitude of
13.0 Nano-Amps,
which is less than 15.0 Nano-Amps. Therefore, a maximum clipping limit 502 for
a present
interval value 506 is set at 13.5 Nano-Amps, which is 0.5 Nano-Amps greater
than the
magnitude of the previous interval value 500. A minimum clipping limit 504 is
set at 12.5 Nano-
11
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
Amps which is 0.5 Nano-Amps below the previous interval value 500. Present
interval value
506, associated with interval N, is between the maximum clipping limit 502 and
the minimum
clipping limit 504 and is therefore acceptable.
In another example shown in FIG. 9, the present interval value 508, associated
with
interval M, has a value of 25.0 Nano-Amps which is outside of the clipping
limit 514 and will
therefore be clipped. The previous interval value 510, associated with
interval M-1, is 26.0
Nano-Amps, which is included in the range from 25.0 up to but not including
50.0 Nano-Amps
as discussed above. Therefore the clipping limits are +1- 2%. The maximum
clipping limit 512 is
2% greater than the previous interval value 510 as follows:
26.0 + 26.0*0.02 = 26.5 Nano-Amps.
Similarly the minimum clipping limit 514 is 2% less than the previous interval
value 510
as follows:
26.0 - 26.0*0.02 = 22.5 Nano-Amps.
Since the present interval value 508 of 25.0 Nano-Amps is less than the
minimum
clipping limit 514 of 25.5 Nano-Amps, it will be clipped, and 25.5 Nano-Amps
will be used in
place of 25.0 Nano-Amps to calculate a memory storage value. For further
illustration, FIG. 8
shows interval value R', which is calculated by averaging sampled values N
through Q, is outside
of the clipping limits 412 and 414, which result from the previous interval
value L'. Therefore,
in this particular example, the magnitude of interval value R' is not used to
calculate memory
value AD", instead R", which is the magnitude of the minimum clipping limit
414, is used.
In other embodiments, clipping limits may be a smaller or larger number of
Nano-Amps
or a smaller or larger percentage of the previous interval value based on the
sensor characteristics
mentioned above. Alternatively, clipping limits may be calculated as plus or
minus the same
percent change from every previous interval value. Other algorithms may use
several interval
values to extrapolate the next interval value and set the clipping limits to a
percentage higher and
lower than the next anticipated interval value. In further alternatives,
clipping may be applied to
the sampled values, interval values, memory values, calculated glucose values,
estimated values
of a measured characteristic, or any combination of such values.
In particular embodiments, interval values are compared to an out-of-range
limit of 200
Nano-Amps. If three consecutive interval values are equal to or exceed the out-
of-range limit,
the sensor sensitivity may be deemed to be too high, and an alarm is activated
to notify the user
that re-calibration is required or the sensor may need replacing. In
alternative embodiments, an
out-of-range limit may be set at higher or lower values depending on the range
of sensor
12
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
sensitivities, the expected working life of the sensor, the range of
acceptable measurements,
and/or the like. In particular embodiments, an out-of range limit is applied
to sampled values. In
other embodiments, an out-of-range limit is applied to the memory storage
values.
In particular embodiments, unstable signal alarm limits may be set to detect
drastic
changes in memory storage values from one to another. Signal alarm limits may
be established
similarly to the clipping limits described above for the interval values, but
allow for a larger
change in value since there is more time between memory storage values than
between interval
values. Re-calibration or replacement of the glucose sensor 12 may be
performed once an
unstable signal alarm is activated. In essence, in a particular
implementation, such an alarm is
therefore activated in the event that glucose monitor 100 detects an
unacceptable level of noise in
the ISIG from glucose sensor 12.
In a particular embodiment, a memory storage value may be considered valid
(Valid ISIG
value) unless one of the following calibration cancellation events occurs: an
unstable signal
alarm (as discussed above); a sensor initialization event (as discussed
above); a sensor
disconnect alarm; a power on/off event; an out-of-range alarm (as discussed
above); or a
calibration error alarm. Here, only Valid ISIG values may be used to calculate
blood glucose
levels by the glucose monitor 100 or post processor 200, as shown in FIG. 10.
Once a
calibration cancellation event occurs, successive memory storage values are
not valid, and
therefore are not used to calculate blood glucose, until glucose monitor 100
or post processor 200
is re-calibrated. FIG. 10 shows an explanatory computer screen shot in which
cell P3 indicates a
sensor disconnect alarm with the abbreviation "SeDi". As shown, blood glucose
values do not
appear in column K, titled "Sensor Value", and Valid ISIG values do not appear
in column J until
after the sensor is initialized, as indicated by the "ESI" flag in cell N17.
One exception however,
is the power on/off event. If glucose monitor 100 is turned off for a short
enough period of time,
up to 30 minutes for example, memory storage values may be considered Valid
ISIG values as
soon as the power is restored. If the power is off for longer than 30 minutes,
for example,
glucose monitor 100 may be re-calibrated before ISIG values are considered
valid. Alternatively,
power may be off for a duration such as 30 minutes or longer and, once power
is restored, the
memory storage values may comprise Valid ISIG values. Here, a sensor
disconnect alarm may
be activated if the glucose monitor 100 does not detect a signal. In preferred
embodiments, when
two or more out of five interval values collected within a given memory
storage rate are less than
1.0 Nano-Amp, a disconnect alarm may be triggered. In alternative embodiments,
greater or
fewer values need be below a particular threshold current level to trigger a
disconnect alarm
13
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
depending of the acceptable range or sensor readings and the stability of an
associated sensor
signal. Two remaining calibration cancellation events, the calibration error
and an alternative
embodiment for the out-of-range alarm, are discussed in conjunction with the
calibration process
below.
Particular implementations are directed to calibration techniques that may be
used by
either glucose monitors during real-time measurements of one or more signals
from a glucose
sensor, or post processors during post-processing of data that has been
previously recorded and
downloaded (as shown in FIG. 10).
To calibrate glucose monitor 100, a calibration factor called a sensitivity
ratio (SR)
(blood glucose level/Valid ISIG value) may be calculated for a particular
glucose sensor 12. The
SR is a calibration factor used to measure/estimate a blood glucose
concentration based, at least
in part on a Valid ISIG value (Nano-Amps) into a blood glucose level (mg/di or
mmo1/1). In
alternative embodiments, units for the SR may vary depending on the type of
signal available
from the sensor (frequency, amplitude, phase shift, delta, current, voltage,
impedance,
capacitance, flux, and the like), the magnitude of the signals, the units to
express the
characteristic being monitored, and/or the like.
In particular implementations, a user may obtain a blood glucose reference
reading from
a common glucose meter, or another blood glucose measuring device, and
immediately enter
such a blood glucose reference reading into glucose monitor 100. Such a blood
glucose
.. reference reading is assumed to be accurate and is used as a reference for
calibration. Glucose
monitor 100, or a post processor 200, may temporally correlate a blood glucose
reference reading
with a Valid ISIG value to establish a "paired calibration data point." Since
a glucose level in an
interstitial body fluid tends to lag behind a blood glucose level, glucose
monitor 100 or post
processor 200 applies a delay time and then pairs the blood glucose reference
reading with a
Valid ISIG value as shown in FIG. 11. In particular embodiments, an
empirically derived ten
minute delay may be used. In a particular implementation where Valid ISIG
values are averaged
and stored every five minutes, glucose monitor 100 may correlate a blood
glucose reference
reading with the third Valid ISIG stored in memory after the blood glucose
reference reading is
entered (resulting in an effective delay of ten to fifteen minutes in this
particular example). FIG.
11 illustrates an example, in which a blood glucose reference reading 600 of
90 mg/di is entered
into the glucose monitor 100 at 127 minutes. The next Valid ISIG value 602 may
be stored at
130 minutes. Given a 10 minute delay, a glucose reference reading 600 may be
paired with
Valid ISIG value 604 which is stored at 140 minutes with a value of 30 Nano-
amps. Note that
14
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
two numbers are needed to establish one paired calibration data point, a blood
glucose reference
reading and a Valid ISIG.
Other delay times may be used depending on a particular user's metabolism,
response
time of the sensor, delay time incurred for the glucose meter to calculate a
reading and for the
.. reading to be entered into the glucose monitor 100, a type of analyte being
measured, the tissue
that the sensor is placed into, environmental factors, whether the previous
glucose Valid ISIG
value (or the trend of the Valid ISIG values) was higher or lower than current
Valid ISIG value,
and/or the like. Once paired calibration data is available, the appropriate
calibration process may
be applied dependent on how many paired calibration data points are available
since the last
calibration, the total period of time that glucose sensor 12 has been in use,
and the number of
times glucose sensor 12 has been calibrated.
In particular embodiments, blood glucose reference readings may be entered
into glucose
monitor 100 periodically through out each day of use. Here, calibration may be
conducted
immediately after the initialization/stabilization of glucose sensor 12 and
once a day thereafter.
However, such calibration may be conducted more or less often depending on
whether glucose
sensor 12 has been replaced, whether a calibration cancellation event has
occurred, the stability
of glucose sensor 12 sensitivity over time, and/or the like.
In preferred embodiments, blood glucose reference readings are collected
several times
per day but a new calibration factor is calculated only once per day.
Therefore, typically more
than one paired calibration data point is collected between calibrations. In
alternative
embodiments, the glucose monitor is calibrated every time a new paired
calibration data point is
collected.
Particular embodiments may use a single-point calibration technique (shown in
a block
diagram of FIG. 13) to calculate the SR if only a single paired calibration
data point is available,
such as immediately after initialization/stabilization. And a modified linear
regression technique
(shown in a block diagram in FIG. 15a) may be used if two or more paired
calibration data points
are available. Particular embodiments may use a single-point calibration
technique whether or
not more than one paired calibration data point is available.
A single-point calibration equation may be based on an assumption that a Valid
ISIG will
be 0 when the blood glucose is 0. As shown in process 750 of FIG. 12, a single
paired
calibration point 700 obtained at block 754 is used with the point (0,0) to
establish a line 702.
The slope of the line from the origin (0,0) and passing through the single
paired calibration point
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
700 provides a single-point sensitivity ratio (SPSR). Here, block 756 may
calculate such an
SPSR as follows:
Blood Glucose Reference Reading
SPSR ¨ -----------------------------------
Valid ISIG
Therefore, the calibrated blood glucose level may be expressed as follows:
Blood Glucose Level = Valid ISIG * SPSR
As an example, using the values of 20.1 Nano-Amps and 102 mg/di from the
paired
calibration data point shown in FIG. 12, calculation of SPSR may be expressed
as follows:
SPSR = 102/20.1 = 5.07 mg/dl per Nano-Amp
To continue with the current example, once calibration is complete, given a
glucose
sensor reading of 15.0 Nano-Amps, calculated blood glucose level may be
determined as
follows:
Blood Glucose Level = 15.0*5.07 = 76.1 mg/dl
Additionally, particular embodiments may use an offset value in a calibration
equation to
compensate for the observation that more sensitive glucose sensors 12 (e.g.,
glucose sensors 12
that generate higher ISIG values compared to other glucose sensors 12 at the
same blood glucose
level, which result in lower SR values) may have a less linear performance at
very high blood
glucose levels in comparison to glucose sensors 12 with lower sensitivity (and
therefore
relatively higher SR values). If the SPSR for a particular glucose sensor 12,
as calculated above,
is less than a sensitivity threshold value, then a modified SPSR (MSPSR) may
be calculated at
block 760 using an offset value selected at block 758. In one particular
implementation, the
threshold value is 7. If the initial calculation of the SPSR (shown above) is
less than 7, for
example, an offset value of 3 may be used to calculate the MSPSR. If the
initial calculation of
SPSR yields a value of 7 or greater, then the offset value may be 0. Thus, the
MSPSR may be
calculated at block 760 using the offset value according to a modified single-
point calibration
expression, as follows:
Blood Glucose Reference Reading
MSPSR = -----------------------------------------------
Valid ISIG - offset
16
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
Accordingly, an initial calibration of sensor 12 may be used to estimate a
blood glucose
from a sensor measurement at block 762 as follows:
Blood Glucose Level = (Valid ISIG ¨ offset) * SPSR
Continuing the above example since the SPSR is 5.07, which is less than 7, the
sensitivity
ratio is recalculated using the MSPSR equation as:
MSPSR = 102/(20.1 ¨3) = 5.96 mg/dl per Nano-Amp
Given a glucose sensor reading of 15.0 Nano-Amps after calibration, the
calculated blood
glucose may be expressed as follows:
Blood Glucose Level = (15.0-3) = 5.96 = 71.5 mg/dl
In another example, given a blood glucose reference reading of 95 from a
typical blood
glucose meter and a Valid ISIG value of 22.1, a resulting SPSR may be
determined as
95/22.1=4.3. Since SR<7 , the offset=3. Therefore, the MSPSR is 95422.1-3]
5Ø Note that if
the SPSR is greater than or equal to 7 the offset value is 0 and therefore the
MSPSR=SPSR.
In alternative embodiments, the offset value may be eliminated from the
expression for
calculating the blood glucose value as follows:
Blood Glucose Level = Valid ISIG*MSPSR
The threshold value of 7 and the associated offset of 3 have been empirically
selected
based on the characteristics observed from testing a particular type of
glucose sensors 12, such as
those described in U.S. Pat. No. 5,391,250 entitled "Method of Fabricating
Thin Film Sensors",
and U.S. Patent No. 6,360,888. Other threshold values may be used in
conjunction with other
offset values to optimize the accuracy of the calculated MSPSR for various
types of glucose
sensors 12 and sensors used to detect other body characteristics. In fact,
many threshold values
may be used to select between many offset values. An example using two
different threshold
values (4 and 7) to select between three different offset values (5, 3 and 0)
follows:
if SPSR <4, offset = 5;
if 4 < SPSR <7, offset = 3; and
if SPSR? 7, offset = 0.
In particular embodiments an MSPSR may be compared to a valid sensitivity
range to
determine whether a newly calculated MSPSR is reasonable. In order to identify
potential
system problems, a valid MSPSR range of 1.5 to 15 may be employed, for
example. However
this is merely an example of such a range and claimed subject matter is not
limited in this
respect. This range may be determined based, at least in part, upon valid
glucose sensor
17
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
sensitivity measurements made in-vitro. MSPSR values outside this range may
result in a
calibration error alarm (CAL ERROR) to notify the user of a potential problem.
Other valid
sensitivity ranges may be applied depending on the types of sensors to be
calibrated, the range of
acceptable sensitivity levels for the various sensor types, the manufacturing
consistency expected
for the sensors, environmental conditions, how long the sensor has been in
use, and/or the like.
Particular embodiments may augment the above described single-point
calibration
technique using a modified linear regression technique (shown in a block
diagram in FIG. 15a) if
more than one paired calibration data point is available. As shown in FIG. 14,
paired calibration
data points 800 may linearly regressed by a least squares method to calculate
a best fit straight
line 802 correlated with paired calibration data points 800. The slope of the
line resulting from
the linear regression may be the linear regression sensitivity ratio (LRSR)
used as the calibration
factor to calibrate the glucose monitor 100.
Linear and nonlinear least squares regression may apply an assumption that
each data
point provides equal information about a deterministic part of a total
variation in a value or
outcome. In such processes a standard deviation of an error associated with a
value would be
constant for all estimated predictions, for example. In some processes this is
not the case. For
example, in real-time continuous glucose monitoring using an enzymatic
minimally invasive
biosensor to estimate plasma glucose concentrations as discussed above, an
unequal error
distribution may exist. Here, a scatter plot of FIG. 15b illustrates several
calibrated glucose
sensor points plotted against paired blood glucose reference values throughout
a large glycemic
range in one particular implementation. It can be observed from the plot that
the accuracy of the
sensor glucose measurements decreases as the reference blood glucose values
increase. Such a
decreasing accuracy may be measured as variance and/or standard deviation of
an error
associated with such measurements that increases with blood glucose
concentration and/or paired
reference blood glucose reference value. Accordingly, in certain circumstances
it may be
advantageous not to treat every observation equally, and apply a weighted
least squares
regression, for example. This may be implemented according to a particular
embodiment by
giving each point an appropriate weight to control an amount of influence over
parameter
determination. In doing this, points with less precise influence may be
weighted less in
computing a linear regression, while points with more influence may be more
heavily weighted.
In a particular implementation, paired calibration points, comprising sample
values
associated with blood-glucose concentration sensor measurements paired with
reference
measurements at block 852, may be linearly regressed at block 854 to determine
an LRSR. As
18
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
pointed out above, in particular embodiments, such a regression may weight
particular pairs
and/or sample values according to a degree of certainty associated with the
accuracy of such
sample values based upon a priori information. Such a linear regression
calibration may be
computed as follows:
Ea, .fi, .isig , .BG ,
_______ LRSR = N
E a ,
,.1
where:
isig, is a value representing a sensor measurement of a blood glucose
concentration for paired
calibration point i;
a, is weighting applied to paired calibration point i based upon the time that
the associated
sample was obtained;
BG, is reference sample of a blood glucose concentration for paired
calibration point i;
fi, is a weighting applied to paired calibration point i based upon a degree
of certainty associated
with accuracy of isig, as a measurement of blood glucose concentration; and
N is a number of paired calibration data points which are to be linearly
regressed.
Accordingly, an estimate of a calibrated blood glucose level may be expressed
as follows:
Blood Glucose Level = Valid ISIG * LRSR
In a particular implementation, a paired calibration point may be weighted
according to a
time associated with when associated sensor measurements and reference values
are obtained.
Here, for example, pairs based on more recent measurements and reference
values may be
associated with an error with a smaller variance than pairs based on
measurements and reference
values obtained in the more distant past. Accordingly, the weight a, applied
to calibration pairs
may decrease the more distant in the past such calibration pairs are obtained.
Also, as pointed out above, variances associated with measurement errors in
calibrating
continuous glucose monitors may not be constant across a dynamic range of
blood glucose
values. Here, in one particular embodiment, weighting may represent an inverse
variance
weighting. In other words, contribution of each data point may be weighted
with the inverse of
the variance for that set of blood glucose values. For example, a set of
sensor current values
were paired (N=90,000 points) and the inverse variance of sensor current
calculated for each
blood glucose reference value as follows:
= [var(isig1)]-1
19
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
Here, application of such an inverse variance to calibration pairs to weight
samples for
linear regression is merely one example of how such calibration pairs may be
weighted based
upon a decreasing accuracy of sensor measurements, and claimed subject matter
is not limited in
this respect. Furthermore, it should be understood that a variance or standard
deviation are
merely examples of how a statistical dispersion of sensor measurement errors
may be quantified,
and that other metrics may be used. In alternative embodiments, for example,
fi, may be derived
as the inverse of an estimate or approximation of the variance of isig,. Also,
as discussed below,
appropriate weights may be derived from other functions for determining a
weight based, at least
in part, on blood glucose reference samples and/or blood glucose
concentration.
In this particular implementation, however, fi, represents an inverse variance
and/or
standard deviation of all sensor samples (isigi) measured at a time
corresponding to when
reference blood glucose sample values i were acquired. In one particular
example, inverse
variance weights are plotted in FIG. 15c for blood glucose values ranging from
40-400 mg/dL.
Again, it should be understood, however, that the use of an inverse variance
is merely one
example of how calibration pairs may be weighted based upon a degree of
certainty associated
with accuracy of sensor measurements and claimed subject matter is not limited
in this respect.
Alternatively, weights (for application to calibration pairs in a linear
regression) may be
obtained from a function based on an inverse variance weights. Here, use of
such a function may
provide a high quality estimate that removes noise present in the inverse
variance weights arising
from sources such as, for example, variability between blood-glucose and a
blood glucose
monitor. This is illustrated in FIG. 15d where a best line fit is produced by
regressing the square
root of the variance or standard deviation. For the particular example of
sensor measurement
samples shown in FIG. 15b, weights may be determined according to the
corresponding function
derived from such a best line fit as follows:
1
w.=
(1.787 + 0.0291.02
FIG. 15e shows a plot of inverse variance fi, and function derived from such a
best line fit
of variance/standard deviation as a function of ISIG weights w, over a range
of blood glucose
concentration range from 0 to 400 mg/d1. An inverse variance is plotted as 902
while a
weighting function is plotted as 900. As can be observed, the weighting
function 900 removes
noise in the inverse variance to provide a weighting function to be applied to
calibration pairs
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
that is a decreasing function of blood glucose concentration and/or associated
blood sample
reference values associated with such calibration pairs.
It should be observed that this particular linear regression uses a fixed
intercept of zero.
In other words, if the Valid ISIG is 0 the blood glucose value is 0.
Accordingly, this particular
linear regression method estimates only one regression parameter, the slope.
In alternative
embodiments, other linear regression methods may be used that estimate
additional regression
parameters such as an offset value.
At block 856, particular embodiments may select an offset value for use in
calculating a
modified linear regression calibration. The purpose of such an offset value,
as described above
for the single-point calibration, is to compensate for an observation that
more sensitive glucose
sensors 12 may have a less linear performance at very high blood glucose
levels. If an LRSR for
a particular glucose sensor 12, as calculated in the linear regression
calibration expression above,
is less than a sensitivity threshold value, then a modified linear regression
sensitivity ratio
(MLRSR) may be calculated using an offset value included in a modified linear
regression
calibration expression. In one particular embodiment, for example, such a
sensitivity threshold
may be 7. Here, if an initial calculation of an LRSR is less than 7, an offset
value of 3 may be
used to calculate an MLRSR. If an initial calculation of LRSR yields a value
of 7 or greater, an
offset value of 0 may be used. Thus, MLRSR may be calculated at block 858
using the selected
offset value in the modified linear regression calibration according to the
following expression:
offsetV3G,
MLRSR = 1=1 N
Ea ¨ offsed2
t=1
Accordingly, a calculated blood glucose level may be estimated at block 860 as
follows:
Blood Glucose Level = (Valid ISIG ¨ offset) * MLRSR
Just as in the case of single-point calibration techniques described above,
other threshold
values may be used at block 856 in conjunction with other offset values in the
modified linear
regression calibration equation to optimize the accuracy of the calculated
MLRSR for various
types of glucose sensors 12 and other characteristic sensors.
In particular embodiments, a newly calculated MLRSR may be compared to a valid
sensitivity range to determine whether the newly calculated MLRSR is
reasonable. To identify
potential system problems, a valid MLRSR range of 2.0 to 10.0 may be employed.
MLRSR values
outside this range may result in a calibration error alarm (CAL ERROR) to
notify a user of a
21
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
potential problem. As described above for the single-point calibration
techniques, other valid
sensitivity ranges may be applied.
In particular embodiments, glucose monitor data (e.g., paired calibration data
points as
discussed above) may be linearly regressed over a 24 hour period (or window),
and new
sensitivity ratios may be used for each 24 hour time period. In other
embodiments, a time period
may be reduced to only a few hours or enlarged to cover the entire monitoring
period with the
glucose sensor (e.g., several days--or even weeks with implanted sensors). In
further
embodiments, such a time window may be fixed at a predetermined size, such as
24 hours, 12
hours, 6 hours, and/or the like, and the window is moved along over the
operational life of the
sensor.
In particular embodiments, paired calibration data points from measurements
taken
before the last calibration may be used to calculate a new sensitivity ratio.
For example, to
calibrate the glucose monitor every 6 hours, a paired calibration data point
may be established
every 6 hours. A linear regression technique described above may be executed
using four paired
calibration data points, the most recently acquired point and points obtained
from six, twelve and
eighteen hours before. Alternatively, a number of paired calibration data
points used in the
calibration may be as few as one or as large as the total number of paired
calibration data points
collected since the glucose sensor was installed. In alternative embodiments,
a number of paired
calibration data points used in a calibration computation may grow or shrink
during the life of
the glucose sensor due to glucose sensor anomalies.
In still other embodiments, decay characteristics of glucose sensor 12 over
time may be
factored into the equation to account for known degradation characteristics of
glucose sensor 12
due to site characteristics, enzyme depletion, body movement, and/or the like.
Considering these
additional parameters in the calibration equation may more accurately tailor
calibration
computations used by the glucose monitor 100 or post processor 200. In
particular
embodiments, other parameters may be measured along with the blood glucose
such as,
temperature, pH, salinity, and/or the like. These other parameters may be used
to calibrate the
glucose sensor using non-linear techniques.
In a particular embodiment, real-time calibration adjustment can be performed
to account
for changes in the sensor sensitivity during the lifespan of the glucose
sensor 12 and to detect
when a sensor fails. FIG. 16 (in conjunction with FIGS. 17, 18a and 18b)
describes the logic of a
self-adjusting calibration technique to adjust the calibration formula or
detect a sensor failure in
accordance with one particular implementation.
22
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
At block 1000, a user may obtain a blood glucose reference from a common
glucose
meter, or another blood glucose measuring device, and immediately enter the
blood glucose
reference reading into glucose monitor 100. For every such meter blood glucose
entry, an
instantaneous calibration check may be performed and compared to an expected
range of the
value of the calibration check, as in block 1010. In particular embodiments, a
Calibration Factor
current is calculated (e.g., CFc = Meter BG/current ISIG value) to determine
if the CFc
(Calibration Factor current) ratio is between 1.5 to 12 ("Criteria 1"), one
criterion for an accurate
ISIG value in a particular implementation. If data is outside this range,
raising a likelihood of a
sensor failure or incorrect determination/entry of a meter BG value, a Cal
Error alarm may be
triggered at block 1030 and the Recalibration Variable (Recal), which is
originally set at
NOFAIL may be changed to FAILC1. At this point, another blood glucose
reference reading
may be requested and entered into the glucose monitor 100 to determine whether
there was
indeed a sensor failure or the Meter Blood Glucose value was incorrectly
inputted. The previous
Metered Blood Glucose value that generated the error can be thrown out
completely. If Criteria
1 is again not satisfied at block 1010, an end of the sensor life message may
be generated at
block 1040 since then the Recal variable would be recognized as FAILC1 at
block 1020.
However, if Criteria 1 is met at block 1010, then block 1200 may determine
whether the Recal
variable is not equal to FAILC2. Here, the Recal variable is set to FAILC2
only if Criteria 2a is
not met, which is discussed below. Given that the Recal variable at this point
may only be set to
a NOFAIL or FAILC1, logic proceeds to block 1210.
Block 1210, a check is performed to determine whether an existing calibration
slope
estimation (Previous Estimated Slope or PES) is much different from the CFc
performed using a
new meter blood glucose value. A significant difference may indicate a sensor
failure, for
example. In a particular embodiment, a difference between a previous estimated
slope (PES) and
a CFc in terms of percentage (threshold 1) and mg/di (threshold 2) may be
performed.
Thresholds 1 and 2 may be set depending on particular sensor characteristics.
In a particular
implementation, an example of checking such changes between the PES and CFc
may be
performed as follows:
11 - PESICFcI*100 > threshold 1; and
ICFc ¨ PESI*isig> threshold 2.
If threshold 1 and/or threshold 2 are exceeded according to the above
expressions
(collectively "Criteria 2a"), then depending on the Recal variable (at block
1220), either trigger
an end of sensor message may be triggered at block 1040 (if the Recal variable
is equal to
23
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
FAILC1 or FAILC2 at block 1220) or a Cal Error alarm may be generated at block
1230 (if the
Recal variable is equal to NOFAIL at block 1220). Here, if a Cal Error alarm
is generated at
block 1230, the Recal variable may be set to FAILC2, the current meter blood
glucose reading
will be stored as MBGp (Meter Blood Glucose previous), and another blood
glucose reference is
requested and entered into the glucose monitor 100 (as MBGc) at block 1000. By
requesting a
new meter blood glucose reading, a comparison can be made between the last
meter blood
glucose reading stored at block 1230, and the new meter blood glucose reading
entered at block
1000 may be used to determine whether there was a sensor failure. The logic
follows the same
paths as described above after block 1000 until the logic reaches block 1200.
At block 1200,
since Recal variable is now set to FAILC2 at block 1230, a difference between
the previous
calibration check (CFp), which generated the FAILC2 alert, and the CFc is
performed at block
1300. In particular implementations, the difference between the previous
calibration check and
the current calibration check in terms of percentage (threshold 1) and mg/di
(threshold 2) may
also be performed. In addition, a check is performed to determine whether
there has been a
directional change between the CFp and CFc (collectively "criteria 2b"). An
example of criteria
2b may be expressed as follows:
11 ¨ CFpICFcl* 100 > threshold 1;
ICFc ¨ CFpl* Isig> threshold 2; and
(CFp ¨ PES)*(CFc ¨ CFp) > 0.
If the percentage and absolute difference exceeds threshold 1 and threshold 2,
and there is
no directional change in the slope with the second blood glucose meter
reading, then an end of
sensor message will be triggered at block 1040. If criteria 2b is met, then
the logic proceeds to
block 1310. At block 1310, the logic then determines whether the difference
between the
previous value and the current value was due to a change in sensitivity of the
sensor or whether
the reading is merely noise. In the preferred embodiment, the determination of
change in
sensitivity versus noise is made by using Criteria 3b. Criteria 3b compares
the difference
between (the PES and CFc) and (the CFp versus the CFc) at block 1420. For
example:
IPES ¨ CFcl < ICFp ¨ CFcl
As illustrated in FIG. 17a, if a difference between PES and CFc is less than a
difference
between CFp and CFc, criteria 3b will be met, indicating that the previous CFp
is an outlier
reading (e.g., an anomaly). Then, the MBGp (Meter Blood Glucose previous) is
removed at
block 1320 and only the MBGc paired with a valid ISIG is used in the slope
calculation, which is
resumed at block 1430 and applied in interpreting the sensor readings at block
1130.
24
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
As illustrated in FIG. 17b, if criteria 3b shows that a difference between the
PES and CFc
is greater than a difference between CFp and CFc, criteria 3b would not be
met, indicating a
change in sensor sensitivity. A slope calculation may then be fine-tuned by
creating a new
(artificial) meter blood glucose value (MBGN) with a paired ISIG according to
the last slope
(Seeding) at block 1330. Using the new paired MBG (MBGN) with the paired MBGp
and
MBGc, the slope calculation may be restarted (or reset) at block 1340, as seen
in FIG. 17b.
Sensor calculation may then be performed using a new slope calculation at
block 1130. By
resetting a slope calculation, such a slope calculation can thus be modified
automatically to
account for changes in sensor sensitivity.
Continuing the logic from block 1210, if the percentage and/or absolute
difference
between the PES and CFc is within threshold 1 and/or threshold 2 at block
1210, indicating a
valid calibration, the Recal variable is again checked at block 1400. If the
Recal variable is
equal to FAILC1 (indicating that the meter BG was checked twice), any fine-
tuning
determination may be skipped and the MBGc may be paired with a valid ISIG for
use in
updating a slope calculation at block 1430 and applied in interpreting sensor
readings at block
1130. If the Recal Variable is not equal to FAILC1, then the logic may decide
whether fine-
tuning the slope calculation is needed at blocks 1410 and 1420. In particular
embodiments, a
decision to fine-tune may be first made by comparing a percentage and/or
absolute difference
between the PES and CFc (as done in block 1210) with a threshold 3 and/or a
threshold 4
("Criteria 4") at block 1410 as follows:
11 - PESICFcI*100 < threshold 3; and
ICFc ¨ PESI*isig < threshold 4.
Again, threshold 3 and 4 may be determined based, at least in part, on
particular sensor
characteristics. If a percentage and/or absolute difference between PES and
CFc is less than
threshold 3 and/or threshold 4 at block 1410 (i.e. Criteria 4 met), then the
slope calculation can
simply be updated with the new MBGc and paired ISIG value at block 1430, and
applied in
interpreting the sensor readings at block 1130.
On the other hand, if the Criteria 4 is not met at block 1410, block 1420 may
determine
whether the difference between the expected value and the current value was
due to a change in
sensitivity of the sensor or whether the reading is merely noise. In one
particular
implementation, such a determination of change in sensitivity versus noise may
be made by
using Criteria 3a. Here, criteria 3a CFc and a CFp at block 1420 as follows:
IPES ¨ CFA <ICFc ¨ CFA
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
As seen in FIG. 18a, if the difference between a PES and CFp is less than a
difference
between CFc and the CFp, criteria 3a may be met, indicating that an error
between predicted and
actual values for the CFc was due to noise in previous calibrations or
beginning of a change in
sensor sensitivity which may be picked up in a subsequent calibration cycle.
Slope calculation
may then be updated with a new paired blood glucose entry (MBGc) at block 1430
and applied
in interpreting sensor readings at block 1130.
As seen in FIG. 18b, if criteria 3a shows that a difference between the PES
and the
previous valid calibration check is greater than a difference between the
previous valid CFp and
the CFc, criteria 3b would not be met, indicating a change in the sensor
sensitivity and fine
tuning is performed. Here, such fine tuning may be performed if two MBG
entries in succession
indicate a change in slope. Slope calculation may be fine-tuned by creating a
new (artificial)
MBGN with a paired 1SIG according to the last slope (Seeding) at block 1330.
Using such a new
paired MBGN with the paired MBGp and MBGc, a slope calculation may be
restarted (or reset)
at block 1340, as seen in FIG. 18b. The sensor calculation may then be
performed using the new
slope calculation at block 1130. Again, by resetting the slope calculation,
the slope calculation
can thus be modified automatically to account for changes in sensor
sensitivity.
Although the above description described the primary calibration techniques in
particular
embodiments, many modifications can be made to the above described calibration
techniques
without deviating from claimed subject matter. For example, in alternative
embodiments, a
calibration factor may be calculated by first using a single-point technique
to calculate an
MSPSR for each paired calibration data point, and then averaging them
together, either
unweighted or weighted by temporal order of by elapsed time.
As discussed above, particular embodiments described herein utilize a least
squares linear
regression computation to calibrate the glucose monitor 100 and/or analyze
sensor data using
post-processor 200, for example. However, alternative embodiments may utilize
a multiple
component linear regression computation with more variables than just the
paired calibration
data points discussed above, to account for additional calibration effecting
parameters, such as
environment, an individual user's characteristics, sensor lifetime,
manufacturing characteristics
(such as lot characteristics), deoxidization, enzyme concentration fluctuation
and/or degradation,
power supply variations, and/or the like.
In particular implementations, after a first calibration is performed on a
particular glucose
sensor 12, subsequent calibrations may employ a weighted average using a
sensitivity ratio
(SPSR, MSPSR, LRSR, or MLRSR) calculated from data collected since the last
calibration, and
26
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
previous sensitivity ratios calculated for previous calibrations. Here, an
initial sensitivity ratio
(SRI) may be calculated immediately after initialization/stabilization using a
paired calibration
data point, and used by glucose monitor 100 or post processor 200 until a
second sensitivity ratio
(SR2) is calculated. Here, second sensitivity ratio SR2 may comprise an
average of SRI and the
sensitivity ratio as calculated using the paired calibration data points since
the initial calibration
(SRday 1) as follows:
SR2 = SRI+ SRdayl
2
The third sensitivity ratio (SR3) is an average of SR2 and the sensitivity
ratio as
calculated using the paired calibration data points since the second
calibration (SRday2). The
equation is as follows:
SR3 = SR2 + SRday2
2
Sensitivity ratios for successive days may be similarly determined as follows:
SR = SR(
SR,"-
1) + SRday(,,_,)
õ
2
where:
SR ,7 is the new sensitivity ratio calculated at the beginning of time period,
n, using data from time
period (n-1), to be used by glucose monitor 100, to convert Valid ISIGs
measurement values to
blood glucose readings throughout time period n;
SR(n_i) is a previous sensitivity ratio calculated at the beginning of time
period n-1, using data
from time period n-2; and
SRday(n_i) is the sensitivity ratio calculated using paired calibration data
points collected since the
last calibration.
Alternatively, previous sensitivity ratios may be ignored and SR may be
calculated using
only the paired calibration data points since the last calibration. In another
alternative, all
previous SRs may be averaged with the latest SR calculated using only the
paired calibration data
points since the last calibration. In other implementations, the paired
calibration data points are
used to establish an equation for a curve representing SR over time. The curve
may then used to
extrapolate SR to be used until the next paired calibration data point is
entered.
27
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
In embodiments that use a post processor 200 to evaluate a sensitivity ratio,
such a
sensitivity ratio may be calculated using paired calibration data points over
a period of time since
a last calibration, and is not averaged with previous sensitivity ratios. A
sensitivity ratio
determined for a period of time may then be applied to the same period of time
over which the
paired calibration data points were collected. This may result in a more
accurate than the real-
time case described above for the glucose monitor 100 because, in the real-
time case, sensitivity
ratios from a previous time period must be used to calculate the blood glucose
level in the
present time period. If the sensitivity ratio has changed over time,
estimation of blood glucose
using an old sensitivity ratio may introduce an error.
In particular embodiments, once calibration is complete, Valid ISIG values may
be
converted to blood glucose readings based on a particular version of the
sensitivity ratio, and the
resulting blood glucose readings are compared to an out-of-range limit. If
such a resulting
calculated blood glucose level is greater than a maximum out-of-range limit of
200 mg/di (or
equivalently 3600 mmo1/1), the out-of-range alarm is activated. This is a
calibration cancellation
event, therefore, ISIG values are no longer valid once this alarm is
activated. Blood glucose
readings are either not calculated, or at least not considered reliable, until
the glucose monitor
100 or post processor 200 is re-calibrated. The user may be notified of the
alarm and that re-
calibration is needed.
In alternative embodiments, higher or lower maximum out-of-range limits may be
used
depending on the sensor characteristics, the characteristic being measured,
the user's body
characteristics, and the like. In particular implementations, a minimum out-of-
range limit may
be used or both a maximum and a minimum out-of-range limits may be used. In
other particular
embodiments, such out-of-range limits may not cause blood glucose readings to
become invalid
and/or re-calibration is not required; however, an alarm could still be
provided. In additional
particular embodiments, an alarm may be activated in response to two or more
ISIG values
exceeding an out-of-range limit. ISIG values that are out-of-range may be
omitted from display.
In alternative embodiments, calibration may be conducted by injecting a fluid
containing
a known value of glucose into the site around the glucose sensor set 10,
followed by sending one
or more glucose sensor readings to glucose monitor 100. The readings may then
be processed
(filtered, smoothed, clipped, averaged, and/or the like) and used along with
the known glucose
value to calculate the SR for the glucose sensor 12. Particular alternative
embodiments may use
a glucose sensor set of the type described in U.S. Pat. No. 5,951,521 entitled
"A Subcutaneous
28
CA 02745090 2011-05-30
WO 2010/077328
PCT/US2009/006677
Implantable Sensor Set Having the Capability To Remove Or Deliver Fluids To An
Insertion
Site".
In other alternative embodiments, glucose sensor 12 may be supplied with a
vessel
containing a solution with a known glucose concentration to be. used as a
reference, and glucose
sensor 12 is immersed into the reference glucose solution during calibration.
Glucose sensor 12
may be shipped in the reference glucose solution, for example. As described
above, glucose
sensor readings may be used to calculate a sensitivity ratio given a known (or
independently
measured) glucose concentration of the solution.
In another alternative embodiment, glucose sensors 12 may be calibrated during
a
manufacturing process. Sensors from the same manufacturing lot have similar
properties may be
calibrated using a sampling of glucose sensors 12 from the population and a
solution with a
known glucose concentration. A sensitivity ratio is provided with the glucose
sensor 12 and is
entered into glucose monitor 100 or post processor 200 by the user or another
individual.
In addition, although the particular process of FIG. 18 includes specific
operations
occurring in a particular order, in alternative embodiments, certain of these
operations may be
performed in a different order, modified, or removed while not deviating from
claimed subject
matter. Moreover, other operations may be added to and/or combined with the
above described
process without deviating from claimed subject matter. For example, although
in the particular
embodiment of FIG. 16 the variable Recal is never reset to no fail,
potentially, an additional
operation may be added to reset Recal to no fail if no cal error alarms are
triggered after a
predetermined number of calibrations.
Unless specifically stated otherwise, as apparent from the following
discussion, it is
appreciated that throughout this specification discussions utilizing terms
such as "processing",
"computing", "calculating", "determining", "estimating", "selecting",
"weighting",
"identifying", "obtaining", "representing", "receiving", "transmitting",
"storing", "analyzing",
"creating", "contracting", "associating", "updating", or the like refer to the
actions or processes
that may be performed by a computing platform, such as a computer or a similar
electronic
computing device, that manipulates or transforms data represented as physical,
electronic or
magnetic quantities or other physical quantities within the computing
platform's processors,
memories, registers, or other information storage, transmission, reception or
display devices.
Accordingly, a computing platform refers to a system or a device that includes
the ability to
process or store data in the form of signals. Thus, a computing platform, in
this context, may
comprise hardware, software, firmware or any combinations thereof. Further,
unless specifically
29
CA 02745090 2016-04-07
WO 2010/077328 PCT/US2009/006677
stated otherwise, a process as described herein, with reference to flow
diagrams or otherwise,
may also be executed or controlled, in whole or in part, by a computing
platform.
It should be noted that, although aspects of the above system, method, or
process have
been described in a particular order, the specific order is merely an example
of a process and
claimed subject matter is of course not limited to the order described. It
should also be noted
that the systems, methods, and processes described herein, may be capable of
being performed
by one or more computing platforms. In addition, the methods or processes
described herein
may be capable of being stored on a storage medium as one or more machine
readable
instructions, that if executed may enable a computing platform to perform one
or more actions.
"Storage medium" as referred to herein relates to media capable of storing
information or
instructions which may be operated on, or executed by, by one or more
machines. For example,
a storage medium may comprise one or more storage devices for storing machine-
readable
instructions or information. Such storage devices may comprise any one of
several media types
including, for example, magnetic, optical or semiconductor storage media. For
further example,
one or more computing platforms may be adapted to perform one or more of the
processed or
methods in accordance with claimed subject matter, such as the methods or
processes described
herein. However, these are merely examples relating to a storage medium and a
computing
platform and claimed subject matter is not limited in these respects.
While there has been illustrated and described what are presently considered
to be
example features, it will be understood by those skilled in the art that
various other modifications
may be made, and equivalents may be substituted, without departing from
claimed subject
matter. Additionally, many modifications may be made to adapt a particular
situation to the
teachings of claimed subject matter without departing from the central concept
described herein.
30