Note: Descriptions are shown in the official language in which they were submitted.
CA 02745229 2011-07-04
TITLE OF INVENTION
Synthesis of Rare Earth Metal Extractant
10
TECHNICAL FIELD
This invention relates to a method for synthesizing an
extractant for extracting and separating a selected rare
earth element from a mixture of rare earth elements,
specifically from a mixture of at least two light rare earth
elements (La, Ce, Pr, Nd, Sm, and Eu) or from a mixture of at
least one light rare earth element and at least one other
rare earth element inclusive of yttrium.
BACKGROUND ART
In the modern society, rare earth elements are used in
a wide variety of applications, for example, as rare earth
magnets, phosphors, and electronic and electric materials in
nickel hydrogen batteries. With respect to the supply of rare
earth elements, a crisis of the rare earth resource is
highlighted because the producers are limited, the price lacks
stability, and the demand is expected to surpass the supply in
the near future. For these reasons, many attempts are made to
reduce the amount of rare earth element used and to develop a
replacement. At the same time, it is desired to establish a
recycle system for recovering rare earth elements as one
valuable from in-process scraps produced during manufacture of
products and municipal wastes like electric and electronic
appliances collected from cities. Also there is an urgent
need for the research and development of new rare earth mines.
-1-
CA 02745229 2011-07-04
Known methods for separating rare earth elements
include column extraction (or solid to liquid extraction)
using ion exchange resins, and solvent extraction (or liquid
to liquid extraction). Although the column extraction (or
solid to liquid extraction) method is simple in apparatus and
easy in operation as compared with the solvent extraction, it
Is small in extraction capacity and discourages rapid
treatment. The column extraction method is thus used in the
removal of a metal when the concentration of a metal to be
lo extracted in a solution is low, that is, when the metal to be
extracted is present as an impurity, as well as in the waste
water treatment. On the other hand, the solvent extraction
(or liquid to liquid extraction) method needs a complex
apparatus and cumbersome operation as compared with the
column extraction, but provides for a large extraction
capacity and rapid treatment. The solvent extraction method
is thus used in industrial separation and purification of
metal elements. For the separation and purification of rare
earth elements that requires efficient treatment of a large
volume through continuous steps, the solvent extraction
method capable of such efficient treatment is often used.
In the solvent extraction method, an aqueous phase
consisting of an aqueous solution containing metal elements
to be separated is contacted with an organic phase consisting
of an extractant for extracting a selected metal element and
an organic solvent for diluting the extractant. Then the
metal element is extracted with the extractant for
separation.
Known extractants used in the art include tributyl
phosphate (TBP), carboxylic acids (e.g., Versatic Acid 10),
phosphoric acid esters, phosphonic acid compounds, and
phosphinic acid compounds. These extractants are
commercially available. A typical phosphoric acid ester is
di-2-ethylhexylphosphoric acid (D2EHPA), a typical phosphonic
acid compound is 2-ethylhexylphosphonic acid-mono-2-
ethylhexyl ester (PC-88A by Daihachi Chemical Industry Co.,
Ltd.), and a typical phosphinic acid compound is bis(2,4,4
-2-
CA 02745229 2011-07-04
trimethylpentyl)phosphinic acid (Cyanex 272 by Cytec
Industries).
The separation efficiency of the solvent extraction
method depends on a separation ability of the metal
extractant, specifically a separation factor. As the
separation factor is higher, the separation efficiency of the
solvent extraction method is higher, which enables
simplification of separating steps and scale-down of the
separation apparatus, making the process efficient and
lo eventually leading to a cost reduction. A low separation
factor, on the other hand, makes the separation process
complex and poses a need for a large-scale separation
apparatus.
Even PC-88A which is known to have a high separation
factor for rare earth elements among the currently
commercially available extractants has a low separation
factor between elements of close atomic numbers, for example,
a separation factor of less than 2, specifically about 1.4
between neodymium and praseodymium which are allegedly most
difficult to separate among rare earth elements. The
separation factor of this value is not sufficient for
separation between neodymium and praseodymium. To separate
them at an acceptable purity, a large-scale apparatus must be
installed at the expense of cost. For more efficient
separation of these elements, there is a desire for the
development of an extractant having a higher separation
factor than in the prior art and an extracting/separating
method using the same.
Dialkyl diglycol amic acids are known from JP-A
2007-327085 as the metal extractant having a high separation
factor with respect to rare earth elements, specifically
light rare earth elements such as lanthanum (La), cerium
(Ce), praseodymium (Pr), neodymium (Nd), and samarium (Sm).
Using this extractant in solvent extraction, the
extraction/separation step of rare earth elements,
specifically light rare earth elements can be made more
efficient. In fact, better results are obtained from the
-3-.
CA 02745229 2011-07-04
extraction/separation step of light rare earth elements using
dialkyl diglycol amic acid on a laboratory scale.
When dialkyl diglycol amic acid was used as the metal
extractant, satisfactory results were confirmed in a light
rare earth element extraction/separation experiment which was
conducted at a rare earth element concentration (CA: 0.01
mol/L s CA 0.7 mol/L) and a corresponding metal extractant
concentration (C,: 0.1 mol/L C, s 1.5 mol/L) which were
practical operating conditions of the rare earth element
lo separating process and in a light rare earth element
extraction/separation experiment using a countercurrent flow
multi-stage mixer/settler of a practically operating
apparatus.
The dialkyl diglycol amic acid exhibits a satisfactory
separation factor in its performance as the metal extractant
for separating light rare earth elements, as mentioned above,
and its operating conditions have been surveyed. However,
its synthesis has not been fully established.
The known method for synthesizing the dialkyl diglycol
amic acid is in accord with the following reaction scheme.
C4H404 + R1R2NH R1R2NCOCH2OCH2COOH
in CH2C12
diglycolic secondary dialkyl diglycol amic acid
anhydride alkyl amine in dichloromethane
0 to 30 C
Herein R1 and R2 are each independently alkyl, and at least
one is a straight or branched alkyl group of at least 6
carbon atoms.
First, diglycolic anhydride is suspended in
dichloromethane. A secondary alkylamine in an amount
slightly less than an equimolar amount to the diglycolic
anhydride is dissolved in dichloromethane and the resulting
solution is mixed with the suspension at 0 to 30 C. As
diglycolic anhydride reacts, the mixed solution becomes
clear. The reaction is completed when the solution becomes
-4-
CA 02745229 2011-07-04
69562-88
clear. This is followed by removal of water-soluble
impurities by washing with deionized water, removal of water
with a dehydrating agent (e.g., sodium sulfate), filtration,
and solvent removal. Recrystallization from hexane is
repeated plural times for purification, yielding the desired
product (see JP-A 2007-327085).
This synthesis method uses as the synthesis medium
dichloromethane which is one of the harmful substances listed
in several environmental pollution control laws, regulations
lo and Pollutant Release and Transfer Register (PRTR) in Japan
and the corresponding regulations in many countries. It is
recommended to avoid the substance.
The above synthesis method allegedly gives a yield of
more than 90% because it is conducted only on a laboratory
scale where the amount of synthesis is several grams.
However, a prominent drop of yield occurs when the synthesis
is enlarged to a scale of several kilograms or more. In
fact, in a synthesis experiment conducted on a scale of
several hundreds of grams, the yield decreases below 80%.
Such a yield drop is unwanted.
Citation List
Patent Document 1: JP-A 2007-327085
SUMMARY OF INVENTION
The invention relates to a method for synthesizing
a rare earth metal extractant without a need for
dichloromethane which is used as reaction medium in the prior
art synthesis, the method being capable of improving the
yield of the reaction product and the efficiency of
synthesis.
The inventors have found that in the synthesis of a
dialkyl diglycol amic acid serving as a rare earth metal
extractant, better results are obtained by reacting
reactants, diglycolic anhydride and a dialkylamine in a
specific synthesis medium. Used as the synthesis medium is a
non-polar or low-polar solvent in which the dialkyl diglycol
amic acid is dissolvable and which will serve as an organic
- 5 -
81684998
solvent to form an organic phase in subsequent solvent
extraction. This method permits the dialkyl diglycol amic acid
to be effectively synthesized in high yields.
The invention relates to a method for synthesizing a rare
earth metal extractant in the form of a dialkyl diglycol amic
acid having the general formula (1):
R1
______________________________________ COCH2OCH2C0011 (1)
R2
wherein Rl and R2 are each independently alkyl, at least one being
a straight or branched alkyl group of at least 6 carbon atoms,
including the step of reacting diglycolic anhydride with a
dialkylamine in a synthesis medium. The dialkylamine (B) and
diglycolic anhydride (A) are present in a molar ratio (B/A) of at
least 1Ø Preferably the molar ratio (B/A) of dialkylamine (B)
to diglycolic anhydride (A) is in a range of 1.0 to 1.2. The
synthesis medium used herein is a non-polar or low-polar solvent
in which the dialkyl diglycol amic acid is dissolvable and which
will serve as an organic solvent to form an organic phase in
subsequent solvent extraction. The non-polar or low-polar solvent
is typically selected from among toluene, xylene, hexane,
isododecane, kerosine, and higher alcohols.
The invention further relates to a method for synthesizing
a rare earth metal extractant in the form of a dialkyl diglycol
amic acid having the general formula (1):
N¨COCH2OCH2COOH ( 1)
R2
- 6 -
CA 2745229 2017-08-24
81684998
wherein Rl and R2 are each independently alkyl, at least one
being a straight or branched alkyl group of 6 to 12 carbon
atoms, comprising the step of reacting diglycolic anhydride
with a dialkylamine in a synthesis medium, wherein dialkylamine
(B) and diglycolic anhydride (A) are present in a molar ratio
(B/A) of at least 1.0, and the synthesis medium is a non-polar
or low-polar solvent in which the dialkyl diglycol amic acid is
dissolvable and which will serve as an organic solvent to form
an organic phase in subsequent solvent extraction.
Also preferably the synthesis medium is used in such an
amount that the reaction solution at the end of reaction may
contain the dialkyl diglycol amic acid in a concentration Cc of
0.1 mol/L to 1.5 mol/L.
ADVANTAGEOUS EFFECTS OF INVENTION
According to the method of the invention, a dialkyl
diglycol amic acid which is an extractant having an improved
separation factor for light rare earth elements can be
effectively synthesized in high yields without a need for a
harmful solvent, dichloromethane. The method is of great worth
in the industry.
- 6a -
CA 2745229 2017-08-24
CA 02745229 2011-07-04
BRIEF DESCRIPTION OF DRAWINGS
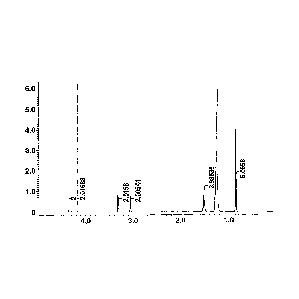
FIG. 1 is a 1H-NMR chart of the reaction product of
Example 1.
FIG. 2 is a 1H-NMR chart of the reaction product of
s Comparative Example 1.
DESCRIPTION OF EMBODIMENTS
The invention pertains to a rare earth metal
extractant which is a dialkyl diglycol amic acid having the
lo general formula (1).
R1
____________________ COCH2OCH2COOH (1)
R'
Herein RI and R2 are each independently alkyl, at least
one of R1 and R2 being a straight or branched alkyl group of
at least 6 carbon atoms, preferably 6 to 18 carbon atoms, and
Is more preferably 7 to 12 carbon atoms. If the carbon count is
less than 6, the compound failing to play the role of
extractant because it is less lipophilic so that the organic
phase lacks stability and exhibits poor separation from the
aqueous phase, and because the dissolution of the extractant
20 itself in aqueous phase becomes noticeable. An excessive
carbon count contributes to no improvements in basic
abilities like extraction and separation abilities despite
the increased cost of extractant manufacture. As long as
lipophilic nature is ensured, if one of R1 and R2 has a
25 carbon count of at least 6, then the other may be of less
than 6 carbon atoms. For example, a compound of formula (1)
wherein two octyl (-C81117) groups are introduced is most
preferred, which is named N,N-diocty1-3-oxapentane-1,5-amic
acid or dioctyl diglycol amic acid (abbreviated as DODGAA,
30 hereinafter).
According to the invention, the dialkyl diglycol amic
acid is synthesized by reacting diglycolic anhydride with a
dialkylamine in a synthesis medium. The synthesis medium
used herein is a non-polar or low-polar solvent in which the
-7-
CA 02745229 2011-07-04
dialkyl diglycol amic acid is dissolvable and which will
serve as an organic solvent to form an organic phase in
subsequent solvent extraction. For example, diglycolic
anhydride is suspended in an organic solvent (which will form
an organic phase in subsequent solvent extraction), and the
dialkylamine is dissolved in an organic solvent (which will
form an organic phase in subsequent solvent extraction). The
suspension and the solution are mixed together for reaction
to take place. The dialkylamine used herein is a secondary
lo alkylamine having alkyl groups corresponding to R' and R2 in
the dialkyl diglycol amic acid of formula (1).
The organic solvent which is used herein as the
synthesis medium and which will form an organic phase in
subsequent solvent extraction is a non-polar or low-polar
solvent in which the dialkyl diglycol amic acid is
dissolvable. The non-polar or low-polar solvent is a solvent
having a dielectric constant of up to 15, for example, having
a low solubility in water, providing an appropriate
solubility for the extractant, having a low specific gravity,
and facilitating an extraction ability. Preferably the
non-polar or low-polar solvent is selected from among
toluene, xylene, hexane, isododecane, kerosine, and higher
alcohols such as straight chain alcohols of 5 to 8 carbon
atoms. Use of such an organic solvent as the synthesis
medium eliminates a need for removal of the synthesis medium
and ensures that the organic solvent present in the reaction
solution may be used as the organic phase for solvent
extraction directly or, if necessary, after an additional
amount of the organic solvent is added so as to provide the
organic phase with a desired metal extractant concentration
for solvent extraction.
If the synthesis medium is a solvent other than the
non-polar or low-polar solvent in which the dialkyl diglycol
amic acid is dissolvable and which will serve as an organic
solvent to form an organic phase in subsequent solvent
extraction, then the synthesis medium must be removed after
the reactants are mixed and reacted therein.
-8-
CA 02745229 2011-07-04
In the reaction step, an amount (B mol) of
dialkylamine and an amount (A mol) of diglycolic anhydride
are used in a molar ratio (B/A) of at least 1.0, preferably
1.0 5 B/A 5 1.2, and more preferably 1.0 5 B/A 5 1.1. The
resulting reaction product contains unreacted dialkylamine as
well as the desired dialkyl diglycol amic acid. In the prior
art method, plural times of recrystallization are necessary
to remove the unreacted dialkylamine. It has been found that
when solvent extraction is carried out using dialkyl diglycol
amic acid having dialkylamine left therein, no problems arise
with respect to separation efficiency and phase separation,
ensuring effective extraction and separation. Specifically,
even if the dialkylamine is left in the metal extractant and
the organic phase during solvent extraction, it does not
become an inhibitory factor to extraction and separation and
there is no need to remove it as an impurity. As a result,
the synthesis process can be simplified. A loss of the
reaction product by recrystallization is minimized. These
contribute to improved yields.
If B/A > 1.2, the resulting reaction product may
contain an excess of unreacted dialkylamine as well as the
desired dialkyl diglycol amic acid. This reaction product
may be used as the extractant because no problems arise with
respect to separation efficiency and phase separation during
solvent extraction. However, use of excess dialkylamine is
meaningless. Also the cost of reactants for synthesis
increases, rendering the method less cost effective.
If B/A < 1.0, which means an excess of diglycolic
anhydride for reaction, the desired dialkyl diglycol amic
acid is obtained as the reaction product, in which unreacted
diglycolic acid may remain. When solvent extraction is
carried out using dialkyl diglycol amic acid having
diglycolic acid left therein, no satisfactory separation
ability is available and the solution becomes white turbid
because clad is formed between organic phase and aqueous
phase. This results in poor phase separation, failing in
normal extraction and separation. This is because the
-9-
CA 02745229 2011-07-04
diglycolic acid remaining along with the metal extractant,
dialkyl diglycol amic acid forms a complex with a rare earth
metal ion, inhibiting satisfactory extraction and separation.
That is, diglycolic acid becomes an inhibitory factor to
extraction. To obtain diglycolic acid-free dialkyl diglycol
amic acid as the rare earth metal extractant capable of
normal extraction and separation, the step of removing
unreacted diglycolic acid is necessary as in the prior art
method. Specifically, the water-soluble diglycolic acid must
lo be removed by removing the synthesis solvent and washing the
reaction product with water. Upon water washing, however,
the dialkyl diglycol amic acid having a very low solubility
in water crystallizes and precipitates in the solvent (for
example, a solubility of DODGAA in water is 6.2x10-6 mol/L).
In order to use the dialkyl diglycol amic acid in
crystallized form as the rare earth metal extractant,
filtration and drying steps are needed. The process becomes
less efficient because extra steps are necessary as compared
with the range of 1.0 s B/A s 1.2.
In a preferred embodiment of the method, the synthesis
medium is used in such an amount that the reaction solution
at the end of reaction may contain the dialkyl diglycol amic
acid in a concentration of 0.1 mol/L to 1.5 mol/L.
Specifically, the amount of dialkyl diglycol amic acid
produced by the synthesis reaction is previously computed
from the amounts of reactants by the stoichlometry in accord
with the reaction scheme, and the amount of the synthesis
medium is adjusted such that the concentration C, of metal
extractant or dialkyl diglycol amic acid in the reaction
solution may fall in a range: 0.1 mol/L s C, S 1.5 mol/L, and
more preferably 0.2 mol/L s C, 5 1.0 mol/L. The reaction
solution obtained in this preferred embodiment may be used as
the organic phase in subsequent solvent extraction directly,
i.e., without a need for concentration adjustment during
subsequent solvent extraction, for example, by adding a
solvent such that the metal extractant in the organic phase
-10-
CA 02745229 2011-07-04
may be present in a predetermined concentration applicable in
the practical extraction step.
In case the extractant concentration C, < 0.1 mol/L,
the dialkyl diglycol amic acid is produced by synthesis.
However, when this reaction product is used in solvent
extraction on an actual operation scale, the metal extractant
concentration in the organic phase is so low that only an
aqueous solution having a concentration of up to 0.03 mol/L
of rare earth elements may be treated. This entails a larger
lo scale of separation apparatus and a cost increase. It is
very difficult, inefficient and impractical to increase the
extractant concentration from such a low level to a high
level for actual operation.
On the other hand, it is often difficult to set an
extractant concentration C, > 1.5 mol/L, from considerations
of the solubility of the dialkyl diglycol amic acid in
organic solvents used in general solvent extraction methods.
After the synthesis reaction, a portion of the dialkyl
diglycol amic acid which is not dissolved in the solvent may
crystallize and precipitate out. Although the extra portion
may be dissolved by adding a solvent, surfactant or
entrainer, the reaction product solution is not efficient as
the organic phase for solvent extraction because the control
of conditions for stable operation becomes more complex.
EXAMPLE
Examples are given below by way of illustration and
not by way of limitation.
Example 1 and Comparative Example 1
Synthesis of rare earth metal extractant and
extraction/separation test
DODGAA was synthesized by the method of the invention.
The DODGAA thus synthesized was examined for an ability to
separate rare earth metals from a mixture thereof by the
solvent extraction method.
-11-
CA 02745229 2011-07-04
First, 34.8 g (0.3 mol) of diglycolic anhydride was
suspended in 400 mL of hexane as synthesis medium.
Separately, 72.4 g (0.3 mol) of dioctylamine was dissolved in
100 mL of hexane. With stirring, the dioctylamine solution
was added dropwise to the diglycolic anhydride suspension.
Stirring was continued at room temperature until it was
confirmed that the solution became clear as a result of
reaction of diglycolic anhydride. The reaction product was
obtained in hexane solution (Example 1).
lo In Comparative Example 1, the same procedure as above
was repeated aside from using acetone as the reaction medium.
The reaction product was obtained in acetone solution.
Samples of the reaction products in Example 1 and
Comparative Example 1 were taken out and vacuum dried for
solvent removal, before they were analyzed by 'H-NMR
spectroscopy as shown in FIGS. 1 and 2, respectively. The
reaction products in Example 1 and Comparative Example 1 were
identified to be DODGAA.
An extraction/separation test was performed. The
concentration of DODGAA in the reaction product solution of
Example 1 or Comparative Example 1 was computed from the
amounts of reactants and synthesis medium. The reaction
product solution was diluted with hexane to form an organic
solution having a DODGAA concentration of 0.3 mol/L, which
might become an organic phase.
An aqueous solution containing mixed rare earth metals
was prepared by dissolving praseodymium chloride and
neodymium chloride in water in a molar ratio Pr:Nd of 1:1 and
a concentration of 0.1 mol/L of Pr+Nd to form an aqueous
solution, which might become an aqueous phase. A separatory
funnel was charged with 100 mL of the organic solution and
100 mL of the aqueous solution and shaken at 20 C for about
20 minutes to effect extraction. After equilibrium was
reached, the liquid was allowed to separate into organic and
aqueous phases. A separatory funnel was charged with 100 mL
of the thus separated organic phase and 100 mL of 5N
hydrochloric acid and shaken at 20 C for about 20 minutes
-12-
CA 02745229 2011-07-04
whereby the rare earth element once extracted into the
organic phase was back-extracted into the aqueous
hydrochloric acid solution. The concentrations of
praseodymium and neodymium in the aqueous phase and the back-
s extracted aqueous hydrochloric acid solution were measured by
an ICP atomic emission spectrometer ICP-7500 (Shimadzu
Corp.). The Nd/Pr separation factor and phase separation are
reported in Table 1.
Table 1
Synthesis medium Nd/Pr Phase
separation
separation factor
Example 1 hexane 2.5 definite
Comparative
acetone 2.5 indefinite
Example 1
For the reaction product obtained in Example 1, its
Nd/Pr separation factor indicative of the separation ability
as a metal extractant was satisfactory, and the phase
separation state was definite. For the reaction product
obtained in Comparative Example 1, its Nd/Pr separation
factor was satisfactory, but it was inadequate for solvent
extraction as demonstrated by an indefinite phase separation
state. It is evident that when a dialkyl diglycol amic acid
is synthesized using as the synthesis medium a non-polar or
low-polar solvent in which the dialkyl diglycol amic acid is
dissolvable and which will serve as an organic solvent to
form an organic phase in subsequent solvent extraction, the
process becomes very efficient due to an eliminated need for
solvent removal.
Examples 2 to 5 and Comparative Example 2
An amount (designated A in Table 2) of diglycolic
anhydride was suspended in 40 mL of hexane. Separately, an
amount (designated B in Table 2) of dioctylamine was
-13-
CA 02745229 2011-07-04
dissolved in 10 mL of hexane. With stirring, the
dioctylamine solution was added dropwise to the diglycolic
anhydride suspension. Stirring was continued at room
temperature until it was confirmed that the solution became
clear as a result of reaction of diglycolic anhydride. The
reaction product was obtained in hexane solution. Table 2
also reports a ratio B/A that is a ratio of the amount (B
mmol) of dioctylamine to the amount (A mmol) of diglycolic
anhydride.
Samples of the reaction products were taken out and
vacuum dried for hexane removal, before they were analyzed by
'H-NMR spectroscopy, with DODGAA detected in all the
products. A minor amount of dioctylamine was detected in
Examples 2, 3 and 5 while a minor amount of diglycolic acid
detected in Comparative Example 2.
An extraction/separation test was performed. The
concentration of DODGAA in the reaction product solution was
computed from the amounts of reactants and synthesis medium.
The reaction product solution was diluted with hexane to form
an organic solution having a DODGAA concentration of 0.3
molt L, which might become an organic phase.
An aqueous solution containing mixed rare earth metals
was prepared by dissolving praseodymium chloride and neodymium
chloride in water in a molar ratio Pr:Nd of 11 and a
concentration of 0.1 mol/L of Pr+Nd to form an aqueous
solution, which might become an aqueous phase. A separatory
funnel was charged with 100 mL of the organic solution and 100
mL of the aqueous solution and shaken at 20 C for about 20
minutes to effect extraction. After equilibrium was reached,
the liquid was allowed to separate into organic and aqueous
phases. A separatory funnel was charged with 100 mL of the
thus separated organic phase and 100 mL of 5N hydrochloric
acid and shaken at 20 C for about 20 minutes whereby the rare
earth element once extracted into the organic phase was back-
extracted into the aqueous hydrochloric acid solution. The
concentrations of praseodymium and neodymium in the aqueous
phase and the back-extracted aqueous hydrochloric acid
-14-
CA 02745229 2011-07-04
solution were measured by an ICP atomic emission spectrometer
ICP-7500 (Shimadzu Corp.). The extractant state, Nd/Pr
separation factor, and phase separation are reported in Table
2.
Table 2
A
Nd/Pr
diglycolic.Extractant Phase
doctylamine B/A separation
anhydride state
separation
factor
(g) (mmol) (g) (mmol)
Example 2 3.5 30.2 8.4 34.8 1.15 liquid 2.5
definite
Example 3 3.5 30.2 8.0 33.1 1.10 liquid 2.5
definite
Example 4 3.5 30.2 7.3 30.2 1.00 liquid 2.5
definite
Example 5 3.5 30.2 9.0 37.3 1.24 solid 2.5
definite
Comparative
3.9 33.6 7.3 30.2 0.90 liquid
indefinite
Example 2
In Examples 2, 3 and 4 wherein a ratio of the amount
(B mmol) of dioctylamine to the amount (A mmol) of diglycolic
anhydride is 1.0 s B/A s 1.2, the Nd/Pr separation factor
indicative of the separation ability of a metal extractant
and the phase separation were satisfactory.
In Example 5 wherein B/A > 1.2, the Nd/Pr separation
factor and the phase separation were satisfactory, but the
reaction product was difficult to handle as compared with the
other products because the excess dioctylamine in the
reaction product solidified. In Comparative Example 2, the
excess diglycolic anhydride became an inhibitory factor to
extraction, causing indefinite phase separation, and the
Nd/Pr separation factor could not be measured.
Examples 6 to 9
Diglycolic anhydride, 46.4 g (0.4 mol), was suspended
in X mL of hexane. Separately, 96.6 g (0.4 mol) of
-15-
CA 02745229 2011-07-04
dioctylamine was dissolved in Y mL of hexane. With stirring,
the dioctylamine solution was added dropwise to the
diglycolic anhydride suspension. Stirring was continued at
room temperature until it was confirmed that the solution
became clear as a result of reaction of diglycolic anhydride.
The reaction product was obtained in hexane solution. The
amounts X and Y of hexane as the reaction medium are shown in
Table 3.
Samples of the reaction products were taken out and
lo vacuum dried for hexane removal, before they were analyzed by
111-NMR spectroscopy, with DODGAA detected in all the
products. The concentration C, of the reaction product
(DODGAA) in the hexane solution is shown in Table 3.
An extraction/separation test was performed using the
hexane solution of the reaction product (DODGAA) directly as
an organic solution which might become an organic phase.
An aqueous solution containing mixed rare earth metals
was prepared by dissolving praseodymium chloride and
neodymium chloride in water in a molar ratio Pr:Nd of 1:1 and
a concentration (mol/L) of Pr+Nd as shown in Table 4 to form
an aqueous solution which might become an aqueous phase. A
separatory funnel was charged with 100 mL of the organic
solution and 100 mL of the aqueous solution and shaken at
20 C for about 20 minutes to effect extraction. After
equilibrium was reached, the liquid was allowed to separate
into organic and aqueous phases. A separatory funnel was
charged with 100 mL of the thus separated organic phase and
100 mL of 5N hydrochloric acid and shaken at 20 C for about
20 minutes whereby the rare earth element once extracted into
the organic phase was back-extracted into the aqueous
hydrochloric acid solution. The concentrations of
praseodymium and neodymium in the aqueous phase and the back-
extracted aqueous hydrochloric acid solution were measured by
an ICP atomic emission spectrometer ICP-7500 (Shimadzu
Corp.). The Nd/Pr separation factor and phase separation are
reported in Table 4.
-16-
CA 02745229 2011-07-04
Table 3
Amount X (mL) Amount Y (mL) DODGAA Concentration Co
of hexane of hexane (mol/L)
Example 6 3200 800 0.1
Example 7 640 160 0.5
Example 8 320 80 1.0
Example 9 214 53 1.5
Table 4
Mixed rare earth metal
concentration Nd/Pr Phase
(mol/L) separation factor separation
Example 6 0.03 2.5 definite
Example 7 0.10 2.5 definite
Example 8 0.10 2.5 definite
Example 9 0.50 2.5 definite
Examples 6 to 9 wherein the concentration C, (mol/L) of
DODGAA was in the range: 0.1 mol/L C, 1.5 mol/L
demonstrated a high separation factor and definite phase
separation.
-17-