Note: Descriptions are shown in the official language in which they were submitted.
CA 02746986 2016-07-13
WATER SOFTENER SYSTEM
USING NANOFILTRATION TO RECLAIM A PORTION
OF THE REGENERATING SODIUM CHLORIDE
10
HELD OF THE INVENTION
The present invention relates to water softening systems. In particular, the
present
invention relates to a water softening system having a filtration system that
separates hardness
ions from brine so that the brine can be recycled instead of being discharged
with the
hardness ions.
BACKGROUND OF THE INVENTION
Among industrialized nations of the world, there is a growing concern for, and
emphasis on, environmentally responsible practices. For example, more and more
governments and communities are interested in minimizing the kinds and
quantities of
chemicals that are deposited into water systems, including wastewater systems.
A common
form of wastewater pollution is the brine solution discharged into sewers or
septic systems
-1-
CA 02746986 2011-07-21
during typical regeneration processes of water softeners.
For the last fifty years or so, water softening has become widely used in
those regions
where water supplies contain high concentrations of calcium and magnesium, and
are
therefore considered "hard". Utilizing a sodium ion exchange process, resin-
based water
softeners are installed on water lines, particularly those leading into
residences, to soften most
if not all of the water used inside such homes. As a water supply passes
through ion
exchange resins inside a water softener, the calcium and magnesium ions bond
to the resins
and are removed from the water flow.
Periodically, these ion exchange resins must be regenerated. Typically this
regeneration is accomplished utilizing a brine solution such as sodium or
potassium chloride.
In a typical regeneration process, the brine solution is slowly pumped through
the resin bed.
Through a chemical exchange process, the calcium and magnesium ions which were
adsorbed
onto the resin are stripped off and replaced with sodium or potassium ions. At
the conclusion
of this process, the "spent" brine solution containing both the hardness ions
and the brine is
discharged into the sewer or septic system. This discharge has serious long-
term effects on
the environment, as the brine salinity, total dissolved solids, and/or
chloride cause corrosion
in the sewage system, and contaminate the planet's fresh water supplies.
Presently, because this pollution problem has defied resolution by
economically
acceptable means, some communities are resorting to banning or limiting water
softening in
2 0 homes. For example, on October 12, 2009, California governor Arnold
Schwarzenegger
signed into law, a bill giving local California water agencies the authority
to restrict or even
to ban the use of water softeners using on-site salt-based regeneration.
Scientific studies such as one conducted by Santa Clarita, California are
finding that
-2-
CA 02746986 2011-07-21
brine solution discharged from water softeners is a significant source of
water pollution. This
finding supports prohibitions of, or restrictions on, current commercially
available water
softening systems. Consequently, removing the salts from the spent brine
solution before the
solution is discharged has become an immediate and real concern both for
communities that
want soft water and for water softener manufacturers.
SUMMARY OF THE INVENTION
The present invention relates to an improved water softener system comprising
apparatus and a process by which the apparatus operates, to separate hardness
ions from brine
solution in a way to allow most of the brine solution to be reclaimed, thereby
reducing the
discharge of brine into the environment.
Nanofiltration (NF) is a pressure driven, membrane separation technology that
separates ionic solute from water supplies based on the ionic charge of the
solute. Preferred
embodiments of the present invention include a pump that supplies the force
required to
effect the separation and the feeding of a brine solution or feed stream into
a housing
containing a nanofilter membrane element.
In the NF process, multivalent salts are rejected to a higher degree than
monovalent
salts. Thus, NF used as part of a water softener system can be used to
selectively remove the
multivalent hardness ions from a brine solution and direct them to a drain
while monovalent
salts that make up the brine solution are recycled to a water softener brine
tank. With the
present invention, approximately 90% or more of the brine solution that
typically is
discharged into a drain can be recovered and recycled, thereby minimizing
water pollution as
well as the cost of water softener salt from which brine solution is prepared.
-3-
CA 02746986 2011-07-21
The softener system of the invention has normal and regeneration modes. During
normal mode, hardness ions exchange positions with salt ions held onto the
resin particles.
When most of the salt on the resin particles has been replaced with hardness
ions, the softener
system goes into regeneration mode.
The invention's regeneration mode has two phases of operation that are
modifications
of the current industry standard. These modifications substantially reduce the
amount of salt
discharged into the drain during regeneration.
In regeneration mode, during a first brine/slow rinse phase, the system uses
modified
diverter valves to direct the effluent during this phase through a nanofilter
to the brine tank.
During a later fast rinse phase, at least a portion of the water in the
softener tank that has
dissolved salt may also be directed to the brine tank.
The system is also compatible with a further improvement that, while the
softener is
in normal mode, allows the nanofilter to process the contents of the brine
tank, removing and
discharging any remaining hardness ions in the brine tank.
The invention in one version comprises a process for regenerating a water
softening
system that recycles a substantial percentage of the softener salt. This
process removes
multivalent ions from water provided by a hard water source.
Such a process operates in a water softening system that conventionally
includes a
softening tank through which the water from the source passes from an upstream
to a
2 0 downstream end. The system also conventionally includes a brine tank
for holding a
monovalent regenerating brine solution.
A functionally conventional first diverter valve supplies liquid selectively
from one of
the water source and the brine tank to the upstream end of the softening tank.
-4-
CA 02746986 2011-07-21
The improved system includes a nanofilter having upstream and downstream sides
allowing monovalent ions to pass to the downstream side and retains
multivalent ions on the
upstream side. Liquid which passes through the NF membrane carries mainly
monovalent
ions and is directed to the brine tank. The liquid that does not pass through
the nanofilter is
rich in bivalent hardness ions and flows to the drain. Most of the salt in the
liquid from the
softening tank, being monovalent, passes through the nanofilter rather than
entering the drain.
A second diverter valve connects between the downstream end of the softening
tank
and selectively to either the upstream side of the nanofilter or to a water
distribution system.
A connection between the downstream side of the nanofilter and the brine tank
allows liquid
carrying mainly salt to return to the brine tank.
The process comprises the steps of operating the first diverter valve to pass
brine
solution from the brine tank through the softening tank of the water softening
system and
operating the second diverter valve to direct liquid from the downstream end
of the softening
tank to the nanofilter. Liquid on the downstream side of the nanofilter flows
to the brine
tank. Liquid on the upstream side of the nanofilter flows to the drain.
A conventional softener system can incorporate the improved NF system with the
following modifications. The softener valve will direct the effluent from the
brine/slow rinse
phase to a buffer tank. The softener valve may direct some or all of the
effluent from the fast
rinse cycle directly back to the brine tank. The amount of fast rinse liquid
directed to the
brine tank may be based on a predetermined degree of salinity of this
solution.
The invention is an improved process and apparatus for regenerating a water
softening
system of the type that removes multivalent (hardness) ions from water. The
improvement
reclaims and reuses brine in the solution carrying the hardness ions by
separating the hardness
-5-
CA 02746986 2011-07-21
ions from that solution.
The water softening system includes a softening tank through which the water
to be
softened passes from an upstream to a downstream end; a brine tank for holding
a
monovalent regenerating salt solution; a first diverter valve connected
between the bottom of
the brine tank and the upstream end of the softening tank; a nanofilter having
upstream and
downstream sides which permits selective passage of monovalent (i.e. salt)
ions and prevents
passage of bivalent (i.e. hardness) ions; and a second diverter valve linking
the downstream
end of the softening tank selectively to the user or building plumbing and to
the upstream side
of the nanofilter;
The improved regeneration process comprises the step of first conventionally
operating the first diverter valve to pass brine solution from the brine tank
through the
softening tank of the water softening system. At the same time, the process
operates the
second diverter valve to direct liquid from the downstream end of the
softening tank to the
upstream side of the nanofilter.
Preferably, the water softening system includes a pump which receives the
liquid
carrying both brine and hardness ions from the softener tank and a throttling
valve connected
between the upstream side of the nanofilter and a drain. The pump pressurizes
the softener
tank liquid and supplies same to the nanofilter. The pump may be powered
concurrently with
operating the second diverter valve to direct liquid from the downstream end
of the softening
tank to the nanofilter.
The throttling valve maintains a relatively high pressure on the upstream side
of the
nanofilter so that a substantial volume of liquid is forced through the
nanofilter. The liquid
that does not pass through the nanofilter and that flows to the drain is rich
in bivalent
-6--
CA 02746986 2011-07-21
hardness ions and has a small amount of salt dissolved therein.
Although the preferred embodiments of the NF water treatment system for water
softeners have been described herein, it should be recognized that numerous
changes and
variations can be made to these embodiments, which changes and variations are
still within
the scope and spirit of the present invention. The present invention should
not be unduly
limited by the illustrative embodiments and examples set forth herein for
exemplary
purposes. Rather, the scope of the present invention is to be defined by the
claims.
BRIEF DESCRIPTION OF THE DRAWING
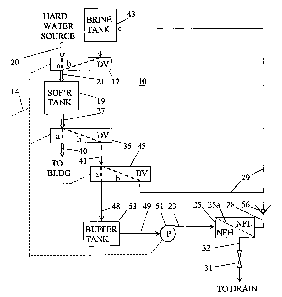
Fig. 1 is a diagram of a water softener system including NF water treatment
allowing
reuse of the softener salt.
DETAILED DESCRIPTION OF THE INVENTION
Referring to Fig. 1, a water softening system 10 according to the invention
conventionally includes a connection to a source 20 of hard water to be
softened such as a
municipal water main or a well. During a normal operating mode or cycle, water
from source
is directed to an input connection of a softener tank 19. Tank 19 contains
resin particles
on which hardness ions in the water adsorb to soften the water as it flows
through tank 19.
The softened water from tank 19 is then supplied to a user such as a building
through a
20 plumbing connector 40.
Also conventionally, system 10 operates in addition to the normal mode, in a
regeneration mode. The regeneration mode has a first slow rinse phase during
which bivalent
hardness ions adsorbed onto the resin particles are replaced by monovalent
salt ions by flow
-7-
CA 02746986 2011-07-21
of brine from a brine tank 43. A fast rinse phase follows the slow rinse
phase, during which
salt remaining in the liquid within softening tank 19 is flushed from tank 19.
First and second diverter valves 17 and 35 control the operating mode in a
conventional softening system. First diverter valve 17 has first and second
inputs to "a" and
"b" paths, from source 20 and brine tank 43 respectively. Valve 17 selectively
directs the
liquid from the selected input to an output 21 as specified by a schematically
shown controller
14. Controller 14 may be nothing more than a clock sequencer or may comprise a
microprocessor.
Second diverter valve 35 receives liquid from softener tank 19 in pipe 27 at
an input,
and selectively directs this liquid along "a" and "b" paths to first and
second outputs
respectively as specified by a schematically shown controller 14. In a
conventional system,
the "b" or second output of valve 35 flows to a drain. In both a conventional
system and in
system 10, water flows from the "a" or first output of valve 35 to users
through connection
40. In conventional systems, valves 17 and 35 are typically combined in a
single physical
unit with functionality as shown.
Diverter valves 17 and 35 each have a normal setting during normal operating
mode
allowing liquid flowing through the path designated as "a". Diverter valve 17,
when set for
normal mode, allows liquid to flow only from source 20 to softener tank 19.
Diverter valve
35, when set for normal mode, allows water to flow only from softener tank 19
to connection
40. Thus, in normal operating mode, water flows from source 20 to softener
tank 19 and
from there to connector 40 for distribution to the building.
Closing the "a" paths and opening the "b" paths of valves 17 and 35 starts the
regeneration mode and shuts off water flow from source 20 to softener tank 19
and to
-8--
CA 02746986 2011-07-21
plumbing connection 40. For this reason, regeneration is preferably done when
little or no
water usage occurs such as nighttime. Alternatively, the design of diverter
valve 17 can
directly connect source 20 to connection 40 during regeneration, to provide
unsoftened water
to users during that time.
The improved softening system 10 shown in Fig. 1 has substantial modifications
compared to current systems in the handling of liquid from softening tank 19
during
regeneration mode. As mentioned, the regeneration mode has a brine/slow rinse
phase and a
fast rinse phase. Controller 14 selects the normal and regeneration modes and
the two phases
of the regeneration mode.
The description hereafter pertains in most to the regeneration mode of system
10. In
system 10, diverter valve 35 directs the effluent from the cycle back to brine
tank 43 through
a nanofilter 25 rather than to a drain. The system 10 also includes additional
elements to
efficiently reuse or reclaim as much of the regeneration salts as is possible.
Thus, system 10
provides for directing much of the liquid flowing from the softening tank 19
during both the
slow and fast rinse phases back to brine tank 43. The flow of liquid to brine
tank 43 during
the fast rinse phase may stop when of salinity of this liquid falls below a
predetermined level.
The operating mode in both conventional systems as well as in system 10 is
determined by settings of first and second diverter valves 17 and 35, as well
that of a newly
added third diverter valve 45. The inputs of valve 17 are conventionally
connected to water
2 0 source
20 and brine tank 43, with the output connection to the input or upstream end
of tank
19.
Valve 35 has its input connection to the output or downstream end of tank 19
and "a"
and "b" paths connected respectively to plumbing connector 40 and to the input
of third
-9-
CA 02746986 2011-07-21
diverter valve 45.
The structure of third diverter valve 45 is functionally very similar to that
of second
diverter valve 35. The second output of second diverter valve 35 connects to
the input of
third diverter valve 45. The first and second outputs of third diverter valve
45 respectively
connect to a buffer tank 53 and to an input pipe 29 to brine tank 43. The
settings for third
diverter valve 45 are controller by controller 14.
During the slow rinse phase, the "b" path of both first and second diverter
valves 17
and 35 is open and the "a" path of valve 45 is open. Brine solution from tank
43 flows
through softener tank 19 picking up divalent hardness ions such as calcium
adsorbed on the
resin particles. Liquid comprising this brine/hardness ions solution then
flows from the
softener tank 19 to buffer tank 53 through valve 35 which then directs the
solution with the
hardness ions through its path "b" to the "a" path of valve 45, pipe 48,
buffer tank 53, and
pump 51.
A pump 51 receives liquid from buffer tank 53 and supplies pressurized
brine/hardness ions solution liquid in pipe 23 to a nanofilter (NF) 25 having
an element 25a.
Pump 51 increases the pressure of the brine/hardness ions solution to perhaps
100 - 150 psig.
A throttling valve 31 connects the upstream side of nanofilter 25 to a drain.
Controller 14
also controls power to pump 51.
Element 25a typically comprises a membrane that blocks a substantial
percentage of
the hardness ions and allows a high percentage of the salt ions to pass
through the membrane.
The NF membrane element 25a has an upstream or high pressure side (indicated
as NFH)
and a downstream, low pressure side (indicated as NFL) that is at essentially
atmospheric
pressure. The liquid on the downstream side of nanofilter 25 comprises a
liquid permeate
-10-
CA 02746986 2011-07-21
stream provided to a pipe 28. The liquid permeate stream has a reduced
concentration of the
hardness ions, and a relatively high concentration of salt.
The permeate stream returns to brine tank 43 through pipe 28, a check valve
56, and a
pipe 29. The absence of the hardness ions in the permeate stream in pipe 28
results from the
flow of the brine solution through the NF membrane element 25a. Check valve 56
may be
integral with nanofilter 25.
Buffer tank 53 may be unnecessary in many systems. In some types of softener
systems whose flow of brine through softener tank 19 during slow rinse is
greater than the
capacity of nanofilter 25, buffer tank 53 may be interposed between valve 45
and pump 51 to
allow a suitable flow rate of brine through softening tank 19. Over a period
of time, pump 51
then draws down any excess liquid in tank 53.
The liquid that does not pass through the NF membrane element 25a has a high
concentration of hardness ions (relative to the NFL side of element 25a). This
concentrate
stream thus contains most of the hardness ions in the liquid flowing in pipe
23 and possibly a
small amount of brine. Liquid not passing through nanofilter 25 flows through
a pipe 32 to
throttling valve 31 and from valve 31, to the drain. Throttling valve 31 and
membrane 25a
cooperate to divide flow between membrane element 25a and throttling valve 31.
Valve 31
may comprise an orifice or other pressure-dropping device.
The high pressure at the NFH side of membrane 25a forces a major portion of
the
pumped liquid through membrane element 25a. The pressure drop across both
membrane
element 25a and throttling valve 31 are each approximately equal to each other
and to the
pump 51 pressure, assuming the brine tank 43 is maintained at approximately
atmospheric
pressure.
-11-
CA 02746986 2011-07-21
In one preferred embodiment, throttling valve 31 has a flow restrictor whose
pressure
drop relative to element 25a divides the fluid flow in pipe 23 so that
approximately 75 -95%
of the liquid flows through element 25a to brine tank 43 and approximately 5 -
25% flows
through throttling valve 31 to the drain. 90% of the flow in pipe 23 reaching
pipe 28 is one
current preferred value.
Almost all of the bivalent hardness ions in the fluid in pipe 23 will flow
through
throttling valve 31. Most of the liquid in pipe 23 passes through membrane 25a
and returns
to brine tank 43, thereby substantially reducing both the volume of drain
water and the total
mass of salt entering the drain. Note that the salt concentration in both
pipes 28 and 32 is
nearly equal. But since the flow through throttling valve 31 is substantially
less than that
through nanofilter 25, most of the salt ions in the flow through pipe 23
returns to brine tank
43.
Causing most of the flow in pipe 23 to flow through nanofilter 25 to brine
tank 43
dramatically reduces the total amount of salt flowing to the drain.
Preferred embodiments may include pressure gauges and flow meters to monitor
performance.
Preferably, the membrane element 25a has a spiral-wound configuration,
although
other configurations are possible, such as capillary fiber, tubular, or plate
and frame. A
common configuration for such a membrane 25a comprises many turns of a strip
of
membrane material with the edges sealed in some manner to cause the majority
of the liquid
entering the NFH side to either pass through the membrane 25a pores or flow to
valve 31.
The following examples, without limitation, are types of NF membrane elements
25a
that are acceptable for use in the present invention, although their
manufacturers may nor may
-12-
CA 02746986 2011-07-21
not have their products evaluated for this application: a spiral wound NF-270
membrane
made by Dow Filmtec; a spiral-wound XN45 membrane made by TriSep Corp.; a
spiral-wound SR2 membrane, by Koch Membrane Systems; a spiral-wound NF
membrane
using a special polymer, by Hydranautics; and a spiral-wound NF membrane using
a special
polymer, by GE Osmonics.
Generally, a suitable NF membrane element 25 has a minimum of approximately
90%
multivalent salts rejection and a maximum of approximately 20% monovalent
salts rejection.
If the concentration of the brine solution in tank 43 is maintained above
approximately 10%,
pH adjustment is usually unnecessary. NF membrane element 25 can remove
hardness ions
from unmodified brine solution in buffer tank 53. The term "unmodified" in
this context
refers to brine solution that has not been subjected to pH adjustment or other
chemical
treatment before passing to NF membrane element 25.
In conventional systems during the fast rinse phase, controller 14 closes the
"b" path
of valve 17 opens and the "a" path for a period of time. Valve 35 remains with
the "a" path
closed and the "b" path open. This allows water from source 20 to flush salt-
containing
liquid from softener tank 19. In a conventional system, this salt-containing
liquid flows
directly to the drain. Eventually controller 14 sets both valves 17 and 35 to
activate their "a"
paths, ending the regeneration mode.
The system of Fig. 1 also includes a further improvement relating to the fast
rinse
phase. During at least the first part of the fast rinse phase of regeneration,
controller 14 closes
the "a" path of valve 45 and opens the "b" path thereof, allowing a
substantial amount of salt-
containing water within softener tank 19 to flow directly to tank 43.
This improvement serves two purposes. First of all, the salt rinsed from
softener tank
-13-
CA 02746986 2016-07-13
19 during the fast rinse cycle returns to brine tank 43, further reducing the
amount of salt sent
to the drain. Secondly, at least some of the water lost to the drain during
the fast rinse phase
of regeneration is replaced, so the overall regeneration process uses less
water.
-14-