Note: Descriptions are shown in the official language in which they were submitted.
81637250
Dialysis Systems and Methods
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority to U.S.
Application Serial No. 12/271,359, filed on November 14, 2008, which claims
the benefit
of U.S. Application Serial No. 61/003,429, filed on November 16, 2007.
TECHNICAL FIELD
This disclosure relates to dialysis systems and methods.
BACKGROUND
Renal dysfunction or failure and, in particular, end-stage renal disease,
causes the
body to lose the ability to remove water and minerals and excrete harmful
metabolites,
maintain acid-base balance and control electrolyte and mineral concentrations
within
physiological ranges. Toxic uremic waste metabolites, including urea,
creatinine, and
uric acid, accumulate in the body's tissues which can result in a person's
death if the
filtration function of the kidney is not replaced.
Dialysis is commonly used to replace kidney function by removing these waste
toxins and excess water. In one type of dialysis treatment--hemodialysis--
toxins are
filtered from a patient's blood externally in a hemodialysis machine. Blood
passes from
the patient through a dialyzer separated by a semi-permeable membrane from a
large
volume of externally-supplied dialysis solution. The waste and toxins dialyze
out of the
blood through the semi-permeable membrane into the, dialysis solution, which
is then
discarded.
Hemodialysis treatments arc typically conducted at a clinic since the
hemodialysis
machines generally require a continuous water source, reverse osmosis
machinery, and
drain lines for discarding the large volumes of water and dialysis solution
used during a
single treatment. Hemodialysis treatment typically, must be performed three or
four times
a week, under supervision of the clinical staff, requirements that
significantly decrease a
patient's autonomy and quality of life.
1
CA 2747606 2017-12-06
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
Certain devices reconstitute used dialysis solution from hemodialysis and/or
peritoneal dialysis as opposed to discarding it. The dialysis solution can be
regenerated
in a machine employing a device that eliminates urea from the solution. For
example, the
original Redy (REcirculating DYalysis) Sorbent System (Blumenkrantz et al.,
Artif
Organs 3(3):230-236, 1978) includes a sorbent cartridge having five layers
through which
dialysis solution containing uremic waste metabolites flows in order to be
regenerated.
SUMMARY
In one aspect of the invention, a dialysis system includes a device configured
so
that a dialysis solution can pass therethrough, a fluid line in fluid
communication with the
o device, and a sodium control system in fluid communication with the fluid
line. The
device is adapted to remove one or more substances from the dialysis solution
as the
dialysis solution passes through the device, and the sodium control system is
adapted to
alter a sodium concentration of solution passing through the fluid line.
In another aspect of the invention, a dialysis system includes a dialysis
machine
and a module connected to the dialysis machine. The module is configured to
retain a
device adapted to remove one or more substances from a dialysis solution as
the dialysis
solution passes through the device after exiting the dialysis machine. The
module is
configured to be releasably fluidly coupled to the dialysis machine and
includes a sodium
control system that is adapted to alter the sodium concentration of the
dialysis solution.
The sodium control system is arranged to alter the sodium concentration of the
dialysis
solution before the dialysis solution passes through the device.
In an additional aspect of the invention, a method includes removing one or
more
substances from spent dialysis solution by passing the spent dialysis solution
through a
device, and altering the sodium concentration of solution entering or exiting
the device.
Embodiments can include one or more of the following features.
In some embodiments, the fluid line is arranged so that solution exiting the
fluid
line enters the device.
In some embodiments, the fluid line is arranged so that solution exiting the
device enters the fluid line.
2
CA 02747606 2011-07-27
Attorney Docket Na: 24275-0028CA1
In some embodiments, the sodium control system is adapted to introduce a
diluent
(e.g., tap water) into the fluid line.
In some embodiments, the sodium control system includes a container that
contains the diluent, and the sodium control system further includes a pump
arranged to
move the diluent from the container to the fluid line.
In some embodiments, the sodium control system is adapted to introduce sodium
(e.g., a sodium chloride solution) into the fluid line.
In some embodiments, the sodium control system includes a container that
contains a sodium solution, and the sodium control system further comprises a
pump
arranged to move the sodium solution from the container to the fluid line.
In some embodiments, the sodium control system further includes a diluent
source, and the pump is arranged to move diluent from the diluent source to
the fluid line.
In some embodiments, the sodium control system further includes one or more
valves that can be actuated to control movement of the sodium solution and the
diluent to
the fluid line.
In some embodiments, the sodium control system is adapted to introduce a
diluent
and sodium into the fluid line.
In some embodiments, the dialysis system further includes a conductivity meter
that is adapted to measure conductivity of the solution passing through the
fluid line, and
the conductivity meter is in communication with the sodium control system.
In some embodiments, the sodium control system is adapted to alter the sodium
concentration of the solution passing through the fluid line based on an
output signal of
the conductivity meter.
In some embodiments, the sodium control system is adapted to decrease the
sodium concentration of the solution passing through the fluid line if the
output signal of
the conductivity meter indicates a conductivity above a predetermined
conductivity.
In some embodiments, the sodium control system is adapted to increase the
sodium concentration of the solution passing through the fluid line if the
output signal of
the conductivity meter indicates a conductivity below a predetermined
conductivity.
In some embodiments, the device is a sorbent cartridge.
3
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
In some embodiments, the sorbent cartridge includes at least one layer of
material
capable of purifying water and/or regenerating spent dialysis solution.
In some embodiments, a layer of the sorbent cartridge comprises sodium
zirconium carbonate.
In some embodiments, the module comprises at least a portion of the sodium
control system, and the device is fluidly coupled to the module.
In some embodiments, the dialysis machine is a hemodialysis machine.
In some embodiments, the module further includes a sodium control system that
is adapted to alter the sodium concentration of the dialysis solution.
In some embodiments, the sodium control system is arranged to alter the sodium
concentration of the dialysis solution before the dialysis solution passes
through the
device.
In some embodiments, the module can be releasably fluidly coupled to any of a
plurality of different dialysis machines.
In some embodiments, the method includes altering the sodium concentration of
solution entering the device.
In some embodiments, the method further includes passing the solution entering
the device through a dialysis machine after altering the sodium concentration
of the
solution entering the device.
In some embodiments, the device is fluidly coupled to a module that is
releasably
fluidly coupled to a dialysis machine.
Embodiments can include one or more of the following advantages.
In some embodiments, the dialysis system can be used in a home environment. In
particular, because the sorbent cartridge allows preparation of dialysate from
tap water
and enables spent dialysis to be recycled, the dialysis system does not
require access to
large volumes of water or dialysis solution, or necessitate expensive reverse
osmosis
devices, or require special plumbing or wiring. Thus, the dialysis system
makes in home
use much more practical compared to certain previous systems.
In some embodiments, the dialysis system controls sodium levels in the
dialysis
solution. In certain embodiments, for example, the spent dialysis solution is
passed
through a sorbent cartridge that removes toxins from the dialysis solution.
The dialysis
4
81637250
system can include a device upstream from the sorbent cartridge that delivers
sodium (e.g.,
sodium chloride (NaC1)) into the dialysis solution to maintain sodium levels
within a desired
range. Maintaining sodium levels in the dialysis solution within a desired
range can help
reduce discomfort experienced by the patient as a result of increased or
decreased sodium
levels in the patient's blood.
In certain embodiments, a sensor (e.g., conductivity meter) is arranged
downstream
of the cartridge and is connected to the sodium control system. The sodium
control system can
make adjustments to the sodium levels in the dialysis solution based on
signals received from
the sensor. As a result, the sensor can ensure the sodium level in the
dialysis solution is within
a desired range before the dialysis solution is delivered to the patient.
In certain embodiments, sensors (e.g., conductivity meters) are located both
upstream
and downstream of the cartridge. The sodium control system can make
adjustments to the
sodium levels in the dialysis solution based on signals received from the
sensors. These
sensors provide information about the conductivity at multiple locations along
the fluid path,
allowing the conductivity to be monitored, adjusted, and controlled more
precisely.
According to one aspect of the present invention, there is provided a dialysis
system,
comprising: a module that is connected to a dialysis machine, the module
comprising: a
device adapted to remove one or more substances from a dialysis solution as
the dialysis
solution passes through the device; a foldable holder configured to retain the
device in a
manner such that the dialysis solution can pass through the device; a fluid
line in fluid
communication with the device; a sodium control system in fluid communication
with the
fluid line, the sodium control system being adapted to alter a sodium
concentration of solution
passing through the fluid line; a first container that stores a diluent, the
diluent being stored
separately from an infusate solution that provides at least a portion of the
dialysis solution; a
first pump that moves dialysis solution from the module into the dialysis
machine; a second
pump that moves diluent from the first container into the module; and a
processor configured
to control the first pump to move dialysis solution from the module into the
dialysis machine
and configured to control the second pump to move diluent from the first
container into the
module.
5
CA 2747606 2017-12-06
=
81637250
According to another aspect of the present invention, there is provided a
dialysis
system, comprising: a dialysis machine; and a module that is configured to be
releasably,
fluidly coupled to the dialysis machine, the module comprising: a device
adapted to remove
one or more substances from a dialysis solution as the dialysis solution
passes through the
device after exiting the dialysis machine, a foldable holder configured to
retain the device in a
manner such that the dialysis solution can pass through the device, a sodium
control system
that is adapted to alter the sodium concentration of the dialysis solution,
and the sodium
control system being arranged to alter the sodium concentration of the
dialysis solution before
the dialysis solution passes through the device, a container that stores a
diluent, the diluent
being stored separately from an infusate solution that provides at least a
portion of the dialysis
solution, a first pump that moves dialysis solution from the module into the
dialysis machine,
a second pump that moves diluent from the container into the module, and a
processor
configured to control the first pump to move dialysis solution from the module
into the
dialysis machine and configured to control the second pump to move diluent
from the
container into the module.
Other aspects, features, and advantages will be apparent from the description
and
drawings, and from the claims.
DESCRIPTION OF DRAWINGS
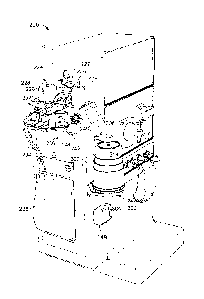
FIG. 1 is a perspective view of a dialysis system that includes a dialysis
machine and
a module with a sorbent cartridge holder that is holding a sorbent cartridge.
FIG. 2 is a cross-sectional view of the sorbent cartridge holder of FIG. 1
with a
sorbent cartridge positioned in the sorbent cartridge holder.
FIG. 3 is a perspective view of the dialysis system of FIG. 1, with the
sorbent
cartridge removed and the sorbent cartridge holder in a folded configuration.
FIG. 4 is a cross-sectional view of the sorbent cartridge holder of FIG. 1
with the
sorbent cartridge removed and the sorbent cartridge holder in a folded
configuration.
5a
CA 2747606 2017-12-06
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA I
FIG 5 is a schematic view of the module of FIG 1, and a front view of the
dialysis machine of FIG 1. The module is configured to introduce sodium
chloride
solution and/or diluent to dialysate entering the sorbent cartridge.
FIG 6 is a schematic view of a module of a dialysis system, and a front view
of a
dialysis machine of the dialysis system. The module of the dialysis system is
configured
to introduce sodium chloride solution and/or diluent to dialysate exiting a
sorbent
cartridge of the module.
DETAILED DESCRIPTION
This disclosure generally relates to dialysis systems and methods. The
dialysis
systems typically include a module that is capable of regenerating dialysis
solution (e.g.,
dialysate). The module typically includes a sorbent device for filtering used
or spent
dialysis solution and a sodium control system located upstream of the sorbent
device for
controlling sodium levels within the dialysis solution. By positioning the
sodium control
system upstream of the sorbent device, tap water can be used to create sodium
chloride
solution and dilution water that is introduced to the dialysis solution to
control sodium
levels.
The systems and methods described herein can advantageously eliminate the high
volume of water usage, expensive and noisy reverse osmosis equipment, and the
need for
a drain line that occur with many known dialysis systems and methods. Thus,
the
systems and methods described herein can enable a hemodialysis machine to be
relatively
easily modified for use in a home environment without requiring the
installation of
special plumbing or wiring in a patient's home. In addition, the systems and
methods
described herein can allow levels of sodium in the dialysis solution to be
maintained
within substantially the same physiological range as is achieved in single-
pass
hemodialysis.
FIG 1 shows a dialysis system 200 that includes a module 220 fluidly coupled
to
a dialysis machine 30. The module 220 includes a sorbent cartridge holder 300
configured to hold a sorbent cartridge 24. The module 220 also includes a
manifold 222
to which fluid lines 224, 226 extending from an infusate container 228 and a
sodium
6
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA I
chloride container 230 are connected, a manifold 232 to which fluid lines 234,
236
extending from a dialysate bag or reservoir 238 are connected, and a manifold
240 to
which fluid lines 242, 244 extending from an ammonium (NH4) sensor 246 are
connected. The module 220 further includes a manifold 248 that can be used to
fluidly
connect other components, such as a priming solution bag, a rinsing solution
bag, a
cleaning solution bag, and/or a drain bag to the module 220. Each of manifolds
222, 232,
240, and 248 can, for example, include projections on which fluid lines can be
positioned
to connect the various components described above to their respective
manifold. Any of
various other suitable connection mechanisms can alternatively or additionally
be used to
connect the fluid lines to the manifolds.
When in an open position, as shown in FIG 1, the manifold 222 permits an
infusate solution (e.g., a solution including magnesium, calcium, and
potassium) and a
sodium chloride solution to be delivered into fluid circulating through the
module 220.
Pumps and valves within the module 220 can, for example, be activated to pump
the
infusate solution and sodium chloride into the fluid circulating within the
module 220.
Similarly, the manifold 232 allows fluid to be transferred from the module 220
to the bag
238 and vice versa. Using pumps and valves within the module 220, fluid can be
pumped
into and suctioned out of the bag 238 via the fluid line 234 connected to the
manifold
232. The manifold 240 permits fluid to be transferred from the module 220 to
the
ammonium sensor 246 and vice versa. By activating pumps and valves within the
module 220 in a desired manner, the fluid can be pumped from the module 220 to
the
ammonium sensor 246 and can be drawn back to the module 220 from the ammonium
sensor 246. The manifold 248 can also be placed in an open configuration
during use and
connected to fluid lines such that by activating pumps and valves within the
module, fluid
can be drawn into the module 220 from a bag (e.g., a priming solution bag, a
rinsing
solution bag, a cleaning solution bag) and/or pumped from the module into a
bag (e.g., a
drain bag). With the sorbent cartridge 24 fluidly connected to the cartridge
holder 300, as
shown in FIG 1, fluid circulating within the module 220 is allowed to pass
through the
sorbent cartridge 24.
During dialysis treatment, the module 220 is configured in the manner shown in
FIG 1 to permit fluid communication between the fluid circulating within the
module 220
7
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
and the sorbent cartridge 24, the infusate container 228, the sodium chloride
container
230, the dialysate bag 238, the ammonium sensor 246, and, in some cases, one
or more
additional bags that can be connected to the module 220 via the manifold 248.
FIG 2 is a cross-sectional view of the cartridge holder 300 holding the
sorbent
cartridge 24. As shown in FIGS. 1 and 2, the cartridge holder 300 includes a
back 302, a
base 304 that is pivotably connected to a bottom portion of the back 302, and
an arm 306
that is pivotably connected to a top portion of the back 302. The sorbent
cartridge 24 can
be positioned between and held in position by the base 304 and the arm 306.
Referring to
FIG 2, fluid passageways 308, 310 extend through the base 304 and the arm 306,
respectively. The base 304 and the arm 306 also include fittings (e.g., male
nipples) 312,
314 that cooperate with the sorbent cartridge 24 to place the fluid
passageways 308, 310
of the base 304 and the arm 306 in fluid communication with an interior
chamber of the
sorbent cartridge 24 and to help retain the sorbent cartridge 24 in position
between the
base 304 and the arm 306. This configuration permits fluid to pass through the
fluid
passageway 308 of the base 304 and into the interior chamber of the sorbent
cartridge 24.
The fluid can pass through the sorbent cartridge 24 and into the fluid
passageway 310 of
the arm 306. The base 304 and/or the arm 306 can be spring loaded. This can
help the
base 304 and the arm 306 to retain the sorbent cartridge 24 while also
permitting the base
304 and the arm 306 to rotate about their hinged axes in the event that the
sorbent
cartridge 24 expands or contracts (e.g., in response to fluid retention and
fluid pressure
therein) during use. The fluid passageways 308, 310 of the base 304 and the
arm 306 are
connected to fluid lines within the module 220 such that the cartridge holder
300 can
receive fluid (e.g., spent dialysis solution) from the module 220 and return
fluid (e.g.,
recycled dialysis solution) to the module 220.
Referring again to FIG. 1, during dialysis, as discussed in greater detail
below,
spent dialysis solution is moved from the dialysis machine 30 into the module
220 where
it passes through the sorbent cartridge 24, and then the recycled dialysis
solution exiting
the sorbent cartridge 24 is moved back to the dialysis machine 30. As the
spent dialysis
solution is passed through the sorbent cartridge 24, toxins, such as urea, and
other
substances, such as calcium, magnesium, and potassium are stripped from the
spent
dialysis solution. Sodium may also be stripped from the spent dialysis
solution during
8
CA 02747606 2011-07-27
Attorney Docket No: 24275-0028CA1
the initial part of the treatment or, in certain cases, added to the spent
dialysis solution as
the spent dialysis solution passes through the sorbent cartridge 24 during the
later part of
the treatment. To compensate for these materials being stripped from the
dialysis
solution, calcium, magnesium, potassium, and sodium levels of the recycled
dialysis
solution can be altered (e.g., by introducing calcium, magnesium, potassium,
sodium,
and/or a diluent into the dialysis solution) to restore concentrations of
those substances to
desired levels. As the recycled dialysis solution then passes through a
dialyzer in the
dialysis machine 30, toxins are transferred from the patient's blood into the
dialysis
solution, forming spent dialysis solution. This spent dialysis solution is
then circulated
through the module 220 again to recycle or regenerate the spent dialysis
solution. This
process can be repeated until a desired amount of toxins have been removed
from the
patient's blood. Because the dialysis solution is recycled during the
treatment as opposed
to simply being discarded, the volume of dialysis solution used during the
treatment can
be substantially reduced relative to certain conventional hemodialysis
techniques. In
addition, maintaining the concentration of the various substances within the
dialysis
solution, such as calcium, magnesium, potassium, and sodium, can help to
prevent the
patient from experiencing discomfort during the treatment.
The sorbent device 24 includes a housing containing a sorbent cartridge
capable
of removing uremic toxins. In some embodiments, the cartridge is disposable.
The
cartridge can, for example, be constructed such that it can be disposed after
use and
removed from the housing. The replaced cartridge could then be replaced with a
similar
cartridge for a subsequent use of the module 20. The cartridge can purify
water and
regenerate spent dialysis solution through the use of a series of layers which
can remove
heavy metals (e.g., lead, mercury, arsenic, cadmium, chromium and thallium),
oxidants
(e.g., chlorine and chloramine), urea, phosphate and other uremic waste
metabolites (e.g.,
creatinine and uric acid) from the solution, without removing or adsorbing
excessive
amounts of cations (e.g., calcium, magnesium, sodium, potassium) or essential
ions.
In some embodiments, the components of the cartridge that perform the afore-
mentioned functions include a purification layer that includes activated
carbon; an ion
exchange layer that includes a polymer phosphate binder or an ion exchange
sorbent; and
a urea removal layer that includes strong acid cation exchange resin and basic
resin(s) or
9
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA I
urea-degrading enzymes and an ion exchange sorbent together with a composition
that
rejects cations (e.g., flat membrane / hollow fibers described further herein,
an ion-
exchange membrane, or an encapsulation surrounding the urea removal
components).
In certain embodiments, the cartridge includes the following layers and
materials:
sodium zirconium carbonate or other alkali metal-Group IV metal-carbonate;
zirconium
phosphate or other ammonia adsorbents; alumina or other like material; alumina
supported urease or other immobilized enzyme layer or other material to
convert urea to
ammonia, such as diatomaceous earth or zirconium oxide; and granular activated
carbon,
such as charcoal, or other adsorbent. The sodium zirconium carbonate component
can act
as a phosphate adsorbent. The zirconium oxide can be capable of acting as a
counter ion
or ion exchanger to remove phosphate, and can be in the form of hydrous
zirconium
oxide (e.g., hydrous zirconium oxide containing acetate). The zirconium oxide
can also
be blended with the sodium zirconium carbonate when positioned in the
cartridge.
Non-limiting examples of urea-degrading enzymes that can be employed in either
embodiment of the sorbent cartridge include enzymes that are naturally
occurring (e.g.
urease from jack beans, other seeds or bacteria), produced by recombinant
technology
(e.g., in bacterial, fungal, insect or mammalian cells that express and/or
secrete urea-
degrading enzymes) or produced synthetically (e.g., synthesized). In some
embodiments,
the enzyme is urease.
In certain embodiments, the sorbent cartridge further includes hollow fibers.
The
hollow fibers can reject positively charged ions, as well as increase the
capacity of the
cartridge. The hollow fibers can be coated with an ion-rejecting material,
which through
a water-purification like mechanism allows the urea through but rejects
positively
charged ions such as calcium and magnesium. The material coating the hollow
fibers can
be any such material known to one of skill in the art (e.g., fatty acids or
polymer chains
like polysulfone) that can effectively reject calcium and magnesium and
therefore retain
the ions in the dialysis solution. Generally, to have this effect the material
itself would be
positively charged. In some embodiments, for example, the material used to
coat the
hollow fibers is cellulose acetate (e.g., cellulose triacetate). The hollow
fibers that are to
be coated are commercially available (e.g., Fresenius Medical Care North
America) and
81637250
can be coated with any desired ion-rejecting material available to one having
skill in the
art.
Alternatively, the hollow fibers can include an ion-selective nanoffitration
membrane. Such membranes are commercially available from a number of sources
(e.g.,
Amerida, Koch, GE, Hoechst and Dow Chemical). These membranes have pores sizes
that prevent ionic substances from diffusing through the membrane. For
example, there
are nanofiltration membranes that have an ability to reject ions with more
than one
negative charge (e.g., sulfate and phosphate) while allowing single-charged
ions to pass
through, with the converse also being the case. In either case, the hollow
fiber devices
are available in a variety of dimensions and need only be small enough to fit
in the
replaceable cartridge, which can be sized for use in an in-home system.
In certain embodiments, the sorbent cartridge can further include a flat
membrane
that is covered with a positively charged material like those described above.
In addition,
the membrane can be an ion exchange (e.g., anion) membrane that limits the
passage of
positively charged ions (e.g., Astrom Neosepta AFX anion exchange membrane,
PCA
GmbH PC-SA anion exchange membrane). Advantageously, this ion exchange
membrane also has an ability to adsorb phosphate.
The cartridge and/or its components or layers can be replaced (e.g., membrane,
urea-degrading enzyme), regenerated (e.g., resin, sorbent) and/or sterilized
for re-use
when necessary (e.g., saturation, damage, depletion). In addition, the entire
cartridge can
be replaceable and thus removed from the dialysis system when there is a
decrease in the
regeneration efficiency of the cartridge (e.g., through layer saturation) or
the cartridge
becomes worn or damaged, for instance.
Further examples of sorbent cartridges are described in U.S. Patent No.
6,878,283; U.S. Patent No. 7,033,498; and in Sorb's REDY cartridge (e.g., see
"Sorbent
Dialysis Primer" COBE Renal Care, Inc. Sep. 4 1993 Edition, and "Rx Guide to
Custom
Dialysis" COBE Renal Care Inc. Revision E. Sep. 1993).
The module 220 is coupled to a hemodialysis machine 30, such as a version of
the
Fresenius Medical Care 2008K design. In hemodialysis, blood flows through an
arterial
channel to an arterial pressure sensor. The arterial pressure sensor includes
a transducer
11
CA 2747606 2017-12-06
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
so that the pressure of the blood flowing through the circuit on the arterial
side can be
monitored. The blood then flows through a portion of the channel that abuts a
pump,
such as a peristaltic pump. The pump forces the blood through the circuit. The
blood
then flows to the dialyzer and then to a venous pressure sensor. The access
ports through
which blood is removed and returned can be at a convenient and appropriate
place on a
patient and can be connected to the hemodialysis machine by any appropriate
medical
tubing.
While the arterial pressure sensor has been described as being positioned
before
the pump, in some embodiments, the arterial pressure sensor is after the pump.
In certain
embodiments, pressure sensors are positioned both before and after the blood
pump.
Referring again to FIG. 2, a bypass component 316 is secured to the back 302
of
the cartridge holder 300. The bypass component 316 includes ports 318 and 320
that are
arranged to receive the fittings 312 and 314 of the base 304 and the arm 306
when the
base 304 and the arrn 306 are pivoted into engagement with the back 302 (after
the
sorbent cartridge 24 has been removed from the cartridge holder 300). The
bypass
component also includes a fluid passage 322 that extends within the back 302
and fluidly
connects the ports 318, 320 to one another. The bypass component 316 allows
fluid to
pass through the cartridge holder 300 even when the sorbent cartridge 24 has
been
removed from the cartridge holder 300.
FIG. 3 shows the system 200 with all external components (e.g., the sorbent
cartridge 24, the infusate container 228, the sodium chloride container 230,
the bag 238,
and their associated fluid lines) disconnected from the module 220 and with
the
manifolds 222, 232, 240, and 248 and the sorbent cartridge holder 300 in a
closed
position. When the manifolds 222, 232, 240, and 248 and the sorbent cartridge
holder
300 are in their closed positions, they inhibit (e.g., prevent) fluid from
exiting the module
220, and thus permit fluid (e.g., a cleaning solution or a rinsing solution)
to be circulated
in a closed circuit within the module 220 and the dialysis machine 30. Each of
the
manifolds can, for example, include a member that abuts or extends into fluid
line
connection ports of the manifolds when the manifolds are in the closed
position to create
a fluid-tight seal.
12
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
FIG 4 is a cross-sectional view of the cartridge holder 300 in its folded or
closed
configuration. Referring to FIG 3 and 4, in this folded configuration, the
base 304 and
the arm 306 are pivoted toward the back 302 and the fittings 312, 314 are
disposed in the
ports 318 and 320 of the bypass component 316. This configuration permits
fluid to pass
through the fluid passageway 308 of the base 304 and into a fluid passageway
322
extending through the bypass component 316. The fluid then passes from the
fluid
passageway 322 of the bypass component 316 to the fluid passageway 310 of the
arm
306. Thus, even when the sorbent cartridge 24 has been removed from the
cartridge
holder 300, a fluid stream can be maintained through the cartridge holder 300.
The external components (e.g., the sorbent cartridge 24, the infusate
container
228, the sodium chloride container 230, the bag 238, and their associated
fluid lines) are
constructed as disposable, single use components and can thus be disconnected
from the
module 220 and discarded after completion of dialysis treatment. The manifolds
and the
sorbent cartridge holder 300 can then be closed and a cleaning and/or rinsing
solution can
be circulated through the module 220 and the dialysis machine 30 to prepare
the module
220 and the dialysis machine 30 for a subsequent use.
FIG. 5 is a schematic view of the module 220 coupled to the dialysis machine
30.
As shown in FIG. 5, in addition to the external components that have been
described as
being connected to the module 220, a dialysate bag 250 and a drain bag 252 are
also
connected to the module 220 via the manifold 248 (shown in FIGS. 1 and 3). In
addition,
a dilution bag 254 is fluidly connected to the dialysis machine 30.
Still referring to FIG 5, a method of performing hemodialysis will now be
described. Prior to beginning the dialysis treatment, dialysate is drawn from
the dialysate
bag 250, passed through the sorbent cartridge 24, and routed to the bag 238.
This can be
done by activating pump 256 with valves 258, 260 opened and valve 262 closed.
After
exiting the bag 250 and passing through the valve 258, the dialysate passes
through a
fluid detector 255, which is adapted to detect the presence or absence of
fluid within the
line. Fluid detectors of this type are available, for example, from Introtek
and Cosense.
The dialysate is drawn from the bag 250 until the fluid detector 255 detects
the absence
of fluid in the line, indicating that all of the dialysate has been forced
from the bag 250
into the module 220. Upon detecting the absence of fluid in the line, the
fluid detector
13
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA I
255 can transmit this information to a control unit (e.g., microprocessor)
that can cause
the valves and pumps throughout the system to operate in a way to cause the
dialysate to
recirculate within the module 220 and/or the dialysis machine 30.
Prior to reaching the sorbent cartridge 24, the dialysate passes through a
flow
meter 261 that is configured to measure the flow rate of the dialysate passing
therethrough. A signal representing the flow rate of the dialysate can be
transmitted from
the flow meter 261 to a control unit (e.g., a microprocessor). As discussed
below, the
detected flow rate of the dialysate can be used to control metering of the
infusate into the
dialysate.
As the dialysate passes through the sorbent cartridge 24, certain substances,
such
as calcium, magnesium, potassium, and sodium may be removed from the
dialysate.
There is generally no sodium uptake by the cartridge while the dialysate is
circulated
through module 220 during treatment except during the prime solution
circulation and in
the initial part of the treatment process. As discussed above, the sorbent
cartridge 24 is
also adapted to remove toxins, such as urea, from fluid flowing therethrough,
but the
dialysate from the dialysate bag 250 would generally not contain any urea.
Upon exiting
the top of the sorbent cartridge 24, the dialysate flows through a bubble trap
264, which
helps to ensure that gases within the dialysate are released. With valve 266
closed, the
dialysate is then forced through fluid line 265. The infusate solution, which
includes
magnesium, calcium, and potassium, is then pumped into the fluid line 265 from
the
infusate solution container 228 by activating a pump 268. The combination of
the
dialysate and the infusate solution are mixed within a mixing chamber 270.
After exiting the mixing chamber 270, the dialysate continues to flow through
the
fluid line 265 and passes through a conductivity meter 273. The conductivity
meter 273
can estimate, based on the conductivity of the fluid passing therethrough, the
concentration of sodium within the fluid. A pump 274 and valves 276, 278 can
then be
activated in a manner to introduce sodium chloride (e.g., a mixture of sodium
chloride
and tap water) into the fluid line 257 from the sodium chloride container 230
if the
conductivity reading indicates that the sodium level in the dialysate is lower
than desired
or to introduce dilution water (e.g., tap water) into the fluid line 257 if
the conductivity
reading indicates that the sodium level in the dialysate is higher than
desired. Tap water
14
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
used as the dilution water is delivered to the dialysate passing through the
fluid lines 257
from the bag 254 connected to the dialysis machine 30. The dialysis machine 30
draws
the tap water used as dilution water from the bag 254 and delivers it to the
module 220
where it passes through a fluid line 277 toward the valve 278. The dilution
water can be
metered into the fluid line 257 by activating the pump 274 and opening the
valve 278.
Similarly, the sodium chloride solution can be metered into the fluid line 257
by
activating the pump 274 and opening the valve 276.
An advantage of connecting the sodium control system, which includes the
sodium chloride container 230, dilution water container 254, and their
respective
pumping and valve mechanisms 274, 276, 278, to the fluid line 257 upstream of
the
sorbent cartridge 24 is that tap water can be used to make the sodium chloride
solution
and tap water can be used as the dilution water. The sorbent cartridge 24
serves to treat
both the spent dialysate received from the dialysis machine 130 and the tap
water added
into the spent dialysate for dilution control. Sodium control systems that are
connected to
a dialysate circuit downstream of a sorbent cartridge typically require the
use of purified
water (e.g., AAMI water) for preparation of sodium chloride solution and for
the dilution
water because the dialysate circuit generally includes no additional filters
capable of
purifying water between the sorbent cartridge and the dialyzer where the
dialysate
contacts the patient's blood. Thus, such systems typically require the storage
and use of
large volumes of purified water. By permitting the use of tap water in the
sodium
chloride solution and the dilution water, the module 220 greatly reduces the
amount of
purified water that the patient must store and use. In some cases, tap water
is also used to
make the dialysate that is used with the system 200. In those cases, the
sorbent cartridge
24 is used to filter the tap water of the dialysate in the same way that the
sorbent cartridge
24 is used to filter the tap water found in the sodium chloride solution and
the dilution
water. As a result, the system 200 can be operated with little or no pre-
packaged purified
water.
Prior to reaching the fluid lines 265 and 257, the infusate solution and the
sodium
chloride solution pass through fluid detectors 269 and 275, which can detect
the presence
or absence of fluid. The fluid detectors 269, 275 can be similar in
construction to the
fluid detector 255 discussed above.
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
A microprocessor is used to control the pumps 268, 274 and the valves 276,
278.
The microprocessor is connected to flow meter 261, the conductivity meter 273,
the
pumps 268, 274, and the valves 276, 278. The measured flow rate of the
dialysate is
transmitted in the form of a signal from the flow meter 261 to the
microprocessor. The
microprocessor controls the pump 268 as a function of the flow rate of the
dialysate
measured by the flow meter 261. This arrangement helps to ensure that a
desired amount
of the infusate is added to the dialysate, and thus helps to ensure a desired
proportion of
the infusate to the dialysate. The conductivity reading is similarly sent in
the form of a
signal from the conductivity meter 273 to the microprocessor, and, in
response, the
microprocessor sends signals to the pumps 268, 274 and the valves 276, 278 to
cause the
infusate solution to be introduced into the fluid line 265, and to cause the
sodium chloride
solution and/or the dilution water to be introduced into the fluid line 257.
The microprocessor is also connected to the fluid detectors 269, 275. Upon
detecting an absence of fluid within their respective lines, the fluid
detectors 269, 275 can
transmit a signal to the microprocessor, which can shut down the system or
provide an
indication (e.g., an audible and/or visual indication) to the user that the
infusate container
228 and/or the sodium chloride container 230 are empty. In response, the user
can, for
example, replace or refill the infusate container 228 and/or the sodium
chloride container
230.
After passing through the conductivity meter 273, the dialysate passes through
a
check valve 279 and into the ammonium sensor 246, which detects ammonium
levels
within the dialysate. If the ammonium levels within the dialysate are within
an
acceptable range, the dialysate is allowed to flow into the bag 238.
After filling the bag 238 to a desired level with dialysate having a desired
concentration of calcium, magnesium, potassium, and sodium, a pump 280 is
activated to
draw the dialysate from the bag 238 into the dialysis machine 30. The
dialysate is
circulated through the dialysis machine 30 where it passes through a dialyzer.
At the
same time, a patient's blood is passed through the dialyzer. As a result,
toxins, such as
urea, are transferred across a permeable membrane of the dialyzer from the
patient's
blood to the dialysate. The spent dialysate exiting the dialyzer is then
routed back to the
module 220.
16
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
The spent dialysate passes through a fluid line 282 in the module 220.
Depending
on the desired volume of dialysate to be cycled back to the dialysis machine,
some of the
spent dialysate can be routed to the bag 238 by opening a valve 284 and
closing a valve
286 as the spent dialysate is forced through the fluid line 282. As a result
of the dialysis,
for example, fluid from the patient may be added to the dialysate as the
dialysate passes
through the dialyzer of the dialysis machine 30. Thus, routing some of the
spent
dialysate to the bag 238 can help to ensure that a substantially constant
volume of
dialysate is circulated through the module 220 and the dialysis machine 30
throughout
treatment. The pump 256 in the fluid line 282 forces the volume of the spent
dialysate
that is not routed to the bag 238 into the sorbent cartridge 24 via the base
304 of the
cartridge holder 300. As the spent dialysate passes through the sorbent
cartridge 24, urea
is removed from the spent dialysate. Calcium, magnesium, and potassium are
also
stripped from the spent dialysate by the sorbent cartridge 24. The recycled
dialysate or
cartridge effluent, upon exiting the sorbent cartridge 24, passes through the
bubble trap
264 where gases that may be produced as a result of chemical reactions within
the
sorbent cartridge 24 can be removed from the recycled dialysate. In the manner
discussed above, after the recycled dialysate exits the sorbent cartridge 24,
the infusate
solution is introduced into the recycled dialysate and based on the
conductivity reading at
the conductivity meter 273, sodium chloride or dilution water can be added to
the
recycled dialysate at the fluid line 257. In the initial stages of treatment,
sodium levels in
the recycled dialysate tend to be lower than desired due to the sorbent
cartridge's
tendency to strip sodium from fluids passing therethrough. Consequently, in
the early
stages of the treatment, sodium chloride will typically be injected into fluid
line 257 to
increase the concentration of sodium in the recycled dialysate. In later
stages of the
treatment, however, the sorbent cartridge may contain higher levels of sodium
and thus
start releasing sodium into the spent dialysate as it passes through the
sorbent cartridge.
This can lead to higher than desired levels of sodium in the recycled
dialysate passing
through the fluid line 265, resulting in an injection of dilution water into
the recycled
dialysate.
The conductivity meter 273 is used to monitor the infusion of dilution water
or
sodium chloride solution into the dialysate to ensure that the conductivity of
the dialysate
17
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
at the output of the module 220 is within a desired range. For example, the
amount of
dilution water added is determined based on the contribution to dialysate
conductivity by
1) sodium released by the sorbent cartridge 24, 2) the amount of infusate
added to the
fluid line 265 and 3) the amount of sodium added to the fluid line 257 from
the sodium
container 230. The conductivity contribution from the sodium released by the
sorbent
cartridge 24 is determined by the difference between the readings of
conductivity meters
271 and 272. The conductivity contribution from the infusate is determined by
the
differences in reading between conductivity meters 271 and 273. For example,
if the
desired conductivity at the output of the module 220 is 14 mS/cm, and the
sorbent
1() cartridge contributes a conductivity of 0.13 mS/cm, and the infusate
contributes a
conductivity of 0.5 mS/cm, then dilution water is added to the dialysate in
the fluid line
257 so that a reading of 13.37 mS/cm is obtained at the conductivity meter
272.
The recycled dialysate, after exiting the conductivity meter 273, then passes
through the check valve 279 and into the ammonium sensor 246. The ammonium
sensor
246 can help to deteimine the state of the sorbent cartridge 24. For example,
as the
sorbent cartridge 24 is used, the ammonium levels in the dialysate will
increase. Upon
reaching a predetermined ammonium level, the treatment can be terminated.
Alternatively, upon reaching the predetermined ammonium level, the sorbent
cartridge 24
can be replaced with a fresh sorbent cartridge and treatment can resume.
After exiting the ammonium sensor, the recycled dialysate is routed to the bag
238 and/or the dialysis machine 30. For example, in order to ensure that an
equal amount
of fluid enters and exits the dialysis machine 30, a T-valve 281 can be
adapted to route a
portion of the dialysate to the dialysis machine 30 via fluid line 283 and to
route any
excess dialysate to the fresh dialysate chamber of the bag 238. If the flow
rate of the
dialysate at the T-valve 281 is greater than the rate at which the dialysate
is being pulled
into the dialysis machine 30, some of the dialysate will be routed to the bag
238. If, on
the other hand, the flow rate of the dialysate at the T-valve 281 is less than
the rate at
which the dialysate is being pulled into the dialysis machine 30, the dialysis
machine 30
will pull some of the dialysate from the bag 238. The bag 238 is formed of a
flexible
material and thus acts as a compliance chamber. In particular, as the
dialysate is added to
18
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA I
the bag 238, the volume of the bag 238 is allowed to increase, and, as the
dialysate is
removed from the bag 238, the volume of the bag 238 is allowed to decrease.
The dialysate that is delivered to the dialysis machine 30 again passes
through the
dialyzer where toxins are transferred from the patient's blood to the
dialysate. The spent
dialysate is then routed back to the module and the process is repeated until
a desired
amount of toxins has been removed from the patient's blood.
During treatment, an ultrafiltration process may also be performed to remove
water from the patient's blood. During ultrafiltration, a pressure gradient is
created
across the membrane between the dialysate side and the blood side of the
dialyzer. As a
result, fluid is drawn across the membrane from the blood side to the
dialysate side. This
fluid exits the dialysis machine 30 and passes though the module 220 via a
fluid line 288
and is routed to a drain bag 252. This ultrafiltration process can be
continued until a
desired volume of fluid has been removed from the patient.
After completing the patient's treatment, the dialysate can be removed from
the
bag 238. For example, the pump 256 can be activated with the valves 262, 263,
284 open
and the valves 260, 286 closed. As a result, the dialysate flows from the bag
238 into the
drain bag 252. Emptying the bag 238 can allow the user to more easily handle
the bag
238 after treatment due to the decreased weight. In some cases, eight liters
or more of
dialysate is removed from the bag 238 prior to disconnecting the bag 238 from
the
module 220.
After draining the bag 238 to a desired level, the external components (e.g.,
the
sorbent cartridge 24, the infusate container 228, the sodium chloride
container 230, the
bag 238, the dialysate bag 250, and their associated fluid lines) are
disconnected from the
module 220 and discarded. The manifolds 222, 232, and 230 (shown in FIGS. 1
and 3) to
which the sorbent cartridge 24, the infusate container 228, the sodium
chloride container
230, and the bag 238 were fluidly connected are closed so that fluid cannot
flow out of
the module 220 through the fluid line connection ports of the manifolds 222,
232, and
230. A bag of rinsing solution is then connected to the fluid connection port
of the
manifold 248 (shown in FIGS. 1 and 3) where the dialysate bag 250 was
previously
connected, and rinsing solution (e.g., water) is circulated through the module
220 and the
dialysis machine 30 to rinse the fluid conduits within the module 220 and the
dialysis
19
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA I
machine 30. The rinsing process is carried out by drawing the rinsing solution
from the
rinsing solution bag into the fluid line 282 by activating the pump 256 with
valves 258
and 260 open. The rinsing solution moves along the fluid line 282 to the
cartridge holder
300, which is in a closed configuration (with the sorbent cartridge 24
removed) such that
the fittings on the base 304 and the arm 306 of the cartridge holder are
connected to the
ports 318 and 320 of the bypass component 316, as shown in FIGS. 3 and 4. The
various
pumps and valves of the module 220 and the dialysis machine 30 are then
activated in a
manner to cause the rinsing solution to pass through the various fluid
conduits of the
module 220 and the dialysis machine 30. The rinsing solution can be collected
in the
drain bag 252 after passing through the various desired fluid conduits of the
module 220
and the dialysis machine 30.
As an alternative to or in addition to passing the rinsing solution through
the
module 220 and the dialysis machine 30, a cleaning solution (e.g., bleach) can
be
circulated though the module 220 and the dialysis machine 30 in a similar
manner to
disinfect the various fluid conduits of the module and the dialysis machine.
In certain embodiments, a fluid (e.g., a rinsing solution, a cleaning
solution, or
dialysate left in the fluid conduits of the module 220 and the dialysis
machine 30 after
treatment) can be passed through the dialysis machine 30 where it is heated to
a
temperature of about 85 degrees Celsius. The heated fluid can be circulated
through the
module 220 and the dialysis machine 30 to disinfect the fluid conduits within
those
devices.
While certain embodiments have been described, other embodiments are possible.
In some embodiments, the bag 238 is connected to the bubble trap 264 via an
additional fluid line extending from the portion of the bag 238 that contains
spent
dialysate to the bubble trap 264. In such embodiments, the fluid line 283 that
leads to the
dialysis machine 30 can extend into the portion of the bag 238 containing the
fresh
dialysate instead of being connected to the T-valve 281. During use, fresh
dialysate is
first forced into the fresh dialysate chamber of the bag 238, and then in a
subsequent
action the fresh dialysate is drawn from the bag 238 into the dialysis machine
230 via the
fluid line 283. Because the pressure is regulated within the bag 238 as a
result of the
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
bag's connection to the bubble trap, the check valve 279 prior to the ammonium
sensor
246 can be removed from the fluid loop.
While the external components (e.g., the sorbent cartridge 24, the infusate
container 228, the sodium chloride container 230, the bag 238, the dialysate
bag 250, the
drain bag 252, the rinsing solution bag, the cleaning solution bag, and their
associated
fluid lines) connected to the module 220 have been described as being
disposable, single
use disposable components, one or more of the external components can
alternatively be
reusable. For example, they can be constructed to withstand disinfection
techniques,
such as autoclave disinfection.
While the module 220 has been described as being connected to the drain bag
252
via the manifold 248, the module can alternatively or additionally be
connected directly
to a drain via the manifold 248.
While the system 200 has been described as being initially primed with
dialysate
from the dialysate bag 250, the system can alternatively or additionally be
attached to a
water source (e.g., a water tap) and can be adapted to convert water (e.g.,
tap water) from
the water source into dialysate. In certain embodiments, for example, the
dialysis
machine 30 is adapted to spike the water passing therethrough with one or more
concentrates to form dialysate. Because the water is passed through the
sorbent cartridge
24 prior to entering the dialysis machine 30, impurities in the water will be
filtered by the
sorbent cartridge 24 prior to contacting the patient's blood in the dialyzer
of the dialysis
machine 30.
While certain methods described above include controlling the rate at which
the
infusate solution is introduced into the fluid line 265 based on the flow rate
of the
dialysate detected at the flow meter 261, other techniques are possible. In
certain
embodiments, for example, the weight of the infusate container 228 and the
weight of the
dialysate bag 238 are measured (e.g., be a weight scale), and the flow rate of
the infusate
is controlled based on these readings. In certain embodiments, the weight of
the drain
bag 252 can also be measured and accounted for when determining the
appropriate flow
rate of the infusate solution.
While the module 220 has been described as including pumps 268, 274 for
moving the infusate solution and the sodium chloride solution from their
respective
21
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
containers to the fluid lines 265 and 257, other techniques can alternatively
or
additionally be used. In certain embodiments, for example, a vacuum is used to
draw the
infusate solution and the sodium chloride solution from their respective
containers into
the fluid lines. The flow rate of the dialysate within the fluid line 265 can,
for example,
create a vacuum that draws the infusate solution into the fluid line 265. In
some
embodiments, venturi tubes are provided along the fluid line 265 at the
locations where
the line extending from the infusate solution container 228 joins the fluid
line 265. The
venturi tubes can help to ensure that a sufficient vacuum is created to draw
the infusate
solution into the fluid line 265 from the infusate container 228. In
embodiments that use
a vacuum to draw the solutions from their respective containers, a valve can
be provided
within the lines leading from the infusate solution container 228 and the
sodium chloride
solution container 230 to control the flow rates of the infusate solution and
the sodium
chloride solution into the fluid lines 265, and 257, respectively. These
valves can be
connected to and controlled by the microprocessor in the module 220.
While the sodium control system 240 of the module 220 has been described as
being connected to the fluid line 257 upstream of the sorbent cartridge 24, in
certain
embodiments, the sodium control system 240 is arranged to introduce sodium
chloride
solution and/or dilution water into the dialysate circuit at a point
downstream of the
sorbent cartridge 24. FIG 6 schematically illustrates an example of such a
system 300.
As shown in FIG 6, the system 300 includes a module 320 that closely resembles
the
module 220 described above. However, in the module 320, the sodium control
system
240 is connected to the fluid line 265 downstream of the sorbent cartridge 24.
Because
the sodium chloride solution and dilution water is not passed through the
sorbent
cartridge 24 prior to passing through the dialyzer 30 and contacting the
patient's blood in
this system, purified water (e.g., AAMI water) is used to make the sodium
chloride
solution and the dilution water. The system 300 otherwise works in generally
the same
manner as the system 200 described above.
As an alternative to or in addition to the sodium control system 240 described
above, other types of sodium control systems can be used. In certain
embodiments, for
example, dilution reservoirs can be used to control the concentration of an
electrolyte,
such as sodium. The dilution reservoir can be of any size or shape that
enables the
22
CA 02747606 2011-07-27
Attorney Docket No.: 24275-0028CA1
reservoir to retain a volume of fluid that is sufficient to provide dilution
of the fluid
circulating through the components of the system. Typically, the diluent
contained in the
dilution reservoir is water. Once the dilution reservoir is filled, the
dialysate is directed to
the dialysis machine 30 where it is spiked with a known concentrated (either
liquid or
powder form) to attain a base sodium and bicarbonate level. This spiking
Process can be
carried out manually or automatically. At this time, fluid continues to be
recirculated
through the dialysis machine 30 and the module 220.
The system for controlling sodium can alternatively or additionally be adapted
to
increase sodium levels in the fluid passing through the system. For example, a
system for
controlling sodium can include a container containing a concentrated sodium
solution in
addition to the dilution reservoir. A flow pump 60 can serve to infuse the
concentrated
sodium solution or the dilution volume into the dialysate. As described, the
sodium
concentrate can be used to adjust and manipulate the sodium levels in the
dialysate and
can thus be used to adjust and manipulate a patient's sodium levels.
Other devices that do not involve adding a diluent (e.g., tap water) to the
fluid
circulating through the system can alternatively or additionally be used to
control sodium
levels within the circulating fluid. For example, a system for controlling
sodium can
include a column containing a strong acid / strong base resin combination that
can be
used to remove sodium from the fluid circulating through the system. The
column can be
formed from a replaceable cartridge. Alternatively, the column can be formed
from a
deionization polisher. The strong acid / strong base resin combinations can
remove
sodium from the dialysis solution and control pH. A three-way valve can be
used is
fluidly connected to the dialysate to the column. Upon detecting excessive
sodium levels
within the fluid circulating through the system, the three-way valve can be
used to divert
the effluent from the sorbent cartridge through the strong acid / strong base
ion exchange
resin mixture in the column to remove sodium in exchange for water.
Advantageously,
this method allows sodium levels to be adjusted without the addition of water
to the fluid
circulating through the system. Thus, additional reservoir volume is not
required to
compensate for the dilution. An exchange program may be used to regenerate the
deionization polisher. The control method for either the dilution or the ion
exchange
systems could be via electronic feedback from the hemodialysis machine, a
separate
23
81637250
conductivity probe or a timed sequence. Further information regarding
alternative
sodium control systems can be found in U.S. Patent Application Publication No.
2009/0127193.
In addition to the components discussed above, the module of the dialysis
system
can further include various fluid detectors to confirm that fluid is passing
through a
particular fluid line or component at a desired time, flow meters to help
ensure that fluid
is passing through a particular fluid line or component at a desired rate, and
filters to
filter fluid passing through a particular fluid line or component. In certain
embodiments,
these additional components can be connected to the microprocessor of the
module such
that other components, such as pumps and valves, of the module can be adjusted
based on
the readings of these additional components.
While the systems described above use the sorbent cartridge 24 to remove
toxins
from the spent dialysate, other types of devices can alternatively or
additionally be used
to remove toxins from the spent dialysate.
While the modules described herein have been described as being coupled to the
dialysis machine 30, other arrangements are possible. In some embodiments, for
example, the module is incorporated into the dialysis apparatus.
Alternatively, the
module can be a stand-alone unit.
While certain devices and methods disclosed herein have been described as
being
used in conjunction with hemodialysis, they can be used in various other renal
treatments.
The principles described herein can be applicable to the particular type of
hemodialysis
apparatus described herein, and to a variety of other dialysis apparatus
having similar
functions.
One skilled in the art will appreciate further features and advantages of the
invention based on the above-described embodiments. Accordingly, the invention
is not
to be limited by what has been particularly shown and described, except as
indicated by
the appended claims.
Other embodiments are within the scope of the following claims.
24
CA 2747606 2017-12-06