Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRRSENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 288
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 288
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
SUBSTITUTED PYRAZOLO [3, 4-B] PYRIDINE COMPOUNDS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims priority to, and the benefit of, U.S.
Provisional
Application No. 61/141,371, filed December 30, 2008. The contents of this
application is
herein incorporated by reference in its entirety.
BACKGROUND OF THE INVENTION
[0002] Cancer is the second leading cause of death in the United States,
exceeded
only by heart disease. (Cancer Facts and Figures 2004, American Cancer
Society, Inc.).
Despite recent advances in cancer diagnosis and treatment, surgery and
radiotherapy may be
curative if a cancer is found early, but current drug therapies for metastatic
disease are mostly
palliative and seldom offer a long-term cure. Even with new chemotherapies
entering the
market, the need continues for new drugs effective in monotherapy or in
combination with
existing agents as first line therapy, and as second and third line therapies
in treatment of
resistant tumors.
[0003] Cancer cells are by definition heterogeneous. For example, within a
single
tissue or cell type, multiple mutational "mechanisms" may lead to the
development of cancer.
As such, heterogeneity frequently exists between cancer cells taken from
tumors of the same
tissue and same type that have originated in different individuals. Frequently
observed
mutational "mechanisms" associated with some cancers may differ between one
tissue type
and another (e.g., frequently observed mutational "mechanisms" leading to
colon cancer may
differ from frequently observed "mechanisms" leading to leukemias). It is
therefore often
difficult to predict whether a particular cancer will respond to a particular
chemotherapeutic
agent (Cancer Medicine, 5th edition, Bast et at., B. C. Decker Inc., Hamilton,
Ontario).
[0004] Components of cellular signal transduction pathways that regulate the
growth
and differentiation of normal cells can, when dysregulated, lead to the
development of
cellular proliferative disorders and cancer. Mutations in cellular signaling
proteins may cause
such proteins to become expressed or activated at inappropriate levels or at
inappropriate
times during the cell cycle, which in turn may lead to uncontrolled cellular
growth or changes
in cell-cell attachment properties. For example, dysregulation of receptor
tyrosine kinases by
1
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
mutation, gene rearrangement, gene amplification, and overexpression of both
receptor and
ligand has been implicated in the development and progression of human
cancers.
[0005] FGFR2 is a member of the fibroblast growth factor receptor family,
where
amino acid sequence is highly conserved between members and throughout
evolution. FGFR
family members differ from one another in their ligand affinities and tissue
distribution. A
full-length representative protein consists of an extracellular region,
composed of three
immunoglobulin-like domains, a single hydrophobic membrane-spanning segment
and a
cytoplasmic tyrosine kinase domain. The extracellular portion of the protein
interacts with
fibroblast growth factors, setting downstream signals, ultimately influencing
mitogenesis and
differentiation.
[0006] Alterations in the activity (expression) of the FGFR2 gene are
associated with
certain cancers. The altered gene expression may enhance several cancer-
related events such
as cell proliferation, cell movement, and the development of new blood vessels
that nourish a
growing tumor. The FGFR2 gene is abnormally active (overexpressed) in certain
types of
stomach cancers, and this amplification is associated with a poorer prognosis
and response to
standard clinical methods. Abnormal expression of FGFR2 is also found in
patients with
prostate cancer. More than 60 percent of women with breast cancer in the
United States carry
at least a single mutation in this gene as well.
[0007] Accordingly, new compounds and methods for modulating FGFR2 and
treating proliferation disorders, including cancer, are needed. The present
invention
addresses these needs.
SUMMARY OF THE INVENTION
[0008] The present invention provides, in part, substituted imidazolyl-5,6-
dihydrobenzo[n]isoquinoline compounds of Formula I, II, III or IV and methods
of preparing
the compounds of Formula I, II, III or IV:
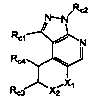
/Rc2 /Rc2
N-N N-N
Rc1 N Rd N
Rc4
X, (Rp)n XI,
Rc3 Xz (I), Xz (II),
2
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
/Rc2
N-N
/Rc2
Rd N-N
:::
Rc6
Rc5 (III), or Rc3 Y Rc6 (IV),
or a salt, solvate, hydrate or prodrug thereof, wherein:
A
is a 5- or 6-member ring which optionally comprises 1-4 heteroatoms selected
from N, 0 and S, and is optionally substituted;
Rcl is H, halogen, unsubstituted or substituted C1-C6 alkyl, unsubstituted or
substituted phenyl, or unsubstituted or substituted heteroaryl comprising one
or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S;
Rc2 is H, or unsubstituted or substituted C1-C6 alkyl;
Rc3 and Rc4 are each independently H, unsubstituted or substituted C1-C6
alkyl, or Rc3
and Rc4, together with the atoms they attach to, form a 5-, 6- or 7-member
ring which
optionally comprises 1-4 heteroatoms selected from N, 0 and S and is
optionally substituted;
Rc5 and Rc6 are each independently H, unsubstituted or substituted C1-C6
alkyl, or
unsubstituted or substituted C6-C1o aryl;
each Rp is independently halogen, hydroxyl, cyan, nitro, amino, unsubstituted
or
substituted C1-C6 alkoxy, unsubstituted or substituted C1-C6 alkyl, -C(O)Raij,
-C(O)ORaij, -
NHC(O)Rai, -NHC(O)ORar;
n is 0, 1, 2, 3, or 4;
Xi-X2 is CH2-CHRR', CH2-NRy, CRR,'-O, CI12-CI2-NRy, or X1'-X2';
X1'-X2' is CHRXa1-CHRXa2, CHRXb-O, O-CHRXb, CHR, CHRxd1-CHRXd2-CHRxd3,
CHRXe1-CHRXe2-O, O-CHRXeI-CHRXe2, CHRXeI-O-CHRXe2, CHRx1-S, S-CHRXf CHRXe1-
CHRXe2-NRy, CRWRW'-O, CHRXe1-NRy-CHRXe2 or NRy-CHRXe1-CHRXe2;
RXai, Rxa2, RXb, RXC, RXd1, RXd2, RXd3, Rxei, Rxe2, or RXf are each
independently H,
unsubstituted or substituted C1-C6 alkyl, or unsubstituted or substituted
phenyl;
Rte, and Rte,' are each independently unsubstituted or substituted C6-Clo
aryl,
unsubstituted or substituted heteroaryl comprising one or two 5- or 6-member
ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring and 1-4
3
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
heteroatoms selected from N, 0 and S, or R, and R,', together with the atom
they attach to,
form a 5-, 6- or 7-member ring which comprises 0-4 heteroatoms selected from
N, 0 and S
and is optionally substituted;
Rakhr is H, unsubstituted or substituted C1-C6 alkyl, unsubstituted or
substituted C6-Cio
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S;
Y is NRy or CHRy';
Ry is H, unsubstituted or substituted C6-Clo aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cg carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -S(O)2R1, -C(O)R1, -C(O)OR1, -(CH2)oR1, -C(=NH)NR2R2', -C(O)NR2R2',
or -
C(S)NR2R2';
o is 0, 1, 2, 3, or 4;
R1 is H, unsubstituted or substituted Ci-C6 alkyl, or -Tyi-Qyi;
R2 and R2' are each independently H, unsubstituted or substituted C1-C6 alkyl,
-Ty3-
Qy3, or R2 and R2', together with the atom they attach to, form a 5-, 6- or 7-
member ring
which comprises 1-4 heteroatoms selected from N, 0 and S and is optionally
substituted;
Ry' is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -NR3R3', -NR3"C(O)NR3R3', -NR3"C(O)R3, -NR3"C(O)OR3, -NR3"S(0)2R3, or
-
C(O)NR3R3';
R3 and R3' are each independently H, or -Ty1'-Qy1';
R3" is H, or unsubstituted or substituted C1-C6 alkyl;
Ty1, Ty1'and Ty3 are each independently a bond, or unsubstituted or
substituted C1-C6
alkyl linker;
Qy' is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -NR21R21';
4
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Qy1' is H, unsubstituted or substituted C6-C15 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -NR21R21';
Qy3 is H, unsubstituted or substituted C6-C1o aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR31, -C(O)R31, -C(O)NR31R31', or -NR31R31'; and
R21, R21', R31 and R31' are each independently H, unsubstituted or substituted
C1-C6
alkyl, unsubstituted or substituted C6-C10 aryl, or unsubstituted or
substituted heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0 and S.
[0009] The present invention also provides pharmaceutical compositions
comprising
one or more compounds of Formula I, II5 III or IV and one or more
pharmaceutically
acceptable carriers.
[00010] The present invention also provides methods of treating a cell
proliferative
disorder by administering to a subject in need thereof, a therapeutically
effective amount of a
compound of Formula I, II5 III or IV, or a pharmaceutically acceptable salt,
prodrug,
metabolite, analog or derivative thereof, in combination with a
pharmaceutically acceptable
carrier, such that the disorder is treated.
[00011] The present invention also provides methods of treating cancer by
administering to a subject in need thereof, a therapeutically effective amount
of a compound
of Formula I, II5 III or IV, or a pharmaceutically acceptable salt, prodrug,
metabolite, analog
or derivative thereof, in combination with a pharmaceutically acceptable
carrier, such that the
cancer is treated.
[00012] The present invention also provides methods of selectively inducing
cell death
in precancerous or cancerous cells by contacting a cell with an effective
amount of a
compound of Formula I, II5 III or IV, or a pharmaceutically acceptable salt,
prodrug,
metabolite, analog or derivative thereof, in combination with a
pharmaceutically acceptable
carrier, such that contacting the cell results in selective induction of cell
death in the
precancerous or cancer cells.
[00013] Unless otherwise defined, all technical and scientific terms used
herein have
the same meaning as commonly understood by one of ordinary skill in the art to
which this
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
invention belongs. In the specification, the singular forms also include the
plural unless the
context clearly dictates otherwise. Although methods and materials similar or
equivalent to
those described herein can be used in the practice or testing of the present
invention, suitable
methods and materials are described below. All publications, patent
applications, patents,
and other references mentioned herein are incorporated by reference. The
references cited
herein are not admitted to be prior art to the claimed invention. In the case
of conflict, the
present specification, including definitions, will control. In addition, the
materials, methods,
and examples are illustrative only and are not intended to be limiting.
[00014] Other features and advantages of the invention will be apparent from
the
following detailed description and claims.
DETAILED DESCRIPTION OF THE INVENTION
1. The Substituted Imidazolyl-5,6-Dihydrobenzo[nlIsoquinoline Compounds
[00015] The present invention provides novel substituted imidazolyl-5,6-
dihydrobenzo[n]isoquinoline compounds, synthetic methods for making the
compounds,
pharmaceutical compositions containing them and the use of the disclosed
compounds.
[00016] The compounds of the present invention include substituted imidazolyl-
5,6-
dihydrobenzo[n]isoquinoline compounds of Formula I:
/Rc2
N-N
Rc1 N
Rc4
"XI
Rc3 X2 (I),
or a salt, solvate, hydrate or prodrug thereof, wherein:
Rcl is H, halogen, unsubstituted or substituted C1-C6 alkyl, unsubstituted or
substituted phenyl, or unsubstituted or substituted heteroaryl comprising one
or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S;
Rc2 is H, or unsubstituted or substituted CI-C6 alkyl;
Rc3 and Rc4 are each independently H, unsubstituted or substituted CI-C6
alkyl, or Rc3
and Rc4, together with the atoms they attach to, form a 5-, 6- or 7-member
ring which
optionally comprises 1-4 heteroatoms selected from N, 0 and S and is
optionally substituted;
Xi-X2 is CHz-CHRR', CI12-NRy, CRR,'-O, CI12-CIz-NRy, or Xi'-X2';
6
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
XI'-X2' is CHRXal-CHRXa2, CHRXb-O, O-CHRXb, CHRXe7 CHRxd1-CHRXd2-CHRXd3,
CHRXeI-CHRXe2-O, O-CHRXeI-CHRXe2, CHRXeI-O-CHRXe2, CHRXf-S, S-CHRXf, CHRXeI-
CHRxe2-NRy, CRR,,,'-O, CHRXeI-NRy-CHRXe2 or NRy-CHRXeI-CHRXe2;
or RXf are each independently H,
RXai, RXa2, RXb, RXe, RXdI, RXd2, RXd3, RXei, R,,,2,
unsubstituted or substituted CI-C6 alkyl, or unsubstituted or substituted
phenyl;
Rte, and Rte,' are each independently unsubstituted or substituted C6-CIO
aryl,
unsubstituted or substituted heteroaryl comprising one or two 5- or 6-member
ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring and 1-4
heteroatoms selected from N, 0 and S, or R, and R,', together with the atom
they attach to,
form a 5-, 6- or 7-member ring which comprises 0-4 heteroatoms selected from
N, 0 and S
and is optionally substituted;
Ry is H, unsubstituted or substituted C6-CI0 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -S(O)2RI, -C(O)RI, -C(O)ORI, -(CH2)oRl, -C(=NH)NR2R2', -C(O)NR2R2',
or -
C(S)NR2R2';
o is 0, 1, 2, 3, or 4;
RI is H, unsubstituted or substituted CI-C6 alkyl, or -Tyl-Qyl;
R2 and R2' are each independently H, unsubstituted or substituted CI-C6 alkyl,
-Ty3-
Qy3, or R2 and R2', together with the atom they attach to, form a 5-, 6- or 7-
member ring
which comprises 1-4 heteroatoms selected from N, 0 and S and is optionally
substituted;
Ry' is H, unsubstituted or substituted C6-CIO aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -NR3R3', -NR3"C(O)NR3R3', -NR3"C(O)R3, -NR3"C(O)OR3, -NR3"S(O)2R3, or
-
C(O)NR3R3';
R3 and R3' are each independently H, or -Tyi'-Qyi';
R3" is H, or unsubstituted or substituted CI-C6 alkyl;
Tyl, Ty1'and Ty3 are each independently a bond, or unsubstituted or
substituted CI-C6
alkyl linker;
7
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Qy' is H, unsubstituted or substituted C6-CIO aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -NR21R21';
Qyl' is H, unsubstituted or substituted C6-C15 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -NR21R21';
Qy3 is H, unsubstituted or substituted C6-CIO aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR31, -C(O)R31, -C(O)NR31R31', or -NR31R31'; and
R21, R21', R31 and R31' are each independently H, unsubstituted or substituted
C1-C6
alkyl, unsubstituted or substituted C6-C1o aryl, or unsubstituted or
substituted heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0 and S.
[00017] For example, R,1 is H.
[00018] For example, R,1 is fluorine, chlorine, bromine, or iodine.
[00019] For example, R,1 is unsubstituted or substituted, straight chain or
branched C1-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[00020] For example, R,1 is methyl.
[00021] For example, R,1 is unsubstituted phenyl.
[00022] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted C1-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)), and -T,l-
Q,l, wherein:
T,1 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
8
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Q,i is H, -NR,,i1R,,11', -C(O)NR,,iiR,,ii', -NHC(O)R,,ii, -NHC(O)OR,,ii, -
NHC(O)NR,,,,R,,,,', or -S(0)2R,11; and
Rill and R,ii' are each independently H, or or unsubstituted or substituted Ci-
C6 alkyl.
[00023] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from -T,1-Q'~1.
[00024] For example, T,1 is a bond.
[00025] For example, T,1 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[00026] For example, T,1 is a methyl linker.
[00027] For example, Q,j is H.
[00028] For example, both R,II and R,11' are H.
[00029] For example, at least one of R,11 and R,11' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[00030] For example, at least one of R,11 and R,11' is methyl, ethyl or t-
butyl.
[00031] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl and
trifluoromethyl.
[00032] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methoxy, t-butoxy and
trifluoromethoxy.
[00033] For example, R,1 is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, cyano, methyl, ethyl, t-
butyl,
trifluoromethyl, methoxy, t-butoxy, trifluoromethoxy, and halogen.
[00034] For example, R,1 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, quinoxalinyl, tetrohyddropyridinyl, pyrrolopyrimidinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl and n-
hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine and iodine)),
9
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
C6-Cio aryl (e.g., phenyl, which is optionally substituted), heteroaryl (e.g.,
pyrrolyl, furanyl,
thiophenyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, and pyrimidinyl, and the like,
and is optionally
substituted), C3-Cs carbocycle (e.g., cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted), or heterocycle (e.g.,
pyrrolidinyl, imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, and morpholinyl, and the like, and is optionally substituted).
[00035] For example, R,1 is heteroaryl selected from furanyl, pyrazolyl,
purinyl,
indolyl, tetrahydropyridinyl, and pyrrolopyrimidinyl, and is optionally
substituted with one or
more groups, each of which can be the same of different, selected from methyl,
ethyl, t-butyl,
amino, and heterocycle (e.g., piperidinyl, which is optionally substituted).
[00036] For example, Rcz is H.
[00037] For example, Rcz is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[00038] For example, Rcz is methyl substituted with unsubstituted phenyl.
[00039] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[00040] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from methyl,
ethyl, t-butyl,
and trifluoromethyl.
[00041] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
methoxy, ethoxy, i-
propyloxy, and t-butoxy.
[00042] For example, Rc3 is H.
[00043] For example, Rc3 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[00044] For example, Rc4 is H.
[00045] For example, Rc4 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[00046] For example, both of Rc3 and Rc4 are H.
[00047] For example, Rc3 and Rc4, together with the atoms they attach to, form
a 5- or
6-member ring selected from:
1) phenyl, which is optionally substituted with one or more groups, each of
which can be the same or different, selected from hydroxyl, halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), cyano, nitro, unsubstituted or substituted amino,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl), unsubstituted or substituted C1-C6 alkoxy
(e.g., methoxy,
ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy), -C(O)Ra , -
C(O)ORai, -
NHC(O)Rar, -NHC(O)ORar, and -C(O)NRa1Ra1');
2) heteroaryl (e.g., pyrrolyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl,
thiazolyl, isothiazolyl, triazolyl, oxadiazolyl, thiadiazolyl, tetrazolyl,
pyridinyl, pyrimidinyl,
pyridazinyl, pyrazinyl, triazinyl, tetrazinyl, furanyl, thienyl, and
pyridinone, and the like, and
is optionally substituted with one or more groups, each of which can be the
same or different,
selected from hydroxyl, halogen (e.g., fluorine, chlorine, bromine, and
iodine), unsubstituted
or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl,
s-butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl), unsubstituted or substituted C1-C6 alkoxy
(e.g., methoxy,
ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy), unsubstituted
or substituted
phenyl, -NRc31Rc32, and -SRai);
3) carbocycle (e.g., cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, or
cycloheptyl, and is optionally substituted); or
4) heterocycle (e.g., pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, tetrahyrofuranyl, piperidinyl, piperazinyl, and
morpholinyl, and
the like, and is optionally substituted),
wherein:
Rakhr and Ralf' are each independently H, unsubstituted or substituted
C1-C6 alkyl, unsubstituted or substituted C6-Clo aryl, or unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S;
11
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Rc31 and Rc32 are each independently H, -Tc3-Qc3, or Rc31 and Rc32,
together with the atom they attach to, form a 5- or 6-member ring which
optionally comprises 1-4 heteroatoms selected from N, 0 and S and is
optionally substituted;
Tc3 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qc3 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, or -ORc33;
and
Rc33 is H, unsubstituted or substituted C1-C6 alkyl, or unsubstituted or
substituted phenyl.
[00048] For example, Rc3 and Rc4, together with the atoms they attach to, form
a
phenyl optionally substituted with one or more groups, each of which can be
the same or
different, selected from hydroxyl, halogen (e.g., fluorine, chlorine, bromine,
and iodine),
cyan, nitro, methyl, ethyl, t-butyl, trifluomethyl, methoxy, ethoxy, t-butoxy,
and -
NHC(O)Rai.
[00049] For example, Rc3 and Rc4, together with the atoms they attach to, form
a
phenyl optionally substituted with two or more groups, each of which can be
the same or
different, selected from hydroxyl, halogen (e.g., fluorine, chlorine, bromine,
and iodine),
cyan, nitro, methyl, ethyl, t-butyl, trifluomethyl, methoxy, ethoxy, t-butoxy,
and -C(O)Ra.
[00050] For example, Rc3 and Rc4, together with the atoms they attach to, form
a
heteroaryl selected from imidazolyl, thiazolyl, pyridinyl, pyridinone,
pyrimidinyl, furanyl, or
thienyl, each of which is optionally substituted with one or more groups, each
of which can
be the same or different, selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
methyl, ethyl, t-butyl, trifluomethyl, methoxy, ethoxy, t-butoxy, phenyl, -
NRc31Rc32, and -
SRakl,,.
[00051 ] For example, both Rain,, and Raitr.' are H.
[00052] For example, at least one of Rakhr and Raic' is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[00053] For example, Ram is H.
12
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[00054] For example, R is unsubstituted or substituted, straight-chain or
branched
C1-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[00055] For example, R,, is methyl.
[00056] For example, both of Rc31 and Rc32 are H.
[00057] For example, at least one of Rc31 and Rc32 is -Tc3-Qc3.
[00058] For example, Tc3 is a bond.
[00059] For example, Tc3 is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[00060] For example, Tc3 is a methyl, ethyl, or propyl linker.
[00061] For example, Qc3 is H.
[00062] For example, Qc3 is unsubstituted phenyl.
[00063] For example, Qc3 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen (e.g., fluorine,
chlorine,
bromine, and iodine), cyano, nitro, unsubstituted or substituted amino,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted C1-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[00064] For example, Qc3 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl,
trifluoromethyl, methoxy,
ethoxy, t-butoxy, and halogen (e.g. fluorine, chlorine, bromine and iodine).
[00065] For example, Qc3 is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl,
trifluoromethyl, methoxy,
ethoxy, t-butoxy, and halogen (e.g. fluorine, chlorine, bromine and iodine).
[00066] For example, Qc3 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[00067] For example, Qc3 is pyridinyl.
13
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[00068] For example, Qc3 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[00069] For example, Qc3 is cycloheptyl.
[00070] For example, Qc3 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
one or more groups, each of which can be the same or different, selected from
hydroxyl,
halogen (e.g., fluorine, chlorine, bromine, and iodine), unsubstituted or
substituted C1-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl).
[00071] For example, Qc3 is pyrrolidinyl, piperidinyl, morpholinyl, or
pyrrolidinone,
and is optionally substituted with methyl, ethyl, or t-butyl.
[00072] For example, Qc3 is -ORc33.
[00073] For example, R,33 is H.
[00074] For example, Rc33 is unsubstituted or substituted, straight-chain or
branched
C1-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[00075] For example, Rc33 is phenyl, and is optionally substituted.
[00076] For example, Rc31 and Rc32, together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with one or more groups, each of which can be the same
or different,
selected from -Tc4-Qc4, wherein:
Tc4 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qc4 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, -C(O)Rc34, -
C(O)ORc34; and
Rc34 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
14
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S.
[00077] For example, Rc31 and Rc32, together with the atom they attach to,
form a 5- or
6-member ring selected from a pyrrolidinyl, piperidinyl, piperazinyl, or
morpholinyl, and is
optionally substituted.
[00078] For example, Tc4 is a bond.
[00079] For example, Tc4 is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[00080] For example, Tc4 is a methyl linker.
[00081] For example, Qc4 is unsubstituted phenyl.
[00082] For example, Qc4 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[00083] For example, Qc4 is pyridinyl.
[00084] For example, Qc4 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[00085] For example, Qc4 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[00086] For example, Qc4 is morpholinyl.
[00087] For example, Qc4 is -C(O)Rc34.
[00088] For example, R,34 is H.
[00089] For example, Rc34 is phenyl and is optionally substituted.
[00090] For example, Rc34 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[00091] For example, Rc34 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[00092] For example, Rc34 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[00093] For example, R,34 is pyrrolidinyl.
[00094] For example, Rc31 and Rc32, together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with two or more groups, any adjacent two of which,
together with the
atoms they attach to form a 5- or 6-member which optionally comprises 1-4
heteroatoms
selected from N, 0 and S and is optionally substituted.
[00095] For example, Rc31 and Rc32, together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with two or more groups, any adjacent two of which,
together with the
atoms they attach to form a phenyl ring, which is optionally substituted.
[00096] For example, Rc3 and Rc4, together with the atoms they attach to, form
a
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, or cycloheptyl, and is
optionally
substituted.
[00097] For example, Rc3 and Rc4, together with the atoms they attach to, form
a
heterocycle selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, tetrahyrofuranyl, piperidinyl, piperazinyl,
morpholinyl, and
pyrrolidinone, and the like, and is optionally substituted.
[00098] For example, XI-X2 is CRR,'-O.
[00099] For example, XI-X2 is CRR,'-O where Rte, and Rte,', together with the
atom
they attach to, form a ring.
[000100] For example, XI-X2 is CRR,'-O where Rte, and Rte,', together with the
atom
they attach to, form an unsubstituted or substituted heterocycle.
[000101] For example, XI-X2 is CRR,'-O where Rte, and Rte,', together with the
atom
they attach to, form an unsubstituted or substituted piperidine ring.
[000102] For example, XI-X2 is CH2-NRy.
[000103] For example, Ry is H.
[000104] For example, Ry is is unsubstituted phenyl.
16
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000105] For example, Ry is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyan,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra,r., -NHC(O)ORa,r., -C(O)Ra,r., and -C(O)OR,,,
wherein
Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000106] For example, Ry is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyan, and -NHC(O)Ra , wherein Rakhr is H, methyl, ethyl, or
t-butyl.
[000107] For example, Ry is unsubstituted naphthyl.
[000108] For example, Ry is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyan,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Ra, -NHC(O)OR, -C(O)Ra, and -C(O)ORa, wherein Ra& is H, or
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000109] For example, Ry is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
[000110] For example, Ry is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
17
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted amino, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra,r., -NHC(O)ORa,r., -C(O)Ra,r., and -C(O)OR,,,
wherein
Rakhr is H, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-C10
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[000111] For example, Ry is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra, wherein Ram is H,
methyl,
ethyl, t-butyl, or phenyl.
[000112] For example, Ry is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
Ci-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000113] For example, Ry is cyclopropyl.
[000114] For example, Ry is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted Ci-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
18
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000115] For example, Ry is -S(O)2R1, -C(O)R1, -C(O)OR1, -(CH2)oR1.
[000116] For example, o is 0.
[000117] For example, o is 1.
[000118] For example, Rl is H.
[000119] For example, Rl is unsubstituted or substituted, straight-chain or
branched C1-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000120] For example, Rl is straight-chain or branched C1-C6 alkyl substituted
with one,
two, three or more groups, each of which can be the same or different,
selected from:
1) phenyl, which is optionally substituted with one or more groups, each of
which can be the same or different, selected from unsubstituted or substituted
C1-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl,
s-pentyl, and n-hexyl), and unsubstituted or substituted CI-C6 alkoxy (e.g.,
methoxy,
ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy);
2) unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy);
3) -C(O)OR11; -C(O)Rii; and
4) -NR12R13, wherein:
R11 is H, or unsubstituted or substituted C1-C6 alkyl;
R12 and R13 are each independently H, -Ty2-Qy2, or R12 and R13,
together with the atom they attach to, form a 5- or 6-member ring;
Ty2 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qy2 is H, hydroxyl, unsubstituted or substituted C1-C6 alkoxy,
unsubstituted or substituted C6-CIO aryl, unsubstituted or substituted
heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from
N, 0 and S, unsubstituted or substituted C3-Cg carbocycle, unsubstituted or
substituted heterocycle comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, -NRa1Rr'5 -C(O)Ra5 -C(O)ORa;
and
Rakhr and Railõ,' are each independently H, or unsubstituted or
substituted C1-C6 alkyl, unsubstituted or substituted C6-CIO aryl, or
unsubstituted or substituted heteroaryl comprising one or two 5- or 6-member
ring and 1-4 heteroatoms selected from N, 0 and S.
[000121] For example, Rl is methyl substitute with unsubstituted phenyl.
19
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000122] For example, Rl is methyl substitute with phenyl substituted with one
or more
groups, each of which can be the same or different, selected from hydroxyl,
halogen, nitro,
cyano, unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000123] For example, Rl is methyl substitute with methoxy, ethoxy, or t-
butoxy.
[000124] For example, Rl is methyl substitute with two or more groups, each of
which
can be the same or different, selected from:
1) phenyl, which is optionally substituted with one or more groups, each of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted
or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-
butoxy, and t-butoxy, each of which is optionally substituted with halogen
(e.g.,
fluorine, chlorine, bromine, and iodine)); and
2) one of the following:
i) -C(O)OR11;
ii) methoxy, ethoxy, i-propyloxy, or t-butoxy; or
iii) -NR12R13.
[000125] For example, Rl is methyl substitute with -C(O)OR11.
[000126] For example, R11 is H.
[000127] For example, R11 is unsubstituted or substituted, straight-chain or
branched C1-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000128] For example, Rl is methyl substitute with -NR12R13=
[000129] For example, both R12 and R13 are H.
[000130] For example, at least one of R12 and R13 is -Ty2-Qy2.
[0001311 For example, Ty2 is a bond.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000132] For example, Tye is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000133] For example, Tye is a methyl, ethyl, or propyl linker.
[000134] For example, Qy2 is H.
[000135] For example, Qy2 is hydroxyl.
[000136] For example, Qy2 is unsubstituted or substituted CI-C6 alkoxy,
including but
not limited to, methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and
t-butoxy.
[000137] For example, Qy2 is methoxy.
[000138] For example, Qy2 is unsubstituted or substituted phenyl or naphthyl.
[000139] For example, Qy2 is unsubstituted phenyl.
[000140] For example, Qyz is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000141] For example, Qy2 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000142] For example, Qyz is indolyl.
[000143] For example, Qy2 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[000144] For example, Qy2 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000145] For example, Qy2 is piperidinyl.
[000146] For example, Qy2 is -NRaRa'.
[000147] For example, Qy2 is -C(O)ORa,r..
[000148] For example, both Rakhr and Ram' are H.
21
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000149] For example, at least one of Rakhr and Ralf' is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000150] For example, at least one of Rakhr and Rai,,.' is methyl or t-butyl.
[000151] For example, R12 and R13, together with the atom they attach to, form
a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C1-C6 alkoxy
(e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy),
unsubstituted or substituted amino, -C(O)Ra,,, or -C(O)ORa, wherein Rakr is H,
or
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000152] For example, R12 and R13, together with the atom they attach to, form
a
piperidinyl or piperazinyl, and is optionally substituted with halogen,
methyl, ethyl, t-butyl,
methoxy, ethoxy, i-propyloxy, t-butoxy, or -C(O)ORa, wherein Rakhr is methyl,
ethyl, or t-
butyl.
[000153] For example, Rl is -Tyl-Qyl.
[000154] For example, Ty, is a bond.
[000155] For example, Ty, is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000156] For example, Ty, is a methyl, ethyl, propyl, or i-propyl linker.
[000157] For example, Qy' is H.
[000158] For example, Qy' is unsubstituted phenyl.
[000159] For example, Qy' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyan,
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted C1-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra, -NHC(O)ORai, -C(O)Ra, and -C(O)ORa, wherein
22
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000160] For example, Qy' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyano, and -NHC(O)Ra, wherein Rakhr is H, methyl, ethyl, or
t-butyl.
[0001611 For example, Qy' is unsubstituted naphthyl.
[000162] For example, Qy' is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Ra, -NHC(O)OR, -C(O)Ra, and -C(O)ORa, wherein Ra& is H, or
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000163] For example, Qy' is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
[000164] For example, Qy' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted amino, unsubstituted or substituted CI-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa, wherein
23
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Rakhr is H, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-C10
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[000165] For example, Qy' is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra, wherein Ram is H,
methyl,
ethyl, t-butyl, or phenyl.
[000166] For example, Qy' is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000167] For example, Qy' is cyclopropyl.
[000168] For example, Qy' is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000169] For example, Ry is -C(=NH)NR2R2'.
[000170] For example, both R2 and R2' are H.
[000171] For example, Ry is -C(S)NR2R2'.
[000172] For example, both R2 and R2' are phenyl.
[000173] For example, Ry is -C(=NH)NR2R2', -C(O)NR2R2', or -C(S)NR2R2'.
[000174] For example, both R2 and R2' are H.
24
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000175] For example, at least one of R2 and R2' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000176] For example, at least one of R2 and R2' is -Ty3-Qy3.
[000177] For example, Ty3 is a bond.
[000178] For example, Ty3 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, n-hexyl, and 2-methylpropyl linker.
[000179] For example, Ty3 is a methyl, ethyl, propyl, or 2-methylpropyl
linker.
[000180] For example, Qy3 is H.
[000181] For example, Qy3 is unsubstituted phenyl.
[000182] For example, Qy3 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from -Ty4-Qy4, wherein:
Ty4, Tys and Ty6 are each independently a bond, or unsubstituted or
substituted
CI-C6 alkyl linker;
Qy4 is H, unsubstituted or substituted CI-C6 alkyl, unsubstituted or
substituted
CI-C6 alkoxy, halogen, nitro, unsubstituted or substituted phenyl,
unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -NR32R32', -NHC(O)R32, -
NHC(O)OR32, -C(O)R32, -C(O)OR32, -OR32, -SR32, -C(O)NR32R32';
R32 and R32' are each independently H, unsubstituted or substituted CI-C6
alkyl, unsubstituted or substituted phenyl, unsubstituted or substituted
heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0
and S. -Tys-Qys, or R32 and R32', together with the atom they attach to, form
a 5- or 6-
member ring which optionally comprises 1-4 heteroatoms selected from N, 0 and
S
and is optionally substituted with -Ty6-Qy6;
Qys is H, hydroxyl, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -ORa,r5 -NRaI,,.Rakh,'5 -
NHC(O)Ra5 -
NHC(O)ORar, -C(O)NRarRa', -C(O)Rar, or -C(O)ORa;
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Qy6 is H, hydroxyl, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -NRairRair', -C(O)NR34R34';
Rakhr and Ram' are each independently H, unsubstituted or substituted CI-C6
alkyl, unsubstituted or substituted C6-Cio aryl, or unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected
from N, 0 and S;
R34 and R34' are each independently H, unsubstituted or substituted CI-C6
alkyl, or R34 and R34', together with the atom they attach to, form a 5- or 6-
member
ring which optionally comprises 1-4 heteroatoms selected from N, 0 and S and
is
optionally substituted.
[000183] For example, Ty4 is a bond.
[000184] For example, Ty4 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000185] For example, Ty4 is a methyl or t-butyl linker.
[000186] For example, Qy4 is H.
[000187] For example, Qy4 is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with halogen
(e.g., fluorine, chlorine, bromine, and iodine).
[000188] For example, Qy4 is methyl, ethyl, t-butyl, or trifluoromethyl.
[000189] For example, Qy4 is unsubstituted or substituted CI-C6 alkoxy,
including but
not limited to, methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
methylenedioxy.
[000190] For example, Qy4 is methoxy, ethoxy, or methylenedioxy.
[000191] For example, Qy4 is fluorine, chlorine, bromine, or iodine.
[000192] For example, Qy4 is unsubstituted phenyl.
[000193] For example, Qy4 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
26
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[000194] For example, Qy4 is heteroaryl selected from heteroaryl selected from
pyrrolyl,
furanyl, thienyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl,
benzoxazolyl,
benzodioxazolyl, benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl,
benzoimidazolyl,
benzothienyl, methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl,
indolyl,
benzofuranyl, purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl,
quinoxalinyl, and
pyrrolopyrimidinyl, and the like, and is optionally substituted with one or
more groups, each
of which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and
n-hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and iodine)),
and unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-propyloxy,
butoxy, i-butoxy, and t-butoxy, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)).
[000195] For example, Qy4 is thiazolyl, isothiazolyl, or oxadiazolyl, each of
which is
optionally substituted with one or more groups, each of which can be the same
or different,
selected from methyl, ethyl, and t-butyl.
[000196] For example, Qy4 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[000197] For example, Qy4 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000198] For example, Qy4 is piperazinyl or piperazinyl, and is optionally
substituted
with methyl, ethyl, or t-butyl.
[000199] For example, Qy4 is -NR32R32'.
27
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000200] For example, both R32 and R32' are H.
[000201] For example, at least one of R32 and R32' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000202] For example, at least one of R32 and R32' is methyl or ethyl.
[000203] For example, R32 and R32' are both methyl or ethyl.
[000204] For example, Qy4 is -OR32 or -SR32.
[000205] For example, R32 is H.
[000206] For example, R32 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with halogen
(e.g., fluorine, chlorine, bromine, and iodine).
[000207] For example, R32 is methyl, ethyl, t-butyl, or trifluoromethyl.
[000208] For example, R32 is unsubstituted phenyl.
[000209] For example, R32 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[000210] For example, R32 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, and quinoxalinyl, and
the like, and is
optionally substituted with one, two or more groups, each of which can be the
same or
different, selected from hydroxyl, halogen, nitro, cyan, unsubstituted or
substituted amino,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
28
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000211] For example, R32 is pyrazinyl, and is optionally substituted with
methyl, ethyl
or t-butyl.
[000212] For example, Qy4 is -C(O)R32, -C(O)OR32.
[000213] For example, R32 is H.
[000214] For example, R32 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000215] For example, R32 is methyl or ethyl.
[000216] For example, Qy4 is -NHC(O)R32, -NHC(O)OR32, or -C(O)NR32R32'.
[000217] For example, Qy4 is -C(O)NR32R32'.
[000218] For example, both R32 and R32' are H.
[000219] For example, at least one of R32 and R32' is -Tys-Qys.
[000220] For example, Tys is a bond.
[000221] For example, Tys is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, n-hexyl, 1,2-dimethylpropyl, and 1-methylbutyl linker.
[000222] For example, Tys is a methyl, ethyl, propyl, i-propyl, butyl, 1,2-
dimethylpropyl, or 1-methylbutyl linker.
[000223] For example, Qys is H.
[000224] For example, Qys is unsubstituted phenyl.
[000225] For example, Qys is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted CI-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted C6-Cio aryloxy (e.g., phenoxy), and -
NHC(O)Ra ,
wherein Rar is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000226] For example, Qys is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from halogen (e.g., fluorine, chlorine,
bromine, and
29
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, t-butoxy,
phenoxy, and -
NHC(O)Ra, wherein Ram is H, methyl or ethyl.
[000227] For example, Qys is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one or more groups, each of
which can be the
same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or substituted
amino, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000228] For example, Qys is pyrazolyl, imidazolyl, isoxazolyl, oxazolyl,
pyridinyl,
furanyl, indolyl, or benzoimidazolyl, each of which is optionally substituted
with one or more
groups, each of which can be the same or different, selected from methyl or
ethyl.
[000229] For example, Qys is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000230] For example, Qys is cyclohexyl or cycloheptyl, which is optionally
substituted
with methyl or ethyl.
[0002311 For example, Qys is heterocycle selected from pyrrolidinyl,
pyrrolidinone,
imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl,
tetrahyrofuranyl,
piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is
optionally
substituted.
[000232] For example, Qys is pyrrolidinyl, pyrrolidinone, piperidinyl,
morpholinyl, or
tetrahydrofuanyl, and is optionally substituted.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000233] For example, Qys is -ORa.
[000234] For example, Ram is H.
[000235] For example, Ram is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000236] For example, Ram is methyl, ethyl, or t-butyl.
[000237] For example, Ram is unsubstituted phenyl.
[000238] For example, Ram is phenyl substituted with one or more groups, each
of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000239] For example, Qy5 is -NRa,r.Ra,r.', -NHC(O)Ra,r, -NHC(O)ORa, -
C(O)NRaRa', -C(O)Ra, or -C(O)ORa.
[000240] For example, Qys is -NRaRa', -NHC(O)Ra, or -C(O)NRaRa'.
[000241] For example, both Rakhr and Ram' are H.
[000242] For example, at least one of Rakhr and Ram' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000243] For example, at least one of Rakhr and Ram' is methyl or ethyl.
[000244] For example, Ram and Ram' are both methyl or ethyl.
[000245] For example, R32 and R32', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl and is
optionally
substituted with -Ty6-Qy6.
[000246] For example, R32 and R32', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, piperidinyl, and piperazinyl, and is
optionally
substituted with -Ty6-Qy6.
[000247] For example, Ty6 is a bond.
31
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000248] For example, Ty6 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000249] For example, Ty6 is a methyl or ethyl linker.
[000250] For example, Qy6 is H.
[000251] For example, Qy6 is unsubstituted phenyl.
[000252] For example, Qy6 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[000253] For example, Qy6 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, and t-butyl.
[000254] For example, Qy6 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl,
benzodihydroimidazolone, and pyrrolopyrimidinyl, and the like, and is
optionally substituted
with one or more groups, each of which can be the same or different, selected
from hydroxyl,
halogen, nitro, cyano, unsubstituted or substituted amino, unsubstituted or
substituted CI-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl, each of which is optionally substituted with halogen (e.g., fluorine,
chlorine,
bromine, and iodine)), and unsubstituted or substituted CI-C6 alkoxy (e.g.,
methoxy, ethoxy,
propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000255] For example, Qy6 is pyridinyl, pyrazinyl, or benzodihydroimidazolone,
each of
which is optionally substituted with one or more groups, each of which can be
the same or
different, selected from methyl or ethyl.
32
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000256] For example, Qy6 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000257] For example, Qy6 is heterocycle selected from pyrrolidinyl,
pyrrolidinone,
imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl,
tetrahyrofuranyl,
piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is
optionally
substituted.
[000258] For example, Qy6 is -NRaI,,.Ra,r.'.
[000259] For example, both Rakhr and Ram' are H.
[000260] For example, at least one of Rakhr and Ram' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000261] For example, at least one of Rakhr and Ram' is methyl or ethyl.
[000262] For example, Ram and Ram' are both methyl or ethyl.
[000263] For example, Qy6 is -C(O)NR34R34'.
[000264] For example, R34 and R34' are both H.
[000265] For example, at least one of R34 and R34' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000266] For example, R34 and R34', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl and is
optionally
substituted.
[000267] For example, R34 and R34', together with the atom they attach to,
form a
pyrrolidinyl ring, and is optionally substituted.
[000268] For example, Qy3 is unsubstituted naphthyl.
[000269] For example, Qy3 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
33
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl,
dihydrobenzofuranyl, and
pyrrolopyrimidinyl, and the like, and is optionally substituted with one, two
or more groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted amino, unsubstituted or substituted C1-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and
n-hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and iodine)),
unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-
propyloxy,
butoxy, i-butoxy, and t-butoxy, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted
phenyl.
[000270] For example, Qy3 is oxazolyl, isoxazolyl, pyridinyl, pyrazinyl,
triazinyl,
thienyl, benzothiadiazolyl, or dihydrobenzofuranyl, each of which is
optionally substituted
with one, two or more groups, each of which can be the same or different,
selected from
halogen (e.g., fluorine, chlorine, bromine, and iodine), methyl, ethyl, t-
butyl, trifluoromethyl,
and phenyl.
[000271] For example, Qy3 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
C1-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000272] For example, Qy3 is cyclohexyl.
[000273] For example, Qy3 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted C1-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000274] For example, Qy3 is -C(O)OR31, -C(O)R31, -C(O)NR31R31', or -NR31R31'.
[000275] For example, Qy3 is -C(O)OR31 or -NR31R31'.
[000276] For example, R31 and R31' are both H.
34
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000277] For example, at least one of R31 and R31' is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine),
hydroxyl, or
unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-
propyloxy,
butoxy, i-butoxy, and t-butoxy).
[000278] For example, at least one of R31 and R31' is methyl or ethyl
optionally
substituted with hydroxyl.
[000279] For example, R2 and R2', together with the atom they attach to, form
a 5-, 6- or
7-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl,
azepanyl, and diazepanyl,
and the like and is optionally substituted with one or more groups, each of
which can be the
same or different, selected from -Ty7-Qy7, wherein:
Tye is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qy7 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, -
C(O)NR33R33', -C(O)ONR33R33', or -NR33R33'; and
R33 and R33' are each independently H, unsubstituted or substituted C1-
C6 alkyl, unsubstituted or substituted phenyl, unsubstituted or substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N, 0 and S, unsubstituted or substitute C3-Cg carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S, or R33 and R33',
together with the atom they attach to, form a 5- or 6-member ring which
optionally comprises 1-4 heteroatoms selected from N, 0 and S and is
optionally substituted.
[000280] For example, R2 and R2', together with the atom they attach to, form
a 5-, 6- or
7-member ring selected from piperidinyl, piperazinyl, pyrrolidinyl, and
diazepanyl, and is
optionally substituted with -Tye-Qy7.
[000281] For example, Tye is a bond.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000282] For example, Tye is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000283] For example, Tye is a methyl, ethyl, or propyl linker.
[000284] For example, Qy7 is H.
[000285] For example, Qy7 is unsubstituted phenyl.
[000286] For example, Qy7 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[000287] For example, Qy7 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from halogen (e.g., fluorine, chlorine,
bromine, and
iodine), methyl, ethyl, -t-butyl, methoxy, ethoxy and t-butoxy.
[000288] For example, Qy7 is -C(O)NR33R33' or -NR33R33'
[000289] For example, R33 and R33' are both H.
[000290] For example, at least one of R33 and R33' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000291] For example, at least one of R33 and R33' is unsubstituted or
substituted phenyl.
[000292] For example, at least one of R33 and R33' is heteroaryl selected from
pyrrolyl,
furanyl, thienyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl,
benzoxazolyl,
benzodioxazolyl, benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl,
benzoimidazolyl,
benzothienyl, methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl,
indolyl,
benzofuranyl, purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl,
quinoxalinyl, and
pyrrolopyrimidinyl, and the like, and is optionally substituted.
[000293] For example, at least one of R33 and R33' is cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, or cycloheptyl, and is optionally substituted.
[000294] For example, at least one of R33 and R33' is heterocycle selected
from
pyrrolidinyl, imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl,
triazolidinyl,
36
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
tetrahyrofuranyl, piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone,
and the like, and is
optionally substituted.
[000295] For example, R33 and R33', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl, and
pyrrolidinone, and the
like, and is optionally substituted.
[000296] For example, R33 and R33', together with the atom they attach to,
form a 5- or
6-member ring selected from morpholinyl.
[000297] For example, Xi-X2 is CH2-CHRy'.
[000298] For example, Ry' is H.
[000299] For example, Ry' is is unsubstituted phenyl.
[000300] For example, Ry' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Rair, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa ,
wherein
Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000301] For example, Ry' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyano, and -NHC(O)R,r., wherein Rakhr is H, methyl, ethyl,
or t-butyl.
[000302] For example, Ry' is unsubstituted naphthyl.
[000303] For example, Ry' is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Ra, -NHC(O)OR, -C(O)Ra, and -C(O)ORa, wherein Ra& is H, or
37
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000304] For example, Ry' is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
[000305] For example, Ry' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted amino, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa , wherein
Rakhr is H, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-Cio
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[000306] For example, Ry' is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra,r., wherein R,, is
H, methyl,
ethyl, t-butyl, or phenyl.
[000307] For example, Ry' is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
Ci-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
38
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
iodine)), unsubstituted or substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000308] For example, Ry' is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted Ci-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000309] For example, R3" is H.
[000310] For example, R3" is unsubstituted or substituted, straight-chain or
branched
Ci-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0003111 For example, both R3 and R3' are H.
[000312] For example, at least one of R3 and R3' is -Ty1'-Qy1'.
[000313] For example, Ty1' is a bond.
[000314] For example, Ty1' is unsubstituted or substituted Ci-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000315] For example, Ty1' is a methyl, ethyl, propyl, or t-butyl linker.
[000316] For example, Qyi' is H.
[000317] For example, Qyi' is unsubstituted phenyl.
[000318] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted Ci-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy,
methylenedioxy, and ethylenedioxy, each of which is optionally substituted
with halogen
(e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted
phenyl,
unsubstituted or substituted C6-Cio aryloxy (e.g., phenoxy), -NRaRa', -
C(O)RaRa', -
S(O)2Ra ,r., and -NHC(O)RaI,,.R ,r.', wherein Ram. and Ram,,.' are each
independently H,
39
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl)
[000319] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from fluorine, chlorine, bromine,
and iodine.
[000320] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from methyl, ethyl, t-butyl, and
trifluoromethyl.
[000321] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from methoxy, ethoxy,
trifluoromethoxy, and
ethylenedioxy.
[000322] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from -NRaRa', -C(O)RaRa', -
S(O)2Ra,
and -NHC(O)R,r.RaI,,.', wherein at least one of Rakhr and Ram,,.' is methyl or
ethyl.
[000323] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from fluorine, chlorine, bromine,
iodine, methyl,
ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, trifluoromethoxy, phenyl,
phenoxy, -
NRa ,,.Ra ,,.', -C(O)Ra ,,Ra ,r', -S(O)2RaI,,., and -NHC(O)RakhrRa ,r',
wherein at least one of
Rakhr and Ram' is methyl or ethyl.
[000324] For example, Qyi' is unsubstituted naphthyl.
[000325] For example, Qyi' is naphthyl substituted with one, two or more
groups, each
of which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted Ci-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000326] For example, Qyi' is naphthyl substituted with one, two or more
groups, each
of which can be the same or different, selected from methyl, ethyl, and
halogen (e.g.,
fluorine, chlorine, bromine, and iodine).
[000327] For example, Qyi' is fluorenyl, and is optionally substituted.
[000328] For example, Qyi' is dihydroindenyl, and is optionally substituted.
[000329] For example, Qyi' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzoisoxazolyl,
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
benzodioxazolyl, benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl,
benzoimidazolyl,
benzothienyl, methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl,
indolyl,
benzofuranyl, purinyl, purinone, deazapurinyl, indolizinyl, imidazolthiazolyl,
dihydrobenzofuranyl, quinoxalinyl, and pyrrolopyrimidinyl, and the like, and
is optionally
substituted with one, two or more groups, each of which can be the same or
different,
selected from hydroxyl, halogen, unsubstituted or substituted amino,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted CI-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)),
unsubstituted or substituted C6-CIO aryloxy (e.g., phenoxy), and unsubstituted
or substituted
phenyl.
[000330] For example, Qyl' is oxazolyl, isoxazolyl, pyridinyl, pyrimidinyl,
triazinyl,
thienyl, purinyl, purinone, quinolinyl, benzothienyl, benzoxazolyl,
benzoisoxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, pyrrolopyrimidinyl, or
dihydrobenzofuranyl, each of which is substituted with one, two or more
groups, each of
which can be the same or different, selected from halogen (e.g., fluorine,
chlorine, bromine,
and iodine), methyl, ethyl, amino, phenyl, and phenoxy.
[000331] For example, Qyl' is -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -
NR21R21'.
[000332] For example, Qyl' is -C(O)OR21.
[000333] For example, R21 is H.
[000334] For example, R21 is unsubstituted or substituted C1-C6 alkyl,
including but not
limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl.
[000335] For example, R21 is methyl or ethyl.
[000336] For example, XI-X2 is Xl'-X2';
[000337] For example, Xl'-X2' is CHRxa1 -CHRxa2, CHRxb-O, O-CHRXb, CHRXe7
CHRxdI-CHRxd2-CHRxd3, CHRxe1-CHRxe2-O, O-CHRxe1-CHRxe2, CHRxe1-O-CHRxe2, CHRxf-
S, or S-CHRxf;
[000338] For example, both Rxai and Rxa2 are H.
[000339] For example, at least one of Rxai and Rxa2 is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
41
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine).
[000340] For example, at least one of Rxal and Rxa2 is methyl, which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine).
[000341] For example, at least one of Rxai and Rxa2 is unsubstituted phenyl.
[000342] For example, at least one of Rxai and Rxa2 is phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000343] For example, at least one of Rxai and Rxa2 is phenyl substituted with
two or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000344] For example, Rxb is H.
[000345] For example, Rxb is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000346] For example, Rxb is unsubstituted phenyl.
[000347] For example, Rxb is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
42
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000348] For example, RXb is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000349] For example, RX, is H.
[000350] For example, RX, is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000351] For example, RX, is methyl.
[000352] For example, RX, is unsubstituted phenyl.
[000353] For example, RX, is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000354] For example, RX, is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000355] For example, all of RXdi, Rxd2 and RXd3 are H.
[000356] For example, at least one of RXdi, RXdz and RXd3 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000357] For example, at least one of RXdi, RXdz and RXd3 is unsubstituted
phenyl.
[000358] For example, at least one of RXdi, RXdz and RXd3 is phenyl
substituted with one
or more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
43
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000359] For example, at least one of Rxdi, Rxd2 and Rxd3 is phenyl
substituted with two
or more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000360] For example, both RXei and Rxe2 are H.
[000361] For example, at least one of RXei and Rxe2 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000362] For example, at least one of RXei and Rxe2 is unsubstituted phenyl.
[000363] For example, at least one of RXei and Rxe2 is phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000364] For example, at least one of RXei and Rxe2 is phenyl substituted with
two or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
44
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000365] For example, Rf is H.
[000366] For example, Rf is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000367] For example, Rf is unsubstituted phenyl.
[000368] For example, Rf is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000369] For example, Rf is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000370] The present invention also provides the compounds of Formula II:
/Rc2
N-N
/
Rc1 N
(Rp)n X,
X2I (II),
or a salt, solvate, hydrate or prodrug thereof, wherein:
R,i is H, halogen, unsubstituted or substituted CI-C6 alkyl, unsubstituted or
substituted phenyl, or unsubstituted or substituted heteroaryl comprising one
or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S;
Rc2 is H, or unsubstituted or substituted CI-C6 alkyl;
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
each Rp is independently halogen, hydroxyl, cyano, nitro, amino, unsubstituted
or
substituted CI-C6 alkoxy, unsubstituted or substituted CI-C6 alkyl, -C(O)Ra, -
C(O)ORa , -
NHC(O)Rar, -NHC(O)ORar;
n is 0, 1, 2, 3, or 4;
Xi'-X2' is CHRxal-CHRxa2, CHRxb-O, O-CHRXb, CHR, CHRxdi-CHRxd2-CHRxd3,
CHRxeI-CHRxe2-O, O-CHRxeI-CHRxe2, CHRxeI-O-CHRxe2, CHRxf-S, S-CHRxf, CHRxel-
CHRxe2-NRy, CHRxeI-NRy-CHRxe2 or NRy-CHRxe1-CHRxe2;
Rxai, Rxa2, Rxb, Rxe, RXd1, Rxd2, Rxd3, Rxei, Rxe2, or Rxf are each
independently H,
unsubstituted or substituted CI-C6 alkyl, or unsubstituted or substituted
phenyl; and
Rakhr is H, unsubstituted or substituted CI-C6 alkyl, unsubstituted or
substituted C6-CIO
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[000371] For example, Ref is H.
[000372] For example, Ref is fluorine, chlorine, bromine, or iodine.
[000373] For example, Ref is unsubstituted or substituted, straight chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000374] For example, Ref is methyl.
[000375] For example, Ref is unsubstituted phenyl.
[000376] For example, Ref is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted CI-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)), and -Tel-
Qei, wherein:
Tel is a bond, or unsubstituted or substituted CI-C6 alkyl linker;
Qei is H, -NR,,i1Reii', -C(O)NR1,i1Reii', -NHC(O)R,,,II, -NHC(O)OR,,,II, -
NHC(O)NReiiR,,ii', or -S(0)2R,11; and
R,II and R,II' are each independently H, or or unsubstituted or substituted Ci-
C6 alkyl.
[000377] For example, Ref is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from -Tel-Qe1.
46
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000378] For example, T,1 is a bond.
[000379] For example, T,1 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000380] For example, T,1 is a methyl linker.
[000381] For example, Q,i is H.
[000382] For example, both R,II and R,11' are H.
[000383] For example, at least one of R,11 and R,11' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000384] For example, at least one of R,11 and R,11' is methyl, ethyl or t-
butyl.
[000385] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl and
trifluoromethyl.
[000386] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methoxy, t-butoxy and
trifluoromethoxy.
[000387] For example, R,1 is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, cyano, methyl, ethyl, t-
butyl,
trifluoromethyl, methoxy, t-butoxy, trifluoromethoxy, and halogen.
[000388] For example, R,1 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, quinoxalinyl, tetrohyddropyridinyl, pyrrolopyrimidinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl and n-
hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine and iodine)),
C6-C10 aryl (e.g., phenyl, which is optionally substituted), heteroaryl (e.g.,
pyrrolyl, furanyl,
thiophenyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, and pyrimidinyl, and the like,
and is optionally
substituted), C3-Cs carbocycle (e.g., cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted), or heterocycle (e.g.,
pyrrolidinyl, imidazolidinyl,
47
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, and morpholinyl, and the like, and is optionally substituted).
[000389] For example, Rc1 is heteroaryl selected from furanyl, pyrazolyl,
purinyl,
indolyl, tetrahydropyridinyl, and pyrrolopyrimidinyl, and is optionally
substituted with one or
more groups, each of which can be the same of different, selected from methyl,
ethyl, t-butyl,
amino, and heterocycle (e.g., piperidinyl, which is optionally substituted
with e.g., C(O)O-
(Ci-C6alkyl)).
[000390] For example, Rc2 is H.
[000391] For example, Rcz is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000392] For example, Rcz is methyl substituted with unsubstituted phenyl.
[000393] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000394] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from methyl,
ethyl, t-butyl,
and trifluoromethyl.
[000395] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
methoxy, ethoxy, i-
propyloxy, and t-butoxy. For example, Rcz is methyl substituted with phenyl
substituted with
one methoxy.
[000396] For example, n is 0.
[000397] For example, n is 1.
[000398] For example, n is 2.
[000399] For example, each Rp is independently fluorine, chlorine, bromine, or
iodine.
[000400] For example, each Rp is independently unsubstituted or substituted,
straight-
chain or branched CI-C6 alkyl, including but not limited to, methyl, ethyl, n-
propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
48
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000401] For example, each Rp is independently unsubstituted or substituted Ci-
C6
alkoxy, including but not limited to, methoxy, ethoxy, n-propyloxy, i-
propyloxy, n-butoxy,
t-butoxy.
[000402] For example, each Rp is independently -NHC(O)Rai,,..
[000403] For example, Rakes is H.
[000404] For example, Rakr is unsubstituted or substituted, straight-chain or
branched
Ci-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000405] For example, Rakes is methyl.
[000406] For example, at least one Rp is hydroxyl, methyl, ethyl, t-butyl,
trifluoromethyl, methoxy, ethoxy, i-propyloxy, t-butoxy, nitro, halogen (e.g.,
fluorine,
chlorine, bromine, and iodine), or -NHC(O)Ra,r, wherein Rakhr is methyl.
[000407] For example, at least two Rp, each of which is independent from the
other, are
hydroxyl, methyl, ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, i-
propyloxy, t-butoxy,
nitro, halogen (e.g., fluorine, chlorine, bromine, and iodine), or -NHC(O)Ra,
wherein Rakhr
is methyl.
[000408] For example, at least three Rp, each of which is independent from the
other,
are hydroxyl, methyl, ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, i-
propyloxy, t-butoxy,
nitro, halogen (e.g., fluorine, chlorine, bromine, and iodine), or -NHC(O)Ra,
wherein Rakhr
is methyl.
[000409] For example, both Rxal and Rxa2 are H.
[000410] For example, at least one of Rxai and Rxaz is unsubstituted or
substituted,
straight-chain or branched Ci-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine).
[000411] For example, at least one of Rxai and Rxaz is methyl, which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine).
[000412] For example, at least one of Rxai and Rxa2 is unsubstituted phenyl.
[000413] For example, at least one of Rxai and Rxa2 is phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyan, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
49
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000414] For example, at least one of Rxal and Rxa2 is phenyl substituted with
two or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000415] For example, Rxb is H.
[000416] For example, Rxb is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000417] For example, Rxb is unsubstituted phenyl.
[000418] For example, Rxb is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000419] For example, Rxb is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000420] For example, Rxa is H.
[000421] For example, Rxa is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000422] For example, Rxa is methyl.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000423] For example, RX, is unsubstituted phenyl.
[000424] For example, RX, is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000425] For example, RX, is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000426] For example, all of RXdi, Rxd2 and RXd3 are H.
[000427] For example, at least one of RXdi, RXdz and RXd3 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000428] For example, at least one of RXdi, RXdz and RXd3 is unsubstituted
phenyl.
[000429] For example, at least one of RXdi, RXdz and RXd3 is phenyl
substituted with one
or more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000430] For example, at least one of RXdi, RXdz and RXd3 is phenyl
substituted with two
or more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
51
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[0004311 For example, both RXei and Rxe2 are H.
[000432] For example, at least one of RXei and Rxe2 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000433] For example, at least one of RXei and Rxe2 is unsubstituted phenyl.
[000434] For example, at least one of RXei and Rxe2 is phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000435] For example, at least one of RXei and Rxe2 is phenyl substituted with
two or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000436] For example, Rxf is H.
[000437] For example, Rxf is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000438] For example, Rxf is unsubstituted phenyl.
[000439] For example, Rxf is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
52
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000440] For example, Rf is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted C1-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000441] For example, Xi'-X2' is CRR,'-O.
[000442] For example, Xi'-X2' is CRR,'-O where Rte, and Rte,', together with
the atom
they attach to, form a ring.
[000443] For example, Xi'-X2' is CRR,'-O where Rte, and Rte,', together with
the atom
they attach to, form an unsubstituted or substituted heterocycle.
[000444] For example, Xi'-X2' is CRR,'-O where Rte, and Rte,', together with
the atom
they attach to, form an unsubstituted or substituted piperidine ring.
[000445] The present invention also provides the compounds of Formulae Hal,
IIa2,
IIb, Ile, IId, Ile and IIf:
53
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
,Rc2
N-N
Rc1 N
N-N Rc2
(Rp)n
Rc1 N
I Rp1 Rp5
(Rp)n
Rxa1 Rp2 Rp4
Rxa2 (IIal), Rp3 (IIa2),
Rc2
N~
,Rc2 N-NRc2 / N
N-N / Rc1 N
Rc1 N Rd N ~R ~ I
p n
(Rp)n Rxd1
O Rxb (IIb), (Rp)n - Rxc (Ile), Rxd3 Rxd2 (IId),
Rc2
N--N N- ,Rc2
N
Rd N Rc1
I N
(Rp)n\
O Rxe1 (Rp)n
S
Rxe2 (Ile), or Rxf (11f),
or a salt, solvate, hydrate or prodrug thereof, wherein:
Rcl is H, halogen, unsubstituted or substituted C1-C6 alkyl, unsubstituted or
substituted phenyl, or unsubstituted or substituted heteroaryl comprising one
or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S;
Rc2 is H, or unsubstituted or substituted C1-C6 alkyl;
each Rp is independently halogen, hydroxyl, cyan, nitro, amino, unsubstituted
or
substituted C1-C6 alkoxy, unsubstituted or substituted C1-C6 alkyl, -C(O)Ra, -
C(O)ORa, -
NHC(O)Rar, or -NHC(O)ORar;
n is 0, 1, 2, 3, or 4;
Rxai, Rxa2, RXb, RXC, RXd1, RXd2, RXd3, Rxei, Rxe2, or RXf are each
independently H,
unsubstituted or substituted C1-C6 alkyl, or unsubstituted or substituted
phenyl;
54
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Rakhr is H, unsubstituted or substituted Ci-C6 alkyl, unsubstituted or
substituted C6-C10
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S; and
Rpl, Rp2, Rp3, Rp4 and Rp5 are each independently H, hydroxyl, halogen, cyano,
nitro,
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl,
unsubstituted or
substituted CI-C6 alkoxy, or unsubstituted or substituted phenyl.
[000446] For example, R,1 is H.
[000447] For example, R,1 is fluorine, chlorine, bromine, or iodine.
[000448] For example, R,1 is unsubstituted or substituted, straight chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000449] For example, R,1 is methyl.
[000450] For example, R,1 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, quinoxalinyl, tetrohyddropyridinyl, pyrrolopyrimidinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl and n-
hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine and iodine)),
C6-C10 aryl (e.g., phenyl, which is optionally substituted), heteroaryl (e.g.,
pyrrolyl, furanyl,
thiophenyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, and pyrimidinyl, and the like,
and is optionally
substituted), C3-Cs carbocycle (e.g., cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted), or heterocycle (e.g.,
pyrrolidinyl, imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, and morpholinyl, and the like, and is optionally substituted).
[000451] For example, R,1 is pyrazolyl, and is optionally substituted with
(e.g.,
piperidinyl, which is optionally substituted).
[000452] For example, Rc2 is H.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000453] For example, Rcz is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000454] For example, Rcz is methyl substituted with unsubstituted phenyl.
[000455] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000456] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from methyl,
ethyl, t-butyl,
and trifluoromethyl.
[000457] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
methoxy, ethoxy, i-
propyloxy, and t-butoxy.
[000458] For example, n is 0.
[000459] For example, n is 1.
[000460] For example, n is 2.
[0004611 For example, each Rp is independently fluorine, chlorine, bromine, or
iodine.
[000462] For example, each Rp is independently unsubstituted or substituted,
straight-
chain or branched CI-C6 alkyl, including but not limited to, methyl, ethyl, n-
propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000463] For example, each Rp is independently unsubstituted or substituted CI-
C6
alkoxy, including but not limited to, methoxy, ethoxy, n-propyloxy, i-
propyloxy, n-butoxy,
t-butoxy.
[000464] For example, each Rp is independently -NHC(O)Rai,,..
[000465] For example, Rar is H.
[000466] For example, Ram is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000467] For example, Rar is methyl.
56
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000468] For example, at least one Rp is hydroxyl, methyl, ethyl, t-butyl,
trifluoromethyl, methoxy, ethoxy, i-propyloxy, t-butoxy, nitro, halogen (e.g.,
fluorine,
chlorine, bromine, and iodine), or -NHC(O)Ra, wherein Rakhr is methyl.
[000469] For example, at least two Rp, each of which is independent from the
other, are
hydroxyl, methyl, ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, i-
propyloxy, t-butoxy,
nitro, halogen (e.g., fluorine, chlorine, bromine, and iodine), or -NHC(O)Ra,
wherein Rakhr
is methyl.
[000470] For example, at least three Rp, each of which is independent from the
other,
are hydroxyl, methyl, ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, i-
propyloxy, t-butoxy,
nitro, halogen (e.g., fluorine, chlorine, bromine, and iodine), or -NHC(O)Ra,
wherein Rakhr
is methyl.
[000471] For example, both Rxal and Rxa2 are H.
[000472] For example, at least one of Rxai and Rxa2 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine).
[000473] For example, at least one of Rxai and Rxa2 is methyl, which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine).
[000474] For example, at least one of Rxai and Rxa2 is unsubstituted phenyl.
[000475] For example, at least one of Rxai and Rxa2 is phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyan, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000476] For example, at least one of Rxai and Rxa2 is phenyl substituted with
two or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyan, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
57
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000477] For example, Rxb is H.
[000478] For example, RXb is unsubstituted phenyl.
[000479] For example, RXb is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000480] For example, RXb is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000481] For example, Rxe is H.
[000482] For example, Rxe is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000483] For example, Rxe is methyl.
[000484] For example, all of RXdi, Rxd2 and Rxd3 are H.
[000485] For example, at least one of RXdi, RXdz and RXd3 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000486] For example, both RXei and Rxe2 are H.
[000487] For example, at least one of RXei and Rxe2 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000488] For example, RXf is H.
58
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000489] For example, Rf is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, -
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000490] For example, all of Rpl, Rp2, Rp3, Rp4 and Rps are H.
[000491] For example, at least one of Rpi, Rpz, Rp3, Rp4 and Rps is fluorine,
chlorine,
bromine, or iodine.
[000492] For example, at least one of Rpi, Rpz, Rp3, Rp4 and Rps is
unsubstituted or
substituted, straight-chain or branched CI-C6 alkyl, including but not limited
to, methyl,
ethyl, n-propyl, i-propyl, -butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-
hexyl.
[000493] For example, at least one of Rpi, Rpz, Rp3, Rp4 and Rps is methyl.
[000494] For example, at least one of Rpi, Rpz, Rp3, Rp4 and Rps is
unsubstituted or
substituted CI-C6 alkoxy, including but not limited to, methoxy, ethoxy, n-
propyloxy,
i-propyloxy, n-butoxy, t-butoxy.
[000495] For example, at least one of Rpi, Rpz, Rp3, Rp4 and Rps is
unsubstituted or
substituted phenyl.
[000496] For example, at least two of Rpl, Rpz, Rp3, Rp4 and Rps, each of
which is
independent from the other, are methyl, ethyl, t-butyl, trifluoromethyl,
methoxy, ethoxy, i-
propyloxy, t-butoxy, hydroxyl, halogen, nitro, or cyano.
[000497] For example, at least three of Rpi, Rpz, Rp3, Rp4 and Rps, each of
which is
independent from the other, are methyl, ethyl, t-butyl, trifluoromethyl,
methoxy, ethoxy, i-
propyloxy, t-butoxy, hydroxyl, halogen, nitro, or cyano.
[000498] The present invention also provides the compounds of Formula III:
/Rc2
N-N
Rd N
A
Rc6
Rc5 (III),
or a salt, solvate, hydrate or prodrug thereof, wherein:
A
is a 5- or 6-member ring which optionally comprises 1-4 heteroatoms selected
from N, 0 and S, and is optionally substituted;
59
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
R,i is H, halogen, unsubstituted or substituted CI-C6 alkyl, unsubstituted or
substituted phenyl, or unsubstituted or substituted heteroaryl comprising one
or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S;
Rc2 is H or unsubstituted or substituted CI-C6 alkyl; and
Rc5 and Rc6 are each independently H, unsubstituted or substituted CI-C6
alkyl, or
unsubstituted or substituted C6-CIO aryl.
[000499] For example, R,1 is H.
[000500] For example, R,1 is fluorine, chlorine, bromine, or iodine.
[000501] For example, R,1 is unsubstituted or substituted, straight chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000502] For example, R,1 is methyl.
[000503] For example, R,1 is unsubstituted phenyl.
[000504] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted CI-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)), and -T,i-
Q,i, wherein:
T,1 is a bond, or unsubstituted or substituted CI-C6 alkyl linker;
Q,i is H, -NR,,i1R,,ii', -C(O)NR,,i1R,,ii', -NHC(O)R,,,ii, -NHC(O)OR,,,ii, -
NHC(O)NR,,,,R,,,,', or -S(0)2R,11; and
R,, 11 and R,, l1' are each independently H, or or unsubstituted or
substituted Ci-
C6 alkyl.
[000505] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from -T,1-Qc1.
[000506] For example, T,1 is a bond.
[000507] For example, T,1 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000508] For example, T,1 is a methyl linker.
[000509] For example, Q,i is H.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000510] For example, both R,II and R,11' are H.
[000511] For example, at least one of R,11 and R,11' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000512] For example, at least one of R,11 and R,11' is methyl, ethyl or t-
butyl.
[000513] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl and
trifluoromethyl.
[000514] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methoxy, t-butoxy and
trifluoromethoxy.
[000515] For example, R,1 is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, cyan, methyl, ethyl, t-
butyl,
trifluoromethyl, methoxy, t-butoxy, trifluoromethoxy, and halogen.
[000516] For example, R,1 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, quinoxalinyl, tetrohyddropyridinyl, pyrrolopyrimidinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl and n-
hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine and iodine)),
C6-Cio aryl (e.g., phenyl, which is optionally substituted), heteroaryl (e.g.,
pyrrolyl, furanyl,
thiophenyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, and pyrimidinyl, and the like,
and is optionally
substituted), C3-Cs carbocycle (e.g., cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted), or heterocycle (e.g.,
pyrrolidinyl, imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, and morpholinyl, and the like, and is optionally substituted).
[000517] For example, R,1 is heteroaryl selected from furanyl, pyrazolyl,
purinyl,
indolyl, tetrahydropyridinyl, and pyrrolopyrimidinyl, and is optionally
substituted with one or
more groups, each of which can be the same of different, selected from methyl,
ethyl, t-butyl,
amino, and heterocycle (e.g., piperidinyl, which is optionally substituted).
[000518] For example, Rc2 is H.
61
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000519] For example, Rcz is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000520] For example, Rcz is methyl substituted with unsubstituted phenyl.
[000521] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000522] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from methyl,
ethyl, t-butyl,
and trifluoromethyl.
[000523] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
methoxy, ethoxy, i-
propyloxy, and t-butoxy.
[000524] For example, both Rc5 and Rc6 are H.
[000525] For example, at least one of Rc5 and Rc6 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000526] For example, at least one of Rc5 and Rc6 is unsubstituted or
substituted phenyl.
A
[000527] For example, is a 5- or 6-member ring selected from:
1) phenyl, which is optionally substituted with one or more groups, each of
which can be the same or different, selected from hydroxyl, halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), cyano, nitro, unsubstituted or substituted amino,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl), unsubstituted or substituted CI-C6 alkoxy
(e.g., methoxy,
ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy), -C(O)Ra , -
C(O)ORa , -
NHC(O)Rar, -NHC(O)ORar, and -C(O)NRaRa');
2) heteroaryl (e.g., pyrrolyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl,
thiazolyl, isothiazolyl, triazolyl, oxadiazolyl, thiadiazolyl, tetrazolyl,
pyridinyl, pyrimidinyl,
62
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
pyridazinyl, pyrazinyl, triazinyl, tetrazinyl, furanyl, thienyl, and
pyridinone, and the like, and
is optionally substituted with one or more groups, each of which can be the
same or different,
selected from hydroxyl, halogen (e.g., fluorine, chlorine, bromine, and
iodine), unsubstituted
or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl,
s-butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl), unsubstituted or substituted C1-C6 alkoxy
(e.g., methoxy,
ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy), unsubstituted
or substituted
phenyl, -NR nnlRnnn2, and -SRal{l,);
3) carbocycle (e.g., cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, or
cycloheptyl, and is optionally substituted); or
4) heterocycle (e.g., pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, tetrahyrofuranyl, piperidinyl, piperazinyl, and
morpholinyl, and
the like, and is optionally substituted),
wherein:
Rakhr and Ralf' are each independently H, unsubstituted or substituted
C1-C6 alkyl, unsubstituted or substituted C6-Clo aryl, or unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S;
Rnnnl and Riiiin2 are each independently H, -Tnnnl-Qnnnl, or Rnnnl and
Rnn2, together with the atom they attach to, form a 5- or 6-member ring which
optionally comprises 1-4 heteroatoms selected from N, 0 and S and is
optionally substituted;
Tnnn1 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qnnni is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, or -ORnnn3;
and
Rnnn3 is H, unsubstituted or substituted C1-C6 alkyl, or unsubstituted or
substituted phenyl.
A
[000528] For example, is a phenyl optionally substituted with one or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen
(e.g., fluorine,
63
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
chlorine, bromine, and iodine), cyano, nitro, methyl, ethyl, t-butyl,
trifluomethyl, methoxy,
ethoxy, t-butoxy, and -NHC(O)Ra.
A
[000529] For example, is a phenyl optionally substituted with two or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen
(e.g., fluorine,
chlorine, bromine, and iodine), cyano, nitro, methyl, ethyl, t-butyl,
trifluomethyl, methoxy,
ethoxy, t-butoxy, and -NHC(O)Ra.
A
[000530] For example, is heteroaryl selected from imidazolyl, thiazolyl,
pyridinyl,
pyrazoyl, pyridinone, pyrimidinyl, furanyl, or thienyl, each of which is
optionally substituted
with one or more groups, each of which can be the same or different, selected
from halogen
(e.g., fluorine, chlorine, bromine, and iodine), methyl, ethyl, t-butyl,
trifluomethyl, methoxy,
ethoxy, t-butoxy, phenyl, -NR,,,,,,1Riiiiii2, and -SRa .
[000531] For example, both Rakhr and Ram' are H.
[000532] For example, at least one of Rakhr and Ram' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000533] For example, Ram is H.
[000534] For example, Ram is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000535] For example, Rte,,. is methyl.
[000536] For example, both of R,,,,,,, and R,,,,,,2 are H.
[000537] For example, at least one of Rnnni and Riiiiiiz is -T,,,,,,1-Qnnnl.
[000538] For example, Tn.1 is a bond.
[000539] For example, Tn.1 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000540] For example, Tn.1 is a methyl, ethyl, or propyl linker.
[000541] For example, Qnnni is H.
[000542] For example, Qnnni is unsubstituted phenyl.
[000543] For example, Qnnni is phenyl substituted with one or more groups,
each of
which can be the same or different, selected from hydroxyl, halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), cyano, nitro, unsubstituted or substituted amino,
unsubstituted or
64
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000544] For example, Q.,,, is phenyl substituted with one or more groups,
each of
which can be the same or different, selected from methyl, ethyl, t-butyl,
trifluoromethyl,
methoxy, ethoxy, t-butoxy, and halogen (e.g. fluorine, chlorine, bromine and
iodine).
[000545] For example, Q.,,, is phenyl substituted with two or more groups,
each of
which can be the same or different, selected from methyl, ethyl, t-butyl,
trifluoromethyl,
methoxy, ethoxy, t-butoxy, and halogen (e.g. fluorine, chlorine, bromine and
iodine).
[000546] For example, Q.,,, is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000547] For example, Qnnni is pyridinyl.
[000548] For example, Qnnni is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
[000549] For example, Qnnni is cycloheptyl.
[000550] For example, Qnnni is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
one or more groups, each of which can be the same or different, selected from
hydroxyl,
halogen (e.g., fluorine, chlorine, bromine, and iodine), and unsubstituted or
substituted CI-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl).
[000551] For example, Q.,,, is pyrrolidinyl, piperidinyl, morpholinyl, or
pyrrolidinone,
and is optionally substituted with methyl, ethyl, or t-butyl.
[000552] For example, Q.,,, is -OR.,,3=
[000553] For example, R,,,,,,3 is H.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000554] For example, R,,,,,,3 is unsubstituted or substituted, straight-chain
or branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000555] For example, R,,,,,,3 is phenyl, and is optionally substituted.
[000556] For example, R,,,,,,1 and R,,,,,,z, together with the atom they
attach to, form a 5-
or 6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with one or more groups, each of which can be the same
or different,
selected from -T,,z-Q,,,,,,z, wherein:
Tiiiiii2 is a bond, or unsubstituted or substituted CI-C6 alkyl linker;
Qnnn2 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, -C(O)R,,,,,,4, -
C(O)OR,,,,,,4; and
Riiiiii4 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S.
[000557] For example, R,,,,,,1 and R,,,,,,z, together with the atom they
attach to, form a 5-
or 6-member ring selected from a pyrrolidinyl, piperidinyl, piperazinyl, or
morpholinyl, and
is optionally substituted.
[000558] For example, T,,,,,,2 is a bond.
[000559] For example, Tiiiiii2 is unsubstituted or substituted CI-C6 alkyl
linker, including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000560] For example, T,,,,,,2 is a methyl linker.
[000561] For example, Qnnn2 is unsubstituted phenyl.
[000562] For example, Qnnn2 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
66
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000563] For example, Q.,,2 is pyridinyl.
[000564] For example, Qnnnz is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
[000565] For example, Qnnnz is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000566] For example, Q.,,2 is morpholinyl.
[000567] For example, Qnnn2 is -C(O)R.,,4=
[000568] For example, Riin4 is H.
[000569] For example, Rnin4 is phenyl and is optionally substituted.
[000570] For example, Rnin4 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000571] For example, Rnin4 is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
[000572] For example, Rnin4 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000573] For example, Riin4 is pyrrolidinyl.
[000574] For example, Rnnn1 and Rnnn2, together with the atom they attach to,
form a 5-
or 6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with two or more groups, any adjacent two of which,
together with the
atoms they attach to form a 5- or 6-member ring which optionally comprises 1-4
heteroatoms
selected from N, 0 and S and is optionally substituted.
[000575] For example, Rnnn1 and Rnnn2, together with the atom they attach to,
form a 5-
or 6-membered ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with two or more groups, any adjacent two of which,
together with the
67
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
atoms they attach to form a phenyl ring, which is optionally substituted e.g.,
with heteroaryl
or heterocycle (e.g., morpholine or pyridine) or with CI-C6 alkyl linker-
unsubstituted phenyl
(e.g., benzyl), CI-C6 alkyl linker-substituted phenyl e.g., substituted with
halogen, CI-C6
alkyl linker-C(O)-heterocycle (e.g., pyrrolidine). For example, the 5- or 6-
membered ring is
uw
N
substituted with a fused aryl ring e.g.,
A
[000576] For example, is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, or
cycloheptyl, and is optionally substituted.
A
[000577] For example, is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000578] The present invention also provides the compounds of Formulae IIIa,
IIIb,
IIIc, IIId, Me I, IIIe2, and IIIf:
/Rc2 /Rc2
N-N N-N
Rd Rcl
Rn4
/N~ Rn3
(Rn1)m I~
Rc6 O N Rc6
Rc5 (IIIa), Rn2 Rc5 (IIIb),
/Rc2 /Rc2
N-N N-N
Rcl Rd
/ N
Rn5
N/ Rn7-
N Rc6 N Rc6
Rn6 Rc5 (IIIc), Rc5 (IIId),
68
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
/Rc2 /Rc2
N-N N-N
Rc1 / Rd N / N
Rn9 Rn9 \
Rng Rng /
S Rc6 O Rc6
Rc5 (IIIel), RC5 (111e2), or
/Rc2
N-N
Rc1
Rnn1
N
Rnn2 N Rc6
Rc5 (III0,
or a salt, solvate, hydrate or prodrug thereof, wherein:
Rcl is H;
Rc2 is H, or unsubstituted or substituted C1-C6 alkyl;
Rc5 and Rc6 are each independently H, unsubstituted or substituted C1-C6
alkyl, or
unsubstituted or substituted C6-CIO aryl;
each Ri1 is each indepenendently unsubstituted or substituted CI-C6 alkyl;
m is 0, 1, 2, or 3;
Ri5 and Ri6 are each indepenendently H, unsubstituted or substituted CI-C6
alkyl, or
unsubstituted or substituted phenyl;
Ri2, Rõ3, and Rõ4 are each indepenendently H, or unsubstituted or substituted
CI-C6
alkyl;
R,,7, Rõ g, and R,,9 are each independently H, unsubstituted or substituted CI-
C6 alkyl,
unsubstituted or substituted C6-C10 aryl, or unsubstituted or substituted
heteroaryl comprising
one or two 5- or 6-member ring and 1-4 heteroatoms selected from N, 0 and S;
R,,,,1 and Riiii2 are each indepenendently H, unsubstituted or substituted C1-
C6 alkyl, -
NR ..IRnnn2, or -SR ;
Rakhr is H, or unsubstituted or substituted C1-C6 alkyl;
R.,,, and R,,,,,,2 are each independently H, -T,,,,,,1-Q,,,n,1, or R,,,,,,1
and R,,,,,,2, together
with the atom they attach to, form a 5- or 6-member ring which optionally
comprises 1-4
heteroatoms selected from N, 0 and S and is optionally substituted;
Tiiiiii1 is a bond, or unsubstituted or substituted CI-C6 alkyl linker;
69
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Q.,,, is H, unsubstituted or substituted phenyl, unsubstituted or substituted
heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0 and S,
unsubstituted or substitute C3-Cg carbocycle, unsubstituted or substituted
heterocycle
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0 and S, or
-OR ..3; and
Riiiiii3 is H, unsubstituted or substituted Ci-C6 alkyl, or unsubstituted or
substituted
phenyl.
[000579] For example, R,1 is H.
[000580] For example, Rcz is H.
[000581] For example, Rcz is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000582] For example, Rcz is methyl substituted with unsubstituted phenyl.
[000583] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000584] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from methyl,
ethyl, t-butyl,
and trifluoromethyl.
[000585] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
methoxy, ethoxy, i-
propyloxy, and t-butoxy.
[000586] For example, both Rc5 and Rc6 are H.
[000587] For example, at least one of Rc5 and Rc6 is unsubstituted or
substituted,
straight-chain or branched Ci-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000588] For example, at least one of Rc5 and Rc6 is unsubstituted or
substituted phenyl.
[000589] For example, m is 0.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000590] For example, each of R,,, is independently unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000591] For example, all of R,,2, Rõ3 and Rõ4 are H.
[000592] For example, at least one of R,,2, Rõ3 and Rõ4 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000593] For example, both of Rõ5 and R,,6 are H.
[000594] For example, at least one of Rõ5 and Rõ6 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000595] For example, at least one of Rõ5 and Rõ6 is unsubstituted phenyl.
[000596] For example, Rõ6 is unsubstituted phenyl.
[000597] For example, R,,7 is H.
[000598] For example, Rõ7 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000599] For example, Rõ7 is methyl.
[000600] For example, both of Rõg and R,,9 are H.
[000601] For example, at least one of Rõg and R,,9 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000602] For example, both of R,,,,1 and R,,,,2 are H.
[000603] For example, at least one of R,,,,1 and R,,,,2 is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000604] For example, at least one of R,,,,1 and Riiii2 is -SRa .
[000605] For example, R,,,,1 is H and Riiii2 is -SRa .
[000606] For example, R,, is H.
[000607] For example, Ram is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000608] For example, R,, is methyl.
[000609] For example, at least one of R,,,,1 and R,,,,2 is -NR,,,,,,iR,,,,,,z.
71
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000610] For example, R,,,,1 is H and R,,,,2 is -NR,,,,,,iR,,,,z.
[000611] For example, both of R,,,,,,, and R,,,,,,2 are H.
[000612] For example, at least one of Rnnni and Riiiiiiz is -T,,,,,,1-Qnnnl.
[000613] For example, T,,,,,,1 is a bond.
[000614] For example, Tn.1 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000615] For example, Tn.1 is a methyl, ethyl, or propyl linker.
[000616] For example, Qnnni is H.
[000617] For example, Qnnni is unsubstituted phenyl.
[000618] For example, Qnnni is phenyl substituted with one or more groups,
each of
which can be the same or different, selected from hydroxyl, halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), cyano, nitro, unsubstituted or substituted amino,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000619] For example, Qnnni is phenyl substituted with one or more groups,
each of
which can be the same or different, selected from methyl, ethyl, t-butyl,
trifluoromethyl,
methoxy, ethoxy, t-butoxy, and halogen (e.g. fluorine, chlorine, bromine and
iodine).
[000620] For example, Qnnni is phenyl substituted with two or more groups,
each of
which can be the same or different, selected from methyl, ethyl, t-butyl,
trifluoromethyl,
methoxy, ethoxy, t-butoxy, and halogen (e.g. fluorine, chlorine, bromine and
iodine).
[000621] For example, Qnnni is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000622] For example, Qnnni is pyridinyl.
[000623] For example, Qnnni is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
[000624] For example, Qnnni is cycloheptyl.
72
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000625] For example, Q,,,,,,1 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
one or more groups, each of which can be the same or different, selected from
hydroxyl,
halogen (e.g., fluorine, chlorine, bromine, and iodine), unsubstituted or
substituted C1-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl).
[000626] For example, Qiiiiii, is pyrrolidinyl, piperidinyl, morpholinyl, or
pyrrolidinone,
and is optionally substituted with methyl, ethyl, or t-butyl.
[000627] For example, Q.,,, is -OR.,,3=
[000628] For example, R,,,,,,3 is H.
[000629] For example, R,,,,,,3 is unsubstituted or substituted, straight-chain
or branched
C1-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000630] For example, R,,,,,,3 is phenyl, and is optionally substituted.
[000631] For example, R,,,,,,1 and R,,,,,,z, together with the atom they
attach to, form a 5-
or 6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with one or more groups, each of which can be the same
or different,
selected from -T,,z-Q,,,,,,z, wherein:
T,,,,,,2 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qnnn2 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, -C(O)R,,,,,,4, -
C(O)ORiiiiii4; and
Riiiiii4 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S.
73
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000632] For example, Rnnn1 and Rnnn2, together with the atom they attach to,
form a 5-
or 6-member ring selected from pyrrolidinyl, piperidinyl, piperazinyl, or
morpholinyl, and is
optionally substituted.
[000633] For example, Tnnn2 is a bond.
[000634] For example, Tnii2 is unsubstituted or substituted CI-C6 alkyl
linker, including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000635] For example, Tnnn2 is a methyl linker.
[000636] For example, Qnnn2 is unsubstituted phenyl.
[000637] For example, Qnnnz is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000638] For example, Q.,,2 is pyridinyl.
[000639] For example, Qnnnz is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
[000640] For example, Qnnnz is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000641] For example, Q.,,2 is morpholinyl.
[000642] For example, Qnnn2 is -C(O)R.,,4=
[000643] For example, R.,A is H.
[000644] For example, R.,A is phenyl and is optionally substituted.
[000645] For example, R.,A is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000646] For example, R.,A is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
74
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000647] For example, R,,,,,,4 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000648] For example, R,,,,,,4 is pyrrolidinyl.
[000649] For example, R,,,,,,1 and R,,,,,,z, together with the atom they
attach to, form a 5-
or 6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with two or more groups, any adjacent two of which,
together with the
atoms they attach to form a 5- or 6-member which optionally comprises 1-4
heteroatoms
selected from N, 0 and S and is optionally substituted.
[000650] For example, R,,,,,,1 and R,,,,,,z, together with the atom they
attach to, form a 5-
or 6-member ring selected from pyrrolidinyl, piperidinyl, piperazinyl, and
morpholinyl, and
is optionally substituted with two or more groups, any adjacent two of which,
together with
the atoms they attach to form a phenyl ring, which is optionally substituted.
[000651] The present invention also provides the compounds of Formula IV':
Rc2
N-N
/
Rc1 N
R4 U
Rc3 Y Rc6 (IV'),
or a salt, solvate, hydrate or prodrug thereof, wherein:
Rc1 is H, halogen, unsubstituted or substituted C1-C6 alkyl, unsubstituted or
substituted phenyl, or unsubstituted or substituted heteroaryl comprising one
or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S;
Rc2 is H, or unsubstituted or substituted C1-C6 alkyl;
Rc3 and Rc4 are each independently H, unsubstituted or substituted C1-C6
alkyl;
Rc6 is H, or unsubstituted or substituted C1-C6 alkyl, or unsubstituted or
substituted
C6-C10 aryl;
Y is NRy or CHRy';
Ry is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
0 and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
O and S, -S(O)2R1, -C(O)R1, -C(O)OR1, -(CH2)0R1, -C(=NH)NR2R2', -C(O)NR2R2',
or -
C(S)NR2R2';
o is 0, 1, 2, 3, or 4;
R1 is H, unsubstituted or substituted C1-C6 alkyl, or -Ty1-Qy1;
R2 and R2' are each independently H, unsubstituted or substituted C1-C6 alkyl,
-Ty3-
Qy3, or R2 and R2', together with the atom they attach to, form a 5-, 6- or 7-
member ring
which comprises 1-4 heteroatoms selected from N, 0 and S and is optionally
substituted;
Ry' is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cg carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -NR3R3', -NR3"C(O)NR3R3', -NR3"C(O)R3, -NR3"C(O)OR3, -NR3"S(0)2R3, or
-
C(O)NR3R3';
R3 and R3' are each independently H, or -Tyi'-Qyi';
R3" is H, or unsubstituted or substituted C1-C6 alkyl;
Ty1, Ty1'and Ty3 are each independently a bond, or unsubstituted or
substituted C1-C6
alkyl linker;
Qy' is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -NR21R21';
Qyl' is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
0 and S, -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -NR21R21';
Qy3 is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
0 and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
0 and S, -C(O)OR31, -C(O)R31, -C(O)NR31R31', or -NR31R31'; and
76
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
R21, R21', R31 and R31' are each independently H, unsubstituted or substituted
CI-C6
alkyl, unsubstituted or substituted C6-C1o aryl, or unsubstituted or
substituted heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0 and S.
[000652] For example, R,1 is H.
[000653] For example, R,1 is fluorine, chlorine, bromine, or iodine.
[000654] For example, R,1 is unsubstituted or substituted, straight chain or
branched C1-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000655] For example, R,1 is methyl.
[000656] For example, R,1 is unsubstituted phenyl.
[000657] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted C1-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)), and -T,I-
Q,I, wherein:
T,1 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qal is H, -NR,,11R,,11', -C(O)NR,,11R,,11', -NHC(O)Rc11, -NHC(O)OR,,,II, -
NHC(O)NR,,,,R,,,,', or -S(0)2R,,,,; and
Rc11 and Rcl1' are each independently H, or or unsubstituted or substituted C1-
C6 alkyl.
[000658] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from -T,I-QaI
[000659] For example, T,1 is a bond.
[000660] For example, T,1 is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[0006611 For example, T,1 is a methyl linker.
[000662] For example, Qal is H.
[000663] For example, both Rc11 and Ra11' are H.
77
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000664] For example, at least one of R,11 and R,11' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000665] For example, at least one of R,11 and R,11' is methyl, ethyl or t-
butyl.
[000666] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl and
trifluoromethyl.
[000667] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methoxy, t-butoxy and
trifluoromethoxy.
[000668] For example, R,1 is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, cyan, methyl, ethyl, t-
butyl,
trifluoromethyl, methoxy, t-butoxy, trifluoromethoxy, and halogen.
[000669] For example, R,1 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, quinoxalinyl, tetrohydropyridinyl, pyrrolopyrimidinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl and n-
hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine and iodine)),
C6-Cio aryl (e.g., phenyl, which is optionally substituted), heteroaryl (e.g.,
pyrrolyl, furanyl,
thiophenyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, and pyrimidinyl, and the like,
and is optionally
substituted), C3-Cs carbocycle (e.g., cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted), or heterocycle (e.g.,
pyrrolidinyl, imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, and morpholinyl, and the like, and is optionally substituted).
[000670] For example, R,1 is heteroaryl selected from furanyl, pyrazolyl,
purinyl,
indolyl, tetrahydropyridinyl, and pyrrolopyrimidinyl, and is optionally
substituted with one or
more groups, each of which can be the same of different, selected from methyl,
ethyl, t-butyl,
amino, and heterocycle (e.g., piperidinyl, which is optionally substituted).
[000671] For example, Rc2 is H.
78
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000672] For example, Rc2 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000673] For example, Rcz is methyl substituted with unsubstituted phenyl.
[000674] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyano, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000675] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from methyl,
ethyl, t-butyl,
and trifluoromethyl.
[000676] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
methoxy, ethoxy, i-
propyloxy, and t-butoxy.
[000677] For example, Rc3 is H.
[000678] For example, Rc3 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000679] For example, Rc4 is H.
[000680] For example, Rc4 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0006811 For example, Rc6 is H.
[000682] For example, Rc6 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000683] For example, all of R,3, R,4 and R,6 are H.
[000684] For example, both Rakhr and Ram' are H.
79
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000685] For example, at least one of Rakhr and Ralf' is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000686] For example, R,, is H.
[000687] For example, Ralr is unsubstituted or substituted, straight-chain or
branched
C1-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000688] For example, R ,r. is methyl.
[000689] For example, both of Rc31 and Rc32 are H.
[000690] For example, at least one of Rc31 and Rc32 is -Tc3-Qc3.
[000691] For example, Tc3 is a bond.
[000692] For example, Tc3 is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000693] For example, Tc3 is a methyl, ethyl, or propyl linker.
[000694] For example, Qc3 is H.
[000695] For example, Qc3 is unsubstituted phenyl.
[000696] For example, Qc3 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen (e.g., fluorine,
chlorine,
bromine, and iodine), cyan, nitro, unsubstituted or substituted amino,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted C1-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000697] For example, Qc3 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl,
trifluoromethyl, methoxy,
ethoxy, t-butoxy, and halogen (e.g. fluorine, chlorine, bromine and iodine).
[000698] For example, Qc3 is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl,
trifluoromethyl, methoxy,
ethoxy, t-butoxy, and halogen (e.g. fluorine, chlorine, bromine and iodine).
[000699] For example, Qc3 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000700] For example, Qc3 is pyridinyl.
[000701] For example, Qc3 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[000702] For example, Qc3 is cycloheptyl.
[000703] For example, Qc3 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
one or more groups, each of which can be the same or different, selected from
hydroxyl,
halogen (e.g., fluorine, chlorine, bromine, and iodine), unsubstituted or
substituted C1-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl).
[000704] For example, Qc3 is pyrrolidinyl, piperidinyl, morpholinyl, or
pyrrolidinone,
and is optionally substituted with methyl, ethyl, or t-butyl.
[000705] For example, Qc3 is -ORc33.
[000706] For example, R,33 is H.
[000707] For example, Rc33 is unsubstituted or substituted, straight-chain or
branched
C1-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000708] For example, Rc33 is phenyl, and is optionally substituted.
[000709] For example, Rc31 and Rc32, together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with one or more groups, each of which can be the same
or different,
selected from -Tc4-Qc4, wherein:
Tc4 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qc4 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, -C(O)Rc34, -
C(O)ORc34; and
81
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Rc34 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S.
[000710] For example, Rc31 and Rc32, together with the atom they attach to,
form a 5- or
6-member ring selected from a pyrrolidinyl, piperidinyl, piperazinyl, or
morpholinyl, and is
optionally substituted.
[000711] For example, Tc4 is a bond.
[000712] For example, Tc4 is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000713] For example, Tc4 is a methyl linker.
[000714] For example, Qc4 is unsubstituted phenyl.
[000715] For example, Qc4 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000716] For example, Qc4 is pyridinyl.
[000717] For example, Qc4 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[000718] For example, Qc4 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000719] For example, Qc4 is morpholinyl.
[000720] For example, Qc4 is -C(O)Rc34.
[000721] For example, R,34 is H.
[000722] For example, Rc34 is phenyl and is optionally substituted.
[000723] For example, Rc34 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
82
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000724] For example, Rc34 is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
[000725] For example, Rc34 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000726] For example, R,34 is pyrrolidinyl.
[000727] For example, Rc31 and Rc32, together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with two or more groups, any adjacent two of which,
together with the
atoms they attach to form a 5- or 6-member which optionally comprises 1-4
heteroatoms
selected from N, 0 and S and is optionally substituted.
[000728] For example, Rc31 and Rc32, together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like and is
optionally substituted with two or more groups, any adjacent two of which,
together with the
atoms they attach to form a phenyl ring, which is optionally substituted.
[000729] For example, Y is NRy.
[000730] For example, Ry is H.
[000731] For example, Ry is is unsubstituted phenyl.
[000732] For example, Ry is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted C1-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Rai, -NHC(O)ORai, -C(O)Rai, and -C(O)ORa ,
wherein
Rakhr is H, or unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000733] For example, Ry is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
83
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyano, and -NHC(O)Ra , wherein Rakhr is H, methyl, ethyl, or
t-butyl.
[000734] For example, Ry is unsubstituted naphthyl.
[000735] For example, Ry is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Ra, -NHC(O)OR, -C(O)Ra, and -C(O)ORa, wherein Ra& is H, or
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000736] For example, Ry is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
[000737] For example, Ry is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted amino, unsubstituted or substituted CI-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa , wherein
Rakhr is H, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-C10
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
84
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000738] For example, Ry is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra, wherein Ram is H,
methyl,
ethyl, t-butyl, or phenyl.
[000739] For example, Ry is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000740] For example, Ry is cyclopropyl.
[000741] For example, Ry is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000742] For example, Ry is -S(O)2R1, -C(O)RI, -C(O)OR1, -(CH2)oR1.
[000743] For example, o is 0.
[000744] For example, o is 1.
[000745] For example, Ri is H.
[000746] For example, Ri is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000747] For example, Ri is straight-chain or branched CI-C6 alkyl substituted
with one,
two, three or more groups, each of which can be the same or different,
selected from:
1) phenyl, which is optionally substituted with one or more groups, each of
which can be the same or different, selected from unsubstituted or substituted
CI-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl,
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
s-pentyl, and n-hexyl), and unsubstituted or substituted CI-C6 alkoxy (e.g.,
methoxy,
ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy);
2) unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy);
3) -C(O)OR11; -C(O)Rii; and
4) -NR12R13, wherein:
R11 is H, or unsubstituted or substituted C1-C6 alkyl;
R12 and R13 are each independently H, -Ty2-Qy2, or R12 and R13,
together with the atom they attach to, form a 5- or 6-member ring, optionally
substituted with e.g., C(O)O(C1-C6alkyl) or CH2-heteroaryl;
Ty2 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qy2 is H, hydroxyl, unsubstituted or substituted C1-C6 alkoxy,
unsubstituted or substituted C6-CIO aryl, unsubstituted or substituted
heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from
N, 0 and S, unsubstituted or substituted C3-Cg carbocycle, unsubstituted or
substituted heterocycle comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, -NRa1Rr'5 -C(O)Ra5 -C(O)ORa;
and
Rakhr and Ralf' are each independently H, unsubstituted or substituted
C1-C6 alkyl, unsubstituted or substituted C6-Clo aryl, or unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S.
[000748] For example, Rl is methyl substitute with unsubstituted phenyl.
[000749] For example, Rl is methyl substitute with phenyl substituted with one
or more
groups, each of which can be the same or different, selected from hydroxyl,
halogen, nitro,
cyano, unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000750] For example, Rl is methyl substitute with methoxy, ethoxy, or t-
butoxy.
[000751] For example, Rl is methyl substitute with two or more groups, each of
which
can be the same or different, selected from:
86
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
1) phenyl, which is optionally substituted with one or more groups, each of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted
or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-
butoxy, and t-butoxy, each of which is optionally substituted with halogen
(e.g.,
fluorine, chlorine, bromine, and iodine)); and
2) one of the following:
i) -C(O)OR11;
ii) methoxy, ethoxy, i-propyloxy, or t-butoxy; or
iii) -NR12R13.
[000752] For example, Rl is methyl substitute with -C(O)OR11.
[000753] For example, R11 is H.
[000754] For example, R11 is unsubstituted or substituted, straight-chain or
branched C1-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000755] For example, Rl is methyl substitute with -NR12R13.
[000756] For example, both R12 and R13 are H.
[000757] For example, at least one of R12 and R13 is -Ty2-Qy2.
[000758] For example, Ty2 is a bond.
[000759] For example, Ty2 is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000760] For example, Ty2 is a methyl, ethyl, or propyl linker.
[000761] For example, Qy2 is H.
[000762] For example, Qy2 is hydroxyl.
[000763] For example, Qy2 is unsubstituted or substituted C1-C6 alkoxy,
including but
not limited to, methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and
t-butoxy.
[000764] For example, Qy2 is methoxy.
[000765] For example, Qy2 is unsubstituted or substituted phenyl or naphthyl.
[000766] For example, Qy2 is unsubstituted phenyl.
[000767] For example, Qy2 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
87
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted C1-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[000768] For example, Qy2 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[000769] For example, Qyz is indolyl.
[000770] For example, Qy2 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[000771] For example, Qy2 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[000772] For example, Qy2 is piperidinyl.
[000773] For example, Qy2 is -NRaItRa'.
[000774] For example, Qy2 is -C(O)ORa.
[000775] For example, both Rakhr and Rah,' are H.
[000776] For example, at least one of Rakhr and Ralf' is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000777] For example, at least one of Rakhr and Ram,,.' is methyl or t-butyl.
[000778] For example, R12 and R13, together with the atom they attach to, form
a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C1-C6 alkoxy
(e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy),
unsubstituted or substituted amino, -C(O)Ra,,, or -C(O)ORS,,., wherein Rte,,
is H, or
88
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000779] For example, R12 and R13, together with the atom they attach to, form
a
piperidinyl or piperazinyl, and is optionally substituted with halogen,
methyl, ethyl, t-butyl,
methoxy, ethoxy, i-propyloxy, t-butoxy, or -C(O)ORai, wherein Rakhr is methyl,
ethyl, or t-
butyl.
[000780] For example, Rl is -Tyl-Qyl.
[000781] For example, Ty, is a bond.
[000782] For example, Ty, is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000783] For example, Ty, is a methyl, ethyl, propyl, or i-propyl linker.
[000784] For example, Qy' is H.
[000785] For example, Qy' is unsubstituted phenyl.
[000786] For example, Qy' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted C1-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Rair, -NHC(O)ORai, -C(O)Rai, and -C(O)ORa,
wherein
Rakhr is H, or unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl). For example, Qy'
is phenyl
substituted with two groups that connect to form a fused six membered ring: '0
[000787] For example, Qy' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyano, and -NHC(O)Ra , wherein Rakhr is H, methyl, ethyl, or
t-butyl.
[000788] For example, Qy' is unsubstituted naphthyl.
[000789] For example, Qy' is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
89
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Ra, -NHC(O)OR, -C(O)Ra, and -C(O)ORa, wherein Ra& is H, or
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000790] For example, Qy' is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
[000791] For example, Qy' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted amino, unsubstituted or substituted CI-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa, wherein
Rakhr is H, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-C10
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[000792] For example, Qy' is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra , wherein Rakh, is
H, methyl,
ethyl, t-butyl, or phenyl.
[000793] For example, Qy' is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000794] For example, Qy' is cyclopropyl.
[000795] For example, Qy' is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000796] For example, Ry is -C(=NH)NR2R2'.
[000797] For example, both R2 and R2' are H.
[000798] For example, Ry is -C(S)NR2R2'.
[000799] For example, both R2 and R2' are phenyl.
[000800] For example, Ry is -C(=NH)NR2R2', -C(O)NR2R2', or -C(S)NR2R2'.
[000801] For example, both R2 and R2' are H.
[000802] For example, at least one of R2 and R2' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000803] For example, at least one of R2 and R2' is -Ty3-Qy3.
[000804] For example, Ty3 is a bond.
[000805] For example, Ty3 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, n-hexyl, and 2-methylpropyl linker.
[000806] For example, Ty3 is a methyl, ethyl, propyl, or 2-methylpropyl
linker.
[000807] For example, Qy3 is H.
[000808] For example, Qy3 is unsubstituted phenyl.
91
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000809] For example, Qy3 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from -Ty4-Qy4, wherein:
Ty4, Tys and Ty6 are each independently a bond, or unsubstituted or
substituted
CI-C6 alkyl linker;
Qy4 is H, unsubstituted or substituted CI-C6 alkyl, unsubstituted or
substituted
CI-C6 alkoxy, halogen, nitro, unsubstituted or substituted phenyl,
unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -NR32R32', -NHC(O)R32, -
NHC(O)OR32, -C(O)R32, -C(O)OR32, -OR32, -SR32, -C(O)NR32R32';
For example, Qy3 is phenyl, wherein phenyl is substituted with two groups that
connect to forma fused 5-membered ring e.g., ' or~
R32 and R32' are each independently H, unsubstituted or substituted CI-C6
alkyl, unsubstituted or substituted phenyl, unsubstituted or substituted
heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0
and S. -Tys-Qys, or R32 and R32', together with the atom they attach to, form
a 5- or 6-
member ring which optionally comprises 1-4 heteroatoms selected from N, 0 and
S
and is optionally substituted with -Ty6-Qy6;
Qys is H, hydroxyl, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -ORa5 -NRaRa'5 -NHC(O)Ra5 -
NHC(O)ORa5 -C(O)NRaRa .'5 -C(O)Rak,r, or -C(O)ORak,,.;
Qy6 is H, hydroxyl, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -NRaRa'5 -C(O)NR34R34';
Rakhr and Ram' are each independently H, unsubstituted or substituted CI-C6
alkyl, unsubstituted or substituted C6-Cio aryl, or unsubstituted or
substituted
92
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected
from N, 0 and S;
R34 and R34' are each independently H, unsubstituted or substituted CI-C6
alkyl, or R34 and R34', together with the atom they attach to, form a 5- or 6-
member
ring which optionally comprises 1-4 heteroatoms selected from N, 0 and S and
is
optionally substituted.
[000810] For example, Ty4 is a bond.
[000811] For example, Ty4 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000812] For example, Ty4 is a methyl or t-butyl linker.
[000813] For example, Qy4 is H.
[000814] For example, Qy4 is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with halogen
(e.g., fluorine, chlorine, bromine, and iodine).
[000815] For example, Qy4 is methyl, ethyl, t-butyl, or trifluoromethyl.
[000816] For example, Qy4 is unsubstituted or substituted CI-C6 alkoxy,
including but
not limited to, methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
methylenedioxy.
[000817] For example, Qy4 is methoxy, ethoxy, or methylenedioxy.
[000818] For example, Qy4 is fluorine, chlorine, bromine, or iodine.
[000819] For example, Qy4 is unsubstituted phenyl.
[000820] For example, Qy4 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[000821] For example, Qy4 is heteroaryl selected from selected from heteroaryl
selected
from pyrrolyl, furanyl, thienyl, thiazolyl, isothiazolyl, imidazolyl,
triazolyl, tetrazolyl,
93
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
pyrazolyl, oxazolyl, isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl,
pyrimidinyl, triazinyl,
benzoxazolyl, benzodioxazolyl, benzoxadiazolyl, benzothiazolyl,
benzothiadiazolyl,
benzoimidazolyl, benzothienyl, methylenedioxyphenyl, quinolinyl,
isoquinolinyl,
naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl, indolizinyl,
imidazolthiazolyl,
quinoxalinyl, oxadiazolyl, and pyrrolopyrimidinyl, and the like, and is
optionally substituted
with one or more groups, each of which can be the same or different, selected
from hydroxyl,
halogen, nitro, cyano, unsubstituted or substituted amino, unsubstituted or
substituted CI-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl, each of which is optionally substituted with halogen (e.g., fluorine,
chlorine,
bromine, and iodine)), and unsubstituted or substituted CI-C6 alkoxy (e.g.,
methoxy, ethoxy,
propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000822] For example, Qy4 is thiazolyl, isothiazolyl, or oxadiazolyl, each of
which is
optionally substituted with one or more groups, each of which can be the same
or different,
selected from methyl, ethyl, and t-butyl.
[000823] For example, Qy4 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted.
[000824] For example, Qy4 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000825] For example, Qy4 is piperazinyl or piperazinyl, and is optionally
substituted
with methyl, ethyl, or t-butyl.
[000826] For example, Qy4 is -NR32R32'.
[000827] For example, both R32 and R32' are H.
[000828] For example, at least one of R32 and R32' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl. For
example, at least one
of R32 and R32' is CI-C6 alkyl substituted with -CH2OH.
[000829] For example, at least one of R32 and R32' is methyl or ethyl.
[000830] For example, R32 and R32' are both methyl or ethyl.
94
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0008311 For example, Qy4 is -OR32 or -SR32.
[000832] For example, R32 is H.
[000833] For example, R32 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with halogen
(e.g., fluorine (e.g., -CF3), chlorine, bromine, and iodine) or hydroxyl.
[000834] For example, R32 is methyl, ethyl, t-butyl, or trifluoromethyl.
[000835] For example, R32 is unsubstituted phenyl.
[000836] For example, R32 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[000837] For example, R32 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, and quinoxalinyl, and
the like, and is
optionally substituted with one, two or more groups, each of which can be the
same or
different, selected from hydroxyl, halogen, nitro, cyano, unsubstituted or
substituted amino,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000838] For example, R32 is pyrazinyl, and is optionally substituted with
methyl, ethyl
or t-butyl.
[000839] For example, Qy4 is -C(O)R32, -C(O)OR32.
[000840] For example, R32 is H.
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000841] For example, R32 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000842] For example, R32 is methyl or ethyl.
[000843] For example, Qy4 is -NHC(O)R32, -NHC(O)OR32, or -C(O)NR32R32'.
[000844] For example, Qy4 is -C(O)NR32R32'.
[000845] For example, both R32 and R32' are H.
[000846] For example, at least one of R32 and R32' is -Tys-Qys=
[000847] For example, Tys is a bond.
[000848] For example, Tys is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, n-hexyl, 1,2-dimethylpropyl, and 1-methylbutyl linker.
[000849] For example, Tys is a methyl, ethyl, propyl, i-propyl, butyl, 1,2-
dimethylpropyl, or 1-methylbutyl linker. For example, Tys is ethyl substituted
with
C(O)NH2.
[000850] For example, Qys is H.
[000851] For example, Qys is unsubstituted phenyl.
[000852] For example, Qys is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted CI-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted C6-Cio aryloxy (e.g., phenoxy), and -
NHC(O)Ra,r,
wherein Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000853] For example, Qys is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from halogen (e.g., fluorine, chlorine,
bromine, and
iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, t-butoxy,
phenoxy, and -
NHC(O)Ra, wherein Rakhr is H, methyl or ethyl.
[000854] For example, Qys is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
96
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one or more groups, each of
which can be the
same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or substituted
amino, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000855] For example, Qys is pyrazolyl, imidazolyl, isoxazolyl, oxazolyl,
pyridinyl,
furanyl, indolyl, or benzoimidazolyl, each of which is optionally substituted
with one or more
groups, each of which can be the same or different, selected from methyl or
ethyl.
[000856] For example, Qys is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
Ci-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000857] For example, Qys is cyclohexyl or cycloheptyl, which is optionally
substituted
with methyl or ethyl.
[000858] For example, Qys is heterocycle selected from pyrrolidinyl,
pyrrolidinone,
imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl,
tetrahydrofuranyl,
piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is
optionally
substituted. For example, Qys is substituted with CH2OH.
[000859] For example, Qys is pyrrolidinyl, pyrrolidinone, piperidinyl,
morpholinyl, or
tetrahydrofuanyl, and is optionally substituted.
[000860] For example, Qys is -ORa .
[0008611 For example, R is H.
[000862] For example, R is unsubstituted or substituted, straight-chain or
branched
Ci-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
97
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000863] For example, Ram is methyl, ethyl, or t-butyl.
[000864] For example, Ram is unsubstituted phenyl.
[000865] For example, Ram is phenyl substituted with one or more groups, each
of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000866] For example, Qy5 is -NRairRair', -NHC(O)Ra , -NHC(O)ORa , -
C(O)NRa Ra ', -C(O)Ra , or -C(O)ORa .
[000867] For example, Qys is -NRaRa', -NHC(O)Ra , or -C(O)NRaRa '.
[000868] For example, both Rakhr and Ram' are H.
[000869] For example, at least one of Rakhr and Ram' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000870] For example, at least one of Rakhr and Ram' is methyl or ethyl.
[000871] For example, Ram and Ram' are both methyl or ethyl.
[000872] For example, R32 and R32', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl and is
optionally
substituted with -Ty6-Qy6.
[000873] For example, R32 and R32', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, piperidinyl, and piperazinyl, and is
optionally
substituted with -Ty6-Qy6.
[000874] For example, Ty6 is a bond.
[000875] For example, Ty6 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000876] For example, Ty6 is a methyl or ethyl linker.
[000877] For example, Qy6 is H.
[000878] For example, Qy6 is unsubstituted phenyl.
98
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000879] For example, Qy6 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[000880] For example, Qy6 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, and t-butyl.
[000881] For example, Qy6 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl,
benzoimidazolonyl,
benzodihydroimidazolone, and pyrrolopyrimidinyl, and the like, and is
optionally substituted
with one or more groups, each of which can be the same or different, selected
from hydroxyl,
halogen, nitro, cyano, unsubstituted or substituted amino, unsubstituted or
substituted CI-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl, each of which is optionally substituted with halogen (e.g., fluorine,
chlorine,
bromine, and iodine)), and unsubstituted or substituted CI-C6 alkoxy (e.g.,
methoxy, ethoxy,
propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000882] For example, Qy6 is pyridinyl, pyrazinyl, or benzodihydroimidazolone,
each of
which is optionally substituted with one or more groups, each of which can be
the same or
different, selected from methyl or ethyl.
[000883] For example, Qy6 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
99
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000884] For example, Qy6 is heterocycle selected from pyrrolidinyl,
pyrrolidinone,
imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl,
tetrahyrofuranyl,
piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is
optionally
substituted.
[000885] For example, Qy6 is -NRaRa'.
[000886] For example, both Rakhr and Ram' are H.
[000887] For example, at least one of Rakhr and Ram' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000888] For example, at least one of Rakhr and Ram' is methyl or ethyl.
[000889] For example, Ram and Ram' are both methyl or ethyl.
[000890] For example, Qy6 is -C(O)NR34R34'.
[000891 ] For example, R34 and R34' are both H.
[000892] For example, at least one of R34 and R34' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000893] For example, R34 and R34', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl and is
optionally
substituted.
[000894] For example, R34 and R34', together with the atom they attach to,
form a
pyrrolidinyl ring, and is optionally substituted.
[000895] For example, Qy3 is unsubstituted naphthyl.
[000896] For example, Qy3 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl,
dihydrobenzofuranyl,and
pyrrolopyrimidinyl, and the like, and is optionally substituted with one, two
or more groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyan,
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and
n-hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and iodine)),
100
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-
propyloxy,
butoxy, i-butoxy, and t-butoxy, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted
phenyl.
[000897] For example, Qy3 is oxazolyl, isoxazolyl, pyridinyl, pyrazinyl,
triazinyl,
thienyl, benzothiadiazolyl, or dihydrobenzofuranyl, each of which is
optionally substituted
with one, two or more groups, each of which can be the same or different,
selected from
halogen (e.g., fluorine, chlorine, bromine, and iodine), methyl, ethyl, t-
butyl, trifluoromethyl,
and phenyl.
[000898] For example, Qy3 is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
C1-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000899] For example, Qy3 is cyclohexyl.
[000900] For example, Qy3 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted C1-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000901] For example, Qy3 is -C(O)OR31, -C(O)R31, -C(O)NR31R31', or -NR31R31'.
[000902] For example, Qy3 is -C(O)OR31 or -NR31R31'.
[000903] For example, R31 and R31' are both H.
[000904] For example, at least one of R31 and R31' is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine),
hydroxyl, or
unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-
propyloxy,
butoxy, i-butoxy, and t-butoxy).
[000905] For example, at least one of R31 and R31' is methyl or ethyl
optionally
substituted with hydroxyl.
101
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000906] For example, R2 and R2', together with the atom they attach to, form
a 5-, 6- or
7-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl,
azepanyl, and diazepanyl,
and the like and is optionally substituted with one or more groups, each of
which can be the
same or different, selected from -Ty7-Qy7, wherein:
Tye is a bond, or unsubstituted or substituted CI-C6 alkyl linker;
Qy7 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, -
C(O)NR33R33', -C(O)ONR33R33', or -NR33R33'; and
R33 and R33' are each independently H, unsubstituted or substituted Ci-
C6 alkyl, unsubstituted or substituted phenyl, unsubstituted or substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N, 0 and S, unsubstituted or substitute C3-Cg carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S, or R33 and R33',
together with the atom they attach to, form a 5- or 6-member ring which
optionally comprises 1-4 heteroatoms selected from N, 0 and S and is
optionally substituted.
[000907] For example, R2 and R2', together with the atom they attach to, form
a 5-, 6- or
7-member ring selected from piperidinyl, piperazinyl, pyrrolidinyl, and
diazepanyl, and is
optionally substituted with -Tye-Qy7.
[000908] For example, Tye is a bond.
[000909] For example, Tye is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000910] For example, Tye is a methyl, ethyl, or propyl linker.
[000911] For example, Qy7 is H.
[000912] For example, Qy7 is unsubstituted phenyl.
[000913] For example, Qy7 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
102
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[000914] For example, Qy7 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from halogen (e.g., fluorine, chlorine,
bromine, and
iodine), methyl, ethyl, -t-butyl, methoxy, ethoxy and t-butoxy.
[000915] For example, Qy7 is heteroaryl e.g., indole.
[000916] For example, Qy7 is -C(O)NR33R33' or -NR33R33'
[000917] For example, R33 and R33' are both H.
[000918] For example, at least one of R33 and R33' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000919] For example, at least one of R33 and R33' is unsubstituted or
substituted phenyl.
[000920] For example, at least one of R33 and R33' is heteroaryl selected from
pyrrolyl,
furanyl, thienyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl,
benzoxazolyl,
benzodioxazolyl, benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl,
benzoimidazolyl,
benzothienyl, methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl,
indolyl,
benzofuranyl, purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl,
quinoxalinyl, and
pyrrolopyrimidinyl, and the like, and is optionally substituted.
[000921] For example, at least one of R33 and R33' is cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, or cycloheptyl, and is optionally substituted.
[000922] For example, at least one of R33 and R33' is heterocycle selected
from
pyrrolidinyl, imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl,
triazolidinyl,
tetrahyrofuranyl, piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone,
and the like, and is
optionally substituted.
[000923] For example, R33 and R33', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl, and
pyrrolidinone, and the
like, and is optionally substituted.
[000924] For example, R33 and R33', together with the atom they attach to,
form a 5- or
6-member ring selected from morpholinyl.
103
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000925] For example, Y is CHRy'.
[000926] For example, Ry' is H.
[000927] For example, Ry' is is unsubstituted phenyl.
[000928] For example, Ry' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Rair, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa, wherein
Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000929] For example, Ry' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyano, and -NHC(O)Ra, wherein Rakhr is H, methyl, ethyl, or
t-butyl.
[000930] For example, Ry' is unsubstituted naphthyl.
[0009311 For example, Ry' is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Ra, -NHC(O)OR, -C(O)Ra, and -C(O)ORa, wherein Ra& is H, or
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[000932] For example, Ry' is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
[000933] For example, Ry' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
104
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted amino, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Rair, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa, wherein
Rakhr is H, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-C10
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[000934] For example, Ry' is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra,r., wherein R,, is
H, methyl,
ethyl, t-butyl, or phenyl.
[000935] For example, Ry' is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
Ci-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[000936] For example, Ry' is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted Ci-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
105
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000937] For example, R3" is H.
[000938] For example, R3" is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000939] For example, both R3 and R3' are H.
[000940] For example, at least one of R3 and R3' is -Ty1'-Qy1'.
[000941] For example, Ty1' is a bond.
[000942] For example, Ty1' is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000943] For example, Ty1' is a methyl, ethyl, propyl, or t-butyl linker.
[000944] For example, Qyi' is H.
[000945] For example, Qyi' is unsubstituted phenyl.
[000946] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyan,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy,
methylenedioxy, and ethylenedioxy, each of which is optionally substituted
with halogen
(e.g., fluorine (e.g., -OCF3), chlorine, bromine, and iodine)), unsubstituted
or substituted
phenyl, unsubstituted or substituted C6-Cio aryloxy (e.g., phenoxy), -NRaRa', -
C(O)RaIjRa ', -C(O)ORa ,r, -S(O)2Ra ,r., and -NHC(O)RaI,,.Ra ,r.', wherein Ral
hr and Ra,r.'
are each independently H, unsubstituted or substituted CI-C6 alkyl (e.g.,
methyl, ethyl,
n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-
hexyl)
[000947] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from fluorine, chlorine, bromine,
and iodine.
[000948] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from methyl, ethyl, t-butyl, and
trifluoromethyl.
[000949] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from methoxy, ethoxy,
trifluoromethoxy,
106
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
phenoxy, and ethylenedioxy. For exmple, Qyi" is phenyl substituted with two
groups that
connect to form a fused 6-membered ring: ' '~o .
[000950] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from -NRaRa', -C(O)RaRa', -
S(O)2Ra,
and -NHC(O)RaRa', wherein at least one of Rakhr and Ram' is methyl or ethyl.
[000951] For example, Qyi' is phenyl substituted with one, two or more groups,
each of
which can be the same or different, selected from fluorine, chlorine, bromine,
iodine, methyl,
ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, trifluoromethoxy, phenyl,
phenoxy, -
NRairRakh,', -C(O)RaRa', -S(O)2Ra]{],,, and -NHC(O)RaRa', wherein at least one
of
Rakhr and Ram,,.' is methyl or ethyl.
[000952] For example, Qyi' is unsubstituted naphthyl.
[000953] For example, Qyi' is naphthyl substituted with one, two or more
groups, each
of which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[000954] For example, Qyi' is naphthyl substituted with one, two or more
groups, each
of which can be the same or different, selected from methyl, ethyl, and
halogen (e.g.,
fluorine, chlorine, bromine, and iodine).
[000955] For example, Qyi' is fluorenyl, and is optionally substituted.
[000956] For example, Qyi' is dihydroindenyl, and is optionally substituted.
[000957] For example, Qyi' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzoisoxazolyl,
benzodioxazolyl, benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl,
benzoimidazolyl,
benzothienyl, methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl,
indolyl,
benzofuranyl, purinyl, purinone, deazapurinyl, indolizinyl, imidazolthiazolyl,
dihydrobenzofuranyl, quinoxalinyl, and pyrrolopyrimidinyl, dihydrobenzofuran,
dihydrobenzothiophene, pyrazolpyrimidinyl, and the like, and is optionally
substituted with
one, two or more groups, each of which can be the same or different, selected
from hydroxyl,
107
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
halogen, unsubstituted or substituted amino, unsubstituted or substituted C1-
C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted C6-C10
aryloxy (e.g., phenoxy), and unsubstituted or substituted phenyl.
[000958] For example, Qyl' is oxazolyl, isoxazolyl, pyridinyl, pyrimidinyl,
triazinyl,
thienyl, purinyl, purinone, quinolinyl, benzothienyl, benzoxazolyl,
benzoisoxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, pyrrolopyrimidinyl, or
dihydrobenzofuranyl, each of which is substituted with one, two or more
groups, each of
which can be the same or different, selected from halogen (e.g., fluorine,
chlorine, bromine,
and iodine), methyl, ethyl, amino, phenyl, and phenoxy.
[000959] For example, Qy" is cyclopropyl.
[000960] For example, Qyl' is -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -
NR21R21'.
[000961] For example, Qyl' is -C(O)OR21.
[000962] For example, R21 is H.
[000963] For example, R21 is unsubstituted or substituted C1-C6 alkyl,
including but not
limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl.
[000964] For example, R21 is methyl or ethyl.
[000965] The present invention also provides the compounds of Formulae IVa and
IVb:
/Rc2 /Rc2
N-N N-N
Rc1 N Rc1 N
Rc4 I Rc4
Rc3 N Rc6 Rc3 Rc6
Y (IVa) or Ry' (IVb),
or a salt, solvate, hydrate or prodrug thereof, wherein:
R,l is H, halogen, unsubstituted or substituted C1-C6 alkyl, unsubstituted or
substituted phenyl, or unsubstituted or substituted heteroaryl comprising one
or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S;
Rc2 is H, or unsubstituted or substituted C1-C6 alkyl;
108
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Rc3 and Rc4 are each independently H, unsubstituted or substituted C1-C6
alkyl, or Rc3
and Rc4, together with the atoms they attach to, form a 5-, 6- or 7-member
ring which
optionally comprises 1-4 heteroatoms selected from N, 0 and S and is
optionally substituted;
Rc6 is H, unsubstituted or substituted C1-C6 alkyl, or unsubstituted or
substituted C6-
Cio aryl;
Ry is H, unsubstituted or substituted C6-Clo aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -S(O)2R1, -C(O)R1, -C(O)OR1, -(CH2)oR1, -C(=NH)NR2R2', -C(O)NR2R2',
or -
C(S)NR2R2';
o is 0, 1, 2, 3, or 4;
R1 is H, unsubstituted or substituted CI-C6 alkyl, or -Tyi-Qyi;
R2 and R2' are each independently H, unsubstituted or substituted C1-C6 alkyl,
-Ty3-
Qy3, or R2 and R2', together with the atom they attach to, form a 5-, 6- or 7-
member ring
which comprises 1-4 heteroatoms selected from N, 0 and S and is optionally
substituted;
Ry' is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -NR3R3', -NR3"C(O)NR3R3', -NR3"C(O)R3, -NR3"C(O)OR3, -NR3"S(0)2R3, or
-
C(O)NR3R3';
R3 and R3' are each independently H, or -Tyi'-Qyi';
R3" is H, or unsubstituted or substituted C1-C6 alkyl;
Ty1, Ty1'and Ty3 are each independently a bond, or unsubstituted or
substituted CI-C6
alkyl linker;
Qy' is H, unsubstituted or substituted C6-C10 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S; -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -NR21R21';
Qyl' is H, unsubstituted or substituted C6-C15 aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
109
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -NR21R21';
Qy3 is H, unsubstituted or substituted C6-CIO aryl, unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, unsubstituted or substituted C3-Cs carbocycle, unsubstituted or
substituted
heterocycle comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N,
O and S, -C(O)OR31, -C(O)R31, -C(O)NR31R31', or -NR31R31'; and
R21, R21', R31 and R31' are each independently H, unsubstituted or substituted
C1-C6
alkyl, unsubstituted or substituted C6-C1o aryl, or unsubstituted or
substituted heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0 and S.
[000966] For example, R,1 is H.
[000967] For example, R,1 is fluorine, chlorine, bromine, or iodine.
[000968] For example, R,1 is unsubstituted or substituted, straight chain or
branched C1-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000969] For example, R,1 is methyl.
[000970] For example, R,1 is unsubstituted phenyl.
[000971] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted C1-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)), and -T,I-
Q,l, wherein:
T,1 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qal is H, -NR,,11R,,11', -C(O)NR,,11R,,11', -NHC(O)Rc11, -NHC(O)OR,,,II, -
NHC(O)NR,,,,R,,,,', or -S(0)2R,11; and
Rcl1 and Rcl1' are each independently H, or or unsubstituted or substituted C1-
C6 alkyl.
[000972] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from -T,I-QaI.
[000973] For example, T,1 is a bond.
110
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000974] For example, T,1 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[000975] For example, T,1 is a methyl linker.
[000976] For example, Q,i is H.
[000977] For example, both R,II and R,11' are H.
[000978] For example, at least one of R,11 and R,11' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000979] For example, at least one of R,11 and R,11' is methyl, ethyl or t-
butyl.
[000980] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, t-butyl and
trifluoromethyl.
[000981] For example, R,1 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methoxy, t-butoxy and
trifluoromethoxy.
[000982] For example, R,1 is phenyl substituted with two or more groups, each
of which
can be the same or different, selected from hydroxyl, cyano, methyl, ethyl, t-
butyl,
trifluoromethyl, methoxy, t-butoxy, trifluoromethoxy, and halogen.
[000983] For example, R,1 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, quinoxalinyl, tetrohyddropyridinyl, pyrrolopyrimidinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl and n-
hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine and iodine)),
C6-Cio aryl (e.g., phenyl, which is optionally substituted), heteroaryl (e.g.,
pyrrolyl, furanyl,
thiophenyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, and pyrimidinyl, and the like,
and is optionally
substituted), C3-Cs carbocycle (e.g., cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted), or heterocycle (e.g.,
pyrrolidinyl, imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, and morpholinyl, and the like, and is optionally substituted).
111
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000984] For example, R,1 is heteroaryl selected from furanyl, pyrazolyl,
purinyl,
indolyl, tetrahydropyridinyl, and pyrrolopyrimidinyl, and is optionally
substituted with one or
more groups, each of which can be the same of different, selected from methyl,
ethyl, t-butyl,
amino, and heterocycle (e.g., piperidinyl, which is optionally substituted).
[000985] For example, Rcz is H.
[000986] For example, Rcz is unsubstituted or substituted CI-C6 alkyl,
including but not
limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl.
[000987] For example, Rcz is methyl substituted with unsubstituted phenyl.
[000988] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
hydroxyl, halogen,
nitro, cyan, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[000989] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from methyl,
ethyl, t-butyl,
and trifluoromethyl.
[000990] For example, Rcz is methyl substituted with phenyl substituted with
one or
more groups, each of which can be the same or different, selected from
methoxy, ethoxy, i-
propyloxy, and t-butoxy.
[000991] For example, Rc3 is H.
[000992] For example, Rc3 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000993] For example, R4 is H.
[000994] For example, R4 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000995] For example, R,6 is H.
112
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[000996] For example, R,6 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[000997] For example, all of R,3, R,4 and R,6 are H.
[000998] For example, Ry is H.
[000999] For example, Ry is is unsubstituted phenyl.
[0001000] For example, Ry is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra , -NHC(O)ORa, -C(O)Ra, and -C(O)ORa , wherein
Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[0001001] For example, Ry is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyano, and -NHC(O)Ra , wherein Rakhr is H, methyl, ethyl, or
t-butyl.
[0001002] For example, Ry is unsubstituted naphthyl.
[0001003] For example, Ry is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Rai,,., -NHC(O)ORa, -C(O)Ra, and -C(O)ORa,,., wherein Ram,,.
is H, or
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[0001004] For example, Ry is naphthyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
113
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001005] For example, Ry is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted amino, unsubstituted or substituted CI-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Rair, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa ,
wherein
Rakhr is H, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-CIO
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[0001006] For example, Ry is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra, wherein Ram is H,
methyl,
ethyl, t-butyl, or phenyl.
[0001007] For example, Ry is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[0001008] For example, Ry is cyclopropyl.
114
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001009] For example, Ry is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[0001010] For example, Ry is -S(O)2R1, -C(O)R1, -C(O)OR1, -(CH2)oR1.
[0001011] For example, o is 0.
[0001012] For example, o is 1.
[0001013] For example, Rl is H.
[0001014] For example, Rl is unsubstituted or substituted, straight-chain or
branched C1-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001015] For example, Rl is straight-chain or branched C1-C6 alkyl
substituted with one,
two, three or more groups, each of which can be the same or different,
selected from:
1) phenyl, which is optionally substituted with one or more groups, each of
which can be the same or different, selected from unsubstituted or substituted
C1-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl,
s-pentyl, and n-hexyl), and unsubstituted or substituted CI-C6 alkoxy (e.g.,
methoxy,
ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy);
2) unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy);
3) -C(O)OR11; -C(O)Rii; and
4) -NR12R13, wherein:
R11 is H, or unsubstituted or substituted C1-C6 alkyl;
R12 and R13 are each independently H, -Ty2-Qy2, or R12 and R13,
together with the atom they attach to, form a 5- or 6-member ring;
Ty2 is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qy2 is H, hydroxyl, unsubstituted or substituted C1-C6 alkoxy,
unsubstituted or substituted C6-C10 aryl, unsubstituted or substituted
heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from
N, 0 and S, unsubstituted or substituted C3-Cg carbocycle, unsubstituted or
substituted heterocycle comprising one or two 5- or 6-member ring and 1-4
115
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
heteroatoms selected from N, 0 and S, -NRa1Ra', -C(O)Ra, -C(O)ORa;
and
Rakhr and Ralf' are each independently H, unsubstituted or substituted
C1-C6 alkyl, unsubstituted or substituted C6-Clo aryl, or unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S.
[0001016] For example, Rl is methyl substitute with unsubstituted phenyl.
[0001017] For example, Rl is methyl substitute with phenyl substituted with
one or more
groups, each of which can be the same or different, selected from hydroxyl,
halogen, nitro,
cyano, unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[0001018] For example, Rl is methyl substitute with methoxy, ethoxy, or t-
butoxy.
[0001019] For example, Rl is methyl substitute with two or more groups, each
of which
can be the same or different, selected from:
1) phenyl, which is optionally substituted with one or more groups, each of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyan,
unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted
or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-
butoxy, and t-butoxy, each of which is optionally substituted with halogen
(e.g.,
fluorine, chlorine, bromine, and iodine)); and
2) one of the following:
i) -C(O)OR11;
ii) methoxy, ethoxy, i-propyloxy, or t-butoxy; or
iii) -NR12R13.
[0001020] For example, Rl is methyl substitute with -C(O)OR11.
[0001021] For example, R11 is H.
116
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001022] For example, R11 is unsubstituted or substituted, straight-chain or
branched C1-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001023] For example, Rl is methyl substitute with -NR12R13.
[0001024] For example, both R12 and R13 are H.
[0001025] For example, at least one of R12 and R13 is -Ty2-Qy2.
[0001026] For example, Ty2 is a bond.
[0001027] For example, Ty2 is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[0001028] For example, Ty2 is a methyl, ethyl, or propyl linker.
[0001029] For example, Qy2 is H.
[0001030] For example, Qy2 is hydroxyl.
[0001031] For example, Qy2 is unsubstituted or substituted C1-C6 alkoxy,
including but
not limited to, methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and
t-butoxy.
[0001032] For example, Qy2 is methoxy.
[0001033] For example, Qy2 is unsubstituted or substituted phenyl or naphthyl.
[0001034] For example, Qy2 is unsubstituted phenyl.
[0001035] For example, Qy2 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted C1-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)).
[0001036] For example, Qy2 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, benzoxazolyl, benzodioxazolyl,
benzothiazolyl, benzoimidazolyl, benzothienyl, methylenedioxyphenyl,
quinolinyl,
isoquinolinyl, naphthrydinyl, indolyl, benzofuranyl, purinyl, deazapurinyl,
indolizinyl,
imidazolthiazolyl, and quinoxalinyl, and the like, and is optionally
substituted.
[0001037] For example, Qy2 is indolyl.
[0001038] For example, Qy2 is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
117
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001039] For example, Qy2 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted.
[0001040] For example, Qy2 is piperidinyl.
[0001041] For example, Qy2 is -NRaItRa' .
[0001042] For example, Qy2 is -C(O)ORai.
[0001043] For example, both Rakhr and Ram' are H.
[0001044] For example, at least one of Rakhr and Ram,,.' is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001045] For example, at least one of Rakhr and Ralf' is methyl or t-butyl.
[0001046] For example, R12 and R13, together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, and morpholinyl, and
the like, and is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine),
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C1-C6 alkoxy
(e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy),
unsubstituted or substituted amino, -C(O)Ra , or -C(O)ORa , wherein Ram is H,
or
unsubstituted or substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[0001047] For example, R12 and R13, together with the atom they attach to,
form a
piperidinyl or piperazinyl, and is optionally substituted with halogen,
methyl, ethyl, t-butyl,
methoxy, ethoxy, i-propyloxy, t-butoxy, or -C(O)ORa, wherein Ram is methyl,
ethyl, or t-
butyl.
[0001048] For example, Rl is -Tyl-Qyl.
[0001049] For example, Ty, is a bond.
[0001050] For example, Ty, is unsubstituted or substituted C1-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[0001051] For example, Ty, is a methyl, ethyl, propyl, or i-propyl linker.
[0001052] For example, Qy' is H.
[0001053] For example, Qy' is unsubstituted phenyl.
118
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001054] For example, Qy' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyan,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra,r., -NHC(O)ORa,r., -C(O)Ra,r., and -C(O)OR,,,
wherein
Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[0001055] For example, Qy' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyan, and -NHC(O)Ra , wherein Rakhr is H, methyl, ethyl, or
t-butyl.
[0001056] For example, Qy' is unsubstituted naphthyl.
[0001057] For example, Qy' is naphthyl substituted with one, two, three or
more groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyan,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Ra, -NHC(O)OR, -C(O)Ra, and -C(O)ORa, wherein Ra& is H, or
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[0001058] For example, Qy' is naphthyl substituted with one, two, three or
more groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
[0001059] For example, Qy' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
119
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted amino, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra,r., -NHC(O)ORa,r., -C(O)Ra,r., and -C(O)OR,,,
wherein
Rakhr is H, unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-C10
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[0001060] For example, Qy' is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra, wherein Ram is H,
methyl,
ethyl, t-butyl, or phenyl.
[0001061] For example, Qy' is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
Ci-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted Ci-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[0001062] For example, Qy' is cyclopropyl.
[0001063] For example, Qy' is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted Ci-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
120
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001064] For example, Ry is -C(=NH)NR2R2'.
[0001065] For example, both R2 and R2' are H.
[0001066] For example, Ry is -C(S)NR2R2'.
[0001067] For example, both R2 and R2' are phenyl.
[0001068] For example, Ry is -C(=NH)NR2R2', -C(O)NR2R2', or -C(S)NR2R2'.
[0001069] For example, both R2 and R2' are H.
[0001070] For example, at least one of R2 and R2' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001071] For example, at least one of R2 and R2' is -Ty3-Qy3.
[0001072] For example, Ty3 is a bond.
[0001073] For example, Ty3 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, n-hexyl, and 2-methylpropyl linker.
[0001074] For example, Ty3 is a methyl, ethyl, propyl, or 2-methylpropyl
linker.
[0001075] For example, Qy3 is H.
[0001076] For example, Qy3 is unsubstituted phenyl.
[0001077] For example, Qy3 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from -Ty4-Qy4, wherein:
Ty4, Tys and Ty6 are each independently a bond, or unsubstituted or
substituted
CI-C6 alkyl linker;
Qy4 is H, unsubstituted or substituted CI-C6 alkyl, unsubstituted or
substituted
CI-C6 alkoxy, halogen, nitro, unsubstituted or substituted phenyl,
unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -NR32R32', -NHC(O)R32, -
NHC(O)OR32, -C(O)R32, -C(O)OR32, -OR32, -SR32, -C(O)NR32R32';
R32 and R32' are each independently H, unsubstituted or substituted CI-C6
alkyl, unsubstituted or substituted phenyl, unsubstituted or substituted
heteroaryl
comprising one or two 5- or 6-member ring and 1-4 heteroatoms selected from N,
0
and S. -Tys-Qy5, or R32 and R32', together with the atom they attach to, form
a 5- or 6-
member ring which optionally comprises 1-4 heteroatoms selected from N, 0 and
S
and is optionally substituted with -Ty6-Qy6;
121
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Qys is H, hydroxyl, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -ORa, -NRaRa', -NHC(O)Ra, -
NHC(O)ORar, -C(O)NRarRa', -C(O)Rar, or -C(O)ORa;
Qy6 is H, hydroxyl, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substituted C3-Cg
carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-member
ring
and 1-4 heteroatoms selected from N, 0 and S, -NRaRa', -C(O)NR34R34';
Rakhr and Ram,,.' are each independently H, unsubstituted or substituted CI-C6
alkyl, unsubstituted or substituted C6-Cio aryl, or unsubstituted or
substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected
from N, 0 and S;
R34 and R34' are each independently H, unsubstituted or substituted CI-C6
alkyl, or R34 and R34', together with the atom they attach to, form a 5- or 6-
member
ring which optionally comprises 1-4 heteroatoms selected from N, 0 and S and
is
optionally substituted.
[0001078] For example, Ty4 is a bond.
[0001079] For example, Ty4 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[0001080] For example, Ty4 is a methyl or t-butyl linker.
[0001081] For example, Qy4 is H.
[0001082] For example, Qy4 is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with halogen
(e.g., fluorine, chlorine, bromine, and iodine).
[0001083] For example, Qy4 is methyl, ethyl, t-butyl, or trifluoromethyl.
[0001084] For example, Qy4 is unsubstituted or substituted CI-C6 alkoxy,
including but
not limited to, methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
methylenedioxy.
122
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001085] For example, Qy4 is methoxy, ethoxy, or methylenedioxy.
[0001086] For example, Qy4 is fluorine, chlorine, bromine, or iodine.
[0001087] For example, Qy4 is unsubstituted phenyl.
[0001088] For example, Qy4 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[0001089] For example, Qy4 is heteroaryl selected from heteroaryl selected
from pyrrolyl,
furanyl, thienyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl,
benzoxazolyl,
benzodioxazolyl, benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl,
benzoimidazolyl,
benzothienyl, methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl,
indolyl,
benzofuranyl, purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl,
quinoxalinyl, and
pyrrolopyrimidinyl, and the like, and is optionally substituted with one or
more groups, each
of which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and
n-hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and iodine)),
and unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-propyloxy,
butoxy, i-butoxy, and t-butoxy, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)).
[0001090] For example, Qy4 is thiazolyl, isothiazolyl, or oxadiazolyl, each of
which is
optionally substituted with one or more groups, each of which can be the same
or different,
selected from methyl, ethyl, and t-butyl.
[0001091] For example, Qy4 is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted.
[0001092] For example, Qy4 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
123
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[0001093] For example, Qy4 is piperazinyl or piperazinyl, and is optionally
substituted
with methyl, ethyl, or t-butyl.
[0001094] For example, Qy4 is -NR32R32'.
[0001095] For example, both R32 and R32' are H.
[0001096] For example, at least one of R32 and R32' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001097] For example, at least one of R32 and R32' is methyl or ethyl.
[0001098] For example, R32 and R32' are both methyl or ethyl.
[0001099] For example, Qy4 is -OR32 or -SR32.
[0001100] For example, R32 is H.
[0001101] For example, R32 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with halogen
(e.g., fluorine, chlorine, bromine, and iodine).
[0001102] For example, R32 is methyl, ethyl, t-butyl, or trifluoromethyl.
[0001103] For example, R32 is unsubstituted phenyl.
[0001104] For example, R32 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[0001105] For example, R32 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, and quinoxalinyl, and
the like, and is
124
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
optionally substituted with one, two or more groups, each of which can be the
same or
different, selected from hydroxyl, halogen, nitro, cyan, unsubstituted or
substituted amino,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[0001106] For example, R32 is pyrazinyl, and is optionally substituted with
methyl, ethyl
or t-butyl.
[0001107] For example, Qy4 is -C(O)R32, -C(O)OR32.
[0001108] For example, R32 is H.
[0001109] For example, R32 is unsubstituted or substituted, straight-chain or
branched Ci-
C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl, n-
butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001110] For example, R32 is methyl or ethyl.
[0001111] For example, Qy4 is -NHC(O)R32, -NHC(O)OR32, or -C(O)NR32R32'.
[0001112] For example, Qy4 is -C(O)NR32R32'.
[0001113] For example, both R32 and R32' are H.
[0001114] For example, at least one of R32 and R32' is -Tys-Qys.
[0001115] For example, Tys is a bond.
[0001116] For example, Tys is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, n-hexyl, 1,2-dimethylpropyl, and 1-methylbutyl linker.
[0001117] For example, Tys is a methyl, ethyl, propyl, i-propyl, butyl, 1,2-
dimethylpropyl, or 1-methylbutyl linker.
[0001118] For example, Qys is H.
[0001119] For example, Qys is unsubstituted phenyl.
[0001120] For example, Qys is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted CI-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
125
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted C6-Cio aryloxy (e.g., phenoxy), and -
NHC(O)Ra,
wherein Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[0001121] For example, Qys is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from halogen (e.g., fluorine, chlorine,
bromine, and
iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, t-butoxy,
phenoxy, and -
NHC(O)Ra,r., wherein Rakhr is H, methyl or ethyl.
[0001122] For example, Qys is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one or more groups, each of
which can be the
same or different, selected from hydroxyl, halogen, nitro, cyan, unsubstituted
or substituted
amino, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)), and
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[0001123] For example, Qys is pyrazolyl, imidazolyl, isoxazolyl, oxazolyl,
pyridinyl,
furanyl, indolyl, or benzoimidazolyl, each of which is optionally substituted
with one or more
groups, each of which can be the same or different, selected from methyl or
ethyl.
[0001124] For example, Qys is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[0001125] For example, Qys is cyclohexyl or cycloheptyl, which is optionally
substituted
with methyl or ethyl.
126
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001126] For example, Qys is heterocycle selected from pyrrolidinyl,
pyrrolidinone,
imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl,
tetrahyrofuranyl,
piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is
optionally
substituted.
[0001127] For example, Qys is pyrrolidinyl, pyrrolidinone, piperidinyl,
morpholinyl, or
tetrahydrofuanyl, and is optionally substituted.
[0001128] For example, Qys is -ORa.
[0001129] For example, R ,r. is H.
[0001130] For example, Ram is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001131] For example, R,, is methyl, ethyl, or t-butyl.
[0001132] For example, Ram is unsubstituted phenyl.
[0001133] For example, Ram is phenyl substituted with one or more groups, each
of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyan,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)).
[0001134] For example, Qy5 is -NRairRair', -NHC(O)Ra , -NHC(O)ORa , -
C(O)NRaRa', -C(O)Ra, or -C(O)ORa.
[0001135] For example, Qys is -NRaRa', -NHC(O)Ra , or -C(O)NRaR'.
[0001136] For example, both Rakhr and Rah,' are H.
[0001137] For example, at least one of Rakhr and Ram' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001138] For example, at least one of Rakhr and Rai,,.' is methyl or ethyl.
[0001139] For example, Ram and Ram' are both methyl or ethyl.
[0001140] For example, R32 and R32', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl and is
optionally
substituted with -Ty6-Qy6.
127
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001141] For example, R32 and R32', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, piperidinyl, and piperazinyl, and is
optionally
substituted with -Ty6-Qy6.
[0001142] For example, Ty6 is a bond.
[0001143] For example, Ty6 is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[0001144] For example, Ty6 is a methyl or ethyl linker.
[0001145] For example, Qy6 is H.
[0001146] For example, Qy6 is unsubstituted phenyl.
[0001147] For example, Qy6 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[0001148] For example, Qy6 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from methyl, ethyl, and t-butyl.
[0001149] For example, Qy6 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl,
benzodihydroimidazolone, and pyrrolopyrimidinyl, and the like, and is
optionally substituted
with one or more groups, each of which can be the same or different, selected
from hydroxyl,
halogen, nitro, cyan, unsubstituted or substituted amino, unsubstituted or
substituted CI-C6
alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl, each of which is optionally substituted with halogen (e.g., fluorine,
chlorine,
bromine, and iodine)), and unsubstituted or substituted CI-C6 alkoxy (e.g.,
methoxy, ethoxy,
propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is
optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)).
128
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001150] For example, Qy6 is pyridinyl, pyrazinyl, or
benzodihydroimidazolone, each of
which is optionally substituted with one or more groups, each of which can be
the same or
different, selected from methyl or ethyl.
[0001151] For example, Qy6 is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[0001152] For example, Qy6 is heterocycle selected from pyrrolidinyl,
pyrrolidinone,
imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl,
tetrahyrofuranyl,
piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is
optionally
substituted.
[0001153] For example, Qy6 is -NRaRa'.
[0001154] For example, both Rakhr and Rah,' are H.
[0001155] For example, at least one of Rakhr and Ram' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001156] For example, at least one of Rakhr and Ram' is methyl or ethyl.
[0001157] For example, Ram and Ram,,.' are both methyl or ethyl.
[0001158] For example, Qy6 is -C(O)NR34R34'.
[0001159] For example, R34 and R34' are both H.
[0001160] For example, at least one of R34 and R34' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001161] For example, R34 and R34', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl and is
optionally
substituted.
[0001162] For example, R34 and R34', together with the atom they attach to,
form a
pyrrolidinyl ring, and is optionally substituted.
[0001163] For example, Qy3 is unsubstituted naphthyl.
129
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001164] For example, Qy3 is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl,
dihydrobenzofuranyl, and
pyrrolopyrimidinyl, and the like, and is optionally substituted with one, two
or more groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted amino, unsubstituted or substituted CI-C6 alkyl
(e.g., methyl,
ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and
n-hexyl, each of
which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and iodine)),
unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-
propyloxy,
butoxy, i-butoxy, and t-butoxy, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted
phenyl.
[0001165] For example, Qy3 is oxazolyl, isoxazolyl, pyridinyl, pyrazinyl,
triazinyl,
thienyl, benzothiadiazolyl, or dihydrobenzofuranyl, each of which is
optionally substituted
with one, two or more groups, each of which can be the same or different,
selected from
halogen (e.g., fluorine, chlorine, bromine, and iodine), methyl, ethyl, t-
butyl, trifluoromethyl,
and phenyl.
[0001166] For example, Qy3 is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[0001167] For example, Qy3 is cyclohexyl.
[0001168] For example, Qy3 is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
130
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001169] For example, Qy3 is -C(O)OR31, -C(O)R31, -C(O)NR31R31', or -
NR31R31'.
[0001170] For example, Qy3 is -C(O)OR31 or -NR31R31'.
[0001171] For example, R31 and R31' are both H.
[0001172] For example, at least one of R31 and R31' is unsubstituted or
substituted,
straight-chain or branched C1-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine),
hydroxyl, or
unsubstituted or substituted C1-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-
propyloxy,
butoxy, i-butoxy, and t-butoxy).
[0001173] For example, at least one of R31 and R31' is methyl or ethyl
optionally
substituted with hydroxyl.
[0001174] For example, R2 and R2', together with the atom they attach to, form
a 5-, 6- or
7-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl,
azepanyl, and diazepanyl,
and the like and is optionally substituted with one or more groups, each of
which can be the
same or different, selected from -Ty7-Qy7, wherein:
Tye is a bond, or unsubstituted or substituted C1-C6 alkyl linker;
Qy7 is H, unsubstituted or substituted phenyl, unsubstituted or
substituted heteroaryl comprising one or two 5- or 6-member ring and 1-4
heteroatoms selected from N, 0 and S, unsubstituted or substitute C3-Cg
carbocycle, unsubstituted or substituted heterocycle comprising one or two 5-
or 6-member ring and 1-4 heteroatoms selected from N, 0 and S, -
C(O)NR33R33', -C(O)ONR33R33', or -NR33R33'; and
R33 and R33' are each independently H, unsubstituted or substituted C1-
C6 alkyl, unsubstituted or substituted phenyl, unsubstituted or substituted
heteroaryl comprising one or two 5- or 6-member ring and 1-4 heteroatoms
selected from N, 0 and S, unsubstituted or substitute C3-Cg carbocycle,
unsubstituted or substituted heterocycle comprising one or two 5- or 6-
member ring and 1-4 heteroatoms selected from N, 0 and S, or R33 and R33',
together with the atom they attach to, form a 5- or 6-member ring which
optionally comprises 1-4 heteroatoms selected from N, 0 and S and is
optionally substituted.
131
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001175] For example, R2 and R2', together with the atom they attach to, form
a 5-, 6- or
7-member ring selected from piperidinyl, piperazinyl, pyrrolidinyl, and
diazepanyl, and is
optionally substituted with -Tye-Qy7.
[0001176] For example, Tye is a bond.
[0001177] For example, Tye is unsubstituted or substituted CI-C6 alkyl linker,
including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[0001178] For example, Tye is a methyl, ethyl, or propyl linker.
[0001179] For example, Qy7 is H.
[0001180] For example, Qy7 is unsubstituted phenyl.
[0001181] For example, Qy7 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyan,
unsubstituted or
substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), and unsubstituted or substituted CI-
C6 alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-butoxy, and
ethylenedioxy,
each of which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)).
[0001182] For example, Qy7 is phenyl substituted with one or more groups, each
of which
can be the same or different, selected from halogen (e.g., fluorine, chlorine,
bromine, and
iodine), methyl, ethyl, -t-butyl, methoxy, ethoxy and t-butoxy.
[0001183] For example, Qy7 is -C(O)NR33R33' or -NR33R33'
[0001184] For example, R33 and R33' are both H.
[0001185] For example, at least one of R33 and R33' is unsubstituted or
substituted,
straight-chain or branched CI-C6 alkyl, including but not limited to, methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001186] For example, at least one of R33 and R33' is unsubstituted or
substituted phenyl.
[0001187] For example, at least one of R33 and R33' is heteroaryl selected
from pyrrolyl,
furanyl, thienyl, thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl,
pyrazolyl, oxazolyl,
isoxazolyl, pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl,
benzoxazolyl,
benzodioxazolyl, benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl,
benzoimidazolyl,
benzothienyl, methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl,
indolyl,
benzofuranyl, purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl,
quinoxalinyl, and
pyrrolopyrimidinyl, and the like, and is optionally substituted.
132
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001188] For example, at least one of R33 and R33' is cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, or cycloheptyl, and is optionally substituted.
[0001189] For example, at least one of R33 and R33' is heterocycle selected
from
pyrrolidinyl, imidazolidinyl, pyrazolidinyl, oxazolidinyl, isoxazolidinyl,
triazolidinyl,
tetrahyrofuranyl, piperidinyl, piperazinyl, morpholinyl, and pyrrolidinone,
and the like, and is
optionally substituted.
[0001190] For example, R33 and R33', together with the atom they attach to,
form a 5- or
6-member ring selected from pyrrolidinyl, imidazolidinyl, pyrazolidinyl,
oxazolidinyl,
isoxazolidinyl, triazolidinyl, piperidinyl, piperazinyl, morpholinyl, and
pyrrolidinone, and the
like, and is optionally substituted.
[0001191] For example, R33 and R33', together with the atom they attach to,
form a 5- or
6-member ring selected from morpholinyl.
[0001192] For example, Ry' is H.
[0001193] For example, Ry' is is unsubstituted phenyl.
[0001194] For example, Ry' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy, and
ethylenedioxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Rair, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa, wherein
Rakhr is H, or unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl,
n-propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[0001195] For example, Ry' is phenyl substituted with one, two, three or more
groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine), methyl, ethyl, t-butyl, trifluoromethyl, methoxy,
ethoxy,
trifluoromethoxy, cyano, and -NHC(O)Ra, wherein Rakhr is H, methyl, ethyl, or
t-butyl.
[0001196] For example, Ry' is unsubstituted naphthyl.
[0001197] For example, Ry' is naphthyl substituted with one, two, three or
more groups,
each of which can be the same or different, selected from hydroxyl, halogen,
nitro, cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
133
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)), -NHC(O)Ra, -NHC(O)OR, -C(O)Ra, and -C(O)ORa, wherein Ra& is H, or
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl).
[0001198] For example, Ry' is naphthyl substituted with one, two, three or
more groups,
each of which can be the same or different, selected from halogen (e.g.,
fluorine, chlorine,
bromine, and iodine).
[0001199] For example, Ry' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzodioxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, benzoimidazolyl,
benzothienyl,
methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl, indolyl,
benzofuranyl,
purinyl, deazapurinyl, indolizinyl, imidazolthiazolyl, quinoxalinyl, and
pyrrolopyrimidinyl,
and the like, and is optionally substituted with one, two, three or more
groups, each of which
can be the same or different, selected from hydroxyl, halogen, nitro, cyano,
unsubstituted or
substituted amino, unsubstituted or substituted CI-C6 alkyl (e.g., methyl,
ethyl, n-propyl,
i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of
which is optionally
substituted with halogen (e.g., fluorine, chlorine, bromine, and iodine)),
unsubstituted or
substituted CI-C6 alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy,
butoxy, i-butoxy,
and t-butoxy, each of which is optionally substituted with halogen (e.g.,
fluorine, chlorine,
bromine, and iodine)), -NHC(O)Ra, -NHC(O)ORa, -C(O)Ra, and -C(O)ORa, wherein
Rakhr is H, unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-
propyl, i-propyl,
n-butyl, s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl), unsubstituted or
substituted C6-C10
aryl, or unsubstituted or substituted heteroaryl comprising one or two 5- or 6-
member ring
and 1-4 heteroatoms selected from N, 0 and S.
[0001200] For example, Ry' is imidazolyl, pyrazolyl, oxazolyl, isoxazolyl,
thiazolyl,
isothiazolyl, pyrimidinyl, triazinyl, quinolinyl, purinyl, thienyl,
quinolinyl, benzoxadiazolyl,
benzothiazolyl, benzothiadiazolyl, benzothienyl, or pyrrolopyrimidiyl, each of
which is
optionallys substituted with one, two, three or more groups, each of which can
be the same or
different, selected from selected from halogen (e.g., fluorine, chlorine,
bromine, and iodine),
amino, methyl, ethyl, t-butyl, trifluoromethyl, and -C(O)Ra, wherein Ram is H,
methyl,
ethyl, t-butyl, or phenyl.
134
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001201] For example, Ry' is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl, and is optionally substituted with unsubstituted or substituted
CI-C6 alkyl (e.g.,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl, and n-hexyl,
each of or which is optionally substituted with halogen (e.g., fluorine,
chlorine, bromine, and
iodine)), unsubstituted or substituted CI-C6 alkoxy (e.g., methoxy, ethoxy,
propyloxy, i-
propyloxy, butoxy, i-butoxy, and t-butoxy, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)).
[0001202] For example, Ry' is heterocycle selected from pyrrolidinyl,
imidazolidinyl,
pyrazolidinyl, oxazolidinyl, isoxazolidinyl, triazolidinyl, tetrahyrofuranyl,
piperidinyl,
piperazinyl, morpholinyl, and pyrrolidinone, and the like, and is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[0001203] For example, R3" is H.
[0001204] For example, R3" is unsubstituted or substituted, straight-chain or
branched
CI-C6 alkyl, including but not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, s-butyl,
t-butyl, n-pentyl, s-pentyl, and n-hexyl.
[0001205] For example, both R3 and R3' are H.
[0001206] For example, at least one of R3 and R3' is -Ty1'-Qy1'.
[0001207] For example, Ty1' is a bond.
[0001208] For example, Ty1' is unsubstituted or substituted CI-C6 alkyl
linker, including
but not limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-
butyl, n-pentyl,
s-pentyl, and n-hexyl linker.
[0001209] For example, Ty1' is a methyl, ethyl, propyl, or t-butyl linker.
[0001210] For example, Qyi' is H.
[0001211] For example, Qyi' is unsubstituted phenyl.
[0001212] For example, Qyi' is phenyl substituted with one, two or more
groups, each of
which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted CI-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted CI-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, t-
butoxy,
methylenedioxy, and ethylenedioxy, each of which is optionally substituted
with halogen
135
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
(e.g., fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted
phenyl,
unsubstituted or substituted C6-Cio aryloxy (e.g., phenoxy), -NRaRa', -
C(O)RaRa', -
S(O)2Ra, and -NHC(O)RaRa', wherein Ra& and Ram' are each independently H,
unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl)
[0001213] For example, Qyi' is phenyl substituted with one, two or more
groups, each of
which can be the same or different, selected from fluorine, chlorine, bromine,
and iodine.
[0001214] For example, Qyi' is phenyl substituted with one, two or more
groups, each of
which can be the same or different, selected from methyl, ethyl, t-butyl, and
trifluoromethyl.
[0001215] For example, Qyi' is phenyl substituted with one, two or more
groups, each of
which can be the same or different, selected from methoxy, ethoxy,
trifluoromethoxy, and
ethylenedioxy.
[0001216] For example, Qyi' is phenyl substituted with one, two or more
groups, each of
which can be the same or different, selected from -NRaRa', -C(O)RaRa', -
S(O)2Ra,
and -NHC(O)RaRa', wherein at least one of Rakhr and Ram' is methyl or ethyl.
[0001217] For example, Qyi' is phenyl substituted with one, two or more
groups, each of
which can be the same or different, selected from fluorine, chlorine, bromine,
iodine, methyl,
ethyl, t-butyl, trifluoromethyl, methoxy, ethoxy, trifluoromethoxy, phenyl,
phenoxy, -
NRairRakh,', -C(O)RaRa', -S(O)2Ra, and -NHC(O)RaRa', wherein at least one of
Rakhr and Ram' is methyl or ethyl.
[0001218] For example, Qyi' is unsubstituted naphthyl.
[0001219] For example, Qyi' is naphthyl substituted with one, two or more
groups, each
of which can be the same or different, selected from hydroxyl, halogen, nitro,
cyano,
unsubstituted or substituted Ci-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-
propyl, n-butyl,
s-butyl, t-butyl, n-pentyl, s-pentyl, and n-hexyl, each of which is optionally
substituted with
halogen (e.g., fluorine, chlorine, bromine, and iodine)), and unsubstituted or
substituted Ci-C6
alkoxy (e.g., methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-
butoxy, each
of which is optionally substituted with halogen (e.g., fluorine, chlorine,
bromine, and
iodine)).
[0001220] For example, Qyi' is naphthyl substituted with one, two or more
groups, each
of which can be the same or different, selected from methyl, ethyl, and
halogen (e.g.,
fluorine, chlorine, bromine, and iodine).
[0001221] For example, Qyi' is fluorenyl, and is optionally substituted.
[0001222] For example, Qyi' is dihydroindenyl, and is optionally substituted.
136
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001223] For example, Qyl' is heteroaryl selected from pyrrolyl, furanyl,
thienyl,
thiazolyl, isothiazolyl, imidazolyl, triazolyl, tetrazolyl, pyrazolyl,
oxazolyl, isoxazolyl,
pyridinyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, benzoxazolyl,
benzoisoxazolyl,
benzodioxazolyl, benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl,
benzoimidazolyl,
benzothienyl, methylenedioxyphenyl, quinolinyl, isoquinolinyl, naphthrydinyl,
indolyl,
benzofuranyl, purinyl, purinone, deazapurinyl, indolizinyl, imidazolthiazolyl,
dihydrobenzofuranyl, quinoxalinyl, and pyrrolopyrimidinyl, and the like, and
is optionally
substituted with one, two or more groups, each of which can be the same or
different,
selected from hydroxyl, halogen, unsubstituted or substituted amino,
unsubstituted or
substituted C1-C6 alkyl (e.g., methyl, ethyl, n-propyl, i-propyl, n-butyl, s-
butyl, t-butyl,
n-pentyl, s-pentyl, and n-hexyl, each of which is optionally substituted with
halogen (e.g.,
fluorine, chlorine, bromine, and iodine)), unsubstituted or substituted C1-C6
alkoxy (e.g.,
methoxy, ethoxy, propyloxy, i-propyloxy, butoxy, i-butoxy, and t-butoxy, each
of which is
optionally substituted with halogen (e.g., fluorine, chlorine, bromine, and
iodine)),
unsubstituted or substituted C6-Clo aryloxy (e.g., phenoxy), and unsubstituted
or substituted
phenyl.
[0001224] For example, Qyl' is oxazolyl, isoxazolyl, pyridinyl, pyrimidinyl,
triazinyl,
thienyl, purinyl, purinone, quinolinyl, benzothienyl, benzoxazolyl,
benzoisoxazolyl,
benzoxadiazolyl, benzothiazolyl, benzothiadiazolyl, pyrrolopyrimidinyl, or
dihydrobenzofuranyl, each of which is substituted with one, two or more
groups, each of
which can be the same or different, selected from halogen (e.g., fluorine,
chlorine, bromine,
and iodine), methyl, ethyl, amino, phenyl, and phenoxy.
[0001225] For example, Qyl' is -C(O)OR21, -C(O)R21, -C(O)NR21R21', or -
NR21R21'.
[0001226] For example, Qyl' is -C(O)OR21.
[0001227] For example, R21 is H.
[0001228] For example, R21 is unsubstituted or substituted C1-C6 alkyl,
including but not
limited to, methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-
pentyl, s-pentyl, and
n-hexyl.
[0001229] For example, R21 is methyl or ethyl.
[0001230] The present invention also provides Formula IVal, IVbl, IVcl, IVdl,
IVel,
and MI.
137
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N-N' Rc2 N-N' Rc2 N-N' Rc2
N-N' H Rc, N Rc, N Rc, N
/
N
N N N
I I I
Y (IVal), Ry (IVbl), C(O)NR2R2' (IVcl), S(O)2R1
N-N' Rc2 N-N' Rc2
Rc1 N Rc1 N
(IVdl), Ry (IVel), and NR3"C(O)NR3R3' (IVfl).
[0001231] Representative compounds of the present invention include compounds
listed
in Tables 1 and 2.
Table 1.
Name & Structure Cmpd Name & Structure Cmpd
7-(3, 4-dichlorophenyl)-6, 7-dihydro-3 H-
7-phenyl-6,7-dihydro-3H- benzo[f]pyrazolo[3,4-c]isoquinoline
benzo[f]pyrazolo[3,4-c]isoquinoline N-N H
N-NH
N
N
v v 2 1
CI
CI
7-(3, 4-difluorophenyl)-6, 7-dihydro-3 H-
benzo [ f] pyrazolo [ 3 , 4 - c ] i so quinoline
N-NH
N
3
F
F
138
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Table 2.
Compound Structure IUPAC Name LC/MS
No. [M+H]
N\
N 3-(4-methoxybenzyl)-6,7,8,9-
4 N N tetrahydro-3H-pyrazolo[3,4- 309
c]isoquinolin-7-amine
,o
N N-[3-(4-methoxybenzyl)-
6,7,8,9-tetrahydro-3H- 413
N NN pyrazolo[3,4-c]isoquinolin-7-
yl]benzamide
N
3-(4-methoxybenzyl)-7-
N
6 (phenylsulfonyl)-6,7,8,9- 435
N tetrahydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridine
NON 3-(4-methoxybenzyl)-N-
phenyl-3,6,8,9-tetrahydro-
7 Y
'N N 7H-pyrazolo[3,4- 414
c] [2,7]naphthyridine-7-
carboxamide
139
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Chiral
(1S)-2-[3-(4-
N methoxybenzyl)-3,6,8,9-
8 N N tetrahydro-7H-pyrazolo[3,4- 428
N
N N c] [2,7]naphthyridin-7-yl]-2-
oxo-l-phenylethanamine
tert-butyl {(IS)-2-[3-(4-
Ch-I methoxybenzyl)-3,6,8,9-
9 tetrahydro-7H-pyrazolo[3,4- 528
~" c][2,7]naphthyridin-7-yl]-2-
oxo-1-
phenylethyl} carbamate
0
N N 3-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4- 294
N c] [2,7]naphthyridin- l -
N N yl)benzamide
0
~/-N /N 3-(4-methoxybenzyl)-3,6-
11 Y -P dihydrochromeno[4,3- 344
N. --~o d]pyrazolo[3,4-b]pyridine
0
3 -(4-methoxyb enzyl)-7-
12 methyl-6,7-dihydro-3H- 356
Y 1 benzo[f]pyrazolo[3,4-
N
c]isoquinoline
140
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N 3-(4-methoxybenzyl)-3,6-
13 ' dihydroindeno[1,2- 328
d]pyrazolo[3,4-b]pyridine
-'o N tz, -
'0 3-(4-methoxybenzyl)-6-
14 N methyl-3,6- 342
dihydroindeno [ 1,2-
d]pyrazolo[3,4-b]pyridine
\0 ~ ~
N-\ N 3-(4-methoxybenzyl)-6,7-
15 N; dihydro-3H- 358
o [1]benzoxepino[5,4-
d]pyrazolo[3,4-b]pyridine
16 N N 3-(4-methoxybenzyl)-8-
d
phenyl-3,6,7,8- 408
8p
~_ ~~
tetrahydrodipyrazolo [3,4-
N _ c:4',3'-f]isoquinoline
0
3-(4-methoxybenzyl)-3,7-
17 dihydroisothiochromeno[4,3- 360
d]pyrazolo[3,4-b]pyridine
141
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
3-(4-methoxybenzyl)-N-
N N, 18 N y methyl-6,7-dihydro-3H- 373
N pyrazolo[4',3':5,6]pyrido[4,3-
f] quinazolin-9-amine
S~ ~ N-
N 3-(4-methoxybenzyl)-6,7-
19 dihydro-3H-pyrazolo[3,4- 348
o c]thieno[3,2-f]isoquinoline
~o
-NN 9,10-dimethoxy-3-(4-
20 N methoxybenzyl)-6,7-dihydro- 402
9 3H-benzo[f]pyrazolo[3,4-
c]isoquinoline
Noe 9-nitro-6,7-dihydro-3H-
21 benzo[f]pyrazolo[3,4- 267
c]isoquinoline
N
N N
0
3-(4-methoxybenzyl)-6,7-
22 N, dihydro-3H- 358
N N benzo[f]pyrazolo[3,4-
c]isoquinolin-8-ol
142
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
3-(4-methoxybenzyl)-6,7-
23 N dihydro-3H-furo[3,2- 332
N f]pyrazolo[3,4-c]isoquinoline
NO2
3-(4-methoxybenzyl)-9-nitro-
24 N 6,7-dihydro-3H- 387
N N benzo[f]pyrazolo[3,4-
c]isoquinoline
0
N-N
3 -(4-methoxybenzyl)-3, 6, 7, 8-
N
25 I , tetrahydro-9H-pyrazolo[4,3- 359
a][3,7]phenanthrolin-9-one
O N
H
[0001232] As used herein, "alkyl", "Cl, C2, C3, C4, C5 or C6 alkyl" or "CI-C 6
alkyl" is
intended to include C1, C2, C3, C4, C5 or C6 straight chain (linear) saturated
aliphatic
hydrocarbon groups and C3, C4, C5 or C6 branched saturated aliphatic
hydrocarbon groups.
For example, C1-C6 alkyl is intended to include CI, C2, C3, C4, C5 and C6
alkyl groups.
For example, C1-C3 alkyl is intended to include CI, C2 and C3 alkyl groups.
Examples of
alkyl include, moieties having from one to six carbon atoms, such as, but not
limited to,
methyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, s-
pentyl or n-hexyl.
[0001233] In certain embodiments, a straight chain or branched alkyl has six
or fewer
carbon atoms (e.g., C1-C6 for straight chain, C3-C6 for branched chain), and
in another
embodiment, a straight chain or branched alkyl has four or fewer carbon atoms.
[0001234] "Heteroalkyl" groups are alkyl groups, as defined above, that have
an oxygen,
nitrogen, sulfur or phosphorous atom replacing one or more hydrocarbon
backbone carbon
atoms.
143
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001235] As used herein, the term "cycloalkyl", "C3, C4, C5, C6, C7 or Cg
cycloalkyl" or
"C3-Cs cycloalkyl" is intended to include hydrocarbon rings having from three
to eight
carbon atoms in their ring structure. In one embodiment, a cycloalkyl group
has five or six
carbons in the ring structure.
[0001236] The term "substituted alkyl" refers to alkyl moieties having
substituents
replacing one or more hydrogen atoms on one or more carbons of the hydrocarbon
backbone.
Such substituents can include, for example, alkyl, alkenyl, alkynyl, halogen,
hydroxyl,
alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy,
carboxylate,
alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, aminocarbonyl,
alkylaminocarbonyl,
dialkylaminocarbonyl, alkylthiocarbonyl, alkoxyl, phosphate, phosphonato,
phosphinato,
amino (including alkylamino, dialkylamino, arylamino, diarylamino and
alkylarylamino),
acylamino (including alkylcarbonylamino, arylcarbonylamino, carbamoyl and
ureido),
amidino, imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates,
alkylsulfinyl,
sulfonato, sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyano, azido,
heterocyclyl,
alkylaryl, or an aromatic or heteroaromatic moiety. Cycloalkyls can be further
substituted,
e.g., with the substituents described above. An "alkylaryl" or an "aralkyl"
moiety is an alkyl
substituted with an aryl (e.g., phenylmethyl (benzyl)).
[0001237] Unless the number of carbons is otherwise specified, "lower alkyl"
includes an
alkyl group, as defined above, having from one to six, or in another
embodiment from one to
four, carbon atoms in its backbone structure. "Lower alkenyl" and "lower
alkynyl" have
chain lengths of, for example, two to six or of two to four carbon atoms.
[0001238] "Alkenyl" includes unsaturated aliphatic groups analogous in length
and
possible substitution to the alkyls described above, but that contain at least
one double bond.
For example, the term "alkenyl" includes straight chain alkenyl groups (e.g.,
ethenyl,
propenyl, butenyl, pentenyl, hexenyl, heptenyl, octenyl, nonenyl, decenyl),
branched alkenyl
groups, cycloalkenyl (e.g., alicyclic) groups (e.g., cyclopropenyl,
cyclopentenyl,
cyclohexenyl, cycloheptenyl, cyclooctenyl), alkyl or alkenyl substituted
cycloalkenyl groups,
and cycloalkyl or cycloalkenyl substituted alkenyl groups. In certain
embodiments, a straight
chain or branched alkenyl group has six or fewer carbon atoms in its backbone
(e.g., C2-C6
for straight chain, C3-C6 for branched chain). Likewise, cycloalkenyl groups
may have from
five to eight carbon atoms in their ring structure, and in one embodiment,
cycloalkenyl
groups have five or six carbons in the ring structure. The term "C2-C6"
includes alkenyl
groups containing two to six carbon atoms. The term "C3-C6" includes alkenyl
groups
containing three to six carbon atoms.
144
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001239] "Heteroalkenyl" includes alkenyl groups, as defined herein, having
an oxygen,
nitrogen, sulfur or phosphorous atom replacing one or more hydrocarbon
backbone carbons.
[0001240] The term "substituted alkenyl" refers to alkenyl moieties having
substituents
replacing one or more hydrogen atoms on one or more hydrocarbon backbone
carbon atoms.
Such substituents can include, for example, alkyl, alkenyl, alkynyl, halogen,
hydroxyl,
alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy,
carboxylate,
alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, aminocarbonyl,
alkylaminocarbonyl,
dialkylaminocarbonyl, alkylthiocarbonyl, alkoxyl, phosphate, phosphonato,
phosphinato,
amino (including alkylamino, dialkylamino, arylamino, diarylamino and
alkylarylamino),
acylamino (including alkylcarbonylamino, arylcarbonylamino, carbamoyl and
ureido),
amidino, imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates,
alkylsulfinyl,
sulfonato, sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyano,
heterocyclyl, alkylaryl, or
an aromatic or heteroaromatic moiety.
[0001241] "Alkynyl" includes unsaturated aliphatic groups analogous in length
and
possible substitution to the alkyls described above, but which contain at
least one triple bond.
For example, "alkynyl" includes straight chain alkynyl groups (e.g., ethynyl,
propynyl,
butynyl, pentynyl, hexynyl, heptynyl, octynyl, nonynyl, decynyl), branched
alkynyl groups,
and cycloalkyl or cycloalkenyl substituted alkynyl groups. In certain
embodiments, a straight
chain or branched alkynyl group has six or fewer carbon atoms in its backbone
(e.g., C2-C6
for straight chain, C3-C6 for branched chain). The term "C2-C6" includes
alkynyl groups
containing two to six carbon atoms. The term "C3-C6" includes alkynyl groups
containing
three to six carbon atoms.
[0001242] "Heteroalkynyl" includes alkynyl groups, as defined herein, having
an
oxygen, nitrogen, sulfur or phosphorous atom replacing one or more hydrocarbon
backbone
carbons.
[0001243] The term "substituted alkynyl" refers to alkynyl moieties having
substituents
replacing one or more hydrogen atoms on one or more hydrocarbon backbone
carbon atoms.
Such substituents can include, for example, alkyl, alkenyl, alkynyl, halogen,
hydroxyl,
alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy,
carboxylate,
alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, aminocarbonyl,
alkylaminocarbonyl,
dialkylaminocarbonyl, alkylthiocarbonyl, alkoxyl, phosphate, phosphonato,
phosphinato,
amino (including alkylamino, dialkylamino, arylamino, diarylamino and
alkylarylamino),
acylamino (including alkylcarbonylamino, arylcarbonylamino, carbamoyl and
ureido),
amidino, imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates,
alkylsulfinyl,
145
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
sulfonato, sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyan, azido,
heterocyclyl,
alkylaryl, or an aromatic or heteroaromatic moiety.
[0001244] "Aryl" includes groups with aromaticity, including "conjugated", or
multicyclic, systems with at least one aromatic ring. Examples include phenyl,
benzyl, etc.
[0001245] "Heteroaryl" groups are aryl groups, as defined above, having from
one to
four heteroatoms in the ring structure, and may also be referred to as "aryl
heterocycles" or
"heteroaromatics". As used herein, the term "heteroaryl" is intended to
include a stable 5-, 6-,
or 7-membered monocyclic or 7-, 8-, 9-, 10-, 11- or 12-membered bicyclic
aromatic
heterocyclic ring which consists of carbon atoms and one or more heteroatoms,
e.g., 1 or 1-2
or 1-3 or 1-4 or 1-5 or 1-6 heteroatoms, independently selected from the group
consisting of
nitrogen, oxygen and sulfur. The nitrogen atom may be substituted or
unsubstituted (i.e., N
or NR wherein R is H or other substituents, as defined). The nitrogen and
sulfur heteroatoms
may optionally be oxidized (i.e., N->O and S(O)p, where p = 1 or 2). It is to
be noted that
total number of S and 0 atoms in the aromatic heterocycle is not more than 1.
[0001246] Examples of heteroaryl groups include pyrrole, furan, thiophene,
thiazole,
isothiazole, imidazole, triazole, tetrazole, pyrazole, oxazole, isoxazole,
pyridine, pyrazine,
pyridazine, pyrimidine, and the like.
[0001247] Furthermore, the terms "aryl" and "heteroaryl" include multicyclic
aryl and
heteroaryl groups, e.g., tricyclic, bicyclic, e.g., naphthalene, benzoxazole,
benzodioxazole,
benzothiazole, benzoimidazole, benzothiophene, methylenedioxyphenyl,
quinoline,
isoquinoline, naphthridine, indole, benzofuran, purine, benzofuran,
deazapurine, indolizine.
[0001248] In the case of multicyclic aromatic rings, only one of the rings
needs to be
aromatic (e.g., 2,3-dihydroindole), although all of the rings may be aromatic
(e.g., quinoline).
The second ring can also be fused or bridged.
[0001249] The aryl or heteroaryl aromatic ring can be substituted at one or
more ring
positions with such substituents as described above, for example, alkyl,
alkenyl, akynyl,
halogen, hydroxyl, alkoxy, alkylcarbonyloxy, arylcarbonyloxy,
alkoxycarbonyloxy,
aryloxycarbonyloxy, carboxylate, alkylcarbonyl, alkylaminocarbonyl,
aralkylaminocarbonyl,
alkenylaminocarbonyl, alkylcarbonyl, arylcarbonyl, aralkylcarbonyl,
alkenylcarbonyl,
alkoxycarbonyl, aminocarbonyl, alkylthiocarbonyl, phosphate, phosphonato,
phosphinato,
amino (including alkylamino, dialkylamino, arylamino, diarylamino and
alkylarylamino),
acylamino (including alkylcarbonylamino, arylcarbonylamino, carbamoyl and
ureido),
amidino, imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates,
alkylsulfinyl,
sulfonato, sulfamoyl, sulfonamido, nitro, trifluoromethyl, cyan, azido,
heterocyclyl,
146
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
alkylaryl, or an aromatic or heteroaromatic moiety. Aryl groups can also be
fused or bridged
with alicyclic or heterocyclic rings, which are not aromatic so as to form a
multicyclic system
(e.g., tetralin, methylenedioxyphenyl).
[0001250] As used herein, "carbocycle" or "carbocyclic ring" is intended to
include any
stable monocyclic, bicyclic or tricyclic ring having the specified number of
carbons, any of
which may be saturated, unsaturated, or aromatic. For example, a C3-C14
carbocycle is
intended to include a monocyclic, bicyclic or tricyclic ring having 3, 4, 5,
6, 7, 8, 9, 10, 11,
12, 13 or 14 carbon atoms. Examples of carbocycles include, but are not
limited to,
cyclopropyl, cyclobutyl, cyclobutenyl, cyclopentyl, cyclopentenyl, cyclohexyl,
cycloheptenyl,
cycloheptyl, cycloheptenyl, adamantyl, cyclooctyl, cyclooctenyl,
cyclooctadienyl, fluorenyl,
phenyl, naphthyl, indanyl, adamantyl and tetrahydronaphthyl. Bridged rings are
also
included in the definition of carbocycle, including, for example,
[3.3.0]bicyclooctane,
[4.3.0]bicyclononane, [4.4.0]bicyclodecane and [2.2.2]bicyclooctane. A bridged
ring occurs
when one or more carbon atoms link two non-adjacent carbon atoms. In one
embodiment,
bridge rings are one or two carbon atoms. It is noted that a bridge always
converts a
monocyclic ring into a tricyclic ring. When a ring is bridged, the
substituents recited for the
ring may also be present on the bridge. Fused (e.g., naphthyl,
tetrahydronaphthyl) and spiro
rings are also included.
[0001251] As used herein, "heterocycle" includes any ring structure (saturated
or
partially unsaturated) which contains at least one ring heteroatom (e.g., N, 0
or S). Examples
of heterocycles include, but are not limited to, morpholine, pyrrolidine,
tetrahydrothiophene,
piperidine, piperazine and tetrahydrofuran.
[0001252] Examples of heterocyclic groups include, but are not limited to,
acridinyl,
azocinyl, benzimidazolyl, benzofuranyl, benzothiofuranyl, benzothiophenyl,
benzoxazolyl,
benzoxazolinyl, benzthiazolyl, benztriazolyl, benztetrazolyl, benzisoxazolyl,
benzisothiazolyl,
benzimidazolinyl, carbazolyl, 4aH-carbazolyl, carbolinyl, chromanyl,
chromenyl, cinnolinyl,
decahydroquinolinyl, 2H,6H-1,5,2-dithiazinyl, dihydrofuro[2,3-
b]tetrahydrofuran, furanyl,
furazanyl, imidazolidinyl, imidazolinyl, imidazolyl, 1H-indazolyl, indolenyl,
indolinyl,
indolizinyl, indolyl, 3H-indolyl, isatinoyl, isobenzofuranyl, isochromanyl,
isoindazolyl,
isoindolinyl, isoindolyl, isoquinolinyl, isothiazolyl, isoxazolyl,
methylenedioxyphenyl,
morpholinyl, naphthyridinyl, octahydroisoquinolinyl, oxadiazolyl, 1,2,3-
oxadiazolyl, 1,2,4-
oxadiazolyl, 1,2,5-oxadiazolyl, 1,3,4-oxadiazolyl, 1,2,4-oxadiazol5(4H)-one,
oxazolidinyl,
oxazolyl, oxindolyl, pyrimidinyl, phenanthridinyl, phenanthrolinyl,
phenazinyl,
phenothiazinyl, phenoxathinyl, phenoxazinyl, phthalazinyl, piperazinyl,
piperidinyl,
147
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
piperidonyl, 4-piperidonyl, piperonyl, pteridinyl, purinyl, pyranyl,
pyrazinyl, pyrazolidinyl,
pyrazolinyl, pyrazolyl, pyridazinyl, pyridooxazole, pyridoimidazole,
pyridothiazole, pyridinyl,
pyridyl, pyrimidinyl, pyrrolidinyl, pyrrolinyl, 2H-pyrrolyl, pyrrolyl,
quinazolinyl, quinolinyl,
4H-quinolizinyl, quinoxalinyl, quinuclidinyl, tetrahydrofuranyl,
tetrahydroisoquinolinyl,
tetrahydroquinolinyl, tetrazolyl, 6H-1,2,5-thiadiazinyl, 1,2,3-thiadiazolyl,
1,2,4-thiadiazolyl,
1,2,5-thiadiazolyl, 1,3,4-thiadiazolyl, thianthrenyl, thiazolyl, thienyl,
thienothiazolyl,
thienooxazolyl, thienoimidazolyl, thiophenyl, triazinyl, 1,2,3-triazolyl,
1,2,4-triazolyl, 1,2,5-
triazolyl, 1,3,4-triazolyl and xanthenyl.
[0001253] The term "substituted", as used herein, means that any one or more
hydrogen
atmos on the designated atom is replaced with a selection from the indicated
groups, provided
that the designated atom's normal valency is not exceeded, and that the
substitution results in
a stable compound. When a substituent is keto(i.e., =0), then 2 hydrogen atoms
on the atom
are replaced. Keto substituents are not present on aromatic moieties. Ring
double bonds, as
used herein, are double bonds that are formed between two adjacent ring atoms
(e.g., C=C,
C=N or N=N). "Stable compound" and "stable structure" are meant to indicate a
compound
that is sufficiently robust to survive isolation to a useful degree of purity
from a reaction
mixture, and formulation into an efficacious therapeutic agent.
[0001254] When a bond to a substituent is shown to cross a bond connecting two
atoms
in a ring, then such substituent may be bonded to any atom in the ring. When a
substituent is
listed without indicating the atom via which such substituent is bonded to the
rest of the
compound of a given formula, then such substituent may be bonded via any atom
in such
formula. Combinations of substituents and/or variables are permissible, but
only if such
combinations result in stable compounds.
[0001255] When any variable (e.g., R1) occurs more than one time in any
constituent or
formula for a compound, its definition at each occurrence is independent of
its definition at
every other occurrence. Thus, for example, if a group is shown to be
substituted with 0-2 Ri
moieties, then the group may optionally be substituted with up to two Ri
moieties and Ri at
each occurrence is selected independently from the definition of R1. Also,
combinations of
substituents and/or variables are permissible, but only if such combinations
result in stable
compounds.
[0001256] The term "hydroxy" or "hydroxyl" includes groups with an -OH or -0-.
[0001257] As used herein, "halo" or "halogen" refers to fluoro, chloro, bromo
and iodo.
The term "perhalogenated" generally refers to a moiety wherein all hydrogen
atoms are
replaced by halogen atoms.
148
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001258] The term "carbonyl" or "carboxy" includes compounds and moieties
which
contain a carbon connected with a double bond to an oxygen atom. Examples of
moieties
containing a carbonyl include, but are not limited to, aldehydes, ketones,
carboxylic acids,
amides, esters, anhydrides, etc.
[0001259] "Acyl" includes moieties that contain the acyl radical (-C(O)-) or a
carbonyl
group. "Substituted acyl" includes acyl groups where one or more of the
hydrogen atoms are
replaced by, for example, alkyl groups, alkynyl groups, halogen, hydroxyl,
alkylcarbonyloxy,
arylcarbonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy, carboxylate,
alkylcarbonyl,
arylcarbonyl, alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl,
alkylthiocarbonyl, alkoxyl, phosphate, phosphonato, phosphinato, amino
(including
alkylamino, dialkylamino, arylamino, diarylamino and alkylarylamino),
acylamino (including
alkylcarbonylamino, arylcarbonylamino, carbamoyl and ureido), amidino, imino,
sulfhydryl,
alkylthio, arylthio, thiocarboxylate, sulfates, alkylsulfinyl, sulfonato,
sulfamoyl, sulfonamido,
nitro, trifluoromethyl, cyano, azido, heterocyclyl, alkylaryl, or an aromatic
or heteroaromatic
moiety.
[0001260] "Aroyl" includes moieties with an aryl or heteroaromatic moiety
bound to a
carbonyl group. Examples of aroyl groups include phenylcarboxy, naphthyl
carboxy, etc.
[0001261] "Alkoxyalkyl", "alkylaminoalkyl" and "thioalkoxyalkyl" include alkyl
groups,
as described above, wherein oxygen, nitrogen or sulfur atoms replace one or
more
hydrocarbon backbone carbon atoms.
[0001262] The term "alkoxy" or "alkoxyl" includes substituted and
unsubstituted alkyl,
alkenyl and alkynyl groups covalently linked to an oxygen atom. Examples of
alkoxy groups
or alkoxyl radicals include, but are not limited to, methoxy, ethoxy,
isopropyloxy, propoxy,
butoxy and pentoxy groups. Examples of substituted alkoxy groups include
halogenated
alkoxy groups. The alkoxy groups can be substituted with groups such as
alkenyl, alkynyl,
halogen, hydroxyl, alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy,
aryloxycarbonyloxy, carboxylate, alkylcarbonyl, arylcarbonyl, alkoxycarbonyl,
aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl, alkylthiocarbonyl,
alkoxyl,
phosphate, phosphonato, phosphinato, amino (including alkylamino,
dialkylamino,
arylamino, diarylamino, and alkylarylamino), acylamino (including
alkylcarbonylamino,
arylcarbonylamino, carbamoyl and ureido), amidino, imino, sulfhydryl,
alkylthio, arylthio,
thiocarboxylate, sulfates, alkylsulfinyl, sulfonato, sulfamoyl, sulfonamido,
nitro,
trifluoromethyl, cyan, azido, heterocyclyl, alkylaryl, or an aromatic or
heteroaromatic
moieties. Examples of halogen substituted alkoxy groups include, but are not
limited to,
149
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
fluoromethoxy, difluoromethoxy, trifluoromethoxy, chloromethoxy,
dichloromethoxy and
trichloromethoxy.
[0001263] The term "ether" or "alkoxy" includes compounds or moieties which
contain
an oxygen bonded to two carbon atoms or heteroatoms. For example, the term
includes
"alkoxyalkyl", which refers to an alkyl, alkenyl, or alkynyl group covalently
bonded to an
oxygen atom which is covalently bonded to an alkyl group.
[0001264] The term "ester" includes compounds or moieties which contain a
carbon or a
heteroatom bound to an oxygen atom which is bonded to the carbon of a carbonyl
group. The
term "ester" includes alkoxycarboxy groups such as methoxycarbonyl,
ethoxycarbonyl,
propoxycarbonyl, butoxycarbonyl, pentoxycarbonyl, etc.
[0001265] The term "thioalkyl" includes compounds or moieties which contain an
alkyl
group connected with a sulfur atom. The thioalkyl groups can be substituted
with groups
such as alkyl, alkenyl, alkynyl, halogen, hydroxyl, alkylcarbonyloxy,
arylcarbonyloxy,
alkoxycarbonyloxy, aryloxycarbonyloxy, carboxylate, carboxyacid,
alkylcarbonyl,
arylcarbonyl, alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl,
alkylthiocarbonyl, alkoxyl, amino (including alkylamino, dialkylamino,
arylamino,
diarylamino and alkylarylamino), acylamino (including alkylcarbonylamino,
arylcarbonylamino, carbamoyl and ureido), amidino, imino, sulfhydryl,
alkylthio, arylthio,
thiocarboxylate, sulfates, alkylsulfinyl, sulfonato, sulfamoyl, sulfonamido,
nitro,
trifluoromethyl, cyan, azido, heterocyclyl, alkylaryl, or an aromatic or
heteroaromatic
moieties.
[0001266] The term "thiocarbonyl" or "thiocarboxy" includes compounds and
moieties
which contain a carbon connected with a double bond to a sulfur atom.
[0001267] The term "thioether" includes moieties which contain a sulfur atom
bonded to
two carbon atoms or heteroatoms. Examples of thioethers include, but are not
limited to
alkthioalkyls, alkthioalkenyls and alkthioalkynyls. The term "alkthioalkyls"
include moieties
with an alkyl, alkenyl or alkynyl group bonded to a sulfur atom which is
bonded to an alkyl
group. Similarly, the term "alkthioalkenyls" refers to moieties wherein an
alkyl, alkenyl or
alkynyl group is bonded to a sulfur atom which is covalently bonded to an
alkenyl group; and
alkthioalkynyls" refers to moieties wherein an alkyl, alkenyl or alkynyl group
is bonded to a
sulfur atom which is covalently bonded to an alkynyl group.
[0001268] As used herein, "amine" or "amino" includes moieties where a
nitrogen atom
is covalently bonded to at least one carbon or heteroatom. "Alkylamino"
includes groups of
compounds wherein nitrogen is bound to at least one alkyl group. Examples of
alkylamino
150
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
groups include benzylamino, methylamino, ethylamino, phenethylamino, etc.
"Dialkylamino" includes groups wherein the nitrogen atom is bound to at least
two additional
alkyl groups. Examples of dialkylamino groups include, but are not limited to,
dimethylamino and diethylamino. "Arylamino" and "diarylamino" include groups
wherein
the nitrogen is bound to at least one or two aryl groups, respectively.
"alkylarylamino",
"alkylaminoaryl" or "arylaminoalkyl" refers to an amino group which is bound
to at least one
alkyl group and at least one aryl group. "Alkaminoalkyl" refers to an alkyl,
alkenyl, or
alkynyl group bound to a nitrogen atom which is also bound to an alkyl group.
"Acylamino"
includes groups wherein nitrogen is bound to an acyl group. Examples of
acylamino include,
but are not limited to, alkylcarbonylamino, arylcarbonylamino, carbamoyl and
ureido groups.
[0001269] The term "amide" or "aminocarboxy" includes compounds or moieties
that
contain a nitrogen atom that is bound to the carbon of a carbonyl or a
thiocarbonyl group.
The term includes "alkaminocarboxy" groups that include alkyl, alkenyl or
alkynyl groups
bound to an amino group which is bound to the carbon of a carbonyl or
thiocarbonyl group.
It also includes "arylaminocarboxy" groups that include aryl or heteroaryl
moieties bound to
an amino group that is bound to the carbon of a carbonyl or thiocarbonyl
group. The terms
"alkylaminocarboxy", "alkenylaminocarboxy", "alkynylaminocarboxy" and
"arylaminocarboxy" include moieties wherein alkyl, alkenyl, alkynyl and aryl
moieties,
respectively, are bound to a nitrogen atom which is in turn bound to the
carbon of a carbonyl
group. Amides can be substituted with substituents such as straight chain
alkyl, branched
alkyl, cycloalkyl, aryl, heteroaryl or heterocycle. Substituents on amide
groups may be
further substituted.
[0001270] Compounds of the present invention that contain nitrogens can be
converted to
N-oxides by treatment with an oxidizing agent (e.g., 3-chloroperoxybenzoic
acid (m-CPBA)
and/or hydrogen peroxides) to afford other compounds of the present invention.
Thus, all
shown and claimed nitrogen-containing compounds are considered, when allowed
by valency
and structure, to include both the compound as shown and its N-oxide
derivative (which can
be designated as N->O or N+-O-). Furthermore, in other instances, the
nitrogens in the
compounds of the present invention can be converted to N-hydroxy or N-alkoxy
compounds.
For example, N-hydroxy compounds can be prepared by oxidation of the parent
amine by an
oxidizing agent such as m-CPBA. All shown and claimed nitrogen-containing
compounds
are also considered, when allowed by valency and structure, to cover both the
compound as
shown and its N-hydroxy (i.e., N-OH) and N-alkoxy (i.e., N-OR, wherein R is
substituted or
151
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
unsubstituted Cl-C 6 alkyl, CI-C6 alkenyl, CI-C6 alkynyl, 3-14-membered
carbocycle or 3-14-
membered heterocycle) derivatives.
[0001271] In the present specification, the structural formula of the compound
represents
a certain isomer for convenience in some cases, but the present invention
includes all
isomers, such as geometrical isomers, optical isomers based on an asymmetrical
carbon,
stereoisomers, tautomers, and the like. In addition, a crystal polymorphism
may be present
for the compounds represented by the formula. It is noted that any crystal
form, crystal form
mixture, or anhydride or hydrate thereof is included in the scope of the
present invention.
Furthermore, so-called metabolite which is produced by degradation of the
present compound
in vivo is included in the scope of the present invention.
[0001272] "Isomerism" means compounds that have identical molecular formulae
but
differ in the sequence of bonding of their atoms or in the arrangement of
their atoms in space.
Isomers that differ in the arrangement of their atoms in space are termed
"stereoisomers".
Stereoisomers that are not mirror images of one another are termed
"diastereoisomers", and
stereoisomers that are non-superimposable mirror images of each other are
termed
"enantiomers" or sometimes optical isomers. A mixture containing equal amounts
of
individual enantiomeric forms of opposite chirality is termed a "racemic
mixture".
[0001273] A carbon atom bonded to four nonidentical substituents is termed a
"chiral
center".
[0001274] "Chiral isomer" means a compound with at least one chiral center.
Compounds with more than one chiral center may exist either as an individual
diastereomer
or as a mixture of diastereomers, termed "diastereomeric mixture". When one
chiral center is
present, a stereoisomer may be characterized by the absolute configuration (R
or S) of that
chiral center. Absolute configuration refers to the arrangement in space of
the substituents
attached to the chiral center. The substituents attached to the chiral center
under
consideration are ranked in accordance with the Sequence Rule of Cahn, Ingold
and Prelog.
(Cahn et al., Angew. Chem. Inter. Edit. 1966, 5, 385; errata 511; Cahn et al.,
Angew. Chem.
1966, 78, 413; Cahn and Ingold, J. Chem. Soc. 1951 (London), 612; Cahn et al.,
Experientia
1956, 12, 81; Cahn, J. Chem. Educ. 1964, 41, 116).
[0001275] "Geometric isomer" means the diastereomers that owe their existence
to
hindered rotation about double bonds. These configurations are differentiated
in their names
by the prefixes cis and trans, or Z and E, which indicate that the groups are
on the same or
opposite side of the double bond in the molecule according to the Cahn-Ingold-
Prelog rules.
152
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001276] Furthermore, the structures and other compounds discussed in this
invention
include all atropic isomers thereof. "Atropic isomers" are a type of
stereoisomer in which the
atoms of two isomers are arranged differently in space. Atropic isomers owe
their existence
to a restricted rotation caused by hindrance of rotation of large groups about
a central bond.
Such atropic isomers typically exist as a mixture, however as a result of
recent advances in
chromatography techniques, it has been possible to separate mixtures of two
atropic isomers
in select cases.
[0001277] "Tautomer" is one of two or more structural isomers that exist in
equilibrium
and is readily converted from one isomeric form to another. This conversion
results in the
formal migration of a hydrogen atom accompanied by a switch of adjacent
conjugated double
bonds. Tautomers exist as a mixture of a tautomeric set in solution. In solid
form, usually
one tautomer predominates. In solutions where tautomerization is possible, a
chemical
equilibrium of the tautomers will be reached. The exact ratio of the tautomers
depends on
several factors, including temperature, solvent and pH. The concept of
tautomers that are
interconvertable by tautomerizations is called tautomerism.
[0001278] Of the various types of tautomerism that are possible, two are
commonly
observed. In keto-enol tautomerism a simultaneous shift of electrons and a
hydrogen atom
occurs. Ring-chain tautomerism arises as a result of the aldehyde group (-CHO)
in a sugar
chain molecule reacting with one of the hydroxy groups (-OH) in the same
molecule to give it
a cyclic (ring-shaped) form as exhibited by glucose.
[0001279] Common tautomeric pairs are: ketone-enol, amide-nitrile, lactam-
lactim,
amide-imidic acid tautomerism in heterocyclic rings (e.g., in nucleobases such
as guanine,
thymine and cytosine), amine-enamine and enamine-enamine.
[0001280] It is to be understood that the compounds of the present invention
may be
depicted as different tautomers. It should also be understood that when
compounds have
tautomeric forms, all tautomeric forms are intended to be included in the
scope of the present
invention, and the naming of the compounds does not exclude any tautomer form.
[0001281] The term "crystal polymorphs", "polymorphs" or "crystal forms" means
crystal structures in which a compound (or a salt or solvate thereof) can
crystallize in
different crystal packing arrangements, all of which have the same elemental
composition.
Different crystal forms usually have different X-ray diffraction patterns,
infrared spectral,
melting points, density hardness, crystal shape, optical and electrical
properties, stability and
solubility. Recrystallization solvent, rate of crystallization, storage
temperature, and other
153
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
factors may cause one crystal form to dominate. Crystal polymorphs of the
compounds can
be prepared by crystallization under different conditions.
[0001282] Additionally, the compounds of the present invention, for example,
the salts of
the compounds, can exist in either hydrated or unhydrated (the anhydrous) form
or as
solvates with other solvent molecules. Nonlimiting examples of hydrates
include
monohydrates, dihydrates, etc. Nonlimiting examples of solvates include
ethanol solvates,
acetone solvates, etc.
[0001283] "Solvate" means solvent addition forms that contain either
stoichiometric or
non stoichiometric amounts of solvent. Some compounds have a tendency to trap
a fixed
molar ratio of solvent molecules in the crystalline solid state, thus forming
a solvate. If the
solvent is water the solvate formed is a hydrate; and if the solvent is
alcohol, the solvate
formed is an alcoholate. Hydrates are formed by the combination of one or more
molecules of
water with one molecule of the substance in which the water retains its
molecular state as
H2O.
[0001284] As used herein, the term "analog" refers to a chemical compound that
is
structurally similar to another but differs slightly in composition (as in the
replacement of one
atom by an atom of a different element or in the presence of a particular
functional group, or
the replacement of one functional group by another functional group). Thus, an
analog is a
compound that is similar or comparable in function and appearance, but not in
structure or
origin to the reference compound.
[0001285] As defined herein, the term "derivative" refers to compounds that
have a
common core structure, and are substituted with various groups as described
herein. For
example, all of the compounds represented by Formula I are 5,6-dihydro-6-
phenylbenzo[f]isoquinolin-2-amine derivatives, and have Formula I as a common
core.
[0001286] The term "bioisostere" refers to a compound resulting from the
exchange of an
atom or of a group of atoms with another, broadly similar, atom or group of
atoms. The
objective of a bioisosteric replacement is to create a new compound with
similar biological
properties to the parent compound. The bioisosteric replacement may be
physicochemically
or topologically based. Examples of carboxylic acid bioisosteres include, but
are not limited
to, acyl sulfonimides, tetrazoles, sulfonates and phosphonates. See, e.g.,
Patani and LaVoie,
Chem. Rev. 96, 3147-3176, 1996.
[0001287] The present invention is intended to include all isotopes of atoms
occurring in
the present compounds. Isotopes include those atoms having the same atomic
number but
154
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
different mass numbers. By way of general example and without limitation,
isotopes of
hydrogen include tritium and deuterium, and isotopes of carbon include C-13
and C-14.
[0001288] It is understood that in the chemical structures or formulae shown
in the
specification hydrogen atoms are assumed to be bonded to atoms (e.g., C, N, 0,
and S) to
complete their valency.
2. Synthesis of Substituted Imidazolyl-5,6-Dihydrobenzo[nilsoguinoline
Compounds
[0001289] The present invention provides methods for the synthesis of the
compounds of
Formula I-IV. The present invention also provides detailed methods for the
synthesis of
various disclosed compounds of the present invention according to the
following schemes as
shown in the Examples.
[0001290] Throughout the description, where compositions are described as
having,
including, or comprising specific components, it is contemplated that
compositions also
consist essentially of, or consist of, the recited components. Similarly,
where methods or
processes are described as having, including, or comprising specific process
steps, the
processes also consist essentially of, or consist of, the recited processing
steps. Further, it
should be understood that the order of steps or order for performing certain
actions is
immaterial so long as the invention remains operable. Moreover, two or more
steps or
actions can be conducted simultaneously.
[0001291] The synthetic processes of the invention can tolerate a wide variety
of
functional groups, therefore various substituted starting materials can be
used. The processes
generally provide the desired final compound at or near the end of the overall
process,
although it may be desirable in certain instances to further convert the
compound to a
pharmaceutically acceptable salt, ester or prodrug thereof.
[0001292] Compounds of the present invention can be prepared in a variety of
ways
using commercially available starting materials, compounds known in the
literature, or from
readily prepared intermediates, by employing standard synthetic methods and
procedures
either known to those skilled in the art, or which will be apparent to the
skilled artisan in light
of the teachings herein. Standard synthetic methods and procedures for the
preparation of
organic molecules and functional group transformations and manipulations can
be obtained
from the relevant scientific literature or from standard textbooks in the
field. Although not
limited to any one or several sources, classic texts such as Smith, M. B.,
March, J., March's
Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 5th edition,
John
Wiley & Sons: New York, 2001; and Greene, T.W., Wuts, P.G. M., Protective
Groups in
155
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Organic Synthesis, 3rd edition, John Wiley & Sons: New York, 1999,
incorporated by
reference herein, are useful and recognized reference textbooks of organic
synthesis known to
those in the art. The following description of synthetic methods are designed
to illustrate, but
not to limit, general procedures for the preparation of compounds of the
present invention.
[0001293] Compounds of the present invention can be conveniently prepared by a
variety
of methods familiar to those skilled in the art. The compounds of this
invention with
Formula I-IV may be prepared according to the following procedures from
commercially
available starting materials or starting materials which can be prepared using
literature
procedures. These procedures show the preparation of representative compounds
of this
invention.
[0001294] The compounds of this invention with Formula I-IV may be also be
prepared
according to the following schemes from commercially available starting
materials or starting
materials, which can be prepared using literature procedures.
[0001295] Compounds of formula (I) can be conveniently prepared by a variety
of
methods familiar to those skilled in the art. One common route is illustrated
in Scheme 1.
Scheme 1
H2N '
NN
N-N O
O O ~
I IV
DMF-DMA I N 0
R2- / R2 / R
2
bR, CF3000H " R,
II III V
N-NH
CF3COOH
R2
R,
[0001296] Ketone compounds such as formula (II) are either available
commercially, can
be prepared by known methods or readily prepared by methods described in the
literature and
known to those skilled in art as shown in Scheme 2. The ketone (II) is treated
with DMF
acetal at 100 C for a period of 12-24 hours to give the enaminone (III).
Alternatively
reagents such as Brederick's reagent and hexamethylmethanetraiamine can also
be used to
prepare the enaminone (III). The enaminone (III) is heated thermally or in a
microwave with
p-methoxy benzyl protected amino pyrazole (IV) in the presence of acids such
as
156
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
trifluoroacetic acid or acetic acid in range between 100 C to 150 C to give
the PMB
protected pyrazolopyridine compounds with formula (V). The p-methoxy benzyl
protecting
group can be removed by heating in trifluoroacetic acid at 100 C for a period
of 12-24 hours
to give the compounds with formula (I). Alternatively conditions outlined in
Protective
Groups in Organic Synthesis, Third Edition, Theodora W. Greene and Peter M.
Wuts, Wiley
Interscience, 1999 can be used to remove the p-methoxybenzyl protecting group.
[0001297] Some ketones (II) used as shown in the scheme 1 to make the
compounds with
formula (I) can be prepared as shown in scheme 2.
Scheme 2
O
~CN' N
0
X R5
S
/>-R6
H S [~'-N
~N ~NHZ
R5 NHz R XI
6
iN NH 0
O O DMF-DMA O R7\S IN
NaOAc, DMF (~C' S"R7
VI VII Ix
R4
`/NH2
R3N ]~~
3
NH
0
N!N'R4
VIII R3
[0001298] Cyclohexane-1,3-dione (VI) can be treated with DMF acetal to give
the
enamonine (VII). The enaminone (VII) can be treated with guanidines such as
methyl
guanidine to give the ketone (VIII). Alternatively, the enaminone (VII) can be
treated with
thiomethyl amidine to give the ketone (IX). Alternatively, the enaminone (VII)
can be treated
with hydrazine to give the ketone (X).Alternatively, the enaminone (VII) can
be treated with
thioamide to give the ketone (XI). These ketones (VIII-XI) can then be
converted to
compounds with the formula (I) as shown in Scheme 1. Many guanidines,
hydrazines,
thioamides are commercially available or readily prepared by methods described
in the
literature (Comprehensive Organic Transformations, Richard C. Larock, Second
Edition,
Wiley- VCH, 1999) and known to those skilled in art.
[0001299] The compounds of formula (XII-XIV) can be prepared as shown in
Scheme 3.
157
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Scheme 3
NH2
O O N Pg'N
DMF-DMA, DMF N ~1O N IV
N
N N N N
Pg Pg
XV XVI
XVII
Pg,
HN Br N Br CF3000H
CF3COOH N H202
CJN CHZCIz N z z
N N N
HN
-0 ~0 \ N
N rc,
XIV XIII
XII
[0001300] The ketone (XV) where Pg is protecting group such as tert-butyl
carbamate (t-
BOC) or benzyloxycarbamate (CBZ) and m and n can be 1-2 is heated with DMF
acetal in
DMF at 100 C for a period of 24-48 hours to give the enaminone (XVI). The
enaminone
(XVI) is heated with p-methoxy benzyl protected amino pyrazole (IV) at 100 C
for a period
of 10-24 hours to give PMB protected pyrazolopyridine (XVII). The tert-butyl
carbamate (t-
BOC) protecting group is removed using trifluoroactic acid at ambient
temperature for a
period of 2-12 hours to give the amine with the formula (XII). The tert-butyl
carbamate (t-
BOC) protecting group can also be removed by using the conditions outlined in
Protective
Groups in Organic Synthesis, Third Edition, Theodora W. Greene and Peter M.
Wuts, Wiley
Interscience, 1999. The n-t-BOC protected pyrazolopyridine (XVII) can also be
bominated
using N-bromosuccinimide (NBS) in solvents such as dichloromethane at ambient
temperatures for a period of 2-12 hours or other conditions outlined in
Comprehensive
Organic Transformations, Richard C. Larock, Second Edition, Wiley- VCH, 1999
to give the
3-bromo-pyrazolopyridine (XIII). The tert-butyl carbamate (t-BOC) protecting
group in the
compound (XIII) is removed using acidic conditions such as trifluoroacetic
acid at ambient
temperatures for a period of 2-12 hours to give the amine with the formula
(XIV). The tert-
butyl carbamate (t-BOC) protecting group can also be removed by using the
conditions
outlined in Protective Groups in Organic Synthesis, Third Edition, Theodora W.
Greene and
Peter M. Wuts, Wiley Interscience, 1999.
[0001301] The compounds of formula (XVIII) can be prepared as shown in Scheme
4.
158
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Scheme 4
NH2 H2N
O O N
DMF-DMA
C r I N~ N~ N
AcOH N N
HN, HN,
Pg Pg \ /
XIX XX -0
XVIII
H
P91N H2N
(BOC)20, THE CF3COOH, CH2CI2
N N
N N N N
-0\ / -0\ /
XXI XVIII
[0001302] The amino ketone (XIX) where Pg is protecting group such as tert-
butyl
carbamate (t-BOC) or benzyloxycarbamate (CBZ) is heated with DMF acetal to
give the
enaminone (XX). The enaminone (XX) is heated with p-methoxy benzyl protected
amino
pyrazole (IV) in acids such as acetic acid at 100 C for a period of 24-48
hours to give PMB
protected pyrazolopyridine (XVIII). Alternatively, trifluoroacetic acid can be
used at 100 C
for a period of 10-24 hours to give the PMB protected pyrazolopyridine
(XVIII). During the
reaction the tert-butyl carbamate (t-BOC) was removed. The amine (XVIII) was
treated with
t-BOC anhydride at ambient temperature for a period of 2-10 hours to protect
the amine with
tert-butyl carbamate (t-BOC) to aid with the purification of the compound
(XXI). The tert-
butyl carbamate (t-BOC) protecting group from the compound (XXI) is removed
using
trifluoroactic acid at ambient temperatures for a period of 2-10 hours to give
the amine
(XVIII). The tert-butyl carbamate (t-BOC) protecting group can also be removed
by using the
conditions outlined in Protective Groups in Organic Synthesis, Third Edition,
Theodora W.
Greene and Peter M. Wuts, Wiley Interscience, 1999.
[0001303] The a-chloroamides of pyrazolopyridines (XXII) can be prepared as
shown in
schemes 5 and 6. The PMB protected pyrazolo pyridine amine (XII) is reacted
with a-
chloroacyl chloride (XXIII) in the presence of tertiary amines bases such as
triethylamine,
diisopropylethyl amine in chlorinated solvents such as dichloromethane at
ambient
temperatures for 1-4 hours. The PMB protecting group of the resulting a-
chloroamide
(XXIV) is then removed by heating in trifluoroacetic acid at 100 C for 16-24
hours to give
the compounds with formula (XXV). The a-chloroamides pyrazolopyridine (XXV) is
then
159
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
bominated in the 3 position using N-bromosuccinimide (NBS) in chlorinated
solvents such as
dichloromethane under ambient temperatures for 1-6 hours to give the 3-bromo-
a-
chloroamides pyrazolopyridine (XXII).
Scheme 5
o
HN\ CI N N
N CI XXIII CI CF3000H CI
N 0-~
N Et3N, CH2CI2 N \ N
N N N H
d XXV
Oxd O
XII XXIV
NBS, CH2CI2 N Br
CI
IN
N N
H
XXII
[0001304] Alternatively the 3-phenyl a-chloroamide of pyrazolopyridine (XXVI)
is
prepared as shown in scheme 6. The PMB protected pyrazolo pyridine (XII) is
first
deprotected by heating in trifluoroacetic acid at 65 C for a period of 16-24
hours. The
piperidine nitrogen of the resulting pyrazolopyridine amine is protected with
tert-butyl
carbamate (t-BOC) using N-t-Boc anhydride, tertiary amine sbases such as
triethylamine,
Hunig's Base at ambient temperature for period of 2-6 hours to give the tert-
butyl carbamate
of the pyrazolo pyridine amine (XXVII). The t-BOC protected pyrazole pyridine
(XXVII) is
brominated on the 3-position using N-bromosuccinimide (NBS) in chlorinated
solvents such
as dichloromethane under ambient temperatures for 1-6 hours to give the t-BOC
protected 3-
bromo-pyrazolopyridine amine (XXVIII). Suzuki coupling reactions are carried
out at 90 C
for a period of 16-24 hours using boronic acids such as phenylboronic acid in
the presence
palladium coupling agents such as dichloro-((bis
diphenylphosphino)ferrocenyl)palladium
(II), aqueous bases such as sodium carbonate and in solvents such as toluene,
and protic
solvents such as ethanol to give the compounds with formula (XXIX). The t-BOC
group on
the compounds with formula (XXIX) is then removed under acidic condition such
as HC1(g)
in dioxane in solvents such as dichloromethane, ethyl acetate, 1,4-dioxane at
ambient
temperatures for a period of 2-24 hours to give the HCl salt of 3-phenyl
pyrazolo pyridine
amine (XXX). The 3-phenyl pyrazolo pyridine amine is reacted with a-chloroacyl
chloride
(XXIII) in the presence of tertiary amines bases such as triethylamine,
diisopropylethyl amine
160
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
in chlorinated solvents such as dichloromethane at ambient temperatures for 1-
2 hours to give
the desired a-chloroamide of 3-phenyl pyrazolopyridine (XXVI).
Scheme 6
HN O O
/ ~O'J~N ~10 N
N N N (i) CF3000H NBS, / Br
(ii) (BOC)20, Et3N, CH2CI2 N N
H \N H
XXVII XXVIII
-O
XII
O O
DtBPFPdC12, PhB(OH)2 ~10 N HCI (g) in Dioxane Cl H2N
Toluene, EtOH,Aq. Na2CO3 \N CH2CI2 \N
N
H
H N N
XXIX XXX
9oci O-T-Z Cl Cl
Hunig's Base, CH2CI2 N
N N
H
XXVI
[0001305] Scheme 7 and 8 illustrates a variety of chemical transformation of
pyrazolopyridine amine (XII, XIII, XVIII) to provide compounds (XXXIa,b-
XXXVIIa,b,
XXXVIII-XXXXIII). The following methods are provided by way of
exemplification.
Alternative methods may be employed and are described in reference text's such
as
Comprehensive Organic Transformations, Richard C. Larock, Second Edition,
Wiley- VCH,
1999; Protective Groups in Organic Synthesis, Third Edition, Theodora W.
Greene and Peter
M. Wuts, Wiley Interscience, 1999; The Practice of Peptide Synthesis, M.
Bodanszky and A.
Bodanzsky, Springer-Verlag, 1984.
[0001306] The pyrazolopyridine amides (XXXIa, b, XXXVIII) can be conveniently
prepared by treating pyrazolopyridine amine (XII, XIII, XVIII) with the
corresponding
carboxylic acid in presence of amide coupling agents such as HBTU (O-
(benzotriazo-l-yl)-
N,N,N',N'-tetramethyluronium hexafluorophosphate), tertiary amine bases such
as
dimethylaminopyridine and solvents such as dimethylformamide at ambient
temperature for
4-24 hours followed by the removal of the PMB protecting group by heating the
intermediate
in trifluoroacetic acid at 65 C for a period of 12-24 hours. Alternatively
amide coupling
agents such as DCC (dicylcohexycarbodiimide), BOP ((benzotriazo-1-
yloxy)tris(dimethylamino) phosphonium hexafluorophosphate, EDCI.HC1(1-(3-
Dimethyl
161
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
aminopropyl)-3-ethylcarbodiimide hydrochloride), DMC (2-chloro-1,3-
dimethylimidazolinium chloride) and tertiary amines bases such as
triethylamine,
diisopropylethyl amine can also be used. Many carboxylic acids are
commercially available
or readily prepared by methods described in the literature and known to those
skilled in art.
[0001307] Alternatively, the pyrazolopyridine amides (XXXIa, b, XXXVIII) can
be
conveniently prepared by treating pyrazolopyridine amine (XII, XIII, XVIII)
with acid
chlorides such as m-trifluoromethylbenzoyl chloride in presence of tertiary
amine bases such
as triethylamine, diisopropylethyl amine and solvents such as dichloromethane
at ambient
temperature for 1-12 hours followed by the removal of the PMB protecting group
by heating
the intermediate in trifluoroacetic acid at 65 C for a period of 12-24 hours.
Many acid
chlorides are commercially available or readily prepared by methods described
in the
literature and known to those skilled in art.
Scheme 7
0
CIAN W R15^N W O
N NN R8 N W
N N H \
~OCH3 XXXVIIa (W=H) \N
XXXIVa (W H) XXXVIIb (W=Br N H
XXXIVb (W=Br)
0~ 2 XXXIa (W=H)
H (i) R15-CHO C0C00 XXXIb (W=Br)
13'N R14 ~~Oho (ii) CF3COOH \\ CF
0 s9 `l
R13,NAN HN R ~S N
s W
R14 \ W (i) R13NCO \ W (I) R9SO Et3N
N I (ii) CF3COOH N N N - (ii) CF3COOH
N H N
N
FIN
XXXllla (W=H) OCH3 CI XXXIIa (W=H)
XXXIIIb (W=Br) C5 XII (W=H) 1 XXXIIb (W=Br)
0 XIII (W = Br) NR
yeN
S
R12 `, (i) R11COCI NON
N H N YN-N (ii) CF3000H N
Rio W
N
N
O ::1 C
H
R11~N W N N
XXXVIa (W=H) H
XXXVIb (W=Br) \ XXXVa (W=H)
N N N XXXVb(W=Br)
H
XXXIa (W=H)
XXXIb (W=Br)
Scheme 8
162
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
H
R151~1 N R H
su
\ II
\N O ~
N H \N
XXXXIII N H
XXXVIII
(i) R15-CHO ~$GOGO
(ii) CF3COOH
l~. GF3
H
NUN HzN R9, N
R1 3 II
O (i) R13NCO (i) R9SO 0
N (ii) CF3000H NN (ii) CF3000H N
N H N N N
XXXX XVIII \ / OCH3 CI XXXIX
H
G pp~ N-"')
R
~ G) R1COCI Msp ` J 10
H H (ii) CF3000 yeah
,N N
R12 II N H
S ~ \N R11 N N N
N H p R10 I ~N
XXXXI I_ N N N H
N H XXXXI
XXXVIII
[0001308] Pyrazolo-pyridine sulfonamide (XXXIIa, b, XXXIX) can be conveniently
prepared by treating pyrazolopyridine amine (XII, XIII, XVIII) with sulfonyl
chlorides such
as methanesulfonyl chloride in presence of tertiary amine bases such as
triethylamine and
solvents such as dichloromethane at ambient temperature for 1-12 hours
followed by the
removal of the PMB protecting group by heating the intermediate in
trifluoroacetic acid at 65
C for a period of 12-24 hours. Alternatively tertiary amines bases such as
diisopropylethyl
amine can also be used. Many sulfonyl chlorides are commercially available or
readily
prepared by methods described in the literature and known to those skilled in
art.
[0001309] Pyrazolopyridine urea (XXXIIIa, b, XXXX) can be conveniently
prepared by
treating pyrazolopyridine amine (XII, XIII, XVIII) with isocyanates such as
phenylisocyanante in solvents such as dichloromethane at ambient temperature
for 1-12 hours
followed by the removal of the PMB protecting group by heating the
intermediate in
trifluoroacetic acid at 65 C for a period of 12-24 hours. Many isocyanates
are commercially
available or readily prepared by methods described in the literature and known
to those
skilled in art. Isocyantes can also be prepared in situ from carboxylic acids
by a variety of
methods familiar to those skilled in the art. Carboxylic acids are treated
with diphenyl
phosphoryl azide in solvents such as toluene at reflux for 1-5 hours. The
isocyanates are used
in situ as solution to react with the pyrazolopyridine amine (XII, XIII) to
prepare pyrazolo-
pyridine ureas (XXXIIIa, b) as described above. Alternatively, the
pyrazolopyridine amine
(XII, XIII) can be converted to pyrazolopyridine amine carbamoyl chloride
(XXXIVa, b)
which can then be reacted with amines followed by the removal of the PMB
protecting group
163
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
by heating the intermediate in trifluoroacetic acid at 65 C for a period of
12-24 hours to give
pyrazolo-pyridine ureas (XXXIIIa, b).
[0001310] Pyrazolopyridine amine heterocycle (XXXVa, b, XXXXI) can be
conveniently prepared by treating pyrazolopyridine amine (XII, XIII, XVIII)
with
heterocyclic halides such as 2-chloropyrazine, tertiary amine bases such as
triethylamine,
diisopropylethyl amies in polar aprotic solvent such as dimethylsulfoxide,
dimethylformamide at 80-110 C for 1-12 hours followed by the removal of the
PMB
protecting group by heating the intermediate in trifluoroacetic acid at 65 C
for a period of
12-24 hours. Many heterocyclic halides are commercially available or readily
prepared by
methods described in the literature and known to those skilled in art.
[0001311] Pyrazolopyridine thiourea (XXXVIa, b, XXXXII) can be conveniently
prepared by treating pyrazolopyridine amine (XII, XIII, XVIII) with
thioisocyanates such as
phenyl thioisocyanante in solvents such as dichloromethane at ambient
temperature for 1-12
hours followed by the removal of the PMB protecting group by heating the
intermediate in
trifluoroacetic acid at 65 C for a period of 12-24 hours. Many isocyanates
are commercially
available or readily prepared by methods described in the literature and known
to those
skilled in art.
[0001312] The compounds of the formula (XXXVIIa, b, XXXXIII) can be
conveniently
prepared by treating pyrazolopyridine amine (XII, XIII, XVIII) with aldehydes
such as
benzaldehyde and reducing agents such sodium triacetoxyborohydride or
tetramethylammoniumborohydride in solvents such as dichloromethane at ambient
temperature for 1-12 hours followed by the removal of the PMB protecting group
by heating
the intermediate in trifluoroacetic acid at 65 C for a period of 12-24 hours.
Many aldehydes
are commercially available or readily prepared by methods described in the
literature and
known to those skilled in art.
[0001313] Pyrazolopyridine urea amides of formula (XXXXIV) formed using
anilines or
heterocyclic amines can be prepared as shown in Scheme 9.
Scheme 9
164
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
0
EtOOC - I Ik
HN EtOOC H N
/ \ NCO NaOH, MeOH
N I NN CH2CI2 N NN
O -O
XII XXXXV
/ 0 Ar-NH / 0
H000- ~
I NIk N 0) DMC, CH2CI2, DMA, Ar-NH2 0U 0 I NIk N
H Hunig's Base H
\N (ii) CF3COOH N
N N H
XXXXIV
XXXXVI /
-O
[0001314] PMB protected pyrazolo pyridine urea ester (XXXXV) can be prepared
by
treating pyrazolopyridine (XII) with isocynates with carboxylic esters in
solvents such as
dichloromethane at ambient temperature for 1-12 hours. Many isocynates with
carboxylic
esters are commercially available or readily prepared by methods described in
the literature
and known to those skilled in art. The ester group on the PMB protected
pyrazolopyridine
urea ester (XXXXV) is converted to carboxylic compound (XXXXVI) by hydrolysis
using
sodium hydroxide, water and protic solvent like methanol, ethanol at ambient
temperatures
for a period of 12-24 hours. The PMB protected pyrazolopyridine urea acid
(XXXXVI) is
then converted to pyrozolopyridine urea amides (XXXXIV) by reacting with
coupling agent
DMC (2-chloro-1,3-dimethylimidazolinium chloride) and amines like anilines,
heterocyclic
amines in solvents such as dichloromethane at ambient temperatures for a
period of 12-24
hours followed by the removal of the PMB protecting group by heating the
intermediate in
trifluoroacetic acid at 65 C for a period of 12-24 hours. Many anilines and
heterocyclic
amines are commercially available or readily prepared by methods described in
the literature
and known to those skilled in art.
[0001315] Alternatively, the pyrazolopyridine urea amide (XXXXVII) of primary
or
secondary amines, which are more reactive than anilines, can be prepared as
shown in
scheme 10.
165
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Scheme 10
HN
/ I N 0) CF3COOH EtOOC / O NaOH, H2O, CH3OH
N NN (ii) OCN N N
I = COOEt
N
Hunig's Base, CH2CI2, DMA N N
H
-p XXXXVIII
XII
R14
IoI H R13-N / IOI
HOOC \ I A R13 N,R14 /\ I J~
N N O N N
H HBTU, Hunig's Base, H
N DMA, I N
N H N H
XXXXIX XXXXVII
[0001316] The PMB protected pyrazolo pyridine amine (XII) is deprotected in
acids such
as trifluoroacetic acid at 65 C for 12-24 hours to give the pyrazolopyridine
amine followed
by reaction with isocynates with carboxylic esters in solvents such as
dichloromethane at
ambient temperature for 1-12 hours to give the pyrazolopyridine urea ester
(XXXXVIII).
Many isocynates with carboxylic esters are commercially available or readily
prepared by
methods described in the literature and known to those skilled in art. The
pyrazolopyridine
urea ester (XXXXVIII) can then be converted to pyrazolopyridine urea
carboxylic acid
(XXXXIX) by hydrolysis using sodium hydroxide, water and protic solvent like
methanol,
ethanol at ambient temperatures for a period of 12-24 hours. The
pyrazolopyridine urea
amide (XXXXVII) can be conveniently prepared by treating pyrazolopyridine urea
acid
(XXXXIX) with the corresponding primary or secondary amines in presence of
amide
coupling agents such as HBTU (O-(benzotriazo-1-yl)-N,N,N',N'-
tetramethyluronium
hexafluorophosphate), tertiary amine bases such as dimethylaminopyridine and
solvents such
as dimethylformamide at ambient temperature for 4-24 hours. Alternatively
amide coupling
agents such as DCC (dicylcohexycarbodiimide), BOP ((benzotriazo-1-
yloxy)tris(dimethylamino) phosphonium hexafluorophosphate, EDCI.HC1(1-(3-
Dimethyl
aminopropyl)-3-ethylcarbodiimide hydrochloride), DMC (2-chloro-1,3-
dimethylimidazolinium chloride) and tertiary amines bases such as
triethylamine,
diisopropylethyl amine can also be used. Many primary and secondary amines are
commercially available or readily prepared by methods described in the
literature and known
to those skilled in art.
166
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001317] The compounds of the formula (XXXXXa-c) can be prepared as shown in
scheme 11.
Scheme 11
O-Ty~ N W H oy N W
N.
\
CI R14 /
R13 R14 R13 N`
N N
N N Condition 1: Hunig's Base, 1,4-Dioxane ~N N
H Condition 2: nBu4N'I-, Hunig's Base, DMSO O H
XXV (W=H) XXXXXa(W=H)
XXII (W=Br) XXXXXb (W=Br)
XXVI (W=Ph) XXXXXc(W=Ph)
H
C) Condition 1
N
BOC
0-Ti N W \ / I O
N W
CN` \N HCI (g) in Dioxane CNJ N
Jl N
BOC N H H N H
XXXXXIa (W=H) XXXXXa (W=H)
XXXXXIb (W=Br) XXXXXb (W=Br)
XXXXXIc (W=Ph) XXXXXc (W=Ph)
[0001318] The compounds of the formula (XXXXXa-c) can be prepared using two
conditions. In condition 1, a primary or secondary amine such as N-Methyl
piperazine or
benzyl amine is heated with the a-chloroamide of pyrazolopyridine amine (XXV,
XXII,
XXVI) in presence of tertiary amines bases such as triethylamine,
diisopropylethyl amine in
solvents such as 1,4-dioxane for at 100 C for a period of 2-18 hours to give
the compound
(XXXXXa-c). If the amine used is a protected diamine such N-t-BOC-piperazine
then a
compound with the formula (XXXXXIa-c) is obtained. The protecting group (N-t-
BOC) can
be removed under acid conditions such as HC1(g) in Dioxane or trifluoroacetic
at ambient
temperatures for a period 2-16 hours to give the desired compounds with the
formula
(XXXXXa-c). Alternatively condition 2 can be utilized where the amino amides
of
pyrazolopyridines (XXXXXa-c) are prepared by heating chloroamide of
pyrazolopyridine
amine (XXV, XXII, XXVI) in presence of phase transfer catalyst such as n-
butylammonium
iodide and tertiary amines bases such as triethylamine, diisopropylethyl amine
in solvents
such as 1,4-dioxane at 100 C for a period of 2-18 hours. Many primary and
secondary
amines are commercially available or readily prepared by methods described in
the literature
and known to those skilled in art.
[0001319] The compounds of the formula (XXXXXII) can be prepared as shown in
scheme 12.
167
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Scheme 12
0
ON / O
H N O 0
HN I N
N XXXXXIOII < ONH N CF3000H
N N CH2CI2 N N' CH2CI2 5 -O -O
XII XXXXXIV
0-0 OO+ N O
CF3000 NH3 CF3COOH N
NN CF3COO NH3
N N
N N
H
XXXXXII
-O
XXXXXV
[0001320] The PMB protected pyrazolopyridine amine (XII) is reacted with the t-
BOC
protected succinimide ester of amino acid (XXXXXIII) such as phenylalanine in
solvent such
as dichloromethane under ambient temperatures for a period of 1-10 hours to
give the
compounds of formula (XXXXXIV). The t-BOC protecting group is then removed
under
acid conditions such as trifluoroacetic acid in dichloromethane or HC1 gas as
a solution in
1,4-dioxane at ambient temperatures for a period 4-18 hours to give the
compounds of
formula (XXXXXV). The PMB protecting of the compound with the formula (XXXXXV)
is
then removed by heating in trifluoroacetic acid at 65 C for a period of 16-24
hours to give
the compound of formula (XXXXXII). Many N-protected succinimyl esters of amino
acids
are commercially available or readily prepared by methods described in the
literature and
known to those skilled in art.
[0001321] The compounds of the formula (XXXXXVI) were prepared as shown in
scheme 13. The thiomethyl pyrimidine pyrazolopyridine (XXXXXXVII) was treated
with an
oxidant such as Oxone in a mixture of water and a protic solvent such as
methanol at ambient
temperature for 12-24 hours to give the corresponding sulfone (XXXXXVIII).
Alternatively,
m-CPBA can be utilized at ambient temperatures for a period 12-24 hours to
prepare
compounds with the formula (XXXXXXVIII), The sulfone (XXXXXVIII) can then be
reacted with the primary or secondary amines or anilines in solvents such as
DMSO at 100 C
for a period of 1- 24 hours followed by the removal of the PMB protecting
group by heating
168
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
the intermediate in trifluoroacetic acid at 65 C for a period of 12-24 hours
to give the desired
compounds with the formula (XXXXXVI). Many primary, secondary amines and
anilines are
commercially available or readily prepared by methods described in the
literature and known
to those skilled in art.
Scheme 13
N-N / O N-N / O
N Oxone , MeOH, H2O / N
INIII \ \ I INII
S N N
O S~0
XXXXXVI I XXXXXVIII
N-NH
H
(I) R13 N`R14 , DMSO N
(i) CF3COOH N
R13-N'k N
R14
XXXXXVI
[0001322] The compounds of formula (XXXXXIX) can be prepared as shown in
Scheme
14.
Scheme 14
NH2 Et02C
O O
N UGH, CH3OH,
DMF-DMA / N 0 / N- IV N THF, H2O
I CH3COOH N N
CO2Et CO2Et
XXXXXX xxxxxxI
-0
XXXXXXI I
O
HO2C NH2 R16 0
R16~ \ 0", N R16
N
N HBTU
I \I \ CF3000H H
r\,," H \
Hunig's Base, N N
DMF N N NN
N H
xxxxxix
xxxxxxI II XXXXXXIV
[0001323] The ketone ethyl ester (XXXXXX) is heated with DMF acetal in DMF at
120
C for a period of 12-48 hours to give the enaminone (XXXXXXI).The enaminone
(XXXXXXI) is heated with p-methoxy benzyl protected amino pyrazole (IV) in
acid such as
glacial acetic acid at 100 C for a period of 12-36 hours to give PMB
protected
169
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
pyrazolopyridine ethyl ester (XXXXXXII). The ethyl ester is hydrolyzed using
base such as
lithium hydroxide in water and protic solvent such as methanol at ambient
temperature for 1
to 12 hours to give the compound with the formula (XXXXXXIII). The
pyrazolopyridine
amide (XXXXXIX) can be conveniently prepared by treating pyrazolo-pyridine
acids
(XXXXXXIII) with the corresponding primary, secondary amine or anilines in
presence of
amide coupling agents such as HBTU (O-(benzotriazo-1-yl)-N,N,N',N'-
tetramethyluronium
hexafluorophosphate), tertiary amine bases such as dimethylaminopyridine and
solvents such
as dimethylformamide at ambient temperature for 4-24 hours followed by the
removal of the
PMB protecting group by heating the intermediate (XXXXXXIV) in trifluoroacetic
acid at 65
C for a period of 12-24 hours. Alternatively amide coupling agents such as DCC
(dicylcohexycarbodiimide), BOP ((benzotriazo-1-yloxy)tris(dimethylamino)
phosphonium
hexafluorophosphate, EDCI.HC1(1-(3-Dimethyl aminopropyl)-3-ethylcarbodiimide
hydrochloride), DMC (2-chloro- 1,3 -dimethylimidazolinium chloride) and
tertiary amines
bases such as triethylamine, diisopropylethyl amine can also be used. Many
amines (primary,
secondary, and aniline) are commercially available or readily prepared by
methods described
in the literature and known to those skilled in art.
[0001324] The compounds of formula (XXXXXXV) can be prepared as shown in
scheme 15.
Scheme 15
B(OH)2 O gR
O 0 N Br = R17 HCI (g), Dioxane
N Pd(Di-tBu ppf)PdCI2, H Na2CO3, Toluene, EtOH, Water N H
XXXXXXVI XXXXXXVII
R18
g17 NCO WR
HI / R8 Et3N, THE H N H N H
XXXXXXVI II XXXXXXV
[0001325] Suzuki reaction is carried out on 3-bromo-pyrazolopyridine
(XXXXXXVI) at
100 C for a period of 16-24 hours using substituted phenyl boronic acids,
palladium
coupling agents such as dichloro-((bis diphenylphosphino)ferrocenyl)palladium,
aqueous
bases such as sodium carbonate and in solvents such as toluene, and protic
solvents such as
ethanol to give the compound with formula (XXXXXXVII). Many boronic acids are
commercially available or readily prepared by methods described in the
literature and known
170
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
to those skilled in art. The t-BOC- protecting group on compounds with formula
(XXXXXXVII) is then removed under acid conditions such as HC1 gas as a
solution in 1,4-
dioxane at 55 C for a period 10-24 hours to give the compounds of formula
(XXXXXXVIII). The amines of the formula (XXXXXXVIII) can then be reacted with
isocyanantes such as phenyl isocyanante in solvents such as tetrahydrofuran at
ambient
temperature for 1-12 hours to give compounds of formula (XXXXXXV). Many
isocyanates
are commercially available or readily prepared by methods described in the
literature and
known to those skilled in art.
3. Methods of Treatment
[0001326] The present invention provides methods for the treatment of a cell
proliferative disorder in a subject in need thereof by administering to a
subject in need of
such treatment, a therapeutically effective amount of a compound of Formula I-
IV. The cell
proliferative disorder can be cancer or a precancerous condition. The present
invention
further provides the use of a compound of Formula I-IV for the preparation of
a medicament
useful for the treatment of a cell proliferative disorder.
[0001327] The present invention also provides methods of protecting against a
cell
proliferative disorder in a subject in need thereof by administering a
therapeutically effective
amount of a compound of Formula I-IV to a subject in need of such treatment.
The cell
proliferative disorder can be cancer or a precancerous condition. The present
invention also
provides the use of a compound of Formula I-IV for the preparation of a
medicament useful
for the prevention of a cell proliferative disorder.
[0001328] As used herein, a "subject in need thereof"is a subject having a
cell
proliferative disorder, or a subject having an increased risk of developing a
cell proliferative
disorder relative to the population at large. A subject in need thereof can
have a precancerous
condition. Preferably, a subject in need thereof has cancer. A "subject"
includes a mammal.
The mammal can be e.g., any mammal, e.g., a human, primate, bird, mouse, rat,
fowl, dog,
cat, cow, horse, goat, camel, sheep or a pig. Preferably, the mammal is a
human.
[0001329] As used herein, the term "cell proliferative disorder" refers to
conditions in
which unregulated or abnormal growth, or both, of cells can lead to the
development of an
unwanted condition or disease, which may or may not be cancerous. Exemplary
cell
proliferative disorders of the invention encompass a variety of conditions
wherein cell
division is deregulated. Exemplary cell proliferative disorder include, but
are not limited to,
neoplasms, benign tumors, malignant tumors, pre-cancerous conditions, in situ
tumors,
171
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
encapsulated tumors, metastatic tumors, liquid tumors, solid tumors,
immunological tumors,
hematological tumors, cancers, carcinomas, leukemias, lymphomas, sarcomas, and
rapidly
dividing cells. The term "rapidly dividing cell" as used herein is defined as
any cell that
divides at a rate that exceeds or is greater than what is expected or observed
among
neighboring or juxtaposed cells within the same tissue. A cell proliferative
disorder includes a
precancer or a precancerous condition. A cell proliferative disorder includes
cancer.
Preferably, the methods provided herein are used to treat or alleviate a
symptom of cancer.
The term "cancer" includes solid tumors, as well as, hematologic tumors and/or
malignancies.
A "precancer cell" or "precancerous cell" is a cell manifesting a cell
proliferative disorder
that is a precancer or a precancerous condition. A "cancer cell" or "cancerous
cell" is a cell
manifesting a cell proliferative disorder that is a cancer. Any reproducible
means of
measurement may be used to identify cancer cells or precancerous cells. Cancer
cells or
precancerous cells can be identified by histological typing or grading of a
tissue sample (e.g.,
a biopsy sample). Cancer cells or precancerous cells can be identified through
the use of
appropriate molecular markers.
[0001330] Exemplary non-cancerous conditions or disorders include, but are not
limited
to, rheumatoid arthritis; inflammation; autoimmune disease;
lymphoproliferative conditions;
acromegaly; rheumatoid spondylitis; osteoarthritis; gout, other arthritic
conditions; sepsis;
septic shock; endotoxic shock; gram-negative sepsis; toxic shock syndrome;
asthma; adult
respiratory distress syndrome; chronic obstructive pulmonary disease; chronic
pulmonary
inflammation; inflammatory bowel disease; Crohn's disease; psoriasis; eczema;
ulcerative
colitis; pancreatic fibrosis; hepatic fibrosis; acute and chronic renal
disease; irritable bowel
syndrome; pyresis; restenosis; cerebral malaria; stroke and ischemic injury;
neural trauma;
Alzheimer's disease; Huntington's disease; Parkinson's disease; acute and
chronic pain;
allergic rhinitis; allergic conjunctivitis; chronic heart failure; acute
coronary syndrome;
cachexia; malaria; leprosy; leishmaniasis; Lyme disease; Reiter's syndrome;
acute synovitis;
muscle degeneration, bursitis; tendonitis; tenosynovitis; herniated, ruptures,
or prolapsed
intervertebral disk syndrome; osteopetrosis; thrombosis; restenosis;
silicosis; pulmonary
sarcosis; bone resorption diseases, such as osteoporosis; graft-versus-host
reaction; Multiple
Sclerosis; lupus; fibromyalgia; AIDS and other viral diseases such as Herpes
Zoster, Herpes
Simplex I or II, influenza virus and cytomegalovirus; and diabetes mellitus.
[0001331] Exemplary cancers include, but are not limited to, adrenocortical
carcinoma,
AIDS-related cancers, AIDS-related lymphoma, anal cancer, anorectal cancer,
cancer of the
anal canal, appendix cancer, childhood cerebellar astrocytoma, childhood
cerebral astrocytoma,
172
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
basal cell carcinoma, skin cancer (non-melanoma), biliary cancer, extrahepatic
bile duct cancer,
intrahepatic bile duct cancer, bladder cancer, uringary bladder cancer, bone
and joint cancer,
osteosarcoma and malignant fibrous histiocytoma, brain cancer, brain tumor,
brain stem glioma,
cerebellar astrocytoma, cerebral astrocytoma/malignant glioma, ependymoma,
medulloblastoma, supratentorial primitive neuroectodeimal tumors, visual
pathway and
hypothalamic glioma, breast cancer, bronchial adenomas/carcinoids, carcinoid
tumor,
gastrointestinal, nervous system cancer, nervous system lymphoma, central
nervous system
cancer, central nervous system lymphoma, cervical cancer, childhood cancers,
chronic
lymphocytic leukemia, chronic myelogenous leukemia, chronic myeloproliferative
disorders,
colon cancer, colorectal cancer, cutaneous T-cell lymphoma, lymphoid neoplasm,
mycosis
fungoides, Seziary Syndrome, endometrial cancer, esophageal cancer,
extracranial germ cell
tumor, extragonadal germ cell tumor, extrahepatic bile duct cancer, eye
cancer, intraocular
melanoma, retinoblastoma, gallbladder cancer, gastric (stomach) cancer,
gastrointestinal
carcinoid tumor, gastrointestinal stromal tumor (GIST), germ cell tumor,
ovarian germ cell
tumor, gestational trophoblastic tumor glioma, head and neck cancer,
hepatocellular (liver)
cancer, Hodgkin lymphoma, hypopharyngeal cancer, intraocular melanoma, ocular
cancer, islet
cell tumors (endocrine pancreas), Kaposi Sarcoma, kidney cancer, renal cancer,
kidney cancer,
laryngeal cancer, acute lymphoblastic leukemia, acute myeloid leukemia,
chronic
lymphocytic leukemia, chronic myelogenous leukemia, hairy cell leukemia, lip
and oral cavity
cancer, liver cancer, lung cancer, non-small cell lung cancer, small cell lung
cancer, AIDS-
related lymphoma, non-Hodgkin lymphoma, primary central nervous system
lymphoma,
Waldenstram macroglobulinemia, medulloblastoma, melanoma, intraocular (eye)
melanoma, merkel cell carcinoma, mesothelioma malignant, mesothelioma,
metastatic
squamous neck cancer, mouth cancer, cancer of the tongue, multiple endocrine
neoplasia
syndrome, mycosis fungoides, myelodysplastic syndromes, myelodysplastic/
myeloproliferative
diseases, chronic myelogenous leukemia, acute myeloid leukemia, multiple
myeloma, chronic
myeloproliferative disorders, nasopharyngeal cancer, neuroblastoma, oral
cancer, oral cavity
cancer, oropharyngeal cancer, ovarian cancer, ovarian epithelial cancer,
ovarian low
malignant potential tumor, pancreatic cancer, islet cell pancreatic cancer,
paranasal sinus and
nasal cavity cancer, parathyroid cancer, penile cancer, pharyngeal cancer,
pheochromocytoma,
pineoblastoma and supratentorial primitive neuroectodermal tumors, pituitary
tumor, plasma cell
neoplasm/multiple myeloma, pleuropulmonary blastoma, prostate cancer, rectal
cancer, renal
pelvis and ureter, transitional cell cancer, retinoblastoma, rhabdomyosarcoma,
salivary gland
cancer, ewing family of sarcoma tumors, Kaposi Sarcoma, soft tissue sarcoma,
uterine
173
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
cancer, uterine sarcoma, skin cancer (non-melanoma), skin cancer (melanoma),
merkel cell
skin carcinoma, small intestine cancer, soft tissue sarcoma, squamous cell
carcinoma,
stomach (gastric) cancer, supratentorial primitive neuroectodermal tumors,
testicular cancer,
throat cancer, thymoma, thymoma and thymic carcinoma, thyroid cancer,
transitional cell
cancer of the renal pelvis and ureter and other urinary organs, gestational
trophoblastic tumor,
urethral cancer, endometrial uterine cancer, uterine sarcoma, uterine corpus
cancer, vaginal
cancer, vulvar cancer, and Wilm's Tumor.
[0001332] A "cell proliferative disorder of the hematologic system" is a cell
proliferative
disorder involving cells of the hematologic system. A cell proliferative
disorder of the
hematologic system can include lymphoma, leukemia, myeloid neoplasms, mast
cell
neoplasms, myelodysplasia, benign monoclonal gammopathy, lymphomatoid
granulomatosis,
lymphomatoid papulosis, polycythemia vera, chronic myelocytic leukemia,
agnogenic
myeloid metaplasia, and essential thrombocythemia. A cell proliferative
disorder of the
hematologic system can include hyperplasia, dysplasia, and metaplasia of cells
of the
hematologic system. Preferably, compositions of the present invention may be
used to treat a
cancer selected from the group consisting of a hematologic cancer of the
present invention or
a hematologic cell proliferative disorder of the present invention. A
hematologic cancer of
the present invention can include multiple myeloma, lymphoma (including
Hodgkin's
lymphoma, non-Hodgkin's lymphoma, childhood lymphomas, and lymphomas of
lymphocytic and cutaneous origin), leukemia (including childhood leukemia,
hairy-cell
leukemia, acute lymphocytic leukemia, acute myelocytic leukemia, chronic
lymphocytic
leukemia, chronic myelocytic leukemia, chronic myelogenous leukemia, and mast
cell
leukemia), myeloid neoplasms and mast cell neoplasms.
[0001333] A "cell proliferative disorder of the lung" is a cell proliferative
disorder
involving cells of the lung. Cell proliferative disorders of the lung can
include all forms of
cell proliferative disorders affecting lung cells. Cell proliferative
disorders of the lung can
include lung cancer, a precancer or precancerous condition of the lung, benign
growths or
lesions of the lung, and malignant growths or lesions of the lung, and
metastatic lesions in
tissue and organs in the body other than the lung. Preferably, compositions of
the present
invention may be used to treat lung cancer or cell proliferative disorders of
the lung. Lung
cancer can include all forms of cancer of the lung. Lung cancer can include
malignant lung
neoplasms, carcinoma in situ, typical carcinoid tumors, and atypical carcinoid
tumors. Lung
cancer can include small cell lung cancer ("SCLC"), non-small cell lung cancer
("NSCLC"),
squamous cell carcinoma, adenocarcinoma, small cell carcinoma, large cell
carcinoma,
174
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
adenosquamous cell carcinoma, and mesothelioma. Lung cancer can include "scar
carcinoma", bronchioalveolar carcinoma, giant cell carcinoma, spindle cell
carcinoma, and
large cell neuroendocrine carcinoma. Lung cancer can include lung neoplasms
having
histologic and ultrastructual heterogeneity (e.g., mixed cell types).
[0001334] Cell proliferative disorders of the lung can include all forms of
cell
proliferative disorders affecting lung cells. Cell proliferative disorders of
the lung can
include lung cancer, precancerous conditions of the lung. Cell proliferative
disorders of the
lung can include hyperplasia, metaplasia, and dysplasia of the lung. Cell
proliferative
disorders of the lung can include asbestos-induced hyperplasia, squamous
metaplasia, and
benign reactive mesothelial metaplasia. Cell proliferative disorders of the
lung can include
replacement of columnar epithelium with stratified squamous epithelium, and
mucosal
dysplasia. Individuals exposed to inhaled injurious environmental agents such
as cigarette
smoke and asbestos may be at increased risk for developing cell proliferative
disorders of the
lung. Prior lung diseases that may predispose individuals to development of
cell proliferative
disorders of the lung can include chronic interstitial lung disease,
necrotizing pulmonary
disease, scleroderma, rheumatoid disease, sarcoidosis, interstitial
pneumonitis, tuberculosis,
repeated pneumonias, idiopathic pulmonary fibrosis, granulomata, asbestosis,
fibrosing
alveolitis, and Hodgkin's disease.
[0001335] A "cell proliferative disorder of the colon" is a cell proliferative
disorder
involving cells of the colon. Preferably, the cell proliferative disorder of
the colon is colon
cancer. Preferably, compositions of the present invention may be used to treat
colon cancer
or cell proliferative disorders of the colon. Colon cancer can include all
forms of cancer of
the colon. Colon cancer can include sporadic and hereditary colon cancers.
Colon cancer
can include malignant colon neoplasms, carcinoma in situ, typical carcinoid
tumors, and
atypical carcinoid tumors. Colon cancer can include adenocarcinoma, squamous
cell
carcinoma, and adenosquamous cell carcinoma. Colon cancer can be associated
with a
hereditary syndrome selected from the group consisting of hereditary
nonpolyposis colorectal
cancer, familial adenomatous polyposis, Gardner's syndrome, Peutz-Jeghers
syndrome,
Turcot's syndrome and juvenile polyposis. Colon cancer can be caused by a
hereditary
syndrome selected from the group consisting of hereditary nonpolyposis
colorectal cancer,
familial adenomatous polyposis, Gardner's syndrome, Peutz-Jeghers syndrome,
Turcot's
syndrome and juvenile polyposis.
[0001336] Cell proliferative disorders of the colon can include all forms of
cell
proliferative disorders affecting colon cells. Cell proliferative disorders of
the colon can
175
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
include colon cancer, precancerous conditions of the colon, adenomatous polyps
of the colon
and metachronous lesions of the colon. A cell proliferative disorder of the
colon can include
adenoma. Cell proliferative disorders of the colon can be characterized by
hyperplasia,
metaplasia, and dysplasia of the colon. Prior colon diseases that may
predispose individuals
to development of cell proliferative disorders of the colon can include prior
colon cancer.
Current disease that may predispose individuals to development of cell
proliferative disorders
of the colon can include Crohn's disease and ulcerative colitis. A cell
proliferative disorder of
the colon can be associated with a mutation in a gene selected from the group
consisting of
p53, ras, FAP and DCC. An individual can have an elevated risk of developing a
cell
proliferative disorder of the colon due to the presence of a mutation in a
gene selected from
the group consisting of p53, ras, FAP and DCC.
[0001337] A "cell proliferative disorder of the pancreas" is a cell
proliferative disorder
involving cells of the pancreas. Cell proliferative disorders of the pancreas
can include all
forms of cell proliferative disorders affecting pancreatic cells. Cell
proliferative disorders of
the pancreas can include pancreas cancer, a precancer or precancerous
condition of the
pancreas, hyperplasia of the pancreas, and dysaplasia of the pancreas, benign
growths or
lesions of the pancreas, and malignant growths or lesions of the pancreas, and
metastatic
lesions in tissue and organs in the body other than the pancreas. Pancreatic
cancer includes
all forms of cancer of the pancreas. Pancreatic cancer can include ductal
adenocarcinoma,
adenosquamous carcinoma, pleomorphic giant cell carcinoma, mucinous
adenocarcinoma,
osteoclast-like giant cell carcinoma, mucinous cystadenocarcinoma, acinar
carcinoma,
unclassified large cell carcinoma, small cell carcinoma, pancreatoblastoma,
papillary
neoplasm, mucinous cystadenoma, papillary cystic neoplasm, and serous
cystadenoma.
Pancreatic cancer can also include pancreatic neoplasms having histologic and
ultrastructual
heterogeneity (e.g., mixed cell types).
[0001338] A "cell proliferative disorder of the prostate" is a cell
proliferative disorder
involving cells of the prostate. Cell proliferative disorders of the prostate
can include all
forms of cell proliferative disorders affecting prostate cells. Cell
proliferative disorders of the
prostate can include prostate cancer, a precancer or precancerous condition of
the prostate,
benign growths or lesions of the prostate, and malignant growths or lesions of
the prostate,
and metastatic lesions in tissue and organs in the body other than the
prostate. Cell
proliferative disorders of the prostate can include hyperplasia, metaplasia,
and dysplasia of
the prostate.
176
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001339] A "cell proliferative disorder of the skin" is a cell proliferative
disorder
involving cells of the skin. Cell proliferative disorders of the skin can
include all forms of
cell proliferative disorders affecting skin cells. Cell proliferative
disorders of the skin can
include a precancer or precancerous condition of the skin, benign growths or
lesions of the
skin, melanoma, malignant melanoma and other malignant growths or lesions of
the skin, and
metastatic lesions in tissue and organs in the body other than the skin. Cell
proliferative
disorders of the skin can include hyperplasia, metaplasia, and dysplasia of
the skin.
[0001340] A "cell proliferative disorder of the ovary" is a cell proliferative
disorder
involving cells of the ovary. Cell proliferative disorders of the ovary can
include all forms of
cell proliferative disorders affecting cells of the ovary. Cell proliferative
disorders of the
ovary can include a precancer or precancerous condition of the ovary, benign
growths or
lesions of the ovary, ovarian cancer, malignant growths or lesions of the
ovary, and
metastatic lesions in tissue and organs in the body other than the ovary. Cell
proliferative
disorders of the skin can include hyperplasia, metaplasia, and dysplasia of
cells of the ovary.
[0001341] A "cell proliferative disorder of the breast" is a cell
proliferative disorder
involving cells of the breast. Cell proliferative disorders of the breast can
include all forms of
cell proliferative disorders affecting breast cells. Cell proliferative
disorders of the breast can
include breast cancer, a precancer or precancerous condition of the breast,
benign growths or
lesions of the breast, and malignant growths or lesions of the breast, and
metastatic lesions in
tissue and organs in the body other than the breast. Cell proliferative
disorders of the breast
can include hyperplasia, metaplasia, and dysplasia of the breast.
[0001342] A cell proliferative disorder of the breast can be a precancerous
condition of
the breast. Compositions of the present invention may be used to treat a
precancerous
condition of the breast. A precancerous condition of the breast can include
atypical
hyperplasia of the breast, ductal carcinoma in situ (DCIS), intraductal
carcinoma, lobular
carcinoma in situ (LCIS), lobular neoplasia, and stage 0 or grade 0 growth or
lesion of the
breast (e.g., stage 0 or grade 0 breast cancer, or carcinoma in situ). A
precancerous condition
of the breast can be staged according to the TNM classification scheme as
accepted by the
American Joint Committee on Cancer (AJCC), where the primary tumor (T) has
been
assigned a stage of TO or Tis; and where the regional lymph nodes (N) have
been assigned a
stage of NO; and where distant metastasis (M) has been assigned a stage of MO.
[0001343] The cell proliferative disorder of the breast can be breast cancer.
Preferably,
compositions of the present invention may be used to treat breast cancer.
Breast cancer
includes all forms of cancer of the breast. Breast cancer can include primary
epithelial breast
177
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
cancers. Breast cancer can include cancers in which the breast is involved by
other tumors
such as lymphoma, sarcoma or melanoma. Breast cancer can include carcinoma of
the breast,
ductal carcinoma of the breast, lobular carcinoma of the breast,
undifferentiated carcinoma of
the breast, cystosarcoma phyllodes of the breast, angiosarcoma of the breast,
and primary
lymphoma of the breast. Breast cancer can include Stage I, II, IIIA, IIIB,
IIIC and IV breast
cancer. Ductal carcinoma of the breast can include invasive carcinoma,
invasive carcinoma
in situ with predominant intraductal component, inflammatory breast cancer,
and a ductal
carcinoma of the breast with a histologic type selected from the group
consisting of comedo,
mucinous (colloid), medullary, medullary with lymphcytic infiltrate,
papillary, scirrhous, and
tubular. Lobular carcinoma of the breast can include invasive lobular
carcinoma with
predominant in situ component, invasive lobular carcinoma, and infiltrating
lobular
carcinoma. Breast cancer can include Paget's disease, Paget's disease with
intraductal
carcinoma, and Paget's disease with invasive ductal carcinoma. Breast cancer
can include
breast neoplasms having histologic and ultrastructual heterogeneity (e.g.,
mixed cell types).
[0001344] Preferably, a compound of the present invention may be used to treat
breast
cancer. A breast cancer that is to be treated can include familial breast
cancer. A breast
cancer that is to be treated can include sporadic breast cancer. A breast
cancer that is to be
treated can arise in a male subject. A breast cancer that is to be treated can
arise in a female
subject. A breast cancer that is to be treated can arise in a premenopausal
female subject or a
postmenopausal female subject. A breast cancer that is to be treated can arise
in a subject
equal to or older than 30 years old, or a subject younger than 30 years old. A
breast cancer
that is to be treated has arisen in a subject equal to or older than 50 years
old, or a subject
younger than 50 years old. A breast cancer that is to be treated can arise in
a subject equal to
or older than 70 years old, or a subject younger than 70 years old.
[0001345] A breast cancer that is to be treated can be typed to identify a
familial or
spontaneous mutation in BRCA1, BRCA2, or p53. A breast cancer that is to be
treated can
be typed as having a HER2/neu gene amplification, as overexpressing HER2/neu,
or as
having a low, intermediate or high level of HER2/neu expression. A breast
cancer that is to
be treated can be typed for a marker selected from the group consisting of
estrogen receptor
(ER), progesterone receptor (PR), human epidermal growth factor receptor-2, Ki-
67, CA15-3,
CA 27-29, and c-Met. A breast cancer that is to be treated can be typed as ER-
unknown, ER-
rich or ER-poor. A breast cancer that is to be treated can be typed as ER-
negative or ER-
positive. ER-typing of a breast cancer may be performed by any reproducible
means. ER-
typing of a breast cancer may be performed as set forth in Onkologie 27: 175-
179 (2004). A
178
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
breast cancer that is to be treated can be typed as PR-unknown, PR-rich or PR-
poor. A breast
cancer that is to be treated can be typed as PR-negative or PR-positive. A
breast cancer that
is to be treated can be typed as receptor positive or receptor negative. A
breast cancer that is
to be treated can be typed as being associated with elevated blood levels of
CA 15-3, or CA
27-29, or both.
[0001346] A breast cancer that is to be treated can include a localized tumor
of the breast.
A breast cancer that is to be treated can include a tumor of the breast that
is associated with a
negative sentinel lymph node (SLN) biopsy. A breast cancer that is to be
treated can include
a tumor of the breast that is associated with a positive sentinel lymph node
(SLN) biopsy. A
breast cancer that is to be treated can include a tumor of the breast that is
associated with one
or more positive axillary lymph nodes, where the axillary lymph nodes have
been staged by
any applicable method. A breast cancer that is to be treated can include a
tumor of the breast
that has been typed as having nodal negative status (e.g., node-negative) or
nodal positive
status (e.g., node-positive). A breast cancer that is to be treated can
include a tumor of the
breast that has metastasized to other locations in the body. A breast cancer
that is to be
treated can be classified as having metastasized to a location selected from
the group
consisting of bone, lung, liver, or brain. A breast cancer that is to be
treated can be classified
according to a characteristic selected from the group consisting of
metastatic, localized,
regional, local-regional, locally advanced, distant, multicentric, bilateral,
ipsilateral,
contralateral, newly diagnosed, recurrent, and inoperable.
[0001347] A compound of the present invention may be used to treat or prevent
a cell
proliferative disorder of the breast, or to treat or prevent breast cancer, in
a subject having an
increased risk of developing breast cancer relative to the population at
large. A subject with
an increased risk of developing breast cancer relative to the population at
large is a female
subject with a family history or personal history of breast cancer. A subject
with an increased
risk of developing breast cancer relative to the population at large is a
female subject having a
germ-line or spontaneous mutation in BRCA1 or BRCA2, or both. A subject with
an
increased risk of developing breast cancer relative to the population at large
is a female
subject with a family history of breast cancer and a germ-line or spontaneous
mutation in
BRCA1 or BRCA2, or both. A subject with an increased risk of developing breast
cancer
relative to the population at large is a female who is greater than 30 years
old, greater than 40
years old, greater than 50 years old, greater than 60 years old, greater than
70 years old,
greater than 80 years old, or greater than 90 years old. A subject with an
increased risk of
developing breast cancer relative to the population at large is a subject with
atypical
179
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
hyperplasia of the breast, ductal carcinoma in situ (DCIS), intraductal
carcinoma, lobular
carcinoma in situ (LCIS), lobular neoplasia, or a stage 0 growth or lesion of
the breast (e.g.,
stage 0 or grade 0 breast cancer, or carcinoma in situ).
[0001348] A breast cancer that is to be treated can histologically graded
according to the
Scarff-Bloom-Richardson system, wherein a breast tumor has been assigned a
mitosis count
score of 1, 2, or 3; a nuclear pleiomorphism score of 1, 2, or 3; a tubule
formation score of 1,
2, or 3; and a total Scarff-Bloom-Richardson score of between 3 and 9. A
breast cancer that
is to be treated can be assigned a tumor grade according to the International
Consensus Panel
on the Treatment of Breast Cancer selected from the group consisting of grade
1, grade 1-2,
grade 2, grade 2-3, or grade 3.
[0001349] A cancer that is to be treated can be staged according to the
American Joint
Committee on Cancer (AJCC) TNM classification system, where the tumor (T) has
been
assigned a stage of TX, Ti, Tlmic, Tla, Tlb, Tlc, T2, T3, T4, T4a, T4b, T4c,
or T4d; and
where the regional lymph nodes (N) have been assigned a stage of NX, NO, Ni,
N2, N2a,
N2b, N3, N3a, N3b, or N3c; and where distant metastasis (M) can be assigned a
stage of MX,
MO, or MI. A cancer that is to be treated can be staged according to an
American Joint
Committee on Cancer (AJCC) classification as Stage I, Stage IIA, Stage IIB,
Stage IIIA,
Stage IIIB, Stage IIIC, or Stage IV. A cancer that is to be treated can be
assigned a grade
according to an AJCC classification as Grade GX (e.g., grade cannot be
assessed), Grade 1,
Grade 2, Grade 3 or Grade 4. A cancer that is to be treated can be staged
according to an
AJCC pathologic classification (pN) of pNX, pNO, PNO (I-), PNO (I+), PNO (mol-
), PNO
(mol+), PN 1, PN 1(mi), PN l a, PN l b, PN l c, pN2, pN2a, pN2b, pN3, pN3 a,
pN3b, or pN3 c.
[0001350] A cancer that is to be treated can include a tumor that has been
determined to
be less than or equal to about 2 centimeters in diameter. A cancer that is to
be treated can
include a tumor that has been determined to be from about 2 to about 5
centimeters in
diameter. A cancer that is to be treated can include a tumor that has been
determined to be
greater than or equal to about 3 centimeters in diameter. A cancer that is to
be treated can
include a tumor that has been determined to be greater than 5 centimeters in
diameter. A
cancer that is to be treated can be classified by microscopic appearance as
well differentiated,
moderately differentiated, poorly differentiated, or undifferentiated. A
cancer that is to be
treated can be classified by microscopic appearance with respect to mitosis
count (e.g.,
amount of cell division) or nuclear pleiomorphism (e.g., change in cells). A
cancer that is to
be treated can be classified by microscopic appearance as being associated
with areas of
necrosis (e.g., areas of dying or degenerating cells). A cancer that is to be
treated can be
180
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
classified as having an abnormal karyotype, having an abnormal number of
chromosomes, or
having one or more chromosomes that are abnormal in appearance. A cancer that
is to be
treated can be classified as being aneuploid, triploid, tetraploid, or as
having an altered ploidy.
A cancer that is to be treated can be classified as having a chromosomal
translocation, or a
deletion or duplication of an entire chromosome, or a region of deletion,
duplication or
amplification of a portion of a chromosome.
[0001351] A cancer that is to be treated can be evaluated by DNA cytometry,
flow
cytometry, or image cytometry. A cancer that is to be treated can be typed as
having 10%,
20%, 30%, 40%, 50%, 60%, 70%, 80%, or 90% of cells in the synthesis stage of
cell division
(e.g., in S phase of cell division). A cancer that is to be treated can be
typed as having a low
S-phase fraction or a high S-phase fraction.
[0001352] As used herein, a "normal cell" is a cell that cannot be classified
as part of a
"cell proliferative disorder". A normal cell lacks unregulated or abnormal
growth, or both,
that can lead to the development of an unwanted condition or disease.
Preferably, a normal
cell possesses normally functioning cell cycle checkpoint control mechanisms.
[0001353] As used herein, "contacting a cell" refers to a condition in which a
compound
or other composition of matter is in direct contact with a cell, or is close
enough to induce a
desired biological effect in a cell.
[0001354] As used herein, "candidate compound" refers to a compound of the
present
invention that has been or will be tested in one or more in vitro or in vivo
biological assays, in
order to determine if that compound is likely to elicit a desired biological
or medical response
in a cell, tissue, system, animal or human that is being sought by a
researcher or clinician. A
candidate compound is a compound of Formula I-IV. The biological or medical
response can
be the treatment of cancer. The biological or medical response can be
treatment or
prevention of a cell proliferative disorder. In vitro or in vivo biological
assays can include,
but are not limited to, enzymatic activity assays, electrophoretic mobility
shift assays,
reporter gene assays, in vitro cell viability assays, and the assays described
herein.
[0001355] As used herein, "monotherapy" refers to the administration of a
single active
or therapeutic compound to a subject in need thereof. Preferably, monotherapy
will involve
administration of a therapeutically effective amount of an active compound.
For example,
cancer monotherapy with one of the compound of the present invention, or a
pharmaceutically acceptable salt, prodrug, metabolite, analog or derivative
thereof, to a
subject in need of treatment of cancer. Monotherapy may be contrasted with
combination
therapy, in which a combination of multiple active compounds is administered,
preferably
181
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
with each component of the combination present in a therapeutically effective
amount. In
one aspect, monotherapy with a compound of the present invention is more
effective than
combination therapy in inducing a desired biological effect.
[0001356] As used herein, "treating" or "treat" describes the management and
care of a
patient for the purpose of combating a disease, condition, or disorder and
includes the
administration of a compound of the present invention to alleviate the
symptoms or
complications of a disease, condition or disorder, or to eliminate the
disease, condition or
disorder.
[0001357] A compound of the present invention, or a pharmaceutically
acceptable salt,
prodrug, metabolite, polymorph or solvate thereof, can also be used to prevent
a disease,
condition or disorder. As used herein, "preventing" or "prevent" describes
reducing or
eliminating the onset of the symptoms or complications of the disease,
condition or disorder.
[0001358] As used herein, the term "alleviate" is meant to describe a process
by which
the severity of a sign or symptom of a disorder is decreased. Importantly, a
sign or symptom
can be alleviated without being eliminated. In a preferred embodiment, the
administration of
pharmaceutical compositions of the invention leads to the elimination of a
sign or symptom,
however, elimination is not required. Effective dosages are expected to
decrease the
severity of a sign or symptom. For instance, a sign or symptom of a disorder
such as cancer,
which can occur in multiple locations, is alleviated if the severity of the
cancer is decreased
within at least one of multiple locations.
[0001359] As used herein, the term "severity" is meant to describe the
potential of cancer
to transform from a precancerous, or benign, state into a malignant state.
Alternatively, or in
addition, severity is meant to describe a cancer stage, for example, according
to the TNM
system (accepted by the International Union Against Cancer (UICC) and the
American Joint
Committee on Cancer (AJCC)) or by other art-recognized methods. Cancer stage
refers to the
extent or severity of the cancer, based on factors such as the location of the
primary tumor,
tumor size, number of tumors, and lymph node involvement (spread of cancer
into lymph
nodes). Alternatively, or in addition, severity is meant to describe the tumor
grade by art-
recognized methods (see, National Cancer Institute, www.cancer.gov). Tumor
grade is a
system used to classify cancer cells in terms of how abnormal they look under
a microscope
and how quickly the tumor is likely to grow and spread. Many factors are
considered when
determining tumor grade, including the structure and growth pattern of the
cells. The specific
factors used to determine tumor grade vary with each type of cancer. Severity
also
describes a histologic grade, also called differentiation, which refers to how
much the tumor
182
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
cells resemble normal cells of the same tissue type (see, National Cancer
Institute,
www.cancer.gov). Furthermore, severity describes a nuclear grade, which refers
to the size
and shape of the nucleus in tumor cells and the percentage of tumor cells that
are dividing
(see, National Cancer Institute, www.cancer.gov).
[0001360] In another aspect of the invention, severity describes the degree to
which a
tumor has secreted growth factors, degraded the extracellular matrix, become
vascularized,
lost adhesion to juxtaposed tissues, or metastasized. Moreover, severity
describes the number
of locations to which a primary tumor has metastasized. Finally, severity
includes the
difficulty of treating tumors of varying types and locations. For example,
inoperable tumors,
those cancers which have greater access to multiple body systems
(hematological and
immunological tumors), and those which are the most resistant to traditional
treatments are
considered most severe. In these situations, prolonging the life expectancy of
the subject
and/or reducing pain, decreasing the proportion of cancerous cells or
restricting cells to one
system, and improving cancer stage/tumor grade/histological grade/nuclear
grade are
considered alleviating a sign or symptom of the cancer.
[0001361] As used herein the term "symptom" is defined as an indication of
disease,
illness, injury, or that something is not right in the body. Symptoms are felt
or noticed by the
individual experiencing the symptom, but may not easily be noticed by others.
Others are defined
as non-health-care professionals.
[0001362] As used herein the term "sign" is also defined as an indication that
something
is not right in the body. But signs are defined as things that can be seen by
a doctor, nurse, or
other health care professional.
[0001363] Cancer is a group of diseases that may cause almost any sign or
symptom. The
signs and symptoms will depend on where the cancer is, the size of the cancer,
and how much
it affects the nearby organs or structures. If a cancer spreads
(metastasizes), then symptoms may
appear in different parts of the body.
[0001364] As a cancer grows, it begins to push on nearby organs, blood
vessels, and
nerves. This pressure creates some of the signs and symptoms of cancer. If the
cancer is in a
critical area, such as certain parts of the brain, even the smallest tumor can
cause early symptoms.
[0001365] But sometimes cancers start in places where it does not cause any
symptoms
until the cancer has grown quite large. Pancreas cancers, for example, do not
usually grow
large enough to be felt from the outside of the body. Some pancreatic cancers
do not cause
symptoms until they begin to grow around nearby nerves (this causes a
backache). Others grow
around the bile duct, which blocks the flow of bile and leads to a yellowing
of the skin known
183
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
as jaundice. By the time a pancreatic cancer causes these signs or symptoms,
it has usually
reached an advanced stage.
[0001366] A cancer may also cause symptoms such as fever, fatigue, or weight
loss. This
may be because cancer cells use up much of the body's energy supply or release
substances
that change the body's metabolism. Or the cancer may cause the immune system
to react in
ways that produce these symptoms.
[0001367] Sometimes, cancer cells release substances into the bloodstream that
cause
symptoms not usually thought to result from cancers. For example, some cancers
of the
pancreas can release substances which cause blood clots to develop in veins of
the legs. Some
lung cancers make hormone-like substances that affect blood calcium levels,
affecting nerves
and muscles and causing weakness and dizziness
[0001368] Cancer presents several general signs or symptoms that occur when a
variety
of subtypes of cancer cells are present. Most people with cancer will lose
weight at some time
with their disease. An unexplained (unintentional) weight loss of 10 pounds or
more may be
the first sign of cancer, particularly cancers of the pancreas, stomach,
esophagus, or lung.
[0001369] Fever is very common with cancer, but is more often seen in advanced
disease.
Almost all patients with cancer will have fever at some time, especially if
the cancer or its
treatment affects the immune system and makes it harder for the body to fight
infection. Less
often, fever may be an early sign of cancer, such as with leukemia or
lymphoma.
[0001370] Fatigue may be an important symptom as cancer progresses. It may
happen
early, though, in cancers such as with leukemia, or if the cancer is causing
an ongoing loss of
blood, as in some colon or stomach cancers.
[0001371] Pain may be an early symptom with some cancers such as bone cancers
or
testicular cancer. But most often pain is a symptom of advanced disease.
[0001372] Along with cancers of the skin (see next section), some internal
cancers can
cause skin signs that can be seen. These changes include the skin looking
darker
(hyperpigmentation), yellow (jaundice), or red (erythema); itching; or
excessive hair growth.
[0001373] Alternatively, or in addition, cancer subtypes present specific
signs or
symptoms. Changes in bowel habits or bladder function could indicate cancer.
Long-term
constipation, diarrhea, or a change in the size of the stool may be a sign of
colon cancer. Pain
with urination, blood in the urine, or a change in bladder function (such as
more frequent or
less frequent urination) could be related to bladder or prostate cancer.
[0001374] Changes in skin condition or appearance of a new skin condition
could
indicate cancer. Skin cancers may bleed and look like sores that do not heal.
A long-lasting
184
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
sore in the mouth could be an oral cancer, especially in patients who smoke,
chew tobacco, or
frequently drink alcohol. Sores on the penis or vagina may either be signs of
infection or an
early cancer.
[0001375] Unusual bleeding or discharge could indicate cancer. Unusual
bleeding can
happen in either early or advanced cancer. Blood in the sputum (phlegm) may be
a sign of
lung cancer. Blood in the stool (or a dark or black stool) could be a sign of
colon or rectal
cancer. Cancer of the cervix or the endometrium (lining of the uterus) can
cause vaginal
bleeding. Blood in the urine may be a sign of bladder or kidney cancer. A
bloody discharge from
the nipple may be a sign of breast cancer.
[0001376] A thickening or lump in the breast or in other parts of the body
could indicate the
presence of a cancer. Many cancers can be felt through the skin, mostly in the
breast, testicle,
lymph nodes (glands), and the soft tissues of the body. A lump or thickening
may be an early
or late sign of cancer. Any lump or thickening could be indicative of cancer,
especially if the
formation is new or has grown in size.
[0001377] Indigestion or trouble swallowing could indicate cancer. While these
symptoms
commonly have other causes, indigestion or swallowing problems may be a sign
of cancer of
the esophagus, stomach, or pharynx (throat).
[0001378] Recent changes in a wart or mole could be indicative of cancer. Any
wart, mole,
or freckle that changes in color, size, or shape, or loses its definite
borders indicates the
potential development of cancer. For example, the skin lesion may be a
melanoma.
[0001379] A persistent cough or hoarseness could be indicative of cancer. A
cough that
does not go away may be a sign of lung cancer. Hoarseness can be a sign of
cancer of the
larynx (voice box) or thyroid.
[0001380] While the signs and symptoms listed above are the more common ones
seen
with cancer, there are many others that are less common and are not listed
here. However, all
art-recognized signs and symptoms of cancer are contemplated and encompassed
by the instant
invention.
[0001381] Treating cancer can result in a reduction in size of a tumor. A
reduction in size
of a tumor may also be referred to as "tumor regression". Preferably, after
treatment, tumor
size is reduced by 5% or greater relative to its size prior to treatment; more
preferably, tumor
size is reduced by 10% or greater; more preferably, reduced by 20% or greater;
more
preferably, reduced by 30% or greater; more preferably, reduced by 40% or
greater; even
more preferably, reduced by 50% or greater; and most preferably, reduced by
greater than
185
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
75% or greater. Size of a tumor may be measured by any reproducible means of
measurement. The size of a tumor may be measured as a diameter of the tumor.
[0001382] Treating cancer can result in a reduction in tumor volume.
Preferably, after
treatment, tumor volume is reduced by 5% or greater relative to its size prior
to treatment;
more preferably, tumor volume is reduced by 10% or greater; more preferably,
reduced by
20% or greater; more preferably, reduced by 30% or greater; more preferably,
reduced by
40% or greater; even more preferably, reduced by 50% or greater; and most
preferably,
reduced by greater than 75% or greater. Tumor volume may be measured by any
reproducible means of measurement.
[0001383] Treating cancer results in a decrease in number of tumors.
Preferably, after
treatment, tumor number is reduced by 5% or greater relative to number prior
to treatment;
more preferably, tumor number is reduced by 10% or greater; more preferably,
reduced by
20% or greater; more preferably, reduced by 30% or greater; more preferably,
reduced by
40% or greater; even more preferably, reduced by 50% or greater; and most
preferably,
reduced by greater than 75%. Number of tumors may be measured by any
reproducible
means of measurement. The number of tumors may be measured by counting tumors
visible
to the naked eye or at a specified magnification. Preferably, the specified
magnification is 2x,
3x, 4x, 5x, lOx, or 50x.
[0001384] Treating cancer can result in a decrease in number of metastatic
lesions in
other tissues or organs distant from the primary tumor site. Preferably, after
treatment, the
number of metastatic lesions is reduced by 5% or greater relative to number
prior to treatment;
more preferably, the number of metastatic lesions is reduced by 10% or
greater; more
preferably, reduced by 20% or greater; more preferably, reduced by 30% or
greater; more
preferably, reduced by 40% or greater; even more preferably, reduced by 50% or
greater; and
most preferably, reduced by greater than 75%. The number of metastatic lesions
may be
measured by any reproducible means of measurement. The number of metastatic
lesions may
be measured by counting metastatic lesions visible to the naked eye or at a
specified
magnification. Preferably, the specified magnification is 2x, 3x, 4x, 5x, l
Ox, or 50x.
[0001385] Treating cancer can result in an increase in average survival time
of a
population of treated subjects in comparison to a population receiving carrier
alone.
Preferably, the average survival time is increased by more than 30 days; more
preferably, by
more than 60 days; more preferably, by more than 90 days; and most preferably,
by more
than 120 days. An increase in average survival time of a population may be
measured by any
reproducible means. An increase in average survival time of a population may
be measured,
186
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
for example, by calculating for a population the average length of survival
following
initiation of treatment with an active compound. An increase in average
survival time of a
population may also be measured, for example, by calculating for a population
the average
length of survival following completion of a first round of treatment with an
active compound.
[0001386] Treating cancer can result in an increase in average survival time
of a
population of treated subjects in comparison to a population of untreated
subjects. Preferably,
the average survival time is increased by more than 30 days; more preferably,
by more than
60 days; more preferably, by more than 90 days; and most preferably, by more
than 120 days.
An increase in average survival time of a population may be measured by any
reproducible
means. An increase in average survival time of a population may be measured,
for example,
by calculating for a population the average length of survival following
initiation of treatment
with an active compound. An increase in average survival time of a population
may also be
measured, for example, by calculating for a population the average length of
survival
following completion of a first round of treatment with an active compound.
[0001387] Treating cancer can result in increase in average survival time of a
population
of treated subjects in comparison to a population receiving monotherapy with a
drug that is
not a compound of the present invention, or a pharmaceutically acceptable
salt, prodrug,
metabolite, analog or derivative thereof. Preferably, the average survival
time is increased by
more than 30 days; more preferably, by more than 60 days; more preferably, by
more than 90
days; and most preferably, by more than 120 days. An increase in average
survival time of a
population may be measured by any reproducible means. An increase in average
survival
time of a population may be measured, for example, by calculating for a
population the
average length of survival following initiation of treatment with an active
compound. An
increase in average survival time of a population may also be measured, for
example, by
calculating for a population the average length of survival following
completion of a first
round of treatment with an active compound.
[0001388] Treating cancer can result in a decrease in the mortality rate of a
population of
treated subjects in comparison to a population receiving carrier alone.
Treating cancer can
result in a decrease in the mortality rate of a population of treated subjects
in comparison to
an untreated population. Treating cancer can result in a decrease in the
mortality rate of a
population of treated subjects in comparison to a population receiving
monotherapy with a
drug that is not a compound of the present invention, or a pharmaceutically
acceptable salt,
prodrug, metabolite, analog or derivative thereof. Preferably, the mortality
rate is decreased
by more than 2%; more preferably, by more than 5%; more preferably, by more
than 10%;
187
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
and most preferably, by more than 25%. A decrease in the mortality rate of a
population of
treated subjects may be measured by any reproducible means. A decrease in the
mortality
rate of a population may be measured, for example, by calculating for a
population the
average number of disease-related deaths per unit time following initiation of
treatment with
an active compound. A decrease in the mortality rate of a population may also
be measured,
for example, by calculating for a population the average number of disease-
related deaths per
unit time following completion of a first round of treatment with an active
compound.
[0001389] Treating cancer can result in a decrease in tumor growth rate.
Preferably, after
treatment, tumor growth rate is reduced by at least 5% relative to number
prior to treatment;
more preferably, tumor growth rate is reduced by at least 10%; more
preferably, reduced by
at least 20%; more preferably, reduced by at least 30%; more preferably,
reduced by at least
40%; more preferably, reduced by at least 50%; even more preferably, reduced
by at least
50%; and most preferably, reduced by at least 75%. Tumor growth rate may be
measured by
any reproducible means of measurement. Tumor growth rate can be measured
according to a
change in tumor diameter per unit time.
[0001390] Treating cancer can result in a decrease in tumor regrowth.
Preferably, after
treatment, tumor regrowth is less than 5%; more preferably, tumor regrowth is
less than 10%;
more preferably, less than 20%; more preferably, less than 30%; more
preferably, less than
40%; more preferably, less than 50%; even more preferably, less than 50%; and
most
preferably, less than 75%. Tumor regrowth may be measured by any reproducible
means of
measurement. Tumor regrowth is measured, for example, by measuring an increase
in the
diameter of a tumor after a prior tumor shrinkage that followed treatment. A
decrease in
tumor regrowth is indicated by failure of tumors to reoccur after treatment
has stopped.
[0001391] Treating or preventing a cell proliferative disorder can result in a
reduction in
the rate of cellular proliferation. Preferably, after treatment, the rate of
cellular proliferation is
reduced by at least 5%; more preferably, by at least 10%; more preferably, by
at least 20%;
more preferably, by at least 30%; more preferably, by at least 40%; more
preferably, by at
least 50%; even more preferably, by at least 50%; and most preferably, by at
least 75%. The
rate of cellular proliferation may be measured by any reproducible means of
measurement.
The rate of cellular proliferation is measured, for example, by measuring the
number of
dividing cells in a tissue sample per unit time.
[0001392] Treating or preventing a cell proliferative disorder can result in a
reduction in
the proportion of proliferating cells. Preferably, after treatment, the
proportion of
proliferating cells is reduced by at least 5%; more preferably, by at least
10%; more
188
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
preferably, by at least 20%; more preferably, by at least 30%; more
preferably, by at least
40%; more preferably, by at least 50%; even more preferably, by at least 50%;
and most
preferably, by at least 75%. The proportion of proliferating cells may be
measured by any
reproducible means of measurement. Preferably, the proportion of proliferating
cells is
measured, for example, by quantifying the number of dividing cells relative to
the number of
nondividing cells in a tissue sample. The proportion of proliferating cells
can be equivalent
to the mitotic index.
[0001393] Treating or preventing a cell proliferative disorder can result in a
decrease in
size of an area or zone of cellular proliferation. Preferably, after
treatment, size of an area or
zone of cellular proliferation is reduced by at least 5% relative to its size
prior to treatment;
more preferably, reduced by at least 10%; more preferably, reduced by at least
20%; more
preferably, reduced by at least 30%; more preferably, reduced by at least 40%;
more
preferably, reduced by at least 50%; even more preferably, reduced by at least
50%; and most
preferably, reduced by at least 75%. Size of an area or zone of cellular
proliferation may be
measured by any reproducible means of measurement. The size of an area or zone
of cellular
proliferation may be measured as a diameter or width of an area or zone of
cellular
proliferation.
[0001394] Treating or preventing a cell proliferative disorder can result in a
decrease in
the number or proportion of cells having an abnormal appearance or morphology.
Preferably,
after treatment, the number of cells having an abnormal morphology is reduced
by at least 5%
relative to its size prior to treatment; more preferably, reduced by at least
10%; more
preferably, reduced by at least 20%; more preferably, reduced by at least 30%;
more
preferably, reduced by at least 40%; more preferably, reduced by at least 50%;
even more
preferably, reduced by at least 50%; and most preferably, reduced by at least
75%. An
abnormal cellular appearance or morphology may be measured by any reproducible
means of
measurement. An abnormal cellular morphology can be measured by microscopy,
e.g., using
an inverted tissue culture microscope. An abnormal cellular morphology can
take the form of
nuclear pleiomorphism.
[0001395] As used herein, the term "selectively" means tending to occur at a
higher
frequency in one population than in another population. The compared
populations can be
cell populations. Preferably, a compound of the present invention, or a
pharmaceutically
acceptable salt, prodrug, metabolite, analog or derivative thereof, acts
selectively on a cancer
or precancerous cell but not on a normal cell. Preferably, a compound of the
present
invention, or a pharmaceutically acceptable salt, prodrug, metabolite, analog
or derivative
189
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
thereof, acts selectively to modulate one molecular target (e.g., a target
kinase) but does not
significantly modulate another molecular target (e.g., a non-target kinase).
The invention
also provides a method for selectively inhibiting the activity of an enzyme,
such as a kinase.
Preferably, an event occurs selectively in population A relative to population
B if it occurs
greater than two times more frequently in population A as compared to
population B. An
event occurs selectively if it occurs greater than five times more frequently
in population A.
An event occurs selectively if it occurs greater than ten times more
frequently in population
A; more preferably, greater than fifty times; even more preferably, greater
than 100 times;
and most preferably, greater than 1000 times more frequently in population A
as compared to
population B. For example, cell death would be said to occur selectively in
cancer cells if it
occurred greater than twice as frequently in cancer cells as compared to
normal cells.
[0001396] A compound of the present invention or a pharmaceutically acceptable
salt,
prodrug, metabolite, analog or derivative thereof, can modulate the activity
of a molecular
target (e.g., a target kinase). Modulating refers to stimulating or inhibiting
an activity of a
molecular target. Preferably, a compound of the present invention modulates
the activity of a
molecular target if it stimulates or inhibits the activity of the molecular
target by at least 2-
fold relative to the activity of the molecular target under the same
conditions but lacking only
the presence of said compound. More preferably, a compound of the present
invention
modulates the activity of a molecular target if it stimulates or inhibits the
activity of the
molecular target by at least 5-fold, at least 10-fold, at least 20-fold, at
least 50-fold, at least
100-fold relative to the activity of the molecular target under the same
conditions but lacking
only the presence of said compound. The activity of a molecular target may be
measured by
any reproducible means. The activity of a molecular target may be measured in
vitro or in
vivo. For example, the activity of a molecular target may be measured in vitro
by an
enzymatic activity assay or a DNA binding assay, or the activity of a
molecular target may be
measured in vivo by assaying for expression of a reporter gene.
[0001397] A compound of the present invention, or a pharmaceutically
acceptable salt,
prodrug, metabolite, analog or derivative thereof, does not significantly
modulate the activity
of a molecular target if the addition of the compound does not stimulate or
inhibit the activity
of the molecular target by greater than 10% relative to the activity of the
molecular target
under the same conditions but lacking only the presence of said compound.
[0001398] As used herein, the term "isozyme selective" means preferential
inhibition or
stimulation of a first isoform of an enzyme in comparison to a second isoform
of an enzyme
(e.g., preferential inhibition or stimulation of a kinase isozyme alpha in
comparison to a
190
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
kinase isozyme beta). Preferably, a compound of the present invention
demonstrates a
minimum of a four fold differential, preferably a ten fold differential, more
preferably a fifty
fold differential, in the dosage required to achieve a biological effect.
Preferably, a
compound of the present invention demonstrates this differential across the
range of
inhibition, and the differential is exemplified at the IC50, i.e., a 50%
inhibition, for a
molecular target of interest.
[0001399] Administering a compound of the present invention, or a
pharmaceutically
acceptable salt, prodrug, metabolite, analog or derivative thereof, to a cell
or a subject in need
thereof can result in modulation (i.e., stimulation or inhibition) of an
activity of a kinase of
interest.
[0001400] The present invention provides methods to assess biological activity
of the
compounds of Formula I-IV. In one method, an assay based on enzymatic activity
can be
utilized. In one specific enzymatic activity assay, the enzymatic activity is
from a kinase. As
used herein, "kinase" refers to a large class of enzymes which catalyze the
transfer of the 7-
phosphate from ATP to the hydroxyl group on the side chain of Ser/Thr or Tyr
in proteins
and peptides and are intimately involved in the control of various important
cell functions,
perhaps most notably: signal transduction, differentiation, and proliferation.
There are
estimated to be about 2,000 distinct protein kinases in the human body, and
although each of
these phosphorylate particular protein/peptide substrates, they all bind the
same second
substrate ATP in a highly conserved pocket. About 50% of the known oncogene
products are
protein tyrosine kinases (PTKs), and their kinase activity has been shown to
lead to cell
transformation. Preferably, the kinase assayed is a tyrosine kinase.
[0001401] A change in enzymatic activity caused by compounds of the present
invention
can be measured in the disclosed assays. The change in enzymatic activity can
be
characterized by the change in the extent of phosphorylation of certain
substrates. As used
herein, "phosphorylation" refers to the addition of phosphate groups to a
substrate, including
proteins and organic molecules; and, plays an important role in regulating the
biological
activities of proteins. Preferably, the phosphorylation assayed and measured
involves the
addition of phosphate groups to tyrosine residues. The substrate can be a
peptide or protein.
[0001402] In some assays, immunological reagents, e.g., antibodies and
antigens, are
employed. Fluorescence can be utilized in the measurement of enzymatic
activity in some
assays. As used herein, "fluorescence" refers to a process through which a
molecule emits a
photon as a result of absorbing an incoming photon of higher energy by the
same molecule.
191
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Specific methods for assessing the biological activity of the disclosed
compounds are
described in the examples.
[0001403] As used herein, an activity of c-Met refers to any biological
function or
activity that is carried out by c-Met. For example, a function of c-Met
includes
phosphorylation of downstream target proteins. Other functions of c-Met
include
autophosphorylation, binding of adaptor proteins such as Gab-1, Grb-2, She,
SHP2 and c-Cbl,
and activation of signal transducers such as Ras, Src, P13K, PLC-y, STATs,
ERK1 and 2 and
FAK.
[0001404] Administering a compound of the present invention, or a
pharmaceutically
acceptable salt, prodrug, metabolite, analog or derivative thereof, to a cell
or a subject in need
thereof results in modulation (i.e., stimulation or inhibition) of an activity
of an intracellular
target (e.g., substrate). Several intracellular targets can be modulated with
the compounds of
the present invention, including, but not limited to, adaptor proteins such as
Gab-1, Grb-2,
She, SHP2 and c-Cbl, and signal transducers such as Ras, Src, P13K, PLC-y,
STATs, ERK1
and 2 and FAK.
[0001405] Activating refers to placing a composition of matter (e.g., protein
or nucleic
acid) in a state suitable for carrying out a desired biological function. A
composition of
matter capable of being activated also has an unactivated state. An activated
composition of
matter may have an inhibitory or stimulatory biological function, or both.
[0001406] Elevation refers to an increase in a desired biological activity of
a composition
of matter (e.g., a protein or a nucleic acid). Elevation may occur through an
increase in
concentration of a composition of matter.
[0001407] As used herein, "a cell cycle checkpoint pathway" refers to a
biochemical
pathway that is involved in modulation of a cell cycle checkpoint. A cell
cycle checkpoint
pathway may have stimulatory or inhibitory effects, or both, on one or more
functions
comprising a cell cycle checkpoint. A cell cycle checkpoint pathway is
comprised of at least
two compositions of matter, preferably proteins, both of which contribute to
modulation of a
cell cycle checkpoint. A cell cycle checkpoint pathway may be activated
through an
activation of one or more members of the cell cycle checkpoint pathway.
Preferably, a cell
cycle checkpoint pathway is a biochemical signaling pathway.
[0001408] As used herein, "cell cycle checkpoint regulator" refers to a
composition of
matter that can function, at least in part, in modulation of a cell cycle
checkpoint. A cell
cycle checkpoint regulator may have stimulatory or inhibitory effects, or
both, on one or
192
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
more functions comprising a cell cycle checkpoint. A cell cycle checkpoint
regulator can be
a protein or not a protein.
[0001409] Treating cancer or a cell proliferative disorder can result in cell
death, and
preferably, cell death results in a decrease of at least 10% in number of
cells in a population.
More preferably, cell death means a decrease of at least 20%; more preferably,
a decrease of
at least 30%; more preferably, a decrease of at least 40%; more preferably, a
decrease of at
least 50%; most preferably, a decrease of at least 75%. Number of cells in a
population may
be measured by any reproducible means. A number of cells in a population can
be measured
by fluorescence activated cell sorting (FACS), immunofluorescence microscopy
and light
microscopy. Methods of measuring cell death are as shown in Li et at., Proc
Natl Acad Sci U
S A. 100(5): 2674-8, 2003. In an aspect, cell death occurs by apoptosis.
[0001410] Preferably, an effective amount of a compound of the present
invention, or a
pharmaceutically acceptable salt, prodrug, metabolite, analog or derivative
thereof is not
significantly cytotoxic to normal cells. A therapeutically effective amount of
a compound is
not significantly cytotoxic to normal cells if administration of the compound
in a
therapeutically effective amount does not induce cell death in greater than
10% of normal
cells. A therapeutically effective amount of a compound does not significantly
affect the
viability of normal cells if administration of the compound in a
therapeutically effective
amount does not induce cell death in greater than 10% of normal cells. In an
aspect, cell
death occurs by apoptosis.
[0001411] Contacting a cell with a compound of the present invention, or a
pharmaceutically acceptable salt, prodrug, metabolite, analog or derivative
thereof, can
induce or activate cell death selectively in cancer cells. Administering to a
subject in need
thereof a compound of the present invention, or a pharmaceutically acceptable
salt, prodrug,
metabolite, analog or derivative thereof, can induce or activate cell death
selectively in cancer
cells. Contacting a cell with a compound of the present invention, or a
pharmaceutically
acceptable salt, prodrug, metabolite, analog or derivative thereof, can induce
cell death
selectively in one or more cells affected by a cell proliferative disorder.
Preferably,
administering to a subject in need thereof a compound of the present
invention, or a
pharmaceutically acceptable salt, prodrug, metabolite, analog or derivative
thereof, induces
cell death selectively in one or more cells affected by a cell proliferative
disorder.
[0001412] The present invention relates to a method of treating or preventing
cancer by
administering a compound of the present invention, or a pharmaceutically
acceptable salt,
prodrug, metabolite, analog or derivative thereof to a subject in need
thereof, where
193
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
administration of the compound of the present invention, or a pharmaceutically
acceptable
salt, prodrug, metabolite, analog or derivative thereof results in one or more
of the following:
accumulation of cells in G1 and/or S phase of the cell cycle, cytotoxicity via
cell death in
cancer cells without a significant amount of cell death in normal cells,
antitumor activity in
animals with a therapeutic index of at least 2, and activation of a cell cycle
checkpoint. As
used herein, "therapeutic index" is the maximum tolerated dose divided by the
efficacious
dose.
[0001413] One skilled in the art may refer to general reference texts for
detailed
descriptions of known techniques discussed herein or equivalent techniques.
These texts
include Ausubel et al., Current Protocols in Molecular Biology, John Wiley and
Sons, Inc.
(2005); Sambrook et at., Molecular Cloning, A Laboratory Manual (3rd edition),
Cold Spring
Harbor Press, Cold Spring Harbor, New York (2000); Coligan et al., Current
Protocols in
Immunology, John Wiley & Sons, N.Y.; Enna et al., Current Protocols in
Pharmacology,
John Wiley & Sons, N.Y.; Fingl et al., The Pharmacological Basis of
Therapeutics (1975),
Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton, PA, 18th
edition (1990).
These texts can, of course, also be referred to in making or using an aspect
of the invention
[0001414] As used herein, "combination therapy" or "co-therapy" includes the
administration of a compound of the present invention and at least a second
agent as part of a
specific treatment regimen intended to provide the beneficial effect from the
co-action of
these therapeutic agents. The beneficial effect of the combination includes,
but is not limited
to, pharmacokinetic or pharmacodynamic co-action resulting from the
combination of
therapeutic agents. Administration of these therapeutic agents in combination
typically is
carried out over a defined time period (usually minutes, hours, days or weeks
depending upon
the combination selected). "Combination therapy" may be, but generally is not,
intended to
encompass the administration of two or more of these therapeutic agents as
part of separate
monotherapy regimens that incidentally and arbitrarily result in the
combinations of the
present invention.
[0001415] "Combination therapy" is intended to embrace administration of these
therapeutic agents in a sequential manner, wherein each therapeutic agent is
administered at a
different time, as well as administration of these therapeutic agents, or at
least two of the
therapeutic agents, in a substantially simultaneous manner. Substantially
simultaneous
administration can be accomplished, for example, by administering to the
subject a single
capsule having a fixed ratio of each therapeutic agent or in multiple, single
capsules for each
of the therapeutic agents. Sequential or substantially simultaneous
administration of each
194
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
therapeutic agent can be effected by any appropriate route including, but not
limited to, oral
routes, intravenous routes, intramuscular routes, and direct absorption
through mucous
membrane tissues. The therapeutic agents can be administered by the same route
or by
different routes. For example, a first therapeutic agent of the combination
selected may be
administered by intravenous injection while the other therapeutic agents of
the combination
may be administered orally. Alternatively, for example, all therapeutic agents
may be
administered orally or all therapeutic agents may be administered by
intravenous injection.
The sequence in which the therapeutic agents are administered is not narrowly
critical.
[0001416] "Combination therapy" also embraces the administration of the
therapeutic
agents as described above in further combination with other biologically
active ingredients
and non-drug therapies (e.g., surgery or radiation treatment). Where the
combination therapy
further comprises a non-drug treatment, the non-drug treatment may be
conducted at any
suitable time so long as a beneficial effect from the co-action of the
combination of the
therapeutic agents and non-drug treatment is achieved. For example, in
appropriate cases, the
beneficial effect is still achieved when the non-drug treatment is temporally
removed from
the administration of the therapeutic agents, perhaps by days or even weeks.
[0001417] A compound of the present invention, or a pharmaceutically
acceptable salt,
prodrug, metabolite, analog or derivative thereof, may be administered in
combination with a
second chemotherapeutic agent. The second chemotherapeutic agent (also
referred to as an
anti-neoplastic agent or anti-proliferative agent) can be an alkylating agent;
an antibiotic; an anti-
metabolite; a detoxifying agent; an interferon; a polyclonal or monoclonal
antibody; an EGFR inhibitor; a
HER2 inhibitor; a histone deacetylase inhibitor, a hormone; a mitotic
inhibitor; an MTOR inhibitor; a multi-
kinase inhibitor, a serine/threonine kinase inhibitor, a tyrosine kinase
inhibitors; a VEGF/VEGFR inhibitor;
a taxane or taxane derivative, an aromatase inhibitor, an anthracycline, a
microtubule
targeting drug, a topoisomerase poison drug, an inhibitor of a molecular
target or enzyme
(e.g., a kinase inhibitor), a cytidine analogue drug or any chemotherapeutic,
anti-neoplastic or
anti-proliferative agent listed in www.cancer.org/docroot/cdg/cdg_O.asp.
[0001418] Exemplary alkylating agents include, but are not limited to,
cyclophosphamide
(Cytoxan; Neosar); chlorambucil (Leukeran); melphalan (Alkeran); carmustine
(BiCNU);
busulfan (Busulfex); lomustine (CeeNU); dacarbazine (DTIC-Dome); oxaliplatin
(Eloxatin);
carmustine (Gliadel); ifosfamide (Ifex); mechlorethamine (Mustargen); busulfan
(Myleran);
carboplatin (Paraplatin); cisplatin (CDDP; Platinol); temozolomide (Temodar);
thiotepa
(Thioplex); bendamustine (Treanda); or streptozocin (Zanosar).
195
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001419] Exemplary antibiotics include, but are not limited to, doxorubicin
(Adriamycin);
doxorubicin liposomal (Doxil); mitoxantrone (Novantrone); bleomycin
(Blenoxane);
daunorubicin (Cerubidine); daunorubicin liposomal (DaunoXome); dactinomycin
(Cosmegen); epirubicin (Ellence); idarubicin (Idamycin); plicamycin
(Mithracin); mitomycin
(Mutamycin); pentostatin (Nipent); or valrubicin (Valstar).
[0001420] Exemplary anti metabolites include, but are not limited to,
fluorouracil (Adrucil);
capecitabine (Xeloda); hydroxyurea (Hydrea); mercaptopurine (Purinethol);
pemetrexed
(Alimta); fludarabine (Fludara); nelarabine (Arranon); cladribine (Cladribine
Novaplus);
clofarabine (Clolar); cytarabine (Cytosar-U); decitabine (Dacogen); cytarabine
liposomal
(DepoCyt); hydroxyurea (Droxia); pralatrexate (Folotyn); floxuridine (FUDR);
gemcitabine
(Gemzar); cladribine (Leustatin); fludarabine (Oforta); methotrexate (MTX;
Rheumatrex);
methotrexate (Trexall); thioguanine (Tabloid); TS-1 or cytarabine (Tarabine
PFS).
[0001421] Exemplary detoxifying agents include, but are not limited to,
amifostine (Ethyol) or
mesna (Mesnex).
[0001422] Exemplary interferon include, but are not limited to, interferon
alfa-2b (Intron A) or
interferon alfa-2a (Roferon-A).
[0001423] Exemplary polyclonal or monoclonal antibodies include, but are not
limited to,
trastuzumab (Herceptin); ofatumumab (Arzerra); bevacizumab (Avastin);
rituximab
(Rituxan); cetuximab (Erbitux); panitumumab (Vectibix); tositumomab/io dine
131
tositumomab (Bexxar); alemtuzumab (Campath); ibritumomab (Zevalin; In-111; Y-
90
Zevalin); gemtuzumab (Mylotarg); eculizumab (Soliris) ordenosumab.
[0001424] Exemplary EGFRinhibitorsinclude, butarenotlimited to,gefitinib
(Iressa); lapatinib
(Tykerb); cetuximab (Erbitux); erlotinib (Tarceva); panitumumab (Vectibix);
PKI-166;
canertinib (CI-1033); matuzumab (Emd7200) or EKB-569.
[0001425] Exemplary HER2inhibitorsinclude, butare not limited to, trastuzumab
(Herceptin);
lapatinib (Tykerb) or AC-480.
[0001426] Histone Deacetylase Inhibitors include, but are not limited to,
vorinostat (Zolinza).
[0001427] Exemplary hormones include, but are not limited to, tamoxifen
(Soltamox;
Nolvadex); raloxifene (Evista); megestrol (Megace); leuprolide (Lupron; Lupron
Depot;
Eligard; Viadur) ; fulvestrant (Faslodex); letrozole (Femara); triptorelin
(Trelstar LA; Trelstar
Depot) ; exemestane (Aromasin) ; goserelin (Zoladex) ; bicalutamide (Casodex);
anastrozole
(Arimidex); fluoxymesterone (Androxy; Halotestin); medroxyprogesterone
(Provera; Depo-
Provera); estramustine (Emcyt); flutamide (Eulexin); toremifene (Fareston);
degarelix
(Firmagon); nilutamide (Nilandron); abarelix (Plenaxis); or testolactone
(Teslac).
196
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001428] Exemplary mitotic inhibitosinclude, butare not limited to,
paclitaxel (Taxol; Onxol;
Abraxane); docetaxel (Taxotere); vincristine (Oncovin; Vincasar PFS);
vinblastine (Velban);
etoposide (Toposar; Etopophos; VePesid); teniposide (Vumon); ixabepilone
(Ixempra);
nocodazole; epothilone; vinorelbine (Navelbine); camptothecin (CPT);
irinotecan
(Camptosar); topotecan (Hycamtin); amsacrine or lamellarin D (LAM-D).
[0001429] Exemplary MTORinhibitors include, butare not limited to, everolimus
(Afinitor) or
temsirolimus (Torisel); rapamune, ridaforolimus; or AP23573.
[0001430] Exemplary multi-kinaseinhibitors include, but are not limited to,
sorafenib (Nexavar);
sunitinib (Sutent); BIBW 2992; E7080; Zd6474; PKC-412; motesanib; or AP24534.
[0001431] Exemplary serine/threonine kinase inhibitors include, but are not
limited to,
ruboxistaurin; eril/easudil hydrochloride; flavopiridol; seliciclib (CYC202;
Roscovitrine);
SNS-032 (BMS-387032); Pkc412; bryostatin; KAI-9803;SF1126; VX-680; Azdl 152;
Arry-
142886 (AZD-6244); SCIO-469; GW681323; CC-401; CEP-1347 or PD 332991.
[0001432] Exemplary tyrosine kinase inhibitors include, but are not limited
to, erlotinib (Tarceva);
gefitinib (Iressa); imatinib (Gleevec); sorafenib (Nexavar); sunitinib
(Sutent); trastuzumab
(Herceptin); bevacizumab (Avastin); rituximab (Rituxan); lapatinib (Tykerb);
cetuximab
(Erbitux); panitumumab (Vectibix); everolimus (Afinitor); alemtuzumab
(Campath);
gemtuzumab (Mylotarg); temsirolimus (Torisel); pazopanib (Votrient); dasatinib
(Sprycel);
nilotinib (Tasigna); vatalanib (Ptk787; ZK222584); CEP-701; SU5614; MLN518;
XL999;
VX-322; Azd0530; BMS-354825; SKI-606 CP-690; AG-490; WHI-P154; WHI-P131; AC-
220; or AMG888.
[0001433] Exemplary VEGF/VEGFRinhibitors include, but are not limited to,
bevacizumab
(Avastin); sorafenib (Nexavar); sunitinib (Sutent); ranibizumab; pegaptanib;
or vandetinib.
[0001434] Exemplary microtubule targeting drugs include, but are not limited
to, paclitaxel,
docetaxel, vincristin, vinblastin, nocodazole, epothilones and navelbine.
[0001435] Exemplary topoisomerase poison drugs include, but are not limited
to, teniposide,
etoposide, adriamycin, camptothecin, daunorubicin, dactinomycin, mitoxantrone,
amsacrine,
epirubicin and idarubicin.
[0001436] Exemplary taxanes or taxane derivatives include, butare
notlimitedto, paclitaxel
and docetaxol.
[0001437] Exemplary general chemotherapeutic, anti neoplastic, anti-
proliferative agents include, but
are not limited to, altretamine (Hexalen); isotretinoin (Accutane; Amnesteem;
Claravis; Sotret);
tretinoin (Vesanoid); azacitidine (Vidaza); bortezomib (Velcade) asparaginase
(Elspar);
levamisole (Ergamisol); mitotane (Lysodren); procarbazine (Matulane);
pegaspargase
197
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
(Oncaspar); denileukin diftitox (Ontak); porfimer (Photofrin); aldesleukin
(Proleukin);
lenalidomide (Revlimid); bexarotene (Targretin); thalidomide (Thalomid);
temsirolimus
(Torisel); arsenic trioxide (Trisenox); verteporfin (Visudyne); mimosine
(Leucenol); (1M
tegafur - 0.4 M 5-chloro-2,4-dihydroxypyrimidine - 1 M potassium oxonate) or
lovastatin.
[0001438] In another aspect, the second chemotherapeutic agent can be a
cytokine such
as G-CSF (granulocyte colony stimulating factor). In another aspect, a
compound of the
present invention, or a pharmaceutically acceptable salt, prodrug, metabolite,
analog or
derivative thereof, may be administered in combination with radiation therapy.
Radiation
therapy can also be administered in combination with a compound of the present
invention
and another chemotherapeutic agent described herein as part of a multiple
agent therapy. In
yet another aspect, a compound of the present invention, or a pharmaceutically
acceptable
salt, prodrug, metabolite, analog or derivative thereof, may be administered
in combination
with standard chemotherapy combinations such as, but not restricted to, CMF
(cyclophosphamide, methotrexate and 5-fluorouracil), CAF (cyclophosphamide,
adriamycin
and 5-fluorouracil), AC (adriamycin and cyclophosphamide), FEC (5-
fluorouracil, epirubicin,
and cyclophosphamide), ACT or ATC (adriamycin, cyclophosphamide, and
paclitaxel),
rituximab, Xeloda (capecitabine), Cisplatin (CDDP), Carboplatin, TS-1
(tegafur, gimestat and
otastat potassium at a molar ratio of 1:0.4:1), Camptothecin-l 1 (CPT-11,
Irinotecan or
CamptosarTM) or CMFP (cyclophosphamide, methotrexate, 5-fluorouracil and
prednisone).
[0001439] In preferred embodiments, a compound of the present invention, or a
pharmaceutically acceptable salt, prodrug, metabolite, polymorph or solvate
thereof, may be
administered with an inhibitor of an enzyme, such as a receptor or non-
receptor kinase.
Receptor and non-receptor kinases of the invention are, for example, tyrosine
kinases or
serine/threonine kinases. Kinase inhibitors of the invention are small
molecules, polynucleic
acids, polypeptides, or antibodies.
[0001440] Exemplary kinase inhibitors include, but are not limited to,
Bevacizumab
(targets VEGF), BIBW 2992 (targets EGFR and Erb2), Cetuximab/Erbitux (targets
Erb I),
Imatinib/Gleevic (targets Bcr-Abl), Trastuzumab (targets Erb2),
Gefitinib/Iressa (targets
EGFR), Ranibizumab (targets VEGF), Pegaptanib (targets VEGF),
Erlotinib/Tarceva (targets
Erb 1), Nilotinib (targets Bcr-Abl), Lapatinib (targets Erb1 and Erb2/Her2),
GW-
572016/lapatinib ditosylate (targets HER2/Erb2), Panitumumab/Vectibix (targets
EGFR),
Vandetinib (targets RET/VEGFR), E7080 (multiple targets including RET and
VEGFR),
Herceptin (targets HER2/Erb2), PKI-166 (targets EGFR), Canertinib/CI-1033
(targets
EGFR), Sunitinib/SU-11464/Sutent (targets EGFR and FLT3), Matuzumab/Emd7200
(targets
198
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
EGFR), EKB-569 (targets EGFR), Zd6474 (targets EGFR and VEGFR), PKC-412
(targets
VEGR and FLT3), Vatalanib/Ptk787/ZK222584 (targets VEGR), CEP-701 (targets
FLT3),
SU5614 (targets FLT3), MLN518 (targets FLT3), XL999 (targets FLT3), VX-322
(targets
FLT3), Azd0530 (targets SRC), BMS-354825 (targets SRC), SKI-606 (targets SRC),
CP-690
(targets JAK), AG-490 (targets JAK), WHI-P 154 (targets JAK), WHI-P 131
(targets JAK),
sorafenib/Nexavar (targets RAF kinase, VEGFR-1, VEGFR-2, VEGFR-3, PDGFR- B,
KIT,
FLT-3, and RET), Dasatinib/Sprycel (BCR/ABL and Src), AC-220 (targets F1t3),
AC-480
(targets all HER proteins, "panHER"), Motesanib diphosphate (targets VEGF1-3,
PDGFR,
and c-kit), Denosumab (targets RANKL, inhibits SRC), AMG888 (targets HER3),
and
AP24534 (multiple targets including F1t3).
[0001441] Exemplary serine/threonine kinase inhibitors include, but are not
limited to,
Rapamune (targets mTOR/FRAP1), Deforolimus (targets mTOR), Certican/Everolimus
(targets mTOR/FRAP1), AP23573 (targets mTOR/FRAP1), Eril/Fasudil hydrochloride
(targets RHO), Flavopiridol (targets CDK), Seliciclib/CYC202/Roscovitrine
(targets CDK),
SNS-032/BMS-387032 (targets CDK), Ruboxistaurin (targets PKC), Pkc412 (targets
PKC),
Bryostatin (targets PKC), KAI-9803 (targets PKC), SF1126 (targets P13K), VX-
680 (targets
Aurora kinase), Azdl 152 (targets Aurora kinase), Arry-142886/AZD-6244
(targets
MAP/MEK), SCIO-469 (targets MAP/MEK), GW681323 (targets MAP/MEK), CC-401
(targets JNK), CEP-1347 (targets JNK), and PD 332991 (targets CDK).
4. Pharmaceutical Compositions
[0001442] The present invention also provides pharmaceutical compositions
comprising
a compound of Formula I-IV in combination with at least one pharmaceutically
acceptable
excipient or carrier.
[0001443] A "pharmaceutical composition" is a formulation containing the
compounds
of the present invention in a form suitable for administration to a subject.
In one
embodiment, the pharmaceutical composition is in bulk or in unit dosage form.
The unit
dosage form is any of a variety of forms, including, for example, a capsule,
an IV bag, a
tablet, a single pump on an aerosol inhaler or a vial. The quantity of active
ingredient (e.g., a
formulation of the disclosed compound or salt, hydrate, solvate or isomer
thereof) in a unit
dose of composition is an effective amount and is varied according to the
particular treatment
involved. One skilled in the art will appreciate that it is sometimes
necessary to make routine
variations to the dosage depending on the age and condition of the patient.
The dosage will
also depend on the route of administration. A variety of routes are
contemplated, including
199
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
oral, pulmonary, rectal, parenteral, transdermal, subcutaneous, intravenous,
intramuscular,
intraperitoneal, inhalational, buccal, sublingual, intrapleural, intrathecal,
intranasal, and the
like. Dosage forms for the topical or transdermal administration of a compound
of this
invention include powders, sprays, ointments, pastes, creams, lotions, gels,
solutions, patches
and inhalants. In one embodiment, the active compound is mixed under sterile
conditions
with a pharmaceutically acceptable carrier, and with any preservatives,
buffers or propellants
that are required.
[0001444] As used herein, the phrase "pharmaceutically acceptable" refers to
those
compounds, materials, compositions, carriers, and/or dosage forms which are,
within the
scope of sound medical judgment, suitable for use in contact with the tissues
of human beings
and animals without excessive toxicity, irritation, allergic response, or
other problem or
complication, commensurate with a reasonable benefit/risk ratio.
[0001445] "Pharmaceutically acceptable excipient" means an excipient that is
useful in
preparing a pharmaceutical composition that is generally safe, non-toxic and
neither
biologically nor otherwise undesirable, and includes excipient that is
acceptable for
veterinary use as well as human pharmaceutical use. A "pharmaceutically
acceptable
excipient" as used in the specification and claims includes both one and more
than one such
excipient.
[0001446] A pharmaceutical composition of the invention is formulated to be
compatible
with its intended route of administration. Examples of routes of
administration include
parenteral, e.g., intravenous, intradermal, subcutaneous, oral (e.g.,
inhalation), transdermal
(topical), and transmucosal administration. Solutions or suspensions used for
parenteral,
intradermal, or subcutaneous application can include the following components:
a sterile
diluent such as water for injection, saline solution, fixed oils, polyethylene
glycols, glycerine,
propylene glycol or other synthetic solvents; antibacterial agents such as
benzyl alcohol or
methyl parabens; antioxidants such as ascorbic acid or sodium bisulfite;
chelating agents such
as ethylenediaminetetraacetic acid; buffers such as acetates, citrates or
phosphates, and agents
for the adjustment of tonicity such as sodium chloride or dextrose. The pH can
be adjusted
with acids or bases, such as hydrochloric acid or sodium hydroxide. The
parenteral
preparation can be enclosed in ampoules, disposable syringes or multiple dose
vials made of
glass or plastic.
[0001447] A compound or pharmaceutical composition of the invention can be
administered to a subject in many of the well-known methods currently used for
chemotherapeutic treatment. For example, for treatment of cancers, a compound
of the
200
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
invention may be injected directly into tumors, injected into the blood stream
or body cavities
or taken orally or applied through the skin with patches. The dose chosen
should be
sufficient to constitute effective treatment but not so high as to cause
unacceptable side
effects. The state of the disease condition (e.g., cancer, precancer, and the
like) and the
health of the patient should preferably be closely monitored during and for a
reasonable
period after treatment.
[0001448] The term "therapeutically effective amount", as used herein, refers
to an
amount of a pharmaceutical agent to treat, ameliorate, or prevent an
identified disease or
condition, or to exhibit a detectable therapeutic or inhibitory effect. The
effect can be
detected by any assay method known in the art. The precise effective amount
for a subject
will depend upon the subject's body weight, size, and health; the nature and
extent of the
condition; and the therapeutic or combination of therapeutics selected for
administration.
Therapeutically effective amounts for a given situation can be determined by
routine
experimentation that is within the skill and judgment of the clinician. In a
preferred aspect,
the disease or condition to be treated is cancer. In another aspect, the
disease or condition to
be treated is a cell proliferative disorder.
[0001449] For any compound, the therapeutically effective amount can be
estimated
initially either in cell culture assays, e.g., of neoplastic cells, or in
animal models, usually rats,
mice, rabbits, dogs, or pigs. The animal model may also be used to determine
the appropriate
concentration range and route of administration. Such information can then be
used to
determine useful doses and routes for administration in humans.
Therapeutic/prophylactic
efficacy and toxicity may be determined by standard pharmaceutical procedures
in cell
cultures or experimental animals, e.g., ED50 (the dose therapeutically
effective in 50% of the
population) and LD50 (the dose lethal to 50% of the population). The dose
ratio between
toxic and therapeutic effects is the therapeutic index, and it can be
expressed as the ratio,
LD50/ED50. Pharmaceutical compositions that exhibit large therapeutic indices
are preferred.
The dosage may vary within this range depending upon the dosage form employed,
sensitivity of the patient, and the route of administration.
[0001450] Dosage and administration are adjusted to provide sufficient levels
of the
active agent(s) or to maintain the desired effect. Factors which may be taken
into account
include the severity of the disease state, general health of the subject, age,
weight, and gender
of the subject, diet, time and frequency of administration, drug
combination(s), reaction
sensitivities, and tolerance/response to therapy. Long-acting pharmaceutical
compositions
201
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
may be administered every 3 to 4 days, every week, or once every two weeks
depending on
half-life and clearance rate of the particular formulation.
[0001451] The pharmaceutical compositions containing active compounds of the
present
invention may be manufactured in a manner that is generally known, e.g., by
means of
conventional mixing, dissolving, granulating, dragee-making, levigating,
emulsifying,
encapsulating, entrapping, or lyophilizing processes. Pharmaceutical
compositions may be
formulated in a conventional manner using one or more pharmaceutically
acceptable carriers
comprising excipients and/or auxiliaries that facilitate processing of the
active compounds
into preparations that can be used pharmaceutically. Of course, the
appropriate formulation is
dependent upon the route of administration chosen.
[0001452] Pharmaceutical compositions suitable for injectable use include
sterile
aqueous solutions (where water soluble) or dispersions and sterile powders for
the
extemporaneous preparation of sterile injectable solutions or dispersion. For
intravenous
administration, suitable carriers include physiological saline, bacteriostatic
water, Cremophor
EL TM (BASF, Parsippany, N.J.) or phosphate buffered saline (PBS). In all
cases, the
composition must be sterile and should be fluid to the extent that easy
syringeability exists. It
must be stable under the conditions of manufacture and storage and must be
preserved against
the contaminating action of microorganisms such as bacteria and fungi. The
carrier can be a
solvent or dispersion medium containing, for example, water, ethanol, polyol
(for example,
glycerol, propylene glycol, and liquid polyethylene glycol, and the like), and
suitable
mixtures thereof. The proper fluidity can be maintained, for example, by the
use of a coating
such as lecithin, by the maintenance of the required particle size in the case
of dispersion and
by the use of surfactants. Prevention of the action of microorganisms can be
achieved by
various antibacterial and antifungal agents, for example, parabens,
chlorobutanol, phenol,
ascorbic acid, thimerosal, and the like. In many cases, it will be preferable
to include isotonic
agents, for example, sugars, polyalcohols such as manitol, sorbitol, sodium
chloride in the
composition. Prolonged absorption of the injectable compositions can be
brought about by
including in the composition an agent which delays absorption, for example,
aluminum
monostearate and gelatin.
[0001453] Sterile injectable solutions can be prepared by incorporating the
active
compound in the required amount in an appropriate solvent with one or a
combination of
ingredients enumerated above, as required, followed by filtered sterilization.
Generally,
dispersions are prepared by incorporating the active compound into a sterile
vehicle that
contains a basic dispersion medium and the required other ingredients from
those enumerated
202
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
above. In the case of sterile powders for the preparation of sterile
injectable solutions,
methods of preparation are vacuum drying and freeze-drying that yields a
powder of the
active ingredient plus any additional desired ingredient from a previously
sterile-filtered
solution thereof.
[0001454] Oral compositions generally include an inert diluent or an edible
pharmaceutically acceptable carrier. They can be enclosed in gelatin capsules
or compressed
into tablets. For the purpose of oral therapeutic administration, the active
compound can be
incorporated with excipients and used in the form of tablets, troches, or
capsules. Oral
compositions can also be prepared using a fluid carrier for use as a
mouthwash, wherein the
compound in the fluid carrier is applied orally and swished and expectorated
or swallowed.
Pharmaceutically compatible binding agents, and/or adjuvant materials can be
included as
part of the composition. The tablets, pills, capsules, troches and the like
can contain any of
the following ingredients, or compounds of a similar nature: a binder such as
microcrystalline
cellulose, gum tragacanth or gelatin; an excipient such as starch or lactose,
a disintegrating
agent such as alginic acid, Primogel, or corn starch; a lubricant such as
magnesium stearate or
Sterotes; a glidant such as colloidal silicon dioxide; a sweetening agent such
as sucrose or
saccharin; or a flavoring agent such as peppermint, methyl salicylate, or
orange flavoring.
[0001455] For administration by inhalation, the compounds are delivered in the
form of
an aerosol spray from pressured container or dispenser, which contains a
suitable propellant,
e.g., a gas such as carbon dioxide, or a nebulizer.
[0001456] Systemic administration can also be by transmucosal or transdermal
means.
For transmucosal or transdermal administration, penetrants appropriate to the
barrier to be
permeated are used in the formulation. Such penetrants are generally known in
the art, and
include, for example, for transmucosal administration, detergents, bile salts,
and fusidic acid
derivatives. Transmucosal administration can be accomplished through the use
of nasal
sprays or suppositories. For transdermal administration, the active compounds
are
formulated into ointments, salves, gels, or creams as generally known in the
art.
[0001457] The active compounds can be prepared with pharmaceutically
acceptable
carriers that will protect the compound against rapid elimination from the
body, such as a
controlled release formulation, including implants and microencapsulated
delivery systems.
Biodegradable, biocompatible polymers can be used, such as ethylene vinyl
acetate,
polyanhydrides, polyglycolic acid, collagen, polyorthoesters, and polylactic
acid. Methods
for preparation of such formulations will be apparent to those skilled in the
art. The materials
can also be obtained commercially from Alza Corporation and Nova
Pharmaceuticals, Inc.
203
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Liposomal suspensions (including liposomes targeted to infected cells with
monoclonal
antibodies to viral antigens) can also be used as pharmaceutically acceptable
carriers. These
can be prepared according to methods known to those skilled in the art, for
example, as
described in U.S. Pat. No. 4,522,811.
[0001458] It is especially advantageous to formulate oral or parenteral
compositions in
dosage unit form for ease of administration and uniformity of dosage. Dosage
unit form as
used herein refers to physically discrete units suited as unitary dosages for
the subject to be
treated; each unit containing a predetermined quantity of active compound
calculated to
produce the desired therapeutic effect in association with the required
pharmaceutical carrier.
The specification for the dosage unit forms of the invention are dictated by
and directly
dependent on the unique characteristics of the active compound and the
particular therapeutic
effect to be achieved.
[0001459] In therapeutic applications, the dosages of the pharmaceutical
compositions
used in accordance with the invention vary depending on the agent, the age,
weight, and
clinical condition of the recipient patient, and the experience and judgment
of the clinician or
practitioner administering the therapy, among other factors affecting the
selected dosage.
Generally, the dose should be sufficient to result in slowing, and preferably
regressing, the
growth of the tumors and also preferably causing complete regression of the
cancer. Dosages
can range from about 0.01 mg/kg per day to about 5000 mg/kg per day. In
preferred aspects,
dosages can range from about 1 mg/kg per day to about 1000 mg/kg per day. In
an aspect,
the dose will be in the range of about 0.1 mg/day to about 50 g/day; about 0.1
mg/day to
about 25 g/day; about 0.1 mg/day to about 10 g/day; about 0.1 mg to about 3
g/day; or about
0.1 mg to about 1 g/day, in single, divided, or continuous doses (which dose
may be adjusted
for the patient's weight in kg, body surface area in m2, and age in years). An
effective
amount of a pharmaceutical agent is that which provides an objectively
identifiable
improvement as noted by the clinician or other qualified observer. For
example, regression
of a tumor in a patient may be measured with reference to the diameter of a
tumor. Decrease
in the diameter of a tumor indicates regression. Regression is also indicated
by failure of
tumors to reoccur after treatment has stopped. As used herein, the term
"dosage effective
manner" refers to amount of an active compound to produce the desired
biological effect in a
subject or cell.
[0001460] The pharmaceutical compositions can be included in a container,
pack, or
dispenser together with instructions for administration.
204
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001461] The compounds of the present invention are capable of further
forming salts.
All of these forms are also contemplated within the scope of the claimed
invention.
[0001462] As used herein, "pharmaceutically acceptable salts" refer to
derivatives of the
compounds of the present invention wherein the parent compound is modified by
making
acid or base salts thereof. Examples of pharmaceutically acceptable salts
include, but are not
limited to, mineral or organic acid salts of basic residues such as amines,
alkali or organic
salts of acidic residues such as carboxylic acids, and the like. The
pharmaceutically
acceptable salts include the conventional non-toxic salts or the quaternary
ammonium salts of
the parent compound formed, for example, from non-toxic inorganic or organic
acids. For
example, such conventional non-toxic salts include, but are not limited to,
those derived from
inorganic and organic acids selected from 2-acetoxybenzoic, 2-hydroxyethane
sulfonic, acetic,
ascorbic, benzene sulfonic, benzoic, bicarbonic, carbonic, citric, edetic,
ethane disulfonic,
1,2-ethane sulfonic, fumaric, glucoheptonic, gluconic, glutamic, glycolic,
glycollyarsanilic,
hexylresorcinic, hydrabamic, hydrobromic, hydrochloric, hydroiodic,
hydroxymaleic,
hydroxynaphthoic, isethionic, lactic, lactobionic, lauryl sulfonic, maleic,
malic, mandelic,
methane sulfonic, napsylic, nitric, oxalic, pamoic, pantothenic, phenylacetic,
phosphoric,
polygalacturonic, propionic, salicyclic, stearic, subacetic, succinic,
sulfamic, sulfanilic,
sulfuric, tannic, tartaric, toluene sulfonic, and the commonly occurring amine
acids, e.g.,
glycine, alanine, phenylalanine, arginine, etc.
[0001463] Other examples of pharmaceutically acceptable salts include hexanoic
acid,
cyclopentane propionic acid, pyruvic acid, malonic acid, 3-(4-
hydroxybenzoyl)benzoic acid,
cinnamic acid, 4-chlorobenzenesulfonic acid, 2-naphthalenesulfonic acid, 4-
toluenesulfonic
acid, camphorsulfonic acid, 4-methylbicyclo-[2.2.2]-oct-2-ene-l-carboxylic
acid, 3-
phenylpropionic acid, trimethylacetic acid, tertiary butylacetic acid, muconic
acid, and the
like. The present invention also encompasses salts formed when an acidic
proton present in
the parent compound either is replaced by a metal ion, e.g., an alkali metal
ion, an alkaline
earth ion, or an aluminum ion; or coordinates with an organic base such as
ethanolamine,
diethanolamine, triethanolamine, tromethamine, N-methylglucamine, and the
like.
[0001464] It should be understood that all references to pharmaceutically
acceptable salts
include solvent addition forms (solvates) or crystal forms (polymorphs) as
defined herein, of
the same salt.
[0001465] The compounds of the present invention can also be prepared as
esters, for
example, pharmaceutically acceptable esters. For example, a carboxylic acid
function group
in a compound can be converted to its corresponding ester, e.g., a methyl,
ethyl or other ester.
205
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Also, an alcohol group in a compound can be converted to its corresponding
ester, e.g., an
acetate, propionate or other ester.
[0001466] The compounds of the present invention can also be prepared as
prodrugs, for
example, pharmaceutically acceptable prodrugs. The terms "pro-drug" and
"prodrug" are
used interchangeably herein and refer to any compound which releases an active
parent drug
in vivo. Since prodrugs are known to enhance numerous desirable qualities of
pharmaceuticals (e.g., solubility, bioavailability, manufacturing, etc.), the
compounds of the
present invention can be delivered in prodrug form. Thus, the present
invention is intended
to cover prodrugs of the presently claimed compounds, methods of delivering
the same and
compositions containing the same. "Prodrugs" are intended to include any
covalently bonded
carriers that release an active parent drug of the present invention in vivo
when such prodrug
is administered to a subject. Prodrugs in the present invention are prepared
by modifying
functional groups present in the compound in such a way that the modifications
are cleaved,
either in routine manipulation or in vivo, to the parent compound. Prodrugs
include
compounds of the present invention wherein a hydroxy, amino, sulfhydryl,
carboxy or
carbonyl group is bonded to any group that may be cleaved in vivo to form a
free hydroxyl,
free amino, free sulfhydryl, free carboxy or free carbonyl group,
respectively.
[0001467] Examples of prodrugs include, but are not limited to, esters (e.g.,
acetate,
dialkylaminoacetates, formates, phosphates, sulfates and benzoate derivatives)
and
carbamates (e.g., N,N-dimethylaminocarbonyl) of hydroxy functional groups,
esters (e.g.,
ethyl esters, morpholinoethanol esters) of carboxyl functional groups, N-acyl
derivatives
(e.g., N-acetyl) N-Mannich bases, Schiff bases and enaminones of amino
functional groups,
oximes, acetals, ketals and enol esters of ketone and aldehyde functional
groups in
compounds of the invention, and the like, See Bundegaard, H., Design of
Prodrugs, p 1-92,
Elesevier, New York-Oxford (1985).
[0001468] The compounds, or pharmaceutically acceptable salts, esters or
prodrugs
thereof, are administered orally, nasally, transdermally, pulmonary,
inhalationally, buccally,
sublingually, intraperintoneally, subcutaneously, intramuscularly,
intravenously, rectally,
intrapleurally, intrathecally and parenterally. In one embodiment, the
compound is
administered orally. One skilled in the art will recognize the advantages of
certain routes of
administration.
[0001469] The dosage regimen utilizing the compounds is selected in accordance
with a
variety of factors including type, species, age, weight, sex and medical
condition of the
patient; the severity of the condition to be treated; the route of
administration; the renal and
206
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
hepatic function of the patient; and the particular compound or salt thereof
employed. An
ordinarily skilled physician or veterinarian can readily determine and
prescribe the effective
amount of the drug required to prevent, counter or arrest the progress of the
condition.
[0001470] Techniques for formulation and administration of the disclosed
compounds of
the invention can be found in Remington: the Science and Practice of Pharmacy,
19th edition,
Mack Publishing Co., Easton, PA (1995). In an embodiment, the compounds
described
herein, and the pharmaceutically acceptable salts thereof, are used in
pharmaceutical
preparations in combination with a pharmaceutically acceptable carrier or
diluent. Suitable
pharmaceutically acceptable carriers include inert solid fillers or diluents
and sterile aqueous
or organic solutions. The compounds will be present in such pharmaceutical
compositions in
amounts sufficient to provide the desired dosage amount in the range described
herein.
[0001471] All percentages and ratios used herein, unless otherwise indicated,
are by
weight. Other features and advantages of the present invention are apparent
from the
different examples. The provided examples illustrate different components and
methodology
useful in practicing the present invention. The examples do not limit the
claimed invention.
Based on the present disclosure the skilled artisan can identify and employ
other components
and methodology useful for practicing the present invention.
5. Examples
[0001472] General Procedure A: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
H 2 N N' N N-N / O
O O N
DMA I N' \O / I \ \
R2 100 C, 24 h R2 ' (i) Hunig's Base, CH3COOH R2 '
110 C, 1 h
R, R, (ii) microwave, 220 C, 90 min R1
N-NH
N
CF3COOH Microwave R2
170 C, 90 min
R1
[0001473] Example 1 or Compound 1 (Table 1): Synthesis of 7-(3,4-
dichlorophenyl)-6,7-
dihydro-3H-benzo[f]pyrazolo[3,4-c]isoquinoline.
207
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N-NH
N
CI
CI
[0001474] Step 1. Synthesis of (E)-4-(3,4-dichlorophenyl)-2-
((dimethylamino)methylene)-3,4-dihydronaphthalen-1(2H)-one. The mixture of 4-
(3,4-
dichlorophenyl)-3,4-dihydronaphthalen-1(2H)-one (10.0 g, 34.5 mmol) in 1,1-
dimethoxy-
N,N-dimethylmethanamine (80 mL) was heated at 110 C for 24 hours. The excess
1,1-
dimethoxy-N,N-dimethylmethanamine was removed under reduced pressure. The
residue
was dried on high vacuum to afford the yellowish desired product (11.5 g, 97
%). The
compound was used in Step-2 without any further purification.
[0001475] Step 2. Synthesis of 7-(3,4-dichlorophenyl)-3-(4-methoxybenzyl)-6,7-
dihydro-3H-benzo[f]pyrazolo[3,4-c]isoquinoline. To the mixture of (E)-4-(3,4-
dichlorophenyl)-2-((dimethylamino)methylene)-3,4-dihydronaphthalen-1(2H)-one
(0.20 g,
0.58 mmol) and 1-(4-methoxybenzyl)-1H-pyrazol-5-amine hydrochloride (0.14 g,
0.58
mmol) was added glacial acetic acid (5 mL) and Hunig's Base (0.4 mL). The
mixture was
heated at 110 C for 1 hour, then transferred to microwave vial and heated at
220 C for 90
minutes. The reaction mixture was evaporated and dissolved in dichloromethane
(100 mL),
washed with water (30 mL), brine (30 mL), dried over sodium sulfate and
concentrated to
dryness. The crude was purified by flash column chromatography (Si02, 10%
EtOAc in
hexane) to give the pure desired product as a brown solid (0.15 g, 53 %). M.p.
= 76-78 C; 'H
NMR (CDC13, 400 MHz) 6 9.53 (d, J= 8 Hz, 1H), 8.32 (s, 1H), 8.02 (s, 1H), 7.54
(t, J= 6.8
Hz, 1H), 7.45 (t, J= 8 Hz, 1H), 7.35-7.26 (m, 4H), 7.06 (d, J= 8 Hz, 1H), 6.96
(d, J= 8 HZ,
1H), 6.843 (d, J= 7.2 Hz, 2H), 4.28 (t, J= 8 Hz, 1H), 4.17 (s, 2H), 3.77 (s,
3H), 3.29-3.26
(m, 2H); LCMS: 486 [M+1].
[0001476] Step 3. Synthesis of 7-(3,4-dichlorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline. To 7-(3,4-dichlorophenyl)-3-(4-
methoxybenzyl)-6,7-
dihydro-3H-benzo[f]pyrazolo[3,4-c]isoquinoline (0.09 g, 0.19 mmol) was added
trifluoroacetic acid (1.5 mL). The reaction was heated in a microwave at 170
C for 90
minutes. Trifluoroacetic acid was then removed under reduced pressure to give
a crude
product. The crude product was purified by flash column chromatographt (Si02,
20% EtOAc
in hexane) to give the pure desired product as a pale yellow solid (0.39 g,
57%). M.p. = 168-
170 C); 'H NMR (CDC13, 400 MHz) 6 9.56 (d, J= 8 Hz, 1H), 8.36 (s, 1H), 8.23
(s, 1H),
208
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
7.56 (t, J= 7.2 Hz, 1H), 7.48 (t, J= 8 Hz, 1H), 7.35-7.26 (m, 2H), 7.08 (d, J=
8 Hz, 1H),
6.96 (d, J = 8 HZ, 1 H), 6.79-6.78 (m, 1 H), 4.29 (t, J = 8 Hz, 1 H), 3.40-
3.23 (m, 2H); LCMS
[M+H]: 366.
[0001477] Example 2 or compound 2 (Table 1): Synthesis of 7-phenyl-6,7-dihydro-
3H-
benzo[f]pyrazolo[3,4-c]isoquinoline.
N--NH
N
[0001478] The compound 7-phenyl-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline
was prepared using 4-phenyl-3,4-dihydronaphthalen-1(2H)-one as described in
the 3 step
general procedure A. The desired product was obtained as a yellow solid (0.047
g, 32%).
Further purification was done by recrystalization from EtOAc. M.p. = 143-145
C; 1H NMR
(DMSO-d6, 400 MHz) 89.47 (m, 1H), 8.54 (s, 1H), 8.32(d, J= 2.4 Hz, 1H), 7.54
(m, 2H),
7.29-7.15 (m, 6H), 6.82 (d, J = 2.0 Hz, 1 H), 4.63 (t, J = 7.2 Hz, 1 H), 3.40
(m, 2H); LCMS
[M+H]: 298; Elemental analysis (%): C 80.64, H 4.76, N 14.17; Calcd for
C20H15N3: C 80.79,
H 5.08, N 14.13.
[0001479] Example 3 or compound 3 (Table 1): Synthesis of 7-(3,4-
difluorophenyl)-6,7-
dihydro-3H-benzo[f]pyrazolo[3,4-c]isoquinoline.
N-NH
N
F
F
[0001480] The compound, 7-(3,4-difluorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 4-(3,4-difluorophenyl)-3,4-dihydronaphthalen-
1(2H)-one
as described in the 3 step general procedure A. The desired product was
obtained as a solid.
M.p. = 195-196 C; 400 MHz 1H NMR (DMSO-d6) 8: 9.45 (m, 1H), 8.53 (s, 1H), 8.31
(d, J=
2.4 Hz, 1H), 7.54 (m, 2H), 7.32 (m, 2H), 7.15 (m, 1H), 6.92 (m, 1H), 6.80 (d,
J = 2.4 Hz,
I H), 4.47 (t, J = 6 Hz, I H), 3.42 (dd, J = 16 and 8.4 Hz, I H), 3.28 (dd, J
= 15.6 and 6 Hz,
1H); LCMS [M+H]: 334.
[0001481] Example 4: Synthesis of 7-(2,4-dichlorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline.
209
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N-NH
N
CI
CI
[0001482] The compound, 7-(2,4-dichlorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 4-(2,4-dichlorophenyl)-3,4-dihydronaphthalen-
1(2H)-one
as described in the 3 step general procedure A. The desired product was
obtained as a solid.
M.p. = 195-196 C; 400 MHz iH NMR (DMSO-d6) 8: 9.45 (m, 1H), 8.53 (s, 1H), 8.31
(d, J=
2.4 Hz, 1H), 7.54 (m, 2H), 7.32 (m, 2H), 7.15 (m, 1H), 6.92 (m, 1H), 6.80 (d,
J = 2.4 Hz,
I H), 4.47 (t, J = 6 Hz, I H), 3.42 (dd, J = 16 and 8.4 Hz, I H), 3.28 (dd, J
= 15.6 and 6 Hz,
1H); LCMS [M+H]: 334.
[0001483] Example 5: Synthesis of 7-(2-dichlorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline.
N-NH
/
N
CI
[0001484] The compound, 7-(2-dichlorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 4-(2,-dichlorophenyl)-3,4-dihydronaphthalen-
1(2H)-one as
described in the 3 step general procedure A. The desired product was obtained
as a solid.
M.p. = 88-91 C; 400 MHz iH NMR (DMSO-d6) 8: 9.49 (d, J = 8 Hz, 1H), 8.49 (s,
1H), 8.33
(d, J = 2.4 Hz, 1 H), 7.5 3 (m, 3 H), 7.27 (t, J = 7.6 Hz, 1 H), 7.19 (t, J =
7.6 Hz, 1 H), 7.01 (d, J
= 7.2 Hz, I H), 6.93 (d, J = 8 Hz, I H), 6.82 (d, J = 2.4 Hz, I H), 4.80 (m, I
H), 3.41 (dd, J =
15.6 and 8.4 Hz, 1H), 3.31 (dd, J = 15.6 and 6 Hz, 1H); LCMS [M+H]: 332.
[0001485] Example 6: Synthesis of 7-(4-fluorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline.
N-NH
N
F
210
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001486] The compound, 7-(4-fluorophenyl)-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 4-(4-fluorophenyl)-3,4-dihydronaphthalen-
1(2H)-one as
described in the 3 step general procedure A. The crude product was purified
using reverse
phase HPLC and the pure desired product was obtained as a yellow solid (0.033
g, 35%).
M.p. = 167-169 C; iH NMR (CDC13, 400 MHz) 89.57 (m, 1H), 8.35 (s, 1H),
8.22(d, J= 2.4
Hz, 1H), 7.50 (m, 2H), 6.99-7.26 (m, 6H), 6.77 (d, J= 2.4 Hz, 1H), 4.35 (m,
1H), 3.30 (m,
2H); LCMS [M+H]: 316.
[0001487] Example 7: Synthesis of 8,10-dimethyl-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline.
N-NH
N
[0001488] The compound, 8,10-dimethyl-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 5,7-dimethyl-3,4-dihydronaphthalen-1(2H)-one
as
described in the 3 step general procedure A. The desired product was obtained
as a solid.
M.p. = 152-155 C. 400 MHz iH NMR (DMSO-d6) 8: 9.05 (s, 1H), 8.55 (s, 1H),
8.30 (d,
J=2.3Hz, 1H), 7.24 (s, 1H), 6.8 (d, J = 2.3Hz, 1H), 2.95-2.8 (m, 4H), 2.45 (s,
3H) 2.4 (s, 3H);
LCMS [M+H]: 250.
[0001489] Example 8: Synthesis of 8-methoxy-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline.
N-NH
N
110
[0001490] The compound 8-methoxy-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 5-methoxy-3,4-dihydronaphthalen-1(2H)-one as
described
in the 3 step general procedure A. The desired product was obtained as a
solid. Mp=85 -
89 C, 400 MHz iH NMR (DMSO-d6) 8: 8.98 (d, J=7.8 Hz, 1H), 8.5 (s, 1H), 8.30
(d, J=2.3,
I H), 7.45 (t, J=8.22 Hz, I H), 7.26 (d, J = 8.22Hz, I H), 6.8 (d, J=2.35 Hz,
I H). 3.8 (s, 3H),
2.9 (s, 4H); LCMS [M+H]: 252.
[0001491] Example 9: Synthesis of 10-methoxy-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline.
211
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N--NH
N
1~0 \ \ I
[0001492] The compound 10-methoxy-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 7-methoxy-3,4-dihydronaphthalen-1(2H)-one as
described
in the 3 step general procedure A. The desired product was obtained as a
solid. Mp=95-97 C,
400 MHz 1H NMR (DMSO- d6) 8: 9.0 (d, J=2.7Hz, 1H), 8.6 (s, 1H), 8.32 (d,
J=2.3, 1H), 7.39
(d, J=8.6lHz, 1H), 7.12 (dd, J = 2.74, 5.87Hz, 1H), 6.82 (d, J=2.35Hz, 1H),
3.85 (s, 3H), 3.0-
2.85 (m, 4H); LCMS [M+H]: 252.
[0001493] Example 10: Synthesis of 6,9-dihydro-5H-pyrazolo[3,4-
k][1,8]phenanthroline.
N--NH
N
N
[0001494] The compound, 6,9-dihydro-5H-pyrazolo[3,4-k][1,8]phenanthroline was
prepared using 6,7-dihydroquinolin-8(5H)-one as described in the 3 step
general procedure
A. The crude product was purified using reverse phase HPLC to give the desired
product as a
yellow solid (0.027 g, 0.12 mmol). M.p. = 263-265 C; 1H NMR (DMSO- d6) 400
MHz 8:
13.67 (s, 1 H), 8.61 (dd, J = 1.5, 4.7 Hz, 1 H), 8.10 (d, J = 3.2 Hz, 2H),
7.73 (d, J = 7.4 Hz,
1H), 7.35 (dd, J= 2.7, 7.4 Hz, 1H), 2.97 (ddt, J = 3.0, 7.2, 9.2 Hz, 4H), LCMS
[M+H]: 223.
Elemental Analysis calculated for C13H10N4: %C: 70.76, %H: 4.54, %N: 25.21,
found
C13H10N4Ø08(CF3CO2H): %C 68.21, %H 4.36, %N 23.66.
[0001495] Example 11: Synthesis of 9-methoxy-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline.
N-NH
N
0
[0001496] The compound, 9-methoxy-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 6-methoxy-3,4-dihydronaphthalen-1(2H)-one as
described
in the 3 step general procedure A. The crude product was purified using
reverse phase HPLC
to give the desired product as a yellow solid (0.022g, 0.088 mmol). 1H NMR
(DMSO- d6)
400 MHz 8: 9.30 (dd, J= 1.5, 4.7 Hz, 1H), 8.48 (s, 1H), 8.25 (d, J= 2.4 Hz,
1H), 7.12-6.92
(m, 2H), 6.90-6.68 (m, 2H), 3.84 (s, 3H), 2.95-2.82 (m, 4H); LCMS [M+H]: 252.
212
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001497] Example 12: Synthesis of 9,10-dimethoxy-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline.
N-NH
N
[0001498] The compound, 9,10-dimethoxy-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 6,7-dimethoxy-3,4-dihydronaphthalen-1(2H)-
one as
described in the 3 step general procedure A. The crude product was purified
using reverse
phase HPLC to give the desired product as a yellow solid (0.072g, 0.26 mmol).
1H NMR
(DMSO- d6) 400 MHz 8: 9.10 (s, 1 H), 8.51 (s, 1 H), 8.31 (d, J = 2.4 Hz, 1 H),
7.11 (s, 1 H),
6.76 (d, J= 2.4 Hz, 2H), 3.89 (s, 3H), 3.84 (s, 3H), 2.95-2.82 (m, 4H); LCMS
[M+H]: 282.
[0001499] Example 13: Synthesis of 10-bromo-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline.
N--NH
N
Br
[0001500] The compound, 10-bromo-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 7-bromo-3,4-dihydronaphthalen-1(2H)-one as
described in
the 3 step general procedure A. The crude product was purified using reverse
phase HPLC to
give the desired product as a yellow solid (0.032 g, 0.11 mmol). 1H NMR (DMSO-
d6) 400
MHz 8: 9.55 (s, I H), 8.59 (d, J= 1.0 Hz, I H), 8.35 (dd, J= 1.2, 2.4 Hz, I
H), 7.71 (dd, J=
2.3, 4.2 Hz, 1H), 7.43 (d, J= 7.9 Hz, 1H), 6.90-6.65 (m, 1H), 2.95-2.82 (m,
4H); LCMS
[M+H] : 299.
[0001501] Example 14: Synthesis of 10-nitro-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline.
N-NH
N
02N
[0001502] The compound, 10-nitroo-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline was prepared using 7-nitro-3,4-dihydronaphthalen-1(2H)-one as
described in
the 3 step general procedure A. The desired product was obtained as a yellow
solid (0.036 g).
M.p.= 214-217 C. 1H NMR (CDC13) 400 MHz 8 10.43 (d, J= 2.0 Hz, 1H), 8.49 (s,
1H),
213
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
8.32 (dd, J= 2.6 and 8.4 Hz, I H), 8.28 (d, J= 2.4 Hz, I H), 6.84 (d, J= 2.4
Hz, I H), 3.15-
3.12 (m, 2H), 3.08-3.03 (m, 2H); LCMS [M+H]: 267.
[0001503] Example 15: Synthesis of 6,7-dihydro-3H-pyrazolo[4,3-
a][3,7]phenanthrolin-
9(8H)-one.
N-NH
N
O N
H
[0001504] The compound, 6,7-dihydro-3H-pyrazolo[4,3-a][3,7]phenanthrolin-9(8H)-
one
was prepared using 2-methoxy-7,8-dihydroquinolin-5(6H)-one as described in the
3 step
general procedure A. The desired product was obtained as a yellow solid (0.005
g). Mp =
290-296 C; iH NMR (DMSO-d6, 400 MHz) 8: 12.46 (brs, 1H), 9.27 (d, J= 9.8 Hz,
1H),
8.42 (s, 1 H), 8.23 (d, J = 2.8 Hz, 1 H), 6.68 (d, J = 2.4 Hz, 1 H), 6.40 (d,
J = 9.8 Hz, 1 H), 2.92
(m, 4H); LC/MS [M+H]: 239.
[0001505] General Procedure B: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
[0001506] Example 16: Synthesis of 1-bromo-7-phenyl-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline.
N-NH N-NH
N Br N
\ I \ I
NBS, CH2CI2
RT, 16 h
[0001507] To a solution of 7-phenyl-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline
(0.018 g, 0.060 mmol) in dichloromethane (2 mL) was added N-bromosuccinimide
(0.011 g,
0.063 mmol). The reaction was stirred at room temperature for 16 hours
followed by addition
of water (2 mL) and dichloromethane (3 mL). The organic layer was separated,
dried using
Na2SO4, filtered and concentrated under reduced pressure. The crude product
was purified
using flash column chromatography (Si02, 10% EtOAc in hexane) to give the pure
desired
product, 1-bromo-7-phenyl-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-c]isoquinoline
as a yellow
solid (0.010 g, 44%). M.p. = 176-178 C; 1H NMR (DMSO-d6, 400 MHz) 8 9.36 (m,
1H),
8.63 (s, 1H), 8.49 (s, 1H), 7.56 (m, 2H), 7.30-7.15 (m, 6H), 4.73 (t, J= 7.2
Hz, 1H), 3.42 (m,
2H); LCMS [M+H]: 377; Elemental analysis (%): C 63.59, H 4.10, N 10.98; Calcd
for
C20H14BrN3: C 63.85, H 3.75, N 11.17.
214
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001508] Example 17: Synthesis of 1-bromo-7-(2-chlorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline.
N-NH
Br / N
CI /
[0001509] The compound, 1-bromo-7-(2-chlorophenyl)-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline was prepared using 7-(2-chlorophenyl)-6,7-
dihydro-3H-
benzo[f]pyrazolo[3,4-c]isoquinoline as described in general procedure B. The
desired product
was obtained as solid. Mp = 180-182 C; 'H NMR (CDC13) 400 MHz 8: 9.47(d,
J=1.2
Hz,1H), 8.38 (s, 1H), 8.22 (s, 1H), 7.43-7.57 (m, 3H), 7.26 (s, 1H), 7.02-7.19
(m, 3H), 6.81
(dd, I H, J=7.8,1.6 Hz), 4.87 (t, I H, J= 6.6 Hz), 3.29-3.43 (m, 2H); LC/MS
[M+H] : 410.
[0001510] Example 18: Synthesis of tert-butyl 4-(4-(7-(2-chlorophenyl)-6,7-
dihydro-3H-
benzo [f]pyrazolo [3,4-c]isoquinolin-1-yl)-1 H-pyrazol-1-yl)piperidine- l -
carboxylate.
0
'O'J~ N
N~ N-NH
N N
Nz~
CI
[0001511] To a solution of 1-bromo-7-(2-chloro-phenyl)-6,7-dihydro-3H-2,3,4-
triaza-
cyclopenta[c]phenanthrene (0.119 g, 0.29 mmol) in toluene (3 mL) and EtOH (1.5
mL) was
added 4-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-pyrazol-1-yl]-
piperidine-l-
carboxylic acid tert-butyl ester-N-acetyl-l-bronic acid (0.110 g, 0.29 mmol)
followed by
aqueous solution of Na2CO3 (0.18 g, 1.74 mmol) in water (1.74 mL) and 1,1-
bis(di-t-
butylphosphino)ferrocene palladium dichloride (BDTBPF-PdC2, CAS#95408-45-0, 15
mg).
The reaction was then purged with nitrogen and heated at 90 C for 16 hours.
On completion
of the reaction, EtOAc (30 mL) and water (20 mL) was added to the reaction
mixture. The
organic layer was separated, washed with brine (2 x 20 mL) and concentrated
under reduced
pressure to give the crude product. The crude product was purified by flash
column
chromatography (Si02, 33% EtOAc in hexane) to give the desired product, 4- {4-
[7-(2-chloro-
phenyl)-6,7-dihydro-3 H-2,3,4-triaza-cyclopenta [c]phenanthren- l -yl]-pyrazol-
l-yl} -
piperidine-l-carboxylic acid tert-butyl ester (0.088 g, 52%), as an orange
solid. M.p. = 139-
215
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
141 C; 400 MHz 1H NMR (DMSO-d6) 8: 9.52-9.47 (, 1H), 8.6 (s, 1H), 8.52 (s,
1H), 8.29 (s,
I H), 8.02 (s, I H), 7.62-7.5 (m, 3H), 7.32-7.25 (m, I H), 7.22-7.15 (m, I H),
7.06-7.02 (m, I H),
6.96-6.92 (m, 1H), 4.82 (t, J = 8 Hz, 1H), 4.48-4.38 (1H), 4.1-4.0 (m, 2H),
3.48-3.32 (m, 2H),
2.92 (br s, 2H), 2.1-2.0 (m, 2H), 1.88-1.76 (m, 2H), 1.42 (s, 9H); LCMS [M+H]:
581.6.
[0001512] Example 19: Synthesis of 7-(2-chlorophenyl)-1 -(1-piperidin-4-yl-lH-
pyrazol-
4-yl)-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-c]isoquinoline.
H N 1-11
N \ N-NH
N~
I N
CI
[0001513] To a solution of 4-{4-[7-(2-chloro-phenyl)-6,7-dihydro-3H-2,3,4-
triaza-
cyclopenta[c]phenanthren-l-yl]-pyrazol-l-yl}-piperidine-l-carboxylic acid tert-
butyl ester
(0.068 g, 0.117 mmol) in dichloromethane (5 mL) was added 4M HC1(g) in dioxane
(0.3
mL). The reaction was shaken overnight at room temperature. A lot of orange
solid were
formed. On completion, toluene (1 mL) was added and the solvent removed under
reduced
pressure. The crude product was purified using reverse phase HPLC to give the
desired
product, 7-(2-chloro-phenyl)-1-(1-piperidin-4-yl-lH-pyrazol-4-yl)-6,7-dihydro-
3H-2,3,4-
triaza-cyclopenta[c]phenanthrene TFA salt, as an orange solid (0.042 g, 60%).
Mp >225 C;
1H NMR (DMSO-d6) 400 MHz 8: 9.46 (d, J=7.43, 1H), 8.61 (s, 1H), 8.5 (brs, 2H),
8.25 (s,
1H), 8.04 (s, 1H), 7.48-7.58 (m, 3H), 7.14-7.25 (m, 2H), 6.90-7.02 (m, 2H),
4.79 (brs, 1H),
3.30-3.41 (m, 2H), 3.03-3.13 (m, 2H), 2.10-2.18 (m, 4H); LC/MS [M+H]: 481.
[0001514] General Procedure C: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
H2N N'N N-N / O
\ N
O O I
~
DMF-DMA I \ / N~ 0 / I \ \
R2 R2 I R2
100 C, 24 h CF,COOH
R1 Step-I R1 100 C, 24 h R1
Step-2
N-NH
N
CF3000H _ I \
100 C, 6 h R2
Step-3 R
1
216
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001515] Example 20: Synthesis of 3,6,7,8-tetrahydrobenzo[6,7]cyclohepta[1,2-
d]pyrazolo[3,4-b]pyridine.
N-NH
~/N
[0001516] Step 1: Synthesis of (E)-6-((dimethylamino)methylene)-6,7,8,9-
tetrahydro-
5H-benzo [7]annulen-5 -one.
o
N
C f \
[0001517] A solution of 6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-one (1.0 g,
6.24
mmol) in 1,1-dimethoxy-N,N-dimethylmethanamine (2.5 mL) was heated at 100 C
for 24
hours. On completion of the reaction, the solvent was removed under reduced
pressure. The
oily residue was triturated with n-hexane (2 x 50 mL) to give the desired pure
product, (E)-6-
((dimethylamino)methylene)-6,7,8,9-tetrahydro-5 H-benzo [7] annulen-5 -one
(1.1 g, 82%) as a
brown solid. 1H NMR (CDC13, 400 MHz): 8 7.75 (s, 1H), 7.62 (d, J = 6.2 Hz,
1H), 7.34-7.25
(m, 2H), 7.09 (d, J = 6.4 Hz, 1H), 3.11 (s, 6H), 2.75 (t, J = 6.8 Hz, 2H),
2.35 (t, J = 6.4 Hz,
2H), 1.83 (quintet, J= 6.8 Hz, 2H). LCMS [M+H]: 216.
[0001518] Step 2: Synthesis of 3-(4-methoxybenzyl)-3,6,7,8-
tetrahydrobenzo [6,7] cyclohepta[ 1,2-d]pyrazolo [3,4-b]pyridine.
0
N-N
N
[0001519] To a mixture of 6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-one (8.5 g,
39.5
mmol) and 1-(4-methoxybenzyl)-1H-pyrazol-5-amine (8.2 g, 44.47 mmol) was added
trifluoroacetic acid (4.25 mL, 54.3 mmol). The reaction mixture was heated at
100 C for 24
hours. On completion of the reaction, the solvent was removed under reduced
pressure and
the crude product was purified by flash column chromatography (Si02, gradient
from 5%
EtOAc in hexanes to 30% EtOAc in hexanes) to give the desired product (4.2g,
30%) as an
off-colored solid. 1H NMR (CDC13, 400 MHz) 8: 7.97 (s, 1H), 7.85 (s, 1H), 7.79
(d, J = 7.2
Hz, I H), 7.46-7.44 (m, I H), 7.38 (d, J = 7.6 Hz, 2H), 7.28 (s, I H), 7.26
(s, I H), 6.84 (d, J =
7.6 Hz, 2H), 5.69 (s, 2H), 3.76 (s, 3H), 2.59 (q, J = 6.0 Hz, 4H), 2.21
(quintet, J = 7.2 Hz,
2H); LCMS [M+H]: 356.
217
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001520] Step 3: Synthesis of 3,6,7,8-tetrahydrobenzo[6,7]cyclohepta[1,2-
d]pyrazolo[3,4-b]pyridine.
N-NH
~/N
[0001521] A solution of 3-(4-methoxybenzyl)-3,6,7,8-
tetrahydrobenzo[6,7]cyclohepta[1,2-d]pyrazolo[3,4-b]pyridine (2.0 g, 5.63
mmol) in
trifluoroacetic acid (10 mL) was heated at 100 C for 6 hours. On completion
of the reaction,
the solvent removed under reduced pressure. The residue was dissolved in water
(20 mL) and
then it was basified to pH=10 using ammonium hydroxide. The aqueous layer was
extracted
with 10% methanol in dichloromethane (2 x 100mL). The organic extracts were
combined,
dried using sodium sulfate and concentrated under reduced pressure. The crude
product was
purified using flash column chromatography (Si02, gradient 100%
dichloromethane to 2%
methanol in dichloromethane) to give the pure desired product (0.9 g, 68%) as
off-colored
solid. 1H NMR (DMSO-d6, 400 MHz): 6 13.58 (s, 1H), 8.14 (s, 1H), 8.10 (s, 1H),
7.66 (t, J=
8.0 Hz, 1H), 7.44-7.36 (m, 3H), 7.32 (t, J= 8.0 Hz, 1H), 2.60-2.38 (m, 4H),
2.15 (quintet,
3H); LCMS [M+H]: 236.
[0001522] Example 21: Synthesis of 3,6-dihydrochromeno[4,3-d]pyrazolo[3,4-
b]pyridine.
N-NH
N
O
[0001523] The compound, 3,6-dihydrochromeno[4,3-d]pyrazolo[3,4-b]pyridine was
prepared using chroman-4-one as described in the 3 step general procedure C.
The desired
product was obtained as a yellow solid (0.230 g, 50%). 1H NMR (DMSO-d6, 400
MHz): 6
13.66 (s, 1 H), 8.21 (d, J = 7.6 Hz, 1 H), 8.14 (s, 1 H), 8.11 (s, 1 H), 7.40
(t, J = 7.6 Hz, 1 H),
7.15 (t, J = 7.6 Hz, 1H), 7.03 (d, J = 8 Hz, 1H), 5.34 (s, 2H); LCMS [M+H]:
224.
[0001524] Example 22: Synthesis of 3,6-dihydropyrazolo[3,4-b]thiochromeno[4,3-
d]pyridine.
N-NH
N
S
218
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001525] The compound 3,6-dihydropyrazolo[3,4-b]thiochromeno[4,3-d]pyridine
was
prepared using thiochroman-4-one as described in the 3 step general procedure
C. The
desired product was obtained as a yellow colored solid (0.400 g, 30%). 'H NMR
(CDC13,
400MHz): 6 10.55 (s, 1H), 8.43 (d, J = 6.8 Hz, 1H), 8.07 (s, 1H), 7.93 (s,
1H), 7.41-7.25 (m,
3H), 4.07 (s, 2H); LCMS [M+H]: 240.
[0001526] Example 23: Synthesis of 7-methyl-6,7-dihydro-3H-
benzo[f]pyrazolo[3,4-
c]isoquinoline.
N-NH
N
[0001527] The compound, 7-methyl-6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinoline
was prepared using 4-methyl-3,4-dihydronaphthalen-1(2H)-one as described in
the 3 step
general procedure C. The desired product was obtained as a yellow colored
solid (0.230 g,
70%). 'H NMR (DMSO-d6, 400MHz): 6 13.53 (s, 1H), 8.30 (dd, J = 8.8, 4.0 Hz,
1H), 8.05
(s, 2H), 7.34-7.42 (m, 3H), 3.09-3.18 (m, 2H), 2.83 (dd, J= 12.0, 5.6 Hz, 1H),
1.17 (s) and
1.15 (s), 3H; LCMS [M+H]: 236.
[0001528] Example 24: Synthesis of 6-methyl-3,6-dihydroindeno[1,2-
d]pyrazolo[3,4-
b]pyridine.
N-NH
N
[0001529] The compound, 6-methyl-3,6-dihydroindeno[1,2-d]pyrazolo[3,4-
b]pyridine
was prepared using 3-methyl-2,3-dihydro-lH-inden-l-one as described in the 3
step general
procedure C. The desired product was obtained as a yellow colored solid (0.400
g, 41 %). 'H
NMR (DMSO-d6, 400MHz): 6 12.84 (bs, 1H), 8.40 (s, 1H), 8.14 (s, 2H), 7.49-7.60
(m, 3H),
4.07 (q, J = 7.2 Hz, 1H), 1.61 (d, J = 7.2 Hz, 1H); LCMS [M+H]: 222.
[0001530] Example 25: Synthesis of 3,7-dihydroisothiochromeno[4,3-
d]pyrazolo[3,4-
b]pyridine.
N--NH
N
S
[0001531] The compound, 3,7-dihydroisothiochromeno[4,3-d]pyrazolo[3,4-
b]pyridine
was prepared using isothiochroman-4-one as described in the 3 step general
procedure C. The
219
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
desired product was obtained as a yellow colored solid (0.500 g, 75%). 'H NMR
(CDC13,
400MHz): 6 13.70 (1H, s), 8.27-8.24 (m, 2H), 8.07 (s, 1H), 7.48-7.39 (m, 3H),
4.05 (s, 2H);
LCMS [M+H]: 240.
[0001532] Example 26: Synthesis of 6,7-dihydro-3H-[1]benzoxepino[5,4-
d]pyrazolo[3,4-
b]pyridine.
N-NH
N
O
[0001533] The compound, 6,7-dihydro-3H-[1]benzoxepino[5,4-d]pyrazolo[3,4-
b]pyridine was prepared using 3,4-dihydrobenzo[b]oxepin-5(2H)-one as described
in the 3
step general procedure C. The desired product was obtained as a yellow colored
solid (0.150
g, 75%). 'H NMR (DMSO-d6, 400MHz): 6 13.65 (s, 1H), 8.17 (s, 1H), 8.14 (s,
1H), 7.77 (d,
J = 7.6 Hz, I H), 7.47 (t, J = 7.6 Hz, I H), 7.32 (t, J = 8.0 Hz, I H), 7.16
(d, J = 8.0 Hz,
1H), 4.49 (t, J = 6.0 Hz, 2H)), 2.89 (t, J = 5.6 Hz, 2H); LCMS [M+H]: 238.
[0001534] Example 27: Synthesis of 6-phenyl-3,6-dihydrochromeno[4,3-
d]pyrazolo[3,4-
b]pyridine.
N-NH
/ N
O
[0001535] The compound, 6-phenyl-3,6-dihydrochromeno[4,3-d]pyrazolo[3,4-
b]pyridine
was prepared using 2-phenylchroman-4-one as described in the 3 step general
procedure C.
The desired product was obtained as a yellow colored solid (0.142 g, 66%). 1H
NMR
(DMSO-d6, 400 MHz): 6 13.72 (s, 1H), 8.22 (t, J = 7.6 Hz, 1H), 8.12 (s, 1H),
7.80 (s, 1H),
7.44-7.30 (m, 6H), 7.14 (t, J = 7.2 Hz, 1H), 7.06 (d, J = 7.6 Hz, 1H), 6.62
(s, 1H); LCMS
[M+H]: 300.
[0001536] Example 28: Synthesis of 3,6-dihydroindeno[1,2-d]pyrazolo[3,4-
b]pyridine.
N-NH
N
[0001537] The compound, 3,6-dihydroindeno[1,2-d]pyrazolo[3,4-b]pyridine was
prepared using 2,3-dihydro-lH-inden-l-one as described in the 3 step general
procedure C.
The desired product was obtained as a solid (0.5 g, 79%). 1H NMR (CD3OD, 400
MHz): 6
220
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
8.30 (s, 1H), 8.15-8.10 (m, 1H), 8.05 (s, 1H), 7.79-7.70 (m, 1H), 7.52-7.48
(m, 2H), 4.00 (s,
2H); LCMS [M+H]: 208.
[0001538] Example 29: Synthesis of 3,6,7,8-
tetrahydropyrazolo[4',3':5,6]pyrido[3,4-
d][1]benzazepine
N-NH
N
N
H
[0001539] The compound, 3,6,7,8-tetrahydropyrazolo[4',3':5,6]pyrido[3,4-
d][1]benzazepine was prepared using 3,4-dihydro-lH-benzo[b]azepin-5(2H)-one as
described
in the 3 step general procedure C. The desired product was obtained as a solid
(0.022 g,
19%). M.p. = 215-217 C, 'H NMR (DMSO-d6, 400MHz): 6 8.05 (s, 1H), 7.9 (s,
1H), 7.8-
7.72 (m, 1H), 6.8-6.7 (m, 2H), 7.2-7.12 (m, 2H), 5.78-5.7 (m, 2H), 3.6-3.52
(m, 2H); LCMS
[M+H]: 237.
[0001540] Example 30: Synthesis of N-(6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinolin-9-yl)acetamide.
N-NH
O O
N
Ac2O, Pyridine O General Procedure C
HZN I/ RT, 3 h N I/ O
Step-1 H AN
H
[0001541] Step 1: Synthesis of N-(5-oxo-5,6,7,8-tetrahydronaphthalen-2-
yl)acetamide.
To a solution of 6-amino-3,4-dihydronaphthalen-1(2H)-one (0.5 g, 3.1 mmol) in
pyridine
(5mL) was added acetic anhydride (0.38 g,3.7 mmol). The solution was stirred
at room
temperature for 3 hours. The reaction was then diluted with dichloromethane
(50 mL). The
organic layer was separated, washed with 2M HC1(2 x 25 mL), brine (50 mL),
dried using
sodium sulfate and concentrated under reduced pressure. The crude was product
was obtained
as an off colored solid (0.5 g, 79%) and used in the next reaction without any
further
purification. 1H NMR (CDC13, 400MHz): 6 8.94 (d, J= 8.0 Hz, 1H), 7.72 (s, 1H),
7.62 (s,
1 H), 7.20 (d, J = 8.0 Hz, 1 HO, 2.96 (t, J = 6.2 Hz, 2H), 2.62 (quintet, J =
6.0 Hz, 2H), 2.12 (s,
3H), 2.11 (t, J= 6.0 Hz, 2H); LCMS [M+H]: 204.
[0001542] Step 2: Synthesis of N-(6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinolin-9-
yl)acetamide.
221
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N-NH
N
O
AN
H
[0001543] The compound, N-(6,7-dihydro-3H-benzo[f]pyrazolo[3,4-c]isoquinolin-9-
yl)acetamide was prepared using N-(5-oxo-5,6,7,8-tetrahydronaphthalen-2-
yl)acetamide as
described in the 3 step general procedure C. The desired product was obtained
as a yellow
colored solid (0.25 g, 99%). 'H NMR (CDC13, 400MHz): 6 13.46 (s, 1H), 10.08
(s, 1H), 8.20
(d, J= 8.0 Hz, 1H), 8.05 (s, 1H), 5.00 (s, 1H), 7.66 (d, J= 8.0 Hz, 1H), 7.60
(s, 1H), 3.00 (t, J
= 6.0 Hz, 2H), 2.84 (t, J= 6.0 Hz, 1H). 2.05 (s, 3H); LCMS [M+H]: 279.
[0001544] General Procedure D: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
[0001545] Example 31: Synthesis of 6,7-dihydro-3H-furo[3,2-f]pyrazolo[3,4-
c]isoquinoline.
N--NH
O O / N
I
I Bredreck's Reagent, Toluene / / i ' General Procedure C / I \
O 100 C, 4 days O O
[0001546] Step 1: Synthesis of (E)-5-((dimethylamino)methylene)-6,7-
dihydrobenzofuran-4(5H)-one. A solution of 6,7-dihydrobenzofuran-4(5H)-one
(1.0 g, 7.3
mmol) and Bredreck's reagent (1.53 g, 8.81 mmol) in toluene (10 mL) was heated
at 100 C
for 4 days. On completion of the reaction, the solvent was removed under
reduced pressure to
give the desired product as a brown oil (0.6 g, 43%). The crude product was
used in the next
step without any further purification. LCMS [M+H]: 192.
[0001547] Step 2: Synthesis of 6,7-dihydro-3H-furo[3,2-f]pyrazolo[3,4-
c]isoquinoline.
The compound, 6,7-dihydro-3H-furo[3,2-f]pyrazolo[3,4-c]isoquinoline was
prepared using
(E)-5-((dimethylamino)methylene)-6,7-dihydrobenzofuran-4(5H)-one as described
in the
general procedure C (Steps 2 and 3). The desired product was obtained as a
yellow colored
solid (0.035 g, 55%). 'H NMR (CDC13, 400MHz): 6 13.4 (s, 1H), 8.00 (s, 2H),
7.70 (s, 1H),
6.90 (s, 1H), 3.09 (t, J = 6.2 Hz, 2H), 2.96 (t, J = 6.2 Hz, 2H); LCMS [M+H]:
212.
[0001548] Example 32: Synthesis of 6,7-dihydro-3H-pyrazolo[3,4-c]thieno[3,2-
f]isoquinoline.
N-NH
N
222
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001549] The compound, 6,7-dihydro-3H-pyrazolo[3,4-c]thieno[3,2-
f]isoquinoline was
prepared using 6,7-dihydrobenzo[b]thiophen-4(5H)-one as described in the 3
step general
procedure D. The desired product was obtained as a yellow colored solid (0.130
g, 55%). 'H
NMR (DMSO-d6, 400 MHz): 6 13.45 (s, 1H), 8.02 (s, 1H), 8.01 (s, 1H), 7.60 (d,
J = 4.8 Hz,
I H), 7.44 (d, J = 4.8 Hz, I H), 3.12 (t, J = 6.8 Hz, 2H), 3.03 (t, J = 7 Hz,
2H); LCMS [M+H] :
228.
[0001550] Example 33: Synthesis of 3H-spiro[chromeno[4,3-d]pyrazolo[3,4-
b]pyridine-
6,4'-piperidine].
N-NH
N
O
NH
[0001551] The compound, 3H-spiro[chromeno[4,3-d]pyrazolo[3,4-b]pyridine-6,4'-
piperidine] was prepared using tert-butyl 4-oxospiro[chroman-2,4'-piperidine]-
l'-carboxylate
as described in the 3 step general procedure D. The desired product was
obtained as a solid
(0.015 g, 42%). 'H NMR (CDC13, 400MHz): 6 8.057 (s, 1H), 8.05 (s, 1H), 7.88
(dd, J= 8.0
Hz, I H), 7.32 (t, J= 8.0 Hz, I H), 7.078 (d, J= 8.0 Hz, I H), 6.90(d, J= 8.0
Hz), 5.94 (s, I H),
3.59 (d, J= 3.2 Hz, 2H), 2.95 (t, J= 5.2 Hz, 2H), 2.10-2.00 (m, 4H); LCMS
[M+H]: 293.
[0001552] Example 34: Synthesis of 6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinolin-
8-01.
N-NH
O O
N
TBDMS-CI, Imidazole General Procedure D
1~q RT, 16 h I
Step 1
OH OTBDMS
OH
[0001553] Step-1: Synthesis of 5-(tert-butyldimethylsilyloxy)-3,4-
dihydronaphthalen-
1(2H)-one. To a solution of 5-hydroxy-3,4-dihydronaphthalen-1(2H)-one (0.5 g,
3.08 mmol)
in DMF (5 mL) was added Imidazole (0.527 g, 7.74 mmol) and tert-
butyldimethylsilyl
chloride (0.581 g, 3.8 mmol). The reaction mixture was stirred at room
temperature for 16
hours. Ice-cooled water (50mL) was then added and the reaction mixture
extracted with
EtOAc (3 x 50mL). The organic extracts were combined, washed with 2N NaOH (2 x
30
mL), water (50 mL), dried using sodium sulfate and concentrated under reduced
pressure to
give (0.6g, 70%) of the desired product as an oil. The crude product was used
in the next step
without further purification. 1H NMR (CDC13, 400MHz): 6 7.96 (d, J = 8.0 Hz,
1H), 7.18 (t,
J = 8.0 Hz, I H), 6.96 (d, J = 8.0 Hz, I H), 2.87 (t, J = 6.0 Hz, 2H), 2.61
(t, J = 6.2 Hz, 2H),
2.14 (quintet, J = 6.0 Hz, 2H), 1.04 (s, 9H), 0.14 (s, 6H); LCMS (M+H): 277.
223
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001554] Step 2: Synthesis of 6,7-dihydro-3H-benzo[f]pyrazolo[3,4-
c]isoquinolin-8-ol.
The compound, 6,7-dihydro-3H-benzo[f]pyrazolo[3,4-c]isoquinolin-8-ol was
prepared using
5-(tert-butyldimethylsilyloxy)-3,4-dihydronaphthalen-1(2H)-one as described in
the 3 step
general procedure D. The desired product was obtained as a solid (0.2g, 37%).
1H NMR
(DMSO-d6, 400MHz): 6 13.50 (s, 1H), 9.48 (s, 1H), 8.10 (s, 2H), 7.82 (d, J = 8
Hz, 1H), 7.22
(t, J = 8 Hz, 1H), 6.90 (d, J = 8 Hz, 1H), 3.16-2.94 (m, 2H), 2.90-2.82 (m,
2H); LCMS
[M+H]: 238.
[0001555] Example 35: Synthesis of 3-(4-methoxybenzyl)-9-(methylsulfanyl)-6,7-
dihydro-3H-pyrazolo[4',3':5,6]pyrido[4,3-f]quinazoline.
iN NH 0
O O DMF-DMA O O SNH2 IN DMF-DMA
L RT, 16 h NaOAc, DMF I NISI Reflux,
100 C, 5 h
Step-1 Step-2 Step-3
N
O HzN -OCH3 NON
N z' N N
NS CF,COOH, 100 C, 16 h N
Step-4 S I N
[0001556] Step 1: Synthesis of 2-((dimethylamino)methylene)cyclohexane- 1,3-
dione. A
mixture of cyclohexane-1,3-dione (10 g, 89 mmol) and DMF-dimethylacetal (21.2
mL, 178
mmol) was stirred at room temperature for 16 hours. On completion of the
reaction, the
reaction mixture was concentrated under reduced pressure and the residue
titurated with
hexane (50 mL) to give the desired product as a solid. 1H NMR (CDC13, 400MHz):
6 8.0 (s,
1H), 3.35 (s, 3H), 3.14 (s, 3H), 2.41 (t, J = 6.2 Hz, 4H), 1.92 (quintet, J =
6.2 Hz, 2H).
LCMS [M+H]: 168.
[0001557] Step 2: Synthesis of 2-(methylthio)-7,8-dihydroquinazolin-5(6H)-one.
To a
solution of 2-((dimethylamino)methylene)cyclohexane-1,3-dione (0.5 g, 2.9
mmol) in DMF
(2mL) was added sodium acetate (0.39 g, 4.7 mmol) and methyl
carbamimidothioate (0.9 g,
3.5 mmol). The reaction mixture was heated at 100 C for 5 hours. On
completion of the
reaction, the reaction mixture was diluted with dichloromethane (30 mL). The
organic layer
was separated and washed with water (2 X 30 mL). The solvent was then removed
under
reduced pressure and the residue dissolved in EtOAc (50 mL) and washed with
water (2 x 30
mL). The organic layer was dried using sodium sulfate and concentrated under
reduced
pressure to give the desired product (0.5 g, 86%) as a solid. 1H NMR (CDC13,
400MHz): 6
224
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
8.94 (s, 1H), 3.01 (t, J = 6 Hz, 2H), 2.64 (t, J = 6.0 Hz, 2H), 2.58 (s, 3H),
2.14 (quintet, J =
6.2 Hz, 2H); LCSM [M+H]: 195.
[0001558] Step 3: Synthesis of (E)-6-((dimethylamino)methylene)-2-(methylthio)-
7,8-
dihydroquinazolin-5(6H)-one. To a solution of 2-(methylthio)-7,8-
dihydroquinazolin-5(6H)-
one (0.2 g, 1.0 mmol) in toluene (3 mL) was added DMFDMA (0.2 mL, 1.5 mmol).
The
reaction mixture was then refluxed for 16 hours. The reaction mixture was then
cooled to
room temperature and concentrated under reduced pressure. The crude compound
was
purified by flash column chromatography (Si02, gradient from 100%
dichloromethane to 2%
methanol in dichloromethane) to give the pure desired product (0.15 g, 60%) as
a solid. 1H
NMR (CDC13, 400MHz): 6 9.0 (s, 1H), 7.72 (s, 1H), 3.18 (s, 6H), 2.98 (t, J =
6.0 Hz, 2H),
2.92 (t, J = 6.0 Hz, 2H), 2.60 (s, 3H); LCMS [M+H]: 250.
[0001559] Step 4: Synthesis of 3-(4-methoxybenzyl)-9-(methylsulfanyl)-6,7-
dihydro-3H-
pyrazolo[4',3':5,6]pyrido[4,3-f]quinazoline. To a mixture of (E)-6-
((dimethylamino)methylene)-2-(methylthio)-7,8-dihydroquinazolin-5(6H)-one (8
g, 32
mmol) and 1-(4-methoxybenzyl)-1H-pyrazol-5-amine (7.2 g, 35 mmol) was added
trifluoroacetic acid (3 mL). The reaction mixture was heated at 100 C for 16
hours. The
reaction was cooled to room temperature and diluted with a 1:1 mixture of
methanol and
isopropanol (40 mL). The mixture was stirred for 30 minutes and filtered. The
solid obtained
was then further purified using flash column chromatography (Si02, gradient
from 100%
chloroform to 2% EtOAc in chloroform) to give the desired product (3.0 g, 24%)
as a yellow
solid. 1H NMR (CDC13, 400MHz): 6 9.52 (s, 1H), 7.98 (s, 1H), 7.82 (s, 1H),
7.40 (d, J = 8.0
Hz, 2H), 6.82 (d, J = 8.0 Hz, 2H), 5.64 (s, 2H), 3.78 (s, 3H), 3.16 (t, J =
6.0 Hz, 2H), 3.10 (t,
J = 6.2 Hz, 2H), 2.65 (s, 3H); LCMS [M+H]: 390.
[0001560] Example 36: Synthesis of 3-(4-methoxybenzyl)-9-(methylsulfonyl)-6,7-
dihydro-3H-pyrazolo[4',3':5,6]pyrido[4,3-f]quinazoline
N_N c / O N_N c / O
N Oxone , MeOH, H2O / N
I RT, 18 h
N N
11 'Jill
S N OS,O N
[0001561] To a suspension of 3-(4-methoxybenzyl)-9-(methylsulfanyl)-6,7-
dihydro-3H-
pyrazolo[4',3':5,6]pyrido[4,3-f]quinazoline (1.9 g, 4.8 mmol) in 2:1 mixture
of methanol-
water (100 mL) was added potassium monopersulfate triple salt (Oxone , 9.2 g,
15 mmol)
and the suspension stirred for 18 hours. On completion of the reaction, the
mixture was
concentrated under reduced pressure. The residue was dissolved in ethyl
acetate (200 mL)
225
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
and washed with water (2 x 20 mL), dried using anhydrous sodium sulfate,
filtered and
concentrated under reduced pressure to give the desired product, 3-(4-
methoxybenzyl)-9-
(methylsulfonyl)-6,7-dihydro-3H-pyrazolo[4',3':5,6]pyrido[4,3-f]quinazoline
(1.5 g, 77%) as
a tan solid. LCMS [M+H]: 422. The compound was used without any further
purification.
[0001562] General Procedure E: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
[0001563] Synthesis of N-substituted 6,7-dihydro-3H-
pyrazolo[4',3':5,6]pyrido[4,3-
f]quinazolin-9-amines.
N-N / O N-NH
H
N (i) Ri N, R2, DMSO, 100 C, 1 h / N
IIII (i) CF3COOH, 60 C, 17 h IN II
OSO N RZ, N N
R1
[0001564] Step 1: To a 0.5M solution of sulfone (0.1 mL, 50 mole) in DMSO was
added a 0.5M solution of amine (0.1 mL, 50 mole) in DMSO. The reaction
mixture was
heated at 100 C for 1 hour. The reaction was then concentrated under reduced
pressure to
give an oily residue, which was used in Step 2 without any further
purification.
[0001565] Step 2: To the oily residue obtained from step (i) was added
trifluoroactic acid
(1 mL). The reaction mixture was then shaken at 60 C for 17 hours. The
reaction was then
concentrated under reduced pressure. The crude product was purified using
reverse phase
HPLC to give the desired product as a solid.
[0001566] Examples 37-67: Compounds in Table 3 are synthesized by using
general
procedure E as described herein.
Table 3.
Structure IUPAC Name LC/MS
[M+1]
N-NH
N N-(2-morpholin-4-ylethyl)-6,7-
dihydro-3H- 352
O") NII pyrazolo[4',3':5,6]pyrido[4,3-
N '-"--"N N f] quinazolin-9-amine
H
N-NH
N N-(4-tert-butylbenzyl)-6,7-
N - dihydro-3H- 385
N N pyrazolo[4',3':5,6]pyrido[4,3-
fJ quinazolin-9-amine
226
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N-NH
9-(4-morpholin-4-ylpiperidin- 1 -
yl)-6,7-dihydro-3H- 392
pyrazolo[4',3':5,6]pyrido[4,3-
~N N f] quinazoline
~N
N-NH
N N-(2-pyrrolidin- 1 -ylethyl)-6,7-
dihydro-3H-
N 336
pyrazolo[4',3':5,6]pyrido[4,3-
ON N N f]quinazolin-9-amine
H
N-NH
N N-(3-morpholin-4-ylpropyl)-6,7-
N dihydro-3H- 366
pyrazolo[4',3':5,6]pyrido[4,3-
N N N f] quinazolin-9-amine
0,-) H
~/ N-NH
N N-(3-fluorobenzyl)-6,7-dihydro-
N 3H-pyrazolo[4',3':5,6]pyrido[4,3- 347
F 1] quinazolin-9-amine
H N
N-NH
9-morpholin-4-yl-6,7-dihydro-
N 3H-pyrazolo[4',3':5,6]pyrido[4,3- 309
f] quinazoline ill N N
O
N-NH
9-(4-pyridin-4-ylpiperazin-l-yl)-
6,7-dihydro-3H- 385
pyrazolo[4',3':5,6]pyrido[4,3-
N N f] quinazoline
N
N
N-NH
N-(2-chlorobenzyl)-6,7-dihydro-
N 3H-pyrazolo[4',3': 5,6]pyrido[4,3- 363
1] quinazolin-9-amine
H N
~ CI
227
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N-NH
N N-(pyridin-3-ylmethyl)-6,7-
dihydro-3H-
N 330
pyrazolo[4',3':5,6]pyrido[4,3-
INI ~ I H N N f]quinazolin-9-amine
`
N--NH
N N-[2-(3-chlorophenyl)ethyl]-6,7-
dihydro-3H- 377
/ N v pyrazolo[4',3':5,6]pyrido[4,3-
CI \ I NN f]quinazolin-9-amine
H
N--NH
N N-(3-phenylpropyl)-6,7-dihydro-
N 3H-pyrazolo[4',3': 5,6]pyrido[4,3- 357
f] quinazolin-9-amine
H
N-NH
N N-(pyridin-2-ylmethyl)-6,7-
dihydro-3H-
N 330
pyrazolo[4', 3': 5,6]pyrido[4,3 -
N N N f]quinazolin-9-amine
H
N--NH
N 9-(4-benzylpiperidin-1-yl)-6,7-
N dihydro-3H- 397
I pyrazolo[4',3':5,6]pyrido[4,3-
N N f] quinazoline
N-NH
N N-[2-(1 -methylpyrrolidin-2-
yl)ethyl] -6,7-dihydro-3H- 350
N N pyrazolo[4',3':5,6]pyrido[4,3-
f]quinazolin-9-amine
N N
H
N--NH
N 1-[3-(6,7-dihydro-3H-
N pyrazolo[4',3':5,6]pyrido[4,3- 364
f]quinazolin-9-
12~N N ylamino)propyl]pyrrolidin-2-one
H
O
N-NH
N N-[2-(4-fluorophenyl)ethyl]-6,7-
F dihydro-3H- 361
/ N pyrazolo[4',3':5,6]pyrido[4,3-
\ I N N f] quinazolin-9-amine
H
228
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N-NH
N-(4-chlorobenzyl)-6,7-dihydro-
NII 3H-pyrazolo[4',3':5,6]pyrido[4,3- 363
H N f] quinazolin-9-amine
CI ""~
N-NH
N
N-(cycloheptylmethyl)-6, 7-
N dihydro-3H- 349
pyrazolo[4',3':5,6]pyrido[4,3-
f] quinazolin-9-amine
N N
H
N-NH
N 9-(3,4-dihydroisoquinolin-2(1H)-
V yl)-6,7-dihydro-3H- 355
IN pyrazolo[4',3':5,6]pyrido[4,3-
N I N fJ quinazoline
N-NH
N N-[3-(trifluoromethyl)benzyl]-
N 6,7-dihydro-3H- 397
pyrazolo[4', 3': 5,6]pyrido[4,3 -
F3C
II N)! N flquinazolin-9-amine
N-NH
N-benzyl-N-methyl-6,7-dihydro-
N 3H-pyrazolo[4',3': 5,6]pyrido[4,3- 343
f]quinazolin-9-amine
\ N N
N-NH
N N-(3,4-dichlorobenzyl)-6,7-
N dihydro-3H- 397
Ill, pyrazolo[4',3':5,6]pyrido[4,3-
CI
\ N f]quinazolin-9-amine
H
CI
N--NH
N N-(2-phenoxyethyl)-6,7-dihydro-
N 3H-pyrazolo[4',3':5,6]pyrido[4,3- 359
\\ 0~, 1]quinazolin-9-amine
N-NH
N N-(3-methoxybenzyl)-6,7-
N dihydro-3H- 359
pyrazolo[4',3':5,6]pyrido[4,3-
iO / II H N flquinazolin-9-amine
229
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N-NH
I N N-(2-chloro-6-fluorobenzyl)-6,7-
F N dihydro-3H- 381
pyrazolo[4',3':5,6]pyrido[4,3-
1] quinazolin-9-amine
I N
CI
N-NH
N 9-pyrrolidin-1-yl-6, 7-dihydro-3 H-
N pyrazolo[4',3':5,6]pyrido[4,3- 293
f] quinazoline
GN N
N-NH
N 9-[4-(2-oxo-2-pyrrolidin-1-
ylethyl)piperazin-1-yl]-6,7-
dihydro-3 H- 419
O N N pyrazolo[4',3':5,6]pyrido[4,3-
GN N J f] quinazoline
N-NH
N 9-(4-benzylpiperazin-1-yl)-6,7-
N dihydro-3H- 398
pyrazolo[4',3':5,6]pyrido[4,3-
- N N f] quinazoline
~ ~ NJ
N-NH
N N-(3-piperidin-1-ylpropyl)-6,7-
N dihydro-3H- 364
pyrazolo[4',3':5,6]pyrido[4,3-
N N N f] quinazolin-9-amine
H
N--NH
N 9-[4-(2-chlorobenzyl)piperazin-1-
N yl]-6,7-dihydro-3H- 432
pyrazolo[4',3':5,6]pyrido[4,3-
cci N IN f] quinazoline
[0001567] Example 68: Synthesis of N-methyl-6,7-dihydro-3H-
pyrazolo[4',3':5,6]pyrido[4,3-f]quinazolin-9-amine.
230
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N NH
~ J~ O O
100 0 H N NH2 IN DMF-DMA -N N
NaOAc, DMF NN Reflux, NN
0-
C,5h
Step-1 H Step-2 H
- NON / O N-NH
H2N OCH3 N CF3COOH N
CF3COOH, 100 C, 16 h N 100100 N
Step-3 N'11 N Step-4 N'ill N
H H
[0001568] Step 1: Synthesis of 2-(methylamino)-7,8-dihydroquinazolin-5(6H)-
one. To
anhydrous ethanol (15 mL) was added sodium metal (0.45 g, 19.5 mmol). After
complete
dissolution of sodium metal in ethanol, 2-
((dimethylamino)methylene)cyclohexane-1,3-dione
(1 g, 5.5 mmol) and N-methylguanidine hydrochloride (1 g, 9.0 mmol) were added
and the
reaction was refluxed for 4 hours. The reaction mixture was cooled to room
temperature and
concentrated under reduced pressure. Ice-cooled water (50 mL) was then added
to the residue
and the aqueous solution extracted with EtOAc (3 x 40 mL). The combined
organic extract
was dried using sodium sulfate and concentrated under reduced pressure to give
the desired
product (0.6 g, 57%) as a solid. The crude product was used in the next step
without further
purification. 1H NMR (CDC13, 400MHz): 6 8.90 and 8.80 (2s, 1H), 5.98 and 5.82
(2s, 1H),
3.11 and 3.09 (2s, 3H), 2.96-2.88 (m, 2H), 2.58 (t, J = 6.0 Hz, 2H), 2.10
(quintet, J = 6.0 Hz,
2H). LCMS [M+H]: 178.
[0001569] Step 2: Synthesis of (E)-6-((dimethylamino)methylene)-2-
(methylamino)-7,8-
dihydroquinazolin-5(6H)-one. To a solution of 2-(methylamino)-7,8-
dihydroquinazolin-
5(6H)-one (0.2 g, 1 mmol) in toluene (3 mL) was added 1, 1-dimethoxy-N,N-
dimethylmethanamine (0.35 mL, 2.9 mmol) and triethylamine (0.15 mL, 1 mmol).
The
reaction mixture was refluxed for 16 hours. The reaction mixture was then
concentrated
under reduced pressure. The crude product was purified by flash column
chromatography
(Si02, gradient from 100% chloroform to 2% methanol in chloroform) to give the
desired
product (0.1 g, 38%) as solid. 1H NMR (CDC13, 400MHz): 6 8.84 (bs, 1H), 7.62
(s, 1H), 5.68
(bs, 1H), 3.15 (s, 6H), 3.12 (d, J = 6.0 Hz, 3H), 3.10-3.05 (m, 2H), 3.03-2.98
(m, 2H). LCMS
[M+H]: 233.
[0001570] Step 3: Synthesis of 3-(4-methoxybenzyl)-N-methyl-6,7-dihydro-3H-
pyrazolo[4',3':5,6]pyrido[4,3-f]quinazolin-9-amine. To a mixture of (E)-6-
((dimethylamino)methylene)-2-(methylamino)-7,8-dihydroquinazolin-5(6H)-one
(2.8 g, 12
231
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
mmol) and 1-(4-methoxybenzyl)-1H-pyrazol-5-amine (2.69 g, 13 mmol) was added
trifluoroacetic (1 mL). The reaction mixture was heated at 100 C for 16
hours. The reaction
was then cooled to room temperature followed by addition of isopropanol (20
mL). The
reaction mixture was the stirred for 30 minutes and the supernatant decanted.
The residue was
dried under reduced pressure. The crude product was purified using flash
column
chromatography (Si02, gradient from 100 hexanes to 15% EtOAc in hexanes) to
give desired
product (1.0 g, 22%) as solid. 1H NMR (CDC13, 400MHz): 6 9.32 (s, 1H), 7.90
(s, 1H), 7.75
(s, 1H), 7.40 (d, J = 8.0 Hz, 2H), 6.82 (d, J = 8.0 Hz, 2H), 5.52-5.48 (m,
1H), 5.62 (s, 2H),
3.76 (s, 3H), 3.18-3.04 (m, 5H), 3.0-2.88 (m, 2H); LCMS [M+H]: 373.
[0001571] Step 4: Synthesis ofN-methyl-6,7-dihydro-3H-
pyrazolo[4',3':5,6]pyrido[4,3-
f]quinazolin-9-amine. A solution of 3-(4-methoxybenzyl)-N-methyl-6,7-dihydro-
3H-
pyrazolo[4',3':5,6]pyrido[4,3-f]quinazolin-9-amine (0.5 g, 1.3 mmol) in
trifluoroacetic acid (2
mL) was heated at 100 C for 16 hours. The reaction mixture was then cooled to
room
temperature and neutralized using 2N NaOH. The reaction mixture was then
extracted with
5% methanol in dichloromethane (3 x 50 mL). The organic extracts were
combined, dried
using sodium sulfate and concentrated under reduced pressure. The crude
product was
purified by flash column chromatography (Si02, gradient from 100%
dichloromethane to 2%
methanol in dichloromethane) to give the pure desired product (0.19 g, 57%) as
a solid. 1H
NMR (DMSO-d6, 400MHz): 6 13.40 (s, 1H), 8.96 (s, 1H), 7.95 (d, J = 2.0 Hz,
1H), 7.45 (s,
1H), 3.70 (bs, 1H), 3.30 (t, J = 6.0 Hz, 2H), 2.82 (t, J = 6.0 Hz, 2H), 2.8
(s, 3H); LCMS
[M+H]: 253.
[0001572] Example 69: Synthesis of 8-phenyl-3,6,7,8-tetrahydrodipyrazolo[3,4-
c:4',3'-
f]isoquinoline.
0 0 0 0 0
Q__NHNH2 I N HOEt H r IN N~
O CH3OH, reflux, 6 h Na, Toluene, EtOH N THF, Toluene
RT, 40 h RT, 40 h
Step-1 Step-2 Step-3
N-N \ / O/ N-NH
N
N O N N
N H2N - OCH3 N I CF3COOH
NI N CF3COOH, 100 C, 2 h 'N 100 C, 2 h 'N
Step-4 6 / Step-5 \ /
[0001573] Step 1: Synthesis of 1-phenyl-6,7-dihydro-lH-indazol-4(5H)-one. To a
stirred
solution of 2-((dimethylamino)methylene)cyclohexane-1,3-dione (14 g, 83 mmol)
in
232
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
methanol (150 mL) was added phenyl hydrazine (8.92 g, 83 mmol). The reaction
mixture was
refluxed for 6 hours. The reaction was then concentrated under reduced
pressure followed by
the addition of n-butanol (130 mL) and glacial acetic acid (2 mL). The
reaction mixture was
refluxed for 3 hours. On the completion of the reaction, the reaction mixture
was
concentrated under reduced pressure. The residue was triturated with ether
(100 mL) to give a
precipitate, which was filtered and dried under reduced pressure to give the
desired product
(4.6 g, 30%) as a solid. 'H NMR (DMSO-d6, 400MHz): 6 9.06 (s, 1H), 7.59-7.51
(m, 4H),
7.45-7.40 (m, 1H), 3.00 (t, J = 6.4 Hz, 2H), 2.58 (t, J = 6.4 Hz, 2H), 2.21
(quintet, J = 6.4 Hz,
2H). LCMS [M+H]: 213.
[0001574] Step 2: Synthesis of 4-oxo-l-phenyl-4,5,6,7-tetrahydro-lH-indazole-5-
carbaldehyde. To a stirred suspension of sodium (0.75 g, 33 mmol) in toluene
(200 mL) was
added 1-phenyl-6,7-dihydro-IH-indazol-4(5H)-one (2.6 g, 123 mmol). The
reaction mixture
was stirred for 30 minutes and a solution of ethyl formate (2.22 mL, 276 mmol)
in toluene
(10 mL) was added over 10 minutes followed by absolute ethanol (1 mL). The
reaction was
stirred at room temperature for 40 hours. The reaction mixture was cautiously
poured into
ice-cooled water (100 mL). The organic layer was separated. The aqueous layer
was
extracted with diethylether (100 mL) and acidified with 6N HCl to a pH between
5 and 6.
The aqueous layer was then extracted with dichloromethane (3 x 75 mL). The
combined
organic layer was dried using sodium sulfate and concentrated under reduced
pressure to give
a residue which on trituration with hexane gave the desired product (1.1 g,
32%) as a solid.
iH NMR (CDC13, 400MHz): 6 10.75 (s, 1H), 8.00 (s, 1H), 7.61-7.54 (m, 5H), 7.48-
7.45 (m,
1H), 3.00-2.96 (m, 2H), 2.76-2.70 (m, 2H); LCMS [M+H]: 241.
[0001575] Step 3: Synthesis of (Z)-5-((dimethylamino)methylene)-l-phenyl-6,7-
dihydro-
1H-indazol-4(5H)-one. To a stirred solution of 4-oxo-l-phenyl-4,5,6,7-
tetrahydro-lH-
indazole-5-carbaldehyde (1.1g, 4.58 mol) in toluene (50 mL) and diethylether
(25 mL) was
added dimethylamine (3 mL, 6 mmol) as a solution in THE (10 mL). The reaction
was stirred
for at room temperature for 40 hours. The reaction was then concentrated under
reduced
pressure to give a brown residue, which was triturated with hexane to give the
desired
product (1 g, 80%) as a solid. 1H NMR (DMSO-d6, 400 MHz): 6 6 7.91 (s, 1H),
7.59-7.53 (m,
4H), 7.45-7.42 (m, 1H), 7.36 (s, 1H), 3.07 (s, 6H), 2.95 (s, 4H); LCMS [M+H]:
267.
[0001576] Step 4: Synthesis of 3-(4-methoxybenzyl)-8-phenyl-3,6,7,8-
tetrahydrodipyrazolo[3,4-c:4',3'-f]isoquinoline. To a mixture of (Z)-5-
((dimethylamino)methylene)-1-phenyl-6,7-dihydro-IH-indazol-4(5H)-one (1 g,
3.74 mmol)
and 1-(4-methoxybenzyl)-1H-pyrazol-5-amine (3.5 g, 17.2 mmol) was added
trifluoroacetic
233
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
acid (0.8 mL, 10.3 mmol). The reaction mixture was heated at 100 C for 2
hours. The
reaction mixture was then poured into ice-cooled water (100 mL) and extracted
with
dichloromethane (3 x 75 mL). The combined organic extracts were dried using
sodium
sulfate and concentrated under reduced pressure to give a dark residue. The
crude product
was purified by flash column chromatography (Si02, gradient from 100%
chloroform to 2%
methanol in chloroform) to a residue, which was further purified by
crystallization from
methanol and isopropanol to give the pure desired product (0.8 g, 15%) as a
solid. 'H NMR
(CDC13, 400 MHz): 6 8.33 (s, 1H), 7.87 (s, 1H), 7.78 (s, 1H), 7.55-7.49 (m,
4H), 7.41-7.39
(m, 1H), 7.38 (d, J = 8.4 Hz, 2H), 6.83 (d, J = 8.4 Hz, 2H), 5.66 (s, 2H),
3.75 (s, 3H), 3.14-
3.09 (m, 4H); LCMS [M+H]: 408.
[0001577] Step 5: Synthesis of 8-phenyl-3,6,7,8-tetrahydrodipyrazolo[3,4-
c:4',3'-
f]isoquinoline. A solution of 3-(4-methoxybenzyl)-8-phenyl-3,6,7,8-
tetrahydrodipyrazolo[3,4-c:4',3'-f]isoquinoline (0.7 g, 1.72 mmol) in
trifluoroacetic acid (4
mL) was heated at 100 C for 2 hours. The reaction mixture was poured into
water (50 mL)
and basified with aqueous potassium carbonate to pH of 11. The reaction
mixture was then
extracted with dichloromethane (3 x 75 mL). The combined organic extract was
dried using
sodium sulfate and concentrated under reduced pressure to give dark residue.
The crude
product was purified by flash column chromatography (Si02, gradient from 100%
chloroform
to 2% methanol in chloroform) to give a residue, which was further purified by
crystallization
from methanol and isopropanol to give the desired product (0.095 g, 18%) as a
solid. 1H
NMR (DMSO-d6, 400 MHz): 6 13.43 (s, 1H), 8.17 (s, 1H), 8.03 (s, 1H), 8.02 (s,
1H), 7.98 (s,
1H), 7.64-7.55 (m, 4H), 7.45 (t, J = 3.2 Hz, 1H), 3.12 (s, 4H); LCMS [M+H]:
288.
[0001578] Example 70: Synthesis of 2-methyl-5,8-dihydro-4H-pyrazolo[3,4-
c]thiazolo[5,4-f]isoquinoline.
O O S O O O O
Ik 1.1 ' 1.1
Br2 CHZCIZ Br NHZ S
H OEt _ H
RT, 1.5 h Pyridine I N>- Na, Toluene, EtOH N
(~Co 100 C, 16 h RT, 40 h
Step-1 Step-2 Step-3
0 Ol/
N-NH
N 0 N,N N,N N
H / I
N~ S NH2 N CF3COOH S THF~ />- CF3COOH S 100 N
RT, 40 h N 100 C, 2 h _<11 Step-4 N Step-6
Step-5
234
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001579] Step 1: Synthesis of 2-bromocyclohexane-1,3-dione. To a stirred
solution of
1,3 Cyclohexandione (10 g, 89 mmol) in dichloromethane (22 mL) at 0 C was
added a
solution of bromine (3.8 mL, 89 mmol) in dichloromethane (3 mL) over a period
of 10
minutes. The reaction mixture was stirred at room temperature for 1.5 hours.
The reaction
mixture was filtered and the residue washed with hexane (200 mL). The residue
was taken
into water (75 mL) and heated at 80 C for 30 minutes. It was cooled to room
temperature,
filtered and dried under reduced pressure to give the desired product (14 g,
82%) as a solid.
ESMS [M+H]: 191.
[0001580] Step 2: Synthesis of 2-methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one.
To a
stirred solution of 2-bromocyclohexane-1,3-dione (8 g, 42 mmol) in pyridine
(50 mL), was
added thioacetamide (3.2 g, 42.5 mmol). The reaction mixture was heated at 100
C for 16
hours. The solvent was then removed under reduced pressure the residue poured
in water
(250 mL). The reaction mixture was extracted with dichloromethane (3 x 100
mL). The
organic extract was dried using anhydrous sodium sulfate, concentrated under
reduced
pressure. The crude product was purified by flash column chromatography
(Silica gel,
gradient from 100% dichloromethane to 2% methanol in dichloromethane) to give
the pure
desired product (3 g, 42%) as a solid. 1H NMR (CDC13, 400MHz): 6 3.01 (t, J =
6.0 Hz, 2H),
2.74 (s, 3H), 2.60 (t, J = 4 Hz, 2H), 2.20 (quintet, J = 4 Hz, 2H); LCMS
[M+H]: 168.
[0001581] Step 3: Synthesis 2-methyl-7-oxo-4,5,6,7-tetrahydrobenzo[d]thiazole-
6-
carbaldehyde. To a stirred suspension of sodium (2 g., 86.9 mmol) in toluene
(150 mL) was
added 2-methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one (7.5 g, 44.9 mmol). The
reaction
mixture was stirred for 30 minutes and a solution of ethyl formate (8.55 g,
115 mmol) in
toluene (15 mL) was added over 10 minutes followed by absolute ethanol (1 mL).
The
reaction was stirred at room temperature for 40 hours. The reaction mixture
was cautiously
poured into ice-cooled water (250 mL). The organic layer was separated. The
aqueous layer
was extracted with diethylether (100 mL) and acidified with 6N HC1 to a pH
between 5 and
6. The aqueous layer was then extracted with dichloromethane (3 x 100 mL). The
combined
organic layer was dried using sodium sulfate and concentrated under reduced
pressure to give
a residue which on trituration with hexane gave the desired product (2.0g,
85%) as a solid. 1H
NMR (DMSO-d6, 400 MHz): 6 10.97 (s, 1H), 7.62 (s, 1H), 2.89 (t, J = 7.0 Hz,
2H), 2.72 (t, J
= 7.0 Hz, 2H), 2.68 (s, 3H); LCMS [M+H]: 196.
[0001582] Step 4: Synthesis of (Z)-6-((dimethylamino)methylene)-2-methyl-5,6-
dihydrobenzo[d]thiazol-7(4H)-one. To a stirred solution of 2-methyl-7-oxo-
4,5,6,7-
tetrahydrobenzo[d]thiazole-6-carbaldehyde (5 g, 25.6 mmol) in toluene (50 mL)
and
235
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
diethylether (25 mL) was added dimethylamine (15 mL, 35 mmol) as a solution in
THF. The
reaction was stirred for at room temperature for 40 hours. The reaction was
then concentrated
under reduced pressure to give a brown residue, which was triturated with
hexane to give the
desired product (5.0 g, 87%) as a solid. 'H NMR (DMSO-d6, 400 MHz): 6 7.35 (s,
1H), 3.10
(s, 6H), 2.98 (t, J = 6.2 Hz, 2H), 2.82 (t, J = 6.2 Hz, 2H), 2.65 (s, 3H);
LCMS [M+H]: 223.
[0001583] Step 5: Synthesis of 8-(4-methoxybenzyl)-2-methyl-5,8-dihydro-4H-
pyrazolo[3,4-c]thiazolo[5,4-f]isoquinoline. To a mixture of (Z)-6-
((dimethylamino)methylene)-2-methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one (3.5
g, 16.9
mmol) and 1-(4-methoxybenzyl)-1H-pyrazol-5-amine (3.5 g, 17.2 mmol) was added
trifluoroacetic acid (2.3 mL, 29.2 mmol). The reaction mixture was heated at
100 C for 2
hours. The reaction mixture was then poured into ice-cooled water (100 mL) and
extracted
with dichloromethane (3 x 75 mL). The combined organic extracts were dried
using sodium
sulfate and concentrated under reduced pressure to give a dark residue. The
crude product
was purified by flash column chromatography (Si02, gradient from 100%
chloroform to 2%
methanol in chloroform) to a residue, which was further purified by
crystallization from
methanol and isopropanol to give the pure desired product (0.8 g, 19%) as a
solid. 'H NMR
(CDC13, 400MHz): 6 7.86 (s, 1H), 7.76 (s, 1H), 7.36 (d, J = 8.0 Hz, 2H), 6.82
(d, J= 8.0 Hz,
2H), 5.59 (s, 2H), 3.75 (s, 3H), 3.17 (t, J = 6.8 Hz, 2H), 3.09 (t, J = 6.8
Hz, 2H), 2.77 (s, 3H);
LCMS [M+H]: 363.
[0001584] Step 6: Synthesis of 2-methyl-5,8-dihydro-4H-pyrazolo[3,4-
c]thiazolo[5,4-
f]isoquinoline. A solution of 8-(4-methoxybenzyl)-2-methyl-5,8-dihydro-4H-
pyrazolo[3,4-
c]thiazolo[5,4-f]isoquinoline (0.6 g, 1.65 mmol) in trifluoroacetic acid (4
mL) was heated at
100 C for 2 hours. The reaction mixture was poured into water (50 mL) and
basified with
aqueous potassium carbonate to pH of 11. The reaction mixture was then
extracted with
dichloromethane (3 x 75 mL). The combined organic extract was dried using
sodium sulfate
and concentrated under reduced pressure to give dark residue. The crude
product was purified
by flash column chromatography (Si02, gradient from 100% chloroform to 2%
methanol in
chloroform) to give a residue, which was further purified by crystallization
from methanol
and isopropanol to give the desired product (0.076 g, 13%) as a solid. 1H NMR
(DMSO-d6,
400 MHz) : 6 13.49 (s, I H), 8.04 (s, I H), 8.02 (s, I H), 3.16 (t, J = 7.6
Hz, 2H), 3.0 (t, J = 7.6
Hz, 2H), 2.70 (s, 3H); LCMS [M+H]: 243.
[0001585] Example 71: Synthesis of 3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c] [2,7]naphthyridine.
236
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
1N NH2 ~ 0
0 0 N
\ N/\\ OA
ce`)
DMF-DMA, DMF / N \O / N~
N 100 C, 48 h N I neat, 130 C, 12 h N NN
O-~-O-~ Step-1 O--~-O Step-2
-O
HN
CF3000H, CH2CI2
RT, 5 h N N N
Step-3
[0001586] Step 1: Synthesis of tert-butyl 3-((dimethylamino)methylene)-4-
oxopiperidine-l-carboxylate. To a solution of tert-butyl 4-oxopiperidine-l-
carboxylate (10 g,
50.25 mmol) in DMF (500 mL) at room temperature was added DMF-DMA (14.9 g,
125.62
mmol). The reaction mixture was heated at 100 C for 48 hours. After
completion of reaction
the solvent was removed under reduced pressure and the resulting residue
dissolved in EtOAc
(300 mL) and extracted with water (2 x 200 mL).This organic layer was dried
using sodium
sulfate and concentrated under reduced pressure to give crude desired product,
tert-butyl 3-
((dimethylamino)methylene)-4-oxopiperidine-1-carboxylate (10.2 g, 79%), which
was used
in the next step without any further purification. LCMS [M+H]:255.
[0001587] Step 2: Synthesis of tert-butyl 3-(4-methoxybenzyl)-8,9-dihydro-3H-
pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxylate. A mixture of tent-butyl-3-
((dimethylamino)methylene)-4-oxopiperidine-l-carboxylate (10.2 g, 40.15 mmol)
and 1-(4-
methoxybenzyl)-1H-pyrzol-5-amine (8.55 g, 42.16 mmol) were heated at 130 C
for 48
hours. The reaction mixture was cooled and the crude product was purified by
flash column
chromatography (Si02, gradient from 100% CH2C12 to 5% MeOH in CH2C12) to give
the
desired product tert-butyl 3-(4-methoxybenzyl)-8,9-dihydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridine-7(6H)-carboxylate as an off white solid (3.5 g, 22%). 'H
NMR (400
MHz, CDC13) 6 8.26 (s, 1 H ), 7.99 (s, 1 H), 7.14 (d, J = 8.4Hz, 2H), 7.81(d,
J = 8.4Hz, 2H) ,
5.62 (s, 2H), 4.71 (s, 2H), 3.82-3.74 (m, 5H), 3.07 (t, J= 6.0Hz, 2H), 1.44
(s, 9H). LCMS
[M+H]: 395.
[0001588] Step 3: Synthesis of 3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-
c][2,7]naphthyridine. To a solution of tert-butyl 3-(4-methoxybenzyl)-8,9-
dihydro-3H-
pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxylate (3.5g, 8.8 mmol) in
dichloromethane
(35 mL) at room temperatuure was added trifluoroacetic acid (5.04 g, 44.4
mmol) in a drop
wise manner. The reaction mixture was stirred at room temperature for 5 hours.
Once the
237
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
reaction was complete the solvent was removed under reduced pressure and the
resulting
residue was triturated with diethyl ether to give a solid. The solid was
filtered, washed with
diethyl ether and dried under reduce pressure. The dried solid was then
dissolved in water and
basified with saturated Na2CO3 solution. The aqueous layer was extracted with
10%
methanol in CH2C12 (3 x 150 mL). The organic extracts were combined, dried
using sodium
sulfate, filtered and concentrated under reduced pressure to give a residue.
The residue was
triturated with hexane to afford the pure desired product 3-(4-methoxy-benzyl)-
6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine as a white solid (2.1 g, 80%).
M.p. = 96-
102 C 'H NMR (400 MHz, CDC13) 6 8.22 (s, 1H), 7.93 (s, 1H), 7.26 (d, J=
8.4Hz, 2H), 6.80
(d, J= 8.4Hz, 2H), 5.61 (s, 2H), 4.11 (s, 2H), 3.73 (s, 3H), 3.219 (t, J= 6Hz,
2H), 3.016 (t, J
= 6Hz, 2H) LCMS [M+H]: 295.
[0001589] General Procedure F: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
[0001590] Example 72: Synthesis of 6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridin-7-ium 2,2,2-trifluoroacetate.
HN
CF3000OH2N
N N CF3COOH
65 C, 16 h N N
N H
-0
[0001591] A solution of 3-(4-methoxy-benzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-
c][2,7]naphthyridine (1.90 g, 6.46 mmol) in trifluoroacetic acid (20 mL) was
heated at 65 C
for 16 hours. On completion of the reaction, toluene (10 mL) was added and the
reaction
mixture evaporated to dryness under reduced pressure to give the desired
product as a dark
brown gel. The crude product was titurated with 1,4-dioxane. The resulting
solid was filtered
to give the desired product, 6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7-ium
2,2,2-trifluoroacetate as a tan powder (2.52 g, 97%)
[0001592] Alternatively the product can be purified using reverse phase HPLC.
M.p. _
224-227 C; 400 MHz 1H NMR (DMSO-d6) 8:13.78 (br s, 1H), 9.33 (s, 1H), 8.39
(s, 1H),
8.23 (s, 1H), 4.43 (s, 2H), 3.52 (t, J = 6 Hz, 2H), 3.30 (t, J = 6 Hz, 2H);
LCMS [M+H]: 175.
[0001593] General Procedure G-1: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
[0001594] Example 73: Synthesis of 1-bromo-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridin-7-ium 2,2,2-trifluoroacetate.
238
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
Boc.N Br CF3000 e (D
H2N Br
CF3000H
IN RT, 30 min N J N N
H
[0001595] To tert-butyl 1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridine-
7(6H)-carboxylate (0.23 g, 0.43 mmol) was added trifluoroacetic acid (2 mL).
The reaction
mixture was stirred at room temperature for 30 minutes. On completion of the
reaction,
toluene (3 mL) and 1,4-dioxane (2 mL) were added and the solvents removed
under reduced
pressure. The crude product, 1-bromo-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridin-7-ium 2,2,2-trifluoroacetate was obtained as brown foam
and was used in
the next step without any further purification. LCMS [M+H]: 253.
[0001596] Example 74: Synthesis of 3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7-amine.
NH2 H2N
O O N
DMF-DMA N~ 0 N~ N
90 C, 15 h / I AcOH,110 C, 48 h N N
HNu O HNu O
Step-1 IIO Step-2
O II
H
OY N H2N
(BOC)20, THE /IT O CF3000H, CH2CI2 / I N
RT, 4 h N N RT, 4 h N
N
Step-3 Step-4 TFA Salt
-0 -0
[0001597] Step 1: Synthesis of (E)-tert-butyl 3-((dimethylamino)methylene)-4-
oxocyclohexylcarbamate. A solution of tert-butyl 4-oxocyclohexylcarbamate (10
g, 0.046
mol) in 1, 1 -dimethoxy-N,N-dimethylmethanamine (50 mL) was heated at 100 C
for 15
hours. 1, 1 -dimethoxy-N,N-dimethylmethanamine was then removed under reduced
pressure.
The crude desired product, (E)-tert-butyl 3-((dimethylamino)methylene)-4-
oxocyclohexylcarbamate obtained as an oil was used in the next reaction
without any
purification. LCMS [M+H}: 269.
[0001598] Step 2: Synthesis of 3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-
c]isoquinolin-7-amine. A mixture of crude (E)-tert-butyl 3-
((dimethylamino)methylene)-4-
oxocyclohexylcarbamate (2 g, 7.87 mmol) (obtained from step-1) and 1-(4-
methoxybenzyl)-
1H-pyrzol-5-amine (1.78 g, 8.66 mmol) in acetic acid (20 mL) were heated at
110 C for 48
239
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
h. The reaction was cooled to room temperature and basified to pH = 11 using
50% aqueous
NaOH solution. The reaction mixture was extracted with EtOAc (2 x100 mL). The
organic
extracts were combined, dried using sodium sulfate, and solvent removed under
reduced
pressure. The desired crude product, 3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7-amine obtained was used in the next reaction
without any
purification. LCMS [M+H]: 309
[0001599] Step 3: Synthesis of tert-butyl 3-(4-methoxybenzyl)-6,7,8,9-
tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7-ylcarbamate. To a solution of crude 3-(4-methoxy-
benzyl)-
6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c]isoquinolin-7-amine (8.0 g, 20.2 mmol)
(obtained from
Step-2) in tetrahydrofuran (80 mL) was added di-tert-butyl dicarbonate (4.84
g, 22.0 mmol).
The reaction mixture was stirred for at room temperature for 4 hours. To the
reaction mixture
was then added water and extracted with EtOAc (2 x 150 mL). The organic
extracts were
combined, dried using sodium sulfate and solvent removed under reduced
pressure. The
crude product was purified using flash column chromatography (Si02, 25% EtOAc
in
hexanes) to give the desired product, tert-butyl 3-(4-methoxybenzyl)-6,7,8,9-
tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7-ylcarbamate (3.5 g, 33%) as a yellow solid. 1H
NMR (400
MHz, CDC13) 6 7.88 (s, 1 H), 7.65 (s, l H), 7.15 (d, J = 8.2 Hz, 2H), 6.91 (d,
J = 8.2 Hz, 2H),
5.6 (s, 2H), 4.63 (bs, 1H), 4.13 (bs, 1H), 3.78 (s, 3H), 3.15-3.27 (m, 3H),
2.79 (dd, J= 9.6,
15.6Hz, 1H), 2.28-2.16 (m, 1H), 1.98-1.84 (m,1H), 1.45 (s, 9H). LC-Mass: m/e
409 (M+H).
[0001600] Step 4: Synthesis of 3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-
c]isoquinolin-7-amine. To a solution of tert-butyl 3-(4-methoxybenzyl)-6,7,8,9-
tetrahydro-
3H-pyrazolo[3,4-c]isoquinolin-7-ylcarbamate (3.0 g, 8.8 mmol) in
dichloromethane (30 mL)
was added trifluoroacetic acid (10 mL). The reaction was stirred at room
temperature for 3
hours. The solvent was then removed under reduced pressure to give an oily
residue that was
triturated in diethyl ether to give a solid. The solid was filtered, washed
with diethyl ether and
dried under reduced pressure to give the desired product 3-(4-methoxy-benzyl)-
6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c]isoquinolin-7-ylamine (TFA salt) as a brown solid
(2.1 g,
92%). 1H NMR (400 MHz, DMSO-d6) 6 8.08 (bs, 2H), 8.07 (s, 1H), 8.02 (s, 1H),
7.161(d,
2H, J= 8.4Hz), 6.84 (d, 2H, J= 8.8 Hz), 5.53 (s, 2H), 3.69 (s, 3H), 3.67-3.52
(m, 1H) 3.23
(dd, J= 4.4, 16Hz, 1H), 3.16-3.04 (m,2H), 2.930 (dd, J= 9.6, 15.6Hz, 1H), 2.19-
2.29
(m,1H), 1.85-1.98 (m,1H). LCMS [M+H]: 309.
[0001601] Example 75: Synthesis of 2-chloro-2-phenyl-l -(1-phenyl-8,9-dihydro-
3H-
pyrazolo[3,4-c] [2,7]naphthyridin-7(6H)-yl)ethanone
240
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
HN O O
O N O N Br
N N N (i) CF3000H, 65 0, 16 h NBS, CH2CI2
(ii) (BOC)20, Et3N, CH2CI2, N RT, 3 h N
RT, 3 h N H N H
/ Step-1 Step-2
-O
O O
DtBPFPdCI2, PhB(OH)2 '1~O~N HCI (g) in Dioxane Cl H2N
Toluene, EtOH, Aq. Na2CO3 I '' N CH2CI2, RT, 16 h I N
90 0, 16 h ~N N 'N N
Step-3 H Step-4 H
CI
O-T-~ N
CI Cl
Hunig's Base, CH2CI2 I \ N
RT, 1 h N N
Step-5
[0001602] Step 1: Example 76: Synthesis of tert-butyl 8,9-dihydro-3H-
pyrazolo[3,4-
c][2,7]naphthyridine-7(6H)-carboxylate. Step (i): A solution of 3-(4-methoxy-
benzyl)-
6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine (0.50 g, 1.70 mmol) in
trifluoroacetic acid (3 mL) was heated in a 40 mL vial at 65 C for 16 hours.
On completion
of the reaction, toluene (2 mL) was added. The solvent was removed under
reduced pressure
to give a dark brown foamy solid (LCMS [M+H]: 175).. M.p. = 224-227 C; 400
MHz 1H
NMR (DMSO-d6) 8: 13.78 (br s, 1H), 9.33 (s, 1H), 8.39 (s, 1H), 8.23 (s, 1H),
4.43 (s, 2H),
3.52 (t, J = 6 Hz, 2H), 3.30 (t, J = 6 Hz, 2H); LCMS [M+H]: 175. Step (ii): To
the residue
obtained above was added dichloromethane (10 mL) and Et3N (2 mL). The mixture
was
vortexed to ensure that dissolution followed by addition of di-tert-butyl
dicarbonate (0.39
mL, 1.70 mmol). The reaction was then stirred at room temperature for 3 h. To
the reaction
mixture was added water (10 mL) and EtOAc (20 mL). The organic layer was
separated,
washed with brine (2 x 20 mL), dried (Na2SO4), filtered and concentrated under
reduced
pressure to give residue. The crude product was purified using flash column
chromatography
(Si02, 50% EtOAc in hexanes) to give the desired product, tert-butyl 8,9-
dihydro-3H-
pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxylate (0.38 g, 82%) as a white
solid. M.p. _
>70 C; 400 MHz iH NMR (DMSO-d6) 8: 13.57 (s, 1H), 8.34 (s, 1H), 8.14 (s, 1H),
4.64 (s,
1H), 4.64 (s, 2H), 3.67 (t, J = 6 Hz, 2H), 3.06 (t, J = 6 Hz, 2H), 1.44 (s,
9H); LCMS [M+H]:
275.
241
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001603] Step 2: Example 77: Synthesis of tert-butyl 1-bromo-8,9-dihydro-3H-
pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxylate. To a solution of tert-
butyl 8,9-
dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxylate (0.301 g, 1.09
mmol) in
dichloromethane (10 mL) was added N-bromosuccinimide (0.293 g, 1.64 mmol). The
reaction mixture was stirred at room temperature for 2 hours. The reaction was
quenched by
adding aqueous Na2CO3. The organic layer was separated, washed with brine (10
mL), dried
(Na2SO4), filtered and concentrated under reduced pressure to give a residue.
The crude
product was purified by flash column chromatography (Si02, 30% EtOAc in
hexanes) to give
the desired product. tert-butyl 1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridine-
7(6H)-carboxylate (0.258 g, 67%) as a foamy white solid. 1H NMR (400 MHz, DMSO-
d6) 8
13.94 (s, 1H), 8.40 (s, 1H), 4.63 (s, 2H), 3.67 (m, 2H), 3.28 (m, 2H), 1.44
(s, 9H); LCMS
[M+H]: 353.
[0001604] Step-3: Synthesis of tert-butyl 1-phenyl-8,9-dihydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridine-7(6H)-carboxylate. To a solution of tert-butyl 1-bromo-
8,9-dihydro-
3H-pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxylate (0.540 g, 1.53 mmol) in
toluene (8
mL) and ethanol (4 mL) was added phenyl bronic acid (0.371 g, 3.07 mmol)
followed by
Na2CO3 (0.96 g, 9.18 mmol) as a solution in water (10 mL). The mixture was
vortexed and
1,l-Bis(Di-t-butylphosphino)Ferrocene Palladium dichloride (BDtPFPdC12, CAS#
95408-45-
0, 0.025 g) was added to the reaction mixture. The reaction was then purged
with nitrogen
and heated at 90 C for 16 hours. On completion of the reaction, EtOAc (50 mL)
and water
(50 mL) was added. The organic layer was separated, washed with brine (2 x 20
mL), dried
(Na2SO4), filtered and concentrated under reduced pressure to give the crude
product. The
crude product was purified using flash column chromatography (Si02, 50% EtOAc
in
hexane) to give the pure desired product, tert-butyl 1-phenyl-8,9-dihydro-3H-
pyrazolo[3,4-
c] [2,7]naphthyridine-7(6H)-carboxylate (0.278 g, 52%) as a white foamy solid.
M.p. = >65
C; 400 MHz iH NMR (DMSO-d6) 8:13.72 (s, 1H), 8.38 (s, 1H), 7.73-7.62 (m, 2H),
7.56-
7.42 (m, 3H), 4.65 (s, 2H), 3.54-3.45 (m, 2H), 2.88-2.8 (m, 2H), 1.44 (s, 9H);
LCMS [M+H]:
351.
[0001605] Step 4: Example 78: Synthesis of 1-phenyl-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c][2,7]naphthyridin-7-ium chloride. To a solution of tert-butyl 1-
phenyl-8,9-
dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxylate (0.21 g, 0.60
mmol) in
dichloromethane (5 mL) was added 4 M solution of HCl gas in 1,4-dioxane (1.5
mL, 6.0
mmol). The reaction was shaken at room temperature for 16 hours. A yellow
solid
precipitated out in the reaction mixture, which was filtered to give the pure
desired product,
242
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
1-phenyl-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7-ium chloride
(0.184 g,
95%) as a yellow solid; M.p. = 265 C decomposition; 400 MHz 1H NMR (DMSO-d6)
8:
13.91 (s, 1H), 9.39 (s, 2H), 8.43 (s, 1H), 7.62-7.46 (m, 5H), 4.44-3.38 (m,
2H), 3.38-3.2 (m,
2H), 3.01 (t, J = 6.4 Hz, 2H); LCMS [M+H]: 251.
[0001606] Step 5: Synthesis of 2-chloro-2-phenyl-l-(1-phenyl-8,9-dihydro-3H-
pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)ethanone. To a suspension of 1-
phenyl-6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7-ium chloride (0.184 g, 0.57
mmol) in
dichloromethane (15 mL) was added Hunig's Base (0.45 mL, 2.56 mmol). The
mixture was
vortexed to ensure dissolution of the reagents followed by the addition of 2-
chloro-2-
phenylacetyl chloride (0.103 mL, 0.64 mmol). Dimethylformamide (6 mL) and 2-
chloro-2-
phenylacetyl chloride (15 L, 0.093 mmol)) was then added and the reaction
stirred for
another 30 minute at room temperature. On completion of the reaction, water
(30 mL) were
added and the aqueous layer extracted with EtOAc (50 mL). The organic layer
was separated,
washed with brine (2 x 10 mL), dried (Na2S04), filtered and concentrated under
reduced
pressure to give the crude product. The crude product was purified using flash
column
chromatography (Si02, gradient from 60% EtOAc in hexane to 66% EtOAc in
hexane) to
give the pure desired product, 2-chloro-2-phenyl-l-(1-phenyl-8,9-dihydro-3H-
pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)ethanone as an off-white solid (0.120 g, 52%). 1H
NMR (400
MHz, DMSO-d6) 8 13.72 (s, 1H), 8.40/8.25 (s, 1H, rotamers), 7.47 (m, 10 H),
6.48 (s, 1H),
4.88 (m, 2H), 3.66 (m, 2H), 2.68 and 2.34 (m, 2H, rotamers); LCMS [M+H]: 403.
[0001607] General Procedure G-2: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
[0001608] Example 79: Synthesis of 1-methyl-4-(2-oxo-l-phenyl-2-(l-phenyl-8,9-
dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)ethyl)piperazin-l-ium
2,2,2-
trifluoroacetate.
\ N ~\ \ gj~\ CI
-NNH Et3N, (n-Bu)4N'I- N
N DMSO, 100 C, 16 h N N H N CF300O N'H N H
[0001609] To a solution of 2-chloro-2-phenyl-l-(1-phenyl-3,6,8,9-tetrahydro-
pyrazolo[3,4-c][2,7]naphthyridin-7-yl)-ethanone (0.031 g, 0.077 mmol) in DMSO
(3 mL)
was added triethylamine (54 L, 0.38 mmol) followed by 1-methyl piperizine (17
L, 0.154
mmol) and catalytic amount tetra-butylammonium iodide. The mixture was heated
at 100 C
for 16 hours. On completion of the reaction, the solvent was removed under
reduced pressure
243
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
to give the crude desired product. The crude product was purified using
reverse phase HPLC
to give the pure desired product, 1-methyl-4-(2-oxo-l-phenyl-2-(l-phenyl-8,9-
dihydro-3H-
pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)ethyl)piperazin-l-ium 2,2,2-
trifluoroacetate
(0.039 g, 58%) was obtained as a pale tan solid. 1H NMR (400 Hz, (DMSO-d6)
13.7 (brs,
I H), 8.40/8.20 (s, I H, rotamers), 7.49 (m, 10 H), 5.03 (m, I H), 4.72 (m,
2H), 3.55 (m, 2H),
3.35 (m, 2H), 3.00 (m, 4H), 2.79 (m, 2H), 2.75/2.51 (s, 3H), 2.18 (m, 2H);
LCMS
[M+H]:467.31 (M+H).
[0001610] Example 80: Synthesis of 2-{[2-oxo-l-phenyl-2-(l-phenyl-3,6,8,9-
tetrahydro-
7H-pyrazolo [3,4-c] [2,7]naphthyridin-7-yl)ethyl] amino } ethanol.
O-T-~ N
HN
Jl N
HO N N
H
[0001611] The compound 2-{[2-oxo-l-phenyl-2-(l-phenyl-3,6,8,9-tetrahydro-7H-
pyrazolo[3,4-c][2,7]naphthyridin-7-yl)ethyl]amino }ethanol was synthesized
using 2-chloro-
2-phenyl-l -(1-phenyl-8,9-dihydro-3H-pyrazolo [3,4-c] [2,7]naphthyridin-7(6H)-
yl)ethanone
and 2-aminoethanol as described in general procedure G-2. The product was
obtained as TFA
salt and as a yellow solid. 1H NMR (400 MHz, DMSO-d6) 813.70 (s, 1H), 9.35
(brs, 1H),
9.22 (brs, I H), 8.42/8.21 (s, I H, rotamers), 7.48 (m, 10 H), 5.71 (m, I H),
5.03-4.76 (m, 2H),
3.66-3.49 (m, 4H), 2.92 (m, 2H), 2.74 (m, 2H); LCMS [M+H]: 428.
[0001612] Example 81: Synthesis ofN,N-dimethyl-N'-[2-oxo-l-phenyl-2-(l-phenyl-
3,6,8,9-tetrahydro-7H-pyrazolo[3,4-c] [2,7]naphthyridin-7-yl)ethyl] ethane-
1,2-diamine.
O-T-~ N
HN
'`
Jl j N
N \N N
H
[0001613] The compound N,N-dimethyl-N'-[2-oxo-l-phenyl-2-(l-phenyl-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridin-7-yl)ethyl]ethane- 1,2-diamine
was
synthesized using 2-chloro-2-phenyl-l-(1-phenyl-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)ethanone andN1,N1-dimethylethane-1,2-diamine as
described
in general procedure procedure G-2. The product was obtained as bis-TFA salt
and as yellow
solid. 1H NMR (400 MHz, DMSO-d6) 813.65 (s, 1H), 8.43/8.18 (s, 1H, rotamers),
7.59-7.32
(m, 10 H), 5.71 (m, 1H), 5.06-4.70 (m, 2H), 3.51-3.00 (m, 8H), 2.81 (s, 3H),
2.54 (s, 3H);
LCMS [M+H]: 455.
244
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001614] Example 82: Synthesis of 1-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridin-7(6H)-yl)-2-chloro-2-phenylethanone.
~ o 0-11o
HN CI I N N
CI CI CF3COOH CI
N N' N Et3N, CH2CI2 N 65 C, 16 h \N
RT, 1 h N N N H
Step-2
Step-1
-0
NBS, CH2CI2 N Br
RT, 16 h CI
J \N
Step-3 , N N
H
[0001615] Step 1: Synthesis of 2-chloro-l-(3-(4-methoxybenzyl)-8,9-dihydro-3H-
pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-phenylethanone. To a solution of
3-(4-
methoxybenzyl)-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine (1.536
g, 5.22
mmol) in anhydrous dichloromethane (20 mL) was added triethylamine (2.18 mL,
15.66
mmol) followed by 2-chloro-2-phenylacetyl chloride (90%, 1.05 mL, 6.52 mmol).
The
mixture was stirred at room temperature for 1 hour. The reaction was quenched
by the
addition of water (10 mL). The reaction mixture was extracted with
dichloromethane (10 mL)
and the organic layer washed with brine (2 x 10 mL), dried (Na2S04), filtered
and
concentrated under reduced pressure to give the crude product. The crude
product was
purified by flash column chromatography (Si02, gradient from 40% EtOAc in
hexanes to
50% EtOAc in hexanes) to give the desired product, 2-chloro- 1 -(3 -(4-
methoxybenzyl)-8,9-
dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-phenylethanone as an
off-white
solid (1.99 g, 85%). 1H NMR (400 MHz, DMSO-d6) 8 8.43/8.24 (s, 1H, rotamers),
8.15/8.12
(s, 1H, rotamers), 7.52 (m, 2H), 7.40 (m, 3H), 7.17 (m, 2H), 6.83 (m, 2H),
6.53/6.48 (s, 1H,
rotamers), 5.56 (s, 2H), 5.02-4.70 (m, 2H), 3.84 (m, 2H), 3.68 (s, 3H),
3.11/2.67 (m, 2H,
rotamers); LCMS [M+H]: 447.
[0001616] Step 2: Synthesis of 2-chloro-l-(8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)-2-phenylethanone. To 2-chloro-l-(3-(4-
methoxybenzyl)-8,9-
dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-phenylethanone (0.60
g, 1.34
mmol) was added trifluoroacetic acid (2 mL). The reaction mixture was heated
at 65 C for
16 hours. On completion of the reaction, 1,4-dioxane (5 ML) and toluene (5 ML)
were added
and the solvent removed under reduced pressure to give the crude product. The
crude product
245
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
was then partitioned between dichloromethane (20 mL) and water (20 mL). The
organic layer
was separated, washed with brine (2 x 10 mL), dried (Na2SO4), filtered and
concentrated
under reduced pressure. The crude product was purified by flash column
chromatography
(Si02, gradient 60% EtOAc in hexane to 80% EtOAc in hexane) to give the pure
desired
product, 2-chloro-l-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-
2-
phenylethanone as a white foamy solid (0.360 g, 83%). LCMS [M+H]: 327.
[0001617] Step 3: Synthesis of 1-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)-2-chloro-2-phenylethanone. To a solution of 2-
chloro- 1 -(8,9-
dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-phenylethanone (0.340
g, 1.04
mmol) in dichloromethane (20 mL) was added N-bromosuccinimide (NBS, 0.195 g,
1.09
mmol). The reaction mixture was stirred for 16 hours at room temperature.
During the
reaction a yellow solid crashed out. The reaction was quenched by addition of
aqueous
NaHCO3. The aqueous layer was extracted with EtOAc (2 x 200 mL). The combined
organic
layer was dried (Na2SO4), filtered and concentrated under reduced pressure to
give crude 1-
(1-bromo-8,9-dihydro-3H-pyrazolo [3,4-c] [2,7]naphthyridin-7(6H)-yl)-2-chloro-
2-
phenylethanone as a pale yellow solid (0.38 g, 90%). 'H NMR (400 MHz, DMSO-d6)
811.08
(brs, 1H), 8.43 (s, 1H), 7.52-7.39 (m, 5 H), 6.52 (s, 1H), 4.80 (m, 2H), 3.85
(m, 2H), 3.28 (m,
2H); LCMS [M+H]: 405. The product was used in the next step without any
further
purification.
[0001618] General Procedure G-3: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
[0001619] Example 83: Synthesis of 2-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)-N-(2-hydroxyethyl)-2-oxo-l-phenylethanaminium
2,2,2-
trifluoroacetate.
0-11Z N Br OeN Br
CI HZNi-____OH , (n-Bu)4N+I_ CF3COOH2N
N I IN
N N Hunig's Base, DMSO, 100 C, 16 h HO N N
H H
[0001620] To a solution of 1-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)-2-chloro-2-phenylethanone (0.096 g, 0.23 mmol)
in DMSO (3
mL) and 1,4-dioxane (3 mL) was added Hunig's Base (0.20 mL, 1.15 mmol)
followed by 2-
aminoethanol (0.029 g, 0.46 mmol) and catalytic amount of tetra-n-
butylammonium iodide.
The reaction mixture was heated at 100 C for 16 hours. The solvent was
removed under
reduced pressure and the crude product was purified using reverse phase HPLC
to give the
246
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
pure desired product, 2-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-
yl)-N-(2-hydroxyethyl)-2-oxo-l-phenylethanaminium 2,2,2-trifluoroacetate as a
tan solid
(0.028 g, 17%). M. p. > 115 C; 1H NMR (400 MHz, DMSO-d6) 813.98 (s, 1H), 9.36
(brs,
I H), 9.18 (brs, I H), 8.45/8.19 (s, I H, rotamers), 7.40-7.62 (m, 5H), 5.75
(m, I H), 4.96 (d, J
= 17.2 Hz, 1 H), 4.76 (d, J = 17.2 Hz, 1 H), 3.65 (m, 4H), 3.25 (m, 1 H), 2.91
(m, 1 H), 2.65
(m, 2H); LCMS [M+H]: 430.
[0001621] Example 84: Synthesis ofN-(2-(1H-indol-3-yl)ethyl)-2-(8,9-dihydro-3H-
pyrazolo[3,4-c] [2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethanaminium 2,2,2-
trifluoroacetate.
o
q_G N
C F30NH2
N
HN \N H
[0001622] The compound, N-(2-(1H-indol-3-yl)ethyl)-2-(8,9-dihydro-3H-
pyrazolo[3,4-
c] [2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethanaminium 2,2,2-
trifluoroacetate was
prepared using 2-(1H-indol-3-yl)ethanamine and 2-chloro-l-(8,9-dihydro-3H-
pyrazolo[3,4-
c] [2,7]naphthyridin-7(6H)-yl)-2-phenylethanone as described in general
procedure G-3. The
product was obtained as a TFA salt and as a tan solid (0.058 g, 19%). M. p. >
164 C; 1H
NMR (400 MHz, DMSO-d6) 810.95 (brs, 1H), 9.53 (brs, 1H), 9.47 (brs, 1H),
8.39/8.12 (s,
1H, rotamers), 8.11/8.05 (s, 1H, rotamers), 7.63-7.32 (m, 6H), 7.20-6.97 (m,
4H), 5.81 (m,
1H), 5.81 (m, 1H), 4.96-4.78 (m, 2H), 4.09/3.69 (m, 2H, rotamers), 3.13-2.89
(m, 5H), 2.30
(m,, 1H); LCMS [M+H]: 451.
[0001623] Example 85: 1-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-
yl)-
2-(3-methoxypropylamino)-2-phenylethanone.
N
N
N N
H
[0001624] The compound, 1-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-
7(6H)-
yl)-2-(3-methoxypropylamino)-2-phenylethanone was prepared using 3-
methoxypropan-l-
amine and 2-chloro-l-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-
yl)-2-
phenylethanone as described in general procedure G-3. The product was obtained
as TFA salt
and was a yellow colored solid. 1H NMR (400 MHz, DMSO-d6) 813.58 (s, 1H), 9.31
(brs,
247
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
2H), 8.38-8.06 (m, 2H), 7.67-7.34 (m, 5H), 5.74 (m, 1H), 5.00-4.75 (m, 2H),
4.39-2.54 (m,
11H), 1.24 (m, 2H); LCMS [M+H]: 380.
[0001625] Example 86: 1-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-
yl)-
2-phenyl-2-(piperidin- l -yl)ethanone.
0-- N
1N
12N
N H
[0001626] The compound, 1-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-
7(6H)-
yl)-2-phenyl-2-(piperidin-l-yl)ethanone was prepared using piperidine and 2-
chloro- 1 -(8,9-
dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-phenylethanone as
described in
general procedure G-3. The product was obtained as a TFA salt and as a tan
hard gel. LCMS
[M+H]: 376.
[0001627] Example 87: 1-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-
yl)-
2-phenyl-2-(4-(pyridin-2-ylmethyl)piperazin- l -yl)ethanone.
OLN
N
N
N N N
N H
[0001628] The compound, 1-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-
7(6H)-
yl)-2-phenyl-2-(4-(pyridin-2-ylmethyl)piperazin-l-yl)ethanone was prepared
using 1-
(pyridin-2-ylmethyl)piperazine and 2-chloro-l-(8,9-dihydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridin-7(6H)-yl)-2-phenylethanone as described in general
procedure G-3. The
product was obtained as a bis-TFA salt and as a tan gel. 1H NMR (400 MHz, DMSO-
d6) 8
8.69 (m, 2H), 8.38/8.14 (s, 1H, rotamers), 8.12/8.08 (s, 1H, rotamers), 8.05
(m, 2H), 7.68-
7.34 (m, 6H), 5.75 (m, 1H), 4.98-4.78 (m, 2H), 4.29 (s, 2H), 3.96-3.69 (m,
4H), 3.39-2.54 (m,
IOH); LCMS [M+H]: 468.
[0001629] Example 88: 1-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-
yl)-
2-phenyl-2-(3-(piperidin-1-yl)propylamino)ethanone.
248
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
0--ry-N
NH
N j
N N N
H
[0001630] The compound, 1-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-
7(6H)-
yl)-2-phenyl-2-(3-(piperidin-l-yl)propylamino)ethanone was prepared using 3-
(piperidin-l-
yl)propan-l-amine and 2-chloro-l-(8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-
yl)-2-phenylethanone as described in general procedure G-3. The product was
obtained as a
bis-TFA salt and as hard tan gel. 1H NMR (400 MHz, DMSO-d6) 813.58 (brs, 1H),
9.54 (brs,
I H), 9.17 (brs, I H), 8.39/8.13 (s, I H, rotamers), 8.09/8.05 (s, I H,
rotamers), 7.57-7.41 (m,
5H), 5.78 (m, 1H), 4.97-4.71 (m, 2H), 3.75-3.59 (m, 2H), 3.27-2.84 (m, 8H),
2.01-1.11 (m,
1OH); LCMS [M+H]: 433.
[0001631] Example 89: 2-(benzylamino)-1-(8,9-dihydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridin-7(6H)-yl)-2-phenylethanone.
ON
NH
\N
N N
H
[0001632] The compound, 2-(benzylamino)-1-(8,9-dihydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridin-7(6H)-yl)-2-phenylethanone was prepared using benzyl
amine and 2-
chloro-l-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-
phenylethanone as
described in general procedure G-3. The product was obtained as a bis-TFA salt
and as a
yellow foamy solid. 1H NMR (400 MHz, DMSO-d6) 8 13.59 (brs, 1H), 9.85 (brs,
1H), 9.60
(brs, 1H), 8.38/8.14 (s, 1H, rotamers), 8.13/8.06 (s, 1H, rotamers), 7.63-7.27
(m, 1OH), 5.81
& 5.76 (m, I H, rotamers), 4.92-4.74 (m, 2H), 4.12 (m, 2H), 3.64 (m, 2H), 2.99
(m, 2H);
LCMS [M+H]: 398.
[0001633] General Procedure H: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
[0001634] Example 90: Synthesis of 4-(2-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethyl)piperazin-l-ium chloride.
249
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
O O
~~ \ I O \ I O
N Br () N Br N Br
CI H N HCI(g) in Dioxane N
N NN Hunig's Base, ~N) N I N N CH2CI2, RT, 16 h 12 O N I N N
H 1,4-Dioxane H H2 CI H
100 C, 16 h p p Step-2
Step-1
[0001635] Step 1: Example 91: Synthesis of tert-butyl 4-(2-(1-bromo-8,9-
dihydro-3H-
pyrazolo [3,4-c] [2,7]naphthyridin-7(6H)-yl)-2-oxo- l -phenylethyl)piperazine-
l -carboxylate.
To a solution of 1-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-
7(6H)-yl)-2-
chloro-2-phenylethanone (0.38 g, 0.94 mmol) in 1,4-dioxane (15 mL) was added
Hunig's
Base (0.50 mL, 2.82 mmol) and tert-butyl piperazine-l-carboxylate (0.349 g,
1.88 mmol).
The reaction mixture was heated at 100 C for 16 hours. The solvent was
removed under
reduced pressure and the crude product was purified using (Si02, gradient from
80% EtOAc
in hexanes to 100% EtOAc in hexane) to give the desired product, tert-butyl 4-
(2-(1-bromo-
8,9-dihydro-3H-pyrazolo [3,4-c] [2,7]naphthyridin-7(6H)-yl)-2-oxo-l -
phenylethyl)piperazine-
1-carboxylate as a white solid (0.188 g, 41%). M.p. = 159-161 C; 400 MHz 1H
NMR
(DMSO-d6) 8: 13.95 (s, 1H), 8.41 (s, 1H), 7.46-7.2 (m, 5H), 5.07-4.65 (m, 3H),
4.0-3.7 (m,
2H), 3.3-3.1 (m, 5H), 2.95-2.85 (m, 1H), 2.53-2.3 (m, 4H), 1.35 (s, 9H); LCMS
[M+H]: 555.
[0001636] Step 2: Synthesis of 4-(2-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethyl)piperazin-l-ium chloride. To
a solution
of tert-butyl 4-(2-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-
7(6H)-yl)-2-
oxo-l-phenylethyl)piperazine-l-carboxylate (0.075 g, 0.17 mmol) in
dichloromethane (12
mL) and 1,4-dioxane (6 mL) was added a 4 M solution of HC1 gas in 1,4-dioxane
(0.82 mL,
3.4 mmol). The reaction was shaken at room temperature for 16 hours. During
the reaction a
pale tan solid precipitate was formed. The solvent was removed under reduced
pressure to
give the pure desired product, 4-(2-(1-bromo-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethyl)piperazin-l-ium chloride
(0.064 g, 71%)
as an tan solid. M. p. > 245 C; 1H NMR (400 MHz, DMSO-d6) 813.98 (s, 1H),
9.92 (brs,
2H), 8.44/8.21 (s, 1H, rotamers), 7.44-7.68 (m, 5H), 6.28 (m, 1H), 4.75-4.92
(m, 2H), 4.11
(m, 8H), 3.90 (m, 1H), 3.66 (m, 1H), 3.29 (m, 1H), 2.62 (m, 1H); LCMS [M+H]:
455.
[0001637] Example 92: Synthesis of 2-(8,9-dihydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridin-7(6H)-yl)-N-(2-hydroxyethyl)-2-oxo-l-phenylethanaminium
2,2,2-
trifluoroacetate.
250
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
OH
O O OH
CI N CF 3000 HZNO N O O 0
CF0 H2N
\ \ I N H2N~~OH N :13c0NN
3000H Hunig's Base, N
DMSO, Step-2 N H
100 C, 16 h \ /
-p Step-1 -0
[0001638] Step 1: Synthesis of N-(2-hydroxyethyl)-2-(3-(4-methoxybenzyl)-8,9-
dihydro-
3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethanaminium 2,2,2-
trifluoroacetate. The compound, N-(2-hydroxyethyl)-2-(3-(4-methoxybenzyl)-8,9-
dihydro-
3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethanaminium 2,2,2-
trifluoroacetate was prepared using 2-aminoethanol and 2-chloro-1-(3-(4-
methoxybenzyl)-
8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-phenylethanone as
described
in general procedure G-3 and was obtained as a yellow foamy solid (0.195 g,
60%). M. p. >
70 C; 1H NMR (400 MHz, DMSO-d6) 8 9.35 (brs, 1H), 9.22 (brs, 1H), 8.45/8.18
(s, 1H,
rotamers), 8.15/8.10 (1H, rotamers), 7.61 (m, 2H), 7.50 (m, 2H), 7.34 (m, 1H),
7.15 (m, 2H),
6.83 (m,2H), 5.75 (m, 1H), 5.56 (s, 2H), 4.87 (m, 2H), 3.96 (m, 1H), 3.70 (m,
1H), 3.69 (s,
3H), 3.65 (m, 2H), 3.02 (m, 2H), 2.71 & 2.36 (m, 2H, rotamers); LCMS [M+H]:
472.
[0001639] Step 2: Synthesis of 2-(8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-
7(6H)-yl)-N-(2-hydroxyethyl)-2-oxo-l-phenylethanaminium 2,2,2-
trifluoroacetate. The
compound, 2-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-N-(2-
hydroxyethyl)-2-oxo-l-phenylethanaminium 2,2,2-trifluoroacetate was prepared
as described
in the general procedure F. The crude product was purified using reverse phase
HPLC and
was obtained as a pale yellow foamy solid (0.049 g, 57%). 1H NMR (400 MHz,
DMSO-d6) 8
13.58 (brs, I H), 8.34 (brs, I H), 8.19 (brs, I H), 8.38/8.14 (s, I H,
rotamers), 8.13/8.07 (s, I H,
rotamers), 7.63-7.38 (m, 5H), 5.76 (m, 1H), 4.85 (m, 2H), 3.79-3.63 (m, 4H),
3.06 (m, 4H);
LCMS [M+H]: 352.
[0001640] Example 93: Synthesis of (S)-2-(8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-7(6H)-yl)-2-oxo-1-phenylethanaminium 2,2,2-
trifluoroacetate.
251
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
O
~ O
/ ON O
HN O 0
HN \ N
N O~ ONH N CF3000H
N N CH2CI2, RT, 1 h O~ N N CH2CI2, RT, 16 h
Step-1 \ / Step-2
N O
0-0
CF3COO NH3 CF3COOH N
O O
N
N ru 65 0, 16 h CF3COO NH3
N
Step-3 N
H
=-O
[0001641] Step 1: Compound 9: Synthesis of (S)-tert-butyl 2-(3-(4-
methoxybenzyl)-8,9-
dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-oxo-l-
phenylethylcarbamate. To a
solution of 3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c][2,7]naphthyridine
(0.188 g, 0.64 mmol) in anhydrous dichloromethane (5 mL) was added (S)-2,5-
dioxopyrrolidin-1-yl 2-(tert-butoxycarbonylamino)-2-phenylacetate (0.333 g,
1.04 mmol).
The reaction mixture was stirred at room temperature for 1 hour. The solvent
was removed
under reduced pressure and the crude product was purified using flash column
chromatography (Si02, gradient from 33% EtOAc in hexane to 50% EtOAc in
hexane) to
give the pure desired product, (S)-tert-butyl 2-(3-(4-methoxybenzyl)-8,9-
dihydro-3H-
pyrazolo[3,4-c] [2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethylcarbamate as a
white solid
(0.258 g, 76%). M. p. 95-110 C; 1H NMR (400 MHz at 60 C, DMSO-d6) 8 8.20 (s,
1H),
9.07 (s, 1H), 7.39-7.15 (m, 7H), 6.82 (m, 2H), 5.69(m, 1H), 5.54 (s, 2H), 4.83
(m, 2H), 3.78
(m, 2H), 3.69 (s, 3H), 3.15 (s, 1H), 3.00 (m, 1H), 1.34 (s, 9H); LCMS [M+H]:
528.
[0001642] Step 2: Compound 8: Synthesis of (S)-2-(3-(4-methoxybenzyl)-8,9-
dihydro-
3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethanaminium 2,2,2-
trifluoroacetate. To a solution of (S)-tert-butyl 2-(3-(4-methoxybenzyl)-8,9-
dihydro-3H-
pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-oxo-l-phenylethylcarbamate (0.23
g, 0.43
mmol) in dichloromethane (10 mL) was added trifluoroacetic acid (3 ML). The
reaction was
stirred at room temperature for 16 hours. The solvent was removed under
reduced pressure
and the crude product was purified using reverse phase HPLC to give the pure
desired
product (S)-2-(3-(4-methoxybenzyl)-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-
252
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
7(6H)-yl)-2-oxo-l-phenylethanaminium 2,2,2-trifluoroacetate as a pale yellow
solid (0.131 g,
50%). M. p. > 115 C; 1H NMR (400 MHz, DMSO-d6) 8 8.60 (brs, 2H), 8.45/8.15
(s, 1H,
rotamers), 8.12/8.08 (s, 1H, rotamers), 7.55-7.33 (m, 5H), 7.17 (m, 2H), 6.82
(m, 2H), 5.75
(m, 1H), 5.55 (s, 2H), 4.97-4.35 (m, 2H), 4.02/3.74 (m, 2H, rotamers),
3.01/2.30 (m, 2H,
rotamers); LC-Mass: m/e 428.57 (M+H). LCMS [M+H]: 428.
[0001643] Step 3: Synthesis of (S)-2-(8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridin-
7(6H)-yl)-2-oxo-l-phenylethanaminium 2,2,2-trifluoroacetate. To (S)-2-(3-(4-
methoxybenzyl)-8,9-dihydro-3H-pyrazolo [3,4-c] [2,7]naphthyridin-7(6H)-yl)-2-
oxo-1-
phenylethanaminium 2,2,2-trifluoroacetate (0.109 g, 0.18 mmol) was added
trifluoroacetic
acid (2 mL). The reaction mixture was heated at 65 C for 16 hours. On
completion of the
reaction, toluene (5 mL) was added followed by removal of the solvent under
reduced
pressure. . The mixture was evaporated to dryness in the Genevac and a dark
brown solid was
obtained. The crude product was purified using reverse phase HPLC to give the
pure desired
product, (S)-2-(8,9-dihydro-3H-pyrazolo[3,4-c][2,7]naphthyridin-7(6H)-yl)-2-
oxo-l-
phenylethanaminium 2,2,2-trifluoroacetate as a pale yellow solid (0.082 g,
84%). M. p. > 100
C; 1H NMR (400 MHz, DMSO-d6) 812.75 (brs, 1H), 8.62 (brs, 2H), 8.39/8.13 (s,
1H,
rotamers), 8.08/8.06 (s, 1H, rotamers), 7.56-7.35 (m, 5H), 5.74 (m, 1H), 4.91
(m, 2H), 3.73
(m, 2H), 3.08 (m, 2H); LCMS [M+H]: 308.
[0001644] Example 93b: Synthesis of 3-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridine-7-carboxamido)benzoic acid
HN
N (i) CF3000H, 65 C, 16 h EtO I NaOH, H2O, CH3OH
N N (ii) OCN C02Et H N N RT, 16 h
N Step-2
Hunig's Base, CH2CI2, DMA N N
O RT, 20 min H
Step-1
aN O
H O
)~ N
Y
O H
N
N
H
[0001645] Step-1: Synthesis of ethyl 3-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c][2,7]naphthyridine-7-carboxamido)benzoate. Step (i): A solution of 3-(4-
methoxy-benzyl)-
6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine (0.945 g, 3.21 mmol)
in
trifluoroacetic acid (10 mL) was heated at 65 C for 16 hours. Once the
reaction was
complete the solvent was removed under reduced pressure to give a dark brown
solid. The
253
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
product was used in the next step without any further purification. Step (ii):
To the dark
brown solid was added dichloromethane (30 mL) and Hunig's Base (5 mL). The
mixture was
vortexed to ensure that the solid had dissolved, then cooled in an ice-bath
followed by the
addition of 3-isocyanato-benzoic acid ethyl ester (0.613 g) as a solution in
anhydrous
dichloromethane (10 mL) in a drop wise fashion. The reaction was then shaken
at room
temperature for 20 minutes. Once the reaction was complete water (20 mL) was
added and
the reaction mixture was then extracted with CH2C12 (60 mL). The organic
extract was
washed with brine (2 x 20 mL), dried using sodium sulfate, filtered and
concentrated to give
desired crude product. The crude product was then purified using flash column
chromatography (Si02, 80% EtOAc in hexanes) to give the pure desired product,
ethyl 3-
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-
carboxamido)benzoate (0.98 g,
83%) as a white solid. M. p. 184-186 C; 1H NMR (400 MHz, DMSO-d6) 813.59 (s,
1H),
8.97 (s, I H), 8.35 (s, I H), 8.17 (s, I H), 8.13 (t, J= 1.6 Hz, I H), 7.83
(m, I H), 7.56 (m, I H),
7.39 (dd, J1= J2= 8.0 Hz, 1H), 4.80 (s, 2H), 4.30 (q, J= 6.8 Hz, 2H), 3.86 (t,
J= 6.0 Hz,
2H), 3.15 (t, J= 5.6 Hz, 2H), 1.32 (t, J= 6.8 Hz, 3H); LCMS [M+H]: 366.
[0001646] Step 2: Synthesis of 3-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c][2,7]naphthyridine-7-carboxamido)benzoic acid. To a solution of ethyl 3-
(6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoate (0.593
g, 1.62
mmol) in methanol (10 mL) was added aqueous NaOH (0.64 g in 6 mL of H20, 16.2
mmol).
The reaction was stirred at room temperature for 16 hours. The solvent was
then removed
under reduced pressure the resulting aqueous solution acidified to pH <2 using
2N HC1. A
white precipitate crashed out, which was filtered, washed with water and dried
to give the
pure desired product, 3-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c][2,7]naphthyridine-7-
carboxamido)benzoic acid (0.539 g, 98%) as a white solid; M.p. = 237-241 C;
400 MHz 1H
NMR (DMSO-d6) 8: 9.0 (s, 1H), 8.41 (s, 1H), 8.26 (s, 1H), 8.14-8.1 (m, 1H),
7.82-7.78 (m,
1H), 7.56-7.51 (m, 1H), 7.4-7.34 (m, 1H), 4.81 (s, 2H), 3.86 (t, J = 6 hz,
2H), 3.18 (t, J = 6
Hz, 2H); LCMS [M+H]: 338.
[0001647] General Procedure I: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
aN 0H` R1 / I 0
Ho i N Ri Rz RzIN \ NAN
0 H HBTU, Hunig's Base, 0 H
N DMA, RT, 16 h N
N H N H
254
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001648] Example 94: Synthesis ofN-(3-(4-(2-oxo-2,3-dihydro-lH-
benzo[d]imidazol-
1-yl)piperidine- l -carbonyl)phenyl)-8,9-dihydro-3H-pyrazolo [3,4-c]
[2,7]naphthyridine-
7(6H)-carboxamide.
_N
NH
O
H
N N N
N
Q~- O
HN4O
[0001649] To a solution of 3-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c][2,7]naphthyridine-
7-carboxamido)benzoic acid (0.080 g, 0.24 mmol) and 1-piperidin-4-yl-1,3-
dihydro-
benzoimidazol-2-one (0.057 g, 0.264 mmol) in dimethylacetamide (5 mL) and
Hunig's Base
(0.124 mL, 0.71 mmol) was added HBTU (0.107 g, 0.284 mmol). The reaction
mixture was
vortexed and then shaken at room temperature for 2 hours. On completion of the
reaction, 1 M
NaHCO3 (20 mL) and EtOAc (30 mL) were added to the reaction mixture. The
organic layer
was separated, washed with brine (2 x 20 mL) and concentrated under reduced
pressure to
give a white solid. The white solid was washed with EtOAc and dried under
reduced pressure
to give the desired product, N-(3-(4-(2-oxo-2,3-dihydro-lH-benzo[d]imidazol-l-
yl)piperidine- l -carbonyl)phenyl)-8,9-dihydro-3H-pyrazolo [3,4-c]
[2,7]naphthyridine-7(6H)-
carboxamide (0.035 g, 27%) as a white solid. Alternatively, the crude product
can be purified
either by flash column chromatography (Si02) or reverse phase HPLC (using TFA
as a
modifier).White solid; M.p. = >215 C; 400 MHz iH NMR (DMSO-d6) 8:13.59 (s,
1H),
10.88 (s, 1H), 8.9 (s, 1H), 8.35 (s, 1H), 8.17 (s, 1H), 7.64-7.56 (m, 2H),
7.38-7.26 (m, 2H),
7.08-6.94 (m, 4H), 4.79 (s, 2H), 4.65 (br s, 1H), 4.52-4.4 (m, 1H), 3.84 (t, J
= 6 Hz, 2H),
3.85-3.7 (m, 1H), 3.4-3.3 (m, 1H), 3.2-3.1 (m, 2H), 3.0-2.85 (m, 1H), 2.4-2.2
(m, 2H), 1.9-1.6
(m, 2H); LCMS [M+H]: 537.
[0001650] Example 95: N-(3-(2-(dimethylamino)ethylcarbamoyl)phenyl)-8,9-
dihydro-
3H-pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxamide (bis TFA salt).
0
H
NN N)~ N
~ H
N
N
H
[0001651] The compound, N-(3-(2-(dimethylamino)ethylcarbamoyl)phenyl)-8,9-
dihydro-
3H-pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxamide was prepared using 3-
(6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine-7-carboxamido)benzoic acid
andN1,N1-
255
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
dimethylethane- 1,2-diamine as described in general procedure I. The product
was obtained as
a white solid and as a bis-TFA salt; M.P. = 184-196 C; 400 MHz iH NMR (DMSO-
d6) 8:
13.59 (s, I H), 9.22 (s, I H), 8.92 (s, I H), 8.58 (t, J = 5.6 Hz, I H), 8.35
(s, I H), 8.17 (d, J = 1.2
Hz, 1H), 8.03-8.0 (m, 1H), 7.68-7.64 (m, 1H), 7.47-7.34 (m, 2H), 4.79 (s, 2H),
3.85 (t, J = 6
Hz, 2H), 3.58 (q, J = 6 Hz, 2H), 3.24 (t, J = 6 hz, 2H), 3.15 (t, J = 6 Hz,
2H), 2.84 (s, 6H);
LCMS [M+H]: 408.
[0001652] Example 96: N-[3-(benzylcarbamoyl)phenyl]-3,6,8,9-tetrahydro-7H-
pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide (TFA salt).
H O
zzz' N I N11~N
O H
N
N N
H
[0001653] The compound N-[3-(benzylcarbamoyl)phenyl]-3,6,8,9-tetrahydro-7H-
pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared using 3-(6,7,8,9-
tetrahydro-
3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic acid and
benzylamine as
described in general procedure I. The product was obtained as a TFA salt and a
pale yellow
solid; M.P. = >135 C; 400 MHz iH NMR (DMSO-d6) 8: 8.96 (t, J = 6 Hz, 1H), 8.9
(s, 1H),
8.35 (s, 1H), 8.18 (s, 1H), 7.96 (t, J = 1.6 Hz, 1H), 7.72-7.78 (m, 1H), 7.5-
7.45 (m, 1H), 7.38-
7.2 (m, 6H), 4.78 (s, 1H), 4.46 (d, J = 6 Hz, 2H), 3.96 (s, 1H), 3.85 (t, J =
6 Hz, 2H), 3.15 (t, J
= 6 Hz, 2H); LCMS [M+H]: 427
[0001654] Example 97: N-{3-[(pyridin-2-ylmethyl)carbamoyl]phenyl}-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide (TFA salt).
NH
O H
N- N NYN N
H O
[0001655] The compound, N-{3-[(pyridin-2-ylmethyl)carbamoyl]phenyl}-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared
using 3-
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic
acid and
pyridin-2-ylmethanamine as described in general procedure I. The product was
obtained as a
TFA salt. LCMS [M+H]: 428
[0001656] Example 98: N-(3-{[2-(1H-indol-3-yl)ethyl]carbamoyl}phenyl)-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide (TFA salt).
256
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
/\ I N p H I
NYN N
p
[0001657] The compound, N-(3 - {[2-(l H-indol-3 -yl)ethyl] carbamoyl }phenyl)-
3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared
using 3-
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic
acid and 2-
(1 H-indol-3-yl)ethanamine as described in general procedure I. The product
was obtained as
a TFA salt. LCMS [M+H]: 480
[0001658] Example 99: N-{3-[(2-hydroxyethyl)carbamoyl]phenyl}-3,6,8,9-
tetrahydro-
7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide (TFA salt).
NH
p H I
HO~~ / N N i N
H \ O
[0001659] The compound N-{3-[(2-hydroxyethyl)carbamoyl]phenyl}-3,6,8,9-
tetrahydro-
7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared using 3-
(6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic acid and
2-
aminoethanol as described in general procedure I. The product was obtained as
a TFA salt.
LCMS [M+H]: 381
[0001660] Example 100: N-{3-[(2-hydroxy-2-phenylethyl)carbamoyl]phenyl}-
3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide (TFA salt).
_N
NH
p H
NYN I iN
OH H O
[0001661] The compound, N-{3-[(2-hydroxy-2-phenylethyl)carbamoyl]phenyl}-
3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared
using 3-
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic
acid and 2-
amino-l-phenylethanol as described in general procedure I. The product was
obtained as a
TFA salt. LCMS [M+H]: 457
[0001662] Example 101: N-{3-[(pyridin-3-ylmethyl)carbamoyl]phenyl}-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide (TFA salt).
H
p H I \
-NI
NyN iN
~ H O
257
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001663] The compound N-{3-[(pyridin-3-ylmethyl)carbamoyl]phenyl}-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared
using 3-
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic
acid and
pyridin-3-ylmethanamine as described in general procedure I. The product was
obtained as a
TFA salt. LCMS [M+H]: 428
[0001664] Example 102: N-{3-[(4-methylpiperazin-1-yl)carbonyl]phenyl}-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide (TFA salt).
NH
0 H
^N N`/N N
r 'N
NJ 0
[0001665] The compound N-{3-[(4-methylpiperazin-1-yl)carbonyl]phenyl}-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared
using 3-
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic
acid and 1-
methylpiperazine as described in general procedure I. The product was obtained
as a TFA
salt. LCMS [M+H]: 420
[0001666] Example 103: N-(3-{[4-(2-hydroxyethyl)piperazin-l-yl]carbonyl
}phenyl)-
3,6,8,9-tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide (TFA
salt).
_N
NH
0 H I
^N N`/N iN
HO---_ N v 0
[0001667] The compound N-(3-{[4-(2-hydroxyethyl)piperazin-l-yl]carbonyl
}phenyl)-
3,6,8,9-tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was
prepared using
3-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine-7-
carboxamido)benzoic acid and
2-(piperazin-l-yl)ethanol as described in general procedure I. The product was
obtained as a
TFA salt. LCMS [M+H]: 450.
[0001668] Examples 104-152: Compounds in Table 4 are synthesized by using
general
procedure I as described herein.
Table 4.
Structure IUPAC Name LC/MS
M1
_N
O NH N-(3-{[2-(hydroxymethyl)piperidin-l-
H N I _- N yl]carbonyl}phenyl)-3,6,8,9-tetrahydro-7H- 435
C N pyrazolo[3,4-c][2,7]naphthyridine-7-
\ I O carboxamide
OH
258
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
H N-{3-[(2-fluorobenl)carbamoyl]phenyl}-
thH
Ny3,6,8,9 tetrahydro 7H pyrazolo[3,4 445 N 5111 H 0 c] [2,7] naphthyridine-7-
carboxamide
.N
NH N-13-[(2-amino-2-
0 H oxoethyl)(methyl)carbamoyl]phenyl}- 408
H2N-~-N)NyN . N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
0 I 0 c] [2,7]naphthyridine-7-carboxamide
thH NN- {3 -[(2-hydroxyethyl)(1-
O H methylethyl)carbamoyl]phenyl}-3,6,8,9- 423
Ho'-'---'N , N tetrahydro-7H-pyrazolo[3,4-
c][2,7]naphthyridine-7-carboxamide
.N
NH N- {3 -[(2-methoxy- l -
0 H methylethyl)carbamoyl]phenyl}-3,6,8,9-
, N N N tetrahydro-7H-pyrazolo[3,4- 409
H , I 0 c] [2,7]naphthyridine-7-carboxamide
NH N-{3-[(4-
0 H methoxybenzyl)carbamoyl]phenyl}-3,6,8,9-
NNyN N tetrahydro 7H pyrazolo[3,4 457
0 H I 0 c][2,7]naphthyridine-7-carboxamide
.N
NH N-{3-[(2-
0 H hydroxyethyl)(methyl)carbamoyl]phenyl}- 395
HO~~N , N N i N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
p c][2,7]naphthyridine-7-carboxamide
.N
N H N- {3 -[(furan-2-
0 H ylmethyl)(methyl)carbamoyl]phenyl}- 431
0 N NyN N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
0 c] [2,7]naphthyridine-7-carboxamide
.N
NH N-(3-{[(3R)-3-(dimethylamino)pyrrolidin-l-
0 H yl]carbonyl}phenyl)-3,6,8,9-tetrahydro-7H- 434
N NN , N Y N N pyrazolo[3,4-c][2,7]naphthyridine-7-
0 carboxamide
.N
0 A NH N-[3-(piperidin-1-ylcarbonyl)phenyl]-
N N N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 405
GN c][2,7]naphthyridine-7-carboxamide
0
.N
NH N-(3-1[2-
0 H (trifluoromethyl)benzyl]carbamoyl}phenyl)-
~NN~N N 3,6,8,9 tetrahydro 7H pyrazolo[3,4 495
OH 0 c] [2,7]naphthyridine-7-carboxamide
F3
_NNH N- {3 -[(3 -piperidin-1-
0 H ylpropyl)carbamoyl]phenyl}-3,6,8,9- 462
CJNN1I N` /N N tetrahydro-7H-pyrazolo[3,4-
0 c] [2,7]naphthyridine-7-carboxamide
NH N-{3-[(4-
0 H N 5,N methylcyclohexyl)carbamoyl]phenyl} 433
N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
H c] [2,7]naphthyridine-7-carboxamide
259
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
.N
NH N-(3-{[3-(2-oxopyrrolidin-l-
0 H N yl)propyl]carbamoyl}phenyl)-3,6,8,9- 462
~rv~N tetrahydro-7H-pyrazolo[3,4-
H o c][2,7]naphthyridine-7-carboxamide
0
.N
NH N-(3-1[2-
0 H \ (dimethylamino)ethyl](ethyl)carbamoyl}phe 436
N 'IN '-'--'N . N nyl)-3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
O c][2,7]naphthyridine-7-carboxamide
HO NH N-(3-{[(1R)-1-
0 H N .N (hydroxymethyl)butyl]carbamoyl}phenyl)- 423
~,N :-l' 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
H v 0 c][2,7]naphthyridine-7-carboxamide
_N
NH N- {3 -[(pyridin-4-
O H N ylmethyl)carbamoyl]phenyl}-3,6,8,9- 428 N v tetrahydro-7H-pyrazolo[3,4-
N H v p c] [2,7]naphthyridine-7-carboxamide
.N
NH N-{3-[(2-morpholin-4-
o'-j o H ylethyl)carbamoyl]phenyl}-3,6,8,9- 450
~,NNYN N tetrahydro-7H-pyrazolo[3,4-
H 0 c] [2,7]naphthyridine-7-carboxamide
N N-(3-{[(1S)-2-amino-1-(1H-indol-3-
HN 0 NH ylmethyl)-2-oxoethyl]carbamoyl}phenyl)- 523
H 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
H2N N NYN N c] [2,7]naphthyridine-7-carboxamide
O H O
NH N- {3 -[(4-chlorobenzyl)carbamoyl]phenylN N 3,6,8,9-tetrahydro-7H-
pyrazolo[3,4- 461
5,N 0
v H c] [2,7]naphthyridine-7-carboxamide
ci \ o
_N
NH N-(3-{[2-(2-
v H methoxyphenyl)ethyl]carbamoyl}phenyl)- 471
i N N~N N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
H v 0 c] [2,7]naphthyridine-7-carboxamide
NH N-{3-[(2-phenoxyethyl)carbamoyl]phenyl}-
p
H
o--,'^~ N N I, N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 457
H Y c][2,7]naphthyridine-7-carboxamide
0
.N
NH N-(3-{[(5-methylisoxazol-3-
0 H yl)methyl]carbamoyl}phenyl)-3,6,8,9- 432
H N y N N tetrahydro-7H-pyrazolo[3,4-
0_N 0 c][2,7]naphthyridine-7-carboxamide
-N NH N-(3-1[2-(3-
0 H chlorophenyl)ethyl]carbamoyl}phenyl)- 475
Ci v N NYN N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
H vJI o c][2,7]naphthyridine-7-carboxamide
_N
0 NH N-{3-[(4-pyridin-2-ylpiperazin-l-
NON N yl)carbonyl]phenyl}-3,6,8,9-tetrahydro-7H- 483
N N N 0 pyrazolo[3,4-c] [2,7]naphthyridine-7-
carboxamide
260
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
-N N-(3-{[4-(pyridin-2-ylmethyl)piperazin-l-
NH
O H yl]carbonyl}phenyl)-3,6,8,9-tetrahydro-7H- 497
N rN NYN N pyrazolo[3,4-c][2,7]naphthyridine-7-
NJ o carboxamide
-,-a
.N
NH N-(3-{[(1-methyl-lH-imidazol-5-
N~ 0 NN yl)methyl]carbamoyl}phenyl)-3,6,8,9- 431
tetrahydro-7H-pyrazolo[3,4-
~N H ~ 0 c][2,7]naphthyridine-7-carboxamide
.N
NH N-(3-{[(2S)-tetrahydrofuran-2-
0 H ylmethyl]carbamoyl}phenyl)-3,6,8,9- 421
0 ,,, N N N N tetrahydro-7H-pyrazolo[3,4-
H o c][2,7]naphthyridine-7-carboxamide
.NH N-(3-{[(1-methyl-lH-benzimidazol-2-
N 0 H N yl)methyl]carbamoyl}phenyl)-3,6,8,9- 481
N i 1 Y tetrahydro-7H-pyrazolo[3,4-
N zzzl' 0 c][2,7]naphthyridine-7-carboxamide
N
NH N-(3-1[2-(3-
0 H methoxyphenyl)ethyl]carbamoyl}phenyl)- 471
N N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
o c][2,7]naphthyridine-7-carboxamide
_ N NH N- {3 -[(2-pyridin-3 -
N 0 H ylethyl)carbamoyl]phenyl}-3,6,8,9- 442
~H NYN N tetrahydro-7H-pyrazolo[3,4-
0 c][2,7]naphthyridine-7-carboxamide
HO NH N-(3-{[(1R)-1-(hydroxymethyl)-2-
0 H methylpropyl]carbamoyl}phenyl)-3,6,8,9- 423
N~N N tetrahydro-7H-pyrazolo[3,4-
H 0 c][2,7]naphthyridine-7-carboxamide
N-{3-[(3-chlorobenzyl)carbamoyl]phenyl}-
O 5,N NH
N N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 461
H o y c][2,7]naphthyridine-7-carboxamide
_N NH N-(3-{[4-(4-methylbenzyl)piperazin-l-
0
NH uN yl]carbonyl}phenyl)-3,6,8,9-tetrahydro-7H- 510
r N II pyrazolo[3,4-c][2,7]naphthyridine-7-
N o carboxamide
.N
NH N-(3-{[4-(2-oxo-2-pyrrolidin-1-
o ~
0 N i N N I ~N ylethyl)piperazin-1-yl]carbonyl}phenyl)- 517 0 3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-
GN~-N~ c][2,7]naphthyridine-7-carboxamide
0 NH N-[3-(cycloheptylcarbamoyl)phenyl]-
H N N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 433
OIN c] [2,7]naphthyridine-7-carboxamide
0
_N
0 NH N-[3-(cyclohexylcarbamoyl)phenyl]-
a H N N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 419
H c] [2,7]naphthyridine-7-carboxamide
0
261
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
0 NH N-{3-[(4-pyrazin-2-ylpiperazin-l-
rN NyN N yl)carbonyl]phenyl}-3,6,8,9-tetrahydro-7H- 484
N N I 0 pyrazolo[3,4-c] [2,7]naphthyridine-7-
C carboxamide
N
J _N
0 NH N- {3 -[(2-methylbenzyl)carbamoyl]phenyl}-
H N N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 441
N c] [2,7]naphthyridine-7-carboxamide N .N
NH N-(3-{[3-
0 H (dimethylamino)propyl] carbamoyl }phenyl)- 422
NyN N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
I H 0 c] [2,7]naphthyridine-7-carboxamide
_N
NH N-(3-{[2-(1-methyl-1 H-imidazol-5-
N4 N 0 H yl)ethyl] carbamoyl }phenyl)-3,6,8,9- 445
N N Y N N tetrahydro-7H-pyrazolo[3,4-
H 0 c] [2,7]naphthyridine-7-carboxamide
.N
NH N-(3 -{[4-(hydroxymethyl)piperidin-1-
0 H N yl]carbonyl}phenyl)-3,6,8,9-tetrahydro-7H- 435
pyrazolo[3,4-c] [2,7]naphthyridine-7-
Ho o carboxamide
NH N-{3-[(3-
0 1 I o H I phenoxyphenyl)carbamoyl]phenyl}-3,6,8,9- 505
i NyN N tetrahydro-7H-pyrazolo[3,4-
o N
-kj
H o c][2,7]naphthyridine-7-carboxamide
.N
NH N-(3-{[3-(hydroxymethyl)piperidin-1-
0 H N yl]carbonyl}phenyl)-3,6,8,9-tetrahydro-7H- 435
HOJN pyrazolo[3,4-c] [2,7]naphthyridine-7-
0 carboxamide
N-(3- { [(3 R)-4-hydroxy-3 -
NH
= 0 H methylbutyl]carbamoyl}phenyl)-3,6,8,9- 423
Ho "N NYN N tetrahydro-7H-pyrazolo[3,4-
H o c][2,7]naphthyridine-7-carboxamide
N
_
NH N-{3-[(2-pyrrolidin-l-
0 H ylethyl)carbamoyl]phenyl}-3,6,8,9- 434
ONN NyN N tetrahydro-7H-pyrazolo[3,4-
0 c][2,7]naphthyridine-7-carboxamide
NH N-{3-[(3-hydroxypyrrolidin-l-
0 H N .N yl)carbonyl]phenyl}-3,6,8,9-tetrahydro-7H- 407
pyrazolo[3,4-c] [2,7]naphthyridine-7-
HON \ o carboxamide
.N
NH N-(3-1[2-
H o (dimethylamino)ethyl] (methyl)carbamoyl }p 422
NyN . N henyl)-3,6,8,9-tetrahydro-7H-pyrazolo[3,4-
I 0 c] [2,7]naphthyridine-7-carboxamide
_N
0 NH N- {3 -[(2-phenylethyl)carbamoyl]phenyl}-
H N N N 3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 441
N o c] [2,7]naphthyridine-7-carboxamide
H)~'a 0
262
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001669] Example 153: Synthesis of 3-(3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-
3H-
pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic acid
/ o
HN EtO NN
H
tO2C / NCO O NaOH, H2O, McOH
E
N N
i2l
N CH2CI2, RT, 30 min N N RT, 16 h
Step-1 Step-2
-O _O
/ O
HO I NAN
O
N
N N
-)~5
[0001670] Step 1: Synthesis of ethyl 3-(3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-
3H-
pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoate. To a solution of 3-
(4-
methoxybenzyl)-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine (0.208
g, 0.707
mmol) in anhydrous dichloromethane (10 mL) was added 3-isocyanato-benzoic acid
ethyl
ester (0.135 g, 0.707 mmol). The reaction was then shaken at room temperature
for 30
minutes. On completion of the reaction water (10 mL) was added and the organic
layer was
separated. The aqueous layer was extracted with dichloromethane (20 mL). The
organic
extracts were combined, washed with brine (2 x 20 mL), dried using sodium
sulfate, filtered
and concentrated under reduced pressure. The crude product was purified using
flash column
chromatography (Si02, gradient from 50% EtOAc in hexanes to 66% EtOAc in
hexane) to
give the pure desired product, ethyl 3-(3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-
3H-
pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoate (0.265 g, 77%) as a
white solid.
M.P. = >80 C; 400 MHz 1H NMR (DMSO-d6) 8: 8.97 (s, 1H), 8.41 (s, 1H), 8.2 (s,
1H), 8.14-
8.1 (m, 1H), 7.85-7.8 (m, 1H), 7.57-7.53 (m, 1H), 7.39 (t, J = 7.6 Hz, 1H),
7.21-7.16 (m, 2H),
6.88-6.82 (m, 2H), 5.59 (s, 2H), 4.81 (s, 2H), 4.31 (q, J = 7.2 Hz, 2H), 3.84
(t, J = 6 Hz, 2H),
3.69 (s, 3H), 3.15 (t, J = 6 Hz, 2H), 1.32 (t, J = 7.2 Hz, 3H); LCMS [M+H]:
486.
[0001671] Step 2: Synthesis of 3-(3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic acid. To a solution of
ethyl 3-(3-
(4-methoxybenzyl)-6,7, 8,9-tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine-7-
carboxamido)benzoate (0.162 g, 0.33 mmol) in methanol (5 mL) was added aqueous
NaOH
(0.13 g in 3 mL H20, 3.3 mmol). The reaction was stirred at room temperature
for 16 hours.
On completion of the reaction the solvent was removed and the resulting
aqueous solution
263
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
was acidified to pH < 2 using 2N HC1. A solid precipitated out, which was
filtered, washed
with water and dried to give the desired product, 3-(3-(4-methoxybenzyl)-
6,7,8,9-tetrahydro-
3H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic acid (0.136 g, 90%)
as a pale
yellow solid. M. p. > 136 C; 'H NMR (400 MHz, DMSO-d6) 812.95 (s, 1H), 8.93
(s, 1H),
8.41 (s, 1 H), 8.19 (s, 1 H), 8.10 (m, 1 H), 7.78 (m, 1 H), 7.53 (m, 1 H),
7.36 (dd, J1= Jz = 8.4
Hz, 1H), 7.18 (m, 2H), 6.84 (m, 2H), 4.86 (m, s, 2H), 3.85 (t, J= 6.0 Hz, 2H),
3.68 (s, 3H),
3.50 (m, 2H), 3.16 (t, J= 6.0 Hz, 2H); LCMS [M+H]: 458.
[0001672] General Procedure J: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
I0I
HO aN H O
0 H N 0) DMC, CH2CI2, DMA, Anilines Ar'N \ I N)~ N
Hunig's Base, RT, 16 h H
N 0
N N (ii) CF3000H, 65 C, 16 h N
N N
d H
-O
[0001673] Example 154: Synthesis of N-(3-(isoxazol-3-ylcarbamoyl)phenyl)-8,9-
dihydro-3H-pyrazolo [3,4-c] [2,7]naphthyridine-7(6H)-carboxamide.
0'N 0 H NH
/ NYN i N
H \~ 0
[0001674] Step 1: To a solution of 3-(3-(4-methoxybenzyl)-6,7,8,9-tetrahydro-
3H-
pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamido)benzoic acid (0.037 g, 80
mol), isoxa-3-
ylamine (8.8 L,120 mol) and Hunig's Base (71 L, 400 mol) in
dimethylacetamide (1
mL) and dichloromethane (1 mL) was added 2-chloro-1,3-dimethylimidazolinium
chloride
(DMC, 0.041 g, 240 mol). The reaction mixture was shaken at room temperature
for 16
hours followed by addition of water (2 mL) and dichloromethane (4 mL) were
added. The
organic layer was separated and the solvent removed under reduced pressure to
give the
desired product, N-(3-(isoxazol-3-ylcarbamoyl)phenyl)-3-(4-methoxybenzyl)-8,9-
dihydro-
3H-pyrazolo[3,4-c][2,7]naphthyridine-7(6H)-carboxamide. LCMS [M+H]: 524. The
crude
product was used in the next step without any further purification.
[0001675] Step 2 To the crude N-(3-(isoxazol-3-ylcarbamoyl)phenyl)-3-(4-
methoxybenzyl)-8,9-dihydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine-7(6H)-
carboxamide
obtained above was added trifluoroacetic acid (1 mL). The reaction mixture was
shaken at 65
C for 16 hours. On completion of the reaction, toluene (1 mL) was added and
solvent
264
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
removed under reduced pressure. The crude product was purified reverse phase
HPLC to give
the desired product, N-(3-(isoxazol-3-ylcarbamoyl)phenyl)-8,9-dihydro-3H-
pyrazolo[3,4-
c][2,7]naphthyridine-7(6H)-carboxamide. LCMS [M+H]: 404.
[0001676] Example 155: Synthesis of N-[3-(phenylcarbamoyl)phenyl]-3,6,8,9-
tetrahydro-7H-pyrazolo [3,4-c] [2,7]naphthyridine-7-carboxamide.
.
NH
/ O H \
\ I NuN I i N
H IOI
[0001677] The compound N-[3-(phenylcarbamoyl)phenyl]-3,6,8,9-tetrahydro-7H-
pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared using 3-(3-(4-
methoxybenzyl)-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine-7-
carboxamido)benzoic acid and aniline as described in general procedure J. The
compound
was obtained as a solid. LCMS [M+H]: 413.
[0001678] Example 156: Synthesis of N-[3-(1H-pyrazol-3-ylcarbamoyl)phenyl]-
3,6,8,9-
tetrahydro-7H-pyrazolo [3,4-c] [2,7]naphthyridine-7-carboxamide.
_N
N NH
HN-N O \
YN N
H O
[0001679] The compoundN-[3-(1H-pyrazol-3-ylcarbamoyl)phenyl]-3,6,8,9-
tetrahydro-
7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared using 3-(3-(4-
methoxybenzyl)-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine-7-
carboxamido)benzoic acid and 1H-pyrazol-3-amine as described in general
procedure J. The
compound was obtained as a solid. LCMS [M+H]: 403.
[0001680] Example 157: N-(3-{[3-(acetylamino)phenyl]carbamoyl}phenyl)-3,6,8,9-
tetrahydro-7H-pyrazolo [3,4-c] [2,7]naphthyridine-7-carboxamide.
-N,
H
0 / I 0 H I
\ N\/N iN
H H 0
[0001681] The compound N-(3-{[3-(acetylamino)phenyl]carbamoyl}phenyl)-3,6,8,9-
tetrahydro-7H-pyrazolo[3,4-c][2,7]naphthyridine-7-carboxamide was prepared
using 3-(3-(4-
methoxybenzyl)-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine-7-
carboxamido)benzoic acid and N-(3-aminophenyl)acetamide as described in
general
procedure J. The compound was obtained as a solid. LCMS [M+H]: 470.
265
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001682] Example 158: Synthesis of 7-(phenylsulfonyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c] [2,7]naphthyridine.
\
o=s'a
HN OSO
N 0) I/, Et3N, CHZCI2, RT, 1 h
N N (ii) CF3000H, 65 C, 16 h N
LG- OCH3 N H
[0001683] Step 1: To a solution of 3-(4-methoxy-benzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c][2,7]naphthyridine (0.129 g, 0.43 mmol) in dichloromethane (8
mL) and Et3N
(0.13 mL, 0.86 mmol) was added benzenesulfonyl chloride (62 L, 0.47 mmol).
The reaction
mixture was shaken at room temperature for 1 hour. The solvent was then
removed under
reduced pressure and the residue obtained was partitioned between EtOAc and
aqueous
NaOH. The organic layer was then separated, washed with brine (2 x 10 mL),
concentrated
under reduced pressure. The crude product was purified using either flash
column
chromatography (Si02, 33% EtOAc in hexane) to give 3-(4-methoxybenzyl)-7-
(phenylsulfonyl)-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine as a
white solid
(0.147 g, 77%) or used in the next step without any further purification. M.p.
= 136-138 C;
400 MHz 1H NMR (DMSO-d6) 8: 8.39 (s, 1H), 8.12 (s, 1H), 7.88-7.83 (m, 2H),
7.72-7.59
(m, 3h), 7.18-7.13 (m, 2H), 6.86-6.8 (m, 2H), 5.55 (s, 2H), 4.36 (s, 2H), 3.68
(s, 3H), 3.43, t,
J = 6.4 Hz, 2H), 3.13 (t, J = 6.4 Hz, 2H), 2.54-2.48 (m, 2H); LCMS [M+H]: 435.
[0001684] Step 2: To 3-(4-methoxybenzyl)-7-(phenylsulfonyl)-6,7,8,9-tetrahydro-
3H-
pyrazolo[3,4-c][2,7]naphthyridine (0.109 g, 2.51 mmol) was added
trifluoroacetic acid (2
mL). The reaction mixture was heated at 65 C for 16 hours. On completion of
the reaction,
toluene (5 mL) was added. The reaction mixture was evaporated to dryness under
reduced
pressure to yield a dark brown solid. The crude product was purified using
reverse phase
HPLC chromatography to give the desired product, 7-(phenylsulfonyl)-6,7,8,9-
tetrahydro-
3H-pyrazolo[3,4-c][2,7]naphthyridine as a white solid (0.043 g, 54%). M.p. =
270-271 C;
400 MHz 1H NMR (DMSO-d6) 8: 13.59 (s, 1H), 8.33 (s, 1H), 8.1 (d, J = 1.2 Hz,
1H), 7.88-
7.84 (m, 2H), 7.73-7.61 (m, 3H), 4.35 (s, 2H), 3.43 (t, J = 5.6 Hz, 2H), 3.14
(t, J = 6.4 Hz,
2H); LCMS [M+H]: 315.
[0001685] Example 158: Synthesis of N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c]isoquinolin-7-yl)benzenesulfonamide.
266
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
'CI
0=s
H2N
H
\
\ N
N 0) I / , Et3N, THF, RT, 1 h S
O O
N N (ii) CF3COOH, 65 C, 16 h I \ "N
L~& OCH3 N H
[0001686] Step 1: To a solution of 3-(4-methoxy-benzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7-ylamine TFA salt (0.156 g, 0.37 mmol) in
anhydrous THE (5
mL) and Et3N (0.15 mL, 1.11 mmol) was added benzenesulfonyl chloride (60 L,
0.46
mmol). The reaction mixture was shaken at room temperature for 3 hours
followed by
addition of EtOAc (20 mL) and 1. OM NaOH (15 mL). The organic layer was then
separated,
washed with brine (2 x 10 mL) and concentrated under reduced pressure to give
the crude
product. The crude product was purified using flash column chromatography
(Si02, 33%
EtOAc in hexane) to give the desired product, N-(3-(4-methoxybenzyl)-6,7,8,9-
tetrahydro-
3H-pyrazolo[3,4-c]isoquinolin-7-yl)benzenesulfonamide as a white solid (0.103
g, 62%).
Alternatively the crude product could be used in the next step without any
further
purification. M. p. >70 C; 'H NMR (400 MHz, DMSO-d6) 87.99 (s, 1H), 7.91 (s,
1H), 7.86
(m, 2H), 7.81 (s, 1H), 7.68-7.58 (m, 3H), 7.14 (m, 2H), 6.83 (m, 2H), 5.50 (s,
2H), 3.68 (s,
3H), 3.48 (m, I H), 2.96 (m, 3H), 2.76 9m, I H), 1.91 (m ,1 H), 1.77 (m, I H);
LCMS [M+H] :
449.
[0001687] Step 2: To N-[3-(4-methoxy-benzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-
c]isoquinolin-7-yl]-benzenesulfonamide (0.082 g, 0.18 mmol) was added
trifluroacetic acid
(2 mL). The reaction mixture was heated at 65 C for 5 h. On the completion of
the reaction,
toluene (5 mL) was added. The solvent was the removed under reduced pressure
to give the
crude product as a dark brown solid. The crude product was purified using
reverse phase
HPLC to give the desired product, N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c]isoquinolin-7-
yl)benzenesulfonamide was obtained as a white solid (0.038 g, 63%). M.p. = 228-
229 C; 400
MHz 1H NMR (DMSO-d6) 8: 13.28 (s, 1H), 7.96 (s, 1H), 7.94-7.84 (m, 3H), 7.78
(s, 1H),
7.69-7.58 (m, 3H), 3.48 (br s, 1H), 3.04-2.72 (m, 4H), 1.95-1.7 (m, 2H); LCMS
[M+H]: 329.
[0001688] General Procedure K: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below.
267
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
HN OõO
0) RSO2CI, Et3N, THF, RT, 16 h R' S `N
N NN
(ii) CF3COOH, 65 C, 16 h I \ "N
OCH3 N H
H2N H
R, N
N (i) RS0201, Et3N, THF, RT, 16 h S
O O
N N (ii) CF3000H, 65 C, 16 h \ "N
LO- OCH3 N H
[0001689] Step 1: To a 0.24M solution of 3-(4-methoxybenzyl)-6,7,8,9-
tetrahydro-3H-
pyrazolo[3,4-c][2,7]naphthyridine (trifluoroacetic acid salt) (0.5 mL, 0.12
mmol) in THF was
added 1M solution of triethylamine (0.5 mL, 0.5 mmol) in THF followd by 0.18M
solution of
sulfonyl chloride (1.0 mL, 0.18 mmol) in THF. The reaction mixture was stirred
at room
temperature for 16 hours. The solvent was then removed under reduced pressure
and the
residue was dissolved in dichloromethane (2 mL). Then organic layer was washed
with water
(2 mL) and the solvent removed under reduced pressure. The crude desired
product was used
in step ii without any further purification.
[0001690] Step 2: To the crude residue from step (i) was added trifluoroactic
acid (1 mL)
and the reaction mixture heated at 65 C for 16 hours. The solvent was removed
under
reduced pressure and crude desried product purified using reverse phase
chromatography (C-
18) using trifluoroactic acid as a modifier.
[0001691] Example 160: Synthesis of 8-(phenylsulfonyl)-3,6,7,8-
tetrahydropyrazolo[4',3':5,6]pyrido[3,4-d] [1 ]benzazepine
N-NH
N
N
O=S
O / j
[0001692] The compound 8-(phenylsulfonyl)-3,6,7,8-
tetrahydropyrazolo[4',3':5,6]pyrido[3,4-d][1]benzazepine was prepared using 3-
(4-
methoxybenzyl)-3,6,7,8-tetrahydropyrazolo[4',3':5,6]pyrido[3,4-
d][1]benzazepine and
phneylsulfonyl chloride as described in the 2 step procedure K. The desired
compound was
obtained as an off-white solid (0.040 g, 18%); 'H NMR (CDC13, 400 MHz) 8: 8.52
(s, 1H),
8.29 (m, 2H), 7.73 (m, 2H), 7.67-7.50 (m, 6H), 7.00 (dd, JI = 1.2 Hz, J2 = 8.8
Hz, 2H), 6.51
(dd, J1= J2 = 8.0 Hz, 2H), 6.20 (dd, J1= J2 = 8.0 Hz, 1H), 4.20 (m, 1H), 3.65
(m, 1H), 2.70
(m, 2H); LCMS [M+H]: 377
268
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001693] Examples 161-317: Compounds in Table 5 are synthesized by using
general
procedure K as described herein.
Table 5.
Structure IUPAC Name LC/MS
[M+1]
C&N NH 4-ethyl-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 357
so N c]isoquinolin-7-yl)benzenesulfonamide
H
J o NH 3,4-dimethyl-N-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7- 357
oS'H N yl)benzenesulfonamide
_N
NH I" N pyrazolo[3,4-c]isoquinolin-7- 378
I d 'H H yl)methanesulfonamide
N
i NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
,0 c]isoquinolin-7-yl)-3- 397
F3c ' N (trifluoromethyl)benzenesulfonamide
S NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 335
c]isoquinolin-7-yl)thiophene-3-sulfonamide
th
HH
CFN-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c]isoquinolin-7-yl)-2- 413
thN ~O
(trifluoromethoxy)benzenesulfonamide
thNH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,- 281
N c]isoquinolin-7-yl)ethanesulfonamide
H
NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
0 c]isoquinolin-7-yl)-2,1,3-benzoxadiazole-4- 371
N /_N N N sulfonamide
O-N H
IN, Ell NH 2-(1-naphthyl)-N-(6,7,8,9-tetrahydro-3H- 408
os N pyrazolo[3,4-c]isoquinolin-7-yl)ethanesulfonamide
N
H
th 3-chloro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3364
c]isoquinolin-7-yl)benzenesulfonamide
H
_N
o NH 3-methoxy-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 359
os N N c]isoquinolin 7 yl)benzenesulfonamide
H
_N
CI F 01NH 4-chloro-2,5-difluoro-N-(6,7,8,9-tetrahydro-3H-
o pyrazolo[3,4-c]isoquinolin-7- 400
F o,H N yl)benzenesulfonamide
th CI 2-chloro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3364
c]isoquinolin-7-yl)benzenesulfonamide
N
H
269
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
4-fluoro-2-methyl-N-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7- 361
thNH N
yl)benzenesulfonamide
_N
ci / I 0 NH 5-chloro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 370
1 , N c]isoquinolin-7-yl)thiophene-2-sulfonamide
s -N
H
C NH N (6,7,8,9 tetrahydro 3H pyrazolo[3,4
,0 c]isoquinolin-7-yl)-2,3-dihydro-1,4-benzodioxine- 387
oS N N 6-sulfonamide
ci ~NH 4-chloro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-::C 1 0 c]isoquinolin-7-
yl)-3- 432
F30 N N (trifluoromethyl)benzenesulfonamide
.N
Cl,aF NH 4-chloro-2-fluoro-N-(6,7,8,9-tetrahydro-3H-
1 pyrazolo[3,4-c]isoquinolin-7- 382
os-N N yl)benzenesulfonamide
N
NH 3,4-dimethoxy-N-(6,7,8,9-tetrahydro-3H-
0 pyrazolo[3,4-c]isoquinolin-7- 389
o-N N yl)benzenesulfonamide
H
?jcNH 4-methyl-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4
0 393
N c]isoquinolin-7-yl)naphthalene-l-sulfonamide
N
H
N
II o NH 3-cyano-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 354
NS N c]isoquinolin-7-yl)benzenesulfonamide
H
_N
NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 293
OS N N c]isoquinolin-7-yl)cyclopropanesulfonamide
H
NH 3-chloro-4-methyl-N-(6,7,8,9-tetrahydro-3H-
1 pyrazolo[3,4-c]isoquinolin-7- 378
a os'N N yl)benzenesulfonamide
H
thNH 1-(2-chlorophenyl)-N-(6,7,8,9-tetrahydro-3H-
sp pyrazolo[3,4-c]isoquinolin-7- 378
co H yl)methanesulfonamide
ci
_N
F
NH 2-fluoro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 347
" N c]isoquinolin-7-yl)benzenesulfonamide
N
H
.N
NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
e0 N c]isoquinolin-7-yl)-2,1,3-benzothiadiazole-4- 387
N / ~~ N sulfonamide
S-N O H
NH 3-chloro-2-methyl-N-(6,7,8,9-tetrahydro-3H-
0 pyrazolo[3,4-c]isoquinolin-7- 378
a os,N N yl)benzenesulfonamide
N
FBr NH 2-bromo-4-fluoro-N-(6,7,8,9-tetrahydro-3H-
s N pyrazolo[3,4 c]isoquinolin 7 426
C? N yl)benzenesulfonamide
H
270
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N N
NH N-{4-[(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 386
s;H N c]isoquinolin 7 ylamino)sulfonyl]phenyl}acetamide
O
ci ~NH 2-chloro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
,0 c]isoquinolin 7 yl) 5 432
F3 os'H C:::
N N (trifluoromethyl)benzenesulfonamide
F3c i NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
I O c]isoquinolin-7-yl)-4- 397
s'H ~S'
( trifluoromethyl)benzenesulfonamide
_N
N H N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 267
0 DSO I N c]isoquinolin-7-yl)methanesulfonamide
N
H
C6N N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
`so c]isoquinolin-7-yl)-1-benzothiophene-3- 385
H sulfonamide
(CF3 'N N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
0 c]isoquinolin-7-yl)-2- 397
N N (trifluoromethyl)benzenesulfonamide
H
H 5-bromo-2-methoxy-N-(6,7,8,9-tetrahydro-3H-
,0 pyrazolo[3,4-c]isoquinolin-7- 438
thN N
Br OSN yl)benzenesulfonamide
H 2,3-dichloro-N-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7- 398
thN N
CI O H yl)benzenesulfonamide
F F NH 2,4-difluoro-N-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7- 365
=S-N N yl)benzenesulfonamide
O H
N
O N
ivH 6 phenoxy N (6,7,8,9 tetrahydro 3H pyrazolo[3,4
os N i N c]isoquinolin-7-yl)pyridine-3-sulfonamide 422
H
_N
N ::a N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
~ O
c] isoquinolin-7-yl)-1,3 -benzothiazole-6- 386
S \ S`N N sulfonamide
H
CI
_N
NH 5-chloro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 414
O c]isoquinolin-7-yl)naphthalene-l-sulfonamide
S= N
N
H
_N
N- NH 3,5-dimethyl-N-(6,7,8,9-tetrahydro-3H-
O O pyrazolo[3,4-c]isoquinolin-7-yl)isoxazole-4- 348
oS, N N sulfonamide
H
271
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N
NH 5-fluoro-2-methyl-N-(6,7,8,9-tetrahydro-3H-
F pyrazolo[3,4-c]isoquinolin-7- 361
S, N
N yl)benzenesulfonamide
H
.N
F3C.CI NH 2-chloro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
0
c]isoquinolin-7-yl)-4- 432
ps H N (trifluoromethyl)benzenesulfonamide
NH 4-methoxy-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 359
O I
~S N c]isoquinolin-7-yl)benzenesulfonamide
HH
_N
CI NH 2,5-dichloro-N-(6,7,8,9-tetrahydro-3H-
CI gO I N pyrazolo[3,4-c]isoquinolin-7- 398
N yl)benzenesulfonamide
H
_N
O'I- NH 2-methoxy-4-methyl-N-(6,7,8,9-tetrahydro-3H-
0
pyrazolo[3,4-c]isoquinolin-7- 373
N yl)benzenesulfonamide
N H
_N
/ NH 2,4,6-trimethyl-N-(6,7,8,9-tetrahydro-3H-
,O pyrazolo[3,4-c]isoquinolin-7- 371
S,N N yl)benzenesulfonamide
H
_N
F3CO , NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
O c]isoquinolin-7-yl)-4- 413
OS N N (trifluoromethoxy)benzenesulfonamide
_N
Br Br NH 2,4-dibromo-N-(6,7,8,9-tetrahydro-3H-
0 pyrazolo[3,4-c]isoquinolin-7- 487
OS`N N yl)benzenesulfonamide
H
_N
O NH 4-methyl-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 343
c]isoquinolin-7-yl)benzenesulfonamide
OS=N N
H
.N
CI NH 2,6-dichloro-N-(6,7,8,9-tetrahydro-3H-
o pyrazolo[3,4-c]isoquinolin-7- 398
C;:~211i N yl)benzenesulfonamide
Cl 0 H
_N
<-;S NH i0 N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 335
// N N c]isoquinolin-7-yl)thiophene-2-sulfonamide
H
_N
F
O NH 4-fluoro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 347
c]isoquinolin-7-yl)benzenesulfonamide
OS. N
N
H
272
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
O NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 329
~ c]isoquinolin-7-yl)benzenesulfonamide
S.N ~ N
O H
NH
O 3-methyl-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4
'S N c]isoquinolin-7-yl)quinoline-8-sulfonamide 394
O H
.N
O NH 2-methyl-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 343
'i c]isoquinolin-7-yl)benzenesulfonamide
OS.N N
H
5,'N N
H -fluoro-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 347
c]isoquinolin-7-yl)benzenesulfonamide
F ON H
_N
F CI NH 2-chloro-4-fluoro-N-(6,7,8,9-tetrahydro-3H-
SO N pyrazolo[3,4-c]isoquinolin-7- 382
-N yl)benzenesulfonamide
H
N
O NH 2,5-dimethoxy-N-(6,7,8,9-tetrahydro-3H-
,O pyrazolo[3,4-c]isoquinolin-7- 389
OS,N N yl)benzenesulfonamide
H
_N
NH 3-chloro-2-fluoro-N-(6,7,8,9-tetrahydro-3H-
O
pyrazolo[3,4-c]isoquinolin-7- 3 82
CI ~S N N yl)benzenesulfonamide
F O H
N
Br F NH 4-bromo-2-fluoro-N-(6,7,8,9-tetrahydro-3H-
0 pyrazolo[3,4-c]isoquinolin-7- 426
OS`N N yl)benzenesulfonamide
H
_N
F NH 5-chloro-2-fluoro-N-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7- 382
CI OS,N N yl)benzenesulfonamide
H
_N
NH
/O N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 295
S, N c]isoquinolin-7-yl)propane- 1 -sulfonamide
O N
H
_N
O NH 3-methyl-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 343
c]isoquinolin-7-yl)benzenesulfonamide
OSN N
H
_N
NH 1-(1,2-benzisoxazol-3-yl)-N-(6,7,8,9-tetrahydro-
3H-pyrazolo[3,4-c]isoquinolin-7- 384
N
yl)methanesulfonamide
I ~. N
N O H
273
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
0
.N
NH 4-(methylsulfonyl)-N-(6,7,8,9-tetrahydro-3H-
I I pyrazolo[3,4-c]isoquinolin-7- 407
OS`N _- N yl)benzenesulfonamide
H
_N
CI v NH 4-chloro-2,5-dimethyl-N-(6,7,8,9-tetrahydro-3H-
/ 0 pyrazolo[3,4-c]isoquinolin-7- 392
OS`N N yl)benzenesulfonamide
H
N
Br CF3 NH 4-bromo-N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
0 c]isoquinolin-7-yl)-2- 476
N N (trifluoromethyl)benzenesulfonamide
H
.N
CI v CI NH 2,4-dichloro-N-(6,7,8,9-tetrahydro-3H-
/ 0 pyrazolo[3,4-c]isoquinolin-7- 398
OS`N N yl)benzenesulfonamide
H
_N
F NH 2,6-difluoro-N-(6,7,8,9-tetrahydro-3H-
iP I pyrazolo[3,4-c]isoquinolin-7- 365
SIN N yl)benzenesulfonamide
F es
H
.N
\ F \ NH 2-fluoro-5-methyl-N-(6,7,8,9-tetrahydro-3H-
/ ~0 N pyrazolo[3,4-c]isoquinolin-7- 361
,N yl)benzenesulfonamide
H
H3,4-difluoro-N-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7- 365
thN N
F ON yl)benzenesulfonamide
H
_N
NH 2,4-dimethyl-N-(6,7,8,9-tetrahydro-3H-
/ O pyrazolo[3,4-c]isoquinolin-7- 357
S`N N yl)benzenesulfonamide
H
NH N-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
O c]isoquinolin-7-yl)-1-benzothiophene-2- 385
S
OS~N N sulfonamide
H
CI ,
NH 3,4-dichloro-N-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7- 398
Cl OS ,N N yl)benzenesulfonamide
H
_N
NH 2,5-dimethyl-N-(6,7,8,9-tetrahydro-3H-
/ ,O pyrazolo[3,4-c]isoquinolin-7- 357
S\N N yl)benzenesulfonamide
H
.N
NH
O ,N N 7-[(4-ethylphenyl)sulfonyl]-6,7,8,9-tetrahydro-3H- 343
\ S~ pyrazolo[3,4-c][2,7]naphthyridine
274
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
7-[(3 -chlorobenzyl)sulfonyl]-6,7,8,9-tetrahydro-3H- 364
0S~N N pyrazolo[3,4 c][2,7]naphthyridine
CI '~
N
$T,NH
O 7-[(2,5-dichloro-3-thienyl)sulfonyl]-6,7,8,9-
\ N N 390
S tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
CI--~s
S CI
_N
NH
O 7- {[3-(trifluoromethyl)phenyl]sulfonyl}-6,7,8,9- 383
F3C / ~s N N tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
_N
NH
O 7-(3-thienylsulfonyl)-6,7,8,9-tetrahydro-3H- 321
N N pyrazolo[3,4-c] [2,7]naphthyridine
Sa
N
NH
O N N 7-[(2,4-dimethyl-1,3-thiazol-5-yl)sulfonyl]-6,7,8,9- 350
S tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
N
N
NH
N N 7-{[2-(trifluoromethoxy)phenyl]sulfonyl}-6,7,8,9- 399
6 tetrahydro 3H-pyrazolo[3,4 c][2,7]naphthyridme
0
CF3
_N
NH
7-(ethylsulfonyl)-6,7,8,9-tetrahydro-3H-
razolo[3 4-c][2 7]naphthYridine 267
N N pY
~~. ~
\is0
_N
NH
N N 7-(2,1,3-benzoxadiazol-4-ylsulfonyl)-6,7,8,9- 357
\ tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
q ~N
N-O
_N
NH
v O 7-{[2-(1-naphthyl)ethyl]sulfonyl}-6,7,8,9-
r- N N tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine 393
275
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
N N 7-[(3 -chlorophenyl)sulfonyl] -6,7,8,9-tetrahydro-3H- 350
CI pyrazolo[3,4-c] [2,7] naphthyridine
.N
NH
7-[(1,3,5-trimethyl-1 H-pyrazol-4-yl)sulfonyl] -
~~ N N 6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 347
-N S c][2,7]naphthyridine
N N
NH
O
7-[(3-methoxyphenyl)sulfonyl]-6,7,8,9-tetrahydro-
345
N N 3H-pyrazolo[3,4-c] [2,7]naphthyridine
_N
NH
O~ N N 7-[(4-chloro-2,5-difluorophenyl)sulfonyl] -6,7,8,9- 386
F \ g~ tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
CI F
NH
N N 7-[(2-chlorophenyl)sulfonyl]-6,7,8,9-tetrahydro-3H- 350
\ S~ pyrazolo[3,4-c] [2,7]naphthyridine
CI
NH
O 7-[(4-fluoro-2-methylphenyl)sulfonyl]-6,7,8,9-
\ N N 347
S tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
F
_N
NH
7-[(5-chloro-2-thienyl)sulfonyl]-6,7,8,9-tetrahydro- 356
~S.N N 3H-pyrazolo[3,4-c] [2,7]naphthyridine
CI
NH
7-(2,3-dihydro-1,4-benzodioxin-6-ylsulfonyl)-
N N 6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 373
CO :cr SD c][2,7]naphthyridine
O
_N
NH
7-{[4-chloro-3-(trifluoromethyl)phenyl]sulfonyl}-
Ovv N N 6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 418
F3C SD c][2,7]naphthyridine
CI
276
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
O~ N N 7-[(4-chloro-2-fluorophenyl)sulfonyl]-6,7,8,9- 368
\ tetrahydro-3H-pyrazolo[3,4-c] [2,7] naphthyridine
CI / F
.N
NH
N N 7-[(3,4-dimethoxyphenyl)sulfonyl] -6,7,8,9- 375
O tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
O
_N
NH
O 3-(3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 340
N~ `g N N c] [2,7]naphthyridin-7-ylsulfonyl)benzonitrile
1 '-Z O
_N
NH
7-(cyclopropylsulfonyl)-6, 7, 8,9-tetrahydro-3 H-
279
0'N N pyrazolo[3,4-c] [2,7]naphthyridine
0
_N
NH
N N 7-[(3 -chloro-4-methylphenyl)sulfonyl]-6,7,8,9- 364
CI tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
_N
NH
CI 7-[(2-chlorobenzyl)sulfonyl]-6,7,8,9-tetrahydro-3H- 364
0N N pyrazolo[3,4 c][2,7]naphthyridine
'O
N
NH
N N 7-[(2-fluorophenyl)sulfonyl]-6,7,8,9-tetrahydro-3H- 333
crb ~ pyrazolo[3,4-c][2,7]naphthyridine
F
NH
H
qy N N 7-(2, 1,3-benzothiadiazol-4 ylsulfonyl)-6,7,8,9-
373
tetrahydro 3H-pyrazolo[3,4 c][2,7]naphthyridine
N
N-S
277
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
N N 7-[(3 -chloro-2-methylphenyl)sulfonyl]-6,7,8,9- 364
tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
CI
_N
NH
CI / 7-[(3,4-dichlorobenzyl)sulfonyl] -6,7,8,9-tetrahydro- 398
QS~N N 3H-pyrazolo[3,4-c] [2,7]naphthyridine
CI 'I
_N
NH
O\ ,N N N-[4-(3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 372
/ S c] [2,7]naphthyridin-7-ylsulfonyl)phenyl]acetamide
N \
H
_N
NH
7-{[2-chloro-5-(trifluoromethyl)phenyl]sulfonyl}-
Ovv N N 6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 418
F3C50 c] [2,7]naphthyridine
1 11:11 CI
NH
O~ .N N 7-{[4-(trifluoromethyl)phenyl]sulfonyl}-6,7,8,9- 383
\ S tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
F3C
NH
N N 7-[(3-fluoro-4-methoxyphenyl)sulfonyl]-6,7,8,9- 363
F / tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
O
N
NH
7-(methylsulfonyl)-6,7,8,9-tetrahydro-3H-
razolo 3 4-c 2 7 na hthYridine 253
O\\ N N pY [ ][ ] p
O
_N
NH
O N N N-[2-chloro-4-(3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 407
OCI SO c] [2,7 ] naphthyridin- 7-ylsulfonyl)phenyl] acetamide
AN
H
_N
NH
7 - (1 -benzothien- 3 -ylsulfonyl) - 6,7,8,9-tetrahydro- 371
`SAN N 3H-pyrazolo[3,4-c] [2,7]naphthyridine
WS, 278
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
O~ N N 7-{[2-(trifluoromethyl)phenyl]sulfonyl}-6,7,8,9- 383
tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
()~CF3
NH
CI OsN N 7-[(3,5-dichlorophenyl)sulfonyl]-6,7,8,9-tetrahydro- 384
\6 3H-pyrazolo[3,4-c] [2,7]naphthyridine
CI
_N
NH
O~ N N 7-[(5-bromo-2-methoxyphenyl)sulfonyl]-6,7,8,9- 424
Br \ S~ tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
_N
NH
N N 7-[(2,3 -dichlorophenyl)sulfonyl] -6,7,8,9-tetrahydro- 384
v6 3H-pyrazolo[3,4-c] [2,7]naphthyridine
CI
CI
NH
O 7-[(2,4-difluorophenyl)sulfonyl]-6,7,8,9-tetrahydro-
\ N N 351
JOE S 3H-pyrazolo[3,4-c] [2,7]naphthyridine
F F
NH
O I 7-(1,3-benzothiazol-6-ylsulfonyl)-6,7,8,9- 372
N N tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
<\ \I O
N
_N
NH
v p 7-[(5-chloro-l-naphthyl)sulfonyl]-6,7,8,9- 400
S- N N tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
CI O
N
NH
O~ N N 7-[(3,5-dimethylisoxazol-4-yl)sulfonyl] -6,7,8,9- 334
tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
N
O
279
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
0 7-[(5-fluoro-2-methylphenyl)sulfonyl]-6,7,8,9-
\ N N 347
tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
F I \\ SO
_N
NH
7-{[2-chloro-4-(trifluoromethyl)phenyl]sulfonyl}-
~SN N 6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 418
~0 c][2,7]naphthyridine
Jaci
F3C NH
N N 7-[(4-bromo-2-methylphenyl)sulfonyl]-6,7,8,9- 408
\ S~ tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
Br /
NH
N N 7-[(4-methoxyphenyl)sulfonyl] -6,7,8,9-tetrahydro- 345
\ g~ 3H-pyrazolo[3,4-c] [2,7]naphthyridine
_N
NH
N N 7-[(2,5-dichlorophenyl)sulfonyl] -6,7,8,9-tetrahydro- 384
CI \ S6 3H-pyrazolo[3,4-c] [2,7]naphthyridine
O
CI
NH
0 7-[(2-methoxy-4-methylphenyl)sulfonyl]-6,7,8,9-
\ N N 359
\ S tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
O
.N
NH
0 7-(mesitylsulfonyl)-6,7,8,9-tetrahydro-3H-
\ N N 357
pyrazolo[3,4-c] [2,7]naphthyridine
So
_N
NH
O~ N N 7-{[4-(trifluoromethoxy)phenyl]sulfonyl}-6,7,8,9- 399
tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
0
F3C,0
NH
0~ N N 7-[(2,4-dibromophenyl)sulfonyl]-6,7,8,9- 473
\ tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
Br' / Br
280
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
O N N 7-[(4-methylphenyl)sulfonyl]-6,7,8,9-tetrahydro-
\ 329
3H-pyrazolo[3,4-c] [2,7]naphthyridine
\ So
_N
NH
CI O~ N N 7-[(2,6-dichlorophenyl)sulfonyl] -6,7,8,9-tetrahydro- 384
\ 3H-pyrazolo[3,4-c] [2,7]naphthyridine
O
CI
_N
NH
O 7-(2-thienylsulfonyl)-6,7,8,9-tetrahydro-3H- 321
%- N N pyrazolo[3,4-c] [2,7]naphthyridine
N
NH
O N N 7-[(4-fluorophenyl)sulfonyl]-6,7,8,9-tetrahydro-3H-
\ 333
S pyrazolo[3,4-c][2,7]naphthyridine
F
_N
NH
O \ 7-(isopropylsulfonyl)-6,7,8,9-tetrahydro-3H- 281
N N pyrazolo[3,4-c][2,7]naphthyridine
O
N
NH
N N 7-(phenylsulfonyl)-6,7,8,9-tetrahydro-3H- 315
pyrazolo[3,4-c] [2,7]naphthyridine
\ So
Cr
NH
N O 7-[(3-methylquinolin-8-yl)sulfonyl]-6,7,8,9- 380
S- N N tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
O
_N
NH
7-[(2-methylphenyl)sulfonyl]-6,7,8,9-tetrahydro- 329
N N 3H-pyrazolo[3,4-c] [2,7]naphthyridine
\ So
281
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
7-[( 1,2-dimethyl-1 H-imidazol-4-yl)sulfonyl] -
~ , N N 6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 333
-N - ~p c][2,7]naphthyridine
N
_N
'
NH
O N N 7-[(3-fluorophenyl)sulfonyl]-6,7,8,9-tetrahydro-3H- 333
F s pyrazolo[3,4-c] [2,7]naphthyridine
\
_N
NH
0 N N 7-[(2-chloro-4-fluorophenyl)sulfonyl]-6,7,8,9- 368
\ S tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
F CI
NH
N N 7-[(4-chorophenyl)sulfonyl]-6,7,8,9-tetrahydro-3H- 350
\ g~ pyrazolo[3,4-c][2,7]naphthyridine
CI
NH
O N N 7-[(2,5-dimethoxyphenyl)sulfonyl] -6,7,8,9- 375
~O \ S~ tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
~ O~
_N
NH
N N 7-[(3 -chloro-2-fluorophenyl)sulfonyl]-6,7,8,9- 368
~~ tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
F
CI
.N
NH
N N 7-[(4-bromo-2-fluorophenyl)sulfonyl]-6,7,8,9- 412
\ S tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
Br F
NH
O~ N N 7-[(5-chloro-2-fluorophenyl)sulfonyl]-6,7,8,9- 368
CI \ S6 tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
O
F
282
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
N %
NH
7-(propylsulfonyl)-6, 7, 8,9-tetrahydro-3 H-
razolo[3 4-c] [2 7]naphthYridine 281
QS.
N N pY
O
_N
NH
O N N 7-[(3-methylphenyl)sulfonyl]-6,7,8,9-tetrahydro-
\ 329
3H-pyrazolo[3,4-c] [2,7]naphthyridine
so
_N
NH
O~ N N 7-[(4-chloro-2,5-dimethylphenyl)sulfonyl]-6,7,8,9- 378
\ S~ tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
CI ~
.N
NH
7-}[4-bromo-2-(trifluoromethyl)phenyl]sulfonyl}-
RN ,N N 6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 462
C~sc][2,7]naphthyridine
Br CF3
NH
O~ N N 7-[(2,4-dichlorophenyl)sulfonyl]-6,7,8,9-tetrahydro- 384
\ 3H-pyrazolo[3,4-c] [2,7]naphthyridine
CI CI
NH
F O~ N N 7-[(2,6-difluorophenyl)sulfonyl] -6,7,8,9-tetrahydro- 351
g~ 3H-pyrazolo[3,4-c] [2,7]naphthyridine
F
_N
NH
O 7-[(2-fluoro-5-methylphenyl)sulfonyl]-6,7,8,9-
\ ,N N 347
g tetrahydro-3H-pyrazolo[3,4-c][2,7]naphthyridine
F
-N
NH
O 7-[(3,4-difluorophenyl)sulfonyl]-6,7,8,9-tetrahydro-
\ N N 351
F / S 3H-pyrazolo[3,4-c] [2,7]naphthyridine
F \
283
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH
O N N 7-[(2,4-dimethylphenyl)sulfonyl]-6,7,8,9-
\ 343
tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
So
_N
NH
N N 7-(1-benzothien-2-ylsulfonyl)-6,7,8,9-tetrahydro-
S6 3H-pyrazolo[3,4-c] [2,7]naphthyridine 371
d2r0 .
N
NH O~ N N 7-[(3,4-dichlorophenyl)sulfonyl]-6,7,8,9-tetrahydro- 384
CI 3H-pyrazolo[3,4-c][2,7]naphthyridine
O
CI
.N
NH
O 7-[(2,5-dimethylphenyl)sulfonyl]-6,7,8,9-
\ N N 343
tetrahydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine
I So
[0001694] General Procedure L: Compounds of the present invention can be
conveniently prepared by the general procedure illustrated below:
HN 0
R,NAN
(i) R-NCO, CH2CI2, RT, 1 h
N H
N N (ii) CF3COOH, 65 C, 16 h N
N N
H
-0
H2N
H H
RAN` /N
N (i) R-NCO, CH2CI2, RT, 1 h 0
N N (ii) CF3COOH, 65 C, 16 h N
N N
H
-0
[0001695] Example 318: Synthesis of N-phenyl-8,9-dihydro-3H-pyrazolo[3,4-
c] [2,7]naphthyridine-7(6H)-carboxamide.
_N
NH
N`/N I iN
/ 0
284
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
[0001696] Step 1: To a solution of 3-(4-methoxy-benzyl)-6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c][2,7]naphthyridine (0.075 g, 0.256 mmol) in dichloromethane (3
mL) was
added phenyl isocyanate (31 L, 0.281 mmol). The reaction mixture was shaken
at room
temperature for 1 hour. The solvent was then removed under reduced pressure to
give 3-(4-
methoxybenzyl)-N-phenyl-8,9-dihydro-3H-pyrazolo[3,4-c] [2,7]naphthyridine-
7(6H)-
carboxamide as a tan solid (LCMS [M+H]:414), which was used for next step
without further
purification. White solid; M.p. = >65 C; 400 MHz iH NMR (DMSO-d6) 8: 8.72 (s,
1H), 8.41
(s, 1H), 8.20 (s, 1H), 7.51-7.45 (m, 2H), 7.28-7.15 (m, 4H), 6.98-6.91 (m,
1H), 6.88-6.82 (m,
2H), 5.59 (s, 2H), 4.79 (s, 2H), 3.82 (t, 6.4 hz, 2H), 3.67 (s, 3H), 3.14 (t,
J = J = 6.4 Hz, 2H);
LCMS [M+H]: 414.6.
[0001697] Step 2: To the solid obtained above was added trifluoroacetic acid
(2 mL). The
reaction mixture was heated at 65 C for 16 hours. On completion of the
reaction, toluene (5
mL) was added. The reaction mixture was evaporated to dryness under reduced
pressure to
yield a dark brown solid. The residue was then partitioned between EtOAc and
aqueous
NaHCO3. The organic layer was then separated, washed with brine (2 x 10 mL)
and
concentrated under reduced pressure to give a residue. The crude product was
purified using
either flash column chromatography (Si02, 1% methanol in EtOAc) or reverse
phase HPLC
to give the desired product, N-phenyl-8,9-dihydro-3H-pyrazolo[3,4-
c][2,7]naphthyridine-
7(6H)-carboxamide as a white solid (0.057 g, 75%). M. p. = 184-186 C; 1H NMR
(400 MHz,
DMSO-d6) 813.58 (s, 1H), 8.71 (s, 1H), 8.34 (s, 1H), 8.17 (s, 1H), 7.49 (m,
2H), 7.24 (m,
2H), 6.32 (m, 1H), 4.77 (s, 2H), 3.81 (t, J= 6.0 Hz, 2H), 3.74 (t, J= 6.0 Hz,
2H); LCMS
[M+H] : 294.
[0001698] Example 319: Synthesis of N-phenyl-6,7-
dihydropyrazolo [4',3':5,6]pyrido [3,4-d] [ 1 ]benzazepine-8(3H)-carboxamide
N-NH
N
N
0 NH
6
[0001699] The compound N-phenyl-6,7-dihydropyrazolo[4',3':5,6]pyrido[3,4-
d][1]benzazepine-8(3H)-carboxamide was prepared using 3-(4-methoxybenzyl)-
3,6,7,8-
tetrahydropyrazolo[4',3':5,6]pyrido[3,4-d][1]benzazepine and phenyl isocyanate
as described
in the two step general procedure L to give the desired compound as an off-
white solid (0.048
285
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
g, 31%). M.p. >140 C; 'H NMR (CDC13, 400 MHz) 8 11.35 (brs, 1H), 8.08 (s,
1H), 8.00 (s,
1H), 7.99 (m, 1H), 7.68-7.60 (m, 2H), 7.46 (dd, J1= 1.6 Hz, J2 = 7.2 Hz, 1H),
7.15-7.06 (m,
4H), 6.90 (m, 1 H), 5.97 (s, 1 H), 4.75 (m, 1 H), 3.77 (m, 1 H), 2.90 (m, 2H);
LCMS [M+H] :
356.
[0001700] Examples 320-400: Compounds in Table 6 are synthesized by using
general
procedure L as described herein.
Table 6.
Structure IUPAC Name LC/MS
M+1
th F3C CINH 1-[2-chloro-4-(trifluoromethyl)phenyl]-3-
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 410
NN c]isoquinolin-7-yl)urea
H H
_N
CIO NH 1-(2-chloro-5-methylphenyl)-3-(6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c]isoquinolin- 356
NN N 7-yl)urea
,,,a
H H
_N
CI NH
/ O A 1-[2-(4-chlorophenyl)ethyl]-3-(6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c]isoquinolin- 370
N N N 7-yl)urea
H H
NH 1-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
0 c]isoquinolin-7-yl)-3-[3- 376
thN
F3C \H N (trifluoromethyl)phenyl]urea
_N
NH
O 1-(3 -methoxybenzyl)-3 -(6,7,8,9-tetrahydro- 352
H H N 3H-pyrazolo[3,4-c]isoquinolin-7-yl)urea
_N
NH
O 1-(2-phenylethyl)-3-(6,7,8,9-tetrahydro- 336
N 3H-pyrazolo[3,4-c]isoquinolin-7-yl)urea
N N
H H
_N
NH
/ O 1-(2-naphthyl)-3-(6,7,8,9-tetrahydro-3H- 358
'N N pyrazolo[3,4-c]isoquinolin-7-yl)urea
H H
_N
F O NH 1-[2-fluoro-6-(trifluoromethyl)phenyl]-3-
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 394
N N N c]isoquinolin-7-yl)urea
CF3 H H
286
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
thNH 1-(2,1,3-benzothiadiazol-4-yl)-3-(6,7,8,9-
O tetrahydro3H-pyrazolo[3,4-c]isoquinolin- 366
N N N 7-yl)urea
%
S,N H H
_N
NH 1-(1-naphthyl)-3-(6,7,8,9-tetrahydro-3H
O 358
N pyrazolo[3,4-c]isoquinolin-7-yl)urea
N N
H H
_N
NH 1 3-chloro-2-fluoro henY1)3 (6 7 8 9-
/ 0 ( p ,,,
\ - N tetrahydro-3H-pyrazolo[3,4-c]isoquinolin- 360
CI N N 7-yl)urea
F H H
N_
HN O ethyl N-(6,7,8,9-tetrahydro-3H-
pyrazolo[3,4-c]isoquinolin-7- 318
N N N O ylcarbamoyl)glycinate
0
_N
NH
N_ 0
O N 1-(3 -methyl-5 -phenylisoxazol-4-yl)-3 -
(6,7,8,9-tetrahydro-3H-pyrazolo[3,4- 389
N N
H H c]isoquinolin-7-yl)urea
th%NH F O 1 (2,6-difluorophenY1)-3 (6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c]isoquinolin- 344
N N 7-yl)urea
F H H
_N
NH 1-(2,3-dihydro-1H-inden-5-yl)-3-(6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c]isoquinolin- 348
CaN p
N i N 7-yl)urea
H H
_N
p 1-[2-(4-methoxyphenyl)ethyl]-3-(6,7,8,9-
NH
1 N tetrahydro-3H-pyrazolo[3,4-c]isoquinolin- 366
N N 7-yl)urea
H H
_N
N H
0 1 -propyl- 3 - (6,7,8,9 -tetrahydro-3 H- 274
N pyrazolo[3,4-c]isoquinolin-7-yl)urea
~~N N
H H
_N
O A NH 1-(9H-fluoren-2-yl)-3-(6,7,8,9-tetrahydro
I N 3H-pyrazolo[3,4-c]isoquinolin-7-yl)urea 396
N N
H H
287
CA 02748276 2011-06-23
WO 2010/078427 PCT/US2009/069821
_N
NH 1-(6,7,8,9-tetrahydro-3H-pyrazolo[3,4-
c]isoquinolin-7-yl)-3-[2-(2- 342
i i
N N N thienyl)ethyl]urea
H H
_N
NH
O 1-(3,4-dichlorobenzyl)-3-(6,7,8,9-
tetrahydro-3H-pyrazolo[3,4-c]isoquinolin- 390
CI H H N 7-yl)urea
CI
.N
CIO NH 1-(2-chlorophenyl)-3-(6,7,8,9-tetrahydro-
N 3H-pyrazolo[3,4-c]isoquinolin-7-yl)urea 342
(:~N N
H H
_N
NH
O 1-benzyl-3-(6,7,8,9-tetrahydro-3H- 322
N1~1 N N pyrazolo[3,4-c]isoquinolin-7-yl)urea
H H
.N
NH
N-[2-chloro-4-(trifluoromethyl)phenyl]-
H N -~J 3,6,8,9-tetrahydro-7H-pyrazolo[3,4- 396
I c][2,7]naphthyridine-7-carboxamide
F3C CIO
N H
N-(2-chloro-5-methylphenyl)-3,6, 8,9-
H tetrahydro-7H-pyrazolo[3,4- 342
NyN N
c] [2,7]naphthyridine-7-carboxamide
CIO
_N
N H
H N-[2-(4-chlorophenyl)ethyl]-3,6,8,9-
N N tetrahydro-7H-pyrazolo[3,4- 356
Cl
y c][2,7]naphthyridine-7-carboxamide
O
_N
N H N-[ 3 - (trifluoromethyl)phenyl] - 3,6,8,9 -
H tetrahydro-7H-pyrazolo[3,4- 362
F3C N y N N
c] [2,7]naphthyridine-7-carboxamide
O
.N
NH N 3-methox bent 1 3 6 8 9-tetrah dro-
H N N 7H-pyrazolo[3,4-c][2,7]naphthyridine-7- 338
carboxamide
0
288
DEMANDE OU BREVET VOLUMINEUX
LA PRRSENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 288
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 288
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: