Note: Descriptions are shown in the official language in which they were submitted.
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
GUI FOR AN IMPLANTABLE DISTENSION DEVICE
AND A DATA LOGGER
This case is related to the following commonly assigned and concurrently filed
U.S.
Applications, all of which are hereby incorporated herein by reference:
U.S. Serial No. [12/261,078] (Attorney Docket Number END6514USNP) titled
DEVICES and METHODS FOR ADJUSTING A SATIATION AND SATIETY-
INDUCING IMPLANTED DEVICE; U.S. Serial No. [12/261,079] (Attorney Docket
Number END6515USNP) titled Sensor Trigger; U.S. Serial No. [12/261,084 ]
(Attorney
Docket Number END6516USNP) titled AUTOMATICALLY ADJUSTING INTRA-
GASTRIC SATIATION AND SATIETY CREATION DEVICE; U.S. Serial No. [
12/162,08] (Attorney Docket Number END6517USNP) titled OPTIMIZING THE
OPERATION OF AN INTRA-GASTRIC SATIETY CREATION DEVICE; U.S. Serial
No. [12/261,089] (Attorney Docket Number END6518USNP) titled POWERING
IMPLANTABLE DISTENSION SYSTEMS USING INTERNAL ENERGY
HARVESTING MEANS; U.S. Serial No. [12/261,092] (Attorney Docket Number
END6519USNP) titled WEARABLE ELEMENTS FOR INTRA-GASTRIC SATIETY
CREATION SYSTEMS; U.S. Serial No. [12/261,093] (Attorney Docket Number
END6520USNP) titled INTRA-GASTRIC SATIETY CREATION DEVICE WITH
DATA HANDLING DEVICES AND METHODS; U.S. Serial No. [ ] (Attorney Docket
Number END6521USNP) titled GUI FOR AN IMPLANTABLE DISTENSION
DEVICE AND A DATA LOGGER; U.S. Serial No. [12/261,095] (Attorney Docket
Number END6522USNP) titled METHODS AND DEVICES FOR FIXING ANTENNA
ORIENTATION IN AN INTRA-GASTRIC SATIETY CREATION SYSTEM; U.S.
Serial No. [12/261096 ] (Attorney Docket Number END6523USNP) titled METHODS
AND DEVICES FOR PREDICTING INTRA-GASTRIC SATIETY CREATION
DEVICE SYSTEM PERFORMANCE; U.S. Serial No. [12/261,099 ] (Attorney Docket
Number END6524USNP) titled CONSTANT FORCE MECHANISMS for Regulating
Distension Devices; U.S. Serial No. [12/261,103 ] (Attorney Docket Number
END6525USNP) titled A METHOD OF REMOTELY ADJUSTING A SATIATION
AND SATIETY-INDUCING IMPLANTED DEVICE. Filed U.S. Serial No.
[12/261,107].
Page 1 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
FIELD OF THE INVENTION
[0001] Embodiments of the present invention relate generally to an implanted
distension
device and, more particularly, to a communication system for monitoring
physiological
parameters related to an implanted stomach distension device.
BACKGROUND OF THE INVENTION
[0002] Obesity is a growing concern, particularly in the United States, as the
number of
obese people continues to increase, and more is learned about the negative
health effects
of obesity. Morbid obesity, in which a person is 100 pounds or more over ideal
body
weight, in particular poses significant risks for severe health problems.
Accordingly, a
great deal of attention is being focused on treating obese patients. One
proposed method
of treating morbid obesity has been to place a distension device, such as a,
spring loaded
coil inside the stomach. Examples of satiation and satiety inducing gastric
implants,
optimal design features, as well as methods for installing and removing them
are
described in commonly owned and pending U.S. Patent Application Serial No.
11/469564, filed September 1, 2006, and pending U.S. Patent Application Serial
No.
11/469,562, filed September 1, 2006, which are hereby incorporated herein by
reference
in their entirety. One effect of the coil is to more rapidly induce feelings
of satiation
defined herein as achieving a level of fullness during a meal that helps
regulate the
amount of food consumed. Another effect of the coil is to prolong the effect
of satiety
which is defined herein as delaying the onset of hunger after a meal which in
turn
regulates the frequency of eating. By way of a non-limiting list of examples,
positive
impacts on satiation and satiety may be achieved by an intragastric coil
through one or
more of the following mechanisms: reduction of stomach capacity, rapid
engagement of
stretch receptors, alterations in gastric motility, pressure induced
alteration in gut
hormone levels, and alterations to the flow of food either into or out of the
stomach.
Page 2 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0003] With each of the above-described food distension devices, safe,
effective
treatment requires that the device be regularly monitored and adjusted to vary
the degree
of distension applied to the stomach.
[0004] During these gastric coil adjustments, it may be difficult to determine
how the
adjustment is proceeding, and whether the adjustment will have the intended
effect. In an
attempt to determine the efficacy of an adjustment, some physicians have
utilized
fluoroscopy with a Barium swallow as the adjustment is being performed,
although
fluoroscopy can be both expensive and raise concerns about radiation dosage. A
physician may simply adopt a "try as you go" method based upon their prior
experience,
and the results of an adjustment may not be discovered until hours or days
later, when the
patient experiences an excessive distension of the stomach cavity, or the coil
induces
erosion of the stomach tissue due to excessive pressure on the tissue walls.
[0005] In addition, tracking or monitoring the long-term performance of the
gastric coil
and/or the patient has been difficult in the past, but promises a wide range
of benefits.
For example, obtaining and displaying data from or related to the gastric coil
over a
period of time (or real-time data) may be useful for adjustment, diagnostic,
monitoring, or
other purposes. It may be further advantageous to store such data, process it
to obtain
other kinds of meaningful data and/or communicate it to a remote location.
Allowing a
physician or patient to manipulate or track such information would add a new
dimension
to obesity treatment or other forms of treatment. The foregoing examples are
merely
illustrative and not exhaustive. While a variety of techniques and devices
have been used
treat obesity, it is believed that no one prior to the inventors has
previously made or used
an invention as described in the appended claims.
[0006] Accordingly, methods and devices are provided for use with an
implantable
distension device, and in particular for logging, displaying, analyzing,
and/or processing
data from or related to an implantable distension device.
Page 3 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
BRIEF DESCRIPTION OF THE DRAWINGS
[0007] While the specification concludes with claims which particularly point
out and
distinctly claim the invention, it is believed the present invention will be
better
understood from the following description of certain examples taken in
conjunction with
the accompanying drawings, in which like reference numerals identify the same
elements
and in which:
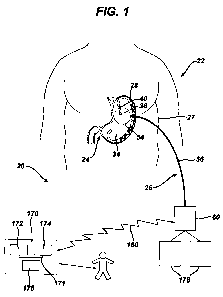
[0008] FIG. 1 is a simplified, schematic diagram of an implanted distension
device and a
bi-directional communication system between the implanted device and a remote
monitoring unit;
[0009] FIG. 2 is a more detailed, perspective view of an implantable portion
of the
stomach distension device shown in FIG. 1;
[0010] FIG. 3 is a side, partially sectioned view of the injection port shown
in FIG. 2;
[0011 ] FIG. 4 is a side, sectional view, taken along line A-A of FIG. 3,
illustrating an
exemplary pressure sensor for measuring fluid pressure in the intake
distension device of
FIG. 2;
[0012] FIG. 5 is a simplified schematic of a variable resistance circuit for
the pressure
sensor shown in FIG. 4;
[0013] FIG. 6 is a cross-sectional view of an alternative bi-directional
infuser for the
stomach distension device of FIG. 2;
[0014] FIG. 7A is a schematic diagram of a mechanically adjustable distension
device
incorporating a pressure transducer;
[0015] FIG. 7B is a cross-sectional view of the mechanically adjustable device
of FIG.
7A taken along line B-B;
[0016] FIG. 8 is a block diagram of the major internal and external components
of the
A-vice shown in FIG. 1;
Page 4 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0017] FIG. 9 is a schematic diagram illustrating a number of different
communication
links between the local and remote units of FIG. 1;
[0018] FIG. 10 is a flow diagram of an exemplary communication protocol
between the
local and remote units for a manually adjustable distension device;
[0019] FIG. 11 is a flow diagram of an exemplary communication protocol
between the
local and remote units for a remotely adjustable distension device;
[0020] FIG. 12 is a flow diagram of an exemplary communication protocol in
which
communication is initiated by the patient;
[0021] FIG. 13 is a simplified schematic diagram of a data logger for
recording pressure
measurements from the implanted distension device;
[0022] FIG. 14 is a block diagram illustrating the major components of the
data logger
shown in FIG. 13;
[0023] FIG. 15 is a graphical representation of a fluid pressure measurement
from the
sensor shown in FIG. 4, as communicated through the system of the present
invention;
[0024] FIG. 16 is a simplified schematic diagram of a data logging system for
recording
pressure measurements from the stomach distension device shown in FIG. 1;
[0025] FIG. 17 is a block diagram illustrating several components of the data
logging
system shown in FIG. 16; and
[0026] FIG. 18 is a simplified schematic diagram showing the data logging
system
shown in FIG. 16 in a docking state with a number of different communication
links.
[0027] FIG. 19A shows an exemplary pressure graph display for a graphical user
interface;
[0028] FIG. 19B shows an exemplary pressure meter display for a graphical user
interface;
Page 5 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0029] FIG. 19C shows an exemplary pulse counter display for a graphical user
interface;
[0030] FIG. 20 shows another exemplary pressure graph display for a graphical
user
interface;
[0031] FIG. 21 shows another exemplary pressure meter display for a graphical
user
interface;
[0032] FIG. 22 shows yet another exemplary pressure meter display for a
graphical user
interface;
[0033] FIG. 23A shows another exemplary pulse counter display for a graphical
user
interface;
[0034] FIG. 23B shows the pulse counter display shown in FIG. 23A over the
course of
a two -pulse sequence;
[0035] FIG. 24A shows an exemplary area distended by a distension device;
[0036] FIG. 24B shows the display of FIG. 24A after a change in pressure
sensed by the
distension device;
[0037] FIG. 25 shows an exemplary graph of pressure over time which can be
correlated
to the displays shown in FIG. 24A-B;
[0038] FIG. 26 shows an exemplary display with one set of data overlaying
another set
of data;
[0039] FIG. 27 shows another exemplary display with one set of data overlaying
another
set of data;
[0040] FIG. 28A shows an exemplary graph of population data related to
distension
devices;
Page 6 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0041] FIG. 28B shows another exemplary graph of population data related to
distension
devices;
[0042] FIG. 29 shows a display device with a screen showing annotated data
values, and
a menu of annotation events;
[0043] FIG. 30 shows a display device with a screen showing data values which
can be
annotated via text entered in a text box via an input device;
[0044] FIG. 31 shows the display device of FIG. 30 with another exemplary
screen of
data values;
[0045] FIG. 32A shows an exemplary plot of pressure values over time collected
from a
distension device at a 100Hz data rate;
[0046] FIG. 32B shows an exemplary plot of pressure values over time from FIG.
32A
which have been converted to a 10Hz data rate;
[0047] FIG. 32C shows an exemplary plot of pressure values over time from FIG.
32A
which have been converted to a 5Hz data rate;
[0048] FIG. 32D shows an exemplary plot of pressure values over time from FIG.
32A
which have been converted to a 3Hz data rate;
[0049] FIG. 32E shows an exemplary plot of pressure values over time from FIG.
32A
which have been converted to a 1 Hz data rate;
[0050] FIG. 32F is an exemplary flow diagram for converting collected data
from a
distension device to other data rates;
[0051] FIG. 33A is an exemplary plot of pressure values over time collected
from a
distension device and overlaid with plots of running averages calculated from
the
pressure values according to a first technique;
Page 7 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0052] FIG. 33B is an exemplary plot of pressure values over time collected
from a
distension device and overlaid with plots of running averages calculated from
the
pressure values according to a second technique;
[0053] FIG. 33C is an exemplary flow diagram for calculating running averages
of data
collected from a distension device;
[0054] FIG. 34A is an exemplary plot of pressure values over time collected
from a
distension device with annotations related to calculating a baseline value;
[0055] FIG. 34B is an exemplary flow diagram for determining the baseline
value of a
parameter from data collected from a distension device;
[0056] FIG. 34C is an exemplary plot of pressure values over time exhibiting a
change in
baseline value;
[0057] FIG. 35A is an exemplary plot of pressure values over time collected
from a
distension device with annotations related to predicting characteristics of a
baseline
value;
[0058] FIG. 35B is an exemplary flow diagram for predicting characteristics
related to a
baseline value of a parameter from data collected from a distension device;
[0059] FIG. 36A is an exemplary plot of pressure values over time collected
from a
distension device exhibiting superimposed pulses of differing frequencies;
[0060] FIG. 36B is another exemplary plot of pressure values over time
collected from a
distension device exhibiting superimposed pulses of differing frequency;
[0061] FIG. 36C is an exemplary flow diagram for determining information about
a
physiological parameter from data collected from a distension device;
[0062] FIG. 36D is another exemplary flow diagram for determining information
about a
physiological parameter from data collected from a distension device;
Page 8 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0063] FIG. 37A is an exemplary plot of pressure values over time collected
from a
distension device with information about a physiological parameter extracted
therefrom;
[0064] FIG. 37B is an exemplary plot of pressure values over time collected
from a
distension device and averaged data overlaid therewith;
[0065] FIG. 37C is an exemplary plot of pressure values over time extracted
from the
data shown in FIG. 37B;
[0066] FIG. 37D is an exemplary flow diagram for determining a physiological
parameter from data collected from a distension device;
[0067] FIG. 38A is an exemplary plot of pressure values over time collected
from a
distension device exhibiting superimposed pulses of differing frequencies;
[0068] FIG. 38B is a detail view of the plot shown in FIG. 38A;
[0069] FIG. 38C is another detail view of the plot shown in FIG. 38A;
[0070] FIG. 39A is an exemplary plot of pressure values over time collected
from a
distension device with annotations related to determining the presence of a
pulse;
[0071] FIG. 39B is an exemplary flow diagram for determining the presence of a
pulse
in data collected from a distension device;
[0072] FIG. 40A is another exemplary plot of pressure values over time
collected from a
distension device with annotations related to determining the presence of a
pulse via
another technique;
[0073] FIG. 40B is another exemplary flow diagram for determining, via the
technique
described in connection with FIG. 40A, the presence of a pulse in data
collected from a
distension device;
Page 9 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0074] FIG. 41A is yet another exemplary plot of pressure values over time
collected
from a distension device with annotations related to determining the presence
of a pulse
via yet another technique;
[0075] FIG. 41B is yet another exemplary flow diagram for determining, via the
technique described in connection with FIG. 41A, the presence of a pulse in
data
collected from a distension device;
[0076] FIG. 42A is another exemplary plot of pressure values over time
collected from a
distension device with annotations related to comparing pulse areas; and,
[0077] FIG. 42B is an exemplary flow diagram for comparing pulses areas using
data
collected from a distension device.
DETAILED DESCRIPTION OF THE INVENTION
[0078] The following description of certain examples of the invention should
not be used
to limit the scope of the present invention. Other examples, features,
aspects,
embodiments, and advantages of the invention will become apparent to those
skilled in
the art from the following description, which is by way of illustration, one
of the best
modes contemplated for carrying out the invention. As will be realized, the
invention is
capable of other different and obvious aspects, all without departing from the
invention.
Accordingly, the drawings and descriptions should be regarded as illustrative
in nature
and not restrictive. The features illustrated or described in connection with
one
exemplary embodiment may be combined with the features of other embodiments.
Such
modifications and variations are intended to be included within the scope of
the present
invention.
[0079] In one aspect, a display for a physiological monitoring device
displaying
information from or related to an implantable distension device is provided.
This
distension device may be adjustable. Exemplary non-limiting examples of
adjustable
implantable distension devices (e.g., satiation and satiety inducing gastric
implants),
optimal design features, as well as methods for installing and removing them
are
Page 10 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
described in commonly owned and pending U.S. Patent Application Serial No. [
], filed
on even date herewith and entitled "Devices and Methods for Adjusting a
Satiation and
Satiety-Inducing Implanted Device" [Atty. Docket No. END6514USNP], which is
hereby
incorporated herein by reference in its entirety. For example, an exemplary
display can
include a simulated graphic of a disposition of a region enclosing an
implantable
distension device, such as an adjustable gastric coil, the simulated graphic
indicating a
size of the disposition through the region. The indicated size can be based at
least in part
on a clinically relevant parameter sensed by the implantable distension device
and
communicated to the physiological monitoring device. Sensed parameters, in
this and
other embodiments described herein, can include a wide variety of parameters
such as
pressure, pulse count, pulse width, pulse duration, pulse amplitude, pulse
frequency,
sensed electrical characteristics, as well as system status parameters and so
on. In some
embodiments, the simulated graphic can include one or more isobars displayed
on the
graphic representation of the enclosed region, the isobars representing sensed
parameter
values so that a perimeter of the disposition in the region is indicative of
the sensed
parameter. The isobars can change color to signal a condition related to the
sensed
parameter values. In other embodiments, the simulated graphic can include an
image of a
cross-section of a coil, an image of the distension device disposed in an
anatomical
lumen, an image of , icons, markings, and/or three dimensional images. The
simulated
graphic also can include a video image for showing a change in the size of the
separation
between ends of the coil in accordance with pressure (or other parameter)
sensed by the
implantable distension device over a time period. The simulated graphic also
can be
based on an image obtained from the body of a patient in which the implantable
distension device is implanted. The display can further include a textual
indicator of a
sensed parameter, sensed parameter data shown on a graph or dial indicator,
and/or an
indication of a distension state of the implantable distension device. By way
of a non-
limiting example, if the distension device is not a fluid filled pressure
based device, then
the parameter being sensed may be the force on a force gauge disposed to
determine the
intragastric forces on the coil. Accordingly, the graphic may display force
vectors or
Page 11 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
deflections on an image of the coil or. Alternatively, the graphic may show
the stomach
affecting coil size, or the coil affecting stomach size.
[0080] In another aspect, an exemplary display can include a graph of a sensed
parameter over time, the graph including a graphic representation of data
representing
parameter values sensed by an implantable distension device, for example an
adjustable
gastric coil, and communicated to the physiological monitoring device. The
display can
also include one or more annotation markers disposed on the graphic
representation to
indicate a presence of an annotation at a selected time, the one or more
annotation
markers each associated with a description, such as text or an image. The
associated
description can include, for example, a description of a medical event,
description of a
physiological state, a system status (device) description of a symptom, a
patient
comment, and/or a physician comment. The graphic representation can include a
curve
plotting sensed pressure values. The display can further include a list of
predefined
annotation events from which a user can select the description.
[0081] In another aspect, an exemplary display can include a plurality of
graphic
representations of parameter/volume datasets (for example, parameter datasets,
such as
pressure, pulse count, pulse width, pulse amplitude, pulse frequency pH,
chemical
content, system fluid levels, system drug or therapeutic levels, and so on),
each
parameter/volume dataset corresponding to an implantable distension device,
such as an
adjustable gastric coil, in a patient and comprising one or more associations
of (a) a fill
volume for the implantable distension device, with (b) a parameter sensed by
the
implantable distension device at the fill volume and communicated to the
physiological
monitoring device. One of the plurality of the graphic representations can
represent a
pressure/volume dataset for a current patient and another of the graphic
representations
can represent a parameter/volume dataset for group of patients as a baseline
for
comparison.
[0082] In some embodiments, one of the plurality of the graphic
representations of a
parameter/volume dataset represents a current patient and the remainder of the
plurality
of the arnnhir representations represent parameter/volume datasets for a
patient
Page 12 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
population. The graphic representations can be, for example, curves plotted on
a graph of
parameter vs. fill volume. The graphic representations also can include curves
plotted on
a graph of parameter vs. fill volume, and wherein one of the plurality of the
graphic
representations represents a parameter/volume dataset for a current patient
and another
graphic representation represents an average parameter/volume dataset for a
patient
population, the average parameter/volume dataset comprising one or more
associations of
(a) a fill volume, and (b) an average of a parameter (such as pressure) sensed
by
implantable distension devices at the fill volume across a patient population.
The display
can further include an upper bound trendline and a lower bound trendline and
defining
surrounding the line plotting the average parameter/volume dataset.
Alternatively, the
parameter being graphed may be presented along the curve of the coil, showing,
for
example, pressure any of a plurality of points along the curve as an
indication of local
pressure conditions.
[0083] In an additional embodiment, the display may show whether the coil is
in chronic
contact with the inner wall of the stomach either as a percentage or as an
absolute value
or series of values. This may be displayed graphically to indicate the
potential for onset
of erosions or internal stomach wall damage.
[0084] A method for monitoring an implantable distension device can also be
provided,
which in one embodiment can include providing a plurality of parameter/volume
datasets,
each corresponding to an implantable distension device in a patient and
comprising one
or more associations of (a) a fill volume for the implantable distension
device, and (b) a
parameter sensed by the implantable distension device at the fill volume and
communicated to an external device. The method can also include displaying a
graphic
representation of a selected parameter/volume dataset corresponding to a
selected
implantable distension device along with one or more other graphic
representations of
one or more other parameter/volume datasets corresponding to one or more other
implantable distension devices. The method also can include calculating an
average
pressure for each volume across the one or more other parameter/volume
datasets to
Page 13 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
create an average parameter/volume dataset, and displaying a graphic
representation of
the average parameter/volume dataset.
[0085] In yet another aspect, an exemplary display can include a graph which
includes a
parameter axis and a pulse count axis for relating a parameter sensed by an
implantable
distension device, such as an adjustable gastric coil, with a pulse count. The
pulse count
can represent a sequence number of a pulse of the sensed parameter within a
sequence of
pulses in a swallowing event. The display can also include a plurality of
discrete
indicators disposed on the graph at an intersection of parameter and pulse
count, wherein
each discrete indicator represents a predetermined parameter amplitude and the
plurality
of discrete indicators thereby represents a total parameter amplitude measured
for each
pulse in a sequence of pulses. In some embodiments, a time stamp can be
displayed for
at least one pulse in the sequence of pulses. The time stamp can indicate the
time at
which the pulse occurred, the duration of the pulse, the intra-pulse time, or
other metrics.
[0086] In yet another aspect, an exemplary display can include a parameter vs.
time
graph, the parameter (such as pressure, or any other parameter, as previously
mentioned)
being sensed by an implantable distension device, a graphic representation
indicating a
value related to the parameter sensed by an implantable distension device,
such as an
adjustable gastric coil, during a first time period, and a graphic
representation indicating a
value related to the parameter sensed by an implantable distension device
during a second
and later time period. In some embodiments, the graphic representation for the
first time
period overlays at least in part the graphic representation for the second
time period. The
first time period can be before a medical action and the second and later time
period can
be after a medical action, and the medical action can be the adjustment of the
implantable
distension device. In some embodiments, the graphic representations for the
first time
period and for the second and later time period comprise curves plotted on the
graph
having one or more parameter pulses there within. The graphic representations
for the
first time period and second time period can be overlaid such that at least
one parameter
pulse in the graphic representations for the first time period overlaps with
at least one
parameter pulse in the graphic representations for the second time period.
Page 14 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0087] In yet another aspect, an exemplary display can include a pressure
screen
displaying a sensed pressure, the sensed pressure being sensed by an
implantable
distension device (such as an adjustable gastric coil) and communicated to the
physiological monitoring device and a pulse count display indicating a number
of pulses
in sensed pressure that occur during a swallowing event, and/or pressure
display having
an indicator for sensed pressure, the indicator falling within one of a
plurality of pressure
ranges corresponding to a condition of the implantable distension device. The
pressure
display can include, for example, a graph displaying pressure over time,
wherein the
sensed pressure is represented by a plotted curve, a linear meter comprising a
plurality of
discrete indicators, wherein in each discrete indicator corresponds to a
predetermined
sensed pressure, an indicator adapted to change color to indicate a condition,
a circular
pressure meter, and/or a textual indicator. The pressure ranges can correspond
to
conditions for a fluid-filled implantable distension device that include
"overfilled,"
"optimal" and "under-filled." In some embodiments, the graph, the linear
meter, the
circular pressure meter, and/or the textual indicator can be configured to
signal a visual
warning or alarm condition. In other embodiments, an audible alarm can be
configured
to activate when any of the graph, the linear meter, the circular pressure
meter, and the
textual indicator indicates a value above a threshold.
[0088] In yet another aspect, an exemplary method can include obtaining a
physiological
monitoring device having any of the foregoing displays or attributes, and
repurposing the
physiological monitoring device and/or the display. Repurposing can include,
for
example, reconstructing the device or display, modifying, reprogramming,
erasing, or
customizing the device or display. Repurposing also can include repairing,
reconditioning, or sterilizing the device or display.
[0089] Data obtained from the implanted device can be used, processed, and/or
analyzed
in a wide variety of ways. For example, one exemplary method of obtaining
information
about a physiological parameter can include collecting data from an
implantable
distension device over a time period, the collected data containing
information about
values of a parameter (such as pressure) sensed within a body during the time
period,
Page 15 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
and, analyzing the data in data processing device to determine information
about a
physiological parameter (e.g., heart rate, breathing rate, rate of pulses
caused by a
peristaltic event, baseline parameter, etc.) for at least a portion of the
time period. The
determined information can include, for example, frequency, value, amplitude,
change in
value over at least a portion of a time period, and average value over a time
period. In
one embodiment, the method can include determining the frequency content of
variations
in the values of the sensed parameter during the time period and identifying
one or more
frequencies in the frequency content as a frequency of the physiological
parameter. The
method can further include comparing one or more frequencies (or an average of
them) to
one or more predetermined frequencies that are designated as frequencies
associated with
the physiological parameter. In some embodiments, the method can include
determining
the frequency content of variations in the values of pressure over at least a
portion of the
time period, selecting one or more frequencies existing in the frequency
content that fall
within a predetermined range of frequencies designated as possible rates of
the
physiological event (e.g., heart rate, breath rate, and so on), and
identifying a rate for the
physiological event based on the one or more selected frequencies. Determining
the
frequency content can further be accomplished by applying Fourier analyses. In
other
embodiments, the method can include calculating a frequency exhibited in the
variations
in the value of pressure over at least a portion of the time period, and
comparing the
frequency to a predetermined range of frequencies designated as possible rates
of the
physiological event to determine if the frequency falls within the range.
Calculating the
frequency can be achieved by, for example, recording at least two times at
which values
of pressure are at a local maximum or minimum; and calculating the frequency
based on
the difference between the at least two times. The method can further include
determining an amplitude of the variations in the values of pressure at the
calculated
frequency, and comparing the amplitude to a predetermined range of amplitudes
designated as possible physiological event amplitudes to determine if the
amplitude falls
within the range. In yet other embodiments, the method can include calculating
the
difference between (i) a value of pressure at a time within the time period,
and (ii) an
average value of pressure at the time, wherein the difference represents a
value
Page 16 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
corresponding to the physiological parameter. The average value can be
calculated, for
example, based on values falling within a window of time. Further, the
determination of
physiological events or rates can lead to alarms, or can cause the data
processing device
to generate reports.
[0090] In another aspect, an exemplary method for analyzing data from an
implantable
distension device to determine a baseline value for a physiological parameter
can include
collecting data from an implantable distension device over a time period, the
collected
data containing information about values of a parameter sensed within a body
over the
time period. The method can also include defining a range of values to
represent a
tolerance range, and comparing one or more values of the sensed parameter
during the
time period to the tolerance range to determine if all of the one or more
values fell within
the tolerance range, and if so, identifying a baseline as having been
established. The
range of values can be defined in a variety of ways, including with respect to
the running
average, or by setting an upper limit that exceeds the running average and a
lower limit
that is less than the running average. The method can further include
calculating a
running average based on the values of the sensed parameter during an
averaging window
within the time period; and, identifying the running average as the baseline
value. In
some embodiments, the method can further include calculating a running average
based
on the values of the sensed parameter during an averaging window within the
time
period; and identifying the running average as the baseline value. In other
embodiments,
the method can include generating an alarm or report upon the occurrence of an
event,
such as (i) identification of the baseline value; (ii) failure to identify the
baseline value
within a threshold time; and (iii) identification of the baseline value and
the baseline
value passes a threshold value. In some embodiments, fluid can be added or
removed
from the implantable distension device, and/or the determined baseline value
can be
correlated to a condition of the implantable distension device, the condition
being one of
optimally-filled, over-filled, or under-filled (or optimally tighted, over-
tightened, and
under-tightened).
Page 17 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[0091] In another aspect, an exemplary method for analyzing data from an
implantable
distension device to determine information about a baseline of a physiological
parameter
can be provided. The method can include collecting data from an implantable
distension
device over a time period, the collected data containing information about
values of a
parameter sensed within a body during the time period. The method can further
include
calculating, based at least in part on one more values of the sensed parameter
during the
time period, a predicted amount of time until the values of the physiological
parameter
will have a rate of change that is about zero. In some embodiments,
calculating the
predicted amount of time can involve calculating a rate of change of the
values of the
sensed parameter for a window within the time period, calculating a rate of
change of the
rate of change of the values of the sensed parameter for the window, and
calculating the
predicted amount of time until the values of the sensed parameter will have a
rate of
change that is about zero, based at least in part on the rate of change and
the rate of
change of the rate of change. In some embodiments, a predicted baseline value
can be
calculated, for example, by extrapolating from one or more values within the
window to
the predicted baseline value of the sensed parameter, and by multiplying the
rate of
change of the values of the sensed parameter for the window within the time
period and
the predicted amount of time. In some embodiments, an alarm or report can be
generated
if the rate of change passes a threshold value. Further, the rate of change
can be
correlated to a condition of the implantable distension device, the condition
being one of:
optimally-filled, over-filled, or under-filled (or optimally tighted, over-
tightened, and
under-tightened).
[0092] In another aspect, an exemplary method for analyzing data from an
implantable
distension device to identify the presence of a pulse can be provided. The
method can
include can include collecting data from an implantable distension device over
a time
period, the collected data containing information about values of a parameter
sensed
within a body over the time period, identifying the presence of a pulse in the
values of the
sensed parameter. Identifying can comprise finding one or more values of the
sensed
parameter that exceeds a first threshold value and finding one or more
subsequent values
of the sensed parameter that fall below the first threshold or a second
threshold (such
Page 18 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
thresholds can be defined relative to a baseline value for the parameter,
and/or can be
different or the same values). In some embodiments, identifying can further
comprise
finding one or more subsequent values of the sensed parameter that fall below
a second
threshold within a time window, the time window being within the time period
and
beginning at a time associated with the one or more values that exceeded the
first
threshold. Another exemplary method for analyzing data from an implantable
distension
device to determine the presence of a pulse can include collecting data from
an
implantable distension device over a time period, the collected data
containing
information about values of a parameter sensed within a body over the time
period, and
identifying the presence of a pulse in the values of the sensed parameter.
Identifying can
comprise finding one or more values of the sensed parameter that exceed a
first threshold
value, finding one or more subsequent values of the sensed parameter that are
followed
by decreasing values, the one or more subsequent values representing a peak
value; and
finding one or more other subsequent values of the sensed parameter that fall
below a
second threshold within a time window. The time window can be within the time
period,
beginning at virtually any time, such as when a peak value occurs, or
otherwise. In some
embodiments, an alarm or report can be generated upon identification of a
pulse or if the
number of pulses passes a threshold value during a predetermined time period.
Further,
such information can be correlated to a condition of the implantable
distension device,
the condition being one of. optimally-filled, over-filled, or under-filled (or
optimally
tighted, over-tightened, and under-tightened).
[0093] In another aspect, an exemplary method for analyzing data from an
implantable
distension device to detect the presence of a physiological condition or a
condition
related to an implantable distension device can be provided. The method can
include
collecting data from an implantable distension device over a time period, the
collected
data containing information about values of a parameter sensed within a body
during the
time period, finding one or more areas corresponding to an area under a
pressure vs. time
curve, and, comparing the areas, the result of the comparison being correlated
to a
condition. In some embodiments, finding one or more areas can include for each
of the
one or more areas, evaluating an integral (including numerical integration in
some
Page 19 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
embodiments) based on values of the sensed parameter over each of a window
within the
time period, the evaluation of the integration producing a result representing
the area
under the pressure vs. time curve (which can be the area under one or more
pulses). The
method can further include correlating a decreasing sequence of areas that
occurs at a
first predetermined rate to an optimally filled implantable distension device,
correlating a
sequence of areas that are substantially equal to an overfilled implantable
distension
device, and/or can include correlating a decreasing sequence of areas that
occurs at a
second predetermined rate to an underfilled implantable distension device..
[0094] In another aspect, an exemplary method of analyzing data from an
implantable
distension device to remove noise in the data can be provided. Such a method
can
include collecting data from an implantable distension device over a time
period, the
collected data containing information about values of a parameter sensed
within a body
over the time period, and conditioning the sensed parameter values for display
or further
analysis. Conditioning can include filtering and/or converting the sensed
parameters
from a first sampling rate to a second and lower sampling rate, and/or can
include
calculating a root mean square of the sensed parameters or performing a
regression
analysis on the sensed parameters. In some embodiments, conditioning can
include
calculating an average value of the sensed parameters at each time in the time
period
based on a group of surrounding sensed parameter values. In other embodiments,
conditioning can include dividing at least a portion of the time period into a
plurality of
averaging windows of a predetermined size; and, calculating the average value
of the
sensed parameter in each averaging window. Conditioned values can be stored as
compressed information.
[0095] In another aspect, an exemplary method for analyzing data from an
implantable
distension device can include collecting data from an implantable distension
device over
a time period, the collected data containing information about values of a
parameter
sensed within a body over the time period. The method can further include
calculating an
average value of the physiological parameter for a time X within the time
period, the
average value being calculated based on one or more values of the sensed
parameter
Page 20 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
within an averaging window in the time period. In some embodiments, the
averaging
window (i) can precede the time X or (ii) can surround the time X. The method
can
further include displaying the average value on a graph of the sensed
parameter vs. time.
[0096] In yet another aspect, an exemplary method can include obtaining a data
processing device for processing data as described in any of the foregoing
embodiments,
and repurposing the device. Repurposing can include, for example,
reconstructing the
device, modifying, reprogramming, erasing, or customizing the device
hardware/software. Repurposing also can include repairing, reconditioning, or
sterilizing
the device.
[0097] Still other examples, features, aspects, embodiments, and advantages of
the
invention will become apparent to those skilled in the art from the following
description,
which includes by way of illustration, one of the best modes contemplated for
carrying
out the invention. As will be realized, the invention is capable of other
different and
obvious aspects, all without departing from the invention. Accordingly, the
drawings and
descriptions should be regarded as illustrative in nature and not restrictive.
[0098] Referring now to the drawings in detail, wherein like numerals indicate
the same
elements throughout the views, FIG. 1 provides a simplified, schematic diagram
of a bi-
directional communication system 20 for transmitting data between an implanted
distensive -opening device and a remotely located monitoring unit. Through
communication system 20, data and command signals may be transmitted between
the
implanted device and a remotely located physician for monitoring and affecting
patient
treatment. The communication system of the invention enables a physician to
control the
distension device and monitor treatment without meeting face-to-face with the
patient.
For purposes of the disclosure herein, the terms "remote" and "remotely
located" are
defined as being at a distance of greater than six feet. In FIG. 1 and the
following
disclosure, the distension device is shown and described as being a stomach
distension
device 22 for use in bariatric treatment. The use of a stomach distension
device is only
renresentntive however, and the present invention may be utilized with other
types of
Page 21 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
implanted distension devices without departing from the scope of the
invention. In
addition, it should be understood that the distension device 22 can be (or
include) any
category of distension device, such as a fluid-fillable distension device,
mechanically
based distension device, and so on.
[0099] As shown in FIG. 1, a first portion 24 of intake distension device 22
is implanted
in the patient's stomach 27, while a second portion 26 is located external to
the patient's
skin. Implanted portion 24 comprises an adjustable distension coil 28 that is
implanted
about the gastrointestinal tract for the treatment of morbid obesity. In this
application,
adjustable coil 28 is placed in the patient's stomach 30 to create a
distension of the
stomach. Adjustable coil 28 may include a cavity made of silicone rubber, or
another type
of biocompatible material, that inflates outwardly against stomach 30 when
filled with a
fluid. Alternatively, coil 28 may comprise a mechanically adjustable device
having a
fluid cavity that experiences pressure changes with coil adjustments, or a
combination
hydraulic/mechanical adjustable coil.
[00100] An injection port 36, which will be described in greater detail below,
is
implanted in a body region accessible for needle injections and telemetry
communication
signals. In the embodiment shown, injection port 36 fluidly communicates with
adjustable coil 28 via a catheter 40. The surgeon may implant injection port
36 in the
stomach of the patient.
[00101] FIG. 2 illustrates adjustable coil 28 in greater detail. In this
embodiment, coil 28
includes a variable volume cavity 42 that expands or contracts against the
inner wall of
the stomach to form a distension for controllably restricting food intake into
the stomach.
A physician may decrease the size of the distension opening by subtracting
fluid to
variable volume cavity 42 or, alternatively, may increase the distension size
by adding
fluid from the cavity. Fluid may be added or withdrawn by inserting a needle
into
injection port 36. The fluid may be, but is not restricted to, a 0.9 percent
saline solution.
[00102] Returning now to FIG. 1, external portion 26 of intake distension
device 22
comprises a hand-held antenna 54 electrically connected (in this embodiment
via an
Page 22 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
electrical cable assembly 56) to a local unit 60. Electrical cable assembly 56
may be
detachably connected to local unit 60 or antenna 54 to facilitate cleaning,
maintenance,
usage, and storage of external portion 26. Local unit 60 is a microprocessor-
controlled
device that communicates with implanted device 22 and a remote unit 170, as
will be
described further below. Through antenna 54, local unit 60 non-invasively
communicates
with implanted injection port 36. Antenna 54 may be held against the patient's
skin near
the location of injection port 36 to transmit telemetry and power signals to
injection port
36.
[00103] Turning now to FIG. 3, which depicts a side, partially sectioned view
of an
exemplary injection port 36. As shown in FIG. 3, injection port 36 comprises a
rigid
housing 70 having an annular flange 72 containing a plurality of attachment
holes 74 for
fastening the injection port to tissue in a patient. A surgeon may attach
injection port 36
to the inner tissue of the stomach, such as the muscular layer, using any one
of numerous
surgical fasteners including suture filaments, staples, and clips. Injection
port 36 further
comprises a septum 76 typically made of a silicone rubber and compressively
retained in
housing 70. Septum 76 is penetrable by a endoscopic Huber-like needle, or a
similar type
of injection instrument, for adding or withdrawing fluid from the port. Septum
76 self-
seals upon withdrawal of the syringe needle to maintain the volume of fluid
inside of
injection port 36. Injection port 36 further comprises a reservoir 80 for
retaining the fluid
and a catheter connector 82. Connector 82 attaches to catheter 40, shown in
FIG. 2, to
form a closed hydraulic circuit between reservoir 80 and cavity 42. Housing 70
and
connector 82 may be integrally molded from a biocompatible polymer or
constructed
from a metal such as titanium or stainless steel.
[00104] Injection port 36 also comprises a pressure sensor 84 for measuring
fluid
pressure within the device. The pressure measured by sensor 84 corresponds to
the
amount of distension applied by coil 28 to the patient's stomach or other body
cavity. The
pressure measurement is transmitted from sensor 84 to local unit 60 via
telemetry signals
using antenna 54. Local unit 60 may display, print and/or transmit the
pressure
measurement to a remote monitoring unit for evaluation, as will be described
in more
Page 23 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
detail below. In the embodiment shown in FIG. 3, pressure sensor 84 is
positioned at the
bottom of fluid reservoir 80 within housing 70. A retaining cover 86 extends
above
pressure sensor 84 to substantially separate the sensor surface from reservoir
80, and
protect the sensor from needle penetration. Retaining cover 86 may be made of
a ceramic
material such as, for example, alumina, which resists needle penetration yet
does not
interfere with electronic communications between pressure sensor 84 and
antenna 54.
Retaining cover 86 includes a vent 90 that allows fluid inside of reservoir 80
to flow to
and impact upon the surface of pressure sensor 84.
[00105] FIG. 4 is a side, sectional view of pressure sensor 84, taken along
line A-A of
FIG. 3, illustrating an exemplary embodiment for measuring fluid pressure.
Pressure
sensor 84 is hermetically sealed within a housing 94 to prevent fluid
infiltrating and
effecting the operation of the sensor. The exterior of pressure sensor 84
includes a
diaphragm 92 having a deformable surface. Diaphragm 92 is formed by thinning
out a
section of the bottom of titanium reservoir 80 to a thickness between 0.001"
and 0.002".
As fluid flows through vent 90 in reservoir 80, the fluid impacts upon the
surface of
diaphragm 92, causing the surface to mechanically displace. The mechanical
displacement of diaphragm 92 is converted to an electrical signal by a pair of
variable
resistance, silicon strain gauges 96, 98. Strain gauges 96, 98 are attached to
diaphragm 92
on the side opposite the working fluid in reservoir 80. Strain gauge 96 is
attached to a
center portion of diaphragm 92 to measure the displacement of the diaphragm.
The
second, matched strain gauge 98 is attached near the outer edge of diaphragm
92. Strain
gauges 96, 98 may be attached to diaphragm 92 by adhesives, or may be diffused
into the
diaphragm structure. As fluid pressure within coil 28 fluctuates, the surface
of diaphragm
92 deforms up or down at the bottom of reservoir 80. The deformation of
diaphragm 92
produces a resistance change in the center strain gauge 96.
[00106] As shown in FIG. 5, strain gauges 96, 98 form the top two resistance
elements
of a half-compensated, Wheatstone bridge circuit 100. As strain gauge 96
reacts to the
mechanical displacements of diaphragm 92, the changing resistance of the gauge
changes
the potential across the top portion of the bridge circuit. Strain gauge 98 is
matched to
Page 24 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
strain gauge 96 and athermalizes the Wheatstone bridge circuit. Differential
amplifiers
102, 104 are connected to bridge circuit 100 to measure the change in
potential within the
bridge circuit due to the variable resistance strain gauges. In particular,
differential
amplifier 102 measures the voltage across the entire bridge circuit, while
differential
amplifier 104 measures the differential voltage across the strain gauge half
of bridge
circuit 100. The greater the differential between the strain gauge voltages,
for a fixed
voltage across the bridge, the greater the pressure difference. If desired, a
fully
compensated Wheatstone bridge circuit could also be used to increase the
sensitivity and
accuracy of the pressure sensor 84. In a fully compensated bridge circuit,
four strain
gauges are attached to the surface of diaphragm 92, rather than only two
strain gauges as
shown in FIG. 4.
[00107] Returning to FIG. 4, the output signals from differential amplifiers
102, 104 are
applied to a microcontroller 106. Microcontroller 106 is integrated into a
circuit board
110 within housing 94. A temperature sensor 112 measures the temperature
within
injection port 36 and inputs a temperature signal to microcontroller 106.
Microcontroller
106 uses the temperature signal from sensor 112 to compensate for variations
in body
temperature and residual temperature errors not accounted for by strain gauge
98.
Compensating the pressure measurement signal for variations in body
temperature
increases the accuracy of the pressure sensor 84. Additionally, a
TET/telemetry coil 114
is located within housing 94. Coil 114 is connected to a capacitor 116 to form
a tuned
tank circuit for receiving power from and transmitting physiological data,
including the
measured fluid pressure, to local unit 60. FIGS. 3-5 illustrate one exemplary
embodiment
for measuring fluid pressure within an intake distension device. Additional
embodiments
for measuring fluid pressure are described in U.S. patent application Ser. No.
11/065,4 10
entitled "Non-invasive Measurement of Fluid Pressure in a Bariatric Device,"
(now
published as U.S. Patent Publication No. 2006/0189888) the disclosure of which
is
incorporated herein by reference.
[00108] As an alternative to injection port 36, implanted portion 24 may
include a bi-
directional infuser for varying the fluid level within the adjustable
distension coil 28.
Page 25 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
With an infuser, fluid can be added or withdrawn from coil 28 via telemetry
command
signals. FIG. 6 is a cross-sectional view of an exemplary infuser 115. As
shown in FIG.
6, infuser 115 includes a pump, designated generally as 118, for non-
invasively
transferring fluid into or out of the coil in response to telemetry command
signals. Pump
118 is encased within a cylindrical outer housing 120 having an annular cover
121
extending across a top portion. A collapsible bellows 122 is securely attached
at a top
peripheral edge to cover 121. Bellows 122 is comprised of a suitable material,
such as
titanium, which is capable of repeated flexure at the folds of the bellows,
but which is
sufficiently rigid so as to be noncompliant to variations in pressure. A lower
peripheral
edge of bellows 122 is secured to an annular bellows cap 123, which translates
vertically
within pump 118. The combination of cover 121, bellows 122 and bellows cap 123
defines the volume of a fluid reservoir 124. A catheter connector 119 attaches
to catheter
40 (shown in FIG. 2) to form a closed hydraulic circuit between the coil and
fluid
reservoir 124. The volume in reservoir 124 may be expanded by moving bellows
cap 123
in a downward direction, away from cover 121. As bellows cap 123 descends, the
folds
of bellows 122 are stretched, creating a vacuum to pull fluid from the coil,
through
catheter 40 and connector 119, and into reservoir 124. Similarly, the volume
in reservoir
124 may be decreased by moving bellows cap 123 in an upward direction towards
cover
121, thereby compressing the folds of bellows 122 and forcing fluid from the
reservoir
through catheter 40 and connector 119 and into coil 28.
[00109] Bellows cap 123 includes an integrally formed lead screw portion 125
that
operatively engages a matching thread on a cylindrical nut 126. The outer
circumference
of nut 126 is securely attached to an axial bore of a rotary drive plate 127.
A cylindrical
drive ring 128 is in turn mounted about the outer annular edge of rotary drive
plate 127.
Nut 126, drive plate 127 and drive ring 128 are all securely attached together
by any
suitable means to form an assembly that rotates as a unit about an axis formed
by screw
portion 125. A bushing frame 129 encloses TET and telemetry coils (not shown)
for
transmitting power and data signals between antenna 54 and pump 118.
Page 26 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[00110] Drive ring 128 is rotatably driven by one or more piezoelectric
harmonic
motors. In the embodiment shown in FIG. 6, two harmonic motors 131 are
positioned so
that a tip 113 of each motor is in frictional contact with the inner
circumference of drive
ring 128. When motors 131 are energized, tips 113 vibrate against drive ring
128,
producing a "walking" motion along the inner circumference of the ring that
rotates the
ring. A microcontroller (not shown) in pump 118 is electrically connected to
the TET and
telemetry coils for receiving power to drive motors 131, as well as receiving
and
transmitting data signals for the pump. To alter the fluid level in coil
cavity 42, an
adjustment prescription is transmitted by telemetry from antenna 54. The
telemetry coil
in infuser 115 detects and transmits the prescription signal to the
microcontroller. The
microcontroller in turn drives motors 131 an appropriate amount to collapse or
expand
bellows 122 and drive the desired amount of fluid to/from coil 28.
[00111 ] In order to measure pressure variations within infuser 115, and,
thus, the size of
the coil, a pressure sensor, indicated by block 84', is included within
bellows 122.
Pressure sensor 84' is similar to pressure sensor 84 described above. As the
pressure
against coil 28 varies due to, for example, peristaltic pressure from
swallowing or
stomach processing of the food, the fluid in coil 28 experiences pressure
changes. These
pressure changes are conveyed back through the fluid in catheter 40 to bellows
122. The
diaphragm in pressure sensor 84' deflects in response to the fluid pressure
changes within
bellows 122. The diaphragm deflections are converted into an electrical signal
indicative
of the applied pressure in the manner described above with respect to FIGS. 4
and 5. The
pressure signal is input to the infuser microcontroller, which transmits the
pressure to a
monitoring unit external to the patient via the telemetry coil. Additional
details regarding
the operation of bi-directional infuser 115 may be found in commonly-assigned,
co-
pending U.S. patent application Ser. No. 11/065,410 entitled "Non-invasive
Measurement
of Fluid Pressure in a Bariatric Device" which has been incorporated herein by
reference.
[00112] FIGS. 7A and 7B depict a mechanically adjustable coil 153 for creating
a
stomach distension in the abdomen of a patient. Mechanical coil 153 may be
used as an
alternative to hydraulically adjustable coil 28 for creating a stoma.
Mechanically
Page 27 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
adjustable coil 153 comprises a substantially circular resilient core 133
having
overlapping end portions 135, 137. Core 133 is substantially enclosed in a
fluid-filled
compliant housing 139.. An implanted motor 141 is spaced from core 133 to
mechanically adjust the overlap of the core end portions 135, 137 and,
accordingly, the
coil size. Motor 141 adjusts the size of core 133 through a drive shaft 143
that is
connected to a drive wheel (not shown) within housing 139. Motor 141 is molded
together with a remote-controlled power supply unit 145 in a body 147
comprised of
silicon rubber, or another similar material.
[00113] As motor 141 changes the size of core 133, the pressure of the fluid
within
housing 139 varies. To measure the pressure variations, a pressure sensor,
similar to that
described above, is placed in communication with the fluid of housing 139. The
pressure
sensor may be placed within housing 139, as shown by block 84", so that the
pressure
variations within the coil are transferred through the fluid in housing 139 to
the
diaphragm of the sensor. Sensor 84" translates the deflections of the
diaphragm into a
pressure measurement signal, which is transmitted to an external unit via
telemetry in the
manner described above. In an alternative scenario, the pressure sensor may be
placed
within the implanted motor body 147, as indicated by block 84"', and fluidly
connected to
housing 139 via a tube 151 extending alongside drive shaft 143. As fluid
pressure varies
in housing 139 due to pressure changes within the coil, the pressure
differentials are
transferred through the fluid in tube 151 to sensor 84"'. Sensor 84"'
generates an electrical
signal indicative of the fluid pressure. This signal is transmitted from the
patient to an
external unit in the manner described above.
[00114] FIG. 8 is a block diagram illustrating the major components of
implanted and
external portions 24, 26 of intake distension device 22. As shown in FIG. 8,
external
portion 26 includes a primary TET coil 130 for transmitting a power signal 132
to
implanted portion 24. A telemetry coil 144 is also included for transmitting
data signals
to implanted portion 24. Primary TET coil 130 and telemetry coil 144 combine
to form
antenna 54 as shown. Local unit 60 of external portion 26 includes a TET drive
circuit
134 for controlling the application of power to primary TET coil 130. TET
drive circuit
Page 28 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
134 is controlled by a microprocessor 136. A graphical user interface 140 is
connected to
microprocessor 136 for inputting patient information and displaying and/or
printing data
and physician instructions. Through user interface 140, the patient or
clinician can
transmit an adjustment request to the physician and also enter reasons for the
request.
Additionally, user interface 140 enables the patient to read and respond to
instructions
from the physician.
[00115] Local unit 60 also includes a primary telemetry transceiver 142 for
transmitting
interrogation commands to and receiving response data, including sensed fluid
pressure,
from implanted microcontroller 106. Primary transceiver 142 is electrically
connected to
microprocessor 136 for inputting and receiving command and data signals.
Primary
transceiver 142 drives telemetry coil 144 to resonate at a selected RF
communication
frequency. The resonating circuit generates a downlink alternating magnetic
field 146
that transmits command data to implanted microcontroller 106. Alternatively,
transceiver
142 may receive telemetry signals transmitted from secondary coil 114. The
received
data may be stored in a memory 138 associated with microprocessor 136. A power
supply 150 supplies energy to local unit 60 in order to power intake
distension device 22.
An ambient pressure sensor 152 is connected to microprocessor 136.
Microprocessor 136
uses the signal from ambient pressure sensor 152 to adjust the received fluid
pressure
measurement for variations in atmospheric pressure due to, for example,
variations in
barometric conditions or altitude.
[00116] FIG. 8 also illustrates the major components of implanted portion 24
of device
22. As shown in FIG. 8, secondary TET/telemetry coil 114 receives power and
communication signals from external antenna 54. Coil 114 forms a tuned tank
circuit that
is inductively coupled with either primary TET coil 130 to power the implant,
or primary
telemetry coil 144 to receive and transmit data. A telemetry transceiver 158
controls data
exchange with coil 114. Additionally, implanted portion 24 includes a
rectifier/power
regulator 160, microcontroller 106 described above, a memory 162 associated
with the
microcontroller, temperature sensor 112, pressure sensor 84 and a signal
conditioning
circuit 164 for amplifying the signal from the pressure sensor. The implanted
components
Page 29 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
transmit the temperature adjusted pressure measurement from sensor 84 to local
unit 60
via antenna 54. The pressure measurement may be stored in memory 138 within
local
unit 60, shown on a display within local unit 60, or transmitted in real time
to a remote
monitoring station.
[00117] As mentioned hereinabove, it is desirable to provide a communication
system
for the remote monitoring and control of an intake distension device. Through
the
communication system, a physician may retrieve a history of fluid pressure
measurements from the distension device to evaluate the efficacy of the
bariatric
treatment. Additionally, a physician may downlink instructions for a device
adjustment.
A remotely located clinician may access the adjustment instructions through
local unit
60. Using the instructions, the clinician may inject a syringe into injection
port 36 and
add or remove saline from fluid reservoir 80 to accomplish the device
adjustment.
Alternatively, the patient may access the instructions through local unit 60,
and non-
invasively execute the instructions in infuser 115 or mechanically adjustable
coil 153
using antenna 54. Real-time pressure measurements may be uplinked to the
physician
during the adjustment for immediate feedback on the effects of the adjustment.
Alternatively, the patient or clinician may uplink pressure measurements to
the physician
after an adjustment for confirmation and evaluation of the adjustment.
[00118] As shown in FIG. 1, communication system 20 includes local unit 60 and
a
remote monitoring unit 170, also referred to herein as a base unit. Remote
unit 170 may
be located at a physician's office, a hospital or clinic, or elsewhere. Remote
unit 170 of
the present example is a personal computer type device comprising a
microprocessor 172,
which may be, for example, an Intel Pentium® or current microprocessor or
the like.
Alternatively, remote unit 170 may comprise a dedicated or non-dedicated
server that is
accessible over a network such as the Internet. In the present example, a
system bus 171
interconnects microprocessor 172 with a memory 174 for storing data such as,
for
example, physiological parameters and patient instructions. A graphical user
interface
176 is also interconnected to microprocessor 172 for displaying data and
inputting
instructions and correspondence to the patient. User interface 176 may
comprise a video
Page 30 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
monitor, a touch screen, or other display device, as well as a keyboard or
stylus for
entering information into remote unit 170. Other devices and configurations
suitable for
providing a remote unit 170 will be apparent to those of ordinary skill in the
art.
[00119] A number of peripheral devices 178 may interface directly with local
unit 60 for
inputting physiological data related to the patient's condition. This
physiological data
may be stored in local unit 60 and uploaded to remote unit 170 during an
interrogation or
other data exchange. Examples of peripheral devices that can be utilized with
the present
invention include a weight scale, blood pressure monitor, thermometer, blood
glucose
monitor, or any other type of device that could be used outside of a
physician's office to
provide input regarding the current physiological condition of the patient. A
weight scale,
for example, can electrically communicate with local unit 60 either directly
or wirelessly
through antenna 54, to generate a weight loss record for the patient. The
weight loss
record can be stored in memory 138 of local unit 60. During a subsequent
interrogation
by remote unit 170, or automatically at prescheduled intervals, the weight
loss record can
be uploaded by microprocessor 136 to remote unit 170. The weight loss record
may be
stored in memory 174 of remote unit 170 until accessed by the physician.
[00120] Also as shown in FIG. 1, a communication link 180 is created between
local unit
60 and remote unit 170 for transmitting data, including voice, video,
instructional
information and command signals, between the units. Communication link 180 may
comprise any of a broad range of data transmission media including web-based
systems
utilizing high-speed cable or dial-up connections, public telephone lines,
wireless RF
networks, satellite, Ti lines or any other type of communication medium
suitable for
transmitting data between remote locations. FIG. 9 illustrates various media
for
communication link 180 in greater detail. As shown in FIG. 9, local and remote
units 60,
170 may communicate through a number of different direct and wireless
connections. In
particular, the units may communicate through the Internet 190 using cable or
telephone
modems 192, 194 or any other suitable device(s). In this instance, data may be
transmitted through any suitable Internet communication medium such as, for
example,
e-mail, instant messaging, web pages, or document transmission. Alternatively,
local and
Page 31 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
remote units 60, 170 may be connected through a public telephone network 196
using
modems 200, 202. Units 60, 170 may also communicate through a microwave or RF
antenna 204 via tunable frequency waves 206, 210. A communication link may
also be
established via a satellite 209 and tunable frequency waves 212, 214. In
addition to the
links described above, it is envisioned that other types of transmission
media, that are
either known in the art or which may be later developed, could also be
utilized to provide
the desired data communication between local and remote units 60, 170 without
departing from the scope of the invention.
[00121] FIG. 10 is a data flow diagram of an exemplary interaction using bi-
directional
communication system 20. In this interaction, a physician may download an
adjustment
prescription that is subsequently manually executed by a clinician present
with the
patient. A physician initiates the communication session between remote unit
170 and
local unit 60 as shown at step 220. The session may be initiated by
transmitting an e-mail
or instant message via the Internet link 190, or through any of the other
communication
links described with respect to FIG. 9. During the communication session, the
physician
may download instructions to memory 138, or may upload previously stored data
obtained from device 22 or peripheral devices 178, as shown at step 222. This
data may
include fluid pressure, a weight history, or a patient compliance report.
After the data is
uploaded, the physician may evaluate the data and determine the need for a
device
adjustment, as shown at step 234. If an adjustment is indicated, the physician
may
download an adjustment prescription command to local unit 60 as shown at step
224.
Local unit 60 stores the prescription in memory 138 for subsequent action by a
clinician,
as shown by step 226. With the patient present, the clinician accesses the
prescription
from memory 138. The clinician then inserts a syringe into septum 76 of
injection port 36
and adds or withdraws the fluid volume specified in the prescription.
Following the
adjustment, the clinician places antenna 54 over the implant and instructs
microcontroller
106 to transmit pressure measurements from sensor 84 to local unit 60. The
pressure
measurements are uploaded by microprocessor 136 in local unit 60 to remote
unit 170, as
shown at step 230, to provide a confirmation to the physician that the
adjustment
instructions were executed, and an indication of the resulting effect on the
patient. In an
Page 32 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
off-line adjustment, the base unit terminates communication with local unit 60
following
the downloading of the adjustment prescription, as shown by line 229, or
following
receipt of the patient data if an adjustment is not indicated, as shown by
line 231.
[00122] In addition to the off-line adjustment session of steps 220-234, a
physician may
initiate a real-time interactive adjustment, as indicated at step 236, in
order to monitor the
patient's condition before, during and after the adjustment. In this instance,
the physician
downloads an adjustment prescription, as shown at step 237, while the patient
is present
with a clinician. The clinician inserts a syringe into septum 76 of injection
port 36 and
adds or withdraws the specified fluid from reservoir 80, as shown at step 238,
to execute
the prescription. After the injection, the physician instructs the clinician
to place antenna
54 over the implant, as shown at step 241, to transmit fluid pressure
measurements from
the implant to local unit 60. The pressure measurements are then up linked to
the
physician through link 180, as shown at step 243. The physician evaluates the
pressure
measurements at step 245. Based upon the evaluation, the physician may provide
further
instructions through link 180 to readjust the coil as indicated by line 242.
Additionally,
the physician may provide instructions for the patient to take a particular
action, such as
eating or drinking, to test the adjustment, as shown at step 244. As the
patient performs
the test, the physician may upload pressure measurements from the implant, as
shown at
step 246, to evaluate the pressure against the coil as the food or liquid
attempts to pass
through the stomach. If the pressure measurements are too high, indicating a
possible
over-distension, the physician may immediately transmit additional command
signals to
the clinician to readjust the coil and relieve the obstruction, as indicated
by line 249.
After the physician is satisfied with the results of the adjustment, the
communication
session is terminated at step 232. As shown in the flow diagram, communication
link 180
enables a physician and patient to interact in a virtual treatment session
during which the
physician can prescribe adjustments and receive real-time fluid pressure
feedback to
evaluate the efficacy of the treatment.
[00123] In a second exemplary interaction, shown in FIG. 11, the physician
downloads
an adjustment prescription for a remotely adjustable device, such as infuser
115 shown in
Page 33 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
FIG. 6. The physician initiates this communication session through link 180 as
shown at
step 220. After initiating communications, the physician uploads previously
stored data,
such as fluid pressure histories, from memory 138 of local unit 60. The
physician
evaluates the data and determines whether an adjustment is indicated. If the
physician
chooses an off-line adjustment, an adjustment command is downloaded to local
unit 60
and stored in memory 138, as indicated in step 224. With the prescription
stored in
memory 138, the patient, at his convenience, places antenna 54 over the
implant area and
initiates the adjustment through local unit 60, as indicated in step 233.
Local unit 60 then
transmits power and command signals to the implanted microcontroller 106 to
execute
the adjustment. After the adjustment, the patient establishes a communication
link with
remote monitoring unit 170 and uploads a series of pressure measurements from
the
implant to the remote unit. These pressure measurements may be stored in
memory 174
of remote unit 170 until accessed by the physician.
[00124] In an alternative scenario, the patient may perform a real-time
adjustment
during a virtual treatment session with the physician. In this situation, the
physician
establishes communication with the patient through link 180. Once connected
through
link 180, the physician instructs the patient to place antenna 54 over the
implant area, as
shown at step 250. After antenna 54 is in position, the physician downloads an
adjustment command to infuser 115 through link 180, as shown at step 252.
During
and/or after the adjustment is executed in infuser 115, a series of pressure
measurements
are up linked from infuser 115 to the physician through link 180, as shown at
step 254.
The physician performs an immediate review of the fluid pressure changes
resulting from
the adjustment. If the resulting fluid pressure levels are too high or too
low, the physician
may immediately readjust the distension coil, as indicated by line 255. The
physician
may also instruct the patient to perform a particular action to test the
adjustment, such as
drinking or eating, as shown at step 256. As the patient performs the test,
the physician
may upload pressure measurements from the pressure sensor, as shown at step
258, to
evaluate the peristaltic pressure against the coil as the patient attempts to
pass food or
liquid through the stoma. If the pressure measurements are too high,
indicating a possible
obstruction, the physician may immediately transmit additional command signals
to
Page 34 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
readjust the coil and relieve the obstruction, as indicated by line 259. After
the physician
is satisfied with the results of the adjustment, the communication session is
terminated at
step 232. In the present invention, local unit 60 is at all times a slave to
remote unit 170
so that only a physician can prescribe adjustments, and the patient is
prevented from
independently executing adjustments through local unit 60.
[00125] In a third exemplary communication session, shown in FIG. 12, a
patient may
initiate an interaction with remote unit 170 by entering a request through
user interface
140, as shown at step 260. This request may be in the form of an e-mail or
other
electronic message. At step 262, the patient's request is transmitted through
communication link 180 to remote unit 170. At remote unit 170, the patient's
request is
stored in memory 174 until retrieved at the physician's convenience (step
264). After the
physician has reviewed the patient's request (step 266), instructions may be
entered
through user interface 176 and downloaded to local unit 60. The physician may
communicate with the patient regarding treatment or the decision to execute or
deny a
particular adjustment request, as shown at step 268. If the physician
determines at step
269 that an adjustment is required, the physician may initiate a communication
session
similar to those shown in the flow diagrams of FIGS. 10 and 11. If an
adjustment is not
indicated, the base unit terminates the session following the responsive
communication of
step 268.
[00126] In addition to the above scenarios, a physician may access local unit
60 at any
time to check on patient compliance with previous adjustment instructions, or
to remind
the patient to perform an adjustment. In these interactions, the physician may
contact
local unit 60 to request a data upload from memory 138, or transmit a reminder
to be
stored in memory 138 and displayed the next time the patient turns on local
unit 60.
Additionally, local unit 60 can include an alarm feature to remind the patient
to perform
regularly scheduled adjustments, such as diurnal relaxations.
[00127] As mentioned above, communication system 20 can be used to uplink a
fluid
pressure history to remote unit 170 to allow the physician to evaluate the
performance of
r1Pv;cP T? n,%7ar n designated time period. FIG. 13 illustrates a data logger
270 that may be
Page 35 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
used in conjunction with communication system 22 of the present invention to
record
fluid pressure measurements over a period of time. In this example, data
logger 270 is
external to the patient, and is positioned over the region under which
injection port 36 is
implanted within the patient. In another embodiment, data logger 270 is also
implanted
within the patient. As shown in FIG. 13, data logger 270 comprises TET and
telemetry
coils 285, 272 which may be worn by the patient so as to lie adjacent to
implanted portion
24. TET coil 285 provides power to the implant, while telemetry coil 272
interrogates the
implant and receives data signals, including fluid pressure measurements,
through
secondary telemetry coil 114. In another embodiment, TET coil 285 and
telemetry coil
272 are consolidated into a single coil, and alternate between TET and
telemetry
functions at any suitable rate for any suitable durations.
[00128] The fluid pressure within the distension coil 28 is repeatedly sensed
and
transmitted to data logger 270 at an update rate sufficient to measure
peristaltic pulses
against the coil. Typically, this update rate is in the range of 10-20
pressure
measurements per second. As shown in FIG. 13, data logger 270 may be worn on a
belt
274 about the patient's waist to position coils 272 adjacent injection port 36
when the port
is implanted in the patient's abdominal area. Alternatively, data logger 270
can be worn
about the patient's neck, as shown by device 270', when injection port 36 is
implanted on
the patient's sternum. Data logger 270 is worn during waking periods to record
fluid
pressure variations during the patient's meals and daily routines. At the end
of the day, or
another set time period, data logger 270 may be removed and the recorded fluid
pressure
data downloaded to memory 138 of local unit 60. The fluid pressure history may
be
uploaded from memory 138 to remote unit 170 during a subsequent communication
session. Alternatively, fluid pressure data may be directly uploaded from data
logger 270
to remote unit 170 using communication link 180.
[00129] FIG. 14 shows data logger 270 in greater detail. As shown in FIG. 14,
data
logger 270 includes a microprocessor 276 for controlling telemetry
communications with
implanted device 24. Microprocessor 276 is connected to a memory 280 for,
among other
functions, storing pressure measurements from device 24. In the present
example,
Page 36 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
memory 280 comprises 40 Mb of SRAM and is configured to store 100 hours of
time
stamped pressure data. Of course, any other type of memory 280 may be used,
and
memory 280 may store any amount of and any other type of data. By way of
example
only, any other type of volatile memory or any type of non-volatile memory may
be used,
including but not limited to flash memory, hard drive memory, etc. While data
logger 270
of the present example is operational, fluid pressure is read and stored in
memory 280 at
a designated data rate controlled by microprocessor 276. Microprocessor 276 is
energized
by a power supply 282. In one embodiment, power supply 282 comprises a
rechargeable
cell (not shown), such as a rechargeable battery. In one version of this
embodiment, the
rechargeable cell is removable and may be recharged using a recharging unit
and
replaced with another rechargeable cell while the spent cell is recharging. In
another
version of this embodiment, the rechargeable cell is recharged by plugging a
recharging
adapter into a data logger 270 and a wall unit. In yet another version of this
embodiment,
the rechargeable cell is recharged wirelessly by a wireless recharging unit.
In another
embodiment, power supply 282 comprises an ultra capacitor, which may also be
recharged. Of course, any other type of power supply 282 may be used.
[00130] To record fluid pressure, microprocessor 276 initially transmits a
power signal
to implanted portion 24 via TET drive circuit 283 and TET coil 285. After the
power
signal, microprocessor 276 transmits an interrogation signal to implanted
portion 24 via
telemetry transceiver 284 and telemetry coil 272. The interrogation signal is
intercepted
by telemetry coil 114 and transmitted to microcontroller 106. Microcontroller
106 sends a
responsive, temperature-adjusted pressure reading from sensor 84 via
transceiver 158 and
secondary telemetry coil 114. The pressure reading is received through coil
272 and
directed by transceiver 284 to microprocessor 276. Microprocessor 276
subsequently
stores the pressure measurement and initiates the next interrogation request.
[00131 ] When the patient is finished measuring and recording fluid pressure,
logger 270
is removed and the recorded pressure data downloaded to local unit 60, or
directly to
remote unit 170. As shown in FIGS. 9 and 14, data logger 270 may comprise a
modem
286 for transmitting the sensed fluid pressure directly to remote unit 170
using a
Page 37 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
telephone line 288. The patient may connect logger modem 286 to a telephone
line, dial
the physician's modem, and select a "send" button on user interface 292. Once
connected,
microprocessor 276 transmits the stored pressure history through the phone
line to
microprocessor 172 in remote unit 170. Alternatively, data logger 270 may
include a
USB port 290 for connecting the logger to local unit 60. Logger USB port 290
may be
connected to a USB port 198 on local unit 60 (shown in FIG. 8), and the "send"
switch
activated to download pressure data to memory 138 in the local unit. After the
pressure
data is downloaded, logger 270 may be turned off through user interface 292,
or reset and
placed back on the patient's body for continued pressure measurement.
[00132] FIG. 15 is a graphical representation of an exemplary pressure signal
294 as
measured by sensor 84 during repeated interrogation by local unit 60 or data
logger 270
over a sampling time period. Pressure signal 294 may be displayed using
graphical user
interface 140 of local unit 60 or graphical user interface 176 of remote unit
170. In the
example shown in FIG. 15, the fluid pressure in coil 28 is initially measured
while the
patient is stable, resulting in a steady pressure reading as shown. Next, an
adjustment is
applied to coil 28 to increase the coil size. During the coil adjustment,
pressure sensor 84
continues to measure the fluid pressure and transmit the pressure readings
through the
patient's skin to local unit 60. As seen in the graph of FIG. 15, fluid
pressure rises
following the coil adjustment.
[00133] In the example shown, the patient is asked to drink a liquid after the
adjustment
to check the accuracy of the adjustment. As the patient drinks, pressure
sensor 84
continues to measure the pressure spikes due to the peristaltic pressure of
swallowing the
liquid. The physician may evaluate these pressure spikes from a remote
location in order
to evaluate and direct the patient's treatment. If the graph indicates
pressure spikes
exceeding desired levels, the physician may immediately take corrective action
through
communication system 20, and view the results of the corrective action, until
the desired
results are achieved. Accordingly, through communication system 20 a physician
can
perform an adjustment and visually see the results of the adjustment, even
when located
at a considerable distance from the patient.
Page 38 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[00134] In addition to adjustments, communication system 20 can be used to
track the
performance of an intake distension device over a period of time. In
particular, a
sampling of pressure measurements from data logger 270 may be uploaded to the
physician's office for evaluation. The physician may visually check a graph of
the
pressure readings to evaluate the performance of the distension device. It
will be
appreciated that long term pressure data may be helpful in seeing when the
patient eats or
drinks during the day and how much. Such data may thus be useful in compliance
management.
[00135] Pressure measurement logs can also be regularly transmitted to remote
monitoring unit 170 to provide a physician with a diagnostic tool to ensure
that a stomach
distension device is operating effectively. For instance, pressure data may be
helpful in
seeing how much coil 28 pressure or tightness varies, and if coil 28 tends to
obstruct at
times. If any abnormalities appear, the physician may use communication system
20 to
contact the patient and request additional physiological data, prescribe an
adjustment, or,
where components permit, administer an adjustment. In particular,
communication
system 20 may be utilized to detect a no pressure condition within coil 28,
indicating a
fluid leakage. Alternatively, system 20 may be used to detect excessive
pressure spikes
within coil 28 or pressure being stuck at a fixed level, which may indicate a
kink in
catheter 40 or another issue.
[00136] Local unit 60, another type of docking station 360, remote unit 170,
or some
other device may further comprise a logic that is configured to process
pressure data and
actively provide an alert to a physician, the patient, or someone else when a
dramatic
change in pressure is detected or under other predefined conditions. Such an
alert may
comprise any of the following: an e-mail, a phone call, an audible signal, or
any other
type of alert. The conditions for and/or type of an alert may also vary
relative to the
recipient of the alert. For instance, with respect to alerts for physicians,
such alerts may
be limited to those provided upon an indication that some component of
implanted
portion 24 has structurally failed (e.g., a kink in catheter 40, a burst coil
28, etc.). With
respect to alerts for patients, such alerts may be limited to those provided
upon an
Page 39 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
indication that the patient is eating too much, eating to quickly, or if the
bite sizes are too
big. A variety of other conditions under which alerts may be directed to a
physician or
patient will be apparent to those of ordinary skill in the art. In addition,
it will be
appreciated that physicians and patients may receive alerts under similar
conditions, or
that either party may simply not receive alerts at all.
[00137] To the extent that local unit 60 has a graphical user interface
permitting the
patient to see pressure data, local unit 60 may be used by the patient to
evaluate pressure
readings at home and notify their physician when the coil 28 pressure drops
below a
specified baseline, indicating the need for an adjustment of the device.
Communication
system 20 thus has benefits as a diagnostic and monitoring tool during patient
treatment
with a bariatric device. The convenience of evaluating an intake distension
device 22
through communication system 20 facilitates more frequent monitoring and,
components
permitting, adjustments of the device.
[00138] The graphical user interface of local unit 60, remote monitoring unit
170, or
another external or physiological monitoring device in the communication
system 20, can
provide a wide variety of displays based on or related to data or information
from the
distension device 22. Further, in some embodiments, the data logger 270 can
have such a
graphical user interface. The displays can include information about
measurements taken
by the distension device 22, such as the measurements of the fluid pressure
sensed within
a fluid-Tillable distension device, pressure in a mechanically-adjustable
distension device,
or other parameters (e.g., pulse widths, pulse durations, pulse amplitude,
pulse count or
pulse frequency, sensed electrical characteristics, etc.), or about
physiological events,
conditions (e.g., of the distension device 22, such as its restricted or fill
state), or trends.
FIG. 19A, for example, shows one exemplary embodiment of a display 1900 that
can be
used as part of a graphical user interface. As shown, the display includes a
plot or graph
1902 of pressure over time, which is shown as a line graph but could also be a
bar graph,
scatter graph, or virtually any other graphic representation. The time scale
along the
horizontal axis 1901 can be automatically sized to the amount of pressure data
available
or can be user-adjustable, e.g., to examine a time period of interest. The
display 1900 can
Page 40 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
also include a textual indicator 1904, which as shown numerically provides a
current or
instantaneous pressure reading. A wide variety of other kinds of information
also can be
presented on display 1900, including a baseline indicator 1906 showing a
steady-state or
baseline value of the pressure and pulse indicators 1908 showing the number of
pulses
(for example, the pulses may be pressure pulses which can represent or be
caused by the
peristaltic contractions of a patient swallowing). In some embodiments, this
information
can be obtained through user input (via the "Set Baseline" button 1912 or by
entering
visually detected pulses, for example), but in many embodiments this
information can be
obtained by analyzing, filtering or otherwise processing pressure or other
data from the
distension device 22 and/or data logger 270 via one or more algorithms, which
will be
discussed in more detail below. The local unit 60, remote monitoring unit 170
or other
device can implement these algorithms and continuously update the display 1900
with the
results. The display 1900 can also include a cluster 1910 of recording
controls to allow a
user to control when pressure is recorded or logged to a file, and the
location of such a
log file can be shown in window 1924. In addition, an annotation function can
be
provided via control 1914. In other embodiments, the display 1900 can include
pressure
readings taken from prior visits (for example, prior visits of the same
patient, or from
previous adjustments of the distension device), and/or pressure readings of
previous
peristaltic events representing swallowing, heart rate, breathing rate, or
virtually any
other physiological parameter. The display 1900 also can include a patient's
name or
other identifying information, along with notes, lists of activities or
guidelines for the
patient, and so on.
[00139] In FIG. 19A, the display 1900 has a menu 1916 that includes three
graphics or
icons 1918, 1920, 1922. Selection of each one of these icons can cause a
different
display screen to be presented. As shown in FIG. 19A, the second icon 1920 is
selected
and the graph 1902 of sensed pressure over time is shown. Selection of the
first icon
1918 can lead to a display 1930 as shown in FIG. 19B, which indicates pressure
via a
meter 1932. In this embodiment the meter 1932 is vertical and linear, however,
a wide
variety of other orientations and shapes can be used, such as a horizontal
meter, circular,
and so on. The meter 1932 can include discrete indicators or bars 1934 which
can be
Page 41 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
divided into one or more zones or ranges 1936a-c. As shown, three discrete
pressure
ranges 1936a-c are provided with limits (in this example, 80 to 140mmHg, 0 to
80
mmHg, and -10 to 0 mmHg), however any number of pressure ranges can be
provided,
and their size and endpoints can be adjustable. As one skilled in the art will
understand,
the ranges 1936a-c can be set by a physician or other user and can vary from
patient to
patient. In some embodiments, the pressure ranges 1936a-c can correspond to
conditions
related to an implantable distension device, for example, the highest range
can indicate
that the distension device is over-filled or over-distended, the middle range
can indicate
an optimally filled or optimally tightened distension device, and the lower
range can
indicate an under-distended or loose distension device. In use, the pressure
can be
indicated by a marker 1937, which can represent current pressure, average
pressure, or
other metrics related to pressure. In some embodiments, the marker 1937 can
move
continuously along the meter 1932, while in other embodiments, the marker 1936
can
move in a discrete fashion from bar 1934 to bar 1934. Display 1930 also can
contain
many of the same or similar interface elements as in display 1900 shown in
FIG. 19A,
such as an cluster 1910 of recording controls, a window 1924 showing the
location of a
log file, and/or an annotation control 1914. Alternatively, the display of the
fill condition
may be represented by a series of colors superposed on an image of the coil in
which one
color such as green may represent an optimally distended coil, red may
represent an over
distended coil and yellow may represent an under distended coil.
[00140] Returning to FIG. 19A, selection of the third icon 1922 can lead to a
pulse count
display 1940, as shown in FIG. 19C, for counting the number of pulses in a
sequence of
pulses. The sequence of pulses can represent a peristaltic event such as
swallowing. The
display 1940 can include a circular meter 1944 with numbering or indicators
around its
periphery. In use, an indicator needle 1932 can rotate within meter 1944 to
provide an
indication of the number of pulses detected in a sequence. Textual indicators
1946, 1948
can also be provided to indicate the number of pulses in the current or a past
sequence of
pulses. Control 1950 can reset the count.
Page 42 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[00141 ] A wide variety of other displays for pressure, pulses, and for other
physiological
parameters and events can be provided. For example, FIG. 20 shows an alternate
waveform display 2000 of pressure vs. time, which provides a time scale
delineated by
textual markers 2002 along the x-axis. The pressure sensed by the distension
device 22
can be plotted as waveform 2004 in this display 2000. In addition, any of the
displays, or
the indicator, meters, graphs, or other display elements within them, can be
configured to
signal an alarm. For example, the pressure graph 1902, the textual indicator
1904, or the
meters 1931, 1944 (or other display elements) can flash when the pressure, or
other
parameter, passes a threshold value. The alarm can also be indicated by an
illumination
change (e.g., the color, intensity, hue, etc. can change) of the display or a
warning
message, or other visual indicator. An audible alarm can also be included in
addition to
or instead of a visual alarm. Any of the displays described herein can use a
green-
yellow-red bar, circle, or other representative geometric figure, graphic
representation or
indicator in which color shift occurs as the parameter being sensed changes.
For
example, the color of an indicator can turn red as the coil nears an
overdistension (e.g., as
indicated by pressure, or otherwise), since this may be health endangering,
but can turn
yellow as the distension device loosens (e.g., as indicated by pressure or
otherwise), as
this may not be considered a life threatening issue. In some embodiments, such
colors
can be achieved using color light emitting diodes (LEDs) or liquid crystal
display (LCD)
screens.
[00142] FIG. 21 shows an alternate embodiment of a display 2100 which
indicates
pressure (for example, current pressure, or pressure at a selected point on
display 2000,
etc.). Display 2100 can include a vertical meter 2103 that is divided into
discrete
segments 2102. Each segment can represent a group of pressures, illuminating
when the
sensed pressure is within the group. As shown in FIG. 21, segment 2114 is
illuminated.
Labels 2104, 2112 can identify the group. The segments 2102 can be grouped
into zones
or ranges which can be differentiated by a color. As shown in FIG. 21, the
meter 2103
includes three ranges 2106, 2108, 2110 (e.g., red, yellow, green) which can
correspond to
high, medium, and low pressure, respectively. The ranges 2106, 2108, 2110 can
be user-
configurable and can correspond to a variety of conditions, for example the
high range
Page 43 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
can correspond to a distension device 22 being too tight, and so on. A medium
range,
which can be designated by green, can correspond to an optimally restricted
adjustment
zone. In use, the meter 2100 can display static and/or dynamic pressure
measurements.
In static measurements, for example, the meter 2100 can present a baseline
pressure or
pressure sensed by the distension device 22, which can be advantageous after
implantation or adjustment of the device 22. In dynamic or instantaneous
measurements,
for example, the meter 2100 can present the pressure detected in the
distension device 22
during a swallowing event. As a result, the illuminated segment 2102 can rise
and fall
along with changes in pressure.
[00143] FIG. 22 shows another alternate embodiment of a display 2200 which
indicates
pressure. In this illustrated embodiment, the display 2200 is in the form of a
circular
meter 2202 with a rotating needle 2206 and labels 2204 located around the
periphery of
the meter 2202. The meter 2002 can be divided in a plurality of zones or
ranges 2208,
which can function as previously described. In use, the needle 2206 can rotate
to point to
the pressure reading, such as baseline pressure, average pressure, static or
dynamic
pressure, and so on.
[00144] FIG. 23A shows an alternate embodiment of a display 2300 which
presents
information about a sequence of pulses in a parameter, such as can occur with
pressure
pulses during a swallowing event. As shown, display 2300 includes a graph 2302
of
pulse amplitude vs. pulse count. In other embodiments, the magnitude of
another
parameter can be displayed instead of pressure. The pulse count can correspond
to the
number of the pulse in a sequence. For example, as shown pulse label 2304
identifies the
sixth pulse in a seven pulse sequence. (It should be noted that although the
example
illustrated in FIGS. 23A shows 7 pulses, any number of pulses may be
determined and
displayed.) In use, vertical bars 2306 can indicate the pulse amplitude of
each pulse in
the pulse sequence. Each vertical bar 2306a-g can be composed of segments or
discrete
indicators 2308, each of which can represent a pressure or group of pressures.
The height
of the vertical bar can represent the magnitude or amplitude of the pressure,
which can be
an absolute pressure reading or a change in pressure from a baseline pressure
or other
Page 44 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
pressure reference. In use, the vertical bars 2306a-g can be displayed as
pulses are
detected. For example, as the pressure detected by the distension device 22
rises, the
display 2300 can present a rising vertical pressure bar 2306a at the left hand
side of the
graph 2302. If that rise in pressure is considered a pulse, which for example
can be
determined via algorithms which will be discussed below, then the vertical bar
2306a can
rise and stop at the peak of the pulse, and a pulse count of "1" can appear on
the bottom
axis 2308. If another pulse occurs, another bar 2306b can appear in similar
fashion,
accompanied by a pulse count under it reading "2." This can continue until the
pressure
no longer exhibits pulse events, until the user indicates that the event is
over, until the
pulses become infrequent (as measured by, for example, inter-pulse periods),
or until
through the expiration of a predetermined timer, and so on. By way of
illustration, FIG.
23B shows a series of displays 2312, as they might appear during the course of
a two-
pulse sequence.
[00145] The display can also include a time stamp for a pulse. For example, as
shown
on FIG. 23A, a time stamp 2314 can be placed near the pulse count number to
indicate
the time at which the pulse was detected (e.g., at a time of 4 seconds within
a time sample
period) or, alternatively, the stamp can indicate the measured duration of the
pulse (e.g.,
the pulse was 4 seconds long), the time since the last pulse (e.g, 4 seconds
since the
onset, peak or, end, other point of a previous pulse), or any of a wide
variety of time
metrics related to the pulses. As one skilled in the art will understand,
although FIG. 23A
shows one time stamp 2314 as an example, time stamps can be associated with
other
pulses as well.
[00146] FIGS. 24-25 show yet other exemplary displays for the graphical user
interface
of the local unit 60, remote monitoring unit 170, data logger 270, or other
device.
Generally, these displays can present a static or dynamic image of the
stomach,
distension device, and/or surrounding physiology which can change or otherwise
be
representative of a parameter (such as pressure) sensed by the distension
device. The
displays can be still images shown in sequence or at appropriate times, video,
or other
kind of image. For example, FIG. 24A shows one exemplary display 2400, which
has a
Page 45 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
simulated graphic of the disposition of a region contacted by a distension
device 2404,
which in this example includes a cross-section of the stomach enclosed by a
distension
device 2404. The graphic can show the size, shape, configuration, effect of
the
distension device 2404 on the region, or other aspect of the region's
disposition. The
illustration of the stomach 2402 region herein is by way of example only, as
virtually any
region within the body and particularly any anatomical lumen, can be
illustrated.
[00147] In use, the display 2400 can change in accordance with pressure sensed
by the
distension device. For example, FIG. 24B shows display 2400 as it might appear
after a
rise in pressure, with the stomach 2402 increasing in size and surrounding
tissue
becoming more distended. In some embodiments, the display 2400 can be
continuously
updating (as in a live display), but in other embodiments it can be composed
of static or
still images which are shown as necessary, each image corresponding to a range
of
pressures. For example, FIG. 25 shows an exemplary plot of pressure over a
time period,
and includes three segments labeled A, B, C, each exhibiting a different
sensed pressure.
FIG. 24A can correspond to segment A, FIG. 24B can correspond to segment C. In
some
embodiments, the segments A,B,C, might correspond to the condition of the
distension
device 2404, such as the distension state or fill state of the distension
device 2404, for
example, segment A might be correlated to the distension device being too
loose or
under-filled, segment B might represent optimal adjustment, and segment C
might
represent an overly tight or over-filled or distension device. In other
embodiments, the
display 2400 can change in accordance with different sensed pulse amplitudes,
pulse
counts, or pulse frequencies, and so on (such pulse information obtained, for
example, in
response to a standardized tests such as a water swallow, or by monitoring
pulses
characteristics over a prescribed amount of time).
[00148] Display 2400 can have a wide variety of other configurations. In some
embodiments, one or more reference lines, isobars, or other indicators can be
shown on
the display 2400. For example, a circle (or one or more concentric circles)
can be shown
on display 2400, allowing a physician or other user to more easily visualize
changes in
the size of the stomach 2402 or other changes in the disposition of the
region. In some
Page 46 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
embodiments, the size of the circles can be chosen and labeled to indicate a
measured
pressure, for example, a label on a circle can represent a sensed pressure,
and when the
size of the stomach or opening 2402 substantially matches the size of the
circle, the
sensed pressure can be substantially equal to that labeled pressure.
Information such as
the sensed pressure and/or the state of the distension device can also be
presented
textually on display 2400, or by using color, for example, the image of the
stomach
turning red as the stomach neared maximal distension, and so on.
[00149] Furthermore, while in FIGS. 24A-B the display 2400 presents a cross-
sectional
image, in other embodiments other two-dimensional images (such as a side view,
a view
of the distension device alone, and so on), or three-dimensional graphics can
be provided.
[00150] As previously mentioned, the graphical user interface of the local
unit 60,
remote monitoring unit 170, or other external device can be suited to
presenting historical
trends or data analysis, for example based on parameter data captured by the
data logger
270. Such functionality can be useful, for example, when a patient visits a
physician to
review progress, to address a complication, and/or to adjust an implanted
distension
device 22. In one exemplary embodiment, shown in FIG. 26, a display 2900 can
present
a graph or plot of pressure over a time period, however other physiological
parameters
such as heart rate, blood pressure, breathing rate, etc., also can be
displayed. The display
2900 can include multiple sets of data, for example, a trendline 2902 or other
graphical
representation of data from a first time period (e.g., a first visit to the
physician) and
another trendline 2904 or graphical representation of data captured at a later
time period
(e.g., a second visit to the physician) overlaid on the trendline 2902 from
the first time
period. The overlay of data from two different time periods can allow a user
to compare
the trend lines. In some embodiments, the later time period can follow some
significant
medical event, such as the adjustment of the distension device 22, and the
overlay of data
allows for the assessment of the adjustment to the distension device 22.
Although FIG.
26 shows an example with pressure over a time period resulting from a water
swallow,
pressure from any source or time period can be used. Additionally, a wide
variety of data
can be plotted in this manner, including weight, weight loss, body mass index,
body
Page 47 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
dimensions, intracoil pressure, heart rate (resting and under exercise),
breathing rate
(resting and under exercise). By way of illustration, FIG. 27 shows an
exemplary display
3000 which overlays a trend line 3002 representing patient's breathing rate
after one
adjustment of a distension device with a second trend line 3004 representing
the
breathing rate after a later adjustment. Different types of data can be
presented in an
overlaid fashion (e.g., pressure trend lines with overlaid heart rate trend
lines).
[00151] FIG. 28A shows one exemplary display 3100 which presents data for a
population or group of patients. The population data can come from a wide
variety of
datasets, including data collected by a physician, regional data, nationwide
data, and/or
data selected from a larger dataset to match the body type (or other
physiological/medically significant characteristics) of a particular patient.
A variety of
parameters can be plotted and compared, but as shown, display 3100 presents a
plot of
pressure vs. fill volume for a fluid-Tillable distension device. Other
parameters such as
pulse count, pulse amplitude, pulse width, pulse amplitude, and pulse
frequency, can also
be plotted against fill volume, and as previously mentioned, such pulse
information can
be obtained, for example, in response to a tests such as a water or bolus
swallow, which
can be of a standardized volume and/or viscosity, or by monitoring pulse
characteristics
over a prescribed amount of time, inclination (body supine, or erect),
acceleration etc..
Display 3100 can also includes several trend lines 3102 (although a bar graph,
scatter
graph, or other graphical representations of the data can be used), each trend
line plotting
data from patient, as shown in the legend 3104. More specifically, the trend
lines 3102
can represent pressure (baseline pressure, average pressure, or any other
pressure
measurement) sensed for each patient for a given fill volumes of their
distension device.
In some embodiment, this data can come from the data logger 270, but in this
example
the trend lines 3102 represent static volume measurements taken by adding a
known
volume of liquid (e.g., 1 ml) at a time to the distension device 22 and
measuring the
resulting pressure. As can be seen, the trend lines 3102 exhibit a range of
pressures at
each volume, which can be due to variability in anatomy or distension device
placement
and fit from patient-to-patient. The display 3100 can be useful to allow a
physician or
other user to visualize how one patient compares to another patient or to a
population.
Page 48 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[00152] FIG. 28B shows another exemplary display 3150 which presents data for
a
population of patients. As shown in FIG. 28B, display 3150 includes a plot of
pressure
vs. fill volume. The display 3150 includes a trend line 3152 representing a
nominal value
of the pressure for a group or population of patients. In this embodiment, the
nominal
value is a mean value, but in other cases it can be a midpoint, weighted
average,
minimum, maximum, range, standard deviation, or the result of any other
mathematical
calculation. The display 3150 also can include an upper bound trend line 3154
and a
lower bound trend line 3156, which collectively can define a range 3158 around
the
nominal value. In some embodiments, a trend line for a particular patient can
be overlaid
onto the display 3152, revealing where the patient falls relative to the
population. In
other embodiments, the display 3152 can be presented without overlaid data for
a
particular patient.
[00153] Displays also can provide the ability to annotate historical data,
particularly data
that is collected over an extended time period (e.g., by the data logger).
FIG. 29 shows
an external device 3200, such as the local unit 60 with a display 3202. It
should be
understood that the external device 3200 can represent any external device for
display
and/or physiological monitoring, including the remote monitoring unit 170. As
shown,
the display 3200 presents a plot of pressure values over a time period and
provides the
ability to annotate the plotted values using a pull-down menu 3204. The menu
3204 can
include a variety of descriptions of predefined events 3206, such as a tests
conducted,
symptoms, observations by a user or physician, and so on. By way of
illustration, in FIG.
29 an annotation 3210 is disposed on the waveform 3208 and includes an
annotation
marker 2310 which indicates that at a particular point in time a "Water
Swallow - 20 ml"
occurred. A user can annotate historical data in a variety of ways. For
example, the
external device 3200 can be adapted for home use, and the patient can annotate
events on
a day-to-day basis. Such an embodiment can be useful if the data logger 270 is
capturing
data over several days, for example. Alternatively, the external device 3200
can be
updated by a physician during patient visits or when the distension device 22
is adjusted.
The physician can annotate the day-to-day data, or can conduct additional
tests (such as a
Water Swallow) to create data logs separate from any day-to-day monitoring. It
should
Page 49 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
be understood that while display 3200 presents predefined events for
annotation, in many
embodiments the user can create their own user-defined events for annotation,
and/or can
enter free-form descriptions about the data values. FIG. 30 shows one
exemplary
embodiment display 3300 on the external device 3200 in which descriptions can
be
entered into a text box 3302. In some embodiments, an image or icon can also
be used
for the description, for example, an icon of a cup can indicate a "Water
Swallow" event.
[00154] The ability to present data with annotations is not limited to
pressure data. For
example, FIG. 31 shows a display 3400 that includes a graphical
representation, in this
case a bar graph, of weight loss over time, with the amplitude of the bars
3402
corresponding to the amount of the weight loss. As shown, a bar 3402 is
provided for a
series of dates 3404. A user can enter comments or annotations associated with
each bar
3402 and/or date 3404 in text box 3406, which can be helpful for tracking
and/or
revealing events in the patient's life that affect weight loss. The external
device 3200 can
include a keypad 3408 or other user input device for this purpose.
[00155] Any or all of the preceding displays can be provided in virtually any
combination to create a graphical user interface for the local unit 60, remote
monitoring
unit 170, data logger 270, or other physiological monitoring device. In some
embodiments, a remote server can be provided to allow users to download
displays
and/or display elements they desire to a local unit 60 or remote monitoring
unit 170. For
example, a library of display screens, display modes, visual skins, desktop
images,
screensavers, and other display configurations can be available for download,
allowing a
user to customize the graphical user interfaces of the devices. In addition,
the remote
server can provide the ability to store and categorize displays and/or display
elements that
were customized or designed and uploaded by users. Such functionality can
allow users
to exchange and to share display elements with one another.
[00156] In addition, any or all of the graphical user interface and/or
displays described
herein can be repurposed by being modified, altered, erased, reprogrammed,
upgraded,
revised, added to, and so on. For example, a device having a graphical user
interface can
hP nhta,narl nnd desired modifications can be made by programming the
appropriate
Page 50 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
software through a data input port or docking station (e.g., USB port 198
shown in FIG.
8) of the local unit 60, remote monitoring unit 170, or other physiological
monitoring
unit. In other embodiments, such modifications can be performed
telemetrically. For
example, additional icons, graphs, indicators and so on can be added, displays
customized
for a particular user, and so on. Use of such techniques, and the resulting
device, are all
within the scope of the present application.
[00157] An alternate embodiment of a data logging system 300 is shown in FIG.
16. In
this example, data logging system 300 comprises a coil head 354 and a data
logger 370.
Coil head 354 and data logger 370 are in communication via a cable 356. Cable
356 is
detachable from coil head 354 and data logger 370. Of course, it will be
appreciated that
cable 356 is merely exemplary, and that any suitable alternative may be used,
including
but not limited to a wireless transmitter/receiver system. In the present
example, coil head
354 is worn around the neck of the patient, and is positioned generally over
injection port
36. Data logger 370 is worn on a belt 274 about the patient's waist. Of
course, these
respective locations are merely exemplary, and it will be appreciated that
coil head 354
and data logger 370 may be positioned elsewhere. By way of example only, where
injection port 36 is implanted in the patient's abdomen, coil head 354 may be
worn on a
belt 274. It will also be appreciated that coil head 354 and data logger 370
are represented
as simple blocks in FIG. 16 for illustrative purposes only, and that either of
coil head 354
or data logger 370 may be provided in a variety of shapes, sizes, and
configurations.
[00158] Exemplary components of data logging system 300 are shown in FIG. 17.
As
shown, data logger 370 comprises a microprocessor 276, a memory 280, a power
supply
282, a USB port 290, and a user interface 292. Coil head 354 comprises a TET
drive
circuit 283, a telemetry transceiver 284, a TET coil 285, and a telemetry coil
272. TET
drive circuit 283 is configured to receive power from power supply 282 via
cable 356.
TET drive circuit is further configured to receive signals from microprocessor
276 via
cable 356. Telemetry transceiver 284 is configured to receive signals from
microprocessor 276, and transmit signals to microprocessor 276, via cable 356.
In
another embodiment, telemetry transceiver 284 is configured to only transmit
signals to
Page 51 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
microprocessor 276. It will be appreciated that many of the components
depicted in FIG.
17 are similar to those depicted in FIG. 14 and described in the accompanying
text.
Accordingly, the above discussion of such components with reference to FIG. 14
may
also be applied to the components shown in FIG. 17. In the present example,
coil head
354 and data logger 370 may be viewed as a separation of components comprising
data
logger 270 (described above) into two physically separate units. It will
further be
appreciated that any of the components shown in FIG. 17, as well as their
relationships,
functions, etc., may be varied in any suitable way.
[00159] In the present example, coil head 354 is configured similar to and
functions in a
manner similar to antenna 54 described above. TET coil 285 of coil head 354 is
configured to provide power to injection port 36. Of course, to the extent
that any other
devices (e.g., a pump, etc.) are implanted in the patient that are configured
to receive
power from a TET coil 285, TET coil 285 may also provide power to such
devices.
Power provided by TET coil 285 may be provided to TET coil 285 by and
regulated by
TET drive circuit 285, which may itself receive power from power supply 282
via cable
356. Such power provided to TET drive circuit 283 may be regulated by
microprocessor
276 via cable 356. In addition, or in the alternative, microprocessor 276 may
regulate the
manner in which TET drive circuit 285 provides power to TET coil 285. Other
suitable
configurations and relationships between these components, as well as
alternative ways in
which they may operate, will be apparent to those of ordinary skill in the
art. It will also
be appreciated that, while the present example contemplates the use of RF
signaling
through TET coil 285, any other type of powering technique, as well as
alternative power
communicators, may be used.
[00160] Telemetry coil 272 of coil head 354 is configured to receive signals
from coil
114 of injection port 36, including signals indicative of the pressure of
fluid within the
implanted device (e.g., pressure of fluid within the injection port 36, within
catheter 40,
and/or within adjustable coil 28, pressure obtained using pressure sensor 84,
etc.) and
signals indicative of temperature. It will be appreciated that telemetry coil
272 may also
receive any other type of signal representing any other type of information
from any other
Page 52 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
source. Signals received by telemetry coil 272 are communicated to telemetry
transceiver
284, which is configured to communicate such signals to microprocessor 276 via
cable
356. Telemetry transceiver 284 may perform any appropriate translation or
processing of
signals received from telemetry coil 272 before communicating signals to
microprocessor
276. Other suitable configurations and relationships between these components,
as well
as alternative ways in which they may operate, will be apparent to those of
ordinary skill
in the art. It will also be appreciated that components may be combined. By
way of
example only, TET coil 285 and telemetry coil 272 may be consolidated into a
single
coil, and alternate between TET and telemetry functions at any suitable rate
for any
suitable durations. In addition, while the present example contemplates the
use of RF
signaling through telemetry coil 272, it will be appreciated that any other
type of
communication technique (e.g., ultrasonic, magnetic, etc.), as well as
alternative
communicators other than a coil, may be used.
[00161] Data logger 370 may receive pressure measurements throughout a given
day,
and store the same in memory 280, thereby recording fluid pressure variations
during the
patient's meals and daily routines. In the present example, memory 280
comprises 40 Mb
of SRAM and is configured to store 100 hours of time stamped pressure data. Of
course,
any other type of memory 280 may be used, and memory 280 may store any amount
of
and any other type of data. By way of example only, any other type of volatile
memory or
any type of non-volatile memory may be used, including but not limited to
flash memory,
hard drive memory, etc. While data logger 370 of the present example is
operational,
fluid pressure is read and stored in memory 280 at a designated data rate
controlled by
microprocessor 276. In one embodiment, fluid pressure is repeatedly sensed and
transmitted to data logger 370, then stored in memory 280, at an update rate
sufficient to
measure peristaltic pulses against adjustable coil 28. By way of example only,
the update
rate may range between approximately 10-20 pressure measurements per second.
Other
suitable update rates may be used.
[00162] In another embodiment, implanted portion 24 comprises a memory (not
shown).
By way of example only, such implanted memory may be located in injection port
36 or
Page 53 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
elsewhere. Such implanted memory may be used for a variety of purposes, to the
extent
that such memory is included. For instance, such implanted memory may store
the same
data as memory 280 of data logger 370, such that implanted memory provides a
backup
for memory 280 of data logger 370. In this version, such data may be further
retained in
implanted memory for archival purposes, may be replaced on a daily basis, may
be
replaced or updated after data logger 370 transmits the same data to remote
unit 170, or
may otherwise be used. It will also be appreciated that an implanted memory
may be used
to store pre-selected information or pre-selected types of information. For
instance, an
implanted memory may store maximum and minimum pressure measurements,
fluoroscopic images or video of a patient swallowing, and/or any other
information.
Other information suitable for storing in an implanted memory will be apparent
to those
of ordinary skill in the art. It will also be appreciated that any type of
memory may be
implanted, including but not limited to volatile (e.g., SRAM, etc.), non-
volatile (e.g.,
flash, hard drive, etc.), or other memory.
[00163] In the present example, microprocessor 276 is energized by a power
supply 282.
In one embodiment, power supply 282 comprises a rechargeable cell (not shown),
such as
a rechargeable battery. In one version of this embodiment, the rechargeable
cell is
removable and may be recharged using a recharging unit and replaced with
another
rechargeable cell while the spent cell is recharging. In another version of
this
embodiment, the rechargeable cell is recharged by plugging a recharging
adapter into a
data logger 370 and a wall unit. In yet another version of this embodiment,
the
rechargeable cell is recharged wirelessly by a wireless recharging unit. In
another
embodiment, power supply 282 comprises an ultra capacitor, which may also be
recharged. Of course, any other type of power supply 282 may be used.
[00164] Data logger 370 of the present example may be configured to provide an
alert to
the patient under a variety of circumstances in a variety of ways. For
instance, data logger
370 may provide an audible and/or visual alert when there is a drastic change
in fluid
pressure. Alternatively, data logger 370 may provide an audible and/or visual
alert upon a
determination, based at least in part on pressure data that the patient is
eating too much,
Page 54 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
too quickly, etc. Data logger 370 may also alert the patient upon a
determination that coil
head 354 is not communicating with injection port 36 properly. Still other
conditions
under which a patient may be alerted by data logger 370 will be apparent to
those of
ordinary skill in the art. It will also be appreciated that user interface 292
may comprise
any number or types of features, including but not limited to a speaker, an
LED, and LCD
display, an on/off switch, etc. In the present example, user interface 292 is
configured to
provide only output to the patient, and does not permit the patient to provide
input to data
logger 370. User interface 292 of the present example thus consists of a green
LED to
show that the power supply 282 is sufficiently charged and a red LED to show
that the
power supply 282 needs to be recharged. Of course, user interface 292 may
alternatively
permit the patient to provide input to data logger 370, and may comprise any
suitable
components and features.
[00165] As shown in FIG. 18, data logging system 300 further comprises a
docking
station 360. Docking station 360 is configured to receive data communications
from data
logger 370, and is further configured to transmit data communications to
remote unit 170.
In the present example, data logger 370 comprises a USB port 290, such that
docking
station 360 may receive communications from data logger 370 via a USB cable
(not
shown) coupled with USB port 290. In one embodiment, docking station 360
comprises
the patient's personal computer. Of course, docking station 360 may receive
communications from data logger 370 in any other suitable way. For instance,
such
communications may be transmitted wirelessly (e.g., via RF signals, Bluetooth,
ultra-
wide coil, etc.).
[00166] In another embodiment, docking station 360 is dedicated to coupling
with data
logger 370, and comprises a cradle-like feature (not shown) configured to
receive data
logger 370. In this example, the cradle-like feature includes contacts
configured to
electrically engage corresponding contacts on data logger 370 to provide
communication
between docking station 360 and data logger 370. Docking station 360 may thus
relate to
data logger 370 in a manner similar to docking systems for personal digital
assistants
(PDAs), BLACKBERRY.RTM devices, cordless telephones, etc. Other suitable ways
in
Page 55 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
which data logger 370 and docking station 360 may communicate or otherwise
engage
will be apparent to those of ordinary skill in the art. It will also be
appreciated that
docking station 360 is depicted in FIG. 18 as a desktop computer for
illustrative purposes
only, and that docking station 360 may be provided in a variety of alternative
shapes,
sizes, and configurations.
[00167] In one embodiment, docking station 360 comprises local unit 60
described
above. Accordingly, it will be appreciated that the above discussion referring
to
components depicted in FIG. 9 may also be applied to components depicted in
FIG. 18.
Similarly, methods such as those shown in FIGS. 10-12 and described in
accompanying
text may also be implemented with docking station 360. In another embodiment,
data
logger 370 comprises local unit 60. In yet another embodiment, data logger 370
is
provided with an AC adapter or similar device operable to recharge power
supply 282,
and data logger 370 further comprises an Ethernet port (not shown) enabling
data logger
370 to be connected directly to a network such as the Internet for
transmitting
information to remote unit 170. It will therefore be appreciated that any of
the features
and functions described herein with respect to local unit 60 and/or docking
station 360
may alternatively be incorporated into data logger 370 or may be otherwise
allocated.
[00168] In one exemplary use, the patient wears coil head 354 and data logger
370
throughout the day to record pressure measurements in memory 280. At night,
the patient
decouples data logger 370 from coil head 354 and couples data logger 370 with
docking
station 360. While data logger 370 and docking station 360 are coupled,
docking station
360 transmits data received from data logger 370 to remote unit 170. To the
extent that
power supply 282 comprises a rechargeable cell, docking station 360 may be
further
configured to recharge the cell while data logger 370 is coupled with docking
station 360.
Of course, it will be immediately apparent to those of ordinary skill in the
art that a
patient need not necessarily decouple data logger 370 from coil head 354 in
order to
couple data logger 370 with docking station 360. It will also be appreciated
that pressure
measurements may be recorded in memory 280 during the night in addition to or
as an
alternative to recording such measurements during the day, and that pressure
Page 56 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
measurements may even be recorded twenty four hours a day. It is thus
contemplated that
the timing of pressure measurement taking and recordation need not be limited
to the
daytime only. It is also contemplated that every pressure measurement that is
taken need
not necessarily be recorded.
[00169] As described above, data logger 370 is configured to receive, store,
and
communicate data relating to the pressure of fluid. However, data logger 370
may
receive, store, and/or communicate a variety of other types of data. By way of
example
only, data logger 370 may also receive, process, store, and/or communicate
data relating
to temperature, EKG measurements, eating frequency of the patient, the size of
meals
eaten by the patient, the amount of walking done by the patient, etc. It will
therefore be
appreciated that data logger 370 may be configured to process received data to
create
additional data for communicating to docking station 360. For instance, data
logger 370
may process pressure data obtained via coil head 354 to create data indicative
of the
eating frequency of the patient. It will also be appreciated that data logger
370 may
comprise additional components to obtain non-pressure data. For instance, data
logger
370 may comprise a pedometer or accelerometer (not shown) to obtain data
relating to
the amount of walking done by the patient. Further, the logger may include a
gravitometer or inclinometer to show the position of the patient for
correlation to eating
habits (while lying down, after going to bed, just before bed time, too long
after waking
up etc. Data obtained by such additional components may be stored in memory
280 and
communicated to docking station 360 in a manner similar to pressure data. Data
logger
370 may also comprise components for obtaining data to be factored in with
internal fluid
pressure measurements to account for effects of various conditions on the
fluid pressure.
For instance, data logger 370 may comprise a barometer for measuring
atmospheric
pressure. In another embodiment, data logger 370 comprises an inclinometer or
similar
device to determine the angle at which the patient is oriented (e.g.,
standing, lying down,
etc.), which may be factored into pressure data to account for hydrostatic
pressure effects
caused by a patient's orientation. Alternatively, an inclinometer or other
device for
obtaining non-pressure data may be physically separate from data logger 370
(e.g.,
Page 57 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
implanted). Still other types of data, ways in which such data may be
obtained, and ways
in which such data may be used will be apparent to those of ordinary skill in
the art.
[00170] The data captured by the data logger 270 (or data logger 370, or any
other data
logger) can be processed and analyzed in a variety of ways. In many
embodiments, the
local unit 60, remote monitoring unit 170, data logger 270, 370 or other
external device,
can be configured to execute one or more data processing algorithms which can
be used
in tracking and analyzing physiological parameters and events, and also can
produce
results that can be presented in the graphical user interface displays
previously described.
It should be understood that the captured and/or logged data can provide
information
about a wide variety of sensed parameters, including without limitation
pressure (e.g., of
a fluid or otherwise). Sensed parameters can also include pulse counts, pulse
widths,
pulse amplitudes, pulse durations, pulse frequency, sensed electrical
characteristics (e.g.,
voltages, capacitances, etc.), and so on.
[00171] Some data processing techniques or algorithms can be generally
directed to
smoothing or conditioning data, (e.g., converting, filtering or other
conditioning) into a
form suitable for later analysis (by computer or by a user) or for display. A
wide variety
of conditioning algorithms are possible. For example, FIG. 32A shows a plot
3500 of
pressure values 3502 sensed by a distension device 22 such as coil 28 and
pressure sensor
84. In this exemplary embodiment, the pressure values 3502 are sensed, or
sampled, over
a period of time, from a pressure signal developed by the pressure sensor 84
in the
distension device 22 (which, as previously mentioned, can be any kind of
distension
device, including fluid-fillable or mechanically based devices). The sensed
values can
be captured by a data logger 270 via repeated interrogation of the distension
device 22. It
should be understood that while pressure values are used as an example, any
sensed
parameter can be used in this algorithm, or any other algorithms described
herein. FIG.
32A shows values that have been collected at a rate of 100 Hz, although
virtually any
sampling rate can be used. The values of the pressure can be converted to a
lower rate,
which can be helpful in presenting phenomena of interest (for example, a pulse
from a
swallowing event might occur on the order of 0.1 Hz), removing noise in the
data, and/or
Page 58 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
compressing the size of the dataset, among other things. The conversion can be
accomplished in a variety of ways, but in one exemplary embodiment, the
pressure values
3502 can be averaged to effectively decrease the sampling rate, the results of
which are
shown in FIG. 32B, which shows a plot 3506 of the pressure values 3502
averaged down
to a 10 Hz rate. The average can be calculated by defining an averaging window
within
the time period on the plot 3500 (for example, by dividing time period into a
sequence of
averaging windows 3504, each 1/10 of a second), and taking the average of the
pressure
values 3502 occurring within each window. The window can be defined by time
(for
example, every 10 seconds) or by the number of data points therein (for
example,
averaging every 10 values or data points). The size of the averaging window
can be user-
defined, and in some embodiments can be defined based on the phenomena or
physiological parameter of interest. As one skilled in the art will
understand, a wide
variety of mathematical techniques can be used, for example, instead of
averaging, the
100 Hz data can be directly converted to 10 Hz data by sampling the pressure
values
3502 at 10 Hz, in other words, downsampling or filtering. FIGS. 32C-E show
three plots
3508, 3510, and 3512 which present the results of converting the pressure
values 3502
plotted in FIG. 32A to lower rates. As shown in FIG. 32E, some lower-frequency
phenomena, such as a pulses 3514, 3516, are still discernible while smaller
amplitude
changes are removed. FIG. 32F shows an exemplary flow diagram illustrating an
averaging algorithm.
[00172] FIGS. 33A-B illustrate the output of an exemplary running average
algorithm
that can be used with data captured by the data logger 270, and FIG. 33C shows
such an
exemplary running average algorithm. A running average algorithm can take a
variety of
forms, but in one embodiment it can include computing each value or data point
for the
running average based on an averaging window, which can be of user-defined
size. The
averaging window can be used to determine the number of data values (the data
values
representing pressure values, for example) that are averaged together to
obtain each
running average value. The averaging window can be shifted as each new data
point is
collected, so the running average value can be updated at the same rate as the
sampling
rate. In one embodiment, the running average value for a particular point in
time can be
Page 59 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
computed by averaging the data values falling within a time window occurring
before
that point in time, in other words a backward-looking running average. The
backward-
looking running average can be defined by the following formula, where RA is
the
running average value, p is the data value, and n is the window sample number:
1 Yi+n-1
RAi=n i A
[00173] In use, for each data value collected, the averaging window can be
applied and
the running average for that point in time can be calculated. The running
average values
can then be displayed, for example alone or with the original data values.
FIG. 33A
illustrates the result of running such an algorithm on pressure data. FIG. 33A
presents a
graph 3600 which includes a plot of raw data values 3602 that have not been
averaged.
Also shown on the graph 3600 are three plots 3604, 3606, 3608 which represent
the data
values following application of a backward-looking average running average
algorithm.
As shown, plot 3604 corresponds to a running average calculated with a 10
second
averaging window, plot 3606 corresponds to a 30 second averaging window, and
plot
3608 corresponds to a 60 second averaging window.
[00174] In another embodiment, the running average for a particular point in
time can be
computed by averaging the data values in an averaging window which includes
data
values both before and after the point in time, in other words a centralized
running
average method. If half of the averaging window precedes the point in time and
half of
the time window follows the averaging window, the centralized running average
can be
defined by the following formula, where RA is the running average value, p is
the data
value, and n is the window sample number:
1 ,+n-1
RAi --~. pi
n a
[00175] FIG. 33B illustrates the result of running such an algorithm on
pressure data.
Graph 3620 includes a plot 3622 of raw data values that have not been
averaged. Also
shown on the graph 3620 are three plots 3624, 3626, 3628 which represent the
raw data
Page 60 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
following the application of the centralized running average algorithm. Plot
3624
corresponds to a running average calculated with a 10 second averaging window,
plot
3626 corresponds to a 30 second averaging window, and plot 3628 corresponds to
a 60
second averaging window. Other variations are possible in which the averaging
window
is not centered on the point of time for which the running average is being
calculated but
surrounds the data value in some other proportion. For example, the running
average for
a point in time can be calculated based on the data values in an averaging
window in
which one-quarter of the time window precedes and three-quarters of the
averaging
window follows the point in time. FIG. 33C shows an exemplary flow diagram
illustrating the above-described exemplary running average algorithm.
[00176] In other embodiments, data conditioning can be performed through a
variety of
statistical and/or mathematical calculations, including root mean square
calculations,
mean absolute deviation calculations, regression analyses to produce fitted
curves (both
linear and non-linear), crest factor and form factor calculations, and so on.
These
approaches can be performed on the parameter data values as described above
for the
running average calculations. The use of other statistical and/or mathematical
calculations can be chosen depending on the particular application. For
example, root
mean square calculations can be particularly advantageous in embodiments in
which the
data parameters produced by the distension device 22 have both positive and
negative
values (such as an electrical voltage).
[00177] The determination of a running average value, or any other value
resulting from
a conditioning calculation, also can trigger a variety of alarms or can be
recorded for
reports maintained by the local unit 60, remote monitoring device 170, and/or
the system
20. For example, an alarm or notification signal can be generated if the
running average
falls within a predetermined range, if it exceeds or falls below a threshold,
if it changes
too quickly (e.g., its rate of change exceeds a threshold), and so on.
Alternatively, the
occurrence of such events can be logged or stored for inclusion in a report or
log
produced by the local unit 60, remote monitoring device 170, and/or the system
20.
Page 61 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[00178] In some embodiments, analog filters can be employed in addition to or
as an
alternative to processing parameter data mathematically. A bank of analog
filters (or
selectable bank of such filters) can be included in one more devices for
removing noise,
or signals at undesired frequencies. For example, the conditioning and
filtering achieved
in the embodiment illustrated in FIGS. 32A-32E can be implanted via
appropriate low-
pass filtering. As one skilled in the art will understand, high-pass and band-
pass filtering
embodiments are also possible and depend on the desired results. The filters
can be
placed in a variety of locations, such as the injection port 36 (e.g., the
injection port 36
that serves as a communication link for the distension device 22), the local
unit 60, the
remote monitoring unit 170, or any other device in the signal path. In some
embodiments, placing the filters in the implant (such as the injection port 36
or in the
distension device 22) can be advantageous because by pre-conditioning the
information it
can reduce the bandwidth and/or power requirements needed for telemetrically
transmitting (or receiving) such data. In addition, by reducing the amount of
data through
analog filtering, the data processing requirements of the devices (for
example, the remote
monitoring device) in analyzing the data can be reduced.
[00179] Data processing algorithms also can be useful for determining baseline
levels of
a physiological parameter represented by the data collected from the
distension device
22. For example, the baseline pressure sensed by a fluid-filled distension
device 22 can
be determined from collected pressure values. A wide variety of methods to
determine a
baseline value can be used. However, in one exemplary embodiment, which is
illustrated
via FIGS. 34A-B, an algorithm for finding a baseline can involve collecting
data from a
distension device (box 3710 of flow diagram FIG. 34B) and calculating a
running
average value based on past data values (box 3712). The data used in the
running
average calculation can be defined by an averaging window (for example, an
averaging
window preceding the point in time for which a running average is being
calculated, or
covering a certain number of data values, e.g., the last ten values.) With the
collection of
each new data value, the running average can be updated. As shown in box 3714,
the
algorithm can determine whether a baseline value has been established by
comparing the
data values within the averaging window to a tolerance range, which can be
defined
Page 62 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
around the running average, to determine if all of the values (or,
alternatively, a portion
of them) were within the tolerance range. If so, at box 3716 the algorithm can
identify
the running average as the baseline value of the parameter. If not, at box
3718 additional
data values can be collected, which can involve the definition of a new
averaging
window, or the collection of a specified number of additional data values. A
new running
average can be computed, and the process repeated until a baseline value is
found. As
one skilled in the art will understand, any or all of the foregoing
thresholds, limits, times,
window sizes, or other variables can be user-defined. FIG. 34A shows a plot of
data
3700 which illustrates the foregoing algorithm applied to collected data, and
shows the
tolerance range 3702 and the averaging window 3704, in the context of pressure
values
measured over a time period 3706.
[00180] In some embodiments, the occurrence of specified events can initiate
an
algorithm to determine or search for a baseline value. For example, it can be
desirable to
check or determine whether a new baseline value exists at the start of data
collection, the
expiration of a timer, or after an adjustment is made to a distension device
22, which can
involve adding or removing fluid. FIG. 34C shows a plot of pressure data 3720
over a
time period which exhibits an upwards baseline shift 3722 due to the addition
of
approximately 7.5 ml to a fluid-filled distension device. The adjustment can
trigger the
execution of a baseline-determining algorithm, such as those described above,
to find the
new baseline value.
[00181 ] Another exemplary algorithm for determining or predicting baseline
levels of a
parameter is illustrated by FIGS. 35A-B. FIG. 35A shows an exemplary plot of
data over
time to illustrate application of the algorithm to a set of data and FIG. 35B
shows an
exemplary flow diagram. In this embodiment, the algorithm generally can
involve
calculating when the rate of change of the parameter values will be zero or
substantially
near zero, and what the parameter value will be at that time. A rate of change
that is zero
or substantially near zero can be treated as indicating that the baseline
value has been
reached. More specifically, with reference to boxes 3802, 3804 and FIG. 35B,
the
algorithm can include collecting parameter data values over a time period, and
Page 63 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
calculating a rate of change at a point of time or for a group of data values
(group A) in a
time window 3820 within the time period. For example, the rate of change can
be
determined by a slope calculation defined by dParameterA With reference to box
3806, the
dtimeA
algorithm can further include calculating how fast the rate of change is
itself changing-
in other words, the rate at which the rate of change is changing.. The rate at
which the
rate of change is changing can be determined for example, by executing two
slope
calculations (e.g., group A in window 3820 and group B in window 3822), and
then
calculating the change in slopes. The windows 3820, 3822, can be defined by
time (a
time window) or by a group of data values, or in any other way suitable for
selecting a
portion of data values. For example:
Slope A A = dParameterA
Slope
Slope B = dParameterB
dtimeB
ASlope = SlopeB - SlopeA
[00182] Furthermore, the rate of change and how fast the rate of change is
itself
changing can be used to determine when the rate of change will be about zero,
and what
the value of the parameter will be at that time. For example, as indicated in
box 3808, the
time needed to reach a rate of change of about zero (which in this example
indicates that
the baseline value has been reached) can be predicted according to the
following formula:
Time to Baseline = SlopeB * PeriodB
ASlope
[00183] The predicted baseline value can be calculated by extrapolation using
a
parameter value and the amount the parameter will change until the Time to
Baseline, as
shown by the following formula:
Baseline Value = (Time to Baseline)*(SlopeB) + (Parameter Value in Group B)
Page 64 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[00184] As one skilled in the art will understand, the foregoing approach can
be varied
widely, without departing from the scope of the technique described herein.
For
example, the Time to Baseline and Baseline Value formulas can be cast in terms
of Slope
A and Period A as well, more than two data windows can be used, and/or the
spacing
between data windows 3820, 3822 can be modified. Further, one skilled in the
art will
understand that the foregoing approach can be described in terms of a
derivative (for
example, to represent a rate of change) and a second derivative (for example,
to represent
a rate at which the rate of change it itself changing).
[00185] The determination of a baseline value can trigger a variety of alarms
or can be
recorded for reports maintained by the local unit 60, remote monitoring device
170,
and/or the system 20. For example, an alarm or notification signal can be
generated if the
baseline pressure exceeds or falls below a threshold (for example, for a
specified time
period), when there is a fluctuation in baseline pressure, when a baseline
cannot be found
after a specified time, when rate of change of the pressure exceeds a
threshold value,
and/or when the baseline pressure is determined. Alternatively, the occurrence
of such
events can be logged or stored for inclusion in a report or log produced by
the local unit
60, remote monitoring device 170, and/or the system 20. In addition, the
baseline value
can be correlated (either alone or in conjunction with other data, as
described herein) to
the condition of the distension device. The baseline value can indicate an
over-tightened,
optimally-tightened, or under-tightened distension device, which for a fluid-
Tillable
distension can represent an over-filled, optimally-filled, or under-filled
condition. For
example, a baseline value that exceeds a predetermined threshold (e.g., a
level considered
to be "too high") can be indicative of an over-filled or over-tightened
distension device,
while a baseline value that falls or remains below a predetermined threshold
(e.g., a level
considered to be "too low") can be indicative of an under-filled or loose
distension
device, and so on. Predetermined thresholds can be obtained using historical
patient data,
group data, or other clinical data. Also, in other embodiments, the rate of
change of the
pressure (as described above with respect to baseline determinations) can be
correlated to
the condition of the distension device. For example, a rate of change that
exceeds a
predetermined rate of change can indicate an over-filled fluid-Tillable
distension coil. A
Page 65 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
rate of change that falls below another threshold can indicate an under-filled
distension
coil.
[00186] Data values collected by the data logger 270 can be used to obtain
information
about physiological parameters of a patient wearing a distension device 22.
For example,
as previously mentioned, the data logger 270 can collect data representing
pressure (or
other parameter) sensed by an implanted distension device 22. Information
about
physiological parameters such as heart rate, breathing rate, and others, can
be determined
from the collected pressure values (or values of another parameter).
Information about
peristaltic or swallowing events, which can manifest themselves as pulses or a
series of
pulses in pressure, can also be determined, and such information can include
the number,
rate, and duration of such pulses. As shown in FIGS. 36A-B, multiple
frequencies can
exist in a set of pressure data (or other data). As shown in FIG. 36A,
relatively high
frequency pulses 3904, which in FIG. 36A represent pressure changes caused by
heartbeats (the heartbeat can exert a detectable force on the distension
device 22), can be
superimposed on low-frequency pulses 3902, which in FIG. 36A represent
swallowing
events. FIG. 36B shows heartbeat pulses 3906 superimposed on pulses 3908
caused by
breathing. As shown the breathing pulses are occurring about once every four
seconds.
[00187] In one exemplary embodiment, the frequency content of pressure data
can be
analyzed. Frequency or frequencies in the data can be selected and identified
as the
frequency of a physiological parameter of interest, for example by comparing
the
frequency to a range of frequencies which are designated as the possible range
for the
particular physiological parameter. The amplitude, or other characteristics of
the
physiological parameter also can be determined by extracting or filtering the
data at the
selected frequencies. A variety of techniques can be used to analyze and
extract
information having a desired frequency content. The following examples refer
to FIGS.
36A-C and sometimes use heart rate as an exemplary physiological parameter,
but as one
skilled in the art will understand, a variety of periodic physiological
parameters can be
analyzed, and data other than pressure data can be used.
Page 66 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
[00188] As illustrated in FIG. 36C, one exemplary algorithm can involve
calculating the
period of pulses or variations in the data values representing the sensed
parameter. With
reference to box 3920, a local maximum or minimum in the data can be
identified, e.g.,
by determining when the slope changes passes through zero. The time can be
recorded at
that point (box 3922), and again at a subsequent maximum or minimum (box
3924). The
period can be calculated based on the time between adjacent maxima and/or
minima, and
this period can be examined to see if it falls within a designated target
range of possible
frequencies associated with the physiological parameter of interest. For
example, a heart
rate might be associated with a frequency of 65 to 150 beats or cycles per
minute, or
about 1.1 to 2.5 Hz. The range can be defined by the device, or user-defined.
If the
calculated frequency falls within the range, at box 3926 the frequency can be
identified or
designated as the frequency of the physiological parameter. In some
embodiments, the
algorithm can include comparing the magnitude of the values at the maxima or
minima to
ensure that they are within a tolerance range of one another. As can be seen
with
reference to FIG. 36A, such an approach can enable the maximum, or peak, of a
swallowing pulse to be distinguished from the maximum or peak of a heart rate
pulse.
Distinguishing between the two can determine the appropriate maxima to use in
calculating the frequency for a particular physiological parameter. In some
embodiments, the value of the parameter at the maximum or minimum also can be
used
to calculate the amplitude of the pulses, and the algorithm can also include
comparing the
amplitude to a predetermined target range associated with the physiological
parameter to
see if it whether it falls within the range. For example, heart rate pulses
can have an
amplitude of about 7-8 mmHg, as shown in FIG. 36B, and a range can be size to
include
at least 7-8 mmHg. As one skilled in the art will understand, the target
frequencies and
amplitudes described above will vary depending on the physiological parameter
about
which information is sought.
[00189] As illustrated in FIG. 36D, in another exemplary embodiment, a
discrete Fourier
transform (in many cases, computed by fast Fourier transform) can be applied
to data
values of a sensed parameter that were logged over a time period. The data
values can
thereby be transformed from time domain values to the frequency domain. The
Page 67 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
frequency content of the data values can be examined to identify a frequency
or
frequencies that exist in the data values that corresponds to a range of
frequencies
associated with a physiological parameter range. In some embodiments, the
frequency
content can be examined to identify one or more frequencies that exist and
exceed a
magnitude threshold, and that correspond to a range of frequencies associated
with a
physiological parameter. If multiple frequencies exist in the range, the
frequency with
the largest magnitude can be selected, or a weighted average of the
frequencies can be
computed, and designated as the frequency of the physiological parameter. The
amplitude can be given by the Fourier coefficients of the identified
frequencies.
Alternatively, frequencies not falling within the target range can be removed
from the
data (for example, by setting the Fourier coefficients of unselected
frequencies to zero),
and the values of the sensed parameter in the time domain can be reconstructed
by
performing an inverse Fourier transform. The data values in the time domain
can be
displayed or analyzed further, e.g., analyzing the amplitude by comparing the
values at
the maxima and minima, etc.
[00190] FIGS. 37A-C illustrate the output of another algorithm which can
extract
information about a physiological parameter from the value of a sensed
parameter (such
as pressure) from a distension device 22 and collected by the data logger 270,
and FIG.
37D shows an exemplary flow diagram of such an algorithm. In this exemplary
embodiment, values of a sensed parameter, such as pressure values 4002, can be
averaged
to create average values 4004. In many embodiments, the average can be
calculated by
averaging the values falling within a averaging window within a time period,
e.g., taking
the average of every X seconds of data values, or computing the average of a
defined
number (a data group) of surrounding data values. The size of the averaging
window can
vary widely, and can be informed by the relationship between the phenomena of
interest.
For example, as shown in FIG. 37A, pressure values have been collected at a
rate of
about 100 Hz, while swallowing events can occur at about 0.1 Hz, and the
average 4004
has been calculated and plotted by averaging every 100 data values, e.g.,
falling within
window 4008. The average values 4004 can be subtracted from the original data,
e.g., the
pressure values 4002 in this example, to produce physiological parameter
values 4006,
Page 68 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
such as values representing heart rate, breath rate, and so on. These
physiological
parameter values 4006 can be displayed. In addition, the frequency, amplitude,
volatility,
or other characteristics of the physiological values 4006 can be further
analyzed, for
example using one or more of the previously described algorithms. The
foregoing
average-and-subtract technique can be repeated on the physiological data 4006
(e.g., with
a smaller averaging window) to extract another set of physiological values
therefrom (for
example, the pulse values can be separated from the breath rate values, then
the breath
rate values can be separated from the heart rate values).
[00191] FIGS. 37B illustrates another set of exemplary pressure values 4010
and average
values 4012 calculated therefrom. The averaged data 4012 also can be useful
for
analyzing physiological phenomena, such as relatively low-frequency phenomena
and/or
swallowing rates. FIG. 37C illustrates physiological values that can be
obtained by
taking the difference between the exemplary pressure values 4010 and the
average values
4012.
[00192] FIGS. 38A-C show another exemplary dataset which illustrates how
pressure
data can be differentiated to reveal information about various physiological
responses.
As shown in FIG. 38A, pressure values 4100 collected over a time period can be
used to
examine the total duration (e.g., examining amplitude and number of pulses) of
a
swallowing event or peristalsis represented by a series of pulses 4102, a
single pulse 4104
from a peristaltic event , and/or superimposed or minor pulses 4106
representing other
physiological parameters. FIG. 38B shows the single pulse 4104 in more detail.
As
shown, a smooth curve can be used (e.g., by calculating an average value) to
analyze the
amplitude, duration, or other characteristics of the pulse 4104. FIG. 38C
shows the minor
pulses 4106 in more detail, which can be converted to a linear (e.g., by one
of the
previously described approaches), as shown under arrow 4108, to measure
frequency,
amplitude or other characteristics.
[00193] The determination of a physiological rate, amplitude or other
parameter can
trigger a variety of alarms or can be recorded for reports maintained by the
local unit 60,
rPmntP mnõitnriõa device 170, and/or the system 20. For example, an alarm or
Page 69 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
notification signal can be generated if the heart rate or breathing rate (or
other rate) is too
high, too low, cannot be detected, is changing drastically (e.g., has a rate
of change that
exceeds a threshold), and so on. Alternatively, the occurrence of such events
or
conditions can be logged or stored for inclusion in a report or log produced
by the local
unit 60, remote monitoring device 170, and/or the system 20.
[00194] A wide variety of algorithms can be used to detect the presence of
pulses in
pressure values or other data values collected by the data logger 270. One
exemplary
embodiment of such an algorithm is illustrated in FIGS. 39A-B. FIG. 39A shows
a plot
4200 of exemplary pressure values over a time period, although any parameter
values can
be used. FIG. 39B shows a flow diagram illustrating exemplary steps of an
algorithm.
As shown, a predetermined threshold value 4202 can be defined relative to the
baseline
value 4212 (boxes 4222, 4224 of FIG. 39B). (For example, the threshold value
can be set
to be 10 mmHg above the baseline value 4212.) At box 4226, the algorithm can
determine the time 4206 at which the parameter value exceeds the threshold
value 4204.
(As the threshold value 4202 can be relative to the baseline value 4212, in
absolute terms,
the time 4206 at which the parameter value exceeds the threshold value 4202
can occur
when the parameter exceeds the baseline value 4212 plus the threshold value
4202.) If
the parameter value decreases such that it no longer exceeds the threshold
value 4202
within a predetermined time 4210, a pulse can be said to have occurred (boxes
4228-
4230). The predetermined time 4210 also can be user-defined.
[00195] FIG. 40A illustrates the application of an alternative embodiment of
an
algorithm that can be used to detect the presence of a pulse to a set of data,
and FIG. 40B
shows an exemplary flow diagram for such an algorithm. As shown, a first
threshold
value 4302 and a second threshold value 4304 can be defined (boxes 4324a,
4324b), both
defined relative to the baseline value 4308, as discussed with respect to
FIGS. 39A-B.
The first threshold value 4302 can apply when the parameter is increasing (for
example,
before the peak of the pulse) and the second threshold 4304 can apply when the
parameter is decreasing (for example, after the peak 4312). At box 4326, the
algorithm
can determine the time 4314 at which the parameter value exceeds the first
threshold
Page 70 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
value 4302. If the parameter value then falls below the second threshold 4304
within a
predetermined time 4306, a pulse can be said to have occurred (boxes 4328-
4330).
[00196] FIG. 41A illustrates the application another alternative embodiment of
an
algorithm that can be used to detect the presence of a pulse in a set of data,
and FIG. 41B
shows an exemplary flow diagram for such an algorithm. In this embodiment, a
first
threshold 4402 can be defined relative to the baseline value 4408, and a
second threshold
4404 can be defined relative to a peak value 4412 (boxes 4424a-b in FIG. 41B).
The
time 4414 at which the parameter exceeds the first threshold 4402 and the time
4412 at
which the parameter reaches a peak (for example, when it has a zero slope) can
be
recorded (boxes 4426, 4428a-b). If the parameter value falls below the second
threshold
4404 within a predetermined time 4406, then a pulse can be said to have
occurred (boxes
4430, 4432). In many embodiments, the second threshold 4404 can be defined as
a
proportion of the peak value 4412 (e.g., 75% of the peak value), which the
algorithm can
then compute when it finds a peak value 4412. In other embodiments, the second
threshold 4404 can be defined directly (e.g., 10 mmHg below the peak value
4412).
[00197] An algorithm for finding a pulse can also trigger a variety of alarms
or can
record pulse events for reports maintained by the local unit 60, remote
monitoring device
170, and/or the system 20. For example, an alarm or notification signal can be
generated
when a pulse is detected, when no pulse can be detected, when a pulse appears
during
certain times (such as outside meal times), when a pulse count exceeds a
threshold value,
when pulses are detected for a specified period of time, when the rate of
change pressure
indicates either a start of a pulse or an end of a pulse, and so on.
Alternatively, the
occurrence of such events can be logged or stored for inclusion in a report or
log
produced by the local unit 60, remote monitoring device 170, and/or the system
20. In
addition, the determination that one or more pulses has occurred can be
correlated (either
alone or in conjunction with other data, as described herein) to the condition
of the
distension device. For example, if pulses continue to occur over a time period
(e.g.,
during a predetermined time period, in some cases such as 5-6 minute window,
although
any time period is possible) can indicate that the distension device is over-
filled or too
Page 71 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
tight. The amplitude of the pulses and the time between pulses (either taken
alone, or in
conjunction with other metrics) can also be used or involved in this
determination, e.g.,
pulses of a threshold amplitude can be considered. In other embodiments, the
number of
pulses in a sequence, or the number of pulses within a time period, can be
used to make a
correlation. Also, the absence of pulses over a predetermined time period can
indicate
that the distension device is too loose or under-filled. Such pulse analysis
can further
involve giving water/food swallows or dry swallow instructions to a patient
who is
wearing a distension coil and monitoring the resulting pulse(s), either to
determine an
appropriate predetermined time period to watch for pulses, to assess the
condition of the
distension device, or otherwise.
[00198] The area under a pulse, or sequence of pulses or other waveform, in
parameter
vs. time data can be used for analytical purposes. FIG. 42A shows an exemplary
plot
4500 of pressure over a time period; FIG. 42B shows a flow diagram
illustrating an
exemplary algorithm for making such an analysis. As shown, the values of the
pressure
are represented by a graphical representation 4502, in this case a waveform,
which
exhibits a series of pulses. The areas under one or more pulses can be
evaluated. The
areas can be calculated by evaluating an integral for each pulse over a
window, such as
time windows 4512, 4514, 4516, 4518. The areas can be calculated with
reference to a
baseline value 4510 or to a zero value. In many embodiments, the window can be
sized
to cover the time of the pulse, for example, by beginning the window when the
parameter
value exceeds a threshold, and ending it when the parameter value falls below
that
threshold value, or by using any of the times discussed in connection with
FIGS. 42-44,
such as times T2 - Ti illustrated in FIG. 40B or Peak Time - Ti in FIG. 41B.
The
results of the integrals can be compared, and the nature of sequence of areas
(increasing,
decreasing, etc.) as well as their magnitude can be correlated to conditions
or events
related to the distension device 22, the patient, and so on. For example, the
presence of
pulses with substantially equivalent areas, generally indicated by bracket
4506 in FIG.
45, can be indicative of a fluid-filled distension device that is overfilled,
or generally a
distension device that is too tight. The presence of pulses with decreasing
areas, or areas
decreasing at a predetermined rate, generally indicated by bracket 4508, can
be indicative
Page 72 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
of an optimally filled or adjusted coil. The decrease of such areas at a
second
predetermined rate (for example, a rate higher than that associated with an
optimally
filled coil) can be correlated to an underfilled distension device. The
presence of a single
pulse without any peaks following, as generally indicated by bracket 4504, can
be
indicative of a distension device that is underfilled, or of coughing or
talking.
[00199] It should be understood that any or all of the foregoing algorithms
and
techniques can be integrated with a graphical user interface to allow a user
to provide
input to the algorithm and to display results, both intermediate and final
results. For
example, plots of pressure over time can be displayed to a user, and the user
can
manually define or select windows for averaging, slope calculations, or for
calculating
the area of a pulse (e.g., by manually marking beginning and ending times). In
other
embodiments, the user can manually mark the baseline value by adjusting a
horizontal
line on the display after viewing pressure values for a timed period. Such
variations are
intended to be within the scope of this disclosure.
[00200] It will be appreciated that several embodiments described herein may
enable
health care providers or others to use pressure data as a feedback mechanism
to identify,
train, and/or prescribe dietary advice to a patient. Such a feedback mechanism
may
provide data or otherwise be used in multiple ways. For instance, pressure
feedback may
be obtained when a patient swallows a particular food portion, and based on
such
pressure feedback, the patient may be taught to eat smaller portions, larger
portions, or
portions equal to the portion tested. Of course, a food portion so prescribed
may be tested
by evaluating pressure feedback obtained when the patient swallows the
prescribed food
portion, such that a food portion prescription may be refined through
reiteration. As
another example, a patient may test desired foods for appropriateness based on
pressure
feedback together with portion size and/or based on any other parameters. It
will also be
appreciated that continuous pressure data monitoring may be used to enable
portion size
monitoring, food consistency monitoring (e.g., liquids vs. solids) and/or
eating frequency.
Still other ways in which pressure data may be used to provide dietary advice
will be
Page 73 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
apparent to those of ordinary skill in the art. It will also be appreciated
that such uses may
be practiced locally, remotely (e.g., via remote unit 170), or combinations
thereof.
[00201] While data logging system 300 is described herein as being implemented
with
injection port 36, it will be appreciated that data logging system 300 may
alternatively be
implemented with any other type of pressure sensing system or other implanted
systems.
By way of example only, data logging system 300 may be combined with any of
the
pressure sensing devices disclosed in U.S. Patent Publication No. 2006-0211914
(Application Serial No. 11/369,682), filed Mar. 7, 2006, and entitled "System
and
Method for Determining Implanted Device Positioning and Obtaining Pressure
Data,"
and U.S. Patent Publication No. filed March 6, 2007, and U.S. Non-Provisional
Patent
Application 11/682,459, entitled "Pressure Sensors for Gastric Band and
Adjacent
Tissue" (Attorney Docket No. END6042USNP and attached hereto as an Appendix),
the
disclosures of both of which are incorporated by reference herein for
illustrative
purposes. For instance, data logging system 300 may receive pressure
measurements
obtained by any of the pressure sensors described in that patent application.
In addition,
the needle guidance sense head described in that patent application may be
used with at
least a portion of data logging system 300 to provide needle guidance for a
local clinician
to adjust fluid pressure in accordance with a remote physician's instructions
that are based
on pressure measurements obtained by the needle guidance sense head and
communicated to the remote physician in substantially real-time. For instance,
the needle
guidance sense head may be coupled with data logger 370, which may connected
directly
to the Internet (or via docking station 360) to provide pressure measurements
to the
remote physician. Still other ways in which devices and components described
herein
may be combined with components described in U.S. Patent Application
Publications US
2006-0211912, US 2006-0211913, and US 2006-0211914, hereby incorporated by
reference, will be apparent to those of ordinary skill in the art.
[00202] Any of the devices disclosed herein can also be designed to be
disposed of after
a single use, or they can be designed to be used multiple times. Devices which
can be
external, such as the local unit, remote monitoring device, data loggers, and
so on, are in
Page 74 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
many cases suitable for reuse. Devices can be reconditioned or reconstructed
for reuse
after at least one use. Reconditioning or reconstructing can include any
combination of
the steps of disassembly of the device, followed by replacement, upgrade,
cleaning, or
modification of particular pieces (including mechanical components, computer
hardware
and software, and so on) and subsequent reassembly. In particular, the device
can be
disassembled, and any number of the particular pieces or parts of the device
can be
selectively replaced or removed in any combination. The device can be
reassembled for
subsequent use either at a reconditioning facility, or by a physician before
using the
device with a patient. Those skilled in the art will appreciate that
reconditioning or
reconstructing of a device can utilize a variety of techniques for
disassembly, cleaning
and/or replacement, and reassembly. Additionally, repairs can be made to
devices and/or
to their individual parts or pieces. Use of such techniques, and the resulting
reconditioned, reconstructed, or repaired device, are all within the scope of
the present
application.
[00203] The devices described herein, particularly including but not limited
to those
devices that can be implanted in or attached to a patient, preferably can be
processed or
sterilized before use. First, a new or used device (or part thereof) is
obtained. The device
can then be sterilized. In one sterilization technique, the device is placed
in a closed and
sealed container, such as a plastic or TYVEK bag. The container and device are
then
placed in a field of radiation that can penetrate the container, such as beta
or gamma
radiation, x-rays, or high-energy electrons. The radiation kills bacteria on
the instrument
and in the container. The sterilized instrument can then be stored in the
sterile container.
The sealed container keeps the instrument sterile until it is opened in a
medical facility.
In other embodiments, ethylene oxide, or steam can be used for sterilization.
[00204] Any patent, publication, application or other disclosure material, in
whole or in
part, that is said to be incorporated by reference herein is incorporated
herein only to the
extent that the incorporated materials does not conflict with existing
definitions,
statements, or other disclosure material set forth in this disclosure. As
such, and to the
extent necessary, the disclosure as explicitly set forth herein supersedes any
conflicting
Page 75 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
material incorporated herein by reference. Any material, or portion thereof,
that is said to
be incorporated by reference herein, but which conflicts with existing
definitions,
statements, or other disclosure material set forth herein will only be
incorporated to the
extent that no conflict arises between that incorporated material and the
existing
disclosure material.
[00205] While preferred embodiments of the present invention have been shown
and
described herein, it will be obvious to those skilled in the art that such
embodiments are
provided by way of example only. Numerous variations, changes, and
substitutions will
now occur to those skilled in the art without departing from the invention.
For example,
as would be apparent to those skilled in the art, the disclosures herein have
equal
application in robotic-assisted surgery. In addition, it should be understood
that every
structure described above has a function and such structure can be referred to
as a means
for performing that function. Accordingly, it is intended that the invention
be limited only
by the spirit and scope of the appended claims.
[00206] While the present invention has been illustrated by description of
several
embodiments, it is not the intention of the applicant to restrict or limit the
spirit and scope
of the appended claims to such detail. Numerous other variations, changes, and
substitutions will occur to those skilled in the art without departing from
the scope of the
invention. For instance, the device and method of the present invention has
been
illustrated with respect to transmitting pressure data from the implant to the
remote
monitoring unit. However, other types of data may also be transmitted to
enable a
physician to monitor a plurality of different aspects of the distension
implant.
Additionally, the present invention is described with respect to a stomach
distension
device for bariatric treatment. The present invention is not limited to this
application, and
may also be utilized with other distension implants or artificial sphincters
without
departing from the scope of the invention. The structure of each element
associated with
the present invention can be alternatively described as a means for providing
the function
performed by the element. It will be understood that the foregoing description
is provided
Page 76 of 81
CA 02749859 2011-07-14
WO 2010/083498 PCT/US2010/021340
by way of example, and that other modifications may occur to those skilled in
the art
without departing from the scope and spirit of the appended Claims.
Page 77 of 81