Note: Descriptions are shown in the official language in which they were submitted.
CA 02751228 2011-08-31
-1-
Method for Producing Thin Silicon Rods
The invention relates to a method for producing thin
silicon rods.
Thin silicon rods are used for the deposition of
polycrystalline silicon.
Polycrystalline silicon (abbreviation: polysilicon) is
used as a starting material for the production of
monocrystalline silicon by means of crucible pulling
(Czochralski or CZ method) or by means of zone melting
(float zone or FZ method). This monocrystalline silicon
is cut into wafers and, after a multiplicity of
mechanical, chemical and chemical-mechanical processing
operations, used in the semiconductor industry to
fabricate electronic components (chips).
In particular, however, polycrystalline silicon is
required to an increased extent for the production of
monocrystalline or polycrystalline silicon by means of
pulling or casting methods, this monocrystalline or
polycrystalline silicon being used to fabricate solar
cells for photovoltaics.
The polycrystalline silicon, often also abbreviated to
polysilicon, is conventionally produced by means of the
Siemens process. In this case, thin rods of silicon are
heated by direct passage of current in a bell-shaped
reactor ("Siemens reactor") and a reaction gas
comprising a silicon-containing component and hydrogen
is introduced.
The thin silicon rods conventionally have an edge
length of from 3 to 15 mm.
As components containing silicon, for example silicon-
halogen compounds such as silicon-chlorine compounds,
in particular chlorosilanes, are suitable. The
ak 02751228 2011-08-31
- 2 -
component containing silicon is introduced together
with hydrogen into the reactor. At temperatures of more
than 1000 C, silicon is deposited on the thin rods.
This finally provides a rod comprising polycrystalline
silicon. DE 1 105 396 describes the basic principles of
the Siemens process.
With respect to the production of thin rods, it is
known from DE 1 177 119 to deposit silicon on a support
body made of silicon (= thin rod), then separate a part
thereof and in turn use this separated part as a
support body for the deposition of silicon. The
separation may be carried out mechanically, for example
by means of sawing, or electrolytically by means of a
liquid jet.
During the mechanical separation of thin rods, however,
their surface becomes contaminated with metals as well
as with boron, phosphorus, aluminum and arsenic
compounds. The surface contamination with metals is for
instance up to 90,000-160,000 pptw (parts per trillion
by weight) after mechanical separation. The average
pollution with B, P, Al and As lies in the range of
from 60 to 700 ppta (parts per trillion atomic).
It is therefore usually necessary to subject the thin
rods to surface cleaning before they can be used for
the deposition of silicon. In this regard, DE 1 177 119
discloses mechanical cleaning, for example by
sandblasting, or chemical cleaning by etching.
By treating the thin rods in an etching tank made of
low-contamination material, for example plastic, by
means of a mixture of HF and HNO3, the surface
contaminations can be reduced significantly: in the
case of metals to as low as 300 pptw or less, and in
the case of B, P, Al and As to less than 15 pptw.
CD, 02751228 2011-08-31
- 3 -
EP 0 548 504 A2 describes a cleaning method in which HF
and HNO3 are used to clean silicon.
Another cleaning method is known from DE 195 29 518 Al.
In this case, polycrystalline silicon is first cleaned
with a mixture of aqua regia (mixture of HC1 and HNO3)
and then is subjected to additional cleaning with HF.
EP 0 905 796 Al discloses a method for producing
semiconductor material which has a low metal
concentration, characterized in that polycrystalline
silicon is washed in precleaning in at least one stage
with an oxidizing cleaning solution, is washed in main
cleaning in a further stage with a cleaning solution
which contains HNO3 and HF, and during hydrophilization
in yet another stage is washed with an oxidizing
cleaning liquid. By this cleaning method, the iron
and/or chromium content on the surface of the silicon
can be reduced from 1.332 x 10-8 g/cm2 (after processing
with a metal tool) to less than 6.66 x1011 g/cm2.
In order to increase the yield in the silicon
deposition, it would also be desirable to be able to
use longer thin rods. Longer thin rods can in principle
be produced by welding shorter thin rods.
WO 02/070184 Al describes a method in which two silicon
workpieces are joined together crack-free by means of
welding. First, the workpieces are heated to a
temperature of at least 600 C, preferably on a heating
plate made of silicon. The workpieces are then joined
together, for example by means of electrical, plasma or
laser welding.
For thin workpieces, however, this method is difficult
to operate. Furthermore, the silicon workpieces are
constantly in direct contact with air during the
CA 02751228 2011-08-31
- 4 -
welding, which is detrimental in respect of
contamination.
US 6,573,471 B1 likewise describes a method by which
two silicon workpieces can be joined together by
welding. The essential difference from the method
according to WO 02/070184 Al is that a reduced pressure
of at most 0.05 Torr is set up before the two
workpieces are joined.
US 6,852,952 Bl describes a method in which two silicon
workpieces are joined together by means of arc welding.
To this end, a plasma is generated between two
electrodes and the silicon workpieces to be joined are
brought into proximity therewith. This is preferably
done in an argon atmosphere.
The method according to US 6,852,952 Bl is however also
elaborate, and disadvantageous for the welding of thin
rods.
Another conceivable method involves induction welding.
By means of this, plastic and metal parts are
conventionally welded in an air atmosphere.
The use of induction welding to join silicon workpieces
would lead to the formation of an SiN layer, since
silicon reacts with nitrogen from the ambient air owing
to the high temperatures of more than 1500 C. Since SiN
does not dissolve in a silicon melt and, as particles,
leads to dislocations in the single crystal, the use of
such polycrystalline silicon is not suitable for the
production of silicon single crystals by means of
crucible pulling or zone melting.
For longer thin rods, the currently available etching
tanks constitute a further problem.
CA 02751228 2012-11-29
- 5 -
This is because the size of the etching tanks for
cleaning systems made of pure plastic is design-
limited. Beyond a certain dimension of the etching
tank, the system becomes unstable. Additional steel
struts could permit enlargement of the etching tanks.
However, the use of steel is critical since it is not
possible to preclude the possibility of acid escaping
from the etching tank in the vicinity of the steel
struts owing to stress cracks, and the acid becoming
contaminated with metals.
It was therefore an object of the invention to avoid
the disadvantages described above and to improve the
prior art.
The object is achieved by a method for producing thin
silicon rods (1), comprising the steps:
a) providing a rod of polycrystalline silicon, from
which at least two thin rods (11, 12) with a reduced
cross section in comparison with the polycrystalline
silicon rod are separated;
b) cleaning the at least two separated thin rods (11,
12) by treatment with a material-eroding liquid medium;
c) welding at least two of the cleaned thin rods (11,
12) to form a longer thin rod (1);
d) packaging the longer thin rod (1) in a tubular film
(100).
The starting point of the method is a rod of
polycrystalline silicon, produced by depositing silicon
on a thin rod, preferably by means of the Siemens
process.
CD, 02751228 2011-08-31
- 6 -
This rod of polycrystalline material is cut into thin
rods. Preferably, the separation of the thin rods is
carried out mechanically by means of sawing.
The separated thin rods are then chemically cleaned.
Preferably, precisely one cleaning step is carried out
before the welding of the thin rods.
This cleaning step is preferably carried out in a
cleanroom of cleanroom class 100 or lower (according to
US FED STD 209E, superseded by ISO 14644-1).
In class 100 (ISO 5), at most 3.5 particles with a
maximum diameter of 0.5 pm may be contained per liter.
The chemical cleaning is preferably carried out by
means of an HF/HNO3 mixture.
The thin rods are then welded.
The welding of the cleaned thin rods is preferably
carried out in an inert gas.
The welding is preferably carried out by means of an
induction method.
The invention will also be explained below with the aid
of figures.
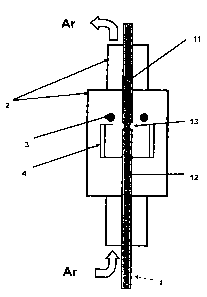
Fig. 1 schematically shows the way in which two thin
rods are welded.
Fig. 2 schematically shows the way in which a welded
thin rod is processed in an etching tank.
List of References Used
CA 02751228 2011-08-31
- 7 -
I welded thin rod
11 first thin rod
12 second thin rod
13 welded joint
2 quartz tube
3 induction coil
4 carbon tube
5 etching container/tank
51 opening
52 opening
6 etching liquid
7 trough
8 pump
81 line
9 drying unit
100 film tube
The welding of the short thin silicon rods 11 and 12 is
carried out in a device in which the two thin rods 11
and 12 are first brought in contact in a protective gas
(particularly preferably argon).
An induction coil 3 heats the two ends of the rods 11
and 12 to above the melting temperature of silicon
(> 1412 C) and a drop of liquid silicon is formed,
which is held in shape by surface tension. After at
most 4 to 5 minutes, the silicon on the ends of the two
rods becomes liquid and the induction coil 3 is
switched off. The two rods 11 and 12 fuse together.
An induction coil 3 is placed over a quartz-
encapsulated tube 4 of carbon (graphite).
The alternating field generated in the induction coil 3
is first coupled into the tube 4 consisting of carbon
and heats it. The thermal radiation subsequently heats
the silicon rods. Beyond a certain temperature, the
alternating field can also be coupled directly into the
CA 02751228 2011-08-31
- 8 -
silicon and heats it further. The actual welding
process can now be started.
Temperatures greatly in excess of 1000 C are set up in
the carbon tube 4. It is therefore necessary to ensure
that this tube is shielded from the external air. It is
expediently encapsulated in quartz. In order to shield
the hot silicon from the ambient air as well, the
entire device is enclosed by a quartz tube 2. Quartz
has, on the one hand, the property that it withstands
high temperatures. On the other hand it is transparent,
so that it makes it possible to observe the welding
process.
The high temperatures inside the quartz tube 2 lead to
a comparatively strong convective flow from the bottom
upward.
If special measures are not implemented here, ambient
air will be sucked in and conveyed to the welding site.
This, however, would entail two disadvantages:
- additional pollution of the welding site,
and
- chemical reactions with the air (nitrogen and
oxygen).
The reaction with nitrogen, in particular, is to be
avoided under all circumstances since the reaction
forms SiN which would cause problems during the
subsequent crystal pulling process. The quartz tube is
therefore supplied from below with a protective gas
(noble gas, argon).
Argon is particularly preferred as a protective gas. In
principle, however, other inert gases may also be used.
CA 02751228 2011-08-31
- 9 -
The protective gas can escape again at the upper
opening. The convective flow, which is caused by the
high temperature of the silicon, ensures that the
ambient air essentially does not come in contact with
the hot silicon.
The welded thin rods are subsequently packaged in
tubular bags 100.
The packaging of the welded thin rods is preferably
carried out in a tubular film of ultrapure PE. The bags
used ideally consist of highly pure PE with a thickness
of from 40 to 100 pm.
During the welding process, the Si surface is easily
contaminated with impurities over the entire thin rod
length.
It has been found that thin rods which are obtained by
this method can be used both to produce polysilicon for
the semiconductor industry (CZ) and for the solar
industry.
Polycrystalline silicon which is deposited by
deposition on thin rods produced in this way can also
be processed further by the zone melting method (FZ) to
form single crystals.
The pulling yield for a resistance of less than 1000
ohm.cm is
however only less than 50% owing to the
impurities which are still present, which is
disadvantageous.
Since high-impedance material is increasingly
necessary, however, it is preferable to increase the
yield. In order to achieve this, it is necessary to
reduce the concentration of metals on the Si surface
CA 02751228 2011-08-31
- 10 -
and in the bulk of the thin rod being used, from about
1012 at/cm2 to about 1011 at/cm2.
It is known of impurities such as iron, copper and
nickel that they drastically reduce the lifetime of the
minority charge carriers in silicon. This has negative
consequences both for the use of such a material in
semiconductor applications (in which case additional
getters for metals must then be used) and in solar
applications (the lifetime then has a major influence
on the efficiency of the solar cell).
An additional cleaning step is therefore preferably
carried out immediately before the packaging.
This additional cleaning step is also preferably
carried out in a cleanroom with a cleanroom class of
100 or lower.
The second chemical cleaning is also preferably carried
out by means of an HF/HNO3 mixture.
If the welded thin rods are cleaned once more after the
welding, then the impurities which have accumulated on
the silicon surface of the thin rod during the welding
can be removed.
Table 1 shows the surface contamination with metals in
pptw after the welding without a second cleaning step.
Table 1
CA 02751228 2011-08-31
¨ 11 -
_____________________________________________________________________ Fe Cr
Ni Na Zn Al. Cu Mo Ti W K Co Mn Ca MV Ag
25%
Quantile 2017 43 138 2908 938 976 77 2 124 14 1707 7 34 3849 728 2 11
Median 2622 98 170 4428 2166 1260 110 5 218 21 2395 9 52 4379 958 3 15
Average 2711
123 160 4551 2645 1221 114 15 339 43 2331 10 56 4653 978 7 16
75%
Quantile 3624 163 185 5169 4870 1667 159 7 305 40 2698 14 77 6655 1389 5 22
Table 2 shows the dopant concentrations in ppta after
the welding without a second cleaning step.
Table 2
Al As
Median 109 104 5 11
Average 132 131 17 18
The second chemical cleaning may be carried out with
very different etching erosions, as shown in the
examples below.
Example 1
In Example 1, at less than 1 pm, the etching erosion in
the second cleaning step is comparatively low.
Conversely, the erosion in the first cleaning step is
30 pm.
The first cleaning step comprises precleaning, main
cleaning, a washing step and hydrophilization.
For the precleaning, the thin rod is cleaned for 5
minutes in a mixture of 11 wt% HCI, 5 wt% HF and 1.5
wt% H202 at a temperature of 20 C.
CA 02751228 2011-08-31
- 12 -
The main cleaning is carried out for 5 minutes at 8 C
in an HF/HNO3 mixture containing 6 wt% HF, 55 wt% HNO3
and 1 wt% Si.
The etching erosion is about 30 pm.
The etched thin rod is subsequently washed for 5
minutes with 18 Mohm ultrapure water heated to 22 C.
Finally, 5 minutes of hydrophilization is carried out
in water heated to 22 C and saturated with 20 ppm of
ozone.
Finally, the thin rod is dried for 60 minutes with
cleanroom class 100 ultrapure air at 80 C.
The welding of the cleaned thin rods is followed by a
second chemical cleaning to remove the particles which
have become attached to the silicon surface owing to
the welding.
The material erosion is less than 1 pm.
For the precleaning, the thin rod is cleaned for 5
minutes in a mixture of 11 wt% HC1, 5 wt% HF and 1.5
wt% H202 at a temperature of 20 C.
The main cleaning is carried out for 0.1 minute at 8 C
in an HF/HNO3 mixture containing 6 wt% HF, 55 wt% HNO3
and 1 wt% Si.
The etching erosion is about 30 pm.
The etched thin rod is subsequently washed for 5
minutes with 18 Mohm ultrapure water heated to 22 C.
Finally, 5 minutes of hydrophilization is carried out
in water heated to 22 C and saturated with 20 ppm of
ozone.
CA 02751228 2011-08-31
- 13 -
Finally, the thin rod is dried for 60 minutes with
cleanroom class 100 ultrapure air at 80 C.
21 thin rods of Example 1 were studied in relation to
the contaminations with metals and dopants.
Table 3 shows the surface contamination with metals in
pptw for Example 1.
Table 3
Fe Cr , Ni Na Zn N = Cu Mo Ti W K Co Mn Ca Mg V Age]
Median 13 1 0 6
1 4 0 0 4 1 8 0 0 49 6 0 2
Average 18 1 0
17 2 6 0 0 4 1 10 0 0 101 12 0 3
75%Quantile 23 1 0 15, 2 7 1 0 5 2 11 0 0 128 13 0
Table 4 shows the dopant concentrations in ppta for
Example 1.
Table 4
Al As
Median 30 25 3 6
Average 35 32 12 11
Significant reductions can be seen both in the metal
contaminations (cf. Table 1) and in the contaminations
with B, P, Al and As (cf. Table 2) by virtue of the
second cleaning step.
Example 2
In Example 2, at about 30 pm, the etching erosion in
the second cleaning step is significantly higher than
in Example 1. The effect of higher etching erosions on
the results is to be studied in more detail.
CA 02751228 2011-08-31
- 14 -
The erosion in the first cleaning step is likewise 30
pm, as in Example 1.
The first cleaning step again comprises precleaning,
main cleaning, a washing step and hydrophilization.
For the precleaning, the thin rod is cleaned for 5
minutes in a mixture of 11 wt% HC1, 5 wt% HF and 1.5
wt% H202 at a temperature of 20 C.
The main cleaning is carried out for 5 minutes at 8 C
in an HF/HNO3 mixture containing 6 wt% HF, 55 wt% HNO3
and 1 wt% Si.
The etching erosion is about 30 pm.
The etched thin rod is subsequently washed for 5
minutes with 18 Mohm ultrapure water heated to 22 C.
Finally, 5 minutes of hydrophilization is carried out
in water heated to 22 C and saturated with 20 ppm of
ozone.
Finally, the thin rod is dried for 60 minutes with
cleanroom class 100 ultrapure air at 80 C.
The welding of the cleaned thin rods is followed by a
second chemical cleaning to remove the particles which
have become attached to the silicon surface owing to
the welding.
The material erosion is about 30 pm.
For the precleaning, the thin rod is cleaned for 5
minutes in a mixture of 11 wt% HC1, 5 wt% HF and 1.5
wt% H202 at a temperature of 20 C.
CA 02751228 2011-08-31
- 15 -
The main cleaning is carried out for 5 minutes at 8 C
in an HF/HNO3 mixture containing 6 wt% HF, 55 wt% HNO3
and 1 wt% Si.
The etching erosion is about 30 pm.
The etched thin rod is subsequently washed for 5
minutes with 18 Mohm ultrapure water heated to 22 C.
Finally, 5 minutes of hydrophilization is carried out
in water heated to 22 C and saturated with 20 ppm of
ozone.
Finally, the thin rod is dried for 60 minutes with
cleanroom class 100 ultrapure air at 80 C.
21 thin rods of Example 2 were studied in relation to
the contaminations with metals and dopants.
Table 5 shows the surface contamination with metals in
pptw for Example 2.
Table 5
____________________________________________________________________ Fe Cr
Ni Na Zn AI Cu Mo Ti W K Co Mn CaML Ag
25%
Quantile 4 0 0 2
0 2 0 0 1 0 2 0 0 8 1 0 1
Median 8 1 0 4
1 4 0 0 2 1 5 0 025 4 0 2
Average 14 1 0 8
2 6 0 0 4 1 7 0 0 55 8 0 3
75%
Quantile 24 1 0
10 2 7 1 0 5 2 8 0 0 65 9 0 4
Table 6 shows the dopant concentrations in ppta for
Example 2.
Table 6
Al As
Median 6 9 1 1
Average 11 12 3 3
CA 02751228 2011-08-31
- 16 -
Compared with Example 1, an improvement in the
contamination can be seen for iron, calcium, magnesium,
potassium, sodium, aluminum, titanium and the dopants
boron, phosphorus, aluminum and arsenic.
The results of Example 2 show that, with respect to the
metal contaminations, higher etching erosions lead to a
further slight improvement for iron and the
environmental elements calcium, magnesium, potassium,
sodium, aluminum, titanium. The concentrations of B, P,
Al and As are likewise reduced.
In the scope of the invention, for the preferred second
cleaning step, however, low etching erosions of less
than 10 pm are preferred. Etching erosions of less than
5 pm are particularly preferred, and etching erosions
of less than 2 pm are more particularly preferred.
For the first cleaning of the thin rods, etching
erosions of 10 pm or more are preferred. Etching
erosions of at least 20 pm are particularly preferred,
and etching erosions of at least 30 pm are more
particularly preferred.
According to previous experience, the etching tanks for
cleaning systems made of pure plastic achieve at most
an external length of 4 m and an internal length of 3.2
m. The cleaning of thin rods with a length of more than
3.2 m is therefore not possible with these etching
tanks. After the welding of two thin rods, however, the
length of the thin rod can reach more than 3.2 m, which
requires a different solution for the application of
the preferred second cleaning step.
The inventors have discovered that even relatively
small etching tanks are suitable for the cleaning of
long thin rods.
CA 02751228 2011-08-31
- 17 -
The previously described brief second step of etching
the very long thin rods 1 can particularly preferably
be carried out in a tank 5 whose length is less than
that of the rod 1. On each of its end faces, this tank
5 has an opening 51 and 52, respectively, through which
the longer =thin rod 1 can be passed. Etching liquid 6
which flows out along the thin rods 1 at these openings
51 and 52 is collected in a trough 7 placed underneath
and pumped back into the etching tank 5 by means of a
pump 8 through a line 81, so that there is an
equilibrium between the outflow and recycling of the
etching liquid 6. After the rod 1 has been passed
through the etching tank 5 and the rod 1 has been
dried, it can be introduced almost immediately into a
film tube 100 for packaging. Further additional
pollution is thereby avoided. The drying may be carried
out with the aid of hot air from which particles have
been removed, and which is blown onto the rod 1.
Corresponding drying units are schematically shown by
9.
The forward drive speed of the rod 1 and the length of
the etching tank 5 determine the residence time in the
etching tank 5 and therefore the etching erosion. The
advantage of this method, compared with etching in
conventional etching tanks 5, is on the one hand the
small space requirement of the system and on the other
hand the more flexible structure. Specifically, with
the principle presented, it is also possible to produce
a cascade of different etching and washing steps, which
can be implemented in a very compact structure.
Hydrophilization steps can also be carried out without
problems in the working sequence.
Grippers such as are used in etching tanks 5 of
conventional design, in order to transport the rods 1
from one tank into another, are not required in this
method. With this very modular design, it is also
ak 02751228 2011-08-31
- 18 -
possible to introduce simple drying units 9 which dry
the thin rod 1 simply with hot air. HF/ozone dryers may
also be envisaged, and are particularly advantageous,
in which the thin rods 1 are pulled in a final etching
bath through a dilute HF/water solution. At the exit
from the container opening 51 or 52, there is still an
HF/water layer on the thin rod 1, which is blown
against the transport direction of the rod 1 by a flow
of ozone. Ozone dissolves in the liquid film on the
thin rod 1 and changes the surface tension of the film,
so that drying according to the Marangoni effect takes
place.
The use of longer thin rods, which satisfy particular
requirements in terms of impurities, offers the
advantage that the yield per run in a deposition
reactor can be increased.
The invention therefore makes it possible to produce
longer thin rods (> 3.2 m) which additionally satisfy
stringent requirements of purity. (Pollution less than
1012 at/cm2 or at/cm3)
Thin rods having a length of more than 3.2 m can be
produced by joining two or more shorter thin rods to
form a longer thin rod.
It has been found that even the use of welded thin rods
having a length of less than 3.2 m offers advantages
during the deposition process. Evidently, the welding
sites modify the stress behavior in the finished rods,
S0 that the rate of collapse when cooling to room
temperature in the Siemens reactor, when the reactor is
turned off, is significantly reduced. This is an
additional unexpected effect of the method according to
the invention.
CA 02751228 2011-08-31
- 19 -
Welding of sawed but not previously cleaned thin rods
increases the metal concentration on the surface to
more than 1016 at/cm2 at the welding site.
Owing to the high temperature of more than 500 C during
the welding, metallic and other particulate impurities
diffuse into the bulk of the thin silicon rod.
Such impurities in the bulk can no longer be removed by
surface cleaning.
This is avoided by the method according to the
invention and the mandatory cleaning of the thin rods
before the welding.