Note: Descriptions are shown in the official language in which they were submitted.
=
CA 02751615 2011-09-08
ENHANCEMENT OF FISCHER-TROPSCH PROCESS FOR
HYDROCARBON FUEL FORMULATION IN A GTL
ENVIRONMENT
[TECHNICAL FIELD]
[0001] The present invention relates to the modification of the Fischer-
Tropsch
sequence of operations including the Fischer-Tropsch process for the
production of
hydrocarbon fuels in an efficient manner.
[BACKGROUND OF THE INVENTION]
[0002] In the prior art, the Fischer-Tropsch process has been used for decades
to
assist in the formulation of hydrocarbons. In the last several years, this has
become a
concern giving the escalating environmental concerns regarding pollution
together
with the increasing costs of hydrocarbon exploration and refining. The major
producers in this area have expanded the art significantly in this
technological area
with a number of patented advances and pending applications in the form of
publications.
[0003] In the art, advances made in terms of the raw materials that have been
progenitor materials for the Fischer-Tropsch process, have included, for
example,
coal-to-liquid (CTL), bio-to-liquid (BTL) and gas-to-liquid (GTL). One of the
more
particularly advantageous features of the gas- to- liquid (GTL) technology is
the fact
that it presents a possibility to formulate a higher value environmentally
beneficial
synthetic diesel product or syndiesel from stranded natural gas reserves,
which would
otherwise have not been commercially feasible to bring to market. As is
generally
known, the Fischer-Tropsch (FT) process converts hydrogen and carbon monoxide
(commonly known as syngas) into liquid hydrocarbon fuels, examples of which
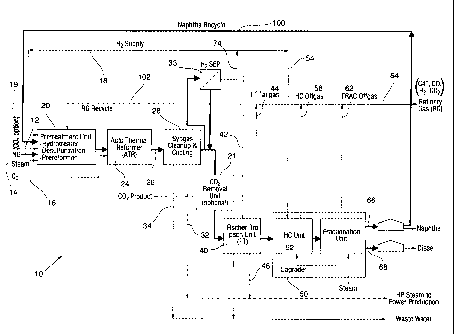
include synthetic diesel, naphtha, kerosene, aviation or jet fuel and
paraffinic wax. As
a precursory step, the natural gas is thermally converted using heat and
pressure in the
presence of catalyst to produce a hydrogen rich syngas containing hydrogen and
carbon monoxide. As a result of the Fischer-Tropsch technique, the synthetic
fuels are
-1-
r ________________________________________
_
CA 02751615 2011-09-08
very appealing from an environmental point of view, since they are paraffinic
in
nature and substantially devoid of contamination. This is particularly true in
the case
of the diesel fuel synthesis where the synthetic product has ideal properties
for diesel
engines, including extremely high cetane rating >70, negligible aromatics and
sulphur
content, in addition to enabling optimum combustion and virtually emission
free
operation. Synthetic diesel or syndiesel fuels significantly reduce nitrous
oxide and
particulate matter when compared with petroleum based diesel fuel.
[0004] One example of recent advances that have been made in this area of
technology includes the features taught in United States Patent No. 6,958,363,
issued
to Espinoza, et al., October 25, 2005. In the document, Espinoza et al.
provide for
hydrogen use in a GTL plant.
[0005] In essence, the patent teaches a process for synthesizing hydrocarbons
where
initially, a synthesis gas stream is formulated in a syngas generator. The
synthesis gas
stream comprises primarily hydrogen and carbon monoxide. The process involves
catalytically converting the synthesis gas stream in a synthesis reaction to
produce
hydrocarbons and water followed by the generation of hydrogen-rich stream in
the
hydrogen generator. The process indicates that the hydrogen generator is
separate
from the syngas generator (supra) and that the hydrogen generator comprises
either a
process for converting hydrocarbons to olefins, a process for catalytically
dehydrogenating hydrocarbons, or a process for refining petroleum, and a
process for
converting hydrocarbons to carbon filaments. The final step in the process in
its
broadest sense, involves consumption of hydrogen from the hydrogen-rich stream
produced in one or more processes that result and increase value of the
hydrocarbons
or the productivity of the conversion of the hydrocarbons from the earlier
second
mentioned step.
[0006] Although a useful process, it is evident from the disclosure of
Espinoza et al.
that there is a clear intent to create olefins such as ethylene and propylene
for
petrochemical use, and aromatics for gasoline production. Additionally, there
is a
reforming step indicated to include the reformation of naphtha feedstock to
generate a
net surplus hydrogen by-product which is then recombined into the process. The
naphtha is subsequently converted to aromatics for high octane gasoline blend
stock.
-2-
......
1Ø=== _________________________________________________________________
CA 02751615 2011-09-08
There is no specific contemplation and therefore no discussion of effectively
destroying the naphtha for purposes of enhancing the Fischer-Tropsch process
which,
in turn, results in the significant augmentation of hydrocarbon synthesis.
[0007] The Espinoza et al. process is an excellent gas to a liquid process
link to
gasoline production from natural gas using naphtha reformation to make the
gasoline
product. In the disclosure, it was discovered that the excess hydrogen could
be used to
enhance the productivity of conversion.
[0008] A further significant advancement in this area of technology is taught
by Bayle
et al., in United States Patent No. 7,214,720, issued May 8, 2007. The
reference is
directed to the production of liquid fuels by a concatenation of processes for
treatment
of a hydrocarbon feedstock.
[0009] It is indicated in the disclosure that the liquid fuels begin with the
organic
material, typically biomass as a solid feedstock. The process involves a stage
for the
gasification of the solid feedstock, a stage for purification of synthesis gas
and
subsequently a stage for transformation of the synthesis gas into a liquid
fuel.
[0010] The patentees indicate in column 2 the essence of the technology:
"A process was found for the production of liquid fuels starting from a solid
feedstock that contains the organic material in which:
a) The solid feedstock is subjected to a gasification stage so as to convert
said
feedstock into synthesis gas comprising carbon monoxide and hydrogen,
b) the synthesis gas that is obtained in stage a) is subjected to a
purification
treatment that comprises an adjustment for increasing the molar ratio of
hydrogen to carbon monoxide, H2/CO, up to a predetermined value,
preferably between 1.8 and 2.2,
c) the purified synthesis gas that is obtained in stage b) is subjected to a
conversion stage that comprises the implementation of a Fischer-Tropsch-type
synthesis so as to convert said synthesis gas into a liquid effluent and a
gaseous effluent,
-3-
.s.........'.,.,
õ-
õ
CA 02751615 2011-09-08
d) the liquid effluent that is obtained in stage c) is fractionated so as to
obtain
at least two fractions that are selected from the group that consists of: a
gaseous fraction, a naphtha fraction, a kerosene fraction, and a gas oil
fraction,
and
e) at least a portion of the naphtha fraction is recycled in gasification
stage.÷
[0011] Although a meritorious procedure, the overall process does not result
in
increased production of hydrocarbons. The naphtha recycle stream that is
generated in
this process is introduced into the gasification stage. This does not directly
augment
the syngas volume to the Fischer-Tropsch reactor which results in increased
volumes
of hydrocarbons being produced giving the fact that the feedstock is required
for the
process. To introduce the naphtha to the gasification stage as taught in Bayle
et al., is
to modify the H2/C0 ratio in the gasification stage using an oxidizing agent
such as
water vapour and gaseous hydrocarbon feedstocks such as natural gas with the
recycled naphtha, while maximizing the mass rate of carbon monoxide and
maintain
sufficient temperature above 1000 C to 1500 C in the gasification stage to
maximize
the conversion of tars and light hydrocarbons.
[0012] In United States Patent No. 6,696,501, issued February 24, 2004, to
Schanke
et al., there is disclosed an optimum integration process for Fischer-Tropsch
synthesis
and syngas production.
100131 Among other features, the process instructs the conversion of natural
gas or
other fossil fuels to higher hydrocarbons where the natural gas or the fossil
fuels is
reacted with steam and oxygenic gas in a reforming zone to produce synthesis
gas
which primarily contains hydrogen, carbon monoxide and carbon dioxide. The
synthesis gas is then passed into a Fischer-Tropsch reactor to produce a crude
synthesis containing lower hydrocarbons, water and non-converted synthesis
gas.
Subsequently, the crude synthesis stream is separated in a recovery zone into
a crude
product stream containing heavier hydrocarbons, a water stream and a tail gas
stream
containing the remaining constituents. It is also taught that the tail gas
stream is
reformed in a separate steam reformer with steam and natural gas and then the
sole
- 4 -
CA 02751615 2011-09-08
reformed tail gas is introduced into the gas stream before being fed into the
Fischer-
Tropsch reactor.
[0014] In the reference, a high carbon dioxide stream is recycled back to an
ATR in
order to maximize the efficiency of the carbon in the process. It is further
taught that
the primary purpose of reforming and recycling the tail gas is to steam reform
the
lower hydrocarbons to carbon monoxide and hydrogen and as there is little in
the way
of light hydrocarbons, adding natural gas will therefore increase the carbon
efficiency.
There is no disclosure regarding the destruction of naphtha in an SMR or ATR
to
generate an excess volume of syngas with subsequent recycle to maximize
hydrocarbon production. In the Schanke et al. reference, the patentees
primarily
focused on the production of the high carbon content syngas in a GTL
environment
using an ATR as crude synthesis stream and reforming the synthesis tail gas in
an
SMR with natural gas addition to create optimum conditions that feed to the
Fischer-
Tropsch reactor.
[0015] In respect of other progress that has been made in this field of
technology, the
art is replete with significant advances in, not only gasification of solid
carbon feeds,
but also methodology for the preparation of syngas, management of hydrogen and
carbon monoxide in a GTL plant, the Fischer-Tropsch reactors management of
hydrogen, and the conversion of biomass feedstock into hydrocarbon liquid
transportation fuels, inter alia. The following is a representative list of
other such
references. This includes: US Patent Nos. 7,776,114; 6,765,025; 6,512,018;
6,147,126; 6,133,328; 7,855,235; 7,846,979; 6,147,126; 7,004,985; 6,048,449;
7,208,530; 6,730,285; 6,872,753, as well as United States Patent Application
Publication Nos. US2010/0113624; US2004/0181313; US2010/0036181;
US2010/0216898; US2008/0021122; US 2008/0115415; and US 2010/0000153.
[SUMMARY OF THE INVENTION]
100161 One object of the present invention is to provide an improved Fischer-
Tropsch
based synthesis process for synthesizing hydrocarbons with a substantially
increased
yield.
-5-
-
_ . õ
" -
CA 02751615 2011-09-08
[0017] In one embodiment of the present invention there is provided a process
for
synthesizing hydrocarbons, comprising:
a) formulating a hydrogen rich stream with a syngas generator;
b) catalytically converting said stream to produce hydrocarbons, containing at
least naphtha;
c) recycling at least a portion of said naphtha to said syngas generator to
form an
enhanced hydrogen rich stream; and
d) re-circulating said enhanced hydrogen rich stream from step (c) for
conversion
in step (b) to enhance the synthesis of hydrocarbons.
[0018] The present technology provides a very elegant solution to ameliorate
the
shortcomings that have been clearly evinced in the prior art references.
Despite the
fact that the prior art, in the form of patent publications, issued patents,
and other
academic publications, all recognize the usefulness of a Fischer-Tropsch
process,
steam methane reforming, autothermal reforming, naphtha recycle, and other
processes, the prior art when taken individually or when mosaiced is deficient
a
process that provides for the synthesis of a hydrogen rich stream in a syngas
generator
and reaction in a Fischer-Tropsch or suitable reactor for the purpose of
enhancing the
production of, as one example, diesel fuel or aviation fuel. As is well known,
the
Fischer-Tropsch process is particularly useful since the resultant synthetic
fuel is
"clean" fuel and does not have the contamination level typically associated
with the
same petroleum based fuel.
[0019] The present invention amalgamates, in a previously unrecognized
combination, a series of known unit operations into a much improved synthesis
route
for production of synthetic hydrocarbon fuels. This process engages a counter-
intuitive step, namely, the removal of a production fraction, namely the
naphtha,
which, despite being a refined product, is then effectively destroyed making
use of the
naphtha as a feedstock for a syngas generator and then recycled into the
Fischer-
Tropsch process. This keystone unit operation is propitious since it works in
concert
with all of the other precursor operations which, of their own right, are
highly
effective.
- 6 -
¨ ________________________________________
CA 02751615 2011-09-08
100201 It has been discovered that by employing the naphtha product fraction
as a
recycled feedstock to the syngas generator, shown in the example and discussed
hereinafter in greater detail, as an autothermal reformer (ATR) or steam
methane
reformer (SMR) or combination thereof, results in an increase in the volume of
diesel,
or as it is more effectively referred to in the art, as syndiesel.
[0021] In accordance with an embodiment of the instant methodology, the
process
may include an autothermal reforming unit (ATR) operation as a syngas
generator. As
is well known to those skilled in the art, autothermal reforming employs
carbon
dioxide and oxygen, or steam, in a reaction with light hydrocarbon gases like
natural
gas to form syngas. This is an exothermic reaction in view of the oxidation
procedure.
When the autothermal reformer employs carbon dioxide, the hydrogen to carbon
monoxide ratio produced is 1:1 and when the autothermal reformer uses steam,
the
ratio produced is approximately 2.5:1. One of the more significant benefits of
using
the ATR is realized in the variability of the hydrogen to carbon monoxide
ratio.
[0022] The reactions that are incorporated in the autothermal reformer are as
follows:
2CH4 + 02 CO2 = 3H2 3C0 + H20 + HEAT.
When steam is employed, the reaction equation is as follows:
4CH4 + 02 + 2H20 + HEAT = 10H2 + 4C0.
[0023] In accordance with a further embodiment of the instant methodology, the
process may include a steam methane reformer (SMR) operation as a syngas
generator. As is well known to those skilled in the art, steam methane
reforming
employs steam in a reaction with light hydrocarbon gases like natural gas and
pre-
reformed naphtha to form syngas in an indirect fired heater configuration.
This is an
endothermic reaction where external heat energy is required to support the
reaction.
[0024] The primary reaction that is incorporated in the steam methane reformer
is as
follows:
Natural Gas + Naphtha + Steam + Heat = CO + nH2 + CO2
[0025] With the steam methane reformer, the hydrogen to carbon monoxide ratio
produced ranges from 3:1 to 5:1. One of the more significant benefits of using
the
-7-
-
_______________________________________________________________________________
__
.õ
CA 02751615 2011-09-08
SMR is realized in the capability of generating relatively high hydrogen to
carbon
monoxide ratios, particularly attractive where excess hydrogen is needed for
other
operations, such as for the hydrocarbon upgrader.
[0026] A further discovery materialized from making use of, for example, light
hydrocarbon gas as by-product from the Fischer-Tropsch reaction and
hydrocarbon
upgrader processing, commonly known as FT Tailgas and Upgrader offgases, or
combined to form a refinery fuel gas, as a recycled feedstock to the ATR, SMR
or
combination thereof together with the naphtha recycle feedstock, resulted in a
significant increase in the volume of syndiesel fuel produced. By way of
example, by
employing the combination of SMR and ATR with naphtha recycle, and the
recycled
refinery fuel gases, the process is capable of converting at least 50% or
greater of all
the carbon introduced to the process to syndiesel with an increase in
production of
syndiesel and synthetic jet fuel, as compared to conventional Fischer-Tropsch
operation and without the production of any hydrocarbon by-products. This
obviously
has significant economic benefits.
[0027] Accordingly, a further aspect of one embodiment of the present
invention is to
provide a process for synthesizing hydrocarbons, comprising the steps of:
providing a source of hydrocarbons at least containing naphtha,
recycling the naphtha to a syngas generator to form hydrogen rich stream; and
catalytically converting the hydrogen rich stream to synthesize hydrocarbons.
[0028] In accordance with a further aspect of one embodiment of the present
invention, there is provided an improved gas to liquids circuit, the
improvement
comprising:
recycling formed naphtha to said syngas generator to form a hydrogen rich
stream
with subsequent catalytic conversion.
[0029] Copious advantages flow from practicing the technology of this
application,
exemplary of which are:
a) high quality diesel product or additive;
b) high quality diesel and jet fuel with an absence of sulfur;
- _-8-
-.,_ .
.
-
CA 02751615 2011-09-08
c) absence of petroleum by-products or low value feedstocks such as naphtha;
d) low emission and clean burning diesel and jet fuel;
e) increased cetane rating with concomitant augmented performance; and
0 significant volume output of diesel/jet fuel compared to conventional
processes using a Fischer-Tropsch reactor.
[0030] Referring now to the drawings as they generally describe the invention,
reference will now be made to the accompanying drawings illustrating preferred
embodiments and in which:
BRIEF DESCRIPTION OF THE DRAWINGS
[0031] Figure 1 is a process flow diagram of methodology known in the prior
art using autothermal reformer technology;
[0032] Figure 2 is a process flow diagram of methodology known in the prior
art
using steam methane reformer technology;
[0033] Figure 3 is a process flow diagram similar to Figure 1, illustrating a
first
embodiment of the present invention;
[0034] Figure 4 is a process flow diagram similar to Figure 2, illustrating a
further
variation of the present invention;
[0035] Figure 5 is a process flow diagram of a still further embodiment of the
present
invention showing the combination of autothermal and steam methane reforming
technologies; and
[0036] Figure 6 is a process flow diagram illustrating a still further
variation of the
present methodology, showing the integration of the autothermal and steam
methane
technologies.
[0037] Similar numerals employed in the figures denote similar elements.
[0038] The dashed lines used in the Figures denote optional operations.
_
-9-
,.,
CA 02751615 2011-09-08
[DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS]
[0039] Referring now to Figure 1, to illustrate prior art, shown is a process
flow
diagram of a circuit for converting gas-to -liquids with the result being the
production
of naphtha and syndiesel. The process is generally denoted by numeral 10 and
begins
with a natural gas supply 12, which feedstock can be in the form of raw field
gas or
pipeline quality treated gas, usually with bulk sulfur and hydrocarbon liquids
removed. The natural gas is then pre-treated in a pre-treatment unit 20 to
which steam
14, hydrogen 18 and optionally carbon dioxide 19 may be added as required. The
pre-
treatment unit may include, as is well known to those skilled in the art, such
unit
operations as a feed gas hydrotreater, sulfur removal and guard operation and
a pre-
reformer to produce a clean vapour feed stream 22 for the syngas generator,
denoted
in Figure 1 as an autothermal reformer (ATR) unit 24. The ATR 24 may be any
suitable catalytic partial oxidization unit, however, as an example, an ATR
that is
useful in this process is that of Haldor Topsoe A/S., Uhde GmbH and CB&I
Lummus
Company. The ATR process and apparatus have been found to be effective in the
methodology of the present invention and will be discussed hereinafter.
=
[0040] Generally, as is known from the ATR process, the same effectively
involves a
thermal catalytic stage which uses an partial oxygen supply 16 to convert the
preconditioned natural gas feed to a syngas 26 containing primarily hydrogen
and
carbon monoxide.
[0041] The so formed syngas is then subjected to cooling and cleaning
operations 28
with subsequent production of steam 32 and removal of produced water at 34.
Common practice in the prior art is to employ the use of a water gas shift
reaction
(WGS) on the clean syngas 30 to condition the hydrogen to carbon dioxide ratio
to
near 2.0:1 for optimum conditions for the Fischer-Tropsch unit 40. It is not
preferred
in this process to include a WGS reaction as all the carbon, primarily as CO
is used to
maximize production of synthesis liquids product. The process may optionally
use the
supplemental addition of hydrogen 42 to maximize the conversion to syndiesel.
The
raw syngas may be further treated, as is well known to those skilled in the
art, in
various steps of scrubbing units and guard units to remove ammonia and sulfur
compounds to create a relatively pure clean syngas 30 suitable for use in a
Fischer-
- 10 -
W.' . __ .
,
CA 02751615 2011-09-08
Tropsch unit. A carbon dioxide removal unit (not shown) may optionally be
included
in the clean syngas stream 30 to reduce the inert load and maximize the carbon
monoxide concentration to the Fischer-Tropsch unit 40. The syngas is then
transferred to a Fischer-Tropsch reactor 40 to produce the hydrocarbons and
water.
The so formed hydrocarbons are then passed on to a product upgrader, generally
denoted as 50, and commonly including a hydrocarbon cracking stage 52, a
product
fractionating stage 60 with naphtha being produced at 66 as a fraction, as
well as
diesel 68 as an additional product. The diesel 68 formulated in this process
is
commonly known as syndiesel. As an example, this process results in the
formulation
of 1000 barrels per day (bbl/day) based on 10 to 15 thousand standard cubic
feet /day
(MSCFD) of natural gas. As is illustrated in the flow diagram, a source of
hydrogen
74 is to be supplemented to the hydrocarbon cracking unit 52 denoted as
streams 54.
Further, energy 32 from the syngas generator 24, typically in the form of
steam, may
be used to generate power and this is equally true of the Fischer-Tropsch
reactor 40
creating energy 46.
[0042] Table 1 establishes a comparison between FT diesel and conventional
petroleum based diesel.
TABLE 1
Specification of FT-diesel in comparison to conventional diesel
Diesel Fuel Specification FT-Diesel Conventional Diesel
Chemical formula Paraffin C12H26
Molecular weight (kg/kmol) 170-200
Cetane number >74 50
Density (kg/I) at 15 C 0.78 0.84
Lower Heating Value (MJ/kg) at 15 C 44.0 42.7
Lower Heating Value (MJ/I) at 15 C 34.3 35.7
Stoichiometric air/fuel ratio (kg air/kg 14.53
fuel)
Oxygen content (%wt) ¨0 0-0.6
Kinematic viscosity (mm2/s) at 20 C 3.57 4
Flash point ( C) 72 77
Source: KMITL Sci. Tech. J. Vol. 6 No. 1 Jan. - Jun. 2006, p. 43
- 11
---õ-
CA 02751615 2011-09-08
[0043] As a further benefit, known to those skilled in the art, the process as
described
by Figure 1 and all configurations of the current invention, the addition of a
further
side stripper column (not shown) off the fractionation in stage 60 may be
included to
produce a new fraction of about 25% of the volume of the syndiesel fuel (200
to 300
barrels per day (bbl/day)), referred to as FT-jet fuel. Table 2 describes a
typical
characteristic of FT jet fuel.
TABLE 2
Typical Specification of FT-Jet Fuel
Typical Product Specification FT Jet Fuel
Acidity mg KOH/g 0.10
Aromatics %vol max <25.0
Sulfur mass% <0.40
Distillation C
50% recovered Min 125 C max 190 C
End Point 270 C
Vapor Pressure kPa max 21
Flash Point C
Density 15 C, kg/m3 750-801
Freezing Point C max -51
Net Heat Combustion MJ/kg min 42.8
Smoke Point mm, min 20
Naphthalenes vol% max <3.0
Copper Corrosion 2hr @ 100 C, max No 1
rating
Thermal Stability
Filter Pressure drop mm Hg, max 25
Visual Tube rating, max <3
Static Test 4hr @ 150 C mg/100m1, max
Existent Gum mg/100m1, max
- 12 -
CA 02751615 2011-09-08
[0044] Naphtha 66 can be generally defined as a distilled fraction of the
Fischer-
Tropsch FT hydrocarbon liquids, categorized by way of example with a typical
boiling range of 30 C to 200 C, and more preferred 80 C to 120 C. The specific
naphtha specification will be optimized for each application to maximize
syndiesel
production, maximize the recovery of light liquid hydrocarbon fractions such
as
propane and butane and partially or fully eliminate the naphtha by-product.
[0045] Suitable examples of FT reactors include fixed bed reactors, such as
tubular
reactors, and multiphase reactors with a stationary catalyst phase and slurry-
bubble
reactors. In a fixed bed reactor, the FT catalyst is held in a fixed bed
contained in
tubes or vessels within the reactor vessel. The syngas flowing through the
reactor
vessel contacts the FT catalyst contained in the fixed bed. The reaction heat
is
removed by passing a cooling medium around the tubes or vessels that contain
the
fixed bed. For the slurry-bubble reactor, the FT catalyst particles are
suspended in a
liquid, e.g., molten hydrocarbon wax, by the motion of bubbles of syngas
sparged into
the bottom of the reactor. As gas bubbles rise through the reactor, the syngas
is
absorbed into the liquid and diffuses to the catalyst for conversion to
hydrocarbons.
Gaseous products and unconverted syngas enter the gas bubbles and are
collected at
the top of the reactor. Liquid products are recovered from the suspending
liquid using
different techniques such as separators, filtration, settling, hydrocyclones,
and
magnetic techniques. Cooling coils immersed in the slurry remove heat
generated by
the reaction. Other possibilities for the reactor will be appreciated by those
skilled.
[0046] In the FT process, H2 and CO combine via polymerization to form
hydrocarbon compounds having varying numbers of carbon atoms. Typically 70%
conversion of syngas to FT liquids takes place in a single pass of the FT
reactor unit.
It is also common practice to arrange the multiple FT reactors in series and
parallel to
achieve conversion levels of 90+%. A supplemental supply of hydrogen 42 may be
provided to each subsequent FT reactor stages to enhance the conversion
performance
of the subsequent FT stages. After the FT reactor, products are sent to the
separation
stage, to divert the unconverted syngas and light hydrocarbons (referred to as
FT
tailgas), FT water and the FT liquids, which are directed to the hydrocarbon
upgrader
unit denoted as 50. The FT tailgas becomes the feed stream for susequent FT
stages or
- 13 -
CA 02751615 2011-09-08
is directed to refinery fuel gas in the final FT stage. The upgrader unit
typically
contains a hydrocracking step 52 and a fractionation step 60.
[0047] Hydrocracking denoted as 52 used herein is referencing the splitting an
organic molecule and adding hydrogen to the resulting molecular fragments to
form
multiple smaller hydrocarbons (e.g., C161122 + H2 = C4H10 and skeletal isomers
+
C61114). Since a hydrocracking catalyst may be active in hydroisomerization,
skeletal
isomerization can occur during the hydrocracking step. Accordingly, isomers of
the
smaller hydrocarbons may be formed. Hydrocracking a hydrocarbon stream derived
from Fischer-Tropsch synthesis preferably takes place over a hydrocracking
catalyst
comprising a noble metal or at least one base metal, such as platinum, cobalt-
molybdenum, cobalt-tungsten, nickel-molybdenum, or nickel-tungsten, at a
temperature of from about 550 F to about 750 F (from about 288 C to about 400
C)
and at a hydrogen partial pressure of about 500 psia to about 1,500 psia
(about 3,400
kPa to about 10,400 kPa).
[0048] The hydrocarbons recovered from the hydrocracker are further
fractionated in
the fractionation unit 60 and refined to contain materials that can be used as
components of mixtures known in the art such as naphtha, diesel, kerosene, jet
fuel,
lube oil, and wax. The combined unit consisting of the hydrocracker 52 and
hydrocarbon fractionator 60 are commonly known as the hydrocarbon upgrader 50.
As is known by those skilled in the art, several hydrocarbon treatment methods
can form part of the upgrader unit depending on the desired refined products,
such as
additional hydrotreating or hydroisomerization steps. The hydrocarbon products
are
essentially free of sulfur. The diesel may be used to produce environmentally
friendly,
sulfur-free fuel and/or blending stock for diesel fuels by using as is or
blending with
higher sulfur fuels created from petroleum sources.
[0049] Unconverted vapour streams, rich in hydrogen and carbon monoxide and
commonly containing inert compounds such as carbon dioxide, nitrogen and argon
are
vented from the process as FT tail gas 44, hydrocracker (HC) offgas 56 and
fractionator (frac) offgas 62. These streams can be commonly collected as
refinery
fuel gas 64 and used as fuel for furnaces and boilers to offset the external
need for
- 14-
..
, , <
CA 02751615 2011-09-08
natural gas. These streams may also be separated and disposed of separately
based on
their unique compositions, well known to those skilled in the art.
[00501 A supplemental supply of hydrogen 74 may be required for the HC unit 54
and
the natural gas hydrotreater 18. This hydrogen supply can be externally
generated or
optionally provided from the syngas stream 30 using a pressure swing
absorption or
membrane unit (not shown), although this feature will increase the volume of
syngas
required to be generated by the syngas generator 24.
100511 Further, useable energy commonly generated as steam from the syngas
stage,
denoted by numeral 32, may be used to generate electric power. This is equally
true of
useable energy that can be drawn from the Fischer-Tropsch unit, owing to the
fact that
the reaction is very exothermic and this represents a useable source of
energy. This is
denoted by numeral 46.
100521 Referring now to Figure 2, to further illustrate the prior art, shown
is an
alternate process flow diagram of a circuit for converting gas-to -liquids
with the
result being the production of naphtha and syndiesel. The components of this
process
are generally the same as that described in Figure 1 with the common elements
denoted with the same numbers. For this process, the syngas generator is
changed to
be a steam methane reformer (SMR) 25. The SMR 25 may be any suitable catalytic
conversion unit , however, as an example, an SMR that is useful in this
process is that
of Haldor Topsoe A/S., Uhde GmbH., CB&I Lununus Company, Lurgi GmbH/Air
Liquide Gruppe, Technip Inc, Foster Wheeler and others. The SMR process and
apparatus have been found to be effective in executing the methodology of the
present
invention to be discussed hereinafter. Generally, as is known from the SMR
process,
the same effectively involves a thermal catalytic stage which uses steam
supply and
heat energy to convert the preconditioned natural gas feed to a syngas 27
containing
primarily hydrogen and carbon dioxide.
100531 An advantage of the SMR technology is that the syngas is very rich in
hydrogen with a ratio of hydrogen to carbon monoxide typically greater than
3.0:1.
This exceeds the typical syngas ratio of 2.0:1 usually preferred for the
Fischer-
Tropsch process. As such, a hydrogen separation unit 33 may be used to provide
the
- 15
CA 02751615 2011-09-08
hydrogen requirement 74 for the GTL process. As discussed previously, well
know to
those skilled in the art, the hydrogen separator may be a pressure swing
adsorption or
a membrane separation unit. Further, although the SMR does not require an
oxygen
source as with the ATR technology, the SMR process requires external heat
energy,
typically provided by natural gas 13 or optionally by use of the excess
refinery gas 76
derived from the FT tail gas 44 or upgrader offgases 56 & 62.
[0054] The SMR 25 may contain any suitable catalyst and be operated at any
suitable
conditions to promote the conversion of the hydrocarbon to hydrogen H2 and
carbon
monoxide. The addition of steam and natural gas may be optimized to suit the
desired
production of hydrogen and carbon monoxide. Generally natural gas or any other
suitable fuel can be used to provide energy to the SMR reaction furnace. The
catalyst
employed for the steam reforming process may include one or more catalytically
active components such as palladium, platinum, rhodium, iridium, osmium,
ruthenium, nickel, chromium, cobalt, cerium, lanthanum, or mixtures thereof.
The
catalytically active component may be supported on a ceramic pellet or a
refractory
metal oxide. Other forms will be readily apparent to those skilled.
[0055] Turning now to Figure 3, shown is a preliminary embodiment of the
technology of the instant invention. As is evinced from Figure 3, many of the
preliminary steps are common with that which is shown in Figure 1. At least a
portion
of the less desirable FT product, naphtha 66 is recycled as ATR 24 feed
through the
pre-treatment unit 20 and is fully destroyed and converted to additional
syngas. Based
on the full recycle and conversion of the naphtha, the diesel production
increase of
greater than 10% can be realized, with the elimination of an undesirable by-
product
stream.
[0056] As a key point, one of the most effective procedures in the instant
technology,
relates to the fact that once the product fractionation stage has been
completed and the
naphtha 66 formulated, it has been found that by recycle and full conversion
of the
naphtha, significant results can be achieved in the production of the
synthetic diesel.
- 16
= 141.P.=
= ,
CA 02751615 2011-09-08
[0057] In the embodiment shown in Figure 3, several other optional features
are
desirable in addition to naphtha recycle, to enhance the production of
syndiesel,
including;
(i) a hydrogen separation unit is added to remove excess hydrogen from the
enhanced syngas for supply to the FT unit 40 and product upgrader 50;
(ii) A portion of hydrogen rich streams not desired to be used as fuel,
separately or combined all together as refinery fuel 64, can be recycled back
102 to
the ATR 24 by way of the pre-treatment unit 20;
(iii) A optional carbon dioxide removal stage 21 may be installed on the FT
syngas feedstream to reduce the inert vapour load on the FT unit 40, and at
least a
portion of the carbon dioxide 12 may be reintroduced into the ATR 24 by way of
the
pre-treatment unit 20 for purposes of reverse shifting and recycling carbon to
enhance
the production of syndiesel.
[0058] As has been discussed herein previously, it is unusual and most
certainly
counter-intuitive to effectively destroy the naphtha in order to generate a
hydrogen
rich stream as the naphtha is commonly desired as primary feedstock for
gasoline
production. Although this is the case, it is particularly advantageous in the
process as
set forth in Figure 3.
[0059] Figure 4 sets forth a further interesting variation on the overall
process that is
set forth in Figure 2 and 3. As is evinced from Figure 4, many of the
preliminary steps
are common with that which is shown in Figure 2. In this variation, and
similar to the
variation described by Figure 3, the process employs the recycle of at least a
portion
of the naphtha 100 to enhance the production of syndiesel using a SMR syngas
generator. Similarly the optional features described for Figure 3 can equally
apply to
Figure 4.
[0060] A further variation of the overall process embraced by the technology
discussed herein is shown in Figure 5. In essence, the process flow as shown
in Figure
combines the unit operations of the SMR 25 and the ATR 24 syngas generators
with
the primary embodiment of this invention, namely the recycle of at least a
portion of
the naphtha, to create the maximum conversion of carbon to syndiesel. Further,
the
- 17 -
, , õ
_
CA 02751615 2014-02-03
optional features as described in Figures 3 and 4, combined with the naphtha
recycle, may
create even further benefits to further enhancement of syndiesel production
without
any nonuseful by-products. The optional addition of CO, at 120 may be added to
pretreatment unit 20. The sizing of the ATR and SMR syngas generators are
specific to
each feed gas compositions and site specific parameters to optimize the
production of
syndiesel. Further the feedstreams for the ATR and SMR may be common or
uniquely prepared in the pre-treatment unit to meet specific syngas
compositions
desired at 26 and 27. Similarlyõ the hydrogen rich syngas stream or portion
thereof, from
the SMR can be optionally preferred as the feed stream to the hydrogen
separation unit
33. By way of example, the preferred steam to carbon ratios at streams 22 and
23 for
the AIR and SMR may be different, thereby requiring separate pre-treatment
steps.
[00611 Turning to Figure 6, as shown is yet another -variation of the overall
process
according to the present invention combining the benefits of Figures 3 and 4.
In this
embodiment, both the SMR and ATR unit operations, combined with the naphtha
recycle
are amalgamated into an integrated unit operation whereby the heat energy
created by the
ATR 24 becomes the indirect heat energy required by the SMR reactor -tubes 25.
This
embodiment allows the integrated ATIVSMR unit, the XTR to be strategically
designed to
maximize the carbon conversion to syndiesel by creating the optimum Fischer-
Tropsch 40
and hydrogen separator 33 syngas -feed with optimum hydrogen to carbon
monoxide ratio
and the minimum quantity of natural gas, steam and oxygen, while maximizing
syndiesel production without -the production of any nonuseful by-product. All
other
optional features remain the same as Figures 3, 4 and 5. As used herein,
"integrated" in
reference to the AIR/SMR means a merged unit where the two distinct operations
are
merged into one.
- '18-