Note: Descriptions are shown in the official language in which they were submitted.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
CLEAN ROOM, CLEAN ROOM SUIT AND ARRANGEMENT THEREFOR
The invention relates to a clean room suit.
Furthermore, the invention relates to a clean room lock.
The invention further relates to a dean room arrangement.
Moreover, the invention relates to a method of managing a
transition of a person between an interior and an exterior of a clean
room.
A clean room is an environment, typically used in manufacturing or
scientific research for instance in the context of semiconductor or
pharmaceutical or medical technology, that has a low level of
environmental pollutants such as dust, airborne microbes, aerosol
particles and chemical vapors. More accurately, a clean room has a
controlled level of contamination that is specified by the number of
particles per cubic meter at a specified particle size.
A clean room suit is an overall garment worn by a user in a clean
room. One common type is an all-in-one coverall worn by semiconductor
and nanotechnology line production workers, technicians, and process
engineers, as well as people in similar roles creating sterile products for
the medical device industry.
However, entry into or exit out of a clean room may be
cumbersome for a user and may be time consuming and cost-intensive.
It is an object of the invention to simplify passage of a person from
an unclean environment to a clean room, or vice versa.
In order to achieve the object defined above, a clean room suit, a
clean room lock, a clean room arrangement, and a method of managing a
transition of a person between an interior and an exterior of a clean room
according to the independent claims are provided.
According to an exemplary embodiment of the invention, a clean
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-g-
room suit for clothing a person (for instance in a clean room) is provided,
the clean room suit comprising a fabric (for instance designed as an
overall) configured to cover at least a part of a (for instance the entire)
body of the person, and a clean room lock adapter (particularly an
adapter configured for coupling to a tube of the clean room lock)
arranged on and/or in (for instance integrated in) the fabric and being
configured for coupling to a clean room lock in such a manner that, in an
operation mode in which the clean room lock is coupled to the clean room
lock adapter and a cleaning procedure is activated (for instance when a
cleaning procedure for cleaning the clean room suit is activated),
particles (such as dust and/or germs) on and/or in the fabric are moved
out (for instance are blown out) of the fabric (for instance from an
interior and/or from a surface of the fabric) towards an exterior (for
example an outer environment) of the clean room suit.
According to another exemplary embodiment of the invention, a
clean room lock for cleaning a person transmitting between an interior
and an exterior of a clean room (for instance for cleaning a person
passing from an unclean environment towards a clean room, or in the
opposite direction) is provided, the clean room lock comprising a clean
room suit adapter (particularly an adapter connected to a tube of the
clean room lock and to be coupled to a corresponding adapter of the
clean room suit) configured for coupling to a clean room suit to clothe the
person and configured so that, in an operation mode in which the clean
room suit adapter is coupled to the clean room suit and a cleaning
procedure is activated (for instance when a cleaning procedure for
cleaning the clean room suit is activated), particles (such as dust and/or
germs) on and/or in the clean room suit are moved out (for instance are
blown out) of the clean room suit (for instance from an interior and/or
from a surface of the clean room suit) towards an exterior of the clean
room suit.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-3-
According to still another exemplary embodiment of the invention,
a clean room arrangement is provided which comprises a clean room suit
having the above mentioned features, and a clean room lock having the
above mentioned features, wherein the clean room suit adapter (of the
clean room lock) and the clean room lock adapter (of the clean room
suit) are configured (to match to one another) for coupling to one
another.
According to yet another exemplary embodiment of the invention,
a method of managing a transition of a person in a clean room lock
between an interior and an exterior of a clean room (for instance for
cleaning a person passing from an unclean environment towards a clean
room, or in the opposite direction) is provided, wherein the method
comprises clothing the person with a clean room suit having a fabric (for
instance designed as an overall) covering at least a part of a (for instance
the entire) body of the person, coupling a clean room lock adapter
arranged on and/or in the fabric to a clean room suit adapter of the clean
room lock, and activating a cleaning procedure controlled by the clean
room lock so that particles on and/or in the fabric are moved out of the
fabric towards an exterior of the clean room suit (for instance activating a
cleaning procedure for cleaning the clean room suit).
In the context of this application, the term "clean room" may
particularly denote an area in which air quality, temperature, humidity,
and particle concentration may be regulated and monitored in order to
protect sensitive equipment from contamination. Air in a clean room may
be repeatedly filtered to remove dust particles and other impurities that
can damage the production of highly sensitive technologies such as the
semiconductor technology. Hence, a clean room may be denoted as a
room in which the concentration of air-born particulate matter may be
controlled at specific limit to facilitate the manufacture of sterile and
highly purified products. In the context of pharmaceutical technology and
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-4-
medical technology, such particles may to be controlled and monitored
and germs may be eliminated which might deteriorate the sterile
properties of such a room.
The term "clean room lock" may particularly denote a device or
chamber permitting the passage of people (and optionally objects)
between a clean room ("clean side") and a surrounding ("unclean side").
Such a lock may comprise a small chamber with for instance two airtight
doors in series which (in the absence of an emergency case) do not open
simultaneously. Hence, a clean room lock can be used to allow passage
of persons and objects between a clean environment and a less clean
environment. Therefore, within such a lock, cleaning procedures may be
performed.
The term "particles" may particularly denote any particulate matter
in the air and/or on objects which might deteriorate sterile and clean
properties. For instance, such particles may include dust, germs, smoke,
etc.
The term "adapter" may particularly denote parts that connect two
devices or systems to enable them to work together. Such an adapter
can be a device, mechanism or fitting usable to make two parts of
different design, shape or size fit to each other. Thus, such an adapter
may achieve operative compatibility between devices. For example, a
clean room suit adapter may be a fastening element forming part of a
clean room lock for fastening to a clean room suit worn by a person in
the clean room lock. Correspondingly, a clean room lock adapter of a
clean room suit may be a fastening element matching to another
fastening element and allowing to couple the clean room suit to a clean
room suit adapter of the clean room lock. Such a coupling may be a
sealed coupling between an interior of the clean room suit and a fluid
supply of the clean room lock. For instance, two correspondingly shaped
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-5-
and dimensioned fastening elements may be engaged to one another in a
reversible or detachable manner to provide the adapter function.
The term "clean room suit" which may also be denoted as a bunny
suit may denote an overall garment to be worn by a person in a clean
room, One example is an all in one cover which may be appropriate for
semiconductor and nanotechnology line production, technicians and
process engineers as well as for people in similar rolls creating sterile
products for the medical device and pharmaceutical industry. A clean
room suit may cover the wearer to prevent skin and hair from being
brought in contact with a clean room environment. A clean room suit may
be integrally formed in one piece or may comprise several separate
garment items worn tightly together.
The term "fabric" may particular denote any flexible material which
can be used as a basis for a garment or a suit. For example, such a fabric
may comprise a textile material which, in turn, may comprise a network
of natural or artificial fibers such as thread or yarn. Thus, a fabric may be
considered as an artifact made by weaving or felting or knitting or
crocheting natural or synthetic fibers,
The term "person" may particularly denote a human being wishing
to enter or leave a clean room. The term "person" should be considered
as a human being of an average size, particularly between 1,50 m and
1,90 m in height and between 40 kg and 120 kg of weight. However, also
children can be considered as persons in the context of the present
application.
The term "fluid" may particularly denote any gas and/or liquid,
wherein optionally also solid particles may be part of such a fluid. Gases
of such fluids may particularly be basically inert gases such as oxygen
molecules, nitrogen molecules, air or noble gases. Fluids may particularly
include water and aqueous solutions and also liquid chemicals for
instance for sterilization purposes.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-6-
According to an exemplary embodiment, an architecture for a clean
room system is provided in which it may be dispensable that a person
entering a clean room from an unclean side has to change clothing
several times and has to pass multiple lock stages. This may be achieved
by providing the person with a (for instance single) clean room suit to be
worn at least over a majority portion of the body and having a clean
room lock adapter allowing to couple a tube or the like of a clean room
lock to the clean room lock adapter. The clean room lock adapter may
guide the tube or the like towards an interior of the clean room suit so
that for instance by the application of pressure a fluid may be pumped in
an interior of the clean room suit so that both particles on an outer
surface of the clean room suit as well as particles within a fabric layer of
the clean room suit are promoted to move apart from the clean room
suit, he. away from the person wearing the clean room suit, so that these
particles may be removed from the person wearing the clean room suit
and can be sucked off subsequently. By taking this measure, a very
simple, save and efficient procedure of cleaning a person wearing a clean
room suit is provided so that it may become possible that a person enters
a clean room from an unclean side by a reduced number of clean room
locks, for instance by only a few clean room locks or by only a single
clean room lock.
In the following, further exemplary embodiments of the clean room
suit will be explained. However, these embodiments also apply to the
clean room lock, to the clean room arrangement and to the method.
The fabric may be configured to cover the entire body of the
person, optionally with the exception of feet, hands and/or a part of the
head of the person (particularly an eye portion may be free of the
garment and may be covered by separate safety glasses). For instance,
the clean room suit may be basically an overall suit, wherein only feet,
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-7-
hands and a part of the head (particularly including the eyes) may
remain free of the fabric and may be protected by other components
such as individual shoe pieces, glove pieces, protection glasses, etc.
The fabric may be configured to sealingly end at feet, hands and/or
a part of the head when these body parts remain uncovered by the
fabric. Therefore, at these end portions of the fabric where other clean
room suit elements such as shoes, gloves or glasses are to be worn by
the person, the fabric may seal the body within the fabric with regard to
an environment, for hygienic reasons and to form a basically air-tight
volume within the fabric which can hence be slightly inflated by pumping
a pressurized gas in this volume via the clean room lock adapter. This
may be achieved by an elastic band or the like which may be provided at
end portions of the garment for instance close to the feet.
The fabric may comprise an inner fabric portion (such as a lining)
to be placed directly on at least a part of the body (for instance, the inner
fabric portion may be placed directly on the skin of the person, wherein it
is possible that the person optionally wears underwear below the inner
fabric portion). The fabric may also comprise an outer fabric portion
(such as a shell fabric) to be exposed to an exterior environment of the
clean room suit. In other words, the inner fabric portion may be arranged
between the body and the outer fabric portion. Thus, the fabric may be
made of two (or more) separate components. Such a construction may
allow each of the components to be specifically adapted to the
corresponding task of a respective component.
Particularly, the inner fabric portion and the outer fabric portion
may be connected to one another (for instance in a sealed manner). For
instance, these two fabric portions may be connected to one another by
sewing, welding, gluing, etc. so that a single piece covering the majority
of the body may be provided which eases the use for a user.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-8-
Furthermore, a fluidic lumen may be formed between the inner
fabric portion and the outer fabric portion which can be in fluid
communication with the clean room lock adapter so that a fluid such as a
gas may be pumped in the lumen between the inner and the outer fabric
portion. The material particularly of the outer fabric portion may be
selected such that on the one hand the lumen within the outer fabric
portion is sufficiently sealed to allow for some inflation of the clean room
suit upon applying a pressurized gas flow to the lumen within the outer
fabric portion. Simultaneously, the material particularly of the outer
fabric portion may be selected such that on the other hand the outer
fabric portion is sufficiently permeable for the pressurized gas to allow
the gas to stream through the outer fabric portion, thereby carrying away
particles within the outer fabric portion and at an outer surface of the
outer fabric portion to clean the fabric.
According to another exemplary embodiment, the fabric of the
clean room suit comprises one or more antimicrobial agents being
capable of controlling microbial growth on clean room equipment,
particularly on the clean room suit, wherein the one or more antimicrobial
agents are of organic origin.
The term "clean room equipment", as used herein, refers to any
goods that are required for a production process in a clean room
environment including inter alia furniture, any types of devices or
apparatuses (e.g., pipettes, robots, centrifuges, work benches, computer
systems, and the like), laboratory supplies (e.g., tubes, culture dishes,
pipette tips, pens, paper, notebooks, packaging containers, and the like),
and particularly clean room clothing such as a clean room suit. The clean
room clothing may further be provided with one or more antimicrobial
agents of inorganic origin, for example gold and/or silver fibers
incorporated in the fabrication materials.
The term "controlling microbial growth", as used herein, denotes
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-9-
any activity for completely inhibiting or at least reducing the growth of
microorganisms such as bacteria, archaea, yeasts, fungi, and viruses. In
particular, the term relates to biologically controlling growth of said
microorganisms, that is, by employing biological means as "growth
inhibitors or growth reducing agents."
The term "inhibiting", as used herein, is to be understood as not
only to include the prevention of further growth but also the killing of
microorganisms. The term "reducing", as used herein, denotes any
decrease in microbial growth (i.e. the growth rate), for example, a
decrease of at least 5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 60%,
70%, 80%, 85%, 90% or 95% as compared to control conditions (i.e. in
the absence of antimicrobial agents).
In specific exemplary embodiments, the antimicrobial agents are
capable of controlling growth of extremo-tolerant microorganisms, that
is, microorganisms specifically adapted to rather harsh environmental
conditions (e.g., depletion of nutrition factors, environmental stresses,
and the like) such as the conditions occurring in clean room
environments. Numerous extremo-tolerant microorganisms are known in
the art (see, for example, La Duc, M.T. et al. (2007) Appl. Environ.
Microbiol. 73, 2600-2611; Moissl, C. et al (2007) FEMS Microbiol. Ecol.
61, 509-521).
The term "antimicrobial agent", as used herein, refers to any
compound or means having an inhibitory (or antagonistic) effect on the
growth of microorganisms, that is, agents that are capable of at least
reducing the growth rate (e.g., bacteriostatic agents with respect to
controlling the growth of bacteria) as well as agents that cause toxic
effects (e.g., bactericide agents killing bacteria) provided that these
compounds or means are of organic origin (i.e. composed of organic
compounds).
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-10-
The one or more antimicrobial agents may be arranged on any
portion(s) of the fabric comprised in the clean room suit. Preferably, said
agents are arranged on the outer portion of the fabric.
The arrangement of said antimicrobial agents may be accomplished
by any physical and/or chemical contacting method known in the art. The
contacting step may comprise a physical contacting method, particularly
a method selected from the group consisting of spraying and gassing of
the fabric with the one or more antimicrobial agents (present, e.g., in
gaseous form, as an aerosol, a solution or a foam), for example by
means of one or more spray heads, nozzles, pump-injectors, spray
valves, and the like.
Additionally and/or alternatively, the contacting step may comprise
a chemical coupling of the one or more antimicrobial agents (present,
e.g., in gaseous form, as an aerosol, a solution or a foam) to the fabric.
Chemical coupling can be achieved either by covalent or non-covalent
bonding between the one or more antimicrobial agents and the fabric.
The term "covalent bonding" refers to a form of chemical bonding
characterized by the sharing of one or more pairs of electrons between
two components, producing a mutual attraction that holds the resultant
chemical linkage (i.e. molecule) together. The term "non-covalent
bonding" refers to a variety of interactions that are not covalent in
nature, between molecules or parts of molecules that provide force to
hold the molecules or parts of molecules together usually in a specific
orientation or conformation. Such non-covalent interactions include inter
alia ionic bonds, hydrophobic interactions, hydrogen bonds, Van-der-
Waals forces, and dipole-dipole bonds.
The one or more antimicrobial agents may either be attached
directly to the fabric or via a given linker molecule. Numerous linkers are
known in the art. A skilled person is well aware of selecting a particular
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-11-
linker molecule appropriate for coupling a given antimicrobial agent to
the fabric to be treated.
The one or more antimicrobial agents may be (chemically) coupled
in a functionally active form or as an inactive precursor molecule that
needs to be activated, e.g., by enzymatic cleavage, photolysis, UV
radiation, and the like. In some exemplary embodiments, the
antimicrobial agent(s) are activated simply be releasing it from the fabric
on which they are aranged, for example via a cleavable linker molecule.
In other exemplary embodiments, the one or more antimicrobial agents
are incorporated or embedded in vesicles, layers, coatings or other
structures arranged on or incorporated in the fabric (e.g., located on the
outer portion of the fabric or between the inner and outer portions of the
fabric) from that they are released, for example, in form of a sustained
release formulation over a given period of time or upon a particular
trigger such as heat, radiation or the presence of microbial contaminants
(i.e. the agents are incorporated in a bioactive layer). All such
configurations are well established in the art.
Preferably, the one or more antimicrobial agents are selected from
the group consisting of microorganisms, preferably of bacteria, yeasts,
and fungi, and metabolites produced by said microorganisms.
"Antagonistic" microorganisms that may be employed herein are
characterized by one or more structural and/or functional properties
conferring to them a selection advantage as compared to the
microorganisms to be treated, thus resulting in a superior growth rate.
Such organisms include inter a/ia (i) microorganisms that compete with
the microorganisms to be treated for any nutrients and/or any trace
elements (e.g., by depleting Fe3-' via the secretion of iron-chelating
siderophores) and/or for living space, (ii) microorganisms that produce
(and optionally secrete in form of exoenzymes) lytic compounds such as
glucanases, chitinases, cellulases, hydrolases (e.g. lysozyme), and
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 12-
lyases, and (iii) microorganisms that produce antimicrobials/antibiotics,
toxins, volatile organic compounds (VOCs; i.e. chemical compounds that
have high enough vapor pressures under normal conditions to
significantly vaporize and to enter the atmosphere) and/or biosurfactants
(i.e. surface-active compounds that enhance the emulsification of
hydrocarbons, have the potential to solubilize hydrocarbons and increase
their availability for microbial degradation).
Examples of antibiotics include inter alia P-lactam antibiotics (e.g.,
penicillin, cephalosporin), tetracycline, aminoglycoside (e.g.,
streptomycin, kanamycin, gentamicin), and macrolides (e.g.,
erythromycin). The term "toxins", as used herein, also includes
bacteriocins, that is, proteinaceous toxins produced by bacteria to inhibit
the growth of similar or closely related bacterial species. Examples of
such toxins are, e.g., colicin, halocin, nisin, sakacin, and vibriocin.
Examples of volatile organic compounds include inter alia methane and
formaldehyde. Finally, examples of biosurfactants include inter alia
mycolic acid, glycolipids, polysaccharide-lipid complexes, lipoproteins or
lipopeptides, and phospholipids.
Instead of and/or in addition to the microorganisms as such one or
more metabolites produced by said microorganisms, preferably the
exoenzymes, antibiotics, volatile organic compounds, and biosurfactants
mentioned above, may be employed herein. The term "produced by said
microorganisms", as used herein, is not to be understood that practicing
it is necessarily required to isolate any "growth inhibitory" metabolites
from said microorganisms. Rather, the term merely refers to the fact that
said metabolites represent "antagonistic factors" of natural origin
produced (and optionally secreted) by microorganisms.
Accordingly, the "antagonistic" metabolites employed herein may
not only be naturally occurring metabolites (i.e. metabolites isolated from
antagonistic microorganisms as defined herein) but also corresponding
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 13-
metabolites that are chemically synthesized or generated by means of
recombinant gene technology (see, for example, Sambrook, J. et at.
(1989) Molecular, Cloning: A Laboratory Manual, 2nd ed., Cold Spring
Harbor Laboratory Press, Cold Spring Harbor, NY).
In some other exemplary embodiments, one or more of the
microorganisms and/or metabolites are isolated or enriched from plants,
preferably from the rhizosphere, endosphere or phyllosphere of plants.
The term "rhizosphere", as used herein, denotes the region (layer) of soil
(including the root surfaces) that is directly influenced by secretions of a
plant's roots and root-associated soil microorganisms. The term
"endosphere", as used herein, denotes the interior of a plant. Finally, the
term "phyllosphere", as used herein, refers to a plant's leaf surfaces or
the total above-ground surfaces of a plant.
Examples of "antagonistic" microorganisms that can be isolated
from the rhizosphere include inter alia Chryseobacterium spec.,
Enterobacter spec., Salmonella typhimurium, Serratia spec.,
Stenotrophomonas maltophilia, Staphylococcus spec., and Pseudomonas
aeruginosa. Examples of "antagonistic" microorganisms that can be
isolated from the endosphere include inter alia Staphylococcus
epidermidis, Pseudomonas aeruginosa, Streptococcus mitts, Serratia
spec., Enterobacter spec., and Stenotrophomonas maltophilia. Finally,
examples of "antagonistic" microorganisms that can be isolated from the
phyllosphere include inter alia Serratia plymuthica, Pseudomonas putida,
and Pantoea agglomerans.
The one or more antimicrobial agents employed in a given setting
may all be isolated or enriched from a single plant species (variety) or
they may be derived from at least two different plant species of the same
plant genus or they may be isolated or enriched from at least two plants
belonging to different genera.
In further particular exemplary embodiments, the plants are
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-14-
selected from the dicotyledon plants (also referred to as "dicotyledons" or
"dicots"). The term "dicotyledon plants", as used herein, denotes the
group of flowering plants (i.e. angiosperms) whose seed typically has two
embryonic leaves or cotyledons. However, in other embodiments
monocotyledon plants (also referred to as "monocotyledons" or
"monocots") having only a single cotyledon may be employed as well.
Examples of dicotyledons to be used herein include inter alia
Fragaria spec. (i.e. strawberry), Brassica napus (i.e. rape or oilseed
rape), Cucurbita pepo (i.e. pumpkin or squash), Beta vulgaris (i.e. beet),
Solanum tuberosum (i.e. potato), Sphagnum spec. (i.e. peat moss),
Viscum album (i.e. mistletoe), and Acer tataricum (i.e. Tatar maple).
The term "isolated", as used herein, denotes that the antimicrobial
agents used herein are present in pure (or almost pure) form, for
example at least 75%, 80%, 85%, 90%, 95% 98% or 99% pure or
completely pure (i.e. 100% pure). The term "enriched", as used herein,
denotes a crude preparation (e.g., a plant extract) subjected to one or
more basic purification steps (e.g., a filtration, a precipitation or a
centrifugation step), thus resulting in the accumulation of the
antimicrobial agents in such preparation which, however, does still
include significant impurities. For example, such enriched preparations
may be at least 20%, 30%, 40%, 50%, 60% or 70% pure.
The one or more antimicrobial agents employed herein may
comprise microorganisms of any one or more microbial species exhibiting
an antagonistic (i.e. growth inhibiting or at least growth reducing)
behavior towards one or more microorganisms inhabiting a clean room
setting. Examples of such "antagonistic" microorganisms include inter alia
any species belonging to the group consisting of the genera
Acinetobacter, Aeromonas, Agrobacterium, Amycolatopsis, Arthrobacter,
Bacillus, Brevundimonas, Burkholderia, Cellulomonas, Citrobacter,
Clavibacter, Chromobacterium, Comamonas, Corynebacterium,
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-15-
Chryseomonas, Curtobacterium, Deiftia, Enterobacter, Enterococcus,
Erwinia, Escherichia, Flavobacterium, Frigoribacterium, Gordonia,
Herbaspirillum, Klebsiella, Kitasatospora, Kocuria, Kytococcus,
Lactobacillus, Leifsonia, Lysobacter, Me thylobacterium, Microbacterium,
Micrococcus, Micromonospora, Morganella, Neisseria, Nocardia,
Oerskovia, Paenibacillus, Pantoea, Pectobacterium, Proteus, Rahnella,
Ralstonia, Rhizomonas, Salmonella, Serratia, Sphingobacterium,
Sphingomonas, Staphylococcus, Streptomyces, Stenotrophomonas,
Streptoverticillium, Tsukamurella, Varlovorax, Xanthobacter, and
Xanthomonas. All these genera are well known in the art (cf., for
example, Garrity, G.M. (ed.) Bergey`s Manual of Systematic Bacteriology,
Vol. II - The Proteobacteria (Brenner, D.J. et al., eds.), 2"d ed. (2005),
Springer Press, New York).
The antimicrobial agents comprised in the fabric of the clean room
suit may include any species of antagonistic microorganisms or any
combination of any one or more species belonging to any one or more
genera of the antagonistic microorganisms referred to herein above. For
example, two or more species of microorganisms belonging to the same
genus (e.g., Bacillus, Microbacterium or Stenotrophomonas) may be
employed as well as two or more species of microorganisms belonging to
at least two different genera (e.g., (Enterobacter and Paenibacillus) or
(Cellulomonas and Neisseria and Xanthobacter)).
Preferably, the one or more antimicrobial agents comprise
microorganisms of the species Serratia plymuthica and/or of the species
Pantoea agglomerans, and/or the one or more antimicrobial agents
comprise one or more metabolites selected from the group consisting of
exoenzymes, antibiotics, volatile organic compounds, and biosurfactants.
The term "one or more" (antimicrobial agents), as used herein,
denotes that a single type of microbial agent may be employed (that is,
e.g., a particular microorganism (i.e. a particular species) or a specific
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 16-
metabolite) or at least two different types of microbial agents, wherein
the at least two different types may be, e.g., at least two different
species of microorganisms (that may or may not belong to the same
genus) or at least two different types of metabolites (that may or may
not belong to the same class of chemical compounds) or at least one
microorganism and at least one metabolite. Accordingly, the one or more
antimicrobial agents employed herein may comprise, for example, three
different species of microorganisms (e.g., Serratia plymuthica, Pantoea
agglomerans, and Stenotrophomonas maltophilia), or four different types
of metabolites (e.g., a glucanase, kanamycin, and two biosurfactants), or
a combination of two different species of microorganisms (e.g., Serratia
plymuthica, and Pantoea agglomerans) with two different types of
metabolites (e.g. two biosurfactants).
The choice of (a) particular antimicrobial agent(s) for a given
scenario may depend inter alia on the extent of microbial growth (i.e. the
quantity) present on or supposed to be present on the clean room
equipment as well as on the nature (i.e. the quality) of the contaminants
present. The skilled person is also well aware how to select one or more
antimicrobial agents for a given scenario.
The clean room lock adapter may be arranged on and/or in the
outer fabric portion. In contrast to this, the inner fabric portion may be
free of the adapter. Therefore, it may be easily possible even for an
unskilled user to connect a clean room suit adapter to the clean room
lock adapter for providing fluid communication between clean room suit
and clean room lock (particularly a tube thereof) in an airtight manner.
In an embodiment, the inner fabric portion may be made of a first
material and the outer fabric portion may be made of a second material
differing from the first materials. Hence, the material selection of the
inner fabric portion and of the outer fabric portion may be different and
may be specifically adjusted with regard to corresponding tasks of these
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 17-
respective fabric portions. A task of the inner fabric portion is to prevent
particles from the human body to reach the outer surrounding of the
fabric, i.e. a cabin of the clean room lock. The outer fabric portion may
have the task to prevent any particles from adhering to this fabric
portion, in order to keep the clean room clean.
Accordingly, the inner fabric portion may be made of a breathable,
eudermic material being impermeable for particles from the body of the
person. It may happen that on a surface of the body of the person, small
particles are present which should not be permeated through the inner
fabric portion towards an outer portion of the clean room suit. A
breathable and eudermic material not only allows for a convenient wear
of the clean room suit by a person, but also prevents or suppresses
sweating or the like which can also be the origin of impurities.
The outer fabric portion may be made of a material being repellent
against particles such as dust and/or germs and being clean room
compatible. Therefore, such an outer fabric portion may be made of a
material which is not prone to particle adhesion to an outer portion
thereof or to pores within the fabric portion. Therefore, by a
corresponding selection of such a material, the performance of the clean
room suit may be further improved.
It should be mentioned that the separation of the fabric into two or
more separate fabric portions may be advantageous, but that in other
embodiments, the fabric may be made of a single component, piece or
material.
The clean room lock adapter of the clean room suit may be
arranged on and/or in the fabric at a position of an abdomen of the
person wearing the clean room suit. For example, the clean room lock
adapter may be approximately positioned close to a navel of the person.
Such a position is an appropriate position enabling even an unskilled user
to easily connect a hose or tube or the like of the clean room lock to the
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 18-
adapter of the clean room suit for providing a pressurized gas beam for
cleaning purposes within the clean room suit.
The clean room lock adapter may comprise a connection flange
configured for mounting on a correspondingly configured clean room lock.
Therefore, the clean room lock adapter and the clean room suit adapter
may be correspondingly shaped sized and dimensioned so as to allow for
a mating connection between these two adapter portions, in a reversible
or detachable way. Preferably, such a connection may result in a gas-
tight configuration so that it may be possible to provide a fluid such as a
gas via the connected clean room lock adapter/clean room suit adapter
without leakage. The assembly of clean room lock adapter and clean
room suit adapter to one another should also be reversible so that, for
instance with a single hand motion of the user, the user may couple or
decouple clean room suit and clean room lock to one another or decouple
them from one another.
Particularly, the connection flange may be configured for mounting
on the correspondingly configured clean room lock by a snap-in
connection, a bayonet connection, a screwing connection, or any other
fluid sealed-connection. With such easily operable connection systems, it
may be ensured that the two adapters may selectively engage one
another or disengage one another by a single hand motion which allows
to operate the system in a short time and without the need of
experienced skills of a user.
The clean room lock adapter may comprise a membrane adapted
for being movable upon applying a pressure on the membrane. Such a
membrane may be made of one or more diaphragms which can provide
some kind of sealing function for sealing an interior of the clean room suit
in the absence of an external pressure, i.e. below a predefined threshold
value. When this threshold value is exceeded, the membrane may be
deformed so as to allow a fluid transfer from a hose and both adapters
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
19-
through the membrane, therefore allowing to pump up an interior of the
clean room suit by applying a pressure via the clean room lock adapter
and the membrane.
According to an exemplary embodiment, it is possible that an
electrical pickup member (for instance a contactor or an activation switch
or any kind of sensor) may be arranged on or at the membrane or at
another portion of the clean room lock adapter. Such an electrical pickup
member may be capable of sensing, for instance, whether the clean room
lock adapter is presently connected to a clean room suit adapter. A
corresponding sensor signal may be a necessary condition before
execution of a cleaning procedure is approved by an automatic control
unit such as a microprocessor. It is also possible that such an electrical
pickup member provides a measurement signal indicative of the presence
or absence of an external pressure applied via the adapter to an interior
of the clean room suit.
The clean room lock adapter may be configured for conveying or
blowing a fluid provided by the clean room lock to an interior of the fabric
and from there through the fabric so that particles on and/or in the fabric
are moved out of the fabric towards the exterior of the clean room suit.
In other words, it may be possible to connect a gas hose to the clean
room lock adapter allowing to provide a gas pressure which may result,
due to some kind of sealing connection of the clean room lock adapter
and the clean room suit adapter, in an overpressure in an interior of the
clean room suit. Such an overpressure may generate a force acting on
potentially present particles on an exterior wall of the clean room suit
and/or within the fabric. Thus, such particles may be blown out of the
clean room suit towards a portion of the clean room lock surrounding the
clean room suit. Such particles can then be sucked off so that an efficient
cleaning of the clean room suit can take place in a very short time.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 20-
It should be mentioned that, as an alternative to an overpressure,
it is also possible that other forces promoting removal of particles from
an interior towards an exterior of the clean room suit can be applied, for
instance the removal of such particles by increasing the temperature
within the clean room suit, by generating concentration differences
between an interior and an exterior of the fabric for promoting
equilibration of the concentration gradient by particle migration or any
other suitable measure such as the use of electric and/or magnetic
forces.
In an embodiment, the clean room suit may comprise goggles
configured to cover at least the eyes of the person. Such a pair of
protection goggles may have a plurality of synergetic effects. Firstly, the
goggles may prevent dust on a face of a person to be exposed to an
environment. Secondly, such goggles may also serve as a protection
against further optional cleaning procedures such as the introduction of a
sterilizing chemical agent in the clean room lock and/or the introduction
of sterilizing ultraviolet radiation (or electromagnetic radiation of any
other appropriate wavelength range) into a cabin of the clean room lock.
Therefore, a corresponding chemical inertness of the material of the
goggles as well as a radiation filter function for filtering potentially
harmful frequencies of such ultraviolet radiation may be considered in the
goggles.
Furthermore, the clean room suit may additionally comprise shoes
configured to cover feet of the person. The clean room suit may also
comprise gloves configured to cover hands of the person. Therefore,
basically the entire body of the person may be covered with a protection
material.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 21 -
Next, further exemplary embodiments of the clean room lock will
be explained. However, these embodiments also apply to the clean room
suit, to the clean room arrangement and to the method.
The clean room suit adapter of the clean room lock may comprise a
connection flange configured for mounting on a correspondingly
configured clean room suit, particularly a correspondingly configured
clean room lock adapter of the clean room suit. In other words, the
adapters of clean room lock and clean room suit may be configured to
correspond to one another.
Such a connection flange may be configured for mounting on the
correspondingly configured clean room suit by a snap-in connection, a
bayonet connection, a screwing connection, or any other fluid sealed-
connection.
The clean room lock may comprise a tube connected to the clean
room suit adapter and configured for supplying a pressurized fluid to the
clean room suit adapter. Such a tube may be anchored in a wall, for
instance a sidewall, enclosing a cabin of the clean room lock. Thus, a
vertical wall accommodating a fluid supply unit may be adapted for
supplying a fluid to the tube. Such a fluid supply unit may for instance be
a gas container for providing an overpressure or may also be a gas
supply system of a hospital, a plant or the like.
Thus, the connection may be such that a fluid supplied via a hose
of the clean room lock (which hose may be mounted on or anchored at a
wall or the like of the clean room lock and may be in fluid communication
with a gas supply) is conducted through the clean room suit adapter and
the clean room lock adapter to an interior of the clean room suit.
The clean room suit adapter may be configured for providing a
pressurized fluid to the clean room suit so that the particles on and/or in
the clean room suit are moved out of the clean room suit towards the
exterior of the clean room suit. For instance, the pressure provided via a
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-22-
fluid conduit or tube towards the clean room suit adapter may be
supplied with an overpressure of more than one atmosphere, for instance
in a range between 1.2 bar and 2 bar, so as to pump up an interior of the
clean room suit to efficiently move particles from the clean room suit
towards a surrounding thereof under the influence of an overpressure
force.
In an embodiment, the clean room lock may comprise a cabin
being spatially delimited by (for instance four) side walls, a bottom wall
and a ceiling, wherein the cabin may be configured so that a standing
person properly fits into the cabin. For instance, a ground area of the
cabin may be in a range between 1 m2 and 4 m2, for instance may be
about 2.5 m2. Within such a cabin, it should be possible that the person
can stand without suffering from claustrophobic feelings. At the same
time, the volume of the cabin should be sufficiently small so that a
cleaning treatment has to be applied only to a small volume, thereby
keeping the system simple and cheap in operation.
The bottom wall of the clean room lock may comprise a marker
indicative of a target position of feet of the person standing in the cabin.
For example, such a marker may comprise any optical (for instance
boundary lines delimiting foot portions in a manner which is intuitive for a
person) and/or haptic (for instance recesses in the floor delimiting foot
portions in a manner which is intuitive for a person) indicia indicating at
which positions a person should put her or his foot or feet on. In view of
a high efficiency of a specific cleaning procedure to be executed, it may
be advantageous that the person is located at the correct position. It is
also possible that a circle (or any other area) is indicated on the ground
within which circle the person should stand. A correct standing positions
of the person may be controlled via one or more appropriate sensors.
For example, the marker may be arranged so that the target
position corresponds to a standing position in which the person stands in
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 23 -
the cabin with (for example slightly) straddled legs. In such a
configuration, the surface of the clean room suit between the legs of the
person may be also exposed to a cleaning procedure which may therefore
be applied to this surface as well, thereby preventing undesired
shadowing effects and ensuring a complete cleaning.
The bottom wall may comprise a transparent material. The
material of the bottom wall may be transparent for electromagnetic
radiation (such as UV) which can be applied from a bottom of the clean
room lock. For example, such an irradiation of the bottom may also allow
to cleaning a shoe sole of the clean room suit. Particularly, the material
of the bottom wall may be transparent for ultraviolet radiation which may
be used for a decontamination or sterilization through the bottom wall.
The clean room lock may further comprise one or more ultraviolet
radiation sources. Such ultraviolet radiation sources may be
accommodated below the bottom and may be configured for irradiating
the clean room suit from below, i.e. vertically, with ultraviolet radiation.
Additionally or alternatively, such ultraviolet radiation sources may be
accommodated on or in side walls of a cabin of the lock room and may be
configured for irradiating the clean room suit laterally or horizontally with
ultraviolet radiation. Additionally or alternatively, such ultraviolet
radiation sources may be accommodated on or in a ceiling wall of a cabin
of the lock room and may be configured for irradiating the clean room
suit vertically from above with ultraviolet radiation. For instance, UV-C
may be a suitable wavelength range for ultraviolet radiation usable for a
decontamination of a surface portion of the clean room suit. Particularly,
shoe soles of the clean room suit and a portion between the legs of the
person can be decontaminated by taking this measure.
A first one of the (for instance four) surrounding side walls
(wherein a circular side wall is possible as well) may comprise a first door
connecting the clean room lock with an exterior of the clean room, i.e. an
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-24-
unclean side. Such a door may be gas-tight when closed and may be
opened to allow a person to leave the clean room lock. Furthermore, a
second one of the side walls may comprise a second door connecting the
clean room lock with an interior of the clean room, i.e. a clean side. The
two side walls accommodating the two doors may be arranged to oppose
one another. Both doors may be gas-tight when closed. It is possible that
the doors include a transparent window such as a glass element so that
the person in the clean room lock may look out of the doors, thereby
preventing any tightness feelings.
The first door and the second door may be lockable, particularly
may be mutually lockable. A corresponding locking mechanism may be a
purely mechanical locking mechanism or may be an electronic
mechanism which automatically locks and unlocks the corresponding
doors in order to prevent undesired misuse of the doors which could
deteriorate the cleaning conditions in the clean room lock or even in the
clean room. However, it may be possible to provide an emergency
opening button allowing a user in the case of an emergency to open the
doors immediately.
The clean room lock may comprise a gas jet unit, particularly an
air jet unit, adapted for blowing a gas to the cabin around an exterior of
the clean room suit. Such a feature may be particularly advantageous in
combination with the application of an overpressure on an interior of the
clean room suit. The latter measure may generate a stream of particles
around the clean room suit which particles have been removed from the
clean room suit due to an applied overpressure. By providing an air jet
around the clean room suit (for instance a gas flow direction of the air jet
can be defined using the assumption that the person stands on markers
indicating a target standing position), such freed particles may be
removed from the surrounding of the clean room suit, thereby promoting
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 25 -
a proper cleaning of the clean room suit and preventing such particles
from a re-deposition on the clean room suit.
For instance, it is possible that such a gas jet unit comprises a
plurality of blowing nozzles, each capable of blowing the gas around an
exterior of the clean room suit. For instance, the plurality of nozzles may
be arranged in a plurality of groups, wherein nozzles of the same group
may be arranged along a vertical row and each blow along a same
direction, and nozzles of different groups are arranged to blow along
different directions. Therefore, it may be possible that nozzles of a group
generate a planar stream removing particles along a respective plane
adjacent the clean room suit. When a plurality of such gas jet planes
intersect one another, an efficient discharge of particles removed from
the clean room suit may be achieved. For instance, it is possible that four
such groups are arranged providing gas jet planes with an intersection
angle of about 90 .
It is possible that the plurality of nozzles are arranged along the
sidewalls of the cabin. Thus, the sidewalls may be used as carriers for gas
tubes connected to these nozzles, and the nozzles may be assembled to
such a sidewall or to a corner between sidewalls.
The clean room lock may further comprise a decontamination unit
(such as a chemical decontamination unit) adapted for ejecting a
decontamination agent (such as a chemical decontamination agent) for
decontaminating the clean room suit. Thus, in addition to the removal of
particles from the clean room suit, a protection against biological germs
may be further increased by for instance chemically decontaminating a
surface of the clean room suit, which may be advantageously for instance
for medical and pharmaceutical applications.
Such a decontamination unit may particularly supply the
decontamination agent to the cabin via the ceiling. However, it is also
possible that other sidewalls or also the bottom wall of the cabin provide
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-26-
nozzles for ejecting a decontamination agent such as diethyl-hexyl-
sebacate (DEHS) or hydrogen peroxide (H202). Therefore, the sterilization
efficiency may be increased. Although the person may be properly
protected against used chemicals by the clean room suit, it might be
advantageous to use a decontamination agent which is not harmful for
human beings.
Moreover, a suction unit may be provided for sucking gas off or
from the clean room lock. Such a suction unit may locally generate a
negative pressure, for instance to remove a chemical decontamination
agent after interaction with the clean room suit, particles, etc. In view of
the gravity force tending to accumulate matter close to the bottom, it
may be advantageous that the suction unit is arranged at or close to a
bottom position of the sidewalls, so that the ejection direction of the
chemical decontamination agent in combination with the gravitational
force result in a removal of a large portion of the injected chemical
decontamination agent by the suction unit.
One or more filter units may be arranged in a suction line or in
fluid communication with the suction unit so that the fluid sucked by the
suction unit is filtered by the filter unit. The suction unit may,
additionally
or alternatively to the removal of the chemical decontamination agent,
also suck gas including particles which have previously been removed
from the clean room suit. These particles may be captured in a filter of
such a filter unit at the entrance of the suction unit. Particularly, a pre-
filter may be arranged at such a position. If desired, such a pre-filter may
be combined with an additional filter for suspended particles.
The clean room lock may further comprise a gas supply unit
adapted for re-supplying filtered gas to the clean room lock after filtering.
By taking this measure, a circulating gas system may be provided which
allows to recycle the gas sucked from the clean room lock to be injected
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 27-
into the clean room lock again after purification or removal of the
particles.
In an embodiment, the clean room lock may comprise a handle
(more particularly two handles, one for each hand of the human being),
particularly a bow-type handle. Such a handle may be arranged above
the person so that hands of the person, when raised above a head, can
grip the handle. By taking this measure, undesired shadowing effects, for
instance of portions below axles of the human, may be prevented and a
large surface of the clean room suit may be exposed to the cleaning
procedure.
The handle may comprise one or more sensors which may be
configured for generating a signal triggering start of a predefined
cleaning procedure upon detecting that the hands of the person grip the
handle. It may be advantageous that the proper gripping of the handle
by the person is detected before the cleaning procedure starts. This
prevents an improper cleaning procedure in which for instance surface
portions below the axle of the person are not exposed to the cleaning
procedure.
The clean room lock may comprise a control unit adapted for
centrally controlling operation of the cleaning procedure. Such a control
unit may have processing and storage capability and may centrally
control operation of the entire system. More particularly, the control unit
may be adapted for automatically executing a predefined cleaning
procedure. It may be possible that a storage unit stores a plurality of
predefined cleaning procedures, each appropriate for a specific
application, so that a corresponding one of these cleaning procedures
may be selected for a specific application.
In an embodiment, the control unit may be configured for
automatically executing the predefined cleaning procedure only when
simultaneously at least the following three conditions are fulfilled:
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-28-
1. It should be ensured that the clean room suit adapter is coupled
to the clean room lock adapter. Only in this case, the cleaning by blowing
out particles from the surface and from an interior of the fabric of the
clean room suit may be ensured. For this purpose, a corresponding
sensor may be arranged at one of the adapters to provide a specific
trigger signal when a corresponding coupling between the two adapter
portions is detected;
2. Feet of the person have to stand at a target position; only when
this condition is fulfilled, a proper cleaning of the shoe soles may be
ensured and also shadowing effects between the legs of the person may
be prevented;
3. Hands of the person have to grip the handle; only when this is
detected, for instance using one or more sensors, it can be ensured that
the axle portions are prevented from being shadowed during a cleaning
procedure.
Next, further exemplary embodiment of the clean room
arrangement will be explained. However, these embodiments also apply
to the clean room suit, to the clean room lock and to the method.
The clean room arrangement may additionally comprise a clean
room. The clean room lock may be spatially arranged between an interior
and an exterior of the clean room. Therefore, when a person comes from
an unclean surrounding, this person first has to pass the clean room lock
via one door thereof, before, after having passed the cleaning procedure
within the clean room lock successfully, the person may open an opposed
door which allows access from the clean room lock towards the clean
room. It is possible that the clean room arrangement is used in either
direction, i.e. either from the unclean side to a clean side or from the
clean side to the unclean side.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
_29-
According to an exemplary embodiment, it is possible that one or
multiple of the described clean room locks is or are provided between the
unclean environment and the clean room. The selection of the number
(one or more) of clean room locks between clean room and unclean
environment may depend on purity requirements of a specific application.
For example, the clean room arrangement may be used in the
context of microtechnology clean rooms, semiconductor technology clean
rooms, pharmaceutical clean rooms, chemical clean rooms, a stock
breeding clean room, a medical clean room, a pharmacy clean room, or a
radioactivity clean room (for instance in a nuclear power plant). Other
applications are of course possible as well.
The aspects defined above and further aspects of the invention are
apparent from the examples of embodiment to be described hereinafter
and are explained with reference to these examples of embodiment.
The invention will be described in more detail hereinafter with
reference to examples of embodiment but to which the invention is not
limited.
Fig. 1 illustrates a perspective view of a clean room lock according
to an exemplary embodiment of the invention in which a person wearing
a clean room suit according to an exemplary embodiment is presently
located.
Fig. 2 illustrates a cross-sectional view of a clean room suit
according to an exemplary embodiment of the invention.
Fig. 3 and Fig. 4 show two partially exploded views of a clean room
suit from a bottom position according to an exemplary embodiment,
wherein Fig. 3 relates to a view from a clean side and Fig. 4 relates to a
view from an unclean side.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-30-
Fig. 5 and Fig. 6 show side views of access doors of a clean room
lock according to an exemplary embodiment, wherein Fig. 5 relates to a
view from a clean side and Fig. 6 relates to a view from an unclean side.
Fig. 7 illustrates a top view of the clean room lock of Fig. 5 and Fig.
6.
Fig. 8 shows a flowchart of a method according to an exemplary
embodiment of the invention.
The illustration in the drawing is schematically. In different
drawings, similar or identical elements are provided with the same
reference signs.
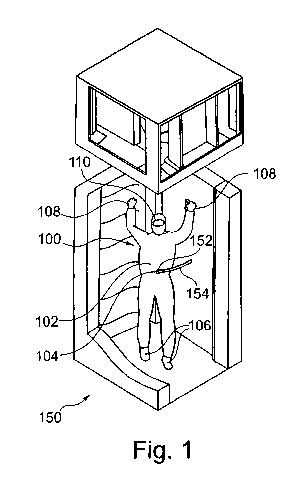
Fig. 1 illustrates a person wearing a clean room suit 100 according
to an exemplary embodiment of the invention and standing within a clean
room lock 150 according to an exemplary embodiment of the invention.
The clean room suit 100 comprises an overall-like fabric 102
covering a major portion of a body of the person, and therefore being
configured as an overall clean room suit. Close to a navel of the person, a
clean room lock adapter 104 is integrated in the fabric 102. In Fig. 1,
clean room lock adapter 104 is detachably coupled to a clean room suit
adapter 152 of the clean room lock 150. Clean room suit adapter 152 is,
in turn, coupled to a gas-tight tube 154. When gas is supplied from a gas
supply container (integrated in a side wall and hence not shown) via the
tube 154 and the adapters 152, 104 providing a sealed connection, the
fluid is supplied to an interior of the fabric 102 which can be therefore
slightly inflated. Consequently, particles which are potentially located on
an outer surface of the fabric 102 and/or in pores within the fabric 102
will be blown out of the fabric 102 towards a surrounding volume of the
clean room lock 150.
As can be taken from Fig. 1, only the hands, the feet and an eye
portion of the person are not covered by the fabric 102. However, the
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
_31_
feet are covered with clean room shoes 106 being sealedly connected
with the fabric 102. Furthermore, the hands of the person are covered by
gloves 108 being sealedly connected with the fabric 102 and the eyes are
covered with protection goggles 110. The goggles 110 may comprise a
wavelength selective filter to provide protection against UV radiation.
Although not shown in Fig. 1, the clean room suit 102 is made of
an inner fabric directly attached on the skin of the person and an outer
fabric exposed to the clean room lock 150. The inner fabric portion and
the outer fabric portion are connected to one another by sewing, wherein
an airtight lumen is formed between the outer and the inner fabric
portion which can be filled with gas via the tube 154 and the adapters
152, 104. The inner fabric portion is breathable, eudermic and
impermeable for particles originating from the body of the person,
whereas the outer fabric portion is repellent against particles and clean
room compatible.
Although not shown in Fig. 1, a membrane and an electrical pickup
are provided at the clean room lock adapter 104 so that an interior of the
clean room suit 100 is only in fluid communication with environment
when a pressure is supplied deforming the membrane. Furthermore, the
electrical pickup can detect the connection of the adapters 152, 104 to
one another and may generate a corresponding sensor signal
transmittable to a control unit 246 (see Fig. 2).
Details regarding the clean room lock 150 will be described in more
detail referring to Fig. 2.
The clean room lock 150 encloses a person cabin 202 having
sidewalls 204, 206, a bottom wall 208 and a ceiling wall 210. The
dimension of the cabin 204 is so that a standing adult person fits properly
into the cabin 202. Markers 212 (for instance a target circle or individual
foot markers) are provided on the transparent bottom wall 210 to
indicate a position where the person should stand in the cabin 202. When
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 32 -
the feet of the person are within the marker portions 212, the person
stands in the cabin 202 with slightly straggled legs, for instance with an
angle of 15 between the legs.
The material of the bottom wall 208 is transparent for UV
radiation, for instance may be a quartz glass plate with an aluminium
reflector. Although not shown in Fig. 2, an ultraviolet radiation source
may be arranged below the transparent bottom 208 so as to irradiate a
surface of the clean room suit 100, particularly a sole of the clean room
shoes 106, with ultraviolet radiation for sterilization purposes. Also close
to the sidewalls 204, 206, a number of UV sources 247 are arranged for
laterally irradiating the cabin 202 with UV radiation for sterilization
purposes.
Sidewall 204 comprises a first door 214, whereas sidewall 206
comprises a second door 216. The doors 214, 216 each have a glass
element 218. The doors 214, 216 also have a corresponding electrical
closure mechanism 220. Grips of the doors 214, 216 are indicated with
reference numerals 240 and 242, respectively.
A gas jet system is provided which comprises nozzle rows each
having eight nozzles 224 for blowing a gas towards the cabin 202,
thereby also directing a gas flow around an exterior of the clean room
suit 100. Via conduits 266, a chemical decontamination medium such as
DENS (diethyl-hexyl-sebacate) may be supplied in the cabin 202. The
chemical decontamination agent may be supplied to the cabin 202 via the
ceiling 210 and/or via the side walls 204, 206.
A suction system may provide an under pressure close to the
bottom wall 208 of the cabin 202 and may suck off gas and/or liquid from
the cabin 202 via pre-filters 228 and filters for suspended particles 230.
Ventilators 252 may provide a corresponding pump performance. After
filtering and hence cleaning, the filtered gas can be supplied back to the
cabin 202.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 33 -
Bow-type handles 232 are arranged in the cabin 202 above the
person so that arms of the person are raised above a head of a person
when the hands of the person grip the handles 232. The handles 232
comprise touch sensors 234, wherein the touch sensors 234 generate a
signal triggering start of a predefined cleaning procedure upon detecting
that the hands of the persons properly grip the handle 232, and therefore
also touch the touch sensors 234. Other kinds of sensors are of course
possible.
A clean side at which a clean room is directly connected is
indicated with reference numeral 236, whereas an unclean side (a
surrounding of the clean room) is indicated with reference numeral 238.
A user interface 246 at the clean side 236 allows a user to control
an operation mode of the cleaning procedure, allows for a control of the
ultraviolet decontamination and may house a central control unit for
controlling the system 100, 150. A further user interface 248 is provided
as well at the unclean side 238. A display panel 250 which, inter alia,
includes a start button and an emergency stop and exit button is shown
in the cabin 202 as well.
A motion sensor 254 is arranged at the ceiling wall 210 and may
detect when the person moves after starting of a cleaning performance in
the cabin 202 and can take corresponding measures, for instance may
terminate the cleaning procedure or may repeat the cleaning procedure.
PDAH units 260 can be seen at various positions. A PDAL unit 262
is shown as well.
Control valves 264 can be controlled individually by the control unit
246 or by a human operator, for instance for activating or deactivating a
DENS injection (which may be measured as well) via fluid conduits 266.
Air delivery can be achieved via a conduit 226, whereas exhaust air
can be conducted via a conduit 270.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-34-
Fig. 3 shows a partially exploded bottom view of the clean room
lock 150 from the clean side 236 without showing the door 214.
In contrast to this, Fig. 4 shows a partially exploded bottom view
of the clean room lock 150 from the unclean side 238 without showing
the door 216. An illumination 400 is shown. Furthermore, an UVC unit
402 is shown in Fig. 4 as well.
Fig. 5 shows a plan view on the clean side 236 showing an
emergency off switch 500 and a signal illumination panel 502.
Fig. 6 shows a plan view on the unclean side 238. An emergency
off button 600, a signal illumination panel 602, an operation panel for
operating an UVC unit 604 and an additional operation panel 606 are
shown. A main switch 608 and measurement ports 610 are shown as
well. Ventilators and filters are integrated in the air unit 612.
Fig. 7 shows a plan view on the ceiling 210 thereby also showing
the clean side 236 and the unclean side 238. The UVC units 247 are
indicated as well.
In the following, some basic recognitions of the present inventor
will be explained based on which exemplary embodiments of the
invention have been developed.
Clean rooms can be distinguished regarding design, technology and
use, as well as operation and regarding legal requirements. All these
characteristics depend on a specific application. For mechanical clean
room production for instance in the field of microelectronics, electronics
and mechanics, particles as solid matter are relevant, whereas for
pharmatechnology, medical applications and chemistry, safety labs and
food technology, microbiological impurities such as viruses, spores and
fungus are relevant.
Material and personal flows have to be strictly separated in a clean
room. Transferring materials into and out of a clean room is usually not
very problematic, whereas the passage of persons into a clean room and
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 35 -
out of a clean room may involve a lot of effort. This usually requires the
user to change the entire clothing. However, this is cumbersome and
involves a danger of introducing impurities. Maintenance, cleaning, and
waste management as well as supply and equipment for such a person
lock is expensive. It further requires a lot of time to introduce people into
a clean room and to guide people out of such a clean room.
In view of these considerations, a clean room arrangement is
provided according to an exemplary embodiment which can be
constructed with a small ground area of for instance about 2.5 m2 and a
combined locking technology. The construction can be considered as
some kind of air shower with gas blow processes. The basic construction
may be a double wall construction with two mutually electrically lockable
doors, a double bottom chamber with defined stand surfaces made of
quartz glass and a ceiling have integrated therein members such as
ventilators and filters. Left and right next to the doors, vertical air
channels may be provided in which air jet nozzles may be integrated. In
side walls, it is possible to integrate back flow openings and pre-filter
units as well as decontamination, irradiation and/or spray systems may
be integrated. For the cleaning of the clothing using a force being
directed out of the clothing and for the implementation of the
decontamination, bow shaped handle mechanisms may be implemented.
A pressure air arrangement may be integrated with corresponding safety
circuitry.
According to an exemplary embodiment it is possible to eliminate
the multiple change of clothing by providing a clean room clothing system
allowing to move between different clean room classes and safety zones
without changing the clothes. Furthermore, a cross-contamination or
undesired carry over of particles may be safely prevented. The lock
ensures that on the one hand the clothing is cleaned and is
decontaminated over its entire surface, and on the other hand provides
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
- 36 -
safety against an uncontrolled access of persons. Regulation of various
pressure levels can be ensured by the clean room lock as well.
Regarding the clean room clothing, a two layer textile system may
be provided with an inner fabric portion and an outer fabric portion. Both
fabric portions may be connected to one another and may be therefore
integrally formed. The interior portion may be close to the body and may
prevent am undesired carryover of particles from the body into the
intermediate zone. The outer fabric may be repellent regarding particles.
At a defined position of the outer fabric (hence, easily accessible by the
user), a membrane with an electrical pickup may be integrated into the
tissue. The end at the hands and feet may be matched with regard to
gloves and shoes and may be gas-tight. The head piece of the fabric may
be fixedly connected with the body clothing and may thus be integrally
formed with it. Exposed portions of the face may be covered by goggles.
The design of the clothing, the tissue, an optional coating of the tissue,
shoes, goggles and the membrane technology are advantageous features
of the clean room suit.
For enabling a person to enter from an unclean region to a clean
region, the person may enter, already wearing the above-described clean
room suit, the clean room lock. The opposing doors may be locked. The
person stands at indicia or markers arranged in the bottom so that the
legs are arranged in a desired position. Afterwards, the person may
attach an air tube to the membrane valve of the clean room adapter. The
person may grip with the hands hand grips arranged close to the ceiling,
so that the arms are in an elongated and straddled position. By contact
members and other kinds of sensors, a correct position of the feet, of the
air tube attached to the clean room suit and a correct use of the hand
grips can be ensured and can be a criteria which has to be fulfilled before
a cleaning procedure is initiated, for instance before a valve is opened for
providing pressurized air. The clean room suit is thereby ventilated or
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-37-
inflated so that particles are blown from an interior to an exterior of the
overall. Consequently, these particles are blown away to leave a surface
of the fabric. In this operation state, the clean room suit may look similar
to a balloon and may be free of folds.
When certain sensor signals indicate a proper positioning of the
person in the clean room lock, an air shower may be automatically
activated and the clean room air streaming away from the clean room
suit can be blown further away via channels with integrated nozzles (for
instance arranged at four sides of side walls or corners, in a diagonal
manner). The released particles can be sucked away and filtered close to
the bottom of the cabin.
It is possible that the entire air circulation is configured as a re-
circulation system. After a defined air rinse phase (for instance 5 to 8
seconds) a disinfection or sterilization system (irradiation with UV-C or
the like and/or application or spraying of chemicals, sterilization agent,
etc.) can be activated. Germs and other biological particles on the clean
room suit can be destroyed. Below the transparent bottom, UV-C
irradiation units may be mounted which may sterilize bottom portions
(soles) of the shoes.
Such a decontamination procedure is not dangerous for human
beings. UV-C rays do not penetrate deeply into the body surface.
Exposed skin portions of the face (for instance close to the eyes) may be
protected by goggles. A safety feature may be provided in connection
with the goggles. After the entire cleaning and decontamination process
(for instance 10 to 20 seconds) the process may be switched off and the
door may be released from the clean side.
Should the person be positioned apart from an expected position
(regarding hands, feet, etc.) or should the person not use the air tube in
a proper way, or if such conditions change during the cleaning cycle, the
process can be terminated immediately and can be started again before
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-38-
allowing access of the person to a clean room. All parameters and
conditions being relevant regarding safety and/or cleanness may be
permanently monitored.
In a similar manner, it is possible to perform a similar cleaning
procedure when transferring a person from the clean to the unclean side.
This may for instance be advantageous in the light of a danger of
carrying over germs from an interior to an exterior position (for instance
in safety labs, stock breeding, etc.). In this scenario, the cleaning
procedure may be performed in both directions, if desired. However, it is
possible that the cleaning procedure is only performed in one direction or
that different cleaning procedures are performed in the two directions, in
dependence on the passing direction.
In the following, referring to Fig. 8, a flow-chart 800 of operating
the described systems will be explained.
Block 802 indicates switching on of a mains supply of the clean
room system. In a block 804, a flushing procedure may be automatically
started for a time of for instance 60 seconds, while both doors are locked.
An LED (light-emitting diode) may be green at the clean and unclean
sides, and the LED may blink green within the lock, as indicated by a
block 806.
Both doors are unlocked in a block 808. Block 810 indicates that an
LED at the clean side is green, as well as LEDs on the unclean side and in
the lock.
Reference numeral 812 indicates an access to the clean room lock
from the unclean side. In this scenario, a block 814 indicates that an
entry via the door on the unclean side takes place. An LED on the clean
side is green, whereas LEDs on the unclean side and in the lock are
switched off, This is indicated by a block 816. In a block 818, the clean
side door is locked. In a block 820, a person is within the lock and both
doors are closed. In a block 822, the person is within the lock. Then a
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
_3g_
decision is made, see block 824 whether a time is larger than 15
seconds.
If no, an air flush is started, see block 826. Both doors are locked
in a block 828. A flush time of 60 seconds is adjusted, see block 830. In
block 832, both doors are unlocked, Block 834 indicates that an LED is
green on the clean side, on the unclean side and within the lock.
Block 836 indicates that a door locking on the unclean side is
unlocked and the person may exit via the unclean side. Therefore,
reference numeral 838 indicates that the clean room lock is left via the
unclean side.
Block 840 indicates that a green LED can be seen on the clean
side, whereas the LED is switched off on the unclean side and in the lock.
A block 842 indicates that an LED blinks green at the clean side,
and blinks green at the unclean side and within the lock.
In a block 844, the door on the clean side is locked. In a block 846,
the door is left via the unclean side; afterwards, the door on the unclear
side is closed. A block 848 is subsequently reached, and is also reached if
the decision in block 824 is yes.
In block 848, an automatic flushing procedure is started with a
flushing time of for instance 60 seconds. As indicated by a block 850, the
LEDs blink green at the clean, unclean and interior side. Both doors are
locked in a block 852, and the procedure continues with the block A.
If the clean room lock is left via the clean side, see reference
numeral 856, the procedure continues with the block 854. In block 854,
the door lock of the clean side is open and the person may exit via the
clean side. In a block 858, the clean door is locked and in a block 860 the
unclean door is locked. In a block 862, the door is left via a clean side,
afterwards the clean door is closed. The procedure then continues with
block A.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-40-
Coming back to block 810, a block 864 illustrates a scenario in
which an emergency button is operated. As can be taken from a block
866, the LED on the clean side is green, and the LED is switched off on
the unclean side and in the interior. In a block 868, the LEDs are red at
the clean side, at the unclean side and in the interior, when emergency
off has been pressed. As can be taken from a block 870, the locked door
on the unclean side is unlocked, and, see block 872, the lock on the clean
side is unlocked. The emergency button may be reset in a block 874.
Again coming back to block 810, now referring to an access to the
lock from the clean side, compare reference numeral 878, whether there
is an access to the door from the clean side. Block 880 corresponds to a
locking of the unclean door. When the person is in the lock, both doors
are closed, see block 882. Block 884 relates to both doors being locked.
Again, two scenarios can be distinguished. In a scenario denoted
with reference numeral 886 (lock is left to the clean side), the door is
opened in a block 888 and the unclean door is locked in a block 890.
Furthermore, in a block 892, the person leaves the clean door, after this
the clean door clean. The procedure then continues with block A.
In an alternative scenario after block 884, the lock may be left via
the unclean side, see reference numeral 894. The door on the unclean
side may be opened, see block 896, and the LED may be green on the
clean side, and may be off at the unclean side and the interior of the
lock, see block 898. The clean door may be locked, see block 900. When
block 902 corresponds to a person leaving the lock via the unclean door,
afterwards the unclean door is closed. A block 904 automatically starts
flushing for a flushing time of for instance 60 seconds, and both doors
are locked. The procedure then continues with block A.
Block 906 indicates that the LED blinks green on the clean side, the
unclean side and in an interior of the lock.
CA 02753683 2011-08-25
WO 2010/097333 PCT/EP2010/052078
-41-
It should be noted that the term "comprising" does not exclude
other elements or features and the "a" or "an" does not exclude a
plurality. Also elements described in association with different
embodiments may be combined.
It should also be noted that reference signs in the claims shall not
be construed as limiting the scope of the claims.