Note: Descriptions are shown in the official language in which they were submitted.
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
1
SEPARATION OF CARBON DIOXIDE AND HYDROGEN
This invention relates to the partial recovery of carbon dioxide from a
synthesis gas
stream comprising hydrogen and carbon dioxide thereby generating a carbon
dioxide
stream that may be used in a chemical process, or may be sequestered or used
for enhanced
oil recovery before being ultimately sequestered, and a hydrogen stream that
may be used
as fuel for a power plant thereby generating electricity or as fuel for a
burner, furnace or
boiler or as a refinery feed stream for upgrading of one or more refinery
streams or as a
hydrogen feed to a chemical process.
US 2007/0221541 relates to a multi-stage autorefrigeration process where the
first
two autorefrigeration stages combined remove about 76% of the total moles of
acid gases
and about the same percentage of moles of CO2 in the starting syngas. It is
said that if the
sulfur had been removed from the syngas initially during syngas production, a
removal
percentage of 76% could quite possibly be, depending on the future of
environmental law,
adequate for sequestration of CO2. In such a case, no additional
autorefrigeration stages
would be needed. However, a disadvantage of the process of US 2007/0221541 is
that the
liquefied acid gases that are separated in the two autorefrigeration stages
are evaporated to
provide the cooling of the syngas. Accordingly, it would be necessary to
pressurize the
acid gas product streams from the stages before they can be sequestered.
It has now been found that at least 70% of the moles of CO2 can be removed
from the
starting synthesis gas using a refrigeration process comprising at least one
refrigeration
stage that employs an external refrigerant by operating the refrigeration
stage(s) under
optimum conditions of temperature and. pressure.
Thus, the present invention provides a process for removing carbon dioxide
from a
synthesis gas feed stream in a cryogenic separation plant that comprises
either a single
cryogenic separation stage or at least two cryogenic separation stages
arranged in series,
with the stages in the series being designated stage 1 through stage N, the
letter N
representing the number of stages in the series, the single stage or each
stage of the series
comprising the steps of (a) condensing carbon dioxide from the synthesis gas
by cooling
the synthesis gas by non-contact heat exchange with an external refrigerant to
produce
liquefied carbon dioxide, and (b) separating the liquefied carbon dioxide from
the synthesis
gas, with the single separation stage discharging a liquefied carbon dioxide
product stream
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
2
and a hydrogen enriched synthesis gas stream or, with each of the stages in
the series
cooling the synthesis gas to a successively lower temperature as the synthesis
gas
progresses from stage 1 to stage N, thereby separately removing a liquefied
carbon dioxide
product stream from each of the stages, with stage N discharging a hydrogen
enriched
synthesis gas vapour stream, wherein:
(i) the synthesis gas feed stream comprises 40 to 65 mole % hydrogen and is
fed to the
single stage or the first stage of the series at a pressure in the range of 46
to 90 bar,
optionally to 76 bar absolute;
(ii) the single stage or stage N of the series is operated at a temperature in
the range of -53
to -48 C and a pressure in the range of 44 to 74 bar absolute such that the
single stage or
the combined stages of the series remove 70 to 80% of the total moles of
carbon dioxide in
the synthesis gas feed stream; and
(iii) the liquefied CO2 product stream(s) removed from the stage(s) of the
cryogenic
separation plant is sequestrated and/or used in a chemical process.
In some aspects of the invention, a higher pressure would be used. For
example, in
some cases, the pressure of the gas feed itself may already be higher than 74
bar.
Alternatively, or in addition, the pressure of the feed may be increased, for
example using a
series of compressors, or a single compressor, before separation.
A further aspect of the invention provides a process for removing carbon
dioxide
from a synthesis gas feed stream in a cryogenic separation plant that
comprises either a
single cryogenic separation stage or at least two cryogenic separation stages
arranged in
series, with the stages in the series being designated stage 1 through stage
N, the letter N
representing the number of stages in the series, the single stage or each
stage of the series
comprising the steps of (a) condensing carbon dioxide from the synthesis gas
by cooling
the synthesis gas by non-contact heat exchange with a refrigerant (optionally
an external
refrigerant) to produce liquefied carbon dioxide, and (b) separating the
liquefied carbon
dioxide from the synthesis gas, with the single separation stage discharging a
liquefied
carbon dioxide product stream and a hydrogen enriched synthesis gas stream or,
with each
of the stages in the series cooling the synthesis gas to a successively lower
temperature as
the synthesis gas progresses from stage 1 to stage N, thereby separately
removing a
liquefied carbon dioxide product stream from each of the stages, with stage N
discharging a
hydrogen enriched synthesis gas vapour stream, wherein:
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
3
(i) the synthesis gas feed stream comprises 40 to 65 mole % hydrogen and is
fed to the
single stage or the first stage of the series at a pressure in the range of 46
to less than 150
bar absolute;
(ii) the single stage or stage N of the series is operated at a temperature in
the range of -53
to -48 C and a pressure in the range of 44 to less than 150 bar absolute; and
(iii) preferably the liquefied CO2 product stream(s) removed from the stage(s)
of the
cryogenic separation plant is preferably sequestrated and/or used in a
chemical process.
In examples, the pressure may be less than 120 bar, 100 bar, or less than
about 80
bar. It will be understood that different % separation of the C02 will be
obtained
depending on the pressure'and temperature of separation.
A further aspect of the invention provides a process for removing carbon
dioxide
from a synthesis gas feed stream in a cryogenic separation plant that
comprises either a
single cryogenic separation stage or at least two cryogenic separation stages
arranged in
series, with the stages in the series being designated stage 1 through stage
N, the letter N
representing the number of stages in the series, the single stage or each
stage of the series
comprising the steps of (a) condensing carbon dioxide from the synthesis gas
by cooling
the synthesis gas by non-contact heat exchange with a refrigerant (optionally
an external
refrigerant) to produce liquefied carbon dioxide, and (b) separating the
liquefied carbon
dioxide from the synthesis gas, with the single separation stage discharging a
liquefied
carbon dioxide product stream and a hydrogen enriched synthesis gas stream or,
with
stages in the series cooling the synthesis gas to a successively lower
temperature as the
synthesis gas progresses from stage I to stage N, thereby separately removing
a liquefied
carbon dioxide product stream from each of the stages, with stage N
discharging a
hydrogen enriched synthesis gas vapour stream, wherein:
(i) the synthesis gas feed stream comprises 40 to 65 mole % hydrogen and is
fed to the
single stage or the first stage of the series at a pressure in the range of 46
to 76 bar
absolute;
(ii) the single stage or first stage of the series is operated at a
temperature in the range of -
53 to -48 C and a pressure in the range of 44 to 74 bar absolute
In some examples, the single stage or the first stage of the series removes 70
to 80%
of the total moles of carbon dioxide in the synthesis gas feed stream. In
other examples,
about 60% to 90% or more may be removed.
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
4
Preferably, the refrigeration process is a multi-stage refrigeration process
having at
least two refrigeration stages arranged in series that each employ an external
refrigerant.
In other examples, other coolants or refrigerants might be used, for example
as described
below. Thus, in a preferred embodiment of the present invention there is
provided a
process for removing carbon dioxide from a synthesis gas feed stream in a
cryogenic
separation plant that comprises at least two cryogenic separation stages
arranged in series,
with the stages in the series being designated stage 1 through stage N, the
letter N
representing the number of stages in the series, each stage comprising the
steps of (a)
condensing carbon dioxide from the synthesis gas by cooling the synthesis gas
by non-
contact heat exchange with an external refrigerant to produce liquefied carbon
dioxide, and
(b) separating the liquefied carbon dioxide from the synthesis gas, with each
of the stages
in the series cooling the synthesis gas to a successively lower temperature as
the synthesis
gas progresses from stage 1 to stage N, thereby separately removing a
liquefied carbon
dioxide product stream from each of the stages, with stage N discharging a
hydrogen
enriched synthesis gas vapour stream, wherein:
(i) the synthesis gas feed stream comprises 40 to 65 mole % hydrogen and is
fed to the first
stage of the cryogenic separation plant at a pressure in the range of 46 to 76
bar absolute;
(ii) stage N of the series is operated at a temperature in the range of -53 to
-48 C and a
pressure in the range of 44 to 74 bar absolute such that the combined stages
of the series
remove 70 to 80% of the total moles of carbon dioxide in the synthesis gas
feed stream;
and
(iii) the liquefied CO2 product streams removed from each stage of the series
are
sequestrated and/or used in a chemical process.
The term "synthesis gas feed stream" used herein refers to a shifted synthesis
gas
stream comprising hydrogen and carbon dioxide. The synthesis gas feed stream
may also
comprise carbon monoxide and hydrogen sulfide.
Where the refrigeration process is a multi-stage refrigeration process,
preferably, the
liquefied CO2 product streams that are removed from each stage of the series
are combined
prior to being sequestrated.
In some examples, 75 to 80% of the total moles of carbon dioxide in the
synthesis gas
feed stream is separated in the cryogenic separation plant. Thus, the process
of the present
invention removes a substantial amount of CO2 from the synthesis gas feed
stream.
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
Typically, the hydrogen enriched synthesis gas stream is fed to the combustor
of a gas
turbine of a power plant. Accordingly, an advantage of the present invention
is that
significantly less CO2 is released to the atmosphere than if the solid fuel or
gaseous
hydrocarbon feedstock that is used to form the synthesis gas was used directly
as fuel for
5 the power plant. A further advantage of the process of the present invention
is that the
hydrogen enriched synthesis gas stream may be obtained at a pressure that is
at or above
the minimum fuel gas feed pressure (inlet pressure) for the combustor(s) of
the gas
turbine(s) of the power plant thereby eliminating the need for compressors to
compress the
fuel gas.
The synthesis gas feed stream may be generated from a solid fuel such as
petroleum
coke or coal in a gasifier or from a gaseous hydrocarbon feedstock in a
reformer. The
synthesis gas stream from the gasifier or reformer contains high amounts of
carbon
monoxide. Accordingly, the synthesis gas stream is typically treated in a
shift converter
unit such that at least a portion, preferably, substantially all of the carbon
monoxide
contained in the synthesis gas stream is converted to carbon dioxide over a
shift catalyst
according to the water gas shift reaction (WGSR):
CO +H2O -+ CO2 + H2.
Where a portion of the carbon monoxide remains in the shifted synthesis gas,
the
majority of this carbon monoxide will be retained in the hydrogen enriched
synthesis gas
stream and will be converted into carbon dioxide when the hydrogen enriched
synthesis
gas stream is used as a fuel.
The shift converter unit may be a single shift reactor containing a shift
catalyst.
However, it is preferred that the shift converter unit comprises a high
temperature shift
reactor containing a high temperature shift catalyst and a low temperature
shift reactor
containing a low temperature shift catalyst. The water gas shift reaction is
exothermic and
results in a significant temperature rise across the shift converter unit.
Accordingly, the
shift converter unit may be cooled by continuously removing a portion of the
shifted
synthesis gas stream and cooling this stream by heat exchange with one or more
process
streams, for example against boiler feed water or against steam (for the
generation of
superheated steam).
The synthesis gas feed stream typically comprises primarily hydrogen, carbon
dioxide and steam and minor amounts of carbon monoxide and methane. Where the
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
6
synthesis gas feed stream is derived from a gasifier, the synthesis gas feed
stream will also
comprise hydrogen sulfide (H2S) that is formed by reaction of COS with steam
in the shift
converter unit. A further advantage of the process of the present invention is
that it allows
the co-capture of the H2S in addition to capture of carbon dioxide (C02).
Thus, H2S will
condense from the synthesis gas in the single stage or each of the stages of
the series and
will be removed from the single stage or each of the stages of the series in
the liquefied
carbon dioxide product stream(s). By operating the single stage or the final
stage of the
series (stage N) at a temperature in the range of -53 to -48 C and a pressure
in the range of
55 to 59 bar absolute, the single stage or the combined stages of the series
will also remove
80 to 90% of the total moles of hydrogen sulfide from the synthesis gas feed
stream.
The synthesis gas feed stream is cooled upstream of the cryogenic separation
plant,
for example, to a temperature in the range of 20 to 50 C, for example, about
40 C to
condense out a condensate (predominantly comprised of water). The condensate
is then
separated from the cooled shifted synthesis gas stream, for example, in a
condensate drum.
Typically, the condensate is cooled against boiler feed water and/or utility
cooling water.
After removal of any condensate, the synthesis gas feed stream is dried prior
to being
passed to the CO2 condensation plant, as any moisture in the synthesis gas
feed stream will
freeze and potentially cause blockages in the plant. The synthesis gas feed
stream may be
dried by being passed through a molecular sieve bed or an absorption tower
that employs
triethylene glycol to selectively absorb the water, preferably a molecular
sieve bed.
Preferably, the dried synthesis gas feed stream-has a water content of less
than 1 ppm (on a
molar basis).
Preferably, the dried synthesis gas feed stream is then passed to a pre-
cooling heat
exchanger of the CO2 condensation plant where the synthesis gas feed stream is
pre-cooled
against a cold stream (for example, a cold process stream such as a liquid CO2
product
stream or a cold H2 enriched synthesis gas vapour stream). Preferably, the pre-
cooling heat
exchanger is a multichannel heat exchanger, for example, a plate fin heat
exchanger or a
printed circuit heat exchanger, with the dried synthesis gas feed stream being
passed
through at least one channel of the multichannel heat exchanger and a
plurality of cold
process streams being passed through further channels of the multichannel heat
exchanger
such that the dried synthesis gas stream is pre-cooled against the cold
process streams.
Alternatively, the dried synthesis gas feed stream may be pre-cooled against a
plurality of
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
7
cold process streams using at least two, preferably, 2 to 8, for example, 4
shell and tube
heat exchangers. These shell and tube heat exchangers may be arranged in
series and/or in
parallel. Where the shell and tube heat exchangers are arranged in parallel,
the synthesis
gas feed stream is divided to form a plurality of sub-streams that are fed to
the heat
exchangers and the cooled sub-streams that exit the heat exchangers are
subsequently
recombined. It is also envisaged that the dried synthesis gas stream may be
pre-cooled
using a combination of a multichannel heat exchanger and one or more shell and
tube heat
exchangers.
As discussed below, the hydrogen enriched synthesis gas vapour stream may be
subjected to isentropic expansion in a turboexpander (resulting in cooling of
the hydrogen
enriched synthesis gas vapour stream) after being used to pre-cool the dried
synthesis gas
feed stream. Cooling of the expanded hydrogen enriched synthesis gas vapour
stream
allows the dried synthesis gas feed stream to be pre-cooled to a temperature
in the range of
-15 to -35 C, for example, about -23 C. Where the hydrogen enriched synthesis
gas
vapour stream is not subjected to isentropic expansion before being used to
pre-cool the
dried synthesis gas stream, the synthesis gas feed stream may typically only
be cooled to a
temperature in the range of 0 to -15 C, for example, about -10 C. Depending
upon the
composition of the synthesis gas feed stream and the amount of pre-cooling,
the pre-cooled
stream may remain in a vapour state or may be cooled to below its dew point
thereby
becoming two phase.
The synthesis gas feed stream is then passed through the cryogenic separation
stage(s) of the cryogenic separation plant. The single cryogenic separation
stage or each
cryogenic separation stage of the series comprises a heat exchanger that
employs an
external refrigerant and a gas-liquid separation vessel. Preferably, the
cryogenic separation
plant comprises 1 to 5, more preferably, 2 to 4, for example, 3 cryogenic
separation stages
arranged in series.
The term "refrigerant" used herein preferably includes any appropriate coolant
or
refrigerant. Furthermore, the term "coolant" preferably includes any
appropriate coolant or
refrigerant.
Preferably the term "internal coolant streams" includes product streams
produced in
the process. For example the internal coolant streams include C02-rich streams
and H2-
rich streams formed in the separation step(s). Preferably, where appropriate,
the term
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
8
"internal coolant streams" includes any appropriate coolant or refrigerant
stream.
Preferably the terms "external refrigerant" or "external coolant" include a
refrigerant
or coolant that is provided in an external refrigeration circuit. Accordingly,
liquid CO2 that
is formed in the process of the present invention will not generally be
regarded as an
external refrigerant. Suitable external refrigerants that may be used as
refrigerant in the
heat exchanger(s) include propane, ethane, propene, ethylene, ammonia,
hydrochlorofluorocarbons (HCFCs) and mixed refrigerants. Typical mixed
refrigerants
comprise at least two refrigerants selected from the group consisting of
butanes, propanes,
ethane, and ethylene. These refrigerants may be cooled to the desired
refrigeration
temperature in external refrigerant circuits using any method known to the
person skilled
in the art including methods known in the production of liquefied natural gas
(LNG) or
natural gas liquids (NGLs).
The operating temperature of each cryogenic separation stage will depend on
the
number of cryogenic separation stages and the desired carbon dioxide capture
level. There
is a limit on the lowest temperature in the final cryogenic separation stage,
as the
temperature must be maintained above a value where solid CO2 will form. This
typically
occurs at a temperature of less than -55 C at pressures of less than 300 barg
(the triple
point for pure CO2 is at 5.18 bar and at a temperature of -56.4 C) although
the presence of
H2S may depress this freezing point.
There is preferably minimal pressure drop across the stages of the cryogenic
separation plant. Typically, the pressure drop across the single stage or the
series of stages
of the cryogenic separation plant is in the range of 2 to 10 bar, preferably,
2 to 5 bar, in
particular, 2 to 3 bar. Preferably, the pressure drop across the single stage
or across each
stage of the series is about I bar. Thus, where the plant comprises at least
two stages
arranged in series, it may be operated with the stages at substantially the
same pressure.
Higher pressure drops across the cryogenic separation stage(s) may be
tolerated (for
example, pressure drops in the range of 10 to 30 bar, preferably 10 to 20 bar)
provided that
the single separation stage or final cryogenic separation stage of the series
is operated at a
pressure in the range of 45 to 59 bar absolute, preferably, 56 to 58 bar
absolute, for
example, 57 bar absolute. An advantage of operating the single or final
cryogenic
separation stage of the series at a pressure in the range of 55 to 59 bar
absolute is that the
H2 enriched synthesis gas stream that is discharged from the separator vessel
of the single
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
9
separation stage or from the separator vessel of the final stage (stage N) of
the series is at
or above the minimum feed gas pressure (minimum inlet pressure) for the
combustor(s) of
the gas turbine(s) of the power plant (see below).
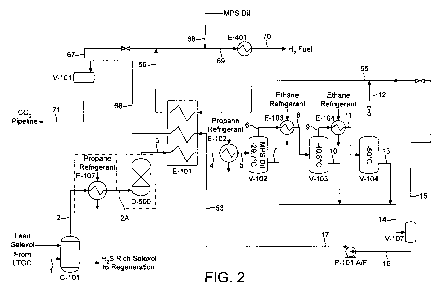
The process of some examples of some aspects of the present invention will now
be
described with respect to a CO2 condensation plant that comprises three
cryogenic
separation stages arranged in series. The synthesis gas feed stream is pre-
cooled against
one or more cold process streams (for example, a hydrogen enriched synthesis
gas vapour
stream and/or a liquefied CO2 stream) before being passed through the heat
exchanger of
the first cryogenic separation stage where the synthesis gas is cooled to a
temperature in
the range of -32 to -28 C against an external refrigerant thereby forming a
two phase
stream comprising a liquid phase (comprising liquid C02) and a vapour phase
comprising
H2 and CO2 (hydrogen enriched synthesis gas). The two phase stream is then
passed to the
gas-liquid separator vessel of the first cryogenic separation stage where the
liquid phase is
separated from the vapour phase. A hydrogen enriched synthesis gas vapour
stream and a
liquid CO2 stream are withdrawn from the separator vessel, preferably, from at
or near the
top and bottom respectively of the separator vessel. The H2 enriched synthesis
gas vapour
stream is then used as feed to the second cryogenic separation stage where it
is passed
through a further heat exchanger and is cooled to a temperature in the range
of -43 to -39 C
against a further external refrigerant. The resulting two phase stream is
passed to the gas-
liquid separator vessel of the second cryogenic separation stage for
separation of the
phases. A vapour stream that is further enriched in H2 and a liquid CO2 stream
are
withdrawn from the separator vessel, preferably, from at or near the top and
bottom
respectively of the separator vessel. The hydrogen enriched synthesis gas
vapour stream
discharged from the second cryogenic separation stage is then used as feed to
the third
cryogenic separation stage where it is passed through a further heat exchanger
and is
cooled to a temperature in the range of -53 to -48 C against a further
external refrigerant.
The resulting two phase stream is passed to the gas-liquid separator vessel of
the third
cryogenic separation stage for separation of the phases. A vapour stream that
is further
enriched in H2 and a liquid CO2 stream are withdrawn from the separator
vessel,
preferably, from at or near the top and bottom respectively of the separator
vessel.
Preferably, the synthesis gas feed stream is fed to the first cryogenic
separation stage at as
high a pressure as possible, which will be dependent upon the source of the
gas. Typically,
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
the synthesis gas feed stream is fed to the first cryogenic separation stage
at a pressure of at
least 50 bar absolute, preferably, 55 to 75 bar absolute, for example, 60 to
70 bar absolute.
If desired, the feed to the first cryogenic separation stage may be compressed
to higher
pressure. Typically, the pressure drop across the three cryogenic separation
stages is
5 minimized such that the third cryogenic separation stage is operated at a
pressure that is
less than 5 bar below the pressure of the first cryogenic separation stage.
For example,
where the synthesis gas feed stream is fed to the first cryogenic separation
stage at a
pressure of 60 bar absolute, the third cryogenic separation stage is typically
operating at a
pressure in the range of 55 to 58 bar absolute.
10 Typically, the hydrogen enriched synthesis vapour stream (non-condensable
stream)
discharged from the final cryogenic separation stage (Stage N) of the
cryogenic separation
plant comprises at least 70 mole % hydrogen, preferably, at least 80 mole %
hydrogen, the
remainder being mostly carbon dioxide. Typically, the amount of CO2 contained
in the H2
enriched synthesis gas vapour stream that is discharged from the final
cryogenic separation
stage (Stage N) of the cryogenic separation plant is less than 30 mole % C02,
preferably,
less than 25 mole % CO2. This hydrogen enriched synthesis gas vapour stream
may also
comprise trace-amounts of carbon monoxide (CO) and methane, for example, less
than 500
ppm on a molar basis (although higher amounts of CO may be tolerated, for
example 2-3
mole% CO). The H2 enriched synthesis gas vapour stream from the final
cryogenic
separation stage of the CO2 condensation plant (Stage N) may be used as a fuel
stream for
the combustor of a gas turbine that drives an electric generator thereby
producing
electricity.
Typically, the fuel gas feed pressure (inlet pressure) for the combustor of
the gas
turbine(s) is in the range of 25 to 45 barg, preferably, 28 to 40 barg, in
particular, 30 to 35
barg. Typically, the combustor of the gas turbine(s) is operated at a pressure
of 15 to 20
bar absolute. Accordingly, the H2 enriched synthesis gas vapour stream may be
obtained
above the minimum fuel gas feed pressure for the combustor(s) of the gas
turbine(s) so that
there is no requirement for a gas compressor to compress the hydrogen enriched
synthesis
gas stream (fuel gas stream) to the inlet pressure for the combustor(s) of the
gas turbine(s).
Typically, the H2 enriched synthesis gas vapour stream may be expanded in at
least one
turboexpander arranged in series down to the inlet pressure of the
combustor(s) of the gas
turbine(s). The isentropic expansion of the hydrogen enriched vapour stream in
the
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
11
turboexpander(s) produces work that may be used to drive at least one turbine
or an
electric motor thereby generating electricity for export or for use within the
process (for
example, for operating the CO2 pumps and/or a compressor of an external
refrigeration
circuit ). Generally, the turboexpanders are mounted on a common shaft.
Typically, the
turboexpanders are operated with substantially the same pressure ratio across
each
turboexpander, for example, a pressure ratio in the range of 0.88 to 0.66. The
H2 enriched
synthesis gas vapour stream is cooled by isentropic expansion in the
turboexpander(s)
thereby allowing additional pre-cooling of the synthesis gas feed stream.
Typically, the H2
enriched synthesis gas vapour stream that exits the separator of the single
cryogenic
separation stage or that exits stage N of the series of cryogenic separation
stages is passed
through a channel of the multichannel heat exchanger in heat exchanger
relationship with
the synthesis gas feed stream and is then cooled by expansion to lower
pressure in a first
turboexpander before being fed to a further channel in the multichannel heat
exchanger.
The hydrogen enriched vapour stream may then be cooled by expansion to a lower
pressure in a second turboexpander before being fed to a further channel of
the
multichannel heat exchanger. Where the H2 enriched synthesis gas vapour stream
is to be
used as fuel gas for the combustor of a gas turbine, it should not be reduced
in pressure to
below the desired fuel gas feed pressure (inlet pressure) for the combustor.
However, it is
also recognised that the hydrogen enriched synthesis gas vapour stream may be
expanded
to pressures below the inlet pressure of the combustor of a gas turbine, if
the hydrogen
enriched synthesis gas vapour stream is to be used for a different purpose,
for example, as
fuel for a low pressure burner of a fired heater, or as fuel for a reformer or
boiler or as a
refinery feed stream for upgrading of one or more refinery streams or as a
hydrogen feed to
a chemical process.
International Patent Application No PCT/GB2009/001810 describes processes in
which H2-enriched gas vapour streams are fed to a turboexpansion system in
which the
hydrogen rich vapour stream is subjected to isentropic expansion in each of a
plurality of
turboexpanders of the series such that hydrogen rich vapour streams are
withdrawn from
the turboexpanders of the series at reduced temperature and at successively
reduced
pressures thereby generating motive power. Additionally, a cooled hydrogen
rich vapour
stream can be passed in heat exchanger relationship with a (higher
temperature) gas feed
stream and thus be used as an internal coolant in the system.
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
12
In examples described in International Patent Application No.
PCT/GB2009/001810,
a synthesis feed gas stream is increased in pressure to a pressure in the
range of 150 to 400
barg before it is cooled and passed to separator for withdrawing of the
hydrogen enriched
gas vapour stream.
As discussed herein, in accordance with aspects of the present invention, it
has been
identified that a turboexpander system can be advantageously be used also
where the
separation step is carried out at pressures lower than 150 barg.
Accordingly, a further aspect of the invention provides a process for
separating a
synthesis gas stream into a hydrogen rich vapour stream and a carbon dioxide
rich stream,
the process including the steps of
a) cooling a synthesis gas stream to a temperature at which at which a two-
phase
mixture is formed, the cooling including feeding synthesis gas to a heat
exchanger system, preferably in heat exchange relationship with an internal
coolant stream that is produced subsequently in the process wherein the
internal
coolant stream is selected from the group consisting of a hydrogen rich vapour
stream and a dense carbon dioxide stream,
b) passing the cooled stream formed in step (a) either directly or indirectly
to a
gas-liquid separator vessel, the feed to the gas-liquid separator vessel
having a
pressure of less than 150 barg
c) withdrawing a hydrogen rich vapour stream from the separator vessel and a
liquid CO2 stream from separator vessel; and
d) feeding a hydrogen-rich stream to an expansion system including at least
one
expander, preferably a plurality of expanders, and reducing the pressure of
the
hydrogen-rich gas at the or each expander.
Preferably the method includes using an expanded hydrogen stream as a coolant
in
the system.
Preferably one or more methods of the present invention of any aspect as
appropriate
includes the step of feeding a hydrogen rich stream to an expansion system
including at
least one expander for reducing the pressure of the hydrogen-rich stream.
Preferably
expansion system includes a plurality of expanders arranged in series.
Preferably the
expansion system includes one or more expanders able to recover work from the
expansion, for example turboexpanders or a series of turboexpanders. In
preferred
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
13
examples, the hydrogen-rich gas is fed to a turboexpansion system including a
plurality of
turboexpanders arranged in series, wherein the hydrogen rich vapour stream is
subjected to
isentropic expansion in each of the turboexpanders of the series such that a
hydrogen rich
vapour stream is withdrawn from each of turboexpanders at reduced temperature
and at
successively reduced pressures and wherein isentropic expansion of the
hydrogen rich
vapour in each of the turboexpanders of the series generates motive power.
Preferably the temperature of the hydrogen-rich stream is reduced by the
expansion
and the cooled expanded hydrogen stream is subsequently used as an internal
coolant in the
system. Preferably the cooled stream from each expander is used as an internal
coolant,
for example prior to being fed to a further expander.
According to a further aspect of the invention there is provided a process for
separating a synthesis gas stream into a hydrogen rich vapour stream and a
carbon dioxide
rich stream, the process including the steps of:
a) cooling a synthesis gas stream to a temperature at which at which a two-
phase
mixture is formed,
b) passing the cooled stream formed in step (a) either directly or indirectly
to a
gas-liquid separator vessel, optionally the feed to the gas-liquid separator
vessel
having a pressure of less than 150 barg
c) withdrawing a hydrogen rich vapour stream from the separator vessel and a
liquid CO2 stream from separator vessel; and
d) feeding a separated hydrogen rich vapour stream to an expansion system
including a plurality of expanders arranged in series, wherein the hydrogen
rich
vapour stream is subjected to expansion in each of the expanders of the series
such that an expanded hydrogen rich vapour stream is withdrawn from each of
the expanders at reduced temperature and at successively reduced pressures;
and
e) using at least one expanded hydrogen-rich vapour stream as a coolant.
In some examples, the expansion system might include only one expander wherein
the hydrogen rich vapour stream is subjected to expansion in the expander of
the system
such that an expanded hydrogen rich vapour stream is withdrawn from the
expander at
reduced temperature and at pressure and used as a coolant. However, in many
examples it
will be preferred for at least two expanders to be used so that improved
temperature and/or
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
14
pressure profiles of the process can be sought. As discussed further below, by
using more
than one expander, in some examples a plurality of relatively cold expanded
hydrogen
streams can be used as internal coolant streams in the system.
The expanded hydrogen-rich vapour stream may be used to cool one or more
streams
selected from a hydrogen-rich gas stream, a carbon dioxide stream and a
synthesis gas
stream.
In preferred examples, a plurality of expanded hydrogen rich vapour streams
are used
as coolant streams in the process. In some examples, all of the expanded
hydrogen rich
streams are used as internal coolants.
Preferably the expanders effect isentropic expansion of the hydrogen rich
vapour in
each of the expanders of the series and generate motive power.
The motive power may for example be further used in the process, leading to
efficiencies. For example, the expander may include an expansion turbine
preferably
connected to a compressor (if used) in the system. Alternatively or in
addition, other
expanders may be used in some examples. For example, the stream may be
expanded
across a valve to reduce the pressure.
The method may further include increasing the pressure of the separated carbon
dioxide stream.
In some examples, the further utilisation of the carbon dioxide stream, for
example
for effecting storage, may require a higher pressure than the pressure of the
stream
withdrawn from the separator. Apparatus, for example a pump, may be provided
downstream of the separator to increase the pressure, for example to above 120
bar, or to
150 bar or above.
The method may further include passing the separated hydrogen rich stream
directly
or indirectly to a further gas-liquid separator vessel and withdrawing a
second separated
hydrogen rich vapour stream from the separator vessel and a second liquid CO2
stream
from separator vessel.
A further aspect of the invention provides a process for separating a
synthesis gas
stream into a hydrogen rich vapour stream and a carbon dioxide rich stream,
the process
including the steps of:
a) cooling a synthesis gas stream to a temperature at which at which a two-
phase
mixture is formed,
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
b) passing the cooled stream formed in step (a) either directly or indirectly
to a
first gas-liquid separator vessel, the feed to the gas-liquid separator vessel
having a pressure of less than 150 barg
c) withdrawing a hydrogen rich vapour stream from the separator vessel and a
5 liquid CO2 stream from separator vessel;
d) passing the hydrogen rich vapour stream formed in step (c) either directly
or
indirectly to a second gas-liquid separator vessel, and withdrawing a second
hydrogen rich vapour stream from the separator vessel and a liquid CO2 stream
from separator vessel; and
10 e) feeding a separated hydrogen rich vapour stream to an expansion system
including at least one expander, wherein the hydrogen rich vapour stream is
subjected to expansion in the expander of the system such that an expanded
hydrogen rich vapour stream is withdrawn from the expander at reduced
temperature and at pressure; and
15 f) using an expanded hydrogen-rich vapour stream as a coolant.
The method may further include cooling the separated hydrogen-rich stream
upstream of the second separator vessel.
An aspect of the invention further provides a process for separating a
synthesis gas
stream into a hydrogen rich vapour stream and a carbon dioxide rich stream,
the process
including the steps of:
a) cooling a synthesis gas stream to a temperature at which at which a two-
phase
mixture is formed, the cooling including feeding synthesis gas to a heat
exchanger system in heat exchange relationship with an internal coolant stream
that is produced subsequently in the process wherein the internal coolant
stream
is selected from the group consisting of a hydrogen rich vapour stream and a
dense carbon dioxide stream,
b) passing the cooled stream formed in step (a) either directly or indirectly
to a
gas-liquid separator vessel, the feed to the gas-liquid separator vessel
having a
pressure of less than 150 barg
c) withdrawing a hydrogen rich vapour stream from the separator vessel and a
dense CO2 stream from separator vessel; and
d) feeding a hydrogen-rich stream to an expansion system including at least
one
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
16
expander, preferably a plurality of expanders, and reducing the pressure of
the
hydrogen-rich gas at the or each expander.
Also provided by the invention is a system for separating a synthesis gas
stream into
a hydrogen rich vapour stream and a carbon dioxide rich stream, the system
including:
a) a cooling system arranged to cool a gas stream to a temperature at which a
two-
phase mixture is formed,
b) a gas-liquid separator vessel arranged to receive the two-phase mixture
either
directly or indirectly from the cooling system, at a pressure of less than 150
bar,
the output of the separator vessel being a hydrogen rich vapour stream and a
liquid CO2 stream; and
c) an expansion system arranged downstream of the separator vessel to receive
a
hydrogen rich vapour stream, the expansion system including a plurality of
expanders arranged in series such that the hydrogen rich vapour stream is
subjected to expansion in each of the expanders of the series such that a
hydrogen rich vapour stream can be withdrawn from each of the expanders at
reduced temperature and at successively reduced pressures
d) a flow path for feeding an expanded hydrogen rich stream to the cooling
system.
Also provided by the invention is a system for separating a synthesis gas
stream into
a hydrogen rich vapour stream and a carbon dioxide rich stream, the system
including:
a) a cooling system arranged to cool a synthesis gas stream to a temperature
at
which at which a two-phase mixture is formed,
b) a first gas-liquid separator vessel arranged to receive the cooled stream
either
directly or indirectly, the feed to the gas-liquid separator vessel having a
pressure of less than 150 barg, and to output a first hydrogen rich stream and
a
liquid CO2 stream;
c) a second gas-liquid separator vessel downstream of the first separator for
receiving the first hydrogen rich stream either directly or indirectly, and
outputting a second hydrogen rich stream from the separator vessel and a
liquid
CO2 stream from the separator vessel; and
d) an expansion system including at least one expander, arranged, preferably
downstream of the second separator vessel, to receive a the hydrogen rich
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
17
vapour stream and subjected it to expansion in the expander of the system such
that an expanded hydrogen rich vapour stream can be withdrawn from the
expander at reduced temperature and at pressure; and
e) a flow path for feeding an expanded hydrogen-rich vapour stream to the
cooling
system.
The system may further include a compressor or pump arranged to increase the
pressure of a separated carbon dioxide stream.
In some examples, the pressure at the separation step will be in the range 80
to
400bar, for example 80 to 250bar.
In some examples, an upstream shift reaction process may generate a shifted
gas
having a pressure in the range 50 to 100 bar. This shifted gas may in some
examples be
fed to the separation stage without significant change in the pressure of the
stream. The
shifted gas leaving a Water Gas Shift unit may for example be at a pressure in
the range 50
to 100bar, for example 60 bar to 95 bar, for example 65 to 90 bar, in some
examples 70 to
80 bar. The shifted gas in some examples may be at a pressure in the range 50
to 250 bar
when cooled such that some preferably most of the carbon dioxide contained
therein
liquefies prior to separation. The pressure at the separation step may be
70bar, 75bar, or
80bar in some cases.
The synthesis gas stream preferably comprises a shifted syngas stream. The
cooled
stream fed to the separator vessel preferably comprises a shifted syngas. It
will be
appreciated that in some arrangements, one or more steps of the process may be
carried out
for example before or after the shifting step.
It has been found that the hydrogen rich vapour stream may be reduced in
pressure to
any desired pressure by passing the hydrogen enriched vapour stream through a
turboexpansion system that comprises a plurality of turboexpanders arranged in
series. In
particular, the hydrogen rich vapour stream may be obtained at the desired
fuel gas feed
pressure for a combustor of a gas turbine of a power plant (for example, at a
pressure of 30
barg). It has also been found that or more expanded H2 rich vapour streams
that exit each
turboexpander of the series may be used as internal coolant streams thereby
providing a
portion, for example a major portion, of the refrigeration duty for the heat
exchanger
system. In some examples, expansion of the H2 rich vapour in the
turboexpanders maybe
used to drive a rotor or shaft of a compressor(s) of a compressor system (if
present) or to
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
18
drive the rotor or shaft of a turbo-electric generator thereby achieving a
reduction of the net
power consumption for the separation of the synthesis gas stream into a
hydrogen rich
vapour stream and liquid CO2 stream
The motive power generated in the turboexpanders may be used for example to
drive
a machine that is a component of the CO2 condensation plant and/or to drive an
alternator
of an electric generator. The machine that is driven by a turboexpander may be
a
compressor in a compression system (for example a compression system used in
the
separation process, if required) and/or a pump.
In some examples, for example as described above, the pressure of the
synthesis gas
feed stream is at least 40 bar.
In particular in examples where a pressurised hydrogen enriched gas is
advantageous,
for example for use as a feed to a turbine, preferably the pressure of the
synthesis gas feed
stream in the process is at least 60 bar, for example at least 80 bar or more.
The pressure
of the stream fed to a separator vessel of the system may be for example 125
bar or less,
for example 110 bar or less, 100 bar or less, or 90 bar.
In some examples, there will be a compression step required to compress the
syngas,
for example, the shifted syngas, to increase the pressure.
Thus the process may include the step of, prior to the separation, and
preferably prior
to the cooling using the internal coolant stream, compressing the synthesis
gas using a
compression system such that the gas is increased in pressure to a pressure in
the range of
60bar to less than 150 bar. Preferably the method further includes the step of
cooling the
resulting high pressure gas against a coolant (for example an external
refrigerant and/or
internal cooling stream) to remove at least part of the heat of compression.
In other arrangements, the syngas feed, for example the shifted syngas feed,
for
example the feed from a Water Gas Shift apparatus, may be such that no further
compression is required. Indeed, as described herein, the separation step may
be carried
out without significant, or any, compression being required upstream of the
separation.
Thus yet a further aspect of the invention provides a process for separating a
synthesis gas stream into a hydrogen rich vapour stream and a carbon dioxide
rich stream,
the process including the steps of:
a) feeding a synthesis gas stream to a cooling system, without substantially
increasing the pressure of the synthesis gas stream immediately upstream of
the
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
19
cooling system;
b) cooling a synthesis gas stream to a temperature at which at which a two-
phase
mixture is formed, the cooling including feeding synthesis gas to a heat
exchanger system, preferably in heat exchange relationship with an internal
coolant stream that is produced subsequently in the process wherein the
internal
coolant stream is selected from the group consisting of a hydrogen rich vapour
stream and a dense carbon dioxide stream,
c) passing the cooled stream formed in step (a) either directly or indirectly,
with
substantially no increase in pressure, to a gas-liquid separator vessel,
d) withdrawing a hydrogen rich vapour stream from the separator vessel and a
dense CO2 stream from separator vessel; and
e) feeding a hydrogen rich vapour stream to an expander system including one,
preferably a plurality of expanders, wherein a hydrogen-rich stream is
subjected
to expansion in the or each expander.
Preferably the expanded hydrogen stream is cool and is used as a coolant in
the
system. Preferably a plurality of expanded hydrogen streams are used as
coolant streams.
The method may include feeding a hydrogen rich vapour stream to a
turboexpansion
system including a plurality of turboexpanders arranged in series, wherein the
hydrogen
rich vapour stream is subjected to isentropic expansion in each of the
turboexpanders of the
series such that a hydrogen rich vapour stream is withdrawn from each of
turboexpanders
at reduced temperature and at successively reduced pressures and wherein
isentropic
expansion of the hydrogen rich vapour in each of the turboexpanders of the
series
generates motive power.
Thus preferably the native feed of synthesis gas, for example shifted
synthesis gas
fed for example from a Water Gas Shift system, is not further compressed prior
to the
separation of the carbon dioxide from the stream.
Thus according to some aspects of the invention, the first separation, and
optionally
subsequent separation steps, is carried out at substantially the pressure of
the feed gas
stream. For example, the feed gas pressure may be between 60bar and 125 bar,
for
example 60 bar to 100 bar.
The temperature of the cooled stream passed to the gas-liquid separator vessel
will
depend in part on the pressure of the stream in the case that it is required
that a two-phase
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
mixture be formed. In examples, the temperature of the feed stream to the
separator
apparatus will generally be between -15 degrees C and -55 degrees C,
preferably less than -
degrees C, preferably less than -40 degrees C, preferably -50 degrees C or
less.
Accordingly, a further aspect of the invention provides a process for
separating a
5 synthesis gas stream into a hydrogen rich vapour stream and a carbon dioxide
rich stream,
the process including the steps of:
a) cooling a synthesis gas stream having a pressure of less than 150 barg to a
temperature between -15 degrees C and -55 degrees C, the cooling including
feeding synthesis gas to a heat exchanger system in heat exchange
10 relationship with an internal coolant stream that is produced subsequently
in
the process wherein the internal coolant stream is selected from the group
consisting of a hydrogen rich vapour stream and a dense carbon dioxide
stream,
b) passing the cooled stream formed in step (a) either directly or indirectly
to a
15 gas-liquid separator vessel, the feed to the gas-liquid separator vessel
having a
pressure of less than 150 barg
c) withdrawing a hydrogen rich vapour stream from the separator vessel and a
dense CO2 stream from separator vessel; and
d) preferably feeding a hydrogen rich vapour stream to a turboexpansion system
20 including a plurality of turboexpanders arranged in series, wherein the
hydrogen rich vapour stream is subjected to isentropic expansion in each of
the turboexpanders of the series such that a hydrogen rich vapour stream is
withdrawn from each of turboexpanders at reduced temperature and at
successively reduced pressures and wherein isentropic expansion of the
25 hydrogen rich vapour in each of the turboexpanders of the series generates
motive power.
In some examples, all or substantially all of the cooling in step (a) may be
carried out
using one or more internal cooling streams. Alternatively, some cooling may
additionally
be provided using an external coolant or refrigerant. For example a
refrigerant such as
30 ethane or propane may be used, although other coolants and refrigerants are
possible.
Preferably cooling using external coolants, if any, is provided downstream of
the internal
cooling, but in other examples it may be preferred to provide external cooling
upstream of
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
21
cooling using internal coolants, or between cooling stages using internal
coolant.
Also provided by an aspect of the invention is provided a process for
separating a
synthesis gas stream into a hydrogen (H2) rich vapour stream and a liquid
carbon dioxide
(C02) stream in a CO2 condensation plant that comprises (a) a heat exchanger
system, (b) a
gas-liquid separator vessel, and (c) a turboexpansion system comprising a
plurality of
turboexpanders arranged in series, the process comprising the steps of:
(A) providing a feed synthesis gas stream having a pressure in the range of 10
to 120
barg;
(B) cooling the synthesis gas stream of step (A) to a temperature in the range
of -15 to -
55 C by passing the synthesis gas stream through the heat exchanger system in
heat
exchange relationship with a plurality of internal refrigerant streams wherein
the internal
refrigerant streams are selected from the group consisting of cold hydrogen
rich vapour
streams and liquid CO2 streams;
(C) passing the cooled synthesis gas stream formed in step (B) either directly
or
indirectly to a gas-liquid separator vessel that is operated at substantially
the same pressure
as the heat exchanger system and withdrawing a hydrogen rich vapour stream
from at or
near the top of the separator vessel and a liquid CO2 stream from at or near
the bottom of
the separator vessel; and
(D) feeding the hydrogen rich vapour stream from step (C) to the
turboexpansion system
wherein the hydrogen rich vapour stream is subjected to isentropic expansion
in each of the
turboexpanders of the series such that hydrogen rich vapour streams are
withdrawn from
the turboexpanders of the series at reduced temperature and at successively
reduced
pressures and wherein isentropic expansion of the hydrogen rich vapour in each
of the
turboexpanders of the series generates motive power thereby driving a machine
that is a
component of the CO2 condensation plant and/or driving an alternator of an
electric
generator.
In some examples, it is preferred for the pressure of the stream at the gas-
liquid
separator vessel to be substantially the same as the pressure of the initial
feed gas, thus for
there to be no or substantially no pressurising of the gas between the feed
and the
separation step.
In other examples, preferably the system includes a compression system
comprising
at least one compressor, the feed gas being fed to the compression system such
that the
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
22
synthesis gas is increased in pressure before the separation step. For
example, the pressure
may be increased to more than 60 bar, more than 70 bar, more than 80 bar or
100 bar or
more. The process may further include cooling the resulting increased pressure
synthesis
gas stream, for example against an external coolant and/or external
refrigerant to remove at
least part of the heat of compression.
A multistage compression system may be preferred in some arrangements where
compression is required, for example multistage compression may be preferred
for higher
discharge pressures from the compression system but is optional, in particular
for lower
discharge pressures from the compression system. Generally, the compressor(s)
of any
compression system used in the apparatus may be mounted on a shaft that may be
driven
by an electric motor, gas turbine or steam turbine. Alternatively, or in
addition,
compressor(s) of a compression system and turboexpanders of a turboexpansion
system
may be mounted on a common shaft so that the isentropic expansion of the
hydrogen rich
vapour in the turboexpanders may be used to drive the compressor(s).
A typical multistage compression system for use in the examples of the present
invention may comprise at least one low pressure (LP) compressor, preferably
two or three
LP compressors mounted on a common drive shaft and at least one high pressure
(HP)
compressor, preferably one or two HP compressors mounted on a further common
drive
shaft (the drive shafts may be connected via a gear system). The LP and HP
compressors
are arranged in series. As would be well known to the person skilled in art,
increased
compression efficiency is achieved by balancing the compression duty across
the
compressors of the series. Thus, it is preferred that the compression ratios
between
successive compressors of the series be substantially the same.
In any of the aspects described herein, the separation process may be a single
stage or
multiple stage process-Further -c-oulirig stages may be-provided-between the
separation-_ _ _
stages. In some examples, after the final stage of a series of separation
steps, the
hydrogen-rich stream will be fed to an expansion device. Preferably the
expansion device
is adapted to reduce the pressure of the hydrogen-rich stream. Preferably the
expansion is
carried out such that the temperature of the gas stream is reduced. Preferably
the
expansion is carried out such that the reduction in pressure is recovered as
work. For
example the expansion may be carried out using a turboexpander, for example as
described
above.
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
23
Preferably, the cooled synthesis gas stream formed in a cooling step may be
passed to
a cryogenic separation system comprising at least one cryogenic separation
stage wherein
the cryogenic separation stage(s) is comprised of a heat exchanger that
employs an external
refrigerant and a gas-liquid separator vessel. Accordingly, the gas-liquid
separator vessel
employed in some examples of the present invention may be either the gas-
liquid separator
vessel of a single cryogenic separation stage that employs an external
refrigerant or is the
final gas-liquid separator vessel of a series of cryogenic separation stages
wherein the
cryogenic separation stages each employ an external refrigerant and are
operated at
progressively lower temperatures. In other examples, cooling will be provided
alternatively, or in addition, using one or more streams of internal coolant.
An advantage of examples of the present invention is that at least 65%, for
example
at least 75%, and in some examples at least 90%, more preferably, at least 95%
of the
carbon dioxide may be separated from the synthesis gas feed stream with the
carbon
dioxide capture level being dependent upon the pressure of the synthesis gas
stream, any
increase of pressure in the system and on the temperature of the cooled gas,
for example
whether or not the cooled synthesis gas stream is subjected to cryogenic
cooling against an
external refrigerant. For example, it has been found that 75 to 85% of the CO2
might be
captured from the synthesis gas feed stream in some examples where an external
refrigerant is not used.
It has been found for examples where the pressure at which the separation step
is
carried out at a pressure of less than about 80bar (including for example
cases where no
initial compression is carried out before the first separation step), the
amount of captured
CO2 is generally between about 65 and 80 % mol. Where higher pressures are
used (for
example by including one or more compressors in the system), higher capture
rates may be
obtained.
The hydrogen rich vapour stream may be reduced in pressure, for example to the
desired inlet pressure for the combustor(s) of the gas turbine(s). For example
by
isentropically expanding the hydrogen rich vapour stream, for example in one
or a series of
turboexpanders cold H2 rich vapour streams (internal coolant or refrigerant
streams) that
may be used to cool the synthesis gas stream. In addition, isentropic
expansion of the
hydrogen rich vapour streams for example in each of the turboexpanders of the
series can
generate motive power that may be used to drive the compressor(s) of a
compression
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
24
system (if present) and/or to drive at least one alternator of an electric
generator thereby
generating electricity for use in the process (for example, for operating one
or more electric
compressors of the compression system) and/or to drive a pump (for example, a
pump for a
liquid CO2 or supercritical CO2 stream). Thus, a major portion of the
compression energy
may be recovered using the turboexpanders thereby increasing the overall
energy
efficiency of the process.
It is recognised that the hydrogen rich vapour stream may be expanded to
pressures
below the inlet pressure of the combustor of a gas turbine, if the hydrogen
rich vapour
stream is to be used for a different purpose, for example, as fuel for a low
pressure burner
of a fired heater, or as fuel for a reformer or boiler or as a refinery feed
stream for
upgrading of one or more refinery streams or as a hydrogen feed to a chemical
process.
The pressure drop across the heat exchanger system may be less than 1.5 bar.
In some preferred examples, the heat exchanger system comprises at least one
multichannel heat exchanger and the synthesis gas stream is passed through a
channel in
the multichannel heat exchanger in heat exchange relationship with a plurality
of internal
refrigerant or coolant streams that are passed through further channels in the
multichannel
heat exchanger. The multichannel heat exchanger may be a diffusion-bonded heat
exchanger, for example, a printed circuit heat exchanger.
The heat exchanger system may comprise a plurality of stand-alone heat
exchangers
arranged in series and the synthesis gas stream may be cooled as it is passed
through the
heat exchangers of the series by heat exchange with a plurality of internal
refrigerant
streams that are fed to the first and successive heat exchangers of the series
at successively
lower temperatures.
Typically, in examples of the invention, the heat exchanger system comprises
at least
one multichannel heat exchanger with the gas stream being passed through a
channel of the
multichannel heat exchanger in heat exchange relationship with a plurality of
internal
refrigerant/coolant streams that are passed through further channels in the
multichannel
heat exchanger. Representative examples of a multichannel heat exchanger
include those
described in US 6622519, WO 2004/016347, EP 212878 and EP 292245 the
disclosures of
which are incorporated herein by reference. As an alternative, or in addition
to pre-cooling
the synthesis gas stream against an external refrigerant in a heat exchanger
of the
compression system, it is envisaged that one or more external refrigerant
streams may be
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
passed through yet further channels in the multichannel heat exchanger thereby
providing
additional cooling duty for the synthesis gas stream. Preferably, the
synthesis gas stream is
passed in a counter-current direction through the multichannel heat exchanger
to the
internal refrigerant streams and optional external refrigerant stream(s).
Preferably in some
5 examples, the heat exchanger system comprises a plurality of refrigeration
stages arranged
in series where each stage in the series comprises either (i) a single
multichannel heat
exchanger, or (ii) a plurality of multichannel heat exchangers arranged in
parallel, for
example, 2 or 3 multichannel heat exchangers arranged in parallel. For
example, the heat
exchanger system may comprise three refrigeration stages arranged in series
with the
10 internal refrigerant streams and optional external refrigerant stream(s)
being fed to each
successive stage of the series at successively lower temperatures. In an
example of a heat
exchanger system, a first refrigeration stage comprises two single-pass
multichannel heat
exchangers arranged in parallel, a second refrigeration stage comprises three
3-pass
multichannel heat exchangers arranged in parallel, and a third refrigeration
stage comprises
15 a single 4-pass multichannel heat exchanger. Thus, the synthesis gas stream
can be divided
and recombined as it passes through the stages of the heat exchanger system
thereby
optimising the heat exchange with the internal refrigerant streams and/or
external
refrigerant stream(s). However, alternative arrangements. for a plurality of
multichannel
heat exchangers may be adopted.
20 Alternatively, or in addition, the heat exchanger system may comprise a
plurality of
refrigeration stages wherein each refrigeration stage comprises either a
single stand-alone
heat exchanger or a plurality of stand-alone heat exchangers arranged in
parallel. Thus, for
example, the synthesis gas stream (or other stream) is cooled as it is passed
through the
refrigeration stages of the heat exchanger system by heat exchange with a
plurality of
25 internal refrigerant streams and optional external refrigerant stream(s)
that are fed to the
stand-alone heat exchanger(s) of each successive refrigeration stage at
successively lower
temperatures. It is preferred that the synthesis gas stream is passed through
the stand-alone
heat exchangers in a counter-current direction to the internal refrigerant
streams and
optional external refrigerant stream(s) that are fed to the stand-alone heat
exchangers.
It is also envisaged that the heat exchanger system may comprise both
multichannel
and stand-alone heat exchangers. Thus, the heat exchanger system may comprise
a
plurality of refrigeration stages arranged in series wherein each
refrigeration stage
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
26
comprises (i) a single multichannel heat exchanger, or (ii) a single stand-
alone heat
exchanger, or (iii) a plurality of multichannel heat exchangers and/or a
plurality of stand-
alone heat exchangers arranged in parallel.
The multichannel heat exchanger(s) of the heat exchanger system may be of the
type
employed in processes for generating liquefied natural gas such as a brazed
aluminium
plate-fin heat exchanger or a diffusion-bonded heat exchanger (for example, a
printed
circuit heat exchanger (PCHE) as supplied by Heatric). Alternatively, the
multichannel
heat exchanger(s) may be a multiple body shell and tube heat exchanger
comprising either
(a) a tube arranged in the shell of the heat exchanger wherein the shell of
the heat
exchanger comprises a plurality of compartments and wherein the synthesis gas
stream is
passed through the tube and an internal refrigerant stream or external
refrigerant stream is
passed through each compartment of the shell in heat exchange relationship
with the
synthesis gas that is flowing through the tube; or (b) a plurality of tubes
arranged in the
shell of the heat exchanger wherein the shell comprises a single compartment
and the
synthesis gas is passed through the compartment and an internal refrigerant
stream or an
external refrigerant stream is passed through each of the tubes in heat
exchange
relationship with the synthesis gas that is flowing through the single
compartment of the
shell. Accordingly, the term "channel" encompasses the channels formed between
the
plates of a brazed aluminium plate-fin heat exchanger or a diffusion-bonded
heat
exchanger and also the compartment(s) and tube(s) of a multiple body shell and
tube heat
exchanger.
The stand-alone heat exchanger(s) of the compression system may be of the
shell and
tube type (a single body shell and tube heat exchanger) with the synthesis gas
stream
passing through the tube side and an internal refrigerant stream or external
refrigerant
stream passing through the shell side of the heat exchanger or vice versa.
However, a
process that employs stand-alone heat exchangers to pre-cool the synthesis gas
stream may
be of reduced efficiency compared with a process that employs a multichannel
heat
exchanger, in whole or in part, to cool the synthesis gas stream in step (B)
of the present
invention.
The cooled stream that exits the heat exchanger system is a two phase stream
comprised of a liquid phase and vapour phase. There is generally a practical
limit on the
temperature to which the gas stream may be cooled in the heat exchanger system
as the
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
27
temperature should normally be maintained above a value where solid CO2 will
form. This
typically occurs at a temperature of -56 C (the triple point for pure CO2 is
at 5.18 bar and
at a temperature of -56.4 C) although the presence of H2 may depress this
freezing point.
The amount of cooling that is achieved in the heat exchanger system owing to
heat
exchange with the plurality of internal refrigerant streams will be dependent
upon the
amount of cooling of the isentropically expanded hydrogen rich vapour streams
that is
achieved in the turboexpansion system which, in turn, is dependent on the
pressure of the
hydrogen rich vapour stream that is formed and the pressure of the H2 rich
vapour stream
that exits a turboexpander or a final turboexpander of the turboexpansion
system. The
amount of electricity generated by turboexpanders of a turboexpansion system
will also be
dependent on the extent to which the hydrogen rich vapour is subjected to
isentropic
expansion in the turboexpansion system which is also dependent on the pressure
of the H2
rich vapour stream formed and the pressure of the H2 rich vapour stream that
exits the final
turboexpander of the turboexpansion system.
The term "refrigerant" used herein preferably includes any appropriate coolant
where
appropriate.
Preferably the term "external refrigerant" includes a refrigerant that is
formed in an
external refrigeration circuit. Accordingly, liquid CO2 that is formed in the
process of the
present invention is not regarded as an external refrigerant. Suitable
external refrigerants
that may be used as refrigerant in the heat exchanger(s) include propane,
ethane, ethylene,
ammonia, hydrochlorofluorocarbons (HCFCs) and mixed refrigerants. Typical
mixed
refrigerants comprise at least two refrigerants selected from the group
consisting of
butanes, propanes, ethane, and ethylene. These refrigerants may be cooled to
the desired
refrigeration temperature in external refrigerant circuits using any method
known to the
person skilled in the art including methods known in the production of
liquefied natural gas
(LNG) or natural gas liquids (NGLs).
These refrigerants may also be cooled to the desired refrigeration temperature
for
example by heat exchange with one or more cold isentropically expanded H2 rich
vapour
streams from the turboexpanders of the turboexpansion system. The external
refrigerant
for the cryogenic separation stage is selected so as to achieve the desired
operating
temperature. For example, propane may be used as refrigerant when the feed
temperature
of the gas stream is in the range of -15 to greater than -30 C and the desired
operating
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
28
temperature of the cryogenic separation stage is in the range of -20 to
greater than -30 C
while ethane and/or ethylene may be used as external refrigerant when the feed
temperature of the gas stream is in the range of -30 to -40 C and the desired
operating
temperature for the cryogenic separation stage is in the range of -40 to -55
C, preferably, -
45 to -50 C. Other arrangements are possible
The CO2 stream that is withdrawn from the gas-liquid separator may in some
examples be obtained at the liquid CO2 export pressure and the liquid CO2
stream may be
passed through the heat exchanger system, for example in heat exchange
relationship with
the synthesis gas stream before being exported from the process and
sequestered and/or
used in a chemical process.
The CO2 stream may in some examples be obtained at a pressure above the liquid
CO2 export pressure and is reduced in pressure to the liquid CO2 export
pressure before
being passed to a flash separation vessel where a hydrogen rich vapour stream
is
withdrawn from at or near the top of the flash separation vessel and a liquid
CO2 stream is
withdrawn from at or near the bottom of the flash separator vessel and the
liquid CO2
stream is then passed through the heat exchanger system in heat exchange
relationship with
the synthesis gas stream before being exported from the process and
sequestered and/or
used in a chemical process.
However, in many examples of aspects of the present invention, the CO2 is
withdrawn from the separator vessel at a pressure which is less than a
required export
pressure. For example, in accordance with some aspects of the invention, the
pressure of
the separated CO2 is less than 150 bar, for example less than 120 bar, less
than 100 bar,
less than 80 bar or even less. Therefore in some arrangements, the method
further includes
the step of pressurizing the separated C02, for example using a pump, although
other
appropriate apparatus could be used, for example a compressor. The pressure to
which the
CO2 is pressurised will of course depend on its intended usage, but in some
examples,
preferably the pressure of the pressurised CO2 is more than 100 bar, more than
120 bar,
preferably 150 bar or more.
Treatment of the liquid carbon dioxide recovered will depend on its intended
use. It
may for example be piped or transported off-site for underground storage. The
liquid
carbon dioxide may if desired be warmed by passing it through one or more of
the cooling
stages, for example one or more of the multichannel heat exchangers to utilise
its cooling
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
29
capacity also.
While the carbon dioxide withdrawn in the separation stage will be in the
liquid
phase, it will be understood that carbon dioxide elsewhere in the process may
be in a
supercritical dense phase. For example, when the liquid carbon dioxide is
warmed as
suggested above, the temperature of the carbon dioxide may rise above the
critical
temperature. References herein to liquid carbon dioxide should be construed
accordingly.
The hydrogen rich vapour stream that is withdrawn from the flash separation
vessel
may be combined with a hydrogen rich vapour stream of similar pressure that is
withdrawn
from one of the turboexpanders and/or is combined with a synthesis gas feed
stream of
similar pressure that is obtained by passing the synthesis gas feed stream
through a
compression system (if present).
The hydrogen rich vapour stream that exits the final turboexpander may be
obtained
at a pressure in the range of 25 to 45 barg, preferably, 30 to 35 barg and may
be passed as
fuel gas to a combustor of at least one gas turbine of a power plant.
The cooled synthesis gas stream cooled in the heat exchanger may have a
temperature in the range of -30 to -40 C and may be subsequently passed to a
cryogenic
separation system that comprises a single cryogenic separation stage comprised
of a heat
exchanger that employs an external refrigerant and a gas-liquid separator
vessel wherein
the pressure drop across the cryogenic separation stage is preferably in the
range of 0.1 to 5
bar; the heat exchanger of the cryogenic separation stage preferably has an
operating
temperature in the range of -40 to -55 C; and wherein the hydrogen rich vapour
stream and
the liquid CO2 stream are withdrawn from the gas-liquid separator vessel of
the cryogenic
separation stage.
The cooled synthesis gas stream may have a temperature in the range of -15 to -
30 C
and may be passed to a cryogenic separation system comprising a plurality of
cryogenic
separation stages that are arranged in series wherein each cryogenic
separation stage of the
series is comprised of a heat exchanger that employs an external refrigerant
and a gas-
liquid separation vessel; the cryogenic separation stages of the series are
operated at
progressively lower temperatures and with a pressure drop across the series of
cryogenic
separation stages preferably in the range of 0.1 to 5 bar; the hydrogen rich
vapour stream
and the liquid CO2 stream being withdrawn from the gas-liquid separator vessel
of the final
cryogenic separation stage in the series; and additional HP liquid CO2 streams
being
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
withdrawn from each of the preceding cryogenic separation stages in the
series.
Where the process includes the step of compressing the synthesis gas,
preferably
synthesis gas is compressed in a multistage compressor system comprising a
plurality of
compressors arranged in series wherein a heat exchanger is provided after each
compressor
5 of the series and wherein the synthesis gas is preferably cooled in each
heat exchanger
against an external coolant for example selected from the group consisting of
air, water or
a against cold process stream selected from the H2 rich vapour stream and the
final H2 rich
vapour stream.
The synthesis gas feed stream may be a sour synthesis gas stream comprising
H2S
10 wherein a major portion of the H2S partitions into the liquid CO2 phase and
is sequestered
with the liquid CO2 stream(s) and residual H2S in the final H2 rich vapour
stream may be
removed downstream of the CO2 condensation plant for example by passing the
final H2
rich vapour stream through a bed comprising a particulate adsorbent material
or through a
scrubber wherein the H2 rich vapour stream contacts a liquid absorbent.
15 The CO2 product stream may be used as injection fluid for an oil reservoir,
for
example by injecting the liquid CO2 down an injection well and into the oil
reservoir
thereby displacing hydrocarbons towards an associated production well.
Also provided by an aspect of the invention is a carbon dioxide condensation
plant
for separating carbon dioxide and hydrogen from a synthesis gas stream, the
plant
20 comprising:
(a) a source of a synthesis gas feed stream;
(b) optionally a compression system;
(c) a heat exchanger system arranged to cool the (optionally compressed)
synthesis gas
stream against at least one (preferably a plurality of) internal coolant or
refrigerant streams
25 thereby partially condensing the synthesis gas stream;
(d) a gas-liquid separator vessel arranged to receive the partially condensed
synthesis gas
stream; and
(e) a turboexpander system comprising a plurality of turboexpanders arranged
in series
to receive a hydrogen rich vapour stream from the gas-liquid separator vessel,
a turbo
30 expander being arranged to expand a hydrogen rich vapour stream and to feed
the
expanded hydrogen rich vapour stream to the heat exchanger system.
In preferred arrangements, the apparatus includes means (for example an
expander)
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
31
for reducing the pressure of the H2-rich fraction downstream of the separator
stage.
Preferably the apparatus is arranged such that the expanded H2-rich fraction
is
subsequently used as an internal coolant elsewhere in the apparatus or in a
related
apparatus. Where the apparatus includes a plurality of expanders, preferably
the apparatus
is such that the H2-rich fraction is used as a coolant after each expansion
step, although
other arrangements are of course possible. Preferably the expander is such
that it recovers
work in expanding the hydrogen-rich gas.
In any of the examples described herein, and any of the aspects of the
invention as
appropriate, other process steps may be included, and further components
included in the
system as required. For example, the process may include a solvent separation
stage, for
example to remove C02, H2S or other component from one or more streams. For
example,
prior to expansion, H2 rich vapour stream may be fed to a solvent extraction
system in
which the vapour stream is contacted with a solvent which absorbs residual CO2
contained
therein. Solvent extraction processes for effecting this separation include
the RectisolTM
and SelexolTM processes which respectively use refrigerated methanol and a
refrigerated
mixture of dimethyl ethers of polyethylene glycol as the absorbent.
Alternatively the
absorbent can be amine based for example monoethanolamine, diethanolamine,
methyldiethanolamine diisopropylamine or the like. Any other appropriate
method may be
used. Alternatively, or in addition, solvent separation stage(s) may be
included at other
parts of the system.
As discussed above, the hydrogen enriched synthesis gas vapour stream may be
used
as fuel gas for the combustor of the gas turbine(s). It is preferred that the
fuel gas contains
35 to 65 mole % hydrogen, more preferably, 45 to 60 mole % hydrogen, for
example, 48 to
52 mole % of hydrogen. An advantage of the present invention is that the
hydrogen
enriched synthesis gas vapour stream that is discharged from the cryogenic
separation plant
contains CO2 as a co-component. It may therefore not be necessary to add a
diluent such
as nitrogen and/or steam to the hydrogen enriched synthesis gas vapour stream
in order to
meet the fuel specification for the combustor of the gas turbine.
Alternatively, the amount
of diluent added to the hydrogen enriched synthesis gas vapour stream may be
reduced.
The exhaust gas from the gas turbine(s) is passed to a heat recovery and steam
generator unit (HRSG) where the exhaust gas may be heat exchanged with various
process
streams. Optionally, the temperature of the exhaust gas from the gas turbine
is increased
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
32
by providing the HRSG with a post-firing system, for example, a post-firing
burner.
Suitably, the post-firing burner is fed with a portion of the hydrogen
enriched synthesis gas
fuel stream which is combusted in the burner using residual oxygen contained
in the
exhaust gas. Suitably, the exhaust gas is raised in temperature in the post-
firing system to
a temperature in the range of 500 to 800 C.
Typically, the HRSG generates and superheats steam for use in at least one
steam
turbine and elsewhere in the process of the present invention. Typically, the
HRSG is
capable of generating high pressure (HP) steam, medium pressure (MP) steam and
low
pressure (LP) steam and of superheating these steam streams. The HRSG may also
be
capable of reheating MP steam that is produced as an exhaust stream from the
high
pressure stage of a multistage steam turbine. In addition, the HRSG may be
used to heat
boiler feed water (for example, boiler feed water that is fed to the waste
heat boiler of a
shift converter unit).
The cooled exhaust gas is discharged from the HRSG to the atmosphere through a
stack. Preferably, the stack is provided with a continuous emission monitoring
system for
monitoring, for example, the NO,, content of the cooled exhaust gas.
The liquid CO2 stream(s) that are withdrawn from the separator vessel(s) of
the
cryogenic separation stage(s) preferably comprises at least 90 mole% C02, in
particular, at
least about 94 mole % CO2, the remainder being mostly hydrogen with some
inerts, for
example, nitrogen and/or CO. Where the cryogenic separation plant comprises a
plurality
of cryogenic separation stages arranged in series, the liquid CO2 streams that
are
withdrawn from the stages are preferably combined. The liquid CO2 stream or
combined
liquid CO2 stream is preferably fed to a to a rectification column for removal
of residual
hydrogen. Typically, the rectification column is a distillation column
comprising a
plurality of distillation trays, for example, 3 to 5 distillation trays. The
liquid CO2 stream
or combined liquid CO2 stream is fed to an intermediate position in the column
while a
hydrogen enriched vapour stream is withdrawn from at or near the top of the
distillation
column and a liquid CO2 stream havinga reduced content of hydrogen is removed
from at
or near the bottom of the distillation column. Typically, the liquid CO2
stream that is
removed from at or near the bottom of rectification column has a hydrogen
content of less
than 1% by volume, preferably, less than 0.05% by volume. Preferably, the
distillation
column is operated with reflux i.e. the hydrogen enriched vapour stream that
is withdrawn
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
33
from at or near the top of the distillation column is cooled to below its dew
point against an
external refrigerant, for example, propane or ethane, to condense out liquid
CO2 and the
condensed liquid CO2 is returned to the upper part of the column, for example,
to the top
tray of the column.
The liquid CO2 stream is then pumped to the desired export pressure, for
example,
the pipeline delivery pressure. The liquid CO2 stream may then be transferred
by pipeline
to a reception facility of an oil field where the stream may be used as an
injection fluid in
the oil field. If necessary, the liquid CO2 stream is further pumped to above
the pressure of
an oil reservoir before being injected down an injection well into the oil
reservoir. The
injected CO2 displaces the hydrocarbons contained in the reservoir rock
towards a
production well for enhanced recovery of hydrocarbons therefrom. If any carbon
dioxide
is produced from the production well together with the hydrocarbons, the
carbon dioxide
may be separated from the hydrocarbons for re-injection into the oil reservoir
such that the
CO2 is sequestered in the oil reservoir. It is also envisaged that the liquid
CO2 stream may
be injected into an aquifer or a depleted oil or gas reservoir for storage
therein.
According to the invention there is also provided a method and/or apparatus
being
substantially as herein described, preferably having reference to one or more
of the
accompanying drawings.
Any one or more of the features described herein may be combined in any
appropriate combination. Features of one aspect of the invention may be
combined, where
appropriate, with features of another aspect of the invention. Method features
may be
provided as apparatus features, and vice versa.
Examples of processes and/or apparatus of aspects of the present invention
will now
be illustrated by reference to the following Figures.
Figure 1 shows a block flow diagram that illustrates the production of a
synthesis gas
stream comprising hydrogen and carbon dioxide and the separation of a hydrogen
enriched
synthesis gas stream from a carbon dioxide stream using a cryogenic separation
plant.
Figure 2 provides a more detailed view a cryogenic separation plant according
to the
present invention while
Figure 3 relates to a cryogenic separation plant according to a preferred
embodiment
of the present invention.
Figures 4a and 4b show the external refrigeration circuits that produce the
external
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
34
refrigerants for the cryogenic separation plants of Figures 2 and 3.
Figure 5 shows schematically elements of a further example of a system for use
in a
method of separation of carbon dioxide from a synthesis gas;
Figure 6 shows schematically elements of a further example of a system for use
in a
method of separation of carbon dioxide from a synthesis gas.
In Figure 1, a shifted synthesis gas stream comprising 30 to 65 mole % H2, 35
to 70
mole % C02, up to 3 mole % of CO, and up to 100 ppm of H2S, is subjected to
Low
Temperature Gas Cooling to knock out water contained in the shifted synthesis
gas stream.
Typically, this is achieved by cooling the shifted synthesis gas stream to a
temperature of
approximately 30 to 40 C in a heat exchanger against boiler feed water thereby
generating
steam. Cooling results in condensation of the majority of the water which is
separated in a
knockout drum. In practice, cooling of the shifted synthesis gas stream
generates two
steam streams, low pressure (LP) steam and medium pressure (MP) steam. These
steam
streams may be used in an upstream plant (for example, a gasifier) or sent to
a steam
turbine for electricity generation. The water that is separated in the knock-
out drum will
contain trace amounts of CO2 and other impurities. These impurities are
stripped from the
condensate in a Condensate Stripper. The remaining condensate (water) is then
used as
boiler feed water.
The shifted synthesis gas from the Low Temperature Gas Cooling Stage may then
sent to an Acid Gas Removal (AGR) plant where the H2S may be stripped out of
the CO2
enriched stream via the use of a physical or chemical absorbent in an
absorption tower.
Typically SelexolTM (a mixture of dimethyl ethers of polyethylene glycol) is
used as
absorbent. The separated H2S may be passed to a Claus plant for the production
of
elemental sulphur, or may be converted to sulphuric acid in a sulphuric acid
plant.
However, where it is desired to co-capture the H2S, the shifted synthesis gas
from the Low
Temperature Gas Cooling Stage an AGR plant may be eliminated with the H2S
partitioning
into the liquefied CO2 in the separator vessel(s) of the cryogenic separation
stage(s) of the
plant. If necessary, the hydrogen enriched synthesis gas vapour stream that is
separated
from the captured CO2 and co-captured H2S is passed through a zinc oxide guard
bed to
remove any residual H2S prior the stream being used as a fuel gas.
Alternatively, H2S may
be removed from the cold hydrogen enriched synthesis gas vapour stream
downstream of
the single cryogenic separation stage or stage N of the series, using a
chemical absorbent in
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
an absorption tower, for example, Rectisol (methanol). Typically, an
absorption tower
that employs Rectisol as absorbent is operated at a temperature of about -40
C.
Accordingly, the hydrogen enriched synthesis gas vapour stream should be
passed to the
absorption tower, prior to the vapour stream being heated to above -40 C
against the dried
5 synthesis gas feed stream.
The synthesis gas feed stream that exits the AGR plant (or by-passes the AGR
plant)
is then dried, as any moisture in the synthesis gas feed stream will cause
freezing and
blockages in downstream processing equipment. Viable options for dehydrating
the
synthesis gas feed stream include passing the gas through a molecular sieve
bed.
10 Typically, the water content of the dried synthesis gas feed stream is less
than 1 ppm
(molar basis).
Once dehydrated, the synthesis gas feed stream is sent at a pressure of 57 bar
to a
cryogenic separation plant. This cryogenic separation plant typically
comprises a
multichannel heat exchanger and at least one, preferably, two or more
cryogenic separation
15 stages arranged in series. In the multichannel heat exchanger, the
synthesis gas feed
stream is cooled against one or more cold product streams. However, it is also
envisaged
that the multichannel heat exchanger may be replaced by two or more shell and
tube heat
exchangers arranged in series and/or in parallel that each employ a cold
product stream as
coolant for the synthesis gas feed stream. Where the shell and tube heat
exchangers are
20 employed in parallel, the synthesis gas feed stream is divided and a
portion of the feed
stream is sent to each heat exchanger and the cooled streams are subsequently
recombined
downstream of the heat exchangers.
Where there is a single cryogenic separation stage, the synthesis gas feed
stream is
cooled to below its dew point against an external refrigerant in a heat
exchanger of the
25 single separation stage that is operated at a temperature in the range of -
53 to -48 C and a
pressure in the range of 55 to 59 bar absolute so that the stream becomes two
phase (a
liquid phase comprising substantially liquid CO2 and a vapour phase that is
enriched in H2
compared with the synthesis gas feed stream). The liquid phase is then
separated from the
vapour phase in a separator vessel of the single cryogenic separation stage
and a liquid
30 CO2 stream and a hydrogen enriched synthesis gas vapour stream are removed
from at or
near the bottom and top of the separator vessel respectively. Where two or
more cryogenic
separation stages are arranged in series, the cryogenic separation stages will
separate at
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
36
least two liquid CO2 streams from the hydrogen enriched synthesis gas vapour
stream that
is discharged from the final stage of the series. Thus, the synthesis gas feed
stream is
cooled to below its dew point against an external refrigerant in a heat
exchanger of a first
cryogenic separation stage of the cryogenic separation plant so that the
stream becomes
two phase. The liquid phase (substantially pure liquid C02) is then separated
from the
vapour phase in a separator vessel of the first cryogenic separation stage and
a liquid CO2
stream and a hydrogen enriched synthesis gas vapour stream are removed from at
or near
the bottom and top of the separator vessel respectively. The hydrogen enriched
synthesis
gas vapour stream is then further cooled to below its dew point against a
further external
refrigerant in a heat exchanger of the second stage of the cryogenic
separation plant so that
the stream becomes two phase and a liquid phase (substantially pure liquid
C02) is then
separated from a vapour phase (that is further enriched in hydrogen) in a
separator vessel
of the second stage. This may be repeated using further cryogenic separation
stages until a
sufficient level of CO2 capture has been achieved. However, the final stage of
the series
should be operated at a temperature in the range of -53 to -48 C and a
pressure in the range
of 55 to 59 bar absolute. An advantage of removing a liquid CO2 stream from
each
cryogenic separation stage of the series is that this reduces the
refrigeration load for the
subsequent cryogenic separation stage(s) of the series by minimizing sub-
cooling of the
liquid. Thus, the liquid CO2 stream that is removed from the first and
intermediate
cryogenic separation stages of the series by-passes the subsequent separation
stage(s) and
is therefore not subjected to additional cooling.
Where there is a single cryogenic separation stage, ethane and/or ethylene is
generally used as refrigerant thereby allowing cooling of the synthesis gas
feed stream to a
temperature in the range of -53 to -48 C.
Where there are two or more cryogenic separation stages arranged in series,
propane
may be used as refrigerant in one or more cryogenic separation stages followed
by the use
of ethane and/or ethylene as refrigerant in one or more further cryogenic
separation stages,
depending on the desired condensation temperatures in the different cryogenic
separation
stages. However, other refrigerants may be used such as ammonia,
hydrochlorofluorocarbons (HCFC's) and mixed refrigerants. Typical mixed
refrigerants
comprises at least two refrigerants selected from the group consisting of
butanes, propanes,
ethane, and ethylene.
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
37
Where the cryogenic separation plant comprises a single cryogenic separation
stage,
the liquid CO2 stream is passed to a pump that increases the pressure of the
stream for
transportation. Where the cryogenic separation plant comprises a plurality of
cryogenic
separation stages arranged in series, the liquid CO2 streams that are
withdrawn from the
separation vessels of the cryogenic stages of the plant are combined before
being passed to
a pump that increases the pressure of the combined liquid CO2 stream for
transportation.
The H2 enriched synthesis gas vapour stream that is discharged from the single
cryogenic separation stage or from the last cryogenic separation stage (Stage
N) of the
series comprises between 75 and 90 mole% H2 and between 10 and 25 mole% CO2.
This
H2 enriched synthesis gas vapour stream is at a high pressure (typically,
approximately 59
barg) as the pressure drop across the cryogenic separation stages are ideally
minimised .
The hydrogen enriched synthesis gas vapour stream is then reduced in pressure
before
being passed to the inlet of-the gas turbines (GTs) of the Power Island,
preferably, using
one or more turboexpanders. It will generally be necessary to warm the
hydrogen enriched
synthesis gas vapour stream before it enters the turboexpander(s) so as to
mitigate the risk
of a fall in temperature to below the temperature at which solid CO2 would
form in the
turboexpander(s). Typically, the hydrogen enriched synthesis gas may be warmed
by
being passed through the multichannel heat exchanger before entering the
turboexpander(s). The expansion energy recovered from the H2 enriched
synthesis gas
vapour stream in the turboexpander(s) can be converted into electrical power
for export or
for use within the plant (e.g. to drive the CO2 pumps or the compressor(s) of
the external
refrigeration circuit(s)). Isentropic expansion of the hydrogen enriched
synthesis gas
vapour stream in the turboexpander(s) results in cooling of the hydrogen
enriched synthesis
gas vapour stream. As discussed above, advantageously, the hydrogen enriched
synthesis
gas vapour stream(s) that exit the turboexpander(s) may be used to cool the
synthesis gas
feed stream in the multichannel heat exchanger or in two or more shell and
tube heat
exchanger(s).
The expanded hydrogen enriched synthesis gas vapour stream is then sent to a
Fuel
Gas Saturation and Dilution Stage (saturation tower) where the hydrogen
enriched
synthesis gas vapour stream is diluted with steam and/or optionally nitrogen
thereby
generating a fuel stream comprising approximately 50 mole % hydrogen. Dilution
of the
fuel stream may be required in order to control NOX emissions and flame
speeds.
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
38
However, the presence of CO2 in the fuel stream may reduce or even eliminate
the need for
added diluent. The fuel stream is then sent to the Power Island, where the
fuel is
combusted in air in the combustor of at least one modified gas turbine (GT).
The GT can
be used to drive an electric motor thereby generating electricity. The exhaust
gas from the
gas turbine is passed to a Heat Recovery Steam Generator (HRSG) where the
exhaust gas
is heat exchanged with boiler feed water thereby generating steam and/or with
steam to
generate superheated steam. Typically, three levels of steam (HP, MP or LP)
can be
generated from boiler feed water. The resulting steam streams may be combined
with the
petroleum coke or coal that is fed to the gasifier and/or may be used in a
steam turbine that
drives an electric generator thereby producing additional electricity. The
exhaust gas from
the HRSG is vented to atmosphere.
Figure 2 shows a detailed process flow diagram for the cryogenic separation
plant of
the block diagram outlined in Figure 1. A synthesis gas feed stream 1 is fed
at a pressure
of 57 bar absolute to a cryogenic separation plant. The synthesis gas feed
stream 1
comprises hydrogen (for example, 40 to 65 mole %, typically 55 mole %), carbon
dioxide
(for example, 35 to 60 mole %, typically 45 mole %), and contaminants such as
water,
inerts (for example nitrogen and/or argon), methane and carbon monoxide. Where
the
synthesis gas feed stream is obtained from a high pressure coal or petroleum
coke gasifier,
it may be a sour shifted synthesis gas stream comprising hydrogen sulfide (0.2
to 1.5 mole
%, typically about 1 mole %). Where the shifted synthesis gas stream is
derived from a
reformer, hydrogen sulfide will have been removed from the feed to the
reformer so as to
avoid poisoning the reforming catalyst. Accordingly, the synthesis gas feed
stream will
not contain any hydrogen sulfide impurity.
Where the synthesis gas feed stream 1 is a sour synthesis gas stream, the
synthesis
gas feed stream may be sent on to an Absorption Tower (C-101), where the
stream 1 is
contacted with a solvent that acts as a selective absorbent for H2S thereby
generating a
desulfurised synthesis gas stream 2. Suitable solvents that can act as
selective absorbent
for H2S include physical solvents, for example, SelexolTM (a mixture of
dimethyl ethers of
polyethylene glycol) or chemical solvents, for example, methyldiethylamine
(MDEA).
However, the desulfurised synthesis gas stream 2 may still retain trace
amounts of H2S.
Optionally, the desulfurised synthesis gas stream 2 is then cooled in heat
exchanger
E-107 against propane refrigerant thereby generating a cold stream 2A. It is
important that
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
39
the cold stream 2A is maintained at a temperature above 0 in order to avoid
the deposition
of ice in the plant. The cooled synthesis gas stream 2A that exits heat
exchanger E107 is
then sent to drier D-500 in order to remove water prior to condensing out the
CO2 in a
cryogenic separation plant. There are many methods known in the art for the
removal of
saturated water from a process stream including absorbent beds (for example,
molecular
sieve beds). The resulting dried synthesis gas stream 3 enters the cryogenic
separation
plant at an elevated pressure of 57 barg and at a temperature above 0 C. If
the plant does
not include optional heat exchanger E- 107, the temperature of the dried
synthesis gas
stream is typically slightly above ambient, for example, 20 to 45 C. The dried
synthesis
gas stream is then cooled in multichannel heat exchanger EX-101, for example,
a plate fin
heat exchanger, against a plurality of cold process streams (see below)
thereby generating a
cooled synthesis gas feed stream 4 having a pressure of 56 bar absolute and a
temperature
of, for example, approximately -27 C. Accordingly, a portion of the CO2 in the
cooled
synthesis gas feed stream that exits multichannel heat exchanger EX-101 will
separate as a
liquid phase from a vapour phase. Optionally, a separator vessel may be
provided
upstream of the first cryogenic separation stage to remove this condensed
liquid phase.
The cooled shifted synthesis gas feed stream 4 then enters the first of a
series of three
cryogenic separation stages each of which comprises a heat exchanger and
separator
vessel. The separator vessels (V-102, V-103 and V-104) are operated at
substantially the
same pressure but at successively lower temperatures. In heat exchanger E- 102
of the first
cryogenic separation stage, the cooled synthesis feed stream 4 is further
cooled to a
temperature of -29.7 C against propane refrigerant to generate a two phase
stream 5 which
is then passed to separator vessel V-102 where a portion of the CO2 in stream
5 separates
as a liquid phase from a vapour phase. A vapour stream 6 that is enriched in
hydrogen and
depleted in CO2 is removed overhead from separator vessel V-102 and is passed
through
heat exchanger E-103 where it is further cooled against propane or ethane
refrigerant to a
temperature of -40.8 C thereby generating a further two phase stream 8 which
is passed to
separator vessel V-103 where a portion of the CO2 in stream 8 separates as a
liquid phase
from a vapour phase. A vapour stream 9 that is further enriched in hydrogen is
withdrawn
overhead from separator vessel V-103 and is passed through heat exchanger E-
104 where
this stream is further cooled to a temperature of -50 C against ethane
refrigerant thereby
generating a two phase stream I 1 that is passed to separator vessel V-104
where a portion
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
of the CO2 in stream 11 separates as a liquid phase from a vapour phase. A
hydrogen
enriched synthesis gas stream 12 is discharged overhead from separator vessel
V-104.
The propane refrigerant that is fed to the shell side of heat exchangers E-
107, and E-
102 and the ethane refrigerant that is fed to the shell side of heat
exchangers E- 103 and E-
5 104 is at successively lower temperatures and may be obtained using any
cryogenic
method known to the person skilled in the art, including cryogenic methods for
producing
refrigerants for liquefying natural gas. The ethane refrigerant for heat
exchangers E-103
and E- 104 may be replaced with ethylene. In addition, the refrigerant for
each of the heat
exchangers E- 107, and E- 102 to E- 104 may be replaced with a mixed
refrigerant stream
10 comprising at least two refrigerants selected from the group consisting of
butanes,
propanes, ethane and ethylene. The composition of the mixed refrigerant
streams that are
fed to the different heat exchangers may be adjusted to achieve the desired
level of cooling.
Although the process of the invention has been described with respect to 3
cryogenic
separation stages, the number of cryogenic separation stages may be increased
or
15 decreased depending predominantly on the different levels of refrigeration
being used, the
desired level of carbon capture, energy efficiency targets and the capital
cost requirements.
Preferably, at least 2 cryogenic separation stages are provided. There is a
limit on the
lowest temperature in the last stage of separation, as the temperature must be
maintained
above a value where solid CO2 will form. This typically occurs at a
temperature of -56 C
20 (the triple point for pure CO2 is at 5.18 bar and at a temperature of 56.4
C) although the
presence of H2S may depress this freezing point. Accordingly, the temperature
of the last
cryogenic separation stage should be above -55 C, preferably, -53 to -48 C.
The pressure
of the final cryogenic separation stage is maintained as high as possible in
order to ensure
the highest possible capture of CO2. Typically, the pressure drop across the
cryogenic
25 separation stages of the plant is at least 1 bar, for example, 1 to 5 bar.
Accordingly, the
pressure of the final cryogenic separation stage may be up to 55 bar absolute.
The liquid CO2 streams 7, 10 and 13 from the separation vessels V-102, V-103,
and
V-104 respectively are at substantially the same pressure and are mixed to
generate a
combined stream 14 that is sent to a separation vessel V-107. A liquid CO2
stream 16 is
30 withdrawn from the bottom of vessel V-107 and is sent to CO2 pump P-101.
The CO2
pump P-101 increases the pressure of the CO2 to the pipeline export pressure,
of
approximately 130 to 200 barg. The high pressure liquid CO2 stream 17 is then
passed
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
41
through the multichannel heat exchanger E-101 before being passed to a further
separator
vessel V-101 where a liquid CO2 stream 71 is withdrawn from at or near the
bottom of
vessel V-101 and is sent to pipeline
Any vapour leaving overhead from vessel V-107 is combined with stream 12
upstream of multichannel heat exchanger E-101 thereby generating stream 55.
Stream 55
is then passed through multichannel heat exchanger E-101 where it is used to
precool the
dried synthesis gas feed stream 3. The hydrogen enriched synthesis gas stream
56 that
exits the multichannel heat exchanger E-101 is combined with a hydrogen
enriched vapour
stream 67 that is withdrawn overhead from separator vessel V-101 thereby
forming stream
68 which is optionally diluted with medium pressure steam to form diluted
stream 69.
Stream 69 is then passed through heat exchanger E-401 before being sent as a
fuel gas
stream 70 to a power plant (not shown). The purpose of heat exchanger E-401 is
to raise
the temperature of the diluted stream 69 to the desired feed temperature for
the GTs of the
power plant.
Figure 3 illustrates a modification to the cryogenic separation plant
described in
Figure 2. In Figure 3, the combined liquid CO2 stream 66 that exits
multichannel heat
exchanger E-101 is passed to a rectification column T-101 for removal of
residual
hydrogen from the liquid CO2 stream. The combined liquid CO2 stream 66 is fed
to an
intermediate position in the column while a hydrogen enriched vapour stream 67
is
withdrawn from at or near the top of the distillation column and a liquid CO2
stream 71
having a reduced content of hydrogen is removed from at or near the bottom of
the
distillation column and is sent to a pipeline. Also, in Figure 3, the hydrogen
enriched
synthesis gas vapour stream 12 that is discharged overhead from separator
vessel V-104 is
passed through a channel of the multichannel heat exchanger E-101 where it is
used to cool
the dried synthesis gas feed stream 3. The hydrogen enriched synthesis gas
vapour stream
that exits the multichannel heat exchanger is at a pressure of about 55 barg
and a
temperature of about -10 C and is fed to turboexpander K-101 where it is
expanded to the
pressure of the hydrogen enriched synthesis gas vapour stream 15 that is
withdrawn
overhead from separator vessel V-107 thereby resulting in cooling of the
stream. The
expanded hydrogen enriched synthesis gas vapour stream exits the turboexpander
K-101 at
a pressure of about 42 bars and a temperature of about -30 C and is then
passed through a
further channel in the multichannel heat exchanger where it provides
additional cooling for
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
42
the dried synthesis gas feed stream 3. The expanded stream that exits the
multichannel
heat exchanger E-101 is then combined with stream 15 and the resulting
combined stream
is fed to turboexpander K- 102 where it is expanded to a pressure of 32 bara
and a
temperature of - 30 C. The cooled stream that exits turboexpander K-102 is
then passed
through yet a further channel of the multichannel heat exchanger E-101 thereby
providing
additional cooling for the dried synthesis gas feed stream 3. The expanded
hydrogen
enriched synthesis gas vapour stream 56 that exits the multichannel heat
exchanger is then
combined with the hydrogen enriched synthesis gas vapour stream 67 that is
withdrawn
overhead from rectification column T-101 thereby forming stream 68.
The propane refrigerant, for use in the cryogenic condensation plants of
Figures 2 and
3, is compressed in three stages by a centrifugal compressor K-301, as shown
in Figure 4a.
Propane vapour stream 301 from the compressor K-301 discharge is desuperheated
in
the air cooled Desuperheater E-301 and is then fully condensed in air cooled
Condenser E-
302. The liquefied propane 305 is collected in a horizontal propane receiver,
V-301. A
liquid propane stream is withdrawn from the bottom of V-301 and a first
portion 306 of
this liquid propane stream is routed to HP heat exchanger E-107 (upstream of
drier D-500).
A second portion 320 of this liquid propane stream is reduced in pressure
across a valve
and is fed to vessel V-302. A liquid propane stream is withdrawn from the
bottom of
vessel V-302 and is reduced in pressure across a further valve thereby forming
stream 310
which is fed to vessel V-303. A liquid propane stream 334 that is withdrawn
from the
bottom of vessel V-303 is divided to form streams 334A and 349 that are routed
to the heat
exchanger (kettle) E- 102 of the first cryogenic separation stage and the
ethane refrigerant
circuit condenser E-201 A-D. The vapour stream 308 exiting the top of heat
exchanger E-
107 and the vapour stream 322 exiting the top of vessel V-302 are combined to
form
stream 308B which is routed to the propane compressor K-301 via propane
compressor
suction drum V-306 and line 311. The vapour stream exiting the top of vessel V-
303 is
routed to the propane compressor K-301 via propane compressor suction drum V-
305 and
the propane vapour exiting the top of heat exchanger E- 102 and ethane
refrigerant circuit
condenser E-201A-D is routed to the propane compressor K-301 via propane
compressor
suction drum V-304. Propane compressor suction drums V-306, V-305 and C304 are
at
successively lower pressures.
The Ethane refrigerant in the CO2 Condensation Circuit is compressed in two
stages
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
43
by centrifugal compressors K-201 and K-202 that operate on a common shaft, as
shown in
Figure 4b. Ethane vapour streams 210 and 216 from the discharge of the
compressors are
combined to form stream 201 that is fully condensed against propane
refrigerant in Ethane
Condenser E-201A-D. The liquefied ethane stream 204 exiting E-201 is then
collected in a
horizontal ethane receiver, V-201. The discharge pressure of the compressors
is governed
by the condensing pressure at the exit of the Ethane Condenser E-201 A-D.
The condensed ethane liquid (stream 205) is routed to the heat exchangers
(kettles)
E-103 and E-104 of the second and third cryogenic separation stages in the HP
and LP
ethane circuit loops respectively. For the HP ethane circuit loop, ethane flow
to kettle E-
103 (stream 207) is controlled by means of an inlet level control valve. The
vapour stream
208 exiting the E-103 kettle is routed to the HP Ethane compressor K-201 via
the HP
ethane suction drum V-202 and line 209. For the LP ethane circuit loop, ethane
flow is via
an Ethane Economiser E-202 to the E-104 kettle, again controlled by means of a
kettle
inlet level control valve. The vapour stream 213A exiting the E-104 kettle is
routed to the
LP Ethane compressor K-202 via the Ethane Economiser E-202, to recover the
cooling
duty, and a LP ethane suction drum V-203.
Figure 5 shows a further example of a system for use in a method of separating
carbon dioxide from a synthesis gas.
In an example of a method using the arrangement in Figure 5, a dry, H2S-free
syngas
feed stream 1 containing about 55.6 mol% H2, and 42.7 mol% CO2 in addition to
other
components including CO, CH4, N2 and at a temperature of 40 degrees C and
pressure of
57 bar is split into two streams 2 and 4.
Stream 2 is then cooled against external coolant or external refrigerant in
heat
exchanger E1 to bring the temperature of stream 3 to about -41 degrees C, and
stream 4 is
cooled in heat exchanger LNG2 against H2 and C02-containing product streams 6
and 9 to
bring the temperature of stream 5 to about -41 degees C. Streams 3 and 5 are
mixed to
form S 1.
Stream S1 enters heat exchanger LNG1 and undergoes further cooling against
internal product streams to bring stream S2 to a temperature of -50 degrees C.
The two-phase mixture in stream S2 is then separated in separation vessel VI
into a
carbon dioxide-rich liquid stream S2L including 98.1 mol% CO2 and capturing
72.4% of
the CO2 in the feed stream 1, and a hydrogen-rich vapor stream SV2 including
80.3 mol%
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
44
H2 and less than 17 mol% CO2 and recovering 98.9% of the H2 in the feed stream
1.
Using the CO2 stream S2L as an internal coolant in heat exchanger LNGI raises
the
temperature of CO2 stream 8 to approximately -38 degrees C, the pressure of
the CO2
liquid stream is boosted in pump P 1 to bring the pressure of stream 9 to
about 150 bar and
achieve the required export pressure in this example for CO2 storage. It will
be understood
that in other applications, a different export pressure for CO2 storage may be
desirable.
The liquid CO2 is used as an internal coolant in heat exchanger LNG2 bringing
the
temperature of stream 10 to approximately 35 degrees C.
The hydrogen rich vapor in stream S2V is used as an internal coolant in heat
exchanger LNG1 and then fed via line IN to series of turboexpanders EX1 and
EX2 where
it is progressively expanded isentropically to lower pressure producing
mechanical work to
aid the compression of feed synthesis gas. The person skilled in the art will
understand
that isentropic expansion of this gas stream will result in it being cooled.
Accordingly the
hydrogen-rich gas exits EX1 at a pressure of 42 bar and a temperature of -53
degrees C and
is routed through heat exchanger LNG1 where it is heat exchanged with the high
pressure
gas stream S 1 to bring stream 2N up to a temperature of approximately -38
degrees C and
then passed to turboexpander EX2 where it is expanded yet again to form stream
2T at a
pressure of 32bar and a temperature of -53 degrees C and is again routed
through heat
exchanger LNG1 where it is heat exchanged with the high pressure gas stream SI
to bring
stream 6 up to a temperature of approximately -38 degrees C. Stream 6 enters
LNG2 where
it exchanges heat with stream 4 to produce stream 7 exiting the apparatus at a
temperature
of approximately 35 degrees C, and a pressure of 30 bar which is a suitable
temperature
and pressure for a fuel feed for a turbine power generator in this example.
Figure 6 shows a further example of a system for use in a method of separating
carbon dioxide from a synthesis gas.
In an example of a method using the arrangement in Figure 6, a dry, H2S-free
syngas
feed stream I containing about 55.6 mol% H2, and 42.7 mol% CO2 in addition to
other
components including CO, CH4, N2 is fed to compressor Cl at a temperature of
40 degrees
C and pressure of 57 bar. This stream I is compressed in two stages with
intercooling to
bring the pressure of stream 2D to 120bar and then cooled in E2 to bring the
temperature
of stream 3 to 40 degrees C.
Stream 3 is then cooled against external coolant or external refrigerant in
heat
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
exchanger E3 before entering heat exchanger LNG 1 where the stream Si is
further cooled
against internal product streams to bring stream S2 to a temperature of -50
degrees C to
form a two-phase mixture. In the present example, there may be for example a
small
pressure drop within the heat exchangers so that the pressure is reduced for
example to
5 about 118 bar.
Stream S2 is then separated in separator VI into a carbon dioxide-rich liquid
stream
S2L including 97.3 mol% CO2 and capturing 83.6% of the CO2 in stream 1, and a
hydrogen-rich vapor stream S2V including 86.2 mol% H2 and recovering 97.3% of
the H2
in stream 1.
10 Using the CO2 stream S2L as an internal coolant in heat exchanger LNG1
raises the
temperature of stream 5 to approximately 10 degrees C, the pressure of the C02
stream is
then boosted in pump P 1 to bring the pressure of stream 6 to 150 bar and
achieve the
required export pressure for CO2 storage.
The hydrogen rich vapor in stream S2V is used as an internal coolant in heat
15 exchanger LNG1 and then fed via line IN to series of turboexpanders EX1,
EX2 and EX3
where it is progressively expanded isentropically to lower pressure producing
mechanical
work to aid the compression of feed synthesis gas. The person skilled in the
art will
understand that isentropic expansion of this gas stream will result in it
being cooled.
Accordingly the hydrogen-rich gas exits EX1 at a pressure of 77 bar and a
temperature of -
20 53 degrees C and is routed through heat exchanger LNG-1 where it is heat
exchanged with
the high pressure gas stream S 1 to bring stream 2N up to a temperature of
approximately -
30 degrees C and then passed to turboexpander EX2 where it is expanded yet
again to form
stream 2T at a pressure of 50bar and a temperature of -53 degrees C and is
again routed
through heat exchanger LNG-1 where it is heat exchanged with the high pressure
gas
25 stream S I to bring stream 3N up to a temperature of approximately -30
degrees C and then
passed to turboexpander EX3 where it is expanded yet again to form stream 3T
at a
pressure of 32bar and a temperature of -53 C and is again routed through heat
exchanger
LNG-1 where it is heat exchanged with the high pressure gas stream Si to
produce stream
4 exiting the apparatus at a temperature of +10 degrees C, and a pressure of
30 bar which is
30 a suitable temperature and pressure for a fuel feed for a turbine power
generator in this
example.
It is to be understood that aspects of the invention are not limited to the
examples
CA 02754135 2011-09-01
WO 2010/103259 PCT/GB2010/000405
46
described herein, and various may be made within the scope of the invention.
In summary, a process is described for removing carbon dioxide from a
synthesis gas
feed stream in a cryogenic separation plant. In an example described the
synthesis gas feed
stream comprises 40 to 65 mole % hydrogen and is fed to a single stage or a
first stage of a
series of separation stages at a pressure in the range of 46 to 90 bar
absolute. The single
stage or a stage of the series is operated at a temperature in the range of -
53 to -48 C and a
pressure in the range of 44 to 90 bar absolute. In some examples, the single
stage or the
combined stages of the series remove 70 to 80% of the total moles of carbon
dioxide in the
synthesis gas feed stream. Liquefied CO2 product stream(s) discharged from the
stage(s)
of the cryogenic separation plant may be sequestrated and/or used in a
chemical process.
Also described is a process for separating a synthesis gas stream into a
hydrogen rich
vapour stream and a carbon dioxide rich stream. In an example, the process
includes the
steps of cooling a synthesis gas stream to a temperature at which at which a
two-phase
mixture is formed, passing the cooled stream formed either directly or
indirectly to a gas-
liquid separator vessel, the feed to the gas-liquid separator vessel having a
pressure of less
than 150 barg, withdrawing a hydrogen rich vapour stream from the separator
vessel and a
liquid CO2 stream from the separator vessel; and feeding a separated hydrogen
rich vapour
stream to an expansion system including a plurality of expanders arranged in
series,
wherein the hydrogen rich vapour stream is subjected to expansion in each of
the
expanders of the series such that an expanded hydrogen rich vapour stream is
withdrawn
from each of the expanders at reduced temperature and at successively reduced
pressures;
and using at least one expanded hydrogen-rich vapour stream as a coolant.
30