Note: Descriptions are shown in the official language in which they were submitted.
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
NEUROTROPHIN MIMETICS AND USES THEREOF
RELATED APPLICATIONS
[0001] This application is a Continuation-in-Part of U.S. Serial No.
11/396,936,
filed on April 3, 2006, which claims priority to U.S. Serial No. 60/671,785,
filed
Apr. 15, 2005, each of which is herein incorporated by reference in its
entirety. This
application also claims priority to U.S. Serial No. 61/158,306, filed March 6,
2009
and U.S. Serial No. 61/164,282, filed March 27, 2009, each of which is herein
incorporated by reference in its entirety.
GOVERNMENT SUPPORT
[0002] These studies were supported by the NIH Grant No. N30687. As such the
U.S. Government has certain rights in the presently disclosed subject matter.
TECHNICAL FIELD
[0003] The present application generally relates to compounds having a binding
specificity for p75NTR molecule and to the use of such compounds in the
treatment of
disorders involving degradation or dysfunction of cells expressing p75,
including,
for example neurodegenerative disorders.
TABLE OF ABBREVIATIONS
2D--two-dimensional
3D--three-dimensional
A(3--amyloid-(3
Ab-antibody
AD--Alzheimer's disease
BCA--bicinchoninic acid
BDNF--brain-derived neurotrophic factor
b.i.d.--twice daily
cm--centimeter
d-day
D--Dalton
DMEM--Dulbecco's Modified Eagle Media
ECL--electrogenerated chemiluminescence
EDTA--ethylenediamine tetraacetic acid
ELISA--Enzyme Linked ImmunoSorbent Assay
-1-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
ERK--extracellular signal-regulated protein kinase
FBS--fetal bovine serum
g--gram
h--hour
HBA--hydrogen bond acceptor
HBD--hydrogen bond donor
HEPES--4-2-hydroxyethyl-l-piperazineethanesulfonic acid
HRP--horseradish peroxidase
IgG--Immunoglobin G
IP--Intraperitoneal
IV-intravenous
K32--lysine residue number 32
kcal--kilocalorie
kg--kilogram
MBP--myelin basic protein
mg--milligram
min--minute
ml--milliliter
mM--millimolar
mol--mole
MTT--3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
MW--molecular weight
NaCl--sodium chloride
ng--nanogram
nM--nanomolar
NS--not significant
NMR--nuclear magnetic resonance
NGF--nerve growth factor
nM-nanomolar
p--probability
p75NTR--p75 neurotrophin receptor
PBS--phosphate-buffered saline
pmol--picomole
PMSF--phenylmethylsulfonyl fluoride
-2-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
PO--per os (by mouth)
pro-NGF--unprocessed precursor of NGF
PVDF--Polyvinylidine Difluoride
SDS--sodium dodecyl sulfate
SE--standard error
s.e.m.--standard error of measurement
Tris--2-Amino-2-(hydroxymethyl)-1, 3 -propanediol
TUNEL--Terminal deoxynucleotidyl transferase-mediated deoxyuridine
triphosphate nick-end labeling
g--microgram
l--microliter
M--micromolar
%--percent
C--degrees Celsius
> --greater than or equal to
>--greater than
< --less than or equal to
<--less than
BACKGROUND
[0004] Neurotrophins are polypeptides that play a role in the development,
function, and/or survival of certain cells, including neurons,
oligodendrocytes,
Schwann cells, hair follicle cells, and other cells. The death or dysfunction
of
neurons and other cell types has been directly implicated in a number of
neurodegenerative disorders. It has been suggested that alterations in
neurotrophin
localization, expression levels of neurotrophins, and/or expression levels of
the
receptors that bind neurotrophins are therefore linked to neuronal
degeneration.
Degeneration occurs in the neurodegenerative disorders Alzheimer's,
Parkinson's
and ALS, among others. Degeneration of oligodendrocytes can occur in central
nervous system injury, multiple sclerosis, and other pathological states.
[0005] A variety of neurotrophins have been identified, including Nerve Growth
Factor (NGF), Neurotrophin-3 (NT-3), Neurotrophin-4/5 (NT-4/5), Neurotrophin 6
(NT-6) and Brain Derived Neurotrophic Factor (BDNF). Neurotrophins are found
in
both precursor form, known as pro-neurotrophins, and in mature form. The
mature
-3-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
forms are proteins of about 120 amino acids in length that exist in
physiological
states as stable, non-covalent approximately 25 kDa homodimers. Each
neurotrophin
monomer includes three solvent-exposed R-hairpin loops, referred to as loops
1, 2,
and 4 that exhibit relatively high degrees of amino acid conservation across
the
neurotrophin family.
[0006] Mature neurotrophins bind preferentially to the receptors Trk and
p75NTR
(p75 neurotrophin receptor, also called the Low Affinity Nerve Growth Factor
Receptor or LNGFR) while pro-neurotrophins, which contain an N-terminal domain
proteolytically removed in mature forms, interact principally with p75 NT' and
through their N-terminal domains, with the sorting receptor sortilin
(Fahnestock, M.,
et al. (2001) Mol Cell Neurosci 18, 210-220; Harrington, A. W. et al. (2004)
Proc
Natl Acad Sci USA 101, 6226-6230; Nykiaer. A. et al., (2004) Nature 427, 843-
848). p75NTR interacts with Trks and modulates Trk signaling, but is also
independently coupled to several signaling systems, including pro-survival
signals,
IRAK/TRAF6/NF.kappa.B, P13/AKT, and pro-apoptotic signals, NRAGE/JNK
(Mamidipudi, V., et al. (2002) J Biol Chem 277, 28010-28018; Roux, P. P., et
al.
(2001) J Biol Chem 276, 23097-23104; Salehi, A. H., et al. (2000) Neuron 27,
279-
288).
[0007] When administered for therapeutic use, neurotrophins exhibit suboptimal
pharmacological properties, including poor stability with low serum half
lives, likely
poor oral bioavailability, and restricted central nervous system penetration
(Podulso,
J. F., Curran, G. L. (1996) Brain Res Mol Brain Res 36, 280-286; Saltzman, W.
M.,
et al (1999) Pharm Res 16, 232-240; Partridge, W. M. (2002) Adv Exp Med Bio
513, 397-430). Additionally, the highly pleiotropic effects of neurotrophins
achieved
through action of the dual receptor signaling network increases the chances of
adverse effects.
[0008] It has been suggested that the unliganded form of p75NTR is
proapoptotic,
and that homodimerization induced by neurotrophin binding eliminates the
effect
(Wang, J. J., et al (2000) J Neurosci Res 60, 587-593), consistent with
studies
showing no effects on survival of monomeric p75NTR ligands, including
monovalent
Fabs (Maliartchouk, S., et al (2000) J Biol Chem 275, 9946-9956) and monomeric
cyclic peptides (Longo, F. M.,. (1997) J Neurosci Res 48, 1-17), while related
bivalent forms in each study promote cell survival. However, these monomeric
-4-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
ligands may not engage the receptor in the same way as the natural ligands.
Though
active NGF is a homodimers containing 2 potential p75NTR binding sites, recent
structural evidence suggests that it engages only one p75NTR molecule,
disallowing
the binding of another (He, X. L., (2004) Science 304, 870-875).
[0009] Unfortunately, technical and ethical considerations have thus far
hampered the development of therapeutic agents based upon neurotrophins. For
example, it is technically difficult to produce sufficient quantities of pure
neurotrophins using recombinant DNA techniques. Additionally, although it is
possible to utilize human fetal cells to produce neurotrophins, the ethical
ramifications raised by the use of such cells (typically obtained from an
aborted
fetus) have all but prevented the utilization of this approach. Accordingly,
there is an
unmet need in the art for the development of small molecule agents with
favorable
drug-like features based upon neurotrophins that are capable of targeting
specific
neurotrophin receptors for use in the treatment of disorders or diseases.
SUMMARY
[0010] This application generally discloses compounds having binding
specificity for p75NTR, as well as to methods for the preparation and use of
such
compounds, and to pharmaceutical compositions containing the same. More
specifically, compounds of the present application are represented by the
general
structures:
R2 2 R3
N
m R4
R5N
n X
R1 R1, I
R3
N R4 r---\N-R3
~N ~N VnN ~R4
VnI N
R1 R1 IA R1 R1 O IB
R10 R11
Y/V
R12 Ni~~ ' p \
N Z
W~N N
R13 II
-5-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
R10 41v R10 RY V
2
R12 P R1 . N P N
N> ~1~)q ' N> R ~R~~
N W N
W I (R6)t R13
IIA IIB
R24 NR22R22'
R21 R21' `
X`R23
O N s X
'
R20' R20 R19 R19
III
R32 R32' V
Y N R35 R35' E
N N
> R33 R36'
W N N R34 34 R36
R31
IV or
R32 R32' V
P N R35 R35' E
N
R33
C1 / R36'
N R34 R34 R36
IVA
including pharmaceutically acceptable salts, esters, solvates, and prodrugs
thereof,
wherein R1, R", R2, R2 , R3, R4, R5, R6, R10, R", R12, R13, R19, R19', R20,
R2o', R21,
R21' R22 R23 R24 R30 R31 R32 R32' R33 R34 R34' R35 R35' R36 R36' E, V, W, X,
Y, Z in, n, p, q, r, s, t, are as defined below.
[00111 Additionally disclosed are stereoisomers of having the structural
formula:
O O
`-/ N
11NH2 H
(2S,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)pentanamide
NN
NH2 H
(2R,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)pentanamide
-6-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
rO
N--iN
NH2 H
(2S,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)pentanamide
Q O
NN
NH2 H
(2R,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)pentanamide
[0012] Generally disclosed herein are methods of treating a neurodegenerative
or other disorder in a subject, comprising administering to the subject an
effective
amount of a compound having binding specificity for a p75NTR Also disclosed
..herein are methods of facilitating neural, oligodendrocyte, or other cell
survival
comprising treating such cells with a compound having binding specificity for
a
p75NTR molecule. Further disclosed herein are methods for treating a disorder
associated with p75 expression.
[0013] One object of the presently disclosed subject matter having been stated
hereinabove, and which is addressed in whole or in part by the present
presently
disclosed subject matter, other objects will become evident as the description
proceeds when taken in connection with the accompanying examples and drawings
as best described hereinbelow.
BRIEF DESCRIPTION OF THE DRAWINGS
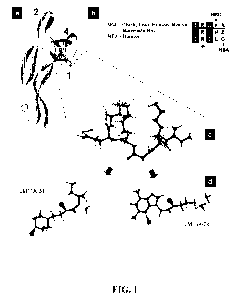
[0014] Figure 1 a is a ribbon representation of the X-ray crystal structure of
human NGF with (3-turn loops 1, 2, and 4 designated. The average side chain
positions for loop 1 are illustrated.
[0015] Figure lb represents the comparison of peptide sequences (SEQ ID
NOs:l-3) of loop 1 from NGF and NT3 from the indicated species and the
assignment of pharmacophores. Positively ionizable groups are signified by
"+".
"HBD" and "HBA" represent hydrogen bond donor and hydrogen bond acceptor,
respectively.
[0016] Figure lc shows application of the pharmacophoric features to a 3D loop
model. Hydrogen bonding features are represented by pairs of spheres with
their
relative positions indicating the locations of the acceptor and the donor. One
of the
spheres of the pair is centered on putative acceptor/donor features in the
model,
while the other indicates the target location of a complementary feature on
any
-7-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
potentially interacting molecule. The diameter of the spheres represents the
spatial
tolerance for chemical feature matching in 3D conformer library scans.
[0017] Figure ld is a 3D loop model disclosing representative fits to the
pharmacophore of two compounds identified by application of the novel
pharmacophore in library screening subsequently found to be active as
disclosed
herein.
[0018] Figure 2a is a series of fluorescence photomicrographs of E16-17 mouse
hippocampal neuronal cultures treated with culture medium only (CM) or medium
containing BDNF or Compound (i) (referred to in the figures as "LMI 1A-28" or
"28"), Compound (ii) (referred to in the figures as "LM11A-7" or "7"),
Compound
(iii) (referred to in the figures as "LM11A-24" or "24"), Compound (iv)
(referred to
in the figures as "LM 11 A-31" or "31 "), or Compound (v) (referred to in the
figures
as "LM11A-36" or "36"). The cultures were stained for expression of the neuron-
specific, growth-associated protein GAP43 at 48 hours post treatment. The 2D
structure of each compound is located adjacent to each image.
[0019] Figure 2b is a series of neuron survival dose-response curves of BDNF,
NGF, and Compounds (i-v), showing similar potency and maximal responses
between NGF and Compounds (i-iv) up to 5 nM, with no response to Compound (v).
BDNF has similar potency, but a higher maximal response. Each of Compounds (i-
v), show a decrementing response above 5 nM. Survival was determined as the
total
number of cells in each well that were both morphologically intact and filled
with
blue formazan MTT-conversion product (Longo, F.M., Manthorpe, M., Xie, Y.M.,
and Varon, S. (1997) JNeurosci Res 48, 1-17). Counts were normalized to
survival
achieved with 25 ng/ml BDNF or to baseline survival. n is 4-18 for all
determinations. Symbols and bars indicate mean +/- s.e.m., and lines are fits
of a
single exponential rise model to the data. Dotted lines in each graph
represent the
fitted NGF response.
[0020] Figure 3a is a series of NGF/p75NTR-Fc binding curves, in the presence
of
increasing concentrations of Compound (iv), as detected by NGF ELISA. Symbols
are mean +/- s.e.m. n > 10 for all determinations. Lines represent fitting to
a
modified Gaddum/Schild equation, with an overall R2 value of 0.93 for Compound
(iv). Also, P<0.0001 by ANOVA with post-hoc Bonferroni/Dunn testing, for
comparisons between binding curves at 0 nM compound and curves with > 500 nM
Compound (iv). KD for NGF in the absence of compounds was 0.8-0.9 DM,
-8-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
.consistent with previous reports of approximately 1 nM (Nykjaer, A. et al.,
(2004)
Nature 427, 843-848). The symbols "o", "a", "0", "A", and "o" represent
Compound (iv) concentrations of zero, 125, 500, 1,500, 4,500, and 10,000
nanomolar, respectively.
[0021] Figure 3b is a series of NGF/p75NTR-Fc binding curves, in the presence
of increasing concentrations of Compound (iii), as detected by NGF ELISA.
Symbols are mean +/- s.e.m. n > 10 for all determinations. Lines represent
fitting to
a modified Gaddum/Schild equation, with an overall R2 value of 0.96 for
Compound
(iii). Also, P<0.0001 by ANOVA with post-hoc Bonferroni/Dunn testing, for
comparisons between binding curves at 0 nM compound and curves with > 125 nM
Compound (iii). KD for NGF in the absence of compounds was 0.8-0.9 nM,
consistent with previous reports of approximately 1 nM (Nykjaer, A. et al.,
(2004)
Nature 427, 843-848). The symbols "e"," ", "a", "0", "A", and "o" represent
Compound (iii) concentrations of zero, 125, 500, 1,500, 4,500, and 10,000
nanomolar, respectively.
[0022] Figure 3c is a series of NGF/TrkA-Fc binding curves in the presence of
increasing concentrations of Compound (v), showing no significant effect up to
10,000 nM. Symbols are mean +/- s.e.m. n > 10 for all determinations. The
symbols "o", ", and "a", represent Compound (v) concentrations of zero,
4,500, and 10,000 nanomolar, respectively.
[0023] Figure 3d is a series of NGF/TrkA-Fc binding curves in the presence of
increasing concentrations of Compound (iv) showing no compound effects up to
10,000 nM. Symbols are mean +/- s.e.m. n > 4 for all determinations. The
symbols
and "a", represent Compound (iv) concentrations of zero, 4,500, and
10,000 nanomolar, respectively.
[0024] Figure 3e is a series of NGF/TrkA-Fc binding curves in the presence of
increasing concentrations of Compound (iii) showing no compound effects up to
10,000 nM. Symbols are mean +/- s.e.m. n > 4 for all determinations. The
symbols
" ", ", and "a", represent Compound (iii) concentrations of zero, 4,500, and
10,000 nanomolar, respectively.
[0025] Figure 3f is a digital image of a western blot showing displacement of
anti-p75 NTR Ab 9651 from anti-p75NTR-expressing 3T3 cells by Compound (iv),
but
not Compound (v). The upper panel represents IgG heavy chain, the lower panel
-9-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
represents R -actin. The graph represents quantitation. The bars represent
mean +/-
s.e.m., normalized to bound antibody (lane 4). n=4 for each condition. A
single
asterisk (*) represents P<0.0005, for comparison with binding in the absence
of
compound, by Student t-test. Antibody and compound treatments are designated
above each lane. Ab 9651 did not bind to p75NTR-negative cells (lanes 1 and
2). Ab
9651 bound to p75NTR-positive cells (lane 4) and was significantly displaced
by
Compound (iv) (lane 5), while Compound (v) had no effect (lane 6).
[0026] Figure 3g is a bar graph showing that Ab 9651 has no effect on baseline
survival (CM), partially inhibits BDNF, and completely inhibits Compound (iii)
and
Compound (iv) promotion of hippocampal neuron survival. The solid bars
represent
non-immune serum treatment. The shaded bars represent Ab 9651 treatment. The
bars represent mean +/- s.e.m. n > 26 for each condition. Double asterisks
(**)
represent P<0.00001 (for comparisons between Ab 9651 and non-immune). NS
represents not significant by Student t-test. Survival in the presence of BDNF
+ Ab
9651 is shown to be significantly greater than CM + Ab 9651 (P<0.00001), while
the differences between CM and Compound (iii), Compound (iv), and Compound
(v) in the presence of antibody are not significant.
[0027] Figure 3h is a bar graph showing that p75NTR-deficiency partially
inhibits
BDNF and completely inhibits NGF, Compound (iii), and Compound (iv) promotion
of hippocampal neuron survival. Neurotrophins were applied at 1.8 nM, and
compounds at 5 nM. The solid bars represent p75NTR+i+ cells. The shaded bars
represent p75NTR-/_ cells. The bars represent mean +/- s.e.m. n > 5 for each
condition. The single asterisk (*) represents P<0.05, the double asterisk (**)
represents P<0.005. NS represents not significant (for comparisons between
knockout and wild type) by Student t-test. In p75NTR-/- cultures, BDNF
treatment
produced greater survival than NGF (P<0.05) or Compounds (iii-v) (P<0.01).
There
was no significant difference in baseline survival between the genotypes.
[0028] Figure 3i shows digital images of western blots of hippocampal neuron
cultures using anti-phosphorylated TrIJ490, compared with total TrkB. BDNF
activated TrkB, while NGF and Compounds (iii-v) resulted in no detectable
activation at 10 or 30 minutes.
[0029] Figure 3j shows digital images of western blots of TrkA-expressing 3T3
cells using anti-phosphorylated TrV490 compared with total TrkA. NGF is shown
to
-10-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
activate TrkA, while Compound (iv) produced no detectable activation. Results
of
two additional independent assays for TrkB and TrkA activation were identical.
[0030] Figures 4a-4d are digital images of western blots of extracts of
hippocampal cultures treated with culture media (C), BDNF (B) at 50 ng/ml, NGF
(N) at 50 nghnl, or Compounds (iii-v) at 20 nM showing representative bands
corresponding to phosphorylated signaling factors (open arrowheads) and the
corresponding total factor (filled arrowheads) and quantitation of the ratio
of
phospho- to total factor, indicating degree of activation. Bars indicate mean
+/-
s.e.m. Solid bars represent sampling at 10 minutes. Shaded bars represent
sampling
at 30 minutes. n=6 independent blots for each determination. Single asterisks
(*)
represent P<0.001 for comparison with CM by Student t-test. Other comparisons
are as indicated, with P values by Student t-test indicated above each
bracket.
[0031] Figure 4a is a digital image of a western blot indicating NFxB-p65
activation analysis, showing similar activation kinetics for all biologically
active
treatments.
[0032] Figure 4b is a digital image of a western blot representing AKT
activation analysis, showing a small lag in activation by the active compounds
relative to NGF.
[0033] Figure 4c is a digital image of a western blot representing ERK44
activation analysis, showing less activation at 10 minutes for the compounds
relative
to NGF.
[0034] Figure 4d is the digital image of a western blot representing ERK42
activation analysis, showing prolonged activation with BDNF treatment relative
to
NGF and Compounds (iii-v).
[0035] Figure 4e is a bar graph indicating survival of hippocampal neurons in
cultures treated with signaling pathway inhibitors and BDNF (25 ng/ml), NGF
(25
ng/ml), or Compounds (iii-v) (5 nM), showing substantial inhibition by NFxB
and
P13K pathway inhibitors, small effects of ERK inhibition on BDNF and NGF
activity, and no effect of ERK inhibition on the activity of Compounds (iii-
v). SN50
is an NFKB translocation inhibitor. LY represents LY294002, a P13K inhibitor.
PD
represents PD98059, an ERK inhibitor. n=18 for each bar, showing mean + s.e.m.
NS represents that the data is not significant. A single asterisk (*)
indicates P <
0.05, double asterisks (**) indicates that P < 0.001 for comparison with
control (no
-11-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
inhibitor) in each group. The open, solid, lighter-shaded, and darker-shaded
bars
represent control, SN50, LY, and PD, respectively.
[0036] Figure 4f is the digital image of a western blot of signaling
activation
analysis of NFxB pathway activation. Bars indicate mean +/- s.e.m. n > 6 for
each
condition. P values are as indicated. Activation is detected between 0 and 0.5
nM
for NFKB, reaching a plateau level at 5 nM.
[0037] Figure 4g is the digital image of a western blot of signaling
activation
analysis of AKT pathway activation. Bars indicate mean +/- s.e.m. n > 6 for
each
condition. P values are as indicated. Activation is detected between 0.5 and 1
nM
for AKT, reaching a plateau level at 5 nM.
[0038] Figure 4h is the digital image of a western blot indicating AKT
activation
by growth factors and compounds in p75NTRa" cells. n > 9 for each condition.
There
are no significant differences between culture medium alone and NGF or
Compounds (iii-v).
[0039] Figure 5a is a bar graph disclosing that Compounds (iii-v) do not
promote death of mature oligondendrocytes and inhibits proNGF-induced death.
Mature oligondendrocytes were treated as indicated and cell death assessed by
determining the proportion of MBP-positive cells that are also TUNEL-positive.
In
the absence of pro-NGF, compounds did not promote cell death. In the presence
of
2.8 ng/ml (0.05 nM) proNGF, Compound (iii) and Compound (iv), but not
Compound (v), blocked cell death. Bars represent mean + s.e.m. n > 2 for each
condition except for 1 nM Compound (v) with proNGF which had a single
determination. P < 0.05, by Student t-test for comparisons with proNGF
treatment
without compounds. The closed, lighter-shaded, and darker-shaded bars
represent
Compound (iii), Compound (iv), and Compound (v), respectively.
[0040] Figure 5b is a line graph showing proNGF displacement from p75NTR by
Compound (iii) and Compound (iv). 100 ng/ml proNGF was incubated with the
indicated concentrations of compounds and detected by ELISA. n = 4 for each
condition. Symbols indicate means +/- s.e.m. The signal from all compound-
treated
samples were significantly less than proNGF alone, with P < 0.01 by Student t-
test.
The symbols "A" and "e" represent Compound (v) and Compound (iii),
respectively.
[0041] Figure 6 demonstrates that Compound (iii) blocks A[3-induced neural
degeneration. Figure 6a is a bar graph representing percentage of surviving
-12-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
hippocampal neuronal cells after addition of API-42 (10 M or 30 M). Addition
of
AP142 resulted in an approximate 40% loss of neurons after 3 days of exposure.
Addition of NGF (100 pg/ml) did not protect against A[31-42. Figure 6b is a
bar graph
representing the percentage of surviving hippocampal neural cells after
addition of
A(31-02 (10 M) with test compounds.
DETAILED DESCRIPTION
[0042] In subjects with disorders related to degeneration or dysfunction of
cells
expressing p75, such as neurodegenerative disorders, alterations in
neurotrophin
localization, expression levels of neurotrophins, expression levels of the
receptors
that bind neurotrophins, and/or receptor signaling and functional outcomes can
occur. In addition within these disorders, alterations in signaling pathways
or other
mechanisms that are linked to p75 receptor mechanisms and that can be
regulated by
p75 signaling can occur. Other disorders involve cells not expressing the p75
receptor however, cells expressing p75 have the ability to compensate for
impairment or loss of non p75-expressing cells. Accordingly, by providing
subjects
suffering from such disorders with a corresponding neurotrophic factor or
mimetic
thereof that modulates p75NTR function or proNGF/NGF binding to prevent
cellular
degeneration or dysfunction, such neural degeneration can be alleviated or
prevented. As disclosed herein, methods of treating neurodegenerative and
other
disorders and/or facilitating cell survival by administering a compound having
binding specificity for a p75NTR molecule are provided.
[0043] The methods and compounds of the present application relate to
compounds having binding specificity for a p75NTR molecule. Compounds having
binding specificity for p75NTR are suitable for positively regulating survival
and/or
inhibiting degeneration of neural and other cells, e.g. inhibition or reversal
of
neuronal spine loss. Particularly, in cells showing trophic responses to
neurotrophins
or cells expressing p75NTR either constitutively or in response to injury or
disease,
the compounds promote survival signaling and/or inhibit degenerative or
dysfunctional signaling. In cells susceptible to neurotrophin-induced death,
the
compounds do not induce apoptosis, but inhibit neurotrophin-mediated death.
Such
mechanisms are relevant beyond the nervous system and include neurotrophin
regulation of p75 receptor-expressing hair follicle cell survival and hair
loss.
[0044] Additional embodiments and advantages of the application will be set
forth in part in the description that follows, and in part will be obvious
from the
-13-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
description, or may be learned by practice of the invention. It is to be
understood
that both the foregoing general description and the following detailed
description are
exemplary and explanatory only and are not restrictive of the invention as
claimed.
Definitions
[0045] It is to be understood that the terminology used herein is for the
purpose
of describing particular embodiments only and is not intended to be limiting.
[0046] Unless defined otherwise, all technical and scientific terms used
herein
have the same meaning as commonly understood to one of ordinary skill in the
art to
which the present application belongs. Although any methods and materials
similar
or equivalent to those described herein can be used in the practice or testing
of the
present application, representative methods and materials are herein
described.
[0047] Following long-standing patent law convention, the terms "a", "an", and
"the" refer to "one or more" when used in this application, including the
claims.
Thus, for example, reference to "a carrier" includes mixtures of one or more
carriers,
two or more carriers, and the like.
[0048] Unless otherwise indicated, all numbers expressing quantities of
ingredients, reaction conditions, and so forth used in the specification and
claims are
to be understood as being modified in all instances by the term "about".
Accordingly, unless indicated to the contrary, the numerical parameters set
forth in
the present specification and attached claims are approximations that can vary
depending upon the desired properties sought to be obtained by the present
application. Generally the term "about", as used herein when referring to a
measurable value such as an amount of weight, time, dose, etc. is meant to
encompass in one example variations of 20% or 10%, in another example
5%,
in another example I%, and in yet another example 0.1 % from the specified
amount, as such variations are appropriate to perform the disclosed method.
[0049] As used herein, the phrase "a disorder involving degeneration or
dysfunction of cells expressing p75" includes, but is not limited to disorders
related
to upregulation of p75. Such disorders include neurodegenerative disorders, as
well
as conditions involving degeneration of p75-expressing cells, such as hair
loss.
Within the nervous system, the p75 receptor is expressed by various cell types
including neurons, oligodendrocytes, astrocytes and microglia. Compounds
targeting
p75 receptors expressed by neurons can be used to prevent loss of function,
degeneration and/or death of neurons in a number of nervous system disorders
-14-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
including, but not limited to, Alzheimer's disease, Parkinson's disease,
Huntington's
disease, stroke, traumatic brain injury, spinal cord injury, epilepsy,
multiple
sclerosis, amyotrophic lateral sclerosis, neuropathies, myopathies and various
forms
of retinal degeneration. In each of these disorders, neurons and other cells
expressing p75 are affected.
[0050] Compounds targeting p75 receptors expressed by oligodendrocytes can
be used to prevent loss of function, degeneration and/or death of
oligodendrocytes in
a number of nervous system disorders including, but not limited to, multiple
sclerosis, spinal cord injury and perinatal anoxia. Compound targeting p75
receptors
expressed by microglia can be used to inhibit deleterious activation of
microglia and
thereby decrease the inflammatory component of neurodegenerative and other
disorders.
[0051] Outside of the nervous system, a number of cell populations express the
p75 receptor. These include hair follicle cells, hepatic cells, vascular
endothelial,
vascular smooth muscle cells, cardiomyocytes. In addition, the p75 receptor is
expressed by certain tumor cells such as those involved in breast or prostate
cancer.
Given this expression pattern, compounds targeting p75 receptors can be used
for
the following indications: to prevent loss of hair follicle cells and thereby
prevent
hair loss; to prevent hepatic cirrhosis and promote liver regeneration; to
regulate
angiogenesis and promote neovascularization in the setting of diabetic wounds
or
other ischemic settings; to prevent cardiomyopathy by preventing myocardial
cell
loss or by stimulating growth of new cardiomyocytes either in the setting of
ischemia or after myocardial infarction; and to inhibit tumor cell growth. In
addition
p75 is expressed by stem cells and is known to regulate stem cell growth;
therefore,
p75 ligands can be used to promote stem cell growth as part of a strategy to
promote
tissue and organ regeneration. P75 receptor ligands can also be used to tag or
identify cells expressing p75 or having upregulated p75 as part of diagnostic
or cell-
harvesting strategy.
[0052] As used herein, the term "neurodegenerative disorder" includes any
disorder characterized by neural damage or dysfunction and includes but is not
limited to Alzheimer's disease, Huntington's disease, Pick's disease,
amyotrophic
lateral sclerosis, epilepsy, Parkinson's disease, spinal cord injury, stroke,
hypoxia,
ischemia, brain injury, diabetic neuropathy, peripheral neuropathy, nerve
transplantation, multiple sclerosis, and peripheral nerve injury.
-15-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[0053] The compounds disclosed herein function as ligands at the p75
neurotrophin receptor and thereby induce intracellular signaling that prevents
cellular degeneration or death and/or upregulates cell function or growth. The
intracellular signaling mechanisms regulated by the p75 receptor are
fundamental
mechanisms present in essentially all cell types; therefore, it is expected
that any cell
or tissue expressing this receptor would be amendable to treatment with these
compounds for the goal of preventing cellular or tissue degeneration,
promoting cell
survival and/or for upregulating function or growth.
[0054] The term "alkyl," alone or in combination, refers to an optionally
substituted straight-chain or branched-chain alkyl radical having from 1 to
about 20
carbon atoms. The term also includes optionally substituted straight-chain or
branched-chain alkyl radicals having from 1 to about 6 carbon atoms as well as
those having from 1 to about 4 carbon atoms. Examples of alkyl radicals
include
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl,
tert-amyl,
pentyl, hexyl, heptyl, octyl and the like. "Branched" refers to an alkyl group
in
which a lower alkyl group, such as methyl, ethyl or propyl, is attached to a
linear
alkyl chain. "Lower alkyl" refers to an alkyl group having 1 to about 8 carbon
atoms
(i.e., a C1_8 alkyl), e.g., 1, 2, 3, 4, 5, 6, 7, or 8 carbon atoms. "Higher
alkyl" refers to
an alkyl group having about 10 to about 20 carbon atoms, e.g., 10, 11, 12, 13,
14,
15, 16, 17, 18, 19, or 20 carbon atoms. In certain embodiments, "alkyl"
refers, in
particular, to C1_8 straight-chain alkyls. In other embodiments, "alkyl"
refers, in
particular, to C1_8 branched-chain alkyls. Alkyl groups can be optionally
substituted.
[0055] The term "heteroalkyl" refers to alkyl groups, as described above, in
which one or more skeletal atoms are oxygen, nitrogen, sulfur or combinations
thereof. The term heteroalkyl also includes alkyl groups in which one 1 to
about 6
skeletal atoms are oxygen, nitrogen, sulfur or combinations thereof, as well
as those
in which 1 to 4 skeletal atoms are oxygen, nitrogen, sulfur or combinations
thereof
and those in which 1 to 2 skeletal atoms are oxygen, nitrogen, sulfur or
combinations thereof. Heteroalkyl groups are optionally substituted.
[0056] The term "alkenyl," alone or in combination, refers to an optionally
substituted straight-chain or branched-chain hydrocarbon radical having one or
more
carbon-carbon double-bonds and having from 2 to about 18 carbon atoms. The
term
also includes optionally substituted straight-chain or branched-chain
hydrocarbon
radicals having one or more carbon-carbon double bonds and having from 2 to
about
-16-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
6 carbon atoms as well as those having from 2 to about 4 carbon atoms.
Examples of
alkenyl radicals include ethenyl, propenyl, butenyl, 1,4-butadienyl and the
like.
Suitable alkenyl groups include allyl. The terms "allylic group" or "allyl"
refer to the
group -CH2HC=CH2 and derivatives thereof formed by substitution. Thus, the
terms
alkenyl and/or substituted alkenyl include allyl groups, such as but not
limited to,
allyl, methylallyl, di-methylallyl, and the like. The term "allylic position"
or "allylic
site" refers to the saturated carbon atom of an allylic group. Thus, a group,
such as a
hydroxyl group or other substituent group, attached at an allylic site can be
referred
to as "allylic." "l-alkenyl" refers to alkenyl groups where the double bond is
between the first and second carbon atom.
[0057] The term "alkynyl," alone or in combination, refers to an optionally
substituted straight-chain or branched-chain hydrocarbon radical having one or
more
carbon-carbon triple-bonds and having from 2 to about 12 carbon atoms. The
term
also includes optionally substituted straight-chain or branched-chain
hydrocarbon
radicals having one or more carbon-carbon triple bonds and having from 2 to
about
6 carbon atoms as well as those having from 2 to about 4 carbon atoms.
Examples of
alkynyl radicals include ethynyl, propynyl, butynyl and the like. "I -alkynyl"
refers
to alkynyl groups where the triple bond is between the first and second carbon
atom.
[0058] "Cyclic alkyl" and "cycloalkyl" refer to a non-aromatic mono- or
multicyclic ring system of about 3 to about 10 carbon atoms, e.g., 3, 4, 5, 6,
7, 8, 9,
or 10 carbon atoms, alternately from about 3 to about 6 carbon atoms. The
cycloalkyl group can be optionally partially unsaturated. The cycloa]_Icyl
group also
can be optionally substituted as defined herein. Representative monocyclic
cycloalkyl rings include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl, cycloheptyl, and the like. Further, the cycloalkyl group can be
optionally
substituted with a linking group, such as an alkylene group as defined
hereinabove,
for example, methylene, ethylene, propylene, and the like. In such cases, the
cycloalkyl group can be referred to as, for example, cyclopropylmethyl,
cyclobutylmethyl, and the like. Additionally, multicyclic cycloalkyl rings
include
adamantyl, octahydronaphthyl, decalin, camphor, camphane, and noradamantyl.
[0059] The term "heterocyclic alkyl" and "heterocycloalkyl" refer to cyclic
groups of 3 to 6 atoms, or 3 to 10 atoms, containing at least one heteroatom.
In one
aspect, these groups contain 1 to 3 heteroatoms. Suitable heteroatoms include
oxygen, sulfur, and nitrogen. Heterocyclic groups may be attached through a
-17-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
nitrogen or through a carbon atom in the ring. Suitable heterocyclic groups
include
pyrrolidinyl, morpholino, morpholinoethyl, and pyridyl. Such groups may be
substituted.
[0060] . The term "aryl" refers to aromatic groups which have 5-14 ring atoms
and at least one ring having a conjugated pi electron system and includes
carbocyclic
aryl, heterocyclic aryl and biaryl groups, all of which may be optionally
substituted.
The term "aryl" is used herein to refer to an aromatic substituent that can be
a single
aromatic ring, or multiple aromatic rings that are fused together, linked
covalently,
or linked to a common group, such as, but not limited to, a methylene or
ethylene
moiety. The common linking group also can be a carbonyl, as in benzophenone,
or
oxygen, as in diphenylether, or nitrogen, as in diphenylamine. The aromatic
ring(s)
can comprise phenyl, naphthyl, biphenyl, diphenylether, diphenylamine and
benzophenone, among others. all of which can be optionally substituted. In
particular embodiments, the term "aryl" means a cyclic aromatic comprising
about 5
to about 10 carbon atoms, e.g., 5, 6, 7, 8, 9, or 10 carbon atoms, and
including 5- and
6-membered hydrocarbon and heterocyclic aromatic rings. Examples of aryl
groups
include, but are not limited to, cyclopentadienyl, phenyl, furan, thiophene,
pyrrole,
pyran, pyridine, imidazole, benzimidazole, isothiazole, isoxazole, pyrazole,
pyrazine, triazine, pyrimidine, quinoline, isoquinoline, indole, carbazole,
and the
like, all optionally substituted.
[0061] The aryl group can be optionally substituted (a "substituted aryl")
with
one. or more aryl group substituents, which can be the same or different,
wherein
"aryl group substituent" includes alkyl, substituted alkyl, aryl, substituted
aryl,
aralkyl, hydroxyl, alkoxyl, aryloxyl, aralkyloxyl, carboxyl, acyl, halo,
nitro,
alkoxycarbonyl, aryloxycarbonyl, aralkoxycarbonyl, acyloxyl, acylamino,
aroylamino, carbamoyl, alkylcarbamoyl, dialkylcarbamoyl, arylthio, alkylthio,
alkylene, and NR'R", wherein R' and R" can each be independently hydrogen,
alkyl, substituted alkyl, aryl, substituted aryl, and aralkyl.
[0062] Thus, as used herein, the term "substituted aryl" includes aryl groups,
as
defined herein, in which one or more atoms or functional groups of the aryl
group
are replaced with another atom or functional group, including for example,
alkyl,
substituted alkyl, halogen, aryl, substituted aryl, alkoxyl, hydroxyl, nitro,
amino,
alkylalnino, dialkylamino, sulfate, and mercapto.
-18-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[0063] Specific examples of aryl groups include, but are not limited to,
cyclopentadienyl, phenyl, furan, thiophene, pyrrole, pyran, pyridine,
imidazole,
benzimidazole, isothiazole, isoxazole, pyrazole, pyrazine, triazine,
pyrimidine,
quinoline, isoquinoline, indole, carbazole, and the like.
[0064] A structure represented generally by a formula such as:
(R)n (R)õ
0010
as used herein refers to a 6-carbon ring structure comprising a substituent R
group,
wherein the R group can be present or absent, and when present, one or more R
groups can each be substituted on one or more available carbon atoms of the
ring
structure. The presence or absence of the R group and number of R groups is
determined by the value of the integer n. Each R group, if more than one, is
substituted on an available carbon of the ring structure rather than on
another R
group. For example, the structure:
(R)n
0
wherein n is an integer from 0 to 2 comprises compound groups including, but
not
limited to:
/ OrR (IR R
\ I
R R and the like.
(R)n
4 3
I ~ \ 2
Y
[0065] The structure: X 6 7 wherein n is one (1) comprises
compound groups including:
-19-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
R
R R
X p Y, ( O Y/ \ Y and
X X O
Y
X J?~O
R
wherein the one (1) R substituent can be attached at any carbon on the
benzofuran
parent structure not occupied by another designated substituent, as in this
case
carbon 6 is substituted by X and carbon 2 is substituted by Y.
[0066] A dashed line representing a bond in a cyclic ring structure indicates
that
the bond can be either present or absent in the ring. That is a dashed line
representing a bond in a cyclic ring structure indicates that the ring
structure is
selected from the group consisting of a saturated ring structure, a partially
saturated
ring structure, and an unsaturated ring structure.
[0067] "Carbocyclic aryl" groups are groups wherein the ring atoms on the
aromatic ring are carbon atoms. Carbocyclic aryl groups include monocyclic
carbocyclic aryl groups and polycyclic or fused compounds such as optionally
substituted naphthyl groups.
[00681 "Heterocyclic aryl" or "heteroaryl" groups are groups having from 1 to
4
heteroatoms as ring atoms in the aromatic ring and the remainder of the ring
atoms
being carbon atoms. Suitable heteroatoms include oxygen, sulfur, nitrogen, and
selenium. Suitable heteroaryl groups include furanyl, thienyl, pyridyl,
pyrrolyl, N-
lower alkyl pyrrolyl, pyridyl-N-oxide, pyrimidyl, pyrazinyl, imidazolyl, and
the like,
all optionally substituted.
[0069] The phrase "carbocyclic ring" refers to a saturated or unsaturated
monocyclic or bicyclic ring in which all atoms of all rings are carbon. Thus,
the term
includes cycloalkyl and carbocyclic aryl rings.
[0070] The phrase "heterocyclic ring" refers to a saturated or unsaturated
monocyclic or bicyclic ring having from 1 to 4 heteroatoms as ring atoms in
the
aromatic ring and the remainder of the ring atoms being carbon atoms. Thus,
the
term includes heterocycloalkyl and heterocyclic aryl rings.
-20-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[0071] The term "optionally substituted" or "substituted" includes groups
substituted by one to four substituents, independently selected from lower
alkyl,
lower aryl, lower aralkyl, lower alicyclic, heterocyclic alkyl, hydroxyl,
lower
alkoxy, lower aryloxy, perhaloalkoxy, aralkoxy, heteroaryl, heteroaryloxy,
heteroarylalkyl, heteroaralkoxy, azido, amino, guanidino, amidino, halo, lower
alkylthio, oxo, acylalkyl, carboxy esters, carboxyl,-carboxamido, nitro,
acyloxy,
aminoalkyl, alkylaminoaryl, alkylaryl, alkylaminoalkyl, alkoxyaryl, arylamino,
aralkylamino, phosphono, sulfonyl, -carboxamidoalkylaryl, -carboxanidoaryl,
hydroxyalkyl, haloalkyl, alkylaminoalkylcarboxy-, aminocarboxamidoalkyl-,
cyano,
lower alkoxyalkyl, lower perhaloalkyl, and arylalkyloxyalkyl.
[0072] In some embodiments, the compounds described by the presently
disclosed subject matter contain a linking group. As used herein, the term
"linking
group" comprises a chemical moiety which is bonded to two or more other
chemical
moieties to form a stable structure. Representative linking groups include but
are
not limited to a furanyl, phenylene, thienyl, or pyrrolyl radical bonded two
or more
aryl groups.
[0073] When a named atom of a ring or chain is defined as being "absent," the
named atom is replaced by a direct bond or is incorporated into double bond
along
with the atom to which it is attached. When the linking group or spacer group
is
defined as being absent, the linking group or spacer group is replaced by a
direct
bond.
[0074] "Alkylene" refers to a straight or branched bivalent aliphatic
hydrocarbon
group having from 1 to about 20 carbon atoms, e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 11,
12, 13, 14, 15, 16, 17, 18, 19, or 20 carbon atoms. The alkylene group can be
straight, branched or cyclic. The alkylene group also can be optionally
unsaturated
and/or substituted with one or more "alkyl group substituents." There can be
optionally inserted along the alkylene group one or more oxygen, sulfur or
substituted or unsubstituted nitrogen atoms (also referred to herein as
"alkylaminoalkyl"), wherein the nitrogen substituent is alkyl as previously
described. Exemplary alkylene groups include methylene (-CH2-); ethylene (-
CH2-CH2-); propylene (-(CH2)3-); cyclohexylene (-C6H10-); -CH=CH-CH=CH-;
-CH=CH-CH2-; -(CH2)a N(R)-(CH2)r , wherein each of q and r is independently
an integer from 0 to about 20, e.g., 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12,
13, 14, 15,
16, 17, 18, 19, or 20, and R is hydrogen or lower alkyl; methylenedioxyl (-O-
CH2-
-21-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
0-); and ethylenedioxyl (-O-(CH2)2-O-). An alkylene group can have about 2 to
about 3 carbon atoms and can further have 6-20 carbons.
[0075] The term "alkenylene" denotes an acyclic carbon chain (i.e., having an
open-chain structure) having a carbon-to-carbon double bond and is represented
by
the formula Cr,H2ri_2, which optionally can be substituted one or more times.
Representative alkenylene groups include, but are not limited to, ethenylene,
propenylene, 1- or 2-butenylene, 1-, or 2-pentylene, and the like.
[0076] As used herein, the term "acyl" refers to an organic acid group wherein
the -OH of the carboxyl group has been replaced with another substituent
(i.e., as
represented by RCO-, wherein R is an alkyl or an aryl group as defined
herein). As
such, the term "acyl" specifically includes arylacyl groups, such as an
acetylfuran
and a phenacyl group. Specific examples of acyl groups include acetyl and
benzoyl.
[0077] "Alkoxyl" or "alkoxyallcyl" refer to an alkyl-O-- group wherein alkyl
is
as previously described. The term "alkoxyl" as used herein can refer to Ci_2o
inclusive, linear, branched, or cyclic, saturated or unsaturated oxo-
hydrocarbon
chains, including, for example, methoxyl, ethoxyl, propoxyl, isopropoxyl,
butoxyl, t-
butoxyl, and pentoxyl.
[0078] "Aryloxyl" refers to an aryl-O-- group wherein the aryl group is as
previously described, including a substituted aryl. The term "aryloxyl" as
used
herein can refer to phenyloxyl or hexyloxyl, and alkyl, substituted alkyl,
halo, or
alkoxyl substituted phenyloxyl or hexyloxyl.
[0079] "Aralkyl" refers to an aryl-alkyl- group wherein aryl and alkyl are as
previously described, and included substituted aryl and substituted alkyl.
Exemplary
aralkyl groups include benzyl, phenylethyl, and naphthylmethyl.
[0080] "Aralkyloxyl" refers to an aralkyl-O-- group wherein the aralkyl group
is
as previously described. An exemplary aralkyloxyl group is benzyloxyl.
[0081] "Dialkylamino" refers to an --NRR' group wherein each of R and R' is
independently an alkyl group and/or a substituted alkyl group as previously
described. Exemplary alkylamino groups include ethylmethylamino,
dimethylamino,
and diethylamino.
[0082] "Alkoxycarbonyl" refers to an alkyl-O--CO-- group. Exemplary
alkoxycarbonyl groups include methoxycarbonyl, ethoxycarbonyl,
butyloxycarbonyl, and t-butyloxycarbonyl.
-22-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
10083] "Aryloxycarbonyl" refers to an aryl-O--CO-- group. Exemplary
aryloxycarbonyl groups include phenoxy- and naphthoxy-carbonyl.
[0084] "Aralkoxycarbonyl" refers to an aralkyl-O--CO-- group. An exemplary
aralkoxycarbonyl group is benzyloxycarbonyl.
[0085] "Carbamoyl" refers to an H2N--CO-- group.
[0086] "Alkylcarbamoyl" refers to a R'RN--CO-- group wherein one of R and R'
is hydrogen and the other of R and R' is alkyl and/or substituted alkyl as
previously
described.
[0087] "Diallcylcarbamoyl" refers to a R'RN--CO-- group wherein each of R and
R' is independently alkyl and/or substituted alkyl as previously described.
[0088] "Acyloxyl" refers to an acyl-O-- group wherein acyl is as previously
described.
[0089] "Acylamino" refers to an acyl-NH-- group wherein acyl is as previously
described.
[0090] "Aroylamino" refers to an aroyl-NH-- group wherein aroyl is as
previously described.
[0091] The term "amino" refers to the --NH2 group.
[0092] The term "carbonyl" refers to the --(C=O)-- group.
[0093] The term "carboxyl" refers to the --COOH group.
[0094] The term "cyano" refers to the --CN group.
[0095] The terms "halo", "halide", or "halogen" as used herein refer to
fluoro,
chloro, bromo, and iodo groups.
[0096] The term "hydroxyl" refers to the --OH group.
[0097] The term "hydroxyalkyl" refers to an alkyl group substituted with an --
OH group.
[0098] The term "mercapto" refers to the --SH group.
[0099] The term "oxo" refers to =0.
[00100] The term "nitro" refers to the NO2 group.
[00101] The term "thio" refers to a compound described previously herein
wherein a carbon or oxygen atom is replaced by a sulfur atom.
[00102] The term "sulfate" refers to the --SO4 group.
[00103] The term "cycloalkenyl" refers to a partially unsaturated cyclic
hydrocarbon group containing one or more rings, for example, one ring, two
rings,
three rings, or four rings, with three or more carbon atoms per ring, for
example, 3,
-23-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
4, 5, 6, 7, or 8 carbon atoms per ring. Exemplary cycloalkenyl groups include,
but
are not limited to, cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl,
and
the like. Cycloalkenyl groups can be optionally substituted, such as with one
or
more substituents, e.g. 1, 2, 3, or 4 substituents, at any available point of
attachment.
Exemplary substituents include, but are not limited to, alkyl, substituted
alkyl, halo,
arylarnino, acyl, hydroxyl, aryloxyl, allcoxyl, alkylthio, arylthio,
aralkyloxyl,
aralkylthio, carboxyl, alkoxycarbonyl, oxo, and cycloalkyl.
[00104] The term "substituted cycloalkenyl" refers to a cycloalkenyl group
substituted with one or more substituents, preferably 1, 2, 3, or 4
substituents, at any
available point of attachment. Exemplary substituents include, but are not
limited
to, alkyl, substituted alkyl, halo, arylamino, acyl, hydroxyl, aryloxyl,
alkoxyl,
alkylthio, arylthio, aralkyloxyl, arallcylthio, carboxyl, alkoxycarbonyl, oxo,
and
cycloalkyl.
[00105] When the term "independently selected" is used, the substituents being
referred to (e.g., R groups, such as groups Rl and R2, or groups X and Y), can
be
identical or different. For example, both Rl and R2 can be substituted alkyls,
or Rl
can be hydrogen and R2 can be a substituted alkyl, and the like.
[00106] A named "R", "R'," "X," "Y," "Y", "A," "A"', "B," "L," or "Z" group
will generally have the structure that is recognized in the art as
corresponding to a
group having that name, unless specified otherwise herein. For the purposes of
illustration, certain representative "R," "X," and "Y" groups as set forth
above are
defined below. These definitions are intended to supplement and illustrate,
not
preclude, the definitions that would be apparent to one of ordinary skill in
the art
upon review of the present disclosure.
[00107] The term "treatment" as used herein covers any treatment of a disease
and/or condition in an animal or mammal, particularly a human, and includes:
(i)
preventing a disease, disorder and/or condition and/or symptoms from occurring
in a
person which can be predisposed to the disease, disorder and/or condition, or
at risk
for being exposed to an agent that can cause the disease, disorder, and/or
condition
and/or symptoms; but, has not yet been diagnosed as having it; (ii) inhibiting
the
disease, disorder and/or condition, and/or symptoms i.e., arresting its
development;
and (iii) relieving the disease, disorder and/or condition, and/or symptoms
i.e.,
causing regression of the disease, disorder and/or condition. iv) the
augmentation of
-24-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
compensatory mechanisms, such as promotion of stem cells, that while not
treating
the primary disease can lead to reduced symptoms and improved function.
[00108] The term "mimetic" refers to a compound having similar functional
and/or structural properties to another known compound or a particular
fragment of
that known compound, such as a known compound of biological origin, e.g., a
polypeptide or fragment thereof.
[00109] "Binding specificity" refers to the ability of a protein or other type
of
molecule capable of recognizing and interacting with a complementary site on
another protein or other type of molecule.
[00110] The term "pharmacophore", as used herein, refers to a specific model
or
representation of a molecular moiety capable of exerting a selected
biochemical
effect, e.g., inhibition of an enzyme, binding to a receptor, chelation of an
ion, and
the like. A selected pharmacophore can have more than one biochemical effect,
e.g.,
can be an inhibitor of one enzyme and an agonist of a second enzyme. A
therapeutic
agent can include one or more pharmacophores, which can have the same or
different biochemical activities.
[00111] The term "derivative" as used herein refers to a compound chemically
modified so as to differentiate it from a parent compound. Such chemical
modifications can include, for example, replacement of hydrogen by an alkyl,
acyl,
or amino group. A derivative compound can be modified by, for example,
glycosylation, pegylation, or any similar process that retains at least one
biological
or immunological function of the compound from which it was derived.
[00112] The term "hydrophilicity" is used in the common manner of the field as
having an affinity for water; readily absorbing and/or dissolving in water.
[00113] The term "lipophilicity" is used in the common manner of the field as
having an affinity for, tending to combine with, or capable of dissolving in
lipids.
[00114] The term "amphipathicity", as used herein, describes a structure
having
discrete hydrophobic and hydrophilic regions. Thus, one portion of the
structure
interacts favorably with aqueous and other polar media, while another portion
of the
structure interacts favorably with non-polar media.
[00115] The term "solubility" as used herein, describes the maximum amount of
solute that will dissolve in a given amount of solvent at a specified
temperature.
[00116] The term "bioavailability" as used herein refers to the systemic
availability (i.e., blood/plasma levels) of a given amount of compound
administered
-25-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
to a subject. The term further encompasses the rate and extent of absorption
of
compound that reaches the site of action.
[00117] As used herein, "solvate" means a complex formed by solvation (the
combination of solvent molecules with molecules or ions of the active agent of
the
present invention), or an aggregate that consists of a solute ion or molecule
(the
active agent of the present invention) with one or more solvent molecules.
Examples of hydrate include, but are not limited to, hemihydrate, monohydrate,
dihydrate, trihydrate, hexahydrate, etc. It should be understood by one of
ordinary
skill in the art that the pharmaceutically acceptable salt of the present
compound
may also exist in a solvate form. The solvate is typically formed via
hydration
which is either part of the preparation of the present compound or through
natural
absorption of moisture by the anhydrous compound of the present invention.
Solvates, including hydrates, may be found in stoichiometric ratios, for
example,
with two, three, four salt molecules per solvate or per hydrate molecule.
Solvents
used for crystallization, such as alcohols, especially methanol and ethanol;
aldehydes; ketones, especially acetone; esters, e.g. ethyl acetate; may be
embedded
in the crystal grating.
[00118] The term "prodrug" as used herein refers to any compound that when
administered to a biological system generates the drug substance (a
biologically
active compound) in or more steps involving spontaneous chemical reaction(s),
enzyme catalyzed chemical reaction(s), or both. Standard prodrugs are formed
using
groups attached to functionality, e.g. HO--, HS--, HOOC--, R2N--, associated
with
the drug substance that cleave in vivo. Prodrugs for these groups are well
known in
the art and are often used to enhance oral bioavailability or other properties
beneficial to the formulation, delivery, or activity of the drug. Standard
prodrugs
include, but are not limited to, carboxylate esters where the group is alkyl,
aryl,
aralkyl, acyloxyalkyl, alkoxycarbonyloxyalkyl as well as esters of hydroxyl,
thiol
and amines where the group attached is an acyl group, an alkoxycarbonyl,
aminocarbonyl, phosphate or sulfate.
[00119] Where the compounds of the present invention have at least one
asymmetric center, they may accordingly exist as enantiomers. Where the
compounds possess two or more asymmetric centers, they may additionally exist
as
diastereoisomers. It is to be understood that all such stereoisomers and
mixtures
thereof in any proportion are encompassed within the scope of the present
invention.
-26-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Where the compounds possess geometrical isomers, all such isomers and mixtures
thereof in any proportion are encompassed within the scope of the present
invention.
Where so indicated in the claims herein, if a single enantiomer of the
potentially
optically active heterocyclic compounds disclosed is desired, for either
health or
efficacy reasons, preferably it is present in an enantiomeric excess of at
least about
80%, or at least about 90%, or at least about 95%, or at least about 98%, or
at least
about 99%, or at least about 99.5%.
[00120] Tautomers of the compounds of the invention are encompassed by the
present application. Thus, for example, a carbonyl includes its hydroxyl
tautomer.
Compounds of the Present Application
[00121] In one aspect, the present application discloses a compound having
binding specificity for a p75NTR molecule.
[00122] In some embodiments, the compound having binding specificity for a
p75NTR molecule is a mimetic of a neurotrophin [3-turn loop.
[00123] In some embodiments, the compound comprises a pharmacophore
substantially identical to the pharmacophore illustrated in Figure 1 c.
[00124] In some embodiments, the compound is a small molecule or a peptide.
[00125] In one aspect, the present application discloses a compound selected
from
the group consisting of-
H
H
H
N
N
Nip
0-11, Compound (i) and
o
- o /
N
( N'S ~S
H' NCI 0 \ / I\ /O N-H
,N
O
1
H Compound (ii).
-27-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
In another aspect, the present application discloses a compound is selected
from the
group consisting of a compound of Formula A or Formula B:
B
N
B2 A4 (CH2)n--~ H
R1, 3
I
B1 A2 A3 D1
R2
(A)
R3
B4
(CH2)n D2
L2 NH
B5 (B)
wherein:
n is an integer from 0 to 8;
L1 and L2 are a linking group selected from the group consisting of alkylene,
substituted alkylene, cycloalkyl, substituted cycloalkyl, cycloalkene,
substituted
cycloalkene, aryl, substituted aryl, alkenylene, and substituted alkenylene;
R1, R2, and R3 are each independently selected from the group consisting of
H, alkyl, substituted alkyl, cycloalkyl, halo, cyano, nitro, mercapto,
hydroxyl,
alkoxyl, aryl, aryloxyl, substituted aryl, and aralkyloxyl;
A1, A2, A3, A4, and A5 are each independently selected from the group
consisting of N and CH;
B1, B2, B3, B4, and B5 are each independently selected from the group
consisting of 0, S, and NR4, wherein R4 is selected from the group consisting
of H,
alkyl, substituted alkyl, cycloalkyl, aryl, and substituted aryl; and
D1 and D2 are selected from the group consisting of:
RS NR ~
AN~RS --N R,
Rs I
R6 R,
NR
_N'J~NR5 YNH
I I
R,o R6 , and
-28-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
wherein:
each R5, R6, R8, R9, and R10 is independently selected from the group
consisting of H, alkyl, substituted alkyl, cycloalkyl, aryl, substituted aryl,
aralkyl, hydroxyalkyl, hydroxycycloalkyl, alkoxycycloalkyl, aminoalkyl,
acyloxyl, alkylaminoalkyl, and alkoxycarbonyl;
each R7 is independently selected from the group consisting of H,
hydroxyl, alkyl, substituted alkyl, aryl, substituted aryl, acyloxyl, and
alkoxyl; or
R7 and R5 or R7 and R9 together represent a C2 to C10 alkyl, C2 to C10
hydroxyalkyl, or C2 to C10 alkene;
or a pharmaceutically acceptable salt thereof.
[00126] In some embodiments, L1 and L2 are each independently -(CH2)m ,
wherein m is an integer from 1 to 8.
[00127] In some embodiments, the compound of Formula (A) has the following
structure:
0
O
N-H
N N
X> (CH2)m
N
I N-R5
R6
wherein:
m is an integer from 1 to 8;
R1 and R2 are each independently selected from the group consisting
of H, alkyl, substituted alkyl, cycloalkyl, aryl, aryloxyl, substituted aryl,
and
aralkyloxyl; and
R5 and R6 are each independently selected from the group consisting
of H, alkyl, substituted alkyl, cycloalkyl, aryl, substituted aryl, aralkyl,
hydroxyl, alkoxyl, hydroxyalkyl, hydroxycycloalkyl, alkoxycycloalkyl,
aminoalkyl, acyloxyl, alkylaminoalkyl, and alkoxycarbonyl.
[00128] In some embodiments, the compound of Formula (A) has the following
structure:
-29-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
0
O
H3C\N N N-H
0 N N
I
CH3 N-CH3
H3C Compound (iii).
[00129] In some embodiments, the compound of Formula (B) has the following
structure:
R3
O NR5
I
/NCH R6
(CH2)m
N
OJ
wherein:
m is an integer from 1 to 8;
R3 is selected from the group consisting of H, alkyl, substituted alkyl,
cycloalkyl, halo, hydroxyl, alkoxyl, aryl, aryloxyl, substituted aryl, and
aralkyloxyl; and
R5 and R6 are each independently selected from the group consisting
of H, alkyl, substituted alkyl, cycloalkyl, aryl, substituted aryl, aralkyl,
hydroxyl, alkoxyl, hydroxyalkyl, hydroxycycloalkyl, alkoxycycloalkyl,
aminoalkyl, acyloxyl, alkylaminoalkyl, and alkoxycarbonyl.
[00130] In some embodiments, the compound of Formula (B) has the following
structure:
CH3
H3C
O NCH
NCH H
f
rN
OJ
Compound (iv).
[00131] In some embodiments, the compound of Formula (A) is not:
-30-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
0
O
H3C\N N N-H
ON N
I
CH3 N-CH3
H3C
Compound (iii);
and the compound of Formula (B) is not:
CH3
H3C
O NCH
I
N H
CH
N
OJ
Compound (iv).
[00132] In some embodiments, the neurotrophin is a nerve growth factor (NGF).
In some embodiments, the (3-turn loop is loop 1 of the NGF.
[00133] In some embodiments, the compound has the formula:
NH NH
NN H NH2
H2NJ~ N'II
H H Y Y
NH NH Compound (vii).
[00134] In some embodiments, the compound has binding specificity for a
neurotrophin binding site of the p75NTR molecule.
[00135] In some embodiments, the compound comprises a derivative of a parent
compound having binding specificity for a p75NTR molecule, wherein the
derivative
also has binding specificity for the p75NTR molecule.
[00136] In some embodiments, the derivative exhibits an enhancement in at
least
one of the characteristics selected from the group consisting of
hydrophilicity,
lipophilicity, amphipathicity, solubility, bioavailability, and resistance to
hepatic
degradation, as compared to the parent compound.
[00137] In one aspect, the present application discloses a compound of Formula
I:
-31-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
3
R2 R2 R
N
rn R4
RN
n x
R1 R1 (I)
or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof,
wherein:
each of R', R", R2, R2', R3, and R4 is independently hydrogen or optionally
substituted alkyl; or R2 and R2' taken together form =0, =S or =CH2; R5 is
heterocycloalkyl; X is CH2, NH, 0 or S; n is 0, 1, 2, 3, 4, or 5; and m is 1
or 2. In
another aspect, in a compound of Formula I each of R', R", R2, R2', R3, and R4
is
independently hydrogen, optionally substituted alkyl, optionally substituted
allcenyl
or optionally substituted alkynyl; or R2 and R2' taken together form =0 or =S;
R5 is
heterocycloalkyl; X is 0 or S; n is 0, 1, 2, 3, or 4; and m is 1 or 2. In one
embodiment of any of the disclosed aspects, X is 0; and m is 1. In another
embodiment R2 and R2' taken together form =0; and each of R3 and R4 is
independently optionally substituted C1-C6 alkyl. In another embodiment, R5 is
morpholinyl, thiomorpholinyl, tetrahydro-2H-pyran, 1-methylpiperazinyl,
piperidinyl, or pyrrolidinyl; and each of Rr and R" is independently hydrogen
or
optionally substituted C1-C4 alkyl. In another embodiment, X is 0; m is 1; R2
and
R2' taken together form =0; each of R3 and R4 is independently optionally
substituted Cl-C6 alkyl; R5 is morpholinyl, thiomorpholinyl, tetrahydro-2H-
pyran, 1
methylpiperazinyl, piperidinyl, or pyrrolidinyl; and each of R1 and R1 is
independently hydrogen or optionally substituted C1-C4 alkyl. In yet another
embodiment, m is 2; X is 0; R2 and R2' each is hydrogen; R3 is optionally
substituted
Cl-C4 alkyl; R5 is a nitrogen-bound morpholinyl, 1-methylpiperazinyl,
piperidinyl,
or pyrrolidinyl; and each of R1 and R" is independently hydrogen or optionally
substituted C1-C4 alkyl. In another aspect, the compound has the structure of
Formula IA:
R3
0~ ON
R4
N
~N
Vn __~
0
R1 R1 (IA)
wherein each of R1, R", R3, and R4 independently is hydrogen or optionally
substituted alkyl; and n is 0, 1, 2, 3, 4, or 5. Another aspect is the
compound having
-32-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
the structure of Formula IA wherein each of R', R", R3, and R4 independently
is
hydrogen, optionally substituted alkyl, optionally substituted alkenyl or
optionally
substituted alkynyl; and n is 1, 2, 3, or 4. In one embodiment of any of the
disclosed
aspects, 'n is 2; each of Rl and R" is hydrogen; R3 is methyl and R4 is sec-
butyl. In
another embodiment, R5 is a heterocycloalkyl bound via a heteroatom; m is 2;
and X
is 0. In another embodiment, R2 and R2' each is hydrogen; and R3 is optionally
substituted C1-C4 alkyl. In yet another embodiment, R5 is a nitrogen-bound
morpholinyl, 1-methylpiperazinyl, piperidinyl, or pyrrolidinyl; and each of R1
and
R" is independently hydrogen or optionally substituted C1-C4 alkyl. In still
another
variation, the compound has the structure of Formula IB:
3
0 ~N_R
(
N
Vn R4
R1 R1, (IB)
One aspect is a compound having the structure of Formula IB wherein each of
R',
R", R3, and R4 independently is hydrogen, optionally substituted alkyl,
optionally
substituted alkenyl or optionally substituted alkynyl; and n is 1, 2, 3, or 4.
In one
embodiment of any of the disclosed aspects, n is 2; each of R1 and R" is
hydrogen;
R3 is methyl and R4 is sec-butyl. In another embodiment, R5 is a
heterocycloalkyl
bound via a heteroatom; m is 2; and X is 0. In another embodiment, R2 and R2'
each
is hydrogen; and R3 is optionally substituted C1-C4 alkyl. In yet another
embodiment, R5 is a nitrogen-bound morpholinyl, 1-methylpiperazinyl,
piperidinyl,
or pyrrolidinyl; and each of R1 and R" is independently hydrogen or optionally
substituted C1-C4 alkyl.
[00138] In another aspect, the present application discloses a compound of
Formula II:
R10 R11
V
R12
N N P Z
W N N
R13 (II)
or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof,
wherein p is
0, 1, 2, 3, 4, 5, or 6; each of Y, V, and W is independently =CH2, NB, =0 or
=S;
each of R10 and R1' is independently hydrogen or optionally substituted alkyl;
each
-33-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
of R12 and R13 is independently hydrogen, -NRaRb, -OH, -C(=O)ORa, -C(=O)NHRa,
-NHC(=O)Ra, -NHS(=0)2Ra, or optionally substituted alkyl; each of Ra and Rb is
independently hydrogen or optionally substituted alkyl; and Z is
heterocycloalkyl or
heteroaryl wherein each heterocycloalkyl or heteroaryl is bound via a
heteroatom
and is optionally substituted. Another aspect is a compound of Formula II
wherein
p is 1, 2, 3, 4, or 5; each of Y, V, and W is independently =0 or =S; each of
R10 and
R" is independently hydrogen, optionally substituted alkyl, optionally
substituted
alkenyl or optionally substituted alkynyl; each of R12 and R13 is
independently
hydrogen, -NRaRb, -OH, optionally substituted alky, optionally substituted
alkenyl
or optionally substituted alkynyl; each of Ra and Rb is independently hydrogen
or
optionally substituted alkyl; and Z is heterocycloalkyl or heteroaryl wherein
each
heterocycloalkyl or heteroaryl is bound via a heteroatom and is optionally
substituted. In one embodiment of any of the disclosed aspects, p is 1, 2 or
3; each
of Y, V, and W is 0; and each of R12 and R13 is independently hydrogen or
optionally substituted C1-C4 alkyl; and Z is an optionally substituted
nitrogen-bound
heterocycloalkyl. In another embodiment, p is 1; each of R10 and R" is
hydrogen;
and each of R12 and R13 is independently C1-C4 alkyl.
[00139] In another embodiment, the compound has the structure of Formula IIA:
R1 R11
V
R12 N P N
WN N~ )a
R13 (R6)t
(IIA)
or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof,
wherein p is
0, 1, 2, 3, 4, 5, or 6;gis 1, 2, 3, or 4; t is 0, 1, 2, or 3; each of Y, V,
and Wis
independently 0 or S; each of R10 and R" is independently hydrogen or
optionally
substituted alkyl; each of R12 and R13 is independently hydrogen, -NRaRb, -OH,
-C(=O)ORa, -C(=O)NHRa, -NHC(=O)Ra, -NHS(=O)2Ra, or optionally substituted
alkyl; each of R6 is independently -NRaRb, -OH, -C(=O)ORa, -C(=O)NHRa,
-NHC(=O)Ra, -NHS(=O)2Ra, or optionally substituted alkyl; and each of Ra and
Rb
is independently hydrogen or optionally substituted alkyl. In one embodiment,
q is
1, 2, 3, or 4; t is 0, 1, 2, or 3; each of Y, V, and W is independently 0 or
S; and each
of R6 is independently -NRaRb, -OH, -C(=O)ORa, -C(=O)NHRa, -NHC(=O)Ra,
-NHS(=O)2Ra, or optionally substituted alkyl. In yet another aspect, the
compound
-34-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
has the structure of Formula IIA wherein p is 1, 2, 3, 4, or 5; q is 2 or 3; t
is 0, 1, 2,
or 3; each of Y, V, and W is independently 0 or S; each of R10 and R' 1 is
independently hydrogen, optionally substituted alkyl, optionally substituted
alkenyl,
or optionally substituted alkynyl; each of R6 is independently -NRaRb, -OH, or
optionally substituted alkyl; and each of Ra and Rb is independently hydrogen
or
optionally substituted alkyl. In another embodiment of any of the disclosed
aspects,
each of Y, V, and W is 0; q is 1; each of R10 and R1' is independently
hydrogen or
C1-C4 alkyl; and each of R12 and R13 is independently C1-C4 alkyl. In yet
another
embodiment, each of R10 and R11 is independently hydrogen; each of R12 and R13
is
independently -Me; and each of R6, R6', R, RT, R8, R", R9, and R9' is
independently
hydrogen, -NRaRb, -OH, or optionally substituted alkyl. In still a further
variation of
any of the disclosed embodiments, each of R10 and R11 is independently -H;
each of
R12 and R13 is independently -Me; q is 2; and each of R6, R6', R, RT, R8, R8',
R9, and
R9, is independently hydrogen, -NRaRb, -OH, or optionally substituted alkyl.
In
another further variation of any of the disclosed embodiments, each of R6,
R6', R,
R7', R8, R", and R9 is hydrogen; and R9' is -N(CH3)2.
[001401 In another embodiment, the compound has the structure of Formula IIB:
RIO R11
Y V
R12
N
N
/ R
W~-N RI
N
R13
(IIB)
or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof,
wherein p is
0, 1, 2, 3, 4, 5, or 6; each of Y, V, and W is independently 0 or S; each of
R'0 and
R" is independently hydrogen or optionally substituted alkyl; each of R12 and
R13 is
independently hydrogen, -NRaRb, -OH, -C(=O)ORa, -C(=O)NHRa, -NHC(=O)Ra,
and R" taken together with the
-NHS(=O)2Ra, or optionally substituted alkyl; and R'
nitrogen to which they are attached form an optionally substituted
heterocyclic aryl.
In yet another aspect, the compound has the structure of Formula IIB wherein p
is 1,
2, 3, 4, or 5; each of Y, V, and W is independently 0 or S; each of R10 and
R11 is
independently hydrogen, optionally substituted alkyl, optionally substituted
alkenyl,
or optionally substituted alkynyl; R and R " taken together with the nitrogen
to which
they are attached form a an optionally substituted pyridyl, an optionally
substituted
-35-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
pyrrolyl, an optionally substituted pyrimidyl or an optionally substituted
pyrazinyl.
In another embodiment of any of the disclosed aspects, each of Y, V, and W is
0;
each of R10 and R" is independently hydrogen or C1-C4 alkyl; and each of R12
and
R13 is independently C1-C4 alkyl. In yet another embodiment, each of R10 and
R" is
independently hydrogen; each of R12 and R13 is independently -Me. In still a
further
variation of any of the disclosed embodiments, each of R10 and Rt 1 is
independently
-H; each of R12 and R13 is independently -Me; q is 2; and R 'and R" taken
together
with the nitrogen to which they are attached form an optionally substituted
pyrrolyl.
In another further variation, the compound has the structural formula
p
0
Or" IN N N___
[001411 In another aspect, the present application discloses a compound of
Formula III:
R24 NR22R22'
R21 R21' NY _R23
O_JN S X
R20' R20 R19 R19'
(III)
or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof,
wherein X is
CH2, NH, 0 or S; s is 0, 1, 2, 3 or 4; each of R19, Rt9', R2 , R2 ', R21,
R21', R22 Rea'
and R24 is independently absent, hydrogen or optionally substituted alkyl; or
R20 and
R20' taken together form =0, =S, or =CH2; or R20 and R21 taken together with
the
atoms to which they are attached form an optionally substituted cycloalkyl; or
R20
and R21 taken together with the atoms to which they are attached form an
optionally
substituted aryl; or R19 and R20 taken together with the atoms to which they
are
attached form an optionally substituted cycloalkyl; or R19 and R20 taken
together
with the atoms to which they are attached form an optionally substituted aryl;
and
R23 is optionally substituted alkyl, optionally substituted cycloalkyl. or
optionally
substituted aryl. In one embodiment of any of the disclosed aspects or
variations,
R23 is hydrogen, optionally substituted alkyl, optionally substituted
cycloalkyl or
optionally substituted aryl. In one embodiment of any of the disclosed aspect
or
variations, the compound of Formula III is not 2-aniino-3-methyl-N-(2-
morpholinoethyl)-butanamide. Yet another aspect is a compound of Formula III
-36-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
wherein X is 0 or S; s is 0, 1, 2, or 3; each of R19, R19', R20, R20', R21,
R21', R22 , R2T
and R24 is independently absent, hydrogen, optionally substituted alkyl,
optionally
substituted alkenyl or optionally substituted alkynyl; or R20 and R20' taken
together
form =0, or =S; or R20 and R21 taken together with the atoms to which they are
attached form an optionally substituted cycloalkyl; or R20 and R21 taken
together
with the atoms to which they are attached form an optionally substituted aryl;
or R19
and R20 taken together with the atoms to which they are attached form an
optionally
substituted cycloalkyl; or R19 and R20 taken together with the atoms to which
they
are attached form an optionally substituted aryl; and R23 is optionally
substituted
alkyl, optionally substituted alkenyl or optionally substituted alkynyl
optionally
substituted cycloalkyl or optionally substituted aryl. In one embodiment of
any of
the disclosed aspects, X is 0; s is 0; R22' is hydrogen; and R22 is optionally
substituted C1-C6 alkyl. In another embodiment, each of R20, R20', R21, and
R2" is
independently hydrogen or optionally substituted C1-C4 alkyl; or R20 and
R20'taken
together form =0. In another embodiment, X is 0; s is 0; each of R22 and R22'
is
hydrogen or optionally substituted C1-C6 alkyl; and each of R20, R20', R21,
and R21' is
independently hydrogen or optionally substituted C1-C4 alkyl; or R20 and
R20'taken
together form =0. In another embodiment R20 and R21 taken together with the
atoms
to which they are attached form an optionally substituted cycloalkyl; or R20
and R2'
taken together with the atoms to which they are attached form an optionally
substituted aryl. In one variation, the compound has the structural formula
Co~ NH
N H 2
0 In another embodiment, s is 2; X is 0; R19 and R20 taken
together with the atoms to which they are attached form an optionally
substituted
cycloalkyl; or R19 and R20 taken together with the atoms to which they are
attached
form an optionally substituted aryl. In one variation, the compound has the
structural
formula:
H NH2
rN yl-0
cr N
-37-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00142] In another variation, the compound has a structural formula selected
from
the group consisting of:
N NH2 H HN O ~~..N NH2
rN H N,'`.,,N rN
o 0 ,- 0 0") o
-'~ H NH2 ,.^. NHZH -,N'
N I N =^\,,,N`~ II OH rN~=~N
0
oJ 0 0 0 0
,)
H NH2 H NH2
0 O
N I N ~
H HN HN H NH2 OH
N~~N Nom'-NH _~ I N
0'-'J o o f o o.,,,,J o
H NH2
N-,_,N O H NH2
o,) O N 'IT ON O
N NH2 IOI H NH2 H NH2
rN~ N~N N
0 0,) o
o y,l p 0")
0
)
N H NH2 H NH2
N C N
0 6,
. and
[00143] In yet another variation of any of the disclosed embodiments, the
compound has a structural formula selected from the group consisting of-
-38-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
r H -1~N NH2 H HN o H NH2
N `f)"`H Namõ-N N
a`..J 0 o, O 61") o
H NH2 ,.^. NH2
N,~Ny H
I N--,~N\~ IIOH
0 O"J O 0
, ,
H N H NH2 H NH2
rl-~N 11-0
0,') o o) 0 0.,,) o
H HN H NH2 OH H rN~,N N~- N HN
0") o 0") 0 0,,,,) 0
H NH2
N,N
(~` O H NH2
o
,.- rN
O~ O
H NH2
0 H NH2
yl,~
rNN N
Y'-
o,,,.~ II
0 0,) and
H NH2
('Ni""N
OJ O
[00144] Further included is a compound having a structural formula selected
from
o H NH-7 H N
rN
O a L.,~ o
the group consisting of o
-39-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
NH
NHg HN O H HN O
0. ICI 0
O 0:,,, 0
, ,
NH 00
H HN S H HN '0 O^ NH2
NN~[s~ N, H
N
O'J O I .-{ a Y
C
ro,
, ,
O O NH2 O NH2 O O NH2
N N Ir N N Trj",C
O
O O
, ,
H NH2 H NH2
NN N-^N
O J O IIO~ O
0
S NH2 H NH2
N N N\
N O C
S
, and Such compounds can be
prepared based on the disclosure herein and chemical synthetic routes familiar
to
one of skill in the art.
[00145] In still another aspect, the present application discloses a compound
of
Formula IV:
R32 F~PA' 32' V
Y NR35 R35' E
R 30
/> 33
N N R
W N N R34 R34 R36 R36'
R31
or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof,
wherein p is
1, 2, 3, 4, 5, or 6; each of Y, V, and W is independently CH2, NH, 0 or S;
each of
R3 , R31, R32, R32' R33, R34, R34', R35, R35 , R36, and R36' is independently
absent,
hydrogen or optionally substituted alkyl; or R34 and R36 taken together with
the
atoms to which they are attached form an optionally substituted carbocyclic
ring; E
-40-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
is -CHR Rd, NR Rd, -OR , and -SR ; and each of R and Rd is independently
hydrogen or optionally substituted alkyl; or R and Rd taken together with the
nitrogen atom to which they are attached form an optionally substituted
heterocyclic
ring or R and Rd taken together with the carbon atom to which they are
attached
form an optionally substituted carbocyclic ring. In one embodiment of any of
the
disclosed aspects or variations, the compound of Formula IV is not N-(3-
(diethylamino)propyl)-2-(4,6-dimethyl-5,7-dioxo-4,5,6,7-tetrahydro-1 H-
benzo[d]imidazol-1-yl)acetamide. In one variation, p is 1, 2, or 3; each of Y,
V,
and W is 0 or S; each of R30 and R31 is independently optionally substituted
C1-C4
alkyl; each of R32, R32' R33, R34, R34', R35, R35', R36, and R36' is
independently
hydrogen or optionally substituted C1-C4 alkyl; and E is -OR , -SR , or NR Rd
wherein R and Rd taken together with the nitrogen atom to which they are
attached
form an optionally substituted heterocycloalkyl.
In one aspect, the compound has the structure of Formula IV wherein p is 1, 2,
3, or
4; each of Y, V, and W is independently 0 or S; each of R30, R31, R32, R32'
R33, R34,
R34', R35, R35', R36, and R36' is independently absent, hydrogen, optionally
substituted
alkyl, optionally substituted alkenyl or optionally substituted alkynyl; or
R34 and R36
taken together with the atoms to which they are attached form an optionally
substituted carbocyclic ring; E is -CHR Rd, NR Rd, -OR , or -SR ; and each of
R
and Rd is independently hydrogen, optionally substituted alkyl, optionally
substituted alkenyl or optionally substituted alkynyl; or R and Rd taken
together
with the nitrogen atom to which they are attached form an optionally
substituted
heterocyclic ring or R and Rd taken together with the carbon atom to which
they are
attached form an optionally substituted carbocyclic ring. In one embodiment of
any
of the disclosed aspects, p is 1, 2, or 3; each of Y, V, and W is 0 or S; E is
-OR or
-SR ; each of R32, R32' R33, R34', R35, R35', and R36' is independently
hydrogen; and
each of R30 and R31 is independently optionally substituted C1-C4 alkyl. In
another
embodiment, p is 1, 2, or 3; each of Y, V, and W is 0 or S; E is NR Rd and R
and
Rd taken together with the nitrogen atom to which they are attached form an
optionally substituted heterocycloalkyl; each of R30 and R31 is independently
optionally substituted C1-C4 alkyl; and each of R32, R32' R33, R34, R34" R35,
R35', R36,
and R36' is independently hydrogen or optionally substituted C1-C4 alkyl. In
yet
another embodiment, p is 1; each of Y, V, and W is 0; each of R30 and R31 is
independently -CH3; R33 is hydrogen; and each of R32, R32' R34, R34', R35,
R35', R36,
-41-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
and R36' is independently hydrogen or Cl-C4 alkyl. In one variation of any of
the
disclosed embodiments, E is NR Rd and each of R and Rd is independently
hydrogen or optionally substituted alkyl. In another embodiment, R34 and R36
taken
together with the atoms to which they are attached form an optionally
substituted
cycloalkyl; or R34 and R36 taken together with the atoms to which they are
attached
form an optionally substituted carbocyclic aryl. In another embodiment, the
O
0
NH
; x N> ON'
compound has the structural formula: or
O
0
N NH
AN~ O b-' ' N N
. In another variation of any of the disclosed embodiments,
E is NR Rd and R and Rd taken together with the nitrogen atom to which they
are
attached form an optionally substituted heterocycloalkyl. In one variation,
the
compound has a structural formula selected from the group consisting of:
O N NH N N NH .N N NH
O~N N O N N O N N
N ( NH O
0
~1(O O O
O / ` "N N NH O NH
`,N N NH ON N N~
O N N N ~ ~
N N N
NH2 \ _O , I and
O
N NH
N I N>
O"i N
-42-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
In yet another embodiment, the compound has a structural formula selected from
the
O O
o O
r NH N NH
N'~ ~N N
O N N O~N N
N--~
NH
group consisting of: ,
0
C7
0 0 /"
NH 1
N> o _.1/ N NH
/ 1 N N N H I
05'N Nib 0-;-'-N N
I O-)- N N N
O 1
NH2 and 00
Alternately, such compounds include:
S O 0
N a O H~`N ~-
N~v NH
NH f _ ~~ ~N
0 N. --J N N- N N
1> NH
N- 0---'-N N
o 0 0
0 0 ~
NH
N ~N NH N
N) N NH -
i> ON N
OWN N O1N N
1-N
r , or
Still further included is included:
0
o w11
N
fl` N ` -N
ht [00146] In another aspect, the present application provides a compound of
Formula IVA:
R32 R32' V
P N R35 R35' E
N R33
~> R36
N R34 R34 R36
(IVA)
-43-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
or a pharmaceutically acceptable salt, ester, prodrug or solvate thereof,
wherein p is
1, 2, 3, 4, 5, or 6; V is CH2, NH, 0 or S; each of R32, R32' R33, R34, R34',
R35, R35"
R36, and R36' is independently absent, hydrogen or optionally substituted
alkyl; or
R34 and R36 taken together with the atoms to which they are attached form an
optionally substituted carbocyclic ring; E is -CHRcRd, NR Rd, -ORc, and -SRS;
and
each of Re and Rd is independently hydrogen or optionally substituted alkyl;
or R
and Rd taken together with the nitrogen atom to which they are attached form
an
optionally substituted heterocyclic ring or R and Rd taken together with the
carbon
atom to which they are attached form an optionally substituted carbocyclic
ring. In
one aspect, the compound has the structure of Formula IVA wherein p is 1, 2,
3, or
4; V is 0 or S; each of R32, R32' R33, R34, R34', R35, R35', R36, and R36 is
independently absent, hydrogen, optionally substituted alkyl, optionally
substituted
alkenyl or optionally substituted alkynyl; or R34 and R36 taken together with
the
atoms to which they are attached form an optionally substituted carbocyclic
ring; E
is -CHRCRd, NR Rd, -OR , or -SRC; and each of Re and Rd is independently
hydrogen, optionally substituted alkyl, optionally substituted alkenyl or
optionally
substituted alkynyl; or R and Rd taken together with the nitrogen atom to
which
they are attached form an optionally substituted heterocyclic ring or R and
Rd taken
together with the carbon atom to which they are attached form an optionally
substituted carbocyclic ring. In one embodiment of any of the disclosed
aspects, p is
1, 2, or 3; V is 0 or S; E is -OR or -SRS; each of R32, R32' R33, R34', R35,
R35', and
R36' is independently hydrogen. In another embodiment, p is 1, 2, or 3; V is 0
or S;
E is NR Rd and R and Rd taken together with the nitrogen atom to which they
are
attached form an optionally substituted heterocycloalkyl; and each of R32,
R32' R33,
R34, R34', R35, R35', R36, and R36' is independently hydrogen or optionally
substituted
C1-C4 alkyl. In yet another embodiment, p is 1; V is 0; R33 is hydrogen; and
each of
R32, R32' R34, R34', R35, R35', R36, and R36' is independently hydrogen or C1-
C4 alkyl-
In one variation of any of the disclosed embodiments, E is NReRd and each of R
and Rd is independently hydrogen or optionally substituted alkyl. In another
embodiment, R34 and R36 taken together with the atoms to which they are
attached
form an optionally substituted cycloalkyl; or R34 and R36 taken together with
the
-44-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
atoms to which they are attached form an optionally substituted carbocyclic
aryl. In
N NH
/>
N
N"
1
one embodiment, the compound has the structural formula
[00147] Since the p75 receptor is upregulated in various pathological states,
compounds disclosed herein can also be linked to molecular markers that can be
detected by imaging or other modalities. Such conjugates can be prepared
according
to synthetic methods known to those of skill in the art and applied in
diagnostic
strategies designed to detect such pathological states.
[00148] In another aspect, the present invention provides a compound selected
from the group consisting of (2R,3R)-2-amino-3-methyl-N-(2-moipholinoethyl)-
pentanamide; (2R,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; and
(2S,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; or a
pharmaceutically acceptable salt, solvate, ester, or prodrug thereof. In one
embodiment, the compound is in a purity of about 80% or more. In another
embodiment, the compound is in a purity of about 85% or more. In another
embodiment, the compound is in a purity of about 90% or more. In another
embodiment, the compound is in a purity of about 95% or more. In another
embodiment, the compound is in a purity of about 96% or more. In another
embodiment, the compound is in a purity of about 97% or more. In another
embodiment, the compound is in a purity of about 98% or more. In another
embodiment, the compound is in a purity of about 99% or more. In another
embodiment, the compound is in a purity of about 99.5% or more.
[00149] In another aspect, the present invention provides a mixture of two or
more compounds selected from the group consisting of (2S,3S)-2-amino-3-methyl-
N-(2-morpholinoethyl)-pentanamide; (2R,3R)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide; (2R,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide; and (2S,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide;
or a pharmaceutically acceptable salt, solvate, ester, or prodrug thereof,
with the
proviso that when the mixture consists of (2S,3S)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide and (2R,3R)-2-amino-3-methyl-N-(2-
-45-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt, solvate,
ester,
or prodrug thereof; then (2S,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide, or a pharmaceutically acceptable salt, solvate, ester, or prodrug
thereof, is in an amount not less than about 5% by weight. In one embodiment,
the
mixture consists of any two of the aforementioned four compounds. In another
embodiment, the mixture consists of any three of the aforementioned four
compounds. In another embodiment, the mixture consists of the aforementioned
four compounds. Subject to the above-mentioned proviso, the individual
compounds in the mixture can be in any ratio or weight percentage. In one
embodiment, any of the two or more compounds in the mixture is in an amount of
about 0.5% by weight or more. In another embodiment, any of the two or more
compounds in the mixture is in an amount of about 5% by weight or more. In
another embodiment, each of the two or more compounds in the mixture is in an
approximately equal amount.
[00150] Scheme A provides the chemical structures of the above-mentioned
compounds.
Scheme A:
O O
__TN --, N
NH2 H
(2S,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)pentanamide
N_~ N
NH2 H
(2R,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)pentanamide
rO
N_~ N
NH2 H
(2S,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)pentanamide
0 r'O
N
NH2 H
(2R,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)pentanamide
[00151] In one embodiment, the present invention provides a mixture of (2R,3R)-
2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide and (2S,3S)-2-amino-3-
-46-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
methyl-N-(2-morpholinoethyl)-pentanamide, or a pharmaceutically acceptable
salt,
solvate, ester, or prodrug thereof, with the proviso that (2S,3S)-2-amino-3-
methyl-
N-(2-morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt,
solvate,
ester, or prodrug thereof, is in an amount not less than about 5% by weight
based on
the total amount of the mixture. Subject to the above-mentioned proviso, the
individual compounds in the mixture can be in any ratio or weight percentage.
In
one specific embodiment, the mixture consists of (2R,3R)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide and (2S,3S)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt, solvate,
ester,
or prodrug thereof, in an approximately equal amount.
[00152] In one embodiment, the present invention provides a mixture of (2R,3S)-
2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide and (2S,3R)-2-amino-3-
methyl-N-(2-morpholinoethyl)-pentanamide, or a pharmaceutically acceptable
salt,
solvate, ester, or prodrug thereof. The individual compounds in the mixture
can be
in any ratio or weight percentage. In one specific embodiment, the mixture
consists
of (2R,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide and (2S,3R)-2-
amino-3-methyl-N-(2-morpholinoethyl)-pentanamide, or a pharmaceutically
acceptable salt, solvate, ester, or prodrug thereof.
[00153] In another aspect, the present invention provides a pharmaceutical
composition comprising the compound selected from the group consisting of
(2R,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; (2R,3S)-2-amino-
3-methyl-N-(2-morpholinoethyl)-pentanamide; and (2S,3R)-2-amino-3-methyl-N-
(2-morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt,
solvate,
ester, or prodrug thereof, and a pharmaceutically acceptable carrier.
[00154] In another aspect, the present invention provides a pharmaceutical
composition comprising a mixture of two or more compounds selected from the
group consisting of (2S,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide;
(2R,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; (2R,3S)-2-amino-
3-methyl-N-(2-morpholinoethyl)-pentanamide; and (2S,3R)-2-amino-3-methyl-N-
(2-morpholinoethyl)-pentanamide; or a pharmaceutically acceptable salt,
solvate,
ester, or prodrug thereof; and a pharmaceutically acceptable carrier, with the
proviso
that when the mixture consists of (2S,3S)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide and (2R,3R)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt, solvate,
ester,
-47-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
or prodrug thereof; then (2S,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide, or a pharmaceutically acceptable salt, solvate, ester, or prodrug
thereof, is in an amount not less than about 5% by weight.
[00155] The application provides compounds having binding specificity for a
p75NTR molecule. These compounds, along with related pharmaceutical compounds
and methods, are useful in the treatment and prevention of neurodegenerative
and
other disorders.
[00156] A set of compounds disclosed herein are labeled as follows:
Table I. Structures of Compounds i-vii
Compound Name
H
NCH
H, f
N Compound (i)
(also referred to herein as
"LM11A-28")
N
ON,O
O O ~, 11 ~N'S S N N` Compound (ii)
H O 0 H (also referred to herein as
O N "LM 11 A-7")
H
0
O
H3C~N N N-H Compound (iii)
(also referred to herein as
N I />
0 N N "LM 11 A-24",
"24", and "C24")
CH3 N-CH3
H3C
CH3
H3C
O NH Compound (iv)
(also referred to herein as
N,H "LM11A-31" and "31")
OJ
-48-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Compound Name
0
0
H3C'N N N"H Compound (v)
I /> (also referred to herein as
0I'll, N N CH3 "LM11A-36",
CH3 N/ "36", and "C36")
CH3
H3
N N~ O Compound (vi)
(also referred to herein as
N I N O
N "LM11A-38" and "C38")
H3C I
O H
NH NH
-',N N NH2 Compound (vii)
H2N N N H H Y Y
NH NH
[00157] One object of the presently disclosed subject matter is to provide
methods of facilitating cell survival using neurotrophin mimetics.
[00158] In accordance with the presently disclosed subject matter, a
representative neurotrophin can include, but is not limited to, NGF. More
particularly, the neurotrophin (3-turn loop having binding specificity for a
p75NTR
molecule includes, but is not limited to, loop 1 of the NGF.
[00159] As disclosed herein, representative structures of the compound or
mimetic having binding specificity for a p75NTR molecule are capable of
binding to
the neurotrophin-binding site of the p75NTR molecule.
[00160] The compounds disclosed herein can also encompass derivatives of a
parent compound, which has binding specificity for a p75NTR molecule, wherein
the
derivative also has binding specificity for the p75NTR The derivative can
exhibit
enhancement in at least one of the characteristics selected from the group
consisting
of hydrophilicity, lipophilicity, amphipathicity, solubility, bioavailability,
and
resistance to hepatic degradation, as compared to the parent compound.
[00161] It is to be understood that in some embodiments the compounds
disclosed herein can encompass a pharmacophore substantially identical to the
-49-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
pharmacophore illustrated in Figure 1 c. Representative such compounds include
but
are not limited to compounds encompassed by Formulas (A) and (B).
[00162] The aforementioned individual compounds or mixtures can be used for
treating a wide range of conditions and diseases described herein.
[00163] In one aspect, there is provided a pharmaceutical composition
comprising
a pharmaceutically acceptable diluent or carrier and a compound of Formula I,
IA,
IB, II, HA, IIB, III, IV or IVA or a pharmaceutically acceptable salt, ester,
prodrug
or solvate thereof.
[00164] In another aspect, there is provided a method for treating a disorder
associated with p75 expression comprising administering to a patient in need
of such
treatment a compound of Formula I, IA, IB, II, IIA, IIB, III, IV or IVA or a
pharmaceutically acceptable salt, ester, solvate or prodrug thereof. In one
embodiment the disorder is associated directly with cells expressing p75; in
another
embodiment, the cells do not express p75, but are affected by p75 expression.
[00165] In another aspect, there is provided a method for activating a p75
receptor comprising contacting a cell containing a p75 receptor with a
compound of
Formula I, IA, IB, II, IIA, IIB, III, IV or IVA or a pharmaceutically
acceptable
salt, ester, solvate or prodrug thereof.
[00166] In another aspect, there is provided a method for the treatment of
disorders involving degeneration or dysfunction of cells expressing p75
comprising
administering to a patient in need of such treatment a compound of Formula I,
IA,
IB, II, IIA, IIB, III, IV or IVA or a pharmaceutically acceptable salt, ester,
solvate
or prodrug thereof. In one embodiment, the method of treatment comprises
facilitating cell survival; in another embodiment, the method of treatment
comprises
inhibiting degenerative p75 signaling; in yet another embodiment, the method
of
treatment comprises inhibiting dysfunctional p75 signaling. In one embodiment,
the
disorder is a neurodegenerative disorder. In another embodiment, the disorder
is
selected from the group consisting of Alzheimer's disease, Huntington's
disease,
Pick's disease, amyotrophic lateral sclerosis, epilepsy, Parkinson's disease,
spinal
cord injury, stroke, hypoxia, ischemia, brain injury, diabetic neuropathy,
peripheral
neuropathy, nerve transplantation, multiple sclerosis, peripheral nerve injury
and
hair loss.
[00167] Compounds of Formula I, IA, IB, II, IIA, IIB, III, IV or IVA or a
pharmaceutically acceptable salt, ester, solvate or prodrug thereof as
disclosed
-50-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
herein that target p75 receptors expressed by neurons are used to prevent loss
of
function, degeneration and/or death of neurons in a number of nervous system
disorders. Such disorders include, but are not limited to, Alzheimer's
disease,
Parkinson's disease, Huntington's disease, stroke, traumatic brain injury,
spinal cord
injury, epilepsy, multiple sclerosis, amyotrophic lateral sclerosis,
neuropathies,
myopathies and various forms of retinal degeneration. In one embodiment,
compounds of the present application are used in the treatment of Alzheimer's
disease. Compounds of Formula I, IA, IB, II, IIA, IIB, III, IV or IVA or a
pharmaceutically acceptable salt, ester, solvate or prodrug thereof as
disclosed
herein that target p75 receptors expressed by oligodendrocytes are used to
prevent
loss of function, degeneration and/or death of oligodendrocytes in a number of
nervous system disorders including, but not limited to, multiple sclerosis,
spinal cord
injury and perinatal anoxia. In another embodiment, compounds of the present
application are used to treat multiple sclerosis.
[00168] Outside of the nervous system, a number of cell populations express
the
p75 receptor. These include hair follicle cells, hepatic cells, vascular
endothelial,
vascular smooth muscle cells, cardiomyocytes. In addition, the p75 receptor is
expressed by certain tumor cells such as those involved in breast or prostate
cancer.
Given this expression pattern, compounds of Formula I, IA, IB, II, IIA, IIB,
III, IV
or IVA or a pharmaceutically acceptable salt, ester, solvate or prodrug
thereof as
disclosed herein that target p75 receptors are used to: prevent loss of hair
follicle
cells and thereby prevent hair loss; prevent hepatic cirrhosis and promote
liver
regeneration; regulate angiogenesis and promote neovascularization in the
setting of
diabetic wounds or other ischemic settings; prevent cardiomyopathy e.g.
preventing
myocardial cell loss or stimulating growth of new cardiomyocytes either in the
setting of ischemia or after myocardial infarction; and inhibit tumor cell
growth. In
addition p75 is expressed by stem cells and is known to regulate stem cell
growth;
therefore, p75 ligands are used to promote stem cell growth as part of a
strategy to
promote tissue and organ regeneration.
[00169] In one variation of any of the disclosed aspects or embodiments, the
compound administered to a patient in need thereof is selected from the group
consisting of:
-51-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
H NH2
^,,,N H HN O
NH N--~~N
O 0 y
, ,
H NH2
rN,"-N
O., ,,) 0
~~H NH2 NH2 H N
N `` rN..-,,N rJ I1 OH rN
O O O O O
H NH2 H NH
^ z
o 0 -' O, J 0
, ,
H HN H NH2 OH
r'`N'.~,'N-," N~ ,N HN I N''~N~O
o.,,.,) 0,,,J o o") 0
, ,
H NH2
Nrt~N O H NH2
0
o') 0 rN,~yNyJ,~
H NH2 0 NH2 H NHZ
r'N-'y N f 'N
o) o 0,,,) o of o
O 0
0 N NH
N H NH2 H NH2 N
\ N1t OWN NrN o Ct~Nyl--( a o
-52-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
p 0 0 O
N NH ~N N NH p r~(/o
N I /? "N N NH
p~N N O N N
NH O O N N
NH2
O
O
N NH
OIN N 0 NH
O N N
O
N 0 r---NH
1.
{j=` N ,14
~ht O O O
O =
O~ O O
N O
,,N ~N N/ Nl~ NN H-\--\
p i> v 'N- ~~ N-
~O O N N I
O
O p
NH
N H~ N />
NJ NH O~~ N N
,and \ .
[001701 In another variation of any of the disclosed embodiments or aspects,
the
compound administered to a patient in need thereof is selected from the group
0 NH "..N
H H
fl "J O
consisting of
~'~` I NH H HN O H HN O
yl_~
a rt,N~N N NN
yl_~ O O
O
-53-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
NH
H HNfS H HNIL-1,0 H NH2
C
O
O O NNH2 N NH2 O O N NH2
~
O O O C
H NH2 H NH2
NN __C rNN
of O OJ O C
0
S NH2 H NH2
N bN S
and
[00171] In another aspect, there is provided a method for treating a disorder
associated with p75 expression comprising administering to a patient in need
of such
treatment a stereoisomer of 2 amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide,
including (2R,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; (2R,3S)-
2-amino-3 -methyl-N-(2-morpholinoethyl)-pentanamide; and (2 S,3 R)-2-amino-3 -
methyl-N-(2-morpholinoethyl)-pentanamide or a pharmaceutically acceptable
salt,
ester, solvate or prodrug thereof. In one embodiment the disorder is
associated
directly with cells expressing p75; in another embodiment, the cells do not
express
p75, but are affected by p75 expression. In another aspect, there is provided
a
method for activating a p75 receptor comprising contacting a cell containing a
p75
receptor with a stereoisomer of 2 amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide, including (2R,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide; (2R,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; and
(2S,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide or a
pharmaceutically acceptable salt, ester, solvate or prodrug thereof.
[00172] In another aspect, there is provided a method for the treatment of
disorders involving degeneration or dysfunction of cells expressing p75
comprising
administering to a patient in need of such treatment a stereoisomer of 2 amino-
3-
methyl-N-(2-morpholinoethyl)-pentanamide, including (2R,3R)-2-amino-3-methyl-
-54-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
N-(2-morpholinoethyl)-pentanarnide; (2R,3 S)-2-amino-3-methyl-N-(2-
moipholinoethyl)-pentanamide; and (2S,3R)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide or a pharmaceutically acceptable salt, ester,
solvate
or prodrug thereof. In one embodiment, the method of treatment comprises
facilitating cell survival; in another embodiment, the method of treatment
comprises
inhibiting degenerative p75 signalling; in yet another embodiment, the method
of
treatment comprises inhibiting dysfunctional p75 signaling. In one embodiment,
the
disorder is a neurodegenerative disorder. In another embodiment, the disorder
is
selected from the group consisting of Alzheimer's disease, Huntington's
disease,
Pick's disease, amyotrophic lateral sclerosis, epilepsy, Parkinson's disease,
spinal
cord injury, stroke, hypoxia, ischemia, brain injury, diabetic neuropathy,
peripheral
neuropathy, nerve transplantation, multiple sclerosis, peripheral nerve injury
and
hair loss.
[00173] Stereoisomers of 2 amino-3-methyl-N-(2-morpholinoethyl)-pentanamide,
including (2R,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; (2R,3S)-
2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; and (2S,3R)-2-amino-3-
methyl-N-(2-morpholinoethyl)-pentanamide or a pharmaceutically acceptable
salt,
ester, solvate or prodrug thereof as disclosed herein that target p75
receptors
expressed by neurons are used to prevent loss of function, degeneration and/or
death
of neurons in a number of nervous system disorders. Such disorders include,
but are
not limited to, Alzheimer's disease, Parkinson's disease, Huntington's
disease,
stroke, traumatic brain injury, spinal cord injury, epilepsy, multiple
sclerosis,
amyotrophic lateral sclerosis, neuropathies, myopathies and various forms of
retinal
degeneration. In one embodiment, compounds of the present application are used
in
the treatment of Alzheimer's disease. Stereoisomers of 2 amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide, including (2R,3R)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide; (2R,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide; and (2S,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide
or a pharmaceutically acceptable salt, ester, solvate or prodrug thereof as
disclosed
herein that target p75 receptors expressed by oligodendrocytes are used to
prevent
loss of function, degeneration and/or death of oligodendrocytes in a number of
nervous system disorders including, but not limited to, multiple sclerosis,
spinal
cord injury and perinatal anoxia. In another embodiment, compounds of the
present
application are used to treat multiple sclerosis.
-55-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00174] Outside of the nervous system, a number of cell populations express
the
p75 receptor. These include hair follicle cells, hepatic cells, vascular
endothelial,
vascular smooth muscle cells, cardiomyocytes. In addition, the p75 receptor is
expressed by certain tumor cells such as those involved in breast or prostate
cancer.
Given this expression pattern, stereoisomers of 2 amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide, including (2R,3R)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide; (2R,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide; and (2S,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide
or a pharmaceutically acceptable salt, ester, solvate or prodrug thereof as
disclosed
herein that target p75 receptors are used to: prevent loss of hair follicle
cells and
thereby prevent hair loss; prevent hepatic cirrhosis and promote liver
regeneration;
regulate angiogenesis and promote neovascularization in the setting of
diabetic
wounds or other ischemic settings; prevent cardiomyopathy e.g. preventing
myocardial cell loss or by stimulating growth of new cardiomyocytes either in
the
setting of ischemia or after myocardial infarction; and inhibit tumor cell
growth. In
addition p75 is expressed by stem cells and is known to regulate stem cell
growth;
therefore, p75 ligands are used to promote stem cell growth as part of a
strategy to
promote tissue and organ regeneration.
[00175] In one variation of any disclosed aspect or embodiment, the method
comprises administering to a patient in need of such treatment a
pharmaceutical
composition comprising a mixture of two or more compounds selected from the
group consisting of (2S,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-
pentanamide;
(2R,3R)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; (2R,3S)-2-amino-
3-methyl-N-(2-morpholinoethyl)-pentanamide; and (2S,3R)-2-amino-3-methyl-N-
(2-morpholinoethyl)-pentanamide; or a pharmaceutically acceptable salt,
solvate,
ester, or prodrug thereof, with the proviso that when the mixture consists of
(2S,3S)-
2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide and (2R,3R)-2-amino-3-
methyl-N-(2-morpholinoethyl)-pentanamide, or a pharmaceutically acceptable
salt,
solvate, ester, or prodrug thereof, then (2S,3S)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt, solvate,
ester,
or prodrug thereof, is in an amount not less than about 5% by weight based on
the
total amount of the mixture. In another variation of any disclosed aspect or
embodiment, the method comprises administering to a patient in need of such
treatment a pharmaceutical composition comprising a mixture of (2R,3R)-2-amino-
-56-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
3-methyl-N-(2-morpholinoethyl)-pentanamide and (2S,3 S)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt, solvate,
ester,
or prodrug thereof, with the proviso that (2S,3S)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt, solvate,
ester,
or prodrug thereof, is in an amount not less than about 5% by weight based on
the
total amount of the mixture. In yet another variation of any disclosed aspect
or
embodiment, the method comprises administering, to a patient in need of such
treatment a pharmaceutical composition comprising a mixture of (2R,3S)-2-amino-
3-methyl-N-(2-morpholinoethyl)-pentanamide and (2S,3R)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide, or a pharmaceutically acceptable salt, solvate,
ester,
or prodrug thereof.
Formulations
[00176] For the purposes of this invention, the compounds may be administered
by a variety of means including orally, parenterally, by inhalation spray,
topically, or
rectally in formulations containing pharmaceutically acceptable carriers,
adjuvants
and vehicles. The term parenteral as used here includes subcutaneous,
intravenous,
intramuscular, and intraarterial injections with a variety of infusion
techniques.
Intraarterial and intravenous injection as used herein includes administration
through
catheters.
[00177] The compounds disclosed herein can be formulated in accordance with
the routine procedures adapted for desired administration route. Accordingly,
the
compounds disclosed herein can take such forms as suspensions, solutions or
emulsions in oily or aqueous vehicles, and can contain formulatory agents such
as
suspending, stabilizing and/or dispersing agents. The compounds disclosed
herein
can also be formulated as a preparation for implantation or injection. Thus,
for
example, the compounds can be formulated with suitable polymeric or
hydrophobic
materials (e.g., as an emulsion in an acceptable oil) or ion exchange resins,
or as
sparingly soluble derivatives (e.g., as a sparingly soluble salt).
Alternatively, the
active ingredient can be in powder form for constitution with a suitable
vehicle, e.g.,
sterile pyrogen-free water, before use. Suitable formulations for each of
these
methods of administration can be found, for example, in Remington: The Science
and Practice of Pharmacy, A. Gennaro, ed., 20th edition, Lippincott, Williams
&
Wilkins, Philadelphia, Pa.
-57-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00178] For example, formulations for parenteral administration can contain as
common excipients sterile water or saline, polyalkylene glycols such as
polyethylene
glycol, oils of vegetable origin, hydrogenated naphthalenes and the like. In
particular, biocompatible, biodegradable lactide polymer, lactide/glycolide
copolymer, or polyoxyethylene-polyoxypropylene copolymers can be useful
excipients to control the release of active compounds. Other potentially
useful
parenteral delivery systems include ethylene-vinyl acetate copolymer
particles,
osmotic pumps, implantable infusion systems, and liposomes. Formulations for
inhalation administration contain as excipients, for example, lactose, or can
be
aqueous solutions containing, for example, polyoxyethylene-9-auryl ether,
glycocholate and deoxycholate, or oily solutions for administration in the
form of
nasal drops, or as a gel to be applied intranasally. Formulations for
parenteral
administration can also include glycocholate for buccal administration,
methoxysalicylate for rectal administration, or citric acid for vaginal
administration.
[00179] The pharmaceutical compositions of the invention may be in the form of
a sterile injectable preparation, such as a sterile injectable aqueous or
oleaginous
suspension. This suspension may be formulated according to the known art using
those suitable dispersing or wetting agents and suspending agents. The sterile
injectable preparation may also be a sterile injectable solution or suspension
in a
non-toxic parenterally acceptable diluent or solvent, such as a solution in
1,3-butane-
diol or prepared as a lyophilized powder. Among the acceptable vehicles and
solvents that may be employed are water, Ringer's solution and isotonic sodium
chloride solution. In addition, sterile fixed oils may conventionally be
employed as a
solvent or suspending medium. For this purpose any bland fixed oil may be
employed including synthetic mono- or diglycerides. In addition, fatty acids
such as
oleic acid may likewise be used in the preparation of injectables.
Formulations for
intravenous administration can comprise solutions in sterile isotonic aqueous
buffer.
Where necessary, the formulations can also include a solubilizing agent and a
local
anesthetic to ease pain at the site of the injection. Generally, the
ingredients are
supplied either separately or mixed together in unit dosage form, for example,
as a
dry lyophilized powder or water free concentrate in a hermetically sealed
container
such as an ampule or sachet indicating the quantity of active agent. Where the
compound is to be administered by infusion, it can be dispensed in a
formulation
with an infusion bottle containing sterile pharmaceutical grade water, saline
or
-58-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
dextrose/water. Where the compound is administered by injection, an ampule of
sterile water for injection or saline can be provided so that the ingredients
can be
mixed prior to administration.
[00180] Suitable formulations further include aqueous and non-aqueous sterile
injection solutions that can contain antioxidants, buffers, bacteriostats,
bactericidal
antibiotics and solutes that render the formulation isotonic with the bodily
fluids of
the intended recipient; and aqueous and non-aqueous sterile suspensions, which
can
include suspending agents and thickening agents.
[00181] The compounds can further be formulated for topical administration.
Suitable topical formulations include one or more compounds in the form of a
liquid, lotion, cream or gel. Topical administration can be accomplished by
application directly on the treatment area. For example, such application can
be
accomplished by rubbing the formulation (such as a lotion or gel) onto the
skin of
the treatment area, or by spray application of a liquid formulation onto the
treatment
area.
[00182] In some formulations, bioimplant materials can be coated with the
compounds so as to improve interaction between cells and the implant.
[00183] Formulations of the compounds can contain minor amounts of wetting or
emulsifying agents, or pH buffering agents. The formulations comprising the
compound can be a liquid solution, suspension, emulsion, tablet, pill,
capsule,
sustained release formulation, or powder.
[00184] The compounds can be formulated as a suppository, with traditional
binders and carriers such as triglycerides.
[00185] Pharmaceutical compositions containing the active ingredient may be in
any form suitable for the intended method of administration. When used for
oral use
for example, tablets, troches, lozenges, aqueous or oil suspensions,
dispersible
powders or granules, emulsions, hard or soft capsules, syrups or elixirs may
be
prepared. Compositions intended for oral use may be prepared according to any
method known to the art for the manufacture of pharmaceutical compositions and
such compositions may contain one or more agents including sweetening agents,
flavoring agents, coloring agents and preserving agents, in order to provide a
palatable preparation. Oral formulations can include standard carriers such as
pharmaceutical grades of mannitol, lactose, starch, magnesium stearate,
polyvinyl
pyrrolidone, sodium saccharine, cellulose, magnesium carbonate, etc. Tablets
-59-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
containing the active ingredient in admixture with non-toxic pharmaceutically
acceptable excipient which are suitable for manufacture of tablets are
acceptable.
These excipients may be, for example, inert diluents, such as calcium or
sodium
carbonate, lactose, calcium or sodium phosphate; granulating and
disintegrating
agents, such as maize starch, or alginic acid; binding agents, such as starch,
gelatin
or acacia; and lubricating agents, such as magnesium stearate, stearic acid or
talc.
Tablets may be uncoated or may be coated by known techniques including
microencapsulation to delay disintegration and adsorption in the
gastrointestinal
tract and thereby provide a sustained action over a longer period. For
example, a
time delay material such as glyceryl monostearate or glyceryl distearate alone
or
with a wax maybe employed.
[00186] Formulations for oral use may be also presented as hard gelatin
capsules
where the active ingredient is mixed with an inert solid diluent, for example
calcium
phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient
is
mixed with water or an oil medium, such as peanut oil, liquid paraffin or
olive oil.
[00187] Aqueous suspensions of the invention contain the active materials in
admixture with excipients suitable for the manufacture of aqueous suspensions.
Such
excipients include a suspending agent, such as sodium carboxymethylcellulose,
methylcellulose, hydroxypropyl methylcellulose, sodium alginate,
polyvinylpyrrolidone, gum tragacanth and gum acacia, and dispersing or wetting
agents such as a naturally occurring phosphatide (e.g., lecithin), a
condensation
product of an alkylene oxide with a fatty acid (e.g., polyoxyethylene
stearate), a
condensation product of ethylene oxide with a long chain aliphatic alcohol
(e.g.,
heptadecaethyleneoxycetanol), a condensation product of ethylene oxide with a
partial ester derived from a fatty acid and a hexitol anhydride (e.g.,
polyoxyethylene
sorbitan monooleate). The aqueous suspension may also contain one or more
preservatives such as ethyl or n-propyl p-hydroxy-benzoate, one or more
coloring
agents, one or more flavoring agents and one or more sweetening agents, such
as
sucrose or saccharin.
[00188] Oil suspensions may be formulated by suspending the active ingredient
in a vegetable oil, such as arachis oil, olive oil, sesame oil or coconut oil,
or in a
mineral oil such as liquid paraffin. The oral suspensions may contain a
thickening
agent, such as beeswax, hard paraffin or cetyl alcohol. Sweetening agents,
such as
those set forth above, and flavoring agents may be added to provide a
palatable oral
-60-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
preparation. These compositions may be preserved by the addition of an
antioxidant
such as ascorbic acid.
[00189] The pharmaceutical formulations comprising the compounds of the
present application can include an agent which controls release of the
compound,
thereby providing a timed or sustained release compound.
Carriers
[00190] Pharmaceutically acceptable carriers are well known to those skilled
in
the art and include, but are not limited to, from about 0.01 to about 0.1 M
and
preferably 0.05M phosphate buffer or 0.8% saline. Such pharmaceutically
acceptable carriers can be aqueous or non-aqueous solutions, suspensions and
emulsions.
[00191] Examples of non-aqueous solvents suitable for use in the present
application include, but are not limited to, propylene glycol, polyethylene
glycol,
vegetable oils such as olive oil, and injectable organic esters such as ethyl
oleate.
[00192] Aqueous carriers suitable for use in the present application include,
but
are not limited to, water, ethanol, alcoholic/aqueous solutions, glycerol,
emulsions or
suspensions, including saline and buffered media. Oral carriers can be
elixirs,
syrups, capsules, tablets and the like.
[00193] Liquid carriers suitable for use in the present application can be
used in
preparing solutions, suspensions, emulsions, syrups, elixirs and pressurized
compounds. The active ingredient can be dissolved or suspended in a
pharmaceutically acceptable liquid carrier such as water, an organic solvent,
a
mixture of both or pharmaceutically acceptable oils or fats. The liquid
carrier can
contain other suitable pharmaceutical additives such as solubilizers,
emulsifiers,
buffers, preservatives, sweeteners, flavoring agents, suspending agents,
thickening
agents, colors, viscosity regulators, stabilizers or osmo-regulators.
[00194] Liquid carriers suitable for use in the present application include,
but are
not limited to, water (partially containing additives as above, e.g. cellulose
derivatives, preferably sodium carboxymethyl cellulose solution), alcohols
(including monohydric alcohols and polyhydric alcohols, e.g. glycols) and
their
derivatives, and oils (e.g. fractionated coconut oil and arachis oil). For
parenteral
administration, the carrier can also include an oily ester such as ethyl
oleate and
isopropyl myristate. Sterile liquid carriers are useful in sterile liquid form
comprising compounds for parenteral administration. The liquid carrier for
-61-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
pressurized compounds disclosed herein can be halogenated hydrocarbon or other
pharmaceutically acceptable propellent.
[00195] Solid carriers suitable for use in the present application include,
but are
not limited to, inert substances such as lactose, starch, glucose, methyl-
cellulose,
magnesium stearate, dicalcium phosphate, mannitol and the like. A solid
carrier can
further include one or more substances acting as flavoring agents, lubricants,
solubilizers, suspending agents, fillers, glidants, compression aids, binders
or tablet-
disintegrating agents; it can also be an encapsulating material. In powders,
the
carrier can be a finely divided solid which is in admixture with the finely
divided
active compound. In tablets, the active compound is mixed with a carrier
having the
necessary compression properties in suitable proportions and compacted in the
shape
and size desired. The powders and tablets preferably contain up to 99% of the
active
compound. Suitable solid carriers include, for example, calcium phosphate,
magnesium stearate, talc, sugars, lactose, dextrin, starch, gelatin,
cellulose,
polyvinylpyrrolidine, low melting waxes and ion exchange resins. A tablet may
be
made by compression or molding, optionally with one or more accessory
ingredients. Compressed tablets may be prepared by compressing in a suitable
machine the active ingredient in a free flowing form such as a powder or
granules,
optionally mixed with a binder (e.g., povidone, gelatin, hydroxypropylmethyl
cellulose), lubricant, inert diluent, preservative, disintegrant (e.g., sodium
starch
glycolate, cross-linked povidone, cross-linked sodium carboxymethyl cellulose)
surface active or dispersing agent. Molded tablets may be made by molding in a
suitable machine a mixture of the powdered compound moistened with an inert
liquid diluent. The tablets may optionally be coated or scored and may be
formulated so as to provide slow or controlled release of the active
ingredient therein
using, for example, hydroxypropyl methylcellulose in varying proportions to
provide the desired release profile. Tablets may optionally be provided with
an
enteric coating, to provide release in parts of the gut other than the
stomach.
[00196] Parenteral carriers suitable for use in the present application
include, but
are not limited to, sodium chloride solution, Ringer's dextrose, dextrose and
sodium
chloride, lactated Ringer's and fixed oils. Intravenous carriers include fluid
and
nutrient replenishers, electrolyte replenishers such as those based on
Ringer's
dextrose and the like. Preservatives and other additives can also be present,
such as,
for example, antimicrobials, antioxidants, chelating agents, inert gases and
the like.
-62-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00197] Carriers suitable for use in the present application can be mixed as
needed with disintegrants, diluents, granulating agents, lubricants, binders
and the
like using conventional techniques known in the art. The carriers can also be
sterilized using methods that do not deleteriously react with the compounds,
as is
generally known in the art.
Salts
[00198] It is also to be understood that the disclosed compounds can further
comprise pharmaceutically acceptable salts.
[00199] Such salts include, but are not limited to, pharmaceutically
acceptable
acid addition salts, pharmaceutically acceptable base addition salts,
pharmaceutically acceptable metal salts, ammonium and allcylated ammonium
salts.
[00200] Acid addition salts include salts of inorganic acids as well as
organic
acids. Representative examples of suitable inorganic acids include
hydrochloric,
hydrobromic, hydroiodic, phosphoric, sulfuric, nitric acids and the like.
Representative examples of suitable organic acids include formic, acetic,
triflhoroacetic, trifluoroacetic, propionic, benzoic, cinnamic, citric,
fumaric,
glycolic, lactic, maleic, malic, malonic, mandelic, oxalic, picric, pyruvic,
salicylic,
succinic, methanesulfonic, ethanesulfonic, tartaric, ascorbic, pamoic,
bismethylene
salicylic, ethanedisulfonic, gluconic, citraconic, aspartic, stearic,
palmitic, EDTA,
glycolic, p-aminobenzoic, glutamic, benzenesulfonic, p-toluenesulfonic acids,
sulphates, nitrates, phosphates, perchlorates, borates, acetates, benzoates,
hydroxynaphthoates, glycerophosphates, ketoglutarates and the like.
[00201] Base addition salts include but are not limited to, ethylenediamine, N-
methyl-glucamine, lysine, arginine, ornithine, choline, N,N'-
dibenzylethylenediamine, chloroprocaine, diethanolamine, procaine, N-
benzylphenethylamine, diethylamine, piperazine, tris-(hydroxymethyl)-
aminomethane, tetramethylammonium hydroxide, triethylamine, dibenzylamine,
ephenamine, dehydroabietylamine, N-ethylpiperidine, benzylamine,
tetramethylammonium, tetraethylammonium, methylamine, dimethylamine,
trimethylamine, ethylamine, basic amino acids, e. g., lysine and arginine
dicyclohexylamine and the like.
[00202] Examples of metal salts include lithium, sodium, potassium, magnesium
salts and the like.
-63-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00203] Examples of ammonium and alkylated ammonium salts include
ammonium, methylammonium, dimethylammonium, trimethylammonium,
ethylammonium, hydroxyethylammonium, diethylammonium, butylammonium,
tetramethylammonium salts and the like. Examples of organic bases include
lysine,
arginine, guanidine, diethanolamine, choline and the like.
[00204] Standard methods for the preparation of pharmaceutically acceptable
salts and their formulations are well known in the art, and are disclosed in
various
references, including for example, "Remington: The Science and Practice of
Pharmacy", A. Gennaro, ed., 20th edition, Lippincott, Williams & Wilkins,
Philadelphia, PA.
Methods of Use
[00205] As disclosed throughout, the present application provides treatment of
disorders associated with p75 expression. Generally, the present application
provides treatment of disorders involving degradation or dysfunction of cells
expressing p75.
[00206] In one aspect, there is provided a method for activating p75 receptors
comprising contacting a cell containing a p75 receptor with one or more
compounds
of the present application or a pharmaceutically acceptable salt, ester,
solvate or
prodrug thereof. Additionally disclosed are methods for treating nervous
system
disorders including, but not limited to, Alzheimer's disease, Parkinson's
disease,
Huntington's disease, stroke, traumatic brain injury, spinal cord injury,
epilepsy,
multiple sclerosis, amyotrophic lateral sclerosis, neuropathies, myopathies
and
various forms of retinal degeneration, based on the ability of the compounds
of the
present application to target p75 receptors expressed by neurons or other
cells.
[00207] Further disclosed are methods for treating nervous system disorders
including, but not limited to, multiple sclerosis, spinal cord injury and
perinatal
anoxia, based on the ability of the compounds of the present application to
target
p75 receptors expressed by oligodendrocytes, microglia or astrocytes.
[00208] Additionally disclosed are methods for treating diseases other than
those
of the nervous system, including methods to: prevent loss of hair follicle
cells and
thereby prevent hair loss; prevent hepatic cirrhosis and promote liver
regeneration;
regulate angiogenesis and promote neovascularization in the setting of
diabetic
wounds or other ischemic settings; prevent cardiomyopathy e.g. preventing
myocardial cell loss or stimulating growth of new cardiomyocytes either in the
-64-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
setting of ischemia or after myocardial infarction; and inhibit tumor cell
growth. In
addition p75 is expressed by stem cells and is known to regulate stem cell
growth;
therefore, p75 ligands disclosed herein can be used to promote stem cell
growth as
part of a strategy to promote tissue and organ regeneration.
[00209] The present application also provides methods of treating
neurodegenerative and other disorders or conditions in a subject. Generally,
the
methods of the present application involve administration of a compound having
binding specificity for a p75NTR molecule in a subject to treat a
neurodegenerative
disorder or other disorder or condition. The compound can be administered in
an
amount effective to induce survival signaling and/or inhibit proNGF-induced
cell
death, which has been determined to be associated with neurodegenerative and
other
disorders.
[00210] The condition to be treated can be any condition which is mediated, at
least in part, by binding of neurotrophins to p75NTR. Such conditions include,
but are
not limited to, Alzheimer's disease, Huntington's disease, Pick's disease,
amyotrophic lateral sclerosis, epilepsy, Parkinson's disease, spinal cord
injury,
stroke, hypoxia, ischemia, brain injury, diabetic neuropathy, peripheral
neuropathy,
nerve transplantation, multiple sclerosis, peripheral nerve injury, and hair
loss.
[00211] In general, the condition to be treated can be any condition which is
mediated, at least in part, by aberrant signaling of the p75 receptor. In one
embodiment, the aberrant signaling is mediated by the presence or absence of
neurotrophin binding; in another embodiment the aberrant signaling is not
mediated
by the presence or absence of neurotrophin binding. In one variation aberrant
signaling occurs in the absence of neurotrophin binding.
[00212] Compounds having binding specificity for p75NTR as disclosed herein
can
be used to treat cell degeneration, including preventing neurodegeneration
such as,
for example, neurodegeneration caused by chemotherapy and/or neurodegenerative
disorders, as well as other conditions such as inducing hair follicle cell
survival after
hair follicle cell degeneration caused by, for example, chemotherapy.
[00213] The present application further provides for novel methods of
facilitating
cell survival. Representative cells include, but are not limited to, septal,
hippocampal, cortical, sensory, sympathetic, motor neurons, hair follicle
cells,
progenitor, and stem cells. Generally, such cells include neurons,
oligodendrocytes
and hair follicle cells. Specifically, the methods comprise treating a cell
with a
-65-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
compound having binding specificity for a p75NTR molecule, whereby the
compound
induces survival signaling and inhibits proNGF-induced cell death.
[00214] The present application also provides for a novel method of optimizing
cell function comprising use of the compounds disclosed herein. In one
embodiment, decreased cell survival is not the primary underlying disease
mechanism; in another embodiment, decreased cell survival is the primary
underlying disease mechanism.
Administration
[00215] The present application discloses a method of administering compounds
having binding specificity for p75NTR in order to ameliorate a condition
mediated by
p75NTR binding in a subject. The method can comprise the step of administering
to a
subject an effective amount of a compound having binding specificity for
p75NTR,
such as any of the compounds disclosed herein.
[00216] As used herein, administering can be effected or performed using any
of
the various methods known to those skilled in the art. The compound can be,
administered, for example, subcutaneously, intravenously, parenterally,
intraperitoneally, intradermally, intramuscularly, topically, enteral (e.g.,
orally),
rectally, nasally, buccally, sublingually, vaginally, by inhalation spray, by
drug
pump or via an implanted reservoir in dosage formulations containing
conventional
non-toxic, physiologically acceptable carriers or vehicles.
[00217] Further, the presently disclosed compounds can be administered to a
localized area in need of treatment. This can be achieved by, for example, and
not by
way of limitation, local infusion during surgery, topical application,
transdermal
patches, by injection, by catheter, by suppository, or by implant (the implant
can
optionally be of a porous, non-porous, or gelatinous material), including
membranes,
such as sialastic membranes or fibers.
[00218] The form in which the compound is administered (e.g., syrup, elixir,
capsule, tablet, solution, foams, emulsion, gel, sol) will depend in part on
the route
by which it is administered. For example, for mucosal (e.g., oral mucosa,
rectal,
intestinal mucosa, bronchial inucosa) administration, nose drops, aerosols,
inhalants,
nebulizers, eye drops or suppositories can be used. The compound can also be
used
to coat bioimplantable materials to enhance neurite outgrowth, neural
survival, or
cellular interaction with the implant surface. The compounds and agents
disclosed
herein can be administered together with other biologically active agents,
such as
-66-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
analgesics, anti-inflammatory agents, anesthetics and other agents which can
control
one or more symptoms or causes of a p75NTR -mediated condition.
[00219] Additionally, administration can comprise administering to the subject
a
plurality of dosages over a suitable period of time. Such administration
regimens can
be determined according to routine methods, upon a review of the instant
disclosure.
[00220] The compounds of the present application can be employed as the sole
active agent in a pharmaceutical or can be used in combination (e.g.,
administered
proximate in time to each other or even in the same formulation) with other
active
ingredients, e.g., neurotrophins, or other factors or drugs which can
facilitate neural
survival or axonal growth in neurodegenerative diseases, including but not
limited to
amyloid-(3 inhibitors, acetylcholinesterase inhibitors, butyrylcholinesterase
inhibitors, and N-methyl-D-aspartate subtype of glutamate receptor
antagonists.
Dosage
[00221] Compounds of the invention are generally administered in a dose of
about 0.01 mg/kg/dose to about 100 mg/kg/dose. Alternately the dose can be
from
about 0.1 mg/kg/dose to about 10 mg/kg/dose; or about 1 mg/kg/dose to
mg/kg/dose. In some dosages, the compounds disclosed herein are administered
at about 5 mg/kg/dose. Time release preparations may be employed or the dose
may
be administered in as many divided doses as is convenient. When other methods
are
used (e.g. intravenous administration), compounds are administered to the
affected
tissue at a rate from about 0.05 to about 10 mg/kg/hour, alternately from
about 0.1 to
about 1 mg/kg/hour. Such rates are easily maintained when these compounds are
intravenously administered as discussed herein. Generally, topically
administered
formulations are administered in a dose of about 0.5 mg/kg/dose to about 10
mg/kg/dose range. Alternately, topical formulations are administered at a dose
of
about 1 mg/kg/dose to about 7.5 mg/kg/dose or even about 1 mg/kg/dose to about
5 mg/kg/dose.
[00222] A range of from about 0.1 to about 100 mg/kg is appropriate for a
single
dose. Continuous administration is appropriate in the range of about 0.05 to
about
10 mg/kg. Topical administration is appropriate for conditions such as hair
loss or
wound revascularization.
[00223] Drug doses can also be given in milligrams per square meter of body
surface area rather than body weight, as this method achieves a good
correlation to
-67-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
certain metabolic and excretionary functions. Moreover, body surface area can
be
used as a common denominator for drug dosage in adults and children as well as
in
different animal species (Freireich et al., (1966) Cancer Chemother Rep. 50,
219-
244). Briefly, to express a mg/kg dose in any given species as the equivalent
mg/sq
m dose, the dosage is multiplied by the appropriate km factor. In an adult
human,
100 mg/kg is equivalent to 100 mg/kgx37 kg/sq m=3700 mg/m2.
[00224] Insofar as the compounds disclosed herein can take the form of a
mimetic
or fragment thereof, it is to be appreciated that the potency, and therefore
dosage of
an effective amount can vary. However, one skilled in the art can readily
assess the
potency of a compound of the type presently envisioned by the present
application.
[00225] In settings of a gradually progressive nervous system disorder,
compounds of the present application are generally administered on an ongoing
basis. In certain settings administration of a compound disclosed herein can
commence prior to the development of disease symptoms as part of a strategy to
delay or prevent the disease. In other settings a compound disclosed herein is
administered after the onset of disease symptoms as part of a strategy to slow
or
reverse the disease process and/or part of a strategy to improve cellular
function and
reduce symptoms. Compounds have been developed that cross the blood brain
barrier and hence would be delivered by oral administration or by other
peripheral
routes. Compounds that do not cross the blood brain barrier are applied for
targets
outside of the central nervous system. For targets and tissues outside of the
nervous
system, compounds are applied in either acute or chronic settings by other
oral or
directed target administration such as by topical application.
[00226] It will be appreciated by one of skill in the art that dosage range
will
depend on the particular compound, and its potency. The dosage range is
understood
to be large enough to produce the desired effect in which the
neurodegenerative or
other disorder and the symptoms associated therewith are ameliorated and/or
survival of the cells is achieved, but not be so large as to cause
unmanageable
adverse side effects. It will be understood, however, that the specific dose
level for
any particular patient will depend on a variety of factors including the
activity of the
specific compound employed; the age, body weight, general health, sex and diet
of
the individual being treated; the time and route of administration; the rate
of
excretion; other drugs which have previously been administered; and the
severity of
the particular disease undergoing therapy, as is well understood by those
skilled in
-68-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
the art. The dosage can also be adjusted by the individual physician in the
event of
any complication. No unacceptable toxicological effects are expected when
compounds disclosed herein are used in accordance with the present
application.
[00227] An effective amount of the compounds disclosed herein comprise
amounts sufficient to produce a measurable biological response. Actual dosage
levels of active ingredients in a therapeutic compound of the present
application can
be varied so as to administer an amount of the active compound that is
effective to
achieve the desired therapeutic response for a particular subject and/or
application.
Preferably, a minimal dose is administered, and the dose is escalated in the
absence
of dose-limiting toxicity to a minimally effective amount. Determination and
adjustment of a therapeutically effective dose, as well as evaluation of when
and
how to make such adjustments, are known to those of ordinary skill in the art.
[00228] Further with respect to the methods of the present application, a
preferred
subject is a vertebrate subject. A preferred vertebrate is warm-blooded; a
preferred
warm-blooded vertebrate is a mammal. The subject treated by the presently
disclosed methods is desirably a human, although it is to be understood that
the
principles of the present application indicate effectiveness with respect to
all
vertebrate species which are to included in the term "subject." In this
context, a
vertebrate is understood to be any vertebrate species in which treatment of a
neurodegenerative disorder is desirable. As used herein, the term "subject"
includes
both human and animal subjects. Thus, veterinary therapeutic uses are provided
in
accordance with the present application.
[00229] As such, the present application provides for the treatment of mammals
such as humans, as well as those mammals of importance due to being
endangered,
such as Siberian tigers; of economic importance, such as animals raised on
farms for
consumption by humans; and/or animals of social importance to humans, such as
animals kept as pets or in zoos. Examples of such animals include but are not
limited
to: carnivores such as cats and dogs; swine, including pigs, hogs, and wild
boars;
ruminants and/or ungulates such as cattle, oxen, sheep, giraffes, deer, goats,
bison,
and camels; and horses. Also provided is the treatment of birds, including the
treatment of those kinds of birds that are endangered and/or kept in zoos, as
well as
fowl, and more particularly domesticated fowl, i.e., poultry, such as turkeys,
chickens, ducks, geese, guinea fowl, and the like, as they are also of
economical
importance to humans. Thus, also provided is the treatment of livestock,
including,
-69-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
but not limited to, domesticated swine, ruminants, ungulates, horses
(including race
horses), poultry, and the like.
EXAMPLES
General Syntheses
[00230] Standard procedures and chemical transformation and related methods
are well known to one skilled in the art, and such methods and procedures have
been
described, for example, in standard references such as Fiesers' Reagents for
Organic
Synthesis, John Wiley and Sons, New York, NY, 2002; Organic Reactions, vols. 1-
83, John Wiley and Sons, New York, NY, 2006; March J. and Smith M., Advanced
Organic Chemistry, 6th ed., John Wiley and Sons, New York, NY; and Larock
R.C.,
Comprehensive Organic Transformations, Wiley-VCH Publishers, New York, 1999.
All texts and references cited herein are incorporated by reference in their
entirety.
[00231] Reactions using compounds having functional groups may be performed
on compounds with functional groups that may be protected. A "protected"
compound or derivatives means derivatives of a compound where one or more
reactive site or sites or functional groups are blocked with protecting
groups.
Protected derivatives are useful in the preparation of the compounds of the
present
invention or in themselves; the protected derivatives may be the biologically
active
agent. An example of a comprehensive text listing suitable protecting groups
may
be found in T. W. Greene, Protecting Groups in Organic Synthesis, 3rd edition,
John
Wiley & Sons, Inc. 1999.
[00232] Preparation of many of the compounds, e.g. Compounds 1-21, can be
illustrated in the general Scheme 1 below:
Scheme 1
0 NHBoc
RIRZNH + N-protected peptide coupling agent R1. N )NH
i R3
R2 O
deprotection R1, 0 NH NH2 HR4
R R3
R2 0
[00233] Generally the protection group for the amino acid is a Boc group. The
coupling agent can be HATU, HBTU, EDC/HOBt, or DCC/DMAP. The
deprotection reagent can be 4 M HCl in MeOH, 4M HCl in water, or TFA in DCM.
[00234] Generally, an amine or aniline is coupled with an N-protected amino
acid
and this coupled intermediate is deprotected to give a final compound or
another
-70-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
intermediate. The second intermediate can be further modified or directly go
through this coupling-deprotection cycle one more time to give the final
compound.
Example 1: Preparation of Compound 1
O NHBoc O NHBoc
r'N-1--_-NH2 + HO\II i EDC/HOBt rN~NH~~
0') HCI O DIEA, DMF OJ 0
0 NH2 TFA Ala
TFA/DCM N)~,NH
r Y
OJ 0
Compound 1
Preparation of Intermediate AI a
S.No. Chemicals/Reagents & Solvents MW mmol E q. Amts
1 Boc-Ile-OH 231.29 1 1.0 231 mg
2 DCM (anhydrous) 5mL
3 DIEA, d=0.742 129.25 2.5 2.5 0.44mL
4 EDC 191.7 1 1.0 192 mg
HOBt 135.13 1 1.0 135 mg
6 Amine 180.63 1 1.0 180 mg
[00235] To a solution of Boc-Ile-OH in DCM were added DIEA, HOBt, EDC,
and amine in that order. The reaction mixture was stirred for 3 hours at room
temperature (RT).
[00236] After the reaction was complete (LC-MS), 10% citric acid was added to
quench the reaction. The DCM layer was separated and washed with saturated
NaHCO3, brine, dried with anhydrous Na2SO4, filtered, and concentrated. The
intermediate Ala was obtained as an off-white solid (303 mg).
Preparation of Compound 1
S.No, Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Ala 300 mg
2 TFA/DCM (1:1) 4 mL
[00237] Cold TFA/DCM was added to the residue of Ala. The reaction mixture
was stirred for 2 hours at room temperature. After the reaction was complete
(LC-
MS), the mixture was concentrated to afford the final compound (272 mg).
Compound 1 was characterized by 'H NMR, LC-MS and HPLC. 'H NMR (MeOD),
8: 4.07-4.21 (m, 2H), 3.77 (d, J=5.84 Hz, 1H), 3.65-3.70 (m, 4H), 3.56-3.58
(m,
2H), 3.45-3.51 (m, 2H), 1.88-1.98 (m, 1H), 1.56-1.66 (m, 1H), 1.21-1.31 (m,
1H),
-71-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
1.05 (d, J=6.78 Hz, 3H), 0.99 (t, J--7.40 Hz, 3H). LC-MS, (M+1), 258. HPLC
(>95%, retention time, 1.99 min).
Example 2: Preparation of Compound 2
NHBoc O O
JNH + HO HBTU, DIEA NN 0 /NHBoc TFA/DCM [ N 0 NHZTFA
~
DMF _ OJ 1 O
O
A2a Alb
0 H NHBBocc 0 H NHZ TFA
TFCM
:::: N Y-1
A2c Compound 2
Preparation of Intermediate A2a
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Ala-OH 189.21 2 1.0 378 mg
2 DMF (anhydrous) 5mL
3 DIEA, d=0.742 129.25 5 2.5 0.87mL
4 HBTU 379 2 1.0 758 mg
Morpholine, d=0.99 87.12 2 1.0 175 uL
[00238] To a solution of Boc-Ala-OH in DMF were added HBTU, DIEA, and
morpholine in that order. The reaction mixture was stirred for 2 hours at room
temperature. After the reaction was complete (LC-MS), the reaction mixture was
subject to prep-HPLC purification to afford the intermediate A2a (333 mg).
Preparation of Intermediate Alb
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A2a 158 mg
2 TFA/DCM (1:1) 4mL
[00239] Cold TFA/DCM was added to the residue of A2a. The reaction mixture
was stirred for 2 hours at room temperature. After the reaction was complete
(LC-
MS), the mixture was concentrated to afford the intermediate A2b (180 mg).
Preparation of Intermediate A2c
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Ile-OH 231.29 0.611 1.0 141 mg
2 DCM (anhydrous) 5mL
3 DIEA, d=0.742 129.25 1.83 3.0 0.32mL
4 HOBt.H20 135.13 0.611 1.0 83 mg
5 EDC 191.7 0.611 1.0 117 mg
6 A2b 0.611 1.0 180 mg
-72-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00240] To the residue of A2b were added DCM, Boc-Ala-OH, HOBt, EDC, and
DIEA in that order. The reaction mixture was stirred for 2 hours at room
temperature. After the reaction was complete (LC-MS), 0.5 HCl was added to
quench the reaction. The DCM layer was separated and washed with saturated
NaHCO3, brine, dried with anhydrous Na2SO4 and filtered. The liquid was
concentrated to afford the intermediate A2c (64 mg).
Preparation of Compound 2
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A2c 60 mg
2 TFA/DCM (1:1) 4mL
[00241] Cold TFA/DCM was added to the residue of A2c. The reaction mixture
was stirred for 3 hours at room temperature. After the reaction was complete
(LC-
MS), the mixture was concentrated to afford the final compound (51 mg).
Compound 2 was characterized by rH NMR, LC-MS and HPLC. 'H NMR (D20), 6:
3.62-3.92 (m, 10H), 1.99-2.05 (m, 1H), 1.54-1.60 (m, 1H), 1.41 (d, J=7.12 Hz,
2H),
1.26-1.33 (m, 1H), 1.06 (d, J6.92 Hz, 3H), 0.99 (t, J7.38 Hz, 3H). LC-MS,
(M+1), 272. HPLC (>95%, retention time, 1.94 min).
Example 3: Preparation of Compound 3
NHBoc NHBoc
O ~N NH2 + HOll HATU, DIEA i NH
0 DMF N 0
NH2A3a
HCl 2 HCl NH
-Or 0
~N
0
Compound 3
Preparation of Intermediate A3a
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Ile-OH 231.29 0.432 1.0 100 mg
2 DMF (anhydrous) 3 mL
3 DIEA, d=0.742 129.25 1.08 2.5 0.20mL
4 HATU 380.2 0.43 1.0 163 mg
4-morpholinoaniline 178.24 0.432 1.0 77 mg
[00242] To a solution of Boc-Ile-OH in DMF were added HATU, 4-
morpholinoaniline, and DIEA in that order. The reaction mixture was stirred
for 1
hour at room temperature.
-73-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[002431 After the reaction was complete (LC-MS), saturated aqueous NaHCO3
was added to quench the reaction and EtOAc was used to extract the product.
The
organic layer was separated and washed with water, brine, dried with anhydrous
Na2SO4, filtered, and concentrated. The intermediate A3a was obtained as an
off-
white solid (166mg).
Preparation of Compound 3
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A3a 160 mg
2 HCl in MeOH 4mL
[002441 Cold HCl solution in MeOH was added to the residue of A3a. The
reaction mixture was stirred for 3 hours at room temperature.
[002451 After the reaction was complete (LC-MS), the mixture was concentrated
to afford the final compound 3 (120mg). Compound 3 was characterized by 1H
NMR, LC-MS and HPLC. 'H NMR (MeOD), 6: 7.88 (d, J=9.16 Hz, 2H), 7.69 (d,
J=9.12 Hz, 2H), 4.09-4.11 (m, 4H), 3.92 (d, J=5.80 Hz, 1 H), 3.66-3.69 (m,
4H),
2.03-2.10 (m, 1H), 1.62-1.69 (m, 1H), 1.24-1.32 (m, 1H), 1.11 (d, J=6.92 Hz,
3H),
1.01 (t, J=7.40 Hz, 3H). LC-MS, (M+1), 292. HPLC (>95%, retention time, 4.86
min).
Example 4: Preparation of Compound 4
H2N NHBoc
0
EDC/HOBt N NHBoc
+ HO NH
0N
0 DCM/DIEA O
O A4a
NH2 2 HCI
HCI N
MeOH &NH
O
Compound 4
Preparation of Intermediate A4a
S.No, Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Ile-OH 231.29 1 1.0 231 mg
2 DCM (anhydrous) 5 mL
3 DIEA, d=0.742 129.25 2.5 2.5 0.44 mL
4 HOBt.H20 135.13 1 1.0 135 mg
EDC 191.7 1 1.0 192 mg
6 2-morpholinoaniline 178.23 0.96 0.96 170 mg
-74-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00246] To the solution of Boc-Ile-OH in DCM were added HOBt, EDC, DIEA,
and 2-morpholinoaniline in that order. The reaction mixture was stirred for
7.5
hours at room temperature.
[00247] The reaction mixture was subject to prep-HPLC purification to afford
the
intermediate A4a (94mg).
Preparation of Compound 4
Savo. Chemicals/Reagents & Solvents MW mmol Eq Amts
1 A4a 60 mg
2 HCI in MeOH 4mL
[00248] Cold HCI solution in MeOH was added to the residue of A4a. The
reaction mixture was stirred for 3 hours at room temperature.
[00249] After the reaction was complete (LC-MS), the mixture was concentrated
to afford the final compound 4 (73mg). Compound 4 was characterized by 1H
NMR, LC-MS and HPLC. 114 NMR (D20), 6: 7.67 (dd, J=7.96 and 1.48 Hz, 1H),
7.49 (dd, J=8.10 and 1.34 Hz, 1H), 7.42 (dt, J=7.75 and 1.53 Hz, 1H), 7.34
(dt,
J=7.67 and 1.43 Hz, 1H), 4.22 (d, J=5.00 Hz, 1H), 3.95-3.97 (m, 4H), 3.13-3.15
(m,
4H), 2.11-2.17 (m, 111), 1.57-1.64 (m, 114), 1.30-1.39 (in, 114), 1.12 (d,
J=6.96 Hz,
3H), 1.00 (t, J7.40 Hz, 3H). LC-MS, (M+1), 292. HPLC (>95%, retention time,
5.31 min).
Example 5: Preparation of Compound 5
NHBoc NHBoc
( LNH2 + HO EDC/HOBt N~NHNy
OJ O DCM/DIEA OJ O
A5a
HCl NH2 2HCI
~N~N H
OJ O =
Compound 5
Preparation of Intermediate A5a
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Ile-OH 231.29 1 1.0 231 mg
2 DCM (anhydrous) 5 mL
3 DIEA, d=0.742 129.25 2.5 2.5 0.44 mL
4 HOBt.H20 135.13 1 1.0 135 mg
EDC 191.7 1 1.0 192 mg
6 2-morpholin-4-yl-propylamine 144.22 0.19 mL
-75-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00250] To the solution of Boc-Ile-OH in DCM were added HOBt, EDC, DIEA,
and 2-morpholin-4-yl-propylamine in that order. The reaction mixture was
stirred
for 3 hours at room temperature.
[00251] The reaction mixture was subject to prep-HPLC purification to afford
the
intermediate A5a (219mg).
Preparation of Compound 5
S.No. Chemicals/Reagents &Solvents MW mmol Eq. Amts
1 A5a 210mg
2 HCl in MeOH 4mL
[00252] Cold HCl solution in MeOH was added to the residue of A4a. The
reaction mixture was stirred for 3 hours at room temperature.
[00253] After the reaction was complete (LC-MS), the mixture was purified by a
prep-HPLC and converted to an HCl salt by a treatment with HCl in MeOH to
afford
final compound 5 (120mg). Compound 5 was characterized by IH N]VIR, LC-MS
and HPLC. 'H NMR (D20), S: 3.30-4.24 (m, 12H), 1.92-2.05 (m, 1H), 1.43-1.56
(m, 1H), 1.36-1.42 (m, 3H), 1.16-1.30 (m, 1H), 1.01 (d, J=6.92 Hz, 3H), 0.94
(d,
J=7.34 Hz, 3H). LC-MS, (M+1), 258. HPLC (>95%, retention time, 1.41 min).
Example 6: Preparation of Compound 6
~INH + HOBoc HBTU, DIEA rN 0
NHBocBH3-THF rN NHBoc
O~/ O DMF O,,j OBI
A6a A6b
HCI H NHBoc
HCI JN1NH2 Boc-Ile-OH ON--T NHCI
EDC/HOBt O =
A6c A6d
ON--IN
H N\II = 1
O
Compound 6
Preparation of Intermediate A6a
S.No. Chemicals/Reagents &`Solvents MW mmol Eq. Amts
1 Boc-Ala-OH 189.21 2 1.0 378 mg
2 DMF (anhydrous) 5mL
3 DIEA, d=0.742 129.25 5 2.5 0.87mL
4 HBTU 379 2 1.0 758 mg
Morpholine, d=0.99 87.12 2 1.0 175 uL
-76-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00254] To a solution of Boc-Ala-OH in DMF were added HBTU, DIEA, and
morpholine in that order. The reaction mixture was stirred for 2 hours at room
temperature.
[00255] After the reaction was complete (LC-MS), the reaction mixture was
subject to prep-HPLC purification to afford the intermediate A6a (333mg).
Preparation of Intermediate A6b
S.No. Chemicals/Reagents & Solvents MW mmol E q. Amts
1 A6a 258.31 1.67 1.0 430 mg
2 THF, anhydrous 10mL
3 BH3-THF, 1M 2.0 3.3 mL
[00256] A6a was dissolved in THF. BH3-THF was added slowly.to the above
solution. Bubbles were observed and the reaction mixture was stirred over
night at
RT.
[00257] After the reaction was complete (LC-MS), the mixture was quenched
carefully with water and concentrated. The mixture was further purified by
prep-
HPLC to afford intermediate A6b (192mg).
Preparation of Intermediate A6c
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A6b 210 mg
2 HCl in MeOH 4mL
[00258] Cold HCl solution in MeOH was added to the residue of A6b. The
reaction mixture was stirred for 3 hours at room temperature.
[00259] After the reaction was complete (LC-MS), the mixture was concentrated
to afford the intermediate A6c (158mg).
Preparation of Intermediate A6d
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Ile-OH 231.29 0.8 1.0 180 mg
2 DMF (anhydrous) 3 mL
3 DIEA, d=0.742 129.25 1.6 2.0 0.28 mL
4 HOBt.H20 135.13 0.8 1.0 108 mg
EDC 191.7 0.8 1.0 153 mg
6 A6c 180 0.83 150 mg
-77-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00260] To the solution of Boc-Ile-OH and A6c in DCM were added HOBt,
EDC, and DIEA in that order. The reaction mixture was stirred for 1 hour at
room
temperature.
[00261] The reaction mixture was subject to prep-HPLC purification to afford
the
intermediate A6d (86mg).
Preparation of compound 6
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A6d 60 mg
2 4 M HC1 in H2O 4mL
[00262] Cold TFAIDCM was added to the residue of A6d. The reaction mixture
was stirred for 3 hours at room temperature.
[00263] After the reaction was complete (LC-MS), the mixture was concentrated
to afford the final compound 6 (89mg). Compound 6 was characterized by 1H
NMR, LC-MS and HPLC. 'H NMR (D20), 6: 4.21-4.32 (m, 1H), 3.88-4.11 (m,
2H), 3.65-3.87 (m, 3H), 3.34-3.53 (m, 2H), 3.07-3.34 (m, 4H), 1.83-1.95 (m,
1H),
1.30-1.41 (m, 1H), 1.21 (d, J=6.80 Hz, 3H), 1.04-1.17 (m, 1H), 0.91 (d, J=6.96
Hz,
3H), 0.82 (t, J=7.38 Hz, 3H). LC-MS, (M+1), 258. HPLC (>95%, retention time,
1.59 min).
Example 7: Preparation of Compound 7
OEt
~OEt
0
(N~ "NH2 + BrCH2CH(OEt)2 70 C (N'om` NH
O--'J O'-'J
Ala
OEt
HBTU EtO-~ NHBoc 1. HCI ~NH 2HCI VD. //~~ N
Boc-Ile-OH IO~ IOI 2.NaHCO3 0
Alb 3. NaBH4 Compound 7
Preparation of Intermediate A 7a
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 2-Morpholin-4-yl-ethylamine 130.11 30 3 4 mL
2 BrCH2CH(OEt)2 197.07 10 1 1.5 mL
[00264] Amine and bromide were mixed and heated up to 70 C for 5 hours with
stirring.
-78-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00265] LC-MS showed that the product contains a mixture of non- mono-, and
bis- alkylated amines. The mixture was used in next step without further
purification
(5.5 nil).
Preparation of Intermediate A 7b
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Ile-OH 231.29 30 1.0 7 g
2 Ala 5.5 mL
2 Acetonitrile (anhydrous) 100 mL
3 DIEA, d=0.742 129.25 75 2.5 13 mL
4 HBTU 379.3 30 1.0 11.4 g
[00266] HBTU was added to a solution of Boc-Ile-OH in acetonitrile and then
DIEA was added. After the mixture was stirred at room temperature for 10
minutes,
Ala was then added. The reaction mixture was stirred for another 2 hours.
[00267] After the reaction was complete (LC-MS), portions of solution were
subjected to prep-HPLC separation, collecting fraction peak with M+ =460 (A7b,
2g).
Preparation of Compound 7
[00268] A7b was dissolved in cold HCl in MeOH. The reaction mixture was
stirred 3 hrs at RT. After the reaction was complete (LC-MS), the mixture was
concentrated to afford an HCl salt intermediate (209 mg).
[00269] The above HCl salt intermediate was dissolved in a mixed solvent of
acetonitrile and water, neutralized with saturated sodium bicarbonate till pH-
7. The
reaction mixture was stirred for 2.5 hrs at RT. Then, excess NaBH4 was added
and
the mixture was stirred overnight. However, not much product was observed by
LC-
MS the second day morning, thus, the reaction mixture was heated to 45 C for
7.5
hrs.
[00270] After the reaction was complete (LC-MS), the mixture was filtered and
subject to prep-HPLC purification to afford the desired final compound. The
final
compound was converted to an HCl salt by treatment with HCl in MeOH (43mg).
Compound 7 was characterized by 'H NMR, LC-MS and HPLC. 1H NMR (D20), 8:
2.90-4.20 (m, 17H), 1.84-2.35 (m, 1H), 1.07-1.53 (m, 2H), 0.80-1.02 (m, 6H).
LC-
MS, (M+1), 270. HPLC (>95%, retention time, 1.27 min).
-79-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Example 8: Preparation of Compound 8
BocN' HATU H Boc
~N~~NH2 + HO N
6-,,j DIEA O p
O
A8a
HCI H NNHH2HCI
r-- 6,N~~N~II
O
Compound 8
Preparation of Intermediate A8a
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 N-methyl-Boc-Ile-OH 245.32 2 2 490 mg
2 2-morpholin-4-yl-ethylamine 130.19 1 1 0.13 mL
3 Acetinitrile (anhydrous) 10 mL
4 DIEA, d=0.742 129.25 3 3 0.52 mL
HATU 380.2 2 2 760 mg
[00271] To a solution of acid and HATU solution in acetonitrile, DIEA was
added with stirring. After 20 minutes, amine was added and the reaction was
continued to stir for 1 hour.
[00272] LC-MS showed the completion of the reaction. The reaction solution was
subjected to prep-HPLC separation (A8a, 65 mg).
Preparation of Compound 8
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A8a 357.49 0.18 1 65 mg
2 Methanol 10 mL
3 12N HCl 5 mL
[00273] To a solution of A8a in methanol, HCl aqueous solution was added and
stirred at room temperature for 2 hours.
[00274] LC-MS showed the completion of the reaction. The solvents were
removed in vacuum to afford compound 8 (60mg). Compound 8 was characterized
by 1H NMR, LC-MS and HPLC. 1H NMR (MeOD), 8: 3.82-4.11 (m, 5H), 3.78 (d,
J=4.52 Hz, 1H), 3.68 (d, J=11.2 Hz, 1H), 3.45-3.59 (m, 2H), 3.11-3.41 (m, 5H),
2.78 (s, 3H), 1.93-2.05 (m, 1H), 1.56-1.68 (m, 1H), 1.15-1.32 (m, 1H) ), 0.96-
1.09
(m, 6H). LC-MS, (M+1), 258. HPLC (>95%, retention time, 1.0 min).
-80-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Example 9: Preparation of Compound 9
H NH 2HCI CDI HC1 O~ N
Et3N ON
~I
O O
CI
H
Compound 8
Compound 9
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Compound 8 330.29 2.27 1 750 mg
2 CDI 160.17 20 8.8 3.2 g
3 Acetinitrile (anhydrous) 15 mL
4 Et3N 101.19 19.7 8.7 2 g
[00275] To a solution of Compound 8 and CDI in acetonitrile, Et3N was added
with stirring. The stirring was continued for three (3) days.
[00276] LC-MS showed the completion of the reaction. Methanol was added to
quench the reaction. After all the volatile solvent was removed, the residue
was
redissolved in methanol and subjected to prep-HPLC separation. After removal
of
the solvent, the product formic acid salt was converted to HCi salt by co-
evaporating
with 25 ml 1.25 N HCl methanol solution three times (76mg). Compound 9 was
characterized by 1H NMR, LC-MS and HPLC. 1H NMR (D2O), 8: 3.68-4.14 (m,
7H), 3.00-3.63 (m, 6H), 2.68 (d, J=8.40 Hz, 3H), 1.91-2.03 (m, 1H), 0.98-1.51
(m,
2H), 0.90 (d, J=7.00 Hz, 1H), 0.79-0.88 (m, 3H), 0.71 (d, J=6.92 Hz, 1H). LC-
MS,
(M+1), 284. HPLC (>95%, retention time, 4.32 min).
Example 10: Preparation of Compound 10
2HC1
----INH2 NHBocEpc H NHBocHCI H NH2
O N + HOIJ DIEA ~N- ~N ) ) INS
J oI of o
Al Oa Compound 10
Preparation of Intermediate AIOa
S:No, Chemicals!Reagents & Solvents MW mmol Eq. __ Amts
1 Boc-Gly-OH 175.18 3 1 526 mg
2 2-morpholin-4-yl-ethylamine 130.19 3 1 0.39 mL
3 Acetinitrile (anhydrous) 10 mL
4 DIEA, d=0.742 129.25 6 2 1.2 mL
EDC 191.7 4.17 1.4 800 mg
-81-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00277] A mixture of Boc-Gly-OH and EDC was suspended in acetonitrile. DIEA
was added, followed by the addition of 2-Morpholino-4-yl-ethylamine. The
reaction
mixture was stirred for 2 hours.
[00278] LC-MS showed the completion of the reaction. The reaction mixture was
subjected to prep-HPLC separation to give pure AlOa (177mg).
Preparation of Compound 10
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 AlOa 357.49 0.18 1 177 mg
2 Methanol 50 mL
3 12N HC1 25 mL
[00279] To a solution of AlOa in methanol, HC1 aqueous solution was added and
stirred at room temperature for 2 hours.
[00280] LC-MS showed the completion of the reaction. The solvents were
removed in vacuum to afford Compound 10 (160mg). Compound 10 was
characterized by 'H NMR, LC-MS and HPLC. 1H NMR (MeOD), 8: 3.93-4.08 (m,
4H), 3.77 (s, 2H), 3.56-3.72 (m, 4H), 3.32-3.36 (m, 3H), 3.09-3.24 (m, 2H). LC-
MS,
(M+1), 188. HPLC (>95%, retention time, 1.0 min).
Example 11: Preparation of Compound 11
NHBoc H NHBoc
N--,__--NH2 HO EDC, HoBt /~N,-~N
I
+ 10 Y,
01) O DIEA OJ 0
Alla
NH2
HCI rNH/N2
O
Compound 11
Preparation of Intermediate All a
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Ala-OH 189.21 1 1 190 mg
2 2-morpholin-4-yl-ethylamine 130.19 1 1 0.13 mL
3 Acetinitrile (anhydrous) 5 mL
4 BtOH=H2O 153.14 1 1 153 mg
DIEA, d=0.742 129.25 2 2 0.38 mL
6 EDC 191.7 1 1 192 mg
-82-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00281] A mixture of Boc-Ala-OH, BtOH H20, and DIEA was suspended in
acetonitrile. EDC was then added with stirring and the solution became clear.
2-
Moipholin-4-yl-ethylamine was added and stirred for 2 hours at room
temperature.
[00282] LC-MS showed the completion of the reaction. The reaction mixture was
subjected to prep-HPLC separation to give pure Al la (66mg).
Preparation of Compound 11
S:No. Chemicals/Reagents & 'Solvents MW mmol 'Eq. Amts
1 Al la 301.38 0.22 1 66 mg
2 Methanol 50 mL
3 12N HC1 25 mL
[00283] To a solution of Al la in methanol, HCl aqueous solution was added and
stirred at room temperature for 2 hours.
[00284] LC-MS showed the completion of the reaction. The solvents were
removed in vacuo to afford Compound 11 (60mg). Compound 11 was characterized
by 1H NMR, LC-MS and HPLC. 1H NMR (D2O), S: 4.10-4.27 (m, 3H), 3.87-4.01
(m, 2H), 3.77-3.88 (m, 1H), 3.60-3.76 (m, 3H) 3.40-3.55 (m, 2H), 3.26-3.39 (m,
2H), 1.60 (d, J=7.16 Hz, 3H). LC-MS, (M+1), 202. HPLC (>95%, retention time,
1.0 min).
Example 12: Preparation of Compound 12
O
H NHBoc H HN
N~~N II _ 1. CF3COZH o N)I
o
0 2. Na2CO3 0
PhCOCI
Compound 12
S.No. Chem icals/Reagents& Solvents MW mmol Eq. Amts
I 2-Boc-amino-3-methyl-N. (2- 343.46 1 1 343 mg
morpholinoethyl)pentamide
2 CF3CO2H 10 mL
3 CH2C12 10 mL
4 THE 10 mL
Water 10 mL
6 Na2CO3 105.99 10 10 1.06 g
7 PhCOCI 140.57 2 2 280 mg
[00285] 2-Boc-amino-3-methyl-N-(2-morpholinoethyl)pentamide was dissolved
in CF3CO2H/CH2C12 and stirred at room temperature for 2 hours. After LC-MS
showed the complete deprotection of the starting material, the solvents were
-83-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
removed under vacuum. The residue was dissolved in THF/H2O solution and
Na2CO3 was added to adjust the solution to basic. Benzoyl chloride was then
added
with stirring for 2 hours.
[00286] LC-MS showed the completion of the reaction. THE was then removed
under vacuum and the aqueous solution was extracted with AcOEt. AcOEt was
dried
over Na2SO4 and removed. The residue was dissolved in methanol and subjected
to
prep-HPLC separation. After removal of the solvent, the product formic acid
salt
was converted to HCl salt by co-evaporating with 50 mL 1.25 HCl methanol
solution three times (91 mg). Compound 12 was characterized by 'H NMR, LC-MS
and HPLC. 1H NMR (MeOD), 8: 7.86-7.91 (m, 2H), 7.56-7.61 (m, 1H), 7.47-7.53
(m, 2H), 4.20 (d, J=7.96 Hz, 1H), 4.00-4.1.0 (m, 2H), 3.82-3.92 (m, 2H), 3.71-
3.80
(m, 1H), 3.59-3.69 (m, 2H), 3.15-3.56 (m, 5H), 1.96-2.08 (m, 1H), 1.63-1.75
(m,
1H), 1.26-1.39 (m, 1H), 0.94-1.06 (m, 6H). LC-MS, (M+1), 348. HPLC (>95%,
retention time, 5.27 min).
Example 13: Preparation of Compound 13
NHBoc H NHBoc
+ HO HBTU f-"-N--,,iN
OJ DIEA O') O
O Ph A13a
2HCI H NH2
4N HCI rN--,----N
OJ O
Compound 13
Preparation of Intermediate A13a
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-Phe-OH 256.31 1 1 256 mg
2 2-morpholin-4-yl-ethylamine 130.19 1 1 0.13 mL
3 Acetonitrile (anhydrous) 5 mL
4 DIEA, d=0.742 129.25 1 1 0.17mL
HBTU 379.25 1 1 379 mg
[00287] To a solution of Boc-Phe-OH, HBTU in acetonitrile was added DIEA.
After stirring for 5 minutes, 2-morpholin-4-yl-ethylamine was added and the
reaction mixture was then stirred for another 2 hours.
[00288] LC-MS showed the completion of the reaction. The reaction mixture was
subjected to prep-HPLC separation to give pure A13a (210mg).
-84-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Preparation of Compound 13
S.No. Chemicals/Reagents & Solvents MW mmol Eq._ Amts
1 A13a 377.48 0.56 1 210 mg
2 Methanol 50 mL
3 12N HCl 25 mL
[00289] To a solution of A13a in methanol, HCl aqueous solution was added and
stirred at room temperature for 2 hours.
[00290] LC-MS showed the completion of the reaction. The solvents were
removed in vacuum to afford Compound 13 (195mg). Compound 13 was
characterized by 1H NMR, LC-MS and HPLC. 'H NMR (D20), 8: 7.32-7.41 (m,
2H), 7.43-7.57 (m, 3H), 4.30 (t, J=7.32 Hz, 1H), 3.94-4.10 (m, 4H), 3.61 (t,
J6.34
Hz, 2H), 3.08-3.44 (m, 9H). LC-MS, (M+1), 278. HPLC (>95%, retention time,
1.21 min).
[00291] A number of compounds, including Compound 14, Compound 15,
Compound 16, Compound 17, and Compound 18, were prepared according to the
method of preparation of Compound 13, substituting the appropriate starting
materials in place of Boc-Phe-OH.
Compound 14
2HCI H NH2
N'-N
OJ AO
[00292] Compound 14 was prepared according to the method of preparation of
Compound 13, except that Boc-Phe-OH was replaced with Boc-Leu-OH.
Compound 14 (175mg) was characterized by 'H NMR, LC-MS and HPLC. 1H NMR
(D20), 8: 4.07-4.20 (m, 2H), 4.03 (d, J=7.22 Hz, 1H), 3.71-3.93 (m, 3H), 3.50-
3.70
(m, 3H), 3.18-3.44 (m, 4H), 1.74 (t, J=7.26 Hz, 2H), 1.61-1.72 (in, 1H), 0.89-
1.03
(m, 6H). LC-MS, (M+1), 244. HPLC (>95%, retention time, 1.0 min).
Compound15
2HCI H NH2
~ ~
J
Compound 15
[00293] Compound 15 was prepared according to the method of preparation of
Compound 13, except that Boc-Phe-OH was replaced with Boc-D-t-butylglycine-
OH. Compound 15 (120mg) was characterized by 'H NMR, LC-MS and HPLC. 1H
-85-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
NMR (D20), 6: 3.91-4.10 (m, 2H), 3.62-3.82 (m, 3H), 3.60 (s, 1H), 3.38-3.58
(m,
3H), 3.05-3.34 (m, 4H), 0.96 (s, 9H). LC-MS, (M+1), 244. HPLC (>95%, retention
time, 1.0 min).
Compound 16
2 HC1 H NH2 OH
N'~ N1O
J O
Compound 16
[00294] Compound 16 was prepared according to the method of preparation of
Compound 13, except that Boc-Phe-OH was replaced with Boc-Asp(OTBU)-OH.
Compound 16 (50mg) was characterized by 1H NMR, LC-MS and HPLC. 'H NMR
(D20), 6: 4.23 (t, J=6.24 Hz, 1H), 3.91-4.11 (m, 2H), 3.67-3.84 (m, 2H), 3.55-
3.67
(m, 2H), 3.41-3.54 (m, 2H), 3.27 (t, J=6.32 Hz, 2H), 3.06-3.22 (m, 2H), 2.85-
3.01
(m, 2H). LC-MS, (M+l), 246. HPLC (>95%, retention time, 1.0 min).
Compound 17
2 HCl H NH2
NN f'_~ OH
OJ 0
Compound 17
[002951 Compound 17 was prepared according to the method of preparation of
Compound 13, except that Boc-Phe-OH was replaced with Boc-Glu(OTBU)-OH.
Compound 17 (60mg) was characterized by 'H NMR, LC-MS and HPLC. 1H NMR
(D20), 8: 3.94-4.10 (m, 2H), 3.97 (t, J=6.56 Hz, 1H), 3.67-3.83 (m, 2H), 3.60-
3.69
(m, 1H), 3.40-3.59 (m, 3H), 3.20 (t, J6.78 Hz, 2H), 3.08-3.23 (m, 2H), 2.45
(t,
J=7.18 Hz, 2H), 2.01-2.17 (m, 2H). LC-MS, (M+1), 260. HPLC (>95%, retention
time, 1.0 min).
Compound 18
2 HC1 H
~N- N
OJ 0
Compound 18
[00296] Compound 17 was prepared according to the method of preparation of
Compound 13, except that Boc-Phe-OH was replaced with Boc-Pro-OH. Compound
18 (80mg) was characterized by 'H NMR, LC-MS and HPLC. 1H NMR (D20), 8:
-86-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
4.27 (t, J=7.38 Hz, 1H), 3.91-4.10 (m, 2H), 3.60-3.81 (m, 3H), 3.37-3.57 (m,
3H),
3.19-3.37 (in, 4H), 3.04-3.19 (m, 2H), 2.25-2.40 (m, 1H), 1.85-2.02 (m, 3H).
LC-
MS, (M+1), 228. HPLC (>95%, retention time, 1.0 min).
Example 19: Preparation of Compound 19
2HC1 H NH2 NaCNBH3 2HC1 H HN~
/N~,NI + CH3CHO CH OH N
o~ O 3 O
Compound 19
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 (2S,3S)-2-amino-3-methyl-N-(2- 316.27 1 1 314 mg
morpholinoethyl)pentanamide
2 CH3CHO 44.05 1 1 44 mg
3 NaCNBH3 (1 M in THF) 1 1 1 mL
4 Methanol 105.99 10 10 1.06 g
[00297] A mixture of (2S,3S)-2-amino-3-methyl-N-(2-
morpholinoethyl)pentanamide and acetaldehyde was dissolved in methanol and
NaCNBH3 was added. The reaction mixture was then stirred overnight.
[00298] LC-MS showed the completion of the reaction. K2C03 (270 mg) was
added to quench the reaction. The precipitate was dissolved by adding 2 mL
water
and then the solution was subjected to prep-HPLC separation. Then the formic
acid
salt was transformed to HCl salt by co- evaporating with HCl/methanol (1N)
solution. Compound 19 (50mg) was characterized by 1H NMR and LC-MS. 1H
NMR (D20), 6: 4.05-4.23 (m, 2H), 3.48-3.94 (m, 7H), 3.38 (t, J=6.82 Hz, 2H),
3.17-
3.34 (m, 2H), 3.08 (q, J=7.32 Hz, 2H), 1.94-2.07 (m, 1H), 1.44-1.60 (m, 1H),
1.30
(t, J=7.32 Hz, 1H), 1.14-1.26 (m, 3H), 0.89-1.06 (m, 6H). LC-MS, (M+1), 272.
Example 20: Preparation of Compound 20
2HC1 H NH2 formaldehyde 2HC1 H \N
~N~iN II = ~N~~N~I
O'--J 0 Pd/C OBI O
Compound 20
S.No. Chemicals/Reagents & Solvents MW mmol, Eq. Amts
1 (2S,3S)-2-amino-3-methyl-N-(2- 316.27 1 1 316 mg
morpholinoethyl)pentanamide
2 Formaldehyde (37% solution) 44.05 16.8 16.8 2 mL
3 Pd/C (10%) 19
4 H2O 10 mL
-87-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00299] A mixture of (2S,3S)-2-amino-3-methyl-N-(2-
morpholinoethyl)pentanamide, formaldehyde, and Pd/C in water was hydrogenated
"at 50 Psi for 5 hours.
[00300] LC-MS showed the completion of the reaction. Pd/C was filtrated off
and
the clear solution was subjected to prep-HPLC separation. The formic acid salt
form
was transformed to HCl salt (220mg) by co-evaporating with HCl/methanol (1N)
solution. Compound 20 (220mg) was characterized by 'H NMR, LC-MS and
HPLC. 'H NMR (D20), S: 3.70-4.13 (m, 4H), 3.64-3.69 (m, 1H), 3.56-3.64 (m,
2H),
3.05-3.52 (m, 6H), 2.80 (s, 6H), 2.02-2.15 (m, 1H), 1.32-1.46 (m, 1H), 1.01-
1.15 (m,
1H), 0.81-0.93 (m, 6H). LC-MS, (M+1), 272. HPLC (>95%, retention time, 1.14
min).
Example 21: Preparation of Compound 21
NHBoc NHBoc
N/---/NH2 + HO HATU, DIEA //////~~~~ NH
O O, DMF OJN O l i
A21 a
NH2 2HCI
HCl NH
JN O
O I /
Compound 21
Preparation of Intermediate A11 a
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Boc-L-alpha-phenylglycine 251.28 1 1.0 251 mg
2 DMF (anhydrous) 6 mL
3 DIEA, d=0.742 129.25 2.0 2.0 0.35 mL
4 HATU 380.2 1 1.0 380 mg
Amine, d=1 130.19 2 2.0 0.13 mL
[00301] To a solution of acid in DMF were added HATU, DIEA, and amine in
that order. The reaction mixture was stirred for 2hr at room temperature.
[00302] After the reaction was complete (LC-MS and TLC), the intermediate
A21 a was purified by prep-HPLC (228mg).
Preparation of Compound 21
S.No. Chemicals/Reagents & Solvents MW -Ijm Eq. Amts
1 A21 a 228 mg
2 4 M HCl in McOH 4mL
-88-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[00303] Cold HCl solution was added to the residue of A21 a. The reaction
mixture was stirred for overnight at room temperature.
[00304] After the reaction was complete (LC-MS), the mixture was concentrated.
It was further purified on prep-HPLC to afford the final compound 21 (206mg).
Compound 21 was characterized by 1H NMR, LC-MS and HPLC. 'H NMR (D20),
S: 7.34-7.49 (m, 5H), 5.04 (s, 1H), 3.86-3.98 (m, 2H), 3.58-3.72 (m, 2H), 3.54
(t,
J=6.22 Hz, 2H), 3.14-3.39 (m, 4H), 2.92-3.07 (m, 2H). LC-MS, (M+1), 264. HPLC
(>95%, retention time, 0.98 min).
[00305] Preparation of lead analogs of a number of compounds of the present
application is illustrated in the general Scheme 2 below:
Scheme 2
o O
N OH O / NHR acid NHR
+ Pd\TH ~N N ~N N
ON N HCl or formate
01 N N 0 N
[00306] In such a synthesis strategy, the coupling agent can be HATU or HBTU.
The acid used to remove a protection group such as Boc can be 4 M HCl in MeOH
or 4M HCl in water.
[00307] Preparation of additional compounds of the present application can be
illustrated in the general Scheme 3 below:
Scheme 3
O O O O O
N OH EtOH N N r% Et RNH O N NHR
-' ~I
/>
N
O N H2SO4 O N N
O N N
[00308] In this synthesis, the starting material acid is first converted to an
ester.
Then the ester is reacted with an amine to afford an amide compound. The amide
compound may undergo further transformation, such as, reductive amination to
afford the final compound.
-89-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Example 22: Preparation of Compound 22
O O
O OH O
+ H2N-\__\ HBTU/DIEA N N H~
N
O N N DMF O N N
Compound 22
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 acid 238.2 1 1.0 238 mg
2 DCM (anhydrous) 5mL
3 DIEA, d=0.742 129.25 2.0 2.0 0.35 mL
4 HBTU 379.25 1 1.0 379 mg
Amine, d=0.871 89.14 1 1.0 0.1 mL
[00309] To a solution of acid in DMF were added HBTU, DIEA, and amine in
that order. The reaction mixture was stirred over night at room temperature.
[00310] After the reaction was complete (LC-MS and TLC), brine and saturated
sodium bicarbonate were added to quench the reaction. 10% MeOH in DCM was
used to extract the aqueous layer (3X). The DCM layer was separated, combined
and washed with brine, dried with anhydrous Na2SO4, filtered, and
concentrated.
The Compound 22 was obtained after silica gel column chromatography as an off-
white solid (138mg). Compound 22 was characterized by 'H NMR, LC-MS and
HPLC. 'H NMR (D20), 8: 7.90 (s, 2H), 5.00 (s, 2H), 3.46 (s, 3H), 3.40 (t,
J=6.40
Hz, 2H), 3.19-3.28 (m, 8H), 1.65-1.75 (in, 2H). LC-MS, (M+1), 310. HPLC (>95%,
retention time, 5.76 min).
Example 23: Preparation of Compound 23
0
O O
0 0 0 ('\
OH HATU/DIEA N H HCI N N
XI~N H) HCOOH
DMF
ON O N NBoc prep HPLC O N N NH
A23a
Compound 23
-90-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Preparation of Intermediate A23a
S.No. Chem' icalslReagents & Solvents MW mmol Eq. Amts
1 acid 238.2 1 1.0 238 mg
2 DMF (anhydrous) 5mL
3 DIEA, d=0.742 129.25 2.0 2.0 0.35 mL
4 HATU 380.2 1 1.0 380 mg
Amine 188.271 1 1.0 0.2 mL
[00311] To a solution of acid in DMF were added HATU, DIEA, and amine in
that order. The reaction mixture was stirred for 1 hr at room temperature.
[00312] After the reaction was complete (LC-MS and TLC), brine and saturated
sodium bicarbonate were added to quench the reaction. 5% MeOH in DCM was
used to extract the aqueous layer (3X). The DCM layer was separated, combined
and washed with brine, dried with anhydrous Na2SO4, filtered, and
concentrated.
The compound was further purified by prep-HPLC (150mg).
Preparation of Compound 23
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A23a 145 mg
2 4 M HCl in H2O 4mL
[00313] Cold HCl solution was added to the residue of C9a. The reaction
mixture
was stirred for 3 hours at room temperature.
[00314] After the reaction was complete (LC-MS), the mixture was concentrated.
It was further purified on prep-HPLC to afford the final compound 23 (73mg).
Compound 23 was characterized by 'H NMR, LC-MS and HPLC. 'H NMR (D20),
6: 7.89 (s, 1H), 4.99 (s, 2H), 3.45 (s, 3H), 3.25 (t, J=6.58 Hz, 2H), 3.22 (s,
3H), 2.96
(t, J=7.66 Hz, 2H), 2.60 (s, 3H), 1.76-1.86 (m, 2H). LC-MS, (M+1), 309. HPLC
(>95%, retention time, 1.20 min).
Example 24: Preparation of Compound 24
0 0 HCOOH
O 2HC1 O ( N,..
N N OH H2N N HATU/DIEA /-HN
OT-N N + \
DMF O--:~N N )
Compound 24
-91-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
SNo. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 acid 238.2 1 1.0 238 mg
2 DMF (anhydrous) 8 mL
3 DIEA, d=0.742 129.25 2.0 2.0 0.7 mL
4 HATU 380.2 1 1.0 380 mg
Aniline 209.12 1 1.0 209 mg
[00315] To a solution of acid in DMF were added HATU, DIEA, and aniline in
that order. The reaction mixture was stirred for 2hr at room temperature.
[00316] After the reaction was complete (LC-MS and TLC), the compound was
purified by prep-HPLC (250mg). Compound 24 was characterized by 1H NMR,
LC-MS and HPLC. 1H NMR (CDC13), S: 9.25 (s, 1H), 7.77 (s, 1H), 7.15 (t, J=8.14
Hz, 1H), 7.05 (s, 1H), 6.74-6.80 (m, 1H), 6.46-6.53 (m, 1H), 4.95 (s, 2H),
3.61 (s,
3H), 3.46 (s, 3H), 2.93 (s, 6H), 1.59 (s, 3H). LC-MS, (M+1), 357. HPLC (>95%,
retention time, 5.79 min).
Example 25: Preparation of Compound 25
N O H2N EtOH O N H HCO2H
O N N N 110 C O N N
Compound 25
.S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 ester 266.25 2.5 1 660 mg
2 3-piperidin-1-yl-propylamine 142.24 3.5 1.4 0.5 mL
3 EtOH 3 mL
[00317] A mixture of ester (prepared by refluxing 18g of Theophylline-7-acetic
acid in 300 ml anhydrous EtOH with 1 mL concentrated H2SO4 as a catalyst) and
3-
piperidin- 1-yl-propylamine was suspended in anhydrous ethanol and sealed in a
high
pressure bottle. The reaction mixture was heated up to 110 C with stirring
for 2
hours.
[00318] LC-MS showed the completion of the reaction. 10 mL methanol was
added and the solution was subjected to prep-HPLC separation (200mg).
Compound 25 was characterized by 1H NMR, LC-MS and HPLC. 'H NMR (D2O),
S: 8.34 (s, 1H), 7.90 (s, 1H), 4.98 (s, 2H), 3.40-3.48 (m, 2H), 3.39 (s, 3H),
3.25 (t,
J=6.48 Hz, 2H), 3.17 (s, 3H), 3.00-3.07 (m, 2H), 2.78-2.88 (m, 2H), 1.53-1.93
(m,
-92-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
7H), 1.31-1.45 (in, 1H). LC-MS, (M+1), 363. HPLC (>95%, retention time, 1.91
min).
Example 26: Preparation of Compound 26
0 0 O
0 0 0 /A
N OEt EtOH \N N H HCOH N N H- J
-(
N H2N NH2 ~~ NH NaCNBH3 0) I N HCOOHN
O N 2 -
O N
A26a Compound 26
Preparation of Intermediate A26a
S.No.' Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Ester 266.2 2.0 1.0 532 mg
2 EtOH, absolute 10 mL
3 1,2 cyclohexane diamine, 114.19 2.25 1.12 270 uL
d=0.95
[00319] The ester and the diamine were suspended in EtOH. The reaction
mixture was refluxed for 2 hours.
[00320] The mixture was concentrated and purified on prep-HPLC. The
compound was triturated with MeOH-Et2O four times to remove trace amount of
diamine (140mg).
Preparation of Compound 26
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A26a 344 0.4 1.0 136 mg
2 HCOH (aq., 37%) 4 mL
3 NaCNBH3 62.84 0.82 2 51 mg
[00321] A26a was dissolved in aqueous HCOH and NaCNBH3 was added. The
reaction mixture was stirred for 2 hours at RT.
[00322] The mixture was concentrated. It was purified on prep-HPLC to afford
the Compound 26 (97mg). Compound 26 was characterized by 'H NMR, LC-MS
and HPLC. 1H NMR (D20), 6: 8.31 (s, 1H), 7.89 (s, 1H), 4.98 (s, 2H), 3.66-3.78
(m,
1 H), 3.47 (s, 3H), 3.18-3.27 (m, 4H), 2.72-2.80 (m, 6H), 2.17-2.25 (m, 1 H),
1.11-
2.04 (m, 8H). LC-MS, (M+1), 363. HPLC (>95%, retention time, 1.95 min).
-93-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Example 27: Preparation of Compound 27
O
O
N N NHCI
i N''
O N N
Compound 27
[00323] Compound 27 (180mg) was prepared by the same method as Compound
26 using an appropriate starting material in place of 1,2-cyclohexane diamine.
The
only difference was that the reaction was carried out at 130 C for 6 hours,
and the
final formic acid salt was converted to HCl salt by co evaporating with 50 mL
of
1.25N HCl methanol solution three times. Compound 27 was characterized by 1H
NMR, LC-MS and HPLC. 111 NMR (D20), 8: 7.85 (s, 1H), 5.19-5.38 (m, 3H), 4.41-
4.50 (m, 1H), 4.00-4.08 (m, 1H), 3.46-3.53 (m, 1H), 3.45 (s, 3H), 3.18-3.28
(m, 4H),
2.80 (s, 6H), 2.68-2.78 (m, 1H), 2.04-2.21 (m, 2H), 1.73-1.86 (m, 1H), 1.53-
1.66 (m,
11-1). LC-MS, (M+1), 349. HPLC (>95%, retention time, 1.71 min).
Example 28: Preparation of Compound 28
0
NCl
O-; NI N H N'
Compound 28
[00324] Compound 28 (130 mg) was prepared by the same method as Compound
27 using a derivatized amine in place of 1,2-cyclohexane diamine, and the
final
formic acid salt was converted to HCl salt by co evaporating with 50 mL 1.25N
HCl
methanol solution three times. Compound 28 was characterized by 'H NMR, LC-
MS and HPLC. 'H NMR (D20), 8: 7.89 (s, 1H), 4.99 (s, 2H), 3.97-4.05 (m, 2H),
3.64-3.75 (m, 2H), 3.40-3.47 (m, 5H), 3.16-3.29 (m, 7H), 3.03-3.16 (m, 4H),
1.83-
1.94 (m, 2H). LC-MS, (M+1), 365. HPLC (>95%, retention time, 1.0 min).
Example 29: Preparation of Compound 29
O /
1--OEt HZN/--\--NH2 -N N H
N I N\ i> HCOOH
// O N
( NH2
O N N EtOH N
Compound 29
-94-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Ester 266.2 1.0 1.0 266 mg
2 EtOH, absolute 5 mL
3 Diamine 74.13 1.0 1.0 84 uL
[00325] Ester and damine were suspended in EtOH. The reaction mixture was
refluxed for 2 hours.
[00326] After the reaction was complete (LC-MS), the mixture was concentrated.
It was purified on prep-HPLC to afford Compound 29. The final compound was
triturated with MeOH-Et2O four times to remove trace amount of damine (155mg).
Compound 29 was characterized by 1H NMR, LC-MS and HPLC. 'H NMR (D20),
6: 8.33 (s, 1H), 7.87 (s, 1H), 4.99 (s, 2H), 3.44 (s, 3H), 3.24 (t, J=6.66 Hz,
2H), 3.20
(s, 3H), 2.91 (t, J=7.62 Hz, 2H), 1.74-1.82 (m, 2H). LC-MS, (M+1), 295. HPLC
(>95%, retention time, 1.12 min).
Example 30: Preparation of Compound 30
O 2HC1
~(O ~ THE
CI+ H2N~~N\--r ~ND H
CI/ Na.N~ '
(JN
Compound 30
S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 Chloroacetyl chloride 112.94 10 1.0 0.8 mL
2 3-(Dimethylamino)-1- 102.18 10 1.0 1.2 mL
propylamine
3 imidazole 68.08 10 1.0 680 mg
4 NaH (60%) 24.00 10 1.0 400 mg
THE 40 mL
[00327] To a solution of chloroacetyl chloride in THE was added 3-
(dimethylamino) -1-propylamine at 0 C with stirring for 20 minutes. LC-MS
showed the formation of amide. To this solution, imidazole sodium salt in THE
(prepared from imidazole and NaH in 10 mL THF) was added. The reaction was
continued for 2 hours with stirring.
[00328] After the reaction was complete (LC-MS), THE was removed. The
residue was dissolved in methanol and subjected to prep-HPLC separation. The
final
compound was still not pure at this stage. Then it was purified again by ISCO
C 18
reverse phase chromatography to give the pure final product. The product was
-95-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
converted to HCl salt by evaporating together with HCl/methanol (1N) solution
(100mg). Compound 30 was characterized by 1H NMR, LC-MS and HPLC. 'H
NMR (D20), S: 8.71 (s, 11-1), 7.36-7.48 (m, 2H), 5.02 (s, 2H), 3.25 (t, J=6.80
Hz,
2H), 3.04-3.10 (in, 2H), 2.78 (s, 6H), 1.83-1.92 (in, 2H). LC-MS, (M+1), 211.
HPLC (>95%, retention time, 1.01 min).
Example 31: Preparation of Compound 31
O O
O+ H N N c THE N N HC1 N H 2HC1
C CI Na. N,.~ ~ N-'~ I N-Boc NH
C"/N A31a Compound 31
Preparation of intermediate A31 a
[00329] Intermediate A31a (250mg) was prepared similarly to Compound 30.
Preparation of Compound 31
'S.No. Chemicals/Reagents & Solvents MW mmol Eq. Amts
1 A31 a 296.37 250 mg
2 Methanol 50 mL
3 12N HCl 25 mL
[00330] Intermediate A3 1 a was dissolved in methanol and HCl aqueous solution
was added and the reaction was stirred for 2 hours.
[00331] After the reaction was complete (LC-MS), the mixture was concentrated.
The residue was dissolved in water and subjected to prep-HPLC separation.
After
removal of solvent, the formic acid salt was converted to HCl salt by
coevaporating
with 50 mL 1.25 HCl methanol solution three times (200mg). Compound 31 was
characterized by 'H NMR, LC-MS and HPLC. 1H NMR (D2O), 8: 8.70 (s, 11-1),
7.33-7.46 (m, 2H), 5.01 (s, 2H), 3.25 (t, J=6.84 Hz, 2H) 2.95 (t, J=7.76 Hz,
2H),
2.60 (s, 3H), 1.77-1.87 (m, 2H). LC-MS, (M+1), 197. HPLC (>95%, retention
time,
0.99 min).
Example 32: Preparation of enantiomerically pure 2-amino-3-methyl-N-(2-
morpholino-ethyl)-pentanamide
[00332] 2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide can be prepared
by a method shown in Scheme 4 below. First, 2-aminoethanol (Compound 1E) is
transformed to its derivative with a leaving group (Compound 2E). Examples of
the
leaving group include halides and alkoxy or other activated hydroxyl group.
-96-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Second, Compound 2E reacts with morpholine at a neutral or basic condition to
yield 2-morpholinoethanamine (Compound 3E). The aforementioned two steps may
also be performed continuously as one step with Compound 2E being generated in
situ. For example, Compound 3E can be prepared from Compound 1E directly
through a Mitsunobu reaction wherein the hydroxyl group of Compound 1E is
activated by diethyl azodicarboxylate (DEAD) before morpholine is added. The
final product, 2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide (Compound
5E), can be obtained by coupling 2-morpholinoethanamine with 2-amino-3-
methylpentanoic acid (Compound 4E) via a peptide coupling agent. Examples of
the peptide coupling agent include 1,1'-carbonyldiimidazole (CDI),
hydroxybenzotriazole (HOBT), 1,3-dicyclohexylcarbodiimide (DCC), 1-
hydroxybenzo-7-azatriazole (HOAt), and the like.
Scheme 4:
H2N,-,,_,OH _ H2NLG
LG: a leaving group
1E 2E
r---~O rO
OH + H2NN,,_) N--, N
4E NH2 3E NH2 5E
[00333] A chiral 2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide
(Compound 5E) can be obtained by using the corresponding chiral 2-amino-3-
methylpentanoic acid (Compound 4E) in the above coupling step. For example,
(2S,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; (2R,3R)-2-amino-
3-methyl-N-(2-morpholinoethyl)-pentanamide; (2R,3 S)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide; and (2S,3R)-2-amino-3-methyl-N-(2-
morpholinoethyl)-pentanamide can be obtained by using (2S,3S)-2-amino-3-
methylpentanoic acid, i.e., L-isoleucine; (2R,3R)-2-amino-3-methylpentanoic
acid,
i.e., D-isoleucine; (2R,3S)-2-amino-3-methylpentanoic acid, i.e., D-
alloisoleucine;
and (2S,3R)-2-amino-3-methylpentanoic acid, i.e., L-alloisoleucine,
respectively.
[00334] The chiral purity, also known as, enantiomeric excess or BE, of a
chiral
Compound 5E can be determined by any method known to one skilled in the art.
For example, a chiral Compound 5E can be hydrolyzed to Compound 3E and the
corresponding chiral Compound 4E. Then, the chiral Compound 4E obtained
-97-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
through hydrolysis can be compared with a standard chiral sample of Compound
4E
to determine the chiral purity of the chiral Compound 5E. The determination
can be
conducted by using a chiral HPLC.
[00335] Each of the isomers demonstrated potent neurotrophic activity,
according
to the measurement of activity relative to BDNF, the details of which are
disclosed
below.
Example 33: Measurement of Activity Relative to BDNF
[00336] Compounds of the present application were tested for their ability to
prevent the degeneration of hippocampal neurons as described in Massa et al J
Neurosci. (2006) 26(20):5288-300. In brief, hippocampal neurons were isolated
from embryological day 16 mice and seeded in 96-well tissue culture plates
under
conditions in which they degenerated in the absence of neurotrophin receptor
ligands. Neuronal degeneration was assessed using morphological criteria 48
hours
following cell seeding. The neurotrophins brain-derived neurotrophic factor
(BDNF)
and nerve growth factor (NGF) served as positive controls. The maximum cell
death
preventing activity of BDNF is defined as 100% neurotrophic activity. The
efficacy
of NGF is 80% of that of BDNF. The neurotrophic activity of the test compounds
at
each applied concentration was quantitated in terms of a percentage of the
maximum
BDNF-supported survival level. In the presence of culture medium (CM) and the
absence of BDNF or compounds, survival is approximately 40% of the BDNF
maximum effect and this is regarded as baseline survival. For each compound,
dose-response curves were generated and the EC50 and maximum survival
percentage are derived. The compounds prepared and characterized as disclosed
herein showed an EC50 between about 1 nM and about 25 nM as well as a maximum
efficacy between about 20% and about 100% of that of BDNF.
Materials and Methods for Examples 34-40
Computational studies
[00337] Computational studies were performed using the Accelrys Catalyse and
InsightlI systems obtained from Accelerys (San Diego, California, United
States of
America).
Antibodies and Proteins
[00338] Polyclonal rabbit anti-NGF antibody was obtained from Chemicon
(Temecula, California, United States of America). Monoclonal anti-phospho-
ERKT202/''204, polyclonal anti-ERK42/44, monoclonal anti-phospho-AKTS473,
-98-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
polyclonal anti-AKT, polyclonal anti-phospho-NFxB-p65(Ser161), and site-
specific
polyclonal anti-TrV490 were obtained from Cell Signaling Technology, Inc.
(Beverly, Massachusetts, United States of America). Monoclonal anti-NFKB-p65
was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, California,
United
States of America). Monoclonal anti-actin was obtained from Sigma-Aldrich
Corp.
(St. Louis, Missouri, United States of America). Polyclonal TrkA and TrkB
antibodies were obtained from Upstate USA, Inc. (Charlottesville, Virginia,
United
States of America). Anti-pan-Trk 1087 and 1088 were previously characterized
(Zhou, Holtzman, D.M., Weiner, R.I., Mobley, W.C. (1994) Proc Natl Acad Sci
USA 91, 3824) and obtained from Dr. William C. Mobley (Stanford University,
California, United States of America). p75NTR polyclonal rabbit antibodies
9651
(Huber, L.J., Chao, M.V. (1995) Dev Bio 167, 227-238) and 9650 raised against
the
neurotrophin-binding region (residues 43-161, cysteine repeat regions II, III,
and IV)
of the extracellular domain of recombinant p75NTR were provided by Dr. Moses
Chao (Skirball Inst., NYU, New York, United States of America). Recombinant
human NGF was obtained from Invitrogen (Carlsbad, California, United States of
America) and BDNF from Sigma-Aldrich (St. Louis, Missouri, United States of
America). P75NTR/Fc and TrkA/Fc chimerae were obtained from R&D Systems
(Minneapolis, Minnesota, United States of America). Furin resistant pro-NGF
was
prepared as previously described (Beattie, M.S. et al. (2002) Neuron 36, 375-
386).
Neural Bioassays
[00339] Hippocampal neurons were prepared from E16-17 mouse embryos as
previously described (Yang, T. et al. (2003) J Neurosci 23, 3353-3363). Low
density cultures were initiated in poly-L-lysine coated A/2 plates by adding
25 l of
cell suspension (2000 neurons/well; 12,500 cells/cm2), 25 1 of DMEM
containing
10% FBS, and different concentrations of recombinant BDNF, NGF, or p75-binding
compounds to each well. For studies employing p75NTR+/+ and p75NTR.- neurons,
mice carrying a mutation in exon 3 of the p75NTR gene (Lee, K.F. et al. (1992)
Cell
69, 737-749) were bred onto a B6 congenic background (>10 B6 backcrosses).
[00340] After 48 hours in culture, cell survival was assessed as previously
described (Longo, F.M., Manthorpe, M., Xie, Y.M., Varon, S. (1997) J Neurosci
Res 48, 1-17) using a combination of standard morphological criteria along
with
visual determination of whether a given cell converted MTT to its blue
formazan
product. The number of surviving neurons was determined by counting the total
-99-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
number of cells in each well that were both morphologically intact and filed
with
blue product (Longo F.M., Manthorpe, M., Xie, Y.M., Varon, S. (1997) JNeurosci
Res 48, 1-17). For each neurotrophin or compound concentration, duplicate
wells
were counted and the resulting values averaged. Activity of each compound was
confirmed by blinded counts. Counts were normalized to survival achieved with
25
ng/ml BDNF or to baseline survival. Fitting of dose-responsive curves was
performed with Sigmaplot obtained from SYSTAT Software Inc. (Richmond,
California, United States of America).
[00341] For signaling pathway inhibitor studies, LY294002, PD98059 (obtained
from EMD Biosciences/Calbiochem, San Diego, California, United States of
America), and SN50 (obtained from Alexis Corp., Lausen, Switzerland) were
added
to cultures at final concentrations of 25 M, 50 M, and 2.5 g/ml
respectively,
concomitantly with BDNF, NGF, or p75-binding compounds. For antibody
inhibition studies, p75NTR antisera and control non-immune serum were used at
a
final dilution of 1:100 in the presence of BDNF, NGF, or p75-binding
compounds.
For all studies applying signaling inhibitors, p75NTR antibodies or p75NTR-1-
neurons,
survival was assessed at 48 hours.
Protein Extraction and Western Blot Analysis
[00342] For assays of Trk, AKT, NFiB, and ERK activation, hippocampal
neurons derived from E16-17 mice were cultured in poly-L-lysine coated six-
well
plates (Corning, Inc., Corning, New York, United States of America) in DMEM
containing 10% FBS, followed by incubation in serum-free DMEM for 2 hours
before addition of neurotrophins or compounds. At the indicated time points,
neurons were harvested in lysis buffer consisting of: 20 mm Tris, pH 8.0, 137
mM
NaCl, 1% Igepal CA-630, 10% glycerol, 1 mM PMSF, 10 g/ml aprotinin, 1 g/ml
leupeptin, 500 M orthovanadate (Zhou, J., Valletta, J.S., Grimes, M.L.,
Mobley,
W.C. (1995) JNeurochem 65, 1146-1156).
[00343] Lysates were centrifuged, the supernatant collected, and protein
concentrations determined using the BCA Protein Assay Reagent obtained from
Pierce (Rockford, Illinois, United States of America). Western blots were
performed as described previously (Yang, T. et al. (2003) J Neurosci 23, 3353-
3363). Western blot signals were detected using the ECL Chemiluminescence
System obtained by Amersham (Piscataway, New Jersey, United States of America)
(Yang, T. et al. (2003) JNeurosci 23, 3353-3363).
-100-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
NGF Displacement from p75NTR and TrkA
[00344] NGF ELISA was performed as previously described (Longo, F.M. et al.
(1999) JNeurosci Res 55, 230-237). Briefly, 96-well plates were incubated with
0.1
pmol (at 1 nM) of p75/Fc or TrkA/Fc recombinant protein obtained from R&D
System (Minneapolis, Minnesota, United States of America) overnight at 4 C
followed by incubation with blocking buffer for 1 hour at room temperature.
ProNGF at 100 ng/ml or different concentrations of NGF, and p75-binding
compounds were diluted in sample buffer, added to the wells, and incubated for
6
hours with shaking at room temperature. Plates were then washed five times
with
Tris-buffered saline (TBS) containing 0.05% Tween-20 and incubated with anti-
NGF rabbit polyclonal antibody overnight at 4 C. Following five washes with
TBS,
wells were incubated for 2.5 hours at room temperature with anti-rabbit IgG
HRP
conjugate and washed five times. 3,3',5,5'-tetramethyl-benzidine substrate was
added and the optical density measured at 450 nm.
p75NTR Antibody Competition
[00345] NIH3T3 fibroblasts expressing either null vector or p75NTR (Huang,
C.S.
et al. (1999) J Biol Chem 274, 36707-36714) were obtained from Dr. William
Mobley (Stanford University, California, United States of America). Cells were
grown in monolayers, harvested in PBS with 2 mM EDTA, pelleted, and
resuspended in ice-cold DMEM HEPES with 1 mg/ml BSA. 6-9 x 106 cells from
one confluent 6-well plate were used for each experimental point.
[00346] For binding analysis, p75NTR antibody (1:100) was allowed to bind in
the
presence or absence of 100 nM p75-binding compounds for 90 minutes at 4 C with
gentle rotation, followed by four washes in PBS. The final cell pellet was
resuspended in lysis buffer.
[00347] Western blots were performed as described above. To detect the
presence of p75NTR antibody, blots were probed with horseradish peroxidase-
linked
goat anti-rabbit IgG obtained from Amersham/Pharmacia Biotech (Piscataway, New
Jersey, United States of America). Signals were detected by the ECL
chemiluminescence system obtained from Amersham Biosciences (Piscataway, New
Jersey, United States of America). To control for variation in protein
loading, the
blot was stripped and reprobed with (3-actin monoclonal antibody obtained from
Sigma (St. Louis, Missouri, United States of America).
-101-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Oligodendrocyte Culture, Pro-Neurotrophin Treatment and Cell Death
Assay
[00348] Cortical oligondendrocytes from rat pups were prepared as previously
described (Yoon et al. (1998) J Neurosci 18, 3273-3281, Harrington, A.W., Kim,
J.Y., Yoon, S.O. (2002) J Neurosci 22, 156-166). Cells were treated with
recombinant, cleavage-resistant proNGF at 0.05 nM (2.8 ng/ml). Controls were
treated with equivalent volumes of proNGF purification buffer containing 350
MM
imidazole. 24 hours after treatment, the cells were fixed and processed for
MBP and
TUNEL staining as previously described (Beattie, M.S. et al. (2002) Neuron 36,
375-386). 200-250 cells were counted per well, for a minimum of 600 cells per
experimental condition.
Example 34: Computational Modeling, Pharmacophore Generation, Virtual
and Functional Screening
[00349] In order to generate a productive pharmacophore emulating a loop
structure likely to interact with a receptor, it was hypothesized that (1) the
degrees of
freedom of the ligand peptide structure are restricted by its residence in the
protein,
and (2) there is little "induced fit" involving changes in loop structure at
the targeted
receptor subsite, or it is accommodated by flexibility of the small molecule
ligand.
When both of these conditions apply, they allow an interacting/activating
small
molecule conformation that interacts with the receptor in a manner similar to
that of
the native ligand.
[00350] Computational studies of active dimeric cyclic peptides mimicking NGF
(3-hairpin loops suggest that energetic and structural constraints would
disallow
simultaneous (3-hairpin folding of both peptide subunits, implying that the
peptides
act in a monomeric fashion. Additionally, based on early virtual screening of
3D
conformer libraries, the presence of many functionally dimeric non-peptide
molecules having a molecular weight of less than 500D was unlikely.
[00351] Therefore, efforts were focused on locating compounds emulating a
single loop 1 structure. Compounds were selected for screening in cell
survival
assays using the protocol outlined in Figure 1. Computational studies
suggested that
in situ, the tethered loop 1 backbone and proximal portions of the side chain
structure had restricted degrees of freedom, and an intermediate structure
chosen
from an ensemble of samples from loop molecular dynamics simulations was
extracted and used to build a novel pharmocophoric model (Figure la). Guidance
-102-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
for the placement of pharmacophore features was obtained from consideration of
loop phylogeny, side chain chemistry, and the inventors' experience with
synthetic
active peptides.
[00352] As a first approximation, it was assumed that analogous loop 1 domains
from neurotrophins of different species and different neurotrophin family
members
should bind similarly to p75NTR The primary structure of BDNF loop 1 diverged
significantly from NGF and NT-3, and in efforts to reduce pharmacophore
complexity, only the latter two were utilized. The propensity of histidine to
act as a
hydrogen bond donor (McDonald, I, Thornton, J.M., (1994) WWW Edition
December 1994) suggested its use at position 34, while the K to R transition
at
position 32 suggested the use of a positively ionizable feature at that
location
(Figures lb and 1c).
[00353] An average of 35 conformers of each of over 800,000 compounds were
screened against the novel pharmacophore, yielding approximately 800 that fit
with
a calculated internal energy of less than 10 kcal/mol (Figure 1 d). This
number was
reduced to approximately 60 by visual inspection on the basis of likely steric
compatibility with a hypothetical shallow receptor binding pocket, and maximal
flexibility of the functional groups. 35 compounds were obtained initially, of
which
23 were soluble in water.
[00354] In preliminary studies using a chicken DRG neuronal survival assay, 4
of
23 compounds tested showed significant activity. Further analysis was carried
out
using embryonic mouse hippocampal neurons. Under conditions of low density and
lack of glial support, the survival of these neurons was dependent, in part,
on the
addition of neurotrophins to the cultures.
[00355] Screening of 23 previously tested compounds in these hippocampal
cultures demonstrated activity of three of the compounds (Compounds( ii-iv))
identified as having activity using DRG cultures, and also identified fourth
and fifth
active compounds (Compounds (i) and (vii)) through DRG and hippocampal assays.
The results corresponded to a 17% yield for the screening procedure. In
further
support for this method, in preliminary studies based on models of NGF loop 4
(LM14A), a high positive rate of identification of active compounds was
achieved (3
positive of 8 compounds screened (37%)).
[00356] A regression analysis of NGF-p75 NTR binding in the presence of the
p75-
binding compounds was performed. The data was fit using the Nonlinear
-103-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Regression module of Sigmaplot, to a modification of the Gadduin/Schild
equation
(adapted from Motulsky, H.J., and Christopoulos, A. (2003) A Practical Guide
to
Curve Fitting, 2nd edn. (San Diego, California, GraphPad Software, Inc.):
Signal top - bottom + bottom
= ([%1ogA] s flcnsiop~ 1+ 101ogEC50 1+ Z
[NGF]
where, top = maximal signal; bottom = basal signal; [C] = compound
concentration;
S = Schild coefficient; HillSlope = Hill coefficient; [NGF] = NGF
concentration,
EC50 = concentration of NGF resulting in 50% maximal (top-bottom) binding; and
A2 = concentration of compound resulting in a doubling of the EC50 from the
unshifted curve. Herein, the calculated Hill slope ranged from 1.0 to 1.6 and
was
generally not significantly different from 1.
Example 35: Compounds Promote Hippocampal Neuron Survival
[00357] High-throughput virtual screening based on neurotrophin loop 1 models
and small-scale in vitro bioassays were used to identify chemically diverse
compounds with potent neurotrophic activity (Figure 1). Approximately 800,000
compounds were screened in silico to produce a high yield of 4 positives out
of 23
compounds submitted to in vitro screening (17%).
[00358] In order to understand the mechanisms of action of the selected
compounds and test the conjecture that they work via the targeted receptor,
p75NTR,
the dose-dependent relationships of the survival-promoting activities of the
p75-
binding compounds compared to NGF and BDNF using embryonic hippocampal
neurons in culture conditions in which NGF promotes neural survival were
examined. In the cultures, neurotrophic activity was mediated by BDNF
principally
through TrkcB and p75NTR, and by NGF primarily through p75NTR, as they express
little TrkA (Brann, A.B., et al. (1999) JNeurosci 19, 8199-8206; Bui, N.T., et
al.
(2002) JNeurochem 81, 594-605).
[00359] Addition of Compounds (i-iv) (Figure 2a, structures) increased the
number of GAP-43 positive neurite-bearing cells, consistent with increased
neuronal
survival (Figure 2a, photomicrographs). Dose-response profiles of the active
compounds (Figure 2b) demonstrated EC50 values in the range of 100-300 pM and
intrinsic activities 80-100% of the NGF response. Independently synthesized
-104-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
preparations of Compound (iii) and Compound (iv) showed similar results.
Compound (v), a compound structurally similar to Compound (iii), showed little
or
no neurotrophic activity. The structures of Compounds (i-iv) are illustrated
in Table
I.
[00360] For each compound, at concentrations greater than 5 nM, survival was
reduced to baseline or below (Figure 2b) as a result of mechanisms unrelated
to
p75NTR, or from modulation of receptor multimer formation and survival
signaling at
higher receptor occupancies, even by a compound without neurotrophic activity
(e.g., Compound (v)). Response curves similar to that of NGF in the
hippocampal
neuron system are consistent with activation of survival signaling through the
NGF
binding region of p75NTR
[00361] Of the four compounds initially identified, the two (Compound (iii), a
derivative of caffeine, and Compound (iv), an amino acid derivative) were
predicted
to have the most "drug-like" character by the Lipinski criteria (Lipinski,
C.A. (2000)
J Pharm Toxicol Methods 44, 235-249) and blood-brain barrier calculations (Fu,
X.C., Chen, C.X., Liang, W.Q., Yu, Q.S. (2001) Acta Pharmacol Sin 22, 663-668;
Clark, D.E. (2002) J Pharm Sci 88, 815-821) were selected for more detailed
mechanistic study. Compound (iv) was prioritized, as preliminary studies
indicated
that it exhibits significant oral uptake and blood-brain barrier penetration.
The
relatively inactive Compound (v) was chosen as a negative control due to its
structural similarity to Compound (iii) (Figure 2a).
Example 36: Compounds Interact with and work through D75 NT Receptors not
Trk Receptors
[00362] In order to assess the interactions of the p75-binding compounds with
neurotrophin receptors, the effects of increasing concentrations of compounds
on
NGF binding to the recombinant chimeric proteins p75NTR-Fc and TrkA-Fc were
examined. In these experiments, Compound (iv) (Figure 3a) and Compound (iii)
(Figure 3b), but not Compound (v) (Figure 3c), shifted the NGF/p75NTR-Fc
biding
curve significantly to the right. The inhibition of NGF binding caused by each
active compound was reversed with increasing NGF concentration, consistent
with a
mechanism that is, at least in part, competitive in nature. When the data was
fit to
the Gaddum-Schild equation that describes ligand binding in the presence of an
inhibitor (Motulsky, H.J., and Christopoulos, A. (2003) A Practical Guide to
Curve
Fitting, 2nd edn. (San Diego, California, GraphPad Software, Inc.)), the
resulting
-105-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Schild coefficients were significantly less than 1.0 for both active compounds
(Compound (iv), 0.58 +/- 0.04; Compound (iii), 0.26 +/- 0.01), suggesting a
more
complex model, e.g., multiple ligand-receptor binding sites (Lutz, M., and
Kenakin,
T. (1999) Quantitative Molecular Pharmacology and Informatics in Drug
Discovery
(Hoboken, New Jersey: John Wiley & Sons); Neubig, R.R., Spedding, M, Kenakin,
T., and Christopoulos, A. (2003) Pharmacol Rev 55, 597-606).
[00363] The results are consistent with models in which the effects of the
compounds are due either to interaction with only a portion of the NGF binding
surface of the receptor, and/or to allosteric effects indirectly affecting NGF
binding.
Gaddum-Schild analysis also yields a measure of potency known as A2 (i.e., the
concentration of compound that shifts the EC50 twofold to the right) that can
be
equated with compound KD when the Schild coefficient is 1. The A2 values
derived
for Compound (iv) and Compound (iii) were 1192 +/- 1.2 and 31.6 +/- 1.3 nM,
respectively. However, since the Schild coefficients are significantly
different from
1, the true KD values are unable to be determined.
[00364] In the case of Compound (iv), the EC50 value for its biologic effect
is
approximately 150 pM, while its A2 is nearly four orders of magnitude greater.
Large differences between biologic potency of small molecules and binding
estimated by ligand displacement are common (Lutz, M., and Kenakin, T. (1999)
Quantitative Molecular Pharmacology and Informatics in Drug Discovery
(Hoboken, New Jersey: John Wiley & Sons)), and may have several causes,
including: differences between receptor states in binding versus functional
assays;
post-receptor signal amplification, such that maximal biologic effects are
seen at
very low receptor occupancies; partial displacement of a multivalent ligand by
a
smaller antagonist; and that the compound works through a mechanism
independent
of the targeted receptor. The latter possibility was addressed using p75NTR
blocking
antibody and p75NTRa- neurons to assess p75NTR dependence. Additionally, the
specificity of p75-binding compounds for p75NTR is supported by the finding
that the
active compounds have no effect on NGF binding to TrkA (Figures 3d, 3e).
[00365] It was then determined that p75-binding compound activity is p75NTR-
dependent. Ab 9651, previously shown to block neurotrophic activity of NGF and
NGF loop 1 peptide mimetics in mouse dorsal root ganglion neurons (Longo,
F.M.,
Manthoipe, M., Xie, Y.M., Varon, S. (1997) J Neurosci Res 48, 1-17), partially
blocked the neurotrophic activity of BDNF (Figure 3g) and completely blocked
the
-106-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
neurotrophic activity of Compound (iii) and Compound (iv), while non-immune
antibody had no effect. Another independently-derived rabbit polyclonal anti-
p75NTR antibody (Ab 9650) gave virtually identical results, further
corroborating the
specificity of the p75NTR blockade. Neither of the antibodies produced changes
in
baseline survival, suggesting that in these cultures the antibody preparations
do not
promote or inhibit survival. These results are consistent with BDNF acting
through
both TrkB and p75NTR while NGF and p75-binding compounds act primarily
through p75NTR
[00366] In addition, the response of p75NTR-deficient (-/-) cells to
neurotrophins
and the p75-binding compounds were examined (Figure 3h). Baseline survival
under these culture conditions was the same in wild type and deficient cells,
while
p75NTR-deficiency was associated with partial responsiveness to BDNF, and lack
of
response to NGF and the p75-binding compounds, a pattern similar to that found
in
the p75NTR-antibody studies. Finally, treatment with 5 nM Compound (iii) or
Compound (iv) along with 50 ng/ml NGF, concentrations of each which induce a
maximal response, produced no additive effect on survival, further supporting
the
hypothesis that the p75-binding compounds act directly through binding to
p75R.
[00367] Despite the observation that Compound (iii) and Compound (iv) did not
affect NGF-TrkA/Fc binding, the question remained whether the p75-binding
compounds activate TrkB, the principal Trk on the hippocampal neurons, or the
nominally expressed TrkA, as their primary mechanism for promoting survival.
Also, ligand binding to p75NTR might influence Trk activation. With these
considerations, it was of interest to determine whether the p75-binding
compounds
promote Trk activation. Compound (iii) and Compound (iv) were assessed for the
ability to activate Trk autophosphorylation, as indicated by TrkY490
phosphorylation,
a well-established marker of Trk activation. In hippocampal cultures, BDNF
exposure resulted in robust Trk activation (Figure 3i), while no activation
was
detected with NGF or the p75-binding compounds. The lack of signal with NGF
confirms that these cultures produce little or no TrkA and supports the idea
that the
trophic effects of NGF are mediated principally by p75NTR. In 3T3-TrkA cells,
NGF
exposure produced the expected TrkA autophosphorylation response, while the
p75-
binding compounds again showed no activity (Figure 3j). These results
suggested
that activation of Trk receptors does not play a primary role in the promotion
of cell
survival by p75-binding compounds.
-107-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Example 37: Compounds work through p75NTR
[00368] Together, the displacement of NGF from p75NTR by the active
compounds, but not with an inactive compound, the dependence of biologic
function
on the presence of an unoccluded p75NTR, lack of Trk interactions and
activation and
lack of additive effects between NGF and compound, strongly suggest that the
p75-
binding compounds directly interact with and work through p75NTR
[00369] This is consistent with the pharmacophoric model used herein to select
the p75-binding compounds (Figure 1). Given fitting to this model as the
principal
initial selection criterion, the identification of a high percentage of
chemically
diverse positives from a small group tested in vitro, and their similar
actions in a
variety of biochemical and biologic assays, the evidence suggests that the p75-
binding compounds interact at a p75NTR neurotrophin binding site rather than
at
other locations in the receptor.
[00370] Pro-survival signaling associated with p75NTR actions include
activation
of P13K and AKT (Roux, P.P., Bhakar, A.L., Kennedy, T.E., Barker, P.A. (2001)
J
Biol Chem 276, 23097-23104; Lachyankar, M.B., et al. (2003) J Neurosci Res 71,
157-172), NFiB (Mamidipudi, V., Li, X., Wooten, M.W. (2002) JBiol Chem 277,
28010-28018; Carter, B.D., et al. (1996) Science 272, 542-545; Gentry, J.J.,
Casaccia-Bonnefil, P., Carter, B.D. (2000) J Biol Chem 275, 7558-7565; Foehr,
E.D., et al. (2003) JNeurosci Res 73, 7556-7563), and ERK (Lad, S.P., Neet,
K.E.
(2003) JNeurosci Res 73, 614-626). Each of these signaling intermediates has
been
shown to be capable of being independently regulated by Trk and p75NTR through
pathways with varying degrees of overlap and crosstalk, and with different
kinetics.
Treatment of hippocampal neurons with 20 nM Compound (iii) and Compound (iv)
(a concentration in the plateau range for acute signaling activation) led to
an
approximately 1.5 fold increase in NFiB-p65 phosphorylation (Figure 4a),
indicative of activation of the NFKB pathway (Sakurai, H., Chiba, H., Miyoshi,
H.,
Sugita, T., Toriumi, W. (1999) J Biol Chem 274, 30353-30356), similar in
extent
and time course to that induced by both neurotrophin proteins.
[00371] Compound (v) at the same concentration did not induce NF-KB-p65
phosphorylation. Consistent with activation of NFKB by Compound (iii) and
Compound (iv), a specific peptide inhibitor of NFxB translocation, SN50 (Lin,
Y.Z.,
Yao, S.Y., Veach, R.A., Torgerson, T.R., Hawiger, J. (1995) J Biol Chem 270,
14255-14258), significantly reduced cell survival promoted by Compound (iii),
-108-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Compound (iv) and the neurotrophins, with no effect on baseline survival
(Figure
4e).
[00372] In studies of AKT activation, Compound (iii) and Compound (iv) at 20
nM showed similar degrees of activation to that found with NGF at 30 minutes,
while BDNF induced a substantially greater response. In addition, the onset of
activation stimulated by Compound (iii) and Compound (iv) was slower than that
of
NGF (Figure 4b). Consistent with these findings, the P13 kinase inhibitor
LY294002, which inhibits AKT activation, markedly decreased survival in all
cases,
including baseline survival under conditions of no treatment or exposure to
Compound (v) (Figure 4e).
[00373] Investigation of ERK signaling showed that ERK44 activation was
induced to a greater extent by the neurotrophins than by the p75-binding
compounds
(Figure 4c), which showed a significant but small response. ERK42 activation
was
more robust overall and greater with BDNF and NGF treatment than with the p75-
binding compounds. There was a prominent loss of signal by 30 minutes but with
greater persistence of the activated form following BDNF treatment (Figure
4d).
Consistent with the finding of greater ERK activation induced by BDNF compared
to NGF and p75-binding compounds, the ERK inhibitor PD98059 significantly
decreased BDNF-stimulated survival while it had a small but significant effect
on
NGF activity, and produced no significant decrease in survival promoted by
either
Compound (iii) or Compound (iv) (Figure 4e).
[00374] These observations suggest that unlike NFKB and P13K, ERK activation
is not a significant factor in the promotion of survival by the p75-binding
compounds. The difference likely relates to the lower levels of ERK activation
observed with the p75-binding compounds relative to the protein ligands. P13K
activation can promote survival through pathways involving and not involving
AKT
(Zhang, Y., et al. (2003) J Neurosci 23, 7385-7394), and so the essential
mechanisms downstream of P13K in this system remain to be determined. The
more robust activation of AKT and ERKs by BDNF likely represents the influence
of TrkB.
[00375] To further examine the relationship between compound-mediated AKT
and NFKB activation, and neural survival, compound dose-activation studies
were
performed (Figures 4f, 4g). The results demonstrate that Compound (iv) induces
activation of both AKT and NFKB over a concentration range of 0.5 nM to 3 nM,
-109-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
which is similar to that required for promotion of survival, and concordant
with a
role for these signaling mechanisms in mediating compound-induced survival.
[00376] Further, it was determined that NGF and compound activation of AKT
signaling was completely absent in cultures of p75NTRa- neurons (Figure 4h),
consistent with the hypothesis that NGF and p75-binding compounds activate AKT
survival signaling through p75NTR Together with the evidence for p75NTR
dependence of compound-induced survival (Figures 3g, 3h), these findings
suggest
that p75-binding compounds induce survival of hippocampal neurons in culture,
at
least in part, through interactions with p75NTR that produce activation of
survival-
promoting signaling pathways involving AKT and NFxB.
Example 38: Compound (iii) and Compound (iv) do not promote cell death of
mature oligodendrocytes, but inhibit pro-NGF-induced death
[00377] Though NGF and the p75-binding compounds promoted cell survival in
the hippocampal cultures used in the studies herein, liganding of p75NTR by
mature
NGF or proNGF, has been associated with cell death rather than promotion of
survival in certain cell types (Lee, R., Kerman, P, Teng, K.K., Hempstead,
B.L.
(2001) Science 294, 1945-1948; Casaccia-Bonnefil, P., Carter, B.D., Dobrowsky,
R.T., Chao, M.V. (1996) Nature 386, 716-719). To determine whether the p75-
binding compounds disclosed herein promote survival or cause death in systems
in
which neurotrophins promote cell death, the survival of mature
oligodendrocytes
treated with p75-binding compounds and proNGF was examined.
[00378] Mature oligodendrocytes express p75NTR but not TrkA, and undergo
apoptotic death on treatment with NGF or proNGF (Beattie, M.S., et al. (2002)
Neuron 36, 375-386; Casaccia-Bonnefil, P., Carter, B.D., Dobrowsky, R.T.,
Chao,
M.V. (1996) Nature 386, 716-719; Yoon, S.O., Casaccia-Bon nefil, P., Carter,
B.,
Chao, M.V. (1998) JNeurosci 18, 3273-3281). Unlike NGF or proNGF, Compound
(iii), Compound (iv) and Compound (iv) alone did not promote cell death
(Figure
5a). In addition, pro-NGF-induced cell death was significantly inhibited by
Compound (iii) and Compound (iv) over a concentration range of 1 to 10 nM, but
not by Compound (v), which appeared to decrease survival at 10 nM (Figure 5a).
[00379] In order to determine whether p75-binding compounds block proNGF
binding to p75NTR, proNGF binding to p75NTR was assessed over a concentration
range of 1500 nM to 10,000 nM. Compound (iii) and Compound (iv) inhibited
proNGF binding equally, up to an approximately 30% decrement at the highest
-110-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
concentration (Figure 5b). The high concentration required to inhibit pro-NGF
binding compared to those blocking proNGF-induced death suggest the
possibilities
that: 1) in the cell-based assay, with native receptor conformation in the
presence of
co-receptors (e.g., sortilin), the proNGF-p75 NTR interaction may be more
susceptible
than in the in vitro assay to disruption by the p75-binding compounds; 2) at
low
concentrations, the compounds qualitatively alter proNGF binding to decrease
the
induction of cell death, but do not decrease the total amount of proNGF
binding to
decrease the induction of cell death but do not decrease the total amount of
proNGF
binding; or 3) that the compounds induce preferential activation of pro-
survival
signaling by p75NTR without affecting proNGF binding. Preferential survival
pathway activation could result from differences in the way the compounds
modulate receptor structure, as well as lack of binding to co-receptors
expressed by
oligodendrocytes, such as sortilin. Indeed, prior studies suggest that
engagement of
both sortilin and p75NTR by proNGF promotes efficient ligand binding, receptor
complex activation and apoptotic actions (Nykjaer, A., Willnow, T.E., and
Petersen,
C.M. (2005) Curr Opin Neurobiol 15, 49-57).
Example 39. Compound (iii) Blocks An-Induced Neural Degeneration
[00380] Using previously well established protocols, A[3 was preincubated for
3
days in water to allow formation of oligomers. E17 hippocampal neurons were
incubated for 5 days to allow for maturation prior to addition of A[3 with
test
compounds. Mature neurons demonstrate high A13 vulnerability.
[00381] Addition of A(342_1 (30 M) as a negative control caused no cell
death.
Addition of A(31_42 at 10 M or 30 M caused an approximate 40% loss of
neurons
after a 3 day exposure (Figure 6a). The results are similar to in vitro A(3-
induced
death levels reported previously (Michaelis, M.L., et al. (2006) J Pharm Exp
Ther
312:659-668). Addition of NGF (100 pg/ml) fails to protect against AQ1-42, a
finding
previously reported (Yankner, B.A., et al. (1990) PNAS 87:9020-9023).
[00382] Addition of API-42 in the absence of compound (NC) resulted in a 40%
loss of neurons (Figure 6b). The presence of inactive Compound (v) and
Compound
(vi) failed to block A[31-42 toxicity. Addition of Compound (iii), however,
blocks
A[3-induced death with a dose-response effect and an EC50 of approximately 10
nM.
Data are expressed as percentage surviving cells over total cells present in a
given
measurement area. Mean +/- SE is shown with at least 20 areas measured per
condition over multiple bioassays. The ability of Compound (iv) to entirely
block
-111-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
A[3-induced degeneration at low nanomolar concentrations, its favorable
molecular
weight (less than 500), and a favorable Lipinski score indicate that it is a
high
priority lead compound for preclinical development in in vivo AD models.
Compound (iv) has also been shown to block A13-induced degeneration of
cortical
and septal neurons.
Example 40. Compound (iv) Prevents Hair Loss in Middle Aged Mice
[00383] In a three-month toxicology trial, the presence of age-related hair
loss
was demonstrated in 3 of 5 vehicle-treated mice and in 0 of 5 Compound (iv)-
treated
male mice. In a follow up study, 4 of 10 vehicle-treated and 0 of 9 Compound
(iv)-
treated middle-aged male mice at the 2-month time point demonstrated hair
loss.
[00384] Taken together, these studies indicate that 7 of 15 vehicle-treated,
and 0
of 14 Compound (iv)-treated mice demonstrate hair loss. The resulting p value
is
0.001 (Fisher's Exact test), supporting the presence of a significant effect
in the
preclinical studies. This data indicates p75NTR regulates the death of hair
follicle
cells and thereby the process of hair loss (known as catagen). These findings,
for the
first time, indicate the efficacy of administering small molecule compounds
targeting p75NTR for the prevention of hair loss occurring during aging or in
pathological states, such as alopecia areata.
Summary of the Examples 34-40
[00385] In targeting one member of a group of receptors that interact with a
given
ligand, activation of a subset of receptor-mediated effects which can or can
not be
naturally occurring was anticipated. Such differences in signaling patterns,
including minimizing activation of Trks, will likely prove clinically useful.
For
example, the compounds disclosed in the Examples can promote survival under
conditions where neurotrophins promote death, and can be less likely to induce
excessive sympathetic fiber sprouting and upregulation of pain transmission
occurring, likely via Trk signaling, with neurotrophin treatments (Walsh,
G.S., Krol,
K.M., Kawaja, M.D. (1999) JNeurosci 19, 258-273).
REFERENCES
Appel, S. H. (1981) Ann Neurol 10, 499.
Beattie, M.S. et al. (2002) Neuron 36, 375-386.
Brann, A.B., et al. (1999) JNeurosci 19, 8199-8206.
-112-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Bui, N.T., et al. (2002) JNeurochem 81, 594-605.
Carter, B.D., et al. (2002) Science 272, 542-545.
Casaccia-Bonnefil, P., Carter, B.D., Dobrowsky, R.T., Chao, M.V. (1996) Nature
386, 716-719.
Clark, D.E. (2002) JPharm Sci 88, 815-821.
Fahnestock, M., Michalski, B., Xu, B., Coughlin, M.D. (2001) Mol Cell Neurosci
18, 210-220.
Foehr, E.D., et al. (2003) JNeurosci Res 73, 7556-7563.
Freireich et al., (1966) Cancer Chemother Rep. 50, 219-244.
Fu, X.C., Chen, C.X., Liang, W.Q., Yu, Q.S. (2001) Acta Pharmacol Sin 22, 663-
668.
Gentry, J.J., Casaccia-Bonnefil, P., Carter, B.D. (2000) JBiol Chem 275, 7558-
7565.
Harrington, A.W. et al. (2004) Proc Natl Acad Sci USA 101, 6226-6230.
Harrington, A.W, Kim, J.Y., Yoon, S.O. (2002) JNeurosci 22, 156-166.
He, X.L., Garcia, K.C. (2004) Science 304, 870-875.
Huang, C.S. et al. (1994) JBiol Chem 274, 36707-36714.
Huber, L.J., Chao, M.V. (1995) Dev Bio 167, 227-23 8.
Lachyankar, M.B., et al. (2003) JNeurosci Res 71, 157-172.
Lad, S.P., Neet, K.E. (2003) JNeurosci Res 73, 614-626.
Lee, K.F. et al. (1992) Cell 69, 737-749.
Lee, R., Kermani, P., Teng, K.K., Hempstead, B.L. (2001) Science 294, 1945-
1948.
Lin, Y.Z., Yao, S.Y., Veach, R.A., Torgerson, T.R., Hawiger, J. (1995) JBiol
Chem
270, 14255-14258.
Lipinski, C.A. (2000) JPharm Toxicol Methods 44, 23 5-249.
Longo, F.M. et al. (1999) JNeurosci Res 55, 230-237.
Longo, F.M., Manthorpe, M., Xie, Y.M., Varon, S. (1997) JNeurosci Res 48, 1-
17.
Lutz, M., and Kenakin, T. (1999) Quantitative Molecular Pharmacology and
Informatics in Drug Discovery (Hoboken, New Jersey: John Wiley & Sons).
Maliartchouk, S., Debeir, T., and Beglova, N. Cuello, A.C., Gehring, K, and
Saragovi, H. U. (2000) JBiol Chem 275, 9946-9956.
Mamidipudi, V., Li, X., Wooten, M.W. (2002) JBiol Chem 277, 28010-28018.
McDonald, I, Thornton, J.M., (1994) WWW Edition December 1994.
-113-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
Michaelis, M.L., Ansar, S., Chen, Y., Reiff, E.R., Seyb, K.I., Himes, R.H.,
Audus,
K.L., and Georg, G.I. (2006) JPharm Exp Ther 312:659-668.
Motulsky, H.J., and Christopoulos, A. (2003) A Practical Guide to Curve
Fitting,
2"d edn. (San Diego, California, GraphPad Software, Inc.).
Neubig, R.R., Spedding, M, Kenakin, T., and Christopoulos, A. (2003) Pharmacol
Rev 55, 597-606.
Nykjaer, A. et al., (2004) Nature 427, 843-848.
Nykjaer, A., Willnow, T.E., and Petersen, C.M. (2005) Curr Opin Neurobiol 15,
49-
57.
Partridge, W.M. (2002) Adv Exp Med Bio 513, 397-430).
Podulso, J.F., Curran, G.L. (1996) Brain Res Mol Brain Res 36, 280-286.
Remington: The Science and Practice of Pharmacy, A. Gennaro, ed., 20th
edition,
Lippincott, Williams & Wilkins, Philadelphia, Pa.
Remington's Pharmaceutical Sciences, Mack Pub. Co., Easton, Pa., 1980.
Roux, P.P., Bhakar, A.L., Kennedy, T.E., Barker, P.A. (2001) JBiol Chem 276,
23097-23104.
Sakurai, H., Chiba, H., Miyoshi, H., Sugita, T., Toriumi, W. (1999) JBiol Chem
274, 30353-30356.
Salehi, A.H., et al. (2000) Neuron 27, 279-288.
Saltzman, W.M., Mak, M.W., Mahoney, M.J., Duenas, E.T., Cleland, J.L. (1999)
Pharm Res 16, 232-240.
Walsh, G.S., Krol, K.M., Kawaja, M.D. (1999) JNeurosci 19, 258-273.
Wang, J.J., Rabizadeh, S., Tasinato, A., Sperandio, S., Ye, X., Green, M.,
Assa-
Munt, N., Spencer, D., and Bredesen, D.E. (2000) JNeurosci Res 60, 587-
593.
Yang, T. et al. (2003) JNeurosci 23, 3353-3363.
Yankner, B.A., Caceres, A., Duffy, L.K. (1990) PNAS 87:9020-9023.
Yoon, S.O., Casaccia-Bonnefil, P., Carter, B., Chao, M.V. (1998) JNeurosci 18,
3273-3281.
Zhang, Y., et al. (2003) JNeurosci 23, 7385-7394.
Zhou, J., Holtzman, D.M., Weiner, R.I., Mobley, W.C. (1994) Proc Natl Acad Sci
USA 91, 3824.
Zhou, J., Valletta, J.S., Grimes, M.L., Mobley, W.C. (1995) JNeurochem 65,
1146-
1156.
-114-
CA 02754570 2011-09-06
WO 2010/102212 PCT/US2010/026372
[003861 The patents and publications listed herein describe the general skill
in the
art and are hereby incorporated by reference in their entireties for all
purposes and to
the same extent as if each was specifically and individually indicated to be
incorporated by reference. In the case of any conflict between a cited
reference and
this specification, the specification shall control. In describing embodiments
of the
present application, specific terminology is employed for the sake of clarity.
However, the invention is not intended to be limited to the specific
terminology so
selected. Nothing in this specification should be considered as limiting the
scope of
the present invention. All examples presented are representative and non-
limiting.
The above-described embodiments may be modified or varied, without departing
from the invention, as appreciated by those skilled in the art in light of the
above
teachings. It is therefore to be understood that, within the scope of the
claims and
their equivalents, the invention may be practiced otherwise than as
specifically
described.
-115-