Note: Descriptions are shown in the official language in which they were submitted.
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 1 -
PROCESS FOR PRODUCING A PURIFIED SYNTHESIS GAS STREAM
The present invention relates to a process for
producing a purified synthesis gas stream from a feed
synthesis gas stream comprising contaminants.
Synthesis gas streams are gaseous streams mainly
comprising carbon monoxide and hydrogen. Synthesis gas
streams are generally produced via partial oxidation or
steam reforming of hydrocarbons including natural gas,
coal bed methane, distillate oils and residual oil, and
by gasification of solid fossil fuels such as biomass or
coal or coke.
There are many solid or very heavy (viscous) fossil
fuels which may be used as feedstock for generating
synthesis gas, including biomass, solid fuels such as
anthracite, brown coal, bitumous coal, sub-bitumous coal,
lignite, petroleum coke, peat and the like, and heavy
residues, e.g. hydrocarbons extracted from tar sands,
residues from refineries such as residual oil fractions
boiling above 360 C, directly derived from crude oil, or
from oil conversion processes such as thermal cracking,
catalytic cracking, hydrocracking etc. All such types of
fuels have different proportions of carbon and hydrogen,
as well as different substances regarded as contaminants.
Depending on the feedstock used to generate
synthesis gas, the synthesis gas will contain
contaminants such as carbon dioxide, hydrogen sulphide,
carbonyl sulphide and carbonyl disulphide while also
nitrogen, nitrogen-containing components (e.g. HCN and
NH3), metals, metal carbonyls (especially nickel carbonyl
and iron carbonyl), and in some cases mercaptans.
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
2 -
Purified synthesis gas can be used in catalytical
chemical conversions or to generate power. A substantial
portion of the world's energy supply is provided by
combustion of fuels, especially natural gas or synthesis
gas, in a power plant. Synthesis gas is combusted with
air in one or more gas turbines and the resulting gas is
used to produce steam. The steam is then used to generate
power.
An especially suitable system for using synthesis
gas in power generation is the Integrated Gasification
Combined Cycle (IGCC) system. IGCC systems were devised
as a way to use coal as the source of fuel in a gas
turbine plant. IGCC is a combination of two systems. The
first system is coal gasification, which uses coal to
create synthesis gas. The syngas is then purified to
remove contaminants. The purified synthesis gas may be
used in the combustion turbine to produce electricity.
The second system in IGCC is a combined-cycle, or
power cycle, which is an efficient method of producing
electricity commercially. A combined cycle includes a
combustion turbine/generator, a heat recovery steam
generator (HRSG), and a steam turbine/generator. The
exhaust heat from the combustion turbine may be recovered
in the HRSG to produce steam. This steam then passes
through a steam turbine to power another generator, which
produces more electricity. A combined cycle is generally
more efficient than conventional power generating systems
because it re-uses waste heat to produce more
electricity. IGCC systems are clean and generally more
efficient than conventional coal plants.
As set out hereinabove, when synthesis gas is used
for power production, removal of contaminants is often
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
3 -
required to avoid deposition of contaminants onto the gas
turbine parts.
When synthesis gas is used in catalytical chemical
conversions, removal of contaminants to low levels is
required to prevent catalyst poisoning.
Processes for producing a purified synthesis gas
stream generally involve the use of expensive line-ups.
For example, cold methanol may be used to remove hydrogen
sulphide and carbon dioxide by physical absorption. The
concentrations of these contaminants in the purified
synthesis gas will still be in the ppmv range. For
applications where the synthesis gas is to be
catalytically converted, contaminant concentrations in
the ppmv range are still too high. Purifying the
synthesis gas streams to a higher degree using a
methanol-based process would be uneconomical due to the
disproportionately large amounts of energy required to
regenerate the methanol. In addition, the absorbed H2S
needs to be removed, usually by contacting the methanol
comprising H2S with a stripping gas at elevated
temperatures, resulting in a stripping gas comprising
H2S. H2S in this stripping gas is then converted to
elemental sulphur, requiring a considerable capital and
operational expenditure.
In US 2007/0072949 a process is disclosed wherein
sulphur species are removed using low-temperature amine-
based absorption processes, followed by a solvent
regeneration Claus/SCOT process unit. The disadvantage of
such a process is that it is limited to the feed gas
composition suitable for a Claus process, which is rather
high in hydrogen sulphide concentration.
It is an object of the present invention to provide
an optimised process for purification of a synthesis gas
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
4 -
stream derived from a range of carbonaceous fuels, such
that the purified synthesis gas is suitable for further
use, especially for power production.
It has now been found that by removing H2S directly
from the feed synthesis gas stream, this object can be
achieved.
Therefore, the invention provides a process for
producing a purified synthesis gas stream from a feed
synthesis gas stream comprising besides the main
constituents carbon monoxide and hydrogen also hydrogen
sulphide, HCN and/or COS, the process comprising the
steps of: (a) removing HCN and/or COS by contacting the
feed synthesis gas stream with a water gas shift catalyst
in a shift reactor in the presence of steam/water to
react at least part of the carbon monoxide to carbon
dioxide, and to obtain a synthesis gas stream depleted in
HCN and/or in COS; (b) removing hydrogen sulphide in the
synthesis gas stream depleted in HCN and/or in COS by
contacting this gas stream in a H2S-removal zone with an
aqueous alkaline washing liquid to obtain a H2S-depleted
synthesis gas stream and a sulphide-comprising aqueous
stream; (c) contacting the sulphide-comprising aqueous
stream with sulphide-oxidizing bacteria in the presence
of oxygen in a bioreactor to obtain a sulphur slurry and
a regenerated aqueous alkaline washing liquid; (d)
removing carbon dioxide from the H2S-depleted synthesis
gas stream, to obtain the purified synthesis gas stream
and a gas stream enriched in CO2.
The process enables removal of hydrogen sulphide,
carbonyl sulphide and/or hydrogen cyanide to low levels.
The purified synthesis gas, because of its low level of
contaminants, is suitable for use as fuel, suitably in
gas turbines, or for use in catalytical chemical
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
-
conversions. The purified synthesis gas is especially
suitable for use in an Integrated Gasification Combined
Cycle (IGCC).
In step (d), a carbon dioxide stream at elevated
5 pressure, suitably in the range of from 4 to 12 bara is
obtained. This carbon dioxide stream can be further
pressurised and used for example for enhanced oil
recovery.
The process is economical because H2S is removed
directly from the synthesis gas stream via conversion
into elemental sulphur. The synthesis gas stream depleted
in H2S has very low concentrations of H2S, enabling the
use of an inexpensive non-selective acid gas removal unit
to remove remaining H2S as well as CO2.
It will be understood that the amount and type of
contaminants in the feed synthesis gas stream can vary
and depends on the amount of these contaminants present
in the feedstock used to generate the feed synthesis gas
stream.
Generally, the feed synthesis gas stream is obtained
by gasification of feedstock.
When using solid fossil fuels such as biomass or
coal as feedstock, generally the amount of H2S and COS in
the synthesis gas stream leaving the gasifier is below 15
volumed, typically below 5 volumed based on the synthesis
gas stream.
When using an oil residue as feedstock, generally
the amount of H2S and COS in the synthesis gas stream
leaving the gasifier will be below 20 volume%, typically
below 10 volumed based on the synthesis gas stream.
The synthesis gas stream generated from a feedstock
may comprise particulate matter, for example fly-ash or
soot particles. Therefore, in a preferred embodiment
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
6 -
synthesis gas exiting a synthesis gas generation unit is
contacted with scrubbing liquid in a soot scrubber to
remove particulate matter, in particular soot, thereby
obtaining the feed synthesis gas stream. The synthesis
gas stream exiting the synthesis gas generating unit is
generally at elevated temperature and/or elevated
pressure. Especially in the event that the synthesis gas
is generated in a gasifier, the synthesis gas stream
exiting the gasifier will be at elevated temperature and
at elevated pressure. To avoid additional cooling and/or
depressurising steps, the scrubbing step in the soot
scrubber is preferably performed at elevated temperature
and/or at elevated pressure. Preferably, the temperature
at which the synthesis gas is contacted with scrubbing
liquid is in the range of from 40 to 160 C, more
preferably from 110 to 150 C. Preferably, the pressure
at which the synthesis gas stream is contacted with
scrubbing liquid is in the range of from 20 to 80 bara,
more preferably from 20 to 60 bara.
The amount of HCN and/or COS in the feed synthesis
gas stream depends on the composition of the feedstock
from which the synthesis gas is derived and the
technology applied for the production of synthesis gas.
Generally, the amount of COS in a feed synthesis gas
stream derived from solid fossil fuel feedstocks,
especially coal, is from about 100 to 3000 ppmv, based
on the feed synthesis gas stream. For biomass, the
amount of COS is generally in the range of from 1 to 100
ppmv.
In step (a), HCN and/or COS is removed from the feed
synthesis gas stream via catalytic conversion.
Catalysts for the hydrolysis of HCN and/or COS are
known to those skilled in the art and include for example
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
7 -
Ti02-based catalysts or catalysts based on alumina and/or
chromium-oxide. Preferred catalysts are Ti02-based
catalysts.
The amount of water/steam is preferably between
5 v/v% and 80 v/v%, more preferably between 10 v/v% and
70 v/v%, still more preferably between 15 v/v% and
50 v/v%, based on steam.
In step (a), the feed synthesis gas stream is
contacted with a water gas shift catalyst in a shift
reactor to remove HCN and/or COS and to additionally
react at least part of the carbon monoxide with water to
form carbon dioxide and hydrogen.
In an especially preferred embodiment of step (a),
carbon monoxide in the feed synthesis gas stream is
converted with a low amount of steam in the presence of a
catalyst as present in one or more fixed bed reactors. A
series of shift reactors may be used wherein in each
reactor a water gas shift conversion step is performed.
The content of carbon monoxide, on a dry basis, in the
feed synthesis gas stream as supplied to the first or
only water gas shift reactor is preferably at least
50 vol.%, more preferably between 55 and 70 vol.%. The
feed synthesis gas stream preferably contains hydrogen
sulphide in order to keep the catalyst sulphided and
active. The minimum content of hydrogen sulphide will
depend on the operating temperature of the shift reactor,
on the space velocity (GHSV) and on the sulphur species
present in the feed synthesis gas stream. Preferably at
least 300 ppm H2S is present in the feed synthesis gas
stream. There is no limitation on the maximum amount of
H2S from a catalyst activity point of view.
In the preferred embodiment of step (a), the
steam/water to carbon monoxide molar ratio in the feed
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 8 -
synthesis gas stream as it enters the first or only
water gas shift reactor is preferably between 0.2:1 and
0.9:1. The temperature of the feed synthesis gas stream
as it enters the shift reactor is preferably between 190
and 230 C. In addition it is preferred that the inlet
temperature is between 10 and 60 C above the dewpoint
of the feed to each water gas shift conversion step. The
space velocity in the reactor is preferably between
6000-9000 h-1. The pressure is preferably between 2 and
5 MPa and more preferably between 3 and 4.5 MPa.
The conversion of carbon monoxide may generally not
be 100% because of the sub-stoichiometric amount of
steam present in the feed of the reactor. In a preferred
embodiment the content of carbon monoxide in the shift
reactor effluent, using a fixed bed reactor, will be
between 35 and 50 vol.% on a dry basis, when starting
from a feed synthesis gas stream comprising between 55
and 70 vol.% carbon monoxide, on a dry basis, and a
steam / CO ratio of 0.2 to 0.3 molar. If a further
conversion of carbon monoxide is desired it is preferred
to subject the shift reactor effluent to a next water
gas shift conversion step.
The preferred steam/water to carbon monoxide molar
ratio, inlet temperature and space velocity for such
subsequent water gas shift conversion steps is as
described for the first water gas shift conversion step.
As described above the feed synthesis gas stream is
suitably obtained from a gasification process and is
suitably subjected to a water scrubbing step. In such a
step water will evaporate and end up in the syngas
mixture. The resultant steam to CO molar ratio in such a
scrubbed syngas will suitably be within the preferred
ranges as described above. This will result in that no
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
9 -
steam or water needs to be added to the syngas as it is
fed to the first water gas shift conversion step. In
order to achieve the desired steam to CO molar ranges
for the subsequent steps steam or boiler feed water will
have to be added to the effluent of each previous step.
The water gas shift step may be repeated to stepwise
lower the carbon monoxide content in the shift reactor
effluent of each next shift reactor to a CO content, on
a dry basis, of below 5 vol.%. It has been found that in
4 to 5 steps, or said otherwise, in 4 to 5 reactors such
a CO conversion can be achieved.
It has been found that it is important to control
the temperature rise in each shift reactor. It is
preferred to operate each shift reactor such that the
maximum temperature in the catalyst bed in a single
reactor does not exceed 440 C and more preferably does
not exceed 400 C. At higher temperatures the exothermal
methanation reaction can take place, resulting in an
uncontrolled temperature rise.
The catalyst used in the shift reactor is preferably
a water gas shift catalyst, which is active at the
preferred low steam to CO molar ratio and active at the
relatively low inlet temperature without favouring side
reactions such as methanation. Suitably the catalyst
comprises a carrier and the oxides or sulphides of
molybdenum (Mo), more preferably a mixture of the oxides
or sulphides of molybdenum (Mo) and cobalt (Co) and even
more preferably also comprising copper (Cu) tungsten (W)
and/or nickel (Ni). The catalyst suitably also comprises
one or more promoters/inhibitors such as potassium (K),
lanthanum (La), manganese (Mn), cerium (Ce) and/or
zirconium (Zr). The carrier may be a refractory material
such as for example alumina, MgA12O4 or MgO-Al2O3-TiO2.
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 10 -
An example of a suitable catalyst comprises an
active y-A1203 carrier and between 1-8 wt% CoO and
between 6-10 wt% Mo03. The catalyst is preferably
present as an extrudate.
In a preferred embodiment of step (a), the feed
synthesis gas stream comprises at least 50 vol.% of
carbon monoxide, and the steam to carbon monoxide molar
ratio in the feed synthesis gas stream as it enters the
shift reactor or reactors is preferably between 0.2:1
and 0.9:1 and the temperature of the feed synthesis gas
stream as it enters the shift reactor or reactors is
between 190 and 230 C.
In the event that step (a) involves the shift
reaction as described hereinabove, preferably, a portion
of the "shifted" synthesis gas stream, optionally after
removal of contaminants, is used for hydrogen
manufacture, such as in a Pressure Swing Adsorption
(PSA) step. The proportion of the shifted synthesis gas
stream used for hydrogen manufacture will generally be
less than 15% by volume, preferably approximately 1-10%
by volume. The hydrogen manufactured in this way can
then be used as the hydrogen source in hydrocracking of
the products of the hydrocarbon synthesis reaction. This
arrangement reduces or even eliminates the need for a
separate source of hydrogen, e.g. from an external
supply, which is otherwise commonly used where
available. Thus, the carbonaceous fuel feedstock is able
to provide a further reactant required in the overall
process of biomass or coal to liquid products
conversion, increasing the self-sufficiency of the
overall process.
In step (a), a synthesis gas stream depleted in
hydrogen cyanide and/or in COS is obtained.
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 11 -
In step (b), the synthesis gas stream depleted in
hydrogen cyanide and/or in COS is contacted with aqueous
alkaline washing liquid to transfer hydrogen sulphide
from the synthesis gas stream depleted in hydrogen
cyanide and/or in COS to the aqueous alkaline washing
liquid.
Suitable aqueous alkaline washing liquids include
aqueous hydroxide solutions, e.g. sodium hydroxide or
potassium hydroxide solutions in water and aqueous
(bi)carbonate solutions.
Suitably, step (b) is performed at a temperature in
the range of from 5 to 70 C, more preferably from 10 to
50 C. Preferably, step (c) is performed at a pressure
in the range of from 1 to 100 bar(g), more preferably
from 1.5 to 80 bar(g).
Optionally, the washing liquid is buffered.
Preferred buffering compounds are carbonates,
bicarbonates phosphates and mixtures thereof, especially
sodium carbonate and/or sodium bicarbonate.
The concentration of the buffering compounds depends
inter alia on the composition of the gas flow and is
generally adjusted in such a way, that the washing liquid
is kept within the preferred pH range.
Preferably, the pH of the washing liquid is in the
range of from 4.5 to 10, more preferably from 5.5 to 9Ø
In step (c) hydrogen sulphide in the scrubbing
medium is converted to elemental sulphur using sulphide-
oxidising bacteria in the presence of oxygen in a
bioreactor.
Reference herein to sulphide-oxidising bacteria is
to bacteria which can oxidise sulphide to elemental
sulphur. Suitable sulphide-oxidising bacteria can be
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 12 -
selected for instance from the known autotropic aerobic
cultures of the genera Thiobacillus and Thiomicrospira.
The main reactions that can take place in the
bioreactor are the microbiological formation of sulphur
and sulphate:
(1a) Sulfur
production
HS- + 1-2 02 - 1/8 S8 + OH-
(1b) Sulfur
production HS5- + lz2 02 5/8 S8 + OH-
(2) Sulphate
production HS- + 202 + OH- -4 5042- + H2O
The sulphur slurry may comprise one or more
products of the main reactions, including elemental
sulphur and sulphates.
The regenerated aqueous alkaline washing liquid may
comprise sulphur particles.
Reference herein to sulphide-oxidising bacteria is
to bacteria which can oxidise sulphide to elemental
sulphur. Suitable sulphide-oxidising bacteria can be
selected for instance from the known autotropic aerobic
cultures of the genera Thiobacillus and Thiomicrospira.
Preferably, the reaction medium in the bioreactor
is buffered. The buffering compounds are chosen in such a
way that they are tolerated by the bacteria present in
the oxidation reactor. Preferred buffering compounds are
carbonates, bicarbonates phosphates and mixtures thereof,
especially sodium carbonate and/or sodium bicarbonate.
The concentration of the buffering compounds depends
inter alia on the composition of the gas flow and is
generally adjusted in such a way, that the pH of the
reaction medium in the oxidation reactor is between 6.0
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 13 -
and 12.0, preferably between 7.0 and 11.0, more
preferably between 8.0 and 10Ø
Typical pressures in the bioreactor are between 0.5
and 2 bar (g) .
Preferably, at least part of the aqueous sulphur
slurry obtained in step (c) is separated from the
regenerated aqueous alkaline washing liquid. Suitably,
the separating step takes place in a solid/liquid
separator. Suitable solid/liquid separators are described
in Perry's Chemical Engineers' Handbook, 7th edition,
section 22 (1997).
The sulphur content of the separated aqueous
sulphur slurry is suitably between 5 w/w% and 50 w/w%,
based on the slurry. Typically, the water of the sulphur
slurry is removed to an extent that a sulphur cake with a
dry solids content of between 55 and 70% is obtained.
Suitably, the sulphur purity of the sulphur cake is
between 90 and 98 w/w%, based on the dry weight of the
sulphur cake. Optionally, the sulphur slurry can be re-
slurried, filtered and dried to obtain a sulphur paste
with a purity of at least 95 wt% sulphur, preferably at
least 99 wt% sulphur. The sulphur paste thus-obtained can
optionally be dried to produce a powder with a dry weight
content of at least 85%, preferably at least 90%. This
powder can suitably be applied as a fungicide, a
fertilizer or as a miticide.
In step (d) carbon dioxide is removed from the
synthesis gas stream depleted in hydrogen sulphide.
In a first embodiment of step (d), carbon dioxide
is removed by contacting the synthesis gas stream
depleted in H2S with absorbing liquid to remove carbon
dioxide and remaining hydrogen sulphide.
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 14 -
Suitable absorbing liquids may comprise chemical
solvents or physical solvents or mixtures thereof.
A preferred absorbing liquid comprises a chemical
solvent and/or a physical solvent, suitably as an aqueous
solution.
Suitable chemical solvents are primary, secondary
and/or tertiary amines, including sterically hindered
amines.
A preferred chemical solvent comprises a secondary
or tertiary amine, preferably an amine compound derived
from ethanolamine, more especially DIPA, DEA, MMEA
(monomethyl-ethanolamine), MDEA (methyldiethanolamine)
TEA (triethanolamine), or DEMEA (diethyl-
monoethanolamine), preferably DIPA or MDEA. It is
believed that these chemical solvents react with acidic
compounds such as H2S.
In a second embodiment of step (d), carbon dioxide
is removed using a membrane.
It is advantageous to use membranes with a high
selectivity for carbon dioxide. The selectivity is
defined as the ratio of the carbon dioxide permeability
over the permeability of carbon monoxide and hydrogen as
measured in single gas experiments. Preferably, the
selectivity of the membrane is between 10 and 200,
preferably between 20 and 150.
Suitably the membrane material is chosen from the
group of polyethylene oxide based materials, preferably
polyethylene oxide based material comprising block-
copolymers, especially PEO 600/5000 T6T6T or a cross
linked PEO, polyimide or polyaramide based materials,
cellulose acetate based materials, zeolite based
materials, preferably silica-alumina phosphate based
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 15 -
materials, more preferably SAPO-34, micro-porous silica
materials and carbon molecular sieves materials.
In a third embodiment of step (d), carbon dioxide
is removed by cooling the gas stream to a temperature at
which carbon dioxide will separate from the gas stream.
Suitably, the gas stream is cooled to a temperature at
which carbon dioxide becomes a liquid or a solid so it
can be separated from the gas stream.
The purified synthesis gas obtained in step (d) has
low levels of contaminants, suitably in the ppmv or even
in the ppbv range.
Suitably, the gas stream enriched in C02 obtained
in step (d) is at a pressure in the range of from 3 to 15
bara, preferably from 5 to 15 bara. This pressurised gas
stream enriched in C02 can advantageously be used for
enhanced oil recovery, with less compression equipment
needed.
In applications where the C02-enriched gas stream
needs to be at a high pressure, for example when it will
be used for injection into a subterranean formation, it
is an advantage that the C02-enriched gas stream is
already at an elevated pressure.
In one embodiment, the C02-enriched gas stream is
further pressurised and used for enhanced oil recovery,
suitably by injecting it into an oil reservoir where it
tends to dissolve into the oil in place, thereby reducing
its viscosity and thus making it more mobile for movement
towards the producing well.
In another embodiment, the C02-enriched gas stream
is further pressurised and pumped into an aquifer
reservoir for storage.
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 16 -
In yet another embodiment, the pressurised C02-
enriched gas stream is further pressurised and pumped
into an empty oil reservoir for storage.
For all the above options, a series of compressors
is needed to pressurise the C02-enriched gas stream to
the desired high pressures. Pressurising the C02-enriched
gas stream from atmospheric pressure to a pressure of
about 10 bara generally requires a large and expensive
compressor. As the process results in a C02-enriched gas
stream which is already at elevated pressure, preferably
above 10 bara, the most extensive compressor is not
needed.
In a preferred embodiment, the purified synthesis
gas is used for power generation, especially in an IGCC
system.
In the IGCC system, typically, fuel and an oxygen-
containing gas are introduced into a combustion section
of a gas turbine. In the combustion section of the gas
turbine, the fuel is combusted to generate a hot
combustion gas. The hot combustion gas is expanded in the
gas turbine, usually via a sequence of expander blades
arranged in rows, and used to generate power via a
generator. Suitable fuels to be combusted in the gas
turbine include natural gas and synthesis gas.
Hot exhaust gas exiting the gas turbine is
introduced into to a heat recovery steam generator unit,
where heat contained in the hot exhaust gas is used to
produce a first amount of steam.
Suitably, the hot exhaust gas has a temperature in
the range of from 350 to 700 C, more preferably from 400
to 650 C. The composition of the hot exhaust gas can
vary, depending on the fuel gas combusted in the gas
turbine and on the conditions in the gas turbine.
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 17 -
The heat recovery steam generator unit is any unit
providing means for recovering heat from the hot exhaust
gas and converting this heat to steam. For example, the
heat recovery steam generator unit can comprise a
plurality of tubes mounted stackwise. Water is pumped and
circulated through the tubes and can be held under high
pressure at high temperatures. The hot exhaust gas heats
up the tubes and is used to produce steam.
The heat recovery steam generator unit can be
designed to produce three types of steam: high pressure
steam, intermediate pressure steam and low pressure
steam.
Preferably, the steam generator is designed to
produce at least a certain amount of high pressure steam,
because high pressure steam can be used to generate
power. Suitably, high-pressure steam has a pressure in
the range of from 90 to 150 bara, preferably from 90 to
125 bara, more preferably from 100 to 115 bara. Suitably,
low-pressure steam is also produced, the low-pressure
steam preferably having a pressure in the range of from 2
to 10 bara, more preferably from to 8 bara, still more
preferably from 4 to 6 bara.
In the heat recovery steam generator unit
preferably high pressure steam is produced in a steam
turbine, which high pressure steam is converted to power,
for example via a generator coupled to the steam turbine.
The purified synthesis gas, because of its low
level of contaminants, is also suitable for use in
catalytic processes, preferably selected from the group
of Fischer-Tropsch synthesis, methanol synthesis, di-
methyl ether synthesis, acetic acid synthesis, ammonia
synthesis, methanation to make substitute natural gas
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 18 -
(SNG) and processes involving carbonylation or
hydroformylation reactions.
Without wishing to be restricted to a particular
embodiment, the invention will now be described in
further detail with reference to the Figures. In
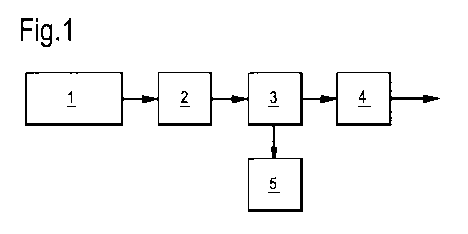
Figure 1, there is shown a process for producing a
purified synthesis gas stream. This starts with the
gasification of biomass or coal with oxygen in a
gasification unit 1 to form a feed synthesis gas stream
comprising hydrogen sulphide, HCN and/or COS. Preferably,
removal of solids such as slag, soot and the like is done
in a solids removal unit (not shown). The resulting feed
synthesis gas stream is led to a shift unit 2, where it
is contacted with a shift catalysis, converting CO to C02
and hydrolysing HCN and COS. The resulting synthesis gas
stream depleted in HCN and COS emanating from unit 2 is
led to H2S-removal zone 3 where H2S is removed by
contacting the synthesis gas stream with an aqueous
alkaline washing liquid. The resulting synthesis gas
stream depleted in H2S is led from H2S-removal zone 3 to
acid gas removal unit 4, where it is contacted with
absorbing liquid to remove C02 and remaining H2S. This
results in a purified synthesis gas stream and a gas
stream enriched in C02. Aqueous alkaline washing liquid
comprising H2S is led from the H2S removal zone to
bioreactor 5, where H2S is converted to elemental
sulphur.
In Figure 2, a preferred embodiment is depicted,
wherein the purified synthesis gas is used for power
production. In Figure 2, a purified synthesis gas stream
as produced in a process according to Figure 1 is led to
a power plant comprising a gas turbine (1) and a heat
CA 02756372 2011-09-22
WO 2010/112502 PCT/EP2010/054188
- 19 -
recovery steam generator unit (2). In the gas turbine, an
oxygen-containing gas is supplied via line 4 to
compressor 5. Purified synthesis gas as produced in a
process described in Figure 1 is supplied via line 6 to
combuster 7 and combusted in the presence of the
compressed oxygen-containing gas. The resulting
combustion gas is expanded in expander 8 and used to
generate power in generator 9. Remaining exhaust gas
comprising C02 and oxygen is led via line 10 to a heat
recovery steam generator unit 2. In the heat recovery
steam generator unit, water is heated against the hot
exhaust gas in in heating section 11 to generate steam.
The steam is led via line 12 into a steam turbine 13 to
produce additional power in generator 14.