Note: Descriptions are shown in the official language in which they were submitted.
CA 02758686 2011-11-21
1 Attorney Docket No. 5049-092780
FLUID TREATMENT SYSTEMS, COMPOSITIONS AND METHODS FOR METAL
ION STABILIZATION IN AQUEOUS SOLUTIONS
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of United States Provisional Patent
Application No.
61/184,471, filed on June 5, 2009, which is incorporated by reference herein.
BACKGROUND OF THE INVENTION
I. FIELD OF THE INVENTION
[0002] The present invention relates to fluid treatment systems and
compositions comprising
carboxylic acid functional and sulfonyl and/or sulfonate functional polymer(s)
and scale control
agent(s), and methods for stabilizing metal ions in aqueous compositions, such
as hydraulic
fracturing compositions, using the fluid treatment systems and compositions.
TECHNICAL CONSIDERATIONS
[0003] Scaling is the precipitation of a salt from a solution that is
supersaturated with respect to
the salt. The salts include but are not limited to salts of calcium,
magnesium, barium, strontium,
iron, aluminum, manganese, and so on. Common scales include but are not
limited to barium
carbonate, barium sulfate, calcium carbonate, calcium sulfate, calcium
phosphate, calcium
silicate, iron carbonate, iron hydroxide/oxide, magnesium silicate, silica,
and strontium sulfate.
In brackish water, sodium chloride can even precipitate.
[0004] The potential to scale, the rate of scale formation, and the crystal
structure or lack thereof
are influenced by factors such as the concentration of ions comprising the
scale, the nature and
concentration of electrolytes in solution, the temperature, residence time,
system cleanliness, and
presence of additives. Mathematically, when the product of ion concentrations,
each raised to a
power equal to its formula coefficient, exceed the solubility product
constant, the solution is
supersaturated. The solubility constant is temperature dependent. Many scales
have inverse
solubility, i.e., the higher the temperature the lower the solubility (hence
solubility product
constant). The concentration of electrolytes in solution also affects
solubility and calculations of
saturation must be corrected accordingly. Dirty systems provide seeding of
crystals and
therefore scaling will occur more quickly in dirty systems than in clean
systems.
26U6545.DOC
CA 02758686 2011-11-21
2 Attorney Docket No. 5049-092780
[0005] As mentioned, residence time impacts scaling. Many inhibitors work by
adsorbing onto
crystallite surfaces, thus retarding further growth and favoring re-
dissolution of crystallite ions.
When crystals do form, growth will be modified and less adherent. Inhibitors
affect the kinetics
of growth and therefore only delay growth. Given enough time, crystals will
form. However,
for practical purposes, proper treatment can control scaling under the right
conditions. That
being said, the longer the residence time, the more likely scaling will occur.
[0006] Threshold inhibitors work on a sub-stoichiometric basis, meaning at
levels substantially
below a molar ratio of inhibitor to ion. Chelants work on a stoichiometric
basis, meaning one
mole of chelant is needed per mole of ion. Thus lower doses of threshold
inhibitors are typically
required than of chelants. =
[0007] A useful index for assessing the scaling potential of a system for
calcium carbonate is the
Langelier Scale Index (LSI). This index models the impact of a combination of
alkalinity,
calcium ion concentration, total dissolved solids (TDS), pH, and temperature
of water for the
potential of calcium carbonate scale formation. More specifically, the LSI is
an equilibrium
model derived from the theoretical concept of saturation and provides an
indicator of the degree
of saturation of water with respect to calcium carbonate. It can be shown that
the LSI
approximates the base 10 logarithm of the calcite saturation level. The
Langelier saturation level
approaches the concept of saturation using pH as a main variable. The LSI can
be interpreted as
the pH change required to bring water to equilibrium. In order to calculate
the LSI, it is
necessary to know the alkalinity (mg/L as CaCO3), the calcium hardness (mg/L
Ca2+ as CaCO3),
the total dissolved solids (mg/L), the actual pH, and the temperature of the
water ( C).
[0008] Solubility product concentrations are exceeded for various reasons,
such as partial
evaporation of the water phase, an increase in pH, or temperature, and the
introduction of
additional cations or anions. Ion concentrations in excess of the solubility
product will tend to
promote precipitation of insoluble compounds. For example, mine pool water
that is pumped to
the surface undergoes degassing of CO2 followed by an increase in solution pH.
The
corresponding LSI shifts from a negative value (corrosive) to a positive value
(scaling). LSI is
often used by water treatment specialists to describe the scaling potential of
a water for
applications such as, for example, in cooling towers.
[0009] As the various reaction products precipitate on surfaces of the water
carrying system,
they form scale or deposits. This accumulation prevents effective heat
transfer, interferes with
26U6545.DOC
CA 02758686 2011-11-21
3 Attorney Docket No. 5049-092780
fluid flow, facilitates corrosive processes, and harbors bacteria. In piping
and tubing, scale can
cause restriction to flow and high friction loss. This scale is an expensive
problem causing
delays and shutdowns for cleaning and removal.
[0010] The presence of iron provides a significant and complex problem in well
stimulation
operations. Ferrous iron under down-hole conditions can form an iron carbonate
scale know as
siderite. Iron in the ferric state can form iron complexes that can block flow
pathways and
inhibit the flow of gas and/or oil therethrough. Also, iron can impair the
performance of
fracturing fluid components, such as the friction reducing additive.
[0011] In oil and gas wells, air can be introduced into water present in the
underground
formation through the borehole or from comingling of underground water with
air-saturated
water which has been pumped from the surface into the well. Ground or well
water typically
exists in a reducing environment. As a result, iron in ground or well water
typically is present as
the ferrous ion (Fe+2) species. The ferrous iron can originate from many
sources, such as the
minerals contained within stratigraphic formations surrounding the water or
from additives
added to the water during oil or gas well drilling or fracturing operations.
Exposure to air
(oxygen) or other oxidants (chlorine, bromine, stabilized bromine, etc.)
causes ferrous ions to be
oxidized to insoluble ferric (Fe+3) ion complexes. Ferric ion complexes, such
as hydrated ferric
oxides (Fe203snH20), are much less soluble than ferrous iron, and once formed
can readily
precipitate. The accumulation of these solids can block pores and flow
pathways (or fracture
conductivity) in the oil or gas well formation, thus causing permeability
impairment with an
associated decline in oil or gas flow. While not intending to be bound by any
theory, it is
believed that when iron is present in soluble or dispersed form, it is less
likely to block the flow
pathways, thus enhancing production potential of the well.
[0012] The formation or precipitation of iron oxides can be inhibited by
stabilization of the
ferrous ion, and/or suspension or dispersion of the iron oxide(s).
Stabilization is the process by
which polymers: (1) form stable complexes with dissolved iron, thus preventing
the formation of
insoluble Fe203.nH20 and (2) absorb onto the surface of particulates that are
forming, thereby
greatly restricting particle growth and thus allowing the particles to remain
suspended. In
contrast, dispersion is the process by which pre-formed iron oxide (Fe203)
particles are
prevented from settling by the action of a polymer. Dispersants are generally
negatively
charged, low molecular weight polymers. Likewise, the surface charge of iron
oxide particles is
26U6545.DOC
CA 02758686 2011-11-21
4 Attorney Docket No. 5049-092780
negative. The repulsion between the negatively charged particle surface and
negatively charged
polymers prevents the particles from agglomerating and settling.
[0013] To prevent clogging of the flow pathways in oil and gas well
formations, chelating agents
have been used. Citric acid, ethylenediaminetetraacetic acid (EDTA) and
nitriloacetic acid
(NTA) are common iron chelating agents used for iron control in fracturing
fluid design.
Chelating agents function on a stoichiometric basis, i.e., one mole of
chelating agent is needed
per mole of iron. Additional chelating agent is needed to drive the reaction,
with the dose
depending on the conditional stability constant (K---
[complex]/[metal][chelating agent], K being
a function of pH). Thus, high doses of chelating agent are needed. The large
dose requirement
of citric acid results in pH depression, which in turn can negatively impact
some friction
reducing additives, such as polyacrylamide-based products. While sulfonated
polymers have
been used to disperse pre-formed ferric iron particulates and/or to stabilize
low levels (<10
mg/L) of ferrous ions in cooling water applications, they have not been used
in oil and gas well
water to stabilize the high levels of ferrous ions and/or ferric oxide
particulates which can exceed
25 mg/L.
[0014] In another aspect of the stimulation process, during the hydraulic
fracturing operation
fluid is pumped at high velocity and high pressure drops are encountered,
resulting in large
energy losses. Pressures at the surface of the well of 3,000 to 15,000 psi are
often required to
overcome the frictional losses and fracture initiation pressure. It is well
known that energy is
lost due to frictional forces encountered during the movement of liquid
through a pipe, tubing or
conduit. The energy loss is reflected in a progressive drop in pressure
measured along the path
between the inlet and discharge point. Factors such as fluid velocity, pipe
diameter, pipe length,
interior surface roughness, fluid density, and fluid viscosity impact the
pressure drop, also known
as differential pressure.
[0015] Well-known laws of fluid dynamics correlate pressure drop as being
proportional to fluid
velocity. The Reynolds' number (Re) is a dimensionless number that gives a
measure of the
ratio of inertial forces (PV212)t0 viscous forces (iAVL), and is used to
describe different flow
regimes, such as laminar or turbulent flow: laminar flow occurs at low
Reynolds numbers, where
viscous forces are dominant, and is characterized by smooth, constant fluid
motion, while
turbulent flow occurs at high Reynolds numbers and is dominated by inertial
forces, which tend
to produce random eddies, vortices and other flow instabilities. As fluid
velocity increases, the
26U6545.DOC
CA 02758686 2011-11-21
5 Attorney Docket No. 5049-092780
conditions change from laminar to transitional to turbulent flow. Under
laminar conditions flow
is smooth and energy loss is minimal, while under turbulent conditions random
impurities and
other flow instabilities contribute to greater energy loss. Generally,
turbulent flow exists when
the Reynolds' number of a fluid is > 5,000. For the most part, hydraulic
fracturing operations
occur in the turbulent flow regime. Therefore, reducing energy loss results in
significant
economic and safety incentives based on lower operating pressures, less
equipment fatigue,
lower horsepower demand and less capital for equipment.
[0016] It is well known that small amounts of high molecular weight polymers
can be very
effective in reducing friction loss of flowing aqueous fluids. Slicicwater
applications have been
effectively applied in the hydraulic fracturing of Barnett Shale and other
unconventional gas
shale applications. Certain metal ions, such as ferrous iron, are known to
degrade
polyacrylamide polymers. The exact mechanism for this degradation is not
completely
understood but is thought to proceed by a free radical mechanism. Since oxygen
is known to
accelerate degradation, it seems plausible that an oxygen-anion radical is
formed when a metal
ion is oxidized. The highly reactive oxygen-anion radical then can attack the
polymer chain,
scission the polymer backbone and result in performance deterioration.
[0017] Also, carbonate and sulfate ions can be present in flowback water from
fracturing
operations. The fracturing fluid in the down-hole environment can release
soluble salts from the
formation that can combine with the fracturing fluid and form precipitates
such as calcium
carbonate, calcium sulfate, barium sulfate, strontium sulfate, and iron
carbonate within the
underground fracture network and cause scale accumulation in perforations or
fissures in the
fractured rock
[0018] There is a long-felt need in the art for alternative metal ion
stabilizers that can be used to
control high levels of ferrous ions and/or ferric oxide particulates which can
exceed 25 mg/L in
aqueous solutions, such as are typically found in hydraulic fracturing
applications, can inhibit
scale formation, and which are compatible with or provide enhanced performance
of friction
reducing agents. A metal ion stabilizer or a metal precipitant dispersant that
would mitigate the
adverse impact of metal ions, such as ferrous iron or calcium or magnesium, on
the friction
reduction additive would be of significant advantage to the well drilling
industry.
26U6545.DOC
CA 02758686 2011-11-21
6 Attorney Docket No. 5049-092780
SUMMARY OF THE INVENTION
[0019] In some non-limiting embodiments, fluid treatment systems and
compositions are
provided comprising: (a) at least one material comprising (1) at least one
carboxylic acid
functional group and (2) at least one sulfur-containing group selected from
the group consisting
of sulfonyl functional groups, sulfonate functional groups, and mixtures
thereof; and (b) at least
one scale control agent.
[0020] In some non-limiting embodiments, aqueous compositions or fracturing
fluids are
provided comprising metal ions, such as calcium ions or ferrous ions, and the
above fluid
treatment system or composition.
[0021] In some non-limiting embodiments, aqueous compositions or fracturing
fluids are
provided comprising at least one acid and the above fluid treatment system or
composition. Such
acid treatments can be used for acid fracturing treatments or combined to use
in part or stages
within a near-neutral pH fracturing fluid system.
[0022] In some non-limiting embodiments, methods of inhibiting formation of
metal oxides in
an aqueous composition comprising metal ions are provided which comprise:
mixing an aqueous
composition comprising metal ions with the above fluid treatment system or
composition.
[0023] In some non-limiting embodiments, methods of inhibiting precipitation
of metal oxides in
an aqueous composition comprising metal ions are provided which comprise:
mixing an aqueous
composition comprising metal ions with the above fluid treatment system or
composition.
[0024] In some non-limiting embodiments, methods of treating a subterranean
formation
penetrated by a well bore are provided which comprise: contacting the
subterranean formation
with the above fracturing fluid.
[0025] In some non-limiting embodiments, methods of inhibiting formation of
metal oxides in
an aqueous composition comprising at least 20 milligrams of metal ions per
liter of aqueous
composition are provided which comprise: mixing an aqueous composition
comprising metal
ions with the above fluid treatment system or composition.
[0026] In some non-limiting embodiments, methods of inhibiting precipitation
of metal oxides in
an aqueous composition comprising at least 20 milligrams of metal ions per
liter of aqueous
composition, comprising: mixing an aqueous composition comprising metal ions
with the above
fluid treatment system or composition.
26U6545.DOC
CA 02758686 2011-11-21
7 Attorney Docket No. 5049-092780
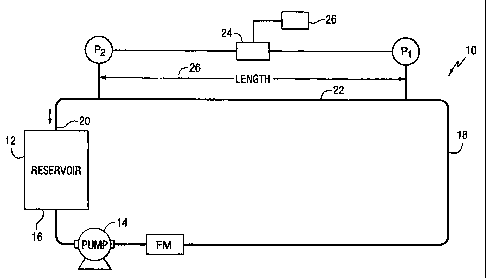
BRIEF DESCRIPTION OF THE DRAWING
[0027] The foregoing summary, as well as the following detailed description,
will be better
understood when read in conjunction with the appended drawing. In the drawing:
[0028] Fig. 1 is a schematic drawing of a closed loop test apparatus for
evaluating friction
reduction of water systems.
DETAILED DESCRIPTION
[0029] For the purposes of this specification, unless otherwise indicated, all
numbers expressing
quantities of ingredients, reaction conditions, dimensions, physical
characteristics, and so forth,
used in the specification and claims are to be understood as being modified in
all instances by the
term "about". Accordingly, unless indicated to the co. ntrary, the numerical
parameters set forth
in the following specification and attached claims are approximations that may
vary depending
upon the desired properties sought to be obtained by the present invention. At
the very least, and
not as an attempt to limit the application of the doctrine of equivalents to
the scope of the claims,
each numerical parameter should at least be construed in light of the number
of reported
significant digits and by applying ordinary rounding techniques.
[0030] Notwithstanding that the numerical ranges and parameters setting forth
the broad scope
of the invention are approximations, the numerical values set forth in the
specific examples are
reported as precisely as possible. Any numerical values, however, inherently
contain certain
errors necessarily resulting from the standard deviation found in their
respective testing
measurements.
[0031] Also, it should be understood that any numerical range recited herein
is intended to
include all sub-ranges subsumed therein. For example, a range of "1 to 10" is
intended to
include any and all sub-ranges between and including the recited minimum value
of 1 and the
recited maximum value of 10, that is, all sub-ranges beginning with a minimum
value equal to or
greater than 1 and ending with a maximum value equal to or less than 10, and
all sub-ranges in
between, e.g., 1 to 6.3, or 5.5 to 10, or 2.7 to 6.1.
[0032] As used herein, the term "substantially free" is meant to indicate that
a material is present
as an incidental impurity. In other words, the material is not intentionally
added to an indicated
composition, but may be present at minor or inconsequential levels because it
was carried over as
an impurity as part of an intended composition component.
26U6545.DOC
CA 02758686 2011-11-21
8 Attorney Docket No. 5049-092780
[0033] It should also be noted that any carbon, as well as heteroatom, with
unsatisfied valences
in the text, schemes, examples and Table herein is assumed to have the
sufficient number of
hydrogen atom(s) to satisfy the valences.
[0034] When any variable (e.g., alkyl, heterocycle, R2, etc.) occurs more than
one time in any
constituent, its definition on each occurrence is independent of its
definition at every other
occurrence.
[0035] As used herein, the term "composition" is intended to encompass a
product comprising
the specified ingredients in the specified amounts, as well as any product
which results, directly
or indirectly, from the combination of the specified ingredients in the
specified amounts.
[0036] As used herein, "formed from" or "prepared from" denotes open, e.g.,
"comprising,"
claim language. As such, it is intended that a composition "formed from" or
"prepared from" a
list of recited components be a composition comprising at least these recited
components or the
reaction product of at least these recited components, and can further
comprise other, non-recited
components, during the composition's formation or preparation.
[0037] As used herein, the phrase "reaction product of' means chemical
reaction product(s) of
the recited components, and can include partial reaction products as well as
fully reacted
products.
[0038] As used herein, the term "polymer" means a substance, typically of
large molecular mass,
comprising structural units or monomers. Examples of polymers include
oligomers,
homopolymers and copolymers. The term "oligomer" means a polymer consisting of
only a few
monomer units up to about ten monomer units, for example a dimer, trimer or
tetramer.
[0039] The compositions and methods of the present invention can be useful in
a wide variety of
applications, non-limiting examples of which include stabilization of metal
ions in aqueous
systems, as well as in petroleum and gas field well drilling or fracturing
operations.
[0040] In some non-limiting embodiments, the fluid treatment systems and
compositions of the
present invention comprise: (a) at least one material comprising (1) at least
one carboxylic acid
functional group and (2) at least one sulfur-containing group selected from
the group consisting
of sulfonyl functional groups, sulfonate functional groups, and mixtures
thereof; and (b) at least
one scale control agent.
[0041] In some non-limiting embodiments, the fluid treatment systems and
compositions of the
present invention consist essentially of or consist of: the at least one
material (a) as described
26U6545.DOC
CA 02758686 2011-11-21
9 Attorney Docket No. 5049-092780
above; the at least one friction reducing agent (b) as described above; and
the at least one scale
control agent (c) as described above.
[0042] In some non-limiting embodiments, the at least one material (a) is a
reaction product or
salt thereof, wherein the reaction product is prepared from reactants
comprising: at least one
ethylenically unsaturated, carboxylic acid functional material or anhydride
thereof; and at least
one ethylenically unsaturated, sulfur-containing material, wherein the
ethylenically unsaturated
sulfur-containing material comprises at least one sulfur-containing group
selected from the group
consisting of sulfonyl functional groups, sulfonate functional groups and
mixtures thereof
(includes at least one sulfonyl functional group and at least one sulfonate
functional group).
[0043] In some non-limiting embodiments, suitable ethylenically unsaturated,
carboxylic acid
functional materials or anhydrides thereof for preparing the reaction product
in the fluid
treatment systems, compositions and methods of the present invention include
those having
acrylic or vinyl functionality. Non-limiting examples of suitable
ethylenically unsaturated,
carboxylic acid functional materials include those selected from the group
consisting of acrylic
acid, methacrylic acid, a-halo acrylic acid, maleic acid, itaconic acid, vinyl
acetic acid, allyl
acetic acid, fumaric acid, 0-carboxyethyl acrylic acid, salts thereof, and
mixtures thereof. A non-
limiting example of a suitable ethylenically unsaturated, carboxylic acid
functional anhydride is
maleic anhydride.
[0044] In some non-limiting embodiments, the at least one ethylenically
unsaturated, carboxylic
acid functional material or anhydride thereof comprises about 10 to about 90
weight percent of
the reactants, or about 20 to about 80 weight percent, or about 30 to about 70
weight percent, on
a basis of total weight of the reactants.
[0045] In some non-limiting embodiments, suitable ethylenically unsaturated,
sulfur-containing
materials for preparing the reaction product in the fluid treatment systems,
compositions and
methods of the present invention include those having vinyl functionality,
acrylic functionality,
acrylamido functionality, acrylamido alkyl functionality and/or acrylarnido
aryl functionality.
Non-limiting examples of suitable ethylenically unsaturated, sulfur-containing
materials include
those selected from the group consisting of 2-acrylamido-2-methylpropyl
sulfonic acid; ally1-2-
hydroxypropyl sulfonic acid ether; ally1-2-hydroxypropyl sulfonate ether;
sulfomethylacrylamide; 2-propene-l-sulfonic acid, 2-methyl; 2-methacrylamido-2-
methylpropyl
sulfonic acid; styrene sulfonic acid; vinyl sulfonic acid; sulfoalkyl
acrylate; sulfoalkyl
26U6545.DOC
CA 02758686 2011-11-21
10 Attorney Docket No. 5049-092780
methacrylate; sulfoalkyl acrylamide; allyl sulfonic acid; methallyl sulfonic
acid; para
methallyloxy benzene sulfonic acid; ally1-2-hydroxypropyl sulfonic acid; 3-
methacrylamido-2-
hydroxypropyl sulfonic acid; sulfonic acid acrylate; sulfonated
phenolmethacrylic ether; salts
thereof and mixtures thereof.
[0046] In some non-limiting embodiments, the at least one ethylenically
unsaturated, sulfur-
containing material comprises about 5 to about 95 weight percent of the
reactants, or about 10 to
about 90 weight percent of the reactants, or about 20 to about 80 weight
percent, or about 30 to
about 70 weight percent, on a basis of total weight of the reactants.
[0047] In some non-limiting embodiments, the weight ratio of ethylenically
unsaturated,
carboxylic acid functional material or anhydride thereof to ethylenically
unsaturated, sulfur-
containing material ranges from about 1:20 to about 20:1, or about 1:10 to
about 10:1, or about
1:5 to about 5:1.
[0048] In some non-limiting embodiments, the reaction product or salt thereof
useful as material
(a) has a weight average molecular weight ranging from about 500 to about
1,000,000 grams per
mole, or about 1,000 to about 100,000 grams per mole, or about 2,000 to about
30,000 grains per
mole.
[0049] In some non-limiting embodiments, the reaction product useful as
material (a) can be
prepared from acrylic acid (AA) and 2-acrylamido-2-methylpropyl sulfonic acid
(AMPS), for
example about 25 to about 95 mole percent of acrylic acid and about 5 to about
75 mole percent
of 2-acrylamido-2-methylpropyl sulfonic acid. Non-limiting examples of
suitable AA/AMPS
copolymers include KR-DP0184 copolymer prepared from about 60 weight percent
AA and
about 40 weight percent AMPS, which is available from Kroff Chemical Co. of
Pittsburgh, PA.
Another useful AAJAMPS copolymer is Acumer 2100 AA/AMPS copolymer prepared
from 60
weight percent AA and 40 weight percent AMPS available from Rohm and Haas Co.,
a
subsidiary of Dow Chemical, of Philadelphia, PA. Also, ICP-1000, a composition
which is
available from Superior Well Services of Indiana, PA, is supplied as a blend
of an AA/AMPS
copolymer and propylene glycol, and the balance being water, sodium ion,
residual sodium
bisu1fite, catalysts and other non-active components. Other useful AA/AMPS
copolymers
include those disclosed in U.S. Patent No. 3,928,196, incorporated by
reference herein.
26U6545.DOC
CA 02758686 2011-11-21
11 Attorney Docket No. 5049-092780
[0050] Other non-limiting examples of suitable copolymers useful as material
(a) include those
prepared from unsaturated mono-carboxylic acids and unsaturated sulfonic acids
include those
disclosed in U.S. Patent No. 4,640,793, incorporated by reference herein.
[0051] Other non-limiting examples of suitable reaction products useful as
material (a) include
copolymers prepared from acrylic acid and sulfonated methacrylic acid ether
(AA/SPME) such
as AQUATREAT AR-540 available from Alco Chemical of Chattanooga, TN or those
disclosed
in U.S. Patent No. 4,892,898, incorporated by reference herein.
[0052] Other non-limiting examples of suitable copolymers useful as material
(a) include
copolymers prepared from acrylic acid and ally1-2-hydroxypropyl sulfonic acid
ether
(AA/AHPSE) such as HPS-1 available from GE Betz, or those disclosed in U.S.
Patent No.
4,560,481, incorporated by reference herein.
[0053] Other non-limiting examples of suitable copolymers useful as material
(a) include those
having repeat units:
R1
H2 I H2 I
C C C C
?H2
R2 oI
- X
R3
Pahl
wherein R1 is H or lower alkyl (Ci-C3); R2 is OH or OM, or NH2; R3 is a
hydroxy substituted
alkyl or alkylene radical having from 1 to 6 carbon atoms or a non-substituted
alkyl or alkylene
radical having from 1 to about 6 carbon atoms; X, when present, is an anionic
radical selected
from the group consisting of SO3, P03, PO4, and COO; Z, when present, is H or
hydrogen or any
water soluble cation or cations which together counterbalance the valence of
the anionic radical;
a is 0 or 1; and the molar ratio of x:y is between about 30:1 to about 1:20,
such as are disclosed
in U.S. Patent Nos. 4,895,663, 4,895,664, 4,944,885, 4,801,387, and 4,869,845,
each
incorporated by reference herein. The number average molecular weight may fall
within the
range of 1,000 to 1,000,000 grams/mole.
[0054] Other non-limiting examples of suitable copolymers useful as material
(a) include those
having as repeat units: (i) at least one sulfonated styrene moiety:
26U6545.DOC
CA 02758686 2011-11-21
12 Attorney Docket No. 5049-092780
H H
I I
C
SO3M
and (ii) at least one moiety derived from maleic anhydride:
H H
I I
¨C ¨C
I I
C=0 C=0
_ om OMI I
wherein M is a water soluble cation, or each M is independently selected from
NH4, H, Na, or K.
Non-limiting examples of such copolymers include VERSA TL-4 sulfonated styrene
copolymer
available from Akzo-Nobel and those disclosed in U.S. Patent No. 4,288,327,
incorporated by
reference herein.
[0055] Other non-limiting examples of suitable copolymers useful as material
(a) include
CARBOSPERSETM K-798 terpolymer of acrylic acid, 2-acrylamido-2-methylpropyl
sulfonic
acid and sulfonated styrene (AA/AMPS/SS) available from Lubrizol Advanced
Materials, Inc. of
Cleveland, OH, and those disclosed in U.S. Patent No. 4,952,327, incorporated
by reference
herein.
[0056] In some non-limiting embodiments, the reactants used to prepare
material (a) further
comprise at least one ethylenically unsaturated material that is different
from (1) the at least one
ethylenically unsaturated, carboxylic acid functional material or anhydride
thereof; and (2) the at
least one ethylenically unsaturated, sulfonyl functional material, and the at
least one ethylenically
unsaturated, sulfonate functional material. The ethylenically unsaturated
material that is
different from (1) and (2) is chemically different from (1) and (2), i.e., has
at least one different
atom or arrangement of atoms from (1) and (2). Non-limiting examples of
suitable ethylenically
unsaturated materials that are different from (1) and (2) include at least one
monomer selected
from the group consisting of acrylamides, vinyl esters, vinyl acetates and
mixtures thereof, for
example tert-butyl acrylamide; 2-propenoic acid, 2-methyl-, methyl ester; and
mixtures thereof.
26U6545.DOC
CA 02758686 2011-11-21
13 Attorney Docket No. 5049-092780
In some non-limiting embodiments, the ethylenically unsaturated materials
different from (1) and
(2) can comprise up to about 60 weight percent of the at least one
ethylenically unsaturated
material (1) above, or about 0.1 to about 60 weight percent, or about 0.5 to
about 30 weight
percent, or about 1 to about 15 weight percent, on a basis of total weight of
the reactants.
[0057] Non-limiting examples of such copolymers useful as material (a) include
ACUMER 3100
terpolymer of acrylic acid, 2-acrylamido-2-methylpropyl sulfonic acid and tert-
butyl acrylamide
(AA/AMPS/TBAM) available from Mid South Chemical Co., Inc. of Ringgold, LA or
Rohm and
Haas Co.; PRISM terpolymer of acrylic acid, sulfomethylacrylamide and
acrylamide
(AA/SMA/AM) available from Nalco Chemical; or those disclosed in U.S. Patent
Nos.
4,711,725, 4,801,388, and 5,282,976, each incorporated by reference herein.
[0058] In some non-limiting embodiments, the reactants used to prepare
material (a) further
comprise at least one ethylenically unsaturated polyalkylene oxide. Non-
limiting examples of
suitable ethylenically unsaturated polyalkylene oxides include those selected
from the group
consisting of allyl polyethylene glycol, methallyl polyethylene glycol,
polyethylene glycol
acrylate, polyethylene glycol methacrylate, methoxy allyl polyethylene oxide,
alkoxyallyl
polyethylene oxide, allyl polypropylene glycol, methallyl polypropylene
glycol, polypropylene
glycol acryl ate, polypropylene glycol methacrylate, methoxy ally!
polypropylene oxide,
alkoxyallyl polypropylene oxide, and mixtures thereof.
[0059] Non-limiting examples of such copolymers useful as material (a) include
TRC-271
copolymer prepared from AA, AMPS and (CH2CH205)H-Methacrylic acid ether (HEM-
5),
available from Nalco Company, or copolymers of unsaturated carboxylic acid,
unsaturated
sulfonic acid and unsaturated polyalkylene oxide, such as are disclosed in
U.S. Patent No.
4,618,448, incorporated by reference herein. The ethylenically unsaturated
polyalkylene
oxide(s) can comprise about 0.1 to about 60 weight percent, or about 0.5 to
about 30 weight
percent, or about 1 to about 15 weight percent, on a basis of total weight of
the reactants.
[0060] The aqueous composition can comprise about 10 parts per million (ppm)
active to about
10,000 ppm of material (a), or about 10 to about 1,000 ppm, or about 10 to
about 1,500 ppm, on
a basis of total weight of the components of the aqueous composition.
[0061] In some non-limiting embodiments, the material (a) is at least
partially water soluble. As
used herein with respect to the material (a), "water soluble" means that the
material (a) is capable
of being at least partially or fully dissolved in water at ambient temperature
(about 25 C). The
26U6545.DOC
CA 02758686 2011-11-21
14 Attorney Docket No. 5049-092780
solubility of a component of the fluid treatment systems or compositions of
the present
invention, for example solubility of the material (a), can be determined by
adding 1.0 weight
percent of the component to water at 25 C and mixing thoroughly (about 5
minutes) with a
magnetic stirrer. The mixture is permitted to stand for 24 hours and the
clarity and separation of
components of the mixture is assessed by visual observation. A clear,
generally haze-free
solution is "water soluble", a hazy/turbid solution is "water dispersible" or
"partially water
soluble", and a mixture that separates into layers or has noticeable solid
particulates is "water
insoluble".
[0062] The fluid treatment systems or compositions comprise at least one scale
control agent (b)
selected from the group consisting of water-soluble polycarboxylates,
phosphonates, phosphates,
polyphosphates, metal salts and sulfonates. The polycarboxylates, metal salts
and sulfonates are
chemically different from material (a) discussed above, i.e., have at least
one different atom or
arrangement of atoms from material (a) discussed above.
[0063] Non-limiting examples of suitable water-soluble polycarboxylates,
phosphonates,
phosphates, polyphosphates, metal salts and sulfonates include disclosed in
U.S. Patent No.
4,640,793, incorporated by reference herein. Non-limiting examples of suitable
water-soluble
polycarboxylates include polymers derived from homo- and/or copolymers
(including
terpolymers, tetra-, etc.) of acrylic acid, methacrylic acid, vinyl acetic
acid, allyl acetic acid,
fumaric acid, phosphinocarboxylic acid, maleic acid or anhydride, itaconic
acid, a-halo acrylic
acid and 0-carboxyethyl acrylic acid. It is possible that the carboxylic acid,
from which the
polycarboxylate is prepared, is the same carboxylic acid used to prepare the
reaction product of
material (a). However, the carboxylic acid used to prepare the polycarboxylate
is not
polymerized with the same ethylenically unsaturated, sulfonyl functional
and/or sulfonate
functional material as used to prepare material (a) as above. Non-limiting
examples of suitable
water-soluble phosphonates include hydroxyphosphono acetic acid (HPA),
diethylenetriaminepenta(methylenephosphonic acid),
hexamethylenediaminetetra-.
(methylenephosphonic acid), 2-phosphono-1,2,4-tricarboxybutane, amino
tri(methylene
phosphonic acid), hydroxyethylidene diphosphonic acid, phosphonosuccinic acid,
benzene
phosphonic acid, 2-aminoethyl phosphonic acid, and polyamino phosphonates, and
salts thereof
where they exist. Other useful phosphonates are disclosed in U.S. Pat. No.
3,837,803,
incorporated by reference herein. Non-limiting examples of suitable water-
soluble phosphates
26U6545.DOC
CA 02758686 2011-11-21
15 Attorney Docket No. 5049-092780
include orthophosphate; condensed phosphates, such as sodium
hexametaphosphate; phosphate
esters; organophosphate esters, such as the lower alkyl mono-, di- and
trialkyl phosphates. The
alkyl group can be selected from C1 to C4 and may be branched or unbranched.
The alkyl group
may be substituted with hydroxy, amino, halide, sulfate or sulfonate, alone or
in combination;
and molecularly dehydrated phosphates. Non-limiting examples of suitable water-
soluble metal
salts include water-soluble salts of zinc, molybdenum, chromate and sodium
silicate and
mixtures thereof. Non-limiting examples of suitable water-soluble sulfonates
include homo-
and/or copolymers of 2-acrylamido-2-methylpropylsulfonic acid, 2-
methacrylamido-2-
methylpropylsulfonic acid, styrene sulfonic acid, vinyl sulfonic acid, sulfo
alkyl acrylate or
methacrylate, allyl or methallyl sulfonic acid, sulfonic acid acrylate, 3-
methacrylamido-2-
hydroxy propyl sulfonic acid, their salts and mixtures thereof.
[0064] In some non-limiting embodiments, the fluid treatment systems or
compositions can
further comprise at least one polyether polyamino phosphonate. Non-limiting
examples of
suitable polyether polyamino methylene phosphonates are disclosed in U.S.
Patent No.
5,262,061, incorporated by reference herein, and include those of the formula:
M203P ¨CH2 R R CH2P03M2
N-C-C H2 H n I
M203 P-C H2 CH2P03M2
and optionally the N-oxides thereof; where n is an integer or fractional
integer which is about 2
to about 12; M is hydrogen or a suitable cation; and each R may be the same or
different and is
independently selected from hydrogen and methyl.
[0065] In some non-limiting embodiments, the scale control agent comprises 2-
phosphono-1,2,4-
tricarboxybutane, polyacrylic acid and a reaction product prepared from
acrylic acid and 2-
acrylamido-2-methylpropyl sulfonic acid.
[0066] The fluid treatment systems or compositions comprise at least one
friction reducing agent
(c) selected from the group consisting of guar gums, hydratable cellulosic
materials,
polyacrylamides, viscoelastic surfactants and mixtures thereof. As used
herein, "friction
reducing agent" means a material that alters fluid rheological properties to
reduce energy loss
aseociated with friction created within the fluid or between the fluid and
tubing or piping as the
fluid flows though the tubing or piping. Generally, friction reducing agents
decrease the =
26U6545.DOC
CA 02758686 2011-11-21
16 Attorney Docket No. 5049-092780
turbulence induced as the fluid flows. The determination of whether a material
is a friction
reducing agent can be made by comparing the pressure drop in turbulent flow in
a closed loop
piping system such as is described in Example 3 using two aqueous compositions
containing the
same components, with one composition replacing a portion of the water
component with the
friction reducing agent. If the pressure drop is less for the composition
containing the proposed
friction reducing agent, then the friction reducing agent is functioning as a
friction reducer. In
some non-limiting embodiments, the percent friction reduction can be at least
5%, or at least
10%, or at least 20% or more. Methods for determining the percent friction
reduction are
described in Example 3 below.
[0067] The friction reducing agent(s) can comprise about 10 parts per million
(ppm) active to
about 20,000 ppm of the components used to prepare the compositions, or about
10 to about
10,000 ppm, or about 10 to about 1,500 ppm on a basis of total weight of the
components of the
composition.
[0068] In some non-limiting embodiments, the friction reducing agent comprises
one or more
guar gums. The guar gum can be non-hydrolyzed or partially hydrolyzed (PHGG),
and can be
produced by the partial enzymatic hydrolysis of guaran, the galactomannan of
the endosperm of
guar seeds (guar gum). PHGG is a neutral polysaccharide consisting of a
mannose backbone
chain with single galactose side units occurring on almost two out of every
three mannose units.
The average molecular weight can be about 25,000 Daltons. Other useful guar
materials include
hydroxypropylguar, carboxymethylhydroxypropylguar, carboxymethylguar and
mixtures thereof.
[0069] In some non-limiting embodiments, the friction reducing agent comprises
one or more
viscoelastic surfactants. As used herein, "viscoelastic" means a viscous
liquid having elastic
properties, i.e., the liquid at least partially returns to its original form
when an applied stress is
released, or that the elastic (or storage) modulus G' of the fluid is greater
than the loss modulus
G" as measured using an oscillatory shear rheometer (such as a Bohlin CVO 50)
at a frequency
of 1 Hz. The measurement of these moduli is described in An Introduction to
Rheology, by H.
A. Barnes, J. F. Hutton, and K. Walters, Elsevier, Amsterdam (1997).
[0070] Non-limiting examples of viscoelastic surfactants (VES), methods for
making the same,
and amounts suitable for use in a fracturing fluid are disclosed in: U.S. Pat.
No. 4,790,958; U.S.
Pat. No. 5,258,137; U.S. Pat. No. 5,551,516; U.S. Pat. No. 5,964,295; U.S.
Pat. No. 5,979,557;
U.S. Pat. No. 6,508,307; U.S. Pat. No. 6,306,800; U.S. Pat. No. 6,140,277;
U.S. Pat. No.
26U6545.DOC
CA 02758686 2011-11-21
( (-
17 Attorney Docket No. 5049-092780
6,412,561; U.S. Pat. No. 6,435,277; U.S. Pat. No. 6,446,727; U.S. Pat. No.
7,196,041; U.S. Pat.
No. 7,343,978 and U.S. Patent Publication No. 2008/0248976. Each of these U.S.
patents and
patent publications is incorporated by reference herein. Additional
information relating to
selected VES-based fluids is found in the SPE article, Polymer-Free Fluids for
Hydraulic
Fracturing, SPE 38622 (1997), incorporated by reference herein.
[0071] For example, U.S. Patent No. 5,551,516 discloses suitable viscoelastic
surfactants, for use
in combination with an organic salt and/or alcohol, as follows:
(a) an amine corresponding to the formula:
R2
I
121-N
i
R3
wherein R1 is at least about a C16 aliphatic group which may be branched or
straight
chained and which may be saturated or unsaturated, R2 and R3 are each
independently, hydrogen or a ci to about C6 aliphatic group which can be
branched or
straight chained, saturated or unsaturated and which may be substituted with a
group
that renders the R2 and/or R3 group more hydrophilic;
(b) salts of the amine corresponding to the formula:
[ R, IT: R.. 3c_
i
R3
wherein RI, R2 and R3 are the same as defined hereinbefore and X- is an
inorganic
anion; and
(c) a quaternary ammonium salt of the amine corresponding to the formula:
R2
RI-74===114+ X-
i
R3
wherein R1, R2 and R3 and X" are the same as defined hereinbefore and R4
independently constitutes a group which has previously been set forth for R3
and R3,
none of RI, R2, R3 or R4 are hydrogen, and the R2, R3 and R4 groups of the
amine salt
and quaternary ammonium salt may be formed into a heterocyclic 5- or 6-member
ring structure which includes the nitrogen atom of the amine.
26U6545.DOC
CA 02758686 2011-11-21
18 Attorney Docket No. 5049-092780
[0072] A non-limiting example of a useful viscoelastic surfactant is a
quaternary ammonium
salt, erucyl methyl bis (2-hydroxyethyl) ammonium chloride.
[0073] The viscoelastic surfactant is capable of forming rod-shaped micelles
as opposed to
typical surfactant materials which tend to form spherical micelles or sheet-
like structures. Non-
limiting examples of suitable viscoelastic surfactants include erucyl
trimethyl ammonium
chloride; N-methyl-N,N-bis(2-hydroxyethyl) rapeseed ammonium chloride; oleyl
methyl
bis(hydroxyethyl) ammonium chloride; octadecyl methyl bis(hydroxyethyl)
ammonium bromide;
octadecyl tris(hydroxyethyl) ammonium bromide; octadecyl dimethyl hydroxyethyl
ammonium
bromide; cetyl dimethyl hydroxyethyl ammonium bromide; cetyl methyl
bis(hydroxyethyl)
ammonium salicylate; cetyl methyl bis(hydroxyethyl) ammonium 3,4,-
dichlorobenzoate; cetyl
tris(hydroxyethyl) ammonium iodide; bis(hydroxyethyl) soya amine; N-methyl, N-
hydroxyethyl
tallow amine; bis(hydroxyethyl) octadecyl amine; cosyl dimethyl hydroxyethyl
ammonium
bromide; cosyl methyl bis(hydroxyethyl) ammonium chloride; cosyl
tris(hydroxyethyl)
ammonium bromide; dicosyl dimethyl hydroxyethyl ammonium bromide; dicosyl
methyl
bis(hydroxyethyl) ammonium chloride; dicosyl tris(hydroxyethyl) ammonium
bromide;
hexadecyl ethyl bis(hydroxyethyl) ammonium chloride; hexadecyl isopropyl
bis(hydroxyethyl)
ammonium iodide; N,N-dihydroxypropyl hexadecyl amine, N-methyl, N-hydroxyethyl
hexadecyl amine; N,N-dihydroxyethyl dihydroxypropyl oleyl amine; N,N-
dihydroxypropyl soya
amine; N,N-dihydroxypropyl tallow amine; N-butyl hexadecyl amine; N-
hydroxyethyl octadecyl
amine; N-hydroxyethyl cosyl amine; cetylamino, N-octadecyl pyridinium
chloride; N-soya-N-
ethyl morpholinium ethosulfate; methyl-1 -oleyl amido ethyl-2-oleyl
imidazolinium-methyl
sulfate; and methyl- 1-tallow amido ethyl-2-tallow imidazolinium-methyl
sulfate.
[0074] Other non-limiting examples of suitable viscoelastic surfactants
include those disclosed
in U.S. Patent No. 6,508,307 having the general formula:
1110H
R4-14+-R3
R201-1
in which R1 and R2 are the same or different and are each short alkyl chains
(i.e., from about two
to about five carbon atoms in length), R3 is an alkyl group of about one to
four carbon atoms, and
R4 is a much longer alkyl chain, that can be unsubstituted.
26U6545.DOC
CA 02758686 2011-11-21
19 Attorney Docket No. 5049-092780
[0075] Other non-limiting examples of suitable viscoelastic surfactants
include those disclosed
in U.S. Patent No. 7,196,041 having the general formula: (Ri--X),2, as defined
therein. R1 is an
aliphatic group comprising a C10-C25 straight chain bonded at a terminal
carbon atom thereof to
X, the straight chain having a length such that a viscoelastic gel is formable
by the surfactant in
aqueous media; and further comprising at least one side C1-C6 side chain
enhancing the solubility
of the surfactant in hydrocarbons, and being sufficiently close to said head
group and sufficiently
short such that the surfactant forms micelles in said viscoelastic gel. X is a
charged head group,
Z is a counterion, and n is an integer which ensures that the surfactant is
charge neutral. X may
be a carboxylate (-000), quaternary ammonium (-NR2R3R4+), sulphate (-0S03), or
sulphonate
(--S03) charged group; N being a nitrogen atom, and R2, R3 and R4 being Ci-C6
aliphatic
groups, or one of R2, R3 and R4 being a C1-C6 aliphatic group and the others
of R2, R3 and R4
forming a five- or six-member heterocyclic ring with the nitrogen atom. When X
is a
carboxylate, sulphate, or sulphonate group, Z may be an alkali metal cation or
an alkaline earth
metal cation, such as Na+ or IC+. When X is a quaternary ammonium group, Z may
be a halide
anion, such as cr or Br", or a small organic anion, such as a salicylate. In
some min-limiting
embodiments, the surfactant is an alkali metal salt of 2-methyl oleic acid or
2-ethyl oleic acid.
[0076] Another non-limiting example of a suitable viscoelastic surfactant is
erucyl-N,N-di-(2-
hydroxyethyl)-N-methylammonium chloride (EHMAC).
[0077] In some non-limiting embodiments, the friction reducing agent comprises
one or more
hydratable cellulosic materials. Non-limiting examples of suitable hydratable
cellulosic
materials include those selected from the group consisting of cellulose,
methyl cellulose,
hydroxyethyl cellulose, grafted hydroxyethyl cellulose, hydroxypropyl
cellulose, hydroxymethyl
cellulose, carboxymethyl cellulose, carboxymethylhydroxyethyl cellulose and
mixtures thereof.
[00781 In some non-limiting embodiments, the friction reducing agent comprises
one or more
polyacrylamides. Non-limiting examples of suitable polyacrylarnides include
water-in-oil
emulsion polymers comprising a polymer or copolymer comprising repeat units
from an
acrylamide monomer, such as are disclosed in U.S. Patent No. 7,482,310,
incorporated herein by
reference herein. For example, suitable polyacrylamides can comprise one or
more repeat units
according to Formula I:
26U6545.DOC
CA 02758686 2011-11-21
20 Attorney Docket No. 5049-092780
---ECHR12¨CR1-1¨
I n
0
ZR2 (I)
wherein each occurrence of RI is independently selected from H, methyl and
ethyl; n is an
integer from 10 to 10,000,000; Z is selected from ¨0¨ and ¨NR2¨; and each
occurrence of
R2 is independently selected from the group consisting of H, C1-C22 linear,
branched or cyclic
alkyl, aryl, alkaryl, aralkyl or alkenyl group, ¨R3¨NR22,¨R3¨N+R23 X, and ¨R3-
803Y,
wherein R2 is as previously defined; R3 is a divalent linking group selected
from the group
consisting of C1-C22 linear, branched or cyclic alkylene, arylene, alkarylene,
aralkylene or
alkenylene, poly(ethyleneoxide) and poly(propyleneoxide); Y is H or an alkali
metal ion; and X
is a halide or methylsulfate.
[0079] As used herein the term "water-in-oil emulsion polymer" refers to a
system or
composition having a hydrophobic liquid as a continuous phase and an aqueous
solution or gel as
a dispersed phase, where the aqueous phase includes one or more water soluble
or water
dispersible polymers. The dispersed phase, present as droplets and/or discrete
microgels, can
have size ranging from at least 10 nm up to 500 microns. The size of the
droplets and/or discrete
microgels can be determined by light scattering and/or scanning electron
microscopy, as is
known in the art.
[0080] As used herein, the phrase "repeat units from an acrylamide monomer" is
meant to
indicate not only the monomer acrylamide, but also analogous repeat units
derived from, for
example, methacrylamide, N-methylacrylamide, and N,N-dimethylacrylamide;
functionalized
acrylamides, such as acrylamidomethylpropane sulfonic acid; hydrolysis
products of acrylamide,
such as acrylic acid and acrylic and methacrylic acid esters.
[0081] In some non-limiting embodiments, the polymer or copolymer contains
repeat units from
an acrylamide monomer can be non-ionic, anionic, cationic, amphoteric, or
ampholytic. As used
herein, the term "anionic polymer or copolymer containing repeat units from an
acrylamide
monomer" refers to polymers containing acrylamide repeat units and repeat
units from a
monomer that can carry a negative charge at an appropriate pH and/or when
neutralized with a
suitable cation, non-limiting examples being acrylic acid, methacrylic acid,
and
26U6545.DOC
CA 02758686 2011-11-21
21 Attorney Docket No. 5049-092780
a.crylamidomethylpropanesulfonic acid. As used herein, the term "cationic
polymer or
copolymer containing repeat units from an acrylamide monomer" refers to
polymers containing
acrylamide repeat units and repeat units from a monomer that carries a
positive charge,
non-limiting examples being methacrylamidopropyltrimethyl ammonium chloride,
methacryloyloxyethyl trimethyl ammonium methylsulfate, and dimethyl diallyl
anunonium
chloride. As used herein, the term "amphoteric polymer or copolymer containing
repeat units
from an acrylamide monomer" refers to polymers containing acrylamide repeat
units and repeat
units from a monomer that carries a positive charge at an appropriate pH and a
monomer that
carries a negative charge at an appropriate pH. Non-limiting examples of the
former are
methacrylamidopropyldimethylamine, methacryloyloxyethyldimethylarnine and
methyl diallyl
amine, and the latter are acrylic acid, methacrylic acid and maleic acid. As
used herein, the term
"ampholytic polymer or copolymer containing repeat units from an acrylamide
monomer" refers
to polymers containing acrylamide repeat units and repeat units from a monomer
that carries a
positive charge and a monomer that carries a negative charge at an appropriate
pH. Non-limiting
examples of the former are methacrylamidopropyltrimethyl ammonium chloride,
methacryloyloxyethyl trimethyl ammonium methylsulfate, acryloyloxyethyl
trimethyl
- ammonium chloride and dimethyl diallyl ammonium chloride, and the latter
are acrylic acid,
methacrylic acid and maleic acid.
[0082] In some non-limiting embodiments, the copolymer containing repeat units
from an
acrylamide monomer can further comprise repeat units derived from one or more
monomers
selected from acrylaraidopropyl trimethyl ammonium chloride (APTAC),
methacrylamidopropyltrimethyl ammonium chloride (MAPTAC), methacryloyloxyethyl
trimethyl ammonium chloride (METAC), methacryloyloxyethyl trimethyl ammonium
methylsulfate (METAMS), acryloyloxyethyl trimethyl ammonium chloride (AETAC),
dimethyl
diallyl ammonium chloride (DMDAAC), acrylic acid (AA), methacrylic acid (MAA),
2-
acrylamido-2-methylpropane sulfonic acid (AMPSA), 2-methacrylamido-2-
methylpropane
sulfonic acid (MAMPSA), C1-C3 alkyl acrylate, C1-C3 alkyl methacrylate, n-
alkyl acrylamide,
methacrylamide, n-alkylmethacrylamide, and/or diacetone acrylamide.
[0083] The molecular weight of the polymer or copolymer containing repeat
units from an
acrylamide monomer is typically approximated by measuring the reduced
viscosity of a solution
of the polymer using an appropriately sized Ubbelohde Capillary Viscometer at
0.05 g/dl in 1N
26U6545.DOC
CA 02758686 2011-11-21
22 Attorney Docket No. 5049-092780
NaC1 at 30 C and pH of 7. In some non-limiting embodiments, the polymer or
copolymer of the
aqueous phase has a reduced viscosity of at least 5 dl/g and up to 50 dl/g.
[0084] Although the molecular weight of the polymer or copolymer containing
repeat units from
an acrylamide monomer can be difficult to determine, it can be measured using
gel permeation
chromatography (GPC) using acrylamide or poly(styrene sulfonate) standards as
is known in the
art. As such, the molecular weight of the polymer or copolymer can be at least
10,000 and up to
1,000,000 as measured using GPC techniques.
[0085] The fluid treatment system(s) or composition(s) of the present
invention can be used to
treat water, for example to inhibit the formation and/or precipitation of
compounds such as metal
oxides. In some non-limiting embodiments, the water can comprise metal ions or
other
contaminants, such as calcium ions, ferrous ions, ferric ions, and/or ferric
compounds, as
described below. In some non-limiting embodiments, the water can be
subterranean water,
surface water or brine water.
[0086] The components of the fluid treatment system, such as material(s) (a)
and scale control
agent(s) (b), can be combined with the water sequentially (in any order
desired) of concurrently.
In some non-limiting embodiments, the friction reducing agent(s), if present,
is added last. The
amount of material (a) added to the water is at least about 0.001%, or about
0.001% to about
1.0%, or about 0.005 to about 1.0%, or about 0.01% to about 0.5% on a basis of
total weight of
the aqueous composition (fluid treatment system, water, and any other
additives). The amount of
scale control agent(s) (b) added to the water can be at least about 0.1 mg/L,
or about 0.2 mg/L to
about 1,000 mg/L, or about 0.2 mg/L to about 100 mg/L on a basis of total
weight of the aqueous
composition (fluid treatment system, water, and any other additives).
[0087] In some non-limiting embodiments, the fluid treatment system or
composition is a
fracturing fluid for treating water in a subterranean formation penetrated by
a well bore. The
fracturing fluid comprises water and the fluid treatment system or composition
described above.
[0088] Suitable water-in-oil emulsion polymers include water-in-oil emulsion
polymers
containing polymers and copolymers of acrylamide, such as are discussed above.
In some non-
limiting embodiments, the water-in-oil emulsion polymer includes a hydrophobic
oil phase, a
surfactant system and a polymer-containing aqueous phase comprising water and
the polymer or
copolymer containing repeat units from an acrylamide monomer. Commercially
available
"water-in-oil emulsion polymers" that can be used in the present invention
include, but are not
261J6545.DOC
CA 02758686 2011-11-21
23 Attorney Docket No. 5049-092780
limited to, WFR-3B, WFR-5, SAS-2 and Cw-3K polyacrylamide-based products
available from
Superior Well Services.
[0089] In some non-limiting embodiments, the water fluid treatment system or
composition
according to the present invention can comprise or consist of (a) ICP-1000
composition; (b)
WFR-3B polyacrylamide; and (c) 2-phosphono-1,2,4-fricarboxybutane, polyacrylic
acid and a
reaction product prepared from acrylic acid and 2-acrylamido-2-methylpropyl
sulfonic acid as
scale control agents, in a weight ratio of (a):(b) of about 0.005:1 to about
50:1 and in a weight
ratio of (b):(c) of about 2:1 to about 1:40. In some non-limiting embodiments,
the fluid
treatment system is the Gamma FRacTm system available from Superior Well
Services.
[0090] In some non-limiting embodiments, the polymer-containing aqueous phase
including
water and the polymer or copolymer containing repeat units from an acrylamide
monomer makes
up at least about 5 to about 90% by weight of the water-in-oil emulsion
polymer.
[0091] As discussed above, the fracturing fluid can comprise one or more
inorganic
microparticles. As used herein, the term "microparticle" means solid particles
with very small
dimensions, which can range from nanometers to microns. Suitable inorganic
microparticles
include, but are not limited to, fumed silica, fumed alumina, precipitated
silica, colloidal silica,
alumina silicates, treated silica, calcium carbonate, silica flour,
diatomites, talc, borosilicates, and
mixtures thereof. Treated silicas can include surface treated or surface
modified silica that has
been treated with organic materials (hydrophobic silica) or alumina (alumina
treated silica) as is
known in the art. In some non-limiting embodiments, the surface area of the
inorganic
microparticle can range from at least about 1 m2/g to about 1,000 m2/g. The
surface area of the
microparticles is determined using BET nitrogen absorption as is known in the
art. In some non-
limiting embodiments, the inorganic microparticles comprise at least about
0.1% to about 10%
by weight of the water-in-oil emulsion composition.
[0092] In an embodiment of the invention, the water-in-oil emulsion
composition comprises at
least about 0.005% up to about 20%, or at least about 0.01% up to about 20%,
by weight of the
fracturing fluid. The water-in-oil emulsion composition can be made as
disclosed in U.S. Patent
No. 7,482,310, incorporated by reference herein.
[0093] The fracturing fluid can further comprise one or more proppant
materials. Suitable
proppant materials include, but are not limited to, resin coated or uncoated
sand, Ottawa type
sand (round), Brady type sand (angular), sintered bauxite, ceramic materials
and glass beads.
26U6545.DOC
CA 02758686 2011-11-21
24 Attorney Docket No. 5049-092780
The particle size of the proppant material can range from about 200 gm to
about 5,000 m. The
particle size is the weight average determined using a series of Tyler Sieves
of various mesh
sizes available from W.S. Tyler, Mento, Ohio. Further description of suitable
proppant
materials, their use and concentrations thereof in the present fracturing
fluid are described in
Glidley et al., Recent Advances in Hydraulic Fracturing, Chapter 6, "Propping
Agents and
Fracture Conductivity", Society of Petroleum Engineers, Richardson, TX, pp.
109-130. In some
non-limiting embodiments, the fracturing fluid can comprise about 0.5% to
about 30% proppant
material based on the weight of the fracturing fluid. In some non-limiting
embodiments, the
fracturing fluid can comprise about 0.1 to about 10 pounds of proppant
material per gallon of
fracturing fluid.
[0094] The water used to make up the fracturing fluid can be selected from
fresh water, recycled
water, water containing high dissolved constituents such as flowback water or
mine drainage
water, unsaturated brine, and saturated brine. Flowback water is the recovered
fracturing fluid
and produced water which flows back to the surface from an oil or gas well
drilling operation
and is extracted. Flowback water may have high salinity and total dissolved
solids (FDS).
[0095] In some non-limiting embodiments, the fracturing fluid can further
comprise an additive
that is a pH adjusting compound selected from sodium hydroxide, potassium
hydroxide, lithium
hydroxide, ammonia, sodium carbonate, potassium carbonate, sodium bicarbonate,
potassium
bicarbonate, sodium diacetate, potassium diacetate, sodium phosphate,
potassium phosphate,
sodium dihydrogen phosphate, potassium dihydrogen phosphate, and mixtures
thereof. These
additives are present at a level sufficient to maintain a desired pH. The
level of pH adjusting
compound can be from about 0.01% to about 1.0% based on the weight of the
fracturing fluid.
[0096] In some non-limiting embodiments, the fracturing fluid can further
comprise a clay
stabilizer selected from the group consisting of potassium chloride, sodium
chloride, ammonium
chloride, tetramethyl ammonium chloride and temporary clay stabilizers. The
level of clay
stabilizers can be from about 0.1% to about 10% based on the weight of the
fracturing fluid.
[0097] In some non-limiting embodiments, the fracturing fluid can further
comprise a fluid loss
control agent selected from the group consisting of silica flour, starches,
waxes and resins. The
level of fluid loss control agent can range from about 0.01% to about 2.0%
based on the weight
of the fracturing fluid.
26U6545.DOC
CA 02758686 2011-11-21
25 Attorney Docket No. 5049-092780
[0098] In some non-limiting embodiments, the fracturing fluid can further
comprise a biocide,
such as 2,2-dibromo-3-nitrilopropionamide, which is available in a 20%
solution as KR-153SL
biocide from Kroff Chemical Co. Other suitable biocides are known to those
skilled in the art.
The amount of biocide can range from about 20 to about 2,000 ppm, based upon
the total weight
of the fracturing fluid.
[0099] In some non-limiting embodiments, the fracturing fluid can further
comprise a delayed
breaker for causing the treating fluid to revert to a thin fluid selected from
the group of oxidizers,
encapsulated oxidizers and enzyme breakers consisting of sodium persulfate,
potassium
persulfate, ammonium persulfate, magnesium peroxide, sodium chlorite, sodium
bromate, alpha
and beta amylases, amyloglucosidase, invertase, maltase, cellulose,
halogenated isocyanurate,
hypochlorites and hemicellulase. The amount of delayed breaker can range from
about 0.01% to
about 2% by weight based on the weight of the fracturing fluid.
[00100] The fracturing fluid can be injected into a formation by first
providing a bore hole
or well hole, which may or may not include a casing or liner and may or may
not have been
shape charged to initiate fractures. The fracturing fluid is pumped into the
bore hole or well hole
to provide a pressure of about 0.1 to about 2 psi/ft. (ft. referring to the
depth of the bore hole or
well hole), depending on the composition of the fracturing fluid and the
nature of the formation
to be fractured. As such, the pressure in the bore hole or well hole can be at
least about 500 psi
up to about 15,000 psi. While not intending to be bound by any single theory,
it is believed that
the pressure drives the fluid into cracks, fissures and fractures in the
formation, forcing such
openings to become larger and propagate. The proppant material tends to wedge
into the
expanded cracks, fissures and fractures to help hold them open when the
pressure is reduced.
However, the pressure can act to force water out of the fluid, in an action
similar to syneresis
(i.e., exudation of the liquid component of a gel). This liquid can then seep
or imbibe through
capillary action into microscopic and larger cracks, fractures and fissures,
thus removing water
from the fluid, increasing the effective polymer concentration and therefore
the viscosity of the
fluid. Such increases in viscosity can limit the ability of the fluid to
penetrate the formation. It
is believed that the microparticles can fill the relatively small cracks,
fractures and fissures,
slowing or limiting water loss, which increases the productivity and
efficiency of the fluid and
the fracturing operation.
26U6545.DOC
CA 02758686 2011-11-21
26 Attorney Docket No. 5049-092780
[001011 As discussed in detail above, calcium and magnesium ions can combine
with
anions, such as carbonate and sulfate, to form a solid. This solid material
will form scale and
may combine with other chemicals in the water. Thus, in some non-limiting
embodiments, the
present invention provides methods of inhibiting formation and/ or
precipitation of calcium salts
in an aqueous composition comprising calcium ions, comprising: mixing an
aqueous
composition comprising calcium ions with any of the above fluid treatment
systems or
compositions.
[001021 As discussed in detail above, the air (oxygen) in the ground water
causes
dissolved ferrous ions contained in the ground water to be oxidized to the
insoluble ferric state.
Ions in the ferric state readily precipitate, clogging flow pathways in the
oil or gas well
formation, thus restricting oil or gas flow. Chelating agents such as citric
acid have been used as
an iron control agent to treat oil and gas well formations. However, improper
dosing of certain
chelating agents such as citric acid can negatively impact friction reducing
additives, for example
certain polyacrylamide-based products, such as acrylic acid/acrylamide
copolymers. The fluid
treatment systems, compositions and fracturing fluids of the present invention
can inhibit the
formation of metal oxides, such as iron oxide, from metal ions present in the
groundwater, such
as ferrous ions, while minimizing the adverse impact of the metal ions on
performance of the
friction reducing agent to enhance the performance of the friction reducing
agent. Thus, when
the fluid treatment systems, compositions or fracturing fluids of the present
invention are mixed
with water comprising metal ions, such as ferrous ions, an aqueous composition
is formed that
inhibits the formation and precipitation of metal oxides. Thus, in some non-
limiting
embodiments, the present invention provides methods of inhibiting formation
and/ or
precipitation of metal oxides in an aqueous composition comprising metal ions,
comprising:
mixing an aqueous composition comprising metal ions with any of the above
fluid treatment
systems or compositions. Non-limiting examples of such metal ions include
ferrous ions,
chromium ions, zinc ions, manganese ions, aluminum ions, and mixtures thereof.
[00103] In some non-limiting embodiments, methods of inhibiting formation
and/or
precipitation of metal oxides in an aqueous composition or ground water
comprising at least 20
milligrams, or at least about 25 mg, or at least about 50 mg, or at least
about 70 mg, of metal ions
(such as calcium ions or ferrous ions) per liter of aqueous composition are
provided which
comprise: (a) mixing the aqueous composition or water with the fluid treatment
system,
26U6545.DOC
CA 02758686 2011-11-21
(-
27 Attorney Docket No. 5049-092780
composition or fracturing fluid of the present invention. In some non-limiting
embodiments, one
or more additional friction reducing agents or other additives as described
above can be included.
[001041 Generally, the amount of fluid treatment system or composition
according to the
present invention administered as a metal stabilizer or iron control agent in
down-hole
applications is at least about 0.1 mg/L reaction product per mg/L metal ion,
or at least about 1.0
mg/L reaction product per mg/L metal ion, or at least about 1.5 mg/L reaction
product per mg/L
metal ion, or at least about 1.6 mg/L reaction product per mg/L metal ion, or
at least about 2.0
mg/L stabilizer per mg/L metal ion, or at least about 10 mg/L stabilizer per
mg/L metal ion, or at
least about 20 mg/L stabilizer per mg/L metal ion, or at least about 40 mg/L
stabilizer per mg/L
metal ion, depending upon such factors as system demand and pH.
[001051 In some non-limiting embodiments, the fracturing fluid can be an acid
fracturing
fluid. The acid fracturing fluids of the present invention can comprise water,
acid, and a fluid
treatment system or composition according to the present invention as
described in detail above.
Non-limiting examples of suitable acids include hydrochloric acid, acetic
acid, formic acid,
hydrofluoric acid, sulfamic acid, chlorinated acetic acid, gelled or
emulsified acids, and mixtures
thereof. The amount of acid in the fracturing fluid can range from about 0.01
weight percent to
about 25 weight percent based upon the total weight of the fracturing fluid.
Such acid fracturing
fluids can be used for matrix acidizing treatments and/or fracture acidizing
treatments or
combined to use in part or stages within a near-neutral pH fracturing fluid
system. In matrix
acidizing, the acid fluid flows through the flow pathways in a formation,
dissolving solids and
fines entrained in pore throats and pore spaces that impede oil or gas flow.
Acid fracturing is an
alternative to hydraulic fracturing with proppant. The acid etches the
fracture face to create
voids and points of support which hold the rock channel open.
1001061 The present invention will further be described by reference to the
following
examples. The following examples are merely illustrative of the invention and
are not intended
to be limiting. Unless otherwise indicated, all percentages are by weight.
26U6545.DOC
CA 02758686 2011-11-21
28 Attorney Docket No. 5049-092780
EXAMPLES
Example 1
[00107] Citric acid functions stoichiometrically (one mole of citric acid
complexes one
mole of iron), however additional citric acid is needed to drive the formation
of the metal
complex, the additional amount being a function of pH (i.e., the conditional
stability constant).
On a stoichiometric basis, 2.0 mg/L Fe+2 would require 6.88 mg/L citric acid.
Interestingly, a
Minimum Effective Dose ("MED") of 7.50 mg/L is close to the stoichiometric
requirement.
[00108] The ability to stabilize iron in an aqueous sample was evaluated using
the "High
Iron Stabilization Test" at 70 mg Fe+2/per liter of water. Samples of
conventional iron
stabilizers, such as citric acid, the tetrasodium salt of
ethylenediaminetetraacetic acid
(NasEDTA), and triethanolamine (TEA) were evaluated and compared to
compositions including
sulfonyl and carboxyl functional reaction products according to the present
invention, such as
KR-DP0184 AA/AMPS copolymer prepared from 60 weight percent AA and 40 weight
percent
AMPS available from Kroff Chemical Co., as discussed in detail below.
High Iron Stabilization Test
[00109] The iron stabilization test evaluates and quantifies the ability of an
iron control
additive to maintain the iron in soluble form and to prevent precipitation.
This is accomplished
by measuring the iron content that remains in solution after a specified time
under test conditions
and comparing to an untreated control. Conditions for the test were:
26U6545.DOC
CA 02758686 2011-11-21
29 Attorney Docket No. 5049-092780
pHi: 8.5
pHf: 8.0 - 8.5
Temperature: Ambient (about 25 C)
Test Duration: 2.0 hr
Mixing Rate: 20 rpm on gang stirrer
Filter: >20-25 micron
Matrix: Synthetic 4X Pittsburgh water
Initial Fe+2 70.0
(ng/L)
The percent stabilization is calculated as follows:
% Stabilization = jilDON
(Febitig - Febtank)
where: Feinitig = Initial concentration of dissolved iron (-70.0 mg,/L)
(This is the value for the "Start" sample.)
Fefing = Final concentration of dissolved iron in treated test
Febtank = Final concentration of dissolved iron in untreated test
The Minimum Effective Dose ("MED") for the scale inhibition tests is defined
as the dosage at
which >90% stabilzation is attained.
V. SAMPLE CALCULATION
[00110] Suppose the final iron concentration, Fefing, for an iron stabilizer
is 60.0 mg/L, the
amount of iron remaining in the blank, Feblank, is 1.3 mg/L, and the initial
Fe(ll) concentration,
Feinittat is 69.5, The % Stabilization would be calculated as follows:
% Stabilization = (60.0 - 1.3) x 100% = 86.1%
(69.5 ¨ 1.3)
[00111] The composition of the 4X Pittsburgh Water matrix and the composition
of the
test water are listed in Table 1 below.
26U6545.DOC
CA 02758686 2011-11-21
30 Attorney Docket No. 5049-092780
Table 1
Ion 4X Pittsburgh Water Test Water*
mg/L
C+ 1111.111111883) 88.3
Mg2+ 24.0 24.0
Na+ 71.0 240.2
Fe+ 70.0
S042" 328.9 689.3
Cl 70.0 70.0
Alkalinity 32.8 151.0
(as CaCO3)
Cations (Meq) 9.47 19.34
Anions (Meq) 9.48 19.35
*Test Water Composition included Fe (II) Stock Solution and the addition of
the 1.0 N
NaOH needed to neutralized the Fe (II) Stock Solution
[001121 To determine the viability of using sulfonyl and carboxylic acid
functional
polymers compositions in down-hole applications, the High Iron Stabilization
Test was
developed with an initial ferrous ion level of 70 mg/L. Control compositions
including Citric
Acid, Na4EDTA, TEA and Test Compositions containing sulfonyl and carboxylic
acid functional
polymers according to the present invention were evaluated.
Composition of Stabilizers:
KR-DP0184: 60/40 AA/AMPS (Acrylic Acid/2-Acrylamido-2-methylpropylsulfonic
Acid), Mw = 17,700; Mn = 5,900
Acumer 3100: ¨ 65/22/13 AA/AMPS/TBAM (Acrylic Acid/2-Acrylamido-2-
methylpropylsulfonic Acid/Tertbutylacrylamide), Mw = 4,500
Prism: 20-80/5-55/5-60 AA/SMA/AM (Acrylic Acid/Sulfomethylacrylamide/
Acrylamide), Mw = 7,000-90,000
HPS-1: ¨52.4:47.6 AA/AHPSE (Acrylic Acid/Ally1-2-hydroxypropylsulfonic acid
ether).
Monomer ratio is 3:1. Mw ¨ 14,000.
Aquatreat AR-540: AA/SPME/Monomer 3/Monomer 4 (Acrylic Acid/Sulfonated Phenol
Methacrylic Ether/2-Propene-1-sulfonic acid, 2-methy1/2-Propenoic Acid, 2-
methyl-, methyl ester) CH2CHCOO]vv-[CH2CH3CCH20C6H4S03]x-
(CH2CH3CCH2S03]y4CH2CH3CCOOCH3]z, Mw = 15,277; Mn = 4,961
26U6545.DOC
CA 02758686 2011-11-21
31 Attorney Docket No. 5049-092780
K-798: ¨60/34/6 AA/AMPS/SS (Acrylic Acid/2-Acrylamido-2-methylpropylsulfonic
Acid/Sulfonated Styrene), MW = 1,000-10,000
Acumer 2000: 75/25 AA/AMPS (Acrylic Acid/2-Acrylamido-2-methylpropylsulfonic
Acid), Mw = 4,500
Note: Monomer Ratios are weight ratios as opposed to mole ratios.
[00113] Tables 2a-2c present the testing results. The first tier of
compositions, containing
sulfonyl and carboxylic acid functional polymers KR-DP0184, Acumer 3100,
Prism, Acumer
2000, and K-798 each required a minimum active dose (MED) of 100-120 mg/L. The
second
tier of compositions, containing sulfonyl and carboxylic acid functional
polymers HP S-1 and
AR-540 each required an active dose of 125-150 mg/L. Citric acid performed the
best, with an
MED of 80 mg/L. The MED for Na4EDTA was 700 mg/L. TEA was ineffective.
[00114] Interestingly, the MED of 80 mg/L for citric acid was lower than even
the
stoichiometric requirement of 241 mg/L (3.4 mg Citric Acid/mg Fe). This would
suggest that
chelation was not the only mechanism of action in effect. In contrast, the MED
of 700 mg/L for
Na4EDTA exceeded the stoichiometric dose of 477 mg/L (6.8 mg Na4EDTA per mg
Fe) as
would be expected.
[00115] The results of the High Iron Stabilization Studies indicate that the
tested sulfonyl
and carboxylic acid functional polymers are effective iron stabilizers even at
the extremely high
iron levels present down hole.
[00116] The results show that as expected sulfonyl and carboxylic acid
functional
polymers provide good Fe(II) stabilization under high iron conditions. Factors
such as the
degree of sulfonation and molecular weight appear to be optimized with each of
the top tier
products. With the top performing polymers, the ratio of mg/L polymer to mg/L
Fe(II) is about
1.6:1.00.
26U6545.DOC
32 Attorney Docket No. 5049-092780
TABLE 2a: High Iron Stabilization Test Results
mg/L Active
Product MED* 50 60 70 75 80 90 100
110
Citric Acid 80 29.0 87.0 94.1 97.5
98.9
82.7 94.1 95.6
KR- 110 1.6 4.4 3.7 31.7
81.8 92.5
DP0184
87.2 89.3
Acumer 100 65.3
88.4
3100
92.0
93.2
Prism 110-120
69.3
HPS1 125-150
0
Ul
AR-540 125-150
K-798 110-150
77.7 0
Acumer 120
66.0
2000
1.)
Na4EDTA 700 9.5 - 6.0 14.0
TEA** None
** Triethanolamine
261J6545.DOC
33 Attorney Docket No. 5049-092780
TABLE 2b: High Iron Stabilization Test Results
mg/L Active
Product MED* 120 125 . 130 150 200 250
300 500
Citric Acid 80
KR- 110 95.1
DP0184
Acumer 100 98.4
3100
M
Prism 110-120 96.5
0
HPS1 125-150 76.6 98.3
0
1.)
87.3
..,
0,
AR-540 125-150 81.4 96.9
co
0,
co
0,
K-798 110-150 94.7
1.)
0
1-,
Acumer 120 88.2
i
2000 92.2
1-,
NatEDTA 700 27.4 35.6 44.2
80.9
1-,
TEA** None 1.1
3.5 4.9
** Triethanolamine
26U6545.DOC
34 Attorney Docket No. 5049-092780
TABLE 2c: High Iron Stabilization Test Results
mg/L Active
Product MED* 600 700
Citric Acid 80
KR- 110
DP0184
Acumer 100
3100
Prism 110-120
HPS1 125-150
0
1.)
AR-540 125-150 co
co
K-798 110-150
1.)
0
Acumer 120
2000
NatEDTA 700 87.4 91.2 1.)
91.4
TEA** None
** Triethanolamine
-1
26U6545.DOC
CA 02758686 2011-11-21
35 Attorney Docket No. 5049-092780
Example 2
[00117] The threshold inhibition test evaluates and quantifies the
ability of a scale inhibitor
to prevent precipitation of a Particular scale. This is accomplished by
measuring the
concentration of cations that remain in solution after a specified time under
supersaturated
conditions and comparing to the concentration in an untreated control. The
percent inhibition is
calculated as follows:
% Inhibition = cc" - cur) x 100%
- cut)
Where:
CT = cation concentration in the treated filtered sample
Cun = cation concentration in the filtered untreated sample
Ci = cation concentration in the initial sample
[00118] The Minimum Effective Dose ("M.E.D.") for the scale inhibition
tests is defined
as the dosage at which >90% inhibition is attained. KR-DP0515 is a 1:1:1
blend, on an active
basis, of (60/40) PAA/AMPS copolymer:PAA:PBTC, respectively.
[00119] For this example, treated and untreated supersaturated solutions
containing calcium
chloride and sodium bicarbonate were prepared at an initial pH of 8.00 to 8.35
in Erlenmeyer flasks.
The flasks were then heated in a water bath for 24 hours at 60 C. After the
incubation period, the samples
were filtered and then the soluble calcium concentration determined by
titration with EDTA. As
shown in Table 3, on an active basis, the M.E.D. for KR-DP0515 is 0.70 mg/L,
"indicating that
KR-DP0515 is an effective calcium carbonate scale inhibitor when compared to
other known
calcium carbonate scale inhibitors.
26U6545.DOC
CA 02758686 2011-11-21
36 Attorney Docket No. 5049-092780
Table 3
Calcium Carbonate Threshold Inhibition Test Results
Percent Inhibition (y,),
Active Dose
AA/AMPS60/40
(mg/L1 KR-DP0515
PBTC PAA copolymer HEDP
0.10
74.4
0.20
63.7
94.4
0.30 72.5
94.1,80.2
100.0
Avg.=87.2
0.40 81.4
101.0,
98.1 Avg.=99.6
0.50 84.6
101.0
46.0
0.60 87.8, 82.0
Avg.=84.9
0.70 89.6,101.1,
92.0 Avg.=94.2
0.75
84.2
0.80 95.6, 90.0
87.9, 27.3
Avg.=92.8
Avg.-57.6
0.90
87.9, 87.8
Avg.=87.9
1.00
96.0 76.0
1.25
97.0
1.50
82.0
2.00
77.0
2.50
70.3
3.00
77.0
4.00
91.5, 75.6
Avg.=83.6
5.00
84.6, 80.2
Avg.=82.4
6.00
102.2
7.00
70.0
8.00
9.00
10.00
97.2
M.E.D.* 0.70
0.40
1.00 For doses 0.20
>1.00,
Avg. %1 =
82.0**
**Average %I is 82%; is not >90% at doses greater or equal to M.E.D.
Conditions:
24 hr @ 60 C (140 F)
pHi: 8.00-8.35
200 mg/L Ca+2, 600 mg/L HCO3-
26U6545.DOC
CA 02758686 2011-11-21
37 Attorney Docket No. 5049-092780
[00120] For this example, treated and untreated supersaturated solutions
containing
calcium chloride and sodium sulfate were prepared and adjusted to an initial
pH of 6.50 to 7.00
in Erlenmeyer flasks. The flasks were then heated in a water bath for 24 hours
at 60 C. After
the incubation period, the samples were filtered and then the soluble calcium
concentration
determined by titration with EDTA. As shown in Table 4, on an active basis,
the M.E.D. for
Super TSC is 2.0 mg/L, indicating that KR-DP0515 is an effective calcium
sulfate scale inhibitor
when compared to other known calcium carbonate scale inhibitors. Synergy of
components was
observed with the KR-DP0515 at a dosage of 2.50 mg/L, active basis. At a
product dose of 2.5
mg/L, the component treatment dosages are: 0.83 mg/L PBTC, 0.83 mg/L PAA, and
0.83 mg/L
60/40 AA/AMPS. The Expected Percent Inhibition, based on the contribution of
components,
would be 7.5% + 22.5% + 15.0% = 45%. The observed inhibition for the blended
composition is
100.0%, indicating a synergy between components.
Table 4
Calcium Sulfate Threshold Inhibition Test Results
Percent Inhibition (%)
60/40
Active Dose AA/AMPS
nfig&) KR-DP0515 PBTC PAA copolymer HEDP
0.50 7.2 3.7
0.83 7.5 25.2, 15.0
19.8
Avg.=22.5
1.00 15.3 97.3
1.50 55.9 99.1
2.00 81.1, 95.3, 27.9 98.2
93.7
Avg.=90.0
2.50 100.0 52.3 9.0
3.00 73.9, 45.9 84.7
Avg.=59.9
3.50 95.5 99.1
4.00 100.0
4.50
5.00 19.8
M.E.D.* 2.00 3.50 1.00 3.50 >5.00
Conditions:
24 hr @ 60 C (140 F)
pHi: 6.5-7.0
2000 mg/L Ca+2, 4800 mg/L SO4-2
26U6545.DOC
CA 02758686 2011-11-21
38 Attorney Docket No. 5049-092780
[00121] For this example, treated and untreated supersaturated solutions
containing barium
chloride and sodium sulfate were prepared and adjusted to an initial pH of
7.00 to 8.00 in plastic
bottles. The bottles were then heated in an oven for 24 hours at 30 C. After
the incubation period, the
samples were filtered and then the soluble barium concentration determined. As
shown in Table 5, on
an active basis, the M.E.D. for KR-DP0515 was 3.0 mg/L, indicating that KR-
DP0515
performed as well or better than its components for barium sulfate scale
inhibition.
Table 5
Barium Sulfate Threshold Inhibition Test Results
Active Dose Percent Inhibition (y)60/40 AA/AMPS
(mg/L) KR-DP0515 PBTC PAA copolymer
1.00 51.2, 0.0 55.8, 31.7 58.1, 24.1,0.0
Avg.=25.6 Avg.=43.8 Avg.=27.4
1.50
2.00 58.4 74.4
2.50
3.00 95.9 39.0 48.8
3.50
4.00 95.3 73.2
5.00
6.00 99.2
7.00
8.00
9.00
10.00 24.1, 56.1
Avg.=40.1
15.00 63.4
20.00 89.9
M.E.D.* 3.00 >4.00 >10.0
Conditions:
24 hr @ 30 C (86 F)
pHi: 7.0-8.0
68.7 mg/L Ba+2, 48.0 mg/L SO4-2
Example 3
[00122] A fluid treatment system according to the present invention was
evaluated for
friction reduction properties on a friction loop test apparatus.
[00123] Referring now to Fig. 1, the friction test apparatus, indicated
generally as 10,
consisted of a reservoir chamber 12, a pump 14 connected to the bottom 16 of
the reservoir
26U6545.DOC
CA 02758686 2011-11-21
39 Attorney Docket No. 5049-092780
chamber 12, and a closed loop piping system 18 which received the test fluid
from the pump and
returned the test fluid to the reservoir chamber 12. The closed loop piping
system 18 includes a
flow meter (FM) for monitoring the flow rate of the test fluid through the
pipe.
[00124] The treatment chemistry was added to the water returning from the
closed loop
piping system 18 as it entered the reservoir chamber 12. High turbulence at
the injection point
20 ensured thorough and rapid dispersion of the treatment in the reservoir
chamber 12. The
friction test apparatus 10 was configured to provide flow through a section of
pipe 22 under a
fixed, steady-state flow rate. For the following examples, the Reynolds'
number of the fluid in
the pipe 22 was calculated to be 58,100 and was well within the turbulent flow
regime. For each
test, a differential pressure gauge 24 was used to measure the pressure
differential between the
pressure in the first position (P1) and the second position (P2) in the piping
system 18. The
length 26 of the pipe 22 between the first position (P1) and the second
position (P2) was at least
times the pipe diameter, or at least about 2 feet. The frictional loss through
the pipe 22
between P1 and P2 was proportional to the pressure drop: P1 minus P2 = AP.
Precision
measurements of flow and pressure are required for an accurate assessment of
the friction
reduction. A datalogger 26 recorded pressure and flow measurements in 1 second
increments
during the test run. After the pressure differential for the base fluid was
recorded, then the
treatment was injected. The treatment rate of the liquid treatment products
are reported in (gpt)
or gallons per 1000 gallons of base fluid.
[00125] The differential pressure reading 60 seconds after injection was used
in the
analysis. The base fluid consisted of the water, dissolved ions and treatment
chemicals prior to
adding the friction reducing agent. The percent of friction reduction achieved
by the water
treatment was determined by measuring the differential pressure for base fluid
and comparing to
differential pressure for the treated fluid, according to the following
formula:
Friction Reduction (%) = (--APbase fluid APtreated fluid)/ APbase fluid*100.
[00126] One skilled in the art would understand that the diameter and length
of the pipe 22
can vary, as long as steady-state turbulent flow conditions are maintained
between P1 and P2.
The pipe diameter can be about 1/4 inch to about 2 inches, and the pipe length
can be about 10
feet to about 200 feet. Space and cost considerations can be taken into
account in determining
the pipe dimensions for the test unit. Since the percent of friction reduction
is determined by
relative pressure drop for tests with and without the fluid treatment, the
actual dimensions of the
26U6545.DOC
CA 02758686 2011-11-21
40 Attorney Docket No. 5049-092780
piping are not important as long as the dimensions fall within the ranges
specified above and
steady-state turbulent is maintained.
[00127] The adverse impact of ferrous iron on the performance the WFR-3B
friction
reducing agent was evaluated. An amount of a 5 % (as Fe+2) solution of ferrous
sulfate solution
was added to the recirculating fresh water in the friction test apparatus to
achieve a desired iron
content in the base fluid. The iron concentration was confirmed using Hach
FerroVer Iron
Reagent (Catalog Number 854-99). The friction reducing agent was introduced
thirty seconds
after the addition of the ferrous compound. For Example 3, the friction
reducing agent was
WFR-3B and was applied at 0.5 gallons per 1000 gallons of base fluid (gpt).
Table 6
Ferrous Content (ppm) % Friction Reduction
0 70%
10 26%
25 25%
= 50 13%
75 0%
[00128] In this example, the effect of using a fluid treatment system
including at least one
material (a) (an iron control agent) and friction reducing agent (b) according
to the present
invention was evaluated. The same apparatus was used and the same flow
conditions were
maintained as above with fresh water. The concentration of ferrous iron was
held constant at 25
ppm. The material (a), ICP-1000, was applied at various treatment rates and
was mixed for 30
seconds prior addition of the ferrous sulfate. For this example, the friction
reducing agent was
WFR-3B and was applied at 1.0 gpt. As observed in Table 7, performance of the
friction
reducing agent was significantly improved in the presence of the material (a)
and surpassed the
performance of the no iron control.
Table 7
ICP -1000 % Friction
(gpt) Reduction
Control ¨ no iron or ICP-1000 64%
26U6545.DOC
CA 02758686 2011-11-21
41 Attorney Docket No. 5049-092780
0.00 44%
0.25 59%
0.50 68%
0.75 68%
1.00 68%
[00129] In this example the same flow conditions were observed as above. The
base fluid
was prepared by adding various inorganic salts to fresh water to simulate well
flowback water.
The chemical makeup of the water is presented below in Table 8:
Table 8
Ion PPm
Na 4,050
Ca 1,000
Mg 500
Cl 9,400
HCO3 145
[00130] In this example, an iron control agent was added and the
concentration of ferrous
iron was held constant at 25 ppm. The iron control agent, ICP-1000, was
applied at various
treatment rates and was mixed for 30 seconds prior addition of the ferrous
sulfate. For this
example, the friction reducing agent was WFR-3B and was applied at 0.5 gpt. As
observed in
Table 9, performance of the friction reducing agent is significantly improved
in the presence of
the iron control agent and performed almost equivalently to the no iron-
containing control.
Table 9
ICP-1000 % Friction
(gpt) Reduction
Control ¨ no iron or ICP-1000 63%
0.00 43%
0.25 59%
0.50 60%
1.00 61%
26U6545.DOC
CA 02758686 2011-11-21
42 Attorney Docket No. 5049-092780
[00131] In this example the same flow conditions were observed as above. The
base fluid
was prepared by adding 10,200 ppm of sodium chloride to fresh water to produce
a high TDS
brine. In this example an iron control agent was added and the concentration
of ferrous iron was
held constant at 25 ppm. The iron control agent, ICP-1000, was applied at
various treatment
rates and was mixed for 30 seconds prior addition of the ferrous sulfate. For
this example, the
friction reducing agent was WFR-3B and was applied at 0.5 gpt. As observed in
Table 10,
performance of the friction reducing agent was significantly improved in the
presence of the iron
control agent and performed almost equivalently to the no iron-containing
control.
Table 10
ICP -1000 % Friction
(gpt) Reduction
Control ¨ no iron or ICP-1000 69%
0.00 53%
0.25 59%
0.50 62%
1.00 70%
[00132] In this example the same flow conditions were observed as above with
fresh
water, an iron control agent was added and the concentration of ferrous iron
was held constant at
25 ppm. For comparison, the performance of ICP-1000 was evaluated against
citric acid and
EDTA which are commonly used in fracturing fluids as iron control agents. The
iron control
agent was applied at various treatment rates and was mixed for 30 seconds
prior addition of the
ferrous sulfate. For this example, the friction reducing agent was WFR-3B and
was applied at
1.0 gpt. As observed in Table 11, the performance of the commonly used iron
control agents in
some instances impaired the performance of the friction reducing agent. EDTA
had a deleterious
effect on the performance of the friction reducing agent over the entire
dosage range. In contrast,
at lower levels of treatment citric acid combined with iron to cause
significant deterioration to
the performance of the friction reducing agent. It is significant to note that
ICP-1000 improved
the performance of the friction reducing agent over the entire treatment
range.
Table 11
Iron Control Additive Friction Reduction (%)
(gpt) ICP-1000 Citric Acid EDTA
26U6545.DOC
CA 02758686 2011-11-21
43 Attorney Docket No. 5049-092780
0.00 44%
44% 44%
0.15
13%
0.25 59%
27% 43%
0.35
48%
0.50 68%
68% 35%
0.75 68%
68% 33%
Example 4
[00133] In this example the same flow conditions were
observed as in Example 3. As in
Table 8, the base fluid was prepared by adding various inorganic salts to
fresh water to simulate
well flowback water. In this example the concentration of ferrous iron was
held constant at 25
ppm. The iron control agent, ICP-1000 and/or the KR-DP0510, a phosphonate-
ester/acrylic acid
copolymer scale inhibitor blend was applied and was mixed for 30 seconds prior
addition of the
. ferrous sulfate. For this example, the friction reducing agent was WFR-3B
and was applied at
0.5 gpt. As observed in Table 12, the performance of the friction reducing
agent with either
treatment was significantly improved over the untreated test condition.
Surprisingly, the
performance of the friction reducing agent also was improved in the presence
of the scale
inhibitor at the same application dosage of the iron control agent.
=
Table 12
ICP- 1000 KR-DP0520
% Friction
(gpt) (gpt)
Reduction
0.00 0.00
43%
0.50 0.00
60%
0.50 2.00
69%
[00134] It will be appreciated by those skilled in
the art that changes could be made to the
embodiments described above without departing from the broad inventive concept
thereof. It is
understood, therefore, that this invention is not limited to the particular
embodiments disclosed,
but it is intended to cover modifications which are within the spirit and
scope of the invention, as
defined by the appended claims.
26U6545.DOC