Note: Descriptions are shown in the official language in which they were submitted.
CA 02758882 2011-10-14
WO 2010/128970 PCT/US2009/043332
FLUORINE EXTRACTION SYSTEMS AND ASSOCIATED
PROCESSES
TECHNICAL FIELD
[0001] The present disclosure is directed generally to fluorine extraction
systems and associated processes.
BACKGROUND
[0002] Fluorine is a chemical element that is most electronegative of all the
chemical elements. Because of this characteristic, fluorine has many unique
applications. For example, fluorine has been used in plasma etching of
semiconductor wafers for producing processors, memory devices, and/or other
microelectronic devices. In another example, compounds of fluorine (e.g.,
fluoropolymers, potassium fluoride, and cryolite) have been used in anti-
reflective
coatings and dichroic mirrors because of their unusually low refractive index.
[0003] Industrial production techniques of fluorine typically include the
electrolysis of hydrogen fluoride (HF) in the presence of potassium fluoride
(KF). The
hydrogen fluoride required for the electrolysis is typically obtained from
phosphate-
containing minerals with significant amounts of calcium fluorides (e.g.,
calcium
fluorite, CaF2). Upon treatment with sulfuric acid (H2SO4), the phosphate-
containing
minerals release hydrogen fluoride as follows:
CaF2 + H2SO4 -4 2 HF + CaSO4
This fluorine production process, however, can be energy intensive because
electrolysis requires a large amount of energy to operate. Also, such
processes can
have high operating costs because of the constant requirement for mineral
extraction.
[0004] Fluorine can also be obtained as a byproduct of the uranium enrichment
process. In nature, uranium exists as about 99.284% of 238U, about 0.711 % of
2350,
and about 0.0058% of 234U. While 235U can be used as a fuel for nuclear
fission, the
other isotopes, 238U and 234U, cannot. Thus, uranium-containing minerals must
first
CA 02758882 2011-10-14
WO 2010/128970 PCT/US2009/043332
be enriched in order to have sufficient concentrations of 235U to support
nuclear
fission. A common byproduct of the uranium enrichment process includes
depleted
uranium hexafluoride (238UF6\234UF6), which is a radioactive and hazardous
compound typically stored at great expense. Accordingly, it may be desirable
to
utilize this source of fluorine to efficiently and cost effectively produce
fluorine on an
industrial scale.
BRIEF DESCRIPTION OF THE DRAWINGS
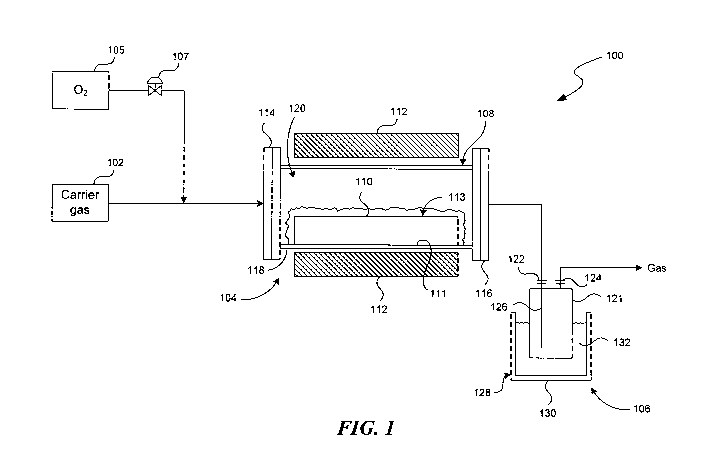
[0005] Figure 1 is a schematic diagram of a fluorine extraction system in
accordance with embodiments of the disclosure.
[0006] Figure 2 is a reaction vessel suitable for the fluorine extraction
system of
Figure 1 in accordance with embodiments of the disclosure.
DETAILED DESCRIPTION
[0007] Various embodiments of fluorine extraction systems and associated
processes are described below. The term "oxidizing agent" generally refers to
a
compound capable of being reduced by being combined with at least one fluorine
atom. In certain embodiments, the oxidizing agent can be in elemental form.
For
example, the oxidizing agent can include silicon (Si) in elemental form. In
other
embodiments, the oxidizing agent can be in a combined form. For example, the
oxidizing agent can include germanium oxide (GeO), germanium dioxide (Ge02),
silicon dioxide (SiO2), arsenic trioxide (As203), titanium oxide (TiO), boron
trioxide
(B203), and/or other compounds that may combine with at least one fluorine
atom.
A person skilled in the relevant art will also understand that the disclosure
may have
additional embodiments, and that the disclosure may be practiced without
several of
the details of the embodiments described below with reference to Figures 1 and
2.
[0008] Figure 1 is a schematic diagram of a fluorine extraction system 100 in
accordance with embodiments of the disclosure. As shown in Figure 1, the
fluorine
extraction system 100 can include a carrier gas storage 102, a reactor 104,
and a
product separator 106 coupled to one another in series. Optionally, the
fluorine
extraction system 100 can also include an oxygen storage 105 coupled to the
reactor 104 via a control valve 107. Even though only certain components of
the
-2-
CA 02758882 2011-10-14
WO 2010/128970 PCT/US2009/043332
fluorine extraction system 100 are shown in Figure 1, in other embodiments,
the
fluorine extraction system 100 can also include valves, actuators, pumps,
compressors, and/or other suitable devices.
[0009] The reactor 104 can include a reaction chamber 108, a reaction vessel
110 positioned in the reaction chamber 108, and a heater 112 at least
proximate to
the reaction chamber 108. The reaction chamber 108 can include an inlet 114
spaced apart from an outlet 116 and a reactor body 118 between the inlet 114
and
the outlet 116. The reaction chamber 108 can be constructed from a
substantially
rigid material (e.g., 316L stainless steel) coated on at least one side with
nickel,
fluoropolymers, and/or other suitable fluorine resistant materials. In the
illustrated
embodiment, the reactor body 118 is shown as having a generally cylindrical
shape
extending between the inlet 114 and the outlet 116. In other embodiments, the
reactor body 118 can also have other suitable shapes and configurations.
[0010] The reaction vessel 110 can be positioned inside the reaction chamber
108 with a head space 120 above the reaction vessel 110. The reaction vessel
110
can include a container configured to hold a reactant or a mixture of
reactants in
solid and/or liquid form. In certain embodiments, the reaction vessel 110
includes a
container having a closed bottom 111 facing an opening 113 to the head space
120,
as described in more detail below with reference to Figure 2. In other
embodiments,
the reaction vessel 110 can have other suitable configurations. In the
illustrated
embodiment, the reaction vessel 110 rests directly on the reactor body 118 of
the
reaction chamber 108. In other embodiments, the reaction vessel 110 can also
include poles, plates, and/or other suitable structural components for
supporting the
reaction vessel 110 inside the reaction chamber 108.
[0011] The heater 112 can include an electric furnace, a microwave radiator,
and/or other suitable types of heater for supplying thermal energy to the
reaction
chamber 108. In the illustrated embodiment, the heater 112 is shown as spaced
apart from the reaction chamber 108. In other embodiments, the heater 112 may
be
at least partially enclosing the reaction chamber 108. In further embodiments,
the
heater 112 may be integrated in the reaction chamber 108.
[0012] The product separator 106 can be configured to collect a gaseous
product having a desired boiling point or a range of desired boiling points.
As shown
-3-
CA 02758882 2011-10-14
WO 2010/128970 PCT/US2009/043332
in Figure 1, the product separator 106 can include a collector vessel 121 at
least
partially enclosed by a cooling bath 128. The collector vessel 121 can include
a
collector inlet 122, a collector outlet 124, and a dip tube 126 coupled to the
collector
inlet 122. In the illustrated embodiment, the cooling bath 128 includes a
chiller bath
130 configured to contain a coolant 132 (e.g., dry ice, water, ice, liquid
nitrogen,
and/or other coolant with a desired cooling temperature). In other
embodiments, the
chiller bath 130 may be omitted, and the collector vessel 121 may be air
cooled. In
further embodiments, the product separator 106 can include a distillation
column, an
adsorption vessel, and/or other suitable separation components in lieu of or
in
addition to the components shown in Figure 1.
[0013] Figure 2 is a reaction vessel 110 suitable for the fluorine extraction
system 100 of Figure 1 in accordance with embodiments of the disclosure. As
shown in Figure 2, the reaction vessel 110 includes a body section 117 welded
to,
adhered to, fastened to, and/or otherwise coupled to end caps 119 (identified
individually as a first end cap 119a and a second end cap 119b). In the
illustrated
embodiment, the body section 117 includes a portion of a half pipe that has a
generally semicircular cross section extending along a longitudinal axis R,
and the
end caps 119 include semicircular plates. At least the inside of the body
section 117
and the end caps 119 can be plated with a nickel film. In other embodiments,
the
body section 117 can also has a rectangular, oval, trapezoidal, and/or other
suitable
cross-sectional shapes and arrangements. The body section 117 and/or the end
caps 119 can be constructed from stainless steel (e.g., 316L), nickel, and/or
other
suitable materials.
[0014] Referring to Figures 1 and 2 together, certain embodiments of the
fluorine extraction system 100 can be used to form non-radioactive fluorine-
containing compounds by reacting at least one uranium fluoride with an
oxidizing
agent. In operation, an operator can first load a reactant and/or a mixture of
reactants 115 in the reaction vessel 110. In certain embodiments, the mixture
of
reactants 115 can include at least one uranium fluoride and an oxidizing agent
in
stoichiametric and/or other suitable proportions. The uranium fluoride can
include
uranium tetrafluoride (UF4), uranium hexafluoride (UF6), uranium oxyfluoride
(U02F2), and/or other suitable uranium fluorides. The oxidizing agent can
include
germanium oxide, (GeO), germanium dioxide (Ge02), silicon (Si), silicon
dioxide
-4-
CA 02758882 2011-10-14
WO 2010/128970 PCT/US2009/043332
(SiO2), arsenic oxides (e.g., As203 or As205), antimony oxides (e.g., Sb203,
Sb204,
and Sb205), titanium oxides (e.g., TiO, Ti02, Ti203, Ti305, and Ti407), boron
oxides
(e.g., B203, B20, and B60), and/or other suitable metal oxides. In other
embodiments, the mixture of reactants 115 can also include additional and/or
different compounds.
[0015] After loading the mixture of reactants 115 in the reaction vessel 110
and
adjusting the depth D based on a desired reaction yield, the operator can then
position the reaction vessel 110 in the reaction chamber 108. The operator can
then
evacuate the headspace 120 (e.g., with a vacuum pump) and purge the reaction
chamber 108 with a carrier gas (e.g., argon, nitrogen, helium, etc.) from the
carrier
gas storage 102. After a desired atmosphere is achieved in the reaction
chamber
108, the operator can energize the heater 112 to supply energy to the mixture
of
reactants 115 in the reaction vessel 110 until a desired temperature is
achieved.
[0016] Without being bound by theory, it is believed that the mixture of
reactants 115 of the uranium oxide and the oxidizing agent can react to form
at least
one uranium oxide and a non-radioactive fluorine-containing compound. For
example, in one embodiment, the mixture of reactants 115 containing uranium
tetrafluoride (UF4) and germanium dioxide (Ge02) can react as follows:
3UF4 + 3GeO2 + 02 -* U308 + 3GeF4
In another example, the mixture of reactants 115 containing uranium
oxyfluoride
(U02F2) and germanium dioxide (Ge02) can react as follows:
2UO2F2 + GeO2 - 2UO2 + GeF4
In yet another example, the mixture of reactants 115 containing uranium
tetrafluoride
(UF4) and silicon dioxide (SiO2) as follows:
3UF4 + Si02 + 02 -) U305 + 3SiF4
In yet further examples, the mixture of reactants 115 containing the uranium
fluoride
can react with other suitable oxidizing agents to produce at least one uranium
oxide
and a non-radioactive fluorine-containing compound.
[0017] Optionally, during the reaction, the operator can introduce oxygen (02)
into the reaction chamber 108 from the oxygen storage 105. The inventors have
recognized that, in addition to the depth D of the mixture of reactants 105, a
flow
-5-
CA 02758882 2011-10-14
WO 2010/128970 PCT/US2009/043332
rate of the introduced oxygen can affect the reaction rate and/or the yield of
the
reaction. Thus, in certain embodiments, the operator can control the flow rate
of the
oxygen into the reaction chamber 108 based on a desired reaction yield by
modulating the control valve 107. In other embodiments, the operator can also
control the flow of the oxygen based on other parameters by utilizing an
orifice plate,
a venturi, and/or other suitable flow elements.
[0018] A product gas containing the carrier gas, the optionally introduced
oxygen, and the gaseous non-radioactive fluorine-containing compound then
flows
to the product separator 106 from the reactor 104. The product separator 106
can
then collect the non-radioactive fluorine-containing compound, for example, by
condensing it as a condensate in the collector vessel 121. In other
embodiments,
multiple collector vessels (not shown) may be used to fractionate the product
gas
into multiple streams.
[0019] As shown in Figure 2, the mixture of reactants 115 can have a depth D
in the reaction vessel relative to the closed bottom 111 of the reaction
vessel 110.
The inventors have recognized that the depth D of the mixture of reactants 115
in
the reaction vessel 110 can be controlled to affect a desired yield of the
reaction
between the reactants. In particular, it was unexpectedly discovered that by
reducing the depth D of the mixture of reactants to have a specific range of
values
(e.g., about 0.25 inches), a surprisingly large improvement in efficiency may
be
obtained, as discussed in more detail below. As a result, the operator may
control
the depth D of the mixture of reactants 115 when loading the mixture into the
reaction vessel 110 based on a desired reaction yield.
[0020] Experiments were conducted in a fluorine extraction system generally
similar in configuration and function as the fluorine extraction system 100 of
Figure
1. A mixture of uranium tetrafluoride (UF4) and germanium dioxide (GeO2) was
heated and reacted in the fluorine extraction system to about 1,600 F with a
purge
gas containing helium (He) and oxygen (02). A gaseous reaction product was
collected in a collector vessel, and the solid residue was observed in the
reaction
vessel. Analyzing the collected gaseous reaction product and the solid residue
provided the following reaction yield data:
-6-
CA 02758882 2011-10-14
WO 2010/128970 PCT/US2009/043332
Bed Depth Efficiency AEfficiency
/ABed Depth
0.25 in 91.5% 76.0%
0.50 in 72.5% 11.2%
0.75 in 69.7 % -1.2%
1.50 in 70.6% -
In the foregoing table, efficiency is defined as the actual yield obtain from
analysis
divided by the theoretical yield, assuming 100% conversion. The parameter
AEfficiency /ABed Depth is calculated as a percentage change per one inch of
bed
depth change.
[0021] As shown in the foregoing table, the efficiency change per bed depth
remained relatively unchanged from 1.50 inches to about 0.50 inches. However,
reducing the bed depth from 0.50 inches to 0.25 inches, the efficiency change
per
bed depth (as represented by AEfficiency /ABed Depth) improved by about 6.8
times. Without being bound by theory, such a surprising efficiency improvement
is
believed to be a result of the improved contact between the oxygen flowing
across
the opening 113 of the reaction vessel 110 and the mixture of reactants 115 in
the
reaction vessel 110. It is believed that the small depth D of the mixture of
reactants
115 may enable the oxygen to penetrate deeper into the mixture of reactants
115 in
the reaction vessel 110, and as a result, improve reaction efficiency between
the
uranium oxide and the oxidizing agent.
[0022] From the foregoing, it will be appreciated that specific embodiments of
the disclosure have been described herein for purposes of illustration, but
that
various modifications may be made without deviating from the disclosure. For
example, many of the elements of one embodiment may be combined with other
embodiments in addition to or in lieu of the elements of the other
embodiments.
Accordingly, the disclosure is not limited except as by the appended claims.
-7-