Note: Descriptions are shown in the official language in which they were submitted.
CA 02759684 2011-10-21
1
METHOD FOR DRAWING POWER FROM ELECTROCHEMICAL CELLS USING
FREQUENCY PULSES, AND USE OF SAID METHOD IN A POWER SOURCE
The use of electrochemical cells for the production of power sources is well
known. Especially
in portable power sources metal-air cells are often used in which a metal
anode and a gas
diffusion cathode and an electrolyte solution are applied. Examples of anode
metals are
magnesium, zinc and aluminum. The electrolyte solutions are predominantly
aqueous alkaline
solutions or sodium chloride solutions.
For mobile use of these power sources the weight and dimensions of the power
source and its
supplies play an important role. Since the required performance of the power
sources are
specified by the application, a more compact design with low weight is
achieved only by the fact
that the efficiency of power sources is optimized. Limiting factors in the
performance of
electrochemical cells are amongst others the formation of reaction products,
insufficient gas
diffusion and insufficient surface activity. Therefore several developments
have been carried out
to improve the portable power sources by means of constructive actions and
change of the
chemical composition of the electrode materials.
U.S. 6,127,061 describes the improvement in the power density of metal-air
cells using a special
air-cathode, which contains a catalytic layer, which is composed of a mixture
of carbon particles,
hydrophobic particles, a metal hydroxide and particle material with a large
surface area. On this
surface an electrically conductive structure is applied, followed by an air-
and water-impermeable
layer.
CA 02759684 2011-10-21
2
In U.S. 5,004,654, an aluminum-air cell is outlined, whose anode material is
alloyed with
magnesium and/or calcium and in whose electrolyte and / or anode material tin
is present. U.S.
5,360,680 describes the user-friendly design of metal-air cell as mechanically
rechargeable power
sources, such as various anode materials, especially activated zinc in slurry
form. Particular
attention is given to use in electric vehicles and the desired properties of a
high current density, a
high current capacity and high set a maximum power.
EP 1843418 Al describes an electrochemical current / power source, in
particular a fuel cell or
battery in which an electrolyte salt water and/or alkali solution, for use in
an electrochemical
metal-air cell suitable anode, and a gas-diffusion cathode or air cathode,
which has at least one
hydrophobic layer is used. Here, the housing on one by one or more liquid-
permeable walls
limited housing space, wherein the housing space can be fed through at least
one opening, air or
oxygen. At least one of the impermeable walls of the housing space is formed
by the cathode or
by the hydrophobic layer of the cathode. The gap is at least partially with an
electrolyte,
particularly sea water, salt water or alkaline solutions, can be filled.
The invention disclosed in claim 1 is based on the problem, the electrical
performance
characteristics and capacity of a power source to improve for a given type of
electrochemical
power source is used.
This problem is solved by a method of removing power from electrochemical
power sources
according to claim 1. Claim 1 describes a method for drawing current from all
kinds of
electrochemical current sources, with the drawing of current occurring by the
consumer (7) via a
current transformer which contains a transformer (4), the primary circuit of
which contains an
CA 02759684 2011-10-21
3
electrochemical current source (1), a shunt circuit (2) and a switching
element with a control unit
(3), and the secondary circuit of which contains an inductive energy storage
unit (5) and the
consumer (7), characterized in that the withdrawal of current occurs by
frequency pulses under
the conditions that the ratio of the capacitance of the shunt circuit (Csh) to
the surface (S) and the
specific differential capacitance (CD,s) of the anode is CSh = 0.5 = (CD,S =
S) to CSh = 5 = (CD,S = S),
and that the minimum frequency fmin of the frequency pulses is chosen
according to the formula
fmin = 1
2i[ C This method serves as a basis for determining the optimum parameters
D,S ' 1.87 fl cmz
for the components of the circuit. The optimization of a power source is
defined by one or more
important parameters for the solution of specific consumer problems. Example
is the
optimization of a DC-DC power converter for a metal-air cell according to the
consumption of
the metal anode on the condition that the output power is not the power source
falls below the set.
Due to the change in frequency of the pulses from the RC-generator in the
controller for the
auxiliary resistance (resistance to adjustable resistance value) can be
experimentally the
dependences of the specific anode consumption (grams per Wh) and the maximum
output power
to build on the pulse frequency. In both curves, one finds a frequency at
which the anode metal
consumption is minimal, provided that the maximum output power not the power
source falls
below the set. Additionally it is observed in the frequency of the structural
parameters of the
planar transformator to optimize the throttle etc.
Claim 2 describes a power source for performing the method of claim 1
containing an
electrochemical current source (1) filled with an electrolytic solution and a
DC-DC current
transformer, characterized in that the DC-DC current transformer contains a
transformer (4), the
CA 02759684 2011-10-21
4
primary circuit of which consists of the electrochemical current source (1), a
shunt circuit (2), and
a switching element with a control unit (3); and its secondary circuit of an
inductive energy
storage unit (5) and a storage capacitor; with the consumer (7) being
connected to the secondary
circuit of the current source and the ratio of the capacitance of the shunt
circuit (Csh) to the
surface (S) and the specific differential capacitance (CD,s) of the anode is
CSh = 0.5 ' (CD,S ' S) to
CSh = 5 ' (CD,S ' S).
The inventive design of the power source (Fig. 1), which operates by a process
according to
claim 1 and 2, all types of electrochemical power sources, an improvement of
the capacity is
reached. In most species, in addition, the capacity of the power source is
increased. This is
achieved through the optimization of through the switching element control
unit (3) of the
electrochemical power source (1) on the transformer (4) generated frequency
pulse to the
subsequent energy storage in the memory (5) and their delivery to the consumer
(7).
Therefore, by the invention, a more compact and lighter power source of the
desired performance
and capacity is realized, based on various electrochemical cells.
Particularly advantageous embodiments of the invention are set forth in the
appended claims. In
claim 3, the optimized use of a switching element presented with a control
unit that is capable of
frequency pulses on the transformer defined leave. An embodiment of the water
board in the
DC-DC power converter will be described hereinafter as the circuit element, a
transistor group is
used. The control unit itself may consist inter alia of the following
components: a controller
(microcircuit) with the required settings, a starter circuit, a recirculation
system which regulates
CA 02759684 2011-10-21
the pulse times and distances depending on the load capacity, microcircuits
for loss reduction and
buffer circuits for shorter switching times of the above transistors.
Claim 4 describes the advantageous use of a planar transformer as the
transformer in the DC-DC
power converter. The advantages are the following special characteristics of
Planar:
5 = Enhanced mutual inductance in planar transformer increases the efficiency
of the DC-DC
power converter.
= The dimensions of the DC-DC power converter is the use of the PIA
nartransformators
much lower.
= The serial production of the planar transformer for the DC-DC power
converter is simpler
and more reliable than conventional types.
Particularly advantageous parameters for the optimal tuning of the
construction components of
the current drain of the electrochemical power source are explained in the
conditions in claim 1
and 2.
Accordingly, the capacitance of the shunt circuit Csh where Csh = 0.5 = (C) to
CSh = 5 = (C), where
C is determined by the following formulas, if multiple anodes exist in the
system:
Parallel circuit: C =CD, s 1 ' S 1 +CD, s2 ' S2 +... +CD,Sn ' Sn
Series circuit: 1 = 1 + i + = = = + 1
C CD S1 ' S1 CD,S2 = S2 CD Sn = Sn
CA 02759684 2011-10-21
6
(n - number of elements; CD, Sn - specific differential capacitance of the
anode of the element n; S,,
- area of the anode of the element n).
In claims 1 and 2 is the formula syntax of a single cell used (C =CDs = S),
but it is understood
that by means of the brackets, the differential capacitance of the anode is
calculated and this, in
case that the electrochemical power source is several cells connected in
parallel or in series to
complete in accordance with the above well-known rules of summation capacity
for parallel and
series connection of elements.
In particular, for the most common case that the electrochemical power source
of N with each
other is in series connected identical cells, there is the capacity of a shunt
circuit Csh according to
claim 6 in the range of Csh = 0.5 = (CDs = S / N) to Csh = 5 = (CDs' S / N).
A portable power sources are particularly suitable embodiment of the electro-
chemical power
source is shown in claim 7 metal-air cell, which allows the electrolyte to be
stored separately
from the battery and bring it to the site.
A further advantageous embodiment of the invention described in claim 8 is the
use of
magnesium as the anode material, which allows for good performance of the
electrochemical cell
is a light weight.
The cell type in claim 7 performed with sodium chloride as the electrolyte has
a decisive
advantage requires that the end user only has to deal with a dangerous
consumer product and not
with alkaline solutions, the special precautions. In addition, the disposal of
waste products is
ecologically clean.
CA 02759684 2011-10-21
7
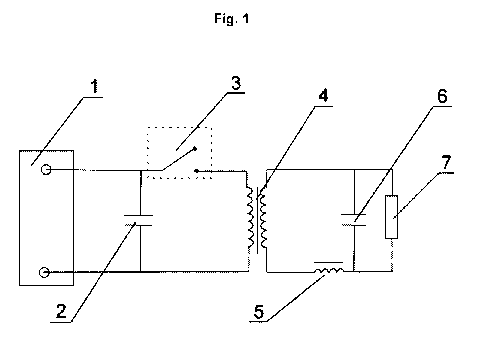
An embodiment of the invention is illustrated in Figure 1, the operation of
the following will be
explained in more detail. The desired technical result of the improved
electrical characteristics of
the current source by using an electrochemical power source (1) is reached,
which is connected
via a switching element with a control unit (3) with a planar transformer (4).
At the
electrochemical power source is also a shunt circuit (shunt) (2) connected
with a capacitance Csh.
The secondary winding of the planar transformer (4) is connected with an
inductive energy
storage (5), a storage capacitor (6) and the load resistor (consumer) (7).
The power source operates in the following way: When the contact closes, the
control unit (3)
the current flows, composed of the sum of the current of electrochemical power
source (1) and
the current of the secondary circuit switching circuit (2) the capacity Csh.
The energy
accumulated in the choke (5) and flows through the storage capacitor (6) to
consumers (7). The
time of the switched-off state is determined by the minimum time of the
transfer of energy that
was stored in the inductive storage (5), to the consumer (7). The maximum
efficiency of
transmission of electrical energy by means of reduction of input resistance of
the CT reaches
below 1 milliohm.
As an electrochemical power source, a metal-air cell is used with a magnesium
anode, a gas
diffusion cathode and an electrolyte of aqueous sodium chloride solution. The
source of internal
resistance R of the electrochemical power source consists of the sum of the
resistances of the
anode, the cathode-en and the electrolyte together (R = RA + RK + RE), where
it makes the
resistance of the anode circuit of Figure 2 (RE =electrolyte resistance, RD
=specific resistance of
the double layer, CD,S =specific differential capacitance of the double layer)
can constitute.
CA 02759684 2011-10-21
8
Below, the anode component summary of the internal resistance of the
electrochemical power
source is studied in two modes: the direct-current regime and the rate of
energy extraction
regime.
In the DC regime of the electrochemical cell is determined by the charging
resistor the double
layer RD the resistance RA. The component RD decreases with increasing current
density due to
the increase in the concentration of reactive elements in the electrical
double layer and the change
in activation energy, which is caused by the potential jump in the dense part
of the bilayer. Fig. 3
shows the dependence of the resistance RD represented by the current density.
In the frequency regime of energy extraction, the anode resistance R is A
determined by the
impedance:
1
R -
2rc=C-f
C =Differential capacitance, f =Frequency.
The capacitance of the double layer depends here on the potential of the
anode.
Fig. 4 shows typical curves of the differential capacity of 0.1 M solutions of
various metals (Hg =
1, 2 =Bi, 3 =Pb, 4 =Sn, 5 =Cd, 6 =In, 7 =In +Ga, 8 =Ga) in C5H11OH shown in
relation to
0.1 N solutions of the surface inactive electrolyte.
In the area of negative potential of the surface-inactive electrolytes be
wearing the differential of
the double layer capacity for all metals as a value of 17 gF / cm2. So you can
put down at a
certain frequency regime of energy extraction, the impedance of the anode so
that the internal
CA 02759684 2011-10-21
9
resistance of the electrochemical power source roughly approximates the
electrolyte resistance.
At a frequency of 100 kHz, for example, the specific impedance of the anode
reached 0.09 ohms =
cm2. The resistivity of the electrolyte at an electrode spacing of 0.5 cm and
a working
temperature of 60-70 C is about 2.5 - 3 ohms = cm2. The resistivity of modern
gas-diffusion
cathode at the same temperature is about 0.8 -1, 0 ohms = cm2. This results in
the summary of the
internal resistance of electrochemical cell in the frequency regime of 100 kHz
(in the case of the
maximum values for RE and RK), a value of about 4.1 ohms = cm2, where RA
approximately 2.5 %
summary of the internal resistance is formed.
In the DC regime of the electrochemical power source, the component RA at
current densities of
50 - 100 mA / cm2, which can be realized in practice, about 6 ohms = cm2 (see
Figure 3).
Accordingly, the summary reaches specific resistance about 10 ohms = cm2,
which is about 2.5-
fold higher than the frequency regime. RA will account for approximately 60%
of total
resistance. The increase in performance compared to the direct current regime
of energy
extraction is the minimum value of the determined intervals between pulses,
which is by the time
of transfer of energy stored in the memory element is limited to the consumer.
The shunt circuit
with a capacitance of Csh = 0.5 = (CD, S = S) to Csh = 5 = (CD,S = S) (S =
surfaces chemically the
anode and CD,S = specific differential capacitance of the anode ) is selected
such that when the
shutdown of the electrochemical power source from the power converter, the
potential of the
anode is no longer in the negative potential decreases as the specific
capacity is higher at less
negative potential values, especially in the adsorption of organic substances
(see Figure 4 ).
CA 02759684 2011-10-21
The determination of the optimum frequencies for practical application is
carried out starting
from the above formula for calculating the impedance of the double layer, the
anode resistor RA
is determined by the removal of electric energy by means of frequency pulses:
1
RA=2TT 'C'f
with: C =differential capacitance of the double layer of the anode, f
=frequency of the pulses
5 As stated above, depends on the capacitance of the double layer on the anode
potential. The
range of values that can assume the capacitance C for different types of
metals, is shown in
Figure 4. In most electrochemical elements, the anode potential is relative to
the zero level of the
reference electrode in the negative range, and runs after turning off the
switching element (3) or
more in the negative zone, i.e., CD,s approaches the known value of 17 gF /
cm2.
10 In order to maintain the anode potential at a higher level and thus the
reduction of RA to be
maintained, the additional capacity Csh (2) is used by the current in the
pauses between the pulses
passes, if the main circuit is turned off. Thus it follows that the minimum
pulse frequency is
determined to implement the method according to claim 1 of the present
application by the
following relationship:
RA= 106
= 1.87 fl ' cm2
27r . 17 fmin
which yields: fmin =5 kHz seen from the above formula is easy to see the
effect of increasing the
frequency to lower the anode impedance RA and thus the total resistance R of
the power source.
CA 02759684 2011-10-21
11
When used in the used electrochemical cell different anode materials as shown
in Figure 4, the
capacity of a different range of values of the differential CD,s has then the
minimum pulse
frequency for performing the method according to claim 1 of the present
application, analogous
to corresponding with a minimum value of CD,S of the anode was determined.
Accordingly, the
definition is the removal of properties in Claim 1 does not have fixed
frequencies, but the
impedance of the anode.
At a pulse frequency of f =100 kHz, the resistance of the anode RA is
considerably smaller than
the resistance of modem cathode (see above) and thus a further increase in
pulse frequency with a
view to further reduction in the resistance portion RA in the total resistance
of an electrochemical
power source uninteresting. Therefore, the possible frequencies are useful for
the
implementation of the method according to claim 1, under the circumstances
called conditions in
the range 5 to 100 kHz.
The selection of the optimum frequency within the specified setting range is
achieved by a
balance between the following physical processes for the purpose of a maximum
efficiency of the
entire system is found under the condition that the value given the maximum
power of the power
source to the consumer is not under a value is:
1) Increase the energy to be removed by increasing the frequency and reduction
of the current
pulse pause ratio (ratio of pulse to pulse pause time).
2) The increase of the electrochemical power source to be removed depends on
power of
increasing the frequency and amplitude, and reduction of the current pulse
pause ratio.
CA 02759684 2011-10-21
12
3) Reduce the resistance of the electrochemical power source with an increase
of the current
pulse frequency.
4) The increase of losses (inductive, resistive, etc.) in DC-DC converter
depends on the change of
frequency, amplitude ratio and the break of the current pulses.
An extension of the frequency bandwidth to 1 MHz appears at the current state
of the art in
practice only be justified if in the future a reduction of the cathode and the
electrolyte resistance
is generally technically possible, and in the event of a further development
of the electronics and
the creation of a new high-frequency transducer for even greater efficiency
and maximum
performance of the entire system.
Consequently, the frequency range defined by claim 1 for most electrochemical
power sources is
basically of 5 kHz to infinity. The calculation of this frequency range for
each electrochemical
power source carried by the above formula for RA in the alignment of the
double layer resistance
RA according to claim 1. The choices regarding a specific frequency will
increase with the state
of the electronics in the current consumption by frequency pulses. The maximum
possible
frequency of the pulses is increased, whereby the pulse pause ratio should be
minimal, but not
less than that for reconstruction (regeneration) of the double layer after
each pulse time required,
which by today's calculations, about 10-7 is specified seconds. Here, the loss
must increase in the
electronic system in the implementation of this method of energy extraction
and transmission to
the consumer less than the higher frequencies and reduction of the current
pulse pause ratio
remains recovered energy.
CA 02759684 2011-10-21
13
As practical example of the above-described embodiment, a single-celled metal-
air cell was used,
the magnesium anode had an area of 280 cm2 and the gas diffusion cathode, an
area of 240 cm2.
The electrode spacing was 0.5 cm. As the electrolyte was an aqueous sodium
chloride solution.
In the non-closed circuit had the cell has a voltage of 1.74 V. A transducer
was manufactured
consisting of a shunt circuit capacity Csh = 10.500 F, a switching element
with control unit and a
planar transformer with a memory element and the storage capacitor in the
secondary circuit.
The power source with the above metal-air cell and the transducer ensured a
charge voltage of
12 V. The input impedance of the current transformer was 1 milliohm.
In the DC regime gave the cell a maximum power of 42 W at a voltage of 0.84 V
and an
operating temperature from 50 C. The current density was 197 mA / cm2. After
40 minutes of
work, the voltage dropped from the cell up to 0.75 V, after which the current
output stopped
because the electrode gap was filled with reaction products. In the DC regime,
the internal
resistance of the cell was 18 milliohms and the current 50 A.
After working in the DC regime, the current transformer is connected to the
cell. At the output of
the current transformer were a charge voltage of 12.05 V, a load current of
3.5A and an output of
41.2 W. Before the experiment, the power converter has been verified. With an
input voltage of
0.9 V and an output of 45-60 W, the efficiency was 0.8. The loss in the
current transformer was
11.5 W. The losses were in the leads, 1.5 W. Thus, the power at the input of
the CT was about
54.2 W. The current strength of the cell in this case was 58 A, the effective
voltage of 0.93 V.
The total internal resistance of the electrochemical cell was calculated to 13
milliohms. After an
estimation in DC regime the sum of the internal resistance was 18 milliohms.
Thus, the
resistance has decreased by 5 milliohms.
CA 02759684 2011-10-21
14
Furthermore, the current transformer is set to a frequency of 77 kHz. The
internal resistance of
the electrochemical cell was reduced by only slightly fell (from 13 milliohms
to 12.5 milliohms),
but the metal consumption by almost 10 %. While working in the 27 kHz regime
of consumption
1.62 Ah/g, while in the 77 kHz regime was 1.78 Ah/g. The voltage across the
electrochemical
cell was while working in the frequency regime of energy extraction from the
electrode and no
gap was not filled with reaction products.
Comparative analysis
The results obtained with the above technical advantages can be illustrated by
a comparison of
the prototype "AKWA MW 12/40" which was developed using the novel process of
the
AKWA GmbH, Frankfurt am Main, with the Russian company of the MVIT, Moscow,
Federation produced, commercially available products, "FLC MVIT 4-800" or "2-
400 MVIT
FLC" show.
At the present time by the Company AKWA GmbH a serial production of
autonomous,
environmentally clean, and compact power sources with salt water functioning
"AKWA 12/40
MW" and "AKWA 12/25 MW" prepared by applying the invention.
a) Maximum capacity, dimensions and weight, a comparison of the maximum power
assets
shows that the prototype "AKWA 12/40 MW" in the power of the power source
"MVIT 400-
800 irC" matches. The dimensions exceed the dimensions of the MVIT AKWA to be
more than
fourfold. A weight comparison results in a similar advantage for the AKWA .
The dry weight
(storage and transport) of the MVIT is more than five times the operating
weight is still more
than four times the amount of AKWA .
CA 02759684 2011-10-21
b) capacity of the replacement cartridge shows a comparison of the replacement
cartridge
capacity, the capacity of "AKWA exceeds MW 12/40" with the capacity of 480 Wh
"MVIT
200-400 itC "with 400 Wh to 80 Wh. It consists of the replacement cartridge
AKWA only two
anodes with a total weight of 0.338 kg, while the implement cartridges MVIT-ge
of four anodes
5 with a total weight of 0.480 kg. This means that the efficiency of metal
utilization factor is
greater of at least 1.7.
A replacement cartridge of AKWA allows among others the following purposes:
= Power a light bulb (12 V, 12 W) for up to 40 hours
= Power a TV (12 V) for up to 25 hours
10 = Power a travel refrigerator (35 W) for up to three days (4800 mAh)
= Up to seven charges of a notebook battery up to 25 charges from mobile
phones, digital
cameras, radios, GPS receivers, radios and portable CD / DVD players
= A charge for a car battery (55 Ah)
The following table compares the data of different power sources.
MVIT 4-800 ztC AKWA MVIT 2-400 ztC
12/40 MW
Nominal tension, [V] 12 12 12
Max. efficiency, [W] 40 40 21
Nominal Capacity per Cartridge, [Wh] 800 480 400
Min. ambient temperature, [.deg.C] -20 -25 20
Dry Weight, [kg] 5.4 1 3
Employment weight, [kg] max. 11.4 max. 2.6 max. 6
CA 02759684 2011-10-21
16
Weight of a spare cartridge, [kg] 0.96 0.338 0.48
Dimensions (LB/H), [mm] 250/420/220 190/100/225 250/230/220
Salt water concentration, [g/1] 100-150
Durability in the dry conditions min. 10 years
Guaranteed Lifetime min. 3000 hours
List of reference numerals
1 Electrochemical power source
2 Capacitive shunt circuit (shunt)
3 Switching element with control unit
4 Transformer
5 Inductive energy storage
6 Storage capacitor
7 Load resistor (consumer)