Note: Descriptions are shown in the official language in which they were submitted.
CA 02762644 2016-05-02
MILDLY ALKALINE THIN INORGANIC CORROSION PROTECTIVE
COATING FOR METAL SUBSTRATES
TECHNICAL FIELD
[0001]
This invention relates generally to corrosion protection of metal substrates,
more particularly to a neutral to mildly alkaline thin inorganic dried in
place coating
composition that can be applied directly to a metal substrate without pre-
treatment such as a
phosphatizing solution and that provides enhanced corrosion protection to the
metal
substrate. The dried in place coatings of the present invention also provide a
unique
morphology when dried in place comprising a continuous inorganic phase and a
discontinuous dispersed polymer phase.
BACKGROUND OF THE INVENTION
[0002]
Untreated metal surfaces are subject to corrosion which can lead to rust
development, weakening, discoloration and failure of the surface. Thus metal
substrates are
typically treated by a variety of methods to make the surface less reactive
and more corrosion
resistant. In addition, metal surfaces are often subsequently coated with
decorative or
additional protective coatings such as resin coatings, primers, paints and
other surface
treatments. Often the initial treatment of the metal surface involves a metal
phosphate
treatment followed by a chrome-containing rinse.
[0003]
Metal objects to which surface treatments and coatings are applied can be
grouped into several categories. In some industrial applications, the metal is
formed into a 3-
dimensional object after which any combination of surface treatments and or
coating
applications may be made. In a second category of industrial applications,
surface treatments
and or coatings are applied to the metal prior to forming when the metal is in
the form of a
flat sheet which is typically rolled into a coil. For many coatings
applications within this
category, special properties are desirable to facilitate rolling and forming
operations. For
coatings such as organic passivates, it may be desirable to have a high degree
of hardness and
block-resistance to facilitate rolling, however conventional coatings of high
hardness
frequently possess poor forming properties in that the integrity of the
coating and ultimately
1
CA 02762644 2016-05-02
its corrosion resistance is compromised by forming operations. It is desirable
to provide
coatings which have both high hardness and good forming properties.
[0004] It
would be beneficial to develop a corrosion resistant coating composition
that was inorganic and that could be used under neutral or mildly alkaline
conditions. It is
also important to provide a coating composition that would not prevent
continued use of the
other decorative surface treatments that have been used in the past. For many
years coatings
for metal such as organic passivate coatings have utilized hexavalent chrome
stemming from
its ability to inhibit corrosion. Hexavalent chrome has become less favored in
the
marketplace due to environmental considerations. Over time, trivalent chrome
containing
coatings have found greater use due to the lower level of environmental
concern relative to
hexavalent chrome based products. In many cases, this change has been made
with a drop in
corrosion resistance. It is always desirable to improve the performance
properties of coatings
such as corrosion resistance. This is true for any coating such as coatings
based on
hexavalent chrome. It would be more desirable to similarly improve performance
properties
such as corrosion resistance for coatings which are not based on hexavalent
chrome, such as
those based on trivalent chrome or non-chrome based coatings. It is also
undesirable for
coatings comprising chrome to leach chrome to the environment
SUMMARY OF THE INVENTION
[0005] In
general terms, this invention provides a neutral or mildly alkaline inorganic
coating composition that can be applied directly to a metal surface without a
phosphatizing pre-treatment and that provides significant corrosion
protection. Coatings
of the present invention also provide a unique morphology when dried in place
of two
distinct phases. The first phase is a continuous inorganic phase derived from
water-
soluble inorganic components. The second phase is a dispersed phase comprising
a
polymer dispersion in the first phase. This morphology provides a number of
desirable
coating attributes.
Such attributes include good forming properties despite high apparent
hardness, outstanding adhesion to metals and alloys such as those based on
iron, zinc and
aluminum, and high chemical and corrosion resistance. Embodiments of the
present
invention which further comprise chrome are not prone to chrome leaching and
show
2
CA 02762644 2016-05-02
significant enhancements in corrosion resistance relative to conventional
chrome based
products.
[0006] The coating compositions prepared according to the present
invention
preferably have a pH of from about 6 to 11 and more preferably from 8 to 10.
In one
embodiment, a coating composition of the present invention comprises a source
of at least
one of the group IVB transition metal elements of the Periodic Table, namely
zirconium,
titanium, and hafnium and, optionally, a source of at least one of the group
VB transition
metal elements of the Periodic Table, namely vanadium, niobium, and tantalum.
Preferably,
the coating composition includes from 9 to 73% by weight, based on the total
dry solids
coating weight, of at least one element from group IVB of the Periodic Table.
A preferred
group IVB element is zirconium, preferably supplied as ammonium zirconium
carbonate. A
preferred group VB element is vanadium supplied as V205. The coating
composition also
includes an organic polymer wherein the weight percentage of organic polymer
active solids
based on total dry solids coating weight is from 1% to 75%.
[0007] In another embodiment, a coating composition of the present
invention
comprises a source of at least one of the group IVB transition metal elements
of the Periodic
Table, namely zirconium, titanium, and hafnium and a source of chrome.
Preferably, the
coating composition includes from 9 to 73% by weight, based on total dry
solids coating
weight, of at least one element from group IVB of the Periodic Table. A
preferred group IVB
element is zirconium, preferably supplied as ammonium zirconium carbonate. In
this
embodiment, the coating composition includes a chrome source such as chromium
trioxide.
The coating composition also includes an organic polymer wherein the weight
percentage of
organic polymer active solids based on total dry solids coating weight is from
1% to 75%.
[0008] The coating compositions according to the present invention are
dry in place
conversion coatings. The coating is very versatile because it can accommodate
addition of a
wide variety of organic polymers which can be added directly to the coating
composition
thus eliminating multistep coating processes, the suitable resin polymers
being ones that are
dispersible or soluble in the inorganic aqueous coating composition. In
addition, the coating
exhibits significant formability and hardness. Being a conversion coating, as
the term is
known in the art, components within the coating composition react with the
metal substrate
during the coating process to produce the final dry in place coating.
3
CA 02762644 2016-05-02
[0009] In one embodiment, there is provided a dried in place corrosion
protective
coating deposited on a metal substrate, the coating having a morphology
comprising:
a continuous phase consisting of an inorganic phase, the inorganic phase
consisting
essentially of at least one element from group IVB of the Periodic Table
present in an amount
of from 9 to 73% by weight, based on total dry solids coating weight; and a
discontinuous
phase consisting of an organic phase, the organic phase consisting essentially
of active solids
of at least one organic polymer, the active solids of the at least one organic
polymer present
in the coating in an amount of from 1% to 75% by weight, based on total dry
solids coating
weight, the active solids of the at least one organic polymer present as
discrete polymer
spheres dispersed in the continuous inorganic phase and wherein the at least
one organic
polymer is selected from the group consisting of a polyvinyl dichloride resin,
an acrylic-
based resin, a methacrylate-based resin, a styrene-based resin, and mixtures
thereof; and
wherein the continuous phase optionally includes at least one element from
group VB of the
Periodic Table, and wherein the coating optionally includes a reducing agent
or a reaction
product thereof for the at least one element from group VB.
[0010] In another embodiment, there is provided a dried in place
corrosion protective
coating for metal substrates having a morphology comprising: a continuous
inorganic phase
consisting of an inorganic phase, the inorganic phase consisting essentially
of from 9 to 73%
by weight, based on total dry solids coating weight, of at least one element
from group IVB
of the Periodic Table and a source of chrome; and a discontinuous phase
consisting of an
organic phase, the organic phase consisting essentially of active solids of at
least one organic
polymer, the active solids of the at least one organic polymer present in an
amount of from
1% to 73% by weight, based on total dry solids coating weight, the active
solids of the at
least one organic polymer present as discrete polymer spheres dispersed in the
continuous
inorganic phase and wherein the at least one organic polymer is selected from
the group
consisting of polyvinyl dichloride resin, an acrylic-resin, a methacrylate-
based resin, a
styrene-based resin, and mixtures thereof; and wherein the continuous phase
optionally
includes at least one element from group VB of the Periodic Table, and wherein
the coating
optionally includes a reducing agent or reaction product thereof for the at
least one element
from group VB.
4
CA 02762644 2016-05-02
[0010A] In another embodiment, there is provided a corrosion protective
conversion
coating composition for metal substrates comprising: a continuous phase
inorganic portion
consisting essentially of from 9 to 73% by weight, based on total dry solids
coating weight,
of at least one element from group IVB of the Periodic Table and a source of
chrome; and a
discontinuous phase organic portion, the organic portion consisting
essentially of from 1% to
75% by weight, based on total dry solids coating weight, of active solids of
at least one
organic polymer, the active solids of the at least one organic polymer present
as discrete
polymer spheres dispersed in the inorganic portion, and wherein the conversion
coating
composition has a pH of from about 6 to 11 and wherein the at least one
organic polymer is
selected from the group consisting of polyvinyl dichloride resin, an acrylic-
resin, a
methacrylate-based resin, a styrene-based resin, and mixtures thereof; and
wherein the
continuous phase optionally includes at least one element from group VB of the
Periodic
Table, and wherein the coating composition optionally includes a reducing
agent or a
reaction product thereof for the at least one element from group VB.
[0011] These and other features and advantages of this invention will
become more
apparent to those skilled in the art from the detailed description of a
preferred embodiment.
The drawings that accompany the detailed description are described below.
BRIEF DESCRIPTION OF THE FIGURES
[00012] Figure 1A is a photograph from a Dark-Field Scanning Transmission
Electron
Microscopy of a chrome-based coating prepared according to the present
invention;
[00013] Figure 1B is a higher magnification of the coating from Figure 1A;
[00014] Figure 2A is a photograph from a Dark-Field Scanning Transmission
Electron Microscopy of a non-chrome coating prepared according to the present
invention;
[00015] Figure 2B is a higher magnification of the coating from Figure 2A;
and
[00016] Figure 3 is a photograph from a Dark-Field Scanning Transmission
Electron
Microscopy of a conventional commercial chrome-based coating not prepared
according to
the present invention.
4a
CA 02762644 2016-05-02
DETAILED DESCRIPTION OF A PREFERRED EMBODIMENT
[00017] The present invention is directed toward treatment of bare metal
surfaces
meaning that the metal surface has not been pre-treated with any metal
phosphate solutions,
chrome-containing rinses, or any other passivating treatments. Metal surfaces
that benefit
from the process of the present invention include steel, cold rolled steel,
hot rolled steel,
stainless steel, aluminum, steel coated with zinc metal or zinc alloys such as
electrogalvanized steel, galvalume0, galvanneal, and hot-dipped galvanized
steel.
[00018] Preferably, the metal surface has been cleaned and degreased prior
to
treatment according to the present invention. Cleaning of metal surfaces is
well known in
the art and can include mild or strongly alkaline cleaners. Examples of two
alkaline
cleaners include Parco0 Cleaner ZX-1 and Parco0 Cleaner 315 both available
from Henkel
Surface Technologies. Following cleaning the surface is preferably rinsed with
water prior
to treatment according to the present invention.
4b
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
[00019] In one embodiment, the corrosion protection coating of the
present
invention comprises a mixture of at least one group IVB element and at least
one group
VB element in deionized water at a pH of from about 6 to 11 and more
preferably at a pH
of from 8 to 10. It is important that the pH of the composition be kept in
this range for the
coating process to work. In one embodiment, preferably the group IVB element
is present
in an amount of from about 1 to 7% by weight, more preferably from about 2 to
5% by
weight and most preferably from 3 to 5% by weight of the composition based on
the total
weight of the composition. The coating composition can include any sub-range
between 1
to 7% by weight based on the total weight. In this embodiment, preferably the
amount of
group VB element in the composition is from about 0.20 to 2.00% by weight and
more
preferably from about 0.40 to 1.00% by weight based on the total weight of the
composition. The coating composition can include any sub-range between 0.20 to
2.00%
by weight based on the total weight. Preferably the coating composition is a
mixture of
zirconium and vanadium. One preferred source of zirconium is ammonium
zirconium
carbonate called Bacote 20 and available from MEI in Flemington New Jersey.
According to the literature from MEI, Bacote 20 is a clear, aqueous alkaline
solution of
stabilized ammonium zirconium carbonate containing anionic hydroxylated
zirconium
polymers. It provides approximately 20% w/w of Zr02. It is sold as a
crosslinking agent
for paper and paperboard applications. The preferred group VB element is
vanadium
provided as V205. Optionally, the present coating can further accommodate the
addition
of organic coating resin polymers of a variety of types including, by way of
example only:
epoxies, polyvinyl dichlorides, acrylic-based resins, methacrylate-based
resins, styrene-
based resins, polyurethane dispersions, and polyurethane dispersion hybrids.
Examples of
these resin polymers include Carboset CR760, Hauthane HD-2120, Hauthane L-
2989,
MaincoteTM PR-15, MaincoteTM PR-71, Avanse MV-100, Rhoplex AC 337N, and
Alberdingk-Boley LV-51136 and M-2959. The coating can also accommodate
addition of
reducing agents for the V205 such as cysteine, Sn2+, ascorbic acid, or
thiosuccinic acid.
Optionally, one could initially start with V+4 from vanadyl sulfate or vanadyl
acetylacetonate. Optionally, the coating can also include processing aids such
as waxes
which aid in formability of the coated substrates. Addition of these optional
agents will be
discussed further below.
[00020] In a first example an inorganic coating composition according to
the
present invention was prepared by combining 83.00% by weight deionized (DI)
water with
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
1.00% by weight V205 and 16.00% by weight of Bacote 20 . This level of Bacote
20
provides 3.2% by weight of Zr02 to the composition. The composition pH was
approximately 9.5. The inorganic coating was applied to a series of hot-dipped
galvanized
(HDG) panels known as ACT HDG panels APR 31893 and U.S. Steel Corp. (USS)
Galvalume panels using the known technique of a draw wire to apply a coating
weight
of 200 milligrams per square foot (200 milligrams per 929.03 square
centimeters).
Galvalume is the trademark name for 55% aluminum-zinc alloy coated sheet
steel. Once
applied the coating was dried in place to a Peak Metal Temperature (PMT) of
210 F (98
C) on the test panels. The panels were then subjected to a Neutral Salt Spray
(NSS)
corrosion test using method ASTM B117-03 with multiple panels for each time
point. In
this testing uncoated panels of either HDG or USS Galvalume showed 100%
corrosion
with in 24 hours in the NSS test. The test results for the average percent
corrosion for
each of the treated panels are shown below in Table 1.
TABLE 1
Time, hours 24 48 144 312 480 649 816 1008
(NSS)
HDG 70.00
USS 0.00 00.00 0.00 4.00 13.00 13.00 22.00 25.00
Galvalume
[00021] The results demonstrate the usefulness of the coating composition
prepared
according to the present invention. The coating composition of the present
invention was
very effective on USS Galvalume steel providing significant corrosion
protection out to
1008 hours as shown. These results are in dramatic difference to uncoated USS
Galvalume which was 100% corroded within 24 hours. The results were also
significant, but not quite as good, using a HDG substrate.
[00022] As discussed above another advantage of the present coating
composition is
that it can easily accommodate the addition of organic resins to further
enhance the
corrosion protection without requiring complex multi-step processing or
applications. The
desired resin can merely be added to the coating composition. In a first
example of
combining the inorganic coating composition with an organic resin use was made
of
polyvinyl dichloride (PVDC) as the organic resin. The PVDC resin used was
Noveon
6
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
XPD-2903. A series of coating compositions were prepared as described below in
Table
2.
TABLE 2
Component Formula 57B Formula 57C Formula 57D
Deionized water 73.50 63.50 53.50
Bacote 20 16.00 16.00 16.00
V205 0.50 0.50 0.50
PVDC 10.00 20.00 30.00
[00023] Each founula was then coated onto a series of HDG panels and a
series of
USS Galvalume panels using the dry in place process described above at a
coating
weight of 200 milligrams per square foot (200 milligrams per 929.03 square
centimeters)
and dried to a PMT of 210 F (98 C). A series of control HDG and USS
Galvalume0
panels were created using the commercially available non-chrome containing
coating
Granocoat 342TM (G342) available from Henkel. The G342 was applied per the
manufacture's instructions. In a first test panels were subjected to a NSS
test as described
above and multiples of each time point were evaluated for the percent
corrosion and the
average calculated. The results are presented below in Table 3 wherein the
abbreviation
Gal. indicates the USS Galvalumee panels.
TABLE 3
Time G342 57B 57C 57D G342 57B 57C 57D
hours Gal. Gal. Gal. Gal. HDG HDG HDG HDG
(NSS)
24 0.10 0.03 0.00 0.00 0.00 1.10 0.13 0.77
48 0.10 0.03 0.00 0.00 0.20 1.10 0.30 2.67
72 0.33 0.33 0.00 0.00 0.67 1.67 4.33 3.00
96 0.67 0.33 0.00 0.00 2.67 3.67 8.67 7.33
168 5.00 1.00 0.00 0.00 17.00 8.67 18.33 20.00
336 13.33 1.00 0.03 0.05 63.33 35.00 56.67 43.33
504 48.67 2.67 0.33 0.50 60.00 75.00 70.00
672 76.67 2.67 2.33 1.00
7
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
840 3.00 4.33 3.00
1200 10.67 9.00 3.00
[00024] The results conclusively demonstrate the enhanced corrosion
protection
provided by the coating composition of the present invention. In viewing the
data on the
USS Galvalume panels one begins to see an improvement in corrosion protection
in all
of the panels compared to the G342 control by 168 hours of testing and the
differences
increase with increased testing time. After 504 hours of testing the panels
coated
according to the present invention have from 18 to 147 fold less corrosion
than the control
G342 panels. By 840 hours the control G342 panels have from 28 to 76 times as
much
corrosion as the panels coated according to the present invention. Even after
1200 hours
of testing the panels coated according to the present invention have only 3 to
11%
corrosion. These results are dramatic and show the power of the coating
composition
prepared according to the present invention. The results also demonstrate that
increasing
the level of polyvinyl dichloride from 10% to 30% had a small effect on the
degree of
corrosion protection at the last time point. Turning to data from the HDG
panels one can
see that coatings according to the present invention also provide enhanced
protection
compared to the G342 up to a point of about 504 hours. The results with the
HDG panels
are not as dramatic as for the USS Galvalume panels. Also, the effect of
increasing the
level of polyvinyl dichloride seems to be the opposite of that seen on the USS
Galvalume panels. The higher the level of polyvinyl dichloride the worse the
coating
seemed to be in protecting from corrosion for the HDG panels.
[00025] In the next series of corrosion testing panels of USS Galvalume
or HDG
were coated as described above using the formulas from Table 2 at 200
milligrams per
square foot (200 milligrams per 929.03 square centimeters) and dried in place
to a PMT of
210 F (98 C) onto the panels. Then a Stack Test was performed to simulate
panels in
contact with each other in a humid environment. The Stack Test was performed
by
spraying deionized water onto a coated side of a first panel, placing a coated
side of a
second panel against the coated side of the first panel and then clamping the
first and
second panels together. The clamped panels are then placed in a humidity test
chamber at
100 F (38 C) and 100% humidity. After various time points multiples of each
condition
are removed and the percent corrosion of each is determined and the results
averaged. The
averaged results are presented below in Table 4.
8
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
TABLE 4
Time G342 57B 57C 57D 0342 57B 57C 57D
hours Gal. Gal. Gal. Gal. HDG HDG HDG HDG
(Stack)
168 3.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
336 5.00 0.00 0.00 0.00 5.00 3.00 1.00 1.00
504 5.00 0.00 0.00 7.00 5.00 3.00 3.00 5.00
672 7.00 0.00 1.00 8.00 5.00 5.00 10.00 16.00
840 8.25 0.50 1.00 12.00 10.00 16.00 25.00 30.00
1200 10.00 2.00 3.00 12.00 50.00 40.00 60.00 60.00
1344 10.00 2.00 3.00 16.00
1512 10.00 2.00 3.00 20.00
1680 10.00 3.00 7.00 23.33
1848 20.00 5.00 7.00 30.00
2016 22.50 5.00 10.00 40.00
[00026] The results demonstrate that for resin levels of 10 and 20% the
coating
composition according to the present invention performed much better than the
G342
coating at all time points by a factors of 16 to 2.2 fold depending on the
time point. The
coating having 30% PVDC, however, did not perform as well as the control G342
panels
after 1200 hours and by 2016 hours it showed about twice as much corrosion as
the
control panel. The reason for this difference is unknown. With respect to the
HDG panels
the results show less difference between the control panels and the coatings
according to
the present invention. The panels all show significant corrosion protection
out to 504
hours. Thereafter the coating compositions with 20 and 30% PVDC performed
worse than
the G342 panels and than the 10% PVDC panels.
[00027] In the next series of corrosion testing panels of USS Galvalume
or HDG
were coated as described above using the formulas from Table 2 at 200
milligrams per
square foot (200 milligrams per 929.03 square centimeters) and dried in place
to a PMT of
210 F (98 C) onto the panels. Then a Cleveland humidity test (CHT) was
performed on
the panels using ASTM method D4585. The results are presented below in Table
5.
TABLE 5
9
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
Time G342 57B 57C 57D G342 57B 57C 57D
hours Gal. Gal. Gal. Gal. HDG HDG HDG HDG
(CHT)
168 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
336 7.00 3.00 0.00 0.00 7.00 3.00 0.00 0.00
504 7.00 3.00 0.00 0.00 10.00 3.00 0.00 0.00
672 7.00 3.00 0.00 0.00 10.00 3.00 0.00
840 7.00 3.00 0.00 0.00 10.00 3.00 1.00
1200 7.00 7.00 1.00 0.3 16.00 5.00 5.00
[00028] The USS Galvalume results demonstrate that coating composition of
the
present invention performs much better than the control G342 coating except
for 1200
hours at 10% PVDC which is equivalent to the control G342. The results also
clearly
demonstrate that increasing the amount of PVDC has a very positive effect on
the
corrosion protection of the coating prepared according to the present
invention. Similar
results are seen on the HDG panels with the coating according to the present
invention
providing significantly enhanced corrosion protection compared to the G342. In
addition,
increasing the amount of PVDC seems to enhance the corrosion protection.
[00029] In the next series of corrosion testing panels of USS Galvalumet
or HDG
were coated as described above using the formulas from Table 2 at 200
milligrams per
square foot (200 milligrams per 929.03 square centimeters) and dried in place
to a PMT of
210 F (98 C) onto the panels. Then a Butler water immersion (BWI) test was
performed
on a series of the panels. Each test panel is supported and immersed in a tank
of distilled
water such that there is one half an inch of water below each panel and three
quarters of an
inch of water above each panel. The tanks with the panels are then placed in a
humidity
chamber set at 100% humidity and 100 F (38 C). Panels are removed at the
selected
time points and evaluated for the percent corrosion. The results are presented
below in
Table 6.
TABLE 6
Time G342 57B 57C 57D G342 57B 57C 57D
hours Gal. Gal. Gal. Gal. HDG HDG HDG HDG
(BWI)
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
168 0.00 0.00 1.00 0.00 0.00 1.00 0.00 0.00
336 0.00 0.00 1.00 1.00 16.00 1.00 0.00 1.00
504 0.00 0.00 1.00 1.00 50.00 1.00 0.00 3.00
672 3.00 0.00 1.00 1.00 1.00 0.00 3.00
840 7.00 7.00 1.00 3.00 7.00 7.00 7.00
1200 16.00 7.00 3.00 10.00 25.00 16.00 10.00
1344 16.00 7.00 3.00 10.00 25.00 16.00 16.00
1572 20.00 7.00 3.00 10.00 30.00 16.00 16.00
1680 20.00 7.00 3.00 10.00 30.00 20.00 20.00
1848 25.00 7.00 3.00 10.00 30.00 20.00 25.00
2016 30.00 7.00 3.00 16.00 40.00 30.00 40.00
[000301 The USS Galvalumee results demonstrate that the coatings prepared
according to the present invention provide significantly more corrosion
protection than the
control G342 coating. The enhanced protection ranges from an approximately 2
fold to 10
fold increased corrosion resistance compared to G342. The effect of PVDC level
on the
corrosion protection appears complex and non-linear with the highest level
appearing less
efficient than levels of from 10 to 20% by weight. The HDG panels also show
the benefit
of the coatings according to the present invention versus G342. All of the
panels coated
according to the present invention showed enhanced corrosion protection
compared to
G342. Again the effect of PVDC level was complex and seemed to show best
results with
20% PVDC.
[000311 As shown above an advantage of the present coating is that it can
easily
accommodate the addition of organic resins to further enhance the corrosion
protection
with out requiring complex multi-step processing or applications. The desired
resin can
merely be added to the coating composition. In a second example of combining
the
inorganic coating with an organic resin use was made of a thermoplastic
styrene-acrylic
copolymer emulsion ,designated Carboset CR-760, as the organic resin. The
Carboset
CR-760 is available from Lubrizol Advanced Materials, Inc. of Cleveland Ohio.
The
Carboset CR-760 has approximately 42% by weight solids. In additional
coatings the
Carboset CR-760 was further combined with the PVDC used above. In additional
formulations the coating composition also included a carnauba wax emulsion to
enhance
foimability of the coating composition. The carnauba wax emulsion used was
Michem
11
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
Lube 160 available from Michelman, Inc. of Cincinnati Ohio. A series of
coating
compositions were prepared as described below in Table 7. Each formula was
then coated
onto a series of HDG panels and a series of USS Galvalume panels using the
dry in
place process described above at a coating weight of 175 to 180 milligrams per
square foot
(175 to 180 milligrams per 929.03 square centimeters) and dried to a PMT of
210 F (98
C). In a first corrosion test panels were subjected to a NSS test as described
above and
multiple panels of each time point were evaluated for the percent corrosion.
The average
results for each time point for the NSS test are presented below in Table 8.
No samples
for NSS for formula 162B were run. Additional panels were used to evaluate the
coatings
using the Butler water immersion test, the Cleveland humidity test, and the
Stack Test
each performed as described above. The results of these tests are present
below in Tables
9, 10 and 11 respectively.
TABLE 7
Component 162A 162B 162C 162D
Deionized 32.50 26.00 39.50 33.00
water
Bacote 16.00 16.00 16.00 16.00
20
V205 0.50 0.50 0.50 0.50
Carboset0 51.00 51.00 26.00 26.00
CR760
PVDC 18.00 18.00
C arnaub a 6.50 6.50
wax
TABLE 8
Time 162A 162B 162C 162D 162A 162B 162C 162D
hours Gal. Gal. Gal Gal. HDG HDG HDG HDG
(NSS)
24 0.00 0.00 0.00 0.00 0.00 7.00 7.00
48 0.00 0.00 0.00 0.00 23.66 16.00 20.00
168 0.00 1.00 0.70 0.00 100.00 86.67 93.33
12
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
336 0.00 3.33 8.67 0.00
504 1.00 5.67 6.00 0.00
672 1.00 8.67 10.00 0.00
840 1.00 8.67 10.00 1.00
1008 1.00 15.00 16.00 1.00
1176 1.00 20.00 25.00 5.00
1344 5.00 25.33 50.00 15.33
1512 5.67 28.67 17.33
1680 6.33 30.00 20.00
1848 6.33 23.33 20.00
2016 6.33 36.67 21.67
[000321 The USS Galvalume results demonstrate that the coatings according
to
the present invention all were more effective than the G342 coating was in the
results
reported in Table 3 above. The coating with just Carboset CR760 was very
effective
even out as far as 2016 hours. The comparison of formula 162A to 162B shows
that
addition of the carnauba wax to this formula appears to reduce the coating
effectiveness as
a corrosion protection coating. The results also show that combining the
Carboset
CR760 with PVDC reduces the effectiveness of the coating composition compared
to use
of Carboset CR760 alone, however, addition of the carnauba wax to the blend
seems to
enhance its effectiveness. None of the coatings appear to be very effective on
the HDG
samples and presence of carnauba wax or PVDC does not seem to affect the
perfoimance
of Carboset CR760 alone.
TABLE 9
Time 162A 162B 162C 162D 162A 162B 162C 162D
hours Gal. Gal. Gal Gal. HDG HDG HDG HDG
(BWI)
168 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
336 1.00 1.00 0.00 0.00 0.00 0.00 0.00 0.00
504 3.00 3.00 1.00 1.00 0.00 3.00 5.00 5.00
672 5.00 3.00 3.00 1.00 1.00 5.00 5.00 5.00
13
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
840 5.00 5.00 3.00 1.00 1.00 7.00 7.00 10.00
1008 5.00 5.00 5.00 1.00 1.00 7.00 7.00 16.00
1176 16.00 10.00 10.00 1.00 1.00 1.00 16.00 20.00
1344 16.00 16.00 16.00 3.00 3.00 7.00 20.00 20.00
1512 16.00 16.00 20.00 3.00 3.00 10.00 25.00 30.00
1680 16.00 16.00 30.00 5.00 7.00 30.00 30.00 30.00
1848 16.00 16.00 30.00 5.00 7.00 30.00 50.00 50.00
2016 16.00 16.00 40.00 5.00 7.00 40.00
[00033] The results with the USS Galvalumee panels demonstrate that with
the
exception of the blend of Carboset CR760 and PVDC all of the coatings
performed
better than did G342 from Table 6. In the BWI test there was not a detrimental
effect on
performance for Carboset CR760 alone. In contrast to the NSS test, the
combination of
Carboset CR760 with PVDC and carnauba wax performed the best in the BWI test.
Again as seen in the NSS test results there is a benefit to including the
camauba wax when
combining the Carboset CR760 with PVDC. The results with the HDG panels also
show that all of the coatings prepared according to the present invention
performed better
than did G342 from Table 6. Significantly better performance was obtained with
the
Carboset CR760 alone compared to addition of camauba wax, PVDC, or camauba
wax
and PVDC.
TABLE 10
Time 162A 162B 162C 162D 162A 162B 162C 162D
hours Gal. Gal. Gal Gal. HDG HDG HDG HDG
(CHT)
168 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
336 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
504 3.00 3.00 3.00 1.00 0.00 3.00 5.00 5.00
672 3.00 3.00 3.00 2.00 0.00 3.00 5.00 5.00
840 3.00 3.00 3.00 3.00 1.00 3.00 5.00 5.00
1008 3.00 3.00 3.00 3.00 3.00 3.00 5.00 5.00
14
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
100034] The results for both the USS Galvalume and HDG show that in the
Cleveland humidity test all of the coatings according to the present invention
performed
equally well irrespective of the substrate and that all performed better than
the results seen
with the control G342 in Table 5.
TABLE 11
Time 162A 162B 162C 162D 162A 162B 162C 162D
hours Gal. Gal. Gal Gal. HDG HDG HDG HDG
(Stack)
168 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
336 0.00 1.00 0.00 0.00 0.00 0.00 1.00 1.00
504 0.00 1.00 1.00 1.00 5.00 5.00 10.00 7.00
672 0.00 3.00 1.00 1.00 10.00 20.00 30.00 16.00
840 1.00 5.00 1.00 3.00 10.00 20.00 30.00 37.50
1008 1.00 5.00 3.00 3.00 20.00 30.00 40.00 40.00
1176 1.00 5.00 3.00 5.00 30.00 40.00
1344 3.00 5.00 3.00 5.00 50.00
1512 3.00 7.00 3.00 5.00
1680 3.00 7.00 3.00 5.00
1848 3.00 7.00 3.00 5.00
2016 5.00 7.00 5.00 5.00
[00035] The USS Galvalume results demonstrate that all of the coatings
according
to the present invention performed equally well in the Stacks Test and that
they performed
better than the control G342 in Table 4. The HDG results were different, the
Carboset
CR760 alone seemed to perform the best with the other coatings performing
worse. None
of the coatings seemed to perform much better than the G342 in Table 4.
[00036] In another series of tests the amount of ammonium zirconium
carbonate in
the coating was varied to vary the amount of Zr02 in the coating composition
and the
effect on corrosion protection was determined. The coating formulas are given
below in
Table 12. In addition, control panels were coated with G342 as described
above. The
coatings were applied to USS Galvalume panels at a coating weight of
approximately
200 milligrams per square foot (200 milligrams per 929.03 square centimeters)
as
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
described above and dried in place to a PMT of 210 F (98 C). The panels were
then
tested in the NSS, Butler water immersion test, and Stack Test and the results
are given
below in Tables 13, 14, and 15 respectively.
TABLE 12
Component 162A 162B 183A/F 183E
Deionized water 32.50 26.00 40.50 42.50
Bacote 20 16.00 16.00 8.00 6.00
V205 0.50 0.50 0.50 0.50
Carboset0 51.00 51.00 51.00 51.00
CR760
Carnauba wax 6.50
TABLE 13
Time hours G342 162A 162B 183A/F 183E
(NSS)
24 0.00 0.00 0.00 0.00 0.00
72 0.00 0.00 0.00 0.00 0.00
168 3.00 0.00 0.00 0.00 1.00
336 31.67 0.00 0.00 3.83 21.67
504 60.00 0.00 1.00 31.00 80.00
672 1.00 1.00 31.50
840 1.00 1.00 25.33
1032 1.00 1.00 35.33
1172 1.00 1.00 30.00
1344 1.67 3.00 40.00
1560 2.00 3.00 40.00
1728 4.00 5.00 50.00
[00037] The
results demonstrate that all of the coatings according to the present
invention were at least as effective as G342 and most were much more
effective. The
results also demonstrate that increasing the level of Zr02 from 1.20% to 3.20%
16
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
dramatically increased the effectiveness of the coatings prepared according to
the present
invention.
TABLE 14
Time hours G342 162A 162B 183AJF 183E
(BWI)
168 0.00 0.00 0.00 0.00 0.00
336 0.00 0.00 0.00 0.00 0.00
504 0.00 0.00 1.00 0.00 1.00
672 0.00 1.00 3.00 0.50 3.00
840 0.00 3.00 3.00 0.50 3.00
1032 0.00 3.00 3.00 3.00 7.00
1176 10.00 5.00 5.00 4.00 10.00
1344 30.00 7.00 7.00 4.00 20.00
1512 50.00 7.00 7.00 5.00 20.00
1680 1.00 1.00 3.00 20.00
1848 3.00 3.00 5.00 20.00
2016 5.00 5.00 7.5 20.00
[00038] The
results again demonstrate that the coatings according to the present
invention all perform much better than G342. In addition, although not as
dramatic as for
the NSS test, the results demonstrate that increasing the amount of Zr02
increases the
effectiveness of the coating in corrosion protection.
TABLE 15
Time hours G342 162A 162B 183A/F 183E
(Stack)
168 0.00 0.00 0.00 0.00 0.00
1 336 0.00 0.00 0.00 0.00 0.00 ____ 1
504 1.00 1.00 0.00 0.00 0.0
________________________________________________________________________ :
672 1.00 3.00 0.00 0.00 1.00
840 3.00 3.00 1.00 2.00 1.00
17
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
1032 3.00 3.00 3.00 2.00 1.00
1176 3.00 5.00 3.00 3.00 3.00
1344 5.00 5.00 5.00 3.00 3.00
1512 7.00 5.00 5.00 4.00 5.00
1680 10.00 5.00 5.00 5.00 5.00
1848 10.00 5.00 5.00 6.00 5.00
2016 10.00 5.00 7.00 13.00 7.00
[00039] The results also demonstrate that the coatings according to the
present
invention perform better than the control G342, however, there was not the
same increase
in effectiveness with increasing Zr02 as was seen in the other tests.
[00040] In the next series of experiments two additional resins 3272-096
and 3272-
103 were prepared as detailed below and then these resins were used to create
coatings
according to the present invention as detailed in Table 16 below.
Resin 3272-096
[00041] The resin 3272-096 included as monomers: acetoacetoxyethyl
methacrylate
(AAEM), n-butyl methacrylate, styrene, methyl methacrylate, 2-ethylhexyl
acrylate, and
ADD APT PolySurf HP which is a mixture of methacrylated mono and di-phosphate
ester.
The total monomer distribution in the resin was as follows: 20.00% AAEM,
12.50% n-
butyl methacrylate, 15.00% styrene, 27.50% methyl methacrylate, 20.00% 2-
ethylhexyl
acrylate, and 5.00% ADD APT PolySurf HP. The resin polymerization reaction was
run
under N2 with stirring and a heat set point of 80 C. The initial charge to
the reaction
vessel was 241.10 grams of DI water, 2.62 grams of ammonium lauryl sulfate
(Rhodapon
L-22 EP), and 2.39 grams of ferrous sulfate 0.5% FeS047H20 (3ppm). This
initial charge
was put into the reaction vessel at time zero and heating to the set point was
begun. After
30 minutes a reactor seed comprising a combination of 5.73 grams of DI water,
0.90
grams of non-ionic surfactant (Tergitol 15-S-20), 0.13 grams of ammonium
lauryl sulfate
(Rhodapon L-22 EP), 2.15 grams of n-butyl methacrylate, 2.57 grams of styrene,
4.74
grams of methyl methacrylate, 3.48 grams of 2-ethylhexyl acrylate, 3.41 grams
of
acetoacetoxyethyl methacrylate (AAEM), and 0.85 grams of ADD APT PolySurf HP
was
added to the reaction vessel and heating to the set point was continued for
another 15
minutes. Then an initial initiator charge was added to the vessel comprising
0.32 grams of
HOCH2S02Na, 4.68 grams of DI water, 0.45 grams of tert-butylhydroperoxide, and
an
18
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
additional 4.54 gams of DI water and the temperature was maintained at the set
point for
another 30 minutes. Then the monomer and initiator co-feeds were added to the
vessel
over a three hour period with the temperature maintained at the set point. The
monomer
co-feed was 106.92 grams of DI water, 17.10 grams of Tergitol 15-S-20, 2.49
grams of
Rhodapon L-22 EP, 40.89 grams of n-butyl methacrylate, 48.83 grams of styrene,
89.97
grams of methyl methacrylate, 66.10 gams of 2-ethylhexyl acrylate, 64.77 grams
of
AAEM, and 16.19 gams of ADD APT PolySurf HP. The initiator co-feed was 0.97
grams of HOCH2S02Na, 14.03 grams of DI water, 1.39 grams of tert-
butylhydroperoxide,
and an additional 13.61 grams of DI water. After the three hours a chaser
charge was
added to the vessel over a 30 minute period. The chaser charge was 0.32 grams
of
HOCH2S02Na, 4.88 grams of DI water, 0.46 grams of tert-butylhydroperoxide, and
an
additional 4.54 grams of DI water. The vessel was then held at the set point
for one hour
and 30 minutes. Then the cool down from the set point was begun and continued
for 2
hours until the temperature was 38 C. Then the buffer co-feed was added to
the vessel.
The buffer co-feed was 5.19 grams of ammonium hydroxide (28%) and 18.48 grams
of DI
water. In this resin formation and that for 3272-103 detailed below another
potential
phosphate containing monomer that could be used in place of the ADD APT
PolySurf HP
is Ebecryl 168 from Radcure Corporation. Additional non-ionic surfactant
stabilizers that
could be used in place of Tergitol 15-S-20, which is a secondary alcohol
ethoxylate, are
other non-ionic stabilizers having a hydrophilic lipophilic balance of from 15
to 18.
Examples of these stabilizers include: other secondary alcohol ethoxylates
such as Tergitol
15-S-15; blends of ethoxylates such as Abex 2515; alkyl polyglycol ether such
as
Emulsogen LCN 118 or 258; tallow fatty alcohol ethoxylate such as Genapol T
200 and T
250; isotridecyl alcohol ethoxylates such as Genapol X 158 and X 250; tridecyl
alcohol
ethoxylates such as Rhodasurf BC-840; and oleyl alcohol ethoxylates such as
Rhoadsurf
ON-877.
Resin 3272-103
[00042] The organic coating resin 3272-103 was prepared as described
below. The
resin includes as monomers: acetoacetoxyethyl methacrylate (AAEM), n-butyl
methacrylate, styrene, methyl methacrylate, 2-ethylhexyl acrylate, and ADD APT
PolySurf HP which is a mixture of methacrylated mono and di-phosphate ester.
The total
monomer distribution in the resin was as follows: 20.00% AAEM, 12.50% n-butyl
19
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
methacrylate, 15.00% styrene, 27.50% methyl methacrylate, 20.00% 2-ethylhexyl
acrylate, and 5.00% ADD APT PolySurf HP. The resin polymerization reaction was
run
under N2 with stirring and a heat set point of 80 C. The initial charge to
the reaction
vessel was 286.10 grams of DI water, 2.47 grams of Rhodapon L-22 EP. This
initial
charge was put into the reaction vessel at time zero and heating to the set
point was begun.
After 30 minutes a reactor seed comprising a combination of 5.44 grams of DI
water, 0.85
grams of Tergitol 15-S-20, 0.12 grams of Rhodapon L-22 EP, 2.04 grams of n-
butyl
methacrylate, 2.44 grams of styrene, 4.49 grams of methyl methacrylate, 3.30
grams of 2-
ethylhexyl acrylate, 3.24 grams of acetoacetoxyethyl methacrylate (AAEM), and
0.81
grams of ADD APT PolySurf HP was added to the reaction vessel and heating to
the set
point was continued for another 15 minutes. Then an initial initiator charge
was added to
the vessel comprising 4.79 grams of DI water and 0.21 grams of (NH4)2S208 and
the
temperature was maintained at 80 C for another 30 minutes. Then the monomer
and
initiator co-feeds were added to the vessel over a three hour period with the
temperature
maintained at the set point. The monomer co-feed was 103.36 grams of DI water,
16.15
grams of Tergitol 15-S-20, 2.35 grams of Rhodapon L-22 EP, 38.81 grams of n-
butyl
methacrylate, 46.34 grams of styrene, 85.38 grams of methyl methacrylate,
62.73 grams of
2-ethylhexyl acrylate, 61.47 grams of AAEM, and 15.37 grams of ADD APT
PolySurf
HP. The initiator co-feed was 14.36 gams of DI water and 0.64 grams of
(NH4)2S208.
After the three hours a chaser charge was added to the vessel over a 30 minute
period.
The chaser charge was 0.35 grams of ascorbic acid, 4.65 gams of DI water, 0.44
grams of
tert-butylhydroperoxide, an additional 4.56 grams of DI water, and 2.39 grams
of ferrous
sulfate 0.5% FeS047H20 (3ppm). The vessel was then held at the set point for
one hour
and 30 minutes. Then the cool down was begun and continued for 2 hours until
the
temperature was 38 C. Then the buffer co-feed was added to the vessel. The
buffer co-
feed was 5.88 grams of ammonium hydroxide (28%) and 18.48 grams of DI water.
[00043] Taking the resins above a series of coatings were created to
examine the
effect of alkaline treatment on the coatings and the benefit of including V205
plus a
reducing agent, cysteine, in the coating. Other reducing agents for the V+5
could include
Sn+2, or ascorbic acid, or thiosuccinic acid, or one could start with V+4 from
vanadyl
sulfate or vanadyl acetylacetonate. The coatings from Table 16 were then
applied to
HDG panels at a coating weight of approximately 200 milligrams per square foot
(200
milligrams per 929.03 square centimeters) to each panel and then dried to a
PMT of either
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
200 F or 300 F (93 or 149 C) and either put directly into the NSS test or
first washed
with the alkaline cleaner PC1 338 and then put into the NSS test. A decrease
in corrosion
protection after pre-treatment with PC1 338 would indicate that the coatings
were not
alkaline resistant. The results of the NSS test are given in Table 17 below.
TABLE 16
Component 8A 8H 9A 9H
Deionized water 66.00 66.00 65.00 65.00
Bacote 20 24.00 24.00 24.00 24.00
V205 0.50 0.50
Cysteine 0.50 0.50
3272-096 10.00 10.00
3272-103 10.00 10.00
TABLE 17
Treatment Time hours 8A 8H 9A 9H
(NSS)
PMT of 200 24 10.00 16.00 0.00 0.00
F(93 C), no 48 30.00 60.00 3.70 1.00
treatment 72 60.00 8.70 1.00
with PC1338 96 11.30 43.00
168 50.00 33.30
336 76.70
PMT of 300 24 80.00 50.00 0.00 0.00
F (149 C), 48 0.00 1.00
no treatment 72 0.00 18.70
with PCI 338 96 1.70 40.00
168 50.00 65.30
336 93.30 I
PMT 200 F 24 20.00 16.00 7.00 3.00
(93 C), pre- 48 50.00 60.00 50.00 30.00
21
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
treat with 72 60.00 50.00 50.00
PC1338 96 50.00
168 50.00
PMT of 300 24 80.00 50.00 3.00 0.00
F (149 C), 48 10.00 20.00
pre-treat with 72 80.00 50.00
PC1338
[00044] The results demonstrate that for either resin the presence of V205
and
cysteine was highly beneficial to the corrosion protection ability. Coatings
prepared
according to the present invention are designed to be applied directly to bare
metal
substrates without the need for any phosphate or other pre-treatments other
than cleaning.
They can be applied at any desired coating weight required by the situation,
preferably
they are applied at a coating weight of from 150 to 400 milligrams per square
foot (150 to
400 milligrams per 929.03 square centimeters), more preferably at from 175 to
300
milligrams per square foot (175 to 300 milligrams per 929.03 square
centimeters) and
most preferably at from 175 to 250 milligrams per square foot (175 to 250
milligrams per
929.03 square centimeters). The coatings of the present invention are dry in
place
conversion coatings as known in the art and are preferably dried to a peak
metal
temperature of from 110 to 350 F (43 to 177 C), more preferably from 180 to
350 F (82
to 177 C), most preferably to a PMT of from 200 to 325 F (93 to 163 C).
[00045] Another series of coating compositions were prepared to
demonstrate the
need for elements both from group IVB and group VB. Initially a resin 3340-082
was
created using the components below in Table 18 as described below.
Table 18
Part Material Wt
added
gms
A Deionized water 245.3
Rhodapon L22 1.7
B1 Deionized water 76.1
22
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
Rhodapon L22 1.7
Tergital 15-S-20 11.9
B2 n-butyl methacrylate 28.6
Styrene 34.1
Methyl methacrylate 62.9
2-ethylhexyl acrylate 46.2
Acetoacetoxyethyl Methacrylate 45.3
Polysurf HP 11.3
C Ammonium persulfate 0.60
Deionized water 11.4
D 70% t-butylhydroperoxide
0.31
Deionized water 9.7
E Ascorbic acid 0.17
Deionized water 9.8
F 0.5% aqueous ferrous sulfate 1.8
G Ammonium hydroxide 28.8%
4.3
Deionized water 10.5
H Deionized water 14.4
[00046] Part A was added to a four-necked 3 liter flask equipped with a
stirrer, a
condenser, a thermocouple and a nitrogen inlet. The contents were heated to
and
maintained at 80 C under nitrogen atmosphere. Parts B1 and B2 were mixed
separately
to form uniform clear compositions. B 1 and B2 were mixed together to form pre-
emulsion B. An amount of 5% of pre-emulsion B and 25% of part C were charged
to the
flask and maintained at 80 C. After 40 minutes the remainder of pre-emulsion B
and part
C were added at a constant rate to the flask over a period of 3 hours after
which part H was
used to flush the pre-emulsion addition pump into the flask. The flask
contents were
cooled to 70 C at which time part F was added to the flask. Parts D and E were
added to
the flask over a period of 30 minutes, after which the mixture was maintained
at 70 C for a
period of 1 hour. The mixture was then cooled to 40 C at which time part G was
added.
The resulting latex had a solids content of 37.2%, a pH of 6.9 , and particle
size of 123
23
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
nanometers. A dihydropyridine function was then added to the resin to form
resin 3340-
83 by combining 300 parts by weight of resin 3340-082 with 0.79 part by weight
of
propionaldehyde. The mixture was sealed in a container and placed in an oven
at 40 C
for a period of 24 hours, thereby founing resin 3340-083. A series of coating
compositions were prepared as described below in Table 19. Coating composition
164Q
is the only one prepared in accordance with the present invention in that it
includes
elements from groups IVB and VB. Coating compositions 164R and 164S are
missing the
group IVB or VB elements respectively. Each coating composition was then
applied to
either HDG or Galvalume (Gal) panels at a coating density of approximately 200
milligrams per square foot (200 milligrams per 929.03 centimeters) and dried
to a peak
metal temperature of 93 C. Multiple panels of each condition were then tested
in the NSS
test as described above and the average results for multiples at each time
point and
condition are reported below in Table 20.
Table 19
Component 164Q 164R 164S
DI Water 62.85 83.95 63.35
Bacote 20 24.0 0.0 24.0
(NH4)2CO3 0.0 2.9 0.0
V205 0.5 0.5 0.0
Resin 3340-083 12.15 12.15 12.15
Cysteine 0.5 0.5 0.5
Table 20
Time 164Q 164R 164S 164Q 164R 164S
hours Gal Gal Gal HDG HDG HDG
(NSS)
24 0 11.0 3.0 0.0 33.3 1.0
48 0 15.3 4.3 0.0 69.0 3.0
72 0 50.0 12.0 0.0 83.3 3.0
96 0.0 3.0
168 1.0 25.0 0.3 4.3
336 9.0 3.0 50.0
24
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
504 10.0 10.0
672 12.0 43.3
840 12.0 83.3
[00047] The results shown in Table 20 clearly demonstrate the benefit of
both IVB
and VB elements in combination. With only one of the elements present the
coating
composition had minimal corrosion protection.
[00048] In another embodiment, coating compositions prepared according to
the
present invention comprise an inorganic portion comprising a source of at
least one of the
group IVB transition metal elements of the Periodic Table, namely zirconium,
titanium,
and hafnium and either at least one element of group VB of the Periodic Table
or a source
of chrome. The coating compositions further include an organic polymer. In
this
embodiment, preferably the coating composition includes from 9% to 73% by
weight of
the group IVB element based on the total dry solids coating weight. A
preferred group
IVB element is zirconium, preferably supplied as ammonium zirconium carbonate.
In this
embodiment, the coating composition also includes either a chrome source such
as
chromium trioxide or a group VB element such as vanadium, niobium, or
tantalum. The
coating composition according to this embodiment is also a dry in place
conversion
coating. The coating also includes at least one of a wide variety of resin
organic polymers,
which can be added directly to the coating composition thus eliminating
multistep coating
processes. Preferably, the weight percentage of organic polymer active solids
based on
total dry solids coating weight is from 1% to 75%, more preferably from 25% to
73% and
most preferably from 40% to 70%. The resin organic polymers that can be
included are of
a variety of types including, by way of example only: epoxies, polyvinyl
dichlorides,
acrylic-based resins, methacrylate-based resins, styrene-based resins,
polyurethane
dispersions, and polyurethane dispersion hybrids. Examples of these resin
polymers
include Carboset0 CR760, Hauthane HD-2120, Hauthane L-2989, MaincoteTM PR-15,
MaincoteTM PR-71, Avanse MV-100, Rhoplex AC 337N, and Alberdingk-Boley LV-
51136 and M-2959. The coating can also accommodate addition of reducing agents
such
as cysteine, Sn2+, ascorbic acid, or thiosuccinic acid and oxidation products
thereof
Optionally, the coating composition can also include processing aids such as
waxes which
aid in formability of the coated substrates. Addition of these optional agents
was
discussed above. Being a conversion coating, as the term is known in the art,
components
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
within the coating composition react with the metal substrate during the
coating process to
produce the final dry in place coating.
[00049] Coating compositions prepared according to the present invention
produce
a dried in place coating having a unique morphology. The dried in place
coating
morphology produced has two phases, unexpectedly the inorganic portion of the
coating
compositions is the continuous phase while the discontinuous phase comprises
the organic
polymer. This is the opposite of conventional coatings and unexpected. A
series of
chrome-based coating compositions prepared according to the present invention
and a
series of comparative coating compositions were prepared according to the
formulas given
below in Table 21. The coating compositions were prepared by adding the
components
together in the order listed with mixing. All compositions were aged for 24
hours after
mixing prior to use in the experiments described below. The Bacote 20 serves
as the
source of the group IVB element in these examples. The weight percentage of
organic
polymer active solids based on total dry solids coating weight is preferably
from 1% to
75%, more preferably from 25% to 73% and most preferably from 40% to 70%. The
useful organic polymers have been described above in the previous examples.
The
organic polymer portion of all of the compositions in this example was the
styrene-acrylic
copolymer latex Carboset CR760. The particle size of the latex was measured
using laser
light scattering measured by a Zetasizer 3000HSA available from Malvern
Instruments.
The average particle size was 111 nanometers with a range from 62 to 116
nanometers.
The chrome content based on active coating solids of compositions 21A and 21B
were the
same as the chrome content of compositions 21C and 21D. In compositions 21B
and 21D,
the comparative examples, the Bacote 20 was not used; however the calculated
ammonium content from the Bacote 20 was added using ammonia. Compositions 21A
and 21B were a bright yellow in color consistent with a characteristic color
of hexavalent
chrome. By way of contrast compositions 21 C and 21D, which include the
reducing
agent ascorbic acid, were a green-brown color consistent with a characteristic
color of a
predominantly trivalent chrome composition. Example 21A has a weight
percentage of
latex polymer active solids of 44% based on the total dry coating solids while
it was 41%
for example 21C. The weight percentage of group IVB element based on total dry
coating
solids was 37.00% for example 21A and 34.50% for example 21C.
Table 21
26
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
Component 21A 21B 21C 21D
Deionized water 65.45 73.85 64.70 74.10
Bacote 20 24.00 0.00 24.00 0.00
Ammonia (29% NH3) 0.00 4.15 0.00 4.15
Chromium trioxide 0.55 0.55 0.55 0.55
Carboset CR760 10.00 21.45 10.00 19.70
Ascorbic acid 0.00 0.00 0.75 1.50
Total 100.00 100.00 100.00 100.00
% Total active solids 9.60 9.60 10.30 10.30
[00050] Another series of coatings was prepared using as the organic
polymer
another acrylic latex polymer Avanse MV100 from Rohm and Haas. Again the
compositions were prepared by mixing the components in the order of Table 22
and then
each was aged for 24 hours prior to use.
[00051] The organic polymer portion of all of the compositions in this
example was
the latex Avanse MV100. The particle size of the latex was measured using
laser light
scattering measured by a Zetasizer 3000HSA available from Malvern Instruments.
The
average particle size was 137 nanometers with a range from 90 to 207
nanometers. The
chrome content based on active coating solids of compositions 22A and 22B were
the
same as the chrome content of compositions 22C and 22D. In compositions 22B
and 22D,
the comparative examples, the Bacote 20 was not used; however the calculated
ammonium content from the Bacote 20 was added using ammonia. Compositions 22A
and 22B were a bright yellow in color consistent with a characteristic color
of hexavalent
chrome. By way of contrast compositions 22 C and 22D, which include the
reducing
agent ascorbic acid, were a green-brown color consistent with a characteristic
color of a
predominantly trivalent chrome composition. Example 22E is a non-chrome based
example prepared according to the present invention. This example is an
embodiment
wherein the inorganic portion includes at least one element from group TVB of
the
Periodic Table and at least one element from group VB of the Periodic Table.
It includes
as the organic polymer Avanse MV100. Example 22A has a weight percentage of
latex
polymer active solids of 67% based on the total dry coating solids while it
was 65% for
example 22C and 65.9% for 22E. The weight percentage of group IVB element
based on
total dry coating solids for example 22A was 21.40%, for example 22C it was
20.50%, and
for example 22E it was 20.80%.
Table 22
27
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
Component 22A 22B 22C 22D 22E
Deionized water 45.05 57.20 44.20 56.70 44.50
Bacote 20 28.15 0.00 28.15 0.00 28.15
Ammonia (29% NH3) 0.00 4.85 0.00 4.85 0.00
Chromium trioxide 0.65 0.65 0.65 0.65 0.00
V205 0.00 0.00 0.00 0.00 0.60
Avanse MV 100 26.15 37.30 26.15 36.60 26.15
Ascorbic acid 0.00 0.00 0.85 1.20 0.60
Total 100.00 100.00 100.00 100.00 100.00
% Total active solids 19.50 19.50 20.30 20.30 20.00
[000521 As a comparative example 23 use was made of the commercial
hexavalent
chrome-based organic coating solution P3000B available from Henkel
Corporation.
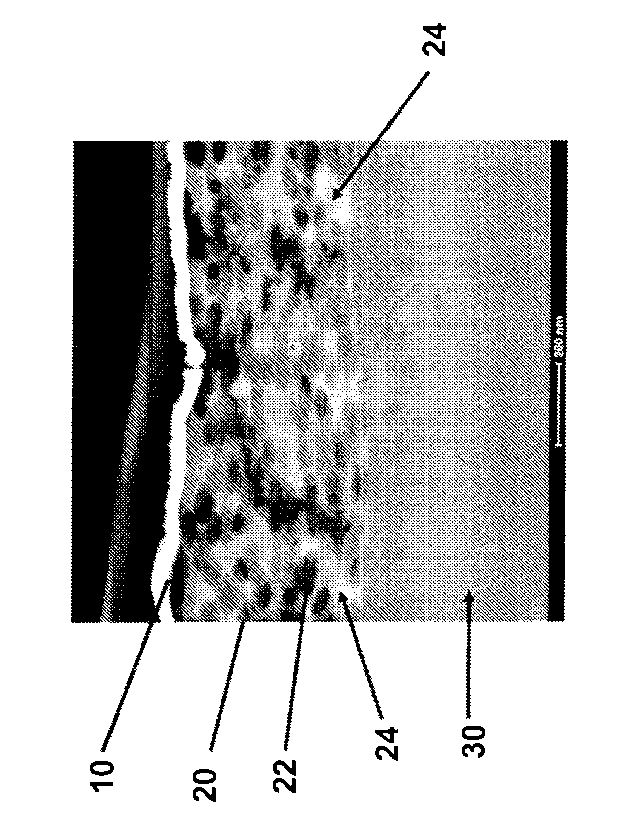
[000531 In example 24 coating composition 21A was applied to a cleaned
aluminum
panel by wire drawbar and dried to a PMT of 93 C to provide a dry coating
weight of 150
25 milligrams/square foot. The coated metal then had a thin layer of gold and
a thin
layer of platinum applied to it to facilitate cross-sectioning. Then it was
cross-sectioned
using a focused ion beam to produce a very thin slice of a cross-section of
the coated
substrate. The cross-section was then characterized by dark-field scanning
transmission
electron microscopy. The novel morphology of coating composition prepared
according
to the present invention is shown in the image obtained from this technique,
shown in
Figures 1A and 1B. In this technique the relative brightness vs. darkness of
regions within
the image reflect the composition with respect to average atomic number (Z) of
the
constituents. Light regions indicate the presence of constituents of higher
average Z
whereas darker regions are indicative of constituents of lower average Z.
Energy
Dispersive X-ray Analysis was performed to verify the elemental composition
within these
regions. The analysis showed that the continuous phase is an inorganic phase
comprising
chrome, zirconium, and oxygen. The technique involves transmission through a
thin slice
of dry coating which contains both continuous and dispersed material phases
which differ
significantly in average Z. As a result an image is provided with 3-dimesional
aspect and
reveals the novel morphology of the invention. After drying of the applied
example 21A
coating, residues from ammonium zirconium carbonate, Bacote 20, contribute to
bright
regions. The acrylic latex polymer, which is largely based on carbon and
oxygen, will be
represented by darker regions. Polymer spheres which overlap at different
depths within
the slice result in the darkest regions of the image. As shown, one observes
that the
invention provides a coating with a continuous inorganic matrix within which
discreet
28
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
dispersed polymer spheres reside. The size of the discreet polymer spheres
within the
image is consistent with the particle size measurement for the acrylic latex
from example
21. Elongation of polymer spheres is attributed to the effects of shrinkage
during drying
of the composite structure. Looking to Figure IA the platinum/gold cap is seen
at 10, the
coating composition 21A is shown at 20 with the dispersed polymer spheres
shown at 22
and the continuous inorganic phase shown at 24. The substrate aluminum is
shown at 30.
Figure 1B is a higher magnification of a region of Figure 1A and clearly shows
the
continuous inorganic phase 24 and the dispersed polymer spheres 22. Clearly,
unlike the
expected in the present invention the polymer latex does not coalesce and
instead stays as
a dispersed phase in the continuous inorganic phase.
[00054] In example 25 composition 22E, a non-chrome example of the present
invention, was applied to a cleaned galvalumet panel by wire drawbar and dried
to a
PMT of 93 C to provide a dry coating weight of 200 + 25 milligrams/square
foot. The
coated metal was cross-sectioned by focused ion beam to produce a thin slice
which was
characterized by dark-field scanning transmission electron microscopy as
described in
example 24. The novel characteristic morphology of the invention, a continuous
inorganic
phase with largely discreet dispersed polymer phase, is demonstrated. Energy
Dispersive
X-ray Analysis was performed to verify the elemental composition within the
continuous
and dispersed phases. Again the continuous phase was inorganic and comprised
zirconium, vanadium, and oxygen. The size of the observed polymer spheres
within the
coating 22E is consistent with the particle size measurements made for the
latex used in
the example 22E formulation. Relative to example 24, one observes a higher
density of
polymer spheres within the coating which is consistent with the difference in
acrylic
content of example 21A and example 22E formulations. The results are shown in
Figures
2A and 2B, which is a higher magnification of a region of shown in Figure 2A.
The
platinum/gold cap is seen at 60, the coating composition 50 includes polymer
spheres 54
and the continuous inorganic phase 52, and the substrate is shown at 40.
1000551 In example 26 comparative example 23 was applied to a cleaned
aluminum
panel by wire drawbar and dried to a PMT of 93 C to provide a dry coating
weight of 150
+ 25 milligrams/square foot. The coated metal was cross-sectioned by focused
ion beam
to produce a thin slice which was characterized by dark-field scanning
transmission
electron microscopy as described in example 24. The image obtained from this
technique
illustrates that the novel morphology of the coatings prepared according to
the present
29
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
invention is not present in a commercial chrome-based coating. What is
observed is a film
comprising a continuous organic phase resulting from coalesced polymer
characteristic of
conventional polymer coatings. Energy Dispersive X-ray Analysis was performed
to
verify the elemental composition within the continuous phase. In Figure 3 the
substrate is
seen at 80, the coalesced polymer at 90 and the platinum/gold cap at 100.
[00056] As discussed above, one of the disadvantages of current chrome-
based
coating compositions is the tendency of the chrome to leach out of the coating
composition after it is applied to a substrate. Thus, the leaching of the
examples in
accordance with the present invention was compared to the comparative samples
in
example 27. In example 27, each of the chrome-containing coating compositions
from
Examples 21 and 22 were applied to clean Hot dipped Galvanized Steel (HDG) and
Galvalume panels by wire drawbar and dried to a PMT of 93 C. Dry coating
weights on
HDG panels were 175+25 milligrams/square foot. Dry coating weights on
Galvalume
panels were 150+25 milligrams/square foot. After coating, the panels were
subjected a
test protocol to characterize the tendency of each to leach chrome with water
exposure.
Panels were immersed in 1.5 liters of warm deionized water at 50 C for 30
seconds after
which they were rinsed for 30 seconds with cold water and dried. Chrome
content of the
coated panels was determined before and after subjecting panels to the test
protocol using
a Portspec X-ray spectrograph model 2501 manufactured by Cianflone Scientific
Instruments Corporation. The difference in chrome content following the
immersion
protocol was calculated. Ratings for the percentage of chrome loss were
assigned a
number from 0 to 5 as follows: 0 is <5%; 1 is 5% to 19.99%; 2 is 20.00% to
39.99%; 3 is
40.00% to 59.99%; 4 is 60.00% to 79.99%; and 5 is 80.00% to 100.00%. The
results are
shown below in Table 28. The results show several significant trends. First,
none of the
coatings prepared according to the present invention showed significant
leaching from
either substrate. Second, virtually all of the comparative examples showed
leaching from
all substrates. Third, the predominantly trivalent chrome comparative examples
showed
significantly more leaching than the predominantly hexavalent comparative
examples.
Finally, all comparative compositions performed better on HDG compared to
Galvalume
Table 28
Example composition Galvalume HDG
21A 0 0
CA 02762644 2011-11-18
WO 2010/134936
PCT/US2009/065663
21B 1 0
21C 0 0
21D 5 4
22A 0 0
22B 2 1
22C 0 0
22D 4 2
[00057] In example 29 compositions 21A, 21C and comparative commercial
example 23 were applied to clean hot-dipped galvanized steel and Galvalume
panels by
wire drawbar and dried to a PMT of 93 C. Dry coating weights achieved were 200
+ 25
milligrams/square foot over HDG and 150 + 25 milligrams/square foot over
Galvalume
panels. Then 3 replicate panels for each composition were placed in Neutral
Salt Spray
test according to ASTM-B117-07A and inspected at regular intervals. At each
interval the
corrosion was rated as % facerust. For the first 168 hours of salt spray
exposure, ratings
were made every 24 hours after which ratings were made at 168 hour intervals.
The
length of exposure time in hours at which % face-rust reached or exceeded
limits of 10%
and 25% were recorded for each of the three replicate panels. The average
exposure times
to reach or exceed the defined limits are summarized below in Table 29. The
results
clearly show that coating compositions prepared according to the present
invention are
significantly better than the tested commercial chrome-based coating
composition. In
addition, all perform significantly better on Galvalume than on HDG.
[00058]
Table 29
Coating Substrate Hours to meet or Hours to meet or
composition exceed 10% face exceed 25% face
rust rust
21A Galvalume 3584 3864
21C Galvalume 2912 3360
Comparative Galvalume 2352 2576
23
21A HDG 1568 1848
21C HDG 1344 1680
Comparative HDG 672 784
23
31
CA 02762644 2011-11-18
WO 2010/134936 PCT/US2009/065663
[00059] In example 30, the coating compositions from example 22 were
compared
to comparative example 23. Examples 22A, 22C and comparative Example 23 were
applied to clean hot-dipped galvanized steel and Galvalume panels by wire
drawbar and
dried to a PMT of 93 C. Dry coating weights achieved were 200 + 25
milligrams/square
foot over HDG and 150 + 25 milligrams/square foot over Galvalume panels. Then
3
replicate panels for each composition were placed in Neutral Salt Spray and
inspected at
168 hour intervals for the entire test period with the exception of two
intervals which were
192 hours and 144 hours. At each interval the corrosion was rated as %
facerust. The
length of exposure time in hours at which % face-rust reached or exceeded a
limit of 3%
was recorded for each of the three replicate panels. The average exposure
times are
summarized below in Table 30. Again the present invention out performed the
comparative example and all were better on Galvalume than on HDG.
[00060]
Table 30
Coating composition Substrate Hours to meet or exceed 3%
face rust
22A Galvalume 1512
22B Galvalume 1080
Comparative 23 Galvalume 1296
22A HDG 848
22C HDG 840
Comparative 23 HDG 504
[00061] The foregoing invention has been described in accordance with the
relevant
legal standards, thus the description is exemplary rather than limiting in
nature. Variations
and modifications to the disclosed embodiment may become apparent to those
skilled in
the art and do come within the scope of the invention. Accordingly, the scope
of legal
protection afforded this invention can only be determined by studying the
following
claims.
32