Note: Descriptions are shown in the official language in which they were submitted.
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
1
METHOD AND PLANT FOR THE THERMAL TREATMENT OF ORGANIC
MATTER IN ORDER TO PRODUCE CHARCOAL OR CHAR.
Background of the Invention
The present invention relates to a method and plant for the thermal treatment
of
organic materials applied to the carbonization of ditto organic materials in
order to
produce charcoal or char.
Any type of organic matter can be used as raw material: log wood in any size,
coconut peel, babassu coconut, rice straw, saw mill wastes, sugar cane, sugar
cane
straw and vegetal wastes in general. In order to simplify wood will be the
reference,
but the text can be applied to any type of biomass.
Brazil is the world greatest charcoal producer with an average annual
production of
8.5 millions of metric tons. This production is only a small fraction of the
actual
potential to produce charcoal from cultivated biomass and agricultural crop
wastes.
The principal aim of this invention in to bring logistic, environmental,
technical,
economic and global energy efficiency when compared to most of the existing
biomass carbonization processes.
Theory of the biomass carbonization
The term "biomass" was created in 1975 in order to describe the natural
materials
suitable to be used as fuels. The term encompasses all organic matter of
vegetal or
animal origin, inclusive the resulting materials of its natural or artificial
transformation (e.g. charcoal). The origin of any type of biomass is the
photosynthesis.
Solar energy stored in the biomass turns it in a renewable energy source.
Growth of
biomass is due to the conversion of atmospheric carbon dioxide through
photosynthesis in organic compounds. Bioenergy is the energy stored in the
plants
and animals or in its residues. Combustion is the simplest way to recover this
energy.
The use of cultivated biomass as an energy source is beneficial to the
environment.
When burning fossil fuel oxygen is consumed and CO2 is emitted to the
atmosphere.
The basic fossil fuel combustion reaction is:
C(s) + 02(g) + 3.76N2(g) C02(g) + 3.76N2(g)
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
2
The CO2 concentration increase in the atmosphere, intensified in the last 200
years, is
one of the reasons for the so called "greenhouse effect", which is supposed to
be the
main responsible for the planet heating. Besides, the corresponding decrease
of the
oxygen concentration in the atmosphere according reaction (1) is followed by a
decrease in the ozone concentration, due to the thermodynamic equilibrium
oxygen-
ozone. The reduction of the ozone concentration increases the ultra violet
radiation
on the earth, and consequently the risk of skin cancer. If nothing is done to
reduce
the consumption of fossil fuels, the future generations will inherit a hostile
planet.
When burning a biomass fuel, CO2 is emitted to the atmosphere in the same way
as a
fossil fuel. But during the growth of the cultivated biomass, CO2 is absorbed
from
the atmosphere and oxygen is emitted through the photosynthesis process. The
final
balance is no reduction of the oxygen concentration in the atmosphere, which
is
extremely beneficial to the environment.
In natural life cycle soon after the biomass death an exothermic decomposition
of its
elementary molecules starts. Biomass conversion with energy liberation
recreates the
natural decomposition process, but in a much faster way, and this energy is a
renewable energy. When burning biomass carbon is recycled, no CO2 being
emitted
to the atmosphere, such as happens when burning fossil fuels. Biomass is the
only
source of renewable energy. Being a renewable source, it should to have in
mind that
fossil fuels are exhaustible.
Organic matter is the primary origin of fossil fuels. Organic matter piled up
on the
sedimentary rocks during the Cambrian geologic period, was transformed in the
absence of oxygen in fossil fuels: coal, oil and natural gas. By its turn,
this organic
matter came from solar energy through the photosynthesis process. This
accumulated
chemical energy during 600 millions years has been increasingly wasted by
humankind.
Energy from biomass can be obtained in several ways. When heated through a
thermo- chemical process, biomass is decomposed in less complex substances.
Any
type of biomass can be submitted in a thermo-chemical conversion process. Due
to
the high productivity, low cost, high density and quality wood is the main
biomass
submitted to thermo-chemical processes. Pyrolysis is the anaerobic (lack of
oxygen
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
3
or air) thermal decomposition process. When oxygen is enough for the complete
biomass chemical reaction, we have combustion or gasification.
Pyrolysis is the thermal biomass conversion at 300 - 800 C temperature range
in the
total absence of air, or with not enough air for combustion. Biomass pyrolysis
is also
called carbonization or wood destructive distillation. Carbonization is the
process
when charcoal is the main product of interest. Heat can be indirectly
supplied, or
produced by burning part of the biomass (direct heating). High temperature
(1,000 C) produces a maximum of fuel gas (gasification), whereas low
temperature
pyrolysis (<500 C) produces a maximum of charcoal.
When heating organic matter in the absence of oxygen, a chemical decomposition
occurs giving a solid product - charcoal -and volatiles products partially
condensable at room temperature. This condensation gives several liquid
products
such as pyrolygenous liquor, acetic acid, methanol, tar, and a significant
amount of
constituents in smaller proportion. With the exception of water vapor the
condensable volatile components are highly polluters. The emission of a heavy
smoke containing those condensable components, is very harmful to the health.
After
condensed those components pollute the soil and the water sources. The
emission of
those condensable components is a characteristic of the primitive brick
carbonizing
kilns.
Carbonizing temperatures when charcoal is the desired main product are in the
range
300 - 500 C. Charcoal is light, has a high low heating value, and burns with
no
smoke, while wood is much denser, its combustion producing a lot of smoke.
Charcoal contains ashes, its content depending on the type of wood, earth
contamination, etc. Besides charcoal, a gaseous phase is emitted to the
atmosphere,
containing condensable and non condensable gases. The non condensable gases
can
be burned in order to generate thermal energy. Basically the components of the
no
condensable gases are: C02, CO, H2, CH4 and CnHm. The condensable gases also
contain combustible components. When not burned, as already mentioned are
strongly polluters and harmful to the health.
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
4
Pyrolysis is the basic biomass thermodynamic conversion process. When heated
in
the absence of air, a decomposition of the biomass in less complex components
occurs. Pyrolysis is a complex process through intermediate radicals, the
final result
being a solid residue rich in carbon (charcoal), and a volatile fraction
composed of
gases, organic vapors and tar components. This volatile fraction if not used
either as
a fuel or for the liquefaction of the condensable components is very polluter.
Although being a rather simple technology for the biomass conversion in solid
fuel,
carbonization is a very complex process.
Carbonization is performed in the following steps.
I - Drying - Before wood could be carbonized, the water in it must be driven
off. It is
a strongly endothermic step. The temperature does not go over 110 C as far as
the
wood moisture is not driven off.
II - Final moisture water driven off, and dehydration reactions, temperature
110 to
175 C, still an endothermic step.
III - Pre-carbonization, temperature range 175 - 270 C, still an endothermic
step.
The wood decomposition starts, with the emission of CO, C02, acetic acid and
methanol. Wood color changes to dark brown. Char is the product obtained in
the
final of this step.
IV - Transition step, temperature 270 - 290 C. Decomposition reactions
continue,
beginning the exothermic step.
V - Carbonization or pyrolysis - It is a term loosely applied when the
chemical
structure of wood breaks down under high temperature and in the absence of
air. The
pyrolysis phase is exothermic and the temperature rises up to 290 - 380 C with
the
emission of hydrocarbon gaseous products, the solid residue becomes charcoal
with a
high volatile matter content. The emitted gases during pyrolysis have a
significant
heating value. Gases species are: CO, C02, H2, CH4, water vapor, hydrocarbon
gases,
and vapors of tar, methanol, acetic acid and pyrolygneous liquor. In the
present text
we use carbonization or pyrolysis for this stage.
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
VI - Final step, with an increase of temperature and fixed carbon, decreasing
the
volatile matter content. The higher the temperature, the higher charcoal fixed
carbon
content.
VII - Cooling - Charcoal produced must be cooled, in order not to bum when
5 opening the kiln or retort, which must be tightly sealed.
Fixed carbon content is a very important metallurgical charcoal property.
Figure 1
shows the relation between carbonization temperature, fixed carbon, and
gravimetric
yield, which is, the ratio. (kg of charcoal)/(ton of anhydrous wood).
Basically the carbonization process starts with a strongly endothermic step
before the
exothermic step. It should be emphasized the difference in phase of the
endothermic
and the exothermic steps of the carbonization process, which starts with the
strongly
endothermic wood drying, followed by the exothermic carbonization step, and
the
emission of combustible gaseous substances. The energy emitted by the wood
during
the carbonization step is more than enough to supply the energy demand of the
drying step. The problem of using the energy emitted by the carbonizing wood
during the drying wood step is the difference in phase of those two steps. If
the
energy emitted during the exothermic step is wasted, part of the wood loaded
into the
carbonization reactor should be burned in order to supply the energy for the
endothermic step, although the energy emitted during the exothermic phase plus
the
energy content of the combustible gases emitted during this step is more than
the
energy demanded by the endothermic reactions of the carbonization process.
Table I is a summary of the theory of the evolution of biomass carbonization.
Table I - Theoretical evolution of the wood carbonization.
Temperature - C > 100 200 - 280 280 - 380 380 -n 500 500 - 700 700 - 900
Period Drying Pre-carbonizing Carbonizing
Water Oxygenated Start of Hydrocarbon gases Charcoal Hydrogen stage
release gases release hydrocarbon stage dissociation
gases release
Fixed carbon 60% 68% 78% 84% 89% 91%
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
6
Non condensable
68,0 66,5 35,5 31,5 12,2 0,5
%C02
%CO 30,0 30,0 20,5 12,3 24,6 9,7
%H2 0,0 0,2 6,5 7,5 42,7 80,9
%Hydrocarbon 2,0 3,3 37,5 48,7 20,5 8,9
LHV Gas Kj/Nm3 4,600 5,000 16,300 19,800 15,300 13,200
Vapor Water Water vapor Acetic acid Heavy tars Tar Little condensation
condensable vapor Acetic acid Methylic alcohol Parafin
components Light tars
Gases Quantity Very small Small Important Important Small Large
Gases C02, CO, C02, CH4, CO, H2, CO, C02,
components CH3OH, H20, H2, Hydrocarbons
CH3CHO, CH3OH,
CH3CO2H CH3CHO,
CH3CO2H
Gravimetric 44.0 38.0 35.0 31.0 26.0
yield - %
Ref. - Uhart, 1971; Doat & Petroff, 1975
Carbonization Technique Actual State
Charcoal is the first wood product used by mankind. Cave men observed that the
residue of burned wood produced a hotter flame with less smoke in the cave.
Later
on, when by hazard a mixture of this residue was fired together with certain
types of
stones, a heavy liquid emerged, starting in that way the age of metals.
The annual charcoal consumption in Brazil is over 8 millions tons.
Approximately
60% of the wood for charcoal production in Brazil comes from the high
productivity
eucalyptus plantations. In Brazil only the kiln method is used for charcoal
making.
Those kilns do not take advantage of the exothermic energy phase, burning part
of
the charged wood in the kiln as the energy source for the carbonization
process.
Besides, brick kilns have no charcoal quality control because the whole
operation is
based on visual observation of the emitted gaseous products color.
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
7
Several types of charcoal producer furnaces were developed since the beginning
of
its utilization. The first charcoal production process was probably the pit
kiln, used
until today in some countries. Wood is slowly burned in a pit covered by
earth.
According to the heat generation process, carbonizing furnaces can be
classified as:
a) - Internal wood combustion by a controlled air input.
b) - External combustion.
c) - Retort with gases recycling.
Internal wood combustion is the more common type. Carbonization starts by a
controlled air input into the furnace in order to burn part of the wood and
heat the
kiln until the carbonization temperature. Air input continues always in a
small
controlled amount, until the complete wood transformation in charcoal. Gases
and
volatiles produced during the carbonization process are emitted to the
atmosphere,
charcoal being the only product. In this type of furnace up to 20% of the
charged
wood is burned. This is one of the oldest processes of charcoal manufacture,
the
more common furnaces being earth mound and pit kilns, portable steel kilns and
brick furnaces. In Brazil until today only brick furnaces are used.
In the external combustion furnaces the hot gases from a combustion chamber
are
introduced into the furnace in order to supply the necessary heat for the
endothermic
carbonization steps. It is possible to burn any kind of fuel in the combustion
chamber, such as agricultural and forestry wastes, charcoal fines, tar, fuel
oil or
natural gas. The investment cost in this system is higher, but it allows a
better
control, producing good quality charcoal with a higher yield.
The retort method uses retort which works on a continuous basis. Wood is
loaded at
the top and as it descends through the retort it is first dried by ascending
hot gases in
the top section, then carbonized by recycling hot gases in the middle section,
and
finally cooled and withdrawn at the bottom. An ingenious system of
recirculation and
combustion of the pyrolysis gases ensures that the maximum advantage is taken
of
their thermal and chemical energy content. The automatic working of the plant
leads
to a reduction of the personnel required.
Retort processes are normally used when the desired main product is the
liquefied
volatiles emitted by the carbonization wood, charcoal being a by product.
Through a
sequence of distillation and condensation operations several important
chemical
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
8
products such as acetic acid, methanol, solvents, food aromatizers, etc, are
recovered
from the carbonizing wood. Due to the much higher investment cost, carbonizing
retorts are not economically feasible when the only desired product is
charcoal.
Besides it is necessary to saw the wood in short logs, not over 30 cm. The
investment
cost is raised by the saw mill and the wood sawing operation raises the
charcoal cost.
As above mentioned, in Brazil only the brick kiln method is used. Brazil is
the
greatest charcoal producer in the world and almost 95% of its production
occurs in
the South East states of Minas Gerais, Sao Paulo, Goias and Bahia located
approximately 3,000 kilometers from the Amazon region. The charcoal main
applications are: as thermal reducer in the iron, ferrous alloys, silicon
metal, calcium
carbide furnaces and as renewable energy source in the lime and cement
industry.
Approximately one third of the pig iron produced in Brazil is based on the
charcoal
as a thermal reducer.
The typical beehive furnace used in Brazil has 10 to 30 cubic meters capacity,
figure
2. Log wood is vertically charged into the kiln through gate 1. Above these
shorter
horizontal logs are placed to the underside of the dome. After charging, the
door
openings are bricked up and sealed with a weak cement and mud mortar. Ignition
is
started through the hole 2 on the top, which is closed when the fire takes
hold. The
beehive kiln has been improved by erecting a chimney aside walls 3.
Carbonization moves downward, with air being drawn in through holes in the
dome
and vertical wall 3, the smoke being emitted by the same holes. As the
combustion
proceeds, all openings are sealed. When the smoke coming from the chimneys
turns
a light blue, all the openings, including the chimneys, are closed and sealed
carefully
with mud. The kiln is then brushed all over with several layers of clay slurry
to close
all leaks and cracks. If this is not done thoroughly, the infiltration of air
will maintain
a certain amount of combustion and slow the cooling. Air leakage into the kiln
would
burn the charcoal from the carbonizing wood. The charcoal cooling, which is
the
final carbonization step is started. Total time for the complete cycle goes
from 9 to
13 days. The beehive kiln has been improved by erecting chimneys aside walls
3.
A very high volume concrete kiln developed and still used in the United States
is
known as Missouri kiln. Those kilns are large permanent structures which were
developed for charcoal making in the deciduous hardwood forest of the state of
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
9
Missouri, from which they get their name. They are rectangular in shape with a
vaulted roof, figure 3. They vary greatly in size normally up to 12 meters
long, 7
meters wide and 4 meters tall. This enables considerable economy of scale, the
entry
of vehicles for direct loading and unloading, but the kiln is difficult to
control.
The concrete walls were replaced by low density refractory silicon-aluminum
bricks.
The walls 4 thickness is normally 25 cm, figure 3. Steel doors gates 5 are
protected
by refractory concrete. The kiln usually has four chimneys 6, along each side.
Air
vents 7 are provided along the base of the furnace walls. Wood is charged upon
a log
basis transversally placed on the soil. The wood pile is lid through a channel
8 under
the central part of the furnace. The furnace operation requires considerable
skill. The
doors in particular are vulnerable to misuse and if the seal is damaged,
operation of
the furnace becomes very difficult.
Such as in the beehive type of brick furnaces, the carbonization is controlled
by the
color of the emitted gases. A light blue color occurs in the end of the
carbonization
process. By closing the air vents 7, the charcoal cooling is started. Like the
beehive
furnaces, the total cycle lasts from 9 to 12 days.
A deficiency of the Missouri furnace is the non homogenous carbonization. In a
certain moment there may be very hot regions where the charcoal bums, together
with regions still in the final wood drying step. An evolution of the furnace
control is
the temperature measurement by infra red pyrometers, which show the
differences in
temperature. Through the control of the air input by the air vents 8, a better
homogenization of the carbonizing wood pile is obtained.
In Brazil, Missouri furnaces were installed by some companies, mainly by the
integrated steel mills. Most of the Brazilian charcoal producers operate with
traditional brick furnaces not equipped with smoke recycling or ways to
burning the
energetic gases emitted by the carbonizing wood.
Brick furnaces do not take advantage of the combustible gases emitted by the
carbonizing wood. As a result, one of the basic characteristic of these
furnaces is the
burning of part of the charged wood. In the brick kilns occurs a certain
overlapping
of the drying and pyrolysis steps. The emission to the atmosphere of harmful
gases
containing up 45 to 50 kg of methane per ton of charcoal is another
characteristic of
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
these kilns. As far as greenhouse effect is concerned, this methane content is
equivalent of one ton of C02-
The humidity content of the charged wood in the traditional brick furnaces
should be
not over 25 - 30% (w.b.). Soon after being cut down, wood moisture content is
an
5 average of 50% (w.b.). It is impossible to carbonize or to use wood as a
fuel with this
moisture content, being necessary to reduce it to 25-30% (w.b.) level, which
is done
by appropriately piling it during 100 to 120 days. This labor intensive piling
requires
the following operations.
Wood cut in logs at least one meter long.
10 Wood piling in an adequate way in order to air dry it.
Load the log wood in the truck.
Transfer of the wood from the truck to the furnace when Missouri kilns are
used, or
to the yard in the case of beehive kilns.
Wood feed into the beehive furnaces.
Transfer of the charcoal from the brick kiln to the truck. During the transfer
of the
lower layers impurities are many times mixed with the charcoal.
Transfer from the truck to the charcoal bin.
This labor intensive sequence of operations is responsible for approximately
60% of
the charcoal from brick furnaces cost. Besides, when contaminated by
impurities the
charcoal ash content is raised.
Gravimetric yield is the relation (kg of charcoal/(ton of anhydrous wood). Due
to the
burning of part of the charged wood brick furnaces gravimetric yield is low,
from 25
to 34%. That is, only 250 to 340 kg of charcoal per ton of anhydrous wood is
obtained. The upper level of this range is obtained in the rectangular brick
furnaces
with internal temperatures measurement.
It follows that the main disadvantages of carbonizing wood in brick furnaces
are:
1 - Low gravimetric yield.
2 - Emission to the environment of harmful gases.
3 - Long cooling time due to the low heat conductivity of the brick walls.
4 - Non homogeneous charcoal quality.
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
11
- Lack of carbonization control, which is made by the smoke color and the
brick
wall temperature.
6 - The carbonization routine can be led in more than one way, depending on
the
operator.
5 7 - Burning part of the charged wood in order to supply energy for the
endothermic
carbonization step.
8 - Low productivity, due to the long cycle time.
9 - Waste of the energetic gaseous (condensable and non condensable) emitted
during the wood carbonization.
10 - Necessity of storing the wood for drying.
Solution of the problems.
The principal aim of the present invention is to solve the above mentioned
problems
by providing a method for carbonizing biomass that considerably simplifies,
with
respect to conventional carbonization methods, the operations for pollutant
removal
and for energy recovery of the products of biomass pyrolysis.
Within the scope of this aim, an object of the invention is to provide a
method that
can be controlled and managed in a very simple manner on the basis of
parameters
preset according to the type of biomass being treated, with high operating
flexibility.
Another object of the invention is to provide a plant that can perform such a
treatment method in a practically continuous manner.
Another object of the invention is to provide a structurally simple plant
requiring
relatively low investments and operating costs.
Another object of the invention is to provide a plant offering adequate
assurances
against a danger of environmental pollution.
This aim, these objects and others which will become apparent hereinafter are
achieved by a wood carbonizing system which uses the gaseous constituents
emitted
by the wood during the pyrolysis step as the supplier of the necessary energy
for the
process. There follows the description of the devised system, which we named
the
DPC Process.
The emitted energy during the exothermic step of the biomass carbonization is
sufficient to meet the thermal demand of the process endothermic phases. But
brick
kilns do not take advantage of this energy because the endothermic stage
occurs
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
12
before the exothermic pyrolysis step. The wood carbonizing process is self
sufficient
in energy. However, this energy is only available after the drying endothermic
stage,
being necessary to burn part of the carbonizing wood in brick kilns. The DPC
Process basic characteristic is the sharp separation of the drying and
pyrolysis stages,
which are performed in independent reactors in such a way that the energy
content in
the emitted gases by the carbonizing wood is used to supply the thermal demand
of
the endothermic steps. The devised DPC Process resolves the drying and
pyrolysis
steps difference in time problem.
The basic concepts of the DPC Process described in this exposition are:
1 - Utilization of the emitted gases - condensable and non condensable - as a
source
of the thermal energy required by the carbonization process.
2 - Utilization of the gases emitted during the pyrolysis step as a heat
carrier for the
endothermic stage of the pyrolysis.
3 - The functions of wood drying, pyrolysis and the charcoal cooling are
performed
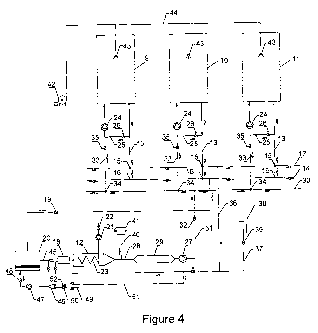
simultaneous and independently into a least three reactors, figure 4. The
emitted
gases during pyrolysis with a significant heat value are burned in a
combustion
chamber, generating hot gases which are transported to the reactor performing
the
wood drying
Any DPC reactor can perform the functions of drying, pyrolysis and charcoal
cooling. The process can be performed in more than three reactors, depending
on the
desired capacity of the charcoal plant.
The disposal of the reactors in the DPC Process, object of the present report,
is
shown in figure 4. The system consists of three independent reactors, 9, 10
and 11,
and an independent combustion chamber 12. Let us suppose that in a certain
moment
the drying step is performed in reactor 10, and charcoal being cooled in
reactor 11. In
reactor 10 condensable and non condensable gases containing combustible
components are emitted by the carbonizing wood. These gases exit reactor 10
through pipe 13. Most of these gases are transported through pipe 13 to the
collecting
pyrolytic gases pipe 14, passing through valves 15 and 16. Valve 15 stays
opened
allowing the pass of the gases to collector pipe 14, but hinders the pass of
the ditto
gases to the diluting gases collector 17. From the collector pipe 14 pyrolytic
gases
are transported to the gas burner 18 situated at the combustion chamber 12,
flow
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
13
controlled by valve 19, passing before through gasifier 20. Combustion air
driven by
fan 21, flow controlled by valve 22, mixed with the fuel gases in the burner
18.
Combustion air is preheated by heat exchanger 23 placed inside the combustion
chamber 12. Hot fuel gases generated by the carbonizingwood return to the
reactor
10 driven by fan 24 through pipe 25, flow controlled by valve 26. The purpose
of this
return is to control the temperature in the carbonizing reactor. The fixed
carbon
content and other metallurgical charcoal properties are functions of the
carbonizing
temperature. Therefore part of the pyrolytic gases flow in a closed circuit, a
looping.
The fuel gases closed circuit with the aim of a precise control of the biomass
carbonizing stage, is a basic characteristic of the DPC Process described in
this text.
The carbonization end is shown by the decreasing pyrolytic gases flow. The
temperature in the carbonizing reactor 10 remains in the range 310 - 350 C,
adequate
to the metallurgical charcoal. The carbonization speed is controlled by the
return
flow of the pyrolytic gases.
Hot flue gases generated by burning pyrolytic gases in the combustion chamber
12
are driven by fan 27 through pipe 28 to mixer 29. Diluting gases coming from
drying
reactor chamber 9 are mixed with hot flue gases coming from combustion chamber
12 inside mixer 29. This gaseous mixture suctioned by fan 27 is driven to the
pipe
collecting hot gases 30 by pipe 31, flow controlled by valve 32. From pipe 30,
hot
flue gases mixed with said diluting gases coming from reactor 9, are suctioned
by fan
24 through pipe 33, passing by valve 34 totally opened, and by control flow
valve 35.
Hot flue gases are then insufflated into the drying reactor 9. Steam from the
drying
wood joins the hot flue gases. This gaseous mixture exits the drying reactor
at
approximately 120 C through pipe 13, being driven to the diluting gases pipe
17,
valve 15 remaining totally opened, and valve 16 totally closed. Pipe 17 is a
diluting
gases distributor. These gases are transported to mixer 29 through pipe 36.
Inside
mixer 29 the diluting gases, which we will name recycle gases, are mixed with
the
hot flue gases coming from the combustion chamber 12. The excess of the
recycle
gases is driven through pipe 37 to the chimney 38, flow controlled by valve
39, and
from said chimney 38 to the atmosphere. The drying reactor 9 inside which wood
drying is performed demands the maximum heat input. In order to avoid high
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
14
temperatures in the drying reactor, hot flue gases exiting combustion chamber
12 are
mixed with the diluting gases flowing through pipe 17. This technique, which
we
named "recirculation", allows the heat input in the drying reactor 9 at the
desired
temperature. The drying reactor 9 ideal entering gas temperature is in the
range 300 -
350 C. Performing in this temperature range undesirable steel containers
overheating
will not occurs. Besides when drying at high temperatures, wood cracks
weakening
the charcoal. The recirculation technique is another basic characteristic of
the DPC
Process described in the present text.
Most of the pyrolytic gases generated during the wood carbonizing step are
driven to
the burner 18 of the combustion chamber 12. A small parcel of ditto gases
return to
the carbonizing reactor 10 in order to control the carbonizing wood
temperature, as
above explained. As a result of this technique no polluting gases are emitted
the
atmosphere. Depending on the initial wood moisture, an excess of flue gases
from
the combustion chamber may occur. Ditto excess is driven by pipe 28, through
valve
40 to the chimney 41, from which they are emitted to the atmosphere.
Charcoal cooling is being performed in reactor 11, which is done in two
stages. The
cooling starts by turning off fan 24 and closing control flow valves 26 and 35
placed
in pipes 25 and 33. Valve 15 remains closed. Reactor 11 should be tightly
sealed,
since any air leakage will burn the hot charcoal. Heat radiation to the
atmosphere
starts the charcoal cooling. During this cooling stage, a small part of the
carbon
contained in the gaseous atmosphere from the carbonization stage, is absorbed
by the
charcoal, slightly raising the charcoal fixed carbon. Besides this gaseous
atmosphere
small pressure hinders atmospheric air entrance. Charcoal cooling continues by
the
injection of a very fine water spray in reactor 11. Water is injected under
high
pressure by pump 42 to atomizer 43 through pipe 44. Water is divided in very
fine
drips, which quickly vaporizes cooling the charcoal. When the cooling reactor
11
temperature falls to 95 C, water injection is stopped. Charcoal cooling is
concluded
by heat radiation to the atmosphere.
During the entire carbonization process in reactors 9, 10 and 11, wood remains
motionless, avoiding charcoal fines generation. Wood drying, carbonization and
charcoal cooling occur simultaneously until the end. When the process is
terminated
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
drying reactor 9 receives hot pyrolytic gases, charcoal cooling starts in
reactor 11,
and reactor 11 is unloaded. A new wood container is placed in reactor 11,
starting the
wood drying. By adequate maneuvers in valves 15, 16 and 34 situated in pipes
14, 17
and 30, reactors 9, 10 and 11 functions are changed. Reactor 9 becomes a
pyrolysis
5 kiln, charcoal cooling is done in reactor 10, and reactor 11 becomes a
drying reactor.
When starting the system, fuel gas to burning in combustion chamber 12 is not
available, as none reactor is in the pyrolysis stage. Therefore an external
heat source
is necessary in order to start and finish the drying of the wood loaded in
reactor 9.
Two options are available for the system start: natural gas utilization in
burner 18, or,
10 which is preferable, to set up a wood gas producer. The first cycle will be
started by
burning wood gas coming from this gas producer. Figure 4 shows the wood gas
producer 20 and pipe 45 which transports the low heating value gas generated
in the
gas producer 20 to the burner 18. The gasifying agent comes from mixer 46,
being
injected in gas producer 20 by fan 47, flow controlled by valve 48.
Atmospheric air
15 is driven to mixer 46 through pipe 49, flow control by valve 50. Diluting
gas is
carried to mixer 40 through pipe 51, flow control by valve 52. A small
proportion of
diluting gas is mixed with the gasifying air in order to avoid too high
temperatures in
the lower part of the gas producer. The gas producer capacity should be
adequate to
the thermal demand by the drying reactor 9. Besides, the gas producer will
assure the
supply of fuel gas in the case of an eventual deficiency of combustible gas
emitted by
the carbonizing wood.
It should be remarked that once started the system by burning a fuel gas from
an
external source, the process is continuous, although wood remains motionless
during
the whole cycle. There is permanently in operation a drying reactor, a
carbonizing
reactor and charcoal cooling in the third one. At the end of the cycle the
function of
each reactor changes, but the process is incessant, continuous. Total wood
carbonization time in the DPC Process is approximately 72 hours. Taking into
account the possibility of loading the wood in the drying reactor soon after
cutting
down the wood, charcoal can be made approximately seven days after the wood
cutting down, while in brick kilns this time goes over 130 days.
In the starting of the carbonization stage and at the end of the wood
carbonization,
emission of combustible gaseous constituents is very small. In order to assure
the
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
16
continuous supply of combustible gas to the combustion chamber 12 it is
necessary a
minimum of six reactors in operation. So, we will have two or more carbonizing
reactors, two or more drying reactors and the correspondent number of cooling
reactors. In order to avoid oscillations in the pyrolytic- gas volume and in
its low
heating value, carbonization in second reactor is started when the first
reactor is near
to maximum gas emission. In that technique of operation when fuel gases
generation
in the first reactor starts decreasing, the second reactor is going towards
the
maximum pyrolytic gases emission, which allows the regularity maintenance. The
system productivity is increased when a minimum of six reactors is running.
Figure 5 shows a system with six reactors.
In figure 5 we can see three master pipes collectors of the different process
gases.
Pipe 52 is the pyrolytic gases collector, flowing in this pipe only this type
of gases.
Pipe 53 carries the pyrolytic gases to pipe 54, flow controlled by valve
55.Combustible gases generated by the carbonizing wood are driven to burner 56
set
at the combustion chamber 57, reaching burner 56 through pipe 58. These gases
were
produced in the wood carbonizing reactors, supposed to be reactors 59 and 60
at the
moment chosen for this description. Gases emitted by the carbonizing wood are
transported to pipe 53 by pipe 61, passing before through valves 62 and 63.
These
valves are fixed in such a way that valve 62 although closed, allows passage
of the
pyrolytic gases only towards pipe 53, crossing valve 57, which at this moment
remains opened. Part of the pyrolytic gases return to reactors 59 and 60
through pipe
64, suctioned by fan 65, flow controlled by valve 66. The purpose of this
return is the
temperature control in reactors 59 and 60 in order to avoid the carbonization
wood
superheating during the pyrolysis exothermic stage. This technique has been
previously expounded in the case of three reactors, figure 4. Having two
carbonizing
reactors, the supply of fuel gas to combustion chamber 57 according the
process
needs is assured.
At the moment chosen for this description, reactors 67 and 68 are processing
the
wood drying. Hot flue gases produced in combustion chamber 57 are suctioned by
fan 69 through pipe 70, which transports ditto hot gases to mixer 71. From
mixer 71
hot flue gases are carried to the collecting flue gases 72, through pipe 73,
flow
controlled by valve 74. From pipe 72, said gases are driven to the drying
reactors
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
17
chambers 67 and 68 through pipe 75, flow controlled by valve 76. During the
wood
drying in reactors 67 and 68 water vapor coming from the wood dehydration
joins
the hot flue gases which entered the drying reactors at 300 t0 350 C. Said hot
flue
gases plus water vapor are driven through pipe 61 to the collecting recycle
gases pipe
77. At this moment valve 62 remains opened and valve 63 remains closed. In
other
words, effluent gases from drying reactor have no way to access pipe 53 going
only
to pipe 70. At this moment valve 66 remains opened for the recirculation.
Diluting
gases are led to pipe 78, flow control valve 79, to the entrance of mixer 71,
where
said gases mix with the hot flue gases coming from combustion chamber 57; with
the
purpose to obtain the correct drying temperature, such as described in the
system
with three reactors, figure 4. Such as in the system described with three
reactors,
figure 4, the excess of hot flue gases produced in the combustion chamber 57,
is
carried through pipe 80, valve 81, to the chimney 82, and from said chimney 82
to
the atmosphere. The excess recycle gases is driven through pipe 83, flow
control
valve 84 to the chimney 85, and from chimney 85 to the atmosphere. Such as in
the
system described for three reactors, figure 4, no polluting substances are
emitted to
the atmosphere.
At this moment reactors 86 and 87 process the charcoal cooling. Such as in the
system with three reactors, charcoal cooling is started with reactors 86 and
87 well
sealed, through heat radiation to the atmosphere; continuing by water spray
injected
by pump 88 through pipe 89 to the water atomizer 90. When cooling reactors
temperature falls to 95 C, charcoal cooling is completed by the heat radiation
to the
atmosphere.
The DPC Process described in the present text provides a precise control of
the wood
carbonization process, producing charcoal according to the metallurgical
properties
specified by the user. The control is made through pyrometers installed at the
gases
entrance and the gases exit of each reactor. When drying starts the
temperature
difference between the entering and the exiting gases is large due to the wood
heating and humidity water vaporization thermal demand. This temperature
difference reduces while drying goes on. The end of the drying period will be
shown
by these temperatures convergence, the drying reactor becoming available for
the
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
18
wood carbonization stage. The control of the process through pyrometers placed
at
the entrance and the exit of each reactor allows the DPC system automation.
During the wood carbonization stage the charcoal fixed carbon is a crescent
function
of the temperature, while the gravimetric yield is a decreasing function of
the
temperature. Figure 1 shows the relation between carbonizing temperature,
charcoal
fixed carbon and gravimetric yield. Steel industry is the main charcoal
consumer in
Brazil for the pig iron blast furnace. Charcoal fixed carbon for the pig iron
blast
furnace is specified in the range 70 - 75%. Figure 1 shows that carbonizing
temperature should be in the range 320 - 350 C for the fixed carbon content in
the
range 70 - 75%.
In the DPC process, object of the present exposition, no combustion occurs in
the
carbonizing reactor; so it is not necessary carbonizing temperatures over 350
C. As
this temperature is compatible with the performance of common carbon steel, it
is
possible to place the wood in metallic containers.
The energy content of the emitted gases by the carbonizing wood is sufficient
to
drying wood soon after cutting down, according to thermodynamic computations.
The cutting down wood humidity is approximately 50% (w.b). One of the
advantages
of the DPC process is the capacity to drying wood soon after cutting down,
avoiding
the wood storing at the atmospheric air in order to reduce the humidity to
water
content in the wood compatible with the carbonization process in brick kilns.
That
storage has an economical cost. However, if convenient to recover the
condensable
products, wood can be dried by adequately placing it in the atmospheric air
during a
minimum of one hundred days. Condensation of the condensable constituents
present
in the pyrolytic gas followed by separation of the various products by the
conventional extraction processes will allow the recover of several wood
liquid
products present in the condensable gases.
Alternatively, the energy of the fuel gases emitted by the carbonizing wood
can be
used for thermal electric generation. It is an economically very attractive
alternative
if the carbonization plant is located in the proximity of the charcoal blast
furnace.
Hot flue gases effluents from auxiliary equipments of the pig iron producer
plant can
be used for the wood drying. In that case, pyrolytic combustible gases or tar
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
19
produced by the condensation of those gases, can be used for thermal electric
generation, which turns the pig iron plant self sufficient in energy.
As previously explained, in the traditional wood carbonization brick kilns, it
is
necessary to store the wood in order to dry it. The wood handling for storing,
drying
and transporting to the brick carbonizing kilns is responsible for
approximately 60%
of the charcoal cost. It should be added the financial cost due to the drying
time,
minimum of one hundred days in the Brazilian weather conditions.
DPC Process described in this exposition gives the opportunity for an
excellent
solution of the drying logistic problem.
Soon after cutting down wood in the forest without any kind of piling, is
sawed in the
length of a roll on truck bucket with no need of several smaller wood cuts.
The cut
wood is manually or mechanically loaded into the bucket of a roll on truck.
The truck
raises the bucket onto its body, and carries the bucket full of wood to the
carbonizing
plant where the bucket is placed into the drying reactor, starting soon after
the wood
drying. At the end of the carbonizing process the truck takes out the bucket
of this
reactor, and transports the charcoal to its store bin. It should be noted that
between
the wood sawing and the charcoal unloading into the bin, no laborer work has
been
necessary. This technique has been possible due the absence of any kind of
combustion in the interior of the carbonizing reactor and due to the
temperature
control. Besides there is not any kind of charcoal contamination by the
impurities
present in the kiln floor. Figure 6 shows the arrangement of the association
DPC
equipment - roll on bucket. This technique is a significant advantage of the
DPC
Process described in this report when compared with the conventional brick
kilns
carbonization.
Due to the independence of the drying, pyrolysis and cooling reactors it is
possible to
halt the process at any stage. So, DPC Process can produce anhydrous wood,
char or
charcoal with a high volatile content. The later is a very convenient fuel,
adequate to
replace fossil fuels in industrial furnaces or in boilers.
A biomass energy concentration is done through anhydrous wood, char or high
volatile charcoal. Due to the distances in large countries like Brazil, the
biomass
energy concentration is very relevant for its transportation.
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
It is possible to carbonize small size biomass such as coconut shells, bones,
babassu
palm coconut, elephant grass, straw, sugar cane and a variety of other biomass
can be
used in the DPC Process. Tests done in a DPC reactor showed the elephant grass
carbonization feasibility, which was achieved by the first time in the world.
5 The following products can be obtained by the DPC Process.
Charcoal with a minimum of 78% fixed carbon content to be used as a reducer in
the
electric furnaces. In comparison with coke the advantages are: no sulfur and
higher
electric resistivity.
Charcoal for the steel industry. Advantages when compared with coke: no
sulfur,
10 higher reactivity, lower ash content, smaller slag volume.
Charcoal with 30 - 50% of volatile matter, the ideal fuel for replacing fossil
fuels in
the industry or for thermoelectric generation.
Charcoal from small size biomass, agricultural wastes in general.
Char.
15 Anhydrous wood.
Table 2 shows the low heating value of these products.
Table 2 - Main wood products low heating value.
PRODUCT LOW HEATING VALUE - KJ/KG
WET WOOD 6,275 - 8,367
AIR DRIED WOOD 11,295 -12,550
ANHYDROUS WOOD 18,425 - 22,000
CHAR 22,590 - 23,000
HIGH VOLATILE CHARCOAL 25,000 - 27,600
BLAST FURNACE CHARCOAL 30,000 - 31,400
The energetic concentration given by the DPC Process, as shown in table 2 is
very
relevant for large countries.
20 Table 3 shows a comparison of the unit energy cost from biomass, and from
fuel oil,
according to current prices in Brazil. The third column of table 3 indicates
the
relation between the biomass energy unit cost and the fuel oil energy unit
cost.
Table 3 - Comparison of the energy unit cost.
PRODUCT LHV - US$/GJ US$BIOMASS/US$FUEL OIL
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
21
GJ/T
AIR DRIED 12.0 5.5 0.25
WOOD
CHAR 22.5 11.1 0.51
HV CHARCOAL 26.0 13.7. 0.64
B. F. CHARCOAL 30.1 14.8 0.69
FUEL OIL 40.1 21.5 1.00
The advantages of the DPC Process object of the present exposition, associated
with
the use of roll on buckets are.
1 - Higher charcoal yield.
2 - No noxious emissions to the atmosphere.
3 - It is not necessary to store wood during a long time in order to reduce
its
humidity.
4 - It is not necessary to saw the wood in small lengths.
5 - The wood remains motionless in the reactors, which eliminates charcoal
fines
generation.
6 - Lower labor cost.
7 - A lower charcoal production cost.
8 - An important logistic advantage.
9 - Able to produce anhydrous wood, char and charcoal with high volatiles
content,
very convenient fuels.
10 - It is a process suitable for any degree of mechanization or automation.
11 - Precise process control.
12 - It is a high productivity process, the residence time of the wet wood in
the
reactor is in the order of 72 hours.
13 - The investment cost expressed in US$ per ton of charcoal is lower than
the
retort processes (continuous carbonization process).
14 - It is possible to carbonize small size dimensions biomass.
There are also significant social advantages given by the DPC Process in the
case of
Brazil. No childish or slave hand labor is used. A DPC Process wood
carbonization
CA 02762863 2011-11-21
WO 2010/132970 PCT/BR2010/000175
22
plant will only employ qualified professionals, adequately trained,
representing a
social gain.
The process is suitable for the carbonization of several high productivity
biomass
crops, such as sugar cane and elephant grass raising a new window of
opportunities
for the strong agricultural sector of the Brazilian or any other large country
economy.
Harvesting sugar cane or elephant grass for energy applications can be an
important
job generator in remote and poor areas of any country, avoiding the migrant
exodus
to the big cities.
The energetic concentration of the biomass given by the DPC Process is very
important for any developing country.
The use of the cultivated biomass by the steel industry can generate a lot of
jobs in
the field, reducing the migration of rural laborers to the big cities. Each
ten hectares
of cultivated forest, generates a job in the field.
The economic advantages of the DPC Process can be resumed as follows.
Approximately 60% of the pig iron cost is due to the charcoal. A significant
reduction in the charcoal cost given by the DPC Process will decrease the pig
iron
and steel cost, raising the competitive conditions of the producer companies
of these
commodities. Besides, pig iron and steel obtained when charcoal is used as a
thermal
reducer have better quality.
The gravimetric yield, that is (kg of charcoal)/(ton of anhydrous wood) of the
DPC
Process is in the range 40 - 42%, while brick kilns range is 28 - 34%. In
other
words, while brick kilns produce 280 - 340 kg of charcoal per ton of anhydrous
wood, the DPC Process obtains 400 to 420 kg of charcoal per ton of anhydrous
wood. That means an increase of 30% in the charcoal production per hectare of
cultivated forest. As a result, keeping constant the charcoal consumption by
the steel
or pig iron plant, the forest will last 30% more time.
Numerous tests, starting in small units later in industrial reactors, have
confirmed all
advantages of the DPC Process, with emphasis in the higher gravimetric yield,
and
no pollution at all.
The remarkable reduction of the charcoal cost and the increase of cultivated
forest
time duration are sufficient to emphasize the important advantages of the DPC
Process, an innovative process integrally developed in Brazil.