Note: Descriptions are shown in the official language in which they were submitted.
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
METHODS FOR IDENTIFYING AN APEX FOR IMPROVED DATA-DEPENDENT
ACQUISITION
BACKGROUND OF THE INVENTION
Field of the Invention
[0001] The present invention relates to the field of data-dependent
acquisition in a
mass spectrometer, and more particularly to the field of tandem mass
spectrometry.
Discussion of the Related Art
[0002] Mass spectrometers are often coupled with chromatography systems in
order to
identify and characterize eluting species from a test sample. In such a
coupled system,
the eluent is ionized and a series of mass spectral scans are obtained at
specified time
intervals for subsequent data analysis. As the test sample may contain many
species or
compounds, it is often desirable to be able to automatically determine or
identify
species or compounds of interest as they elute and perform tandem mass
spectrometry
analysis to characterize them.
[0003] Tandem mass spectrometry is a mode of operation that utilizes multiple
stages of
mass analysis with a collision or reaction process between each stage of mass
analysis.
The coupling of multiple stages of mass analysis provides the ability to
determine or
identify species or compounds of interest by providing additional information
on the
fragmentation or reaction characteristics of the compound. Tandem mass
spectrometry
having two stages of mass analysis is typically referred to as mass
spectrometry/ mass
spectrometry (MS/MS). In data-dependent mode, the eluting sample is
automatically
selected for further analysis by MS/ MS when the signal intensity of a mass
spectral
1
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
peak is above a user specified intensity. But, direct intensity-based
triggering is far from
ideal, for several reasons. First, high baselines will cause triggering at all
masses.
Second, tandem mass spectra will be collected as soon as an eluting
chromatographic
peak exceeds the threshold value, and not at the ideal point, which is at the
top of the
chromatographic peak where it is at its greatest intensity. Collecting a
sample for
MS/ MS at the beginning of the elution of the chromatographic peak produces a
lower
quality spectrum, due to a limited number of sample ions being combined with a
high
percentage of background contaminants. For ion accumulating mass
spectrometers,
which use automatic gain control, such as quadrupole ion traps and Fourier
transform
mass spectrometers, performing MS/MS at the beginning of elution also requires
the
largest amount of time, thus slowing analysis. Third, direct intensity-based
triggering
results in redundant MS/ MS acquisition of compounds during the entire elution
time.
[00041 United States Patent Application No. 7,009,174 describes a method of
dynamic
background signal exclusion in chromatography/ mass spectrometry data-
dependent
data acquisition to detect species eluting at a low level of concentration
that elute
simultaneously with a number of other major components by identifying ions
having a
fast rising mass signal. The ions having the fastest rising mass signal may be
identified
by subtracting a previously acquired mass spectrum, or an average of
previously
acquired mass spectra, from the current mass spectrum. The disadvantage of
taking a
sample of the eluted ionized species at the fastest rising mass signal is that
MS/ MS can
often be triggered on noise spikes in the spectrum, caused by either charged
droplets
from the ion source or electrical noise in the detection system.
2
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
SUMMARY OF THE INVENTION
[0005] Accordingly, the present invention provides a method of enabling data-
dependent data acquisition in a mass spectrometer, including: obtaining a
plurality of
mass spectra from a sample; selecting from the plurality of mass spectra, a
set of eluting
m/ z peaks of interest from a weighted mass spectrum by way of a correlation
technique; obtaining phase data resulting from portions of extracted ion
chromatographs (XIC) from the selected set of eluting m/z peaks of interests
so that a
subset of the set of eluting m/z peaks of interest can be identified; and
triggering off of
the identified subset of the eluting m/ z peaks of interest based on the
obtained phase
data so that real-time data is acquired that corresponds to one or more
desired eluting
m/ z peaks of interest.
[0006] Another aspect of the present invention includes a computer readable
medium
that provides instructions. When the instructions are executed on a processor,
such
instructions can cause the processor to perform a method of controlling a mass
spectrometer that includes: obtaining a plurality of mass spectra from a
sample;
selecting from the plurality of mass spectra, a set of eluting m/z peaks of
interest from a
weighted mass spectrum by way of a correlation technique; obtaining phase data
resulting from portions of extracted ion chromatographs (XIC) from the
selected set of
eluting m/z peaks of interests so that a subset of the set of eluting m/z
peaks of interest
can be identified; and triggering off of the identified subset of the eluting
m/z peaks of
interest based on the obtained phase data so that real-time data is acquired
that
corresponds to one or more desired eluting m/ z peaks of interest.
[0007] In another aspect of the present invention, embodiments can include a
sample
processing apparatus for data-dependent acquisition. The apparatus may include
a
3
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
mass spectrometer, a system controller for controlling the mass spectrometer,
and a
machine-readable medium coupled to the system controller. The machine-readable
medium may have a memory that stores a set of instructions that controls data-
dependent acquisition by the mass spectrometer. The set of instructions may
control
parameters of the data-dependent acquisition of the mass spectrometer by
obtaining a
plurality of mass spectra from a sample; selecting from the plurality of mass
spectra, a
set of eluting m/z peaks of interest from a weighted mass spectrum by way of a
correlation technique; obtaining phase data resulting from portions of
extracted ion
chromatographs (XIC) from the selected set of eluting m/ z peaks of interests
so that a
subset of the set of eluting m/z peaks of interest can be identified; and
triggering off of
the identified subset of the eluting m/z peaks of interest based on the
obtained phase
data so that real-time data is acquired that corresponds to one or more
eluting m/z
peaks of interest.
[00081 Accordingly, the present invention is directed to data-dependent peak
selection
techniques and apparatus that enable the identification of eluting compounds
in
tandem mass spectrometer systems. Such methods and apparatus, as disclosed
herein,
are beneficial in applications, such as, but not limited to, in-vitro sample
analysis,
proteomics analysis and/or complex sample analysis wherein identification of
desired
eluting peaks requires optimal performance for identification.
BRIEF DESCRIPTION OF THE DRAWINGS
[00091 Fig. 1A is a block diagram of a combination method in accordance with
embodiments of the present invention.
[00101 Fig. 1B is a block diagram of a sample processing apparatus that
embodies
aspects of the present invention.
4
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
[0011] Fig. 2 is a block diagram of the correlation method to determine when
to make a
data-dependent acquisition.
[0012] Fig. 3A illustrates an example of five mass spectra taken at different
times.
[0013] Fig. 3B illustrates extracted ion chromatograms created for each of the
m/ z data
points within measured mass spectra.
[0014] Fig. 3C illustrates weighted extracted ion chromatograms for the m/z
data
points obtained in Fig. 3A.
[0015] Fig. 3D illustrates a weighted mass spectrum based on XIC correlation
values of
the extracted ion chromatograms.
[0016] Fig. 4 is a block diagram of methods of selecting mass spectral m/ z
data points
near the apex of the chromatographic peak for tandem mass spectrometry.
[0017] Fig. 5 is a graph showing a curve that is part of an extracted ion
chromatogram
(XIC) for a selected mass-to-charge ratio, and showing a further curve
representing a
portion of the XIC curve that has been rotated in preparation for
transformation from
the time domain to the frequency domain.
[0018] Fig. 6 is a graph showing a curve that represents the magnitude mode
frequency
domain data obtained by taking rotated time domain data and transforming it
into the
frequency domain.
[0019] Fig. 7 is a graph showing more of the XIC curve of Fig. 5, showing a
further
curve representing the magnitude of a single-cycle component of the frequency
data in
Fig. 6, and showing a phase curve that represents the variation over time of
the phase
(in radians) of the single-cycle frequency component.
[0020] Fig. 8 is a flowchart showing a sequence of operations carried out by a
processor
of the present invention.
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
DETAILED DESCRIPTION
[0021] In the description of the invention herein, it is understood that a
word
appearing in the singular encompasses its plural counterpart, and a word
appearing in
the plural encompasses its singular counterpart, unless implicitly or
explicitly
understood or stated otherwise. Furthermore, it is understood that for any
given
component or embodiment described herein, any of the possible candidates or
alternatives listed for that component may generally be used individually or
in
combination with one another, unless implicitly or explicitly understood or
stated
otherwise. Additionally, it will be understood that any list of such
candidates or
alternatives is merely illustrative, not limiting, unless implicitly or
explicitly understood
or stated otherwise.
[0022] Moreover, unless otherwise indicated, numbers expressing quantities of
ingredients, constituents, reaction conditions and so forth used in the
specification and
claims are to be understood as being modified by the term "about."
Accordingly, unless
indicated to the contrary, the numerical parameters set forth in the
specification and
attached claims are approximations that may vary depending upon the desired
properties sought to be obtained by the subject matter presented herein. At
the very
least, and not as an attempt to limit the application of the doctrine of
equivalents to the
scope of the claims, each numerical parameter should at least be construed in
light of
the number of reported significant digits and by applying ordinary rounding
techniques. Notwithstanding that the numerical ranges and parameters setting
forth the
broad scope of the subject matter presented herein are approximations, the
numerical
values set forth in the specific examples are reported as precisely as
possible. Any
6
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
numerical values, however, inherently contain certain errors necessarily
resulting from
the standard deviation found in their respective testing measurements.
General Description
[0023] Where data-dependent acquisition is encompassed within the performance
of
mass spectrometry, such as, successive stages of mass spectrometry, often
tandem mass
spectrometry, it is beneficial to perform mass spectrometry on a precursor ion
near the
apex of the chromatographic peak containing the precursor ion. In tandem mass
spectrometry, for example, precursor ions are further fragmented by collision
or
reaction within a separate chamber or within the ion trap itself. The further
fragmentation of the precursor ions on which mass spectra are obtained creates
more
information for the characterization or identification of compounds eluting
from the
chromatographic column. The apex of the chromatographic peak is an
advantageous
place to collect the precursor ions for tandem mass spectrometry because the
flux of
ions into the ion trap near the apex of the peak is at its greatest.
Therefore, the ion trap
may be quickly filled to capacity with the ions of interest, resulting in the
faster
production of mass spectra and improved signal to noise ratios. The signal to
noise ratio
may be enhanced because the time to fill the ion trap to capacity is minimized
so that
any contaminants, if present within the sample, may be present in
insignificant
quantities. Additionally, filling the ion trap to capacity is valuable because
it provides
more precursor ions within the resulting mass spectrum.
[0024] Accordingly, the present invention is generally directed to methods of
determining when to make a data-dependent acquisition in real-time. In
particular, the
embodiments disclosed herein apply to the determination of when to execute
tandem
7
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
mass spectrometry on a precursor. As an unexpected result, combining the two
methods of the present invention for data-dependent peak selection provides a
superior
performance in making such a determination in contradistinction to utilizing
alone any
of the methods discussed herein.
[0025] One of the methods, described herein as the "correlation" method,
includes
taking the data collected from a mass spectrometer and correlating the data to
a model
function. Such a similar method is described in United States Patent No.
7,297,941,
entitled, "Methods For Improved Data dependent Acquisition," to Senko et al.,
the
disclosure of which is incorporated by reference in its entirety.
[0026] In particular, one compares an extracted pattern of separated
substances, i.e.,
an ion chromatogram, to the front half of a peak, such as, for example, a
model
Gaussian peak. The observed abundance may then be multiplied by the
correlation. In
this way, masses that are initially eluting are unaffected, masses that
constantly elute
are multiplied by zero, and masses on the tail of the elution are multiplied
by minus
one. Such a method includes applying a weighting function to the correlated
data to
obtain a reconstructed weighted mass spectrum to make a real-time decision for
a data-
dependent acquisition. This weighting step helps emphasize masses of interest
from
constantly eluting chemical noise or background. In order to trigger near the
top of the
chromatographic peak, the correlation processing can be combined with a simple
apex
detection which looks for a decrease in the abundance at any mass in the
chromatogram, but more often, as disclosed herein, it is desired to combine
the
correlation method with a second method (i.e., the phase method as disclosed
in greater
8
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
detail hereinafter) for a more accurate data-dependent determination of
eluting m/z
peaks of interest.
[0027] The second method, in particular, provides for improved data-dependent
peak detection based upon analysis of extracted ion chromatograms that have
been
transformed from the time domain into the frequency domain. Such a similar
method is
described in Pending U.S Application Serial No. 11/644,180, entitled, "Method
And
Apparatus For Identifying The Apex of A Chromatogram," to Senko, the
disclosure of
which is incorporated by reference in its entirety. While other data
characteristics can be
used as indicators, it has been found that the phase of the transformed data
is a good
indicator for identifying an elution peak. Thus, a "phase" method in
accordance with
embodiments of the present invention transforms at least portions of extracted
ion
chromatograms or portions of the chromatogram representing raw data from the
time
domain to the frequency domain. This transformation is beneficial in that it
separates
the signal of interest at low frequencies from the noise and signal
instabilities at high
frequency. The signal and corresponding data at low frequencies is of greater
interest
because masses of interest in the sample will correspond to low frequency
components
in the chromatogram. However, instead of just looking at the abundance of the
low
frequency signals, it has been found that the phase of these signals is
actually more
useful. This is especially so because there may be a mass of interest that has
a low
abundance such that its peak is hidden by noise or background. In particular,
the phase
of the single cycle component has been found to be an excellent indicator of
the current
position on the chromatographic peak. Limiting peak selection to a narrow
window of
phases allows for triggering near the apex of chromatographic peaks while
9
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
simultaneously reducing random peak selection by greater than about sixty-five
percent.
[0028] To reiterate, it is to be appreciated that a beneficial processing
technique of
the present invention is a combination of the two methods discussed above,
wherein the
first method provides the noise filtering for a baseline set of m/ z of
interest while the
second method provides the apex detection of a subset of the provided for
baseline m/z
of interest. This is because the first method used by itself utilizes a simple
apex
detection method by looking for a decrease in abundance. This simple apex
method
may erroneously identify a shoulder as an apex. The second method, on the
other hand,
used by itself, uses a simple intensity threshold which may erroneously
identify a
continuously eluting background ion. However, by using the correlation method
to
provide a weighted mass spectrum and then by providing a correlated set of m/
z of
interest enables the apex detection arrangement of the second method to
provide results
that are better than either method utilized alone.
[0029] Combining the two methods, as described herein, however, does result in
an
increase of the processing time greater than the processing time for any of
the
individual methods operating alone. In particular, the first and second
methods
described above, and in greater detail below, often require about 10
milliseconds each
when executed separately using unit resolution bins and a scan width of about
1500
m/z. It is to be appreciated, however, that the combined execution time
requires less
than the sum of the individual processing steps since the second step of phase
analysis
needs to be performed only on the bins that pass a correlation processing
threshold. In
other words, the correlation or first method helps to narrow in on a portion
of the
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
chromatogram or the weighted chromatogram of interest such that the phase
method
need only be performed on a smaller data set, i.e., a subset. Simple estimates
indicate
that the combined method increases the processing time by less than about
twenty-five
percent. Thus, a twenty-five percent or less increase can result in a
processing time
increase from about 10 milliseconds to less than about 12.5 milliseconds.
[0030] Accordingly, utilizing such combined methods improves the ability to
pick
peaks with greater accuracy in a data-dependent fashion during a mass
spectrometry
analysis, such as, but not limited to, a Liquid Chromatography/ Mass
Spectrometry
(LC/MS) experiment.
Specific Description
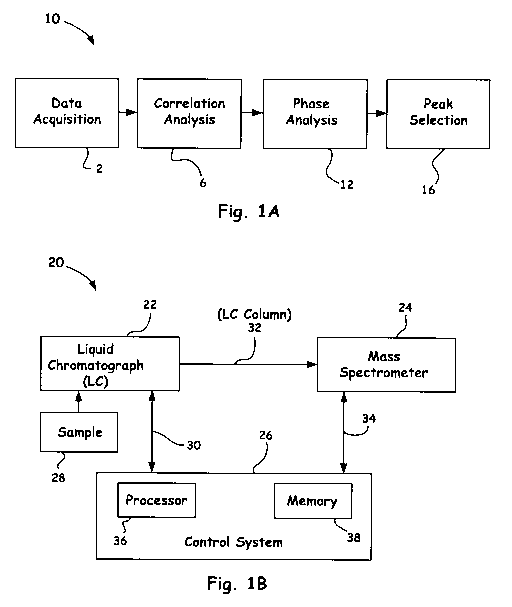
[0031] Turning now to the drawings, Fig. 1A shows a block diagram, generally
designated by the reference numeral 10, which illustrates a combination method
of a
first and a second method of the present invention. In particular, as shown in
Fig. 1A, a
set of data is acquired at block 2, the first method (i.e., a correlation
method) is
implemented at block 6, the second method (i.e., a phase method) is
implemented at
block 12, and a desired peak is identified and selected, as shown in block 16.
[0032] Fig. 1B shows a simple block diagram of an apparatus, generally
designated
by the reference numeral 20 that incorporates the general aspects shown in
Fig. 1A.
Such an apparatus 20 generally includes a liquid chromatograph 22, a mass
spectrometer 24, and a controller or control system 26. The liquid
chromatograph 22
often is a known type of device, as understood by those of ordinary skill in
the art, and
thus can be any of a number of commercially-available devices. The liquid
chromatograph 22 is designed to receive a sample 28 of a material to be
analyzed so that
11
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
particles of that material, i.e., analytes, can be produced. In particular,
the liquid
chromatograph 22 outputs analytes (not shown) that are atoms or molecules of
the
sample 28. The resultant analytes produced by the liquid chromatograph 22 are
delivered to the mass spectrometer 24 through a liquid chromatograph (LC)
column 32
of a known type, such as, for example, a plastic column, a glass column (e.g.,
fused silica
capillary tube) or a high-performance (HPLC) stainless steel tube.
[0033] In an example disclosed embodiment herein, the utilized mass
spectrometer
24, while not detailed in the present application, can be arranged from
commercially-
available devices known and understood by those of ordinary skill in the art,
such as,
but not limited to, a triple quadrupole, a quadrupole time-of-flight (q-TOF),
an ion trap,
an ion trap-FT, or an ion trap-Orbitrap. Such compatible mass spectrometers 24
can be
configured to perform tandem mass spectrometry, which involves two (e.g., mass
spectrometry/ mass spectrometry (MS/MS), or more successive stages of mass
analysis
with a collision or reaction process often occurring between each stage of
mass analysis
to enhance the ability to determine or identify species or compounds of
interest from
the sample 28.
[0034] In a data-dependent approach of the present invention, using for
example, an
MS/ MS configuration, analytes from the Liquid Chromatograph (LC) column 32
can be
processed in the first stage of mass spectrometry in order to identify mass
spectral
peaks and when a mass spectral peak is identified, the analytes associated
with the
identified peak(s) are subjected to further evaluation in the second stage of
analysis.
[0035] The control system 26, shown operatively coupled in Fig. IB to the
liquid
chromatograph 22 and the mass spectrometer 24 (note: coupling denoted by
reference
12
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
numerals 30 and 34 respectively), often includes a processor 36, e.g., a
microprocessor,
and a machine readable medium (a form that can be accessed by an automated
sensing
device) or memory 38. The memory 38 collectively represents two or more
different
types of memory, such as, for example, a read only memory (ROM) that can store
static
data and a program executed by the processor 26 in addition to random access
memory
(RAM) that is used by the processor 26 to store data that changes dynamically
during
program execution. While such an arrangement is beneficial in the present
invention,
the processor 26 and the memory 38 can optionally be implemented as respective
portions of a known device that is commonly referred to as a microcontroller.
[0036] As stated above, during, for example, tandem mass spectrometry, data
from a
first stage of mass spectrometry can be monitored to identify mass spectral
peaks, and
the identification of a mass spectral peak can trigger a second stage of mass
spectrometry with respect to analytes that correspond to the peak. Pre-
existing
techniques for identifying a mass spectral peak have been generally adequate
for their
intended purposes, but have not been entirely satisfactory in all respects.
The apparatus
20, as shown in Fig. 1B, however, takes a different approach to the
identification of
mass spectral peaks.
[00371 Specifically, in describing the coupled method embodiment of the
present
invention, the first method of analyzing data from a mass spectrometer for a
data-
dependent acquisition in real-time comprises first taking a series of mass
spectral scans
of a sample that has eluted from the liquid chromatography (LC) column 32, as
shown
in Fig. 1B. After eluting from the (LC) column 32, the sample may be ionized
by
electrospray ionization to put the liquid sample into an ionized gas phase. It
is to be
13
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
noted that while such ionization means are beneficial, other ionization
methods may
alternately be used, such as, atmospheric pressure chemical ionization,
particle beam
ionization, and thermospray ionization. After the sample is ionized, the
ionized sample
can be steered into an ion trap by using electrodynamic and electrostatic
forces as
known and understood by those of ordinary skill in the art. In an exemplary
embodiment, the ion trap is the mass analyzer of the mass spectrometer 24
shown in
Fig. 1B.
[00381 The amount of ionized sample within the ion trap can be manipulated for
each mass spectrum scan by using automatic gain control (AGC) (see for
example, U.S.
Pat. No. 5,107,109 titled "Method of Increasing The Dynamic Range And
Sensitivity Of
A Quadrupole Ion Trap Mass Spectrometer" by Stafford et al., and U.S. Pat. No.
5,572,022 titled "Method And Apparatus of Increasing Dynamic Range And
Sensitivity
Of A Mass Spectrometer" by Schwartz et al., the disclosures of which are
herein
incorporated by reference in their entirety.
[00391 In general, automatic gain control is a method whereby the rate of ion
flow
into the trap is measured by a prescan to determine the amount of time to fill
the ion
trap to contain the same amount of ions before each mass spectral microscan.
Several
mass spectral microscans may be taken before being averaged into a single mass
spectral scan. The frequency at which the average mass spectral scan is formed
is very
dependent on the specific instrument type and the operating mode. Typically,
instruments may take one scan per second, although there are some that are
capable of
up to 100 scans per second. The number of microscans acquired is user
selectable. A
standard Thermo Finnigan ion trap scans the full mass range 5-6 times per
second, with
14
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
most users operating with a single microscan. The choice of the number of
microscans
determines a trade-off between speed and spectral quality, i.e., the fewer the
number of
scans, the faster the speed, the greater the number of scans, the higher the
spectral
quality.
[0040] It is to be appreciated that while a tandem mass spectrometer with a
single
analyzer (known as tandem in time) is a beneficial arrangement with the
present
invention, other single stage analyzer systems capable of tandem mass
spectrometry are
also within the scope and spirit of the present invention, such as, for
example, a linear
ion trap (LIT), ion cyclotron resonance (ICR), an orbitrap or a Fourier
Transform Mass
Spectrometer (FTMS).
[0041] Moreover, the embodiments of the present invention can also be utilized
in a
tandem mass spectrometer with more than one analyzer (known as tandem in
space.)
For example, one mass analyzer can isolate one precursor from many precursors
entering a mass analyzer, after which the isolated precursor is collided with
a gas
within a collision cell causing fragmentation of the isolated precursor. A
second mass
analyzer then catalogs the fragments produced from the fragmented isolated
precursor.
Such a process is called collision-induced dissociation and is used for many
experiments
in proteomics. Multiple stage mass analyzers are utilized for such
applications, such as
a Quadrupole/ oa-time-of-flight (TOF), LIT-TOF, LIT-orbitrap, Quadrupole-ICR,
IT-
ICR, LIT-oa-TOF, or a LIT-orbitrap mass analyzer.
[0042] Fig. 2 shows a block diagram of the steps of the first method, as
disclosed
herein, applied to one or more mass spectral scans resulting from accumulated
precursor ions of an ionized sample within, for example, an ion trap. Once
mass spectra,
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
as shown in block 40, have been collected for a predetermined amount of time,
an
extracted ion chromatogram (XIC) is created, as shown in block 41, for each m/
z data
point within the mass spectrum. The optimal duration of the predetermined
amount of
time over which the mass spectra are collected to create such an XIC is
dependent on
the width of peaks eluting from the chromatograph. In a beneficial embodiment,
the
predetermined amount of time can be fixed or automatically adjusted based upon
observed chromatographic peak widths, such as, for example, between one-half
chromatographic peak widths and three chromatographic peak widths, as measured
at
half maximum height of the collected chromatographic peaks. To minimize
processing
time, two or more m/z points, or m/z points falling within defined ranges, can
be
combined before creating each XIC.
[0043] Fig. 3A illustrates an example of five mass spectra taken
(corresponding to
block 40 of Fig. 2) at different times (obtained at predetermined time frames
ti, t2, t3, t4,
and t5), which for purposes of explanation contain only three m/z data points
a, b, and
c. In each of the mass spectra scans illustrated in Fig. 3A, the ion counts
are plotted on
the y-axis and the m/z (mass to charge ratio) data points are plotted on the x-
axis. The
m/z data points are a measure of the mass (m) divided by charge (z) of each of
the ions
detected by, for example, a mass spectrometer having contents of an ion trap.
The m/ z
data points used for processing may be raw data or a copy of the raw data.
Using a
temporary copy of the raw data may allow for enhanced data-dependent
performance
without actually altering the data that is returned to the data system and
stored in the
memory of the controller 26, as shown in Fig. 1B.
16
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
[0044] The number of mass spectra used to form the extracted ion chromatograms
(as implemented in block 41, as shown in Fig. 2) and the number of m/z data
points
within the mass spectra may vary. The number of mass spectra used to form the
extracted ion chromatograms may be within the approximate range of 3 and 20,
and the
number of m/z data points may be up to about 1,000,000 in a mass spectrum,
often in
the range of about 5,000 up to about 1,000,000 in a mass spectrum. The wide
range of
m/ z data points that may be collected is due to the variation among different
instruments. For example, an ion trap instrument may acquire up to about
15,000 data
points and a Fourier transform instrument may acquire up to about 1,000,000
data
points.
[0045] Fig. 3B illustrates extracted ion chromatograms created for each of the
m/ z
data points (a, b, and c) within the mass spectra of Fig. 3A. The extracted
ion
chromatogram for each of the m/z data points is then correlated to a model
function,
such as, but not limited to, a monotonically increasing function, or a
gaussian function,
to obtain a XIC correlation value, as shown at block 42 of Fig. 2. The model
function is a
function (a set of time vs. intensity pairs) that matches the expected elution
profile of an
analyte from a chromatograph. The gaussian function works well for correlation
because the chromatographic peaks often have a gaussian shape, but any
monotonically
increasing function may be used. Other monotonically increasing functions that
may be
used include, but are not limited to, a Lorentzian function or a linear
function. Using a
gaussian function in the present example, the XIC correlation value r obtained
for the
m/z data point c at time point ts, as shown in the bottom left portion of Fig.
3B, is
approximately +1 because the XIC for c matches the front half of a gaussian
peak. The
17
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
correlation value r is a measure of how closely the XIC correlates to the
first half of a
gaussian function or a monotonically increasing function. The XIC correlation
value
may be any value between -1 and +1. A weighting function is then applied (as
shown in
block 43 of Fig. 2) to the most recent value of the XIC to obtain a current
weighted
intensity.
[0046] Fig. 3C illustrates such weighted extracted ion chromatograms for the
m/ z
data points a, b, and c, as shown in Fig. 3A. From such example weighted
chromatograms, the current weighted intensity for m/ z data point a is
approximately 0
because the XIC for a is neither increasing nor decreasing. The current
weighted
intensity for the m/z data point b is negative because the XIC for b is
decreasing and
the current weighted intensity for the m/z data point c is positive because
the XIC for c
is increasing
[0047] The weighting function provides a scaling factor to the raw intensities
to
reflect how well the XIC represents the expected elution profile. The
weighting function
may be the product of the XIC correlation value and the most recent time
point, the
product of a square of the XIC correlation value (while maintaining initial
sign) and the
most recent time point, or the most recent time point raised to the power of
an XIC
correlation value. The weighting function serves to emphasize the mass
spectral peaks
that occur at the actual apex of the chromatographic peak and to also prevent
triggering
on a tail of a chromatographic peak. This is because once the apex of a
chromatographic
peak has been passed, the XIC correlation value will be negative.
[0048] The weighting function also serves to improve the signal-to-noise in
real-time
because when the XIC correlation values are near zero, then it is likely that
signal is
18
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
primarily noise. The low intensity peaks may be picked out by this process
even when
eluting at the same time as a peak of much higher intensity or as a shoulder
of another
peak. With this method, it is also possible to differentiate eluting peaks
from one
another when there are many overlapping peaks eluting at the same time and to
identify peaks that are much smaller in comparison to other peaks eluting at
the same
time. This is because the intensity of each m/z data point is independently
monitored
over time, so even a peak hidden beneath another peak or within noise may be
reliably
identified. In this way, the correlation method may be used for identifying a
baseline of
m/z values, e.g., a set of identified ions. That is, identifying a set of ions
may include
fitting an extracted ion chromatogram taken from the mass spectrum to a
function that
approximates the front half of a chromatographic peak in preparation of
identifying a
subset of ions by the second method.
[0049] As an example embodiment, a threshold value may be applied to the XIC
correlation values to eliminate values below a certain threshold value. For
example, the
threshold value may be used to de-emphasize a very strong background peak that
is
not eluting (i.e., it is at a constant level) but may occasionally have a weak
correlation,
e.g., a correlation of approximately 0.1. The weighted intensity for this very
strong
background peak may still result in a strong signal. Restricting the
correlation value to
something such as greater than about 0.5 places a stronger emphasis on the XIC
elution
profile than on the very strong background peak. Additionally, a threshold
value may
be used because it is mathematically easier and faster to be able to disregard
all of the
values below the threshold value.
19
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
[0050] Although the above indicates that the correlation coefficient method is
the
technique used to compare the similarity between the XIC data points and the
model
function, to provide a value that represents how similar they are, the use of
the
correlation technique is only one aspect of the invention. Other techniques or
methods
of determining how well the XIC data points fit the model function may also be
utilized
to accomplish this aim. In the present application, the term "correlation" is
intended to
cover such alternative techniques.
[0051] Turning back to Fig. 2, at block 44, a weighted mass spectrum is then
reconstructed using the current weighted intensities for each m/z data point
to make a
real-time decision for the data-dependent acquisition.
[0052] Fig. 3D illustrates such a weighted mass spectrum based on XIC
correlation
values of the extracted ion chromatograms of Fig. 3B and 3C. The current
weighted
intensities for each of the different m/ z data points within the
reconstructed weighted
mass spectrum give an indication of whether the precursor ions from which the
m/ z
data points are derived are increasing, constant, or decreasing in intensity
and therefore
whether a chromatographic elution peak of the precursor ions exists and
whether it is
approaching or has already been passed. Whether the chromatographic elution
peak is
approaching or has already been passed is valuable information for determining
when
to make a data-dependent acquisition. The data-dependent acquisition may be,
for
example, the performance of tandem mass spectrometry, the collection of a
particular
isolated compound eluted from the liquid chromatography column, a diversion to
a
nuclear magnetic resonance (NMR) analysis, or the spotting of the compound
onto a
matrix assisted laser desorption and ionization (MALDI) plate.
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
[0053] Fig. 4 shows a flow chart illustrating a process capable of performing
data-
dependent acquisition near the apex of a chromatographic peak. In such a
process, the
method of analyzing data from a mass spectrometer for data-dependent
acquisition is
expanded upon after reconstructing the weighted mass spectrum, for example,
the
weighted mass spectrum shown in Fig. 3D. As shown in Fig. 4, at block 45, the
weighted intensity for each m/z data point in the current weighted mass
spectrum, for
example, the weighted mass spectrum shown of Fig. 3D, is compared to the
weighted
intensity for each m/z data point in a previous weighted mass spectrum.
[0054] At block 46, an m/z data point (precursor ion) can be selected if its
weighted
intensity has decreased in the current weighted mass spectrum from the
previous
weighted mass spectrum. In the embodiment where tandem mass spectrometry is
performed, the precursor ions corresponding to the selected weighted
intensities is
sampled at block 47 and tandem mass spectrometry is performed on the precursor
ion
near the apex of the chromatographic peak containing the precursor ion at
block 48.
[0055] Returning to the discussion involving Fig. 4, it is to be noted that a
simple
decrease in the weighted intensity of a particular m/z in the current weighted
mass
spectrum as compared to the previous weighted mass spectrum may not always
accurately identify the highest apex, such as when the decrease in intensity
corresponds
to a shoulder in the mass spectrum.
[0056] Accordingly, another beneficial technique for determining a triggering
time
and of which is beneficially combined with the correlation method discussed
above, is
set forth below. To illustrate principles of the present invention, the
discussion that
follows assumes that the apparatus 20, as shown in Fig. 1B, is being utilized
to
21
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
determine whether the sample 28 is a compound having a particular mass-to-
charge
ratio (m/ z) that coincides with an apex of the analyte of interest in the
mass spectrum.
[0057] In a method of operation, data from the first stage of mass
spectrometry is
supplied from the mass spectrometer 24 to the control system 26 either without
the
application of the correlation method or after application of the correlation
method so,
as provide a set of m/ z data of interest. The control system 26 uses this
data to generate
an extracted ion chromatogram (XIC) for the particular mass-to-charge ratio of
interest,
as has been described above. Generally, suitable techniques for generating an
XIC are
well-known in the art, and are therefore not further explained in detail here.
[0058] Fig. 5 shows a plot having a curve 51 that is part of an XIC for a
desired mass-
to-charge ratio that is the focus of the present example. As illustrated in
Fig. 5, the
horizontal axis represents time in minutes, and the vertical axis represents
ion counts, in
arbitrary units. According to an example aspect of the present invention, a
portion of
the data from the XIC curve 51 is capable of being converted from the time
domain into
the frequency domain. Reference numeral 53 (denoted with a double arrow and
dashed
lines) designates a sliding window that is designed to end with the most
recently
acquired data with a time window selected to have a length that is
approximately equal
to the expected width of a chosen elution peak, as measured at the base of
such an
elution peak.
[0059] With respect to transformation methods, the present invention is
designed to
use any suitable means, such as, but not limited to, a fast Fourier transform
method, a
wavelet transform method, a Hadamard transform method, a Hilbert transform
method, or a Laplace transform method to convert data from the time domain to
the
22
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
frequency domain. Often however, the present invention utilizes a discrete
Fourier
transform (DFT) method to do such a conversion. A problem can exist, however,
if the
data being converted using any of the desired methods discussed above, has
starting
and ending points with different values, then the transformation can
distribute power
into the frequency domain spectrum, which is undesirable. Therefore, before
effecting a
conversion from the time domain to the frequency domain, the data to be
transformed
is first adjusted or "rotated".
[0060] In particular, as shown in Fig. 5, assume that an imaginary line 56 is
drawn
from the starting point 57 to the ending point 58 of a selected data within
the sliding
window 53, up to and including the most recently acquired point. Then, within
the
sliding window 53, the value of each point on the line 56 is subtracted from
the
corresponding point on the curve 51. For example, the value at point 61 on the
line 56 is
subtracted from the value at the corresponding point 62 on the XIC curve 51,
thereby
yielding a value indicated at point 63. This procedure yields rotated data,
which is
represented in Fig. 5 by the curve 66 (now shown as a dashed curve). As shown
in Fig.
5, curve 66 now has starting and ending points 68 and 69 that have the same
value and
are both on the horizontal axis. Next, the rotated XIC data represented by the
curve 66
is transformed from the time domain into the frequency domain, often by using
standard DFT processing techniques.
[0061] Fig. 6 shows a graph having a curve 81 that represents the magnitude
mode
frequency domain data obtained by taking the time domain data from the curve
66 of
Fig. 5 and transforming it into the frequency domain. In the frequency domain,
high
and low frequency components can be separately identified. In the illustrated
example,
23
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
the source is unstable, as reflected by a relatively large magnitude at 82 for
the highest-
frequency component. For purposes of the disclosed technique, the component of
primary interest is a low-frequency component. The lowest or zero-frequency
component, represented by the point 83, is just a measure of the average
offset of the
data, and can be ignored. The next-lowest component, represented by the point
84, is
the "single cycle" frequency. This represents a signal that rotates through
one complete
cycle over the duration of the transformed time-domain data. This frequency is
of
primary interest for characterizing and identifying a chromatographic peak.
Similarly,
the raw data may be transformed from the time domain into the frequency
domain. In
either case, the frequency domain data includes information about the phase of
the
single-cycle frequency. Conceptually, a sine or cosine curve can be fitted to
the
frequency domain data for the single-cycle frequency. The phase of the single-
cycle
frequency is the phase of the point on the fitted sine or cosine curve that
corresponds to
the starting point of the single-cycle frequency data.
[0062] Fig. 7 shows a graph re-illustrating curve 51, as shown in Fig. 5. In
particular,
Fig. 5 shows only the left portion of the curve, now denoted by the reference
numeral
51' in Fig. 7. As discussed in association with Fig. 5, the curve 51' of Fig.
7, shown as a
solid line, is the XIC for the particular mass-to-charge ratio of interest,
and represents
time-domain data. Fig. 7 also depicts a further curve 101 (shown as a dashed
line) that
represents the variation over time of the phase (in radians) of the single-
cycle frequency
corresponding to the point 84 in Fig. 6. With reference to the vertical axis
on the right
side of Fig. 7, the phase varies between approximately 17 (3.1416) and -U (-
3.1416).
Although the curve 51' in Fig. 7 represents time domain data, the curve 101
represents
24
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
frequency domain data. The curve 51' shows there is a chromatographic peak at
a time
of approximately 10.9 minutes, and the curve 101 shows that, at this point in
time, the
phase has a value of approximately II/ 2.
[00631 With reference to the curve 101, it is noted that the phase data is
very noisy at
the start of the peak, or in other words before a time of approximately 10.7
minutes.
This is primarily because a low-frequency component is of low magnitude. With
significant magnitude, the phase varies smoothly from -II to II. At 11.1
minutes, there is
an apparent discontinuity in the phase 101, but this is actually the phase
wrapping
around from IT back down to -IT, rather than a true discontinuity. This phase
data can
be used to help identify a chromatographic peak. In particular, if the phase
has a
current value that is within a certain window, for example between about I71/4
and
about 3U/4, then the selected mass-to-charge ratio is eligible for data-
dependent
selection.
[0064] Turning back to Fig. 7, it is noted that there are several different
points in
time where the phase falls within the window of II/4 to 3I71/4. Consequently,
to
accurately identify a chromatographic peak, a further selection criterion is
used. To
illustrate such a criterion, Fig. 7 shows a user-selected threshold 107
(denoted with a
dashed line). In Fig. 7, the threshold 107 corresponds to an intensity of
approximately
23,000. However, this is purely by way of example, and a user can select the
threshold
107 to be either higher or lower. If the curve 51' is below the threshold,
then the phase
101 is ignored. In other words, if the curve 51' is below the threshold 107,
then the
system does not need to prepare the rotated data (curve 66 as shown in Fig.
5), and does
not need to convert this rotated data from the time domain to the frequency
domain to
2s
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
obtain phase data. On the other hand, when the curve 51' is above the
threshold 107,
then the system prepares the rotated data (curve 66 as shown in Fig. 5),
converts this
rotated data from the time domain to the frequency domain to obtain phase
data, and
then evaluates the phase data. Thus, in Fig. 7, the portion of the phase data
101 that is
actually calculated and taken into account is the portion within a time window
111
when the curve 51' is above the threshold 107. This has the beneficial aspect
that the
processor (26 as shown in Fig. 1B) does not waste time carrying out complex
calculations of data that will be ignored.
[0065] Proper selection of the length of the sliding time window (reference
numeral
53, as shown in Fig. 5) can improve the accuracy with which the foregoing
technique
identifies an elution peak. In the disclosed embodiment, the sliding window
53, as
shown in Fig. 5, as discussed above, is selected to have a length that is
approximately
equal to the expected width of the elution peak, as measured at the base of
the elution
peak.
[0066] Fig. 8 is a flowchart that summarizes the technique described above. In
particular, the processor 36, as shown in Fig. 1B, begins in block 401, and
proceeds to
block 402, where time domain data from the mass spectrometer 24, as shown in
Fig. 1B,
is used to generate XIC data. Control then proceeds to block 403, where a
determination
is made as to whether the current value of the XIC data is above a threshold.
If not, then
control returns to block 402, in order to continue to receive time domain data
and
calculate further XIC data for the mass-to-charge ratio of interest, or for a
range of mass-
to-charge ratios of interest. On the other hand, if it is determined in block
403, as shown
in Fig. 8, that the current XIC data is above the threshold, then control
proceeds to block
26
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
406, where a window of XIC data is rotated in the manner discussed earlier, in
order to
obtain rotated data such as that shown by reference numeral 66, as shown in
Fig. 5.
Control then proceeds to block 407, where a discrete Fourier transform is used
to
transform the rotated data from the time domain into the frequency domain, and
to
then identify the phase of a single-cycle component of the rotated data. In
the case of
the example discussed above, this corresponds to a point on the phase curve
101 in Fig.
7.
[0067] Control then proceeds to block 408, where a determination is made on
whether the current phase value is within a selected window or range, such as
11/4 to
3I1/4 radians, or 0 to 2 radians. If not, then control returns to block 402.
Otherwise,
control proceeds to block 411, where the system flags the identification of a
chromatographic peak, so that this can be subsequently used for processing
material of
a chromatographic output. For example, the accurate identification of a
chromatographic peak can be used to carry out a second mass analysis in tandem
mass
spectrometry. Alternatively, the accurate identification of a chromatographic
peak can
be saved, and then used to carry out mass spectrometry in a later scan of the
same sample.
[0068] In comparison to existing techniques, the technique(s) disclosed herein
is
beneficial for identifying the apex of a chromatographic peak, and is
beneficial for
eliminating problems caused by source instability. The disclosed
transformation
technique(s) to enable the phase information to be derived often, as discussed
above,
uses a discrete Fourier transform (DFT). Alternatively, however, the rotated
data can be
converted from the time domain to the frequency domain using any other
suitable type
27
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
of transform, including a fast Fourier transform (FFT). In the case of an FFT,
the FFT
expects input data in the form of a number of data points that are a power of
two. Since
the sliding window 53, as shown in Fig. 5, has a length that is selected based
on a time
criteria, rather than the amount of data, the number of data points associated
with the
sliding window is typically not automatically a power of two. In such a case,
the time-
domain data associated with the sliding window can be supplemented with
"dummy"
points up to the next power of two, where the dummy points represent a set of
values
that can maintain continuity between the beginning and end of the rotated
data. In the
case of an FFT, a further consideration is that the FFT normally expects data
points that
are spaced equally in time. The disclosed technique produces data points that
may not
be spaced equally in time. But in most cases, the unequal spacing of data
points do not
have a significant effect on the results.
[0069] The foregoing transformation discussion can thus often include two
selection
criteria for determining if the data is eligible for data dependent selection,
e.g., whether
the current value of XIC data is above a threshold, and then determining
whether the
current value of the phase for a single-cycle frequency is within a selected
phase
window. However, it is also possible to use other criteria, either in addition
to these
criteria, or in place of one or both of these two criteria. One such criterion
is monitoring
the trend of the phase information, for example by looking for three points in
a row
where the phase is progressively increasing. As another alternative, the
technique
described above contemplates that the real and imaginary components produced
by the
Fourier transform be combined. However, as another example arrangement, one
can
28
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
utilize either the real component or the imaginary component, without first
combining
the two.
[0070] Still another alternative selection criterion can be based on the
magnitude of
the single-cycle frequency component. For example, in Fig. 7, reference
numeral 431
designates a curve representing part of the frequency domain data obtained
with the
Fourier transform. More specifically, the curve 431 represents the variation
over time of
the magnitude of the single-cycle component in the frequency domain. Instead
of
comparing the XIC curve 51' to the threshold 107 in order to determine whether
or not
to consider phase data, the magnitude of the curve 431, as shown in Fig. 7,
can be
compared to an appropriate threshold in order to determine whether or not to
consider
phase data.
[0071] Another example selection criterion involves use of the rotation angle
of the
rotated data. For example, with reference to Fig. 5, if the portion of the XIC
curve 51
that is to be rotated has a starting point, as denoted by reference numeral
57, with a
value lower than the value of the end point 58, then rotation followed by
Fourier
transformation is carried out. In contrast, if the starting point 57 had a
value higher than
the value of the end point 58, then rotation and Fourier transformation is not
carried
out. This rotation angle criterion can, for example, be used in combination
with the
above-mentioned phase criterion, and the above-mentioned threshold criterion
for the
curve 431 as shown in Fig. 7.
[0072] Further, as discussed above in association with Fig. 5, the XIC curve
51 is
generated using known techniques. These known techniques typically involve
"binning" of mass-to-charge ratios detected by the mass spectrometer. For the
purpose
29
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
of the techniques described herein, binning is typically done at unit
resolution, i.e., with
bin widths of 1 m/ z per bin. However, the present invention can be arranged
to use
wider bins, and such a technique is still effective in spotting eluting peaks
despite the
frequency data obtained being slightly noisier. But where this noise is
tolerated, then
initial binning can occur using a wider width such as 5 m/ z per bin. Then,
any of the
wider bins that met all selection criteria can be further analyzed at a higher
binning
resolution such as 1 m/ z per bin, in order to more accurately identify a mass
of interest.
For instruments with high resolution capabilities, such as FTICR and orbitrap,
narrower
bins can be used to assist in separating distinct masses. A further factor
that can
influence bin width is the particular type of sample material 28, as shown in
Fig. 1B,
that is being analyzed. For example, samples with more complex mixtures might
require finer binning than relatively simple samples.
[0073] As described above, the first and second method may be combined. For
example, once a set of m/ z data has been identified such as by a correlation
method,
narrowing in on a subset of m/ z data of interest may include identifying data
corresponding to a low frequency portion of the corresponding XIC's. As an
alternate
arrangement, the combination of the first and second methods may include
narrowing
in on the subset of m/z data of interest by identifying data corresponding to
a single
cycle component of the low frequency portion of the XIC's. Selecting data
based on this
method results in acquiring data at a time corresponding to elution of at
least one m/ z
peak in a subset of data left after initial baseline filtering or thresh-
holding.
[0074] The identifying of a set of m/z values and the selecting of a subset of
m/z
values may be executed under instructions stored on a machine-readable medium
(e.g.,
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
a computer readable medium) coupled to a sample processing apparatus. A
computer-
readable medium, in accordance with aspects of the present invention, refers
to
mediums known and understood by those of ordinary skill in the art, which have
encoded information provided in a form that can be read (i.e., scanned/sensed)
by a
machine/ computer and interpreted by the machine's/computer's hardware and/or
software. When, for example, mass spectra data of a mass spectrum is received
by the
apparatus disclosed herein, the information embedded in a computer program of
the
present invention can be utilized, for example, to extract data (e.g., a
weighted mass
spectrum) from the mass spectral data, which corresponds to a selected set of
mass-to-
charge ratios. In addition, the information embedded in a computer program of
the
present invention can be utilized to carry out methods for identification of
an elution
peak in a manner that includes determining whether phase information in the
frequency domain data meets a phase criterion. The computer program may also
identify, as a function of the frequency domain data, one or more elution
peaks of m/z
of interest. The computer program, when executed, may carry out the extraction
in a
manner so that the resulting data represents a period of time approximately
equal to the
expected width of the elution peak. The width of the elution peak may be
measured in
the region of the base thereof for this purpose. When executed, the computer
program
may also cause processing of material generated from a chromatographic output
as a
function of an identified elution peak.
[0075] It is to be understood that features described with regard to the
various
embodiments herein may be mixed and matched in any combination without
departing
from the spirit and scope of the invention. Although different selected
embodiments
31
CA 02763261 2011-11-23
WO 2010/138120 PCT/US2009/045348
have been illustrated and described in detail, it is to be appreciated that
they are
exemplary, and that a variety of substitutions and alterations are possible
without
departing from the spirit and scope of the present invention, as defined by
the
following claims.
32