Note: Descriptions are shown in the official language in which they were submitted.
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
ADSORPTION-ENHANCED COMPRESSED AIR ENERGY STORAGE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This patent application is a continuation-in-part of International
Application No. PCT/US2009/001656 filed March 16, 2009, which claims the
benefit of
U.S. Provisional Application No. Serial No. 61/036,587, filed on March 14,
2008. This
patent application claims the benefit of U.S. Provisional Application Serial
No.
61/161,492 filed on May 27, 2009, U. S. Provisional Application Serial No.
61/225,399
tiled on July 14, 2009 and U.S. Provisional Application Serial No. 61/248,057
filed on
October 2, 2009, by Timothy F'. Havel. The contents of each of these
applications being
incorporated herein by reference in their entireties.
BACKGROUND
1. Technical Field
[0002] The present disclosure relates to the field of energy storage. In
particular, the present disclosure is directed to an energy storage device
that includes a
pressure chamber containing a porous material that adsorbs air.
2. Description of the Related Art
[0003] Compressed air energy storage is commonly known by its acronym
"CAES." In some CAES devices, the air compressor is driven by an electric
motor, and
subsequently used to drive an air motor or turbine connected to an
electromagnetic
generator, thereby forming the functional equivalent of an electrochemical
battery. If the
charge-discharge cycle is carried out slowly enough to be approximately
isothermal,
meaning that the heat generated by compression dissipates without raising the
temperature of the air appreciably during compression, and the heat drawn in
from the
environment likewise keeps the air from cooling appreciably during expansion,
this form
of electricity storage can have good efficiency.
[0004] CAES systems can also be engineered to have higher reliability, lower
maintenance and longer operating lifetimes than chemical batteries, and their
cost can
1
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
be comparable to battery-based systems providing that an inexpensive means of
storing
the compressed air is available. Unfortunately, the high cost, weight and
large size of
manufactured pressure vessels in which to store the air, such as steel tanks,
prevents
ORES devices from competing with batteries in all of their usual applications.
[0005] To date CAES has been used for three commercial purposes. The first
and most widespread use is not as a means of energy storage per se, but to
power
pneumatic tools and machines in shops and factories. Pneumatic tools have
higher
weight-to-power ratios than electrically powered tools, and the small electric
motors in
such tools also tend to be inefficient compared to the larger motors that
drive air
compressors. The compressed air is stored in a tank big enough to serve as a
buffer
and ensure that the pressure in the system stays constant. The overall
efficiency of
these systems is limited by the fact that they discard the heat of compression
and do
not reheat the air during its rapid expansion. This inefficiency is limited by
using modest
pressures, usually less than ten atmospheres, which also reduces the capital
costs of
such ORES systems.
[0006] The second use of ORES is for temporary backup power to keep
essential machinery running in the event of a power failure, for example in
computer
data centers or hospitals. In such cases floor space is at a premium,
necessitating the
use of pressures of a hundred or more atmospheres to attain a relatively high
energy
density, but the cost of the high-pressure steel storage tanks for the
compressed air is
justified by the high reliability of the system and the high power it can
immediately
deliver in the event of a power failure, Subsequently a longer-term backup
system like
a diesel generator can be brought online if need be. Although the same
functionality
could be obtained from electrochemical batteries, a battery system that could
deliver
enough power would also have to store more energy than was needed while
waiting for
the long-term backup system to come online, making batteries a relatively
expensive
solution. A ORES system also requires less maintenance, has a longer lifetime,
and
does not have the disposal costs associated with environmentally hazardous
chemicals.
Other such short-term backup power solutions include supercapacitors and
flywheels,
which are likewise relatively costly.
[0007] The third commercial use to which CAES has been put is to lower the
cost of generating and/or distributing electric power by utility companies.
This can be
2
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
done in several ways, the most common of which is to enhance central
generation
capacity. Large central power plants such as coal and nuclear are expensive to
stop
and start, while smaller plants such as gas-fired turbines are readily turned
off and on
but are comparatively expensive to operate. Hence, if the energy from large
plants can
be stored when demand is low and used to produce electricity when demand is
high,
the need to install and operate small peak-load plants can be reduced, thereby
also
reducing the average or "levelized" cost of producing electricity.
SUMMARY OF THE INVENTION
[0008] In an embodiment of the present disclosure, an energy storage device
is presented. The energy storage device includes a porous material that
adsorbs air
and a compressor. The compressor converts mechanical energy into pressurized
air
and heat, and the pressurized air is cooled and adsorbed by the porous
material. The
energy storage device also includes a tank used to store the pressurized and
adsorbed
air and a motor. The motor is driven to recover the energy stored as
compressed and
adsorbed air by allowing the air to desorb and expand while driving the motor.
[0009] In another embodiment of the present disclosure, another energy
storage device is presented. The energy storage device includes a porous
material,
where a suitable fluid has been adsorbed. The device also includes a
compressor that
converts mechanical energy into pressurized air and heat and a barrier. The
pressurized air is cooled by allowing the heat to flow through the barrier,
the heat is
transported to the porous material to which a fluid has been adsorbed, and the
heat
raises the temperature of the porous material, causing the fluid to desorb
from it. The
heat is recovered, and used to keep the temperature of the expanding air from
failing
and lowering the work done while driving a motor, by allowing the fluid to re-
adsorb to
the porous material.
[0010] In yet another embodiment, another energy storage device is
presented. The energy storage device includes a porous material that adsorbs
air and
a thermal energy storage system that stores heat. The device further includes
a
compressor that converts mechanical energy into pressurized air and heat. The
pressurized air is cooled and adsorbed by the porous material and the
temperature of
the porous material and surrounding air is controlled by allowing the heat to
flow
3
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
through a barrier that prevents the pressurized and adsorbed air from
escaping. The
heat is directed to the thermal energy system and is stored there. Further,
the device
includes a tank that stores the pressurized and adsorbed air, and the energy
it contains
is recovered when needed by directing the heat stored in the thermal energy
storage
system back through the barrier, causing the air to desorb, and allowing it to
expand
and do work in the process.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] An exemplary embodiment and related extrapolated experimental data
are illustrated in Figures 1 through 11. A second exemplary embodiment and
additional
extrapolations of experimental data are illustrated in Figures 12 through 23.
[0012] Figure 1 plots adsorption isotherms for the principal constituents of
air
on the zeollte NaX;
[0013] Figure 2 plots the ratio of the number of nitrogen to the number of
oxygen molecules versus nitrogen pressure where the ratio of nitrogen to
oxygen
pressures has a fixed value of 4,0;
[0014] Figure 3 is a schematic diagram of mass and energy flow in an
adsorption-enhanced compressed air energy storage embodiment, showing these
flows
during the first half of the charging process;
[0015] Figure 4 is a schematic diagram of mass and energy flow in an
adsorption-enhanced compressed air energy storage embodiment, showing these
flows
during the second half of the charging process;
[0016] Figure 5 is a schematic diagram of mass and energy flow in an
adsorption-enhanced compressed air energy storage embodiment, showing these
flows
during the first half of the discharging process;
[0017] Figure 6 is a schematic diagram of mass and energy flow in an
adsorption-enhanced compressed air energy storage embodiment, showing these
flows
during the second half of the discharging process;
[0018] Figure 7 is a process flow diagram which illustrates in greater detail
how an adsorption-enhanced compressed air energy storage embodiment operates
during the first half of the charging process;
4
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
[0019] Figure 8 is a process flow diagram which illustrates in greater detail
how an adsorption-enhanced compressed air energy storage embodiment operates
during the second half of the discharging process.
[0020] Figure 9 is a three-dimensional drawing of an array of air adsorption
cylinders in a temperature-control chamber;
[0021] Figure 10 is a three-dimensional drawing of the adsorption heat pump
that is primed and used to upgrade stored heat during the first half of the
charging and
second half of the discharging processes, respectively;
[0022] Figure 11 is a three-dimensional drawing of the mixer-elector air
turbine used to recover the energy stored as compressed airõ adsorbed air, and
heat
during the discharging process;
[0023] Figure 12 plots the adsorption isotherms for air on the zeolite NaX at
four different temperatures, which were extrapolated from the published data;
[0024] Figure 13 plots the density with which a bed of NaX pellets is expected
to store energy, based on the isotherms of Fig. 12 over a ---40-to-100 C
temperature
swing as a function of the fixed working pressure;
[0025] Figure 14 depicts the four legs of the storage cycle of a second
adsorption-enhanced compressed air energy storage embodiment, along with the
flows
of heat among the principal thermal reservoirs of the embodiment;
[0026] Figure 15 is a simplified process flow diagram illustrating the mass
and
energy flows in the second adsorption-enhanced compressed air energy storage
embodiment during the first leg of the storage cycle (or first half of the
charging
process);
[0027] Figure 16 is a simplified process flow diagram illustrating the mass
and
energy flows in the second adsorption-enhanced compressed air energy storage
embodiment during the second leg of the storage cycle (or second half of the
charging
process);
[0028] Figure 17 is a simplified process flow diagram illustrating the mass
and
energy flows in the second adsorption-enhanced compressed air energy storage
embodiment during the third leg of the storage cycle (or first half of the
discharging
process);
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
[0029] Figure 18 is a simplified process flow diagram illustrating the mass
and
energy flows in the second adsorption-enhanced compressed air energy storage
embodiment during the fourth leg of the storage cycle (or second half of the
discharging
process);
[0030] Figure 19 Is a detailed process flow diagram which shows the internal
structures of the key subsystems of the second adsorption-enhanced compressed
air
energy storage embodiment and mass flows among them during the first leg of
the
storage cycle;
[0031] Figure 20 Is a detailed process flow diagram which shows the internal
structures of the key subsystems of the second adsorption-enhanced compressed
air
energy storage embodiment and mass flows among them during the second leg of
the
storage cycle;
[0032] Figure 21 Is a detailed process flow diagram which shows the internal
structures of the key subsystems of the second adsorption-enhanced compressed
air
energy storage embodiment and mass flows among them during the third leg of
the
storage cycle;
[0033] Figure 22 Is a detailed process flow diagram which shows the internal
structures of the key subsystems of the second adsorption-enhanced compressed
air
energy storage embodiment and mass flows among them during the fourth leg of
the
storage cycle; and
[0034] Figure 23 depicts the pressure-volume diagram of an alternative
storage cycle in which some external heat is captured by heating the fully
charged NaX
bed at constant volume prior to expansion, thereby compensating for the energy
losses
in a three-stage adiabatic compression and expansion process where each stage
is
followed by isobaric cooling and heating, respectively.
DETAILED DESCRIPTION OF THE INVENTION
[0035] The present disclosure provides uses for the physical process of
adsorption in porous materials, which greatly improve the economics of
compressed air
energy storage (CAES). Further the present disclosure provides several
improvements
to devices that store energy in the form of compressed air, and that may also
store
some of the energy in the form of sensible or latent heat.
6
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
[0036] In order to make the use of CAES for central generation capacity cost
effective, the compressed air is presently stored in underground geological
reservoirs
such as natural aquifers or man-made depleted gas or oil wells, rather than in
manufactured tanks. The economics is further improved by using the compressed
air to
turbo-charge a gas-fired turbine, thereby saving the turbine from having to
expend
energy compressing the air itself. This allows the energy stored in the
compressed air
to be recovered while at the same time generating additional energy from
natural gas.
Although the pressures required for turbo-charging are fairly high, of order
50 or so
atmospheres, turbocharging allows the stored energy to be delivered at a high
power
level and recovered with an overall efficiency of about 70%.
[0037] A somewhat different approach to using CAES for utility purposes,
which has yet to be commercially deployed, is known as "advanced adiabatic
CAES."
In AA-CAE S, the heat extracted from the air during compression is stored and
used to
reheat the air during expansion as it powers an air motor or turbine. In
principle, this
allows both the energy stored as heat and stored as compressed air to be
recovered, so
the efficiency of. -CAES can approach 100% in principle. In practice, it is
difficult to
store and recover the heat of compression without significant losses
especially at high
power levels. In all the proposed embodiments of M-CAES to date, the air is
again to
be stored in underground reservoirs at high pressure, and the heat is to be
stored in
sensible rather than latent form, usually at temperatures well above 200 C.
[0038] Energy storage has the potential to reduce the operating costs of
electric utilities in several other ways as well, although none have yet come
into
widespread use. These include transmission capacity deferral and congestion
reduction, various ancillary services, bulk electricity price arbitrage, and
load shifting or
leveling at the end-user level. In the future, however, the most valuable use
of energy
storage is likely to be renewable capacity firming. Renewable energy sources
such as
wind and soar tend to be intermittent, so that their capacity varies in time
and is often
not sufficient to satisfy the demand for electricity. If the energy can be
stored at times
when capacity exceeds demand and used to produce electricity when demand
exceeds
capacity, these renewable energy sources will become much more cost effective.
[0039] The main drawback of existing CAES systems in any of the foregoing
applications is that suitable underground reservoirs are neither common nor
7
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
transportable. A modular system that could be assembled anywhere and scaled to
the
size of the power plant there would, if cost effective, be much more useful
for central
generation capacity as well as renewable capacity firming. In addition, if it
were
possible to deliver inexpensive, self-contained CAES systems to well-chosen
locations
on the grid, nearer to substations or end users, CAES could provide some or
all of the
other cost reduction services mentioned above. The main reason that such CAES
systems are not presently cost-effective is, once again, the high cost of
manufactured
storage tanks for compressed air. It should be noted that, to a first-order
approximation,
the cost of the tank is independent of the pressure at which the air is
stored, since
raising the pressure allows the tank to be made smaller but requires its walls
to become
proportionately thicker, and vice versa.
[0040] One approach to making CAES systems more economical, which has
not received much attention, is to take advantage of the fact that the
compression and
expansion of air is a facile means of pumping heat from one place to another.
This
means that a CAES system could easily be developed to provide combined heat,
cooling and power to end users. If such a CAES system were installed in a home
or
business where time of day electricity pricing is available, for example, it
could be
charged during the night when the electricity is relatively inexpensive while
simultaneously providing heat to the building, and the electricity it produced
used or sold
back to the grid during peak daytime hours while also providing air
conditioning. During
the winter, when the cooling was not needed, a flat-plate solar collector
could be used
to heat water, and this hot water used to provide heat for the air during
expansion,
increasing the power output significantly with only a modest increase in cost.
The
economics of such a system would depend on many factors including the utility
tariffs,
the prevailing climate, and of course the cost of the air storage tank.
[0041] The storage of gases and of heat can be accomplished by adsorption
in suitable porous materials such as activated carbon, silica gel or zeolites.
Gases are
more easily stored in the presence of such a material because the adsorbed
phase is
much denser than the free gas, thus reducing the volume of the tank required
to store a
given mass of the gas at a given pressure, or equivalently the pressure
required at a
given volume. In addition, heat may be stored in latent form using adsorbent
materials
because the process of desorption consumes heat. The heat may subsequently be
8
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
regenerated by allowing the adsorbate (e.g. water vapor) to be re-adsorbed by
the
adsorbent. Additionally, the heat released upon condensation of the desorbed
vapor
may be stored in sensible form, and recovered by using it to promote the
evaporation of
the condensate and then allowing the resulting vapor to re-adsorb. Such a
device can
include an adsorption refrigerator or heat pump. Nevertheless there have been
no
attempts to use the process of adsorption in any of these ways to make CAES
systems
less expensive, more efficient or transportable, better suited to combined
heat-and-
power applications, and / or safer to deploy.
[0042] The present disclosure improves upon the economics of compressed
air energy storage in four interrelated ways. The first is the use of an
adsorbent for air in
order to reduce the pressure in and/or volume of the vessel needed to store a
given
quantity of energy in the form of compressed air. The second is the desorption
of water
or some other suitable fluid, possibly combined with storage of the low-grade
sensible
heat released upon condensation of the vapor thereby produced, as a means of
storing
the heat of compression so as to make AA-CAES more economical. The third is to
store
the heat generated by adsorption of the air, possibly along with the heat of
compression, and to recover this energy at a later time by using it to raise
the
temperature of the adsorbent material and/or the compressed air as it expands.
The
fourth is a new thermodynamic cycle for CAES, in which the temperature of the
compressed air is varied so as to keep the pressure of the stored air
approximately
constant over the charge/discharge cycle. This "temperature-swing" cycle is
especially
advantageous when an adsorbent for air is utilized, as just described, and it
is also
applicable when the heat of compression and/or adsorption is stored for
subsequent
use, for example by means of an adsorbent for water or some other suitable
fluid. The
use of a temperature-swing cycle in adsorption-based gas separation processes
is well
established (see, for example, USPTO Pub. No. 2006/0230930).
[00431 It should be noted that energy can be stored by compressing gases
other than air, and that a regenerative braking system has been proposed that
utilizes
adsorbent materials to enhance this process (see, for example, US Patent No.
7,152,932). This has the advantage that other gases may be more compressible
and
also more strongly taken up by common adsorbents than is air, allowing energy
to be
stored more densely than could be done when using air as the working fluid.
The main
9
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
difference between this kind of system and those under consideration here is
that the
use of any fluid other than air necessitates a closed system in which the
fluid can be
recycled and reused. In contrast, air can be taken freely from the environment
and
released again without environmental consequences. This leads to an open
system
which is much more economical for large-scale energy storage at the end user,
electric
substation or power plant level. The present disclosure describes the use of
adsorbents
for air in large-scale, stationary energy storage applications, the desorption
of water or
some other suitable fluid as a means of storing the heat of compression and 1
or
adsorption of the air, and CAES systems that use a temperature-swing cycle.
None of
these processes are suitable for small-scale, mobile applications such as
regenerative
braking.
[0044] Although several kinds of porous materials are known that adsorb the
nitrogen and oxygen constituents of air to some degree, an adsorption-enhanced
CAES
embodiment of the present invention utilizes a zeolite material for this
purpose. At
modest pressures and ambient temperatures, zeolites adsorb nitrogen more
strongly
than oxygen, and so have been extensively utilized to separate the oxygen and
nitrogen
constituents of air for industrial and medical purposes. Nevertheless, there
have been
few detailed studies of the adsorption of air to zeolites or other porous
materials at the
relatively high pressures of interest for CAES. For example, the temperature-
pressure
boundary at which the air in zeolites liquefies has not been mapped out in any
detail.
This process, also called capillary condensation, is not normally observed at
temperatures well above the critical point of the adsorbate gas, or about -
140CC in the
case of air. Such a low temperature would be difficult to achieve in a cost-
effective
adsorption-enhanced CAES device.
[0045] Thus a new use of adsorption in porous materials provided by the
present disclosure is as a means of reducing the volume of the tank needed to
store a
given mass of air at a given pressure and temperature, or alternatively, of
reducing the
thickness of the walls of the tank or the strength of the materials of which
it is made, by
reducing the pressure needed to store a given mass of air in a given volume
and at a
given temperature. Either of these two alternatives may be achieved by placing
a
suitable porous material inside the pressure chamber that holds the compressed
air,
where said porous material adsorbs a greater volume of air than the material
itself
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
occupies at the temperature and pressure of the compressed air in the chamber.
Such
porous materials exist by virtue of the fact that, at equilibrium with the
temperature and
pressure fixed at suitable values, air molecules in an adsorbed state have
greatly
reduced mobility and a much higher density than those in the gaseous air
around them.
[0046] Likewise, another new use of adsorption in porous materials is as a
means of storing the heat generated by the process of compressing the air,
and/or the
heat generated by the process of adsorption of the air as In the first new use
above.
This second new use is achieved by placing a porous material to which water or
some
other suitable fluid is adsorbed in thermal contact with, but outside of, the
air
compressor and/or pressure chamber. The porous material of the second new use
need not be the same kind of material as that of the first new use. The heat
increases
the temperature of this porous material and so promotes desorption of the
water or
other fluid from it. At the molecular level, this process converts kinetic
energy into
potential energy, which may then be stored indefinitely by preventing the
vapor
produced by desorption from coming back into contact with the porous material
and
being re-adsorbed. This may be described by saying that the heat has been
stored in
latent form. The transfer of heat from the compressed air to the porous
material of the
second new use reduces the temperature of the compressed air, thereby also
reducing
the work needed to further compress it, as well as the size or strength of the
tank in
which it is stored. Similarly, the cooling of the porous material of the first
new use, which
is concomitant upon transferring the heat of adsorption from it, increases the
amount of
air that it adsorbs at any given pressure.
[0047] In order to recover the stored latent heat in sensible form, the vapor
produced by desorption of the fluid must be available for re-adsorption when
needed.
Unfortunately, the large volume occupied by the vapor makes it difficult to
store in that
form, and compressing or condensing it releases a smaller but still
significant amount
energy in the form of sensible heat. It is nevertheless possible to store this
sensible
heat, and to subsequently use the process of expansion of the vapor or
evaporation of
the liquid to harvest this heat and so regenerate the vapor. The advantage of
doing
this, instead of storing the heat generated by compression and / or adsorption
of the air
directly in sensible form, lies in the fact that in the former case the
sensible heat is
contained in a material at a lower temperature that can be more easily
insulated against
11
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
losses. While such low-grade heat is normally difficult to harvest, i.e. to
convey to
where it is needed, the process of expansion or evaporation serves to
refrigerate this
material and so pump the heat from it much more rapidly and efficiently than
could
otherwise be done. This could also, in principle, be done directly by using
the
compressed air as a refrigerant, but it is difficult to both transfer large
quantities of low-
grade heat from a solid or liquid material into the expanding air and at the
same time to
capture the mechanical energy generated. It also takes energy to convert low-
grade
heat to the high-grade heat needed to facilitate the rapid expansion and / or
promote
desorption of the air.
[0048] Regardless of how the vapor needed is obtained, the latent heat may
be recovered, along with the energy stored as compressed and / or adsorbed
air, in
mechanical form by placing the porous material of the second new use in
thermal
contact with the air motor or turbine and at the same time allowing the water
or other
fluid vapor to re-adsorb to it. The sensible heat generated as the water or
other fluid re-
adsorbs is conducted or otherwise transferred to the compressed air as it
expands in
the air motor or turbine, raising its temperature and pressure so that it does
more useful
work. At the same time this transfer of heat cools the porous material of the
second
new use and so further promotes the spontaneous re-adsorption of water or some
other
suitable fluid to it. Similarly, the transfer of heat from this porous
material to the porous
material of the first new use promotes the desorption of air from it at the
pressure in the
chamber, and this compressed air may then be converted back to mechanical
energy
via the air motor or turbine as just described.
[0049] When porous materials are incorporated into a CAES device for either
of these two new uses, we shall refer to the resulting process as adsorption-
enhanced
CAES, or AE-CAES, and to the energy storage device itself as an AE-CAES device
or
AE-CAES system.
[0050] This disclosure further provides a new use for the industrial process
of
temperature-swing adsorption, which has been widely employed as a means of
separating mixtures of fluids. In this process, the temperature of the air and
of the
porous material to which air is adsorbed is lowered when charging the CAES
device
with energy, and raised again when discharging it, all the while pumping air
in or
12
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
allowing air to escape from the pressure chamber at a rate that keeps the
pressure of
the compressed air therein approximately constant.
[0051] A constant air pressure will simplify the construction and operation of
any CAES device, but more important for the purposes of the present invention
is the
fact that the temperature-swing process is a convenient means of increasing
the
amount of air stored and released by any given quantity of porous material as
in the first
new use. It does this because the quantity of a gas adsorbed by the vast
majority of
known porous materials decreases rapidly as the temperature thereof is raised,
and
vice versa. It follows that if the minimum temperature, attained when the AE-
CAES
device is in its charged state, is low enough to ensure that the porous
material is largely
saturated by air at the working pressure of the device, while the maximum
temperature,
attained when the AE-CAES device is in its discharged state, is high enough to
ensure
that most of the air is desorbed from the material at the working pressure of
the device,
then one will obtain a greater benefit from the chosen porous material of the
first new
use than if a pressure-swing cycle had been utilized, at least without the
costly and
energy consuming expedient of going to subatmospheric pressures. This includes
a
pressure-swing cycle with either a constant temperature, or with the
spontaneous
temperature variation of the pressure-swing cycle which reaches its minimum
temperature in the discharged state and its maximum in the charged state.
[0052] For each of the two new uses of the physical process of adsorption
given above, a variety of porous materials are available by which useful
embodiments of
the invention may be constructed. In an AE-CAES embodiment that will now be
described in detail, the first new use is implemented by a zeolite known as
NaX. This is
a widely available Faujasite-type zeolite containing sodium ions, which is
commonly
sold under the commercial name of 13X.
[0053] Dry air is about 78% nitrogen, 21 % oxygen and 1 % argon by mole
fraction. Like most naturally and/or commercially available zeolites, NaX
adsorbs
nitrogen more strongly than oxygen or argon, i.e. on a molar basis it adsorbs
more
nitrogen than oxygen or argon when placed under these pure gases at a given
pressure
and temperature - at least at the relatively low pressures usually considered
for the
purpose of purifying oxygen or nitrogen. Furthermore, oxygen and argon are
largely
adsorbed at chemically identical sites on the NaX pore walls and also have
similar
13
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
adsorption isotherms, while nitrogen is largely adsorbed at distinct sites
which do not
overlap with those of oxygen and argon. Because of these facts, we may
simplify our
analysis by treating the argon fraction of air as if it were oxygen in the
following without
making any errors large enough to invalidate the principles that an AE CAES
embodiment is intended to exemplify. Furthermore, the above observations
together
with experimental data presented by F. A. Ustinov (Russ. J. Chem. 81, 246,
2007) show
that we may assume that the amount of nitrogen adsorbed is independent of the
amount of oxygen (and argon) adsorbed, and vice versa.
[0054] Complete isotherms for nitrogen, oxygen (and argon) adsorption to
NaX have been measured at pressures of up to about 4 atmospheres and at four
widely
separated temperatures between -70 and 50*G (see G. W. Miller, AlChE Syrup.
Ser.
83, 28, 1987). The values of the parameters in the Sips and Langmuir isotherm
equations, as determined by fitting these data, were also given in that paper,
and may
be used to extrapolate these measurements to higher pressures.
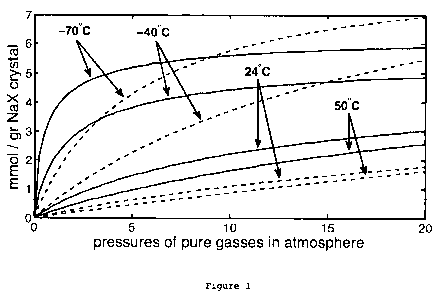
[0055] Figure 1 plots adsorption isotherms for the principal constituents of
air,
namely nitrogen and oxygen, with the commercially available zeolite widely
known as
NaX or 13X, at four different temperatures and at pressures of up to 20
atmospheres.
The isotherms for nitrogen, obtained from the Sips isotherm formula, are
plotted with
soled lines, while those for oxygen are obtained from the Langmuir isotherm, a
special
case of the Sips, and are plotted with dashed lines. The plots shown thus
extrapolate
Millers data to the higher pressures needed for a cost-effective adsorption-
enhanced
compressed air energy storage device,
[0056] Figure 2 plots the ratio of the number of nitrogen molecules to the
number of oxygen molecules adsorbed to NaX against pressure at the same four
temperatures as in Figure 1, where the pressure of oxygen at each point on the
plot is
25% that of nitrogen and hence approximately equal to the partial pressure of
oxygen in
air at 125% of the nitrogen pressure. These ratios are calculated using the
extrapolated
isotherms shown in Figure 1. The dashed horizontal line shows where this ratio
has the
value 4.0, so that the ratio adsorbed is approximately equal to the ratio of
the partial
pressures of nitrogen and oxygen in air. The corresponding pressure at a
temperature
of -40 C, indicated by the dashed vertical line, is expected to be a
reasonably cost-
effective nitrogen partial pressure for an embodiment of adsorption-enhanced
14
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
compressed air energy storage based on a temperature-swing cycle with a
minimum
temperature of ---40 C. This is because going to higher pressures or lower
temperatures
would increase the amount of air adsorbed at a lower rate than had been
achieved at
lower pressures and higher temperatures, so that the cost-benefit ratio
obtained from
the use of the NaX adsorbent would become less favorable.
[0057] Figures 3 through 8 show schematic diagrams of the complete AE-
CAES (adsorption-enhanced compressed air energy storage) embodiment. These
diagrams are graphic versions of the well-known process flow diagrams and the
associated symbols for the common mechanical, fluidic and electrical
components of
chemical and materials processing systems, which are widely used by the
engineering
community. Process flow diagrams are not intended as blue-prints for a
specific design,
but rather to allow one skilled in the art of chemical and materials
processing to design
a system that can reproduce a specific process using such standard components.
The
diagrams thus provide a suitable means of describing the invention, which
provides
processes by which CAES systems may be enhanced using adsorption in porous
materials, rather than a specific device or design. In those parts of the
embodiment in
which the components employed are not perfectly standard, more detailed
drawings are
given, and these have been enlarged in Figures 9 through 11.
[0058] Figures 3 through 6 give high-level views of the principal mass and
energy fluxes through an exemplary embodiment of an AE-CAES system at four
points
in its charge - discharge cycle. Figure 3 shows these fluxes at the beginning
of the
charging process, when the pressurized NaX bed 1 is near 100 C and so has the
minimum quantity of air adsorbed to it, while the unpressurized NaX bed 41 is
largely
saturated with water. Figure 4 shows how the fluxes are altered about halfway
through
the charging process, when the temperature of the pressurized NaX bed I has
fallen to
the prevailing ambient air temperature and the unpressurized NaX bed 41 is has
lost
most of its water. Figure 5 shows the fluxes at the beginning of the
discharging
process, when the pressurized NaX bed 1 is at 0 C and so has the maximum
amount
of air adsorbed to it, while the unpressurized NaX bed 41 is still hot and
dry. Figure 6
shows how these fluxes are altered about halfway through the discharging
process,
when the temperature of the pressurized NaX bed 1 is approaching the ambient
air
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
temperature and water vapor is now being carried into the unpressurized NaX
bed 41 to
produce the heat needed for complete discharge.
[0059] Figure 7 shows a more detailed view of an AE-CAES embodiment in
the beginning of the process of being charged with energy (cf. Fig. 3), when
the
unpressurized NaX bed 41 of the adsorption heat pump is being heated to drive
off the
adsorbed water. Figure 8 shows the same embodiment following the halfway point
of
the discharging process (cf. Fig. 6), when water vapor is being passed through
the
unpressurized NaX bed 41 to generate the high temperatures needed for full
discharge.
Figure 9 shows a cutaway enlargement of a compressed air storage module, which
contains cylinders 2 packed with zeolite pellets 1, within the condensation /
vaporization
chamber 4 used to control the temperature. Figure 10 shows an enlargement of
the
adsorption heat pump 40 containing the zeolite bed 41 used to store the heat
generated
by the compression and adsorption of air, including the baffles 42 used to
ensure that
the atmospheric air, which carries water vapor out of it during charging,
roughly
reverses the flow of the air, which carries water vapor into it during
discharging, for
maximum efficiency. Figure 11 shows an enlargement of the mixer / ejector air
turbine,
including the components labeled 53, 54 and 55, used to efficiently convert
both the
energy stored as pressure and as heat back into mechanical energy during the
discharging process.
[0060] The foregoing assumptions, together with extrapolations graphed in
Fig. 1, imply that at -40 C and 10 atmospheres the ratio of the quantities of
nitrogen to
oxygen adsorbed will be about 4 (Fig. 2). Since this is also about the ratio
of the partial
pressures of nitrogen to oxygen in air and NaX is largely saturated by
nitrogen at this
temperature and 8 atmospheres, the amount of air adsorbed should not increase
greatly
at higher pressures or lower temperatures. An AE-CAES embodiment thus utilizes
a
working pressure of 10 atmospheres and a minimum temperature, obtained when
the
device is fully charged with energy, of -40 C.
[0061] Similarly, the approximations and the extrapolations shown in Fig. I
imply that at 10 atmospheres and 24 C, about 34.5% of the nitrogen and 74.5%
oxygen
adsorbed at -40 C has been desorbed, while at 50 C these percentages are 53.5%
and
82.5% respectively. Thus if one goes up to 100 C at 10 atmospheres, at least
75% of
nitrogen and essentially all of the oxygen will have been ddsorbed. This in
turn implies
16
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
that at least 80% of the total air that is adsorbed at -40 C will be desorbed
at 100 C.
Because going beyond 100 C would make the device more complicated and
expensive,
an AE-CAES embodiment utilizes a maximum temperature, attained when the device
is
fully discharged, of 100 C, which as just argued impiles a duty cycle of at
least 80% in
an AE-CAES embodiment.
[0062] Under dry air at - 40 C and 10 atmospheres, our approximations and
the extrapolated isotherms further indicate that NaX will have adsorbed 4.24
and 1.14
moles of nitrogen and oxygen, respectively, per kilogram of anhydrous
crystalline Na.
With a molar volume for ambient air of 24.8 liters and a density for
crystalline NaX of
1.53 Kgr / L (Kgr / L = Kilogram / Liter), this implies about 204 L of ambient
air will be
adsorbed per liter of NaX under these conditions. This is about 160 L of air
at -40 C
and one atmosphere, or 16.0 L for air at this temperature and 10 atmospheres.
[0063] Rather than working with a microcrystalline powder, however, it is
necessary to form the NaX into pellets that will allow air to flow readily
through the
zeollte beds used in the device, by means of a thermally conducting binder
that will also
enable rapid heat transfer through the beds. Typically these pellets are about
20% by
volume of the binder, and can be packed with a density of about 80% by volume,
thus
reducing the volume of air adsorbed at the working pressure and minimum
temperature
to about 0.82 x 16.0 = 10.25 L per liter of NaX pellets. Taking the 20% void
fraction into
account, at equilibrium the total quantity of air in a tank packed with a bed
of NaX
pellets and filled with air at - 40 C and 10 atmospheres will thus be 10.45
times the
amount that could be stored in the same tank at the same temperature and
pressure.
Together with the 80% duty cycle conservatively estimated above, this gives us
an 8.35
fold reduction in the amount of structural material needed to make a tank that
can store
and release a given quantity of air at the working pressure and minimum
temperature of
an AE CAES embodiment.
[0064] The foregoing calculations show that when fully charged each cubic
meter of the NaX pellet bed in an AE-CAES embodiment will store about 133
cubic
meters of ambient air. Assuming that we perfectly store and recover the heat
while
operating the device, but assuming once again an 80% duty cycle, the work
needed to
isothermally compress this much air to 10 atmospheres comes out to 24.5 MJ /
M3, or
6.8 kilowatt-hours in each cubic meter of the bed. The volumetft energy
density of the
17
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
zeolite pellet bed in an AE-CAES embodiment is thus about one tenth that of
typical
lead acid batteries. The efficiency with which this energy can be recovered in
practice
is discussed in what follows.
[0065] Before moving on to discuss the rest of an AE-CAES embodiment, we
will estimate the heat released by the adsorption of air to the NaX bed, as
well as the
amount of heat that must be taken from it simply to lower its temperature by
140 C.
Miller (b e. cit.) has estimated that the heat of adsorption of nitrogen to
NaX over the
range of loadings utilized in an embodiment is 18.87 KJ / (mol K), while that
of oxygen is
about 13.09 KJ / (mol K). It follows that the energy released on adsorbing
4.24 moles of
nitrogen and 1.14 moles of oxygen is 94.9 KJ (ICJ = Kilo-Joules). Taking into
account
the reductions due to our use of a packed bed of NaX pellets and assuming an
80%
duty cycle as before, this comes out to about 48.6 MJ (Mega-Joules) or 13.5
KWHr I M3
(Kilo-Watt-Hours per cubic Meter). This is about twice the amount of energy
that could
be stored and recovered per cubic meter. Although E. A. Ustinov (16c. cit.)
found a
slightly lower heat of adsorption oxygen to NaX and also some fall off in that
of nitrogen
at 10 atmospheres, it is clear that the most of the heat of adsorption must be
stored and
recovered in any reasonable efficient embodiment of AE-CAES.
[0066] The heat of adsorption, however, will be considerably smaller than the
sensible heat needed to cool and reheat the NaX bed itself over the 140 C
temperature
swing. The specific heat capacity of the bed will vary with the how the
pellets are
prepared and to some extent with temperature, but is typically of order 1 ICJ
/ (Kgr K),
which together with the above assumptions concerning the pellets' packing
density
implies a volumetric heat capacity of about 1 MJ / (M3 K). Multiplying this by
140 and
converting to kilowatt-hours gives 38.9, which is much larger than the energy
to be
stored and recovered per cubic meter. Fortunately, as we shall see, the
relatively high-
grade heat needed to raise the temperature of the NaX bed from ambient to 100
C is
easily recovered, and it is of course not necessary to keep the temperature
high once
the air has been removed from the pressure chamber and the valve leading into
it has
been closed. Similarly, the relatively low-grade heat that must be removed to
take the
temperature of the bed from ambient down to - 40 C does not need to be stored
and
recovered, since that heat can readily be obtained from the environment while
18
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
discharging the device. We now turn to the mechanisms used in an AE-CAE S
embodiment to accomplish all of the above tasks.
[0067] Referring now to the schematic diagrams shown in Fig. 7 and 8, we
first point out that the parallel dashed lines separated by white space which
cut the
diagrams in two are meant to indicate that the scale of the device is somewhat
arbitrary,
and will be determined in practice largely by how it is transported to its
site and utilized.
Purely for the sake of discussion, however, we will often use one megawatt-
hour as
amount of energy stored per module in what follows. This would require about
145 M3
of NaX pellets (horizontal-vertical cross-hatching in the diagrams.
[0066] As may be seen in the drawing of Fig. 9, the NaX zeolite pellets 1 of
an
AE-CAES embodiment are packed into cylinders 2, with a perforated hollow tube
3
extending from a hole at the bottom of each cylinder all the way to the other
end of the
cylinder. This tube allows the compressed air (left-to-right upwards-slanted
hatching in
the diagrams) to pass rapidly from the vent at the bottom of the cylinder
through its
entire length when charging the AE-CAES device, and back out again when
discharging
it. As a result, the length of the cylinders is not cdtical, but their
diameters should be
small enough to allow the rapid diffusion of air from the holes in the tube 3
through the
NaX bed 1 to the surface of the cylinder 2, as well as the rapid diffusion of
the heat
generated as the air is adsorbed.
[0069] Primarily because they are mass produced and hence available for a
low cost, an AE CAES embodiment uses cylinders similar to, but longer than,
the
aluminum cans in which beverages like Coca Cola' are commonly packaged.
Aluminum is more costly than steel, but is more easily formed into such
cylinders, more
corrosion resistant and has a higher thermal conductivity, although slightly
thicker walls
than those of typical aluminum cans will be needed in order to contain ten
atmospheres
of pressure. As such, the diameter of the cylinders 2 in an embodiment will be
6.0
centimeters, while the perforated tubes 3 down their centers need be no more
than 0.5
centimeters in inner diameter and are made from steel in order to provide
structural
support to the packed cylinders. The distance through which air and heat must
diffuse
in order to reach the surface of the cylinders is thus only about 2.75
centimeters. Of
course neither the exact dimensions of the cylinders, the material of which
they are
19
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
made, nor even a cylindrical form for the pressure vessels that contain the
bed of pellets
of NaX or other porous material is essential to the invention.
[0070] The cylinders 2 in turn are contained in a chamber with thermally
insulated walls 4 that can withstand modest pressures and be evacuated over
the
temperature swing of an AE-CAS embodiment. This chamber serves to contain a
heat
transfer fluid, which in turn is used to control the temperature of the
compressed air and
NaX bed 1 inside the cylinders 2 and so implement the temperature-swing cycle
utilized.
Neither the geometry of the chamber nor the way in which the cylinders 2 are
arranged
within it are critical, but for the sake of economy the packing should be as
dense as
possible while allowing the heat transfer fluid to flow freely around the
cylinders. In Fig.
9 a temperature-control chamber 1.25 M in diameter is shown, which contains
108
cylinders each 1.0 M long and arranged on a square grid with its points 0.1 M
apart, for
a total of about 0.21 M3 of NaX bed per chamber. Six hundred ninety such
chambers
would be needed to store a megawatt-hour of energy.
[00711 In this AE-CAES embodiment, the fluid that carries heat to and from
the chamber with walls 4 is methanol. This is a liquid at ambient pressures
and -40 C,
the lowest temperature reached over the temperature-swing cycle, while it is a
gas at
ambient pressures and 100 C, the highest temperature reached. It also has a
high heat
of vaporization, averaging about 36 kJ 1 mole over this temperature range, and
its exact
boiling point can be set to any value between -40 and 100 C by controlling the
pressure
in the chamber with walls 4. Specifically, the boiling point of methanol at a
pressure of
one atmosphere is 64.7 C, and if we assume that its heat of vaporization does
not
depend on pressure, we may use the Clausius-Clapyron equation to show that its
boiling point will be 100 C at 3.6 atmospheres and ---40 C at 231.5 Pascal
(about 0.2%
of an atmosphere). These modest temperatures and pressures allow the walls 4
of the
chamber to be made out of an inexpensive fiberglass composite formed from a
heat-
resistant phenolic resin or epoxy, which will also provide some of the
requisite thermal
insulation. Of course other embodiments are possible in which fluids besides
methanol
are utilized to transfer the heat, and / or other materials are used for the
walls 4 of the
chamber.
[0072] When charging an AE-CAES embodiment, liquid methanol (heavier
left-to-right downwards-slanted hatching) is sucked from a hermetically sealed
and
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
thermally insulated tank 15 through the control valve 10 and sprayed at a
programmed
rate from nozzles 8 in the top of the chamber with walls 4, as indicated in
Fig. 7. A
portion of this methanol vaporizes and exits the chamber through vents 9
interspersed
with the nozzles while the remaining liquid methanol, now at its boiling point
for the
pressure in the chamber, flows down the sides of the cylinders 2 and boils off
of them
as it does so, thereby cooling them along with the NaX beds I which they
contain. The
additional methanol vapor (lighter left-to-right downwards-slanted hatching)
generated
by this process rises and exits the chamber through the vents 9 as before,
while any
liquid methanol that makes it to the bottom of the chamber flows into a drain
6 in the
bottom and thence back to a small sealed holding tank 7 for reuse.
[0073] In contrast, when discharging an AE-CAES embodiment, the valve 10
is closed, another control valve 11 opened, and the methanol in the storage
tank 15 is
heated by the passage of hot water (heavier diagonal cross-hatching in the
diagrams)
through a heat exchanger 16 inside the tank. The resulting methanol vapor
exits the
tank 15 through a vent 14 in its top and flows through a pipe that leads to a
network of
perforated tubes 5 at the bottom of the chamber with walls 4. The methanol
vapor then
rises and condenses on the surfaces of the cylinders 2, transferring its heat
of
vaporization to them at the temperature determined by the prevailing pressure
in the
chamber. This in turn increases the temperature of the NaX bed I towards its
desired
value, while the condensed liquid methanol again flows out of the chamber
through the
drain 6 and into the holding tank 7. A simple positive-displacement pump 12
then
returns it to the tank 15 via the now-open valve 13 for reuse, as indicated in
Fig. 8.
[0074] While charging an AE-CAES embodiment, the pressure in the chamber
with walls 4 is reduced via a compressor 19 into which the methanol vapor
flows from
the vents 9 through the valve 18, as indicated in Fig. 7. It exits the
compressor 19 at a
high pressure and temperature, and flows into a heat exchanger 21 in a
thermally
insulated tank 20, where it is cooled by a stream of water at ambient pressure
to a
temperature of about 100 C. The methanol vapor then passes through the
pressure-
reducing valve 24, which allows it to expand, further cool and largely
condense, and
from there back through the open valve 17 to the storage tank 15 for reuse. In
this way,
the heat generated by adsorption of the air to the NaX bed I is transferred to
the water
or steam (diagonal cross-hatching in the diagrams) passing through the tank
20. Marry
21
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
kinds of compressors could be used for 19, with the exact choice to be
determined
mainly on economic grounds, in accord with the following technical
considerations.
[0075] For efficient heat transfer to boiling water, the compressed methanol
vapor should have a temperature well above that, say 150 C. With an adiabatic
index
for methanol of 1 .3 it follows that early in the charging process, when the
methanol
vapor enters the compressor 19 with a pressure of 3.6 atmospheres and a
temperature
of 100 C, it will only need to increase the pressure by a factor of about 1.7,
or to 6.2
atmospheres. Late in the charging process, however, as the pressure and
temperature
in the chamber with walls 4 fall to 231.5 Pascal and to -40 C, respectively,
it would
need to increase the methanol vapor pressure by a factor of almost 13.3,
resulting in a
pressure that is still only 0.03 atmosphere. The Carnot limit on the
coefficient of
performance of this cooling system is infinite at the beginning when the
temperature in
the chamber with walls 4 is 100 C, but only 1.66 at the end of the charging
process
when it has fallen to - 40 C. In accord with our earlier discussion of the
large quantity of
sensible heat that must also be removed from the NaX beds 1 during charging,
once the
theoretical coefficient of performance falls below about 3, which happens when
the NaX
bed temperature reaches 7 C, it will no longer be profitable to try to store
this heat, nor
the smaller amount of heat released by adsorption, in a form that can
subsequently be
used to generate high temperatures. This issue will be taken up again
presently (cf.
Figs. 3 and 4).
[0076] Before describing where the heat goes next, we first consider the
process by which the air is compressed to ten atmospheres when charging an AEm
CAES embodiment, and at the same time much of the heat of compression is
removed
from it. Due to their high efficiency, in the AE-CAES embodiment this is done
by two
standard centrifugal compressors 26 and 28 in tandem, each of which increases
the
pressure of the air by a factor of 3.16 after cooling back to ambient (the
square root of
ten). An air filter and desiccator 25 is used to remove particulate matter and
water
vapor from the air prior to entering the first compressor 26. Using an
adiabatic index for
air of 1.4, it may be shown that each compression stage will increase the
absolute
temperature of the air by a factor of 1.39, or to about 141 C starting from
ambient
temperatures. With a heat capacity at constant volume for air of 20.77 J Keel
K), the
22
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
heat of compression over the two stages is thus 54 watt hours per cubic meter
of
ambient air compressed to ten atmospheres, or 83% of the total energy to be
stored.
[0077] The air is cooled as it exits the each of the two compressors 26 and
28. This is done using the pump 39 to drive a stream of cool water through the
countercurrent heat exchangers 27 and 29 in the exits of the compressors 26
and 28,
respectively. In this way the heat of compression preheats the water, which in
turn is
directed through a pipe to the nozzle 22 where, during the first half of the
charging
process (see Fig. 3), it is boiled by the compressed methanol vapor, as
previously
described. Later in the charging process, i.e. once the theoretical
coefficient of
performance of the methanol heat pump has fallen below 3 or so, the
compression ratio
of the compressor 19 is lowed so that the methanol vapor is raised to at most
100"C. At
the same time the rate of water flow through the air compressors 26 and 28 is
increased
so that it is not preheated as much, with the net result that now the water is
not boiled
but instead merely heated and recirculated (as indicated in Fig. 4). The
compressed air
itself is directed through the open valve 30 to the NaX beds 1, as indicated
in Fig. 7.
Any residual heat of compression remaining in it will subsequently be removed
in the
course of cooling the NaX beds 1 and wind up in the steam or water exiting the
tank 20
as well. This steam or water thus contains most of the heat of compression and
of
adsorption of the air, as well as the sensible heat removed from the NaX beds
I to cool
them.
[0078] During the first half of the charging process (Fig. 3), the high-grade
heat contained in the steam exiting the tank 20 is used to prime an adsorption
heat
pump that uses NaX-water as its adsorbent-adsorbate pair. This open adsorption
system is modeled after one recently demonstrated by Andreas Hauer in the
Federal
Republic of Germany, where it was used to reduce the cost of heating buildings
by
desorbing water from the NaX at night and using the re-adsorption of water
vapor to
upgrade waste heat during the day when the demand for heating is greater (see
section
2 of chapter 25 by A. Hauer, pp. 409--27 in "Thermal Energy Storage for
Sustainable
Energy Consumption," NATO Sci. Ser. II; Math., Fhys, and Chem., vol. 234, H.
0.
Paksoy, ed., Springer, 2007). This open adsorption heat pump is simply a
thermally
insulated tank 40, constructed in an embodiment from a heat-resistant
fiberglass
23
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
composite as before, which is filled with NaX pellets 41 similar, but not
necessarily
identical in form, to those used to adsorb the air.
[0079] Thus an AE-CAES embodiment also utilizes the NaX zeolite for the
second new use of adsorption in porous materials of the invention. It should
nevertheless be emphasized that a great many other porous materials, such as
silica
gel, are available that can also be used to pump heat via the adsorption of
water, or
indeed any other suitable fluid. The meter NaX adsorbate-adsorbent pair used
here is
chosen because, like the air-NaX pair, the adsorbate is inexpensive and
environmentally benign, while the adsorbent is well understood, not prone to
degradation with repeated use (when a suitable binder is used for the pellets;
see O.
Storch, O. Reichenauer, F. Scheffler and A. Hauer, Adsorption 14, 275, 2008),
and
commercially available. A further advantage of the water-NaX system lies in
the fact that
the differential heat of adsorption of water vapor to NaX increases from a
value close of
that of the heat of vaporation of water, or 44 KJ / mole, to about twice that
value as the
amount of water adsorbed to the NaX falls from 30 to 0% by weight. This means
that in
addition to providing a means of upgrading heat to higher temperatures, the
NaX bed 41
of the heat pump will also store a significant amount of heat in latent (as
well as
sensible) form, even after deducting the heat needed to evaporate water during
discharge. Because the heat of adsorption of water vapor to NaX is so much
larger
than the heat of adsorption of air to NaX, the amount of NaX needed for this
adsorption
heat pump is only a fraction of that which is required to adsorb the air
itself.
[0080] Once again during the first half of the charging process (Fig. 3), the
steam from the tank 20 passes through vents 23 in its top to another
compressor 31,
which raises the steam's pressure by a factor of 2.8 and, since the adiabatic
index of
water is also about 1.3, its temperature to about 200 C. It then passes via
the open
valve 32 to a heat exchanger 36, where the steam is cooled by a countercurrent
stream
of atmospheric air which is blown over the heat exchanger by the fan 37,
heating the air
to a temperature of about 150 C in the process. The Carnot limit on the
coefficient of
performance for this heat pump is 7.5, which should be comparable to the
average
coefficient of performance of the methanol compressor 19 over the first half
of the
charging process. It should be noted that the energy needed by the compressors
19
and 31 also winds up as stored heat, and may subsequently be recovered thereby
24
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
making up for losses elsewhere in the system; the energy needed to run the fan
37 is
not significant by comparison.
[0081] The hot air from the heat exchanger 36 flows into the thermally
insulated tank 40 and through the unpressurized bed of NaX zeolite pellets 41,
which
initially have about 30% of their weight in water adsorbed to them (see Fig.
10). The hot
air raises the temperature of the NaX pellets 41 a causing this water to
desorb from them
in the form of water vapor and cooling the air in the process. This water
vapor is carried
by the air through the NaX-pellet-packed container 40 and exits from its other
end in the
form of moist air at a temperature of about 40 C. The steam used to heat the
air
entering the NaX bed 41 exits from the heat exchanger 36 through the pressure-
reducing valve 38, whereupon it also cools down well below the normal boiling
point of
water and largely condenses. Because no heat transfer is ever complete, this
water still
holds a portion of the heat it contained entering the heat exchanger. The
energy
contained in this sensible heat is stored by returning the water to the
surface of the
reservoir 43 from which it originated.
[0082] similarly, the warm moist air exiting from the NaX bed 41 passes over
a condenser 47 through which water is passed via the action of the pump 44.
This
water flows from the cool bottom of the reservoir 43 through the condenser 47
and back
through the open valve 50 to the warm surface of the reservoir 43. The heat of
condensation is thereby likewise transferred to the surface water of the
reservoir. The
need to use the heat of condensation for efficiency's sake has been stressed
by A.
Hauer (loc. cit.), and the option to store it in a reservoir has also been
claimed in a more
recent patent (US 6,820,441). The condensed water itself collects in the basin
49, and
may be discarded or added to the reservoir 43 once an AE-CAES embodiment is
fully
charged.
[00831 In contrast, during the latter half of the charging period (Fig. 4),
the fan
37 is turned off and the container 40 sealed so that moisture cannot
prematurely re-
adsorb to the MaX bed 41 it contains. Instead of steam at 200 C, hot water at
well
below its boiling point flows directly from the tank 20, where it has picked
up heat from
the hot compressed methanol vapor, through the now-open valve 35 which by-
passes
the now-passive compressor 31, and on to the surface of the reservoir 43
without
further cooling. In this way the heat generated by the compression and
adsorption of
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
the air during the latter half of the charging period, as well as the
remaining sensible
heat in the NaX bed 1, also winds up in the reservoir 43. How this heat is
subsequently
recovered will be described below.
[0084) Once an ASE-CAES embodiment has been fully charged, the majority of
the mechanical energy put into it is stored largely in the form of adsorbed
air in the NaX
pellet bed I within the cylinders 2. As previously noted, about 83% of this
energy is
also stored as heat, primarily in the water reservoir 43. In addition, several
times more
energy has been taken out of the NaX bed I in the form of heat, the majority
of which
was sensible heat with a smaller but significant contribution from the heat
generated by
adsorption of the air. Most of this heat will likewise be stored as sensible
heat in the
water reservoir 43, although a significant amount will also be stored as both
latent and
sensible heat in the NaX bed 41 of the adsorption heat pump.
(0085] As long as the valves 30 and 56 are kept closed to trap the
compressed and adsorbed air, essentially none of the energy stored in this
form will be
lost prior to discharge. Similarly, as long as the container 40 is kept sealed
from
moisture, none of the energy stored as latent heat in the NaX bed 41 will leak
from it
prior to discharge. As shown above, a considerably larger quantity of heat
will be stored
as sensible heat in the water reservoir 43, but the rate at which this heat
leaks from the
reservoir will not be large because the temperature difference between the
water and
the reservoir's environment will not be large (well under OO'C even in cold
weather).
Another, less direct, form of loss would be from heat leaking into the chamber
with walls
4, raising the temperature of the NaX beds 1 therein and forcing release of
some of the
compressed air to keep the pressure from rising beyond that which the
cylinders 2 are
able to withstand. Once again, however, the AE CAES embodiment strives to keep
these temperature differences low by using minimum and maximum temperatures
symmetrically placed about 70 C below and above normal ambient temperatures.
For
such modest temperature gradients, standard low-cost insulation such as
polyurethane
foam should keep all of the loses due to sensible heat leakage down to an
acceptable
level over the anticipated storage period of a day or less.
[0086] When the time comes to recover the mechanical energy stored in an
AE CAES embodiment, warm water from the surface of the reservoir is directed
through
the heat exchanger 16 by closing the valve 50 and opening the valve 51. At the
same
26
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
time the fan 37 is used to blow ambient air through the NaX bed 41 of the
adsorption
heat pump, where it picks up sensible heat from the bed but not much of the
latent heat
because it does not contain much moisture to re-adsorb. Some of this heat will
be
transferred to the water flowing through the heat exchanger 47 at the exit,
whence it
continues to the heat exchanger 16, but most of the heat will be carried along
with the
air into the exit chamber 48 at a still elevated temperature. This warm air is
directed via
the duct 52 to an air turbine, which includes components 53, 54 and 55, by
rearranging
the baffling in the exit chamber 48, as indicated schematically in Figs. 7 and
8. It will
be used there to keep the expanding compressed air from cooling, as will be
described
presently.
[0087] Meanwhile, the warm water flowing through the heat exchanger 16
boils the methanol in the storage tank 15, which is initially under a pressure
of a fraction
of an atmosphere. The resulting methanol vapor is then used to heat the
cylinders 2
containing the NaX pellet beds I to which air is adsorbed, as previously
described. This
converts the adsorbed air to compressed air at a rate that is controlled by
controlling the
rate at which methanol vapor enters the chamber with walls 4. This compressed
air is
also directed as it is generated by desorption through the now-open valve 56
to the air
turbine with components 53, 54 and 55, as shown in Fig. 6. The mass and energy
fluxes during this first half of the discharging process are illustrated in
Fig. 5.
[0088] Once about half the stored energy has been recovered and the
temperature of the pressurized NaX bed I is approaching ambient temperatures,
the
valve 45 is opened to let warm water from the surface of the reservoir 43 pass
through a
vaporizer 46, which dispenses it as a mist over the heat exchanger 36. At the
same
time warm water from the reservoir 43 is driven by the pump 39 through the
heat
exchanger 36 via the open valve 34, and prevented from getting to the air
compressors
26 and 28 by closing valves 32, 33 and 35, so as to keep the evaporating water
from
cooling the air around it. In this way the air from the fan 37 is saturated
with water
vapor prior to entering the unpressurized NaX bed 41, and heated by the
process of
adsorption of the water vapor as it passes through the unpressurized NaX bed.
The
mass and energy fluxes during this second half of the discharging process are
illustrated in Fig. 6. Of course the use of a simple vaporizer such as 46 is
not essential
27
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
to the invention, and could easily be replaced by an impeller or ultrasonic
humidifier if so
desired.
[0089] Hauer (brae. cit.) has shown that the air will exit the far end of the
adsorption heat pump container 40 at a temperature in excess of 100 C. As it
does so,
a portion of the heat it contains will be transferred via the heat exchanger
47 to the
countercurrent stream of warm water from the surface of the reservoir 43,
heating it
gradually towards 100 C as the discharge process progresses. This will raise
the
temperature and pressure of the methanol vapor generated in the tank 15 to
ever higher
levels, thereby heating the NaX beds I in the cylinders 2 to 100 C at the end
of the
discharge process. At the same time the water passing through the heat
exchanger 36
has been cooled and is returned to the bottom of the reservoir 43 to be used
the next
time the device is charged.
[0090] The efficiency of the AE-CAES embodiment is also improved by
passing the air during discharge through the unpressurized NaX pellet bed 41
in
approximately the reverse of the direction in which hot air was passed through
it in order
to desorb moisture from the unpressurized NaX bed during charging. This
increases
the efficiency because otherwise some of the sensible heat picked up by the
air entering
the bed during the first half of the discharge process, or generated by the
adsorption of
moisture from the air during the second half, will be lost to the cooler and /
or less dry
NaX bed before it reaches the far end. This approximate reversal of the flow
is
accomplished by a system of internal baffles 42, depicted by heavy solid lines
in the
drawings, which are arranged so that during charging the air enters the near
end
through the center of the bed but exits the far end around the periphery, and
then
rearranged during discharging so that the air enters the periphery on the near
end but
exits through the center on the far end, as indicated schematically in Figs. 7
and 8 (see
also Fig. 10). Of course other embodiments are possible in which the far end
includes a
second fan, enabling the air to take exactly the opposite path back through
the NaX bed
41 while the roles of the heat exchangers 36 and 47 are swapped while
discharging the
device.
[0091] Finally, we describe how the warm air entering the exit chamber 48
and passing via the duct 52 is used to heat the expanding compressed air from
the NaX
bed 1 and thereby recover the heat of compression throughout both halves of
the
28
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
discharging process. This air turbine, which includes the components labeled
53, 54
and 55 in Figs. 7 and 8, is designed so that the stream of compressed air
entering it
expands and accelerates through a venturi with twisted vanes running in
parallel along
its length (see Fig. 11). This creates a vortex which generates a vacuum
behind it,
which in tarn draws the warm air from the duct 52 through a larger-in-diameter
annulus
of static blades 54 Tightly up-wind of the blades 53. This second vortex of
warm air
merges with the vortex of cold expanding air from the blades 53 and is rapidly
and
thoroughly mixed with it by this process. The now rapidly moving air vortex
hits the
blades of the air turbine rotor 55 and thereby converts the energy stored in
the
compressed air and a portion of the energy stored as heat into mechanical form
for
external use. Of course many other devices are available, such as
reciprocating air
motors, by which heat and compressed air may be converted into mechanical
energy in
various alternative embodiments, although these will generally not be as
efficient as the
mixer-ejector air turbine just described.
[0092] Assuming that the AE-CAES embodiment releases one megawatt-hour
of energy at a constant rate over a six hour period and that the compressed
air is
heated back to ambient temperatures in the process, the compressed air must be
released at flow rate of about 700 M3 per hour, measured at ambient
temperature and
pressure. The actual temperature of the compressed air will start out at --40
C and
gradually rise to near 100 C over the six hour period, and air at ---40 C is
1.6 times more
dense than air at 100 C at any given pressure. It follows that the air at ten
atmospheres
must be released at a rate of 54 M3 per hour at the beginning of discharge
period and
86 M3 per hour at the end. Under adiabatic conditions, this air would cool as
it expands
to -152 C at the beginning and -30 C at the end of the discharge period, which
in turn
would reduce the flow due to the release of compressed air to 283 and 454 M3
per hour
respectively. To return air at those temperatures to ambient temperatures, it
must be
mixed with about 8.87 and 5.25 times the same mass of air at a temperature of
45 C,
the approximate temperature of the air entering the air turbine through the
duct 52. The
required flow rate of 45 C air through the duct thus varies from 6628 to 3920
M3 per
hour over the six hour discharge period,
[0093] Using a 7000 kilogram NaX pellet bed, A. Hauer (loc. cit.) was able to
heat an air flow of 6000 M3 per hour to between 120 and 100 , also over a six
hour
29
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
period, which corresponds to about 120 kilowatts of heat. Because only 83% of
the
energy is stored as heat, it follows that about 0.83 x 1000 16 = 138 kilowatts
of heat will
be needed by the turbine during the assumed 6 hour discharge period for one
megawatt
hour. Early in the discharge process it will not be necessary to heat the
methanol by
very much, so the rate of non-humidified air flow through the NaX pellet bed
41 can kept
relatively high, and water can be pumped through the heat exchanger 47 at a
high
speed. The resulting air will enter the duct 52 at a temperature somewhat
below the
45 C assumed above, but its flow rate into the turbine will also be greater
than the 6628
M3 per hour found above at 45"C. As the discharge progresses, the pump 44 is
slowed
so that by the end of the discharge period the temperature of the water
exiting the heat
exchanger 47 approaches that of the air passing over it, or 100 C. At the same
time the
rate of humidified air flow through the NaX pellet bed 41 is gradually slowed,
so that
near the end of the discharging process the temperature of the air entering
the turbine
through the duct 52 will be somewhat larger than 45CC while its flow rate will
also be
less than the 3920 M3 per hour estimated above at 45 C.
[0094] The components of the AE-CAES embodiment presented above
include the water-NaX adsorption heat pump, the NaX zeolite bed that stores
compressed air in adsorbed form, and advanced air turbines based on mixer-
ejector
principles. It also includes the control systems needed to make all these
components
work in synchrony, as described above. In particular, the pressure in the
chamber with
walls 4 and the rate at which methanol enters it during charging and
discharging must
be regulated so that compressed air is converted to and from adsorbed air at
the same
rate that it is produced by the compressors 26 and 28 or fed to the turbine
including the
components labeled 53, 54 and 55, respectively, thereby keeping the pressure
of the
gaseous air in the cylinders 2 approximately constant throughout. This task,
although
not trivial, is nevertheless a perfectly standard systems integration problem
in chemical
process engineering that can be accomplished by one skilled in that art.
[00951 Numerous substitutes may be employed for the mechanical and fluid
components of the AE-CAES embodiment as well as for the materials it employs,
all of
which were chosen only the illustrate the advantages to be obtained through
the use of
adsorbents to facilitate the storage of compressed air and heat, along with
the
complementary temperature-swing cycle. Because the energy needed to run the
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
pumps and compressors must be subtracted from the energy released in
calculating the
overall efficiency of an AE-CAES device, it is entirely possible that modest
improvements to an embodiment could be attained by such substitutions,
although they
must still be subject to the Carnot limits given above. It should be noted, in
particular,
that we have refrained from saying where the motive force that drives the
compressors
19, 26, 28 and 31 comes from, or what the mechanical force generated by the
air
turbine including components 53, 54 and 55 is used for. Normally compressors
are
driven by electric motors, but at a coal or nuclear power plant it would be
more
economical to drive them directly, for example via a hydraulic system, from
the steam
turbines of the power plant than it would to convert the mechanical energy
from the
turbines into electricity and then back to mechanical energy in the
compressors. The
same, of course, is true of an AE-CAES device installed at a wind turbine
farm.
Similarly, it could under some circumstances be more economical to use the
compressed air released while discharging an AE-CAES device to power pneumatic
tools or machinery, rather than to generate electricity.
[0096] The AE-CAES device, and/or a temperature-swing CAES device, could
also employ a variety of other established chemical processes without
materially
deviating from the intent of the inventors. For example, the water-NaX heat
pump 40
and 41 of an embodiment could be based on other adsorbate-adsorbent pairs, the
absorption of a gas in a liquid medium, or even be replaced by a wide variety
of solid-
liquid phase-change materials, which can also store heat in latent form. It is
further
possible to supplement or replace the heat storage subsystem entirely by waste
heat
recovery or thermal energy harvesting in a variety of ways. If, for example,
an AE-
CAES device were located at a power plant that produces heat as a by-product,
such as
a coal or nuclear power plant, then this heat could be used to reheat the
expanding air
and/or the adsorbent for air. Alternatively, a flat-plate solar thermal
collector could also
readily generate the modest temperatures needed when discharging an AE-CAES
device, installed for example at a wind turbine farm. The main point is that
the heat
utilized by any component of an AE-CAES device during discharge need not have
been
produced by the inverse process while charging it.
[0097] Given a suitable inexpensive source of heat, it would also be possible
to use it to regenerate an adsorbent refrigeration system during the storage
or
.
31
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
discharge period, which could be utilized instead of the vapor compression
refrigeration
system of an embodiment to cool the NaX bed while it adsorbed air during the
charging
period. In cases where such environmental heat sources are not always
available at
the time they are needed, the heat could be stored when available in either
sensible or
latent form along with the heat generated while charging the device, and used
to make
up for any energy loses due to incomplete heat transfer. It should also be
possible to
reduce the size of the temperature swing needed for a high duty cycle, and
hence the
amount of heat that must be taken from and returned to the adsorbent for air,
by using
some combination of a temperature and pressure swing instead of a pure
temperature
swing as in the above AE-CAES embodiment, These variations could significantly
improve the economics of building and / or operating an AE-CAES device in many
of its
diverse potential applications.
[0098] In a second embodiment, an adsorption heat pump is used to
refrigerate the porous material that adsorbs air while charging the system
with
compressed air, as an alternative to heating that porous material during
discharge. This
has the advantage that it can reduce the amount of energy that must be
expended
running vapor-compression heat pumps, because the temperature difference over
which the heat is pumped may be considerably reduced. This temperature
difference
depends on a number of factors such as the adsorbent-adsorbate pair that is
utilized by
the adsorption heat pump, the availability and temperature of inexpensive
waste or solar
heat, the temperature at which sensible heat is stored in the water reservoir
or other
thermal energy storage subsystem, the temperature of the external environment,
and
the other operating parameters of the energy storage device. The amount of
extra
mechanical energy that must be expended to transfer a given quantity of heat
via a
vapor-compression heat pump, in turn, falls off rapidly as this temperature
difference
decreases. Since this extra energy cannot be recovered like the mechanical
energy that
is stored in the form of compressed and adsorbed air, it must be deducted from
the
recovered energy in order to calculate the round-trip efficiency of the energy
storage
system. It follows that the second embodiment may under some circumstances
provide
a more efficient energy storage device.
[0099] Before describing the second embodiment in detail, however, a more
refined estimate of the density with which air and energy can be stored in a
packed bed
32
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
of NaX pellets will be given. This estimate improves upon those given earlier
in the
following respects. First, instead of assuming that the adsorption of nitrogen
and oxygen
from air are independent processes, the Sipps multi-component isotherm formula
will be
used to extrapolate the number of air molecules adsorbed as a function of
pressure
from the pure gas N2, 02 and Ar isotherm formula [G. W. Miller, AIChE Symp.
Ser. 83,
28, 1987]. Second, instead of estimating a "duty cycle" over a temperature
swing of -40
to +100 C by extrapolating from the estimated quantities of air adsorbed at -
40, 24 and
50 C, explicit pure gas isotherms at 100 C were extrapolated from those at
these three
lower temperatures by a least squares fit of the logarithms of the
coefficients in the
Langmuire (or Sipps, for N2) isotherms to the inverse absolute temperatures,
and
setting the exponent in the Sipps isotherm for N2 to its high-temperature
asymptote of
unity. Such a linear dependence is implied by the van't Hoff equation of
thermodynamics, and the resulting pure gas isotherms can then be used to
estimate the
mixed gas isotherm at 100 C via the extended S ipps formula, just as at the
three lower
temperatures. Even though the van't Hoff equation will be only approximate at
the
temperatures and pressures of interest here and the fits, although reasonably
precise,
were based on only three points each, such an objective procedure was deemed
more
rigorous than the previous ad hoc estimates. Third, the stored energy
densities
associated with the quantities of air adsorbed over the range of operating
pressures
considered were estimated using an isothermal expansion from the assumed
working
pressure to one atmosphere, instead of to zero pressure as in the simpler
formula used
previously. In addition, the work done by the air as it is desorbed at the
working
pressure is included. It turns out that these last two refinements in our
model of the
expansion process largely cancel one another, so the resulting energy density
estimates are similar to those obtained by our previous, less rigorous
procedures.
[0100] Figure 12 plots the graphs of the mixed gas air isotherms for NaX at
the temperatures of --40, 24, 50 and 100 C, derived as described above.
Assuming as
before that the NaX pellets are 20% inert binder by volume, that the volume of
the intra-
granular macro pores is negligible, and that the pellets are packed into an
adsorbent bed
with a volumetric density of 80%, these isotherms imply the quantities of air
shown in
Table I below for various temperatures and pressures. The dimensionless
numbers in
the table are the volumes which the air contained in a unit volume of
adsorbent bed
33
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
would occupy in the form of a free gas at the standard temperature and
pressure (STP)
of 25 C and one atmosphere, assuming an STP molar volume of 24.8 liters.
Table I
gauge pressure (bar): 0 5 10 15 20 25 30
volume of air at STP
stored per unit volume
NaX bed at ---40 C: 45.0 96.7 111.8 119.9 125.3 129.4 132.7
volume of air at STP
stored per unit volume
WaX bed at 24 C: 9.0 37.3 54.2 65.9 74.7 81.5 87.1
volume of air at STIR
stored per unit volume
NaX bed at 50 C: 5.2 25.6 41.2 53.3 64.4 73.5 81.3
volume of air at STP
stored per unit volume
NaX bed at 100 C: 1.9 10.7 18.6 25.8 32.4 38.4 44.0
[0101] Note that at 10 bar we obtain a duty cycle over a -40 to 100 C
temperature swing of (111.8 -13.6) / 111.8 = 83%, in agreement with our
earlier
estimate. The results in Table I also lead directly to those in Table 2 below,
where we
compare the quantities of air released from a unit volume of NaX bed over
various
temperature and pressure swings with those released from a unit volume tank
devoid of
NaX over a simple pressure swing starting from the working pressure given in
the
column heading and decreasing to atmospheric pressure, all at 25 C.
Table 2
gauge pressure (bar): 0 5 10 15 20 25 30
P-swing at 24 C in an NaX
bed over P-swing w/o 13.E at
25 C: N/A 5.7 4.5 3.8 3.3 2.9 2.6
24 to 100 C T-swing in 13X
bed over P-swing w/o 13X at
25 C: N/A 5.3 3.6 2.7 2.1 1.7 1.4
(T, P)-swing of (24, X) to
(100, 0) over P--swing w/o at
25 C: N/A 7.1 5.2 4.3 3.6 3.2 2.8
34
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
-40 to 100 C T-swing at P =X
in 13X bed over P-swing w/o
at 25 C: N/A 17.2 9.3 6.3 4.6 3.6 3.0
(T, P)-swing of (-40, X) to
(100, 0) over P-swing w/o at
25 C: N/A 19.0 11.0 7.9 6.2 5.1 4.4
[0102] It may be seen that the improvement in the duty cycle when NaX is
used in conjunction with a temperature swing between ---40 and 100 C, relative
to a
simple pressure swing at 25 C without NaX, is 17.2 at 5 bar and falls off by
about a
factor of two for every doubling of the pressure. The amount of NaX needed to
release a
given quantity of air, however, will fall off more slowly beyond about 10 bar
because it is
largely saturated with air at that pressure and ---40 C (cf. Fig. 12).
Similarly, since NaX
holds less than 20% of that air at 10 bar and 100 C, the improvements to be
gained by
lowering the pressure below 10 bar are also fairly limited. These observations
support
our earlier conclusion that an operating pressure of about 10 bar will be
optimal for the
system when a -40 to 100 C temperature swing is employed. The density with
which air
is stored relative to a simple pressure swing may be increased from 9.3 to
11.0 when
this same temperature swing is combined with a pressure swing (see last row of
Table
2), but such a mere 18% improvement is probably not worth the additional
expense of
the hardware needed maintain a constant output power over such a large
pressure
variation.
[0103] Accordingly, we assume as before that the air is desorbed from NaX at
constant pressure by means of a -40 to 100 C temperature swing, and
subsequently
expanded in an isothermal process at 25 C. This allows the mechanical work
done
while discharging the system to be divided into two parts. The first is the
work done by
the air as it is desorbed and allowed to expand as necessary to keep the
pressure
constant as the NaX bed is warmed from -40 to 100 C, and the second is the
work
done by the air during isothermal expansion back to atmospheric pressure at 25
C
(which is approximately the average temperature of the NaX bed over the
cycle). Figure
13 plots these two contributions to the total PV work done as a function of
the operating
pressure, keeping the temperature swing at -40 to 100 C throughout. The work
done
during isobaric desorption and expansion of the air is essentially constant
beyond 10
bar, at which pressure it is also about 75% of the work done during the
subsequent
.........
...................................................................
............................
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
isothermal expansion. These observations further support our earlier
conclusion that
this pressure roughly maximizes the benefit derived from using a bed of NaX to
adsorb
the air.
[0104] Due to the above-mentioned cancellations in our more refined but still
idealized expansion model, the estimated density with which energy is stored
in the
NaX bed at a (gauge) pressure of 10 bar comes out to 6.9 kWhr / M3, almost
exactly as
in our earlier estimate. The heat of adsorption remains about twice the
mechanical
energy stored, and the sensible heat that must be taken from and returned to
the NaX
bed over the storage cycle remains several times larger yet, In principle, all
this heat
can be stored while charging the system with compressed air and recovered
again while
discharging it, which would allow an AE-CAES system to be operated as a "pure"
energy storage device. For ease of presentation both the original as well as
the second
embodiment presented below were designed to operate, to the maximum extent
possible, in this fashion. In practice however the expense of such a highly
efficient
thermal energy storage subsystem would be substantial, and the additional
energy used
by the vapor-compression heat pumps needed to move this heat around preclude a
highly efficient energy storage system in any case. A less expensive AE-CAES
device
could be obtained by using a less efficient thermal energy storage subsystem
while
making up for the resulting thermal energy losses with an external heat source
of some
kind. in the simplest case, this external heat could just be added to the hot
water
reservoir, which both the original as well as second embodiment already use
for thermal
energy storage.
[0105] One caveat that must be noted is that this additional thermal energy
must be deducted in calculating the physical round-trip efficiency of an
adsorption-
enhanced CAES system, regarded as a pure energy storage device. Fortunately,
this
additional heat does not need to be at a temperature much above 100 C in order
to
heat the NaX bed to that temperature while discharging the stored mechanical
energy.
Moreover, the methanol-and-activated-carbon-based adsorption refrigerator used
in the
second embodiment to cool the bed back to -40 C (see below) can also be
regenerated
using heat at similar modest temperatures. a result, an AE-CAES system can be
economically efficient even if it is not "efficient" in the strict physical
sense of the word.
By this we mean that the cost of the additional thermal energy needed can be
quite
36
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
trivial in comparison to the value of the stored mechanical energy itself.
Indeed heat at
such modest temperatures is often regarded as "waste" and discharged directly
into the
environment, and even when such a waste stream is not available heat at these
same
modest temperatures can often be obtained from inexpensive solar thermal
collectors.
[0106] Turning now to the second embodiment, we begin with the overview of
the energy storage cycle shown in Fig. 14. The state of the system at the
beginning of
each of the four legs of the cycle is described in the boxes at the bottom,
left, top and
right of the figure, while the diagrams in the four corners indicate the heat
flows between
the various components of the system during each leg. In greater detail, these
legs of
the cycle are:
The first half of the charging process, which is labeled "spontaneous cooling"
because the temperature of the NaX bed will exceed that of the cold (or near-
ambient temperature) water reservoir, so that heat flows spontaneously from
the
NaX to the water. In this embodiment, the heat is carried from the NaX to the
water
by actively circulating methanol between these two thermal reservoirs. At the
same
time air is compressed by the input of mechanical energy, the heat of
compression
transferred to the water reservoir, and the cooled and compressed air adsorbed
by
the NaX bed.
The second half of the charging process, labeled as "adsorption refrigeration"
because during this leg of the cycle methanol vapor is adsorbed in an
activated
carbon bed as it evaporates and carries heat from the NaX bed. This heat,
together
with the heat of adsorption of the methanol vapor to it, is transferred from
the
activated carbon to the water reservoir as before. Meanwhile air continues to
be
compressed by mechanical energy, the heat of compression transferred to the
water
reservoir, and the air adsorbed by the NaX until it has reached its minimum
temperature over the cycle.
The first half of the discharging process, labeled as "spontaneous heating"
because
now the temperature of the NaX bed is below ambient so that heat would flow
spontaneously into it from the cold water reservoir. In order to attain the
higher
temperatures needed to desorb the methanol from the activated carbon and so
regenerate it for use in the next cycle, however, the heat is first
transferred from the
37
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
hot water reservoir to the activated carbon. From there the heat is carried by
the
methanol vapor to a heat exchanger in contact with the NaX bed, where it
condenses, and the resulting liquid is stored for use in the next cycle. This
in turn
warms the NaX bed from its minimum temperature back to approximately ambient
temperatures, causing a portion of the air it contains to desorb. The desorbed
air is
allowed to expand back to atmospheric pressure while also taking up heat from
the
hot water reservoir and producing the output mechanical energy.
The second half of the discharging process, labeled "active heating" because
during
this leg of the cycle the NaX bed is actively heated back to its maximum
temperature over the cycle, and this temperature will be at least that of the
unpressurized hot water reservoir. In this embodiment, the heat is moved from
the
hot water reservoir to the NaX again using methanol as a heat transfer fluid.
As a
result the NaX bed desorbs its remaining air, which expands taking up
additional
heat from the water reservoir and producing additional output mechanical
energy in
the process.
[0107] As in the first embodiment, heat is actively transferred between its
thermal reservoirs using vapor-compression heat pumps. Two such heat pumps are
utilized by the second embodiment, one of which uses methanol as its working
fluid and
the other of which uses a conventional halocarbon refrigerant. For
completeness, we
further note that when external sources of heat at 100 C or more are
available, they can
be used instead of active heat pumping thereby saving the energy overhead
associated
with vapor-compression heat pumps. Such external heat sources can also be used
to
regenerate the activated carbon bed, in which case the cold in the NaX bed
could be
used for refrigeration or air conditioning in a building. Either of these uses
of external
heat could also make up for thermal loses from the hot water reservoir or
during the
various heat transfers in the cycle. They could even free up enough of the
heat stored in
the hot water reservoir to allow it to be used for space heating or hot water
in a building.
Once again, for simplicity's sake we will not consider all these alternatives
to running an
AE-CAES system as a "pure" energy storage device here, although in many
situations
this may be the most economical way to use it.
38
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
[0108] Figures 15 through 18 show more detailed but still schematic views of
the second embodiment at the beginning of each one of the four legs of the
storage
cycle, in the same order as given above. The parallel lines depict the piping
of the
system, while the sizes of the dashes between them indicate the kind of fluid
flowing
through the pipe. Air is indicated by an intermediate length normal dash,
while a long
bold dash indicates water, an intermediate bold dash methanol, and a short
bold dash a
conventional halocarbon refrigerant. in these four figures, open valves are
depicted by
hour-glass shapes parallel but behind the "pipes, and closed valves by hour-
glass
shapes which cover the pipes. The pressure-reducing expansion valves of the
vapor-
compression heat pumps are asymmetrical hour-glass shapes, which should be
understood to include a by-pass that allows the flow through them to be
reversed
without any effect upon pressure. The four-way valves which determine the
direction of
heat flow in the two heat pumps are depicted by circles with a diagonal line
through
them, with the fluid flow passing through the pairs of ports not cut off by
the line. The
compressors of the two heat pumps are depicted as isosceles trapezoids which
receive
their low-pressure input stream in the large end and eject their high-pressure
output
stream from the narrow end, as is traditional in engineering diagrams.
Positive-
displacement liquid pumps are shown as circles, with a filled triangle in them
indicating
the direction of flow when they are operating, or which simply sit on top of
the pipe
without a triangle when not operating. Heat exchanger subsystems are indicated
by
zigzags in the piping, as in the two that are contained in the air compressor
and
expander on the left-hand sides of the four figures. These are likewise drawn
as
isosceles trapezoids, which however take their air in and out through pipes in
their
sides, as indicated.
[0109] The thermal energy storage subsystem of the second uses separate
reservoirs for the cold and hot water, rather than keeping the cold water at
the bottom
and the hot water at the top of a single reservoir. This should improve the
efficiency of
the subsystem, but is not critical to its operation. As mentioned above,
methanol is the
working fluid used to move heat from the air-adsorbing NaX bed to the water as
it is
pumped from the cold reservoir to the hot while charging, and back from the
water to
the NaX bed as it is pumped from the hot reservoir to the cold while
discharging the AE-
CAES system. This is done using the methanol vapor compression heat pump H.P.
#1
39
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
during the first half of the charging and second half of the discharging
processes. During
the second half of the charging and first half of the discharging processes,
however,
heat is moved to and from the NaX bed by an adsorption heat pump based on
methanol
and activated carbon, which constitute the adsorbate and adsorbent,
respectively. The
heat in the activated carbon bed, in turn, is transferred to and from the
water reservoir
by a second vapor compression heat pump H.P. #2, which is based on a
conventional
halocarbon refrigerant such as dichloromethane. This second heat pump is also
used to
cool and to heat the air during compression and expansion, respectively, as
well as to
heat and to cool the methanol reservoir when H.P. #1 is in use and the
adsorption heat
pump is not.
[0110] The arrows adjacent the piping in Fig. 15 indicate the direction of
flow
of the various working fluids therein, in some instances labeled by the heat
these carry
between the various thermal reservoirs, during the first leg of the storage
cycle (or initial
half of the charging process). The heat produced by the compression of the air
is
labeled as Qi, while the heat taken from the methanol reservoir is labeled as
Q4. The
heat produced by adsorption of the air to the NaX is labeled as Q2, and the
additional
sensible heat taken from the NaX bed as it cools down towards ambient
temperatures is
labeled as Q3. Similarly, the arrows in Fig. 16 indicate the flows of the
various working
fluids, where the labels Qi, 02 and Q3 stand for these same components of the
overall
heat transferred to the hot water reservoir during the second leg of the
storage cycle,
and Q5 stands for the heat of adsorption of the methanol to the activated
carbon bed.
The arrows in Figs. 17 and 18 likewise indicate the direction of flow in the
adjacent
pipes, and the labels stand for these same components of the overall heat
transferred
back from the hot water reservoir to the rest of the system during the third
and fourth
legs (discharging portion) of the storage cycle, respectively. As previously
emphasized,
for ease of presentation we are disregarding the thermal energy losses
concomitant
upon all these heat transfers which, in most practical applications, must be
made up for
using an external heat source of some kind.
(0111] Figures 19 through 22 show much more detailed process flow
diagrams of the AE-CAES system of the second embodiment at the same four
points of
the overall charge-discharge cycle as Figs. 15 through 18, respectively. The
numbers of
the components in Figs. 19 through 22 are the same as in the corresponding
Figs. 7
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
and 8 of the first embodiment in those cases in which the components serve
similar
functions, and otherwise the numbers continue consecutively from those of the
first
embodiment. Note also that, just as in Figs. 7 and 8, Figs. 19 through 22 have
a parallel
pair of dashed lines with white space between them extending from top to
bottom, which
are intended to indicate that the scale of the embodiment is to some extent
arbitrary,
and that the relative sizes of the various subsystems, the number of repeated
components in them and the like are not essential to the embodiment, but could
be
varied substantially without altering the embodiment's ability to store and
regenerate
mechanical energy.
[01121 Specifically it may be seen that, just as in the first embodiment, the
NaX pellet beds I (heavy rectangular hatching) which adsorb the compressed air
are
contained in an array of cylinders with walls 2 formed from aluminum or other
pressure-
resistant, heat-conductive material, each with a perforated rigid tube 3
extending
through its length to provide structural support and to facilitate the flow of
air through the
bed. Note however that in Figs. 19 through 22 the compressed air is indicated
by
covering the space it fills with a pattern of heavy square dots, instead of
the left-to-right
upwards-slanted hatching that was used for this purpose in Figs. 7 and 8 of
the first
embodiment. The array of cylinders with walls 2 is once again contained in a
larger tank
with a thermally insulated (as indicated by the brick-like hatching) wall 4
that is used to
confine the methanol heat transfer fluid (left-to-right downwards slanted
hatching) by
which the cylinders and the NaX beds in them are cooled or heated while
charging or
discharging the system with compressed air, respectively. When charging the
system,
methanol liquid (heavy hatching) is sprayed through the nozzles 9 onto the
tops of the
cylinders in order to cool them as it flows down their sides and evaporates,
whereas
when charging the system methanol vapor (light hatching) is sucked into the
tank with
wall 4 through the perforated tubes 5 below the cylinders in order to heat
them as it
condenses on their sides. The methanol vapor produced by evaporation exits the
tank
with wall 4 through the vents 9 in its roof, while the methanol liquid from
condensation
exits through a drain 6 in its floor. The wall 4 of the temperature-control
tank could be
economically formed from fiberglass thick enough to withstand the pressure
variations
within it, which may range from several atmospheres to a few hundred torn,
depending
on the temperature in the tank at any given point in the cycle.
41
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
[0113] Other subsystems of the second embodiment that are similar to those
of the first embodiment are the methanol holding tank and pump (components 7 &
12),
the thermally insulated methanol reservoir with embedded heat exchanger
(components
14, 15 & 16), the methanol-based vapor-compression heat pump and heat
exchanger
(components 18, 19 and 20, 21), the tandem pair of centrifugal air compressors
(components 25 through 29), and an expansion turbine that uses the mixer
ejector
principle to keep the compressed air from cooling as it expands and regenerate
the
stored mechanical energy by efficiently mixing it with warm unpressurized air
(indicated
by filling the space it occupies with a pattern of light square dots in the
figures) in the
process (components 52 through 56). One small but significant refinement in
this last
subsystem is its use of a converging-diverging (or de Laval) nozzle to improve
the
suction efficiency, where the diverging portion is numbered 57 in Figs. 19
through 22.
This arrangement is an instance of a constant-pressure ejector (see e.g., J.
M.
Abdulateef, K. Sopian, M. A. Alghoul and M. Y. Sulaiman, Renew. Sustain.
Energy Rev.
13, 1336-1349, 2009).
[0114] Looking now specifically at Fig. 19, the charging process begins with
the NaX beds 1 in the cylinders with walls 2 at 10000 and the air pressure in
them at 10
bar gauge. All the water is in the cold (ambient temperature) water reservoir
with
thermally insulated walls 66, while essentially all the methanol is in the
reservoir with
walls 15. The pumps 64 and 65 are turned on to move water from the cold to the
hot
water reservoir with walls 67 at a controlled rate, passing through the heat
exchangers'
thermally insulated tanks with walls 20 and 62 as it does so. At the same time
the
compressors 19 and 69 of the vapor-compression heat pumps (H.P. #1 and M.F. #2
respectively in Figs. 15 through 18) are turned on, and the four-way valves 71
and 70
are set so that the heat is transferred to the water via the heat exchangers
21 and 63 in
the tanks with walls 20 and 62, respectively, as it is pumped through them.
The control
valve 10 is opened to allow liquid methanol to flow from the reservoir with
walls 15
through the nozzles 8 onto the cylinders with walls 2 which contain the hot
NaX beds 1,
where it cools the NaX beds 1 by evaporation off the walls 2 and exits the
thermally
insulated tank with walls 4 via the vents 9 in its top as previously
described. From there
it is sucked through the open valves 76 into the compressor 19, and the hot
compressed vapor exiting it is cooled by the water as the vapor passes through
the heat
42
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
exchanger 21. The vapor then partially liquefies as it passes through the
pressure-
reducing valve 24, and the liquid-vapor mixture returns to the reservoir with
wall 15 via
the port 14 in its top. Similarly, the hot compressed halocarbon refrigerant
vapor exiting
the compressor 69 is cooled by the water as it passes through the heat
exchanger 63,
and partially liquefies as it passes through the pressure-reducing valve 78.
This liquid-
vapor mixture then passes through the heat exchangers 27 and 29 of the
compressors
26 and 28, where it cools the air following the corresponding two stages of
compression
to 10 bar gauge. The air passes through the filter and dryer 25 before
entering the first
stage of compression, and is directed to the NaX beds 1 in the cylinders with
walls 2
after exiting the second stage, Meanwhile the still partially liquid
refrigerant exiting the
heat exchangers 27 and 29 continues on to the heat exchanger 16 in the
methanol
reservoir with walls 15, where completely vaporizes taking heat from the
methanol
reservoir as it does so and cooling it for more effective use in the next leg
of the cycle,
which will now be described.
[0115] Turning next to Fig. 20, the second leg of the cycle begins with the
NaX beds I at near-ambient temperatures (-25 C) and with roughly equal amounts
of
water in the cold and hot water reservoirs with walls 66 and 67, respectively.
The
methanol compressor 19 and corresponding water pump 64 are turned off, and the
valve 68 is closed to make sure water does not flow through that pathway.
Similarly the
valve 18 is shut, and the valves 75 leading to the thermally insulated tank
with wall 72
containing the activated carbon 74 opened. As a result the methanol vapor,
instead of
returning to the reservoir with wall 15, is adsorbed by the activated carbon,
which in turn
is cooled by the conventional halocarbon refrigerant as it passes through the
heat
exchanger 73. This is done by closing the valves 80 and 81 leading to the
methanol
reservoirs heat exchanger 16 and opening the valves 79 and 83 instead. The
other
subsystems continue to operate exactly as in the first leg of the cycle
described above.
It should be noted that in order for the adsorption refrigeration subsystem to
attain a
sufficient specific cooling power as the temperature drops to -40 C, it may be
necessary to blow a carrier gas such as air between the insulated tanks with
walls 4 and
72, although the fan and other components needed to achieve this have been
omitted
for simplicity.
43
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
[0116] The black diagonal bands signifying the activated carbon 74 in Figs. 19
through 22 are intended to indicate that it is formed into a fibrous ribbon
which is
wrapped around the heat exchanger 73 so as to improve the thermal contact
between
the activated carbon and heat exchanger, as described for example in [Hamamoto
et
atop Intnl. J. Refrl 29 (2006), 305]. The exact form of the activated carbon
is however
not essential to the embodiment, and many other forms such as a monolith or
granules
of carbon could be utilized. It is also possible that another adsorbent
entirely, such as a
zeohte or silica gel, could be employed. Neither is the use of methanol as the
primary
refrigerant in any way essential to the invention, and indeed a greater
specific cooling
power would be expected from a more volatile refrigerant such as ammonia at
low
temperatures, albeit at the expense of much higher pressures in the tank with
walls 4
during the high-temperature portion of the cycle. A mixture of refrigerants
such as
methanol and ammonia may also provide the optimum compromise in other
embodiments which similarly utilize an adsorption refrigerator of some kind to
cool the
porous material to which air is adsorbed. The existence of these and many
other well-
known variations serves to emphasize that the exact implementation of the
adsorption
refrigerator utilized is not essential to the invention, and it is also
possible that other
kinds of heat-driven refrigerators such as absorption systems or thereto-
compressors
could be advantageous in some applications of AE-CAES.
[0117] Looking now at Fig. 21, the discharging process begins with the NaX
beds 1 in the cylinders with walls 2 at -40 C but still under an air pressure
of 10 bar
gauge. All the water is in the hot water reservoir with wall 67, and all the
methanol that
was in the methanol reservoir with wall 15 has been adsorbed by the activated
carbon
74 in the thermally insulated tank with wall 72. The compressed air is
desorbed from the
NaX beds I by increasing their temperature in a controlled fashion. This is
done by
closing the control valve 10 and setting the four-way valve 70 so that the
hot,
pressurized vapor exiting the heat pump compressor 69 passes through the heat
exchanger 73 in thermal contact with the activated carbon 74, thereby raising
the latter's
temperature and causing methanol vapor to desorb from it. The valves 76
leading to
ports at the top of the temperature-control tank with wal14 are closed, and
the valve 11
is opened so that this methanol vapor now flows down the pressure gradient
leading to
the perforated tubing 5 at the bottom of the temperature-control tank, where
it rises by
44
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
virtue of its higher temperature and hence lower density. As it encounters the
cold
cylinders with walls 2, it condenses on them and releases its heat of
condensation in the
process. The liquid methanol runs down the sides of the cylinders and exits
the
temperature-control tank through the drain 6 in its bottom, from which it is
directed to
the holding tank 7. The positive-displacement pump 12 then drives it back
through the
now open valve 13 to the methanol reservoir tank with wai115. The heat that is
imparted
to the activated carbon 74 by the heat exchanger 73 comes from the hot water
reservoir
with wall 67. This heat is transferred to the conventional halocarbon
refrigerant flowing
through the heat exchanger 63 as the water is driven through the surrounding
tank with
wall 62 by the pump 65 to the cold water reservoir with wall 66. This process
causes the
halocarbon refrigerant to boll under the reduced pressure in the heat
exchanger 62, and
the resulting vapor is sucked into the compressor 69, from which it exits at
an elevated
temperature and pressure. This same hot pressurized halocarbon refrigerant is
also
used to heat the expanding air, as will now be described.
[0118] Continuing with the first part of the discharge process and Fig. 21,
the
air compressor subsystem 25 through 29 is turned off and the valve 30 shut to
isolate it
from the rest of the system. The air expander subsystem with components 52
though 59
is turned on by opening the valve 56 leading to the compressed air storage
subsystem
including components I through 4. In addition, the fan 60 is turned on to
bring additional
ambient air into the expander subsystem, passing as it does so over the heat
exchanger
61 through which the conventional halocarbon vapor exiting the heat exchanger
73 is
directed by opening the valves 84 and 85 while closing the valve 82 to prevent
flow
through the air compressor heat exchangers 27 and 29. This warm unpressurized
air
(indicated by filling the space it occupies with a pattern of light square
dots) passes via
the duct 52 to the stator blades 54, which impart vorticity to the warm air as
it is sucked
through them. This suction is generated by the compressed air as it passes
through the
converging-diverging nozzle, reaching Mach speed as it exits the converging
region 53
and supersonic speed as it exits the diverging region 57 with a pressure which
is at that
point well below that of the warm unpressurized air. This supersonic stream of
cold air
erupts into vortices as it exits the nozzle and entrains the warm air passing
through the
stator 54 in the converging region 58 of the elector, where the pressure
remains below
ambient. The two still incompletely mixed air streams enter the constant-area
region 59
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
at high velocity, where the vortices dissipate as they proceed to thoroughly
mix the two
air streams in a largely energy and momentum conserving process. Near the end
of the
constant area region 59, a shock wave forms that abruptly brings the air's
pressure
back above ambient and further reduces its speed. The ratio of the mass flow
rates of
the warm unpressured air and cold expanding air entering the expander
subsystem is
tailored so as to ensure that this rotating, subsonic but still rapidly
moving, stream of air
exits the constant area section 59 at a pressure slightly above ambient and
also at a
temperature near the normal ambient value of 25 C. This in turn ensures that
the
additional cooling that occurs as the air stream imparts its energy to the
rotor 55 will be
modest, since the pressure energy has already been largely converted into
kinetic
energy by the mixer-ejector subsystem with components 53, 54, 57, 58 and 59,
as
desired.
[0119] Finally, we come to the last leg of the cycle as illustrated in Fig.
22. At
the beginning of this leg essentially all the methanol has been driven from
the activated
carbon by heating it, condensed back to a liquid by the initially cold NaX,
and returned
to the methanol reservoir with wall 15. The valves 75 are closed to isolate
the activated
carbon from the rest of the system, the valve 18 is opened, the methanol
compressor 19
is turned on and the four-way valve 71 of the methanol heat pump is set so
that the
compressed, high-temperature methanol vapor exiting the compressor is driven
through
the valve 11 into the perforated tubing 5 at the bottom of the temperature-
control tank
with wall 4, just as it was during the previous leg of the cycle. In this way
the NaX beds
1 continue to be heated towards their maximum temperature over the cycle of
100 C,
while the resulting liquid methanol exiting the temperature-control tank
through the drain
6 is recycled back to the methanol reservoir by the pump 12. The heat again
comes
from the hot water reservoir, but it is passed directly to the methanol as it
boils in the
heat exchanger 21 and as the hot water is driven by the pump 64 through the
surrounding tank with wall 20 on its way to the cold reservoir. The methanol
exits the
reservoir as a vapor through the port 14 in its ceiling, and is partially
liquefied by
passage through the pressure-reducing valve 17 on its way to the heat
exchanger 21.
The methanol in the reservoir is heated by the conventional halocarbon
refrigerant to
promote vaporization as it is driven by the compressor 69 through the heat
exchanger
16. The halocarbon vapor then continues on to the heat exchanger 61 to warm
the
46
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
unpressurized air going into the mixer-ejector expansion turbine, as in the
previous leg.
The heat carried by the halocarbon vapor also comes from the hot water
reservoir as it
is driven by the pump 65 through the tank with wall 62 containing the heat
exchanger 63
on its way to the cold reservoir. By the end of this leg of the cycle the NaX
beds I have
been heated by to 100 C, and essentially all of the water has been returned to
the cold
water reservoir. The AE-CAES system is then ready to be recharged.
[0120] To keep the Camot limits on the efficiency of the vapor-compression
heat pumps above 90% (or coefficient of performance above 10), it is necessary
to
restrict the temperature lift to 35 C for heating or 30 0 for cooling. This
means that
when using the methanol-based heat pump to raise the temperature of the NaX
beds to
100 C at the end of the fourth leg of the cycle, the water passing into the
cold water
reservoir from the heat exchanger tank with wall 20 cannot be less than 65 C,
and
similarly, we can cool the NaX beds down as far as 35 C during the first leg
of the cycle
using the methanol-based heat pump while heating the water passing into the
hot water
reservoir to at most 65 C. Fortunately, during most of the fourth leg of the
cycle the
temperature of the NaX beds will be well below 1 00 C, allowing us cool the
water going
into the cold water reservoir quite a bit below 65"C, and similarly, during
most of the first
leg the NaX beds will be well above 35 C allowing us to heat the water passing
into the
hot water reservoir well above 65 C. The temperature of the cold water
reservoir will be
no more than 25 0 while that of the hot water reservoir will be no less than
75 C, once
a steady state has been reached over many charge-discharge cycles.
[0121] In order to obtain a round-trip efficiency greater than 80% for the
storage and recovery of mechanical energy, the halocarbon-based heat pump
should
also be at least 90% efficient in both directions, with similar restrictions
on the
temperature lifts it can achieve. In this case, however, the maximum and
minimum
temperatures it must attain are less precisely defined by the embodiment, and
these
details may vary significantly without substantially changing the nature of
the
embodiment. For example, the regeneration temperature of the activated carbon
will
depend on the precise preparation that is utilized, even assuming its physical
form is
that of a fibrous ribbon. Most activated carbon preparations would be expected
to lead
to regeneration temperatures in the range of 60 to 90 C at the reduced
methanol
pressures present while the NaX beds are below normal ambient temperatures,
which is
47
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
less demanding than the OO'C assumed for the NaX beds. Similarly, it is not
necessary
to cool the activated carbon much below 25 C in order to cool the NaX beds to
0 C.
The specific activated carbon preparation utilized however, has no effect on
the
principles which this AE-CAES embodiment is intended to illustrate, and it is
sufficient to
note that those skilled in the art of adsorption refrigeration will recognize
that both the
cooling and heating requirements for the activated carbon should be less
demanding
than those assumed here for the NaX beds. Similarly, the cooling and heating
requirements for the air as it is compressed to and expanded from 10 bar
should be less
demanding than for the NaX, especially given the mixer-ejector turbine used
for the
latter purpose and the fact that the air will be further cooled after it is
adsorbed by the
NaX beds.
10122] In the operation of an AE-CAES system, it is possible to use the
processes of adsorption and desoprtion to harvest additional energy from a low-
grade
heat source. In an analogous process, boiling water in a Rankine cycle power
generator
converts a certain amount of the heat of vaporization directly into PV
(pressure-volume)
work, even before the steam has been run through a turbine. A similar process
is also
operative in desorption, in that a certain fraction of the heat of desorption
is converted
directly into PV work prior to expanding the desorbed air. If an AE-CAES
system is run
using a symmetric PV cycle, this stores a modest of amount of additional
energy in the
AE-CAES system, as was explicitly illustrated in Fig. 13. Figure 23 shows an
idealized
PV-cycle that illustrates how a clockwise loop can be added to the overall
cycle,
allowing an AE-CAES system to also harvest a certain amount of heat energy
(subject,
of course, to the Carnot limits). In the idealized cycle shown, there are
three stages of
adiabatic compression and expansion to and from 13 bar (12 bar gauge),
separated by
isobaric cooling and heating to 25 C, respectively, which approximates a
practical (less-
than-isothermal) compression and expansion cycle, The compression stages are
followed by isobaric adsorption of the air in an NaX bed as it is cooled to ---
40 C, greatly
reducing its volume for storage. Rather than desorbing the air by the inverse
isobaric
process, however, the bed is allowed to warm up to -6"C at constant volume,
which
raises its pressure to 30.5 bar, followed by isobaric heating to 107 C and
adiabatic
expansion back to 13 bar. The rest of the expansion process then proceeds as
it would
in a pure storage cycle. The energy harvested is equal to the area of the
enclosed by
48
CA 02763642 2011-11-25
WO 2010/138677 PCT/US2010/036334
the upper left-hand loop, and is approximately equal to the areas enclosed by
the three
lower right-hand loops which represent the energy lost in the compression-and-
expansion processes.
49