Note: Descriptions are shown in the official language in which they were submitted.
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
HYDROCARBON GAS PROCESSING
SPECIFICATION
BACKGROUND OF THE INVENTION
[0001] This invention relates to a process and apparatus for the
separation of a gas
containing hydrocarbons. The applicants claim the benefits under Title 35,
United States
Code, Section 119(e) of prior U.S. Provisional Application Number 61/186,361
which was
filed on June 11, 2009. The applicants also claim the benefits under Title 35,
United States
Code, Section 120 as a continuation-in-part of U.S. Patent Application No.
12/750,862 which
was filed on March 31, 2010, and as a continuation-in-part of U.S. Patent
Application No.
12/717,394 which was filed on March 4, 2010, and as a continuation-in-part of
U.S. Patent
Application No. 12/689,616 which was filed on January 19, 2010, and as a
continuation-in-part of U.S. Patent Application No. 12/372,604 which was filed
on
-1-
CA 02763714 2011-11-28
WO 2010/144186
PCT/US2010/033374
February 17, 2009. Assignees S.M.E. Products LP and Ortloff Engineers, Ltd.
were parties
to a joint research agreement that was in effect before the invention of this
application was
made.
[0002] Ethylene, ethane, propylene, propane, and/or heavier hydrocarbons
can be
recovered from a variety of gases, such as natural gas, refinery gas, and
synthetic gas streams
obtained from other hydrocarbon materials such as coal, crude oil, naphtha,
oil shale, tar
sands, and lignite. Natural gas usually has a major proportion of methane and
ethane, i.e.,
methane and ethane together comprise at least 50 mole percent of the gas. The
gas also
contains relatively lesser amounts of heavier hydrocarbons such as propane,
butanes,
pentanes, and the like, as well as hydrogen, nitrogen, carbon dioxide, and
other gases.
[0003] The present invention is generally concerned with the recovery of
ethylene,
ethane, propylene, propane, and heavier hydrocarbons from such gas streams. A
typical
analysis of a gas stream to be processed in accordance with this invention
would be, in
approximate mole percent, 90.3% methane, 4.0% ethane and other C2 components,
1.7%
propane and other C3 components, 0.3% iso-butane, 0.5% normal butane, and 0.8%
pentanes
plus, with the balance made up of nitrogen and carbon dioxide. Sulfur
containing gases are
also sometimes present.
[0004] The historically cyclic fluctuations in the prices of both natural
gas and its
natural gas liquid (NGL) constituents have at times reduced the incremental
value of ethane,
ethylene, propane, propylene, and heavier components as liquid products. This
has resulted
in a demand for processes that can provide more efficient recoveries of these
products, for
processes that can provide efficient recoveries with lower capital investment,
and for
processes that can be easily adapted or adjusted to vary the recovery of a
specific component
over a broad range. Available processes for separating these materials include
those based
upon cooling and refrigeration of gas, oil absorption, and refrigerated oil
absorption.
-2-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
Additionally, cryogenic processes have become popular because of the
availability of
economical equipment that produces power while simultaneously expanding and
extracting
heat from the gas being processed. Depending upon the pressure of the gas
source, the
richness (ethane, ethylene, and heavier hydrocarbons content) of the gas, and
the desired end
products, each of these processes or a combination thereof may be employed.
[0005] The cryogenic expansion process is now generally preferred for
natural gas
liquids recovery because it provides maximum simplicity with ease of startup,
operating
flexibility, good efficiency, safety, and good reliability. U.S. Patent Nos.
3,292,380;
4,061,481; 4,140,504; 4,157,904; 4,171,964; 4,185,978; 4,251,249; 4,278,457;
4,519,824;
4,617,039; 4,687,499; 4,689,063; 4,690,702; 4,854,955; 4,869,740; 4,889,545;
5,275,005;
5,555,748; 5,566,554; 5,568,737; 5,771,712; 5,799,507; 5,881,569; 5,890,378;
5,983,664;
6,182,469; 6,578,379; 6,712,880; 6,915,662; 7,191,617; 7,219,513; reissue U.S.
Patent No.
33,408; and co-pending application nos. 11/430,412; 11/839,693; 11/971,491;
and
12/206,230 describe relevant processes (although the description of the
present invention in
some cases is based on different processing conditions than those described in
the cited U.S.
Patents).
[0006] In a typical cryogenic expansion recovery process, a feed gas
stream under
pressure is cooled by heat exchange with other streams of the process and/or
external sources
of refrigeration such as a propane compression-refrigeration system. As the
gas is cooled,
liquids may be condensed and collected in one or more separators as high-
pressure liquids
containing some of the desired C2+ components. Depending on the richness of
the gas and
the amount of liquids formed, the high-pressure liquids may be expanded to a
lower pressure
and fractionated. The vaporization occurring during expansion of the liquids
results in
further cooling of the stream. Under some conditions, pre-cooling the high
pressure liquids
prior to the expansion may be desirable in order to further lower the
temperature resulting
-3-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
from the expansion. The expanded stream, comprising a mixture of liquid and
vapor, is
fractionated in a distillation (demethanizer or deethanizer) column. In the
column, the
expansion cooled stream(s) is (are) distilled to separate residual methane,
nitrogen, and other
volatile gases as overhead vapor from the desired C2 components, C3
components, and
heavier hydrocarbon components as bottom liquid product, or to separate
residual methane,
C2 components, nitrogen, and other volatile gases as overhead vapor from the
desired C3
components and heavier hydrocarbon components as bottom liquid product.
[0007] If the feed gas is not totally condensed (typically it is not),
the vapor
remaining from the partial condensation can be split into two streams. One
portion of the
vapor is passed through a work expansion machine or engine, or an expansion
valve, to a
lower pressure at which additional liquids are condensed as a result of
further cooling of the
stream. The pressure after expansion is essentially the same as the pressure
at which the
distillation column is operated. The combined vapor-liquid phases resulting
from the
expansion are supplied as feed to the column.
[0008] The remaining portion of the vapor is cooled to substantial
condensation by
heat exchange with other process streams, e.g., the cold fractionation tower
overhead. Some
or all of the high-pressure liquid may be combined with this vapor portion
prior to cooling.
The resulting cooled stream is then expanded through an appropriate expansion
device, such
as an expansion valve, to the pressure at which the demethanizer is operated.
During
expansion, a portion of the liquid will vaporize, resulting in cooling of the
total stream. The
flash expanded stream is then supplied as top feed to the demethanizer.
Typically, the vapor
portion of the flash expanded stream and the demethanizer overhead vapor
combine in an
upper separator section in the fractionation tower as residual methane product
gas.
Alternatively, the cooled and expanded stream may be supplied to a separator
to provide
-4-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
vapor and liquid streams. The vapor is combined with the tower overhead and
the liquid is
supplied to the column as a top column feed.
100091 In the ideal operation of such a separation process, the residue
gas leaving the
process will contain substantially all of the methane in the feed gas with
essentially none of
the heavier hydrocarbon components and the bottoms fraction leaving the
demethanizer will
contain substantially all of the heavier hydrocarbon components with
essentially no methane
or more volatile components. In practice, however, this ideal situation is not
obtained
because the conventional demethanizer is operated largely as a stripping
column. The
methane product of the process, therefore, typically comprises vapors leaving
the top
fractionation stage of the column, together with vapors not subjected to any
rectification step.
Considerable losses of C2, C3, and C4+ components occur because the top liquid
feed contains
substantial quantities of these components and heavier hydrocarbon components,
resulting in
corresponding equilibrium quantities of C2 components, C3 components, C4
components, and
heavier hydrocarbon components in the vapors leaving the top fractionation
stage of the
demethanizer. The loss of these desirable components could be significantly
reduced if the
rising vapors could be brought into contact with a significant quantity of
liquid (reflux)
capable of absorbing the C2 components, C3 components, C4 components, and
heavier
hydrocarbon components from the vapors.
[0010] In recent years, the preferred processes for hydrocarbon
separation use an
upper absorber section to provide additional rectification of the rising
vapors. One method of
generating a reflux stream for the upper rectification section is to use the
flash expanded
substantially condensed stream to cool and partially condense the column
overhead vapor,
with the heated flash expanded stream then directed to a mid-column feed point
on the
demethanizer. The liquid condensed from the column overhead vapor is separated
and
supplied as top feed to the demethanizer, while the uncondensed vapor is
discharged as the
-5-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
residual methane product gas. The heated flash expanded stream is only
partially vaporized,
and so contains a substantial quantity of liquid that serves as supplemental
reflux for the
demethanizer, so that the top reflux feed can then rectify the vapors leaving
the lower section
of the column. U.S. Patent No. 4,854,955 is an example of a process of this
type.
[0011] The present invention employs a novel means of performing the
various steps
described above more efficiently and using fewer pieces of equipment. This is
accomplished
by combining what heretofore have been individual equipment items into a
common housing,
thereby reducing the plot space required for the processing plant and reducing
the capital cost
of the facility. Surprisingly, applicants have found that the more compact
arrangement also
significantly reduces the power consumption required to achieve a given
recovery level,
thereby increasing the process efficiency and reducing the operating cost of
the facility. In
addition, the more compact arrangement also eliminates much of the piping used
to
interconnect the individual equipment items in traditional plant designs,
further reducing
capital cost and also eliminating the associated flanged piping connections.
Since piping
flanges are a potential leak source for hydrocarbons (which are volatile
organic compounds,
VOCs, that contribute to greenhouse gases and may also be precursors to
atmospheric ozone
famiation), eliminating these flanges reduces the potential for atmospheric
emissions that can
damage the environment.
[0012] In accordance with the present invention, it has been found that
C2 recoveries
in excess of 86% can be obtained. Similarly, in those instances where recovery
of C2
components is not desired, C3 recoveries in excess of 99% can be obtained
while providing
essentially complete rejection of C2 components to the residue gas stream. In
addition, the
present invention makes possible essentially 100% separation of methane (or C2
components)
and lighter components from the C2 components (or C3 components) and heavier
components
at lower energy requirements compared to the prior art while maintaining the
same recovery
-6-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
level. The present invention, although applicable at lower pressures and
warmer
temperatures, is particularly advantageous when processing feed gases in the
range of 400 to
1500 psia [2,758 to 10,342 kPa(a)] or higher under conditions requiring NGL
recovery
column overhead temperatures of -50 F [-46 C] or colder.
[0013] For a better understanding of the present invention, reference is
made to the
following examples and drawings. Referring to the drawings:
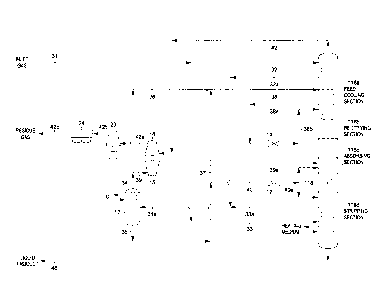
[0014] FIGS. 1 and 2 are flow diagrams of prior art natural gas
processing plants in
accordance with United States Patent No. 4,854,955;
[0015] FIG. 3 is a flow diagram of a natural gas processing plant in
accordance with
the present invention; and
[0016] FIGS. 4 through 6 are flow diagrams illustrating alternative means
of
application of the present invention to a natural gas stream.
[0017] In the following explanation of the above figures, tables are
provided
summarizing flow rates calculated for representative process conditions. In
the tables
appearing herein, the values for flow rates (in moles per hour) have been
rounded to the
nearest whole number for convenience. The total stream rates shown in the
tables include all
non-hydrocarbon components and hence are generally larger than the sum of the
stream flow
rates for the hydrocarbon components. Temperatures indicated are approximate
values
rounded to the nearest degree. It should also be noted that the process design
calculations
performed for the purpose of comparing the processes depicted in the figures
are based on the
assumption of no heat leak from (or to) the surroundings to (or from) the
process. The quality
of commercially available insulating materials makes this a very reasonable
assumption and
one that is typically made by those skilled in the art.
[0018] For convenience, process parameters are reported in both the
traditional
British units and in the units of the Systeme International d'Unites (SI). The
molar flow rates
-7-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
given in the tables may be interpreted as either pound moles per hour or
kilogram moles per
hour. The energy consumptions reported as horsepower (HP) and/or thousand
British
Thermal Units per hour (MBTU/Hr) correspond to the stated molar flow rates in
pound moles
per hour. The energy consumptions reported as kilowatts (kW) correspond to the
stated
molar flow rates in kilogram moles per hour.
DESCRIPTION OF THE PRIOR ART
[0019] FIG. 1 is a process flow diagram showing the design of a
processing plant to
recover C2+ components from natural gas using prior art according to U.S. Pat.
No.
4,854,955. In this simulation of the process, inlet gas enters the plant at
110 F [43 C] and
915 psia [6,307 kPa(a)] as stream 31. If the inlet gas contains a
concentration of sulfur
compounds which would prevent the product streams from meeting specifications,
the sulfur
compounds are removed by appropriate pretreatment of the feed gas (not
illustrated). In
addition, the feed stream is usually dehydrated to prevent hydrate (ice)
formation under
cryogenic conditions. Solid desiccant has typically been used for this
purpose.
[0020] The feed stream 31 is divided into two portions, streams 32 and
33. Stream 32
is cooled to -34 F [-37 C] in heat exchanger 10 by heat exchange with cool
residue gas
stream 42a, while stream 33 is cooled to -13 F [-25 C] in heat exchanger 11 by
heat
exchange with demethanizer reboiler liquids at 52 F [11 C] (stream 45) and
side reboiler
liquids at -49 F [-45 C] (stream 44). Streams 32a and 33a recombine to form
stream 31a,
which enters separator 12 at -28 F [-33 C] and 893 psia [6,155 kPa(a)] where
the vapor
(stream 34) is separated from the condensed liquid (stream 35).
[0021] The vapor (stream 34) from separator 12 is divided into two
streams, 36 and
39. Stream 36, containing about 27% of the total vapor, is combined with the
separator liquid
(stream 35), and the combined stream 38 passes through heat exchanger 13 in
heat exchange
-8-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
relation with cold residue gas stream 42 where it is cooled to substantial
condensation. The
resulting substantially condensed stream 38a at -135 F [-93 C] is then flash
expanded
through expansion valve 14 to slightly above the operating pressure
(approximately 396 psia
[2,730 kPa(a)]) of fractionation tower 18. During expansion a portion of the
stream is
vaporized, resulting in cooling of the total stream. In the process
illustrated in FIG. 1, the
expanded stream 38b leaving expansion valve 14 reaches a temperature of -138 F
[-94 C]
before entering heat exchanger 20. In heat exchanger 20, the flash expanded
stream is heated
and partially vaporized as it provides cooling and partial condensation of
column overhead
stream 41, with the heated stream 38c at -139 F [-95 C] thereafter supplied to
fractionation
tower 18 at an upper mid-column feed point. (Note that the temperature of
stream 38b/38c
drops slightly as it is heated, due to the pressure drop through heat
exchanger 20 and the
resulting vaporization of some of the liquid methane contained in the stream.)
[0022] The remaining 73% of the vapor from separator 12 (stream 39)
enters a work
expansion machine 15 in which mechanical energy is extracted from this portion
of the high
pressure feed. The machine 15 expands the vapor substantially isentropically
to the tower
operating pressure, with the work expansion cooling the expanded stream 39a to
a
temperature of approximately -95 F [-71 C]. The typical commercially available
expanders
are capable of recovering on the order of 80-85% of the work theoretically
available in an
ideal isentropic expansion. The work recovered is often used to drive a
centrifugal
compressor (such as item 16) that can be used to re-compress the heated
residue gas stream
(stream 42b), for example. The partially condensed expanded stream 39a is
thereafter
supplied as feed to fractionation tower 18 at a lower mid-column feed point.
[0023] The column overhead vapor (stream 41) is withdrawn from the top of
demethanizer 18 and cooled from -136 F [-93 C] to -138 F [-94 C] and partially
condensed
(stream 41a) in heat exchanger 20 by heat exchange with the flash expanded
substantially
-9-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
condensed stream 38b as previously described. The operating pressure in reflux
separator 21
(391 psia [2,696 kPa(a)]) is maintained slightly below the operating pressure
of demethanizer
18. This provides the driving force which causes overhead vapor stream 41 to
flow through
heat exchanger 20 and thence into the reflux separator 21 wherein the
condensed liquid
(stream 43) is separated from the uncondensed vapor (stream 42). The liquid
stream 43 from
reflux separator 21 is pumped by pump 22 to a pressure slightly above the
operating pressure
of demethanizer 18, and stream 43a is then supplied as cold top column feed
(reflux) to
demethanizer 18. This cold liquid reflux absorbs and condenses the C2
components, C3
components, and heavier components in the vapors rising through the upper
region of
absorbing section 18a of demethanizer 18.
[0024] The demethanizer in tower 18 is a conventional distillation column
containing
a plurality of vertically spaced trays, one or more packed beds, or some
combination of trays
and packing. As is often the case in natural gas processing plants, the
demethanizer tower
consists of two sections: an upper absorbing (rectification) section 18a that
contains the trays
and/or packing to provide the necessary contact between the vapor portion of
expanded
stream 39a rising upward and cold liquid falling downward to condense and
absorb the C2
components, C3 components, and heavier components; and a lower stripping
(demethanizing)
section 18b that contains the trays and/or packing to provide the necessary
contact between
the liquids falling downward and the vapors rising upward. The demethanizing
section 18b
also includes reboilers (such as the reboiler and the side reboiler described
previously) which
heat and vaporize a portion of the liquids flowing down the column to provide
the stripping
vapors which flow up the column to strip the liquid product (stream 46) of
methane and
lighter components. The liquid product stream 46 exits the bottom of the tower
at 77 F
[25 C], based on a typical specification of a methane to ethane ratio of
0.010:1 on a mass
basis in the bottom product.
-10-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
[0025] Vapor stream 42 from reflux separator 21 is the cold residue gas
stream. It
passes countercurrently to the incoming feed gas in heat exchanger 13 where it
is heated to
-54 F [-48 C] (stream 42a) and in heat exchanger 10 where it is heated to 98 F
[37 C]
(stream 42b) as it provides cooling as previously described. The residue gas
is then
re-compressed in two stages. The first stage is compressor 16 driven by
expansion machine
15. The second stage is compressor 23 driven by a supplemental power source
which
compresses the residue gas (stream 42d) to sales line pressure. After cooling
to 110 F [43 C]
in discharge cooler 24, residue gas stream 42e flows to the sales gas pipeline
at 915 psia
[6,307 kPa(a)], sufficient to meet line requirements (usually on the order of
the inlet
pressure).
[0026] A summary of stream flow rates and energy consumption for the
process
illustrated in FIG. 1 is set forth in the following table:
-11-
CA 02763714 2011-11-28
WO 2010/144186
PCT/US2010/033374
Table I
(FIG. 1)
Stream Flow Summary - Lb. Moles/Hr [kg moles/Hr]
Stream Methane Ethane Propane Butanes+ Total
31 12,398 546 233 229 13,726
32 8,431 371 159 156 9,334
33 3,967 175 74 73 4,392
34 12,195 501 179 77 13,261
35 203 45 54 152 465
36 3,317 136 49 21 3,607
38 3,520 181 103 173 4,072
39 8,878 365 130 56 9,654
41 12,449 86 7 1 12,788
43 60 4 2 1 69
42 12,389 82 5 0 12,719
46 9 464 228 229 1,007
-12-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
Recoveries*
Ethane 84.99%
Propane 97.74%
Butanes+ 99.83%
Power
Residue Gas Compression 5,505 HP 9,050 kW]
* (Based on un-rounded flow rates)
[0027] FIG. 2 is a process flow diagram showing one manner in which the
design of
the processing plant in FIG. 1 can be adapted to operate at a lower C2
component recovery
level. This is a common requirement when the relative values of natural gas
and liquid
hydrocarbons are variable, causing recovery of the C2 components to be
unprofitable at times.
The process of FIG. 2 has been applied to the same feed gas composition and
conditions as
described previously for FIG. 1. However, in the simulation of the process of
FIG. 2, the
process operating conditions have been adjusted to reject nearly all of C2
components to the
residue gas rather than recovering them in the bottom liquid product from the
fractionation
tower.
[0028] In this simulation of the process, inlet gas enters the plant at
110 F [43 C] and
915 psia [6,307 kPa(a)] as stream 31 and is cooled in heat exchanger 10 by
heat exchange
with cool residue gas stream 42a. Cooled stream 31a enters separator 12 at 15
F [-9 C] and
900 psia [6,203 kPa(a)] where the vapor (stream 34) is separated from the
condensed liquid
(stream 35).
[0029] The vapor (stream 34) from separator 12 is divided into two
streams, 36 and
39. Stream 36, containing about 28% of the total vapor, is combined with the
separator liquid
-13-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
(stream 35), and the combined stream 38 passes through heat exchanger 13 in
heat exchange
relation with cold residue gas stream 42 where it is cooled to substantial
condensation. The
resulting substantially condensed stream 38a at -114 F [-81 C] is then flash
expanded
through expansion valve 14 to slightly above the operating pressure
(approximately 400 psia
[2,758 kPa(a)]) of fractionation tower 18. During expansion a portion of the
stream is
vaporized, resulting in cooling of the total stream. In the process
illustrated in FIG. 2, the
expanded stream 38b leaving expansion valve 14 reaches a temperature of -137 F
[-94 C]
before entering heat exchanger 20. In heat exchanger 20, the flash expanded
stream is heated
and partially vaporized as it provides cooling and partial condensation of
column overhead
stream 41, with the heated stream 38c at -107 F [-77 C] thereafter supplied to
fractionation
tower 18 at an upper mid-column feed point.
[0030] The remaining 72% of the vapor from separator 12 (stream 39)
enters a work
expansion machine 15 in which mechanical energy is extracted from this portion
of the high
pressure feed. The machine 15 expands the vapor substantially isentropically
to the tower
operating pressure, with the work expansion cooling the expanded stream 39a to
a
temperature of approximately -58 F [-50 C] before it is supplied as feed to
fractionation
tower 18 at a lower mid-column feed point.
[0031] The column overhead vapor (stream 41) is withdrawn from the top of
deethanizer 18 and cooled from -102 F [-74 C] to -117 F [-83 C] and partially
condensed
(stream 41a) in heat exchanger 20 by heat exchange with the flash expanded
substantially
condensed stream 38b as previously described. The partially condensed stream
41a enters
reflux separator 21, operating at 395 psia [2,723 kPa(a)], where the condensed
liquid (stream
43) is separated from the uncondensed vapor (stream 42). The liquid stream 43
from reflux
separator 21 is pumped by pump 22 to a pressure slightly above the operating
pressure of
-14-
CA 02763714 2011-11-28
deethanizer 18, and stream 43a is then supplied as cold top column feed
(reflux) to
deethanizer 18.
100321 The liquid product stream 46 exits the bottom of the tower at 223 F
[106 C],
based on atypical specification of an ethane to propane ratio of 0.050:1 on a
molar basis in
the bottom product. The cold residue gas (vapor stream 42 from reflux
separator 21) passes
countercurrently to the incoming feed gas in heat exchanger 13 where it is
heated to -25 F
[-31 C] (stream 42a) and in heat exchanger 10 where it is heated to 105 F [41
C] (stream
42b) as it provides cooling as previously described. The residue gas is then
re-compressed in
two stages, compressor 16 driven by expansion machine 15 and compressor 23
driven by a
supplemental power source. After stream 42d is cooled to 110 F [43 C] in
discharge cooler
24, the residue gas product (stream 42e) flows to the sales gas pipeline at
915 psia
[6,307 kPa(a)].
100331 A summary of stream flow rates and energy consumption for the
process
illustrated in FIG. 2 is set forth in the following table:
-15-
CA 02763714 2011-11-28
WO 2010/144186
PCT/US2010/033374
Table II
(FIG. 2)
Stream Flow Summary - Lb. Moles/Hr [kg moles/Hr]
Stream Methane Ethane Propane Butanes+ Total
31 12,398 546 233 229 13,726
34 12,332 532 215 128 13,523
35 66 14 18 101 203
36 3,502 151 61 36 3,841
38 3,568 165 79 137 4,044
39 8,830 381 154 92 9,682
41 13,441 1,033 7 0 14,877
43 1,043 498 6 0 1,624
42 12,398 535 1 0 13,253
46 0 11 232 229 473
Recoveries*
Propane 99.50%
Butanes+ 100.00%
Power
Residue Gas Compression 5,595 HP [ 9,198 kW]
* (Based on un-rounded flow rates)
-16-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
DESCRIPTION OF THE INVENTION
Example 1
[0034] FIG. 3 illustrates a flow diagram of a process in accordance with
the present
invention. The feed gas composition and conditions considered in the process
presented in
FIG. 3 are the same as those in FIG. 1. Accordingly, the FIG. 3 process can be
compared
with that of the FIG. 1 process to illustrate the advantages of the present
invention.
[0035] In the simulation of the FIG. 3 process, inlet gas enters the
plant as stream 31
and is divided into two portions, streams 32 and 33. The first portion, stream
32, enters a
heat exchange means in the upper region of feed cooling section 118a inside
processing
assembly 118. This heat exchange means may be comprised of a fin and tube type
heat
exchanger, a plate type heat exchanger, a brazed aluminum type heat exchanger,
or other type
of heat transfer device, including multi-pass and/or multi-service heat
exchangers. The heat
exchange means is configured to provide heat exchange between stream 32
flowing through
one pass of the heat exchange means and a distillation vapor stream arising
from rectifying
section 118b inside processing assembly 118 that has been heated in a heat
exchange means
in the lower region of feed cooling section 118a. Stream 32 is cooled while
further heating
the distillation vapor stream, with stream 32a leaving the heat exchange means
at -29 F
[-34 C].
[0036] The second portion, stream 33, enters a heat and mass transfer
means in
stripping section 118d inside processing assembly 118. This heat and mass
transfer means
may also be comprised of a fin and tube type heat exchanger, a plate type heat
exchanger, a
brazed aluminum type heat exchanger, or other type of heat transfer device,
including
multi-pass and/or multi-service heat exchangers. The heat and mass transfer
means is
configured to provide heat exchange between stream 33 flowing through one pass
of the heat
and mass transfer means and a distillation liquid stream flowing downward from
an
-17-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
absorbing means above the heat and mass transfer means in stripping section
118d, so that
stream 33 is cooled while heating the distillation liquid stream, cooling
stream 33a to -10 F
[-23 C] before it leaves the heat and mass transfer means. As the distillation
liquid stream is
heated, a portion of it is vaporized to form stripping vapors that rise upward
as the remaining
liquid continues flowing downward through the heat and mass transfer means.
The heat and
mass transfer means provides continuous contact between the stripping vapors
and the
distillation liquid stream so that it also functions to provide mass transfer
between the vapor
and liquid phases, stripping the liquid product stream 46 of methane and
lighter components.
[0037] Streams 32a and 33a recombine to form stream 31a, which enters
separator
section 118e inside processing assembly 118 at -23 F [-31 C] and 900 psia
[6,203 kPa(a)],
whereupon the vapor (stream 34) is separated from the condensed liquid (stream
35).
Separator section 118e has an internal head or other means to divide it from
stripping section
118d, so that the two sections inside processing assembly 118 can operate at
different
pressures.
[0038] The vapor (stream 34) from separator section 118e is divided into
two streams,
36 and 39. Stream 36, containing about 29% of the total vapor, is combined
with the
separated liquid (stream 35, via stream 37), and the combined stream 38 enters
a heat
exchange means in the lower region of feed cooling section 118a inside
processing assembly
118. This heat exchange means may likewise be comprised of a fin and tube type
heat
exchanger, a plate type heat exchanger, a brazed aluminum type heat exchanger,
or other type
of heat transfer device, including multi-pass and/or multi-service heat
exchangers. The heat
exchange means is configured to provide heat exchange between stream 38
flowing through
one pass of the heat exchange means and the distillation vapor stream arising
from rectifying
section 118b inside processing assembly 118, so that stream 38 is cooled to
substantial
condensation while heating the distillation vapor stream.
-18-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
[0039] The resulting substantially condensed stream 38a at -135 F [-93 C]
is then
flash expanded through expansion valve 14 to slightly above the operating
pressure
(approximately 388 psia [2,675 kPa(a)]) of rectifying section 118b and
absorbing section
118c (an absorbing means) inside processing assembly 118. During expansion a
portion of
the stream may be vaporized, resulting in cooling of the total stream. In the
process
illustrated in FIG. 3, the expanded stream 38b leaving expansion valve 14
reaches a
temperature of -139 F [-95 C] before it is directed into a heat and mass
transfer means inside
rectifying section 118b. This heat and mass transfer means may also be
comprised of a fin
and tube type heat exchanger, a plate type heat exchanger, a brazed aluminum
type heat
exchanger, or other type of heat transfer device, including multi-pass and/or
multi-service
heat exchangers. The heat and mass transfer means is configured to provide
heat exchange
between the distillation vapor stream arising from absorbing section 118c
flowing upward
through one pass of the heat and mass transfer means and the expanded stream
38b flowing
downward, so that the distillation vapor is cooled while heating the expanded
stream. As the
distillation vapor stream is cooled, a portion of it is condensed and falls
downward while the
remaining distillation vapor continues flowing upward through the heat and
mass transfer
means. The heat and mass transfer means provides continuous contact between
the
condensed liquid and the distillation vapor so that it also functions to
provide mass transfer
between the vapor and liquid phases, thereby providing rectification of the
distillation vapor.
The condensed liquid is collected from the bottom of the heat and mass
transfer means and
directed to absorbing section 118c.
[0040] The flash expanded stream 38b is partially vaporized as it
provides cooling
and partial condensation of the distillation vapor stream, and exits the heat
and mass transfer
means in rectifying section 118b at -140 F [-96 C]. (Note that the temperature
of stream 38b
drops slightly as it is heated, due to the pressure drop through the heat and
mass transfer
-19-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
means and the resulting vaporization of some of the liquid methane contained
in the stream.)
The heated flash expanded stream is separated into its respective vapor and
liquid phases,
with the vapor phase combining with the vapor arising from absorbing section
118c to form
the distillation vapor stream that enters the heat and mass transfer means in
rectifying section
118b as previously described. The liquid phase is directed to the upper region
of absorbing
section 118c to join with the liquid condensed from the distillation vapor
stream in rectifying
section 118b.
100411 The remaining 71% of the vapor from separator section 118e (stream
39)
enters a work expansion machine 15 in which mechanical energy is extracted
from this
portion of the high pressure feed. The machine 15 expands the vapor
substantially
isentropically to the operating pressure of absorbing section 118c, with the
work expansion
cooling the expanded stream 39a to a temperature of approximately -93 F [-70
C]. The
partially condensed expanded stream 39a is thereafter supplied as feed to the
lower region of
absorbing section 118c inside processing assembly 118 to be contacted by the
liquids
supplied to the upper region of absorbing section 118c.
[0042] Absorbing section 118c and stripping section 118d each contain an
absorbing
means consisting of a plurality of vertically spaced trays, one or more packed
beds, or some
combination of trays and packing. The trays and/or packing in absorbing
section 118c and
stripping section 118d provide the necessary contact between the vapors rising
upward and
cold liquid falling downward. The liquid portion of the expanded stream 39a
commingles
with liquids falling downward from absorbing section 118c and the combined
liquid
continues downward into stripping section 118d. The vapors arising from
stripping section
118d combine with the vapor portion of the expanded stream 39a and rise upward
through
absorbing section 118c, to be contacted with the cold liquid falling downward
to condense
and absorb most of the C2 components, C3 components, and heavier components
from these
-20-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
vapors. The vapors arising from absorbing section 118c combine with the vapor
portion of
the heated expanded stream 38b and rise upward through rectifying section
118b, to be
cooled and rectified to remove most of the C2 components, C3 components, and
heavier
components remaining in these vapors as previously described. The liquid
portion of the
heated expanded stream 38b commingles with liquids falling downward from
rectifying
section 118b and the combined liquid continues downward into absorbing section
118c.
[0043] The distillation liquid flowing downward from the heat and mass
transfer
means in stripping section 118d inside processing assembly 118 has been
stripped of methane
and lighter components. The resulting liquid product (stream 46) exits the
lower region of
stripping section 118d and leaves processing assembly 118 at 73 F [23 C]. The
distillation
vapor stream arising from rectifying section 118b is warmed in feed cooling
section 118a as
it provides cooling to streams 32 and 38 as previously described, and the
resulting residue gas
stream 42 leaves processing assembly 118 at 99 F [37 C]. The residue gas
stream is then
re-compressed in two stages, compressor 16 driven by expansion machine 15 and
compressor
23 driven by a supplemental power source. After stream 42b is cooled to 110 F
[43 C] in
discharge cooler 24, the residue gas product (stream 42c) flows to the sales
gas pipeline at
915 psia [6,307 kPa(a)].
[0044] A summary of stream flow rates and energy consumption for the
process
illustrated in FIG. 3 is set forth in the following table:
-21-
CA 02763714 2011-11-28
WO 2010/144186
PCT/US2010/033374
Table III
(FIG. 3)
Stream Flow Summary - Lb. Moles/Hr [kg moles/Hr]
Stream Methane Ethane Propane Butanes+ Total
31 12,398 546 233 229 13,726
32 8,431 371 159 156 9,334
33 3,967 175 74 73 4,392
34 12,221 507 186 83 13,308
35 177 39 47 146 418
36 3,544 147 54 24 3,859
37 177 39 47 146 418
38 3,721 186 101 170 4,277
39 8,677 360 132 59 9,449
42 12,389 73 5 0 12,700
46 9 473 228 229 1,026
-22-
, CA 02763714 2011-11-28
=
Recoveries*
Ethane 86.66%
Propane 98.01%
Butanes+ 99.81%
Power
Residue Gas Compression 5,299 HP [ 8,711
kW]
* (Based on un-rounded flow rates)
100451 A comparison of Tables I and III shows that, compared
to the prior art, the
present invention improves ethane recovery from 84.99% to 86.66% and propane
recovery
from 97.74% to 98.01%, and maintains essentially the same butanes+ recovery
(99.81%
versus 99.83% for the prior art). Comparison of Tables I and III further shows
that the
product yields were achieved using significantly less power than the prior
art. In terms of the
recovery efficiency (defined by the quantity of ethane recovered per unit of
power), the
present invention represents nearly a 6% improvement over the prior art of the
FIG. 1
process.
100461 The improvement in recovery efficiency provided by the
present invention
over that of the prior art of the FIG. 1 process is primarily due to three
factors. First, the
compact arrangement of the heat exchange means in feed cooling section 118a
and rectifying
section 118b inside processing assembly 118 eliminates the pressure drop
imposed by the
interconnecting piping found in conventional processing plants. The result is
that the residue
gas flowing to compressor 16 is at higher pressure for the present invention
compared to the
prior art, so that the residue gas entering compressor 23 is at significantly
higher pressure,
-23-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
thereby reducing the power required by the present invention to restore the
residue gas to
pipeline pressure.
[0047] Second, using the heat and mass transfer means in stripping
section 118d to
simultaneously heat the distillation liquid leaving the absorbing means in
stripping section
118d while allowing the resulting vapors to contact the liquid and strip its
volatile
components is more efficient than using a conventional distillation column
with external
reboilers. The volatile components are stripped out of the liquid
continuously, reducing the
concentration of the volatile components in the stripping vapors more quickly
and thereby
improving the stripping efficiency for the present invention.
[0048] Third, using the heat and mass transfer means in rectifying
section 118b to
simultaneously cool the distillation vapor stream arising from absorbing
section 118c while
condensing the heavier hydrocarbon components from the distillation vapor
stream provides
more efficient rectification than using reflux in a conventional distillation
column. As a
result, more of the C2 components, C3 components, and heavier hydrocarbon
components can
be removed from the distillation vapor stream using the refrigeration
available in the
expanded stream 38b compared to the prior art of the FIG. I process.
[0049] The present invention offers two other advantages over the prior
art in addition
to the increase in processing efficiency. First, the compact arrangement of
processing
assembly 118 of the present invention replaces eight separate equipment items
in the prior art
(heat exchangers 10, 11, 13, and 20, separator 12, reflux separator 21, reflux
pump 22, and
fractionation tower 18 in FIG. I) with a single equipment item (processing
assembly 118 in
FIG. 3). This reduces the plot space requirements, eliminates the
interconnecting piping, and
eliminates the power consumed by the reflux pump, reducing the capital cost
and operating
cost of a process plant utilizing the present invention over that of the prior
art. Second,
elimination of the interconnecting piping means that a processing plant
utilizing the present
-24-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
invention has far fewer flanged connections compared to the prior art,
reducing the number of
potential leak sources in the plant. Hydrocarbons are volatile organic
compounds (VOCs),
some of which are classified as greenhouse gases and some of which may be
precursors to
atmospheric ozone formation, which means the present invention reduces the
potential for
atmospheric releases that can damage the environment.
Example 2
[0050] In those cases where the C2 component recovery level in the liquid
product
must be reduced (as in the FIG. 2 prior art process described previously, for
instance), the
present invention offers significant efficiency advantages over the prior art
process depicted
in FIG. 2. The operating conditions of the FIG. 3 process can be altered as
illustrated in
FIG. 4 to reduce the ethane content in the liquid product of the present
invention to the same
level as for the FIG. 2 prior art process. The feed gas composition and
conditions considered
in the process presented in FIG. 4 are the same as those in FIG. 2.
Accordingly, the FIG. 4
process can be compared with that of the FIG. 2 process to further illustrate
the advantages of
the present invention.
[0051] In the simulation of the FIG. 4 process, inlet gas stream 31
enters a heat
exchange means in the upper region of feed cooling section 118a inside
processing assembly
118. The heat exchange means is configured to provide heat exchange between
stream 31
flowing through one pass of the heat exchange means and a distillation vapor
stream arising
from rectifying section 118b inside processing assembly 118 that has been
heated in a heat
exchange means in the lower region of feed cooling section 118a. Stream 31 is
cooled while
further heating the distillation vapor stream, with stream 31a leaving the
heat exchange
means and thereafter entering separator section 118e inside processing
assembly 118 at 15 F
-25-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
[-9 C] and 900 psia [6,203 kPa(a)], whereupon the vapor (stream 34) is
separated from the
condensed liquid (stream 35).
[0052] The vapor (stream 34) from separator section 118e is divided into
two streams,
36 and 39. Stream 36, containing about 28% of the total vapor, is combined
with the
separated liquid (stream 35, via stream 37), and the combined stream 38 enters
a heat
exchange means in the lower region of feed cooling section 118a inside
processing assembly
118. The heat exchange means is configured to provide heat exchange between
stream 38
flowing through one pass of the heat exchange means and the distillation vapor
stream arising
from rectifying section 118b inside processing assembly 118, so that stream 38
is cooled to
substantial condensation while heating the distillation vapor stream.
[0053] The resulting substantially condensed stream 38a at -114 F [-81 C]
is then
flash expanded through expansion valve 14 to slightly above the operating
pressure
(approximately 393 psia [2,710 kPa(a)]) of rectifying section 118b and
absorbing section
118c inside processing assembly 118. During expansion a portion of the stream
may be
vaporized, resulting in cooling of the total stream. In the process
illustrated in FIG. 4, the
expanded stream 38b leaving expansion valve 14 reaches a temperature of -138 F
[-94 C]
before it is directed into a heat and mass transfer means inside rectifying
section 118b. The
heat and mass transfer means is configured to provide heat exchange between
the distillation
vapor stream arising from absorbing section 118c flowing upward through one
pass of the
heat and mass transfer means and the expanded stream 38b flowing downward, so
that the
distillation vapor is cooled while heating the expanded stream. As the
distillation vapor
stream is cooled, a portion of it is condensed and falls downward while the
remaining
distillation vapor continues flowing upward through the heat and mass transfer
means. The
heat and mass transfer means provides continuous contact between the condensed
liquid and
the distillation vapor so that it also functions to provide mass transfer
between the vapor and
-26-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
liquid phases, thereby providing rectification of the distillation vapor. The
condensed liquid
is collected from the bottom of the heat and mass transfer means and directed
to absorbing
section 118c.
[0054] The flash expanded stream 38b is partially vaporized as it
provides cooling
and partial condensation of the distillation vapor stream, then exits the heat
and mass transfer
means in rectifying section 118b at -104 F [-75 C] and is separated into its
respective vapor
and liquid phases. The vapor phase combines with the vapor arising from
absorbing section
118c to form the distillation vapor stream that enters the heat and mass
transfer means in
rectifying section 118b as previously described. The liquid phase is directed
to the upper
region of absorbing section 118c to join with the liquid condensed from the
distillation vapor
stream in rectifying section 118b.
[0055] The remaining 72% of the vapor from separator section 118e (stream
39)
enters a work expansion machine 15 in which mechanical energy is extracted
from this
portion of the high pressure feed. The machine 15 expands the vapor
substantially
isentropically to the operating pressure of absorbing section 118c, with the
work expansion
cooling the expanded stream 39a to a temperature of approximately -60 F [-51
C]. The
partially condensed expanded stream 39a is thereafter supplied as feed to the
lower region of
absorbing section 118c inside processing assembly 118 to be contacted by the
liquids
supplied to the upper region of absorbing section 118c.
[0056] Absorbing section 118c and stripping section 118d each contain an
absorbing
means. Stripping section 118d also includes a heat and mass transfer means
beneath its
absorbing means which is configured to provide heat exchange between a heating
medium
flowing through one pass of the heat and mass transfer means and a
distillation liquid stream
flowing downward from the absorbing means, so that the distillation liquid
stream is heated.
As the distillation liquid stream is heated, a portion of it is vaporized to
form stripping vapors
-27-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
that rise upward as the remaining liquid continues flowing downward through
the heat and
mass transfer means. The heat and mass transfer means provides continuous
contact between
the stripping vapors and the distillation liquid stream so that it also
functions to provide mass
transfer between the vapor and liquid phases, stripping the liquid product
stream 46 of
methane, C2 components, and lighter components. The resulting liquid product
(stream 46)
exits the lower region of stripping section 118d and leaves processing
assembly 118 at 221 F
[105 C].
100571 The distillation vapor stream arising from rectifying section 118b
is warmed
in feed cooling section 118a as it provides cooling to streams 31 and 38 as
previously
described, and the resulting residue gas stream 42 leaves processing assembly
118 at 106 F
[41 C]. The residue gas stream is then re-compressed in two stages, compressor
16 driven by
expansion machine 15 and compressor 23 driven by a supplemental power source.
After
stream 42b is cooled to 110 F [43 C] in discharge cooler 24, the residue gas
product (stream
42c) flows to the sales gas pipeline at 915 psia [6,307 kPa(a)].
[0058] A summary of stream flow rates and energy consumption for the
process
illustrated in FIG. 4 is set forth in the following table:
-28-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
Table IV
(FIG. 4)
Stream Flow Summary - Lb. Moles/Hr [kg moles/Hr]
Stream Methane Ethane Propane Butanes+ Total
31 12,398 546 233 229 13,726
34 12,332 532 215 128 13,523
35 66 14 18 101 203
36 3,515 152 61 36 3,854
37 66 14 18 101 203
38 3,581 166 79 137 4,057
39 8,817 380 154 92 9,669
42 12,398 535 1 0 13,253
46 0 11 232 229 473
Recoveries*
Propane 99.50%
Butanes+ 100.00%
Power
Residue Gas Compression 5,384 HP [ 8,851 kW]
* (Based on un-rounded flow rates)
[0059] A comparison of Tables II and IV shows that the present invention
maintains
essentially the same recoveries as the prior art. However, further comparison
of Tables II and
-29-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
IV shows that the product yields were achieved using significantly less power
than the prior
art. In terms of the recovery efficiency (defined by the quantity of propane
recovered per unit
of power), the present invention represents nearly a 4% improvement over the
prior art of the
FIG. 2 process.
[0060] The FIG. 4 embodiment of the present invention provides the same
advantages
related to the compact arrangement of processing assembly 118 as the FIG. 3
embodiment.
The FIG. 4 embodiment of the present invention replaces seven separate
equipment items in
the prior art (heat exchangers 10, 13, and 20, separator 12, reflux separator
21, reflux pump
22, and fractionation tower 18 in FIG. 2) with a single equipment item
(processing assembly
118 in FIG. 4). This reduces the plot space requirements, eliminates the
interconnecting
piping, and eliminates the power consumed by the reflux pump, reducing the
capital cost and
operating cost of a process plant utilizing this embodiment of the present
invention over that
of the prior art, while also reducing the potential for atmospheric releases
that can damage the
environment.
Other Embodiments
[0061] Some circumstances may favor supplying liquid stream 35 directly
to stripping
section 118d via stream 40 as shown in FIGS. 3 through 6. In such cases, an
appropriate
expansion device (such as expansion valve 17) is used to expand the liquid to
the operating
pressure of stripping section 118d and the resulting expanded liquid stream
40a is supplied as
feed to stripping section 118d above the absorbing means, above the heat and
mass transfer
means, or to both such feed points (as shown by the dashed lines). Some
circumstances may
favor combining a portion of liquid stream 35 (stream 37) with the vapor in
stream 36 to form
combined stream 38 and routing the remaining portion of liquid stream 35 to
stripping section
118d via streams 40/40a. Some circumstances may favor combining the expanded
liquid
-30-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
stream 40a with expanded stream 39a and thereafter supplying the combined
stream to the
lower region of absorbing section 118c as a single feed.
[0062] Some circumstances may favor using the cooled second portion
(stream 33a in
FIGS. 3 and 5) in lieu of the first portion (stream 36) of vapor stream 34 to
form stream 38
flowing to the heat exchange means in the lower region of feed cooling section
118a. In such
cases, only the cooled first portion (stream 32a) is supplied to separator
section 118e (FIG. 3)
or separator 12 (FIG. 5), and all of the resulting vapor stream 34 is supplied
to work
expansion machine 15.
[0063] In some circumstances, it may be advantageous to use an external
separator
vessel to separate cooled feed stream 31a, rather than including separator
section 118e in
processing assembly 118. As shown in FIGS. 5 and 6, separator 12 can be used
to separate
cooled feed stream 31a into vapor stream 34 and liquid stream 35.
[0064] Depending on the quantity of heavier hydrocarbons in the feed gas
and the
feed gas pressure, the cooled feed stream 31a entering separator section 118e
in FIGS. 3 and
4 or separator 12 in FIGS. 5 and 6 may not contain any liquid (because it is
above its
dewpoint, or because it is above its cricondenbar). In such cases, there is no
liquid in streams
35 and 37 (as shown by the dashed lines), so only the vapor from separator
section 118e in
stream 36 (FIGS. 3 and 4) or the vapor from separator 12 in stream 36 (FIGS. 5
and 6) flows
to stream 38 to become the expanded substantially condensed stream 38b
supplied to the heat
and mass transfer means in rectifying section 118b. In such circumstances,
separator section
118e in processing assembly 118 (FIGS. 3 and 4) or separator 12 (FIGS. 5 and
6) may not be
required.
[0065] Feed gas conditions, plant size, available equipment, or other
factors may
indicate that elimination of work expansion machine 15, or replacement with an
alternate
expansion device (such as an expansion valve), is feasible. Although
individual stream
-31-
CA 02763714 2011-11-28
expansion is depicted in particular expansion devices, alternative expansion
means may be
employed where appropriate. For example, conditions may warrant work expansion
of the
substantially condensed portion of the feed stream (stream 38a).
100661 In accordance with the present invention, the use of external
refrigeration to
supplement the cooling available to the inlet gas from the distillation vapor
and liquid streams
may be employed, particularly in the case of a rich inlet gas. In such cases,
a heat and mass
transfer means may be included in separator section 118e (or a gas collecting
means in such
cases when the cooled feed stream 31a contains no liquid) as shown by the
dashed lines in
FIGS. 3 and 4, or a heat and mass transfer means may be included in separator
12 as shown
by the dashed lines in FIGS. 5 and 6. This heat and mass transfer means may be
comprised
of a fin and tube type heat exchanger, a plate type heat exchanger, a brazed
aluminum type
heat exchanger, or other type of heat transfer device, including multi-pass
and/or
multi-service heat exchangers. The heat and mass transfer means is configured
to provide
heat exchange between a refrigerant stream (e.g., propane) flowing through one
pass of the
heat and mass transfer means and the vapor portion of stream 31a flowing
upward, so that the
refrigerant further cools the vapor and condenses additional liquid, which
falls downward to
become part of the liquid removed in stream 35. Alternatively, conventional
gas chiller(s)
could be used to cool stream 32a, stream 33a, and/or stream 31a with
refrigerant before
stream 31a enters separator section 118e (FIGS. 3 and 4) or separator 12
(FIGS. 5 and 6).
100671 Depending on the temperature and richness of the feed gas and the
amount of
C2 components to be recovered in liquid product stream 46, there may not be
sufficient
heating available from stream 33 to cause the liquid leaving stripping section
118d to meet
the product specifications. In such cases, the heat and mass transfer means in
stripping
section 118d may include provisions for providing supplemental heating with
heating
medium as shown by the dashed lines in FIGS. 3 and 5. Alternatively, another
heat and mass
-32-
CA 02763714 2011-11-28
transfer means can be included in the lower region of stripping section 118d
for providing
supplemental heating, or stream 33 can be heated with heating medium before it
is supplied
to the heat and mass transfer means in stripping section 118d.
100681 Depending on the type of heat transfer devices selected for the
heat exchange
means in the upper and lower regions of feed cooling section 118a, it may be
possible to
combine these heat exchange means in a single multi-pass and/or multi-service
heat transfer
device. In such cases, the multi-pass and/or multi-service heat transfer
device will include
appropriate means for distributing, segregating, and collecting stream 32,
stream 38, and the
distillation vapor stream in order to accomplish the desired cooling and
heating. Likewise,
the type of heat and mass transfer device selected for the heat and mass
transfer means in
rectifying section 118b may allow combining it with the heat exchange means in
the lower
region of feed cooling section 118a (and possibly with the heat exchange means
in the upper
region of feed cooling section 118a as well) in a single multi-pass and/or
multi-service heat
and mass transfer device. In such cases, the multi-pass and/or multi-service
heat and mass
transfer device will include appropriate means for distributing, segregating,
and collecting
stream 38, stream 38b, and the distillation vapor stream (and optionally
stream 32) in order to
accomplish the desired cooling and heating.
100691 Some circumstances may favor not providing an absorbing means in
the upper
region of stripping section 118d. In such cases, a distillation liquid stream
is collected from
the lower region of absorbing section 118c and directed to the heat and mass
transfer means
in stripping section 118d.
100701 A less preferred option for the FIGS. 3 and 5 embodiments of the
present
invention is providing a separator vessel for cooled first portion 32a and a
separator vessel
for cooled second portion 33a, combining the vapor streams separated therein
to form vapor
stream 34, and combining the liquid streams separated therein to form liquid
stream 35.
-33-
CA 02763714 2011-11-28
WO 2010/144186 PCT/US2010/033374
Another less preferred option for the present invention is cooling stream 37
in a separate heat
exchange means inside feed cooling section 118a (rather than combining stream
37 with
stream 36 to form combined stream 38), expanding the cooled stream in a
separate expansion
device, and supplying the expanded stream either to the heat and mass transfer
means in
rectifying section 118b or to the upper region of absorbing section 118c.
[0071] It will be recognized that the relative amount of feed found in
each branch of
the split vapor feed will depend on several factors, including gas pressure,
feed gas
composition, the amount of heat which can economically be extracted from the
feed, and the
quantity of horsepower available. More feed above absorbing section 118c may
increase
recovery while decreasing power recovered from the expander and thereby
increasing the
recompression horsepower requirements. Increasing feed below absorbing section
118c
reduces the horsepower consumption but may also reduce product recovery.
[0072] The present invention provides improved recovery of C2 components,
C3
components, and heavier hydrocarbon components or of C3 components and heavier
hydrocarbon components per amount of utility consumption required to operate
the process.
An improvement in utility consumption required for operating the process may
appear in the
form of reduced power requirements for compression or re-compression, reduced
power
requirements for external refrigeration, reduced energy requirements for
supplemental
heating, reduced energy requirements for tower reboiling, or a combination
thereof.
[0073] While there have been described what are believed to be preferred
embodiments of the invention, those skilled in the art will recognize that
other and further
modifications may be made thereto, e.g. to adapt the invention to various
conditions, types of
feed, or other requirements without departing from the spirit of the present
invention as
defined by the following claims.
-34-