Note: Descriptions are shown in the official language in which they were submitted.
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
PROCESS FOR PRODUCING CEMENTED AND SKINNED ACICULAR
MULLITE HONEYCOMB STUCTURES
This application claims benefit of United States Provisional Patent
Application
No. 61/221,422, filed 29 June 2009.
The present invention relates to a process for producing acicular mullite
honeycomb structures having an inorganic cement layer or an inorganic skin.
Acicular mullite honeycomb structures are often used as filters in high
temperature applications. These honeycombs are often used as particulate
filters to
remove soot particles or droplets from diesel engine exhaust. Filters of these
types are
frequently exposed to large, rapid changes in temperature. The temperature
changes
can occur during the normal operation of the vehicle, but they are especially
pronounced
when the filter is thermally regenerated to burn out the captured soot. These
large,
rapid temperature changes are sometimes referred to as "thermal shock" events.
These rapid temperature changes usually create temporary but significant
temperature gradients within the honeycomb structure, which in turn lead to
the
creation of large localized stresses due to non-uniform thermal expansion (or
thermal
contraction) within the part. When these localized stresses exceed the
strength of the
part, the structure will relieve the stress by cracking, which can lead to
part failure.
Various approaches have been tried to improve the thermal shock resistance of
these honeycomb structures. In one approach, the honeycomb is made up of
multiple
smaller honeycombs which are cemented together. Another approach focuses on
the
peripheral "skin" of the honeycomb. The periphery of the part is often
subjected to the
highest thermally-induced stresses, especially during rapid temperature
increases. As a
result, cracking often initiates at the skin, from which the cracks can
propagate
throughout the structure and destroy the part. This skin can be removed and
replaced
with another ceramic material that is more compliant than the original
acicular mullite
skin of the honeycomb. The cement and skins are made by applying and firing a
cement
composition that contains a colloidal silica or alumina, filler particles and
a carrier fluid.
For example, USP 7,083,842 describes a ceramic honeycomb structure in which
the
original peripheral region of the structure is removed and replaced with an
inorganic
coating that is fired to form a replacement skin. The coating composition
includes an
inorganic binder, ceramic fibers of up to 100 microns in length, and particles
having a
diameter of from 0.5 to 100 microns. USP 5,914,187 describes a cement that
includes an
-1-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
inorganic binder such as a glassy silica phase, as well as both ceramic fibers
and other
inorganic powders or whiskers. The powders or whiskers are used to increase
the
thermal conductivity of the cement. USP 7,112,233 describes a similar cement,
which in
this case is formulated to have a specific thermal conductivity. The cement
described in
USP 7,112,233 includes silica-alumina fibers which are at least 1 mm in
length.
According to USP 7,112,233, shorter fibers do not permit an "elastic"
structure to be
formed. The needed thermal conductivity is provided by including silicon
carbide,
silicon nitride or boron nitride particles in the cement formulation.
In one aspect, this invention is a process comprising the steps of (a) forming
a
ceramic honeycomb containing multiple axially-extending cells defined by
intersecting
walls, (b) applying to at least one surface of the ceramic honeycomb a cement
composition that contains both aluminum and silicon atoms and includes (1) at
least one
inorganic filler, (2) a colloidal silica, colloidal alumina or mixture thereof
which forms a
binding phase upon firing, and (3) a carrier fluid and then (c) firing the
honeycomb and
cement composition at a temperature of at least 1000 C in the presence of a
fluorine
source.
The resulting ceramic honeycomb structure often has greater thermal shock
resistance, compared to when those steps are performed sequentially. Although
the
invention is not limited to any theory, it is believed that mullite forms in
the cement
composition when it is fired in the presence of the fluorine source. Some
mullite can
form when the cement is fired, even in the absence of the fluorine source.
However, it
has been found that mullite forms faster and to a greater extent in the cement
composition when a fluorine source is present. The higher mullite content of
the fired
cement in some cases can more closely match the coefficient of thermal
expansion (CTE)
of the fired cement to that of the underlying honeycomb, especially in
preferred cases in
which the honeycomb is acicular mullite. This closer match in CTE is believed
to
account for the greater shock resistance of the honeycomb structure.
The cement composition can function as a cement which adheres the honeycomb
to another part of the final structure. For example, the honeycomb may be
composed of
two or more smaller honeycombs, which are cemented together using the cement
composition to produce a larger honeycomb. The cement composition may perform
such
a cementing function. The cement composition of the invention may also serve
to
cement a honeycomb to some other structure. The cement composition may
instead, or
in addition, be used to produce a peripheral skin for the honeycomb structure.
-2-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
In a particularly preferred embodiment, this invention is a process comprising
the steps of (a) forming a ceramic honeycomb containing multiple axially-
extending cells
defined by intersecting walls, wherein at least a portion of the ceramic
honeycomb is an
acicular mullite that contains at least 0.5 weight percent residual fluorine,
based on the
weight of the acicular mullite in the honeycomb (b) applying to at least one
surface of
the ceramic honeycomb a cement composition that contains both aluminum and
silicon
atoms and includes (1) at least one inorganic filler, (2) a colloidal silica,
colloidal
alumina or mixture thereof which forms a binding phase upon being fired, and
(3) a
carrier fluid, and then (c) exposing the honeycomb and cement composition to a
temperature of at least 1200 C.
An additional advantage of this embodiment of this invention is that two
normally distinct steps in the manufacture of a ceramic honeycomb structure
can be
combined into one operation.
Honeycomb structures made in accordance with the invention are useful in a
variety of filtration, heat exchange and catalytic applications. Because those
honeycomb
structures tend to have good thermal shock resistance, they are particularly
useful in
applications in which the structure is exposed to rapid and large changes in
temperature.
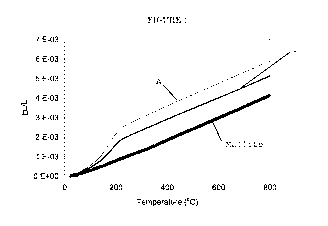
Figure 1 is graph showing the coefficient of thermal expansion of an acicular
mullite honeycomb, a cement (Example 1) formed in accordance with this
invention, and
of a comparative cement (Comparative Sample A) that is not formed in the
presence of a
fluorine source.
Figure 2 is a graph showing the coefficient of thermal expansion of an
acicular
mullite honeycomb, a cement (Example 2) formed in accordance with this
invention, and
of a comparative cement (Comparative Sample B) that is not formed in the
presence of a
fluorine source.
The ceramic honeycomb is characterized in having multiple cells that extend
axially throughout the length of the honeycomb body. The cells are defined by
multiple
intersecting walls. The walls and the intersection points define the number of
cells, as
well as their cross-sectional shape and dimensions. A typical honeycomb for
many
filtration or catalysis applications will contain from 25 to 1000 cells/square
inch (about 4
to 150 cells/square centimeter) of cross-sectional area (i.e., transverse to
the longitudinal
extension). Wall thicknesses are typically from 0.05 to 10 mm, preferably from
0.2 to 1
mm, although larger or smaller wall thicknesses might be used.
-3-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
The ceramic honeycomb may be monolithic (i.e., formed in a single piece), or
may
be an assembly of smaller honeycombs which are manufactured separately and
then
assembled together, usually using a ceramic cement. The ceramic cement in such
an
assembly is in some embodiments a fired cement composition as described
herein.
The walls of the honeycomb preferably are porous, and a fluid can pass through
the pores from one cell to one or more adjacent cells. The ceramic making up
the
honeycomb generally has a porosity of about 30% to 85%. Preferably, the porous
ceramic has a porosity of at least about 40%, more preferably at least about
45%, even
more preferably at least about 50%, and most preferably at least about 55% to
preferably at most about 80%, more preferably at most about 75%, and most
preferably
at most about 70%. Porosities are determined by water immersion methods.
The ceramic honeycomb may be made from an inorganic material such as
alumina, zirconia, silicon carbide, silicon nitride, aluminum nitride, silicon
oxynitride,
silicon carbonitride, mullite, cordierite, beta spodumene, aluminum titanate,
strontium
aluminum silicates, lithium aluminum silicates. In preferred embodiments, at
least a
portion of the ceramic honeycomb is an acicular mullite that contains at least
0.5 weight
percent residual fluorine. If the ceramic honeycomb is monolithic, then the
entire
honeycomb preferably is such an acicular mullite. In cases in which the
ceramic
honeycomb is a cemented assembly of smaller honeycombs, at least one of the
smaller
honeycombs preferably is such an acicular mullite. It is preferred that all of
the smaller
honeycombs are acicular mullite containing at least 0.5 weight percent
residual fluorine.
Acicular mullite honeycomb structures can be prepared by forming a clay from a
mullite precursor, shaping the clay into the honeycomb configuration
(typically by
extrusion) and then mullitizing the clay. Mullitization is performed by
exposing the clay
to a fluorine-containing compound under conditions that the mullite precursors
react
with the fluorine-containing compound to form a fluorotopaz which then
decomposes to
form acicular mullite needles. Suitable methods for preparing acicular mullite
honeycombs are described, for example, in WO 92/11219, WO 03/082773 and WO
04/096729.
As fluorotopaz decomposes to form mullite, a mass of interconnected needle-
like
crystals is created. The crystals are comprised mainly of a crystalline
mullite, although
it is possible for small quantities of other crystalline and/or glassy phases
to be present.
For example, the crystals may contain up to about 2 volume percent of a
crystalline
silica phase such as cristobalite, as described in WO 03/082773, or up to
about 10
-4-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
volume percent of a glassy oxide phase that may contain silicon and/or
aluminum as
well as one or more metals contributed by a sintering aid and/or other
compounds as
may be present.
The acicular mullite crystals are bonded together at points of contact to form
a
porous mass having essentially the same overall geometry and dimensions as the
clay
honeycomb. The aspect ratio of the mullite crystals is typically at least 5,
preferably at
least 10, more preferably at least 20. The crystals may have a mean grain
diameter of
from 5 to 50 microns.
Acicular mullite bodies prepared as described above tend to contain some
residual fluorine. The amount of fluorine may constitute from 0.5 to about 3
weight
percent of the weight of the acicular mullite. More typically, the fluorine
constitutes
from about 0.8 to 2 weight percent of the acicular mullite. In conventional
processes,
this residual fluorine is removed by heating the honeycomb to a temperature of
at least
1200 C, preferably at least 1400 C, preferably in air or the presence of
oxygen. In this
invention, however, it is preferred that at least a portion of this residual
fluorine
remains in the acicular mullite honeycomb until the cement composition is
applied, as
described more fully below. The acicular mullite in the honeycomb should
contain at
least 0.5 weight percent fluorine.
The cement composition is applied to one surface of the ceramic honeycomb. As
already mentioned, the cement composition may perform a cementing function,
adhering the ceramic honeycomb to another honeycomb or to some other
structure. The
cement composition may instead, or in addition, serve as a peripheral skin for
the
honeycomb structure.
The cement composition contains both silicon and aluminum atoms. Its
constituent components include (1) inorganic filler particles, (2) a colloidal
silica,
colloidal alumina or mixture thereof which forms a binding phase upon firing,
and (3) a
carrier fluid. The inorganic filler particles are materials which do not form
a binding
phase when the cement composition is fired, and thus are distinguished from
the
colloidal silica and/or colloidal alumina component of the composition. The
inorganic
filler particles instead retain their particulate nature throughout the firing
process,
although they may become bound by the binding phase to other particles or to
the
inorganic fibers. Other components may be present in the cement composition,
as
described more fully below.
-5-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
Colloidal silica and colloidal aluminum are of course sources of silicon and
aluminum atoms, respectively. If colloidal silica is used by itself to form
the binder
phase, the cement composition must contain some additional source of aluminum
atoms.
This source is typically the inorganic filler particles, which may contain
silicon atoms in
addition to the necessary aluminum atoms. Similarly, if colloidal alumina is
used by
itself to form the binder phase, the cement composition must contain some
additional
source, of silicon atoms, which again typically will be the inorganic filler
particles. In
this second case, the inorganic filler may contain aluminum atoms in addition
to the
needed silicon atoms.
If both colloidal silica and colloidal alumina are present in the cement,
another
source of silicon and aluminum atoms is not needed. Nonetheless, it is
preferred even in
this case that the inorganic filler particles contain aluminum atoms, silicon
atoms or
both aluminum and silicon atoms.
The preferred inorganic filler particles are therefore aluminate, silicate or
aluminosilicate materials. The filler particles may be amorphous, partially
crystalline
or fully crystalline. The inorganic filler particles may contain a crystalline
phase that is
surrounded by glass. The inorganic filler particles may also contain other
elements such
as rare earths, zirconium, iron, boron and alkaline earths. Examples of
silicon- and/or
aluminum-containing materials that can be used as the inorganic filler
particles are
alumina, borosilicate glass, quartz, e-glass, s-glass, silicon carbide,
silicon nitride,
mullite, cordierite, alumina silicates, alumina-zirconia-silicates,
wollastonite, basalt and
aluminum titanate. If no silicon or aluminum atoms are needed in the inorganic
filler
particles, other materials such as boron nitride or carbon nitride particles
can be used.
It is preferred to use an aluminosilicate material that can be at least
partially
converted to mullite as the inorganic filler particles.
Preferably, at least a portion of the inorganic filler particles are in the
form of
fibers that have a diameter of from 100 nanometers to 20 microns and an aspect
ratio
(longest dimension divided by shortest dimension) of at least 10, preferably
at least 20.
A preferred fiber diameter is from 0.5 to 10 microns. A more preferred fiber
diameter is
from 3 to 10 microns.
The number average length of the inorganic fibers may range from 100 microns
to 130 millimeters or more. The number average length is preferably at least
100
microns and more preferably at least 200 microns. The number average length is
preferably no greater than 10 millimeters. The number average length may be no
-6-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
greater than 5 millimeters or no greater than 2 millimeters. Longer fibers,
such as
those having lengths of 10 mm or more, often tend to form bundles during
processing.
These bundles cause difficulties in applying the skin and also lead to
inconsistencies in
the skin composition. Therefore, longer fibers preferably are used somewhat
sparingly if
at all.
In some embodiments of the invention, essentially all of the fibers have a
length
of less than 1 mm. In other embodiments, the fibers have a bimodal or
multimodal
length distribution, in which one portion of the fibers are shorter fibers
having a number
average length of from 100 to 1000 microns, and at least one other portion of
the fibers
are longer fibers having a number average length of at least 1 millimeter,
preferably
from 1 to 100 millimeters, more preferably from 2 to 100 millimeters and even
more
preferably from 5 to 30 millimeters. In such embodiments, the longer fibers
preferably
constitute from 1 to 50, more preferably from 3 to 30 and even more preferably
from 5 to
25 percent of the total weight of the inorganic fibers. Mixed length fibers
provide
certain advantages. The presence of a minor proportion of longer fibers tends
to
increase the viscosity of the cement composition, at a given fiber content in
the
composition. The viscosity of the cement composition should be somewhat high,
so it
can be applied and shaped readily without sagging or flowing off of the
honeycomb
before it can dry. The presence of a minor proportion of longer fibers can
allow a good
working viscosity to be achieved without unduly increasing the fiber content.
If the
fiber content becomes too high, there may not be enough colloidal silica
and/or colloidal
alumina in the composition to adequately bind the fibers to each other or to
the
underlying honeycomb. Typically, the strength of the fired cement composition
tends to
decrease with increasing fiber length, because the number of fibers decreases
as their
length increases, and fewer fibers means fewer points of intersection where
they can be
bound together. When a mixture of shorter and longer fibers is used, the
strength of the
fired cement composition is often comparable to that of a cement that contains
an
equivalent proportion of only short fibers. Thus, a mixture of shorter fibers
and a minor
proportion of longer fibers can provide significant processing benefits with
little or no
corresponding disadvantages.
Examples of useful organic fibers include mullite fibers, such as are
available
from Unifrax; alumina-zirconium-silicate fibers, such as are available from
Unifrax;
alumina fibers containing up to 10% by weight silica, such as are available
from Saffil; y
-alumina and a-alumina + mullite fibers such as Nextel 312 or Nextel 610
fibers from
-7-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
3M; y-alumina + mullite + amorphous SiO2 fibers such as Nextel 440 fibers from
3M; 7-
alumina + amorphous SiO2 fibers such as Nextel 550 fibers from 3M; quartz
fibers such
as are available from Saint Gobain; e-glass or s-glass fibers; borosilicate
fibers such as
are available from Mo-SiC Corporation; basalt fibers such as are available
from
Albarrie, wollastonite fibers such as are available from Fibertec, and the
like.
The cement composition may contain low aspect ratio inorganic filler particles
in
addition to or instead of the inorganic fibers described above. "Low aspect
ratio" refers
to an aspect ratio of less than 10. These inorganic filler particles are
different from and
do not include the colloidal silica and/or colloidal alumina component of the
cement
composition. The low aspect ratio inorganic filler particles do not form a
binding phase
when the cement composition is fired. The low aspect ratio inorganic filler
particles
instead retain their particulate nature throughout the firing process,
although they may
become bound by the glassy binding phase to other particles or to the
inorganic fibers.
A mixtue of inorganic fibers and low aspect ratio inorganic filler particles
may be
present. In such cases, these low aspect ratio inorganic filler particles can
be classified
into two types. The first type is particles that have the same CTE or very
nearly the
same CTE as the inorganic fiber (i.e., differing by no more than 1 ppm/ C in
the
temperature range of from 100 to 600 C), after the firing step is completed.
The
comparison is performed on the basis of the fired skin composition to account
for
changes in CTE that may occur to the fibers and/or other particles during the
firing
step, due to, for example, changes in crystallinity and/or composition that
may occur.
Particles of this type generally have the same or nearly the same chemical
composition
as the inorganic fiber. A common source of this type of particle is so-called
"shot"
material, which is a by-product of the fiber manufacturing process and is
included in
many commercial grades of inorganic fibers. However, this type of particle may
be
supplied from other sources as well. This first type of inorganic filler
particle may
constitute from 0 to as much as 60% of the total weight of the inorganic
filler.
Preferably, this type of inorganic filler particle constitutes no more than
50%, more
preferably no more than 25% and still more preferably no more than 10% to the
total
weight of the inorganic fillers.
The second type of inorganic filler particles have a CTE which is
significantly
different (i.e., different by more than 1 ppm/ C, preferably by at least 2
ppm/ C in the
temperature range from 100 to 600 C) than that of the inorganic fibers, after
the firing
step is completed. Inorganic filler particles of this type, if present at all,
constitute no
-8-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
more than 5% by weight of the solids of the cement composition. For purposes
of this
calculation, the "solids" are constituted by the inorganic materials in the
cement
composition that remain in the skin after the firing step is completed,
including fillers
and inorganic binding phase. One advantage of this invention is that it is not
necessary
to add fillers or otherwise attempt to "match" the coefficient of thermal
expansion of the
cement to that of the underlying honeycomb. Accordingly, the cement
composition may
contain no inorganic filler particles of the second type at all, or may
contain only very
small proportions thereof, such as, for example, from 0 to 3% or from 0 to 2%
or from 0
to 1% of the solids of the cementcomposition. Examples of this second type of
inorganic
filler particles are alumina, silicon carbide, silicon nitride, mullite,
cordierite and
aluminum titanate.
In one preferred embodiment, the inorganic filler contains only the inorganic
fiber, "shot" material from the inorganic fiber, and optionally the second
type of
inorganic filler particle, which may be present in an amount from 0 to 5% by
weight of
the solids of the cement composition, but essentially no (less than 5 weight
percent,
preferably no more than 1%) other organic filler particles of the first type.
In such an
embodiment, it is more preferred that the inorganic fibers constitute at least
50, at least
75 or at least 90% of the total weight of the inorganic filler, and that the
"shot" material
constitutes no more than 50, no more than 25 or no more than 10% of the total
weight of
the inorganic filler. An especially inorganic filler of this type includes
only inorganic
fiber and "shot" material.
In another preferred embodiment, the inorganic filler contains only the
inorganic
fiber and from 0-5 weight percent of the second type of inorganic filler, but
no "shot"
material or other inorganic filler of the first type.
The inorganic filler particles in the aggregate may constitute from about 30
to
90% by weight of the solids in the cement. A preferred amount is from 50 to
85% by
weight of the solids and a more preferred amount is from 60 to 80% by weight
of the
solids. As mentioned before, the "solids" in the composition are those
inorganic
materials that remain after the firing step is completed. In most cases, the
solids will be
made up of the inorganic filler particles and the colloidal silica and/or
colloidal alumina.
Carrier fluids and organic materials generally are lost from the cement during
the firing
step(s). The "solids" therefore do not include any amounts of those materials.
-9-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
The colloidal silica and/or colloidal alumina may constitute from 10 to 70%,
preferably from 15 to 50% and more preferably from 20 to 40% of the weight of
the solids
portion of the cement composition.
The cement composition also includes a carrier liquid. The mixture of carrier
fluid and colloidal silica and/or alumina particles forms a paste or viscous
fluid in which
the inorganic fibers are dispersed. The fluid or semi-fluid nature of the
cement
composition permits it to be applied easily and to adhere well to the
underlying
honeycomb until the firing step is completed. The carrier liquid may be, for
example,
water, or an organic liquid. Suitable organic liquids include alcohols,
glycols, ketones,
ethers, aldehydes, esters, carboxylic acids, carboxylic acid chlorides,
amides, amines,
nitriles, nitro compounds, sulfides, sulfoxides, sulfones and the like.
Hydrocarbons,
including aliphatic, unsaturated aliphatic (including alkenes and alkynes)
and/or
aromatic hydrocarbons, are useful carriers. Organometallic compounds are also
useful
carriers. Preferably, the carrier fluid is an alcohol, water or combination
thereof. When
an alcohol is used it is preferably methanol, propanol, ethanol or
combinations thereof.
Water is the most preferred carrier fluid.
The cement composition contains enough of the carrier fluid to wet the
colloidal
silica and/or alumina and produce a paste or viscous fluid, in which the
inorganic filler
particles are dispersed. A useful Brookfield viscosity, as measured at 25 C
using a #6
spindle at 5 rpm, is typically at least about 5, 10, 25, 50, 75 or even 100 Pa-
s. The
cement composition may exhibit shear-thinning behavior, such that its
viscosity
becomes lower at higher shear. The total amount of carrier fluid in the cement
composition (including any carrier fluid that may be brought in with the
colloidal silica
and/or colloidal alumina) is generally from about 25% by weight to at most
about 90% by
weight of the entire composition. A preferred amount of carrier fluid is from
40 to 70%
by weight of the entire composition.
The cement may contain other useful components in addition to the inorganic
filler particles, colloidal silica and/or colloidal alumina and carrier fluid.
An organic
binder or plasticizer can provide desirable rheological properties to the
cement
composition, and therefore preferably is present. Preferably, the binder
dissolves in the
carrier liquid. Examples of suitable binders and organic plasticizers include
cellulose
ethers, such as methyl cellulose, ethyl cellulose, hydroxypropylmethyl
cellulose,
hydroxypropyl cellulose, carboxylmethyl cellulose and the like; polyethylene
glycol, fatty
acids, fatty acid esters and the like.
-10-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
Other optional components include dispersants, deflocculants, flocculants,
defoamers, lubricants and preservatives, such as those described in Chapters
10-12 of
Introduction to the Principles of Ceramic Processing, J. Reed, John Wiley and
Sons, NY,
1988. The cement composition also may contain one or more porogens. Porogens
are
materials specifically added to create voids in the skin after being heated to
form the
amorphous phase. Typically these are any particulates that decompose,
evaporate or in
some way volatilize during a heating or firing step to leave a void. Examples
include
flour, wood flour, carbon particulates (amorphous or graphitic), nut shell
flour or
combinations thereof.
Organic materials such as binders, plasticizers and porogens typically
constitute,
in the aggregate, from 0 to 15%, preferably from 1 to 10% of the total weight
of the
cement composition.
The cement composition is applied to at least one surface of the honeycomb.
The
manner of applying the cement composition not critical, and any suitable
method by
which the composition can be applied at the desired thickness is suitable. The
cement
can be applied manually or through the use of various types of mechanical
apparatus.
The cement composition may be applied under sub-atmospheric pressures to
facilitate
removal of the carrier fluid during the application process. If the cement is
used to
assemble multiple parts (such as multiple honeycombs) into a larger assembly,
the
cement is applied in any convenient manner to a surface of one or more of the
parts that
are being assembled, and the parts are then joined with the cement interposed
between
the parts.
If the cement composition is to be used to form a skin on the honeycomb (or an
assembly containing the honeycomb), the composition is applied to at least a
portion of
the periphery of the honeycomb. Ceramic honeycombs as manufactured typically
have
an outer peripheral "skin", which may be simply the exterior cell walls of the
peripheral
cells of the honeycomb structure. It is generally preferable to remove such a
skin before
applying a replacement skin in accordance with this invention. At least the
exterior
walls of the peripheral cells of the honeycomb are removed. More typically,
the removal
of the "skin" is only part of a more general shaping process, in which outer
portions of
the ceramic honeycomb are removed to bring its cross-sectional shape and size
to
necessary specifications. This step of removing peripheral portions of the
ceramic
honeycomb exposes the interior of the axially-extending cells that remain on
the
periphery of the honeycomb after the removal step is completed. The cement
-11-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
composition is then applied to at least a portion of the newly exposed
periphery of the
honeycomb.
The periphery of the honeycomb usually is not smooth, and in most cases a
certain proportion of the axially-extending cells around the periphery of the
honeycomb
will be open before the cement composition is applied to form a skin. The
cement
composition typically will be applied in such a manner as to fill those open
cells and to
form a somewhat smooth exterior surface. Therefore, the thickness of the skin
usually
will vary. At its thinnest points, the applied skin should be at least 1 mm in
thickness,
and may be as much as 25 mm thick.
The cement composition is fired after it is applied to the honeycomb. The
firing
step removes the carrier fluid and any organic materials (including any
porogen) from
the cement. The colloidal silica and/or colloidal alumina form a binding phase
during
the firing step.
In this invention, at least a portion of the firing step is performed at a
temperature of at least 1000 C in the presence of a fluorine source. The
temperature
may be as high as 1600 C, and preferably is up to 1500 C. The fluorine source
may be,
for example, SiF4, A1F3, HF, Na2SiF6, NaF, NH4F, a fluorinated polymer such a
fluorinated polyethylene or polytetrafluoroethene or some mixture of any two
or more
thereof.
In preferred embodiments, the fluorine source is residual fluorine contained
in an
acicular mullite honeycomb or a mixture of that residual fluorine and an
additional
fluorine source as described in the preceding paragraph. In such a case, the
firing
temperature preferably is at least 1200 C and is more preferably at least 1400
C. At
this high firing temperature, residual fluorine is released from the acicular
mullite
honeycomb, possibly in the form of SiF4. The released fluorine or SiF4 is
believed to
contribute to mullite formation in the cement under the firing conditions. As
a result of
the high temperature firing step, a significant quantity of mullite tends to
form from the
silicon- and aluminum-containing components of the cement. Although some
mullite
formation is common, even when the cement is fired at lower temperatures
(i.e., below
1400 C, especially below 1200 C), when the higher firing temperatures are
used, more
mullite tends to form in the cement than is seen when lower temperatures are
used, or
when the acicular mullite honeycomb does not contain residual fluorine. In
addition,
mullite formation may occur more rapidly when the firing step is performed in
accordance with this invention.
-12-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
In a preferred firing regimen, the honeycomb and applied cement composition
are
heated at a rate of no greater than 20 C/minute, preferably no greater than 10
C/minute
and still more preferably no greater than 5 C/minute, from ambient temperature
up to
at least 1000 C (or at least 1200 C, when the fluorine source is an acicular
mullite
honeycomb). The gradual heating rate is intended to help prevent thermal
shocks and
also to provide time for the carrier fluid and any organic materials to be
removed. It
desired, the assembly may be held at one or more intermediate temperatures for
a
period. This may be desirable, for example, to remove the carrier fluid,
organic binders
and/or porogens in some predetermined sequence, to allow some chemical
reaction to
take place, or for some other reason. Once the assembly reaches the necessary
temperature, it is preferably held at or above that that temperature for a
period of from
minutes to 10 hours. This allows time for the fluorine source to react with
the cement
composition to produce mullite, and, in preferred embodiments, allows time for
residual
fluorine to escape from the acicular mullite honeycomb. In the preferred
embodiments,
it is preferred to reduce the residual fluorine in the acicular honeycomb to
less than 0.5
weight percent, more preferably less than 0.1 weight percent, of the acicular
mullite.
The assembly, with the cement now being fired, is then cooled to ambient
temperature,
preferably at some gradual cooling rate (such as no greater than 10 or 20
C/minute) to
prevent damage from thermal shock.
The mullite content of the fired cement will of course depend somewhat on the
amount of silicon atoms, aluminum atoms and fluorine that were available in
the
starting materials. The fired cement may contain as much as 85% by weight
mullite.
More typically, the fired cement contains from about 45 to 80% mullite, or
from 45 to
75% mullite. The mullite formation has little effect on the morphology of the
fired
cement. The inorganic filler particles largely maintain their particulate or
fibrous
nature, and are bound together via a binding phase that is mainly formed from
colloidal
the silica and/or colloidal alumina component of the cement. Mullite may be
present in
the filler particles or fibers, in the binding phase, or both.
The fired cement is usually porous. The porosity of the fired cement may be
from
to 90%, and is more typically from 40 to 70%.
The fired cement typically has a modulus that is significantly lower than that
of
the honeycomb. The modulus of the fired cement may be, for example in the
range of 3
to 25% of that of the ceramic material in the honeycomb. It is believed that
this lower
modulus imparts high crack resistance of the fired cement. The modulus of the
fired
-13-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
cement can be measured by forming 8 mm X 4 mm X 40 mm test bars from the
cement
composition, firing the blocks and measuring modulus using the Grindosonic
impulse
excitation apparatus following ASTM Standard C 1259-98, Standard Test Method
for
Dynamic Young's Modulus, Shear Modulus, and Poisson's Ratio for Advanced
Ceramics
by Impulse Excitation of Vibration.
Because the fired cement tends to resist cracking, a honeycomb made in
accordance with the invention tends to exhibit excellent thermal shock
resistance,
whether the cement is used as a skin, to adhere constituent parts of the
honeycomb
together to form an assembly, or both. A suitable method of evaluating thermal
shock
resistance is described in the following examples. In this method, the
structure is
subjected to increasingly harsh thermal cycles, and inspected for cracking
after each of
the cycles.
A honeycomb produced in accordance with the invention can be used as a
particulate filter, especially for removing particulate matter from power
plant (mobile or
stationary) exhaust gases. A specific application of this type is a soot
filter for an
internal combustion engine, especially a diesel engine.
Functional materials can be applied to the honeycomb, before or after applying
and firing the cement composition, using various methods. The functional
materials
may be organic or inorganic. Inorganic functional materials, particularly
metals and
metal oxides, are of interest as many of these have desirable catalytic
properties,
function as sorbents or perform some other needed function. One method of
introducing
a metal or metal oxide onto the composite body is by impregnating the
honeycomb with
a solution of a salt or acid of the metal, and then heating or otherwise
removing the
solvent and, if necessary calcining or otherwise decomposing the salt or acid
to form the
desired metal or metal oxide.
Thus, for example, an alumina coating or a coating of another metal oxide is
often applied in order to provide a higher surface area upon which a catalytic
or sorbent
material can be deposited. Alumina can be deposited by impregnating the
honeycomb
with colloidal alumina, followed by drying, typically by passing a gas through
the
impregnated body. This procedure can be repeated as necessary to deposit a
desired
amount of alumina. Other ceramic coatings such as titania can be applied in an
analogous manner.
Metals such as barium, platinum, palladium, silver, gold and the like can be
deposited on the composite body by impregnating the honeycomb (the internal
walls of
-14-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
which are preferably coated with alumina or other metal oxide) with a soluble
salt of the
metal, such as, for example, platinum nitrate, gold chloride, rhodium nitrate,
tetraamine palladium nitrate, barium formate, followed by drying and
preferably
calcination. Catalytic converters for power plant exhaust streams, especially
for
vehicles, can be prepared from the skinned honeycomb in that manner.
Suitable methods for depositing various inorganic materials onto a honeycomb
structure are described, for example, in US 205/0113249 and W02001045828.
These
processes are generally in relation to the skinned honeycomb of this
invention.
In an especially preferred embodiment, alumina and platinum, alumina and
barium or alumina, barium and platinum can be deposited onto the honeycomb in
one or
more steps to from a filter that is simultaneously capable of removing
particulates such
as soot, NOX compounds, carbon monoxide and hydrocarbons from a power plant
exhaust, such as from vehicle engines.
The following examples are provided to illustrate the invention, but are not
intended to limit the scope thereof. All parts and percentages are by weight
unless
otherwise indicated.
Example 1
A cement composition is prepared by mixing 42.0 wt% of ball milled aluminum
silicate fiber (HP-95-SAB-T60, available from Thermal Ceramics Inc., Augusta,
GA),
13.5 wt% of colloidal alumina (AL20SD, available from Nyacol Nano
Technologies, Inc.,
Ashland, MA), 40.5 wt% of water, 2 wt% methyl cellulose (METHOCEL A15LV,
available from The Dow Chemical Co. Midland, MI), and 2 wt% polyethylene
glycol 400
(available from Alfa Aesar, Ward Hill, MA) to obtain a uniform mixture.
A portion of the cement composition is applied onto as-mullitized acicular
mullite
honeycomb segments, which are joined together using the mixture as a cement to
form a
larger honeycomb assembly. The as-mullitized acicular mullite honeycombs
contain 1-
1.4% by weight residual fluorine.
Another portion of the cement composition is applied onto the periphery an as-
mullitized acicular mullite honeycomb to form a skin coating.
A third portion of the cement composition is formed into blocks for material
property measurements.
The honeycomb assembly, coated honeycombs and cement blocks are fired
together by heating them to1400 C at a rate of 2 C/minute, holding at 1400 C
for 6 hours
-15-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
and then cooling slowly to room temperature. Residual fluorine is removed from
the
acicular mullite honeycombs during this firing step, and a binding phase is
simultaneously formed. The resulting materials are referred to collectively as
Example
1.
A fourth portion of the cement composition is applied to heat-treated acicular
mullite honeycombs that contain less than 0.5% by weight residual fluorine.
The
acicular mullite honeycombs are then joined together to form a larger
honeycomb
assembly. A fifth portion of the cement composition is applied as a skin over
a heat-
treated acicular mullite honeycomb. A sixth portion of the cement composition
is formed
into blocks. The honeycomb assembly, skinned honeycomb and blocks are fired
together in the same manner as Example 1. The fired materials are referred to
collectively as Comparative Sample A.
X-ray diffraction (XRD) on the cement and skin of Example 1 shows that they
contain 69.7% mullite, 16.4% cristobalite and 13.9% aluminum oxide. The cement
and
skin of Comparative Sample A contain only 47.4% mullite, wheres the
cristobalite and
aluminum oxide phases are larger (being 26.0% and 26.6%, respectively).
Therefore,
firing in the presence of an acicular mullite having residual fluorine
increases mullite
formation by about 47%.
The higher mullite content of the fired cement and skin results in a closer
chemical composition match of with the acicular mullite honeycomb, and in a
closer
match of CTE, as shown in Figure 1. In Figure 1, the CTE of Example 1 over the
temperature range from about 25 C to 800 C is indicated by line 1, whereas
that of
Comparative Sample A is indicated by line A. The thermal expansion of cement
and skin
fired in presence of an acicular mullite honeycomb containing residual
fluorine is closer
to that of mullite substrate, compared to when the acicular mullite honeycomb
has little
residual fluorine, as in Comparative Sample A. The closer thermal expansion
match
with mullite substrate is due to the increase of mullite phase in the cement
and skin
fired in presence of fluorine and can result in improved thermal shock
performance.
The porosity of the cement and the skin of Example 1 are measured by water
intrusion methods and found to be 64%, which is nearly identical to that of
Comparative
Sample A. Therefore, the co-firing of the cement and skin with the removal of
residual
fluorine from the acicular mullite honeycomb does not result in changes in the
microstructure or porosity of the cement and the skin.
-16-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
The elastic modulus of the fired cement composition is measured by Grindosonic
method. Cement bars with 8 mm x 4 mm x 40 mm dimensions are cut from fired
cement
blocks. Cement bars fired in presence of fluorine (Example 1) has an elastic
modulus of
4.9 GPa, whereas the elastic modulus of the Comparative Sample A cement bars
is
nearly the same (4.7 GPa). The acicular mullite in the honeycombs has an
elastic
modulus of 23.6 GPa. The fired cement composition is more compliant than the
underlying honeycomb and therefore helps relieve the thermomechanical stresses
generated in thermal shock situation.
Example 2 and Comparative Sample B
Example 1 and Comparative Sample A are repeated, this time using a cement
composition prepared by mixing 48.8 wt% of ball milled aluminum silicate fiber
(PS3400
fiber, available from Unifrax LLC, Niagara Falls, NY), 11.9 wt% of colloidal
alumina
(AL20SD, available from Nyacol Nano Technologies, Inc, Ashland, MA), 35.9 wt%
of
water, 1.7 wt% methyl cellulose (METHOCEL A15LV, available from The Dow
Chemical Co., Midland, MI), and 1.7 wt% polyethylene glycol 400 (available
from Alfa
Aesar, Ward Hill, MA). For Example 2, this cement composition is used to join
and skin
acicular mullite honeycombs that contain 1-1.4% residual fluorine, and to form
cement
blocks. For Comparative Sample B, the honeycombs are previously heat-treated
to
reduce residual fluorine to below 0.1%. The materials are then fired as
described with
respect to Example 1 and Comparative Sample A, to form Example 2 and
Comparative
Sample B, respectively.
The cement and skin of Example 2 contain 76.7% mullite, 8.1% cristobalite and
15.3% aluminum oxide by XRD, compared to only 69.2% mullite, 4.9% cristobalite
and
25.9% aluminum oxide for Comparative Sample B. This indicates that mullite
content
in the cement and skin increase by 10.8% when the acicular mullite honeycomb
contains
residual fluorine when the cement composition is fired. The thermal expansion
of
cement and skin for both Example 2 and Comparative Sample B are shown in
Figure 2.
In Figure 1, the CTE of Example 2 over the temperature range from about 25 C
to 800 C
is indicated by line 2, whereas that of Comparative Sample B is indicated by
line B. The
thermal expansion of the Example 2 cement and skin is closer to that of the
acicular
mullite honeycomb substrate than are the cement and skin of Comparative Sample
B.
The closer thermal expansion match with mullite substrate is due to the
increased
mullite content of Example 2 and can result in improved thermal shock
performance.
-17-
CA 02766653 2011-12-22
WO 2011/008463 PCT/US2010/039842
Example 3 and Comparative Sample C
Example 1 and Comparative Sample A are again repeated, this time using a
cement composition prepared by mixing 27.5 wt% of ball milled aluminum
zirconium
silicate fiber (Z-95-SAB-T30, available from Thermal Ceramics Inc., Augusta,
GA), 16.9
wt% of colloidal alumina (AL20SD, available from Nyacol Nano Technologies,
Inc.,
Ashland, MA), 50.6 wt% of water, 2.5 wt% methyl cellulose (METHOCEL A15LV,
available from The Dow Chemical Co. Midland, MI), and 2.5 wt% polyethylene
glycol
400 (available from Alfa Aesar, Ward Hill, MA).
For Example 3, this cement composition is used to join and skin acicular
mullite
honeycombs that contain 1-1.4% residual fluorine, and to form cement blocks.
For
Comparative Sample C, the honeycombs are previously heat-treated to reduce
residual
fluorine to below 0.5%. The materials are then fired as described with respect
to
Example 1 and Comparative Sample A to form Example 3 and Comparative Sample C,
respectively.
The cement and skin of Example 3 contain 53.0% mullite, 13.7% crystobalite,
24.6% aluminum oxide and 8.6% zirconium oxide by XRD, compared to only 42.0%
mullite, 18.0% cristobalite, 32.1% aluminum oxide and 7.9% zirconium oxide for
Comparative Sample C. This indicates that mullite content in the cement and
skin
increase by 26.2% when the acicular mullite honeycomb contains residual
fluorine when
the cement composition is fired, even when the fibers in the cement
composition are
doped with zirconium. Example 3 cement and skin has an elastic modulus of 3.3
GPa,which is nearly unchanged from that of Comparative Sample C (3.6 GPa).
These
values are much lower that that of the underlying acicular mullite honeycomb,
indicating greater compliance and ability to relieve thermomechanical
stresses.
-18-