Note: Descriptions are shown in the official language in which they were submitted.
CA 02772315 2017-01-19
WO 2011/4131557 PCTAUS2010/047011
AN ACCOMMODATING INTRAOCULAR LENS
WITH A SURFACE ADHERENT
Field of the Invention
[0002] The present invention relates to ophthalmic implants and related
methods, and
more particularly to intraocular lenses and glaucoma shunts with improved
fixation and/or
control of cellular growth.
Background of the Inventiou
[0003] A human eye can suffer diseases that impair a patient's vision. For
instance, a
cataract may increase the opacity of the lens, causing blindness. To restore
the patient's vision,
the diseased lens may be surgically removed and replaced with an artificial
lens, known as an
intraocular lens, or 10L. In other cases, glaucoma may result in a gradual and
undesirable
increase of intraocular pressure (LOP), hi such instances, a shunt may be
implanted to help
control pressure within the eye. In either case, it is generally desirable to
maintain the ocular
device at a fixed location within the eye.
[0004] The simplest 10Ls are monofoc:al 10Ls that are fixed within the eye and
have a
single focal length or power. Unlike the eye's natural lens, which can adjust
its focal length
within a particular range in a process known as accommodation, these 10Ls
cannot generally
accommodate. As a result, objects at a particular position away from the eye
appear in focus,
while objects at increasing distances away from that position appear
increasingly blurred.
Bifocal or multifocal 10Ls, which are also generally fixed within the eye,
produce two or more
foci in order to simulate the accommodation produced by the eye's natural
lens. For example,
one of the foci may be selected to provide distant vision, while a second
focus is selected to
provide near vision. While multifocal IOLs improve the ability of a subject to
focus on objects
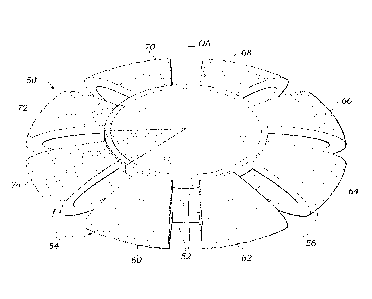
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
over a range of distances, the presence of more than one focus generally
results in reduced
contrast sensitivity compared to monofocal IOLs.
[0005] An IOL may also be used for presbyopic lens exchange. Presbyopia is the
condition where the eye exhibits a progressively diminished ability to focus
on objects over a
range of distances. It is caused by a gradual loss of "accommodation" in the
natural lens inside
the eye due to age-related changes that make the lens harder and less elastic
with the years.
[0006] An improvement over the fixed IOLs (either monofocal or multifocal) is
an
accommodating IOL, or aIOL, which can adjust its power and/or axial position
within a
particular range. As a result, the patient can clearly focus on objects over a
range of distances
from the eye in a way that is similar to that provided by the natural lens.
This ability to
accommodate may be of tremendous benefit for the patient, and more closely
approximates the
patient's natural vision than monofocal or multifocal IOLs. Such artificial
implantable lenses
can take the form of injectable IOLs (polymer material injected into the
capsular bag),
Deformable IOLs (the lens' optic shape change creates optical power change),
axially moving
IOLs, Dual Optics IOLs, etc, or some combination thereof. Alignment of aIOLs
within the eye
may be particularly important. Thus, reliable attachment means may be
especially useful in
assuring quality optical performance for aIOLs.
[0007] The human eye contains a structure known as the capsular bag, which
surrounds
the natural lens. The capsular bag is transparent, and serves to hold the
lens. In the natural eye,
accommodation is initiated in part by the ciliary muscle and a series of
zonular fibers, also
known as zonules. The zonules are located in a relatively thick band mostly
around the equator
of the lens, and impart a largely radial force to the capsular bag that can
alter the shape and/or
the location of the natural lens and thereby change its effective power and/or
focal distance.
[0008] In a typical surgery in which the natural lens is removed from the eye,
the lens
material is typically broken up and vacuumed out of the eye, but the capsular
bag is left
generally intact. The remaining capsular bag is extremely useful in that it
may be used to house
an aIOL, which is acted on by the zonules to change shape and/or shift in some
manner to affect
the lens power and/or the axial location of the image.
[0009] The aIOL has an optic, which refracts light that passes through it and
forms an
image on the retina, and may also include a haptic, which mechanically couples
the optic to the
capsular bag or holds the aIOL in contact with the capsular bag. During
accommodation, the
-2-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
zonules exert a force on the capsular bag, which in turn exerts a force on the
optic. The force
may be transmitted from the capsular bag directly to the optic, or from the
capsular bag through
a haptic to the optic. In either case, the lens changes shape and/or position
dynamically to keep
an object in focus on the retina as its distance from the eye varies.
[0010] Desirably, the design of the aIOLs effectively translates the ocular
forces of the
natural accommodative mechanism of the eye [ciliary muscle ¨ zonules ¨
capsular bag] to
maximize accommodation amplitude or range. Also, aIOLs may take into account
the problem
of lens epithelial cell (LECs) proliferation which can cause opacification and
stiffening of the
capsular bag over time. This phenomenon is caused by the wound healing
reactions of the
natural lens epithelial cells that remain on the inside of the capsular bag,
often in the narrow ring
around the equatorial region. Several methods to prevent the LECs from
proliferating have been
tried, including removing the LECs as much as possible, mechanically as well
as
pharmaceutically. Alternatively, design features such as a square edge and
spacers have been
incorporated into the aIOLs.
[0011] As mentioned above, ocular implants may also be used in long-term
glaucoma
treatment. Glaucoma is a progressive disease of the eye characterized by a
gradual increase of
intraocular pressure (TOP). This increase in pressure is most commonly caused
by stenosis or
blockage of the aqueous outflow channel, resulting in excessive buildup of
aqueous fluid within
the eye. The implant solution typically involves suturing a small plate to the
sclera in the
anterior segment of the eye at the limbus, and inserting a drainage tube into
the anterior chamber
of the eye, which may also be secured via a suture to the sclera. Once
implanted, the body forms
scar tissue around the plate. Aqueous humor flow through the tube causes the
tissues above the
plate to lift and form a bleb. A bleb is a fluid filled space surrounded by
scar tissue, somewhat
akin to a blister. The fluid within the bleb then flows through the scar
tissue at a rate which
desirably regulates TOP. More recently, U.S. Patent Nos. 5,476,445 and
6,050,970 to Dr.
George Baerveldt, et al. disclose glaucoma implants or shunts featuring a
flexible plate that
attaches to the sclera and a drainage tube positioned for insertion into the
anterior chamber of the
eye. This type of shunt is sold under the tradename Baerveldt BG Series of
glaucoma implants
by Advanced Medical Optics (AMO) of Santa Ana, CA. The Baerveldt device has
an open
tube without flow restricting elements. Temporary sutures are used to restrict
fluid flow for a
predetermined period, after which the bleb forms and fluid drainage is
properly regulated. The
-3-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
temporary sutures are either biodegradable or removed in a separate procedure.
This method
works well, but the timing of suture dissolution is necessarily inexact, and a
second procedure
undesirable.
[0012] In these and other situations, ocular devices and methods are needed
for securely
attaching ocular implants in an eye. In some instances, reversal of the
attachment means is
desirable, for example, to allow the device to be more readily explanted. In
addition, there exists
a need for an aIOL with increased efficiency in converting an ocular force to
a change in power
and/or a change in axial location of the image, preferably in a way which also
reduces the
problem of lens epithelial cell proliferation. There is also a need for an
alternative to suturing
glaucoma shunts in place.
Brief Description of the Drawings
[0013] Features and advantages of the present invention will become
appreciated as the
same become better understood with reference to the specification, claims, and
appended
drawings wherein:
[0014] Figure 1 is a vertical sectional view of a human eye.
[0015] Figure 2A is a vertical sectional view of a portion of an eye having an
implanted
intraocular lens, in an accommodative or "near" state.
[0016] Figure 2B is a vertical sectional view of the eye of Figure 2A, in a
disaccommodative or "far" state.
[0017] Figure 3 is a perspective view of an intraocular lens having a pair of
axially
spaced-apart and centered optics, and a plurality of convex haptic legs
connecting the optics and
radiating outward therefrom;
[0018] Figure 4 is an elevational view of the intraocular lens of Figure 3;
[0019] Figure 5 is a sectional view of the intraocular lens of Figure 3;
[0020] Figure 6A and 6B are vertical sectional views through an eye showing
the
implanted exemplary aIOL of Figures 3-5 in two states of accommodation;
[0021] Figure 7 is a perspective view of an intraocular lens having an optic
within which
is embedded a portion of an accommodative haptic, the accommodative haptic
including a
central vaulted portion, a plurality of spokes each having a unitary outer
end, axially spaced
-4-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
apart bifurcated inner ends connected in two axially spaced planes, and
central throughholes in
the central vaulted portion;
[0022] Figure 8A is a vertical sectional view through an eye showing
preparation of the
inner surface of the capsular bag by application of a bio-adhesive;
[0023] Figure 8B is a vertical sectional view through an eye showing
introduction of an
injectable polymer aIOL into the capsular bag prepared as in Figure 8A;
[0024] Figure 9 is a perspective view of an exemplary glaucoma shunt that may
be fixed
in place using the principles described herein; and
[0025] Figure 10 is a bottom plan view of the glaucoma shunt of Figure 9
showing an
exemplary distribution of an adhering surface.
Detailed Description of the Preferred Embodiments
[0026] Embodiments of the present invention are generally directed to devices,
substances, and methods for attaching ophthalmic devices and/or controlling
cellular growth
after implantation of an ocular device. Embodiments of the present invention
are particularly
useful when used in conjunction with IOLs. For example, embodiments of the
present invention
may provide immediate and/or reversible adhesion of an IOL within the capsular
bag of an
animal or human subject. Surface adherents according to embodiments of the
present invention
are generally reversible, thus allowing an IOL to be explanted or readjusted
subsequent to initial
attachment within the eye. While potentially applicable to a variety of
ophthalmic devices and
IOLs, surface adherents according to embodiments of the present invention may
find particular
use with accommodating IOLs, which may have attachment and alignment
requirements that are
especially critical.
[0027] In a healthy human eye, the natural lens is housed in a structure known
as the
capsular bag. The capsular bag is driven by a ciliary muscle and zonular
fibers (also known as
zonules) in the eye, which can alternately pull on or release on the capsular
bag to change its
shape. The motions of the capsular bag change the shape of the natural lens in
order to change its
power and/or the location of the lens, so that the eye can focus on objects at
varying distances
away from the eye in a process known as accommodation.
-5-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
[0028] For some people suffering from cataracts, the natural lens of the eye
becomes
clouded or opaque. If left untreated, the vision of the eye becomes degraded
and blindness can
occur in the eye. A standard treatment is surgery, during which the natural
lens is broken up,
removed, and replaced with a manufactured intraocular lens. Typically, the
capsular bag is left
intact in the eye, so that it may house the implanted intraocular lens.
[0029] Because the capsular bag is capable of shape change, initiated by the
capsular bag
resiliency, ciliary muscle, and/or zonules, it is desirable that the implanted
intraocular lens be
configured to utilize the ocular forces produced thereby to change its power
and/or location in
the eye in a manner similar to that of the natural lens. Such an accommodating
lens may produce
improved vision over conventional monofocal or multifocal IOLs.
[0030] A desirable optic for an accommodating IOL is one that changes shape in
response to an ocular force, for example, a squeezing or expanding radial
force applied largely to
the equator of the optic (e.g., by pushing or pulling on or near the edge of
the optic,
circumferentially around the optic axis). Under the influence of an ocular
force, the optic of the
IOL may bulge slightly in the axial direction, producing more steeply curved
anterior and/or
posterior faces, and producing an increase in the power of the optic.
Likewise, an expanding
radial force produces a decrease in the optic power by flattening the optic.
This change in power
is accomplished in a manner similar to that of the natural eye and is well
adapted to
accommodation.
[0031] Figure 1 shows a human eye 10 in vertical section. Light enters from
the left of
Figure 1, and passes through the cornea 11, the anterior chamber 12, the iris
13, and enters the
capsular bag 14. Prior to surgery, the natural lens occupies essentially the
entire interior of the
capsular bag 14. After surgery, the capsular bag 14 houses the intraocular
lens. The intraocular
lens is described in more detail below. After passing through the natural
lens, light exits the
posterior wall 15 of the capsular bag 14, passes through the posterior chamber
24, and is focused
onto the retina 16, which detects the light and converts it to a signal
transmitted through the
optic nerve 17 to the brain.
[0032] Figure 2A shows the eye 10 after an accommodating intraocular lens has
been
implanted. A well-corrected eye forms an image at the retina 16. If the lens
system (cornea +
IOL) has too much or too little power, the image shifts axially along the
optical axis away from
the retina. The power required to focus on a close or near object is more than
the power required
-6-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
to focus on a distant or far object. The difference between the "near" and
"far" powers is known
typically as the add power or as the range of accommodation. A normal range of
accommodation is about 2 to 4 diopters, which is considered sufficient for
most patients, but
some have a range of about 1 to 8 diopters. As used herein, the term "about"
means within plus
or minus 0.25 Diopters, when used in reference to an optical power.
[0033] The capsular bag is acted upon by the ciliary muscle 25 via the zonules
18, which
change the shape of the capsular bag 14 by releasing or stretching it radially
in a relatively thick
band about its equator. Experimentally, it is found that the ciliary muscle 25
and/or the zonules
18 typically exert a total ocular force of up to about 10 grams of force,
which is distributed
generally uniformly around the equator of the capsular bag 14. As used herein,
the term "about"
means within plus or minus 0.5 grams of force, when used in reference to an
ocular force. As
used herein, an "ocular force" is a force produced by a human or animal eye to
provide
accommodation, for example, a force produce by the ciliary muscle, zonules,
and/or capsular
bag of an eye. In human eyes, an ocular force is generally be considered to be
a force that is in a
range from 0.5 gram force to 20 grams force, 0.5 gram force to 10 grams force,
or 0.5 gram force
to 6 grams force. Although the range of ocular force may vary from patient to
patient, it should
be noted that for each patient, the range of accommodation is limited by the
total ocular force
that can be exerted. It may be desirable that the intraocular lens be
configured to vary its power
over the full range of accommodation, in response to this limited range of
ocular forces. In other
words, it is desirable to have a relatively large change in power for a
relatively small driving
force. As used herein, the term "full range of accommodation" means a
variation in optical
power of an optic, lens, or lens system that is able to provide both distant
and near vision, for
example, a change in optical power of at least 3 Diopters or at least 4
Diopters.
[0034] Note that the lens may be designed so that its relaxed state (i.e., in
the absence of
outside forces other than gravity) is a "far" condition for providing far
vision (sometimes
referred to as "disaccommodative biased"), a "near" condition for providing
near vision
("accommodative biased"), or some condition in between the two.
[0035] The intraocular lens itself generally has two components, an optic 21,
which is
made of a transparent, deformable and/or elastic material, and a haptic 23,
which holds the optic
21 in place and mechanically transfers forces on the capsular bag 14 to the
optic 21. The haptic
23 may have an engagement member with a central recess that is sized to
receive the peripheral
-7-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
edge of the optic 21. The haptic and optic may be refractive index matched,
though if at least
some of the haptic is embedded in or otherwise overlapping the optic the two
materials must be
index matched.
[0036] The lens desirably has a surface adherent thereon, either on just the
haptic 23 or
also on the optic 21. Various surface adherents are described herein, and any
combination and
placement of such adherents may be applied to the lens in Figures 2A and 2B to
facilitate
accommodation, as will be described.
[0037] When the eye 10 focuses on a relatively close object, as shown in
Figure 2A, the
zonules 18 relax and permit the capsular bag 14 to return to its natural shape
in which it is
relatively thick at its center and has more steeply curved sides. As a result
of this action, the
power of the lens increases (i.e., one or both of the radii of curvature can
decrease, and/or the
lens can become thicker, and/or the lens may also move axially), placing the
image of the
relatively close object at the retina 16. Note that if the lens could not
accommodate, the image
of the relatively close object would be located behind the retina, and would
appear blurred.
[0038] Figure 2B shows a portion of an eye 20 that is focused on a relatively
distant
object. The cornea 11 and anterior chamber 12 are typically unaffected by
accommodation, and
are substantially identical to the corresponding elements in Figure 2A. To
focus on the distant
object, the ciliary muscle 25 contracts and the zonules 18 retract and change
the shape of the
capsular bag 14, which becomes thinner at its center and has less steeply
curved sides. This
reduces the lens power by flattening (i.e., lengthening radii of curvature
and/or thinning) the
lens, placing the image of the relatively distant object at the retina (not
shown).
[0039] For both the "near" case of Figure 2A and the "far" case of Figure 2B,
the
intraocular lens itself changes shape in response to ocular forces provided by
the ciliary muscles
and/or the capsular bag. For a "near" object, the haptic 23 compresses the
optic 21 at its edge,
increasing the thickness of the optic 21 at its center and increasing the
curvature of at least a
portion of its anterior face 19 and/or its posterior face 15. As a result, the
power of the optic 21
increases. For the "far" object, the haptic 30 expands, pulling on the optic
21 at its edge, and
thereby decreasing the thickness of the optic 21 at its center and decreasing
the curvature of at
least a portion of its anterior face 19 and/or its posterior face 15. As a
result, the lens power
decreases.
-8-
CA 02772315 2017-01-19
WO 2011/031557 PCT/US2010/047011
[0040] Note that the specific degrees of change in curvature of the anterior
and posterior
faces may depend on the nominal curvatures. Although the optic 21 is drawn as
biconvex, it
may also be piano-convex, meniscus or other lens shapes. In all of these
cases, the optic is
compressed or expanded by forces applied by the haptic to the edge and/or
faces of the optic. In
addition, there may be some axial movement of the optic. In some embodiments,
the haptic is
configured to transfer the generally symmetric radial forces symmetrically to
the optic to change
the shape or surface curvature of the optic in an axisymmetric way. However,
in alternate
embodiments the haptic is configured non-uniformly (e.g., having different
material properties,
thickness, dimensions, spacing, angles or curvatures), to allow for non-
uniform transfer of forces
by the haptic to the optic. For example, this could be used to combat
astigmatism, coma or other
asymmetric aberrations of the eye/lens system. The optic may optionally have
one or more
diffractive elements, one or more multifocal elements, and/or one or more
aspheric elements.
[0041] Certain exemplary embodiments herein provide a haptic partly embedded
within
an adjustable or accommodative central optic. The haptic transmits forces to
alter at least one of
the shape and the thickness of the adjustable optic. The materials of the
haptic and optic may
have similar compressive or spring moduli, to encourage direct transfer of
forces and reduce
uneven expansion/contraction and accompanying tension therebetween, though the
haptics are
generally somewhat stiffer to be capable of transmitting capsular forces.
Additionally, similar
material stiffness may reduce the mismatch in shrinkage rates during molding
or post-
processing, which mismatch may ultimately negatively impact lens optical
resolution. In one
embodiment, the haptic is stiffer than the optic. Moreover, the two materials
have the same or
similar refractive indices to reduce any unwanted glare or reflection from
light passing across
adjacent surfaces. A number of such embedded optics may be seen in U.S. Patent
Publications
2008-0161913 and 2008-0161914.
[0042] A number of intraocular lenses may be adapted to the concepts described
herein
to improve the accommodative performance of the haptic or 10L, such that
compressive/tensile
forces may be more efficiently transferred from the haptic to the optic. It
should be understood
that any combination of individual haptic or IOL features described herein,
where appropriate,
may be formed even if not explicitly described or shown. It should also be
noted that while
described in relation to aIOLs, surface adherents according to embodiments of
the present
-9-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
invention may be used with a variety of types of IOLs or other ophthalmic
devices (e.g., shunts).
For instance, any monofocal or multifocal IOL may benefit from a surface
adherent on its haptic
and/or optic to fix the lens in position, enhance stability, and/or prevent
PCO. For example, a
thermo-reversible adhesive, which solidifies at body temperature, may be
useful to initially
attach an IOL and subsequently reverse the attachment temporarily to readjust
the IOL position
by flowing a cold BSS solution through the eye. Likewise, both phakic IOLs
(PIOL) may be
adapted with the surface adherents described herein. For instance, a phakic
anterior chamber
IOL may have microfibers on its haptics for better fixation.
[0043] Figure 3 is a perspective view of an accommodative IOL 50 having a pair
of
axially spaced-apart optics 52 centered on an optical axis OA, and a plurality
of convex haptic
legs 54 connect the optics and radiating outward therefrom. The haptic legs 54
are configured to
transmit forces from the surrounding capsular bag/zonules to alter the spacing
between the optics
52.
[0044] In some embodiments, the aIOL 50 is symmetric across a midplane
perpendicular
to the optical axis OA such that there are matching legs 54 connected to each
optic 52.
Preferably, each pair of matching legs 54 joins together at their outer ends
in a convex outer
curve 56 that may be configured to generally match the shape of a capsular bag
of an eye into
which the intraocular lens is inserted. As illustrated, there may be eight
pairs of matching legs
54, though more and as few as three are contemplated. The convex outer ends of
the haptic legs
54 provides a capsular bag-filling outer profile to the aIOL 50 that
effectively couples the bag
forces to the dual optics 52 to either axially expand or contract the spacing
therebetween. That
is, forces exerted on the outer ends of the haptic legs 54 are transmitted
through the legs to cause
the spaced optics 52 to move apart or toward each other, thus changing the
dual lens focal
length. Although movement between the two optics 52 may be configured to
amplify a change
in power (accommodative range), in some embodiments the aIOL 50 includes only
one of the
lenses 52, for example, to reduce criticality of alignment of the aIOL within
the eye.
[0045] In accordance with the principles described herein, varying degrees of
a surface
adherent may be provided to the exterior of the aIOL 50. As seen in Figures 3
and 4, gradually
larger regions of stippling are shown around the aIOL 50 and on succeeding
haptic legs 54. A
thin band of stippling 60 is shown on a leg 54 at the lower left in Figure 3,
with gradually larger
regions of stippling shown at 62-70 in a CCW direction around the aIOL 50. The
largest region
-10-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
of stippling in this series at 70 covers the entire haptic leg 54. Continuing
CCW, two other
regions of stippling 72, 74 extend partway and all the way radially inward
onto sectors on the
optics 52 (the lower half shall be considered to be symmetric with the upper
half, though such is
not strictly necessary).
[0046] The regions of stippling 60-74 represent application locations for a
number of
different potential surface adherents according to embodiments of the present
invention. In
general, surface adherents according to embodiments of the present invention
are
advantageously provide adhesion within a relatively short period of time
(e.g., less than or equal
to one second, less than 1 to 5 minutes, or less than 1 to 5 hours), help to
prevent or control cell
growth (e.g., PCO), are reversible, and/or otherwise provide mechanism for
easily detaching a
device after adhesion to a part of an eye. For instance, the regions of
stippling 60-74 could be a
thermo-reversible bioadhesive polymer such as polymerized N-isopropyl
acrylamide (pNIPAM)
(also known as NIPAAm (poly(N-isopropylacrylamide)). Alternatively, the
regions of stippling 60-
74 could comprise a plurality of microfibers, for example, having physical
surface texturing
designed to mimic the feet of certain lizards and insects. Each of these
alternatives will be
discussed in more detail below, including their preferred sites of application
on the aIOL.
Preferably, the amount of surface adherent is sufficient to hold the aIOL in
place under normal
ocular forces after insertion into an eye. In some embodiments, reversible
adhesion is provided
by a substance that changes its adhesion characteristic with an intensity or
wavelength of light,
vibration of the adhesion interface, application or concentration of a
chemical substance,
exposure or intensity of an electric or magnetic field, or the like.
[0047] Polymeric systems that may modify adhesive properties in response to
changes in
the physical and chemical characteristics of the physiological medium are
promising candidates
to achieve reversible tissue adhesion. Several groups have explored the use of
dynamic
stimulus-responsive surface chemistries for cell patterning, thermo-active,
electrical-active, and
photo-active chemistries have been defined for cellular adhesion. In general,
all of these
chemistries operate under the same principle. These substances can be switched
from a state that
prevents cellular attachment to a state that promotes it. In the context of
the present application,
a reversible adhesive means one which can change state depending on certain
stimulus, such as
temperature for a thermo-reversible adhesive. Other possible stimuli include
mechanical (e.g.,
vibration), light, radiation, chemical, or others.
-11-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
[0048] A particularly useful composition for use in the present invention is a
thermo-
reversible bioadhesive polymer, such as a composition which is liquid at or
below room
temperature and forms a high viscosity layer or gel at body temperature.
[0049] Polymers having bioadhesive properties are for instance water-soluble
cellulose
derivatives, such as sodium carboxymethyl cellulose, and polyacrylic acids,
which are used in
many pharmaceutical preparations to improve the contact between drug and body.
Improved
uptake of ophthalmic drugs has been achieved by using vehicles containing
viscosity-increasing
polymers such as the cellulose derivatives, polyvinyl alcohol and
polyvinylpyrrolidone.
Thermogelling pharmaceutical preparations are described in U.S. Pat. Nos.
4,478,822,
4,474,751, 4,474,752 and 4,474,753, which refer to a drug delivery system
which at room
temperature has the properties of a liquid, but forms a semi-solid gel at
human body
temperatures. The compositions to be administered comprise 10 to 50% by weight
of a polymer,
which is a tetra-substituted derivative of certain diamines containing
approximately 40 to 80%
poly(oxyethylene) and approximately 20 to 60% poly(oxypropylene), as a drug
delivery vehicle.
In this system the gel transition temperature and/or the rigidity of the gel
can be modified by
adjustment of the pH. Other systems are known in which the gelling is induced
by an increase in
the amount of electrolytes or a change in pH. Further, certain water-soluble
nonionic cellulose
ethers in combination with a charged surfactant and optional additives in
water have the property
of being liquid at room temperature and forming a gel when warmed to body
temperature, and
the process is reversible.
[0050] An ideal thermo-reversible bioadhesive polymer for intraocular use
should be
nontoxic and biocompatible. Polymerized N-isopropyl acrylamide (pNIPAM) has
been shown
not to be toxic to neural tissue and is commonly used in cell and tissue
cultures for its reversible
cell adhesion properties. Previous reports showed that cells may be attached
and detached from
pNIPAM coated culture dishes without exhibiting any changes in morphology.
Some studies
show that pNIPAM has a lower critical solution temperature of 31 C in an
aqueous environment.
This may indicate that the reversible thermoresponsive adhesive or hydrogel
(pNIPAM) exhibits
decreased solubility or swelling in water as the temperature is increased, due
to a phase
transformation at the lower critical solution temperature. Thus, pNIPAM may be
switched from
a state that promotes cellular attachment to a state that prevents cellular
attachment, as the
temperature of the surface is decreased. A particular characteristic of this
material is the ability
-12-
CA 02772315 2017-01-19
WO 2011/031557 PCT/US2010/047011
to he adhesive at body temperature (37C) and not adhesive at room temperature.
Various
applications for such a bioadhesive are disclosed in US Patent Publication No.
2008-0140192,
assigned to the University of Southern California.
[0051] The use of this type of thermo-reversible, or some other type of
reversible,
bioadhesive polymer with accommodating 10Ls (001.$) may resolve two key issues
currently
challenging the use of al0Ls technologies (that is, prevention of LECs from
proliferating
("PCO") and optimization of the coupling of the capsular bag to the aIOLs) by
fully adhering the
alOL to the capsular bag once the alOL is in place. Further, cold or room
temperature saline
could be injected at the device and/or into the capsular bag to release the
adhesive to allow for
re-position of the alOL or its explantation.
[0052] If applied to a lens of an IOL or alOL, the lens could be coated with
the thermo-
reversible bioadhesive polymer. In this case, the lens could be handled in a
manner consistent
with current standard cataract surgical procedures and inserted at operating
room temperatures.
Once the lens is implanted in the eye, the thermo-reversible polymer (such as
pNIPAM)
properties will allow the IOL to adhere to the capsular bag. The coating can
be selective
(specific areas of the alOL) or on all surfaces of the alOL as required by the
MI. design to
prevent LECs proliferation and to optimize capsular bag coupling. Also, as
mentioned above,
the adhesive may be reversible based on some other stimulus than a temperature
change.
[0053] In a preferred embodiment, a thermo-reversible bioadhesive polymer is
coated on
the exterior of the aIOL 50 prior to implant, and remains in a state that
prevents cellular
attachment (less adherent) while outside the body. After implant into the
capsular bag, and a rise
in temperature to match the body's, the thermo-reversible bioadhesive polymer
undergoes a
change of state to one that that promotes cellular attachment (more adherent).
Postsurgically,
should the alOL 50 require removal, replacement, or re-positioning, a cold
saline or other such
solution may be used to cause the thenno-reversible bioadhesive polymer to
revert back to its
less adherent state. Preferably, the amount of thermo-reversible bioadhesive
polymer is
sufficient to hold the alOL 50 in place under normal ocular forces after
insertion into an eye.
[0054] With reference to Figures 3 and 4, one or more of the varying sizes
shown of the
stippled regions 60-74 may be reproduced on all haptic legs 54 of the aIOL 50.
In a preferred
embodiment, the surface adherent is provided in thin bands, as in the small
band 60, on the outer
-13-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
end of each haptic leg 54. One benefit from providing the thin surface
adherent bands 60 is that
the equatorial region of the haptic legs 54 adheres better within the area of
the capsular bag
where the zonular fibers attach to the bag. Also, providing adhesive between
the haptic legs 54
and the capsular bag may prevent cell migration over these contact areas. Lens
epithelial cell
(LECs) often remain in the tight equatorial corner inside the capsular bag
after attempts at
removal. Adhering the haptic legs 54 to the capsular bag in these areas
effectively eliminates
any gap therebetween and thus inhibits further overgrowth. In some
embodiments, a surface
adherent is applied to selectively provide adhesion in a region where the
zonules attach to the
capsular bag, for example, to provide enhanced transfer of ocular forces to
the capsular bag and
aIOL. In such embodiments, other surface portions of the haptic and/or optic
may be free of the
bioadhesive polymer, for example, to allow relative motion between the
capsular bag and the
aIOL.
[0055] Alternatively, larger bands of a surface adherent as the band 62 may be
used, or
even larger bands as seen at 64-68, moving CCW around the aIOL 50. Ultimately,
the entirety
of each haptic leg 54 may be covered with the surface adherent, as seen at 70.
[0056] Depending on the effect on the optical performance, surface adherent
may also
cover a portion or all of the external surface of the optics 52 (or just one
of the optics). For
instance, region 72 shows the surface adherent extending inward beyond the
corresponding
haptic leg 54 and onto the outer rim of the optic 52. Likewise, region 74
shows the surface
adherent extending inward beyond the corresponding haptic leg 54, over the
outer rim of the
optic 52, and onto the surface of the optic to its center. The stippling 74
has been drawn to
indicate that if all of the sectors were so configured that the entire
exterior surface of the aIOL
50 - that is, both the optics 52 and the haptic legs 54 ¨ would be covered
with a surface adherent.
In some embodiments, a surface adherent is located on at least portions of one
or both optics 52,
but no, or little, surface adherent is located on the haptics legs 54, for
example, to hold the aIOL
in place and allow relative motion between the capsular bag and haptic legs
54.
[0057] As mentioned above, the regions of stippling 60-74 could be physical
surface
texturing designed to mimic the feet of certain lizards and insects. The
ability of geckos, spiders
and flies to adhere to seemingly shear surfaces has long fascinated
researchers. For instance,
geckos' exhibit a remarkable ability to stick to surfaces without the use of
an adhesive substance
(such as a polymer, etc.). Geckos foot surfaces are characterized by a
plurality of microfibers
-14-
CA 02772315 2017-01-19
WO 2011/031557 PCT/US2010/047011
that in some aspects are similar to synthetic microfibers. The adherent
principle (i.e., adhesion
through physical surface structure rather than exuded polymers, or other
similar contact
adhesives, etc.) is believed to be due to van der Waals forces.
[0058] A van der Waals force is the attractive or repulsive force between
molecules (or
between parts of the same molecule) other than those due to covalent bonds or
to
the electrostatic interaction of ions with one another or with neutral
molecules. The term
includes permanent dipole¨permanent dipole forces, induced dipole¨induced
dipole forces, and
instantaneous induced dipole-induced dipole (London dispersion forces). It is
also sometimes
used loosely as a synonym for the totality of intermolecular forces. Van der
Waals forces are
relatively weak compared to normal chemical bonds.
[00591 Through various molding processes and techniques, it is possible to
mimic the
microfiber structure found on gecko feet that provides such an adherent
surface. Consequently,
one "surface adherent" as defined herein is a surface having a plurality of
microfibers thereon.
Microfibers, in this context, will be defined as fibers having a diameter of
between 3-5 microns
(micrometers, ).tm). The microfibers will be provided in sufficient
numbers/density over a
particular area of the alOL to provide adhesion between the aIOL and the
surrounding capsular
bag. This would provide immediate IOL-to-capsular bag fixation after implant
as well as an
easy detachment process through pealing. Preferably, the microfibers will be
provided in
sufficient numbers/density over a sufficient area so as to hold the aIOL 50 in
place under normal
ocular forces after insertion into an eye.
[00601 For instance, microfibers may be molded in sufficient quantities along
the
perimeter of the haptic (such as in the thin bands 60, 62, or 64 in Figures 3
and 4) so that the
existing capsular bag could adhere to them. Again, this adhesion will allow
the haptic legs 54 to
he more effectively pulled bringing the two optics closer (during dis-
accommodation, reducing
power) and pushed forcing the optics apart (during accommodation, increasing
power).
Locating these fibers primarily along the equator of the haptic legs 54 within
the band where the
zonular fibers attach to the bag provides excellent results in terms of
improved force transfer
during accommodation. Proper shape and sizing of the haptic structure would be
necessary, as
described below.
[00611 An exemplary discussion of a variety of microfiber configurations is
given in
U.S. Patent No. 7,344,617 to Dubrow.
-15-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
[0062] Different embodiments of the invention comprise a range of densities
(e.g.,
number of microfibers per unit area of a substrate to which microfibers are
attached or
associated) The number of microfibers per unit area can optionally range from
about 1
microfiber per 10 micron2 up to about 200 or more microfibers per micron2;
from about 1
microfiber per micron2 up to about 150 or more microfibers per micron2; from
about 10
microfibers per micron2 up to about 100 or more microfibers per micron2; or
from about 25
microfibers per micron2 up to about 75 or more microfibers per micron2 In yet
other
embodiments, the density can optionally range from about 1 to 3 microfibers
per square micron
to up to approximately 2,500 or more microfibers per square micron
[0063] In terms of individual fiber dimensions, it will be appreciated that by
increasing
the thickness or diameter of each individual fiber, one will again,
automatically increase the area
of the fiber that is able to make intimate contact with another surface,
whether such contact is
with a fiber that is directly orthogonal to the second surface or is parallel
or tangential with that
other surface Preferred fiber thicknesses are optionally between from about 3-
5 microns.
Choice of microfiber thickness can also be influenced by compliance of such
microfibers (e.g.,
taking into account that microfiber' s composition, etc.) Thus, since some
compositions can
produce a less compliant microfiber at greater diameter such changes can
optionally influence
the choice of microfiber diameter
[0064] In the case of parallel or tangential contact between fibers from one
surface and a
second surface, it will be appreciated that by providing fibers of varying
lengths, one can
enhance the amount of contact between a fiber, e.g., on an edge, and the
second surface, thereby
increasing adhesion Of course, it will also be understood that for some fiber
materials,
increasing length may yield increasing fragility Accordingly, preferred fiber
lengths will
typically be between about 30 microns or less up to about 130 microns.
[0065] In terms of the aIOL 50 illustrated in Figures 3-5, the microfibers
mimicking
gecko feet are desirably provided only on the haptic legs 54, and not on the
optics 52, as the
physical surface irregularities thus presented may interfere with the optical
transmission quality.
However, as with other surface roughening treatments, microfibers may be
provided on an outer
portion of the optics 52 without deterioration of vision, such as in regions
like 72 around the
aIOL 50.
-16-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
[0066] It is also possible to combine different surface adherents on a single
lens, such as
a bioadhesive (e.g., pNIPAM) and microfibers (e.g., gecko feet). For example,
microfibers may
be provided on the IOL haptics, while a bioadhesive is coated on at least a
portion of the optic
for lower interference with the optical transmission through the lens. One
contemplated
embodiment is for microfibers on the IOL haptics to be coated with a
bioadhesive which is
reversible so as to be relatively thick at room temperature and liquid at body
temperature. This
configuration prevents the microfibers from sticking to surrounding structures
and instruments
prior to implant, but exposes the microfibers after implant for good adherence
to the capsular
bag.
[0067] Figure 6A and 6B are vertical sectional views through an eye showing
the
implanted exemplary aIOL of Figures 3-5 in two states of accommodation. In
Figure 6A the
zonules pull on the equatorial region of the capsular bag and cause elongation
of the aIOL 50,
such that the two optics 52 are brought closer together, thus decreasing the
optic power. In
Figure 6B the zonules push radially inward on the equatorial region of the
capsular bag and
cause a squeezing of the aIOL 50', such that the two optics 52 are separated
in the axial
direction, producing an increase in the power of the optic. Again, these
reactions to the muscle
movement of the zonules are accentuated by the intimate and adherent contact
between at least
the equatorial region of the exemplary aIOL haptics with the capsular bag.
[0068] Another embodiment of aIOL 80 into which the benefits of the present
application may be incorporated is shown in Figure 7. The aIOL 80 includes a
haptic 82
embedded within a relatively softer optic 84. As was described in U.S. Patent
Publications
2008-0161913 and 2008-0161914, mentioned above, various aIOL embodiments
provide a
haptic partly embedded within an adjustable or accommodative central optic.
The haptic
transmits forces to alter at least one of the shape and the thickness of the
adjustable optic. The
materials of the haptic 82 and optic 84 have similar refractive indices to
reduce any unwanted
glare or reflection from light passing across adjacent surfaces.
[0069] The haptic 82 includes a plurality of spoke-like legs 86 that each
terminate at an
outer end in a convex surface and include bifurcated segments that converge in
two axially-
spaced inner rings 88 surrounding central apertures 90. The resulting
structure is a series of
vaulted legs 86 joined in the middle. Each leg 86 further includes a
cylindrical strut 92
-17-
CA 02772315 2017-01-19
WO 2011/031557 PCT/US2010/047011
extending outward from its outer end that ends in an enlarged disk-shaped head
94. Each strut
92 and head 94 combination resembles a combustion. engine cylinder valve.
[0070] The outermost face of each head 94 has a surface adherent 96 thereon,
indicated
by stippling. Although the entire outer face of each head 94 is shown covered
with the surface
adherent 96, only portions thereof may be covered, such as, for instance, the
peripheral edge.
The aIOL 80 of Figure 7 relies on the same capsular bag fixation technique as
described above,
with adhesion along the capsular bag equator to push and pull on the single
optic 84. In this
case, instead of relying on power change from dual optic movement, the forces
are transferred
via the haptic 82 towards the center of the soft optic body 84, thus inducing
power by changing
the shape or curvature of the optic surface. In one version, each head 94 has
an oval shape and is
formed of a material and thickness that easily conforms to the existing
capsular bag geometry
once placed in the eye.
[0071] Various configuration of surface adherent 96 are contemplated for the
aIOL 80,
including an adhesive such as the thermo-reversible bioadhesive polymer
described above, or
microfibers. In the case of microtibers, the fibers would desirably be formed
normal to the oval-
shaped haptic heads 94.
[0072] It should be understood that the al0I, embodiments of Figures 3 and 7
are only
two of a myriad of lens designs that could benefit from direct attachment to
the capsular bag
using the surface adherents described herein. Again, the principle attachment
area would at least
be along the equator of the capsular bag, though other designs may benefit
from anterior or
posterior capsular bag attachments as well.
[0073] Figures 8A and 8B show a modified technique for implanting an
injectable
polymer alOL in accordance with the principles described herein. Injectable
al0Ls are known
in the art, such as in U.S. Patent Nos. 4542542, 4608050, 6589550, 6598606,
and 7182780
In general, these
patents describe techniques for removing a cataracteous and/or presbyopic
natural lens from the
capsular bag of the eye and replacing it by a lens-forming liquid material
injected directly into
the capsular bag. The liquid material is a partially polymerized material,
which can undergo a
curing process in the eye and thereby form a solid lens implant. The lens
implant acts as a
substitute for the natural lens and aims to substantially restore the features
of the natural lens of
the young eye. The defective natural lens matrix can be removed by a
conventional surgical
-18-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
method involving an ultrasound probe, such as a phacoemulsification method
involving
aspiration. In order to facilitate the removal of the lens matrix and
refilling with lens forming
liquid material, a capsulotomy, i.e. a capsulorhexis, is prepared from a
circular or essentially
circular capsulotomy in the capsular bag wall, typically with a diameter of
from about 0.5 to
about 2.5 mm. An injection syringe needle is inserted through an incision in
the eye and through
the capsulorhexis into the capsular bag so the lens-forming liquid material
can be injected into
the capsular bag.
[0074] A preferred technique is to "coat" the capsular bag with a layer of the
thermo-
reversible polymer just prior to the aIOL implantation/capsular bag filling
with polymer material
injected into it (for Injectable IOLs) during the cataract surgery procedure.
This can be achieved
for example by manually applying the thermo-reversible polymer by the surgeon
using adjunct
instrumentation, by implanting a temporary IOL, device or "bag-filling
balloon" that will
transfer the layer to the capsular bag and then be removed. Once again, a
reversible adhesive in
general may be used, the thermo-reversible polymer being particularly useful.
[0075] For instance, Figure 8A illustrates a cannula 100 inserted into the
previously
evacuated capsular bag space and inflating a balloon 102. The balloon 102 has
been coated with
a preferred bioadhesive, such as pNIPAM as described above. Eventually, the
balloon 102 fills
the space within the capsular bag and the adhesive transfers to the bag. The
balloon 102 is then
deflated and the cannula 100 removed.
[0076] Subsequently, the surgeon advances the needle of a syringe 110 into the
capsular
bag and injects a polymer material 112 that will form the aIOL. The material
112 fills the space
within the capsular bag and comes into intimate contact with the adhesive
previously applied.
This arrangement fully adheres the aIOL to the capsular bag and will
effectively couple the
forces of the natural accommodative mechanism of the eye to the aIOL to
maximize
accommodation amplitude for years with no expected degradation over time. Full
adhesion of
the aIOL/Injectable Polymer to the capsular bag will also prevent lens
epithelial cell (LECs)
migration over those areas.
[0077] Rather than injecting an amorphous mass into the capsular bag, an
injectable IOL
could be encapsulated within a flexible structure like a balloon which is then
inflated to fill the
capsular bag. Such a configuration may be better received by the immune system
of the eye. In
such a case, an adhesive layer may be provided on the outside of balloon
rather than on the
-19-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
inside of the capsular bag. The balloon could be partly inflated prior to
implant or fully inflated
after implant, though obviously the latter reduces the size of the capsulotomy
necessary.
[0078] Another use for the surface adherents described herein is with glaucoma
shunts,
such as shown at 120 in Figures 9 and 10. The shunt 120 includes a large
curved plate 122 that
will conform around the sclera and typically has a small tab 124 extending
from one side. An
elongated flexible drainage tube 126 opens at one end over the plate 122, and
another end is free.
The free end will be inserted into the inner fluid chamber of the eye to
initiate fluid drainage
therefrom.
[0079] The underside of the plate 122 preferably is covered with a surface
adherent,
shown as stippling in Figure 10. Again, the entire surface may be covered, or
at least those
portions in between fenestration holes. Alternatively, only a peripheral edge
or some other
portion of the plate underside may be covered. In any event, the surface
adherent will bond to
the sclera, thus eliminating the need for temporary sutures, and perhaps also
the need for the tab
124 that typically was used for a suture anchor. A preferred surface adherent
for the glaucoma
shunt 120 is microfibers as described above.
[0080] In addition to securing IOLs in the eye, such as in the capsular bag,
certain of the
adhesives described herein are suitable for other ophthalmic uses. For
instance, as described
previously the procedure for injecting polymer type of IOL requires formation
of an essentially
circular capsulotomy in the capsular bag wall, typically with a diameter of
from about 0.5 to
about 2.5 mm. One application of the reversible adhesives described herein is
in plugging this
capsulorhexis. A small amount of pNIPAM, for example, deposited into the
capsulorhexis may
be sufficient to close it. The instrument that deposits the adhesive may
include some form of
shaper that spreads the adhesive in a thin layer across the capsulorhexis, and
may linger for a
sufficient time for a thermo-responsive adhesive to set up. Alternatively, a
light-sensitive
adhesive may be used which sets up on absorbing light from an LED or other
such source.
[0081] Another potential application for the adhesives described herein is in
fixing
capsular bag ruptures after implant of an IOL, PIOL or aIOL. Again, an
adhesive responsive to
an external stimulus such as a temperature change may be deposited at a tear
in the capsular bag
and held in place long enough to gel or otherwise harden.
[0082] Still another application is in repair of at least small tears between
the zonules and
the capsular bag.
-20-
CA 02772315 2012-02-24
WO 2011/031557 PCT/US2010/047011
[0083] Finally, the adhesives may be used to seal a surgical incision through
the
cornea/sclera after cataract surgery.
[0084] While the invention has been described in its preferred embodiments, it
is to be
understood that the words which have been used are words of description and
not of limitation.
Therefore, changes may be made within the appended claims without departing
from the true
scope of the invention.
-21-