Note: Descriptions are shown in the official language in which they were submitted.
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-1-
HYDROPROCESSING FEEDSTOCK CONTAINING LIPID MATERIAL
TO PRODUCE TRANSPORTATION FUEL
FIELD OF THE INVENTION
[0001] This invention relates to the production of a fuel composition from a
feedstock that comprises lipid material and mineral oil. More particularly,
this
invention relates to the production of at least one transportation fuel
composition from
a feedstock that comprises lipid material selected from the group consisting
of
triglyceride, fatty acid alkyl ester and a combination thereof, and mineral
oil, wherein
the transportation fuel is produced by hydroprocessing the feedstock.
BACKGROUND OF THE INVENTION
[0002] An increased demand for fuel has generated interest in finding
feedstock
other than crude oil or mineral oil. Various biological oils have been under
study for
their potential use as feedstock to product fuel, particularly transportation
fuel. For
example, plant oils such as corn, rapeseed, canola and soybean oils and
greases, such as
inedible tallow, yellow, and brown greases, have been under study. A common
feature
of these types of oils is that they are composed of triglycerides and free
fatty acids that
generally have hydrocarbon chains from 8-20 carbons, which is also a common
characteristic of crude oil.
[0003] U. S. Patent No. 7,511,181 discloses a process for producing a
hydrocarbon
component useful as diesel fuel from biorenewable feedstocks such as plant
oils and
greases. The process involves hydrogenating and deoxygenating, i.e.,
decarboxylating
and/or hydrodeoxygenating, the feedstock to provide a hydrocarbon fraction
useful as a
diesel fuel. An optional pretreatment step to remove contaminants such as
alkali metals
from the feedstock can also be carried out. The hydrocarbon fraction can be
isomerized
to improve cold flow properties.
[0004] U.S. Patent No. 7,232,935 discloses a process for producing a
hydrocarbon
component of biological origin. The process comprises at least two steps, the
first of
which is a hydrodeoxygenation step, and the second of which is an
isomerization step
operated using a counter-current flow principle. A biological raw material
containing
fatty acids and/or fatty acid esters serves as the feed stock.
[0005] In spite of the ongoing efforts to produce fuels using biological
materials
as the feedstock, significant improvements still need to be sought as there
are many
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-2-
problems that must be addressed. For example, transportation fuels such as
diesel and
various jet fuels must meet tight specifications. Biological materials alone
cannot meet
such specifications, without hydroprocessing. However, hydroprocessing
biological
materials is problematic to the extent that processing the biological
materials using
current processes quite often result in excess heats of reaction, a reduction
in catalyst
activity, and significant shifts in co-product formation. Accordingly, there
is a need for
additional improvement in producing fuels, particularly transportation fuels,
from
feedstock containing biologically derived material.
SUMMARY OF THE INVENTION
[0006] This invention provides processes for producing fuel, particularly
transportation fuel, from biological material, e.g., lipid material. The
product can
advantageously include one or more high quality transportation fuels, such as
gasoline,
kerosene, jet fuel, and diesel.
[0007] According to one aspect of the invention, there is provided a method of
producing transportation fuel, including providing a feedstock containing
lipid material
and mineral oil. Preferably, the lipid material can be selected from the group
consisting
of triglycerides, fatty acid alkyl esters and combinations thereof.
[0008] The feedstock can be hydroprocessed in a hydroprocessing zone to
produce
the transportation fuel. Preferably, the hydroprocessing zone can be
maintained at not
greater than 1000 vppm CO, based on total vapor content of the hydroprocessing
zone.
[0009] In one embodiment of the invention, a hydrogen-containing stream that
contains not greater than 200 vppm CO, based on total volume of the hydrogen-
containing stream, is added to the hydroprocessing zone during
hydroprocessing.
Preferably, the hydrogen-containing stream that is added to the
hydroprocessing zone
during hydroprocessing contains greater than 60 vol% H2, based on total volume
of the
hydrogen-containing stream.
[0010] In another embodiment, the hydroprocessing zone contains a CoMo or a
NiMo hydroprocessing catalyst.
[0011] In yet another embodiment, the hydroprocessing can produce a
hydroprocessed product comprised of a liquid fraction and a gas fraction, and
the gas
fraction can advantageously be separated from the liquid fraction, with at
least a portion
of the liquid fraction forming the transportation fuel. Preferably, the
separated gas
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-3-
fraction can be treated or contacted with a membrane or an adsorbent to remove
at least
a majority of the CO from the gas stream to form a treated gas stream.
[0012] In one embodiment, the treated gas stream can contain greater than
60 vol% H2 and not greater than 200 vppm CO, based on total volume of the
treated gas
stream. In another embodiment, the separated gas fraction can be treated or
contacted
with an adsorbent contained in a pressure swing adsorption system or a rapid
cycle
pressure swing adsorption system to form the treated gas stream. Preferably,
at least a
portion of the treated gas stream can be added to the hydroprocessing zone
during
hydroprocessing. Additionally or alternately, at least a portion of the
separated gas
fraction can be acid gas treated.
[0013] In general, the feedstock can include at least 0.05 wt % lipid
material,
based on total weight of the feedstock. Additionally or alternately, the lipid
material
portion of the feedstock can be comprised of at least 20 wt % fatty acid alkyl
ester,
based on total weight of the lipid material in the feedstock.
[0014] According to another aspect of the invention, there is provided a
process
for producing a transportation fuel that includes hydroprocessing feedstock
containing
lipid material and mineral oil in a hydroprocessing zone to produce a
hydroprocessed
product comprised of a liquid fraction and a gas fraction. At least a portion
of the gas
fraction can be separated from the hydroprocessed product, and at least a
portion of the
liquid fraction can be recovered as the transportation fuel.
[0015] In one embodiment, at least a majority of CO contained in the separated
gas fraction can be removed from the separated gas fraction to form a treated
gas
stream, which can be provided or recycled to the hydroprocessing zone. In
another
embodiment, at least a portion of the gas fraction separated from the
hydroprocessed
product can be acid gas treated prior to removing the CO.
BRIEF DESCRIPTION OF THE DRAWINGS
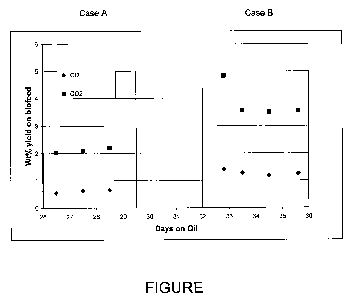
[0016] FIGURE demonstrates two Cases (A and B) according to the invention in
which differing levels of hydrogen are added with a combined mineral and
biocomponent feed to hydrotreat the combined feed.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0017] This invention can increase, or maximize, the amount of lipid or bio-
material that can be converted to transportation fuel. One aspect of this
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-4-
increase/maximization involves hydroprocessing a feedstock that comprises the
lipid
material, while controlling the process to limit undesirable side reactions
that can cause
substantial increases in heats of reaction and that can produce compounds that
negatively impact catalyst efficiency.
Feedstock
[0018] The feedstock that is used in the invention is preferably a combination
feed
containing both lipid material and mineral oil. By "mineral oil" is meant a
fossil/mineral fuel source, such as crude oil, and not the commercial organic
product,
such as sold under the CAS number 8020-83-5, e.g., by Aldrich. In one
embodiment,
the lipid material and mineral oil are mixed together prior to processing. In
another
embodiment, the lipid material and mineral oil are provided as separate
streams into
appropriate processing unit(s) or vessel(s).
[0019] The term "lipid material" as used according to the invention is a
composition comprised of biological materials. Generally, these biological
materials
include vegetable fats/oils, animal fats/oils, fish oils, pyrolysis oils, and
algae
lipids/oils, as well as components of such materials. More specifically, the
lipid
material includes one or more type of lipid compounds. Lipid compounds are
typically
biological compounds that are insoluble in water, but soluble in nonpolar (or
fat)
solvents. Non-limiting examples of such solvents include alcohols, ethers,
chloroform,
alkyl acetates, benzene, and combinations thereof.
[0020] Major classes of lipids include, but are not necessarily limited to,
fatty
acids, glycerol-derived lipids (including fats, oils and phospholipids),
sphingosine-
derived lipids (including ceramides, cerebrosides, gangliosides, and
sphingomyelins),
steroids and their derivatives, terpenes and their derivatives, fat-soluble
vitamins,
certain aromatic compounds, and long-chain alcohols and waxes.
[0021] In living organisms, lipids generally serve as the basis for cell
membranes
and as a form of fuel storage. Lipids can also be found conjugated with
proteins or
carbohydrates, such as in the form of lipoproteins and lipopolysaccharides.
[0022] Examples of vegetable oils that can be used in accordance with this
invention include, but are not limited to rapeseed (canola) oil, soybean oil,
coconut oil,
sunflower oil, palm oil, palm kernel oil, peanut oil, linseed oil, tall oil,
corn oil, castor
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-5-
oil, jatropha oil, jojoba oil, olive oil, flaxseed oil, camelina oil,
safflower oil, babassu
oil, tallow oil and rice bran oil.
[0023] Vegetable oils as referred to herein can also include processed
vegetable
oil material. Non-limiting examples of processed vegetable oil material
include fatty
acids and fatty acid alkyl esters. Alkyl esters typically include CI-C5 alkyl
esters. One
or more of methyl, ethyl, and propyl esters are preferred.
[0024] Examples of animal fats that can be used in accordance with the
invention
include, but are not limited to, beef fat (tallow), hog fat (lard), turkey
fat, fish fat/oil,
and chicken fat. The animal fats can be obtained from any suitable source
including
restaurants and meat production facilities.
[0025] Animal fats as referred to herein also include processed animal fat
material. Non-limiting examples of processed animal fat material include fatty
acids
and fatty acid alkyl esters. Alkyl esters typically include CI-C5 alkyl
esters. One or
more of methyl, ethyl, and propyl esters are preferred.
[0026] Algae oils or lipids are typically contained in algae in the form of
membrane components, storage products, and metabolites. Certain algal strains,
particularly microalgae such as diatoms and cyanobacteria, contain
proportionally high
levels of lipids. Algal sources for the algae oils can contain varying
amounts, e.g., from
2 wt% to 40 wt% of lipids, based on total weight of the biomass itself.
[0027] Algal sources for algae oils include, but are not limited to,
unicellular and
multicellular algae. Examples of such algae include a rhodophyte, chlorophyte,
heterokontophyte, tribophyte, glaucophyte, chlorarachniophyte, euglenoid,
haptophyte,
cryptomonad, dinoflagellum, phytoplankton, and the like, and combinations
thereof. In
one embodiment, algae can be of the classes Chlorophyceae and/or Haptophyta.
Specific species can include, but are not limited to, Neochloris oleoabundans,
Scenedesmus dimorphus, Euglena gracilis, Phaeodactylum tricornutum,
Pleurochrysis
carterae, Prymnesium parvum, Tetraselmis chui, and Chlamydomonas reinhardtii.
[0028] The lipid material portion of the feedstock is preferably comprised of
triglycerides, fatty acid alkyl esters, or preferably combinations thereof In
one
embodiment, the feedstock includes at least 0.05 wt%, for example at least 0.1
wt%, at
least 0.5 wt%, or at least 1 wt% lipid material, based on total weight of the
feedstock
provided for processing into fuel.
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-6-
[0029] In a particular embodiment of the invention, the feedstock includes not
more than 40 wt%, preferably not more than 30 wt%, for example not more than
20 wt%, not more than 10 wt%, or not more than 5 wt% lipid material, based on
total
weight of the feedstock.
[0030] In one embodiment, the lipid material contains one or more
triglycerides.
Types of triglycerides can be determined according to their fatty acid
constituents. The
fatty acid constituents can be readily determined using Gas Chromatography
(GC)
analysis. This analysis involves extracting the fat or oil, saponifying
(hydrolyzing) the
fat or oil, preparing an alkyl (e.g., methyl) ester of the saponified fat or
oil, and
determining the type of (methyl) ester using GC analysis. In one embodiment, a
majority (i.e., greater than 50%) of the triglyceride present in the lipid
material can be
comprised of C8 to C22 fatty acid constituents, based on total triglyceride
present in the
lipid material. For clarity, when a fatty acid or fatty acid ester molecule is
specified as
a "CXX" fatty acid, fatty acid constituent, or fatty acid ester, what is meant
is that "xx" is
the number of carbons on the carbon side of the carboxylate linkage, i.e.,
including the
carboxylate carbon, whereas, in fatty acid esters, the ester carbons are not
included in
the "CXX" and are the carbons on the oxygen side of the carboxylate linkage,
i.e.,
stopping at the carboxylate oxygen. Further, a triglyceride is a molecule
having a
structure identical to the reaction product of glycerol and three fatty acids.
Thus,
although a triglyceride is described herein as being comprised of fatty acids,
it should
be understood that the fatty acid component does not necessarily contain a
carboxylic
acid hydrogen. In the processes of the present invention, a majority of the
triglyceride
present in the lipid material can preferably be comprised of Cio to CIS, for
example C12
to C18, fatty acid constituents, based on total triglyceride present in the
lipid material.
[0031] In a particular embodiment, the lipid material includes triglyceride,
with at
least 20 wt%, preferably at least 30 wt%, for example at least 40 wt%, of the
triglyceride being comprised of lauric acid (C 12:0) constituents. Using the
notation "C
xx:yy" indicates a compound having "xx" carbons on the main chain, i.e., on
the carbon
side of the carboxylate group including the carboxylate carbon, and having
"yy" double
bonds on that main chain. Additionally or alternately, the lipid material
includes
triglyceride, with 40 wt% to 60 wt%, for example from 42 wt% to 58 wt% or from
44
wt% to 55 wt%, of the triglyceride being comprised of lauric acid
constituents. Unless
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-7-
otherwise unambiguously specified, percentages expressed herein are
percentages
based on a number total of elements or constituents.
[0032] Additionally or alternately, the lipid material includes triglyceride,
with at
least 2 wt%, preferably at least 5 wt%, for example at least 10 wt%, of the
triglyceride
being comprised of myristic acid (C 14:0) constituents. Additionally or
alternately, the
lipid material includes triglyceride, with 10 wt% to 28 wt%, for example 12
wt% to 26
wt% or 14 wt% to 24 wt%, of the triglyceride being comprised of myristic acid
constituents.
[0033] Additionally or alternately, the lipid material includes triglyceride,
with at
least 2 wt%, preferably at least 3 wt%, for example at least 5 wt%, of the
triglyceride
being comprised of palmitic acid (C 16:0) constituents. Additionally or
alternately, the
lipid material includes triglyceride, with 2 wt% to 12 wt%, for example 3 wt%
to 10
wt% or 5 wt% to 8 wt%, of the triglyceride being comprised of palmitic acid
constituents.
[0034] Additionally or alternately, the lipid material includes triglyceride,
with at
least 0.5 wt%, preferably at least 1 wt%, for example at least 2 wt%, of the
triglyceride
being comprised of stearic acid (C 18:0) constituents. Additionally or
alternately, the
lipid material includes triglyceride, with 0.5 wt% to 60 wt%, for example 1
wt% to 55
wt% or 2 wt% to 50 wt%, of the triglyceride being comprised of stearic acid
constituents.
[0035] Additionally or alternately, the lipid material includes triglyceride,
with at
least 5 wt%, preferably at least 6 wt%, for example at least 7 wt%, of the
triglyceride
being comprised of oleic acid (C 18: 1) constituents. Additionally or
alternately, the
lipid material includes triglyceride, with 5 wt% to 30 wt%, for example 6 wt%
to 25
wt% or 7 wt% to 20 wt%, of the triglyceride being comprised of oleic acid
constituents.
[0036] Additionally or alternately, the lipid material includes triglyceride,
with at
least 2 wt%, preferably at least 3 wt%, for example at least 4 wt%, of the
triglyceride
being comprised of erucic acid (C 22:1) constituents. Additionally or
alternately, the
lipid material includes triglyceride, with 2 wt% to 70 wt%, for example 3 wt%
to 65
wt% or 4 wt% to 60 wt% of the triglyceride being comprised of erucic acid
constituents.
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-8-
[0037] In one embodiment, the lipid material comprises fatty acid alkyl ester.
Preferably, the fatty acid alkyl ester comprises fatty acid methyl esters
(FAME), fatty
acid ethyl esters (FAEE), and/or fatty acid propyl esters.
[0038] In a particular embodiment of the invention, the lipid material portion
of
the feedstock comprises fatty acid alkyl ester, and a majority of the fatty
acid alkyl ester
present in the lipid material is preferably FAME.
[0039] In another embodiment of the invention, the lipid material portion of
the
feedstock can comprise at least 20 wt%, preferably at least 30 wt%, for
example at least
40 wt% fatty acid alkyl ester, preferably FAME, based on total weight of the
lipid
material. Preferably, a majority of the fatty acid constituents of the fatty
acid alkyl
ester, preferably FAME, can be selected from the group consisting of caprylic
acid
(C 8:0), capric acid (C 10:0), lauric acid (C 12:0), myristic acid (C 14:0),
palmitic acid
(C 16:0), palmitoleic acid (C 16:1), stearic acid (C 18:0), oleic acid (C
18:1), linoleic
acid (C 18:2), linolenic acid (C 18:3), erucic acid (C22:1), and combinations
thereof.
In a particular embodiment, a majority of the fatty acid constituents of the
FAME
present in the lipid material portion can be selected from the group
consisting of lauric
acid (C 12:0), myristic acid (C 14:0), palmitic acid (C 16:0), palmitoleic
acid (C 16:1),
stearic acid (C 18:0), oleic acid (C 18:1), and combinations thereof, based on
total
amount of FAME present in the lipid material portion.
[0040] The feedstock provided according to this invention comprises a mineral
oil. Examples of mineral oils can include, but are not limited to, straight
run
(atmospheric) gas oils, vacuum gas oils, demetallized oils, coker distillates,
cat cracker
distillates, heavy naphthas (optionally but preferably at least partially
denitrogenated
and/or at least partially desulfurized), diesel boiling range distillate
fraction (optionally
but preferably at least partially denitrogenated and/or at least partially
desulfurized), jet
fuel boiling range distillate fraction (optionally but preferably at least
partially
denitrogenated and/or at least partially desulfurized), kerosene boiling range
distillate
fraction (optionally but preferably at least partially denitrogenated and/or
at least
partially desulfurized), and coal liquids. The mineral oil that is included as
a part of the
feedstock can comprise any one of these example streams or any combination
thereof
that would be suitable for hydrocracking with the lipid material portion.
Preferably, the
feedstock does not contain any appreciable asphaltenes. In one embodiment, the
mineral oil can be mixed with the lipid material portion and then hydrotreated
to form a
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-9-
hydrotreated material. In another embodiment, the mineral oil can be
hydrotreated to
reduce the nitrogen and/or sulfur content before being mixed with the lipid
material
portion.
[0041] The mineral oil component can contain nitrogen-containing compounds
(abbreviated as "nitrogen"). For example, the mineral oil can contain at least
5 wppm
nitrogen, based on total weight of the mineral oil component. Preferably, the
mineral
oil will contain not greater than 1.0 wt% nitrogen, based on total weight of
the mineral
oil component. In general, at least a majority of the nitrogen will be in the
form of
organonitrogen compounds.
[0042] The mineral oil will typically contain sulfur-containing compounds
(abbreviated as "sulfur" or "sulfur content"). Such compounds can typically be
present
in the mineral oil at a sulfur content greater than 500 wppm, or often greater
than 0.1
wt%, based on total weight of the mineral oil. Preferably, the sulfur content
of the
mineral oil will not be greater than 6 wt%, preferably not greater than 4 wt%,
based on
total weight of the mineral oil.
[0043] In one embodiment, the feedstock can include not greater than 99.5 wt%,
for example not greater than 99 wt%, not greater than 98 wt%, not greater than
95 wt%,
not greater than 90 wt%, or not greater than 85 wt% mineral oil, based on
total weight
of the feedstock.
[0044] Additionally or alternately, the feedstock can include at least 50 wt%
mineral oil, based on total weight of the feedstock. Preferably, the feedstock
can
include at least 60 wt%, for example at least 70 wt%, at least 75 wt%, at
least 80 wt%,
or at least 85 wt% mineral oil, based on total weight of the feedstock.
[0045] According to one aspect of the invention, the feedstock that is
hydrocracked can have an initial boiling point of at least 100 C, preferably
at least
150 C, for example at least 180 C or at least 200 C. The basic test method of
determining the boiling points or ranges of such feedstock, as well as the
fuel
compositions produced according to this invention, can be by performing batch
distillation according to ASTM D86-09e1, Standard Test Method for Distillation
of
Petroleum Products at Atmospheric Pressure.
[0046] Additionally or alternately, the feedstock can have a final boiling
point of
not greater than 500 C, preferably not greater than 450 C, for example not
greater than
400 C.
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-10-
[0047] The feedstock can preferably be converted to a product by
hydroprocessing, preferably in a continuous operation process. In one
embodiment,
hydroprocessing can be carried out at a liquid hourly space velocity (LHSV)
from 0.1
hr-1 to 20 hr 1, for example from 0.1 hr -1 to 5 hr 1.
Hydroprocessing
[0048] The transportation fuel produced according to this invention can
advantageously include hydroprocessing of the desired feedstock in a
hydroprocessing
zone. Hydroprocessing is a process in which feed material is treated or
contacted with
hydrogen, optionally but preferably in the presence of a hydroprocessing
catalyst. The
hydrogen (and/or catalyst) in the process serves to reduce or remove hetero-
(non-
carbon) atoms from the feed such as nitrogen, sulfur, and oxygen. The hydrogen
(and/or catalyst) in the process can also be used to saturate carbon compounds
and/or to
increase the ratio of isoparaffins to normal paraffins in the product
composition.
Examples of hydroprocessing processes for lipid (bio-) material include, but
are not
limited to, hydrodeoxygenation, hydrotreating, hydrocracking, hydrogenation
(including dearomatization), dewaxing, hydroisomerization, and hydrofinishing.
Additional (or alternate) hydroprocessing processes for mineral feeds can
include
hydrodenitrogenation, hydrodesulfurization, and the like.
CO Control
[0049] The hydroprocessing zone can be maintained during the process at
conditions that promote the efficiency of converting the lipid-containing
feedstock into
transportation fuel. In one embodiment, the hydroprocessing zone can be
maintained at
not greater than 1000 vppm CO (carbon monoxide), for example not greater than
900
vppm CO, not greater than 800 vppm CO, or not greater than 700 vppm CO, based
on
total vapor content of the hydroprocessing zone.
[0050] It may also be preferred that, during hydroprocessing, a hydrogen-
containing stream be added to the hydroprocessing zone. Preferably, the
hydrogen-
containing stream contains greater than 60 vol% H2, more preferably at least
70 vol%
H2, for example at least 80 vol% H2 or at least 90 vol % H2, based on total
volume of
the hydrogen-containing stream.
[0051] The hydrogen-containing stream can also be referred to by other names
such as a treat gas stream, a hydrogen stream, or a hydrogen treat gas stream.
It is not
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-11-
necessary that the stream be pure H2, as long as the stream does not contain
levels of
impurities that would substantially negatively impact hydroprocessing of the
feedstock
to efficiently form transportation fuel.
[0052] In one embodiment of the invention, the hydrogen-containing stream
added
to the hydroprocessing zone contains not greater than 200 vppm CO, preferably
not
greater than 100 vppm CO, for example not greater than 50 vppm CO, not greater
than
vppm CO, or not greater than 5 vppm CO, based on total volume of the hydrogen-
containing stream added to the hydroprocessing zone during hydroprocessing.
[0053] The amount of hydrogen added to the hydroprocessing zone should be
sufficient to reduce one or more of nitrogen, sulfur, and oxygen atoms in the
liquid
product portion or fraction by at least a desired amount. In one embodiment,
the
hydrogen-containing stream added to the hydroprocessing zone can be added at
volume
ratio of hydrogen-containing stream to feedstock (i.e., treat gas rate) from
300 scf/bbl
(53 Nm3/m3) to 5000 scf/bbl (890 Nm3/m3). Preferably, the hydrogen-containing
treat
gas rate can be from 2000 scf/bbl (360 Nm3/m3) to 4000 scf/bbl (710 Nm3/m3).
Hydrodeoxygenation
[0054] According to an aspect of the invention, at least a portion of the
feedstock
can be hydrodeoxygenated during hydroprocessing or as a part of the
hydroprocessing
process. Hydrodeoxygenation refers to oxygen reduction and/or removal from a
compound by means of hydrogen. Water is typically liberated in the reaction,
olefinic
(double) bonds may be hydrogenated (saturated), and various sulfur and
nitrogen
compounds may be removed, if present. Hydrodeoxygenation reactions are
typically
exothermic.
[0055] In one embodiment, hydrodeoxygenation can be carried out in a
hydroprocessing zone at a pressure from 0.1 MPa to 20 MPa, for example from 1
MPa
to 15 MPa. Additionally or alternately, hydrodeoxygenation can be carried out
in a
hydroprocessing zone at a temperature from 100 C to 500 C, for example from
150 C
to 350 C.
[0056] In a preferred embodiment, hydrodeoxygenation can be carried out by
treating the feedstock in the presence of a catalyst containing at least one
Group VIII
metal, at least one Group VIB metal, or a combination thereof. In one
embodiment, the
catalyst can preferably comprise Pd, Pt, Ru, Rh, Ni, NiMo, or CoMo metals, for
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-12-
example in the form of a supported catalyst, with a preferred support
comprising
activated carbon, alumina, silica or a combination thereof
[0057] Preferably, feedstock can be brought into contact or can be treated
with the
catalyst in the presence of hydrogen at operating temperatures and pressures
sufficient
to hydrodeoxygenate at least a majority (i.e., more than 50 wt%) of any
alcohols and to
saturate at least a majority of any olefins present in the feed. The reaction
temperature
used in the hydroprocessing zone can be in the range from 100 C to 350 C,
preferably
from 150 C to 300 C or from 150 C to 275 C. Additionally or alternately, the
reaction
pressure within the hydroprocessing zone can be in the range from 5 bara to
150 bara
(0.5 MPag to 15 MPag), preferably from 10 bara to 100 bara (1.0 MPag to
lOMPag),
for example from 10 bara to 90 bara (1.0 MPag to 9.0 MPag).
Hydrotreating
[0058] According to an aspect of the invention, at least a portion of the
feedstock
can be hydrotreated during hydroprocessing or as a part of the hydroprocessing
process.
Hydrotreating typically results in the reduction and/or removal of hetero-
(non-carbon)
atoms, such as nitrogen and/or sulfur, from the feedstock.
[0059] In one embodiment, hydrotreating can be carried out at a temperature
from
400 F to 900 F (204 C to 482 C), for example from 650 F to 850 F (343 C to 454
C).
Additionally or alternately, hydrotreating can be carried out at a pressure
from 500 psig
to 5000 psig (3.5 MPag to 34.6 MPag), for example from 1000 psig to 3000 psig
(7.0
MPag to 20.8 MPag).
[0060] A preferred hydrotreating catalyst can comprise at least one Group VIB
metal and at least one Group VIII metal, either as a bulk catalyst or
optionally
supported on a porous refractory base material. The Groups referred to herein
are
Groups found in the Periodic Table of the Elements in Hawley's Condensed
Chemical
Dictionary, 13th Edition. Examples of such base materials include, but are not
limited
to, alumina, silica, alumina-silica, zirconia, and combinations thereof.
Preferred
catalyst metals useful in the process of this invention can include, but are
not limited to,
cobalt-molybdenum, nickel, nickel-tungsten, cobalt-tungsten, nickel-
molybdenum,
nickel-cobalt-molybdenum, nickel-cobalt-tungsten, nickel-molybdenum-tungsten,
and
cobalt-molybdenum-tungsten, optionally but preferably supported with activated
carbon, alumina, silica, or a combination thereof.
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
- 13 -
Hydrocracking
[0061] According to an aspect of the invention, at least a portion of the
feedstock
can be hydrocracked during hydroprocessing or as a part of the hydroprocessing
process. Hydrocracking is a particular hydroprocessing process that includes
cracking
or breaking larger carbon number molecules into smaller carbon number
molecules.
[0062] In one embodiment, hydrocracking of the feedstock can be carried out in
the hydroprocessing zone at a temperature in the range from 600 F to 900 F
(316 C to
482 C), for example from 650 F to 850 F (343 C to 454 C). Additionally or
alternately, hydrocracking of the feedstock can be carried out in the
hydroprocessing
zone at a pressure in the range from 200 psia to 4000 psia (13 atm to 270 atm,
or 1.4
MPaa to 27.6 MPaa), for example from 500 psia to 3000 psia (34 atm to 200 atm,
or 3.4
MPaa to 20.7 MPaa).
[0063] Hydroprocessing can typically be carried out in a hydroprocessing zone
that includes a catalyst capable of carrying out a cracking reaction, which
catalyst, in
one embodiment, is comprised of an amorphous or zeolitic base and one or more
Group
VIII and/or Group VIB metal hydrogenation components. In another embodiment,
the
hydrocracking catalyst can comprise a crystalline zeolite cracking base upon
which is
deposited at least one Group VIII or Group VIB metal hydrogenating component.
The
Groups referred to herein are Groups found in the Periodic Table of the
Elements in
Hawley's Condensed Chemical Dictionary, 13th Edition. Examples of Group VIII
metals can include Fe, Co, and Ni, preferably Co and/or Ni; examples of Group
VIB
metals include Mo and/or W.
[0064] The zeolite cracking bases, which can be used as a component of the
hydrocracking catalyst, are also generally referred to as molecular sieves.
These
materials can be composed of silica, alumina, and one or more exchangeable
cations,
such as sodium, magnesium, calcium, and one or more rare earth metals.
[0065] In one embodiment, a large pore crystalline molecular sieve can be
used.
Preferably, the crystalline molecular sieve has a Constraint Index of less
than 2, more
preferably less than 1. The method by which the Constraint Index is determined
is fully
described in U.S. Patent No. 4,016,218, which is incorporated herein by
reference.
[0066] In another embodiment, the hydrocracking catalyst can be comprised of a
molecular sieve having a pore size of at least 7 angstroms, preferably at
least 7.4
angstroms, for example at least 8 angstroms. Additionally or alternately, the
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-14-
hydrocracking catalyst can be comprised of a molecular sieve having a pore
size of not
greater than 15 angstroms, for example not greater than 12 angstroms.
[0067] Examples of zeolite molecular sieves that can be used in the
hydrocracking
catalyst can include, but are not limited to, Zeolite Beta, Zeolite X, Zeolite
Y, faujasite,
Ultrastable Y (USY), Dealuminized Y (Deal Y), Mordenite, ZSM-3, ZSM-4, ZSM-18,
ZSM-20, and the like, and combinations thereof.
[0068] Typically, the hydrocracking catalyst has at least some acidity.
Preferably,
the hydrocracking catalyst has an alpha value greater than 1, for example
greater than 5
or greater than 10. The alpha value is a measure of zeolite acidic
functionality and is
described in greater detail in U.S. Patent No. 4,016,218 and in J Catalysis,
Vol. VI,
pages 278-287 (1966).
[0069] It is not necessary that the hydrocracking catalyst be highly acidic,
although a highly acidic catalyst can be used. In one embodiment, the
hydrocracking
catalyst has an alpha value of not greater than 200, for example not greater
than 100.
[0070] Hydrocracking can be carried out under conditions effective for
producing
the desired fuel product. Preferably, hydrocracking can be carried out at an
average
reaction temperature from 300 F to 900 F (149 C to 482 C), for example from
550 F
to 800 F (289 C to 427 C).
[0071] Hydrocracking can also be preferably carried out at an average reaction
pressure from 400 psia to 3000 psia (27 atm to 200 atm, or 2.8 MPaa to 20.7
MPaa),
preferably from 500 psia to 2000 psia (34 atm to 136 atm, or 3.5 MPaa to 13.8
MPaa).
Hydrogenation
[0072] According to an aspect of the invention, at least a portion of the
feedstock
can be hydrogenated during hydroprocessing or as a part of the hydroprocessing
process. Hydrogenation generally involves saturating unsaturated carbon bonds,
including saturating aromatic rings. Preferably, hydrogenation can be carried
out in the
hydroprocessing zone at a temperature in the range from 300 F to 800 F (149 C
to
427 C), for example from 400 F to 600 F (204 C to 316 C).
[0073] In one embodiment, hydrogenation can be carried out in the
hydroprocessing zone at a pressure from 50 psig to 2000 psig (0.34 MPag to
13.8
MPag), for example from 100 psig to 500 psig (0.69 MPag to 3.4 MPag).
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-15-
[0074] In one embodiment, hydroprocessing can be carried out in a
hydroprocessing zone that includes a catalyst capable of carrying out a
hydrogenation
reaction. Catalysts that are useful in carrying out hydrotreating reactions
are generally
also useful in hydrogenation reactions. Exemplary catalysts can include non-
sulfided
catalysts containing one or more of Pt and Pd, preferably dispersed on a
support, such
as alumina, silica, silica-alumina, carbon, or the like, or a combination
thereof. A
particularly preferred support is silica-alumina.
Dewaxing
[0075] According to an aspect of the invention, at least a portion of the
feedstock
can be dewaxed during hydroprocessing or as a part of the hydroprocessing
process.
Dewaxing in this invention refers to catalytic dewaxing in which a heavier
hydrocarbon
reacts with hydrogen in the presence of a dewaxing catalyst at dewaxing
reaction
conditions. The catalytic dewaxing process is, in essence, a type of
hydrocracking
process. Dewaxing is more particularly based on selective hydrocracking of
predominantly normal paraffins.
[0076] Dewaxing typically incorporates the use of a molecular sieve-based
catalyst in which active hydrocracking sites are accessible to contact with
the paraffin
molecules and preferably not accessible to aromatic type compounds. The
reactions
conditions in dewaxing are preferably effective to improve at least one of
freeze point,
cloud point, pour point, and cold filter plug point of the desired
transportation fuel
produced according to the invention.
[0077] Any catalyst effective in dewaxing hydrocarbon can be used. In one
embodiment, a hydrotreating catalyst can be used as a dewaxing catalyst,
particularly
those that include one or more of Co, Ni, and Fe, in combination with one or
more of
Mo or W. In another embodiment, a hydrodeoxygenation catalyst or a
hydrogenation
catalyst can be used as a dewaxing catalyst, such as Pt and/or Pd noble metals
on an
acidic support. Additionally or alternately, the dewaxing catalyst can include
an acidic
oxide support or carrier. Non-limiting examples of such carrier can include,
but are not
limited to, silica, alumina, silica-alumina, shape selective molecular sieves,
and the
like, and combinations thereof. Preferably, the carrier can be combined with
at least
one catalytic component such as titania, zirconia, vanadia, other Group IIA,
IVB, VB,
or VIB oxides, ferrierite, mordenite, ZSM-5, ZSM-11, ZSM-23, ZSM-35, ZSM-22
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-16-
(also known as theta one or TON), ZSM-48, silicoaluminophosphates (SAPOs)
including SAPO-11, -36, -37 and -40, zeolite Y sieves such as ultrastable Y,
and the
like, as well as combinations thereof If stripping is not available prior to
dewaxing
and/or if the sulfur content of the hydrotreated and separated heavy fraction
is high
enough to result in dewaxing catalyst activity reduction or loss, zeolites
containing
framework transition metals having improved sulfur resistance (see, e.g., U.S.
Patent
Nos. 5,185,136, 5,185,137, and 5,185,13 8) maybe employed.
[0078] The dewaxing can be carried out at reaction conditions which include an
average hydroprocessing zone temperature from 300 F to 900 F (149 C to 482 C),
for
example from 550 F to 800 F (289 C to 427 C). Dewaxing can also be carried out
at
an average reaction pressure from 400 psia to 2000 psia (27 atm to 136 atm, or
2.8
MPaa to 13.8 MPaa).
Hydroisomerization
[0079] According to an aspect of invention, at least a portion of the
feedstock can
be hydroisomerized during hydroprocessing or as a part of the hydroprocessing
process.
The terms "hydroisomerize," "hydroisomerized," and "hydroisomerization," as
used
herein, all refer to a catalytic process in which feedstock is contacted with
catalyst in
the presence of hydrogen and in which a substantial portion of waxy paraffin
compounds in the feedstock is converted to non-waxy (e.g., branched and/or iso-
)
paraffins, while at the same time minimizing conversion of normal paraffins (n-
paraffins) by cracking. Hydroisomerization can effectively increase the volume
of
transportation fuel formed in the overall process. In particular, a
hydroprocessing
process in which at least a portion of the feedstock is hydroisomerized can
reduce the
heavier portion of the feedstock by transforming that component into an
additional
volume of transportation fuel.
[0080] Hydroisomerization can be carried out using a shape selective molecular
sieve catalyst. Large pore crystalline molecular sieves or intermediate pore
molecular
sieves are particularly effective.
[0081] Large pore crystalline molecular sieves useful in the
hydroisomerization
aspect this invention preferably have a Constraint Index of less than 2.
Intermediate
pore crystalline molecular sieves useful in the hydroisomerization step of
this invention
preferably have a Constraint Index of at least 2. The method by which the
Constraint
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
- 17-
Index is determined is fully described in U.S. Patent No. 4,016,218, which is
incorporated herein by reference.
[0082] In one embodiment, the molecular sieves used in the hydroisomerization
aspect of this invention have an alpha value of less than 100. The alpha value
is an
approximate indication of the catalytic cracking activity of the catalyst
compared to a
standard catalyst. The alpha test gives the relative rate constant (rate of
normal hexane
conversion per volume of catalyst per unit time) of the test catalyst relative
to the
standard catalyst which is taken as an alpha of 1 (Rate Constant = 0.0 16 see-
'). The
alpha test is described in U.S. Patent No. 3,354,078 and in J Catalysis, 4,
527 (1965);
6, 278 (1966); and 61, 395 (1980), to which reference is made for a
description of the
test. The experimental conditions of the test used to determine the alpha
values
referred to in this specification include a constant temperature of 538 C and
a variable
flow rate as described in detail in J. Catalysis, 61, 395 (1980).
[0083] Non-limiting examples of large pore molecular sieve catalysts can
include,
but are not limited to, molecular sieves selected from the group consisting of
zeolite
beta, mordenite, zeolite Y, ZSM-20, ZSM-4 (omega), zeolite L, VPI-5, SAPO-37,
MeAPO-37, A1PO-8, cloverite, and combinations thereof. Non-limiting examples
of
intermediate pore molecular sieves can include, but are not limited to, ZSM-
22, ZSM-
23, ZSM-48, SAPO-11, SAPO-5, MeAPO-11, MeAPO-5, and combinations thereof;
and an example of a non-intersecting two-dimensional intermediate pore
molecular
sieve is ZSM-35 (synthetic ferrierite).
[0084] Catalysts useful in the hydroisomerization step preferably contain a
hydrogenation metal, which can be one or more noble metals, one or more non-
noble
metals, or a combination thereof. Suitable noble metals include Group VIII
noble
metals, such as platinum and other members of the platinum group, such as
iridium,
palladium, and rhodium, and combinations of these metals. Suitable non-noble
metals
include those of Groups VB, VIB, and (the non-noble metals of) VIII of the
Periodic
Table. Preferred non-noble metals include, but are not limited to, chromium,
molybdenum, tungsten, cobalt, nickel, and combinations of these metals,
including
cobalt-molybdenum, nickel-tungsten, nickel-molybdenum, cobalt-nickel-
molybdenum,
nickel-molybdenum-tungsten, cobalt-molybdenum-tungsten, and cobalt-nickel-
tungsten. The non-noble metals can be pre-sulfided prior to use by exposure to
a
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-18-
sulfur-containing gas such as hydrogen sulfide at an elevated temperature to
effect
conversion (e.g., of the oxide form) to the corresponding sulfide form of the
metal.
[0085] The metal can be incorporated into the catalyst by any suitable method
or
combination of methods, such as by impregnation or ion exchange into the
zeolite. The
metal can be incorporated in the form of a cationic, anionic, or neutral
complex.
Cationic complexes of the type Pt(NH3)4++ can be used for exchanging metals
onto the
zeolite. Anionic complexes such as the molybdate or metatungstate ions can
also be
useful for impregnating metals into the catalysts.
[0086] In one embodiment, the hydroisomerization catalyst can comprise a
zeolite
and a hydrogenation metal. In one preferred embodiment, the catalyst can
comprise
from 0.01 wt% to 20 wt%, for example from 0.1 wt% to 15 wt%, of hydrogenation
metal, based on total weight of the catalyst.
[0087] The molecular sieve, in one embodiment, can include a binder (or
matrix)
material. Binder materials are preferably metal oxides. Non-limiting examples
of
metal oxide binders can include, but are not limited to, alumina, silica-
alumina, silica-
magnesia, silica-zirconia, silica-thoria, silica-beryllia, silica-titania, as
well as ternary
compositions such as silica-alumina-thoria, silica-alumina-zirconia, silica-
alumina-
magnesia, and silica-magnesia-zirconia, and the like, and combinations
thereof. In one
embodiment, the catalysts are ZSM-23, ZSM-48 or SAPO-11, and zeolite beta,
which
are combined with alumina, and formed into a useable shape by methods such as
extrusion or tabletting.
[0088] Hydroisomerization can be carried out in the presence of hydrogen gas
under hydroprocessing conditions of elevated temperature and pressure.
Particular
reaction conditions for hydroisomerization can depend on the feed used, the
catalyst
used, whether or not the catalyst is sulfided, the desired yield, and the
desired
properties of the desired product, inter alia. Conditions under which the
hydroisomerization process of this invention can be carried out include
temperatures
from 600 F to 750 F (315 C to 399 C), for example from 600 F to 700 F (315 C
to
371 C), and pressures from 1.7 atm to 204 atm (25 psia to 3000 psia, or 170
kPaa to
20.7 MPaa), for example 6.8 atm to 170 atm (100 psia to 2500 psia, or 1.4 MPaa
to
17.3 MPaa). Hydroisomerization pressures in this context refer to the hydrogen
partial
pressure within the hydroisomerization reactor, although the hydrogen partial
pressure
is the same as or substantially the same as the total pressure when the treat
gas is 100%
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
- 19-
or substantially 100% hydrogen. However, the total pressure will be greater
than the
hydrogen partial pressure when the treat gas contains hydrogen and other
usually
relatively inert gases.
Hydrofinishing
[0089] According to an aspect of invention, at least a portion of the
feedstock can
be hydrofinished during hydroprocessing or as a part of the hydroprocessing
process.
Hydrofinishing refers to treating the feedstock with hydrogen to saturate at
least a
portion of the feedstock for improved (oxidative) stability.
[0090] Hydroprocessing catalysts that can accomplish a hydrofinishing aspect
can
include catalysts containing at least one Group VIB metal and at least one
Group VIII
metal. Such a catalyst can include at least one noble metal having a strong
hydrogenation function, such as platinum and/or palladium. A mixture of metals
can
be present as bulk metal catalysts, wherein the amount of metal is 50 wt% or
greater,
for example 60 wt% or greater or 70 wt% or greater, based on the catalyst.
Suitable
metal oxide supports, when present, can include relatively low acidic oxides,
such as
silica, alumina, silica-alumina, titania, and combinations thereof, preferably
at least
including alumina. Non-noble metal content of the catalyst can be up to 20
wt%, but is
preferably not greater than about 1 wt%.
[0091] In one embodiment, the catalyst can be a mesoporous material belonging
to
the M4 IS class or family of catalysts. Examples can include, but are not
limited to,
MCM-41, MCM-48, MCM-50, and the like, and combinations thereof. The term
"mesoporous" refers to catalysts having pore sizes ranging from 15 to 100
angstroms.
The mesoporous materials can include a metal hydrogenation component, which
can be
at least one Group VIII metal. Preferred are noble Group VIII metals,
particularly Pt
and/or Pd.
[0092] In one embodiment, the hydrofinishing aspect can be carried out at a
temperature in a range from 150 C to 350 C, for example 180 C to 250 C. Total
pressure in the hydroprocessing zone can be in a range from 400 psig to 3000
psig (2.8
MPag to 20.7 MPag).
Separation of Liquid and Gas Streams Formed in Hydroprocessing
[0093] Hydroprocessing the feedstock in this invention can advantageously
produce a hydroprocessed product comprised of a liquid fraction and a gas
fraction.
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-20-
The gas fraction can be separated from the liquid fraction, with at least a
portion of the
liquid fraction preferably forming a transportation fuel. Separation can be
accomplished by any suitable means. Such means can include, but are not
limited to,
flash separation, distillation, and the like.
Treatment of Gas Stream to Form Hydrogen Stream
[0094] In one embodiment, the separated gas fraction can be further processed
to
remove CO or to reduce the CO content in order to form a treated gas fraction.
Preferably, the hydrogen-containing stream added to the hydroprocessing zone
can
comprise at least a portion of this treated gas fraction.
[0095] In a particular embodiment, a gas fraction can be separated from the
hydroprocessed product and can then be treated or contacted with a membrane or
an
adsorbent to remove at least a majority (i.e., at least 50%) of the CO from
the gas
stream to form a treated gas stream. Treatment/contact/adsorption can be
carried out to
recover a treated gas stream comprised of not greater than 50 vppm CO,
preferably not
greater then 20 vppm CO, for example not greater than 10 vppm CO or not
greater than
vppm CO, based on total volume of the treated gas stream.
[0096] In one embodiment, at least a portion of the gas fraction of the
hydroprocessed product can be contacted with a membrane to remove at least a
portion
of the CO from the gas stream form a treated gas stream. The membrane can
preferably be a membrane preferential for permeation of hydrogen gas over
carbon
monoxide (and optionally carbon dioxide). Examples of such membranes can
include,
but are not limited to, membranes comprised of silicon rubber, butyl rubber,
polycarbonate, poly(phenylene oxide), nylon 6,6, polystyrene, polysulfones,
polyamides, polyimides, polyethers, polyarylene oxides, polyurethanes,
polyesters, and
combinations and copolymers thereof. In one preferred embodiment, the hydrogen
gas
permeation membrane can be of hollow fiber construction.
[0097] In a particular embodiment, at least a portion of the gas fraction of
the
hydroprocessed product can be contacted with the preferential hydrogen gas
permeation
membrane at a pressure at which the non-permeate pressure can remain
sufficiently
high to allow downstream use without further compression. Preferably, at least
a
portion of the gas fraction of the hydroprocessed product can be passed along
or across
the preferential hydrogen gas permeation membrane at a pressure in the range
from 500
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-21-
psig to 2000 psig (34 atm to 140 atm, or 3.4 MPag to 13.8 MPag), for example
from
800 psig to 1200 psig (54 atm to 82 atm, or 5.5 MPag to 8.3 MPag). A hydrogen-
rich
gas can permeate through the membrane, and the permeate can generally
experience a
pressure drop in the range from about 300 psig to 700 psig (3.1 MPag to 4.7
MPag) as
it passes through the membrane. After membrane separation, the permeate can
generally be at a pressure in the range from 200 psig to 1500 psig (1.4 MPag
to 10.3
MPag).
[0098] In another embodiment of the invention, an adsorbent material can be
used
to remove at least a majority of the CO from the gas stream to form the
treated gas
stream. In this embodiment, the adsorbent material can be an adsorbent having
a
greater affinity for carbon monoxide (and optionally but preferably also a
greater
affinity for carbon dioxide and/or for methane) than for hydrogen. The
adsorbent
material may be a molecular sieve, activated carbon, or a combination thereof.
Additionally or alternately, one or more of calcium and sodium aluminosilicate
zeolites
can be employed. Carbon molecular sieves and silica molecular sieves can
additionally
or alternately be used. Suitable zeolite sieves can include, but are not
limited to, types
5A, 10X, and 13X zeolite molecular sieves, mordenites, and the like. Preferred
zeolite
sieves can include type 5A zeolite sieves and molecular sieves with comparable
pore
size and molecular attraction affinity.
[0099] In another embodiment, the adsorbent material can be an adsorbent
having
a greater affinity for carbon monoxide than for hydrogen, carbon dioxide, and
methane.
Non-limiting examples of such materials can include copper-exchanged
substrates,
such as copper-exchanged Y-type aluminosilicate zeolite molecular sieves,
copper-
exchanged alumina, copper-exchanged activated carbon, and combinations
thereof. In
one particular embodiment, the adsorbent material can be or include copper
aluminosilicate zeolite molecular sieve material, such as commercially
available under
the tradename NKK type adsorbent from Nippon Kokan K.K. of Tokyo, Japan.
[00100] According to an aspect of the invention, adsorption of the CO from the
gas
stream can be carried out in pressure swing adsorption system. In a pressure
swing
adsorption system, a gas stream can be passed through a bed of an adsorbent
material
which selectively adsorbs one or more of the components of the gas stream.
Treated
gas, enriched in the unadsorbed gaseous component(s), can then be withdrawn
from the
bed and either further treated or in some other way recycled or utilized.
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-22-
[00101] A pressure swing adsorption system typically employs at least two
adsorbent beds operated on cycles that are sequenced to be out of phase with
one
another, so that, when at least one bed is in the adsorption or production
step, at least
one other bed can be in a regeneration step. Multiple adsorption beds may be
connected in series or in parallel. Generally, series arrangements of beds are
preferred
for obtaining a high purity gas product, with parallel arrangements of beds
typically
being preferred for purifying a large quantity of a gaseous mixture in a short
time cycle.
[00102] In a preferred embodiment, adsorption of the CO from the gas stream is
carried out in a rapid cycle pressure swing adsorption system. Rapid cycle
pressure
swing adsorption (RCPSA) is primarily characterized relative to standard or
conventional pressure swing adsorption (PSA) by relatively shorter, or more
rapid,
cycles. For example, RCPSA cycle times are typically less than a minute,
preferably
not greater than 30 seconds, for example not greater than 15 seconds, not
greater than
seconds, or not greater than 5 seconds, while PSA cycle times are typically 2-
4
minutes or greater. Hardware (e.g., valves, piping, configuration of vessels)
to perform
these cycles also tends to differ considerably, and commercial vendors of
equipment for
both PSA and RCPSA exist.
[00103] An example of an RCPSA apparatus that can be used according to this
invention is described in U.S. Patent Publication Application No.
2009/0071332. In an
embodiment, the CO can be removed using an RCPSA having a rotary valving
system
to conduct the gas flow through a rotary sorber module that contains a number
of
separate adsorbent bed compartments or "tubes," each of which can be
successively
cycled through the sorption and desorption steps as the rotary module
completes the
cycle of operations. In this embodiment, the rotary sorber module can
preferably be
comprised of multiple tubes held between two seal plates on either end of the
rotary
sorber module, which seal plates can be in contact with a stator comprised of
separate
manifolds, wherein the inlet gas can be conducted to the RCPSA tubes, and
wherein
processed purified product gas and the tail gas exiting the RCPSA tubes can be
conducted away from rotary sorber module. By suitable arrangement of the seal
plates
and manifolds, a number of individual compartments or tubes may pass through
the
characteristic steps of the complete cycle at any one time.
[00104] In RCPSA, flow and pressure variations required for the
sorption/desorption cycle can change in a number of separate increments on the
order
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
- 23 -
of seconds per cycle, which can smooth out the pressure and flow rate
pulsations
encountered by the compression and valving machinery. In this form, the RCPSA
module can include valving elements angularly spaced around the circular path
taken
by the rotating sorption module, so that each compartment can be successively
passed
to a gas flow path in the appropriate direction and pressure to achieve one of
the
incremental pressure/flow direction steps in the complete RCPSA cycle. Example
RCPSA modules and valving arrangements that can be used according to this
invention
are described in U.S. Reissue Patent Nos. RE 40,006 and RE 38,493.
[00105] Without being limited by theory, one significant advantage of the
RCPSA
technology includes a highly efficient use of the adsorbent material. The
quantity of
adsorbent required with RCPSA technology is typically a fraction of that
required for
standard PSA technology to achieve the same separation quantities and
qualities.
RCPSA technology also tends to have a small foot print, which allows
technology to be
deployed closer to the hydroprocessing units in an efficient manner. Because
of the
relatively fast cycle times, RCPSA technology can also exhibit added
capability to
produce treat gas hydrogen with low CO content in a steady manner (i.e.,
steady state
can be achieved relatively quickly, e.g., in less than about an hour).
Acid Gas Treating
[00106] According to an aspect of the invention, the gas stream separated from
the
hydroprocessed product can be acid gas treated. Acid gas treatment refers to
treating a
gas stream to remove or to lower acid gas components. Acid gas components can
include one or more acid gases, such as those selected from the group
consisting of
C02, H2S, SO2, CS2, HCN, COS, and mercaptans.
[00107] Acid gas treatment can preferably comprise contacting the gas stream
containing at least one of the acid gas components with an organic solvent or
an
aqueous solution of an organic solvent in a gas scrub or a liquid-liquid
extraction. The
solvent can be a physical solvent or a chemical solvent. Physical solvents
generally
rely on a physical absorption process. In such a process the acid gases can
dissolve in a
physical solvent. Examples of physical solvents can include, but are not
limited to,
cyclotetramethylene sulfone (sulfolane) and its derivatives, aliphatic acid
amides, NMP
(N-methylpyrrolidone), N-alkylated pyrrolidones and corresponding piperidones,
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-24-
methanol, mixtures of dialkylethers of polyethylene glycols (e.g., SELEXOL
from
Union Carbide of Danbury, Connecticut), and combinations thereof.
[00108] Chemical solvents can tend to work on the basis of chemical reactions
in
which the acid gases are converted into compounds that are simpler to remove.
Examples of such chemical solvents can include aqueous solutions of one or
more
amines, preferably alkanolamines. Preferred amines include those that form
salts when
acid gases pass through a solution containing the amine. These salts can then
preferably be decomposed by heating and/or be stripped off using steam. The
amine
solution can be regenerated by heating or stripping and can be re-used.
Preferred
alkanolamines can include, but are not limited to, monoethanolamine (MEA),
diethanolamine (DEA), triethanolamine (TEA), diisopropylamine (DIPA),
aminoethoxyethanol (AEE), methyldiethanolamine (MDEA), and combinations
thereof.
Reactor Type
[00109] Any reactor or catalyst arrangement suitable for hydroprocessing the
feedstock of this invention can be used. For example, the feedstock can be
provided to
a hydroprocessing zone so as to contact a fixed bed of catalyst, a fluidized
bed, or an
ebullating bed. An example of one type of configuration includes a trickle-bed
operation in which a liquid feedstock trickles through a stationary fixed bed.
Another
example of a reactor configuration includes a countercurrent process, i.e.,
the
hydrocarbon feed flows down over a fixed catalyst bed while H2 flows in the
upward
(opposite) direction.
Transportation Fuel Recovery
[00110] Light or heavy fractions of the hydroprocessed product (typically a
liquid
portion of the hydroprocessed product) can be removed to produce or recover
the
desired transportation fuel. In one embodiment, separation of light or heavy
components or fractions of the hydroprocessed product can be carried out to
positively
affect fuel quality, and in particular to provide at least one jet fuel or
diesel fuel having
high quality characteristics. Separation can be carried out using any
appropriate means.
Fractionation or distillation may be preferred. Atmospheric distillation,
vacuum
distillation, or a combination thereof can be used.
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-25-
Additional Embodiments
[00111] Additionally or alternately, the invention includes one or more of the
following embodiments.
[00112] Embodiment 1. A method for producing transportation fuel, comprising:
providing a feedstock containing lipid material and mineral oil, wherein the
lipid
material is selected from the group consisting of triglycerides, fatty acid
alkyl esters,
and combinations thereof; and hydroprocessing the feedstock in a
hydroprocessing
zone to produce the transportation fuel, wherein the hydroprocessing zone is
maintained at not greater than 1000 vppm CO, based on total vapor content of
the
hydroprocessing zone.
[00113] Embodiment 2. The method of embodiment 1, wherein a hydrogen-
containing stream that contains not greater than 200 vppm CO, based on total
volume
of the hydrogen-containing stream, is added to the hydroprocessing zone during
hydroprocessing, which hydrogen-containing stream optionally contains greater
than 60
vol% H2, based on total volume of the hydrogen-containing stream.
[00114] Embodiment 3. The method of embodiment 1 or embodiment 2, wherein
the hydroprocessing zone contains a CoMo or a NiMo hydroprocessing catalyst.
[00115] Embodiment 4. The method of any of the previous embodiments, wherein
the hydroprocessing produces a hydroprocessed product comprised of a liquid
fraction
and a gas fraction, and the gas fraction is separated from the liquid
fraction, with at
least a portion of the liquid fraction forming the transportation fuel,
wherein the
separated gas fraction is optionally treated or contacted with a membrane or
an
adsorbent to remove at least a majority of the CO from the gas stream to form
a treated
gas stream.
[00116] Embodiment 5. The method of embodiment 4, wherein (i) the separated
gas fraction is treated or contacted with an adsorbent that is contained in a
pressure
swing adsorption system or a rapid cycle pressure swing adsorption system to
form the
treated gas stream, (ii) at least a portion of the treated gas stream is added
to the
hydroprocessing zone during hydroprocessing, or (iii) both (i) and (ii).
[00117] Embodiment 6. The method of embodiment 4 or embodiment 5, wherein
at least a portion of the separated gas fraction is acid gas treated.
[00118] Embodiment 7. The method of any of the previous embodiments, wherein
(i) the feedstock includes at least 0.05 wt% lipid material, based on total
weight of the
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-26-
feedstock, (ii) the lipid material portion of the feedstock is comprised of at
least 20 wt
% fatty acid alkyl ester, based on total weight of the lipid material in the
feedstock, or
(iii) both (i) and (ii).
[00119] Embodiment 8. A process for producing a transportation fuel,
comprising:
providing a feedstock containing lipid material and mineral oil;
hydroprocessing the
feedstock in a hydroprocessing zone to produce a hydroprocessed product
comprised of
a liquid fraction and a gas fraction; separating at least a portion of the gas
fraction from
the hydroprocessed product; removing at least a majority of CO contained in
the
separated gas fraction to form a treated gas stream; providing at least a
portion of the
treated gas stream to the hydroprocessing zone; and recovering at least a
portion of the
liquid fraction as the transportation fuel.
[00120] Embodiment 9. The method of embodiment 8, wherein at least a portion
of the gas fraction separated from the hydroprocessed product is acid gas
treated prior
to removing the CO.
[00121] Embodiment 10. The method of embodiment 8 or embodiment 9, wherein
the hydroprocessing zone is maintained at not greater than 1000 vppm CO, based
on
total vapor content of the hydroprocessing zone.
[00122] Embodiment 11. The method of any one of embodiments 8-10, wherein (i)
the separated gas fraction is treated or contacted with an adsorbent that is
contained in a
pressure swing adsorption system or a rapid cycle pressure swing adsorption
system to
form the treated gas stream, (ii) at least a portion of the treated gas stream
is added to
the hydroprocessing zone during hydroprocessing, or (iii) both (i) and (ii).
[00123] Embodiment 12. The method of any one of embodiments 8-11, wherein
the hydroprocessing zone contains a CoMo or a NiMo hydroprocessing catalyst.
[00124] Embodiment 13. The method of any one of embodiments 8-12, wherein (i)
the feedstock includes at least 0.05 wt% lipid material selected from the
group
consisting of triglycerides, fatty acid alkyl esters, and combinations
thereof, based on
total weight of the feedstock, (ii) the lipid material portion of the
feedstock is
comprised of at least 20 wt % fatty acid alkyl ester, based on total weight of
the lipid
material in the feedstock, or (iii) both (i) and (ii).
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-27-
EXAMPLES
Example 1
[00125] A feedstock was prepared, with about 1.5 wt% of the total feedstock
being
comprised of a fatty acid methyl ester and the remainder being a light gas
oil. The
feedstock was hydroprocessed over a reactor containing about 100 cm3 of a CoMo
hydroprocessing catalyst at a liquid hourly space velocity of about 0.76 hr 1.
The
reaction was carried out at a reactor temperature of about 630 F (about 322 C)
and a
reactor pressure of about 230 psig (about 1.6MPag), with the reactor operating
over a
period of several days. At about 26.6-29.2 days of operation, hydrogen treat
gas was
added to the reactor. The treat gas contained about 80% H2, and was added at a
relative
treat gas rate (TGR) of about 370 scf/bbl (about 300 scf/bbl of H2). The
result of the
run is shown in the Figure as Case A.
[00126] At about 32.8-35.6 days, the treat gas was changed to contain about
60%
H2, and was added at a relative rate of about 550 scf/bbl (about 330 scf/bbl
of H2). The
result of the run is shown in the Figure as Case B.
[00127] Comparing Case A to Case B in the Figure, it can be seen that Case B
produces substantially greater CO and CO2 relative to Case A.
Example 2
[00128] A Run 2A is performed as in Example 1, except that the hydrogen treat
gas
contains 100% H2 to obtain a base case run.
[00129] A Run 2B is performed as in Run 2A, except that the hydrogen treat gas
contains 200 vppm CO, with the remainder of the treat gas being 1-12-
[00130] A Run 2C is performed as in Run 2A, except that the hydrogen treat gas
contains 1000 vppm CO, with the remainder of the treat gas being 1-12-
[00131] An analysis of the gas phase of the hydroprocessed product of Run 2B
should show a reduction in catalyst activity, as compared to Run 2A.
[00132] An analysis of the gas phase of the hydroprocessed product of Run 2C
should show a significant reduction in catalyst activity, relative to Runs 2A
and 2B.
[00133] The above Examples indicate that the H2 and/or CO content of a treat
gas
to a hydroprocessing zone can significantly impact the conversion of feedstock
to fuel
product when the feedstock contains even a small amount of lipid material. In
one
embodiment, a combination of membranes, adsorbents, or both can be utilized to
CA 02777795 2012-04-13
WO 2011/056543 PCT/US2010/054043
-28-
increase H2 purity to a desired, predetermined amount, as well as to
selectively adsorb
CO to thereby reduce the CO content of the treat gas to a desired,
predetermined
amount.
[00134] The principles and modes of operation of this invention have been
described above with reference to various exemplary and preferred embodiments.
As
understood by those of skill in the art, the overall invention, as defined by
the claims,
may encompass other embodiments not specifically enumerated herein.