Note: Descriptions are shown in the official language in which they were submitted.
CA 02778010 2012-05-23
File No. P1754CA00
Title: A NOVEL BLOOD SUBSTITUTE WITH COMPLETE RED BLOOD CELL
FUNCTIONS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority of US provisional patent
application
61/490,304, filed on May 26, 2012.
BACKGROUND
(a) Field
The subject matter disclosed generally relates to artificial blood substitutes
and method of using and making the same. Particularly, the artificial blood
substitutes comprises soluble complexes made by crosslinking stroma free
hemoglobin (SFHb) or hemoglobin (Hb) with antioxidant enzymes [superoxide
dismutase (SOD) and catalase (CAT)] and an enzyme with acid-base and carbon
dioxide transport (carbonic anhydrase, CA) to form soluble complexes of Poly-
[SFHb-SOD-CAT-CA]
(b) Related Prior Art
[0002] Increased demand for blood transfusions in emergency and
surgical settings has led to urgent need for red blood cell (RBC) substitutes.
Different types of oxygen-carrying blood substitutes have been investigated.
One
of such products is the nanobiotechnology based polyhemoglobin (polyHb).
Crosslinking haemoglobin (Hb) with bifunctional agents such as diacid or
glutaraldehyde results in the formation of polyHb. These complex of 4-5
hemoglobin (Hb) molecules have been shown to replace blood loss by
maintaining Hb levels in a safe range during surgery and perform adequate
oxygen transport. Two of these nanobiotechnological products had reached
Phase III clinical trials [Jahr JS et at, J Trauma 64: 1484-97 (2008) and
Moore
EE et al, J Am Coll Surg. 208: 1-13 (2009)], but the US FDA did not approve
these products because of side effects. These side effects include a 3%
incidence of myocardial infarction in ambulance trauma patients infused with
polyhemoglobin, as compared to only 0.6% in patients receiving only saline
1
CA 02778010 2012-05-23
File No. P1754CA00
solution. This is most likely due to ischemia-reperfusion injury especially in
those
patients with prior ischemic heart conditions. Furthermore, in all cases of
hemorrhagic shock there is also accumulation of carbon dioxide and acid base
changes [Moore EE et al, J Am Coll Surg. 208: 1-13 (2009)].
[0003] The advantages of hemoglobin-based blood substitutes
such as
polyHb compared to donor blood are:
[0004] (1) They do not have blood group antigens. Thus, they
can be
transfused immediately on the spot without the need for the patients to be
transported to the hospital for cross-matching and typing as in the case of
donor
blood. This is particularly important in emergency or during natural or man
made
disasters where transportation is difficult.
= [0005] (2) Unlike RBC, polyHb can be sterilized and therefore do
not have
the potential of infective agents in case of epidemics (earlier examples of
AIDS
and the resulting HIV contaminated donor blood can now be avoided in future
epidemics of other types of infective agents),
[0006] (3) Donor blood has to be stored at 4 C and is only
good for about
42 days. PolyHb can be stored at room temperature for more than a year. This
allows for PolyHb in plastic bags to be kept anywhere that has potential need
for
this product for at least 1 year and longer if kept in lower temperature.
[0007] However, polyHb only has only one of the three
important functions
of RBC. The three important functions of RBC are: (1) transport and supply
oxygen, (2) transport and remove carbon dioxide, and (3) remove harmful
oxygen radicals. At present, industrial RBC substitutes in the form of polyHb
only
transport oxygen, and they were not approved by the US FDA because of side
effects. Furthermore, the antioxidant enzyme activities of SOD and CAT of RBC
extracts are not sufficient to prevent ischemia-reperfusion injury in severe
hemorrhagic shock, stroke, myocardial infarction and other conditions. SOD and
CAT have been complexed with polyHb to form polyHb-SOD-CAT (US patent
2
CA 02778010 2012-05-23
File No. P1754CA00
No. 5,606,025 to D'Agnillo and Chang. However, this complex still lacks one of
the important function of RBC in that it does not transport and remove carbon
dioxide from blood and will therefore cause side effects. Therefore, there is
a
need for more complete RBC substitutes with all 3 functions of RBC to treat
blood loss.
[0008] Furthermore, there is a need for more complete RBC substitutes to
prevent ischemia-reperfusion injuries.
SUMMARY
[0009] According to an embodiment, there is provided a soluble complex
comprising hemoglobin cross-linked with an antioxidant enzyme for reduction of
reactive oxygen species and an acid-base and carbon dioxide transport enzyme
for CO2 and 02 transport.
[0010] The antioxidant enzyme may be chosen from super oxide
dismutase (SOD), catalase (CAT), or combinations thereof.
[0011] The antioxidant enzyme may be a combination of super oxide
dismutase (SOD) and catalase (CAT).
[0012] The ratio of hemoglobin to super oxide dismutase (SOD) may be
from about 1 g : 4 000 U to about 1 g : 25 000 U.
[0013] The ratio of hemoglobin to super oxide dismutase (SOD) may be
about 1 g : 18 000 U.
[0014] The ratio of hemoglobin to catalase (CAT) may be from about 1 g:
25,000 U to about 1 g : 310,000 U.
[0015] The ratio of hemoglobin to catalase (CAT) may be about 1 g :
310 000 U.
[0016] The acid-base and carbon dioxide transport enzyme may comprise
carbonic anhydrase (CA).
3
CA 02778010 2012-05-23
File No. P1754CA00
[0017] The ratio of hemoglobin to carbonic anhydrase (CA) may be from
about 1 g : 80 000 U to about 1 g : 250 000 U.
[0018] The ratio of hemoglobin to carbonic anhydrase (CA) may be about
1 g : 130 000 U.
[0019] The hemoglobin may comprise stroma free hemoglobin (SFHb) or
hemoglobin (Hb).
[0020] The soluble complex may have a molecular weight of more than
100 kDa.
[0021] The soluble complex may have a molecular weight from about
more than 100 kDa to more than 450 kDa.
[0022] According to another embodiment, there is provided an artificial
blood substitute comprising a soluble complex according to the present
invention,
in a suitable pharmaceutical fluid.
[0023] According to another embodiment, there is provided a method of
treating a medical condition comprising administering a therapeutically
effective
amount of a soluble complex according to the present invention, or an
artificial
blood substitute according to the present invention to a patient in need
thereof.
[0024] The medical condition may be chosen from a blood loss, a
condition with accumulation of carbon dioxide, an ischemia-reperfusion injury,
and a diver's disease.
[0025] The ischemia-reperfusion injury may comprise a severe
hemorrhagic shock, a stroke, a myocardial infarction, or combinations thereof.
[0026] According to another embodiment, there is provided a method for
removing carbon dioxide from a fluid comprising treating the fluid with an
effective amount of a soluble complex according to the present invention, or
an
artificial blood substitute according to the present invention.
4
CA 02778010 2012-05-23
File No. P1754CA00
[0027] The fluid may be chosen from blood and air. The fluid may be blood
in a dialysis. The fluid may be blood in an artificial lung. The fluid may be
air
undergoing environmental removal of CO2.
[0028] According to another embodiment, there is provided a use of a
soluble complex according to the present invention for the preparation of a
medicament for the treatment of a medical condition.
[0029] According to another embodiment, there is provided a use of a
soluble complex according to the present invention for the treatment of a
medical
condition.
[0030] According to another embodiment, there is provided a use of an
artificial blood substitute according to the present invention for the
preparation of
a medicament for the treatment of a medical condition.
[0031] According to another embodiment, there is provided a use of an
artificial blood substitute according to the present invention for the
treatment of a
medical condition.
[0032] The condition may be chosen from a blood loss, a condition with
accumulation of carbon dioxide, an ischemia-reperfusion injury, a diver's
disease.
[0033] The ischemia-reperfusion injury may comprise a severe
hemorrhagic shock, a stroke, a myocardial infarction, or combinations thereof.
[0034] According to another embodiment, there is provided a use of a
soluble complex according to the present invention or an artificial blood
substitute
according to the present invention for treating a fluid for removing carbon
dioxide
therefrom.
[0035] The fluid may be chosen from blood and air. The fluid may be blood
in an artificial lung. The fluid may be blood in a dialysis.
CA 02778010 2012-05-23
File No. P1754CA00
[0036] According to another embodiment, there is provided a method of
preparing a soluble complex comprising the steps of:
a) cross-linking a mixture comprising
= a stroma-free hemoglobin or hemoglobin;
= at least one antioxidant enzyme;
= an acid-base and carbon dioxide transport enzyme, and
= lysine
b) purifying and concentrating said mixture to obtain said
soluble complex.
[0037] The molar ratio of lysine to the stroma-free hemoglobin may be
from about 7 : 1 to about 12 : 1. Preferably the molar ratio of lysine to the
stroma-
free hemoglobin may be 7:1.
[0038] The step a) may be performed with glutaraldehyde.
[0039] The molar ratio of glutaraldehyde to stroma-free hemoglobin may
be from about 8:1 to about 32:1. Preferably, the molar ratio of glutaraldehyde
to
the stroma-free hemoglobin may be 16:1.
[0040] The step of cross-linking may be performed for 24h at 4 C.
[0041] The step of cross-linking may be stopped with lysine at molar ratio
to the stroma-free hemoglobin of 200:1.
[0042] The step of purifying and concentrating may be performed by
filtering the mixture, dialyzing the mixture, microconcentrating the mixture,
or
combinations thereof.
[0043] The following terms are defined below.
[0044] The term "pharmaceutically acceptable fluid" is intended to mean a
preservative solution, a saline solution, an isotonic (about 0.9%) saline
solution,
or about a 5% albumin solution, suspension, sterile water, phosphate buffered
saline, and the like. Other buffering agents, dispersing agents, and inert non-
toxic
6
CA 02778010 2012-05-23
File No. P1754CA00
substances suitable for delivery to a patient may be included in the
compositions
of the present invention. The compositions may be solutions, suspensions or
any
appropriate formulation suitable for administration, and are typically sterile
and
free of undesirable particulate matter. The compositions may be sterilized by
conventional sterilization techniques.
[0045] Features and advantages of the subject matter hereof will become
more apparent in light of the following detailed description of selected
embodiments, as illustrated in the accompanying figures. As will be realized,
the
subject matter disclosed and claimed is capable of modifications in various
respects, all without departing from the scope of the claims. Accordingly, the
drawings and the description are to be regarded as illustrative in nature, and
not
as restrictive and the full scope of the subject matter is set forth in the
claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0046] Further features and advantages of the present disclosure will
become apparent from the following detailed description, taken in combination
with the appended drawings, in which:
[0047] Fig. 1 illustrates the carbonic anhydrase activity (CA): (1) SFHb ¨
content of red blood cells (SFHb) with CA activity; (2) PolySFHb ¨ content of
red
blood cells crosslinked into polySFHb with very low CA activity;(3) SFHb + SOD
+ CAT + CA ¨ enzymes added to extracts of red blood cell content without
crosslinking, and (4) Poly-[Hb-SOD-CAT-CA] represent the previous components
crosslinked into Poly-[Hb-SOD-CAT-CA] according to an embodiment of the
present invention with resulting good CA activity. SFHb: stroma free
hemoglobin;
PolySFHb: Crosslinked SFHb; SFHb+SOD+CAT+CA: add 15000 U SOD,
300,000 U CAT and 15,300 U CA to each gram of SFHb; Poly-[SFHb-SOD-CAT-
CA] : crosslinked SFHb+SOD+CAT+CA.
[0048] Fig. 2 illustrates the superoxide dismutase (SOD): SFHb ¨ content
of red blood cells (SFHb) with very low SOD activity; PolySFHb ¨ content of
red
7
CA 02778010 2012-05-23
File No. P1754CA00
blood cells crosslinked into polySFHb with very low SOD activity; SFHb + SOD +
CAT + CA ¨ enzymes added to extracts of red blood cell content without
crosslinking, and Poly-[SFHb-SOD-CAT-CA] represent the previous components
crosslinked into Poly-[SFHb-SOD-CAT-CA] according to an embodiment of the
present invention with resulting greatly enhanced SOD activity. SFHb: stroma
free hemoglobin; PolySFHb: Crosslinked SFHb; SFHb+SOD+CAT+CA : Add
1500 U SOD, 300,000 U CAT and 15,300 U CA to each gram of SFHb; Poly-
[SFHb-SOD-CAT-CA]: crosslinked SFHb+SOD+CAT+CA.
[0049] Fig. 3 illustrates the catalase activity (CAT): SFHb ¨ content of
red
blood cells (SFHb) with very low SOD activity; PolySFHb ¨ content of red blood
cells crosslinked into polySFHb with very low SOD activity; SFHb + SOD + CAT
+ CA ¨ enzymes added to extracts of red blood cell content without
crosslinking,
and Poly-[SFHb-SOD-CAT-CA] represent the previous components crosslinked
into Poly-[SFHb-SOD-CAT-CA] according to an embodiment of the present
invention with resulting markedly enhanced CAT activity. SFHb: stroma free
hemoglobin; PolySFHb: Crosslinked SFHb; SFHb+SOD+CAT+CA : Add 1500 U
SOD, 300,000 U CAT and 15,300 U CA to each gram of SFHb; Poly-[SFHb-
SOD-CAT-CA] : crosslinked SFHb+SOD+CAT+CA.
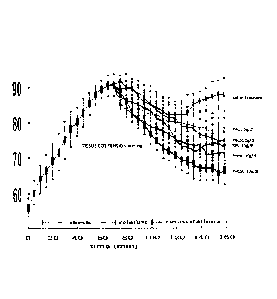
[0050] Fig. 4 illustrates an in vivo trial of the soluble complex
according to
an embodiment of the present invention. RBC: whole blood, PHcsc PolyHb with
low enzyme, PHCSC: PolyHb with high enzyme = PolyHbCAT-SOD-CA.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0051] In embodiments there is disclosed a soluble complex formed by
crosslinking hemoglobin with antioxidant enzyme and acid-base and carbon
dioxide enzyme. Extracts from red blood cell contains hemoglobin contain very
low antioxidant enzymes activity (e.g. SOD and CAT) and an acid-base and
carbon dioxide transport activity (CA). However, when direct cross-linking of
these enzymes is performed, it significantly decreases the SOD, CAT and CA
enzyme activity to levels much below the optimal levels. Furthermore, the
8
CA 02778010 2012-05-23
File No. P1754CA00
antioxidant enzyme (e.g. of SOD and CAT) present in red blood cell extracts
are
insufficient to prevent ischemia-reperfusion injuries that occur in severe
hemorrhagic shock, stroke, myocardial infarction and other conditions.
Furthermore, after crosslinking, the CA level is no longer sufficient for acid-
base
and carbon dioxide transport functions, especially in severe ischemia.
[0052] Therefore, according to an embodiment of the present invention,
the soluble complex (e.g. Poly-[SFHb-SOD-CAT-CA]) of the present invention
comprises the three functions of RBC: It is an oxygen carrier (PolySFHb) with
antioxidant activity (SOD, CAT) and also the ability to facilitate the
transport and
acid-base functions (CA activity). It also comprises enhanced SOD, CAT and CA
activities to enhance the function of this novel red blood cell substitute.
The SOD
and CAT levels in this Poly-[SFHb-SOD-CAT-CA] cross-linked polyhemoglobin
are much higher than those in RBC. Therefore, it is believed that it may also
be
used as a therapeutic agent for the treatment or prevention of ischemia-
reperfusion injuries and to facilitate the removal of accumulated carbon
dioxide in
the case of severe hemorrhagic shock and other conditions.
[0053] According to an embodiment of the present invention, the
hemoglobin component of the soluble complex acts as an oxygen carrier. The
hemoglobin component of the soluble complex may be prepared from any
suitable hemoglobin extracted from red blood cells, from human or animals
(bovine, Porcine etc) or recombinant Hb or other types of organisms and even
synthetic hemoglobin.
[0054] The soluble complex of the present invention also includes a
therapeutically effective amount of an antioxidant enzyme. This antioxidant
enzyme may be any suitable antioxidant enzymes. The antioxidant enzyme may
be either super oxide dismutase (SOD), catalase (CAT), or combinations
thereof.
Preferably, the antioxidant enzyme is a combination of super oxide dismutase
(SOD) and catalase (CAT). These enzymes may be from sources exogenous
from the hemoglobin. The SOD enzyme may be present in a ratio with the
9
CA 02778010 2012-05-23
File No. P1754CA00
hemoglobin component corresponding to from about 1g of hemoglobin to 4 000
U to 25 000 U of SOD. Preferably, the optimal ratio of the-hemoglobin to the
super oxide dismutase (SOD) is about 1 g :18 000 U. The CAT enzyme may be
present in a ratio with the hemoglobin corresponding from about 1g of
hemoglobin to 25 000 U to 310,000 U of CAT. Preferably, the ratio of
hemoglobin
preparation to said catalase (CAT) is about 1 g : 310 000 U.
[0055] The soluble complex of the present invention also includes a
therapeutically effective amount of an acid-base and carbon dioxide transport
enzyme. Preferably, the acid-base and carbon dioxide transport enzyme
comprises carbonic anhydrase (CA), but synthetic enzymes (e.g. synthetic
enzyme bearing the active sites necessary to provide CA activity) with the
same
activity may also be used. The CA enzyme may be from an exogenous source.
The CA enzyme may be present in a ratio with the hemoglobin component
corresponding from about 1g of hemoglobin to 80 000 U to 250 000 U of CA.
Preferably, the ratio of hemoglobin to said carbonic anhydrase (CA) is about 1
g:
130 000 U.
[0056] The components of the soluble complex are chemically bonded
because hemoglobin and the enzymes, when in the free form, are rapidly
removed after infusion and they may also cause harmful effects. Especially
sensitivity, allergic and immunological types of reactions. Thus, according to
one
embodiment of the present invention, the soluble complex may be Poly-[SFHb-
SOD-CAT-CA], where the SFHb, SOD, CAT and CA are cross-linked to each
other.
[0057] The SOD and CAT activity of the present invention is much higher
than that normally present in the RBC and can therefore enhance the
antioxidant
activity. This enhanced ability to prevent the harmful effects of oxygen
radicals is
important in ischemia-reperfusion encountered in severe hemorrhagic shock,
stroke, myocardial infarction. The CA activity is important because in any
CA 02778010 2012-05-23
File No. P1754CA00
hemorrhagic shock or ischemic condition, there is also accumulation of carbon
dioxide that also needs to be removed.
[0058] According to another embodiment, the soluble complex of Poly-
[SFHb-SOD-CAT-CA] may have a molecular weight of at least 100 kDa.
Preferably, the soluble complex of Poly-[SFHb-SOD-CAT-CA] has a molecular
weight from about 100 kDa to more than 450 kDa, and most preferably, the
soluble complex of Poly-[SFHb-SOD-CAT-CA] has a molecular weight of more
than 450 kDa. Table I below, shows the molecular distribution of Poly-[SFHb-
SOD-CAT-CA] and enzyme activities. Poly-[SFHb-SOD-CAT-CA] shows three
molecular components: (1) low (<100 kDa), (3) intermediate (100-450 kDa), and
(3) high molecular weight (>450kDa). The sample contains about 86%
components with molecular weight higher than 100 kDa. The low molecular
weight fraction (<100 kDa) is discarded. Most of the Hb, SOD, CAT and CA
activity (70%, 82%, 90%, and 84% respectively) are in the Poly-[SFHb-SOD-
CAT-CA] fraction with a molecular weight of greater than 450 kDa. The fraction
with molecular weight between 100 kDa and 450 kDa contains Hb, SOD, CAT
and CA activities of 16%, 11%, 9%, and 13% respectively. In the fraction with
molecular weight of less than 100 kDa the activities of SOD, CAT and CA
activities are even lower (14%, 5%, 0.5%, and 0.05%) ¨ this fraction contains
the
uncrosslinked Hb and enzymes and is discarded.
Table I. Molecular Distribution of PolySFHb-SOD-CAT-CA and enzyme activities
Molecular MW Component SOD Activity CAT Activity CA Activity
Weight (%) (%) (%) (%)
>450 kDa 70 82 + 3 90 1 84 2
100-450 kDa 16 11 1 9 1 13 2
<100kDa 14 5 1 0.5 10. 05 3 1
11
CA 02778010 2012-05-23
File No. P1754CA00
[0059] According to another embodiment of the present invention, there
are disclosed methods of treating medical conditions by administering a
therapeutically effective amount of a soluble complex according to the present
invention.
[0060] According to some embodiment the medical condition may be
blood loss, conditions with accumulation of carbon dioxide, ischemia-
reperfusion
injuries, and diver's diseases.
[0061] The ischemia-reperfusion injury may comprise a severe
hemorrhagic shock, a stroke, a myocardial infarction, or combinations thereof.
[0062] According to another embodiment, there are disclosed methods of
removing carbon dioxide from a fluid with an effective amount of a soluble
complex according to the present invention, or with an artificial blood
substitute
according to the present invention.
[0063] The fluid may be for example blood and air, but could be any fluid
from which carbon dioxide may be advantageously be removed. For example,
the fluid may be blood in a dialysis or in artificial lung. According to
another
embodiment, the fluid may be air undergoing environmental removal of 002.
[0064] According to another embodiment of the present invention, there is
disclosed a method of preparing a soluble complex according to the present
invention. The method comprises the steps of cross-linking a mixture which
comprises stroma-free hemoglobin, antioxidant enzymes, an acid-base and
carbon dioxide transport enzyme, and lysine.
[0065] The molar ratio lysine to the stroma-free hemoglobin may be from
about 7: 1 to about 12 : 1, or from about 8:1 to about 12:1, or from about 9:1
to
about 12:1, or from about 10:1 to about 12:1 or from about 11:1 to about 12:1,
or
from about 7:1 to about 8:1, or from about 7:1 to about 9:1, or from about 7:1
to
about 10:1, or from about 7:1 to about 11:1, or from about 8:1 to about 9:1,
or
from about 8:1 to about 10:1, or from about 8:1 to about 11:1, or from about
8:1
12
CA 02778010 2012-05-23
File No. P1754CA00
to about 12:1, or from about 9:1 to about 10:1, or from about 9:1 to about
11:1, or
from about 9:1 to about 12:1, or from about 10:1 to about 11:1, or from about
11:
to about 12:1. Preferably, the molar ratio of lysine to the stroma-free
hemoglobin
is 7:1.
[0066] According to an embodiment, the cross-linking is performed with
glutaraldehyde. The molar ratio of glutaraldehyde to the stroma-free
hemoglobin
may be from about 8:1 to about 32:1, or from about 8:1 to about 12:1, or from
about 8:1 to about 16:1, or from about 8:1 to about 20:1, or from about 8:1 to
about 24:1, or from about 8:1 to about 28:1, or from about 12:1 to about 16:1,
or
from about 12:1 to about 20:1, or from about 12:1 to about 24:1, or from about
12:1 to about 28:1, or from about 12:1 to about 32:1, or from about 16:1 to
about
20:1, or from about 16:1 to about 24:1, or from about 16:1 to about 28:1, or
from
about 16:1 to about 32:1, or from about 20:1 to about 24:1, or from about 20:1
to
about 28:1, or from about 20:1 to about 32:1, or from about 24:1 to about
28:1, or
from about 24:1 to about 32:1, ,or from about 28: to about 32:1. The molar
ratio
of glutaraldehyde to the stroma-free hemoglobin will be varied depending on
the
length of the incubation for cross linking. Preferably, the molar ratio will
be 16:1,
and the incubation period may be for example about 24h at 4 C. The reaction
may be stoped by addition of an excess lysine (e.g. 200:1) relative to the
stroma-
free hemoglobin. The mixture may be subsequently purified, dialyzed and
concentrated to obtain the soluble complex.
[0067] The present invention will be more readily understood by referring
to the following examples which are given to illustrate the invention rather
than to
limit its scope.
13
File No. P1754CA00
EXAMPLE 1
Preparation of soluble complexes of Poly-[SFHb-SOD-CAT-CA]
MATERIALS
[0068] The Hb used is Stroma Free Hb extracted from red blood cells.
SOD, CAT and CA (EC 4.2.1.1, 2720 units/mg solid manufacturer's stated
activity) were purchased from Sigma-Aldrich (Ontario, Canada). All other
chemicals or reagents of analytical grade were purchased from Sigma-Aldrich.
METHOD
[0069] SOD (1050 units/mL), catalase (21,000 units/mL) and carbonic
anhydrase (1070 units/mL) are added to the 20 mL solution containing stoma-
free hemoglobin (7 g/dI) in 50mM sodium phosphate buffer (pH 7.4). Before the
initiation of the crosslinking reaction, 1.3 M lysine is added at a molar
ratio of 7:1
lysine/Hb. Nitrogen gas is used to flush the reaction vessel to prevent
formation
of methemoglobin. Crosslinking is started with the addition of 5%
glutaraldehyde
at a molar ratio of 16:1 glutaraldehyde/Hb. Glutaraldehyde is added in four
equal
aliquots over a period of 15 minutes, and the reaction is allowed to crosslink
for
24 hours at 4 C with constant stirring under aerobic conditions. Crosslinking
is
stopped by adding 2.0 M lysine at a molar ratio of 200:1 lysine/Hb. The
preparation is then filtered using a sterile 0.45 pM filter, and subsequently
dialyzed overnight using molecular porous dialysis membrane (MWCO: 12 000-
14 000) against Ringer's lactated solution. 100 kDa microconcentrators
(Amicon,
Beverly, MA) are used to concentrate 500 pL aliquots of the crosslinked
solution
by centrifuging at 2500 g for 55 minutes at 23 C.
EXAMPLE 2
Molecular Weight Distribution
[0070] For the analysis of molecular weight distribution of polyHb-
SOD-
CAT-CA a Sephacry1-300 HRTColumn is used (Vtotai=560 ml) at a flow rate of 36
14
CA 2778010 2018-07-23
File No. P1754CA00
ml/hour. This column is equilibrated with 0.1M Tris-HCI and 0.15M NaCl (pH
7.4)
elution buffer. The molecular weight distribution is recorded by a 280nm UV
detector
at a recording velocity of 1mm/min. Fractions collected are: (1) molecular
weights
higher than 450kDa, (2) 100-450kDa and (3) less than 100kDa. For animal
studies, the less than 100 kDa fraction are discarded. Dialysis membrane
(MWCO:
12 000-14 000) and Spectra/gel absorbent are used to concentrate the sample
and
the hemoglobin concentration are determined by Drabkin's method. The aliquoted
samples are stored at -80 C.
EXAMPLE 3
Quantitative Determination of Hb Concentration
[0071) The Hb concentration in the polySFHb and polySFHb-CA
preparations is colorimetrically determined by reacting the samples with
Drabkin's
reagent (Sigma-Aldrich), then measuring the concentration of the resulting
cyanmethemoglobin solution by spectrophotometry at 540 nm.
EXAMPLE 4
Determination of CA Activity
(0072) The hydration activity of carbon dioxide by CA is determined
by an
electrometric delta pH assay based on the methods of Henry (14), and of Wilbur
and Anderson (15). One Wilbur- Anderson (W-A) unit of CA activity is defined
as
the amount of enzyme that causes the pH of a 0.02 M Tris buffer to drop from
pH
8.3 to 6.3 per minute. The reagent solution consisted of test samples
containing
CA, and 0.02 M Tris. HCl buffer (pH 8.0) kept between 0-4 C before use.
Dissolved
CO2 is prepared by bubbling CO2 through distilled water to obtain the
substrate for
the assay. The reaction is initiated by the addition of substrate, and the
time (T)
needed for the pH of the reaction mixture to drop from pH 8.3 to 6.3 is
recorded. A
Fisher AccumetTM Basic pH meter (Fisher Scientific, Pittsburgh, PA) with MI-
407 (P)
Needle pH electrode (Microelectrodes Inc., Bedford, NH) is used to measure the
change in pH caused by the hydration reaction of CO2 catalyzed
CA 2778010 2020-02-25
CA 02778010 2012-05-23
File No. P1754CA00
by CA. The control (To) for the assay consists of the same mixture without the
test sample. The measurements in seconds are converted into W-A units
according to the following formula:
1 W-A unit = [2 x (To ¨ T)]/T.
[0073] The units are then plotted versus the Hb/CA concentration
(mg/mL).
EXAMPLE 5
Determination of CAT Activity
[0074] For catalase measurement, a UV 240nm spectrophotometer is
used to measure the rate of disappearance of H202. The reaction mixture
contains 2mL of 50mM phosphate buffer, pH 7.0, and 1mL of 30mM H202. The
decrease of the H202 level is monitored at 240nm for 15s. The same
concentrations of blood samples or phosphate buffer mixtures without H202 are
applied as blank. The catalase activity is expressed in units per grams of
haemoglobin.
EXAMPLE 6
Determination of SOD Activity
[0075] The measurement for SOD activity is based on the reduction of
cytochrome C by superoxide. The reagent solution consists of xantine (50 pM),
cytochrome C (10pM) and CAT (500 units/ mL) in 50 mL potassium phosphate
buffer, 0.1 mM EDTA, pH 7.8. The reaction system consists of test samples and
reagent solution. Xanthine oxidase is added to start the reaction. 0.154 M
NaCI is
used as blank. The cytochrome C reduction is recorded at 550nm by a
spectrophotometer.
EXAMPLE 7
Enzyme Activity of SFHb, polySFHb and soluble complex
16
CA 02778010 2012-05-23
File No. P1754CA00
of Poly-[SFHb-SOD-CAT-CA]
[0076] Now referring to Figs. 1-3. PolySFHb-SOD-CAT-CA was formed by
nanobiotechnology using glutaraldehyde as crosslink reagent. The enzymes
activities of (1) SFHb, (2) PolySFHb, (3) PolySFHb+SOD+CAT+CA (SOD, CAT
and CA added in solution to PolySFHb) and (4) Poly-[SFHb-SOD-CAT-CA]
(PolySFHb crosslinked with SOD, CAT and CA) are assessed. To eliminate
errors, three batches of polySFHb and Poly-[SFHb-SOD-CAT-CA] are prepared
and tested separately. Crosslinking reactions significantly decreased the SOD,
CAT and CA enzyme activities. Thus, PolySFHb alone did not have the same
enzyme activities as RBC. The amount of SOD and CAT used in the crosslinking
are much higher than those found in the RBC. This is required in order to have
a
therapeutic level of SOD and CAT for ischemia-reperfusion injuries. CA (1070
units/mL) also has to be added in the crosslink of PolySFHb and CA to emulate
the CO2 hydration activity of SFHb in RBC. Enrichment of enzymes highly
improves the activities of SOD, CAT and CA in Poly-[SFHb-SOD-CAT-CA]. An
optimal Poly-[SFHb-SOD-CAT-CA] with the following addition of enzymes
requires a Hb: SOD: CAT: CA ratio of 1g: 18,000:310,000:130,000U.
EXAMPLE 8
Animal Study
[0077] Increase in tissue CO2 in hemorrhagic shock is correlated with poor
recovery in patients. Thus, the effectiveness of the novel approach in
lowering
the elevated CO2 in hemorrhagic shock in analyzed in rats.
[0078] Study design
[0079] Rats of average body weight of 300 grams. 6 groups of rats with 3
in each group. Phenobarbital Anesthesia. Cannulate femoral artery and vein on
left side. The mean blood pressure (mmHg) and tissue CO2 tension (mmHg) are
continuously recorded. The animals are bled 50% of their total blood volume. A
17
CA 02778010 2012-05-23
File No. P1754CA00
mean blood pressure of 30 mmHg for is maintained for 1 hour. Then each rat
receives one of the following intravenous infusions.
[0080] Group 1: Saline at 3 times the volume of lost blood;
[0081] Group 2: Red blood cells (rbc 15gm/dI): reinfuse the shed loss of
blood (half the total blood volume);
[0082] Group 3: PHcsc: (5 gm/di of polyhemoglobin-csc prepared by
crosslinking stroma-free hemoglobin;
[0083] Group 4: PHCSC: (5 gm/di prepared by crosslinking stroma-free
hemoglobin PLUS the addition of more enzymes at the concentration stated
above: C = catalase; S= superoxide dismutase and C= carbonic anhydrase.
Specifically, SOD (1050 units/mL), catalase (21,000 units/mL), and carbonic
anhydrase (1070 units/mL) are added to stroma-free hemoglobin (7 g/dI), then
polymerized into PolySFHb-SOD-CAT-CA resulting in HB:SOD ratio of 1g: 8,000
U; Hb:CAT ratio of 1g:310,000 U after crosslinking and lib:CA ratio of
1g:130,000 U after crosslinking.
[0084] Group 5: PHcsc: (10 gm/dl prepared as in Group 3 by crosslinking
stroma-free hemoglobin. Then concentrating the preparation to 10 gm/di
[0085] Group 6: PHCSC: (10 gm/di prepared as in group 4 but ,then
concentrating the preparation to 10gm/d1,
[0086] Results
[0087] Group 1: Saline at 3 times the volume of lost blood is the least
effective since CO2 even increases after infusion of saline.
[0088] Group 4: 5 gm/di of the Polyhemoglobin-Catalase-
Superoxidedismutase-carbonic anhydrase (PHCSC) is slightly more effective
than triple the amount of red blood cells (15gm/dI).
18
CA 02778010 2012-05-23
File No. P1754CA00
[0089] Group 6: 10 gm/dl of
Polyhemoglobin-Catalase-
Superoxidedismutase-carbonic anhydrase (PHCSC) as shown in the lowest line
is much more effective than the larger amount, 15gm/d1, of red blood cells.
[0090] Conclusion
[0091] This novel approach results in a preparation that is much more
effective than red blood cells in lowering the elevated tissue CO2 level.
Elevated
CO2 level in hemorrhagic shock if not lowered can lead to poor recovery from
shock.
[0092] While preferred embodiments have been described above and
illustrated in the accompanying drawings, it will be evident to those skilled
in the
art that modifications may be made without departing from this disclosure.
Such
modifications are considered as possible variants comprised in the scope of
the
disclosure.
19