Note: Descriptions are shown in the official language in which they were submitted.
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
PRECIPITATED MAGNESIUM CARBONATE
The present invention relates to a process for preparing hydromagnesite in an
aqueous environment. The invention further relates to such hydromagnesite
having a
platy-like morphology in combination with a specific average particle size and
to
their use as minerals, fillers and pigments in the paper, paint, rubber and
plastics
industries and to the use as flame-retardant.
Hydromagnesite or basic magnesium carbonate, which is the standard industrial
name for hydromagnesite, is a naturally occurring mineral which is found in
magnesium rich minerals such as serpentine and altered magnesium rich igneous
rocks, but also as an alteration product of brucite in periclase marbles.
Hydromagnesite is described as having the following formula:
Mg5(CO3)4(OH)2 = 4H20
It should be appreciated that hydromagnesite is a very specific mineral form
of
magnesium carbonate and occurs naturally as small needle-like crystals or
crusts of
acicular or bladed crystals. In addition thereto, it should be noted that
hydromagnesite is a distinct and unique form of magnesium carbonate and is
chemically, physically and structurally different from other forms of
magnesium
carbonate. Hydromagnesite can readily be distinguished from other magnesium
carbonates by x-ray diffraction analysis, thermogravimetric analysis or
elemental
analysis. Unless specifically described as hydromagnesite, all other forms of
magnesium carbonates (e.g. artinite (Mg2(CO3)(OH)2 = 3H20), dypingite
(Mg5(CO3)4(OH)2 = 5H20), giorgiosite (Mg5(CO3)4(OH)2 = 5H20), pokrovskite
(Mg2(CO3)(OH)2 = 0.5H20), magnesite (MgCO3), barringtonite (MgCO3 = 2H20),
lansfordite (MgCO3 = 5H20) and nesquehonite (MgCO3 = 3H20)) are not
hydromagnesite within the meaning of the present invention and do not
correspond
chemically to the formula described above.
1
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
Besides the natural hydromagnesite, synthetic hydromagnesites (or precipitated
magnesium carbonates) can be prepared. For instance, US 1,361,324, US 935,418,
GB 548,197 and GB 544,907 generally describe the formation of aqueous
solutions
of magnesium bicarbonate (typically described as "Mg(HCO3)2"), which is then
transformed by the action of a base, e.g., magnesium hydroxide, to form
hydromagnesite. Other processes described in the art suggest to prepare
compositions
containing both, hydromagnesite and magnesium hydroxide, wherein magnesium
hydroxide is mixed with water to form a suspension which is further contacted
with
carbon dioxide and an aqueous basic solution to form the corresponding
mixture; cf.
for example US 5,979,461.
Additionally, general processes for preparing magnesium carbonate are
described in
the art. For example, EP 0 526 121 describes a calcium-magnesium carbonate
composite consisting of calcium carbonate and magnesium carbonate hydroxide
and
a method for the preparation thereof. Furthermore, GB 594,262 relates to a
method
and apparatus for treating magnesia-containing materials, such as magnesium
and
calcium carbonate materials for obtaining respective carbonates in discrete
and
separate forms, by controlled carbonation such that the magnesium and calcium
carbonates may be separated by mechanical means and with attainment of special
utilities in separated products. US 2007194276 describes a method of
reductively
bleaching a mineral slurry comprising adding in the mineral slurry an
effective
amount of a formamidine sulfinic acid (FAS) and an effective amount of a
borohydride to reductively bleach the mineral slurry.
In practice, hydromagnesite is used in huge quantities in the paper, rubber
and
plastics industries for various purposes such as coatings, fillers, extenders
and
pigments for papermaking as well as flame-retardants in electrical wires and
cables
but also to impart resistance to chemicals in fibers. For example, EP 0 543
262, EP 0
393 813, JP 21 50436, JP 22 55 843, JP 51 70 984, JP 50 98 085 and
KR 2003/0040953 describe flame-retardant compositions comprising
2
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
hydromagnesite in admixture with other magnesium compounds such as huntite,
dolomite and/or magnesium hydroxide. In this context, hydromagnesite in
combination with various magnesium compounds is usually added into a resin
composition for providing flame resistance and high mechanical strength so
that such
compositions can be used as a covering or insulation material for electric
wires or
cables, flame arresting materials, wall materials for various areas such as
the
automotive sector, for the production of housings for electrical appliances or
in the
building sector.
Another application for hydromagnesite is described in WO 2009/008600 which
relates to a spandex fiber containing hydromagnesite and having resistance to
chlorine without affecting intrinsic properties of the polyurethane polymer.
Furthermore, WO 97/09473 describes spandex containing particles of a mineral
mixture of huntite and hydromagnesite, wherein the spandex is described as
having
decreased tackiness and increased resistance to chlorine-induced degradation.
Additionally, hydromagnesite in combination with other magnesium compounds is
used in the paper industries in order to impart printability, a high
brightness at high
opacity but also suitable smoothness and gloss to paper products such as
magazines.
In this respect, JP 2003/293291 describes coated paper produced by disposing
an
adhesive and a coating layer consisting mainly of at least one of huntite and
hydromagnesite on base paper, wherein the resulting coated paper has high
brightness, a high surface-masking effect and excellent printing suitability.
Hydromagnesite and other magnesium compounds, e.g. magnesium carbonate and
magnesium hydroxide, can also be incorporated as a filler in wrapping papers
of
smoking articles such as cigarettes or cigars in order to control many
physical
properties or characteristics such as the tar delivery per puff, burn rate,
puff count,
etc. One particularly important aspect of a smoking article that can be
controlled by
such wrapping paper is the sidestream smoke, which is the smoke given off by
the
3
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
burning end of a smoking article between puffs. However, as such smoke may be
objectionable to other people near the smoker several attempts have been made
to
reduce such sidestream smoke through the use of various magnesium compounds.
For example, US 5,092,306 relates to a smoking article wrapper, and in
particular,
cigarette paper which uses magnesite as a filler composition. Others have used
physical mixtures of magnesium hydroxide and hydromagnesite, e.g. US 5,927,288
and US 5,979,461, while others have made attempts for developing compositions
wherein the amount of magnesium hydroxide is reduced by replacing this
hydroxide
with other magnesium compounds. For example, US 5,253,660 discloses a
cigarette
or cigar wrapper wherein the paper filler consists of two homogeneously
intermixed
minerals, namely huntite and hydromagnesite, alone, or admixed with calcium
carbonate or magnesium hydroxide or calcium carbonate and magnesium hydroxide
and carbon.
However, with respect to the aforementioned possible applications of
hydromagnesite, it is to be noted that there are significant constrains
regarding the
suitability of the corresponding filler particles or their application field.
Hydromagnesite obtained from natural sources or prepared by processes
described in
the prior art for use as filler or coatings in paper applications, in smoking
articles
and/or as flame retardant usually has an average particle size of about 5 gm
or more.
In this context, it is to be noted that the thickness of, for example,
wrapping papers
for smoking articles is generally in the range of about 30 gm, so that the
incorporation of hydromagnesite into smoking articles often do not impart the
desired properties such as smoothness onto the surface of such articles and,
thus, the
physical and optical properties of the obtained products are not always
satisfactory.
Additionally, as well known from kaolin and its use as surface coating and
filler in
the field of paper applications, the morphology of the particles plays also a
decisive
role for imparting the desired optical and physical properties such as good
printability, high brightness at high opacity, moderate porosity and a
favorable
smoothness and gloss into paper products such as magazines. For many
applications,
4
CA 02778928 2015-07-27
a platy-like morphology of the particles is highly favorable for obtaining
said properties. The
provision of platy-like particles having a small particle size would be
especially advantageous.
In this context, it is further to be noted that a mechanical comminution of
particles is usually
not a suitable and effective method for obtaining smaller particles of
hydromagnesite.
Thus, there is still a need in the art for providing alternative processes for
preparing
hydromagnesite, wherein such process should be simple and inexpensive and
should provide
the possibility of controlling particular parameters such as the particle size
in combination with
the morphology and the density of the obtained particles.
Accordingly, it is an objective of the present invention to provide an
alternative process for
preparing hydromagnesite preferably hydromagnesite having a specific platy-
like morphology
in combination with decreased particle sizes. Another objective of the present
invention may
be seen in the provision of a process for preparing hydromagnesite having a
high absolute
density. A further objective of the present invention may be seen in the
provision of a process
for preparing hydromagnesite having improved optical properties and especially
a high degree
of whiteness R457. Even a further objective of the present invention may be
seen in the
provision of a process which can be carried out in a simple way. A still
further objective of the
present invention may be seen in the provision of a process which can be
carried out under
mild conditions and the obtained hydromagnesite can be used directly without
further
complex and costly processing steps. Even another objective of the invention
may be seen in
the provision of a process, in which hydromagnesite material can be prepared
in high yield.
Further objects can be gathered from the following description of the
invention,
In order to fulfill the foregoing need(s), the present invention provides a
process for preparing
hydromagnesite in an aqueous environment, the process comprising the steps of:
a) providing and calcining at least one magnesium oxide source to obtain a
calcined
magnesium oxide source comprising magnesium oxide; wherein the at least one
magnesium
oxide source is selected from the group consisting of dolomite, huntite and
mixtures thereof;
5
CA 02778928 2015-07-27
b) providing gaseous CO2 and/or carbonate-comprising anions;
c) slaking of said calcined magnesium oxide source of step a) to convert the
magnesium oxide at
least partially into magnesium hydroxide, wherein a first aqueous suspension
having a solids
content of between 1 wt% and 20 wt% based on the total weight of the
suspension is obtained;
d) contacting the magnesium hydroxide obtained in step c) with said gaseous
CO2 and/or
carbonate-comprising anions of step b) to convert the magnesium hydroxide at
least partially
into precipitated nesquehonite, wherein the precipitated nesquehonite is
obtained in the form
of a second aqueous suspension further comprising precipitated calcium
carbonate; and
e) treating the precipitated nesquehonite and precipitated calcium carbonate
of step d) in a
heat-ageing step to obtain the hydromagnesite, wherein the hydromagnesite has
particles
having an average particle size elso of less than 10 mm, and wherein the heat-
ageing step is
carried out at a temperature in the range between 90 C and 150 C and for a
period of time of
to 60 minutes.
The inventors surprisingly found that the foregoing process allows for the
efficient and
controlled production of hydromagnesite. According to the process of the
present invention
hydromagnesite having a platy-like morphology as well as decreased particle
sizes can be
provided or prepared directly. More precisely, the inventors found that the
morphology as
well as the physical values of hydromagnesite being obtained by said process
can be
improved by specifically controlling or adjusting the process conditions
during the
20 preparation of said hydromagnesite. The process involves slaking a
magnesium oxide
source, like "pure" magnesium oxide, a magnesium oxide containing mineral or
another
source containing magnesium compounds such as dolomite, which can be used for
preparing the magnesium oxide. The resulting magnesium hydroxide in a further
process
step undergoes a reaction with gaseous carbon dioxide and/or carbonate-
comprising
anions resulting in precipitated nesquehonite as an intermediate product. The
carbonization
temperature according to one embodiment of the invention should be controlled
and
preferably should be below 35 C. Finally, hydromagnesite is directly obtained
after
6
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
transforming said precipitated intermediate product by a heat treatment step.
According to one embodiment of the invention, the thus obtained precipitated
intermediate product is ground prior to further processing. The hydromagnesite
obtained by the inventive process provides several advantageous
characteristics, like
small particle size in combination with a platy-like morphology and high
absolute
density.
It should be understood that for the purposes of the present invention, the
following
terms have the following meanings:
"Hydromagnesite", "basic magnesium carbonate" or "magnesium carbonate
hydroxide" in the meaning of the present invention defines a synthetically
prepared
material of magnesium carbonate with the chemical formula Mg5(CO3)4(0F1)2 =
4H20.
"Nesquehonite" in the meaning of the present invention defines a synthetically
prepared material of magnesium carbonate with the chemical formula MgCO3 =
3H20 or Mg(HCO3)(OH) = 2H20.
The term "precipitation" in the meaning of the present invention refers to the
formation of a solid material in a solution during a chemical reaction.
A "suspension" or "slurry" in the meaning of the present invention comprises
insoluble solids and water and optionally further additives and usually
contains large
amounts of solids and, thus, is more viscous and generally of higher density
than the
liquid from which it is formed.
The term "slaking" or "slake" in the meaning of the present invention refers
to the
hydration of magnesium oxide by contacting said compounds with water or
moisture.
7
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
The term "calcining" in the meaning of the present invention refers to a
thermal
treatment process applied to solid materials causing loss of moisture,
reduction or
oxidation, and the decomposition of carbonates and other compounds resulting
in an
oxide of the corresponding solid material.
The term "carbonation" in the meaning of the present invention refers to a
process in
which at least one hydroxide group is replaced by carbonate.
The term "heat-ageing" in the meaning of the present invention relates to a
thermal
treatment process in which crystals having initially a higher internal energy
state
undergo a phase transformation by dissolving and redepositing into crystals
having a
lower internal energy state.
According to another aspect of the present invention, hydromagnesite is
provided,
wherein said hydromagnesite is obtainable by the inventive process for
preparing
hydromagnesite. The hydromagnesite preferably has a platy-like morphology in
combination with a decreased particle size and improved physical and optical
properties. According to a further aspect, said hydromagnesite obtainable by
the
process of the present invention has an increased density. According to
another
aspect, the present invention refers to the use of said hydromagnesite as
mineral,
filler and pigment in paper, paint, rubber and plastics applications and to
its use as
flame-retardant.
According to one preferred embodiment of the present invention, the at least
one
magnesium oxide source comprises magnesium oxide, magnesite, dolomite,
huntite,
magnesium carbonate, magnesium hydroxide, brucite or mixtures thereof.
According to another preferred embodiment of the inventive process, the
gaseous
CO2 comes from an external CO2 supply or from the recirculation of CO2 or
both.
8
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
According to yet another preferred embodiment of the inventive process, the
carbonate-comprising anions are selected from the group consisting of sodium
carbonate, potassium carbonate, sodium hydrogen carbonate, potassium hydrogen
carbonate or mixtures thereof.
According to one preferred embodiment of the inventive process, the starting
temperature of step d) is adjusted to a temperature of between 5 C and 35 C
and
most preferably to a temperature of between 10 C and 30 C.
According to another preferred embodiment of the present invention, the heat-
ageing
step of step e) is carried out at a temperature of at least 90 C, preferably
in the range
between 90 C and 150 C, more preferably at a temperature of between 110 C and
140 C, even more preferably at a temperature of between 120 C to 135 C and
most
preferably at a temperature of about 130 C.
According to yet another preferred embodiment of the present invention, the
heat-
ageing step is carried out for a period of time of 20 min to 60 min,
preferably for a
period of time of 20 min to 40 min and most preferably for a period of time of
25 to
35 min.
According to one preferred embodiment of the present invention, the
precipitated
nesquehonite obtained in step d) is ground prior to the heat-ageing step of
step e).
According to another preferred embodiment of the present invention, the
precipitated
nesquehonite obtainable after the grinding comprises particles of which at
least 50 %
by weight have an an average particle size of less than 25 gm, more preferably
of
less than 20 gm, even more preferably of less than 15 gm and most preferably
of less
than 10 gm.
9
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
According to yet another preferred embodiment of the present invention, the
hydromagnesite obtainable by the process has a specific BET surface area of 10
m2/g
to 150 m2/g, more preferably of 10 m2/g to 100 m2/g and most preferably of 20
m2/g
to 70 m2/g.
According to one preferred embodiment of the present invention, the
hydromagnesite
obtainable by the process has a degree of whiteness R457 of at least 80 %,
more
preferably of at least 85 %, even more preferably of between 85 % and 99 % and
most preferably of between 85 % and 99 %.
According to another preferred embodiment of the present invention, the
hydromagnesite obtained by the process comprises particles having an average
particle size c/50 of less than 20 gm, preferably of less than 15 gm, more
preferably of
less than 10, and most preferably of less than 5 gm.
According to yet another preferred embodiment of the present invention, the
hydromagnesite obtained by the process is further treated with fatty acids,
preferably
selected from the group consisting of one or more fatty acids selected from
stearic
acid, palmitic acid, behenic acid, montanic acid, capric acid, lauric acid,
myristic
acid, isostearic acid and cerotic acid.
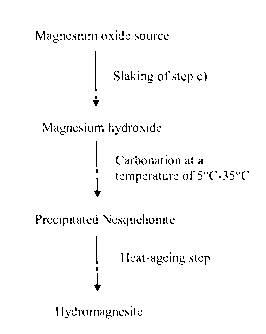
Figure 1 shows a simplified flow-chart illustrating the process for preparing
hydromagnesite according to the process of the present invention.
As set out above, the inventive process for preparing hydromagnesite having
improved optical and physical properties comprises the steps a), b), c), d)
and e). In
the following, it is referred to further details of the present invention and
especially
the foregoing steps of the inventive process for preparing magnesium carbonate
suspensions having a specific morphology in combination with decreased
particle
size as well as excellent optical and physical properties. Those skilled in
the art will
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
understand that many embodiments described herein can be combined or applied
together.
Characterization of step a): provision of a magnesium oxide source
According to step a) of the process of the present invention, at least one
magnesium
oxide source is provided.
The at least one magnesium oxide source in the meaning of the present
invention
refers to
- magnesium oxide; and/or
- a material in which magnesium oxide naturally occurs; and/or
- a material in which at least one magnesium compound occurs which can be
converted into magnesium oxide.
Accordingly, the at least one magnesium oxide source is preferably selected
from
magnesium oxide, magnesium oxide containing minerals, magnesium containing
materials and mixtures thereof. Preferred magnesium oxide sources are
magnesite
and dolomite, for which a calcining step is needed to convert the contained
magnesium compounds into the desired magnesium oxide.
In the case where the at least one magnesium oxide source is selected from
magnesium oxide, said magnesium oxide is preferably in the form of a powder
having a magnesium oxide content of more than 95 wt.-% and more preferably of
more than 98 wt.-%, based on the weight of the powder. In a preferred
embodiment,
the particles of magnesium oxide in the powder are of small particle sizes;
i.e. the
particles of magnesium oxide have an average particle size d99 value of less
than 100
gm and a d95 value of less than 75 gm, as determined by laser diffraction
using the
instrument CILAS 920 particle-size-analyzer of CILAS, Orleans, France.
11
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
A material in which a form of magnesium oxide naturally occurs is understood
to be
a magnesium oxide containing mineral. An example of such magnesium oxide rich
mineral is represented for example by periclase, which occurs naturally in
contact
metamorphic rocks and is a major component of most basic refractory bricks.
By contrast, a material from which magnesium oxide can be synthetically
obtained,
i.e. can be converted into magnesium oxide may be any magnesium containing
material, for example, materials comprising magnesium hydroxide and/or
magnesium carbonate. If the at least one magnesium oxide source is a magnesium
containing material, said material comprises the magnesium compound(s)
preferably
in an amount of at least 15 wt.-%, more preferably of at least 25 wt.-% and
most
preferably of at least 40 wt.-% based on the total weight of the magnesium
containing material.
In the case where the at least one magnesium oxide source is a magnesium
containing material, said material is preferably selected from magnesium
carbonate,
magnesium hydroxide, magnesite, brucite, dolomite, huntite, magnesium chloride
rich brine, seawater from which magnesium oxide can be obtained and mixtures
thereof. In this context, the term "magnesium carbonate" according to the
present
invention comprises anhydrous magnesium carbonate as well as forms of
magnesium
carbonate comprising crystal water (hydrate).
According to the present invention, the magnesium oxide source of step a) of
the
process of the present invention is not restricted to the specific minerals
and/or
materials described above. Rather any mineral and/or material can be used
provided
that said mineral and/or material comprises a sufficient amount of magnesium
oxide
and/or a corresponding magnesium oxide containing mineral and/or a material or
mineral which can be at least partially converted into magnesium oxide.
12
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
Said at least partial conversion of a magnesium compound into magnesium oxide
is
preferably carried out by calcining said materials. Said calcining step can be
carried
out by any conventional calcining process known to the skilled person.
In one preferred embodiment, the at least one magnesium oxide source is
magnesite
(MgCO3), which is a frequently occurring mineral merely exclusively consisting
of
anhydrous magnesium carbonate. Such material in the meaning of the present
invention would be regarded as magnesium containing material. Said magnesite
is
preferably calcined at temperatures of about 600 C to 900 C in order to obtain
magnesium oxide in the form of caustic calcined magnesite. The obtained
particles of
caustic calcined magnesite are characterized by their high porosity and
possess a
high reactivity due to their large inner surface and, thus, are especially
suitable for
the purposes of the present invention. Alternatively or additionally, other
forms of
magnesium carbonate, for example, synthetically prepared magnesium carbonate
in
the anhydrous form and/or forms comprising crystal water and/or other
naturally
occurring forms of magnesium carbonate may also be used for providing the at
least
one magnesium oxide source. The magnesium carbonates may also be calcined
under
the same conditions as applied for magnesite in order to convert these
magnesium
carbonates at least partially into magnesium oxide.
Preferably, the obtained caustic calcined magnesite has a content of magnesium
oxide of more than 85 wt.-%, more preferably more than 90 wt.-% and most
preferably of more than 92 wt.-%, based on the total weight of the caustic
calcined
magnesite. In a preferred embodiment, said caustic calcined magnesite has a
content
of magnesium oxide of between 90 wt.-% and 99 wt.-% and most preferably of
between 92 wt.-% and 98 wt.-%, based on the total weight of the caustic
calcined
magnesite. Magnesium oxide obtained from other magnesium carbonates has
preferably a content of magnesium oxide of more than 90 wt.-%, for example in
the
range between 95 wt.-% and 99 wt.-%, based on the total weight of the
magnesium
oxide.
13
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
The particles of the caustic calcined magnesite and/or magnesium oxide
obtained
from other forms of magnesium carbonate preferably have a particle size
distribution
as conventionally employed for the material(s) involved in a corresponding
process
for converting a magnesium oxide source at least partially into magnesium
hydroxide
as used in step c) of the process of the present invention. In general, the
caustic
calcined magnesite particles and/or particles of magnesium oxide obtained from
other forms of magnesium carbonate have an average particle size d50 value of
from
1 gm to 100 gm, preferably from 5 gm to 50 gm and most preferably from 10 gm
to
25 gm, for example between 15 gm and 20 gm, as determined by laser diffraction
using the instrument CILAS 920 particle-size-analyzer of CILAS, Orleans,
France.
As used herein and as generally defined in the art, the d50 value is defined
as the size
at which 50 % (the median point) of the particle mass is accounted for by
particles
having a particle size equal to the specified value.
In the case where the magnesium containing material is magnesium hydroxide or
a
material comprising a high magnesium hydroxide content, said material may be
directly subjected to step d) of the process of the present invention.
Alternatively or
additionally, said magnesium containing material may be first subjected to the
slaking of step c) of the process of the present invention.
In another preferred embodiment, the at least one magnesium oxide source is
dolomite which is merely composed of calcium magnesium carbonate (CaMg(CO3)2)
and thus representing a magnesium containing mineral. For the purposes of the
present invention, any available variety of dolomite can be used as the at
least one
magnesium oxide source. However, in one preferred embodiment, the dolomite is
white dolomite representing a relatively pure dolomite which may be e.g.
obtained or
extracted from Norwegian Talc's Hammerfall deposits. In order to convert the
magnesium compound, i.e. the carbonates, contained in said dolomite at least
14
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
partially into magnesium oxide, the dolomite is preferably calcined at high
temperatures, wherein said calcining step can be carried out by any
conventional
calcining process known to the skilled person.
In one preferred embodiment, the dolomite is calcined at temperatures of
between
900 C and 1200 C and more preferably at a temperature of between 1000 C and
1100 C in order to obtain burned dolomite (MgO = CaO). Alternatively, the
dolomite
is calcined at temperatures of between 600 C and 900 C, more preferably of
between
600 C and 800 C and most preferably at a temperature of about 750 C in order
to
obtain half-burned dolomite (MgO = CaCO3). The chemical characteristics such
as
reactivity of the half-burned or burned dolomite, i.e. of the obtained
magnesium
oxide, depend mainly on the temperatures and calcining processes used which
are
well known to the skilled person. For instance, if the dolomite is calcined at
a
temperature in the range of 1000 C to 1100 C in order to obtain burned
dolomite,
said temperature is preferably maintained for a period of time of between 30
min to
120 min and most preferably for a period of time of between 45 min and 90 min,
for
example for about 60 min.
The dolomite subjected to the calcining process has preferably a size
distribution as
conventionally employed for the material(s) involved in a corresponding
calcining
process for obtaining burned or half-burned dolomite. In this context, it is
to be noted
that dolomite as well as other magnesium containing materials such as for
example
magnesite used as the at least one magnesium oxide source according to the
present
invention are usually in the form of rocks and grains of various sizes. For
obtaining
a sufficient amount of magnesium oxide having a sufficient high reactivity
and/or a
sufficient specific surface area, said rocks and/or grains are preferably
comminuted
by a mechanical processing step prior to calcining said magnesium rich
materials
resulting in a reduction of the original grain size. As a result of the
comminution, the
mean grain size of the obtained particles are preferably in the range of 1 mm
to 250
mm, preferably in the range of 1 mm to 150 mm, even more preferably in the
range
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
of 1 mm to 100 mm and most preferably in the range of 1 mm to 75 mm. In one
preferred embodiment, said particles have preferably a mean grain size in the
range
between 10 mm and 50 mm, as measured by screening on analytical test sieves
from
Retsch , Germany and determining mass fractions of selected size ranges.
Such comminution steps may, for example, be performed under conditions such
that
refinement predominantly results from impacts with a secondary body, i.e. in
one or
more of a vertical bead mill, a ball mill, a rod mill, a vibrating mill, a
roll crusher, a
centrifugal impact mill, an attrition mill, a pin mill, a hammer mill, a
pulveriser, a
shredder, a de-clumper, a knife cutter, or other such equipment known to the
skilled
person, or may be performed under conditions such that autogenous grinding
takes
place. In one preferred embodiment, such comminution step is carried out by
grinding rocks and grains of, for example, dolomite by using a ball mill.
In the case where magnesium containing materials have been calcined and are
subsequently used as the corresponding magnesium oxide source in step c) of
the
process of the present invention, said step of comminution may, alternatively
or
additionally, be carried out after said magnesium containing material has been
calcined, i.e. prior to step c) of the process of the present invention. If
magnesium
oxide and/or magnesium oxide containing minerals are used as the at least one
magnesium oxide source, such step of comminution may be also performed prior
to
step c) of the process of the present invention. Such comminution prior to
step c) of
the process of the present invention is preferably carried out if there is a
particle size
distribution of the magnesium oxide source which is considered too broad
and/or if
the median diameter of the magnesium oxide source is above 150 gm.
Accordingly,
the particles of the magnesium oxide source used in step c) of the process of
the
present invention are preferably of an average particle size d50 value of from
1 gm to
150 gm, more preferably from 5 gm to 100 gm and most preferably from 10 gm to
75 gm, as measured by laser diffraction using the instrument CILAS 920
particle-
size-analyzer of CILAS, Orleans, France
16
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
Characterization of step b): providing gaseous CO2 and/or carbonate-
comprising
anions
According to step b) of the process of the present invention, gaseous CO2
and/or
carbonate-comprising anions are provided.
The carbon dioxide provided in step b) may be any form of gaseous CO2, for
example, carbon dioxide, a carbon dioxide containing gas or carbonic acid,
i.e.
carbon dioxide dissolved in water.
In one preferred embodiment, the gaseous CO2 is derived from the gaseous
effluent
obtained by calcining various magnesium oxide sources provided in step a) of
the
same process, i.e. the gaseous carbon dioxide is supplied by the recirculation
of CO2.
By calcining of such sources such as magnesium carbonate, for example, in the
form
of magnesite, a rising temperature during the calcining process increases the
amount
of carbon dioxide gas released during the process. Preferably, the generated
carbon
dioxide is vented out of the reaction vessel as the obtained magnesium oxide
has an
affinity to absorb moisture as well as carbon dioxide, so that said compound
undergoes the reaction back to the magnesium carbonate. Such gases contain
approximately 5 % to 40 % by volume of CO2 and are preferably used after
purification and, optionally up-concentration or dilution of the gaseous
effluent.
Additionally or alternatively, the carbon dioxide can be supplied from an
external
source such as from a steel cylinder or from flue gases and/or exhaust gases
of
industrial processes using furnaces and kilns and/or from suitable reactions
of
carbonate salts with acids etc.. However, it is to be noted that the carbon
dioxide
source provided in step b) of the inventive process is not particularly
limited
provided said source contains no reactive gas.
17
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
The gaseous carbon dioxide of step b) may be provided in a concentrated form
or in
a diluted form. If the gaseous carbon dioxide is provided in a diluted form,
the
carbon dioxide is preferably provided in admixture with air or the like.
In this case, the gaseous carbon dioxide provided in step b) of the process of
the
present invention has, in terms of volume, a concentration in, for example,
air of less
than 40 vol.-%, more preferably less than 35 vol.-% and most preferably
between 10
vol.-% and 30 vol.-% based on the total volume of the gaseous composition. The
minimum carbon dioxide content in the carbon dioxide source may be about 8
vol.-
%, based on the total volume of the gaseous composition.
Additionally or alternatively, carbonate-comprising anions are provided in
step b) of
the process of the present invention. The carbonate-comprising anions of step
b) may
be provided in any form of carbonate salts which are soluble in water, i.e.
dissolve in
water to form a homogeneous solution. In one preferred embodiment, carbonate-
comprising anions refer to carbonate salts, which when mixed with deionised
water
provide a solubility of more than 50 g/1 at 20 C, preferably of more than 100
g/1 at
C, more preferably of more than 150 g/1 at 20 C and most preferably of more
than 200 g/1 at 20 C.
Accordingly, the carbonate-comprising anions of step b) are preferably
selected from
the group comprising alkali carbonates and/or alkali hydrogen carbonates,
wherein
the alkali ion of the alkali carbonate and/or alkali hydrogen carbonate is
selected
from sodium, potassium and mixtures thereof. Sodium carbonate, potassium
carbonate, sodium hydrogen carbonate, potassium hydrogen carbonate and
mixtures
thereof are preferred carbonate-comprising anions of step b) of the process of
the
present invention. In one preferred embodiment, the carbonate-comprising
anions are
sodium carbonate.
18
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
In the context of the present invention, the term "sodium carbonate" shall
include
sodium carbonate in the anhydrous form as well as forms comprising crystal
water
(hydrate). In one preferred embodiment, the sodium carbonate of the present
invention is anhydrous sodium carbonate (Na2CO3) or sodium carbonate
decahydrate
(Na2CO3 = 10 H20).
The term "potassium carbonate" also refers to potassium carbonate in the
anhydrous
form as well as forms comprising crystal water (hydrate). Preferably, the
potassium
carbonate of the present invention is anhydrous potassium carbonate (K2CO3).
In the context of the present invention, the term "sodium hydrogen carbonate"
shall
include sodium hydrogen carbonate in the anhydrous form as well as forms
comprising crystal water (hydrate). Preferably, the sodium hydrogen carbonate
of the
present invention is anhydrous sodium hydrogen carbonate (NaHCO3).
In the context of the present invention, the term "potassium hydrogen
carbonate"
shall also include potassium hydrogen carbonate in the anhydrous form as well
as
forms comprising crystal water (hydrate). Preferably, the potassium hydrogen
of the
present invention is anhydrous potassium hydrogen carbonate (KHCO3).
In case carbonate-comprising anions are provided in step b) of the process of
the
present invention, said carbonate-comprising anions can be provided in any
appropriate solid form, e.g. in the form of granules or a powder.
Alternatively, said
carbonate-comprising anions can be provided in the form of a suspension or
solution.
19
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
Characterization of step c): slaking of said magnesium oxide source of step a)
According to step c) of the process of the present invention, said at least
one
magnesium oxide source of step a) is slaked to convert at least a part of the
magnesium oxide into magnesium hydroxide.
"Slaking" in the meaning of the present invention refers to a process in which
magnesium oxide is hydrated. Thus, the term "slaking" refers to a process of
adding
water to magnesium oxide to produce magnesium hydroxide. Accordingly, the
particles of magnesium oxide of the at least one magnesium oxide source
provided in
step a) are hydrated in a slaking process, which is carried out by contacting
the
magnesium oxide of the at least one magnesium oxide source with water.
According
to the inventive process the magnesium oxide is at least partially converted
into
magnesium hydroxide.
The water to be used in the slaking process of step c) may be any water
available
such as tap water and/or deionised water. Preferably, the water used for
slaking the at
least one magnesium oxide source of step a) is tap water.
In one preferred embodiment of the present invention, the at least one
magnesium
oxide source of step a) is added to the water in one portion and/or
continuously over
a period of 1 h or less, preferably over a period of 45 min or less, more
preferably
over a period of 30 min or less and most preferably over a period of 15 min or
less to
yield or provide a suitable solid content in the resulting suspension. In
another
preferred embodiment, the at least one magnesium oxide source of step a) is
added to
water over a period of 10 min or less to a suitable solid content in the
resulting
suspension. In a further preferred embodiment of the present invention, the at
least
one magnesium oxide source of step a) is added to the water in several
portions to a
suitable solid content in the resulting suspension, preferably in two to five
portions,
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
more preferably in two to four portions, even more preferably in two to three
portions and most preferably in two portions.
In the case where the at least one magnesium oxide source of step a) is added
to the
water in several portions, the at least one magnesium oxide source of step a)
is
preferably added in about equal portions to the water. As an alternative, it
is also
possible to add the at least one magnesium oxide source of step a) in unequal
portions to the water, i.e. in larger and smaller portions. In one preferred
embodiment, the larger portion is added first followed by the addition of the
smaller
portion of the at least one magnesium oxide source to the resulting suspension
in
order to slake or hydrate the at least one magnesium oxide source provided in
step a).
In another preferred embodiment, the smaller portion of the at least one
magnesium
oxide source of step a) is added first followed by the addition of the larger
portion to
the water in order to convert the at least one magnesium oxide source of step
a) at
least partially into magnesium hydroxide.
The ratio of the at least one magnesium oxide source of step a) to water is
preferably
adjusted in such a way that the suspension develops a sufficient or suitable
viscosity.
In one preferred embodiment, a considerable excess of water is used, so that
the ratio
of water to the at least one magnesium oxide source in the suspension is such
that the
ratio (volume of water): (volume of the at least one magnesium oxide source)
is from
40:1 to 3:1, more preferably from 30:1 to 3:1 and most preferably from 20:1 to
3:1.
For example, if the at least one magnesium oxide source is selected from
caustic
calcined magnesite, the ratio of water to the caustic calcined magnesite in
the
suspension may be such that the ratio (volume of water): (volume of caustic
calcined
magnesite) is from 40:1 to 5:1, more preferably from 30:1 to 10:1 and most
preferably from 20:1 to 15:1. In case where the at least one magnesium oxide
source
of step a) is selected from burned dolomite, the ratio of water to the burned
dolomite
in the suspension may be such that the ratio (volume of water):(volume of
burned
dolomite) is from 30:1 to 3:1, more preferably from 20:1 to 3:1 and most
preferably
21
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
from 10:1 to 3:1. In another preferred embodiment, the resulting aqueous
suspension
comprising water and the at least one magnesium oxide source provided in step
a)
has a solid content of between 1 wt.-% and 20 wt.-% and most preferably
between
1.5 wt.-% and 17.5 wt.-%, based on the total weight of the suspension.
In one preferred embodiment, said suspension has a lower solid content if the
at least
one magnesium oxide source provided in step a) has higher magnesium oxide
content. The term "high content" in the meaning of the present invention
refers to an
amount of magnesium oxide or magnesium compound which can be converted into
magnesium oxide in the corresponding magnesium oxide source of at least 70 wt.-
%,
based on the total dry weight of the magnesium oxide source. For example, if
the at
least one magnesium oxide source provided in step a) is magnesium oxide and/or
a
magnesium oxide containing mineral having a high content of magnesium oxide
and/or a magnesium containing material having a high content of a magnesium
compound which can be converted into magnesium oxide, the solid content in the
suspension may be in the range between 1 wt.-% and 15 wt.-%, more preferably
in
the range between 1.5 wt.-% and 12.5 wt.-% and most preferably in the range
between 2 wt.-% and 10 wt.-% based on the total weight of the suspension. Such
solid content is preferably adjusted if the at least one magnesium oxide
source
provided in step a) is selected from magnesium oxide, periclase, magnesite,
magnesium carbonate, magnesium hydroxide and mixtures thereof. For instance,
if
the at least one magnesium oxide source provided in step a) is caustic
calcined
magnesite obtained by calcining magnesite, the solid content in the suspension
is
preferably in the range between 1 wt.-% and 12.5 wt.-%, more preferably in the
range between 1.5 wt.-% and 10 wt.-%, even more preferably in the range
between 2
wt.-% and 7.5 wt.-% and most preferably in the range between 4 wt.-% and 6 wt.-
%,
for example between 4.5 wt.-% and 5.5 wt.-% based on the total weight of the
suspension.
22
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
In another preferred embodiment, said suspension may have a higher solid
content if
the at least one magnesium oxide source provided in step a) has a lower
magnesium
oxide content. The term "low content" in the meaning of the present invention
refers
to an amount of magnesium oxide or magnesium compound which can be converted
into magnesium oxide in the corresponding magnesium oxide of less than 70 wt.-
%,
based on the total dry weight of the magnesium oxide source. For example, if
the at
least one magnesium oxide source provided in step a) is a magnesium oxide
containing mineral having a low content of magnesium oxide and/or a magnesium
containing material having a low content of a magnesium compound which can be
converted into magnesium oxide, the solid content in the suspension may be in
the
range between 10 wt.-% and 20 wt.-%, more preferably in the range between 10
wt.-
% and 17.5 wt.-% and most preferably in the range between 12.5 wt.-% and 17.5
wt.-
% based on the total weight of the suspension. Such solid content is
preferably
adjusted if the at least one magnesium oxide source provided in step a) is
selected
from dolomite, huntite and mixtures thereof. For example, if the at least one
magnesium oxide source provided in step a) is burned dolomite obtained by
calcining dolomite, the solid content in the suspension is preferably in the
range
between 10 wt.-% and 20 wt.-%, more preferably in the range between 12.5 wt.-%
and 17.5 wt.-% and most preferably in the range between 15 wt.-% and 17.5 wt.-
%,
for example between 16 wt.-% and 17 wt.-% based on the total weight of the
suspension.
Depending on equipment and site conditions, the slaking process is preferably
carried out with water having an elevated temperature in order to provide
small
particles of magnesium hydroxide having a high specific surface and
additionally or
alternatively in order to obtain a sufficient reaction rate. Furthermore, as
the
incoming water temperature may inversely affect the time required for carrying
out
the slaking process, a contacting of the at least one magnesium oxide source
with
cool water should preferably be avoided. In the case in which cool water and
magnesium oxide come in contact a condition called "drowning" may take place,
23
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
resulting in particles of magnesium hydroxide which are very coarse and not
very
reactive. Therefore, the temperature of the water used in the slaking process
should
preferably be above room temperature but below the boiling point of water.
In one preferred embodiment, the temperature of the water added into the
reaction
vessel for slaking said at least one magnesium oxide source provided in step
a) is
preferably in the range between 20 C and 90 C, more preferably in the range
between 30 C and 60 C and most preferably in the range between 35 C and 55 C,
for example 40 C or 50 C.
For example, if the at least one magnesium oxide source provided in step a) is
caustic
calcined magnesite obtained by calcining magnesite, said temperature is
preferably in
the range between 20 C and 45 C, more preferably in the range between 25 C and
45 C and most preferably in the range between 35 C and 45 C, for example about
40 C. In the case where the at least one magnesium oxide source provided in
step a)
is burned dolomite obtained by calcining dolomite, said temperature is
preferably in
the range between 35 C and 60 C, more preferably in the range between 40 C and
55 C and most preferably in the range between 45 C and 55 C, for example about
50 C.
During the slaking process the temperature in the reaction vessel varies due
to
variation in water temperature, magnesium oxide reactivity, and quality of
water and,
thus, the temperature of the suspension may be adjusted frequently.
Preferably, the
temperature is controlled continuously. Alternatively, the temperature may be
controlled repeatedly. In another preferred embodiment, the temperature of the
suspension is not adjusted during step c) of the process of the present
invention is
carried out.
In one preferred embodiment, the slaking process of step c) is carried out by
agitation
of the suspension. In this respect, agitation can be carried out continuously
or
24
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
discontinuously. However, as the degree of agitation during the slaking
process may
have an impact on the obtained magnesium hydroxide, the suspension is
preferably
agitated continuously. In this respect, too little agitation may result in
uneven
temperature within the suspension resulting in hot and cold spots. Such uneven
temperature may result in crystals of large size and reduced surface area and
agglomeration of particles, while cold spots will result in either drowning or
higher
amounts of unhydrated particles of magnesium oxide.
The slaking process of step c) of the process of the present invention is
preferably
conducted to the point where at least a part of the magnesium oxide contained
in the
at least one magnesium oxide source is converted to its respective hydroxides,
i.e.
magnesium hydroxide. In this respect, it is to be noted that only a portion of
the
magnesium oxide contained in the at least one magnesium oxide source is
converted
into the respective magnesium hydroxide during the slaking process of step c).
For
example, if the at least one magnesium oxide source is contacted for about 15
min
with the slaking water having a temperature of about 40 C, the amount of
magnesium oxide converted into magnesium hydroxide is in the range between 5
wt.-% and 15 wt.-%, for example about 10 wt.-%, i.e. the magnesium oxide
content
is in the range between 85 wt.-% and 95 wt.-%, for example about 90 wt.-%,
based
on the total weight of magnesium oxide and magnesium hydroxide. By contrast,
if
the at least one magnesium oxide source is contacted for about 30 min with the
slaking water having a temperature of about 40 C, the amount of magnesium
oxide
converted into magnesium hydroxide is in the range between 15 wt.-% and 25 wt.-
%,
for example about 20 wt.-%, i.e. the magnesium oxide content is in the range
between 75 wt.-% and 85 wt.-%, for example about 80 wt.-%, based on the total
weight of magnesium oxide and magnesium hydroxide.
The mixture of the at least one magnesium oxide source and magnesium hydroxide
obtained by slaking said magnesium oxide source of step a) at a water
temperature of
40 C and a slaking period of 30 min may have a ratio (weight of magnesium
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
oxide): (weight of magnesium hydroxide) which is preferably from 10:1 to 2:1,
more
preferably from 8:1 to 3:1 and most preferably from 6:1 to 3:1. In this
context, it is to
be noted that after conversion or reaction of the obtained magnesium hydroxide
with
the gaseous carbon dioxide and/or carbonate-comprising anions of step b),
further
magnesium oxide of the at least one magnesium oxide source of step a) in the
mixture is converted into magnesium hydroxide which then can also be reacted
with
carbon dioxide and/or carbonate-comprising anions. In other words, the
inventive
process can be carried out with a mixture of magnesium oxide and magnesium
hydroxide (which may be obtained by a partial slaking reaction) since the
remaining
magnesium oxide is successively converted into the magnesium hydroxide after
the
magnesium hydroxide already contained in the starting mixture reacted with the
gaseous carbon dioxide and/or carbonate-comprising anions of step b). The at
least
one magnesium oxide source of step a) may be added into the water for carrying
out
the slaking of step c) of the present invention in several portions and/or
continuously
over the time desired for carrying out the process of the present invention
and/or
until the desired amount of resulting product is obtained. In said process the
amount
of water may be adjusted frequently in order to obtain a solid content and/or
viscosity suitable for carrying out the process of the present invention.
It is to be noted that in the case where a magnesium oxide containing mineral
having
a low content of magnesium oxide and/or a magnesium containing material having
a
low content of a magnesium compound which can be converted into magnesium
oxide is used as the magnesium oxide source provided in step a), said mineral
and/or
material usually comprises a content of magnesium oxide and/or of a magnesium
compound which can be converted into magnesium oxide of less than 70 wt.-%,
based on the total dry weight of the magnesium oxide source; i.e. the original
material further comprises other compounds such as carbonates, oxides,
hydroxides
etc. of alkali metals and/or alkaline earth metals.
26
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
For example, if the at least one magnesium oxide source provided in step a) is
dolomite, said mineral is merely made of magnesium carbonate and calcium
carbonate. However, natural products of dolomite possess not only varying
compositions regarding the specific compounds but also varying compositions
regarding the ratios of said magnesium and calcium carbonates which occur in a
wide range. The ratio of magnesium carbonate to calcium carbonate in naturally
occurring dolomite is usually such that the ratio (weight of magnesium
carbonate):(weight of calcium carbonate) is from 2:1 to 1:2, more preferably
1.5:1 to
1:1.5 and most preferably about 1:1.
Thus, by using dolomite as the magnesium oxide source of step a), the
calcining step
carried out prior to the slaking of step c) does not only result in the
conversion of
magnesium carbonate into magnesium oxide but also in the conversion of calcium
carbonate into calcium oxide in the corresponding ratios depending on the
dolomite
used. In the case where such a mixture of obtained magnesium oxide and calcium
oxide is slaked by step c), said slaking converts the magnesium oxide at least
partially into magnesium hydroxide and in addition thereto, the calcium oxide
is
almost completely converted into calcium hydroxide; i.e. by slaking of burned
dolomite in step c) of the process of the present invention, a mixture
comprising
magnesium hydroxide, calcium hydroxide, magnesium oxide and calcium oxide is
obtained. The term "almost completely converted" in the meaning of the present
invention refers to a reaction in which at least 99 wt.-%, more preferably at
least 99.2
wt.-% and most preferably at least 99.5 wt.-% of a compound is converted into
the
respective reaction product. For example, if a mixture of magnesium oxide and
calcium oxide is slaked by step c), said slaking converts at least 99 wt.-% of
calcium
oxide, more preferably at least 99.2 wt.-% and most preferably at least 99.5
wt.-%,
based on the total dry weight of the calcium oxide, into calcium hydroxide,
while the
magnesium oxide is only partially converted into magnesium hydroxide.
27
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
The time required for carrying out the slaking process of step c) is the time
required
to obtain a sufficient amount of magnesium hydroxide by the hydration/slaking
of
the at least one magnesium oxide source for carrying out step d) of the
process of the
present invention. This time depends mainly on the at least one magnesium
oxide
source provided in step a).
In a preferred embodiment, the at least one magnesium oxide source provided in
step
a) is slaked for a shorter period of time if the at least one magnesium oxide
source of
step a) is in the form of magnesium oxide and/or a magnesium oxide containing
mineral having a high content of magnesium oxide and/or a magnesium containing
material having a high content of a magnesium compound which can be converted
into magnesium oxide. Said period of time is preferably in the range between 5
min
and 30 min, more preferably in the range between 5 min and 20 min and most
preferably in the range between 10 min and 20 min. Such shorter period of time
is
preferably applied for the slaking step if the at least one magnesium oxide
source
provided in step a) is selected from magnesium oxide, periclase, magnesite,
magnesium carbonate, magnesium hydroxide and mixtures thereof. For instance,
if
the at least one magnesium oxide source provided in step a) is caustic
calcined
magnesite obtained by calcining magnesite, said period of time is preferably
in the
range between 10 min and 30 min, more preferably in the range between 10 min
and
min and most preferably in the range between 10 min and 20 min, for example
about 15 min.
In another preferred embodiment, the at least one magnesium oxide source
provided
25 in step a) is slaked for a longer period of time if the at least one
magnesium oxide
source provided in step a) is a magnesium oxide containing mineral having a
low
content of magnesium oxide and/or a magnesium containing material having a low
content of a magnesium compound which can be converted into magnesium oxide.
Said period of time is preferably in the range between 5 min and 60 min, more
preferably in the range between 10 min and 45 min and most preferably in the
range
28
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
between 20 min and 40 min. Such longer period of time is preferably applied if
the at
least one magnesium oxide source provided in step a) is selected from
dolomite,
huntite and mixtures thereof. For example, if the at least one magnesium oxide
source provided in step a) is burned dolomite obtained by calcining dolomite,
said
period of time is preferably in the range between 15 min and 50 min, more
preferably
in the range between 15 min and 45 min and most preferably in the range
between 25
min and 40 min, for example about 30 min.
After carrying out step c) of the present invention, the obtained mixture of
magnesium hydroxide and magnesium oxide is formed into a suitable suspension
for
carrying out step d). The overall solid content of this suspension is
preferably in the
range between 1 wt.-% and 20 wt.-%, more preferably between 1 wt.-% and 15 wt.-
% and most preferably between 2 wt.-% and 10 wt.-%, based on the total weight
of
the suspension obtained in step c). For example, if the magnesium hydroxide is
obtained from magnesite as the at least one magnesium oxide source, said
overall
solid content of the suspension is preferably in the range between 2 wt.-% and
8 wt.-
%, more preferably in the range between 3 wt.-% and 7 wt.-% and most
preferably in
the range between 4 wt.-% and 6 wt.-%, for example about 5 wt.-%, based on the
total weight of the suspension obtained in step c). If the magnesium hydroxide
is
obtained from dolomite as the at least one magnesium oxide source, said
overall
solid content of the suspension is preferably in the range between 5 wt.-% and
10
wt.-%, more preferably in the range between 6 wt.-% and 10 wt.-% and most
preferably in the range between 7 wt.-% and 9 wt.-%, for example about 8 wt.-
%,
based on the total weight of the suspension obtained in step c).
Additionally or alternatively, the obtained suspension comprising magnesium
hydroxide and magnesium oxide has preferably a viscosity of less than 1.000
mPa.s
and more preferably of less than 100 mPa.s, as measured with a Brookfield DV-
II
Viscometer at a speed of 100 rpm and equipped with a LV-3 spindle. In the case
where the obtained suspension has a solid content above or below the desired
range
29
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
and/or the viscosity of said suspension is too high or low the suspension may
be
diluted with water or up-concentrated by any conventional process known to the
skilled person to obtain a suspension of said desired solid content and/or
viscosity for
the further process steps.
The obtained suspension comprising magnesium hydroxide and magnesium oxide
has preferably a pH of more than 8, more preferably of more than 9 and most
preferably of more than 10, as measured according to the measurement method
described in the Examples section here below.
Characterization of step d): contacting the obtained magnesium hydroxide with
said
gaseous CO2 and/or carbonate-comprising anions
According to step d) of the process of the present invention, said obtained
magnesium hydroxide of step c) is contacted with said gaseous carbon dioxide
and/or
carbonate-comprising anions of step b) to convert at least a part of the
magnesium
hydroxide into precipitated nesquehonite.
The magnesium hydroxide is preferably in the form of a suspension, and
consists of
water, magnesium hydroxide, unreacted magnesium oxide and impurities normally
associated with magnesium hydroxide suspensions, for example, silica, calcium
oxide, calcium hydroxide and other magnesium compounds such as magnesium
carbonate etc.
In a preferred embodiment, said suspension has an overall solids content of at
most
20 wt.-%, preferably of at most 15 wt.-%, more preferably of at most 10 wt.-%
and
most preferably of between 1 wt.-% and 8.5 wt.-%, based on the total weight of
the
suspension.
30
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
In the case where the suspension comprising magnesium hydroxide and magnesium
oxide is obtained from magnesite as the at least one magnesium oxide source,
the
solid content of said suspension is preferably in the range between 2 wt.-%
and 8 wt.-
%, more preferably in the range between 3 wt.-% and 7 wt.-% and most
preferably in
the range between 4 wt.-% and 6 wt.-%, for example in the range between 4.5
wt.-%
and 5.5 wt.-%, based on the total weight of the suspension obtained in step
c). If the
suspension comprising magnesium hydroxide and magnesium oxide is obtained from
dolomite as the at least one magnesium oxide source of step a), the solid
content of
magnesium hydroxide and magnesium oxide in said suspension is preferably in
the
range between 1 wt.-% and 10 wt.-%, more preferably in the range between 2.5
wt.-
% and 5 wt.-% and most preferably in the range between 3 wt.-% and 5 wt.-%,
for
example in the range between 3.5 wt.-% and 4.5 wt.-%, based on the total
weight of
the suspension obtained in step c).
In one preferred embodiment, said suspension obtained by slaking of dolomite
as the
at least one magnesium oxide source provided in step a) further comprises
calcium
hydroxide. In this case, the solid content of calcium hydroxide in the
suspension is
preferably in the range between 1 wt.-% and 10 wt.-%, more preferably in the
range
between 2.5 wt.-% and 5 wt.-% and most preferably in the range between 3 wt.-%
and 5 wt.-%, for example in the range between 3.5 wt.-% and 4.5 wt.-%, based
on the
total weight of the suspension obtained in step c). In another preferred
embodiment,
the solid content of said suspension comprising magnesium hydroxide, magnesium
oxide and calcium hydroxide is preferably in the range between 2 wt.-% and 20
wt.-
%, more preferably in the range between 2 wt.-% and 10 wt.-%, even more
preferably in the range between 5 wt.-% and 10 wt.-% and most preferably in
the
range between 6 wt.-% and 10 wt.-%, for example in the range between 7 wt.-%
and
9 wt.-%, based on the total weight of the suspension obtained in step c).
Additionally or alternatively, the ratio of magnesium oxide and magnesium
hydroxide to calcium hydroxide in the suspension obtained by slaking of burned
31
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
dolomite as the at least one magnesium oxide source in step c) of the process
of the
present invention may vary in a wide range. However, in case the suspension of
step
c) is obtained from burned dolomite as the at least one magnesium oxide source
provided in step a), the ratio of magnesium oxide and magnesium hydroxide to
calcium hydroxide in the obtained suspension is preferably such that the ratio
(weight of magnesium oxide and magnesium hydroxide): (weight of calcium
hydroxide) is from 2:1 to 1:2, more preferably 1.5:1 to 1:1.5 and most
preferably
about 1:1.
In an optional embodiment, the particles of the obtained mixture of magnesium
hydroxide and magnesium oxide in the suspension may be separated by their
particle
size or from impurities prior to process step d). Preferably, the magnesium
hydroxide
is separated from particles having a particle size larger than 300 gm and more
preferably from particles having a particle size larger than 200 gm by
separation
technologies known to the skilled person, for example, by vibrating screens
and the
like.
Step d) involves contacting the suspension of magnesium hydroxide and
magnesium
oxide obtained in step c) with sufficient gaseous CO2 and/or carbonate-
comprising
anions provided in step b) until at least a part of the provided magnesium
hydroxide
is converted to a crystalline magnesium carbonate precipitate (precipitated
magnesium carbonate). In this context, it is to be noted, that the formation
of said
crystalline magnesium carbonate precipitate may lead to a conversion of
remaining
magnesium oxide in the suspension to magnesium hydroxide, which may be further
converted to said crystalline magnesium carbonate precipitate by contacting
the
obtained magnesium hydroxide with sufficient gaseous CO2 and/or carbonate-
comprising anions provided in step b). The carbonation is continued until
substantially all of the magnesium is precipitated, so that the suspension is
composed
almost entirely of a crystalline magnesium carbonate precipitate. Said
crystalline
magnesium carbonate precipitate is characterized as being nesquehonite having
the
32
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
formula Mg(HCO3)(OH) = 2H20, which may also be described as being MgCO3 =
3H20. The precipitated nesquehonite crystals obtained are of a prismatic
elongated
type being typical for nesquehonite.
For contacting the suspension comprising magnesium hydroxide and magnesium
oxide obtained in step c) with said gaseous CO2 of step b), the gas is
preferably
bubbled through the suspension. By bubbling the gaseous CO2 through the
suspension, a sufficient mixing may be achieved by the flow of the gas in the
suspension, so that an additional agitation is not required. Additionally or
alternatively, the suspension comprising magnesium hydroxide and magnesium
oxide is agitated, which may provide a more thorough mixing and thus a shorter
period of time for completing the conversion of magnesium hydroxide into
magnesium carbonate, namely precipitated nesquehonite. In a preferred
embodiment,
the suspension comprising magnesium hydroxide and magnesium oxide is
additionally agitated to ensure a thorough mixing of the particles in order to
provide
a sufficient amount of unreacted magnesium hydroxide particles for contacting
the
particles with said CO2. Such agitation can be carried out continuously or
discontinuously as long as the mixing provides a sufficient conversion of
magnesium
hydroxide into magnesium carbonate. In one preferred embodiment, the
suspension
is preferably agitated continuously.
In one preferred embodiment, said gaseous CO2 is preferably added to the
suspension
comprising magnesium hydroxide and magnesium oxide by bubbling the carbon
dioxide through the suspension in a constant rate. Said rate is preferably in
the range
between 0.1 and 10 kg CO2/h per kg magnesium oxide, more preferably in the
range
between 0.2 and 5 kg CO2/h per kg magnesium oxide and most preferably in the
range between 0.5 and 2 kg CO2/h per kg magnesium oxide.
33
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
Preferably, the ratio of suspension comprising magnesium hydroxide and
magnesium
oxide to gaseous CO2 in the aqueous suspension is, in terms of volume, such
that the
ratio (volume of suspension):(volume of gaseous CO2) is from 1:0.5 to 1:10 and
more preferably 1:0.5 to 1:5. In one preferred embodiment, the ratio of
magnesium
hydroxide in the suspension to gaseous CO2 is, in terms of volume, such that
the ratio
(volume of magnesium hydroxide): (volume of gaseous CO2) is from 1:2 to 1:100
and
more preferably 1:5 to 1:50.
In one preferred embodiment, the carbonation; i.e. the conversion of magnesium
hydroxide is monitored by the change of the pH value and/or the electrical
conductivity and/or temperature and/or CO2 content in the offgas in order to
control
the progress or completion of the reaction.
For instance, if said crystalline magnesium carbonate precipitate is obtained
from
caustic calcined magnesite as the at least one magnesium oxide source provided
in
step a), the pH of the suspension comprising magnesium oxide and magnesium
hydroxide prior to step d) of the process of the present invention is
preferably in the
range between pH 10 and 12, approximately about pH 11. In one preferred
embodiment, the pH of said suspension decreases during contacting the obtained
magnesium hydroxide of step c) with said gaseous CO2 of step b) such that the
obtained suspension after carrying out process step d) has a pH in the range
between
7 and 8, approximately between pH 7.5 and 8.
By contrast, if said crystalline magnesium carbonate precipitate is obtained
from
burned dolomite as the at least one magnesium oxide source provided in step
a), the
pH of the suspension comprising magnesium oxide and magnesium hydroxide and
calcium hydroxide prior to step d) of the process of the present invention is
preferably above pH 11, approximately about pH 12. In one preferred
embodiment,
the pH of said suspension decreases during contacting the obtained magnesium
hydroxide of step c) with said gaseous CO2 of step b) such that the obtained
34
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
suspension after carrying out process step d) has a pH in the range between 7
and 8,
approximately between pH 7.5 and 8.
The temperature provided at the beginning of step d) of the present invention
is
decisive for controlling the formation of the resulting precipitated
nesquehonite or its
properties even though the temperature employed may vary within a specific
range.
For example, the starting temperature of the carbonation step provided in step
d) may
be adjusted to a temperature in the range between 5 C and 35 C and most
preferably
in the range between 10 C and 30 C.
The temperature in the suspension may preferably be controlled and maintained
at
said starting temperature while step d) is carried out. In this respect, it is
to be noted
that the term "the temperature is maintained" during said process step in the
meaning
of the present invention relates to a temperature which does preferably not
exceed the
starting temperature by more than 5 C; i.e. if the starting temperature is for
example
adjusted to a temperature of 25 C, the temperature during the process step may
not
exceed 30 C. For example, if the at least one magnesium oxide source provided
in
step a) is caustic calcined magnesite obtained by calcining magnesite, said
starting
temperature at the beginning of process step d) is preferably in the range
between
20 C and 28 C and most preferably in the range between 24 C and 26 C. During
step d) is carried out, the temperature is preferably controlled and
maintained
between 20 C and 25 C. As another example, if the at least one magnesium oxide
source provided in step a) is burned dolomite obtained by calcining dolomite,
said
starting temperature at the beginning of process step d) is preferably in the
range
between 20 C and 28 C and most preferably in the range between 24 C and 26 C.
During step d) is carried out, the temperature is preferably controlled and
maintained
between 20 C and 30 C.
In another preferred embodiment, the starting temperature of process step d)
is
allowed to rise while step d) is carried out. However, due to the exothermic
reaction
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
the temperature of the reaction mixture may rise to temperatures of 50 C and
more.
The maximum temperature in this embodiment of the process is preferably not
more
than 50 C and most preferably the maximum temperature reached during step d)
is
not more than about 45 C. If the temperature is allowed to rise during step d)
is
carried out, the starting temperature adjusted is preferably in the range of
between
5 C and 15 C. Such setting is preferably applied if the at least one magnesium
oxide
source provided in step a) is selected from dolomite, huntite and mixtures
thereof.
For example, if the at least one magnesium oxide source provided in step a) is
burned
dolomite obtained by calcining dolomite, said starting temperature during step
d) of
the process of the present invention is preferably in the range between 7 C
and 15 C,
more preferably in the range between 10 C and 15 C and most preferably in the
range between 11 C and 13 C. During step d) is carried out, the temperature is
allowed to rise such that the temperature rises to a maximum temperature of at
most
50 C, preferably between 40 C and 45 C.
In case the suspension comprising magnesium hydroxide and magnesium oxide
obtained in step c) is contacted with said carbonate-comprising anions of step
b), the
carbonate-comprising anions are preferably added to said suspension in any
appropriate solid form, e.g. in the form of granules or a powder or in the
form of a
suspension or solution. In one preferred embodiment, the suspension comprising
magnesium hydroxide and magnesium oxide is agitated during the addition of the
carbonate-comprising anions, which may provide a more thorough mixing and thus
a
shorter period of time for completing the conversion of magnesium hydroxide
into
magnesium carbonate, namely precipitated nesquehonite. Such agitation can be
carried out continuously or discontinuously as long as the mixing provides a
sufficient conversion of magnesium hydroxide into magnesium carbonate. In one
preferred embodiment, the suspension is preferably agitated continuously.
36
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
Preferably, the concentration of the carbonate-comprising anions in the
suspension
comprising magnesium oxide and magnesium hydroxide is such that the weight
ratio
of said suspension: carbonate-comprising anions is from 300:1 to 10:1, more
preferably 250:1 to 25:1, and even more preferably 200:1 to 50:1.
By carrying out step d) of the process of the present invention a precipitated
intermediate product is obtained by contacting the obtained suspension of
magnesium hydroxide of step c) with the gaseous CO2 and/or carbonate-
comprising
anions of step b). Said precipitated intermediate product is characterized as
being
nesquehonite having the formula Mg(HCO3)(OH) = 2H20, which may also be
described as being MgCO3 = 3H20. The precipitated nesquehonite crystals
obtained
are of a prismatic elongated type being typical for nesquehonite.
Accordingly, the time required for carrying out the carbonation of step d) is
the time
required to almost complete the conversion of the magnesium hydroxide obtained
in
step c) into precipitated nesquehonite. Such almost complete conversion of
magnesium hydroxide into precipitated nesquehonite is preferably obtained
within 4
hours, more preferably within 3 hours, even more preferably within 2 hours and
most
preferably within 90 min, calculated from the start of contacting the at least
partially
obtained magnesium hydroxide of step c) with said gaseous CO2 and/or carbonate-
comprising anions.
The precipitated nesquehonite obtained is preferably in the form of an aqueous
suspension. It has been found that a suspension of precipitated nesquehonite
having a
solid content in the suspension of up to 50 wt.-%, preferably between 1 and 50
wt.-
%, more preferably between 1 and 25 wt.-% and most preferably between 5 and 15
wt.-%, based on the total weight of the suspension, is preferred. In the case
where the
obtained suspension has a solid content above or below the desired range the
suspension may be diluted with water or up-concentrated by any conventional
37
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
process known to the skilled person to obtain a suspension of said desired
solid
content for the further process step.
In an optional embodiment, the particles of the obtained precipitated
nesquehonite in
the suspension may be separated by their particle size or from impurities
prior to the
heat-ageing step. In one preferred embodiment, the precipitated nesquehonite
is
separated from particles having an average particle size c150 value of more
than 200
gm, more preferably from particles having an average particle size d50 value
of more
than 150 gm and most preferably from particles having an average particle size
clso
value of more than 100 gm, as measured by screening and determining mass
fractions of selected size ranges.
In this context, it is to be noted that the average particle size d50 value of
the obtained
precipitated nesquehonite may vary in a broad range but, in general, the
particles of
the obtained precipitated nesquehonite have an average particle size d50 value
of less
than 50 gm, more preferably of less than 35 gm, even more preferably of less
than
gm and most preferably of less than 15 gm, as determined by laser diffraction
using the instrument CILAS 920 particle-size-analyzer of CILAS, Orleans,
France.
20 For example, if the at least one magnesium oxide source is derived from
magnesite in
the form of caustic calcined magnesite, the particles of the obtained
precipitated
nesquehonite in the suspension preferably have an average particle size d50
value of
less than 30 gm, more preferably of less than 25 gm, even more preferably of
less
than 20 gm and most preferably between 10 gm and 15 gm, as determined by laser
diffraction using the instrument CILAS 920 particle-size-analyzer of CILAS,
Orleans, France.
By contrast, if the precipitated nesquehonite of step d) is obtained from
burned
dolomite as the at least one magnesium oxide source provided in step a), the
suspension obtained in step d) may further comprise precipitated calcium
carbonate
38
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
(PCC). In this case, the mixture of the obtained particles of precipitated
nesquehonite
and precipitated calcium carbonate in the suspension preferably have an
overall
average particle size d50 value of less than 20 gm, more preferably of less
than 15
gm, even more preferably of less than 10 gm and most preferably of less than
7.5
gm, for example less than 5 gm, as determined by laser diffraction by using
the
instrument CILAS 920 particle-size-analyzer of CILAS, Orleans, France.
In an especially preferred embodiment, the particles of the obtained
precipitated
nesquehonite in the suspension are ground prior to the heat-ageing step in
order to
provide particles having a reduced particle size and/or in order to provide
particles of
about equal diameter. The grinding step can be carried out with any
conventional
grinding device such as a grinding mill known to the skilled person. In one
preferred
embodiment, the precipitated nesquehonite particles in the aqueous suspension
are
wet-ground in a vertical bead mill. Preferably, said wet-grinding is carried
out at a
specific energy input during grinding in the range between 10 kWh/dry ton to
500
kWh/dry ton, more preferably at a specific energy input during grinding in the
range
between 20 kWh/dry ton to 300 kWh/dry ton and most preferably at a specific
energy input during grinding in the range between 50 kWh/dry ton to 200
kWh/dry
ton, for example at a specific energy input during grinding of about 100
kWh/dry
ton.
The intermediate grinding step is especially advantageous if the magnesium
oxide
source is derived from magnesite in the form of caustic calcined magnesite.
Accordingly, if the precipitated nesquehonite particles are ground prior to
the heat-
ageing of step e) of the process of the present invention, the nesquehonite
particles
obtained from caustic calcined magnesite as the at least one magnesium oxide
source
of step a) preferably have an average particle size d50 value of less than 25
gm, more
preferably of less than 20 gm, even more preferably of less than 15 gm and
most
preferably of less than 10 gm, as determined by laser diffraction using the
instrument
CILAS 920 particle-size-analyzer of CILAS, Orleans, France.
39
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
However, in case where the mixture of particles of precipitated nesquehonite
and
precipitated calcium carbonate obtained from burned dolomite as the at least
one
magnesium oxide source of step a) is ground prior to the heat-ageing step, the
particles in the obtained mixture of precipitated nesquehonite and
precipitated
calcium carbonate preferably have an overall average particle size d50 value
of less
than 10 gm, more preferably of less than 7.5 gm, even more preferably of less
than
5 gm and most preferably of less than 3 gm, for example less than 2.5 gm, as
determined by laser diffraction by using the instrument CILAS 920 particle-
size-
analyzer of CILAS, Orleans, France.
It is further to be noted that if the precipitated nesquehonite of step d) is
obtained
from burned dolomite as the at least one magnesium oxide source provided in
step a),
the ratio of precipitated nesquehonite and precipitated calcium carbonate in
the
suspension obtained in step d) of the process of the present invention may
vary in a
wide range. Preferably, the ratio of precipitated nesquehonite to precipitated
calcium
carbonate in the obtained suspension is preferably such that the ratio (weight
of
precipitated nesquehonite):(weight of precipitated calcium carbonate) is from
3:1 to
1:3, more preferably 2:1 to 1:2 and most preferably from 1.5:1 to 1:1.5.
Additionally or alternatively, a polysaccharide may be added into the
suspension
before step d) of the process of the present invention is carried out; i.e.
the
suspension containing the at least one magnesium oxide source contains said
polysaccharide during the carbonation of step d). The polysaccharide is
preferably
selected from the group consisting of sorbitol, mannitol, sucrose and mixtures
thereof. In one preferred embodiment, the polysaccharide is sorbitol.
The polysaccharide is preferably added into the suspension in a quantity so
that it is
contained in the resulting suspension in a concentration between 0.001 wt.-%
and 5
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
wt.-%, more preferably between 0.01 wt.-% and 0.1 wt.-% and most preferably
between 0.05 wt.-% and 0.75 wt.-%, based on the total weight of the
suspension.
The polysaccharide can be added to the suspension in any appropriate solid
form,
e.g. in the form of granules or a powder. Alternatively, the polysaccharide
can be
added to the suspension in the form of a suspension or solution.
Characterization of step e): treating the obtained precipitated nesquehonite
in a heat-
ageing step
According to step e) of the process of the present invention, said obtained
precipitated nesquehonite of step d) is treated by a heat-ageing in order to
obtain the
hydromagnesite.
The term "heat-ageing" in the meaning of the present invention relates to a
thermal
process in which crystals, such as of nesquehonite, having initially a higher
internal
energy state, undergo a phase transformation by dissolving and redepositing
into
crystals having a lower internal energy state. The process results in a final
crystal
product characterized by greater perfection of its crystal lattice structure,
a narrower
particle size distribution, greater degree of particle discreteness and lower
surface
energy.
The heat-ageing of the precipitated nesquehonite obtained in step d) may be
carried
out at temperatures of above 90 C and most preferably at a temperature in the
range
between 90 C and 150 C, wherein said temperature range reflects the period
required for converting the obtained precipitated nesquehonite into
hydromagnesite;
i.e. the higher the temperature at which the heat-ageing is carried out the
lower the
time required for achieving an almost complete conversion of precipitated
nesquehonite into hydromagnesite or the lower the temperature at which the
heat-
41
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
ageing is carried out the higher the time required for achieving an almost
complete
conversion of precipitated nesquehonite into hydromagnesite. Preferably, the
precipitated nesquehonite is maintained at the heat-ageing temperature for a
sufficient time to cause the morphology of the nesquehonite to rearrange to
the final
form of the final product hydromagnesite. In this respect, the period required
for
achieving an almost complete conversion into hydromagnesite starting from the
precipitated nesquehonite may vary between 10 min and several hours depending
on
the temperature applied during said heat-ageing step.
The period of time the precipitated nesquehonite should be maintained at the
heat-
ageing temperature in order to recrystallize to the new morphology having
decreased
particle size is determined by both the initial morphology of the precipitated
nesquehonite and the nature and extent of any impurities present in the
magnesium
carbonate. For example, where the precipitated nesquehonite material has a
small
initial average particle size, the period of time for said ageing step at
about 130 C is
as short as, for example, about 30 minutes.
In order to arrive at specifically small particles of hydromagnesite the heat-
ageing
process for converting the precipitated nesquehonite into hydromagnesite is
preferably carried out at temperatures is in the range between 90 C and 150 C,
preferably in the range between 110 C and 140 C, more preferably in the range
between 120 C to 135 C and most preferably at a temperature of about 130 C.
For instance, if the heat-ageing temperature of the precipitated nesquehonite
is
adjusted to a temperature of about 130 C, said temperature is preferably
maintained
for a period of time of more than 10 min and more preferably for a period of
time of
between 20 min and 60 min. In one preferred embodiment, the heat-ageing
temperature is maintained for a period of time of between 20 min and 40 min,
more
preferably for a period of time of between 25 and 35 min and most preferably
for
42
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
about 30 min. The heat-ageing reaction can be monitored by measuring the
surface
area and/or conductivity of the hydromagnesite at specific intervals.
In an optional embodiment, a bleaching agent is added into the suspension of
nesquehonite obtained in step d); i.e. said bleaching agent is added prior the
heat-
ageing of step e) is carried out. Additionally or alternatively, the bleaching
agent may
be added during the carbonation step, i.e. during the step in which the
obtained
magnesium hydroxide of step c) is contacted with the gaseous CO2 of step b).
In one preferred embodiment, the bleaching agent is sodium dithionite. In a
further
preferred embodiment, the bleaching agent is formamidine sulfinic acid.
Alternatively or additionally other suitable bleaching agents may be used.
The bleaching agent is preferably added into the corresponding suspension in a
quantity so that it is contained in the resulting suspension of magnesium
hydroxide
and precipitated nesquehonite, respectively, in a concentration between 0.001
wt.-%
and 10 wt.-%, more preferably between 0.01 wt.-% and 1 wt.-% and most
preferably
between 0.05 wt.-% and 0.5 wt.-%, based on the total weight of the suspension.
The bleaching agent can be added to the corresponding suspension in any
appropriate
solid form, e.g. in the form of granules or a powder. Alternatively, the
bleaching
agent can be added to the corresponding suspension in the form of a suspension
or
solution.
By using the process of the present invention, it is possible to provide
hydromagnesite particles having a specifically decreased particle size.
Preferably the
obtained hydromagnesite particles have an average particle size d50 value in
the
range of less than 20 gm, preferably of less than 15 gm, more preferably of
less than
10 gm and most preferably of less than 5 gm, as determined by laser
diffraction
using the instrument CILAS 920 particle-size-analyzer of CILAS, Orleans,
France.
43
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
In one preferred embodiments, it is possible to obtain a hydromagnesite
suspension
having a high content of hydromagnesite if said hydromagnesite is obtained
from a
magnesium oxide source in the form of magnesium oxide and/or a magnesium oxide
containing mineral having a high content of magnesium oxide and/or a magnesium
containing material having a high content of a magnesium compound which can be
converted into magnesium oxide. Said content of hydromagnesite is preferably
above
85 wt.-%, more preferably above 90 wt.-% and most preferably above 95 wt.-%,
based on the total weight of the solid content in the suspension. For example,
if
magnesite is used as the at least one magnesium oxide source provided in step
a), the
content of hydromagnesite is preferably above 90 wt.-%, more preferably above
93.5 wt.-% and most preferably above 97 wt.-%, based on the total weight of
the
solid content in the suspension.
Furthermore, if said hydromagnesite is obtained from a magnesium oxide source
in
the form of magnesium oxide and/or a magnesium oxide containing mineral having
a
high content of magnesium oxide and/or a magnesium containing material having
a
high content of a magnesium compound which can be converted into magnesium
oxide, said process provides hydromagnesite particles having a specifically
decreased particle size. Preferably the obtained hydromagnesite particles have
an
average particle size d50 value in the range of less than 20 gm, preferably in
the range
of 0.1 gm to 15 gm, more preferably in the range of 0.5 gm to 10 gm, and most
preferably in the range of 1 gm to 5 gm, for example in the range between 4.75
gm
to 5 gm, as determined by laser diffraction using the instrument CILAS 920
particle-
size-analyzer of CILAS, Orleans, France. Additionally, by using the process of
the
present invention, the particles obtained are preferably of a platy-like
morphology.
Particles having the foregoing characteristics are preferably obtained if the
at least
one magnesium oxide source of step a) in the form of magnesium oxide and/or a
magnesium oxide containing mineral having a high content of magnesium oxide
and/or a magnesium containing material having a high content of a magnesium
44
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
compound which can be converted into magnesium oxide is selected from
magnesium oxide, periclase, magnesite, magnesium carbonate, magnesium
hydroxide and mixtures thereof.
In a preferred embodiment, particles having the foregoing characteristics are
preferably obtained if the at least one magnesium oxide source of step a) is
magnesite and most preferably magnesite in the form of caustic calcined
magnesite.
If said hydromagnesite is obtained from a magnesium oxide source in the form
of a
magnesium oxide containing mineral having a low content of magnesium oxide
and/or a magnesium containing material having a low content of a magnesium
compound which can be converted into magnesium oxide, the content of the
hydromagnesite in the resulting suspension is preferably in the range between
wt.-% and 70 wt.-%, based on the total weight of the solid content in the
15 suspension. In the case where the hydromagnesite is obtained from
dolomite, the
content of the hydromagnesite in the resulting suspension may be for example
in the
range between 30 wt.-% and 60 wt.-% and more preferably in the range between
35 wt.-% and 50 wt.-%, based on the total weight of the solid content in the
suspension.
Additionally, if the at least one magnesium oxide source provided in step a)
is
dolomite, it is to be noted that by using the process of the present
invention,
hydromagnesite is obtained in admixture with precipitated calcium carbonate,
wherein the obtained composition comprises hydromagnesite having a platy-like
morphology and precipitated calcium carbonate having a colloidal morphology.
In the case where hydromagnesite is obtained in admixture with precipitated
calcium
carbonate, the content of the precipitated calcium carbonate in the resulting
suspension may be for example in the range between 40 wt.-% and 70 wt.-% and
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
more preferably in the range between 50 wt.-% and 65 wt.-%, based on the total
weight of the solid content in the suspension.
Furthermore, by using the inventive process, it is possible to obtain
hydromagnesite
particles in admixture with other particles having a decreased particle size
if said
hydromagnesite is obtained from a magnesium oxide source in the form of a
magnesium oxide containing mineral having a low content of magnesium oxide
and/or a magnesium containing material having a low content of a magnesium
compound which can be converted into magnesium oxide. Said process preferably
provides hydromagnesite particles in admixture with other particles having an
average particle size d50 value in the range of up to 15 gm, preferably in the
range of
0.1 gm to 10 gm, more preferably in the range of 0.5 gm to 5 gm and most
preferably in the range of 1 gm to 4 gm, for example, in the range between
3.25 gm
and 3.5 gm, as determined by laser diffraction using the instrument CILAS 920
particle-size-analyzer of CILAS, Orleans, France. Particles having said
characteristics are preferably obtained if the at least one magnesium oxide
source of
step a) is selected from dolomite, huntite and mixtures thereof.
In a preferred embodiment, particles having the foregoing characteristics are
preferably obtained if the at least one magnesium oxide source of step a) is
dolomite
and most preferably dolomite in the form of burned dolomite and/or half-burned
dolomite.
In a preferred embodiment, the obtained hydromagnesite of the present
invention is
preferably in the form of a suspension, wherein the solid content can be
adjusted to
any solid content suitable for application in the paper, paint, rubber and
plastics
industries. In this respect, it is to be noted that the obtained
hydromagnesite can be
used directly without carrying out further treatment steps.
46
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
In one preferred embodiment, hydromagnesite in the form of a suspension has a
solid
content of up to 30 wt.-%, preferably between 1 wt.-% and 20 wt.-%, more
preferably between 5 wt.-% and 15 wt.-% and most preferably between 7 wt.-%
and
11 wt.-%, based on the total weight of the suspension.
In another preferred embodiment, said suspension preferably has a pH value in
the
range of 6 to 11, preferably a pH value of 7 to 10.5 and more preferably a pH
value
of 8,5 to 10.5. The viscosity is preferably less than 2.500 mPa.s, more
preferably less
than 2.000 mPa.s and most preferably less than 1.750 mPa.s, as measured with a
Brookfield DV-II Viscometer at a speed of 100 rpm and equipped with a LV-3
spindle.
In a preferred embodiment, the aqueous phase of the obtained hydromagnesite
suspension may be replaced with deionised water. In an optional embodiment,
the
obtained hydromagnesite suspension may be concentrated, optionally up to the
point
of obtaining a dry hydromagnesite product. If the aqueous suspension described
above is dried, the obtained solids (i.e. dry or containing as little water
that it is not in
a fluid form) of hydromagnesite may be in the form of granules or a powder. In
the
case of a dry product, this product may additionally be treated with fatty
acids during
and/or before and/or after drying. Said fatty acids are preferably selected
from stearic
acid, palmitic acid, behenic acid, montanic acid, capric acid, lauric acid,
myristic
acid, isostearic acid and cerotic acid.
The hydromagnesite obtained from magnesium oxide sources by the process of the
present invention are of distinct platy-like morphology in combination with
specifically decreased particle size and, thus, allows for easy and economic
applications in the paper, paint, rubber and plastics industries. The
particles of the
hydromagnesite obtained according to the present invention, have a particle
size
distribution, wherein the obtained particles have an average particle size d50
value in
the range of less than 20 gm, preferably of less than 15 gm, more preferably
of less
47
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
than 10 gm and most preferably of less than 5 gm. In a preferred embodiment,
the
process of the present invention may provide hydromagnesite particles having
an
average particle size d50 value of less than 20 gm, preferably in the range of
0.1 gm
to 15 gm, more preferably in the range of 0.5 gm to 10 gm, and most preferably
in
the range of 1 gm to 5 gm, for example in the range between 4.75 gm to 5 gm.
In
another preferred embodiment, the process of the present invention may provide
hydromagnesite particles having an average particle size d50 value in the
range of up
to 15 gm, preferably in the range of 0.1 gm to 10 gm, more preferably in the
range of
0.5 gm to 5 gm and most preferably in the range of 1 gm to 4 gm, for example,
in
the range between 3.25 gm and 3.5 gm, as determined by laser diffraction using
the
instrument CILAS 920 particle-size-analyzer of CILAS, Orleans, France.
In one preferred embodiment, the obtained hydromagnesite provides or shows an
absolute density of above 2.25 g/cm3, more preferably the density is between
2.26 g/cm3 and 2.40 g/cm3, even more preferably the density is between 2.26
g/cm3
and 2.35 g/cm3 and most preferably the density is between 2.26 g/cm3 and
2.32 g/cm3. For example, if the hydromagnesite is obtained from caustic
calcined
magnesite as the at least one magnesium oxide source provided in step a), the
density
of the hydromagnesite may be about 2.29 g/cm3.
In another preferred embodiment, the obtained hydromagnesite provides a
specific
BET surface area of 10 m2/g to 150 m2/g, more preferably 10 m2/g to 100 m2/g
and
most preferably 20 m2/g to 70 m2/g, as measured using nitrogen and the BET
method
according to ISO 9277.
For example, if the hydromagnesite is obtained from caustic calcined magnesite
as
the at least one magnesium oxide source provided in step a) said
hydromagnesite
preferably features a specific BET surface area of 10 m2/g to 70 m2/g, more
preferably of 20 m2/g to 50 m2/g and most preferably of 25 m2/g to 40 m2/g,
for
example of 30 m2/g to 35 m2/g, as measured using nitrogen and the BET method
48
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
according to ISO 9277. In the case where the hydromagnesite is obtained from
burned dolomite as the at least one magnesium oxide source provided in step
a), i.e.
the resulting suspension of step e) also comprises precipitated calcium
carbonate,
said composition preferably features a specific BET surface area of 40 m2/g to
100
m2/g, more preferably of 45 m2/g to 80 m2/g and most preferably of 50 m2/g to
70
m2/g, for example of 55 m2/g to 65 m2/g, as measured using nitrogen and the
BET
method according to ISO 9277.
In another preferred embodiment, the obtained hydromagnesite has a specific
BET
surface area within the range of 20 to 50 m2/g and the particles have an
average
particle size d50 value of less than 20 gm, preferably in the range of 0.1 gm
to 15 gm,
more preferably in the range of 0.5 gm to 10 gm, and most preferably in the
range of
1 gm to 5 gm, for example, in the range between 4.75 gm to 5 gm, as determined
by
laser diffraction using the instrument CILAS 920 particle-size-analyzer of
CILAS,
Orleans, France.
Even more preferably the specific BET surface area is within the range of 45
to
80 m2/g and the particles have a average particle size d50 value in the range
of up to
15 gm, preferably in the range of 0.1 gm to 10 gm, more preferably in the
range of
0.5 gm to 5 gm and most preferably in the range of 1 gm to 4 gm, for example,
in
the range between 3.25 gm and 3.5 gm, as determined by laser diffraction using
the
instrument CILAS 920 particle-size-analyzer of CILAS, Orleans, France.
Furthermore, it is preferred that the obtained hydromagnesite has a degree of
whiteness R457, measured in accordance with the ISO 2469 Standard, of at least
80 %, more preferably of at least 85 %, even more preferably of between 85 %
and
99 % and most preferably of between 85 % and 99 %. In another preferred
embodiment, the obtained hydromagnesite has a degree of whiteness R457,
measured in accordance with the ISO 2469 Standard, of at least 89 % and more
preferably between 89 % and 99 %. In a further preferred embodiment, the
obtained
49
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
hydromagnesite has a degree of whiteness R457, measured in accordance with the
ISO 2469 Standard, of at least 93 %. Additionally or alternatively, the
hydromagnesite obtained by the process of the process of the present invention
has a
Yellowness Index according to DIN 6167 of less than 5, more preferably of less
than
4 and most preferably of less than 3.
If the hydromagnesite is provided in the form of a suspension, said
hydromagnesite
is optionally dispersed. Conventional dispersants known to the skilled person
can be
used. The dispersant can be anionic or cationic. A preferred dispersant is one
based
on polyacrylic acid. Such dispersants are preferably dosed so as to account
for about
0.3 wt.-% to about 3 wt.-%, based on the total weight of said hydromagnesite.
The hydromagnesite thus obtained may be used in paper, tissue paper, plastics
or
paints. In particular, said hydromagnesite can be used as mineral filler
and/or for
coating of paper and in particular as mineral filler in paper wrappers for
smoking
articles. In particular, coating compositions and/or mineral filler
compositions
according to the invention are characterized in that they contain
hydromagnesite
obtained by the process of the present invention and in that they provide
improved
optical properties in comparison to compositions comprising hydromagnesite of
the
prior art. Papers and in particular paper wrapper for smoking articles
manufactured
and/or coated are characterized in that they contain said hydromagnesite
obtained by
the process of the present invention. As another advantage, the hydromagnesite
obtained by the process of the present invention can be used directly in a
paper
making application without the removal of, for example, impurities such as
other
salts or colored compounds. Furthermore, the obtained hydromagnesite may be
used
as flame-retardants having non-conductive properties and further functions as
electrical insulators. Such flame-retardants may be incorporated in electric
and
electronic parts, constructional materials, waste pipes, gutter, automobile
parts,
cabinets for televisions, computers and similar equipments, profiles and
fittings such
as fittings for cables, electric switches, sealants, plasters and paints.
Flame-retardants
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
comprising hydromagnesite may thus preferably used in building industry,
ships,
aircrafts, trains and vehicles.
The following examples may additionally illustrate the invention, but are not
meant
to restrict the invention to the exemplified embodiments. The examples below
show
the good optical properties such as opacity of the calcium carbonate
suspensions
according to the present invention:
EXAMPLES
Measurement methods
The following measurement methods are used to evaluate the parameters given in
the
examples and claims.
Brookfield viscosity
The Brookfield-viscosity of a slurry was determined with a Brookfield
Viscometer
type RVT equipped with a LV-3 spindle at a speed of 100 rpm and room
temperature
(20 3 C).
BET specific surface area of a material
The BET specific surface area is measured via the BET method according to ISO
4652 using nitrogen, following conditioning of the sample by heating at 250 C
for a
period of 30 minutes. Prior to such measurements, the sample is filtered,
rinsed and
dried at 110 C in an oven for at least 12 hours.
Particle size distribution (mass % particles with a size <X) and average
particle
size (d50) of a particulate material
The average particle size and the average particle size mass distribution of a
particulate material are determined via laser diffraction, i.e. the light from
a laser
51
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
passes though a suspension and the particle size distribution is calculated
from the
resulting diffraction pattern. The measurement is made with a CILAS 920
particle-
size-analyzer of CILAS, Orleans, France.
The method is well known to the skilled person and is commonly used to
determine
the particle size distribution of particulate materials. The measurement is
carried out
by diluting the corresponding suspension (deionised water; solution of 0.1 wt.-
% of
sodium pyrophosphate). The samples were dispersed using a high speed stirrer
and
ultrasonic.
pH of an aqueous suspension
The pH of the aqueous suspension is measured using a standard pH-meter at
approximately 22 C.
Density of solid particles
The density of the product is measured using the standard density analyzer
Micromeritics AccuPycO commercialized by Micromeritics.
Solids content of an aqueous suspension
The suspension solids content (also known as "dry weight") is determined using
a
Moisture Analyser HR73 commercialized by Mettler-Toledo with the following
settings: temperature of 120 C, automatic switch off 3, standard drying, 5-20
g of
suspension.
Comparative Example:
The following comparative example illustrates the preparation of
hydromagnesite by
a process of the prior art. Said process is carried out by slaking caustic
calcined
magnesite and contacting the obtained magnesium hydroxide with gaseous CO2 to
convert the obtained magnesium hydroxide into hydromagnesite, wherein the
carbonation is carried out at a starting temperature of about 60 C.
52
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
90 kg of caustic calcined magnesite (Van Mannekus M95) were slaked by adding
said magnesite to 1 700 litres of 40 C tap water in a stirred reactor. The
magnesite
was slaked for 15 min under continuous stirring and the resulting suspension
was
adjusted to about 5 % solids content via dilution with water. The carbonation
was
conducted in a 1 800 litre baffled cylindrical stainless steel reactor
equipped with a
gasing agitator, a stainless steel carbonation tube to direct a carbon
dioxide/air gas
stream to the impeller and probes for monitoring the pH and conductivity of
the
suspension. The suspension obtained in the slaking step was adjusted to a
temperature of 60 C and added to the carbonating reactor. A gas of 26 % by
volume
of CO2 in air was then bubbled upwards through the suspension at a rate of 200
m3/h
under a slurry agitation of 240 rpm. During the carbonation, the temperature
of the
reaction mixture was not controlled. After 85 minutes (calculated from start
of
introduction of said gas) the introduction of gas was stopped. The product was
recovered as an aqueous suspension. Characteristics and physical properties
are
given in column A of table 1.
Example 1:
The following illustrative example of the invention involves the preparation
of
hydromagnesite by slaking caustic calcined magnesite and contacting the
obtained
magnesium hydroxide with gaseous CO2 to convert the obtained magnesium
hydroxide into hydromagnesite, wherein the carbonation is carried out with a
starting
temperature of about 20 C to 25 C and a subsequent heat-ageing step.
90 kg of caustic calcined magnesite (Van Mannekus M95) were slaked by adding
said magnesite to 1 700 litres of 40 C tap water in a stirred reactor. The
magnesite
was slaked for 15 min under continuous stirring and the resulting suspension
was
adjusted to about 5 % solids content via dilution with water. The carbonation
was
conducted in a 1800 litre baffled cylindrical stainless steel reactor equipped
with a
gasing agitator, a stainless steel carbonation tube to direct a carbon
dioxide/air gas
53
CA 02778928 2012-04-25
WO 2011/054831 PCT/EP2010/066664
stream to the impeller and probes for monitoring the pH and conductivity of
the
suspension. The suspension obtained in the slaking step was adjusted to a
temperature of about 25 C and added to the carbonating reactor. A gas of 26 %
by
volume of CO2 in air was then bubbled upwards through the slurry at a rate of
200
m3/h under a slurry agitation of 240 rpm. During the carbonation, the
temperature of
the reaction mix was controlled and maintained between 20-25 C. After 85 min
(calculated from start of introduction of said gas) the introduction of gas
was
stopped. Immediately after carbonation, the resulting suspension was wet-
ground in a
vertical bead mill at a flow rate of 320 litres/h, resulting in a specific
grinding energy
consumption of about 100 kWh/dry ton. To the resulting suspension, 1,8 kg of
Formamidine Sulfinic Acid (DegaFAS from Degussa-Hills) was added. The slurry
was then transferred to a pressurized vessel and heated to about 130 C for 30
min.
The product was recovered as an aqueous suspension. Characteristics and
physical
properties are given in column B of table 1.
Example 2:
The following illustrative example of the invention involves the preparation
of
hydromagnesite by calcining and slaking white dolomite stone and contacting
the
obtained magnesium hydroxide with gaseous CO2 to convert the obtained
magnesium hydroxide into hydromagnesite, wherein the carbonation is carried
out
with a starting temperature of about 12 C and a subsequent ageing step.
White Dolomite stones (Hammerfall A/S) were crushed to yield a grain size of
10 ¨ 50 mm and calcined in a rotary kiln at 1050 C for 60 min. The resulting
burned
Dolomite (CaO = MgO) was ground in a ball mill to obtain a powder with mean
particle size of about 40 gm (CILAS laser diffraction method).
200 kg of said burned Dolomite were slaked by adding to 1 000 liters of 50 C
tap
water in a stirred reactor. The burned Dolomite was slaked for 30 min under
continuous stirring and the resulting suspension was adjusted to about 8 %
solids
content via dilution with water. The carbonation was conducted in a 1 800
litre
54
CA 02778928 2012-04-25
WO 2011/054831
PCT/EP2010/066664
baffled cylindrical stainless steel reactor equipped with a gasing agitator, a
stainless
steel carbonation tube to direct a carbon dioxide/air gas stream to the
impeller and
probes for monitoring the pH and conductivity of the suspension. 1 800 litres
of the
suspension obtained in the slaking step was adjusted to a temperature of 12 C
and
added to the carbonating reactor. A gas of 26 % by volume of CO2 in air was
then
bubbled upwards through the slurry at a rate of 200 m3/h under a slurry
agitation of
240 rpm. During the carbonation, the temperature of the reaction mix was not
controlled and allowed to rise due to heat generated in the exothermic
reaction. After
85 min (calculated from start of introduction of said gas) the introduction of
gas was
stopped. The suspension was then transferred to a pressurized vessel and
heated to
about 130 C for 30 min. The product was recovered as an aqueous slurry.
Characteristics and physical properties are given in column C of table 1.
column A B C
example comparative 1 2
specific surface area BET m2/g 42.6 34.4 55.9
PSD
avg. particle size clso
CILAS 920 [tm 14.34 4.9 3.39
brightness (DIN 53140)
R457 (ISO 2469) % 82.9 89.9 93.3
yellow index (DIN 6167) 7.4 0.5 1.1
solids content % 9.4 9.7 7.8
viscosity (Brookfield 100 rpm) mPas 30 1600 780
pH Slurry 7,3 9.4 10.2
mineralogical composition XRD
Hydromagnesite Mg5(CO3)4(OH)2.4(H20) % 90.8 98.3 35.7
Nesquehonite Mg(HCO3)(OH).2(H20) %
Calcite CaCO3 % 1.1 1.6 58.2
Dolomite CaMg[CO3]2 %
Brucite Mg(OH)2 % 3.1
Periclase MgO % 5 0.1
Table 1: physical data
As can be gathered from the data shown in table 1, the inventive method
especially
leads to products having a significantly lower particle size (average particle
size d50).