Note: Descriptions are shown in the official language in which they were submitted.
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
COPPER ALLOY ENCLOSURES
FIELD OF THE INVENTION
[0001] The present application relates to materials and methods for the
prevention of
biofouling, or the undesired accumulation of one or more organisms on a
surface. Marine
biofouling is commonplace in lakes, seas, oceans, bays, ponds, reservoirs,
estuaries and
rivers. Marine biofouling may involve any of a wide variety of organisms,
including animals,
plants, and microorganisms, such as, but not limited to, algae, seaweeds,
anemones, and
barnacles. Biofouling is most widespread in warmer waters with low velocity
water and high
nutrient content. However, biofouling can be problematic in cooler waters as
well as
nutrient-poor waters. Biofouling is detrimental to marine surfaces because it
increases drag
and weight, weakens the underlying materials, and, in some cases, harbors
toxins,
microorganisms, and viruses.
[0002] Biofouling has become a particular concern to commercial fisheries,
especially
those fisheries that rely on enclosures such as fish pens, lobster traps, and
crab traps, which
are exposed to seawater for long periods of time. In some cases the biofouling
is merely a
nuisance, requiring the traps to be cleaned regularly to prevent the build-up
of algaes and
slime, which degrade the materials. In other case, biofouling encourages the
growth of
organisms such as barnacles and algae on the harvested animals, resulting in a
less-
appealing products that fetch a lower market price. In still other cases,
biofouling can
provide a breeding ground for harboring and transmitting bacteria or viruses
that kill the
harvested animals, or make the harvested animals toxic to humans.
[0003] Enclosures that diminish biofouling may also pose a risk to the animals
that are
restrained within the enclosures, however. For example, high-copper content
alloys that
have innate antimicrobial properties have been shown to be effective at
diminishing
biofouling in marine environments. See, e.g., Huguenin, "The Advantages and
Limitations of
Using Copper Materials in Marine Aquaculture," IEEE Ocean '75, p. 444-453
(1975),
incorporated herein by reference in its entirety. However, these same alloys
may kill or
sicken the animals within. Shellfish and mollusks, such as lobsters, crabs,
crayfish, oysters,
scallops, clams, and mussels, are especially susceptible to harm from copper
poisonining
when placed in enclosures constructed from alloys having high copper content.
SUMMARY OF THE INVENTION
[0004] The invention provides, among other things, a welded wire mesh of a
silicon
bronze alloy comprising about 0.5% to about 3.8% silicon (wt/wt alloy) and
greater than
1
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
about 90% copper (wt/wt alloy). In some embodiments, the silicon bronze alloy
additionally
includes from about 0.05% to about 1.3% manganese (wt/wt alloy). The silicon
bronze may
additionally comprise a naturally-occurring silicon oxide coating. An
enclosure may be
constructed from the welded wire mesh of the invention.
[0005] The invention additionally provides, among other things, a method of
restraining a
marine animal with reduced biofouling, comprising restraining the marine
animal in an
enclosure comprising a welded wire mesh of a silicon bronze alloy, the silicon
bronze alloy
comprising about 0.5% to about 3.8% silicon (wt/wt alloy) and greater than
about 90%
copper (wt/wt alloy). In some embodiments, the silicon bronze alloy
additionally includes
from about 0.05% to about 1.3% manganese (wt/wt alloy). The silicon bronze may
additionally comprise a naturally-occurring silicon oxide coating. The marine
animal may be
a lobster, a crab, a crayfish, a shrimp, an oyster, a clam, a scallop, an eel,
or a fish.
[0006] The invention additionally provides, among other things, an antifouling
barrier
comprising a silicon bronze alloy including about 0.5% to about 3.8% silicon
(wt/wt alloy) and
greater than about 90% copper (wt/wt alloy). In some embodiments, the silicon
bronze alloy
additionally includes from about 0.05% to about 1.3% manganese (wt/wt alloy).
The silicon
bronze may further comprise a naturally-occurring silicon oxide coating. The
antifouling
barrier may be, but is not limited to, a screen, chain-link, chain-mail, grid,
weave, perforated
sheet, or chicken wire. Animal enclosures, comprising the barrier of the
invention, may be
constructed. Such enclosures include, but need not be limited to, nets, pens,
traps, kennels,
buckets, boxes, stalls, trays, and paddocks.
[0007] The invention additionally provides, among other things, a method of
reducing the
growth of an organism on an animal enclosure, comprising contacting at least a
portion of
the animal enclosure with an antifouling barrier comprising a silicon bronze
alloy comprising
about 0.5% to about 3.8% silicon (wt/wt alloy) and greater than about 90%
copper (wt/wt
alloy). In some embodiments, the silicon bronze alloy may additionally
comprise from about
0.05% to about 1.3% manganese (wt/wt alloy). The organism whose growth is
reduced may
be an animal, a plant, or a microorganism. In some embodiments, the growth of
Staphylococcus epidermidis, Escherichia coli, Navicula incerta, Cellulophaga
lytica,
Halomonas pacifica, Pseudoalteromonas atlantica, Cobetia marina, Clostridium
difficile, or
Listeria monocytogenes may be reduced. In some embodiments, the growth of
infectious
salmon anemia virus (ISAV), viral hemorrhagic septicemia (VHS), epizootic
hematopoietic
necrosis virus (EHNV), infectious hematopoietic necrosis virus (IHNV), or koi
herpes virus
may be reduced.
2
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
[0008] The invention additionally provides, among other things, a method for
reducing
the growth of an organism on a structure, comprising contacting at least a
portion of the
structure with an antifouling barrier comprising a silicon bronze alloy
comprising about 0.5%
to about 3.8% silicon (wt/wt alloy) and greater than about 90% copper (wt/wt
alloy). In some
embodiments, the silicon bronze alloy may additionally comprise from about
0.05% to about
1.3% manganese (wt/wt alloy). The silicon bronze may further comprise a
naturally-
occurring silicon oxide coating. The structure may be an offshore platform, a
seawall, a
piling, a pier, a wharf, a dock, or a buoy.
[0009] Other aspects of the invention will become apparent by consideration of
the
detailed description and accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] FIG. 1 shows an embodiment of a barrier comprising a silicon bronze
alloy.
[0011] FIG. 2 shows another embodiment of a barrier comprising a silicon
bronze alloy.
[0012] FIG. 3 shows another embodiment of a barrier comprising a silicon
bronze alloy.
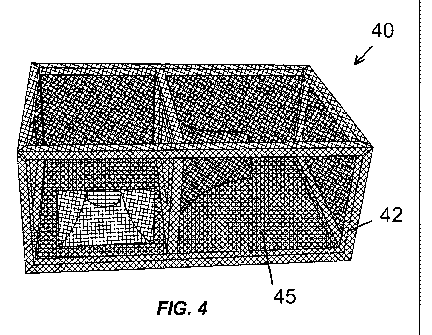
[0013] FIG. 4 shows a lobster trap comprising a silicon bronze alloy netting.
[0014] FIG. 5 shows a crab trap comprising a silicon bronze alloy weave.
[0015] FIG. 6 shows a fish pen comprising a silicon bronze alloy weave.
DETAILED DESCRIPTION
[0016] The invention provides copper alloys that are effective in reducing the
growth of
organisms that contact, or grow on, the alloys. Silicon bronze alloys,
typically having about
0.5% to about 3.8% silicon (wt/wt alloy) and greater than about 90 % copper
(wt/wt alloy),
are suitable for reducing the growth of organisms, especially animals, plants,
and
microorganisms. The silicon bronze alloys may additionally comprise about
0.05% to about
1.3% manganese (wt/wt alloy), as well as up to 1.5% zinc, up to 0.8% iron, 0.8
% lead, and
0.6% nickel. In many embodiments, the lead will be present in only trace
amounts (less than
0.05%). Some embodiments will comprise about 2.0% silicon, about 1.0%
manganese,
about 1.0% zinc, and about 96% copper.
[0017] High-copper content alloys have been touted for their antibacterial
activity, and
the U.S. EPA has recently certified several copper alloys as public health
antimicrobial
3
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
products. (See http://www.epa.gov/pesticides/factsheets/copper-alloy-
products.htm) While
the exact reasons for the antibacterial activity of high copper-content alloys
are unknown, it
is suspected that the predominantly copper surfaces disrupt the outer membrane
or cell wall
of an organism that contacts the alloy. When the cellular architecture is
disrupted, the
cytoplasm is compromised, in many cases resulting in the death of the cell.
Consequently, it
is difficult for biofilms or colonies of microorganisms to populate the high
copper-content
alloys.
[0018] Copper alloys having appreciable silicon content (i.e., silicon
bronzes) are known
to produce a protective silicon oxide layer upon exposure to water, and it was
believed that
the silicon oxide layer would limit the effectiveness of silicon bronze alloys
as anti-fouling
barriers. Recent testing has shown that silicon bronze alloys do maintain
their antibacterial
properties upon exposure to water, however. The mechanism is unknown, but it
is
hypothesized that the silicon oxide coating is semi-permeable to copper atoms
within the
allow. More surprisingly, silicon bronzes have been found to be generally
effective
antifouling agents, reducing the growth of a wide variety of organisms,
including animals,
plants, and microorganisms. In particular, silicon bronze alloys are effective
antifouling
agents when used in marine environments.
[0019] As discussed above, antifouling alloys can pose a risk to animals that
are
exposed to the alloys. Surprisingly, silicon bronze alloys, such as those
described in the
invention, do not appear to affect the health of animals restrained within an
enclosure made
from the silicon bronze, however. This property makes silicon bronze alloys of
the invention
suitable for constructing enclosures for shellfish and mollusks, among other
animals.
Additionally, the mechanical properties of the silicon bronze alloys
facilitates the fabrication
of barriers such as welded-wire mesh, screen, chain-link, chain-mail, grid,
weave, or chicken
wire. The silicon bronze alloys are also strong and stiff while exhibiting
good cold-worked
and hot-formed workability. Thus it is possible to make entire enclosures,
including, but not
limited to, nets, pens, traps, kennels, buckets, boxes, stalls, trays, and
paddocks from silicon
bronze alloys.
[0020] Other copper alloys may exhibit antifouling properties while not
harming an
animal restrained in an enclosure constructed from the copper alloy. These
alloys may
include tin bronzes having 1-8% (wt/wt) tin content and 0.3% to 0.35% (wt/wt)
phosphorus
content, and aluminum bronzes having 4-9% (wt/wt) aluminum content.
[0021] As used herein, animals include any species from the kingdoms Animalia
or
Metazoa. Animals may include, but need not be limited to, crustaceans, such as
barnacles
4
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
and sea lice, slugs, and anemone. Animals may also includes insects, such as
fleas, and
lice, as well as arachnids, such as ticks. As used herein, plants includes any
species from
the kingdom Plantae. Plants may include, but need not be limited to seaweeds,
algae,
mosses, and kelp. As used herein, microorganisms includes single-cell and
multi-cell
bacteria, fungi, parasites, protozoans, archaea, protests, amoeba, viruses,
diatoms, and
algae. Microorganisms whose growth may be inhibited by silicon bronze alloys
of the
invention include, but are not limited to, Staphylococcus aureus,
Staphylococcus
epidermidis,, Streptococcus faecalis, Bacillus subtilis, Salmonella
chloraesius, Salmonella
typhosa, Escherichia coli, Mycobacterium tuberculosis, Pseudomonas aeruginosa,
Aerobacter aerogenes Saccharomyces cerevisiae, Candida albicans, Aspergillus
niger,
Aspergillus flares, Aspergillus terreus, Aspergillus verrucaria, Aureobasidium
pullulans,
Chaetomium globosum, Penicillum funiculosum, Trichophyton interdigital,
Pullularia
pullulans, Trichoderm sp. madison P-42, and Cephaldascus fragans; Chrysophyta,
Oscillatoria borneti, Anabaena cylindrical, Selenastrum gracile, Pleurococcus
sp., Gonium
sp., Volvox sp., Klebsiella pneumoniae, Pseudomonas fluorescens, Proteus
mirabilis,
Enterobacteriaceae, Acinetobacter spp., Pseudomonas spp., Candida spp.,
Candida
tropicalis, Streptococcus salivarius, Rothia dentocariosa, Micrococcus lute
us, Sarcina lutea,
Salmonella typhimurium, Serratia marcescens, Candida utilis, Hansenula
anomala,
Kluyveromyces marxianus, Listeria monocytogenes, Serratia liquefasciens,
Micrococcus
lysodeikticus, Alicyclobacillus acidoterrestris, MRSA, Bacillus megaterium,
Desulfovibrio
sulfuricans, Streptococcus mutans, Cobetia marina, Enterobacter aerogenes,
Enterobacter
cloacae, Proteus vulgaris, Proteus mirabilis, Lactobacillus plantarum,
Halomonas pacifica,
Ulva linza, and Clostridium difficile. Additionally, silicon bronze alloys of
the invention my
inhibit the growth of viruses such as infectious salmon anemia virus (ISAV),
viral
hemorrhagic septicemia (VHS), epizootic hematopoietic necrosis virus (EHNV),
infectious
hematopoietic necrosis virus (IHNV), koi herpes virus, or avian flu virus.
Silicon bronze
alloys of the invention may reduce the growth of small colonies of
microorganisms, in
addition to reducing the growth of biofilms.
[0022] The biocidal properties of the silicon bronze alloys of the invention
lend
themselves to the fabrication and installation of barriers made from the
silicon bronze alloys.
The barriers may protect nearly any structure that would otherwise be at risk
for the growth
of organisms on the structure. Marine structures include, but need not be
limited to, offshore
platforms, seawalls, pilings, piers, wharfs, docks, or buoys. Other structures
suitable for
protection with a silicon bronze alloy of the invention can be found
throughout the globe,
including but not limited to structures within hospitals, homes, factories,
laboratories, food
processing facilities, farms, dairies, subways, airports, and bathrooms. For
example, silicon
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
bronze alloys of the invention may be used to reduce the growth of organisms
on knobs,
handles, rails, poles, countertops, sinks, faucets, urinals, dispensers, pots,
pans, and
utensils.
[0023] Barriers formed from silicon bronze alloys of the invention may be of
any suitable
shape depending upon the mechanical needs (e.g., strength, flow-through,
weight, etc.) of
the associated structure. Barriers of the invention may suitably be formed
from sheets,
strips, wires, plates, rods, bars, ingots, or tubes of the alloy. The barriers
may be formed
using any suitable mechanical process including, but not limited to, rolling,
welding, drawing,
twisting, extruding, machining, lathing, stamping, pulling, or cutting. The
final barrier may be
a welded-wire mesh, sheet, tube, screen, chain-link, chain-mail, grid, weave,
perforated
sheet, or chicken wire, however other structures would be within the purview
of one of skill in
the art. As shown in FIG. 1, silicon bronze alloy wire may be formed into a
chain link, which,
depending upon the gauge of the wire, will have some amount of flexibility. As
shown in
FIG. 2, silicon bronze alloy wire may be formed into a mesh or weave, which
may have a
varying amount of open space between the wires, depending upon the end
application. In
some embodiments, intersections 20 between wires may be mechanically fixed,
e.g., with
welding, lashing, or fasteners. The arrangement shown in FIG. 2 with welded
intersections
20 may be described as a welded-wire mesh. Intersections 20 may be resistively
welded,
oxyacetylene welded, arc welded, soldered, or brazed. In other embodiments,
the woven
wires are capable of freely moving past one another. As shown in FIG. 3,
silicon bronze
alloy wire may also be formed into a repeating hexagonal structure, also known
as chicken
wire. Other open barrier structures may also be used, including chain mail or
ring mail.
[0024] Different barrier structures offer differing degrees of rigidity in the
ultimate barrier.
For example a weave of thicker wire may be directly bent or formed to form
structures such
as a box, a trap or a pen. In contrast, flexible materials, such as mails may
be useful as
netting. In some embodiments, more rigid barrier materials may not need
additional
structural support, however, in other embodiments less rigid barriers may need
additional
support, e.g., a frame. The overall resistance to corrosion of the barrier may
depend upon
the physical structure and use, however, as repeated abrasion may remove the
protective
silicon oxide layer, allowing for corrosion of the underlying alloy.
Typically, copper alloys
fabricated into relatively inflexible forms such as expanded metal and welded
mesh can
withstand repetitive motions over a prolonged time better than flexible forms,
such as chain
link or woven mesh.
6
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
[0025] In addition to barriers formed from the silicon bronze alloys,
antifouling protection
may be provided by coatings comprising silicon bronze alloys of the
inventions. Such
coatings may be coated onto metal, rock, cement, plastic, glass, or ceramic to
reduce the
growth of organisms on those surfaces. The coatings may be applied with
electrospray.
Antifouling protection may also be provided by incorporating microscopic or
nanoscopic
particles of silicon bronze alloys into plastics, glasses, paints or fabrics.
[0026] The silicon bronze barriers of the invention may be used to construct a
variety of
animal enclosures, including, but not limited to, nets, pens, traps, kennels,
buckets, trays,
boxes, stalls, and paddocks. Animals suitable to be placed in the enclosures
include any
wild or domesticated animal that may be captured, harvested, raised, or bred
for human
benefit. (In the case of traps, the animals place themselves in the
enclosures.) Once the
animals are placed or trapped in the enclosures, the animals are considered to
be
restrained. Animals suitable to be placed in enclosures include, but need not
be limited to
fish, eels, lobsters, crab, shrimp, crawfish, mussels, clams, oysters,
scallops, rabbits,
chickens, turkeys, ferrets, guinea pigs, hamsters, mice, rats, cows, horses,
pigs, goats,
sheep, deer, dogs, cats, and birds.
[0027] Because of the biocidal activity of the silicon bronze barriers of the
invention,
animal enclosures comprising silicon bronze barriers offer increased
resistance to biofouling,
including the growth of animals, plants, or microorganisms on the enclosure.
Biofouling is
known to increase the risk of disease transmission, especially from bacteria
and viruses, and
may result in harvested or domesticated animals that are sick, unproductive,
or unappealing.
For example, the prevalence of infectious salmon anemia virus (ISAV) in farmed
Atlantic
salmon has been linked to biofouling of fish pens. ISAV is characterized by
high mortality
with exophthalmia, pale gills, ascites, hemorrhagic liver necrosis, renal
interstitial
hemorrhage and tubular nephritis. ISAV is known to cause overt and fatal
systemic infection
in farmed Atlantic salmon and asymptomatic infection in feral fish, a
situation analogous to
that caused by avian influenza viruses in domestic poultry and feral birds.
Once a fish pen
has been exposed to an ISAV outbreak, it may be necessary to destroy or
sanitize the fish
pen to avoid spreading the virus to subsequent populations of fish. Using the
silicon bronze
alloys of the invention, however, a fish farmer experiencing an outbreak of
ISAV need only
remove the infected stock from the pen and restock the pen to resume farming.
[0028] The combination of biocidal activity and corrosion resistance will make
silicon
bronze alloys of the invention excellent materials for the construction of
marine enclosures
such as fish pens and traps. As shown in FIG. 4, lobster trap 40 may be
constructed of
7
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
silicon bronze frame 42 and silicon bronze mesh 45. Mechanically, lobster trap
40 is
identical to lobster traps known in the industry, and will be equally
effective in trapping
lobsters. The use of silicon bronze alloys results in lobster trap 40 having
less biofouling and
superior corrosion resistance to other traps known in the industry, however.
While not
shown, suitable lobster traps can also be constructed from welded wire mesh of
copper
alloys. As shown in FIG. 5, crab trap 50 may be constructed of silicon bronze
frame 52 and
silicon bronze mesh 55. Mechanically, crab trap 50 is identical to crab traps
known in the
industry, and will be equally effective in trapping crabs. The use of silicon
bronze alloys
results in crab trap 50 having less biofouling and superior corrosion
resistance to other traps
known in the industry, however.
[0029] Silicon bronze alloys may also be used to construct any of a number of
enclosures for farmed fish. In one embodiment, illustrated in FIG. 6, a fish
pen is made in
the shape of a geodesic dome. Geodesic fish pen 60 may be constructed from
polyethylene
supports 62, which provide a rigid structure and some buoyancy. (Additional
buoyancy may
be provided by floats 63 as needed). The fish are retained within geodesic
fish pen 60 by
silicon bronze ring mail 65 which serves the dual purpose of keeping the
farmed fish in and
keeping predators (e.g., sharks) out. Geodesic fish pen 60 is tethered with
lines 67 to
anchor 69, which is connected to a weight on the seabed (not shown). Geodesic
fish pen 60
is particularly well suited for cultivation of larger ocean-borne fish.
[0030] The copper alloys of the invention additionally provide a method for
reducing the
growth of an organism on a structure, comprising contacting at least a portion
of the
structure with an antifouling barrier comprising a silicon bronze alloy
comprising about 0.5%
to about 3.8% silicon (wt/wt alloy) and greater than about 90% copper (wt/wt
alloy). In some
embodiments, the silicon bronze alloy comprises about 2.0% to about 3.5%
silicon (wt/wt
alloy). In some embodiments, the silicon bronze alloy additionally comprises
from about
0.05% to about 1.3% manganese (wt/wt alloy). In some embodiments, the silicon
bronze
alloy composition is (wt/wt alloy): 0.5 - 3.8% silicon; 0.05 - 1.3% manganese;
maximum
1.5% zinc; maximum 0.8% iron; maximum 0.8% lead; maximum 0.6% nickel; and
balance
copper. In some embodiments, the silicon bronze alloy additionally comprises a
silicon-
oxide coating. When used to reduce the growth of an organism on a structure,
the alloys are
effective in reducing the growth of organisms such as barnacles, algae,
seaweed, and kelp.
Structures suitable for having the growth of organisms reduced include
offshore platforms,
seawalls, pilings, piers, wharfs, docks, and buoys.
8
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
[0031] It is to be understood that the invention is not limited in its
application to the
details of construction and the arrangement of components set forth in the
following
description. The invention is capable of other embodiments and of being
practiced or of
being carried out in various ways. Also it is to be understood that the
phraseology and
terminology used herein is for the purpose of description and should not be
regarded as
limiting.
[0032] Recitation of ranges of values herein are merely intended to serve as a
shorthand
method of referring individually to each separate value falling within the
range, unless
otherwise indicated herein, and each separate value is incorporated into the
specification as
if it were individually recited herein. All methods described herein can be
performed in any
suitable order unless otherwise indicated herein or otherwise clearly
contradicted by context.
The use of any and all examples, or exemplary language (e.g., "such as")
provided herein, is
intended merely to better illuminate the invention and does not pose a
limitation on the
scope of the invention unless otherwise claimed. No language in the
specification should be
construed as indicating any nonclaimed element as essential to the practice of
the invention.
[0033] It also is understood that any numerical range recited herein includes
all values
from the lower value to the upper value. For example, if a concentration range
is stated as
1 % to 50%, it is intended that values such as 2% to 40%, 10% to 30%, or 1 %
to 3%, etc.,
are expressly enumerated in this specification. These are only examples of
what is
specifically intended, and all possible combinations of numerical values
between and
including the lowest value and the highest value enumerated are to be
considered to be
expressly stated in this application.
[0034] Further, no admission is made that any reference, including any patent
or patent
document, cited in this specification constitutes prior art. In particular, it
will be understood
that, unless otherwise stated, reference to any document herein does not
constitute an
admission that any of these documents forms part of the common general
knowledge in the
art in the United States or in any other country. Any discussion of the
references states what
their authors assert, and the applicant reserves the right to challenge the
accuracy and
pertinency of any of the documents cited herein.
PROPHETIC EXAMPLES
[0035] EXAMPLE 1 - Silicon Bronze wire weave
9
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
[0036] Silicon bronze alloy wire having a diameter of 4 mm, and having the
following
composition will be obtained from a commercial source (Luvata Appleton, LLC,
Kimberly,
WI).
[0037] Composition of silicon bronze alloy.
Element % composition wt/wt alloy)
Silicon 3.0
Manganese 1.0
Copper Balance
(The silicon bronze alloy may have trace amounts of lead, iron, zinc, and
nickel.) The 4 mm
wire will be fabricated into a mesh similar to FIG. 2, with spot welding at
the junctions of the
wire.
[0038] The silicon bronze wire weave will be submerged in Atlantic Ocean water
off the
coast of Massachusetts for three months, with weekly observation to quantify
(observable)
corrosion and biofouling. As a control, a 4 mm welded galvanized steel mesh,
and a 4 mm
welded 90/10 copper/nickel alloy mesh also will be submerged in nearby water,
and
observed on the same schedule. The weaves will not be cleaned until the end of
the trial.
[0039] Within weeks of placement in the water, both the 90/10 copper/nickel
alloy and
galvanized materials will have developed a thin coating of algae. The silicon
bronze alloy,
however, will not develop an algae coating until about one month into the
trial. With time,
the galvanized sample will grow a thick coating of algae with stingy
attachments. The 90/10
copper nickel alloy will stabilize after about one month, but will
consistently have more algae
than the silicon bronze alloy.
[0040] After three months, the weaves will be removed from the ocean and
cleaned with
high-pressure fresh water. The degree of corrosion will then be quantified.
The silicon
bronze formulation will show a build-up of a silicon oxide layer, resulting in
a material that is
duller in luster than the original alloy, but otherwise, there will be no
other outward sign of
corrosion. Similar to the silicon bronze alloy, the 90/10 copper/nickel alloy
will have less
luster, but will not show appreciable corrosion. The galvanized steel weave,
however, will
show discoloration and pitting across the surface and of the metal, as well as
rust at the
junctions of the wire.
[0041] EXAMPLE 2 -Crab pens of welded wire silicon bronze alloy and 90/10
copper
nickel alloy.
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
[0042] Two 30 cm x 100 cm x 100 cm crab pens will be fabricated. Pen 1 will be
fabricated from welded wire silicon bronze alloy using the alloy of EXAMPLE 1.
Pen 2 will
be fabricated from a 90/10 copper nickel alloy with welding. Both pens will
have 1.5 cm
spacing between wires, and be of identical construction save the alloy
composition. Five
Lake Pontchartrain Blue Crabs (Callinectes sapidus) will be placed in each
pen, and the
pens will be placed in approximately 2 meters of water in Lake Pontchartrain
(Louisiana,
U.S.A.) for two months for observation. The crabs will be able to feed on
their normal diet.
After about three weeks, the crabs in Pen 2 (90/10 copper nickel) will begin
to die, with all of
the crabs in Pen 2 dead by week five. All five crabs in Pen 1 (silicon bronze
alloy) will be
alive and healthy at the end of the two month trial, and at least one of the
crabs will have
molted.
[0043] EXAMPLE 3 - Lobster Trap with Silicon Bronze wire weave
[0044] Five standard Atlantic Lobster wire-type traps, will be purchased from
a
commercial trap supplier (e.g., Rainbow Net Rigging, Ltd., Dartmouth, Nova
Scotia) and the
polyvinyl-coated steel mesh will be replaced with silicon bronze wire mesh
from EXAMPLE
1. The test traps will have identically sized kitchens and parlors and use the
same bait bags.
The traps will be placed in service with 200 standard polyvinyl-coated steel
weave lobster
traps from the same manufacturer. After two months of service, the silicon
bronze traps will
be notably less fouled than the polyvinyl-coated steel weave traps.
Additionally, the lobsters
harvested from the silicon bronze traps will have fewer shell blemishes and
appear healthier
upon harvest.
[0045] EXAMPLE 4 - Crab Trap with Silicon Bronze wire weave
[0046] Ten standard Alaskan King crab traps, similar to FIG. 4, will be
purchased from a
commercial trap supplier (e.g., Dungeness Gear Works, Everitt, WA) and the
polyvinyl-
coated steel chain-link will be replaced with silicon bronze chain-link formed
from the alloy of
EXAMPLE 1. The traps will be placed in service with 500 standard polyvinyl-
coated steel
weave crab traps from the same manufacturer. After a season of service, the
silicon bronze
traps will be notably less fouled than the polyvinyl-coated steel weave traps.
Additionally,
the crabs harvested from the silicon bronze traps will have fewer shell
blemishes and appear
healthier upon harvest.
[0047] EXAMPLE 5 - Fish pen with Silicon Bronze chain mail
[0048] A geodesic dome fish pen, similar to FIG. 6, will be constructed using
a silicon
bronze chain mail having the same composition as the silicon bronze weave of
EXAMPLE 1.
11
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
The geodesic fish pen will have a frame constructed of reinforced
polyethylene, and the
silicon bronze chain mail will be fastened to the frame using silicon bronze
wire. The
completed pen will have a volumetric capacity of 1000 cubic meters.
[0049] The pen will be anchored in approximately 50 feet of water in a
protected bay in
Hawaii, U.S.A. The pen will have approximately 2000 small mahi mahi
(Coryphaena
hippurus) placed inside the pen. The fish will be regularly fed hydraulically
from a feed boat
via a hose linkage, allowing for the transfer of water-borne squid and smaller
fish. After eight
months, the mahi-mahi will be harvested by removing the pen from the ocean.
Upon
removal, there will be little biofouling of the silicon bronze chain mail, and
the fish will be
healthy and marketable.
[0050] EXAMPLE 6 - Survival rates for Clostridium difficile on silicon bronze
surface
[0051] A 10 mm x 10 mm sample of the alloy of EXAMPLE 1 ("sample") will be cut
from
3 mm thick sheet stock. The sample will be degreased and cleaned by vortexing
the sample
in acetone along with 2 mm glass beads and then immersing the sample in 200
proof
ethanol. Prior to testing, excess ethanol will be burned off with a Bunsen
burner. As a
control, a 10 mm x 10 mm piece of 3 mm thick stainless steel ("control") will
also be
degreased and immersed in ethanol, and the excess ethanol burned off.
[0052] Clostridium difficile on glycerol protected beads (Fisher Scientific)
will be
incubated anaerobically with brain heart infusion broth (Oxoid) at 37 C for 3-
5 days to
produce a culture of vegetative cells and spores for testing. Both the control
and sample will
have 20 pL of the Clostridium difficile culture pipetted onto their respective
surfaces, and the
control and sample will be incubated at room temperature for 2 hours. After
two hours of
incubation, 20 pL of a 5mM solution of CTC (5-Cyano-2,3-ditolyl tetrazolium
chloride; Sigma-
Aldrich) will be deposited on the sample and the control, and the sample and
control will be
incubated in a dark, humid chamber for at 37 C for 8 hours.
[0053] After rinsing the sample and control with sterile DI water to remove
excess CTC
stain, the sample and control will be imaged using epifluorescent microscopy,
and a series of
field views will be collected with a digital camera. A count of cells or
spores in these field
views will show that after two hours of incubation, the control sample had a
great number of
metabolically active cells or spore (e.g., CTC-stained) while the sample had
less than 1% of
the metabolically active cells or spores that were found on the control. The
data will thus
confirm that the alloy of EXAMPLE 1 kills at least 99% of Clostridium
difficile within two
hours.
12
CA 02779604 2012-05-01
WO 2011/065939 PCT/US2009/065773
[0054] EXAMPLE 7 - Survival rates for Listeria monocytogenes on silicon bronze
surface
[0055] As in EXAMPLE 4, a 10 mm x 10 mm sample of the alloy of EXAMPLE 1
("sample") will be cut from 3 mm thick sheet stock. The sample will be
degreased and
cleaned by vortexing the sample in acetone along with 2 mm glass beads and
then
immersing the sample in 200 proof ethanol. Prior to testing, excess ethanol
will be burned
off with a Bunsen burner. As a control, a 10 mm x 10 mm piece of 3 mm thick
stainless
steel ("control") will also be degreased and immersed in ethanol, and the
excess ethanol
burned off.
[0056] Listeria monocytogenes Scott A from previously frozen microbeads
(Centre for
Applied Microbiology Research, Porton Down, UK) will be incubated with brain
heart infusion
broth (Oxoid) at 37 C for 15-20 hours to produce an active culture for
testing. Both the
control and sample will have 20 pL of the Listeria monocytogenes culture
pipetted onto their
respective surfaces, and the control and sample will be incubated at room
temperature for 2
hours. After two hours of incubation, 20 pL of a 5mM solution of CTC (5-Cyano-
2,3-ditolyl
tetrazolium chloride; Sigma-Aldrich) will be deposited on the sample and the
control, and the
sample and control will be incubated in a dark, humid chamber for at 37 C for
2 hours.
[0057] After rinsing the sample and control with sterile DI water to remove
excess CTC
stain, the sample and control will be imaged using epifluorescent microscopy,
and a series of
field views will be collected with a digital camera. A count of cells or in
these field views will
show that after two hours of incubation, the control sample had a great number
of
metabolically active cells (e.g., CTC-stained) while the sample had less than
1% of the
metabolically active cells that were found on the control. The data will thus
confirm that the
alloy of EXAMPLE 1 kills at least 99% of Listeria monocytogenes within two
hours.
[0058] Thus, the invention provides, among other things, barriers comprising
silicon
bronze alloys and animal enclosures incorporating the barriers. Various
features and
advantages of the invention are set forth in the following claims.
13