Note: Descriptions are shown in the official language in which they were submitted.
WO 2011/068488 PCT/US2009/006320
-1-
IMPROVED SELECTIVE CRACKED NAPHTHA DESULFURIZATION
USING ARSENIC TRAP CATALYSTS
FIELD OF THE INVENTION
[0001] This invention provides a process for the manufacture of a naphtha
boiling range product with improved properties.
BACKGROUND OF THE INVENTION
[0002] One conventional technique for processing of cracked naphthas
involves performing a selective hydrodesulfurization of the cracked naphtha. A
selective hydrodesulfurization refers to a process where sulfur is removed
from
the naphtha while minimizing the amount of olefin saturation that occurs in
the
reaction. Avoiding olefin saturation can be valuable, as it leads to a higher
octane naphtha product. Retaining a higher octane value allows a selectively
hydrodesulfurized feed to be used as a naphtha fuel stock without having to
use a
reforming step.
[0003] One type of naphtha feed with a suitable octane rating for use
without reforming is a naphtha feed produced by a fluid catalytic cracking
(FCC)
process. FCC naphtha feeds can contain a substantial amount of olefins, making
a selective hydrodesulfurization process an attractive option. However,
depending on the type of feed provided to the FCC process, the resulting FCC
naphtha feed can also contain substantial amounts of arsenic. Arsenic is a
known catalyst poison for many hydrodesulfurization catalysts.
[0004] Arsenic trap catalysts are commercially available for mitigating the
effects of arsenic in a feed. In the present invention, such arsenic trap
catalysts
can be loaded at or near the top of the catalyst bed(s) for a
hydrodesulfurization
process. The arsenic trap catalyst can function to sequester arsenic from the
feed, thereby reducing or even preventing the arsenic from reaching, and
subsequently poisoning the hydrodesulfurization catalyst.
WO 2011/068488 PCT/US2009/006320
-2-
SUMMARY OF THE INVENTION
[00051 One aspect of the invention relates to a method for selectively
hydrotreating a naphtha boiling range feed that includes providing a naphtha
boiling range feed containing at least about 5 wt% olefins and at least about
1
ppb of arsenic. A run length and product sulfur content for the selective
hydrodesulfurization process can then be identified. Additionally, first
effective
selective hydrodesulfurization conditions can be determined for selectively
hydrodesulfurizing the naphtha boiling range feed in the presence of a first
volume of hydrodesulfurization catalyst, with the first effective conditions
including a first start of run catalyst bed temperature and a first space
velocity.
The naphtha boiling range feed can then be contacted with an arsenic trap
catalyst. This can be followed by contacting the naphtha boiling range feed
with
a second volume of hydrodesulfurization catalyst that is about 95% or less of
the
first volume under the second effective selective hydrodesulfurization
conditions, with the second effective selective hydrodesulfurization
conditions
including (i) a second start of run catalyst bed temperature that is at least
about
1.5 C higher than the first start of run catalyst bed temperature and (ii) a
second
space velocity that is greater than the first space velocity. In this aspect
of the
invention, the naphtha boiling range feed can contact the arsenic trap
catalyst
prior to contacting the second volume of hydrodesulfurization catalyst. The
contacting of the naphtha boiling range feed with the arsenic trap catalyst
and
the second volume of hydrodesulfurization catalyst can be continued for the
identified run length while maintaining the identified product sulfur content
in
the hydrodesulfurized naphtha feed.
BRIEF DESCRIPTION OF THE FIGURES
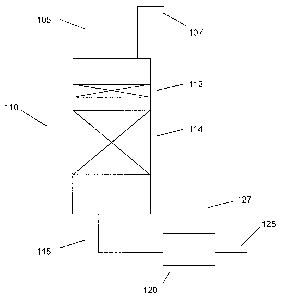
[00061 FIG. 1 schematically shows an example of a reactor suitable for
performing an embodiment of the invention.
WO 2011/068488 PCT/US2009/006320
-3-
DETAILED DESCRIPTION OF THE EMBODIMENTS
[00071 In an embodiment, a low cost process is provided for producing
naphtha boiling range products with at least comparable, and preferably
improved, octane while achieving a desired process run length. The improved
octane preservation can be achieved by using a combination of a reduced
hydrodesulfurization catalyst load with a sufficient load of As trap catalyst
to
match a desired run length. This can allow the hydrodesulfurization reactor to
be operated at a higher initial temperature, which can enhance octane
preservation during the early portions of the run length for a
hydrodesulfurization process.
[00081 In a selective hydrodesulfurization process, a variety of
considerations can be balanced in order to choose the size of the catalyst
load
and the processing temperature. It can often be desirable to remove sulfur to
a
level that corresponds to the current requirements for low sulfur fuels. For
example, production of a naphtha product with about 15 wppm or less, for
example about 10 wppm or less, of sulfur is often desirable. Another
consideration can include maintaining the activity of the catalyst. Typically,
a
catalyst should deactivate more quickly during higher temperature operation.
Thus, lower operating temperatures can be preferred, particularly during the
initial processing period after new catalyst has been added to a
hydroprocessing
reactor. Still another consideration can include preservation of olefins in
the
resulting naphtha product. Often, processing a feed at a temperature that is
higher than necessary to meet a desired sulfur specification can result in
additional saturation of olefins. This consideration would tend to suggest
that
lower reaction temperatures are preferable, to avoid overprocessing of a feed.
However, the selectivity of a catalyst can increase with increasing
temperature.
Here, selectivity refers to the relative activity for hydrodesulfurization
versus
activity for olefin saturation. Thus, there are factors that can favor both
lower
and higher temperature processing.
WO 2011/068488 PCT/US2009/006320
-4-
[0009] Contaminants in a feed can provide another set of challenges for
consideration. A catalyst poison such as arsenic can reduce the activity of a
hydrodesulfurization catalyst during the course of a hydrodesulfurization
process. The arsenic can often cause the catalyst to deactivate at a much
faster
rate than would typically be expected. One method to combat this deactivation
includes increasing the overall catalyst load. Due to practical
considerations,
end of run temperatures above about 800 F (about 427 C) are typically not
preferred, and preferably the end of run temperature can be less than about
675 F (about 357 C). Increasing the amount of hydrodesulfurization catalyst
can reduce the temperature required to effectively hydrodesulfurize a given
flow
rate of a naphtha feed. By increasing the amount of hydrodesulfurization
catalyst, a portion of the catalyst can be deactivated while still leaving
sufficient
higher activity catalyst to stay below a desired temperature for a desired run
length.
[0010] Conventionally, increased catalyst loads have been used in
conjunction with arsenic trap catalysts. An arsenic trap catalyst can be
loaded
into a catalyst bed so that the feed contacts the arsenic trap catalyst prior
to
contacting the hydrodesulfurization catalyst(s) in the reactor. Without
wishing
to be bound by theory, it is believed that the hydrodesulfurization catalyst
binds
with the arsenic, thus reducing the amount of arsenic from reaching the
hydrodesulfurization catalyst and extending the run length for a reactor, as
the
hydrodesulfurization catalyst would undergo only typical deactivation from
processing, and not more rapid deactivation due to the presence of arsenic.
[0011] In contrast to a conventional use of an arsenic trap catalyst, various
embodiments of the invention make use of an arsenic trap catalyst to maintain,
and preferably enhance, the octane value of the desulfurized naphtha product.
This can be achieved by reducing the amount of hydrodesulfurization catalyst
used. By using less catalyst, the start of run temperature for the reaction
can be
increased, which can allow for greater octane retention. In an embodiment, use
WO 2011/068488 PCT/US2009/006320
-5-
of an arsenic trap catalyst can allow the volume of hydrodesulfurization
catalyst
to be reduced to about 95% or less of the volume required without the arsenic
trap catalyst, for example to about 90% or less or to about 85% or less. By
reducing the volume of hydrodesulfurization catalyst, the corresponding space
velocity for feed contacting the hydrodesulfurization catalyst can be
increased
while still processing a similar flow rate of feed. In an embodiment, use of
an
arsenic trap catalyst can allow a space velocity that is greater than the
space
velocity without the use of an arsenic trap catalyst. Preferably, the space
velocity with the arsenic trap catalyst can be at least about 105% of the
space
velocity without the arsenic trap catalyst, for example at least about 110% of
the
space velocity without the arsenic trap catalyst.
Feedstocks
[00121 In various embodiments, the feedstock for a selective
hydrodesulfurization process can be a naphtha boiling range feed, particularly
an
olefinic naphtha boiling range feed. Suitable feedstocks can typically boil in
the
range from about 50 F (about 10 C) to about 450 F (about 232 C). With regard
to olefin content, suitable feedstocks can advantageously include feedstocks
having an olefin content of at least about 5 wt%. Non-limiting examples of
such
suitable feedstocks can include, but are by no means limited to, fluid
catalytic
cracking unit naphtha (FCC catalytic naphtha or cat naphtha), steam cracked
naphtha, coker naphtha, or a combination thereof. Also included are blends of
olefinic naphthas with non-olefinic naphthas, so long as the blend has an
olefin
content of at least about 5 wt%.
[00131 Olefinic naphtha refinery streams generally contain not only
paraffins, naphthenes, and aromatics, but also unsaturates, such as open-chain
and cyclic olefins, dienes, and cyclic hydrocarbons with olefinic side chains.
The olefinic naphtha feedstock can contain an overall olefins concentration of
about 60 wt% or less, for example about 50 wt% or less or about 40 wt% or
less.
Additionally or alternately, the olefin concentration can be at least about 5
wt%,
WO 2011/068488 PCT/US2009/006320
-6-
for example at least about 10 wt% or at least about 20 wt%. The olefinic
naphtha feedstock can also have a diene concentration up to about 15 wt%, but
more typically less than about 5 wt%, based on the total weight of the
feedstock.
High diene concentrations are typically undesirable, since they can result in
a
gasoline product having poor stability and color.
100141 The sulfur content of the olefinic naphtha can be at least about 100
wppm, for example at least about 500 wppm, at least about 1000 wppm, or at
least about 1500 wppm. Additionally or alternately, the sulfur content can be
about 7000 wppm or less, for example about 6000 wppm or less, about 5000
wppm or less, or about 3000 wppm or less. The sulfur can typically be present
as organically bound sulfur, i.e., as sulfur compounds such as simple
aliphatic,
naphthenic, and aromatic mercaptans, sulfides, di- and poly- sulfides, and the
like. Other organically bound sulfur compounds can include the class of
heterocyclic sulfur compounds such as thiophene and its higher
homologs/analogs (including dibenzodithiophene et al.).
[00151 Nitrogen can also be present in the feed. In an embodiment, the
amount of nitrogen can be at least about 5 wppm, for example at least about 10
wppm, at least about 20 wppm, or at least about 40 wppm. Additionally or
alternately, the nitrogen content can be about 250 wppm or less, for example
about 150 wppm or less, about 100 wppm or less, or about 50 wppm or less.
[00161 Arsenic can also be present in the feed. In an embodiment, the
amount of arsenic can be at least about I wppb, for example at least about 5
wppb, at least about 10 wppb, at least about 20 wppb, or at least about 40
wppb.
Additionally or alternately, the arsenic content can be about 100 wppb or
less,
for example about 75 wppb or less or about 50 wppb or less.
WO 2011/068488 PCT/US2009/006320
-7-
Catalysts
[0017] A selective hydrodesulfurization can be performed by exposing an
olefinic naphtha feed to one or more beds of hydrodesulfurization catalyst
under
effective selective hydrodesulfurization conditions. In an embodiment, an
arsenic trap catalyst can be used in a separate bed, such as a bed that is
upstream
of the hydrodesulfurization catalyst bed(s), or the arsenic trap catalyst can
be
loaded into the top of a bed that also includes a hydrodesulfurization
catalyst.
[0018] Typically, arsenic trap catalysts are catalysts with sufficient
activity
to sequester (adsorb) arsenic, but with otherwise a relatively low catalytic
activity that has a reduced or minimal impact on the desired reaction, such as
hydrodesulfurization. Typical arsenic trap catalysts can be relatively low
activity supported nickel-based catalysts. For example, a catalyst could
include
from about 5 wt% to about 20 wt% of Ni on an alumina support. A
commercially available example of such an arsenic trap catalyst includes TK-
47,
which is commercially available from Haldor Topsoe.
[0019] In an embodiment, the amount of arsenic trap catalyst to include in
the catalyst beds can be dependent on the amount of arsenic present in the
feed,
as well as on the desired run length. Preferably, the amount of arsenic trap
catalyst can be sufficient to prevent substantial arsenic contact with the
hydrodesulfurization catalyst. It is noted that having an excess of arsenic
trap
catalyst can have little or no effect (other than increased catalyst cost) on
hydrodesulfurization activity and/or selectivity, as the arsenic trap catalyst
can
typically have a relatively low activity for hydrodesulfurization and/or
olefin
saturation.
[0020] In various embodiments, suitable selective hydrodesulfurization
catalysts can include catalysts that are comprised of. at least one Group VIII
metal oxide, for example an oxide Co and/or Ni, preferably at least containing
Co; and at least one Group VIB metal oxide, for example an oxide of Mo and/or
WO 2011/068488 PCT/US2009/006320
-8-
W, preferably at least containing Mo; on a support material, such as silica,
alumina, or a combination thereof. Other suitable hydrotreating catalysts can
include zeolitic catalysts, as well as noble metal catalysts (e.g., where the
noble
metal comprises Pd and/or Pt). It is within the scope of the present invention
that more than one type of hydrotreating catalyst be used in the same reaction
vessel. The Group VIII metal oxide of a selective hydrodesulfurization
catalyst
can be present in an amount ranging from about 0.1 wt% to about 20 wt%,
preferably from about 1 wt% to about 12%. Additionally or alternately, the
Group VIB metal oxide can be present in an amount ranging from about 1 wt%
to about 50 wt%, preferably from about 2 wt% to about 20 wt%. All metal oxide
weight percents are on support. By "on support," it is meant that the percents
are based on the weight of the support. For example, if the support were to
weigh 100 grams, then 20 wt% Group VIII metal oxide would mean that 20
grams of Group VIII metal oxide is on the support.
[00211 The hydrodesulfurization catalysts used in the practice of the present
invention can preferably be supported catalysts. Any suitable refractory
catalyst
support material, preferably inorganic oxide support materials, can be used as
supports for the catalyst of the present invention. Non-limiting examples of
suitable support materials can include zeolites, alumina, silica, titania,
calcium
oxide, strontium oxide, barium oxide, carbon, zirconia, magnesia, diatomaceous
earth, lanthanide oxides (including cerium oxide, lanthanum oxide, neodymium
oxide, yttrium oxide, and praesodymium oxide), chromia, thorium oxide, urania,
niobia, tantala, tin oxide, zinc oxide, aluminum phosphates, and the like, and
combinations thereof. Preferred supports include alumina, silica, and silica-
alumina. It is to be understood that the support material can also contain
small
amounts of contaminants, such as Fe, sulfates, silica, and/or various metal
oxides
that can be introduced during the preparation of the support material. These
contaminants can often be present in the raw materials used to prepare the
support and can preferably be present in amounts less than about 1 wt%, based
WO 2011/068488 PCT/US2009/006320
-9-
on the total weight of the support. It is more preferred that the support
material
be substantially free (e.g., containing not more than about 0.1 wt%,
preferably
not more than about 0.05 wt%, not more than about 0.01 wt%, or no detectable
amount) of such contaminants. Additionally or alternately, about 0 wt% to
about
wt%, for example from about 0.5 wt% to about 4 wt% or from about 1 wt% to
about 3 wt%, of an additive can be present in/on the support, which additive
can
be selected from the group consisting of phosphorus and metals or metal oxides
from Group IA (alkali metals) of the (CAS version of the) Periodic Table of
the
Elements.
Reaction conditions and environment
[0022] The selective hydrodesulfurization can be performed in any suitable
reaction system, for instance in one or more fixed bed reactors, each of which
can comprise one or more catalyst beds of the same, or different,
hydrodesulfurization catalyst. Optionally, more than one type of catalyst can
be
used in a single bed. Although other types of catalyst beds can be used, fixed
beds are preferred. Non-limiting examples of such other types of catalyst beds
that may be used in the practice of the present invention can include, but are
not
limited to, fluidized beds, ebullating beds, slurry beds, moving beds, and the
like, and combinations thereof. Interstage cooling between reactors, or
between
catalyst beds in the same reactor, can be employed in some embodiments, since
some olefin saturation can take place, and since olefin saturation, as well as
desulfurization, are generally exothermic. A portion of the heat generated
during hydrodesulfurization can be recovered, e.g., by conventional
techniques.
Where this heat recovery option is not available, conventional cooling may be
performed through cooling utilities such as cooling water or air, and/or by
use of
a hydrogen quench stream. In this manner, optimum reaction temperatures can
be more easily maintained.
[00231 Generally, selective hydrodesulfurization conditions can include a
temperature from about 425 F (about 218 C) to about 800 F (about 427 C),
WO 2011/068488 PCT/US2009/006320
-10-
preferably from about 500 F (about 260 C) to about 675 F (about 357 C). In an
embodiment, the temperature at the start of a reaction run can be at least
about
450 F (about 232 C), for example at least about 475 F (about 246 C), at least
about 500 F (about 260 C), or at least about 510 F (about 266 C). Additionally
or alternately, the temperature at the start of a run can be about 575 F
(about
302 C) or less, for example about 540 F (about 282 C) or less or about 525 F
(about 274 C) or less.
[00241 Independently, or in combination with the embodiments describing
the start of run temperature, the temperature at the end of a processing run
can be
about 800 F (about 427 C) or less, for example about 750 F (about 399 C) or
less, about 700 F (about 371 C) or less, about 675 F (about 357 C) or less, or
about 650 F (about 343 C) or less. Additionally or alternately, the
temperature
at the end of a processing run can be at least about 550 F (about 288 C), for
example at least about 575 F (about 302 C), at least about 600 F (about 316
C),
or at least about 625 F (about 329 C).
[00251 In various embodiments, the temperature selected as the end of a
processing run can be dependent on a variety of factors. For example, it could
be desirable to operate the reactor and other equipment in a reaction system
at
temperatures below a certain value. This could be due to equipment
limitations,
a desired temperature in another upstream or downstream process, or for other
reasons. Another consideration can be the rate of catalyst deactivation. As a
catalyst deactivates, the number of remaining active sites on catalyst can be
reduced. When many of the active sites on a catalyst are deactivated, the
process
stability for using the catalyst can be reduced. This could be reflected, for
example, in a need to increase temperature at a faster rate in order to
maintain a
substantially constant sulfur level. Additionally, as noted above, some types
of
catalysts generally deactivate more quickly at higher temperatures.
[00261 In an embodiment, the temperature differential between the
beginning of a hydrodesulfurization process and the end of the process can be
at
WO 2011/068488 PCT/US2009/006320
-11-
least about 25 F (about 14 C), for example at least about 50 F (about 28 C),
at
least about 75 F (about 42 C), or at least about 100 F (about 56 C).
Additionally or alternately, the temperature differential between the start of
a run
and the end of a run can be about 300 F (about 167 C) or less, for example
about 200 F (about 111 C) or less, about 150 F (about 83 C) or less, about
100 F (about 56 C) or less, or about 75 F (about 42 C) or less.
[00271 Other selective hydrodesulfurization conditions can include a
pressure from about 60 psig (about 400 kPag) to about 800 psig (about 5.5
MPag), for example from about 200 psig (about 1.4 MPag) to about 500 psig
(about 3.4 MPag) or from about 250 psig (about 1.7 MPag) to about 400 psig
(about 2.8 MPag). The hydrogen feed rate can be from about 500 scf/b (about
84 Nm3/m3) to about 6000 scf/b (about 1000 Nm3/m3), for example from about
1000 scf/b (about 170 Nm3/m3) to about 3000 scf/b (about 510 Nm3/m3). The
liquid hourly space velocity can be from about 0.5 hr-' to about 15 hr"', for
example from about 0.5 hr-' to about 10 hr-' or from about 1 hf' to about 5
hf' .
[00281 FIG. 1 schematically shows an example of a reactor suitable for
performing an embodiment of the invention. In FIG. 1, an arsenic-containing
naphtha feed 105 and a hydrogen feed 107 are introduced into a reactor 110.
Reactor 110 is shown as including a separate arsenic trap catalyst bed 112 and
a
separate hydrodesulfurization catalyst bed 114. Alternately, the arsenic trap
catalyst and hydrodesulfurization catalyst can be in a single bed, with the
arsenic
trap catalyst loaded at the top of the bed. Optionally, additional
hydrodesulfurization catalyst beds 114 could also be included. After treatment
in reactor 110, the hydrodesulfurized feed 115 can be passed to a separator
120.
In the embodiment shown in FIG. 1, separator 120 can advantageously remove a
stream 127 comprising H2, H2S, and other gas phase products from the rest of
the separated, desulfurized naphtha feed 125.
WO 2011/068488 PCT/US2009/006320
-12-
Product Characterization and Control of Reaction Conditions
100291 In various embodiments, a hydrotreated naphtha can be produced
with reduced or preferably no loss of octane, as compared to a hydrotreated
naphtha formed from a similar process that does not employ an arsenic trap
catalyst. By allowing use of reduced amount of catalyst, and therefore an
increased start of run temperature, olefin saturation can be reduced. This can
lead to higher values for the road octane number (RON) and/or the motor octane
number (MON) for the resulting hydrotreated naphtha.
[00301 In various embodiments, a goal of a selective hydrodesulfurization
process can be to produce a naphtha product having a substantially constant
level
of sulfur. In an embodiment, the substantially constant level of sulfur can be
at
least about 5 wppm, for example at least about 10 wppm, at least about 15
wppm, at least about 20 wppm, or at least about 30 wppm. Additionally or
alternately, the substantially constant level of sulfur can be about 150 wppm
or
less, for example about 100 wppm or less, about 75 wppm or less, about 50
wppm or less, or about 30 wppm or less. As used herein, maintaining a
substantially constant level of sulfur in a hydrodesulfurized product can be
defined as maintaining the sulfur content to within about 5 wppm (e.g., to
within
about 3 wppm) of the target level.
[00311 It can be desirable to maintain a substantially constant level of
sulfur
in the naphtha product for a variety of reasons. Maintaining a constant level
of
sulfur can allow for process control, as a gasoline formulator will be able to
rely
on the specifications for the naphtha product. For this purpose, maintaining a
substantially constant sulfur level can be beneficial, because the sulfur
content
does not increase. It can also be desirable to provide a constant sulfur level
to
prevent the sulfur level from being too low. At the product sulfur levels
described for embodiments of this invention, removing additional sulfur can
indicate that the reaction conditions may be too severe. Using more severe
hydrodesulfurization conditions can sometimes result in increased saturation
of
WO 2011/068488 PCT/US2009/006320
-13-
olefin bonds, which may be undesirable. Thus, achieving a sulfur level that is
lower than the target level can actually be detrimental in some instances, as
the
processing used to achieve the lower sulfur level may also further reduce the
RON and or MON of the naphtha product.
[00321 In various embodiments, another goal can be to provide a naphtha
product with an improved octane number. By operating at a higher start of run
temperature but with a reduced catalyst amount (e.g., so that the feed is not
over-
processed), fewer olefin bonds can be saturated in the feed and/or converted
into
mercaptans. Such preservation of olefins can lead to reduced octane loss
during
hydrodesulfurization. In an embodiment, the octane loss due to
hydrodesulfurization can be reduced by about 0.05 RON or more, for example
by about 0.1 RON or more, relative to the octane loss due to
hydrodesulfurization under similar conditions without an arsenic trap
catalyst.
[00331 One or both of the aforementioned goals may be attained according
to the invention, or neither of the goals may be attained.
[00341 One way to maintain a desired sulfur level can be to use the product
sulfur level to provide feedback for the process conditions. Various methods
are
available for detecting product sulfur levels. One option for monitoring
sulfur
levels can be to withdraw samples of the hydrodesulfurized naphtha and analyze
the sample for sulfur. Due to the time scales involved in catalyst
deactivation
during processing, off-line analysis of a naphtha sample can be sufficient to
allow for maintaining a substantially constant level. Alternately, techniques
for
in-line monitoring of sulfur content levels in a hydrodesulfurized naphtha
product may also be available. While in certain circumstances it may be
desirable to use a system containing an arsenic trap catalyst in order to
reduce
the product sulfur content, in other circumstances feedback based on the
sulfur
level in the naphtha product can be used to adjust reaction conditions so that
a
substantially constant level of product sulfur can be maintained. In various
embodiments, adjusting the reaction conditions can include adjusting the
WO 2011/068488 PCT/US2009/006320
-14-
temperature of the catalyst bed (the Weighted Average Bed Temperature), inter
alia.
Example - Simulation of Selective Hydrotreating with and without Arsenic Trap
[00351 Process simulations for a selective hydrodesulfurization process
were developed to illustrate an advantage of using arsenic trap catalyst. The
simulation results are shown in Table 1. The conditions for these simulations
included: about 5500 barrels/day (about 870 m3/day) of FCC naphtha feed;
about 4200 wppm feed sulfur content; about 45 centigrams/gram feed bromine
number; and about 40 ppb feed arsenic content. One process objective was to
lower the sulfur content to at least about 120 wppm, while meeting an
approximate six year run length. The treat gas rate was about 620000 scf/hr
(about 18000 Nm3/hr), with about 72% hydrogen and about 10 wppm CO
(remainder inert gas). The catalyst being simulated represented commercially
available CoMo catalyst on a refractory support.
Table 1
Units Zero As Trap Full Run Length As Trap
As Trap Volume m N/A 2.0
As Trap LHSV hf N/A 18
As Trap run length years N/A 6.1
(based on 40 b feed)
Catalyst Volume m 57.9 48.6
Catalyst LHSV hf 0.63 0.75
End of Run Catalyst As wt% 0.2 0.0
(based on 40 b feed)
Catalyst Bed WABT C 261 264
(SOR)
RON loss 8.52 8.40
Product sulfur m 120 120
[00361 The first data column in Table 1 (Zero As Trap) shows that a
catalyst volume of about 2043 ft3 (about 57.9 m3) was required to meet the run
length objective of approximately six years. The expected start of run (SOR)
temperature was about 502 F (about 261 C). The second data column shows
that, by adding about 71.5 ft3 (about 2.0 m3) of arsenic trap catalyst, the
same run
length could be achieved with a lower catalyst volume of about 1716 ft3 (about
WO 2011/068488 PCT/US2009/006320
- 15-
48.6 m3) while reducing octane loss by about 0.12 RON (road octane number).
The higher SOR temperature for the second case of 508 F (about 264 C)
appeared to reduce mercaptan formation and to improve octane loss. The higher
SOR temperature would ordinarily result in faster deactivation, but this is
balanced out by the presence of arsenic trap catalyst, which mitigates arsenic-
based deactivation. It is noted that the amount of arsenic trap catalyst in
the
second data column was roughly equivalent to that needed to minimize the
arsenic from reaching the main catalyst bed. This was indicated by the
expected
arsenic trap run length of about 6.1 years.