Note: Descriptions are shown in the official language in which they were submitted.
20 02780901 2012-05-14
WO 2011/062934 PC T/ U
S2010/056935
METHOD AND APPARATUS FOR VERIFICATION
OF MEDICATION ADMINISTRATION ADHERENCE
Field of the Invention
100011 This invention relates generally to patient compliance in clinical drug
trials and more
particularly to the monitoring, instruction and intervention of patients in
clinical trials in order to
improve compliance with required drug protocols in accordance with those
trials. This invention
further relates generally to patient compliance in clinical drug trials or
other medication
administration protocol scenarios, and more particularly to an apparatus for
the collection, analysis
and transmission of data related to monitoring, instruction and intervention
of patients in clinical trials
or other medication administration protocol scenarios in order to improve
adherence with prescribed
drug protocols in accordance therewith.
Background of the Invention
[0002] Dr Lars Osterberg, M.D. and Dr, Terence Blaschke have reported in the
New England
Journal of Medicine, Adherence to Medication, (N Engl J Med 2005; 353:487-97)
2005 an alarming
lack of adherence to required medication protocol, further noting that while
the average rates of
adherence in even clinical trials is "high", this number still comprises only
rates of 43 to 78 percent.
Most importantly, the authors note "The ability of physicians to recognize
nonadherence is poor, and
interventions to improve adherence have had mixed results." Adherence, p.
487.The authors conclude
"Poor adherence to medication regimens is common, contributing to substantial
worsening of disease,
death and increased healthcare costs." Adherence, p. 494. The Trend Repot
Series, 2008 Patient
Adherence Update: New Approaches for Success, October 2008, report similar
discouraging statistics.
It is against this backdrop of poor adherence, and potential danger to
patients, that the present
invention operates.
[0003] A number of systems exist that provide instructions to a user regarding
when to take a
medication and records when the user indicates that a medication has been
taken. US Patent No.
7,359,214 describes such a system. A device is provided that provides
instruction to a patient
regarding medications to take. Furthermore, the system may provide a method
for determining that
the prescription is appropriate given the patient's conditions, and other
medications he or she may
already be taking. The system may also provide a method for monitoring
compliance of the patient
with such a regimen, through the dispensing of medicine in accordance with a
predetermined
treatment protocol. While such a system provides many improvements for easing
a burden on the
patient, this system suffers in many ways, and in particular in ways relevant
to clinical trials.
[0004] Most importantly, this system provides no mechanism for actually
confirming that a
patient is in fact ingesting or otherwise properly administering required
medication as required in a
clinical drug trial. While the system may be sufficient for one who is in full
possession of their
mental faculties, any individual who may have difficulty following directions,
or one who is actively
avoiding medication may still not be taking required medication after it is
dispensed. Thus,
1
CA 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
participants may be forgetful, visually impaired, or otherwise do not believe
in the benefit of taking
such medication, and may thus not properly log medication administration.
Furthermore, the system
requires preloading of various medications into a dispenser, and thus likely
requires regular visits by
an administering manager to be sure appropriate medications are in fact
properly loaded therein. It is
surely possible that an inexperienced user may place incorrect medications
into the device, or may
somehow provide incorrect dosages into the device. Additionally, for
potentially more complex
regimens, there is no method provided for insuring that a user is able to
follow such a protocol, and to
thereafter confirm that the user has in fact taken all required medications in
accordance with any
provided instructions or the like, or has taken the medications according to
one or more specifications
or followed suggested procedures. Furthermore, this system is expensive and
requires constant
maintenance to confirm that the various mechanical parts are in working order.
[0005] US Patent Application Serial No. 11/839,723, filed August 16, 2007,
titled Mobile
Wireless Medication Management System provides a medication management system
employing
mobile devices and an imaging technology so that a user is able to show a pill
to be taken to the
system, and the system can then identify the medication. Patient histories are
available to an
administrator, including various vital signs as measured by the system. Images
may also be taken of
the patient, provider, medication container or the like. While the system
professes to ensure
adherence to a protocol, the system only provides such help if requested by a
user. There is in fact no
particular manner in which to ensure actual adherence or the relationship of
adherence to the efficacy
of the drug over time. When customizing a medication regimen or monitoring a
personal medication
regimen, this is particularly relevant.
[0006] Additionally, existing systems fail to maintain an audit trail for post
administration
review by a medical official, and further cannot therefore confirm
confirmation of proper medication
administration. Existing systems are further generally impractical in that
they fail to address many of
a patient's other healthcare needs, including billing, insurance paperwork and
filing, education
regarding healthy living and proper use of medications, easing obtaining
refills for medications, and
providing feedback to a medical service provider, for example, in advance of
an appointment so that
the medical provider can be prepared for such an appointment.
[0007] It has been widely recognized that methods and systems for insuring
proper
medication ingestion or administration by individuals are very important in
defending against
unnecessary sickness, deaths and other problems. Giving instructions and then
letting patients fend
for themselves has been shown not to work particularly well. This is because
it is not only the
improper ingestion of medicines that is the primary cause of medical danger.
Rather, an overall lack
of sufficient patient guidance is also part of the problem. Further, the
inability to confirm a proper
prescription regimen being provided to a user in the first place may cause a
number of other problems
with the use of such medication.
2
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[0008] These issues are even more problematic in a clinical trial setting
where a lack of
adherence to a particular assigned protocol may influence eventual approval of
a particular drug
therapy, potentially denying a valuable drug to the public and resulting in
possible rejection of drugs
that should be on the market that have been backed by pharmaceutical companies
for hundreds of
millions of dollars. Additionally, failure to adhere to prescribed protocols
in clinical drug trials may
result in poor data collection and evaluation, thus resulting in the above
mentioned deaths from failure
for trials to identify potentially life-threatening side effects. Poor
adherence to prescribed protocols in
clinical drug trials may refer to any deviation in a patient's behavior from
that recommended by the
trial designers, including, in addition to improper medication administration,
improper timing of
administration, such areas as dietary advice, advice on smoking, or even
advice about attendance for
further investigation or follow-up. Specifically in clinical trials, any such
poor pharmaceutical
compliance by the patient may result in inadequate results or outcomes.
[0009] Corrupt or incomplete clinical trial data, inefficiency in following
drug regimens and
the inherent liability involved with clinical trials further complicate an
already time consuming,
complex, and expensive approval process which may take between 8 to 12 years
and cost over $900
million. Even after the initial clinical trial testing has been completed,
there is generally no system for
monitoring further use in Phase IV testing, or monitoring patients after
launch. Given the possibility
of failure of the clinical trial testing to ensure drug safety, such
additional testing may be quite
important. Inherent to the clinical trial process are a number of common
mistakes that have not yet
been addressed with existing clinical trial protocols.
[00010] Traditionally, participants attend introductions and follow ups for
clinical trials in-
person. Data collection is similarly limited to patient visits, rather than on
a daily basis. Old methods
such as patient questioning and pill counting have been proven to be
inadequate measures of
adherence and offer no information on dose timing and drug holidays (omission
of medication for
three or more sequential days).
1000111 Compliance technologies can increase the statistical power of clinical
trials. Through
the use of such technology, clinical events can be precisely linked to
medication use history. Captured
data can be linked to other sources such as EDC, patient diaries and data
collected by the physician.
Technologies can create many possibilities for remote visits and data capture.
While smart packaging
technologies exist such as RFID-enabled computer chip technology, smart
blister packs and MEMS
caps (microprocessor in a bottle cap), they are: a) invasive and need to be
physically attached to the
medications; b) are non-conclusive regarding compliance ¨ a patient may
activate the technology
without ingestion of the medication; c) remain largely unadopted in clinical
trials by the
pharmaceutical and biotech companies due to their high cost; and d) take a
longer time to implement.
[00012] Jo Carol et al. stated that "The most reliable method for research
purposes, although
not practical in a clinical setting, may be a combination approach that
includes pill counts, patient
self-report, and electronic monitoring." (Carol J. et al, Patterns to
Antiretroviral Medication, The
3
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
Value of Electronic Monitoring, AIDS, 17 (12), ppl, 763-767, Oct 2003. To
date, technologies alone
have only been used to monitor compliance rather than to encourage it.
Furthermore, there has been
no comprehensive system provided that allows for the management of multiple
patients and multiple
patient populations. While current technology may allow poor compliers to be
recognized, as will be
described below, the proposed system of the present invention will help to
encourage pharmaceutical
compliance and tackle some of the problems that are encountered in the
clinical trial process.
1000131 Another problem is the issue of informed consent and the protection
many clinical
trials attempt to implement, often unsuccessfully, to protect themselves from
potential lawsuits. A
survey was conducted by CenterWatch (Getz, K.A. (2002). Informed consent
process: A survey of
subjects assesses strengths and weaknesses Applied Clinical Trials, 11(11), 30-
36) to assess subjects'
understanding of the consent documents further supports the concept that
patients may not understand
the forms they are signing. The survey reported that 14% of patients signed a
consent document
without reading the form. In the same survey, 30% of patients reported that
they did not understand
that their trial could carry more risk and discomfort than standard treatment.
Nearly 40% of patients
did not know that they could call an ethics board or IRB representative with
questions about problems
or concerns. The survey reported that 41% of patients did have study nurses
review the consent form
with them. The survey finally concluded that 70% of the participants may not
have known what
questions to ask at the outset of the informed consent process. And, this is
with the current
information provided and consent received from these participants. With
approximately 77,967 trials
in as many as 172 countries registered with ClinicalTrials.gov as of the date
of this application, the
logistical challenge of ensuring consistency is overwhelming.
1000141In patients with psychiatric illness, adherence may be even more
difficult and
technology solutions such as the one proposed in accordance with the present
invention may offer
accurate ways to authenticate adherence. According to Cramer and Rosenhek
(Compliance with
Medication regimens for mental and physical disorders, Psychiatr Sery 1998),
among patients with
physical disorders, the mean rate of medication adherence was 76%, whereas
among those with
psychoses the mean rate was 58% and among those with depression the mean rate
was 65%. Similar
compliance issues might be expected to be present when dealing with child
patients, or others who
may have difficulty following potentially confusing instructions.
1000151 The logistical challenge of medication adherence and auditing in
clinical trials is
certainly increasing, with more than 70,000 clinical trials under way
throughout the world, growing at
a rate of 8 to 10 percent a year, according to CenterWatch in Boston, which
lists clinical trials. In
2003, there were 3.6 million individuals enrolled in clinical trials in the
United States, according to
the center. Self-regulation in the industry is necessary as the FDA inspected
only 1% of clinical trial
sites during the 2000 to 2005 period (Department of Health and Human Services,
Food and Drug
Administration's Oversight of Clinical Trials, September 2007). As the
requirements for drugs
increase, one can expect that the ability for the FDA to inspect such clinical
trial sites to decrease even
4
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
further. Furthermore, the average cost of $6533 to recruit a patient for a
trial, and three times that
amount to recruit a new patient if one is lost due to noncompliance is high so
pre-screening subjects
and monitoring them on an ongoing basis is critical. These numbers are
significant given the fact that
it has been calculated that if 30% of patients in a clinical trial had
inadequate compliance, double the
number of patients would need to be studied to create the same alpha and beta
levels (Pledger G W.
Compliance in clinical trials: impact on design, analysis and interpretation.
Epilepsy Research 1988; 2
(suppl): 125-33).
[00016] Tufts CSDD Impact Report stated that within three years, major
sponsors project that
up to 65% of FDA-regulated clinical trials will be conducted outside the
U.S.¨primarily in Central
and Eastern Europe, Latin America, India, and Asia¨due to economic advantages
and ready access to
well-trained physicians and large numbers of treatment-naive patients. In
addition, contract clinical
services account for more than 17% of total drug development spending. The
growth of sponsor
spending on CRO (Clinical Research Organization) services will outpace overall
growth in spending
on global drug development for the foreseeable future, reflecting increasing
reliance on contract
providers to provide added capacity, more flexibility, and greater efficiency.
[00017] With greater amounts of clinical trials being conducted abroad,
technology can aid
standardization and provide greater communication where deficiencies in
existing infrastructure such
as physician and nurse training, may exist.
[00018]A number of systems exist that provide instructions to a user regarding
when to take a
medication and records when the user indicates that a medication has been
taken. US Patent No.
7,359,214 describes such a system. A device is provided that provides
instruction to a patient
regarding medications to take. Furthermore, the system may provide a method
for determining that
the prescription is appropriate given the patient's conditions, and other
medications he or she may
already be taking. The system may monitor the dispensing of medicine in
accordance with a
predetermined treatment protocol. While such a system provides many
improvements for easing a
burden on the patient, this system suffers in many ways and in particular in
ways relevant to the
administration of clinical trials.
[00019] Therefore, it would be desirable to provide a method and apparatus
that overcomes
the drawbacks of the prior art.
Summary of the Invention
1000201In accordance with the present invention, a system and method are
provided that
allow for complete control and verification of adherence to a prescribed
medication protocol, whether
is a health care provider's care, or when self administered in a homecare
situation by a patient. The
system and method preferably includes a means for receiving user medication
information, including
a medication profile and history, providing indications to a user regarding
when to take a medication,
and which medication to take, imaging medication either in pill or container
form to confirm a correct
medication is being taken, confirming, in accordance with the user medication
history that a new
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
medication will not conflict with an already being taken medication (including
non-prescription
medication that may be used by a patient), scan a group of medication
containers or pills to inform a
user which is the correct medication through the use of visual, verbal or
other prompts, providing
assistance information to aid a user in properly taking the medication,
imaging, employing still
photos, video sequences or other activity or gesture recognition techniques,
the user to confirm that
the medication is being actually and properly taken, providing any appropriate
additional or special
instructions, using the imaging to note any possible adverse effects
associated with taking of the
medication, accumulating statistics about adherence to a prescribed protocol,
assisting in reordering
medication on an as-needed basis and notifying a medical professional if the
user is not properly
following a prescribed protocol.
1000211From beginning to end, a user is provided with a system and method that
aids the user
in properly following a protocol, while informing an administrator of any
deviations from the
protocol, either innocent or purposeful, by a user so that an early
interaction may be provided. The
present invention is the only medication adherence verification system that
may determine whether a
user is actually following a protocol, and provide additional assistance to a
user, starting with
instructions, video instructions, and the like, and moving up to contact from
a medication
administrator through phone, email video conferencing or the like, if it is
determined that the user
may benefit from such assistance. Upon prescription of a medication to a user
and entry of
appropriate medical information into the system, the pharmacist or other
medical personnel may allow
the system to image the medication or enter medication information in any
number of ways, thus
recalling one or more preferred protocols for administration of the
medication. An image of the
medication may be further provided to the pharmacist upon this or any
subsequent refills of the
medication and act as an added visual check to confirm proper medication
administration. The
personnel may then select a desired protocol, thus removing a likely point of
error, incorrect
instructions to a user. Further, a medication image may be shown to a patient
when they are to take a
medication, to thus further ensure proper medication is administered.
1000221Further in accordance with the invention, access to such medication
information may
be made available to emergency responders, hospital or other medical service
providers or the like. It
is contemplated that such information may be available by entering a
predefined sequence into a
patient's cellular telephone, other mobile device, or home based system.
Further access may be
provided at a predetermined website or the like.
1000231In accordance with the present invention, a system and method are
provided that
allow for complete control and verification of adherence to a prescribed
medication protocol or
machine or apparatus use in a clinical trial setting, whether in a health care
provider's care, or when
self administered in a homecare situation by a patient.
[00024] The present invention is intended for the clinical trial market as a
full audit and
tracking tool for pharmaceutical compliance in subjects. The invention
provides the only medication
6
20 02780901 2012-05-14
WO 2011/062934 PC T/ U
S2010/056935
management system that may determine whether a user is actually following a
protocol, provide
additional assistance to a user, starting with instructions, video
instructions, and the like, and moving
up to contact from a medication administrator if it is determined that the
user would need such
assistance. Upon prescription of a medication to a user in a clinical trial,
the trial designers may then
select a desired protocol for the user to follow, and therefore, not only may
a particular drug be tested,
but also test the ease of adherence given a particular set of instructions.
[0002511n recent years, the United States Depainnent of Health and Human
Services, Office
of the Inspector General ("OIG") has issued publications to make it clear to
members of the boards of
directors of health care companies conducting business with federal health
care programs (directly or
indirectly) that these fiduciary duties include an obligation at the board
level to assure that the health
care company maintains an effective corporate compliance program. Due to
increased compliance
obligations by the office of the inspector general (OIG), chief compliance
officers of pharmaceutical
companies must, to best of their luiowledge, ensure that their company is
compliant with any required
regulations. Failure may result in personal liability. Thus, the ability to
maintain an audit trail in a
clinical trial setting may aid in meeting these requirements.
[00026] The novel combination of text, graphical, pictorial, and video allows
the compliance
problem within clinical trials to be tackled in a holistic manner. The system
in accordance with the
present invention offers a number of solutions. First, the system helps to
ensure that the patient is
giving informed consent. The system and method may provide step-by-step image
and video
instructions to the user or care provider on how to administer the medication,
what the side effects of
the medication are and the benefits that have been found. This avoids
misunderstandings resulting
from prescribing instructions and may allow an approach that may be utilized
internationally, thus
overcoming possible language barrier issues and the like.
1000271A specific medication regimen may be programmed into a medication
calendar to
alert the user when medication should be taken and provide clear video
instruction for taking the
medication. These reminders minimize forgetfulness by the subject in clinical
trials and help the
logistical challenges involved in complex medication regimen such as double
blind studies, for
example. Furthermore, the medication calendar and the compliance results are
accessible to the
clinical trial organizers.
[00028] Once an alert is made and the patient has confirmed that they are
ready to take the
medication, the system and method of the invention record the type of
medication and quantity
through image recognition. The subject shows the medication to an imaging
device which then
authenticates the medication if in the correct dosage and offer administration
instructions. Other types
of recognition of the bottles may also be used, including RFID tags, bar code
reading, text
recognition, or other confirmation through a provided graphical user
interface, preferably in
conjunction with the image recognition. Once the medication type and quantity
has been
authenticated, the system and method of the invention records or otherwise
visually analyzes the
7
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
patient actually administering the medication to him or herself, using any of
the above recognition
methods, and further including facial recognition, badge identification, or
any other method for
confirming the identity of the individual. A real-time log for audit trails
and further analysis is thereby
created. Additional information may be captured using a brief questionnaire on
the device which may
help to highlight problems when administering the medication ¨ such as
difficulty in swallowing
tablets or opening packages - or adverse reactions from which the patient is
suffering. Whatever the
reason a patient misses a reminder or medication prompt, the system and method
of the invention
alerts the clinical trial organizers. Data from all the population can be
captured and presented in an
aggregated manner online in real-time, giving real-time data results and flag
problems or results early.
1000291As clinical trials begin, it will quickly become evident if a clinical
trial is designed
well and difficulties or non-compliance will be highlighted by the system and
method of the
invention. Often inconvenient or restrictive precautions prevent the
medication regimen from being
followed accurately and this type of feedback can be very useful. The system
of the invention
minimizes delinquent or inaccurate data submission by linking clinical events
to medication use. The
system also tracks the actions taken when a problem has been highlighted. This
audit trail is then
stored. Finally, if a patient is determined to be unable to follow a
prescribed regimen, that patient may
be identified and removed from the clinical trial population, or at least
flagged, thus removing a
confounding factor from the determination of whether a drug is safe and
effective.
1000301 The system and method preferably includes a means for receiving user
medication
information, including a medication profile and history, providing indications
to a user regarding
when to take a medication in accordance with a prescribed regimen in
accordance with a clinical trial,
and which medication to take, imaging medication either in pill or container
form to confirm that a
correct medication is being taken, confirming, in accordance with the user
medication, that a new
medication prescribed in accordance with a clinical trial will not conflict
with another medication the
user is already taking (including non-prescription medication that may be used
by a patient), scan a
group of medication containers or pills to inform a user which is the correct
medication through the
use of visual, verbal or other prompts, providing assistance information to
aid a user in properly
taking the medication, imaging, employing still photos, video sequences or
other activity or gesture
recognition techniques, the user to confirm that the medication is being
actually and properly taken,
providing any appropriate additional or special instructions so that the user
is able to comply with the
instructions of the clinical trial, using the imaging to note any possible
adverse effects associated with
taking of the medication, accumulating statistics about adherence to a
prescribed protocol, assisting in
reordering medication on an as-needed basis and notifying a clinical trial
manager if the user is not
properly following a prescribed protocol. If desired, a patient's face or
other identifying features may
be hidden so that the recorded sequences may be stored, displayed and
otherwise used without
allowing the identity of any particular patient to be released.
8
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
1000311 From beginning to end, a user is provided with a system and method
that aids the user
in properly following a protocol as defined in a clinical trial setting, while
informing an administrator
of any deviations from the protocol, either innocent or purposeful, by a user
so that an early
intervention may be provided. The present invention is the only clinical trial
medication adherence
verification system that may determine whether a user is actually following a
protocol, and provide
additional assistance to a user, starting with instructions, video
instructions, and the like, and moving
up to intervention from a medication administrator through phone, email video
conferencing or the
like, if it is determined that the user may benefit from such assistance.
Through such improved
provision of care, potential clinical trial participants may be more likely to
participate in such a
clinical trial. The system may also identify individuals to be removed from a
clinical trial population,
and also possibly identify sets of instructions that may not be working to get
people to follow the
prescribed regimen.
1000321In accordance with the present invention, an apparatus is provided that
facilitates
information presentation to a patient, information capture of medication
administration at home in a
homecare setting, in a hospital setting, in a clinical trial setting, or in
any other setting in which
medication adherence is potentially an issue, and other aspects described as
part of the method and
system of the pending applications noted above. Therefore, in accordance with
the present invention,
a video capture device is provided including a memory for storing captured
video and other patient
data, analyzing such captured data, transmitting such captured data to a
remote location, receiving
information from a remote location and providing information to the patient as
preferred in
accordance with the present invention. In accordance with a preferred
embodiment of the invention,
an apparatus is provided comprising a video capture device, an audio capture
device, memory for
storing such captured data, a processor adapted to operate analysis software
for analyzing the captured
data, a transmitter for transmitting the captured data, or other versions of
the data or analysis results to
a remote location, for receiving data and further instructions or
communication from the remote
location, and a display for providing such data or further instructions to the
patient. Such apparatus
may preferably interface with management software adapted to manage multiple
patients, and thereby
providing a full monitoring and data collection procedure.
1000331It is therefore contemplated that the apparatus in accordance with the
present
invention be applicable to settings including clinical trials, as well as in
more general healthcare
settings, such as with home care provided by a healthcare assistant, or self
administered by a patient,
in a hospital or other clinic setting, or in other locations where medication
management would be
beneficial. The ability for the apparatus constructed in accordance with the
invention to capture
patient and medication administration data in a more controlled environment
will allow for more
consistent data to be gathered, thus easing the burden of insuring adherence
to one or more prescribed
medication administration regimens.
9
20 02780901 2012-05-14
WO 2011/062934 PCT/U
S2010/056935
[00034] Furthermore, while the apparatus constructed in accordance with the
application has
been described as implementing a method and system as described above, it is
intended that the
apparatus be available as a standalone apparatus, for use and implementation
in systems other than
those described in accordance with this application.
[00035] Still other objects and advantages of the invention will in part be
obvious and will in
part be apparent from the specification and drawings.
[00036] The invention accordingly comprises the several steps and the relation
of one or more
of such steps with respect to each of the others, and the apparatus embodying
features of construction,
combinations of elements and arrangement of parts that are adapted to affect
such steps, all as
exemplified in the following detailed disclosure, and the scope of the
invention will be indicated in
the claims.
1000371 Brief Description of the Drawings
[00038] For a more complete understanding of the invention, reference is made
to the
following description and accompanying drawings, in which:
[00039] Figure 1 is a flow chart diagram depicting top level functionality in
accordance with
an embodiment of the present invention;
[00040] Figure 2 is a flow chart diagram describing a data entry and
prescription assignment
process in accordance with an embodiment of the present invention;
[00041] Figure 3 is a flow chart diagram describing a medical compliance
regimen from in
accordance with an embodiment of the invention;
[00042] Figure 4 is a flow chart diagram depicting top level functionality in
accordance with
an embodiment of the present invention;
1000431 Figure 5 is a flow chart diagram describing a data entry and
prescription assignment
process in accordance with an embodiment of the present invention;
[00044] Figure 6 is a flow chart diagram describing a medical compliance
regimen from in
accordance with an embodiment of the invention;
[00045] Figure 7 is a representation of a summary page of a dashboard in
accordance with a
preferred embodiment of the invention;
[00046] Figure 8 is a representation of a zoomed in view of the summary page
of Figure 7;
[00047] Figure 9 is a representation of information provided in accordance
with an individual
clinical trial participant indicator in accordance with a preferred embodiment
of the invention;
[00048] Figure 10 is a flow chart diagram depicting top level functionality in
accordance with
an embodiment of the present invention;
[00049] Figure 11 is a flow chart diagram describing a data entry and
prescription assignment
process in accordance with an embodiment of the present invention;
[00050] Figure 12 is a flow chart diagram describing a medical compliance
regimen from in
accordance with an embodiment of the invention;
20 02780901 2012-05-14
WO 2011/062934 PCT/U
S2010/056935
[00051] Figure 13 is a representation of a summary page of a dashboard in
accordance with a
preferred embodiment of the invention;
[00052] Figure 14 is a representation of a zoomed in view of the summary page
of Figure 13;
1000531 Figure 15 is a representation of information provided in accordance
with an
individual clinical trial participant indicator in accordance with a preferred
embodiment of the
invention;
[00054] Figure 16 is a block diagram depicting an embodiment of the invention;
[00055] Figure 17 is a block diagram depicting details of systems associated
with an
embodiment of the invention;
[00056] Figure 18 is a flow chart diagram depicting a method in accordance
with an
embodiment of the invention; and
1000571 Figure 19 is a flow chart diagram depicting a method in accordance
with an
embodiment of the invention.
[00058] Detailed Description of the Preferred Embodiments
1000591In accordance with the invention, a system and process are provided
that improve
adherence to medical protocol, and give administrators a tangible and concrete
manner in which to
confirm compliance or lack thereof, and the ability to intervene early in the
process to ensure that
patients are properly taking their medication. The system and method of the
invention provide for
prescription selection, instructions to patients on the use of any
prescription medications, and
verification to a doctor or other service provider of patient adherence to the
prescribed protocol.
[00060] Referring first to Figure 1, a data flow overview is shown. In
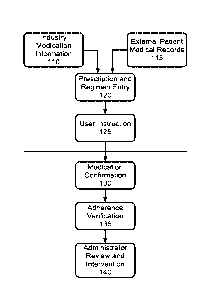
accordance with the
invention, existing industry medication information databases 110 are employed
to access
prescription, interaction, application and other available information about
any number of proposed
prescription and non-prescription medications. Further, patient medical
records 115 are used, and as
will be described below, are used in conjunction with the industry medical
information and a medical
professional's prescribing expertise to prescribe a medicine regimen, and
enter such a regimen into the
system of the invention at 120. Once entered into the system, a particular
prescription regimen causes
a set of user instructions 125 to be generated. Such user instructions may
include general instructions
about a particular medication, methods for ingestion, concerns about side
effects, concerns about drug
interactions with common substances or medications, or other medications
prescribed to the patient by
the system or by another medical service provider. It is contemplated in
accordance with the
invention that such set of user instructions may be interactive, allowing a
user to view additional
information about such instructions or prescriptions as desired. These
instructions may comprise
written, audio or video instructions. Furthermore, it is contemplated in
accordance with the invention
that at various points during the instruction set, for example when a patient
asks a particular type of
question, or asks to receive additional information about a particular aspect
of the medication or
prescription regimen, that the system may reach out and contact a
representative of a medical service
11
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
provider to provide the patient with additional, personal help as necessary,
if it is determined that such
intervention by the medical professional would be desirable to the patient.
Thus, such a patient may
be assisted in properly taking medication so that various errors do not take
place. Indeed, in more
traditional scenarios, it is only after perhaps finishing a prescription
regimen and a return to a doctor
that it is discovered that the medication may have been taken incon-ectly. In
accordance with the
present invention, early intervention with such issues can be exercised to
deter any possible
unfortunate outcomes from improper administration of medication. It is
contemplated in accordance
with the invention that a medical service provide or one of the individuals
noted above be provided
with a patient dashboard for managing prescription regimes for one or more
patients. Such a
dashboard allows the medical professional to monitor any number of patients in
a manner that will be
described below, allow statistical analyses of patient adherence and other
patient reactions, provide
links to information, including recorded activity sequences for one or more
patients, and generally
allow the medical professional to monitor and administer medication to all of
their patients form a
convenient single access point. Such a dashboard also allows for monitoring of
any health care
providers that may be administering medication to a number of patients, or
review of one or more
administration sequences or the like.
1000611Additionally, it is contemplated in accordance with the invention that
the patient be
provided with a user interface dashboard or the like allowing of modification
of prescription regimen
and instruction information by the patient. Thus, by way of example, if a user
is to take a particular
medication before eating in the morning, the user may be able to determine
when a reminder for such
medication will be given. If the patient is an early riser, the reminder may
be provided by cell phone
or email at 6:00AM. Conversely, if the patient sleeps late and normally does
not eat an early meal,
reminders can be set for later in the morning, thus matching a patient's
schedule. Of course, any such
patient adjustments must be set within broader prescription regimen as defined
by the prescribing
medical provider. It is contemplated in accordance with the invention that a
touch or other user
friendly graphical user interface be provided so that the user can easily
manipulate any number of
prescription factors, and perhaps enter additional information that may be
useful to a prescribing
medical provider, such as level of fatigue, level of hunger, jitter inducing
medications, etc. All of
these data collection points allow for a smoother administration of medication
to a patient, and
therefore a more likely chance of adherence to a prescribed protocol.
1000621Referring to the portion of Figure 1, the horizontal line indicates a
time for patient
ingestion or other administration of medication. In accordance with the
invention, confirmation of
patient adherence to the prescribed administration schedule for the medication
is determined. While
such confirmation may take a number of forms, in accordance with the
invention, a preferred method
for such confirmation may include capturing a video sequence of the patient
actually administering
the medication. In a further preferred method, such a sequence for such
confirmation may include
employing a facial recognition sequence or other biometric confirmation that a
particular patient is in
12
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
fact receiving treatment. Activity recognition, gesture recognition or other
feature for determining
whether a particular subject movement meets a predefined movement sequence may
be employed to
be sure that the patient is properly taking prescribed medication. The patient
may then display a
medication container and/or an actual pill or other medication form to an
imaging apparatus, and the
apparatus confirming that the medication is correct and is the currently
prescribed medication to be
taken through the user of text recognition, pill recognition, or other
appropriate medication
recognition scheme. This sequence of steps therefore acts as an audit trail
each time a medication is
taken, that can be reviewed later, to ensure that a patient is properly
following a regimen.
1000631 Furthermore, in accordance with the present invention, a video image
of the patient
actually administering or ingesting the medication may be taken and stored so
that actual confirmation
may be achieved, rather than simply relying on the patient to state that a
particular medication was
administered. Such a video image may be captured or stored in any appropriate
format given a
selected type of activity or gesture recognition that is employed in
accordance with a particular
embodiment of the invention. Such may include full video, biometric data
points, recording of
movement of an article, such as a bracelet or the like, affixed to the patient
or administrator, use of
mapping to provide a stick figure or other body movement tracking technique,
or gesture or activity
recognition to determine movement or the like. Finally, in accordance with the
invention, if recording
of a video of a patient having the medication administered thereto is not
possible, the system of the
invention will recognize such an issue and request audio confirmation as a
next best option. If the
audio confirmation is also not possible, then a less reliable method of
confirmation, such as a
keyboard confirmation by the patient may be accepted. If higher reliability
methods of confirmation
are not available for an extended period of time, an alarm is preferably
forwarded to a medical
professional to inquire as to reasons and to remedy any situation that might
be wrong in the
administration situation.
[000641These steps of confirming identity, confirming medication and
confirming
administration are then reviewed to verify adherence to the prescribed
protocol at 135. Such review is
preferably performed automatically by a computing system that is able to align
the actual recorded
images with ideal or expected images, or through the user of other activity or
gesture recognition as
mentioned above. In the case of facial recognition and bottle or pill
recognition, such techniques arc
known in the art. With regard to video confirmation of adherence to prescribed
medicine
administration procedures, such processing may include various stick figure
comparison analyses,
motion recognition analysis, or other schemes as noted above able to determine
whether appropriate
actions have been performed by the patient.
[00065] The ability to provide automated determination of adherence to proper
administration
procedures allows for a large number of such images to be review in a short
period of time. Even if
actual and complete lack of adherence is not able to be determined 100% in
each possible situation,
the ability to pre screen the administration video captures to remove from
further consideration
13
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
administration situations that are clearly compliant may reduce a number of
compliance situations to
be reviewed by a medical professional substantially.
1000661 In an additional embodiment of the invention the imaged sequences used
for activity
recognition to determine regimen adherence may be further used to check for
adverse or other
reactions to taking of the medication. Thus, in addition to simply determining
proper adherence to a
protocol, such activity or gesture recognition may determine any number of
different actions that may
have been taken by a patient. Thus, actions taken before medication
administration, or actions taken
after medication administration may give insight into reasons for particular
responses, etc. Thus,
before administration, in accordance with the invention, activity recognition
may determine a current
activity of a user. Any subsequent reminders to take a medication may in part
be based upon this
determined activity. By way of example, if a user is putting on a coat, or is
detei mined to be leaving a
residence or other facility or the like, a reminder to take a medication
before leaving may be provided,
even if earlier than normal, or if medication is portable, the user may be
reminded to take the
medication with them, and then subsequently reminded to administer the
medication via notification
on a mobile device. By way of further example, if the user is cooking, a
reminder may be given to
take the medication a predetermined time before eating. Other scenarios may be
possible, thus
allowing greater response from the system to ensure proper medication
administration by a patient.
Additionally, various patient consent issues may be prompted and recorded in
accordance with the
invention. Patients may ask further additional questions regarding such
consent, thus insuring that
patients have all of the information they need to make informed consent
decisions, and medication
providers have proper evidence of such consent.
[00067] Similarly, actions after taking medication may give insight into
patient responses.
Notice of fainting, falling down, lack of motion, facial gestures,
gastrointestinal distress or the like
may all be logged as adverse reactions to a particular medication regimen, and
may allow for
adjustment of dosage or prescription instructions in the future for the
patient. If adverse reactions are
severe, an immediate medication review and contact from a medical professional
may be provided to
cure the issue. Additionally, the system in accordance with the present
invention may be directly tied
and be interoperable with a pharmacy or medical provider's systems, thus
allowing such
recommendations for dosage changes, regimen changes and the like to be
forwarded to these
professionals automatically. Through such links, reordering medication, dosage
changes, medication
changes and the like may be automatically provided. Furthermore, ease of
providing additional
prescriptions can be enhanced as patient, medication and regimen information
will already be
available to the pharmacist or medical service provider.
1000681After such automatic, or combination of automatic and manual, adherence
verification is performed allowing a health care provider or other medical
professional to review and
verify results of the automatic comparison or direct review of captured
activity sequences, and
indication of variation from a desired identity, medication or application
procedure may request
14
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
administrator review of the situation, and intervention as may be determined
necessary at 140. Such
review may be required immediately as an emergency situation may exist, or
such review may be less
urgent, perhaps requiring an electronic communication with suggestions or the
like from such an
administrator or the like. Additionally, such adherence review may be stored
over time for a particular
patient, thus allowing for various medication trends to be determined, such as
if a patient misses
medication at a same time each week, or an indication that one particular
health care worker aiding
the patient may occasionally give an incorrect medication dosage amount. Thus,
in addition to
allowing for immediate notice of problems in medication administration, an
audit trail for tracking the
actions of various health care providers is generated.
1000691 Therefore, in accordance with the invention as set forth in Figure 1,
a method and
system are provided in which patient adherence to a prescribed medication
regimen can be reviewed,
acutely for a particular instance, or over time to determine any changes in
behavior of a patient.
Because all aspects of such adherence are monitored preferably visually, and
do not rely on patient
confirmation of medication administration, desires of the patient are taken
out of the equation, and a
true review of actually procedures used in the medication administration can
be studied.
1000701 Referring next to Figure 2, a more precise description of the data,
medical record and
prescription regimen entry will now be described. As is shown in Figure 2, a
number of entities may
be able to enter information into a system in accordance with the invention.
Such individuals may
include a doctor 202, a pharmacist 204, a patient 206 and a caregiver 208.
Each of these individuals
may be provided with different rights for data entry. For example, a doctor
may be provided with the
ability to alter patient information, basic medical statistics, and virtually
all other medical information
included in a database. A pharmacist may be limited to entering information
about a particular
prescription, including when delivered to a patient, type of pill or
medication, dosage size, refill
availability, etc. A patient may be limited to providing personal information,
and perhaps other
relevant information, such as non-prescription medication usage, alcohol
consumption, recent
symptoms, reaction to use of particular prescription medications and the like.
A caregiver may be
limited to entering information about patient reactions, times of
administration of medication and the
like. Such data entry allows for the tracking of an audit trail at this step
of administration as well.
1000711 Information from each user of the system is combined, where available,
and formed
into a prescription medication entry 210. In particular, a preferred list of
information that may first be
provided in accordance with the invention in order to organize the system may
comprise a patient
name, a user name, if that user is not the patient, and information to be used
to provide various alerts,
such as a patient email for prescription reminders, contact information for a
doctor or caregiver for
emergency contact, or the like. In addition, in accordance with the invention,
facial images of the
patient and other people interacting with the system are stored, or other
biometric identifiers, such as
fingerprint identification, retinal identification, voice recognition, various
provided RFID tags or the
20 02780901 2012-05-14
WO 2011/062934 PC T/ U
S2010/056935
like may be employed. Of course, if the patient is a returning patient, such
information may be
extracted from an existing database stored with another, previous
prescription.
1000721Next, a user such as the doctor 202 or the pharmacist 204 may indicate
a particular
new or recurring prescription medication to be provided to a user. An external
medication database
215 is accessed and information regarding such medication is provided,
including medication name or
names if multiple brand and generic names exist, suggestions for appropriate
prescription dosages
based upon patient information and the particular version of the medication to
be administered, and
usage instructions to be provided to the patient, these instructions being
modified or supplemented as
necessary by the prescribing entity. These usage
instructions preferably include detailed
administration instructions, including time of day, patient status (i.e.
before or after eating, after
waking, before sleeping, etc.), precise method of application, and other
useful instructions for a user.
These instructions also may include video sequences to describe particular
details of the medication or
administration procedure thereof. They may also comprise alternative versions
of instructions so that
if a user is unclear regarding a particular set of instructions, an
alternative set of instructions may be
provided to the patient to aid in adherence to the prescription regimen.
1000731After selection of such a medication, a patient medical history 217 is
preferably
accessed to provide additional medication and patient information to the
prescribing individual. Such
information may include, other prescriptions to the patient so that adverse
drug interaction may be
determined (although if such other prescriptions were implemented in
accordance with this invention,
such prescriptions will already be known to the system), patient indication of
use of non-prescription
drugs, patient allergies, patient activity level, past diseases and
procedures, and any other information
that may be relevant to the prescription of medication.
1000741 After all of the information has been entered about the user, and
information from the
medication and patient database has been accumulated, various medication
interactions are checked
automatically by the system at step 220. Any dangerous interactions are noted
to the prescribing
individual, and may preclude entry of the prescription into the system. Other
interactions may be
noted to the prescribing individual so that the individual may make the
patient aware of such
situations. These may include, for example a notification that the taking of
two medications together
may result in stomach pains, so that the patient should take one in the
morning and one in the
afternoon. Such interactions check will then result in a set of instructions
that will be provided to the
user, in addition to the more generic medication instructions. Finally, the
prescribing individual may
review such instructions and supplement them as desired. It should be noted
that in accordance with
the invention it is contemplated that various instructions provided to a user
may comprise hot links to
additional audio and visual information that may be provided to a patient to
further assist in their
adherence to any particular prescribed protocol, or may provide various
information regarding the
particular medication being taken as will be further described below.
16
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
1000751 Finally, after all interactions and instructions have been reviewed
and approved, the
prescription information is stored at step 225. The storing of such a
prescription makes the
prescription, alerts, help information and the like accessible to a patient
and other system users.
Furthermore, such completion may also transmit the prescription to a pharmacy
or other medication
provision facility so that the user is able to simply swing by to pick up the
medication or to have the
medication delivered to the patient.
1000761Referring next to Figure 3, a user implementation of the method in
accordance with
the invention will be described. When a time for receiving or administering a
medication is reached, a
patient, and any other necessary user may be provided a notification 310 in
accordance with
notification addresses entered in the system as noted above. Thus, in a home
situation, only the
patient may receive notification. If there is a home health care provider,
such provider may also
receive separate independent verification. In the case of hospital or other in-
patient care facility,
various medical service providers may similarly receive such notification.
After notification, or in the
absence of such notification, system initiation takes place at 315. In
accordance with such system
initiation, one or more users are preferably recognized by the system.
Therefore, at step 320, a user
recognition sequence takes place. In a preferred embodiment, such a user has a
still or video image
captured of their face, and facial recognition techniques are employed to
confirm the identity of the
user. Such capture may be performed by fixed camera, mobile camera, mobile
communication device
such as a cellular phone, or any other appropriate video or image capture
device. Alternative
recognition techniques, such as retinal, fingerprint, voice or other biometric
measurements may be
employed, in addition to a more common password query.
1000771At step 325 it is determined whether all necessary users have been
recognized and
authenticated. In a situation where a nurse, doctor or other caregiver is to
administer medication, it
may be preferable to have the patient and caregiver to be recognized by the
system to further confirm
that the appropriate procedure is followed, and to allow the system to keep
track of people using the
medication so it can track if any one person, for example, is improperly using
the medication, as will
be evident from the generated audit trail. After step 325, if all users are
not recognized, control passes
back to step 320 and any additional users are recognized by the system.
[00078] Once all users are recognized, control passes to step 327 where the
medication to be
administered in accordance with the prescribed prescription regimen is
confirmed. Thus, a user is
prompted to allow a still or video image, text recognition image, or other
method of identifying a
medication to be captured of the medication bottle or other container, a pill
of the medication, or other
form of medication, and is also able to determine appropriate quantities,
dosage, and any potentially
required or dangerous medication combinations. As noted above, if video
confirmation is for some
reason not available, the user may be prompted by the system to provide audio
or other indication of
medication and other desired information. This image, video sequence or other
received confirmation
information is then compared to an image associated with the prescription as
noted above in Figure 2.
17
:A 027809012012-05-14
WO 2011/062934
PCT/US2010/056935
If the medication is determined to be incorrect, a warning may be provided to
the user that the
medication is incorrect. The user may then be prompted to choose another
medication for imaging.
1000791 Alternatively, the invention contemplates a user displaying a number
of medications
to the image capture apparatus and allowing the apparatus to suggest which
medication is correct.
Thus, the user may be able to scan a medicine cabinet with such a video
imaging apparatus and have
the system indicate which is the correct medication. This may prove valuable
when sequence of
ingesting medication is important, or when two people have similar medications
and may have
difficulty in distinguishing between medications for each. Once a correct
medication has been
identified, control passes to step 330.
1000801In step 330 user prompts and other instructions are provided to the
patient, and
present caregivers, as to how to administer the medication according to the
prescription guidelines
outlined above. These instructions allow for a user to receive further
information or instructions as
necessary through asking the system for additional help. Especially in
situations where an elaborate
scheme may be required, it is contemplated that video samples and instructions
may be provided to
the user. Further, in accordance with the invention, for complicated
administration procedures, it may
be possible to set up a two way video conference employing traditional video
conferencing, VOIP
conferencing, traditional telephone conferencing, or any other appropriate
communication system
with an expert in such administration so that a caregiver or patient may
receive live coaching
regarding such administration.
[00081] When following such instruction prompts, the actual act of
administration is
preferably captured as a video sequence at step 335. This captured video
sequence may be utilized in
accordance with the invention in a number of ways. First, the actions of
administration of the
medication is reviewed in real time and compared to an ideal or desired video
sequence. If a
determination is made that the medication is being administered in an
incorrect manner, and in a way
that may be detrimental to the patient, immediate warnings may be provided at
step 340 advising the
caregiver or patient to stop administration at once. Furthermore, in extreme
cases, a doctor or other
caregiver may be notified, or in the most dangerous cases, an ambulance or
other emergency
personnel may be dispatched to provide immediate care. If video recording is
not available, other
confirmation methods as noted above may be employed and be subject to
automatic confirmation as
with the video recorded sequences.
1000821If such immediate care or warning is not required, control then passes
to step 345
where the video images are more formally captured and analyzed for various
other non-critical issues.
The images may be captured and stored locally, being provided to a central
server in a batch
processing, or images may be captured and sent to the remote server for
immediate analysis and
storage. Such transmission may take place over a well known Internet
connection, wireless
connection, or other proprietary communication system. Such analysis may
consider suggestions to a
caregiver to improve dosage accuracy, reduce pain in administration, or the
like. Furthermore, as such
18
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
video sequences may be available from multiple patients and/or caregivers, the
effectiveness of
various sets of instructions and the like can be tested and reviewed, and
changes thereto made if
consistent problems are encountered. This type of study is nearly impossible
without the present
invention, because in any type of clinical setting, individuals are far more
likely to be careful in
administration of medication, and therefore not cause errors. However, in
accordance with the
invention, responses to instructions can be analyzed, and lack of adherence
based upon confusing or
difficult to follow instructions can be remedied, providing better or more
usable instructions, and
therefore improving regimen adherence.
[00083] In any event, after such analysis, any warnings or suggestions for
instruction issues
may generate a warning at step 350, suggesting areas of instruction that may
be problematic. These
video sequences are also stored for longer term analysis if desired at step
355, and processing ends.
10008411t is further contemplated in accordance with the invention that the
system and
method thereof act as an overall prescription management tool. For example,
luiowing the
prescription and the number of actual times the medication has been
administered, the system can
order prescriptions to be refilled and sent to the patient. Trends of a
patient can be monitored, such as
blood pressure or other measurable quantities of the patient, and correlation
between such measured
quantities and medication administration may be observed, potentially allowing
for a more
customized solution of medication to be applied to the patient, possible
modifying dosage or
frequency of administration based upon individual reactions to a particular
prescription regimen.
Further, insurance administration can be simplified by providing the
prescription information directly
to the insurance company, and allowing reimbursement for patient costs and
other billing issues to be
taken care of over a computer network, by phone or the like. Additionally,
features of the invention
noted above allowing for user interaction and recordation of activities of a
user, adverse effects and
the like may be incorporated into the system to provide further information
for determining alternative
instruction sets, modification of medications and the like.
[00085] A system provided in accordance with the invention includes imaging
technology and
hardware, communication hardware, computer systems including storage memory
and remote
communication via the Internet or other communication network for remote
storage and analysis,
databases of patient information and medication information sufficient to
implement the method as
described above.
[00086] Therefore, in accordance with the invention, a method and system are
provided that
allow for the automated administration of medication, and provide for a most
sophisticated method for
confirming and studying methods of administration of such prescription
medication.
[00087] In accordance with an alternative embodiment of the invention, a
system and process
are provided that improve adherence to medical protocol in a clinical trial
setting, and give
administrators a tangible and concrete manner in which to confirm compliance
or lack thereof, and the
ability to intervene early in the process to ensure that patients enrolled in
such a clinical trial study are
19
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
properly taking their medication. The system and method of the invention
provide for instructions to
patients on the use of the prescription medication under trial, verification
to a doctor or other service
provider of patient adherence to the prescribed protocol, and statistical and
individual analysis of
adherence rates to ensure proper clinical trial administration. The system and
method further improve
a level of care received by a patient in a clinical trial, and other setting,
as well as improve the
perception of that level of care by the patient. The system and method of the
invention further
provide the ability to track adverse events in a clinical trial or other
setting, thus allowing for these
events to perhaps be correlated with other events to aid in determination of
the effectiveness and/or
potential danger of a particular drug that is the subject of the trial.
Furthermore, the clinical trial
management application in accordance with this invention is contemplated to be
equally applicable to
the trial of medical devices or other apparatuses, as well as any method of
administration of
medication, including ingestion, injection, topical or other appropriate
delivery method.
[00088] Referring first to Figure 4, a data flow overview is shown. In
accordance with the
invention, information about a particular drug to be the subject of a clinical
trial is provided in a
database 405, and existing industry medication information databases 410 are
employed to access
prescription, interaction, application and other available information about
any number of proposed
prescription and non-prescription medications and their possible interaction
with the clinical trial
medication. Further, patient medical records 415 are used, and as will be
described below, are used in
conjunction with the industry medical information and a medical professional's
prescribing expertise
to confirm that a patient is a good candidate for such a clinical trial. Once
confirmed, a medicine
regimen in accordance with the clinical trial requirements is prescribed and
entered into the system of
the invention at 420. Once entered into the system, a particular prescription
regimen causes a set of
user instructions 425 to be generated. In accordance with the clinical trial,
such instructions may be
varied for different users to determine the best set of instructions, or may
be varied based upon
demographics, experience, or other factors that may require different types of
instructions to be
provided. It is further contemplated in accordance with the invention that
multiple clinical trials may
be managed by a manager in accordance with the invention so that the invention
contemplates a
clinical trial administration system that allows for a single point of
management for all clinical trials
associated with a particular manager or the like.
1000891 Such user instructions may include general instructions about the
particular
medication subject to the current trial, methods for ingestion, warnings about
side effects, concerns
about drug interactions with common substances or medications, or other
medications prescribed to
the patient by the system or by another medical service provider. It is
contemplated in accordance
with the invention that such set of user instructions may be interactive,
allowing a user to view
additional information about such instructions or prescriptions as desired.
These instructions may
comprise written, audio or video instructions. Furthermore, it is contemplated
in accordance with the
invention that at various points during the instruction set, for example when
a patient asks a particular
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
type of question, or asks to receive additional information about a particular
aspect of the medication
or prescription regimen, that the system may reach out and contact a
representative of a medical
service provider to provide the patient with additional, personal help as
necessary, if it is determined
that such intervention by the medical professional would be desirable to the
patient. Thus, such a
patient may be assisted in properly taking medication so that various errors
do not take place. Indeed,
in more traditional scenarios, it is only after perhaps finishing a
prescription regimen and a return to a
doctor in accordance with a predetermined clinical trial schedule that it is
discovered that the
medication may have been taken incorrectly. In accordance with the present
invention, early
intervention with such issues can be exercised to deter any possible
unfortunate outcomes from
improper administration of medication, and to ensure that the particular
patient is able to remain in the
study and provide accurate data to the study.
1000901 It is contemplated in accordance with the invention that a clinical
trial manager is
provided with a patient dashboard for managing regimes for one or more of the
patients taking part in
the clinical trial, and as noted above, for managing multiple clinical trials
taking place and managed
by the particular manager. Such a dashboard allows the clinical trial manager
to monitor any number
of patients in a manner that will be described below, allow statistical
analyses of patient adherence
and other patient reactions, provide links to information, including recorded
activity sequences for one
or more patients, record and be made aware of any adverse events taking place
during the trial, and
generally allow the medical professional to monitor and administer medication
to all of trial
participants from a convenient single access point. Such a dashboard also
allows for monitoring of
any health care providers that may be administering medication to a number of
the clinical trial
participants, or review of one or more administration sequences or the like.
1000911 Additionally, it is contemplated in accordance with the invention that
the patient is
provided with a user interface dashboard or the like allowing of modification
of prescription regimen
and instruction information by the patient, limited of course to within
guidelines established by the
prescribing medical provider. Thus, by way of example, if a user is to take a
particular medication
before eating in the morning, the user may be able to determine when a
reminder for such medication
will be given. If the patient is an early riser, the reminder may be provided
by cell phone or email at
6:00AM. Conversely, if the patient sleeps late and normally does not eat an
early meal, reminders can
be set for later in the morning, thus matching a patient's schedule. Of
course, any such patient
adjustments must be set within broader prescription regimen as defined by the
prescribing medical
provider. These requested modifications may give additional information to the
trial managers
regarding easier protocols for drug administration, or may lead one to
investigate a particular
individual for non-adherence to the trial protocol.
[00092] It is contemplated in accordance with the invention that a touch or
other user friendly
graphical user interface be provided so that the user can easily manipulate
any number of prescription
factors, and perhaps enter additional information that may be useful to a
prescribing medical provider,
21
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
such as level of fatigue, level of hunger, jitter inducing medications, etc.
All of these data collection
points allow for a smoother administration of medication to a patient, and
therefore a more likely
chance of adherence to a prescribed protocol.
1000931Referring to the lower portion of Figure 4, the horizontal line
indicates a time for
patient ingestion or other administration of medication. The patient may
display a medication
container and/or an actual pill or other medication form to an imaging
apparatus, and the apparatus
confirming that the medication is correct and is the currently prescribed
medication to be taken
through the user of text recognition, pill recognition, or other appropriate
medication recognition
scheme. This sequence of steps therefore acts as an audit trail each time a
medication is taken, that
can be reviewed later, to ensure that a patient is properly following a
regimen.
1000941 In accordance with the invention, confirmation of patient adherence to
the prescribed
administration schedule for the medication as prescribed by the clinical trial
regimen is determined.
While such confirmation may take a number of forms, in accordance with the
invention, a preferred
method for such confirmation may include capturing a video sequence of the
patient actually
administering the medication. In a further preferred method, such a sequence
for such confirmation
may include employing a facial recognition sequence or other biometric
confirmation that a particular
patient is in fact receiving treatment, but may also provide for the ability
to obscure the face or other
identifying feature of a user to allow for the storage and use of such images
while protecting the
identity of the patient, a technique that may be beneficial when a manager is
providing a general
report about the clinical trial, and not trying to remedy a situation with a
particular patient. Activity
recognition, gesture recognition or other feature for determining whether a
particular subject
movement meets a predefined movement sequence may be employed to be sure that
the patient is
properly taking prescribed medication.
1000951 Furthermore, in accordance with the present invention, a video image
of the patient
actually administering or ingesting the medication may be taken and stored so
that actual confirmation
may be achieved, rather than simply relying on the patient to state that a
particular medication was
administered. Such a video image may be captured or stored in any appropriate
format given a
selected type of activity or gesture recognition that is employed in
accordance with a particular
embodiment of the invention. Such may include full video, biometric data
points, recording of
movement of an article, such as a bracelet or the like, affixed to the patient
or administrator, use of
mapping to provide a stick figure or other body movement tracking technique,
or gesture or activity
recognition to determine movement or the like. The user may be encouraged to
use a particular
sequence of movement to be confirmed that they are properly administering the
medication according
to the protocol, thus reducing the possibility of the potential appropriate
movements considered to be
"correct." Indeed, various instructional videos or other appropriate training
may be provided to a user
to insure they properly administer the medication. Finally, in accordance with
the invention, if
recording of a video of a patient having the medication administered thereto
is not possible, the
22
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
system of the invention will recognize such an issue and request audio
confirmation as a next best
option. If the audio confirmation is also not possible, then a less reliable
method of confirmation,
such as a keyboard confirmation by the patient may be accepted. If higher
reliability methods of
confirmation are not available for an extended period of time, an alarm is
preferably forwarded to a
medical professional to inquire as to reasons and to remedy any situation that
might be wrong in the
administration situation.
[00096] These steps of confirming identity, confirming medication and
confirming
administration are then reviewed to verify adherence to the prescribed
protocol at 435. Such review is
preferably performed automatically by a computing system that is able to align
the actual recorded
images with ideal or expected images, or through the user of other activity or
gesture recognition as
mentioned above. In the case of facial recognition and bottle or pill
recognition, such techniques are
known in the art. With regard to video confirmation of adherence to prescribed
medicine
administration procedures, such processing may include various stick figure
comparison analyses,
activity recognition analysis, or other schemes as noted above able to
determine whether appropriate
actions have been performed by the patient.
[00097] The ability to provide automated determination of adherence to proper
administration
procedures allows for a large number of such images to be review in a short
period of time. Even if
actual and complete lack of adherence is not able to be determined 100% in
each possible situation,
the ability to pre screen the administration video captures to remove from
further consideration
administration situations that are clearly compliant may reduce a number of
compliance situations to
be reviewed by a medical professional substantially. Additional human review
of indications of
failure of adherence may be provided, thus insuring proper review of all
potentially dangerous
situations while greatly reducing the number of images necessary to be
reviewed by a human. Thus,
multiple benefits of such an automated system are realized, including reducing
time to review such
images, reducing costs of such review, and improving patient privacy by
limiting the number of
humans viewing such data, while improving quality.
1000981 In an additional embodiment of the invention the imaged sequences used
for activity
recognition to determine regimen adherence may be further used to check for
adverse or other
reactions to taking of the medication. Thus, in addition to simply determining
proper adherence to a
protocol, such activity or gesture recognition may determine any number of
different actions that may
have been taken by a patient. Thus, actions taken before medication
administration, or actions taken
after medication administration may give insight into reasons for particular
responses, etc. Thus,
before administration, in accordance with the invention, activity recognition
may determine a current
activity of a user. Any subsequent reminders to take a medication may in part
be based upon this
determined activity. By way of example, if a user is putting on a coat, or is
determined to be leaving a
residence or other facility or the like, a reminder to take a medication
before leaving may be provided,
even if earlier than normal, or if medication is portable, the user may be
reminded to take the
23
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
medication with them, and then subsequently reminded to administer the
medication via notification
on a mobile device. By way of further example, if the user is cooking, a
reminder may be given to
take the medication a predetermined time before eating. Other scenarios may be
possible, thus
allowing greater response from the system to ensure proper medication
administration by a patient.
Additionally, various patient consent issues may be prompted and recorded in
accordance with the
invention. Patients may ask further additional questions regarding such
consent, thus insuring that
patients have all of the information they need to make informed consent
decisions, and medication
providers have proper evidence of such consent.
[00099] Similarly, actions after taking medication may give insight into
patient responses.
Notice of fainting, falling down, lack of motion, facial gestures,
gastrointestinal distress or the like
may all be logged as adverse reactions to a particular medication regimen, and
may allow for
adjustment of dosage or prescription instructions in the future for the
patient. If adverse reactions are
severe, an immediate medication review and contact from a medical professional
may be provided to
cure the issue. Additionally, the system in accordance with the present
invention may be directly tied
and be interoperable with a pharmacy or medical provider's systems as
administered by the clinical
trial manager, thus allowing such recommendations for dosage changes, regimen
changes and the like
to be forwarded to these professionals automatically. Through such links,
reordering medication,
dosage changes, medication changes and the like may be automatically provided.
Furthermore, ease
of providing additional prescriptions can be enhanced as patient, medication
and regimen information
will already be available to the pharmacist or medical service provider.
[000100] After such
automatic, or combination of automatic and manual, adherence
verification is performed allowing a health care provider or other medical
professional to review and
verify results of the automatic comparison or direct review of captured
activity sequences, and
indication of variation from a desired identity, medication or application
procedure may request
administrator review of the situation, and intervention as may be determined
necessary at 440. Such
review may be required immediately as an emergency situation may exist, or the
patient may gesture
or otherwise indicate that help is necessary, or such review may be less
urgent, perhaps requiring an
electronic communication with suggestions or the like from such an
administrator or the like.
Additionally, such adherence review may be stored over time for a particular
patient, thus allowing for
various medication trends to be determined, such as if a patient misses
medication at a same time each
week, or an indication that one particular health care worker aiding the
patient may occasionally give
an incorrect medication dosage amount. Thus, in addition to allowing for
immediate notice of
problems in medication administration, an audit trail for tracking the actions
of various health care
providers is generated. Such an audit trail allows clinical trial
administrators or other medical
professional to determine immediately, or upon review of a complete medication
regimen, whether a
particular patient or group of patients has followed the protocol to the level
that allows their results to
be used as part of the study. Additionally, levels of adherence may give
insight as to the ease of use of
24
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
a particular regimen, and whether instructions provided to the patient are
sufficient to allow for
adherence to the prescribed medication regimen.
[000101] Therefore,
in accordance with the invention as set forth in Figure 4, a method
and system are provided in which patient adherence to a prescribed medication
regimen as set forth in
a clinical trial scenario can be reviewed, acutely for a particular instance,
or over time to determine
any changes in behavior of a patient. Because all aspects of such adherence
are monitored preferably
visually, and do not rely on patient confirmation of medication
administration, desires of the patient
are taken out of the equation, and a true review of actually procedures used
in the medication
administration can be studied. Trial managers are therefore able to keep
patients on their prescribed
regimens, thus reducing the overall cost associated with the clinical trials,
and remove patients from
the test population when they cannot maintain adherence to the prescribed
regimen, thus increasing
the accuracy and repeatability of the results of the trial. Through the
management of multiple clinical
trials, patient interaction is eased, and any similarities across trials may
also be determined, thus
improving quality of care generally and allowing for consistency across any
number of trials.
[000102] Referring
next to Figure 5, a more precise description of the data, medical
record and prescription regimen entry will now be described. As is shown in
Figure 5, a clinical trial
manager 502 enters information into a system in accordance with the invention,
and in particular
enters information regarding medication regimen, dosage, instructions sets and
the like. A patient 506
may also enter information relevant to the patient. The trial manager 502 may
further enter or alter
patient information, basic medical statistics, and virtually all other medical
information included in a
database. The patient 506 may be limited to providing personal information,
and perhaps other
relevant information, such as non-prescription medication usage, alcohol
consumption, recent
symptoms, reaction to use of particular prescription medications and the like.
Such data entry allows
for the tracking of an audit trail at this step of administration as well. Of
course, any of this
information may be automatically entered into a system from a database having
been previously
entered or otherwise obtained from an existing data storage area. Also, if
particular information is
omitted by the patient, or is otherwise unavailable, it might be possible to
request provision of this
information, or otherwise remove the patient from the clinical trial.
Additionally, determination of
failure of proper procedure of the information collector may be determined so
that additional training
or removal of this individual may take place before a large number of patients
are entered into a
clinical trial with insufficient information collection.
[000103] Information
from each user of the system is combined, where available, and
formed into a clinical trial medication entry 510. In particular, a preferred
list of information that may
first be provided in accordance with the invention in order to organize the
system may comprise a
patient name, a user name, if that user is not the patient, and information to
be used to provide various
alerts, such as a patient email for prescription reminders, contact
information for a doctor or caregiver
for emergency contact, mailing address for various bulk and non-urgent
communications, and cellular
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
telephone or landline telephone information for patient contact, or the like.
Further notifications may
be provided to the trial manager 502 so that the manager can monitor patient
use of the medication
and adherence to the proposed and prescribed medication administration. In
addition, in accordance
with the invention, facial images of the patient and other people interacting
with the system are stored,
or other biometric identifiers, such as fingerprint identification, retinal
identification, voice
recognition, various provided RFID tags or the like may be employed along with
more traditional
passwords and user names or the like. Of course, if the patient is a returning
patient, such information
may be extracted from an existing database stored with another, previous
prescription.
[000104] Next, a user
such as the doctor 502 or the pharmacist 504 or nurse, may
indicate a particular new or recurring prescription medication to be provided
to a user in accordance
with the clinical trial. An external medication database 515 is accessed and
infoimation regarding
such medication is provided, including medication name, suggestions for
appropriate prescription
dosages based upon patient information and the particular version of the
medication to be
administered, and usage instructions to be provided to the patient, these
instructions being modified or
supplemented as necessary by the trial manager. These usage instructions
preferably include detailed
administration instructions, including time of day, patient status (i.e.
before or after eating, after
waking, before sleeping, etc.), precise method of application, and other
useful instructions for a user.
These instructions also may include video sequences to describe particular
details of the medication or
administration procedure thereof and in order to properly indicate gestures to
be implemented by the
patient. They may also comprise alternative versions of instructions so that
if a user is unclear
regarding a particular set of instructions, an alternative set of instructions
may be provided to the
patient to aid in adherence to the prescription regimen.
10001051 After
selection of such a medication, a patient medical history 517 is
preferably accessed to provide additional medication and patient information
to the trial manager.
Such information may include, other prescriptions to the patient so that
adverse drug interaction may
be determined (although if such other prescriptions were implemented in
accordance with this
invention, such prescriptions will already be known to the system), patient
indication of use of non-
prescription drugs, patient allergies, patient activity level, past diseases
and procedures, and any other
information that may be relevant to the prescription of medication.
[000106] After all of
the information has been entered about the user, and information
from the medication and patient database has been accumulated, various
medication interactions are
checked automatically by the system at step 520. Any dangerous interactions
are noted to the trial
manager, and may preclude entry of the prescription into the system and
therefore preclude the
particular patient from participating in the clinical trial. Other
interactions may be noted to the trial
manager so that the individual may make the patient aware of such situations.
These may include, for
example a notification that the taking of two medications together may result
in stomach pains, so that
the patient should take one in the morning and one in the afternoon. Such
interactions check will then
26
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
result in a set of instructions that will be provided to the user, in addition
to the more generic
medication instructions. Finally, the trial manager may review such
instructions and supplement them
as desired. It should be noted that in accordance with the invention it is
contemplated that various
instructions provided to a user may comprise hot links to additional audio and
visual information that
may be provided to a patient to further assist in their adherence to any
particular prescribed protocol,
or may provide various information regarding the particular medication being
taken as will be further
described below. Such instructions may indicate methods by which a user is to
administer one or
more medications, and thus requests use of a particular gesture or the like
during administration.
[000107] Finally,
after all interactions and instructions have been reviewed and
approved, the prescription information is stored at step 525. The storing of
such a prescription makes
the prescription, alerts, help information and the like accessible to a
patient and other system users.
Furthermore, such completion may also transmit the prescription to a pharmacy
or other medication
provision facility so that the user is able to simply swing by to pick up the
medication or to have the
medication delivered to the patient.
[000108] Referring
next to Figure 6, a user implementation of the method in
accordance with the invention will be described. When a time for receiving or
administering a
medication in accordance with a clinical trial regimen is reached, a patient,
and any other necessary
user may be provided a notification 610 in accordance with notification
addresses entered in the
system as noted above. Thus, in a home situation, only the patient may receive
notification. If there
is a home health care provider, such provider may also receive separate
independent verification. In
the case of hospital or other in-patient care facility, various medical
service providers may similarly
receive such notification. After notification, or in the absence of such
notification, system initiation
takes place at 615. In accordance with such system initiation, one or more
users are preferably
recognized by the system. Therefore, at step 620, a user recognition sequence
takes place. In a
preferred embodiment, such a user has a still or video image captured of their
face, and facial
recognition techniques are employed to confirm the identity of the user. Such
capture may be
performed by fixed camera, mobile camera, mobile communication device such as
a cellular phone, or
any other appropriate video or image capture device. Alternative recognition
techniques, such as
retinal, fingerprint, voice or other biometric measurements may be employed,
in addition to a more
common password query. Any other appropriate identification technique may be
employed, and any
unique individual identifiers may be obscured, as noted above, when the images
are to be used as a
more general report regarding adherence, rather than an individual patient
response.
[000109] At step 625
it is determined whether all necessary users have been recognized
and authenticated. In a situation where a nurse, doctor or other caregiver is
to administer medication,
it may be preferable to have the patient and caregiver to be recognized by the
system to further
confirm that the appropriate procedure is followed, and to allow the system to
keep track of people
using the medication so it can track if any one person, for example, is
improperly using the
27
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
medication, as will be evident from the generated audit trail. After step 625,
if all users are not
recognized, control passes back to step 320 and any additional users are
recognized by the system.
[000110] Once all
users are recognized, control passes to step 627 where the
medication to be administered in accordance with the prescribed prescription
regimen of the clinical
trial is confirmed. Thus, a user is prompted to allow a still or video image,
text recognition image, or
other method of identifying a medication to be captured of the medication
bottle or other container, a
pill of the medication, or other form of medication, and is also able to
determine appropriate
quantities, dosage, and any potentially required or dangerous medication
combinations. As noted
above, if video confirmation is for some reason not available, the user may be
prompted by the system
to provide audio or other indication of medication and other desired
information. This image, video
sequence or other received confirmation infomiation is then compared to an
image associated with the
prescription as noted above in Figure 5. If the medication is determined to be
incorrect, a warning
may be provided to the user that the medication is incorrect. The user may
then be prompted to
choose another medication for imaging.
[000111]
Alternatively, the invention contemplates a user displaying a number of
medications to the image capture apparatus and allowing the apparatus to
suggest which medication is
correct. Thus, the user may be able to scan a medicine cabinet with such a
video imaging apparatus
and have the system indicate which is the correct medication. This may prove
valuable when
sequence of ingesting medication is important, or when two people have similar
medications and may
have difficulty in distinguishing between medications for each. Once a correct
medication has been
identified, control passes to step 630.
[000112] In step 630
user prompts and other instructions are provided to the patient,
and present caregivers, as to how to administer the medication according to
the prescription guidelines
outlined above as determined in accordance with the particular clinical trial
under consideration.
These instructions allow for a user to receive further information or
instructions as necessary through
asking the system for additional help. Especially in situations where an
elaborate scheme may be
required, it is contemplated that video samples and instructions may be
provided to the user. Further,
in accordance with the invention, for complicated administration procedures,
it may be possible to set
up a two way video conference employing traditional video conferencing, VOIP
conferencing,
traditional telephone conferencing, or any other appropriate communication
system with an expert in
such administration so that a caregiver or patient may receive live coaching
regarding such
administration. Such instructions and prompts may be determined by the
clinical trial manager to
determine the success or failure of particular sets of instructions. Thus, not
only are medications
tested in such a clinical trial, but also sets of instructions are tested to
determine which are best for all,
or for giver demographic groups or the like for eventual user when the
medication is released to the
public, thereby allowing for a better adherence rate by the public. As the
number of clinical trials
grows, and the locations of such trials becomes more international, such
administration through a
28
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
system such as that set forth in accordance with the invention may become far
more important to
various clinical trial managers.
[000113] When
following such instruction prompts, the actual act of administration is
preferably captured as a video sequence at step 635. Thus administration
preferably includes one or
more identifiable gestures as suggested in accordance with the instructions
above. Thus, a patient or
administrator may be provided with one or more images or sequences for method
of application or
administration, and thus the following of these sequences is used to determine
compliance with a
particular prescribed regimen. Further, long gaps or pauses may be determined
to give further insight
into areas of administration that may be giving problems to administrators of
trial participants. This
captured video sequence may be utilized in accordance with the invention in a
number of ways. First,
the actions of administration of the medication is reviewed in real time and
compared to an ideal or
desired video sequence. If a determination is made that the medication is
being administered in an
incorrect manner, and in a way that may be detrimental to the patient,
immediate warnings may be
provided at step 340 advising the caregiver or patient to stop administration
at once. Furthermore, in
extreme cases, a doctor or other caregiver may be notified, or in the most
dangerous cases, an
ambulance or other emergency personnel may be dispatched to provide immediate
care. Notification
may also be provided to the clinical trial manager so that this person is
warned that one or more
patients are having problems with adherence with the protocol. If such
problems turn out to be
isolated, alternative instructions, or personal help may remedy the situation,
thus allowing the person
to provide meaningful data to the trial. If such lack of adherence is far more
widespread, the clinical
trial manager may change instructions for all participants, or may even ask
all participants to come
back in for further live instructions. In either situation, the cost of the
clinical trial is greatly reduced
as participants are able to remain in the study, and major failures of studies
for lack of protocol
adherence may be avoided. Reports based upon such widespread lack of adherence
may provide a
manager with a report, using images or the like with identifying features
removed so that the report
may show precisely how a medication is being administered while maintaining
patient privacy and
confidentiality. If video recording is not available, other confirmation
methods as noted above may be
employed and be subject to automatic confirmation as with the video recorded
sequences.
[000114] If such
immediate care or warning is not required, control then passes to step
645 where the video images are more formally captured and analyzed for various
other non-critical
issues. The images may be captured and stored locally, being provided to a
central server in a batch
processing, or images may be captured and sent to the remote server for
immediate analysis and
storage. Such transmission may take place over a well known Internet
connection, wireless
connection, or other proprietary communication system. Such analysis may
consider suggestions to a
caregiver to improve dosage accuracy, reduce pain in administration, or the
like. These suggestions
may be implemented as tweaks to various instruction or training sequences
before the medication is
released to the general public. Furthermore, as such video sequences may be
available from multiple
29
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
patients and/or caregivers taking part in the current or multiple clinical
trials, the effectiveness of
various sets of instructions and the like can be tested and reviewed, and
changes thereto made if
consistent problems are encountered. This type of study is nearly impossible
without the present
invention, because in any type of clinical setting, individuals are far more
likely to be careful in
administration of medication, and therefore not cause errors. When they return
home or back to their
regular lives, this is when adherence and administration issues arise. In
accordance with the
invention, responses to instructions can be analyzed, and lack of adherence
based upon confusing or
difficult to follow instructions can be remedied, providing better or more
usable instructions, and
therefore improving regimen adherence. Without the present invention, such a
clinical trial manager
may be unaware of such issues. With the invention, these issues can be
addressed as they arise, thus
ensuring the integrity of the clinical trial.
[0001151 In any
event, after such analysis, any warnings or suggestions for instruction
issues may generate a warning at step 650, suggesting areas of instruction
that may be problematic.
These video sequences are also stored for longer term analysis if desired at
step 655, and processing
ends. Furthermore, in order to encourage patients in the clinical trial,
notices of lack of adherence
may be used to change remuneration received by a patient in the clinical
trial. Thus, adherence to the
protocol results in higher payments to the patient, a great incentive for the
patient to adhere to the
protocol.
[000116] Trends of a
patient taking part in the clinical trial can be monitored, such as
blood pressure or other measurable quantities of the patient, and correlation
between such measured
quantities and medication administration may be observed, potentially allowing
for a more
customized solution of medication to be applied to the patient, possible
modifying dosage or
frequency of administration based upon individual reactions to a particular
prescription regimen.
Additionally, features of the invention noted above allowing for user
interaction and recordation of
activities of a user, adverse effects and the like may be incorporated into
the system to provide further
information for determining alternative instruction sets, modification of
medications and the like.
[000117] It is
further contemplated that the clinical trial manager be provided with a
manager dashboard allowing for summary review of all patients taking part in
one or more clinical
trials. Details of an exemplary embodiment of such a dashboard will now be
described making
reference to Figures 7-9. As is shown in Figure 7, a summary page dashboard is
shown at 700. Each
different clinical trial is contemplated to have one or more summary pages (as
more than one page
may be necessary for a particular clinical trial given the number of
participants). Each summary page
includes indicators 710 for each participant taking place in a particular
clinical trial. In Figure 7 such
indicators are shown as icons representing each person. Such icons may be
replaced with summary
identification information or the like, or any other desired indicator, such a
demographic indicators
(i.e. age group, sex, medical condition, individual characteristics, etc.)
Also included in dashboard
700 is an indicator legend including information that may be applied to any
one or more of the
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
indicators 710. In this particular preferred embodiment of the invention, the
indicator legend includes
indicator modifiers representing that a particular trial participant asked a
question, has a report of poor
compliance, or requested clarification of some instruction or other facet of
the clinical trial. As is
shown, each indicator modifier may be applied to a particular indicator 710 to
which the modifier
applies. Thus, as is shown in Figure 7, indicator 712 has been flagged for
poor compliance, while
indication 714 has been flagged as asking a question. Thus, in this manner, a
clinical trial manager is
provided with an overview of the status of the patients in a clinical trial.
Major compliance problems
would be easily determined by the clinical trial manager viewing a dashboard
with many of the
indicators 710 being modified by the indicator modifier related to poor
compliance. Similarly,
problems with instructions might be apparent if the summary display indicates
a large number of
questions or clarification requests. Of course indicator modifiers for any
number of other situations
may be provided. Statistical analysis of the population of the clinical trial
may also be provided, thus
allowing for the clinical trial manager to determine adherence rates and other
metrics of the entire or
subsection of the clinical trial population. Such modifiers are preferably
color indicators, but may
comprise any other appropriate indication that allows the clinical trial
manager to determine status.
[000118] Dashboard
700 further preferably includes a zoom indicator that allows the
clinical trial manager down to each patient or a smaller number of patients to
view their overall
compliance statistics, or to view individual recorded video sequences,
requests for additional
information, or any other information stored as the audit trail for patients
in accordance with the
invention. Thus, if the zoom indicator is zoomed all of the way out, the
clinical trial manager is
presented with the largest number of indicators 710 on the dashboard. A medium
zoom preferably
provides the clinical trial manager with a view such as that shown in Figure
8. As is shown, indicator
712 includes additional information adjacent the indicator. In the displayed
situation, the name for the
indicator modifier is shown. This may be beneficial when a large number of
indicator modifiers may
be provided. Alternatively, this text might include a most critical piece of
information, or a user
selected piece of information. The zoom feature may also allow the clinical
trial manager to zoom
down by demographic information of the like, thus viewing indicators 710
representing clinical trial
participants having similar characteristics in one form or another.
[000119] In addition,
it is contemplated that the clinical trial manager may use the
summary dashboard, at any desired level of zoom, to select and move, modify,
or otherwise act upon a
subset of indicators included in a clinical trial. Thus, one or more
participants may be assigned to
different populations, grouped as desired to receive different instructions,
dosages, or have any other
modification of medication administration applied to the selected group of
participants. Participants
may also be grouped based upon prescribing medical provider, clinic, medical
group, insurance
company, or in any other manner that might be considered useful to the
clinical trial manager. By
grouping by these groups, it may be possible to determine if any particular
clinic, doctor or the like
results in poor compliance for that group. Thus, poor compliance linked to a
particular such group
31
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
can also be addressed through, perhaps, additional training for the clinic or
doctor. Participants may
also be added to, or deleted from a particular trial or other epidemiological
study or the like, if
desired, or otherwise manipulated. Groupings may also be automated, such as
grouping all patients
by age, gender, or other demographic characteristic, thus providing a quick
useful view to the clinical
trial manager.
[000120] Further
zooming in allows the clinical trial manager to view a smaller number
of indicators, down to a single indicator. As the number of indicators
decreases, the amount of
information available to the clinical trial manager for each indicator
increases. Thus, the clinical trial
manager may zoom down to one of the possible levels as shown in Figure 9. As
is shown in Figure 9,
the first zoom level shows the level of information of Figure 8, while a great
number of indicators 710
are still visible. A more detailed zoom level preferably displays The same
poor compliance indicator,
but further shows that this poor compliance is related to the medication
schedule, and in particular a
supply shortage of the medication, thus providing a more complete compliance
check of the clinical
trial participant. The information further provides poor compliance
information in that the participant
represented by the indicator has not yet reviewed a required (or suggested)
training video, and is also
having difficulty with a fifth process in the defined confirmation scheme.
Finally, a further zoom
level, after indication a desire to have more information regarding the poor
compliance indicates that
the participant has only had 7% of their actions confirmed by video, 10%
confirmed by voice, and the
remaining 83% confirmed by GUI interface (which is patient dependent and
therefore is not
determined to be as reliable as the other two methods). Furthermore, the
participant has asked a
particular question. From this particular zoom level, it is contemplated that
the clinical trial manager
may directly link to the stored video, audio or GUI provided information.
Preferably, a timeline or the
like is provided to the clinical trial manager indicating when the various,
video, audio and/or GUI
entries have been stored. The clinical trial manager is able to view an even
more useful picture of the
sequence of compliance, and is then able to select any of the stored sequences
for review. The
manager may also answer the question, or forward the question to a physician
or the like who is
appropriate to answer the question. The clinical trial manager may also opt to
change instructions
provided to one or more patients in accordance with their record of adherence
when being exposed to
a first set of instructions. This level of zoom may also provide links for
allowing communication with
a particular participant, and method for suggesting additional training,
instructions or help, and a
process for ordering or providing additional medication to a participant.
Specific instructions to a
patient, preferences, or comments or the like provided by a patient may also
be reviewed. Finally, the
clinical trial manager may view upcoming prescription information, including
times for taking a
prescription or other medication administration applicable to one or more
patients.
[000121] It is
therefore through such a dashboard that the clinical trial manager
receives notice of any problematic failures on the part of patients warranting
intervention, either by
direct contact, changes in regimen or instruction, or recall of the patient
for retraining. As noted
32
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
above, rather than waiting for the patient to come back in and report poor
adherence, or worse yet,
allowing the patient data to become part of the clinical resultant data even
with poor adherence, the
clinical trial manager may use the dashboard of the invention to ensure that
poor compliance is
recognized early and dealt with. Furthermore, emergency situations may be
indicated to the clinical
trial manager in an immediate manner.
[000122] Such a
dashboard is preferably provided with patient management features in
addition to the notification features noted above. Thus, the clinical trial
manager is able to manage
individual participants, subsets of the population, or the entire population
through drop and/or drag
functionality, and is able to broadcast information to these groups as
necessary, including the
broadcast of video, text, or voice instructions. The clinical trial manager
may also preferably remind
participants of upcoming doctor appointments, meetings regarding the clinical
trial, and also provide
such notices to the patient's prescribing physician. Additional medication or
supplies may be
provided to a participant upon notice that they have run out, or merely
tracking of medication actually
taken by the participant. Thus, there is no guesswork. The manager may further
review patient
medical records, as necessary, to further aid in trial procedure compliance.
[000123] The
dashboard also preferably provides access to medical professionals
associated with one or more of the clinical trial participants. In a situation
where the trial provides all
of the prescribing doctors or other medication administrators, for those
patients that may need
assistance, this feature may be trivial, but when multiple prescribing doctors
are involved in a trial,
being able to forward information and interact with these doctors may prove
very valuable. Such
contact may be made through a link or connection provided associated with each
patient identifier
icon on the dashboard. Thus, the clinical trial manager may be able to review
videos or other
compliance indicia with such a prescribing physician, perform remote triage
regarding any
complications that might arise with a patient during the course of the trial,
or request prescription
changes, etc. Furthermore, the clinical trial manager may be able to audit
these very medical
professionals looking after, and administering medication in accordance with
the prescribed clinical
trial procedure. Through such an audit, the manager may be able to determine
issues related to
actions of a particular administrator.
[000124] A user
dashboard may also be provided to allow a participant to enter
information, such as GUI entered compliance information as noted above, but
also to ask questions,
view additional instructions, order supplies, or the like. The user may view
more specific prescription
regimen information, such as when a next medication is scheduled to be taken,
or be provided with a
calendar for a predetermined future time period to allow for future planning.
Through such a user
dashboard, the user may view particular information relevant to their
treatment, but preferably not
enough information to influence their participation in the trial. If in use, a
participant may also view
whether they have achieved various adherence levels resulting in payments to
be made to them in
accordance with the invention. Furthermore, the user may be provided with a
method for determining
33
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
a level of data transmission, including identity information that may be
provided to various level of
administrator. Thus, while all information may be provided to a clinical trial
manager, perhaps more
limited identifying information may be made available to other researchers or
the like. The
participant may be notified as to potential uses, and an opt in or opt out set
of choices for such
different levels of disclosure. The user may be provided with a privacy
setting indicator to indicate a
level of availability of recorded information and attachment of identifying
information thereto.
[000125] Thus,
provision of such a dashboard in conjunction with the operative clinical
trial verification process and system described above eases use, increases
effectiveness and allows for
better control over such a clinical trial. Difficulties in following protocols
may be determined in near
real time. Changes in a trial may be easily implemented and conveyed to trial
participants and their
prescribing doctors. New training modules, including video, written, or other
instructions may be
provided during a trial in order to improve adherence. Inventory management is
eased and all trial
participants are sure to have the appropriate amounts of medication and other
supplies. Further, a full
audit trail of who has accessed information for any particular patient, groups
of patients, or one or
more clinical trials is maintained. This dashboard, however, is not limited to
use only in a clinical
trial. Rather, the system can be used by a single medical provider, doctor
group or network, insurance
company, governmental agency, nursing home, hospice, home care provider or the
like, or other group
in order to track any number of patients and associated home care workers,
doctors, or other
medication administrators. In such scenarios, the ability to view, group, and
retrieve significant
information about particular patients allows for greater control over these
patients, resulting in
reduced likelihood of a patient continuing to take improper medication, or to
improperly administer
such medication, and therefore in turn reducing the possibility of an adverse
reaction or death from a
particular medication.
[000126] A system
provided in accordance with the invention includes imaging
technology and hardware, communication hardware, computer systems including
storage memory and
remote communication via the Internet or other communication network for
remote storage and
analysis, databases of patient information and medication information
sufficient to implement the
method as described above. All communications are encrypted or otherwise
protected during
transmission and storage at both local and remote mass storage location to
meet any security issues
and any regulations required for the storage and maintenance of medical and
patient health care
information.
[000127] Therefore,
in accordance with the invention, a method and system are
provided that allow for the automated confirmation of adherence to
administration protocol for
medication in a clinical trial environment, and provide for a most
sophisticated method for confirming
and studying methods of administration of such prescription medication.
[000128] In
accordance with another embodiment of the invention, the invention, a
system and process are provided that improve adherence to medical protocol in
a clinical trial setting,
34
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
and give administrators a tangible and concrete manner in which to confirm
compliance or lack
thereof, and the ability to intervene early in the process to ensure that
patients enrolled in such a
clinical trial study are properly taking their medication. The system and
method of the invention
provide for instructions to patients on the use of the prescription medication
under trial, verification to
a doctor or other service provider of patient adherence to the prescribed
protocol, and statistical and
individual analysis of adherence rates to ensure proper clinical trial
administration. The system and
method further improve a level of care received by a patient in a clinical
trial, and other setting, as
well as improve the perception of that level of care by the patient. The
system and method of the
invention further provide the ability to track adverse events in a clinical
trial or other setting, thus
allowing for these events to perhaps be correlated with other events to aid in
determination of the
effectiveness and/or potential danger of a particular drug that is the subject
of the trial. Furthel more,
the clinical trial management application in accordance with this invention is
contemplated to be
equally applicable to the trial of medical devices or other apparatuses, as
well as any method o
administration of medication, including ingestion, injection, topical or other
appropriate delivery
method.
[000129] Referring
first to Figure 10, a data flow overview is shown. In accordance
with the invention, information about a particular drug to be the subject of a
clinical trial is provided
in a database 1005, and existing industry medication information databases
1010 are employed to
access prescription, interaction, application and other available information
about any number of
proposed prescription and non-prescription medications and their possible
interaction with the clinical
trial medication. Further, patient medical records 1015 are used, and as will
be described below, are
used in conjunction with the industry medical information and a medical
professional's prescribing
expertise to confirm that a patient is a good candidate for such a clinical
trial. Once confirmed, a
medicine regimen in accordance with the clinical trial requirements is
prescribed and entered into the
system of the invention at 1020. Once entered into the system, a particular
prescription regimen
causes a set of user instructions 1025 to be generated. In accordance with the
clinical trial, such
instructions may be varied for different users to determine the best set of
instructions, or may be
varied based upon demographics, experience, or other factors that may require
different types of
instructions to be provided. It is further contemplated in accordance with the
invention that multiple
clinical trials may be managed by a manager in accordance with the invention
so that the invention
contemplates a clinical trial administration system that allows for a single
point of management for all
clinical trials associated with a particular manager or the like.
[000130] Such user
instructions may include general instructions about the particular
medication subject to the current trial, methods for ingestion, warnings about
side effects, concerns
about drug interactions with common substances or medications, or other
medications prescribed to
the patient by the system or by another medical service provider. It is
contemplated in accordance
with the invention that such set of user instructions may be interactive,
allowing a user to view
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
additional information about such instructions or prescriptions as desired.
These instructions may
comprise vvritten, audio or video instructions. Furthermore, it is
contemplated in accordance with the
invention that at various points during the instruction set, for example when
a patient asks a particular
type of question, or asks to receive additional information about a particular
aspect of the medication
or prescription regimen, that the system may reach out and contact a
representative of a medical
service provider to provide the patient with additional, personal help as
necessary, if it is determined
that such intervention by the medical professional would be desirable to the
patient. Thus, such a
patient may be assisted in properly taking medication so that various errors
do not take place. Indeed,
in more traditional scenarios, it is only after perhaps finishing a
prescription regimen and a return to a
doctor in accordance with a predetermined clinical trial schedule that it is
discovered that the
medication may have been taken incorrectly. In accordance with the present
invention, early
intervention with such issues can be exercised to deter any possible
unfortunate outcomes from
improper administration of medication, and to ensure that the particular
patient is able to remain in the
study and provide accurate data to the study.
[000131] It is
contemplated in accordance with the invention that a clinical trial
manager is provided with a patient dashboard for managing regimes for one or
more of the patients
taking part in the clinical trial, and as noted above, for managing multiple
clinical trials taking place
and managed by the particular manager. Such a dashboard allows the clinical
trial manager to
monitor any number of patients in a manner that will be described below, allow
statistical analyses of
patient adherence and other patient reactions, provide links to information,
including recorded activity
sequences for one or more patients, record and be made aware of any adverse
events taking place
during the trial, and generally allow the medical professional to monitor and
administer medication to
all of trial participants from a convenient single access point. Such a
dashboard also allows for
monitoring of any health care providers that may be administering medication
to a number of the
clinical trial participants, or review of one or more administration sequences
or the like.
[000132]
Additionally, it is contemplated in accordance with the invention that the
patient is provided with a user interface dashboard or the like allowing of
modification of prescription
regimen and instruction information by the patient, limited of course to
within guidelines established
by the prescribing medical provider. Thus, by way of example, if a user is to
take a particular
medication before eating in the morning, the user may be able to determine
when a reminder for such
medication will be given. If the patient is an early riser, the reminder may
be provided by cell phone
or email at 6:00AM. Conversely, if the patient sleeps late and normally does
not eat an early meal,
reminders can be set for later in the morning, thus matching a patient's
schedule. Of course, any such
patient adjustments must be set within broader prescription regimen as defined
by the prescribing
medical provider. These requested modifications may give additional
information to the trial
managers regarding easier protocols for drug administration, or may lead one
to investigate a
particular individual for non-adherence to the trial protocol.
36
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[000133] It is
contemplated in accordance with the invention that a touch or other user
friendly graphical user interface be provided so that the user can easily
manipulate any number of
prescription factors, and perhaps enter additional information that may be
useful to a prescribing
medical provider, such as level of fatigue, level of hunger, jitter inducing
medications, etc. All of
these data collection points allow for a smoother administration of medication
to a patient, and
therefore a more likely chance of adherence to a prescribed protocol.
[000134] Referring to
the lower portion of Figure 10, the horizontal line indicates a
time for patient ingestion or other administration of medication. The patient
may display a medication
container and/or an actual pill or other medication form to an imaging
apparatus, and the apparatus
confirming that the medication is correct and is the currently prescribed
medication to be taken
through the user of text recognition, pill recognition, or other appropriate
medication recognition
scheme. This sequence of steps therefore acts as an audit trail each time a
medication is taken, that
can be reviewed later, to ensure that a patient is properly following a
regimen.
[000135] In
accordance with the invention, confirmation of patient adherence to the
prescribed administration schedule for the medication as prescribed by the
clinical trial regimen is
determined. While such confirmation may take a number of forms, in accordance
with the invention,
a preferred method for such confirmation may include capturing a video
sequence of the patient
actually administering the medication. In a further preferred method, such a
sequence for such
confirmation may include employing a facial recognition sequence or other
biometric confirmation
that a particular patient is in fact receiving treatment, but may also provide
for the ability to obscure
the face or other identifying feature of a user to allow for the storage and
use of such images while
protecting the identity of the patient, a technique that may be beneficial
when a manager is providing
a general report about the clinical trial, and not trying to remedy a
situation with a particular patient.
Activity recognition, gesture recognition or other feature for determining
whether a particular subject
movement meets a predefined movement sequence may be employed to be sure that
the patient is
properly taking prescribed medication.
[000136] Furthermore,
in accordance with the present invention, a video image of the
patient actually administering or ingesting the medication may be taken and
stored so that actual
confirmation may be achieved, rather than simply relying on the patient to
state that a particular
medication was administered. Such a video image may be captured or stored in
any appropriate
format given a selected type of activity or gesture recognition that is
employed in accordance with a
particular embodiment of the invention. Such may include full video, biometric
data points, recording
of movement of an article, such as a bracelet or the like, affixed to the
patient or administrator, use of
mapping to provide a stick figure or other body movement tracking technique,
or gesture or activity
recognition to determine movement or the like. The user may be encouraged to
use a particular
sequence of movement to be confirmed that they are properly administering the
medication according
to the protocol, thus reducing the possibility of the potential appropriate
movements considered to be
37
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
"correct." Indeed, various instructional videos or other appropriate training
may be provided to a user
to insure they properly administer the medication. Finally, in accordance with
the invention, if
recording of a video of a patient having the medication administered thereto
is not possible, the
system of the invention will recognize such an issue and request audio
confirmation as a next best
option. If the audio confirmation is also not possible, then a less reliable
method of confirmation,
such as a keyboard confirmation by the patient may be accepted. If higher
reliability methods of
confirmation are not available for an extended period of time, an alarm is
preferably forwarded to a
medical professional to inquire as to reasons and to remedy any situation that
might be wrong in the
administration situation.
[000137] These steps
of confirming identity, confirming medication and confirming
administration are then reviewed to verify adherence to the prescribed
protocol at 1035. Such review
is preferably performed automatically by a computing system that is able to
align the actual recorded
images with ideal or expected images, or through the user of other activity or
gesture recognition as
mentioned above. In the case of facial recognition and bottle or pill
recognition, such techniques are
known in the art. With regard to video confirmation of adherence to prescribed
medicine
administration procedures, such processing may include various stick figure
comparison analyses,
activity recognition analysis, or other schemes as noted above able to
determine whether appropriate
actions have been performed by the patient.
[000138] The ability
to provide automated determination of adherence to proper
administration procedures allows for a large number of such images to be
reviewed in a short period
of time. Even if actual and complete lack of adherence is not able to be
determined 100% in each
possible situation, the ability to pre screen the administration video
captures to remove from further
consideration administration situations that are clearly compliant may reduce
a number of compliance
situations to be reviewed by a medical professional substantially. Additional
human review of
indications of failure of adherence may be provided, thus insuring proper
review of all potentially
dangerous situations while greatly reducing the number of images necessary to
be reviewed by a
human. Thus, multiple benefits of such an automated system are realized,
including reducing time to
review such images, reducing costs of such review, and improving patient
privacy by limiting the
number of humans viewing such data, while improving quality.
[000139] In an
additional embodiment of the invention the imaged sequences used for
activity recognition to determine regimen adherence may be further used to
check for adverse or other
reactions to taking of the medication. Thus, in addition to simply determining
proper adherence to a
protocol, such activity or gesture recognition may determine any number of
different actions that may
have been taken by a patient. Thus, actions taken before medication
administration, or actions taken
after medication administration may give insight into reasons for particular
responses, etc. Thus,
before administration, in accordance with the invention, activity recognition
may determine a current
activity of a user. Any subsequent reminders to take a medication may in part
be based upon this
38
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
determined activity. By way of example, if a user is putting on a coat, or is
determined to be leaving a
residence or other facility or the like, a reminder to take a medication
before leaving may be provided,
even if earlier than normal, or if medication is portable, the user may be
reminded to take the
medication with them, and then subsequently reminded to administer the
medication via notification
on a mobile device. By way of further example, if the user is cooking, a
reminder may be given to
take the medication a predetermined time before eating. Other scenarios may be
possible, thus
allowing greater response from the system to ensure proper medication
administration by a patient.
Additionally, various patient consent issues may be prompted and recorded in
accordance with the
invention. Patients may ask further additional questions regarding such
consent, thus insuring that
patients have all of the information they need to make informed consent
decisions, and medication
providers have proper evidence of such consent.
10001401 Similarly,
actions after taking medication may give insight into patient
responses. Notice of fainting, falling down, lack of motion, facial gestures,
gastrointestinal distress or
the like may all be logged as adverse reactions to a particular medication
regimen, and may allow for
adjustment of dosage or prescription instructions in the future for the
patient. If adverse reactions are
severe, an immediate medication review and contact from a medical professional
may be provided to
cure the issue. Additionally, the system in accordance with the present
invention may be directly tied
and be intcroperable with a pharmacy or medical provider's systems as
administered by the clinical
trial manager, thus allowing such recommendations for dosage changes, regimen
changes and the like
to be forwarded to these professionals automatically. Through such links,
reordering medication,
dosage changes, medication changes and the like may be automatically provided.
Furthermore, ease
of providing additional prescriptions can be enhanced as patient, medication
and regimen information
will already be available to the pharmacist or medical service provider.
[000141] After such
automatic, or combination of automatic and manual, adherence
verification is performed allowing a health care provider or other medical
professional to review and
verify results of the automatic comparison or direct review of captured
activity sequences, and
indication of variation from a desired identity, medication or application
procedure may request
administrator review of the situation, and intervention as may be determined
necessary at 1040. Such
review may be required immediately as an emergency situation may exist, or the
patient may gesture
or otherwise indicate that help is necessary, or such review may be less
urgent, perhaps requiring an
electronic communication with suggestions or the like from such an
administrator or the like.
Additionally, such adherence review may be stored over time for a particular
patient, thus allowing for
various medication trends to be determined, such as if a patient misses
medication at a same time each
week, or an indication that one particular health care worker aiding the
patient may occasionally give
an incon-ect medication dosage amount. Thus, in addition to allowing for
immediate notice of
problems in medication administration, an audit trail for tracking the actions
of various health care
providers is generated. Such an audit trail allows clinical trial
administrators or other medical
39
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
professional to determine immediately, or upon review of a complete medication
regimen, whether a
particular patient or group of patients has followed the protocol to the level
that allows their results to
be used as part of the study. Additionally, levels of adherence may give
insight as to the ease of use of
a particular regimen, and whether instructions provided to the patient are
sufficient to allow for
adherence to the prescribed medication regimen.
[000142] Therefore,
in accordance with the invention as set forth in Figure 10, a
method and system are provided in which patient adherence to a prescribed
medication regimen as set
forth in a clinical trial scenario can be reviewed, acutely for a particular
instance, or over time to
determine any changes in behavior of a patient. Because all aspects of such
adherence are monitored
preferably visually, and do not rely on patient confirmation of medication
administration, desires of
the patient are taken out of the equation, and a true review of actually
procedures used in the
medication administration can be studied. Trial managers are therefore able to
keep patients on their
prescribed regimens, thus reducing the overall cost associated with the
clinical trials, and remove
patients from the test population when they cannot maintain adherence to the
prescribed regimen, thus
increasing the accuracy and repeatability of the results of the trial. Through
the management of
multiple clinical trials, patient interaction is eased, and any similarities
across trials may also be
determined, thus improving quality of care generally and allowing for
consistency across any number
of trials.
[000143] Referring
next to Figure 11, a more precise description of the data, medical
record and prescription regimen entry will now be described. As is shown in
Figure 11, a clinical trial
manager 1102 enters information into a system in accordance with the
invention, and in particular
enters information regarding medication regimen, dosage, instructions sets and
the like. A patient
1106 may also enter information relevant to the patient. The trial manager
1102 may further enter or
alter patient information, basic medical statistics, and virtually all other
medical information included
in a database. The patient 1106 may be limited to providing personal
information, and perhaps other
relevant information, such as non-prescription medication usage, alcohol
consumption, recent
symptoms, reaction to use of particular prescription medications and the like.
Such data entry allows
for the tracking of an audit trail at this step of administration as well. Of
course, any of this
information may be automatically entered into a system from a database having
been previously
entered or otherwise obtained from an existing data storage area. Also, if
particular information is
omitted by the patient, or is otherwise unavailable, it might be possible to
request provision of this
information, or otherwise remove the patient from the clinical trial.
Additionally, determination of
failure of proper procedure of the information collector may be determined so
that additional training
or removal of this individual may take place before a large number of patients
are entered into a
clinical trial with insufficient information collection.
[000144] Information
from each user of the system is combined, where available, and
formed into a clinical trial medication entry 1110. In particular, a preferred
list of information that
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
may first be provided in accordance with the invention in order to organize
the system may comprise
a patient name, a user name, if that user is not the patient, and information
to be used to provide
various alerts, such as a patient email for prescription reminders, contact
information for a doctor or
caregiver for emergency contact, mailing address for various bulk and non-
urgent communications,
and cellular telephone or landline telephone information for patient contact,
or the like. Further
notifications may be provided to the trial manager 1102 so that the manager
can monitor patient use of
the medication and adherence to the proposed and prescribed medication
administration. In addition,
in accordance with the invention, facial images of the patient and other
people interacting with the
system are stored, or other biometrie identifiers, such as fingerprint
identification, retinal
identification, voice recognition, various provided RFID tags or the like may
be employed along with
more traditional passwords and user names or the like. Of course, if the
patient is a returning patient,
such information may be extracted from an existing database stored with
another, previous
prescription.
[000145] Next, a user
such as the doctor 1102 or the pharmacist 1104 or nurse, may
indicate a particular new or recurring prescription medication to be provided
to a user in accordance
with the clinical trial. An external medication database 1115 is accessed and
information regarding
such medication is provided, including medication name, suggestions for
appropriate prescription
dosages based upon patient information and the particular version of the
medication to be
administered, and usage instructions to be provided to the patient, these
instructions being modified or
supplemented as necessary by the trial manager. These usage instructions
preferably include detailed
administration instructions, including time of day, patient status (i.e.
before or after eating, after
waking, before sleeping, etc.), precise method of application, and other
useful instructions for a user.
These instructions also may include video sequences to describe particular
details of the medication or
administration procedure thereof and in order to properly indicate gestures to
be implemented by the
patient. They may also comprise alternative versions of instructions so that
if a user is unclear
regarding a particular set of instructions, an alternative set of instructions
may be provided to the
patient to aid in adherence to the prescription regimen.
[000146] After
selection of such a medication, a patient medical history 1117 is
preferably accessed to provide additional medication and patient information
to the trial manager.
Such information may include, other prescriptions to the patient so that
adverse drug interaction may
be determined (although if such other prescriptions were implemented in
accordance with this
invention, such prescriptions will already be known to the system), patient
indication of use of non-
prescription drugs, patient allergies, patient activity level, past diseases
and procedures, and any other
information that may be relevant to the prescription of medication.
[000147] After all of
the information has been entered about the user, and information
from the medication and patient database has been accumulated, various
medication interactions are
checked automatically by the system at step 1120. Any dangerous interactions
are noted to the trial
41
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
manager, and may preclude entry of the prescription into the system and
therefore preclude the
particular patient from participating in the clinical trial. Other
interactions may be noted to the trial
manager so that the individual may make the patient aware of such situations.
These may include, for
example a notification that the taking of two medications together may result
in stomach pains, so that
the patient should take one in the morning and one in the afternoon. Such
interactions check will then
result in a set of instructions that will be provided to the user, in addition
to the more generic
medication instructions. Finally, the trial manager may review such
instructions and supplement them
as desired. It should be noted that in accordance with the invention it is
contemplated that various
instructions provided to a user may comprise hot links to additional audio and
visual information that
may be provided to a patient to further assist in their adherence to any
particular prescribed protocol,
or may provide various infoimation regarding the particular medication being
taken as will be further
described below. Such instructions may indicate methods by which a user is to
administer one or
more medications, and thus requests use of a particular gesture or the like
during administration.
[000148] Finally,
after all interactions and instructions have been reviewed and
approved, the prescription information is stored at step 1125. The storing of
such a prescription
makes the prescription, alerts, help information and the like accessible to a
patient and other system
users. Furthermore, such completion may also transmit the prescription to a
pharmacy or other
medication provision facility so that the user is able to simply swing by to
pick up the medication or
to have the medication delivered to the patient.
[000149] Referring
next to Figure 12, a user implementation of the method in
accordance with the invention will be described. When a time for receiving or
administering a
medication in accordance with a clinical trial regimen is reached, a patient,
and any other necessary
user may be provided a notification 1210 in accordance with notification
addresses entered in the
system as noted above. Thus, in a home situation, only the patient may receive
notification. If there
is a home health care provider, such provider may also receive separate
independent verification. In
the case of hospital or other in-patient care facility, various medical
service providers may similarly
receive such notification. After notification, or in the absence of such
notification, system initiation
takes place at 1215. In accordance with such system initiation, one or more
users are preferably
recognized by the system. Therefore, at step 1220, a user recognition sequence
takes place. In a
preferred embodiment, such a user has a still or video image captured of their
face, and facial
recognition techniques are employed to confirm the identity of the user. Such
capture may be
performed by fixed camera, mobile camera, mobile communication device such as
a cellular phone, or
any other appropriate video or image capture device. Alternative recognition
techniques, such as
retinal, fingerprint, voice or other biometric measurements may be employed,
in addition to a more
common password query. Any other appropriate identification technique may be
employed, and any
unique individual identifiers may be obscured, as noted above, when the images
are to be used as a
more general report regarding adherence, rather than an individual patient
response.
42
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[000150] At step 1225
it is determined whether all necessary users have been
recognized and authenticated. In a situation where a nurse, doctor or other
caregiver is to administer
medication, it may be preferable to have the patient and caregiver to be
recognized by the system to
further confirm that the appropriate procedure is followed, and to allow the
system to keep track of
people using the medication so it can track if any one person, for example, is
improperly using the
medication, as will be evident from the generated audit trail. After step
1225, if all users are not
recognized, control passes back to step 320 and any additional users are
recognized by the system.
[000151] Once all
users are recognized, control passes to step 1227 where the
medication to be administered in accordance with the prescribed prescription
regimen of the clinical
trial is confirmed. Thus, a user is prompted to allow a still or video image,
text recognition image, or
other method of identifying a medication to be captured of the medication
bottle or other container, a
pill of the medication, or other form of medication, and is also able to
determine appropriate
quantities, dosage, and any potentially required or dangerous medication
combinations. As noted
above, if video confirmation is for some reason not available, the user may be
prompted by the system
to provide audio or other indication of medication and other desired
information. This image, video
sequence or other received confirmation information is then compared to an
image associated with the
prescription as noted above in Figure 11. If the medication is determined to
be incorrect, a warning
may be provided to the user that the medication is incorrect. The user may
then be prompted to
choose another medication for imaging.
[000152]
Alternatively, the invention contemplates a user displaying a number of
medications to the image capture apparatus and allowing the apparatus to
suggest which medication is
correct. Thus, the user may be able to scan a medicine cabinet with such a
video imaging apparatus
and have the system indicate which is the correct medication. This may prove
valuable when
sequence of ingesting medication is important, or when two people have similar
medications and may
have difficulty in distinguishing between medications for each. Once a correct
medication has been
identified, control passes to step 330.
[000153] In step 1230
user prompts and other instructions are provided to the patient,
and present caregivers, as to how to administer the medication according to
the prescription guidelines
outlined above as determined in accordance with the particular clinical trial
under consideration.
These instructions allow for a user to receive further information or
instructions as necessary through
asking the system for additional help. Especially in situations where an
elaborate scheme may be
required, it is contemplated that video samples and instructions may be
provided to the user. Further,
in accordance with the invention, for complicated administration procedures,
it may be possible to set
up a two way video conference employing traditional video conferencing, VOIP
conferencing,
traditional telephone conferencing, or any other appropriate communication
system with an expert in
such administration so that a caregiver or patient may receive live coaching
regarding such
administration. Such instructions and prompts may be determined by the
clinical trial manager to
43
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
determine the success or failure of particular sets of instructions. Thus, not
only are medications
tested in such a clinical trial, but also sets of instructions are tested to
determine which are best for all,
or for given demographic groups or the like for eventual user when the
medication is released to the
public, thereby allowing for a better adherence rate by the public. As the
number of clinical trials
grows, and the locations of such trials becomes more international, such
administration through a
system such as that set forth in accordance with the invention may become far
more important to
various clinical trial managers.
[000154] When
following such instruction prompts, the actual act of administration is
preferably captured as a video sequence at step 1235. Thus administration
preferably includes one or
more identifiable gestures as suggested in accordance with the instructions
above. Thus, a patient or
administrator may be provided with one or more images or sequences for method
of application or
administration, and thus the following of these sequences is used to determine
compliance with a
particular prescribed regimen. Further, long gaps or pauses may be determined
to give further insight
into areas of administration that may be giving problems to administrators of
trial participants. This
captured video sequence may be utilized in accordance with the invention in a
number of ways. First,
the actions of administration of the medication is reviewed in real time and
compared to an ideal or
desired video sequence. If a determination is made that the medication is
being administered in an
incorrect manner, and in a way that may be detrimental to the patient,
immediate warnings may be
provided at step 1240 advising the caregiver or patient to stop administration
at once. Furthermore, in
extreme cases, a doctor or other caregiver may be notified, or in the most
dangerous cases, an
ambulance or other emergency personnel may be dispatched to provide immediate
care. Notification
may also be provided to the clinical trial manager so that this person is
warned that one or more
patients are having problems with adherence with the protocol. If such
problems turn out to be
isolated, alternative instructions, or personal help may remedy the situation,
thus allowing the person
to provide meaningful data to the trial. If such lack of adherence is far more
widespread, the clinical
trial manager may change instructions for all participants, or may even ask
all participants to come
back in for further live instructions. In either situation, the cost of the
clinical trial is greatly reduced
as participants are able to remain in the study, and major failures of studies
for lack of protocol
adherence may be avoided. Reports based upon such widespread lack of adherence
may provide a
manager with a report, using images or the like with identifying features
removed so that the report
may show precisely how a medication is being administered while maintaining
patient privacy and
confidentiality. If video recording is not available, other confirmation
methods as noted above may be
employed and be subject to automatic confirmation as with the video recorded
sequences.
[000155] If such
immediate care or warning is not required, control then passes to step
1245 where the video images are more formally captured and analyzed for
various other non-critical
issues. The images may be captured and stored locally, being provided to a
central server in a batch
processing, or images may be captured and sent to the remote server for
immediate analysis and
44
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
storage. Such transmission may take place over a well known Internet
connection, wireless
connection, or other proprietary communication system. Such analysis may
consider suggestions to a
caregiver to improve dosage accuracy, reduce pain in administration, or the
like. These suggestions
may be implemented as tweaks to various instruction or training sequences
before the medication is
released to the general public. Furthermore, as such video sequences may be
available from multiple
patients and/or caregivers taking part in the current or multiple clinical
trials, the effectiveness of
various sets of instructions and the like can be tested and reviewed, and
changes thereto made if
consistent problems are encountered. This type of study is nearly impossible
without the present
invention, because in any type of clinical setting, individuals are far more
likely to be careful in
administration of medication, and therefore not cause errors. When they return
home or back to their
regular lives, this is when adherence and administration issues arise. In
accordance with the
invention, responses to instructions can be analyzed, and lack of adherence
based upon confusing or
difficult to follow instructions can be remedied, providing better or more
usable instructions, and
therefore improving regimen adherence. Without the present invention, such a
clinical trial manager
may be unaware of such issues. With the invention, these issues can be
addressed as they arise, thus
ensuring the integrity of the clinical trial.
[000156] In any
event, after such analysis, any warnings or suggestions for instruction
issues may generate a warning at step 1250, suggesting areas of instruction
that may be problematic.
These video sequences are also stored for longer term analysis if desired at
step 1255, and processing
ends. Furthermore, in order to encourage patients in the clinical trial,
notices of lack of adherence
may be used to change remuneration received by a patient in the clinical
trial. Thus, adherence to the
protocol results in higher payments to the patient, a great incentive for the
patient to adhere to the
protocol.
[000157] Trends of a
patient taking part in the clinical trial can be monitored, such as
blood pressure or other measurable quantities of the patient, and correlation
between such measured
quantities and medication administration may be observed, potentially allowing
for a more
customized solution of medication to be applied to the patient, possible
modifying dosage or
frequency of administration based upon individual reactions to a particular
prescription regimen.
Additionally, features of the invention noted above allowing for user
interaction and recordation of
activities of a user, adverse effects and the like may be incorporated into
the system to provide further
information for determining alternative instruction sets, modification of
medications and the like.
[000158] It is
further contemplated that the clinical trial manager be provided with a
manager dashboard allowing for summary review of all patients taking part in
one or more clinical
trials. Details of an exemplary embodiment of such a dashboard will now be
described making
reference to Figures 13-15. As is shown in Figure 13, a summary page dashboard
is shown at 1300.
Each different clinical trial is contemplated to have one or more summary
pages (as more than one
page may be necessary for a particular clinical trial given the number of
participants). Each summary
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
page includes indicators 1310 for each participant taking place in a
particular clinical trial. In Figure
13 such indicators are shown as icons representing each person. Such icons may
be replaced with
summary identification information or the like, or any other desired
indicator, such a demographic
indicators (i.e. age group, sex, medical condition, individual
characteristics, etc.) Also included in
dashboard 1300 is an indicator legend including information that may be
applied to any one or more
of the indicators 1310. In this particular preferred embodiment of the
invention, the indicator legend
includes indicator modifiers representing that a particular trial participant
asked a question, has a
report of poor compliance, or requested clarification of some instruction or
other facet of the clinical
trial. As is shown, each indicator modifier may be applied to a particular
indicator 1310 to which the
modifier applies. Thus, as is shown in Figure 13, indicator 1312 has been
flagged for poor
compliance, while indication 1314 has been flagged as asking a question. Thus,
in this manner, a
clinical trial manager is provided with an overview of the status of the
patients in a clinical trial.
Major compliance problems would be easily determined by the clinical trial
manager viewing a
dashboard with many of the indicators 1310 being modified by the indicator
modifier related to poor
compliance. Similarly, problems with instructions might be apparent if the
summary display indicates
a large number of questions or clarification requests. Of course indicator
modifiers for any number of
other situations may be provided. Statistical analysis of the population of
the clinical trial may also be
provided, thus allowing for the clinical trial manager to determine adherence
rates and other metrics
of the entire or subsection of the clinical trial population. Such modifiers
are preferably color
indicators, but may comprise any other appropriate indication that allows the
clinical trial manager to
determine status.
[000159] Dashboard
1300 further preferably includes a zoom indicator that allows the
clinical trial manager down to each patient or a smaller number of patients to
view their overall
compliance statistics, or to view individual recorded video sequences,
requests for additional
information, or any other information stored as the audit trail for patients
in accordance with the
invention. Thus, if the zoom indicator is zoomed all of the way out, the
clinical trial manager is
presented with the largest number of indicators 1310 on the dashboard. A
medium zoom preferably
provides the clinical trial manager with a view such as that shown in Figure
14. As is shown,
indicator 1312 includes additional information adjacent the indicator. In the
displayed situation, the
name for the indicator modifier is shown. This may be beneficial when a large
number of indicator
modifiers may be provided. Alternatively, this text might include a most
critical piece of information,
or a user selected piece of information. The zoom feature may also allow the
clinical trial manager to
zoom down by demographic information of the like, thus viewing indicators 1310
representing
clinical trial participants having similar characteristics in one form or
another.
[000160] In addition,
it is contemplated that the clinical trial manager may use the
summary dashboard, at any desired level of zoom, to select and move, modify,
or otherwise act upon a
subset of indicators included in a clinical trial. Thus, one or more
participants may be assigned to
46
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
different populations, grouped as desired to receive different instructions,
dosages, or have any other
modification of medication administration applied to the selected group of
participants. Participants
may also be grouped based upon prescribing medical provider, clinic, medical
group, insurance
company, or in any other manner that might be considered useful to the
clinical trial manager. By
grouping by these groups, it may be possible to determine if any particular
clinic, doctor or the like
results in poor compliance for that group. Thus, poor compliance linked to a
particular such group
can also be addressed through, perhaps, additional training for the clinic or
doctor. Participants may
also be added to, or deleted from a particular trial or other epidemiological
study or the like, if
desired, or otherwise manipulated. Groupings may also be automated, such as
grouping all patients
by age, gender, or other demographic characteristic, thus providing a quick
useful view to the clinical
trial manager.
10001611 Further
zooming in allows the clinical trial manager to view a smaller number
of indicators, down to a single indicator. As the number of indicators
decreases, the amount of
information available to the clinical trial manager for each indicator
increases. Thus, the clinical trial
manager may zoom down to one of the possible levels as shown in Figure 15. As
is shown in Figure
15, the first zoom level shows the level of information of Figure 14, while a
great number of
indicators 1310 are still visible. A more detailed zoom level preferably
displays The same poor
compliance indicator, but further shows that this poor compliance is related
to the medication
schedule, and in particular a supply shortage of the medication, thus
providing a more complete
compliance check of the clinical trial participant. The information further
provides poor compliance
information in that the participant represented by the indicator has not yet
reviewed a required (or
suggested) training video, and is also having difficulty with a fifth process
in the defined confirmation
scheme. Finally, a further zoom level, after indication a desire to have more
information regarding the
poor compliance indicates that the participant has only had 7% of their
actions confirmed by video,
10% confirmed by voice, and the remaining 83% confirmed by GUI interface
(which is patient
dependent and therefore is not determined to be as reliable as the other two
methods). Furthermore,
the participant has asked a particular question. From this particular zoom
level, it is contemplated that
the clinical trial manager may directly link to the stored video, audio or GUI
provided information.
Preferably, a timeline or the like is provided to the clinical trial manager
indicating when the various,
video, audio and/or GUI entries have been stored. The clinical trial manager
is able to view an even
more useful picture of the sequence of compliance, and is then able to select
any of the stored
sequences for review. The manager may also answer the question, or forward the
question to a
physician or the like who is appropriate to answer the question. The clinical
trial manager may also
opt to change instructions provided to one or more patients in accordance with
their record of
adherence when being exposed to a first set of instructions. This level of
zoom may also provide links
for allowing communication with a particular participant, and method for
suggesting additional
training, instructions or help, and a process for ordering or providing
additional medication to a
47
20 02780901 2012-05-14
WO 2011/062934
PCT/ES2010/056935
participant. Specific instructions to a patient, preferences, or comments or
the like provided by a
patient may also be reviewed. Finally, the clinical trial manager may view
upcoming prescription
information, including times for taking a prescription or other medication
administration applicable to
one or more patients.
[000162] It is
therefore through such a dashboard that the clinical trial manager
receives notice of any problematic failures on the part of patients warranting
intervention, either by
direct contact, changes in regimen or instruction, or recall of the patient
for retraining. As noted
above, rather than waiting for the patient to come back in and report poor
adherence, or worse yet,
allowing the patient data to become part of the clinical resultant data even
with poor adherence, the
clinical trial manager may use the dashboard of the invention to ensure that
poor compliance is
recognized early and dealt with. Furthermore, emergency situations may be
indicated to the clinical
trial manager in an immediate manner.
[000163] Such a
dashboard is preferably provided with patient management features in
addition to the notification features noted above. Thus, the clinical trial
manager is able to manage
individual participants, subsets of the population, or the entire population
through drop and/or drag
functionality, and is able to broadcast information to these groups as
necessary, including the
broadcast of video, text, or voice instructions. The clinical trial manager
may also preferably remind
participants of upcoming doctor appointments, meetings regarding the clinical
trial, and also provide
such notices to the patient's prescribing physician. Additional medication or
supplies may be
provided to a participant upon notice that they have run out, or merely
tracking of medication actually
taken by the participant. Thus, there is no guesswork. The manager may further
review patient
medical records, as necessary, to further aid in trial procedure compliance.
10001641 The
dashboard also preferably provides access to medical professionals
associated with one or more of the clinical trial participants. In a situation
where the trial provides all
of the prescribing doctors or other medication administrators, for those
patients that may need
assistance, this feature may be trivial, but when multiple prescribing doctors
are involved in a trial,
being able to forward information and interact with these doctors may prove
very valuable. Such
contact may be made through a link or connection provided associated with each
patient identifier
icon on the dashboard. Thus, the clinical trial manager may be able to review
videos or other
compliance indicia with such a prescribing physician, perfolut remote triage
regarding any
complications that might arise with a patient during the course of the trial,
or request prescription
changes, etc. Furthermore, the clinical trial manager may be able to audit
these very medical
professionals looking after, and administering medication in accordance with
the prescribed clinical
trial procedure. Through such an audit, the manager may be able to determine
issues related to
actions of a particular administrator.
[000165] A user
dashboard may also be provided to allow a participant to enter
information, such as GUI entered compliance information as noted above, but
also to ask questions,
48
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
view additional instructions, order supplies, or the like. The user may view
more specific prescription
regimen information, such as when a next medication is scheduled to be taken,
or be provided with a
calendar for a predetermined future time period to allow for future planning.
Through such a user
dashboard, the user may view particular information relevant to their
treatment, but preferably not
enough information to influence their participation in the trial. If in use, a
participant may also view
whether they have achieved various adherence levels resulting in payments to
be made to them in
accordance with the invention. Furthermore, the user may be provided with a
method for determining
a level of data transmission, including identity information that may be
provided to various level of
administrator. Thus, while all information may be provided to a clinical trial
manager, perhaps more
limited identifying information may be made available to other researchers or
the like. The
participant may be notified as to potential uses, and an opt in or opt out set
of choices for such
different levels of disclosure. The user may be provided with a privacy
setting indicator to indicate a
level of availability of recorded information and attachment of identifying
information thereto.
[000166] Thus,
provision of such a dashboard in conjunction with the operative clinical
trial verification process and system described above eases use, increases
effectiveness and allows for
better control over such a clinical trial. Difficulties in following protocols
may be determined in near
real time. Changes in a trial may be easily implemented and conveyed to trial
participants and their
prescribing doctors. New training modules, including video, written, or other
instructions may be
provided during a trial in order to improve adherence. Inventory management is
eased and all trial
participants are sure to have the appropriate amounts of medication and other
supplies. Further, a full
audit trail of who has accessed information for any particular patient, groups
of patients, or one or
more clinical trials is maintained. This dashboard, however, is not limited to
use only in a clinical
trial. Rather, the system can be used by a single medical provider, doctor
group or network, insurance
company, governmental agency, nursing home, hospice, home care provider or the
like, or other group
in order to track any number of patients and associated home care workers,
doctors, or other
medication administrators. In such scenarios, the ability to view, group, and
retrieve significant
information about particular patients allows for greater control over these
patients, resulting in
reduced likelihood of a patient continuing to take improper medication, or to
improperly administer
such medication, and therefore in turn reducing the possibility of an adverse
reaction or death from a
particular medication.
[000167] A system
provided in accordance with the invention includes imaging
technology and hardware, communication hardware, computer systems including
storage memory and
remote communication via the Internet or other communication network for
remote storage and
analysis, databases of patient information and medication information
sufficient to implement the
method as described above. All communications are encrypted or otherwise
protected during
transmission and storage at both local and remote mass storage location to
meet any security issues
49
WO 2011/062934
PCT/US2010/056935
and any regulations required for the storage and maintenance of medical and
patient health care
information.
10001681 Therefore,
in accordance with the invention, a method and system are
provided that allow for the automated confirmation of adherence to
administration protocol for
medication in a clinical trial environment, and provide for a most
sophisticated method for confirming
and studying methods of administration of such prescription medication.
[000169] Referring
first to Figure 16, a remote information capture apparatus 1600 is
shown. Such information capture apparatus 1600 is placed in communication with
a remote data
and computing location 1800 via a communication system 1700, preferably the
Internet or other
communication system. Via communication system 1700, information captured by
apparatus 1600 is
transmitted to remote data and computing location 1800, and analysis
information or other
instructions may be provided from remote data and computing location 1800 to
apparatus 1600. It is
further contemplated that a plurality such information capture apparatuses
1600 may be coordinated to
monitor a larger space than a space that can be covered by a single such
apparatus. Thus, the
apparatuses can be made aware of the presence of the other apparatuses, and
may operate by
transmitting all information to one of the apparatuses 1600, or these
apparatuses may each
independently communicate with remote data and computing location, which is
adapted to piece
together the various information received from the plurality of devices 1600.
[000170] Referring
next to Figure 17, a more detailed view of a preferred embodiment
of remote information capture apparatus 1600 and remote data and computing
location 1800. As is
shown in Figure 17, apparatus 1600 comprises an information capture device
1610 for capturing video
and audio data as desired. A motion detector 1615 or other appropriate trigger
device may be
provided associated with capture device 1610 to allow for the initiation and
completion of data
capture. Information capture device 1610 may comprise a visual data capture
device, or may be
provided with an infrared, night vision, or other appropriate information
capture device. A storage
location 1620 is further provided for storing captured information, and a
processor 1630 is provided to
control such capture and storage, as well as other functions associated with
the operation of remote
information capture apparatus 1600. An analysis module 1635 is provided in
accordance with
processor 1630 to perform a portion of analysis of any captured information at
the remote information
capture apparatus 1600. Apparatus 1600 is further provided with a display
1640, and a data
transmission and receipt system 1650 and 1660 for displaying information, and
for communicating
with remote data and computing location 1800. Remote data and computing
location 1800 comprises
system management functions 1830, and a transmission and reception system 1850
and 1860 for
communicating with apparatus 1600.
CA 2780901 2017-12-18
WO 2011/062934
PCT/US2010/056935
Transmission and reception system 1850 and 1860 may further comprise various
GPS modules so
that a location of the device can be determined at any time, and may further
allow for a message to be
sent to one or more individual apparatuses, broadcast to all apparatuses in a
particular trial, or being
used for administration of a particular prescription regimen, of broadcast to
all available apparatuses.
[000171] In
accordance with the invention, apparatus 1600 is adapted to be part of a
system that improves adherence to medical protocol in both a clinical trial
and other medication
administration setting. Users of apparatus 1600 in accordance with this system
give administrators a
tangible and concrete manner in which to confirm compliance or lack thereof,
and the ability to
intervene early in the process to ensure that patients enrolled in such a
clinical trial study are properly
taking their medication. Apparatus 1600 of the invention is adapted to receive
instructions for
patients from remote data and computing location 1800 and provide these
instructions to patients on
the use of the prescription medication. Such instructions may comprise
written, audio or audio visual
instructions for guiding a user to adhere with a prescribed protocol.
Apparatus 1600 further provides
verification to a doctor or other service provider of patient adherence to the
prescribed protocol, and
statistical and individual analysis of adherence rates to ensure proper
medication administration.
[000172] Referring
next to Figure 18, a data flow overview on accordance with the
operation of apparatus 1600 is shown. In accordance with the invention,
information about a
particular drug to be the subject of a clinical trial or other medication
administration program or
prescription is provided in a database 1805, and existing industry medication
information databases
1810 are employed to access prescription, interaction, application and other
available information
about any number of proposed prescription and non-prescription medications and
their possible
interaction with the clinical trial medication. Further, patient medical
records 1815 are used, and as
will be described below, are used in conjunction with the industry medical
information and a medical
professional's prescribing expertise to confirm that a patient is a good
candidate for such a clinical
trial. These databases may be accessed via apparatus 1600, or from remote data
and computing
location 1800. Once confirmed, a medicine regimen in accordance with the
clinical trial or other
prescription requirements is prescribed and entered into the system of the
invention at 1820. Once
entered into the system, a particular prescription regimen causes a set of
user instructions 1825 to be
generated and transmitted to apparatus 1600. In a clinical trial setting, such
instructions may be
varied for different users to determine the best set of instructions, or may
be varied based upon
demographics, experience, or other factors that may require different types of
instructions to be
provided. It is further contemplated in accordance with the invention that
multiple clinical trials or
patient populations may be managed by a manager in accordance with the
invention so that the
invention contemplates a medication administration system that allows for a
single point of
management for all clinical trials or patient management groups associated
with a particular manager
or the like.
51
CA 2780901 2017-12-18
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[000173] Such user
instructions may include general instructions about the particular
medication subject to the current trial or medication administration protocol,
methods for ingestion,
warnings about side effects, concerns about drug interactions with common
substances or
medications, or other medications prescribed to the patient by the system or
by another medical
service provider. It is contemplated in accordance with the invention that
such set of user instructions
may be interactive, allowing a user to view additional information about such
instructions or
prescriptions as desired. These instructions may comprise written, audio or
video instructions
provided to the user on display 1640 of apparatus 1600. Furthermore, it is
contemplated in
accordance with the invention that at various points during the instruction
set, for example when a
patient asks a particular type of question, or asks to receive additional
information about a particular
aspect of the medication or prescription regimen through entry of information
into apparatus 1600,
that apparatus 1600 may reach out and contact a representative of a medical
service provider via the
transmission and reception systems 1650 and 1660 to provide the patient with
additional, personal
help as necessary, if it is determined that such intervention by the medical
professional would be
desirable to the patient. Thus, such a patient may be assisted in properly
taking medication so that
various errors do not take place. Indeed, in more traditional scenarios, it is
only after perhaps
finishing a prescription regimen and a return to a doctor in accordance with a
predetermined clinical
trial schedule that it is discovered that the medication may have been taken
incorrectly. In accordance
with the present invention, early intervention with such issues can be
exercised to deter any possible
unfortunate outcomes from improper administration of medication, and to ensure
that the particular
patient is able to remain in the study and provide accurate data to the study.
[000174] It is
contemplated in accordance with the invention that a touch or other user
friendly graphical user interface be provided associated with apparatus 1600
so that the user can easily
manipulate any number of prescription factors, and perhaps enter additional
information that may be
useful to a prescribing medical provider, such as level of fatigue, level of
hunger, jitter inducing
medications, etc. All of these data collection points allow for a smoother
administration of medication
to a patient, and therefore a more likely chance of adherence to a prescribed
protocol.
[000175] Referring to
the lower portion of Figure 18, the horizontal line indicates a
time for patient ingestion or other administration of medication. The patient
may display a medication
container and/or an actual pill or other medication form to imaging capture
device 1610 of apparatus
1600 at step 1830, which in turn may perform local processing and analysis via
processor 1630 and
associated analysis module 1635. Apparatus 1600 confirms that the medication
is correct and is the
currently prescribed medication to be taken through the use of text
recognition, pill recognition, or
other appropriate medication recognition scheme. This sequence of steps
therefore acts as an audit
trail each time a medication is taken, that can be reviewed later, to ensure
that a patient is properly
following a regimen.
52
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[000176] In
accordance with the invention, confirmation of patient adherence to the
prescribed administration schedule for the medication as prescribed by the
clinical trial or other
prescription regimen is determined. While such confirmation may take a number
of forms, in
accordance with the invention, a preferred method for such confirmation may
include capturing a
video sequence of the patient actually administering the medication by
apparatus 1600. In a further
preferred method, such a sequence for such confirmation may include employing
a facial recognition
sequence or other biometric confirmation that a particular patient is in fact
receiving treatment, but
may also provide for the ability to obscure the face or other identifying
feature of a user to allow for
the storage and use of such images while protecting the identity of the
patient, a technique that may be
beneficial when a manager is providing a general report about the clinical
trial, and not trying to
remedy a situation with a particular patient. Activity recognition, gesture
recognition or other feature
for determining whether a particular subject movement meets a predefined
movement sequence may
be employed to be sure that the patient is properly taking prescribed
medication.
[000177] Furthermore,
in accordance with the present invention, a video image of the
patient actually administering or ingesting the medication may be taken in
accordance with apparatus
1600 and stored in memory 1620 so that actual confirmation may be achieved,
rather than simply
relying on the patient to state that a particular medication was administered.
Such a video image may
be captured or stored in any appropriate format given a selected type of
activity or gesture recognition
that is employed in accordance with a particular embodiment of the invention.
Such may include full
video, biometric data points, recording of movement of an article, such as a
bracelet or the like,
affixed to the patient or administrator, use of mapping to provide a stick
figure or other body
movement tracking technique, or gesture or activity recognition to determine
movement or the like.
The user may be encouraged to use a particular sequence of movement to be
confirmed that they are
properly administering the medication according to the protocol, thus reducing
the possibility of the
potential appropriate movements considered to be "correct." Indeed, various
instructional videos or
other appropriate training may be provided to a user to insure they properly
administer the
medication. Finally, in accordance with the invention, if recording of a video
of a patient having the
medication administered thereto is not possible, the system of the invention
will recognize such an
issue and request audio confirmation via apparatus 1600 as a next best option.
If the audio
confirmation is also not possible, then a less reliable method of
confimiation, such as a keyboard
confirmation by the patient may be accepted. If higher reliability methods of
confirmation are not
available for an extended period of time, an alarm is preferably forwarded to
a medical professional to
inquire as to reasons and to remedy any situation that might be wrong in the
administration situation.
[000178] These steps
of confirming identity, confirming medication and confirming
administration are then reviewed to verify adherence to the prescribed
protocol at 1835. Such review
is preferably performed automatically first by processor 1630 and analysis
component 1635 thereof
that is able to align the actual recorded images with ideal or expected
images, or through the use of
53
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
other activity or gesture recognition as mentioned above. In the case of
facial recognition and bottle
or pill recognition, such techniques are known in the art. With regard to
video confirmation of
adherence to prescribed medicine administration procedures, such processing
may include various
stick figure comparison analyses, activity recognition analysis, or other
schemes as noted above able
to determine whether appropriate actions have been performed by the patient.
[000179] The ability
to provide automated determination of adherence to proper
administration procedures at the apparatus 1600 allows for a first, quick
review of any such images to
determine whether any immediate attention may be warranted. As will be
described below, further
review at remote data and computing location 1800 allows for a large number of
such images to be
reviewed in a short period of time. Even if actual and complete lack of
adherence is not able to be
determined 100% in each possible situation, the ability to pre screen the
administration video captures
to remove from further consideration administration situations that are
clearly compliant may reduce a
number of compliance situations to be reviewed by a medical professional
substantially. Additional
human review of indications of failure of adherence may be provided, thus
insuring proper review of
all potentially dangerous situations while greatly reducing the number of
images necessary to be
reviewed by a human. Thus, multiple benefits of such an automated system are
realized, including
reducing time to review such images, reducing costs of such review, and
improving patient privacy by
limiting the number of humans viewing such data, while improving quality.
[000180] In an
additional embodiment of the invention the imaged sequences used for
activity recognition to determine regimen adherence may be further used to
check for adverse or other
reactions to taking of the medication. Thus, in addition to simply determining
proper adherence to a
protocol at apparatus 1600, such activity or gesture recognition may determine
any number of
different actions that may have been taken by a patient. Thus, actions taken
before medication
administration, or actions taken after medication administration may give
insight into reasons for
particular responses, etc. Thus, before administration, in accordance with the
invention, activity
recognition may determine a current activity of a user. Any subsequent
reminders to take a
medication may in part be based upon this determined activity. By way of
example, if a user is
putting on a coat, or is determined to be leaving a residence or other
facility or the like, a reminder to
take a medication before leaving may be provided, even if earlier than normal,
or if medication is
portable, the user may be reminded to take the medication with them, and then
subsequently reminded
to administer the medication via notification on a mobile device. By way of
further example, if the
user is cooking, a reminder may be given to take the medication a
predetermined time before eating.
Other scenarios may be possible, thus allowing greater response from the
system to ensure proper
medication administration by a patient. Additionally, various patient consent
issues may be prompted
and recorded in accordance with the invention. Patients may ask further
additional questions
regarding such consent, thus insuring that patients have all of the
information they need to make
informed consent decisions, and medication providers have proper evidence of
such consent.
54
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[000181] Similarly,
actions after taking medication may give insight into patient
responses. Notice of fainting, falling down, lack of motion, facial gestures,
gastrointestinal distress or
the like may all be logged as adverse reactions to a particular medication
regimen, and may allow for
adjustment of dosage or prescription instructions in the future for the
patient. If adverse reactions are
severe, an immediate medication review and contact from a medical professional
may be provided to
cure the issue. Additionally, the system in accordance with the present
invention may be directly tied
and be interoperable with a pharmacy or medical provider's systems as
administered by the clinical
trial manager, thus allowing such recommendations for dosage changes, regimen
changes and the like
to be forwarded to these professionals automatically. Through such links,
reordering medication,
dosage changes, medication changes and the like may be automatically provided.
Furthermore, ease
of providing additional prescriptions can be enhanced as patient, medication
and regimen information
will already be available to the pharmacist or medical service provider.
[000182] After such
automatic, or combination of automatic and manual, adherence
verification is performed allowing a health care provider or other medical
professional to review and
verify results of the automatic comparison or direct review of captured
activity sequences, and
indication of variation from a desired identity, medication or application
procedure may request
administrator review of the situation, and intervention as may be determined
necessary at 1840. Such
review may be required immediately as an emergency situation may exist, or the
patient may gesture
or otherwise indicate that help is necessary, or such review may be less
urgent, perhaps requiring an
electronic communication with suggestions or the like from such an
administrator or the like.
Additionally, such adherence review may be stored over time for a particular
patient, thus allowing for
various medication trends to be determined, such as if a patient misses
medication at a same time each
week, or an indication that one particular health care worker aiding the
patient may occasionally give
an incon-ect medication dosage amount. Thus, in addition to allowing for
immediate notice of
problems in medication administration, an audit trail for tracking the actions
of various health care
providers is generated. Such an audit trail allows clinical trial
administrators or other medical
professional to determine immediately, or upon review of a complete medication
regimen, whether a
particular patient or group of patients has followed the protocol to the level
that allows their results to
be used as part of the study. Additionally, levels of adherence may give
insight as to the ease of use of
a particular regimen, and whether instructions provided to the patient are
sufficient to allow for
adherence to the prescribed medication regimen.
[000183] Refen-ing
next to Figure 19, a user implementation of the method in
accordance with the invention will be described. When a time for receiving or
administering a
medication in accordance with a clinical trial regimen is reached, a patient,
and any other necessary
user may be provided a notification 1910 from apparatus 1600. Thus, in a home
situation, only the
patient may receive notification. If there is a home health care provider,
such provider may also
receive separate independent verification. In the case of hospital or other in-
patient care facility,
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
various medical service providers may similarly receive such notification.
After notification, or in the
absence of such notification, system initiation preferably takes place at
1915. In accordance with such
system initiation, one or more users are preferably recognized by the system.
Therefore, at step 1920,
a user recognition sequence may be employed. In a preferred embodiment, such a
user has a still or
video image captured of their face by apparatus 1600, and facial recognition
techniques are employed
to confirm the identity of the user in analysis module 1635. Alternative
recognition techniques, such
as retinal, fingerprint, voice or other biometric measurements may be
employed, in addition to a more
common password query. Any other appropriate identification technique may be
employed, and any
unique individual identifiers may be obscured, as noted above, when the images
are to be used as a
more general report regarding adherence, rather than an individual patient
response.
[000184] At step 1925
it is determined whether all necessary users have been
recognized and authenticated. In a situation where a nurse, doctor or other
caregiver is to administer
medication, it may be preferable to have the patient and caregiver to be
recognized by the system to
further confirm that the appropriate procedure is followed, and to allow the
system to keep track of
people using the medication so it can track if any one person, for example, is
improperly using the
medication, as will be evident from the generated audit trail. After step
1925, if all users are not
recognized, control passes back to step 1920 and any additional users are
recognized by the system.
[000185] Once all
users are recognized, control passes to step 1927 where the
medication to be administered in accordance with the prescribed prescription
regimen of the clinical
trial is confirmed. Thus, a user is prompted by apparatus 1600 to allow a
still or video image, text
recognition image, or other method of identifying a medication to be captured
of the medication bottle
or other container, a pill of the medication, or other form of medication, and
is also able to determine
appropriate quantities, dosage, and any potentially required or dangerous
medication combinations.
As noted above, if video confirmation is for some reason not available, the
user may be prompted by
the system to provide audio or other indication of medication and other
desired information. This
image, video sequence or other received confirmation information is then
compared to an image
associated with a prescribed prescription. If the medication is determined to
be incorrect after
analysis by analysis module 1635, a warning may be provided from apparatus
1600 to the user that
the medication is incorrect. The user may then be prompted to choose another
medication for
imaging.
[000186]
Alternatively, the invention contemplates a user displaying a number of
medications to apparatus 1600 and allowing the apparatus to suggest which
medication is correct.
Thus, the user may be able to scan a medicine cabinet with such a video
imaging apparatus and have
the system indicate which the correct medication is. This may prove valuable
when sequence of
ingesting medication is important, or when two people have similar medications
and may have
difficulty in distinguishing between medications for each. Once a correct
medication has been
identified, control passes to step 1930.
56
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[000187] In step 1930
user prompts and other instructions are provided to the patient
from apparatus 1600, and present caregivers, as to how to administer the
medication according to the
prescription guidelines outlined above as determined in accordance with the
particular clinical trial
under consideration. These instructions allow for a user to receive further
information or instructions
as necessary through asking the system for additional help. Especially in
situations where an
elaborate scheme may be required, it is contemplated that video samples and
instructions may be
provided to the user. Further, in accordance with the invention, for
complicated administration
procedures, it may be possible to set up a two way video conference employing
traditional video
conferencing, VOIP conferencing, traditional telephone conferencing, or any
other appropriate
communication system with an expert in such administration so that a caregiver
or patient may
receive live coaching regarding such administration, all via apparatus 1600,
perhaps with additional
features and elements provided to allow for such additional functionality.
Such instructions and
prompts may be determined by the medication manager to determine the success
or failure of
particular sets of instructions. Thus, not only may medications tested in a
clinical trial, but also sets of
instructions are tested to determine which are best for all, or for giver
demographic groups or the like
for eventual user when the medication is released to the public, thereby
allowing for a better
adherence rate by the public. As the number of clinical trials and other
patient populations grows, and
the locations of such trials groups become more international, such
administration through a system
such as that set forth in accordance with the invention may become far more
important to various
medication administration managers.
[000188] When
following such instruction prompts, the actual act of administration is
preferably captured as a video sequence at step 1935 by apparatus 1600 via
capture device 1610 and
stored to storage 1620. Administration preferably includes one or more
identifiable gestures as
suggested in accordance with the instructions above. A patient or
administrator may be provided with
one or more images or sequences for method of application or administration,
and thus the following
of these sequences is used to determine compliance with a particular
prescribed regimen. Further,
long gaps or pauses may be determined to give further insight into areas of
administration that may be
giving problems to administrators of trial participants. This captured video
sequence may be utilized
in accordance with the invention in a number of ways. First, the actions of
administration of the
medication is reviewed in real time and compared to an ideal or desired video
sequence by processor
1630 and analysis module 1635. If a determination is made that the medication
is being administered
in an incorrect manner, and in a way that may be detrimental to the patient,
immediate warnings may
be provided at step 1940 via apparatus 1600 advising the caregiver or patient
to stop administration at
once. Furthermore, in extreme cases, a doctor or other caregiver may be
notified, or in the most
dangerous cases, an ambulance or other emergency personnel may be dispatched
to provide
immediate care.
57
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[000189] Notification
may also be provided to the clinical trial manager or other
medication administrator so that this person is warned that one or more
patients are having problems
with adherence with the protocol. If such problems turn out to be isolated,
alternative instructions, or
personal help may remedy the situation, thus allowing the person to provide
meaningful data to the
trial. If such lack of adherence is far more widespread, a clinical trial
manager or other medication
administrator may change instructions for all participants, or may even ask
all participants to come
back in for further live instructions. In either situation, the cost of
administration is greatly reduced as
participants are able to remain in a study, and major failures of studies for
lack of protocol adherence
may be avoided. Reports based upon such widespread lack of adherence may
provide a manager with
a report, using images or the like with identifying features removed so that
the report may show
precisely how a medication is being administered while maintaining patient
privacy and
confidentiality. If video recording is not available, other confirmation
methods as noted above may be
employed and be subject to automatic confirmation as with the video recorded
sequences.
[000190] If such
immediate care or warning is not required, control then passes to step
1945 where the video images are more formally captured, transmitted to remote
data and computing
location 1800 and analyzed for various other non-critical issues. The images
may be captured and
stored locally, being provided to a central server in a batch processing, or
images may be captured and
sent to the remote server location 1800 for immediate analysis and storage.
Such analysis may
consider suggestions to a caregiver to improve dosage accuracy, reduce pain in
administration, or the
like. Furthermore, as such video sequences may be available from multiple
patients and/or caregivers
taking part in a current or multiple clinical trials, the effectiveness of
various sets of instructions and
the like can be tested and reviewed, and changes thereto made if consistent
problems are encountered.
This type of study is nearly impossible without the present invention, because
in any type of clinical
setting, individuals are far more likely to be careful in administration of
medication, and therefore not
cause errors. When they return home or back to their regular lives, this is
when adherence and
administration issues arise. In accordance with the invention, responses to
instructions can be
analyzed, and lack of adherence based upon confusing or difficult to follow
instructions can be
remedied, providing better or more usable instructions, and therefore
improving regimen adherence.
Without the present invention, such a clinical trial manager may be unaware of
such issues. With the
invention, these issues can be addressed as they arise, thus ensuring the
integrity of the clinical trial.
[000191] In any
event, after such analysis, any warnings or suggestions for instruction
issues may generate a warning at step 1950, suggesting areas of instruction
that may be problematic.
These video sequences are also stored for longer term analysis if desired at
step 1955, and processing
ends. Furthermore, in order to encourage patients in the clinical trial,
notices of lack of adherence
may be used to change remuneration received by a patient in the clinical
trial. Thus, adherence to the
protocol results in higher payments to the patient, a great incentive for the
patient to adhere to the
protocol.
58
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
[000192] Trends of a
patient taking part in the clinical trial or other medication
administration program can be monitored by apparatus 1600 in accordance with
the provision of
additional diagnostic attachments, such as blood pressure or other measurable
quantities of the patient,
and correlation between such measured quantities and medication administration
may be observed,
potentially allowing for a more customized solution of medication to be
applied to the patient,
possible modifying dosage or frequency of administration based upon individual
reactions to a
particular prescription regimen. Additionally, features of the invention noted
above allowing for user
interaction and recordation of activities of a user, adverse effects and the
like may be incorporated into
the system to provide further information for determining alternative
instruction sets, modification of
medications and the like.
[000193] Thus,
provision of such an apparatus 1600 increases effectiveness and allows
for better control over medication administration. Difficulties in following
protocols may be
determined in near real time. Changes in a medication protocol may be easily
implemented and
conveyed to patients and their prescribing doctors. New training modules,
including video, written, or
other instructions may be provided improve adherence. Inventory management is
eased and all
patients are sure to have the appropriate amounts of medication and other
supplies. The system and
apparatus 100 can be used by a single medical provider, doctor group or
network, insurance company,
governmental agency, nursing home, hospice, home care provider or the like, or
other group in order
to track any number of patients and associated home care workers, doctors, or
other medication
administrators.
[000194] All
communications in accordance with the invention are preferably
encrypted or otherwise protected during transmission and storage at both local
and remote mass
storage location to meet any security issues and any regulations required for
the storage and
maintenance of medical and patient health care information.
[000195] Alternative
embodiments of the invention may provide one or more of the
following additional features. Rather than simply providing a video capture in
a traditional sense,
apparatus 1600 may be provided with night vision, infrared vision, or other
desired capture scheme.
Apparatus 1600 is also contemplated to be provided with a number of apparatus
management features,
including an ability to choose between color and black and white video storage
and transmission, auto
focus on a patient or the like, and including tracking of such an individual
as they move about a room,
the ability to strip any identifying information from transmitted video so
that anonymity can be
maintained, if desired. Apparatus 1600 may include mechanisms to indicate a
need for recharging,
and include sleep modes for extending battery life. It is further contemplated
that apparatus 1600 may
act as a monitoring device for a patient, and in a standby mode the apparatus
may "listen" or "look"
for sounds from the patient, including suggestions of a fall, scream or the
like. Automated responses
may be provided in order to access patient status via integrated speakers or
the like. The transmission
59
20 02780901 2012-05-14
WO 2011/062934
PCT/US2010/056935
system of apparatus 100 may then notify appropriate personnel. Thus, the
portability and multiple use
areas provide an additional benefit to the user of this apparatus.
[000196] Therefore,
in accordance with the invention, a method and apparatus are
provided that allow for the automated confirmation of adherence to
administration protocol for
medication, and provide for a most sophisticated method for confirming and
studying methods of
administration of such prescription medication.
[000197] It will thus
be seen that the objects set forth above, among those made
apparent from the preceding description, are efficiently attained and, because
certain changes may be
made in carrying out the above method and in the construction(s) set forth
without departing from the
spirit and scope of the invention, it is intended that all matter contained in
the above description and
shown in the accompanying drawings shall be interpreted as illustrative and
not in a limiting sense.
10001981 It is also
to be understood that this description is intended to cover all of the
generic and specific features of the invention herein described and all
statements of the scope of the
invention which, as a matter of language, might be said to fall there between.