Note: Descriptions are shown in the official language in which they were submitted.
CA 02781273 2012-06-28
DILUTING AGENT FOR DILUTING VISCOUS OIL
FIELD OF THE INVENTION
[0001] The present invention relates generally to diluting agents
for use in diluting
viscous oil, for instance bitumen.
BACKGROUND OF THE INVENTION
[0002] Bitumen and heavy oil (collectively referred to herein as
"viscous oil" as further
defined below) reserves exist at varying depths beneath the earth's surface.
More shallow
reserves are often mined followed by surface extraction. Deeper reserves are
often
exploited by in situ processes.
[0003] Diluting agents have been used for both in situ and surface
extraction
processes to dilute viscous oil. The term "solvent" is often used in the
industry and literature
in place of "diluting agent".
[0004] Surface Extraction
[0005] Oil sand deposits near the surface may be recovered by open-
pit mining
techniques, using powered shovels to remove the oil sand and load the trucks
for transport to
an extraction plant. Because the bitumen itself is a highly viscous material,
separating it from
the sands poses certain practical difficulties. The extraction of bitumen from
oil sands mined
in such a manner involves the liberation and separation of bitumen from the
associated
sands in a form that is suitable for further processing to produce a
marketable product.
Among several processes for bitumen extraction, the Clark Hot Water Extraction
(CHWE)
process represents a well-developed commercial recovery technique. In the CHWE
process,
mined oil sands are mixed with hot water to create slurry suitable for
extraction. Caustic may
be added to adjust the slurry pH to a desired level and thereby enhance the
efficiency of the
separation of bitumen. Recent industry developments have shown the feasibility
of operating
at lower temperatures and without caustic addition in the slurryfying process.
[0006] The result of most of the CHWE processes is an extract that
typically
comprises two parts: a hydrocarbon predominant phase (known as a bitumen froth
stream),
and a tailings stream made up of coarse solids, some fine solids, and water.
The specific
properties of the tailings will vary depending on the extraction method used,
but the tailings
essentially comprise spent water, reagents (e.g. surfactants), and waste ore
once the
recovered bitumen has been removed. A typical composition of the bitumen froth
stream is
1
CA 02781273 2012-06-28
about 60 wt% bitumen, 30 wt% water and 10 wt% mineral matter (solids), with
some
variations to account for the extraction and processing conditions. The water
and mineral
matter in the froth are considered as contaminants and must be either
essentially eliminated
or reduced to a level suitable for pipeline transportation, feed to an oil
refinery or an
upgrading facility.
[0007]
The processes to reject the water and mineral matter contaminants are known
as froth treatment processes. Due to the high viscosity of bitumen, the first
step in such
processes is usually the introduction of a solvent. There are two major
commercial
approaches to reject the froth contaminants, namely naphtha solvent-based
froth treatment
and paraffinic solvent-based froth treatment. Solvent addition increases the
density
differential between bitumen and water and mineral matter and enables
contaminants
rejection, which can be carried out by any number of methods, such as
centrifugation or
gravity separation using multi-stage gravity settling units. The separation
schemes generally
result in a product effluent stream of diluted bitumen ("dilbit") and a reject
or tailings stream,
commonly referred to as the froth treatment tailings, comprising mineral
matter, water,
residual solvent, and some residual bitumen. More specifically, in a
paraffinic froth treatment
process the solvent dilution induces the precipitation of asphaltenes from the
bitumen as an
additional contaminant that results in an improvement in the efficiency of the
contaminant
rejection process.
[0008] An
example of naphtha froth treatment (NFT) is described in U.S. Patent No.
5,236,577. Addition of naphtha and separation may yield a bitumen product
containing 1 to 3
wt% water and < 1.0 wt% solids. Such product composition does not meet
pipeline
specifications and renders the NFT product stream unsuitable for
transportation through a
common pipeline carrier.
[0009]
Examples of paraffinic froth treatment (PFT) are described in Canadian
Patents Nos. 2,149,737 and 2,217,300. The addition of sufficient amounts of
paraffinic
solvent results in asphaltene precipitation, formation of aggregates with the
contaminants
(entrained water and carryover solids in the froth), and settling.
Conventional treaters which
separate water and mineral matter will not remove very fine particulate
("fines") from the
froth. Therefore, PFT settling vessels are sized to allow gravity settling of
fines and other
contaminants to provide a solids-free dry bitumen product (< 300 wppm solids,
< 0.5%
BS&W) suitable for transportation in a common carrier to refineries. Bitumen
of such quality
2
CA 02781273 2012-06-28
is termed "fungible" because it can be processed in conventional refinery
processes, such as
hydroprocessing, without dramatically fouling the refinery equipment.
[0010] The CHWE process, described above, is the most commonly
employed water-
based extraction process. In the case of water-based extraction, water is the
dominant liquid
in the process and the extraction occurs by having water displace the bitumen
on the surface
of the solids.
[0011] Solvent-based extraction processes for the recovery of the
hydrocarbons have
been proposed as an alternative to water-based extraction of mined oil sands.
In the case of
solvent-based extraction, the solvent is the dominant liquid and the
extraction of the bitumen
occurs by dissolving bitumen into the solvent. A challenge of certain solvent-
based
extraction of oil sands can be the tendency of fine particles within the oil
sands to hamper the
separation of solids from the hydrocarbon extract. Solvent extraction with
solids
agglomeration is a technique that has been proposed to deal with this
challenge. The
original application of this technology was coined Solvent Extraction
Spherical Agglomeration
(SESA). A more recent description of the SESA process can be found in Sparks
et al., Fuel
1992(71); pp 1349-1353.
[0012] Previously described methodologies for SESA have not been
commercially
adopted. In general, the SESA process involves mixing oil sands with a
hydrocarbon
solvent, adding a bridging liquid to the oil sands slurry, agitating the
mixture in a slow and
controlled manner to nucleate particles, and continuing such agitation to
permit these
nucleated particles to form larger multi-particle spherical agglomerates for
removal. The
bridging liquid is preferably water or an aqueous solution since the solids of
oil sands are
mostly hydrophilic and water is immiscible with hydrocarbon solvents.
[0013] The SESA process described by Meadus et al. in U.S. Patent No.
4,057,486,
involves combining solvent extraction with solids agglomeration to achieve dry
tailings
suitable for direct mine refill. In the process, organic material is separated
from oil sands by
mixing the oil sands material with an organic solvent to form a slurry, after
which an aqueous
bridging liquid is added in the amount of 8 to 50 wt% of the feed mixture. By
using controlled
agitation, solid particles from oil sands come into contact with the aqueous
bridging liquid
and adhere to each other to form macro-agglomerates of a mean diameter of 2 mm
or
greater. The formed agglomerates are more easily separated from the organic
extract
compared to un-agglomerated solids. This process permitted a significant
decrease in water
3
CA 02781273 2012-06-28
use, as compared with conventional water-based extraction processes.
Furthermore, the
organic extract produced has significantly lower amounts of solids entrained
within compared
to previously described solvent-based extraction methods.
[0014] Solvent extracted bitumen has a much lower solids and water
content than that
of bitumen froth produced in the water-based extraction process. However, the
residual
amounts of water and solids contained in solvent extracted bitumen may
nevertheless render
the bitumen unsuitable for marketing. Removing contaminants from solvent
extracted bitumen
is difficult using conventional separation methods such as gravity settling,
centrifugation or
filtering.
[0015] Another example of a solvent-based extraction process is described
in
Canadian Patent Application Serial No. 2,724,806 ("Adeyinka et al."),
published June 30, 2011
and entitled "Processes and Systems for Solvent Extraction of Bitumen from Oil
Sands".
[0016] In Situ Processes
[0017] Where deposits lie well below the surface, viscous oil may
be extracted using
in situ ("in place") techniques. For in situ recovery processes, diluting
agents have been
injected alone and in combination with steam. Diluting agents reduce the
viscosity of viscous
oil by dilution, while steam reduces the viscosity of viscous oil by raising
the viscous oil
temperature. Reducing the viscosity of in situ viscous oil is done to permit
or facilitate its
production.
[0018] One example of an in situ technique is the steam-assisted gravity
drainage
method (SAGD). In SAGD, directional drilling is employed to place two
horizontal wells in the
oil sands ¨ a lower well and an upper well positioned above it. Steam is
injected into the
upper well to heat the bitumen and lower its viscosity. The bitumen and
condensed steam
will then drain downward through the reservoir under the action of gravity and
flow into the
lower production well, whereby these liquids can be pumped to the surface. At
the surface of
the well, the condensed steam and bitumen are separated, and the bitumen is
diluted with
appropriate light hydrocarbons for transport to a refinery or an upgrader. An
example of
SAGD is described in U.S. Patent No. 4,344,485 (Butler).
[0019] In other processes, such as in Cyclic Steam Stimulation
(CSS), the same well
is used both for injecting a fluid and for producing oil. In CSS, cycles of
steam injection,
soak, and oil production are employed. Once the production rate falls to a
given level, the
4
CA 02781273 2012-06-28
well is put through another cycle of injection, soak, and production. An
example of CSS is
described in U.S. Patent No. 4,280,559 (Best).
[0020] Steam Flooding (SF) involves injecting steam into the
formation through an
injection well. Steam moves through the formation, mobilizing oil as it flows
toward the
production well. Mobilized oil is swept to the production well by the steam
drive. An
example of steam flooding is described in U.S. Patent No. 3,705,625 (Whitten).
[0021] Other thermal processes include Solvent-Assisted Steam
Assisted Gravity
Drainage (SA-SAGD), an example of which is described in Canadian Patent No.
1,246,993
(Vogel); Vapour Extraction (VAPEX), an example of which is described in U.S.
Patent No.
5,899,274 (Frauenfeld); Liquid Addition to Steam for Enhanced Recovery
(LASER), an
example of which is described in U.S. Patent No. 6,708,759 (Leaute et al.);
and Combined
Steam and Vapour Extraction Process (SAVEX), an example of which is described
in U.S.
Patent No. 6,662,872 (Gutek), and derivatives thereof. These processes employ
a "diluting
agent".
[0022] Solvent-dominated recovery processes (SDRPs) are another category
of in
situ processes where solvent is used to reduce the viscosity of the viscous
oil. At the present
time, solvent-dominated recovery processes (SDRPs) are rarely used to produce
highly
viscous oil. In certain described SDRPs, the solvent is heated.
[0023] Cyclic solvent-dominated recovery processes (CSDRPs) have
also been
proposed. CSDRPs are a subset of SDRPs. A CSDRP may be, but is not
necessarily, a
generally non-thermal recovery method that uses a solvent (or "diluting
agent") to mobilize
viscous oil by cycles of injection and production. In a CSDRP, a viscosity-
reducing solvent is
injected through a well into a subterranean viscous-oil reservoir, causing the
pressure to
increase. Next, the pressure is lowered and reduced-viscosity oil is produced
to the surface
through the same well through which the solvent was injected. Multiple cycles
of injection
and production are used. In some instances, a well may not undergo cycles of
injection and
production, but only cycles of injection or only cycles of production. CSDRPs
may be
particularly attractive for thinner or lower-oil-saturation reservoirs. In
such reservoirs, thermal
methods utilizing heat to reduce viscous oil viscosity may be inefficient due
to excessive heat
loss to the overburden and/or underburden and/or reservoir with low oil
content. References
describing specific CSDRPs include: Canadian Patent No. 2,349,234 (Lim et
al.); G. B. Lim
et al., "Three-dimensional Scaled Physical Modeling of Solvent Vapour
Extraction of Cold
5
CA 02781273 2012-06-28
Lake Bitumen", The Journal of Canadian Petroleum Technology, 35(4), pp. 32-40,
April
1996; G. B. Urn et al., "Cyclic Stimulation of Cold Lake Oil Sand with
Supercritical Ethane",
SPE Paper 30298, 1995; US Patent No. 3,954,141 (Allen et al.); and M. Feali et
al.,
"Feasibility Study of the Cyclic VAPEX Process for Low Permeable Carbonate
Systems",
International Petroleum Technology Conference Paper 12833, 2008. The family of
processes within the Lim et al. references describe embodiments of a
particular SDRP that is
also a cyclic solvent-dominated recovery process (CSDRP). These processes
relate to the
recovery of heavy oil and bitumen from subterranean reservoirs using cyclic
injection of a
solvent in the liquid state which vaporizes upon production. The family of
processes within
the Lim et al. references may be referred to as CSPTM processes. Another
example of a
CSDRP is described in Canadian Patent Document No. 2,688,392 (Lebel et al.,
published
June 9, 2011). In certain CSDRPs, there may be the formation of a second
liquid phase
whose high viscosity may affect the mobility of heavy oil and bitumen, and may
thereby
impact their recovery.
[0024] In certain predominantly non-thermal CSDRPs, while heat is not used
to
reduce the viscosity of the viscous oil, the use of heat is not excluded.
Heating may be
beneficial to improve performance or start-up. For start-up, low-level heating
(for example,
less than 100 C) may be appropriate. Low-level heating of the solvent prior to
injection may
also be performed to prevent hydrate formation in tubulars and in the
reservoir. Heating to
higher temperatures may also benefit recovery.
[0025] Canadian Patent No. 2,652,930 (Ignasiak et al., published
July 20, 2010)
describes, according to the Abstract, a method "for energy efficient,
environmentally friendly,
in-situ recovery of viscous oil or heavy oil by injecting di-methyl ether
(DME) into the
reservoir. The method includes the steps of heating the reservoir utilizing
the condensation
and latent heats of injected DME liquids and/or vapours, mobilizing the
viscous oil/heavy oil
by lowering its viscosity, dissolving the water and some of the components of
the viscous
oil/heavy oil in the DME, recovering from the reservoir the mixture of viscous
oil and DME
containing the dissolved components of the viscous oil, separating the DME
from the mixture
by depressurization followed by pressurizing, heating and re-injecting the
recovered DME,
into the reservoir." Potential disadvantages of DME alone may include DME loss
in water in
the reservoir, and compatibility issues with seal materials in process
equipment, pump and
vessels.
6
CA 02781273 2012-06-28
=
[0026] It is desirable to provide an improved or alternative
diluting agent for diluting
viscous oil for use in surface extraction or in situ processes.
SUMMARY OF THE INVENTION
[0027] Generally, described herein is a diluting agent comprising: (a) an
ether with 2
to 8 carbon atoms; and (b) a non-polar hydrocarbon with 2 to 30 carbon atoms,
for diluting
viscous oil. The dilution may be in situ or at surface.
[0028] Other aspects and features of the present invention will
become apparent to
those ordinarily skilled in the art upon review of the following description
of specific
embodiments of the invention in conjunction with the accompanying figures.
BRIEF DESCRIPTION OF THE DRAWINGS
[0029] Embodiments of the present invention will now be
described, by way of
example only, with reference to the attached Figures, wherein:
[0030] Fig. 1 is a graph illustrating hydrogen bonding solubility parameter
versus
polar solubility parameter of various diluting agents.
[0031] Fig. 2 is a graph illustrating bitumen recovery using
various diluting agents.
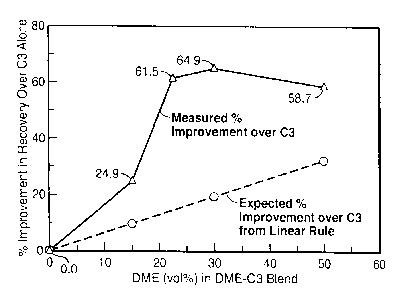
[0032] Fig. 3 is a graph illustrating the percentage improvement
in bitumen recovery
by various blends of di-methyl ether and propane over propane alone.
DETAILED DESCRIPTION
[0033] The term "viscous oil" as used herein means a hydrocarbon,
or mixture of
hydrocarbons, that occurs naturally and that has a viscosity of at least 10 cP
(centipoise) at
initial reservoir conditions. Viscous oil includes oils generally defined as
"heavy oil" or
"bitumen". Bitumen oil is classified as an extra heavy oil, with an API
gravity of about 10 or
less, referring to its gravity as measured in degrees on the American
Petroleum Institute
(API) Scale. Heavy oil has an API gravity in the range of about 22.3 to about
10 . The terms
viscous oil, heavy oil, and viscous oil are used interchangeably herein since
they may be
extracted using similar processes. Viscous oil also includes hydrocarbon
refinery
intermediates and residual hydrocarbon waste streams resulting from both
surface
extraction, for instance solvent based extraction and water-based extraction,
as well as from
in situ recovery processes, for instance SAGD, SA-SAGD, CSS, SDRP, CSDRP, and
other
processes described herein.
7
CA 02781273 2012-06-28
[0034] In situ is a Latin phrase for "in the place" and, in the
context of hydrocarbon
recovery, refers generally to a subsurface hydrocarbon-bearing reservoir. For
example, an in
situ oil recovery technique is one that recovers oil from a reservoir within
the earth.
[0035] The term "formation" as used herein refers to a subterranean
body of rock that
is distinct and continuous. The terms "reservoir" and "formation" may be used
herein
interchangeably.
[0036] "Diluting Agent" means a fluid of a lower viscosity and
lower density than
those of a viscous oil with which it is mixed or blended. Its viscosity may,
for example, be 0.2
to 5 cp at room temperature and at a pressure high enough to make it liquid.
Its density may
be, for example, 450 to 750 kg/m3 at 15 C and at a pressure high enough to
make it liquid.
The mixture or the blend of diluting agent and viscous oil has a viscosity and
a density that is
in between those of the diluting agent and the viscous oil. The diluting agent
may or may not
precipitate asphaltenes if its concentration exceeds a critical concentration.
In addition to
reducing viscosity for the purpose of extracting bitumen at surface or in
situ, the diluting
agent can also be used to reduce viscosity and density for the purpose of
separating
emulsified water droplets from viscous oil.
[0037] Diluting agents that have previously been suggested for
viscous oil recovery
include, but are not limited to, n-alkanes, such as ethane, propane, butane,
normal- and iso-
pentane, cycloalkanes like cyclopentane and cyclohexane, and gas plant
condensates (a
mixture of n-alkanes, naphthenes and aromatics). These diluting agents can
cause
asphaltenes precipitation when their concentrations exceed certain limits. The
precipitated
asphaltenes may adversely affect the permeability of the reservoir. Avoiding
asphaltenes
precipitation is not necessarily a requirement of embodiments described
herein. Aromatic
diluting agents, such as toluene and xylene, are excellent diluting agents for
viscous oil by
being miscible with viscous oil in all proportions and dissolving all four
components of
viscous oil: saturates, aromatics, resins and asphaltenes (SARA). The aromatic
diluting
agents, however, are not generally considered for viscous oil recovery because
of their cost,
material safety, and relatively higher boiling points (for example 110 to 144
C), the latter
leading to poor diluting agent recovery from the reservoir. Ideally, a
diluting agent would
possess good solvency power (as do aromatic diluting agents) but have a lower
boiling point
than do aromatics.
8
CA 02781273 2012-06-28
[0038] As described above in the Summary section, described herein
is a diluting
agent comprising: (a) an ether with 2 to 8 carbon atoms; and (b) a non-polar
hydrocarbon
with 2 to 30 carbon atoms, for diluting viscous oil. The dilution may be in
situ or at surface.
[0039] The ether may have 2 to 4 carbon atoms. The ether may be di-
methyl ether,
methyl ethyl ether, di-ethyl ether, methyl iso-propyl ether, methyl propyl
ether, di-isopropyl
ether, di-propyl ether, methyl iso-butyl ether, methyl butyl ether, ethyl iso-
butyl ether, ethyl
butyl ether, iso-propyl butyl ether, propyl butyl ether, di-isobutyl ether, or
di-butyl ether. The
ether may be di-methyl ether. The ether may be di-ethyl ether.
[0040] The non-polar hydrocarbon may be a C2-C30 alkane, a C2-C30 n-
alkane, C2-
C20 alkane, a C2-C20 n-alkane, a C2-05 alkane, propane, a C5-C7 cycloalkane,
or
cylcohexane.
[0041] The non-polar hydrocarbon may be a mixture of non-polar
hydrocarbons, the
non-polar hydrocarbons being C2-C30 alkanes, the mixture being substantially
aliphatic and
substantially non-halogenated. "Substantially aliphatic and substantially non-
halogenated"
means less than 10% by weight of aromaticity and with no more than 1 mole
percent halogen
atoms. In other embodiments, the level of aromaticity is less than 5, less
than 3, less than 1,
or 0 % by weight.
[0042] The non-polar hydrocarbon may be a mixture of non-polar
hydrocarbons and
may be a gas plant condensate comprising at least one C3-C17 n-alkane, at
least one C5-C7
cycloalkane, and at least one C6-C8 aromatic hydrocarbon.
[0043] The non-polar hydrocarbon may be an alkane, for example
ethane, propane,
butane, normal- and iso-pentane, hexane, heptane, or other higher molecular
weight
alkanes. In one embodiment, the alkane has up to 10 carbon atoms per molecule.
In one
embodiment, the alkane has up to 20 carbon atoms per molecule. In one
embodiment, the
alkane has up to 30 carbon atoms per molecule. In one embodiment, the non-
polar
component of the blend has a low-boiling point of less than 125 C that can be
easily
separated from in situ or surface-extracted solvent-diluted bitumen. Alkanes
have been
previously suggested as bitumen solvents. Compared to toluene and xylene,
which dissolve
all the four components of bitumen, namely saturates, aromatics, resins and
asphaltenes
(SARA), and are miscible with bitumen in all proportions, alkanes do not
dissolve
asphaltenes and are not miscible with bitumen at high solvent concentrations.
Alkanes can
be quite slow acting in that they penetrate an oil sands matrix at a slow
pace, which can
affect economic recovery.
9
CA 02781273 2012-06-28
[0044] The non-polar component may alternatively be another known
diluting agent,
such as one used in processes described in the above background section.
[0045] The diluting agent may be di-methyl ether and propane.
[0046] The hydrogen bonding parameter and the polarity parameter of
the non-polar
component may be each less than 1 MPa 5.
[0047] The diluting agent may have a volume ratio of (a):(b) of
10:90 to 90:10; or
20:80 to 70:30; or 22.5:77.5 to 50:50.
[0048] The diluting agent may have a viscosity of 0.2 to 5 cp at
room temperature.
[0049] The diluting agent may have a density of 450 to 750 kg/m3 at
15 C.
[0050] The diluting agent may be in liquid form.
[0051] The diluting agent may have a Hansen hydrogen bonding
solubility parameter
of 0.7 to 6.2.
[0052] The diluting agent may have a Hansen polar solubility
parameter of 0.5 to 6.5.
[0053] As described in the Background section, Canadian Patent No.
2,652,930
(Ignasiak et al., published July 20, 2010) describes the use of heated di-
methyl ether (DME)
for in situ extraction. Potential disadvantages of DME alone may include DME
loss in the
reservoir water, and compatibility issues with seal materials.
[0054] As described below in the Examples section, combining DME
with propane
may improve oil recovery over the use of propane alone and over what would be
expected
based on a linear blending relationship. A blend of DME and propane may also
mitigate
diluting agent loss and/or be more compatible with seal materials than DME
alone. A blend
of DME and propane may also reduce the formation of the viscous second liquid
phase that
may form when propane is used alone.
[0055] Surface Extraction
[0056] The present diluting agent may be used for surface extraction of
viscous oil.
After extraction, the diluting agent may be recovered from the extracted sands
and recycled.
Diluting agent may also be recovered from the diluting agent-diluted extracted
viscous oil for
recycling.
[0057] The present diluting agent may provide a non-aqueous route to
dilute viscous
oil to eliminate, or reduce, the need for the tailings ponds. "Non-aqueous" is
used herein to
refer to extraction that is effected by diluting viscous oil using a diluting
agent other than
water, however the use of water is not necessarily excluded from the entire
process as
CA 02781273 2012-06-28
detailed in other solvent-based extraction processes described above in the
Background
section.
[0058] The use of the present diluting agent may extract more
viscous oil in less time
than at least certain conventional diluting agents.
[0059] Various solvent-based extraction methods are discussed above in the
background section. The instant diluting agent may be used in such processes.
For
instance, the instant diluting agent may be used in a Solvent Extraction
Spherical
Agglomeration (SESA), descriptions of which are provided in Sparks et al.,
Fuel 1992(71); pp
1349-1353; and U.S. Patent No. 4,057,486 (Meadus at al.), as well as a solvent-
based
extraction process as described in Canadian Patent Application Serial No.
2,724,806
("Adeyinka et al."), published June 30, 2011 and entitled "Processes and
Systems for Solvent
Extraction of Bitumen from Oil Sands". Examples of solvents used in Adeyinka
are heptane,
iso-heptane, hexane, iso-hexane, pentane, iso-pentane, a cycloalkane of 4 to 9
carbon
atoms, cyclohexane, cyclopentane, and mixtures thereof.
[0060] In order to make DME a liquid, the system can be under pressure
since DME
boils at -24 C at atmospheric pressure. DME should be easier to recover than
cyclohexane or
heptane which boil at 81 C and 97 C, respectively. The recovery of the
diluting agent may be
accomplished by reduction of pressure at or above room temperature.
[0061] In Situ
[0062] The present diluting agent may be used for in situ dilution to
reduce the
viscosity of viscous oil in an underground reservoir. The particular process
of injection and
production (including well configuration) may be a known process (with the new
diluting
agent), for instance one described above in the background section which uses
a diluting
agent (often referred to in the literature as a solvent), optionally with
other fluids.
[0063] The process may be, for instance, solvent-assisted steam-assisted
gravity
drainage (SA-SAGD), a cyclic solvent dominated recovery process (CSDRP), a
liquid
addition to steam for enhanced recovery process (LASER), a vapour extraction
process
(VAPEX), Cyclic Steam Stimulation (CSS), or a heated solvent process.
[0064] The injection well may be horizontal, vertical, or otherwise.
[0065] The diluting agent may be injected as a liquid, as a heated liquid,
as a vapor,
or as a supercritical fluid.
[0066] The process may be thermal or non-thermal. "Non-thermal" means
that
11
CA 02781273 2012-06-28
heat is not generally used to reduce the viscosity of the viscous oil, while
the use of heat is
not excluded. For instance in certain non-thermal CSDRPs, heating may be
beneficial to
improve performance or start-up. For start-up, low-level heating (for example,
less than
100 C) may be appropriate. Low-level heating of the solvent prior to injection
may also be
performed to prevent hydrate formation in tubulars and in the reservoir.
Heating to higher
temperatures may also benefit recovery.
[0067] The diluting agent may be injected with other fluids, for
instance in certain
CSDRPs, co-injectants may include: diesel, viscous oil, bitumen, or diluent,
to provide flow
assurance, or CO2, natural gas, C3+ hydrocarbons, ketones, or alcohols.
[0068] The diluting may be injected with a gas plant condensate to
potentially
improve the effectiveness of the latter in recovering viscous oil. A gas plant
condensate
comprises alkanes, naphthenes, and aromatics, and is commonly used in viscous
oil
extraction partially because of its availability.
[0069] In one embodiment, the diluting agent may be used to dilute
viscous oil
between a horizontal injector well and a horizontal producer well to establish
fluid
communication between the two wells, prior to steam injection to start a SAGD
process.
[0070] In another embodiment, the production is continuous from a
neighboring
horizontal or vertical well which is at some distance from the injection well.
[0071] In another embodiment, the diluting agent is injected from a
horizontal well
and the diluted viscous oil is produced from a horizontal well spaced at a
certain depth below
the injector. Injection and production from this well pair is either
continuous or cyclical.
[0072] A combination of known in situ processes may also be used.
[0073] In another embodiment, the diluting agent may be used to
reduce the
formation of the viscous second liquid phase. In yet another embodiment, the
proportion of
ether and nonpolar component in the diluting agent injected can be varied over
time to
optimize diluent finger growth and/or minimize the viscous second liquid phase
formation.
[0074] The diluent storing system can be pressurized to liquefy a
lower boiling ether
like DME. The separation of DME from produced fluids is simpler than that with
prior
solvents. The present diluting agent may be particularly suitable for cyclic
processes and
lower pressure SA-SAGD. It may also be particularly suitable for processes
where the
viscous second liquid phase formation occurs and hinders mobility of bitumen
and/or causes
plugging in tubular, pump or process equipment. While C3 has been exemplified
below as
being the non-polar component in the diluting agent, other non-polar C2-C20
hydrocarbons
12
CA 02781273 2012-06-28
are also expected to function as they are non-polar molecules with Hansen
solubility
parameters similar to that of C3. While DME has been exemplified below as
being the polar
component in the diluting agent, other C2-C8 ethers are also expected to
function as these
are polar molecules with Hansen solubility parameters similar to that of DME.
[0075] Additional Embodiments and Potential Advantages
[0076] Fig. 1 is a graph of hydrogen bonding solubility parameter
versus polar
solubility parameter of various diluting agents (e.g. propane and hexane) and
bitumen
solvents (e.g., toluene and xylene). Ideally, the solubility parameters of a
solvent and those
of the viscous oil should be similar. The oval region in Fig.1 is a non-
limiting preferred solvent
region for bitumen based on experimental data. While DME and propane do not
individually
fall within this region, their blend (shown as SynSolv in Fig. 1) does.
[0077] The diluting agent may have a Hansen hydrogen bonding
parameter of 0.7 to
6.2 and a Hansen polar solubility parameter of 0.5 to 6.5.
[0078] Potential advantages of embodiments described herein may
include fewer
tailings than aqueous based extraction and/or a lower energy requirement to
separate the
diluting agent from the viscous oil as compared to certain solvent-based
extraction
processes.
[0079] Potential advantages of embodiments described herein over
certain
conventional diluting agents in diluting viscous oil may include faster
dilution, more efficient
diluting agent separation from diluting agent-diluted viscous oil, improved
environmental
conditions, improved safety conditions, and/or reduced diluting agent cost.
[0080] The diluting agent may also be used to clean viscous oil-
coated equipment or
vessels used in extraction processes. This may be environmentally advantageous
over
aromatic diluting agents, for instance toluene or xylene.
[0081] Example
[0082] Tests were conducted in a sand pack saturated with live Cold
Lake bitumen at
room temperature using various fluids using the following test conditions:
[0083] - Sand Pack Permeability: 5.4 D,
[0084] - Live Cold Lake Bitumen (10 CH4 GOR),
[0085] - Confining Pressure: 7.5 MPaa; T: 21 C,
[0086] - Diluting Agents: C3, diesel, acetone, DME,
[0087] - Test protocol:
13
CA 02781273 2012-06-28
[0088] Injecting a diluting agent at a constant rate of 2.7 mL/min
until the pressure
increases to 9.5 MPaa.
[0089] Injecting at a constant pressure of 9.5 MPaa and at an
outlet pressure of 5
MPaa, and collecting produced bitumen in a sample cylinder until the solvent
breakthrough at
the end of the pack occurs.
[0090] Stopping injection at solvent breakthrough and closing
outlet valve and
allowing sand pack pressure to equilibrate.
[0091] Switching sample cylinder and injecting diluting agent at a
constant rate of
2.73 ml/min for a predetermined pore volume (PV) of injection and collecting
produced
diluted bitumen in another sample cylinder. One PV is the total volume of the
pores in the
porous sand pack.
[0092] Monitoring pressure differential across the sand pack during
diluting agent
injection and recording the density of the diluted produced bitumen.
[0093] Switching sample cylinder again after the predetermined PV
of diluting agent
injection and continuing injection and production after several samples of
produced diluted
bitumen have been collected in different sample cylinders.
[0094] Determining the bitumen produced in different sample bottle
and cylinders
after removing the diluting agent from the diluted bitumen.
[0095] Cleaning the sand pack with a series of solvent injection
and reusing it for the
next test.
[0096] As illustrated in Figure 2, the tests showed higher recovery
using 85:15,
77.5:22.5, 70:30 and 50:50 (all v/v) blends of 03 (propane) and DME (di-methyl
ether) than
by C3 alone. The Hansen hydrogen bonding parameters for the blends ranged from
0.70 to
5.7 and the Hansen polar solubility parameter ranged from 0.5 to 6.10. The
blends were
even more effective at a higher PV. For instance, at 2.3 PV, the recovery by
the 50:50 (v/v)
DME:C3 blend was 65% of original bitumen in place (OBIP) as compared to a
recovery of
41%. This level of recovery uplift over C3 alone seems to persist at as low as
22.5 vol%
DME. At 15 vol% DME, the recovery uplift falls off but is significant
(recovery of 51% OBIP
for the blend vs. 42% OBIP for C3 alone.
[0097] Figure 3 compares the percentage improvement in bitumen recovery by
blends of DME and C3 over C3 alone. The improvement varies from 25% (for 15
vol% DME)
to 65% (at 30 vol% DME). Shown in Fig. 3 is also the expected improvement in
recovery
over C3 based on a linear mixing rule using the measured recovery at 100% DME.
For each
14
CA 02781273 2012-06-28
of the four DME-C3 blends, the measured recovery is significantly higher than
that calculated
from the linear rule. This indicates some unexpected synergy takes place when
C3 is
blended with DME. Having a wide effective or acceptable blending ratio (for
instance, 22.5 to
100 vol% DME) may be useful for mitigating the effects of a variable supply
and price of
diluent agent(s).
[0098] The water production was also significantly lower (in the ppm
range) when the
DME is blended with C3, in the few hundred ppm at lower PV and increasing to a
few
thousand ppm at higher PV. The seal compatibility should also improve by
blending DME
with C3. DME alone is not compatible with seal materials but its 30:70 (VN)
blend is
compatible with seal materials. The viscous second liquid phase formation
observed with C3
injection was also suppressed significantly by blending DME with C3.
[0099] Numbered Embodiments
[00100] Paragraph 1. A diluting agent for diluting viscous oil, the
diluting agent
comprising:
(a) an ether with 2 to 8 carbon atoms; and
(b) a non-polar hydrocarbon with 2 to 30 carbon atoms.
[00101] Paragraph 2. The diluting agent according to Paragraph 1,
wherein (a) has 2
to 4 carbon atoms.
[00102] Paragraph 3. The diluting agent according to Paragraph 1,
wherein (a) is di-
methyl ether, methyl ethyl ether, di-ethyl ether, methyl iso-propyl ether,
methyl propyl ether,
di-isopropyl ether, di-propyl ether, methyl iso-butyl ether, methyl butyl
ether, ethyl iso-butyl
ether, ethyl butyl ether, iso-propyl butyl ether, propyl butyl ether, di-
isobutyl ether, or di-butyl
ether.
[00103] Paragraph 4. The diluting agent according to Paragraph 1,
wherein (a) is di-
methyl ether.
[00104] Paragraph 5. The diluting agent according to Paragraph 1,
wherein (a) is di-
ethyl ether.
[00105] Paragraph 6. The diluting agent according to any one of
Paragraphs 1 to 5,
wherein (b) is a C2-C30 alkane.
[00106] Paragraph 7. The diluting agent according to any one of Paragraphs
1 to 5,
wherein (b) is a C2-C30 n-alkane.
[00107] Paragraph 8. The diluting agent according to any one of
Paragraphs 1 to 5,
wherein (b) is a C2-C20 alkane.
CA 02781273 2012-06-28
[00108] Paragraph 9. The diluting agent according to any one of
Paragraphs 1 to 5,
wherein (b) is a C2-C20 n-alkane.
[00109] Paragraph 10. The diluting agent according to any one of
Paragraphs 1 to 5,
wherein (b) is a C2-05 alkane.
[00110] Paragraph 11. The diluting agent according to any one of Paragraphs
1 to 5,
wherein (b) is propane.
[00111] Paragraph 12. The diluting agent according to any one of
Paragraphs 1 to 5,
wherein (b) is a C5-C7 cycloalkane.
[00112] Paragraph 13. The diluting agent according to any one of
Paragraphs 1 to 5,
wherein (b) is cyclohexane.
[00113] Paragraph 14. The diluting agent according to any one of
Paragraphs 1 to 5,
wherein (b) is a mixture of non-polar hydrocarbons, the non-polar hydrocarbons
being C2-
C30 alkanes, the mixture being substantially aliphatic and substantially non-
halogenated.
[00114] Paragraph 15. The diluting agent according to any one of
Paragraphs Ito 5,
wherein (b) is a mixture of non-polar hydrocarbons and is a gas plant
condensate comprising
at least one C3-C17 n-alkane, at least one C5-C7 cycloalkane, and at least one
C6-C8
aromatic hydrocarbon.
[00115] Paragraph 16. The diluting agent according to Paragraph 1,
wherein (a) is di-
methyl ether and (b) is propane.
[00116] Paragraph 17. The diluting agent according to any one of Paragraphs
Ito 16,
wherein (b) has a hydrogen bonding parameter of less than 1 MPa" and a
polarity
parameter of less than 1 MPa".
[00117] Paragraph 18. The diluting agent according to any one of
Paragraphs Ito 17,
wherein the diluting agent has a volume ratio (a):(b) of 10:90 to 90:10.
[00118] Paragraph 19. The diluting agent according to Paragraph 18, wherein
the
volume ratio is 20:80 to 70:30.
[00119] Paragraph 20. The diluting agent according to Paragraph 18 or
19, wherein
the volume ratio is 22.5:77.5 to 50:50.
[00120] Paragraph 21. The diluting agent according to any one of
Paragraphs 1 to 20,
wherein the diluting agent has a viscosity of 0.2 to 5 cp at room temperature.
[00121] Paragraph 22. The diluting agent according to any one of
Paragraphs 1 to 21,
wherein the diluting agent has a density of 450 to 750 kg/m3 at 15 C.
16
CA 02781273 2012-06-28
[00122] Paragraph 23. The diluting agent according to any one of
Paragraphs 1 to 22,
wherein the diluting agent is in liquid form.
[00123] Paragraph 24. The diluting agent according to any one of
Paragraphs 1 to 23,
wherein the diluting agent has a Hansen hydrogen bonding solubility parameter
of 0.7 to 6.2.
[00124] Paragraph 25. The diluting agent according to any one of Paragraphs
1 to 24,
wherein the diluting agent has a Hansen polar solubility parameter of 0.5 to
6.5.
[00125] Paragraph 26. A use of the diluting agent according to any
one of Paragraphs
1 to 25, for diluting viscous oil.
[00126] Paragraph 27. The use according to Paragraph 26, wherein the
diluting
viscous oil is in situ dilution of the viscous oil within an underground
viscous oil reservoir.
[00127] Paragraph 28. The use according to Paragraph 26 or 27,
wherein the use is
for injecting the diluting agent into a well completed in the underground
viscous oil reservoir
to reduce a viscosity of the in situ viscous oil.
[00128] Paragraph 29. The use according to any one of Paragraphs 26
to 28, wherein
the use is for in situ viscous oil dilution by a solvent-assisted steam-
assisted gravity drainage
process, a cyclic solvent dominated recovery process, a liquid addition to
steam for
enhanced recovery process, a vapour extraction process, or a heated solvent
process.
[00129] Paragraph 30. The use according to any one of Paragraphs 26
to 29, wherein
the use is for in situ viscous oil dilution by a cyclic solvent dominated
recovery process.
[00130] Paragraph 31. The use according to Paragraph 26, for establishing
fluid
communication in an underground viscous oil reservoir between injector and
producer wells
in a solvent assisted gravity drainage process prior to steam injection.
[00131] Paragraph 32. The use according to any one of Paragraphs 26
to 28, wherein
the use of the diluting agent is together with steam.
[00132] Paragraph 33. The use according to Paragraph 26, wherein the
diluting
viscous oil is surface dilution.
[00133] Paragraph 34. The use according to Paragraph 33, wherein the
diluting
viscous oil is a solvent-based extraction process.
[00134] Paragraph 35. The use according to Paragraph 26, for cleaning
a viscous oil-
coated surface.
[00135] Paragraph 36. A process for recovering viscous oil from an
underground
reservoir, the process comprising:
17
CA 02781273 2012-06-28
=
(i) injecting the diluting agent according to any one of Paragraphs 1 to 25
into the reservoir to reduce the viscosity of the viscous oil; and
(ii) producing at least a fraction of the diluting agent and the viscous
oil.
[00136] Paragraph 37. The process of Paragraph 36, wherein the
process is a solvent-
assisted steam-assisted gravity drainage, a cyclic solvent dominated recovery
process, a
liquid addition to steam for enhanced recovery process, a vapour extraction
process, or a
heated solvent process.
[00137] Paragraph 38. The process according to Paragraph 36 or 37,
wherein the
process is a cyclic solvent dominated recovery process.
[00138] Paragraph 39. A process for establishing fluid communication in an
underground viscous oil reservoir between injector and producer wells in a
solvent assisted
gravity drainage process prior to steam injection, the process comprising:
(i) providing the diluting agent according to any one of
Paragraphs 1 to
25; and
(ii) injecting the diluting agent into the reservoir to establish the fluid
communication.
[00139] Paragraph 40. A process for diluting viscous oil at surface,
the process
comprising:
providing viscous oil; and
(ii) combining the diluting agent according to any one of Paragraphs 1 to
with the viscous oil to dilute the viscous oil.
[00140] Paragraph 41. A process for cleaning a viscous oil-coated
surface, the
process comprising:
(i) providing the diluting agent according to any one of Paragraphs 1 to
25 25; and
(ii) applying the diluting agent to the viscous oil-coated surface to clean
the viscous oil-coated surface.
[00141] In the preceding description, for purposes of explanation,
numerous details
are set forth in order to provide a thorough understanding of the embodiments
of the
invention. However, it will be apparent to one skilled in the art that these
specific details are
not required in order to practice the invention.
[00142] The above-described embodiments of the invention are intended
to be
examples only. Alterations, modifications and variations can be effected to
the particular
18
CA 02781273 2012-06-28
embodiments by those of skill in the art without departing from the scope of
the invention,
which is defined solely by the claims appended hereto.
19