Note: Descriptions are shown in the official language in which they were submitted.
CA 02781727 2012-05-23
WO 2011/064308 _ PCT/EP2010/068235
Method for predicting the response of a tumor disease
to a therapeutic measure
The invention relates to a method for predicting the
response of a tumor disease in a patient, caused by a
solid epithelial tumor, to a therapeutic measure. A
solid tumor is understood as meaning a tumor whose
cells grow in the cell assemblage, forming a solid,
locally circumscribed tissue. It is known that cells
become detached from such a tumor and are distributed
in the body via the blood or the lymph.
It is furthermore known that tumors have different
sensitivities to therapeutic measures such as, for
example, a chemotherapy or an irradiation. The
sensitivity of cancer cells to growth-inhibitory
chemotherapeutics, also known as cytostatics, which are
administered in the context of a chemotherapy is
referred to as chemosensitivity. The success of a
chemotherapy depends, inter alia, on the
chemosensitivity of the cancer cells. For example, the
chemosensitivity to cytostatics from the group of
alkylants, which act via a modification of the
hereditary material of the tumor cells, may be reduced
as the result of the activation of DNA repair enzymes
in tumor cells. Likewise, a reduced transport of
cytostatics into the interior of the cell, the
inactivation of the cytostatics or else the lack of
expression of activity-mediating receptors may result
in a reduced chemosensitivity of the tumor cells. A
reduced chemosensitivity of the tumor cells may lead to
failure of the therapy.
It is assumed that each patient reacts individually to
a certain chemotherapeutic as the result of his genetic
background and the development of subclones within a
tumor. To determine the most efficient chemotherapeutic
for a patient, the current procedure is, before
treating the tumor, first to obtain cells from tissue
CA 02781727 2012-05-23
WO 2011/064308 - 2 - PCT/EP2010/068235
portions of the tumor by digesting the tumor tissue of
the tissue portions with proteolytically active
enzymes. Then, the chemosensitivity to various
chemotherapeutics, of the cells which have been
obtained from the tumor tissue and which have been
cultured over a prolonged period, is determined in
vitro. To this end, it is tested whether cultured cells
multiply in the presence of a specific chemotherapeutic
or whether they die off in the presence of the latter.
The procedure is intended largely to prevent a patient
from being treated with a chemotherapeutics which shows
no, or only poor, activity for this patient.
An in-vitro chemosensitivity test for hematological
tumors is known from Bird, M.C. et al., Leuk. Res.
1986, 10(4); 445-449. Here, leukocytes are isolated
from the blood or from bone marrow, exposed to a
therapeutic agent and incubated for 4 days. The
destruction of tumor cells by the therapeutic agent is
determined by differential staining of live and dead
cells, with the live cells being identified in
morphological terms. The disadvantage of this method is
that it is only suitable for testing the
chemosensitivity of hematological tumors.
It is known from Pachmann, K. et al., J. Clin. Oncol.
2008, 26:1208-1215 that the risk of breast cancer
recurring in female patients which are given an
adjuvant chemotherapy can be predicted with a certain
degree of probability by determining the number of
epithelial tumor cells (CETCs) which circulate in the
blood during and at the end of the chemotherapy. It is
assumed that the increase in the number of CETCs is due
to already growing metastases which release cells into
the circulation. Monitoring the number of CETCs is
considered to be a valuable tool for monitoring the
therapy.
CA 02781727 2012-05-23
WO 2011/064308 - 3 - PCT/EP2010/068235
It is known from Veneziani, B.M. et al., Mol. Cancer
Ther. 2007, 6(12), pages 3091 to 3099, to remove tissue
from solid epithelial tumors, to isolate stromal cells
and epithelial cells and to coculture these cells for
at least 15 days. After passaging the cells, the latter
were exposed to various therapeutic agents and the
inhibition of the tumor cell growth was studied so as
to obtain indications regarding the efficiency of the
therapeutic agents. The disadvantage of this method is
that it takes a relative long time to carry out.
It is an object of the present invention to provide an
alternative method which can be carried out rapidly and
which, before carrying out a therapeutic measure
against a tumor disease caused by a solid epithelial
tumor, permits a prediction of whether the tumor is
sensitive to the therapeutic measure.
This object is achieved by a method according to claim
1. Expedient configurations can be seen from the
features of patent claims 2 to 11.
According to the invention, there is provided a method
for predicting the response of a tumor disease in a
patient, caused by a solid epithelial tumor, to a
therapeutic measure, wherein epithelial tumor cells
from a body fluid of the patient are taken up in a cell
culture medium in each case. The epithelial tumor cells
which are present in the body fluid are cells which
have become detached from the solid tumor.
The cell culture medium is a medium which retains the
viability of the tumor cells in cell culture. A large
number of such media are known, such as, for example,
Ham's F12 or RPMI. Preferably, the cell culture medium
does not comprise any added growth factor or any serum
comprising growth factors, such as, for example, fetal
calf serum. This brings about a selection of the
CA 02781727 2012-05-23
WO 2011/064308 - 4 - PCT/EP2010/068235
epithelial tumor cells over any other cells which may
be present because the tumor cells, in contrast to the
other cells, do not require any external growth factors
for their survival and/or multiplication.
In the method, the tumor cells from a sample of the
cell culture medium comprising the tumor cells are
exposed to the therapeutic measure, while the tumor
cells from a control sample of the cell culture medium
comprising the tumor cells remain untreated. Then, the
proportion of dying-off and dead epithelial tumor cells
in the total number of epithelial tumor cells is
determined for the sample and the control sample,
respectively, and is used to determine a dying-off rate
of the epithelial tumor cells which is caused by the
therapeutic measure as a measure of the response. The
proportion of dying-off and dead epithelial tumor cells
can also be determined indirectly by determining the
proportion of live and non-dying-off epithelial tumor
cells in the total number of epithelial tumor cells and
subtracting it from the total (100% or 1) which
corresponds to the total number. The proportion of
dying-off and dead and/or of live and non-dying-off
epithelial tumor cells can be determined with the aid
of cytometric methods or methods of image analysis.
The dying-off rate of the epithelial tumor cells, which
is caused by the therapeutic measure, is determined by
subtracting the proportion of dying-off and dead
epithelial tumor cells in the control sample from the
proportion of dying-off and dead epithelial tumor cells
in the sample. The dying-off rate can be determined as
a function of time and/or as a function of the
concentration of a chemotherapeutic agent or of the
intensity of a therapeutic measure, for example of an
irradiation.
CA 02781727 2012-05-23
WO 2011/064308 - 5 - PCT/EP2010/068235
A big advantage with respect to the exposure of the
patient and to a rapid execution of the method is that
the execution of the method does not require any
material to be removed from the solid epithelial tumor.
Furthermore, the method according to the invention does
not require the culturing of the epithelial tumor cells
before they are exposed to the therapeutic measure. The
tumor cells can be exposed to the therapeutic measure
directly after being taken up in the cell culture
medium.
A period of from one hour to a few days, in particular
of 2 hours, 3 hours, 1 day, 2 days or 3 days, may
elapse between the therapeutic measure and the
determination of the proportion of dying-off and dead
epithelial tumor cells in the total number of
epithelial tumor cells. During this time interval, the
tumor cells are kept under customary cell culture
conditions, which permit a survival of the tumor cells
in the cell culture medium without therapeutic measure.
Usually, such cell culture conditions comprise a CO2
content of 5% - 10% in the atmosphere and a temperature
of 37 C. The cell culture medium preferably does not
comprise any added growth factor and preferably no
added serum which comprises growth factors.
The inventors have found that the reaction to a
therapeutic measure of cells of a solid epithelial
tumor which are present in a body fluid of a patient is
suitable for predicting the response of a tumor disease
caused by this tumor to the therapeutic measure. This
is surprising because it has been assumed to date that
most of the tumor cells present in the body fluid are
present in the body fluid because they have lost their
ability to grow in association with other cells of the
tumor. One has therefore assumed that they are not
representative for a solid tumor. However, the
CA 02781727 2012-05-23
WO 2011/064308 - 6 - PCT/EP2010/068235
inventors have found that the epithelial tumor cells
which are present in a body fluid can undergo changes
in such a way that they are again capable of colonizing
and growing within the cell assemblage. Therefore, they
play a decisive role in the formation of metastases,
which is frequently life-threatening.
The tumor disease may also take the form of a minimum
residual disease or of a recurrence of the tumor
disease in the form of metastases after the complete
removal of a primary tumor. Epithelial tumor cells
often may still be present in the body fluid even years
after the removal of a solid epithelial primary tumor.
In such a case, the removal of tissue from the solid
tumor as described by Veneziani et al. is not possible
in the first place.
The method according to the invention makes it
possible, before the beginning of a therapy and without
removing a tissue sample from the solid tumor of a
patient, to test whether an envisaged therapeutic
measure is suitable for the therapy of the tumor
disease. It permits an individual patient-specific
therapy using that therapeutic measure to which the
response of the patient in question is best. At the
same time, the method according to the invention makes
it possible to avoid the patient being treated by an
expensive therapy which, for this patient, has no or
little activity and a multiplicity of side effects.
Apart from the individual advantages for the individual
patient, the method according to the invention allows
money to be saved in the health care system.
The method according to the invention manages without
concentration methods which are specific for epithelial
tumor cells, for example by means of antibody-coated
magnetic particles. This rules out distortion of the
determined dying-off rate as the result of the
CA 02781727 2012-05-23
WO 2011/064308 - 7 - PCT/EP2010/068235
concentration process being different, or incomplete,
for various cells. Moreover, the method according to
the invention manages without the cells being isolated
from the tumor tissue by means of enzymatic digestion,
which might change the tumor cells' properties, and
without the long-term culturing of the tumor cells
which has previously been customary in chemosensitivity
tests. The properties such as, for example, the
sensitivity of the tumor cells to the therapeutic
measure, may change during the long-term culture. The
isolation of the cells and the long-term culture may,
therefore, lead to a distortion of the results. In
contrast, the method according to the invention allows
the sensitivity of the tumor to the therapeutic measure
to be determined specifically and selectively for the
epithelial tumor cells obtained from the body fluid.
Therefore, the method according to the invention can be
performed considerably more rapidly, and leads to a
result more rapidly than for example the method known
from Veneziani et al. It is not necessary to remove
tissue of the solid epithelial tumor from the patient
or to culture the cells obtained therefrom before they
are exposed to the therapeutic measure. After the cells
have been exposed to the therapeutic measure, no cell
growth is required because in the method according to
the invention it is not necessary to determine a growth
inhibition for assessing the efficacy of the
therapeutic measure.
The body fluid may take the form of blood, ascites
fluid, lymph, pleural exudate, liquor or urine. The
epithelial tumor cells in this context may be in
particular circulating epithelial tumor cells (CETCs)
which occur in the blood.
Before the tumor cells are taken up in the culture
medium, they may be concentrated solely exploiting the
CA 02781727 2012-05-23
WO 2011/064308 - 8 - PCT/EP2010/068235
fact that their specific weight is higher than that of
the body fluid surrounding them, in particular by
allowing them to settle or by centrifugation. If the
body fluid takes the form of blood, the red blood cells
present therein may be lyzed before the tumor cells are
concentrated. This facilitates the optical
identification, in particular by image analysis, of the
epithelial tumor cells, at a later point in time.
So as to distinguish the epithelial tumor cells from
other cells which are present in the body fluid, the
former may be brought into contact with a substance
which specifically labels the tumor cells, in
particular an antibody with specificity for epithelial
cells. The antibody may, for example, take the form of
an antibody which recognizes the human epithelial
antigen, for example the monocular antibody HEA-125.
The human epithelial antigen, which is also referred to
as HEA or as CD326, does not occur on blood cells.
Since epithelial cells are usually not present in the
body fluid, an antibody which specifically labels
epithelial cells, in particular a staining antibody,
suffices to identify the former as epithelial tumor
cells. The substance which specifically labels the
tumor cells is a substance which does not, or at least
not essentially, affect the viability of the epithelial
tumor cells.
To identify the dying-off and dead epithelial tumor
cells and/or the live and non-dying-off tumor cells, a
first indicator which specifically indicates dying-off
and/or dead cells, in particular propidium iodide, or a
second indicator which specifically indicates live
cells, may be added to the epithelial tumor cells. Upon
addition of propidium iodide, the live cells remain
unstained by virtue of the fact that their cell
membrane is intact, while the dead and dying-off cells
CA 02781727 2012-05-23
WO 2011/064308 - 9 - PCT/EP2010/068235
are stained by the propidium iodide as it enters the
cells.
The therapeutic measure may comprise an exposure to
radiation or heat or a bringing into contact with a
cytostatic, in particular 5-fluorouracil, or with any
other therapeutic agent which is directed against the
tumor. The solid epithelial tumor may take the form of
a mammary carcinoma or of a bronchial carcinoma.
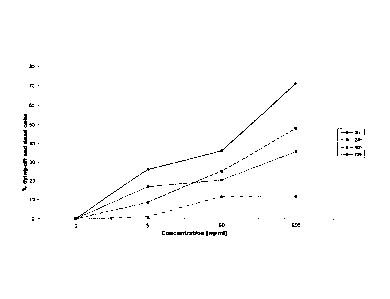
The invention is illustrated hereinbelow with reference
to a use example and to figure 1.
Fig. 1 shows the dying-off rate of cells which bear the
human epithelial antigen (HEA), following incubation
with 5-fluorouracil and staining with propidium iodide,
as a function of the concentration of the
5-fluorouracil employed and of the incubation time.
3 ml of blood treated with EDTA as anticoagulant are
treated with 30 ml of ammonium chloride lysis reagent
for red blood cells (Qiagen, 40724 Hilden, Germany) and
incubated for 10 minutes at 8 to 12 C. The leukocytes
and the epithelial tumor cells are sedimented by
centrifugation at 700 x g for 7 minutes. After the
supernatant comprising the lyzed red blood cells has
been decanted off, the sediment is resuspended in 50 ml
of phosphate-buffered saline (PBS) and recentrifuged at
700 x g for 7 minutes. Thereafter, the sediment is
taken up in 300 p1 of Ham's F12 medium without Phenol
Red supplemented with 1 mM L-glutamine, 100 units/ml
penicillin, 100 units/ml streptomycin, 50 pg/ml
gentamicin and 1 pg/ml fungizone (= amphotericin B), pH
7.4.
To delimit the tumor cells from the remaining blood
cells, 30 pl of the ready-to-use prediluted
fluorochrome-labeled antibody anti-CD326-FITC (Miltenyi
CA 02781727 2012-05-23
WO 2011/064308 - 10 - PCT/EP2010/068235
Biotec GmbH, 51429 Bergisch Gladbach, Germany) are
added to the cell suspension and the mixture is
incubated for 15 minutes in the dark at room
temperature. Thereafter, 6 ml of Ham's F12 medium of
the above-described composition are added and the cells
are suspended therein. In each case 100 pl of PBS for a
control sample or 100 pl of PBS with the test
therapeutic 5-fluorouracil (Medac Gesellschaft fur
klinische Spezialpraparate mbH, Theaterstr. 6, 22880
Wedel, Germany) in concentrations of 5 ng/ml, 50 ng/ml
and 500 ng/ml are placed into the wells of a microtiter
plate. Additionally, in each case 10 pl of a 5 pg/ml
propidium iodide solution are added to act as an
indicator for dead cells. Thereafter, in each case
100 pl of the cell suspension are added.
The cell suspension present in each well is analyzed
cytometrically at the beginning of the incubation and
after 1 hour, 3 hours, 24 hours, 48 hours and 72 hours.
To do so, the number of the tumor cells present in
total in a specific volume and the proportion of the
dying-off and dead tumor cells, i.e. the tumor cells
stained intracellularly by propidium iodide, present in
total is determined in each case. Thereafter, the
proportion of dead cells determined for the control
sample is subtracted from the proportion of dead cells
determined for each of the samples, thereby determining
the time- and concentration-dependent dying-off rate of
the tumor cells which can be attributed to the
therapeutic agent.
Fig. 1 shows typical graphs for different
concentrations of the cytostatic 5-fluorouracil after
different incubation times.