Note: Descriptions are shown in the official language in which they were submitted.
CA 02781995 2012-05-25
Invention Title
NON-FLAMMABLE MAGNESIUM ALLOY WITH EXCELLENT MECHANICAL
PROPERTIES, AND PREPARATION METHOD THEREOF
Technical Field
The present invention relates to a magnesium alloy having
excellent ignition resistance or nonflammability, and more
particularly, to a magnesium alloy that can be melted and cast
in the air as well as in a common inert atmosphere due to the
presence of a stable protective film formed on the surface of
the molten metal, has excellent ignition resistance or
nonflammability in order to prevent spontaneous ignition of
chips, and is excellent in both strength and ductility.
Background Art
Magnesium alloys, which have a high specific strength,
are the lightest of alloys, are applicable in a variety of
casting and machining processes, and have a wide range of
application, and are thereby used in almost all fields in which
light weight is required, such as parts for vehicles and
electronic parts. However, magnesium (Mg) is a metallic
element that has a low electrochemical potential and is very
active. Mg still has limitations in terms of the stability and
reliability of the material, since it undergoes a strong
1
CA 02781995 2012-05-25
reaction when it comes into contact with oxygen or water, and
sometimes causes fires. Therefore, the fields in which Mg can
be applied are still limited compared to its potential
applicability. In particular, it cannot be used in
applications in which safety is important.
Because of this activity of Mg alloys, it is necessary to
create an inert atmosphere using an inert mixture gas, such as
a flux or CO2 + SF6. Since the flux that is used in melting and
refining is a chlorinated substance, there is a problem in that
chlorine atoms reside inside a material, thereby significantly
decreasing corrosion resistance when the conditions for
processing the molten metal are not fulfilled. In order to
solve this problem, it is effective to perform melting and
casting in an atmosphere in which SF6, CO2 and air are mixed,
instead of using the flux. However, SF6 is classified as a
greenhouse gas, the global-warming potential (GWP) of which is
24 times that of C02, so that the use thereof is expected to be
regulated in the future time.
In order to more fundamentally solve this problem,
studies for improving the oxidation resistance of Mg alloys, in
particular, studies intended to increase the ignition
temperature of Mg alloys by adding Ca, Be or rare-earth metals,
have been carried out. Traditionally, Ca has been a main
choice among the alloying elements that are added to Mg alloys
that are oxidation resistant because Ca is cheaper than other
2
CA 02781995 2012-05-25
rare-earth metals, is nontoxic, and greatly increases the
ignition temperature in consideration of the amount that is
added.
According to previous studies on magnesium alloys that
contain Ca, it is known that the ignition temperature increases
by about 250L when 3wt% or greater of Ca is added. Therefore,
in order to realize an ignition temperature of 700L or higher,
at which casting is possible in the condition of being exposed
to the air without a protective gas, or an ignition temperature
of 650L or higher, at which casting is possible in the
condition of including the protective gas, Ca must be added to
Mg alloys, preferably in an amount of 3wt% or greater, and in a
minimum amount of 2wt% or greater. However, when Ca is added
in an amount greater than 2wt%, the tensile properties of Mg
alloys are generally degraded, with the decrease in elongation
being particularly significant. This is because a great
quantity of coarse and brittle eutectic phases is formed,
thereby resulting in cracks. As such, increasing the amount of
Ca that is added has the merit of increasing the ignition
resistance, but also has a drawback in that the tensile
properties are significantly degraded. Therefore, there is the
demand for the development of a magnesium alloy that can
satisfy both the ignition resistance and the tensile properties.
Disclosure
Technical Problem
3
CA 02781995 2012-05-25
Therefore, an object of the present invention is to
provide a magnesium alloy that is intended to solve the
foregoing problem of the related art.
Specifically, an object of the present invention is to
provide a magnesium alloy that contains Ca therein, and more
particularly, has excellent ignition resistance and excellent
tensile properties.
In addition, an object of the present invention is to
provide a magnesium alloy that enables an environment-friendly
manufacturing process, which uses a minimum amount of Ca and
does not use a protective gas such as SF6, which is an
environmental pollutant.
Technical Solution
In order to realize the foregoing object, according to
the present invention, provided is a magnesium (Mg) alloy,
which is manufactured by melt casting. The Mg alloy includes,
by weight, 1.0% or greater but less than 7.0% of Al, 0.05% to
2.0% of Ca, 0.05% to 2.0% of Y, greater than 0% but not greater
than 6.0% of Zn, and the balance of Mg, and the other
unavoidable impurities. The total content of the Ca and the Y
is equal to or greater than 0.1% but less than 2.5% of the
total weight of the magnesium alloy.
In addition, it is preferable that the content of the Ca
range, by weight, from 0.2% to 1.5%.
Furthermore, it is preferable that the content of the Y
4
CA 02781995 2012-05-25
range, by weight, from 0.1% to 1.5%.
In addition, it is preferable that the contents of the Ca
and the Y range from 0.3% to 2.0% of a total weight of the
magnesium alloy.
Furthermore, it is preferable that the magnesium alloy
further include, by weight, greater than 0% but not greater
than 1.0% of Mn.
In addition, it is preferable that the magnesium alloy
further include, by weight, 0.1% to 1.0% of Zr.
According to the present invention, provided is a method
of manufacturing a magnesium alloy. The method includes the
following steps of: forming a magnesium alloy molten metal,
which contains Mg, Al and Zn; adding raw materials of Ca and Y
into the magnesium alloy molten metal; producing a magnesium
alloy cast material from the magnesium alloy molten metal, in
which the raw materials of Ca and Y are added, using a fusion
casting method. A magnesium alloy produced as described above
includes, by weight, 1.0% or greater but less than 7.0% of Al,
0.05% to 2.0% of Ca, 0.05% to 2.0% of Y, greater than 0% but
not greater than 6.0% of Zn, the balance of Mg, and the other
unavoidable impurities.
In addition, it is preferable that the step of adding the
raw materials of Ca and Y into the magnesium alloy molten metal
include the step of adding the raw materials of Ca and Y at a
temperature higher than 800 C.
CA 02781995 2012-05-25
According to the present invention, provided is a method
of manufacturing a magnesium alloy. The method includes the
following steps of: forming a magnesium alloy molten metal,
which contains Mg, Al and Zn; forming a master alloy ingot,
which contains Mg, Al, Zn, Ca and Y, and is soluble at 750 C or
lower; inputting the master alloy ingot, which is soluble at
750 C or lower, into the magnesium alloy molten metal; and
producing a magnesium alloy cast material from the molten metal,
which contains the master alloy ingot, using a fusion casting
method. A magnesium alloy produced as described above includes,
by weight, 1.0% or greater but less than 7.0% of Al, 0.05% to
2.0% of Ca, 0.05% to 2.0% of Y, greater than 0% but not greater
than 6.0% of Zn, the balance of Mg, and the other unavoidable
impurities.
In addition, it is preferable that the master alloy ingot,
which contains Mg, Al, Zn, Ca and Y, is soluble at 750 C or
lower, and is input into the magnesium alloy molten metal at a
temperature lower than 750 C.
According to the present invention, provided is a method
of manufacturing a magnesium alloy. The method includes the
following steps of: forming a magnesium alloy molten metal,
which contains Mg, Al and Zn; adding a Ca compound and a Y
compound into the magnesium alloy molten metal; and producing a
magnesium alloy cast material from the magnesium alloy molten
metal, in which the Ca compound and the Y compound are added,
6
CA 02781995 2012-05-25
using a fusion casting method. A magnesium alloy produced as
described above includes, by weight, 1.0% or greater but less
than 7.0% of Al, 0.05% to 2.0% of Ca, 0.05% to 2.0% of Y,
greater than 0% but not greater than 6.0% of Zn, the balance of
Mg, and the other unavoidable impurities.
In addition, it is preferable that the step of inputting
the raw materials of Ca and Y, the master alloy ingot, which
contains Mg, Al, Zn, Ca and Y, or the Ca compound and the Y
compound into the magnesium alloy molten metal further include
the step of periodically stirring the magnesium alloy molten
metal.
Furthermore, it is preferable that the casting method be
one selected from the group consisting of mold casting, sand
casting, gravity casting, squeeze casting, continuous casting,
strip casting, die casting, precision casting, lost foam
casting, spray casting, and semi-solid casting.
In addition, it is preferable that the method further
include the step of carrying out hot working on the magnesium
alloy cast material produced by the casting method.
The reasons why the content of respective components in
the magnesium alloy of the present invention is limited are as
follows.
Aluminum (Al)
7
CA 02781995 2012-05-25
Al is an element that increases the strength, flowability
and solidification range of a magnesium alloy, thereby
improving castability. In general, the fraction of the
eutectic phase increases in response to an increase in the
content of Al that is added. In addition, as will be described
later, according to the results of experiments of the present
invention, it can be appreciated that the ignition resistance
increases in response to an increase in the content of Al when
Al is added in combination with other alloying elements. When
the content of Al is less than lwt%, the effect of the
increased strength and ignition resistance does not occur, and
when the content of Al is equal to or greater than 7wt%,
tensile properties are degraded due to a coarse Mg17A112
eutectic phase. Therefore, it is preferred that Al is
contained in the range equal to or greater than lwt% and less
than 7wt%.
Calcium (Ca)
Ca improves the strength and thermal resistance
properties of a Mg-Al-based alloy by forming an intermetallic
compound as well as reducing the oxidation of a molten metal by
forming a thin and dense oxide layer of CaO on the surface of
the molten metal, thereby improving the ignition resistance of
the Mg alloy. However, when the content of Ca is less than
0.05wt%, the effect of the improved ignition resistance is not
8
CA 02781995 2012-05-25
significant. On the other hand, when the content of Ca is
greater than 2wt%, the castability of the molten metal
decreases, hot cracking occurs, die sticking increases, and
elongation significantly decreases, which are problematic.
Therefore, in the Mg alloy of the present invention, Ca is
added in an amount ranging preferably from 0.05wt% to 2.Owt%,
and more preferably from 0.2wt% to 1.5wt%.
Yttrium (Y)
Y is generally used as an element that increases high-
temperature creep resistance due to precipitation strengthening,
since it has a high solubility limit. When Y is added in
combination with Ca to the magnesium alloy, the fraction of the
coarse Ca-containing eutectic phase decreases. When Y is added
in an amount of 0.5wt% or greater, there is an effect in that
A12Y particles, which form microscopic grains of a cast
material, are formed, thereby improving tensile properties. In
addition, an oxide layer of Y2O3 is formed on the surface of a
molten metal to form a mixed layer with MgO and CaO, thereby
increasing ignition resistance. When Y is contained in an
amount of less than 0.05wt% in the Mg alloy, the increase in
the ignition temperature is not significant. When Y is
contained in an amount greater than 2wt%, the price of the Mg
alloy rises, and the effect of micronization is lost due to the
coarsening of A12Y particles. Therefore, in the Mg alloy of the
9
CA 02781995 2012-05-25
present invention, Y is included in an amount ranging
preferably from 0.05wt% to 2.Owt%, and more preferably from
O.lwt% to 1.5wt%.
Zinc (Zn)
Zn has an effect of refining grains and increasing
strength when added together with Al. In addition, the maximum
solubility limit of Zn in the Mg alloy is 6.2wt%. When an
amount of Zn greater than this limit is added, a coarse
eutectic phase that is created during casting weakens the
mechanical properties of the cast material, and a considerable
amount of coarse eutectic phase resides even after
homogenization heat treatment, thereby becoming a factor that
weakens the mechanical properties, in particular, elongation.
Therefore, it is preferred that Zn be added in an amount equal
to or less than 6wt%.
Manganese (Mn)
In the Mg-Al-based alloy, Mn improves corrosion
resistance due to its bonding with Fe, which is an impurity
element that impedes corrosion resistance, and increases
strength by forming an Al-Mn intermetallic compound at a rapid
cooling speed. However, when Mn is added in an amount greater
than l.Owt%, a coarse (3-Mn or Al8Mn5 phase is formed in the Mg
alloy, thereby deteriorating the mechanical properties.
CA 02781995 2012-05-25
Therefore, it is preferred that Mn be included in an amount
equal to or less than l.Owt%.
Zirconium (Zr)
Zr is generally added for the purpose of micronization of
grains due to the non-homogeneous nucleation of Mg crystals in
primary Zr because the primary Zr, the crystal lattice of which
is very similar to Mg crystals, is created during
solidification when Zr is added to a Mg alloy that does not
contains some elements, such as Al and Mn. When Zr is added in
an amount less than O.lwt%, its effect is not sufficient. When
Zr is added in an amount that is greater than 1.Owt%,
elongation decreases due to the formation of the coarse primary
Zr. Therefore, it is preferred that Zr be added in an amount
ranging from O.lwt% to l.Owt%.
Other Unavoidable Impurities
The Mg alloy of the present invention may contain
impurities that are unavoidably mixed from raw materials
thereof or during the process of manufacture. Among the
impurities that can be contained in the Mg alloy of the
invention, iron (Fe), silicon (Si) and nickel (Ni) are
components that particularly worsen the corrosion resistance of
the Mg alloy. Therefore, it is preferred that the content of
Fe be maintained at 0.004wt% or less, the content of Si be
11
CA 02781995 2012-05-25
maintained at 0.04wt% or less, and the content of Ni be
maintained at 0.001wt% or less.
Total Amount of Ca and Y
When Ca and Y are added in combination, a dense combined
oxide layer of CaO/Y203 is formed on the surface of a solid or
liquid Mg alloy, so that the ignition resistance of the Mg
alloy is superior to that of a Mg alloy to which Ca or Y is
separately added. In addition, when Ca or Y is separately
added, an amount of 3wt% or greater is generally added in order
to obtain excellent ignition resistance. In this case, however,
there is a problem in that the tensile properties are greatly
degraded because a coarse intermetallic compound is formed. In
contrast, the addition of Ca and Y in combination can
advantageously improve tensile properties by decreasing the
fraction and size of the intermetallic compound while obtaining
excellent ignition resistance. When Ca and Y are added to the
Mg alloy such that the total content thereof is less than
O.lwt%, the effect of the combined addition of Ca and Y does
not appear. This results in a low ignition temperature of
650 C, thereby making it impossible to perform melting in the
air or a common inert gas atmosphere. In addition, when the
total content of Ca and Y is 2.5wt% or greater, an increase in
the cost of the alloy results without any significant advantage
related to the additional increase in the ignition temperature.
12
CA 02781995 2012-05-25
Therefore, in the Mg alloy of the invention, it is preferred
that the total content of Ca and Y that are added be in the
range preferably equal to or greater than 0.lwt% and less than
2.5wt, and more preferably from 0.2wt% to 2.Owt%.
Advantageous Effects
The Mg alloy according to the invention forms a dense
composite oxide layer that acts as a protective film. Thus the
Mg alloy has very excellent oxidation and ignition resistance,
can be melted, cast and machined in the air or a common inert
atmosphere (Ar or N2), and can reduce the spontaneous ignition
of chips that are accumulated during the process of machining.
In addition, the Mg alloy according to the invention is
adapted to reduce costs, protect the health of workers, and
prevent environmental pollution since it does not use a
protective gas such as SF6.
Furthermore, the Mg alloy according to the invention is
applicable as a material for structural components, since its
ignition resistance is superior to that of common alloys, with
the ignition temperature thereof being 50 C higher than the
melting point thereof, and it also has excellent strength and
ductility.
Moreover, the Mg alloy according to the invention can be
variously used as a processing material or a cast material, and
in particular, can be manufactured as an extruded material, a
sheet material, a forged material, a cast material, and the
13
CA 02781995 2012-05-25
like, which can be practically applied to next-generation
vehicles, high-speed rail systems, and the like, in which high-
strength, high-elongation and safety characteristics are
required.
Description of Drawings
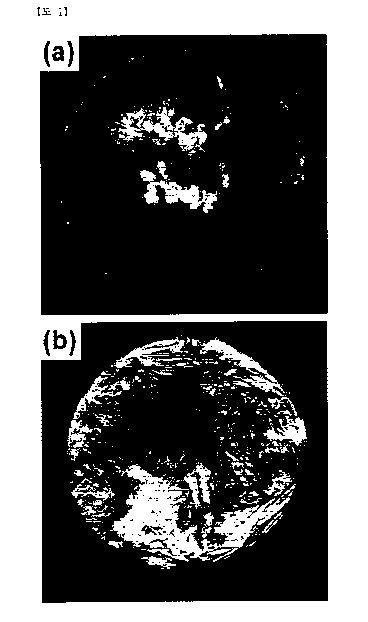
FIG. 1 (a) is a picture showing the surface of an alloy
cast material according to comparative example 1, which is
manufactured in the air according to an exemplary embodiment of
the invention;
FIG. 1 (b) is a picture showing the surface of an alloy
cast material according to comparative example 2, which is
manufactured in the air according to an exemplary embodiment of
the invention;
FIG. 2 is a view illustrating a method of measuring the
ignition temperature of a magnesium alloy, which is
manufactured according to an exemplary embodiment of the
invention;
FIG. 3 is a view showing the results of electron probe
micro-analysis (EPMA) on an oxide layer on the surface of a
molten metal after a magnesium alloy according to example 5,
which was cast according to an exemplary embodiment of the
invention, was maintained at 670 C for 10 minutes;
FIG. 4 is a view schematically showing the structure of
double composite oxide layers formed on the surface of a solid
or liquid phase in an alloy in which Ca and Y are added in
14
CA 02781995 2012-05-25
combination, the double composite oxide layers serving to block
the penetration of external oxygen;
FIG. 5 (a) is an optical picture showing the microscopic
structure of an alloy of comparative example 3, which is cast
according to an exemplary embodiment of the invention;
FIG. 5 (b) is an optical picture showing the microscopic
structure of an alloy of example 2, which is cast according to
an exemplary embodiment of the invention;
FIG. 6 (a) is an optical picture showing the microscopic
structure of an alloy of comparative example 1, which is
extruded according to an exemplary embodiment of the invention;
FIG. 6 (b) is an optical picture showing the microscopic
structure of an alloy of comparative example 2, which is
extruded according to an exemplary embodiment of the invention;
FIG. 6 (c) is an optical picture showing the microscopic
structure of an alloy of comparative example 3, which is
extruded according to an exemplary embodiment of the invention;
FIG. 6 (d) is an optical picture showing the microscopic
structure of an alloy of example 1, which is extruded according
to an exemplary embodiment of the invention;
FIG. 7 is a picture showing variation in the ignition
temperature depending on the total amount of Ca and Y that is
added in the comparative examples and examples, which are
manufactured according to an exemplary embodiment of the
invention; and
CA 02781995 2012-05-25
FIG. 8 is a picture showing variations in the value of
tensile strength x uniform elongation depending on the total
amount of Ca and Y that is added in the comparative examples
and examples, which are manufactured according to an exemplary
embodiment of the invention.
Best Mode
Reference will now be made in detail to exemplary
embodiments of a Mg alloy and a method of manufacturing the
same according to the present invention. However, it is to be
understood that the following embodiments are illustrative but
do not limited the invention.
As the results of studies that were carried out on a
thermodynamically calculated alloy design in order to solve the
foregoing problem of the related art and realize the object of
the invention, the inventors of the invention found that, when
Ca and Y are added in combination to a Mg-Al-based alloy or a
Mg-Al-Zn-based alloy, as presented in Table 1 below, the
fraction of a hard eutectic phase (eutectic phase I)
significantly decreases compared to the case in which Ca is
added alone, and at the same time, the formation of a A12Y
phase, i.e. particles that form micronized grains, is induced,
so that not only ignition resistance but also tensile strength
can be improved.
16
CA 02781995 2012-05-25
Table 1
A12Y Eutectic phase I Eutectic phase
(A12Ca, Mg2Ca) II (Mg1-7CAL12)
Mg-3A1-1Zn-1Ca - 2.241
Mg-3A1-1Zn-2Ca - 3.733
Mg-3A1-1Zn-1Ca-0.6Y 0.845 2.169
Mg-6A1-1Zn-1Ca - 2.303 4.183
Mg-6Al-lZn-2Ca - 4.600 1.776
Mg-6Al-1Zn-1Ca-0.6Y 0.890 2.272 3.505
The inventors of the invention manufactured Mg alloys
having a variety of compositions based on the above data. The
method of manufacturing a Mg alloy according to an exemplary
embodiment of the invention is as follows.
First, raw materials that include Mg (99.9%), Al (99.9%),
Zn (99.990), Ca (99.90), Y (99.9%) and selectively Mn (99.90)
were prepared, and were then melted. Then, Mg alloy cast
materials having the alloy compositions described in example 1
to example 17 and comparative example 1 to comparative example
9 in Table 2 below were formed from the raw materials using a
gravity casting method. Specifically, the temperature of a
molten metal was increased up to a temperature between 850 C
and 900 C, so that these elements were completely melted, in
order to produce an alloy by directly inputting Ca and Y, which
have high melting points of 842 C and 1525 C, respectively,
17
CA 02781995 2012-05-25
into the molten metal. After that, the molten metal was
gradually cooled down to a casting temperature, and then the Mg
alloy cast materials were produced by casting the molten metal.
Alternatively, according to an exemplary embodiment of
the invention, it is possible to manufacture a Mg alloy by a
variety of methods in addition to the method in which casting
is performed after a molten metal is formed by simultaneously
melting raw materials including Mg (99.9%), Al (99.9%), Zn
(99.99%), Ca (99.9%) and Y (99.9%). In an example, it is
possible to first form a Mg alloy molten metal using the raw
materials of Mg, Al and Zn or alloys thereof, input the raw
materials of Ca and Y, or a Ca compound and a Y compound into
the Mg alloy molten metal, and then produce a Mg alloy cast
material by a suitable casting method. It is also possible to
produce a Mg alloy cast material by preparing a Mg, Al, Zn, Ca
and Y alloy (master alloy ingot) of which the contents of Ca
and Y are higher than final target values, forming a Mg alloy
molten metal using raw materials of Mg, Al and Zn or alloys
thereof, and then inputting the master alloy ingot into the Mg
alloy molten metal. This method is particularly advantageous
in that the master alloy ingot can be input at a temperature
that is lower than the temperature at which the raw materials
of Ca and Y are directly input into the Mg alloy molten metal,
since the melting point of the master alloy ingot is lower than
those of the raw materials of Ca and Y. In addition, the
18
CA 02781995 2012-05-25
formation of a Mg alloy according to the invention can be
realized by a variety of methods, and all methods of forming a
Mg alloy that are well-known in the art to which the invention
belongs are included as part of the invention.
In this embodiment, a graphite crucible was used for
induction melting, and a mixture gas of SF6 and CO2 was applied
on the upper portion of the molten metal, so that the molten
metal did not come into contact with the air, in order to
prevent the molten metal from being oxidized before the
alloying process was finished. In addition, after the melting
was completed, mold casting was performed using a steel mold
without a protective gas. A sheet-shaped cast material having
a width of 100mm, a length of 150mm and a thickness of 15mm was
manufactured for a rolling test, a cylindrical billet having a
diameter of 80mm and a length of 150mm was manufactured for an
extrusion test, and a cylindrical billet having a diameter of
55mm and a length of 100mm was manufactured for an ignition
test of the alloy cast material. Although the Mg alloy was
cast by a mold casting method in this embodiment, a variety of
casting methods, such as sand casting, gravity casting, squeeze
casting, continuous casting, strip casting, die casting,
precision casting, spray casting, semi-solid casting, and the
like, may also be used. Although the Mg alloy according to the
invention is not necessarily limited to a specific casting
method, fusion casting is more preferable.
19
CA 02781995 2012-05-25
Afterwards, the slabs that were prepared above were
subjected to homogenization heat treatment at 400 C for 15
hours. In sequence, the materials of comparative example 1 to
comparative example 6 and example 1 to example 7 in Table 2,
which were subjected to homogenization heat treatment, were
machined into sheet materials having a final thickness of lmm
via hot working, in which the respective materials were rolled
under conditions of a roll temperature of 200 C, a roll
diameter of 210mm, a roll speed of 5.74mpm, and reduction
ratios of each roll of 30%/pass and 72%/pass. Here, when the
reduction ratio of each roll was 30%/pass, rolling was
performed a total of 7 times until the final thickness of lmm
was realized.
In addition, in comparative example 7, comparative
example 8 and example 8 in Table 2, rod-shaped extruded
materials having a final diameter of 16mm were manufactured by
extruding the materials that were subjected to homogenization
heat treatment under conditions including an extrusion speed of
5m/min, an extrusion ratio of 25:1, and an extrusion
temperature of 250. The extruded materials had a good surface
state.
Although rolling and extrusion were performed after
casting and homogenization heat treatment in this embodiment,
the materials may be manufactured by a variety of machining
methods, such as forging and drawing, without being necessarily
CA 02781995 2012-05-25
limited to a specific machining method.
Measurement of Ignition Temperature of Mg Alloy
In order to measure the ignition temperature of the Mg
alloys, chips having a predetermined size were produced by
machining the outer portion of the cylindrical billets, which
were manufactured above, in conditions including a depth of
0.5mm, a pitch of 0.1mm, and a constant speed of 350rpm. 0.1g
chips that were produced by the foregoing method were heated by
loading them at a constant speed into a heating furnace, which
was maintained at 1000 C. The temperatures at which a sudden
rise in temperature begins during this process were determined
as ignition temperatures, as shown in FIG. 3, and the results
are presented in Table 2.
As can be seen from comparative example 1 to comparative
example 6 in Table 2, the ignition temperature of Mg alloys
suddenly increases in response to the addition of Ca. When the
same amount of Ca was added, the ignition temperature of the
alloys tends to increase in response to an increase in the
content of Al therein.
Table 2
Alloy Alloy Composition (wt%) Ignition Test Atm.
Symbol Al Zn Ca Y Mn Zr Temp ( C)
21
CA 02781995 2012-05-25
Comp. Ex. 1 AZ31 3 1 490 Air
554 Air + Ar
Comp. Ex. 2 AZX311 3 1 1 708 Air
Comp. Ex. 3 AZX312 3 1 2 747 Air
Comp. Ex. 4 AZ61 6 1 507 Air
602 Air + Ar
Comp. Ex. 5 AZX611 6 1 1 703 Air
Comp. Ex. 6 AZX612 6 1 2 755 Air
Comp. Ex. 7 ZX61 6 1 672 Air
Comp. Ex. 8 ZX62 6 2 704 Air
Comp. Ex. 9 ZK60 5.5 0.45 553 Air
Example 1 Alloy 1 3 0.8 1 1 0.3 807 Air
Example 2 Alloy 2 3 1 1 0.6 768 Air
776 Air + Ar
Example 3 Alloy 3 3 1 0.7 0.6 714 Air
707 Air + Ar
Example 4 Alloy 4 3 0.8 0.3 0.3 0.25 698 Air
705 Air + Ar
Example 5 Alloy 5 6 1 1 0.6 774 Air
Example 6 Alloy 6 6 1 0.7 0.6 745 Air
749 Air + Ar
Example 7 Alloy 7 6 1 0.3 0.3 705 Air
717 Air + Ar
Example 8 Alloy 8 6 1 0.1 0.1 677 Air
22
CA 02781995 2012-05-25
Example 9 Alloy 9 6 2 1 0.6 783 Air
Example 10 Alloy 10 4 4 0.7 0.6 658 Air
711 Air + Ar
Example 11 Alloy 11 4 4 0.1 0.1 771 Air
Example 12 Alloy 12 1 6 1 1 653 Air
676 Air + Ar
Example 13 Alloy 13 1 6 0.7 0.6 744 Air
Example 14 Alloy 14 1 6 1 0.3 689 Air
Example 15 Alloy 15 2 6 1 1 659 Air
Example 16 Alloy 16 1 6 0.7 0.6 0.2 755 Air
Example 17 Alloy 17 698 Air
In Table 2, comparing each ignition temperature of
example 2 and example 5 with the respective ignition
temperature of comparative example 2 and comparative example 5,
it can be appreciated that the ignition temperature is much
higher when Y was also added to the Mg alloys than when Ca was
added alone to the Mg alloys. This is because a mixed layer of
CaO and Y203 was formed in the portion that was in contact with
molten metal due to the addition of Y, as can be seen from the
result of electron probe micro-analysis (EPMA) of FIG. 4, and
that this layer was able to effectively reduce the oxygen in
the air from penetrating into and reacting with the molten
metal. In addition, a mixed layer of CaO and MgO was present
23
CA 02781995 2012-05-25
in the outer portion of the mixed layer of CaO and Y203. These
double mixed layers help the molten metal remain stable even at
high temperatures.
In addition, comparing comparative example 3 with example
2 and comparative example 6 with example 5, it can be
appreciated that the ignition temperature was higher when Ca
and Y were added in combination than when Ca was added alone,
even though the total content of Ca and Y was less than the
content of Ca. This shows that a more excellent effect can be
realized in terms of increasing ignition resistance when Ca and
Y are added in combination than when Ca is used alone in order
to increase the ignition temperature of the Mg alloy.
In addition, Table 2 presents that the Mg alloy according
to example 1 has a very high ignition temperature of 807 C.
This is because Y has a very high content of lwt%. Thus, it
can be appreciated that ignition resistance can significantly
increase in response to an increase in the content of Y that is
added. Furthermore, in Table 2, the Mg alloy according to
example 8 has a very high ignition resistance of 811 C. This
shows that the ignition temperature of the Mg alloy, in which
6wt% of Zn is added, significantly increases when Ca and Y are
added respectively in an amount of lwt%.
Evaluation of Tensile Properties of Mg Alloy
The sheet materials, which were manufactured by the
24
CA 02781995 2012-05-25
above-described method, were heat-treated at 250 C for 30
minutes, and then sub-size sheet-shaped samples according to
the ASTM-E-8M standard, in which the length of a gauge was 25mm,
were produced. A tensile test was carried out at room
temperature under a strain of 1x10-3s-1 using a common tensile
tester, and the results are presented in Table 3.
In addition, samples of a rod-shaped extruded material,
in which the length of a gauge was 25mm, were manufactured, and
tensile test was carried out under the same conditions as for
the sheet-shaped samples.
As presented in Table 3, comparing comparative example 2
with comparative example 3, comparative example 5 with
comparative example 6, and comparative example 7 with
comparative example 8, it can be appreciated that the yield
strength and tensile strength increased but elongation
significantly decreased in response to the increase in the
content of Ca from lwt% to 2wt%. This decrease in the
elongation is because the fraction of a microscopic precipitate
phase of A12Ca as well as the fraction of a coarse and hard
ternary eutectic phase of Mg-Al-Ca increased, as shown in FIG.
(a), when the content of Ca that was added was increased to
2wt%. In contrast, as shown in FIG. 5 (b), when the content of
Ca that was added was lwt%, even though 0.6wt% of Y was
included, no coarse and hard ternary eutectic phase of Mg-Al-Ca
was observed and thus elongation was not decreased. Likewise,
CA 02781995 2012-05-25
comparing the microscopic structures of the extruded materials
of comparative example 1 to comparative example 3 and example 1
with reference to FIG. 6, when the content of Ca that was added
was increased to lwt% and 2wt%, large amounts of black second
phases, indicated by the arrows in FIG. 6 (b) and FIG. 6 (c) ,
respectively, were observed, and elongation decreased since the
hard second phases were vulnerable to defects.
Table 3
Alloy Tensile Properties
Symbol YS1) TS2 E131 UE1" TS X UE1 Remarks
(MPa) (MPa) (%) (%) (MPa=%)
Comp. Ex. 1 AZ31 176.4 274.5 25.2 17.4 4788 RAM5)
176.8 270.4 26.0 15.3 4142 EM6)
Comp. Ex. 2 AZX311 191.1 276.1 24.3 16.9 4658 RAM
239.4 294.5 17.1 11.8 3479 EM
Comp. Ex. 3 AZX312 255.2 303.6 16.5 9.7 2943 RAM
346.0 355.4 9.0 5.8 2045 EM
Comp. Ex. 4 AZ61 218.7 324.0 22.0 17.2 5565 RAM
166.5 298.1 26.4 21.1 6292 EM
Comp. Ex. 5 AZX611 204.4 306.2 19.7 16.0 4909 RAM
150.8 276.9 21.2 19.3 5337 EM
Comp. Ex. 6 AZX612 230.0 321.0 16.7 14.1 4536 RAM
169.9 275.6 19.2 16.4 4533 EM
26
CA 02781995 2012-05-25
Comp. Ex. 7 ZX61-F 191.4 268.1 25.4 17.2 4606 EM
Comp. Ex. 8 ZX62-F 294.9 298.5 13.7 9.4 2791 EM
Comp. Ex. 9 ZK60-F 238.4 318.4 24.1 13.5 4298 EM
Example 2 Alloy 2 175.8 265.2 24.7 18.4 4880 RAM
Example 3 Alloy 3 171.1 264.3 26.5 18.4 4856 RAM
Example 4 Alloy 4 175.1 267.4 27.8 16.8 4483 EM
Example 5 Alloy 5 225.7 323.4 19.6 15.5 5020 RAM
Example 8 Alloy 8 156.3 297.6 26.8 22.6 6738 EM
Example 9 Alloy 9 242.1 337.0 16.8 15.3 5157 RAM
Example 10 Alloy 10 215.1 340.2 21.7 19.5 6633 EM
Example 11 Alloy 11 152.0 302.1 33.5 29.1 8780 EM
Example 12 Alloy 12 189.8 323.7 27.3 22.7 7338 EM
Example 13 Alloy 13 161.5 276.1 26.8 22.4 6196 RAM
Example 14 Alloy 14 165.9 288.3 30.3 25.9 7467 EM
Example 15 Alloy 15 167.5 280.8 31.3 23.9 6711 EM
Example 16 Alloy 16 175.3 285.7 26.5 22.3 6363 EM
Notes)
YS1): Yield Strength, TS2): Tensile Strength, E13): Elongation,
UE1C: Uniform Elongation, RAMS: Rolled and Annealed Material,
EM6J: Extruded Material
On the other hand, as shown in FIG. 6 (d), no hard second
phase that decreases elongation was observed in the extruded
material of an alloy in which each of Ca and Y was added in an
27
CA 02781995 2012-05-25
amount of lwt%. This result is more apparent when comparing
example 2 with comparative example 3, example 5 with
comparative example 6, and example 13 with comparative example
8. Specifically, it can be appreciated that, even though only
lwt% of Ca and 0.6wt% of Y were added in example 2 and example
5, their elongation was very high and their ignition resistance
and tensile strength were at levels similar to those of
comparative example 3 and comparative example 6, in which 2wt%
of Ca was added. Likewise, in example 13, it can be
appreciated that the ignition resistance was greatly increased
and that the tensile properties, particularly the value of
tensile strength x uniform elongation, were greatly increased
when lwt% of Ca and lwt% of Y were added to the Mg-6Zn-lAl
alloy. That is, due to the addition of a small amount of Y, it
was possible to produce a Mg alloy of this embodiment in which
the content of Ca was maintained low, on the order of lwt%, but
in which the fraction of the coarse and hard ternary eutectic
phase was greatly decreased, such that both strength and
elongation were improved.
In addition, comparing example 2 with comparative example
2 and example 5 with comparative example 5, it can be
appreciated that example 2 and example 5 contain the same
content of Ca that was added in relation to the addition of Y,
and have ignition resistance that is superior to that of the
case in which Y was not added. At the same time, the value of
28
CA 02781995 2012-05-25
tensile strength x uniform elongation is further increased.
This tendency can be appreciated from FIG. 7 and FIG. 8,
which show variations in the ignition temperature and tensile
properties thereof in response to the total amount of Ca and Y
that was added. As shown in FIG. 7, the ignition temperature
tends to gradually increase in response to an increase in the
total amount of Ca and Y that was added. In particular, it can
be appreciated that the slope of the increase in the ignition
temperature is further increased when Y is added than when Y is
not added. In contrast, as shown in FIG. 8, when Ca is added
alone, the value of tensile strength x uniform elongation tends
to greatly decrease in response to an increase in the content
of Ca that is added, irrespective of the type of hot working.
However, when both Ca and Y are added, the mechanical strength
thereof is improved more than that of an alloy in which neither
Ca nor Y is added. From these results, it can be appreciated
that the ignition resistance is greatly increased, and at the
same time that tensile properties are greatly improved due to
the addition of a small content of Ca and Y at the same time.
The Mg alloy and the method of manufacturing the same
according to exemplary embodiments of the present invention
have been described above in detail with reference to the
accompanying drawings. However, it will be apparent to a
person having ordinary skilled in the art to which the present
invention belongs that the foregoing embodiments are merely
29
CA 02781995 2012-05-25
examples of the invention and various modifications and
variations are possible. Therefore, it should be understood
that the scope of the invention shall be defined only by the
appended claims.