Note: Descriptions are shown in the official language in which they were submitted.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-1-
PROCESS AND SYSTEM TO CONVERT OLEFINS
TO DIESEL AND OTHER DISTILLATES
FIELD OF THE INVENTION
[00011 The present invention relates to processes and systems that provide
for the conversion of olefins to diesel and/or other distillate products.
BACKGROUND OF THE INVENTION
[00021 It is believed by some that, in the future, the increase in demand for
diesel and other distillate products will outpace the increase in demand for
gasoline. Accordingly, there is a need for additional techniques for obtaining
diesel and other distillate fuels.
[00031 Light olefins are produced in typical hydrocarbon refining operations
that also produce mogas and distillate products. There is a desire to obtain
higher amounts of mogas and diesel end products per unit volume of crude oil
extracted upstream. To supplement obtaining diesel from newly-extracted crude
oil, and to meet the rising demand for diesel and other distillates, it is
desirable
to make use of light olefins to yield additional diesel and other distillate
products.
[00041 It is also possible to obtain olefins from natural gas and coal sources
via
conversion of methanol and other oxygenates via the use of zeolite catalysts.
While
processes exist to convert olefins to gasoline, it would also be advantageous
to provide
more economically efficient methods of converting olefins to diesel and other
distillate
products. There is a thus desire to provide an economically feasible process
to move
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-2-
conversions based on light olefins out of the mogas pool and into the diesel
pool by
oligomerization and aromatic alkylation reactions.
SUMMARY OF THE INVENTION
[00051 One aspect of the present invention provides a process for producing
a hydrocarbon fuel composition that includes introducing an olefin feed
composition including light olefins (e.g., C2 to C6 olefins) to an
oligomerization
catalyst to yield an intermediate composition including olefins having at
least
four carbon atoms, introducing the intermediate composition and a second feed
of aromatic compounds (e.g., a feed containing from 2 to 99.9% alkylatable
aromatics) to an aromatic alkylation catalyst to yield a hydrocarbon fuel
composition.
[00061 Another aspect of the present invention provides a system for
producing a hydrocarbon fuel composition that includes an olefin feed
including
light olefins (e.g., C2 to C6 olefins), a first reaction vessel containing an
oligomerization catalyst in fluid communication with the first feed to yield
an
intermediate composition including olefins having at least four carbon atoms,
a
second reaction vessel containing an aromatic alkylation catalyst in fluid
communication with a second feed of aromatic compounds and the intermediate
composition to yield a hydrocarbon fuel composition, and a collection assembly
in fluid communication with the second reaction vessel to recover the
hydrocarbon fuel composition from the stream exiting the reaction vessel
containing the aromatic alkylation catalyst.
BRIEF DESCRIPTION OF THE DRAWINGS
[00071 The invention will now be described in conjunction with the
accompanying drawings in which:
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-3-
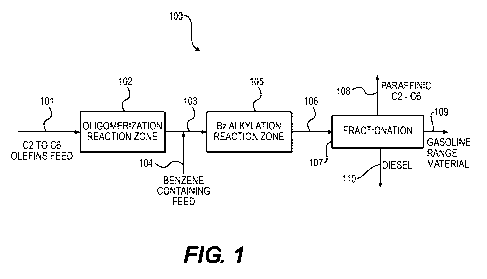
[00081 Figure 1 is a conceptual process flow diagram demonstrating
conversion of a C2-C6 olefin feed to a diesel and gasoline fuel composition,
and a
C2-C6 paraffinic composition.
[00091 Figure 2 is a conceptual process flow diagram depicting reformate
alkylation within a diesel reactor system.
[00101 Figure 3 is a conceptual process flow diagram for a FCC naptha and
scanfinate alkylation process in accordance with a single feed embodiment of
the
present invention.
[00111 Figure 4 is a plot demonstrating the conversion of benzene, 1-hexene
and toluene as described in Example 1.
[00121 Figure 5 is a plot based on the GC analysis of the feed and product,
as described in Example 1.
[00131 Figure 6 depicts an ASTM D86 test method analysis of the aromatic
feed and alkylated product after reaction with hexene, as described in
Example 1.
[00141 Figure 7 depicts an ASTM D86 test method analysis of an alkylated
product after reaction with propylene and an alkylated aromatic product after
reaction with hexane.
[00151 Figure 8 is a second GC analysis of the feed and product of
Example 1.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-4-
DETAILED DESCRIPTION OF THE INVENTION
Definitions
[00161 As used herein, the term "produced in an industrial scale" refers to a
production scheme in which gasoline and/or distillate end products are
produced
on a continuous basis (with the exception of necessary outages for plant
maintenance) over an extended period of time (e.g., over at least a week, or a
month, or a year) with the expectation of generating revenues from the sale or
distribution of the gas and/or distillate. Production at an industrial scale
is
distinguished from laboratory or pilot plant settings which are typically
maintained only for the limited period of the experiment or investigation, and
are
conducted for research purposes and not with the expectation of generating
revenue from the sale or distribution of the gasoline or distillate produced
thereby.
[00171 As used herein, and unless specified otherwise, "gasoline" or
"gasoline boiling range components" refers to a composition containing at
least
predominantly C5-C12 hydrocarbons. In one embodiment, gasoline or gasoline
boiling range components is further defined to refer to a composition
containing
at least predominantly C5-C12 hydrocarbons and further having a boiling range
of
from about 100 F to about 360 F. In an alternative embodiment, gasoline or
gasoline boiling range components is defined to refer to a composition
containing at least predominantly C5-C12 hydrocarbons, having a boiling range
of
from about 100 F to about 360 F, and further defined to meet AS TM standard
D439.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-5-
[00181 As used herein, and unless specified otherwise, the term "distillate"
or "distillate boiling range components" refers to a composition containing
predominately C10-C40 hydrocarbons. In one embodiment, distillate or
distillate
boiling range components is further defined to refer to a composition
containing
at least predominately C10-C40 hydrocarbons and further having a boiling range
of from about 300 F to about 1100 F. Examples of distillates or distillate
boiling range components include, but are not limited to, naphtha, jet fuel,
diesel, kerosene, aviation gas, fuel oil, and blends thereof.
[00191 As used herein, and unless specified otherwise, the term "diesel"
refers to middle distillate fuels containing at least predominantly C12-C25
hydrocarbons. In one embodiment, diesel is further defined to refer to a
composition containing at least predominantly C12-C25 hydrocarbons, and
further
having a boiling range of from about 330 F to about 700 F. In an alternative
embodiment, diesel is as defined above to refer to a composition containing at
least predominantly C12-C25 hydrocarbons, having a boiling range of from about
330 F to about 700 F, and further defined to meet ASTM standard D975.
[00201 For those embodiments of the presently disclosed subject matter in
which the hydrocarbon fuel composition includes diesel, the cetane value for
the
recovered diesel can vary. In one embodiment the recovered diesel has a cetane
number of at least 35, or alternatively has a cetane value of at least 40, or
still
alternatively has a cetane value of at least 45.
[00211 As used herein, a feed is rich in a certain component if it contains at
least 50 wt% of that component. In certain embodiments, a feed is rich in a
certain component contains at least 75 wt%, or at least 90 wt%, at least 95
wt%
or at least 99 wt% of that component.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-6-
[00221 As used herein, a SPA-type catalyst refers to a catalyst which
contains as one of its principal raw ingredients an acid of phosphorus such as
ortho-, pyro- or tetraphosphoric acid.
[00231 As used herein, a MWW-type catalyst is a catalyst having the MWW
framework topology, as classified by the Structure Commission of the
International Zeolite Association according to the rules of the IUPAC
Commission on Zeolite Nomenclature, and includes, for example, zeolites PSH-
3, MCM-22, MCM-49, MCM-56, SSZ 25, ERB-1 and ITQ-1 catalysts.
[00241 As used herein, the term "alkylatable aromatics" refers to aromatic
compounds that can be alkylated under suitable alkylation conditions. While
benzene is the prototypical alkylatable aromatic, it is understood that
alkylatable
aromatics can also include - in addition to benzene - toluene, xylenes and
lower
alkyl benzenes (e.g., ethylbenzene). It should also be understood that
reference
to benzene in this application in the context of alkylation reactions also
encompasses other alkylatable aromatics in addition to benzene, such as those
compounds described above.
[00251 Reference will now be made to various aspects and embodiments of
the presently disclosed subject matter in view of the definitions above.
[00261 One aspect of the present invention provides a process for producing
a hydrocarbon fuel composition (e.g., diesel or other distillate) that
includes
introducing an olefin feed composition including light olefins (e.g., a
composition containing C2 to C6 olefins) to an oligomerization catalyst (e.g.,
a
MCM-22, ZSM-22 or ZSM-57 catalyst) to yield an intermediate composition
including olefins having at least four carbon atoms (e.g., a composition that
includes at least 1 wt%, or at least 5 wt%, or at least 10 wt%, or at least 25
wt%,
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-7-
or at least 50 wt% C5-C16 olefin oligomers), introducing the intermediate
composition and a second feed of aromatic compounds (e.g., a feed including
from 2 to 99.9% of alkylatable aromatics) to an aromatic alkylation catalyst
(e.g., a MCM-22 type catalyst) to yield a fractionation feed to provide a
composition which can be further refined to provide one or more hydrocarbon
fuel compositions (e.g., C2-C6 paraffins, gasoline and a distillate (e.g.,
diesel)).
In one embodiment the hydrocarbon fuel composition is produced in an
industrial scale.
[00271 The olefin feed composition can be obtained utilizing existing process
streams within a hydrocarbon refining plant, from chemical grade olefin
sources,
or a mixture thereof. In one embodiment, the olefin feed composition is
obtained from fuel gas, chemical grade propylene, refinery grade propylene,
polymer grade propylene, liquefied petroleum gas (LPG), light cracked naptha
(LCN) process streams, scafinate (hydroprocessed LCN) process streams, de-
hydrogenated LVN process streams (light virgin naptha), and butylene or
butylene-containing process streams (e.g., an alkylation feed). In another
embodiment, the olefin feed composition is obtained from a FCC coking
operation, such as a FCC off-gas or coker off-gas stream, or from a steam
cracking operation.
[00281 The olefin oligomer content in the intermediate stream can vary
depending on the olefin content in the olefin feed stream, which in turn may
vary
depending on the source of the olefin feed stream. While the intermediate
stream in some embodiments of the presently disclosed subject matter contains
at least 50wt% olefins oligomers (e.g., at least 50 wt% C5-C16 olefin
oligomers),
other embodiments that employ a more dilute olefin feed stream will provide an
intermediate with a lower concentration of olefins oligomers (e.g., at least 5
wt%, or at leastl0 wt%, or at least 25 wt% C5-C16 olefin oligomers).
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-8-
[00291 Similarly, the feed of aromatic compounds can be obtained from
existing process streams within a hydrocarbon refining plant. In one
embodiment, the aromatic compounds are obtained from light reformate, a
benzene heart-cut reformate, heavy reformate, full reformate or catalytic
cracked
naptha (cat naphtha), virgin naptha, or hydrocracked naptha process streams.
[00301 The oligomerization catalyst can be a solid phosphoric acid (SPA)
catalyst, a MWW type catalysts or a ZSM-type catalyst. The oligomerization
catalyst can be selected from, for example, a MCM-22 catalyst, a ZSM-22
catalyst or a ZSM-57 catalyst, or a combination thereof. In one embodiment the
aromatic catalyst is a MCM-22 catalyst. Other solid acid catalysts can be
employed and optimized to provide desired product properties.
[00311 The oligomerization catalyst can be contained in a reaction vessel. In
one embodiment, the reaction vessel containing the oligomerization catalyst is
a
fixed bed reaction vessel. The fixed bed reaction vessel can be of a chamber
design or a tubular design. In one embodiment, the reaction vessel containing
the oligomerization catalyst is maintained at a pressure of from about 200
psig to
about 1500 psig and/or at a temperature of from about 100 F to about 600 F.
[00321 The aromatic alkylation catalyst can also be contained in a reaction
vessel. In one embodiment, the vessel containing the aromatic alkylation
catalyst is a fixed bed reaction vessel. The fixed bed reaction vessel can be
of a
chamber design or a tubular design. In one embodiment, the vessel containing
the aromatic alkylation catalyst is maintained at a pressure from about 50 or
100
psig to about 1000 or 1500 psig and at a temperature of from about 80 or 100 F
to about 600 F.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-9-
[00331 Another aspect of the present invention provides a system for
producing a hydrocarbon fuel composition that includes an olefin feed
including
C2 to C6 olefins, a first reaction vessel containing an oligomerization
catalyst in
fluid communication with the olefin feed to yield an intermediate composition
including olefins having at least four carbon atoms, a second reaction vessel
containing an aromatic alkylation catalyst in fluid communication with a
second
feed of aromatic compounds and the intermediate composition to yield a
hydrocarbon fuel composition, and a collection assembly in fluid communication
with the second reaction vessel to recover the hydrocarbon fuel composition
from the stream exiting the reaction vessel containing the aromatic alkylation
catalyst.
[00341 Exemplary further embodiments of the present invention are provided
below for illustrative purposes, and not for purposes of limitation. Reference
to
the system will be made in conjunction with and understood from the method
disclosed herein.
[00351 An exemplary process flow diagram (100) is shown in Figure 1. An
olefin feed composition (101) containing C2 to C6 olefins is introduced to an
oligomerization reaction zone (102), which can include an oligomerization
catalyst housed in a reaction vessel (e.g., a fixed bed reactor containing an
oligomerization catalyst). Besides olefins, the olefin feed composition can
also
contain paraffins, hydrogen, and/or other inert compounds.
[00361 Referring still to Figure 1, an intermediate composition (103),
containing C9-C16 olefins is combined with a benzene containing feed (104),
and
the combined stream is introduced to an benzyl (or aromatic) reaction zone
(105), which can include an aromatic alkylation catalyst housed in a reaction
vessel (e.g., a fixed bed reaction vessel). The product (106) of the benzyl
(or
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-10-
aromatic) reaction zone is then introduced to a fractionation operation (107),
in
which a C2-C6 paraffinic composition (108), a gasoline boiling range material
(109) and a diesel boiling range material (110) is provided as end products.
The
fractionation operation can include fractionation columns or stills, which can
be
operated under reaction conditions known to those of ordinary skill in the
art.
[00371 Figure 2 provides another exemplary embodiment of the present
invention, in which a conceptual process configuration (200) is shown which
produces diesel end-product with a cetane rating of 45-55+. A feed (201) is
provided which can be rich in C3 olefins, or rich in C4 olefins, or
alternatively
can contain a mixture of C3 and C4 olefins. The feed is introduced to a fixed
bed
reaction vessel (202) containing an oligomerization catalyst. In this
embodiment, the vessel (202) is maintained a temperature of about 150-200 C
and a pressure of about 500 to about 1200 psig. The LHSV is from about 0.1 to
10hr-i, preferably about 1 hr-1, based on the total amount of olefin feed.
[00381 Referring still to Figure 2, the oligomerized olefin stream exiting the
reaction vessel (202) is combined with a reformate stream or feed (203) of
benzene, toluene, and xylenes, and the combined stream (204) is introduced to
fixed bed reaction vessel (205) containing a aromatic alkylation catalyst. In
this
embodiment, the vessel (205) is maintained at a temperature of about 200 C and
a pressure of from about 250 psig to about 500 psig. The LHSV is about 1 hr 1,
based on the amount of olefin feed. The product (206) leaving the reaction
vessel will contain alkylated aromatic compounds that can be recovered to
obtain a diesel fuel composition with a cetane rating of 45-55+. For example,
the end product can contain n-nonylbenzene and/or n-dodecylbenzene, which
have cetane ratings of 49-51 and 55-68 respectively. It is expected that
different
product isomers will be formed having a range of cetane numbers.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-11-
[00391 The heat generated by reaction vessels (202) and (205) can be
managed by interstage cooling or by recycle streams. Reaction vessels 202 and
205 can exist as two physical reactors, or alternatively they can be combined
into
a single vessel.
[00401 Olefin feeds containing rich in near linear olefins with a minimum of
five carbon atoms are, in certain embodiments, preferred in order to provide a
diesel fuel composition with higher cetane ratings. Benzene rings with an n-
alkyl substituent from 6 to 9 carbon atoms have a cetane rating between about
40
and 50.
O1i2omerization Reaction Zone
[00411 As noted above, an olefin feed composition is introduced to an
oligomerization catalyst to provide an intermediate composition that includes
oligomerized olefins. In certain embodiments of the present invention, the
oligomerization catalyst will be contained within a vessel (e.g., a reactor),
which
is referred to herein as the first reaction vessel. A person of ordinary skill
in the
art can determine the proper reaction conditions, and thus the proper
conditions
for the first reaction vessel, in order to convert a feed containing, for
example,
C2-C6 olefins to yield an intermediate composition containing at least four
carbon atoms (e.g., a composition containing C4-C16 olefins).
[00421 In certain embodiments the vessel containing the oligomerization
catalyst (i.e. the first reaction vessel) is maintained at a temperature
ranging from
about 100 F to about 600 F more preferably from about 200 to 400 F. In certain
embodiments, the vessel containing the oligomerization catalyst is maintained
at
a pressure ranging from about 200 psig to about 1500 psig, more preferably
from
about 400 to about 1100 psig.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-12-
[00431 In certain embodiments, the conversion of the olefin feed
composition after being contacted with the oligomerization catalyst ranges
from
about 50 to 100%, or from about 70 to 99%, or from about 80 to 95%. The
process can be operated at a lower conversion if necessary, for example if the
refinery were economically balancing the production of LPG. A person of
ordinary skill in the art can adjust the flow rate and operating temperature
of the
olefin feed composition in order to operate at the desired oligomerization
conversion. In one embodiment, the olefin feed can be operated over a range of
0.1 to 10 LHSV and over a temperature range of 200-400 F.
[00441 Suitable oligomerization reaction conditions are also disclosed in
U.S. Published Patent Application No. 2007/0173676, which is hereby
incorporated by reference in its entirety.
Olefin Feed Composition
[00451 The ultimate product distribution can change based on the olefin feed
composition entering the oligomerization reaction zone. If the olefin feed
composition is rich in C3 olefins, the first reactor will yield an
intermediate
composition rich in C6-C12+ olefins. Alternatively, if the olefin feed
composition is rich in C4, olefins, the product produced in the largest
quantity
will be Cg-C16+ olefins. If the feed contains a mixture of C3 and C4 olefins,
the
product produced in the largest quantity will be C6-C16 olefins. Generally
higher
oligomers are preferred such that they produce molecules within the distillate
boiling range as these higher oligomers tend to produce alkylaromatics with
higher cetane values. It is preferred to select an oligomerization catalyst
that
provided near linear oligomers as increasing linearity of the oligomer
corresponds to increasing cetane of the resulting alkylaromatic.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-13-
[00461 Accordingly, one embodiment includes selecting a feed rich in C3
olefins for use in the process of the present invention, as described anywhere
in
this application, in order to obtain a hydrocarbon fuel composition rich in C6-
C12+ olefins. An alternative embodiment includes selecting a feed rich in C4
olefins for use in the process of the present invention, as described anywhere
in
this application, in order to obtain a hydrocarbon fuel composition rich in Cg-
C16+ olefins.
Aromatic Alkylation Reaction Zone
[00471 The intermediate composition obtained from the oligomerization
reaction zone, and a second feed of aromatic compounds is introduced to an
aromatic alkylation catalyst to provide a hydrocarbon fuel composition. The
intermediate composition can be combined with the second feed of aromatic
compounds upstream from the aromatic alkylation catalyst such that one feed
containing both the intermediate composition and aromatic compounds is
introduced to the aromatic alkylation catalyst. Alternatively, the
intermediate
composition and the second feed of aromatic compounds can be introduced
separately to the aromatic alkylation catalyst. In certain embodiments of the
present invention, the aromatic alkylation catalyst will be contained within a
vessel (e.g., a reactor), which is referred to herein as the second reaction
vessel.
[00481 A person of ordinary skill in the art can determine the proper reaction
conditions, and thus the proper conditions for the second reaction vessel, in
order
to convert a feed that includes, for example, an intermediate composition
(e.g., a
feed containing C9-C16 olefins) and a second feed of aromatic compounds (e.g.,
a
feed containing 2-99.9% benzene and other alkylatable aromatics) to yield a
composition which includes a hydrocarbon fuel composition. The hydrocarbon
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-14-
fuel composition can be recovered (i.e., further isolated) using refining and
separation techniques known to those of ordinary skill in the art.
[00491 The amount of alkylatable aromatics in the second feed of aromatic
compounds can vary. For example, the second feed of aromatic compounds can
include at least 1 wt%, or at least 5 wt%, or at least 10 wt% of alkylatable
aromatics, based on the total weight of the second feed of aromatic compounds.
[00501 In certain embodiments the vessel containing the aromatic alkylation
catalyst (i.e., the second reaction vessel) is maintained at a temperature
ranging
from about 80 F to about 600 F, or from about 100 F to about 400 F. In certain
embodiments, the vessel containing the aromatic alkylation catalyst is
maintained at a pressure ranging from about 50 psig to about 1500 psig, or
from
about 100 psig to about 1000 psig.
[00511 The conversion of aromatic compounds can vary. In one embodiment,
the conversion of aromatic compounds ranges from about 50% to about 100%.
Higher aromatic conversions are preferred to maximize the amount of distillate
produced.
[00521 The feed amount of aromatic compounds and intermediate
composition to the aromatic alkylation reaction zone can also vary. It is
desirable to operate with a molar ratio of Olefin:Aromatic of 0.5 to 3, more
preferably about 1.
[00531 In certain embodiments, the oligomerization catalyst and aromatic
alkylation catalyst are housed in separate vessels. Alternatively, the
oligomerization catalyst and aromatic alkylation catalyst can be housed in the
same vessel. In embodiments in which the oligomerization catalyst and aromatic
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
- 15-
alkylation catalyst are housed in the same vessel, it is understood that
reaction
conditions for the respective vessels refer to reaction conditions for that
portion
of the vessel that contains the oligomerization catalyst, or aromatic
alkylation
catalyst, as appropriate.
Sin21e Feed Option
[00541 In certain embodiments of the present invention, the pre-
oligomerization step is eliminated and a composition containing olefins having
at least three carbon atoms is combined with an aromatic feed, and the
combined
stream is introduced to an aromatic alkylation catalyst (e.g., a MCM-22 type
catalyst) to yield a hydrocarbon fuel composition. For example, existing
streams
within a hydrocarbon refinery that contain both olefins and aromatics (an FCC
Naptha stream and/or a scanfinate stream) can be introduced to an aromatic
alkylation catalyst to yield diesel fuel.
[00551 An exemplary single feed embodiment is shown in Figure 3. A FCC
Naptha stream (401) is combined with a scanfinate stream (402), and the
combined stream (403) is introduced to a fixed bed reactor (404) containing
MCM-22 catalyst. Prior to being introduced to the catalyst, nitrogen and
sulphur
containing compounds are removed from the FCC Naptha stream, since these
components cause detrimental effects on the catalyst. In this example, the FCC
Naphtha stream (401) contains about 20-30% linear olefins (as a percentage of
total olefin content), with the balance being primarily mono-branched olefins.
The resulting product stream (405) contains a diesel fuel composition.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-16-
Oligomerization Catalysts
[00561 As disclosed in U.S. Patent No. 7,361,798, which is hereby
incorporated by reference, zeolites are classified by the Structure Commission
of
the International Zeolite Association according to the rules of the IUPAC
Commission on Zeolite Nomenclature. A framework-type describes the
topology and connectivity of the tetrahedrally coordinated atoms constituting
the
framework and makes an abstraction of the specific properties for those
materials. Molecular sieves for which a structure has been established are
assigned a three letter code and are described in the Atlas of Zeolite
Framework
Types, 5th edition, Elsevier, London, England (2001), which is hereby
incorporated by reference in its entirety.
[00571 Unless specified otherwise, the oligomerization catalysts of the
present invention is without limitation so long as it facilitates the
oligomerization of an olefin feed composition. In one embodiment, the
oligomerization catalyst is selected from a solid phosphoric acid catalyst
(SPA),
a MWW type catalyst and a ZSM-type catalyst.
[00581 Solid phosphoric acid (SPA) catalysts are known in the art and are
commercially available, for example, from UOP LLC (Des Plaines, IL). Further
details regarding the composition and production of SPA catalysts can be
obtained from U.S. Patent Nos. 3,050,472; 3,050,473; and 3,132,109, which are
each hereby incorporated by reference in their entirety.
[00591 As disclosed in U.S. Published Application No. 2007/0173676, which
is hereby incorporated by reference in its entirety, the SPA catalyst can be
provided with a carrier, such as a naturally occurring porous silica-
containing
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
- 17-
materials (e.g., kieselguhr, kaolin, infusorial earth and diatomaceous earth).
As
disclosed therein, the SPA catalyst can also be employed in conjunction with
crystalline molecular sieve catalysts, such as, for example, ZSM-22, ZSM-23,
SAPO-11, ZSM-48 or other molecular sieve catalysts described herein or
otherwise known in the art.
[0060] MWW type catalysts are also known in the art and can be
commercially obtained from, for example, ExxonMobil Catalyst Technologies
LLC (Baytown, TX). As disclosed in U.S. Published Application No.
2006/0194999, which is hereby incorporated by reference, the MWW family of
zeolite materials has achieved recognition as having a characteristic
framework
structure which presents unique and interesting catalytic properties. The MWW
topology consists of two independent pore systems: a sinusoidal ten-member
ring [10 MR] two dimensional channel separated from each other by a second,
two dimensional pore system comprised of 12 MR super cages connected to
each other through 10 MR windows. The crystal system of the MWW
framework is hexagonal and the molecules diffuse along the directions in the
zeolite, i.e., there is no communication along the c direction between the
pores.
In the hexagonal plate-like crystals of the MWW type zeolites, the crystals
are
formed of relatively small number of units along the c direction as a result
of
which, much of the catalytic activity is due to active sites located on the
external
surface of the crystals in the form of the cup-shaped cavities. MWW-type
catalysts that can be used in connection with the presently disclosed subject
matter include, but are not limited to, PSH-3, MCM-22, MCM-36, MCM-49,
MCM-56, SSZ-25, ERB-1, EMM-1, EMM-2, and ITQ-1 catalysts.
[0061] In one embodiment, the MWW type catalyst is selected from a MCM
catalyst (e.g., MCM-22, MCM-36, MCM-49, and MCM-56 catalyst). MCM
catalysts are known in the art, and can be obtained from, for example from
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-18-
ExxonMobil Catalyst Technologies LLC (Baytown, TX). MCM type catalysts,
including synthesis details, are described in, for example, U.S. Patent Nos.
7,198,711; 5,639,931; 5,296,428; 5,1460,29; and U.S. Published Application No.
2006/0194998. Each of these references are hereby incorporated by reference in
their entirety.
[00621 In one embodiment, the MWW type catalyst is a MCM-22 catalyst.
MCM-22 is described in U.S. Pat. No. 4,954,325 as well as in U.S. Pat. Nos.
5,250,777; 5,284,643 and 5,382,742. MCM-49 is described in U.S. Pat. No.
5,236,575; MCM-36 in U.S. Pat. No. 5,229,341 and MCM-56 in U.S. Pat. No.
5,362,697. Each of these patents are hereby incorporated by reference in their
entirety.
[00631 In another embodiment, the oligomerization catalyst is a EMM
catalyst (e.g., EMM-1 or EMM-2 catalyst). EMM catalysts are known in the art
and are preferably obtained from ExxonMobil Catalyst Technologies LLC
(Baytown, TX). Synthesis details regarding EMM catalysts can be found, for
example, in U.S. Patent Nos. 7,255,849 and 6,787,124 and U.S. Published
Application Nos. 2006/0079723, 2009/0163753, each of which are hereby
incorporated by reference in its entirety.
[00641 In one embodiment, the oligomerization catalyst is a ZSM-type
catalyst. ZSM (Zeolite Socony Mobil) catalysts are known in the art and can be
commercially obtained or synthesized. Commercially available ZSM-type
catalysts can be obtained from, for example, Zeolyst International Corporation
(Valley Forge, PA), BASF Catalysts LLC (Iselin, NJ), Sud-Chemie Incorporated
(Louisville, KY), and, preferably, from ExxonMobil Catalyst Technologies LLC
(Baytown, TX). ZSM catalysts, including synthesis details, are generally
described, for example, in U.S. Patent Nos. 5,367,100; 4,845,063; 4,872,968;
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-19-
4,076,842; 4,046,859; 4,035,430; 4,021,331; 4,016,245; 3,972,983; 3,965,205;
3,832,449; 3,709,979; 3,702,886; 3,303,069; and Re. 28,341. The contents of
each of these patents is hereby incorporated by reference in their entirety.
[00651 In one embodiment, the oligomerization catalyst is a ZSM-type
catalyst selected from ZSM-5, ZSM-11, ZSM-12, ZSM-22, ZSM-23, ZSM-35,
ZSM-48, ZSM-50, ZSM-57 catalysts. In one embodiment, the ZSM-type
catalyst is selected from ZSM-23 and ZSM-57, or a combination thereof. In one
embodiment the oligomerization catalyst is a combination of a ZSM-23 and
ZSM-57 catalyst, since this combination yields a high amount of linear
olefins.
[00661 In one embodiment, the oligomerization catalyst is an ITQ type
catalysts. ITQ type catalysts, including synthesis details, are described in,
for
example, U.S. Patent Nos. 7,449,169; 7,081,556; 6,709,572; and 6,469,226, as
well as published U.S. Application No. 2008/0021253. Each of these references
are hereby incorporated by reference in their entirety.
[00671 In one embodiment, the ITQ type catalyst is ITQ-13. ITQ-13
structure is 1Ox10x9-member rings. Pore sizes of the ITQ-13 are 4.8 x 5.3 A;
4.8 x 5.1 A; 4.0 x 4.8 A (9-member ring).
[00681 Other molecular sieves catalysts can be used as the oligomerization
catalyst. These catalysts include those described in R. Szostak, Handbook of
Molecular Sieves, Van Nostrand Reinhold, New York, N.Y. (1992), which is
hereby incorporated by reference in its entirety.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-20-
Aromatic Alkylation Catalysts
[00691 Unless specified otherwise, the aromatic alkylation catalysts of the
present invention is without limitation so long as it facilitates the aromatic
alkylation of an intermediate olefin composition. In one embodiment, the
aromatic alkylation catalyst is a MWW framework type catalyst, including the
MWW type catalyst described above. In one embodiment, the MWW type
catalyst is a MCM-22 catalyst. It is also contemplated that zeolites beta
catalyst
and USY catalysts may be used.
Examples
[00701 The present application is further described by means of the
examples, presented below. The use of such examples is illustrative only and
in
no way limits the scope and meaning of the invention or of any exemplified
term. Likewise, the invention is not limited to any particular preferred
embodiments described herein. Indeed, many modifications and variations of
the invention will be apparent to those skilled in the art upon reading this
specification. The invention is therefore to be limited only by the terms of
the
appended claims along with the full scope of equivalents to which the claims
are
entitled.
Example 1
[00711 A feed including 30.8 wt% 1-hexene, 17.0 wt% benzene, 3.4 wt%
toluene and the additional components identified below in Table 1 was
prepared.
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-21-
4000 n-Butane 0.0726
4001 Ico-Butane 0.0101
4098 Other C4 Paraffinc 0.0008
4101 C-2-Butene 0.0000
4102 T-2-Butene 0.0000
4104 1-Butene + Ico-Butene 0.0000
5000 n-Pentane 1.0945
5001 I co-Pentane 1.0712
5098 Other C5 Paraffinc 0.0088
5100 1- entene 0.0000
5101 cic-2-entene 0.0046
5102 T Pentene 0.0075
5103 2M But-e-1 0.0000
5104 methyl-1-butene 0.0024
5105 2M-Butene-2 0.0282
5200 Cyclo entane 0.3506
0000 n-Hexane 8.2458
0001 2M Pentane 7.5930
0002 3M Pentane 6.2668
0003 2 2 DM Butane 1.2242
0004 20 DM Butane 1.4955
0098 Other C0 Paraffinc 0.5906
0100 1-hexene 30.8069
0101 cic-2-hexene 0.0447
0102 trans-'-hexene 0.0873
01074-method entene-1 0.0093
0103 2-methylentene-2 0.1220
0181 1M Cclo entene 0.0126
0200 Methcclo entane 1.9176
0201 Cyclohexane 0.3778
1 C ~CC'7
7000 n-He tane 2.8404
70012M Hexane 3.9279
ruu
2 3M Hexane 4.6536
7004 2.2 DM Pentane 0.6836
7005 2.3 DM Pentane 1.4432
7000 2 4 DM Pentane 0.5797
70n8 2 2 3 TM Butane 0.0998
7098 Other C7 Paraffinc 0.7355
7100 1-he tene 0.0000
7101 eic-2-he tene 0.0240
7102 trans 2-he tene 0.0154
7108 eic-8-he tene 0.0612
7104 trans-8-he tene 0.0000
7'_00 Ethydcdo entane 0.1381
7208 1-T= DM C elo entane 0.0193
7204 1-C3 DM Cyclo entane 0.1804
-205 1-TC DM Cclo entane 0.1682
7200 MethI777 lohexane 0.1428
3.3618
(Table 1 continues to next page)
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-22-
3.3618
8000 n-Octane 0.1878
8002 3M-He Mane 0.2465
8005 2 4 DM Hexane 0.0062
8016 2.34 TM Pentane 0.0072
8098 Other C8 Parafhnc 1.4233
8100 I -oc to n e 0.0034
8101 cls-2-octene 0.0112
8102 trans-2-octene 0.0107
8300 EtIvIBenzene 0.1003
8301 O-XVIene 0.0347
8302 M-X VIene 0.1485
8303 P-XVIene 0.0820
8320 Styrene 0.0000
9000 n-Nonane 0.0000
9098 Other C9 Paraffin- 0.0513
9100 I-nonene 0.0000
9300 NC3 Benzene 0.0056
0.0016
9302 I rd12ET Benzene 0.0000
9303 1 N13ET Benzene 0.0000
9304 1 N14ET Benzene 0.0094
9305 123Trd Benzene 0.0000
9306 124TH Benzene 0.0140
9307 135TH Benzene 0.0045
9370 Indane 0.0000
9398 Other C9 Aromatics 0.0318
10000 n-Decane 0.0000
10098 Other C10+ Paraffins 0.0070
100 I -decene 0.0000
10300 N-butyl Benzene 0.0000
10301 I;o-butyl Benzene 0.0049
10302 Sec-butyl Benzene 0.0862
10304 IM2NP Benzene 0.0026
10305 1M3NP Benzene 0.0000
10306 IM4NP Benzene 0.0000
I03071M21P Benzene 0.0017
I03081M31P Benzene 0.0037
I03091M41P Benzene 0.0000
10310 12DET Benzene 0.0036
10311 13DET Benzene 0.0000
10312 14DET Benzene 0.0000
10313 12DM3ET Benzene 0.0000
10314 I2DM4ET Benzene 0.0033
10315 13DM2ET Benzene 0.0000
10316 13DM4ET Benzene 0.0023
10317 13DM5ET Benzene 0.0000
10318 14DM2ET Benzene 0.0000
10319 1234TH Benzene 0.0000
10320 1235TH Benzene 0.0014
10321 1245TH Bz 0.0000
10360 Naphthalene 0.0000
10370 M-Indane 0.0000
10398 Other C 10 Aromatic- 0.0163
Table 1. Composition of Feed composition
[00721 The feed was passed over a MCM-49 catalyst containing a 80/20
zeolite:binder ratio and 1/20" quadrulube in a fixed bed reactor about 1" in
diameter. 177g/hr of feed was passed over 63g of the catalyst at around 400 F
and 600 psig.
[00731 The resulting product was analyzed by gas chromatography ("GC").
The conversion of the feed is shown in Figure 4 and Figure 8. The weight
CA 02782929 2012-06-04
WO 2011/075523 PCT/US2010/060491
-23-
percentage of the feed and product, as analyzed by GC is shown in Figure 2.
The majority of the product was Cio+, shown in Fig. 5.
[00741 An ASTM D86 analysis of feed and typical product is shown in
Figures 6 and 7. ASTM D86 is a standard test method known to those skilled in
the art. There, the movement in MW of the feed from the mogas boiling range
to the distillate boiling range can be seen. The y-axis represents the boiling
point in degrees F and the x-axis represents the liquid volume % off the
sample
at each corresponding boiling point temperature.
[00751 The present invention is not to be limited in scope by the specific
embodiments described herein. Indeed, various modifications of the invention
in
addition to those described herein will become apparent to those skilled in
the art
from the foregoing description and the accompanying figures. Such
modifications are intended to fall within the scope of the appended claims.
[00761 It is further to be understood that all values are approximate, and are
provided for description.
[00771 Patents, patent applications, publications, product descriptions, and
protocols are cited throughout this application, the disclosures of each of
which
is incorporated herein by reference in its entirety for all purposes.