Note: Descriptions are shown in the official language in which they were submitted.
CA 02783190 2012-07-06
1
PROCESS AND APPARATUS FOR THE TREATMENT OF CO2-CONTAINING GAS
USING CARBONIC ANHYDRASE
FIELD OF THE INVENTION
The present invention relates generally to processes for the treatment of gas
effluents
with a view to cleaning or purifying such effluents. More particularly, it
relates to a
process and an apparatus using a spray absorber for the biocatalytic treatment
of gases.
BACKGROUND OF THE INVENTION
Contemporary industrial activities generate gaseous effluents containing a
multitude of
chemical compounds and contaminants which interfere with the equilibrium of
elements
in nature and affect the environment at different levels. Acid rain, the
greenhouse effect,
smog and the deterioration of the ozone layer are examples that speak volumes
about
this problem. Reduction of noxious emissions is therefore not surprisingly the
subject of
more and more legislation and regulation. Industrial activities and
applications which
must contend with stricter environmental regulatory standards in order to
expect any
long-term commercial viability will turn more and more to biological and
environmentally
safe methods. Consequently, there is a real need for new apparatuses and
methods
aimed at the treatment of gaseous waste or effluents.
There already exists a vast array of technologies aimed at the separation and
recovery
of individual or mixed gases and a number of different biological methods is
known to
treat gaseous waste or effluents: bacterial degradation (JP 2000-287679; JP
2000-
236870), fermentation by anaerobic bacteria (WO 98/00558), photosynthesis
through
either plants (CA 2,029,101 A1; JP 04-190782) or microorganisms (JP 03-
216180).
Among the more popular are those gained through the harnessing of biological
processes such as peat biofilters sprinkled with a flora of microorganisms in
an aqueous
phase, or biofilter columns comprising immobilized resident microorganisms
(Deshusses
et al. (1996) Biotechnol. Bioeng. 49, 587-598). Although such biofilters have
contributed
to technological advances within the field of
_________________________________
CA 02783190 2012-07-06
2
gaseous waste biopurification, the main drawbacks associated with their use
are their
difficult maintenance and upkeep, lack of versatility, as well as time
consuming bacterial
acclimation and response to perturbation periods (Deshusses et al.).
A number of biological sanitation/purification methods and products is known
to use
enzymatic processes, coupled or not to filtration membranes (US 5,250,305;
US 4,033,822; JP 63-129987). However, these are neither intended nor adequate
for the
cleansing of gaseous waste or effluents. The main reason for this is that, in
such
systems, contaminants are generally already in solution (US 5,130,237; US
4,033,822;
US 4,758,417; US 5,250,305; WO 97/19196; JP 63-129987). Efficient enzymatic
conversion and treatability itself of gaseous waste or effluents in liquids
therefore depend
on adequate and sufficient dissolution of the gaseous phase in the liquid
phase.
However, the adequate dissolution of gaseous waste or effluents into liquids
for
enzymatic conversion poses a real problem which constitutes the first of a
series of
important limitations which compound the problem of further technological
advances in
the field of gas biopurification.
Although triphasic Gas-Liquid-Solid (GLS) reactors are commonly used in a
large
variety of industrial applications, their utilization remains quite limited in
the area of
biochemical gas treatment (US 6,245,304; US 4,743,545). Also known in the
prior art are
the GLS bioprocesses abundantly reported in the literature. A majority of
these concerns
wastewater treatment (JP 09057289). These GLS processes are characterized in
that
the gaseous intake serves the sole purpose of satisfying the specific metabdic
requirements of the particular organism selected for the wastewater treatment
process.
Such GLS treatment processes are therefore not aimed at reducing gaseous
emissions.
As previously mentioned, these systems are neither intended nor adequate for
the
treatment of gaseous waste or effluents. An additional problem associated with
the use
of these systems is the non retention of the solid phase within the reactor.
Biocatalysts
are in fact washed right out of the reactors along with the liquid phase.
Different
concepts are, nonetheless, based on this principle for the reduction of
gaseous
emissions, namely carbon dioxide. Certain bioreactors allow the uptake of CO2
by
photosynthetic organisms (JP 03-216180) and similar processes bind CO2 through
algae
CA 02783190 2012-07-06
3
(CA 2,232,707; JP 08-116965; JP 04-190782; JP 04-075537). However, the
biocatalyst
retention problem remains largely unaddressed and constitutes another serious
limitation, along with gaseous effluent dissolution, to further technological
advancements.
The main argument against the use of ultrafiltration membranes to solve this
biocatalyst
retention problem is their propensity to clogging. Clogging renders them
unattractive and
so, their use is rather limited for the retention of catalysts within
reactors. However, a
photobioreactor for medical applications as an artificial lung (WO 92/00380;
US 5,614,378) and an oxygen recovery system (US 4,602,987; US 4,761,209) are
notable exceptions making use of carbonic anhydrase and an ultrafiltration
unit.
The patent applications held by the assignee, CO2 Solution Inc., via Les
Systemes
Envirobio Inc. (EP 0 991 462; WO 98/55210; CA 2,291,785) propose a packed
column
for the treatment of carbon dioxide using immobilized carbonic anhydrase.
Carbonic
anhydrase is a readily available and highly reactive enzyme that is used in
other systems
for the reduction of carbon dioxide emissions (US 4,602,987; US 4,743,545;
US 5,614,378; US 6,257,335). In the system described by Trachtenberg for the
carbonic
anhydrase treatment of gaseous effluents (US 6,143,556; CA 2,222,030),
biocatalyst
retention occurs through a porous wall or through enzyme immobilization.
However,
important drawbacks are associated with the use of enzyme immobilization, as
will be
discussed below.
Other major drawbacks are associated with the use of enzymatic systems. One of
these
stems from systems where enzymatic activity is specifically and locally
concentrated.
This is the case with systems where enzymes are immobilized at a particular
site or on a
specific part of an apparatus. Examples in point of such systems are those
where
enzymes are immobilized on a filtration membrane (JP 60014900008A2; US
4,033,822;
US 5,130,237; US 5,250,305; JP 54-132291; JP 63-129987; JP 02-109986;
DE 3,937,892) or even, at a gas-liquid phase boundary (WO 96/40414; US
6,143,556).
The limited surface contact area obtainable between the dissolved gas
substrate, the
liquid and the enzyme active site poses an important problem. Hence, these
systems
generate significantly greater waste of input material, such as expensive
purified
CA 02783190 2012-07-06
,
4
enzymes, because the contact surface with the gaseous phase is far from
optimal and
limits productive reaction rates. Therefore, as mentioned previously,
overcoming the
contact surface area difficulty should yield further technological advances.
Other examples of prior art apparatuses or methods for the treatment of gas or
liquid
effluents are given in the following documents: CA 2,160,311; CA 2,238,323;
CA 2,259,492; CA 2,268,641; JP 2000-236870; JP 2000-287679; JP 2000-202239;
US 4,758,417; US 5,593,886; US 5,807,722; US 6,136,577; and US 6,245,304.
SUMMARY OF THE INVENTION
An object of the present invention is to provide an apparatus that is distinct
from and
overcomes several disadvantages of the prior art bioreactor for the treatment
of gas
effluent, as will be discussed in detail below.
Another object is to provide a process for the biocatalytic treatment of
gases, which is
more efficient with respect to the reaction rate of the reactants.
In accordance with the present invention, that object is achieved with a
process
characterized in that it comprises a step of contacting a gas phase,
containing a
particular gas to be treated, to spray droplets of liquid phase containing
biocatalysts.
More particularly, the present invention proposes a process for the
biocatalytic treatment
of gases, comprising the steps of:
a) contacting a gas phase, containing a gas to be treated, to a liquid phase
containing a
reactant capable of absorbing and/or chemically reacting with the gas, thereby
producing
a spent liquid phase containing at least one reaction product and a treated
gas phase
substantially free of the gas, such step being performed in the presence of
biocatalysts
suitable for catalyzing the chemical reaction between the gas and the
reactant, the
process being characterized in that:
- it comprises, prior to step a) of contacting, the step of mixing the
biocatalysts to the
liquid phase; and
CA 02783190 2012-07-06
- the step of contacting comprises the step of spraying droplets of the liquid
phase
containing the biocatalysts.
The liquid phase may be aqueous or non aqueous and the gas to be treated is
usually
soluble in the liquid. Contact between the gas and liquid phases results in
the absorption
5 of the gas to be treated and thus to its extraction from the gas phase.
The use of droplets of liquid containing the biocatalysts, sprayed into the
reaction
chamber, enables to increase the gas-liquid interface, thereby allowing a high
mass
transfer rate of the gas to be treated from the gas phase to the liquid phase.
These
conditions of high mass transfer enable to transform the gas with a maximum
reaction
rate.
Then, the dissolved gas is transformed in presence of appropriate biocatalysts
into one
or more products. The role of biocatalysts is to accelerate the transformation
reaction of
the dissolved gas in the liquid phase environment. In addition to
biocatalysts, the liquid
phase may contain reactants required for the transformation of the dissolved
gas or for a
reaction with one or more reaction products of the dissolved gas. Depending on
the
reactants and biocatalysts present in the liquid phase, products are in a
soluble or solid
form. Reaction products are preferably further treated to give useful products
to be used
in other applications or to be disposed of.
Absorption and biocatalytic transformation of the gas take place in the
reaction chamber
of a spray absorber bioreactor. The gas phase, containing the selected gas to
be
treated, is fed into the reaction chamber where it is contacted to spray
droplets of a liquid
phase containing the biocatalysts. Droplets are obtained using atomizers. The
gas phase
in contact to the liquid phase has one or more components absorbed in the
liquid phase.
Then, the dissolved gas is transformed because of biocatalysts activity and
presence of
reactants, if required. The gas phase is almost free of one or more components
and
exits the reaction chamber purified. The liquid phase containing the
biocatalysts and
reaction products (dissolved and/or solid) exits from the reaction chamber. In
accordance with a preferred aspect of the invention, the gas is further
treated to remove
droplets in suspension or to decrease its humidity.
CA 02783190 2012-07-06
6
Hence, the present invention is also directed to a biocatalytic treatment unit
for the
biocatalytic treatment of gases, comprising:
- a bioreactor comprising a reaction chamber having a liquid inlet for
receiving a
liquid, a gas inlet for receiving a gas to be treated, a liquid outlet for
discharging a spent
liquid and a gas outlet for releasing a treated gas;
the treatment unit being characterized in that it comprises;
a mixing unit for mixing biocatalysts to the liquid;
means for conveying the liquid from the mixing unit to the liquid inlet of the
reaction
chamber; and
the liquid inlet comprises at least one atomizer mounted within the reaction
chamber to
spray droplets of liquid containing the biocatalyst.
Biocatalysts are usually very costly. Therefore, it is preferable to recycle
the spent liquid
phase containing biocatalysts and reactants. However, recycling requires that
reaction
products be removed from the liquid phase. Therefore, in accordance with a
preferred
aspect of the invention, the process further comprises the steps of:
b) removing from the spent liquid phase the at least one reaction product,
thereby
obtaining a recycled liquid phase free of the reaction product and containing
the
biocatalysts; and
c) recycling the recycled liquid phase to step a) of contacting.
In accordance with that aspect of the invention, the treatment unit further
comprises a
separation unit in fluid communication with the liquid outlet for removing the
reaction
products contained in the spent liquid. The separation unit has a first liquid
outlet for
discharging a liquid fraction substantially free of the reaction products and
a second
outlet for discharging the liquid fraction containing the reaction products.
Conveying
means are provided for conveying the fraction substantially free of the
reaction products
to the mixing unit.
CA 02783190 2012-07-06
7
Removal assures that a maximum mass transfer rate of the gas from the gas
phase to
the liquid phase is achieved at each pass of the liquid phase in the reaction
chamber of
the spray absorber bioreactor. Removal of dissolved reaction products is
preferably
obtained using membrane processes such as ultrafiltration, microfiltration
processes
and/or ion exchange and/or adsorption processes. Removal may also be obtained
by
first precipitating the reaction products with appropriate reactant (s) and
then by
removing the particles using separation processes. Solid particles originating
from solid
reaction products or subsequently precipitated products are preferably removed
by using
separation processes such as settling, filtration or expression. Moreover,
agents
facilitating removal of particles, such as coagulants or flocculants or filter
aids, may be
added to the liquid effluent prior to particle removal units or to the liquid
phase entering
the spray absorber bioreactor.
A biocatalyst is a biological entity, which can transform a substrate in one
or more
products. The biocatalysts used in the process are preferably enzymes,
cellular
organelles (mitochondrion, membranes), animal, vegetal or human cells. The
biocatalysts can be used free or immobilized. Immobilization is preferably the
result of
fixation to a solid support, entrapment inside a solid matrix. Immobilization
can also be
obtained by using intermolecular binding of biocatalysts molecules or
structures. In all
these cases, the solid support, the solid matrix and the intermolecular
binding must be in
the form of micro particles sufficiently small to pass through the atomizer
with the liquid
phase.
Depending on patterns of spray, droplet size, uniformity of spray, turndown
ratio and/or
power consumption, available atomizers may fall in three categories: pressure
nozzles,
two-fluid nozzles or rotary devices. However, any other type of atomizer may
be used.
Membrane processes for removal of dissolved products or solid particles may
include
the use of flat or tubular membranes. Those membranes are preferably involved
in
CA 02783190 2012-07-06
,
8
different modules such as plate-and-frame, spiral-wound, tubular capillary and
hollow
fiber module. The operation of those modules may be dead-end or cross-flow (co-
current, countercurrent, cross-flow with perfect permeate mixing and perfect
mixing).
Those filtration units may be used in a single-stage or in a multi-stage
process in a
single-pass system or a recirculation system.
The present invention also provides a process for the biocatalytic treatment
of a CO2-
containing gas, comprising the steps of:
a) contacting the CO2-containing gas phase, containing CO2 gas to be treated,
to
a liquid phase containing carbonic anhydrase capable of catalyzing the
hydration
reaction of CO2 into bicarbonate and hydrogen ions, thereby producing a spent
liquid phase containing the bicarbonate and hydrogen ions and a treated gas
phase substantially free of the CO2, the process being characterized in that:
it comprises, prior to step a) of contacting, the step of mixing the carbonic
anhydrase to the liquid phase; and
the step of contacting comprises the step of spraying droplets of the liquid
phase
containing the carbonic anhydrase.
The present invention also provides a carbonic anhydrase treatment unit for
the
treatment of CO2-containing gas, comprising:
a bioreactor comprising a reaction chamber having a liquid inlet for receiving
a
liquid, a gas inlet for receiving the CO2-containing gas to be treated, a
liquid
outlet for discharging a spent liquid and a gas outlet for releasing a treated
gas;
the treatment unit being characterized in that it comprises;
a mixing unit for mixing carbonic anhydrase to said liquid;
means for conveying the liquid from the mixing unit to the liquid inlet of the
reaction chamber; and
the liquid inlet comprises at least one atomizer mounted within the reaction
chamber to spray droplets of liquid containing the carbonic anhydrase.
CA 02783190 2012-07-06
8a
The present invention also provides a process for enzymatic treatment of a CO2-
containing gas, comprising:
mixing enzymatic particles comprising carbonic anhydrase with a liquid
solution to
form a sprayable mixture;
spraying the sprayable mixture to form droplets thereof comprising the
enzymatic
particles;
contacting the CO2-containing gas with the droplets such that the carbonic
anhydrase catalyzes the hydration reaction of CO2 into bicarbonate and
hydrogen
ions, thereby producing a spent liquid phase containing the bicarbonate and
hydrogen ions and a treated gas phase substantially free of the CO2.
The present invention also provides a process for treatment of a fluid by
enzymatic
catalysis of reaction (I) with carbonic anhydrase, wherein the reaction (I) is
as follows:
CO2 +H20 erbonic anhydral HCO+H+ (I)
the process comprising:
feeding the fluid into a reaction zone in the presence of enzymatic particles
comprising carbonic anhydrase, the enzymatic particles being sized to be mixed
and sprayed with a liquid solution thereby forming droplets comprising the
enzymatic particles;
allowing the reaction (I) to occur within the droplets in the reaction zone,
to
produce a gas stream and a liquid stream; and
releasing the gas stream and the liquid stream from the reaction zone. In one
embodiment, the process as described above, wherein the fluid is a CO2-
containing gas; the process comprises spraying the droplets comprising the
enzymatic particles into the reaction zone to contact the CO2-containg gas so
as
CA 02783190 2012-07-06
8b
to dissolve CO2 from the CO2-containing effluent gas into the droplets; the
reaction (l) is a forward reaction catalyzing the hydration of dissolved CO2
into the
bicarbonate and hydrogen ions; and the gas stream is a treated gas phase
substantially free of the CO2 and the liquid stream is a spent liquid phase
containing the bicarbonate and hydrogen ions.
The present invention further provides a carbonic anhydrase treatment unit for
the
treatment of CO2-containing gas, comprising:
a bioreactor comprising a reaction chamber having a liquid inlet for receiving
a
liquid, a gas inlet for receiving the CO2-containing gas to be treated, a
liquid
outlet for discharging a spent liquid and a gas outlet for releasing a treated
gas;
a mixing unit for mixing enzymatic particles comprising carbonic anhydrase
with
the liquid to form a sprayable mixture;
means for conveying the sprayable mixture from the mixing unit to the liquid
inlet
of the reaction chamber; and
the liquid inlet comprises at least one atomizer mounted within the reaction
chamber to spray droplets of sprayable mixture containing the enzymatic
particles
into the reaction chamber.
The present invention further provides a carbonic anhydrase treatment unit for
the
treatment of a fluid by enzymatic catalysis of reaction (l) with carbonic
anhydrase,
wherein the reaction (l) is as follows:
CO2 +H20 tlrh Inc anhYdral HCO3 +H (l)
the treatment unit comprising:
CA 02783190 2014-02-12
'
8c
a bioreactor comprising a reaction chamber for accommodating the reaction (I),
a
liquid inlet, a liquid outlet for discharging a liquid stream and a gas outlet
for
releasing a gas stream;
a mixing unit for mixing enzymatic particles comprising carbonic anhydrase
with a
liquid solution thereby forming a sprayable mixture;
means for conveying the sprayable mixture from the mixing unit to a liquid
inlet of
the reaction chamber; and
the liquid inlet comprises at least one atomizer mounted within the reaction
chamber to
spray droplets of the sprayable mixture containing the enzymatic particles
into the
reaction chamber such that the reaction (I) to occur within the droplets. In
one
embodiment the carbonic anhydrase treatment unit as described above, wherein
the
fluid is a CO2-containing gas; the bioreactor comprises a gas inlet for
feeding the CO2-
containing gas into the reaction chamber; the droplets comprising the
enzymatic
particles; the reaction (I) is a forward reaction catalyzing the hydration of
dissolved CO2
into the bicarbonate and hydrogen ions; and the gas is a treated gas phase
substantially
free of the CO2 and the liquid stream is a spent liquid phase containing the
bicarbonate
and hydrogen ions.
The present invention also provides a carbonic anhydrase treatment unit
comprising a
reaction chamber having a liquid inlet for receiving a liquid containing micro-
particles
comprising carbonic anhydrase, a gas inlet for receiving a CO2-containing gas
to be
treated, a liquid outlet for discharging a spent liquid and a gas outlet for
releasing a
treated gas, the liquid inlet being sized and configured to feed the liquid
and micro-
particles into the reaction chamber.
CA 02783190 2014-09-18
8d
The present invention also provides a process for enzymatic treatment of a CO2-
containing gas, comprising:
a process for enzymatic treatment of a CO2-containing gas, comprising:
spraying a mixture comprising:
an aqueous liquid solution comprising 2-amino-2-hydroxymethy1-1,3-
propanediol; and
carbonic anhydrase;
in order to form droplets of the mixture; and
contacting the CO2-containing gas with the droplets of the mixture such that
the carbonic anhydrase catalyzes the hydration reaction of CO2 into
bicarbonate and hydrogen ions, thereby producing a liquid phase containing
the bicarbonate and hydrogen ions and a treated CO2 depleted gas phase.
The present invention also provides a process for enzymatic treatment of a CO2-
containing gas, comprising:
a process for enzymatic treatment of a CO2-containing gas, comprising:
spraying a mixture comprising:
an aqueous liquid solution comprising 2-amino-2-hydroxymethy1-1,3-
propanediol; and
carbonic anhydrase;
into a reaction chamber;
contacting the CO2-containing gas with the mixture within the reaction
chamber in presence of the carbonic anhydrase that catalyzes the hydration
reaction of CO2 into bicarbonate and hydrogen ions, thereby producing a
CA 02783190 2014-09-18
8e
liquid phase containing the bicarbonate and hydrogen ions and a treated CO2
depleted gas phase comprising mist of the aqueous liquid solution; and
removing the mist from the CO2 depleted gas phase to produce a mist
depleted gas.
The present invention also provides a process for enzymatic treatment of a CO2-
containing gas, comprising:
a process for enzymatic treatment of a CO2-containing gas, comprising:
spraying a mixture comprising:
an aqueous liquid solution comprising 2-amino-2-hydroxymethy1-1,3-
propanediol; and
carbonic anhydrase;
into a reaction chamber;
contacting the CO2-containing gas with the mixture in presence of the
carbonic anhydrase, the carbonic anhydrase catalyzing the hydration reaction
of CO2 into bicarbonate and hydrogen ions, thereby producing a loaded liquid
phase containing the bicarbonate and hydrogen ions and a treated CO2
depleted gas phase;
releasing the loaded liquid phase from the reaction chamber;
precipitating solid particles in the loaded liquid phase;
removing the precipitated solid particles from the loaded liquid phase to
produce a precipitate removed liquid; and
recycling the precipitate removed liquid back into the reaction chamber as the
aqueous liquid solution.
CA 02783190 2014-09-18
8f
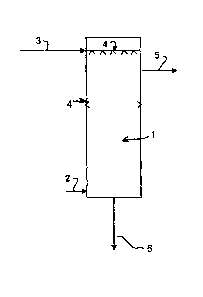
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic representation of a spray absorber bioreactor
according to a
preferred embodiment of the present invention.
Figure 2 is a schematic flow chart of a second preferred embodiment of the
process
according to the present invention.
Figures 3a and 3b are schematic flow charts of a third preferred embodiment of
the
process according to the present invention.
DESCRIPTION OF PREFERRED EMBODIMENTS
Referring to figure 1, a gas phase containing CO2 is fed to a reaction chamber
(1) of a
spray absorber bioreactor. The gas phase is preferably fed at the bottom (2)
of the
reaction chamber (1) of the spray absorber bioreactor. The aqueous liquid
phase
containing biocatalysts is preferably fed at the top of the reaction chamber
(3) through
atomizers (4) where the liquid phase forms droplets. Additional atomizers may
be
found at the side (4) of the reaction chamber. In this preferred embodiment,
the gas
phase flows upward and contact spray droplets of the liquid phase. During the
contact, CO2 is absorbed and then transformed into bicarbonate and hydrogen
ions.
This transformation is catalyzed by a biocatalyst accelerating CO2
transformation. The
biocatalyst is preferably the enzyme carbonic anhydrase but may be any
biological
catalyst enabling CO2 transformation. CO2 transformation reaction is the
following:
CO2 +1120 karborne anhydra, Hco H+ Equation 1
CA 02783190 2012-07-06
9
This reaction is natural. It is at the basis of CO2 transportation and removal
phenomenon
in the human body and in most living beings. Reaction products are ions in
solution in
the aqueous liquid phase (6). The treated gas phase (5) exits at the top of
the spray
absorber bioreactor and is almost free of CO2. However, droplets of the liquid
phase may
remain in suspension in the purified gas exiting the bioreactor (5). An
additional
equipment, such as a mist eliminator, is thus preferably added to the spray
absorber
bioreactor to remove those droplets. Both gas and liquid phases are preferably
further
treated for proper disposal or reuse.
It is well known that under specific conditions, bicarbonate and/or carbonate
ions may
react with some cations (Na, Ca2+, Mg2+, Ba2+) to precipitate. For example, if
the liquid
phase contains calcium ions and the pH is around 9,0, bicarbonate and
carbonate ions
are present because of natural physicochemical equilibrium reactions. These
carbonate
ions may react with calcium ions (see Eq. 2 below), for example, by adding
Ca(OH)2 to
the solution, thus leading to the formation of solid particles of calcium
carbonate in the
liquid phase. Consequently, bicarbonate ions transform into carbonate ions,
because of
equilibrium between both species. Those newly formed carbonate ions will then
lead to
the formation of calcium carbonate. In this case, an agitation device is
preferably
required to facilitate discharge of solid particles. Moreover, the bottom of
the bioreactor
preferably has a conical shape.
C032- + Ca2+= CaCO3 Equation 2
Biocatalysts are usually costly material. Therefore, it is interesting to
recycle the liquid
phase containing the biocatalysts. Figure 2 shows a process implying steps
described
previously in figure 1. However, for the recycling of the liquid phase,
additional
considerations have to be made. CO2 removal process is based on absorption,
thus
removal of the reaction products (HCO3- and H+) is required in order to
maintain optimal
conditions for CO2 mass transfer. In this case, bicarbonate and hydrogen ions
are
soluble. Bicarbonate ions are preferably removed in a product removal process
(11) such
CA 02783190 2012-07-06
as membrane separation processes (ultrafiltration, nanofiltration), ion
exchange or
adsorption units separately or in combination.
Referring now to figure 2, the liquid phase containing biocatalysts and rich
in reaction
products (8) is fed to a product removal process (11). Two effluents (12,10)
are
5 generated: one (12) rich in reaction product, bicarbonate ions and one
(10) containing a
very low level of bicarbonate ions. This latter liquid phase is preferably
enriched in fresh
liquid phase (9) or biocatalysts to compensate for possible loss of those two
components. Acid or alkali may also be added to the liquid phase in order to
control pH
of the liquid phase. pH control may also be done inside the product removal
process. In
10 the present application, alkali such as NaOH might be added to the liquid
phase to
neutralize protons produced during the biological transformation of CO2
(Equation 1).
The resultant liquid phase (7) enters at the top of the bioreactor. The
effluent (12) is
preferably further treated for proper disposal or use in another process.
Turning now to figures 3a and 3b, when solid particles are present in the
liquid phase
(14) because of a precipitation reaction (Eq. 2), solid particles have to be
removed for
recycling of the liquid phase. The particles removal process (15) may consist
of one or
more separation units, as shown in figure 3a. Separation is preferably
performed by
settling (by gravity, centrifugal force, heavy media, flotation, magnetic
force) and/or
filtration (on screens or on filters (by gravity, pressure, vacuum or
centrifugal force))
and/or pression (batch presses or continuous presses (screw presses, rolls or
belt
presses)). Moreover, agents facilitating removal of particles such as
coagulants or
flocculants or filter aids may be added to the liquid effluent (16) prior to
or in the particle
removal process or to the liquid phase entering the spray absorber bioreactor
(13).The
liquid phase (18) exiting the particles removal process is preferably
supplemented in
fresh liquid phase and/or biocatalysts (9). Moreover, acid or alkali may also
be added to
liquid phase for pH control. The liquid phase (13) is then fed to the
bioreactor. Solid
particles (17) obtained may further be treated or be disposed of.
CA 02783190 2012-07-06
11
The separation unit may, in a particular case, be integrated to the
bioreactor. Figure 3b
shows a process where separation by gravity is integrated to the bioreactor.
In this
particular case, an agitation device is preferably added at the bottom of the
bioreactor
and the bottom of the bioreactor preferably has a conical shape.
Example
An experiment was conducted to validate the concept of a spray absorber for
removal of
CO2. The process diagram of the spray absorber for the test was similar to the
one
shown in figure 1. The spray absorber consists of a column having a 7,5 cm
diameter
and a 70 cm height. A pressure nozzle atomizer was mounted within the reaction
chamber. Five litres of a 12 mM Tris solution with 20 mg carbonic anhydrase
per litre of
solution were pumped into the spray absorber at a flow rate of 1,5 l/min.
Carbonic
anhydrase was used free within the Tris Solution. The Tris solution is a
buffer consisting
of 2-amino-2-hydroxymethy1-1,3-propanediol. The solution was used in a closed
loop
operation, until the Tris solution was saturated with dissolved CO2.The gas
flow rate was
6,0 g/min at a CO2 concentration of 52000 ppm. Gas and solution were at room
temperature. The pressure inside the spray absorber was set at 5 psig.
The results obtained showed that the CO2 contained in the gas phase was
removed at a
rate of 2,3 x 10-3 mol of CO2 /min.
These results were compared with the ones obtained from experiments conducted
with a
conventional bioreactor using a reaction chamber filled with carbonic
anhydrase
immobilized on rashig supports. The following table provides a comparison of
these
results.
CA 02783190 2012-07-06
,
12
Parameters Spray absorber Prior art
packed
according to the bioreactor
invention
Concentration of CO2 in 52000 pm 50000 ppm
the gas phase
Liquid flow rate 1,5 l/min 0,5 l/min
Biocatalyst Carbonic anhydrase free Carbonic
anhydrase
in liquid immobilized in
the
bioreactor
Gas flow rate 6,0 g/min 1,5 g/min
Ratio of liquid flow rate to 0,25 1/g 0,5 1/g
gas flow rate
Mass of enzyme within the Less than 100 mg* 275 mg
reactor
Removal rate of CO2 2,3 x iO3 mol of CO2 /min 1,3 x 10-3 mol of CO2
/min
* Five liters of Tris solution contains 100 mg. However, for the absoption of
CO2, only a
portion of the enzyme ends in the reaction chamber.
These results show that the process according to the invention is surprisingly
more
efficient than the process known in the prior art, since 1) at all times, the
enzyme
participating in the removal of CO2 is less, therefore the removal of CO2
requires less
enzyme, and 2) even though the ratio of liquid flow rate to gas flow rate is
lower with the
process according to the invention, the removal rate of CO2 is greater.
Indeed, it is well
known for a person skilled in the art that in a process for absorbing a gas,
if that ratio
decreases, the performance of the process also decreases. In other words, the
performance of the packed bioreactor would have been even less if the ratio of
liquid
flow rate to gas flow rate used had been 0,25 1/g, as for the experiments with
the spray
absorber of the invention.
CA 02783190 2012-07-06
13
Although the present invention has been explained hereinabove by way of
preferred
embodiments thereof, it should be understood that the invention is not limited
to these
precise embodiments and that various changes and modifications may be effected
therein without departing from the scope or spirit of the invention.